User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Intensive outpatient PTSD treatment linked to fewer emergency encounters
NEW ORLEANS – , according to a new study released at the annual meeting of the American Psychiatric Association.
In an analysis of 256 individuals, over the 12 months before they joined the IOP, 28.7% and 24.8% had inpatient and emergency department encounters, respectively, according to the researchers. Afterward, those numbers fell to 15.9% (P < .01) and 18.2% (P = .04), respectively.
“Engagement in IOP for patients with PTSD may help avoid the need for higher levels of care such as residential or inpatient treatment,” Nathan Lingafelter, MD, a psychiatrist and researcher at Kaiser Permanente in Oakland, Calif., said in an interview.
Dr. Lingafelter described IOP programs as typically “offering patients a combination of individual therapy, group therapy, and medication management all at an increased frequency of about 3 half-days per week. IOPs are thought to be helpful in helping patients with severe symptoms while they are still in the community – i.e., living in their homes, with their families, occasionally still working at reduced time.”
While other studies have examined the effects of IOP, “the existing literature focuses on how IOP reduces symptoms, rather than looking at how IOP involvement might be associated with patients utilizing different acute care resources,” he said. “Prior studies have also been conducted mostly in veteran populations and in populations with less diversity than our population in Oakland.”
For the new study, researchers tracked 256 IOP participants (83% female; mean age = 39; 44% White, 27% Black, 14% Hispanic, and 7% Asian). The wide majority – 85% – had comorbid depressive disorders.
“Patients are assigned a case manager when they enter the program who they can meet with individually, and they spend time attending group therapy sessions. Patients are also able to meet with a psychiatrist to discuss medications,” Dr. Lingafelter said. “A major component in both the group and individual therapy is helping patients identify which kind of interventions work for them and what we can do now that will help. IOP can really help clarify for patients what their trauma responses are and how to start treatments that actually fit their symptoms.”
The subjects had a mean 0.3 psychiatric encounters in the year before joining the program and 0.2 in the year after (P < .01). Their mean emergency department visits related to mental health fell from 0.5 to 0.3 (P = .03).
The study has limitations. Participants took part in IOP therapy from 2017 to 2018, before the pandemic disrupted mental health treatment. It does not examine whether medication use changed after IOP treatment. It is retrospective and doesn’t confirm that IOP had any positive effect.
Multiple benefits of IOP
In an interview, Deborah C. Beidel, PhD, director of UCF RESTORES at the University of Central Florida, Orlando, said IOP has several advantages as a treatment for PTSD. Her clinic, which focuses on PTSD treatment for military veterans, has used the approach to treat hundreds of people.
“First, IOPs can address the stigma that surrounds mental health treatment. If you have a physical injury, you take time off from work to go to physical therapy, which is time-limited. If you have a stress injury, why not do the same? Take a few weeks, get it treated, and get back to work,” she said. “The second reason is that the most effective treatment for PTSD is exposure therapy, which is more effective when treatment sessions occur in a daily as opposed to a weekly or monthly time frame. Third, from a cost and feasibility perspective, an intensive program could reduce overall medical costs and get people back to work sooner.”
The new study is “definitely useful” since it examines the impact of IOP over a longer term, Dr. Beidel said. This kind of data “can influence policy, particularly with insurance companies. If we can build the evidence that short, intensive treatment produces better long-term outcomes, insurance companies will be more likely to pay for the IOP.”
The University of Central Florida program is funded by federal research grants and state funding, she said. “When we calculate the cost, it comes to about $10,000 in therapy time plus an average of about $3,000 in travel related costs – transportation, lodging, meals – for those who travel from out of state for our program.”
What’s next? “Further study is needed to characterize whether these findings are applicable to other practice settings, including virtual treatment programs; the long-term durability of these findings; and whether similar patterns of reduced resource use extend to non–mental health–specific care utilization,” said Dr. Lingafelter, the study’s lead author.
No study funding and no author disclosures were reported. Dr. Beidel disclosed IOP-related research support from the U.S. Army Medical Research and Development Command–Military Operational Medicine Research Program.
NEW ORLEANS – , according to a new study released at the annual meeting of the American Psychiatric Association.
In an analysis of 256 individuals, over the 12 months before they joined the IOP, 28.7% and 24.8% had inpatient and emergency department encounters, respectively, according to the researchers. Afterward, those numbers fell to 15.9% (P < .01) and 18.2% (P = .04), respectively.
“Engagement in IOP for patients with PTSD may help avoid the need for higher levels of care such as residential or inpatient treatment,” Nathan Lingafelter, MD, a psychiatrist and researcher at Kaiser Permanente in Oakland, Calif., said in an interview.
Dr. Lingafelter described IOP programs as typically “offering patients a combination of individual therapy, group therapy, and medication management all at an increased frequency of about 3 half-days per week. IOPs are thought to be helpful in helping patients with severe symptoms while they are still in the community – i.e., living in their homes, with their families, occasionally still working at reduced time.”
While other studies have examined the effects of IOP, “the existing literature focuses on how IOP reduces symptoms, rather than looking at how IOP involvement might be associated with patients utilizing different acute care resources,” he said. “Prior studies have also been conducted mostly in veteran populations and in populations with less diversity than our population in Oakland.”
For the new study, researchers tracked 256 IOP participants (83% female; mean age = 39; 44% White, 27% Black, 14% Hispanic, and 7% Asian). The wide majority – 85% – had comorbid depressive disorders.
“Patients are assigned a case manager when they enter the program who they can meet with individually, and they spend time attending group therapy sessions. Patients are also able to meet with a psychiatrist to discuss medications,” Dr. Lingafelter said. “A major component in both the group and individual therapy is helping patients identify which kind of interventions work for them and what we can do now that will help. IOP can really help clarify for patients what their trauma responses are and how to start treatments that actually fit their symptoms.”
The subjects had a mean 0.3 psychiatric encounters in the year before joining the program and 0.2 in the year after (P < .01). Their mean emergency department visits related to mental health fell from 0.5 to 0.3 (P = .03).
The study has limitations. Participants took part in IOP therapy from 2017 to 2018, before the pandemic disrupted mental health treatment. It does not examine whether medication use changed after IOP treatment. It is retrospective and doesn’t confirm that IOP had any positive effect.
Multiple benefits of IOP
In an interview, Deborah C. Beidel, PhD, director of UCF RESTORES at the University of Central Florida, Orlando, said IOP has several advantages as a treatment for PTSD. Her clinic, which focuses on PTSD treatment for military veterans, has used the approach to treat hundreds of people.
“First, IOPs can address the stigma that surrounds mental health treatment. If you have a physical injury, you take time off from work to go to physical therapy, which is time-limited. If you have a stress injury, why not do the same? Take a few weeks, get it treated, and get back to work,” she said. “The second reason is that the most effective treatment for PTSD is exposure therapy, which is more effective when treatment sessions occur in a daily as opposed to a weekly or monthly time frame. Third, from a cost and feasibility perspective, an intensive program could reduce overall medical costs and get people back to work sooner.”
The new study is “definitely useful” since it examines the impact of IOP over a longer term, Dr. Beidel said. This kind of data “can influence policy, particularly with insurance companies. If we can build the evidence that short, intensive treatment produces better long-term outcomes, insurance companies will be more likely to pay for the IOP.”
The University of Central Florida program is funded by federal research grants and state funding, she said. “When we calculate the cost, it comes to about $10,000 in therapy time plus an average of about $3,000 in travel related costs – transportation, lodging, meals – for those who travel from out of state for our program.”
What’s next? “Further study is needed to characterize whether these findings are applicable to other practice settings, including virtual treatment programs; the long-term durability of these findings; and whether similar patterns of reduced resource use extend to non–mental health–specific care utilization,” said Dr. Lingafelter, the study’s lead author.
No study funding and no author disclosures were reported. Dr. Beidel disclosed IOP-related research support from the U.S. Army Medical Research and Development Command–Military Operational Medicine Research Program.
NEW ORLEANS – , according to a new study released at the annual meeting of the American Psychiatric Association.
In an analysis of 256 individuals, over the 12 months before they joined the IOP, 28.7% and 24.8% had inpatient and emergency department encounters, respectively, according to the researchers. Afterward, those numbers fell to 15.9% (P < .01) and 18.2% (P = .04), respectively.
“Engagement in IOP for patients with PTSD may help avoid the need for higher levels of care such as residential or inpatient treatment,” Nathan Lingafelter, MD, a psychiatrist and researcher at Kaiser Permanente in Oakland, Calif., said in an interview.
Dr. Lingafelter described IOP programs as typically “offering patients a combination of individual therapy, group therapy, and medication management all at an increased frequency of about 3 half-days per week. IOPs are thought to be helpful in helping patients with severe symptoms while they are still in the community – i.e., living in their homes, with their families, occasionally still working at reduced time.”
While other studies have examined the effects of IOP, “the existing literature focuses on how IOP reduces symptoms, rather than looking at how IOP involvement might be associated with patients utilizing different acute care resources,” he said. “Prior studies have also been conducted mostly in veteran populations and in populations with less diversity than our population in Oakland.”
For the new study, researchers tracked 256 IOP participants (83% female; mean age = 39; 44% White, 27% Black, 14% Hispanic, and 7% Asian). The wide majority – 85% – had comorbid depressive disorders.
“Patients are assigned a case manager when they enter the program who they can meet with individually, and they spend time attending group therapy sessions. Patients are also able to meet with a psychiatrist to discuss medications,” Dr. Lingafelter said. “A major component in both the group and individual therapy is helping patients identify which kind of interventions work for them and what we can do now that will help. IOP can really help clarify for patients what their trauma responses are and how to start treatments that actually fit their symptoms.”
