User login
A clash of expectations
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Machine learning can predict primary progressive MS progression
BOSTON – , according to a proof-of-concept study presented at the 2023 annual meeting of the American Academy of Neurology.
The accuracy was sufficient for the lead author of the study, Michael Gurevich, PhD, head of the Neuroimmunological Laboratory in the Multiple Sclerosis Center of Sheba Medical Center in Ramat Gan, Israel, to say that it is already clinically viable even as the tool is still evolving.
“We are looking at larger sample sizes to improve the accuracy and generalizability of our model, but we can use it now to inform treatment decisions,” Dr. Gurevich said.
In patients with PPMS who have a highly variable course, the model predicts disability progression with an accuracy of approximately 70%, according to the data he presented. He said he believes this is already at a level that it is meaningful for a risk-to-benefit calculation when considering treatment.
Machine learning analyzes blood samples
The study pursues the concept that the genetic information governing highly complex pathophysiological processes is contained in RNA sequencing. While multimodal omics generate data that are too complex for human pattern recognition, there is a growing body of evidence, including that provided by this study, to suggest that machine learning can employ these same RNA profiles and predict disease activity.
In this study, blood samples were collected from patients who participated in the phase 3 clinical ORATORIO trial that led to approval of ocrelizumab for PPMS. Analyses were conducted only on blood samples from those randomized to placebo, who, like those in the active treatment arm, were evaluated at baseline and at 12-week intervals for more than 2 years.
After development of a prediction model and creation of a training dataset, machine learning was applied to the deep sequencing of the blood transcriptome data for predicting two endpoints. One was disease progression at 120 weeks defined as a 1 point or more increase in the Expanded Disability Status Scale (EDSS) among patients with confirmed disability progression for at least 12 weeks (12W-CDP).
The other was change at 120 weeks in brain morphology defined as a 1% or more reduction in brain volume (120W PBVC).
The peripheral blood samples were subjected to RNA sequencing analysis (RNA-Seq) using commercially available analysis techniques. The prediction model for the disability endpoint was based on data generated from the blood transcriptome of 135 patients of which 53 (39%) met the endpoint at 120 weeks. The prediction model for the change in brain morphology was based on the blood transcriptome from 94 patients of which 63 (67%) met the endpoint.
On the basis of 10 genes that most significantly differentiated those who met the disability endpoint from those who did not, machine recognition of patterns after training was able to predict correctly the outcome in 70.9%. The sensitivity was 55.6%, and the specificity was 79.0%. The positive and negative predictive values were 59.0% and 76.8%, respectively.
On the basis of the 12 genes the most significantly differentiated those that reached the 120W PBVC endpoint from those who did not, machine learning resulted in a correct prediction of outcomes in 75.1%. The sensitivity was 78.1%, and the specificity was 66.7%. The positive and negative predictive values were 83.3% and 58.8%, respectively
Typical of a PPMS trial population, the mean age of the patients was about 44 years. The mean disease duration was about 6 years. The majority of patients had an EDSS score below 5.5 at baseline. The baseline T2 lesion number was approximately 50.
If further validated by others and in larger studies, this type of information could play a valuable role in PPMS management, according to Dr. Gurevich. Now that there is an approved therapy for PPMS, it can help clinicians and patients determine whether to initiate treatment early to address the high risk of progression or delay treatment that might not be needed.
A useful tool
In the field of MS, most of the studies performed with machine learning have focused on the analysis of radiological images. However, others are now looking at the blood transcriptome as a potential path to better classifying a highly complex disease with substantial heterogeneity in presentation, progression, and outcome.
For example, machine learning of the blood transcriptome has also shown high accuracy in the diagnosis and classification of MS in patients with clinically isolated syndrome (CIS). One study, published in Cell Reports Medicine, was led by Cinthia Farina, PhD, Institute of Experimental Neurology, IRCCS San Raffaele Scientific Institute, Milan.
Although she did not hear the presentation by Dr. Gurevich, Dr. Farina is enthusiastic about the potential for machine learning to help manage MS through the analysis of the blood transcriptome. “I do believe that transcriptomics in peripheral immune cells may become a useful tool for MS diagnosis and prognosis,” she said.
In her own study, in which machine learning algorithms were developed and trained on the basis of peripheral blood from patients with CIS, the tool proved accurate with a strong potential for being incorporated into routine clinical management.
“Machine learning applied to the blood transcriptomes was extremely efficient with a 95.6% accuracy in discriminating PPMS from RRMS [relapsing-remitting] MS,” she reported.
Dr. Gurevich has no potential financial conflicts of interest to report. He reported funding for the study was provided by Roche. Dr. Farina reports financial relationships with Merck-Serono, Novartis, and Teva.
BOSTON – , according to a proof-of-concept study presented at the 2023 annual meeting of the American Academy of Neurology.
The accuracy was sufficient for the lead author of the study, Michael Gurevich, PhD, head of the Neuroimmunological Laboratory in the Multiple Sclerosis Center of Sheba Medical Center in Ramat Gan, Israel, to say that it is already clinically viable even as the tool is still evolving.
“We are looking at larger sample sizes to improve the accuracy and generalizability of our model, but we can use it now to inform treatment decisions,” Dr. Gurevich said.
In patients with PPMS who have a highly variable course, the model predicts disability progression with an accuracy of approximately 70%, according to the data he presented. He said he believes this is already at a level that it is meaningful for a risk-to-benefit calculation when considering treatment.
Machine learning analyzes blood samples
The study pursues the concept that the genetic information governing highly complex pathophysiological processes is contained in RNA sequencing. While multimodal omics generate data that are too complex for human pattern recognition, there is a growing body of evidence, including that provided by this study, to suggest that machine learning can employ these same RNA profiles and predict disease activity.
In this study, blood samples were collected from patients who participated in the phase 3 clinical ORATORIO trial that led to approval of ocrelizumab for PPMS. Analyses were conducted only on blood samples from those randomized to placebo, who, like those in the active treatment arm, were evaluated at baseline and at 12-week intervals for more than 2 years.
After development of a prediction model and creation of a training dataset, machine learning was applied to the deep sequencing of the blood transcriptome data for predicting two endpoints. One was disease progression at 120 weeks defined as a 1 point or more increase in the Expanded Disability Status Scale (EDSS) among patients with confirmed disability progression for at least 12 weeks (12W-CDP).
The other was change at 120 weeks in brain morphology defined as a 1% or more reduction in brain volume (120W PBVC).
The peripheral blood samples were subjected to RNA sequencing analysis (RNA-Seq) using commercially available analysis techniques. The prediction model for the disability endpoint was based on data generated from the blood transcriptome of 135 patients of which 53 (39%) met the endpoint at 120 weeks. The prediction model for the change in brain morphology was based on the blood transcriptome from 94 patients of which 63 (67%) met the endpoint.
On the basis of 10 genes that most significantly differentiated those who met the disability endpoint from those who did not, machine recognition of patterns after training was able to predict correctly the outcome in 70.9%. The sensitivity was 55.6%, and the specificity was 79.0%. The positive and negative predictive values were 59.0% and 76.8%, respectively.
On the basis of the 12 genes the most significantly differentiated those that reached the 120W PBVC endpoint from those who did not, machine learning resulted in a correct prediction of outcomes in 75.1%. The sensitivity was 78.1%, and the specificity was 66.7%. The positive and negative predictive values were 83.3% and 58.8%, respectively
Typical of a PPMS trial population, the mean age of the patients was about 44 years. The mean disease duration was about 6 years. The majority of patients had an EDSS score below 5.5 at baseline. The baseline T2 lesion number was approximately 50.
If further validated by others and in larger studies, this type of information could play a valuable role in PPMS management, according to Dr. Gurevich. Now that there is an approved therapy for PPMS, it can help clinicians and patients determine whether to initiate treatment early to address the high risk of progression or delay treatment that might not be needed.
A useful tool
In the field of MS, most of the studies performed with machine learning have focused on the analysis of radiological images. However, others are now looking at the blood transcriptome as a potential path to better classifying a highly complex disease with substantial heterogeneity in presentation, progression, and outcome.
For example, machine learning of the blood transcriptome has also shown high accuracy in the diagnosis and classification of MS in patients with clinically isolated syndrome (CIS). One study, published in Cell Reports Medicine, was led by Cinthia Farina, PhD, Institute of Experimental Neurology, IRCCS San Raffaele Scientific Institute, Milan.
Although she did not hear the presentation by Dr. Gurevich, Dr. Farina is enthusiastic about the potential for machine learning to help manage MS through the analysis of the blood transcriptome. “I do believe that transcriptomics in peripheral immune cells may become a useful tool for MS diagnosis and prognosis,” she said.
In her own study, in which machine learning algorithms were developed and trained on the basis of peripheral blood from patients with CIS, the tool proved accurate with a strong potential for being incorporated into routine clinical management.
“Machine learning applied to the blood transcriptomes was extremely efficient with a 95.6% accuracy in discriminating PPMS from RRMS [relapsing-remitting] MS,” she reported.
Dr. Gurevich has no potential financial conflicts of interest to report. He reported funding for the study was provided by Roche. Dr. Farina reports financial relationships with Merck-Serono, Novartis, and Teva.
BOSTON – , according to a proof-of-concept study presented at the 2023 annual meeting of the American Academy of Neurology.
