User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Are there long-term benefits to infants born to patients after bariatric surgery?
Rives-Lange C, Poghosyan T, Phan A, et al. Risk-benefit balance associated with obstetric, neonatal, and child outcomes after metabolic and bariatric surgery. JAMA Surg. 2023;158:36-44. doi:10.1001/jamasurg.2022.5450.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise, with approximately 40% of reproductive-aged patients having a body mass index greater than 30 kg/m2.1 Several adverse perinatal outcomes are more common in pregnant patients with obesity.2 In addition, their infants have a higher risk of obesity, insulin resistance, hypertension, and neurodevelopmental disorders in the long term.
Bariatric surgery is an effective procedure for weight loss and has been shown to lower adverse pregnancy outcomes, such as hypertensive disorders of pregnancy and gestational diabetes.5,6 Benefits to newborns, however, have been debated.5 In addition, long-term benefits to infants were unknown until a recent study evaluated neonatal and child outcomes up to 2 years after pregnancy among patients who had undergone bariatric surgery.
Details of the study
Using the French nationwide database, Rives-Lange and colleagues performed a population-based study that included patients who had at least 1 pregnancy before and 1 pregnancy after bariatric surgery. Their objective was to compare pregnancy, neonatal, and child outcomes between pregnancies pre- and post-bariatric surgery.
Results. Among 3,686 patients who had at least 1 pregnancy before and after bariatric surgery, the authors found that pregnancies after bariatric surgery had lower rates of several adverse pregnancy outcomes, including preeclampsia (OR, 0.19), gestational hypertension (OR, 0.16), and gestational diabetes (OR, 0.39), compared with pregnancies before bariatric surgery. Regarding neonatal and child outcomes up to 2 years after pregnancy, there were lower rates of birth injuries (OR, 0.27), convulsions (OR, 0.43), newborn carbohydrate metabolism disorders (OR, 0.54),and viral intestinal infections (OR, 0.56) in pregnancies after bariatric surgery compared with those before surgery.
Notably, respiratory failure rates associated with bronchiolitis increased in pregnancies after bariatric surgery (OR, 2.42). This finding remained associated after adjusting for prematurity and small for gestational age as well as including 2 successive pregnancies before bariatric surgery (OR, 1.37).
Study strengths and limitations
A limitation of this study is the use of an administrative database, which may be biased and missing relevant variables. However, the study’s major strength was the large sample of patients serving as their own control to compare outcomes from pre-bariatric surgery with those of post-bariatric surgery. In addition, to account for confounders such as age and parity, the authors also evaluated for associations between 2 consecutive pregnancies among patients before bariatric surgery. They did not consider diagnoses found to be associated with bariatric surgery if they were also significant in the analysis between 2 consecutive pregnancies before bariatric surgery.
The finding of increased risk of respiratory failure from bronchiolitis after bariatric surgery is surprising given that obesity is a risk factor for the severity of bronchiolitis.7 Although this risk remained significant after including the analysis that used 2 consecutive pregnancies pre-bariatric surgery, the risk was lower (from an OR of 2.42 to an OR of 1.37). Thus, more data are required to confirm this potential risk. Despite this concerning finding, the overwhelming pregnancy, neonatal, and child benefits found and confirmed in this large, well-designed study support the continued practice of counseling on the benefits of bariatric surgery to our obese patients. ●
Bariatric surgery remains an effective procedure for weight loss, and it lowers the risks of several important perinatal, neonatal, and child outcomes, including hypertensive disorders, birth injuries, convulsions, and viral intestinal infections. Clinicians should include the benefits of neonatal and child outcomes in their counseling of bariatric surgery for their obese patients who are planning pregnancy.
RODNEY A. MCLAREN JR, MD
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Sagi-Dain L. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;138:489. doi:10.1097 /AOG.0000000000004527.
- O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf). 2013;78:9-16. doi:10.1111/cen.12055.
- Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37:95-110. doi:10.1002/pd.4932.
