User login
FDA Approves Brentuximab for Two Lymphomas
The Food and Drug Administration on Aug. 19 approved brentuximab, a CD30-directed antibody drug-conjugate, for the treatment of Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma, after other treatments have failed.
Specifically, brentuximab was approved for the treatment of patients with Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multiagent chemotherapy regimens in patients who are not ASCT candidates. It is the first new treatment approved for HL by the FDA since 1977, according to the FDA statement. The indications are based on response rates, and there are no available data that show "improvement in patient reported outcomes or survival" with treatment, according to the prescribing information.
The FDA also approved brentuximab for patients with systemic anaplastic large-cell lymphoma (ALCL), a rare form of lymphoma, after failure of at least one prior multiagent chemotherapy regimen. It is the first treatment approved for this rare lymphoma; approximately 2,000 new cases are diagnosed yearly in the United States.
Both types of lymphomas express the CD30 antigen. Brentuximab is a combination of an anti-CD30 antibody and a drug, monomethyl auristatin E (MMAE), a microtubule disrupting agent. The antibody directs the drug to C30-expressing tumor cells, where it is released.
Brentuximab is administered intravenously every 3 weeks; it will be marketed in the United States as Adcetris by Seattle Genetics. It will be available the week of Aug. 22, according to a company spokesperson.
The product was approved under the FDA’s accelerated approval program, which allows the agency to approve a drug for a serious disease based on data showing the drug is effective on a surrogate end point that is "reasonably likely" to predict clinical benefit. Accelerated approvals provide patients with access to promising treatments, but companies are required to provide confirmatory clinical data in order for the drug to be converted to a full approval and remain on the market.
"Early clinical data suggest that patients who received Adcetris for Hodgkin’s lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy," Dr. Richard Pazdur, director of the Office of Oncology Drug Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
In a study of 102 patients with HL treated with brentuximab, 73% of the patients had either a complete or partial response to treatment. In a similarly designed study of 58 patients with ALCL, 86% achieved a partial or complete response to treatment. Neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper-respiratory infection, diarrhea, fever, cough, vomiting, and thrombocytopenia were among the most common adverse effects associated with treatment, according to the FDA.
At a meeting in July, an FDA advisory panel recommended the accelerated approval of both indications.
Brentuximab is under review for the same indications in Europe; a decision on approval is expected in the first half of 2012, according to a spokesperson for Millennium: the Takeda Oncology Co., which has rights to commercialize brentuximab outside the United States and Canada.
The Food and Drug Administration on Aug. 19 approved brentuximab, a CD30-directed antibody drug-conjugate, for the treatment of Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma, after other treatments have failed.
Specifically, brentuximab was approved for the treatment of patients with Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multiagent chemotherapy regimens in patients who are not ASCT candidates. It is the first new treatment approved for HL by the FDA since 1977, according to the FDA statement. The indications are based on response rates, and there are no available data that show "improvement in patient reported outcomes or survival" with treatment, according to the prescribing information.
The FDA also approved brentuximab for patients with systemic anaplastic large-cell lymphoma (ALCL), a rare form of lymphoma, after failure of at least one prior multiagent chemotherapy regimen. It is the first treatment approved for this rare lymphoma; approximately 2,000 new cases are diagnosed yearly in the United States.
Both types of lymphomas express the CD30 antigen. Brentuximab is a combination of an anti-CD30 antibody and a drug, monomethyl auristatin E (MMAE), a microtubule disrupting agent. The antibody directs the drug to C30-expressing tumor cells, where it is released.
Brentuximab is administered intravenously every 3 weeks; it will be marketed in the United States as Adcetris by Seattle Genetics. It will be available the week of Aug. 22, according to a company spokesperson.
The product was approved under the FDA’s accelerated approval program, which allows the agency to approve a drug for a serious disease based on data showing the drug is effective on a surrogate end point that is "reasonably likely" to predict clinical benefit. Accelerated approvals provide patients with access to promising treatments, but companies are required to provide confirmatory clinical data in order for the drug to be converted to a full approval and remain on the market.
"Early clinical data suggest that patients who received Adcetris for Hodgkin’s lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy," Dr. Richard Pazdur, director of the Office of Oncology Drug Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
In a study of 102 patients with HL treated with brentuximab, 73% of the patients had either a complete or partial response to treatment. In a similarly designed study of 58 patients with ALCL, 86% achieved a partial or complete response to treatment. Neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper-respiratory infection, diarrhea, fever, cough, vomiting, and thrombocytopenia were among the most common adverse effects associated with treatment, according to the FDA.
At a meeting in July, an FDA advisory panel recommended the accelerated approval of both indications.
Brentuximab is under review for the same indications in Europe; a decision on approval is expected in the first half of 2012, according to a spokesperson for Millennium: the Takeda Oncology Co., which has rights to commercialize brentuximab outside the United States and Canada.
The Food and Drug Administration on Aug. 19 approved brentuximab, a CD30-directed antibody drug-conjugate, for the treatment of Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma, after other treatments have failed.
Specifically, brentuximab was approved for the treatment of patients with Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multiagent chemotherapy regimens in patients who are not ASCT candidates. It is the first new treatment approved for HL by the FDA since 1977, according to the FDA statement. The indications are based on response rates, and there are no available data that show "improvement in patient reported outcomes or survival" with treatment, according to the prescribing information.
The FDA also approved brentuximab for patients with systemic anaplastic large-cell lymphoma (ALCL), a rare form of lymphoma, after failure of at least one prior multiagent chemotherapy regimen. It is the first treatment approved for this rare lymphoma; approximately 2,000 new cases are diagnosed yearly in the United States.
Both types of lymphomas express the CD30 antigen. Brentuximab is a combination of an anti-CD30 antibody and a drug, monomethyl auristatin E (MMAE), a microtubule disrupting agent. The antibody directs the drug to C30-expressing tumor cells, where it is released.
Brentuximab is administered intravenously every 3 weeks; it will be marketed in the United States as Adcetris by Seattle Genetics. It will be available the week of Aug. 22, according to a company spokesperson.
The product was approved under the FDA’s accelerated approval program, which allows the agency to approve a drug for a serious disease based on data showing the drug is effective on a surrogate end point that is "reasonably likely" to predict clinical benefit. Accelerated approvals provide patients with access to promising treatments, but companies are required to provide confirmatory clinical data in order for the drug to be converted to a full approval and remain on the market.
"Early clinical data suggest that patients who received Adcetris for Hodgkin’s lymphoma and systemic anaplastic lymphoma experienced a significant response to the therapy," Dr. Richard Pazdur, director of the Office of Oncology Drug Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
In a study of 102 patients with HL treated with brentuximab, 73% of the patients had either a complete or partial response to treatment. In a similarly designed study of 58 patients with ALCL, 86% achieved a partial or complete response to treatment. Neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper-respiratory infection, diarrhea, fever, cough, vomiting, and thrombocytopenia were among the most common adverse effects associated with treatment, according to the FDA.
At a meeting in July, an FDA advisory panel recommended the accelerated approval of both indications.
Brentuximab is under review for the same indications in Europe; a decision on approval is expected in the first half of 2012, according to a spokesperson for Millennium: the Takeda Oncology Co., which has rights to commercialize brentuximab outside the United States and Canada.
FROM THE FOOD AND DRUG ADMINISTRATION
Investment Strategies for Early Career Hospitalists
There are no hard and fast rules for crafting an investment strategy, especially early in your career. As with so many domains, the devil’s in the details, according to two top financial advisors.
Disability Coverage Checkup
Protecting your income is job one when creating a sound investment strategy. “Your biggest asset is your earning power,” says Bob Wacker, CFP, president of R.E. Wacker Associates Inc. in San Luis Obispo, Calif. That’s why you need to examine your disability coverage as soon as you start working.
Joel Greenwald, MD, CFP, partner at Sterling Retirement Resources in St. Louis Park, Minn., who often presents financial workshops to groups of residents at the University of Minnesota Medical School, agrees. Many physicians assume that group disability coverage offered through the hospital or physicians group is adequate. But it might not be.
Group insurance policies differ from individual policies in key ways. For example, group carriers might supply disability benefits for two to five years, based on one definition of your occupation, and then, according to their definition of “reasonable” occupation, stop paying if you do not take work in a related capacity.
What you want, says Dr. Greenwald, is a policy—often an individual one—that ties the definition of disability to the performance of activities specific to your specialty. A financial advisor or an insurance agent who specializes in disability insurance can review your coverage and help tailor it to your needs.
For more on this issue, visit http://issuu.com/metrodoctors/docs/julyaugust2010.
Analyze and Prioritize Debt
One of the first questions high-income earners have to ask themselves is: Should I pay off my debt right away or start saving? “There is no right answer,” Dr. Greenwald says.
High-interest credit card debt is bad, so physicians should eliminate it as soon as possible.
School loans? The experts suggest paying off the higher-interest loans first. But if you can, you should resist the urge to pay down low-interest loans. The interest on your debt, Wacker explains, might be tax-deductible. Look for a financial planner who has a “robust” tax background to help you weigh the tax consequences of debt payoff versus savings.
Investment Literacy
When you choose a financial planner (see “Finding an Advisor”), expect to examine your disability and debt portfolios, as well as create an estate plan. Make sure you fully fund your retirement plan at work. A 401(k) allows you to contribute $16,500 per year, and most plans match your contributions at 25%, 50%, or 100%, which automatically boosts your balance sheet. It’s also a good idea to establish 529 plans for your children’s education.
Remember that a financial plan is not static; it will require revisiting on a regular basis. And that will entail learning about a whole new body of knowledge. Choose your guides wisely.
Gretchen Henkel is a freelance writer based in California.
There are no hard and fast rules for crafting an investment strategy, especially early in your career. As with so many domains, the devil’s in the details, according to two top financial advisors.
Disability Coverage Checkup
Protecting your income is job one when creating a sound investment strategy. “Your biggest asset is your earning power,” says Bob Wacker, CFP, president of R.E. Wacker Associates Inc. in San Luis Obispo, Calif. That’s why you need to examine your disability coverage as soon as you start working.
Joel Greenwald, MD, CFP, partner at Sterling Retirement Resources in St. Louis Park, Minn., who often presents financial workshops to groups of residents at the University of Minnesota Medical School, agrees. Many physicians assume that group disability coverage offered through the hospital or physicians group is adequate. But it might not be.
Group insurance policies differ from individual policies in key ways. For example, group carriers might supply disability benefits for two to five years, based on one definition of your occupation, and then, according to their definition of “reasonable” occupation, stop paying if you do not take work in a related capacity.
What you want, says Dr. Greenwald, is a policy—often an individual one—that ties the definition of disability to the performance of activities specific to your specialty. A financial advisor or an insurance agent who specializes in disability insurance can review your coverage and help tailor it to your needs.
For more on this issue, visit http://issuu.com/metrodoctors/docs/julyaugust2010.
Analyze and Prioritize Debt
One of the first questions high-income earners have to ask themselves is: Should I pay off my debt right away or start saving? “There is no right answer,” Dr. Greenwald says.
High-interest credit card debt is bad, so physicians should eliminate it as soon as possible.
School loans? The experts suggest paying off the higher-interest loans first. But if you can, you should resist the urge to pay down low-interest loans. The interest on your debt, Wacker explains, might be tax-deductible. Look for a financial planner who has a “robust” tax background to help you weigh the tax consequences of debt payoff versus savings.
Investment Literacy
When you choose a financial planner (see “Finding an Advisor”), expect to examine your disability and debt portfolios, as well as create an estate plan. Make sure you fully fund your retirement plan at work. A 401(k) allows you to contribute $16,500 per year, and most plans match your contributions at 25%, 50%, or 100%, which automatically boosts your balance sheet. It’s also a good idea to establish 529 plans for your children’s education.
Remember that a financial plan is not static; it will require revisiting on a regular basis. And that will entail learning about a whole new body of knowledge. Choose your guides wisely.
Gretchen Henkel is a freelance writer based in California.
There are no hard and fast rules for crafting an investment strategy, especially early in your career. As with so many domains, the devil’s in the details, according to two top financial advisors.
Disability Coverage Checkup
Protecting your income is job one when creating a sound investment strategy. “Your biggest asset is your earning power,” says Bob Wacker, CFP, president of R.E. Wacker Associates Inc. in San Luis Obispo, Calif. That’s why you need to examine your disability coverage as soon as you start working.
Joel Greenwald, MD, CFP, partner at Sterling Retirement Resources in St. Louis Park, Minn., who often presents financial workshops to groups of residents at the University of Minnesota Medical School, agrees. Many physicians assume that group disability coverage offered through the hospital or physicians group is adequate. But it might not be.
Group insurance policies differ from individual policies in key ways. For example, group carriers might supply disability benefits for two to five years, based on one definition of your occupation, and then, according to their definition of “reasonable” occupation, stop paying if you do not take work in a related capacity.
What you want, says Dr. Greenwald, is a policy—often an individual one—that ties the definition of disability to the performance of activities specific to your specialty. A financial advisor or an insurance agent who specializes in disability insurance can review your coverage and help tailor it to your needs.
For more on this issue, visit http://issuu.com/metrodoctors/docs/julyaugust2010.
Analyze and Prioritize Debt
One of the first questions high-income earners have to ask themselves is: Should I pay off my debt right away or start saving? “There is no right answer,” Dr. Greenwald says.
High-interest credit card debt is bad, so physicians should eliminate it as soon as possible.
School loans? The experts suggest paying off the higher-interest loans first. But if you can, you should resist the urge to pay down low-interest loans. The interest on your debt, Wacker explains, might be tax-deductible. Look for a financial planner who has a “robust” tax background to help you weigh the tax consequences of debt payoff versus savings.
Investment Literacy
When you choose a financial planner (see “Finding an Advisor”), expect to examine your disability and debt portfolios, as well as create an estate plan. Make sure you fully fund your retirement plan at work. A 401(k) allows you to contribute $16,500 per year, and most plans match your contributions at 25%, 50%, or 100%, which automatically boosts your balance sheet. It’s also a good idea to establish 529 plans for your children’s education.
Remember that a financial plan is not static; it will require revisiting on a regular basis. And that will entail learning about a whole new body of knowledge. Choose your guides wisely.
Gretchen Henkel is a freelance writer based in California.
Neuro-HM Gains Numbers, Momentum
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter
David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter
David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter
David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
Act Early With Pediatric Acne
As a pediatrician, you are on the front line of acne treatment for neonates, children, and adolescents. Acne is a very common condition that will affect 80% of your patients at some point in their lives. It can be easy to diagnose, but acne is often difficult to evaluate and manage. Presentations vary from mild to severe, and you’re likely to see a wide range of acne severity as you treat babies, children, and adolescents through office consultations and regular wellness checks.