The accuracy was sufficient for the lead author of the study, Michael Gurevich, PhD, head of the Neuroimmunological Laboratory in the Multiple Sclerosis Center of Sheba Medical Center in Ramat Gan, Israel, to say that it is already clinically viable even as the tool is still evolving.
“We are looking at larger sample sizes to improve the accuracy and generalizability of our model, but we can use it now to inform treatment decisions,” Dr. Gurevich said.
In patients with PPMS who have a highly variable course, the model predicts disability progression with an accuracy of approximately 70%, according to the data he presented. He said he believes this is already at a level that it is meaningful for a risk-to-benefit calculation when considering treatment.
Machine learning analyzes blood samples
The study pursues the concept that the genetic information governing highly complex pathophysiological processes is contained in RNA sequencing. While multimodal omics generate data that are too complex for human pattern recognition, there is a growing body of evidence, including that provided by this study, to suggest that machine learning can employ these same RNA profiles and predict disease activity.
In this study, blood samples were collected from patients who participated in the phase 3 clinical ORATORIO trial that led to approval of ocrelizumab for PPMS. Analyses were conducted only on blood samples from those randomized to placebo, who, like those in the active treatment arm, were evaluated at baseline and at 12-week intervals for more than 2 years.
After development of a prediction model and creation of a training dataset, machine learning was applied to the deep sequencing of the blood transcriptome data for predicting two endpoints. One was disease progression at 120 weeks defined as a 1 point or more increase in the Expanded Disability Status Scale (EDSS) among patients with confirmed disability progression for at least 12 weeks (12W-CDP).
The other was change at 120 weeks in brain morphology defined as a 1% or more reduction in brain volume (120W PBVC).
The peripheral blood samples were subjected to RNA sequencing analysis (RNA-Seq) using commercially available analysis techniques. The prediction model for the disability endpoint was based on data generated from the blood transcriptome of 135 patients of which 53 (39%) met the endpoint at 120 weeks. The prediction model for the change in brain morphology was based on the blood transcriptome from 94 patients of which 63 (67%) met the endpoint.
On the basis of 10 genes that most significantly differentiated those who met the disability endpoint from those who did not, machine recognition of patterns after training was able to predict correctly the outcome in 70.9%. The sensitivity was 55.6%, and the specificity was 79.0%. The positive and negative predictive values were 59.0% and 76.8%, respectively.
On the basis of the 12 genes the most significantly differentiated those that reached the 120W PBVC endpoint from those who did not, machine learning resulted in a correct prediction of outcomes in 75.1%. The sensitivity was 78.1%, and the specificity was 66.7%. The positive and negative predictive values were 83.3% and 58.8%, respectively
Typical of a PPMS trial population, the mean age of the patients was about 44 years. The mean disease duration was about 6 years. The majority of patients had an EDSS score below 5.5 at baseline. The baseline T2 lesion number was approximately 50.
If further validated by others and in larger studies, this type of information could play a valuable role in PPMS management, according to Dr. Gurevich. Now that there is an approved therapy for PPMS, it can help clinicians and patients determine whether to initiate treatment early to address the high risk of progression or delay treatment that might not be needed.
A useful tool
In the field of MS, most of the studies performed with machine learning have focused on the analysis of radiological images. However, others are now looking at the blood transcriptome as a potential path to better classifying a highly complex disease with substantial heterogeneity in presentation, progression, and outcome.
For example, machine learning of the blood transcriptome has also shown high accuracy in the diagnosis and classification of MS in patients with clinically isolated syndrome (CIS). One study, published in Cell Reports Medicine, was led by Cinthia Farina, PhD, Institute of Experimental Neurology, IRCCS San Raffaele Scientific Institute, Milan.
Although she did not hear the presentation by Dr. Gurevich, Dr. Farina is enthusiastic about the potential for machine learning to help manage MS through the analysis of the blood transcriptome. “I do believe that transcriptomics in peripheral immune cells may become a useful tool for MS diagnosis and prognosis,” she said.
In her own study, in which machine learning algorithms were developed and trained on the basis of peripheral blood from patients with CIS, the tool proved accurate with a strong potential for being incorporated into routine clinical management.
“Machine learning applied to the blood transcriptomes was extremely efficient with a 95.6% accuracy in discriminating PPMS from RRMS [relapsing-remitting] MS,” she reported.
Dr. Gurevich has no potential financial conflicts of interest to report. He reported funding for the study was provided by Roche. Dr. Farina reports financial relationships with Merck-Serono, Novartis, and Teva.
FROM AAN 2023
Personalizing treatment plans for older patients with T2D
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Smoking cessation has many benefits in diabetes
MONTPELLIER, FRANCE – The first expert consensus on smoking and diabetes, coauthored by the Francophone Diabetes Society (SFD) and the French Society for the Study of Nicotine Addiction (SFT), was presented at the SFD’s annual conference.
Alexia Rouland, MD, an endocrinologist at Dijon Bourgogne University Hospital, Dijon, France, took the conference as an opportunity to list the many benefits of smoking cessation for patients with diabetes, despite the “slight and temporary” risk for blood sugar imbalance.
Societies target smoking
Diabetes societies around Europe have set their sights on the topic of smoking. Indeed, the guidelines published in 2019 by the European Association for the Study of Diabetes and the European Society of Cardiology state that “smoking cessation is obligatory for all prediabetic and diabetic patients” (class I, level A).
This year, the France-based SFD and SFT dedicated an expert consensus to the major problem of smoking in patients with diabetes. The aim was to provide health care professionals with convincing, well-supported arguments in favor of smoking cessation in their patients with type 1 and type 2 diabetes.
“Before anything else, diabetic patients need to be made aware of the risks of smoking,” said Dr. Rouland. “It’s not just about the fear factor, though. It’s also about providing a positive incentive – they need to be told about the ways they’ll benefit from quitting smoking. For example, you have all-cause mortality, macro- and microangiopathic complications, and so on.”
Duration of abstinence
“Diabetic patients who have stopped smoking have a relative risk for all-cause mortality of 1.28 (1.09-1.51), which is less than what you see in active smokers (relative risk = 1.58; 1.42-1.77), but still above that of nonsmokers,” said Dr. Rouland.
A previous study revealed that although the risk does indeed go down after stopping smoking, it is linked to how long ago the person stopped. Patients who stopped smoking less than 10 years ago still had a slightly raised all-cause mortality risk, and this was even higher if they had smoked for 20 years or more.
After 10 years of not smoking, however, the greater all-cause mortality risk was no longer significant in any of the groups monitored (smoking duration, number of cigarettes/day). Concrete evidence of the link between all-cause mortality and the length of time since a person stopped smoking also emerged from the large cohort in the American Nurses’ Health Study.
The relative risk for all-cause mortality in women who stopped smoking less than 5 years ago remained high (RR = 1.96, 1.47-2.67), then decreased over time. After 10 years, it was no longer significant (RR = 1.11, 0.92-1.35).
Macro- and microangiopathic risks
Smoking cessation also has a real benefit in terms of the increased macro- and microangiopathic risks. In type 2 diabetes, a study found an increased relative risk for macro- and microalbuminuria of 1.86 (95% confidence interval, 1.37-2.52) in former smokers, compared with an increased relative risk of 2.61 (95% CI, 1.86-2.64) in current smokers.
In type 1 diabetes, the cumulative risk for microalbuminuria in former smokers was 15.1% vs. 18.9% in smokers and 10% in nonsmokers.
A 2019 meta-analysis of prospective cohort studies determined that smoking is an independent risk factor for diabetic nephropathy, especially in patients with type 1 diabetes.
Yet, most of the data for this condition come from subjects with type 2 diabetes. One publication estimated its prevalence after a 1-year follow-up of the smoking cessation program as 10.9% in former smokers and 15% in those who continued smoking.
In regard to macroangiopathy in the context of type 2 diabetes, the aforementioned 2019 meta-analysis focused on coronary artery disease, cerebrovascular accident (CVA), cardiovascular mortality, and myocardial infarction (MI). It found that smokers face an increased risk for all these outcomes.
The relative risks wavered between 1.53 and 1.66 and decreased after smoking cessation. For coronary artery disease and MI, they became insignificant. There was still a risk for CVA (RR = 1.34; 1.07-1.67) and fatal cardiovascular events (RR = 1.19; 0.02-1.39).
The data are slightly more heterogeneous for type 1 diabetes, where, despite smoking cessation, the increased risk for heart failure and CVA persists in men, yet the same risk for coronary heart disease and CVA drops in women.
Risk for weight gain
Dr. Rouland tried to reassure patients about the risk for gaining weight. “Weight gain is not inevitable. There is a risk for this, but it’s temporary. And, even with some weight gain, the cardiovascular benefits are still indisputable.”
A study carried out in 2013 focused specifically on this point, with an average post-cessation weight gain of 3.8 kg (8.4 lb) seen in diabetic individuals in the first 4 years after stopping smoking and of 0.1 kg (0.2 lb) thereafter. A time-based effect was observed with regulation of excess weight post-cessation over time, as seen in the general population (3 kg [6.6 lb] on average in nondiabetic individuals).
Weight gain tends to occur mainly in the immediate post-cessation period, essentially in the first 3 months, and there is a large variation in weight change. Some people gain a lot (from 5 to 10 kg [11 to 22 lb], or even more than 10 kg); others lose weight (20% of diabetic former smokers in the first month, 7% after 12 months), and 25% gain less than 5 kg (11 lb).
