User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
A purple warrior rises in the battle against diabetes
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
Trends in HPV vaccination among adults aged 27 to 45 years
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?
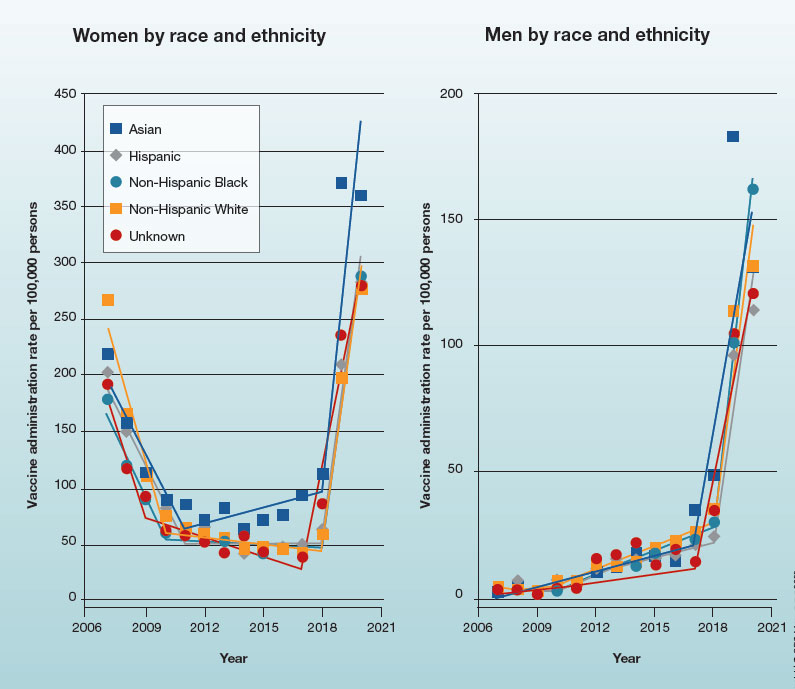
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

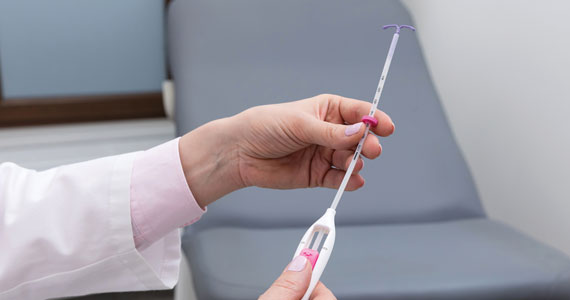
How to place an IUD with minimal patient discomfort
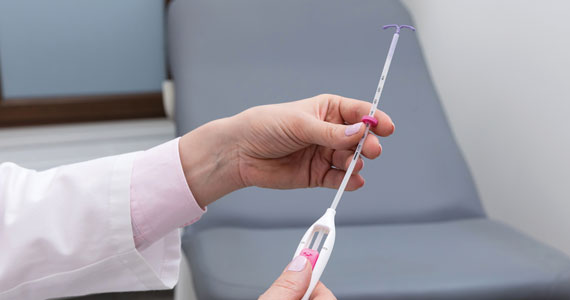
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
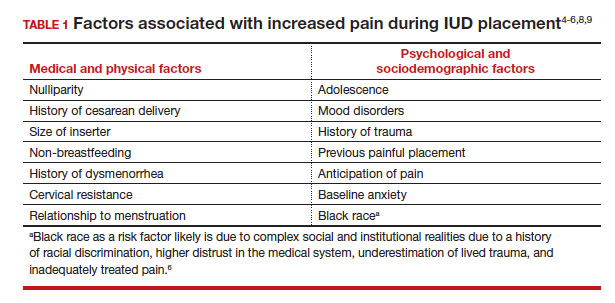
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
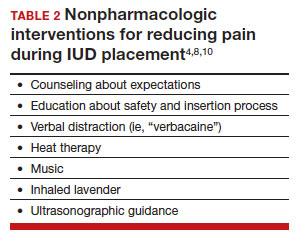
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
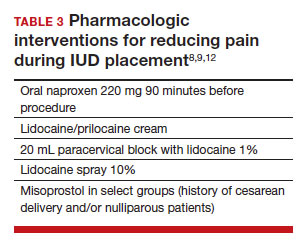
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
Postpartum urinary retention: Intermittent catheterization may be best
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
FROM THE PREGNANCY MEETING
Physician group staffing down, expenses up, new reports show
Physician groups saw staff-to-physician ratios decline even as their workforce expenses rose between 2019 and 2021, according to recent reports from the American Medical Group Association (AMGA) and the Medical Group Management Association (MGMA).
As patients started to return to doctors’ offices as the pandemic eased in 2021, physician groups found it increasingly difficult to recruit and retain lower-level clinicians, including medical assistants and LPNs, officials from both associations told this news organization. Many clinics had to raise their pay scales to be competitive with employers in other fields, and some had to hire higher-priced RNs to keep their practices running.
The AMGA report was based largely on data from groups of over 500 physicians, mostly affiliated with health systems. According to a news release accompanying the report, the ratio between full-time equivalent (FTE) clinic staff and health care professionals in direct patient care dropped by 11.3% between 2019 and 2021. The ratio of medical assistants (MAs) to clinicians declined by a greater percentage.
In the MGMA report, which represented about 4,000 practices ranging from very small (two doctors) to very large groups, total support staff per FTE primary-care physician dropped by 18% from 2019 to 2021 in independent groups and by 13% in hospital-affiliated groups. The ratios decreased by smaller amounts in surgical practices.
In contrast, nonsurgical specialty groups under both types of ownership saw their staffing ratios rise slightly.
Although it’s unclear why medical specialties increased their staff while other types of specialties lost employees, Ron Holder, MHA, chief operating officer of MGMA, said that some specialists may have opened more ancillary facilities and hired new employees to recoup revenue lost during the pandemic.
Expenses rise sharply
The AMGA report found that staffing expenses for the surveyed groups increased by 15% between 2019 and 2021.
“We saw a decrease in staff and an increase in expenses during that time period, and there are a few reasons for that,” Rose Wagner, RN, chief operating officer of AMGA, said. “Groups increased salaries to maintain staff. We also saw lower-paid staff find other jobs outside of health care. For example, medical assistants and receptionists could find jobs outside of health care that paid more. [Open positions] got back-filled with other higher paid staff, such as RNs, doing lower skilled jobs.”
Mr. Holder added that rising wages in other sectors made leaving physician groups more attractive for employees.
“Three years ago, there weren’t many positions in a medical practice where you were competing with Chick-fil-A or Taco Bell,” he said. In Denver, where Mr. Holder is based, “every restaurant in town is now advertising $17-$19 [hourly] starting pay just to do fast food. That causes practices to either lose employees or pay more for the employees they have. So that raises per-employee expense significantly,” he said.
Mr. Holder noted that inflation also has driven up wages as employees demand higher pay to keep up with the cost of living.
Unusual exodus of employees
Fred Horton, MHA, president of AMGA Consulting, said he has never seen so many people leaving health care for other occupations.
Some exits resulted from practices laying people off early in the pandemic, but most staff members who left practices were seeking higher pay, he said. In addition, Ms. Wagner noted, some staff members didn’t want to be exposed to COVID at work.
“There was an exodus from health care that was different from what we’d experienced in the past,” Mr. Horton added. “It’s still extremely challenging to get up to the staffing levels that are appropriate.”
Mr. Holder, however, said that the situation is slowly improving. “Health care is fairly recession-proof, because people need it. So when you see companies in other industries closing shop or reducing their head count, that actually helps health care recruiting in some jobs. And people are coming back to the workplace who previously were worried about COVID or didn’t want to get the vaccine.”
