User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
12 state boards have disciplined docs for COVID misinformation
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
Major COVID-19 case growth expected in coming weeks
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
Beta-thalassemia gene therapy achieves lasting transfusion independence
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
FROM ASH 2021
What is the diagnosis?
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
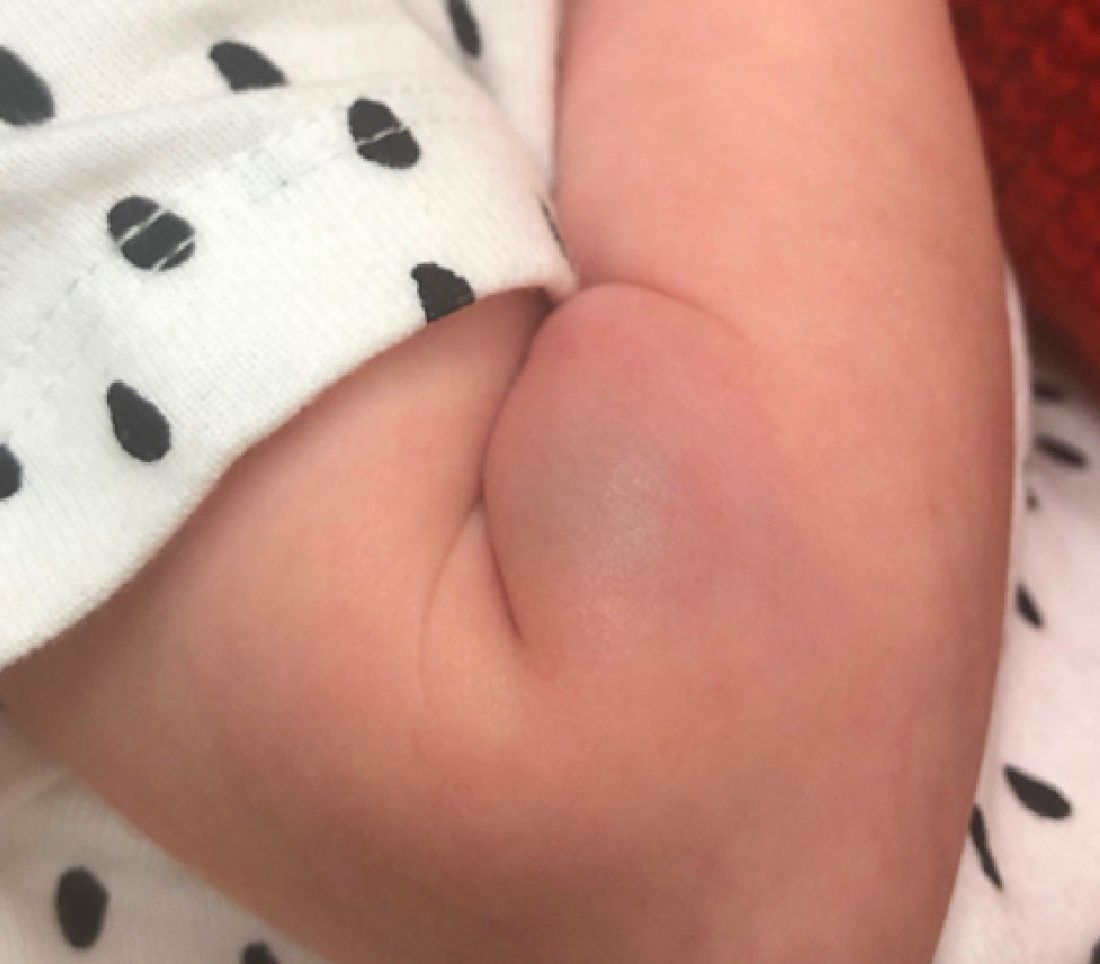
A 35-day-old female was referred to our pediatric dermatology clinic for evaluation of a red lesion on the right arm. The lesion presented at about 4 days of life as a red plaque (image 1 at 8 days of life).
