User login
Chronic itch on the upper back, with pain
A 47-year-old man had had a chronic itch on his back for 2 years. He had no history of trauma to the site, nor did he recall applying topical products to that area.
He was otherwise healthy. He worked as an electrician and said he occasionally experienced cervical and back pain while working.
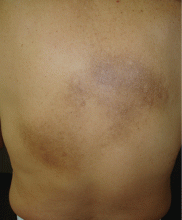
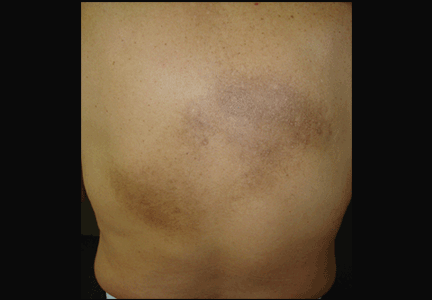
An examination revealed two grayish-brown ovoid patches on the upper back, each 5 cm to 7 cm in diameter (Figure 1).
DIAGNOSIS: NOTALGIA PARESTHETICA
Chronic, brown-gray, itching patches on the back in an adult patient are characteristic of notalgia paresthetica.
Conditions that may be included in the differential diagnosis but that do not match the presentation in this patient include the following:
- Cutaneous sarcoidosis, which may exhibit several morphologies, but itching would be unusual
- Chronic discoid lupus erythematosus, characterized by scarring and atrophic plaques, but mainly on the face and scalp
- Contact dermatitis, an itchy eczematous condition, characterized by scaly erythematous plaques
- Lichen amyloidosis, a variant of cutaneous amyloidosis characterized by the deposition of amyloid or amyloid-like proteins in the dermis, resulting in red-brown hyperkeratotic lichenoid papules, usually on the pretibial surfaces.
CAUSES AND MANAGEMENT
Notalgia paresthetica is a neuropathic syndrome of the skin of the middle of the back characterized by localized pruritus.1–3 Although common, it often goes undiagnosed.1,3,4 It tends to be chronic, with periodic remissions and exacerbations.
Notalgia paresthetica is thought to be a sensory neuropathy and may result from compression of the posterior rami of spinal nerve segments T2 to T6. Slight degenerative changes are often but not always observed, and their clinical significance is uncertain.1,2,4 The condition affects people of all races and both sexes, usually adults ages 40 to 80.
Clinically, it presents as localized pruritus on the back, usually within the dermatomes T2 to T6.5 Examination reveals a hyperpigmented patch, sometimes with excoriations.5
Diagnosis is based on clinical findings. Laboratory tests are not useful. Imaging is not needed, but magnetic resonance imaging and evaluation by an orthopedic surgeon are appropriate when there is chronic focal pain. Skin biopsy is usually not necessary, although it may be useful in some patients to exclude other conditions. When biopsy is done, macular amyloidosis or postinflammatory hyperpigmentation is seen.
Treatment is difficult. Topical steroids and oral antihistamines are usually ineffective,5 but topical capsaicin may provide temporary relief.3 The most recommended treatment in patients with notalgia paresthetica and underlying spinal disease is evaluation and conservative management of the spinal disease, including progressive exercise and rehabilitation.2 Other therapies include oxcarbazepine, gabapentin, transcutaneous electrical nerve stimulation, phototherapy,6 and botulinum toxin injection.
TREATMENT OF OUR PATIENT
In our patient, an orthopedic evaluation revealed cervicothoracic scoliosis. He underwent 6 months of conservative treatment under the care of his family physician and a dermatologist. Treatment consisted of exercise and rehabilitation for his scoliosis, and daily application of topical mometasone. The pain and itch gradual improved.
- Pérez-Pérez LC. General features and treatment of notalgia paresthetica. Skinmed 2011; 9:353–358.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol 2011; 91:356–357.
- Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol 1995; 32:287–289.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
- Raison-Peyron N, Meunier L, Acevedo M, Meynadier J. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol 1999; 12:215–221.
- Pérez-Pérez L, Allegue F, Fabeiro JM, Caeiro JL, Zulaica A. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol 2010; 24:730–732.
A 47-year-old man had had a chronic itch on his back for 2 years. He had no history of trauma to the site, nor did he recall applying topical products to that area.
He was otherwise healthy. He worked as an electrician and said he occasionally experienced cervical and back pain while working.
An examination revealed two grayish-brown ovoid patches on the upper back, each 5 cm to 7 cm in diameter (Figure 1).
DIAGNOSIS: NOTALGIA PARESTHETICA
Chronic, brown-gray, itching patches on the back in an adult patient are characteristic of notalgia paresthetica.
Conditions that may be included in the differential diagnosis but that do not match the presentation in this patient include the following:
- Cutaneous sarcoidosis, which may exhibit several morphologies, but itching would be unusual
- Chronic discoid lupus erythematosus, characterized by scarring and atrophic plaques, but mainly on the face and scalp
- Contact dermatitis, an itchy eczematous condition, characterized by scaly erythematous plaques
- Lichen amyloidosis, a variant of cutaneous amyloidosis characterized by the deposition of amyloid or amyloid-like proteins in the dermis, resulting in red-brown hyperkeratotic lichenoid papules, usually on the pretibial surfaces.
CAUSES AND MANAGEMENT
Notalgia paresthetica is a neuropathic syndrome of the skin of the middle of the back characterized by localized pruritus.1–3 Although common, it often goes undiagnosed.1,3,4 It tends to be chronic, with periodic remissions and exacerbations.
Notalgia paresthetica is thought to be a sensory neuropathy and may result from compression of the posterior rami of spinal nerve segments T2 to T6. Slight degenerative changes are often but not always observed, and their clinical significance is uncertain.1,2,4 The condition affects people of all races and both sexes, usually adults ages 40 to 80.
Clinically, it presents as localized pruritus on the back, usually within the dermatomes T2 to T6.5 Examination reveals a hyperpigmented patch, sometimes with excoriations.5
Diagnosis is based on clinical findings. Laboratory tests are not useful. Imaging is not needed, but magnetic resonance imaging and evaluation by an orthopedic surgeon are appropriate when there is chronic focal pain. Skin biopsy is usually not necessary, although it may be useful in some patients to exclude other conditions. When biopsy is done, macular amyloidosis or postinflammatory hyperpigmentation is seen.
Treatment is difficult. Topical steroids and oral antihistamines are usually ineffective,5 but topical capsaicin may provide temporary relief.3 The most recommended treatment in patients with notalgia paresthetica and underlying spinal disease is evaluation and conservative management of the spinal disease, including progressive exercise and rehabilitation.2 Other therapies include oxcarbazepine, gabapentin, transcutaneous electrical nerve stimulation, phototherapy,6 and botulinum toxin injection.
TREATMENT OF OUR PATIENT
In our patient, an orthopedic evaluation revealed cervicothoracic scoliosis. He underwent 6 months of conservative treatment under the care of his family physician and a dermatologist. Treatment consisted of exercise and rehabilitation for his scoliosis, and daily application of topical mometasone. The pain and itch gradual improved.
A 47-year-old man had had a chronic itch on his back for 2 years. He had no history of trauma to the site, nor did he recall applying topical products to that area.
He was otherwise healthy. He worked as an electrician and said he occasionally experienced cervical and back pain while working.
An examination revealed two grayish-brown ovoid patches on the upper back, each 5 cm to 7 cm in diameter (Figure 1).
DIAGNOSIS: NOTALGIA PARESTHETICA
Chronic, brown-gray, itching patches on the back in an adult patient are characteristic of notalgia paresthetica.
Conditions that may be included in the differential diagnosis but that do not match the presentation in this patient include the following:
- Cutaneous sarcoidosis, which may exhibit several morphologies, but itching would be unusual
- Chronic discoid lupus erythematosus, characterized by scarring and atrophic plaques, but mainly on the face and scalp
- Contact dermatitis, an itchy eczematous condition, characterized by scaly erythematous plaques
- Lichen amyloidosis, a variant of cutaneous amyloidosis characterized by the deposition of amyloid or amyloid-like proteins in the dermis, resulting in red-brown hyperkeratotic lichenoid papules, usually on the pretibial surfaces.
CAUSES AND MANAGEMENT
Notalgia paresthetica is a neuropathic syndrome of the skin of the middle of the back characterized by localized pruritus.1–3 Although common, it often goes undiagnosed.1,3,4 It tends to be chronic, with periodic remissions and exacerbations.
Notalgia paresthetica is thought to be a sensory neuropathy and may result from compression of the posterior rami of spinal nerve segments T2 to T6. Slight degenerative changes are often but not always observed, and their clinical significance is uncertain.1,2,4 The condition affects people of all races and both sexes, usually adults ages 40 to 80.
Clinically, it presents as localized pruritus on the back, usually within the dermatomes T2 to T6.5 Examination reveals a hyperpigmented patch, sometimes with excoriations.5
Diagnosis is based on clinical findings. Laboratory tests are not useful. Imaging is not needed, but magnetic resonance imaging and evaluation by an orthopedic surgeon are appropriate when there is chronic focal pain. Skin biopsy is usually not necessary, although it may be useful in some patients to exclude other conditions. When biopsy is done, macular amyloidosis or postinflammatory hyperpigmentation is seen.
Treatment is difficult. Topical steroids and oral antihistamines are usually ineffective,5 but topical capsaicin may provide temporary relief.3 The most recommended treatment in patients with notalgia paresthetica and underlying spinal disease is evaluation and conservative management of the spinal disease, including progressive exercise and rehabilitation.2 Other therapies include oxcarbazepine, gabapentin, transcutaneous electrical nerve stimulation, phototherapy,6 and botulinum toxin injection.
TREATMENT OF OUR PATIENT
In our patient, an orthopedic evaluation revealed cervicothoracic scoliosis. He underwent 6 months of conservative treatment under the care of his family physician and a dermatologist. Treatment consisted of exercise and rehabilitation for his scoliosis, and daily application of topical mometasone. The pain and itch gradual improved.
- Pérez-Pérez LC. General features and treatment of notalgia paresthetica. Skinmed 2011; 9:353–358.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol 2011; 91:356–357.
- Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol 1995; 32:287–289.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
- Raison-Peyron N, Meunier L, Acevedo M, Meynadier J. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol 1999; 12:215–221.
- Pérez-Pérez L, Allegue F, Fabeiro JM, Caeiro JL, Zulaica A. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol 2010; 24:730–732.
- Pérez-Pérez LC. General features and treatment of notalgia paresthetica. Skinmed 2011; 9:353–358.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol 2011; 91:356–357.
- Wallengren J, Klinker M. Successful treatment of notalgia paresthetica with topical capsaicin: vehicle-controlled, double-blind, crossover study. J Am Acad Dermatol 1995; 32:287–289.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
- Raison-Peyron N, Meunier L, Acevedo M, Meynadier J. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. A study of 12 cases. J Eur Acad Dermatol Venereol 1999; 12:215–221.
- Pérez-Pérez L, Allegue F, Fabeiro JM, Caeiro JL, Zulaica A. Notalgia paresthesica successfully treated with narrow-band UVB: report of five cases. J Eur Acad Dermatol Venereol 2010; 24:730–732.
Which lower-extremity DVTs should be removed early?
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
Azithromycin and risk of sudden cardiac death: Guilty as charged or falsely accused?
A March 2013 warning by the US Food and Drug Administration that azithromycin (Zithromax, Zmax, Z-pak) may increase the risk of sudden cardiac death does not mean we must abandon using it. We should, however, try to determine if our patients have cardiovascular risk factors for this extreme side effect and take appropriate precautions.
AZITHROMYCIN: THE SAFEST OF THE MACROLIDES?
Azithromycin, a broad-spectrum macrolide antibiotic, is used to treat or prevent a range of common bacterial infections, including upper and lower respiratory tract infections and certain sexually transmitted diseases.
In terms of overall toxicity, azithromycin has been considered the safest of the macrolides, as it neither undergoes CYP3A4 metabolism nor inhibits CYP3A4 to any clinically meaningful degree, and therefore does not interfere with the array of commonly used medications that undergo CYP3A4 metabolism.
Also, in vitro, azithromycin shows only limited blockade of the potassium channel hERG. This channel is critically involved in cardiomyocyte repolarization, and if it is blocked or otherwise malfunctioning, the result can be a prolonged QT interval, ventricular arrhythmias, and even sudden cardiac death.1–4 Therefore, lack of blockade, as reflected by a high inhibitory concentration (Table 1), boded well for the safety of azithromycin in terms of QT liability. However, we should be cautious in interpreting in vitro data.
With its broad antibiotic spectrum and perceived favorable safety profile, azithromycin has become one of the top 15 most prescribed drugs and the best-selling antibiotic in the United States, accounting for 55.4 million prescriptions in 2012, according to the IMS Institute for Healthcare Informatics.
THE FDA RECEIVES 203 REPORTS OF ADVERSE EVENTS IN 8 YEARS
However, beginning with a report of azithromycin-triggered torsades de pointes in 2001,5 a growing body of evidence, derived from postmarketing surveillance, has linked azithromycin to cardiac arrhythmias such as pronounced QT interval prolongation and associated torsades de pointes (which can progress to life-threatening ventricular fibrillation). Other, closely related macrolides such as clarithromycin and erythromycin are also linked to these effects.
Furthermore, in the 8-year period from 2004 to 2011, the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) received a total of 203 reports of azithromycin-associated QT prolongation, torsades de pointes, ventricular arrhythmia, or, in 65 cases, sudden cardiac death (Table 1).6
At face value, the number of FAERS reports appears to be similar between the various macrolide antibiotics. However, it is important to remember that these drugs differ substantially in the number of prescriptions written for them, with azithromycin being prescribed more often. Also, the FAERS numbers are subject to a number of well-known limitations such as confounding variables, uneven quality and completeness of reports, duplication, and underreporting. These limitations preclude the use of such adverse reporting databases in calculating and thereby comparing the true incidence of adverse events associated with the various macrolide antibiotics.6–9
RAY ET AL FIND A HIGHER RISK OF CARDIOVASCULAR DEATH
Despite these inherent flaws, initial postmarketing surveillance reports cast enough doubt on the long-standing notion that azithromycin is the safest macrolide antibiotic to prompt Ray et al10 to assess its safety in an observational, nonrandomized study of people enrolled in the Tennessee Medicaid program.
They found that, over the typical 5 days of therapy, people taking azithromycin had a rate of cardiovascular death 2.88 times higher than in people taking no antibiotic, and 2.49 times higher than in people taking amoxicillin (Table 2).
However, the absolute excess risk compared with amoxicillin varied considerably according to baseline risk score for cardiovascular disease, with 1 excess cardiovascular death per 4,100 in the highest-risk decile compared with 1 excess cardiovascular death per 100,000 in the lowest-risk decile.10,11
Moreover, the increase in deaths did not persist after the 5 days of therapy. This time-limited pattern directly correlated with expected peak azithromycin plasma levels during a standard 5-day course.
Ray et al used appropriate analytic methods to attempt to correct for any confounding bias intrinsic to the observational, nonrandomized study design. Nevertheless, the patients were Medicaid beneficiaries, who have a higher prevalence of comorbid conditions and higher mortality rates than the general population. Therefore, legitimate questions were raised about whether the results of the study could be generalized to populations with substantially lower baseline risk of cardiovascular disease and if differences in the baseline characteristics of the treatment groups were adequately controlled.12,13
THE FDA REVISES AZITHROMYCIN’S WARNINGS AND PRECAUTIONS
The striking observations by Ray et al,10 coupled with the concerns raised by postmarketing surveillance reports, compelled the FDA to review the labels of azithromycin and other macrolide antibiotics.
Ultimately, the FDA opted to revise the “warning and precautions” section of the azithromycin drug label to include a warning about the potential risk of fatal arrhythmias, specifically QT interval prolongation and torsades de pointes. In a March 2013 safety announcement, it also urged health care professionals to use caution when prescribing azithromycin to patients known to have risk factors for drug-related arrhythmias, including congenital long QT syndrome, acquired QT interval prolongation, hypokalemia, hypomagnesia, bradycardia, and concurrent use of other medications known to prolong the QT interval, specifically the class IA (eg, quinidine and procainamide) and class III (eg, amiodarone, sotalol, and dofetilide) antiarrhythmics.
SVANSTRÖM ET AL FIND NO INCREASED RISK
However, just when the medical community appeared ready to accept that azithromycin may not be as safe as we thought it was, a large prospective study by Svanström et al, published in early May 2013, found no increased risk of cardiovascular death associated with azithromycin (Table 2).14
The patients were a representative population of young to middle-aged Danish adults at low baseline risk of underlying cardiovascular disease.
Interestingly, Svanström et al were careful to point out that their study was only powered to rule out a moderate-to-high (> 55%) increase in the relative risk of cardiovascular death. Furthermore, profound differences existed in the baseline risk of death and cardiovascular risk factors between their patients and the Tennessee Medicaid patients studied by Ray et al.14 Therefore, the authors suggested that their study complements rather than contradicts the study by Ray et al. They attributed the differences in the findings to treatment-effect heterogeneity, in which the risk of azithromycin-associated cardiovascular mortality is largely limited to high-risk patients, namely those with multiple preexisting cardiovascular risk factors.14
ACC/AHA RECOMMENDATION: IDENTIFY THOSE AT RISK
Collectively, the data reviewed above provide compelling evidence that azithromycin is not completely free of the QT-prolonging and torsadogenic effects that have long been associated with other macrolide antibiotics. However, the findings from both the study by Ray et al and that of Svanström et al suggest that preexisting cardiovascular risk factors play a prominent role in determining the incidence of azithromycin-associated cardiovascular death in a given population (Table 2).10,14
These findings should prompt physicians to carefully reassess the risks and benefits of azithromycin use in their clinical practices. They also reinforce a recent call by the American Heart Association (AHA) and American College of Cardiology (ACC) to better identify, early on, patients at risk of drug-induced ventricular arrhythmias and sudden death and to subsequently improve how these patients are monitored when the use of QT-prolonging and torsadogenic drugs is medically necessary.15
AN ELECTRONIC MEDICAL RECORD FLAGS QTc ≥ 500 MS
On the heels of these AHA/ACC suggestions, our hospital has adopted an institution-wide QT alert system. Here, the electronic medical record system (Centricity EMR; GE Healthcare) uses a proprietary algorithm to detect and electronically alert ordering physicians when a patient has a prolonged QT interval, and gives information about the potential clinical significance of this electrocardiographic finding.16 Physicians also receive a warning when ordering QT-prolonging drugs in patients at risk.
This system is still in its infancy, but it has already confirmed that a prolonged QT interval (QTc ≥ 500 ms) is a powerful predictor of death from any cause and has demonstrated that mortality rates in those with prolonged QT intervals increase in a dose-dependent fashion with the patient’s number of modifiable risk factors (eg, electrolyte disturbances or QT-prolonging medications) and nonmodifiable risk factors (eg, genetic disposition, female sex, structural heart disease, diabetes mellitus).16 We have also found evidence that modifiable risk factors may have a more pronounced effect on mortality risk than non-modifiable risk factors.16
These findings suggest that information technology-based QT alert systems may one day provide physicians with an important tool to efficiently identify and possibly even modify the risk of cardiovascular death in patients at high risk, for example, by correcting electrolyte abnormalities or reducing the burden of QT-prolonging medications.
CONSIDER RISK OF QT PROLONGATION WHEN PRESCRIBING AZITHROMYCIN
For most institutions and clinical practices, such electronic QT alert systems are still years if not decades away. However, in light of the information summarized above, all physicians should begin considering risk factors for QT prolongation and torsades de pointes (summarized in Table 3) and weighing the risks and benefits of prescribing azithromycin vs alternative antibiotics with minimal QT liability. This should be relatively simple to do. Things to keep in mind:
- Although azithromycin may increase the relative risk of a cardiovascular event, for most otherwise-healthy patients, the absolute risk is miniscule.
- In a patient at risk (eg, with baseline QT prolongation or multiple risk factors for it), if azithromycin or another QT-prolonging antibiotic such as a macrolide or fluoroquinolone is medically necessary due to preferential bacterial susceptibility or patient allergies, every effort should be made to correct modifiable risk factors (eg, electrolyte abnormalities) and, if possible, to avoid polypharmacy with multiple QT-prolonging drugs.
- For patients who have multiple risk factors for QT prolongation in whom treatment with a known QT-prolonging medication is still deemed in the patient’s best interest, strong consideration should be given to inpatient administration and monitoring until the treatment has been completed.
With careful consideration of modifiable and nonmodifiable risk factors as well as a little extra caution when prescribing potential QT-prolonging medications such as azithromycin, the clinical benefit of these often-advantageous medications can be maximized and the incidence of these tragic but rare drug-induced sudden cardiac deaths can be reduced.
- Hopkins S. Clinical toleration and safety of azithromycin. Am J Med 1991; 91:40S–45S.
- Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther 2002; 303:218–225.
- Ioannidis JP, Contopoulos-Ioannidis DG, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for upper respiratory tract infections. J Antimicrob Chemother 2001; 48:677–689.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43:1603–1611.
- Arellano-Rodrigo E, García A, Mont L, Roqué M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. (Article in Spanish.) Med Clin (Barc) 2001; 117:118–119.
- Raschi E, Poluzzi E, Koci A, Moretti U, Sturkenboom M, De Ponti F. Macrolides and torsadogenic risk: emerging issues from the fda pharmacovigilance database. J Pharmacovigilance 2013; 1:104.
- Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002; 35:197–200.
- Stephenson WP, Hauben M. Data mining for signals in spontaneous reporting databases: proceed with caution. Pharmacoepidemiol Drug Saf 2007; 16:359–365.
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009; 18:427–436.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Mosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med 2013; 368:1665–1668.
- Louie R. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Koga T, Imaoka H. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Drew BJ, Ackerman MJ, Funk M, et al; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121:1047–1060.
- Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 2013; 88:315–325.
A March 2013 warning by the US Food and Drug Administration that azithromycin (Zithromax, Zmax, Z-pak) may increase the risk of sudden cardiac death does not mean we must abandon using it. We should, however, try to determine if our patients have cardiovascular risk factors for this extreme side effect and take appropriate precautions.
AZITHROMYCIN: THE SAFEST OF THE MACROLIDES?
Azithromycin, a broad-spectrum macrolide antibiotic, is used to treat or prevent a range of common bacterial infections, including upper and lower respiratory tract infections and certain sexually transmitted diseases.
In terms of overall toxicity, azithromycin has been considered the safest of the macrolides, as it neither undergoes CYP3A4 metabolism nor inhibits CYP3A4 to any clinically meaningful degree, and therefore does not interfere with the array of commonly used medications that undergo CYP3A4 metabolism.
Also, in vitro, azithromycin shows only limited blockade of the potassium channel hERG. This channel is critically involved in cardiomyocyte repolarization, and if it is blocked or otherwise malfunctioning, the result can be a prolonged QT interval, ventricular arrhythmias, and even sudden cardiac death.1–4 Therefore, lack of blockade, as reflected by a high inhibitory concentration (Table 1), boded well for the safety of azithromycin in terms of QT liability. However, we should be cautious in interpreting in vitro data.
With its broad antibiotic spectrum and perceived favorable safety profile, azithromycin has become one of the top 15 most prescribed drugs and the best-selling antibiotic in the United States, accounting for 55.4 million prescriptions in 2012, according to the IMS Institute for Healthcare Informatics.
