User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Dexmedetomidine sublingual film for agitation
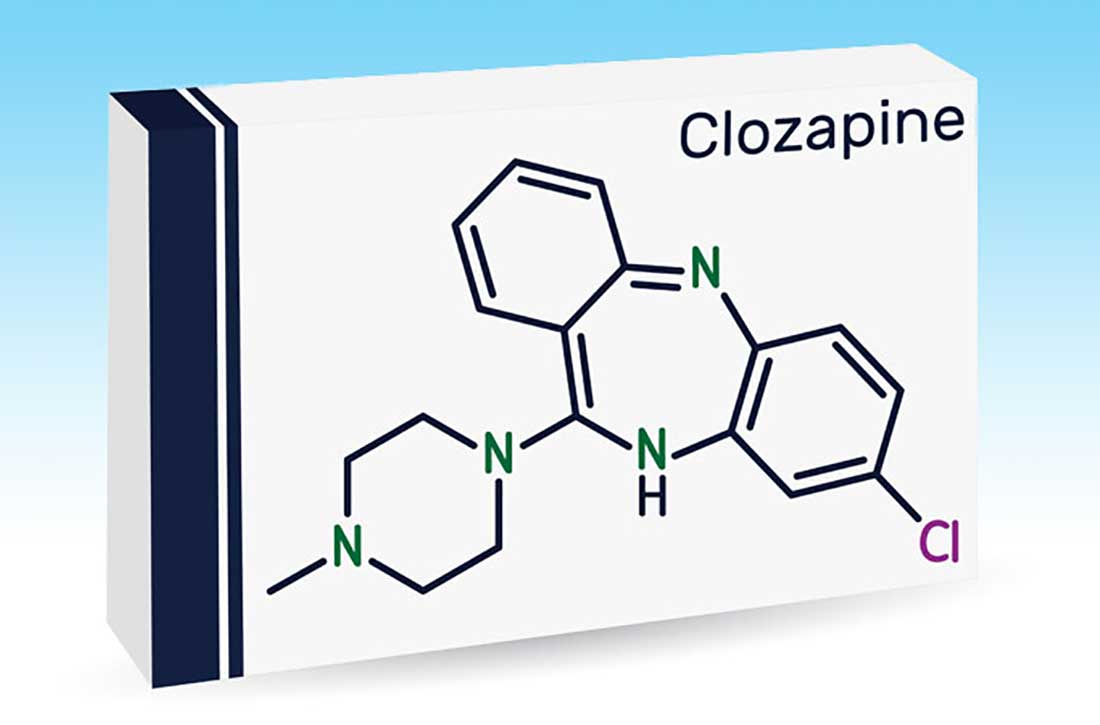
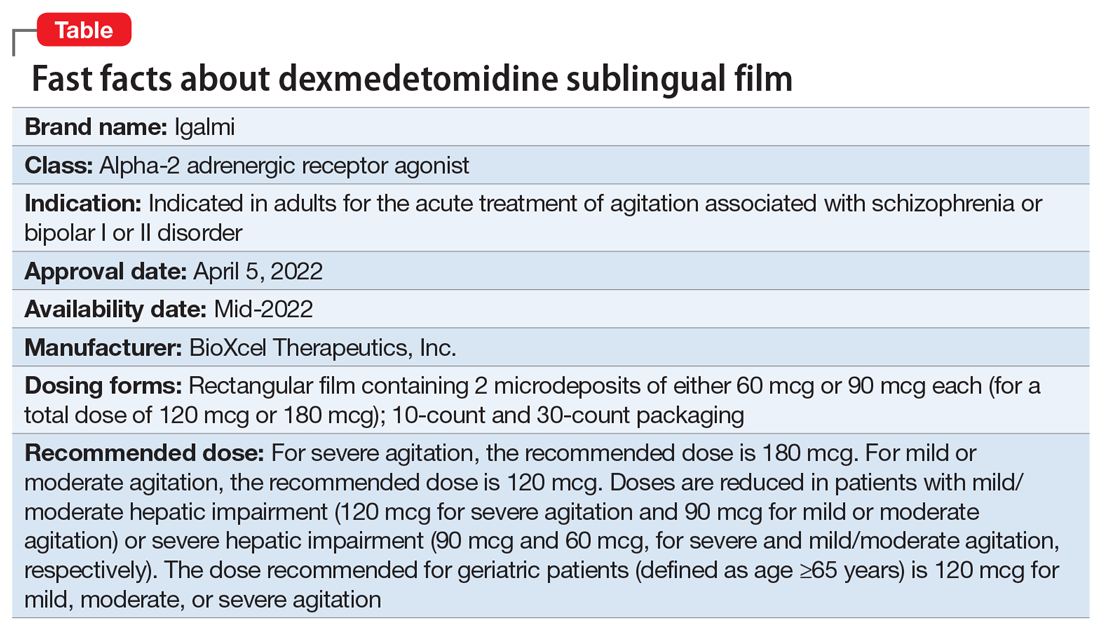
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2

Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Sublingual buprenorphine plus buprenorphine XR for opioid use disorder
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
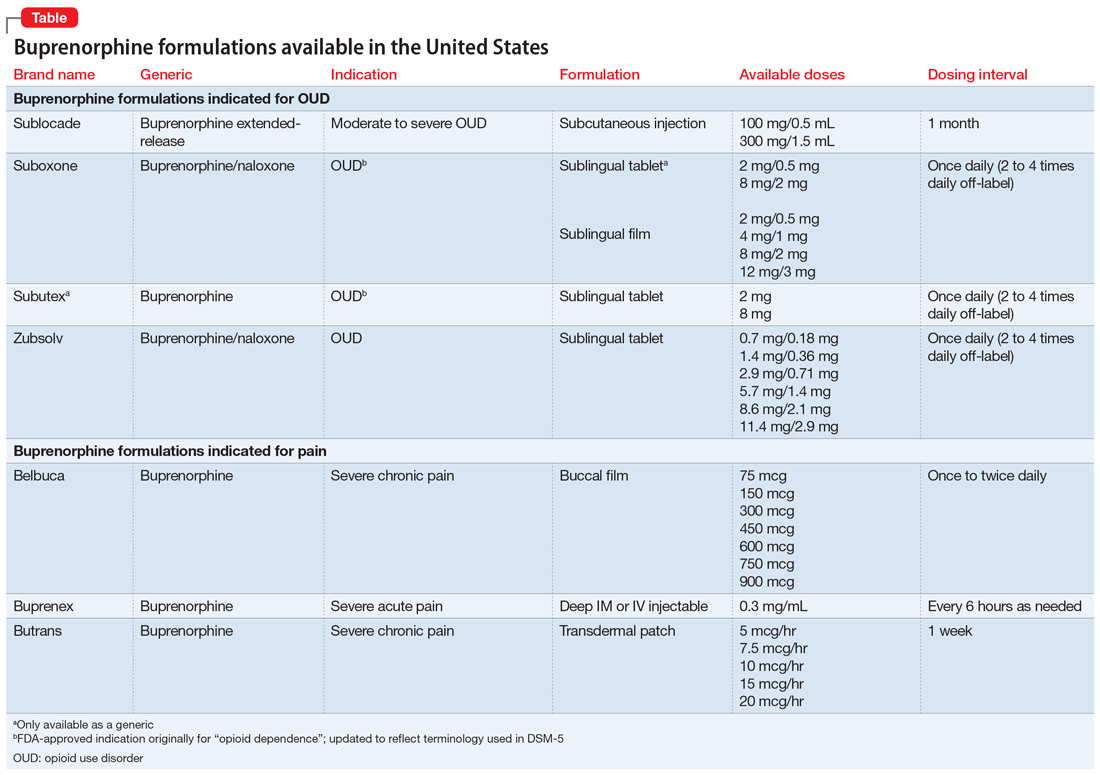
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Should clozapine be discontinued in a patient receiving chemotherapy?