- Johansson K, Cnattinguius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372:814-824. doi:10.1056/NEJMoa1405789.
- Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2022;226:121.e1-121.e16. doi:10.1016/j.ajog.2021.06.087.
- James T, Samakar K, Martin MJ. Special delivery—metabolic bariatric surgery as a key component of maternal-fetal health care. JAMA Surg. 2023;158:44-45. doi:10.1001 /jamasurg.2022.5458.
Rives-Lange C, Poghosyan T, Phan A, et al. Risk-benefit balance associated with obstetric, neonatal, and child outcomes after metabolic and bariatric surgery. JAMA Surg. 2023;158:36-44. doi:10.1001/jamasurg.2022.5450.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise, with approximately 40% of reproductive-aged patients having a body mass index greater than 30 kg/m2.1 Several adverse perinatal outcomes are more common in pregnant patients with obesity.2 In addition, their infants have a higher risk of obesity, insulin resistance, hypertension, and neurodevelopmental disorders in the long term.
Bariatric surgery is an effective procedure for weight loss and has been shown to lower adverse pregnancy outcomes, such as hypertensive disorders of pregnancy and gestational diabetes.5,6 Benefits to newborns, however, have been debated.5 In addition, long-term benefits to infants were unknown until a recent study evaluated neonatal and child outcomes up to 2 years after pregnancy among patients who had undergone bariatric surgery.
Details of the study
Using the French nationwide database, Rives-Lange and colleagues performed a population-based study that included patients who had at least 1 pregnancy before and 1 pregnancy after bariatric surgery. Their objective was to compare pregnancy, neonatal, and child outcomes between pregnancies pre- and post-bariatric surgery.
Results. Among 3,686 patients who had at least 1 pregnancy before and after bariatric surgery, the authors found that pregnancies after bariatric surgery had lower rates of several adverse pregnancy outcomes, including preeclampsia (OR, 0.19), gestational hypertension (OR, 0.16), and gestational diabetes (OR, 0.39), compared with pregnancies before bariatric surgery. Regarding neonatal and child outcomes up to 2 years after pregnancy, there were lower rates of birth injuries (OR, 0.27), convulsions (OR, 0.43), newborn carbohydrate metabolism disorders (OR, 0.54),and viral intestinal infections (OR, 0.56) in pregnancies after bariatric surgery compared with those before surgery.
Notably, respiratory failure rates associated with bronchiolitis increased in pregnancies after bariatric surgery (OR, 2.42). This finding remained associated after adjusting for prematurity and small for gestational age as well as including 2 successive pregnancies before bariatric surgery (OR, 1.37).
Study strengths and limitations
A limitation of this study is the use of an administrative database, which may be biased and missing relevant variables. However, the study’s major strength was the large sample of patients serving as their own control to compare outcomes from pre-bariatric surgery with those of post-bariatric surgery. In addition, to account for confounders such as age and parity, the authors also evaluated for associations between 2 consecutive pregnancies among patients before bariatric surgery. They did not consider diagnoses found to be associated with bariatric surgery if they were also significant in the analysis between 2 consecutive pregnancies before bariatric surgery.
The finding of increased risk of respiratory failure from bronchiolitis after bariatric surgery is surprising given that obesity is a risk factor for the severity of bronchiolitis.7 Although this risk remained significant after including the analysis that used 2 consecutive pregnancies pre-bariatric surgery, the risk was lower (from an OR of 2.42 to an OR of 1.37). Thus, more data are required to confirm this potential risk. Despite this concerning finding, the overwhelming pregnancy, neonatal, and child benefits found and confirmed in this large, well-designed study support the continued practice of counseling on the benefits of bariatric surgery to our obese patients. ●

Bariatric surgery remains an effective procedure for weight loss, and it lowers the risks of several important perinatal, neonatal, and child outcomes, including hypertensive disorders, birth injuries, convulsions, and viral intestinal infections. Clinicians should include the benefits of neonatal and child outcomes in their counseling of bariatric surgery for their obese patients who are planning pregnancy.
