User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
New treatment meets unmet need in breast cancer
CHICAGO -- An antibody drug conjugate that targets a cell-surface antigen found on most breast and bladder cancers demonstrated improved progression-free survival over standard chemotherapy in patients with endocrine-resistant hormone receptor positive/HER2 negative metastatic breast cancer.
The agent, called sacituzumab govitecan (Trodelvy, Gilead), was approved on an accelerated basis in 2020 by the Food and Drug Administration for patients with unresectable locally advanced or metastatic triple-negative breast cancer. It received regular approval in 2021.
The conjugate includes an antibody that targets the Trop-2 protein. The antibody is bound to govitecan, which is the active metabolite of the topoisomerase inhibitor 1 irinotecan.
“Sacituzumab demonstrated significant and clinically meaningful benefit, compared with chemotherapy in patients with heavily pretreated endocrine resistant hormone receptor positive, HER2 negative, advanced breast cancer and should be considered a potential treatment in this heavily pretreated patient population,” said lead author Hope S. Rugo, MD, during a press conference held June 4 in Chicago at the annual meeting of the American Society of Clinical Oncology. Dr. Rugo is director of Breast Oncology and Clinical Trials Education at the University of California, San Francisco comprehensive cancer center.
The results drew praise from ASCO spokesperson and breast cancer expert Jane Lowe Meisel, MD, since patients with HR+/HER2- metastatic breast cancer who become resistant to endocrine therapy are left with only sequential, single-agent chemotherapy. “We’ve all been eagerly awaiting the results of this trial. These estrogen positive endocrine negative resistant patients really are an area of great unmet clinical need, and their cancers can be very difficult to treat,” Dr. Meisel said during the press conference.
Approximately, 74% of all breast cancers are HR positive/HER2 negative. And, of these, 92% of patients live beyond five years, according to the American Cancer Society.
The study found a relatively small 1.5 months difference in median progression-free survival, but the results are nevertheless clinically important, especially given that 21% of patients were progression-free at one year, compared with 7% in the chemotherapy arm. “When you look at the patients who do respond on sacituzumab govitecan, it seems that they tend to respond better and longer. The idea that someone with such heavily pretreated disease could walk into your clinic and you could offer them an option that would allow them a one in five chance of still not having progressed at one year is really huge from a clinical standpoint,” Dr. Meisel said.
“This is what we need, incremental options that may be different or better than chemotherapy, so I think this really represents a step forward for the field,” he said.
Two other antibody-drug conjugates that are FDA approved for HER2-positive breast cancer include ado-trastuzumab emtansine (Kadcyla, Genentech) and fam-trastuzumab deruxtecan (Enhertu, AstraZeneca, and Daiichi Sankyo). This new wave of therapies is exciting, according to Julie Gralow, MD, who is chief medical officer and executive vice president of ASCO. “I think this way of delivering chemotherapy inside the cancer cell by having an antibody directed to something on the cell surface and then internalization is really, really very interesting,” Dr. Gralow said during the press conference.
The study included 543 patients from 113 international centers who had previously received endocrine therapy, CDK4/6 inhibitors, and at least two previous regimens of chemotherapy. Median progression-free survival (PFS) was 5.5 months in the sacituzumab govitecan group and 4.0 months in the chemotherapy group (hazard ratio, 0.66; P <.001). PFS was more frequent at 6 months (46% vs. 30%) and 12 months (21% vs. 7%). There was no significant improvement in overall survival (13.9 months vs. 12.3 months). The sacituzumab govitecan group had higher rates of overall response (21% vs. 14%) and clinical benefit (34% vs. 22%), as well as a longer median duration of response (7.4 vs. 5.6 months).
Adverse events were more common with sacituzumab govitecan (74% vs. 60%), including low white blood cell counts (51% vs. 39%) and diarrhea (10% vs. 1%). Both groups had low rates of treatment discontinuation due to adverse events (6% in sacituzumab govitecan vs. 4% in chemotherapy).
Dr. Rugo has received honoraria from Puma Biotechnology and Samsung Bioepis, has consulted for Napo Pharmaceuticals, and has received funding from Astellas Pharma, AstraZeneca, Ayala Pharmaceuticals, Daiichi Sankyo, Genentech, Gilead Sciences, Lilly, Merck, Novartis, OBI Pharma, Odonate Therapeutics, Pfizer, and Sermonix Pharmaceuticals. Dr. Meisel has advised or consulted for Medscape and Total Health Conferencing. She has advised or consulted for AstraZeneca, Curio Science, Genentech, GlaxoSmithKline, Novartis, and SeaGen. She has received research funding from Pfizer and Seattle Genetics. She has received travel, accommodation, or expenses from Pfizer, Puma Biotechnology, and Total Health Conferencing.
CHICAGO -- An antibody drug conjugate that targets a cell-surface antigen found on most breast and bladder cancers demonstrated improved progression-free survival over standard chemotherapy in patients with endocrine-resistant hormone receptor positive/HER2 negative metastatic breast cancer.
The agent, called sacituzumab govitecan (Trodelvy, Gilead), was approved on an accelerated basis in 2020 by the Food and Drug Administration for patients with unresectable locally advanced or metastatic triple-negative breast cancer. It received regular approval in 2021.
The conjugate includes an antibody that targets the Trop-2 protein. The antibody is bound to govitecan, which is the active metabolite of the topoisomerase inhibitor 1 irinotecan.
“Sacituzumab demonstrated significant and clinically meaningful benefit, compared with chemotherapy in patients with heavily pretreated endocrine resistant hormone receptor positive, HER2 negative, advanced breast cancer and should be considered a potential treatment in this heavily pretreated patient population,” said lead author Hope S. Rugo, MD, during a press conference held June 4 in Chicago at the annual meeting of the American Society of Clinical Oncology. Dr. Rugo is director of Breast Oncology and Clinical Trials Education at the University of California, San Francisco comprehensive cancer center.
The results drew praise from ASCO spokesperson and breast cancer expert Jane Lowe Meisel, MD, since patients with HR+/HER2- metastatic breast cancer who become resistant to endocrine therapy are left with only sequential, single-agent chemotherapy. “We’ve all been eagerly awaiting the results of this trial. These estrogen positive endocrine negative resistant patients really are an area of great unmet clinical need, and their cancers can be very difficult to treat,” Dr. Meisel said during the press conference.
Approximately, 74% of all breast cancers are HR positive/HER2 negative. And, of these, 92% of patients live beyond five years, according to the American Cancer Society.
The study found a relatively small 1.5 months difference in median progression-free survival, but the results are nevertheless clinically important, especially given that 21% of patients were progression-free at one year, compared with 7% in the chemotherapy arm. “When you look at the patients who do respond on sacituzumab govitecan, it seems that they tend to respond better and longer. The idea that someone with such heavily pretreated disease could walk into your clinic and you could offer them an option that would allow them a one in five chance of still not having progressed at one year is really huge from a clinical standpoint,” Dr. Meisel said.
“This is what we need, incremental options that may be different or better than chemotherapy, so I think this really represents a step forward for the field,” he said.
Two other antibody-drug conjugates that are FDA approved for HER2-positive breast cancer include ado-trastuzumab emtansine (Kadcyla, Genentech) and fam-trastuzumab deruxtecan (Enhertu, AstraZeneca, and Daiichi Sankyo). This new wave of therapies is exciting, according to Julie Gralow, MD, who is chief medical officer and executive vice president of ASCO. “I think this way of delivering chemotherapy inside the cancer cell by having an antibody directed to something on the cell surface and then internalization is really, really very interesting,” Dr. Gralow said during the press conference.
The study included 543 patients from 113 international centers who had previously received endocrine therapy, CDK4/6 inhibitors, and at least two previous regimens of chemotherapy. Median progression-free survival (PFS) was 5.5 months in the sacituzumab govitecan group and 4.0 months in the chemotherapy group (hazard ratio, 0.66; P <.001). PFS was more frequent at 6 months (46% vs. 30%) and 12 months (21% vs. 7%). There was no significant improvement in overall survival (13.9 months vs. 12.3 months). The sacituzumab govitecan group had higher rates of overall response (21% vs. 14%) and clinical benefit (34% vs. 22%), as well as a longer median duration of response (7.4 vs. 5.6 months).
