User login
FDA OKs pancreatic islet cell therapy for type 1 diabetes
The Food and Drug Administration has approved donislecel (Lantidra, CellTrans), a pancreatic islet cell therapy developed from cadaver donors, for the treatment of people with type 1 diabetes who are unable to achieve target glucose levels owing to severe hypoglycemic episodes.
The product is given as a single infusion via the hepatic portal vein into the liver. A second infusion is given if necessary. Immunosuppression is required to maintain cell viability, just as it is required to support a transplanted kidney or other organ, as these all represent “foreign” tissues to the recipient.
“Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in an FDA statement.
The product was approved despite concerns from the American Society of Transplant Surgeons, the American Society of Transplantation, and an organization of more than 50 transplant surgeons – the Islets for U.S. Collaborative – whose members argue that cadaver-derived (allogeneic) pancreatic islets should be regulated as transplanted organs rather than as biologic drugs, as is done in many other parts of the world.
Lantidra differs from stem cell therapy being developed by Vertex Pharmaceuticals. In the latter, beta cells are grown from allogeneic stem cells using a proprietary technology. So far, six patients have received the therapy, and it has been successful in all of them to varying degrees, as reported at last week’s American Diabetes Association meeting. So while this is a promising technology, with talk of a “cure” for type 1 diabetes, it’s important to remember that this is very early in the development phase, says Anne Peters, MD, of the University of California, Los Angeles.
Approval based on small studies, with adverse events
The approval of Lantidra, following a 12-4 vote in favor by the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee in April 2021, was based on two nonrandomized, single-arm studies that included a total of 30 individuals with type 1 diabetes who had hypoglycemic unawareness and who received between one and three infusions of donislecel.
Insulin independence was achieved at 1 year by 21 participants; 11 were still insulin independent at 5 years, and 10 remained so more than 5 years. Five participants were unable to discontinue insulin treatment at all.
Adverse events included nausea, fatigue, anemia, diarrhea, and abdominal pain. Most of the participants experienced at least one serious adverse reaction related to the method of infusion and/or the use of immunosuppression. Some of these reactions required discontinuation of the immunosuppressive medications, resulting in the loss of islet cell function and return to insulin dependence.
“These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra,” according to the FDA statement.
U.S. transplant physicians had expressed concern, bill introduced
The transplant surgery organizations had written letters to the FDA, as well as to several other government agencies, to ask that the regulatory framework for Lantidra be shifted from the FDA to the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
They also wrote to members of Congress. On June 22, 2023, U.S. Senators Mike Lee (R-UT), Ted Budd (R-NC), and Marsha Blackburn (R-TN) introduced the Islet Transplantation Bill, which would shift the regulatory framework for cadaveric islets from that of biologic drugs to transplanted organs.
Asked for comment, Piotr Witkowski, MD, PhD, the leader of the Islets for U.S. Collaborative, told this news organization: “We were really happy about the introduction of the islet bill. Now, we’re concerned about negative downstream effects of granting a licence to a private company for distribution of the cadaveric islets.”
During the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee’s discussion in 2021, several panel members noted that the target patient population for this treatment with the current indication will likely be smaller today than it was when the two studies were initiated, in 2004 and 2007, given current automated diabetes technology – such as insulin pumps, continuous glucose monitors, and hybrid closed-loop systems in which the two are linked together as a so-called artificial pancreas – that reduces hypoglycemia risk.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved donislecel (Lantidra, CellTrans), a pancreatic islet cell therapy developed from cadaver donors, for the treatment of people with type 1 diabetes who are unable to achieve target glucose levels owing to severe hypoglycemic episodes.
The product is given as a single infusion via the hepatic portal vein into the liver. A second infusion is given if necessary. Immunosuppression is required to maintain cell viability, just as it is required to support a transplanted kidney or other organ, as these all represent “foreign” tissues to the recipient.
“Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in an FDA statement.
The product was approved despite concerns from the American Society of Transplant Surgeons, the American Society of Transplantation, and an organization of more than 50 transplant surgeons – the Islets for U.S. Collaborative – whose members argue that cadaver-derived (allogeneic) pancreatic islets should be regulated as transplanted organs rather than as biologic drugs, as is done in many other parts of the world.
Lantidra differs from stem cell therapy being developed by Vertex Pharmaceuticals. In the latter, beta cells are grown from allogeneic stem cells using a proprietary technology. So far, six patients have received the therapy, and it has been successful in all of them to varying degrees, as reported at last week’s American Diabetes Association meeting. So while this is a promising technology, with talk of a “cure” for type 1 diabetes, it’s important to remember that this is very early in the development phase, says Anne Peters, MD, of the University of California, Los Angeles.
Approval based on small studies, with adverse events
The approval of Lantidra, following a 12-4 vote in favor by the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee in April 2021, was based on two nonrandomized, single-arm studies that included a total of 30 individuals with type 1 diabetes who had hypoglycemic unawareness and who received between one and three infusions of donislecel.
Insulin independence was achieved at 1 year by 21 participants; 11 were still insulin independent at 5 years, and 10 remained so more than 5 years. Five participants were unable to discontinue insulin treatment at all.
Adverse events included nausea, fatigue, anemia, diarrhea, and abdominal pain. Most of the participants experienced at least one serious adverse reaction related to the method of infusion and/or the use of immunosuppression. Some of these reactions required discontinuation of the immunosuppressive medications, resulting in the loss of islet cell function and return to insulin dependence.
“These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra,” according to the FDA statement.
U.S. transplant physicians had expressed concern, bill introduced
The transplant surgery organizations had written letters to the FDA, as well as to several other government agencies, to ask that the regulatory framework for Lantidra be shifted from the FDA to the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
They also wrote to members of Congress. On June 22, 2023, U.S. Senators Mike Lee (R-UT), Ted Budd (R-NC), and Marsha Blackburn (R-TN) introduced the Islet Transplantation Bill, which would shift the regulatory framework for cadaveric islets from that of biologic drugs to transplanted organs.
Asked for comment, Piotr Witkowski, MD, PhD, the leader of the Islets for U.S. Collaborative, told this news organization: “We were really happy about the introduction of the islet bill. Now, we’re concerned about negative downstream effects of granting a licence to a private company for distribution of the cadaveric islets.”