RODNEY A. MCLAREN JR, MD
Rives-Lange C, Poghosyan T, Phan A, et al. Risk-benefit balance associated with obstetric, neonatal, and child outcomes after metabolic and bariatric surgery. JAMA Surg. 2023;158:36-44. doi:10.1001/jamasurg.2022.5450.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise, with approximately 40% of reproductive-aged patients having a body mass index greater than 30 kg/m2.1 Several adverse perinatal outcomes are more common in pregnant patients with obesity.2 In addition, their infants have a higher risk of obesity, insulin resistance, hypertension, and neurodevelopmental disorders in the long term.
Bariatric surgery is an effective procedure for weight loss and has been shown to lower adverse pregnancy outcomes, such as hypertensive disorders of pregnancy and gestational diabetes.5,6 Benefits to newborns, however, have been debated.5 In addition, long-term benefits to infants were unknown until a recent study evaluated neonatal and child outcomes up to 2 years after pregnancy among patients who had undergone bariatric surgery.
Details of the study
Using the French nationwide database, Rives-Lange and colleagues performed a population-based study that included patients who had at least 1 pregnancy before and 1 pregnancy after bariatric surgery. Their objective was to compare pregnancy, neonatal, and child outcomes between pregnancies pre- and post-bariatric surgery.
Results. Among 3,686 patients who had at least 1 pregnancy before and after bariatric surgery, the authors found that pregnancies after bariatric surgery had lower rates of several adverse pregnancy outcomes, including preeclampsia (OR, 0.19), gestational hypertension (OR, 0.16), and gestational diabetes (OR, 0.39), compared with pregnancies before bariatric surgery. Regarding neonatal and child outcomes up to 2 years after pregnancy, there were lower rates of birth injuries (OR, 0.27), convulsions (OR, 0.43), newborn carbohydrate metabolism disorders (OR, 0.54),and viral intestinal infections (OR, 0.56) in pregnancies after bariatric surgery compared with those before surgery.
Notably, respiratory failure rates associated with bronchiolitis increased in pregnancies after bariatric surgery (OR, 2.42). This finding remained associated after adjusting for prematurity and small for gestational age as well as including 2 successive pregnancies before bariatric surgery (OR, 1.37).
Study strengths and limitations
A limitation of this study is the use of an administrative database, which may be biased and missing relevant variables. However, the study’s major strength was the large sample of patients serving as their own control to compare outcomes from pre-bariatric surgery with those of post-bariatric surgery. In addition, to account for confounders such as age and parity, the authors also evaluated for associations between 2 consecutive pregnancies among patients before bariatric surgery. They did not consider diagnoses found to be associated with bariatric surgery if they were also significant in the analysis between 2 consecutive pregnancies before bariatric surgery.
The finding of increased risk of respiratory failure from bronchiolitis after bariatric surgery is surprising given that obesity is a risk factor for the severity of bronchiolitis.7 Although this risk remained significant after including the analysis that used 2 consecutive pregnancies pre-bariatric surgery, the risk was lower (from an OR of 2.42 to an OR of 1.37). Thus, more data are required to confirm this potential risk. Despite this concerning finding, the overwhelming pregnancy, neonatal, and child benefits found and confirmed in this large, well-designed study support the continued practice of counseling on the benefits of bariatric surgery to our obese patients. ●

Bariatric surgery remains an effective procedure for weight loss, and it lowers the risks of several important perinatal, neonatal, and child outcomes, including hypertensive disorders, birth injuries, convulsions, and viral intestinal infections. Clinicians should include the benefits of neonatal and child outcomes in their counseling of bariatric surgery for their obese patients who are planning pregnancy.
RODNEY A. MCLAREN JR, MD
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Sagi-Dain L. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;138:489. doi:10.1097 /AOG.0000000000004527.
