User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Elective surgery should be delayed 7 weeks after COVID-19 infection for unvaccinated patients, statement recommends
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
Phthalate exposure via maternal and cord blood affects infant outcomes
Exposure to phthalates through maternal blood and cord blood affected outcomes including head circumference and anogenital index for male and female infants, according to data from 65 mother-infant pairs.
Phthalates are recognized endocrine disruptors that have been associated with adverse birth outcomes, but the specific relationship between maternal phthalate exposure and birth outcomes has not been well studied, wrote Hsiao-Lin Hwa, MD, of National Taiwan University, Taipei, and colleagues.
Previous research suggests that trace exposure to hazardous chemicals during the fetal period “may cause fetal metabolic dysfunction and adversely change the morphology of body systems,” they said. In 2011, “the Taiwan Food and Drug Administration found that di‐2‐ethylhexyl phthalate (DEHP) and DiNP [di‐isononyl phthalate] had been illegally added as emulsifiers to replace palm oil in beverages and food,” they added. The researchers sought to examine the association between infant birth outcomes and phthalate exposure levels in the Taiwanese population after 2011. In a study published in Environmental Toxicology and Chemistry, the researchers recruited 65 pregnant women in Taiwan between 2016 and 2017. Birth length, birth weight, head circumference, anogenital distance (AGD), anoscrotal distance (ASD), and anofourchette distance (AFD) were measured for each newborn at the time of delivery. The average age of the women was 33.6 years, and the rate of low birth weight was 13.7%. The mean measures of birth length, birth weight, head circumference, and chest circumference were 47.6 cm, 3022 g, 32.9 cm, and 30.8 mm, respectively. The mean AFD and ASD were 14.2 mm and 22.3 mm, respectively.
The researchers tested for 12 phthalates in maternal blood and cord blood samples. Of these, the six most frequently detected phthalate metabolites were mono‐ethyl phthalate (MEP), mono‐isobutyl phthalate (MiBP), mono‐n‐butyl phthalate (MnBP), mono‐(2‐ethyl‐5‐oxohexyl)‐phthalate (MEOHP), mono‐(2‐ethyl‐5‐hydroxyhexyl) phthalate (MEHHP), and mono‐n‐octyl phthalate (MOP); these six were present in 80%–100% of the maternal blood samples.
Overall, the mean levels of MEP, MiBP, MnBP, and MEHP were relatively higher in both maternal and infant blood than other phthalates, the researchers noted. The mean concentrations of metabolites in maternal blood and infant cord blood were 0.03-2.27 ng/mL and 0.01-3.74 ng/mL, respectively.
Among male infants, levels of MMP, MiBP, and MEHP in maternal blood were inversely related to anogenital index (AGI), with P values for regression coefficients ranging from .011 to .033. In addition, the total concentration of MEHP, MEOHP, and MEHHP (designated as Σdi‐2‐ethylhexyl phthalate, ΣDEHP) was inversely related to AGI in males.
Among female infants, however, phthalates in cord blood, rather than maternal blood, were positively related to AGI, including MMP, MibP, MnBP, and MOP, with P values for regression coefficients ranging from .001 to .034.
Cord blood levels of MnBP, MEOHP, MEHP, and ΣDEHP were inversely associated with gestational age-adjusted head circumference in all infants, with beta coefficients of –0.15, –0.12, –0.01, and –0.01, respectively (P < .05 for all).
“The detection rates of MEHHP, MEOHP, and MEHP in the cord blood were lower than those in the maternal blood, particularly those of MEHHP and MEOHP, which were approximately 25% lower,” which may be caused by slow placental transfer, the researchers wrote in their discussion section. “The high detection rate of phthalate metabolites indicated that our subjects may continue to be exposed to these phthalates even after the 2011 Taiwan DEHP incident,” they noted.
The study findings were limited by several factors including the possibility for contamination of samples and other environmental confounders, the researchers noted. However, the results support the role of phthalates as endocrine disruptors, and the distinction in effects between males and females “may suggest that phthalate monoesters are potentially estrogenic and antiandrogenic chemicals,” they added.
“Further investigations involving multiple phthalate analyses during pregnancy and measurements throughout childhood are necessary to confirm our findings,” they concluded.
Direct clinical implications remain uncertain
“Phthalates are a group of chemicals that are used to make plastic more durable; they are found in multiple everyday materials, food products, and common household products,” Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “It is known that we are exposed to phthalates on a routine basis but the long-term effects of this exposure are unclear,” she said.
The current study findings “were not entirely surprising given data from prior animal studies because they do imply that there is some placental transfer of the phthalate metabolites that can cause adverse effects on the developing fetus,” said Dr. Platner. “However, they also demonstrate that the placenta acts as a filter for certain larger molecules to protect the fetus,” she said.
“This study was based on a small sample size, therefore the clinical implications are not clear,” Dr. Platner noted. “However it may be worthwhile after further research to encourage our pregnant patients to try to decrease their exposure to phthalates,” she said.
Dr. Platner identified two areas for additional research to explore the role of phthalate exposure.
“The first would be to assess the level of maternal phthalate exposure throughout the pregnancy instead of just at one point in time, and the second would be to assess how the reproductive system differences at birth translate to long-term outcomes in children, such as early puberty in females or decreased fertility in males,” she said.
