User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
More than half of U.S. children under 6 years show detectable blood lead levels
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Lead poisoning remains a significant threat to the health of young children in the United States, based on data from blood tests of more than 1 million children.
Any level of lead is potentially harmful, although blood lead levels have decreased over the past several decades in part because of the elimination of lead from many consumer products, as well as from gas, paint, and plumbing fixtures, wrote Marissa Hauptman, MD, of Boston Children’s Hospital and colleagues.
However, “numerous environmental sources of legacy lead still exist,” and children living in poverty and in older housing in particular remain at increased risk for lead exposure, they noted.
In a study published in JAMA Pediatrics, the researchers analyzed deidentified results from blood lead tests performed at a single clinical laboratory for 1,141,441 children younger than 6 years between Oct. 1, 2018, and Feb. 29, 2020. The mean age of the children was 2.3 years; approximately half were boys.
Overall, 50.5% of the children tested (576,092 children) had detectable blood lead levels (BLLs), defined as 1.0 mcg/dL or higher, and 1.9% (21,172 children) had elevated BLLs, defined as 5.0 mcg/dL or higher.
In multivariate analysis, both detectable BLLs and elevated BLLs were significantly more common among children with public insurance (adjusted odds ratios, 2.01 and 1.08, respectively).
Children in the highest vs. lowest quintile of pre-1950s housing had significantly greater odds of both detectable and elevated BLLs (aOR, 1.65 and aOR, 3.06); those in the highest vs. lowest quintiles of poverty showed similarly increased risk of detectable and elevated BLLs (aOR, 1.89 and aOR, 1.99, respectively; P < .001 for all).
When the data were broken out by ZIP code, children in predominantly Black non-Hispanic and non-Latino neighborhoods were more likely than those living in other ZIP codes to have detectable BLLs (aOR, 1.13), but less likely to have elevated BLLs (aOR, 0.83). States with the highest overall proportions of children with detectable BLLs were Nebraska (83%), Missouri (82%), and Michigan (78%).
The study findings were limited by several factors, especially the potential for selection bias because of the use of a single reference laboratory (Quest Diagnostics), that does not perform all lead testing in the United States, the researchers noted. Other limitations included variability in testing at the state level, and the use of ZIP code–level data to estimate race, ethnicity, housing, and poverty, they said.
However, the results suggest that lead exposure remains a problem in young children, with significant disparities at the individual and community level, and national efforts must focus on further reductions of lead exposure in areas of highest risk, they concluded.
Step up lead elimination efforts
“The removal of lead from gasoline and new paint produced a precipitous decrease in blood lead levels from a population mean of 17 mcg/dL (all ages) in 1976 to 4 mcg/dL in the early 1990s to less than 2 mcg/dL today,” wrote Philip J. Landrigan, MD, of Boston College and David Bellinger, PhD, of Harvard University, Boston, in an accompanying editorial. However, “The findings from this study underscore the urgent need to eliminate all sources of lead exposure from U.S. children’s environments,” and highlight the persistent disparities in children’s lead exposure, they said.
The authors emphasized the need to remove existing lead paint from U.S. homes, as not only the paint itself, but the dust that enters the environment as the pain wears over time, continue to account for most detectable and elevated BLLs in children. A comprehensive lead paint removal effort would be an investment that would protect children now and would protect future generations, they emphasized. They proposed “creating a lead paint removal workforce through federally supported partnerships between city governments and major unions,” that would not only protect children from disease and disability, but could potentially provide jobs and vocational programs that would have a significant impact on communities.
Elevated lead levels may be underreported
In fact, the situation of children’s lead exposure in the United States may be more severe than indicated by the study findings, given the variation in testing at the state and local levels, said Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn.
“There are no available lead test kits in our offices, so I do worry that many elevated lead levels will be missed,” she said.
“The recent case of elevated lead levels in drinking water in Flint, Michigan, was largely detected through pediatric clinic screening and showed that elevated lead levels may remain a major issue in some communities,” said Tim Joos, MD, a clinician in combined internal medicine/pediatrics in Seattle, Wash., in an interview.
