User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
PAP sensor may cut real-world heart failure hospitalization
Implantation of a pulmonary artery pressure sensor to guide care in chronic heart failure was associated with a significant 45% reduction in HF hospitalization and its attendant substantial costs in a real-world patient population, Akshay S. Desai, MD, said at the annual meeting of the American College of Cardiology.
The PAP sensor is used to monitor pulmonary artery filling pressure, which rises in many HF patients during the weeks preceding an HF exacerbation. This early detection of progressing congestion allows clinicians to intervene earlier and head off hospitalization for the exacerbation.
Their intention was to determine whether the positive results of the CHAMPION clinical trial, which prompted FDA approval of the device as a means to reduce HF-associated hospitalizations, could be replicated in a real-world population, said Dr. Desai of Brigham and Women’s Hospital, Boston.
The results of their study were presented March 19 at the annual meeting of the American College of Cardiology and simultaneously published online in the Journal of the American College of Cardiology (2017 Mar 19. doi: 10.1016/j.jacc.2017.03.009).
The mean age of the study cohort was 71 years, and 40% of the participants were at least 75 years of age. Women composed 40% of the cohort. There was a high burden of comorbid illness, including diabetes, hypertension, and chronic obstructive pulmonary disease. This represents a broader sample than was enrolled in the CHAMPION trial, he noted.
There were 1,020 HF hospitalizations before implantation and 381 afterward. A total of 59% of patients had at least one HF hospitalization before the PAP implantation, compared with 22% afterward. The median number of HF hospitalizations was 0.92 per patient before implantation and 0.37 per patient afterward.
Further analysis showed that the cumulative rate of HF hospitalization was 45% lower during the 6 months after implantation than during the 6 months preceding it (hazard ratio, 0.55). This finding remained robust across several subgroups of patients and in several sensitivity analyses.
These reductions were associated with a corresponding decline in costs related to HF care, which dropped by $7,433 per patient.
In addition to HF-related hospitalizations, all-cause hospitalizations also declined by roughly 30% after implantation of a PAP sensor (HR, 0.69).
These findings suggest that the reduction in hospitalizations, along with attendant reductions in the costs of care, may be achievable in real-world practice. The 45% drop in HF hospitalizations in this study “compares favorably with the 28% reduction seen with PAP-guided therapy over the same time period in the randomized CHAMPION study that supported the initial FDA approval,” Dr. Desai said.
Moreover, a subgroup of 480 patients had data for 12 months preceding and 12 months following implantation. Analysis of those data showed that the benefits of PAP monitoring to guide HF care “were consistent over longer-term follow-up, with a 34% reduction in HF hospitalizations sustained at 12 months,” he added.
The study had several limitations. It excluded Medicare Part D data, so medication changes related to implantation could not be examined and may have exerted substantial influence on study outcomes.
It also didn’t include the actual PAP-sensor data, “which makes it challenging to confirm that physicians intervened to treat elevated PAPs” and that that intervention is the reason for the study outcomes.
“We were unable to definitively ascertain whether reduced HF hospitalizations are related to undertreatment in the preimplant period or improved treatment in the postimplant period,” Dr. Desai said.
The study was sponsored by Abbott, maker of the CardioMEMS PAP sensor. Dr. Desai and his associates reported ties to Abbott and St. Jude Medical.
Real-world evidence about the PAP sensor is particularly important, because the FDA approved the device amid considerable controversy over the CHAMPION trial and its sponsor. Employees of the sponsor were considered to have contaminated the initial data, and the FDA initially rejected the device. Substantial uncertainty still persists concerning the sensor’s effectiveness in reducing HF hospitalizations.
In addition, the percentage of HF patients who have received the device is extremely small, given that the study and the highly selected patient sample make both causal inferences and generalizability of the findings difficult.
The net effect of these important limitations is that the study by Desai et al. lacks the strength to change prior assumptions about the PAP sensor’s benefits in any direction. Unfortunately, this study doesn’t have the evidentiary robustness to inform clinical decisions.
Harlan M. Krumholz, MD, and Sanket S. Dhruva, MD, are both in the Robert Wood Johnson Foundation Clinical Scholars Program and at the Yale School of Medicine and Yale School of Public Health in New Haven, Conn. Dr. Krumholz reported ties to Johnson & Johnson and Medtronic; Dr. Dhruva reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the study (J Am Coll Cardiol. 2017 Mar 19. doi:10.1016/j.jacc.2017.03.019).
Real-world evidence about the PAP sensor is particularly important, because the FDA approved the device amid considerable controversy over the CHAMPION trial and its sponsor. Employees of the sponsor were considered to have contaminated the initial data, and the FDA initially rejected the device. Substantial uncertainty still persists concerning the sensor’s effectiveness in reducing HF hospitalizations.