The study was funded by the Ministry of Science and Technology of Taiwan and the Far Eastern Memorial Hospital‐National Taiwan University Hospital. The researchers and Dr. Platner had no financial conflicts to disclose.
Exposure to phthalates through maternal blood and cord blood affected outcomes including head circumference and anogenital index for male and female infants, according to data from 65 mother-infant pairs.
Phthalates are recognized endocrine disruptors that have been associated with adverse birth outcomes, but the specific relationship between maternal phthalate exposure and birth outcomes has not been well studied, wrote Hsiao-Lin Hwa, MD, of National Taiwan University, Taipei, and colleagues.
Previous research suggests that trace exposure to hazardous chemicals during the fetal period “may cause fetal metabolic dysfunction and adversely change the morphology of body systems,” they said. In 2011, “the Taiwan Food and Drug Administration found that di‐2‐ethylhexyl phthalate (DEHP) and DiNP [di‐isononyl phthalate] had been illegally added as emulsifiers to replace palm oil in beverages and food,” they added. The researchers sought to examine the association between infant birth outcomes and phthalate exposure levels in the Taiwanese population after 2011. In a study published in Environmental Toxicology and Chemistry, the researchers recruited 65 pregnant women in Taiwan between 2016 and 2017. Birth length, birth weight, head circumference, anogenital distance (AGD), anoscrotal distance (ASD), and anofourchette distance (AFD) were measured for each newborn at the time of delivery. The average age of the women was 33.6 years, and the rate of low birth weight was 13.7%. The mean measures of birth length, birth weight, head circumference, and chest circumference were 47.6 cm, 3022 g, 32.9 cm, and 30.8 mm, respectively. The mean AFD and ASD were 14.2 mm and 22.3 mm, respectively.
The researchers tested for 12 phthalates in maternal blood and cord blood samples. Of these, the six most frequently detected phthalate metabolites were mono‐ethyl phthalate (MEP), mono‐isobutyl phthalate (MiBP), mono‐n‐butyl phthalate (MnBP), mono‐(2‐ethyl‐5‐oxohexyl)‐phthalate (MEOHP), mono‐(2‐ethyl‐5‐hydroxyhexyl) phthalate (MEHHP), and mono‐n‐octyl phthalate (MOP); these six were present in 80%–100% of the maternal blood samples.
Overall, the mean levels of MEP, MiBP, MnBP, and MEHP were relatively higher in both maternal and infant blood than other phthalates, the researchers noted. The mean concentrations of metabolites in maternal blood and infant cord blood were 0.03-2.27 ng/mL and 0.01-3.74 ng/mL, respectively.
Among male infants, levels of MMP, MiBP, and MEHP in maternal blood were inversely related to anogenital index (AGI), with P values for regression coefficients ranging from .011 to .033. In addition, the total concentration of MEHP, MEOHP, and MEHHP (designated as Σdi‐2‐ethylhexyl phthalate, ΣDEHP) was inversely related to AGI in males.
Among female infants, however, phthalates in cord blood, rather than maternal blood, were positively related to AGI, including MMP, MibP, MnBP, and MOP, with P values for regression coefficients ranging from .001 to .034.
Cord blood levels of MnBP, MEOHP, MEHP, and ΣDEHP were inversely associated with gestational age-adjusted head circumference in all infants, with beta coefficients of –0.15, –0.12, –0.01, and –0.01, respectively (P < .05 for all).
“The detection rates of MEHHP, MEOHP, and MEHP in the cord blood were lower than those in the maternal blood, particularly those of MEHHP and MEOHP, which were approximately 25% lower,” which may be caused by slow placental transfer, the researchers wrote in their discussion section. “The high detection rate of phthalate metabolites indicated that our subjects may continue to be exposed to these phthalates even after the 2011 Taiwan DEHP incident,” they noted.
The study findings were limited by several factors including the possibility for contamination of samples and other environmental confounders, the researchers noted. However, the results support the role of phthalates as endocrine disruptors, and the distinction in effects between males and females “may suggest that phthalate monoesters are potentially estrogenic and antiandrogenic chemicals,” they added.
“Further investigations involving multiple phthalate analyses during pregnancy and measurements throughout childhood are necessary to confirm our findings,” they concluded.
Direct clinical implications remain uncertain
“Phthalates are a group of chemicals that are used to make plastic more durable; they are found in multiple everyday materials, food products, and common household products,” Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “It is known that we are exposed to phthalates on a routine basis but the long-term effects of this exposure are unclear,” she said.
The current study findings “were not entirely surprising given data from prior animal studies because they do imply that there is some placental transfer of the phthalate metabolites that can cause adverse effects on the developing fetus,” said Dr. Platner. “However, they also demonstrate that the placenta acts as a filter for certain larger molecules to protect the fetus,” she said.
“This study was based on a small sample size, therefore the clinical implications are not clear,” Dr. Platner noted. “However it may be worthwhile after further research to encourage our pregnant patients to try to decrease their exposure to phthalates,” she said.
Dr. Platner identified two areas for additional research to explore the role of phthalate exposure.
“The first would be to assess the level of maternal phthalate exposure throughout the pregnancy instead of just at one point in time, and the second would be to assess how the reproductive system differences at birth translate to long-term outcomes in children, such as early puberty in females or decreased fertility in males,” she said.
The study was funded by the Ministry of Science and Technology of Taiwan and the Far Eastern Memorial Hospital‐National Taiwan University Hospital. The researchers and Dr. Platner had no financial conflicts to disclose.