Paying more for nurses
In 2021, groups adopted a variety of tactics to adapt to the pandemic and respond to patient demand, the AMGA survey shows. Forty percent of system-affiliated groups and 18% of independent practices changed registered nurses’ responsibilities, in many cases having them do the work of medical assistants who were in short supply.
Some practices hired RNs, who have historically been utilized less by primary care than by surgical specialties, Mr. Holder noted. Other clinics paid temp agencies to supply nurses at a steep cost.
“When you’re short staffed, you end up paying more overtime, you end up paying temporary agencies at higher dollars, and you hire higher skilled people to do lower-skilled work,” Ms. Wagner said.
Meanwhile, many physician groups tried to cope with the physician shortage by bringing on more advanced practice clinicians (APCs), including nurse practitioners (NPs) and physician assistants (PAs). Seventy percent of the AMGA groups used this strategy, the report revealed.
“The use of APCs has been steadily increasing as groups try to adopt a lower-cost care model in the midst of a nationwide physician shortage,” Ms. Wagner said in the press release.
Changes in patient care
About half of the groups in the AMGA survey said they changed their staff structure to allow APCs to carry their own patient panels. Although most of these clinicians were probably under physician supervision, nearly half of the states now allow NPs to practice autonomously.
Mr. Horton cautioned that APCs can’t fully substitute for physicians and require the same support staff that doctors do if they have their own panels. In primary care groups, Mr. Holder noted, the average salary of an APC “is continuing to rise, and there isn’t a huge difference between what they and doctors make.”
Nevertheless, he added, “there are more NPs and PAs being added to the marketplace all the time, whereas [physician] residency programs aren’t really growing. There are caps on the number of residency positions, and some physicians are retiring. So the clock is ticking to the point where someday doctors will be grossly outnumbered by NPs.”
A version of this article first appeared on Medscape.com.
Physician groups saw staff-to-physician ratios decline even as their workforce expenses rose between 2019 and 2021, according to recent reports from the American Medical Group Association (AMGA) and the Medical Group Management Association (MGMA).
As patients started to return to doctors’ offices as the pandemic eased in 2021, physician groups found it increasingly difficult to recruit and retain lower-level clinicians, including medical assistants and LPNs, officials from both associations told this news organization. Many clinics had to raise their pay scales to be competitive with employers in other fields, and some had to hire higher-priced RNs to keep their practices running.
The AMGA report was based largely on data from groups of over 500 physicians, mostly affiliated with health systems. According to a news release accompanying the report, the ratio between full-time equivalent (FTE) clinic staff and health care professionals in direct patient care dropped by 11.3% between 2019 and 2021. The ratio of medical assistants (MAs) to clinicians declined by a greater percentage.
In the MGMA report, which represented about 4,000 practices ranging from very small (two doctors) to very large groups, total support staff per FTE primary-care physician dropped by 18% from 2019 to 2021 in independent groups and by 13% in hospital-affiliated groups. The ratios decreased by smaller amounts in surgical practices.
In contrast, nonsurgical specialty groups under both types of ownership saw their staffing ratios rise slightly.
Although it’s unclear why medical specialties increased their staff while other types of specialties lost employees, Ron Holder, MHA, chief operating officer of MGMA, said that some specialists may have opened more ancillary facilities and hired new employees to recoup revenue lost during the pandemic.
Expenses rise sharply
The AMGA report found that staffing expenses for the surveyed groups increased by 15% between 2019 and 2021.
“We saw a decrease in staff and an increase in expenses during that time period, and there are a few reasons for that,” Rose Wagner, RN, chief operating officer of AMGA, said. “Groups increased salaries to maintain staff. We also saw lower-paid staff find other jobs outside of health care. For example, medical assistants and receptionists could find jobs outside of health care that paid more. [Open positions] got back-filled with other higher paid staff, such as RNs, doing lower skilled jobs.”
Mr. Holder added that rising wages in other sectors made leaving physician groups more attractive for employees.
“Three years ago, there weren’t many positions in a medical practice where you were competing with Chick-fil-A or Taco Bell,” he said. In Denver, where Mr. Holder is based, “every restaurant in town is now advertising $17-$19 [hourly] starting pay just to do fast food. That causes practices to either lose employees or pay more for the employees they have. So that raises per-employee expense significantly,” he said.
Mr. Holder noted that inflation also has driven up wages as employees demand higher pay to keep up with the cost of living.
Unusual exodus of employees
Fred Horton, MHA, president of AMGA Consulting, said he has never seen so many people leaving health care for other occupations.
Some exits resulted from practices laying people off early in the pandemic, but most staff members who left practices were seeking higher pay, he said. In addition, Ms. Wagner noted, some staff members didn’t want to be exposed to COVID at work.
“There was an exodus from health care that was different from what we’d experienced in the past,” Mr. Horton added. “It’s still extremely challenging to get up to the staffing levels that are appropriate.”
Mr. Holder, however, said that the situation is slowly improving. “Health care is fairly recession-proof, because people need it. So when you see companies in other industries closing shop or reducing their head count, that actually helps health care recruiting in some jobs. And people are coming back to the workplace who previously were worried about COVID or didn’t want to get the vaccine.”
Paying more for nurses
In 2021, groups adopted a variety of tactics to adapt to the pandemic and respond to patient demand, the AMGA survey shows. Forty percent of system-affiliated groups and 18% of independent practices changed registered nurses’ responsibilities, in many cases having them do the work of medical assistants who were in short supply.
Some practices hired RNs, who have historically been utilized less by primary care than by surgical specialties, Mr. Holder noted. Other clinics paid temp agencies to supply nurses at a steep cost.
“When you’re short staffed, you end up paying more overtime, you end up paying temporary agencies at higher dollars, and you hire higher skilled people to do lower-skilled work,” Ms. Wagner said.
Meanwhile, many physician groups tried to cope with the physician shortage by bringing on more advanced practice clinicians (APCs), including nurse practitioners (NPs) and physician assistants (PAs). Seventy percent of the AMGA groups used this strategy, the report revealed.
“The use of APCs has been steadily increasing as groups try to adopt a lower-cost care model in the midst of a nationwide physician shortage,” Ms. Wagner said in the press release.
Changes in patient care
About half of the groups in the AMGA survey said they changed their staff structure to allow APCs to carry their own patient panels. Although most of these clinicians were probably under physician supervision, nearly half of the states now allow NPs to practice autonomously.
Mr. Horton cautioned that APCs can’t fully substitute for physicians and require the same support staff that doctors do if they have their own panels. In primary care groups, Mr. Holder noted, the average salary of an APC “is continuing to rise, and there isn’t a huge difference between what they and doctors make.”
Nevertheless, he added, “there are more NPs and PAs being added to the marketplace all the time, whereas [physician] residency programs aren’t really growing. There are caps on the number of residency positions, and some physicians are retiring. So the clock is ticking to the point where someday doctors will be grossly outnumbered by NPs.”
A version of this article first appeared on Medscape.com.
Physician groups saw staff-to-physician ratios decline even as their workforce expenses rose between 2019 and 2021, according to recent reports from the American Medical Group Association (AMGA) and the Medical Group Management Association (MGMA).
As patients started to return to doctors’ offices as the pandemic eased in 2021, physician groups found it increasingly difficult to recruit and retain lower-level clinicians, including medical assistants and LPNs, officials from both associations told this news organization. Many clinics had to raise their pay scales to be competitive with employers in other fields, and some had to hire higher-priced RNs to keep their practices running.
