User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
NYC switching children’s COVID vaccine sites to monkeypox
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
#ShowMeYourBuns: Social media outrage over nurses’ messy hair policy
Social media comic Blake Lynch, BSN, RN, known to his millions of followers as “Nurse Blake,” took to his online platforms recently to voice outrage over a Nebraska health care system’s personal appearance policy.
His posts included a screenshot explanation that was presented at a Bryan Health clinical manager meeting featuring an image of women’s hair in buns and the statement: “There is emphasis on hair being clean, neatly managed, therefore ‘no messy buns.’ “
“If you really want to make a difference, don’t worry about hair,” Mr. Lynch said in his social media posts. “Let’s talk safe staffing. Let’s talk mandatory breaks, uninterrupted breaks. Since when is hair an indication if a nurse is a good nurse or a bad nurse? ... Nurses are running around over 12 hours, sweating in patient rooms, putting on PPE, taking off PPE, saving lives, doing CPR. They don’t even have time for beaks, so what nurse is going to be worrying about what their hair looks like?”
Mr. Lynch’s video response to the statement and image attracted more than 560,000 views on Facebook. He subsequently encouraged followers to post photos of their messy buns under the hashtags #showmeyourbuns and #messybunhairday.
Mr. Lynch, who tours the country as a comedian and leads continuing nurse education programs, told this news organization he was not surprised by the reaction to his “messy buns” video. “I think this particular post got so much attention because it resonated with so many nurses,” he said.
He reiterated that
Bryan Health, based in Lincoln, Neb., responded on Twitter and in a more extensive statement to this news organization that in his “messy bun” post, Mr. Lynch misrepresented a long-standing health system policy on personal appearance and cleanliness.
The health system’s dress code policy does not mention “messy buns,” the health system stated. The policy mirrors those of other health systems and industries that try to maintain safety and sanitation, the statement continued.
The portion of the policy that sparked interest was not about securing hair but eliminating previous language pertaining to unnatural hair colors, Bryan Health stated.
The relaxed language reads: “Haircuts and colors will not be restricted, but all hair is to be clean, neatly managed, and appropriately secured out of the face. Headbands worn should be simple and professional in color or pattern.”
The health system’s statement continued: “The policy does and will continue to reference clean, neatly managed hair, appropriately secured out of the face. Appropriately secured hair is important for a number of safety reasons.”
A pediatric nurse who goes by “CB” on Twitter responded to Mr. Lynch’s post by indicating that she worked at the Nebraska hospital. “What a joke!!!” Earlier in her response, she said, “You realize most hospitals are dealing with severely understaffed units and nurse burnout. How about you worry about your staff ratios, not your nurses’ hair.”
Mr. Lynch said a nurse sent him a screenshot of “messy buns,” like other followers who send him items for discussion on his social media page. Since the post went viral, Mr. Lynch said he’s had followers inform him of how hair policies such as Bryan Health’s have targeted people of color for more than a decade. And a Nebraska health system told him they’d welcome any nurses with messy hair to offset their nursing shortage.
A version of this article first appeared on Medscape.com.
Social media comic Blake Lynch, BSN, RN, known to his millions of followers as “Nurse Blake,” took to his online platforms recently to voice outrage over a Nebraska health care system’s personal appearance policy.
His posts included a screenshot explanation that was presented at a Bryan Health clinical manager meeting featuring an image of women’s hair in buns and the statement: “There is emphasis on hair being clean, neatly managed, therefore ‘no messy buns.’ “
“If you really want to make a difference, don’t worry about hair,” Mr. Lynch said in his social media posts. “Let’s talk safe staffing. Let’s talk mandatory breaks, uninterrupted breaks. Since when is hair an indication if a nurse is a good nurse or a bad nurse? ... Nurses are running around over 12 hours, sweating in patient rooms, putting on PPE, taking off PPE, saving lives, doing CPR. They don’t even have time for beaks, so what nurse is going to be worrying about what their hair looks like?”