Exposure to phthalates through maternal blood and cord blood affected outcomes including head circumference and anogenital index for male and female infants, according to data from 65 mother-infant pairs.
Phthalates are recognized endocrine disruptors that have been associated with adverse birth outcomes, but the specific relationship between maternal phthalate exposure and birth outcomes has not been well studied, wrote Hsiao-Lin Hwa, MD, of National Taiwan University, Taipei, and colleagues.
Previous research suggests that trace exposure to hazardous chemicals during the fetal period “may cause fetal metabolic dysfunction and adversely change the morphology of body systems,” they said. In 2011, “the Taiwan Food and Drug Administration found that di‐2‐ethylhexyl phthalate (DEHP) and DiNP [di‐isononyl phthalate] had been illegally added as emulsifiers to replace palm oil in beverages and food,” they added. The researchers sought to examine the association between infant birth outcomes and phthalate exposure levels in the Taiwanese population after 2011. In a study published in Environmental Toxicology and Chemistry, the researchers recruited 65 pregnant women in Taiwan between 2016 and 2017. Birth length, birth weight, head circumference, anogenital distance (AGD), anoscrotal distance (ASD), and anofourchette distance (AFD) were measured for each newborn at the time of delivery. The average age of the women was 33.6 years, and the rate of low birth weight was 13.7%. The mean measures of birth length, birth weight, head circumference, and chest circumference were 47.6 cm, 3022 g, 32.9 cm, and 30.8 mm, respectively. The mean AFD and ASD were 14.2 mm and 22.3 mm, respectively.
The researchers tested for 12 phthalates in maternal blood and cord blood samples. Of these, the six most frequently detected phthalate metabolites were mono‐ethyl phthalate (MEP), mono‐isobutyl phthalate (MiBP), mono‐n‐butyl phthalate (MnBP), mono‐(2‐ethyl‐5‐oxohexyl)‐phthalate (MEOHP), mono‐(2‐ethyl‐5‐hydroxyhexyl) phthalate (MEHHP), and mono‐n‐octyl phthalate (MOP); these six were present in 80%–100% of the maternal blood samples.
Overall, the mean levels of MEP, MiBP, MnBP, and MEHP were relatively higher in both maternal and infant blood than other phthalates, the researchers noted. The mean concentrations of metabolites in maternal blood and infant cord blood were 0.03-2.27 ng/mL and 0.01-3.74 ng/mL, respectively.
Among male infants, levels of MMP, MiBP, and MEHP in maternal blood were inversely related to anogenital index (AGI), with P values for regression coefficients ranging from .011 to .033. In addition, the total concentration of MEHP, MEOHP, and MEHHP (designated as Σdi‐2‐ethylhexyl phthalate, ΣDEHP) was inversely related to AGI in males.
Among female infants, however, phthalates in cord blood, rather than maternal blood, were positively related to AGI, including MMP, MibP, MnBP, and MOP, with P values for regression coefficients ranging from .001 to .034.
Cord blood levels of MnBP, MEOHP, MEHP, and ΣDEHP were inversely associated with gestational age-adjusted head circumference in all infants, with beta coefficients of –0.15, –0.12, –0.01, and –0.01, respectively (P < .05 for all).
“The detection rates of MEHHP, MEOHP, and MEHP in the cord blood were lower than those in the maternal blood, particularly those of MEHHP and MEOHP, which were approximately 25% lower,” which may be caused by slow placental transfer, the researchers wrote in their discussion section. “The high detection rate of phthalate metabolites indicated that our subjects may continue to be exposed to these phthalates even after the 2011 Taiwan DEHP incident,” they noted.
The study findings were limited by several factors including the possibility for contamination of samples and other environmental confounders, the researchers noted. However, the results support the role of phthalates as endocrine disruptors, and the distinction in effects between males and females “may suggest that phthalate monoesters are potentially estrogenic and antiandrogenic chemicals,” they added.
“Further investigations involving multiple phthalate analyses during pregnancy and measurements throughout childhood are necessary to confirm our findings,” they concluded.
Direct clinical implications remain uncertain
“Phthalates are a group of chemicals that are used to make plastic more durable; they are found in multiple everyday materials, food products, and common household products,” Marissa Platner, MD, of Emory University, Atlanta, said in an interview. “It is known that we are exposed to phthalates on a routine basis but the long-term effects of this exposure are unclear,” she said.
The current study findings “were not entirely surprising given data from prior animal studies because they do imply that there is some placental transfer of the phthalate metabolites that can cause adverse effects on the developing fetus,” said Dr. Platner. “However, they also demonstrate that the placenta acts as a filter for certain larger molecules to protect the fetus,” she said.
“This study was based on a small sample size, therefore the clinical implications are not clear,” Dr. Platner noted. “However it may be worthwhile after further research to encourage our pregnant patients to try to decrease their exposure to phthalates,” she said.
Dr. Platner identified two areas for additional research to explore the role of phthalate exposure.
“The first would be to assess the level of maternal phthalate exposure throughout the pregnancy instead of just at one point in time, and the second would be to assess how the reproductive system differences at birth translate to long-term outcomes in children, such as early puberty in females or decreased fertility in males,” she said.
The study was funded by the Ministry of Science and Technology of Taiwan and the Far Eastern Memorial Hospital‐National Taiwan University Hospital. The researchers and Dr. Platner had no financial conflicts to disclose.