- O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf). 2013;78:9-16. doi:10.1111/cen.12055.
- Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37:95-110. doi:10.1002/pd.4932.
- Johansson K, Cnattinguius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372:814-824. doi:10.1056/NEJMoa1405789.
- Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2022;226:121.e1-121.e16. doi:10.1016/j.ajog.2021.06.087.
- James T, Samakar K, Martin MJ. Special delivery—metabolic bariatric surgery as a key component of maternal-fetal health care. JAMA Surg. 2023;158:44-45. doi:10.1001 /jamasurg.2022.5458.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Sagi-Dain L. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;138:489. doi:10.1097 /AOG.0000000000004527.
- O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf). 2013;78:9-16. doi:10.1111/cen.12055.
- Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37:95-110. doi:10.1002/pd.4932.
- Johansson K, Cnattinguius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372:814-824. doi:10.1056/NEJMoa1405789.
- Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2022;226:121.e1-121.e16. doi:10.1016/j.ajog.2021.06.087.
- James T, Samakar K, Martin MJ. Special delivery—metabolic bariatric surgery as a key component of maternal-fetal health care. JAMA Surg. 2023;158:44-45. doi:10.1001 /jamasurg.2022.5458.
‘Financial toxicity’ from breast cancer is a worldwide phenomenon
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
FROM JAMA NETWORK OPEN
Hormonal contraception and lactation: Reset your practices based on the evidence
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.
Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8
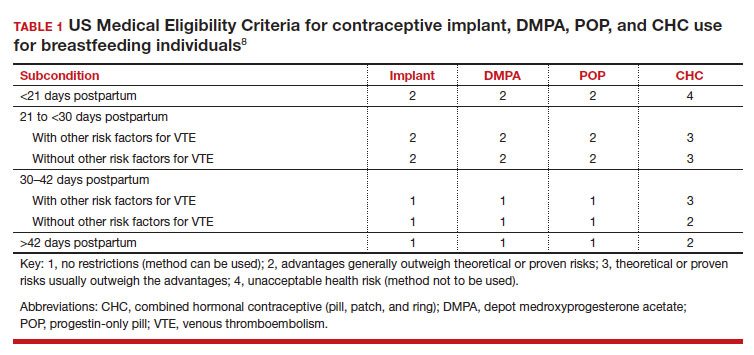
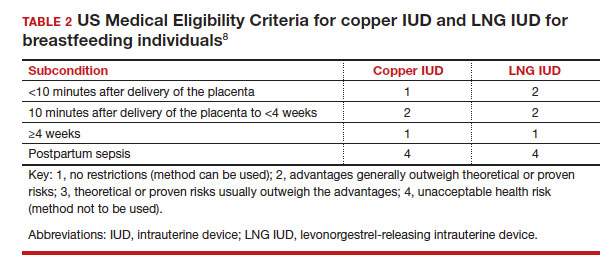
Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
Is it time to reconsider Rh testing and Rh D immune globulin treatment for miscarriage and abortion care in early pregnancy?
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
Progress in breast cancer screening over the past 50 years: A remarkable story, but still work to do
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3
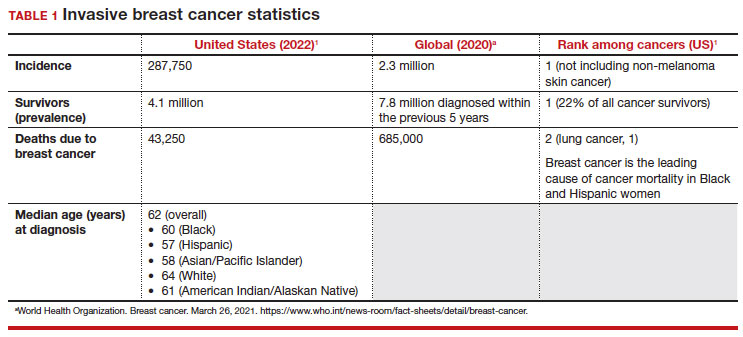
While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.
