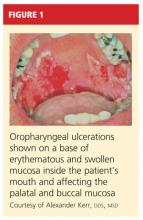
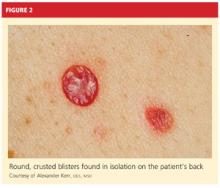
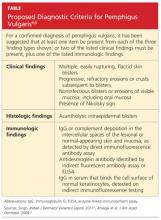
User login
Exploring the human genome, and relearning genetics by necessity
The ability to scan the entire human genome and to recognize variations in specific nucleotides within recognized genes is more than a technologic feat. It is now possible to assess the risk of some genetic diseases before they are phenotypically expressed. We are increasingly able to predict whether specific drugs will be effective or pose higher risks of adverse effects in individual patients, a field called pharmacogenomics. How much pharmacogenomics can and should be incorporated into our practice as part of personalized medicine remains to be determined,
Genome-wide association studies can answer certain research questions, but also raise additional ones. In some ways, these studies are like molecular epidemiology—they can demonstrate a statistical association between a risk factor and a clinical event such as a heart attack, but just as in traditional epidemiologic studies, association does not always equate with causation.
As discussed by Drs. Manace and Babyatsky in this issue of the Journal, additional techniques can be used to try to sort out the issue of association vs causation—in this case, whether C-reactive protein (CRP) is merely associated with cardiovascular events or is a cause of them. Using the tools of traditional clinical research, it would be ideal to demonstrate that the use of a highly specific inhibitor of the risk factor (CRP) prevents the disease. CRP levels can be lowered with statins, but these drugs also reduce levels of low-density lipoprotein cholesterol, which will lower the risk of cardiac events. Thus, statins do not have the specificity to prove that CRP causes myocardial infarction.
This paper is one of the first in the Journal to discuss advances in genomics that may affect our practice. Beginning in May, the Journal will begin a new series on personalized medicine to highlight the role that genetics and molecular medicine can play in our clinical practice and in our understanding of pathophysiology.
The ability to scan the entire human genome and to recognize variations in specific nucleotides within recognized genes is more than a technologic feat. It is now possible to assess the risk of some genetic diseases before they are phenotypically expressed. We are increasingly able to predict whether specific drugs will be effective or pose higher risks of adverse effects in individual patients, a field called pharmacogenomics. How much pharmacogenomics can and should be incorporated into our practice as part of personalized medicine remains to be determined,
Genome-wide association studies can answer certain research questions, but also raise additional ones. In some ways, these studies are like molecular epidemiology—they can demonstrate a statistical association between a risk factor and a clinical event such as a heart attack, but just as in traditional epidemiologic studies, association does not always equate with causation.
As discussed by Drs. Manace and Babyatsky in this issue of the Journal, additional techniques can be used to try to sort out the issue of association vs causation—in this case, whether C-reactive protein (CRP) is merely associated with cardiovascular events or is a cause of them. Using the tools of traditional clinical research, it would be ideal to demonstrate that the use of a highly specific inhibitor of the risk factor (CRP) prevents the disease. CRP levels can be lowered with statins, but these drugs also reduce levels of low-density lipoprotein cholesterol, which will lower the risk of cardiac events. Thus, statins do not have the specificity to prove that CRP causes myocardial infarction.
This paper is one of the first in the Journal to discuss advances in genomics that may affect our practice. Beginning in May, the Journal will begin a new series on personalized medicine to highlight the role that genetics and molecular medicine can play in our clinical practice and in our understanding of pathophysiology.
The ability to scan the entire human genome and to recognize variations in specific nucleotides within recognized genes is more than a technologic feat. It is now possible to assess the risk of some genetic diseases before they are phenotypically expressed. We are increasingly able to predict whether specific drugs will be effective or pose higher risks of adverse effects in individual patients, a field called pharmacogenomics. How much pharmacogenomics can and should be incorporated into our practice as part of personalized medicine remains to be determined,
Genome-wide association studies can answer certain research questions, but also raise additional ones. In some ways, these studies are like molecular epidemiology—they can demonstrate a statistical association between a risk factor and a clinical event such as a heart attack, but just as in traditional epidemiologic studies, association does not always equate with causation.
As discussed by Drs. Manace and Babyatsky in this issue of the Journal, additional techniques can be used to try to sort out the issue of association vs causation—in this case, whether C-reactive protein (CRP) is merely associated with cardiovascular events or is a cause of them. Using the tools of traditional clinical research, it would be ideal to demonstrate that the use of a highly specific inhibitor of the risk factor (CRP) prevents the disease. CRP levels can be lowered with statins, but these drugs also reduce levels of low-density lipoprotein cholesterol, which will lower the risk of cardiac events. Thus, statins do not have the specificity to prove that CRP causes myocardial infarction.
This paper is one of the first in the Journal to discuss advances in genomics that may affect our practice. Beginning in May, the Journal will begin a new series on personalized medicine to highlight the role that genetics and molecular medicine can play in our clinical practice and in our understanding of pathophysiology.
Putting genome analysis to good use: Lessons from C-reactive protein and cardiovascular disease
Genomics research is paying off, not only by identifying people at risk of rare inherited diseases but also by clarifying the pathogenic mechanisms of important, common ones.
Thanks to advances in technology, we can now, at a reasonable cost, simultaneously screen for millions of genetic variants in thousands of people to find variants that are more common in people with a given disease than without the disease, a fruitful method called a genome-wide association study. Moreover, an epidemiologic method called mendelian randomization takes advantage of the natural reshuffling of the genetic deck that occurs with each generation to give an estimate of whether certain gene products are mediators—or merely markers—of disease.
In a landmark study published in 2009, Elliott et al1 used mendelian randomization to evaluate the role of C-reactive protein (CRP) in coronary artery disease.
Here, we review the use of genetic tools in a clinical context, highlighting CRP to illustrate some of the potential uses and limitations of applied genomics in clinical investigation.
NATURE VS NURTURE: AN AGE-OLD DEBATE
The relative contributions of genetic and environmental factors to human health and disease— nature vs nurture—is an age-old debate in which interest has been renewed in this era of intensive research in molecular genetics.
In the 19th century, Charles Darwin proposed that evolution proceeds through natural selection of variations in inherited traits. His contemporary, Gregor Mendel, showed that traits are inherited in discrete units, later named genes. Just what genes were and how they worked had to await the discovery of the structure of DNA in 1953, by Watson and Crick.2
Since then, progress has accelerated. Advances in recombinant DNA and DNA-sequencing technologies enabled sequencing of the entire human genome only 50 years later. More recently, we have seen automated rapid sequencing, the HapMap project (more on this below), and the advent of genome-wide association studies that uncover genetic variants correlated with or predisposing to common, complex human diseases.
Until recent years, medical genetics was mostly confined to the study of rare syndromes, such as Huntington disease, that are due either to a change in a single gene or to abnormal quantities of large swaths of chromosomes containing many genes. It had little application to most of the common disorders seen by primary care physicians. However, the genes and pathways implicated in rare monogenic disorders have provided key insights into common diseases. For example, defining the genes and mutations underlying familial hypercholesterolemia highlighted the role of low-density lipoprotein cholesterol (LDL-C) in the pathogenesis of atherosclerotic disease.
3.4 BILLION BASE PAIRS, 23,000 GENES
The DNA molecule consists of two strings of the nucleotides guanine (G), cytosine (C), thymine (T), and adenine (A). The human genome contains about 3.4 billion of these nucleotides, also called base pairs, as they bind G to C and A to T across the length of the double helix of the DNA molecule.
Only about 2% of these 3.4 billion base pairs make up genes, ie, sequences that are transcribed into RNA and then translated into protein. Humans have only about 23,000 genes, which is less than in some plant species.
What about the rest of the human genome, ie, most of it? Previously dismissed as “junk,” these regions likely possess more elusive regulatory functions, controlling gene expression (ultimately, the production of protein), which varies considerably from tissue to tissue and over a person’s lifetime.
It is the orchestration of gene expression over time and cell type that gives the human body its intricate complexity. The study of how all our genes and gene products interact is called genomics and is part of the larger topic of the network of protein interactions (proteomics) and of the integration of various protein pathways (metabolomics).
We are all 99% identical—or 12 million nucleotides different
Human genome sequences are 99% identical across populations. But the remaining 1% is still a big number: there are more than 12 million variants between any two individuals’ genomes. These variants include:
- Single-nucleotide polymorphisms (SNPs), ie, a single-nucleotide change that is present in at least 1% of the population
- Copy number variants (CNVs), ie, a stretch of DNA that is either missing or duplicated
- Repeating patterns of DNA that vary in the number of repeated sequences.
THE EVOLUTION OF GENOMICS RESEARCH
Much of the initial focus of research in the genomics era consisted of identifying these variants and discovering associations between them and particular human diseases or clinical outcomes. In this way, we uncovered a multitude of potential new biomarkers and therapeutic targets, requiring further investigation into the connection between the DNA variant and the clinical state.
At the close of the 20th century, genetic factors were correlated with human disease by linkage analysis (a method of mapping patterns of markers that congregate in relatively narrow regions of DNA in families with specific diseases), and candidate gene approaches, whereby genes were investigated on the basis of their postulated biology and of previous studies. These techniques were relatively low-yield and cumbersome; years of work uncovered only a handful of genes proven to be associated with diseases.
Newer tools can look at scores of genes linked to common diseases. Researchers now rely on sophisticated DNA sequencing tools and interpretation software to sift masses of data to find meaningful markers (DNA variants or mutations).
Genomics research in the past few years has been mostly hypothesis-independent. Investigators are no longer limited to the small cache of genes whose corresponding proteins are well characterized, but can instead probe the entire genome for connections between our DNA and our physiology.
The rise of genome-wide association studies
Over the past decade, much clinically useful information has been gathered in genome-wide association studies.
The rise of this type of study rested on our emerging understanding of the architecture of our genome. When the genomes of multiple humans were fully sequenced, we discovered that specific variants do not occur randomly in relation to each other. Rather, they tend to be inherited in particular blocks called haplotypes, and some SNPs or combinations of SNPS are very rare or essentially never seen.
In its first phase, the HapMap project organized these useful blocks of variants, genotyping 1 million SNPs for each of 270 individuals from mother-father-offspring trios from distinct geographic regions of the world.3 The second phase of the HapMap project extended the analysis to more than 3 million SNPs and to other populations.4
While the HapMap should be generally applicable to other populations not yet studied, limitations of the first two HapMap phases include rare SNPs or CNVs, or variants outside of haplotype regions.
The 1,000 Genomes Project, now under way, will develop an even more comprehensive catalog of human genetic variants in much broader populations.
The success of genome-wide association studies is also partly attributable to progress in DNA-sequencing technology. Using microarray chips, we can now look at millions of SNPs per patient or the entire coding sequence of the genome (termed the exome) in a single experiment that is both time-effecient and cost-effective.
What is a genome-wide association study?
A genome-wide association study generally compares genetic variants between patients with a particular clinical condition (cases) and people without the condition (controls), looking for statistically significant differences. As a tool for genetic discovery, these studies have revealed many avenues for further investigation in the pathogenesis of disease, as well as potential targets of therapy.
Using these studies, research groups around the world have found reproducible correlations between genetic variants and susceptibility to common adult-onset diseases.
Although many of the variants identified in these studies are associated with only a slightly higher risk of disease, the method is free of many of the inherent biases associated with clinical research. These studies permit a comprehensive, hypothesis-independent and unbiased scan of the genome to identify novel susceptibility factors, whereas earlier genetic epidemiology studies could take on only a handful of variables to evaluate at a time. Additionally, they are powered to detect very small increases (or decreases) in disease risk, previously outside the reach of linkage analysis. Polymorphisms (or, presumably, non-disease-causing DNA changes) discovered using these studies often correlate with clinical phenotypes or with levels of biomarkers, even if the genetic variants are not necessarily pathologic in themselves.
Thus, genome-wide association studies have led to important insights into the pathogenesis of multiple common diseases, such as inflammatory bowel disease and diabetes mellitus, and they are facilitating new treatment approaches. For instance, multiple studies have reproduced an association between Crohn disease and variation in the gene NOD2, whose protein product is implicated in bacterial product recognition, autophagy, and apoptosis.5 This discovery led to the investigation of new potential therapies for Crohn disease, ie, the tyrosine kinase inhibitors gefitinib (Iressa) and erlotinib (Tarceva), known to inhibit NOD2 activity, and to the prognostic use of the NOD2 genotype in Crohn disease (a field of study known as genotype-phenotype correlation).
Future advances will likely come from looking at combinations of variants, which may carry a higher risk of disease than single variants.
CORONARY HEART DISEASE: FRESH INSIGHT INTO AN OLD PROBLEM
Cardiovascular disease accounts for 30% of deaths worldwide.6 Of all the cardiovascular disorders, coronary heart disease is rising most rapidly in incidence, as the rest of the world adopts Western practices such as a high-calorie, high-fat, high-glycemic diet.
Hundreds of risk factors for coronary heart disease have been described.7 Three of them are clearly modifiable participants in the pathogenesis of atherosclerosis: hypertension, smoking, and elevated LDL-C. These and others form the basis for risk-assessment tools such as the Framingham risk score and the Prospective Cardiovascular Münster (PROCAM) study score. Other possible markers require further evaluation as to whether they are clinically useful and are direct mediators of coronary heart disease.
Because up to 40% of coronary deaths occur in people who lack conventional risk factors for it (eg, they do not smoke and they have normal levels of LDL-C and blood pressure), researchers are searching hard for new, potentially treatable risk factors.8 Of particular interest are components of inflammatory pathways linked with atherosclerosis and coronary heart disease. The identity of the key inflammatory factors that cause arterial plaque formation and rupture continues to be studied.
CRP, an acute-phase reactant produced by the liver in response to inflammation, has received much attention, as serum CRP levels correlate strongly with coronary events. Researchers have used modifiers of CRP to try to alter the course of coronary heart disease, but traditional research has so far failed to establish a causal relationship between CRP and coronary heart disease.9
How we know that LDL-C is a mediator, not just a marker
As a risk factor, LDL-C resembles CRP in that its levels correlate with a number of other, confounding risk factors. Therefore, much basic research and clinical observation had to be done before we could say that LDL-C plays a role in the pathogenesis of coronary heart disease.
Initially an association between LDL-C and heart disease was noted.10 Then, studies of familial hypercholesterolemia uncovered genetic abnormalities that increase LDL-C levels and, thereby, the risk of coronary heart disease—eg, mutations in the LDL receptor gene,11–14 the apolipoprotein B (APOB) gene at its LDL receptor-binding domain,15LDL-RAP1 (a gene encoding an accessory adaptor protein that interacts with the LDL receptor),16 and PCSK9 (a gene that codes for proprotein convertase subtilisin-kexin type 9 protease).17
Conversely, specific loss-of-function truncating mutations of PCSK9 that reduce LDL-C levels are associated with strong protection against coronary heart disease.18 Other gene mutations that reduce LDL-C also lower the risk.19,20
Further, a genome-wide association study21 identified multiple genetic variations associated with different forms of dyslipidemia, uncovering additional links between LDL-C and coronary heart disease.
Finally, randomized controlled trials of niacin, fibrates, and statins showed that these potent LDL-C-lowering agents reduce the rate of development or progression of coronary heart disease.22,23
C-reactive protein: Marker or mediator?
Unlike LDL-C, no familial syndromes of coronary heart disease have been recognized in patients who have isolated high serum levels of CRP.
Since many substances in addition to CRP increase in concentration in both acute and chronic inflammatory states, agents that lower CRP in a targeted manner would be needed for large prospective, randomized trials to show whether CRP plays a direct role in coronary heart disease. A specific CRP inhibitor, 1,6-bis(phosphocholine)-hexane, may aid in these efforts, although it is not orally bioavailable and has a very short serum half-life.24
The JUPITER trial. Statins lower levels of both LDL-C and CRP. The Justification for the Use of Statins in Primary Prevention: an Intervention Evaluating Rosuvastatin (JUPITER) trial was designed to find out whether statins alter coronary risk in patients with “normal” LDL-C levels (< 130 mg/dL) and elevated CRP levels (> 2 g/L).25
In this prospective, randomized trial, statin treatment resulted in a dramatic risk reduction of 40% to 50% in multiple coronary end points, as well as a reduction in CRP levels of 37% compared with placebo. However, LDL-C levels fell by 50%, confounding the effect on CRP, as the lower coronary event rate could alternatively be explained by the effect of lower-than-normal LDL-C levels. Thus, a causative link between CRP and coronary heart disease could not be proved.26
Though ongoing trials may further illuminate the role of inflammation in the development of coronary heart disease, and specific CRP inhibitors are in development, we have few tools to answer the fundamental question of whether CRP itself is an active participant in cardiovascular disease progression or if it is a bystander marker, helping to define risk for patients who develop coronary heart disease without other known risk factors.
Of note, adding CRP to the Framingham risk score does not improve its predictive power very much in any age group.27,28 Nevertheless, for certain end points, such as the long-term rate of death after percutaneous coronary intervention29 or of cardiovascular death immediately after coronary artery bypass grafting,30 CRP levels predict coronary events reliably.
BIOMARKERS AND MENDELIAN RANDOMIZATION
Further insight into the CRP-coronary association may lie in the genes. Intriguingly, while mutations have been found that alter the serum concentration of CRP, these isolated changes in CRP levels have not yet been shown to affect heart disease risk.9,31,32
If one were to design a prospective, interventional study to evaluate the role of CRP in coronary heart disease, it would be very difficult to tease apart the specific impact of CRP from that of other variables that are often present in people with high CRP, such as obesity and hyperlipidemia. The technique of mendelian randomization offers a way to evaluate the correlation between coronary heart disease development and CRP levels independent of other risk factors.
How many heart attacks in people with or without polymorphisms?
Mendelian randomization takes advantage of a basic genetic principle, ie, the independent assortment of traits. According to Mendel’s second law, alleles for different traits are inherited independently of one another. Therefore, the gene that encodes CRP and other genes that influence its circulating level are presumably inherited independently from other genes that influence coronary risk.
In typical studies of CRP, participants are grouped according to whether they have high or low CRP levels. In these studies, confounding variables congregate in these two groups. For example, people with high CRP may be more likely to smoke and to have a higher body mass index and higher lipid levels—all of which influence cardiovascular outcomes. It is therefore difficult to tease out the effect of CRP levels from other background risk factors.
In contrast, in studies using mendelian randomization, patients are grouped according to whether they have a variant that affects the substance being studied (eg, CRP), and outcomes are compared between the two genetic groups.
Strengths and limitations of this method
By randomizing research subjects by gene variants affecting CRP levels, it is theoretically possible to achieve more equal stratification and minimize confounding between subgroups.33
Mendelian randomization should also address the possibility of “reverse causality,” when the intermediate trait with a potential role in disease development (eg, CRP) is actually regulated by the disease state itself (ie, “inflammation of atherosclerotic cardiovascular disease”).34
A limitation of mendelian randomization is that different genes influencing the biomarker under investigation must be proven to be truly randomly assorted among populations. It cannot be assumed that levels of a biomarker are equally distributed across cases and controls when there may in fact be non-random genetic associations.
For instance, if SNPs in various genes that affect creatine kinase levels were being compared to cardiovascular outcome, it would be important to take into account that baseline creatine kinase levels are higher in African Americans as well as in men in interpreting the study data.35
THE ELLIOTT STUDY (2009)
In a study published in 2009, Elliott et al1 mined genome-wide data collected over the last decade to bring more clarity to the issue of causality between elevated CRP and heart disease.
To accomplish mendelian randomization, the authors assessed SNPs that affect circulating CRP levels in combined sets of 28,000 cases and 100,000 controls—robust population sizes. The SNP variants included were associated with approximately 20% lower CRP levels. This degree of CRP reduction should correspond to a 6% reduction in coronary risk as predicted by meta-analysis of observational studies.
No association between low-CRP variants and heart disease
The authors found significant associations between these SNPs and CRP levels and between CRP levels and coronary heart disease, but not between the SNPs and coronary disease when results for three SNPs were combined and standardized to a 20% lower CRP level (odds ratio 1.00, 95% confidence interval 0.97–1.02).1
In view of the lack of association between coronary heart disease and SNPs that affect CRP levels, the authors suggested that the observational data linking CRP levels and coronary disease may have been confounded by other risk factors, or that the trend is due to reverse causation (the inflammatory response associated with atherosclerosis elevates CRP) rather than CRP’s directly causing heart disease.