The AMGA report was based largely on data from groups of over 500 physicians, mostly affiliated with health systems. According to a news release accompanying the report, the ratio between full-time equivalent (FTE) clinic staff and health care professionals in direct patient care dropped by 11.3% between 2019 and 2021. The ratio of medical assistants (MAs) to clinicians declined by a greater percentage.
In the MGMA report, which represented about 4,000 practices ranging from very small (two doctors) to very large groups, total support staff per FTE primary-care physician dropped by 18% from 2019 to 2021 in independent groups and by 13% in hospital-affiliated groups. The ratios decreased by smaller amounts in surgical practices.
In contrast, nonsurgical specialty groups under both types of ownership saw their staffing ratios rise slightly.
Although it’s unclear why medical specialties increased their staff while other types of specialties lost employees, Ron Holder, MHA, chief operating officer of MGMA, said that some specialists may have opened more ancillary facilities and hired new employees to recoup revenue lost during the pandemic.
Expenses rise sharply
The AMGA report found that staffing expenses for the surveyed groups increased by 15% between 2019 and 2021.
“We saw a decrease in staff and an increase in expenses during that time period, and there are a few reasons for that,” Rose Wagner, RN, chief operating officer of AMGA, said. “Groups increased salaries to maintain staff. We also saw lower-paid staff find other jobs outside of health care. For example, medical assistants and receptionists could find jobs outside of health care that paid more. [Open positions] got back-filled with other higher paid staff, such as RNs, doing lower skilled jobs.”
Mr. Holder added that rising wages in other sectors made leaving physician groups more attractive for employees.
“Three years ago, there weren’t many positions in a medical practice where you were competing with Chick-fil-A or Taco Bell,” he said. In Denver, where Mr. Holder is based, “every restaurant in town is now advertising $17-$19 [hourly] starting pay just to do fast food. That causes practices to either lose employees or pay more for the employees they have. So that raises per-employee expense significantly,” he said.
Mr. Holder noted that inflation also has driven up wages as employees demand higher pay to keep up with the cost of living.
Unusual exodus of employees
Fred Horton, MHA, president of AMGA Consulting, said he has never seen so many people leaving health care for other occupations.
Some exits resulted from practices laying people off early in the pandemic, but most staff members who left practices were seeking higher pay, he said. In addition, Ms. Wagner noted, some staff members didn’t want to be exposed to COVID at work.
“There was an exodus from health care that was different from what we’d experienced in the past,” Mr. Horton added. “It’s still extremely challenging to get up to the staffing levels that are appropriate.”
Mr. Holder, however, said that the situation is slowly improving. “Health care is fairly recession-proof, because people need it. So when you see companies in other industries closing shop or reducing their head count, that actually helps health care recruiting in some jobs. And people are coming back to the workplace who previously were worried about COVID or didn’t want to get the vaccine.”
Paying more for nurses
In 2021, groups adopted a variety of tactics to adapt to the pandemic and respond to patient demand, the AMGA survey shows. Forty percent of system-affiliated groups and 18% of independent practices changed registered nurses’ responsibilities, in many cases having them do the work of medical assistants who were in short supply.
Some practices hired RNs, who have historically been utilized less by primary care than by surgical specialties, Mr. Holder noted. Other clinics paid temp agencies to supply nurses at a steep cost.
“When you’re short staffed, you end up paying more overtime, you end up paying temporary agencies at higher dollars, and you hire higher skilled people to do lower-skilled work,” Ms. Wagner said.
Meanwhile, many physician groups tried to cope with the physician shortage by bringing on more advanced practice clinicians (APCs), including nurse practitioners (NPs) and physician assistants (PAs). Seventy percent of the AMGA groups used this strategy, the report revealed.
“The use of APCs has been steadily increasing as groups try to adopt a lower-cost care model in the midst of a nationwide physician shortage,” Ms. Wagner said in the press release.
Changes in patient care
About half of the groups in the AMGA survey said they changed their staff structure to allow APCs to carry their own patient panels. Although most of these clinicians were probably under physician supervision, nearly half of the states now allow NPs to practice autonomously.
Mr. Horton cautioned that APCs can’t fully substitute for physicians and require the same support staff that doctors do if they have their own panels. In primary care groups, Mr. Holder noted, the average salary of an APC “is continuing to rise, and there isn’t a huge difference between what they and doctors make.”
Nevertheless, he added, “there are more NPs and PAs being added to the marketplace all the time, whereas [physician] residency programs aren’t really growing. There are caps on the number of residency positions, and some physicians are retiring. So the clock is ticking to the point where someday doctors will be grossly outnumbered by NPs.”
A version of this article first appeared on Medscape.com.
Doctors and their families tend to ignore medical guidelines
according to a study by economic professors from the Massachusetts Institute of Technology, Cambridge; Stanford (Calif.) University; and the George Gund Professor of Economics and Business Administration at Harvard University, Boston.
What to know
- Doctors’ medical knowledge may influence them and their families to often ignore medical advice while the rest of the population adheres to general medication guidelines.
- Of the 63 guidelines used in the study, doctors and their families followed the standards less than a third of the time.
- The difference in adherence to guidelines between experts and nonexperts is largest with respect to antibiotics, in which doctors and their families are 5.2 percentage points less in compliance than everyone else.
- Doctors could be more likely to prescribe broader-spectrum antibiotics for themselves and their families, whereas most patients receive more narrow-spectrum antibiotics.
- Many members of the general public don’t understand medical guidelines, finding them too complex to follow, and many people don’t trust their doctors.
This is a summary of the article, “A Taste of Their Own Medicine: Guideline Adherence and Access to Expertise,” published in the American Economic Review: Insights on December 13, 2022. The full article can be found on aeaweb.org.
A version of this article first appeared on Medscape.com.
according to a study by economic professors from the Massachusetts Institute of Technology, Cambridge; Stanford (Calif.) University; and the George Gund Professor of Economics and Business Administration at Harvard University, Boston.
What to know
- Doctors’ medical knowledge may influence them and their families to often ignore medical advice while the rest of the population adheres to general medication guidelines.
- Of the 63 guidelines used in the study, doctors and their families followed the standards less than a third of the time.
- The difference in adherence to guidelines between experts and nonexperts is largest with respect to antibiotics, in which doctors and their families are 5.2 percentage points less in compliance than everyone else.
- Doctors could be more likely to prescribe broader-spectrum antibiotics for themselves and their families, whereas most patients receive more narrow-spectrum antibiotics.
- Many members of the general public don’t understand medical guidelines, finding them too complex to follow, and many people don’t trust their doctors.
This is a summary of the article, “A Taste of Their Own Medicine: Guideline Adherence and Access to Expertise,” published in the American Economic Review: Insights on December 13, 2022. The full article can be found on aeaweb.org.
A version of this article first appeared on Medscape.com.
according to a study by economic professors from the Massachusetts Institute of Technology, Cambridge; Stanford (Calif.) University; and the George Gund Professor of Economics and Business Administration at Harvard University, Boston.
What to know
- Doctors’ medical knowledge may influence them and their families to often ignore medical advice while the rest of the population adheres to general medication guidelines.
- Of the 63 guidelines used in the study, doctors and their families followed the standards less than a third of the time.
- The difference in adherence to guidelines between experts and nonexperts is largest with respect to antibiotics, in which doctors and their families are 5.2 percentage points less in compliance than everyone else.
- Doctors could be more likely to prescribe broader-spectrum antibiotics for themselves and their families, whereas most patients receive more narrow-spectrum antibiotics.
- Many members of the general public don’t understand medical guidelines, finding them too complex to follow, and many people don’t trust their doctors.