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.
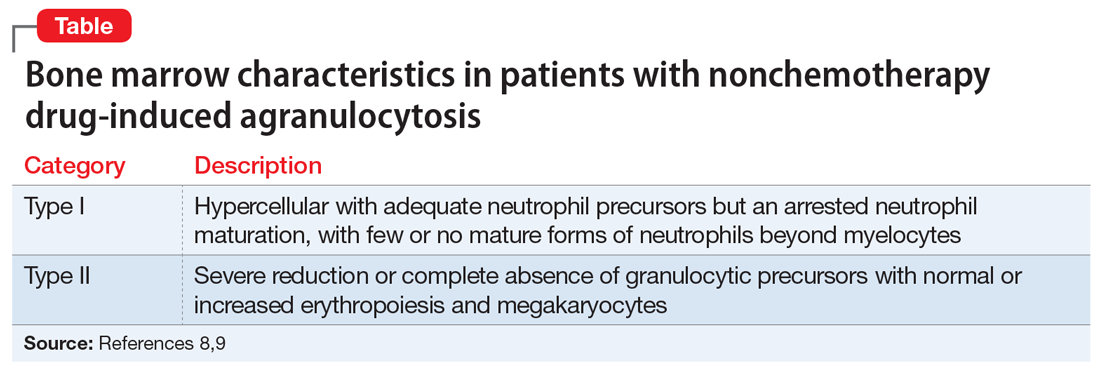
Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.
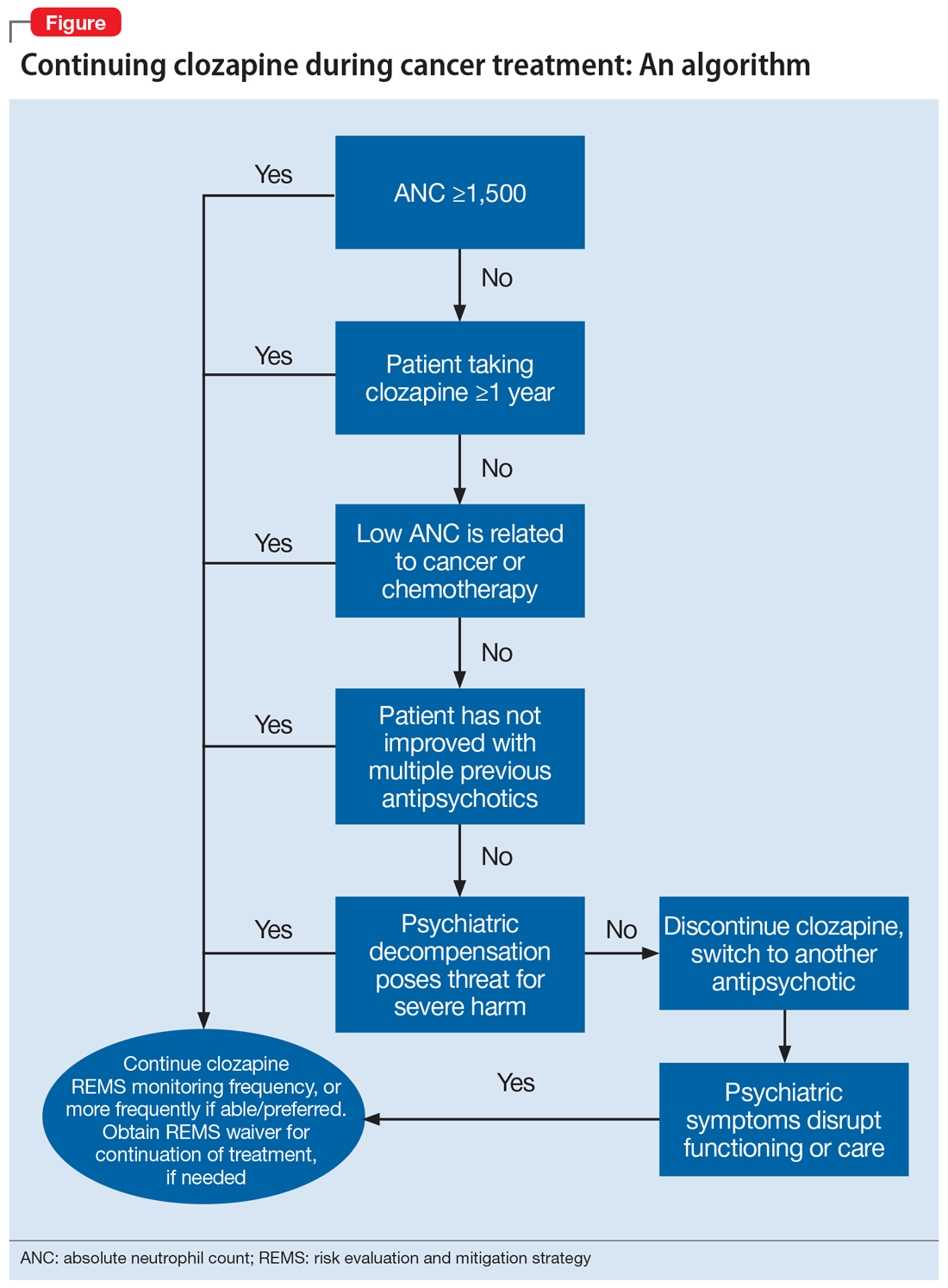
OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
CASE Schizophrenia, leukemia, and chemotherapy
Mr. A, age 30, has schizophrenia but has been stable on clozapine 600 mg/d. He presents to the emergency department with generalized pain that started in his right scapula, arm, elbow, and back. Laboratory tests and a diagnostic examination reveal severe leukocytosis, thrombocytopenia, and anemia, and clinicians diagnose Mr. A with B-cell acute lymphocytic leukemia (B-ALL). Upon admission, Mr. A is neutropenic with an absolute neutrophil count (ANC) of 1,420 µL (reference range 2,500 to 6,000 µL). The hematology team recommends chemotherapy. The treating clinicians also consult the psychiatry team for recommendations on how to best manage Mr. A’s schizophrenia during chemotherapy, including whether clozapine should be discontinued.