These findings have important implications for management of cardiovascular disease, as therapeutic strategies to reduce plasma CRP levels are less likely to be beneficial.
The authors also described other genetic variants that may affect coronary heart disease. Carriers of minor alleles of SNPs in the gene for the leptin receptor LEPR and the APOE-CI-CII cluster showed a significantly higher risk of coronary heart disease.1 However, both variants were associated with lower levels of CRP (and, for the SNP in LEPR, lower body weight and body mass index), suggesting that the links with coronary heart disease are not mediated by CRP. These findings illustrate the ability of genome-wide association studies to identify novel susceptibility loci for complex disease without limiting investigation to genes previously thought to take part in coronary heart disease.
In view of the evidence from this study, it seems that the benefits accruing to patients with high CRP from lipid-lowering therapy as demonstrated in the JUPITER trial are likely not the result of CRP-lowering per se, but rather are the result of action on the underlying pathology that leads to elevation of inflammatory markers, including CRP. As an editorial accompanying the study by Elliot et al pointed out, the work not only provides important information in the effort to identify genetic markers associated with complex disease, but it also helps discern the role of the genes and their products in the progress and treatment of common diseases.36
Subsequent studies of CRP and the “directionality” of its role in coronary disease,37 as well as in other conditions such as obesity and cancer,38,39 have carried on the strategy of Elliott et al, providing further evidence for the function of CRP as a bystander in the inflammatory response and complex disease progression.
IMPLICATIONS OF THESE FINDINGS
Tools now exist to leapfrog the randomized controlled trials that have been the primary way of examining the role of potential mediators of common diseases. Mendelian randomization aids in determining whether biomarkers are involved in disease pathogenesis, are simply bystanders, or are secondary markers caused by the disease itself. While randomized controlled trials will still be important, this new approach offers the power of evaluating much larger sample sizes and more equally stratifying confounding factors between study groups by relying on independent assortment of genetic traits.
In medical care today, the prevention of coronary heart disease entails aggressive treatment of hypertension and hyperlipidemia, along with lifestyle modifications such as balanced diet, routine exercise, and smoking cessation. Given the large numbers of patients at risk, even with low risk scores using currently identified risk factors, more specific and sensitive markers (or panels of such markers) of cardiovascular risk are needed.
In the personalized medicine of the future, we will rely on markers that not only identify people at higher risk, but also tell us who would benefit from certain therapies. From the JUPITER trial, we understand that patients with elevated CRP levels may be appropriate candidates for statin therapy even if they have normal levels of LDL-C.36 The study by Elliott et al steers us away from using CRP-affecting SNPs in predicting the course of disease and also from the belief that targeting CRP alone would be a worthwhile therapeutic strategy.
The inflammatory hypothesis of coronary heart disease remains a very important area of investigation, and CRP may turn out to be one of the best biomarkers we have to predict the progression of coronary diseases. But the study by Elliott et al demonstrates that CRP-lowering drugs are unlikely to be magic bullets.
Most importantly, geneticists will partner with clinical researchers to answer important questions about biomarkers and genes, capitalizing on large sets of population data.
- Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009; 302:37–48.
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953; 171:737–738.
- International HapMap Consortium. A haplotype map of the human genome. Nature 2005; 437:1299–1320.
- International HapMap Consortium; Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007; 449:851–861.
- Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411:599–603.
- Anderson GF, Chu E. Expanding priorities—confronting chronic disease in countries with low income. N Engl J Med 2007; 356:209–211.
- Hingorani AD, Shah T, Casas JP, Humphries SE, Talmud PJ. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem 2009; 55:239–255.
- Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol 2006; 97:28A–32A.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986; 256:2823–2838.
- Lehrman MA, Schneider WJ, Südhof TC, Brown MS, Goldstein JL, Russell DW. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 1985; 227:140–146.
- Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 1990; 24:133–170.
- Südhof TC, Goldstein JL, Brown MS, Russell DW. The LDL receptor gene: a mosaic of exons shared with different proteins. Science 1985; 228:815–822.
- Villéger L, Abifadel M, Allard D, et al. The UMD-LDLR database: additions to the software and 490 new entries to the database. Hum Mutat 2002; 20:81–87.
- Soria LF, Ludwig EH, Clarke HR, Vega GL, Grundy SM, McCarthy BJ. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc Natl Acad Sci U S A 1989; 86:587–591.
- Garcia CK, Wilund K, Arca M, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 2001; 292:1394–1398.
- Sun XM, Eden ER, Tosi I, et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet 2005; 14:1161–1169.
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Linsel-Nitschke P, Götz A, Erdmann J, et al; Wellcome Trust Case Control Consortium (WTCCC). Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—a Mendelian Randomisation study. PLoS One 2008; 3:e2986.
- Linsel-Nitschke P, Heeren J, Aherrahrou Z, et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis 2010; 208:183–189.
- Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41:56–65.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006; 440:1217–1221.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. Jupiter to earth: a statin helps people with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009; 38:217–231.
- Hamer M, Chida Y, Stamatakis E. Utility of C-reactive protein for cardiovascular risk stratification across three age groups in subjects without existing cardiovascular diseases. Am J Cardiol 2009; 104:538–542.
- Razzouk L, Muntner P, Bansilal S, et al. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J 2009; 158:277–283.
- Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive C-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesth 2009; 12:127–132.
- Hunter DJ, Altshuler D, Rader DJ. From Darwin’s finches to canaries in the coal mine—mining the genome for new biology. N Engl J Med 2008; 358:2760–2763.
- Lawlor DA, Harbord RM, Timpson NJ, et al. The association of C-reactive protein and CRP genotype with coronary heart disease: findings from five studies with 4,610 cases amongst 18,637 participants. PLoS One 2008; 3:e3011.
- Lange LA, Carlson CS, Hindorff LA, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 2006; 296:2703–2711.
- Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 2008; 5:e177.
- Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med 2009; 122:73–78.
- Shah SH, de Lemos JA. Biomarkers and cardiovascular disease: determining causality and quantifying contribution to risk assessment. JAMA 2009; 302:92–93.
- Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovasc Dis 2009; 19:521–524.
- Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 2010; 95:93–99.
- Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst 2010; 102:202–206.
Genomics research is paying off, not only by identifying people at risk of rare inherited diseases but also by clarifying the pathogenic mechanisms of important, common ones.
Thanks to advances in technology, we can now, at a reasonable cost, simultaneously screen for millions of genetic variants in thousands of people to find variants that are more common in people with a given disease than without the disease, a fruitful method called a genome-wide association study. Moreover, an epidemiologic method called mendelian randomization takes advantage of the natural reshuffling of the genetic deck that occurs with each generation to give an estimate of whether certain gene products are mediators—or merely markers—of disease.
In a landmark study published in 2009, Elliott et al1 used mendelian randomization to evaluate the role of C-reactive protein (CRP) in coronary artery disease.
Here, we review the use of genetic tools in a clinical context, highlighting CRP to illustrate some of the potential uses and limitations of applied genomics in clinical investigation.
NATURE VS NURTURE: AN AGE-OLD DEBATE
The relative contributions of genetic and environmental factors to human health and disease— nature vs nurture—is an age-old debate in which interest has been renewed in this era of intensive research in molecular genetics.
In the 19th century, Charles Darwin proposed that evolution proceeds through natural selection of variations in inherited traits. His contemporary, Gregor Mendel, showed that traits are inherited in discrete units, later named genes. Just what genes were and how they worked had to await the discovery of the structure of DNA in 1953, by Watson and Crick.2
Since then, progress has accelerated. Advances in recombinant DNA and DNA-sequencing technologies enabled sequencing of the entire human genome only 50 years later. More recently, we have seen automated rapid sequencing, the HapMap project (more on this below), and the advent of genome-wide association studies that uncover genetic variants correlated with or predisposing to common, complex human diseases.
Until recent years, medical genetics was mostly confined to the study of rare syndromes, such as Huntington disease, that are due either to a change in a single gene or to abnormal quantities of large swaths of chromosomes containing many genes. It had little application to most of the common disorders seen by primary care physicians. However, the genes and pathways implicated in rare monogenic disorders have provided key insights into common diseases. For example, defining the genes and mutations underlying familial hypercholesterolemia highlighted the role of low-density lipoprotein cholesterol (LDL-C) in the pathogenesis of atherosclerotic disease.
3.4 BILLION BASE PAIRS, 23,000 GENES
The DNA molecule consists of two strings of the nucleotides guanine (G), cytosine (C), thymine (T), and adenine (A). The human genome contains about 3.4 billion of these nucleotides, also called base pairs, as they bind G to C and A to T across the length of the double helix of the DNA molecule.
Only about 2% of these 3.4 billion base pairs make up genes, ie, sequences that are transcribed into RNA and then translated into protein. Humans have only about 23,000 genes, which is less than in some plant species.
What about the rest of the human genome, ie, most of it? Previously dismissed as “junk,” these regions likely possess more elusive regulatory functions, controlling gene expression (ultimately, the production of protein), which varies considerably from tissue to tissue and over a person’s lifetime.
It is the orchestration of gene expression over time and cell type that gives the human body its intricate complexity. The study of how all our genes and gene products interact is called genomics and is part of the larger topic of the network of protein interactions (proteomics) and of the integration of various protein pathways (metabolomics).
We are all 99% identical—or 12 million nucleotides different
Human genome sequences are 99% identical across populations. But the remaining 1% is still a big number: there are more than 12 million variants between any two individuals’ genomes. These variants include:
- Single-nucleotide polymorphisms (SNPs), ie, a single-nucleotide change that is present in at least 1% of the population
- Copy number variants (CNVs), ie, a stretch of DNA that is either missing or duplicated
- Repeating patterns of DNA that vary in the number of repeated sequences.
THE EVOLUTION OF GENOMICS RESEARCH
Much of the initial focus of research in the genomics era consisted of identifying these variants and discovering associations between them and particular human diseases or clinical outcomes. In this way, we uncovered a multitude of potential new biomarkers and therapeutic targets, requiring further investigation into the connection between the DNA variant and the clinical state.
At the close of the 20th century, genetic factors were correlated with human disease by linkage analysis (a method of mapping patterns of markers that congregate in relatively narrow regions of DNA in families with specific diseases), and candidate gene approaches, whereby genes were investigated on the basis of their postulated biology and of previous studies. These techniques were relatively low-yield and cumbersome; years of work uncovered only a handful of genes proven to be associated with diseases.
Newer tools can look at scores of genes linked to common diseases. Researchers now rely on sophisticated DNA sequencing tools and interpretation software to sift masses of data to find meaningful markers (DNA variants or mutations).
Genomics research in the past few years has been mostly hypothesis-independent. Investigators are no longer limited to the small cache of genes whose corresponding proteins are well characterized, but can instead probe the entire genome for connections between our DNA and our physiology.
The rise of genome-wide association studies
Over the past decade, much clinically useful information has been gathered in genome-wide association studies.
The rise of this type of study rested on our emerging understanding of the architecture of our genome. When the genomes of multiple humans were fully sequenced, we discovered that specific variants do not occur randomly in relation to each other. Rather, they tend to be inherited in particular blocks called haplotypes, and some SNPs or combinations of SNPS are very rare or essentially never seen.
In its first phase, the HapMap project organized these useful blocks of variants, genotyping 1 million SNPs for each of 270 individuals from mother-father-offspring trios from distinct geographic regions of the world.3 The second phase of the HapMap project extended the analysis to more than 3 million SNPs and to other populations.4
While the HapMap should be generally applicable to other populations not yet studied, limitations of the first two HapMap phases include rare SNPs or CNVs, or variants outside of haplotype regions.
The 1,000 Genomes Project, now under way, will develop an even more comprehensive catalog of human genetic variants in much broader populations.
The success of genome-wide association studies is also partly attributable to progress in DNA-sequencing technology. Using microarray chips, we can now look at millions of SNPs per patient or the entire coding sequence of the genome (termed the exome) in a single experiment that is both time-effecient and cost-effective.
What is a genome-wide association study?
A genome-wide association study generally compares genetic variants between patients with a particular clinical condition (cases) and people without the condition (controls), looking for statistically significant differences. As a tool for genetic discovery, these studies have revealed many avenues for further investigation in the pathogenesis of disease, as well as potential targets of therapy.
Using these studies, research groups around the world have found reproducible correlations between genetic variants and susceptibility to common adult-onset diseases.
Although many of the variants identified in these studies are associated with only a slightly higher risk of disease, the method is free of many of the inherent biases associated with clinical research. These studies permit a comprehensive, hypothesis-independent and unbiased scan of the genome to identify novel susceptibility factors, whereas earlier genetic epidemiology studies could take on only a handful of variables to evaluate at a time. Additionally, they are powered to detect very small increases (or decreases) in disease risk, previously outside the reach of linkage analysis. Polymorphisms (or, presumably, non-disease-causing DNA changes) discovered using these studies often correlate with clinical phenotypes or with levels of biomarkers, even if the genetic variants are not necessarily pathologic in themselves.
Thus, genome-wide association studies have led to important insights into the pathogenesis of multiple common diseases, such as inflammatory bowel disease and diabetes mellitus, and they are facilitating new treatment approaches. For instance, multiple studies have reproduced an association between Crohn disease and variation in the gene NOD2, whose protein product is implicated in bacterial product recognition, autophagy, and apoptosis.5 This discovery led to the investigation of new potential therapies for Crohn disease, ie, the tyrosine kinase inhibitors gefitinib (Iressa) and erlotinib (Tarceva), known to inhibit NOD2 activity, and to the prognostic use of the NOD2 genotype in Crohn disease (a field of study known as genotype-phenotype correlation).
Future advances will likely come from looking at combinations of variants, which may carry a higher risk of disease than single variants.
CORONARY HEART DISEASE: FRESH INSIGHT INTO AN OLD PROBLEM
Cardiovascular disease accounts for 30% of deaths worldwide.6 Of all the cardiovascular disorders, coronary heart disease is rising most rapidly in incidence, as the rest of the world adopts Western practices such as a high-calorie, high-fat, high-glycemic diet.
Hundreds of risk factors for coronary heart disease have been described.7 Three of them are clearly modifiable participants in the pathogenesis of atherosclerosis: hypertension, smoking, and elevated LDL-C. These and others form the basis for risk-assessment tools such as the Framingham risk score and the Prospective Cardiovascular Münster (PROCAM) study score. Other possible markers require further evaluation as to whether they are clinically useful and are direct mediators of coronary heart disease.
Because up to 40% of coronary deaths occur in people who lack conventional risk factors for it (eg, they do not smoke and they have normal levels of LDL-C and blood pressure), researchers are searching hard for new, potentially treatable risk factors.8 Of particular interest are components of inflammatory pathways linked with atherosclerosis and coronary heart disease. The identity of the key inflammatory factors that cause arterial plaque formation and rupture continues to be studied.
CRP, an acute-phase reactant produced by the liver in response to inflammation, has received much attention, as serum CRP levels correlate strongly with coronary events. Researchers have used modifiers of CRP to try to alter the course of coronary heart disease, but traditional research has so far failed to establish a causal relationship between CRP and coronary heart disease.9
How we know that LDL-C is a mediator, not just a marker
As a risk factor, LDL-C resembles CRP in that its levels correlate with a number of other, confounding risk factors. Therefore, much basic research and clinical observation had to be done before we could say that LDL-C plays a role in the pathogenesis of coronary heart disease.
Initially an association between LDL-C and heart disease was noted.10 Then, studies of familial hypercholesterolemia uncovered genetic abnormalities that increase LDL-C levels and, thereby, the risk of coronary heart disease—eg, mutations in the LDL receptor gene,11–14 the apolipoprotein B (APOB) gene at its LDL receptor-binding domain,15LDL-RAP1 (a gene encoding an accessory adaptor protein that interacts with the LDL receptor),16 and PCSK9 (a gene that codes for proprotein convertase subtilisin-kexin type 9 protease).17
Conversely, specific loss-of-function truncating mutations of PCSK9 that reduce LDL-C levels are associated with strong protection against coronary heart disease.18 Other gene mutations that reduce LDL-C also lower the risk.19,20
Further, a genome-wide association study21 identified multiple genetic variations associated with different forms of dyslipidemia, uncovering additional links between LDL-C and coronary heart disease.
Finally, randomized controlled trials of niacin, fibrates, and statins showed that these potent LDL-C-lowering agents reduce the rate of development or progression of coronary heart disease.22,23
C-reactive protein: Marker or mediator?
Unlike LDL-C, no familial syndromes of coronary heart disease have been recognized in patients who have isolated high serum levels of CRP.
Since many substances in addition to CRP increase in concentration in both acute and chronic inflammatory states, agents that lower CRP in a targeted manner would be needed for large prospective, randomized trials to show whether CRP plays a direct role in coronary heart disease. A specific CRP inhibitor, 1,6-bis(phosphocholine)-hexane, may aid in these efforts, although it is not orally bioavailable and has a very short serum half-life.24
The JUPITER trial. Statins lower levels of both LDL-C and CRP. The Justification for the Use of Statins in Primary Prevention: an Intervention Evaluating Rosuvastatin (JUPITER) trial was designed to find out whether statins alter coronary risk in patients with “normal” LDL-C levels (< 130 mg/dL) and elevated CRP levels (> 2 g/L).25
In this prospective, randomized trial, statin treatment resulted in a dramatic risk reduction of 40% to 50% in multiple coronary end points, as well as a reduction in CRP levels of 37% compared with placebo. However, LDL-C levels fell by 50%, confounding the effect on CRP, as the lower coronary event rate could alternatively be explained by the effect of lower-than-normal LDL-C levels. Thus, a causative link between CRP and coronary heart disease could not be proved.26
Though ongoing trials may further illuminate the role of inflammation in the development of coronary heart disease, and specific CRP inhibitors are in development, we have few tools to answer the fundamental question of whether CRP itself is an active participant in cardiovascular disease progression or if it is a bystander marker, helping to define risk for patients who develop coronary heart disease without other known risk factors.
Of note, adding CRP to the Framingham risk score does not improve its predictive power very much in any age group.27,28 Nevertheless, for certain end points, such as the long-term rate of death after percutaneous coronary intervention29 or of cardiovascular death immediately after coronary artery bypass grafting,30 CRP levels predict coronary events reliably.
BIOMARKERS AND MENDELIAN RANDOMIZATION
Further insight into the CRP-coronary association may lie in the genes. Intriguingly, while mutations have been found that alter the serum concentration of CRP, these isolated changes in CRP levels have not yet been shown to affect heart disease risk.9,31,32
If one were to design a prospective, interventional study to evaluate the role of CRP in coronary heart disease, it would be very difficult to tease apart the specific impact of CRP from that of other variables that are often present in people with high CRP, such as obesity and hyperlipidemia. The technique of mendelian randomization offers a way to evaluate the correlation between coronary heart disease development and CRP levels independent of other risk factors.
How many heart attacks in people with or without polymorphisms?
Mendelian randomization takes advantage of a basic genetic principle, ie, the independent assortment of traits. According to Mendel’s second law, alleles for different traits are inherited independently of one another. Therefore, the gene that encodes CRP and other genes that influence its circulating level are presumably inherited independently from other genes that influence coronary risk.
In typical studies of CRP, participants are grouped according to whether they have high or low CRP levels. In these studies, confounding variables congregate in these two groups. For example, people with high CRP may be more likely to smoke and to have a higher body mass index and higher lipid levels—all of which influence cardiovascular outcomes. It is therefore difficult to tease out the effect of CRP levels from other background risk factors.
In contrast, in studies using mendelian randomization, patients are grouped according to whether they have a variant that affects the substance being studied (eg, CRP), and outcomes are compared between the two genetic groups.
Strengths and limitations of this method
By randomizing research subjects by gene variants affecting CRP levels, it is theoretically possible to achieve more equal stratification and minimize confounding between subgroups.33
Mendelian randomization should also address the possibility of “reverse causality,” when the intermediate trait with a potential role in disease development (eg, CRP) is actually regulated by the disease state itself (ie, “inflammation of atherosclerotic cardiovascular disease”).34
A limitation of mendelian randomization is that different genes influencing the biomarker under investigation must be proven to be truly randomly assorted among populations. It cannot be assumed that levels of a biomarker are equally distributed across cases and controls when there may in fact be non-random genetic associations.
