User login
Investigational uricase-based gout drug meets primary endpoints in phase 3 trials
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
MILAN – Serum uric acid of less than 6 mg/dL was achieved and maintained for a substantial period of time with a once-monthly infusion of SEL-212 in patients with refractory gout, according to results of the two phase 3 DISSOLVE I and II trials.
Both trials met their primary endpoints. In DISSOLVE I – the U.S. study – 56% of patients on SEL-212 at 0.15 mg/kg (high dose) achieved a response, defined as achievement and maintenance of a reduction in serum urate to less than 6 mg/dL for at least 80% of the time during month 6 of treatment. In DISSOLVE II – the global study – 46% of patients on SEL-212 on the 0.15-mg/kg dose achieved response.
In participants aged 50 years or older, there was a statistically significant higher response rate at the high dose of SEL-212 in both DISSOLVE I and II of 65% and 47%, respectively, compared with placebo.
Herbert S.B. Baraf, MD, clinical professor of medicine at George Washington University, Washington, and principal investigator of the DISSOLVE program, presented results of the two phase 3 trials during a late-breaking session at the annual European Congress of Rheumatology.
“The top-line data from the two SEL-212 phase 3 studies are encouraging. They show that induction of immunotolerance with an infusion of a rapamycin-containing nanoparticle (SEL-110), followed immediately by an infusion of pegadricase, a potent but immunogenic uricase, allows for a strong and sustained uric acid–lowering effect without the development of anti-drug antibodies,” Dr. Baraf said in an interview.
SEL-212 is a monthly two-part infusion therapy – a combination of Selecta Biosciences’s ImmTOR immune tolerance platform, and a therapeutic uricase enzyme (pegadricase), designed to treat refractory gout. SEL-110 (ImmTOR) is an immune-tolerizing, nanoencapsulated rapamycin administered 30 minutes before pegadricase and inhibits anti-pegadricase antibodies. SEL-37 is a pegylated uricase (pegadricase) that converts uric acid to excretable allantoin.
SEL-212 was originally developed by Selecta. Swedish Orphan Biovitrum (Sobi) licensed SEL-212 from Selecta in June 2020 and is responsible for development, regulatory, and commercial activities in all markets outside of China. Selecta is responsible for ImmTOR manufacturing. The phase 3 program for SEL-212 was run by Selecta and funded by Sobi.
It is understood that a biologic license application will be submitted to the Food and Drug Administration, most likely next year, and if approved, “the SEL-212 two-component infusion treatment would provide a monthly alternative to twice-monthly pegloticase, for patients with refractory gout,” Dr. Baraf added.
Details of the trials
The two DISSOLVE studies replicate double-blind, placebo-controlled trials in patients with chronic refractory gout. DISSOLVE I was carried out in 112 patients across 29 sites in the United States, and DISSOLVE II tested the two-part treatment in 153 patients across 37 sites in the United States, Russia, Ukraine, Georgia, and Serbia.
Both studies randomized patients 1:1:1 to a high dose (SEL-110 of 0.15 mg/kg plus SEL-037 of 0.2 mg/kg), low dose (SEL-110 of 0.1 mg/kg plus SEL-037 of 0.2 mg/kg), or placebo (saline) infused every 28 days for 6 months. Prophylaxis against infusion reactions and gout flares were given to all participants.
Adult patients had a 10- to 14-year history of symptomatic gout, with three or more flares over the 18 months prior to screening, or one or more tophus, or a diagnosis of gouty arthritis. They were also required to have chronic refractory gout with a failure to normalize serum uric acid with any xanthine oxidase inhibitor (for example, allopurinol) and to have not been previously exposed to uricase-based therapy. Serum uric acid had to be at least 7 mg/dL. Participants were balanced for age, body mass index, and sex across treatment groups. Gout severity was greater in DISSOLVE II, Dr. Baraf reported.
Both studies treated patients for 6 months, but DISSOLVE 1 continued with a 6-month, blinded safety extension. The primary endpoint in both studies was serum urate control during month 6, and secondary endpoints included tender and swollen joint counts, tophus burden, patient-reported outcomes of activity limitation, quality of life, and gout flare incidence.
In DISSOLVE I, patients on SEL-212 had a statistically significant higher response rate during month 6 of 56% with the high dose (P < .0001) and 48% with the low dose (P < .0001), compared with 4% of patients randomized to receive placebo. In DISSOLVE II, participants on SEL-212 had a statistically significant higher response rate during month 6 of 46% with the high dose (P = .0002) and 40% with the low dose (P = .0008), compared with 11% of patients randomized to receive placebo.
“We also saw significant reductions in serum uric acid for all treatment groups, compared with placebo,” Dr. Baraf reported. Mean percentage change was –62.3% and –58.3% in the high- and low-dose groups, respectively, in DISSOLVE I, and –58.1% and –52.2% in DISSOLVE II, respectively.
SEL-212 had a favorable safety profile with adverse events as expected across both doses, including mild to moderate stomatitis (3.4% in the low-dose group and 9.2% in the high-dose group versus 0% in the placebo group), and a greater number of infusion reactions at 24 hours and 1 hour after drug administration in both treatment groups versus placebo. Six patients had treatment-related serious adverse events, including two cases of anaphylaxis and one gout flare in both the high- and low-dose treatment groups. The 6-month extension period in the DISSOLVE I trial showed that the majority (75%) of patients who completed 6 months of SEL-212 treatment as a responder continued to be successfully treated through 12 months with no infusion reactions or safety signals.
“I expect more data will be forthcoming on the important clinical secondary endpoints targeted by SEL-212 therapy,” Dr. Baraf noted.
Need control arm taking allopurinol?
Roy Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center and codirector of the Metroplex Clinical Research Center, both in Dallas, commented on the study methods after the presentation. “The major problem with this study is that they say the patients had had insufficient response to allopurinol, and my guess is most had received 100-200 mg of allopurinol but were not titrated up to the maximum tolerated dose,” he said, adding: “they should have had a control arm of patients on allopurinol and titrated to the maximum tolerated dose. So, I don’t know what this is really telling us with respect to allopurinol, which is a relatively cheap drug.”
Dr. Baraf reported consulting with Horizon, Sobi, and Selecta; serving on Horizon’s speakers bureau, and receiving grant/research support from Horizon and Sobi. Dr. Fleischmann reported no financial relationship of relevance to this study.
AT EULAR 2023
Racial, ethnic disparities persist in access to MS care
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
At CMSC 2023
Why Is There a Lack of Representation of Skin of Color in the COVID-19 Literature?
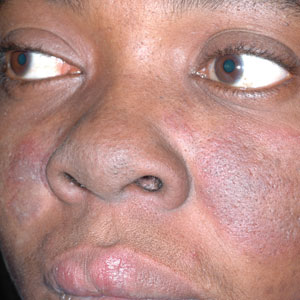
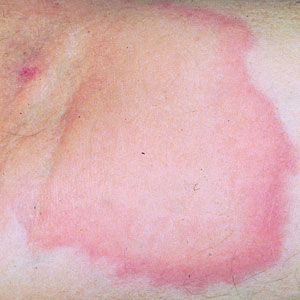
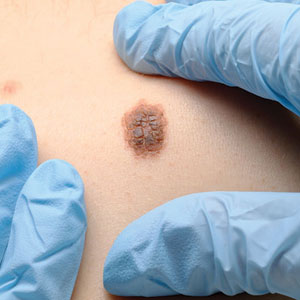
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
Music therapy helps motivate patients with schizophrenia
SAN FRANCISCO –
Although the study had conflicting results regarding the effects of music therapy on positive symptoms of schizophrenia, such as hallucinations, delusions, and disordered thoughts, it consistently shows that music therapy improves negative symptoms, poster presenter Amy Agrawal, MD, VA Boston Healthcare System and instructor of psychiatry at Harvard Medical School, Boston, said in an interview.
