User login
A new way to gauge suicide risk?
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Risk for MS in children often missed
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
New coalition aims to revolutionize stalled lupus research
Clinical research into lupus has long been hampered by failures of medications that initially seemed promising. Now, a coalition of drugmakers, federal regulators, and activists has come together to forge a path toward better-designed studies and – potentially – groundbreaking new drugs.
“We have an opportunity to work collaboratively in lupus to address the challenges in drug development,” Teodora Staeva, PhD, vice president and chief scientific officer of the Lupus Research Alliance, said in an interview.
The alliance held a press conference on March 29 to announce the formation of the public-private Lupus Accelerating Breakthroughs Consortium. Coalition members include several major drugmakers, lupus organizations such as the LRA, the American College of Rheumatology, the Food and Drug Administration, and other federal agencies. Academic researchers, people living with lupus, caregivers and family members, and other members of the lupus community are also on board.
As Dr. Staeva explained, research into lupus has been marked by a high rate of failure. “Often, phase 2 trial successes have not translated into phase 3 successes,” she said.
But researchers, she said, don’t tend to think this is because the drugs themselves are useless.
Instead, it appears that “trial designs are not adequate to capture meaningful readouts of the drug effects, and that may have contributed to the multiple failures,” she said.
According to her, this may because the trials aren’t yet designed to fully detect whether drugs are useful. This is difficult to accomplish since patients have so many manifestations of the disease and trial participants already take a variety of existing drugs.
“Another major limitation has been the lack of integration of the patient’s voice and needs in the drug development process,” she said. It’s also challenging to recruit patients with the most severe lupus to participate in studies, especially since the trials often last 52 weeks.
The new coalition will not directly develop or favor specific drugs. Instead, it will focus on clinical research priorities. “It’s all open and collaborative,” Dr. Staeva explained, and a patient council will provide input. “We have a unique opportunity to bring the voice of people [living with lupus] to the table for the first time and be able to integrate their needs and priorities into the infrastructure.”
The new coalition was inspired by existing public-private partnerships such as the Kidney Health Initiative, she said. That initiative was founded in 2012 by the FDA and the American Society of Nephrology and has dozens of members, including multiple drugmakers and medical societies.
The leadership of the Lupus ABC coalition will include three nonvoting members from the FDA. They’ll offer guidance, Dr. Staeva said. At the press conference, Albert T. Roy, president and CEO of the LRA, said drug companies will appreciate the opportunity to speak with FDA representatives “in a space that is not competitive with respect to intellectual property or anything like that.”
The coalition will meet later in spring 2023, Dr. Staeva said. She hopes it will launch a couple of projects by the end of 2023 and be able to release preliminary results by the end of 2024.
One challenge will be figuring out how to stratify trial subjects so drug studies will more easily detect medications that may work in smaller populations of patients, Hoang Nguyen, PhD, director of scientific partnerships at the LRA, said in an interview. “Now we lump [patients] all together, and that’s not the optimal way to test drugs on patients who have a lot of differences.”
According to Dr. Staeva, the LRA funded the development of the coalition, and drugmakers will primarily provide financial support going forward. The pharmaceutical company members of the coalition are Biogen, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Genentech, Gilead, GlaxoSmithKline, Merck, and Takeda.
Dr. Staeva, Dr. Nguyen, and Mr. Roy have no disclosures.
Clinical research into lupus has long been hampered by failures of medications that initially seemed promising. Now, a coalition of drugmakers, federal regulators, and activists has come together to forge a path toward better-designed studies and – potentially – groundbreaking new drugs.
“We have an opportunity to work collaboratively in lupus to address the challenges in drug development,” Teodora Staeva, PhD, vice president and chief scientific officer of the Lupus Research Alliance, said in an interview.
The alliance held a press conference on March 29 to announce the formation of the public-private Lupus Accelerating Breakthroughs Consortium. Coalition members include several major drugmakers, lupus organizations such as the LRA, the American College of Rheumatology, the Food and Drug Administration, and other federal agencies. Academic researchers, people living with lupus, caregivers and family members, and other members of the lupus community are also on board.
As Dr. Staeva explained, research into lupus has been marked by a high rate of failure. “Often, phase 2 trial successes have not translated into phase 3 successes,” she said.
But researchers, she said, don’t tend to think this is because the drugs themselves are useless.
Instead, it appears that “trial designs are not adequate to capture meaningful readouts of the drug effects, and that may have contributed to the multiple failures,” she said.
According to her, this may because the trials aren’t yet designed to fully detect whether drugs are useful. This is difficult to accomplish since patients have so many manifestations of the disease and trial participants already take a variety of existing drugs.