HISTORY Stable on clozapine for >10 years
Mr. A was diagnosed with schizophrenia at age 15 after developing paranoia and auditory hallucinations of people talking to him and to each other. He had been hospitalized multiple times for worsened auditory hallucinations and paranoia that led to significant agitation and violence. Previous treatment with multiple antipsychotics, including haloperidol, quetiapine, aripiprazole, olanzapine, risperidone, and ziprasidone, was not successful. Mr. A began clozapine >10 years ago, and his symptoms have been stable since, without any further psychiatric hospitalizations. Mr. A takes clozapine 600 mg/d and divalproex sodium 1,500 mg/d, which he tolerates well and without significant adverse effects. Though he continues to have intermittent auditory hallucinations, they are mild and manageable. Mr. A lives with his mother, who reports he occasionally talks to himself but when he does not take clozapine, the auditory hallucinations worsen and cause him to become paranoid and aggressive. His ANC is monitored monthly and had been normal for several years until he was diagnosed with B-ALL.
[polldaddy:11125941]
The authors’ observations
The decision to continue clozapine during chemotherapy is challenging and should weigh the risk of agranulocytosis against that of psychiatric destabilization. Because clozapine and chemotherapy are both associated with agranulocytosis, there is concern that concurrent treatment could increase this risk in an additive or synergistic manner. To the best of our knowledge, there are currently no controlled studies investigating the interactions between clozapine and chemotherapeutic agents. Evidence on the hematopoietic consequences of concurrent clozapine and chemotherapy treatment has been limited to case reports because the topic does not lend itself well to randomized controlled trials.
A recent systematic review found no adverse outcomes among the 27 published cases in which clozapine was continued during myelosuppressive chemotherapy.1 The most notable finding was an association between clozapine discontinuation and psychiatric decompensation, which was reported in 12 of 13 cases in which clozapine was prophylactically discontinued to minimize the risk of agranulocytosis.
Patient-specific factors must also be considered, such as the likelihood that psychotic symptoms will recur or worsen if clozapine is discontinued, as well as the extent to which symptom recurrence would interfere with cancer treatment. Clinicians should evaluate the feasibility of switching to another antipsychotic by obtaining a thorough history of the patient’s previous antipsychotics, doses, treatment duration, and response. However, many patients are treated with clozapine because their psychotic symptoms did not improve with other treatments. The character and severity of the patient’s psychotic symptoms when untreated or prior to clozapine treatment can provide a clearer understanding of how a recurrence of symptoms may interfere with cancer treatment. To formulate an accurate assessment of risks and benefits, it is necessary to consider both available evidence and patient-specific factors. The significant agitation and paranoia that Mr. A experienced when not taking clozapine was likely to disrupt chemotherapy. Thus, the adverse consequences of discontinuing clozapine were both severe and likely.
TREATMENT Continuing clozapine
After an extensive discussion of risks, benefits, and alternative treatments with the hematology and psychiatry teams, Mr. A and his family decide to continue clozapine with increased ANC monitoring during chemotherapy. Concurrent treatment was pursued with close collaboration among the patient, the patient’s family, and the hematology and pharmacy teams, and in careful consideration of the clozapine risk evaluation and mitigation strategy. Mr. A’s ANC was monitored daily during chemotherapy treatments and weekly in the intervals between treatments.
As expected, chemotherapy resulted in bone marrow suppression and pancytopenia. Mr. A’s ANC steadily decreased during the next 10 days until it reached 0 µL. This was consistent with the predicted ANC nadir between Day 10 and Day 14, after which recovery was expected. However, Mr. A’s ANC remained at 0 µL on Day 15.
[polldaddy:11125947]
Continue to: The authors' observations
The authors’ observations
Temporary decreases in ANC are expected during chemotherapy, and the timing of onset and recovery is often well characterized. Prior to Day 15, the observed progressive marrow suppression was solely due to chemotherapy. However, because Mr. A’s ANC remained 0 µL longer than anticipated, reevaluation of clozapine’s effects was warranted.
Timing, clinical course, and comprehensive hematologic monitoring can provide important clues as to whether clozapine may be responsible for prolonged neutropenia. Though a prolonged ANC of 0 µL raised concern for clozapine-induced agranulocytosis (CIAG), comprehensive monitoring of hematologic cell lines was reassuring because CIAG selectively targets granulocytic cells (neutrophils).2 In contrast, chemotherapy can affect other cell lineages, including lymphocytes, red blood cells, and platelets, which causes pancytopenia.3 For Mr. A, though the clinical presentation of pancytopenia was significant and concerning, it was inconsistent with CIAG.
Additionally, the patient’s baseline risk of CIAG should be considered. After 18 weeks of clozapine treatment, the risk of CIAG decreases to a level similar to that associated with other antipsychotics.4,5 Therefore, CIAG would be unlikely in a patient treated with clozapine for more than 1 year and who did not have a history of neutropenia, as was the case with Mr. A.
While bone marrow biopsy can help differentiate between the causes of agranulocytosis,6 it is highly invasive and may not be necessary if laboratory evidence is sufficient. However, if a treatment team is strongly considering discontinuing clozapine and there are no suitable alternatives, a biopsy may provide additional clarification.
TREATMENT CAR T-cell therapy and cancer remission
Clozapine is continued with daily monitoring. On Day 19, Mr. A’s ANC increases, reaching 2,600 µL by discharge on Day 40. Mr. A remains psychiatrically stable throughout his hospitalization and does not experience any complications associated with neutropenia, despite its prolonged duration.
Continue to: Unfortunately, multiple cycles of...
Unfortunately, multiple cycles of chemotherapy fail to induce remission. Mr. A is referred for CD19/CD22 chimeric antigen receptor (CAR) T-cell therapy, which helps achieve remission. Allogeneic hematopoietic stem cell transplant (HSCT) is recommended to maximize the likelihood of sustained remission.7 As with chemotherapy, Mr. A and his family agree with the multidisciplinary treatment recommendation to continue clozapine during both CAR T-cell therapy and HSCT, because the risks associated with psychiatric decompensation were greater than a potential increased risk of agranulocytosis. Clozapine treatment is continued throughout both therapies without issue.
Four months after HSCT, Mr. A is admitted for neutropenic fever and left face cellulitis. Upon admission, his ANC is 30 µL and subsequently decreases to 0 µL. In addition to neutropenia, Mr. A is also anemic and thrombocytopenic. He undergoes a bone marrow biopsy.