During the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee’s discussion in 2021, several panel members noted that the target patient population for this treatment with the current indication will likely be smaller today than it was when the two studies were initiated, in 2004 and 2007, given current automated diabetes technology – such as insulin pumps, continuous glucose monitors, and hybrid closed-loop systems in which the two are linked together as a so-called artificial pancreas – that reduces hypoglycemia risk.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved donislecel (Lantidra, CellTrans), a pancreatic islet cell therapy developed from cadaver donors, for the treatment of people with type 1 diabetes who are unable to achieve target glucose levels owing to severe hypoglycemic episodes.
The product is given as a single infusion via the hepatic portal vein into the liver. A second infusion is given if necessary. Immunosuppression is required to maintain cell viability, just as it is required to support a transplanted kidney or other organ, as these all represent “foreign” tissues to the recipient.
“Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in an FDA statement.
The product was approved despite concerns from the American Society of Transplant Surgeons, the American Society of Transplantation, and an organization of more than 50 transplant surgeons – the Islets for U.S. Collaborative – whose members argue that cadaver-derived (allogeneic) pancreatic islets should be regulated as transplanted organs rather than as biologic drugs, as is done in many other parts of the world.
Lantidra differs from stem cell therapy being developed by Vertex Pharmaceuticals. In the latter, beta cells are grown from allogeneic stem cells using a proprietary technology. So far, six patients have received the therapy, and it has been successful in all of them to varying degrees, as reported at last week’s American Diabetes Association meeting. So while this is a promising technology, with talk of a “cure” for type 1 diabetes, it’s important to remember that this is very early in the development phase, says Anne Peters, MD, of the University of California, Los Angeles.
Approval based on small studies, with adverse events
The approval of Lantidra, following a 12-4 vote in favor by the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee in April 2021, was based on two nonrandomized, single-arm studies that included a total of 30 individuals with type 1 diabetes who had hypoglycemic unawareness and who received between one and three infusions of donislecel.
Insulin independence was achieved at 1 year by 21 participants; 11 were still insulin independent at 5 years, and 10 remained so more than 5 years. Five participants were unable to discontinue insulin treatment at all.
Adverse events included nausea, fatigue, anemia, diarrhea, and abdominal pain. Most of the participants experienced at least one serious adverse reaction related to the method of infusion and/or the use of immunosuppression. Some of these reactions required discontinuation of the immunosuppressive medications, resulting in the loss of islet cell function and return to insulin dependence.
“These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra,” according to the FDA statement.
U.S. transplant physicians had expressed concern, bill introduced
The transplant surgery organizations had written letters to the FDA, as well as to several other government agencies, to ask that the regulatory framework for Lantidra be shifted from the FDA to the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
They also wrote to members of Congress. On June 22, 2023, U.S. Senators Mike Lee (R-UT), Ted Budd (R-NC), and Marsha Blackburn (R-TN) introduced the Islet Transplantation Bill, which would shift the regulatory framework for cadaveric islets from that of biologic drugs to transplanted organs.
Asked for comment, Piotr Witkowski, MD, PhD, the leader of the Islets for U.S. Collaborative, told this news organization: “We were really happy about the introduction of the islet bill. Now, we’re concerned about negative downstream effects of granting a licence to a private company for distribution of the cadaveric islets.”
During the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee’s discussion in 2021, several panel members noted that the target patient population for this treatment with the current indication will likely be smaller today than it was when the two studies were initiated, in 2004 and 2007, given current automated diabetes technology – such as insulin pumps, continuous glucose monitors, and hybrid closed-loop systems in which the two are linked together as a so-called artificial pancreas – that reduces hypoglycemia risk.
A version of this article originally appeared on Medscape.com.
Genetic counseling enhances patient empowerment in familial colorectal cancer
TOPLINE:
, decreased depression, and reduced emotional distress.
METHODOLOGY:
- The researchers enrolled 82 patients (mean age, 44 years; 52% women) who were affected by or at risk for fCRC (Lynch syndrome, associated polyposis conditions, other risk-associated pathogenic variants, and clinically defined fCRC).
- Participants were randomly assigned to receive either standard care or standard care plus genetic counseling.
- Measures included empowerment, anxiety, depression, knowledge, risk perception, emotional distress, screening/surveillance behaviors, perceived social support, decisional conflict, and quality of life.
TAKEAWAY:
- Genetic counseling had a significant effect on patient empowerment after the researchers controlled for precounseling empowerment scores (P = .0043) and depression scores (P = .025).
- Genetic counseling also led to significant improvement in anxiety (P = .04), depression (P = .03), emotional distress (P = .03), and knowledge about fCRC (P = .025).
- Emotional distress appeared to have a moderating effect; those with lower initial levels of emotional distress benefited more from genetic counseling in terms of empowerment (P = .016).
IN PRACTICE:
“Empowerment is particularly important for these patients, since not only does it help them feel they can make real, informed choices, but it also aids their ability to manage their feelings and make plans for the future,” Andrada Ciuca, PhD, with Babes-Bolyai University, Cluj-Napoca, Romania, said in a statement. “An interesting finding was that the more anxiety decreased after their counseling session, the greater the impact was on their empowerment. This highlights the importance of addressing emotional distress during genetic counselling.”
STUDY DETAILS:
The study was conducted by Dr. Ciuca and colleagues. The results were presented at the European Society of Human Genetics 2023 annual conference on June 11.
LIMITATIONS:
The study comprised 82 participants and focused specifically on fCRC.
DISCLOSURES:
No conflicts of interest were disclosed.
A version of this article originally appeared on Medscape.com.
TOPLINE:
, decreased depression, and reduced emotional distress.
METHODOLOGY:
- The researchers enrolled 82 patients (mean age, 44 years; 52% women) who were affected by or at risk for fCRC (Lynch syndrome, associated polyposis conditions, other risk-associated pathogenic variants, and clinically defined fCRC).
- Participants were randomly assigned to receive either standard care or standard care plus genetic counseling.
- Measures included empowerment, anxiety, depression, knowledge, risk perception, emotional distress, screening/surveillance behaviors, perceived social support, decisional conflict, and quality of life.