Minimal intervention is reasonable for children with mild, comedonal acne. Most are unaware and unconcerned about their acne. It is important to stress they should avoid aggressive facial scrubbing and "popping zits." A discussion of acne physiology that dispels common myths – for example, that junk foods and poor hygiene cause acne in children and adolescents – also is useful.
Early intervention is essential to successful management. Prompt initiation of acne therapy can prevent sequelae that, if left untreated, can include significant scarring and emotional distress for your patients.
Refer your patient to a dermatologic surgeon early if the child’s acne is recalcitrant to treatment or shows early signs of scarring. Dermatologic surgeons understand the science behind healthy skin and can help your patients with the special needs of skin through every stage of life.
The differential diagnosis for acneiform eruptions varies by age. Neonatal acne (or neonatal cephalic pustulosis, as it is sometimes called) can affect about one in five babies. It is usually self-limited and requires no treatment, although topical ketoconazole can be prescribed if the parents are concerned or the presentation is extensive.
Infantile acne is less common. This occurs between the ages of 6 months and 1 year. Typical lesions include comedones or more inflammatory lesions. Benzoyl peroxide products and/or topical retinoids can be used to treat infantile acne if it is comedonal.
Acne that appears at age 1-7 years is very rare. Toddlers and children with this early childhood acne also should be evaluated further and/or referred to a specialist. A careful history and physical examination are warranted. Measure height and weight, and plot them on the growth chart. Also look for signs of virilization or precocious sexual development.
An abnormal blood pressure can point to congenital adrenal hyperplasia in the neonatal period. Rule out hyperandrogenism, particularly with severe or persistent infantile acne or sudden onset childhood acne. Refer patients to an endocrinologist if you are uncertain, or if any of the following screening tests are abnormal: bone age, serum DHEA (dehydroepiandrosterone), and free testosterone levels. (Total testosterone can be checked if the free testosterone test is unavailable.) Also consider serologic measures of follicle stimulating hormone, luteinizing hormone, prolactin, and 17 alpha-hydroxyprogesterone.
Performance of an exhaustive search for hyperandrogenism in your office is unnecessary in the majority of neonates, infants, children, and adolescents. It is important to know when these screening tests should be ordered and when to refer to a specialist for further evaluation and/or management.
Prepubertal or adolescent acne can occur earlier than parents might expect (at around 8 years of age), and can be the first sign of pubertal maturation. Distinguish comedonal from inflammatory acne to determine appropriate therapy.
Treatment with topical retinoids is the best for comedonal acne. Take the time to educate parents and the child on proper application of a topical retinoid. Instruct them to apply a pea-sized amount to dry skin every other night (or even every third night) for the first 2-4 weeks. This initial small dose can be titrated up gradually over time to minimize adverse effects. Improper use can lead to significant irritation and dryness, and contribute to the lack of treatment compliance.
As with any disease process, patient education is extremely important and can have a great impact on outcomes. Extensively counsel patients and parents on therapy options, and stress the importance of compliance with your recommended treatment regimen.
If the child has inflammatory acne lesions, a combination of benzoyl peroxide and topical antibiotic therapy (erythromycin or clindamycin) is more effective than either agent alone. With more severe acne, oral antibiotics may be warranted. Keep in mind that the tetracycline family of antibiotics can interfere with bone and teeth development, and is contraindicated in children younger than 8 years. Treatment with erythromycin or with Mutual Pharmaceutical’s Bactrim (a combination of sulfamethoxazole and trimethoprim) is appropriate for this age group. For older children with fully developed teeth, oral tetracycline, minocycline, and doxycycline are often the antibiotics of choice.
Infants, children, or adolescents with severe, recalcitrant, or scarring acne should be referred to a specialist for more aggressive intervention. For adolescents with nodulocystic acne (severe acne characterized by inflammation, nodular breakouts, and cysts), early intervention with systemic therapies, including isotretinoin, is important to prevent scarring. Also refer patients to dermatologic surgeons for further evaluation and management if their acne causes them psychological distress, whether or not their clinical presentation is severe.
Dr. Sikora is a private practice dermatologist in Chestnut Hill, Mass., and a member of many professional organizations, including the American Society for Dermatologic Surgery (www.ASDS.net) and the American Academy of Dermatology. Dr. Sikora said she had no relevant financial disclosures.
As a pediatrician, you are on the front line of acne treatment for neonates, children, and adolescents. Acne is a very common condition that will affect 80% of your patients at some point in their lives. It can be easy to diagnose, but acne is often difficult to evaluate and manage. Presentations vary from mild to severe, and you’re likely to see a wide range of acne severity as you treat babies, children, and adolescents through office consultations and regular wellness checks.
Minimal intervention is reasonable for children with mild, comedonal acne. Most are unaware and unconcerned about their acne. It is important to stress they should avoid aggressive facial scrubbing and "popping zits." A discussion of acne physiology that dispels common myths – for example, that junk foods and poor hygiene cause acne in children and adolescents – also is useful.
Early intervention is essential to successful management. Prompt initiation of acne therapy can prevent sequelae that, if left untreated, can include significant scarring and emotional distress for your patients.
Refer your patient to a dermatologic surgeon early if the child’s acne is recalcitrant to treatment or shows early signs of scarring. Dermatologic surgeons understand the science behind healthy skin and can help your patients with the special needs of skin through every stage of life.
The differential diagnosis for acneiform eruptions varies by age. Neonatal acne (or neonatal cephalic pustulosis, as it is sometimes called) can affect about one in five babies. It is usually self-limited and requires no treatment, although topical ketoconazole can be prescribed if the parents are concerned or the presentation is extensive.
Infantile acne is less common. This occurs between the ages of 6 months and 1 year. Typical lesions include comedones or more inflammatory lesions. Benzoyl peroxide products and/or topical retinoids can be used to treat infantile acne if it is comedonal.
Acne that appears at age 1-7 years is very rare. Toddlers and children with this early childhood acne also should be evaluated further and/or referred to a specialist. A careful history and physical examination are warranted. Measure height and weight, and plot them on the growth chart. Also look for signs of virilization or precocious sexual development.
An abnormal blood pressure can point to congenital adrenal hyperplasia in the neonatal period. Rule out hyperandrogenism, particularly with severe or persistent infantile acne or sudden onset childhood acne. Refer patients to an endocrinologist if you are uncertain, or if any of the following screening tests are abnormal: bone age, serum DHEA (dehydroepiandrosterone), and free testosterone levels. (Total testosterone can be checked if the free testosterone test is unavailable.) Also consider serologic measures of follicle stimulating hormone, luteinizing hormone, prolactin, and 17 alpha-hydroxyprogesterone.
Performance of an exhaustive search for hyperandrogenism in your office is unnecessary in the majority of neonates, infants, children, and adolescents. It is important to know when these screening tests should be ordered and when to refer to a specialist for further evaluation and/or management.
Prepubertal or adolescent acne can occur earlier than parents might expect (at around 8 years of age), and can be the first sign of pubertal maturation. Distinguish comedonal from inflammatory acne to determine appropriate therapy.
Treatment with topical retinoids is the best for comedonal acne. Take the time to educate parents and the child on proper application of a topical retinoid. Instruct them to apply a pea-sized amount to dry skin every other night (or even every third night) for the first 2-4 weeks. This initial small dose can be titrated up gradually over time to minimize adverse effects. Improper use can lead to significant irritation and dryness, and contribute to the lack of treatment compliance.
As with any disease process, patient education is extremely important and can have a great impact on outcomes. Extensively counsel patients and parents on therapy options, and stress the importance of compliance with your recommended treatment regimen.
If the child has inflammatory acne lesions, a combination of benzoyl peroxide and topical antibiotic therapy (erythromycin or clindamycin) is more effective than either agent alone. With more severe acne, oral antibiotics may be warranted. Keep in mind that the tetracycline family of antibiotics can interfere with bone and teeth development, and is contraindicated in children younger than 8 years. Treatment with erythromycin or with Mutual Pharmaceutical’s Bactrim (a combination of sulfamethoxazole and trimethoprim) is appropriate for this age group. For older children with fully developed teeth, oral tetracycline, minocycline, and doxycycline are often the antibiotics of choice.
Infants, children, or adolescents with severe, recalcitrant, or scarring acne should be referred to a specialist for more aggressive intervention. For adolescents with nodulocystic acne (severe acne characterized by inflammation, nodular breakouts, and cysts), early intervention with systemic therapies, including isotretinoin, is important to prevent scarring. Also refer patients to dermatologic surgeons for further evaluation and management if their acne causes them psychological distress, whether or not their clinical presentation is severe.
Dr. Sikora is a private practice dermatologist in Chestnut Hill, Mass., and a member of many professional organizations, including the American Society for Dermatologic Surgery (www.ASDS.net) and the American Academy of Dermatology. Dr. Sikora said she had no relevant financial disclosures.
As a pediatrician, you are on the front line of acne treatment for neonates, children, and adolescents. Acne is a very common condition that will affect 80% of your patients at some point in their lives. It can be easy to diagnose, but acne is often difficult to evaluate and manage. Presentations vary from mild to severe, and you’re likely to see a wide range of acne severity as you treat babies, children, and adolescents through office consultations and regular wellness checks.
Minimal intervention is reasonable for children with mild, comedonal acne. Most are unaware and unconcerned about their acne. It is important to stress they should avoid aggressive facial scrubbing and "popping zits." A discussion of acne physiology that dispels common myths – for example, that junk foods and poor hygiene cause acne in children and adolescents – also is useful.
Early intervention is essential to successful management. Prompt initiation of acne therapy can prevent sequelae that, if left untreated, can include significant scarring and emotional distress for your patients.
Refer your patient to a dermatologic surgeon early if the child’s acne is recalcitrant to treatment or shows early signs of scarring. Dermatologic surgeons understand the science behind healthy skin and can help your patients with the special needs of skin through every stage of life.
The differential diagnosis for acneiform eruptions varies by age. Neonatal acne (or neonatal cephalic pustulosis, as it is sometimes called) can affect about one in five babies. It is usually self-limited and requires no treatment, although topical ketoconazole can be prescribed if the parents are concerned or the presentation is extensive.
Infantile acne is less common. This occurs between the ages of 6 months and 1 year. Typical lesions include comedones or more inflammatory lesions. Benzoyl peroxide products and/or topical retinoids can be used to treat infantile acne if it is comedonal.
Acne that appears at age 1-7 years is very rare. Toddlers and children with this early childhood acne also should be evaluated further and/or referred to a specialist. A careful history and physical examination are warranted. Measure height and weight, and plot them on the growth chart. Also look for signs of virilization or precocious sexual development.
An abnormal blood pressure can point to congenital adrenal hyperplasia in the neonatal period. Rule out hyperandrogenism, particularly with severe or persistent infantile acne or sudden onset childhood acne. Refer patients to an endocrinologist if you are uncertain, or if any of the following screening tests are abnormal: bone age, serum DHEA (dehydroepiandrosterone), and free testosterone levels. (Total testosterone can be checked if the free testosterone test is unavailable.) Also consider serologic measures of follicle stimulating hormone, luteinizing hormone, prolactin, and 17 alpha-hydroxyprogesterone.
Performance of an exhaustive search for hyperandrogenism in your office is unnecessary in the majority of neonates, infants, children, and adolescents. It is important to know when these screening tests should be ordered and when to refer to a specialist for further evaluation and/or management.
Prepubertal or adolescent acne can occur earlier than parents might expect (at around 8 years of age), and can be the first sign of pubertal maturation. Distinguish comedonal from inflammatory acne to determine appropriate therapy.
Treatment with topical retinoids is the best for comedonal acne. Take the time to educate parents and the child on proper application of a topical retinoid. Instruct them to apply a pea-sized amount to dry skin every other night (or even every third night) for the first 2-4 weeks. This initial small dose can be titrated up gradually over time to minimize adverse effects. Improper use can lead to significant irritation and dryness, and contribute to the lack of treatment compliance.
As with any disease process, patient education is extremely important and can have a great impact on outcomes. Extensively counsel patients and parents on therapy options, and stress the importance of compliance with your recommended treatment regimen.
If the child has inflammatory acne lesions, a combination of benzoyl peroxide and topical antibiotic therapy (erythromycin or clindamycin) is more effective than either agent alone. With more severe acne, oral antibiotics may be warranted. Keep in mind that the tetracycline family of antibiotics can interfere with bone and teeth development, and is contraindicated in children younger than 8 years. Treatment with erythromycin or with Mutual Pharmaceutical’s Bactrim (a combination of sulfamethoxazole and trimethoprim) is appropriate for this age group. For older children with fully developed teeth, oral tetracycline, minocycline, and doxycycline are often the antibiotics of choice.
Infants, children, or adolescents with severe, recalcitrant, or scarring acne should be referred to a specialist for more aggressive intervention. For adolescents with nodulocystic acne (severe acne characterized by inflammation, nodular breakouts, and cysts), early intervention with systemic therapies, including isotretinoin, is important to prevent scarring. Also refer patients to dermatologic surgeons for further evaluation and management if their acne causes them psychological distress, whether or not their clinical presentation is severe.
Dr. Sikora is a private practice dermatologist in Chestnut Hill, Mass., and a member of many professional organizations, including the American Society for Dermatologic Surgery (www.ASDS.net) and the American Academy of Dermatology. Dr. Sikora said she had no relevant financial disclosures.
Patient Acuity Rating
Recently released Accreditation Council for Graduate Medical Education (ACGME) recommendations, set to take effect in 2011, place further limits on resident duty hours, which are expected to result in additional handoffs of patients between inpatient providers.1 The increase in these handoffs following the prior set of ACGME recommendations in 2003 has been cited as a potential etiology for the underwhelming effects of the duty hour restrictions on patient outcomes, whereby the benefits of well‐rested physicians are theorized to be offset by increased harm associated with discontinuous care, especially in high‐risk patients.2 In 2007, an Institute of Medicine committee on the topic recommended improving handovers to make the transfer of patient responsibility and information more effective and less error prone.3
Several strategies have been proposed, but an ideal way to quickly transfer complex medical information on numerous patients remains to be identified. A standardized metric of a patient's risk level, if accurate, has the potential to summarize how stable or unstable a patient might be. We hypothesized that clinicians would be able to quantify their judgments regarding the stability of their inpatients and that this measure would correlate with impending clinical deterioration as determined by cardiac arrest or intensive care unit (ICU) transfer within the next 24 hours.