This is a summary of the article, “A Taste of Their Own Medicine: Guideline Adherence and Access to Expertise,” published in the American Economic Review: Insights on December 13, 2022. The full article can be found on aeaweb.org.
A version of this article first appeared on Medscape.com.
Docs with one paid malpractice claim are four times more likely to have another
In this retrospective case-control study, law and public health researchers from Georgetown University, the National Opinion Research Center, the University of Colorado, and Northwestern University analyzed paid malpractice claims for all licensed U.S. physicians.
The findings suggest that a single malpractice claim may not be a random stroke of bad luck but instead holds some predictive power into the risk for future paid claims.
“A four times increase in risk is huge, particularly since we observe a similar increase in both high-risk and lower-risk specialties,” David Hyman, JD, MD, professor of health law and policy at Georgetown University, Washington, and lead researcher on the study, told this news organization. “There are surely some false positives, but there must be lots of actual negligence too, or we would not see these results.”
For the 881,876 physicians analyzed, researchers looked at malpractice claims paid during two 5-year periods: 2009-2013 and 2014-2018. Nearly 96% of physicians had no paid malpractice claims between 2009 and 2013; 3% had one, and less than 1% had multiple claims. The proportion of physicians with paid claims between 2014 and 2018 was similar.
Compared with physicians with no 2009-2013 claims, a physician with just one paid claim in that time period had a 3.7 times higher risk for a future paid claim. Physicians with two paid claims were nearly 7 times more likely to have a future paid claim, and those with three or more paid claims were more than 11 times more likely to have one.
Approximately 3% of physicians with no paid claims between 2009 and 2013 had a future paid claim, growing to 12.4% of those with one paid claim during that time.
The study’s findings may have implications for medical licensing boards and hospitals granting staff privileges.
“After some number of paid claims, there should be an official response” from these entities, such as a hands-on assessment of technical skills or assignment of a peer mentor, said Dr. Hyman, who is also coauthor of a book titled “Medical Malpractice Litigation: How It Works, Why Tort Reform Hasn’t Helped.” A graduated set of interventions, whether voluntary or mandatory, can reduce future claim risk and patient harm, Dr. Hyman added.
Interventions may include error avoidance and post-error communication training, counseling to improve bedside skills, and encouragement to move into nonclinical practice. Either way, Dr. Hyman says a nuanced intervention strategy would be a welcome shift away from the current “all or nothing approach” that too often ends in the revocation of a physician’s medical license.
Although there are strategies to proactively identify physicians with excess risk for malpractice claims and implement preventive measures – like Vanderbilt University’s Patient Advocacy Reporting System, for example – most hospitals and physician groups fail to initiate even informal interventions after a malpractice settlement or verdict, which is a missed opportunity, Dr. Hyman said.
A version of this article first appeared on Medscape.com.
In this retrospective case-control study, law and public health researchers from Georgetown University, the National Opinion Research Center, the University of Colorado, and Northwestern University analyzed paid malpractice claims for all licensed U.S. physicians.
The findings suggest that a single malpractice claim may not be a random stroke of bad luck but instead holds some predictive power into the risk for future paid claims.
“A four times increase in risk is huge, particularly since we observe a similar increase in both high-risk and lower-risk specialties,” David Hyman, JD, MD, professor of health law and policy at Georgetown University, Washington, and lead researcher on the study, told this news organization. “There are surely some false positives, but there must be lots of actual negligence too, or we would not see these results.”
For the 881,876 physicians analyzed, researchers looked at malpractice claims paid during two 5-year periods: 2009-2013 and 2014-2018. Nearly 96% of physicians had no paid malpractice claims between 2009 and 2013; 3% had one, and less than 1% had multiple claims. The proportion of physicians with paid claims between 2014 and 2018 was similar.
Compared with physicians with no 2009-2013 claims, a physician with just one paid claim in that time period had a 3.7 times higher risk for a future paid claim. Physicians with two paid claims were nearly 7 times more likely to have a future paid claim, and those with three or more paid claims were more than 11 times more likely to have one.
Approximately 3% of physicians with no paid claims between 2009 and 2013 had a future paid claim, growing to 12.4% of those with one paid claim during that time.
The study’s findings may have implications for medical licensing boards and hospitals granting staff privileges.
“After some number of paid claims, there should be an official response” from these entities, such as a hands-on assessment of technical skills or assignment of a peer mentor, said Dr. Hyman, who is also coauthor of a book titled “Medical Malpractice Litigation: How It Works, Why Tort Reform Hasn’t Helped.” A graduated set of interventions, whether voluntary or mandatory, can reduce future claim risk and patient harm, Dr. Hyman added.
Interventions may include error avoidance and post-error communication training, counseling to improve bedside skills, and encouragement to move into nonclinical practice. Either way, Dr. Hyman says a nuanced intervention strategy would be a welcome shift away from the current “all or nothing approach” that too often ends in the revocation of a physician’s medical license.
Although there are strategies to proactively identify physicians with excess risk for malpractice claims and implement preventive measures – like Vanderbilt University’s Patient Advocacy Reporting System, for example – most hospitals and physician groups fail to initiate even informal interventions after a malpractice settlement or verdict, which is a missed opportunity, Dr. Hyman said.
A version of this article first appeared on Medscape.com.
In this retrospective case-control study, law and public health researchers from Georgetown University, the National Opinion Research Center, the University of Colorado, and Northwestern University analyzed paid malpractice claims for all licensed U.S. physicians.
The findings suggest that a single malpractice claim may not be a random stroke of bad luck but instead holds some predictive power into the risk for future paid claims.
“A four times increase in risk is huge, particularly since we observe a similar increase in both high-risk and lower-risk specialties,” David Hyman, JD, MD, professor of health law and policy at Georgetown University, Washington, and lead researcher on the study, told this news organization. “There are surely some false positives, but there must be lots of actual negligence too, or we would not see these results.”
For the 881,876 physicians analyzed, researchers looked at malpractice claims paid during two 5-year periods: 2009-2013 and 2014-2018. Nearly 96% of physicians had no paid malpractice claims between 2009 and 2013; 3% had one, and less than 1% had multiple claims. The proportion of physicians with paid claims between 2014 and 2018 was similar.
Compared with physicians with no 2009-2013 claims, a physician with just one paid claim in that time period had a 3.7 times higher risk for a future paid claim. Physicians with two paid claims were nearly 7 times more likely to have a future paid claim, and those with three or more paid claims were more than 11 times more likely to have one.
Approximately 3% of physicians with no paid claims between 2009 and 2013 had a future paid claim, growing to 12.4% of those with one paid claim during that time.
The study’s findings may have implications for medical licensing boards and hospitals granting staff privileges.
“After some number of paid claims, there should be an official response” from these entities, such as a hands-on assessment of technical skills or assignment of a peer mentor, said Dr. Hyman, who is also coauthor of a book titled “Medical Malpractice Litigation: How It Works, Why Tort Reform Hasn’t Helped.” A graduated set of interventions, whether voluntary or mandatory, can reduce future claim risk and patient harm, Dr. Hyman added.
Interventions may include error avoidance and post-error communication training, counseling to improve bedside skills, and encouragement to move into nonclinical practice. Either way, Dr. Hyman says a nuanced intervention strategy would be a welcome shift away from the current “all or nothing approach” that too often ends in the revocation of a physician’s medical license.
Although there are strategies to proactively identify physicians with excess risk for malpractice claims and implement preventive measures – like Vanderbilt University’s Patient Advocacy Reporting System, for example – most hospitals and physician groups fail to initiate even informal interventions after a malpractice settlement or verdict, which is a missed opportunity, Dr. Hyman said.
A version of this article first appeared on Medscape.com.