Mr. Lynch’s video response to the statement and image attracted more than 560,000 views on Facebook. He subsequently encouraged followers to post photos of their messy buns under the hashtags #showmeyourbuns and #messybunhairday.
Mr. Lynch, who tours the country as a comedian and leads continuing nurse education programs, told this news organization he was not surprised by the reaction to his “messy buns” video. “I think this particular post got so much attention because it resonated with so many nurses,” he said.
He reiterated that
Bryan Health, based in Lincoln, Neb., responded on Twitter and in a more extensive statement to this news organization that in his “messy bun” post, Mr. Lynch misrepresented a long-standing health system policy on personal appearance and cleanliness.
The health system’s dress code policy does not mention “messy buns,” the health system stated. The policy mirrors those of other health systems and industries that try to maintain safety and sanitation, the statement continued.
The portion of the policy that sparked interest was not about securing hair but eliminating previous language pertaining to unnatural hair colors, Bryan Health stated.
The relaxed language reads: “Haircuts and colors will not be restricted, but all hair is to be clean, neatly managed, and appropriately secured out of the face. Headbands worn should be simple and professional in color or pattern.”
The health system’s statement continued: “The policy does and will continue to reference clean, neatly managed hair, appropriately secured out of the face. Appropriately secured hair is important for a number of safety reasons.”
A pediatric nurse who goes by “CB” on Twitter responded to Mr. Lynch’s post by indicating that she worked at the Nebraska hospital. “What a joke!!!” Earlier in her response, she said, “You realize most hospitals are dealing with severely understaffed units and nurse burnout. How about you worry about your staff ratios, not your nurses’ hair.”
Mr. Lynch said a nurse sent him a screenshot of “messy buns,” like other followers who send him items for discussion on his social media page. Since the post went viral, Mr. Lynch said he’s had followers inform him of how hair policies such as Bryan Health’s have targeted people of color for more than a decade. And a Nebraska health system told him they’d welcome any nurses with messy hair to offset their nursing shortage.
A version of this article first appeared on Medscape.com.
Social media comic Blake Lynch, BSN, RN, known to his millions of followers as “Nurse Blake,” took to his online platforms recently to voice outrage over a Nebraska health care system’s personal appearance policy.
His posts included a screenshot explanation that was presented at a Bryan Health clinical manager meeting featuring an image of women’s hair in buns and the statement: “There is emphasis on hair being clean, neatly managed, therefore ‘no messy buns.’ “
“If you really want to make a difference, don’t worry about hair,” Mr. Lynch said in his social media posts. “Let’s talk safe staffing. Let’s talk mandatory breaks, uninterrupted breaks. Since when is hair an indication if a nurse is a good nurse or a bad nurse? ... Nurses are running around over 12 hours, sweating in patient rooms, putting on PPE, taking off PPE, saving lives, doing CPR. They don’t even have time for beaks, so what nurse is going to be worrying about what their hair looks like?”
Mr. Lynch’s video response to the statement and image attracted more than 560,000 views on Facebook. He subsequently encouraged followers to post photos of their messy buns under the hashtags #showmeyourbuns and #messybunhairday.
Mr. Lynch, who tours the country as a comedian and leads continuing nurse education programs, told this news organization he was not surprised by the reaction to his “messy buns” video. “I think this particular post got so much attention because it resonated with so many nurses,” he said.
He reiterated that
Bryan Health, based in Lincoln, Neb., responded on Twitter and in a more extensive statement to this news organization that in his “messy bun” post, Mr. Lynch misrepresented a long-standing health system policy on personal appearance and cleanliness.
The health system’s dress code policy does not mention “messy buns,” the health system stated. The policy mirrors those of other health systems and industries that try to maintain safety and sanitation, the statement continued.
The portion of the policy that sparked interest was not about securing hair but eliminating previous language pertaining to unnatural hair colors, Bryan Health stated.