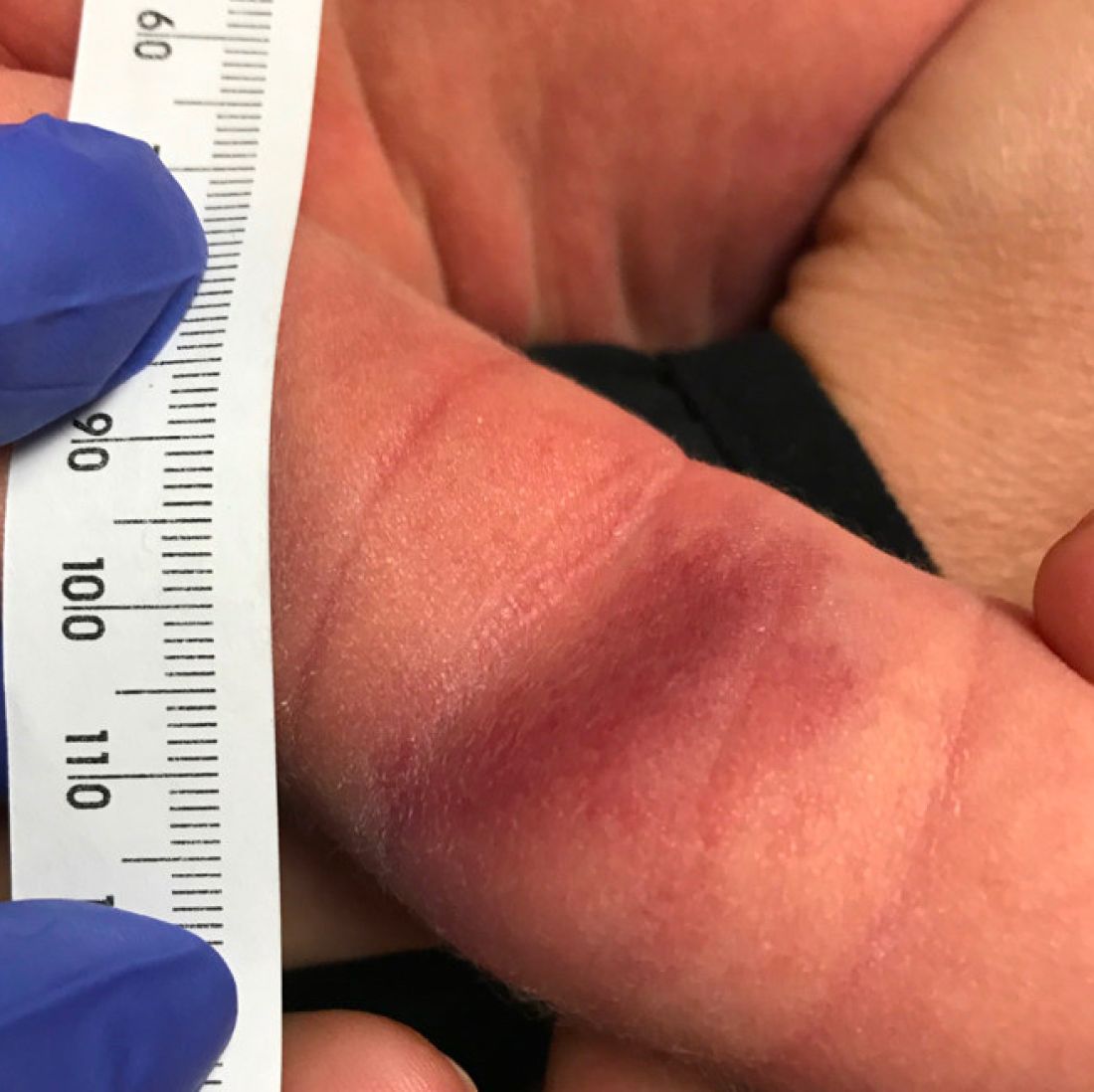
On the following days, the lesion started growing but it didn't seem to be tender or bothersome to the patient (image 2, at 35 days of life).