Current antipsychotic drugs aren’t very effective in addressing negative symptoms of schizophrenia, and many patients are noncompliant with these drug regimens because of side effects.
“We need to target the negative symptoms of schizophrenia better,” said Dr. Agrawal. “The antipsychotic medications we have are not enough, so why don’t we start incorporating music therapy groups into the inpatient psychiatry setting as a standard of care?”
The findings were presented at the annual meeting of the American Psychiatric Association.
Dr. Agrawal has long been interested in music. As a child, she was a member of a state choir, but she hadn’t sung for years. After receiving several medals for her clarinet playing during her youth, she stopped playing while in medical school.
Instant boost
During the pandemic, though, she turned back to music and started singing regularly. “I noticed an instant boost in my mood and wondered why I stopped making music for so long, as it made me feel so much happier and calmer.”
She also noticed how music affected her sister, who has paranoid schizophrenia. She described an incident in which her sibling became so loud and paranoid at a restaurant that Dr. Agrawal thought they would be asked to leave.
Then her sister started singing a song she’d sung during a beauty contest years before. “With the music, she calmed right down; she was smiling; she was happy,” said Dr. Agrawal.
That incident made Dr. Agrawal feel, “I had my sister back.” She decided to bring music therapy to her inpatient psychiatry unit and soon noted the benefits for individual patients.
For this new study, Dr. Agrawal and her mentor carried out a literature search. “I was surprised at how many articles popped up, because the field of psychiatry can be very heavily medication based, but people are now getting very interested in this topic,” said Dr. Agrawal.
The review included seven articles, most of which were published within the past 3 years. Some articles specified that the therapy was conducted on an inpatient psychiatric unit, but others didn’t indicate the setting. Studies also didn’t specify whether the therapy was delivered by a trained music therapist.
There was an overall lack of clear measures, graphs, or statistics quantifying the benefits of music therapy on schizophrenia, noted Dr. Agrawal. “But from general statements in the articles, music therapy helped treat sleep disturbances and improved negative symptoms.”
Gets patients socializing
The music, she said, led patients to start socializing, talking about their emotions, and opening up to their clinicians about their mental health symptoms. “Some patients just did not engage at all, and then when the music came on, they would actively participate with the clinician.”
Dancing to music also tended to motivate patients to participate in their treatment, she added. Different forms of movement, such as rhythmic movements and creative exercises, can be added during music therapy.
In addition to improving negative schizophrenia symptoms, music therapy helps with sleep disturbances, depression, and regulating emotional behavior, the research shows. “When patients were agitated or upset, certain music would help them regulate their own affect,” said Dr. Agrawal.
However, it’s not clear from these studies what type of music – classical, rock, country, etc. – was most effective for people.
One article discussed the positive impact of music on patients with schizophrenia while at work. “They seem to have improved work performance,” Dr. Agrawal said.
The length of exposure to music therapy did not seem to make a difference in terms of whether the therapy had a positive effect, she added.
Key research wave
A “key next wave” of research should be to determine whether music therapy decreases the hospital readmission rate, said Dr. Agrawal.
There are several barriers to implementing music therapy programs in hospitals, including cost, the availability of trained therapists, and time constraints, she said.
“Regardless of the barriers, hospital administrators and psychiatrists need to know about this research so they will invest more efforts in recruiting music therapists and incorporating music group therapy into standard clinical practice for psychiatric patients so there are better clinical outcomes.”
Commenting on the research, Michelle B. Riba, MD, professor, department of psychiatry, University of Michigan, Ann Arbor, said the study adds to the literature “and helps us think about adjunctive treatments in a very difficult population.”
She added, “It’s good to see physicians get interested in this topic.”
Difficult topic to study
Although she found the review “very limited,” she recognizes the difficulty of studying music therapy on in-patient psychiatry units.
“Patients are there for short stays, most are getting other treatments, and it’s hard to segment people into negative vs. positive. Also, the ages and genders are different, and their previous treatments are different.”
While it’s sometimes difficult to conduct major research on a topic, “that doesn’t mean we can’t help people,” said Dr. Riba.
She noted that music therapy is beneficial not only for patients with schizophrenia but also is “soothing and relaxing” for those with other conditions. She runs a psychiatric oncology program at her institution’s cancer center, which offers music therapy along with art therapy.
Kevin M. Malone, MD, of University College Dublin, also has firsthand experience with music therapy. “We had a terrific music therapist as part of our clinical psychosis team,” he said in an interview.
The music therapist is no longer there, but, he said, “as far as I’m concerned, every clinical psychosis team should have a music therapist as an essential team member.”
Dr. Agrawal, Dr. Riba, and Dr. Malone had no reported disclosures.
A version of this article was first published on Medscape.com.
SAN FRANCISCO –
Although the study had conflicting results regarding the effects of music therapy on positive symptoms of schizophrenia, such as hallucinations, delusions, and disordered thoughts, it consistently shows that music therapy improves negative symptoms, poster presenter Amy Agrawal, MD, VA Boston Healthcare System and instructor of psychiatry at Harvard Medical School, Boston, said in an interview.
Current antipsychotic drugs aren’t very effective in addressing negative symptoms of schizophrenia, and many patients are noncompliant with these drug regimens because of side effects.
“We need to target the negative symptoms of schizophrenia better,” said Dr. Agrawal. “The antipsychotic medications we have are not enough, so why don’t we start incorporating music therapy groups into the inpatient psychiatry setting as a standard of care?”
The findings were presented at the annual meeting of the American Psychiatric Association.
Dr. Agrawal has long been interested in music. As a child, she was a member of a state choir, but she hadn’t sung for years. After receiving several medals for her clarinet playing during her youth, she stopped playing while in medical school.
Instant boost
During the pandemic, though, she turned back to music and started singing regularly. “I noticed an instant boost in my mood and wondered why I stopped making music for so long, as it made me feel so much happier and calmer.”
She also noticed how music affected her sister, who has paranoid schizophrenia. She described an incident in which her sibling became so loud and paranoid at a restaurant that Dr. Agrawal thought they would be asked to leave.
Then her sister started singing a song she’d sung during a beauty contest years before. “With the music, she calmed right down; she was smiling; she was happy,” said Dr. Agrawal.
That incident made Dr. Agrawal feel, “I had my sister back.” She decided to bring music therapy to her inpatient psychiatry unit and soon noted the benefits for individual patients.
For this new study, Dr. Agrawal and her mentor carried out a literature search. “I was surprised at how many articles popped up, because the field of psychiatry can be very heavily medication based, but people are now getting very interested in this topic,” said Dr. Agrawal.
The review included seven articles, most of which were published within the past 3 years. Some articles specified that the therapy was conducted on an inpatient psychiatric unit, but others didn’t indicate the setting. Studies also didn’t specify whether the therapy was delivered by a trained music therapist.
There was an overall lack of clear measures, graphs, or statistics quantifying the benefits of music therapy on schizophrenia, noted Dr. Agrawal. “But from general statements in the articles, music therapy helped treat sleep disturbances and improved negative symptoms.”