“Another major limitation has been the lack of integration of the patient’s voice and needs in the drug development process,” she said. It’s also challenging to recruit patients with the most severe lupus to participate in studies, especially since the trials often last 52 weeks.
The new coalition will not directly develop or favor specific drugs. Instead, it will focus on clinical research priorities. “It’s all open and collaborative,” Dr. Staeva explained, and a patient council will provide input. “We have a unique opportunity to bring the voice of people [living with lupus] to the table for the first time and be able to integrate their needs and priorities into the infrastructure.”
The new coalition was inspired by existing public-private partnerships such as the Kidney Health Initiative, she said. That initiative was founded in 2012 by the FDA and the American Society of Nephrology and has dozens of members, including multiple drugmakers and medical societies.
The leadership of the Lupus ABC coalition will include three nonvoting members from the FDA. They’ll offer guidance, Dr. Staeva said. At the press conference, Albert T. Roy, president and CEO of the LRA, said drug companies will appreciate the opportunity to speak with FDA representatives “in a space that is not competitive with respect to intellectual property or anything like that.”
The coalition will meet later in spring 2023, Dr. Staeva said. She hopes it will launch a couple of projects by the end of 2023 and be able to release preliminary results by the end of 2024.
One challenge will be figuring out how to stratify trial subjects so drug studies will more easily detect medications that may work in smaller populations of patients, Hoang Nguyen, PhD, director of scientific partnerships at the LRA, said in an interview. “Now we lump [patients] all together, and that’s not the optimal way to test drugs on patients who have a lot of differences.”
According to Dr. Staeva, the LRA funded the development of the coalition, and drugmakers will primarily provide financial support going forward. The pharmaceutical company members of the coalition are Biogen, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Genentech, Gilead, GlaxoSmithKline, Merck, and Takeda.
Dr. Staeva, Dr. Nguyen, and Mr. Roy have no disclosures.
Clinical research into lupus has long been hampered by failures of medications that initially seemed promising. Now, a coalition of drugmakers, federal regulators, and activists has come together to forge a path toward better-designed studies and – potentially – groundbreaking new drugs.
“We have an opportunity to work collaboratively in lupus to address the challenges in drug development,” Teodora Staeva, PhD, vice president and chief scientific officer of the Lupus Research Alliance, said in an interview.
The alliance held a press conference on March 29 to announce the formation of the public-private Lupus Accelerating Breakthroughs Consortium. Coalition members include several major drugmakers, lupus organizations such as the LRA, the American College of Rheumatology, the Food and Drug Administration, and other federal agencies. Academic researchers, people living with lupus, caregivers and family members, and other members of the lupus community are also on board.
As Dr. Staeva explained, research into lupus has been marked by a high rate of failure. “Often, phase 2 trial successes have not translated into phase 3 successes,” she said.
But researchers, she said, don’t tend to think this is because the drugs themselves are useless.
Instead, it appears that “trial designs are not adequate to capture meaningful readouts of the drug effects, and that may have contributed to the multiple failures,” she said.
According to her, this may because the trials aren’t yet designed to fully detect whether drugs are useful. This is difficult to accomplish since patients have so many manifestations of the disease and trial participants already take a variety of existing drugs.
“Another major limitation has been the lack of integration of the patient’s voice and needs in the drug development process,” she said. It’s also challenging to recruit patients with the most severe lupus to participate in studies, especially since the trials often last 52 weeks.
The new coalition will not directly develop or favor specific drugs. Instead, it will focus on clinical research priorities. “It’s all open and collaborative,” Dr. Staeva explained, and a patient council will provide input. “We have a unique opportunity to bring the voice of people [living with lupus] to the table for the first time and be able to integrate their needs and priorities into the infrastructure.”
The new coalition was inspired by existing public-private partnerships such as the Kidney Health Initiative, she said. That initiative was founded in 2012 by the FDA and the American Society of Nephrology and has dozens of members, including multiple drugmakers and medical societies.
The leadership of the Lupus ABC coalition will include three nonvoting members from the FDA. They’ll offer guidance, Dr. Staeva said. At the press conference, Albert T. Roy, president and CEO of the LRA, said drug companies will appreciate the opportunity to speak with FDA representatives “in a space that is not competitive with respect to intellectual property or anything like that.”
The coalition will meet later in spring 2023, Dr. Staeva said. She hopes it will launch a couple of projects by the end of 2023 and be able to release preliminary results by the end of 2024.
One challenge will be figuring out how to stratify trial subjects so drug studies will more easily detect medications that may work in smaller populations of patients, Hoang Nguyen, PhD, director of scientific partnerships at the LRA, said in an interview. “Now we lump [patients] all together, and that’s not the optimal way to test drugs on patients who have a lot of differences.”