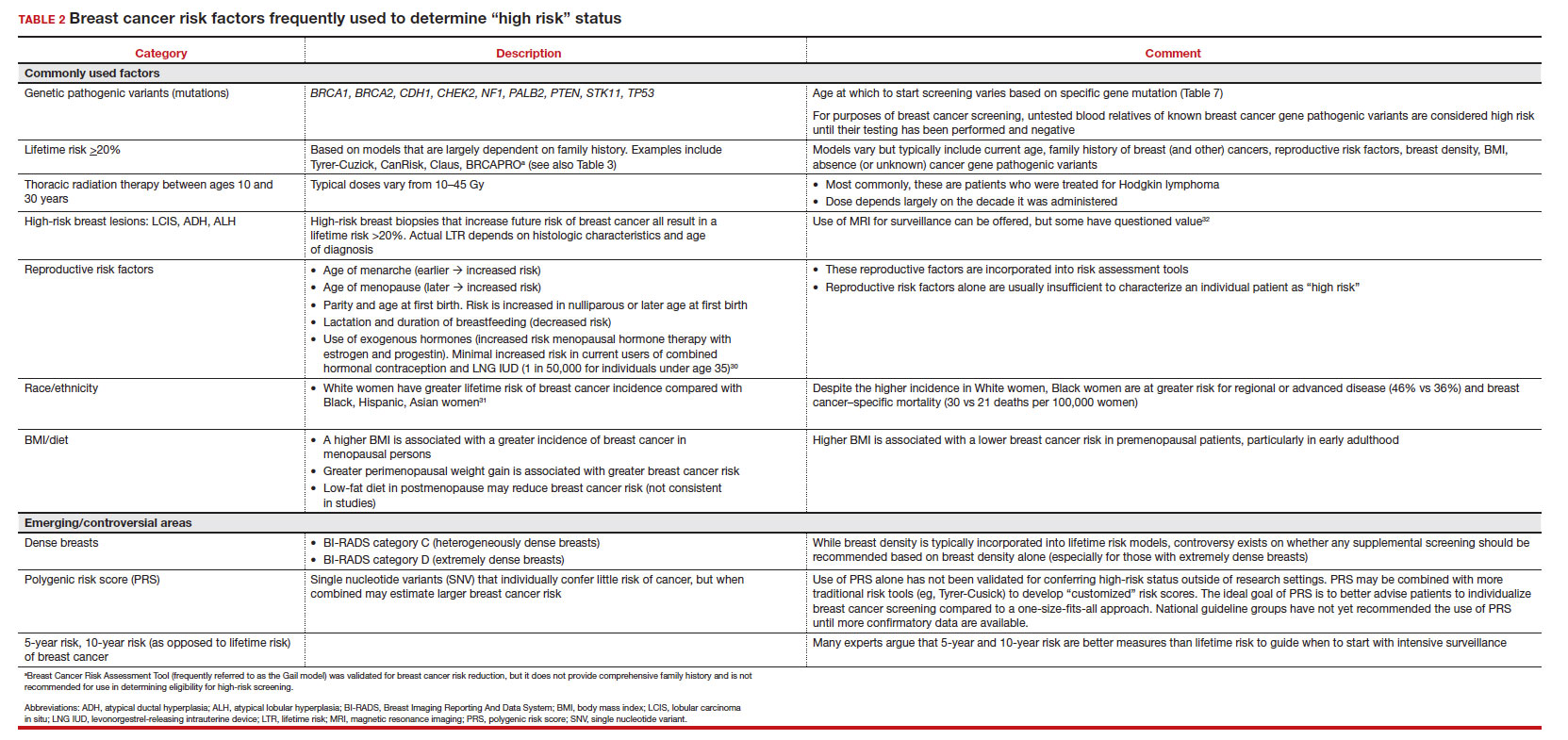
Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7
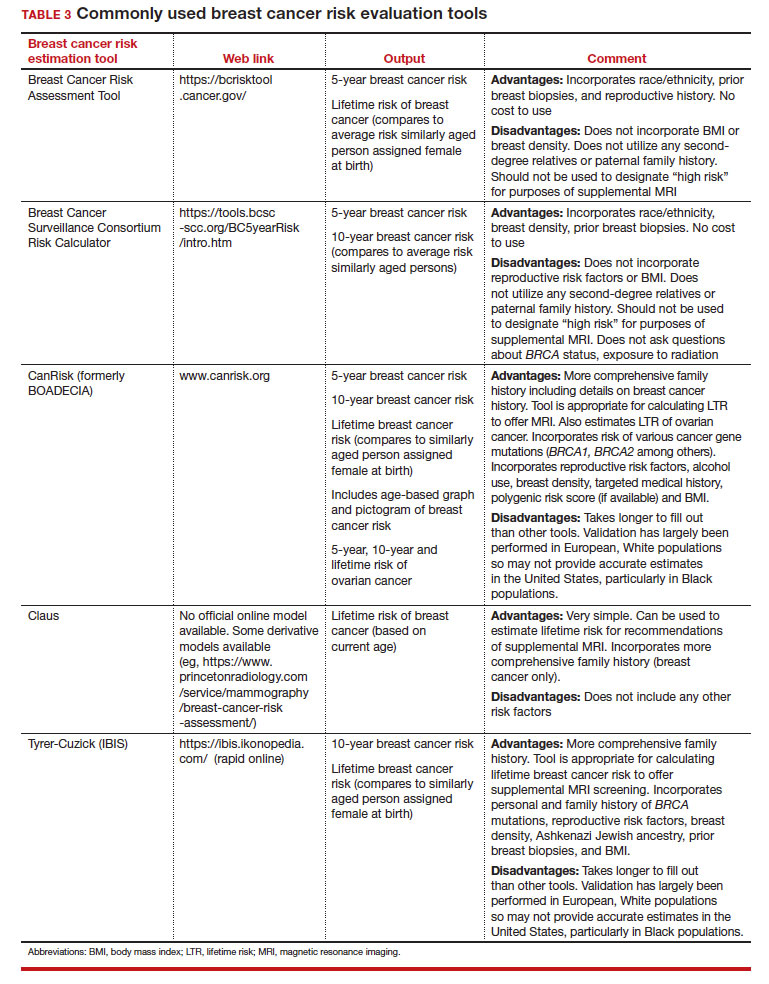
Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10
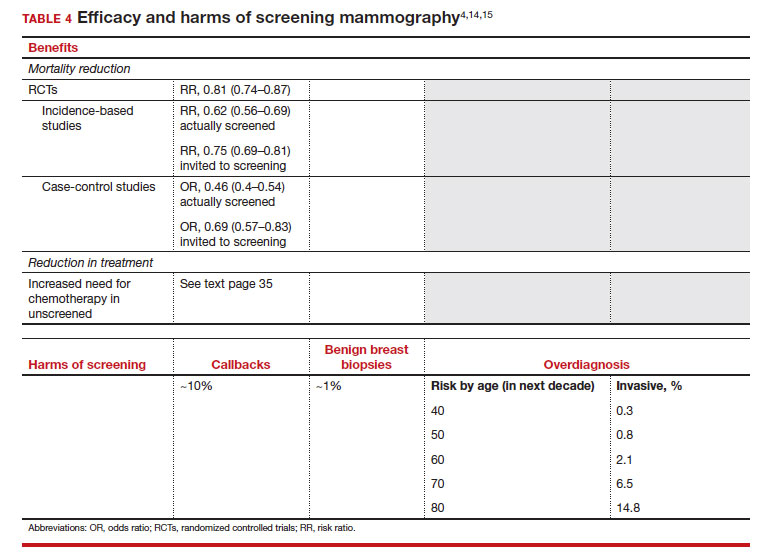
With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).