Blood glucose imbalance
“A risk for blood glucose imbalance has been reported after smoking cessation, although this is very slight and only temporary,” said Dr. Rouland.
A British retrospective study examined this question, focusing on glycated hemoglobin in patients with type 2 diabetes. Hemoglobin A1c increased by 0.21% (95% CI, 0.17-0.25; P < .001) within the first year after quitting. A1c decreased as abstinence continued and became comparable to that of continual smokers after 3 years. This increase in A1c was not mediated by weight change.
Another study published in 2018 on the topic of type 2 diabetes also reported on the risk for poor glycemic control (defined as A1c > 7%) persisting for 10 years after smoking cessation (odds ratio, 1.23; 95% CI, 1.06-1.42). Thereafter, between 10 and 19 years post-cessation, the OR decreased to 0.97 (95% CI, 0.80-1.19, NS). Beyond 20 years post-cessation, the OR was 1.14 (95% CI, 0.89-1.44, NS) and was therefore no longer significant.
Regardless, “the risk for poor glycemic control is lower in quitters than in active smokers,” said Dr. Rouland.
Quitting and diabetes risk
Will a smoker’s increased risk for diabetes drop when he or she stops smoking? “This is essentially what happens,” Dr. Rouland confirmed, “and his or her increased risk for metabolic syndrome also drops. One meta-analysis revealed a time-based effect.
“Patients who had stopped smoking less than 5 years previously had an increased relative risk for type 2 diabetes, and this risk dropped to 1.11 after more than 10 years of not smoking. Moreover, this relative risk for type 2 diabetes remained lower than that of active smokers, at between 1.19 and 1.60, depending on tobacco use.”
In regard to the risk for metabolic syndrome, those who quit smoking seem to have an increased risk of 10%, compared with nonsmokers (RR = 1.10, 1.08-1.11; P < .001). “But yet again, this increased risk is much lower than that of active smokers, whose risk is between 37% (less than 20 cigarettes/day) and 71% (more than 20 cigarettes/day).”
Women with diabetes
“The benefits of quitting appear identical, regardless of the sex of the diabetic person,” said Dr. Rouland. “As in the general population, weight gain after smoking cessation is greater in women. Furthermore, while smoking increases the risk for gestational diabetes (RR, 1.4-1.9) and for the use of insulin in this context, stopping smoking reduces these risks.
“Moreover, smoking during pregnancy not only increases the risk for pregnancy-related complications (early miscarriage, ectopic pregnancy, birth defects, placental abruption, premature birth, intrauterine fetal demise, cesarean birth, low birth weight), but it also increases the risk of type 2 diabetes in the newborn. The risk to the newborn is said to be around 34% in cases in which the mother smokes during pregnancy and 22% in cases in which the mother is a passive smoker, thereby justifying the use of measures to help the mother’s family members to stop smoking.”
Dr. Rouland reports no relevant financial relationships.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
MONTPELLIER, FRANCE – The first expert consensus on smoking and diabetes, coauthored by the Francophone Diabetes Society (SFD) and the French Society for the Study of Nicotine Addiction (SFT), was presented at the SFD’s annual conference.
Alexia Rouland, MD, an endocrinologist at Dijon Bourgogne University Hospital, Dijon, France, took the conference as an opportunity to list the many benefits of smoking cessation for patients with diabetes, despite the “slight and temporary” risk for blood sugar imbalance.
Societies target smoking
Diabetes societies around Europe have set their sights on the topic of smoking. Indeed, the guidelines published in 2019 by the European Association for the Study of Diabetes and the European Society of Cardiology state that “smoking cessation is obligatory for all prediabetic and diabetic patients” (class I, level A).
This year, the France-based SFD and SFT dedicated an expert consensus to the major problem of smoking in patients with diabetes. The aim was to provide health care professionals with convincing, well-supported arguments in favor of smoking cessation in their patients with type 1 and type 2 diabetes.
“Before anything else, diabetic patients need to be made aware of the risks of smoking,” said Dr. Rouland. “It’s not just about the fear factor, though. It’s also about providing a positive incentive – they need to be told about the ways they’ll benefit from quitting smoking. For example, you have all-cause mortality, macro- and microangiopathic complications, and so on.”
Duration of abstinence
“Diabetic patients who have stopped smoking have a relative risk for all-cause mortality of 1.28 (1.09-1.51), which is less than what you see in active smokers (relative risk = 1.58; 1.42-1.77), but still above that of nonsmokers,” said Dr. Rouland.
A previous study revealed that although the risk does indeed go down after stopping smoking, it is linked to how long ago the person stopped. Patients who stopped smoking less than 10 years ago still had a slightly raised all-cause mortality risk, and this was even higher if they had smoked for 20 years or more.
After 10 years of not smoking, however, the greater all-cause mortality risk was no longer significant in any of the groups monitored (smoking duration, number of cigarettes/day). Concrete evidence of the link between all-cause mortality and the length of time since a person stopped smoking also emerged from the large cohort in the American Nurses’ Health Study.
The relative risk for all-cause mortality in women who stopped smoking less than 5 years ago remained high (RR = 1.96, 1.47-2.67), then decreased over time. After 10 years, it was no longer significant (RR = 1.11, 0.92-1.35).
Macro- and microangiopathic risks
Smoking cessation also has a real benefit in terms of the increased macro- and microangiopathic risks. In type 2 diabetes, a study found an increased relative risk for macro- and microalbuminuria of 1.86 (95% confidence interval, 1.37-2.52) in former smokers, compared with an increased relative risk of 2.61 (95% CI, 1.86-2.64) in current smokers.
In type 1 diabetes, the cumulative risk for microalbuminuria in former smokers was 15.1% vs. 18.9% in smokers and 10% in nonsmokers.
A 2019 meta-analysis of prospective cohort studies determined that smoking is an independent risk factor for diabetic nephropathy, especially in patients with type 1 diabetes.
Yet, most of the data for this condition come from subjects with type 2 diabetes. One publication estimated its prevalence after a 1-year follow-up of the smoking cessation program as 10.9% in former smokers and 15% in those who continued smoking.
In regard to macroangiopathy in the context of type 2 diabetes, the aforementioned 2019 meta-analysis focused on coronary artery disease, cerebrovascular accident (CVA), cardiovascular mortality, and myocardial infarction (MI). It found that smokers face an increased risk for all these outcomes.
The relative risks wavered between 1.53 and 1.66 and decreased after smoking cessation. For coronary artery disease and MI, they became insignificant. There was still a risk for CVA (RR = 1.34; 1.07-1.67) and fatal cardiovascular events (RR = 1.19; 0.02-1.39).
The data are slightly more heterogeneous for type 1 diabetes, where, despite smoking cessation, the increased risk for heart failure and CVA persists in men, yet the same risk for coronary heart disease and CVA drops in women.
Risk for weight gain
Dr. Rouland tried to reassure patients about the risk for gaining weight. “Weight gain is not inevitable. There is a risk for this, but it’s temporary. And, even with some weight gain, the cardiovascular benefits are still indisputable.”
A study carried out in 2013 focused specifically on this point, with an average post-cessation weight gain of 3.8 kg (8.4 lb) seen in diabetic individuals in the first 4 years after stopping smoking and of 0.1 kg (0.2 lb) thereafter. A time-based effect was observed with regulation of excess weight post-cessation over time, as seen in the general population (3 kg [6.6 lb] on average in nondiabetic individuals).
Weight gain tends to occur mainly in the immediate post-cessation period, essentially in the first 3 months, and there is a large variation in weight change. Some people gain a lot (from 5 to 10 kg [11 to 22 lb], or even more than 10 kg); others lose weight (20% of diabetic former smokers in the first month, 7% after 12 months), and 25% gain less than 5 kg (11 lb).
Blood glucose imbalance
“A risk for blood glucose imbalance has been reported after smoking cessation, although this is very slight and only temporary,” said Dr. Rouland.
A British retrospective study examined this question, focusing on glycated hemoglobin in patients with type 2 diabetes. Hemoglobin A1c increased by 0.21% (95% CI, 0.17-0.25; P < .001) within the first year after quitting. A1c decreased as abstinence continued and became comparable to that of continual smokers after 3 years. This increase in A1c was not mediated by weight change.
Another study published in 2018 on the topic of type 2 diabetes also reported on the risk for poor glycemic control (defined as A1c > 7%) persisting for 10 years after smoking cessation (odds ratio, 1.23; 95% CI, 1.06-1.42). Thereafter, between 10 and 19 years post-cessation, the OR decreased to 0.97 (95% CI, 0.80-1.19, NS). Beyond 20 years post-cessation, the OR was 1.14 (95% CI, 0.89-1.44, NS) and was therefore no longer significant.
Regardless, “the risk for poor glycemic control is lower in quitters than in active smokers,” said Dr. Rouland.
Quitting and diabetes risk
Will a smoker’s increased risk for diabetes drop when he or she stops smoking? “This is essentially what happens,” Dr. Rouland confirmed, “and his or her increased risk for metabolic syndrome also drops. One meta-analysis revealed a time-based effect.
“Patients who had stopped smoking less than 5 years previously had an increased relative risk for type 2 diabetes, and this risk dropped to 1.11 after more than 10 years of not smoking. Moreover, this relative risk for type 2 diabetes remained lower than that of active smokers, at between 1.19 and 1.60, depending on tobacco use.”