Adverse events were more common with sacituzumab govitecan (74% vs. 60%), including low white blood cell counts (51% vs. 39%) and diarrhea (10% vs. 1%). Both groups had low rates of treatment discontinuation due to adverse events (6% in sacituzumab govitecan vs. 4% in chemotherapy).
Dr. Rugo has received honoraria from Puma Biotechnology and Samsung Bioepis, has consulted for Napo Pharmaceuticals, and has received funding from Astellas Pharma, AstraZeneca, Ayala Pharmaceuticals, Daiichi Sankyo, Genentech, Gilead Sciences, Lilly, Merck, Novartis, OBI Pharma, Odonate Therapeutics, Pfizer, and Sermonix Pharmaceuticals. Dr. Meisel has advised or consulted for Medscape and Total Health Conferencing. She has advised or consulted for AstraZeneca, Curio Science, Genentech, GlaxoSmithKline, Novartis, and SeaGen. She has received research funding from Pfizer and Seattle Genetics. She has received travel, accommodation, or expenses from Pfizer, Puma Biotechnology, and Total Health Conferencing.
CHICAGO -- An antibody drug conjugate that targets a cell-surface antigen found on most breast and bladder cancers demonstrated improved progression-free survival over standard chemotherapy in patients with endocrine-resistant hormone receptor positive/HER2 negative metastatic breast cancer.
The agent, called sacituzumab govitecan (Trodelvy, Gilead), was approved on an accelerated basis in 2020 by the Food and Drug Administration for patients with unresectable locally advanced or metastatic triple-negative breast cancer. It received regular approval in 2021.
The conjugate includes an antibody that targets the Trop-2 protein. The antibody is bound to govitecan, which is the active metabolite of the topoisomerase inhibitor 1 irinotecan.
“Sacituzumab demonstrated significant and clinically meaningful benefit, compared with chemotherapy in patients with heavily pretreated endocrine resistant hormone receptor positive, HER2 negative, advanced breast cancer and should be considered a potential treatment in this heavily pretreated patient population,” said lead author Hope S. Rugo, MD, during a press conference held June 4 in Chicago at the annual meeting of the American Society of Clinical Oncology. Dr. Rugo is director of Breast Oncology and Clinical Trials Education at the University of California, San Francisco comprehensive cancer center.
The results drew praise from ASCO spokesperson and breast cancer expert Jane Lowe Meisel, MD, since patients with HR+/HER2- metastatic breast cancer who become resistant to endocrine therapy are left with only sequential, single-agent chemotherapy. “We’ve all been eagerly awaiting the results of this trial. These estrogen positive endocrine negative resistant patients really are an area of great unmet clinical need, and their cancers can be very difficult to treat,” Dr. Meisel said during the press conference.
Approximately, 74% of all breast cancers are HR positive/HER2 negative. And, of these, 92% of patients live beyond five years, according to the American Cancer Society.
The study found a relatively small 1.5 months difference in median progression-free survival, but the results are nevertheless clinically important, especially given that 21% of patients were progression-free at one year, compared with 7% in the chemotherapy arm. “When you look at the patients who do respond on sacituzumab govitecan, it seems that they tend to respond better and longer. The idea that someone with such heavily pretreated disease could walk into your clinic and you could offer them an option that would allow them a one in five chance of still not having progressed at one year is really huge from a clinical standpoint,” Dr. Meisel said.
“This is what we need, incremental options that may be different or better than chemotherapy, so I think this really represents a step forward for the field,” he said.
Two other antibody-drug conjugates that are FDA approved for HER2-positive breast cancer include ado-trastuzumab emtansine (Kadcyla, Genentech) and fam-trastuzumab deruxtecan (Enhertu, AstraZeneca, and Daiichi Sankyo). This new wave of therapies is exciting, according to Julie Gralow, MD, who is chief medical officer and executive vice president of ASCO. “I think this way of delivering chemotherapy inside the cancer cell by having an antibody directed to something on the cell surface and then internalization is really, really very interesting,” Dr. Gralow said during the press conference.
The study included 543 patients from 113 international centers who had previously received endocrine therapy, CDK4/6 inhibitors, and at least two previous regimens of chemotherapy. Median progression-free survival (PFS) was 5.5 months in the sacituzumab govitecan group and 4.0 months in the chemotherapy group (hazard ratio, 0.66; P <.001). PFS was more frequent at 6 months (46% vs. 30%) and 12 months (21% vs. 7%). There was no significant improvement in overall survival (13.9 months vs. 12.3 months). The sacituzumab govitecan group had higher rates of overall response (21% vs. 14%) and clinical benefit (34% vs. 22%), as well as a longer median duration of response (7.4 vs. 5.6 months).
Adverse events were more common with sacituzumab govitecan (74% vs. 60%), including low white blood cell counts (51% vs. 39%) and diarrhea (10% vs. 1%). Both groups had low rates of treatment discontinuation due to adverse events (6% in sacituzumab govitecan vs. 4% in chemotherapy).
Dr. Rugo has received honoraria from Puma Biotechnology and Samsung Bioepis, has consulted for Napo Pharmaceuticals, and has received funding from Astellas Pharma, AstraZeneca, Ayala Pharmaceuticals, Daiichi Sankyo, Genentech, Gilead Sciences, Lilly, Merck, Novartis, OBI Pharma, Odonate Therapeutics, Pfizer, and Sermonix Pharmaceuticals. Dr. Meisel has advised or consulted for Medscape and Total Health Conferencing. She has advised or consulted for AstraZeneca, Curio Science, Genentech, GlaxoSmithKline, Novartis, and SeaGen. She has received research funding from Pfizer and Seattle Genetics. She has received travel, accommodation, or expenses from Pfizer, Puma Biotechnology, and Total Health Conferencing.
AT ASCO 2022
TNF placental transfer makes little difference in offspring infections
COPENHAGEN – Here’s reassuring news for pregnant women with rheumatic diseases treated with tumor necrosis factor (TNF)–alpha inhibitors: Although the drugs vary widely in their transmissibility across the placenta, there appears to be no excess risk for serious infections in children exposed in utero to TNF inhibitors with high, compared with low, placental transfer.
That’s according to investigators at McGill University in Montreal, who studied outcomes for nearly 3,000 infants who were exposed to TNF inhibitors during gestation.
“Our data are reassuring as we saw no strong signal, which suggests that there is no need to switch the mother’s drugs. More studies are needed, but this is a step in the right direction to reduce maternal stress and reassure physicians,” said Leah K. Flatman, MSc, a PhD candidate in epidemiology at McGill.
Ms. Flatman presented the findings in an oral abstract session at the annual European Congress of Rheumatology.
Not without risks
Approximately 20% of pregnant women with chronic inflammatory diseases are prescribed a TNF inhibitor, a class of drug that is effective for disease control but also increases risk for infection because of immunosuppressive effects.
“Similarly, offspring exposed in utero to TNF inhibitors may also experience immunosuppression and subsequent serious infections in their first year of life. This is the result of the TNF inhibitor entering the fetal bloodstream at different concentrations,» Ms. Flatman said.
Anti-TNF monoclonal immunoglobulins, such as infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), and golimumab (Simponi) have the highest placental transfer, reaching higher levels in fetal circulation than in maternal circulation, she noted.
In contrast, certolizumab (Cimzia), a pegylated humanized antigen-binding fragment, and etanercept (Enbrel and biosimilars), a fusion protein, have the lowest placental penetration, Ms. Flatman said.
Population study
The investigators conducted a population cohort study using the IBM MarketScan database of commercial claims from employer-provided health insurance plans in the United States.
They looked at data on offspring of mothers with rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and/or inflammatory bowel diseases (IBD; Crohn’s disease, and ulcerative colitis). The children were born from Jan. 1, 2011 through Dec. 31, 2019.
The exposure was at least one filled prescription and/or infusion procedure claim for TNF inhibitors in the 6 months before delivery. The exposures were divided into high and low placental-transfer categories.
A total of 26,088 offspring were identified, of whom 2,902 (11.1%) were exposed to a TNF inhibitor in utero. A little more than half of these children were born to mothers treated with TNF inhibitors for IBD.
For the primary outcome of serious infections (based on at least one hospitalization with infection in the first year of life), the investigators plotted Kaplan-Meier curves, which showed that the survival probability of serious infections in the high and low groups overlapped, indicating no large differences.
Of 2,105 offspring of mothers treated with a high–placental-transfer drug, 38 (1.8%) had serious infections, compared with 10 of 797 offspring (1.3%) of mothers who received low–placental-transfer drugs.