FROM JAMA
Zika virus still calls for preparedness and vaccine development
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Are ‘Momi Pods’ the future of postnatal care?
Mindi Rosen met Seuli Brill, MD, at just the right time.
Ms. Rosen’s firstborn son was in the neointensive natal unit at The Ohio State University Wexner Medical Center in Columbus, and she didn’t have a pediatrician picked out yet for the baby. Nor did she have a primary care physician who could help her manage the gestational diabetes she developed during her pregnancy.
Dr. Brill, a clinical associate professor of internal medicine and pediatrics at Ohio State, suggested Ms. Rosen visit her at the new clinic she was piloting in Columbus. There, she provided pediatric care for newborns and primary care for mothers who had developed gestational diabetes.
“I looked at my husband, my husband looked at me, and I said: ‘Why not?’ “ Ms. Rosen, 38, recalled of that 2019 meeting. “I’m so glad she walked in at that moment.”
The mother of two is still part of the rapidly growing program at the medical facility that provides care for more than 200 mothers and babies.
Launched in 2018, the clinic – called the Multi-Modal Maternal Infant Perinatal Outpatient Delivery System, or “Momi Pods,” started with a focus on helping women with gestational diabetes, which occurs in up to 10% of pregnancies.
The program allows moms to book regular checkups for their baby, and then a follow-up appointment immediately after for themselves. Women are seen for the first 1,000 days (just under 3 years) after giving birth.
The idea was simple. Dr. Brill wanted to develop a more formalized program for the work she was already doing as a primary care physician and pediatrician. At the time, she was fielding referrals from specialists for young women who didn’t have a physician. She’d often develop a relationship with the patient over the years, go on to help oversee their care during pregnancy, then new mothers would select her as their newborn’s pediatrician.
“I would have a relationship with the mom when they did have the newborn, and then I would see the baby because I’m a pediatrician,” Dr. Brill said.
Dr. Brill was serving on the Ohio Gestational Diabetes Mellitus Collaborative, a state-backed program that aims to raise awareness about the condition and encourage more preventative care for patients. She presented her proposal to launch the program to the Ohio Department of Medicaid, which helped to fund the pilot.
The idea, she hoped, would improve postpartum follow-up care for mothers diagnosed with the condition.
Follow-up care is especially important for women who develop gestational diabetes because the condition raises their lifetime risk of developing type 2 diabetes up to 10-fold.
Yet most of those mothers don’t get the appropriate follow-up care during the crucial postpartum period, said Maya Subbalakshmi Venkataramani, MD, MPH, an assistant professor of medicine at Johns Hopkins University in Baltimore, who has researched parental care.
“Things get very busy after you have a child. There’s just the general logistics of a mom having to take care of a newborn and thinking about themselves,” Dr. Venkataramani, a primary care clinician and pediatrician, said. “A lot of parents in general may not put a lot of emphasis on their own health.”
Seeking care may be especially difficult for low-income mothers who might not have consistent health care coverage, she added.
In fact, only half of women who developed gestational diabetes received primary follow-up care, according to a study published in JAMA Network Open. The study, which examined more than 280,000 insurance claims between 2015 and 2018, found only 36% of women with gestational diabetes received the recommended blood glucose testing in the first 12 weeks of the postpartum period.
In the Momi Pods program, Dr. Brill checked in on Ms. Rosen’s gestational diabetes regularly during pediatric office visits for her newborn’s care. Ms. Rosen said whenever she brought her baby in for a visit during the postpartum period, Dr. Brill measured her blood sugar.
Dr. Brill and her team also asked how Ms. Rosen was doing physically and mentally during each visit. The screenings helped to catch a bout of postpartum depression Ms. Rosen experienced after the birth of her first son.
“I thought it was great, because honestly as a new mom I wouldn’t have followed up with myself so much,” Ms. Rosen said. “Every time you went into the doctor appointments, they’d ask you how you are doing. As a new mom, it’s so much easier to do it at the same time.”
Those who participate in the program are also more likely to complete postpartum visits with their ob.gyn. (95% vs. 58%, respectively; P < .001) than those who don’t participate, according to research Dr. Brill and colleagues published.
Dr. Brill began expanding the program’s reach nearly 2 years after its launch, targeting the services for women who are at risk for poor postpartum outcomes, including those with a history of depression, preterm labor, diabetes and congenital heart disease. Ob.gyns. in Ohio State’s network can refer their patients to the program, which now has 43 doctors trained to provide primary and pediatric care through Momi Pods. Soon-to-be moms can be referred to the program as early as the second trimester, Dr. Brill said.
Many of the mothers referred to the program don’t have a primary care clinician when they talk to Paola Beamon, RN, at Ohio State. Ms. Beamon reaches out to each referred patient over voicemail, a MyChart message, and even regular mail in hopes of helping them navigate the postpartum period. She also provides education on what a primary care clinician can offer new moms.
“Really, we’re pursuing these moms and doing everything we can so there’s less of a burden for them,” Ms. Beamon said. “A lot of them don’t even know what a primary care office does.”
One of the biggest perks to the program for new moms is that they don’t have to spend time and money traveling to a different doctor’s office, take time off work, or secure childcare in order to schedule a separate appointment for themselves, she said.
The program, which receives funding from the university and the state, even helps women get bus passes to a doctor’s appointment if needed.
Dyad programs targeting women with substance abuse disorders or mental health conditions have existed for many years. But catering to women with gestational diabetes or other medical conditions appears to be new. In part, Dr. Venkataramani said, because scheduling and space can be big hurdles to launch such a program, as well as finding doctors who can care for both baby and mother.
“There are logistical challenges to even doing this that makes it less common,” she said.
Dr. Brill said she is not aware of any other programs that are structured like the tandem care clinic at Ohio State. She hopes, however, that the program can be a model for other hospital systems to consider, and she is working to expand the program regionally. Her team is collecting data – including on the best way to schedule patients – to help other clinics develop something similar.
“We really want to leverage that expertise to make it easier for moms to get care with their infants and remove barriers to care,” she said.
A version of this article first appeared on Medscape.com.
Mindi Rosen met Seuli Brill, MD, at just the right time.
Ms. Rosen’s firstborn son was in the neointensive natal unit at The Ohio State University Wexner Medical Center in Columbus, and she didn’t have a pediatrician picked out yet for the baby. Nor did she have a primary care physician who could help her manage the gestational diabetes she developed during her pregnancy.
Dr. Brill, a clinical associate professor of internal medicine and pediatrics at Ohio State, suggested Ms. Rosen visit her at the new clinic she was piloting in Columbus. There, she provided pediatric care for newborns and primary care for mothers who had developed gestational diabetes.
“I looked at my husband, my husband looked at me, and I said: ‘Why not?’ “ Ms. Rosen, 38, recalled of that 2019 meeting. “I’m so glad she walked in at that moment.”
The mother of two is still part of the rapidly growing program at the medical facility that provides care for more than 200 mothers and babies.
Launched in 2018, the clinic – called the Multi-Modal Maternal Infant Perinatal Outpatient Delivery System, or “Momi Pods,” started with a focus on helping women with gestational diabetes, which occurs in up to 10% of pregnancies.
The program allows moms to book regular checkups for their baby, and then a follow-up appointment immediately after for themselves. Women are seen for the first 1,000 days (just under 3 years) after giving birth.
The idea was simple. Dr. Brill wanted to develop a more formalized program for the work she was already doing as a primary care physician and pediatrician. At the time, she was fielding referrals from specialists for young women who didn’t have a physician. She’d often develop a relationship with the patient over the years, go on to help oversee their care during pregnancy, then new mothers would select her as their newborn’s pediatrician.