[polldaddy:11125950]
The authors’ observations
While no published cases have examined the bone marrow of patients experiencing CIAG, 2 retrospective studies have characterized 2 classes of bone marrow findings associated with drug-induced agranulocytosis resulting from nonchemotherapeutic agents (Table).8,9 Type I marrow appears hypercellular with adequate neutrophil precursors but an arrested neutrophil maturation, with few or no mature forms of neutrophils beyond myelocytes.8,9 Type II demonstrates a severe reduction or complete absence of granulocytic precursors with normal or increased erythropoiesis and megakaryocytes.8,9 These findings have been used to accurately differentiate between chemotherapy and nonchemotherapy drug-induced agranulocytosis,6 resulting in successful identification and discontinuation of the responsible agent.

Mr. A’s bone marrow biopsy showed severe pancytopenia with profound neutropenia and normocytic anemia, without evidence of residual leukemia, inconsistent with Type I or Type II. Findings were suggestive of a myelodysplastic syndrome, consistent with secondary graft failure. Symptoms resolved after treatment with antibiotics, granulocyte colony-stimulating factor, epoetin alfa, and thrombopoietin. Mr. A’s ANC remained 0 µL for 22 days before returning to normal (>1,500 µL) by Day 29. He had no secondary complications resulting from neutropenia. As the clinical evidence suggested, Mr. A’s neutropenia was unlikely to be due to clozapine. Clozapine was continued throughout his cancer treatment, and he remained psychiatrically stable.
Clozapine, cancer treatments, and agranulocytosis
This case demonstrates that clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even with the development of agranulocytosis and prolonged neutropenia. Evidence to guide psychiatric clinicians to evaluate the likelihood that agranulocytosis is clozapine-induced is limited.
Continue to: We offer an algorithm...
We offer an algorithm to assist clinicians faced with this challenging clinical dilemma (Figure). Based on our experience and limited current evidence, we recommend continuing clozapine during cancer treatment unless there is clear evidence to suggest otherwise. Presently, no evidence in published literature suggests worsened outcomes in patients treated concurrently with clozapine and cancer therapies.

OUTCOME Cancer-free and psychiatrically stable
Mr. A continues clozapine therapy throughout all phases of treatment, without interruption. No adverse effects are determined to be secondary to clozapine. He remains psychiatrically stable throughout treatment, and able to participate and engage in his oncologic therapy. Mr. A is now more than 1 year in remission with no recurrence of graft failure, and his psychiatric symptoms continue to be well controlled with clozapine.
Bottom Line
Clozapine can be safely continued during a variety of cancer treatments (ie, chemotherapy, CAR T-cell therapy, HSCT), even in patients who develop agranulocytosis and prolonged neutropenia. Based on our experience and limited evidence, we offer an algorithm to assist clinicians faced with this challenging clinical dilemma.
Related Resources
- Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286. doi:10.1111/ejh.13285
- Daniel JS, Gross T. Managing clozapine-induced neutropenia and agranulocytosis. Current Psychiatry. 2016;15(12):51-53.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Divalproex sodium • Depakote
Epoetin alfa • Epogen
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
1. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol. 2019;103(4):277-286.
2. Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014:27(5):447-452.
3. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606-3618.
4. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
5. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483-488.
6. Azadeh N, Kelemen K, Fonseca R. Amitriptyline-induced agranulocytosis with bone marrow confirmation. Clin Lymphoma Myeloma Leuk. 2014;14(5):e183-e185.
7. Liu J, Zhang X, Zhong JF, et al. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9(13):1115-1125.
8. Apinantriyo B, Lekhakula A, Rujirojindakul P. Incidence, etiology and bone marrow characteristics of non-chemotherapy-induced agranulocytosis. Hematology. 2011;16(1):50-53.
9. Yang J, Zhong J, Xiao XH, et al. The relationship between bone marrow characteristics and the clinical prognosis of antithyroid drug-induced agranulocytosis. Endocr J. 2013;60(2):185-189.
BOARDING psychiatric patients in the ED: Key strategies
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
Boarding of psychiatric patients in the emergency department (ED) has been well documented.1 Numerous researchers have discussed ways to address this public health crisis. In this Pearl, I use the acronym BOARDING to provide key strategies for psychiatric clinicians managing psychiatric patients who are boarding in an ED.
Be vigilant. As a patient’s time waiting in the ED increases, watch for clinical blind spots. New medical problems,2 psychiatric issues, or medication errors3 may unexpectedly arise since the patient was originally stabilized by emergency medicine clinicians.
Orders. Since the patient could be waiting in the ED for 24 hours or longer, consider starting orders (eg, precautions, medications, diet, vital sign checks, labs, etc) as you would for a patient in an inpatient psychiatric unit or a dedicated psychiatric ED.
AWOL. Unlike inpatient psychiatric units, EDs generally are not locked. Extra resources (eg, sitter, safety alarm bracelet) may be needed to help prevent patients from leaving this setting unnoticed, especially those on involuntary psychiatric holds.
Re-evaluate. Ideally, re-evaluate the patient every shift. Does the patient still need an inpatient psychiatric setting? Can the involuntary psychiatric hold be discontinued?
Disposition. Is there a family member or reliable caregiver to whom the patient can be discharged? Can the patient go to a shelter or be stabilized in a short-term residential program, instead of an inpatient psychiatric unit?
Inpatient. If the patient waits 24 hours or longer, begin thinking like an inpatient psychiatric clinician. Are there any interventions you can reasonably begin in the ED that you would otherwise begin on an inpatient psychiatric unit?
Nursing. Work with ED nursing staff to familiarize them with the patient’s specific needs.
Guidelines. With the input of clinical and administrative leadership, establish local hospital-based guidelines for managing psychiatric patients who are boarding in the ED.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
1. Nordstrom K, Berlin JS, Nash SS, et al. Boarding of mentally ill patients in emergency departments: American Psychiatric Association Resource Document. West J Emerg Med. 2019;20(5):690-695.
2. Garfinkel E, Rose D, Strouse K, et al. Psychiatric emergency department boarding: from catatonia to cardiac arrest. Am J Emerg Med. 2019;37(3):543-544.
3. Bakhsh HT, Perona SJ, Shields WA, et al. Medication errors in psychiatric patients boarded in the emergency department. Int J Risk Saf Med. 2014;26(4):191-198.
Caring for Muslim patients who fast during Ramadan
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
Ramadan is one of the obligatory pillars in Islam during which healthy Muslims are required to fast from dawn until sunset every day for 1 month. There are an estimated 3.45 million Muslims in the United States, and this population will continue to grow by 100,000 per year.1 With the increased growth of the Muslim population, it is important for clinicians to be aware of how patients of Muslim faith are affected during Ramadan. In this article, we explore the potential risks, as well as the benefits, the month of Ramadan brings to patients. We will also explain how being religiously aware is necessary to provide optimal care for these individuals.