The subjects had a mean 0.3 psychiatric encounters in the year before joining the program and 0.2 in the year after (P < .01). Their mean emergency department visits related to mental health fell from 0.5 to 0.3 (P = .03).
The study has limitations. Participants took part in IOP therapy from 2017 to 2018, before the pandemic disrupted mental health treatment. It does not examine whether medication use changed after IOP treatment. It is retrospective and doesn’t confirm that IOP had any positive effect.
Multiple benefits of IOP
In an interview, Deborah C. Beidel, PhD, director of UCF RESTORES at the University of Central Florida, Orlando, said IOP has several advantages as a treatment for PTSD. Her clinic, which focuses on PTSD treatment for military veterans, has used the approach to treat hundreds of people.
“First, IOPs can address the stigma that surrounds mental health treatment. If you have a physical injury, you take time off from work to go to physical therapy, which is time-limited. If you have a stress injury, why not do the same? Take a few weeks, get it treated, and get back to work,” she said. “The second reason is that the most effective treatment for PTSD is exposure therapy, which is more effective when treatment sessions occur in a daily as opposed to a weekly or monthly time frame. Third, from a cost and feasibility perspective, an intensive program could reduce overall medical costs and get people back to work sooner.”
The new study is “definitely useful” since it examines the impact of IOP over a longer term, Dr. Beidel said. This kind of data “can influence policy, particularly with insurance companies. If we can build the evidence that short, intensive treatment produces better long-term outcomes, insurance companies will be more likely to pay for the IOP.”
The University of Central Florida program is funded by federal research grants and state funding, she said. “When we calculate the cost, it comes to about $10,000 in therapy time plus an average of about $3,000 in travel related costs – transportation, lodging, meals – for those who travel from out of state for our program.”
What’s next? “Further study is needed to characterize whether these findings are applicable to other practice settings, including virtual treatment programs; the long-term durability of these findings; and whether similar patterns of reduced resource use extend to non–mental health–specific care utilization,” said Dr. Lingafelter, the study’s lead author.
No study funding and no author disclosures were reported. Dr. Beidel disclosed IOP-related research support from the U.S. Army Medical Research and Development Command–Military Operational Medicine Research Program.
AT APA 2022
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
Today’s medical oxymoron: Healthy overconfidence
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”
Early metformin minimizes antipsychotic-induced weight gain
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Refugees have a high burden of chronic pain associated with mental illness
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
Psilocybin benefits in severe depression observed up to 12 weeks
NEW ORLEANS –
“This is easily the largest study of a psychedelic drug employing modern randomized controlled trial methodology [with] 22 sites and 10 countries, so it’s not your typical phase 2 trial,” the study’s lead author, Guy M. Goodwin, MD, emeritus professor of psychiatry at the University of Oxford, England, said in an interview.
“Importantly, 94% of the patients in the study were psilocybin naive, which is very important for generalizability,” Dr. Goodwin noted.
Long used as psychedelic ‘magic mushrooms,’ psilocybin has gained increased interest in psychiatry in recent years as a potential treatment for severe depression after showing benefits in patients with life-threatening cancers and others with major depressive disorder (MDD).
To put the therapy to test in a more rigorous, randomized trial, Dr. Goodwin and colleagues conducted the phase 2b study of a proprietary synthetic formulation of psilocybin, COMP360 (COMPASS Pathways), recruiting 233 patients with treatment-resistant depression at 22 centers.
The study was presented at the annual meeting of the American Psychiatric Association.
After a 2-week washout period following the discontinuation of antidepressants, the patients were randomized to one of three groups: A single dose of 25 mg (n = 79), 10 mg (n = 75), or a subtherapeutic comparison of 1 mg (n = 79).
The psilocybin was administered in the presence of specially trained therapists who provided psychological support before, during, and after the 6- to 8-hour session.
Patients were then asked to refrain from antidepressant use for at least 3 weeks following the session, and had periodic follow-up for 12 weeks.
For the primary endpoint, those in the 25-mg group, but not the 10-mg dose, showed a significantly greater reduction in depression from baseline versus the 1-mg group on the Montgomery-Åsberg Depression Rating Scale at week 3 (MADRS; -6.6; P < .001).
The benefit was observed at day 2 and week 1 following administration, “confirming the rapid-acting character of the effect,” the investigators reported.
Sustained responses, defined as at least a 50% change from baseline in MADRS total score, were further observed up to week 12 among 20.3% in the 25-mg group and among 5.3% in the 10-mg groups versus 10.1% in the 1-mg group.
On the day of psilocybin treatment, the treatment-emergent side effects that were reported were headache, nausea, and dizziness, with event rates of 83.5% in the 25-mg group, 74.7% in the 10-mg group, and 72.2% in the 1-mg group.
One participant in the 25-mg group experienced acute anxiety and was treated with lorazepam.
The incidence of treatment-emergent serious adverse events from day 2 to week 3 was 6.3% (five patients) in the 25-mg group, 8.0% (six patients) in the 10-mg group, and 1.3% (one patient) in the 1-mg group.
Serious AEs included suicidal ideation and intentional self-injury among two patients each in the 25-mg group, while in the 10-mg group, two had suicidal ideation and one had hospitalization for severe depression.
There were no significant differences between the groups in terms of vital signs or clinical laboratory tests.
Of note, two patients in the 25-mg group had a change from baseline in QTcF >60 msec on day 2. For one patient, the increase was within normal range, and the other had a QTcF interval duration >500 msec on day 2, but levels returned to normal by day 9.
Improvements in context
Dr. Goodwin noted that the improvements were swift and impressive when compared with those of the STAR*D trial, which is the largest prospective study of treatment outcomes in patients with MDD.
“In the STAR*D trial, third- and fourth-step treatments showed low response rates of under 15% and high relapse rates,” Dr. Goodwin said. “By comparison, our response rates at 12 weeks were 20%-25%, so almost double that seen for probably equivalent treatment steps in STAR*D, with a single treatment with 25 mg, and no additional antidepressant, so no side effect burden.
“We hope to follow up enough of these patients [in the new study] to get some idea of relapse rates,” Dr. Goodwin added. “These have been low in comparable studies with MDD patients: We will see.”
Commenting on the research, Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, Rochester, Minn., said the study represents a valuable addition to needed evidence on psilocybin – with some caveats.
“This study adds to the emerging evidence base of psilocybin for treatment-resistant depression, at least in the short term,” he said in an interview. “I think the challenge in the real world would be to have access to 6-8 hours of therapy with psilocybin when patients struggle to find good therapists who could provide even weekly therapy for an hour.”
In addition, Dr. Singh questioned the durability of a single dose of psilocybin in the long term, noting a recent study (N Engl J Med. 2021 Apr 15. doi: 10.1056/NEJMoa2032994) that evaluated two doses of psilocybin (25 mg) 3 weeks apart, and failed to show any significant difference compared with the serotonergic antidepressant escitalopram at 6 weeks.
He further expressed concern about the emergence of suicidal behaviors in some patients, as well as the prolongation of QTc > 60 msec reported in the two patients.
“This is something to be carefully assessed in future studies, due to the risk of arrhythmias,” Dr. Singh said.
The study was sponsored by COMPASS Pathfinder Limited. Dr. Goodwin is chief medical officer for COMPASS Pathways. Dr. Singh had no disclosures to report.
NEW ORLEANS –
“This is easily the largest study of a psychedelic drug employing modern randomized controlled trial methodology [with] 22 sites and 10 countries, so it’s not your typical phase 2 trial,” the study’s lead author, Guy M. Goodwin, MD, emeritus professor of psychiatry at the University of Oxford, England, said in an interview.
“Importantly, 94% of the patients in the study were psilocybin naive, which is very important for generalizability,” Dr. Goodwin noted.
Long used as psychedelic ‘magic mushrooms,’ psilocybin has gained increased interest in psychiatry in recent years as a potential treatment for severe depression after showing benefits in patients with life-threatening cancers and others with major depressive disorder (MDD).
To put the therapy to test in a more rigorous, randomized trial, Dr. Goodwin and colleagues conducted the phase 2b study of a proprietary synthetic formulation of psilocybin, COMP360 (COMPASS Pathways), recruiting 233 patients with treatment-resistant depression at 22 centers.
The study was presented at the annual meeting of the American Psychiatric Association.
After a 2-week washout period following the discontinuation of antidepressants, the patients were randomized to one of three groups: A single dose of 25 mg (n = 79), 10 mg (n = 75), or a subtherapeutic comparison of 1 mg (n = 79).
The psilocybin was administered in the presence of specially trained therapists who provided psychological support before, during, and after the 6- to 8-hour session.
Patients were then asked to refrain from antidepressant use for at least 3 weeks following the session, and had periodic follow-up for 12 weeks.
For the primary endpoint, those in the 25-mg group, but not the 10-mg dose, showed a significantly greater reduction in depression from baseline versus the 1-mg group on the Montgomery-Åsberg Depression Rating Scale at week 3 (MADRS; -6.6; P < .001).
The benefit was observed at day 2 and week 1 following administration, “confirming the rapid-acting character of the effect,” the investigators reported.
Sustained responses, defined as at least a 50% change from baseline in MADRS total score, were further observed up to week 12 among 20.3% in the 25-mg group and among 5.3% in the 10-mg groups versus 10.1% in the 1-mg group.
On the day of psilocybin treatment, the treatment-emergent side effects that were reported were headache, nausea, and dizziness, with event rates of 83.5% in the 25-mg group, 74.7% in the 10-mg group, and 72.2% in the 1-mg group.
One participant in the 25-mg group experienced acute anxiety and was treated with lorazepam.
