User login
Does Ozempic cause hair loss?
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
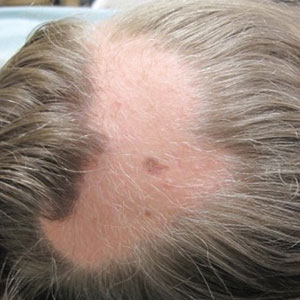
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ChatGPT bot flunks gastroenterology exam
Versions 3 and 4 of the chatbot scored only 65% and 62%, respectively, on the American College of Gastroenterology Self-Assessment Test. The minimum passing grade is 70%.
“You might expect a physician to score 99%, or at least 95%,” lead author Arvind J. Trindade, MD, regional director of endoscopy at Northwell Health (Central Region) in New Hyde Park, New York, said in an interview.
The study was published online in the American Journal of Gastroenterology.
Dr. Trindade and colleagues undertook the study amid growing reports of students using the tool across many academic areas, including law and medicine, and growing interest in the chatbot’s potential in medical education.
“I saw gastroenterology students typing questions into it. I wanted to know how accurate it was in gastroenterology – if it was going to be used in medical education and patient care,” said Dr. Trindade, who is also an associate professor at Feinstein Institutes for Medical Research in Manhasset, New York. “Based on our research, ChatGPT should not be used for medical education in gastroenterology at this time, and it has a way to go before it should be implemented into the health care field.”
Poor showing
The researchers tested the two versions of ChatGPT on both the 2021 and 2022 online ACG Self-Assessment Test, a multiple-choice exam designed to gauge how well a trainee would do on the American Board of Internal Medicine Gastroenterology board examination.
Questions that involved image selection were excluded from the study. For those that remained, the questions and answer choices were copied and pasted directly into ChatGPT, which returned answers and explanations. The corresponding answer was selected on the ACG website based on the chatbot’s response.
Of the 455 questions posed, ChatGPT-3 correctly answered 296, and ChatGPT-4 got 284 right. There was no discernible pattern in the type of question that the chatbot answered incorrectly, but questions on surveillance timing for various disease states, diagnosis, and pharmaceutical regimens were all answered incorrectly.
The reasons for the tool’s poor performance could lie with the large language model underpinning ChatGPT, the researchers write. The model was trained on freely available information – not specifically on medical literature and not on materials that require paid journal subscriptions – to be a general-purpose interactive program.
Additionally, the chatbot may use information from a variety of sources, including non- or quasi-medical sources, or out-of-date sources, which can lead to errors, they note. ChatGPT-3 was last updated in June 2021 and ChatGPT-4 in September 2021.
“ChatGPT does not have an intrinsic understanding of an issue,” Dr. Trindade said. “Its basic function is to predict the next word in a string of text to produce an expected response, regardless of whether such a response is factually correct or not.”
Previous research
In a previous study, ChatGPT was able to pass parts of the U.S. Medical Licensing Examination.
The chatbot may have performed better on the USMLE because the information tested on the exam may have been more widely available for ChatGPT’s language training, Dr. Trindade said. “In addition, the threshold for passing [the USMLE] is lower with regard to the percentage of questions correctly answered,” he said.
ChatGPT seems to fare better at helping to inform patients than it does on medical exams. The chatbot provided generally satisfactory answers to common patient queries about colonoscopy in one study and about hepatocellular carcinoma and liver cirrhosis in another study.
For ChatGPT to be valuable in medical education, “future versions would need to be updated with medical resources such as journal articles, society guidelines, and medical databases, such as UpToDate,” Dr. Trindade said. “With directed medical training in gastroenterology, it may be a future tool for education or patient use in this field, but not currently as it is now. Before it can be used in gastroenterology, it should be validated.”
That said, he noted, medical education has evolved from being based on textbooks and print journals to include Internet-based journal data and practice guidelines on specialty websites. If properly primed, resources such as ChatGPT may be the next logical step.
This study received no funding. Dr. Trindade is a consultant for Pentax Medical, Boston Scientific, Lucid Diagnostic, and Exact Science and receives research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
Versions 3 and 4 of the chatbot scored only 65% and 62%, respectively, on the American College of Gastroenterology Self-Assessment Test. The minimum passing grade is 70%.
“You might expect a physician to score 99%, or at least 95%,” lead author Arvind J. Trindade, MD, regional director of endoscopy at Northwell Health (Central Region) in New Hyde Park, New York, said in an interview.
The study was published online in the American Journal of Gastroenterology.
Dr. Trindade and colleagues undertook the study amid growing reports of students using the tool across many academic areas, including law and medicine, and growing interest in the chatbot’s potential in medical education.
“I saw gastroenterology students typing questions into it. I wanted to know how accurate it was in gastroenterology – if it was going to be used in medical education and patient care,” said Dr. Trindade, who is also an associate professor at Feinstein Institutes for Medical Research in Manhasset, New York. “Based on our research, ChatGPT should not be used for medical education in gastroenterology at this time, and it has a way to go before it should be implemented into the health care field.”
Poor showing
The researchers tested the two versions of ChatGPT on both the 2021 and 2022 online ACG Self-Assessment Test, a multiple-choice exam designed to gauge how well a trainee would do on the American Board of Internal Medicine Gastroenterology board examination.
Questions that involved image selection were excluded from the study. For those that remained, the questions and answer choices were copied and pasted directly into ChatGPT, which returned answers and explanations. The corresponding answer was selected on the ACG website based on the chatbot’s response.
Of the 455 questions posed, ChatGPT-3 correctly answered 296, and ChatGPT-4 got 284 right. There was no discernible pattern in the type of question that the chatbot answered incorrectly, but questions on surveillance timing for various disease states, diagnosis, and pharmaceutical regimens were all answered incorrectly.
