User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.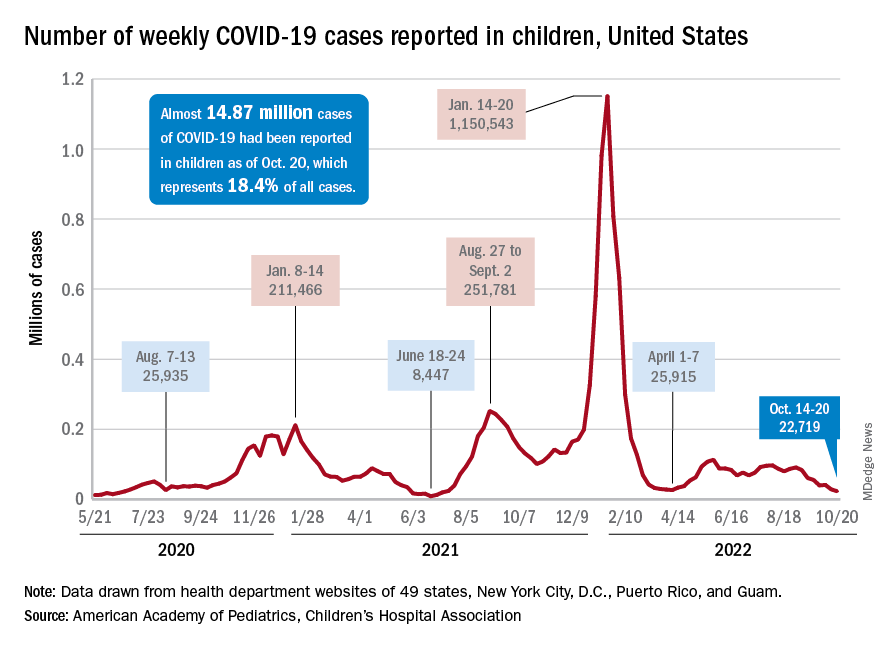
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.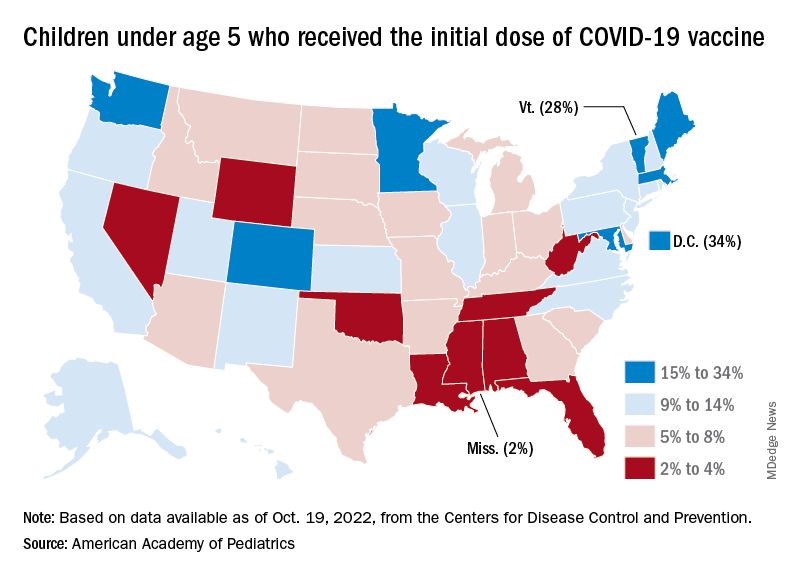
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
Sexual health care for disabled youth: Tough and getting tougher
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab-associated ocular surface disease in patients with AD: Unraveling the link
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
FROM ISAD 2022
Doctors favor euphemisms and jargon in discussions of death
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Psychiatric comorbidities in the pediatric neurology clinic
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
FROM CNS 2022
Two-year dupilumab data: Continued response for moderate to severe pediatric asthma
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
AT CHEST 2022
Achieving diversity, equity and inclusion: Invite everyone and build a team
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
FROM CHEST 2022
Diazepam nasal spray effective in Lennox-Gastaut syndrome
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
AT CNS 2022
Few transgender teens discontinue hormones in young adulthood
Most adolescents with gender dysphoria who took puberty-blocking drugs for at least 3 months and then progressed to cross-sex hormone treatment were still taking hormones as they entered adulthood, new research of patients at a pioneering Dutch clinic shows.
