User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Percentage of doctors who are Black barely changed in 120 years
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA expands use of SLIT pollen allergy treatment to children
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new indication for ALK’s under-the-tongue immunotherapy tablet Ragwitek (Ambrosia artemisiifolia) to treat ragweed pollen–induced hay fever in children aged 5-17 years.
Ragwitek received FDA approval in 2014 to treat short ragweed pollen–induced hay fever, with or without allergic rhinoconjunctivitis, in adults aged 18-65 years.
The approval for Ragwitek comes with a boxed warning regarding a risk for life-threatening allergic reactions associated with the immunotherapy treatment, including anaphylaxis and severe laryngopharyngeal restriction. The package insert specifies that physicians should prescribe autoinjectable epinephrine with the drug.
“Ragwitek tablets provide a new immunotherapy treatment option for children and adolescents with seasonal ragweed allergies which often causes uncomfortable nasal symptoms and red, itchy eyes during the late summer and early fall,” David I. Bernstein, MD, University of Cincinnati, Bernstein Clinical Research, said in a company press release.
Short ragweed pollen is one of the most common weed allergies. Allergic rhinitis, or hay fever, affects 10%-30% of the population worldwide, according to the American Academy of Allergy Asthma & Immunology. In the United States, approximately 7.7% of adults and 7.2% of children were diagnosed with it annually, according to the Centers for Disease Control and Prevention.
The new indication was based partly on data from a phase 3 clinical trial in children with short ragweed–induced allergic rhinitis, or hay fever, published in the Journal of Allergy and Clinical Immunology. In the study, researchers evaluated the efficacy and safety of the treatment in 1,022 participants aged 5-17 years with a history of ragweed-induced rhinoconjunctivitis and sensitivity to ragweed over a 20- to 28-week treatment period.
Researchers found that Ragwitek improved symptoms in children and adolescents and decreased their use of symptom-relieving medication, compared with placebo.
Among children and adolescents aged 5-17 years, the most common adverse reactions reported were throat irritation/tickle (48.3% in the Ragwitek group vs. 17.7% in the placebo group), itching in the mouth (47.8% vs. 11.2%), itching in the ear (33.9% vs. 6.3%), mouth pain (18.9% vs. 4.5%), swelling of the lips (13.8% vs. 1.2%), nausea (11.5% vs. 3.3%), swelling of the tongue (11.3% vs. 0.8%), throat swelling (10.7% vs. 1.6%), and stomach pain (10.1% vs. 4.5%).
The FDA also recommends that Ragwitek not be prescribed to people with severe, unstable, or uncontrolled asthma, those with a history of severe systemic allergic reactions, and those with a history of eosinophilic esophagitis. The immunotherapy treatment also may not be suitable for people who are unresponsive to epinephrine or inhaled bronchodilators.
In addition, the treatment is not approved for the immediate relief of allergic symptoms in children or adults. The once-daily treatment, which contains an extract from short ragweed pollen, should begin 12 weeks before the start of ragweed pollen season and continue throughout the season, according to the FDA.
Dr. Bernstein said that the under-the-tongue immunotherapy works by targeting the specific allergy trigger and reducing allergy symptoms by “stimulating the immune system.”
A version of this article first appeared on Medscape.com.