TAKEAWAY:
- Genetic counseling had a significant effect on patient empowerment after the researchers controlled for precounseling empowerment scores (P = .0043) and depression scores (P = .025).
- Genetic counseling also led to significant improvement in anxiety (P = .04), depression (P = .03), emotional distress (P = .03), and knowledge about fCRC (P = .025).
- Emotional distress appeared to have a moderating effect; those with lower initial levels of emotional distress benefited more from genetic counseling in terms of empowerment (P = .016).
IN PRACTICE:
“Empowerment is particularly important for these patients, since not only does it help them feel they can make real, informed choices, but it also aids their ability to manage their feelings and make plans for the future,” Andrada Ciuca, PhD, with Babes-Bolyai University, Cluj-Napoca, Romania, said in a statement. “An interesting finding was that the more anxiety decreased after their counseling session, the greater the impact was on their empowerment. This highlights the importance of addressing emotional distress during genetic counselling.”
STUDY DETAILS:
The study was conducted by Dr. Ciuca and colleagues. The results were presented at the European Society of Human Genetics 2023 annual conference on June 11.
LIMITATIONS:
The study comprised 82 participants and focused specifically on fCRC.
DISCLOSURES:
No conflicts of interest were disclosed.
A version of this article originally appeared on Medscape.com.
TOPLINE:
, decreased depression, and reduced emotional distress.
METHODOLOGY:
- The researchers enrolled 82 patients (mean age, 44 years; 52% women) who were affected by or at risk for fCRC (Lynch syndrome, associated polyposis conditions, other risk-associated pathogenic variants, and clinically defined fCRC).
- Participants were randomly assigned to receive either standard care or standard care plus genetic counseling.
- Measures included empowerment, anxiety, depression, knowledge, risk perception, emotional distress, screening/surveillance behaviors, perceived social support, decisional conflict, and quality of life.
TAKEAWAY:
- Genetic counseling had a significant effect on patient empowerment after the researchers controlled for precounseling empowerment scores (P = .0043) and depression scores (P = .025).
- Genetic counseling also led to significant improvement in anxiety (P = .04), depression (P = .03), emotional distress (P = .03), and knowledge about fCRC (P = .025).
- Emotional distress appeared to have a moderating effect; those with lower initial levels of emotional distress benefited more from genetic counseling in terms of empowerment (P = .016).
IN PRACTICE:
“Empowerment is particularly important for these patients, since not only does it help them feel they can make real, informed choices, but it also aids their ability to manage their feelings and make plans for the future,” Andrada Ciuca, PhD, with Babes-Bolyai University, Cluj-Napoca, Romania, said in a statement. “An interesting finding was that the more anxiety decreased after their counseling session, the greater the impact was on their empowerment. This highlights the importance of addressing emotional distress during genetic counselling.”
STUDY DETAILS:
The study was conducted by Dr. Ciuca and colleagues. The results were presented at the European Society of Human Genetics 2023 annual conference on June 11.
LIMITATIONS:
The study comprised 82 participants and focused specifically on fCRC.
DISCLOSURES:
No conflicts of interest were disclosed.
A version of this article originally appeared on Medscape.com.
Offering blood test ups CRC screening for people who first declined colonoscopy, FIT
, researchers report.
However, the number of people in the study who subsequently underwent timely colonoscopy after a positive blood test did not increase, signaling a continuing challenge in CRC prevention and treatment.
“The main message is that the blood test can do what it’s meant to do, which is increase screening uptake,” first author Peter Liang, MD, MPH, told this news organization. Dr. Liang is a gastroenterologist and researcher at NYU Langone Health and the VA New York Harbor Health Care System in New York.
The study was published online in Clinical Gastroenterology and Hepatology.
In the United States, the rate of use of first-line screening has for years been stuck at about 70% or lower for eligible people, Dr. Liang said. A different modality is needed to help raise the numbers.
The blood test is easy to perform and requires only a few tubes of blood, he noted. No diet restrictions, test prep, or contact with stool is necessary.
We are all searching for ways to get that first-line screening rate up from 60% to 70% to 80% to 90%, noted Folasade P. May, MD, PhD, who was not involved in the study.
“A lot of people think these blood tests are the promised land,” said Dr. May, associate professor of medicine at the University of California, Los Angeles, and director of the gastroenterology quality improvement program at UCLA Health. “We want to see that, when we offer these blood tests, the uptake is 20%-25% higher, which would get us closer to the national goal of 80% screened.”
Blood test as a second-line screening option
The study enrolled 359 veterans at a Veterans Affairs medical center. Participants were 50-75 years old and were eligible for screening but had declined a colonoscopy and a stool test within the previous 6 months.
They were randomly assigned to one of two groups. The control group received a letter and telephone outreach in which participants were again offered screening with colonoscopy or FIT only. The intervention group was additionally offered the blood test as a second-line option.
The primary outcome was completion of any screening test within 6 months. The secondary outcome was completion of a full screening strategy within 6 months, including colonoscopy for those with a positive noninvasive test result.
Of the people who had declined first-line tests and were reoffered first-line tests and the blood test, 17.1% completed screening within 6 months, compared with 9.6% of those who were only reoffered the first-line tests. The uptake of colonoscopy and FIT was similar between the two groups. The full-screening strategy was completed by 14.9% in the intervention group, compared with 9% in the control group.
At first glance, the results for uptake seem a bit disappointing, Dr. May said. However, the numbers in this study may not reflect the true potential of the blood tests – which are relatively new and have not yet been incorporated into routine care – because they had to be conducted in a separate appointment at a lab, she said.
If blood tests for CRC were part of the workflow, Dr. May explained, patients could undergo them with a routine blood draw already scheduled to check for diabetes or high cholesterol, for instance, and the numbers presumably would go up.
“I think this study underestimates the proportion of people who will participate,” she said. “We need a study that tests this strategy in a more real-world scenario.”
Nonetheless, “This is the first trial that’s looking at this question, and it’s an important question,” Dr. May added.
Dr. Liang and colleagues acknowledge that a limitation of the study is that it was performed in only one VA center among an older, predominantly male population, so it will be important to make the comparisons in more diverse study populations.