Gets patients socializing
The music, she said, led patients to start socializing, talking about their emotions, and opening up to their clinicians about their mental health symptoms. “Some patients just did not engage at all, and then when the music came on, they would actively participate with the clinician.”
Dancing to music also tended to motivate patients to participate in their treatment, she added. Different forms of movement, such as rhythmic movements and creative exercises, can be added during music therapy.
In addition to improving negative schizophrenia symptoms, music therapy helps with sleep disturbances, depression, and regulating emotional behavior, the research shows. “When patients were agitated or upset, certain music would help them regulate their own affect,” said Dr. Agrawal.
However, it’s not clear from these studies what type of music – classical, rock, country, etc. – was most effective for people.
One article discussed the positive impact of music on patients with schizophrenia while at work. “They seem to have improved work performance,” Dr. Agrawal said.
The length of exposure to music therapy did not seem to make a difference in terms of whether the therapy had a positive effect, she added.
Key research wave
A “key next wave” of research should be to determine whether music therapy decreases the hospital readmission rate, said Dr. Agrawal.
There are several barriers to implementing music therapy programs in hospitals, including cost, the availability of trained therapists, and time constraints, she said.
“Regardless of the barriers, hospital administrators and psychiatrists need to know about this research so they will invest more efforts in recruiting music therapists and incorporating music group therapy into standard clinical practice for psychiatric patients so there are better clinical outcomes.”
Commenting on the research, Michelle B. Riba, MD, professor, department of psychiatry, University of Michigan, Ann Arbor, said the study adds to the literature “and helps us think about adjunctive treatments in a very difficult population.”
She added, “It’s good to see physicians get interested in this topic.”
Difficult topic to study
Although she found the review “very limited,” she recognizes the difficulty of studying music therapy on in-patient psychiatry units.
“Patients are there for short stays, most are getting other treatments, and it’s hard to segment people into negative vs. positive. Also, the ages and genders are different, and their previous treatments are different.”
While it’s sometimes difficult to conduct major research on a topic, “that doesn’t mean we can’t help people,” said Dr. Riba.
She noted that music therapy is beneficial not only for patients with schizophrenia but also is “soothing and relaxing” for those with other conditions. She runs a psychiatric oncology program at her institution’s cancer center, which offers music therapy along with art therapy.
Kevin M. Malone, MD, of University College Dublin, also has firsthand experience with music therapy. “We had a terrific music therapist as part of our clinical psychosis team,” he said in an interview.
The music therapist is no longer there, but, he said, “as far as I’m concerned, every clinical psychosis team should have a music therapist as an essential team member.”
Dr. Agrawal, Dr. Riba, and Dr. Malone had no reported disclosures.
A version of this article was first published on Medscape.com.
SAN FRANCISCO –
Although the study had conflicting results regarding the effects of music therapy on positive symptoms of schizophrenia, such as hallucinations, delusions, and disordered thoughts, it consistently shows that music therapy improves negative symptoms, poster presenter Amy Agrawal, MD, VA Boston Healthcare System and instructor of psychiatry at Harvard Medical School, Boston, said in an interview.
Current antipsychotic drugs aren’t very effective in addressing negative symptoms of schizophrenia, and many patients are noncompliant with these drug regimens because of side effects.
“We need to target the negative symptoms of schizophrenia better,” said Dr. Agrawal. “The antipsychotic medications we have are not enough, so why don’t we start incorporating music therapy groups into the inpatient psychiatry setting as a standard of care?”
The findings were presented at the annual meeting of the American Psychiatric Association.
Dr. Agrawal has long been interested in music. As a child, she was a member of a state choir, but she hadn’t sung for years. After receiving several medals for her clarinet playing during her youth, she stopped playing while in medical school.
Instant boost
During the pandemic, though, she turned back to music and started singing regularly. “I noticed an instant boost in my mood and wondered why I stopped making music for so long, as it made me feel so much happier and calmer.”
She also noticed how music affected her sister, who has paranoid schizophrenia. She described an incident in which her sibling became so loud and paranoid at a restaurant that Dr. Agrawal thought they would be asked to leave.
Then her sister started singing a song she’d sung during a beauty contest years before. “With the music, she calmed right down; she was smiling; she was happy,” said Dr. Agrawal.
That incident made Dr. Agrawal feel, “I had my sister back.” She decided to bring music therapy to her inpatient psychiatry unit and soon noted the benefits for individual patients.
For this new study, Dr. Agrawal and her mentor carried out a literature search. “I was surprised at how many articles popped up, because the field of psychiatry can be very heavily medication based, but people are now getting very interested in this topic,” said Dr. Agrawal.
The review included seven articles, most of which were published within the past 3 years. Some articles specified that the therapy was conducted on an inpatient psychiatric unit, but others didn’t indicate the setting. Studies also didn’t specify whether the therapy was delivered by a trained music therapist.
There was an overall lack of clear measures, graphs, or statistics quantifying the benefits of music therapy on schizophrenia, noted Dr. Agrawal. “But from general statements in the articles, music therapy helped treat sleep disturbances and improved negative symptoms.”
Gets patients socializing
The music, she said, led patients to start socializing, talking about their emotions, and opening up to their clinicians about their mental health symptoms. “Some patients just did not engage at all, and then when the music came on, they would actively participate with the clinician.”
Dancing to music also tended to motivate patients to participate in their treatment, she added. Different forms of movement, such as rhythmic movements and creative exercises, can be added during music therapy.
In addition to improving negative schizophrenia symptoms, music therapy helps with sleep disturbances, depression, and regulating emotional behavior, the research shows. “When patients were agitated or upset, certain music would help them regulate their own affect,” said Dr. Agrawal.
However, it’s not clear from these studies what type of music – classical, rock, country, etc. – was most effective for people.
One article discussed the positive impact of music on patients with schizophrenia while at work. “They seem to have improved work performance,” Dr. Agrawal said.
The length of exposure to music therapy did not seem to make a difference in terms of whether the therapy had a positive effect, she added.
Key research wave
A “key next wave” of research should be to determine whether music therapy decreases the hospital readmission rate, said Dr. Agrawal.
There are several barriers to implementing music therapy programs in hospitals, including cost, the availability of trained therapists, and time constraints, she said.
“Regardless of the barriers, hospital administrators and psychiatrists need to know about this research so they will invest more efforts in recruiting music therapists and incorporating music group therapy into standard clinical practice for psychiatric patients so there are better clinical outcomes.”
Commenting on the research, Michelle B. Riba, MD, professor, department of psychiatry, University of Michigan, Ann Arbor, said the study adds to the literature “and helps us think about adjunctive treatments in a very difficult population.”
She added, “It’s good to see physicians get interested in this topic.”
Difficult topic to study
Although she found the review “very limited,” she recognizes the difficulty of studying music therapy on in-patient psychiatry units.
“Patients are there for short stays, most are getting other treatments, and it’s hard to segment people into negative vs. positive. Also, the ages and genders are different, and their previous treatments are different.”
While it’s sometimes difficult to conduct major research on a topic, “that doesn’t mean we can’t help people,” said Dr. Riba.
She noted that music therapy is beneficial not only for patients with schizophrenia but also is “soothing and relaxing” for those with other conditions. She runs a psychiatric oncology program at her institution’s cancer center, which offers music therapy along with art therapy.
Kevin M. Malone, MD, of University College Dublin, also has firsthand experience with music therapy. “We had a terrific music therapist as part of our clinical psychosis team,” he said in an interview.