In multivariable analysis that controlled for maternal age at delivery, any RA diagnosis without an IBD diagnosis, and IBD diagnosis, gestational or pregestational diabetes, maternal asthma, preterm delivery, corticosteroid use, and disease-modifying antirheumatic drug use, the investigators saw that the hazard ratio for risk for serious infection in the high–, compared with the low–placental-transfer group was 1.20, with a confidence interval crossing 1, indicating nonsignificance.
Similar results reported
Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented, told this news organization that the findings were in line with those of a recent meta-analysis looking at the safety of biologic agents in pregnant women with IBD.
She added, however, that although the meta-analysis also showed little difference in outcomes for the children of women treated with high– compared with low–placental-transfer drugs, “we need more data to be sure about this.”
Comoderator Gabriela Riemekasten, MD, director of the clinic for rheumatology and clinical immunology at University Hospital in Lübeck, Germany, told this news organization that she was surprised to see that more women received high– than low–placental-transfer drugs.
Although there was a 20% difference between the groups, the numbers were relatively low, and “I would consider this in my practice and give my patients the advice of these data,” she said.
The study was supported by an Arthritis Society PhD Salary Award, and a Canadian Institutes of Health Project grant. Ms. Flatman, Dr. Förger, and Dr. Riemekasten reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Here’s reassuring news for pregnant women with rheumatic diseases treated with tumor necrosis factor (TNF)–alpha inhibitors: Although the drugs vary widely in their transmissibility across the placenta, there appears to be no excess risk for serious infections in children exposed in utero to TNF inhibitors with high, compared with low, placental transfer.
That’s according to investigators at McGill University in Montreal, who studied outcomes for nearly 3,000 infants who were exposed to TNF inhibitors during gestation.
“Our data are reassuring as we saw no strong signal, which suggests that there is no need to switch the mother’s drugs. More studies are needed, but this is a step in the right direction to reduce maternal stress and reassure physicians,” said Leah K. Flatman, MSc, a PhD candidate in epidemiology at McGill.
Ms. Flatman presented the findings in an oral abstract session at the annual European Congress of Rheumatology.
Not without risks
Approximately 20% of pregnant women with chronic inflammatory diseases are prescribed a TNF inhibitor, a class of drug that is effective for disease control but also increases risk for infection because of immunosuppressive effects.
“Similarly, offspring exposed in utero to TNF inhibitors may also experience immunosuppression and subsequent serious infections in their first year of life. This is the result of the TNF inhibitor entering the fetal bloodstream at different concentrations,» Ms. Flatman said.
Anti-TNF monoclonal immunoglobulins, such as infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), and golimumab (Simponi) have the highest placental transfer, reaching higher levels in fetal circulation than in maternal circulation, she noted.
In contrast, certolizumab (Cimzia), a pegylated humanized antigen-binding fragment, and etanercept (Enbrel and biosimilars), a fusion protein, have the lowest placental penetration, Ms. Flatman said.
Population study
The investigators conducted a population cohort study using the IBM MarketScan database of commercial claims from employer-provided health insurance plans in the United States.
They looked at data on offspring of mothers with rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and/or inflammatory bowel diseases (IBD; Crohn’s disease, and ulcerative colitis). The children were born from Jan. 1, 2011 through Dec. 31, 2019.
The exposure was at least one filled prescription and/or infusion procedure claim for TNF inhibitors in the 6 months before delivery. The exposures were divided into high and low placental-transfer categories.
A total of 26,088 offspring were identified, of whom 2,902 (11.1%) were exposed to a TNF inhibitor in utero. A little more than half of these children were born to mothers treated with TNF inhibitors for IBD.
For the primary outcome of serious infections (based on at least one hospitalization with infection in the first year of life), the investigators plotted Kaplan-Meier curves, which showed that the survival probability of serious infections in the high and low groups overlapped, indicating no large differences.
Of 2,105 offspring of mothers treated with a high–placental-transfer drug, 38 (1.8%) had serious infections, compared with 10 of 797 offspring (1.3%) of mothers who received low–placental-transfer drugs.
In multivariable analysis that controlled for maternal age at delivery, any RA diagnosis without an IBD diagnosis, and IBD diagnosis, gestational or pregestational diabetes, maternal asthma, preterm delivery, corticosteroid use, and disease-modifying antirheumatic drug use, the investigators saw that the hazard ratio for risk for serious infection in the high–, compared with the low–placental-transfer group was 1.20, with a confidence interval crossing 1, indicating nonsignificance.
Similar results reported
Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented, told this news organization that the findings were in line with those of a recent meta-analysis looking at the safety of biologic agents in pregnant women with IBD.
She added, however, that although the meta-analysis also showed little difference in outcomes for the children of women treated with high– compared with low–placental-transfer drugs, “we need more data to be sure about this.”
Comoderator Gabriela Riemekasten, MD, director of the clinic for rheumatology and clinical immunology at University Hospital in Lübeck, Germany, told this news organization that she was surprised to see that more women received high– than low–placental-transfer drugs.
Although there was a 20% difference between the groups, the numbers were relatively low, and “I would consider this in my practice and give my patients the advice of these data,” she said.
The study was supported by an Arthritis Society PhD Salary Award, and a Canadian Institutes of Health Project grant. Ms. Flatman, Dr. Förger, and Dr. Riemekasten reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Here’s reassuring news for pregnant women with rheumatic diseases treated with tumor necrosis factor (TNF)–alpha inhibitors: Although the drugs vary widely in their transmissibility across the placenta, there appears to be no excess risk for serious infections in children exposed in utero to TNF inhibitors with high, compared with low, placental transfer.
That’s according to investigators at McGill University in Montreal, who studied outcomes for nearly 3,000 infants who were exposed to TNF inhibitors during gestation.
“Our data are reassuring as we saw no strong signal, which suggests that there is no need to switch the mother’s drugs. More studies are needed, but this is a step in the right direction to reduce maternal stress and reassure physicians,” said Leah K. Flatman, MSc, a PhD candidate in epidemiology at McGill.
Ms. Flatman presented the findings in an oral abstract session at the annual European Congress of Rheumatology.
Not without risks
Approximately 20% of pregnant women with chronic inflammatory diseases are prescribed a TNF inhibitor, a class of drug that is effective for disease control but also increases risk for infection because of immunosuppressive effects.
“Similarly, offspring exposed in utero to TNF inhibitors may also experience immunosuppression and subsequent serious infections in their first year of life. This is the result of the TNF inhibitor entering the fetal bloodstream at different concentrations,» Ms. Flatman said.
Anti-TNF monoclonal immunoglobulins, such as infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), and golimumab (Simponi) have the highest placental transfer, reaching higher levels in fetal circulation than in maternal circulation, she noted.
In contrast, certolizumab (Cimzia), a pegylated humanized antigen-binding fragment, and etanercept (Enbrel and biosimilars), a fusion protein, have the lowest placental penetration, Ms. Flatman said.
Population study
The investigators conducted a population cohort study using the IBM MarketScan database of commercial claims from employer-provided health insurance plans in the United States.
They looked at data on offspring of mothers with rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and/or inflammatory bowel diseases (IBD; Crohn’s disease, and ulcerative colitis). The children were born from Jan. 1, 2011 through Dec. 31, 2019.
The exposure was at least one filled prescription and/or infusion procedure claim for TNF inhibitors in the 6 months before delivery. The exposures were divided into high and low placental-transfer categories.
A total of 26,088 offspring were identified, of whom 2,902 (11.1%) were exposed to a TNF inhibitor in utero. A little more than half of these children were born to mothers treated with TNF inhibitors for IBD.
For the primary outcome of serious infections (based on at least one hospitalization with infection in the first year of life), the investigators plotted Kaplan-Meier curves, which showed that the survival probability of serious infections in the high and low groups overlapped, indicating no large differences.
Of 2,105 offspring of mothers treated with a high–placental-transfer drug, 38 (1.8%) had serious infections, compared with 10 of 797 offspring (1.3%) of mothers who received low–placental-transfer drugs.
In multivariable analysis that controlled for maternal age at delivery, any RA diagnosis without an IBD diagnosis, and IBD diagnosis, gestational or pregestational diabetes, maternal asthma, preterm delivery, corticosteroid use, and disease-modifying antirheumatic drug use, the investigators saw that the hazard ratio for risk for serious infection in the high–, compared with the low–placental-transfer group was 1.20, with a confidence interval crossing 1, indicating nonsignificance.