The study negates past findings that large numbers of youth regret transitioning, say Maria Anna Theodora Catharina van der Loos, MD, and colleagues from the Centre of Expertise on Gender Dysphoria, Amsterdam, in their article published online in The Lancet Child & Adolescent Health. They believe the difference between their findings and those of other studies lies in proper diagnostic evaluation.
“The study aims to demonstrate, with a methodology that is more than adequate, that transgender people who begin their transition in childhood-adolescence do not give up,” Adrián Carrasco Munera, MD, a specialist in family and community medicine and member of the LGTBIQ+ Health Group of the Madrid Society of Family and Community Medicine told the UK Science Media Centre.
The cohort included 720 youth: 220 (31%) were assigned male at birth (AMAB) and 500 (69%) were assigned female at birth (AFAB). At the start of puberty-blocking treatment with a gonadotrophin-releasing hormone agonist, the median age of patients was 14.1 years for AMAB and 16.0 years for AFAB.
Of that cohort, 704 (98%) continued hormone therapy to the end of data collection (Dec. 31, 2018), at which point the median age of patients was 20 years for AMAB and 19 years for AFAB.
Careful consideration of patient needs
All the patients received care at the “Dutch Clinic,” which more than 20 years ago pioneered the approach of giving puberty-blocking drugs to children looking to transition, followed by cross-sex hormones. The study includes the “complete adolescent population” at the facility who met the inclusion criteria.
A similar U.S. study published earlier this year found that 74.4% of individuals who had started gender-affirming hormones before age 18 were still on them 4 years after starting medical treatment.
“However, it is unclear how many of these adolescents [in the U.S. study] used puberty-suppressing treatment before gender-affirming hormone treatment and to what extent they underwent diagnostic evaluation before initiation of medical treatment,” say Dr. van der Loos and colleagues.
She told this news organization that her clinic provides “a thorough diagnostic and mental health assessment” and discussion of fertility preservation prior to any youth being prescribed puberty blockers or cross-sex hormones.
About 40% of adolescents assessed by the gender clinic in Amsterdam go on to receive hormonal treatment.
“The gender identity unit of the Amsterdam UMC is a world leader in all aspects of transgender medicine and is governed by protocolized actions. This is reflected in the quality of the data and methodology of the study, and therefore of its conclusions,” endocrinologist Gilberto Pérez López, MD, Gregorio Marañón General University Hospital, Madrid, told the UK Science Media Centre.
“These findings can and should help and guide the current public and legal debate on the initiation of medical treatment in transgender minors.”
However, he cautioned the study is limited by the fact that the data come from a registry and they looked at only prescriptions issued and not compliance.
Another interesting thing to note in the research is that almost 70% of patients were born girls and they presented at the gender clinics later in adolescence than the natal boys.
“We don’t have a sound reason for this,” Dr. van der Loos noted.
Study limitations
She also acknowledges that the short follow-up data in some individuals make it difficult to draw conclusions about regret, to some extent.
The average use of cross-sex hormones in their study was 3.5 years for males transitioning to females and 2.3 years for females transitioning to males, so on average, this wouldn’t be long enough to see regret, she acknowledged.
Prior research shows that if youth decide to detransition to their natal sex, this can take, on average, 5 years from the start of medical therapy among born females and 7 years among born males.
However, some born males in the study had been taking hormones for 20 years and some natal females for 15 years, said Dr. van der Loos.
Another limitation is that the research only followed individuals until the end of 2018 while some government data estimate that the number of teens identifying as transgender has nearly doubled over the past 5 years.
The authors, Dr. Munera, and Dr. Lopez have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most adolescents with gender dysphoria who took puberty-blocking drugs for at least 3 months and then progressed to cross-sex hormone treatment were still taking hormones as they entered adulthood, new research of patients at a pioneering Dutch clinic shows.
The study negates past findings that large numbers of youth regret transitioning, say Maria Anna Theodora Catharina van der Loos, MD, and colleagues from the Centre of Expertise on Gender Dysphoria, Amsterdam, in their article published online in The Lancet Child & Adolescent Health. They believe the difference between their findings and those of other studies lies in proper diagnostic evaluation.