In addition, the percentage of HF patients who have received the device is extremely small, given that the study and the highly selected patient sample make both causal inferences and generalizability of the findings difficult.
The net effect of these important limitations is that the study by Desai et al. lacks the strength to change prior assumptions about the PAP sensor’s benefits in any direction. Unfortunately, this study doesn’t have the evidentiary robustness to inform clinical decisions.
Harlan M. Krumholz, MD, and Sanket S. Dhruva, MD, are both in the Robert Wood Johnson Foundation Clinical Scholars Program and at the Yale School of Medicine and Yale School of Public Health in New Haven, Conn. Dr. Krumholz reported ties to Johnson & Johnson and Medtronic; Dr. Dhruva reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the study (J Am Coll Cardiol. 2017 Mar 19. doi:10.1016/j.jacc.2017.03.019).
Real-world evidence about the PAP sensor is particularly important, because the FDA approved the device amid considerable controversy over the CHAMPION trial and its sponsor. Employees of the sponsor were considered to have contaminated the initial data, and the FDA initially rejected the device. Substantial uncertainty still persists concerning the sensor’s effectiveness in reducing HF hospitalizations.
In addition, the percentage of HF patients who have received the device is extremely small, given that the study and the highly selected patient sample make both causal inferences and generalizability of the findings difficult.
The net effect of these important limitations is that the study by Desai et al. lacks the strength to change prior assumptions about the PAP sensor’s benefits in any direction. Unfortunately, this study doesn’t have the evidentiary robustness to inform clinical decisions.
Harlan M. Krumholz, MD, and Sanket S. Dhruva, MD, are both in the Robert Wood Johnson Foundation Clinical Scholars Program and at the Yale School of Medicine and Yale School of Public Health in New Haven, Conn. Dr. Krumholz reported ties to Johnson & Johnson and Medtronic; Dr. Dhruva reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the study (J Am Coll Cardiol. 2017 Mar 19. doi:10.1016/j.jacc.2017.03.019).
Implantation of a pulmonary artery pressure sensor to guide care in chronic heart failure was associated with a significant 45% reduction in HF hospitalization and its attendant substantial costs in a real-world patient population, Akshay S. Desai, MD, said at the annual meeting of the American College of Cardiology.
The PAP sensor is used to monitor pulmonary artery filling pressure, which rises in many HF patients during the weeks preceding an HF exacerbation. This early detection of progressing congestion allows clinicians to intervene earlier and head off hospitalization for the exacerbation.
Their intention was to determine whether the positive results of the CHAMPION clinical trial, which prompted FDA approval of the device as a means to reduce HF-associated hospitalizations, could be replicated in a real-world population, said Dr. Desai of Brigham and Women’s Hospital, Boston.
The results of their study were presented March 19 at the annual meeting of the American College of Cardiology and simultaneously published online in the Journal of the American College of Cardiology (2017 Mar 19. doi: 10.1016/j.jacc.2017.03.009).
The mean age of the study cohort was 71 years, and 40% of the participants were at least 75 years of age. Women composed 40% of the cohort. There was a high burden of comorbid illness, including diabetes, hypertension, and chronic obstructive pulmonary disease. This represents a broader sample than was enrolled in the CHAMPION trial, he noted.
There were 1,020 HF hospitalizations before implantation and 381 afterward. A total of 59% of patients had at least one HF hospitalization before the PAP implantation, compared with 22% afterward. The median number of HF hospitalizations was 0.92 per patient before implantation and 0.37 per patient afterward.
Further analysis showed that the cumulative rate of HF hospitalization was 45% lower during the 6 months after implantation than during the 6 months preceding it (hazard ratio, 0.55). This finding remained robust across several subgroups of patients and in several sensitivity analyses.
These reductions were associated with a corresponding decline in costs related to HF care, which dropped by $7,433 per patient.
In addition to HF-related hospitalizations, all-cause hospitalizations also declined by roughly 30% after implantation of a PAP sensor (HR, 0.69).
These findings suggest that the reduction in hospitalizations, along with attendant reductions in the costs of care, may be achievable in real-world practice. The 45% drop in HF hospitalizations in this study “compares favorably with the 28% reduction seen with PAP-guided therapy over the same time period in the randomized CHAMPION study that supported the initial FDA approval,” Dr. Desai said.
Moreover, a subgroup of 480 patients had data for 12 months preceding and 12 months following implantation. Analysis of those data showed that the benefits of PAP monitoring to guide HF care “were consistent over longer-term follow-up, with a 34% reduction in HF hospitalizations sustained at 12 months,” he added.