FROM ENVIRONMENTAL TOXICOLOGY AND CHEMISTRY
Mycoplasma genitalium: The Smallest Pathogen Becoming a Big Concern
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
This supplement reviews key aspects of Mycoplasma genitalium and further testing and treatment options for the STI. To read more about this click the link below.
Click Here to Read More
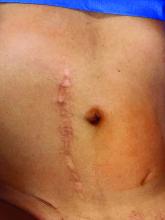
Left upper quadrant entry is often a reliable alternative to umbilicus
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.
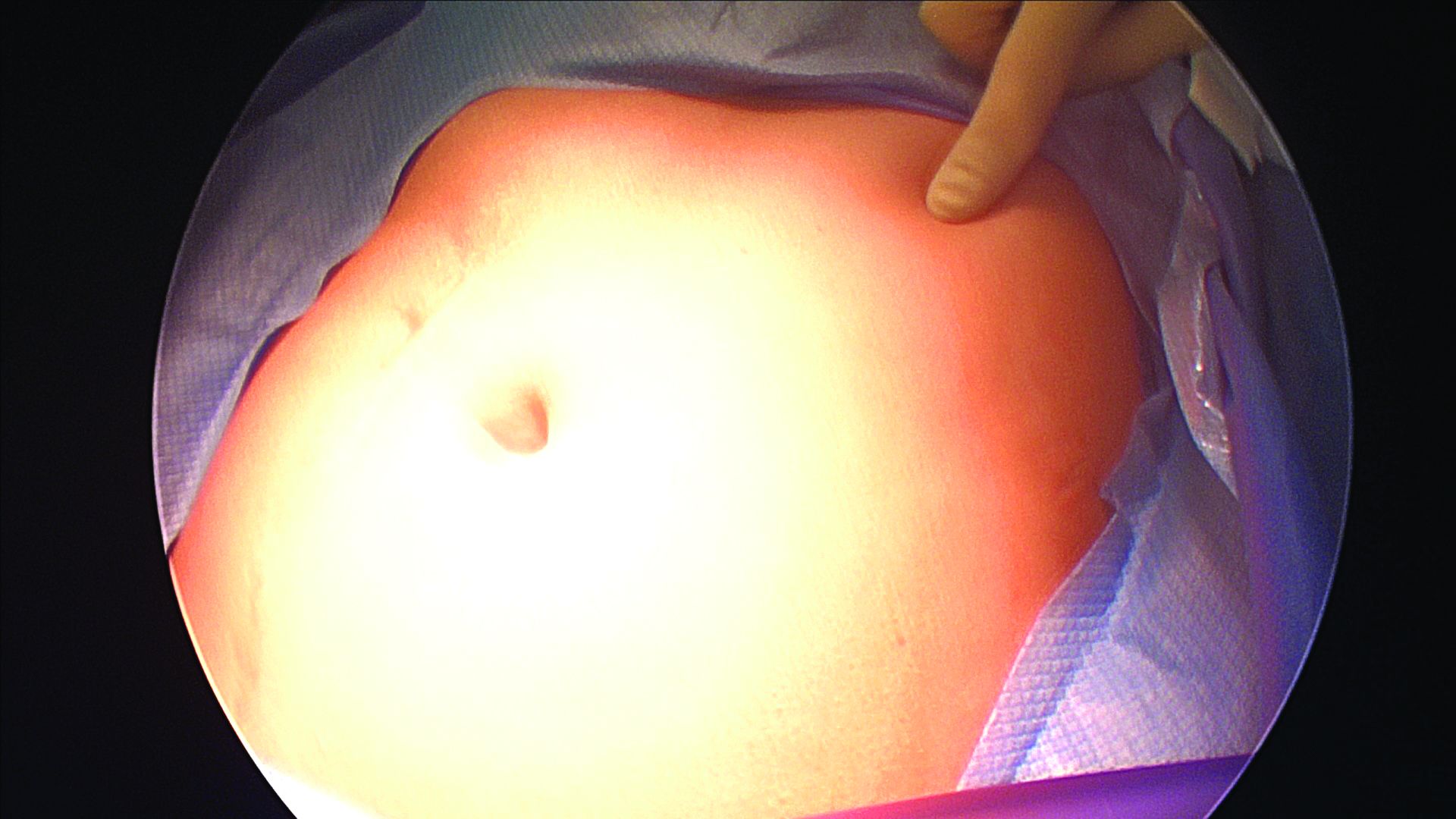
The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.