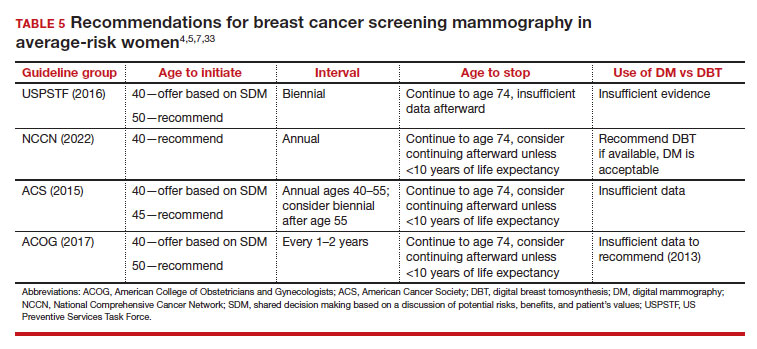
Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.
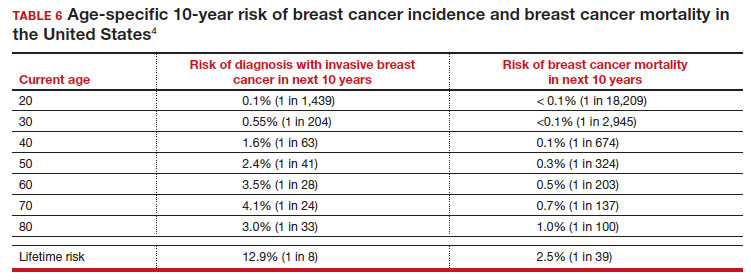
For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.
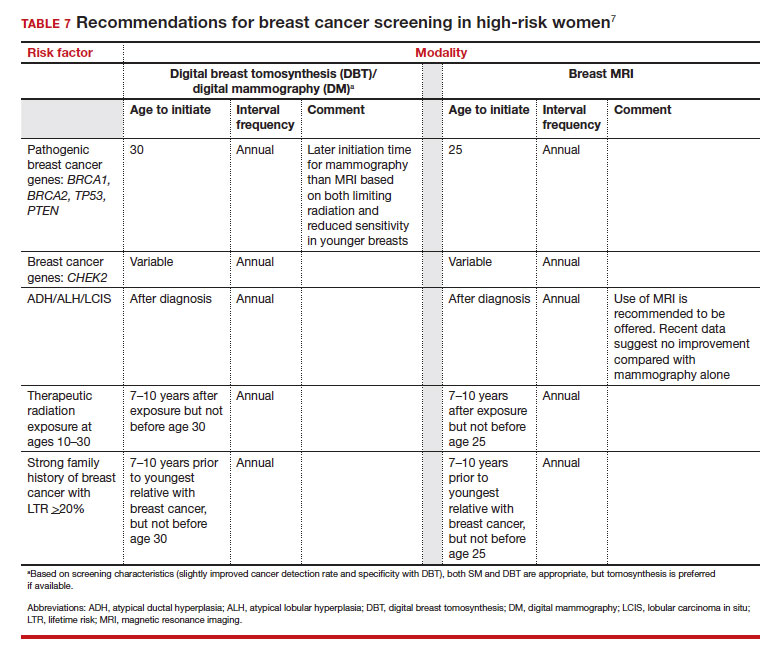
Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).