“The study aims to demonstrate, with a methodology that is more than adequate, that transgender people who begin their transition in childhood-adolescence do not give up,” Adrián Carrasco Munera, MD, a specialist in family and community medicine and member of the LGTBIQ+ Health Group of the Madrid Society of Family and Community Medicine told the UK Science Media Centre.
The cohort included 720 youth: 220 (31%) were assigned male at birth (AMAB) and 500 (69%) were assigned female at birth (AFAB). At the start of puberty-blocking treatment with a gonadotrophin-releasing hormone agonist, the median age of patients was 14.1 years for AMAB and 16.0 years for AFAB.
Of that cohort, 704 (98%) continued hormone therapy to the end of data collection (Dec. 31, 2018), at which point the median age of patients was 20 years for AMAB and 19 years for AFAB.
Careful consideration of patient needs
All the patients received care at the “Dutch Clinic,” which more than 20 years ago pioneered the approach of giving puberty-blocking drugs to children looking to transition, followed by cross-sex hormones. The study includes the “complete adolescent population” at the facility who met the inclusion criteria.
A similar U.S. study published earlier this year found that 74.4% of individuals who had started gender-affirming hormones before age 18 were still on them 4 years after starting medical treatment.
“However, it is unclear how many of these adolescents [in the U.S. study] used puberty-suppressing treatment before gender-affirming hormone treatment and to what extent they underwent diagnostic evaluation before initiation of medical treatment,” say Dr. van der Loos and colleagues.
She told this news organization that her clinic provides “a thorough diagnostic and mental health assessment” and discussion of fertility preservation prior to any youth being prescribed puberty blockers or cross-sex hormones.
About 40% of adolescents assessed by the gender clinic in Amsterdam go on to receive hormonal treatment.
“The gender identity unit of the Amsterdam UMC is a world leader in all aspects of transgender medicine and is governed by protocolized actions. This is reflected in the quality of the data and methodology of the study, and therefore of its conclusions,” endocrinologist Gilberto Pérez López, MD, Gregorio Marañón General University Hospital, Madrid, told the UK Science Media Centre.
“These findings can and should help and guide the current public and legal debate on the initiation of medical treatment in transgender minors.”
However, he cautioned the study is limited by the fact that the data come from a registry and they looked at only prescriptions issued and not compliance.
Another interesting thing to note in the research is that almost 70% of patients were born girls and they presented at the gender clinics later in adolescence than the natal boys.
“We don’t have a sound reason for this,” Dr. van der Loos noted.
Study limitations
She also acknowledges that the short follow-up data in some individuals make it difficult to draw conclusions about regret, to some extent.
The average use of cross-sex hormones in their study was 3.5 years for males transitioning to females and 2.3 years for females transitioning to males, so on average, this wouldn’t be long enough to see regret, she acknowledged.
Prior research shows that if youth decide to detransition to their natal sex, this can take, on average, 5 years from the start of medical therapy among born females and 7 years among born males.
However, some born males in the study had been taking hormones for 20 years and some natal females for 15 years, said Dr. van der Loos.
Another limitation is that the research only followed individuals until the end of 2018 while some government data estimate that the number of teens identifying as transgender has nearly doubled over the past 5 years.
The authors, Dr. Munera, and Dr. Lopez have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most adolescents with gender dysphoria who took puberty-blocking drugs for at least 3 months and then progressed to cross-sex hormone treatment were still taking hormones as they entered adulthood, new research of patients at a pioneering Dutch clinic shows.
The study negates past findings that large numbers of youth regret transitioning, say Maria Anna Theodora Catharina van der Loos, MD, and colleagues from the Centre of Expertise on Gender Dysphoria, Amsterdam, in their article published online in The Lancet Child & Adolescent Health. They believe the difference between their findings and those of other studies lies in proper diagnostic evaluation.
“The study aims to demonstrate, with a methodology that is more than adequate, that transgender people who begin their transition in childhood-adolescence do not give up,” Adrián Carrasco Munera, MD, a specialist in family and community medicine and member of the LGTBIQ+ Health Group of the Madrid Society of Family and Community Medicine told the UK Science Media Centre.