Similar results reported
Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented, told this news organization that the findings were in line with those of a recent meta-analysis looking at the safety of biologic agents in pregnant women with IBD.
She added, however, that although the meta-analysis also showed little difference in outcomes for the children of women treated with high– compared with low–placental-transfer drugs, “we need more data to be sure about this.”
Comoderator Gabriela Riemekasten, MD, director of the clinic for rheumatology and clinical immunology at University Hospital in Lübeck, Germany, told this news organization that she was surprised to see that more women received high– than low–placental-transfer drugs.
Although there was a 20% difference between the groups, the numbers were relatively low, and “I would consider this in my practice and give my patients the advice of these data,” she said.
The study was supported by an Arthritis Society PhD Salary Award, and a Canadian Institutes of Health Project grant. Ms. Flatman, Dr. Förger, and Dr. Riemekasten reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
THE EULAR 2022 CONGRESS
Müllerian anomalies: Operative considerations
Surgeons, who see it up close, offer ways to stop gun violence
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
High maternal, fetal morbidity rates in SLE pregnancies
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.
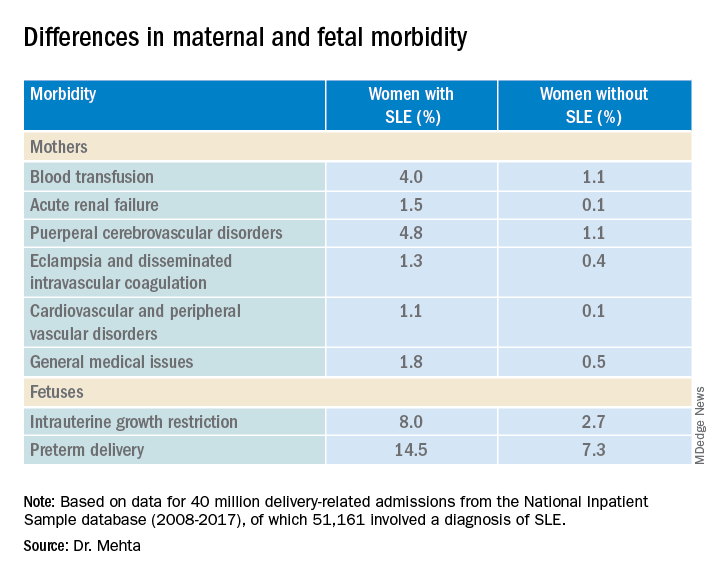
Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
mTOR inhibitor shows early promise in endometrial cancer
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
FROM JAMA ONCOLOGY
Informal human-milk donation: How to counsel patients
I have become obsessed with the reality that the unprecedented national shortage of formula is keeping some families from adequately feeding their infants and young children. I am deeply concerned, both as a family medicine physician and a new mother, about the heartbreaking stories that I’ve heard from parents of all socioeconomic backgrounds. New mothers, unable to breastfeed for a multitude of reasons, find themselves standing in front of empty store shelves, in tears.
In recent months, many health care providers have had patients disclose that they are diluting ready-to-feed formula or mixing powdered formula with more water than instructed to make it go further. Some parents are giving cow’s milk to their children at too young an age because they can’t find formula. Others are foregoing milk altogether and feeding their children beverages such as juice or soda. All of these practices can threaten a child’s life, growth, and development.
When breastfeeding isn’t possible
We all know that human milk is the optimal, most nutritionally complete food source for newborn babies and infants. It can improve dental health and neurodevelopmental outcomes, as well as reduce the risk for asthma, eczema, diabetes, and obesity. An added benefit during the COVID-19 pandemic has been providing newborn infants with a boost of immunity before they are able to be vaccinated against SARS-CoV-2 infection.
But lactation and breastfeeding aren’t possible for everyone. Earlier this year, when my daughter was born more than a month prematurely, I worried that I would be unable to breastfeed her. The complications of prematurity can interfere with establishing lactation, and my daughter spent some time in the neonatal intensive care unit (NICU), requiring frequent feedings to treat hypoglycemia. She also lacked the muscle strength or coordination to latch on to the breast, so she was fed my colostrum and donor breast milk by bottle.
Not knowing when my mature milk would come in, my family scoured the retail stores for formula while I was still recovering from delivery. My daughter needed a specific type of high-calorie formula for premature infants. Eventually, my mother found one can of this powdered formula. The hospital also sent us home with 16 oz of ready-to-feed samples and enough donor breastmilk to last 24 hours at home. We considered ourselves lucky. The fear and anxiety about being able to feed my baby still stands out in my mind.
Pumping and sharing
Over the next few months, out of necessity, I became an “exclusively pumping” mother. My daughter, unable to latch, drank my pumped milk from a bottle. My body started to produce more milk than she needed in a day. In an effort to pay it forward and to put my extra milk to use, I became a human-milk donor. I underwent rigorous screening, including testing for infectious diseases such as HIV and hepatitis C. I was approved to donate to our local hospital’s milk bank, helping other families in the NICU feed their babies. Through informal connections on the internet, I also provide expressed milk to another mother in the community who is unable to lactate. To date, I’ve donated more than 1,500 oz of human milk (and counting).
The practice of human-milk donation dates back millennia with wet-nursing, when children were breastfed by someone other than their biological mothers: relatives, friends, or even strangers. The first milk bank in the United States opened in Boston in the early 20th century. In 1980, the World Health Organization and the United Nations Children’s Fund released a joint statement supporting the use of human-donor milk as the first alternative if the biological mother is unable to breastfeed. Donor milk is a safe option for families who cannot provide their own human milk to their children.
Human-milk banks
More than 30 nonprofit milk banks now operate in the United States. Because their mission is primarily to meet the needs of sick and hospitalized children rather than the general public, these milk banks are an impractical solution to the national formula shortage. Although families with healthy children can purchase donor milk with a prescription, supplies are scarce, and insurance doesn’t cover the cost.
Milk provided by formal human-milk banks is considered safe. Certain infections such as HIV and hepatitis can be transmitted through human milk. However, milk banks screen their donors and safely pasteurize and store donated breastmilk, following standard protocols. The risk of contracting an illness from banked donor milk is very low. The American Academy of Pediatrics recommends accepting donor milk only from a milk bank.
Informal human-milk donation
An increasingly popular alternative to formal human-milk banks is informal human-milk sharing. But many people, including health care professionals, hold misconceptions about how informal milk donation works. Today’s informal milk donation looks very different from age-old wet-nursing: Moms in support groups, often via social media, are requesting pumped milk from one another. (Note that this definition of “informal human-milk donation” does not include selling or purchasing human milk.)
Although the safety of sharing pumped human milk this way cannot be guaranteed, a harm-reduction approach is warranted, especially in view of the current formula scarcity.
I believe that medical professionals have a responsibility to raise awareness and dispel myths about donor breast milk. Many physicians acknowledge that informal milk sharing is common but rarely recommend it to patients. Whether they are donors or recipients, families who choose to participate need to be educated about how to go about the process as safely as possible.
Patients who are considering accepting informally donated human milk should ask key questions of the donor to gauge the risk of pathogens or other harmful substances being passed to their babies:
- What medications do you take?
- What supplements do you take?
- What recreational drugs do you take?
- Any recent travel?
- Any tattoos and if so, how recent?
- How much alcohol do you drink and how often?
- Have you been diagnosed with any infections?
- Any recent illness?
- How do you pump your breast milk?
- How do you store your breast milk?
- When was the available milk pumped?
We can help families by offering our medical expertise, allowing them to make an informed decision about whether to accept donated human milk. Clinicians can encourage patients and their families to use resources like the Infant Risk Center, which provides evidence-based information about medication safety and breast milk.
If your lactating patient is considering donating milk through informal channels to a family in need, encourage them to be open and honest about their medical history and lifestyle habits. If they cannot be transparent, they should not donate. A mutual level of respect and honesty can ensure the safety of those they hope to help. It is also important to counsel prospective milk donors to notify their milk recipients of any new illnesses, substance use, medications, travel, tattoos, or changes to their medical history.
Finally, encourage lactating patients who are able to do so to donate their extra milk to local nonprofit milk banks to increase the availability of screened, pasteurized breast milk in the community.