METHODS
Study Design
We developed the Patient Acuity Rating (PAR), a 7‐point Likert scale to quantify clinician judgment regarding the stability of inpatients outside the ICU, and conducted a prospective study of its diagnostic accuracy for predicting impending clinical deterioration in an academic tertiary care hospital. Providers were prospectively surveyed once per call‐cycle, on the day after patient admission, and asked to rate each of their patients on their likelihood of suffering a cardiac arrest or being transferred to the ICU. The scale was anchored at both ends, with a PAR of 1 corresponding to extreme unlikelihood of suffering a cardiac arrest or requiring emergent ICU transfer within the next 24 hours, and a PAR of 7 corresponding with extreme likelihood (Figure 1). A score of 4 suggested neither likely nor unlikely to experience an event. No further anchors were provided.

Study Setting and Participants
This study was conducted at The University of Chicago Medical Center, an academic, tertiary care facility with approximately 600 inpatient beds. Subjects involved both the clinicians who provided PAR scores and the patients upon whom the PAR scores and outcomes were based. The clinicians included internal medicine interns, residents, and attending physicians, as well as midlevel providers (nurse practitioners or physician assistants). Clinicians were eligible for inclusion if they cared for patients on one of nine adult ward services between January and June 2008. They were included in the study if they consented to participate. Housestaff, with medicine attending supervision, covered patients on seven general medicine services, while midlevel practitioners, also with medicine attending supervision, covered patients on two hepatology and transplant services.
Providers were independently surveyed once per call‐cycle (every 2 to 4 days depending on the service) by study personnel regarding each of their patients, and instructed not to consult with other members of the team regarding their PAR score assignments. All patients for whom a participating clinician provided a PAR score were included in the analysis. Clinician subjects were carefully surveyed at the end of their work day, just prior to, or immediately following, their handover to the cross‐covering physician, so as to minimize the risk that they might alter their plan and transfer a patient to the ICU based on the PAR score.
Data Analysis
PAR scores were entered into a database (Excel, Microsoft Corporation, Redmond, WA) and then linked to patient demographic and outcome data obtained from hospital administrative databases. Weighted kappa statistics were used to evaluate inter‐rater reliability. Ordinal trend testing was used to correlate the PAR with patient outcomes by provider. In addition, receiver operator characteristics (ROC) curves were constructed, and area under the curve (AUC) calculated and compared among providers using paired chi‐squared statistics. Sensitivities and specificities were determined for each theoretical PAR cutoff. Clustered multivariate logistic regression was used to adjust for provider, service, and individual patient. All calculations were performed using a statistical software application (Stata, College Station, TX).
Approval
The study protocol, consent, and data collection mechanisms were approved by the Institutional Review Board of the University of Chicago Medical Center. Waiver of consent provisions were used for patients on the basis of minimal harm and general impracticability, while a written consent process was used for patient care providers. Collection of patient information was designed to comply with the Health Insurance Portability and Accountability Act of 1996 regulations.
RESULTS
During the study period, 140/159 (88.1%) eligible clinicians consented to participate. Of these clinicians, 45 (32.1%) were intern physicians, 40 (28.6%) were resident physicians, 51 (36.4%) were attending physicians, and 4 (2.9%) were midlevel providers. They provided PAR scores on 1663 distinct patients over the course of 2019 separate admissions. Table 1 shows the patient and admission demographics grouped by the type of medical service: general medicine teaching or multispecialty non‐teaching. Severity of illness assignments were determined using All Patient Refined Diagnosis Related Group (APR‐DRG) methodology, which incorporates features such as principle diagnosis at admission, co‐morbidities, complications during admission, age, and gender.4, 5 The multispecialty patients were more likely to be male, have a higher severity of illness, and die during the hospitalization, when compared to general medicine patients.
| Characteristic | General Medicine Teaching Services | Multispecialty Non‐Teaching Services | P‐Value |
|---|---|---|---|
| |||
| Patients (n) | 1,373 | 290 | NA |
| Admissions (n) | 1,660 | 359 | NA |
| Age, mean (SD) years | 57 (21) | 57 (13) | 0.73 |
| Women, n (%) | 1,006 (61) | 173 (48) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 203 (12) | 133 (37) | |
| Black | 1,129 (68) | 125 (35) | |
| Hispanic | 26 (2) | 34 (9) | |
| Asian | 11 (1) | 10 (3) | |
| Other/unknown | 291 (18) | 57 (16) | |
| Severity of illness, n (%) | <0.001 | ||
| Minor | 121 (7) | 2 (1) | |
| Moderate | 461 (28) | 44 (12) | |
| Major | 677 (41) | 179 (50) | |
| Extreme | 329 (20) | 123 (34) | |
| N/A | 77 (4) | 11 (3) | |
| Discharged home, n (%) | 1,347 (81) | 282 (79) | 0.25 |
| Expired (not hospice), n (%) | 25 (2) | 28 (8) | <0.001 |
A total of 6034 individual PAR scores from 3419 patient‐days were obtained, which represented a response rate of 74.3%. The average PAR was 2.9 1.4. Table 2 shows the inter‐rater reliability between providers. Weighted kappa statistics ranged from 0.32 (for interns and attendings) to 0.43 (for midlevels and attendings), representing moderate inter‐rater reliability. No comparison was made between midlevel providers and interns or residents, as these participants never cared for the same patients on the same day.
| Provider Pair | Observations (n) | Agreement (%) | Weighted Kappa |
|---|---|---|---|
| Interns vs residents | 1,006 | 87.1 | 0.42 |
| Residents vs attendings | 1,012 | 82.5 | 0.35 |
| Interns vs attendings | 1,026 | 84.4 | 0.32 |
| Midlevels vs attendings | 208 | 85.0 | 0.43 |
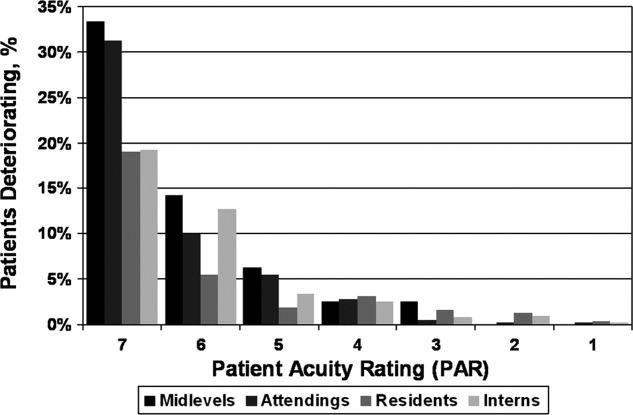
Seventy‐four of the 3419 patient‐days (2.2%) ended in cardiac arrest or unplanned ICU transfer. The distribution of clinical deterioration by average PAR, along with sensitivity and specificity values, are shown in Table 3. Using a cutoff value of 5 yielded a sensitivity of 62.2% and a specificity of 84.6%. Lowering the threshold to 4 increased the sensitivity to 82.4% but decreased the specificity to 68.3%. This corresponded with a combined AUC of 0.82 [95% CI 0.77, 0.87] (Table 4). Provider‐specific AUC values ranged from a low of 0.69 [95% CI 0.59, 0.78] for residents to a high of 0.84 [95% CI 0.78, 0.90] for attendings on general medicine (P = 0.01). The remaining values were not statistically different from one another. Figure 2 shows the provider‐specific percentage of patients deteriorating by PAR. The risk of clinical deterioration decreased in logarithmic fashion as the PAR decreased for all provider types (P < 0.001). These results were confirmed using multivariate logistic regression adjusting for provider, service, and individual patient (data not shown). In addition, we found no significant differences in AUC values between attendings in terms of years in practice or specialty, however, the study was not powered to detect such differences.
| PAR | All Patients (n) | Decompensating Patients (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 7 | 40 | 12 | 16.2 | 99.2 |
| 6 | 184 | 30 | 40.5 | 95.4 |
| 5 | 561 | 46 | 62.2 | 84.6 |
| 4 | 1,120 | 61 | 82.4 | 68.3 |
| 3 | 2,044 | 69 | 93.2 | 41.0 |
| 2 | 3,005 | 73 | 98.6 | 12.3 |
| 1 | 3,419 | 74 | 100.0 | 0.0 |
| Service | Provider | Observations (n) | PAR, median (IQR) | AUROC (95% CI) |
|---|---|---|---|---|
| ||||
| General medicine | Interns | 1,567 | 3 (2‐4) | 0.79 (0.70, 0.88) |
| General medicine | Residents | 1,611 | 3 (2‐4) | 0.69 (0.59, 0.78)* |
| General medicine | Attendings | 1,791 | 3 (2‐4) | 0.84 (0.78, 0.90)* |
| Multispecialty | Attendings | 823 | 3 (2‐4) | 0.88 (0.79, 0.97) |
| Multispecialty | Midlevels | 242 | 3 (2‐4) | 0.80 (0.64, 0.95) |
| Combined | All | 3,419 | 3 (2‐4) | 0.82 (0.77, 0.87) |
DISCUSSION
Physicians frequently depend on subjective judgments in their decision making.6 However, these judgments are difficult to communicate succinctly and hard to compare among clinicians. We have developed a simple tool for quantifying provider judgment, which yields moderate inter‐rater reliability, and good accuracy in predicting which floor patients may suffer cardiac arrest or emergent ICU transfer in the next 24 hours at an academic medical center.
Physicians routinely use written sign‐outs to convey important information to covering physicians during the handoff process, with the result being loss of information and decreased communication.7, 8 A recent study found that sign‐outs are frequently lacking comprehensive data, with the least commonly conveyed information being the patient's current clinical condition.9 The PAR has the potential to improve clinician handoffs by succinctly summarizing a patient's risk level. This need is made even more pressing by the ACGME's new resident duty hour restrictions and impending further increase in handoffs, a known correlate with inpatient morbidity and mortality.10 The PAR could be added to the sign‐out and updated nightly to readily summarize the judgments of the primary inpatient providers for the covering physician who has little, if any, personal knowledge of the patient at hand.
While ours is the first to examine the correlation between physician judgment and clinical deterioration on the floors, several studies have evaluated the accuracy of clinical judgment in predicting mortality of critically ill hospitalized patients. In the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT), for example, physicians accurately predicted 180‐day mortality of ICU patients with an AUC of 0.78.11 Similar studies in the neonatal intensive care setting revealed greater than 80% predictive ability to identify those patients who would be intubated or fail to survive.12 These intuitive judgments have faired well when compared to previously validated ICU‐based physiologic scoring systems, such as the Acute Physiology, Age, Chronic Health Evaluation (APACHE) system. A meta‐analysis of studies which compared physician intuition to various physiologic scoring systems found intuition to be significantly better at predicting ICU mortality with an AUC of 0.85 compared to 0.63, P = 0.002.13
Physiology‐based scoring systems, relying on routine vital signs, have been developed for non‐ICU inpatients. Smith and colleagues14 recently conducted a systematic review and identified 33 distinct scoring systems, which they independently validated on a single data set for the ability of the admission score to predict overall hospital mortality. The resulting AUC values ranged from 0.66 to 0.78.14 In a prospective study, Kho and colleagues used an electronic medical record (EMR) to generate real‐time risk scores for use in surveillance rounds by their Rapid Response Team (RRT).15 Their scoring system relied on systolic blood pressure, heart rate, respiratory rate, temperature, oxygen saturation, age, and body mass index. The resulting score yielded an AUC of 0.72, with a sensitivity of 88% and specificity of 48% using a cutoff of 3, or 34% and 86% using a cutoff of 5, for predicting code call, cardiopulmonary arrest, or transfer to an ICU. Similar to the latter study, using the PAR for RRT surveillance would allow an institution to set its threshold according to available resources. For example, the team could first evaluate all the patients with a PAR score of 7, followed by those who received a score of 6 and so on. Using the data from the current study, evaluating all the patients with scores of 5, 6, or 7 would require assessing 16% of the patients in order to identify 62% of the events. Adding patients with a score of 4 would require assessing one‐third of the floor patients, but would identify 82% of subsequent deteriorations.
Although the objective nature of physiology‐based scoring systems makes them very appealing, they have two significant limitations. The first is that these scoring systems either require manual vital sign data entry and score calculation, which can be labor intensive and impractical, or technological solutions such as an EMR, which are costly and therefore cannot be applied broadly to all hospitalized patients. In fact, in a recent survey of U.S. hospitals, only 1.5% were found to have a comprehensive EMR on all units.16 Additionally, they are limited by the quality of the data input. This is particularly true for the case of respiratory rate and mental status, which are frequently unreliably measured and documented in current practice.17, 18 The PAR score has the benefit of being readily generated in minimal time by a broad range of providers, as we have demonstrated.
Furthermore, it is well known that vital signs do not capture the full clinical picture, which is why most RRT activation criteria include a vague catch‐all trigger for provider worry or concern.19, 20 Interestingly, this trigger is frequently one of the top cited reasons for activating the RRT,21, 22 and is missed by any automated track‐and‐trigger scoring system which relies only on quantitative clinical assessments such as vital signs. The PAR allows this concern to be quantified, either for addition to a physiology‐based track‐and‐trigger system, or for use on its own, as we have done here.
It is interesting to note that, in this study, attending physician judgment was most predictive and resident judgment the least. One explanation may be that clinical judgment optimally requires both experience and at‐the‐bedside data. While attendings have the most experience, the amount of time interns spend at the bedside collecting data may offset their relative inexperience.23, 24 In contrast, residents generally spend less time at the bedside than interns and have only marginally more experience,25 suggesting that either strong clinical experience or a good amount of time at the bedside are required for the best assessments of risk. This is supported by the close agreement between the attendings and midlevels, who likely spend a comparable amount of time at the bedside as interns.
There are several imitations to this study. First, there may be respondent bias in those who chose to participate and the days in which they provided scores. We would expect this bias to work against the null hypothesis if providers with better clinical judgment were more inclined to participate, and were less likely to provide scores when they were very busy, and thus may have had less time to assess patients. However, the enrollment and response rates were quite good (88% and 74%, respectively) which likely mitigates against this bias. Another limitation is that the study was conducted at a single institution, and only on medical patients, which may limit its generalizability to other institutions and patient populations. Also, intern performance during this January through June period may not reflect their performance earlier in their training year. In addition, we did not have physiologic data available for the patients, and thus were not able to compare the PAR directly to a physiology‐based scoring system. Finally, it is theoretically possible that a provider could decide on the PAR and then transfer the patient to the ICU based on their score. However, we carefully surveyed physicians and nurse practitioners at the time of sign‐out, when they had finished their clinical work for the day, to minimize this risk. We also instructed providers not to share their PAR score with the covering physicians to avoid introduction of bias on the part of the cross‐covering physician.