For instance, if SNPs in various genes that affect creatine kinase levels were being compared to cardiovascular outcome, it would be important to take into account that baseline creatine kinase levels are higher in African Americans as well as in men in interpreting the study data.35
THE ELLIOTT STUDY (2009)
In a study published in 2009, Elliott et al1 mined genome-wide data collected over the last decade to bring more clarity to the issue of causality between elevated CRP and heart disease.
To accomplish mendelian randomization, the authors assessed SNPs that affect circulating CRP levels in combined sets of 28,000 cases and 100,000 controls—robust population sizes. The SNP variants included were associated with approximately 20% lower CRP levels. This degree of CRP reduction should correspond to a 6% reduction in coronary risk as predicted by meta-analysis of observational studies.
No association between low-CRP variants and heart disease
The authors found significant associations between these SNPs and CRP levels and between CRP levels and coronary heart disease, but not between the SNPs and coronary disease when results for three SNPs were combined and standardized to a 20% lower CRP level (odds ratio 1.00, 95% confidence interval 0.97–1.02).1
In view of the lack of association between coronary heart disease and SNPs that affect CRP levels, the authors suggested that the observational data linking CRP levels and coronary disease may have been confounded by other risk factors, or that the trend is due to reverse causation (the inflammatory response associated with atherosclerosis elevates CRP) rather than CRP’s directly causing heart disease.
These findings have important implications for management of cardiovascular disease, as therapeutic strategies to reduce plasma CRP levels are less likely to be beneficial.
The authors also described other genetic variants that may affect coronary heart disease. Carriers of minor alleles of SNPs in the gene for the leptin receptor LEPR and the APOE-CI-CII cluster showed a significantly higher risk of coronary heart disease.1 However, both variants were associated with lower levels of CRP (and, for the SNP in LEPR, lower body weight and body mass index), suggesting that the links with coronary heart disease are not mediated by CRP. These findings illustrate the ability of genome-wide association studies to identify novel susceptibility loci for complex disease without limiting investigation to genes previously thought to take part in coronary heart disease.
In view of the evidence from this study, it seems that the benefits accruing to patients with high CRP from lipid-lowering therapy as demonstrated in the JUPITER trial are likely not the result of CRP-lowering per se, but rather are the result of action on the underlying pathology that leads to elevation of inflammatory markers, including CRP. As an editorial accompanying the study by Elliot et al pointed out, the work not only provides important information in the effort to identify genetic markers associated with complex disease, but it also helps discern the role of the genes and their products in the progress and treatment of common diseases.36
Subsequent studies of CRP and the “directionality” of its role in coronary disease,37 as well as in other conditions such as obesity and cancer,38,39 have carried on the strategy of Elliott et al, providing further evidence for the function of CRP as a bystander in the inflammatory response and complex disease progression.
IMPLICATIONS OF THESE FINDINGS
Tools now exist to leapfrog the randomized controlled trials that have been the primary way of examining the role of potential mediators of common diseases. Mendelian randomization aids in determining whether biomarkers are involved in disease pathogenesis, are simply bystanders, or are secondary markers caused by the disease itself. While randomized controlled trials will still be important, this new approach offers the power of evaluating much larger sample sizes and more equally stratifying confounding factors between study groups by relying on independent assortment of genetic traits.
In medical care today, the prevention of coronary heart disease entails aggressive treatment of hypertension and hyperlipidemia, along with lifestyle modifications such as balanced diet, routine exercise, and smoking cessation. Given the large numbers of patients at risk, even with low risk scores using currently identified risk factors, more specific and sensitive markers (or panels of such markers) of cardiovascular risk are needed.
In the personalized medicine of the future, we will rely on markers that not only identify people at higher risk, but also tell us who would benefit from certain therapies. From the JUPITER trial, we understand that patients with elevated CRP levels may be appropriate candidates for statin therapy even if they have normal levels of LDL-C.36 The study by Elliott et al steers us away from using CRP-affecting SNPs in predicting the course of disease and also from the belief that targeting CRP alone would be a worthwhile therapeutic strategy.
The inflammatory hypothesis of coronary heart disease remains a very important area of investigation, and CRP may turn out to be one of the best biomarkers we have to predict the progression of coronary diseases. But the study by Elliott et al demonstrates that CRP-lowering drugs are unlikely to be magic bullets.
Most importantly, geneticists will partner with clinical researchers to answer important questions about biomarkers and genes, capitalizing on large sets of population data.
Genomics research is paying off, not only by identifying people at risk of rare inherited diseases but also by clarifying the pathogenic mechanisms of important, common ones.
Thanks to advances in technology, we can now, at a reasonable cost, simultaneously screen for millions of genetic variants in thousands of people to find variants that are more common in people with a given disease than without the disease, a fruitful method called a genome-wide association study. Moreover, an epidemiologic method called mendelian randomization takes advantage of the natural reshuffling of the genetic deck that occurs with each generation to give an estimate of whether certain gene products are mediators—or merely markers—of disease.
In a landmark study published in 2009, Elliott et al1 used mendelian randomization to evaluate the role of C-reactive protein (CRP) in coronary artery disease.
Here, we review the use of genetic tools in a clinical context, highlighting CRP to illustrate some of the potential uses and limitations of applied genomics in clinical investigation.
NATURE VS NURTURE: AN AGE-OLD DEBATE
The relative contributions of genetic and environmental factors to human health and disease— nature vs nurture—is an age-old debate in which interest has been renewed in this era of intensive research in molecular genetics.
In the 19th century, Charles Darwin proposed that evolution proceeds through natural selection of variations in inherited traits. His contemporary, Gregor Mendel, showed that traits are inherited in discrete units, later named genes. Just what genes were and how they worked had to await the discovery of the structure of DNA in 1953, by Watson and Crick.2
Since then, progress has accelerated. Advances in recombinant DNA and DNA-sequencing technologies enabled sequencing of the entire human genome only 50 years later. More recently, we have seen automated rapid sequencing, the HapMap project (more on this below), and the advent of genome-wide association studies that uncover genetic variants correlated with or predisposing to common, complex human diseases.
Until recent years, medical genetics was mostly confined to the study of rare syndromes, such as Huntington disease, that are due either to a change in a single gene or to abnormal quantities of large swaths of chromosomes containing many genes. It had little application to most of the common disorders seen by primary care physicians. However, the genes and pathways implicated in rare monogenic disorders have provided key insights into common diseases. For example, defining the genes and mutations underlying familial hypercholesterolemia highlighted the role of low-density lipoprotein cholesterol (LDL-C) in the pathogenesis of atherosclerotic disease.
3.4 BILLION BASE PAIRS, 23,000 GENES
The DNA molecule consists of two strings of the nucleotides guanine (G), cytosine (C), thymine (T), and adenine (A). The human genome contains about 3.4 billion of these nucleotides, also called base pairs, as they bind G to C and A to T across the length of the double helix of the DNA molecule.
Only about 2% of these 3.4 billion base pairs make up genes, ie, sequences that are transcribed into RNA and then translated into protein. Humans have only about 23,000 genes, which is less than in some plant species.
What about the rest of the human genome, ie, most of it? Previously dismissed as “junk,” these regions likely possess more elusive regulatory functions, controlling gene expression (ultimately, the production of protein), which varies considerably from tissue to tissue and over a person’s lifetime.
It is the orchestration of gene expression over time and cell type that gives the human body its intricate complexity. The study of how all our genes and gene products interact is called genomics and is part of the larger topic of the network of protein interactions (proteomics) and of the integration of various protein pathways (metabolomics).
We are all 99% identical—or 12 million nucleotides different
Human genome sequences are 99% identical across populations. But the remaining 1% is still a big number: there are more than 12 million variants between any two individuals’ genomes. These variants include:
- Single-nucleotide polymorphisms (SNPs), ie, a single-nucleotide change that is present in at least 1% of the population
- Copy number variants (CNVs), ie, a stretch of DNA that is either missing or duplicated
- Repeating patterns of DNA that vary in the number of repeated sequences.
THE EVOLUTION OF GENOMICS RESEARCH
Much of the initial focus of research in the genomics era consisted of identifying these variants and discovering associations between them and particular human diseases or clinical outcomes. In this way, we uncovered a multitude of potential new biomarkers and therapeutic targets, requiring further investigation into the connection between the DNA variant and the clinical state.
At the close of the 20th century, genetic factors were correlated with human disease by linkage analysis (a method of mapping patterns of markers that congregate in relatively narrow regions of DNA in families with specific diseases), and candidate gene approaches, whereby genes were investigated on the basis of their postulated biology and of previous studies. These techniques were relatively low-yield and cumbersome; years of work uncovered only a handful of genes proven to be associated with diseases.
Newer tools can look at scores of genes linked to common diseases. Researchers now rely on sophisticated DNA sequencing tools and interpretation software to sift masses of data to find meaningful markers (DNA variants or mutations).
Genomics research in the past few years has been mostly hypothesis-independent. Investigators are no longer limited to the small cache of genes whose corresponding proteins are well characterized, but can instead probe the entire genome for connections between our DNA and our physiology.
The rise of genome-wide association studies
Over the past decade, much clinically useful information has been gathered in genome-wide association studies.
The rise of this type of study rested on our emerging understanding of the architecture of our genome. When the genomes of multiple humans were fully sequenced, we discovered that specific variants do not occur randomly in relation to each other. Rather, they tend to be inherited in particular blocks called haplotypes, and some SNPs or combinations of SNPS are very rare or essentially never seen.
In its first phase, the HapMap project organized these useful blocks of variants, genotyping 1 million SNPs for each of 270 individuals from mother-father-offspring trios from distinct geographic regions of the world.3 The second phase of the HapMap project extended the analysis to more than 3 million SNPs and to other populations.4
While the HapMap should be generally applicable to other populations not yet studied, limitations of the first two HapMap phases include rare SNPs or CNVs, or variants outside of haplotype regions.
The 1,000 Genomes Project, now under way, will develop an even more comprehensive catalog of human genetic variants in much broader populations.
The success of genome-wide association studies is also partly attributable to progress in DNA-sequencing technology. Using microarray chips, we can now look at millions of SNPs per patient or the entire coding sequence of the genome (termed the exome) in a single experiment that is both time-effecient and cost-effective.
What is a genome-wide association study?
A genome-wide association study generally compares genetic variants between patients with a particular clinical condition (cases) and people without the condition (controls), looking for statistically significant differences. As a tool for genetic discovery, these studies have revealed many avenues for further investigation in the pathogenesis of disease, as well as potential targets of therapy.
Using these studies, research groups around the world have found reproducible correlations between genetic variants and susceptibility to common adult-onset diseases.
Although many of the variants identified in these studies are associated with only a slightly higher risk of disease, the method is free of many of the inherent biases associated with clinical research. These studies permit a comprehensive, hypothesis-independent and unbiased scan of the genome to identify novel susceptibility factors, whereas earlier genetic epidemiology studies could take on only a handful of variables to evaluate at a time. Additionally, they are powered to detect very small increases (or decreases) in disease risk, previously outside the reach of linkage analysis. Polymorphisms (or, presumably, non-disease-causing DNA changes) discovered using these studies often correlate with clinical phenotypes or with levels of biomarkers, even if the genetic variants are not necessarily pathologic in themselves.
Thus, genome-wide association studies have led to important insights into the pathogenesis of multiple common diseases, such as inflammatory bowel disease and diabetes mellitus, and they are facilitating new treatment approaches. For instance, multiple studies have reproduced an association between Crohn disease and variation in the gene NOD2, whose protein product is implicated in bacterial product recognition, autophagy, and apoptosis.5 This discovery led to the investigation of new potential therapies for Crohn disease, ie, the tyrosine kinase inhibitors gefitinib (Iressa) and erlotinib (Tarceva), known to inhibit NOD2 activity, and to the prognostic use of the NOD2 genotype in Crohn disease (a field of study known as genotype-phenotype correlation).
Future advances will likely come from looking at combinations of variants, which may carry a higher risk of disease than single variants.
CORONARY HEART DISEASE: FRESH INSIGHT INTO AN OLD PROBLEM
Cardiovascular disease accounts for 30% of deaths worldwide.6 Of all the cardiovascular disorders, coronary heart disease is rising most rapidly in incidence, as the rest of the world adopts Western practices such as a high-calorie, high-fat, high-glycemic diet.
Hundreds of risk factors for coronary heart disease have been described.7 Three of them are clearly modifiable participants in the pathogenesis of atherosclerosis: hypertension, smoking, and elevated LDL-C. These and others form the basis for risk-assessment tools such as the Framingham risk score and the Prospective Cardiovascular Münster (PROCAM) study score. Other possible markers require further evaluation as to whether they are clinically useful and are direct mediators of coronary heart disease.
Because up to 40% of coronary deaths occur in people who lack conventional risk factors for it (eg, they do not smoke and they have normal levels of LDL-C and blood pressure), researchers are searching hard for new, potentially treatable risk factors.8 Of particular interest are components of inflammatory pathways linked with atherosclerosis and coronary heart disease. The identity of the key inflammatory factors that cause arterial plaque formation and rupture continues to be studied.
CRP, an acute-phase reactant produced by the liver in response to inflammation, has received much attention, as serum CRP levels correlate strongly with coronary events. Researchers have used modifiers of CRP to try to alter the course of coronary heart disease, but traditional research has so far failed to establish a causal relationship between CRP and coronary heart disease.9
How we know that LDL-C is a mediator, not just a marker
As a risk factor, LDL-C resembles CRP in that its levels correlate with a number of other, confounding risk factors. Therefore, much basic research and clinical observation had to be done before we could say that LDL-C plays a role in the pathogenesis of coronary heart disease.
Initially an association between LDL-C and heart disease was noted.10 Then, studies of familial hypercholesterolemia uncovered genetic abnormalities that increase LDL-C levels and, thereby, the risk of coronary heart disease—eg, mutations in the LDL receptor gene,11–14 the apolipoprotein B (APOB) gene at its LDL receptor-binding domain,15LDL-RAP1 (a gene encoding an accessory adaptor protein that interacts with the LDL receptor),16 and PCSK9 (a gene that codes for proprotein convertase subtilisin-kexin type 9 protease).17
Conversely, specific loss-of-function truncating mutations of PCSK9 that reduce LDL-C levels are associated with strong protection against coronary heart disease.18 Other gene mutations that reduce LDL-C also lower the risk.19,20
Further, a genome-wide association study21 identified multiple genetic variations associated with different forms of dyslipidemia, uncovering additional links between LDL-C and coronary heart disease.
Finally, randomized controlled trials of niacin, fibrates, and statins showed that these potent LDL-C-lowering agents reduce the rate of development or progression of coronary heart disease.22,23
C-reactive protein: Marker or mediator?
Unlike LDL-C, no familial syndromes of coronary heart disease have been recognized in patients who have isolated high serum levels of CRP.
Since many substances in addition to CRP increase in concentration in both acute and chronic inflammatory states, agents that lower CRP in a targeted manner would be needed for large prospective, randomized trials to show whether CRP plays a direct role in coronary heart disease. A specific CRP inhibitor, 1,6-bis(phosphocholine)-hexane, may aid in these efforts, although it is not orally bioavailable and has a very short serum half-life.24
The JUPITER trial. Statins lower levels of both LDL-C and CRP. The Justification for the Use of Statins in Primary Prevention: an Intervention Evaluating Rosuvastatin (JUPITER) trial was designed to find out whether statins alter coronary risk in patients with “normal” LDL-C levels (< 130 mg/dL) and elevated CRP levels (> 2 g/L).25
In this prospective, randomized trial, statin treatment resulted in a dramatic risk reduction of 40% to 50% in multiple coronary end points, as well as a reduction in CRP levels of 37% compared with placebo. However, LDL-C levels fell by 50%, confounding the effect on CRP, as the lower coronary event rate could alternatively be explained by the effect of lower-than-normal LDL-C levels. Thus, a causative link between CRP and coronary heart disease could not be proved.26
Though ongoing trials may further illuminate the role of inflammation in the development of coronary heart disease, and specific CRP inhibitors are in development, we have few tools to answer the fundamental question of whether CRP itself is an active participant in cardiovascular disease progression or if it is a bystander marker, helping to define risk for patients who develop coronary heart disease without other known risk factors.
Of note, adding CRP to the Framingham risk score does not improve its predictive power very much in any age group.27,28 Nevertheless, for certain end points, such as the long-term rate of death after percutaneous coronary intervention29 or of cardiovascular death immediately after coronary artery bypass grafting,30 CRP levels predict coronary events reliably.
BIOMARKERS AND MENDELIAN RANDOMIZATION
Further insight into the CRP-coronary association may lie in the genes. Intriguingly, while mutations have been found that alter the serum concentration of CRP, these isolated changes in CRP levels have not yet been shown to affect heart disease risk.9,31,32
If one were to design a prospective, interventional study to evaluate the role of CRP in coronary heart disease, it would be very difficult to tease apart the specific impact of CRP from that of other variables that are often present in people with high CRP, such as obesity and hyperlipidemia. The technique of mendelian randomization offers a way to evaluate the correlation between coronary heart disease development and CRP levels independent of other risk factors.
How many heart attacks in people with or without polymorphisms?
Mendelian randomization takes advantage of a basic genetic principle, ie, the independent assortment of traits. According to Mendel’s second law, alleles for different traits are inherited independently of one another. Therefore, the gene that encodes CRP and other genes that influence its circulating level are presumably inherited independently from other genes that influence coronary risk.
In typical studies of CRP, participants are grouped according to whether they have high or low CRP levels. In these studies, confounding variables congregate in these two groups. For example, people with high CRP may be more likely to smoke and to have a higher body mass index and higher lipid levels—all of which influence cardiovascular outcomes. It is therefore difficult to tease out the effect of CRP levels from other background risk factors.
In contrast, in studies using mendelian randomization, patients are grouped according to whether they have a variant that affects the substance being studied (eg, CRP), and outcomes are compared between the two genetic groups.
Strengths and limitations of this method
By randomizing research subjects by gene variants affecting CRP levels, it is theoretically possible to achieve more equal stratification and minimize confounding between subgroups.33
Mendelian randomization should also address the possibility of “reverse causality,” when the intermediate trait with a potential role in disease development (eg, CRP) is actually regulated by the disease state itself (ie, “inflammation of atherosclerotic cardiovascular disease”).34
A limitation of mendelian randomization is that different genes influencing the biomarker under investigation must be proven to be truly randomly assorted among populations. It cannot be assumed that levels of a biomarker are equally distributed across cases and controls when there may in fact be non-random genetic associations.
For instance, if SNPs in various genes that affect creatine kinase levels were being compared to cardiovascular outcome, it would be important to take into account that baseline creatine kinase levels are higher in African Americans as well as in men in interpreting the study data.35
THE ELLIOTT STUDY (2009)
In a study published in 2009, Elliott et al1 mined genome-wide data collected over the last decade to bring more clarity to the issue of causality between elevated CRP and heart disease.
To accomplish mendelian randomization, the authors assessed SNPs that affect circulating CRP levels in combined sets of 28,000 cases and 100,000 controls—robust population sizes. The SNP variants included were associated with approximately 20% lower CRP levels. This degree of CRP reduction should correspond to a 6% reduction in coronary risk as predicted by meta-analysis of observational studies.
No association between low-CRP variants and heart disease
The authors found significant associations between these SNPs and CRP levels and between CRP levels and coronary heart disease, but not between the SNPs and coronary disease when results for three SNPs were combined and standardized to a 20% lower CRP level (odds ratio 1.00, 95% confidence interval 0.97–1.02).1
In view of the lack of association between coronary heart disease and SNPs that affect CRP levels, the authors suggested that the observational data linking CRP levels and coronary disease may have been confounded by other risk factors, or that the trend is due to reverse causation (the inflammatory response associated with atherosclerosis elevates CRP) rather than CRP’s directly causing heart disease.
These findings have important implications for management of cardiovascular disease, as therapeutic strategies to reduce plasma CRP levels are less likely to be beneficial.