The reasons for the tool’s poor performance could lie with the large language model underpinning ChatGPT, the researchers write. The model was trained on freely available information – not specifically on medical literature and not on materials that require paid journal subscriptions – to be a general-purpose interactive program.
Additionally, the chatbot may use information from a variety of sources, including non- or quasi-medical sources, or out-of-date sources, which can lead to errors, they note. ChatGPT-3 was last updated in June 2021 and ChatGPT-4 in September 2021.
“ChatGPT does not have an intrinsic understanding of an issue,” Dr. Trindade said. “Its basic function is to predict the next word in a string of text to produce an expected response, regardless of whether such a response is factually correct or not.”
Previous research
In a previous study, ChatGPT was able to pass parts of the U.S. Medical Licensing Examination.
The chatbot may have performed better on the USMLE because the information tested on the exam may have been more widely available for ChatGPT’s language training, Dr. Trindade said. “In addition, the threshold for passing [the USMLE] is lower with regard to the percentage of questions correctly answered,” he said.
ChatGPT seems to fare better at helping to inform patients than it does on medical exams. The chatbot provided generally satisfactory answers to common patient queries about colonoscopy in one study and about hepatocellular carcinoma and liver cirrhosis in another study.
For ChatGPT to be valuable in medical education, “future versions would need to be updated with medical resources such as journal articles, society guidelines, and medical databases, such as UpToDate,” Dr. Trindade said. “With directed medical training in gastroenterology, it may be a future tool for education or patient use in this field, but not currently as it is now. Before it can be used in gastroenterology, it should be validated.”
That said, he noted, medical education has evolved from being based on textbooks and print journals to include Internet-based journal data and practice guidelines on specialty websites. If properly primed, resources such as ChatGPT may be the next logical step.
This study received no funding. Dr. Trindade is a consultant for Pentax Medical, Boston Scientific, Lucid Diagnostic, and Exact Science and receives research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
Versions 3 and 4 of the chatbot scored only 65% and 62%, respectively, on the American College of Gastroenterology Self-Assessment Test. The minimum passing grade is 70%.
“You might expect a physician to score 99%, or at least 95%,” lead author Arvind J. Trindade, MD, regional director of endoscopy at Northwell Health (Central Region) in New Hyde Park, New York, said in an interview.
The study was published online in the American Journal of Gastroenterology.
Dr. Trindade and colleagues undertook the study amid growing reports of students using the tool across many academic areas, including law and medicine, and growing interest in the chatbot’s potential in medical education.
“I saw gastroenterology students typing questions into it. I wanted to know how accurate it was in gastroenterology – if it was going to be used in medical education and patient care,” said Dr. Trindade, who is also an associate professor at Feinstein Institutes for Medical Research in Manhasset, New York. “Based on our research, ChatGPT should not be used for medical education in gastroenterology at this time, and it has a way to go before it should be implemented into the health care field.”
Poor showing
The researchers tested the two versions of ChatGPT on both the 2021 and 2022 online ACG Self-Assessment Test, a multiple-choice exam designed to gauge how well a trainee would do on the American Board of Internal Medicine Gastroenterology board examination.
Questions that involved image selection were excluded from the study. For those that remained, the questions and answer choices were copied and pasted directly into ChatGPT, which returned answers and explanations. The corresponding answer was selected on the ACG website based on the chatbot’s response.
Of the 455 questions posed, ChatGPT-3 correctly answered 296, and ChatGPT-4 got 284 right. There was no discernible pattern in the type of question that the chatbot answered incorrectly, but questions on surveillance timing for various disease states, diagnosis, and pharmaceutical regimens were all answered incorrectly.
The reasons for the tool’s poor performance could lie with the large language model underpinning ChatGPT, the researchers write. The model was trained on freely available information – not specifically on medical literature and not on materials that require paid journal subscriptions – to be a general-purpose interactive program.
Additionally, the chatbot may use information from a variety of sources, including non- or quasi-medical sources, or out-of-date sources, which can lead to errors, they note. ChatGPT-3 was last updated in June 2021 and ChatGPT-4 in September 2021.
“ChatGPT does not have an intrinsic understanding of an issue,” Dr. Trindade said. “Its basic function is to predict the next word in a string of text to produce an expected response, regardless of whether such a response is factually correct or not.”
Previous research
In a previous study, ChatGPT was able to pass parts of the U.S. Medical Licensing Examination.
The chatbot may have performed better on the USMLE because the information tested on the exam may have been more widely available for ChatGPT’s language training, Dr. Trindade said. “In addition, the threshold for passing [the USMLE] is lower with regard to the percentage of questions correctly answered,” he said.
ChatGPT seems to fare better at helping to inform patients than it does on medical exams. The chatbot provided generally satisfactory answers to common patient queries about colonoscopy in one study and about hepatocellular carcinoma and liver cirrhosis in another study.
For ChatGPT to be valuable in medical education, “future versions would need to be updated with medical resources such as journal articles, society guidelines, and medical databases, such as UpToDate,” Dr. Trindade said. “With directed medical training in gastroenterology, it may be a future tool for education or patient use in this field, but not currently as it is now. Before it can be used in gastroenterology, it should be validated.”
That said, he noted, medical education has evolved from being based on textbooks and print journals to include Internet-based journal data and practice guidelines on specialty websites. If properly primed, resources such as ChatGPT may be the next logical step.