This was a pilot study designed to measure the correlation between PAR scores and patient outcomes. The PAR has the potential to be added to any handoff system as a way to convey individual severity of illness for patients. In addition, it has the potential for use in risk stratifying patients for interventions, such as increased vital sign monitoring or heightened surveillance by cross‐covering physicians or Rapid Response Teams. One could imagine instructing interns to have a low threshold of concern for patients with high PAR scores, and even formalizing procedures for rounding on such patients a second time during the day or overnight, when on call. Future studies will be required to test its use in clinical practice, which would ideally include a randomized‐controlled trial.
We conclude that clinical judgment regarding floor patient stability is quantifiable in a readily obtained, low‐technology score that has moderate inter‐rater reliability and a good ability to distinguish patients who will suffer a cardiac arrest or require ICU transfer within the next 24 hours. Due to its simple and easy to administer nature, the PAR has the potential to be a useful tool for efficiently conveying complex assessments from one member of the healthcare team to another, thereby improving handoffs and identifying patients at risk of clinical deterioration who might benefit from earlier intervention.
Acknowledgements
The authors are grateful for the support and dedication of the residents, faculty, and staff in the Department of Medicine at the University of Chicago, without whom this study would not have been possible. They also thank Trevor C. Yuen for statistical support, David Beiser, MD, MS, and Kenneth Rasinski, PhD, for scientific advice, Kate Weaver for expert administrative support and Deborah Walsh, RN, MS, Jefferson Cua, and Amanda Schmitz for assistance with data collection.
- Accreditation Council for Graduate Medical Education (ACGME).Task Force on Quality Care and Professionalism.Proposed standards: common program requirements.2007. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,year="2010"2010.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- Institute of Medicine (IOM).Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety.Report brief on resident duty hours: enhancing sleep, supervision, and safety.Washington, DC:National Academy Press;2008. Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2008/Resident‐Duty‐Hours/residency%20hours%20revised% 20for%20web.pdf. Accessed August 16,year="2010"2010.
- ,,,,.Determining benchmarks for evaluation and management coding in an academic division of general surgery.J Am Coll Surg.2004;199(1):124–130.
- .Applying the 3M all patient refined diagnosis related groups grouper to measure inpatient severity in the VA.Med Care.2003;41(6):103–110.
- ,,.Non‐analytical models of clinical reasoning: the role of experience.Med Educ.2007;41(12):1140–1145.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,,.What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff.Qual Saf Health Care.2009;18(4):248–255.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,, et al.The SUPPORT prognostic model—objective estimates of survival for seriously ill hospitalized adults.Ann Intern Med.1995;122(3):191–203.
- ,,,,,.Serial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consent.Pediatrics.2002;109(5):878–886.
- ,,, et al.Mortality predictions in the intensive care unit: comparing physicians with scoring systems.Crit Care Med.2006;34(3):878–885.
- ,,,.Review and performance evaluation of aggregate weighted ‘track and trigger’ systems.Resuscitation.2008;77(2):170–179.
- ,,, et al.Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration.AMIA Annu Symp Proc.2007:404–408.
- ,,, et al.Use of electronic health records in U.S. hospitals.N Engl J Med.2009;360(16):1628–1638.
- ,,,.The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage.Ann Emerg Med.2005;45(1):68–76.
- ,,,.Detection and documentation of dementia and delirium in acute geriatric wards.Gen Hosp Psychiatry.2004;26(1):31–35.
- ,,,,.Redefining in‐hospital resuscitation: the concept of the medical emergency team.Resuscitation.2001;48(2):105–110.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54(2):125–131.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90(11):1148–1152.
- ,,.Time analysis of a general medicine service: results from a random work sampling study.J Gen Intern Med.1994;9(5):272–277.
- ,,, et al.The on‐call experience of interns in internal medicine. Medical Education Task Force of Henry Ford Hospital.Arch Intern Med.1990;150(11):2294–2297.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
Recently released Accreditation Council for Graduate Medical Education (ACGME) recommendations, set to take effect in 2011, place further limits on resident duty hours, which are expected to result in additional handoffs of patients between inpatient providers.1 The increase in these handoffs following the prior set of ACGME recommendations in 2003 has been cited as a potential etiology for the underwhelming effects of the duty hour restrictions on patient outcomes, whereby the benefits of well‐rested physicians are theorized to be offset by increased harm associated with discontinuous care, especially in high‐risk patients.2 In 2007, an Institute of Medicine committee on the topic recommended improving handovers to make the transfer of patient responsibility and information more effective and less error prone.3
Several strategies have been proposed, but an ideal way to quickly transfer complex medical information on numerous patients remains to be identified. A standardized metric of a patient's risk level, if accurate, has the potential to summarize how stable or unstable a patient might be. We hypothesized that clinicians would be able to quantify their judgments regarding the stability of their inpatients and that this measure would correlate with impending clinical deterioration as determined by cardiac arrest or intensive care unit (ICU) transfer within the next 24 hours.
METHODS
Study Design
We developed the Patient Acuity Rating (PAR), a 7‐point Likert scale to quantify clinician judgment regarding the stability of inpatients outside the ICU, and conducted a prospective study of its diagnostic accuracy for predicting impending clinical deterioration in an academic tertiary care hospital. Providers were prospectively surveyed once per call‐cycle, on the day after patient admission, and asked to rate each of their patients on their likelihood of suffering a cardiac arrest or being transferred to the ICU. The scale was anchored at both ends, with a PAR of 1 corresponding to extreme unlikelihood of suffering a cardiac arrest or requiring emergent ICU transfer within the next 24 hours, and a PAR of 7 corresponding with extreme likelihood (Figure 1). A score of 4 suggested neither likely nor unlikely to experience an event. No further anchors were provided.

Study Setting and Participants
This study was conducted at The University of Chicago Medical Center, an academic, tertiary care facility with approximately 600 inpatient beds. Subjects involved both the clinicians who provided PAR scores and the patients upon whom the PAR scores and outcomes were based. The clinicians included internal medicine interns, residents, and attending physicians, as well as midlevel providers (nurse practitioners or physician assistants). Clinicians were eligible for inclusion if they cared for patients on one of nine adult ward services between January and June 2008. They were included in the study if they consented to participate. Housestaff, with medicine attending supervision, covered patients on seven general medicine services, while midlevel practitioners, also with medicine attending supervision, covered patients on two hepatology and transplant services.
Providers were independently surveyed once per call‐cycle (every 2 to 4 days depending on the service) by study personnel regarding each of their patients, and instructed not to consult with other members of the team regarding their PAR score assignments. All patients for whom a participating clinician provided a PAR score were included in the analysis. Clinician subjects were carefully surveyed at the end of their work day, just prior to, or immediately following, their handover to the cross‐covering physician, so as to minimize the risk that they might alter their plan and transfer a patient to the ICU based on the PAR score.
Data Analysis
PAR scores were entered into a database (Excel, Microsoft Corporation, Redmond, WA) and then linked to patient demographic and outcome data obtained from hospital administrative databases. Weighted kappa statistics were used to evaluate inter‐rater reliability. Ordinal trend testing was used to correlate the PAR with patient outcomes by provider. In addition, receiver operator characteristics (ROC) curves were constructed, and area under the curve (AUC) calculated and compared among providers using paired chi‐squared statistics. Sensitivities and specificities were determined for each theoretical PAR cutoff. Clustered multivariate logistic regression was used to adjust for provider, service, and individual patient. All calculations were performed using a statistical software application (Stata, College Station, TX).
Approval
The study protocol, consent, and data collection mechanisms were approved by the Institutional Review Board of the University of Chicago Medical Center. Waiver of consent provisions were used for patients on the basis of minimal harm and general impracticability, while a written consent process was used for patient care providers. Collection of patient information was designed to comply with the Health Insurance Portability and Accountability Act of 1996 regulations.
RESULTS
During the study period, 140/159 (88.1%) eligible clinicians consented to participate. Of these clinicians, 45 (32.1%) were intern physicians, 40 (28.6%) were resident physicians, 51 (36.4%) were attending physicians, and 4 (2.9%) were midlevel providers. They provided PAR scores on 1663 distinct patients over the course of 2019 separate admissions. Table 1 shows the patient and admission demographics grouped by the type of medical service: general medicine teaching or multispecialty non‐teaching. Severity of illness assignments were determined using All Patient Refined Diagnosis Related Group (APR‐DRG) methodology, which incorporates features such as principle diagnosis at admission, co‐morbidities, complications during admission, age, and gender.4, 5 The multispecialty patients were more likely to be male, have a higher severity of illness, and die during the hospitalization, when compared to general medicine patients.
| Characteristic | General Medicine Teaching Services | Multispecialty Non‐Teaching Services | P‐Value |
|---|---|---|---|
| |||
| Patients (n) | 1,373 | 290 | NA |
| Admissions (n) | 1,660 | 359 | NA |
| Age, mean (SD) years | 57 (21) | 57 (13) | 0.73 |
| Women, n (%) | 1,006 (61) | 173 (48) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 203 (12) | 133 (37) | |
| Black | 1,129 (68) | 125 (35) | |
| Hispanic | 26 (2) | 34 (9) | |
| Asian | 11 (1) | 10 (3) | |
| Other/unknown | 291 (18) | 57 (16) | |
| Severity of illness, n (%) | <0.001 | ||
| Minor | 121 (7) | 2 (1) | |
| Moderate | 461 (28) | 44 (12) | |
| Major | 677 (41) | 179 (50) | |
| Extreme | 329 (20) | 123 (34) | |
| N/A | 77 (4) | 11 (3) | |
| Discharged home, n (%) | 1,347 (81) | 282 (79) | 0.25 |
| Expired (not hospice), n (%) | 25 (2) | 28 (8) | <0.001 |
A total of 6034 individual PAR scores from 3419 patient‐days were obtained, which represented a response rate of 74.3%. The average PAR was 2.9 1.4. Table 2 shows the inter‐rater reliability between providers. Weighted kappa statistics ranged from 0.32 (for interns and attendings) to 0.43 (for midlevels and attendings), representing moderate inter‐rater reliability. No comparison was made between midlevel providers and interns or residents, as these participants never cared for the same patients on the same day.
| Provider Pair | Observations (n) | Agreement (%) | Weighted Kappa |
|---|---|---|---|
| Interns vs residents | 1,006 | 87.1 | 0.42 |
| Residents vs attendings | 1,012 | 82.5 | 0.35 |
| Interns vs attendings | 1,026 | 84.4 | 0.32 |
| Midlevels vs attendings | 208 | 85.0 | 0.43 |
Seventy‐four of the 3419 patient‐days (2.2%) ended in cardiac arrest or unplanned ICU transfer. The distribution of clinical deterioration by average PAR, along with sensitivity and specificity values, are shown in Table 3. Using a cutoff value of 5 yielded a sensitivity of 62.2% and a specificity of 84.6%. Lowering the threshold to 4 increased the sensitivity to 82.4% but decreased the specificity to 68.3%. This corresponded with a combined AUC of 0.82 [95% CI 0.77, 0.87] (Table 4). Provider‐specific AUC values ranged from a low of 0.69 [95% CI 0.59, 0.78] for residents to a high of 0.84 [95% CI 0.78, 0.90] for attendings on general medicine (P = 0.01). The remaining values were not statistically different from one another. Figure 2 shows the provider‐specific percentage of patients deteriorating by PAR. The risk of clinical deterioration decreased in logarithmic fashion as the PAR decreased for all provider types (P < 0.001). These results were confirmed using multivariate logistic regression adjusting for provider, service, and individual patient (data not shown). In addition, we found no significant differences in AUC values between attendings in terms of years in practice or specialty, however, the study was not powered to detect such differences.
| PAR | All Patients (n) | Decompensating Patients (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 7 | 40 | 12 | 16.2 | 99.2 |
| 6 | 184 | 30 | 40.5 | 95.4 |
| 5 | 561 | 46 | 62.2 | 84.6 |
| 4 | 1,120 | 61 | 82.4 | 68.3 |
| 3 | 2,044 | 69 | 93.2 | 41.0 |
| 2 | 3,005 | 73 | 98.6 | 12.3 |
| 1 | 3,419 | 74 | 100.0 | 0.0 |
| Service | Provider | Observations (n) | PAR, median (IQR) | AUROC (95% CI) |
|---|---|---|---|---|
| ||||
| General medicine | Interns | 1,567 | 3 (2‐4) | 0.79 (0.70, 0.88) |
| General medicine | Residents | 1,611 | 3 (2‐4) | 0.69 (0.59, 0.78)* |
| General medicine | Attendings | 1,791 | 3 (2‐4) | 0.84 (0.78, 0.90)* |
| Multispecialty | Attendings | 823 | 3 (2‐4) | 0.88 (0.79, 0.97) |
| Multispecialty | Midlevels | 242 | 3 (2‐4) | 0.80 (0.64, 0.95) |
| Combined | All | 3,419 | 3 (2‐4) | 0.82 (0.77, 0.87) |

DISCUSSION
Physicians frequently depend on subjective judgments in their decision making.6 However, these judgments are difficult to communicate succinctly and hard to compare among clinicians. We have developed a simple tool for quantifying provider judgment, which yields moderate inter‐rater reliability, and good accuracy in predicting which floor patients may suffer cardiac arrest or emergent ICU transfer in the next 24 hours at an academic medical center.
Physicians routinely use written sign‐outs to convey important information to covering physicians during the handoff process, with the result being loss of information and decreased communication.7, 8 A recent study found that sign‐outs are frequently lacking comprehensive data, with the least commonly conveyed information being the patient's current clinical condition.9 The PAR has the potential to improve clinician handoffs by succinctly summarizing a patient's risk level. This need is made even more pressing by the ACGME's new resident duty hour restrictions and impending further increase in handoffs, a known correlate with inpatient morbidity and mortality.10 The PAR could be added to the sign‐out and updated nightly to readily summarize the judgments of the primary inpatient providers for the covering physician who has little, if any, personal knowledge of the patient at hand.