COVID-19 infection conveys imperfect immunity in young adults
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
FROM THE LANCET RESPIRATORY MEDICINE
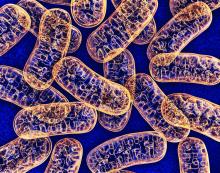
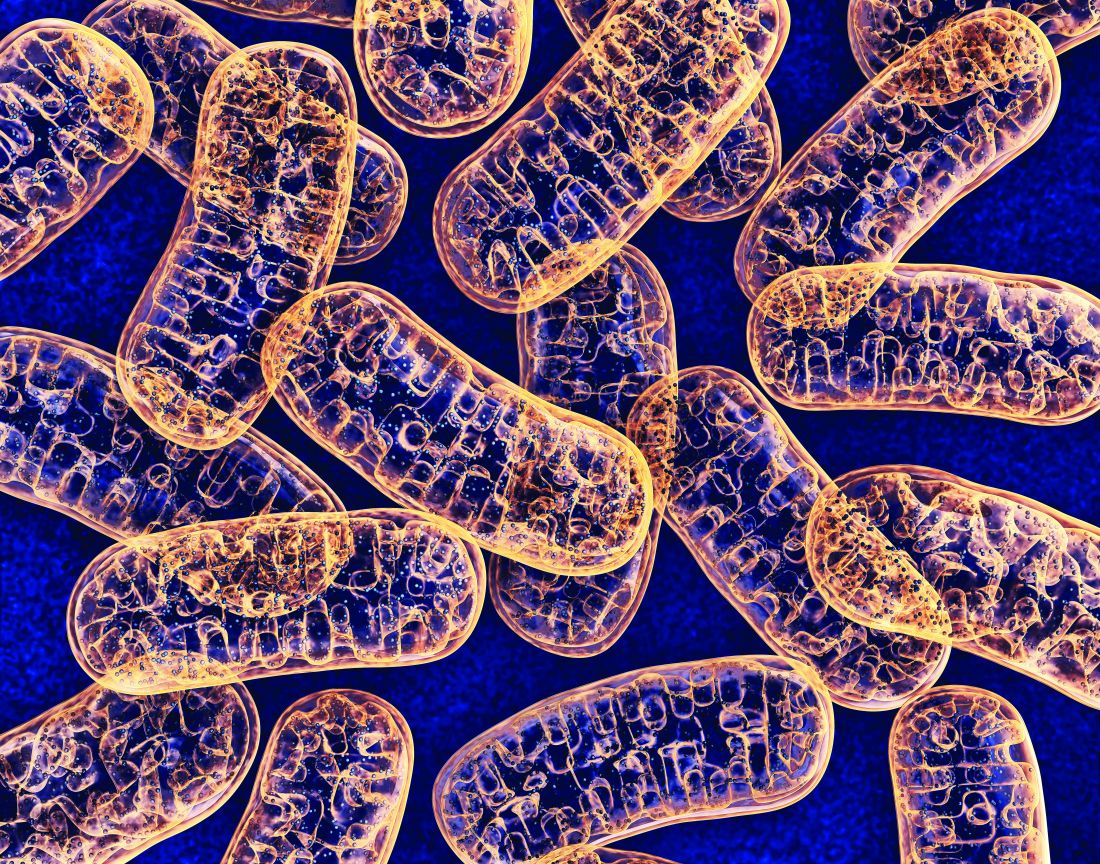
Mitochondrial DNA predicts survival in pediatric acute myeloid leukemia
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
FROM MITOCHONDRION
Asian children less likely to receive ADHD treatment
A study of U.S. children across ethnic and racial groups found that Asians were least likely to receive therapy for ADHD, compared with White children – who had the highest odds of getting some kind of treatment over other groups.
Other studies have identified disparity problems in ADHD diagnosis, although results have varied on inequality metrics. Few studies have looked at Asians separately, according to the study’s lead author, Yu Shi, MD, MPH. “They were usually just classified as ‘other’ or as non-White,” Dr. Shi, a consultant with the Mayo Clinic’s division of pediatric anesthesia in Rochester, Minn., said in an interview.
, and the way in which clinicians interpret behavior and apply diagnostic criteria.
“Further understanding of how treatment patterns for ADHD may differ based on race, at the time of initial diagnosis and in the early stages of treatment, may help all children receive appropriate evidence-based care,” Dr. Shi and colleagues reported in JAMA Network Open.
Researchers develop large birth cohort
Dr. Shi and colleagues hypothesized that non-Hispanic White children had a greater chance of getting diagnosed and treated within the first year of diagnosis than that of other ethnic and racial cohorts. Using administrative claims data with socioeconomic status information from a national commercial insurance warehouse, they constructed a retrospective birth cohort of children born between Jan. 1, 2006, and Dec. 31, 2012. The children had continuous insurance coverage for at least 4 years, and represented non-Hispanic Whites, Blacks, Hispanics, and Asians. Self-reporting identified the race/ethnicity groups.
Investigators analyzed ADHD diagnosis and treatment data on 238,011 children between October 2019 and December 2020, using a multivariate Cox regression model to adjust for sex, region, and household income. Primary and secondary outcomes included ADHD diagnosis as defined by recent ICD codes, ADHD behavior, and medication therapies in the clinical setting after initial diagnosis, respectively.
Whites made up most of the cohort (72.7%), followed by Hispanics (9.8%), Asians (6.7%), and Blacks (6.2%). Nearly half the population was female (48.8%). During the follow-up period with these children, 11,401, or 4.8%, had received an ADHD diagnosis. Mean age of diagnosis was 6.5 years, and overall incidence of ADHD was 69 per 10,000 person years (95% confidence interval, 68-70).
Pediatricians were most likely to make an ADHD diagnosis, although the study cited other clinicians, such as psychiatrists, neurologists, psychologists, and family practice clinicians, as responsible for these decisions.
Children diagnosed with ADHD had more years of coverage in the data set, and were more likely to be White and male. The Southern census region had a higher representation of diagnoses (50.6%) than did the Northeast region (11.8%).
Asians at highest odds for no treatment
Taking a closer look at race and ethnicity, Whites had the highest cumulative incidence of ADHD (14.19%), versus Asian children, who had lowest incidence (6.08%). “The curves for Black and Hispanic children were similar in shape and slightly lower than that for White children,” reported the investigators.