“I would have a relationship with the mom when they did have the newborn, and then I would see the baby because I’m a pediatrician,” Dr. Brill said.
Dr. Brill was serving on the Ohio Gestational Diabetes Mellitus Collaborative, a state-backed program that aims to raise awareness about the condition and encourage more preventative care for patients. She presented her proposal to launch the program to the Ohio Department of Medicaid, which helped to fund the pilot.
The idea, she hoped, would improve postpartum follow-up care for mothers diagnosed with the condition.
Follow-up care is especially important for women who develop gestational diabetes because the condition raises their lifetime risk of developing type 2 diabetes up to 10-fold.
Yet most of those mothers don’t get the appropriate follow-up care during the crucial postpartum period, said Maya Subbalakshmi Venkataramani, MD, MPH, an assistant professor of medicine at Johns Hopkins University in Baltimore, who has researched parental care.
“Things get very busy after you have a child. There’s just the general logistics of a mom having to take care of a newborn and thinking about themselves,” Dr. Venkataramani, a primary care clinician and pediatrician, said. “A lot of parents in general may not put a lot of emphasis on their own health.”
Seeking care may be especially difficult for low-income mothers who might not have consistent health care coverage, she added.
In fact, only half of women who developed gestational diabetes received primary follow-up care, according to a study published in JAMA Network Open. The study, which examined more than 280,000 insurance claims between 2015 and 2018, found only 36% of women with gestational diabetes received the recommended blood glucose testing in the first 12 weeks of the postpartum period.
In the Momi Pods program, Dr. Brill checked in on Ms. Rosen’s gestational diabetes regularly during pediatric office visits for her newborn’s care. Ms. Rosen said whenever she brought her baby in for a visit during the postpartum period, Dr. Brill measured her blood sugar.
Dr. Brill and her team also asked how Ms. Rosen was doing physically and mentally during each visit. The screenings helped to catch a bout of postpartum depression Ms. Rosen experienced after the birth of her first son.
“I thought it was great, because honestly as a new mom I wouldn’t have followed up with myself so much,” Ms. Rosen said. “Every time you went into the doctor appointments, they’d ask you how you are doing. As a new mom, it’s so much easier to do it at the same time.”
Those who participate in the program are also more likely to complete postpartum visits with their ob.gyn. (95% vs. 58%, respectively; P < .001) than those who don’t participate, according to research Dr. Brill and colleagues published.
Dr. Brill began expanding the program’s reach nearly 2 years after its launch, targeting the services for women who are at risk for poor postpartum outcomes, including those with a history of depression, preterm labor, diabetes and congenital heart disease. Ob.gyns. in Ohio State’s network can refer their patients to the program, which now has 43 doctors trained to provide primary and pediatric care through Momi Pods. Soon-to-be moms can be referred to the program as early as the second trimester, Dr. Brill said.
Many of the mothers referred to the program don’t have a primary care clinician when they talk to Paola Beamon, RN, at Ohio State. Ms. Beamon reaches out to each referred patient over voicemail, a MyChart message, and even regular mail in hopes of helping them navigate the postpartum period. She also provides education on what a primary care clinician can offer new moms.
“Really, we’re pursuing these moms and doing everything we can so there’s less of a burden for them,” Ms. Beamon said. “A lot of them don’t even know what a primary care office does.”
One of the biggest perks to the program for new moms is that they don’t have to spend time and money traveling to a different doctor’s office, take time off work, or secure childcare in order to schedule a separate appointment for themselves, she said.
The program, which receives funding from the university and the state, even helps women get bus passes to a doctor’s appointment if needed.
Dyad programs targeting women with substance abuse disorders or mental health conditions have existed for many years. But catering to women with gestational diabetes or other medical conditions appears to be new. In part, Dr. Venkataramani said, because scheduling and space can be big hurdles to launch such a program, as well as finding doctors who can care for both baby and mother.
“There are logistical challenges to even doing this that makes it less common,” she said.
Dr. Brill said she is not aware of any other programs that are structured like the tandem care clinic at Ohio State. She hopes, however, that the program can be a model for other hospital systems to consider, and she is working to expand the program regionally. Her team is collecting data – including on the best way to schedule patients – to help other clinics develop something similar.
“We really want to leverage that expertise to make it easier for moms to get care with their infants and remove barriers to care,” she said.
A version of this article first appeared on Medscape.com.
Mindi Rosen met Seuli Brill, MD, at just the right time.
Ms. Rosen’s firstborn son was in the neointensive natal unit at The Ohio State University Wexner Medical Center in Columbus, and she didn’t have a pediatrician picked out yet for the baby. Nor did she have a primary care physician who could help her manage the gestational diabetes she developed during her pregnancy.
Dr. Brill, a clinical associate professor of internal medicine and pediatrics at Ohio State, suggested Ms. Rosen visit her at the new clinic she was piloting in Columbus. There, she provided pediatric care for newborns and primary care for mothers who had developed gestational diabetes.
“I looked at my husband, my husband looked at me, and I said: ‘Why not?’ “ Ms. Rosen, 38, recalled of that 2019 meeting. “I’m so glad she walked in at that moment.”
The mother of two is still part of the rapidly growing program at the medical facility that provides care for more than 200 mothers and babies.
Launched in 2018, the clinic – called the Multi-Modal Maternal Infant Perinatal Outpatient Delivery System, or “Momi Pods,” started with a focus on helping women with gestational diabetes, which occurs in up to 10% of pregnancies.
The program allows moms to book regular checkups for their baby, and then a follow-up appointment immediately after for themselves. Women are seen for the first 1,000 days (just under 3 years) after giving birth.
The idea was simple. Dr. Brill wanted to develop a more formalized program for the work she was already doing as a primary care physician and pediatrician. At the time, she was fielding referrals from specialists for young women who didn’t have a physician. She’d often develop a relationship with the patient over the years, go on to help oversee their care during pregnancy, then new mothers would select her as their newborn’s pediatrician.
“I would have a relationship with the mom when they did have the newborn, and then I would see the baby because I’m a pediatrician,” Dr. Brill said.
Dr. Brill was serving on the Ohio Gestational Diabetes Mellitus Collaborative, a state-backed program that aims to raise awareness about the condition and encourage more preventative care for patients. She presented her proposal to launch the program to the Ohio Department of Medicaid, which helped to fund the pilot.
The idea, she hoped, would improve postpartum follow-up care for mothers diagnosed with the condition.
Follow-up care is especially important for women who develop gestational diabetes because the condition raises their lifetime risk of developing type 2 diabetes up to 10-fold.
Yet most of those mothers don’t get the appropriate follow-up care during the crucial postpartum period, said Maya Subbalakshmi Venkataramani, MD, MPH, an assistant professor of medicine at Johns Hopkins University in Baltimore, who has researched parental care.
“Things get very busy after you have a child. There’s just the general logistics of a mom having to take care of a newborn and thinking about themselves,” Dr. Venkataramani, a primary care clinician and pediatrician, said. “A lot of parents in general may not put a lot of emphasis on their own health.”
Seeking care may be especially difficult for low-income mothers who might not have consistent health care coverage, she added.
In fact, only half of women who developed gestational diabetes received primary follow-up care, according to a study published in JAMA Network Open. The study, which examined more than 280,000 insurance claims between 2015 and 2018, found only 36% of women with gestational diabetes received the recommended blood glucose testing in the first 12 weeks of the postpartum period.
In the Momi Pods program, Dr. Brill checked in on Ms. Rosen’s gestational diabetes regularly during pediatric office visits for her newborn’s care. Ms. Rosen said whenever she brought her baby in for a visit during the postpartum period, Dr. Brill measured her blood sugar.