While ours is the first to examine the correlation between physician judgment and clinical deterioration on the floors, several studies have evaluated the accuracy of clinical judgment in predicting mortality of critically ill hospitalized patients. In the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT), for example, physicians accurately predicted 180‐day mortality of ICU patients with an AUC of 0.78.11 Similar studies in the neonatal intensive care setting revealed greater than 80% predictive ability to identify those patients who would be intubated or fail to survive.12 These intuitive judgments have faired well when compared to previously validated ICU‐based physiologic scoring systems, such as the Acute Physiology, Age, Chronic Health Evaluation (APACHE) system. A meta‐analysis of studies which compared physician intuition to various physiologic scoring systems found intuition to be significantly better at predicting ICU mortality with an AUC of 0.85 compared to 0.63, P = 0.002.13
Physiology‐based scoring systems, relying on routine vital signs, have been developed for non‐ICU inpatients. Smith and colleagues14 recently conducted a systematic review and identified 33 distinct scoring systems, which they independently validated on a single data set for the ability of the admission score to predict overall hospital mortality. The resulting AUC values ranged from 0.66 to 0.78.14 In a prospective study, Kho and colleagues used an electronic medical record (EMR) to generate real‐time risk scores for use in surveillance rounds by their Rapid Response Team (RRT).15 Their scoring system relied on systolic blood pressure, heart rate, respiratory rate, temperature, oxygen saturation, age, and body mass index. The resulting score yielded an AUC of 0.72, with a sensitivity of 88% and specificity of 48% using a cutoff of 3, or 34% and 86% using a cutoff of 5, for predicting code call, cardiopulmonary arrest, or transfer to an ICU. Similar to the latter study, using the PAR for RRT surveillance would allow an institution to set its threshold according to available resources. For example, the team could first evaluate all the patients with a PAR score of 7, followed by those who received a score of 6 and so on. Using the data from the current study, evaluating all the patients with scores of 5, 6, or 7 would require assessing 16% of the patients in order to identify 62% of the events. Adding patients with a score of 4 would require assessing one‐third of the floor patients, but would identify 82% of subsequent deteriorations.
Although the objective nature of physiology‐based scoring systems makes them very appealing, they have two significant limitations. The first is that these scoring systems either require manual vital sign data entry and score calculation, which can be labor intensive and impractical, or technological solutions such as an EMR, which are costly and therefore cannot be applied broadly to all hospitalized patients. In fact, in a recent survey of U.S. hospitals, only 1.5% were found to have a comprehensive EMR on all units.16 Additionally, they are limited by the quality of the data input. This is particularly true for the case of respiratory rate and mental status, which are frequently unreliably measured and documented in current practice.17, 18 The PAR score has the benefit of being readily generated in minimal time by a broad range of providers, as we have demonstrated.
Furthermore, it is well known that vital signs do not capture the full clinical picture, which is why most RRT activation criteria include a vague catch‐all trigger for provider worry or concern.19, 20 Interestingly, this trigger is frequently one of the top cited reasons for activating the RRT,21, 22 and is missed by any automated track‐and‐trigger scoring system which relies only on quantitative clinical assessments such as vital signs. The PAR allows this concern to be quantified, either for addition to a physiology‐based track‐and‐trigger system, or for use on its own, as we have done here.
It is interesting to note that, in this study, attending physician judgment was most predictive and resident judgment the least. One explanation may be that clinical judgment optimally requires both experience and at‐the‐bedside data. While attendings have the most experience, the amount of time interns spend at the bedside collecting data may offset their relative inexperience.23, 24 In contrast, residents generally spend less time at the bedside than interns and have only marginally more experience,25 suggesting that either strong clinical experience or a good amount of time at the bedside are required for the best assessments of risk. This is supported by the close agreement between the attendings and midlevels, who likely spend a comparable amount of time at the bedside as interns.
There are several imitations to this study. First, there may be respondent bias in those who chose to participate and the days in which they provided scores. We would expect this bias to work against the null hypothesis if providers with better clinical judgment were more inclined to participate, and were less likely to provide scores when they were very busy, and thus may have had less time to assess patients. However, the enrollment and response rates were quite good (88% and 74%, respectively) which likely mitigates against this bias. Another limitation is that the study was conducted at a single institution, and only on medical patients, which may limit its generalizability to other institutions and patient populations. Also, intern performance during this January through June period may not reflect their performance earlier in their training year. In addition, we did not have physiologic data available for the patients, and thus were not able to compare the PAR directly to a physiology‐based scoring system. Finally, it is theoretically possible that a provider could decide on the PAR and then transfer the patient to the ICU based on their score. However, we carefully surveyed physicians and nurse practitioners at the time of sign‐out, when they had finished their clinical work for the day, to minimize this risk. We also instructed providers not to share their PAR score with the covering physicians to avoid introduction of bias on the part of the cross‐covering physician.
This was a pilot study designed to measure the correlation between PAR scores and patient outcomes. The PAR has the potential to be added to any handoff system as a way to convey individual severity of illness for patients. In addition, it has the potential for use in risk stratifying patients for interventions, such as increased vital sign monitoring or heightened surveillance by cross‐covering physicians or Rapid Response Teams. One could imagine instructing interns to have a low threshold of concern for patients with high PAR scores, and even formalizing procedures for rounding on such patients a second time during the day or overnight, when on call. Future studies will be required to test its use in clinical practice, which would ideally include a randomized‐controlled trial.
We conclude that clinical judgment regarding floor patient stability is quantifiable in a readily obtained, low‐technology score that has moderate inter‐rater reliability and a good ability to distinguish patients who will suffer a cardiac arrest or require ICU transfer within the next 24 hours. Due to its simple and easy to administer nature, the PAR has the potential to be a useful tool for efficiently conveying complex assessments from one member of the healthcare team to another, thereby improving handoffs and identifying patients at risk of clinical deterioration who might benefit from earlier intervention.
Acknowledgements
The authors are grateful for the support and dedication of the residents, faculty, and staff in the Department of Medicine at the University of Chicago, without whom this study would not have been possible. They also thank Trevor C. Yuen for statistical support, David Beiser, MD, MS, and Kenneth Rasinski, PhD, for scientific advice, Kate Weaver for expert administrative support and Deborah Walsh, RN, MS, Jefferson Cua, and Amanda Schmitz for assistance with data collection.
Recently released Accreditation Council for Graduate Medical Education (ACGME) recommendations, set to take effect in 2011, place further limits on resident duty hours, which are expected to result in additional handoffs of patients between inpatient providers.1 The increase in these handoffs following the prior set of ACGME recommendations in 2003 has been cited as a potential etiology for the underwhelming effects of the duty hour restrictions on patient outcomes, whereby the benefits of well‐rested physicians are theorized to be offset by increased harm associated with discontinuous care, especially in high‐risk patients.2 In 2007, an Institute of Medicine committee on the topic recommended improving handovers to make the transfer of patient responsibility and information more effective and less error prone.3
Several strategies have been proposed, but an ideal way to quickly transfer complex medical information on numerous patients remains to be identified. A standardized metric of a patient's risk level, if accurate, has the potential to summarize how stable or unstable a patient might be. We hypothesized that clinicians would be able to quantify their judgments regarding the stability of their inpatients and that this measure would correlate with impending clinical deterioration as determined by cardiac arrest or intensive care unit (ICU) transfer within the next 24 hours.
METHODS
Study Design
We developed the Patient Acuity Rating (PAR), a 7‐point Likert scale to quantify clinician judgment regarding the stability of inpatients outside the ICU, and conducted a prospective study of its diagnostic accuracy for predicting impending clinical deterioration in an academic tertiary care hospital. Providers were prospectively surveyed once per call‐cycle, on the day after patient admission, and asked to rate each of their patients on their likelihood of suffering a cardiac arrest or being transferred to the ICU. The scale was anchored at both ends, with a PAR of 1 corresponding to extreme unlikelihood of suffering a cardiac arrest or requiring emergent ICU transfer within the next 24 hours, and a PAR of 7 corresponding with extreme likelihood (Figure 1). A score of 4 suggested neither likely nor unlikely to experience an event. No further anchors were provided.

Study Setting and Participants
This study was conducted at The University of Chicago Medical Center, an academic, tertiary care facility with approximately 600 inpatient beds. Subjects involved both the clinicians who provided PAR scores and the patients upon whom the PAR scores and outcomes were based. The clinicians included internal medicine interns, residents, and attending physicians, as well as midlevel providers (nurse practitioners or physician assistants). Clinicians were eligible for inclusion if they cared for patients on one of nine adult ward services between January and June 2008. They were included in the study if they consented to participate. Housestaff, with medicine attending supervision, covered patients on seven general medicine services, while midlevel practitioners, also with medicine attending supervision, covered patients on two hepatology and transplant services.
Providers were independently surveyed once per call‐cycle (every 2 to 4 days depending on the service) by study personnel regarding each of their patients, and instructed not to consult with other members of the team regarding their PAR score assignments. All patients for whom a participating clinician provided a PAR score were included in the analysis. Clinician subjects were carefully surveyed at the end of their work day, just prior to, or immediately following, their handover to the cross‐covering physician, so as to minimize the risk that they might alter their plan and transfer a patient to the ICU based on the PAR score.
Data Analysis
PAR scores were entered into a database (Excel, Microsoft Corporation, Redmond, WA) and then linked to patient demographic and outcome data obtained from hospital administrative databases. Weighted kappa statistics were used to evaluate inter‐rater reliability. Ordinal trend testing was used to correlate the PAR with patient outcomes by provider. In addition, receiver operator characteristics (ROC) curves were constructed, and area under the curve (AUC) calculated and compared among providers using paired chi‐squared statistics. Sensitivities and specificities were determined for each theoretical PAR cutoff. Clustered multivariate logistic regression was used to adjust for provider, service, and individual patient. All calculations were performed using a statistical software application (Stata, College Station, TX).
Approval
The study protocol, consent, and data collection mechanisms were approved by the Institutional Review Board of the University of Chicago Medical Center. Waiver of consent provisions were used for patients on the basis of minimal harm and general impracticability, while a written consent process was used for patient care providers. Collection of patient information was designed to comply with the Health Insurance Portability and Accountability Act of 1996 regulations.
RESULTS
During the study period, 140/159 (88.1%) eligible clinicians consented to participate. Of these clinicians, 45 (32.1%) were intern physicians, 40 (28.6%) were resident physicians, 51 (36.4%) were attending physicians, and 4 (2.9%) were midlevel providers. They provided PAR scores on 1663 distinct patients over the course of 2019 separate admissions. Table 1 shows the patient and admission demographics grouped by the type of medical service: general medicine teaching or multispecialty non‐teaching. Severity of illness assignments were determined using All Patient Refined Diagnosis Related Group (APR‐DRG) methodology, which incorporates features such as principle diagnosis at admission, co‐morbidities, complications during admission, age, and gender.4, 5 The multispecialty patients were more likely to be male, have a higher severity of illness, and die during the hospitalization, when compared to general medicine patients.
| Characteristic | General Medicine Teaching Services | Multispecialty Non‐Teaching Services | P‐Value |
|---|---|---|---|
| |||
| Patients (n) | 1,373 | 290 | NA |
| Admissions (n) | 1,660 | 359 | NA |
| Age, mean (SD) years | 57 (21) | 57 (13) | 0.73 |
| Women, n (%) | 1,006 (61) | 173 (48) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 203 (12) | 133 (37) | |
| Black | 1,129 (68) | 125 (35) | |
| Hispanic | 26 (2) | 34 (9) | |
| Asian | 11 (1) | 10 (3) | |
| Other/unknown | 291 (18) | 57 (16) | |
| Severity of illness, n (%) | <0.001 | ||
| Minor | 121 (7) | 2 (1) | |
| Moderate | 461 (28) | 44 (12) | |
| Major | 677 (41) | 179 (50) | |
| Extreme | 329 (20) | 123 (34) | |
| N/A | 77 (4) | 11 (3) | |
| Discharged home, n (%) | 1,347 (81) | 282 (79) | 0.25 |
| Expired (not hospice), n (%) | 25 (2) | 28 (8) | <0.001 |
A total of 6034 individual PAR scores from 3419 patient‐days were obtained, which represented a response rate of 74.3%. The average PAR was 2.9 1.4. Table 2 shows the inter‐rater reliability between providers. Weighted kappa statistics ranged from 0.32 (for interns and attendings) to 0.43 (for midlevels and attendings), representing moderate inter‐rater reliability. No comparison was made between midlevel providers and interns or residents, as these participants never cared for the same patients on the same day.
| Provider Pair | Observations (n) | Agreement (%) | Weighted Kappa |
|---|---|---|---|
| Interns vs residents | 1,006 | 87.1 | 0.42 |
| Residents vs attendings | 1,012 | 82.5 | 0.35 |
| Interns vs attendings | 1,026 | 84.4 | 0.32 |
| Midlevels vs attendings | 208 | 85.0 | 0.43 |
Seventy‐four of the 3419 patient‐days (2.2%) ended in cardiac arrest or unplanned ICU transfer. The distribution of clinical deterioration by average PAR, along with sensitivity and specificity values, are shown in Table 3. Using a cutoff value of 5 yielded a sensitivity of 62.2% and a specificity of 84.6%. Lowering the threshold to 4 increased the sensitivity to 82.4% but decreased the specificity to 68.3%. This corresponded with a combined AUC of 0.82 [95% CI 0.77, 0.87] (Table 4). Provider‐specific AUC values ranged from a low of 0.69 [95% CI 0.59, 0.78] for residents to a high of 0.84 [95% CI 0.78, 0.90] for attendings on general medicine (P = 0.01). The remaining values were not statistically different from one another. Figure 2 shows the provider‐specific percentage of patients deteriorating by PAR. The risk of clinical deterioration decreased in logarithmic fashion as the PAR decreased for all provider types (P < 0.001). These results were confirmed using multivariate logistic regression adjusting for provider, service, and individual patient (data not shown). In addition, we found no significant differences in AUC values between attendings in terms of years in practice or specialty, however, the study was not powered to detect such differences.
| PAR | All Patients (n) | Decompensating Patients (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 7 | 40 | 12 | 16.2 | 99.2 |
| 6 | 184 | 30 | 40.5 | 95.4 |
| 5 | 561 | 46 | 62.2 | 84.6 |
| 4 | 1,120 | 61 | 82.4 | 68.3 |
| 3 | 2,044 | 69 | 93.2 | 41.0 |
| 2 | 3,005 | 73 | 98.6 | 12.3 |
| 1 | 3,419 | 74 | 100.0 | 0.0 |
| Service | Provider | Observations (n) | PAR, median (IQR) | AUROC (95% CI) |
|---|---|---|---|---|
| ||||
| General medicine | Interns | 1,567 | 3 (2‐4) | 0.79 (0.70, 0.88) |
| General medicine | Residents | 1,611 | 3 (2‐4) | 0.69 (0.59, 0.78)* |
| General medicine | Attendings | 1,791 | 3 (2‐4) | 0.84 (0.78, 0.90)* |
| Multispecialty | Attendings | 823 | 3 (2‐4) | 0.88 (0.79, 0.97) |
| Multispecialty | Midlevels | 242 | 3 (2‐4) | 0.80 (0.64, 0.95) |
| Combined | All | 3,419 | 3 (2‐4) | 0.82 (0.77, 0.87) |

DISCUSSION
Physicians frequently depend on subjective judgments in their decision making.6 However, these judgments are difficult to communicate succinctly and hard to compare among clinicians. We have developed a simple tool for quantifying provider judgment, which yields moderate inter‐rater reliability, and good accuracy in predicting which floor patients may suffer cardiac arrest or emergent ICU transfer in the next 24 hours at an academic medical center.