The incidence of treatment-emergent serious adverse events from day 2 to week 3 was 6.3% (five patients) in the 25-mg group, 8.0% (six patients) in the 10-mg group, and 1.3% (one patient) in the 1-mg group.
Serious AEs included suicidal ideation and intentional self-injury among two patients each in the 25-mg group, while in the 10-mg group, two had suicidal ideation and one had hospitalization for severe depression.
There were no significant differences between the groups in terms of vital signs or clinical laboratory tests.
Of note, two patients in the 25-mg group had a change from baseline in QTcF >60 msec on day 2. For one patient, the increase was within normal range, and the other had a QTcF interval duration >500 msec on day 2, but levels returned to normal by day 9.
Improvements in context
Dr. Goodwin noted that the improvements were swift and impressive when compared with those of the STAR*D trial, which is the largest prospective study of treatment outcomes in patients with MDD.
“In the STAR*D trial, third- and fourth-step treatments showed low response rates of under 15% and high relapse rates,” Dr. Goodwin said. “By comparison, our response rates at 12 weeks were 20%-25%, so almost double that seen for probably equivalent treatment steps in STAR*D, with a single treatment with 25 mg, and no additional antidepressant, so no side effect burden.
“We hope to follow up enough of these patients [in the new study] to get some idea of relapse rates,” Dr. Goodwin added. “These have been low in comparable studies with MDD patients: We will see.”
Commenting on the research, Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, Rochester, Minn., said the study represents a valuable addition to needed evidence on psilocybin – with some caveats.
“This study adds to the emerging evidence base of psilocybin for treatment-resistant depression, at least in the short term,” he said in an interview. “I think the challenge in the real world would be to have access to 6-8 hours of therapy with psilocybin when patients struggle to find good therapists who could provide even weekly therapy for an hour.”
In addition, Dr. Singh questioned the durability of a single dose of psilocybin in the long term, noting a recent study (N Engl J Med. 2021 Apr 15. doi: 10.1056/NEJMoa2032994) that evaluated two doses of psilocybin (25 mg) 3 weeks apart, and failed to show any significant difference compared with the serotonergic antidepressant escitalopram at 6 weeks.
He further expressed concern about the emergence of suicidal behaviors in some patients, as well as the prolongation of QTc > 60 msec reported in the two patients.
“This is something to be carefully assessed in future studies, due to the risk of arrhythmias,” Dr. Singh said.
The study was sponsored by COMPASS Pathfinder Limited. Dr. Goodwin is chief medical officer for COMPASS Pathways. Dr. Singh had no disclosures to report.
NEW ORLEANS –
“This is easily the largest study of a psychedelic drug employing modern randomized controlled trial methodology [with] 22 sites and 10 countries, so it’s not your typical phase 2 trial,” the study’s lead author, Guy M. Goodwin, MD, emeritus professor of psychiatry at the University of Oxford, England, said in an interview.
“Importantly, 94% of the patients in the study were psilocybin naive, which is very important for generalizability,” Dr. Goodwin noted.
Long used as psychedelic ‘magic mushrooms,’ psilocybin has gained increased interest in psychiatry in recent years as a potential treatment for severe depression after showing benefits in patients with life-threatening cancers and others with major depressive disorder (MDD).
To put the therapy to test in a more rigorous, randomized trial, Dr. Goodwin and colleagues conducted the phase 2b study of a proprietary synthetic formulation of psilocybin, COMP360 (COMPASS Pathways), recruiting 233 patients with treatment-resistant depression at 22 centers.
The study was presented at the annual meeting of the American Psychiatric Association.
After a 2-week washout period following the discontinuation of antidepressants, the patients were randomized to one of three groups: A single dose of 25 mg (n = 79), 10 mg (n = 75), or a subtherapeutic comparison of 1 mg (n = 79).
The psilocybin was administered in the presence of specially trained therapists who provided psychological support before, during, and after the 6- to 8-hour session.
Patients were then asked to refrain from antidepressant use for at least 3 weeks following the session, and had periodic follow-up for 12 weeks.
For the primary endpoint, those in the 25-mg group, but not the 10-mg dose, showed a significantly greater reduction in depression from baseline versus the 1-mg group on the Montgomery-Åsberg Depression Rating Scale at week 3 (MADRS; -6.6; P < .001).
The benefit was observed at day 2 and week 1 following administration, “confirming the rapid-acting character of the effect,” the investigators reported.
Sustained responses, defined as at least a 50% change from baseline in MADRS total score, were further observed up to week 12 among 20.3% in the 25-mg group and among 5.3% in the 10-mg groups versus 10.1% in the 1-mg group.
On the day of psilocybin treatment, the treatment-emergent side effects that were reported were headache, nausea, and dizziness, with event rates of 83.5% in the 25-mg group, 74.7% in the 10-mg group, and 72.2% in the 1-mg group.
One participant in the 25-mg group experienced acute anxiety and was treated with lorazepam.
The incidence of treatment-emergent serious adverse events from day 2 to week 3 was 6.3% (five patients) in the 25-mg group, 8.0% (six patients) in the 10-mg group, and 1.3% (one patient) in the 1-mg group.
Serious AEs included suicidal ideation and intentional self-injury among two patients each in the 25-mg group, while in the 10-mg group, two had suicidal ideation and one had hospitalization for severe depression.
There were no significant differences between the groups in terms of vital signs or clinical laboratory tests.
Of note, two patients in the 25-mg group had a change from baseline in QTcF >60 msec on day 2. For one patient, the increase was within normal range, and the other had a QTcF interval duration >500 msec on day 2, but levels returned to normal by day 9.
Improvements in context
Dr. Goodwin noted that the improvements were swift and impressive when compared with those of the STAR*D trial, which is the largest prospective study of treatment outcomes in patients with MDD.
“In the STAR*D trial, third- and fourth-step treatments showed low response rates of under 15% and high relapse rates,” Dr. Goodwin said. “By comparison, our response rates at 12 weeks were 20%-25%, so almost double that seen for probably equivalent treatment steps in STAR*D, with a single treatment with 25 mg, and no additional antidepressant, so no side effect burden.
“We hope to follow up enough of these patients [in the new study] to get some idea of relapse rates,” Dr. Goodwin added. “These have been low in comparable studies with MDD patients: We will see.”
Commenting on the research, Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, Rochester, Minn., said the study represents a valuable addition to needed evidence on psilocybin – with some caveats.
“This study adds to the emerging evidence base of psilocybin for treatment-resistant depression, at least in the short term,” he said in an interview. “I think the challenge in the real world would be to have access to 6-8 hours of therapy with psilocybin when patients struggle to find good therapists who could provide even weekly therapy for an hour.”
In addition, Dr. Singh questioned the durability of a single dose of psilocybin in the long term, noting a recent study (N Engl J Med. 2021 Apr 15. doi: 10.1056/NEJMoa2032994) that evaluated two doses of psilocybin (25 mg) 3 weeks apart, and failed to show any significant difference compared with the serotonergic antidepressant escitalopram at 6 weeks.
He further expressed concern about the emergence of suicidal behaviors in some patients, as well as the prolongation of QTc > 60 msec reported in the two patients.
“This is something to be carefully assessed in future studies, due to the risk of arrhythmias,” Dr. Singh said.
The study was sponsored by COMPASS Pathfinder Limited. Dr. Goodwin is chief medical officer for COMPASS Pathways. Dr. Singh had no disclosures to report.
AT APA 2022
Paraphilic disorders and sexual criminality
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5
Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.
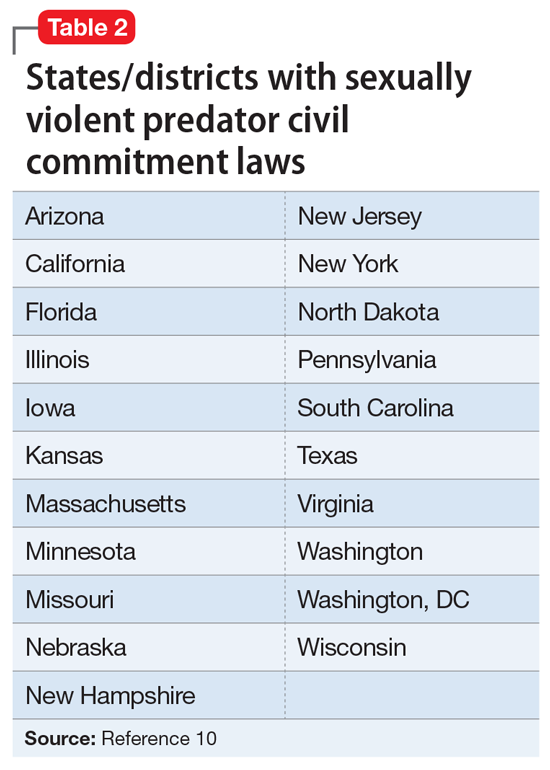
A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).