The study had several limitations. It excluded Medicare Part D data, so medication changes related to implantation could not be examined and may have exerted substantial influence on study outcomes.
It also didn’t include the actual PAP-sensor data, “which makes it challenging to confirm that physicians intervened to treat elevated PAPs” and that that intervention is the reason for the study outcomes.
“We were unable to definitively ascertain whether reduced HF hospitalizations are related to undertreatment in the preimplant period or improved treatment in the postimplant period,” Dr. Desai said.
The study was sponsored by Abbott, maker of the CardioMEMS PAP sensor. Dr. Desai and his associates reported ties to Abbott and St. Jude Medical.
Implantation of a pulmonary artery pressure sensor to guide care in chronic heart failure was associated with a significant 45% reduction in HF hospitalization and its attendant substantial costs in a real-world patient population, Akshay S. Desai, MD, said at the annual meeting of the American College of Cardiology.
The PAP sensor is used to monitor pulmonary artery filling pressure, which rises in many HF patients during the weeks preceding an HF exacerbation. This early detection of progressing congestion allows clinicians to intervene earlier and head off hospitalization for the exacerbation.
Their intention was to determine whether the positive results of the CHAMPION clinical trial, which prompted FDA approval of the device as a means to reduce HF-associated hospitalizations, could be replicated in a real-world population, said Dr. Desai of Brigham and Women’s Hospital, Boston.
The results of their study were presented March 19 at the annual meeting of the American College of Cardiology and simultaneously published online in the Journal of the American College of Cardiology (2017 Mar 19. doi: 10.1016/j.jacc.2017.03.009).
The mean age of the study cohort was 71 years, and 40% of the participants were at least 75 years of age. Women composed 40% of the cohort. There was a high burden of comorbid illness, including diabetes, hypertension, and chronic obstructive pulmonary disease. This represents a broader sample than was enrolled in the CHAMPION trial, he noted.
There were 1,020 HF hospitalizations before implantation and 381 afterward. A total of 59% of patients had at least one HF hospitalization before the PAP implantation, compared with 22% afterward. The median number of HF hospitalizations was 0.92 per patient before implantation and 0.37 per patient afterward.
Further analysis showed that the cumulative rate of HF hospitalization was 45% lower during the 6 months after implantation than during the 6 months preceding it (hazard ratio, 0.55). This finding remained robust across several subgroups of patients and in several sensitivity analyses.
These reductions were associated with a corresponding decline in costs related to HF care, which dropped by $7,433 per patient.
In addition to HF-related hospitalizations, all-cause hospitalizations also declined by roughly 30% after implantation of a PAP sensor (HR, 0.69).
These findings suggest that the reduction in hospitalizations, along with attendant reductions in the costs of care, may be achievable in real-world practice. The 45% drop in HF hospitalizations in this study “compares favorably with the 28% reduction seen with PAP-guided therapy over the same time period in the randomized CHAMPION study that supported the initial FDA approval,” Dr. Desai said.
Moreover, a subgroup of 480 patients had data for 12 months preceding and 12 months following implantation. Analysis of those data showed that the benefits of PAP monitoring to guide HF care “were consistent over longer-term follow-up, with a 34% reduction in HF hospitalizations sustained at 12 months,” he added.
The study had several limitations. It excluded Medicare Part D data, so medication changes related to implantation could not be examined and may have exerted substantial influence on study outcomes.
It also didn’t include the actual PAP-sensor data, “which makes it challenging to confirm that physicians intervened to treat elevated PAPs” and that that intervention is the reason for the study outcomes.
“We were unable to definitively ascertain whether reduced HF hospitalizations are related to undertreatment in the preimplant period or improved treatment in the postimplant period,” Dr. Desai said.
The study was sponsored by Abbott, maker of the CardioMEMS PAP sensor. Dr. Desai and his associates reported ties to Abbott and St. Jude Medical.
FROM ACC 17
Key clinical point: Implantation of a pulmonary artery pressure sensor to guide care in chronic heart failure was associated with a significant reduction in HF hospitalizations and associated substantial costs.
Major finding: The cumulative rate of HF hospitalization was 45% lower during the 6 months after implantation than during the 6 months preceding it (hazard ratio, 0.55).
Data source: A retrospective observational cohort study comparing HF hospitalizations during the 6 months before and the 6 months after PAP sensor implantation in 1,114 Medicare patients.
Disclosures: The study was sponsored by Abbott, maker of the CardioMEMS PAP sensor. Dr. Desai and his associates reported ties to Abbott and St. Jude Medical.