This study received no funding. Dr. Trindade is a consultant for Pentax Medical, Boston Scientific, Lucid Diagnostic, and Exact Science and receives research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Earlier anticoagulation safe in stroke with AFib: ELAN
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Half of deaths from homozygous FH occur before age 32 years
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Maternal health clinic teams with legal services to aid patients
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ACOG 2023
Harmful emotional hit of antidepressants underappreciated
SAN FRANCISCO – , new research shows.
Emotional blunting can be described as feeling emotionally flat and incapable of finding pleasure. The patient may feel less sadness, guilt, or hopelessness, but that may come at the cost of feeling less joy, surprise, and happiness. Some people with SSRI-induced blunting even report caring less about important relationships.
It’s an issue that needs greater attention, study investigator Mujeeb U. Shad, MD, with Valley Health Services and University of Nevada, Las Vegas, said in an interview.
“Patients may come to the clinic and report feeling emotionally and cognitively flat and not be taken seriously by their provider, but they are genuinely reporting something that is happening to them and decreasing their quality of life,” Dr. Shad explained.
The study was presented at the annual meeting of the American Psychiatric Association.
Something ‘missing’
Dr. Shad said that the genesis for the study came from a resident who noticed that many patients receiving SSRIs reported feeling better and not as bothered by the depression, yet, at the same time, they felt something was “missing. Their families would say, ‘You’re better but you’re not the same person.’ ”
To investigate further, the researchers did a “scoping review” of 25 original studies that assessed antidepressant-related emotional blunting. Until now, there hasn’t been a systematic review of this issue, Dr. Shad said.
Ten of the studies looked at the role of SSRIs in emotional blunting, whereas the other 15 looked at serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and second-generation antipsychotic medications.
The results of the review show that emotional blunting is a significant patient-reported concern. It often presents as a subjective complaint of changed personality, feeling a lesser intensity of overall emotions, and the manifestation of not being oneself often attributed to antidepressant use, the researchers found. Emotional blunting was more commonly associated with SSRIs than with the other medications in the studies.
Common clinical strategies to manage antidepressant-induced emotional blunting reported in the literature include dose reduction or switching to a different antidepressant class; however, the literature did not make the distinction between emotional blunting as a primary symptom of depression or an adverse effect of antidepressants.
Dr. Shad said that there is a need to develop valid and reliable measures to assess emotional blunting related to antidepressants.
He noted that optimal patient care should include pre- and posttreatment assessment of emotional blunting. One useful tool is the Oxford Questionnaire on the Emotional Side-Effects of Antidepressants.
Can’t get to the top
Jacob Cross, MD, who wasn’t involved in the study, said that he has seen the impact of antidepressant-related emotional blunting first-hand.
“I’ve had multiple patients report emotional blunting on antidepressant therapy,” Dr. Cross, with the department of psychiatry, Rush Medical College, Chicago, said.
“These patients feel like their emotions are not as high and not as low; so they experience directional improvement, but they’re still not feeling like they can get that top peak emotion. It’s kind of similar to anhedonia. They’re just feeling like a little cut off, like they’re climbing a cliff and just can’t get to that top,” Dr. Cross said.
For a patient with emotional blunting, Dr. Cross said he might “switch to an antidepressant that’s more stimulating like an SNRI from an SSRI. You could also lower the dose and see if that helps, but I usually change the drug class.”
The study had no specific funding. Dr. Shad and Dr. Cross have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , new research shows.
Emotional blunting can be described as feeling emotionally flat and incapable of finding pleasure. The patient may feel less sadness, guilt, or hopelessness, but that may come at the cost of feeling less joy, surprise, and happiness. Some people with SSRI-induced blunting even report caring less about important relationships.
It’s an issue that needs greater attention, study investigator Mujeeb U. Shad, MD, with Valley Health Services and University of Nevada, Las Vegas, said in an interview.
“Patients may come to the clinic and report feeling emotionally and cognitively flat and not be taken seriously by their provider, but they are genuinely reporting something that is happening to them and decreasing their quality of life,” Dr. Shad explained.
The study was presented at the annual meeting of the American Psychiatric Association.
Something ‘missing’
Dr. Shad said that the genesis for the study came from a resident who noticed that many patients receiving SSRIs reported feeling better and not as bothered by the depression, yet, at the same time, they felt something was “missing. Their families would say, ‘You’re better but you’re not the same person.’ ”
To investigate further, the researchers did a “scoping review” of 25 original studies that assessed antidepressant-related emotional blunting. Until now, there hasn’t been a systematic review of this issue, Dr. Shad said.
Ten of the studies looked at the role of SSRIs in emotional blunting, whereas the other 15 looked at serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and second-generation antipsychotic medications.
The results of the review show that emotional blunting is a significant patient-reported concern. It often presents as a subjective complaint of changed personality, feeling a lesser intensity of overall emotions, and the manifestation of not being oneself often attributed to antidepressant use, the researchers found. Emotional blunting was more commonly associated with SSRIs than with the other medications in the studies.
Common clinical strategies to manage antidepressant-induced emotional blunting reported in the literature include dose reduction or switching to a different antidepressant class; however, the literature did not make the distinction between emotional blunting as a primary symptom of depression or an adverse effect of antidepressants.
Dr. Shad said that there is a need to develop valid and reliable measures to assess emotional blunting related to antidepressants.
He noted that optimal patient care should include pre- and posttreatment assessment of emotional blunting. One useful tool is the Oxford Questionnaire on the Emotional Side-Effects of Antidepressants.
Can’t get to the top
Jacob Cross, MD, who wasn’t involved in the study, said that he has seen the impact of antidepressant-related emotional blunting first-hand.
“I’ve had multiple patients report emotional blunting on antidepressant therapy,” Dr. Cross, with the department of psychiatry, Rush Medical College, Chicago, said.