For some patients, fasting may pose risks
Similar to other communities in the United States, individuals who are Muslim experience mood disorders, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, substance use disorders, and other psychiatric illnesses.2 During the month of Ramadan, Muslims are to abstain completely from eating and drinking from dawn until sunset. This includes medications as well as food and drink.
Due to these circumstances, patients will often change the timing, frequency, and dosing of their medications to allow them to fast. One study found 60% of Muslims made medication adjustments during Ramadan without seeking medical advice.3 It is possible that such alterations may be detrimental. During Ramadan, some Muslims wake up early in the morning to eat a pre-dawn meal, and often go back to sleep. This has been reported to cause a delay in sleep-wake times and to reduce rapid eye movement sleep.4 These circadian rhythm changes can be detrimental to patients with bipolar disorder. One study found higher rates of relapse to depression and mania in patients with bipolar disorder who were fasting during Ramadan.5 Circadian rhythm disturbances also may worsen depression.6 Another point of concern is patients with eating disorders. One small case series (N = 6) found that fasting during Ramadan exacerbated symptoms in patients with eating disorders.7
Another concern is that dehydration while fasting can lead to lithium toxicity. However, one study found lithium levels remained stable while fasting for 10 to 12 hours.5 Another showed that changing lithium dosing from twice a day to once a day allowed for easier administration without causing a subtherapeutic change in blood lithium levels.8
The practice also may have benefits for mental health
For many Muslims, Ramadan is the best time of the year, where they reconnect with their religion and experience the utmost spiritual growth. Studies have shown that the incidence of suicide is lowest during Ramadan compared to other months.9 A study of older men found that intermittent fasting and calorie restriction (not during Ramadan) resulted in decreases in tension, confusion, anger, and mood disturbance.10 Another study found that fasting during Ramadan had a positive impact on depression, anxiety, stress, and cognitive function.11
Clinical considerations
To provide the best care for Muslim patients during Ramadan, clinicians should take a holistic approach and take all factors into consideration. It is common for circadian rhythm disruptions to exacerbate mood disorders, so encourage patients to maintain healthy sleep hygiene to their best ability during this month. Another important consideration is medication timing and dosing.12 For patients prescribed a medication that typically is taken twice a day, determine if this dosing can be changed to once a day, or if both doses can be taken when it is permissible to eat (sunset to dawn). For medications that are absorbed with food, consider how these medications might be adjusted and maintained while a patient is fasting. Some medications may be sedating or activating, so the timing of administration may need to be adjusted to meet the patient’s needs. Lastly, keep in mind that certain medications can have withdrawal effects, and the likelihood of this occurring while a patient is fasting.
One vital point is that if a patient is at high risk of clinically decompensating due to fasting or medication adjustments or discontinuation, advise them to not fast. Muslims with physical or mental illnesses are excused from fasting. Bear in mind that because Ramadan is meant to be a month of heightened spirituality, many Muslims will prefer to fast.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
1. Pew Research Center. Demographic portrait of Muslim Americans. Published July 26, 2017. Accessed January 15, 2019. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans
2. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
3. Aslam M, Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100(1):49-53.
4. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577-586.
5. Eddahby S, Kadri N, Moussaoui D. Fasting during Ramadan is associated with a higher recurrence rate in patients with bipolar disorder. World Psychiatry. 2014;13(1):97.
6. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571-585.
7. Akgül S, Derman O, Kanbur NÖ. Fasting during Ramadan: a religious factor as a possible trigger or exacerbator for eating disorders in adolescents. Int J Eat Disord. 2014;47(8):905-910.
8. Kadri N, Mouchtaq N, Hakkou F, et al. Relapses in bipolar patients: changes in social rhythm? Int J Neuropsychopharmacol. 2000;3(1):45-49.
9. Taktak S, Kumral B, Unsal A, et al. Evidence for an association between suicide and religion: a 33-year retrospective autopsy analysis of suicide by hanging during the month of Ramadan in Istanbul. Aust J Forensic Sci. 2016;48(2):121-131.
10. Hussin NM, Shahar S, Teng NI, et al. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. J Nutr Health Aging. 2013;17(8):674-680.
11. Amin A, Sai Sailesh K, Mishra S, et al. Effects of fasting during Ramadan month on depression, anxiety and stress and cognition. Int J Med Res Rev. 2016;4(5):771-774.
12. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
Hearing, vision loss combo a colossal risk for cognitive decline
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).
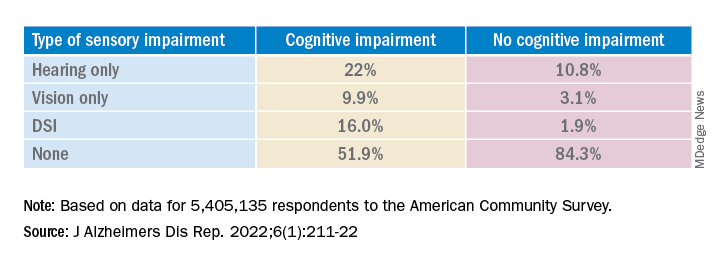
The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.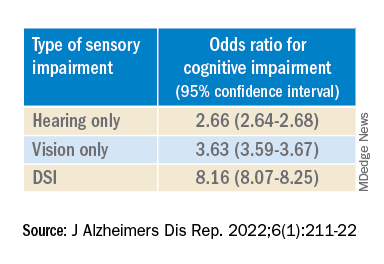
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.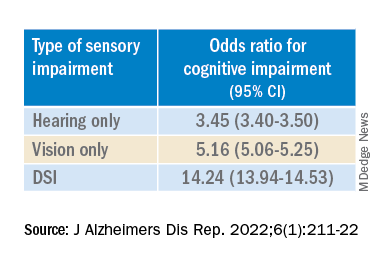
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.
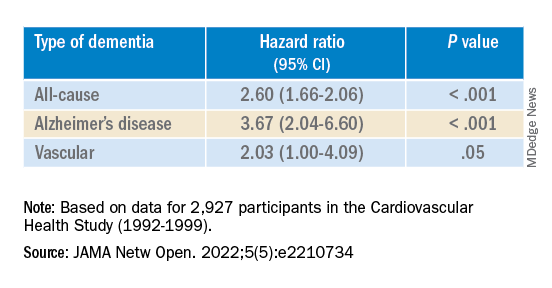
They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE REPORTS
Long COVID neuropsychiatric deficits greater than expected
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
AT APA 2022
Don’t equate mass shootings with mental illness
Here we go again, and again, and again.