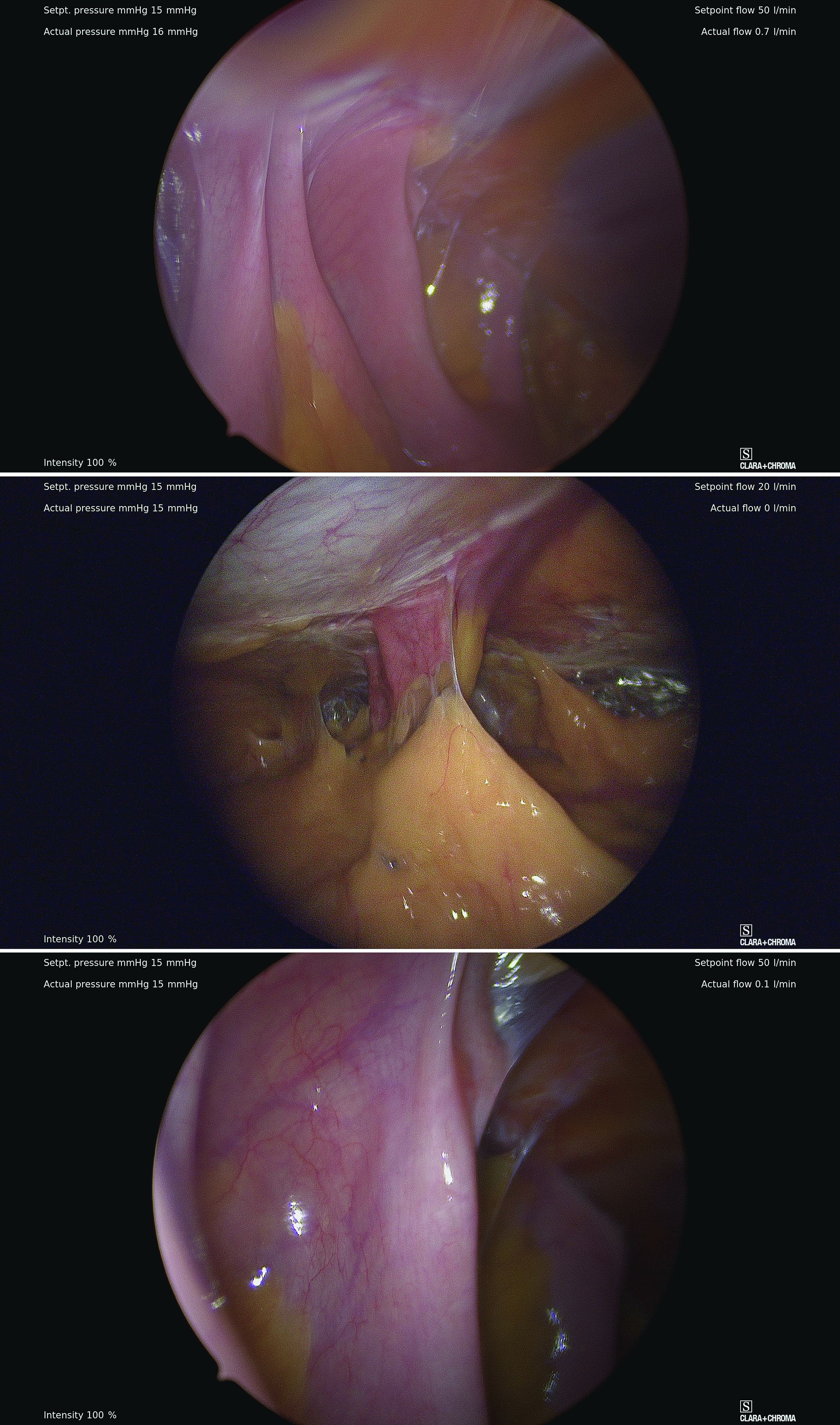
However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.

The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.

The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
Safe abdominal laparoscopic entry
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
COVID-19 vaccines do not trigger sudden hearing loss: Study
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY – HEAD AND NECK SURGERY
Why challenging patients can trigger resentment
I have a secret. It’s one I think many physicians and nurses share. Sometimes, when I’m stretched too thin — overbooked, hungry, tired, fielding yet another appeal to an insurance company in the middle of a clinic day —
As soon as this happens, I feel immediate guilt. These are the worst moments of my day. Why the heck would I resent my patients? They’re the entire reason I’m there. I wouldn’t be a physician without patients to care for. I became a physician, and completed subspecialty training, to help patients. People.
Recently, I started thinking more about this emotion of resentment. What exactly is it, and where does it come from? Is what I’m feeling actually resentment? Or is it something else?
Two books I’ve recently read have helped me explore the complicated emotion of resentment and how it might play a role in burnout for both physicians and nurses.
First, Brené Brown’s most recent book, Atlas of the Heart: Mapping Meaningful Connection and the Language of Human Experience, provides a roadmap for 87 of our human emotions. (That’s right — 87!)
One emotion of the 87 that she shares has been a particular struggle for her has been our good old friend, resentment.
In her book, Dr Brown shares that she initially considered resentment to belong to the anger family of emotion. As I read this, I agreed. When I feel resentful, I associate that with feeling angry.
But she then writes about her discovery that resentment, in fact, belongs to the envy family. She explains how this discovery shook her world. I had to close the book for a moment at this point.
Wait a minute, I thought. If resentment is in the envy family, why do we (physicians) often find ourselves resenting patients who take up our time? What are we envious of?
I took some time to think about how this might be true. Could it be that I’m envious they have the time I don’t have? I want to have all the time in the world to answer their questions, but the reality is I don’t.
Or maybe it’s because sometimes I feel the patient is expecting me to offer them something more than is available. A cure when there might be none.
But is this actually true? Or is this my unrealistic expectation of myself?
Here’s how Brené Brown defines resentment in her book: “Resentment is the feeling of frustration, judgment, anger, ‘better than,’ and/or hidden envy related to perceived unfairness or injustice. It’s an emotion that we often experience when we fail to set boundaries or ask for what we need, or when expectations let us down because they were based on things we can’t control, like what other people think, what they feel, or how they’re going to react.”
Wow, I thought, Healthcare checks all of these boxes.