White children had higher odds of receiving some kind of treatment, compared with the other groups.
Incidence of medication treatment was lower among Asians and Hispanics. In a striking finding, Asians were most likely to receive no treatment at all (odds ratio compared with White children, 0.54; 95% confidence interval, 0.42-0.70). “However, the percentage of Asian children receiving psychotherapy was not significantly lower than other groups, which is different than a 2013 study finding that Asian children with ADHD were less likely to use mental health services,” they noted.
Most of the patients received medication (32.4%) in the first year after diagnosis, whereas (19.4%) received behavioral therapy only. Nineteen percent had both. More than 29% of these cases had no claims associated with either treatment. Among school-aged children, 65.5% were prescribed medications, compared with just 14.4% who received therapy. Twenty percent had no treatment.
Diagnosis with another disorder often preceded ADHD diagnosis. Results varied among racial groups. White children were more likely than were Black children to be diagnosed with an anxiety or adjustment disorder. Relative to White children, Asians were more likely to be diagnosed with autism spectrum disorder, speech sound disorders, or unspecified neurodevelopmental disorders. Even after an ADHD diagnosis, clinicians were more likely to diagnose Asian children with autism.
Parents may influence treatment decisions
Parental views and preferences may explain some of the variations in diagnosis and treatment among the racial/ethnic groups.
“In order for a diagnosis of ADHD to happen, a parent has first to recognize a problem and bring a child for clinical evaluation,” said Dr. Shi. “A certain behavior could be viewed as normal or a problem depending on a person’s cultural or racial background.” It’s unclear whether clinicians played any role in diagnosis disparities, he added. Patients’ concerns about racism might also influence the desire to get treated in health care systems.
Overall, the findings underscore the presence of racial and ethnic disparities in ADHD diagnosis and treatment. Future research should explore the underlying mechanisms, Dr. Shi suggested. While he and his colleagues have no immediate plans to do another ADHD study, “we’re planning on research to understand disparities in surgery in children,” he said.
The authors cited numerous limitations with their study. Use of ICD codes to identify cases might not have represented true clinical diagnosis, since the data were collected for billing, not research purposes. Investigators drew participants from a commercial insurance database, which did not necessarily reflect all U.S. children. The results might not represent a large number of children covered by Medicaid, for example, noted Dr. Shi. “It is more difficult to work with Medicaid data because there’s no national-level Medicaid data for research. Only state-level data is available.”
Because of other data gaps, Dr. Shi and colleagues might have underestimated the number of children in therapy.
A need for ‘culturally sensitive care’
The findings “ultimately demonstrate the need for culturally sensitive care in the diagnosis and treatment of children and adolescents,” said Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., who was not involved with the study. She specializes in child and adolescent psychiatry.
The exact cause for racial disparity in diagnosis and treatment of ADHD is unknown and likely multifaceted, she continued. “It may be due to differences in the way that disruptive behaviors are interrupted based on factors such as race. This study found that Asian parents often brought their children in for evaluation for reasons other than ADHD. Concerns surrounding the stigma of mental health treatment and racism also could contribute to the disparity in diagnosis and treatment,” she said.
Dr. Bell said she hopes to see future studies that address the impact of social determinants of health on mental illness and investigate underlying causes that contribute to disparities in treatment and diagnosis.
The Mayo Clinic supported the study but had no role in its design or research methods. The authors reported no conflicts of interest.
A study of U.S. children across ethnic and racial groups found that Asians were least likely to receive therapy for ADHD, compared with White children – who had the highest odds of getting some kind of treatment over other groups.
Other studies have identified disparity problems in ADHD diagnosis, although results have varied on inequality metrics. Few studies have looked at Asians separately, according to the study’s lead author, Yu Shi, MD, MPH. “They were usually just classified as ‘other’ or as non-White,” Dr. Shi, a consultant with the Mayo Clinic’s division of pediatric anesthesia in Rochester, Minn., said in an interview.
, and the way in which clinicians interpret behavior and apply diagnostic criteria.
“Further understanding of how treatment patterns for ADHD may differ based on race, at the time of initial diagnosis and in the early stages of treatment, may help all children receive appropriate evidence-based care,” Dr. Shi and colleagues reported in JAMA Network Open.
Researchers develop large birth cohort
Dr. Shi and colleagues hypothesized that non-Hispanic White children had a greater chance of getting diagnosed and treated within the first year of diagnosis than that of other ethnic and racial cohorts. Using administrative claims data with socioeconomic status information from a national commercial insurance warehouse, they constructed a retrospective birth cohort of children born between Jan. 1, 2006, and Dec. 31, 2012. The children had continuous insurance coverage for at least 4 years, and represented non-Hispanic Whites, Blacks, Hispanics, and Asians. Self-reporting identified the race/ethnicity groups.