As a physician and mother, I hope that U.S. families will be less vulnerable to future formula shortages. Human milk is an ideal food source, but not everyone can lactate. Though not perfect, human milk donated outside of formal milk banks offers a safer alternative to diluting formula or feeding other unsuitable beverages to infants and children. As health care professionals, we need to counsel our patients about how to engage in this practice safely.
Dr. Mieses Malchuk is assistant professor in the department of family medicine at the University of North Carolina at Chapel Hill and a board-certified family physician and attending physician at UNC Health in Chapel Hill. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have become obsessed with the reality that the unprecedented national shortage of formula is keeping some families from adequately feeding their infants and young children. I am deeply concerned, both as a family medicine physician and a new mother, about the heartbreaking stories that I’ve heard from parents of all socioeconomic backgrounds. New mothers, unable to breastfeed for a multitude of reasons, find themselves standing in front of empty store shelves, in tears.
In recent months, many health care providers have had patients disclose that they are diluting ready-to-feed formula or mixing powdered formula with more water than instructed to make it go further. Some parents are giving cow’s milk to their children at too young an age because they can’t find formula. Others are foregoing milk altogether and feeding their children beverages such as juice or soda. All of these practices can threaten a child’s life, growth, and development.
When breastfeeding isn’t possible
We all know that human milk is the optimal, most nutritionally complete food source for newborn babies and infants. It can improve dental health and neurodevelopmental outcomes, as well as reduce the risk for asthma, eczema, diabetes, and obesity. An added benefit during the COVID-19 pandemic has been providing newborn infants with a boost of immunity before they are able to be vaccinated against SARS-CoV-2 infection.
But lactation and breastfeeding aren’t possible for everyone. Earlier this year, when my daughter was born more than a month prematurely, I worried that I would be unable to breastfeed her. The complications of prematurity can interfere with establishing lactation, and my daughter spent some time in the neonatal intensive care unit (NICU), requiring frequent feedings to treat hypoglycemia. She also lacked the muscle strength or coordination to latch on to the breast, so she was fed my colostrum and donor breast milk by bottle.
Not knowing when my mature milk would come in, my family scoured the retail stores for formula while I was still recovering from delivery. My daughter needed a specific type of high-calorie formula for premature infants. Eventually, my mother found one can of this powdered formula. The hospital also sent us home with 16 oz of ready-to-feed samples and enough donor breastmilk to last 24 hours at home. We considered ourselves lucky. The fear and anxiety about being able to feed my baby still stands out in my mind.
Pumping and sharing
Over the next few months, out of necessity, I became an “exclusively pumping” mother. My daughter, unable to latch, drank my pumped milk from a bottle. My body started to produce more milk than she needed in a day. In an effort to pay it forward and to put my extra milk to use, I became a human-milk donor. I underwent rigorous screening, including testing for infectious diseases such as HIV and hepatitis C. I was approved to donate to our local hospital’s milk bank, helping other families in the NICU feed their babies. Through informal connections on the internet, I also provide expressed milk to another mother in the community who is unable to lactate. To date, I’ve donated more than 1,500 oz of human milk (and counting).
The practice of human-milk donation dates back millennia with wet-nursing, when children were breastfed by someone other than their biological mothers: relatives, friends, or even strangers. The first milk bank in the United States opened in Boston in the early 20th century. In 1980, the World Health Organization and the United Nations Children’s Fund released a joint statement supporting the use of human-donor milk as the first alternative if the biological mother is unable to breastfeed. Donor milk is a safe option for families who cannot provide their own human milk to their children.
Human-milk banks
More than 30 nonprofit milk banks now operate in the United States. Because their mission is primarily to meet the needs of sick and hospitalized children rather than the general public, these milk banks are an impractical solution to the national formula shortage. Although families with healthy children can purchase donor milk with a prescription, supplies are scarce, and insurance doesn’t cover the cost.
Milk provided by formal human-milk banks is considered safe. Certain infections such as HIV and hepatitis can be transmitted through human milk. However, milk banks screen their donors and safely pasteurize and store donated breastmilk, following standard protocols. The risk of contracting an illness from banked donor milk is very low. The American Academy of Pediatrics recommends accepting donor milk only from a milk bank.
Informal human-milk donation
An increasingly popular alternative to formal human-milk banks is informal human-milk sharing. But many people, including health care professionals, hold misconceptions about how informal milk donation works. Today’s informal milk donation looks very different from age-old wet-nursing: Moms in support groups, often via social media, are requesting pumped milk from one another. (Note that this definition of “informal human-milk donation” does not include selling or purchasing human milk.)
Although the safety of sharing pumped human milk this way cannot be guaranteed, a harm-reduction approach is warranted, especially in view of the current formula scarcity.
I believe that medical professionals have a responsibility to raise awareness and dispel myths about donor breast milk. Many physicians acknowledge that informal milk sharing is common but rarely recommend it to patients. Whether they are donors or recipients, families who choose to participate need to be educated about how to go about the process as safely as possible.
Patients who are considering accepting informally donated human milk should ask key questions of the donor to gauge the risk of pathogens or other harmful substances being passed to their babies:
- What medications do you take?
- What supplements do you take?
- What recreational drugs do you take?
- Any recent travel?
- Any tattoos and if so, how recent?
- How much alcohol do you drink and how often?
- Have you been diagnosed with any infections?
- Any recent illness?
- How do you pump your breast milk?
- How do you store your breast milk?
- When was the available milk pumped?
We can help families by offering our medical expertise, allowing them to make an informed decision about whether to accept donated human milk. Clinicians can encourage patients and their families to use resources like the Infant Risk Center, which provides evidence-based information about medication safety and breast milk.
If your lactating patient is considering donating milk through informal channels to a family in need, encourage them to be open and honest about their medical history and lifestyle habits. If they cannot be transparent, they should not donate. A mutual level of respect and honesty can ensure the safety of those they hope to help. It is also important to counsel prospective milk donors to notify their milk recipients of any new illnesses, substance use, medications, travel, tattoos, or changes to their medical history.
Finally, encourage lactating patients who are able to do so to donate their extra milk to local nonprofit milk banks to increase the availability of screened, pasteurized breast milk in the community.
As a physician and mother, I hope that U.S. families will be less vulnerable to future formula shortages. Human milk is an ideal food source, but not everyone can lactate. Though not perfect, human milk donated outside of formal milk banks offers a safer alternative to diluting formula or feeding other unsuitable beverages to infants and children. As health care professionals, we need to counsel our patients about how to engage in this practice safely.
Dr. Mieses Malchuk is assistant professor in the department of family medicine at the University of North Carolina at Chapel Hill and a board-certified family physician and attending physician at UNC Health in Chapel Hill. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have become obsessed with the reality that the unprecedented national shortage of formula is keeping some families from adequately feeding their infants and young children. I am deeply concerned, both as a family medicine physician and a new mother, about the heartbreaking stories that I’ve heard from parents of all socioeconomic backgrounds. New mothers, unable to breastfeed for a multitude of reasons, find themselves standing in front of empty store shelves, in tears.
In recent months, many health care providers have had patients disclose that they are diluting ready-to-feed formula or mixing powdered formula with more water than instructed to make it go further. Some parents are giving cow’s milk to their children at too young an age because they can’t find formula. Others are foregoing milk altogether and feeding their children beverages such as juice or soda. All of these practices can threaten a child’s life, growth, and development.
When breastfeeding isn’t possible
We all know that human milk is the optimal, most nutritionally complete food source for newborn babies and infants. It can improve dental health and neurodevelopmental outcomes, as well as reduce the risk for asthma, eczema, diabetes, and obesity. An added benefit during the COVID-19 pandemic has been providing newborn infants with a boost of immunity before they are able to be vaccinated against SARS-CoV-2 infection.
But lactation and breastfeeding aren’t possible for everyone. Earlier this year, when my daughter was born more than a month prematurely, I worried that I would be unable to breastfeed her. The complications of prematurity can interfere with establishing lactation, and my daughter spent some time in the neonatal intensive care unit (NICU), requiring frequent feedings to treat hypoglycemia. She also lacked the muscle strength or coordination to latch on to the breast, so she was fed my colostrum and donor breast milk by bottle.
Not knowing when my mature milk would come in, my family scoured the retail stores for formula while I was still recovering from delivery. My daughter needed a specific type of high-calorie formula for premature infants. Eventually, my mother found one can of this powdered formula. The hospital also sent us home with 16 oz of ready-to-feed samples and enough donor breastmilk to last 24 hours at home. We considered ourselves lucky. The fear and anxiety about being able to feed my baby still stands out in my mind.