Blood test reliability
The septin 9 blood test is the only blood test approved by the U.S. Food and Drug Administration for CRC screening and is indicated for those who have declined first-line tests. However, the extent of usage of the blood test in this context is unclear, as it has only been approved since 2016.
Because the blood test is not covered by Medicare, Dr. Liang said, accessibility has been limited. One of the reasons for the lack of coverage is that the blood tests are less reliable than first-line tests, he said.
The test detects methylated septin 9 DNA, a biomarker for CRC. The FDA-approved version of the test has a sensitivity of 68% and a specificity of 79% for CRC.
Dr. May said she’d have more confidence in the blood tests if those numbers were higher, at 80%-90%.
Dr. Liang told this news organization that previous research has compared test use when colonoscopy, FIT, and a blood test are considered equally, but because the blood test is indicated only after a person declines first-line screening, his team designed an approach in which the blood test was a second-line option for its target population.
Other blood tests now on the market or under development appear to have higher sensitivity and specificity, he added.
“We think our results are actually applicable to a blood test in general,” Dr. Liang said.
Blood tests are only the first step, though. Getting people who screen positive to follow up with a diagnostic colonoscopy is critical, Dr. May and the authors agree.
“That’s something we, as a nation, just haven’t figured out,” Dr. May said. “It has to become a priority.”
The study was supported in part by the Veterans Health Administration and was funded by Epigenomics and a grant from the New York Society for Gastrointestinal Endoscopy’s Florence Lefcourt Endoscopy Research Award to Dr. Laing, who is also supported by the National Cancer Institute. Dr. Liang has received research support from Epigenomics and Freenome and is on the advisory board for Guardant Health. The remaining authors disclosed no relevant financial relationships. Dr. May is a consultant for Exact Sciences and Geneoscopy, both of which are developing stool tests, and for Freenome, which is developing stool and blood tests.
A version of this article originally appeared on Medscape.com.
, researchers report.
However, the number of people in the study who subsequently underwent timely colonoscopy after a positive blood test did not increase, signaling a continuing challenge in CRC prevention and treatment.
“The main message is that the blood test can do what it’s meant to do, which is increase screening uptake,” first author Peter Liang, MD, MPH, told this news organization. Dr. Liang is a gastroenterologist and researcher at NYU Langone Health and the VA New York Harbor Health Care System in New York.
The study was published online in Clinical Gastroenterology and Hepatology.
In the United States, the rate of use of first-line screening has for years been stuck at about 70% or lower for eligible people, Dr. Liang said. A different modality is needed to help raise the numbers.
The blood test is easy to perform and requires only a few tubes of blood, he noted. No diet restrictions, test prep, or contact with stool is necessary.
We are all searching for ways to get that first-line screening rate up from 60% to 70% to 80% to 90%, noted Folasade P. May, MD, PhD, who was not involved in the study.
“A lot of people think these blood tests are the promised land,” said Dr. May, associate professor of medicine at the University of California, Los Angeles, and director of the gastroenterology quality improvement program at UCLA Health. “We want to see that, when we offer these blood tests, the uptake is 20%-25% higher, which would get us closer to the national goal of 80% screened.”
Blood test as a second-line screening option
The study enrolled 359 veterans at a Veterans Affairs medical center. Participants were 50-75 years old and were eligible for screening but had declined a colonoscopy and a stool test within the previous 6 months.
They were randomly assigned to one of two groups. The control group received a letter and telephone outreach in which participants were again offered screening with colonoscopy or FIT only. The intervention group was additionally offered the blood test as a second-line option.
The primary outcome was completion of any screening test within 6 months. The secondary outcome was completion of a full screening strategy within 6 months, including colonoscopy for those with a positive noninvasive test result.
Of the people who had declined first-line tests and were reoffered first-line tests and the blood test, 17.1% completed screening within 6 months, compared with 9.6% of those who were only reoffered the first-line tests. The uptake of colonoscopy and FIT was similar between the two groups. The full-screening strategy was completed by 14.9% in the intervention group, compared with 9% in the control group.
At first glance, the results for uptake seem a bit disappointing, Dr. May said. However, the numbers in this study may not reflect the true potential of the blood tests – which are relatively new and have not yet been incorporated into routine care – because they had to be conducted in a separate appointment at a lab, she said.
If blood tests for CRC were part of the workflow, Dr. May explained, patients could undergo them with a routine blood draw already scheduled to check for diabetes or high cholesterol, for instance, and the numbers presumably would go up.
“I think this study underestimates the proportion of people who will participate,” she said. “We need a study that tests this strategy in a more real-world scenario.”
Nonetheless, “This is the first trial that’s looking at this question, and it’s an important question,” Dr. May added.
Dr. Liang and colleagues acknowledge that a limitation of the study is that it was performed in only one VA center among an older, predominantly male population, so it will be important to make the comparisons in more diverse study populations.
Blood test reliability
The septin 9 blood test is the only blood test approved by the U.S. Food and Drug Administration for CRC screening and is indicated for those who have declined first-line tests. However, the extent of usage of the blood test in this context is unclear, as it has only been approved since 2016.
Because the blood test is not covered by Medicare, Dr. Liang said, accessibility has been limited. One of the reasons for the lack of coverage is that the blood tests are less reliable than first-line tests, he said.
The test detects methylated septin 9 DNA, a biomarker for CRC. The FDA-approved version of the test has a sensitivity of 68% and a specificity of 79% for CRC.
Dr. May said she’d have more confidence in the blood tests if those numbers were higher, at 80%-90%.
Dr. Liang told this news organization that previous research has compared test use when colonoscopy, FIT, and a blood test are considered equally, but because the blood test is indicated only after a person declines first-line screening, his team designed an approach in which the blood test was a second-line option for its target population.
Other blood tests now on the market or under development appear to have higher sensitivity and specificity, he added.
“We think our results are actually applicable to a blood test in general,” Dr. Liang said.
Blood tests are only the first step, though. Getting people who screen positive to follow up with a diagnostic colonoscopy is critical, Dr. May and the authors agree.
“That’s something we, as a nation, just haven’t figured out,” Dr. May said. “It has to become a priority.”