The authors also described other genetic variants that may affect coronary heart disease. Carriers of minor alleles of SNPs in the gene for the leptin receptor LEPR and the APOE-CI-CII cluster showed a significantly higher risk of coronary heart disease.1 However, both variants were associated with lower levels of CRP (and, for the SNP in LEPR, lower body weight and body mass index), suggesting that the links with coronary heart disease are not mediated by CRP. These findings illustrate the ability of genome-wide association studies to identify novel susceptibility loci for complex disease without limiting investigation to genes previously thought to take part in coronary heart disease.
In view of the evidence from this study, it seems that the benefits accruing to patients with high CRP from lipid-lowering therapy as demonstrated in the JUPITER trial are likely not the result of CRP-lowering per se, but rather are the result of action on the underlying pathology that leads to elevation of inflammatory markers, including CRP. As an editorial accompanying the study by Elliot et al pointed out, the work not only provides important information in the effort to identify genetic markers associated with complex disease, but it also helps discern the role of the genes and their products in the progress and treatment of common diseases.36
Subsequent studies of CRP and the “directionality” of its role in coronary disease,37 as well as in other conditions such as obesity and cancer,38,39 have carried on the strategy of Elliott et al, providing further evidence for the function of CRP as a bystander in the inflammatory response and complex disease progression.
IMPLICATIONS OF THESE FINDINGS
Tools now exist to leapfrog the randomized controlled trials that have been the primary way of examining the role of potential mediators of common diseases. Mendelian randomization aids in determining whether biomarkers are involved in disease pathogenesis, are simply bystanders, or are secondary markers caused by the disease itself. While randomized controlled trials will still be important, this new approach offers the power of evaluating much larger sample sizes and more equally stratifying confounding factors between study groups by relying on independent assortment of genetic traits.
In medical care today, the prevention of coronary heart disease entails aggressive treatment of hypertension and hyperlipidemia, along with lifestyle modifications such as balanced diet, routine exercise, and smoking cessation. Given the large numbers of patients at risk, even with low risk scores using currently identified risk factors, more specific and sensitive markers (or panels of such markers) of cardiovascular risk are needed.
In the personalized medicine of the future, we will rely on markers that not only identify people at higher risk, but also tell us who would benefit from certain therapies. From the JUPITER trial, we understand that patients with elevated CRP levels may be appropriate candidates for statin therapy even if they have normal levels of LDL-C.36 The study by Elliott et al steers us away from using CRP-affecting SNPs in predicting the course of disease and also from the belief that targeting CRP alone would be a worthwhile therapeutic strategy.
The inflammatory hypothesis of coronary heart disease remains a very important area of investigation, and CRP may turn out to be one of the best biomarkers we have to predict the progression of coronary diseases. But the study by Elliott et al demonstrates that CRP-lowering drugs are unlikely to be magic bullets.
Most importantly, geneticists will partner with clinical researchers to answer important questions about biomarkers and genes, capitalizing on large sets of population data.
- Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009; 302:37–48.
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953; 171:737–738.
- International HapMap Consortium. A haplotype map of the human genome. Nature 2005; 437:1299–1320.
- International HapMap Consortium; Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007; 449:851–861.
- Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411:599–603.
- Anderson GF, Chu E. Expanding priorities—confronting chronic disease in countries with low income. N Engl J Med 2007; 356:209–211.
- Hingorani AD, Shah T, Casas JP, Humphries SE, Talmud PJ. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem 2009; 55:239–255.
- Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol 2006; 97:28A–32A.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986; 256:2823–2838.
- Lehrman MA, Schneider WJ, Südhof TC, Brown MS, Goldstein JL, Russell DW. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 1985; 227:140–146.
- Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 1990; 24:133–170.
- Südhof TC, Goldstein JL, Brown MS, Russell DW. The LDL receptor gene: a mosaic of exons shared with different proteins. Science 1985; 228:815–822.
- Villéger L, Abifadel M, Allard D, et al. The UMD-LDLR database: additions to the software and 490 new entries to the database. Hum Mutat 2002; 20:81–87.
- Soria LF, Ludwig EH, Clarke HR, Vega GL, Grundy SM, McCarthy BJ. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc Natl Acad Sci U S A 1989; 86:587–591.
- Garcia CK, Wilund K, Arca M, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 2001; 292:1394–1398.
- Sun XM, Eden ER, Tosi I, et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet 2005; 14:1161–1169.
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Linsel-Nitschke P, Götz A, Erdmann J, et al; Wellcome Trust Case Control Consortium (WTCCC). Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—a Mendelian Randomisation study. PLoS One 2008; 3:e2986.
- Linsel-Nitschke P, Heeren J, Aherrahrou Z, et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis 2010; 208:183–189.
- Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41:56–65.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006; 440:1217–1221.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. Jupiter to earth: a statin helps people with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009; 38:217–231.
- Hamer M, Chida Y, Stamatakis E. Utility of C-reactive protein for cardiovascular risk stratification across three age groups in subjects without existing cardiovascular diseases. Am J Cardiol 2009; 104:538–542.
- Razzouk L, Muntner P, Bansilal S, et al. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J 2009; 158:277–283.
- Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive C-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesth 2009; 12:127–132.
- Hunter DJ, Altshuler D, Rader DJ. From Darwin’s finches to canaries in the coal mine—mining the genome for new biology. N Engl J Med 2008; 358:2760–2763.
- Lawlor DA, Harbord RM, Timpson NJ, et al. The association of C-reactive protein and CRP genotype with coronary heart disease: findings from five studies with 4,610 cases amongst 18,637 participants. PLoS One 2008; 3:e3011.
- Lange LA, Carlson CS, Hindorff LA, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 2006; 296:2703–2711.
- Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 2008; 5:e177.
- Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med 2009; 122:73–78.
- Shah SH, de Lemos JA. Biomarkers and cardiovascular disease: determining causality and quantifying contribution to risk assessment. JAMA 2009; 302:92–93.
- Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovasc Dis 2009; 19:521–524.
- Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 2010; 95:93–99.
- Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst 2010; 102:202–206.
- Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009; 302:37–48.
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953; 171:737–738.
- International HapMap Consortium. A haplotype map of the human genome. Nature 2005; 437:1299–1320.
- International HapMap Consortium; Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007; 449:851–861.
- Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411:599–603.
- Anderson GF, Chu E. Expanding priorities—confronting chronic disease in countries with low income. N Engl J Med 2007; 356:209–211.
- Hingorani AD, Shah T, Casas JP, Humphries SE, Talmud PJ. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem 2009; 55:239–255.
- Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol 2006; 97:28A–32A.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986; 256:2823–2838.
- Lehrman MA, Schneider WJ, Südhof TC, Brown MS, Goldstein JL, Russell DW. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 1985; 227:140–146.
- Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 1990; 24:133–170.
- Südhof TC, Goldstein JL, Brown MS, Russell DW. The LDL receptor gene: a mosaic of exons shared with different proteins. Science 1985; 228:815–822.
- Villéger L, Abifadel M, Allard D, et al. The UMD-LDLR database: additions to the software and 490 new entries to the database. Hum Mutat 2002; 20:81–87.
- Soria LF, Ludwig EH, Clarke HR, Vega GL, Grundy SM, McCarthy BJ. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc Natl Acad Sci U S A 1989; 86:587–591.
- Garcia CK, Wilund K, Arca M, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 2001; 292:1394–1398.
- Sun XM, Eden ER, Tosi I, et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet 2005; 14:1161–1169.
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Linsel-Nitschke P, Götz A, Erdmann J, et al; Wellcome Trust Case Control Consortium (WTCCC). Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—a Mendelian Randomisation study. PLoS One 2008; 3:e2986.
- Linsel-Nitschke P, Heeren J, Aherrahrou Z, et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis 2010; 208:183–189.
- Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41:56–65.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006; 440:1217–1221.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. Jupiter to earth: a statin helps people with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009; 38:217–231.
- Hamer M, Chida Y, Stamatakis E. Utility of C-reactive protein for cardiovascular risk stratification across three age groups in subjects without existing cardiovascular diseases. Am J Cardiol 2009; 104:538–542.
- Razzouk L, Muntner P, Bansilal S, et al. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J 2009; 158:277–283.
- Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive C-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: a prospective observational study. Ann Card Anaesth 2009; 12:127–132.
- Hunter DJ, Altshuler D, Rader DJ. From Darwin’s finches to canaries in the coal mine—mining the genome for new biology. N Engl J Med 2008; 358:2760–2763.
- Lawlor DA, Harbord RM, Timpson NJ, et al. The association of C-reactive protein and CRP genotype with coronary heart disease: findings from five studies with 4,610 cases amongst 18,637 participants. PLoS One 2008; 3:e3011.
- Lange LA, Carlson CS, Hindorff LA, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA 2006; 296:2703–2711.
- Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 2008; 5:e177.
- Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med 2009; 122:73–78.
- Shah SH, de Lemos JA. Biomarkers and cardiovascular disease: determining causality and quantifying contribution to risk assessment. JAMA 2009; 302:92–93.
- Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovasc Dis 2009; 19:521–524.
- Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 2010; 95:93–99.
- Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst 2010; 102:202–206.
KEY POINTS
- Genome-wide association studies can uncover associations between genetic markers and medical conditions, but they fall short of establishing causality or even clear biologic interactions between a genetic variant and a disease state.
- Mendelian randomization is a method for addressing the relationship between genetic variants and disease, ie, whether a biomarker affected by the variant is a cause of the disease or merely a bystander.
- CRP, an acute-phase reactant produced by the liver in response to inflammation, is one of many inflammatory markers whose levels correlate with coronary disease and which has been suggested to play a role in its pathogenesis.
- The findings of Elliott et al suggest that therapies that specifically lower CRP levels are not likely to affect coronary artery disease.
Correction: Presumed premature ventricular contractions
In the article “Presumed premature ventricular contractions” by Drs. Moises Auron and Donald Underwood (Cleve Clin J Med 2011; 78:812–813), Figure 1 was incorrectly labelled. The corrected figure and legend appear below. The authors wish to thank Philippe Akhrass, MD, from the State University of New York, Brooklyn, and Shahrokh Rafii, MD, from Brookdale University Hospital and Medical Center, Brooklyn, NY, for pointing out this error.
In the article “Presumed premature ventricular contractions” by Drs. Moises Auron and Donald Underwood (Cleve Clin J Med 2011; 78:812–813), Figure 1 was incorrectly labelled. The corrected figure and legend appear below. The authors wish to thank Philippe Akhrass, MD, from the State University of New York, Brooklyn, and Shahrokh Rafii, MD, from Brookdale University Hospital and Medical Center, Brooklyn, NY, for pointing out this error.

In the article “Presumed premature ventricular contractions” by Drs. Moises Auron and Donald Underwood (Cleve Clin J Med 2011; 78:812–813), Figure 1 was incorrectly labelled. The corrected figure and legend appear below. The authors wish to thank Philippe Akhrass, MD, from the State University of New York, Brooklyn, and Shahrokh Rafii, MD, from Brookdale University Hospital and Medical Center, Brooklyn, NY, for pointing out this error.

In reply: Cervical cancer screening
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
Dabigatran
To the Editor: In their response to a letter to the editor (December 2011), Drs. Wartak and Bartholomew suggested the use of recombinant activated factor VIIa (NovoSeven) for bleeding in patients on dabigatran. They based this recommendation on a review by Stangier and Clemens,1 which was based on phase II and III data on the efficacy and safety of dabigatran. There have been no controlled trials or prospective data on the use of this agent for this indication, nor are there data on its use in bleeding after intracranial hemorrhage, bleeding related to cardiac surgery, or trauma-related bleeding. In a systematic review, Yank et al2 found that there is no lower mortality rate and an increased risk of thromboembolism when activated factor VIIa is used off-label. This agent is approved for use only in patients with hemophilia, and in fact Novo Nordisk paid a $25 million settlement for off-label promotion of this drug for nonapproved indications.3 Recombinant factor VIIa costs up to $10,000 per vial, and if it is used off-label, that cost is not reimbursed to the hospital.
Just because we can do something does not mean that we should do it. The use of recombinant factor VIIa for dabigatran-related bleeding needs to be studied in a controlled trial before it is routinely used. As seen in the cited review, indication drift can lead to adverse patient outcomes and will certainly lead to financial peril in hospitals across the country.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011; 154:529–540.
- Silverman E. Novo Nordisk pays $25M for off-label marketing. Pharmalot. www.pharmalot.com/2011/06/novo-nordisk-pays-25m-for-off-label-marketing. Accessed February 9, 2012.
To the Editor: In their response to a letter to the editor (December 2011), Drs. Wartak and Bartholomew suggested the use of recombinant activated factor VIIa (NovoSeven) for bleeding in patients on dabigatran. They based this recommendation on a review by Stangier and Clemens,1 which was based on phase II and III data on the efficacy and safety of dabigatran. There have been no controlled trials or prospective data on the use of this agent for this indication, nor are there data on its use in bleeding after intracranial hemorrhage, bleeding related to cardiac surgery, or trauma-related bleeding. In a systematic review, Yank et al2 found that there is no lower mortality rate and an increased risk of thromboembolism when activated factor VIIa is used off-label. This agent is approved for use only in patients with hemophilia, and in fact Novo Nordisk paid a $25 million settlement for off-label promotion of this drug for nonapproved indications.3 Recombinant factor VIIa costs up to $10,000 per vial, and if it is used off-label, that cost is not reimbursed to the hospital.
Just because we can do something does not mean that we should do it. The use of recombinant factor VIIa for dabigatran-related bleeding needs to be studied in a controlled trial before it is routinely used. As seen in the cited review, indication drift can lead to adverse patient outcomes and will certainly lead to financial peril in hospitals across the country.
To the Editor: In their response to a letter to the editor (December 2011), Drs. Wartak and Bartholomew suggested the use of recombinant activated factor VIIa (NovoSeven) for bleeding in patients on dabigatran. They based this recommendation on a review by Stangier and Clemens,1 which was based on phase II and III data on the efficacy and safety of dabigatran. There have been no controlled trials or prospective data on the use of this agent for this indication, nor are there data on its use in bleeding after intracranial hemorrhage, bleeding related to cardiac surgery, or trauma-related bleeding. In a systematic review, Yank et al2 found that there is no lower mortality rate and an increased risk of thromboembolism when activated factor VIIa is used off-label. This agent is approved for use only in patients with hemophilia, and in fact Novo Nordisk paid a $25 million settlement for off-label promotion of this drug for nonapproved indications.3 Recombinant factor VIIa costs up to $10,000 per vial, and if it is used off-label, that cost is not reimbursed to the hospital.
Just because we can do something does not mean that we should do it. The use of recombinant factor VIIa for dabigatran-related bleeding needs to be studied in a controlled trial before it is routinely used. As seen in the cited review, indication drift can lead to adverse patient outcomes and will certainly lead to financial peril in hospitals across the country.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011; 154:529–540.
- Silverman E. Novo Nordisk pays $25M for off-label marketing. Pharmalot. www.pharmalot.com/2011/06/novo-nordisk-pays-25m-for-off-label-marketing. Accessed February 9, 2012.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011; 154:529–540.
- Silverman E. Novo Nordisk pays $25M for off-label marketing. Pharmalot. www.pharmalot.com/2011/06/novo-nordisk-pays-25m-for-off-label-marketing. Accessed February 9, 2012.
Prenatal Exposure to Valproate Is Associated With Increased Risk of Autism and Lower IQ
Researchers advise physicians to discuss the risks with women who have epilepsy and are of childbearing potential.
BALTIMORE—Fetal exposure to the antiepileptic drug (AED) valproate increases a child’s risk of autism and impairs his or her IQ until the age of 6, according to two studies presented at the 65th Annual Meeting of the American Epilepsy Society.
Children born to mothers on valproate monotherapy have a risk of childhood autism that is five times greater than that of children without prenatal exposure to the drug. In addition, children’s IQs are negatively associated with valproate dose, but not with carbamazepine, lamotrigine, or phenytoin.
Increased Risk of Autism
Previous research in animals and small studies involving humans have suggested that valproate treatment during pregnancy was associated with an increased risk of autism in the child.
To investigate this link, Jakob Christensen, PhD, a consultant neurologist at Aarhus University Hospital in Denmark, carried out a population-based cohort study. Using the Danish Civil Registration System, he identified 655,691 children born to 428,431 mothers between 1996 and 2006.
He looked to the Danish Prescription Register to identify women who had filled prescriptions for valproate from 30 days before the estimated date of conception to the day of birth.
Dr. Christensen also used the Danish Psychiatric Register to identify children diagnosed with an autism spectrum disorder and focused particularly on the subgroup that had been diagnosed with childhood autism. He and his colleagues then estimated the risk of autism in children born to mothers who used valproate during pregnancy and adjusted the risk estimates for parental psychiatric history, maternal age, and gender of the child.
Children born after prenatal exposure to valproate had more than twice the risk of an autism spectrum disorder than those without such exposure, according to the investigators. The risk of an autism spectrum disorder was 2.6 following valproate monotherapy and 2.5 following valproate polytherapy. The risk of childhood autism in children with prenatal exposure to valproate was 4.1 following valproate monotherapy and 6.8 following valproate polytherapy.
"Stopping any anticonvulsant medication poses a danger,” Dr. Christensen commented. “Women taking valproate who are contemplating pregnancy should consult with their doctors about the possibility of transitioning to another drug or reducing the dosage of their present medication when that isn’t possible.”
NeurodevelopmentalEffects of AEDs
Kimford Meador, MD, Director of the Emory Epilepsy Center and Professor of Neurology at Emory University in Atlanta, has directed the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study. His preliminary analysis indicated that fetal exposure to valproate impaired children’s IQ at age 3. Dr. Meador and his colleagues conducted a new analysis to determine whether valproate’s effects on IQ continued until age 6.
The NEAD study enrolled pregnant women with epilepsy on AED monotherapy from 1999 to 2004. It aims to examine the long-term neurodevelopmental effects of four common AEDs: carbamazepine, lamotrigine, phenytoin, and valproate. The primary outcome for Dr. Meador’s new analysis was IQ at age 6, as measured by the Differential Ability Scale (DAS). The team also performed a secondary analysis of verbal and nonverbal cluster scores from the DAS. The sample size was 310 children.
Dr. Meador found that child IQ was lower with valproate exposure than it was with exposure to any of the other AEDs. The adjusted mean IQ for children born after valproate exposure was 97. By contrast, the adjusted mean IQ for carbamazepine was 105, the adjusted mean IQ for lamotrigine was 108, and the adjusted mean IQ for phenytoin was 108. The verbal cluster score was less than the nonverbal cluster score for lamotrigine and valproate.
In children born after fetal exposure to valproate, the risks of low IQ and decreased verbal and nonverbal abilities are dose-dependent. However, “the dose-dependent effect is not seen with other AEDs on any of these factors,” said Dr. Meador.
Women of childbearing potential need to understand the risks that valproate entails, said Dr. Meador. “In my mind, it’s a very poor first choice for women of childbearing potential.
“The quandary is that there’s a subgroup of women with primary generalized epilepsy who will only respond to valproate,” he added. “Because it is not possible to predict which women are in this category, neurologists should treat them with another AED before trying valproate,” said Dr. Meador.
To hear an audiocast related to this news article, please click here.
Suggested Reading
Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
Sun Y, Vestergaard M, Christensen J, et al. Intake of marine n-3 fatty acids during pregnancy and risk for epilepsy in the offspring: a population-based cohort study. Epilepsy Res. 2010;91(2-3):267-272.
Researchers advise physicians to discuss the risks with women who have epilepsy and are of childbearing potential.
BALTIMORE—Fetal exposure to the antiepileptic drug (AED) valproate increases a child’s risk of autism and impairs his or her IQ until the age of 6, according to two studies presented at the 65th Annual Meeting of the American Epilepsy Society.
Children born to mothers on valproate monotherapy have a risk of childhood autism that is five times greater than that of children without prenatal exposure to the drug. In addition, children’s IQs are negatively associated with valproate dose, but not with carbamazepine, lamotrigine, or phenytoin.
Increased Risk of Autism
Previous research in animals and small studies involving humans have suggested that valproate treatment during pregnancy was associated with an increased risk of autism in the child.