Evidence for medical marijuana largely up in smoke
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
SAN DIEGO – Despite the popularity of medical marijuana, robust evidence for its use is limited or nonexistent for most medical conditions.
“This is a tough subject to study,” Ellie Grossman, MD, said at the annual meeting of the American College of Physicians. “There is federal money that can only be used in very limited ways to study it. Our science is way behind the times in terms of what our patients are doing and using.”
Typical limitations of marijuana studies include self-report of quantity/duration used and the fact that biochemical/quantifiable measures are lacking. “For inhaled marijuana, there is variability in how much is inhaled and how deeply it’s being inhaled,” said Dr. Grossman, an internist who practices in Somerville, Mass.
Then there’s the issue of recall bias and the question as to whether oral cannabinoids equate to the plant-derived forms of medical marijuana that patients obtain from their local dispensaries.
That matters, because the majority of published studies on the topic have evaluated oral cannabinoids, not the plant form. “So, what we’re studying is vastly different from what our patients are using,” she said.
The most solid indication clinicians have for recommending medical marijuana is for chronic pain, and the most common condition studied has been neuropathy.
“Most evidence compares cannabinoid to placebo,” said Dr. Grossman, primary care lead for behavioral health integration at Cambridge (Mass.) Health Alliance. “There’s almost nothing out there comparing cannabinoid to any other pain-relieving agent that a patient might choose to use.
“A lot of the literature comes from oral synthesized agents,” she continued. “There’s a little bit of science about inhaled forms, but a lot of this is very different from what my patient got last week in a medical marijuana dispensary in Massachusetts.”
Results from a systematic review of 79 studies of cannabinoids for medical use in 6,462 study participants showed that, compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs. 20%; odds ratio, 3.82), reduction in pain (37% vs. 31%; OR, 1.41), a greater average reduction in numerical rating scale pain assessment (on a 0- to 10-point scale; weighted mean difference of –0.46), and average reduction in the Ashworth spasticity scale (–0.36) (JAMA 2015 Jun 23-30;313[24]:2456-73).
A separate meta-analysis of studies compared inhaled cannabis sativa to placebo for chronic painful neuropathy. The researchers found that those patients who used inhaled cannabis sativa were 3.2 times more likely to achieve a 30% or greater reduction in pain, compared with those in the placebo group (J. Pain 2015 Dec;16[12]:1221-32).
However, Dr. Grossman cautioned that the number of patients studied was fewer than 200, “so, you could argue that this is a body of knowledge where the jury is still out.”
According to a 2017 report from the National Academy of Sciences titled, “The Health Effects of Cannabis and Cannabinoids,” another area in which the knowledge base is less solid is the use of oral cannabinoids for chemotherapy-induced nausea and vomiting.
“There’s a reasonable amount of evidence showing that some of these are better than placebo for relief of these symptoms,” Dr. Grossman said. “That said, the jury’s out as to whether they are any better than our other antiemetic agents. And there are no studies comparing them to neurokinin-1 inhibitors, which are the newest class of drug often used by oncologists for this indication. There is also no good evidence about inhaled plant cannabis.”
Studies of oral cannabinoids for multiple-sclerosis–related spasticity have demonstrated a small improvement on patient-reported spasticity (less than 1 point on a 10-point scale), but there was no improvement in clinician-reported outcomes. At the same time, their use for weight loss/anorexia in HIV “is very limited, and there are no studies of plant-derived cannabis,” Dr. Grossman said.
According to the National Academy of Sciences report, some evidence supports the use of oral cannabinoids for short-term sleep outcomes in patients with chronic diseases such as fibromyalgia and MS. One small study of oral cannabinoid for anxiety found that it improved social anxiety symptoms on the public speaking test, but there have been no studies using inhaled cannabinoids/marijuana.
The health risks of medical marijuana are largely unknown, Dr. Grossman said, noting that most evidence on longer‐term health risks comes from epidemiologic studies of recreational cannabis users.
“Medical marijuana users tend to be older and tend to be sicker,” she said. “We don’t know anything about the long-term effects in that sicker population.”
Among healthier people, Dr. Grossman continued, cannabis use is associated with increased risk of cough, wheeze, and sputum/phlegm. “There’s also an increased risk of motor vehicle accidents,” she said. “That is certainly a concern in places where they’re legalizing marijuana.”
Cannabis use is associated with lower neonatal birth weight, case reports/series of unintentional pediatric ingestions, and a possible increase in suicidal ideation, suicide attempts, and completed suicides.
“The evidence is very limited regarding associations with myocardial infarction, stroke, COPD, and mortality,” she added. “We don’t really know.”