According to Dr. Staeva, the LRA funded the development of the coalition, and drugmakers will primarily provide financial support going forward. The pharmaceutical company members of the coalition are Biogen, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Genentech, Gilead, GlaxoSmithKline, Merck, and Takeda.
Dr. Staeva, Dr. Nguyen, and Mr. Roy have no disclosures.
COAPT 5-year results ‘remarkable,’ but patient selection issues remain
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.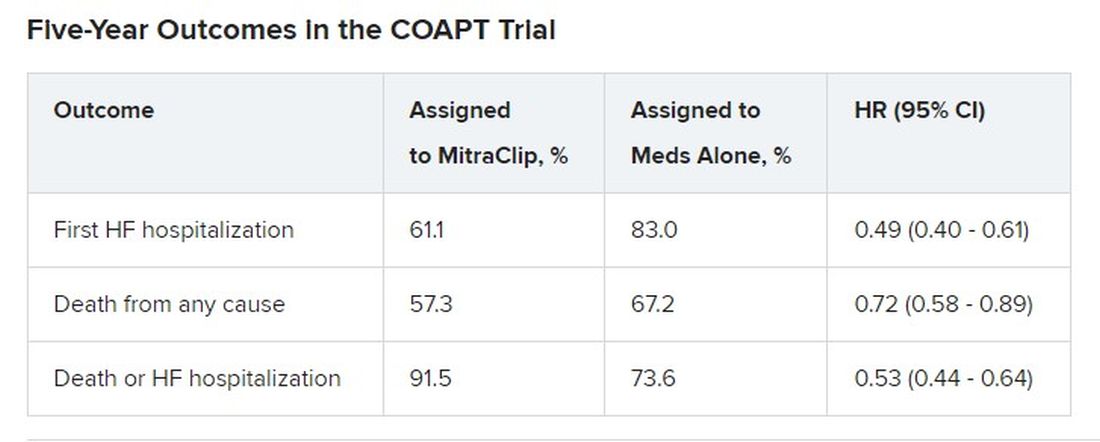
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
FROM ACC 2023
Exercise tied to reduced Parkinson’s motor symptoms and increased well-being
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Telehealth services tied to a major reduction in opioid overdose deaths
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Biosimilars and patients: Discussions should address safety, cost, and anxiety about change
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Meta-analysis compares efficacy of lasmiditan, rimegepant, and ubrogepant for acute treatment of migraine
Key clinical point: Lasmiditan, rimegepant, and ubrogepant demonstrated superior efficacy over placebo for the acute treatment of migraine attacks, with lasmiditan being the most effective at high doses but with higher odds of adverse events.
Major finding: Compared with placebo, 200 mg lasmiditan (odds ratio [OR] 2.88; 95% CI 2.22-3.73), followed by 100 mg lasmiditan (OR 2.28; 95% CI 1.75-2.96), rimegepant (OR 2.0; 95% CI 1.45-2.75), and 100 mg ubrogepant (OR 1.97; 95% CI 1.27-3.07) were more efficacious for achieving pain freedom at 2 hours post-dose before the use of any rescue medication. However, the odds of dizziness, nausea, and somnolence were greater with all doses of lasmiditan.
Study details: This network meta-analysis of seven phase 3 randomized controlled trials included 12,859 patients with migraine
Disclosures: This study did not report the funding source. PJ Goadsby and C Tassorelli declared receiving grants or personal fees or participating in advisory boards or lecturing at symposia for various sources.
Source: Puledda F et al. Efficacy, safety and indirect comparisons of lasmiditan, rimegepant, and ubrogepant for the acute treatment of migraine: A systematic review and network meta-analysis of the literature. Cephalalgia. 2023;43(3): 03331024231151419 (Feb 14). Doi: 10.1177/03331024231151419
Key clinical point: Lasmiditan, rimegepant, and ubrogepant demonstrated superior efficacy over placebo for the acute treatment of migraine attacks, with lasmiditan being the most effective at high doses but with higher odds of adverse events.
Major finding: Compared with placebo, 200 mg lasmiditan (odds ratio [OR] 2.88; 95% CI 2.22-3.73), followed by 100 mg lasmiditan (OR 2.28; 95% CI 1.75-2.96), rimegepant (OR 2.0; 95% CI 1.45-2.75), and 100 mg ubrogepant (OR 1.97; 95% CI 1.27-3.07) were more efficacious for achieving pain freedom at 2 hours post-dose before the use of any rescue medication. However, the odds of dizziness, nausea, and somnolence were greater with all doses of lasmiditan.