The music therapist is no longer there, but, he said, “as far as I’m concerned, every clinical psychosis team should have a music therapist as an essential team member.”
Dr. Agrawal, Dr. Riba, and Dr. Malone had no reported disclosures.
A version of this article was first published on Medscape.com.
AT APA 2023
Systemic Lupus Erythematosus
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
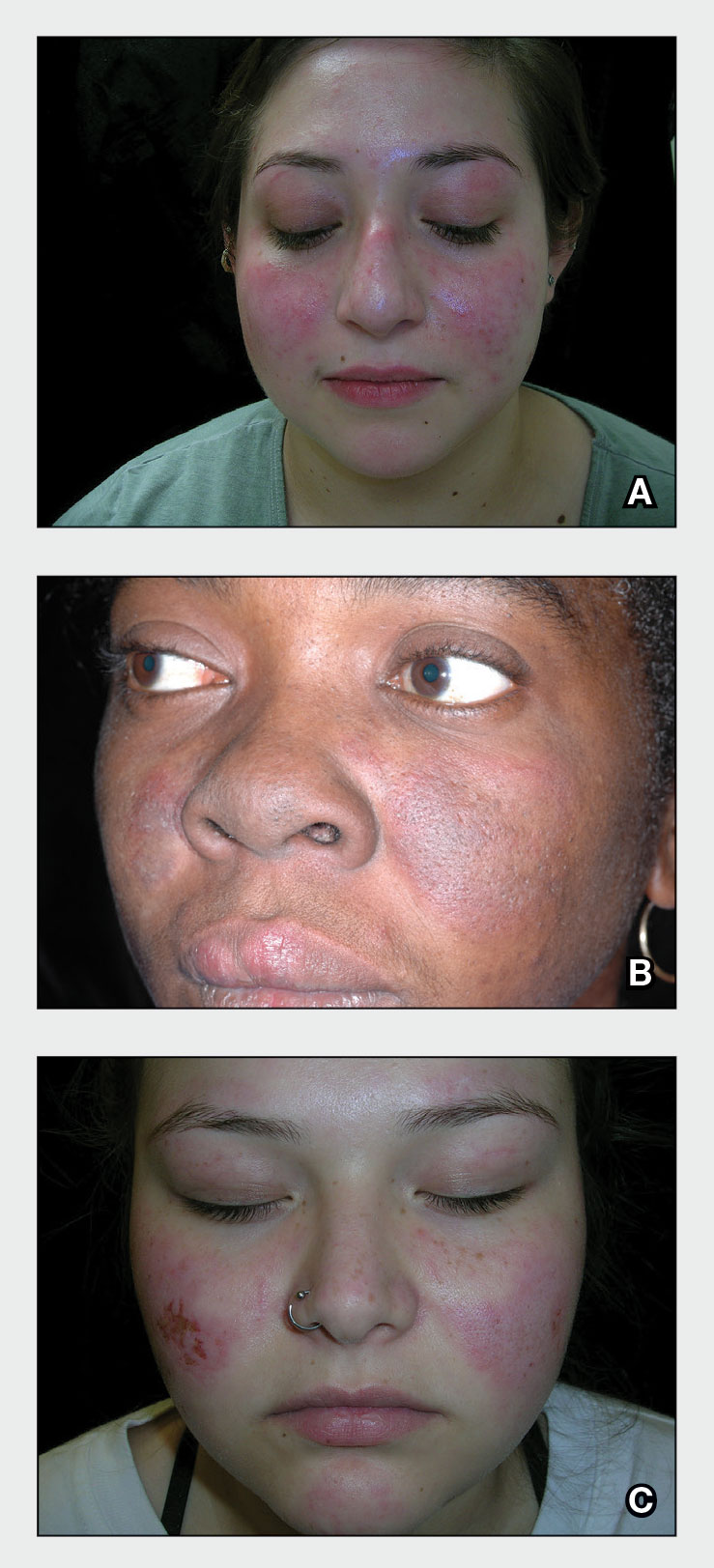
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
Cross-sectional Analysis of Matched Dermatology Residency Applicants Without US Home Programs
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.
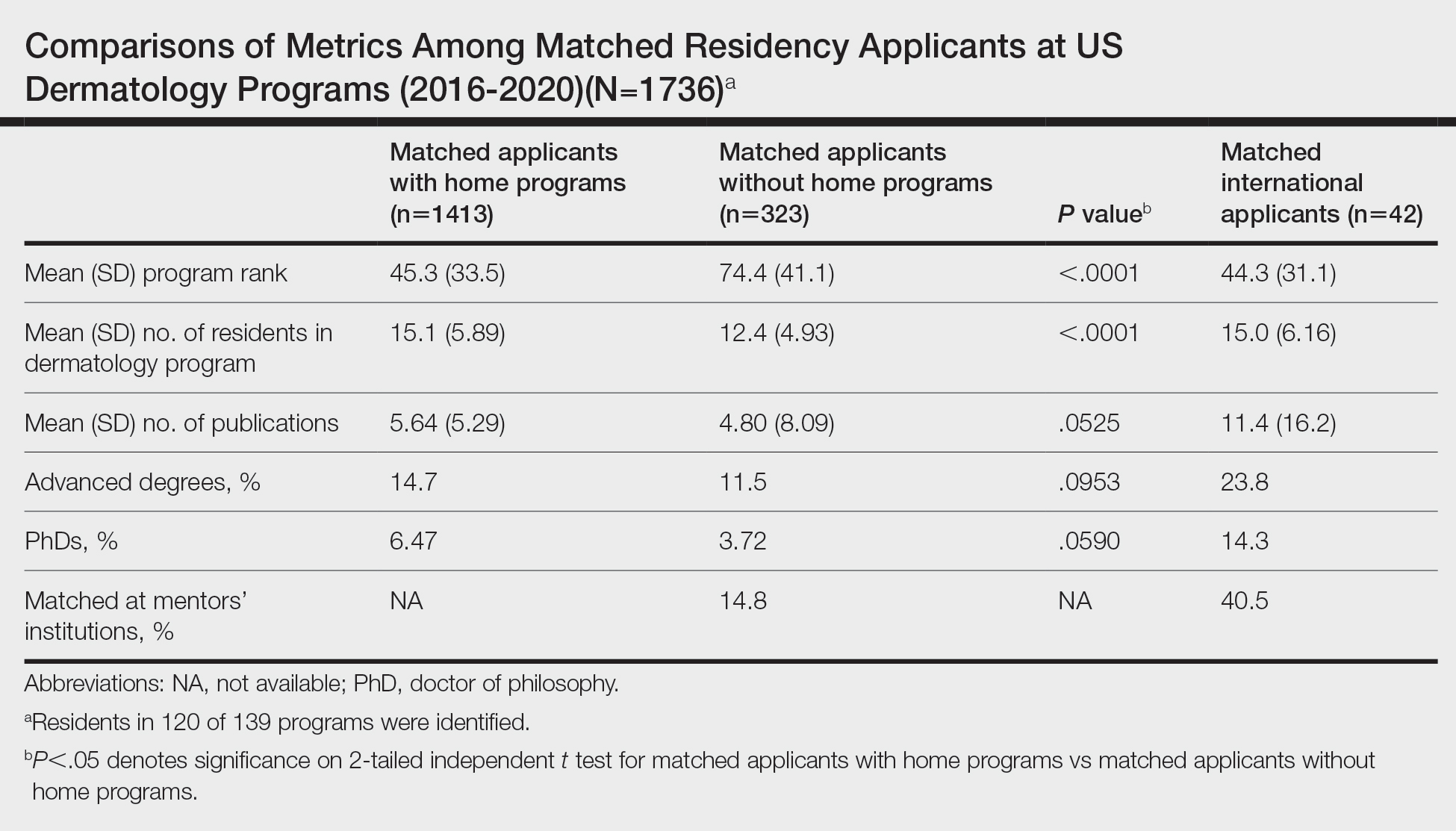
Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
Practice Points
- Our study suggests that matched dermatology applicants with and without home programs (HPs) had similar achievements, on average, for number of publications and holding advanced degrees.