In regard to the risk for metabolic syndrome, those who quit smoking seem to have an increased risk of 10%, compared with nonsmokers (RR = 1.10, 1.08-1.11; P < .001). “But yet again, this increased risk is much lower than that of active smokers, whose risk is between 37% (less than 20 cigarettes/day) and 71% (more than 20 cigarettes/day).”
Women with diabetes
“The benefits of quitting appear identical, regardless of the sex of the diabetic person,” said Dr. Rouland. “As in the general population, weight gain after smoking cessation is greater in women. Furthermore, while smoking increases the risk for gestational diabetes (RR, 1.4-1.9) and for the use of insulin in this context, stopping smoking reduces these risks.
“Moreover, smoking during pregnancy not only increases the risk for pregnancy-related complications (early miscarriage, ectopic pregnancy, birth defects, placental abruption, premature birth, intrauterine fetal demise, cesarean birth, low birth weight), but it also increases the risk of type 2 diabetes in the newborn. The risk to the newborn is said to be around 34% in cases in which the mother smokes during pregnancy and 22% in cases in which the mother is a passive smoker, thereby justifying the use of measures to help the mother’s family members to stop smoking.”
Dr. Rouland reports no relevant financial relationships.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
MONTPELLIER, FRANCE – The first expert consensus on smoking and diabetes, coauthored by the Francophone Diabetes Society (SFD) and the French Society for the Study of Nicotine Addiction (SFT), was presented at the SFD’s annual conference.
Alexia Rouland, MD, an endocrinologist at Dijon Bourgogne University Hospital, Dijon, France, took the conference as an opportunity to list the many benefits of smoking cessation for patients with diabetes, despite the “slight and temporary” risk for blood sugar imbalance.
Societies target smoking
Diabetes societies around Europe have set their sights on the topic of smoking. Indeed, the guidelines published in 2019 by the European Association for the Study of Diabetes and the European Society of Cardiology state that “smoking cessation is obligatory for all prediabetic and diabetic patients” (class I, level A).
This year, the France-based SFD and SFT dedicated an expert consensus to the major problem of smoking in patients with diabetes. The aim was to provide health care professionals with convincing, well-supported arguments in favor of smoking cessation in their patients with type 1 and type 2 diabetes.
“Before anything else, diabetic patients need to be made aware of the risks of smoking,” said Dr. Rouland. “It’s not just about the fear factor, though. It’s also about providing a positive incentive – they need to be told about the ways they’ll benefit from quitting smoking. For example, you have all-cause mortality, macro- and microangiopathic complications, and so on.”
Duration of abstinence
“Diabetic patients who have stopped smoking have a relative risk for all-cause mortality of 1.28 (1.09-1.51), which is less than what you see in active smokers (relative risk = 1.58; 1.42-1.77), but still above that of nonsmokers,” said Dr. Rouland.
A previous study revealed that although the risk does indeed go down after stopping smoking, it is linked to how long ago the person stopped. Patients who stopped smoking less than 10 years ago still had a slightly raised all-cause mortality risk, and this was even higher if they had smoked for 20 years or more.
After 10 years of not smoking, however, the greater all-cause mortality risk was no longer significant in any of the groups monitored (smoking duration, number of cigarettes/day). Concrete evidence of the link between all-cause mortality and the length of time since a person stopped smoking also emerged from the large cohort in the American Nurses’ Health Study.
The relative risk for all-cause mortality in women who stopped smoking less than 5 years ago remained high (RR = 1.96, 1.47-2.67), then decreased over time. After 10 years, it was no longer significant (RR = 1.11, 0.92-1.35).
Macro- and microangiopathic risks
Smoking cessation also has a real benefit in terms of the increased macro- and microangiopathic risks. In type 2 diabetes, a study found an increased relative risk for macro- and microalbuminuria of 1.86 (95% confidence interval, 1.37-2.52) in former smokers, compared with an increased relative risk of 2.61 (95% CI, 1.86-2.64) in current smokers.
In type 1 diabetes, the cumulative risk for microalbuminuria in former smokers was 15.1% vs. 18.9% in smokers and 10% in nonsmokers.
A 2019 meta-analysis of prospective cohort studies determined that smoking is an independent risk factor for diabetic nephropathy, especially in patients with type 1 diabetes.
Yet, most of the data for this condition come from subjects with type 2 diabetes. One publication estimated its prevalence after a 1-year follow-up of the smoking cessation program as 10.9% in former smokers and 15% in those who continued smoking.
In regard to macroangiopathy in the context of type 2 diabetes, the aforementioned 2019 meta-analysis focused on coronary artery disease, cerebrovascular accident (CVA), cardiovascular mortality, and myocardial infarction (MI). It found that smokers face an increased risk for all these outcomes.
The relative risks wavered between 1.53 and 1.66 and decreased after smoking cessation. For coronary artery disease and MI, they became insignificant. There was still a risk for CVA (RR = 1.34; 1.07-1.67) and fatal cardiovascular events (RR = 1.19; 0.02-1.39).
The data are slightly more heterogeneous for type 1 diabetes, where, despite smoking cessation, the increased risk for heart failure and CVA persists in men, yet the same risk for coronary heart disease and CVA drops in women.
Risk for weight gain
Dr. Rouland tried to reassure patients about the risk for gaining weight. “Weight gain is not inevitable. There is a risk for this, but it’s temporary. And, even with some weight gain, the cardiovascular benefits are still indisputable.”
A study carried out in 2013 focused specifically on this point, with an average post-cessation weight gain of 3.8 kg (8.4 lb) seen in diabetic individuals in the first 4 years after stopping smoking and of 0.1 kg (0.2 lb) thereafter. A time-based effect was observed with regulation of excess weight post-cessation over time, as seen in the general population (3 kg [6.6 lb] on average in nondiabetic individuals).
Weight gain tends to occur mainly in the immediate post-cessation period, essentially in the first 3 months, and there is a large variation in weight change. Some people gain a lot (from 5 to 10 kg [11 to 22 lb], or even more than 10 kg); others lose weight (20% of diabetic former smokers in the first month, 7% after 12 months), and 25% gain less than 5 kg (11 lb).
Blood glucose imbalance
“A risk for blood glucose imbalance has been reported after smoking cessation, although this is very slight and only temporary,” said Dr. Rouland.
A British retrospective study examined this question, focusing on glycated hemoglobin in patients with type 2 diabetes. Hemoglobin A1c increased by 0.21% (95% CI, 0.17-0.25; P < .001) within the first year after quitting. A1c decreased as abstinence continued and became comparable to that of continual smokers after 3 years. This increase in A1c was not mediated by weight change.
Another study published in 2018 on the topic of type 2 diabetes also reported on the risk for poor glycemic control (defined as A1c > 7%) persisting for 10 years after smoking cessation (odds ratio, 1.23; 95% CI, 1.06-1.42). Thereafter, between 10 and 19 years post-cessation, the OR decreased to 0.97 (95% CI, 0.80-1.19, NS). Beyond 20 years post-cessation, the OR was 1.14 (95% CI, 0.89-1.44, NS) and was therefore no longer significant.
Regardless, “the risk for poor glycemic control is lower in quitters than in active smokers,” said Dr. Rouland.
Quitting and diabetes risk
Will a smoker’s increased risk for diabetes drop when he or she stops smoking? “This is essentially what happens,” Dr. Rouland confirmed, “and his or her increased risk for metabolic syndrome also drops. One meta-analysis revealed a time-based effect.
“Patients who had stopped smoking less than 5 years previously had an increased relative risk for type 2 diabetes, and this risk dropped to 1.11 after more than 10 years of not smoking. Moreover, this relative risk for type 2 diabetes remained lower than that of active smokers, at between 1.19 and 1.60, depending on tobacco use.”
In regard to the risk for metabolic syndrome, those who quit smoking seem to have an increased risk of 10%, compared with nonsmokers (RR = 1.10, 1.08-1.11; P < .001). “But yet again, this increased risk is much lower than that of active smokers, whose risk is between 37% (less than 20 cigarettes/day) and 71% (more than 20 cigarettes/day).”
Women with diabetes
“The benefits of quitting appear identical, regardless of the sex of the diabetic person,” said Dr. Rouland. “As in the general population, weight gain after smoking cessation is greater in women. Furthermore, while smoking increases the risk for gestational diabetes (RR, 1.4-1.9) and for the use of insulin in this context, stopping smoking reduces these risks.
“Moreover, smoking during pregnancy not only increases the risk for pregnancy-related complications (early miscarriage, ectopic pregnancy, birth defects, placental abruption, premature birth, intrauterine fetal demise, cesarean birth, low birth weight), but it also increases the risk of type 2 diabetes in the newborn. The risk to the newborn is said to be around 34% in cases in which the mother smokes during pregnancy and 22% in cases in which the mother is a passive smoker, thereby justifying the use of measures to help the mother’s family members to stop smoking.”
Dr. Rouland reports no relevant financial relationships.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
FDA approves injectable treatment for cheek lines, wrinkles
The treatment, marketed as Sculptra, is the first FDA-approved PLLA collagen stimulator that, “when injected into the cheek area, helps stimulate natural collagen production to smooth wrinkles and improve skin quality such as firmness and glow,” according to a press release from the manufacturer, Galderma. Sculptra was first approved for aesthetic use in 2009 in the United States and is now available in more than 40 countries.
With this expanded approval, PLLA-SCA is now indicated for use in people with healthy immune systems for correcting shallow to deep nasolabial fold contour deficiencies, fine lines, and wrinkles in the cheeks and other facial areas.