THE FDA RECEIVES 203 REPORTS OF ADVERSE EVENTS IN 8 YEARS
However, beginning with a report of azithromycin-triggered torsades de pointes in 2001,5 a growing body of evidence, derived from postmarketing surveillance, has linked azithromycin to cardiac arrhythmias such as pronounced QT interval prolongation and associated torsades de pointes (which can progress to life-threatening ventricular fibrillation). Other, closely related macrolides such as clarithromycin and erythromycin are also linked to these effects.
Furthermore, in the 8-year period from 2004 to 2011, the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) received a total of 203 reports of azithromycin-associated QT prolongation, torsades de pointes, ventricular arrhythmia, or, in 65 cases, sudden cardiac death (Table 1).6
At face value, the number of FAERS reports appears to be similar between the various macrolide antibiotics. However, it is important to remember that these drugs differ substantially in the number of prescriptions written for them, with azithromycin being prescribed more often. Also, the FAERS numbers are subject to a number of well-known limitations such as confounding variables, uneven quality and completeness of reports, duplication, and underreporting. These limitations preclude the use of such adverse reporting databases in calculating and thereby comparing the true incidence of adverse events associated with the various macrolide antibiotics.6–9
RAY ET AL FIND A HIGHER RISK OF CARDIOVASCULAR DEATH
Despite these inherent flaws, initial postmarketing surveillance reports cast enough doubt on the long-standing notion that azithromycin is the safest macrolide antibiotic to prompt Ray et al10 to assess its safety in an observational, nonrandomized study of people enrolled in the Tennessee Medicaid program.
They found that, over the typical 5 days of therapy, people taking azithromycin had a rate of cardiovascular death 2.88 times higher than in people taking no antibiotic, and 2.49 times higher than in people taking amoxicillin (Table 2).
However, the absolute excess risk compared with amoxicillin varied considerably according to baseline risk score for cardiovascular disease, with 1 excess cardiovascular death per 4,100 in the highest-risk decile compared with 1 excess cardiovascular death per 100,000 in the lowest-risk decile.10,11
Moreover, the increase in deaths did not persist after the 5 days of therapy. This time-limited pattern directly correlated with expected peak azithromycin plasma levels during a standard 5-day course.
Ray et al used appropriate analytic methods to attempt to correct for any confounding bias intrinsic to the observational, nonrandomized study design. Nevertheless, the patients were Medicaid beneficiaries, who have a higher prevalence of comorbid conditions and higher mortality rates than the general population. Therefore, legitimate questions were raised about whether the results of the study could be generalized to populations with substantially lower baseline risk of cardiovascular disease and if differences in the baseline characteristics of the treatment groups were adequately controlled.12,13
THE FDA REVISES AZITHROMYCIN’S WARNINGS AND PRECAUTIONS
The striking observations by Ray et al,10 coupled with the concerns raised by postmarketing surveillance reports, compelled the FDA to review the labels of azithromycin and other macrolide antibiotics.
Ultimately, the FDA opted to revise the “warning and precautions” section of the azithromycin drug label to include a warning about the potential risk of fatal arrhythmias, specifically QT interval prolongation and torsades de pointes. In a March 2013 safety announcement, it also urged health care professionals to use caution when prescribing azithromycin to patients known to have risk factors for drug-related arrhythmias, including congenital long QT syndrome, acquired QT interval prolongation, hypokalemia, hypomagnesia, bradycardia, and concurrent use of other medications known to prolong the QT interval, specifically the class IA (eg, quinidine and procainamide) and class III (eg, amiodarone, sotalol, and dofetilide) antiarrhythmics.
SVANSTRÖM ET AL FIND NO INCREASED RISK
However, just when the medical community appeared ready to accept that azithromycin may not be as safe as we thought it was, a large prospective study by Svanström et al, published in early May 2013, found no increased risk of cardiovascular death associated with azithromycin (Table 2).14
The patients were a representative population of young to middle-aged Danish adults at low baseline risk of underlying cardiovascular disease.
Interestingly, Svanström et al were careful to point out that their study was only powered to rule out a moderate-to-high (> 55%) increase in the relative risk of cardiovascular death. Furthermore, profound differences existed in the baseline risk of death and cardiovascular risk factors between their patients and the Tennessee Medicaid patients studied by Ray et al.14 Therefore, the authors suggested that their study complements rather than contradicts the study by Ray et al. They attributed the differences in the findings to treatment-effect heterogeneity, in which the risk of azithromycin-associated cardiovascular mortality is largely limited to high-risk patients, namely those with multiple preexisting cardiovascular risk factors.14
ACC/AHA RECOMMENDATION: IDENTIFY THOSE AT RISK
Collectively, the data reviewed above provide compelling evidence that azithromycin is not completely free of the QT-prolonging and torsadogenic effects that have long been associated with other macrolide antibiotics. However, the findings from both the study by Ray et al and that of Svanström et al suggest that preexisting cardiovascular risk factors play a prominent role in determining the incidence of azithromycin-associated cardiovascular death in a given population (Table 2).10,14
These findings should prompt physicians to carefully reassess the risks and benefits of azithromycin use in their clinical practices. They also reinforce a recent call by the American Heart Association (AHA) and American College of Cardiology (ACC) to better identify, early on, patients at risk of drug-induced ventricular arrhythmias and sudden death and to subsequently improve how these patients are monitored when the use of QT-prolonging and torsadogenic drugs is medically necessary.15
AN ELECTRONIC MEDICAL RECORD FLAGS QTc ≥ 500 MS
On the heels of these AHA/ACC suggestions, our hospital has adopted an institution-wide QT alert system. Here, the electronic medical record system (Centricity EMR; GE Healthcare) uses a proprietary algorithm to detect and electronically alert ordering physicians when a patient has a prolonged QT interval, and gives information about the potential clinical significance of this electrocardiographic finding.16 Physicians also receive a warning when ordering QT-prolonging drugs in patients at risk.
This system is still in its infancy, but it has already confirmed that a prolonged QT interval (QTc ≥ 500 ms) is a powerful predictor of death from any cause and has demonstrated that mortality rates in those with prolonged QT intervals increase in a dose-dependent fashion with the patient’s number of modifiable risk factors (eg, electrolyte disturbances or QT-prolonging medications) and nonmodifiable risk factors (eg, genetic disposition, female sex, structural heart disease, diabetes mellitus).16 We have also found evidence that modifiable risk factors may have a more pronounced effect on mortality risk than non-modifiable risk factors.16
These findings suggest that information technology-based QT alert systems may one day provide physicians with an important tool to efficiently identify and possibly even modify the risk of cardiovascular death in patients at high risk, for example, by correcting electrolyte abnormalities or reducing the burden of QT-prolonging medications.
CONSIDER RISK OF QT PROLONGATION WHEN PRESCRIBING AZITHROMYCIN
For most institutions and clinical practices, such electronic QT alert systems are still years if not decades away. However, in light of the information summarized above, all physicians should begin considering risk factors for QT prolongation and torsades de pointes (summarized in Table 3) and weighing the risks and benefits of prescribing azithromycin vs alternative antibiotics with minimal QT liability. This should be relatively simple to do. Things to keep in mind:
- Although azithromycin may increase the relative risk of a cardiovascular event, for most otherwise-healthy patients, the absolute risk is miniscule.
- In a patient at risk (eg, with baseline QT prolongation or multiple risk factors for it), if azithromycin or another QT-prolonging antibiotic such as a macrolide or fluoroquinolone is medically necessary due to preferential bacterial susceptibility or patient allergies, every effort should be made to correct modifiable risk factors (eg, electrolyte abnormalities) and, if possible, to avoid polypharmacy with multiple QT-prolonging drugs.
- For patients who have multiple risk factors for QT prolongation in whom treatment with a known QT-prolonging medication is still deemed in the patient’s best interest, strong consideration should be given to inpatient administration and monitoring until the treatment has been completed.
With careful consideration of modifiable and nonmodifiable risk factors as well as a little extra caution when prescribing potential QT-prolonging medications such as azithromycin, the clinical benefit of these often-advantageous medications can be maximized and the incidence of these tragic but rare drug-induced sudden cardiac deaths can be reduced.
A March 2013 warning by the US Food and Drug Administration that azithromycin (Zithromax, Zmax, Z-pak) may increase the risk of sudden cardiac death does not mean we must abandon using it. We should, however, try to determine if our patients have cardiovascular risk factors for this extreme side effect and take appropriate precautions.
AZITHROMYCIN: THE SAFEST OF THE MACROLIDES?
Azithromycin, a broad-spectrum macrolide antibiotic, is used to treat or prevent a range of common bacterial infections, including upper and lower respiratory tract infections and certain sexually transmitted diseases.
In terms of overall toxicity, azithromycin has been considered the safest of the macrolides, as it neither undergoes CYP3A4 metabolism nor inhibits CYP3A4 to any clinically meaningful degree, and therefore does not interfere with the array of commonly used medications that undergo CYP3A4 metabolism.
Also, in vitro, azithromycin shows only limited blockade of the potassium channel hERG. This channel is critically involved in cardiomyocyte repolarization, and if it is blocked or otherwise malfunctioning, the result can be a prolonged QT interval, ventricular arrhythmias, and even sudden cardiac death.1–4 Therefore, lack of blockade, as reflected by a high inhibitory concentration (Table 1), boded well for the safety of azithromycin in terms of QT liability. However, we should be cautious in interpreting in vitro data.
With its broad antibiotic spectrum and perceived favorable safety profile, azithromycin has become one of the top 15 most prescribed drugs and the best-selling antibiotic in the United States, accounting for 55.4 million prescriptions in 2012, according to the IMS Institute for Healthcare Informatics.
THE FDA RECEIVES 203 REPORTS OF ADVERSE EVENTS IN 8 YEARS
However, beginning with a report of azithromycin-triggered torsades de pointes in 2001,5 a growing body of evidence, derived from postmarketing surveillance, has linked azithromycin to cardiac arrhythmias such as pronounced QT interval prolongation and associated torsades de pointes (which can progress to life-threatening ventricular fibrillation). Other, closely related macrolides such as clarithromycin and erythromycin are also linked to these effects.
Furthermore, in the 8-year period from 2004 to 2011, the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) received a total of 203 reports of azithromycin-associated QT prolongation, torsades de pointes, ventricular arrhythmia, or, in 65 cases, sudden cardiac death (Table 1).6
At face value, the number of FAERS reports appears to be similar between the various macrolide antibiotics. However, it is important to remember that these drugs differ substantially in the number of prescriptions written for them, with azithromycin being prescribed more often. Also, the FAERS numbers are subject to a number of well-known limitations such as confounding variables, uneven quality and completeness of reports, duplication, and underreporting. These limitations preclude the use of such adverse reporting databases in calculating and thereby comparing the true incidence of adverse events associated with the various macrolide antibiotics.6–9
RAY ET AL FIND A HIGHER RISK OF CARDIOVASCULAR DEATH
Despite these inherent flaws, initial postmarketing surveillance reports cast enough doubt on the long-standing notion that azithromycin is the safest macrolide antibiotic to prompt Ray et al10 to assess its safety in an observational, nonrandomized study of people enrolled in the Tennessee Medicaid program.
They found that, over the typical 5 days of therapy, people taking azithromycin had a rate of cardiovascular death 2.88 times higher than in people taking no antibiotic, and 2.49 times higher than in people taking amoxicillin (Table 2).
However, the absolute excess risk compared with amoxicillin varied considerably according to baseline risk score for cardiovascular disease, with 1 excess cardiovascular death per 4,100 in the highest-risk decile compared with 1 excess cardiovascular death per 100,000 in the lowest-risk decile.10,11
Moreover, the increase in deaths did not persist after the 5 days of therapy. This time-limited pattern directly correlated with expected peak azithromycin plasma levels during a standard 5-day course.
Ray et al used appropriate analytic methods to attempt to correct for any confounding bias intrinsic to the observational, nonrandomized study design. Nevertheless, the patients were Medicaid beneficiaries, who have a higher prevalence of comorbid conditions and higher mortality rates than the general population. Therefore, legitimate questions were raised about whether the results of the study could be generalized to populations with substantially lower baseline risk of cardiovascular disease and if differences in the baseline characteristics of the treatment groups were adequately controlled.12,13
THE FDA REVISES AZITHROMYCIN’S WARNINGS AND PRECAUTIONS
The striking observations by Ray et al,10 coupled with the concerns raised by postmarketing surveillance reports, compelled the FDA to review the labels of azithromycin and other macrolide antibiotics.
Ultimately, the FDA opted to revise the “warning and precautions” section of the azithromycin drug label to include a warning about the potential risk of fatal arrhythmias, specifically QT interval prolongation and torsades de pointes. In a March 2013 safety announcement, it also urged health care professionals to use caution when prescribing azithromycin to patients known to have risk factors for drug-related arrhythmias, including congenital long QT syndrome, acquired QT interval prolongation, hypokalemia, hypomagnesia, bradycardia, and concurrent use of other medications known to prolong the QT interval, specifically the class IA (eg, quinidine and procainamide) and class III (eg, amiodarone, sotalol, and dofetilide) antiarrhythmics.
SVANSTRÖM ET AL FIND NO INCREASED RISK
However, just when the medical community appeared ready to accept that azithromycin may not be as safe as we thought it was, a large prospective study by Svanström et al, published in early May 2013, found no increased risk of cardiovascular death associated with azithromycin (Table 2).14
The patients were a representative population of young to middle-aged Danish adults at low baseline risk of underlying cardiovascular disease.
Interestingly, Svanström et al were careful to point out that their study was only powered to rule out a moderate-to-high (> 55%) increase in the relative risk of cardiovascular death. Furthermore, profound differences existed in the baseline risk of death and cardiovascular risk factors between their patients and the Tennessee Medicaid patients studied by Ray et al.14 Therefore, the authors suggested that their study complements rather than contradicts the study by Ray et al. They attributed the differences in the findings to treatment-effect heterogeneity, in which the risk of azithromycin-associated cardiovascular mortality is largely limited to high-risk patients, namely those with multiple preexisting cardiovascular risk factors.14
ACC/AHA RECOMMENDATION: IDENTIFY THOSE AT RISK
Collectively, the data reviewed above provide compelling evidence that azithromycin is not completely free of the QT-prolonging and torsadogenic effects that have long been associated with other macrolide antibiotics. However, the findings from both the study by Ray et al and that of Svanström et al suggest that preexisting cardiovascular risk factors play a prominent role in determining the incidence of azithromycin-associated cardiovascular death in a given population (Table 2).10,14
These findings should prompt physicians to carefully reassess the risks and benefits of azithromycin use in their clinical practices. They also reinforce a recent call by the American Heart Association (AHA) and American College of Cardiology (ACC) to better identify, early on, patients at risk of drug-induced ventricular arrhythmias and sudden death and to subsequently improve how these patients are monitored when the use of QT-prolonging and torsadogenic drugs is medically necessary.15
AN ELECTRONIC MEDICAL RECORD FLAGS QTc ≥ 500 MS
On the heels of these AHA/ACC suggestions, our hospital has adopted an institution-wide QT alert system. Here, the electronic medical record system (Centricity EMR; GE Healthcare) uses a proprietary algorithm to detect and electronically alert ordering physicians when a patient has a prolonged QT interval, and gives information about the potential clinical significance of this electrocardiographic finding.16 Physicians also receive a warning when ordering QT-prolonging drugs in patients at risk.
This system is still in its infancy, but it has already confirmed that a prolonged QT interval (QTc ≥ 500 ms) is a powerful predictor of death from any cause and has demonstrated that mortality rates in those with prolonged QT intervals increase in a dose-dependent fashion with the patient’s number of modifiable risk factors (eg, electrolyte disturbances or QT-prolonging medications) and nonmodifiable risk factors (eg, genetic disposition, female sex, structural heart disease, diabetes mellitus).16 We have also found evidence that modifiable risk factors may have a more pronounced effect on mortality risk than non-modifiable risk factors.16
These findings suggest that information technology-based QT alert systems may one day provide physicians with an important tool to efficiently identify and possibly even modify the risk of cardiovascular death in patients at high risk, for example, by correcting electrolyte abnormalities or reducing the burden of QT-prolonging medications.
CONSIDER RISK OF QT PROLONGATION WHEN PRESCRIBING AZITHROMYCIN
For most institutions and clinical practices, such electronic QT alert systems are still years if not decades away. However, in light of the information summarized above, all physicians should begin considering risk factors for QT prolongation and torsades de pointes (summarized in Table 3) and weighing the risks and benefits of prescribing azithromycin vs alternative antibiotics with minimal QT liability. This should be relatively simple to do. Things to keep in mind:
- Although azithromycin may increase the relative risk of a cardiovascular event, for most otherwise-healthy patients, the absolute risk is miniscule.
- In a patient at risk (eg, with baseline QT prolongation or multiple risk factors for it), if azithromycin or another QT-prolonging antibiotic such as a macrolide or fluoroquinolone is medically necessary due to preferential bacterial susceptibility or patient allergies, every effort should be made to correct modifiable risk factors (eg, electrolyte abnormalities) and, if possible, to avoid polypharmacy with multiple QT-prolonging drugs.
- For patients who have multiple risk factors for QT prolongation in whom treatment with a known QT-prolonging medication is still deemed in the patient’s best interest, strong consideration should be given to inpatient administration and monitoring until the treatment has been completed.
With careful consideration of modifiable and nonmodifiable risk factors as well as a little extra caution when prescribing potential QT-prolonging medications such as azithromycin, the clinical benefit of these often-advantageous medications can be maximized and the incidence of these tragic but rare drug-induced sudden cardiac deaths can be reduced.
- Hopkins S. Clinical toleration and safety of azithromycin. Am J Med 1991; 91:40S–45S.
- Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther 2002; 303:218–225.
- Ioannidis JP, Contopoulos-Ioannidis DG, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for upper respiratory tract infections. J Antimicrob Chemother 2001; 48:677–689.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43:1603–1611.
- Arellano-Rodrigo E, García A, Mont L, Roqué M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. (Article in Spanish.) Med Clin (Barc) 2001; 117:118–119.
- Raschi E, Poluzzi E, Koci A, Moretti U, Sturkenboom M, De Ponti F. Macrolides and torsadogenic risk: emerging issues from the fda pharmacovigilance database. J Pharmacovigilance 2013; 1:104.
- Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002; 35:197–200.
- Stephenson WP, Hauben M. Data mining for signals in spontaneous reporting databases: proceed with caution. Pharmacoepidemiol Drug Saf 2007; 16:359–365.
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009; 18:427–436.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Mosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med 2013; 368:1665–1668.
- Louie R. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Koga T, Imaoka H. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Drew BJ, Ackerman MJ, Funk M, et al; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121:1047–1060.
- Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 2013; 88:315–325.
- Hopkins S. Clinical toleration and safety of azithromycin. Am J Med 1991; 91:40S–45S.
- Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther 2002; 303:218–225.
- Ioannidis JP, Contopoulos-Ioannidis DG, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for upper respiratory tract infections. J Antimicrob Chemother 2001; 48:677–689.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43:1603–1611.
- Arellano-Rodrigo E, García A, Mont L, Roqué M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. (Article in Spanish.) Med Clin (Barc) 2001; 117:118–119.
- Raschi E, Poluzzi E, Koci A, Moretti U, Sturkenboom M, De Ponti F. Macrolides and torsadogenic risk: emerging issues from the fda pharmacovigilance database. J Pharmacovigilance 2013; 1:104.
- Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002; 35:197–200.
- Stephenson WP, Hauben M. Data mining for signals in spontaneous reporting databases: proceed with caution. Pharmacoepidemiol Drug Saf 2007; 16:359–365.
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009; 18:427–436.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Mosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med 2013; 368:1665–1668.
- Louie R. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Koga T, Imaoka H. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 367:774–775.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Drew BJ, Ackerman MJ, Funk M, et al; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121:1047–1060.
- Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 2013; 88:315–325.
It is not a ‘mini’-stroke, it is a call to action
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
Transient ischemic attack: Omen and opportunity
A transient ischemic attack (TIA), like an episode of unstable angina, is an ominous portent of future morbidity and death even though, by definition, the event leaves no residual neurologic deficit.
But there is a positive side. When a patient presents with a TIA, the physician has the rare opportunity to reduce the risk of a disabling outcome—in this case, stroke. Therefore, patients deserve a rapid and thorough evaluation and appropriate stroke-preventive treatment.
MANY ‘TIAs’ ARE ACTUALLY STROKES
TIA has traditionally been described as a sudden focal neurologic deficit that lasts less than 24 hours, is presumed to be of vascular origin, and is confined to an area of the brain, spinal cord, or eye perfused by a specific artery. This symptom-based definition was based on the arbitrary and inaccurate assumption that brief symptoms would not be associated with damage to brain parenchyma.
The definition has since been updated and made more rational based on new concepts of brain ischemia informed by imaging, especially diffusion-weighted magnetic resonance imaging (MRI).1 One-third of episodes characterized as a TIA according to the classic definition would be considered an infarction on the basis of diffusion-weighted MRI.2 The new tissue-based definition characterizes TIA as a brief episode of neurologic dysfunction caused by focal ischemia of the brain, spinal cord, or retina, with clinical symptoms lasting less than 24 hours and without evidence of acute infarction.3
AN OPPORTUNITY TO INTERVENE
Most TIAs resolve in less than 30 minutes. The US National Institute of Neurological Disorders and Stroke trial of tissue plasminogen activator found that if symptoms of cerebral ischemia had not resolved by 1 hour or had not rapidly improved within 3 hours, complete resolution was rare (only 2% at 24 hours).4 Hence, physicians evaluating and treating patients with TIAs should treat these episodes with the urgency they deserve.
Moreover, half of the strokes that follow TIAs occur within 48 hours.5 A rapid and thorough evaluation and the initiation of secondary preventive treatments have been shown to reduce the early occurrence of stroke by up to 80%.6 Hence, the correct diagnosis of TIA gives the clinician the best opportunity to prevent stroke and its personal, social, and sometimes fatal consequences.
STROKES OUTNUMBER TIAs, BUT TIAs ARE UNDERREPORTED
According to 2012 statistics, nearly 795,000 strokes occur in the United States each year, 610,000 of which are first attacks and 185,000 are recurrences. Every 40 seconds, someone in the United States has a stroke.7
In comparison, the incidence of TIA in the United States is estimated at 200,000 to 500,000 per year, though the true number is difficult to know because of underreporting.8,9 About half of patients who experience a TIA fail to report it to their health care provider—a lost opportunity for intervention and stroke prevention.10,11
A meta-analysis showed that the risk of stroke after TIA was 9.9% at 2 days, 13.4% at 30 days, and 17.3% at 90 days.12
Interestingly, the risk of stroke after TIA exceeds the risk of recurrent stroke after a first stroke. This was shown in a study that found that patients who had made a substantial recovery within 24 hours (ie, patients with a TIA) were more likely to suffer neurologic deterioration in the next 3 months than were those who did not have significant early improvement.13
RISK FACTORS FOR TIA ARE THE SAME AS FOR STROKE
The risk of cerebrovascular disease increases with age and is higher in men14 and in blacks and Hispanics.15
The risk factors and clinical presentation do not differ between TIA and stroke, so the evaluation and treatment should not differ either. These two events represent a continuum of the same disease entity.