- Because of the potential benefits of having program connections for matching in dermatology, applicants without HPs should seek dermatology research mentors.
What’s Eating You? Triatoma and Arilus cristatus Bugs
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.
Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.

Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
Classification
Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are part of the family Reduviidae (order Hemiptera, a name that describes the sucking proboscis on the front of the insect’s head).1,2 Both arthropods are found in multiple countries and are especially common in warmer areas, including in the United States, where they can be seen from Texas to California.3,4 Because blood-feeding triatomines need a blood meal to survive while laying eggs and then throughout their 5 developmental nymph stages to undergo molting, they feed on mammals, such as opossums, raccoons, pack rats, and armadillos, whereas wheel bugs mainly prey on soft-bodied insects.1,4-6
Triatoma bugs seek cutaneous blood vessels using thermosensors in their antennae to locate blood flow under the skin for feeding. After inserting the proboscis, they release nitric oxide and an anticoagulant that allows for continuous blood flow while feeding.7 It has been reported that triatomine bugs are not able to bite through clothing, instead seeking exposed skin, particularly near mucous membranes, such as the hands, arms, feet, head, and trunk. The name kissing bug for triatomines was coined because bites near the mouth are common.6 The bite typically is painless and occurs mainly at night when the insect is most active. After obtaining a blood meal, triatomine bugs seek shelter and hide in mud and daub structures, cracks, crevices, and furniture.1,8
Unlike Triatoma species, A cristatus does not require a blood meal for development and survival, leading it to prey on soft-bodied insects. Piercing prey with the proboscis, wheel bugs inject a toxin to digest the contents and suck the digested contents through this apparatus.4 Because the wheel bug does not require a blood meal, it typically bites a human only for defense if it feels threatened. Unlike the painless bite of a triatomine bug, the bite of A cristatus is extremely painful; it has been described as the worst arthropod bite—worse than a hornet’s sting. The pain from the bite is caused by the toxin being injected into the skin; possible retention of the proboscis makes the pain worse.4,9 In addition, when A cristatus is disturbed, it exudes pungent material from a pair of bright orange subrectal glands while stridulating to repulse predators.9
Although Triatoma species and A cristatus have separate roles in nature and vastly different impacts on health, they often are mistaken for the same arthropod when seen in nature. Features that members of Reduviidae share include large bodies (relative to their overall length); long thin legs; a narrow head; wings; and a long sucking proboscis on the front of the head.10

Characteristics that differentiate Triatoma and A cristatus species include size, color, and distinctive markings. Most triatomine bugs are 12- to 36-mm long; are dark brown or black; and have what are called tiger-stripe orange markings on the peripheral two-thirds of the body (Figure 1).11 In contrast, wheel bugs commonly are bigger—measuring longer than 1.25 inches—and gray, with a cogwheel-like structure on the thorax (Figure 2).10

Dermatologic Presentation and Clinical Symptoms
The area of involved skin on patients presenting with Triatoma or A cristatus bites may resemble other insect bites. Many bites from Triatoma bugs and A cristatus initially present as an erythematous, raised, pruritic papule with a central punctum that is visible because of the involvement of the proboscis. However, other presentations of bites from both arthropods have been reported4,6,7: grouped vesicles on an erythematous base; indurated, giant, urticarial-type wheels measuring 10 to 15 mm in diameter; and hemorrhagic bullous nodules (Figure 3). Associated lymphangitis or lymphadenitis is typical of the latter 2 variations.6 These variations in presentation can be mistaken for other causes of similarly presenting lesions, such as shingles or spider bites, leading to delayed or missed diagnosis.

Patients may present with a single bite or multiple bites due to the feeding pattern of Triatoma bugs; if the host moves or disrupts its feeding, the arthropod takes multiple bites to finish feeding.8 In comparison, 4 common variations of wheel bug bites have been reported: (1) a painful bite without complications; (2) a cutaneous horn and papilloma at the site of toxin injection; (3) a necrotic ulcer around the central punctum caused by injected toxin; and (4) an abscess under the central punctum due to secondary infection.4
Anaphylaxis—Although the bites of Triatoma and A cristatus present differently, both can cause anaphylaxis. Triatoma is implicated more often than A cristatus as the cause of anaphylaxis.12 In fact, Triatoma bites are among the more common causes of anaphylaxis from bug bites, with multiple cases of these reactions reported in the literature.12,13
Symptoms of Triatoma anaphylaxis include acute-onset urticarial rash, flushing, dyspnea, wheezing, nausea, vomiting, and localized edema.2 The cause of anaphylaxis is proteins in Triatoma saliva, including 20-kDa procalin, which incites the systemic reaction. Other potential causes of anaphylaxis include serine protease, which has similarities to salivary protein and desmoglein in humans.11
The degree of reaction to a bite depends on the patient's sensitization to antigenic proteins in each insect’s saliva.4,6 Patients who have a bite from a triatomine bug are at risk for subsequent bites, as household infestation is likely due to the pliability of the insect, allowing it to hide in small spaces unnoticed.8 In the case of a bite from Triatoma or A cristatus, sensitization may lead to severe and worsening reactions with subsequent bites, which ultimately can result in life-threatening anaphylaxis.1,6
Treatment and Prevention
Treatment of Triatoma and A cristatus bites depends on the severity of the patient’s reaction to the bite. A local reaction to a bite from either insect can be treated supportively with local corticosteroids and antihistamines.3 If the patient is sensitized to proteins associated with a bite, standard anaphylaxis treatment such as epinephrine and intravenous antihistamines may be indicated.14 Secondary infection can be treated with antibiotics; a formed abscess might need to be drained or debrided.15
There’s No Place Like Home—Because Triatoma bugs have a pliable exoskeleton and can squeeze into small spaces, they commonly infest dwellings where they find multiple attractants: light, heat, carbon dioxide, and lactic acid.8 The more household occupants (including pets), the higher the levels of carbon dioxide and lactic acid, thus the greater the attraction. Infestation of a home can lead to the spread of diseases harbored by Triatoma, including Chagas disease, which is caused by the parasite Trypanosoma cruzi.5
Preventive measures can be taken to reduce the risk and extent of home infestation by Triatoma bugs, including insecticides, a solid foundation, window screens, air conditioning, sealing of cracks and crevices, outdoor light management, and removal of clutter throughout the house.8 Because Triatoma bugs cannot bite through clothing, protective clothing and bug repellent on exposed skin can be employed. Another degree of protection is offered by pest management, especially control of rodents by removing food, water, and nests in areas where triatomine bugs feed off of that population.8,14
Unlike triatomine bugs, wheel bugs tend not to invade houses; therefore, these preventive measures are unnecessary. If a wheel bug is identified, do not engage the arthropod due to the defensive nature of its attack.4,9 Such deliberate avoidance should ensure protection from the wheel bug’s painful bite.