Investigators analyzed ADHD diagnosis and treatment data on 238,011 children between October 2019 and December 2020, using a multivariate Cox regression model to adjust for sex, region, and household income. Primary and secondary outcomes included ADHD diagnosis as defined by recent ICD codes, ADHD behavior, and medication therapies in the clinical setting after initial diagnosis, respectively.
Whites made up most of the cohort (72.7%), followed by Hispanics (9.8%), Asians (6.7%), and Blacks (6.2%). Nearly half the population was female (48.8%). During the follow-up period with these children, 11,401, or 4.8%, had received an ADHD diagnosis. Mean age of diagnosis was 6.5 years, and overall incidence of ADHD was 69 per 10,000 person years (95% confidence interval, 68-70).
Pediatricians were most likely to make an ADHD diagnosis, although the study cited other clinicians, such as psychiatrists, neurologists, psychologists, and family practice clinicians, as responsible for these decisions.
Children diagnosed with ADHD had more years of coverage in the data set, and were more likely to be White and male. The Southern census region had a higher representation of diagnoses (50.6%) than did the Northeast region (11.8%).
Asians at highest odds for no treatment
Taking a closer look at race and ethnicity, Whites had the highest cumulative incidence of ADHD (14.19%), versus Asian children, who had lowest incidence (6.08%). “The curves for Black and Hispanic children were similar in shape and slightly lower than that for White children,” reported the investigators.
White children had higher odds of receiving some kind of treatment, compared with the other groups.
Incidence of medication treatment was lower among Asians and Hispanics. In a striking finding, Asians were most likely to receive no treatment at all (odds ratio compared with White children, 0.54; 95% confidence interval, 0.42-0.70). “However, the percentage of Asian children receiving psychotherapy was not significantly lower than other groups, which is different than a 2013 study finding that Asian children with ADHD were less likely to use mental health services,” they noted.
Most of the patients received medication (32.4%) in the first year after diagnosis, whereas (19.4%) received behavioral therapy only. Nineteen percent had both. More than 29% of these cases had no claims associated with either treatment. Among school-aged children, 65.5% were prescribed medications, compared with just 14.4% who received therapy. Twenty percent had no treatment.
Diagnosis with another disorder often preceded ADHD diagnosis. Results varied among racial groups. White children were more likely than were Black children to be diagnosed with an anxiety or adjustment disorder. Relative to White children, Asians were more likely to be diagnosed with autism spectrum disorder, speech sound disorders, or unspecified neurodevelopmental disorders. Even after an ADHD diagnosis, clinicians were more likely to diagnose Asian children with autism.
Parents may influence treatment decisions
Parental views and preferences may explain some of the variations in diagnosis and treatment among the racial/ethnic groups.
“In order for a diagnosis of ADHD to happen, a parent has first to recognize a problem and bring a child for clinical evaluation,” said Dr. Shi. “A certain behavior could be viewed as normal or a problem depending on a person’s cultural or racial background.” It’s unclear whether clinicians played any role in diagnosis disparities, he added. Patients’ concerns about racism might also influence the desire to get treated in health care systems.
Overall, the findings underscore the presence of racial and ethnic disparities in ADHD diagnosis and treatment. Future research should explore the underlying mechanisms, Dr. Shi suggested. While he and his colleagues have no immediate plans to do another ADHD study, “we’re planning on research to understand disparities in surgery in children,” he said.
The authors cited numerous limitations with their study. Use of ICD codes to identify cases might not have represented true clinical diagnosis, since the data were collected for billing, not research purposes. Investigators drew participants from a commercial insurance database, which did not necessarily reflect all U.S. children. The results might not represent a large number of children covered by Medicaid, for example, noted Dr. Shi. “It is more difficult to work with Medicaid data because there’s no national-level Medicaid data for research. Only state-level data is available.”
Because of other data gaps, Dr. Shi and colleagues might have underestimated the number of children in therapy.
A need for ‘culturally sensitive care’
The findings “ultimately demonstrate the need for culturally sensitive care in the diagnosis and treatment of children and adolescents,” said Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., who was not involved with the study. She specializes in child and adolescent psychiatry.
The exact cause for racial disparity in diagnosis and treatment of ADHD is unknown and likely multifaceted, she continued. “It may be due to differences in the way that disruptive behaviors are interrupted based on factors such as race. This study found that Asian parents often brought their children in for evaluation for reasons other than ADHD. Concerns surrounding the stigma of mental health treatment and racism also could contribute to the disparity in diagnosis and treatment,” she said.
Dr. Bell said she hopes to see future studies that address the impact of social determinants of health on mental illness and investigate underlying causes that contribute to disparities in treatment and diagnosis.
The Mayo Clinic supported the study but had no role in its design or research methods. The authors reported no conflicts of interest.