Some risk factors for TIA are modifiable, others are not.
Nonmodifiable risk factors
Nonmodifiable risk factors for TIA include older age, male sex, African American race, low birth weight, Hispanic ethnicity, and family history. If the patient has nonmodifiable risk factors, we should try all the harder to correct the modifiable ones.
Older age. The risk of ischemic stroke and intracranial hemorrhage doubles with each decade after age 55 in both sexes.16
Sex. Men have a significantly higher incidence of TIA than women,11 whereas the opposite is true for stroke: women have a higher lifetime risk of stroke than men.17
African Americans have an incidence of stroke (all types) 38% higher than that of whites,18 and an incidence of TIA (inpatient and out-of-hospital) 40% higher than the overall age- and sex-adjusted rate in the white population.11
Low birth weight. The odds of stroke are more than twice as high in people who weighed less than 2,500 g at birth compared with those who weighed 4,000 g or more, probably because of a correlation between low birth weight and hypertension.19
A family history of stroke increases the risk of stroke by nearly 30%, the association being stronger with large-vessel and smallvessel stroke than with cardioembolic stroke.20
Modifiable risk factors
Modifiable risk factors include cigarette smoking, hypertension, diabetes, lipid abnormalities, atrial fibrillation, carotid stenosis, and dietary and hormonal factors. Detecting these factors, which often coexist, is the first step in trying to modify them and reduce the patient’s risk.
Cigarette smoking approximately doubles the risk of ischemic stroke.21–23
Hypertension has a relationship with stroke risk that is strong, continuous, graded, consistent, and significant.24
Diabetes increases stroke risk nearly six times.25
Lipid abnormalities. Most studies have found an association between lipid levels (total cholesterol and low-density lipoprotein cholesterol) and the risk of death from ischemic stroke,26–28 and an inverse relationship between high-density lipoprotein cholesterol levels and stroke risk.29
Atrial fibrillation increases the risk of ischemic stroke up to fivefold, even in the absence of cardiac valvular disease. The mechanism is embolism of stasis-induced thrombi that form in the left atrial appendage.30
Carotid stenosis. Asymptomatic carotid atherosclerotic stenotic lesions in the extracranial internal carotid artery or carotid bulb are associated with a higher risk of stroke.24,31
Lifestyle factors. Diets that lower blood pressure have been found to decrease stroke risk.24 Exercise in men and women reduces the risk of stroke or death by 25% to 30% compared with inactive people.32 Weight reduction has been found to lower blood pressure and reduce stroke risk.24
Other potentially modifiable risk factors include migraine with aura, metabolic syndrome, excess alcohol consumption (and, paradoxically, complete abstinence from alcohol), drug abuse, sleep-disordered breathing, hyperhomocysteinemia, high lipoprotein (a) levels, hypercoagulability, infection with organisms such as Chlamydia pneumoniae, cytomegalovirus, and Helicobacter pylori, and acute infections such as respiratory and urinary infections.26
Conditions in certain demographic groups
Patients in certain demographic groups present with rarer conditions associated with stroke and TIA.
Sickle cell disease. Eleven percent of patients with sickle cell disease have clinical strokes, and a substantial number have “silent” infarcts identified on neuroimaging.33,34
Postmenopausal hormone replacement therapy with any product containing conjugated equine estrogen carries a risk of cerebrovascular events,35 and the higher the dose, the higher the risk.36 Also, oral contraceptives may be harmful in women who have additional risk factors such as cigarette smoking, prior thromboembolic events, or migraine with aura.37,38
THREE CAUSES OF STROKE AND TIA
Stroke and TIA should not be considered diagnoses in themselves, but rather the end point of many other diseases. The diagnosis lies in identifying the mechanism of the cerebrovascular event. The three main mechanisms are thrombosis, embolism, and decreased perfusion.
Thrombosis is caused by obstruction of blood flow within one or more blood vessels, the most common cause being atherosclerosis. Large-artery atherosclerosis, such as in the carotid bifurcation or extracranial internal carotid, causes TIAs that occur over a period of weeks or months with a variety of presentations in that vascular territory, from years of gradual accumulation of atherosclerotic plaque.39
In patients with small-artery or penetrating artery disease, hypertension is the primary risk factor and the pathology, specific to small arterioles, is lipohyalinosis rather than atherosclerosis. These patients may present with a stuttering clinical course, and episodes are more stereotypical.
Less common obstructive vascular pathologies include fibromuscular dysplasia, arteritides, and dissection.
Embolism can occur from a proximal source such as the heart or from proximal vessels such as the aorta, carotid, or vertebral arteries. The embolic particle may form on heart valves or lesions within the heart (eg, clot, tumor), or in the venous circulation and paradoxically cross over to the arterial side through an intracardiac or transpulmonary shunt. Embolism may also be due to a hypercoagulable state.40 Embolic stroke is suspected when multiple vascular territories within the brain are clinically or radiographically affected.
Decreased systemic perfusion caused by severe heart failure or systemic hypotension can cause ischemia to the brain diffusely and bilaterally, limiting the ability of the blood-stream to wash out microemboli, especially in the border zones (also known as “watershed areas”), thus leading to ischemia or infarction.41 Decreased perfusion can also be local, due to a fixed vessel stenosis.
Using another classification system, a study in Rochester, MN, found the following incident rates of stroke subtypes, adjusted for age and sex, per 100,000 population42:
- Large-vessel cervical or intracranial atherosclerosis with more than 50% stenosis—27
- Cardioembolism—40
- Lacunar, small-vessel disease—25
- Uncertain cause—52
- Other identifiable cause—4.
THREE CLINICAL FEATURES SUGGEST TIA
TIAs can be hard to distinguish from nonischemic neurologic events in the acute setting such as an emergency room. Up to 60% of patients suspected of having a TIA actually have a nonischemic cause of their symptoms.43
Three clinical features suggest a TIA during the emergency room evaluation:
- Rapid onset of symptoms—“like lightning” or “in seconds,” in contrast to migraine and seizures, which develop over minutes
- No history of similar episodes in the past
- Absence of nonspecific symptoms—eg, stomach upset or tightness in the chest.
CLINICAL DIAGNOSIS
Because most TIA symptoms and signs have already resolved by the time of evaluation, the diagnosis depends on a careful history with special attention to the pace of onset and resolution, the duration and nature of the symptoms, circumstances at the time of symptom onset, previous similar episodes, associated features, vascular risk factors, and family history (Table 1).44,45
A detailed neurologic examination is imperative and should include fundoscopy. A cardiovascular assessment should include cardiac rhythm, bruits in the neck, orbits, and groin, peripheral pulses, and electrocardiography.
Do neurologists do a better job at diagnosing TIA and stroke?
Primary care physicians, internists, and emergency department physicians are often the ones to carry out the clinical assessment of possible TIA.
Determining if transient neurologic symptoms are caused by ischemia can be a challenge. When in doubt, referral to a neurologist with subspecialty training in cerebrovascular disease should be considered.
But do neurologists really do a better job? A recent study sought to compare the accuracy of diagnosis of TIA made by general practitioners, emergency physicians, and neurologists. The nonneurologists considered “confusion” and “unexplained fall” suggestive of TIA and “lower facial palsy” and “monocular blindness” less suggestive of TIA—whereas the opposite is true. This shows that nonneurologists often label minor strokes and several nonvascular transient neurologic disturbances as TIAs, and up to half of patients could be mislabeled as a result.46
Differences in diagnosing cerebrovascular events between emergency room physicians and attending neurologists have been tested,47 with an accuracy of diagnosis as low as 38% by emergency department physicians in one study.48 However, other studies did not show such a trend.49,50
A study at a university-based teaching hospital found the sensitivity of emergency room physician diagnosis to be 98.6% with a positive predictive value of 94.8%,49 showing that at a large teaching hospital with a comprehensive stroke intervention program, emergency physicians could identify patients with stroke, particularly hemorrhagic stroke, very accurately.
Improving the diagnosis of stroke and TIA
Routine use of imaging and involvement of a neurologist increase the sensitivity and accuracy of diagnosis. Education and written guidelines for acute stroke treatment both in the emergency department and in out-of-hospital settings seem to dramatically improve the rates of diagnostic accuracy and appropriate treatment.50
Emergency medical service personnel use two screening tools in the field to identify TIA and stroke symptoms:
- The Cincinnati Prehospital Stroke Scale, a three-item scale based on three signs: facial droop, arm drift, and slurring of speech51
- The Los Angeles Prehospital Stroke Screen, which uses screening questions and asymmetry in the face, hand grip strength, and arm drift.52
Knowing that the patient is having a minor stroke or TIA is important. Urgent treatment of these conditions decreases the risk of stroke in the next 90 days, which was 10.5% in one study.5 Urgent assessment and early intervention could reduce this risk of subsequent stroke down to 2%.6
ASSESSING RISK OF STROKE AFTER TIA
There is a practical need for prediction of stroke during the first few days after the event. The ABCD and ABCD2 scores were developed to stratify the short-term risk of stroke in patients with recent TIA.
The ABCD score
The ABCD score53 was derived to allow primary care physicians and other physicians to identify which patients with a suspected diagnosis of TIA should be referred for emergency assessment, to allow secondary-care physicians to determine which patients with probable or definite TIA need emergency investigation and treatment, to allow public education about the need for medical attention after a TIA, and to identify people at high risk.
The ABCD2 score
The ABCD2 score predicts the short-term risk of stroke following a TIA.54 Points are assigned as follows:
- Age > 60 years: 1 point
- Blood pressure (systolic) > 140 mm Hg or diastolic blood pressure > 90 mm Hg: 1 point
- Clinical factors: unilateral weakness with or without speech impairment: 2 points (1 point for speech impairment without weakness)
- Duration of symptoms > 60 minutes: 2 points (1 point for 10–59 minutes)
- Diabetes: 1 point.
Thus, the possible total ranges from 0 to 7 points. Higher scores indicate a greater risk of stroke at 2, 7, 30, and 90 days:
- Total score 0, 1, 2, or 3: 2-day stroke risk 1.0% (low risk)
- Total score 4 or 5: 2-day stroke risk 4.1% (moderate risk)
- Total score 6 or 7: 2-day stroke risk 8.1% (high risk).
WHO SHOULD BE HOSPITALIZED?
It has been suggested that the ABCD2 score can help in triaging patients to hospital admission or outpatient care, though no randomized trial has actually evaluated the utility of the ABCD2 score in this way.3
A study of consecutive TIA patients admitted over 12 months55 found that patients with an ABCD2 score of 3 or less had the same chance of requiring hospitalization (based on positive diffusion-weighted MRI studies, risk factor identification, and treatment initiation) as those with a score of 4 to 7. Hence, admitting TIA patients on the basis of the ABCD2 score alone requires further study. However, such decisions, though informed by clinical data, depend heavily on societal input (eg, from insurance companies, national health protocols) and may be outside the purview of clinical investigation.
The benefits of hospitalization include the ability to rapidly carry out tests such as cardiac monitoring for atrial fibrillation; to detect atherosclerosis, aortic arch atheroma, and paradoxical emboli; and to quickly start secondary prevention treatments and education about the importance of adhering to them. Early endarterectomy in the case of carotid stenosis can be offered. Additionally, if stroke symptoms recur, thrombolytic drug therapy can be started quickly.
Nguyen-Huynh et al56 analyzed the cost utility of 24-hour hospitalization for patients diagnosed with a recent TIA who were candidates for tissue plasminogen activator if a stroke occurred. They found hospitalization to be borderline cost-effective on the whole, with definite cost-effectiveness found in patients with higher stroke risk.
If patients come to medical attention several days after the TIA, then assessing risk with the ABCD2 score may no longer be reliable.57
INVESTIGATIONS
Parenchymal neuroimaging
Computed tomography (CT) without contrast is the most widely used neuroimaging test in the acute setting, since it is widely available, fast, and relatively low-cost. It will not show any abnormality in TIA or early ischemic stroke. However, it is helpful as a screening tool to rule out intracranial lesions such as hemorrhage or tumor. It may also show evidence of established infarction, which would indicate that the ischemia probably had been present for at least 6 to 12 hours.
MRI is clearly superior to noncontrast CT for detecting small areas of ischemia in patients with TIA, and it should be used unless the patient has a contraindication to it. Roughly one-third of TIA patients have lesions detectable on diffusion-weighted imaging, which helps to confirm that the episode was caused by cerebral ischemia, but nearly half of the diffusion MRI changes may be fully reversible.58 Evidence of prior stroke, leukoaraiosis, or white matter disease on fluid-attenuated inversion recovery and T2 sequences and microhemorrhages (on gradient echo sequences) help to determine a mechanistic diagnosis.
Subcategorizing TIA patients on the basis of the findings on diffusion-weighted MRI and the ABCD2 score is prognostically helpful.59 It can help to determine which patients need hospitalization and aggressive treatment, and in the case of identified diffusion-weighted MRI-positive stroke, it helps to localize and elucidate the mechanism of stroke. Hence, MRI is the preferred neuroimaging study for evaluating patients with TIA.3
Vascular imaging
Establishing the status of both intracranial and extracranial vessels is important for understanding the etiology, estimating the risk of future ischemic events, and formulating a treatment plan—eg, carotid endarterectomy in cases of significant stenosis (70% to 99%), which reduces the risk of ipsilateral stroke.60 Imaging studies include CT angiography, magnetic resonance angiography, extracranial and transcranial ultrasonography, and conventional catheter-based angiography.
CT angiography has higher spatial resolution, but vessels may be obscured by calcification associated with atherosclerotic plaque. It has the advantage of wide availability, low cost, short scanning time, and excellent patient tolerability.
Magnetic resonance angiography with gadolinium enhancement offers good quality imaging from the great vessels in the chest to the medium-sized vessels distal to the circle of Willis.
The contrast agents used in MRI and CT can have negative consequences in patients with renal disease. MRI contrast has been associated with nephrogenic fibrosing dermopathy, 61 and CT contrast can cause contrast-induced nephropathy.62
Carotid ultrasonography and transcranial Doppler ultrasonography are noninvasive and are not associated with significant adverse events. They can be used safely in patients with renal dysfunction, and they provide physiologic information that cannot be obtained with MRI and CT, which are static imaging techniques. Detecting microemboli on transcranial Doppler is an independent predictor of recurrent ischemic events.63,64
Catheter-based angiography is occasionally needed in confusing or more complicated cases, but it is invasive and occasionally is associated with iatrogenic stroke and other vascular complications.
Cardiac and aortic imaging
Echocardiography is used to detect lesions that can be sources of embolism such as regional wall-motion abnormalities, cardiac thrombus or mass, endocarditis, aortic arch atheroma, and patent foramen ovale. In patients with cryptogenic TIA or stroke, those with patent foramen ovale alone were found to have a lower risk of recurrent stroke than those who had both atrial septal aneurysm and patent foramen ovale.65
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting cardioembolic lesions, especially patent foramen ovale.66 In patients with cerebral ischemia and normal transthoracic findings, cardiac sources of embolism may be detected in about 40% of patients with transesophageal echocardiography.67
Cardiac rhythm monitoring
Electrocardiography and prolonged telemetry are recommended in patients with cryptogenic TIA to detect cardiac ischemia and paroxysmal atrial fibrillation. In one study, Holter monitoring detected atrial fibrillation in 6% of patients hospitalized with ischemic stroke or TIA.68 In another study, atrial fibrillation was detected after a median of 21 days of outpatient cardiac monitoring in 23% of patients.69
The optimal duration of outpatient telemetry has not yet been established, but studies have found significant increases in detection of paroxysms of atrial fibrillation with monitoring for 7 or longer.70
Laboratory tests in the acute setting
These include lipid profile, hemoglobin A1c, and cardiac enzymes. The advantages of hospitalization are early detection of these modifiable risk factors and early initiation of treatment.
Tests for rarer disorders
Tests for rarer disorders are sometimes indicated in unusual cases, such as ischemic symptoms occurring in young patients without other common risk factors. This includes testing for prothrombotic states, toxicology, blood cultures, inflammatory markers, hemoglobin electrophoresis, and lumbar puncture. The benefit of routine testing for thrombophilic disorders in cerebrovascular disease remains uncertain, with no clear association demonstrated with arterial stroke, but testing is more relevant in the case of venous (and paradoxical) thromboembolism.71
TREAT THE UNDERLYING DISORDER
Treatment depends on the mechanism that is thought to be responsible for the ischemic event. Vascular risk factors are important to identify and modify for all stroke subtypes.
Illustrating the importance of treating TIA and minor stroke, one study72 found that for antiplatelet therapy (aspirin, dipyridamole, or aspirin plus dipyridamole), the number needed to treat for 2 years was around 18.
Anticoagulation for cardioembolism
Atrial fibrillation, especially following a cerebrovascular ischemic event, should be treated with long-term anticoagulation with warfarin (Coumadin), dabigatran (Pradaxa), rivaroxaban (Xarelto), or apixaban (Eliquis).73 If the patient cannot tolerate anticoagulation, aspirin is recommended, and if he or she cannot tolerate aspirin, clopidogrel (Plavix) is recommended.
Antiplatelet therapy for large-vessel atherosclerosis and small-vessel disease
In the acute phase, aspirin 81 mg to 325 mg orally can be given. If the patient is allergic to aspirin, a loading dose of clopidogrel 300 mg and then 75 mg daily may be given.
A pilot study of loading with aspirin 325 mg or clopidogrel 375 mg in acute ischemic stroke and TIA patients showed that these treatments were safe when given within 36 hours and decreased the risk of neurologic deterioration.74 The patient should continue on aspirin 81 mg or clopidogrel 75 mg, as suggested by the Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER) trial.75 In the long term, an antiplatelet drug such as aspirin or clopidogrel or the combination of aspirin and extended-release dipyridamole is reasonable.76
Cilostazol (Pletal) is not inferior and is possibly superior to aspirin in preventing noncardioembolic ischemic stroke. It is used off-label for secondary prevention of stroke of noncardioembolic origin.77
Statins
In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, high-dose atorvastatin (Lipitor)—80 mg daily—was found to reduce the risk of subsequent stroke and other cardiovascular events in patients with recent stroke irrespective of low-density lipoprotein cholesterol (LDL-C) level, but there was a small increase in the risk of hemorrhagic stroke.78
In patients with hyperlipidemia, current recommendations suggest a target LDL-C level lower than 100 mg/dL in patients with atherosclerotic stroke or TIA, and lower than 70 mg/dL in those with concomitant diabetes.79
Antihypertensive therapy
In the acute period, ie, the first 24 hours after symptoms, guidelines have advocated allowing high blood pressure to remain high (“permissive hypertension”) unless the systolic pressure is greater than 200 mm Hg or the diastolic pressure is greater than 120 mm Hg or the patient is receiving thrombolytic therapy.80 However, this has recently been challenged by findings in randomized trials.81 Permissive hypertension and avoidance of dehydration with intravenous normal saline may improve cerebral perfusion, which is especially important in patients with high-grade intracranial or extracranial stenosis. Within the parameters outlined above, we recommend against aggressively treating high blood pressure in the acute phase.
In the long term, antihypertensive therapy reduces the risk of recurrent stroke or TIA.82 The goal is to keep blood pressure lower than 140/90 mm Hg, or lower than 130/80 mm Hg in patients with diabetes. A study of patients with ischemic noncardioembolic stroke showed a higher risk of recurrent stroke if the systolic blood pressure was lower than 120 or higher than 140 mm Hg.83
Some classes of antihypertensive medication may be more beneficial than others. There is some evidence that angiotensin-converting enzyme (ACE) inhibitors alone or in combination with a diuretic or an angiotensin receptor blocker are superior to other regimens, possibly because of neuroprotective mechanisms.84 A recent meta-analysis found angiotensin receptor blockers to be more effective than either ACE inhibitors or beta-blockers in stroke prevention; however, calcium channel blockers were superior to renin-angiotensin system blockers (ACE inhibitors and angiotensin receptor blockers).85
Lifestyle modifications
Smoking cessation and cardiovascular exercise for more than 10 minutes more than 3 times per week is strongly recommended.
For patients with diabetes, the goal is to keep the fasting blood glucose level lower than 126 mg/dL.
Moderate alcohol intake has been shown to decrease stroke risk compared with excessive intake or none at all.86
Carotid endarterectomy
Carotid endarterectomy has been recommended within 2 weeks of cerebral or retinal TIA in those cases attributable to high-grade internal carotid artery stenosis in patients who have low surgical risk.87 This risk can be estimated on the basis of patient factors, surgeon factors, and hospital volume. The specific recommendations are as follows:
- 70% to 99% carotid stenosis: carotid endarterectomy recommended
- 50% to 69% carotid stenosis: carotid endarterectomy recommended in select patients with a perioperative complication rate < 6%
- < 50% carotid stenosis: carotid endarterectomy not routinely recommended.
Carotid artery angioplasty and stenting with distal embolic protection device
Data from the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and European stenting trials indicate that in patients over age 70, carotid endarterectomy appears to be superior to carotid artery stenting, whereas in younger patients the periprocedural risks of stroke and death are similar. Hence, carotid artery stenting performed by an interventionist with a low complication rate is a reasonable alternative to carotid endarterectomy.88,89
- Albers GW, Caplan LR, Easton JD, et al; TIA Working Group. Transient ischemic attack—proposal for a new definition. N Engl J Med 2002; 347:1713–1716.
- Ovbiagele B, Kidwell CS, Saver JL. Epidemiological impact in the United States of a tissue-based definition of transient ischemic attack. Stroke 2003; 34:919–924.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000; 55:1649–1655.
- Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284:2901–2906.
- Rothwell PM, Giles MF, Chandratheva A, et al; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370:1432–1442.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Johnston SC. Clinical practice. Transient ischemic attack. N Engl J Med 2002; 347:1687–1692.
- Johnston SC, Fayad PB, Gorelick PB, et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology 2003; 60:1429–1434.
- Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJ; North American Symptomatic Carotid Endarterectomy Trial Group. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ 2004; 170:1105–1109.
- Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005; 36:720–723.
- Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007; 167:2417–2422.
- Johnston SC, Leira EC, Hansen MD, Adams HP. Early recovery after cerebral ischemia risk of subsequent neurological deterioration. Ann Neurol 2003; 54:439–444.
- Bots ML, van der Wilk EC, Koudstaal PJ, Hofman A, Grobbee DE. Transient neurological attacks in the general population. Prevalence, risk factors, and clinical relevance. Stroke 1997; 28:768–773.
- White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111:1327–1331.
- Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27:373–380.
- Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006; 37:345–350.
- Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999; 30:736–743.
- Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertens (Greenwich) 2003; 5:133–136.
- Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke 2004; 35:212–227.
- Rodriguez BL, D’Agostino R, Abbott RD, et al. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke 2002; 33:230–236.
- Manolio TA, Kronmal RA, Burke GL, O’Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke 1996; 27:1479–1486.
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22:312–318.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Allen TW. Guide to clinical preventive services. Report of the US Preventive Services Task Force. J Am Osteopath Assoc 1991; 91:281–289.
- Goldstein LB, Bushnell CD, Adams RJ, et al; American Heart Association Stroke Council. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:517–584.
- Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989; 320:904–910.
- Zhang X, Patel A, Horibe H, et al; Asia Pacific Cohort Studies Collaboration. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol 2003; 32:563–572.