The study was supported in part by the Veterans Health Administration and was funded by Epigenomics and a grant from the New York Society for Gastrointestinal Endoscopy’s Florence Lefcourt Endoscopy Research Award to Dr. Laing, who is also supported by the National Cancer Institute. Dr. Liang has received research support from Epigenomics and Freenome and is on the advisory board for Guardant Health. The remaining authors disclosed no relevant financial relationships. Dr. May is a consultant for Exact Sciences and Geneoscopy, both of which are developing stool tests, and for Freenome, which is developing stool and blood tests.
A version of this article originally appeared on Medscape.com.
, researchers report.
However, the number of people in the study who subsequently underwent timely colonoscopy after a positive blood test did not increase, signaling a continuing challenge in CRC prevention and treatment.
“The main message is that the blood test can do what it’s meant to do, which is increase screening uptake,” first author Peter Liang, MD, MPH, told this news organization. Dr. Liang is a gastroenterologist and researcher at NYU Langone Health and the VA New York Harbor Health Care System in New York.
The study was published online in Clinical Gastroenterology and Hepatology.
In the United States, the rate of use of first-line screening has for years been stuck at about 70% or lower for eligible people, Dr. Liang said. A different modality is needed to help raise the numbers.
The blood test is easy to perform and requires only a few tubes of blood, he noted. No diet restrictions, test prep, or contact with stool is necessary.
We are all searching for ways to get that first-line screening rate up from 60% to 70% to 80% to 90%, noted Folasade P. May, MD, PhD, who was not involved in the study.
“A lot of people think these blood tests are the promised land,” said Dr. May, associate professor of medicine at the University of California, Los Angeles, and director of the gastroenterology quality improvement program at UCLA Health. “We want to see that, when we offer these blood tests, the uptake is 20%-25% higher, which would get us closer to the national goal of 80% screened.”
Blood test as a second-line screening option
The study enrolled 359 veterans at a Veterans Affairs medical center. Participants were 50-75 years old and were eligible for screening but had declined a colonoscopy and a stool test within the previous 6 months.
They were randomly assigned to one of two groups. The control group received a letter and telephone outreach in which participants were again offered screening with colonoscopy or FIT only. The intervention group was additionally offered the blood test as a second-line option.
The primary outcome was completion of any screening test within 6 months. The secondary outcome was completion of a full screening strategy within 6 months, including colonoscopy for those with a positive noninvasive test result.
Of the people who had declined first-line tests and were reoffered first-line tests and the blood test, 17.1% completed screening within 6 months, compared with 9.6% of those who were only reoffered the first-line tests. The uptake of colonoscopy and FIT was similar between the two groups. The full-screening strategy was completed by 14.9% in the intervention group, compared with 9% in the control group.
At first glance, the results for uptake seem a bit disappointing, Dr. May said. However, the numbers in this study may not reflect the true potential of the blood tests – which are relatively new and have not yet been incorporated into routine care – because they had to be conducted in a separate appointment at a lab, she said.
If blood tests for CRC were part of the workflow, Dr. May explained, patients could undergo them with a routine blood draw already scheduled to check for diabetes or high cholesterol, for instance, and the numbers presumably would go up.
“I think this study underestimates the proportion of people who will participate,” she said. “We need a study that tests this strategy in a more real-world scenario.”
Nonetheless, “This is the first trial that’s looking at this question, and it’s an important question,” Dr. May added.
Dr. Liang and colleagues acknowledge that a limitation of the study is that it was performed in only one VA center among an older, predominantly male population, so it will be important to make the comparisons in more diverse study populations.
Blood test reliability
The septin 9 blood test is the only blood test approved by the U.S. Food and Drug Administration for CRC screening and is indicated for those who have declined first-line tests. However, the extent of usage of the blood test in this context is unclear, as it has only been approved since 2016.
Because the blood test is not covered by Medicare, Dr. Liang said, accessibility has been limited. One of the reasons for the lack of coverage is that the blood tests are less reliable than first-line tests, he said.
The test detects methylated septin 9 DNA, a biomarker for CRC. The FDA-approved version of the test has a sensitivity of 68% and a specificity of 79% for CRC.
Dr. May said she’d have more confidence in the blood tests if those numbers were higher, at 80%-90%.
Dr. Liang told this news organization that previous research has compared test use when colonoscopy, FIT, and a blood test are considered equally, but because the blood test is indicated only after a person declines first-line screening, his team designed an approach in which the blood test was a second-line option for its target population.
Other blood tests now on the market or under development appear to have higher sensitivity and specificity, he added.
“We think our results are actually applicable to a blood test in general,” Dr. Liang said.
Blood tests are only the first step, though. Getting people who screen positive to follow up with a diagnostic colonoscopy is critical, Dr. May and the authors agree.
“That’s something we, as a nation, just haven’t figured out,” Dr. May said. “It has to become a priority.”
The study was supported in part by the Veterans Health Administration and was funded by Epigenomics and a grant from the New York Society for Gastrointestinal Endoscopy’s Florence Lefcourt Endoscopy Research Award to Dr. Laing, who is also supported by the National Cancer Institute. Dr. Liang has received research support from Epigenomics and Freenome and is on the advisory board for Guardant Health. The remaining authors disclosed no relevant financial relationships. Dr. May is a consultant for Exact Sciences and Geneoscopy, both of which are developing stool tests, and for Freenome, which is developing stool and blood tests.
A version of this article originally appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Indefinite anticoagulation likely not cost effective after unprovoked VTE
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
FROM ANNALS OF INTERNAL MEDICINE
Placebo effect can be found in a cup of coffee
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
In head and neck cancer, better outcomes seen in patients with overweight
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
FROM JAMA NETWORK OPEN
Eccrine Porocarcinoma in 2 Patients
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.