The cohort included 720 youth: 220 (31%) were assigned male at birth (AMAB) and 500 (69%) were assigned female at birth (AFAB). At the start of puberty-blocking treatment with a gonadotrophin-releasing hormone agonist, the median age of patients was 14.1 years for AMAB and 16.0 years for AFAB.
Of that cohort, 704 (98%) continued hormone therapy to the end of data collection (Dec. 31, 2018), at which point the median age of patients was 20 years for AMAB and 19 years for AFAB.
Careful consideration of patient needs
All the patients received care at the “Dutch Clinic,” which more than 20 years ago pioneered the approach of giving puberty-blocking drugs to children looking to transition, followed by cross-sex hormones. The study includes the “complete adolescent population” at the facility who met the inclusion criteria.
A similar U.S. study published earlier this year found that 74.4% of individuals who had started gender-affirming hormones before age 18 were still on them 4 years after starting medical treatment.
“However, it is unclear how many of these adolescents [in the U.S. study] used puberty-suppressing treatment before gender-affirming hormone treatment and to what extent they underwent diagnostic evaluation before initiation of medical treatment,” say Dr. van der Loos and colleagues.
She told this news organization that her clinic provides “a thorough diagnostic and mental health assessment” and discussion of fertility preservation prior to any youth being prescribed puberty blockers or cross-sex hormones.
About 40% of adolescents assessed by the gender clinic in Amsterdam go on to receive hormonal treatment.
“The gender identity unit of the Amsterdam UMC is a world leader in all aspects of transgender medicine and is governed by protocolized actions. This is reflected in the quality of the data and methodology of the study, and therefore of its conclusions,” endocrinologist Gilberto Pérez López, MD, Gregorio Marañón General University Hospital, Madrid, told the UK Science Media Centre.
“These findings can and should help and guide the current public and legal debate on the initiation of medical treatment in transgender minors.”
However, he cautioned the study is limited by the fact that the data come from a registry and they looked at only prescriptions issued and not compliance.
Another interesting thing to note in the research is that almost 70% of patients were born girls and they presented at the gender clinics later in adolescence than the natal boys.
“We don’t have a sound reason for this,” Dr. van der Loos noted.
Study limitations
She also acknowledges that the short follow-up data in some individuals make it difficult to draw conclusions about regret, to some extent.
The average use of cross-sex hormones in their study was 3.5 years for males transitioning to females and 2.3 years for females transitioning to males, so on average, this wouldn’t be long enough to see regret, she acknowledged.
Prior research shows that if youth decide to detransition to their natal sex, this can take, on average, 5 years from the start of medical therapy among born females and 7 years among born males.
However, some born males in the study had been taking hormones for 20 years and some natal females for 15 years, said Dr. van der Loos.
Another limitation is that the research only followed individuals until the end of 2018 while some government data estimate that the number of teens identifying as transgender has nearly doubled over the past 5 years.
The authors, Dr. Munera, and Dr. Lopez have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evidence mounting that full-body emollients don’t prevent AD in at-risk babies
MONTREAL – , according to 5-year results of the BEEP randomized trial, reported at the annual meeting of the International Society of Atopic Dermatitis.
“So far, the science does not look convincing, and I am concerned about the possible harms,” commented senior investigator Hywel C. Williams, DSc, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
The rate of AD at 2 years – the primary outcome of the BEEP trial – have already shown no benefit of either Diprobase cream or DoubleBase gel plus standard skin-care advice versus standard skin-care advice alone among 1,394 infants at high risk for developing AD. “These are children born to parents with a first-degree relative with eczema,” Dr. Williams explained.
At 2 years, 23% of the emollient group versus 25% of the control group developed eczema (adjusted relative risk, 0.95), and the parent-reported clinical skin infection rate was statistically increased (incidence rate ratio, 1.55). Despite these results, follow-up of BEEP was extended to 5 years to determine if there was a delayed benefit of emollients, both in AD prevention but also with other related disorders, he explained.
“Prevention is so much more logical than treating sick individuals with severe disease who present after a long chain of pathological events with expensive drugs. And even if you can’t primarily prevent eczema, even a small shift in the severity of distribution to the left has major public health implications,” Dr. Williams added. “And if you believe in the atopic march, then if you could prevent eczema, you might be able to prevent subsequent food allergy, asthma, and allergic rhinitis.”