The relaxed language reads: “Haircuts and colors will not be restricted, but all hair is to be clean, neatly managed, and appropriately secured out of the face. Headbands worn should be simple and professional in color or pattern.”
The health system’s statement continued: “The policy does and will continue to reference clean, neatly managed hair, appropriately secured out of the face. Appropriately secured hair is important for a number of safety reasons.”
A pediatric nurse who goes by “CB” on Twitter responded to Mr. Lynch’s post by indicating that she worked at the Nebraska hospital. “What a joke!!!” Earlier in her response, she said, “You realize most hospitals are dealing with severely understaffed units and nurse burnout. How about you worry about your staff ratios, not your nurses’ hair.”
Mr. Lynch said a nurse sent him a screenshot of “messy buns,” like other followers who send him items for discussion on his social media page. Since the post went viral, Mr. Lynch said he’s had followers inform him of how hair policies such as Bryan Health’s have targeted people of color for more than a decade. And a Nebraska health system told him they’d welcome any nurses with messy hair to offset their nursing shortage.
A version of this article first appeared on Medscape.com.
Should you sell your practice to a private equity firm?
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
More and more physicians are being wooed by private equity firms that want to buy their practices. The total value of private equity deals in health care in 2019 is estimated at about $120 billion, and it’s expected to grow over the coming years.
While the potential profit may seem alluring, physicians have mixed feelings as to whether this will be a boon or a disappointment.
Angelo Falcone, MD, a former emergency physician in Rockville, Md., found that a private equity investment transformed his career path.
For 19 years, Dr. Falcone was CEO of an emergency medicine group with 35 partners that staffed 10 emergency departments, mostly in Maryland. “We were a pretty small operation looking to get bigger, but to do that would require a substantial investment,” he said.
In 2015, after checking out all their options, the partners decided to sell to US Acute Care Solutions (USACS), a new private equity company founded by Welsh, Carson, Anderson & Stowe, an investment firm in New York. Private equity can be used to expand practices and pay for new equipment. Dr. Falcone, serving as a USACS board member and its operational president, helped spur the company’s astounding growth. Today, USACS has about 5,000 physicians and other clinicians operating in 30 states.
In 2019, Dr. Falcone stepped down from his management post at USACS, took training in integrative medicine, and 2 years later opened a solo integrative medicine practice in Rockville. The new practice, which operates on a concierge model, is not connected with USACS, but Dr, Falcone still sits on the USACS board.
“I had a great experience at USACS. I believe in the power of private equity to support our patients and physicians,” Dr. Falcone said. “Now, at age 58, I have a second career in integrative medicine.”
Private equity is still controversial
David Fleeger, MD, has a different opinion of private equity. “I get offers from private equity firms fairly often, but I’m not seriously interested,” said Dr. Fleeger, a surgeon with Central Texas Colon and Rectal Surgeons in Austin.
“We don’t want to sell to anybody; we want to control our destiny,” he said. “We don’t have to borrow money or repay loans, and we don’t expect to get a windfall for the practice. The profits in medicine are too narrow for that to be realistic. There is no free lunch.”
Some of the doctors who sign up for private equity deals become dissatisfied and want to end the arrangement, according to John Pinto, an ophthalmic practice management consultant in San Diego.
“I get calls about once a month from doctors who want to get out of a private equity deal or revise the terms,” he said. “Some complaints are that the PE firm was too tight with the budget, wouldn’t hire needed staff, mismanaged operations, or otherwise mishandled their investment in the practice.”
It’s difficult for disgruntled physicians to exit a private equity deal, Mr. Pinto said. They commonly have to give up part of the payment they had received for their practice if they leave prematurely, and depending on the jurisdiction, stiff noncompete clauses in their contract won’t allow them to practice nearby.
Disillusioned physicians – and even many physicians who had good experiences with private equity – usually don’t want to air their complaints in public. One reason most of these doctors keep silent is that they have signed nondisclosure and nondisparagement agreements that are part of most private equity deals.