Physicians routinely use written sign‐outs to convey important information to covering physicians during the handoff process, with the result being loss of information and decreased communication.7, 8 A recent study found that sign‐outs are frequently lacking comprehensive data, with the least commonly conveyed information being the patient's current clinical condition.9 The PAR has the potential to improve clinician handoffs by succinctly summarizing a patient's risk level. This need is made even more pressing by the ACGME's new resident duty hour restrictions and impending further increase in handoffs, a known correlate with inpatient morbidity and mortality.10 The PAR could be added to the sign‐out and updated nightly to readily summarize the judgments of the primary inpatient providers for the covering physician who has little, if any, personal knowledge of the patient at hand.
While ours is the first to examine the correlation between physician judgment and clinical deterioration on the floors, several studies have evaluated the accuracy of clinical judgment in predicting mortality of critically ill hospitalized patients. In the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT), for example, physicians accurately predicted 180‐day mortality of ICU patients with an AUC of 0.78.11 Similar studies in the neonatal intensive care setting revealed greater than 80% predictive ability to identify those patients who would be intubated or fail to survive.12 These intuitive judgments have faired well when compared to previously validated ICU‐based physiologic scoring systems, such as the Acute Physiology, Age, Chronic Health Evaluation (APACHE) system. A meta‐analysis of studies which compared physician intuition to various physiologic scoring systems found intuition to be significantly better at predicting ICU mortality with an AUC of 0.85 compared to 0.63, P = 0.002.13
Physiology‐based scoring systems, relying on routine vital signs, have been developed for non‐ICU inpatients. Smith and colleagues14 recently conducted a systematic review and identified 33 distinct scoring systems, which they independently validated on a single data set for the ability of the admission score to predict overall hospital mortality. The resulting AUC values ranged from 0.66 to 0.78.14 In a prospective study, Kho and colleagues used an electronic medical record (EMR) to generate real‐time risk scores for use in surveillance rounds by their Rapid Response Team (RRT).15 Their scoring system relied on systolic blood pressure, heart rate, respiratory rate, temperature, oxygen saturation, age, and body mass index. The resulting score yielded an AUC of 0.72, with a sensitivity of 88% and specificity of 48% using a cutoff of 3, or 34% and 86% using a cutoff of 5, for predicting code call, cardiopulmonary arrest, or transfer to an ICU. Similar to the latter study, using the PAR for RRT surveillance would allow an institution to set its threshold according to available resources. For example, the team could first evaluate all the patients with a PAR score of 7, followed by those who received a score of 6 and so on. Using the data from the current study, evaluating all the patients with scores of 5, 6, or 7 would require assessing 16% of the patients in order to identify 62% of the events. Adding patients with a score of 4 would require assessing one‐third of the floor patients, but would identify 82% of subsequent deteriorations.
Although the objective nature of physiology‐based scoring systems makes them very appealing, they have two significant limitations. The first is that these scoring systems either require manual vital sign data entry and score calculation, which can be labor intensive and impractical, or technological solutions such as an EMR, which are costly and therefore cannot be applied broadly to all hospitalized patients. In fact, in a recent survey of U.S. hospitals, only 1.5% were found to have a comprehensive EMR on all units.16 Additionally, they are limited by the quality of the data input. This is particularly true for the case of respiratory rate and mental status, which are frequently unreliably measured and documented in current practice.17, 18 The PAR score has the benefit of being readily generated in minimal time by a broad range of providers, as we have demonstrated.
Furthermore, it is well known that vital signs do not capture the full clinical picture, which is why most RRT activation criteria include a vague catch‐all trigger for provider worry or concern.19, 20 Interestingly, this trigger is frequently one of the top cited reasons for activating the RRT,21, 22 and is missed by any automated track‐and‐trigger scoring system which relies only on quantitative clinical assessments such as vital signs. The PAR allows this concern to be quantified, either for addition to a physiology‐based track‐and‐trigger system, or for use on its own, as we have done here.
It is interesting to note that, in this study, attending physician judgment was most predictive and resident judgment the least. One explanation may be that clinical judgment optimally requires both experience and at‐the‐bedside data. While attendings have the most experience, the amount of time interns spend at the bedside collecting data may offset their relative inexperience.23, 24 In contrast, residents generally spend less time at the bedside than interns and have only marginally more experience,25 suggesting that either strong clinical experience or a good amount of time at the bedside are required for the best assessments of risk. This is supported by the close agreement between the attendings and midlevels, who likely spend a comparable amount of time at the bedside as interns.
There are several imitations to this study. First, there may be respondent bias in those who chose to participate and the days in which they provided scores. We would expect this bias to work against the null hypothesis if providers with better clinical judgment were more inclined to participate, and were less likely to provide scores when they were very busy, and thus may have had less time to assess patients. However, the enrollment and response rates were quite good (88% and 74%, respectively) which likely mitigates against this bias. Another limitation is that the study was conducted at a single institution, and only on medical patients, which may limit its generalizability to other institutions and patient populations. Also, intern performance during this January through June period may not reflect their performance earlier in their training year. In addition, we did not have physiologic data available for the patients, and thus were not able to compare the PAR directly to a physiology‐based scoring system. Finally, it is theoretically possible that a provider could decide on the PAR and then transfer the patient to the ICU based on their score. However, we carefully surveyed physicians and nurse practitioners at the time of sign‐out, when they had finished their clinical work for the day, to minimize this risk. We also instructed providers not to share their PAR score with the covering physicians to avoid introduction of bias on the part of the cross‐covering physician.
This was a pilot study designed to measure the correlation between PAR scores and patient outcomes. The PAR has the potential to be added to any handoff system as a way to convey individual severity of illness for patients. In addition, it has the potential for use in risk stratifying patients for interventions, such as increased vital sign monitoring or heightened surveillance by cross‐covering physicians or Rapid Response Teams. One could imagine instructing interns to have a low threshold of concern for patients with high PAR scores, and even formalizing procedures for rounding on such patients a second time during the day or overnight, when on call. Future studies will be required to test its use in clinical practice, which would ideally include a randomized‐controlled trial.
We conclude that clinical judgment regarding floor patient stability is quantifiable in a readily obtained, low‐technology score that has moderate inter‐rater reliability and a good ability to distinguish patients who will suffer a cardiac arrest or require ICU transfer within the next 24 hours. Due to its simple and easy to administer nature, the PAR has the potential to be a useful tool for efficiently conveying complex assessments from one member of the healthcare team to another, thereby improving handoffs and identifying patients at risk of clinical deterioration who might benefit from earlier intervention.
Acknowledgements
The authors are grateful for the support and dedication of the residents, faculty, and staff in the Department of Medicine at the University of Chicago, without whom this study would not have been possible. They also thank Trevor C. Yuen for statistical support, David Beiser, MD, MS, and Kenneth Rasinski, PhD, for scientific advice, Kate Weaver for expert administrative support and Deborah Walsh, RN, MS, Jefferson Cua, and Amanda Schmitz for assistance with data collection.
- Accreditation Council for Graduate Medical Education (ACGME).Task Force on Quality Care and Professionalism.Proposed standards: common program requirements.2007. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,year="2010"2010.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- Institute of Medicine (IOM).Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety.Report brief on resident duty hours: enhancing sleep, supervision, and safety.Washington, DC:National Academy Press;2008. Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2008/Resident‐Duty‐Hours/residency%20hours%20revised% 20for%20web.pdf. Accessed August 16,year="2010"2010.
- ,,,,.Determining benchmarks for evaluation and management coding in an academic division of general surgery.J Am Coll Surg.2004;199(1):124–130.
- .Applying the 3M all patient refined diagnosis related groups grouper to measure inpatient severity in the VA.Med Care.2003;41(6):103–110.
- ,,.Non‐analytical models of clinical reasoning: the role of experience.Med Educ.2007;41(12):1140–1145.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,,.What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff.Qual Saf Health Care.2009;18(4):248–255.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,, et al.The SUPPORT prognostic model—objective estimates of survival for seriously ill hospitalized adults.Ann Intern Med.1995;122(3):191–203.
- ,,,,,.Serial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consent.Pediatrics.2002;109(5):878–886.
- ,,, et al.Mortality predictions in the intensive care unit: comparing physicians with scoring systems.Crit Care Med.2006;34(3):878–885.
- ,,,.Review and performance evaluation of aggregate weighted ‘track and trigger’ systems.Resuscitation.2008;77(2):170–179.
- ,,, et al.Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration.AMIA Annu Symp Proc.2007:404–408.
- ,,, et al.Use of electronic health records in U.S. hospitals.N Engl J Med.2009;360(16):1628–1638.
- ,,,.The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage.Ann Emerg Med.2005;45(1):68–76.
- ,,,.Detection and documentation of dementia and delirium in acute geriatric wards.Gen Hosp Psychiatry.2004;26(1):31–35.
- ,,,,.Redefining in‐hospital resuscitation: the concept of the medical emergency team.Resuscitation.2001;48(2):105–110.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54(2):125–131.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90(11):1148–1152.
- ,,.Time analysis of a general medicine service: results from a random work sampling study.J Gen Intern Med.1994;9(5):272–277.
- ,,, et al.The on‐call experience of interns in internal medicine. Medical Education Task Force of Henry Ford Hospital.Arch Intern Med.1990;150(11):2294–2297.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
- Accreditation Council for Graduate Medical Education (ACGME).Task Force on Quality Care and Professionalism.Proposed standards: common program requirements.2007. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards.pdf. Accessed August 16,year="2010"2010.
- ,,, et al.Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities.JAMA.2008;300(10):1146–1153.
- Institute of Medicine (IOM).Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety.Report brief on resident duty hours: enhancing sleep, supervision, and safety.Washington, DC:National Academy Press;2008. Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2008/Resident‐Duty‐Hours/residency%20hours%20revised% 20for%20web.pdf. Accessed August 16,year="2010"2010.
- ,,,,.Determining benchmarks for evaluation and management coding in an academic division of general surgery.J Am Coll Surg.2004;199(1):124–130.
- .Applying the 3M all patient refined diagnosis related groups grouper to measure inpatient severity in the VA.Med Care.2003;41(6):103–110.
- ,,.Non‐analytical models of clinical reasoning: the role of experience.Med Educ.2007;41(12):1140–1145.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,,.What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff.Qual Saf Health Care.2009;18(4):248–255.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,, et al.The SUPPORT prognostic model—objective estimates of survival for seriously ill hospitalized adults.Ann Intern Med.1995;122(3):191–203.
- ,,,,,.Serial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consent.Pediatrics.2002;109(5):878–886.
- ,,, et al.Mortality predictions in the intensive care unit: comparing physicians with scoring systems.Crit Care Med.2006;34(3):878–885.
- ,,,.Review and performance evaluation of aggregate weighted ‘track and trigger’ systems.Resuscitation.2008;77(2):170–179.
- ,,, et al.Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration.AMIA Annu Symp Proc.2007:404–408.
- ,,, et al.Use of electronic health records in U.S. hospitals.N Engl J Med.2009;360(16):1628–1638.
- ,,,.The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage.Ann Emerg Med.2005;45(1):68–76.
- ,,,.Detection and documentation of dementia and delirium in acute geriatric wards.Gen Hosp Psychiatry.2004;26(1):31–35.
- ,,,,.Redefining in‐hospital resuscitation: the concept of the medical emergency team.Resuscitation.2001;48(2):105–110.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54(2):125–131.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90(11):1148–1152.
- ,,.Time analysis of a general medicine service: results from a random work sampling study.J Gen Intern Med.1994;9(5):272–277.
- ,,, et al.The on‐call experience of interns in internal medicine. Medical Education Task Force of Henry Ford Hospital.Arch Intern Med.1990;150(11):2294–2297.
- ,,,,,.Analyzing the time and value of housestaff inpatient work.J Gen Intern Med.1998;13(8):534–540.
Copyright © 2011 Society of Hospital Medicine
False Assumptions
Hospitalists unfamiliar with palliative care might think that older patients are the ones generating higher costs per day and longer length of stay (LOS). But new research from a fellow hospitalist suggests that's not the case.
A report in this month's Journal of Hospital Medicine found that patients 65 years and older had a significantly lower cost per day ($811; P=0.02) and LOS (-1.8 days; P=0.003) for each decade increase in age (J Hosp Med. 2011;6(6):338-343). The data also show patients on surgical specialty services generate higher costs and higher LOS, a likely nod to the complexity of cases that land on those services, says University of Colorado Denver hospitalist Jean Youngwerth, MD.
"We were anticipating more from the start that patients who were older were going to have higher costs per day and LOS," says Dr. Youngwerth, who also serves as associate program director for the Colorado Palliative Medicine Fellowship and Director of the University of Colorado Hospital Palliative Care Consult Service. "A lot of times, it is younger patients who are the sickest of the sick.
"People will go full force on them and they won't necessarily have a lot of the conversations they'd be having with older patients. The assumption is 'They're young, they shouldn't be dying' … even though they're a very sick person."
Dr. Youngwerth says hospitalists working without the aid of institutional support can still make palliative-care discussions a priority.
"You don't need a specialist for that," she adds, "[for] sitting down and setting goals of care, learning about the person, and then making sure that we're matching what their goals and values are to their plan of care."
Hospitalists unfamiliar with palliative care might think that older patients are the ones generating higher costs per day and longer length of stay (LOS). But new research from a fellow hospitalist suggests that's not the case.
A report in this month's Journal of Hospital Medicine found that patients 65 years and older had a significantly lower cost per day ($811; P=0.02) and LOS (-1.8 days; P=0.003) for each decade increase in age (J Hosp Med. 2011;6(6):338-343). The data also show patients on surgical specialty services generate higher costs and higher LOS, a likely nod to the complexity of cases that land on those services, says University of Colorado Denver hospitalist Jean Youngwerth, MD.
"We were anticipating more from the start that patients who were older were going to have higher costs per day and LOS," says Dr. Youngwerth, who also serves as associate program director for the Colorado Palliative Medicine Fellowship and Director of the University of Colorado Hospital Palliative Care Consult Service. "A lot of times, it is younger patients who are the sickest of the sick.
"People will go full force on them and they won't necessarily have a lot of the conversations they'd be having with older patients. The assumption is 'They're young, they shouldn't be dying' … even though they're a very sick person."
Dr. Youngwerth says hospitalists working without the aid of institutional support can still make palliative-care discussions a priority.