“It is important to highlight to what extent baseline and point-source lead contamination still exists, monitor progress towards lowering levels, and identify communities at high risk,” Dr. Joos emphasized. “The exact prevalence of elevated lead levels among the general pediatric populations is hard to estimate from this study because of the methodology, which looked at demographic characteristics of the subset of the pediatric population that had venous samples sent to Quest Lab,” he noted.
“As the authors pointed out, it is hard to know what biases went into deciding whether to screen or not, and whether these were confirmatory tests for elevated point of care testing done earlier in the clinic,” said Dr. Joos. “Nonetheless, it does point to the role of poverty and pre-1950s housing in elevated blood lead levels,” he added. “The study also highlights that, as the CDC considers lowering the level for what is considered an ‘elevated blood lead level’ from 5.0 to perhaps 3.5 mcg/dL, we still have a lot more work to do,” he said.
The study was funded by Quest Diagnostics and the company provided salaries to several coauthors during the study. Dr. Hauptmann disclosed support from the National Institutes of Health/National Institute of Environmental Health Sciences during the current study and support from the Agency for Toxic Substances and Disease Registry and the U.S. Environmental Protection Agency unrelated to the current study. Dr. Landrigan had no financial conflicts to disclose. Dr. Bellinger disclosed fees from attorneys for testimony in cases unrelated to the editorial. Dr. Kinsella had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Pediatric News. Dr. Joos had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
A female toddler presents with an itchy yellow nodule
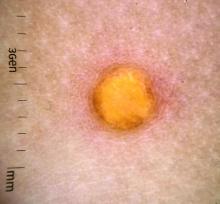
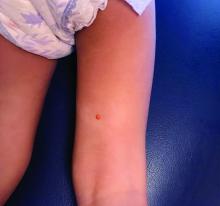
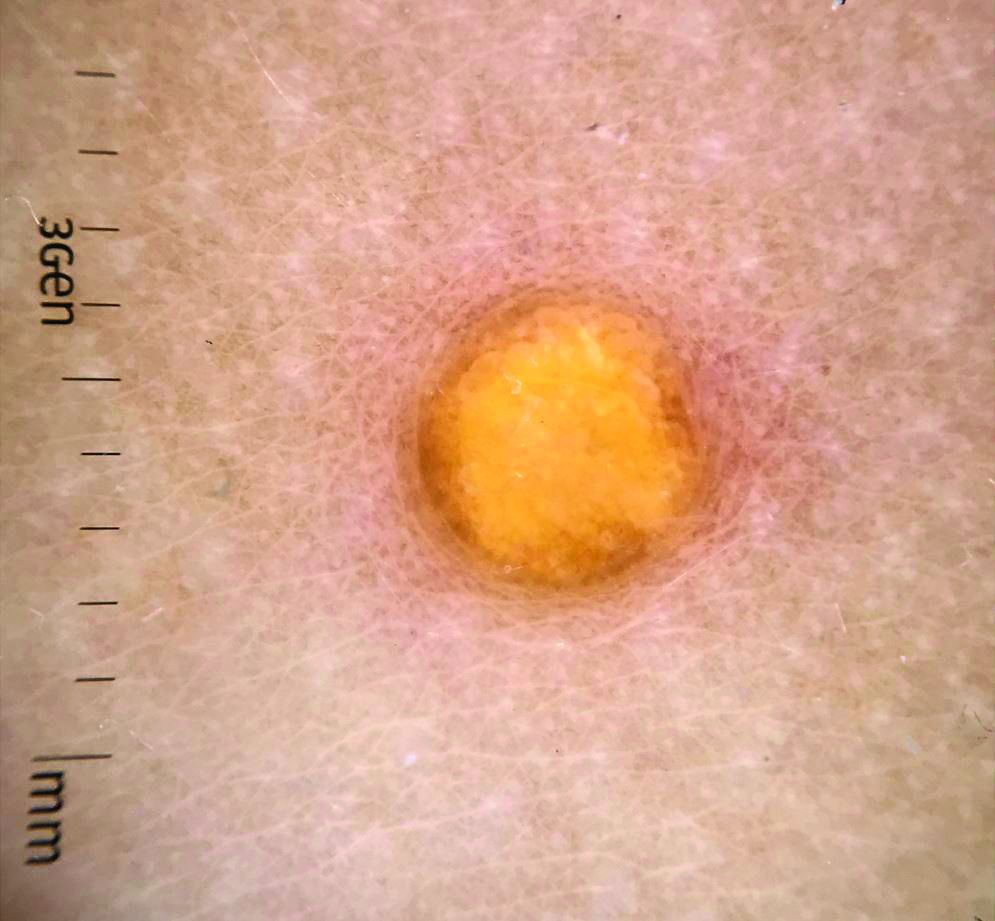
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
Juvenile xanthogranuloma (JXG) is a benign disorder presenting as firm, yellow-red skin papules or nodules, usually in infancy or early childhood. It derives its name based on its yellowish color and the histologic finding of lipid-filled histiocytes. In fact, it is a form of non-Langerhans’ cell histiocytosis. It most commonly presents on the head, neck, and trunk, but can arise anywhere on the body as demonstrated by this case. While often pink to reddish early on, the characteristic yellow or orange, brown appearance over time is common, occasionally with overlying telangiectasia, and ranging in size from 1 mm to 2 cm. While typically asymptomatic, it is possible for lesions to itch. JXG is usually self-limiting, and spontaneously resolves over several years. On dermoscopy (with polarized light), it has a characteristic “setting sun” appearance because of its central yellow area surrounded by a reddish periphery.