94% have enduring improvement at 2 years
In a clinical trial, PLLA-SCA achieved the primary efficacy endpoint of at least a 1-grade improvement in wrinkles on both cheeks concurrently at rest and its secondary endpoint of improving cheek wrinkles when smiling for up to 2 years, the company states.
According to Galderma, patients showed aesthetic improvement in cheek wrinkles throughout the study; 96% showed improvement at 3 months, 94% showed improvement at 1 year, and 94% showed improvement at 2 years.
The most common side effects after initial treatment are injection site swelling, tenderness, redness, pain, bruising, bleeding, itching, and lumps, according to the company. Other side effects may include small lumps under the skin that are sometimes noticeable when pressing on the treated area.
PLLA-SCA is available only through a licensed practitioner and should not be used by people allergic to any ingredient of the product or who have a history of keloid formation or hypertrophic scarring. The company notes that safety has not been established in patients who are pregnant, lactating, breastfeeding, or younger than 18.
In its instruction to clinicians, the company warns the treatment should not be injected into the blood vessels “as it may cause vascular occlusion, infarction, or embolic phenomena.”
Skin sores, cysts, pimples, rashes, hives, or infection should be healed completely before injecting the treatment, the company cautions. PLLA-SCA should not be injected into the red area of the lip or in the periorbital area.
A version of this article first appeared on Medscape.com.
The treatment, marketed as Sculptra, is the first FDA-approved PLLA collagen stimulator that, “when injected into the cheek area, helps stimulate natural collagen production to smooth wrinkles and improve skin quality such as firmness and glow,” according to a press release from the manufacturer, Galderma. Sculptra was first approved for aesthetic use in 2009 in the United States and is now available in more than 40 countries.
With this expanded approval, PLLA-SCA is now indicated for use in people with healthy immune systems for correcting shallow to deep nasolabial fold contour deficiencies, fine lines, and wrinkles in the cheeks and other facial areas.
94% have enduring improvement at 2 years
In a clinical trial, PLLA-SCA achieved the primary efficacy endpoint of at least a 1-grade improvement in wrinkles on both cheeks concurrently at rest and its secondary endpoint of improving cheek wrinkles when smiling for up to 2 years, the company states.
According to Galderma, patients showed aesthetic improvement in cheek wrinkles throughout the study; 96% showed improvement at 3 months, 94% showed improvement at 1 year, and 94% showed improvement at 2 years.
The most common side effects after initial treatment are injection site swelling, tenderness, redness, pain, bruising, bleeding, itching, and lumps, according to the company. Other side effects may include small lumps under the skin that are sometimes noticeable when pressing on the treated area.
PLLA-SCA is available only through a licensed practitioner and should not be used by people allergic to any ingredient of the product or who have a history of keloid formation or hypertrophic scarring. The company notes that safety has not been established in patients who are pregnant, lactating, breastfeeding, or younger than 18.
In its instruction to clinicians, the company warns the treatment should not be injected into the blood vessels “as it may cause vascular occlusion, infarction, or embolic phenomena.”
Skin sores, cysts, pimples, rashes, hives, or infection should be healed completely before injecting the treatment, the company cautions. PLLA-SCA should not be injected into the red area of the lip or in the periorbital area.
A version of this article first appeared on Medscape.com.
The treatment, marketed as Sculptra, is the first FDA-approved PLLA collagen stimulator that, “when injected into the cheek area, helps stimulate natural collagen production to smooth wrinkles and improve skin quality such as firmness and glow,” according to a press release from the manufacturer, Galderma. Sculptra was first approved for aesthetic use in 2009 in the United States and is now available in more than 40 countries.
With this expanded approval, PLLA-SCA is now indicated for use in people with healthy immune systems for correcting shallow to deep nasolabial fold contour deficiencies, fine lines, and wrinkles in the cheeks and other facial areas.
94% have enduring improvement at 2 years
In a clinical trial, PLLA-SCA achieved the primary efficacy endpoint of at least a 1-grade improvement in wrinkles on both cheeks concurrently at rest and its secondary endpoint of improving cheek wrinkles when smiling for up to 2 years, the company states.
According to Galderma, patients showed aesthetic improvement in cheek wrinkles throughout the study; 96% showed improvement at 3 months, 94% showed improvement at 1 year, and 94% showed improvement at 2 years.
The most common side effects after initial treatment are injection site swelling, tenderness, redness, pain, bruising, bleeding, itching, and lumps, according to the company. Other side effects may include small lumps under the skin that are sometimes noticeable when pressing on the treated area.
PLLA-SCA is available only through a licensed practitioner and should not be used by people allergic to any ingredient of the product or who have a history of keloid formation or hypertrophic scarring. The company notes that safety has not been established in patients who are pregnant, lactating, breastfeeding, or younger than 18.
In its instruction to clinicians, the company warns the treatment should not be injected into the blood vessels “as it may cause vascular occlusion, infarction, or embolic phenomena.”
Skin sores, cysts, pimples, rashes, hives, or infection should be healed completely before injecting the treatment, the company cautions. PLLA-SCA should not be injected into the red area of the lip or in the periorbital area.
A version of this article first appeared on Medscape.com.
Should youth with type 1 diabetes use closed-loop systems?
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Musculoskeletal disorders prevalent in orchestra musicians
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
Osteoporosis and osteopenia: Latest treatment recommendations
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today’s topic is the new osteoporosis treatment guidelines issued by the American College of Physicians (ACP). The focus of the guidelines is treatment of osteoporosis. But first, I want to discuss screening.
In its 2018 statement, the U.S. Preventive Services Task Force (USPSTF) says that osteoporosis should be screened for in women older than 65 years of age, and those who are younger who are at increased risk based on a risk assessment tool (usually the FRAX tool). There is not enough evidence to weigh in for or against screening men. The other large organization that weighs in on screening is the Bone Health & Osteoporosis Foundation, which agrees with the USPSTF, but in addition says that we should be screening men over age 70 and men who are younger (age 50 to 69) who have risk factors. We should also screen anyone who has a fracture after low impact or no trauma.
Let’s now go on to the ACP treatment guidelines. Osteoporosis is defined as bone mineral density at the femoral neck or the lumbar spine, or both, with a T score less than -2.5.
For postmenopausal women with osteoporosis, you should use a bisphosphonate as first-line treatment to reduce the risk for future fractures. This is given a strong recommendation based on a high certainty of evidence. Bisphosphonates vs. placebo over 3 years leads to one fewer hip fracture per 150 patients treated and one fewer vertebral fracture per 50 people treated.
All the other recommendations in the guidelines are considered “conditional recommendations” that are correct for most people. But whether they make sense for an individual patient depends upon other details, as well as their values and preferences. For instance, treatment of osteoporosis in men is given a conditional recommendation, not because the evidence suggests that it’s not as effective, but because there is not as much evidence. Initial treatment for a man with osteoporosis is with bisphosphonates. Men do get osteoporosis and account for about 30% of hip fractures. This is not a surprise to anyone who takes care of older adults.
For postmenopausal women or men who you would want to treat but who can’t tolerate a bisphosphonate, then the recommendation is to use a RANK ligand inhibitor. Denosumab can be used as second-line treatment to reduce the risk for fractures. Remember, bisphosphonates and denosumab are antiresorptive drugs, meaning they slow the progression of osteoporosis. The anabolic drugs, on the other hand, such as the sclerostin inhibitor romosozumab and recombinant human parathyroid hormone (PTH) teriparatide, increase bone density. The anabolic agents should be used only in women with primary osteoporosis who are at very high risk for fractures, and use of these agents always needs to be followed by an antiresorptive agent, because otherwise there’s a risk for rebound osteoporosis and an increased risk for vertebral fractures.
Now, how about osteopenia? The guidelines recommend that for women over 65 with osteopenia, use an individualized approach influenced by the level of risk for fracture, including increased age, low body weight, current smoking, hip fracture in a parent, fall risk, and a personal history of fracture. The guidelines note that increasing the duration of bisphosphonate therapy beyond 3-5 years does reduce the risk for new vertebral fractures, but it doesn’t reduce the risk for other fractures and it increases the risk for osteonecrosis of the jaw and atypical hip fractures. Therefore, the guidelines say that we should use bisphosphonates only for 3-5 years unless someone is at extremely high risk. It’s also important to note that there’s a fivefold higher risk for atypical femoral fractures among Asian women.
Don’t forget about adequate vitamin D and calcium. And most importantly, don’t forget about exercise, particularly exercise aimed at improving balance and quadriceps strength, which helps prevent falls.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today’s topic is the new osteoporosis treatment guidelines issued by the American College of Physicians (ACP). The focus of the guidelines is treatment of osteoporosis. But first, I want to discuss screening.
In its 2018 statement, the U.S. Preventive Services Task Force (USPSTF) says that osteoporosis should be screened for in women older than 65 years of age, and those who are younger who are at increased risk based on a risk assessment tool (usually the FRAX tool). There is not enough evidence to weigh in for or against screening men. The other large organization that weighs in on screening is the Bone Health & Osteoporosis Foundation, which agrees with the USPSTF, but in addition says that we should be screening men over age 70 and men who are younger (age 50 to 69) who have risk factors. We should also screen anyone who has a fracture after low impact or no trauma.