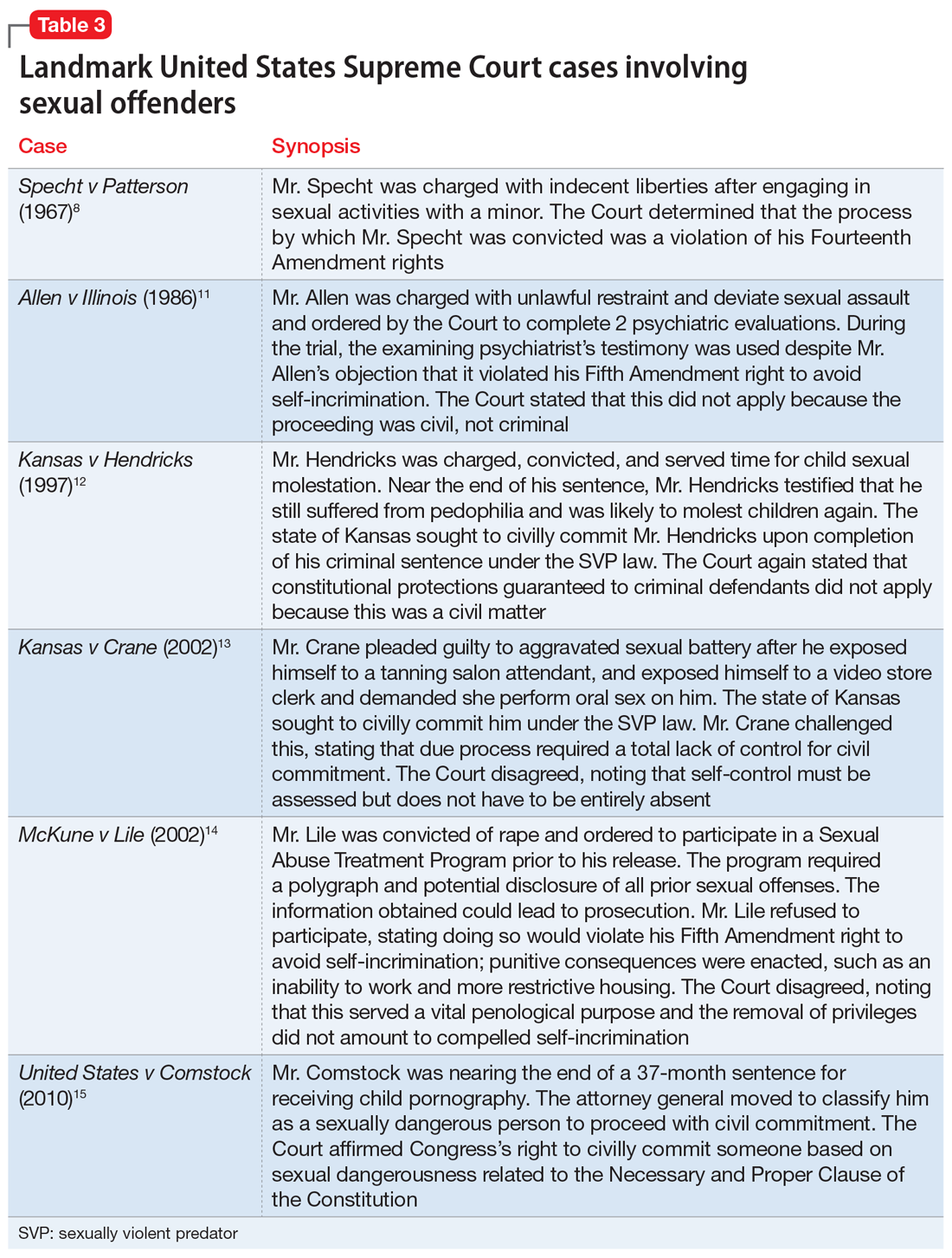
Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5

Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.

A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).

Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
Mr. J, age 23, presents to an outpatient mental health clinic for treatment of anxiety. He has no psychiatric history, is dressed neatly, and recently finished graduate school with a degree in accounting. Mr. J is reserved during the initial psychiatric evaluation and provides only basic facts about his developmental history.
Mr. J comes from a middle-class household with no history of trauma or substance use. He does not report any symptoms consistent with anxiety, but discloses a history of sexual preoccupations. Mr. J says that during adolescence he developed a predilection for observing others engage in sexual activity. In his late teens, he began following couples to their homes in the hope of witnessing sexual intimacy. In the rare instance that his voyeuristic fantasy comes to fruition, he masturbates and achieves sexual gratification he is incapable of experiencing otherwise. Mr. J notes that he has not yet been caught, but he expresses concern and embarrassment related to his actions. He concludes by noting that he seeks help because the frequency of this behavior has steadily increased.
How would you treat Mr. J? Where does the line exist between a normophilic sexual interest, fantasy or urge, and a paraphilia? Does Mr. J qualify as a sexually violent predator?
From The Rocky Horror Picture Show to Fifty Shades of Grey, sensationalized portrayals of sexual deviancy have long been present in popular culture. The continued popularity of serial killers years after their crimes seems in part related to the extreme sexual torture their victims often endure. However, a sexual offense does not always qualify as a paraphilic disorder.1 In fact, many individuals with paraphilic disorders never engage in illegal activity. Additionally, experiencing sexually deviant thoughts alone does not qualify as a paraphilic disorder.1
A thorough psychiatric evaluation should include a discussion of the patient’s sexual history, including the potential of sexual dysfunction and abnormal desires or behaviors. Most individuals with sexual dysfunction do not have a paraphilic disorder.2 DSM-5 and ICD-11 classify sexual dysfunction and paraphilic disorders in different categories. However, previous editions grouped them together under sexual and gender identity disorders. Individuals with paraphilic disorders may not originally present to the outpatient setting for a paraphilic disorder, but instead may first seek treatment for a more common comorbid disorder, such as a mood disorder, personality disorder, or substance use disorder.3
Diagnostically speaking, if individuals do not experience distress or issues with functionality and lack legal charges (suggesting that they have not violated the rights of others), they are categorized as having an atypical sexual interest but do not necessarily meet the criteria for a disorder.4 This article provides an overview of paraphilic disorders as well as forensic considerations when examining individuals with sexually deviant behaviors.
Overview of paraphilic disorders
DSM-5 characterizes a paraphilic disorder as “recurrent, intense sexually arousing fantasies, sexual urges, or behaviors generally involving nonhuman objects or nonconsenting partners for at least 6 months. The individual must have acted on the thought and/or it caused clinically significant distress or impairment in social, occupational, or other important areas of functioning.” DSM-5 outlines 9 categories of paraphilic disorders, which are described in Table 1.4,5

Continue to: Paraphilic disorders are more common...
Paraphilic disorders are more common in men than in women; the 2 most prevalent are voyeuristic disorder and frotteuristic disorder.6 The incidence of paraphilias in the general outpatient setting varies by disorder. Approximately 45% of individuals with pedophilic disorder seek treatment, whereas only 1% of individuals with zoophilia seek treatment.6 The incidence of paraphilic acts also varies drastically; individuals with exhibitionistic disorder engaged in an average of 50 acts vs only 3 for individuals with sexual sadism.6 Not all individuals with paraphilic disorders commit crimes. Approximately 58% of sexual offenders meet the criteria for a paraphilic disorder, but antisocial personality disorder is a far more common diagnosis.7
Sexual psychopath statutes: Phase 1
In 1937, Michigan became the first state to enact sexual psychopath statutes, allowing for indeterminate sentencing and the civil commitment/treatment of sex offenders with repeated convictions. By the 1970s, more than 30 states had enacted similar statutes. It was not until 1967, in Specht v Patterson,8 that the United States Supreme Court unanimously ruled that the Fourteenth Amendment Due Process Clause was violated when Francis Eddie Specht faced life in prison following his conviction for indecent liberties under the Colorado Sex Offenders Act.
Specht was convicted in 1959 for indecent liberties after pleading guilty to enticing a child younger than age 16 into an office and engaging in sexual activities with them. At the time of Specht’s conviction, the crime of indecent liberties carried a punishment of 10 years. However, Specht was sentenced under the Sexual Offenders Act, which allowed for an indeterminate sentence of 1 day to life in prison. The Supreme Court noted that Specht was denied the right to be present with counsel, to confront the evidence against him, to cross-examine witnesses, and to offer his own evidence, which was a violation of his constitutionally guaranteed Fourteenth Amendment right to Procedural Due Process. The decision led most states to repeal early sexual psychopath statutes.8
Sexually violent predator laws: Phase 2
After early sexual psychopath statutes were repealed, many states pushed to update sex offender laws in response to the Earl Shriner case.9 In 1989, Shriner was released from prison after serving a 10-year sentence for sexually assaulting 2 teenage girls. At the time, he did not meet the criteria for civil commitment in the state of Washington. On the day he was released, Shriner cut off a young boy’s penis and left him to die. Washington subsequently became the first of many states to enact sexually violent predator (SVP) laws. Table 210 shows states and districts that have SVP civil commitment laws.

A series of United States Supreme Court cases solidified current sexual offender civil commitment laws (Table 38,11-15).

Continue to: Allen v Illinois
Allen v Illinois (1986).11 The Court ruled that forcing an individual to participate in a psychiatric evaluation prior to a sexually dangerous person’s commitment hearing did not violate the individual’s Fifth Amendment right against self-incrimination because the purpose of the evaluation was to provide treatment, not punishment.
Kansas v Hendricks (1997).12 The Court upheld that the Kansas Sexually Violent Predator Act was constitutional and noted that the use of the broad term “mental abnormality” (in lieu of the more specific term “mental illness”) does not violate an individual’s Fourteenth Amendment right to substantive due process. Additionally, the Court opined that the constitutional ban on double jeopardy and ex post facto lawmaking does not apply because the procedures are civil, not criminal.
Kansas v Crane (2002).13 The Court upheld the Kansas Sexually Violent Predator Act, stating that mental illness and dangerousness are essential elements to meet the criteria for civil commitment. The Court added that proof of partial (not total) “volitional impairment” is all that is required to meet the threshold of sexual dangerousness.
McKune v Lile (2002).14 The Court ruled that a policy requiring participation in polygraph testing, which would lead to the disclosure of sexual crimes (even those that have not been prosecuted), does not violate an individual’s Fifth Amendment rights because it serves a vital penological purpose.
Adam Walsh Child Protection and Safety Act of 200616; United States v Comstock (2010).15 This act and subsequent case reinforced the federal government’s right to civilly commit sexually dangerous persons approaching the end of their prison sentences.
Continue to: What is requiried for civil commitment?