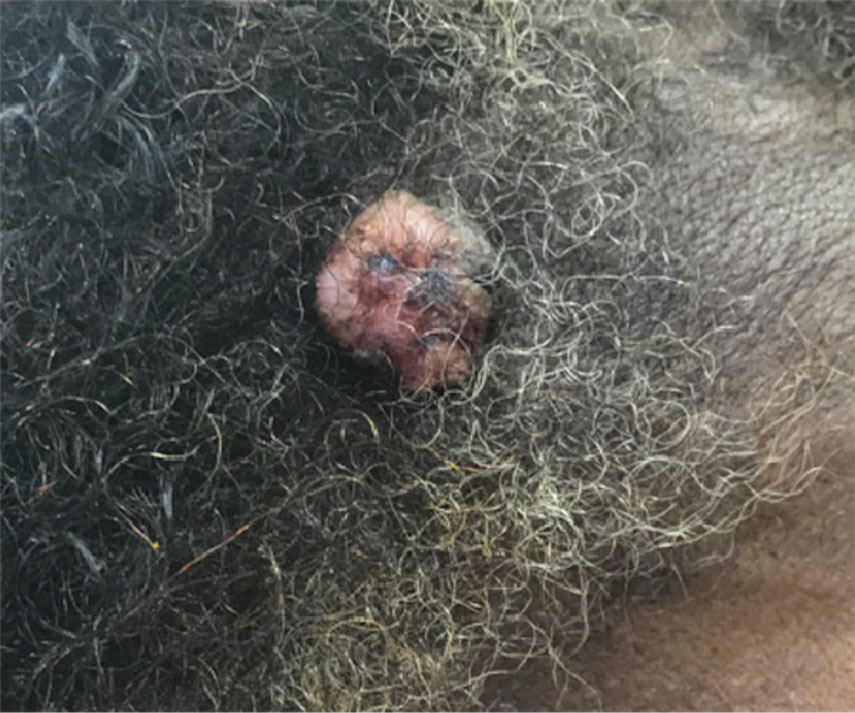
A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.
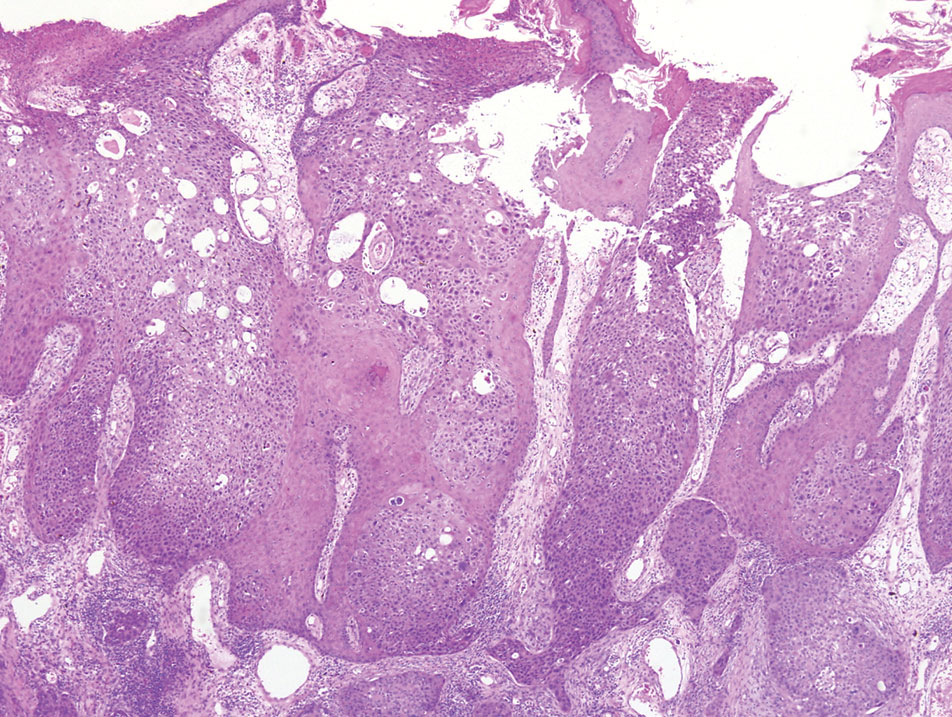
A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.
Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.
Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
Practice Points
- Eccrine porocarcinoma is a rare malignancy that clinically mimics other cutaneous malignancies.
- Early histologic diagnosis is essential, as lymphatic metastasis is common and carries a 65% to 67% mortality rate.
FDA rejects NASH drug for the second time
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
In response, the company has decided to discontinue all NASH-related investment.
Intercept first sought FDA approval for OCA in treatment of NASH in 2019 and received a complete response letter. The company refiled for a new drug application in December 2022. A second resubmission would require a completion of the long-term outcomes phase of an ongoing clinical trial, according to an Intercept press release.
The FDA decision follows the recommendation from May’s FDA Gastrointestinal Drugs Advisory Committee meeting. During the meeting, members voted 15 to 1 to advise deferring approval until clinical outcome data became available. Intercept’s clinical trial data demonstrated that OCA showed moderate benefit over placebo in improving fibrosis in NASH patients, but “there is uncertainty how the magnitude of changes in these surrogate endpoints may translate to meaningful changes in clinical outcomes,” an FDA meeting briefing document stated. There were also notable safety concerns including an increased risk for drug-induced liver injury.
An estimated 16.8 million Americans have NASH, and there are no FDA-approved medications for the condition.
Intercept plans to promptly begin closing out their NASH clinical trial and restructuring to focus on rare and serious liver diseases.
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” said Jerry Durso, the president and CEO of Intercept, in a statement. “Intercept thanks the scientists, clinicians, and patients whose contributions to the clinical development of OCA in NASH have significantly advanced the understanding of this deadly disease.”
A version of this article originally appeared on Medscape.com.
ESMO helps hematologists assess new cancer drugs
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
FROM ANNALS OF ONCOLOGY
Should breast cancer screening start at a younger age?
The US Preventive Services Task Force (USPSTF) recently issued draft recommendations on breast cancer screening that lower the starting age for routine mammography screening for those at average risk.1 The proposed recommendations are an update to USPSTF’s 2016 guidance on this topic.
What’s different. There are 2 major differences in the new recommendations:
- Recommendation for routine mammography starting at age 40 for women at average risk for breast cancer (eg, no personal or family history or genetic risk factors). This is a “B” recommendation (offer or provide the service). Previously, the recommended age to start routine mammography was 50 years, with a “C” recommendation (individual decision-making) for those ages 40 to 49 years.