A study of U.S. children across ethnic and racial groups found that Asians were least likely to receive therapy for ADHD, compared with White children – who had the highest odds of getting some kind of treatment over other groups.
Other studies have identified disparity problems in ADHD diagnosis, although results have varied on inequality metrics. Few studies have looked at Asians separately, according to the study’s lead author, Yu Shi, MD, MPH. “They were usually just classified as ‘other’ or as non-White,” Dr. Shi, a consultant with the Mayo Clinic’s division of pediatric anesthesia in Rochester, Minn., said in an interview.
, and the way in which clinicians interpret behavior and apply diagnostic criteria.
“Further understanding of how treatment patterns for ADHD may differ based on race, at the time of initial diagnosis and in the early stages of treatment, may help all children receive appropriate evidence-based care,” Dr. Shi and colleagues reported in JAMA Network Open.
Researchers develop large birth cohort
Dr. Shi and colleagues hypothesized that non-Hispanic White children had a greater chance of getting diagnosed and treated within the first year of diagnosis than that of other ethnic and racial cohorts. Using administrative claims data with socioeconomic status information from a national commercial insurance warehouse, they constructed a retrospective birth cohort of children born between Jan. 1, 2006, and Dec. 31, 2012. The children had continuous insurance coverage for at least 4 years, and represented non-Hispanic Whites, Blacks, Hispanics, and Asians. Self-reporting identified the race/ethnicity groups.
Investigators analyzed ADHD diagnosis and treatment data on 238,011 children between October 2019 and December 2020, using a multivariate Cox regression model to adjust for sex, region, and household income. Primary and secondary outcomes included ADHD diagnosis as defined by recent ICD codes, ADHD behavior, and medication therapies in the clinical setting after initial diagnosis, respectively.
Whites made up most of the cohort (72.7%), followed by Hispanics (9.8%), Asians (6.7%), and Blacks (6.2%). Nearly half the population was female (48.8%). During the follow-up period with these children, 11,401, or 4.8%, had received an ADHD diagnosis. Mean age of diagnosis was 6.5 years, and overall incidence of ADHD was 69 per 10,000 person years (95% confidence interval, 68-70).
Pediatricians were most likely to make an ADHD diagnosis, although the study cited other clinicians, such as psychiatrists, neurologists, psychologists, and family practice clinicians, as responsible for these decisions.
Children diagnosed with ADHD had more years of coverage in the data set, and were more likely to be White and male. The Southern census region had a higher representation of diagnoses (50.6%) than did the Northeast region (11.8%).
Asians at highest odds for no treatment
Taking a closer look at race and ethnicity, Whites had the highest cumulative incidence of ADHD (14.19%), versus Asian children, who had lowest incidence (6.08%). “The curves for Black and Hispanic children were similar in shape and slightly lower than that for White children,” reported the investigators.
White children had higher odds of receiving some kind of treatment, compared with the other groups.
Incidence of medication treatment was lower among Asians and Hispanics. In a striking finding, Asians were most likely to receive no treatment at all (odds ratio compared with White children, 0.54; 95% confidence interval, 0.42-0.70). “However, the percentage of Asian children receiving psychotherapy was not significantly lower than other groups, which is different than a 2013 study finding that Asian children with ADHD were less likely to use mental health services,” they noted.
Most of the patients received medication (32.4%) in the first year after diagnosis, whereas (19.4%) received behavioral therapy only. Nineteen percent had both. More than 29% of these cases had no claims associated with either treatment. Among school-aged children, 65.5% were prescribed medications, compared with just 14.4% who received therapy. Twenty percent had no treatment.
Diagnosis with another disorder often preceded ADHD diagnosis. Results varied among racial groups. White children were more likely than were Black children to be diagnosed with an anxiety or adjustment disorder. Relative to White children, Asians were more likely to be diagnosed with autism spectrum disorder, speech sound disorders, or unspecified neurodevelopmental disorders. Even after an ADHD diagnosis, clinicians were more likely to diagnose Asian children with autism.
Parents may influence treatment decisions
Parental views and preferences may explain some of the variations in diagnosis and treatment among the racial/ethnic groups.
“In order for a diagnosis of ADHD to happen, a parent has first to recognize a problem and bring a child for clinical evaluation,” said Dr. Shi. “A certain behavior could be viewed as normal or a problem depending on a person’s cultural or racial background.” It’s unclear whether clinicians played any role in diagnosis disparities, he added. Patients’ concerns about racism might also influence the desire to get treated in health care systems.
Overall, the findings underscore the presence of racial and ethnic disparities in ADHD diagnosis and treatment. Future research should explore the underlying mechanisms, Dr. Shi suggested. While he and his colleagues have no immediate plans to do another ADHD study, “we’re planning on research to understand disparities in surgery in children,” he said.