To investigate this link, Jakob Christensen, PhD, a consultant neurologist at Aarhus University Hospital in Denmark, carried out a population-based cohort study. Using the Danish Civil Registration System, he identified 655,691 children born to 428,431 mothers between 1996 and 2006.
He looked to the Danish Prescription Register to identify women who had filled prescriptions for valproate from 30 days before the estimated date of conception to the day of birth.
Dr. Christensen also used the Danish Psychiatric Register to identify children diagnosed with an autism spectrum disorder and focused particularly on the subgroup that had been diagnosed with childhood autism. He and his colleagues then estimated the risk of autism in children born to mothers who used valproate during pregnancy and adjusted the risk estimates for parental psychiatric history, maternal age, and gender of the child.
Children born after prenatal exposure to valproate had more than twice the risk of an autism spectrum disorder than those without such exposure, according to the investigators. The risk of an autism spectrum disorder was 2.6 following valproate monotherapy and 2.5 following valproate polytherapy. The risk of childhood autism in children with prenatal exposure to valproate was 4.1 following valproate monotherapy and 6.8 following valproate polytherapy.
"Stopping any anticonvulsant medication poses a danger,” Dr. Christensen commented. “Women taking valproate who are contemplating pregnancy should consult with their doctors about the possibility of transitioning to another drug or reducing the dosage of their present medication when that isn’t possible.”
NeurodevelopmentalEffects of AEDs
Kimford Meador, MD, Director of the Emory Epilepsy Center and Professor of Neurology at Emory University in Atlanta, has directed the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study. His preliminary analysis indicated that fetal exposure to valproate impaired children’s IQ at age 3. Dr. Meador and his colleagues conducted a new analysis to determine whether valproate’s effects on IQ continued until age 6.
The NEAD study enrolled pregnant women with epilepsy on AED monotherapy from 1999 to 2004. It aims to examine the long-term neurodevelopmental effects of four common AEDs: carbamazepine, lamotrigine, phenytoin, and valproate. The primary outcome for Dr. Meador’s new analysis was IQ at age 6, as measured by the Differential Ability Scale (DAS). The team also performed a secondary analysis of verbal and nonverbal cluster scores from the DAS. The sample size was 310 children.
Dr. Meador found that child IQ was lower with valproate exposure than it was with exposure to any of the other AEDs. The adjusted mean IQ for children born after valproate exposure was 97. By contrast, the adjusted mean IQ for carbamazepine was 105, the adjusted mean IQ for lamotrigine was 108, and the adjusted mean IQ for phenytoin was 108. The verbal cluster score was less than the nonverbal cluster score for lamotrigine and valproate.
In children born after fetal exposure to valproate, the risks of low IQ and decreased verbal and nonverbal abilities are dose-dependent. However, “the dose-dependent effect is not seen with other AEDs on any of these factors,” said Dr. Meador.
Women of childbearing potential need to understand the risks that valproate entails, said Dr. Meador. “In my mind, it’s a very poor first choice for women of childbearing potential.
“The quandary is that there’s a subgroup of women with primary generalized epilepsy who will only respond to valproate,” he added. “Because it is not possible to predict which women are in this category, neurologists should treat them with another AED before trying valproate,” said Dr. Meador.
To hear an audiocast related to this news article, please click here.
Researchers advise physicians to discuss the risks with women who have epilepsy and are of childbearing potential.
BALTIMORE—Fetal exposure to the antiepileptic drug (AED) valproate increases a child’s risk of autism and impairs his or her IQ until the age of 6, according to two studies presented at the 65th Annual Meeting of the American Epilepsy Society.
Children born to mothers on valproate monotherapy have a risk of childhood autism that is five times greater than that of children without prenatal exposure to the drug. In addition, children’s IQs are negatively associated with valproate dose, but not with carbamazepine, lamotrigine, or phenytoin.
Increased Risk of Autism
Previous research in animals and small studies involving humans have suggested that valproate treatment during pregnancy was associated with an increased risk of autism in the child.
To investigate this link, Jakob Christensen, PhD, a consultant neurologist at Aarhus University Hospital in Denmark, carried out a population-based cohort study. Using the Danish Civil Registration System, he identified 655,691 children born to 428,431 mothers between 1996 and 2006.
He looked to the Danish Prescription Register to identify women who had filled prescriptions for valproate from 30 days before the estimated date of conception to the day of birth.
Dr. Christensen also used the Danish Psychiatric Register to identify children diagnosed with an autism spectrum disorder and focused particularly on the subgroup that had been diagnosed with childhood autism. He and his colleagues then estimated the risk of autism in children born to mothers who used valproate during pregnancy and adjusted the risk estimates for parental psychiatric history, maternal age, and gender of the child.
Children born after prenatal exposure to valproate had more than twice the risk of an autism spectrum disorder than those without such exposure, according to the investigators. The risk of an autism spectrum disorder was 2.6 following valproate monotherapy and 2.5 following valproate polytherapy. The risk of childhood autism in children with prenatal exposure to valproate was 4.1 following valproate monotherapy and 6.8 following valproate polytherapy.
"Stopping any anticonvulsant medication poses a danger,” Dr. Christensen commented. “Women taking valproate who are contemplating pregnancy should consult with their doctors about the possibility of transitioning to another drug or reducing the dosage of their present medication when that isn’t possible.”
NeurodevelopmentalEffects of AEDs
Kimford Meador, MD, Director of the Emory Epilepsy Center and Professor of Neurology at Emory University in Atlanta, has directed the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study. His preliminary analysis indicated that fetal exposure to valproate impaired children’s IQ at age 3. Dr. Meador and his colleagues conducted a new analysis to determine whether valproate’s effects on IQ continued until age 6.
The NEAD study enrolled pregnant women with epilepsy on AED monotherapy from 1999 to 2004. It aims to examine the long-term neurodevelopmental effects of four common AEDs: carbamazepine, lamotrigine, phenytoin, and valproate. The primary outcome for Dr. Meador’s new analysis was IQ at age 6, as measured by the Differential Ability Scale (DAS). The team also performed a secondary analysis of verbal and nonverbal cluster scores from the DAS. The sample size was 310 children.
Dr. Meador found that child IQ was lower with valproate exposure than it was with exposure to any of the other AEDs. The adjusted mean IQ for children born after valproate exposure was 97. By contrast, the adjusted mean IQ for carbamazepine was 105, the adjusted mean IQ for lamotrigine was 108, and the adjusted mean IQ for phenytoin was 108. The verbal cluster score was less than the nonverbal cluster score for lamotrigine and valproate.
In children born after fetal exposure to valproate, the risks of low IQ and decreased verbal and nonverbal abilities are dose-dependent. However, “the dose-dependent effect is not seen with other AEDs on any of these factors,” said Dr. Meador.
Women of childbearing potential need to understand the risks that valproate entails, said Dr. Meador. “In my mind, it’s a very poor first choice for women of childbearing potential.
“The quandary is that there’s a subgroup of women with primary generalized epilepsy who will only respond to valproate,” he added. “Because it is not possible to predict which women are in this category, neurologists should treat them with another AED before trying valproate,” said Dr. Meador.
To hear an audiocast related to this news article, please click here.
Suggested Reading
Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
Sun Y, Vestergaard M, Christensen J, et al. Intake of marine n-3 fatty acids during pregnancy and risk for epilepsy in the offspring: a population-based cohort study. Epilepsy Res. 2010;91(2-3):267-272.
Suggested Reading
Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
Sun Y, Vestergaard M, Christensen J, et al. Intake of marine n-3 fatty acids during pregnancy and risk for epilepsy in the offspring: a population-based cohort study. Epilepsy Res. 2010;91(2-3):267-272.
How Safe Is 3D TV for Children With Epilepsy?
When 150 children, 84 with epilepsy, were exposed to 15 minutes of three-dimensional TV, no seizures occurred—but some important EEG changes were observed.
BALTIMORE—Preliminary results from an ongoing study indicate that three-dimensional (3D) TV does not pose a significant safety hazard for children with epilepsy, researchers reported at the 65th Annual Meeting of the American Epilepsy Society.
The risk of suffering a seizure by watching 3D TV is very low, but not zero, and we think that it’s probably basically linked not to the 3D technique itself but to the content of the video,” said study coauthor Gerhard Kluger, MD, of Schön Klinik, Vogtareuth, Germany, and Paracelsus Medical University, Salzburg, Austria.
Although the electronics manufacturer Samsung has warned consumers that 3D TV may cause epileptic seizures, no previous research has investigated this possibility in children, especially in children with epilepsy, Dr. Kluger said. His study team, together with Herbert Plischke, MD, Managing Director of the Generation Research Program at the University of Munich, and other researchers, are doing so through a study that will eventually include at least 200 children with the condition.
The researchers are providing each study participant with two EEGs—a 20-minute, routine EEG that includes photo stimulation, and an EEG performed while the participant views 15 minutes of 3D video footage from the film Ice Age 3. Participants view this footage on a 50-inch 3D plasma TV while sitting about 2 meters away and wearing 3D shutter glasses.
The researchers have compiled data on 150 children and adolescents—84 with epilepsy and 66 with other neurologic conditions, such as headache, that suggested the risk of epilepsy. Participants had a mean age of 12, and 77 (51%) were male.
“We did not observe a single patient suffering a seizure during the 15 minutes” of 3D viewing, Dr. Kluger said. However, one participant with epilepsy, who typically experiences seizures four times per day, had a seizure two minutes following the viewing. “We cannot be sure it was linked to the 3D viewing, but we cannot exclude that a longer observation period might cause harm,” said Dr. Kluger.
In addition, three participants—all of whom were between the ages of 14 and 16 and had idiopathic generalized epilepsy—experienced a paroxysm increase of more than 100% during the 3D viewing. None of these adolescents made any complaints, showed any clinical signs, or experienced any subclinical seizures during the viewing.
“Interestingly, one boy with the diagnosis of ADHD (under treatment with methylphenidate) without a history of seizures showed a normal routine EEG with no epileptiform spikes but some spike during 3D viewing,” Dr. Kluger told Neurology Reviews.
It is likely that the observed paroxysm increases were triggered by non-3D content of the film, such as surprises and motion, according to Dr. Kluger. Laboratory time constraints necessitated the 15-minute period for 3D viewing and prevented researchers from comparing children’s reactions to 3D TV with their reactions to normal TV, he added. The study team is planning to perform research with a longer 3D viewing period and different video material in the future.
Most participants experienced a significant increase in lambda waves during the viewing period. The researchers attributed this increase to normal augmentation of saccadic eye movements.
Although 15 children with no history of seizures were photosensitive, as indicated by photoparoxysmal reactions during the pre-viewing EEG, no child showed any paroxysmal increase while viewing 3D. “3D seems to be no special problem for this population—at least with the 3D technology and the method we have used so far,” Dr. Kluger said.
In 10 patients with epilepsy, there was a reduction in the frequency of epileptiform activity during the 3D viewing. “This is not unusual, and it may be easily explained by an increase in alertness” during the viewing, Dr. Kluger noted. “Typically, if a child with epilepsy is alert, then he or she doesn’t seize so often.”
Seventeen percent of the children complained of dizziness, headache, or nausea. “That’s quite a lot,” Dr. Kluger said. “And my impression is that the older the children, the more likely they didn’t like the 3D.
“I think most of the children can go home and go to the video and watch it, but in some there’s doubt,” Dr. Kluger concluded. “Maybe when we study 200 children with epilepsy, we can answer these questions more precisely.”
Apart from the study, Dr. Kluger said, “I think we will continue offering this simulation for the parents, because they go home happy there was no reaction.” He also emphasized, “Children with epilepsy should do the same as children without epilepsy as much as possible.”
—Jack Baney
When 150 children, 84 with epilepsy, were exposed to 15 minutes of three-dimensional TV, no seizures occurred—but some important EEG changes were observed.
BALTIMORE—Preliminary results from an ongoing study indicate that three-dimensional (3D) TV does not pose a significant safety hazard for children with epilepsy, researchers reported at the 65th Annual Meeting of the American Epilepsy Society.
The risk of suffering a seizure by watching 3D TV is very low, but not zero, and we think that it’s probably basically linked not to the 3D technique itself but to the content of the video,” said study coauthor Gerhard Kluger, MD, of Schön Klinik, Vogtareuth, Germany, and Paracelsus Medical University, Salzburg, Austria.
Although the electronics manufacturer Samsung has warned consumers that 3D TV may cause epileptic seizures, no previous research has investigated this possibility in children, especially in children with epilepsy, Dr. Kluger said. His study team, together with Herbert Plischke, MD, Managing Director of the Generation Research Program at the University of Munich, and other researchers, are doing so through a study that will eventually include at least 200 children with the condition.
The researchers are providing each study participant with two EEGs—a 20-minute, routine EEG that includes photo stimulation, and an EEG performed while the participant views 15 minutes of 3D video footage from the film Ice Age 3. Participants view this footage on a 50-inch 3D plasma TV while sitting about 2 meters away and wearing 3D shutter glasses.
The researchers have compiled data on 150 children and adolescents—84 with epilepsy and 66 with other neurologic conditions, such as headache, that suggested the risk of epilepsy. Participants had a mean age of 12, and 77 (51%) were male.
“We did not observe a single patient suffering a seizure during the 15 minutes” of 3D viewing, Dr. Kluger said. However, one participant with epilepsy, who typically experiences seizures four times per day, had a seizure two minutes following the viewing. “We cannot be sure it was linked to the 3D viewing, but we cannot exclude that a longer observation period might cause harm,” said Dr. Kluger.
In addition, three participants—all of whom were between the ages of 14 and 16 and had idiopathic generalized epilepsy—experienced a paroxysm increase of more than 100% during the 3D viewing. None of these adolescents made any complaints, showed any clinical signs, or experienced any subclinical seizures during the viewing.
“Interestingly, one boy with the diagnosis of ADHD (under treatment with methylphenidate) without a history of seizures showed a normal routine EEG with no epileptiform spikes but some spike during 3D viewing,” Dr. Kluger told Neurology Reviews.
It is likely that the observed paroxysm increases were triggered by non-3D content of the film, such as surprises and motion, according to Dr. Kluger. Laboratory time constraints necessitated the 15-minute period for 3D viewing and prevented researchers from comparing children’s reactions to 3D TV with their reactions to normal TV, he added. The study team is planning to perform research with a longer 3D viewing period and different video material in the future.
Most participants experienced a significant increase in lambda waves during the viewing period. The researchers attributed this increase to normal augmentation of saccadic eye movements.
Although 15 children with no history of seizures were photosensitive, as indicated by photoparoxysmal reactions during the pre-viewing EEG, no child showed any paroxysmal increase while viewing 3D. “3D seems to be no special problem for this population—at least with the 3D technology and the method we have used so far,” Dr. Kluger said.
In 10 patients with epilepsy, there was a reduction in the frequency of epileptiform activity during the 3D viewing. “This is not unusual, and it may be easily explained by an increase in alertness” during the viewing, Dr. Kluger noted. “Typically, if a child with epilepsy is alert, then he or she doesn’t seize so often.”
Seventeen percent of the children complained of dizziness, headache, or nausea. “That’s quite a lot,” Dr. Kluger said. “And my impression is that the older the children, the more likely they didn’t like the 3D.
“I think most of the children can go home and go to the video and watch it, but in some there’s doubt,” Dr. Kluger concluded. “Maybe when we study 200 children with epilepsy, we can answer these questions more precisely.”
Apart from the study, Dr. Kluger said, “I think we will continue offering this simulation for the parents, because they go home happy there was no reaction.” He also emphasized, “Children with epilepsy should do the same as children without epilepsy as much as possible.”
—Jack Baney
When 150 children, 84 with epilepsy, were exposed to 15 minutes of three-dimensional TV, no seizures occurred—but some important EEG changes were observed.
BALTIMORE—Preliminary results from an ongoing study indicate that three-dimensional (3D) TV does not pose a significant safety hazard for children with epilepsy, researchers reported at the 65th Annual Meeting of the American Epilepsy Society.
The risk of suffering a seizure by watching 3D TV is very low, but not zero, and we think that it’s probably basically linked not to the 3D technique itself but to the content of the video,” said study coauthor Gerhard Kluger, MD, of Schön Klinik, Vogtareuth, Germany, and Paracelsus Medical University, Salzburg, Austria.
Although the electronics manufacturer Samsung has warned consumers that 3D TV may cause epileptic seizures, no previous research has investigated this possibility in children, especially in children with epilepsy, Dr. Kluger said. His study team, together with Herbert Plischke, MD, Managing Director of the Generation Research Program at the University of Munich, and other researchers, are doing so through a study that will eventually include at least 200 children with the condition.
The researchers are providing each study participant with two EEGs—a 20-minute, routine EEG that includes photo stimulation, and an EEG performed while the participant views 15 minutes of 3D video footage from the film Ice Age 3. Participants view this footage on a 50-inch 3D plasma TV while sitting about 2 meters away and wearing 3D shutter glasses.
The researchers have compiled data on 150 children and adolescents—84 with epilepsy and 66 with other neurologic conditions, such as headache, that suggested the risk of epilepsy. Participants had a mean age of 12, and 77 (51%) were male.
“We did not observe a single patient suffering a seizure during the 15 minutes” of 3D viewing, Dr. Kluger said. However, one participant with epilepsy, who typically experiences seizures four times per day, had a seizure two minutes following the viewing. “We cannot be sure it was linked to the 3D viewing, but we cannot exclude that a longer observation period might cause harm,” said Dr. Kluger.
In addition, three participants—all of whom were between the ages of 14 and 16 and had idiopathic generalized epilepsy—experienced a paroxysm increase of more than 100% during the 3D viewing. None of these adolescents made any complaints, showed any clinical signs, or experienced any subclinical seizures during the viewing.
“Interestingly, one boy with the diagnosis of ADHD (under treatment with methylphenidate) without a history of seizures showed a normal routine EEG with no epileptiform spikes but some spike during 3D viewing,” Dr. Kluger told Neurology Reviews.
It is likely that the observed paroxysm increases were triggered by non-3D content of the film, such as surprises and motion, according to Dr. Kluger. Laboratory time constraints necessitated the 15-minute period for 3D viewing and prevented researchers from comparing children’s reactions to 3D TV with their reactions to normal TV, he added. The study team is planning to perform research with a longer 3D viewing period and different video material in the future.
Most participants experienced a significant increase in lambda waves during the viewing period. The researchers attributed this increase to normal augmentation of saccadic eye movements.
Although 15 children with no history of seizures were photosensitive, as indicated by photoparoxysmal reactions during the pre-viewing EEG, no child showed any paroxysmal increase while viewing 3D. “3D seems to be no special problem for this population—at least with the 3D technology and the method we have used so far,” Dr. Kluger said.
In 10 patients with epilepsy, there was a reduction in the frequency of epileptiform activity during the 3D viewing. “This is not unusual, and it may be easily explained by an increase in alertness” during the viewing, Dr. Kluger noted. “Typically, if a child with epilepsy is alert, then he or she doesn’t seize so often.”
Seventeen percent of the children complained of dizziness, headache, or nausea. “That’s quite a lot,” Dr. Kluger said. “And my impression is that the older the children, the more likely they didn’t like the 3D.
“I think most of the children can go home and go to the video and watch it, but in some there’s doubt,” Dr. Kluger concluded. “Maybe when we study 200 children with epilepsy, we can answer these questions more precisely.”
Apart from the study, Dr. Kluger said, “I think we will continue offering this simulation for the parents, because they go home happy there was no reaction.” He also emphasized, “Children with epilepsy should do the same as children without epilepsy as much as possible.”
—Jack Baney
New and Noteworthy Information for March 2012
Men show higher rates of amnestic mild cognitive impairment (aMCI) and nonamnestic mild cognitive impairment (naMCI) than women, researchers reported in the January 31 Neurology. Starting in 2004, a cohort of Olmsted County, Minnesota, residents ages 70 to 89 underwent baseline and 15-month interval evaluations to assess their cognitive status. Of the 1,450 participants who were cognitively normal at baseline, 296 developed MCI, with an age- and sex-standardized incidence rate of 63.6 (per 1,000 person years) overall. Men (72.4) showed higher rates of MCI than women (57.3), and this trend continued for both aMCI and naMCI. Participants with fewer years of education had higher rates of MCI. “Differences in incidence rates by clinical subtype and by sex suggest that risk factors for MCI should be investigated separately for aMCI and naMCI, and in men and women,” the study authors stated.