Dr. Brill and her team also asked how Ms. Rosen was doing physically and mentally during each visit. The screenings helped to catch a bout of postpartum depression Ms. Rosen experienced after the birth of her first son.
“I thought it was great, because honestly as a new mom I wouldn’t have followed up with myself so much,” Ms. Rosen said. “Every time you went into the doctor appointments, they’d ask you how you are doing. As a new mom, it’s so much easier to do it at the same time.”
Those who participate in the program are also more likely to complete postpartum visits with their ob.gyn. (95% vs. 58%, respectively; P < .001) than those who don’t participate, according to research Dr. Brill and colleagues published.
Dr. Brill began expanding the program’s reach nearly 2 years after its launch, targeting the services for women who are at risk for poor postpartum outcomes, including those with a history of depression, preterm labor, diabetes and congenital heart disease. Ob.gyns. in Ohio State’s network can refer their patients to the program, which now has 43 doctors trained to provide primary and pediatric care through Momi Pods. Soon-to-be moms can be referred to the program as early as the second trimester, Dr. Brill said.
Many of the mothers referred to the program don’t have a primary care clinician when they talk to Paola Beamon, RN, at Ohio State. Ms. Beamon reaches out to each referred patient over voicemail, a MyChart message, and even regular mail in hopes of helping them navigate the postpartum period. She also provides education on what a primary care clinician can offer new moms.
“Really, we’re pursuing these moms and doing everything we can so there’s less of a burden for them,” Ms. Beamon said. “A lot of them don’t even know what a primary care office does.”
One of the biggest perks to the program for new moms is that they don’t have to spend time and money traveling to a different doctor’s office, take time off work, or secure childcare in order to schedule a separate appointment for themselves, she said.
The program, which receives funding from the university and the state, even helps women get bus passes to a doctor’s appointment if needed.
Dyad programs targeting women with substance abuse disorders or mental health conditions have existed for many years. But catering to women with gestational diabetes or other medical conditions appears to be new. In part, Dr. Venkataramani said, because scheduling and space can be big hurdles to launch such a program, as well as finding doctors who can care for both baby and mother.
“There are logistical challenges to even doing this that makes it less common,” she said.
Dr. Brill said she is not aware of any other programs that are structured like the tandem care clinic at Ohio State. She hopes, however, that the program can be a model for other hospital systems to consider, and she is working to expand the program regionally. Her team is collecting data – including on the best way to schedule patients – to help other clinics develop something similar.
“We really want to leverage that expertise to make it easier for moms to get care with their infants and remove barriers to care,” she said.
A version of this article first appeared on Medscape.com.
Maternal infection in pregnancy ups risk for childhood leukemia?
Children born to mothers who had urinary or genital tract infections during pregnancy appear to have an increased risk for childhood leukemia, said researchers reporting a Danish registry analysis that may point to preventive strategies for the disease.
The research was published online in JAMA Network Open.
The team studied more than 2.2 million children born in Denmark over more than 3 decades, linking their records across multiple national registries to examine both later cancer risk and maternal infection rates.
They found that, overall, at least one maternal infection during pregnancy was associated with a 35% increased risk for leukemia in the children, rising to 65% for urinary tract infections, and 142% for genital infections.
“The findings of this large population-based cohort study suggest that maternal urinary and genital tract infections during pregnancy are associated with a higher risk of childhood leukemia in offspring,” said lead author Jian-Rong He, DPhil, division of birth cohort study, Guangzhou (China) Women and Children’s Medical Center.
However, he added, “the associated absolute risk remained small given the rarity” of the disease. In absolute terms, the risk difference between exposed and unexposed children was 1.8 cases per 100,000 person-years for any infection, 3.4 cases per 100,000 person-years for urinary traction infection, and 7.1 cases per 100,000 person-years for genital tract infection.
Maternal infections during pregnancy may be associated with chromosomal and immunologic alterations in the fetus, the authors speculated.
“Given that little is known about the etiology of childhood leukemia,” these results “suggest an important direction for research on the etiology of childhood leukemia as well as development of potential preventive measures,” they wrote.
In many countries, pregnant women are tested for urinary tract infection and bacterial vaginosis, and treated with antibiotics in antenatal care, as these infections are linked to adverse perinatal outcomes, they pointed out.
Study details
The team conducted a large population-based study that included all live births in Denmark between 1978 and 2015.
After exclusions, they gathered information on 2,222,797 children, linking data from several national registries, including the Danish Medical Birth Register, the Danish National Patient Registry, and the Danish National Cancer Registry, to identify cases of childhood cancers and maternal infection during pregnancy.
The results were then validated by comparing them with those in 2.6 million live births in Sweden between 1988 and 2014, for whom similar data were available through linkage with several Swedish registries.
The Danish cohort was followed up for a mean of 12 years per person, yielding a total of 27 million person-years. Just over half (51.3%) were boys.
Cancer was diagnosed in 4,362 children before 15 years of age, of whom 1,307 had leukemia (1,050 had acute lymphocytic leukemia), 1,267 had a brain tumor, 224 had lymphoma, and 1,564 had other cancers.
At least one infection during pregnancy was diagnosed in 81,717 mothers (3.7%). Urinary tract infections were the most common (in 1.7% of women), followed by genital tract infection (in 0.7%), digestive system infection (in 0.5%), and respiratory tract infection (in 0.3%).
Women with any infection during pregnancy were more likely to be younger and primiparous than were women who did not have infections, and they were also more likely to have fewer years of education, higher prepregnancy BMI, diabetes, and to smoke during early pregnancy.
Preterm delivery and low-birth-weight infants were also more common in women with infections during pregnancy.
Cox proportional hazards regression models revealed that, after adjustment for confounders, any maternal infection was associated with a hazard ratio of childhood leukemia of 1.35.
Further analysis revealed that the association was driven by genital tract infection, at a hazard ratio for childhood leukemia of 2.42, and urinary tract infection, at a hazard ratio 1.65.
Moreover, children born to women who had a sexually transmitted infection during pregnancy had a hazard ratio for developing leukemia of 3.13 compared with unexposed children.
There were no associations between other maternal infections and childhood leukemia.
The patterns of association between maternal infections and childhood leukemia were similar when looking at disease subtypes, as well as in the Swedish validation cohort, they added.
When interpreting the results, the researchers caution that, as data on maternal infection were drawn from hospital data, “milder infections and those not diagnosed or treated in specialized health care facilities were not captured.”
“Also, some infections could be captured because the mother sought care for other, more serious conditions, which might bias the association of maternal infections and childhood leukemia.”
The study was supported by grants from the China Scholarship Council–University of Oxford; National Natural Science Foundation of China; Danish Council for Independent Research; Nordic Cancer Union; Novo Nordisk Fonden; and the Swedish Council for Working Life and Social Research. Dr He reported receiving a PhD scholarship from the China Scholarship Council during the conduct of the study. Several other coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.
Children born to mothers who had urinary or genital tract infections during pregnancy appear to have an increased risk for childhood leukemia, said researchers reporting a Danish registry analysis that may point to preventive strategies for the disease.
The research was published online in JAMA Network Open.
The team studied more than 2.2 million children born in Denmark over more than 3 decades, linking their records across multiple national registries to examine both later cancer risk and maternal infection rates.
They found that, overall, at least one maternal infection during pregnancy was associated with a 35% increased risk for leukemia in the children, rising to 65% for urinary tract infections, and 142% for genital infections.
“The findings of this large population-based cohort study suggest that maternal urinary and genital tract infections during pregnancy are associated with a higher risk of childhood leukemia in offspring,” said lead author Jian-Rong He, DPhil, division of birth cohort study, Guangzhou (China) Women and Children’s Medical Center.