The authors cited numerous limitations with their study. Use of ICD codes to identify cases might not have represented true clinical diagnosis, since the data were collected for billing, not research purposes. Investigators drew participants from a commercial insurance database, which did not necessarily reflect all U.S. children. The results might not represent a large number of children covered by Medicaid, for example, noted Dr. Shi. “It is more difficult to work with Medicaid data because there’s no national-level Medicaid data for research. Only state-level data is available.”
Because of other data gaps, Dr. Shi and colleagues might have underestimated the number of children in therapy.
A need for ‘culturally sensitive care’
The findings “ultimately demonstrate the need for culturally sensitive care in the diagnosis and treatment of children and adolescents,” said Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., who was not involved with the study. She specializes in child and adolescent psychiatry.
The exact cause for racial disparity in diagnosis and treatment of ADHD is unknown and likely multifaceted, she continued. “It may be due to differences in the way that disruptive behaviors are interrupted based on factors such as race. This study found that Asian parents often brought their children in for evaluation for reasons other than ADHD. Concerns surrounding the stigma of mental health treatment and racism also could contribute to the disparity in diagnosis and treatment,” she said.
Dr. Bell said she hopes to see future studies that address the impact of social determinants of health on mental illness and investigate underlying causes that contribute to disparities in treatment and diagnosis.
The Mayo Clinic supported the study but had no role in its design or research methods. The authors reported no conflicts of interest.
FROM JAMA NETWORK OPEN
Vaccinating homebound patients is an uphill battle

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.
“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.
Pros and cons of proposed recommendation for prediabetes and T2D screening
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
Gene therapy shows promise for Sanfilippo syndrome
. Most of the benefit from the treatment came in patients who began treatment at younger age, but comparisons to natural history controls showed profound improvement among many recipients, some of whom attained normal developmental trajectories.
The study was presented at the American Academy of Neurology’s 2021 annual meeting by Kevin Flanigan, MD, an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. He highlighted the improved developmental outcomes. “There’s been nothing shown to change the cognitive pathway of the disease. This is the first time it’s been seen as a treatment effect,” Dr. Flanigan said during a follow-up Q&A session.
The therapy was delivered using an adeno-associated virus-9 (AAV-9) vector, which led one questioner to ask about potential safety concerns, since AAV-associated risks date back to the death of Jesse Gelsinger in 1999. “There is concern about AAV therapies related to immune responses to potentially complement-mediated activation and thrombocytopenic syndrome, which has led to clinical holds on some other AAV-9 products related to muscular dystrophies. We’ve not seen signals of anything reminiscent of that, and we’re at AAV-9 dosages that are quite similar to what’s been used elsewhere in the field,” said Dr. Flanigan.
The results have him optimistic about the therapy. “I do think if it continues to be increasing divergent from the natural history, it will be questionable as to whether a subsequent trial will be necessary for this. That’s a decision for the [Food and Drug Administration] and the company to decide. Each observation point that goes by, each patient treated, and each time we get more data, I get more and more confident. It’s really gratifying to watch,” said Dr. Flanigan.
The study confirms the potential of gene replacement therapy autosomal recessive conditions, according to Nicholas Johnson, MD, associate professor of neurology at Virginia Commonwealth University, Richmond, as well as a fellow of the American Academy of Neurology. “Where the genetic problem is loss of gene function, the ability to replace that gene using a viral approach is going to be transformative across the board for many of these different conditions, including Sanfilippo syndrome,” said Dr. Johnson, who attended the session but was not involved in the research.
Toxicity could remain an issue, even in the absence of AAV-based safety concerns. “The rate limiting step in terms of gene replacement therapy development likely relates to the ability to provide those therapies to larger adults, because many approaches are weight based and it’s unclear what the upper limit of toxicity would be for adults,” said Dr. Johnson.
Transpher A study results
Dr. Flanigan presented results from Transpher A, a phase 1/2 clinical trial that has enrolled 20 patients to date in three cohorts: Cohort 1, with 3 patients, received 5 x 1,012 vg/kg, and had a mean follow-up of 58 months; cohort 2, with 3 patients, received 1 x 1,013 vg/kg, and had a mean follow-up of 49 months; and cohort 3, with 14 patients, received 3 x 1,013 vg/kg, with a mean follow-up of 24 months. Included patients ranged from birth to age 2, or older than age 2 with a development quotient of 60 or higher on the Bayley Scale.
Dr. Flanigan showed a plot of developmental progress compared with natural history controls, which showed that patients treated before age 2 or with a developmental quotient of 60 or higher had improved outcomes compared to other patients in the high dose cohort. They continued to show normal developmental progression at 30-36 months post treatment, at a time when the natural history data suggested they would suffer cognitive decline. Two years after administration, this group had cerebral spinal fluid levels of heparan sulfate that fell below the lower limit of detection. Patients in the high-dose cohort had normalized CSF levels of GM2 and GM3 gangliosides, and there were reductions in plasma heparan sulfate and urinary glycosaminoglycans. There was also a sustained decrease in liver volume.