Pumping and sharing
Over the next few months, out of necessity, I became an “exclusively pumping” mother. My daughter, unable to latch, drank my pumped milk from a bottle. My body started to produce more milk than she needed in a day. In an effort to pay it forward and to put my extra milk to use, I became a human-milk donor. I underwent rigorous screening, including testing for infectious diseases such as HIV and hepatitis C. I was approved to donate to our local hospital’s milk bank, helping other families in the NICU feed their babies. Through informal connections on the internet, I also provide expressed milk to another mother in the community who is unable to lactate. To date, I’ve donated more than 1,500 oz of human milk (and counting).
The practice of human-milk donation dates back millennia with wet-nursing, when children were breastfed by someone other than their biological mothers: relatives, friends, or even strangers. The first milk bank in the United States opened in Boston in the early 20th century. In 1980, the World Health Organization and the United Nations Children’s Fund released a joint statement supporting the use of human-donor milk as the first alternative if the biological mother is unable to breastfeed. Donor milk is a safe option for families who cannot provide their own human milk to their children.
Human-milk banks
More than 30 nonprofit milk banks now operate in the United States. Because their mission is primarily to meet the needs of sick and hospitalized children rather than the general public, these milk banks are an impractical solution to the national formula shortage. Although families with healthy children can purchase donor milk with a prescription, supplies are scarce, and insurance doesn’t cover the cost.
Milk provided by formal human-milk banks is considered safe. Certain infections such as HIV and hepatitis can be transmitted through human milk. However, milk banks screen their donors and safely pasteurize and store donated breastmilk, following standard protocols. The risk of contracting an illness from banked donor milk is very low. The American Academy of Pediatrics recommends accepting donor milk only from a milk bank.
Informal human-milk donation
An increasingly popular alternative to formal human-milk banks is informal human-milk sharing. But many people, including health care professionals, hold misconceptions about how informal milk donation works. Today’s informal milk donation looks very different from age-old wet-nursing: Moms in support groups, often via social media, are requesting pumped milk from one another. (Note that this definition of “informal human-milk donation” does not include selling or purchasing human milk.)
Although the safety of sharing pumped human milk this way cannot be guaranteed, a harm-reduction approach is warranted, especially in view of the current formula scarcity.
I believe that medical professionals have a responsibility to raise awareness and dispel myths about donor breast milk. Many physicians acknowledge that informal milk sharing is common but rarely recommend it to patients. Whether they are donors or recipients, families who choose to participate need to be educated about how to go about the process as safely as possible.
Patients who are considering accepting informally donated human milk should ask key questions of the donor to gauge the risk of pathogens or other harmful substances being passed to their babies:
- What medications do you take?
- What supplements do you take?
- What recreational drugs do you take?
- Any recent travel?
- Any tattoos and if so, how recent?
- How much alcohol do you drink and how often?
- Have you been diagnosed with any infections?
- Any recent illness?
- How do you pump your breast milk?
- How do you store your breast milk?
- When was the available milk pumped?
We can help families by offering our medical expertise, allowing them to make an informed decision about whether to accept donated human milk. Clinicians can encourage patients and their families to use resources like the Infant Risk Center, which provides evidence-based information about medication safety and breast milk.
If your lactating patient is considering donating milk through informal channels to a family in need, encourage them to be open and honest about their medical history and lifestyle habits. If they cannot be transparent, they should not donate. A mutual level of respect and honesty can ensure the safety of those they hope to help. It is also important to counsel prospective milk donors to notify their milk recipients of any new illnesses, substance use, medications, travel, tattoos, or changes to their medical history.
Finally, encourage lactating patients who are able to do so to donate their extra milk to local nonprofit milk banks to increase the availability of screened, pasteurized breast milk in the community.
As a physician and mother, I hope that U.S. families will be less vulnerable to future formula shortages. Human milk is an ideal food source, but not everyone can lactate. Though not perfect, human milk donated outside of formal milk banks offers a safer alternative to diluting formula or feeding other unsuitable beverages to infants and children. As health care professionals, we need to counsel our patients about how to engage in this practice safely.
Dr. Mieses Malchuk is assistant professor in the department of family medicine at the University of North Carolina at Chapel Hill and a board-certified family physician and attending physician at UNC Health in Chapel Hill. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
High rates of med student burnout during COVID
NEW ORLEANS –
Researchers surveyed 613 medical students representing all years of a medical program during the last week of the Spring semester of 2021.
Based on the Maslach Burnout Inventory-Student Survey (MBI-SS), more than half (54%) of the students had symptoms of burnout.
Eighty percent of students scored high on emotional exhaustion, 57% scored high on cynicism, and 36% scored low on academic effectiveness.
Compared with male medical students, female medical students were more apt to exhibit signs of burnout (60% vs. 44%), emotional exhaustion (80% vs. 73%), and cynicism (62% vs. 49%).
After adjusting for associated factors, female medical students were significantly more likely to suffer from burnout than male students (odds ratio, 1.90; 95% confidence interval, 1.34-2.70; P < .001).
Smoking was also linked to higher likelihood of burnout among medical students (OR, 2.12; 95% CI, 1.18-3.81; P < .05). The death of a family member from COVID-19 also put medical students at heightened risk for burnout (OR, 1.60; 95% CI, 1.08-2.36; P < .05).
The survey results were presented at the American Psychiatric Association (APA) Annual Meeting.
The findings point to the need to study burnout prevalence in universities and develop strategies to promote the mental health of future physicians, presenter Sofia Jezzini-Martínez, fourth-year medical student, Autonomous University of Nuevo Leon, Monterrey, Mexico, wrote in her conference abstract.
In related research presented at the APA meeting, researchers surveyed second-, third-, and fourth-year medical students from California during the pandemic.
Roughly 80% exhibited symptoms of anxiety and 68% exhibited depressive symptoms, of whom about 18% also reported having thoughts of suicide.
Yet only about half of the medical students exhibiting anxiety or depressive symptoms sought help from a mental health professional, and 20% reported using substances to cope with stress.
“Given that the pandemic is ongoing, we hope to draw attention to mental health needs of medical students and influence medical schools to direct appropriate and timely resources to this group,” presenter Sarthak Angal, MD, psychiatry resident, Kaiser Permanente San Jose Medical Center, California, wrote in his conference abstract.
Managing expectations
Weighing in on medical student burnout, Ihuoma Njoku, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, noted that, “particularly for women in multiple fields, including medicine, there’s a lot of burden placed on them.”
“Women are pulled in a lot of different directions and have increased demands, which may help explain their higher rate of burnout,” Dr. Njoku commented.
She noted that these surveys were conducted during the COVID-19 pandemic, “a period when students’ education experience was a lot different than what they expected and maybe what they wanted.”
Dr. Njoku noted that the challenges of the pandemic are particularly hard on fourth-year medical students.
“A big part of fourth year is applying to residency, and many were doing virtual interviews for residency. That makes it hard to really get an appreciation of the place you will spend the next three to eight years of your life,” she told this news organization.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
Researchers surveyed 613 medical students representing all years of a medical program during the last week of the Spring semester of 2021.
Based on the Maslach Burnout Inventory-Student Survey (MBI-SS), more than half (54%) of the students had symptoms of burnout.
Eighty percent of students scored high on emotional exhaustion, 57% scored high on cynicism, and 36% scored low on academic effectiveness.
Compared with male medical students, female medical students were more apt to exhibit signs of burnout (60% vs. 44%), emotional exhaustion (80% vs. 73%), and cynicism (62% vs. 49%).
After adjusting for associated factors, female medical students were significantly more likely to suffer from burnout than male students (odds ratio, 1.90; 95% confidence interval, 1.34-2.70; P < .001).
Smoking was also linked to higher likelihood of burnout among medical students (OR, 2.12; 95% CI, 1.18-3.81; P < .05). The death of a family member from COVID-19 also put medical students at heightened risk for burnout (OR, 1.60; 95% CI, 1.08-2.36; P < .05).
The survey results were presented at the American Psychiatric Association (APA) Annual Meeting.
The findings point to the need to study burnout prevalence in universities and develop strategies to promote the mental health of future physicians, presenter Sofia Jezzini-Martínez, fourth-year medical student, Autonomous University of Nuevo Leon, Monterrey, Mexico, wrote in her conference abstract.