Let’s now go on to the ACP treatment guidelines. Osteoporosis is defined as bone mineral density at the femoral neck or the lumbar spine, or both, with a T score less than -2.5.
For postmenopausal women with osteoporosis, you should use a bisphosphonate as first-line treatment to reduce the risk for future fractures. This is given a strong recommendation based on a high certainty of evidence. Bisphosphonates vs. placebo over 3 years leads to one fewer hip fracture per 150 patients treated and one fewer vertebral fracture per 50 people treated.
All the other recommendations in the guidelines are considered “conditional recommendations” that are correct for most people. But whether they make sense for an individual patient depends upon other details, as well as their values and preferences. For instance, treatment of osteoporosis in men is given a conditional recommendation, not because the evidence suggests that it’s not as effective, but because there is not as much evidence. Initial treatment for a man with osteoporosis is with bisphosphonates. Men do get osteoporosis and account for about 30% of hip fractures. This is not a surprise to anyone who takes care of older adults.
For postmenopausal women or men who you would want to treat but who can’t tolerate a bisphosphonate, then the recommendation is to use a RANK ligand inhibitor. Denosumab can be used as second-line treatment to reduce the risk for fractures. Remember, bisphosphonates and denosumab are antiresorptive drugs, meaning they slow the progression of osteoporosis. The anabolic drugs, on the other hand, such as the sclerostin inhibitor romosozumab and recombinant human parathyroid hormone (PTH) teriparatide, increase bone density. The anabolic agents should be used only in women with primary osteoporosis who are at very high risk for fractures, and use of these agents always needs to be followed by an antiresorptive agent, because otherwise there’s a risk for rebound osteoporosis and an increased risk for vertebral fractures.
Now, how about osteopenia? The guidelines recommend that for women over 65 with osteopenia, use an individualized approach influenced by the level of risk for fracture, including increased age, low body weight, current smoking, hip fracture in a parent, fall risk, and a personal history of fracture. The guidelines note that increasing the duration of bisphosphonate therapy beyond 3-5 years does reduce the risk for new vertebral fractures, but it doesn’t reduce the risk for other fractures and it increases the risk for osteonecrosis of the jaw and atypical hip fractures. Therefore, the guidelines say that we should use bisphosphonates only for 3-5 years unless someone is at extremely high risk. It’s also important to note that there’s a fivefold higher risk for atypical femoral fractures among Asian women.
Don’t forget about adequate vitamin D and calcium. And most importantly, don’t forget about exercise, particularly exercise aimed at improving balance and quadriceps strength, which helps prevent falls.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today’s topic is the new osteoporosis treatment guidelines issued by the American College of Physicians (ACP). The focus of the guidelines is treatment of osteoporosis. But first, I want to discuss screening.
In its 2018 statement, the U.S. Preventive Services Task Force (USPSTF) says that osteoporosis should be screened for in women older than 65 years of age, and those who are younger who are at increased risk based on a risk assessment tool (usually the FRAX tool). There is not enough evidence to weigh in for or against screening men. The other large organization that weighs in on screening is the Bone Health & Osteoporosis Foundation, which agrees with the USPSTF, but in addition says that we should be screening men over age 70 and men who are younger (age 50 to 69) who have risk factors. We should also screen anyone who has a fracture after low impact or no trauma.
Let’s now go on to the ACP treatment guidelines. Osteoporosis is defined as bone mineral density at the femoral neck or the lumbar spine, or both, with a T score less than -2.5.
For postmenopausal women with osteoporosis, you should use a bisphosphonate as first-line treatment to reduce the risk for future fractures. This is given a strong recommendation based on a high certainty of evidence. Bisphosphonates vs. placebo over 3 years leads to one fewer hip fracture per 150 patients treated and one fewer vertebral fracture per 50 people treated.
All the other recommendations in the guidelines are considered “conditional recommendations” that are correct for most people. But whether they make sense for an individual patient depends upon other details, as well as their values and preferences. For instance, treatment of osteoporosis in men is given a conditional recommendation, not because the evidence suggests that it’s not as effective, but because there is not as much evidence. Initial treatment for a man with osteoporosis is with bisphosphonates. Men do get osteoporosis and account for about 30% of hip fractures. This is not a surprise to anyone who takes care of older adults.
For postmenopausal women or men who you would want to treat but who can’t tolerate a bisphosphonate, then the recommendation is to use a RANK ligand inhibitor. Denosumab can be used as second-line treatment to reduce the risk for fractures. Remember, bisphosphonates and denosumab are antiresorptive drugs, meaning they slow the progression of osteoporosis. The anabolic drugs, on the other hand, such as the sclerostin inhibitor romosozumab and recombinant human parathyroid hormone (PTH) teriparatide, increase bone density. The anabolic agents should be used only in women with primary osteoporosis who are at very high risk for fractures, and use of these agents always needs to be followed by an antiresorptive agent, because otherwise there’s a risk for rebound osteoporosis and an increased risk for vertebral fractures.
Now, how about osteopenia? The guidelines recommend that for women over 65 with osteopenia, use an individualized approach influenced by the level of risk for fracture, including increased age, low body weight, current smoking, hip fracture in a parent, fall risk, and a personal history of fracture. The guidelines note that increasing the duration of bisphosphonate therapy beyond 3-5 years does reduce the risk for new vertebral fractures, but it doesn’t reduce the risk for other fractures and it increases the risk for osteonecrosis of the jaw and atypical hip fractures. Therefore, the guidelines say that we should use bisphosphonates only for 3-5 years unless someone is at extremely high risk. It’s also important to note that there’s a fivefold higher risk for atypical femoral fractures among Asian women.
Don’t forget about adequate vitamin D and calcium. And most importantly, don’t forget about exercise, particularly exercise aimed at improving balance and quadriceps strength, which helps prevent falls.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article originally appeared on Medscape.com.
Obesity drugs overpriced, change needed to tackle issue
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).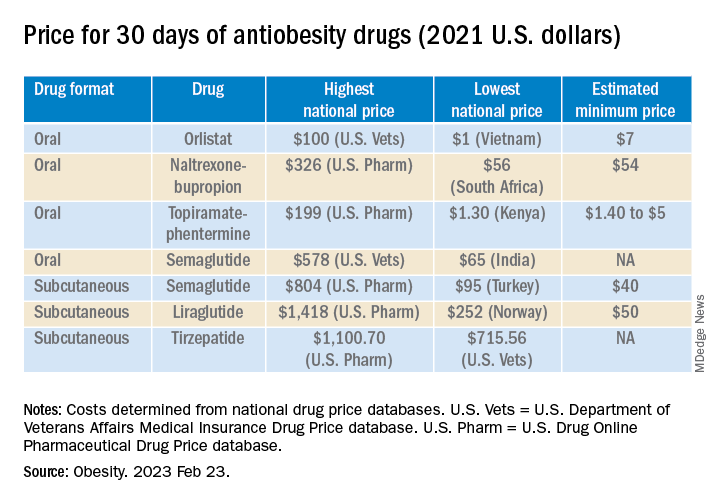
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Novel strategy could improve heart transplant allocation
Prediction models that incorporate more than just treatment status could rank order heart transplant candidates by urgency more effectively than the current system, a modeling study suggests.
Since 2018, the U.S. heart transplant allocation system has ranked heart candidates according to six treatment-based “statuses” (up from three used previously), ignoring many objective patient characteristics, the authors write.
Their study showed no significant difference in survival between statuses four and six, and status five had lower survival than status four.
“We expected multivariable prediction models to outperform the six-status system when it comes to rank ordering patients by how likely they are to die on the wait list (medical urgency),” William F. Parker, MD, MS, PhD, of the University of Chicago, told this news organization.
“However, we were surprised to see that the statuses were out of order,” he said. “Status five patients are more urgent than status three or status four patients,” mainly because most are in renal failure and listed for multiorgan transplantation with a kidney.
Objective physiologic measurements, such as glomerular filtration rate (GFR), had high variable importance, offering a minimally invasive measurement with predictive power in assessing medical urgency. Therefore, including GFR and other variables such as extracorporeal membrane oxygenation (ECMO) could improve the accuracy of the allocation system in identifying the most medically urgent candidates, Dr. Parker and colleagues suggest.
The study was published online in JACC: Heart Failure.
‘Moderate ability’ to rank order
The investigators assessed the effectiveness of the standard six-status ranking system and several novel prediction models in identifying the most urgent heart transplant candidates. The primary outcome was death before receipt of a heart transplant.
The final data set contained 32,294 candidates (mean age, 53 years; 74%, men); 27,200 made up the prepolicy training set and 5,094 were included in the postpolicy test set.
The team evaluated the accuracy of the six-status system using Harrell’s C-index and log-rank tests of Kaplan-Meier estimated survival by status for candidates listed after the policy change (November 2018 to March 2020) in the Scientific Registry of Transplant Recipients data set.
They then developed Cox proportional hazards models and random survival forest models using prepolicy data (2010-2017). Predictor variables included age, diagnosis, laboratory measurements, hemodynamics, and supportive treatment at the time of listing.
They found that the six-status ranking at listing has had “moderate ability” to rank order candidates.
As Dr. Parker indicated, statuses four and six had no significant difference in survival, and status five had lower survival than status four.
The investigators’ multivariable prediction models derived with prepolicy data ranked candidates correctly more often than the six-status rankings. Objective physiologic measurements, such as GFR and ECMO, were identified as having significant importance with regard to ranking by urgency.
