User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Fast foods contain endocrine-disrupting chemicals
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF EXPOSURE SCIENCE & ENVIRONMENTAL EPIDEMIOLOGY
Unvaccinated people 20 times more likely to die from COVID: Texas study
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
Drug combo at outset of polyarticular JIA benefits patients most
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
FROM ACR 2021
Cities, states offer to pay kids to get vaccinated
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.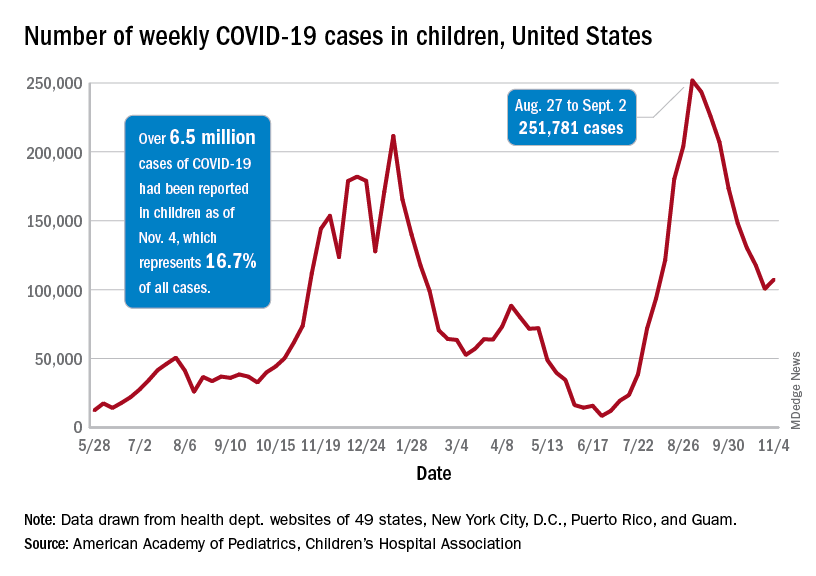
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
Breast milk of COVID-19–infected mothers helps build infant’s immune defenses
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
FROM JAMA NETWORK OPEN
More eczema in children exposed to toxic metals in utero
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Early peanut feeding guidelines still not reaching families
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Expected spike in acute flaccid myelitis did not occur in 2020
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
FROM MMWR
Detransitioners received poor evaluation when transitioning
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.