The private equity proposition
Private equity firms typically pay a great deal more for practices than hospitals or even many large private practices, according to James D. Wall, an attorney in Winston-Salem, N.C., who has handled many private equity deals. Mr. Wall said private equity often organizes physicians around one specialty. One advantage these physicians have over hospital-employed physicians is that they aren’t under pressure to refer within a network.
Private equity companies set values for practices on the basis of their earnings before interest, taxes, depreciation, and amortization (EBITDA), said Howard Bogard, an attorney with Burr & Forman in Raleigh, N.C., who has handled many deals. Mr. Bogard said the amount physicians are paid is usually between 4 and 12 times’ EBITDA, so if your practice is earning $1 million a year in EBITDA, you would get $4 million to $12 million for it.
Of the total price tag, “Doctors get a hefty immediate payment when they sell,” Mr. Bogard said. “It might be 70% of the purchase price up front, and the 30% left over is equity in the buyer. The private equity firm then sells the practice 5-7 years later, and at that time, the physician’s equity is converted to cash and equity in the new buyer, often at the same 70/30 ratio. The idea is to keep the doctor interested in staying.”
Private equity firms expand practices to receive more favorable reimbursements and achieve economies of scale, according to Jane Zhu, MD, an assistant professor of medicine at Oregon Health & Science University, Portland, who has studied the phenomenon. Dr. Zhu said these firms may enhance profits by contracting with Medicare Advantage plans, joining accountable care organizations (ACOs), having their physicians work longer hours, and using advanced-practice clinicians instead of physicians.
“They want to make a large return in the order of 20% per year over several years, but they don’t want to strip the practice of value, because they’ll need to sell it to a new investor,” Dr. Zhu said.
When doctors sell to a private equity firm, they become employees and often have to take a pay cut, but their pay may rise again as new efficiencies are instituted. This occurred for partners in Minnesota Eye Consultants (MEC), an 11-member ophthalmology practice in Bloomington, Minn., that helped found Unifeye Vision Partners (UVP), a private equity company financed by Chicago-based Waud Capital Partners.
“When we sold the practice in 2017, we expected to see a 30% cut in the partners’ personal income,” said Richard L. Lindstrom, MD, who headed MEC until he retired last year. “Now, coming into the 6th year, all of the former partners who are still working are earning 10% above presale levels, except for one doctor who wanted to work fewer hours.” These doctors aren’t working longer hours but rather are benefiting from efficiencies, such as adding scribes and improving scheduling, he said.
Private equity brought discipline to the practice, said Dr. Lindstrom, who still sits on the Unifeye board. “In an independent practice, the partners may decide on a new piece of equipment because it would be fun to have, not because they’ve done a financial analysis,” he said. “We don’t wing it anymore.”
On the other hand, according to Dr. Zhu, some private equity firms may use draconian methods to improve efficiency. “Doctors may be expected to order or perform more services or work faster or longer to reach a certain threshold,” she said.
Can private equity uphold your interests?
To win over doctors, a private equity firm may agree to finance projects that the doctors want. For example, Dr. Lindstrom said after his group joined Unifeye, Waud Capital agreed to finance the doctors’ plan to open a new $6 million office. Before the deal, the partners would have had to take out a $6 million loan and personally guarantee it, he said.
A private equity firm may even agree to support the selling doctors’ practice philosophy, such as serving low-income patients – as long as it has a revenue stream. Luis Benavides, MD, is part of a seven-physician family medicine practice that treats many low-income patients in Laredo, Tex. “There is a lot of poverty here,” he said. This March, the group sold to a large private equity company, whose name Dr. Benavides preferred not to reveal.
One reason they made the new arrangement, Dr. Benavides said, was to qualify for ACO REACH, a new Medicare payment program that is mostly used in underserved areas and that allows more distribution of shared savings payments. “Our goal has always been better care,” he said. “We want to know how we can best serve our community.”