“The novel prediction models we developed … could be implemented by the Organ Procurement and Transplantation Network (OPTN) as allocation policy and would be better than the status quo,” Dr. Parker said. “However, I think we could do even better using the newer data collected after 2018.”
Modifications underway
The OPTN Heart Transplantation Committee is currently working on developing a new framework for allocating deceased donor hearts called Continuous Distribution.
“The six-tiered system works well, and it better stratifies the most medically urgent candidates than the previous allocation framework,” the leadership of the United Network for Organ Sharing Heart Transplantation Committee, including Chair Richard C. Daly, MD, Mayo Clinic; Vice-Chair Jondavid Menteer, MD, University of Southern California, Los Angeles; and former Chair Shelley Hall, MD, Baylor University Medical Center, told this news organization.
“That said, it is always appropriate to review and adjust variables that affect the medical urgency attribute for heart allocation.”
The new framework will change how patients are prioritized, they said. “Continuous distribution will consider all patient factors, including medical urgency, together to determine the order of an organ offer, and no single factor will decide an organ match.
“The goal is to increase fairness by moving to a points-based allocation framework that allows candidates to be compared using a single score composed of multiple factors.
“Furthermore,” they added, “continuous distribution provides a framework that will allow modifications of the criteria defining medical urgency (and other attributes of allocation) to a finer degree than the current policy. … Once continuous distribution is in place and the OPTN has policy monitoring data, the committee may consider and model different ways of defining medical urgency.”
Kiran K. Khush, MD, of Stanford (Calif.) University School of Medicine, coauthor of a related commentary, elaborated. “The composite allocation score (CAS) will consist of a ‘points-based system,’ in which candidates will be assigned points based on (1) medical urgency, (2) anticipated posttransplant survival, (3) candidate biology (eg., special characteristics that may result in higher prioritization, such as blood type O and allosensitization), (4) access (eg., prior living donor, pediatric patient), and (5) placement efficacy (travel, proximity).”
Candidates will be assigned points based on these categories, and will be rank ordered for each donor offer.
Dr. Khush and colleagues propose that a multivariable model – such as the ones described in the study – would be the best way to assign points for medical urgency.
“This system will be more equitable than the current system,” Dr. Khush said, “because it will better prioritize the sickest candidates while improving access for patients who are currently at a disadvantage [for example, blood O, highly sensitized patients], and will also remove artificial geographic boundaries [for example, the current 500-mile rule for heart allocation].”
Going further
Jesse D. Schold, PhD, of the University of Colorado at Denver, Aurora, raises concerns about other aspects of the heart allocation system in another related commentary.
“One big issue with our data in transplantation … is that, while it is very comprehensive for capturing transplant candidates and recipients, there is no data collection for patients and processes of care for patients prior to wait list placement,” he told this news organization. This phase of care is subject to wide variation in practice, he said, “and is likely as important as any to patients – the ability to be referred, evaluated, and placed on a waiting list.”
Report cards that measure quality of care after wait list placement ignore key phases prior to wait list placement, he said. “This may have the unintended consequences of limiting access to care and to the waiting list for patients perceived to be at higher risk, or the use of higher-risk donors, despite their potential survival advantage.
“In contrast,” he said, “quality report cards that incentivize treatment for all patients who may benefit would likely have a greater beneficial impact on patients with end-organ disease.”
There is also significant risk of underlying differences in patient populations between centers, despite the use of multivariable models, he added. This heterogeneity “may not be reflected accurately in the report cards [which] have significant impact for regulatory review, private payer contracting, and center reputation.”
Some of these concerns may be addressed in the new OPTN Modernization Initiative, according to David Bowman, a public affairs specialist at the Health Resources and Services Administration. One of the goals of the initiative “is to ensure that the OPTN Board of Directors is high functioning, has greater independence, and represents the diversity of communities served by the OPTN,” he told this news organization. “Strengthened governance will lead to effective policy development and implementation, and enhanced transparency and accountability of the process.”
Addressing another concern about the system, Savitri Fedson, MD, of the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, Houston, wonders in a related editorial whether organ donors and recipients should know more about each other, and if so, could that reverse the ongoing downward trend in organ acceptance?
Although some organizations are in favor of sharing more information, Dr. Fedson notes that “less information may have the greater benefit.” She writes, “We might realize that the simplest approach is often the best: a fulsome thank you for the donor’s gift that is willingly given to a stranger without expectation of payment, and on the recipient side, the knowledge that an organ is of good quality.
“The transplant patient can be comforted with the understanding that the risk of disease transmission, while not zero, is low, and that their survival following acceptance of an organ is better than languishing on a waiting list.”
The study received no commercial funding. Dr. Parker, Dr. Khush, Dr. Schold, and Dr. Fedson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prediction models that incorporate more than just treatment status could rank order heart transplant candidates by urgency more effectively than the current system, a modeling study suggests.
Since 2018, the U.S. heart transplant allocation system has ranked heart candidates according to six treatment-based “statuses” (up from three used previously), ignoring many objective patient characteristics, the authors write.
Their study showed no significant difference in survival between statuses four and six, and status five had lower survival than status four.
“We expected multivariable prediction models to outperform the six-status system when it comes to rank ordering patients by how likely they are to die on the wait list (medical urgency),” William F. Parker, MD, MS, PhD, of the University of Chicago, told this news organization.
“However, we were surprised to see that the statuses were out of order,” he said. “Status five patients are more urgent than status three or status four patients,” mainly because most are in renal failure and listed for multiorgan transplantation with a kidney.
Objective physiologic measurements, such as glomerular filtration rate (GFR), had high variable importance, offering a minimally invasive measurement with predictive power in assessing medical urgency. Therefore, including GFR and other variables such as extracorporeal membrane oxygenation (ECMO) could improve the accuracy of the allocation system in identifying the most medically urgent candidates, Dr. Parker and colleagues suggest.
The study was published online in JACC: Heart Failure.
‘Moderate ability’ to rank order
The investigators assessed the effectiveness of the standard six-status ranking system and several novel prediction models in identifying the most urgent heart transplant candidates. The primary outcome was death before receipt of a heart transplant.
The final data set contained 32,294 candidates (mean age, 53 years; 74%, men); 27,200 made up the prepolicy training set and 5,094 were included in the postpolicy test set.
The team evaluated the accuracy of the six-status system using Harrell’s C-index and log-rank tests of Kaplan-Meier estimated survival by status for candidates listed after the policy change (November 2018 to March 2020) in the Scientific Registry of Transplant Recipients data set.
They then developed Cox proportional hazards models and random survival forest models using prepolicy data (2010-2017). Predictor variables included age, diagnosis, laboratory measurements, hemodynamics, and supportive treatment at the time of listing.
They found that the six-status ranking at listing has had “moderate ability” to rank order candidates.
As Dr. Parker indicated, statuses four and six had no significant difference in survival, and status five had lower survival than status four.
The investigators’ multivariable prediction models derived with prepolicy data ranked candidates correctly more often than the six-status rankings. Objective physiologic measurements, such as GFR and ECMO, were identified as having significant importance with regard to ranking by urgency.
“The novel prediction models we developed … could be implemented by the Organ Procurement and Transplantation Network (OPTN) as allocation policy and would be better than the status quo,” Dr. Parker said. “However, I think we could do even better using the newer data collected after 2018.”
Modifications underway
The OPTN Heart Transplantation Committee is currently working on developing a new framework for allocating deceased donor hearts called Continuous Distribution.
“The six-tiered system works well, and it better stratifies the most medically urgent candidates than the previous allocation framework,” the leadership of the United Network for Organ Sharing Heart Transplantation Committee, including Chair Richard C. Daly, MD, Mayo Clinic; Vice-Chair Jondavid Menteer, MD, University of Southern California, Los Angeles; and former Chair Shelley Hall, MD, Baylor University Medical Center, told this news organization.
“That said, it is always appropriate to review and adjust variables that affect the medical urgency attribute for heart allocation.”
The new framework will change how patients are prioritized, they said. “Continuous distribution will consider all patient factors, including medical urgency, together to determine the order of an organ offer, and no single factor will decide an organ match.
“The goal is to increase fairness by moving to a points-based allocation framework that allows candidates to be compared using a single score composed of multiple factors.
“Furthermore,” they added, “continuous distribution provides a framework that will allow modifications of the criteria defining medical urgency (and other attributes of allocation) to a finer degree than the current policy. … Once continuous distribution is in place and the OPTN has policy monitoring data, the committee may consider and model different ways of defining medical urgency.”
Kiran K. Khush, MD, of Stanford (Calif.) University School of Medicine, coauthor of a related commentary, elaborated. “The composite allocation score (CAS) will consist of a ‘points-based system,’ in which candidates will be assigned points based on (1) medical urgency, (2) anticipated posttransplant survival, (3) candidate biology (eg., special characteristics that may result in higher prioritization, such as blood type O and allosensitization), (4) access (eg., prior living donor, pediatric patient), and (5) placement efficacy (travel, proximity).”
Candidates will be assigned points based on these categories, and will be rank ordered for each donor offer.
Dr. Khush and colleagues propose that a multivariable model – such as the ones described in the study – would be the best way to assign points for medical urgency.
“This system will be more equitable than the current system,” Dr. Khush said, “because it will better prioritize the sickest candidates while improving access for patients who are currently at a disadvantage [for example, blood O, highly sensitized patients], and will also remove artificial geographic boundaries [for example, the current 500-mile rule for heart allocation].”