Medical Complications
Although triatomine bugs and wheel bugs are in the same taxonomic family, subsequent infection is unique to Triatoma bugs because they need a blood meal to survive. Because Triatoma bugs feed on mammals, they present an increased opportunity for transmitting the causative agents of infection from hosts on which they have fed.12 The principal parasite transmitted by triatomines is T cruzi, which causes Chagas disease and lives in the gastrointestinal (GI) tract of the insect.5 When a triatomine bug seeks out a mucosal surface to bite, including the mouth, it defecates and urinates during or shortly after feeding, leading to contamination of the initial wound or mucosal surfaces. In addition, Triatoma bugs are vectors for transmission of Serratia marcescans, Bartonella henselae, and Mycobacterium leprae.16
Chagas Disease—This infection has 3 stages: acute, intermediate, and chronic.5 The acute stage can present with symptoms of conjunctivitis, fever, lymphadenopathy, hepatosplenomegaly, and anemia. The intermediate stage typically is asymptomatic. The chronic stage usually involves the heart and GI tract and causes cardiac aneurysms, cardiomegaly, megacolon, and megaesophagus. Initial symptoms can be a localized nodule (chagoma) at the inoculation site, fever, fatigue, lymphadenopathy, and hepatosplenomegaly.2 Unilateral palpebral edema with associated lymphadenopathy (Romaña sign) also can be seen—not to be confused with bilateral swelling in an acute reaction to an insect bite. Romaña sign is pathognomonic of T cruzi infection; bilateral palpebral swelling is typical of an allergic reaction.12
Identification of a triatomine bite is the first step in diagnosing Chagas disease, which can be life-threatening. Among chronic carriers of Chagas disease, 30% develop GI and cardiac symptoms, of which 20% to 30% develop cardiomyopathy, with serious symptoms that present 10 to 20 years after the asymptomatic intermediate phase.2
Chagas disease is endemic to Central and South America but is also seen in North America; 28,000 new cases are reported annually in South America and North America combined. Human migration from endemic areas and from rural to urban areas has promoted the spread of Chagas disease.2 However, patients in the United States have a relatively low risk for Chagas disease, largely because of the quality of housing construction and use of insecticides.
Treatment options for Chagas disease include nifurtimox and benznidazole. Without treatment, the host immune response typically controls acute replication of the parasite but will lead to a chronic state, ultimately involving the heart and GI tract.5
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7:6.
- Zemore ZM, Wills BK. Kissing bug bite. StatPearls [Internet]. StatPearlsPublishing; 2023.
- Edwards L, Lynch PJ. Anaphylactic reaction to kissing bug bites. Ariz Med. 1984;41:159-161.
- Smith FD, Miller NG, Carnazzo SJ, et al. Insect bite by Arilus cristatus, a North American reduviid. AMA Arch Derm. 1958;77:324-330. doi:10.1001/archderm.1958.01560030070011
- Nguyen T, Waseem M. Chagas disease. StatPearls [Internet]. StatPearls Publishing; 2022.
- Shields TL, Walsh EN. Kissing bug bite. AMA Arch Derm. 1956;74:14-21. doi:10.1001/archderm.1956.01550070016004
- Beatty NL, Klotz SA. The midnight bite! a kissing bug nightmare. Am J Med. 2018;131:E43-E44. doi:10.1016/j.amjmed.2017.10.013
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. doi:10.3390/insects12070654
- Aldrich JR, Chauhan KR, Zhang A, et al. Exocrine secretions of wheel bugs (Heteroptera: Reduviidae: Arilus spp.): clarification and chemistry. Z Naturforsch C J Biosci. 2013;68:522-526.
- Boggs J. They’re wheel bugs, NOT kissing bugs. Buckeye Yard and Garden onLine [Internet]. September 17, 2020. Accessed May 25, 2023. https://bygl.osu.edu/node/1688
- Weber RW. Allergen of the month—assassin bug. Ann Allergy Asthma Immunol. 2015;115:A9.
- Klotz JH, Dorn PL, Logan JL, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis 2010;50:1629-1634. doi:10.1086/652769
- Anderson C, Belnap C. The kiss of death: a rare case of anaphylaxis to the bite of the “red margined kissing bug”. Hawaii J Med Public Health. 2015;74(9 suppl 2):33-35.
- Moffitt JE, Venarske D, Goddard J, et al. Allergic reactions to Triatoma bites. Ann Allergy Asthma Immunol. 2003;91:122-128. doi:10.1016/s1081-1206(10)62165-5
- Burnett JW, Calton GJ, Morgan RJ. Triatoma: the “kissing bug”. Cutis. 1987;39:399.
- Vieira CB, Praça YR, Bentes K, et al. Triatomines: Trypanosomatids, bacteria, and viruses potential vectors? Front Cell Infect Microbiol. 2018;8:405. doi:10.3389/fcimb.2018.00405
Practice Points
- Triatomine bugs (Triatoma) and the wheel bug (Arilus cristatus) are found throughout North America with a concentration in southern regions.
- Bites of triatomine bugs can cause anaphylaxis; prevention of bites to diminish household infestation is important because sensitization can result in increased severity of anaphylaxis upon subsequent exposure.
- Chagas disease—caused by transmission of the parasite Trypanosoma cruzi—can be a complication of a Triatoma bite in endemic areas; treatments include nifurtimox and benznidazole.
- Left undiagnosed and untreated, Chagas disease can have long-lasting implications for cardiac and gastrointestinal pathology.
Coding the “Spot Check”: Part 2
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Extensive Erosions and Ulcerations on the Trunk and Extremities in a Neonate
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5
Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).
Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12
When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
A neonate was born with extensive erosions and ulcerations on the trunk and extremities. The eroded areas had a beefy red appearance. A biopsy taken from a small blister was stained for type VII collagen by indirect immunofluorescence.
Guidelines on Away Rotations in Dermatology Programs
Medical students often perform away rotations (also called visiting electives) to gain exposure to educational experiences in a particular specialty, learn about a program, and show interest in a certain program. Away rotations also allow applicants to meet and form relationships with mentors and faculty outside of their home institution. For residency programs, away rotations provide an opportunity for a holistic review of applicants by allowing program directors to get to know potential residency applicants and assess their performance in the clinical environment and among the program’s team. In a National Resident Matching Program survey, program directors (n=17) reported that prior knowledge of an applicant is an important factor in selecting applicants to interview (82.4%) and rank (58.8%).1
In this article, we discuss the importance of away rotations in dermatology and provide an overview of the Organization of Program Director Associations (OPDA) and Association of Professors of Dermatology (APD) guidelines for away rotations.