JXGs have been associated with neurofibromatosis-1 and a “triple association” of NF-1, JXG, and juvenile myelomonocytic leukemia (JMML) has been debated. Many cases are diagnosed on clinical grounds without histologic confirmation, so while the absolute incidence is unknown, they are not uncommon.
What is on the differential?
Spitz nevus is a melanocytic lesion which typically presents as a sharply circumscribed, dome-shaped, pink-red or brown papule or nodule, and is composed of large epithelioid and/or spindled cells. These nevi can present with a spectrum of morphology and biologic activity; commonly with benign melanocytic proliferations and a symmetric appearance or, rarely, with atypical tumors or lesions, characterized as Spitzoid melanomas. The yellowish color of JXG is distinct from the appearance of Spitz tumors.
Molluscum contagiosum is a common pox viral infection seen in children that presents with round, flat-topped firm papules on the skin and distinctive whitish centers with or without umbilication. Like JXG, molluscum contagiosum papules may grow over time and cause pruritus. However, this diagnosis is less likely given the absence of other lesions on the skin, lack of known contacts with similar lesions, and yellowish color without a more typical appearance of molluscum.
Dermatofibromas occur in people of all ages, although more commonly between the ages of 20 and 40 and in those with a history of trauma at the lesion. Like JXGs, dermatofibromas tend to be firm, solitary papules or nodules. They usually are hyperpigmented, and classically “dimple when pinched” as they are fixed to the subcutaneous tissue. However, this patient’s age, lack of trauma, and the lesion morphology are not consistent with dermatofibromas.
Like XJGs, mastocytomas commonly present in the first 2 years of life with maculopapular or nodular lesions that itch. However, the history of new-onset itch in recent months as the lesion grew larger and the yellow color on dermoscopy are more consistent with JXG.
Eruptive xanthomas typically appear suddenly as multiple erythematous yellow, dome-shaped papules on the extensor surfaces of the extremities, buttocks, and hands. They are usually present with hypertriglyceridemia and are very rare in young children. The presence of a solitary lesion in a 6-month-old patient without a history of lipid abnormalities favors the diagnosis of XJG.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Kleinman is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Kleinman have no relevant financial disclosures.
References
Hernandez-Martin A et al. J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):355-67.