At a 2-week follow up the lesion was getting fuller and more violaceous. There was no history of fever and the lesion didn't appear tender to the touch.
She was born via normal spontaneous vaginal delivery. There were no complications and the mother received prenatal care.
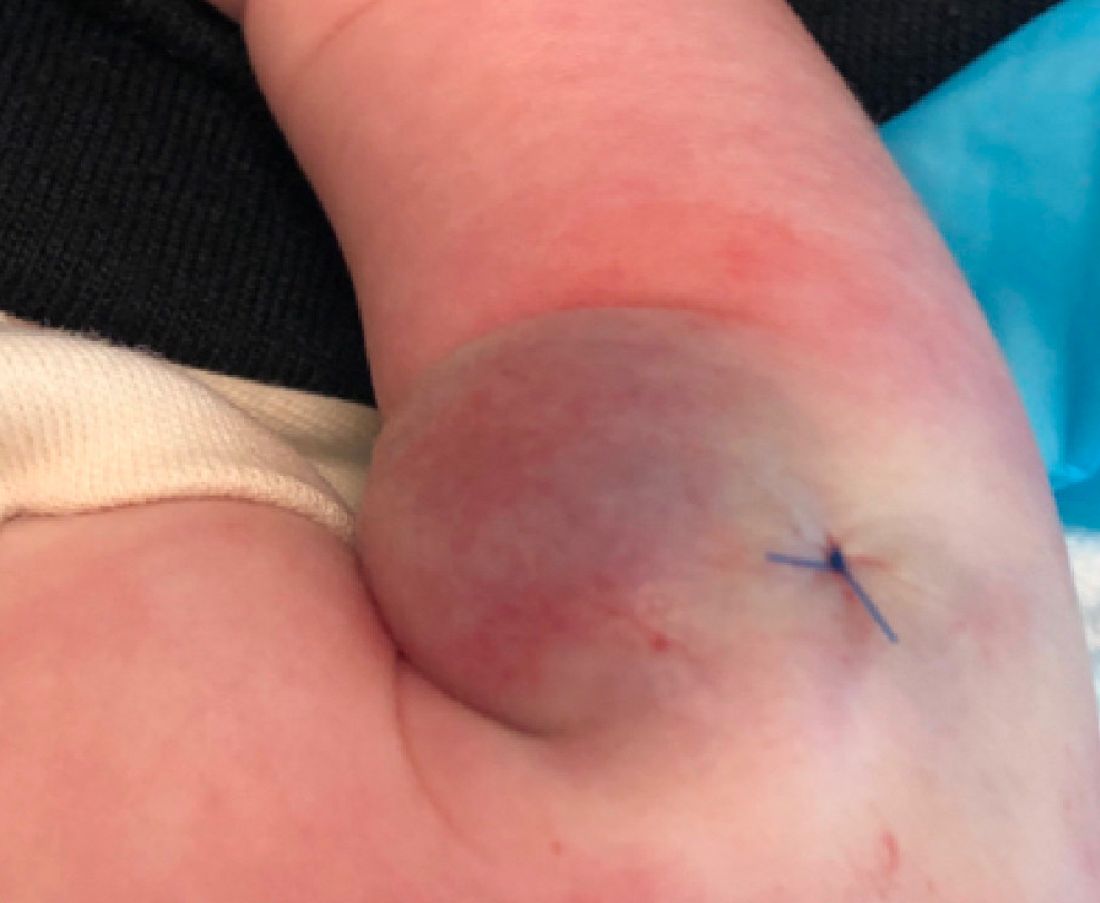
On exam she had a red to violaceous nodule on the right arm (image 3 at 45 days of life).
IDF Atlas: 1 in 10 adults worldwide now has diabetes
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer boosters for 16- and 17-year-olds
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
Medical board stops warning docs against giving false COVID information
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.