Importance of the Away Rotation in the Match
According to the Association of American Medical Colleges, 86.7% of dermatology applicants (N=345) completed one or more away rotations (mean, 2.7) in 2020.2 Winterton et al3 reported that 47% of dermatology applicants (N=45) matched at a program where they completed an away rotation. Prior to the COVID-19 pandemic, the number of applicants matching to their home program was reported as 26.7% (N=641), which jumped to 40.3% (N=231) in the 2020-2021 cycle.4 Given that the majority of dermatology applicants reportedly match either at their home program or at programs where they completed an away rotation, the benefits of away rotations are high, particularly in a competitive specialty such as dermatology and particularly for applicants without a dermatology program at their home institution. However, it must be acknowledged that correlation does not necessarily mean causation, as away rotations have not necessarily been shown to increase applicants’ chances of matching for the most competitive specialties.5
OPDA Guidelines for Away Rotations
In 2021, the Coalition of Physician Accountability’s Undergraduate Medical Education-Graduate Medical Education Review Committee recommended creating a workgroup to explore the function and value of away rotations for medical students, programs, and institutions, with a particular focus on issues of equity (eg, accessibility, assessment, opportunity) for underrepresented in medicine students and those with financial disadvantages.6 The OPDA workgroup evaluated the advantages and disadvantages of away rotations across specialties. The disadvantages included that away rotations may decrease resources to students at their own institution, particularly if faculty time and energy are funneled/dedicated to away rotators instead of internal rotators, and may impart bias into the recruitment process. Additionally, there is a consideration of equity given the considerable cost and time commitment of travel and housing for students at another institution. In 2022, the estimated cost of an away rotation in dermatology ranged from $1390 to $5500 per rotation.7 Visiting scholarships may be available at some institutions but typically are reserved for underrepresented in medicine students.8 Virtual rotations offered at some programs offset the cost-prohibitiveness of an in-person away rotation; however, they are not universally offered and may be limited in allowing for meaningful interactions between students and program faculty and residents.
The OPDA away rotation workgroup recommended that (1) each specialty publish guidelines regarding the necessity and number of recommended away rotations; (2) specialties publish explicit language regarding the use of program preference signals to programs where students rotated; (3) programs be transparent about the purpose and value of an away rotation, including explicitly stating whether a formal interview is guaranteed; and (4) the Association of American Medical Colleges create a repository of these specialty-specific recommendations.9
APD Guidelines for Away Rotations
In response to the OPDA recommendations, the APD Residency Program Directors Section developed dermatology-specific guidelines for away rotations and established guidelines in other specialties.10 The APD recommends completing up to 2 away rotations, or 3 for those without a home program, if desired. This number was chosen in acknowledgment of the importance of external program experiences, along with the recognition of the financial and time restrictions associated with away rotations as well as the limited number of spots for rotating students. Away rotations are not mandatory. The APD guidelines explain the purpose and value of an away rotation while also noting that these rotations do not necessarily guarantee a formal interview and recommending that programs be transparent about their policies on interview invitations, which may vary.10
Final Thoughts
Publishing specialty-specific guidelines on away rotations is one step toward streamlining the process as well as increasing transparency on the importance of these external program experiences in the application process and residency match. Ideally, away rotations provide a valuable educational experience in which students and program directors get to know each other in a mutually beneficial manner; however, away rotations are not required for securing an interview or matching at a program, and there also are recognized disadvantages to away rotations, particularly with regard to equity, that we must continue to weigh as a specialty. The APD will continue its collaborative work to evaluate our application processes to support a sustainable and equitable system.
- National Resident Matching Program. Results of the 2021 NRMP program director survey. Published August 2021. Accessed May 17, 2023. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 17, 2023. https://students-residents.aamc.org/media/9496/download
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186/s12909-016-0805-z
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Griffith M, DeMasi SC, McGrath AJ, et al. Time to reevaluate the away rotation: improving return on investment for students and schools. Acad Med. 2019;94:496-500. doi:10.1097/ACM.0000000000002505
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medication Education-Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement in the UME-GME transition. Published August 26, 2021. Accessed May 18, 2023. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540.
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis [published online November 15, 2022]. J Am Acad Dermatol. 2023;88:941-943.
- Council of Medical Specialty Societies. The Organization of Program Director Associations (OPDA): away rotations workgroup. Published July 26, 2022. Accessed May 18, 2023. https://cmss.org/wp-content/uploads/2022/08/OPDA-Work-Group-on-Away-Rotations-7.26.2022-1.pdf
- Association of Professors of Dermatology. Recommendations regarding away electives. Published December 14, 2022. Accessed May 18, 2023. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
Medical students often perform away rotations (also called visiting electives) to gain exposure to educational experiences in a particular specialty, learn about a program, and show interest in a certain program. Away rotations also allow applicants to meet and form relationships with mentors and faculty outside of their home institution. For residency programs, away rotations provide an opportunity for a holistic review of applicants by allowing program directors to get to know potential residency applicants and assess their performance in the clinical environment and among the program’s team. In a National Resident Matching Program survey, program directors (n=17) reported that prior knowledge of an applicant is an important factor in selecting applicants to interview (82.4%) and rank (58.8%).1
In this article, we discuss the importance of away rotations in dermatology and provide an overview of the Organization of Program Director Associations (OPDA) and Association of Professors of Dermatology (APD) guidelines for away rotations.
Importance of the Away Rotation in the Match
According to the Association of American Medical Colleges, 86.7% of dermatology applicants (N=345) completed one or more away rotations (mean, 2.7) in 2020.2 Winterton et al3 reported that 47% of dermatology applicants (N=45) matched at a program where they completed an away rotation. Prior to the COVID-19 pandemic, the number of applicants matching to their home program was reported as 26.7% (N=641), which jumped to 40.3% (N=231) in the 2020-2021 cycle.4 Given that the majority of dermatology applicants reportedly match either at their home program or at programs where they completed an away rotation, the benefits of away rotations are high, particularly in a competitive specialty such as dermatology and particularly for applicants without a dermatology program at their home institution. However, it must be acknowledged that correlation does not necessarily mean causation, as away rotations have not necessarily been shown to increase applicants’ chances of matching for the most competitive specialties.5
OPDA Guidelines for Away Rotations
In 2021, the Coalition of Physician Accountability’s Undergraduate Medical Education-Graduate Medical Education Review Committee recommended creating a workgroup to explore the function and value of away rotations for medical students, programs, and institutions, with a particular focus on issues of equity (eg, accessibility, assessment, opportunity) for underrepresented in medicine students and those with financial disadvantages.6 The OPDA workgroup evaluated the advantages and disadvantages of away rotations across specialties. The disadvantages included that away rotations may decrease resources to students at their own institution, particularly if faculty time and energy are funneled/dedicated to away rotators instead of internal rotators, and may impart bias into the recruitment process. Additionally, there is a consideration of equity given the considerable cost and time commitment of travel and housing for students at another institution. In 2022, the estimated cost of an away rotation in dermatology ranged from $1390 to $5500 per rotation.7 Visiting scholarships may be available at some institutions but typically are reserved for underrepresented in medicine students.8 Virtual rotations offered at some programs offset the cost-prohibitiveness of an in-person away rotation; however, they are not universally offered and may be limited in allowing for meaningful interactions between students and program faculty and residents.
The OPDA away rotation workgroup recommended that (1) each specialty publish guidelines regarding the necessity and number of recommended away rotations; (2) specialties publish explicit language regarding the use of program preference signals to programs where students rotated; (3) programs be transparent about the purpose and value of an away rotation, including explicitly stating whether a formal interview is guaranteed; and (4) the Association of American Medical Colleges create a repository of these specialty-specific recommendations.9
APD Guidelines for Away Rotations
In response to the OPDA recommendations, the APD Residency Program Directors Section developed dermatology-specific guidelines for away rotations and established guidelines in other specialties.10 The APD recommends completing up to 2 away rotations, or 3 for those without a home program, if desired. This number was chosen in acknowledgment of the importance of external program experiences, along with the recognition of the financial and time restrictions associated with away rotations as well as the limited number of spots for rotating students. Away rotations are not mandatory. The APD guidelines explain the purpose and value of an away rotation while also noting that these rotations do not necessarily guarantee a formal interview and recommending that programs be transparent about their policies on interview invitations, which may vary.10
Final Thoughts
Publishing specialty-specific guidelines on away rotations is one step toward streamlining the process as well as increasing transparency on the importance of these external program experiences in the application process and residency match. Ideally, away rotations provide a valuable educational experience in which students and program directors get to know each other in a mutually beneficial manner; however, away rotations are not required for securing an interview or matching at a program, and there also are recognized disadvantages to away rotations, particularly with regard to equity, that we must continue to weigh as a specialty. The APD will continue its collaborative work to evaluate our application processes to support a sustainable and equitable system.