However, he added, “the associated absolute risk remained small given the rarity” of the disease. In absolute terms, the risk difference between exposed and unexposed children was 1.8 cases per 100,000 person-years for any infection, 3.4 cases per 100,000 person-years for urinary traction infection, and 7.1 cases per 100,000 person-years for genital tract infection.
Maternal infections during pregnancy may be associated with chromosomal and immunologic alterations in the fetus, the authors speculated.
“Given that little is known about the etiology of childhood leukemia,” these results “suggest an important direction for research on the etiology of childhood leukemia as well as development of potential preventive measures,” they wrote.
In many countries, pregnant women are tested for urinary tract infection and bacterial vaginosis, and treated with antibiotics in antenatal care, as these infections are linked to adverse perinatal outcomes, they pointed out.
Study details
The team conducted a large population-based study that included all live births in Denmark between 1978 and 2015.
After exclusions, they gathered information on 2,222,797 children, linking data from several national registries, including the Danish Medical Birth Register, the Danish National Patient Registry, and the Danish National Cancer Registry, to identify cases of childhood cancers and maternal infection during pregnancy.
The results were then validated by comparing them with those in 2.6 million live births in Sweden between 1988 and 2014, for whom similar data were available through linkage with several Swedish registries.
The Danish cohort was followed up for a mean of 12 years per person, yielding a total of 27 million person-years. Just over half (51.3%) were boys.
Cancer was diagnosed in 4,362 children before 15 years of age, of whom 1,307 had leukemia (1,050 had acute lymphocytic leukemia), 1,267 had a brain tumor, 224 had lymphoma, and 1,564 had other cancers.
At least one infection during pregnancy was diagnosed in 81,717 mothers (3.7%). Urinary tract infections were the most common (in 1.7% of women), followed by genital tract infection (in 0.7%), digestive system infection (in 0.5%), and respiratory tract infection (in 0.3%).
Women with any infection during pregnancy were more likely to be younger and primiparous than were women who did not have infections, and they were also more likely to have fewer years of education, higher prepregnancy BMI, diabetes, and to smoke during early pregnancy.
Preterm delivery and low-birth-weight infants were also more common in women with infections during pregnancy.
Cox proportional hazards regression models revealed that, after adjustment for confounders, any maternal infection was associated with a hazard ratio of childhood leukemia of 1.35.
Further analysis revealed that the association was driven by genital tract infection, at a hazard ratio for childhood leukemia of 2.42, and urinary tract infection, at a hazard ratio 1.65.
Moreover, children born to women who had a sexually transmitted infection during pregnancy had a hazard ratio for developing leukemia of 3.13 compared with unexposed children.
There were no associations between other maternal infections and childhood leukemia.
The patterns of association between maternal infections and childhood leukemia were similar when looking at disease subtypes, as well as in the Swedish validation cohort, they added.
When interpreting the results, the researchers caution that, as data on maternal infection were drawn from hospital data, “milder infections and those not diagnosed or treated in specialized health care facilities were not captured.”
“Also, some infections could be captured because the mother sought care for other, more serious conditions, which might bias the association of maternal infections and childhood leukemia.”
The study was supported by grants from the China Scholarship Council–University of Oxford; National Natural Science Foundation of China; Danish Council for Independent Research; Nordic Cancer Union; Novo Nordisk Fonden; and the Swedish Council for Working Life and Social Research. Dr He reported receiving a PhD scholarship from the China Scholarship Council during the conduct of the study. Several other coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.
Children born to mothers who had urinary or genital tract infections during pregnancy appear to have an increased risk for childhood leukemia, said researchers reporting a Danish registry analysis that may point to preventive strategies for the disease.
The research was published online in JAMA Network Open.
The team studied more than 2.2 million children born in Denmark over more than 3 decades, linking their records across multiple national registries to examine both later cancer risk and maternal infection rates.
They found that, overall, at least one maternal infection during pregnancy was associated with a 35% increased risk for leukemia in the children, rising to 65% for urinary tract infections, and 142% for genital infections.
“The findings of this large population-based cohort study suggest that maternal urinary and genital tract infections during pregnancy are associated with a higher risk of childhood leukemia in offspring,” said lead author Jian-Rong He, DPhil, division of birth cohort study, Guangzhou (China) Women and Children’s Medical Center.
However, he added, “the associated absolute risk remained small given the rarity” of the disease. In absolute terms, the risk difference between exposed and unexposed children was 1.8 cases per 100,000 person-years for any infection, 3.4 cases per 100,000 person-years for urinary traction infection, and 7.1 cases per 100,000 person-years for genital tract infection.
Maternal infections during pregnancy may be associated with chromosomal and immunologic alterations in the fetus, the authors speculated.
“Given that little is known about the etiology of childhood leukemia,” these results “suggest an important direction for research on the etiology of childhood leukemia as well as development of potential preventive measures,” they wrote.
In many countries, pregnant women are tested for urinary tract infection and bacterial vaginosis, and treated with antibiotics in antenatal care, as these infections are linked to adverse perinatal outcomes, they pointed out.
Study details
The team conducted a large population-based study that included all live births in Denmark between 1978 and 2015.
After exclusions, they gathered information on 2,222,797 children, linking data from several national registries, including the Danish Medical Birth Register, the Danish National Patient Registry, and the Danish National Cancer Registry, to identify cases of childhood cancers and maternal infection during pregnancy.
The results were then validated by comparing them with those in 2.6 million live births in Sweden between 1988 and 2014, for whom similar data were available through linkage with several Swedish registries.
The Danish cohort was followed up for a mean of 12 years per person, yielding a total of 27 million person-years. Just over half (51.3%) were boys.
Cancer was diagnosed in 4,362 children before 15 years of age, of whom 1,307 had leukemia (1,050 had acute lymphocytic leukemia), 1,267 had a brain tumor, 224 had lymphoma, and 1,564 had other cancers.
At least one infection during pregnancy was diagnosed in 81,717 mothers (3.7%). Urinary tract infections were the most common (in 1.7% of women), followed by genital tract infection (in 0.7%), digestive system infection (in 0.5%), and respiratory tract infection (in 0.3%).
Women with any infection during pregnancy were more likely to be younger and primiparous than were women who did not have infections, and they were also more likely to have fewer years of education, higher prepregnancy BMI, diabetes, and to smoke during early pregnancy.
Preterm delivery and low-birth-weight infants were also more common in women with infections during pregnancy.
Cox proportional hazards regression models revealed that, after adjustment for confounders, any maternal infection was associated with a hazard ratio of childhood leukemia of 1.35.
Further analysis revealed that the association was driven by genital tract infection, at a hazard ratio for childhood leukemia of 2.42, and urinary tract infection, at a hazard ratio 1.65.
Moreover, children born to women who had a sexually transmitted infection during pregnancy had a hazard ratio for developing leukemia of 3.13 compared with unexposed children.
There were no associations between other maternal infections and childhood leukemia.
The patterns of association between maternal infections and childhood leukemia were similar when looking at disease subtypes, as well as in the Swedish validation cohort, they added.
When interpreting the results, the researchers caution that, as data on maternal infection were drawn from hospital data, “milder infections and those not diagnosed or treated in specialized health care facilities were not captured.”
“Also, some infections could be captured because the mother sought care for other, more serious conditions, which might bias the association of maternal infections and childhood leukemia.”
The study was supported by grants from the China Scholarship Council–University of Oxford; National Natural Science Foundation of China; Danish Council for Independent Research; Nordic Cancer Union; Novo Nordisk Fonden; and the Swedish Council for Working Life and Social Research. Dr He reported receiving a PhD scholarship from the China Scholarship Council during the conduct of the study. Several other coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.