Prendiville J. Lumps, bumps and hamartomas in “Neonatal and Infant Dermatology,” 3rd ed. (Philadelphia: Elsevier, 2015).
Püttgen KB. Juvenile xanthogranuloma. UpToDate, 2021.
Schaffer JV. Am J Clin Dermatol. 2021 Mar;22(2):205-20.
Drug cocktail significantly reduced severe COVID, death in outpatients
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
Flu shot highly recommended this year
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
With the Delta variant of COVID-19 still raging in the United States and ICUs in parts of the country filled with patients with the coronavirus, experts are voicing concern about the added risk of a difficult flu season.
Two mathematical models are predicting a big rebound in the number and severity of flu cases in the 2021-22 season after 2020-2021’s flu season failed to show up when public health measures brought in to control COVID-19 seemed to have the added benefit of stopping the flu.
But both analyses, posted to the medRxiv preprint server and not yet peer reviewed by other experts, have come to the same conclusion: The flu could make a comeback this year.
In the worst-case scenario, the United States could see an extra 300,000-400,000 hospitalizations from the flu – almost double the usual number – according to senior study author Mark Roberts, MD, director of the Public Health Dynamics Laboratory at the University of Pittsburgh. These numbers could be a disaster in areas where hospitals are already filled with COVID-19 patients.
Waning natural immunity in the public because of 2020-2021’s missing flu season could make people, especially young children, more likely to get the virus.
“Usually, a combination of natural immunity and vaccination helps tamp down seasonal influenza,” said Dr. Roberts. “If we don’t have the first part, we’ll have to rely more on the vaccine.”
In a typical year, about half of Americans get the flu shot. The new mathematical models predict that the vaccination rate would need to rise to about 75% to avoid the extra hospitalizations. But even a 10% increase in vaccination rates could reduce hospitalizations by 6%-46%, depending on what strains are dominant.
Usually, the Southern Hemisphere flu season, from February to August, helps show what the Northern Hemisphere can expect over the coming winter. But with strict COVID-19 measures and limits on international travel still in place in countries like Australia and New Zealand and much of South America, it has been another record-low year for flu infections, said Ian Barr, PhD, deputy director of the World Health Organization’s Collaborating Center for Reference and Research on Influenza in Melbourne.
Australia detected only around 500 cases in 2021, compared with about 300,000 in a normal year, and recorded no hospitalizations or deaths from the flu. New Zealand recorded just two cases.
“I’ve never seen anything like this,” Dr. Barr said.
In Australia, the mild flu season led to fewer people getting their flu shot than usual. The rate fell from around 50% to just 33%, said Dr. Barr. “If that happens in the U.S., the population will be even more vulnerable because there has been almost no flu for more than 12 months,” he said.
Both Dr. Roberts and Dr. Barr say it is vital that as many people as possible get vaccinated during the upcoming flu season, especially children who will have almost no natural immunity to the virus.
“The vaccine is our best weapon against the flu, especially for the most at-risk groups,” said Dr. Barr.
Other parts of the world had mixed results. India saw a high number of flu cases, while neighboring Sri Lanka had very few. West Africa also saw quite a high level of circulating virus. Overall, the flu was detected in 45 countries during the Southern Hemisphere season, less than half of what might be expected in a normal year, said Dr. Barr.
Despite the overall low numbers, the WHO saw enough in the data to make two changes to 2022’s Southern Hemisphere vaccine formulation at its meeting on Sept. 24, after changing just one of the strains for the Northern Hemisphere vaccine at its meeting in February.
The CDC recommends that everyone 6 months or older get the flu shot, with few exceptions.
A version of this article first appeared on WebMD.com.
Management of pediatric food allergies evolving
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
New data illustrate pandemic pivot to telehealth by patients, physicians
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Worried parents scramble to vaccinate kids despite FDA guidance
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
Predicted pandemic retirement of many physicians hasn’t happened
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
Study finds paying people to participate in clinical trials is not unethical
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Dr. Judy C. Washington shows URM physicians how to lead
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”