“These patients feel like their emotions are not as high and not as low; so they experience directional improvement, but they’re still not feeling like they can get that top peak emotion. It’s kind of similar to anhedonia. They’re just feeling like a little cut off, like they’re climbing a cliff and just can’t get to that top,” Dr. Cross said.
For a patient with emotional blunting, Dr. Cross said he might “switch to an antidepressant that’s more stimulating like an SNRI from an SSRI. You could also lower the dose and see if that helps, but I usually change the drug class.”
The study had no specific funding. Dr. Shad and Dr. Cross have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , new research shows.
Emotional blunting can be described as feeling emotionally flat and incapable of finding pleasure. The patient may feel less sadness, guilt, or hopelessness, but that may come at the cost of feeling less joy, surprise, and happiness. Some people with SSRI-induced blunting even report caring less about important relationships.
It’s an issue that needs greater attention, study investigator Mujeeb U. Shad, MD, with Valley Health Services and University of Nevada, Las Vegas, said in an interview.
“Patients may come to the clinic and report feeling emotionally and cognitively flat and not be taken seriously by their provider, but they are genuinely reporting something that is happening to them and decreasing their quality of life,” Dr. Shad explained.
The study was presented at the annual meeting of the American Psychiatric Association.
Something ‘missing’
Dr. Shad said that the genesis for the study came from a resident who noticed that many patients receiving SSRIs reported feeling better and not as bothered by the depression, yet, at the same time, they felt something was “missing. Their families would say, ‘You’re better but you’re not the same person.’ ”
To investigate further, the researchers did a “scoping review” of 25 original studies that assessed antidepressant-related emotional blunting. Until now, there hasn’t been a systematic review of this issue, Dr. Shad said.
Ten of the studies looked at the role of SSRIs in emotional blunting, whereas the other 15 looked at serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and second-generation antipsychotic medications.
The results of the review show that emotional blunting is a significant patient-reported concern. It often presents as a subjective complaint of changed personality, feeling a lesser intensity of overall emotions, and the manifestation of not being oneself often attributed to antidepressant use, the researchers found. Emotional blunting was more commonly associated with SSRIs than with the other medications in the studies.
Common clinical strategies to manage antidepressant-induced emotional blunting reported in the literature include dose reduction or switching to a different antidepressant class; however, the literature did not make the distinction between emotional blunting as a primary symptom of depression or an adverse effect of antidepressants.
Dr. Shad said that there is a need to develop valid and reliable measures to assess emotional blunting related to antidepressants.
He noted that optimal patient care should include pre- and posttreatment assessment of emotional blunting. One useful tool is the Oxford Questionnaire on the Emotional Side-Effects of Antidepressants.
Can’t get to the top
Jacob Cross, MD, who wasn’t involved in the study, said that he has seen the impact of antidepressant-related emotional blunting first-hand.
“I’ve had multiple patients report emotional blunting on antidepressant therapy,” Dr. Cross, with the department of psychiatry, Rush Medical College, Chicago, said.
“These patients feel like their emotions are not as high and not as low; so they experience directional improvement, but they’re still not feeling like they can get that top peak emotion. It’s kind of similar to anhedonia. They’re just feeling like a little cut off, like they’re climbing a cliff and just can’t get to that top,” Dr. Cross said.
For a patient with emotional blunting, Dr. Cross said he might “switch to an antidepressant that’s more stimulating like an SNRI from an SSRI. You could also lower the dose and see if that helps, but I usually change the drug class.”
The study had no specific funding. Dr. Shad and Dr. Cross have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
AT APA 2023
People still want their medical intelligence in human form
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
AxSpA remission on TNFi seen in half of patients with comorbid IBD
CLEVELAND – About half (52%) of patients living with both axial spondyloarthritis and inflammatory bowel disease (IBD) reached clinical remission of axSpA at 12 months after starting a tumor necrosis factor inhibitor (TNFi), researchers have found.
The disease course for axSpA among patients with IBD who start anti-TNF agents is not well understood.
Rahul S. Dalal, MD, an advanced fellow in IBD with the division of gastroenterology, hepatology, and endoscopy at Brigham and Women’s Hospital, Boston, and colleagues studied whether certain clinical factors were associated with remission of axSpA after patients with axSpA, who also had Crohn’s disease (CD) or ulcerative colitis (UC), started anti-TNF therapy.
Short IBD duration, adalimumab linked with higher remission odds
They found that those who had IBD for less than 5 years and those taking the TNFi adalimumab (Humira and biosimilars), as opposed to another TNFi, had a higher likelihood of reaching axSpA remission at 1 year. The odds ratios calculated for those factors were statistically significant.
Dr. Dalal said that most of the patients in the study (70%) were prescribed adalimumab, and because the study didn’t compare TNFis head to head, it’s hard to say whether adalimumab should be the preferred treatment for these patients.
“But it’s an interesting question that should be addressed in a bigger study,” he said.
Other TNFis included infliximab (Remicade and biosimilars) in 27%, golimumab (Simponi) in 2%, and certolizumab pegol (Cimzia) in 1%.
He presented the results at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Study details
Included in the retrospective cohort study were 82 adults with IBD and either ankylosing spondylitis or sacroiliitis who started anti-TNF agents approved for IBD between January 2012 and October 2021 at a large academic center.
Clinical remission of axSpA was the primary outcome, defined as the absence or adequate control of pain and/or stiffness related to axSpA as documented in the rheumatology note 1 year (+/– 2 months) after starting anti-TNF agents.
The secondary outcome was clinical remission of IBD, defined as 2 or less on the simple clinical colitis activity index, a score of less than 5 on the Harvey-Bradshaw Index, or provider assessment with no use of oral or intravenous glucocorticoids for 30 days. Dr. Dalal said 74% in the study reached that endpoint in the study period.