- Sanossian N, Saver JL, Navab M, Ovbiagele B. High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke 2007; 38:1104–1109.
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008; 92:17–40.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97:864–870.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998; 91:288–294.
- Mosca L, Banka CL, Benjamin EJ, et al; Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women's Association; Centers for Disease Control and Prevention; Office of Research on Women's Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med 2004; 164:741–747.
- Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia 2000; 20:155–156.
- Mohr JP, Caplan LR, Melski JW, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology 1978; 28:754–762.
- Caplan LR. Brain embolism, revisited. Neurology 1993; 43:1281–1287.
- Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998; 55:1475–1482.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999; 30:2513–2516.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Baquis GD, Pessin MS, Scott RM. Limb shaking—a carotid TIA. Stroke 1985; 16:444–448.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol 2009; 8:235–243.
- Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996; 27:2225–2229.
- Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet 1982; 1:328–331.
- Ferro JM, Pinto AN, Falcão I, et al. Diagnosis of stroke by the nonneurologist. A validation study. Stroke 1998; 29:1106–1109.
- Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians. Accuracy in the diagnosis of stroke. Stroke 1995; 26:2238–2241.
- Artto V, Putaala J, Strbian D, et al; Helsinki Stroke Thrombolysis Registry Group. Stroke mimics and intravenous thrombolysis. Ann Emerg Med 2012; 59:27–32.
- Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 1999; 33:373–378.
- Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke 2000; 31:71–76.
- Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005; 366:29–36.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Lou M, Safdar A, Edlow JA, et al. Can ABCD score predict the need for in-hospital intervention in patients with transient ischemic attacks? Int J Emerg Med 2010; 3:75–80.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology 2005; 65:1799–1801.
- Calvet D, Lamy C, Touzé E, Oppenheim C, Meder JF, Mas JL. Management and outcome of patients with transient ischemic attack admitted to a stroke unit. Cerebrovasc Dis 2007; 24:80–85.
- Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999; 30:1174–1180.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 2011; 77:1222–1228.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am 2009; 47:827–831.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- Valton L, Larrue V, le Traon AP, Massabuau P, Géraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke 1998; 29:2125–2128.
- Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke 2004; 35:2832–2836.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993; 24:1020–1024.
- de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006; 37:2531–2534.
- Lazzaro MA, Krishnan K, Prabhakaran S. Detection of atrial fibrillation with concurrent Holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2012; 21:89–93.
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71:1696–1701.
- Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011; 124:477–486.
- Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke 2010; 41:2985–2990.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Meyer DM, Albright KC, Allison TA, Grotta JC. LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2008; 17:26–29.
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM; FASTER Investigators. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007; 6:961–969.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Koenig MA, Geocadin RG, de Grouchy M, et al. Safety of induced hypertension therapy in patients with acute ischemic stroke. Neurocrit Care 2006; 4:3–7.
- Elijovich F, Laffer CL. Acute stroke: lower blood pressure looks better and better. Hypertension 2010; 56:808–810.
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004; 35:1024.
- Ovbiagele B, Diener HC, Yusuf S, et al; PROFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011; 306:2137–2144.
- Chrysant SG. The pathophysiologic role of the brain renin-angiotensin system in stroke protection: clinical implications. J Clin Hypertens (Greenwich) 2007; 9:454–459.
- Verdecchia P, Gentile G, Angeli F, Reboldi G. Beyond blood pressure: evidence for cardiovascular, cerebrovascular, and renal protective effects of renin-angiotensin system blockers. Ther Adv Cardiovasc Dis 2012; 6:81–91.
- Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006; 37:13–19.
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ; Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363:915–924.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Carotid Stenting Trialists’ Collaboration; Bonati LH, Dobson J, Algra A, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376:1062–1073.
A transient ischemic attack (TIA), like an episode of unstable angina, is an ominous portent of future morbidity and death even though, by definition, the event leaves no residual neurologic deficit.
But there is a positive side. When a patient presents with a TIA, the physician has the rare opportunity to reduce the risk of a disabling outcome—in this case, stroke. Therefore, patients deserve a rapid and thorough evaluation and appropriate stroke-preventive treatment.
MANY ‘TIAs’ ARE ACTUALLY STROKES
TIA has traditionally been described as a sudden focal neurologic deficit that lasts less than 24 hours, is presumed to be of vascular origin, and is confined to an area of the brain, spinal cord, or eye perfused by a specific artery. This symptom-based definition was based on the arbitrary and inaccurate assumption that brief symptoms would not be associated with damage to brain parenchyma.
The definition has since been updated and made more rational based on new concepts of brain ischemia informed by imaging, especially diffusion-weighted magnetic resonance imaging (MRI).1 One-third of episodes characterized as a TIA according to the classic definition would be considered an infarction on the basis of diffusion-weighted MRI.2 The new tissue-based definition characterizes TIA as a brief episode of neurologic dysfunction caused by focal ischemia of the brain, spinal cord, or retina, with clinical symptoms lasting less than 24 hours and without evidence of acute infarction.3
AN OPPORTUNITY TO INTERVENE
Most TIAs resolve in less than 30 minutes. The US National Institute of Neurological Disorders and Stroke trial of tissue plasminogen activator found that if symptoms of cerebral ischemia had not resolved by 1 hour or had not rapidly improved within 3 hours, complete resolution was rare (only 2% at 24 hours).4 Hence, physicians evaluating and treating patients with TIAs should treat these episodes with the urgency they deserve.
Moreover, half of the strokes that follow TIAs occur within 48 hours.5 A rapid and thorough evaluation and the initiation of secondary preventive treatments have been shown to reduce the early occurrence of stroke by up to 80%.6 Hence, the correct diagnosis of TIA gives the clinician the best opportunity to prevent stroke and its personal, social, and sometimes fatal consequences.
STROKES OUTNUMBER TIAs, BUT TIAs ARE UNDERREPORTED
According to 2012 statistics, nearly 795,000 strokes occur in the United States each year, 610,000 of which are first attacks and 185,000 are recurrences. Every 40 seconds, someone in the United States has a stroke.7
In comparison, the incidence of TIA in the United States is estimated at 200,000 to 500,000 per year, though the true number is difficult to know because of underreporting.8,9 About half of patients who experience a TIA fail to report it to their health care provider—a lost opportunity for intervention and stroke prevention.10,11
A meta-analysis showed that the risk of stroke after TIA was 9.9% at 2 days, 13.4% at 30 days, and 17.3% at 90 days.12
Interestingly, the risk of stroke after TIA exceeds the risk of recurrent stroke after a first stroke. This was shown in a study that found that patients who had made a substantial recovery within 24 hours (ie, patients with a TIA) were more likely to suffer neurologic deterioration in the next 3 months than were those who did not have significant early improvement.13
RISK FACTORS FOR TIA ARE THE SAME AS FOR STROKE
The risk of cerebrovascular disease increases with age and is higher in men14 and in blacks and Hispanics.15
The risk factors and clinical presentation do not differ between TIA and stroke, so the evaluation and treatment should not differ either. These two events represent a continuum of the same disease entity.
Some risk factors for TIA are modifiable, others are not.
Nonmodifiable risk factors
Nonmodifiable risk factors for TIA include older age, male sex, African American race, low birth weight, Hispanic ethnicity, and family history. If the patient has nonmodifiable risk factors, we should try all the harder to correct the modifiable ones.
Older age. The risk of ischemic stroke and intracranial hemorrhage doubles with each decade after age 55 in both sexes.16
Sex. Men have a significantly higher incidence of TIA than women,11 whereas the opposite is true for stroke: women have a higher lifetime risk of stroke than men.17
African Americans have an incidence of stroke (all types) 38% higher than that of whites,18 and an incidence of TIA (inpatient and out-of-hospital) 40% higher than the overall age- and sex-adjusted rate in the white population.11
Low birth weight. The odds of stroke are more than twice as high in people who weighed less than 2,500 g at birth compared with those who weighed 4,000 g or more, probably because of a correlation between low birth weight and hypertension.19
A family history of stroke increases the risk of stroke by nearly 30%, the association being stronger with large-vessel and smallvessel stroke than with cardioembolic stroke.20
Modifiable risk factors
Modifiable risk factors include cigarette smoking, hypertension, diabetes, lipid abnormalities, atrial fibrillation, carotid stenosis, and dietary and hormonal factors. Detecting these factors, which often coexist, is the first step in trying to modify them and reduce the patient’s risk.
Cigarette smoking approximately doubles the risk of ischemic stroke.21–23
Hypertension has a relationship with stroke risk that is strong, continuous, graded, consistent, and significant.24
Diabetes increases stroke risk nearly six times.25
Lipid abnormalities. Most studies have found an association between lipid levels (total cholesterol and low-density lipoprotein cholesterol) and the risk of death from ischemic stroke,26–28 and an inverse relationship between high-density lipoprotein cholesterol levels and stroke risk.29
Atrial fibrillation increases the risk of ischemic stroke up to fivefold, even in the absence of cardiac valvular disease. The mechanism is embolism of stasis-induced thrombi that form in the left atrial appendage.30
Carotid stenosis. Asymptomatic carotid atherosclerotic stenotic lesions in the extracranial internal carotid artery or carotid bulb are associated with a higher risk of stroke.24,31
Lifestyle factors. Diets that lower blood pressure have been found to decrease stroke risk.24 Exercise in men and women reduces the risk of stroke or death by 25% to 30% compared with inactive people.32 Weight reduction has been found to lower blood pressure and reduce stroke risk.24
Other potentially modifiable risk factors include migraine with aura, metabolic syndrome, excess alcohol consumption (and, paradoxically, complete abstinence from alcohol), drug abuse, sleep-disordered breathing, hyperhomocysteinemia, high lipoprotein (a) levels, hypercoagulability, infection with organisms such as Chlamydia pneumoniae, cytomegalovirus, and Helicobacter pylori, and acute infections such as respiratory and urinary infections.26
Conditions in certain demographic groups
Patients in certain demographic groups present with rarer conditions associated with stroke and TIA.
Sickle cell disease. Eleven percent of patients with sickle cell disease have clinical strokes, and a substantial number have “silent” infarcts identified on neuroimaging.33,34
Postmenopausal hormone replacement therapy with any product containing conjugated equine estrogen carries a risk of cerebrovascular events,35 and the higher the dose, the higher the risk.36 Also, oral contraceptives may be harmful in women who have additional risk factors such as cigarette smoking, prior thromboembolic events, or migraine with aura.37,38
THREE CAUSES OF STROKE AND TIA
Stroke and TIA should not be considered diagnoses in themselves, but rather the end point of many other diseases. The diagnosis lies in identifying the mechanism of the cerebrovascular event. The three main mechanisms are thrombosis, embolism, and decreased perfusion.
Thrombosis is caused by obstruction of blood flow within one or more blood vessels, the most common cause being atherosclerosis. Large-artery atherosclerosis, such as in the carotid bifurcation or extracranial internal carotid, causes TIAs that occur over a period of weeks or months with a variety of presentations in that vascular territory, from years of gradual accumulation of atherosclerotic plaque.39
In patients with small-artery or penetrating artery disease, hypertension is the primary risk factor and the pathology, specific to small arterioles, is lipohyalinosis rather than atherosclerosis. These patients may present with a stuttering clinical course, and episodes are more stereotypical.
Less common obstructive vascular pathologies include fibromuscular dysplasia, arteritides, and dissection.
Embolism can occur from a proximal source such as the heart or from proximal vessels such as the aorta, carotid, or vertebral arteries. The embolic particle may form on heart valves or lesions within the heart (eg, clot, tumor), or in the venous circulation and paradoxically cross over to the arterial side through an intracardiac or transpulmonary shunt. Embolism may also be due to a hypercoagulable state.40 Embolic stroke is suspected when multiple vascular territories within the brain are clinically or radiographically affected.
Decreased systemic perfusion caused by severe heart failure or systemic hypotension can cause ischemia to the brain diffusely and bilaterally, limiting the ability of the blood-stream to wash out microemboli, especially in the border zones (also known as “watershed areas”), thus leading to ischemia or infarction.41 Decreased perfusion can also be local, due to a fixed vessel stenosis.
Using another classification system, a study in Rochester, MN, found the following incident rates of stroke subtypes, adjusted for age and sex, per 100,000 population42:
- Large-vessel cervical or intracranial atherosclerosis with more than 50% stenosis—27
- Cardioembolism—40
- Lacunar, small-vessel disease—25
- Uncertain cause—52
- Other identifiable cause—4.
THREE CLINICAL FEATURES SUGGEST TIA
TIAs can be hard to distinguish from nonischemic neurologic events in the acute setting such as an emergency room. Up to 60% of patients suspected of having a TIA actually have a nonischemic cause of their symptoms.43
Three clinical features suggest a TIA during the emergency room evaluation:
- Rapid onset of symptoms—“like lightning” or “in seconds,” in contrast to migraine and seizures, which develop over minutes
- No history of similar episodes in the past
- Absence of nonspecific symptoms—eg, stomach upset or tightness in the chest.
CLINICAL DIAGNOSIS
Because most TIA symptoms and signs have already resolved by the time of evaluation, the diagnosis depends on a careful history with special attention to the pace of onset and resolution, the duration and nature of the symptoms, circumstances at the time of symptom onset, previous similar episodes, associated features, vascular risk factors, and family history (Table 1).44,45
A detailed neurologic examination is imperative and should include fundoscopy. A cardiovascular assessment should include cardiac rhythm, bruits in the neck, orbits, and groin, peripheral pulses, and electrocardiography.
Do neurologists do a better job at diagnosing TIA and stroke?
Primary care physicians, internists, and emergency department physicians are often the ones to carry out the clinical assessment of possible TIA.
Determining if transient neurologic symptoms are caused by ischemia can be a challenge. When in doubt, referral to a neurologist with subspecialty training in cerebrovascular disease should be considered.
But do neurologists really do a better job? A recent study sought to compare the accuracy of diagnosis of TIA made by general practitioners, emergency physicians, and neurologists. The nonneurologists considered “confusion” and “unexplained fall” suggestive of TIA and “lower facial palsy” and “monocular blindness” less suggestive of TIA—whereas the opposite is true. This shows that nonneurologists often label minor strokes and several nonvascular transient neurologic disturbances as TIAs, and up to half of patients could be mislabeled as a result.46
Differences in diagnosing cerebrovascular events between emergency room physicians and attending neurologists have been tested,47 with an accuracy of diagnosis as low as 38% by emergency department physicians in one study.48 However, other studies did not show such a trend.49,50
A study at a university-based teaching hospital found the sensitivity of emergency room physician diagnosis to be 98.6% with a positive predictive value of 94.8%,49 showing that at a large teaching hospital with a comprehensive stroke intervention program, emergency physicians could identify patients with stroke, particularly hemorrhagic stroke, very accurately.
Improving the diagnosis of stroke and TIA
Routine use of imaging and involvement of a neurologist increase the sensitivity and accuracy of diagnosis. Education and written guidelines for acute stroke treatment both in the emergency department and in out-of-hospital settings seem to dramatically improve the rates of diagnostic accuracy and appropriate treatment.50
Emergency medical service personnel use two screening tools in the field to identify TIA and stroke symptoms:
- The Cincinnati Prehospital Stroke Scale, a three-item scale based on three signs: facial droop, arm drift, and slurring of speech51
- The Los Angeles Prehospital Stroke Screen, which uses screening questions and asymmetry in the face, hand grip strength, and arm drift.52
Knowing that the patient is having a minor stroke or TIA is important. Urgent treatment of these conditions decreases the risk of stroke in the next 90 days, which was 10.5% in one study.5 Urgent assessment and early intervention could reduce this risk of subsequent stroke down to 2%.6
ASSESSING RISK OF STROKE AFTER TIA
There is a practical need for prediction of stroke during the first few days after the event. The ABCD and ABCD2 scores were developed to stratify the short-term risk of stroke in patients with recent TIA.
The ABCD score
The ABCD score53 was derived to allow primary care physicians and other physicians to identify which patients with a suspected diagnosis of TIA should be referred for emergency assessment, to allow secondary-care physicians to determine which patients with probable or definite TIA need emergency investigation and treatment, to allow public education about the need for medical attention after a TIA, and to identify people at high risk.
The ABCD2 score
The ABCD2 score predicts the short-term risk of stroke following a TIA.54 Points are assigned as follows:
- Age > 60 years: 1 point
- Blood pressure (systolic) > 140 mm Hg or diastolic blood pressure > 90 mm Hg: 1 point
- Clinical factors: unilateral weakness with or without speech impairment: 2 points (1 point for speech impairment without weakness)
- Duration of symptoms > 60 minutes: 2 points (1 point for 10–59 minutes)
- Diabetes: 1 point.
Thus, the possible total ranges from 0 to 7 points. Higher scores indicate a greater risk of stroke at 2, 7, 30, and 90 days:
- Total score 0, 1, 2, or 3: 2-day stroke risk 1.0% (low risk)
- Total score 4 or 5: 2-day stroke risk 4.1% (moderate risk)
- Total score 6 or 7: 2-day stroke risk 8.1% (high risk).
WHO SHOULD BE HOSPITALIZED?
It has been suggested that the ABCD2 score can help in triaging patients to hospital admission or outpatient care, though no randomized trial has actually evaluated the utility of the ABCD2 score in this way.3
A study of consecutive TIA patients admitted over 12 months55 found that patients with an ABCD2 score of 3 or less had the same chance of requiring hospitalization (based on positive diffusion-weighted MRI studies, risk factor identification, and treatment initiation) as those with a score of 4 to 7. Hence, admitting TIA patients on the basis of the ABCD2 score alone requires further study. However, such decisions, though informed by clinical data, depend heavily on societal input (eg, from insurance companies, national health protocols) and may be outside the purview of clinical investigation.
The benefits of hospitalization include the ability to rapidly carry out tests such as cardiac monitoring for atrial fibrillation; to detect atherosclerosis, aortic arch atheroma, and paradoxical emboli; and to quickly start secondary prevention treatments and education about the importance of adhering to them. Early endarterectomy in the case of carotid stenosis can be offered. Additionally, if stroke symptoms recur, thrombolytic drug therapy can be started quickly.
Nguyen-Huynh et al56 analyzed the cost utility of 24-hour hospitalization for patients diagnosed with a recent TIA who were candidates for tissue plasminogen activator if a stroke occurred. They found hospitalization to be borderline cost-effective on the whole, with definite cost-effectiveness found in patients with higher stroke risk.
If patients come to medical attention several days after the TIA, then assessing risk with the ABCD2 score may no longer be reliable.57
INVESTIGATIONS
Parenchymal neuroimaging
Computed tomography (CT) without contrast is the most widely used neuroimaging test in the acute setting, since it is widely available, fast, and relatively low-cost. It will not show any abnormality in TIA or early ischemic stroke. However, it is helpful as a screening tool to rule out intracranial lesions such as hemorrhage or tumor. It may also show evidence of established infarction, which would indicate that the ischemia probably had been present for at least 6 to 12 hours.
MRI is clearly superior to noncontrast CT for detecting small areas of ischemia in patients with TIA, and it should be used unless the patient has a contraindication to it. Roughly one-third of TIA patients have lesions detectable on diffusion-weighted imaging, which helps to confirm that the episode was caused by cerebral ischemia, but nearly half of the diffusion MRI changes may be fully reversible.58 Evidence of prior stroke, leukoaraiosis, or white matter disease on fluid-attenuated inversion recovery and T2 sequences and microhemorrhages (on gradient echo sequences) help to determine a mechanistic diagnosis.
Subcategorizing TIA patients on the basis of the findings on diffusion-weighted MRI and the ABCD2 score is prognostically helpful.59 It can help to determine which patients need hospitalization and aggressive treatment, and in the case of identified diffusion-weighted MRI-positive stroke, it helps to localize and elucidate the mechanism of stroke. Hence, MRI is the preferred neuroimaging study for evaluating patients with TIA.3
Vascular imaging
Establishing the status of both intracranial and extracranial vessels is important for understanding the etiology, estimating the risk of future ischemic events, and formulating a treatment plan—eg, carotid endarterectomy in cases of significant stenosis (70% to 99%), which reduces the risk of ipsilateral stroke.60 Imaging studies include CT angiography, magnetic resonance angiography, extracranial and transcranial ultrasonography, and conventional catheter-based angiography.
CT angiography has higher spatial resolution, but vessels may be obscured by calcification associated with atherosclerotic plaque. It has the advantage of wide availability, low cost, short scanning time, and excellent patient tolerability.
Magnetic resonance angiography with gadolinium enhancement offers good quality imaging from the great vessels in the chest to the medium-sized vessels distal to the circle of Willis.
The contrast agents used in MRI and CT can have negative consequences in patients with renal disease. MRI contrast has been associated with nephrogenic fibrosing dermopathy, 61 and CT contrast can cause contrast-induced nephropathy.62
Carotid ultrasonography and transcranial Doppler ultrasonography are noninvasive and are not associated with significant adverse events. They can be used safely in patients with renal dysfunction, and they provide physiologic information that cannot be obtained with MRI and CT, which are static imaging techniques. Detecting microemboli on transcranial Doppler is an independent predictor of recurrent ischemic events.63,64
Catheter-based angiography is occasionally needed in confusing or more complicated cases, but it is invasive and occasionally is associated with iatrogenic stroke and other vascular complications.
Cardiac and aortic imaging
Echocardiography is used to detect lesions that can be sources of embolism such as regional wall-motion abnormalities, cardiac thrombus or mass, endocarditis, aortic arch atheroma, and patent foramen ovale. In patients with cryptogenic TIA or stroke, those with patent foramen ovale alone were found to have a lower risk of recurrent stroke than those who had both atrial septal aneurysm and patent foramen ovale.65
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting cardioembolic lesions, especially patent foramen ovale.66 In patients with cerebral ischemia and normal transthoracic findings, cardiac sources of embolism may be detected in about 40% of patients with transesophageal echocardiography.67
Cardiac rhythm monitoring
Electrocardiography and prolonged telemetry are recommended in patients with cryptogenic TIA to detect cardiac ischemia and paroxysmal atrial fibrillation. In one study, Holter monitoring detected atrial fibrillation in 6% of patients hospitalized with ischemic stroke or TIA.68 In another study, atrial fibrillation was detected after a median of 21 days of outpatient cardiac monitoring in 23% of patients.69
The optimal duration of outpatient telemetry has not yet been established, but studies have found significant increases in detection of paroxysms of atrial fibrillation with monitoring for 7 or longer.70
Laboratory tests in the acute setting
These include lipid profile, hemoglobin A1c, and cardiac enzymes. The advantages of hospitalization are early detection of these modifiable risk factors and early initiation of treatment.
Tests for rarer disorders
Tests for rarer disorders are sometimes indicated in unusual cases, such as ischemic symptoms occurring in young patients without other common risk factors. This includes testing for prothrombotic states, toxicology, blood cultures, inflammatory markers, hemoglobin electrophoresis, and lumbar puncture. The benefit of routine testing for thrombophilic disorders in cerebrovascular disease remains uncertain, with no clear association demonstrated with arterial stroke, but testing is more relevant in the case of venous (and paradoxical) thromboembolism.71
TREAT THE UNDERLYING DISORDER
Treatment depends on the mechanism that is thought to be responsible for the ischemic event. Vascular risk factors are important to identify and modify for all stroke subtypes.