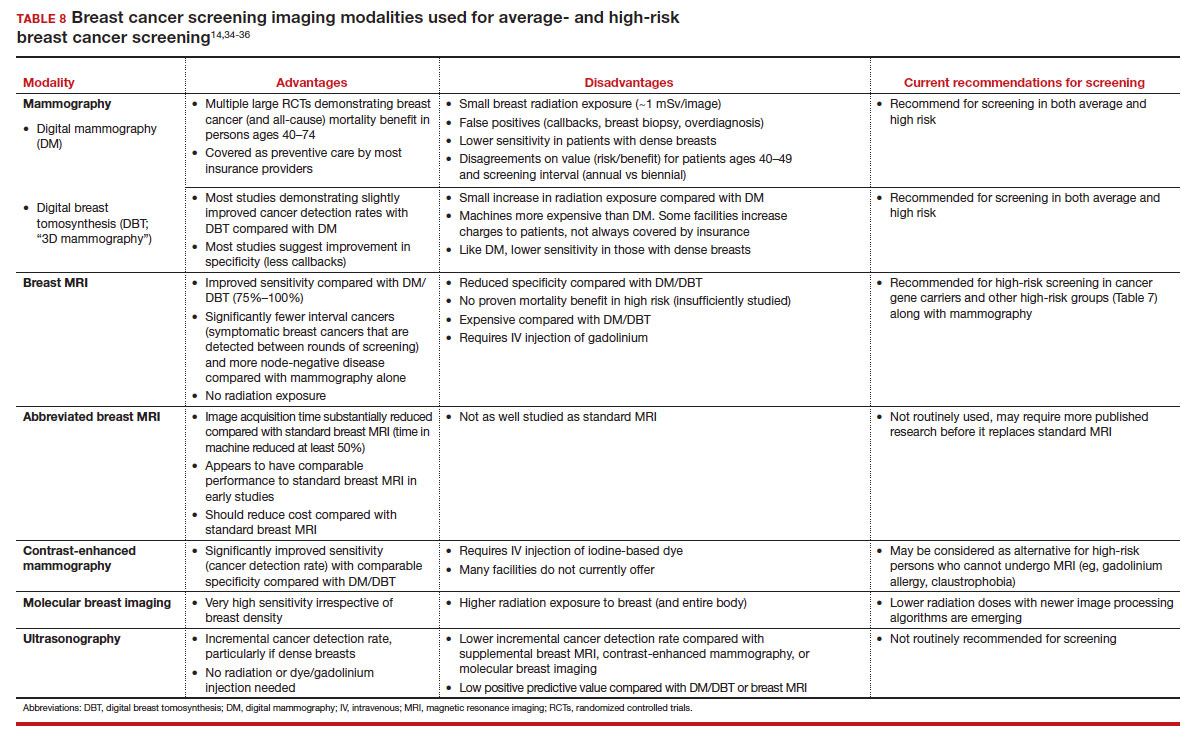
Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Omit radiation in older women with low-risk, ER+ breast cancer
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
‘Only a sociopath could work for a large health system,’ doc says sardonically
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
Not always implemented or enforced: Harassment policies at work
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.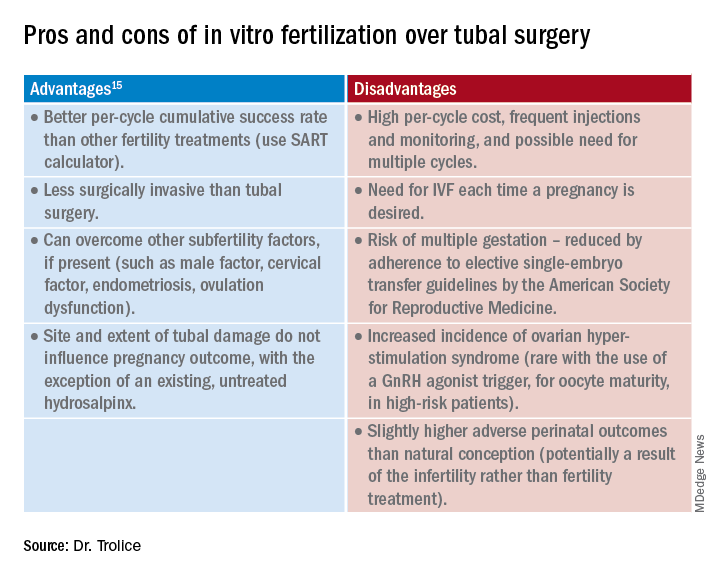
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.