“Some patients had good response to anti-TNF treatments for their IBD but not necessarily for their spondyloarthritis,” he explained.
There were insufficient observations to calculate odds ratios for the variables, including Hispanic ethnicity, endoscopic inflammation, and prior history of using vedolizumab (Entyvio), secukinumab (Cosentyx), and ustekinumab (Stelara), the authors noted.
Dr. Dalal said it’s important to study this population because patients with IBD and axSpA take some of the same medications, but it’s not known how each medication acts in patients.
“We don’t have much data to tell us who’s going to respond to treatments from both diseases simultaneously,” he said.
Conclusions called ‘reassuring’
Jean Liew, MD, a spondyloarthritis specialist at Boston University, who was not part of the study, noted that the team reported univariate associations of clinical factors with achievement of clinical axSpA remission, but no multivariable analyses with adjustment for potential confounders.
She said the finding of half the patients achieving clinical remission is “reassuring, as anecdotally we may find that patients with IBD-associated spondyloarthritis tend to have more difficult-to-treat symptoms as well as more limited treatment options. For example, they cannot use [interleukin]-17 inhibitors.”
She noted the study is small and descriptive and further analyses are limited by the small number of patients.
“I think if a study of the same type could be performed at a larger scale with larger numbers, it could generate more data on which type of patient with IBD-associated spondyloarthritis is more likely to have a good response after starting a TNF inhibitor,” she said. “Of course, the other question is how long those patients would have good disease control while on the TNF inhibitor. What is the persistence of the medication? This study doesn’t ask or answer that question.”
Dr. Dalal added that in future research it will be important to look at response to medications beyond TNFis, especially Janus kinase inhibitors.
That will help show “whether there is a treatment algorithm that can be tailored to this population in terms of what agents to choose first,” he said. “I think we need multicenter studies to do this.”
Dr. Dalal has received grant funding from Pfizer and Janssen and has served as a consultant for Centaur Labs and Janssen. Dr. Liew has no relevant financial relationships.
CLEVELAND – About half (52%) of patients living with both axial spondyloarthritis and inflammatory bowel disease (IBD) reached clinical remission of axSpA at 12 months after starting a tumor necrosis factor inhibitor (TNFi), researchers have found.
The disease course for axSpA among patients with IBD who start anti-TNF agents is not well understood.
Rahul S. Dalal, MD, an advanced fellow in IBD with the division of gastroenterology, hepatology, and endoscopy at Brigham and Women’s Hospital, Boston, and colleagues studied whether certain clinical factors were associated with remission of axSpA after patients with axSpA, who also had Crohn’s disease (CD) or ulcerative colitis (UC), started anti-TNF therapy.
Short IBD duration, adalimumab linked with higher remission odds
They found that those who had IBD for less than 5 years and those taking the TNFi adalimumab (Humira and biosimilars), as opposed to another TNFi, had a higher likelihood of reaching axSpA remission at 1 year. The odds ratios calculated for those factors were statistically significant.
Dr. Dalal said that most of the patients in the study (70%) were prescribed adalimumab, and because the study didn’t compare TNFis head to head, it’s hard to say whether adalimumab should be the preferred treatment for these patients.
“But it’s an interesting question that should be addressed in a bigger study,” he said.
Other TNFis included infliximab (Remicade and biosimilars) in 27%, golimumab (Simponi) in 2%, and certolizumab pegol (Cimzia) in 1%.
He presented the results at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Study details
Included in the retrospective cohort study were 82 adults with IBD and either ankylosing spondylitis or sacroiliitis who started anti-TNF agents approved for IBD between January 2012 and October 2021 at a large academic center.
Clinical remission of axSpA was the primary outcome, defined as the absence or adequate control of pain and/or stiffness related to axSpA as documented in the rheumatology note 1 year (+/– 2 months) after starting anti-TNF agents.
The secondary outcome was clinical remission of IBD, defined as 2 or less on the simple clinical colitis activity index, a score of less than 5 on the Harvey-Bradshaw Index, or provider assessment with no use of oral or intravenous glucocorticoids for 30 days. Dr. Dalal said 74% in the study reached that endpoint in the study period.
“Some patients had good response to anti-TNF treatments for their IBD but not necessarily for their spondyloarthritis,” he explained.
There were insufficient observations to calculate odds ratios for the variables, including Hispanic ethnicity, endoscopic inflammation, and prior history of using vedolizumab (Entyvio), secukinumab (Cosentyx), and ustekinumab (Stelara), the authors noted.
Dr. Dalal said it’s important to study this population because patients with IBD and axSpA take some of the same medications, but it’s not known how each medication acts in patients.
“We don’t have much data to tell us who’s going to respond to treatments from both diseases simultaneously,” he said.
Conclusions called ‘reassuring’
Jean Liew, MD, a spondyloarthritis specialist at Boston University, who was not part of the study, noted that the team reported univariate associations of clinical factors with achievement of clinical axSpA remission, but no multivariable analyses with adjustment for potential confounders.
She said the finding of half the patients achieving clinical remission is “reassuring, as anecdotally we may find that patients with IBD-associated spondyloarthritis tend to have more difficult-to-treat symptoms as well as more limited treatment options. For example, they cannot use [interleukin]-17 inhibitors.”
She noted the study is small and descriptive and further analyses are limited by the small number of patients.
“I think if a study of the same type could be performed at a larger scale with larger numbers, it could generate more data on which type of patient with IBD-associated spondyloarthritis is more likely to have a good response after starting a TNF inhibitor,” she said. “Of course, the other question is how long those patients would have good disease control while on the TNF inhibitor. What is the persistence of the medication? This study doesn’t ask or answer that question.”