Dr. Grossman reported having no financial disclosures.
EXPERT ANALYSIS AT ACP INTERNAL MEDICINE
Vaccination reduces risk of flu-associated pediatric deaths
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
FROM PEDIATRICS
Latest weekly flu data show no decline in visits
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.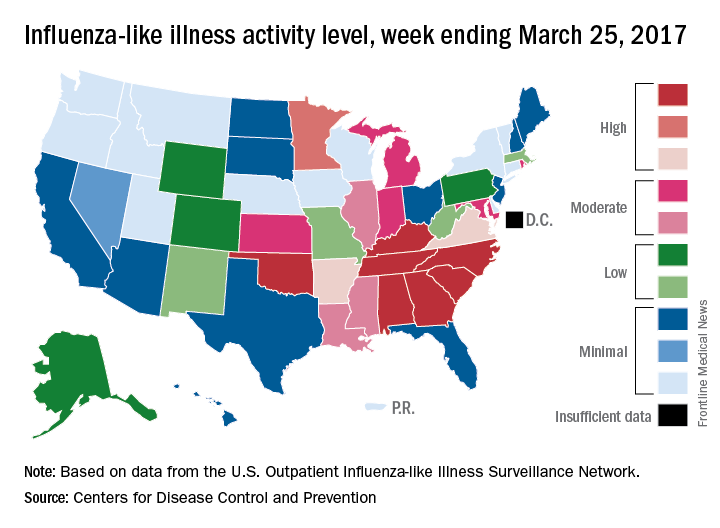
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
Osimertinib receives full approval for advanced EGFR-mutated NSCLC
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
The Food and Drug Administration has converted accelerated approval of osimertinib to full approval for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer (NSCLC), as detected by an FDA-approved test.
Also included in the indication, disease must have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy, the FDA said in a statement.
Full approval was based on an improvement in progression-free survival (PFS) in the phase III AURA3 study, which randomized 419 patients (2:1) to receive osimertinib (n = 279) 80 mg orally once daily or platinum-based doublet chemotherapy (n = 140). The hazard ratio for the investigator-assessed PFS was .30 (95% confidence interval: 0.23, 0.41; P less than .001).
The estimated median PFS was 10.1 months in the osimertinib arm and 4.4 months in the chemotherapy arm. Confirmed ORR was 65% (95% CI: 59%, 70%) and 29% (95% CI: 21%, 37%) in the osimertinib and chemotherapy arms, respectively (P less than .0001). Estimated median response durations were 11 months (95% CI: 8.6, 12.6) and 4.2 months (95% CI: 3.9, 5.9) in the osimertinib and chemotherapy arms, respectively, according to the FDA statement.
Overall survival data are immature, the FDA said.
All patients had metastatic EGFR T790M mutation–positive NSCLC, identified by the cobas EGFR mutation test performed in a central laboratory, and progressive disease following first-line EGFR TKI therapy. Patients in the chemotherapy arm received either pemetrexed, 500 mg/m2 with carboplatin AUC5, or pemetrexed, 500mg/m2 with cisplatin 75 mg/m2), on day 1 of every 21-day cycle for up to six cycles followed by pemetrexed maintenance therapy.
The most serious adverse reactions, evaluated in 833 patients receiving osimertinib, were interstitial lung disease/pneumonitis (3.5%), QTc interval prolongation (0.7%), cardiomyopathy (1.9%), and keratitis (0.7%). The most common adverse reactions were diarrhea, rash, dry skin, nail toxicity, and fatigue.
The recommended dose of osimertinib, to be marketed as Tagrisso by AstraZeneca, is 80 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. The presence of an EGFR T790M mutation in a tumor specimen, or plasma specimen (if tumor tissue is unavailable), should be confirmed by an FDA-approved test prior to initiation of treatment.
Full prescribing information is available here.
Many patients’ severe asthma remains uncontrolled
ATLANTA – More than half of patients severe asthma have disease that remains uncontrolled at their index date of treatment, and 39% remained uncontrolled at 12 months of follow-up, results from a large single specialty practice study showed.
“Severe asthma accounts for only 5%-10% of all asthma [in] patients but at least half of the health care costs, and it’s a significant burden to those who suffer from it,” study author Brian D. Stone, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The mean age of patients was 44.4 years, 67% were female, 78% were white, 77% had concomitant rhinitis, 21% had sinusitis, and 44% had allergic sensitivities. The mean baseline prebronchodilation FEV1 and FEV1% predicted were 2.45 L and 79.7%, respectively. The mean baseline ACT score was 17, with 61% of patients having ACT scores of 19 or lower, indicating poor symptom control.