The extension data was based on questionnaires at 3, 4, and 5 years documenting parental reports of doctor-diagnosed eczema and eczema severity, wheezing, allergic rhinitis, food allergy symptoms, and clinical diagnosis, as well as 5-year clinical diagnoses of asthma or allergic rhinitis. About 70% of parents returned their questionnaires at each point, showing no significant difference at 5 years for a clinical diagnosis of eczema (31% in the emollient group vs. 28% in controls), clinical diagnosis of food allergy (15% vs. 14%, respectively), or other outcomes.
“It’s a lovely hypothesis, but did we use the wrong emollients, or did we start it too late? Or should we start facing the possibility that maybe emollients really do not prevent eczema?” Dr. Williams commented, adding that he does not recommend use of emollients for AD prevention.
“There’s more research needed,” agreed panelist Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, whose AD primary prevention CASCADE trial is expected to shed more light on the role of emollients in the near future. “And we can’t just ignore [another] randomized controlled trial that was done really well ... showing a positive effect,” he added, referring to the small, single-center STOP-AD trial.
“We’re always hoping, and it’s scientifically incredibly frustrating that none of this has borne out,” Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, told this news organization. “It’s so appealing that emollients early in life would improve the skin barrier and then decrease likelihood of getting eczema. It’s great that there’s a new, large study from Dr. Simpson that is going to be coming out soon, so we’ll have another piece of this puzzle.”
Dr. Drucker said that although it sounds simple, there is much nuance in the question of emollients and skin barrier protection: “Who is the population that you ought to use the emollients in? What kind of emollient? How often and where? All of these things can influence potentially what the results of a trial might be. That’s where there’s still hope. I think the hope fades more and more as more evidence piles up.”
He added that although there currently is not enough evidence to recommend emollients for AD prevention, there is also not enough evidence of harm. “It’s nothing we should be afraid of,” Dr. Drucker advised.
Dr. Williams and Dr. Drucker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to 5-year results of the BEEP randomized trial, reported at the annual meeting of the International Society of Atopic Dermatitis.
“So far, the science does not look convincing, and I am concerned about the possible harms,” commented senior investigator Hywel C. Williams, DSc, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
The rate of AD at 2 years – the primary outcome of the BEEP trial – have already shown no benefit of either Diprobase cream or DoubleBase gel plus standard skin-care advice versus standard skin-care advice alone among 1,394 infants at high risk for developing AD. “These are children born to parents with a first-degree relative with eczema,” Dr. Williams explained.
At 2 years, 23% of the emollient group versus 25% of the control group developed eczema (adjusted relative risk, 0.95), and the parent-reported clinical skin infection rate was statistically increased (incidence rate ratio, 1.55). Despite these results, follow-up of BEEP was extended to 5 years to determine if there was a delayed benefit of emollients, both in AD prevention but also with other related disorders, he explained.
“Prevention is so much more logical than treating sick individuals with severe disease who present after a long chain of pathological events with expensive drugs. And even if you can’t primarily prevent eczema, even a small shift in the severity of distribution to the left has major public health implications,” Dr. Williams added. “And if you believe in the atopic march, then if you could prevent eczema, you might be able to prevent subsequent food allergy, asthma, and allergic rhinitis.”
The extension data was based on questionnaires at 3, 4, and 5 years documenting parental reports of doctor-diagnosed eczema and eczema severity, wheezing, allergic rhinitis, food allergy symptoms, and clinical diagnosis, as well as 5-year clinical diagnoses of asthma or allergic rhinitis. About 70% of parents returned their questionnaires at each point, showing no significant difference at 5 years for a clinical diagnosis of eczema (31% in the emollient group vs. 28% in controls), clinical diagnosis of food allergy (15% vs. 14%, respectively), or other outcomes.
“It’s a lovely hypothesis, but did we use the wrong emollients, or did we start it too late? Or should we start facing the possibility that maybe emollients really do not prevent eczema?” Dr. Williams commented, adding that he does not recommend use of emollients for AD prevention.
“There’s more research needed,” agreed panelist Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, whose AD primary prevention CASCADE trial is expected to shed more light on the role of emollients in the near future. “And we can’t just ignore [another] randomized controlled trial that was done really well ... showing a positive effect,” he added, referring to the small, single-center STOP-AD trial.