There just aren’t enough tears, and before the bodies of 19 small children are identified, the political noise starts up. Mass shootings are a part of the American landscape, but when they happen at schools, we all feel a distinct sense of violation and gaping grief. Those children are so innocent, so deserving of a right to live their lives, hold their place with their families, create their own legacies, and die of natural causes at a ripe old age. And those children could have been our children. There was nothing special about them; they were just sent to school that day like every child who is sent to school every day.
Here is how the politics goes: The Republicans will blame the Democrats and the Democrats will blame the Republicans. Is Rachel Maddow at fault, or is it Tucker Carlson? Social media accounts blamed both of them for the racially motivated mass murder in a Buffalo grocery store on May 14.
Mass murders were previously defined as a shooting where four or more victims are killed, excluding the shooter, in a public place that is not related to the commission of another crime. In 2012, the definition was changed to include events with three victims. This definition excludes gang violence and the murder of family members.
When it comes to explaining mass murder, the camps divide: They are the result of some combination of mental illness, easy access to firearms, and terrorism and hate. For psychiatry, there is a unique place in the argument – half of all mass shooters have exhibited signs or symptoms of psychiatric illness, and for those who want to deflect the issue away from issues related to the regulation of firearms, it becomes easy to blame “mental illness,” as though that explains it all. Either the gunman “snapped” in such a way that no one could have predicted, or the mental health system is at fault for not preventing it.
There are many ways to be emotionally disturbed; mental illness is only one of them, and there is no psychiatric diagnosis that includes the symptom of shooting strangers, or shooting children. The vast majority of people, including nearly all psychiatrists, will never know someone who perpetrates a mass shooting.
Take John Hinckley Jr., who shot President Ronald Reagan as a means to impress actress Jodie Foster. Sometimes these killings are motivated by delusional beliefs. But the planning and preparation that goes into most mass shootings involves a degree of organization and forethought that we don’t typically see in those with severe psychotic disorders.
The other psychological explanation that satisfies some of a nonmedical population is that these killers “just snap.” This, too, is a term that is not included in our diagnostic vocabulary, but it remains a way for some to explain that which can’t be explained. If mental illness, however, is the cause of mass murders, then more stringent gun control is unnecessary. Every state already has a mechanism to prevent those with criminal and specified psychiatric histories from buying legal firearms, and it may be inevitable that these screens are not perfect.
The next line of political thinking moves to the psychiatric “if only.” If only there were more state hospital beds and if only it were easier to compel people with psychiatric disorders to get treatment against their will, then we could eliminate these crimes. The Virginia tech shooter was mandated to get outpatient psychiatric treatment after a brief hospitalization, yet he never went and there was no mechanism in place to track him.
In cases where a person with a psychotic illness has a history of repeated violent episodes after stopping medications, it does make sense to mandate treatment, not because they are likely to shoot strangers, but because some people do become violent when they are ill and mental illness is believed to play a role in 10% of murders.
Mass murders remain rare, and while advocates for legislation that would make it easier to mandate involuntary care have cited violence prevention as a reason, it is hard to imagine that we would force people to get care because they “might” commit such a crime – unless there was convincing evidence that someone was at risk of committing such a heinous act.
For those who oppose stronger gun control laws, the “what if” may circulate around the need for even more firearms. What if teachers carried guns? What if schools were more heavily policed? What if the criminals were made to be afraid?
We are left with the fact that other countries do not see these numbers of mass shooting events, yet mental illness is ubiquitous. While the presence of psychiatric disorders does little to explain school shootings, we still have no understanding of what motivated the Sandy Hook killer, and it remains to be seen what we will come to understand about the gunman in Uvalde, Texas.
Mental illness is not unique to the United States; however, the number of available firearms is. In a country of 323 million people (including children and people who live in institutions where they have no access to firearms), there are estimated to be over 400 million guns in the United States, 98% of which are owned by civilians.
Hate crimes and terrorism are another explanation for mass murders. In these instances, the gunman makes his motive obvious: There are social media announcements, or the site of the shooting is a synagogue, a mosque, or a location where the victims are of a specific race or religion. But hate may come out of a psychotic illness, and easy access to firearms allows for these crimes to continue.
Firearms are now the No. 1 cause of mortality in children. Very few of these deaths are the result of mass murders. Many more are from accidental deaths, targeted crime, or suicide. Still, school shootings rip at our hearts. Neither the victims nor their grieving families have any role in the act, and suffering leaves its mark on families, communities, and all of us.
Are there answers?
In many states, physicians can now request emergency removal of firearms from the home of someone who is both mentally ill and threatening either suicide or homicide. During the era when high-capacity firearms were banned, from 1994 to 2004, mass murders decreased in our country. While most gunmen use legal firearms they have purchased, I would contend that “smart guns” – firearms that allow only the legal owner to operate them based on biometrics – would prevent some mass shootings and many accidents, crimes, and suicides. Universal background checks and tracking gun purchases in the way we monitor controlled medications, or even Sudafed, might allow authorities to predict who might be at risk of committing these heinous acts.
In his newly released book, Trigger Points: Inside the Mission to Stop Mass Murders in America, journalist Mark Follman argues for a proactive community approach using threat assessment methods and providing wraparound services to those who are deemed to be at risk for violent acts. Mr. Follman’s voice is one of the few out there saying that these events are not random and are, in fact, preventable.
In psychiatry, we struggle with school shootings such as the one we just saw in Uvalde. Our own hearts ache as we hold our children close and empathize with the loss of strangers who have been through the unthinkable. We help our patients as they process their emotions. And we wonder whether any of our patients might ever do anything so horrific. The feelings get complicated, the sadness and anger intermingle while the frustration builds, and we are left with our fears and the hope that if that very rare person were to walk through our office door, we would know what to do.
Dr. Miller is a coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. A version of this article first appeared on Medscape.com.
Here we go again, and again, and again.
There just aren’t enough tears, and before the bodies of 19 small children are identified, the political noise starts up. Mass shootings are a part of the American landscape, but when they happen at schools, we all feel a distinct sense of violation and gaping grief. Those children are so innocent, so deserving of a right to live their lives, hold their place with their families, create their own legacies, and die of natural causes at a ripe old age. And those children could have been our children. There was nothing special about them; they were just sent to school that day like every child who is sent to school every day.
Here is how the politics goes: The Republicans will blame the Democrats and the Democrats will blame the Republicans. Is Rachel Maddow at fault, or is it Tucker Carlson? Social media accounts blamed both of them for the racially motivated mass murder in a Buffalo grocery store on May 14.
Mass murders were previously defined as a shooting where four or more victims are killed, excluding the shooter, in a public place that is not related to the commission of another crime. In 2012, the definition was changed to include events with three victims. This definition excludes gang violence and the murder of family members.