- Perceived unfairness of work schedules? Check.
- Perceived injustice? Of course — we see that in our dealings with insurance company denials every day.
But those are both extrinsic. What about the intrinsic factors she’s calling us out on here?
- Do we, as physicians, fail to set boundaries?
- Do we fail to ask for what we need?
Hard yes and yes. (Do we even know, as physicians, what our own boundaries are?)
And the last one:
- Do our expectations of how our clinic day will go let us down every day because they’re based on things we can’t control?
My brain had to repeat the critical parts of that: Expectations let us down when they’re based on things we can’t control.
But wait, my brain argued back; I’m the physician, I thought I was supposed to get to control things.
Next, the revelation: Could it be that a key to experiencing less resentment is accepting how much control we don’t have in a typical day?
And a corollary: How much does resentment factor into burnout? (To read more on my personal journey with burnout, see this piece).
It so happens that around this same time, I was reading another excellent book, Changing How We Think About Difficult Patients: A Guide for Physicians and Healthcare Professionals, by Joan Naidorf, DO.
Dr Naidorf is an emergency medicine physician of 30 years who wrote the book to “provid[e] insight and tools to manage our negative thoughts about difficult patients” and help “beleaguered colleagues…return to their benevolent guiding principles and find more enjoyment in their vitally important careers.”
As I read Dr Naidorf’s book, I thus did so with the mindset of wanting to further understand for myself where this specific emotion of resentment toward our “difficult” patients could come from and how to best understand it in order to get past it.
Dr. Naidorf writes, “Challenging patients will never stop appearing… You cannot change them or control them—the only person you can control is you.”
I wondered how much the resentment we might involuntarily feel at being asked to see a “difficult” patient has nothing to do with the patient but everything to do with it making us feel not in control of the situation.
Dr. Naidorf also writes, “Negative thoughts about challenging patients can cause, in otherwise capable clinicians, a sense of inadequacy and incompetence.”
Do we perhaps resent our challenging patients because of the negative thoughts they sometimes trigger in us? If so, how does this relate to envy, as Dr. Brown asserts resentment is tied to? Is it triggering us to feel inadequate?
“[Difficult patients] often make us question ourselves,” Dr. Naidorf writes, “and we need to feel comfortable with the answers.”
Again, the discrepancy between expectations and reality creates the negative emotion.
Or, as Dr. Naidorf writes, “What if you could stop judging others so harshly and accept them exactly as they are?”
Hmmm, I thought, then the cessation of harsh judgment and implementation of acceptance would have to apply to us too. The elusive concept of self-compassion.
Maybe the resentment/envy comes from us not allowing ourselves to behave in this way because to do so would allow too much vulnerability. Something most of us were conditioned to avoid to survive medical training.
Dr. Brown also writes about an “aha” moment she had in her struggle to understand resentment. “I’m not mad because you’re resting. I’m mad because I’m so bone tired and I want to rest. But, unlike you, I’m going to pretend that I don’t need to.”
I felt all too seen in that passage. Could it be my old nemesis, perfectionism, creeping its way back in? Is resentment the ugly stepsister to perfectionism?
Perhaps challenging patients can engender resentment because they make us feel like we’re not living up to our own unrealistic expectations. And in that case, we need to change our unrealistic expectations for ourselves.
Dr Naidorf’s book explores much more on the complex matter of what makes a “difficult” patient, but I chose to focus here only on the resentment piece as a tie-in to Dr. Brown’s book. I highly recommend both books for further reading to help physicians and nurses navigate the complex emotions our jobs can trigger.
Most importantly, recognizing that we have these transient negative emotions does not make us bad people or healthcare professionals. It only makes us human.
Dr. Lycette is medical director, Providence Oncology and Hematology Care Clinic, Seaside, Ore. She has disclosed having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have a secret. It’s one I think many physicians and nurses share. Sometimes, when I’m stretched too thin — overbooked, hungry, tired, fielding yet another appeal to an insurance company in the middle of a clinic day —
As soon as this happens, I feel immediate guilt. These are the worst moments of my day. Why the heck would I resent my patients? They’re the entire reason I’m there. I wouldn’t be a physician without patients to care for. I became a physician, and completed subspecialty training, to help patients. People.
Recently, I started thinking more about this emotion of resentment. What exactly is it, and where does it come from? Is what I’m feeling actually resentment? Or is it something else?
Two books I’ve recently read have helped me explore the complicated emotion of resentment and how it might play a role in burnout for both physicians and nurses.
First, Brené Brown’s most recent book, Atlas of the Heart: Mapping Meaningful Connection and the Language of Human Experience, provides a roadmap for 87 of our human emotions. (That’s right — 87!)
One emotion of the 87 that she shares has been a particular struggle for her has been our good old friend, resentment.
In her book, Dr Brown shares that she initially considered resentment to belong to the anger family of emotion. As I read this, I agreed. When I feel resentful, I associate that with feeling angry.
But she then writes about her discovery that resentment, in fact, belongs to the envy family. She explains how this discovery shook her world. I had to close the book for a moment at this point.
Wait a minute, I thought. If resentment is in the envy family, why do we (physicians) often find ourselves resenting patients who take up our time? What are we envious of?
I took some time to think about how this might be true. Could it be that I’m envious they have the time I don’t have? I want to have all the time in the world to answer their questions, but the reality is I don’t.