“We’re always hoping, and it’s scientifically incredibly frustrating that none of this has borne out,” Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, told this news organization. “It’s so appealing that emollients early in life would improve the skin barrier and then decrease likelihood of getting eczema. It’s great that there’s a new, large study from Dr. Simpson that is going to be coming out soon, so we’ll have another piece of this puzzle.”
Dr. Drucker said that although it sounds simple, there is much nuance in the question of emollients and skin barrier protection: “Who is the population that you ought to use the emollients in? What kind of emollient? How often and where? All of these things can influence potentially what the results of a trial might be. That’s where there’s still hope. I think the hope fades more and more as more evidence piles up.”
He added that although there currently is not enough evidence to recommend emollients for AD prevention, there is also not enough evidence of harm. “It’s nothing we should be afraid of,” Dr. Drucker advised.
Dr. Williams and Dr. Drucker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to 5-year results of the BEEP randomized trial, reported at the annual meeting of the International Society of Atopic Dermatitis.
“So far, the science does not look convincing, and I am concerned about the possible harms,” commented senior investigator Hywel C. Williams, DSc, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
The rate of AD at 2 years – the primary outcome of the BEEP trial – have already shown no benefit of either Diprobase cream or DoubleBase gel plus standard skin-care advice versus standard skin-care advice alone among 1,394 infants at high risk for developing AD. “These are children born to parents with a first-degree relative with eczema,” Dr. Williams explained.
At 2 years, 23% of the emollient group versus 25% of the control group developed eczema (adjusted relative risk, 0.95), and the parent-reported clinical skin infection rate was statistically increased (incidence rate ratio, 1.55). Despite these results, follow-up of BEEP was extended to 5 years to determine if there was a delayed benefit of emollients, both in AD prevention but also with other related disorders, he explained.
“Prevention is so much more logical than treating sick individuals with severe disease who present after a long chain of pathological events with expensive drugs. And even if you can’t primarily prevent eczema, even a small shift in the severity of distribution to the left has major public health implications,” Dr. Williams added. “And if you believe in the atopic march, then if you could prevent eczema, you might be able to prevent subsequent food allergy, asthma, and allergic rhinitis.”
The extension data was based on questionnaires at 3, 4, and 5 years documenting parental reports of doctor-diagnosed eczema and eczema severity, wheezing, allergic rhinitis, food allergy symptoms, and clinical diagnosis, as well as 5-year clinical diagnoses of asthma or allergic rhinitis. About 70% of parents returned their questionnaires at each point, showing no significant difference at 5 years for a clinical diagnosis of eczema (31% in the emollient group vs. 28% in controls), clinical diagnosis of food allergy (15% vs. 14%, respectively), or other outcomes.
“It’s a lovely hypothesis, but did we use the wrong emollients, or did we start it too late? Or should we start facing the possibility that maybe emollients really do not prevent eczema?” Dr. Williams commented, adding that he does not recommend use of emollients for AD prevention.
“There’s more research needed,” agreed panelist Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, whose AD primary prevention CASCADE trial is expected to shed more light on the role of emollients in the near future. “And we can’t just ignore [another] randomized controlled trial that was done really well ... showing a positive effect,” he added, referring to the small, single-center STOP-AD trial.
“We’re always hoping, and it’s scientifically incredibly frustrating that none of this has borne out,” Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, told this news organization. “It’s so appealing that emollients early in life would improve the skin barrier and then decrease likelihood of getting eczema. It’s great that there’s a new, large study from Dr. Simpson that is going to be coming out soon, so we’ll have another piece of this puzzle.”
Dr. Drucker said that although it sounds simple, there is much nuance in the question of emollients and skin barrier protection: “Who is the population that you ought to use the emollients in? What kind of emollient? How often and where? All of these things can influence potentially what the results of a trial might be. That’s where there’s still hope. I think the hope fades more and more as more evidence piles up.”
He added that although there currently is not enough evidence to recommend emollients for AD prevention, there is also not enough evidence of harm. “It’s nothing we should be afraid of,” Dr. Drucker advised.
Dr. Williams and Dr. Drucker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ISAD 2022