Study details: This network meta-analysis of seven phase 3 randomized controlled trials included 12,859 patients with migraine
Disclosures: This study did not report the funding source. PJ Goadsby and C Tassorelli declared receiving grants or personal fees or participating in advisory boards or lecturing at symposia for various sources.
Source: Puledda F et al. Efficacy, safety and indirect comparisons of lasmiditan, rimegepant, and ubrogepant for the acute treatment of migraine: A systematic review and network meta-analysis of the literature. Cephalalgia. 2023;43(3): 03331024231151419 (Feb 14). Doi: 10.1177/03331024231151419
Key clinical point: Lasmiditan, rimegepant, and ubrogepant demonstrated superior efficacy over placebo for the acute treatment of migraine attacks, with lasmiditan being the most effective at high doses but with higher odds of adverse events.
Major finding: Compared with placebo, 200 mg lasmiditan (odds ratio [OR] 2.88; 95% CI 2.22-3.73), followed by 100 mg lasmiditan (OR 2.28; 95% CI 1.75-2.96), rimegepant (OR 2.0; 95% CI 1.45-2.75), and 100 mg ubrogepant (OR 1.97; 95% CI 1.27-3.07) were more efficacious for achieving pain freedom at 2 hours post-dose before the use of any rescue medication. However, the odds of dizziness, nausea, and somnolence were greater with all doses of lasmiditan.
Study details: This network meta-analysis of seven phase 3 randomized controlled trials included 12,859 patients with migraine
Disclosures: This study did not report the funding source. PJ Goadsby and C Tassorelli declared receiving grants or personal fees or participating in advisory boards or lecturing at symposia for various sources.
Source: Puledda F et al. Efficacy, safety and indirect comparisons of lasmiditan, rimegepant, and ubrogepant for the acute treatment of migraine: A systematic review and network meta-analysis of the literature. Cephalalgia. 2023;43(3): 03331024231151419 (Feb 14). Doi: 10.1177/03331024231151419
Real-world study compares benefits for patients with migraine of mAb against CGRP and its receptor
Key clinical point: Both types of anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), those against CGRP ligand (anti-CGRP) and those against CGRP receptor (anti-CGRP-R), showed benefits as preventive treatments for migraine; however, the proportion of anti-CGRP super responders was higher.
Major finding: Patients receiving anti-CGRP vs anti-CGRP-R had a significantly lower Migraine Disability Assessment scale score at 1 (P = .040) and 3 (P = .048) months and mean migraine days at 3 months (P = .01). The proportion of super responders were significantly higher in the anti-CGRP vs anti-CGRP-R group at 3-month (P = .041) and 6-month (P = .047) follow-ups.
Study details: This retrospective observational study included 152 patients with high-frequency episodic migraine or chronic migraine, of whom 68 were treated with anti-CGRP mAbs and 84 were treated with anti-CGRP-R mAbs.
Disclosures: This study did not receive any specific funding. Three authors declared serving as consultants and on scientific advisory boards or receiving speaker honoraria from various sources.
Source: Schiano di Cola F et al. An observational study on monoclonal antibodies against CGRP and its receptor. Eur J Neurol. 2023 (Mar 1). Doi: 10.1111/ene.15761
Key clinical point: Both types of anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), those against CGRP ligand (anti-CGRP) and those against CGRP receptor (anti-CGRP-R), showed benefits as preventive treatments for migraine; however, the proportion of anti-CGRP super responders was higher.
Major finding: Patients receiving anti-CGRP vs anti-CGRP-R had a significantly lower Migraine Disability Assessment scale score at 1 (P = .040) and 3 (P = .048) months and mean migraine days at 3 months (P = .01). The proportion of super responders were significantly higher in the anti-CGRP vs anti-CGRP-R group at 3-month (P = .041) and 6-month (P = .047) follow-ups.
Study details: This retrospective observational study included 152 patients with high-frequency episodic migraine or chronic migraine, of whom 68 were treated with anti-CGRP mAbs and 84 were treated with anti-CGRP-R mAbs.
Disclosures: This study did not receive any specific funding. Three authors declared serving as consultants and on scientific advisory boards or receiving speaker honoraria from various sources.
Source: Schiano di Cola F et al. An observational study on monoclonal antibodies against CGRP and its receptor. Eur J Neurol. 2023 (Mar 1). Doi: 10.1111/ene.15761
Key clinical point: Both types of anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), those against CGRP ligand (anti-CGRP) and those against CGRP receptor (anti-CGRP-R), showed benefits as preventive treatments for migraine; however, the proportion of anti-CGRP super responders was higher.