What is required for civil commitment?
SVP laws require 4 conditions to be met for the civil commitment of sexual offenders (Table 417). In criteria 1, “charges” is a key word, because this allows individuals found Not Guilty by Reason of Insanity or Incompetent to Stand Trial to be civilly committed. Criteria 2 defines “mental abnormality” as a “congenital or acquired condition affecting the emotional or volitional capacity which predisposes the person to commit criminal sexual acts in a degree constituting such person a menace to the health and safety of others.”18 This is a broad definition, and allows individuals with personality disorders to be civilly committed (although most sexual offenders are committed for having a paraphilic disorder). To determine risk, various actuarial instruments are used to assess for sexually violent recidivism, including (but not limited to) the Static-99R, Sexual Violence Risk-20, and the Sex Offender Risk Appraisal Guide.19
Although the percentages vary, sex offenders rarely are civilly committed following their criminal sentence. In California, approximately 1.5% of sex offenders are civilly committed.17 The standard of proof for civil commitment varies by state between “clear and convincing evidence” and “beyond a reasonable doubt.” As sex offenders approach the end of their sentence, sexually violent offenders are identified to the general population and referred for a psychiatric evaluation. If the individual meets the 4 criteria for commitment (Table 417), their case is sent to the prosecuting attorney’s office. If accepted, the court holds a probable cause hearing, followed by a full trial.
Pornography and sex offenders
Pornography has long been considered a risk factor for sexual offending, and the role of pornography in influencing sexual behavior has drawn recent interest in research towards predicting future offenses. However, a 2019 systematic review by Mellor et al20 on the relationship between pornography and sexual offending suggested that early exposure to pornography is not a risk factor for sexual offending, nor is the risk of offending increased shortly after pornography exposure. Additionally, pornography use did not predict recidivism in low-risk sexual offenders, but did in high-risk offenders.
The use of child pornography presents a set of new risk factors. Prohibited by federal and state law, child pornography is defined under Section 2256 of Title 18, United States Code, as any visual depiction of sexually explicit conduct involving a minor (someone <age 18). Visual depictions include photographs, videos, digital or computer-generated images indistinguishable from an actual minor, and images created to depict a minor. The law does not require an image of a child engaging in sexual activity for the image to be characterized as child pornography. Offenders are also commonly charged with the distribution of child pornography. A conviction of child pornography possession carries a 15- to 30-year sentence, and distribution carries a 5- to 20-year sentence.21 The individual must also file for the sex offender registry, which may restrict their employment and place of residency.
It is unclear what percentage of individuals charged with child pornography have a history of prior sexual offenses. Numerous studies suggest there is a low risk of online offenders without prior offenses becoming contact offenders. Characteristics of online-only offenders include being White, a single male, age 20 to 30, well-educated, and employed, and having antisocial traits and a history of sexual deviancy.22 Contact offenders tend to be married with easy access to children, unemployed, uneducated, and to have a history of mental illness or criminal offenses.22
Continue to: Recidivism and treatment
Recidivism and treatment
The recidivism rate among sexual offenders averages 13.7% at 3- to 6-year follow-up,although rates vary by type of sexual offense.23 Individuals who committed rape have the highest rate of recidivism, while those who engaged in incest have the lowest. Three key points about sexual offender recidivism are:
- it declines over time and with increased age.
- sexual offenders are more like to commit a nonsexual offense than a sexual offense.
- sexual offenders who have undergone treatment are 26.3% less likely to reoffend.23
Although there is no standard of treatment, current interventions include external control, reduction of sexual drive, treatment of comorbid conditions, cognitive-behavioral therapy (CBT), and dynamic psychotherapy. External control relies on an outside entity that affects the individual’s behavior. For sexually deviant behaviors, simply making the act illegal or involving the law may inhibit many individuals from acting on a thought. Additional external control may include pharmacotherapy, which ranges from nonhormonal options such as selective serotonin reuptake inhibitors (SSRIs) to hormonal options. Therapy tends to focus on social skills training, sex education, cognitive restructuring, and identifying triggers, as well as victim empathy. The best indicators for successful treatment include an absence of comorbidities, increased age, and adult interpersonal relationships.24
Treatment choice may be predicated on the severity of the paraphilia. Psychotherapy alone is recommended for individuals able to maintain functioning if it does not affect their conventional sexual activity. Common treatment for low-risk individuals is psychotherapy and an SSRI. As risk increases, so does treatment with pharmacologic agents. Beyond SSRIs, moderate offenders may be treated with an SSRI and a low-dose antiandrogen. This is escalated in high-risk violent offenders to long-acting gonadotropin-releasing hormone analogs and synthetic steroidal analogs.25
An evolving class of disorders
With the evolution and accessibility of pornography, uncommon sexual practices have become more common, gaining notoriety and increased social acceptance. As a result, mental health professionals may be tasked with evaluating patients for possible paraphilic disorders. A common misconception is that individuals with sexually deviant thoughts, sexual offenders, and patients with paraphilic disorders are all the same. However, more commonly, sexual offenders do not have a paraphilic disorder. In the case of SVPs, outside of imprisonment, civil commitment remains a consideration for possible treatment. To meet the threshold of civil commitment, a sexual offender must have a “mental abnormality,” which is most commonly a paraphilic disorder. The treatment of paraphilic disorders remains a difficult task and includes a mixture of psychotherapy and medication options.
CASE CONTINUED
Mr. J begins weekly CBT to gain control of his voyeuristic fantasies without impacting his conventional sexual activity and desire. He responds well to treatment, and after 18 months, begins a typical sexual relationship with a woman. Although his voyeuristic thoughts remain, the urge to act on the thoughts decreases as Mr. J develops coping mechanisms. He does not require pharmacologic treatment.
Bottom Line
Individuals with paraphilic disorders are too often portrayed as sexual deviants or criminals. Psychiatrists must review each case with careful consideration of individual risk factors, such as the patient’s sexual history, to evaluate potential treatment options while determining if they pose a threat to the public.
Related Resources
- Sorrentino R, Abramowitz J. Minor-attracted persons: a neglected population. Current Psychiatry. 2021;20(7):21-27. doi:10.12788/cp.0149
- Berlin FS. Paraphilic disorders: a better understanding. Current Psychiatry. 2019;18(4):22-26,28.
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
1. Federoff JP. The paraphilias. In: Gelder MG, Andreasen NC, López-Ibor JJ Jr, Geddes JR, eds. New Oxford Textbook of Psychiatry. 2nd ed. Oxford University Press; 2012:832-842.
2. Grubin D. Medical models and interventions in sexual deviance. In: Laws R, O’Donohue WT, eds. Sexual Deviance: Theory, Assessment and Treatment. 2nd ed. Guilford Press; 2008:594-610.
3. Guidry LL, Saleh FM. Clinical considerations of paraphilic sex offenders with comorbid psychiatric conditions. Sex Addict Compulsivity. 2004;11(1-2):21-34.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Balon R. Paraphilic disorders. In: Roberts LW, Hales RE, Yudofsky SC, eds. The American Psychiatric Association Publishing Textbook of Psychiatry. 7th ed. American Psychiatric Association Publishing; 2019:749-770.
6. Sadock BJ, Sadock VA, Ruiz P. Paraphilic disorders. Kaplan and Sadock’s Synopsis of Psychiatry. 11th ed. Wolters Kluwer; 2015:593-599.
7. First MB, Halon RL. Use of DSM paraphilia diagnosis in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
8. Specht v Patterson, 386 US 605 (1967).
9. Ra EP. The civil confinement of sexual predators: a delicate balance. J Civ Rts Econ Dev. 2007;22(1):335-372.
10. Felthous AR, Ko J. Sexually violent predator law in the United States. East Asian Arch Psychiatry. 2018;28(4):159-173.
11. Allen v Illinois, 478 US 364 (1986).
12. Kansas v Hendricks, 521 US 346 (1997).
13. Kansas v Crane, 534 US 407 (2002).
14. McKune v Lile, 536 US 24 (2002).
15. United States v Comstock, 560 US 126 (2010).
16. Adam Walsh Child Protection and Safety Act of 2006, HR 4472, 109th Cong (2006). Accessed April 25, 2022. https://www.congress.gov/bill/109th-congress/house-bill/4472
17. Tucker DE, Brakel SJ. Sexually violent predator laws. In: Rosner R, Scott C, eds. Principles and Practice of Forensic Psychiatry. 3rd ed. CRC Press; 2017:823-831.
18. Wash. Rev. Code. Ann. §71.09.020(8)
19. Bradford J, de Amorim Levin GV, Booth BD, et al. Forensic assessment of sex offenders. In: Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2017:382-397.
20. Mellor E, Duff S. The use of pornography and the relationship between pornography exposure and sexual offending in males: a systematic review. Aggress Violent Beh. 2019;46:116-126.
21. Failure To Register, 18 USC § 2250 (2012). Accessed April 25, 2022. https://www.govinfo.gov/app/details/USCODE-2011-title18/USCODE-2011-title18-partI-chap109B-sec2250
22. Hirschtritt ME, Tucker D, Binder RL. Risk assessment of online child sexual exploitation offenders. J Am Acad Psychiatry Law. 2019;47(2):155-164.
23. Blasko BL. Overview of sexual offender typologies, recidivism, and treatment. In: Jeglic EL, Calkins C, eds. Sexual Violence: Evidence Based Policy and Prevention. Springer; 2016:11-29.
24. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490.
25. Holoyda B. Paraphilias: from diagnosis to treatment. Psychiatric Times. 2019;36(12).
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 2)
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (

Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16

Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (

Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16

Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
There is a need to connect mental and physical symptoms in the diagnosis and treatment of psychiatric disorders. Obviously, we are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. The science of defining the underlying mechanisms is lagging behind the clinical needs. However, in this article, we present a few hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. Our descriptions do not reflect the entire set of symptoms caused by these neurotransmitters; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypotheses we present.