Dr. Dalal added that in future research it will be important to look at response to medications beyond TNFis, especially Janus kinase inhibitors.
That will help show “whether there is a treatment algorithm that can be tailored to this population in terms of what agents to choose first,” he said. “I think we need multicenter studies to do this.”
Dr. Dalal has received grant funding from Pfizer and Janssen and has served as a consultant for Centaur Labs and Janssen. Dr. Liew has no relevant financial relationships.
CLEVELAND – About half (52%) of patients living with both axial spondyloarthritis and inflammatory bowel disease (IBD) reached clinical remission of axSpA at 12 months after starting a tumor necrosis factor inhibitor (TNFi), researchers have found.
The disease course for axSpA among patients with IBD who start anti-TNF agents is not well understood.
Rahul S. Dalal, MD, an advanced fellow in IBD with the division of gastroenterology, hepatology, and endoscopy at Brigham and Women’s Hospital, Boston, and colleagues studied whether certain clinical factors were associated with remission of axSpA after patients with axSpA, who also had Crohn’s disease (CD) or ulcerative colitis (UC), started anti-TNF therapy.
Short IBD duration, adalimumab linked with higher remission odds
They found that those who had IBD for less than 5 years and those taking the TNFi adalimumab (Humira and biosimilars), as opposed to another TNFi, had a higher likelihood of reaching axSpA remission at 1 year. The odds ratios calculated for those factors were statistically significant.
Dr. Dalal said that most of the patients in the study (70%) were prescribed adalimumab, and because the study didn’t compare TNFis head to head, it’s hard to say whether adalimumab should be the preferred treatment for these patients.
“But it’s an interesting question that should be addressed in a bigger study,” he said.
Other TNFis included infliximab (Remicade and biosimilars) in 27%, golimumab (Simponi) in 2%, and certolizumab pegol (Cimzia) in 1%.
He presented the results at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Study details
Included in the retrospective cohort study were 82 adults with IBD and either ankylosing spondylitis or sacroiliitis who started anti-TNF agents approved for IBD between January 2012 and October 2021 at a large academic center.
Clinical remission of axSpA was the primary outcome, defined as the absence or adequate control of pain and/or stiffness related to axSpA as documented in the rheumatology note 1 year (+/– 2 months) after starting anti-TNF agents.
The secondary outcome was clinical remission of IBD, defined as 2 or less on the simple clinical colitis activity index, a score of less than 5 on the Harvey-Bradshaw Index, or provider assessment with no use of oral or intravenous glucocorticoids for 30 days. Dr. Dalal said 74% in the study reached that endpoint in the study period.
“Some patients had good response to anti-TNF treatments for their IBD but not necessarily for their spondyloarthritis,” he explained.
There were insufficient observations to calculate odds ratios for the variables, including Hispanic ethnicity, endoscopic inflammation, and prior history of using vedolizumab (Entyvio), secukinumab (Cosentyx), and ustekinumab (Stelara), the authors noted.
Dr. Dalal said it’s important to study this population because patients with IBD and axSpA take some of the same medications, but it’s not known how each medication acts in patients.
“We don’t have much data to tell us who’s going to respond to treatments from both diseases simultaneously,” he said.
Conclusions called ‘reassuring’
Jean Liew, MD, a spondyloarthritis specialist at Boston University, who was not part of the study, noted that the team reported univariate associations of clinical factors with achievement of clinical axSpA remission, but no multivariable analyses with adjustment for potential confounders.
She said the finding of half the patients achieving clinical remission is “reassuring, as anecdotally we may find that patients with IBD-associated spondyloarthritis tend to have more difficult-to-treat symptoms as well as more limited treatment options. For example, they cannot use [interleukin]-17 inhibitors.”
She noted the study is small and descriptive and further analyses are limited by the small number of patients.
“I think if a study of the same type could be performed at a larger scale with larger numbers, it could generate more data on which type of patient with IBD-associated spondyloarthritis is more likely to have a good response after starting a TNF inhibitor,” she said. “Of course, the other question is how long those patients would have good disease control while on the TNF inhibitor. What is the persistence of the medication? This study doesn’t ask or answer that question.”
Dr. Dalal added that in future research it will be important to look at response to medications beyond TNFis, especially Janus kinase inhibitors.
That will help show “whether there is a treatment algorithm that can be tailored to this population in terms of what agents to choose first,” he said. “I think we need multicenter studies to do this.”
Dr. Dalal has received grant funding from Pfizer and Janssen and has served as a consultant for Centaur Labs and Janssen. Dr. Liew has no relevant financial relationships.
AT SPARTAN 2023
Extracellular Matrix–Based Collagen Dressings for Scalp Repair Following Mohs Micrographic Surgery
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.