Using National Asthma Education and Prevention Program criteria, the researchers found that 52% of patients had uncontrolled asthma at the index date and 39% remained uncontrolled at 12 months of follow-up. In an effort to better manage their severe asthma, more than one-third of patients on Step 4 therapy and 60% of patients on Step 5 therapy changed their asthma controller medications during follow-up.
“As an asthma specialist, I hoped that more [of these patients] would have come under control. These are the most difficult patients to treat,” said Dr. Stone, who practices at Allergy Partners of San Diego. “It’s a population that deserves special attention, and interventions are probably going to be on multiple levels, depending on what type of severe persistent asthma they have. More therapies are needed.”
He acknowledged certain limitations of the study, including the fact that patients were treated by specialists in allergy, asthma, and immunology and, therefore, may not represent the general population with asthma.
The study was supported by AstraZeneca. Dr. Stone reported having no relevant financial disclosures.
ATLANTA – More than half of patients severe asthma have disease that remains uncontrolled at their index date of treatment, and 39% remained uncontrolled at 12 months of follow-up, results from a large single specialty practice study showed.
“Severe asthma accounts for only 5%-10% of all asthma [in] patients but at least half of the health care costs, and it’s a significant burden to those who suffer from it,” study author Brian D. Stone, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The mean age of patients was 44.4 years, 67% were female, 78% were white, 77% had concomitant rhinitis, 21% had sinusitis, and 44% had allergic sensitivities. The mean baseline prebronchodilation FEV1 and FEV1% predicted were 2.45 L and 79.7%, respectively. The mean baseline ACT score was 17, with 61% of patients having ACT scores of 19 or lower, indicating poor symptom control.
Using National Asthma Education and Prevention Program criteria, the researchers found that 52% of patients had uncontrolled asthma at the index date and 39% remained uncontrolled at 12 months of follow-up. In an effort to better manage their severe asthma, more than one-third of patients on Step 4 therapy and 60% of patients on Step 5 therapy changed their asthma controller medications during follow-up.
“As an asthma specialist, I hoped that more [of these patients] would have come under control. These are the most difficult patients to treat,” said Dr. Stone, who practices at Allergy Partners of San Diego. “It’s a population that deserves special attention, and interventions are probably going to be on multiple levels, depending on what type of severe persistent asthma they have. More therapies are needed.”
He acknowledged certain limitations of the study, including the fact that patients were treated by specialists in allergy, asthma, and immunology and, therefore, may not represent the general population with asthma.
The study was supported by AstraZeneca. Dr. Stone reported having no relevant financial disclosures.
ATLANTA – More than half of patients severe asthma have disease that remains uncontrolled at their index date of treatment, and 39% remained uncontrolled at 12 months of follow-up, results from a large single specialty practice study showed.
“Severe asthma accounts for only 5%-10% of all asthma [in] patients but at least half of the health care costs, and it’s a significant burden to those who suffer from it,” study author Brian D. Stone, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The mean age of patients was 44.4 years, 67% were female, 78% were white, 77% had concomitant rhinitis, 21% had sinusitis, and 44% had allergic sensitivities. The mean baseline prebronchodilation FEV1 and FEV1% predicted were 2.45 L and 79.7%, respectively. The mean baseline ACT score was 17, with 61% of patients having ACT scores of 19 or lower, indicating poor symptom control.
Using National Asthma Education and Prevention Program criteria, the researchers found that 52% of patients had uncontrolled asthma at the index date and 39% remained uncontrolled at 12 months of follow-up. In an effort to better manage their severe asthma, more than one-third of patients on Step 4 therapy and 60% of patients on Step 5 therapy changed their asthma controller medications during follow-up.
“As an asthma specialist, I hoped that more [of these patients] would have come under control. These are the most difficult patients to treat,” said Dr. Stone, who practices at Allergy Partners of San Diego. “It’s a population that deserves special attention, and interventions are probably going to be on multiple levels, depending on what type of severe persistent asthma they have. More therapies are needed.”
He acknowledged certain limitations of the study, including the fact that patients were treated by specialists in allergy, asthma, and immunology and, therefore, may not represent the general population with asthma.
The study was supported by AstraZeneca. Dr. Stone reported having no relevant financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: More than half of patients with severe asthma (52%) had uncontrolled disease at the index date of treatment, and 39% remained uncontrolled at 12 months of follow-up.
Data source: A retrospective review of 12,922 patients aged 12 years and older with severe asthma who were treated between Jan. 1, 2010, and April 30, 2016.