Dr. Benavides acknowledges that he has less independence in the new arrangement, but “I already lost my independence when I went from solo practice to a group,” he said. “The upside of a larger organization is that other people may have better ideas than you have.”
Private equity firms often set up governance structures to give physicians some measure of control. Dr. Lindstrom said the governing board of his former practice is solely made up of physicians and deals with local issues such as what office doctors will work in and how many patients they will see. Waud Capital has control of the Unifeye board of directors, but it mainly deals with larger issues, such as acquisition of more practices, he said.
In rare instances, private equity gives doctors control. Dr. Falcone said that from the start of USACS, doctors owned 65% of the company, and in 2020, the physician partners bought out Welsh Carson. “Then we engaged the private equity firm Apollo Global Management, which lent us money for the buyout and became our capital partner, with the doctors now owning 98% of the company,” he said.
On the other hand, some private equity arrangements reportedly have little regard for doctors’ well-being, especially if they are new doctors who didn’t participate in the deal and don’t have equity in it. Dr. Zhu recalled that a new physician was recruited by a practice and was promised a partnership track, but she wasn’t told that the partners were negotiating a private equity deal. “She didn’t find out until the practice was sold months later,” Dr. Zhu said. “The chances of her getting any equity now are unclear.”
Making sure that you pick a company that has your interests at heart requires a lot of digging. Dr. Lindstrom said he and his partners took 3 years to make a decision. They hired a broker to pick the 10 best private equity firms. Then they met with those companies and hired a law firm and an accounting firm to assess them. As the partners inched toward a deal, they voted on each of five critical steps in the decision-making process, he said. He noted that each vote was unanimous.
Impact of private equity
“Private equity deals are changing the health care landscape,” Mr. Wall said. “They are creating large, independent practices that help physicians remain independent from hospital systems and potentially have the clout to get more favorable reimbursements.”
“There is a lot of misunderstanding and mistrust among physicians about private equity,” Dr. Benavides said. “I imagine it will take a while for it to be accepted.”
Until the COVID pandemic, the annual number of private equity deals for doctors had been rising. Will it recover that pace? Mr. Pinto said rising interest rates may dampen activity in the near future.
“The private equity firm often performs a leveraged buyout using borrowed money,” he explained. “This works better when interest rates are low, but interest rates are trending higher. Private equity firms aren’t going away, but they may have to be less generous as the cost of money rises.”
A version of this article first appeared on Medscape.com.
What are growing pains? Turns out no one really knows
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Two deaths from liver failure linked to spinal muscular atrophy drug
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
First weeks back to school: An uneasy transition
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Meet a champion climber with type 1 diabetes
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Saddled with med school debt, yet left out of loan forgiveness plans
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.
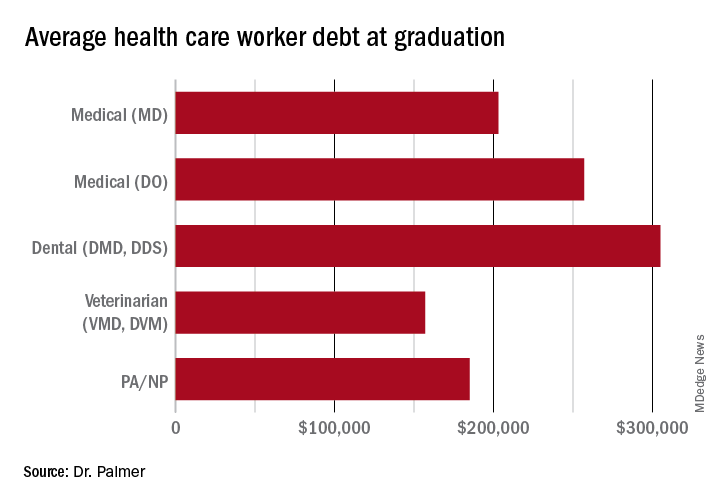
The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.