Illustrating the importance of treating TIA and minor stroke, one study72 found that for antiplatelet therapy (aspirin, dipyridamole, or aspirin plus dipyridamole), the number needed to treat for 2 years was around 18.
Anticoagulation for cardioembolism
Atrial fibrillation, especially following a cerebrovascular ischemic event, should be treated with long-term anticoagulation with warfarin (Coumadin), dabigatran (Pradaxa), rivaroxaban (Xarelto), or apixaban (Eliquis).73 If the patient cannot tolerate anticoagulation, aspirin is recommended, and if he or she cannot tolerate aspirin, clopidogrel (Plavix) is recommended.
Antiplatelet therapy for large-vessel atherosclerosis and small-vessel disease
In the acute phase, aspirin 81 mg to 325 mg orally can be given. If the patient is allergic to aspirin, a loading dose of clopidogrel 300 mg and then 75 mg daily may be given.
A pilot study of loading with aspirin 325 mg or clopidogrel 375 mg in acute ischemic stroke and TIA patients showed that these treatments were safe when given within 36 hours and decreased the risk of neurologic deterioration.74 The patient should continue on aspirin 81 mg or clopidogrel 75 mg, as suggested by the Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER) trial.75 In the long term, an antiplatelet drug such as aspirin or clopidogrel or the combination of aspirin and extended-release dipyridamole is reasonable.76
Cilostazol (Pletal) is not inferior and is possibly superior to aspirin in preventing noncardioembolic ischemic stroke. It is used off-label for secondary prevention of stroke of noncardioembolic origin.77
Statins
In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, high-dose atorvastatin (Lipitor)—80 mg daily—was found to reduce the risk of subsequent stroke and other cardiovascular events in patients with recent stroke irrespective of low-density lipoprotein cholesterol (LDL-C) level, but there was a small increase in the risk of hemorrhagic stroke.78
In patients with hyperlipidemia, current recommendations suggest a target LDL-C level lower than 100 mg/dL in patients with atherosclerotic stroke or TIA, and lower than 70 mg/dL in those with concomitant diabetes.79
Antihypertensive therapy
In the acute period, ie, the first 24 hours after symptoms, guidelines have advocated allowing high blood pressure to remain high (“permissive hypertension”) unless the systolic pressure is greater than 200 mm Hg or the diastolic pressure is greater than 120 mm Hg or the patient is receiving thrombolytic therapy.80 However, this has recently been challenged by findings in randomized trials.81 Permissive hypertension and avoidance of dehydration with intravenous normal saline may improve cerebral perfusion, which is especially important in patients with high-grade intracranial or extracranial stenosis. Within the parameters outlined above, we recommend against aggressively treating high blood pressure in the acute phase.
In the long term, antihypertensive therapy reduces the risk of recurrent stroke or TIA.82 The goal is to keep blood pressure lower than 140/90 mm Hg, or lower than 130/80 mm Hg in patients with diabetes. A study of patients with ischemic noncardioembolic stroke showed a higher risk of recurrent stroke if the systolic blood pressure was lower than 120 or higher than 140 mm Hg.83
Some classes of antihypertensive medication may be more beneficial than others. There is some evidence that angiotensin-converting enzyme (ACE) inhibitors alone or in combination with a diuretic or an angiotensin receptor blocker are superior to other regimens, possibly because of neuroprotective mechanisms.84 A recent meta-analysis found angiotensin receptor blockers to be more effective than either ACE inhibitors or beta-blockers in stroke prevention; however, calcium channel blockers were superior to renin-angiotensin system blockers (ACE inhibitors and angiotensin receptor blockers).85
Lifestyle modifications
Smoking cessation and cardiovascular exercise for more than 10 minutes more than 3 times per week is strongly recommended.
For patients with diabetes, the goal is to keep the fasting blood glucose level lower than 126 mg/dL.
Moderate alcohol intake has been shown to decrease stroke risk compared with excessive intake or none at all.86
Carotid endarterectomy
Carotid endarterectomy has been recommended within 2 weeks of cerebral or retinal TIA in those cases attributable to high-grade internal carotid artery stenosis in patients who have low surgical risk.87 This risk can be estimated on the basis of patient factors, surgeon factors, and hospital volume. The specific recommendations are as follows:
- 70% to 99% carotid stenosis: carotid endarterectomy recommended
- 50% to 69% carotid stenosis: carotid endarterectomy recommended in select patients with a perioperative complication rate < 6%
- < 50% carotid stenosis: carotid endarterectomy not routinely recommended.
Carotid artery angioplasty and stenting with distal embolic protection device
Data from the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and European stenting trials indicate that in patients over age 70, carotid endarterectomy appears to be superior to carotid artery stenting, whereas in younger patients the periprocedural risks of stroke and death are similar. Hence, carotid artery stenting performed by an interventionist with a low complication rate is a reasonable alternative to carotid endarterectomy.88,89
A transient ischemic attack (TIA), like an episode of unstable angina, is an ominous portent of future morbidity and death even though, by definition, the event leaves no residual neurologic deficit.
But there is a positive side. When a patient presents with a TIA, the physician has the rare opportunity to reduce the risk of a disabling outcome—in this case, stroke. Therefore, patients deserve a rapid and thorough evaluation and appropriate stroke-preventive treatment.
MANY ‘TIAs’ ARE ACTUALLY STROKES
TIA has traditionally been described as a sudden focal neurologic deficit that lasts less than 24 hours, is presumed to be of vascular origin, and is confined to an area of the brain, spinal cord, or eye perfused by a specific artery. This symptom-based definition was based on the arbitrary and inaccurate assumption that brief symptoms would not be associated with damage to brain parenchyma.
The definition has since been updated and made more rational based on new concepts of brain ischemia informed by imaging, especially diffusion-weighted magnetic resonance imaging (MRI).1 One-third of episodes characterized as a TIA according to the classic definition would be considered an infarction on the basis of diffusion-weighted MRI.2 The new tissue-based definition characterizes TIA as a brief episode of neurologic dysfunction caused by focal ischemia of the brain, spinal cord, or retina, with clinical symptoms lasting less than 24 hours and without evidence of acute infarction.3
AN OPPORTUNITY TO INTERVENE
Most TIAs resolve in less than 30 minutes. The US National Institute of Neurological Disorders and Stroke trial of tissue plasminogen activator found that if symptoms of cerebral ischemia had not resolved by 1 hour or had not rapidly improved within 3 hours, complete resolution was rare (only 2% at 24 hours).4 Hence, physicians evaluating and treating patients with TIAs should treat these episodes with the urgency they deserve.
Moreover, half of the strokes that follow TIAs occur within 48 hours.5 A rapid and thorough evaluation and the initiation of secondary preventive treatments have been shown to reduce the early occurrence of stroke by up to 80%.6 Hence, the correct diagnosis of TIA gives the clinician the best opportunity to prevent stroke and its personal, social, and sometimes fatal consequences.
STROKES OUTNUMBER TIAs, BUT TIAs ARE UNDERREPORTED
According to 2012 statistics, nearly 795,000 strokes occur in the United States each year, 610,000 of which are first attacks and 185,000 are recurrences. Every 40 seconds, someone in the United States has a stroke.7
In comparison, the incidence of TIA in the United States is estimated at 200,000 to 500,000 per year, though the true number is difficult to know because of underreporting.8,9 About half of patients who experience a TIA fail to report it to their health care provider—a lost opportunity for intervention and stroke prevention.10,11
A meta-analysis showed that the risk of stroke after TIA was 9.9% at 2 days, 13.4% at 30 days, and 17.3% at 90 days.12
Interestingly, the risk of stroke after TIA exceeds the risk of recurrent stroke after a first stroke. This was shown in a study that found that patients who had made a substantial recovery within 24 hours (ie, patients with a TIA) were more likely to suffer neurologic deterioration in the next 3 months than were those who did not have significant early improvement.13
RISK FACTORS FOR TIA ARE THE SAME AS FOR STROKE
The risk of cerebrovascular disease increases with age and is higher in men14 and in blacks and Hispanics.15
The risk factors and clinical presentation do not differ between TIA and stroke, so the evaluation and treatment should not differ either. These two events represent a continuum of the same disease entity.
Some risk factors for TIA are modifiable, others are not.
Nonmodifiable risk factors
Nonmodifiable risk factors for TIA include older age, male sex, African American race, low birth weight, Hispanic ethnicity, and family history. If the patient has nonmodifiable risk factors, we should try all the harder to correct the modifiable ones.
Older age. The risk of ischemic stroke and intracranial hemorrhage doubles with each decade after age 55 in both sexes.16
Sex. Men have a significantly higher incidence of TIA than women,11 whereas the opposite is true for stroke: women have a higher lifetime risk of stroke than men.17
African Americans have an incidence of stroke (all types) 38% higher than that of whites,18 and an incidence of TIA (inpatient and out-of-hospital) 40% higher than the overall age- and sex-adjusted rate in the white population.11
Low birth weight. The odds of stroke are more than twice as high in people who weighed less than 2,500 g at birth compared with those who weighed 4,000 g or more, probably because of a correlation between low birth weight and hypertension.19
A family history of stroke increases the risk of stroke by nearly 30%, the association being stronger with large-vessel and smallvessel stroke than with cardioembolic stroke.20
Modifiable risk factors
Modifiable risk factors include cigarette smoking, hypertension, diabetes, lipid abnormalities, atrial fibrillation, carotid stenosis, and dietary and hormonal factors. Detecting these factors, which often coexist, is the first step in trying to modify them and reduce the patient’s risk.
Cigarette smoking approximately doubles the risk of ischemic stroke.21–23
Hypertension has a relationship with stroke risk that is strong, continuous, graded, consistent, and significant.24
Diabetes increases stroke risk nearly six times.25
Lipid abnormalities. Most studies have found an association between lipid levels (total cholesterol and low-density lipoprotein cholesterol) and the risk of death from ischemic stroke,26–28 and an inverse relationship between high-density lipoprotein cholesterol levels and stroke risk.29
Atrial fibrillation increases the risk of ischemic stroke up to fivefold, even in the absence of cardiac valvular disease. The mechanism is embolism of stasis-induced thrombi that form in the left atrial appendage.30
Carotid stenosis. Asymptomatic carotid atherosclerotic stenotic lesions in the extracranial internal carotid artery or carotid bulb are associated with a higher risk of stroke.24,31
Lifestyle factors. Diets that lower blood pressure have been found to decrease stroke risk.24 Exercise in men and women reduces the risk of stroke or death by 25% to 30% compared with inactive people.32 Weight reduction has been found to lower blood pressure and reduce stroke risk.24
Other potentially modifiable risk factors include migraine with aura, metabolic syndrome, excess alcohol consumption (and, paradoxically, complete abstinence from alcohol), drug abuse, sleep-disordered breathing, hyperhomocysteinemia, high lipoprotein (a) levels, hypercoagulability, infection with organisms such as Chlamydia pneumoniae, cytomegalovirus, and Helicobacter pylori, and acute infections such as respiratory and urinary infections.26
Conditions in certain demographic groups
Patients in certain demographic groups present with rarer conditions associated with stroke and TIA.
Sickle cell disease. Eleven percent of patients with sickle cell disease have clinical strokes, and a substantial number have “silent” infarcts identified on neuroimaging.33,34
Postmenopausal hormone replacement therapy with any product containing conjugated equine estrogen carries a risk of cerebrovascular events,35 and the higher the dose, the higher the risk.36 Also, oral contraceptives may be harmful in women who have additional risk factors such as cigarette smoking, prior thromboembolic events, or migraine with aura.37,38
THREE CAUSES OF STROKE AND TIA
Stroke and TIA should not be considered diagnoses in themselves, but rather the end point of many other diseases. The diagnosis lies in identifying the mechanism of the cerebrovascular event. The three main mechanisms are thrombosis, embolism, and decreased perfusion.
Thrombosis is caused by obstruction of blood flow within one or more blood vessels, the most common cause being atherosclerosis. Large-artery atherosclerosis, such as in the carotid bifurcation or extracranial internal carotid, causes TIAs that occur over a period of weeks or months with a variety of presentations in that vascular territory, from years of gradual accumulation of atherosclerotic plaque.39
In patients with small-artery or penetrating artery disease, hypertension is the primary risk factor and the pathology, specific to small arterioles, is lipohyalinosis rather than atherosclerosis. These patients may present with a stuttering clinical course, and episodes are more stereotypical.
Less common obstructive vascular pathologies include fibromuscular dysplasia, arteritides, and dissection.
Embolism can occur from a proximal source such as the heart or from proximal vessels such as the aorta, carotid, or vertebral arteries. The embolic particle may form on heart valves or lesions within the heart (eg, clot, tumor), or in the venous circulation and paradoxically cross over to the arterial side through an intracardiac or transpulmonary shunt. Embolism may also be due to a hypercoagulable state.40 Embolic stroke is suspected when multiple vascular territories within the brain are clinically or radiographically affected.
Decreased systemic perfusion caused by severe heart failure or systemic hypotension can cause ischemia to the brain diffusely and bilaterally, limiting the ability of the blood-stream to wash out microemboli, especially in the border zones (also known as “watershed areas”), thus leading to ischemia or infarction.41 Decreased perfusion can also be local, due to a fixed vessel stenosis.
Using another classification system, a study in Rochester, MN, found the following incident rates of stroke subtypes, adjusted for age and sex, per 100,000 population42:
- Large-vessel cervical or intracranial atherosclerosis with more than 50% stenosis—27
- Cardioembolism—40
- Lacunar, small-vessel disease—25
- Uncertain cause—52
- Other identifiable cause—4.
THREE CLINICAL FEATURES SUGGEST TIA
TIAs can be hard to distinguish from nonischemic neurologic events in the acute setting such as an emergency room. Up to 60% of patients suspected of having a TIA actually have a nonischemic cause of their symptoms.43
Three clinical features suggest a TIA during the emergency room evaluation:
- Rapid onset of symptoms—“like lightning” or “in seconds,” in contrast to migraine and seizures, which develop over minutes
- No history of similar episodes in the past
- Absence of nonspecific symptoms—eg, stomach upset or tightness in the chest.
CLINICAL DIAGNOSIS
Because most TIA symptoms and signs have already resolved by the time of evaluation, the diagnosis depends on a careful history with special attention to the pace of onset and resolution, the duration and nature of the symptoms, circumstances at the time of symptom onset, previous similar episodes, associated features, vascular risk factors, and family history (Table 1).44,45
A detailed neurologic examination is imperative and should include fundoscopy. A cardiovascular assessment should include cardiac rhythm, bruits in the neck, orbits, and groin, peripheral pulses, and electrocardiography.
Do neurologists do a better job at diagnosing TIA and stroke?
Primary care physicians, internists, and emergency department physicians are often the ones to carry out the clinical assessment of possible TIA.
Determining if transient neurologic symptoms are caused by ischemia can be a challenge. When in doubt, referral to a neurologist with subspecialty training in cerebrovascular disease should be considered.
But do neurologists really do a better job? A recent study sought to compare the accuracy of diagnosis of TIA made by general practitioners, emergency physicians, and neurologists. The nonneurologists considered “confusion” and “unexplained fall” suggestive of TIA and “lower facial palsy” and “monocular blindness” less suggestive of TIA—whereas the opposite is true. This shows that nonneurologists often label minor strokes and several nonvascular transient neurologic disturbances as TIAs, and up to half of patients could be mislabeled as a result.46
Differences in diagnosing cerebrovascular events between emergency room physicians and attending neurologists have been tested,47 with an accuracy of diagnosis as low as 38% by emergency department physicians in one study.48 However, other studies did not show such a trend.49,50
A study at a university-based teaching hospital found the sensitivity of emergency room physician diagnosis to be 98.6% with a positive predictive value of 94.8%,49 showing that at a large teaching hospital with a comprehensive stroke intervention program, emergency physicians could identify patients with stroke, particularly hemorrhagic stroke, very accurately.
Improving the diagnosis of stroke and TIA
Routine use of imaging and involvement of a neurologist increase the sensitivity and accuracy of diagnosis. Education and written guidelines for acute stroke treatment both in the emergency department and in out-of-hospital settings seem to dramatically improve the rates of diagnostic accuracy and appropriate treatment.50
Emergency medical service personnel use two screening tools in the field to identify TIA and stroke symptoms:
- The Cincinnati Prehospital Stroke Scale, a three-item scale based on three signs: facial droop, arm drift, and slurring of speech51
- The Los Angeles Prehospital Stroke Screen, which uses screening questions and asymmetry in the face, hand grip strength, and arm drift.52
Knowing that the patient is having a minor stroke or TIA is important. Urgent treatment of these conditions decreases the risk of stroke in the next 90 days, which was 10.5% in one study.5 Urgent assessment and early intervention could reduce this risk of subsequent stroke down to 2%.6
ASSESSING RISK OF STROKE AFTER TIA
There is a practical need for prediction of stroke during the first few days after the event. The ABCD and ABCD2 scores were developed to stratify the short-term risk of stroke in patients with recent TIA.
The ABCD score
The ABCD score53 was derived to allow primary care physicians and other physicians to identify which patients with a suspected diagnosis of TIA should be referred for emergency assessment, to allow secondary-care physicians to determine which patients with probable or definite TIA need emergency investigation and treatment, to allow public education about the need for medical attention after a TIA, and to identify people at high risk.
The ABCD2 score
The ABCD2 score predicts the short-term risk of stroke following a TIA.54 Points are assigned as follows:
- Age > 60 years: 1 point
- Blood pressure (systolic) > 140 mm Hg or diastolic blood pressure > 90 mm Hg: 1 point
- Clinical factors: unilateral weakness with or without speech impairment: 2 points (1 point for speech impairment without weakness)
- Duration of symptoms > 60 minutes: 2 points (1 point for 10–59 minutes)
- Diabetes: 1 point.
Thus, the possible total ranges from 0 to 7 points. Higher scores indicate a greater risk of stroke at 2, 7, 30, and 90 days:
- Total score 0, 1, 2, or 3: 2-day stroke risk 1.0% (low risk)
- Total score 4 or 5: 2-day stroke risk 4.1% (moderate risk)
- Total score 6 or 7: 2-day stroke risk 8.1% (high risk).
WHO SHOULD BE HOSPITALIZED?
It has been suggested that the ABCD2 score can help in triaging patients to hospital admission or outpatient care, though no randomized trial has actually evaluated the utility of the ABCD2 score in this way.3
A study of consecutive TIA patients admitted over 12 months55 found that patients with an ABCD2 score of 3 or less had the same chance of requiring hospitalization (based on positive diffusion-weighted MRI studies, risk factor identification, and treatment initiation) as those with a score of 4 to 7. Hence, admitting TIA patients on the basis of the ABCD2 score alone requires further study. However, such decisions, though informed by clinical data, depend heavily on societal input (eg, from insurance companies, national health protocols) and may be outside the purview of clinical investigation.
The benefits of hospitalization include the ability to rapidly carry out tests such as cardiac monitoring for atrial fibrillation; to detect atherosclerosis, aortic arch atheroma, and paradoxical emboli; and to quickly start secondary prevention treatments and education about the importance of adhering to them. Early endarterectomy in the case of carotid stenosis can be offered. Additionally, if stroke symptoms recur, thrombolytic drug therapy can be started quickly.
Nguyen-Huynh et al56 analyzed the cost utility of 24-hour hospitalization for patients diagnosed with a recent TIA who were candidates for tissue plasminogen activator if a stroke occurred. They found hospitalization to be borderline cost-effective on the whole, with definite cost-effectiveness found in patients with higher stroke risk.
If patients come to medical attention several days after the TIA, then assessing risk with the ABCD2 score may no longer be reliable.57
INVESTIGATIONS
Parenchymal neuroimaging
Computed tomography (CT) without contrast is the most widely used neuroimaging test in the acute setting, since it is widely available, fast, and relatively low-cost. It will not show any abnormality in TIA or early ischemic stroke. However, it is helpful as a screening tool to rule out intracranial lesions such as hemorrhage or tumor. It may also show evidence of established infarction, which would indicate that the ischemia probably had been present for at least 6 to 12 hours.
MRI is clearly superior to noncontrast CT for detecting small areas of ischemia in patients with TIA, and it should be used unless the patient has a contraindication to it. Roughly one-third of TIA patients have lesions detectable on diffusion-weighted imaging, which helps to confirm that the episode was caused by cerebral ischemia, but nearly half of the diffusion MRI changes may be fully reversible.58 Evidence of prior stroke, leukoaraiosis, or white matter disease on fluid-attenuated inversion recovery and T2 sequences and microhemorrhages (on gradient echo sequences) help to determine a mechanistic diagnosis.
Subcategorizing TIA patients on the basis of the findings on diffusion-weighted MRI and the ABCD2 score is prognostically helpful.59 It can help to determine which patients need hospitalization and aggressive treatment, and in the case of identified diffusion-weighted MRI-positive stroke, it helps to localize and elucidate the mechanism of stroke. Hence, MRI is the preferred neuroimaging study for evaluating patients with TIA.3
Vascular imaging
Establishing the status of both intracranial and extracranial vessels is important for understanding the etiology, estimating the risk of future ischemic events, and formulating a treatment plan—eg, carotid endarterectomy in cases of significant stenosis (70% to 99%), which reduces the risk of ipsilateral stroke.60 Imaging studies include CT angiography, magnetic resonance angiography, extracranial and transcranial ultrasonography, and conventional catheter-based angiography.
CT angiography has higher spatial resolution, but vessels may be obscured by calcification associated with atherosclerotic plaque. It has the advantage of wide availability, low cost, short scanning time, and excellent patient tolerability.
Magnetic resonance angiography with gadolinium enhancement offers good quality imaging from the great vessels in the chest to the medium-sized vessels distal to the circle of Willis.
The contrast agents used in MRI and CT can have negative consequences in patients with renal disease. MRI contrast has been associated with nephrogenic fibrosing dermopathy, 61 and CT contrast can cause contrast-induced nephropathy.62
Carotid ultrasonography and transcranial Doppler ultrasonography are noninvasive and are not associated with significant adverse events. They can be used safely in patients with renal dysfunction, and they provide physiologic information that cannot be obtained with MRI and CT, which are static imaging techniques. Detecting microemboli on transcranial Doppler is an independent predictor of recurrent ischemic events.63,64
Catheter-based angiography is occasionally needed in confusing or more complicated cases, but it is invasive and occasionally is associated with iatrogenic stroke and other vascular complications.
Cardiac and aortic imaging
Echocardiography is used to detect lesions that can be sources of embolism such as regional wall-motion abnormalities, cardiac thrombus or mass, endocarditis, aortic arch atheroma, and patent foramen ovale. In patients with cryptogenic TIA or stroke, those with patent foramen ovale alone were found to have a lower risk of recurrent stroke than those who had both atrial septal aneurysm and patent foramen ovale.65
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting cardioembolic lesions, especially patent foramen ovale.66 In patients with cerebral ischemia and normal transthoracic findings, cardiac sources of embolism may be detected in about 40% of patients with transesophageal echocardiography.67
Cardiac rhythm monitoring
Electrocardiography and prolonged telemetry are recommended in patients with cryptogenic TIA to detect cardiac ischemia and paroxysmal atrial fibrillation. In one study, Holter monitoring detected atrial fibrillation in 6% of patients hospitalized with ischemic stroke or TIA.68 In another study, atrial fibrillation was detected after a median of 21 days of outpatient cardiac monitoring in 23% of patients.69
The optimal duration of outpatient telemetry has not yet been established, but studies have found significant increases in detection of paroxysms of atrial fibrillation with monitoring for 7 or longer.70
Laboratory tests in the acute setting
These include lipid profile, hemoglobin A1c, and cardiac enzymes. The advantages of hospitalization are early detection of these modifiable risk factors and early initiation of treatment.