Disclosures: The study was supported by AstraZeneca. Dr. Stone reported no relevant financial disclosures.
What happens when a baked egg oral challenge is negative?
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Following a negative physician-supervised baked egg oral challenge 94% of patients have had continued exposure to egg while 83% have shown tolerance to cooked egg.
Data source: A retrospective review of 22 patients who underwent a physician-supervised negative oral baked egg challenge.
Disclosures: Dr. Peng reported having no relevant financial disclosures.
Omalizumab may help chronic rhinosinusitis symptoms
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point: In patients with severe chronic rhinosinusitis, omalizumab treatment provided significant improvement in every major clinical symptom of the condition.
Major finding: Among patients in the omalizumab treatment group, overall symptoms improved by 70%.
Data source: A study that evaluated the clinical effect of omalizumab in 25 patients with recalcitrant chronic rhinosinusitis who were receiving anti-IgE therapy as part of their asthma treatment and 5 control patients.
Disclosures: Dr. Kilty reported having no relevant financial disclosures.
A history of asthma exacerbations predicts future exacerbations
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
Survey eyes trends in care of severe pediatric asthma
ATLANTA – The treatment of pediatric severe acute asthma has changed over the past 21 years, but interspecialty differences in the management of these patients persist, results from a national survey suggest.
“I think it’s good for every ER and ICU department to have a conversation with providers about what to do when these kinds of patients come in,” lead study author Roua Azmeh, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “A lot of ERs are establishing protocols. I think that’s going to be the wave of the future.”
The National Heart, Blood, and Lung Institute Asthma Guidelines, first published in 1991, were most recently revised in 2007. In an effort to observe changes in asthma management in pediatric EDs and ICUs over the past 21 years, and to compare common management strategies, Dr. Azmeh and her associates distributed a 16-question online survey to 144 current program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care. Results were compared to a similar survey that was sent by snail mail to program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care in 1995.
Dr. Azmeh, a fellow in allergy and immunology at the Saint Louis University, reported results from 62 respondents who completed the 2016 questionnaire (43%). For initial management of pediatric acute severe asthma, a greater proportion of program directors in pediatric critical care reported using parenteral corticosteroids, compared with their counterparts in pediatric emergency medicine (85% vs. 32%, respectively; P less than .0001), as well as continuous beta 2-agonists (73% vs. 56%; P less than .05). A majority of overall respondents (98%) did not use theophylline for initial management, but more program directors in pediatric critical care reported using it for treatment failure, compared with their counterparts in pediatric emergency medicine (56% vs. 20%, respectively; P less than .0071). There was a trend among all respondents for more use of heliox for treatment failure than for initial management (13% vs. 6%).
When the researchers compared current survey responses to responses from the 1995 survey, they observed that program training directors across both specialties increased the use of nebulized ipratropium bromide in initial management and treatment failure (17% vs. 69%; P less than .0001 and 33% vs. 42%; P less than .05) and decreased use of theophylline for initial management of severe acute asthma (17% vs. 3%; P less than .05). However, theophylline is still used in treatment failure.
Among respondents to the 2016 survey, program directors in pediatric emergency medicine were less likely than were those in pediatric critical care to use continuous nebulized beta-2 agonists for initial management or to add parenteral selective beta-2 agonists (56% vs. 73% and 12% vs. 21%, respectively; P less than .05). They also were less likely to use theophylline in treatment failure (20% vs. 56%; P less than .05).
Dr. Azmeh reported having no relevant financial disclosures.
Surveys are interesting to establish a trend for what residents and fellows are being taught in emergency rooms and critical care units. The parenteral steroid use difference for the two groups in 2016 may be related to the fact that the emergency room hasn’t decide
Surveys are interesting to establish a trend for what residents and fellows are being taught in emergency rooms and critical care units. The parenteral steroid use difference for the two groups in 2016 may be related to the fact that the emergency room hasn’t decide
Surveys are interesting to establish a trend for what residents and fellows are being taught in emergency rooms and critical care units. The parenteral steroid use difference for the two groups in 2016 may be related to the fact that the emergency room hasn’t decide
ATLANTA – The treatment of pediatric severe acute asthma has changed over the past 21 years, but interspecialty differences in the management of these patients persist, results from a national survey suggest.
“I think it’s good for every ER and ICU department to have a conversation with providers about what to do when these kinds of patients come in,” lead study author Roua Azmeh, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “A lot of ERs are establishing protocols. I think that’s going to be the wave of the future.”