Infants who are eventually diagnosed with autism spectrum disorders (ASD) show abnormal development of white matter pathways starting as early as 6 months of age, according to a study published in the February 17 online American Journal of Psychiatry. Researchers analyzed imaging results from 92 high-risk infants who had siblings with autism. The participants underwent diffusion tensor imaging at 6 months, 12 months, and 24 months. After characterizing the white matter fiber tracts of the 28 infants who met criteria for ASD at 24 months and the 64 infants who did not meet the criteria, the investigators found notable differences in the infants’ development. Compared with the 64 infants who did not develop ASD, infants who had ASD had different white matter development for 12 of the 15 brain pathways studied. “These results suggest that aberrant development of white matter pathways may precede the manifestation of autistic symptoms in the first year of life,” the researchers concluded.
Epilepsy surgery has long-term beneficial effects on patients’ seizure control and overall quality of life, according to research published in the February 7 online Epilepsia. Patients who underwent epilepsy surgery by Sidney Goldring, MD, from 1967 to 1990 were followed up for a mean duration of 26 years. Of the 361 patients who had epilepsy surgery, 117 completed follow-up interviews, and 80% reported a higher quality of life on the Quality of Life in Epilepsy (QOLIE-31) questionnaire after surgery. In addition, an association was observed between seizure freedom and better quality of life, and patients who underwent temporal lobe resection showed better seizure outcomes than those who had different procedures. Considering that the positive outcomes of epilepsy surgery from decades ago seem sustainable, the researchers said they are “optimistic that the outcomes from modern epilepsy surgery will be even better and that our present enthusiasm for this treatment modality is not misplaced.”
Elderly nursing home residents show an increased mortality risk with higher doses of antipsychotic drugs, according to a study in the February 23 online BMJ. All 75,445 study participants were age 65 or older, lived in nursing homes from 2001 to 2005, and were new users of antipsychotic drugs (haloperidol, aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone). After comparing 180-day risks of all-cause and cause-specific mortality by individual drug, the researchers found that users of haloperidol had an increased rate of mortality and users of quetiapine had a decreased risk of mortality, compared with users of risperidone. These effects were seen for all causes of mortality examined, remained after adjustment for dose, and were strongest immediately following the start of treatment. “There was no evidence that the treatment effect differed for patients with a diagnosis of dementia or behavioral disturbances,” noted the researchers. They added that although their findings do not prove causality, “….they provide more evidence of the risk of using these drugs in older patients, reinforcing the concept that they should not be used in the absence of clear need.”
—Lauren LeBano
Men show higher rates of amnestic mild cognitive impairment (aMCI) and nonamnestic mild cognitive impairment (naMCI) than women, researchers reported in the January 31 Neurology. Starting in 2004, a cohort of Olmsted County, Minnesota, residents ages 70 to 89 underwent baseline and 15-month interval evaluations to assess their cognitive status. Of the 1,450 participants who were cognitively normal at baseline, 296 developed MCI, with an age- and sex-standardized incidence rate of 63.6 (per 1,000 person years) overall. Men (72.4) showed higher rates of MCI than women (57.3), and this trend continued for both aMCI and naMCI. Participants with fewer years of education had higher rates of MCI. “Differences in incidence rates by clinical subtype and by sex suggest that risk factors for MCI should be investigated separately for aMCI and naMCI, and in men and women,” the study authors stated.
Infants who are eventually diagnosed with autism spectrum disorders (ASD) show abnormal development of white matter pathways starting as early as 6 months of age, according to a study published in the February 17 online American Journal of Psychiatry. Researchers analyzed imaging results from 92 high-risk infants who had siblings with autism. The participants underwent diffusion tensor imaging at 6 months, 12 months, and 24 months. After characterizing the white matter fiber tracts of the 28 infants who met criteria for ASD at 24 months and the 64 infants who did not meet the criteria, the investigators found notable differences in the infants’ development. Compared with the 64 infants who did not develop ASD, infants who had ASD had different white matter development for 12 of the 15 brain pathways studied. “These results suggest that aberrant development of white matter pathways may precede the manifestation of autistic symptoms in the first year of life,” the researchers concluded.
Epilepsy surgery has long-term beneficial effects on patients’ seizure control and overall quality of life, according to research published in the February 7 online Epilepsia. Patients who underwent epilepsy surgery by Sidney Goldring, MD, from 1967 to 1990 were followed up for a mean duration of 26 years. Of the 361 patients who had epilepsy surgery, 117 completed follow-up interviews, and 80% reported a higher quality of life on the Quality of Life in Epilepsy (QOLIE-31) questionnaire after surgery. In addition, an association was observed between seizure freedom and better quality of life, and patients who underwent temporal lobe resection showed better seizure outcomes than those who had different procedures. Considering that the positive outcomes of epilepsy surgery from decades ago seem sustainable, the researchers said they are “optimistic that the outcomes from modern epilepsy surgery will be even better and that our present enthusiasm for this treatment modality is not misplaced.”
Elderly nursing home residents show an increased mortality risk with higher doses of antipsychotic drugs, according to a study in the February 23 online BMJ. All 75,445 study participants were age 65 or older, lived in nursing homes from 2001 to 2005, and were new users of antipsychotic drugs (haloperidol, aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone). After comparing 180-day risks of all-cause and cause-specific mortality by individual drug, the researchers found that users of haloperidol had an increased rate of mortality and users of quetiapine had a decreased risk of mortality, compared with users of risperidone. These effects were seen for all causes of mortality examined, remained after adjustment for dose, and were strongest immediately following the start of treatment. “There was no evidence that the treatment effect differed for patients with a diagnosis of dementia or behavioral disturbances,” noted the researchers. They added that although their findings do not prove causality, “….they provide more evidence of the risk of using these drugs in older patients, reinforcing the concept that they should not be used in the absence of clear need.”
—Lauren LeBano
Men show higher rates of amnestic mild cognitive impairment (aMCI) and nonamnestic mild cognitive impairment (naMCI) than women, researchers reported in the January 31 Neurology. Starting in 2004, a cohort of Olmsted County, Minnesota, residents ages 70 to 89 underwent baseline and 15-month interval evaluations to assess their cognitive status. Of the 1,450 participants who were cognitively normal at baseline, 296 developed MCI, with an age- and sex-standardized incidence rate of 63.6 (per 1,000 person years) overall. Men (72.4) showed higher rates of MCI than women (57.3), and this trend continued for both aMCI and naMCI. Participants with fewer years of education had higher rates of MCI. “Differences in incidence rates by clinical subtype and by sex suggest that risk factors for MCI should be investigated separately for aMCI and naMCI, and in men and women,” the study authors stated.
Infants who are eventually diagnosed with autism spectrum disorders (ASD) show abnormal development of white matter pathways starting as early as 6 months of age, according to a study published in the February 17 online American Journal of Psychiatry. Researchers analyzed imaging results from 92 high-risk infants who had siblings with autism. The participants underwent diffusion tensor imaging at 6 months, 12 months, and 24 months. After characterizing the white matter fiber tracts of the 28 infants who met criteria for ASD at 24 months and the 64 infants who did not meet the criteria, the investigators found notable differences in the infants’ development. Compared with the 64 infants who did not develop ASD, infants who had ASD had different white matter development for 12 of the 15 brain pathways studied. “These results suggest that aberrant development of white matter pathways may precede the manifestation of autistic symptoms in the first year of life,” the researchers concluded.
Epilepsy surgery has long-term beneficial effects on patients’ seizure control and overall quality of life, according to research published in the February 7 online Epilepsia. Patients who underwent epilepsy surgery by Sidney Goldring, MD, from 1967 to 1990 were followed up for a mean duration of 26 years. Of the 361 patients who had epilepsy surgery, 117 completed follow-up interviews, and 80% reported a higher quality of life on the Quality of Life in Epilepsy (QOLIE-31) questionnaire after surgery. In addition, an association was observed between seizure freedom and better quality of life, and patients who underwent temporal lobe resection showed better seizure outcomes than those who had different procedures. Considering that the positive outcomes of epilepsy surgery from decades ago seem sustainable, the researchers said they are “optimistic that the outcomes from modern epilepsy surgery will be even better and that our present enthusiasm for this treatment modality is not misplaced.”
Elderly nursing home residents show an increased mortality risk with higher doses of antipsychotic drugs, according to a study in the February 23 online BMJ. All 75,445 study participants were age 65 or older, lived in nursing homes from 2001 to 2005, and were new users of antipsychotic drugs (haloperidol, aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone). After comparing 180-day risks of all-cause and cause-specific mortality by individual drug, the researchers found that users of haloperidol had an increased rate of mortality and users of quetiapine had a decreased risk of mortality, compared with users of risperidone. These effects were seen for all causes of mortality examined, remained after adjustment for dose, and were strongest immediately following the start of treatment. “There was no evidence that the treatment effect differed for patients with a diagnosis of dementia or behavioral disturbances,” noted the researchers. They added that although their findings do not prove causality, “….they provide more evidence of the risk of using these drugs in older patients, reinforcing the concept that they should not be used in the absence of clear need.”
—Lauren LeBano
Photoparoxysmal Response Is High Among Teenagers With Autism and Epilepsy
A recent finding may indicate brain irritability or hypersensitivity in children with autism spectrum disorders.
BALTIMORE—The incidence of a photoparoxysmal response among children older than 15 with autism spectrum disorders (ASD) is 25%, which is much higher than that for younger children with ASD, according to a study presented at the 65th Annual Meeting of the American Epilepsy Society. When children in the sample population without epilepsy are excluded, the incidence of a photoparoxysmal response among children older than 15 with ASD is 29.4%.
Epilepsy and frequent interictal discharges are common among children with ASD. Photoparoxysmal responses are interictal EEG discharges correlated with generalized, photosensitive epilepsy. A flashing strobe light, for example, can trigger a photoparoxysmal response in a predisposed individual. “The brain picks up that stimulus through the eye, and for some children, it can cause an abnormal brainwave pattern,” explained Jill Miller-Horn, MD, an epilepsy fellow at Children’s Hospital Boston.
“We already know that abnormal brainwave responses to flashing light can be associated with epilepsy. You might be familiar with what happened in Japan with the Pokemon cartoon, where there was bright, flashing colored light on the screen and hundreds of children then had seizures,” she added. Dr. Miller-Horn and her colleagues decided to study photoparoxysmal responses to photic stimulation in children with ASD, a topic that she said no researchers had previously investigated.
Identifying a Cohort of Children With ASD
The team conducted a retrospective pilot study of children with ASD to determine the rate of the photoparoxysmal response caused by intermittent photic stimulation during EEG studies at Children’s Hospital Boston. The investigators searched medical records that identified 333 children with ASD who were treated at the hospital between December 2010 and May 2011.
Of these children, 206 had had EEGs. In the group of 206 children, 118 had comorbid ASD and epilepsy, and 88 had ASD without seizures. Intermittent photic stimulation was part of 177 children’s EEG studies. The group of 177 included 138 boys and 39 girls, and the children’s average age was 9.
Photoparoxysmal Response Increases in Adolescence
A photoparoxysmal response was elicited in 13 of the 177 children who received photic stimulation during their EEGs. The 7.3% incidence of a photoparoxysmal response in children with ASD was within the range previously reported in the normal population, according to Dr. Miller-Horn. “Our study found that in the ASD population, there is an association between the photoparoxysmal response and epilepsy, as has been previously reported in children with epilepsy without ASD,” she said.
“When we subdivided these children with autism by age, we found that there’s an increase in the photoparoxysmal response as they entered adolescence,” commented Dr. Miller-Horn. The meaning of the result is unclear, “but it’s a difference from the normal population, and it’s a difference from other children who have epilepsy,” she added.
“This is a new finding that may be a clue to the pathophysiology for the high rate of ASD and epilepsy comorbidity,” Dr. Miller-Horn continued. “There may be irritability or hypersensitivity in the brain for children with autism that’s being revealed with the clue that they are more photosensitive.”
Large-scale and prospective studies are needed to confirm the trend, according to the investigators. Further studies could reveal the findings’ significance in the pathophysiology of epilepsy in children with ASD.
—Erik Greb
Suggested Reading
Lo C, Shorvon S, Davis M, et al. Genetic linkage analysis of a large family with photoparoxysmal response. Epilepsy Res. 2011 Nov 7; [Epub ahead of print]
Lopes da Silva FH, Harding GF. Transition to seizure in photosensitive epilepsy. Epilepsy Res. 2011;97(3):278-282.
A recent finding may indicate brain irritability or hypersensitivity in children with autism spectrum disorders.
BALTIMORE—The incidence of a photoparoxysmal response among children older than 15 with autism spectrum disorders (ASD) is 25%, which is much higher than that for younger children with ASD, according to a study presented at the 65th Annual Meeting of the American Epilepsy Society. When children in the sample population without epilepsy are excluded, the incidence of a photoparoxysmal response among children older than 15 with ASD is 29.4%.
Epilepsy and frequent interictal discharges are common among children with ASD. Photoparoxysmal responses are interictal EEG discharges correlated with generalized, photosensitive epilepsy. A flashing strobe light, for example, can trigger a photoparoxysmal response in a predisposed individual. “The brain picks up that stimulus through the eye, and for some children, it can cause an abnormal brainwave pattern,” explained Jill Miller-Horn, MD, an epilepsy fellow at Children’s Hospital Boston.
“We already know that abnormal brainwave responses to flashing light can be associated with epilepsy. You might be familiar with what happened in Japan with the Pokemon cartoon, where there was bright, flashing colored light on the screen and hundreds of children then had seizures,” she added. Dr. Miller-Horn and her colleagues decided to study photoparoxysmal responses to photic stimulation in children with ASD, a topic that she said no researchers had previously investigated.
Identifying a Cohort of Children With ASD
The team conducted a retrospective pilot study of children with ASD to determine the rate of the photoparoxysmal response caused by intermittent photic stimulation during EEG studies at Children’s Hospital Boston. The investigators searched medical records that identified 333 children with ASD who were treated at the hospital between December 2010 and May 2011.
Of these children, 206 had had EEGs. In the group of 206 children, 118 had comorbid ASD and epilepsy, and 88 had ASD without seizures. Intermittent photic stimulation was part of 177 children’s EEG studies. The group of 177 included 138 boys and 39 girls, and the children’s average age was 9.
Photoparoxysmal Response Increases in Adolescence
A photoparoxysmal response was elicited in 13 of the 177 children who received photic stimulation during their EEGs. The 7.3% incidence of a photoparoxysmal response in children with ASD was within the range previously reported in the normal population, according to Dr. Miller-Horn. “Our study found that in the ASD population, there is an association between the photoparoxysmal response and epilepsy, as has been previously reported in children with epilepsy without ASD,” she said.
“When we subdivided these children with autism by age, we found that there’s an increase in the photoparoxysmal response as they entered adolescence,” commented Dr. Miller-Horn. The meaning of the result is unclear, “but it’s a difference from the normal population, and it’s a difference from other children who have epilepsy,” she added.
“This is a new finding that may be a clue to the pathophysiology for the high rate of ASD and epilepsy comorbidity,” Dr. Miller-Horn continued. “There may be irritability or hypersensitivity in the brain for children with autism that’s being revealed with the clue that they are more photosensitive.”
Large-scale and prospective studies are needed to confirm the trend, according to the investigators. Further studies could reveal the findings’ significance in the pathophysiology of epilepsy in children with ASD.
—Erik Greb
A recent finding may indicate brain irritability or hypersensitivity in children with autism spectrum disorders.
BALTIMORE—The incidence of a photoparoxysmal response among children older than 15 with autism spectrum disorders (ASD) is 25%, which is much higher than that for younger children with ASD, according to a study presented at the 65th Annual Meeting of the American Epilepsy Society. When children in the sample population without epilepsy are excluded, the incidence of a photoparoxysmal response among children older than 15 with ASD is 29.4%.
Epilepsy and frequent interictal discharges are common among children with ASD. Photoparoxysmal responses are interictal EEG discharges correlated with generalized, photosensitive epilepsy. A flashing strobe light, for example, can trigger a photoparoxysmal response in a predisposed individual. “The brain picks up that stimulus through the eye, and for some children, it can cause an abnormal brainwave pattern,” explained Jill Miller-Horn, MD, an epilepsy fellow at Children’s Hospital Boston.
“We already know that abnormal brainwave responses to flashing light can be associated with epilepsy. You might be familiar with what happened in Japan with the Pokemon cartoon, where there was bright, flashing colored light on the screen and hundreds of children then had seizures,” she added. Dr. Miller-Horn and her colleagues decided to study photoparoxysmal responses to photic stimulation in children with ASD, a topic that she said no researchers had previously investigated.
Identifying a Cohort of Children With ASD
The team conducted a retrospective pilot study of children with ASD to determine the rate of the photoparoxysmal response caused by intermittent photic stimulation during EEG studies at Children’s Hospital Boston. The investigators searched medical records that identified 333 children with ASD who were treated at the hospital between December 2010 and May 2011.
Of these children, 206 had had EEGs. In the group of 206 children, 118 had comorbid ASD and epilepsy, and 88 had ASD without seizures. Intermittent photic stimulation was part of 177 children’s EEG studies. The group of 177 included 138 boys and 39 girls, and the children’s average age was 9.
Photoparoxysmal Response Increases in Adolescence
A photoparoxysmal response was elicited in 13 of the 177 children who received photic stimulation during their EEGs. The 7.3% incidence of a photoparoxysmal response in children with ASD was within the range previously reported in the normal population, according to Dr. Miller-Horn. “Our study found that in the ASD population, there is an association between the photoparoxysmal response and epilepsy, as has been previously reported in children with epilepsy without ASD,” she said.
“When we subdivided these children with autism by age, we found that there’s an increase in the photoparoxysmal response as they entered adolescence,” commented Dr. Miller-Horn. The meaning of the result is unclear, “but it’s a difference from the normal population, and it’s a difference from other children who have epilepsy,” she added.
“This is a new finding that may be a clue to the pathophysiology for the high rate of ASD and epilepsy comorbidity,” Dr. Miller-Horn continued. “There may be irritability or hypersensitivity in the brain for children with autism that’s being revealed with the clue that they are more photosensitive.”
Large-scale and prospective studies are needed to confirm the trend, according to the investigators. Further studies could reveal the findings’ significance in the pathophysiology of epilepsy in children with ASD.
—Erik Greb
Suggested Reading
Lo C, Shorvon S, Davis M, et al. Genetic linkage analysis of a large family with photoparoxysmal response. Epilepsy Res. 2011 Nov 7; [Epub ahead of print]
Lopes da Silva FH, Harding GF. Transition to seizure in photosensitive epilepsy. Epilepsy Res. 2011;97(3):278-282.
Suggested Reading
Lo C, Shorvon S, Davis M, et al. Genetic linkage analysis of a large family with photoparoxysmal response. Epilepsy Res. 2011 Nov 7; [Epub ahead of print]
Lopes da Silva FH, Harding GF. Transition to seizure in photosensitive epilepsy. Epilepsy Res. 2011;97(3):278-282.
Grand Rounds: Man, 61, With Painful Oral Ulcerations
A 61-year-old man, who had recently emigrated from the Ukraine, presented to his primary care provider with a chief complaint of painful oral lesions and weight loss. The patient described the gradual onset of a severe sore throat and mouth pain three months earlier. Originally, he attributed his symptoms to an upper respiratory infection but became concerned when his symptoms did not resolve.
He reported that the pain had worsened over time and that he was now barely able to swallow solid food or tolerate acidic beverages due to considerable discomfort. His son, who accompanied him to the appointment, had also noted weight loss.
The patient denied any concomitant symptoms, including fever, cough, night sweats, fatigue, lymphadenopathy, abdominal pain, diarrhea, melena, or concomitant rash. His medical history was remarkable only for stage 1 hypertension, which had been well controlled on hydrochlorothiazide 12.5 mg/d for the previous three years. However, the patient had received only minimal preventive health care while living in the Ukraine. His family history was unknown.
One week earlier, the patient had seen a dentist complaining of mouth pain, and was referred to an oral medicine specialist; this specialist, in turn, referred the patient to a primary care nurse practitioner for lab work to confirm the suspected diagnosis of pemphigus vulgaris.