In Part 1 (

Endorphin excess (Table 11-16)
Ms. R is a frustrated chronic pain patient who bitterly complains that despite having seen more than 20 physicians, she does not have an answer to what causes her “all over” pain and headache.4,5,11 She does not believe that all her laboratory test are normal, and insists that “something is missing.” She aches all over but says she can actually tolerate more pain than others and experiences only a little discomfort during an electromyogram or dental interventions. Though Ms. R is not very susceptible to acute pain,4,5,9,16 pain all over without an identifiable cause is part of her life.4,5,11 She says that listening to music and social interactions help decrease her pain.4,5,10 Ms. R states that opioid medications do not help her pain, though she has a history of opioid overuse and opioid-induced hyperalgesia.6,11,16

Ms. R tends to overdo pleasureful activities to achieve satisfaction.2 She says exercise is particularly satisfying, to the point that she experiences euphoria and a loss of time.9 She is angry that her neurologist suggested she see a psychiatrist. Her depression bothers her more than her anxiety.2,5,7
Ms. R clearly has a self-image problem, alternating between high and low self-esteem. She has a low appetite1,12,14-16 and sleeps excessively.2,4,7,9,10 Her mother privately tells you that Ms. R has a history of childhood sexual abuse and lagged in life due to a lack of motivation. Ms. R used to self-mutilate “to feel normal.”12 Her primary care physician chronically addresses Ms. R’s poorly explained cholestasis and pruritus8 as well as dysregulation of blood pressure and heart rate, both of which tend to be low.12,13,16
Impression. Ms. R shows multiple symptoms associated with endorphin excess. A trial of an opioid antagonist may be reasonable. Dopamine blockade helps with endorphin suppression and also may be used for this patient. Using a low starting dose and a slow titration of such medications would be beneficial due to frequent intolerance issues, especially nausea. Gamma aminobutyric acid-ergic medications modulate the opioid system and may be considered. A serotonin-norepinephrine reuptake inhibitor (SNRI) or mirtazapine may help patients such as Ms. R to control mood and pain through norepinephrine’s influence on endorphins.
Endorphin deficiency (Table 11,16-24)
Mr. J complains of low back pain, diffuse body pain, depression, and moodiness.19,20,24 He is sluggish and plagued by psychomotor retardation.24 All his life, a heightened perception of pain has caused him problems,19,20 but has not stopped him from engaging in self-mutilation
Continue to: Mr. J responds to treatment...
Mr. J responds to treatment with opioids16,20 but comments that his mood, and not necessarily his pain, improves when he takes these medications.20 He tends to overuse his pain medications, and had run into trouble with his previous pain management physician. Nitrous oxide is remarkably effective during dental procedures.19 Acupuncture helps to control his pain and mood.17 Exercise is also rewarding.18
Mr. J has difficulty achieving orgasm, a decreased sexual drive, and emotional sensitivity.24 He is impulsive.19,20,24 His baseline mood is low-grade; anxiety bothers him more than depression.23,24 Mr. J is thin, has a poor appetite,1,16 and sleeps poorly.24 His primary care physician struggles to help Mr. J to control dysregulation of his heart rate, blood pressure,21 and urinary retention,16,22 as well as episodes of hypoglycemia.1,16 He reluctantly admits to abusing alcohol, but explains that it helps with his mood and pain better than his prescribed medications.18,23
Impression. Mr. J exhibits multiple symptoms associated with endorphin deficiency. Short-term use of opioids is warranted, but he should avoid long-term opioid use, and he and his physician should work together to establish strict control of their intake. Buprenorphine would be the opioid of choice for such a patient. Psychiatric treatment, including for alcohol use disorder, should be a mandatory part of his treatment regimen. Behavioral therapy with a focus on finding healthy ways to achieve gratification would be effective. Alternative treatments such as acupuncture may be of value.
Norepinephrine excess (Table 216,25-30)
Mr. G comes to the office irritable and angry28,30 because no one can help him with his intractable headaches.
Comment. Norepinephrine and dopamine functions are connected through common neuronal and glial uptake mechanisms. This is a foundation of norepinephrine excess symptoms crossing over with symptoms of dopamine deficiency.
Continue to: Impression
Impression. Mr. G shows multiple symptoms associated with norepinephrine excess. It is important to avoid caffeine intake in patients with clinical signs of excessive norepinephrine. Beta-blockers and alpha-2 agonists work well in patients such as Mr. G. Benzodiazepines indirectly decrease norepinephrine activity, but need to be used carefully due to the potential for misuse and addiction. In particular, short-acting benzodiazepines such as alprazolam and lorazepam must be avoided due to the induction of CNS instability with rapidly changing medication blood levels. Chlordiazepoxide may be a good choice for a patient such as Mr. G because it has the fewest adverse effects and the lowest abuse potential compared with other benzodiazepines. Avoid SNRIs in such a patient. Using mood-stabilizing antipsychotic medications may be especially warranted in treating Mr. G’s depression and pain.
Norepinephrine deficiency (Table 216,26,31-39)
Two years ago, Ms. A was diagnosed with chronic fatigue31 and fibromyalgia. She also had been diagnosed with depression and attention-deficit/hyperactivity disorder (ADHD). She presents with concerns of “brain fog,” no energy, low sex drive, and daytime sleepiness.33,35 Allodynia is widespread.16,36,37 Ms. A suffers from bulimia; she eats once a day but is still overweight.26 She has orthostatic hypotension in addition to baseline low blood pressure and bradycardia.16,38,39 Her pupils are almost pinpoint, even when she does not take opioid medications.
Comment. As mentioned earlier, because of the norepinephrine/dopamine relationship, symptoms of excess dopamine overlap with symptoms of norepinephrine deficiency.
Impression. Ms. A shows multiple symptoms associated with norepinephrine deficiency. The use of noradrenergic antidepressants (such as SNRIs and mirtazapine)26 and stimulants may be warranted. Physical exercise, participating in social activities, massage, acupuncture, and family support may help with Ms. A’s pain as well as her depression, as might vasopressors.
In Part 3, we will address gamma aminobutyric acid and glutamate.
Bottom Line
Both high and low levels of endorphins and norepinephrine may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (Part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Lorazepam • Ativan
Mirtazapine • Remeron
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
1. Applyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760.
2. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104-111.
3. Dabo F, Nyberg F, Qin Zhou, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17(8):742-747.
4. Dunbar RI, Kaskatis K, MacDonald I, et al. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688-702.
5. Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
6. Grisel JE, Bartels JL, Allen SA, et al. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl). 2008;200(1):105-115.
7. Zorrilla EP, DeRubeis RJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20(6):591-601.
8. Li X, Zhu J, Tao Y, et al. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-121.
9. Hicks SD, Jacob P, Perez O, et al. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970-978.
10. Dunbar RIM. The anatomy of friendship. Trends Cogn Sci. 2018;22(1):32-51.
11. Stephan BC, Parsa FD. Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood, and their relation to postoperative pain. Hawaii J Med Public Health. 2016;75(3):63-70.
12. Cuthbert BN, Holaday JW, Meyerhoff J, et al. Intravenous beta-endorphin: behavioral and physiological effects in conscious monkeys. Peptides. 1989;10(4):729-734.
13. Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;(6):977-981.
14. Davis JM, Lowy MT, Yim GK, et al. Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides. 1983;4(1):79-83.
15. Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3): 421-428.
16. Hall JE, Guyton AC. Textbook of Medical Physiology. 12th ed. Spanish version. Elsevier; 2011:587-588.
17. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1-3):258-261.
18. Harte JL, Eifert GH, Smith R. The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol. 1995;40(3):251-265.
19. Petrizzo R, Mohr J, Mantione K, et al. The role of endogenous morphine and nitric oxide in pain management. Pract Pain Manag. 2014;14(9).
20. Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69(3):70-100.
21. Dontsov AV. The influence of deficit of endogenous neuropeptides on the clinical course of coronary artery disease. Klin Med (Mosk). 2017;95(2):127-131. In Russian.
22. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides. 1984;5(3):645-647.
23. Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26(4):701-705.
24. McLay RN, Pan W, Kastin AJ. Effects of peptides on animal and human behavior: a review of studies published in the first twenty years of the journal Peptides. Peptides. 2001;22(12):2181-2255.
25. Wong-Riley MT. Neuroscience Secrets. 1st ed. Spanish version. Hanley & Belfus; 1999:424-428.
26. Brewerton TD. Clinical Handbook of Eating Disorders: An Integrated Approach. CRC Press; 2004:257-281.
27. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, et al. Stress response, brain noradrenergic system and cognition. Adv Exp Med Biol. 2017;980:67-74.
28. McCall JG, Al-Hasani R, Siuda ER, et al. Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620.
29. Wszedybyl-Winklewska M, Wolf J, Szarmach A, et al. Central sympathetic nervous system reinforcement in obstructive sleep apnoea. Sleep Med Rev. 2018;39:143-154.
30. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;201(68):1-20.
31. Stone EA, Lin Y, Sarfraz Y, et al. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1-2):193-208.
32. Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861-871.
33. De Carvalho D, Patrone LG, Taxini CL, et al. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front Physiol. 2014;5(288):1-13.
34. Markianos M, Evangelopoulos ME, Koutsis G, et al. Evidence for involvement of central noradrenergic activity in crying proneness. J Neuropsychiatry Clin Neurosci. 2011;23:403-408.
35. Cao S, Fisher DW, Yu T, et al. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;(16):204-215.
36. Caraci F, Merlo S, Drago F, et al. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front Pharmacol. 2019;(10):1024.
37. Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20(4):822.
38. Kur’yanova EV, Tryasuchev AV, Stupin VO, et al. Effect of atropine on adrenergic responsiveness of erythrocyte and heart rhythm variability in outbred rats with stimulation of the central neurotransmitter systems. Bull Exp Biol Med. 2018;165(5):165(5):597-601.
39. Peterson AC, Li CR. Noradrenergic dysfunction in Alzheimer’s and Parkinson’s disease: an overview of imaging studies. Front Aging Neurosci. 2018;(10):127.
A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
Having witnessed the devastating impact of stigma on patients with mental illness throughout my psychiatric career, I am fed up and disgusted with this malevolent scourge.
I regard the stigma that engulfs neuropsychiatric disorders as a malignancy that mutilates patients’ souls and hastens their mortality.
Stigma is hate speech
How would you feel if you had a serious medical illness, a disabling brain disorder such as schizophrenia, depression, or anxiety, and people refer to you with pejorative and insulting terms such as crazy, deranged, lunatic, unhinged, nutty, insane, wacky, berserk, cuckoo, bonkers, flaky, screwball, or unglued? This is hate speech generated by stigma against people with mental illness. Individuals with heart disease, cancer, or diabetes never get called such disgraceful and stigmatizing terms that shame, stain, besmirch, and scar them, which happens daily to persons with psychiatric brain disorders.
The damage and harm of the discriminatory stigma on our patients is multifaceted. It is painful, detrimental, pernicious, and deleterious. It is corrosive to their spirits, crippling to their self-image, and subversive to their self-confidence. Hate speech is not simply words, but a menacing weapon that assaults the core humanity of medically ill psychiatric patients.
Although hate speech is punishable by law, there are rarely any legal actions against those who hurl hate speech at psychiatric patients every day. Society has institutionalized the stigma of mental illness and takes it in stride instead of recognizing it as an illegal, harmful act.
Long before the stresses of the COVID-19 pandemic, 43% of the population had been shown to experience a diagnosable psychiatric disorder over the course of their life.1 Thus, tens of millions of people are burdened by stigma and the hate speech associated with it. This is directly related to massive ignorance about mental illness being the result of a neurobiological condition due to either genetic or intrauterine adverse events that disrupt brain development. Delusions and hallucinations are symptoms of a malfunctioning brain, depression is not a sign of personal weakness, anxiety is the most prevalent mental disorder in the world, and obsessive-compulsive disorder (OCD) is not odd behavior but the result of dysfunction of neural circuits. Correcting public misperceptions about psychiatric brain disorders can mitigate stigma, but it has yet to happen.
Stigma is a hate crime
Stigma can accelerate physical death and premature mortality. Many studies have confirmed that persons with schizophrenia do not receive basic primary care treatments for the life-shortening medical conditions that often afflict them, such as diabetes, dyslipidemia, and hypertension.2 Stigma is responsible for a significant disparity of medical3-5 and intensive care6 among individuals with mental illness compared to the general population. It’s no wonder most psychiatric disorders are associated with accelerated mortality.7 A recent study during the pandemic by Balasuriya et al8 reported that patients with depression had poor access to care. Stigma interferes with or delays necessary medical care, leading to clinical deterioration and unnecessary, preventable death. Stigma shortens life and is a hate crime.
Continue to: The extremely high suicide rates...
The extremely high suicide rates among individuals with serious mental illness, who live under the oppressiveness of stigma, is another example of how stigma is a hate crime that can cause patients with psychiatric disorders to give up and end their lives. Zaheer et al9 found that young patients with schizophrenia had an astronomical suicide rate compared to the general population (1 in 52 in individuals with schizophrenia, compared to 12 in 100,000 in the general population, roughly a 200-fold increase!). This is clearly a consequence of stigma and discrimination,10 which leads to demoralization, shame, loneliness, distress, and hopelessness. Stigma can be fatal, and that makes it a hate crime.
Stigma also limits vocational opportunities for individuals with mental illness. They are either not hired, or quickly fired. Even highly educated professionals such as physicians, nurses, lawyers, or teachers can lose their jobs if they divulge a history of a psychiatric disorder or alcohol or substance abuse, regardless of whether they are receiving treatment and are medically in remission. Even highly qualified politicians have been deemed “ineligible” for higher office if they disclose a history of psychiatric treatment. Stigma is loaded with outrageous discrimination that deprives our patients of “the pursuit of happiness,” a fundamental constitutional right.
Stigma surrounding the mental health professions
Stigma also engulfs mental health professionals, simply because they deal with psychiatric patients every day. In a classic article titled “The Enigma of Stigma,”11 Dr. Paul Fink, past president of the American Psychiatric Association (1988-1989), described how psychiatrists are perceived as “different” from other physicians by the public and by the media. He said psychiatrists are tarred by the same brush as their patients as “undesirables” in society. And movies such as Psycho and One Flew Over the Cuckoo’s Nest reinforce the stigma against both psychiatric patients and the psychiatrists and nurses who treat them. The health care system that carves out “behavioral health” from the umbrella of “medical care” further accentuates the stigma by portraying the “separateness” of psychiatry, a genuine medical specialty, from its fellow medical disciplines. This becomes fodder for the antipsychiatry movement at every turn and can even lead to questioning the existence of mental illness, as Thomas Szasz12 did by declaring that mental illness is a myth and describing psychiatry as “the science of lies.” No other medical specialty endures abuse and insults like psychiatry, and that’s a direct result of stigma.
Extinguishing stigma is a societal imperative
So what can be done to squelch stigma and defeat it once and for all, so that psychiatric patients can be treated with dignity and compassion, like people with cancer, heart attacks, diabetes, or brain tumors? The pandemic, terrible as it has been for the entire world, did have the silver lining of raising awareness about the ubiquity of psychiatric symptoms, such as anxiety and depression, across all ages, genders, educational and religious backgrounds, and socioeconomic classes. But there should also be a robust legal battle against the damaging effects of stigma. There are laws to sanction and penalize hate speech and hate crimes that must be implemented when stigma is documented. There are also parity laws, but they have no teeth and have not ameliorated the insurance discrepancies and economic burden of psychiatric disorders. A bold step would be to reclassify serious psychiatric brain disorders (schizophrenia, bipolar disorder, major depressive disorder, OCD, attention-deficit/hyperactivity disorder, generalized anxiety disorder/panic attacks, and borderline personality disorder) as neurologic disorders, which would automatically give patients with these disorders broad access to medical care, which happened when autism was reclassified as a neurologic disorder. Finally, a much more intensive public education must be disseminated about the neurobiological etiologies, brain structure, and function in psychiatric disorders, and the psychiatric symptoms associated with all neurologic disorders. Regrettably, empathy can be difficult to teach.
Stigma is hate speech and a hate crime. It must be permanently eliminated by effective laws and by erasing the widespread ignorance about the medical and neurologic roots of mental disorders, and by emphasizing the fact that they are as treatable as other general medical conditions.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
3. Druss BG, Rosenheck RA. Use of medical services by veterans with mental disorders. Psychosomatics. 1997;38(5):451-458.
4. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry. 1998;155(12):1775-1777.
5. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
6. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
7. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
8. Balasuriya L, Quinton JK, Canavan ME, et al. The association between history of depression and access to care among Medicare beneficiaries during the COVID-19 pandemic. J Gen Intern Med. 2021;36(12):3778-3785.
9. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
10. Brohan E, Thornicroft G, Rüsch N, et al. Measuring discrimination experienced by people with a mental illness: replication of the short-form DISCUS in six world regions. Psychol Med. 2022:1-11. doi:10.1017/S0033291722000630
11. Fink P. The enigma of stigma and its relation to psychiatric education. Psychiatric Annals. 1983;13(9):669-690.
12. Szasz T. The Myth of Mental Illness. Harper Collins; 1960.
The brain’s Twitter system: Neuronal extracellular vesicles
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.