The National Heart, Blood, and Lung Institute Asthma Guidelines, first published in 1991, were most recently revised in 2007. In an effort to observe changes in asthma management in pediatric EDs and ICUs over the past 21 years, and to compare common management strategies, Dr. Azmeh and her associates distributed a 16-question online survey to 144 current program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care. Results were compared to a similar survey that was sent by snail mail to program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care in 1995.
Dr. Azmeh, a fellow in allergy and immunology at the Saint Louis University, reported results from 62 respondents who completed the 2016 questionnaire (43%). For initial management of pediatric acute severe asthma, a greater proportion of program directors in pediatric critical care reported using parenteral corticosteroids, compared with their counterparts in pediatric emergency medicine (85% vs. 32%, respectively; P less than .0001), as well as continuous beta 2-agonists (73% vs. 56%; P less than .05). A majority of overall respondents (98%) did not use theophylline for initial management, but more program directors in pediatric critical care reported using it for treatment failure, compared with their counterparts in pediatric emergency medicine (56% vs. 20%, respectively; P less than .0071). There was a trend among all respondents for more use of heliox for treatment failure than for initial management (13% vs. 6%).
When the researchers compared current survey responses to responses from the 1995 survey, they observed that program training directors across both specialties increased the use of nebulized ipratropium bromide in initial management and treatment failure (17% vs. 69%; P less than .0001 and 33% vs. 42%; P less than .05) and decreased use of theophylline for initial management of severe acute asthma (17% vs. 3%; P less than .05). However, theophylline is still used in treatment failure.
Among respondents to the 2016 survey, program directors in pediatric emergency medicine were less likely than were those in pediatric critical care to use continuous nebulized beta-2 agonists for initial management or to add parenteral selective beta-2 agonists (56% vs. 73% and 12% vs. 21%, respectively; P less than .05). They also were less likely to use theophylline in treatment failure (20% vs. 56%; P less than .05).
Dr. Azmeh reported having no relevant financial disclosures.
ATLANTA – The treatment of pediatric severe acute asthma has changed over the past 21 years, but interspecialty differences in the management of these patients persist, results from a national survey suggest.
“I think it’s good for every ER and ICU department to have a conversation with providers about what to do when these kinds of patients come in,” lead study author Roua Azmeh, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “A lot of ERs are establishing protocols. I think that’s going to be the wave of the future.”
The National Heart, Blood, and Lung Institute Asthma Guidelines, first published in 1991, were most recently revised in 2007. In an effort to observe changes in asthma management in pediatric EDs and ICUs over the past 21 years, and to compare common management strategies, Dr. Azmeh and her associates distributed a 16-question online survey to 144 current program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care. Results were compared to a similar survey that was sent by snail mail to program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care in 1995.
Dr. Azmeh, a fellow in allergy and immunology at the Saint Louis University, reported results from 62 respondents who completed the 2016 questionnaire (43%). For initial management of pediatric acute severe asthma, a greater proportion of program directors in pediatric critical care reported using parenteral corticosteroids, compared with their counterparts in pediatric emergency medicine (85% vs. 32%, respectively; P less than .0001), as well as continuous beta 2-agonists (73% vs. 56%; P less than .05). A majority of overall respondents (98%) did not use theophylline for initial management, but more program directors in pediatric critical care reported using it for treatment failure, compared with their counterparts in pediatric emergency medicine (56% vs. 20%, respectively; P less than .0071). There was a trend among all respondents for more use of heliox for treatment failure than for initial management (13% vs. 6%).
When the researchers compared current survey responses to responses from the 1995 survey, they observed that program training directors across both specialties increased the use of nebulized ipratropium bromide in initial management and treatment failure (17% vs. 69%; P less than .0001 and 33% vs. 42%; P less than .05) and decreased use of theophylline for initial management of severe acute asthma (17% vs. 3%; P less than .05). However, theophylline is still used in treatment failure.
Among respondents to the 2016 survey, program directors in pediatric emergency medicine were less likely than were those in pediatric critical care to use continuous nebulized beta-2 agonists for initial management or to add parenteral selective beta-2 agonists (56% vs. 73% and 12% vs. 21%, respectively; P less than .05). They also were less likely to use theophylline in treatment failure (20% vs. 56%; P less than .05).
Dr. Azmeh reported having no relevant financial disclosures.
Key clinical point:
Major finding: For initial management of pediatric acute severe asthma, a greater proportion of program directors in pediatric critical care reported using parenteral corticosteroids, compared with their counterparts in pediatric emergency medicine (85% vs. 32%, respectively; P less than .0001).
Data source: Results from a 16-question online survey sent to 144 current program directors of U.S. training programs in pediatric emergency medicine and pediatric critical care.
Disclosures: Dr. Azmeh reported having no relevant financial disclosures.