Major finding: Patients receiving anti-CGRP vs anti-CGRP-R had a significantly lower Migraine Disability Assessment scale score at 1 (P = .040) and 3 (P = .048) months and mean migraine days at 3 months (P = .01). The proportion of super responders were significantly higher in the anti-CGRP vs anti-CGRP-R group at 3-month (P = .041) and 6-month (P = .047) follow-ups.
Study details: This retrospective observational study included 152 patients with high-frequency episodic migraine or chronic migraine, of whom 68 were treated with anti-CGRP mAbs and 84 were treated with anti-CGRP-R mAbs.
Disclosures: This study did not receive any specific funding. Three authors declared serving as consultants and on scientific advisory boards or receiving speaker honoraria from various sources.
Source: Schiano di Cola F et al. An observational study on monoclonal antibodies against CGRP and its receptor. Eur J Neurol. 2023 (Mar 1). Doi: 10.1111/ene.15761
Fremanezumab shows favorable benefit-risk profile in difficult-to-treat migraine
Key clinical point: Real-world data support fremanezumab as an effective, safe, and well-tolerated treatment option in patients with difficult-to-treat migraine and multiple preventive treatment failures.
Major finding: Overall, 83.5% and 62.6% of patients with high-frequency episodic migraine (HFEM) and chronic migraine (CM) receiving fremanezumab achieved ≥50% reduction in monthly headache days (MHD), respectively, along with a significant improvement in mean MHD, MHD with peak headache intensity of ≤5, intake of any abortive medications, migraine-related disability, and quality of life (all P < .001). Only 36 cases of mild adverse events were reported.
Study details: This open-label, single-arm, prospective, multicenter, clinical study included 204 patients with HFEM (n = 97) or CM (n = 107) who received ≥3 monthly courses of fremanezumab.
Disclosures: This study did not receive any funding. Some authors declared receiving investigator fees or travel grants from, or serving as consultants or advisory board members for various sources.
Source: Argyriou AA et al. Efficacy and safety of fremanezumab for migraine prophylaxis in patients with at least three previous preventive failures: Prospective, multicenter, real-world data from a Greek registry. Eur J Neurol. 2023 (Feb 11). Doi: 10.1111/ene.15740
Key clinical point: Real-world data support fremanezumab as an effective, safe, and well-tolerated treatment option in patients with difficult-to-treat migraine and multiple preventive treatment failures.
Major finding: Overall, 83.5% and 62.6% of patients with high-frequency episodic migraine (HFEM) and chronic migraine (CM) receiving fremanezumab achieved ≥50% reduction in monthly headache days (MHD), respectively, along with a significant improvement in mean MHD, MHD with peak headache intensity of ≤5, intake of any abortive medications, migraine-related disability, and quality of life (all P < .001). Only 36 cases of mild adverse events were reported.
Study details: This open-label, single-arm, prospective, multicenter, clinical study included 204 patients with HFEM (n = 97) or CM (n = 107) who received ≥3 monthly courses of fremanezumab.
Disclosures: This study did not receive any funding. Some authors declared receiving investigator fees or travel grants from, or serving as consultants or advisory board members for various sources.
Source: Argyriou AA et al. Efficacy and safety of fremanezumab for migraine prophylaxis in patients with at least three previous preventive failures: Prospective, multicenter, real-world data from a Greek registry. Eur J Neurol. 2023 (Feb 11). Doi: 10.1111/ene.15740
Key clinical point: Real-world data support fremanezumab as an effective, safe, and well-tolerated treatment option in patients with difficult-to-treat migraine and multiple preventive treatment failures.
Major finding: Overall, 83.5% and 62.6% of patients with high-frequency episodic migraine (HFEM) and chronic migraine (CM) receiving fremanezumab achieved ≥50% reduction in monthly headache days (MHD), respectively, along with a significant improvement in mean MHD, MHD with peak headache intensity of ≤5, intake of any abortive medications, migraine-related disability, and quality of life (all P < .001). Only 36 cases of mild adverse events were reported.
Study details: This open-label, single-arm, prospective, multicenter, clinical study included 204 patients with HFEM (n = 97) or CM (n = 107) who received ≥3 monthly courses of fremanezumab.
Disclosures: This study did not receive any funding. Some authors declared receiving investigator fees or travel grants from, or serving as consultants or advisory board members for various sources.
Source: Argyriou AA et al. Efficacy and safety of fremanezumab for migraine prophylaxis in patients with at least three previous preventive failures: Prospective, multicenter, real-world data from a Greek registry. Eur J Neurol. 2023 (Feb 11). Doi: 10.1111/ene.15740