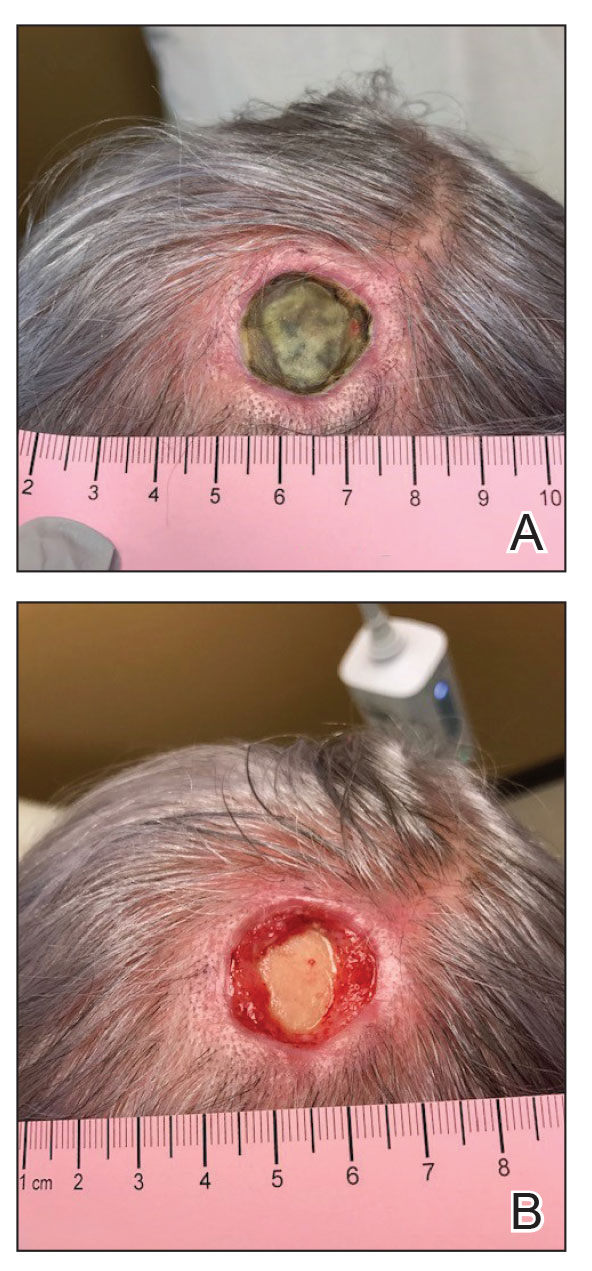
A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
Practice Points
- Patients who undergo Mohs micrographic surgery on the scalp are prone to developing complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.
- Use of extracellular matrix–based dressings may assist with deep wound healing on the scalp.
Nevus Sebaceus With Novel HRAS Sequence Variant Mutation Misdiagnosed as Alopecia Areata
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).
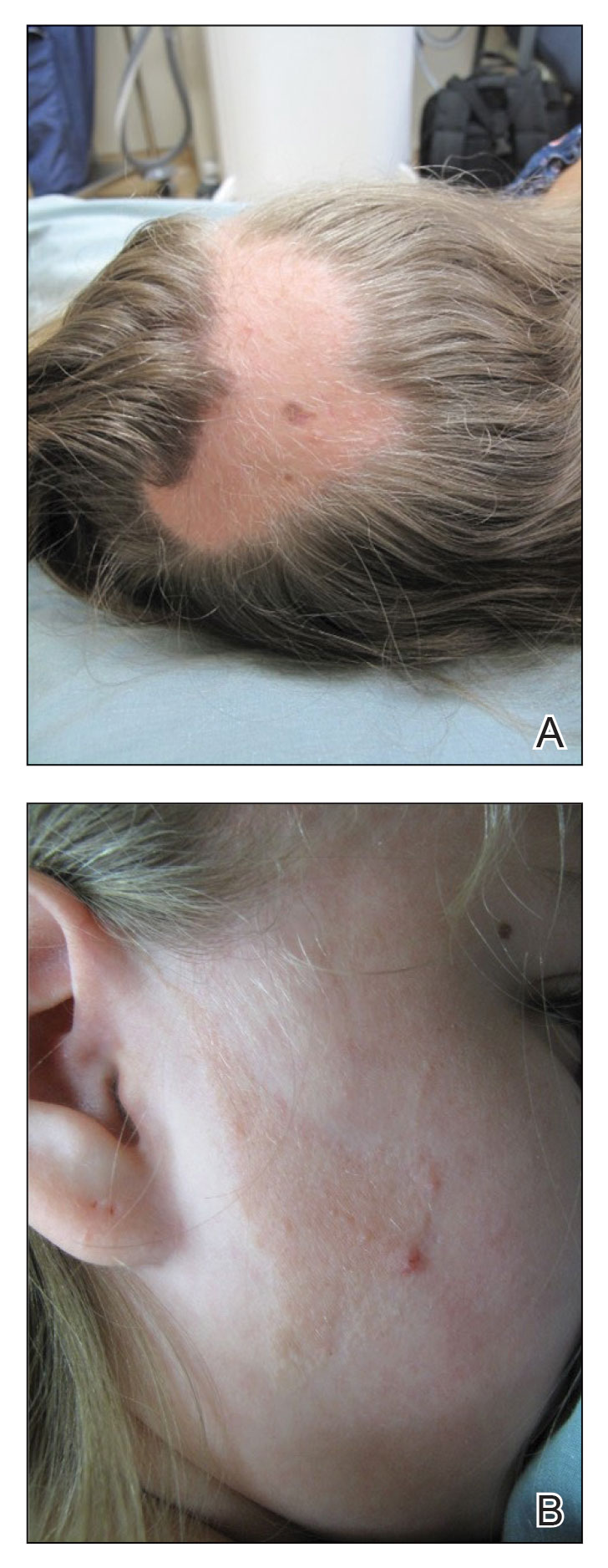
The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.