"You don't need a specialist for that," she adds, "[for] sitting down and setting goals of care, learning about the person, and then making sure that we're matching what their goals and values are to their plan of care."
Hospitalists unfamiliar with palliative care might think that older patients are the ones generating higher costs per day and longer length of stay (LOS). But new research from a fellow hospitalist suggests that's not the case.
A report in this month's Journal of Hospital Medicine found that patients 65 years and older had a significantly lower cost per day ($811; P=0.02) and LOS (-1.8 days; P=0.003) for each decade increase in age (J Hosp Med. 2011;6(6):338-343). The data also show patients on surgical specialty services generate higher costs and higher LOS, a likely nod to the complexity of cases that land on those services, says University of Colorado Denver hospitalist Jean Youngwerth, MD.
"We were anticipating more from the start that patients who were older were going to have higher costs per day and LOS," says Dr. Youngwerth, who also serves as associate program director for the Colorado Palliative Medicine Fellowship and Director of the University of Colorado Hospital Palliative Care Consult Service. "A lot of times, it is younger patients who are the sickest of the sick.
"People will go full force on them and they won't necessarily have a lot of the conversations they'd be having with older patients. The assumption is 'They're young, they shouldn't be dying' … even though they're a very sick person."
Dr. Youngwerth says hospitalists working without the aid of institutional support can still make palliative-care discussions a priority.
"You don't need a specialist for that," she adds, "[for] sitting down and setting goals of care, learning about the person, and then making sure that we're matching what their goals and values are to their plan of care."
In the Literature: Research You Need to Know
Clinical question: Is decreased nursing staffing and increased patient turnover across various inpatient adult hospital units associated with higher patient mortality?
Background: Studies that have shown an association between lower nurse staffing and higher inpatient mortality have been limited by methodological issues. These limitations include the use of hospital-level administrative data that do not fully capture actual staffing levels and the lack of control for expected nursing requirements for patients.
Study design: Retrospective observational study.
Setting: Forty-three hospital units on both medical and surgical services at a single institution.
Synopsis: The authors examined whether patients who were cared for during shifts that had nursing staffing that was eight hours or more below the staffing target had a higher-than-expected mortality compared with predicted mortality, based on risk-adjusted DRG-related mortality. They also assessed if increased patient turnover during a patient-care shift was associated with a higher-than-expected mortality.
The authors analyzed mortality outcomes of 197,961 patients who were cared for across 176,696 staffed unit-shifts. The risk of death increased with the number of shifts a patient was cared for when the nursing staffing was eight hours below target, with a hazard ratio per below-target shift of 1.02 (95% CI: 1.01 to 1.03). There was also an association between a higher mortality and a greater number of high-turnover shifts, with a hazard ratio of 1.04 (95% CI 1.02 to 1.03).
Bottom line: Patients cared for during shifts with below-target levels of nurse staffing and during shifts with increased patient turnover had an increased mortality.
Citation: Needleman J, Buerhaus P, Pankratz S, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045.
For more physician reviews of HM-related literature, visit our website.
Clinical question: Is decreased nursing staffing and increased patient turnover across various inpatient adult hospital units associated with higher patient mortality?
Background: Studies that have shown an association between lower nurse staffing and higher inpatient mortality have been limited by methodological issues. These limitations include the use of hospital-level administrative data that do not fully capture actual staffing levels and the lack of control for expected nursing requirements for patients.
Study design: Retrospective observational study.
Setting: Forty-three hospital units on both medical and surgical services at a single institution.
Synopsis: The authors examined whether patients who were cared for during shifts that had nursing staffing that was eight hours or more below the staffing target had a higher-than-expected mortality compared with predicted mortality, based on risk-adjusted DRG-related mortality. They also assessed if increased patient turnover during a patient-care shift was associated with a higher-than-expected mortality.
The authors analyzed mortality outcomes of 197,961 patients who were cared for across 176,696 staffed unit-shifts. The risk of death increased with the number of shifts a patient was cared for when the nursing staffing was eight hours below target, with a hazard ratio per below-target shift of 1.02 (95% CI: 1.01 to 1.03). There was also an association between a higher mortality and a greater number of high-turnover shifts, with a hazard ratio of 1.04 (95% CI 1.02 to 1.03).
Bottom line: Patients cared for during shifts with below-target levels of nurse staffing and during shifts with increased patient turnover had an increased mortality.
Citation: Needleman J, Buerhaus P, Pankratz S, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045.
For more physician reviews of HM-related literature, visit our website.
Clinical question: Is decreased nursing staffing and increased patient turnover across various inpatient adult hospital units associated with higher patient mortality?
Background: Studies that have shown an association between lower nurse staffing and higher inpatient mortality have been limited by methodological issues. These limitations include the use of hospital-level administrative data that do not fully capture actual staffing levels and the lack of control for expected nursing requirements for patients.
Study design: Retrospective observational study.
Setting: Forty-three hospital units on both medical and surgical services at a single institution.
Synopsis: The authors examined whether patients who were cared for during shifts that had nursing staffing that was eight hours or more below the staffing target had a higher-than-expected mortality compared with predicted mortality, based on risk-adjusted DRG-related mortality. They also assessed if increased patient turnover during a patient-care shift was associated with a higher-than-expected mortality.
The authors analyzed mortality outcomes of 197,961 patients who were cared for across 176,696 staffed unit-shifts. The risk of death increased with the number of shifts a patient was cared for when the nursing staffing was eight hours below target, with a hazard ratio per below-target shift of 1.02 (95% CI: 1.01 to 1.03). There was also an association between a higher mortality and a greater number of high-turnover shifts, with a hazard ratio of 1.04 (95% CI 1.02 to 1.03).
Bottom line: Patients cared for during shifts with below-target levels of nurse staffing and during shifts with increased patient turnover had an increased mortality.
Citation: Needleman J, Buerhaus P, Pankratz S, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364(11):1037-1045.
For more physician reviews of HM-related literature, visit our website.
Noninvasive Scan Genotypes Non-Small Cell Lung Cancer
AMSTERDAM – An experimental combination of PET scanning and a positron-emitting form of erlotinib appeared to work as a noninvasive way of identifying patients with advanced non–small cell lung cancer tumors that have the right genotype to receive erlotinib therapy.
"[11C]erlotinib PET shows promise as a noninvasive, in vivo means of selecting patients who may benefit from thymidine kinase inhibitor therapy," Dr. Idris Bahce said, reporting on a pilot study of 10 patients. Erlotinib (Tarceva) is from the thymidine kinase inhibitor drug class.
In his study, uptake of 11C-labeled erlotinib was significantly linked to the patients’ having an activating mutation in their epidermal growth factor receptor (EGFR) gene, specifically an exon 19 deletion.
Patients positive for erlotinib uptake on the PET scan also showed a tendency for better clinical responses to a therapeutic erlotinib regimen, reported Dr. Bahce, a pulmonologist at VU University, Amsterdam, during the World Conference on Lung Cancer.
Until now, the only way to identify advanced non–small cell lung cancer (NSCLC) tumors that are candidates for treatment with a tyrosine kinase inhibitor has been to biopsy the tumor and run an in vitro genetic analysis on the tumor cells. That can be challenging in some patients, such as when the tumor is not easy to biopsy, a limited amount of tissue is available, or the tumor is genetically heterogeneous. To get a reliable result from biopsy and testing, at least 30% of the specimen must contain malignant cells, Dr. Bahce said at the conference, sponsored by the International Association for the Study of Lung Cancer.
"It is a very early study, but ... it’s important because personalized treatment [for cancer] has gone to the next level, where we use new agents and match them to the right patients by doing biopsies," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven. "The PET method also allows physicians to assess the volume of cancer carrying the EGFR mutation following treatment, a way to track treatment efficacy," said Dr. Herbst in an interview.
"Instead of getting tissue at one point in time, you can image more frequently. It’s a way to track the course of treatment noninvasively," and in real time, he said.
He also predicted that the [11C]erlotinib PET test will become commercialized, although currently Dr. Bahce’s studies do not have any commercial funding.
"This is a proof of concept study," commented Dr. Luis Paz-Ares, chief of medical oncology at University Hospital Virgin del Rocio in Seville, Spain. "We need to define the positive predictive value and the negative predictive value" of the test, he added. The long-term future of a test like this may also be limited because future testing will probably need to look at multiple biomarkers, Dr. Paz-Ares said.
The study enrolled five patients with advanced NSCLC who had exon 19 deletion EGFR mutations, and five advanced NSCLC patients with wild-type EGFR genes. Each patient underwent a pair of [11C]erlotinib PET scans, each preceded by a [15O]water PET scan to assess blood perfusion of the tumors. A 4-hour interval separated the two sets of scans.
The scan results showed that the volume of distribution of the tagged erlotinib in the patients with EGFR mutations ran about 50% higher than in the patients with wild-type tumors, a difference that was significant (P = .03).
Clinically, two of the five wild-type patients had nonetheless received erlotinib treatment prior to testing, and neither patient responded, with both showing progressive disease.
Three of the five patients with an EGFR mutation began receiving erlotinib treatment after testing and responded. In one of these patients, the tumor remained in check for 13 months. In a second patient, the tumor began to progress after 17 months of no progression on treatment. In the third patient, the tumor began to progress again after about 4 weeks of no progression on erlotinib treatment, Dr. Bahce said. A fourth patient went on erlotinib treatment before testing, and did not respond and continued to have progressive disease.
The two patient subgroups showed no difference in blood perfusion into the tumors, or in EGFR expression in cell membranes.
Dr. Bahce said he had no disclosures.
AMSTERDAM – An experimental combination of PET scanning and a positron-emitting form of erlotinib appeared to work as a noninvasive way of identifying patients with advanced non–small cell lung cancer tumors that have the right genotype to receive erlotinib therapy.
"[11C]erlotinib PET shows promise as a noninvasive, in vivo means of selecting patients who may benefit from thymidine kinase inhibitor therapy," Dr. Idris Bahce said, reporting on a pilot study of 10 patients. Erlotinib (Tarceva) is from the thymidine kinase inhibitor drug class.
In his study, uptake of 11C-labeled erlotinib was significantly linked to the patients’ having an activating mutation in their epidermal growth factor receptor (EGFR) gene, specifically an exon 19 deletion.
Patients positive for erlotinib uptake on the PET scan also showed a tendency for better clinical responses to a therapeutic erlotinib regimen, reported Dr. Bahce, a pulmonologist at VU University, Amsterdam, during the World Conference on Lung Cancer.
Until now, the only way to identify advanced non–small cell lung cancer (NSCLC) tumors that are candidates for treatment with a tyrosine kinase inhibitor has been to biopsy the tumor and run an in vitro genetic analysis on the tumor cells. That can be challenging in some patients, such as when the tumor is not easy to biopsy, a limited amount of tissue is available, or the tumor is genetically heterogeneous. To get a reliable result from biopsy and testing, at least 30% of the specimen must contain malignant cells, Dr. Bahce said at the conference, sponsored by the International Association for the Study of Lung Cancer.
"It is a very early study, but ... it’s important because personalized treatment [for cancer] has gone to the next level, where we use new agents and match them to the right patients by doing biopsies," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven. "The PET method also allows physicians to assess the volume of cancer carrying the EGFR mutation following treatment, a way to track treatment efficacy," said Dr. Herbst in an interview.
"Instead of getting tissue at one point in time, you can image more frequently. It’s a way to track the course of treatment noninvasively," and in real time, he said.
He also predicted that the [11C]erlotinib PET test will become commercialized, although currently Dr. Bahce’s studies do not have any commercial funding.
"This is a proof of concept study," commented Dr. Luis Paz-Ares, chief of medical oncology at University Hospital Virgin del Rocio in Seville, Spain. "We need to define the positive predictive value and the negative predictive value" of the test, he added. The long-term future of a test like this may also be limited because future testing will probably need to look at multiple biomarkers, Dr. Paz-Ares said.
The study enrolled five patients with advanced NSCLC who had exon 19 deletion EGFR mutations, and five advanced NSCLC patients with wild-type EGFR genes. Each patient underwent a pair of [11C]erlotinib PET scans, each preceded by a [15O]water PET scan to assess blood perfusion of the tumors. A 4-hour interval separated the two sets of scans.
The scan results showed that the volume of distribution of the tagged erlotinib in the patients with EGFR mutations ran about 50% higher than in the patients with wild-type tumors, a difference that was significant (P = .03).
Clinically, two of the five wild-type patients had nonetheless received erlotinib treatment prior to testing, and neither patient responded, with both showing progressive disease.
Three of the five patients with an EGFR mutation began receiving erlotinib treatment after testing and responded. In one of these patients, the tumor remained in check for 13 months. In a second patient, the tumor began to progress after 17 months of no progression on treatment. In the third patient, the tumor began to progress again after about 4 weeks of no progression on erlotinib treatment, Dr. Bahce said. A fourth patient went on erlotinib treatment before testing, and did not respond and continued to have progressive disease.
The two patient subgroups showed no difference in blood perfusion into the tumors, or in EGFR expression in cell membranes.
Dr. Bahce said he had no disclosures.
AMSTERDAM – An experimental combination of PET scanning and a positron-emitting form of erlotinib appeared to work as a noninvasive way of identifying patients with advanced non–small cell lung cancer tumors that have the right genotype to receive erlotinib therapy.
"[11C]erlotinib PET shows promise as a noninvasive, in vivo means of selecting patients who may benefit from thymidine kinase inhibitor therapy," Dr. Idris Bahce said, reporting on a pilot study of 10 patients. Erlotinib (Tarceva) is from the thymidine kinase inhibitor drug class.
In his study, uptake of 11C-labeled erlotinib was significantly linked to the patients’ having an activating mutation in their epidermal growth factor receptor (EGFR) gene, specifically an exon 19 deletion.
Patients positive for erlotinib uptake on the PET scan also showed a tendency for better clinical responses to a therapeutic erlotinib regimen, reported Dr. Bahce, a pulmonologist at VU University, Amsterdam, during the World Conference on Lung Cancer.
Until now, the only way to identify advanced non–small cell lung cancer (NSCLC) tumors that are candidates for treatment with a tyrosine kinase inhibitor has been to biopsy the tumor and run an in vitro genetic analysis on the tumor cells. That can be challenging in some patients, such as when the tumor is not easy to biopsy, a limited amount of tissue is available, or the tumor is genetically heterogeneous. To get a reliable result from biopsy and testing, at least 30% of the specimen must contain malignant cells, Dr. Bahce said at the conference, sponsored by the International Association for the Study of Lung Cancer.