- No mention of digital tomosynthesis. Previously, this screening modality was rated as an “I” (insufficient evidence to assess). While the new draft recommendation does not mention tomosynthesis, the related evidence report concludes that there is still insufficient evidence to assess it.2
What’s the same. Several important recommendations have not changed. The USPSTF continues to state that the evidence is insufficient to assess the value of (1) supplemental screening with breast ultrasonography and magnetic resonance imaging in women with dense breasts and negative mammograms and (2) mammography in women ages 75 years and older.
And, most importantly, the USPSTF continues to recommend biennial, rather than annual, mammography screening. This recommendation is based on studies that show very little difference in outcomes between these strategies but higher rates of false-positive tests and subsequent biopsies with annual testing.2
What others say. USPSTF’s draft recommendations continue to differ from those of the American Cancer Society, which for average-risk women recommend individual decision-making from ages 40 to 45 years; routine annual mammography for those ages 45 to 54 years; annual or biennial mammography for those ages 55 years and older; and continued screening for women older than 75 years who are in good health and have a life expectancy ≥ 10 years.3
The USPSTF’s rationale for lowering the age at which to start routine mammography is a little puzzling. Several conclusions in the draft evidence report seem to contradict this recommendation:
In the summary of screening effectiveness, the report states “For women ages 39 to 49 years, the combined [relative risk] for breast cancer mortality was 0.92 (95% CI, 0.75 to 1.02; 9 trials); absolute breast cancer mortality reduction was 2.9 (95% CI, –0.6 to 8.9) deaths prevented per 10,000 women over 10 years. None of the trials indicated statistically significantly reduced breast cancer mortality with screening….”2
And in a summary of screening harms, it states that for “every case of invasive breast cancer detected by mammography screening in women age[s] 40 to 49 years, 464 women had screening mammography, 58 were recommended for additional diagnostic imaging, and 10 were recommended for biopsies.”2
The USPSTF apparently based its decision on a modeling study conducted by the Cancer Intervention and Surveillance Modeling Network (CISNET) at USPSTF’s request. This analysis found that screening biennially from ages 50 to 74 years resulted in about 7 breast cancer deaths averted over the lifetimes of 1000 females and that 1 additional death was averted if the starting age for screening was 40 years.4
Financial implications. The USPSTF’s change from a “C” to a “B” recommendation for women ages 40 to 49 years has financial implications. The Affordable Care Act mandates that all “A” and “B” recommendations by the USPSTF have to be provided by commercial health plans with no out-of-pocket costs. (This is currently being challenged in the courts.) However, any follow-up testing for abnormal results is not subject to this provision—so false-positive work-ups and biopsies may result in out-of-pocket costs.
What to discuss with your patients. For women ages 40 to 50 years, discuss the differences in mammography recommendations and the potential risks and benefits of the procedure, as well as financial implications; respect the patient’s decision.
For those ages 50 to 74 years, recommend biennial mammography.
For those older than 74 years, assess life expectancy and other health problems. Discuss the potential risks and benefits of the procedure and respect the patient’s decision.
For all patients, document all discussions and decisions.
1. USPSTF. Breast cancer: screening. Draft recommendation statement. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults
2. Henderson JT, Webber, EM, Weyrich M, et al. Screening for breast cancer: a comparative effectiveness review for the US Preventive Services Task Force. Evidence Synthesis No. 231. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/breast-cancer-screening-adults
3. American Cancer Society. American Cancer Society recommendations for the early detection of breast cancer. Revised January 14, 2022. Accessed June 20, 2023. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
4. Trentham Dietz A, Chapman CH, Jayasekera J, et al. Breast cancer screening with mammography: an updated decision analysis for the US Preventive Services Task Force. Technical report. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-modeling-report/breast-cancer-screening-adults
The US Preventive Services Task Force (USPSTF) recently issued draft recommendations on breast cancer screening that lower the starting age for routine mammography screening for those at average risk.1 The proposed recommendations are an update to USPSTF’s 2016 guidance on this topic.
What’s different. There are 2 major differences in the new recommendations:
- Recommendation for routine mammography starting at age 40 for women at average risk for breast cancer (eg, no personal or family history or genetic risk factors). This is a “B” recommendation (offer or provide the service). Previously, the recommended age to start routine mammography was 50 years, with a “C” recommendation (individual decision-making) for those ages 40 to 49 years.
- No mention of digital tomosynthesis. Previously, this screening modality was rated as an “I” (insufficient evidence to assess). While the new draft recommendation does not mention tomosynthesis, the related evidence report concludes that there is still insufficient evidence to assess it.2
What’s the same. Several important recommendations have not changed. The USPSTF continues to state that the evidence is insufficient to assess the value of (1) supplemental screening with breast ultrasonography and magnetic resonance imaging in women with dense breasts and negative mammograms and (2) mammography in women ages 75 years and older.
And, most importantly, the USPSTF continues to recommend biennial, rather than annual, mammography screening. This recommendation is based on studies that show very little difference in outcomes between these strategies but higher rates of false-positive tests and subsequent biopsies with annual testing.2
What others say. USPSTF’s draft recommendations continue to differ from those of the American Cancer Society, which for average-risk women recommend individual decision-making from ages 40 to 45 years; routine annual mammography for those ages 45 to 54 years; annual or biennial mammography for those ages 55 years and older; and continued screening for women older than 75 years who are in good health and have a life expectancy ≥ 10 years.3
The USPSTF’s rationale for lowering the age at which to start routine mammography is a little puzzling. Several conclusions in the draft evidence report seem to contradict this recommendation:
In the summary of screening effectiveness, the report states “For women ages 39 to 49 years, the combined [relative risk] for breast cancer mortality was 0.92 (95% CI, 0.75 to 1.02; 9 trials); absolute breast cancer mortality reduction was 2.9 (95% CI, –0.6 to 8.9) deaths prevented per 10,000 women over 10 years. None of the trials indicated statistically significantly reduced breast cancer mortality with screening….”2
And in a summary of screening harms, it states that for “every case of invasive breast cancer detected by mammography screening in women age[s] 40 to 49 years, 464 women had screening mammography, 58 were recommended for additional diagnostic imaging, and 10 were recommended for biopsies.”2
The USPSTF apparently based its decision on a modeling study conducted by the Cancer Intervention and Surveillance Modeling Network (CISNET) at USPSTF’s request. This analysis found that screening biennially from ages 50 to 74 years resulted in about 7 breast cancer deaths averted over the lifetimes of 1000 females and that 1 additional death was averted if the starting age for screening was 40 years.4
Financial implications. The USPSTF’s change from a “C” to a “B” recommendation for women ages 40 to 49 years has financial implications. The Affordable Care Act mandates that all “A” and “B” recommendations by the USPSTF have to be provided by commercial health plans with no out-of-pocket costs. (This is currently being challenged in the courts.) However, any follow-up testing for abnormal results is not subject to this provision—so false-positive work-ups and biopsies may result in out-of-pocket costs.