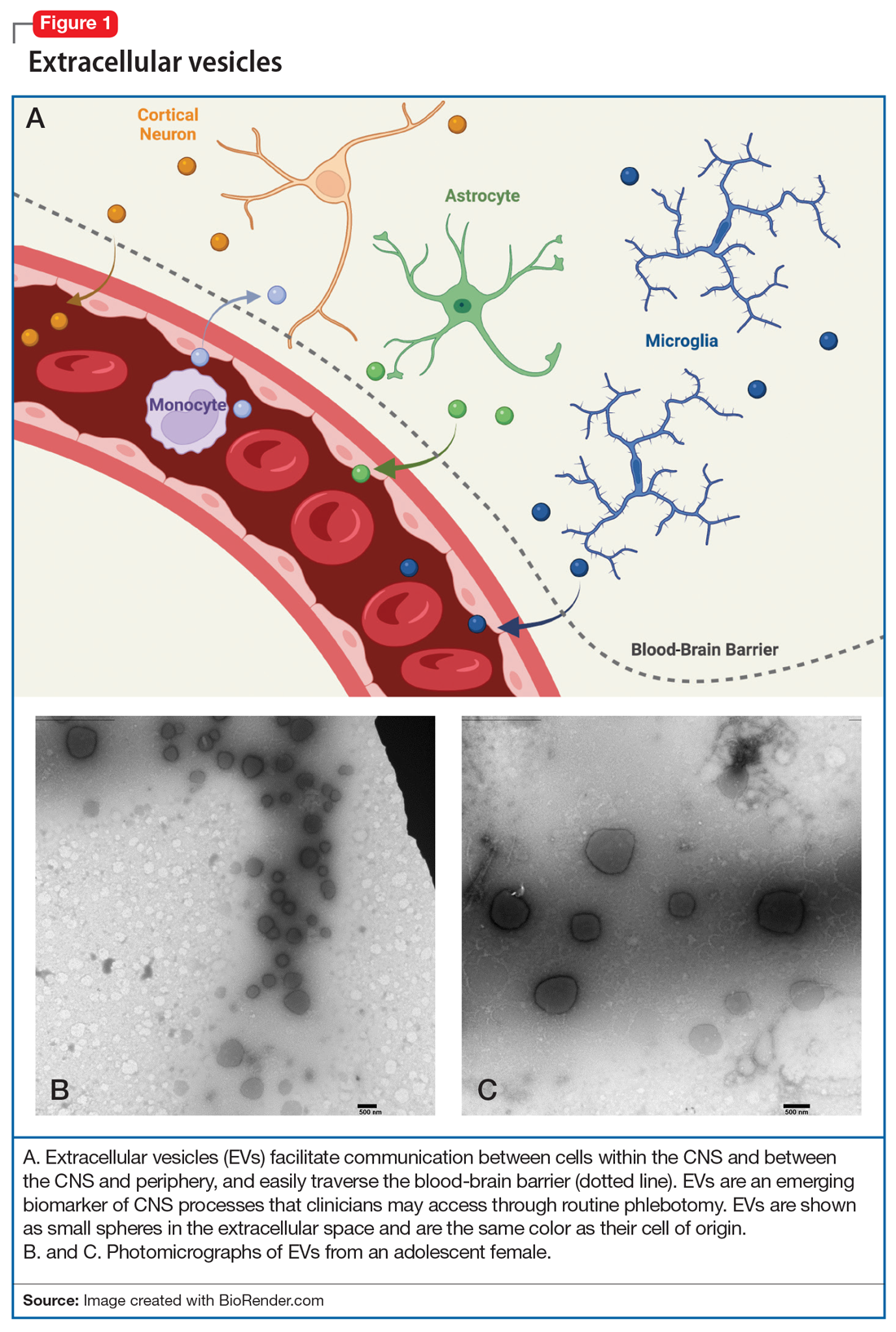
Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.
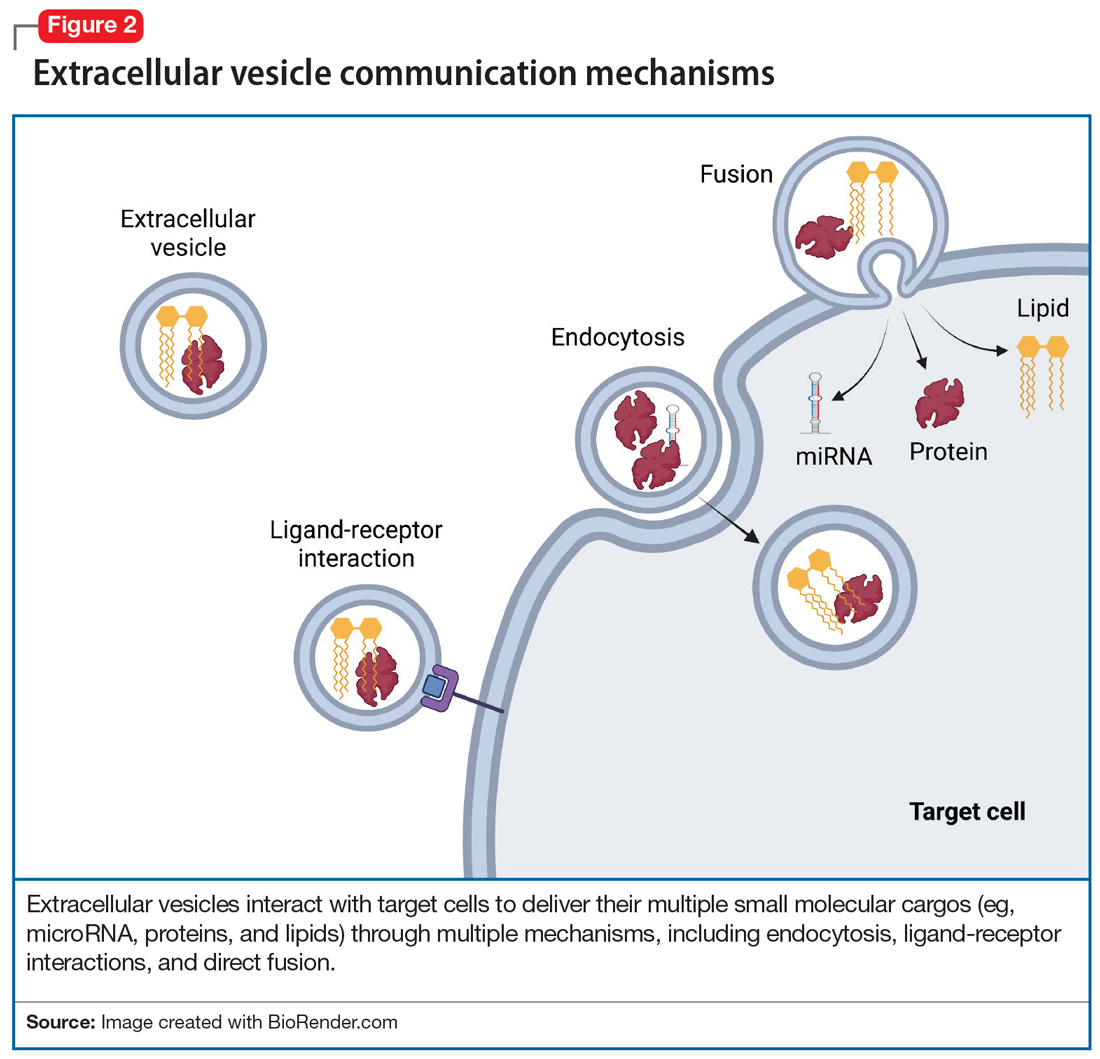
EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.
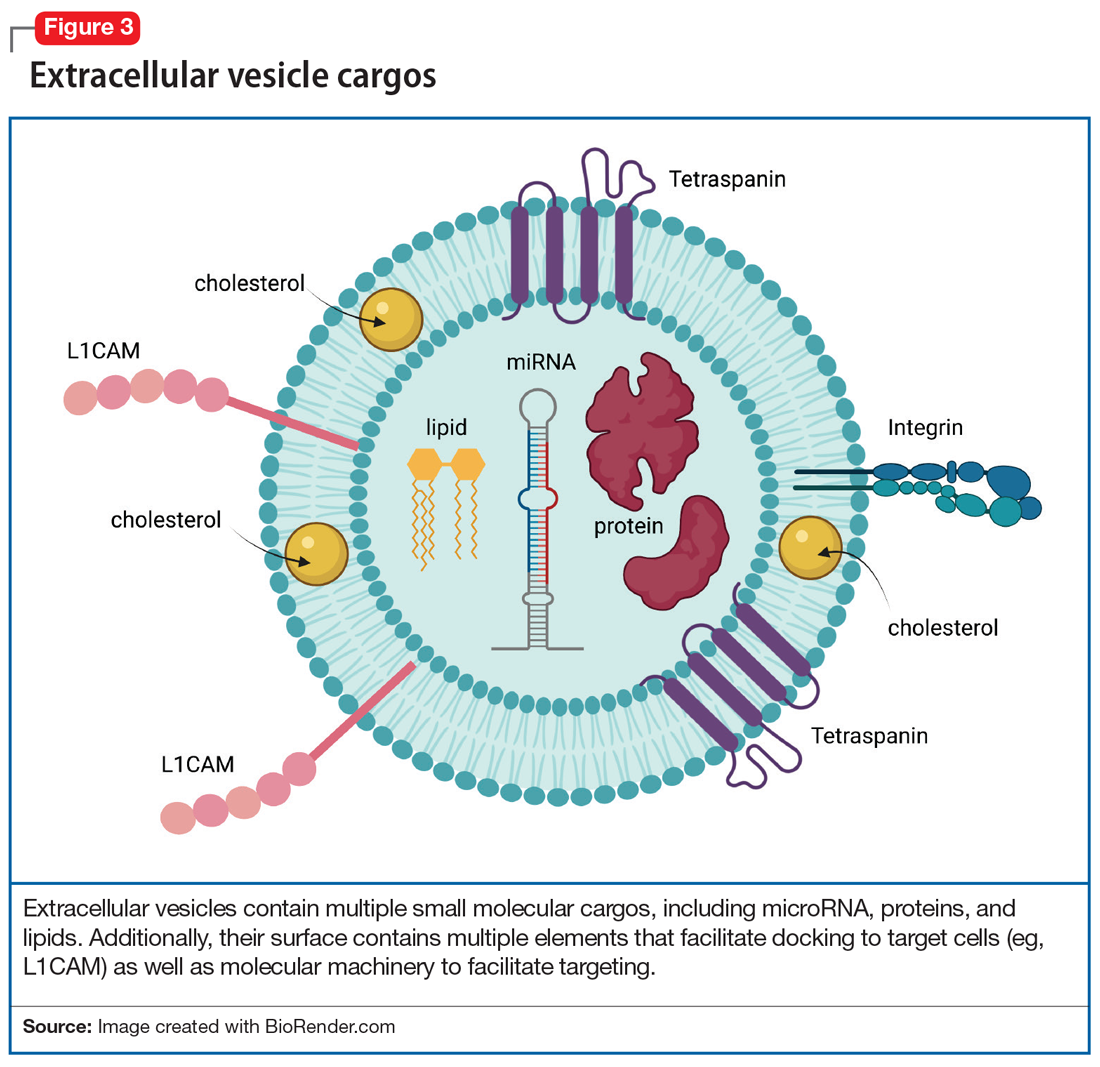
EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758