When it comes to explaining mass murder, the camps divide: They are the result of some combination of mental illness, easy access to firearms, and terrorism and hate. For psychiatry, there is a unique place in the argument – half of all mass shooters have exhibited signs or symptoms of psychiatric illness, and for those who want to deflect the issue away from issues related to the regulation of firearms, it becomes easy to blame “mental illness,” as though that explains it all. Either the gunman “snapped” in such a way that no one could have predicted, or the mental health system is at fault for not preventing it.
There are many ways to be emotionally disturbed; mental illness is only one of them, and there is no psychiatric diagnosis that includes the symptom of shooting strangers, or shooting children. The vast majority of people, including nearly all psychiatrists, will never know someone who perpetrates a mass shooting.
Take John Hinckley Jr., who shot President Ronald Reagan as a means to impress actress Jodie Foster. Sometimes these killings are motivated by delusional beliefs. But the planning and preparation that goes into most mass shootings involves a degree of organization and forethought that we don’t typically see in those with severe psychotic disorders.
The other psychological explanation that satisfies some of a nonmedical population is that these killers “just snap.” This, too, is a term that is not included in our diagnostic vocabulary, but it remains a way for some to explain that which can’t be explained. If mental illness, however, is the cause of mass murders, then more stringent gun control is unnecessary. Every state already has a mechanism to prevent those with criminal and specified psychiatric histories from buying legal firearms, and it may be inevitable that these screens are not perfect.
The next line of political thinking moves to the psychiatric “if only.” If only there were more state hospital beds and if only it were easier to compel people with psychiatric disorders to get treatment against their will, then we could eliminate these crimes. The Virginia tech shooter was mandated to get outpatient psychiatric treatment after a brief hospitalization, yet he never went and there was no mechanism in place to track him.
In cases where a person with a psychotic illness has a history of repeated violent episodes after stopping medications, it does make sense to mandate treatment, not because they are likely to shoot strangers, but because some people do become violent when they are ill and mental illness is believed to play a role in 10% of murders.
Mass murders remain rare, and while advocates for legislation that would make it easier to mandate involuntary care have cited violence prevention as a reason, it is hard to imagine that we would force people to get care because they “might” commit such a crime – unless there was convincing evidence that someone was at risk of committing such a heinous act.
For those who oppose stronger gun control laws, the “what if” may circulate around the need for even more firearms. What if teachers carried guns? What if schools were more heavily policed? What if the criminals were made to be afraid?
We are left with the fact that other countries do not see these numbers of mass shooting events, yet mental illness is ubiquitous. While the presence of psychiatric disorders does little to explain school shootings, we still have no understanding of what motivated the Sandy Hook killer, and it remains to be seen what we will come to understand about the gunman in Uvalde, Texas.
Mental illness is not unique to the United States; however, the number of available firearms is. In a country of 323 million people (including children and people who live in institutions where they have no access to firearms), there are estimated to be over 400 million guns in the United States, 98% of which are owned by civilians.
Hate crimes and terrorism are another explanation for mass murders. In these instances, the gunman makes his motive obvious: There are social media announcements, or the site of the shooting is a synagogue, a mosque, or a location where the victims are of a specific race or religion. But hate may come out of a psychotic illness, and easy access to firearms allows for these crimes to continue.
Firearms are now the No. 1 cause of mortality in children. Very few of these deaths are the result of mass murders. Many more are from accidental deaths, targeted crime, or suicide. Still, school shootings rip at our hearts. Neither the victims nor their grieving families have any role in the act, and suffering leaves its mark on families, communities, and all of us.
Are there answers?
In many states, physicians can now request emergency removal of firearms from the home of someone who is both mentally ill and threatening either suicide or homicide. During the era when high-capacity firearms were banned, from 1994 to 2004, mass murders decreased in our country. While most gunmen use legal firearms they have purchased, I would contend that “smart guns” – firearms that allow only the legal owner to operate them based on biometrics – would prevent some mass shootings and many accidents, crimes, and suicides. Universal background checks and tracking gun purchases in the way we monitor controlled medications, or even Sudafed, might allow authorities to predict who might be at risk of committing these heinous acts.
In his newly released book, Trigger Points: Inside the Mission to Stop Mass Murders in America, journalist Mark Follman argues for a proactive community approach using threat assessment methods and providing wraparound services to those who are deemed to be at risk for violent acts. Mr. Follman’s voice is one of the few out there saying that these events are not random and are, in fact, preventable.
In psychiatry, we struggle with school shootings such as the one we just saw in Uvalde. Our own hearts ache as we hold our children close and empathize with the loss of strangers who have been through the unthinkable. We help our patients as they process their emotions. And we wonder whether any of our patients might ever do anything so horrific. The feelings get complicated, the sadness and anger intermingle while the frustration builds, and we are left with our fears and the hope that if that very rare person were to walk through our office door, we would know what to do.
Dr. Miller is a coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. A version of this article first appeared on Medscape.com.
Here we go again, and again, and again.
There just aren’t enough tears, and before the bodies of 19 small children are identified, the political noise starts up. Mass shootings are a part of the American landscape, but when they happen at schools, we all feel a distinct sense of violation and gaping grief. Those children are so innocent, so deserving of a right to live their lives, hold their place with their families, create their own legacies, and die of natural causes at a ripe old age. And those children could have been our children. There was nothing special about them; they were just sent to school that day like every child who is sent to school every day.
Here is how the politics goes: The Republicans will blame the Democrats and the Democrats will blame the Republicans. Is Rachel Maddow at fault, or is it Tucker Carlson? Social media accounts blamed both of them for the racially motivated mass murder in a Buffalo grocery store on May 14.
Mass murders were previously defined as a shooting where four or more victims are killed, excluding the shooter, in a public place that is not related to the commission of another crime. In 2012, the definition was changed to include events with three victims. This definition excludes gang violence and the murder of family members.
When it comes to explaining mass murder, the camps divide: They are the result of some combination of mental illness, easy access to firearms, and terrorism and hate. For psychiatry, there is a unique place in the argument – half of all mass shooters have exhibited signs or symptoms of psychiatric illness, and for those who want to deflect the issue away from issues related to the regulation of firearms, it becomes easy to blame “mental illness,” as though that explains it all. Either the gunman “snapped” in such a way that no one could have predicted, or the mental health system is at fault for not preventing it.
There are many ways to be emotionally disturbed; mental illness is only one of them, and there is no psychiatric diagnosis that includes the symptom of shooting strangers, or shooting children. The vast majority of people, including nearly all psychiatrists, will never know someone who perpetrates a mass shooting.
Take John Hinckley Jr., who shot President Ronald Reagan as a means to impress actress Jodie Foster. Sometimes these killings are motivated by delusional beliefs. But the planning and preparation that goes into most mass shootings involves a degree of organization and forethought that we don’t typically see in those with severe psychotic disorders.
