User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cold viruses thrived in kids as other viruses faded in 2020
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Web of antimicrobials doesn’t hold water
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Handheld device highly sensitive in detecting amblyopia; can be used in children as young as 2 years of age
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA clears first mobile rapid test for concussion
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
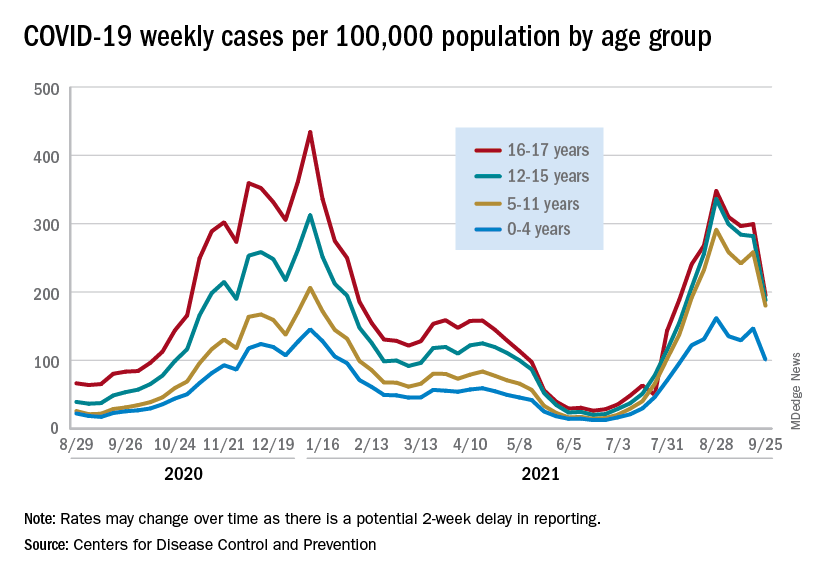
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
Ruxolitinib cream meets primary endpoints in phase 3 vitiligo trial
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
COVID-19: Two more cases of mucosal skin ulcers reported in male teens
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Extension study finds dupilumab effective for up to 1 year in teens with AD
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.