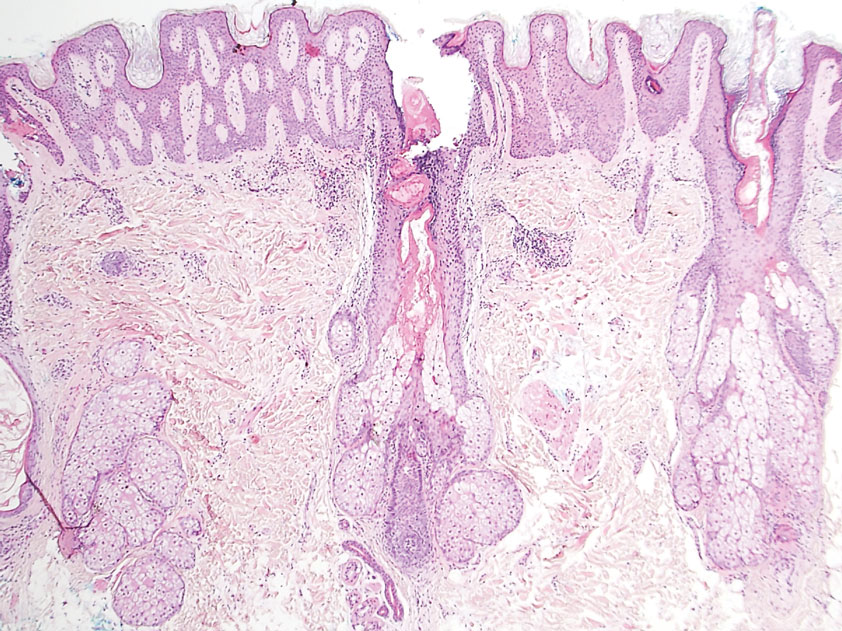
A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.
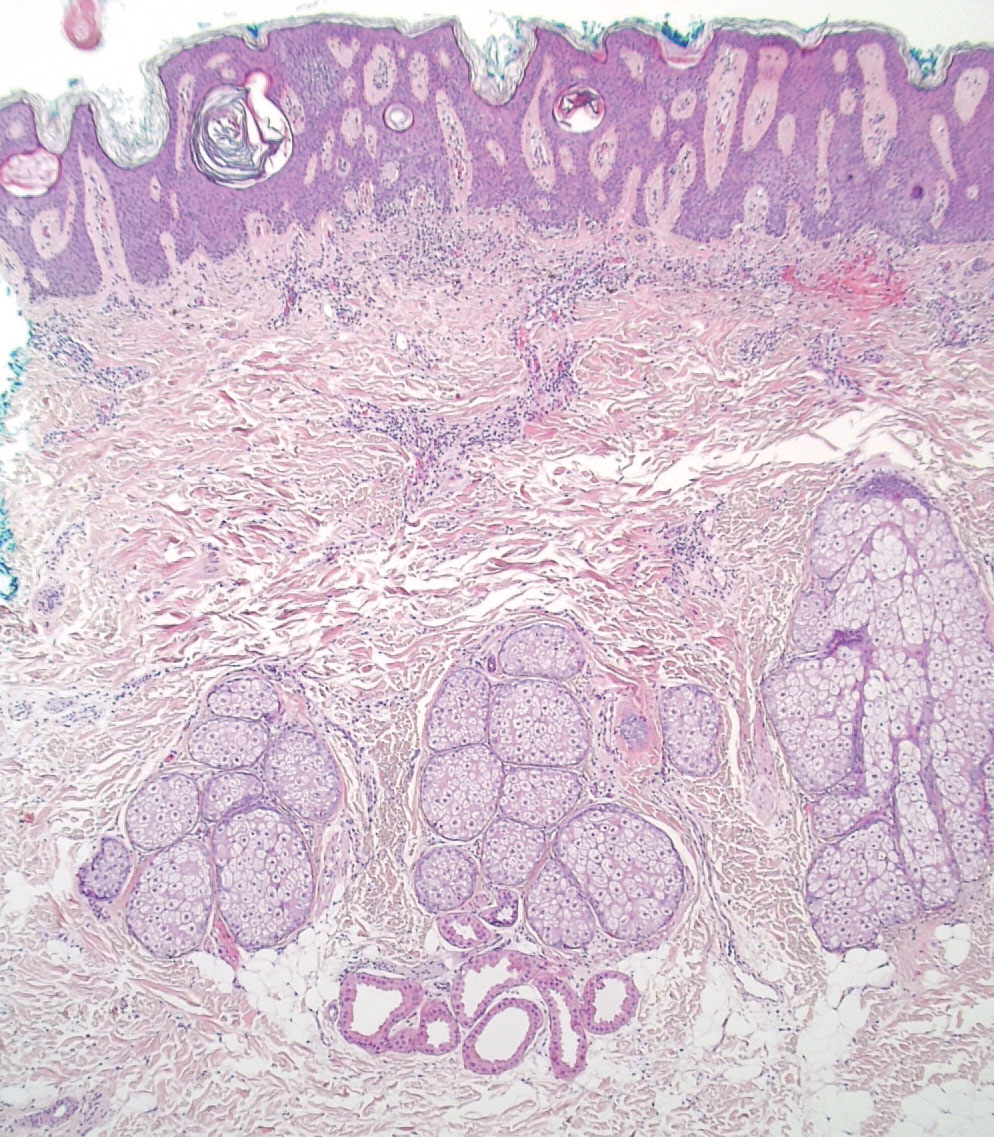
Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
Practice Points
- Nevus sebaceus (NS), commonly referred to as NS of Jadassohn or organoid nevus, is a benign skin hamartoma that consists of epidermal, sebaceous, and apocrine elements and is caused by a congenital defect in the pilosebaceous follicular unit.
- Early stages of NS can be mistaken for alopecia areata.
- Once the diagnosis of NS is confirmed, the presence of associated syndromes should be evaluated.
- The definitive treatment of NS is surgical excision; however, multiple variables must be considered when determining treatment, including patient age, risk for developing malignancy, and surgery-associated risks.











![An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_300x300.jpg)