The other psychological explanation that satisfies some of a nonmedical population is that these killers “just snap.” This, too, is a term that is not included in our diagnostic vocabulary, but it remains a way for some to explain that which can’t be explained. If mental illness, however, is the cause of mass murders, then more stringent gun control is unnecessary. Every state already has a mechanism to prevent those with criminal and specified psychiatric histories from buying legal firearms, and it may be inevitable that these screens are not perfect.
The next line of political thinking moves to the psychiatric “if only.” If only there were more state hospital beds and if only it were easier to compel people with psychiatric disorders to get treatment against their will, then we could eliminate these crimes. The Virginia tech shooter was mandated to get outpatient psychiatric treatment after a brief hospitalization, yet he never went and there was no mechanism in place to track him.
In cases where a person with a psychotic illness has a history of repeated violent episodes after stopping medications, it does make sense to mandate treatment, not because they are likely to shoot strangers, but because some people do become violent when they are ill and mental illness is believed to play a role in 10% of murders.
Mass murders remain rare, and while advocates for legislation that would make it easier to mandate involuntary care have cited violence prevention as a reason, it is hard to imagine that we would force people to get care because they “might” commit such a crime – unless there was convincing evidence that someone was at risk of committing such a heinous act.
For those who oppose stronger gun control laws, the “what if” may circulate around the need for even more firearms. What if teachers carried guns? What if schools were more heavily policed? What if the criminals were made to be afraid?
We are left with the fact that other countries do not see these numbers of mass shooting events, yet mental illness is ubiquitous. While the presence of psychiatric disorders does little to explain school shootings, we still have no understanding of what motivated the Sandy Hook killer, and it remains to be seen what we will come to understand about the gunman in Uvalde, Texas.
Mental illness is not unique to the United States; however, the number of available firearms is. In a country of 323 million people (including children and people who live in institutions where they have no access to firearms), there are estimated to be over 400 million guns in the United States, 98% of which are owned by civilians.
Hate crimes and terrorism are another explanation for mass murders. In these instances, the gunman makes his motive obvious: There are social media announcements, or the site of the shooting is a synagogue, a mosque, or a location where the victims are of a specific race or religion. But hate may come out of a psychotic illness, and easy access to firearms allows for these crimes to continue.
Firearms are now the No. 1 cause of mortality in children. Very few of these deaths are the result of mass murders. Many more are from accidental deaths, targeted crime, or suicide. Still, school shootings rip at our hearts. Neither the victims nor their grieving families have any role in the act, and suffering leaves its mark on families, communities, and all of us.
Are there answers?
In many states, physicians can now request emergency removal of firearms from the home of someone who is both mentally ill and threatening either suicide or homicide. During the era when high-capacity firearms were banned, from 1994 to 2004, mass murders decreased in our country. While most gunmen use legal firearms they have purchased, I would contend that “smart guns” – firearms that allow only the legal owner to operate them based on biometrics – would prevent some mass shootings and many accidents, crimes, and suicides. Universal background checks and tracking gun purchases in the way we monitor controlled medications, or even Sudafed, might allow authorities to predict who might be at risk of committing these heinous acts.
In his newly released book, Trigger Points: Inside the Mission to Stop Mass Murders in America, journalist Mark Follman argues for a proactive community approach using threat assessment methods and providing wraparound services to those who are deemed to be at risk for violent acts. Mr. Follman’s voice is one of the few out there saying that these events are not random and are, in fact, preventable.
In psychiatry, we struggle with school shootings such as the one we just saw in Uvalde. Our own hearts ache as we hold our children close and empathize with the loss of strangers who have been through the unthinkable. We help our patients as they process their emotions. And we wonder whether any of our patients might ever do anything so horrific. The feelings get complicated, the sadness and anger intermingle while the frustration builds, and we are left with our fears and the hope that if that very rare person were to walk through our office door, we would know what to do.
Dr. Miller is a coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. A version of this article first appeared on Medscape.com.
What can we do about mass shootings?
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.
“It must be mental illness. My mind cannot possibly conceive of an alternative. A rational healthy mind cannot be capable of this, Doc.”
These were the opening words of one of many discussions that I had with patients in the wake of yet another gut-wrenching tragedy where we saw innocent children and their teachers murdered in school.
This narrative is appealing, regardless of whether or not it is true, because we find some measure of solace in it. We are now at a point in our nation where we are not ashamed to say that we live in a mental health crisis. It is inconceivable to us that a “healthy” brain could plot and premeditate the cold-blooded execution of children.
But just because something feels true does not mean that it actually is.
I personally felt this after a shooter walked into my hospital and shot my coworkers, murdering one and injuring several others. How can this be? It didn’t make a whole lot of sense then. I don’t know if it makes any more sense now. But he had no mental illness that we knew of.
Do any mass shooters have untreated mental illness?
Could we have diagnosed those cases earlier? Intervened sooner? Offered more effective treatment? Certainly. Would that have explain away the rest of the cases? Unfortunately, no.
What is it, then?
The scary answer is that the people who are capable of doing this are not so far away. They are not the folks that we would image locking up in a “psych ward” and throwing away the key. They are, rather, people who are lonely, neglected, rejected, bullied, and broken down by life. Anger, hatred, racism, and evil may be ailments of the soul, but they are not mental illnesses. The carnage they produce is just as tangible. As a psychiatrist, I must admit to you that I do not have a good medication to treat these manifestations of the human condition.
What do we do as a society?
Gun reform is the first obvious and essential answer, without which little else is truly as impactful. We must advocate for it and fight tirelessly.
But at the time you will read this article, your disgruntled coworker will be able to walk into a local store in a moment of despair, anguish, and hopelessness and purchase a semiautomatic weapon of war.
What if we were to start seeing, as a society, that our lives are interwoven? What if we saw that our health is truly interdependent? The COVID-19 pandemic shattered many things in our lives, but one element in particular is our radical individualism. We saw that the choices you make certainly affect me and vice versa. We saw that public health is just that – a public matter, not a private one. We saw that there are some areas of our lives that force us to come together for our own survival.
Perhaps politicians will not save us here. Perhaps kindness will. Empathy can be as potent as legislation, and compassion as impactful as a Twitter hashtag. We each know a lonely coworker, an isolated neighbor, a bullied student, or someone beaten down by life.
What if some of the prevention is in fact in our hands? Together.
“Darkness cannot drive out darkness. Only light can do that. Hate cannot drive out hate; only love can do that.” – Reverend Dr. Martin Luther King, Jr.
Mena Mirhom, MD, is an assistant professor of psychiatry at Columbia University and teaches writing to public psychiatry fellows. He is a board-certified psychiatrist and a consultant for the National Basketball Players Association, treating NBA players and staff.
A version of this article first appeared on Medscape.com.