User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.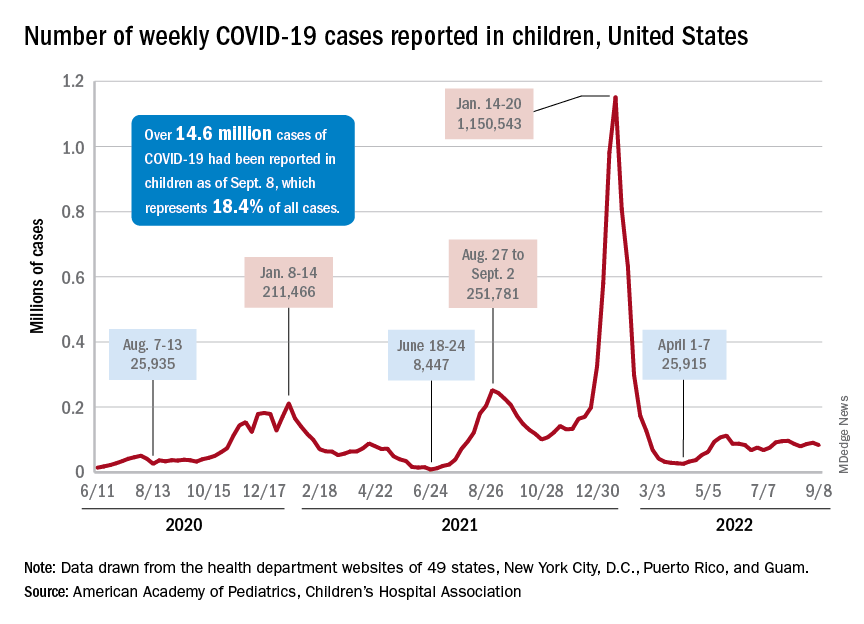
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
CDC warns of enterovirus strain linked to polio-like condition
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
N.Y. governor declares state disaster emergency to boost polio vaccination
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Congenital cytomegalovirus declined in wake of COVID-19
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Pediatric vaccines & infectious diseases, 2022
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
Introduction
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
By Christopher J. Harrison, MD
Table of contents
Antibiotics use and vaccine antibody levels
Commentary
Emerging tick-borne pathogen has spread to state of Georgia
Commentary
WHO, UNICEF warn about increased risk of measles outbreaks
Commentary
Babies die as congenital syphilis continues a decade-long surge across the U.S.
Commentary
Meningococcal vaccine shows moderate protective effect against gonorrhea
Commentary
Adolescents are undertested for STIs
Commentary
TB treatment can be shortened for most children: Study
Commentary
Nirsevimab protects healthy infants from RSV
Commentary
Norovirus vaccine candidates employ different approaches
Commentary
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
The potential problem(s) with a once-a-year COVID vaccine
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Flashy, blingy doc sabotages his own malpractice trial in rural farm town
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.