Medical students often perform away rotations (also called visiting electives) to gain exposure to educational experiences in a particular specialty, learn about a program, and show interest in a certain program. Away rotations also allow applicants to meet and form relationships with mentors and faculty outside of their home institution. For residency programs, away rotations provide an opportunity for a holistic review of applicants by allowing program directors to get to know potential residency applicants and assess their performance in the clinical environment and among the program’s team. In a National Resident Matching Program survey, program directors (n=17) reported that prior knowledge of an applicant is an important factor in selecting applicants to interview (82.4%) and rank (58.8%).1
In this article, we discuss the importance of away rotations in dermatology and provide an overview of the Organization of Program Director Associations (OPDA) and Association of Professors of Dermatology (APD) guidelines for away rotations.
Importance of the Away Rotation in the Match
According to the Association of American Medical Colleges, 86.7% of dermatology applicants (N=345) completed one or more away rotations (mean, 2.7) in 2020.2 Winterton et al3 reported that 47% of dermatology applicants (N=45) matched at a program where they completed an away rotation. Prior to the COVID-19 pandemic, the number of applicants matching to their home program was reported as 26.7% (N=641), which jumped to 40.3% (N=231) in the 2020-2021 cycle.4 Given that the majority of dermatology applicants reportedly match either at their home program or at programs where they completed an away rotation, the benefits of away rotations are high, particularly in a competitive specialty such as dermatology and particularly for applicants without a dermatology program at their home institution. However, it must be acknowledged that correlation does not necessarily mean causation, as away rotations have not necessarily been shown to increase applicants’ chances of matching for the most competitive specialties.5
OPDA Guidelines for Away Rotations
In 2021, the Coalition of Physician Accountability’s Undergraduate Medical Education-Graduate Medical Education Review Committee recommended creating a workgroup to explore the function and value of away rotations for medical students, programs, and institutions, with a particular focus on issues of equity (eg, accessibility, assessment, opportunity) for underrepresented in medicine students and those with financial disadvantages.6 The OPDA workgroup evaluated the advantages and disadvantages of away rotations across specialties. The disadvantages included that away rotations may decrease resources to students at their own institution, particularly if faculty time and energy are funneled/dedicated to away rotators instead of internal rotators, and may impart bias into the recruitment process. Additionally, there is a consideration of equity given the considerable cost and time commitment of travel and housing for students at another institution. In 2022, the estimated cost of an away rotation in dermatology ranged from $1390 to $5500 per rotation.7 Visiting scholarships may be available at some institutions but typically are reserved for underrepresented in medicine students.8 Virtual rotations offered at some programs offset the cost-prohibitiveness of an in-person away rotation; however, they are not universally offered and may be limited in allowing for meaningful interactions between students and program faculty and residents.
The OPDA away rotation workgroup recommended that (1) each specialty publish guidelines regarding the necessity and number of recommended away rotations; (2) specialties publish explicit language regarding the use of program preference signals to programs where students rotated; (3) programs be transparent about the purpose and value of an away rotation, including explicitly stating whether a formal interview is guaranteed; and (4) the Association of American Medical Colleges create a repository of these specialty-specific recommendations.9
APD Guidelines for Away Rotations
In response to the OPDA recommendations, the APD Residency Program Directors Section developed dermatology-specific guidelines for away rotations and established guidelines in other specialties.10 The APD recommends completing up to 2 away rotations, or 3 for those without a home program, if desired. This number was chosen in acknowledgment of the importance of external program experiences, along with the recognition of the financial and time restrictions associated with away rotations as well as the limited number of spots for rotating students. Away rotations are not mandatory. The APD guidelines explain the purpose and value of an away rotation while also noting that these rotations do not necessarily guarantee a formal interview and recommending that programs be transparent about their policies on interview invitations, which may vary.10
Final Thoughts
Publishing specialty-specific guidelines on away rotations is one step toward streamlining the process as well as increasing transparency on the importance of these external program experiences in the application process and residency match. Ideally, away rotations provide a valuable educational experience in which students and program directors get to know each other in a mutually beneficial manner; however, away rotations are not required for securing an interview or matching at a program, and there also are recognized disadvantages to away rotations, particularly with regard to equity, that we must continue to weigh as a specialty. The APD will continue its collaborative work to evaluate our application processes to support a sustainable and equitable system.
- National Resident Matching Program. Results of the 2021 NRMP program director survey. Published August 2021. Accessed May 17, 2023. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 17, 2023. https://students-residents.aamc.org/media/9496/download
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186/s12909-016-0805-z
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Griffith M, DeMasi SC, McGrath AJ, et al. Time to reevaluate the away rotation: improving return on investment for students and schools. Acad Med. 2019;94:496-500. doi:10.1097/ACM.0000000000002505
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medication Education-Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement in the UME-GME transition. Published August 26, 2021. Accessed May 18, 2023. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540.
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis [published online November 15, 2022]. J Am Acad Dermatol. 2023;88:941-943.
- Council of Medical Specialty Societies. The Organization of Program Director Associations (OPDA): away rotations workgroup. Published July 26, 2022. Accessed May 18, 2023. https://cmss.org/wp-content/uploads/2022/08/OPDA-Work-Group-on-Away-Rotations-7.26.2022-1.pdf
- Association of Professors of Dermatology. Recommendations regarding away electives. Published December 14, 2022. Accessed May 18, 2023. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- National Resident Matching Program. Results of the 2021 NRMP program director survey. Published August 2021. Accessed May 17, 2023. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 17, 2023. https://students-residents.aamc.org/media/9496/download
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186/s12909-016-0805-z
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Griffith M, DeMasi SC, McGrath AJ, et al. Time to reevaluate the away rotation: improving return on investment for students and schools. Acad Med. 2019;94:496-500. doi:10.1097/ACM.0000000000002505
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medication Education-Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement in the UME-GME transition. Published August 26, 2021. Accessed May 18, 2023. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540.
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis [published online November 15, 2022]. J Am Acad Dermatol. 2023;88:941-943.
- Council of Medical Specialty Societies. The Organization of Program Director Associations (OPDA): away rotations workgroup. Published July 26, 2022. Accessed May 18, 2023. https://cmss.org/wp-content/uploads/2022/08/OPDA-Work-Group-on-Away-Rotations-7.26.2022-1.pdf
- Association of Professors of Dermatology. Recommendations regarding away electives. Published December 14, 2022. Accessed May 18, 2023. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
Practice Points
- Away rotations are an important tool for both applicants and residency programs during the application process.
- The Association of Professors of Dermatology (APD) recommends completing up to 2 external program experiences, or 3 if the student has no home program, ideally to be completed early in the fourth year of medical school prior to interview invitations.
- Away rotations may have considerable cost and time restrictions on applicants, which the APD recognizes and weighs in its recommendations. There may be program-specific scholarships and opportunities available to help with the cost of away rotations.