The highest dose group was originally given to older patients, and most were similar to the natural history cohort, though some did stabilize. “More compellingly, patients (in the high-dose group) who were treated younger actually showed continued increase in development. One individual follows the normal development quotient line, and we would say that these are really quite distinct from what we typically see in patients,” said Dr. Flanigan.
The treatment was well tolerated. There were no deaths or treatment-related serious adverse events, and no clinically-significant adverse events within the first 5 years of follow-up.
The study was funded by Abeona Therapeutics. Dr. Flanigan has been on advisory boards for Apic Bio and 4D Molecular Therapeutics, consulted for Encoded Therapeutics, and has received royalties from Audentes Therapeutics. Dr. Flanigan has received funding from and been a consultant for Avidity.
. Most of the benefit from the treatment came in patients who began treatment at younger age, but comparisons to natural history controls showed profound improvement among many recipients, some of whom attained normal developmental trajectories.
The study was presented at the American Academy of Neurology’s 2021 annual meeting by Kevin Flanigan, MD, an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. He highlighted the improved developmental outcomes. “There’s been nothing shown to change the cognitive pathway of the disease. This is the first time it’s been seen as a treatment effect,” Dr. Flanigan said during a follow-up Q&A session.
The therapy was delivered using an adeno-associated virus-9 (AAV-9) vector, which led one questioner to ask about potential safety concerns, since AAV-associated risks date back to the death of Jesse Gelsinger in 1999. “There is concern about AAV therapies related to immune responses to potentially complement-mediated activation and thrombocytopenic syndrome, which has led to clinical holds on some other AAV-9 products related to muscular dystrophies. We’ve not seen signals of anything reminiscent of that, and we’re at AAV-9 dosages that are quite similar to what’s been used elsewhere in the field,” said Dr. Flanigan.
The results have him optimistic about the therapy. “I do think if it continues to be increasing divergent from the natural history, it will be questionable as to whether a subsequent trial will be necessary for this. That’s a decision for the [Food and Drug Administration] and the company to decide. Each observation point that goes by, each patient treated, and each time we get more data, I get more and more confident. It’s really gratifying to watch,” said Dr. Flanigan.
The study confirms the potential of gene replacement therapy autosomal recessive conditions, according to Nicholas Johnson, MD, associate professor of neurology at Virginia Commonwealth University, Richmond, as well as a fellow of the American Academy of Neurology. “Where the genetic problem is loss of gene function, the ability to replace that gene using a viral approach is going to be transformative across the board for many of these different conditions, including Sanfilippo syndrome,” said Dr. Johnson, who attended the session but was not involved in the research.
Toxicity could remain an issue, even in the absence of AAV-based safety concerns. “The rate limiting step in terms of gene replacement therapy development likely relates to the ability to provide those therapies to larger adults, because many approaches are weight based and it’s unclear what the upper limit of toxicity would be for adults,” said Dr. Johnson.
Transpher A study results
Dr. Flanigan presented results from Transpher A, a phase 1/2 clinical trial that has enrolled 20 patients to date in three cohorts: Cohort 1, with 3 patients, received 5 x 1,012 vg/kg, and had a mean follow-up of 58 months; cohort 2, with 3 patients, received 1 x 1,013 vg/kg, and had a mean follow-up of 49 months; and cohort 3, with 14 patients, received 3 x 1,013 vg/kg, with a mean follow-up of 24 months. Included patients ranged from birth to age 2, or older than age 2 with a development quotient of 60 or higher on the Bayley Scale.
Dr. Flanigan showed a plot of developmental progress compared with natural history controls, which showed that patients treated before age 2 or with a developmental quotient of 60 or higher had improved outcomes compared to other patients in the high dose cohort. They continued to show normal developmental progression at 30-36 months post treatment, at a time when the natural history data suggested they would suffer cognitive decline. Two years after administration, this group had cerebral spinal fluid levels of heparan sulfate that fell below the lower limit of detection. Patients in the high-dose cohort had normalized CSF levels of GM2 and GM3 gangliosides, and there were reductions in plasma heparan sulfate and urinary glycosaminoglycans. There was also a sustained decrease in liver volume.
The highest dose group was originally given to older patients, and most were similar to the natural history cohort, though some did stabilize. “More compellingly, patients (in the high-dose group) who were treated younger actually showed continued increase in development. One individual follows the normal development quotient line, and we would say that these are really quite distinct from what we typically see in patients,” said Dr. Flanigan.
The treatment was well tolerated. There were no deaths or treatment-related serious adverse events, and no clinically-significant adverse events within the first 5 years of follow-up.