What to discuss with your patients. For women ages 40 to 50 years, discuss the differences in mammography recommendations and the potential risks and benefits of the procedure, as well as financial implications; respect the patient’s decision.
For those ages 50 to 74 years, recommend biennial mammography.
For those older than 74 years, assess life expectancy and other health problems. Discuss the potential risks and benefits of the procedure and respect the patient’s decision.
For all patients, document all discussions and decisions.
The US Preventive Services Task Force (USPSTF) recently issued draft recommendations on breast cancer screening that lower the starting age for routine mammography screening for those at average risk.1 The proposed recommendations are an update to USPSTF’s 2016 guidance on this topic.
What’s different. There are 2 major differences in the new recommendations:
- Recommendation for routine mammography starting at age 40 for women at average risk for breast cancer (eg, no personal or family history or genetic risk factors). This is a “B” recommendation (offer or provide the service). Previously, the recommended age to start routine mammography was 50 years, with a “C” recommendation (individual decision-making) for those ages 40 to 49 years.
- No mention of digital tomosynthesis. Previously, this screening modality was rated as an “I” (insufficient evidence to assess). While the new draft recommendation does not mention tomosynthesis, the related evidence report concludes that there is still insufficient evidence to assess it.2
What’s the same. Several important recommendations have not changed. The USPSTF continues to state that the evidence is insufficient to assess the value of (1) supplemental screening with breast ultrasonography and magnetic resonance imaging in women with dense breasts and negative mammograms and (2) mammography in women ages 75 years and older.
And, most importantly, the USPSTF continues to recommend biennial, rather than annual, mammography screening. This recommendation is based on studies that show very little difference in outcomes between these strategies but higher rates of false-positive tests and subsequent biopsies with annual testing.2
What others say. USPSTF’s draft recommendations continue to differ from those of the American Cancer Society, which for average-risk women recommend individual decision-making from ages 40 to 45 years; routine annual mammography for those ages 45 to 54 years; annual or biennial mammography for those ages 55 years and older; and continued screening for women older than 75 years who are in good health and have a life expectancy ≥ 10 years.3
The USPSTF’s rationale for lowering the age at which to start routine mammography is a little puzzling. Several conclusions in the draft evidence report seem to contradict this recommendation:
In the summary of screening effectiveness, the report states “For women ages 39 to 49 years, the combined [relative risk] for breast cancer mortality was 0.92 (95% CI, 0.75 to 1.02; 9 trials); absolute breast cancer mortality reduction was 2.9 (95% CI, –0.6 to 8.9) deaths prevented per 10,000 women over 10 years. None of the trials indicated statistically significantly reduced breast cancer mortality with screening….”2
And in a summary of screening harms, it states that for “every case of invasive breast cancer detected by mammography screening in women age[s] 40 to 49 years, 464 women had screening mammography, 58 were recommended for additional diagnostic imaging, and 10 were recommended for biopsies.”2
The USPSTF apparently based its decision on a modeling study conducted by the Cancer Intervention and Surveillance Modeling Network (CISNET) at USPSTF’s request. This analysis found that screening biennially from ages 50 to 74 years resulted in about 7 breast cancer deaths averted over the lifetimes of 1000 females and that 1 additional death was averted if the starting age for screening was 40 years.4
Financial implications. The USPSTF’s change from a “C” to a “B” recommendation for women ages 40 to 49 years has financial implications. The Affordable Care Act mandates that all “A” and “B” recommendations by the USPSTF have to be provided by commercial health plans with no out-of-pocket costs. (This is currently being challenged in the courts.) However, any follow-up testing for abnormal results is not subject to this provision—so false-positive work-ups and biopsies may result in out-of-pocket costs.
What to discuss with your patients. For women ages 40 to 50 years, discuss the differences in mammography recommendations and the potential risks and benefits of the procedure, as well as financial implications; respect the patient’s decision.
For those ages 50 to 74 years, recommend biennial mammography.
For those older than 74 years, assess life expectancy and other health problems. Discuss the potential risks and benefits of the procedure and respect the patient’s decision.
For all patients, document all discussions and decisions.
1. USPSTF. Breast cancer: screening. Draft recommendation statement. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults
2. Henderson JT, Webber, EM, Weyrich M, et al. Screening for breast cancer: a comparative effectiveness review for the US Preventive Services Task Force. Evidence Synthesis No. 231. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/breast-cancer-screening-adults
3. American Cancer Society. American Cancer Society recommendations for the early detection of breast cancer. Revised January 14, 2022. Accessed June 20, 2023. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
4. Trentham Dietz A, Chapman CH, Jayasekera J, et al. Breast cancer screening with mammography: an updated decision analysis for the US Preventive Services Task Force. Technical report. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-modeling-report/breast-cancer-screening-adults
1. USPSTF. Breast cancer: screening. Draft recommendation statement. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults
2. Henderson JT, Webber, EM, Weyrich M, et al. Screening for breast cancer: a comparative effectiveness review for the US Preventive Services Task Force. Evidence Synthesis No. 231. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/breast-cancer-screening-adults
3. American Cancer Society. American Cancer Society recommendations for the early detection of breast cancer. Revised January 14, 2022. Accessed June 20, 2023. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
4. Trentham Dietz A, Chapman CH, Jayasekera J, et al. Breast cancer screening with mammography: an updated decision analysis for the US Preventive Services Task Force. Technical report. Agency for Healthcare Research and Quality; 2023. Published May 9, 2023. Accessed June 19, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-modeling-report/breast-cancer-screening-adults