Tests for rarer disorders
Tests for rarer disorders are sometimes indicated in unusual cases, such as ischemic symptoms occurring in young patients without other common risk factors. This includes testing for prothrombotic states, toxicology, blood cultures, inflammatory markers, hemoglobin electrophoresis, and lumbar puncture. The benefit of routine testing for thrombophilic disorders in cerebrovascular disease remains uncertain, with no clear association demonstrated with arterial stroke, but testing is more relevant in the case of venous (and paradoxical) thromboembolism.71
TREAT THE UNDERLYING DISORDER
Treatment depends on the mechanism that is thought to be responsible for the ischemic event. Vascular risk factors are important to identify and modify for all stroke subtypes.
Illustrating the importance of treating TIA and minor stroke, one study72 found that for antiplatelet therapy (aspirin, dipyridamole, or aspirin plus dipyridamole), the number needed to treat for 2 years was around 18.
Anticoagulation for cardioembolism
Atrial fibrillation, especially following a cerebrovascular ischemic event, should be treated with long-term anticoagulation with warfarin (Coumadin), dabigatran (Pradaxa), rivaroxaban (Xarelto), or apixaban (Eliquis).73 If the patient cannot tolerate anticoagulation, aspirin is recommended, and if he or she cannot tolerate aspirin, clopidogrel (Plavix) is recommended.
Antiplatelet therapy for large-vessel atherosclerosis and small-vessel disease
In the acute phase, aspirin 81 mg to 325 mg orally can be given. If the patient is allergic to aspirin, a loading dose of clopidogrel 300 mg and then 75 mg daily may be given.
A pilot study of loading with aspirin 325 mg or clopidogrel 375 mg in acute ischemic stroke and TIA patients showed that these treatments were safe when given within 36 hours and decreased the risk of neurologic deterioration.74 The patient should continue on aspirin 81 mg or clopidogrel 75 mg, as suggested by the Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER) trial.75 In the long term, an antiplatelet drug such as aspirin or clopidogrel or the combination of aspirin and extended-release dipyridamole is reasonable.76
Cilostazol (Pletal) is not inferior and is possibly superior to aspirin in preventing noncardioembolic ischemic stroke. It is used off-label for secondary prevention of stroke of noncardioembolic origin.77
Statins
In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, high-dose atorvastatin (Lipitor)—80 mg daily—was found to reduce the risk of subsequent stroke and other cardiovascular events in patients with recent stroke irrespective of low-density lipoprotein cholesterol (LDL-C) level, but there was a small increase in the risk of hemorrhagic stroke.78
In patients with hyperlipidemia, current recommendations suggest a target LDL-C level lower than 100 mg/dL in patients with atherosclerotic stroke or TIA, and lower than 70 mg/dL in those with concomitant diabetes.79
Antihypertensive therapy
In the acute period, ie, the first 24 hours after symptoms, guidelines have advocated allowing high blood pressure to remain high (“permissive hypertension”) unless the systolic pressure is greater than 200 mm Hg or the diastolic pressure is greater than 120 mm Hg or the patient is receiving thrombolytic therapy.80 However, this has recently been challenged by findings in randomized trials.81 Permissive hypertension and avoidance of dehydration with intravenous normal saline may improve cerebral perfusion, which is especially important in patients with high-grade intracranial or extracranial stenosis. Within the parameters outlined above, we recommend against aggressively treating high blood pressure in the acute phase.
In the long term, antihypertensive therapy reduces the risk of recurrent stroke or TIA.82 The goal is to keep blood pressure lower than 140/90 mm Hg, or lower than 130/80 mm Hg in patients with diabetes. A study of patients with ischemic noncardioembolic stroke showed a higher risk of recurrent stroke if the systolic blood pressure was lower than 120 or higher than 140 mm Hg.83
Some classes of antihypertensive medication may be more beneficial than others. There is some evidence that angiotensin-converting enzyme (ACE) inhibitors alone or in combination with a diuretic or an angiotensin receptor blocker are superior to other regimens, possibly because of neuroprotective mechanisms.84 A recent meta-analysis found angiotensin receptor blockers to be more effective than either ACE inhibitors or beta-blockers in stroke prevention; however, calcium channel blockers were superior to renin-angiotensin system blockers (ACE inhibitors and angiotensin receptor blockers).85
Lifestyle modifications
Smoking cessation and cardiovascular exercise for more than 10 minutes more than 3 times per week is strongly recommended.
For patients with diabetes, the goal is to keep the fasting blood glucose level lower than 126 mg/dL.
Moderate alcohol intake has been shown to decrease stroke risk compared with excessive intake or none at all.86
Carotid endarterectomy
Carotid endarterectomy has been recommended within 2 weeks of cerebral or retinal TIA in those cases attributable to high-grade internal carotid artery stenosis in patients who have low surgical risk.87 This risk can be estimated on the basis of patient factors, surgeon factors, and hospital volume. The specific recommendations are as follows:
- 70% to 99% carotid stenosis: carotid endarterectomy recommended
- 50% to 69% carotid stenosis: carotid endarterectomy recommended in select patients with a perioperative complication rate < 6%
- < 50% carotid stenosis: carotid endarterectomy not routinely recommended.
Carotid artery angioplasty and stenting with distal embolic protection device
Data from the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and European stenting trials indicate that in patients over age 70, carotid endarterectomy appears to be superior to carotid artery stenting, whereas in younger patients the periprocedural risks of stroke and death are similar. Hence, carotid artery stenting performed by an interventionist with a low complication rate is a reasonable alternative to carotid endarterectomy.88,89
- Albers GW, Caplan LR, Easton JD, et al; TIA Working Group. Transient ischemic attack—proposal for a new definition. N Engl J Med 2002; 347:1713–1716.
- Ovbiagele B, Kidwell CS, Saver JL. Epidemiological impact in the United States of a tissue-based definition of transient ischemic attack. Stroke 2003; 34:919–924.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000; 55:1649–1655.
- Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284:2901–2906.
- Rothwell PM, Giles MF, Chandratheva A, et al; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370:1432–1442.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Johnston SC. Clinical practice. Transient ischemic attack. N Engl J Med 2002; 347:1687–1692.
- Johnston SC, Fayad PB, Gorelick PB, et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology 2003; 60:1429–1434.
- Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJ; North American Symptomatic Carotid Endarterectomy Trial Group. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ 2004; 170:1105–1109.
- Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005; 36:720–723.
- Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007; 167:2417–2422.
- Johnston SC, Leira EC, Hansen MD, Adams HP. Early recovery after cerebral ischemia risk of subsequent neurological deterioration. Ann Neurol 2003; 54:439–444.
- Bots ML, van der Wilk EC, Koudstaal PJ, Hofman A, Grobbee DE. Transient neurological attacks in the general population. Prevalence, risk factors, and clinical relevance. Stroke 1997; 28:768–773.
- White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111:1327–1331.
- Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27:373–380.
- Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006; 37:345–350.
- Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999; 30:736–743.
- Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertens (Greenwich) 2003; 5:133–136.
- Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke 2004; 35:212–227.
- Rodriguez BL, D’Agostino R, Abbott RD, et al. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke 2002; 33:230–236.
- Manolio TA, Kronmal RA, Burke GL, O’Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke 1996; 27:1479–1486.
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22:312–318.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Allen TW. Guide to clinical preventive services. Report of the US Preventive Services Task Force. J Am Osteopath Assoc 1991; 91:281–289.
- Goldstein LB, Bushnell CD, Adams RJ, et al; American Heart Association Stroke Council. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:517–584.
- Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989; 320:904–910.
- Zhang X, Patel A, Horibe H, et al; Asia Pacific Cohort Studies Collaboration. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol 2003; 32:563–572.
- Sanossian N, Saver JL, Navab M, Ovbiagele B. High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke 2007; 38:1104–1109.
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008; 92:17–40.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97:864–870.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998; 91:288–294.
- Mosca L, Banka CL, Benjamin EJ, et al; Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women's Association; Centers for Disease Control and Prevention; Office of Research on Women's Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med 2004; 164:741–747.
- Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia 2000; 20:155–156.
- Mohr JP, Caplan LR, Melski JW, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology 1978; 28:754–762.
- Caplan LR. Brain embolism, revisited. Neurology 1993; 43:1281–1287.
- Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998; 55:1475–1482.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999; 30:2513–2516.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Baquis GD, Pessin MS, Scott RM. Limb shaking—a carotid TIA. Stroke 1985; 16:444–448.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol 2009; 8:235–243.
- Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996; 27:2225–2229.
- Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet 1982; 1:328–331.
- Ferro JM, Pinto AN, Falcão I, et al. Diagnosis of stroke by the nonneurologist. A validation study. Stroke 1998; 29:1106–1109.
- Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians. Accuracy in the diagnosis of stroke. Stroke 1995; 26:2238–2241.
- Artto V, Putaala J, Strbian D, et al; Helsinki Stroke Thrombolysis Registry Group. Stroke mimics and intravenous thrombolysis. Ann Emerg Med 2012; 59:27–32.
- Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 1999; 33:373–378.
- Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke 2000; 31:71–76.
- Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005; 366:29–36.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Lou M, Safdar A, Edlow JA, et al. Can ABCD score predict the need for in-hospital intervention in patients with transient ischemic attacks? Int J Emerg Med 2010; 3:75–80.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology 2005; 65:1799–1801.
- Calvet D, Lamy C, Touzé E, Oppenheim C, Meder JF, Mas JL. Management and outcome of patients with transient ischemic attack admitted to a stroke unit. Cerebrovasc Dis 2007; 24:80–85.
- Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999; 30:1174–1180.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 2011; 77:1222–1228.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am 2009; 47:827–831.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- Valton L, Larrue V, le Traon AP, Massabuau P, Géraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke 1998; 29:2125–2128.
- Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke 2004; 35:2832–2836.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993; 24:1020–1024.
- de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006; 37:2531–2534.
- Lazzaro MA, Krishnan K, Prabhakaran S. Detection of atrial fibrillation with concurrent Holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2012; 21:89–93.
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71:1696–1701.
- Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011; 124:477–486.
- Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke 2010; 41:2985–2990.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Meyer DM, Albright KC, Allison TA, Grotta JC. LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2008; 17:26–29.
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM; FASTER Investigators. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007; 6:961–969.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Koenig MA, Geocadin RG, de Grouchy M, et al. Safety of induced hypertension therapy in patients with acute ischemic stroke. Neurocrit Care 2006; 4:3–7.
- Elijovich F, Laffer CL. Acute stroke: lower blood pressure looks better and better. Hypertension 2010; 56:808–810.
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004; 35:1024.
- Ovbiagele B, Diener HC, Yusuf S, et al; PROFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011; 306:2137–2144.
- Chrysant SG. The pathophysiologic role of the brain renin-angiotensin system in stroke protection: clinical implications. J Clin Hypertens (Greenwich) 2007; 9:454–459.
- Verdecchia P, Gentile G, Angeli F, Reboldi G. Beyond blood pressure: evidence for cardiovascular, cerebrovascular, and renal protective effects of renin-angiotensin system blockers. Ther Adv Cardiovasc Dis 2012; 6:81–91.
- Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006; 37:13–19.
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ; Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363:915–924.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Carotid Stenting Trialists’ Collaboration; Bonati LH, Dobson J, Algra A, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376:1062–1073.
- Albers GW, Caplan LR, Easton JD, et al; TIA Working Group. Transient ischemic attack—proposal for a new definition. N Engl J Med 2002; 347:1713–1716.
- Ovbiagele B, Kidwell CS, Saver JL. Epidemiological impact in the United States of a tissue-based definition of transient ischemic attack. Stroke 2003; 34:919–924.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000; 55:1649–1655.
- Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284:2901–2906.
- Rothwell PM, Giles MF, Chandratheva A, et al; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370:1432–1442.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Johnston SC. Clinical practice. Transient ischemic attack. N Engl J Med 2002; 347:1687–1692.
- Johnston SC, Fayad PB, Gorelick PB, et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology 2003; 60:1429–1434.
- Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJ; North American Symptomatic Carotid Endarterectomy Trial Group. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ 2004; 170:1105–1109.
- Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005; 36:720–723.
- Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007; 167:2417–2422.
- Johnston SC, Leira EC, Hansen MD, Adams HP. Early recovery after cerebral ischemia risk of subsequent neurological deterioration. Ann Neurol 2003; 54:439–444.
- Bots ML, van der Wilk EC, Koudstaal PJ, Hofman A, Grobbee DE. Transient neurological attacks in the general population. Prevalence, risk factors, and clinical relevance. Stroke 1997; 28:768–773.
- White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111:1327–1331.
- Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27:373–380.
- Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006; 37:345–350.
- Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999; 30:736–743.
- Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertens (Greenwich) 2003; 5:133–136.
- Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke 2004; 35:212–227.
- Rodriguez BL, D’Agostino R, Abbott RD, et al. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke 2002; 33:230–236.
- Manolio TA, Kronmal RA, Burke GL, O’Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke 1996; 27:1479–1486.
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22:312–318.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Allen TW. Guide to clinical preventive services. Report of the US Preventive Services Task Force. J Am Osteopath Assoc 1991; 91:281–289.
- Goldstein LB, Bushnell CD, Adams RJ, et al; American Heart Association Stroke Council. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:517–584.
- Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989; 320:904–910.
- Zhang X, Patel A, Horibe H, et al; Asia Pacific Cohort Studies Collaboration. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol 2003; 32:563–572.
- Sanossian N, Saver JL, Navab M, Ovbiagele B. High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke 2007; 38:1104–1109.
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008; 92:17–40.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 2009; 67:114–120.
- Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97:864–870.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998; 91:288–294.
- Mosca L, Banka CL, Benjamin EJ, et al; Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women's Association; Centers for Disease Control and Prevention; Office of Research on Women's Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366:2257–2266.
- Chan WS, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med 2004; 164:741–747.
- Bousser MG, Conard J, Kittner S, et al. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. The International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Cephalalgia 2000; 20:155–156.
- Mohr JP, Caplan LR, Melski JW, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology 1978; 28:754–762.
- Caplan LR. Brain embolism, revisited. Neurology 1993; 43:1281–1287.
- Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998; 55:1475–1482.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999; 30:2513–2516.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Baquis GD, Pessin MS, Scott RM. Limb shaking—a carotid TIA. Stroke 1985; 16:444–448.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol 2009; 8:235–243.
- Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996; 27:2225–2229.
- Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet 1982; 1:328–331.
- Ferro JM, Pinto AN, Falcão I, et al. Diagnosis of stroke by the nonneurologist. A validation study. Stroke 1998; 29:1106–1109.
- Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians. Accuracy in the diagnosis of stroke. Stroke 1995; 26:2238–2241.
- Artto V, Putaala J, Strbian D, et al; Helsinki Stroke Thrombolysis Registry Group. Stroke mimics and intravenous thrombolysis. Ann Emerg Med 2012; 59:27–32.
- Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 1999; 33:373–378.
- Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke 2000; 31:71–76.
- Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005; 366:29–36.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Lou M, Safdar A, Edlow JA, et al. Can ABCD score predict the need for in-hospital intervention in patients with transient ischemic attacks? Int J Emerg Med 2010; 3:75–80.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology 2005; 65:1799–1801.
- Calvet D, Lamy C, Touzé E, Oppenheim C, Meder JF, Mas JL. Management and outcome of patients with transient ischemic attack admitted to a stroke unit. Cerebrovasc Dis 2007; 24:80–85.
- Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999; 30:1174–1180.
- Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 2011; 77:1222–1228.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am 2009; 47:827–831.
- Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006; 354:379–386.
- Valton L, Larrue V, le Traon AP, Massabuau P, Géraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke 1998; 29:2125–2128.
- Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke 2004; 35:2832–2836.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993; 24:1020–1024.
- de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006; 37:2531–2534.
- Lazzaro MA, Krishnan K, Prabhakaran S. Detection of atrial fibrillation with concurrent Holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2012; 21:89–93.
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71:1696–1701.
- Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011; 124:477–486.
- Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke 2010; 41:2985–2990.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Meyer DM, Albright KC, Allison TA, Grotta JC. LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2008; 17:26–29.
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM; FASTER Investigators. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007; 6:961–969.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Amarenco P, Bogousslavsky J, Callahan A, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Koenig MA, Geocadin RG, de Grouchy M, et al. Safety of induced hypertension therapy in patients with acute ischemic stroke. Neurocrit Care 2006; 4:3–7.
- Elijovich F, Laffer CL. Acute stroke: lower blood pressure looks better and better. Hypertension 2010; 56:808–810.
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004; 35:1024.
- Ovbiagele B, Diener HC, Yusuf S, et al; PROFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011; 306:2137–2144.
- Chrysant SG. The pathophysiologic role of the brain renin-angiotensin system in stroke protection: clinical implications. J Clin Hypertens (Greenwich) 2007; 9:454–459.
- Verdecchia P, Gentile G, Angeli F, Reboldi G. Beyond blood pressure: evidence for cardiovascular, cerebrovascular, and renal protective effects of renin-angiotensin system blockers. Ther Adv Cardiovasc Dis 2012; 6:81–91.
- Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006; 37:13–19.
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ; Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363:915–924.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Carotid Stenting Trialists’ Collaboration; Bonati LH, Dobson J, Algra A, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376:1062–1073.
KEY POINTS
- Modifiable risk factors for stroke and TIA include cigarette smoking, hypertension, diabetes, lipid abnormalities, atrial fibrillation, carotid stenosis, and dietary and hormonal factors.
- The three major mechanisms of stroke and TIA are thrombosis, embolism, and decreased systemic perfusion.
- Typical symptoms of TIA include hemiparesis, hemisensory loss, aphasia, vision loss, ataxia, and diplopia. Three clinical features that suggest TIA are rapid onset of symptoms, no history of similar episodes in the past, and the absence of nonspecific symptoms.
- In suspected TIA, magnetic resonance imaging is clearly superior to noncontrast computed tomography (CT) for detecting small areas of ischemia; this test should be used unless contraindicated.
- Imaging studies of the blood vessels include CT angiography, magnetic resonance angiography, conventional angiography, and extracranial and transcranial ultrasonography.
Should you report a patient who misuses a prescription?
Dear Dr. Mossman:
My patient, Ms. X, returned to see me after she had spent 3 months in jail. When I accessed her medication history in our state’s prescription registry, I discovered that, during her incarceration, a local pharmacy continued to fill her prescription for clonazepam. After anxiously explaining that her roommate had filled the prescriptions, Ms. X pleaded with me not to tell anyone. Do I have to report this to legal authorities? If I do, will I be breaching confidentiality?
Submitted by Dr. L
Preserving the confidentiality of patient encounters is an ethical responsibility as old as the Hippocratic Oath,1 but protecting privacy is not an absolute duty. As psychiatrists familiar with the Tarasoff case2 know, clinical events sometimes create moral and legal obligations that outweigh our confidentiality obligations.
What Dr. L should do may hinge on specific details of Ms. X’s previous and current treatment, but in this article, we’ll examine some general issues that affect Dr. L’s choices. These include:
• reporting a past crime
• liability risks associated with violating confidentiality.
Monitoring controlled substances
Dr. L’s clinical situation probably would not have arisen 10 years ago because until recently, she would have had no easy way to learn that Ms. X’s prescription had been filled. In 2002, Congress responded to increasing concern about “epidemic” abuse of controlled substances—especially opioids—by authorizing state grants for prescription drug monitoring programs (PDMPs).3
PDMPs are internet-based registries that let physicians quickly find out when and where their patients have filled prescriptions for controlled substances (defined in the Table).4,5 As the rate of opioid-related deaths has risen,6 at least 43 states have initiated PDMPs; soon, all U.S. jurisdictions likely will have such programs.7 Data about the impact of PDMPs, although limited, suggest that PDMPs reduce “doctor shopping” and prescription drug abuse.8
The U.S. Department of Health and Human Services is promoting the development of electronic architecture standards to facilitate information exchange across jurisdictions,9 but states currently run their own PDMPs independently and have varying regulations about how physicians should use PDMPs.10 Excerpts from the rules used in Ohio’s prescription reporting system appear in the Box.11
Reporting past crimes
What Ms. X told Dr. L implies that someone—the patient, her roommate, or both—misused a prescription to obtain a controlled substance. Simple improper possession of a scheduled drug is a federal misdemeanor offense,12 and deception and conspiracy to obtain a scheduled drug are federal-level felonies.13 Such actions also violate state laws. Dr. L therefore knows that a crime has occurred.
Are doctors obligated or legally required to breach confidentiality and tell authorities about a patient’s past criminal acts? Writing several years ago, Appelbaum and Meisel14 and Goldman and Gutheil15 said the answer, in general, is “no.”
In recent years, state legislatures have modified criminal codes to encourage people to disclose their knowledge of certain crimes to police. For example, failures to report environmental offenses and financial misdealings have become criminal acts.16 A minority of states now punish failure to report other kinds of illegal behavior, but these laws focus mainly on violent crimes (often involving harm to vulnerable persons).17 Although Ohio has a law that obligates everyone to report knowledge of any felony, it makes exceptions when the information is learned during a customarily confidential relationship—including a physician’s treatment of a patient.18 Unless Dr. L herself has aided or concealed a crime (both illegal acts19), concerns about possible prosecution should not affect her decision to report what she has learned thus far.14
Deciding how to proceed
If Dr. L still feels inclined to do something about the misused prescription, what are her options? What clinical, legal, and moral obligations to act should she consider?
Obtain the facts. First, Dr. L should try to learn more about what happened. Jails are reluctant to give inmates benzodiazepines20; did Ms. X receive clonazepam while in jail? When and how did Ms. X learn about her roommate’s actions? Did Ms. X obtain previous prescriptions from Dr. L with the intention of letting her roommate use them? Answers to these questions can help Dr. L determine whether her patient participated in prescription misuse, an important factor in deciding what clinical or legal actions to take.
Should the patient take the lead? Learning more about the situation might suggest that Ms. X should report what has happened herself. If, for example, the roommate has coerced Ms. X to engage in illegal conduct, Dr. L might help Ms. X figure out how to tell police what has happened—preferably after Ms. X has obtained legal advice.14
Consider implications for treatment. Last, what Ms. X reveals might significantly alter her future interactions with Dr. L. This is particularly true if Dr. L concluded that Ms. X would likely divert drugs in the future, or that the patient had established her relationship with Dr. L for purposes of improperly obtaining drugs. Federal regulations require that doctors prescribe drugs only for “legitimate medical purposes,” and issuing prescriptions to a patient who is known to be delivering the drugs to others violates this law.22
Bottom Line
Growing concern about prescription drug misuse has led to nationwide implementation of systems for monitoring patients’ access to, and receipt of, controlled substances. Psychiatrists are expected to be more vigilant about patients’ use of scheduled drugs and, when they believe that a prescription has been misused, to take appropriate clinical or legal action.
Related Resources
- Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. www.whitehouse.gov/sites/default/files/ondcp/issues-content/ prescription-drugs/rx_abuse_plan.pdf.