In related research presented at the APA meeting, researchers surveyed second-, third-, and fourth-year medical students from California during the pandemic.
Roughly 80% exhibited symptoms of anxiety and 68% exhibited depressive symptoms, of whom about 18% also reported having thoughts of suicide.
Yet only about half of the medical students exhibiting anxiety or depressive symptoms sought help from a mental health professional, and 20% reported using substances to cope with stress.
“Given that the pandemic is ongoing, we hope to draw attention to mental health needs of medical students and influence medical schools to direct appropriate and timely resources to this group,” presenter Sarthak Angal, MD, psychiatry resident, Kaiser Permanente San Jose Medical Center, California, wrote in his conference abstract.
Managing expectations
Weighing in on medical student burnout, Ihuoma Njoku, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, noted that, “particularly for women in multiple fields, including medicine, there’s a lot of burden placed on them.”
“Women are pulled in a lot of different directions and have increased demands, which may help explain their higher rate of burnout,” Dr. Njoku commented.
She noted that these surveys were conducted during the COVID-19 pandemic, “a period when students’ education experience was a lot different than what they expected and maybe what they wanted.”
Dr. Njoku noted that the challenges of the pandemic are particularly hard on fourth-year medical students.
“A big part of fourth year is applying to residency, and many were doing virtual interviews for residency. That makes it hard to really get an appreciation of the place you will spend the next three to eight years of your life,” she told this news organization.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
Researchers surveyed 613 medical students representing all years of a medical program during the last week of the Spring semester of 2021.
Based on the Maslach Burnout Inventory-Student Survey (MBI-SS), more than half (54%) of the students had symptoms of burnout.
Eighty percent of students scored high on emotional exhaustion, 57% scored high on cynicism, and 36% scored low on academic effectiveness.
Compared with male medical students, female medical students were more apt to exhibit signs of burnout (60% vs. 44%), emotional exhaustion (80% vs. 73%), and cynicism (62% vs. 49%).
After adjusting for associated factors, female medical students were significantly more likely to suffer from burnout than male students (odds ratio, 1.90; 95% confidence interval, 1.34-2.70; P < .001).
Smoking was also linked to higher likelihood of burnout among medical students (OR, 2.12; 95% CI, 1.18-3.81; P < .05). The death of a family member from COVID-19 also put medical students at heightened risk for burnout (OR, 1.60; 95% CI, 1.08-2.36; P < .05).
The survey results were presented at the American Psychiatric Association (APA) Annual Meeting.
The findings point to the need to study burnout prevalence in universities and develop strategies to promote the mental health of future physicians, presenter Sofia Jezzini-Martínez, fourth-year medical student, Autonomous University of Nuevo Leon, Monterrey, Mexico, wrote in her conference abstract.
In related research presented at the APA meeting, researchers surveyed second-, third-, and fourth-year medical students from California during the pandemic.
Roughly 80% exhibited symptoms of anxiety and 68% exhibited depressive symptoms, of whom about 18% also reported having thoughts of suicide.
Yet only about half of the medical students exhibiting anxiety or depressive symptoms sought help from a mental health professional, and 20% reported using substances to cope with stress.
“Given that the pandemic is ongoing, we hope to draw attention to mental health needs of medical students and influence medical schools to direct appropriate and timely resources to this group,” presenter Sarthak Angal, MD, psychiatry resident, Kaiser Permanente San Jose Medical Center, California, wrote in his conference abstract.
Managing expectations
Weighing in on medical student burnout, Ihuoma Njoku, MD, department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville, noted that, “particularly for women in multiple fields, including medicine, there’s a lot of burden placed on them.”
“Women are pulled in a lot of different directions and have increased demands, which may help explain their higher rate of burnout,” Dr. Njoku commented.
She noted that these surveys were conducted during the COVID-19 pandemic, “a period when students’ education experience was a lot different than what they expected and maybe what they wanted.”
Dr. Njoku noted that the challenges of the pandemic are particularly hard on fourth-year medical students.
“A big part of fourth year is applying to residency, and many were doing virtual interviews for residency. That makes it hard to really get an appreciation of the place you will spend the next three to eight years of your life,” she told this news organization.
A version of this article first appeared on Medscape.com.
FROM APA 2022
At-home vagus nerve stimulation promising for postpartum depression
At-home, noninvasive auricular vagus nerve stimulation (aVNS) therapy is well-tolerated and associated with a significant reduction in postpartum depressive and anxiety symptoms, new research suggests.
In a small proof-of-concept pilot study of 25 women with postpartum depression receiving 6 weeks of daily aVNS treatment, results showed that 74% achieved response and 61% achieved remission, as shown in reduced scores on the Hamilton Rating Scale for Depression (HAM-D17).
Although invasive electrical stimulation of the vagus nerve was approved by the U.S. Food and Drug Administration for treatment-resistant depression in 2005, it involves risk for implantation, infection, and significant side effects, coinvestigator Kristina M. Deligiannidis, MD, director, Women’s Behavioral Health, Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
“This newer approach, transcutaneous auricular VNS, is non-invasive, is well tolerated, and has shown initial efficacy in major depression in men and women,” she said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) Annual Meeting.
Potential alternative to meds
“Given that aVNS is a non-invasive treatment which can be administered at home, we wanted to test if this approach was safe, feasible, and could reduce depressive symptoms in women with postpartum depression, as many of these women have barriers to accessing current treatments,” Dr. Deligiannidis said.
Auricular VNS uses surface skin electrodes to stimulate nerve endings of a branch of the vagus nerve, located on the surface of the outer ear. Those nerve endings travel to the brain where they have been shown to modulate brain communication in areas important for mood and anxiety regulation, she said.
Dr. Deligiannidis noted that evidence-based treatments for postpartum depression include psychotherapies and antidepressants. However, some women have difficulty accessing weekly psychotherapy, and, when antidepressants are indicated, many are reluctant to take them if they are breastfeeding because of concerns about the medications getting into their breast milk, she said.
Although most antidepressants are safe in lactation, many women postpone antidepressant treatment until they have finished breastfeeding, which can postpone their postpartum depression treatment, Dr. Deligiannidis added.
“At home treatments reduce many barriers women have to current treatments, and this intervention [of aVNS] does not impact breastfeeding, as it is not a medication approach,” she said.
The researchers enrolled 25 women (mean age, 33.7 years) diagnosed with postpartum depression. Ten of the women (40%) were on a stable dose of antidepressant medication.
The participants self-administered 6 weeks of open-label aVNS for 15 minutes daily at home. They were then observed without intervention for an additional 2 weeks. The women also completed medical, psychiatric, and safety interviews throughout the study period.
Promising findings
At baseline, the mean HAM-D17 was 18.4 and was similar for those on (17.8) and off (18.9) antidepressants.
By week 6, the mean HAM-D17 total score decreased by 9.7 points overall, compared with baseline score. For participants on antidepressants, the HAM-D17 decreased by 8.7 points; for women off antidepressants, it decreased by 10.3 points.
In addition, 74% of the women achieved a response to the therapy, and 61% achieved remission of their depressive symptoms.
The most common adverse effects were discomfort (n = 5 patients), headache (n = 3), and dizziness (n = 2). All resolved without intervention.
Commenting on the findings, Anita Clayton, MD, professor and chair, department of psychiatry and neurobehavioral sciences, University of Virginia School of Medicine, Charlottesville, said the study was “quite interesting.”
Dr. Clayton, who was not involved with the research, also noted the “pretty high” response and remission rates.
“So, I think this does have promise, and it would be worth doing a study where you look at placebo versus this treatment,” she said.
“Many women are fearful of taking medicines postpartum, even peripartum, unless they have had pre-existing severe depression. This is not a medicine, and it sounds like it could be useful even in people who are pregnant, although it’s harder to do studies in pregnant women,” Dr. Clayton added.
The study was funded by Nesos Corporation. Dr. Deligiannidis received contracted research funds from Nesos Corporation to conduct this study. She also serves as a consultant to Sage Therapeutics, Brii Biosciences, and GH Research. Dr. Clayton reports financial relationships with Dare Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, AbbVie, Brii Biosciences, Fabre-Kramer, Field Trip Health, Mind Cure Health, Ovoca Bio, PureTech Health, S1 Biopharma, Takeda/Lundbeck, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, Guilford Publications, Euthymics Bioscience, and Mediflix.