Or maybe it’s because sometimes I feel the patient is expecting me to offer them something more than is available. A cure when there might be none.
But is this actually true? Or is this my unrealistic expectation of myself?
Here’s how Brené Brown defines resentment in her book: “Resentment is the feeling of frustration, judgment, anger, ‘better than,’ and/or hidden envy related to perceived unfairness or injustice. It’s an emotion that we often experience when we fail to set boundaries or ask for what we need, or when expectations let us down because they were based on things we can’t control, like what other people think, what they feel, or how they’re going to react.”
Wow, I thought, Healthcare checks all of these boxes.
- Perceived unfairness of work schedules? Check.
- Perceived injustice? Of course — we see that in our dealings with insurance company denials every day.
But those are both extrinsic. What about the intrinsic factors she’s calling us out on here?
- Do we, as physicians, fail to set boundaries?
- Do we fail to ask for what we need?
Hard yes and yes. (Do we even know, as physicians, what our own boundaries are?)
And the last one:
- Do our expectations of how our clinic day will go let us down every day because they’re based on things we can’t control?
My brain had to repeat the critical parts of that: Expectations let us down when they’re based on things we can’t control.
But wait, my brain argued back; I’m the physician, I thought I was supposed to get to control things.
Next, the revelation: Could it be that a key to experiencing less resentment is accepting how much control we don’t have in a typical day?
And a corollary: How much does resentment factor into burnout? (To read more on my personal journey with burnout, see this piece).
It so happens that around this same time, I was reading another excellent book, Changing How We Think About Difficult Patients: A Guide for Physicians and Healthcare Professionals, by Joan Naidorf, DO.
Dr Naidorf is an emergency medicine physician of 30 years who wrote the book to “provid[e] insight and tools to manage our negative thoughts about difficult patients” and help “beleaguered colleagues…return to their benevolent guiding principles and find more enjoyment in their vitally important careers.”
As I read Dr Naidorf’s book, I thus did so with the mindset of wanting to further understand for myself where this specific emotion of resentment toward our “difficult” patients could come from and how to best understand it in order to get past it.
Dr. Naidorf writes, “Challenging patients will never stop appearing… You cannot change them or control them—the only person you can control is you.”
I wondered how much the resentment we might involuntarily feel at being asked to see a “difficult” patient has nothing to do with the patient but everything to do with it making us feel not in control of the situation.
Dr. Naidorf also writes, “Negative thoughts about challenging patients can cause, in otherwise capable clinicians, a sense of inadequacy and incompetence.”
Do we perhaps resent our challenging patients because of the negative thoughts they sometimes trigger in us? If so, how does this relate to envy, as Dr. Brown asserts resentment is tied to? Is it triggering us to feel inadequate?
“[Difficult patients] often make us question ourselves,” Dr. Naidorf writes, “and we need to feel comfortable with the answers.”
Again, the discrepancy between expectations and reality creates the negative emotion.
Or, as Dr. Naidorf writes, “What if you could stop judging others so harshly and accept them exactly as they are?”
Hmmm, I thought, then the cessation of harsh judgment and implementation of acceptance would have to apply to us too. The elusive concept of self-compassion.
Maybe the resentment/envy comes from us not allowing ourselves to behave in this way because to do so would allow too much vulnerability. Something most of us were conditioned to avoid to survive medical training.
Dr. Brown also writes about an “aha” moment she had in her struggle to understand resentment. “I’m not mad because you’re resting. I’m mad because I’m so bone tired and I want to rest. But, unlike you, I’m going to pretend that I don’t need to.”
I felt all too seen in that passage. Could it be my old nemesis, perfectionism, creeping its way back in? Is resentment the ugly stepsister to perfectionism?
Perhaps challenging patients can engender resentment because they make us feel like we’re not living up to our own unrealistic expectations. And in that case, we need to change our unrealistic expectations for ourselves.
Dr Naidorf’s book explores much more on the complex matter of what makes a “difficult” patient, but I chose to focus here only on the resentment piece as a tie-in to Dr. Brown’s book. I highly recommend both books for further reading to help physicians and nurses navigate the complex emotions our jobs can trigger.
Most importantly, recognizing that we have these transient negative emotions does not make us bad people or healthcare professionals. It only makes us human.
Dr. Lycette is medical director, Providence Oncology and Hematology Care Clinic, Seaside, Ore. She has disclosed having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have a secret. It’s one I think many physicians and nurses share. Sometimes, when I’m stretched too thin — overbooked, hungry, tired, fielding yet another appeal to an insurance company in the middle of a clinic day —
As soon as this happens, I feel immediate guilt. These are the worst moments of my day. Why the heck would I resent my patients? They’re the entire reason I’m there. I wouldn’t be a physician without patients to care for. I became a physician, and completed subspecialty training, to help patients. People.
Recently, I started thinking more about this emotion of resentment. What exactly is it, and where does it come from? Is what I’m feeling actually resentment? Or is it something else?
Two books I’ve recently read have helped me explore the complicated emotion of resentment and how it might play a role in burnout for both physicians and nurses.
First, Brené Brown’s most recent book, Atlas of the Heart: Mapping Meaningful Connection and the Language of Human Experience, provides a roadmap for 87 of our human emotions. (That’s right — 87!)
One emotion of the 87 that she shares has been a particular struggle for her has been our good old friend, resentment.