- California Department of Alcohol and Drug Misuse. Preventing prescription drug misuse. www.prescriptiondrugmisuse.org.
- U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm.
Drug Brand Names
Clonazepam • Klonopin Hydrocodone/acetaminophen • Vicodin
Methylphenidate • Ritalin Hydromorphone • Dilaudid
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
2. Tarasoff v Regents of the University of California, 17 Cal.3d 425, 551 P.2d 334, 131 Cal Rptr 14 (Cal 1976).
3. PubLNo.107-177,115Stat748.
4. ControlledSubstancesAct,21USC§812(b)(2007).
5. Schedules of Controlled Substances, 21 CFR. § 1308.11– 1308.15 (2013).
6. Dowell D, Kunins HV, Farley TA. Opioid analgesics— risky drugs, not risky patients. JAMA. 2013;309: 2219-2220.
7. US Department of Justice. Harold Rogers Prescription Drug Monitoring Program FY 2013 Competitive Grant Announcement. Washington, DC: Bureau of Justice Assistance, Office of Justice Programs; 2013. OMB No. 1121-0329.
8. Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319-328.
9. PubLNo.112-144,126Stat993.
10. Finklea KM, Bagalman E, Sacco L. Prescription Drug Monitoring Programs. Washington, DC: Library of Congress, Congressional Research Service; 2013. Report No. R42593.
11. Ohio State Medical Association. 4731-11-11 Standards and procedures for review of Ohio Automated Rx Reporting System (OARRS). http://www.osma.org/files/pdf/sept- 2011-draft-4731-11-11-ph-of-n-ru-20110520-1541.pdf. Accessed August 5, 2013.
12. Prohibited Acts C, 21 USC §843(a)(3) (2007).
13. PenaltyforSimplePossession,21USC§844(a)(2007).
14. Appelbaum PS, Meisel A. Therapists’ obligations to report their patients’ criminal acts. Bull Am Acad Psychiatry Law. 1986;14(3):221-230.
15. Goldman MJ, Gutheil TG. The misperceived duty to report patients’ past crimes. Bull Am Acad Psychiatry Law. 1994; 22(3):407-410.
16. Thompson SG. The white-collar police force: “duty to report” statutes in criminal law theory. William Mary Bill Rights J. 2002;11(1):3-65.
17. Trombley B. No stitches for snitches: the need for a duty-to-report law in Arkansas. Univ Ark Little Rock Law J. 2012; 34:813-832.
18. OhioRevisedCode§2921.22.
19. Section2:Principals,18USC§2(a).
20. Reeves R. Guideline, education, and peer comparison to reduce prescriptions of benzodiazepines and low-dose quetiapine in prison. J Correct Health Care. 2012;18(1): 45-52.
21. Appelbaum PS. Suits against clinicians for warning of patients’ violence. Psychiatr Serv. 1996;47(7):683-684.
22. UnitedStatesvRosen,582F2d1032(5thCir1978).
23. State Medical Board of Ohio. Regarding the duty of a physician to report criminal behavior to law enforcement. http://www.med.ohio.gov/pdf/NEWS/Duty%20to%20Report_March%202013.pdf. Adopted March 2013. Accessed July 1, 2013.
24. Missouri Department of Health & Senior Services. Preventing Prescription Fraud. http://health.mo.gov/ safety/bndd/publications.php. Accessed July 1, 2013.
Dear Dr. Mossman:
My patient, Ms. X, returned to see me after she had spent 3 months in jail. When I accessed her medication history in our state’s prescription registry, I discovered that, during her incarceration, a local pharmacy continued to fill her prescription for clonazepam. After anxiously explaining that her roommate had filled the prescriptions, Ms. X pleaded with me not to tell anyone. Do I have to report this to legal authorities? If I do, will I be breaching confidentiality?
Submitted by Dr. L
Preserving the confidentiality of patient encounters is an ethical responsibility as old as the Hippocratic Oath,1 but protecting privacy is not an absolute duty. As psychiatrists familiar with the Tarasoff case2 know, clinical events sometimes create moral and legal obligations that outweigh our confidentiality obligations.
What Dr. L should do may hinge on specific details of Ms. X’s previous and current treatment, but in this article, we’ll examine some general issues that affect Dr. L’s choices. These include:
• reporting a past crime
• liability risks associated with violating confidentiality.
Monitoring controlled substances
Dr. L’s clinical situation probably would not have arisen 10 years ago because until recently, she would have had no easy way to learn that Ms. X’s prescription had been filled. In 2002, Congress responded to increasing concern about “epidemic” abuse of controlled substances—especially opioids—by authorizing state grants for prescription drug monitoring programs (PDMPs).3
PDMPs are internet-based registries that let physicians quickly find out when and where their patients have filled prescriptions for controlled substances (defined in the Table).4,5 As the rate of opioid-related deaths has risen,6 at least 43 states have initiated PDMPs; soon, all U.S. jurisdictions likely will have such programs.7 Data about the impact of PDMPs, although limited, suggest that PDMPs reduce “doctor shopping” and prescription drug abuse.8
The U.S. Department of Health and Human Services is promoting the development of electronic architecture standards to facilitate information exchange across jurisdictions,9 but states currently run their own PDMPs independently and have varying regulations about how physicians should use PDMPs.10 Excerpts from the rules used in Ohio’s prescription reporting system appear in the Box.11
Reporting past crimes
What Ms. X told Dr. L implies that someone—the patient, her roommate, or both—misused a prescription to obtain a controlled substance. Simple improper possession of a scheduled drug is a federal misdemeanor offense,12 and deception and conspiracy to obtain a scheduled drug are federal-level felonies.13 Such actions also violate state laws. Dr. L therefore knows that a crime has occurred.
Are doctors obligated or legally required to breach confidentiality and tell authorities about a patient’s past criminal acts? Writing several years ago, Appelbaum and Meisel14 and Goldman and Gutheil15 said the answer, in general, is “no.”
In recent years, state legislatures have modified criminal codes to encourage people to disclose their knowledge of certain crimes to police. For example, failures to report environmental offenses and financial misdealings have become criminal acts.16 A minority of states now punish failure to report other kinds of illegal behavior, but these laws focus mainly on violent crimes (often involving harm to vulnerable persons).17 Although Ohio has a law that obligates everyone to report knowledge of any felony, it makes exceptions when the information is learned during a customarily confidential relationship—including a physician’s treatment of a patient.18 Unless Dr. L herself has aided or concealed a crime (both illegal acts19), concerns about possible prosecution should not affect her decision to report what she has learned thus far.14
Deciding how to proceed
If Dr. L still feels inclined to do something about the misused prescription, what are her options? What clinical, legal, and moral obligations to act should she consider?
Obtain the facts. First, Dr. L should try to learn more about what happened. Jails are reluctant to give inmates benzodiazepines20; did Ms. X receive clonazepam while in jail? When and how did Ms. X learn about her roommate’s actions? Did Ms. X obtain previous prescriptions from Dr. L with the intention of letting her roommate use them? Answers to these questions can help Dr. L determine whether her patient participated in prescription misuse, an important factor in deciding what clinical or legal actions to take.
Should the patient take the lead? Learning more about the situation might suggest that Ms. X should report what has happened herself. If, for example, the roommate has coerced Ms. X to engage in illegal conduct, Dr. L might help Ms. X figure out how to tell police what has happened—preferably after Ms. X has obtained legal advice.14
Consider implications for treatment. Last, what Ms. X reveals might significantly alter her future interactions with Dr. L. This is particularly true if Dr. L concluded that Ms. X would likely divert drugs in the future, or that the patient had established her relationship with Dr. L for purposes of improperly obtaining drugs. Federal regulations require that doctors prescribe drugs only for “legitimate medical purposes,” and issuing prescriptions to a patient who is known to be delivering the drugs to others violates this law.22
Bottom Line
Growing concern about prescription drug misuse has led to nationwide implementation of systems for monitoring patients’ access to, and receipt of, controlled substances. Psychiatrists are expected to be more vigilant about patients’ use of scheduled drugs and, when they believe that a prescription has been misused, to take appropriate clinical or legal action.
Related Resources
- Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. www.whitehouse.gov/sites/default/files/ondcp/issues-content/ prescription-drugs/rx_abuse_plan.pdf.
- California Department of Alcohol and Drug Misuse. Preventing prescription drug misuse. www.prescriptiondrugmisuse.org.
- U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm.
Drug Brand Names
Clonazepam • Klonopin Hydrocodone/acetaminophen • Vicodin
Methylphenidate • Ritalin Hydromorphone • Dilaudid
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman:
My patient, Ms. X, returned to see me after she had spent 3 months in jail. When I accessed her medication history in our state’s prescription registry, I discovered that, during her incarceration, a local pharmacy continued to fill her prescription for clonazepam. After anxiously explaining that her roommate had filled the prescriptions, Ms. X pleaded with me not to tell anyone. Do I have to report this to legal authorities? If I do, will I be breaching confidentiality?
Submitted by Dr. L
Preserving the confidentiality of patient encounters is an ethical responsibility as old as the Hippocratic Oath,1 but protecting privacy is not an absolute duty. As psychiatrists familiar with the Tarasoff case2 know, clinical events sometimes create moral and legal obligations that outweigh our confidentiality obligations.
What Dr. L should do may hinge on specific details of Ms. X’s previous and current treatment, but in this article, we’ll examine some general issues that affect Dr. L’s choices. These include:
• reporting a past crime
• liability risks associated with violating confidentiality.
Monitoring controlled substances
Dr. L’s clinical situation probably would not have arisen 10 years ago because until recently, she would have had no easy way to learn that Ms. X’s prescription had been filled. In 2002, Congress responded to increasing concern about “epidemic” abuse of controlled substances—especially opioids—by authorizing state grants for prescription drug monitoring programs (PDMPs).3
PDMPs are internet-based registries that let physicians quickly find out when and where their patients have filled prescriptions for controlled substances (defined in the Table).4,5 As the rate of opioid-related deaths has risen,6 at least 43 states have initiated PDMPs; soon, all U.S. jurisdictions likely will have such programs.7 Data about the impact of PDMPs, although limited, suggest that PDMPs reduce “doctor shopping” and prescription drug abuse.8
The U.S. Department of Health and Human Services is promoting the development of electronic architecture standards to facilitate information exchange across jurisdictions,9 but states currently run their own PDMPs independently and have varying regulations about how physicians should use PDMPs.10 Excerpts from the rules used in Ohio’s prescription reporting system appear in the Box.11
Reporting past crimes
What Ms. X told Dr. L implies that someone—the patient, her roommate, or both—misused a prescription to obtain a controlled substance. Simple improper possession of a scheduled drug is a federal misdemeanor offense,12 and deception and conspiracy to obtain a scheduled drug are federal-level felonies.13 Such actions also violate state laws. Dr. L therefore knows that a crime has occurred.
Are doctors obligated or legally required to breach confidentiality and tell authorities about a patient’s past criminal acts? Writing several years ago, Appelbaum and Meisel14 and Goldman and Gutheil15 said the answer, in general, is “no.”
In recent years, state legislatures have modified criminal codes to encourage people to disclose their knowledge of certain crimes to police. For example, failures to report environmental offenses and financial misdealings have become criminal acts.16 A minority of states now punish failure to report other kinds of illegal behavior, but these laws focus mainly on violent crimes (often involving harm to vulnerable persons).17 Although Ohio has a law that obligates everyone to report knowledge of any felony, it makes exceptions when the information is learned during a customarily confidential relationship—including a physician’s treatment of a patient.18 Unless Dr. L herself has aided or concealed a crime (both illegal acts19), concerns about possible prosecution should not affect her decision to report what she has learned thus far.14
Deciding how to proceed
If Dr. L still feels inclined to do something about the misused prescription, what are her options? What clinical, legal, and moral obligations to act should she consider?
Obtain the facts. First, Dr. L should try to learn more about what happened. Jails are reluctant to give inmates benzodiazepines20; did Ms. X receive clonazepam while in jail? When and how did Ms. X learn about her roommate’s actions? Did Ms. X obtain previous prescriptions from Dr. L with the intention of letting her roommate use them? Answers to these questions can help Dr. L determine whether her patient participated in prescription misuse, an important factor in deciding what clinical or legal actions to take.
Should the patient take the lead? Learning more about the situation might suggest that Ms. X should report what has happened herself. If, for example, the roommate has coerced Ms. X to engage in illegal conduct, Dr. L might help Ms. X figure out how to tell police what has happened—preferably after Ms. X has obtained legal advice.14
Consider implications for treatment. Last, what Ms. X reveals might significantly alter her future interactions with Dr. L. This is particularly true if Dr. L concluded that Ms. X would likely divert drugs in the future, or that the patient had established her relationship with Dr. L for purposes of improperly obtaining drugs. Federal regulations require that doctors prescribe drugs only for “legitimate medical purposes,” and issuing prescriptions to a patient who is known to be delivering the drugs to others violates this law.22
Bottom Line
Growing concern about prescription drug misuse has led to nationwide implementation of systems for monitoring patients’ access to, and receipt of, controlled substances. Psychiatrists are expected to be more vigilant about patients’ use of scheduled drugs and, when they believe that a prescription has been misused, to take appropriate clinical or legal action.
Related Resources
- Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. www.whitehouse.gov/sites/default/files/ondcp/issues-content/ prescription-drugs/rx_abuse_plan.pdf.
- California Department of Alcohol and Drug Misuse. Preventing prescription drug misuse. www.prescriptiondrugmisuse.org.
- U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm.
Drug Brand Names
Clonazepam • Klonopin Hydrocodone/acetaminophen • Vicodin
Methylphenidate • Ritalin Hydromorphone • Dilaudid
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
2. Tarasoff v Regents of the University of California, 17 Cal.3d 425, 551 P.2d 334, 131 Cal Rptr 14 (Cal 1976).
3. PubLNo.107-177,115Stat748.
4. ControlledSubstancesAct,21USC§812(b)(2007).
5. Schedules of Controlled Substances, 21 CFR. § 1308.11– 1308.15 (2013).
6. Dowell D, Kunins HV, Farley TA. Opioid analgesics— risky drugs, not risky patients. JAMA. 2013;309: 2219-2220.
7. US Department of Justice. Harold Rogers Prescription Drug Monitoring Program FY 2013 Competitive Grant Announcement. Washington, DC: Bureau of Justice Assistance, Office of Justice Programs; 2013. OMB No. 1121-0329.
8. Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319-328.
9. PubLNo.112-144,126Stat993.
10. Finklea KM, Bagalman E, Sacco L. Prescription Drug Monitoring Programs. Washington, DC: Library of Congress, Congressional Research Service; 2013. Report No. R42593.
11. Ohio State Medical Association. 4731-11-11 Standards and procedures for review of Ohio Automated Rx Reporting System (OARRS). http://www.osma.org/files/pdf/sept- 2011-draft-4731-11-11-ph-of-n-ru-20110520-1541.pdf. Accessed August 5, 2013.
12. Prohibited Acts C, 21 USC §843(a)(3) (2007).
13. PenaltyforSimplePossession,21USC§844(a)(2007).
14. Appelbaum PS, Meisel A. Therapists’ obligations to report their patients’ criminal acts. Bull Am Acad Psychiatry Law. 1986;14(3):221-230.
15. Goldman MJ, Gutheil TG. The misperceived duty to report patients’ past crimes. Bull Am Acad Psychiatry Law. 1994; 22(3):407-410.
16. Thompson SG. The white-collar police force: “duty to report” statutes in criminal law theory. William Mary Bill Rights J. 2002;11(1):3-65.
17. Trombley B. No stitches for snitches: the need for a duty-to-report law in Arkansas. Univ Ark Little Rock Law J. 2012; 34:813-832.
18. OhioRevisedCode§2921.22.
19. Section2:Principals,18USC§2(a).
20. Reeves R. Guideline, education, and peer comparison to reduce prescriptions of benzodiazepines and low-dose quetiapine in prison. J Correct Health Care. 2012;18(1): 45-52.
21. Appelbaum PS. Suits against clinicians for warning of patients’ violence. Psychiatr Serv. 1996;47(7):683-684.
22. UnitedStatesvRosen,582F2d1032(5thCir1978).
23. State Medical Board of Ohio. Regarding the duty of a physician to report criminal behavior to law enforcement. http://www.med.ohio.gov/pdf/NEWS/Duty%20to%20Report_March%202013.pdf. Adopted March 2013. Accessed July 1, 2013.
24. Missouri Department of Health & Senior Services. Preventing Prescription Fraud. http://health.mo.gov/ safety/bndd/publications.php. Accessed July 1, 2013.
1. von Staden H. “In a pure and holy way”: personal and professional conduct in the Hippocratic Oath? J Hist Med Allied Sci. 1996;51(4):404-437.
2. Tarasoff v Regents of the University of California, 17 Cal.3d 425, 551 P.2d 334, 131 Cal Rptr 14 (Cal 1976).
3. PubLNo.107-177,115Stat748.
4. ControlledSubstancesAct,21USC§812(b)(2007).
5. Schedules of Controlled Substances, 21 CFR. § 1308.11– 1308.15 (2013).
6. Dowell D, Kunins HV, Farley TA. Opioid analgesics— risky drugs, not risky patients. JAMA. 2013;309: 2219-2220.
7. US Department of Justice. Harold Rogers Prescription Drug Monitoring Program FY 2013 Competitive Grant Announcement. Washington, DC: Bureau of Justice Assistance, Office of Justice Programs; 2013. OMB No. 1121-0329.
8. Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319-328.
9. PubLNo.112-144,126Stat993.
10. Finklea KM, Bagalman E, Sacco L. Prescription Drug Monitoring Programs. Washington, DC: Library of Congress, Congressional Research Service; 2013. Report No. R42593.
11. Ohio State Medical Association. 4731-11-11 Standards and procedures for review of Ohio Automated Rx Reporting System (OARRS). http://www.osma.org/files/pdf/sept- 2011-draft-4731-11-11-ph-of-n-ru-20110520-1541.pdf. Accessed August 5, 2013.
12. Prohibited Acts C, 21 USC §843(a)(3) (2007).
13. PenaltyforSimplePossession,21USC§844(a)(2007).
14. Appelbaum PS, Meisel A. Therapists’ obligations to report their patients’ criminal acts. Bull Am Acad Psychiatry Law. 1986;14(3):221-230.
15. Goldman MJ, Gutheil TG. The misperceived duty to report patients’ past crimes. Bull Am Acad Psychiatry Law. 1994; 22(3):407-410.
16. Thompson SG. The white-collar police force: “duty to report” statutes in criminal law theory. William Mary Bill Rights J. 2002;11(1):3-65.
17. Trombley B. No stitches for snitches: the need for a duty-to-report law in Arkansas. Univ Ark Little Rock Law J. 2012; 34:813-832.
18. OhioRevisedCode§2921.22.
19. Section2:Principals,18USC§2(a).
20. Reeves R. Guideline, education, and peer comparison to reduce prescriptions of benzodiazepines and low-dose quetiapine in prison. J Correct Health Care. 2012;18(1): 45-52.
21. Appelbaum PS. Suits against clinicians for warning of patients’ violence. Psychiatr Serv. 1996;47(7):683-684.
22. UnitedStatesvRosen,582F2d1032(5thCir1978).
23. State Medical Board of Ohio. Regarding the duty of a physician to report criminal behavior to law enforcement. http://www.med.ohio.gov/pdf/NEWS/Duty%20to%20Report_March%202013.pdf. Adopted March 2013. Accessed July 1, 2013.
24. Missouri Department of Health & Senior Services. Preventing Prescription Fraud. http://health.mo.gov/ safety/bndd/publications.php. Accessed July 1, 2013.
Ill-advised genetic counseling: $1M verdict
A mother had given birth to two children with thalamic abnormalities that resulted in seizures, developmental delays, and death. Before getting pregnant again, the parents sought genetic counseling and were told that identifying the specific defective gene would be impossible. The geneticist advised them that a child conceived with a donor egg and father’s sperm would have essentially the same risk as the general population. The parents asked in writing if it would be safer to
use both donor egg and donor sperm; the geneticist responded that the difference in risk was negligible.
The mother gave birth in June 2007 to a child conceived with a donated egg and the father’s sperm. After the child began to show the same symptoms as the others, an MRI of the child’s brain revealed a thalamic abnormality, and testing revealed Alpers syndrome caused by POLG gene mutations. The third child died in September 2008.
PARENTS’ CLAIM The chances of having a child with Alpers syndrome are
about 1:200,000 in the general population; if one parent is a known carrier, the chance is 1:1,000. If the parents had known this risk, they would have used donor egg and donor sperm to conceive or adopted. They were not told about Alpers syndrome and its relationship to the POLG gene until after their third child was born. The geneticist was negligent in failing to provide this information.
PHYSICIAN’S DEFENSE The parents received appropriate and accurate genetic counseling.
VERDICT A $1 million Florida verdict was returned.
What caused a delay in breast cancer diagnosis?
A 39-year-old woman underwent mammography in October 2004. After recommending a spotcompression film of a left-breast lesion, and then ultrasonography, the radiologist concluded that the lesion was benign, and suggested a 1-year follow-up. Reports were sent to the patient and her primary care physician.
In August 2006, when mammography was suspicious for breast cancer, a biopsy diagnosed infiltrating ductal carcinoma of the left breast. After undergoing a mastectomy, radiation therapy, and chemotherapy, the patient was cancer-free at the time of the trial.
patient’s CLAIM The radiologist failed to properly interpret the 2004 mammography.
physician’s DEFENSE The radiologist’s interpretations of the 2004 tests were correct. The patient failed to follow up in 1 year, as recommended, and this delayed the cancer diagnosis. The patient’s survival indicated that she had been cured of her breast cancer.
VERDICT A confidential settlement was reached with the hospital before the trial. An Illinois defense verdict was returned for the radiologist.
Heparin overdose for preemie
At 27 weeks' gestation, a woman went to a clinic with preeclampsia. After she was stabilized, the baby was born by emergency cesarean delivery.
At birth, the baby was thrombocytopenic (platelet count, 37,000/mL) with a heart rate of 60 bpm. The child’s cord blood pH was 7.27, indicating no significant hypoxia. At 1 minute of life, the child’s heart rate had not improved. After trying three times to place an endotracheal tube, chest compressions were begun at 10 minutes of life. An umbilical vein catheter (UVC) was placed at 22 minutes. Heparin was used to flush the UVC. After 40 minutes, the baby’s pH was 6.88, indicating severe acidosis. The infant was transferred to another hospital 3 hours after birth.
Head ultrasonography at 5 days of life revealed hemorrhagic and ischemic changes in the baby’s brain. The child suffered massive brain damage, is ventilator-dependent, and has a G-tube for feeding. She cannot sit up, walk, or speak, and will require specialized care for life.
Parent's claim Emergency resuscitation was not performed at birth: the low heart rate and thrombocytopenia were not treated; the UVC was not immediately placed. Twice, adult doses of heparin were used instead of normal saline to flush the UVC; heparin caused bleeding in the baby’s brain.
Defendant's Defense The case was settled during trial.
Verdict A $3 million Maryland settlement was reached.