A version of this article first appeared on Medscape.com.
At-home, noninvasive auricular vagus nerve stimulation (aVNS) therapy is well-tolerated and associated with a significant reduction in postpartum depressive and anxiety symptoms, new research suggests.
In a small proof-of-concept pilot study of 25 women with postpartum depression receiving 6 weeks of daily aVNS treatment, results showed that 74% achieved response and 61% achieved remission, as shown in reduced scores on the Hamilton Rating Scale for Depression (HAM-D17).
Although invasive electrical stimulation of the vagus nerve was approved by the U.S. Food and Drug Administration for treatment-resistant depression in 2005, it involves risk for implantation, infection, and significant side effects, coinvestigator Kristina M. Deligiannidis, MD, director, Women’s Behavioral Health, Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
“This newer approach, transcutaneous auricular VNS, is non-invasive, is well tolerated, and has shown initial efficacy in major depression in men and women,” she said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) Annual Meeting.
Potential alternative to meds
“Given that aVNS is a non-invasive treatment which can be administered at home, we wanted to test if this approach was safe, feasible, and could reduce depressive symptoms in women with postpartum depression, as many of these women have barriers to accessing current treatments,” Dr. Deligiannidis said.
Auricular VNS uses surface skin electrodes to stimulate nerve endings of a branch of the vagus nerve, located on the surface of the outer ear. Those nerve endings travel to the brain where they have been shown to modulate brain communication in areas important for mood and anxiety regulation, she said.
Dr. Deligiannidis noted that evidence-based treatments for postpartum depression include psychotherapies and antidepressants. However, some women have difficulty accessing weekly psychotherapy, and, when antidepressants are indicated, many are reluctant to take them if they are breastfeeding because of concerns about the medications getting into their breast milk, she said.
Although most antidepressants are safe in lactation, many women postpone antidepressant treatment until they have finished breastfeeding, which can postpone their postpartum depression treatment, Dr. Deligiannidis added.
“At home treatments reduce many barriers women have to current treatments, and this intervention [of aVNS] does not impact breastfeeding, as it is not a medication approach,” she said.
The researchers enrolled 25 women (mean age, 33.7 years) diagnosed with postpartum depression. Ten of the women (40%) were on a stable dose of antidepressant medication.
The participants self-administered 6 weeks of open-label aVNS for 15 minutes daily at home. They were then observed without intervention for an additional 2 weeks. The women also completed medical, psychiatric, and safety interviews throughout the study period.
Promising findings
At baseline, the mean HAM-D17 was 18.4 and was similar for those on (17.8) and off (18.9) antidepressants.
By week 6, the mean HAM-D17 total score decreased by 9.7 points overall, compared with baseline score. For participants on antidepressants, the HAM-D17 decreased by 8.7 points; for women off antidepressants, it decreased by 10.3 points.
In addition, 74% of the women achieved a response to the therapy, and 61% achieved remission of their depressive symptoms.
The most common adverse effects were discomfort (n = 5 patients), headache (n = 3), and dizziness (n = 2). All resolved without intervention.
Commenting on the findings, Anita Clayton, MD, professor and chair, department of psychiatry and neurobehavioral sciences, University of Virginia School of Medicine, Charlottesville, said the study was “quite interesting.”
Dr. Clayton, who was not involved with the research, also noted the “pretty high” response and remission rates.
“So, I think this does have promise, and it would be worth doing a study where you look at placebo versus this treatment,” she said.
“Many women are fearful of taking medicines postpartum, even peripartum, unless they have had pre-existing severe depression. This is not a medicine, and it sounds like it could be useful even in people who are pregnant, although it’s harder to do studies in pregnant women,” Dr. Clayton added.
The study was funded by Nesos Corporation. Dr. Deligiannidis received contracted research funds from Nesos Corporation to conduct this study. She also serves as a consultant to Sage Therapeutics, Brii Biosciences, and GH Research. Dr. Clayton reports financial relationships with Dare Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, AbbVie, Brii Biosciences, Fabre-Kramer, Field Trip Health, Mind Cure Health, Ovoca Bio, PureTech Health, S1 Biopharma, Takeda/Lundbeck, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, Guilford Publications, Euthymics Bioscience, and Mediflix.
A version of this article first appeared on Medscape.com.
At-home, noninvasive auricular vagus nerve stimulation (aVNS) therapy is well-tolerated and associated with a significant reduction in postpartum depressive and anxiety symptoms, new research suggests.
In a small proof-of-concept pilot study of 25 women with postpartum depression receiving 6 weeks of daily aVNS treatment, results showed that 74% achieved response and 61% achieved remission, as shown in reduced scores on the Hamilton Rating Scale for Depression (HAM-D17).
Although invasive electrical stimulation of the vagus nerve was approved by the U.S. Food and Drug Administration for treatment-resistant depression in 2005, it involves risk for implantation, infection, and significant side effects, coinvestigator Kristina M. Deligiannidis, MD, director, Women’s Behavioral Health, Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
“This newer approach, transcutaneous auricular VNS, is non-invasive, is well tolerated, and has shown initial efficacy in major depression in men and women,” she said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) Annual Meeting.
Potential alternative to meds
“Given that aVNS is a non-invasive treatment which can be administered at home, we wanted to test if this approach was safe, feasible, and could reduce depressive symptoms in women with postpartum depression, as many of these women have barriers to accessing current treatments,” Dr. Deligiannidis said.
Auricular VNS uses surface skin electrodes to stimulate nerve endings of a branch of the vagus nerve, located on the surface of the outer ear. Those nerve endings travel to the brain where they have been shown to modulate brain communication in areas important for mood and anxiety regulation, she said.
Dr. Deligiannidis noted that evidence-based treatments for postpartum depression include psychotherapies and antidepressants. However, some women have difficulty accessing weekly psychotherapy, and, when antidepressants are indicated, many are reluctant to take them if they are breastfeeding because of concerns about the medications getting into their breast milk, she said.
Although most antidepressants are safe in lactation, many women postpone antidepressant treatment until they have finished breastfeeding, which can postpone their postpartum depression treatment, Dr. Deligiannidis added.
“At home treatments reduce many barriers women have to current treatments, and this intervention [of aVNS] does not impact breastfeeding, as it is not a medication approach,” she said.
The researchers enrolled 25 women (mean age, 33.7 years) diagnosed with postpartum depression. Ten of the women (40%) were on a stable dose of antidepressant medication.
The participants self-administered 6 weeks of open-label aVNS for 15 minutes daily at home. They were then observed without intervention for an additional 2 weeks. The women also completed medical, psychiatric, and safety interviews throughout the study period.
Promising findings
At baseline, the mean HAM-D17 was 18.4 and was similar for those on (17.8) and off (18.9) antidepressants.
By week 6, the mean HAM-D17 total score decreased by 9.7 points overall, compared with baseline score. For participants on antidepressants, the HAM-D17 decreased by 8.7 points; for women off antidepressants, it decreased by 10.3 points.
In addition, 74% of the women achieved a response to the therapy, and 61% achieved remission of their depressive symptoms.
The most common adverse effects were discomfort (n = 5 patients), headache (n = 3), and dizziness (n = 2). All resolved without intervention.
Commenting on the findings, Anita Clayton, MD, professor and chair, department of psychiatry and neurobehavioral sciences, University of Virginia School of Medicine, Charlottesville, said the study was “quite interesting.”
Dr. Clayton, who was not involved with the research, also noted the “pretty high” response and remission rates.
“So, I think this does have promise, and it would be worth doing a study where you look at placebo versus this treatment,” she said.
“Many women are fearful of taking medicines postpartum, even peripartum, unless they have had pre-existing severe depression. This is not a medicine, and it sounds like it could be useful even in people who are pregnant, although it’s harder to do studies in pregnant women,” Dr. Clayton added.
The study was funded by Nesos Corporation. Dr. Deligiannidis received contracted research funds from Nesos Corporation to conduct this study. She also serves as a consultant to Sage Therapeutics, Brii Biosciences, and GH Research. Dr. Clayton reports financial relationships with Dare Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, AbbVie, Brii Biosciences, Fabre-Kramer, Field Trip Health, Mind Cure Health, Ovoca Bio, PureTech Health, S1 Biopharma, Takeda/Lundbeck, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, Guilford Publications, Euthymics Bioscience, and Mediflix.
A version of this article first appeared on Medscape.com.
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.