"It is a very early study, but ... it’s important because personalized treatment [for cancer] has gone to the next level, where we use new agents and match them to the right patients by doing biopsies," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven. "The PET method also allows physicians to assess the volume of cancer carrying the EGFR mutation following treatment, a way to track treatment efficacy," said Dr. Herbst in an interview.
"Instead of getting tissue at one point in time, you can image more frequently. It’s a way to track the course of treatment noninvasively," and in real time, he said.
He also predicted that the [11C]erlotinib PET test will become commercialized, although currently Dr. Bahce’s studies do not have any commercial funding.
"This is a proof of concept study," commented Dr. Luis Paz-Ares, chief of medical oncology at University Hospital Virgin del Rocio in Seville, Spain. "We need to define the positive predictive value and the negative predictive value" of the test, he added. The long-term future of a test like this may also be limited because future testing will probably need to look at multiple biomarkers, Dr. Paz-Ares said.
The study enrolled five patients with advanced NSCLC who had exon 19 deletion EGFR mutations, and five advanced NSCLC patients with wild-type EGFR genes. Each patient underwent a pair of [11C]erlotinib PET scans, each preceded by a [15O]water PET scan to assess blood perfusion of the tumors. A 4-hour interval separated the two sets of scans.
The scan results showed that the volume of distribution of the tagged erlotinib in the patients with EGFR mutations ran about 50% higher than in the patients with wild-type tumors, a difference that was significant (P = .03).
Clinically, two of the five wild-type patients had nonetheless received erlotinib treatment prior to testing, and neither patient responded, with both showing progressive disease.
Three of the five patients with an EGFR mutation began receiving erlotinib treatment after testing and responded. In one of these patients, the tumor remained in check for 13 months. In a second patient, the tumor began to progress after 17 months of no progression on treatment. In the third patient, the tumor began to progress again after about 4 weeks of no progression on erlotinib treatment, Dr. Bahce said. A fourth patient went on erlotinib treatment before testing, and did not respond and continued to have progressive disease.
The two patient subgroups showed no difference in blood perfusion into the tumors, or in EGFR expression in cell membranes.
Dr. Bahce said he had no disclosures.
FROM THE WORLD CONFERENCE ON LUNG CANCER
Major Finding: Advanced non–small cell lung cancer tumors with an epidermal growth factor receptor (EGFR)–activating mutation bound significantly more radiolabeled erlotinib than did tumors with wild-type EGFR genes (P = .03).
Data Source: A pilot study in 10 patients.
Disclosures: Dr. Bahce said he had no disclosures.
Rivaroxaban noninferior to warfarin in AF patients
A large, multicenter, randomized study of 14, 264 patients at risk for stroke with nonvalvular atrial fibrillation (AF) found the factor Xa inhibitor rivaroxaban to be noninferior to warfarin for preventing stroke or systemic embolism.
The ROCKET AF investigators, who reported the results online August 10 in The New England Journal of Medicine, detected no significant difference between rivaroxaban and warfarin in the rates of major or nonmajor clinically relevant bleeding.
Investigators at 1178 study sites in 45 countries randomly assigned the patients to receive either fixed-dose rivaroxaban at 20 mg daily or adjusted-dose warfarin to a target of INR 2.0 – 3.0. Patients with a creatinine clearance of 30-49 mL/minute received a rivaroxaban dose of 15 mg daily.
Patients in both arms of the intent-to-treat population were a median age of 73 years and about 40% were women. The patients had considerable rates of coexisting conditions, including 90.5% with hypertension, 62.5% with heart failure, and 54.8% who had had a previous stroke, embolism, or transient ischemic attack.
After a median treatment duration of 590 days, the primary efficacy analysis showed188 patients (1.7% per year) in the rivaroxaban group had a stroke or systemic embolism, compared with 241 patients (2.2% per year) in the warfarin group (P<0.001 for noninferiority).
Rates of major bleeding were similar in the 2 groups—3.6% with rivaroxaban and 3.4% with warfarin (P=0.58). Major and clinically relevant nonmajor bleeding occurred in 1475 (14.9%) rivaroxaban-treated patients and 1449 (14.5%) warfarin-treated patients (P=0.44). Intracranial and fatal bleeding occurred less often in the rivaroxaban group.
The investigators noted that the warfarin-treated patients were in therapeutic range a mean of 55% of the time. However, the efficacy of rivaroxaban was as favorable in those centers with the best INR control as it was in those with inferior control.
Lead author Manesh R. Patel, MD, of Duke University School of Medicine in North Carolina, said, “Warfarin has been a standard treatment for decades, but requires a rigorous monitoring schedule to ensure therapeutic dosing levels, and is subject to the potential of food and drug interactions that present treatment obstacles for patients and doctors alike.”
He indicated that the result of the trial “have convincingly shown rivaroxaban to be an alternative to warfarin in treating patients with atrial fibrillation, and importantly, with no increase in bleeding.”
The study was funded by Johnson & Johnson and Bayer.
ROCKET AF stands for Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation. ![]()
A large, multicenter, randomized study of 14, 264 patients at risk for stroke with nonvalvular atrial fibrillation (AF) found the factor Xa inhibitor rivaroxaban to be noninferior to warfarin for preventing stroke or systemic embolism.
The ROCKET AF investigators, who reported the results online August 10 in The New England Journal of Medicine, detected no significant difference between rivaroxaban and warfarin in the rates of major or nonmajor clinically relevant bleeding.
Investigators at 1178 study sites in 45 countries randomly assigned the patients to receive either fixed-dose rivaroxaban at 20 mg daily or adjusted-dose warfarin to a target of INR 2.0 – 3.0. Patients with a creatinine clearance of 30-49 mL/minute received a rivaroxaban dose of 15 mg daily.
Patients in both arms of the intent-to-treat population were a median age of 73 years and about 40% were women. The patients had considerable rates of coexisting conditions, including 90.5% with hypertension, 62.5% with heart failure, and 54.8% who had had a previous stroke, embolism, or transient ischemic attack.
After a median treatment duration of 590 days, the primary efficacy analysis showed188 patients (1.7% per year) in the rivaroxaban group had a stroke or systemic embolism, compared with 241 patients (2.2% per year) in the warfarin group (P<0.001 for noninferiority).
Rates of major bleeding were similar in the 2 groups—3.6% with rivaroxaban and 3.4% with warfarin (P=0.58). Major and clinically relevant nonmajor bleeding occurred in 1475 (14.9%) rivaroxaban-treated patients and 1449 (14.5%) warfarin-treated patients (P=0.44). Intracranial and fatal bleeding occurred less often in the rivaroxaban group.
The investigators noted that the warfarin-treated patients were in therapeutic range a mean of 55% of the time. However, the efficacy of rivaroxaban was as favorable in those centers with the best INR control as it was in those with inferior control.
Lead author Manesh R. Patel, MD, of Duke University School of Medicine in North Carolina, said, “Warfarin has been a standard treatment for decades, but requires a rigorous monitoring schedule to ensure therapeutic dosing levels, and is subject to the potential of food and drug interactions that present treatment obstacles for patients and doctors alike.”
He indicated that the result of the trial “have convincingly shown rivaroxaban to be an alternative to warfarin in treating patients with atrial fibrillation, and importantly, with no increase in bleeding.”
The study was funded by Johnson & Johnson and Bayer.
ROCKET AF stands for Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation. ![]()
A large, multicenter, randomized study of 14, 264 patients at risk for stroke with nonvalvular atrial fibrillation (AF) found the factor Xa inhibitor rivaroxaban to be noninferior to warfarin for preventing stroke or systemic embolism.
The ROCKET AF investigators, who reported the results online August 10 in The New England Journal of Medicine, detected no significant difference between rivaroxaban and warfarin in the rates of major or nonmajor clinically relevant bleeding.
Investigators at 1178 study sites in 45 countries randomly assigned the patients to receive either fixed-dose rivaroxaban at 20 mg daily or adjusted-dose warfarin to a target of INR 2.0 – 3.0. Patients with a creatinine clearance of 30-49 mL/minute received a rivaroxaban dose of 15 mg daily.
Patients in both arms of the intent-to-treat population were a median age of 73 years and about 40% were women. The patients had considerable rates of coexisting conditions, including 90.5% with hypertension, 62.5% with heart failure, and 54.8% who had had a previous stroke, embolism, or transient ischemic attack.
After a median treatment duration of 590 days, the primary efficacy analysis showed188 patients (1.7% per year) in the rivaroxaban group had a stroke or systemic embolism, compared with 241 patients (2.2% per year) in the warfarin group (P<0.001 for noninferiority).
Rates of major bleeding were similar in the 2 groups—3.6% with rivaroxaban and 3.4% with warfarin (P=0.58). Major and clinically relevant nonmajor bleeding occurred in 1475 (14.9%) rivaroxaban-treated patients and 1449 (14.5%) warfarin-treated patients (P=0.44). Intracranial and fatal bleeding occurred less often in the rivaroxaban group.
The investigators noted that the warfarin-treated patients were in therapeutic range a mean of 55% of the time. However, the efficacy of rivaroxaban was as favorable in those centers with the best INR control as it was in those with inferior control.
Lead author Manesh R. Patel, MD, of Duke University School of Medicine in North Carolina, said, “Warfarin has been a standard treatment for decades, but requires a rigorous monitoring schedule to ensure therapeutic dosing levels, and is subject to the potential of food and drug interactions that present treatment obstacles for patients and doctors alike.”
He indicated that the result of the trial “have convincingly shown rivaroxaban to be an alternative to warfarin in treating patients with atrial fibrillation, and importantly, with no increase in bleeding.”
The study was funded by Johnson & Johnson and Bayer.
ROCKET AF stands for Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation. ![]()
Out of Control
That the largest-ever study of glucose control in U.S. hospitals found roughly 1 in 3 patients are hyperglycemic (<180 mg/dL) during their hospital stay is no surprise to hospitalist Cheryl O'Malley, MD, FACP, program director of internal medicine at the Banner Good Samaritan Medical Center, Phoenix.
The data (PDF), based on point-of-care bedside glucose tests at 575 hospitals, showed hyperglycemia in 32.2% of ICU patients and 32% in non-ICU patients. Dr. O'Malley says the findings are further evidence that HM leaders have a duty to focus on glycemic control because so many of their patients are hyperglycemic.
Dr. O'Malley does her part as a mentor for SHM's Glycemic Control Mentored Initiative (GCMI) program, which recently expanded to a second cohort of 96 sites. The mentoring program has branched out to include nurses, physician assistants, and even two leading endocrinologists as mentors: Emory University School of Medicine's Guillermo Umpierrez, MD, FACP, FACE, and HealthPartners' John MacIndoe, MD.
SHM also has launched a microsite, dubbed eQUIPS (Electronic Quality Improvement Programs), which gives HM groups not involved in the mentoring program access to data analysis, benchmarking tools, and other services.
Kendall M. Rogers, MD, CPE, FACP, SFHM, associate professor of medicine and hospital medicine division chief at the University of New Mexico Health Sciences Center's Department of Internal Medicine, says SHM has always wanted to broaden the program to as many hospitals and physicians as possible to battle glycemic-control issues. And bringing in nationally respected endocrinologists as mentors furthers the goal to build "teams of experts within local hospitals."
"Hospitalists, endocrinologists, and other specialists have to work together," Dr. O'Malley adds. "The volume of work is just too much for any one group to bear."
That the largest-ever study of glucose control in U.S. hospitals found roughly 1 in 3 patients are hyperglycemic (<180 mg/dL) during their hospital stay is no surprise to hospitalist Cheryl O'Malley, MD, FACP, program director of internal medicine at the Banner Good Samaritan Medical Center, Phoenix.
The data (PDF), based on point-of-care bedside glucose tests at 575 hospitals, showed hyperglycemia in 32.2% of ICU patients and 32% in non-ICU patients. Dr. O'Malley says the findings are further evidence that HM leaders have a duty to focus on glycemic control because so many of their patients are hyperglycemic.
Dr. O'Malley does her part as a mentor for SHM's Glycemic Control Mentored Initiative (GCMI) program, which recently expanded to a second cohort of 96 sites. The mentoring program has branched out to include nurses, physician assistants, and even two leading endocrinologists as mentors: Emory University School of Medicine's Guillermo Umpierrez, MD, FACP, FACE, and HealthPartners' John MacIndoe, MD.
SHM also has launched a microsite, dubbed eQUIPS (Electronic Quality Improvement Programs), which gives HM groups not involved in the mentoring program access to data analysis, benchmarking tools, and other services.
Kendall M. Rogers, MD, CPE, FACP, SFHM, associate professor of medicine and hospital medicine division chief at the University of New Mexico Health Sciences Center's Department of Internal Medicine, says SHM has always wanted to broaden the program to as many hospitals and physicians as possible to battle glycemic-control issues. And bringing in nationally respected endocrinologists as mentors furthers the goal to build "teams of experts within local hospitals."
"Hospitalists, endocrinologists, and other specialists have to work together," Dr. O'Malley adds. "The volume of work is just too much for any one group to bear."
That the largest-ever study of glucose control in U.S. hospitals found roughly 1 in 3 patients are hyperglycemic (<180 mg/dL) during their hospital stay is no surprise to hospitalist Cheryl O'Malley, MD, FACP, program director of internal medicine at the Banner Good Samaritan Medical Center, Phoenix.
The data (PDF), based on point-of-care bedside glucose tests at 575 hospitals, showed hyperglycemia in 32.2% of ICU patients and 32% in non-ICU patients. Dr. O'Malley says the findings are further evidence that HM leaders have a duty to focus on glycemic control because so many of their patients are hyperglycemic.
Dr. O'Malley does her part as a mentor for SHM's Glycemic Control Mentored Initiative (GCMI) program, which recently expanded to a second cohort of 96 sites. The mentoring program has branched out to include nurses, physician assistants, and even two leading endocrinologists as mentors: Emory University School of Medicine's Guillermo Umpierrez, MD, FACP, FACE, and HealthPartners' John MacIndoe, MD.
SHM also has launched a microsite, dubbed eQUIPS (Electronic Quality Improvement Programs), which gives HM groups not involved in the mentoring program access to data analysis, benchmarking tools, and other services.
Kendall M. Rogers, MD, CPE, FACP, SFHM, associate professor of medicine and hospital medicine division chief at the University of New Mexico Health Sciences Center's Department of Internal Medicine, says SHM has always wanted to broaden the program to as many hospitals and physicians as possible to battle glycemic-control issues. And bringing in nationally respected endocrinologists as mentors furthers the goal to build "teams of experts within local hospitals."
"Hospitalists, endocrinologists, and other specialists have to work together," Dr. O'Malley adds. "The volume of work is just too much for any one group to bear."