In her book, Dr Brown shares that she initially considered resentment to belong to the anger family of emotion. As I read this, I agreed. When I feel resentful, I associate that with feeling angry.
But she then writes about her discovery that resentment, in fact, belongs to the envy family. She explains how this discovery shook her world. I had to close the book for a moment at this point.
Wait a minute, I thought. If resentment is in the envy family, why do we (physicians) often find ourselves resenting patients who take up our time? What are we envious of?
I took some time to think about how this might be true. Could it be that I’m envious they have the time I don’t have? I want to have all the time in the world to answer their questions, but the reality is I don’t.
Or maybe it’s because sometimes I feel the patient is expecting me to offer them something more than is available. A cure when there might be none.
But is this actually true? Or is this my unrealistic expectation of myself?
Here’s how Brené Brown defines resentment in her book: “Resentment is the feeling of frustration, judgment, anger, ‘better than,’ and/or hidden envy related to perceived unfairness or injustice. It’s an emotion that we often experience when we fail to set boundaries or ask for what we need, or when expectations let us down because they were based on things we can’t control, like what other people think, what they feel, or how they’re going to react.”
Wow, I thought, Healthcare checks all of these boxes.
- Perceived unfairness of work schedules? Check.
- Perceived injustice? Of course — we see that in our dealings with insurance company denials every day.
But those are both extrinsic. What about the intrinsic factors she’s calling us out on here?
- Do we, as physicians, fail to set boundaries?
- Do we fail to ask for what we need?
Hard yes and yes. (Do we even know, as physicians, what our own boundaries are?)
And the last one:
- Do our expectations of how our clinic day will go let us down every day because they’re based on things we can’t control?
My brain had to repeat the critical parts of that: Expectations let us down when they’re based on things we can’t control.
But wait, my brain argued back; I’m the physician, I thought I was supposed to get to control things.
Next, the revelation: Could it be that a key to experiencing less resentment is accepting how much control we don’t have in a typical day?
And a corollary: How much does resentment factor into burnout? (To read more on my personal journey with burnout, see this piece).
It so happens that around this same time, I was reading another excellent book, Changing How We Think About Difficult Patients: A Guide for Physicians and Healthcare Professionals, by Joan Naidorf, DO.
Dr Naidorf is an emergency medicine physician of 30 years who wrote the book to “provid[e] insight and tools to manage our negative thoughts about difficult patients” and help “beleaguered colleagues…return to their benevolent guiding principles and find more enjoyment in their vitally important careers.”
As I read Dr Naidorf’s book, I thus did so with the mindset of wanting to further understand for myself where this specific emotion of resentment toward our “difficult” patients could come from and how to best understand it in order to get past it.
Dr. Naidorf writes, “Challenging patients will never stop appearing… You cannot change them or control them—the only person you can control is you.”
I wondered how much the resentment we might involuntarily feel at being asked to see a “difficult” patient has nothing to do with the patient but everything to do with it making us feel not in control of the situation.
Dr. Naidorf also writes, “Negative thoughts about challenging patients can cause, in otherwise capable clinicians, a sense of inadequacy and incompetence.”
Do we perhaps resent our challenging patients because of the negative thoughts they sometimes trigger in us? If so, how does this relate to envy, as Dr. Brown asserts resentment is tied to? Is it triggering us to feel inadequate?
“[Difficult patients] often make us question ourselves,” Dr. Naidorf writes, “and we need to feel comfortable with the answers.”
Again, the discrepancy between expectations and reality creates the negative emotion.
Or, as Dr. Naidorf writes, “What if you could stop judging others so harshly and accept them exactly as they are?”
Hmmm, I thought, then the cessation of harsh judgment and implementation of acceptance would have to apply to us too. The elusive concept of self-compassion.
Maybe the resentment/envy comes from us not allowing ourselves to behave in this way because to do so would allow too much vulnerability. Something most of us were conditioned to avoid to survive medical training.
Dr. Brown also writes about an “aha” moment she had in her struggle to understand resentment. “I’m not mad because you’re resting. I’m mad because I’m so bone tired and I want to rest. But, unlike you, I’m going to pretend that I don’t need to.”
I felt all too seen in that passage. Could it be my old nemesis, perfectionism, creeping its way back in? Is resentment the ugly stepsister to perfectionism?
Perhaps challenging patients can engender resentment because they make us feel like we’re not living up to our own unrealistic expectations. And in that case, we need to change our unrealistic expectations for ourselves.
Dr Naidorf’s book explores much more on the complex matter of what makes a “difficult” patient, but I chose to focus here only on the resentment piece as a tie-in to Dr. Brown’s book. I highly recommend both books for further reading to help physicians and nurses navigate the complex emotions our jobs can trigger.
Most importantly, recognizing that we have these transient negative emotions does not make us bad people or healthcare professionals. It only makes us human.
Dr. Lycette is medical director, Providence Oncology and Hematology Care Clinic, Seaside, Ore. She has disclosed having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Infectious disease pop quiz: Clinical challenge #16 for the ObGyn
What is the best test for the diagnosis of acute hepatitis A infection?
Continue to the answer...
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What is the best test for the diagnosis of acute hepatitis A infection?
Continue to the answer...
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
What is the best test for the diagnosis of acute hepatitis A infection?
Continue to the answer...
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Ukrainian physicians ‘ready to die for their freedom’
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Older age for menopause raises risk for lung cancer
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.