On physical examination, the patient appeared older than his stated age. He was a thin, mildly ill–appearing man, afebrile and normotensive, with heart rate and respirations within normal limits. However, intraoral examination revealed multiple oropharyngeal ulcerations of varying size on a base of erythematous and swollen mucosa on the inside of the man’s cheek and palatal and buccal mucosa (see Figure 1). On his upper back, two round, crusted blisters were noted in isolation (Figure 2). The remaining findings in the physical examination were unremarkable.
Based on the patient’s physical exam findings and clinical guideline recommendations regarding chronic oral ulcerations of unknown etiology,1,2 the patient was scheduled for a cytologic smear to be performed by oral medicine, followed by a gingival biopsy for a direct immunofluorescence test and routine histopathology.3 Unfortunately, due to extensive involvement and concern for possible mucosal shredding, an oral biopsy was not deemed possible.
However, the oral medicine specialist, because he strongly suspected pemphigus vulgaris, recommended testing for circulating autoantibodies against the antigens desmogleins 1 and/or 3 in the epidermis, which are responsible for cellular adhesion. (A positive test result supports, but does not confirm, a diagnosis of pemphigus vulgaris.4)
Additionally, baseline labs were performed for signs of systemic illness, including infection, anemia, and liver and kidney disease. Frequent monitoring was conducted for steroid-induced symptoms of elevated blood sugars; the primary care provider was responsible for monitoring the patient for weight gain and steroid-induced psychosis. The patient was referred to gastroenterology for a colonoscopy to ensure that his weight loss and anorexia were not the result of gastrointestinal malignancy. However, the patient declined this test.
DISCUSSION
Painful oral lesions can have numerous etiologies of varying severity and complexity, including herpes simplex virus infection, aphthae, lichen planus, erythema multiforme, squamous cell and other oral carcinomas, primary HIV infection, lupus, and pemphigus. Differentiating among these conditions requires a careful medical history and complete physical exam.5
Pemphigus vulgaris (PV) is the most common variant of pemphigus, a group of chronic autoimmune diseases that cause blistering and ulceration of the mucous membranes and the skin.6 From the Greek pemphix (bubble), PV is more common in people of Ashkenazi Jewish or Mediterranean descent,6,7 usually occurs in middle-aged and older persons, and occurs about 1.5 times more commonly in women than men.5,7 Until the introduction of systemic steroids, pemphigus was often a fatal disease. Significant mortality still exists, mainly as a result of infection or adverse reactions to medication therapy.5
In patients with PV, flaccid bullae are formed on the skin in a process called acantholysis, in which epidermal cells lose their ability to adhere to one another. This results in rapidly expanding, thin-walled blisters on the oral mucosa, scalp, face, axillae, and groin. The blisters burst easily, leaving irregularly shaped, painful ulcerations.4 Painful oral mucosal membrane erosions are the first presenting sign of PV and often the only sign for an average of five months before other skin lesions develop.3 These lesions are noninfectious.
To make a definitive diagnosis of PV, clinical lesions must be present, with a confirmation of histologic findings, acantholysis on biopsy, and a confirmation of autoantibodies present in tissue and/or serum.4 (For proposed detailed diagnostic criteria, see table4,8.)
Initial misdiagnoses, which often lead to delayed or incorrect treatment, usually include aphthous stomatitis, gingivostomatitis, erythema multiforme, erosive lichen planus, herpes simplex virus, and/or oral candidiasis.3
Common Differentials
Herpes simplex virus. Affecting between 15% and 45% of the population, herpes simplex virus (HSV) infection, also known as cold sores, is the most common cause of recurrent oral ulcers.9 HSV is transmitted through direct contact with lesions or via viral shedding. Primary infection, which may occur with flu-like symptoms, causes the sudden onset of multiple clustered vesicles on an erythematous base that quickly ulcerate and crust. Recurrent infections tend to be less severe and are accompanied by minimal systemic symptoms.10
Diagnosis is usually made through history and physical exam. However, diagnostic tests, including Tzanck smears, biopsy, polymerase chain reaction (PCR) assay, and/or viral isolation in culture, are sometimes used to confirm a suspected case.10
Oral lichen planus (OLP). This is a common, chronic, mucocutaneous inflammatory disease of unknown etiology that affects skin and mucous membranes of the mouth, including the buccal mucosa, tongue, and/or gums. These lesions are noninfectious and are an immunologically mediated disease. Stress, anxiety, genetic predisposition, NSAID use, antihypertensive medications (eg, captopril, enalapril, propranolol; considered an oral lichenoid drug reaction), and altered cell-mediated immune response have been considered possible causative factors.11,12 Recent reports suggest an association between hepatitis C virus and OLP.13
Affecting about 4% of the general population, and more predominate in perimenopausal women, OLP lesions appear as white, lacey patches; red, swollen tissues; or open sores, most commonly on the inside of the mouth bilaterally. Patients will present with complaints of burning, roughness, or pain in the mouth, dry mouth, sensitivity to hot or spicy foods, and difficulty swallowing if the throat is involved. Diagnosis is based on history and physical examination and often a confirmatory biopsy. Topical high-potency corticosteroids are generally first-line therapy, with systemic medications such as oral prednisone used to treat severe cases.14,15
Oral candidiasis. Up to 80% of healthy individuals carry Candida albicans in their mouths16; this pathogen accounts for about half of all cases of oral candidiasis (oral thrush). Oral infections occur only with an underlying predisposing condition in the host. Oral thrush presents as creamy white lesions on the oral mucosa; a diagnostic feature is that the plaques can be removed to reveal an erythematous base.16,17
In the chronic form of candidiasis, the mucosal surface is bright red and smooth. When the tongue is involved, it may appear dry, fissured, or cracked. Patients may report a dry mouth, burning pain, and difficulty eating. Infection can be confirmed with periodic acid-Schiff staining of a smear to detect candidal hyphae.9
Use of antifungal creams and lozenges, as well as improved oral hygiene, will often lead to resolution of symptoms.9 Management of any associated underlying conditions, such as diabetes, asthma requiring long-term use of steroid inhalers, or infection with HIV/AIDS, is essential.18
Oral aphthae. Recurrent aphthous ulcers (commonly called canker sores; also referred to as recurrent aphthous stomatitis [RAS]) are a common oral condition. Etiology is unknown and most likely multifactorial, with a strong genetic tendency and multiple predisposing factors, including trauma, stress, food allergies, hormones, and smoking.19 Certain chronic illnesses, including celiac disease, inflammatory bowel disease (IBD), HIV, and neutropenia may also predispose patients to RAS or RAS-like syndromes.
Aphthous ulcers are classified as minor or major. Minor aphthae, which account for 90% of RAS cases, present as single or multiple, small, oval or round ulcers with an erythematous halo on the buccal or labial mucosa or tongue.19 The ulcers last 7 to 10 days and heal spontaneously without scarring.
Diagnosis, based on history and clinical presentation, may include evaluation for systemic causes of oral ulcers. Treatment for both minor and major apthae is palliative, with mainstays including topical corticosteroids, mouth rinses, and, in severe cases, thalidomide, although randomized controlled trials have not shown this agent to be of benefit.9
Treatment for Pemphigus Vulgaris
The outcome goal for management of pemphigus is to achieve and maintain remission. This includes the epithelialization of all skin and mucosal lesions, prevention of relapse, minimization of adverse treatment effects, and successful withdrawal of therapeutic medications.20
The response to treatment varies greatly among patients, as the optimal therapeutic regimen for pemphigus is unknown.20 Systemic glucocorticoids are considered the gold standard of treatment and management, but their use has been associated with several adverse effects, including weight gain and elevated blood sugar levels. Recently, the combination of IV immune globulin and biological therapies (eg, rituximab) that target specific molecules in the inflammatory process have been demonstrated as effective in cases of refractory pemphigus.21,22
PATIENT MANAGEMENT AND OUTCOME
Several referrals were made, including dermatology, for its familiarity with autoimmune diseases of the skin. There, the patient was fully examined and found to have a small truncal lesion compatible with PV. He was referred to an otolaryngologist for a nasal endoscopy to determine the extent of the lesions. They were found to extend far beyond his oral cavity into his esophagus.
Based on a positive enzyme-linked immunosorbent assay (ELISA) for PV antibodies, a cytologic smear with acantholytic cells, and a classic clinical presentation of PV, the patient was started on prednisone 80 mg/d with azathioprine 50 mg/d for the first 14 days.23,24 He responded quickly to these oral medications and underwent a confirmatory oral biopsy within a few weeks.
After several months, the patient was slowly titrated down to lower maintenance doses of prednisone and azathioprine. Now in remission, he continues to receive collaborative management from oral medicine, dermatology, and a nurse practitioner–managed primary care practice. Health care maintenance has included appropriate vaccination and discussion regarding prostate cancer screening, per 2010 guidelines from the US Preventive Services Task Force.25
CONCLUSION
Since the differential diagnosis for pemphigus vulgaris is extensive and the diagnostic criteria are exacting, many affected patients are undiagnosed or misdiagnosed, with a resulting delay in effective treatment. It is important for the primary care clinician to undertake a frequent review of common oral infections, particularly those with similar presentations.
The authors extend their thanks to Alexander Kerr, DDS, MSD, Clinical Associate Professor, Department of Oral and Maxillary Pathology, Radiology and Medicine, New York University College of Dentistry, for the images included in this article and for Dr. Kerr’s clinical expertise and partnership.
REFERENCES
1. Sciubba JJ. Oral mucosal diseases in the office setting. Part II. Oral lichen planus, pemphigus vulgaris, and mucosal pemphigoid. Gen Dent. 2007;55(5):464-476.
2. Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part II. Chronic ulcers. Clin Exp Dermatol. 2009; 34(4):456-461.
3. Dagistan S, Goregen M, Miloglu O, Cakur B. Oral pemphigus vulgaris: a case report with review of the literature. J Oral Sci. 2008;50(3):359-362.
4. Singh S. Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus and bullous pemphigoid: a systematic review. Indian J Dermatol Venereol Leprol. 2011;77(4):456-469.
5. Ohta M, Osawa S, Endo H, et al. Pemphigus vulgaris confined to the gingiva: a case report. Int J Dent. 2011;2011:207153. Epub 2011 May 11.
6. Mignona MD, Fortuna G, Leuci S. Oral pemphigus. Minerva Stomatol. 2009;58(10):501-518.
7. Mimouni D, Bar H, Gdalevich M, et al. Pemphigus: analysis of epidemiological factors in 155 patients. J Eur Acad Dermatol Venereol. 2008; 22(10):1232-1235.
8. Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60(4):595-603.
9. Gonsalves WC, Chi AC, Neville BW. Common oral lesions: Part I. Superficial mucosal lesions. Am Fam Physician. 2007;75(4):501-507.
10. Fatahzadeh M, Schwartz R. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737-763.
11. Sugerman PB, Savage NW. Oral lichen planus: causes, diagnosis and management. Aust Dent J. 2002;47(4):290-297.
12. Kaomongkolgit R. Oral lichenoid drug reaction associated with antihypertensive and hypoglycemic drugs. J Drugs Dermatol. 2010;9(1):73-75.
13. Petti S, Rabiei M, De Luca M, Scully C. The magnitude of the association between hepatitis C virus infection and oral lichen planus: meta-analysis and case control study. Odontology. 2011;99(2):168-178.
14. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84(1): 53-60.
15. Thongprasom K, Carrozzo M, Furness S, Lodi G. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011 Jul 6; (7):CD001168.
16. Giannini PJ, Shetty KV. Diagnosis and management of oral candidiasis. Otolaryngol Clin North Am. 2011;44(1):231-240, vii.
17. Lynch DP. Oral candidiasis. History, classification, and clinical presentation. Oral Surg Oral Med Oral Pathol. 1994;78(2):189-193.
18. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol. 2011 Jan 28;3. doi: 10.3402/jom.v3i0.5771.
19. Scully C, Challacombe SJ. Pemphigus vulgaris: update on etiopathogenesis, oral manifestations, and management. Crit Rev Oral Biol Med. 2002;13(5):397-408.
20. Martin LK, Werth V, Villanueva E, Murrell DF. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 2011;64(5):903-908.
21. Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545-552.
22. Diaz LA. Rituximab and pemphigus: a therapeutic advance. N Engl J Med. 2007;357(6):605-607.
23. Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151(6):1123-1132.
24. Chams-Davatchi C, Daneshpazhooh M. Prednisolone dosage in pemphigus vulgaris. J Am Acad Dermatol. 2005;53(3):547.
25. Agency for Healthcare Research and Quality. Guide to Clinical Preventive Services, 2010-2011: recommendations of the US Preventive Services Task Force. AHRQ Publication No. 10-05145, September 2010. www.ahrq.gov/clinic/pocketgd1011/pocketgd1011.pdf. Accessed January 23, 2012.
A 61-year-old man, who had recently emigrated from the Ukraine, presented to his primary care provider with a chief complaint of painful oral lesions and weight loss. The patient described the gradual onset of a severe sore throat and mouth pain three months earlier. Originally, he attributed his symptoms to an upper respiratory infection but became concerned when his symptoms did not resolve.
He reported that the pain had worsened over time and that he was now barely able to swallow solid food or tolerate acidic beverages due to considerable discomfort. His son, who accompanied him to the appointment, had also noted weight loss.
The patient denied any concomitant symptoms, including fever, cough, night sweats, fatigue, lymphadenopathy, abdominal pain, diarrhea, melena, or concomitant rash. His medical history was remarkable only for stage 1 hypertension, which had been well controlled on hydrochlorothiazide 12.5 mg/d for the previous three years. However, the patient had received only minimal preventive health care while living in the Ukraine. His family history was unknown.
One week earlier, the patient had seen a dentist complaining of mouth pain, and was referred to an oral medicine specialist; this specialist, in turn, referred the patient to a primary care nurse practitioner for lab work to confirm the suspected diagnosis of pemphigus vulgaris.
On physical examination, the patient appeared older than his stated age. He was a thin, mildly ill–appearing man, afebrile and normotensive, with heart rate and respirations within normal limits. However, intraoral examination revealed multiple oropharyngeal ulcerations of varying size on a base of erythematous and swollen mucosa on the inside of the man’s cheek and palatal and buccal mucosa (see Figure 1). On his upper back, two round, crusted blisters were noted in isolation (Figure 2). The remaining findings in the physical examination were unremarkable.
Based on the patient’s physical exam findings and clinical guideline recommendations regarding chronic oral ulcerations of unknown etiology,1,2 the patient was scheduled for a cytologic smear to be performed by oral medicine, followed by a gingival biopsy for a direct immunofluorescence test and routine histopathology.3 Unfortunately, due to extensive involvement and concern for possible mucosal shredding, an oral biopsy was not deemed possible.
However, the oral medicine specialist, because he strongly suspected pemphigus vulgaris, recommended testing for circulating autoantibodies against the antigens desmogleins 1 and/or 3 in the epidermis, which are responsible for cellular adhesion. (A positive test result supports, but does not confirm, a diagnosis of pemphigus vulgaris.4)
Additionally, baseline labs were performed for signs of systemic illness, including infection, anemia, and liver and kidney disease. Frequent monitoring was conducted for steroid-induced symptoms of elevated blood sugars; the primary care provider was responsible for monitoring the patient for weight gain and steroid-induced psychosis. The patient was referred to gastroenterology for a colonoscopy to ensure that his weight loss and anorexia were not the result of gastrointestinal malignancy. However, the patient declined this test.
DISCUSSION
Painful oral lesions can have numerous etiologies of varying severity and complexity, including herpes simplex virus infection, aphthae, lichen planus, erythema multiforme, squamous cell and other oral carcinomas, primary HIV infection, lupus, and pemphigus. Differentiating among these conditions requires a careful medical history and complete physical exam.5
Pemphigus vulgaris (PV) is the most common variant of pemphigus, a group of chronic autoimmune diseases that cause blistering and ulceration of the mucous membranes and the skin.6 From the Greek pemphix (bubble), PV is more common in people of Ashkenazi Jewish or Mediterranean descent,6,7 usually occurs in middle-aged and older persons, and occurs about 1.5 times more commonly in women than men.5,7 Until the introduction of systemic steroids, pemphigus was often a fatal disease. Significant mortality still exists, mainly as a result of infection or adverse reactions to medication therapy.5
In patients with PV, flaccid bullae are formed on the skin in a process called acantholysis, in which epidermal cells lose their ability to adhere to one another. This results in rapidly expanding, thin-walled blisters on the oral mucosa, scalp, face, axillae, and groin. The blisters burst easily, leaving irregularly shaped, painful ulcerations.4 Painful oral mucosal membrane erosions are the first presenting sign of PV and often the only sign for an average of five months before other skin lesions develop.3 These lesions are noninfectious.
To make a definitive diagnosis of PV, clinical lesions must be present, with a confirmation of histologic findings, acantholysis on biopsy, and a confirmation of autoantibodies present in tissue and/or serum.4 (For proposed detailed diagnostic criteria, see table4,8.)
Initial misdiagnoses, which often lead to delayed or incorrect treatment, usually include aphthous stomatitis, gingivostomatitis, erythema multiforme, erosive lichen planus, herpes simplex virus, and/or oral candidiasis.3
Common Differentials
Herpes simplex virus. Affecting between 15% and 45% of the population, herpes simplex virus (HSV) infection, also known as cold sores, is the most common cause of recurrent oral ulcers.9 HSV is transmitted through direct contact with lesions or via viral shedding. Primary infection, which may occur with flu-like symptoms, causes the sudden onset of multiple clustered vesicles on an erythematous base that quickly ulcerate and crust. Recurrent infections tend to be less severe and are accompanied by minimal systemic symptoms.10
Diagnosis is usually made through history and physical exam. However, diagnostic tests, including Tzanck smears, biopsy, polymerase chain reaction (PCR) assay, and/or viral isolation in culture, are sometimes used to confirm a suspected case.10
Oral lichen planus (OLP). This is a common, chronic, mucocutaneous inflammatory disease of unknown etiology that affects skin and mucous membranes of the mouth, including the buccal mucosa, tongue, and/or gums. These lesions are noninfectious and are an immunologically mediated disease. Stress, anxiety, genetic predisposition, NSAID use, antihypertensive medications (eg, captopril, enalapril, propranolol; considered an oral lichenoid drug reaction), and altered cell-mediated immune response have been considered possible causative factors.11,12 Recent reports suggest an association between hepatitis C virus and OLP.13
Affecting about 4% of the general population, and more predominate in perimenopausal women, OLP lesions appear as white, lacey patches; red, swollen tissues; or open sores, most commonly on the inside of the mouth bilaterally. Patients will present with complaints of burning, roughness, or pain in the mouth, dry mouth, sensitivity to hot or spicy foods, and difficulty swallowing if the throat is involved. Diagnosis is based on history and physical examination and often a confirmatory biopsy. Topical high-potency corticosteroids are generally first-line therapy, with systemic medications such as oral prednisone used to treat severe cases.14,15
Oral candidiasis. Up to 80% of healthy individuals carry Candida albicans in their mouths16; this pathogen accounts for about half of all cases of oral candidiasis (oral thrush). Oral infections occur only with an underlying predisposing condition in the host. Oral thrush presents as creamy white lesions on the oral mucosa; a diagnostic feature is that the plaques can be removed to reveal an erythematous base.16,17
In the chronic form of candidiasis, the mucosal surface is bright red and smooth. When the tongue is involved, it may appear dry, fissured, or cracked. Patients may report a dry mouth, burning pain, and difficulty eating. Infection can be confirmed with periodic acid-Schiff staining of a smear to detect candidal hyphae.9
Use of antifungal creams and lozenges, as well as improved oral hygiene, will often lead to resolution of symptoms.9 Management of any associated underlying conditions, such as diabetes, asthma requiring long-term use of steroid inhalers, or infection with HIV/AIDS, is essential.18
Oral aphthae. Recurrent aphthous ulcers (commonly called canker sores; also referred to as recurrent aphthous stomatitis [RAS]) are a common oral condition. Etiology is unknown and most likely multifactorial, with a strong genetic tendency and multiple predisposing factors, including trauma, stress, food allergies, hormones, and smoking.19 Certain chronic illnesses, including celiac disease, inflammatory bowel disease (IBD), HIV, and neutropenia may also predispose patients to RAS or RAS-like syndromes.