The study was funded by Abeona Therapeutics. Dr. Flanigan has been on advisory boards for Apic Bio and 4D Molecular Therapeutics, consulted for Encoded Therapeutics, and has received royalties from Audentes Therapeutics. Dr. Flanigan has received funding from and been a consultant for Avidity.
. Most of the benefit from the treatment came in patients who began treatment at younger age, but comparisons to natural history controls showed profound improvement among many recipients, some of whom attained normal developmental trajectories.
The study was presented at the American Academy of Neurology’s 2021 annual meeting by Kevin Flanigan, MD, an attending neurologist at Nationwide Children’s Hospital in Columbus, Ohio. He highlighted the improved developmental outcomes. “There’s been nothing shown to change the cognitive pathway of the disease. This is the first time it’s been seen as a treatment effect,” Dr. Flanigan said during a follow-up Q&A session.
The therapy was delivered using an adeno-associated virus-9 (AAV-9) vector, which led one questioner to ask about potential safety concerns, since AAV-associated risks date back to the death of Jesse Gelsinger in 1999. “There is concern about AAV therapies related to immune responses to potentially complement-mediated activation and thrombocytopenic syndrome, which has led to clinical holds on some other AAV-9 products related to muscular dystrophies. We’ve not seen signals of anything reminiscent of that, and we’re at AAV-9 dosages that are quite similar to what’s been used elsewhere in the field,” said Dr. Flanigan.
The results have him optimistic about the therapy. “I do think if it continues to be increasing divergent from the natural history, it will be questionable as to whether a subsequent trial will be necessary for this. That’s a decision for the [Food and Drug Administration] and the company to decide. Each observation point that goes by, each patient treated, and each time we get more data, I get more and more confident. It’s really gratifying to watch,” said Dr. Flanigan.
The study confirms the potential of gene replacement therapy autosomal recessive conditions, according to Nicholas Johnson, MD, associate professor of neurology at Virginia Commonwealth University, Richmond, as well as a fellow of the American Academy of Neurology. “Where the genetic problem is loss of gene function, the ability to replace that gene using a viral approach is going to be transformative across the board for many of these different conditions, including Sanfilippo syndrome,” said Dr. Johnson, who attended the session but was not involved in the research.
Toxicity could remain an issue, even in the absence of AAV-based safety concerns. “The rate limiting step in terms of gene replacement therapy development likely relates to the ability to provide those therapies to larger adults, because many approaches are weight based and it’s unclear what the upper limit of toxicity would be for adults,” said Dr. Johnson.
Transpher A study results
Dr. Flanigan presented results from Transpher A, a phase 1/2 clinical trial that has enrolled 20 patients to date in three cohorts: Cohort 1, with 3 patients, received 5 x 1,012 vg/kg, and had a mean follow-up of 58 months; cohort 2, with 3 patients, received 1 x 1,013 vg/kg, and had a mean follow-up of 49 months; and cohort 3, with 14 patients, received 3 x 1,013 vg/kg, with a mean follow-up of 24 months. Included patients ranged from birth to age 2, or older than age 2 with a development quotient of 60 or higher on the Bayley Scale.
Dr. Flanigan showed a plot of developmental progress compared with natural history controls, which showed that patients treated before age 2 or with a developmental quotient of 60 or higher had improved outcomes compared to other patients in the high dose cohort. They continued to show normal developmental progression at 30-36 months post treatment, at a time when the natural history data suggested they would suffer cognitive decline. Two years after administration, this group had cerebral spinal fluid levels of heparan sulfate that fell below the lower limit of detection. Patients in the high-dose cohort had normalized CSF levels of GM2 and GM3 gangliosides, and there were reductions in plasma heparan sulfate and urinary glycosaminoglycans. There was also a sustained decrease in liver volume.
The highest dose group was originally given to older patients, and most were similar to the natural history cohort, though some did stabilize. “More compellingly, patients (in the high-dose group) who were treated younger actually showed continued increase in development. One individual follows the normal development quotient line, and we would say that these are really quite distinct from what we typically see in patients,” said Dr. Flanigan.
The treatment was well tolerated. There were no deaths or treatment-related serious adverse events, and no clinically-significant adverse events within the first 5 years of follow-up.
The study was funded by Abeona Therapeutics. Dr. Flanigan has been on advisory boards for Apic Bio and 4D Molecular Therapeutics, consulted for Encoded Therapeutics, and has received royalties from Audentes Therapeutics. Dr. Flanigan has received funding from and been a consultant for Avidity.
FROM AAN 2021
Transgender hormone therapy linked to blood pressure changes
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Frontline brentuximab vedotin shows promise in high-risk pediatric Hodgkin lymphoma
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
FROM THE JOURNAL OF CLINICAL ONCOLOGY