Uterine rupture: $130M verdict
After a woman's first child was born by cesarean delivery, vaginal birth after cesarean (VBAC) was planned for her second pregnancy. When a nurse recognized a ruptured uterus, the ObGyn ordered a cesarean delivery. The newborn suffered severe brain damage, with seizures. She has cerebral palsy with near-normal intelligence, but cannot talk or walk and continues to have seizures.
Parents' claim The baby’s injuries occurred due to a failure to respond to fetal distress. When the intrauterine pressure catheter (IUPC) stopped working for 27 minutes, the nurse did not notify the ObGyn or apply an external monitor. Fetal heart decelerations occurred, including a prolonged deceleration for 3 minutes; the nurse did not notify the ObGyn, reposition the mother, provide oxygen and extra fluids, or discontinue oxytocin. A cesarean delivery should have occurred 30 to 60 minutes earlier.
Defendants' defense The fetal heart rates were what typically occur during the second stage of labor. The hospital’s accepted practices were followed. When the IUPC failed, the nurse measured contractions by hand and analyzed the fetal heartbeat from audible sounds; therefore, it was not necessary to notify the ObGyn. The physician was promptly called when uterine rupture was suspected. Uterine rupture and placental abruption caused the child’s injury. Uterine rupture cannot be predicted or prevented and is a known complication of VBAC.
Verdict After the parents declined an $8 million settlement, the matter was tried to a defense verdict. That decision was overturned on appeal, and, at a second trial, a $130 million New York verdict was returned against the hospital that employed the ObGyn and nurse.
Uterus, small bowel injured during D&C
A 65-year-old woman underwent dilation and curettage (D&C) to screen for uterine cancer performed by an ObGyn and a general surgeon. Her uterus and small intestine were perforated during the procedure, and a second operation was required to repair the damage.
Patient's claim Both physicians were negligent in performing D&C.
Physician's defense The ObGyn denied negligence and countered that the injuries are known complications of the procedure.
Verdict The surgeon settled for a confidential amount before trial. A New Jersey defense verdict was returned for the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A mother had given birth to two children with thalamic abnormalities that resulted in seizures, developmental delays, and death. Before getting pregnant again, the parents sought genetic counseling and were told that identifying the specific defective gene would be impossible. The geneticist advised them that a child conceived with a donor egg and father’s sperm would have essentially the same risk as the general population. The parents asked in writing if it would be safer to
use both donor egg and donor sperm; the geneticist responded that the difference in risk was negligible.
The mother gave birth in June 2007 to a child conceived with a donated egg and the father’s sperm. After the child began to show the same symptoms as the others, an MRI of the child’s brain revealed a thalamic abnormality, and testing revealed Alpers syndrome caused by POLG gene mutations. The third child died in September 2008.
PARENTS’ CLAIM The chances of having a child with Alpers syndrome are
about 1:200,000 in the general population; if one parent is a known carrier, the chance is 1:1,000. If the parents had known this risk, they would have used donor egg and donor sperm to conceive or adopted. They were not told about Alpers syndrome and its relationship to the POLG gene until after their third child was born. The geneticist was negligent in failing to provide this information.
PHYSICIAN’S DEFENSE The parents received appropriate and accurate genetic counseling.
VERDICT A $1 million Florida verdict was returned.
What caused a delay in breast cancer diagnosis?
A 39-year-old woman underwent mammography in October 2004. After recommending a spotcompression film of a left-breast lesion, and then ultrasonography, the radiologist concluded that the lesion was benign, and suggested a 1-year follow-up. Reports were sent to the patient and her primary care physician.
In August 2006, when mammography was suspicious for breast cancer, a biopsy diagnosed infiltrating ductal carcinoma of the left breast. After undergoing a mastectomy, radiation therapy, and chemotherapy, the patient was cancer-free at the time of the trial.
patient’s CLAIM The radiologist failed to properly interpret the 2004 mammography.
physician’s DEFENSE The radiologist’s interpretations of the 2004 tests were correct. The patient failed to follow up in 1 year, as recommended, and this delayed the cancer diagnosis. The patient’s survival indicated that she had been cured of her breast cancer.
VERDICT A confidential settlement was reached with the hospital before the trial. An Illinois defense verdict was returned for the radiologist.
Heparin overdose for preemie
At 27 weeks' gestation, a woman went to a clinic with preeclampsia. After she was stabilized, the baby was born by emergency cesarean delivery.
At birth, the baby was thrombocytopenic (platelet count, 37,000/mL) with a heart rate of 60 bpm. The child’s cord blood pH was 7.27, indicating no significant hypoxia. At 1 minute of life, the child’s heart rate had not improved. After trying three times to place an endotracheal tube, chest compressions were begun at 10 minutes of life. An umbilical vein catheter (UVC) was placed at 22 minutes. Heparin was used to flush the UVC. After 40 minutes, the baby’s pH was 6.88, indicating severe acidosis. The infant was transferred to another hospital 3 hours after birth.
Head ultrasonography at 5 days of life revealed hemorrhagic and ischemic changes in the baby’s brain. The child suffered massive brain damage, is ventilator-dependent, and has a G-tube for feeding. She cannot sit up, walk, or speak, and will require specialized care for life.
Parent's claim Emergency resuscitation was not performed at birth: the low heart rate and thrombocytopenia were not treated; the UVC was not immediately placed. Twice, adult doses of heparin were used instead of normal saline to flush the UVC; heparin caused bleeding in the baby’s brain.
Defendant's Defense The case was settled during trial.
Verdict A $3 million Maryland settlement was reached.
Uterine rupture: $130M verdict
After a woman's first child was born by cesarean delivery, vaginal birth after cesarean (VBAC) was planned for her second pregnancy. When a nurse recognized a ruptured uterus, the ObGyn ordered a cesarean delivery. The newborn suffered severe brain damage, with seizures. She has cerebral palsy with near-normal intelligence, but cannot talk or walk and continues to have seizures.
Parents' claim The baby’s injuries occurred due to a failure to respond to fetal distress. When the intrauterine pressure catheter (IUPC) stopped working for 27 minutes, the nurse did not notify the ObGyn or apply an external monitor. Fetal heart decelerations occurred, including a prolonged deceleration for 3 minutes; the nurse did not notify the ObGyn, reposition the mother, provide oxygen and extra fluids, or discontinue oxytocin. A cesarean delivery should have occurred 30 to 60 minutes earlier.
Defendants' defense The fetal heart rates were what typically occur during the second stage of labor. The hospital’s accepted practices were followed. When the IUPC failed, the nurse measured contractions by hand and analyzed the fetal heartbeat from audible sounds; therefore, it was not necessary to notify the ObGyn. The physician was promptly called when uterine rupture was suspected. Uterine rupture and placental abruption caused the child’s injury. Uterine rupture cannot be predicted or prevented and is a known complication of VBAC.
Verdict After the parents declined an $8 million settlement, the matter was tried to a defense verdict. That decision was overturned on appeal, and, at a second trial, a $130 million New York verdict was returned against the hospital that employed the ObGyn and nurse.
Uterus, small bowel injured during D&C
A 65-year-old woman underwent dilation and curettage (D&C) to screen for uterine cancer performed by an ObGyn and a general surgeon. Her uterus and small intestine were perforated during the procedure, and a second operation was required to repair the damage.
Patient's claim Both physicians were negligent in performing D&C.
Physician's defense The ObGyn denied negligence and countered that the injuries are known complications of the procedure.
Verdict The surgeon settled for a confidential amount before trial. A New Jersey defense verdict was returned for the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
A mother had given birth to two children with thalamic abnormalities that resulted in seizures, developmental delays, and death. Before getting pregnant again, the parents sought genetic counseling and were told that identifying the specific defective gene would be impossible. The geneticist advised them that a child conceived with a donor egg and father’s sperm would have essentially the same risk as the general population. The parents asked in writing if it would be safer to
use both donor egg and donor sperm; the geneticist responded that the difference in risk was negligible.
The mother gave birth in June 2007 to a child conceived with a donated egg and the father’s sperm. After the child began to show the same symptoms as the others, an MRI of the child’s brain revealed a thalamic abnormality, and testing revealed Alpers syndrome caused by POLG gene mutations. The third child died in September 2008.
PARENTS’ CLAIM The chances of having a child with Alpers syndrome are
about 1:200,000 in the general population; if one parent is a known carrier, the chance is 1:1,000. If the parents had known this risk, they would have used donor egg and donor sperm to conceive or adopted. They were not told about Alpers syndrome and its relationship to the POLG gene until after their third child was born. The geneticist was negligent in failing to provide this information.
PHYSICIAN’S DEFENSE The parents received appropriate and accurate genetic counseling.
VERDICT A $1 million Florida verdict was returned.
What caused a delay in breast cancer diagnosis?
A 39-year-old woman underwent mammography in October 2004. After recommending a spotcompression film of a left-breast lesion, and then ultrasonography, the radiologist concluded that the lesion was benign, and suggested a 1-year follow-up. Reports were sent to the patient and her primary care physician.
In August 2006, when mammography was suspicious for breast cancer, a biopsy diagnosed infiltrating ductal carcinoma of the left breast. After undergoing a mastectomy, radiation therapy, and chemotherapy, the patient was cancer-free at the time of the trial.
patient’s CLAIM The radiologist failed to properly interpret the 2004 mammography.
physician’s DEFENSE The radiologist’s interpretations of the 2004 tests were correct. The patient failed to follow up in 1 year, as recommended, and this delayed the cancer diagnosis. The patient’s survival indicated that she had been cured of her breast cancer.
VERDICT A confidential settlement was reached with the hospital before the trial. An Illinois defense verdict was returned for the radiologist.
Heparin overdose for preemie
At 27 weeks' gestation, a woman went to a clinic with preeclampsia. After she was stabilized, the baby was born by emergency cesarean delivery.
At birth, the baby was thrombocytopenic (platelet count, 37,000/mL) with a heart rate of 60 bpm. The child’s cord blood pH was 7.27, indicating no significant hypoxia. At 1 minute of life, the child’s heart rate had not improved. After trying three times to place an endotracheal tube, chest compressions were begun at 10 minutes of life. An umbilical vein catheter (UVC) was placed at 22 minutes. Heparin was used to flush the UVC. After 40 minutes, the baby’s pH was 6.88, indicating severe acidosis. The infant was transferred to another hospital 3 hours after birth.
Head ultrasonography at 5 days of life revealed hemorrhagic and ischemic changes in the baby’s brain. The child suffered massive brain damage, is ventilator-dependent, and has a G-tube for feeding. She cannot sit up, walk, or speak, and will require specialized care for life.
Parent's claim Emergency resuscitation was not performed at birth: the low heart rate and thrombocytopenia were not treated; the UVC was not immediately placed. Twice, adult doses of heparin were used instead of normal saline to flush the UVC; heparin caused bleeding in the baby’s brain.
Defendant's Defense The case was settled during trial.
Verdict A $3 million Maryland settlement was reached.
Uterine rupture: $130M verdict
After a woman's first child was born by cesarean delivery, vaginal birth after cesarean (VBAC) was planned for her second pregnancy. When a nurse recognized a ruptured uterus, the ObGyn ordered a cesarean delivery. The newborn suffered severe brain damage, with seizures. She has cerebral palsy with near-normal intelligence, but cannot talk or walk and continues to have seizures.
Parents' claim The baby’s injuries occurred due to a failure to respond to fetal distress. When the intrauterine pressure catheter (IUPC) stopped working for 27 minutes, the nurse did not notify the ObGyn or apply an external monitor. Fetal heart decelerations occurred, including a prolonged deceleration for 3 minutes; the nurse did not notify the ObGyn, reposition the mother, provide oxygen and extra fluids, or discontinue oxytocin. A cesarean delivery should have occurred 30 to 60 minutes earlier.
Defendants' defense The fetal heart rates were what typically occur during the second stage of labor. The hospital’s accepted practices were followed. When the IUPC failed, the nurse measured contractions by hand and analyzed the fetal heartbeat from audible sounds; therefore, it was not necessary to notify the ObGyn. The physician was promptly called when uterine rupture was suspected. Uterine rupture and placental abruption caused the child’s injury. Uterine rupture cannot be predicted or prevented and is a known complication of VBAC.
Verdict After the parents declined an $8 million settlement, the matter was tried to a defense verdict. That decision was overturned on appeal, and, at a second trial, a $130 million New York verdict was returned against the hospital that employed the ObGyn and nurse.
Uterus, small bowel injured during D&C
A 65-year-old woman underwent dilation and curettage (D&C) to screen for uterine cancer performed by an ObGyn and a general surgeon. Her uterus and small intestine were perforated during the procedure, and a second operation was required to repair the damage.
Patient's claim Both physicians were negligent in performing D&C.
Physician's defense The ObGyn denied negligence and countered that the injuries are known complications of the procedure.
Verdict The surgeon settled for a confidential amount before trial. A New Jersey defense verdict was returned for the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Which abnormal ovarian findings can be followed by serial TVUS?
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
Investigational treatments for cognitive impairment in schizophrenia
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
Obsessed with Facebook
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Paranoid and online
Mr. M, age 22, is brought to the emergency department by family because they are concerned about his paranoia and increasing agitation related to Facebook posts by friends and siblings. At age 8, Mr. M was diagnosed with depression, attention-deficit/hyperactivity disorder (ADHD), and anger management problems, which were well controlled with fluoxetine until last year, when he discontinued psychiatric follow-up. Mr. M’s girlfriend ended their relationship 1 month ago, although it is unclear whether the break-up was caused by his depressive symptoms or exacerbated them. In the last 2 days, his parents have noticed an increase in his delusional thoughts and aggressive behavior.
Family psychiatric history is not significant. Five years ago, Mr. M suffered a head injury in a motor vehicle collision, but completed high school without evidence of cognitive impairment or behavioral changes.
Mr. M appears disheveled and irritable. He reports his mood as “depressed,” but denies suicidal or homicidal ideations. He has no history of violence or antisocial behavior.
Mr. M is alert and oriented with clear speech, intact language, and grossly intact memory and concentration—although, he admits, “I just obsess over certain thoughts.” He endorses feelings of anxiety, insomnia, low energy, lack of sleep secondary to his paranoia, and claims that “something was said on Facebook about a girl and everyone is in on it.” He explains that his Facebook friends talk in “analogies” about him, and reports that, “I can just tell that’s what they are talking about even if they don’t say it directly.”
a) impulse control disorder
b) brief psychotic episode
c) psychotic depression
d) bipolar disorder
The authors’ observations
The last decade has seen a rise in the creation and use of social networking sites such as Facebook, Myspace, and Twitter. Facebook has 1.15 billion monthly active users.1 Seventy-five percent of teenagers own cell phones, and 25% report using their phones to access social media outlets.2 More than 50% of teenagers visit a social networking site daily, with 22% logging in to their favorite social media network more than 10 times a day.3 The easy accessibility of social media outlets has prompted study of the association of that accessibility with anxiety, depression, and self-esteem.3-7
Although not a DSM-5 or ICD-10 diagnosis, internet addiction has been correlated with depression.8 Similarly, O’Keefe and colleagues describe Facebook depression in teens who spend a large amount of time on social networking sites.4 The recently developed Bergen Facebook Addiction Scale (BFAS)9 evaluates the six core elements of addiction (salience, mood modification, tolerance, withdrawal, conflict, and relapse) in Facebook users.
Facebook certainly provides a valuable mechanism for friends to stay connected in an increasingly global society, and has acknowledged the potential it has to address mental illness. In 2011, Facebook partnered with the National Suicide Prevention Lifeline to allow users to report observed suicidal content, thereby utilizing the online community to facilitate delivery of mental health resources.10,11
HISTORY: Sibling rivalry
Mr. M had a romantic relationship with “Ms. B” in high school that he describes as “on and off,” beginning during his sophomore year. He describes himself as a “quick learner” who is task-oriented. He says he was outgoing in high school but became more introverted during his last year there. After high school, Mr. M worked as an electrician and discontinued psychiatric follow-up because he “felt fine.” He lives at home with his parents, two older sisters, and twin brother, who he identifies as being a lifelong “rival.”
After Ms. B ended her relationship with Mr. M, he began to suspect that she had become romantically involved with his twin brother. After Mr. M observed his brother leaving the house one night, he confronted his twin, who denied any involvement with Ms. B. After his brother left, Mr. M became enraged and punched a wall, fracturing his hand.
Two weeks before admission, Mr. M became increasingly preoccupied with suspicions of his brother’s involvement with Ms. B and looked for evidence on Facebook. Mr. M intensely monitored his Facebook news feed, which constantly updates to show public posts made by a user’s Facebook friends. He interpreted his friends’ posts as either directly relating to him or to a new relationship between Ms. B and his twin brother, stating that his friends were “talking in analogies” rather than directly using names.
Mr. M’s Facebook use rapidly increased to 3 or more hours a day. He can access Facebook from his laptop or cell phone, and reports logging in more than 10 times throughout the day. He says that, on Facebook, “it’s easier to talk trash” because people can say things they would not normally say face to face. He also states that Facebook is “ruining personal relationships,” and that it is “so easy to be in touch with everyone without really being in touch.”
The authors’ observations
In Mr. M’s case, Facebook served as a vehicle through which he could pursue a non-bizarre delusion. Mr. M openly admitted to viewing his twin brother as a rival; it is not surprising, therefore, that his delusions targeted his brother and ex-girlfriend.
Before social networks, the perseveration of this delusion might have been limited to internal thinking, or gathering corroborative information by means of stalking. Social media outlets have provided a means to perseverate and implicate others remotely, however, and Mr. M soon expanded his delusions to include more peers.
After beginning to suspect that friends and family are commenting on or criticizing him through Facebook, Mr. M experienced an irresistible impulse to repeatedly check the social network, which may have provided short-term relief of anticipatory anxiety, but that perpetuated the cycle. Constant access to the internet facilitated and intensified Mr. M’s cycle of paranoia, anxiety, and dysphoria. He called this process an “addiction.” A conceptual framework of the development of Mr. M’s maladaptive use of Facebook is illustrated in Figure 1.
Risk factors
Insecurity with one’s self-worth also may be a warning sign. Online social networking circumvents the need for physical interaction. A Facebook profile allows a person to selectively portray himself (herself) to the world, which may not be congruous with how his peers see him in everyday life. Patients who fear criticism or judgment may be more prone to maladaptive Facebook use, because they might feel empowered by the control they have over how others see them—online, at least.
Limited or, in Mr. M’s case, singular romantic experience may have influenced the course of his illness. Mr. M described his romantic involvement as a single, tumultuous relationship that lasted several years. Young patients with limited romantic experience may struggle to develop healthy protective mechanisms and may become preoccupied with the details of the situation, such that it interferes with functioning.
Mr. M’s history of ADHD might be a risk factor for abnormal patterns of internet use. Patients with ADHD have increased attentiveness with visually stimulating tasks—specifically, computers and video games.12
Last, it is unclear how, or if, Mr. M’s history of head injury contributed to his symptoms. There were no clear, temporal changes in cognition or emotion associated with the head injury, and he did not receive regular follow-up. Significant cognitive impairment does not appear to be a factor.
a) restart fluoxetine
b) begin an atypical antipsychotic
c) begin a mood stabilizer and atypical antipsychotic
d) encourage Mr. M to deactivate his Facebook account
TREATMENT: Observed use
Quetiapine is selected to target psychosis, agitation, and insomnia characterized by difficulty with sleep initiation. Risperidone is added as a short-term agent to boost antipsychotic effect during the day when Mr. M is not fully responsive to quetiapine alone. Valproic acid is added on admission as a mood stabilizer to target emotional lability, impulsiveness, and possible mania.
After several days of treatment, and without access to a computer, Mr. M is calmer. We begin to assess the challenges of self-limiting time spent on Facebook; Mr. M explains that, before hospitalization, he had deactivated his Facebook account several times to try to rid himself of what he describes as an “addiction to social media”; soon afterward, however, he experienced overwhelming anxiety that led him to reactivate his account.
We sit with Mr. M as he logs in to Facebook and discuss the range of alternative explanations that specific public messages on his news feed could have. Explicitly listing alternative explanations is a technique used in cognitive-behavioral therapy. Mr. M begins to demonstrate increased insight regarding his paranoia and possible misinterpretation of information gleaned via Facebook; however, he still believes that masked references to him had existed. During his hospital stay he begins to acknowledge the problems that online interactions pose compared with face-to-face interactions, stating that, “There’s no emotion in [Facebook], so you can easily misinterpret what someone says.”
The authors’ observations
Mr. M was discharged after 7 days of treatment and has been seen weekly as an outpatient for 3 months without need for further hospitalization.
Bottom Line
Pervasive access to social media represents a vehicle for relapse of many psychiatric conditions. Younger patients may be especially at risk because they are more likely to use social media and are in the age range for onset of psychiatric illness. Although some degree of dependence on online networks can be considered normal, patients suffering from mental illness represent a vulnerable population for maladaptive online interactions.
Related Resources
• Sandler EP. If you’re in crisis, go online. Psychology Today. www.psychologytoday.com/blog/promoting-hope-preventing-suicide/201110/if-you-re-in-crisis-go-online. Published October 26, 2011.
• Nitzan U, Shoshan E, Lev-Ran S, et al. Internet-related psychosis−a sign of the times. Isr J Psychiatry Relat Sci. 2011;48(3):207-211.
• Martin EA, Bailey DH, Cicero DC, et al. Social networking profile correlates of schizotypy. Psychiatry Res. 2012;200(2-3):641-646.
Drug Brand Names
Fluoxetine • Prozac Quetiapine • Seroquel
Risperidone • Risperdal Valproic acid • Depakote
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.
1. Facebook. Facebook reports second quarter 2013 results. http://investor.fb.com/releasedetail.cfm?ReleaseID= 780093. Updated July 24, 2013. Accessed July 29, 2013.
2. Hinduja S, Patchin JW. Offline consequences of online victimization: school violence and delinquency. Journal of School Violence. 2007;6(3):89-112.
3. Pantic I, Damjanovic A, Todorovic J, et al. Associations between online social networking and depression in high school students: behavioral physiology viewpoint. Psychiatr Danub. 2012;24(1):90-93.
4. O’Keeffe GS, Clarke-Pearson K; Council on Communications and Media. The impact of social media on children, adolescents, and families. Pediatrics. 2011;127(4):800-804.
5. Gonzales AL, Hancock JT. Mirror, mirror on my Facebook wall: effects of exposure to Facebook on self-esteem. Cyberpsychol Behav Soc Netw. 2011;14(1-2):79-83.
6. Hinduja S, Patchin JW. Bullying, cyberbullying, and suicide. Arch Suicide Res. 2010;14(3):206-221.
7. Selfhout MH, Branje SJ, Delsing M, et al. Different types of Internet use, depression, and social anxiety: the role of perceived friendship quality. J Adolesc. 2009;32(4):819-833.
8. Morrison CM, Gore H. The relationship between excessive internet use and depression: a questionnaire-based study of 1,319 young people and adults. Psychopathology. 2010; 43:121-126.
9. Andreassen CS, Torsheim T, Brunborg GS, et al. Development of a Facebook Addiction Scale. Psychol Rep. 2012;110(2):501-517.
10. SAMHSA News. Suicide prevention: a national priority. vol 20, no 3. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
11. Facebook. New partnership between Facebook and the National Suicide Prevention Lifeline http://www.facebook.com/note.php?note_id=310287485658707. Accessed July 25, 2013.
12. Weinstein A, Weizman A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2012;14(5):590-597.