Aphthous ulcers are classified as minor or major. Minor aphthae, which account for 90% of RAS cases, present as single or multiple, small, oval or round ulcers with an erythematous halo on the buccal or labial mucosa or tongue.19 The ulcers last 7 to 10 days and heal spontaneously without scarring.
Diagnosis, based on history and clinical presentation, may include evaluation for systemic causes of oral ulcers. Treatment for both minor and major apthae is palliative, with mainstays including topical corticosteroids, mouth rinses, and, in severe cases, thalidomide, although randomized controlled trials have not shown this agent to be of benefit.9
Treatment for Pemphigus Vulgaris
The outcome goal for management of pemphigus is to achieve and maintain remission. This includes the epithelialization of all skin and mucosal lesions, prevention of relapse, minimization of adverse treatment effects, and successful withdrawal of therapeutic medications.20
The response to treatment varies greatly among patients, as the optimal therapeutic regimen for pemphigus is unknown.20 Systemic glucocorticoids are considered the gold standard of treatment and management, but their use has been associated with several adverse effects, including weight gain and elevated blood sugar levels. Recently, the combination of IV immune globulin and biological therapies (eg, rituximab) that target specific molecules in the inflammatory process have been demonstrated as effective in cases of refractory pemphigus.21,22
PATIENT MANAGEMENT AND OUTCOME
Several referrals were made, including dermatology, for its familiarity with autoimmune diseases of the skin. There, the patient was fully examined and found to have a small truncal lesion compatible with PV. He was referred to an otolaryngologist for a nasal endoscopy to determine the extent of the lesions. They were found to extend far beyond his oral cavity into his esophagus.
Based on a positive enzyme-linked immunosorbent assay (ELISA) for PV antibodies, a cytologic smear with acantholytic cells, and a classic clinical presentation of PV, the patient was started on prednisone 80 mg/d with azathioprine 50 mg/d for the first 14 days.23,24 He responded quickly to these oral medications and underwent a confirmatory oral biopsy within a few weeks.
After several months, the patient was slowly titrated down to lower maintenance doses of prednisone and azathioprine. Now in remission, he continues to receive collaborative management from oral medicine, dermatology, and a nurse practitioner–managed primary care practice. Health care maintenance has included appropriate vaccination and discussion regarding prostate cancer screening, per 2010 guidelines from the US Preventive Services Task Force.25
CONCLUSION
Since the differential diagnosis for pemphigus vulgaris is extensive and the diagnostic criteria are exacting, many affected patients are undiagnosed or misdiagnosed, with a resulting delay in effective treatment. It is important for the primary care clinician to undertake a frequent review of common oral infections, particularly those with similar presentations.
The authors extend their thanks to Alexander Kerr, DDS, MSD, Clinical Associate Professor, Department of Oral and Maxillary Pathology, Radiology and Medicine, New York University College of Dentistry, for the images included in this article and for Dr. Kerr’s clinical expertise and partnership.
REFERENCES
1. Sciubba JJ. Oral mucosal diseases in the office setting. Part II. Oral lichen planus, pemphigus vulgaris, and mucosal pemphigoid. Gen Dent. 2007;55(5):464-476.
2. Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part II. Chronic ulcers. Clin Exp Dermatol. 2009; 34(4):456-461.
3. Dagistan S, Goregen M, Miloglu O, Cakur B. Oral pemphigus vulgaris: a case report with review of the literature. J Oral Sci. 2008;50(3):359-362.
4. Singh S. Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus and bullous pemphigoid: a systematic review. Indian J Dermatol Venereol Leprol. 2011;77(4):456-469.
5. Ohta M, Osawa S, Endo H, et al. Pemphigus vulgaris confined to the gingiva: a case report. Int J Dent. 2011;2011:207153. Epub 2011 May 11.
6. Mignona MD, Fortuna G, Leuci S. Oral pemphigus. Minerva Stomatol. 2009;58(10):501-518.
7. Mimouni D, Bar H, Gdalevich M, et al. Pemphigus: analysis of epidemiological factors in 155 patients. J Eur Acad Dermatol Venereol. 2008; 22(10):1232-1235.
8. Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60(4):595-603.
9. Gonsalves WC, Chi AC, Neville BW. Common oral lesions: Part I. Superficial mucosal lesions. Am Fam Physician. 2007;75(4):501-507.
10. Fatahzadeh M, Schwartz R. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737-763.
11. Sugerman PB, Savage NW. Oral lichen planus: causes, diagnosis and management. Aust Dent J. 2002;47(4):290-297.
12. Kaomongkolgit R. Oral lichenoid drug reaction associated with antihypertensive and hypoglycemic drugs. J Drugs Dermatol. 2010;9(1):73-75.
13. Petti S, Rabiei M, De Luca M, Scully C. The magnitude of the association between hepatitis C virus infection and oral lichen planus: meta-analysis and case control study. Odontology. 2011;99(2):168-178.
14. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84(1): 53-60.
15. Thongprasom K, Carrozzo M, Furness S, Lodi G. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011 Jul 6; (7):CD001168.
16. Giannini PJ, Shetty KV. Diagnosis and management of oral candidiasis. Otolaryngol Clin North Am. 2011;44(1):231-240, vii.
17. Lynch DP. Oral candidiasis. History, classification, and clinical presentation. Oral Surg Oral Med Oral Pathol. 1994;78(2):189-193.
18. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol. 2011 Jan 28;3. doi: 10.3402/jom.v3i0.5771.
19. Scully C, Challacombe SJ. Pemphigus vulgaris: update on etiopathogenesis, oral manifestations, and management. Crit Rev Oral Biol Med. 2002;13(5):397-408.
20. Martin LK, Werth V, Villanueva E, Murrell DF. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 2011;64(5):903-908.
21. Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545-552.
22. Diaz LA. Rituximab and pemphigus: a therapeutic advance. N Engl J Med. 2007;357(6):605-607.
23. Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151(6):1123-1132.
24. Chams-Davatchi C, Daneshpazhooh M. Prednisolone dosage in pemphigus vulgaris. J Am Acad Dermatol. 2005;53(3):547.
25. Agency for Healthcare Research and Quality. Guide to Clinical Preventive Services, 2010-2011: recommendations of the US Preventive Services Task Force. AHRQ Publication No. 10-05145, September 2010. www.ahrq.gov/clinic/pocketgd1011/pocketgd1011.pdf. Accessed January 23, 2012.
A 61-year-old man, who had recently emigrated from the Ukraine, presented to his primary care provider with a chief complaint of painful oral lesions and weight loss. The patient described the gradual onset of a severe sore throat and mouth pain three months earlier. Originally, he attributed his symptoms to an upper respiratory infection but became concerned when his symptoms did not resolve.
He reported that the pain had worsened over time and that he was now barely able to swallow solid food or tolerate acidic beverages due to considerable discomfort. His son, who accompanied him to the appointment, had also noted weight loss.
The patient denied any concomitant symptoms, including fever, cough, night sweats, fatigue, lymphadenopathy, abdominal pain, diarrhea, melena, or concomitant rash. His medical history was remarkable only for stage 1 hypertension, which had been well controlled on hydrochlorothiazide 12.5 mg/d for the previous three years. However, the patient had received only minimal preventive health care while living in the Ukraine. His family history was unknown.
One week earlier, the patient had seen a dentist complaining of mouth pain, and was referred to an oral medicine specialist; this specialist, in turn, referred the patient to a primary care nurse practitioner for lab work to confirm the suspected diagnosis of pemphigus vulgaris.
On physical examination, the patient appeared older than his stated age. He was a thin, mildly ill–appearing man, afebrile and normotensive, with heart rate and respirations within normal limits. However, intraoral examination revealed multiple oropharyngeal ulcerations of varying size on a base of erythematous and swollen mucosa on the inside of the man’s cheek and palatal and buccal mucosa (see Figure 1). On his upper back, two round, crusted blisters were noted in isolation (Figure 2). The remaining findings in the physical examination were unremarkable.
Based on the patient’s physical exam findings and clinical guideline recommendations regarding chronic oral ulcerations of unknown etiology,1,2 the patient was scheduled for a cytologic smear to be performed by oral medicine, followed by a gingival biopsy for a direct immunofluorescence test and routine histopathology.3 Unfortunately, due to extensive involvement and concern for possible mucosal shredding, an oral biopsy was not deemed possible.
However, the oral medicine specialist, because he strongly suspected pemphigus vulgaris, recommended testing for circulating autoantibodies against the antigens desmogleins 1 and/or 3 in the epidermis, which are responsible for cellular adhesion. (A positive test result supports, but does not confirm, a diagnosis of pemphigus vulgaris.4)
Additionally, baseline labs were performed for signs of systemic illness, including infection, anemia, and liver and kidney disease. Frequent monitoring was conducted for steroid-induced symptoms of elevated blood sugars; the primary care provider was responsible for monitoring the patient for weight gain and steroid-induced psychosis. The patient was referred to gastroenterology for a colonoscopy to ensure that his weight loss and anorexia were not the result of gastrointestinal malignancy. However, the patient declined this test.
DISCUSSION
Painful oral lesions can have numerous etiologies of varying severity and complexity, including herpes simplex virus infection, aphthae, lichen planus, erythema multiforme, squamous cell and other oral carcinomas, primary HIV infection, lupus, and pemphigus. Differentiating among these conditions requires a careful medical history and complete physical exam.5
Pemphigus vulgaris (PV) is the most common variant of pemphigus, a group of chronic autoimmune diseases that cause blistering and ulceration of the mucous membranes and the skin.6 From the Greek pemphix (bubble), PV is more common in people of Ashkenazi Jewish or Mediterranean descent,6,7 usually occurs in middle-aged and older persons, and occurs about 1.5 times more commonly in women than men.5,7 Until the introduction of systemic steroids, pemphigus was often a fatal disease. Significant mortality still exists, mainly as a result of infection or adverse reactions to medication therapy.5
In patients with PV, flaccid bullae are formed on the skin in a process called acantholysis, in which epidermal cells lose their ability to adhere to one another. This results in rapidly expanding, thin-walled blisters on the oral mucosa, scalp, face, axillae, and groin. The blisters burst easily, leaving irregularly shaped, painful ulcerations.4 Painful oral mucosal membrane erosions are the first presenting sign of PV and often the only sign for an average of five months before other skin lesions develop.3 These lesions are noninfectious.
To make a definitive diagnosis of PV, clinical lesions must be present, with a confirmation of histologic findings, acantholysis on biopsy, and a confirmation of autoantibodies present in tissue and/or serum.4 (For proposed detailed diagnostic criteria, see table4,8.)
Initial misdiagnoses, which often lead to delayed or incorrect treatment, usually include aphthous stomatitis, gingivostomatitis, erythema multiforme, erosive lichen planus, herpes simplex virus, and/or oral candidiasis.3
Common Differentials
Herpes simplex virus. Affecting between 15% and 45% of the population, herpes simplex virus (HSV) infection, also known as cold sores, is the most common cause of recurrent oral ulcers.9 HSV is transmitted through direct contact with lesions or via viral shedding. Primary infection, which may occur with flu-like symptoms, causes the sudden onset of multiple clustered vesicles on an erythematous base that quickly ulcerate and crust. Recurrent infections tend to be less severe and are accompanied by minimal systemic symptoms.10
Diagnosis is usually made through history and physical exam. However, diagnostic tests, including Tzanck smears, biopsy, polymerase chain reaction (PCR) assay, and/or viral isolation in culture, are sometimes used to confirm a suspected case.10
Oral lichen planus (OLP). This is a common, chronic, mucocutaneous inflammatory disease of unknown etiology that affects skin and mucous membranes of the mouth, including the buccal mucosa, tongue, and/or gums. These lesions are noninfectious and are an immunologically mediated disease. Stress, anxiety, genetic predisposition, NSAID use, antihypertensive medications (eg, captopril, enalapril, propranolol; considered an oral lichenoid drug reaction), and altered cell-mediated immune response have been considered possible causative factors.11,12 Recent reports suggest an association between hepatitis C virus and OLP.13
Affecting about 4% of the general population, and more predominate in perimenopausal women, OLP lesions appear as white, lacey patches; red, swollen tissues; or open sores, most commonly on the inside of the mouth bilaterally. Patients will present with complaints of burning, roughness, or pain in the mouth, dry mouth, sensitivity to hot or spicy foods, and difficulty swallowing if the throat is involved. Diagnosis is based on history and physical examination and often a confirmatory biopsy. Topical high-potency corticosteroids are generally first-line therapy, with systemic medications such as oral prednisone used to treat severe cases.14,15
Oral candidiasis. Up to 80% of healthy individuals carry Candida albicans in their mouths16; this pathogen accounts for about half of all cases of oral candidiasis (oral thrush). Oral infections occur only with an underlying predisposing condition in the host. Oral thrush presents as creamy white lesions on the oral mucosa; a diagnostic feature is that the plaques can be removed to reveal an erythematous base.16,17
In the chronic form of candidiasis, the mucosal surface is bright red and smooth. When the tongue is involved, it may appear dry, fissured, or cracked. Patients may report a dry mouth, burning pain, and difficulty eating. Infection can be confirmed with periodic acid-Schiff staining of a smear to detect candidal hyphae.9
Use of antifungal creams and lozenges, as well as improved oral hygiene, will often lead to resolution of symptoms.9 Management of any associated underlying conditions, such as diabetes, asthma requiring long-term use of steroid inhalers, or infection with HIV/AIDS, is essential.18
Oral aphthae. Recurrent aphthous ulcers (commonly called canker sores; also referred to as recurrent aphthous stomatitis [RAS]) are a common oral condition. Etiology is unknown and most likely multifactorial, with a strong genetic tendency and multiple predisposing factors, including trauma, stress, food allergies, hormones, and smoking.19 Certain chronic illnesses, including celiac disease, inflammatory bowel disease (IBD), HIV, and neutropenia may also predispose patients to RAS or RAS-like syndromes.
Aphthous ulcers are classified as minor or major. Minor aphthae, which account for 90% of RAS cases, present as single or multiple, small, oval or round ulcers with an erythematous halo on the buccal or labial mucosa or tongue.19 The ulcers last 7 to 10 days and heal spontaneously without scarring.
Diagnosis, based on history and clinical presentation, may include evaluation for systemic causes of oral ulcers. Treatment for both minor and major apthae is palliative, with mainstays including topical corticosteroids, mouth rinses, and, in severe cases, thalidomide, although randomized controlled trials have not shown this agent to be of benefit.9
Treatment for Pemphigus Vulgaris
The outcome goal for management of pemphigus is to achieve and maintain remission. This includes the epithelialization of all skin and mucosal lesions, prevention of relapse, minimization of adverse treatment effects, and successful withdrawal of therapeutic medications.20
The response to treatment varies greatly among patients, as the optimal therapeutic regimen for pemphigus is unknown.20 Systemic glucocorticoids are considered the gold standard of treatment and management, but their use has been associated with several adverse effects, including weight gain and elevated blood sugar levels. Recently, the combination of IV immune globulin and biological therapies (eg, rituximab) that target specific molecules in the inflammatory process have been demonstrated as effective in cases of refractory pemphigus.21,22
PATIENT MANAGEMENT AND OUTCOME
Several referrals were made, including dermatology, for its familiarity with autoimmune diseases of the skin. There, the patient was fully examined and found to have a small truncal lesion compatible with PV. He was referred to an otolaryngologist for a nasal endoscopy to determine the extent of the lesions. They were found to extend far beyond his oral cavity into his esophagus.
Based on a positive enzyme-linked immunosorbent assay (ELISA) for PV antibodies, a cytologic smear with acantholytic cells, and a classic clinical presentation of PV, the patient was started on prednisone 80 mg/d with azathioprine 50 mg/d for the first 14 days.23,24 He responded quickly to these oral medications and underwent a confirmatory oral biopsy within a few weeks.
After several months, the patient was slowly titrated down to lower maintenance doses of prednisone and azathioprine. Now in remission, he continues to receive collaborative management from oral medicine, dermatology, and a nurse practitioner–managed primary care practice. Health care maintenance has included appropriate vaccination and discussion regarding prostate cancer screening, per 2010 guidelines from the US Preventive Services Task Force.25
CONCLUSION
Since the differential diagnosis for pemphigus vulgaris is extensive and the diagnostic criteria are exacting, many affected patients are undiagnosed or misdiagnosed, with a resulting delay in effective treatment. It is important for the primary care clinician to undertake a frequent review of common oral infections, particularly those with similar presentations.
The authors extend their thanks to Alexander Kerr, DDS, MSD, Clinical Associate Professor, Department of Oral and Maxillary Pathology, Radiology and Medicine, New York University College of Dentistry, for the images included in this article and for Dr. Kerr’s clinical expertise and partnership.
REFERENCES
1. Sciubba JJ. Oral mucosal diseases in the office setting. Part II. Oral lichen planus, pemphigus vulgaris, and mucosal pemphigoid. Gen Dent. 2007;55(5):464-476.
2. Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part II. Chronic ulcers. Clin Exp Dermatol. 2009; 34(4):456-461.
3. Dagistan S, Goregen M, Miloglu O, Cakur B. Oral pemphigus vulgaris: a case report with review of the literature. J Oral Sci. 2008;50(3):359-362.
4. Singh S. Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus and bullous pemphigoid: a systematic review. Indian J Dermatol Venereol Leprol. 2011;77(4):456-469.
5. Ohta M, Osawa S, Endo H, et al. Pemphigus vulgaris confined to the gingiva: a case report. Int J Dent. 2011;2011:207153. Epub 2011 May 11.
6. Mignona MD, Fortuna G, Leuci S. Oral pemphigus. Minerva Stomatol. 2009;58(10):501-518.
7. Mimouni D, Bar H, Gdalevich M, et al. Pemphigus: analysis of epidemiological factors in 155 patients. J Eur Acad Dermatol Venereol. 2008; 22(10):1232-1235.
8. Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60(4):595-603.
9. Gonsalves WC, Chi AC, Neville BW. Common oral lesions: Part I. Superficial mucosal lesions. Am Fam Physician. 2007;75(4):501-507.
10. Fatahzadeh M, Schwartz R. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737-763.
11. Sugerman PB, Savage NW. Oral lichen planus: causes, diagnosis and management. Aust Dent J. 2002;47(4):290-297.
12. Kaomongkolgit R. Oral lichenoid drug reaction associated with antihypertensive and hypoglycemic drugs. J Drugs Dermatol. 2010;9(1):73-75.
13. Petti S, Rabiei M, De Luca M, Scully C. The magnitude of the association between hepatitis C virus infection and oral lichen planus: meta-analysis and case control study. Odontology. 2011;99(2):168-178.
14. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84(1): 53-60.
15. Thongprasom K, Carrozzo M, Furness S, Lodi G. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011 Jul 6; (7):CD001168.
16. Giannini PJ, Shetty KV. Diagnosis and management of oral candidiasis. Otolaryngol Clin North Am. 2011;44(1):231-240, vii.
17. Lynch DP. Oral candidiasis. History, classification, and clinical presentation. Oral Surg Oral Med Oral Pathol. 1994;78(2):189-193.
18. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. J Oral Microbiol. 2011 Jan 28;3. doi: 10.3402/jom.v3i0.5771.
19. Scully C, Challacombe SJ. Pemphigus vulgaris: update on etiopathogenesis, oral manifestations, and management. Crit Rev Oral Biol Med. 2002;13(5):397-408.
20. Martin LK, Werth V, Villanueva E, Murrell DF. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 2011;64(5):903-908.
21. Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545-552.
22. Diaz LA. Rituximab and pemphigus: a therapeutic advance. N Engl J Med. 2007;357(6):605-607.
23. Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151(6):1123-1132.
24. Chams-Davatchi C, Daneshpazhooh M. Prednisolone dosage in pemphigus vulgaris. J Am Acad Dermatol. 2005;53(3):547.
25. Agency for Healthcare Research and Quality. Guide to Clinical Preventive Services, 2010-2011: recommendations of the US Preventive Services Task Force. AHRQ Publication No. 10-05145, September 2010. www.ahrq.gov/clinic/pocketgd1011/pocketgd1011.pdf. Accessed January 23, 2012.