Going further
Jesse D. Schold, PhD, of the University of Colorado at Denver, Aurora, raises concerns about other aspects of the heart allocation system in another related commentary.
“One big issue with our data in transplantation … is that, while it is very comprehensive for capturing transplant candidates and recipients, there is no data collection for patients and processes of care for patients prior to wait list placement,” he told this news organization. This phase of care is subject to wide variation in practice, he said, “and is likely as important as any to patients – the ability to be referred, evaluated, and placed on a waiting list.”
Report cards that measure quality of care after wait list placement ignore key phases prior to wait list placement, he said. “This may have the unintended consequences of limiting access to care and to the waiting list for patients perceived to be at higher risk, or the use of higher-risk donors, despite their potential survival advantage.
“In contrast,” he said, “quality report cards that incentivize treatment for all patients who may benefit would likely have a greater beneficial impact on patients with end-organ disease.”
There is also significant risk of underlying differences in patient populations between centers, despite the use of multivariable models, he added. This heterogeneity “may not be reflected accurately in the report cards [which] have significant impact for regulatory review, private payer contracting, and center reputation.”
Some of these concerns may be addressed in the new OPTN Modernization Initiative, according to David Bowman, a public affairs specialist at the Health Resources and Services Administration. One of the goals of the initiative “is to ensure that the OPTN Board of Directors is high functioning, has greater independence, and represents the diversity of communities served by the OPTN,” he told this news organization. “Strengthened governance will lead to effective policy development and implementation, and enhanced transparency and accountability of the process.”
Addressing another concern about the system, Savitri Fedson, MD, of the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, Houston, wonders in a related editorial whether organ donors and recipients should know more about each other, and if so, could that reverse the ongoing downward trend in organ acceptance?
Although some organizations are in favor of sharing more information, Dr. Fedson notes that “less information may have the greater benefit.” She writes, “We might realize that the simplest approach is often the best: a fulsome thank you for the donor’s gift that is willingly given to a stranger without expectation of payment, and on the recipient side, the knowledge that an organ is of good quality.
“The transplant patient can be comforted with the understanding that the risk of disease transmission, while not zero, is low, and that their survival following acceptance of an organ is better than languishing on a waiting list.”
The study received no commercial funding. Dr. Parker, Dr. Khush, Dr. Schold, and Dr. Fedson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prediction models that incorporate more than just treatment status could rank order heart transplant candidates by urgency more effectively than the current system, a modeling study suggests.
Since 2018, the U.S. heart transplant allocation system has ranked heart candidates according to six treatment-based “statuses” (up from three used previously), ignoring many objective patient characteristics, the authors write.
Their study showed no significant difference in survival between statuses four and six, and status five had lower survival than status four.
“We expected multivariable prediction models to outperform the six-status system when it comes to rank ordering patients by how likely they are to die on the wait list (medical urgency),” William F. Parker, MD, MS, PhD, of the University of Chicago, told this news organization.
“However, we were surprised to see that the statuses were out of order,” he said. “Status five patients are more urgent than status three or status four patients,” mainly because most are in renal failure and listed for multiorgan transplantation with a kidney.
Objective physiologic measurements, such as glomerular filtration rate (GFR), had high variable importance, offering a minimally invasive measurement with predictive power in assessing medical urgency. Therefore, including GFR and other variables such as extracorporeal membrane oxygenation (ECMO) could improve the accuracy of the allocation system in identifying the most medically urgent candidates, Dr. Parker and colleagues suggest.
The study was published online in JACC: Heart Failure.
‘Moderate ability’ to rank order
The investigators assessed the effectiveness of the standard six-status ranking system and several novel prediction models in identifying the most urgent heart transplant candidates. The primary outcome was death before receipt of a heart transplant.
The final data set contained 32,294 candidates (mean age, 53 years; 74%, men); 27,200 made up the prepolicy training set and 5,094 were included in the postpolicy test set.
The team evaluated the accuracy of the six-status system using Harrell’s C-index and log-rank tests of Kaplan-Meier estimated survival by status for candidates listed after the policy change (November 2018 to March 2020) in the Scientific Registry of Transplant Recipients data set.
They then developed Cox proportional hazards models and random survival forest models using prepolicy data (2010-2017). Predictor variables included age, diagnosis, laboratory measurements, hemodynamics, and supportive treatment at the time of listing.
They found that the six-status ranking at listing has had “moderate ability” to rank order candidates.
As Dr. Parker indicated, statuses four and six had no significant difference in survival, and status five had lower survival than status four.
The investigators’ multivariable prediction models derived with prepolicy data ranked candidates correctly more often than the six-status rankings. Objective physiologic measurements, such as GFR and ECMO, were identified as having significant importance with regard to ranking by urgency.
“The novel prediction models we developed … could be implemented by the Organ Procurement and Transplantation Network (OPTN) as allocation policy and would be better than the status quo,” Dr. Parker said. “However, I think we could do even better using the newer data collected after 2018.”
Modifications underway
The OPTN Heart Transplantation Committee is currently working on developing a new framework for allocating deceased donor hearts called Continuous Distribution.
“The six-tiered system works well, and it better stratifies the most medically urgent candidates than the previous allocation framework,” the leadership of the United Network for Organ Sharing Heart Transplantation Committee, including Chair Richard C. Daly, MD, Mayo Clinic; Vice-Chair Jondavid Menteer, MD, University of Southern California, Los Angeles; and former Chair Shelley Hall, MD, Baylor University Medical Center, told this news organization.
“That said, it is always appropriate to review and adjust variables that affect the medical urgency attribute for heart allocation.”
The new framework will change how patients are prioritized, they said. “Continuous distribution will consider all patient factors, including medical urgency, together to determine the order of an organ offer, and no single factor will decide an organ match.
“The goal is to increase fairness by moving to a points-based allocation framework that allows candidates to be compared using a single score composed of multiple factors.
“Furthermore,” they added, “continuous distribution provides a framework that will allow modifications of the criteria defining medical urgency (and other attributes of allocation) to a finer degree than the current policy. … Once continuous distribution is in place and the OPTN has policy monitoring data, the committee may consider and model different ways of defining medical urgency.”
Kiran K. Khush, MD, of Stanford (Calif.) University School of Medicine, coauthor of a related commentary, elaborated. “The composite allocation score (CAS) will consist of a ‘points-based system,’ in which candidates will be assigned points based on (1) medical urgency, (2) anticipated posttransplant survival, (3) candidate biology (eg., special characteristics that may result in higher prioritization, such as blood type O and allosensitization), (4) access (eg., prior living donor, pediatric patient), and (5) placement efficacy (travel, proximity).”
Candidates will be assigned points based on these categories, and will be rank ordered for each donor offer.
Dr. Khush and colleagues propose that a multivariable model – such as the ones described in the study – would be the best way to assign points for medical urgency.
“This system will be more equitable than the current system,” Dr. Khush said, “because it will better prioritize the sickest candidates while improving access for patients who are currently at a disadvantage [for example, blood O, highly sensitized patients], and will also remove artificial geographic boundaries [for example, the current 500-mile rule for heart allocation].”
Going further
Jesse D. Schold, PhD, of the University of Colorado at Denver, Aurora, raises concerns about other aspects of the heart allocation system in another related commentary.
“One big issue with our data in transplantation … is that, while it is very comprehensive for capturing transplant candidates and recipients, there is no data collection for patients and processes of care for patients prior to wait list placement,” he told this news organization. This phase of care is subject to wide variation in practice, he said, “and is likely as important as any to patients – the ability to be referred, evaluated, and placed on a waiting list.”
Report cards that measure quality of care after wait list placement ignore key phases prior to wait list placement, he said. “This may have the unintended consequences of limiting access to care and to the waiting list for patients perceived to be at higher risk, or the use of higher-risk donors, despite their potential survival advantage.
“In contrast,” he said, “quality report cards that incentivize treatment for all patients who may benefit would likely have a greater beneficial impact on patients with end-organ disease.”
There is also significant risk of underlying differences in patient populations between centers, despite the use of multivariable models, he added. This heterogeneity “may not be reflected accurately in the report cards [which] have significant impact for regulatory review, private payer contracting, and center reputation.”
Some of these concerns may be addressed in the new OPTN Modernization Initiative, according to David Bowman, a public affairs specialist at the Health Resources and Services Administration. One of the goals of the initiative “is to ensure that the OPTN Board of Directors is high functioning, has greater independence, and represents the diversity of communities served by the OPTN,” he told this news organization. “Strengthened governance will lead to effective policy development and implementation, and enhanced transparency and accountability of the process.”
Addressing another concern about the system, Savitri Fedson, MD, of the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, Houston, wonders in a related editorial whether organ donors and recipients should know more about each other, and if so, could that reverse the ongoing downward trend in organ acceptance?
Although some organizations are in favor of sharing more information, Dr. Fedson notes that “less information may have the greater benefit.” She writes, “We might realize that the simplest approach is often the best: a fulsome thank you for the donor’s gift that is willingly given to a stranger without expectation of payment, and on the recipient side, the knowledge that an organ is of good quality.
“The transplant patient can be comforted with the understanding that the risk of disease transmission, while not zero, is low, and that their survival following acceptance of an organ is better than languishing on a waiting list.”
The study received no commercial funding. Dr. Parker, Dr. Khush, Dr. Schold, and Dr. Fedson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE



