User login
Proactive Approaches Necessary to Offset Primary-Care Physician Shortage
As I was reading my departmental, end-of-the-academic-year newsletter, I was pondering my own group’s hospitalist pipeline. Each year, I earnestly read the list of internal-medicine-program graduates, focusing on what and where they are going to practice. I first selfishly scan the list for “hospital medicine, MUSC.” Then I go back and reread the list to see who I can now send my discharges to or who I can refer any new friends or relatives who move to town, scanning the list for “primary care, MUSC.”
This year, similar to recent years, the list for “primary care” is slim.
SHM has long been motivated to think about the pipeline, about how to get the best and the brightest interested in practicing HM, and practicing primary care, as they are vital partners in the spectrum of generalist care. We need to know and understand our pipeline: Where will they train, how will they be trained, will they be prepared to function and thrive in the medical industry of tomorrow? Regardless of how or where you practice, all of us should be thinking about our pipeline.
As such, all of us should be thinking about graduate medical education (GME), how it is funded, how much it is funded, and what regulations control the types of specialties that come out of U.S. training programs.1 This is especially true given the projected need for more hospitalists in all areas of the hospital of the future, the ever-expanding role of “specialty hospitalists,” and the need for hospitalists during the “peri-hospital” stay (from pre-operative clinics to post-discharge clinics). And this is especially true given the ongoing projected expanse of the primary-care shortage.
The career path for physicians starts long before medical school and is heavily shaped by what types of physicians they are exposed to, when they are exposed to them, and what their experience was. The periods of medical school and graduate medical education training can have a profound impact on the “health” of the U.S. health-care system and whether it is equipped to care for the needs of its citizens.
American taxpayers have long been in the business of funding the physician pipeline. The federal government invests $13 billion annually on graduate medical education subsidies. The money flows directly to teaching hospitals to pay for the salaries of the trainees and the salaries of the attendings who supervise their work, as well as the hospital overhead that has to be invested to house these trainees during their tenure.
Federal subsidies for apprenticeships are relatively unheard of in other industries; this funding stream was initiated with the passage of Medicare almost 50 years ago, under the provision that additional training for medical students would result in better and safer medical care for all Americans. However, what was not set up as a tagline to these federal subsidies was any type of accountability on process or outcome measures, such as how exactly do teaching hospitals invest their GME money, and how will they produce the types and amounts of physicians that the U.S. needs?
Cold, Hard Facts
Source: U.S. Department of Health and Human Resources, Health Resources and Services Administration, http://datawarehouse.hrsa.gov/hpsadetail.aspx
So what do Americans get for that annual $13 billion investment? We get what we should expect out of the “free will” of graduating residents: We get an oversupply of specialists in areas of abundance and an undersupply of generalists in most areas. The system “produces” the most appealing specialties (those handsomely reimbursed and highly prestigious), leaving a dwindling number of generalists to be spread thinly. And the most prestigious and top-ranked academic medical centers are the least likely to produce generalists. In many of these highly ranked training programs, less than 10% of their graduates go on to work in primary care, and even fewer work in rural or public health facilities. More than 20% of all residency programs produce no primary-care physicians (PCPs) at all. Despite the $13 billion annual investment, the American Association of Medical Colleges (AAMC) predicts a shortage of 45,000 primary-care physicians by 2020.2
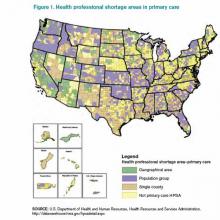
Because of this shortage, even fully insured Americans find the act of securing a generalist to be problematic: Almost 1 in 5 of us live in a federally designated primary-care-shortage area (see Figure 1).3 It is estimated that our current training programs will produce 40% fewer PCPs than will be needed to keep pace with the baby boomers and the insurance expansion of the Affordable Care Act. Attempts at using GME subsidies as a lever to increase the number of generalists have failed for decades. Almost 30 years ago, Dan Quayle petitioned Medicare to forgo any subsidies to training programs that did not commit to graduating at least 70% of trainees to primary-care careers, to no avail. Years later, the Institute of Medicine appealed to the federal government to reduce the training of specialists, and increase the training pool for generalists, to no avail. To reduce the financial burden of GME training, about 15 years ago, Congress threw in place a stop-gap measure, putting a freeze on the total number of residency slots that would be funded, but it did not put any measures in place to ensure that the allocation of slots would match what the U.S. health-care system needs. This has left us in a global shortage of physicians, the most grotesque of which is among generalists in regions of greatest need.
The Good News
So where does this leave hospitalists? Fortunately for our specialty, hospital medicine remains very appealing to new graduates and to the health-care system. For new graduates, it offers a competitive salary and work-life balance, without additional fellowship training. For the health-care system, we are generalists who can enhance the “value equation,” having proven to enhance quality while simultaneously reducing cost. As generalists, our specialty remains relatively undifferentiated and flexible to meet the needs of the system, including caring for patients at many stages of an acute or chronic illness; pre-operative care; post-discharge transitions of care; and assisting in some stages of “specialty care” (e.g. the medical care of the neurologic emergency, the pregnant patient, comanagement with a variety of surgical subspecialists).
As a progressive specialty, we should continue to focus on the pipeline, not only to ensure we recruit our “favorite picks” to hospital medicine, but also to support the reform needed to enhance the appeal of generalist practices and reduce the irresistible appeal of specialty care. In this way, we can add yet another meaningful contribution to meeting the needs of the U.S. health-care system.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Longman P. First teach no harm. The Washington Monthly website. Available at: http://www.washingtonmonthly.com/magazine/july_august_2013/features/first_teach_no_harm045361.php?page=all. Accessed Aug. 4, 2013.
- Association of American Medical Colleges. Physician shortages to worsen without increases in residency training. Association of American Medical Colleges website. Available at: https://www.aamc.org/download/150612/data/md-shortage.pdf. Accessed Aug. 4, 2013.
As I was reading my departmental, end-of-the-academic-year newsletter, I was pondering my own group’s hospitalist pipeline. Each year, I earnestly read the list of internal-medicine-program graduates, focusing on what and where they are going to practice. I first selfishly scan the list for “hospital medicine, MUSC.” Then I go back and reread the list to see who I can now send my discharges to or who I can refer any new friends or relatives who move to town, scanning the list for “primary care, MUSC.”
This year, similar to recent years, the list for “primary care” is slim.
SHM has long been motivated to think about the pipeline, about how to get the best and the brightest interested in practicing HM, and practicing primary care, as they are vital partners in the spectrum of generalist care. We need to know and understand our pipeline: Where will they train, how will they be trained, will they be prepared to function and thrive in the medical industry of tomorrow? Regardless of how or where you practice, all of us should be thinking about our pipeline.
As such, all of us should be thinking about graduate medical education (GME), how it is funded, how much it is funded, and what regulations control the types of specialties that come out of U.S. training programs.1 This is especially true given the projected need for more hospitalists in all areas of the hospital of the future, the ever-expanding role of “specialty hospitalists,” and the need for hospitalists during the “peri-hospital” stay (from pre-operative clinics to post-discharge clinics). And this is especially true given the ongoing projected expanse of the primary-care shortage.
The career path for physicians starts long before medical school and is heavily shaped by what types of physicians they are exposed to, when they are exposed to them, and what their experience was. The periods of medical school and graduate medical education training can have a profound impact on the “health” of the U.S. health-care system and whether it is equipped to care for the needs of its citizens.
American taxpayers have long been in the business of funding the physician pipeline. The federal government invests $13 billion annually on graduate medical education subsidies. The money flows directly to teaching hospitals to pay for the salaries of the trainees and the salaries of the attendings who supervise their work, as well as the hospital overhead that has to be invested to house these trainees during their tenure.
Federal subsidies for apprenticeships are relatively unheard of in other industries; this funding stream was initiated with the passage of Medicare almost 50 years ago, under the provision that additional training for medical students would result in better and safer medical care for all Americans. However, what was not set up as a tagline to these federal subsidies was any type of accountability on process or outcome measures, such as how exactly do teaching hospitals invest their GME money, and how will they produce the types and amounts of physicians that the U.S. needs?
Cold, Hard Facts
Source: U.S. Department of Health and Human Resources, Health Resources and Services Administration, http://datawarehouse.hrsa.gov/hpsadetail.aspx
So what do Americans get for that annual $13 billion investment? We get what we should expect out of the “free will” of graduating residents: We get an oversupply of specialists in areas of abundance and an undersupply of generalists in most areas. The system “produces” the most appealing specialties (those handsomely reimbursed and highly prestigious), leaving a dwindling number of generalists to be spread thinly. And the most prestigious and top-ranked academic medical centers are the least likely to produce generalists. In many of these highly ranked training programs, less than 10% of their graduates go on to work in primary care, and even fewer work in rural or public health facilities. More than 20% of all residency programs produce no primary-care physicians (PCPs) at all. Despite the $13 billion annual investment, the American Association of Medical Colleges (AAMC) predicts a shortage of 45,000 primary-care physicians by 2020.2
Because of this shortage, even fully insured Americans find the act of securing a generalist to be problematic: Almost 1 in 5 of us live in a federally designated primary-care-shortage area (see Figure 1).3 It is estimated that our current training programs will produce 40% fewer PCPs than will be needed to keep pace with the baby boomers and the insurance expansion of the Affordable Care Act. Attempts at using GME subsidies as a lever to increase the number of generalists have failed for decades. Almost 30 years ago, Dan Quayle petitioned Medicare to forgo any subsidies to training programs that did not commit to graduating at least 70% of trainees to primary-care careers, to no avail. Years later, the Institute of Medicine appealed to the federal government to reduce the training of specialists, and increase the training pool for generalists, to no avail. To reduce the financial burden of GME training, about 15 years ago, Congress threw in place a stop-gap measure, putting a freeze on the total number of residency slots that would be funded, but it did not put any measures in place to ensure that the allocation of slots would match what the U.S. health-care system needs. This has left us in a global shortage of physicians, the most grotesque of which is among generalists in regions of greatest need.
The Good News
So where does this leave hospitalists? Fortunately for our specialty, hospital medicine remains very appealing to new graduates and to the health-care system. For new graduates, it offers a competitive salary and work-life balance, without additional fellowship training. For the health-care system, we are generalists who can enhance the “value equation,” having proven to enhance quality while simultaneously reducing cost. As generalists, our specialty remains relatively undifferentiated and flexible to meet the needs of the system, including caring for patients at many stages of an acute or chronic illness; pre-operative care; post-discharge transitions of care; and assisting in some stages of “specialty care” (e.g. the medical care of the neurologic emergency, the pregnant patient, comanagement with a variety of surgical subspecialists).
As a progressive specialty, we should continue to focus on the pipeline, not only to ensure we recruit our “favorite picks” to hospital medicine, but also to support the reform needed to enhance the appeal of generalist practices and reduce the irresistible appeal of specialty care. In this way, we can add yet another meaningful contribution to meeting the needs of the U.S. health-care system.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Longman P. First teach no harm. The Washington Monthly website. Available at: http://www.washingtonmonthly.com/magazine/july_august_2013/features/first_teach_no_harm045361.php?page=all. Accessed Aug. 4, 2013.
- Association of American Medical Colleges. Physician shortages to worsen without increases in residency training. Association of American Medical Colleges website. Available at: https://www.aamc.org/download/150612/data/md-shortage.pdf. Accessed Aug. 4, 2013.
As I was reading my departmental, end-of-the-academic-year newsletter, I was pondering my own group’s hospitalist pipeline. Each year, I earnestly read the list of internal-medicine-program graduates, focusing on what and where they are going to practice. I first selfishly scan the list for “hospital medicine, MUSC.” Then I go back and reread the list to see who I can now send my discharges to or who I can refer any new friends or relatives who move to town, scanning the list for “primary care, MUSC.”
This year, similar to recent years, the list for “primary care” is slim.
SHM has long been motivated to think about the pipeline, about how to get the best and the brightest interested in practicing HM, and practicing primary care, as they are vital partners in the spectrum of generalist care. We need to know and understand our pipeline: Where will they train, how will they be trained, will they be prepared to function and thrive in the medical industry of tomorrow? Regardless of how or where you practice, all of us should be thinking about our pipeline.
As such, all of us should be thinking about graduate medical education (GME), how it is funded, how much it is funded, and what regulations control the types of specialties that come out of U.S. training programs.1 This is especially true given the projected need for more hospitalists in all areas of the hospital of the future, the ever-expanding role of “specialty hospitalists,” and the need for hospitalists during the “peri-hospital” stay (from pre-operative clinics to post-discharge clinics). And this is especially true given the ongoing projected expanse of the primary-care shortage.
The career path for physicians starts long before medical school and is heavily shaped by what types of physicians they are exposed to, when they are exposed to them, and what their experience was. The periods of medical school and graduate medical education training can have a profound impact on the “health” of the U.S. health-care system and whether it is equipped to care for the needs of its citizens.
American taxpayers have long been in the business of funding the physician pipeline. The federal government invests $13 billion annually on graduate medical education subsidies. The money flows directly to teaching hospitals to pay for the salaries of the trainees and the salaries of the attendings who supervise their work, as well as the hospital overhead that has to be invested to house these trainees during their tenure.
Federal subsidies for apprenticeships are relatively unheard of in other industries; this funding stream was initiated with the passage of Medicare almost 50 years ago, under the provision that additional training for medical students would result in better and safer medical care for all Americans. However, what was not set up as a tagline to these federal subsidies was any type of accountability on process or outcome measures, such as how exactly do teaching hospitals invest their GME money, and how will they produce the types and amounts of physicians that the U.S. needs?
Cold, Hard Facts
Source: U.S. Department of Health and Human Resources, Health Resources and Services Administration, http://datawarehouse.hrsa.gov/hpsadetail.aspx
So what do Americans get for that annual $13 billion investment? We get what we should expect out of the “free will” of graduating residents: We get an oversupply of specialists in areas of abundance and an undersupply of generalists in most areas. The system “produces” the most appealing specialties (those handsomely reimbursed and highly prestigious), leaving a dwindling number of generalists to be spread thinly. And the most prestigious and top-ranked academic medical centers are the least likely to produce generalists. In many of these highly ranked training programs, less than 10% of their graduates go on to work in primary care, and even fewer work in rural or public health facilities. More than 20% of all residency programs produce no primary-care physicians (PCPs) at all. Despite the $13 billion annual investment, the American Association of Medical Colleges (AAMC) predicts a shortage of 45,000 primary-care physicians by 2020.2
Because of this shortage, even fully insured Americans find the act of securing a generalist to be problematic: Almost 1 in 5 of us live in a federally designated primary-care-shortage area (see Figure 1).3 It is estimated that our current training programs will produce 40% fewer PCPs than will be needed to keep pace with the baby boomers and the insurance expansion of the Affordable Care Act. Attempts at using GME subsidies as a lever to increase the number of generalists have failed for decades. Almost 30 years ago, Dan Quayle petitioned Medicare to forgo any subsidies to training programs that did not commit to graduating at least 70% of trainees to primary-care careers, to no avail. Years later, the Institute of Medicine appealed to the federal government to reduce the training of specialists, and increase the training pool for generalists, to no avail. To reduce the financial burden of GME training, about 15 years ago, Congress threw in place a stop-gap measure, putting a freeze on the total number of residency slots that would be funded, but it did not put any measures in place to ensure that the allocation of slots would match what the U.S. health-care system needs. This has left us in a global shortage of physicians, the most grotesque of which is among generalists in regions of greatest need.
The Good News
So where does this leave hospitalists? Fortunately for our specialty, hospital medicine remains very appealing to new graduates and to the health-care system. For new graduates, it offers a competitive salary and work-life balance, without additional fellowship training. For the health-care system, we are generalists who can enhance the “value equation,” having proven to enhance quality while simultaneously reducing cost. As generalists, our specialty remains relatively undifferentiated and flexible to meet the needs of the system, including caring for patients at many stages of an acute or chronic illness; pre-operative care; post-discharge transitions of care; and assisting in some stages of “specialty care” (e.g. the medical care of the neurologic emergency, the pregnant patient, comanagement with a variety of surgical subspecialists).
As a progressive specialty, we should continue to focus on the pipeline, not only to ensure we recruit our “favorite picks” to hospital medicine, but also to support the reform needed to enhance the appeal of generalist practices and reduce the irresistible appeal of specialty care. In this way, we can add yet another meaningful contribution to meeting the needs of the U.S. health-care system.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Longman P. First teach no harm. The Washington Monthly website. Available at: http://www.washingtonmonthly.com/magazine/july_august_2013/features/first_teach_no_harm045361.php?page=all. Accessed Aug. 4, 2013.
- Association of American Medical Colleges. Physician shortages to worsen without increases in residency training. Association of American Medical Colleges website. Available at: https://www.aamc.org/download/150612/data/md-shortage.pdf. Accessed Aug. 4, 2013.
New Thinking, Higher Expectations Needed to Solve Hospital Readmission Problem
As we enter a new era of health-care and payment reform, we are all keenly aware of the need to limit unnecessary readmissions. We have been given tools and tips on how to most efficiently and effectively transition patients from the hospital setting to the outpatient world in order to limit the chances that they will “bounce back” to us, resulting in penalties to our hospital or health-care system. Tools such as Project BOOST and others help us to educate patients, transfer information effectively, follow up on patients post-discharge, and reconcile medications safely across the continuum of care. But without a competent and committed provider of care to “catch” the patient on the other side, we might just be dropping the ball yet again.
It is imperative as we look to safely transition patients into the next level of care that we, as hospitalists, get outside the box and begin to engage the community of care providers outside our walls, and yes, even outside of our control. We have been down this road before with other quality initiatives, which at first glance appeared to be outside our sphere of influence—such projects as post-operative antibiotic use and hospitalwide DVT prophylaxis. Given the right hospitalist leader, with the right set of leadership tools, these quality-improvement (QI) projects have been widely successful in many environments.
I would suggest that the issue of safe transitions for our patients is no different, and maybe more important, to the health and safety of our patients.
Solving the readmission problem on a local level requires an analytical approach, much like a “root-cause analysis.” We need to begin to examine the sources of our readmitted patients, as well as the routes of our discharged patients, and we need to ask ourselves if we are continuing to feed patients into the vicious circle that results in readmissions. Are there post-acute-care facilities in your area that are responsible for more than their fair share of patients returning to your service? If so, why do we continue to discharge patients to their care? Is it because we are pressured to lower length of stay, and any bed at the next level of care is better than another day in the acute-care hospital? At some point, this reasoning fails, and given the penalties coming soon, it may be better to begin to more discriminately discharge patients to facilities that provide higher-quality care and assist us in our goals to reduce unnecessary readmissions. Leading the charge in this endeavor also necessitates that we begin to engage those providers on the other side, making them aware of the quality data related to their facility and providing education and resources to assist them in improving their performance.
Realities of the Care Continuum
Several options pertaining to hospitalist groups are available. The first, already a large movement in our current marketplace, is to extend the current hospitalist group across the chasm and begin to deliver care in those post-acute facilities. Long-term acute care (LTAC) and skilled nursing facilities (SNF) are prime examples of this movement; the obvious advantage lies in the effective control of quality and efficient transfer of information that a single group can achieve when it extends to these facilities. Obviously, manpower issues and financial support are drawbacks in a model such as this.
More realistically, a group might consider taking a less aggressive approach to this problem. Educating care providers and assisting these facilities with QI projects would require fewer resources and might provide a higher return on investment (ROI) for your group and hospital. Engaging these physicians, nonphysician providers, and facility administrators is key to our ability to impact this problem. Demanding quality care for our discharged patients in terms of timeliness of follow-up, adherence to care paths, and responsiveness to changes in condition should be non-negotiable and factored into our development of referral patterns.
As our population of patients continues to be more acutely ill, and the level of care provided at post-acute care facilities continues to rise, our current reality is that a majority of these patients, at any given time, meet hospital admissions criteria. Preventing readmissions requires that post-acute care providers have mechanisms in place to stop the “knee-jerk” transfer to the emergency department, rather than attempt to evaluate and treat the patient in the facility. Interact II (http://interact2.net/index.aspx) is a resource that provides tools for post-acute-care facilities to use in monitoring their own internal data around acute-care transfers. It also provides tracking tools, communication strategies, advanced-care-planning tools, and clinical pathways for limiting the number of acute-care transfers. The reality is, once these patients end up in the emergency department, they are likely to be referred to us for consideration of readmission. The best way to stop this is to stop the transfer before it happens.
Demand Better
We, as hospitalists, need to begin to leverage our own “buying power” as it relates to the care of our patients post-discharge. We can start by educating and assisting care providers on a local level to improve compliance with well-known standards of care that prevent unnecessary readmissions. We need to be prepared to wield our collective weight as a specialty to demand from our post-acute care colleagues what has been demanded of us over the last several years: quality and value. Make no mistake—hospitalists have to get outside the box.
Dr. Harrington is an SHM board member and chief medical officer of Locum Leaders in Alpharetta, Ga.
As we enter a new era of health-care and payment reform, we are all keenly aware of the need to limit unnecessary readmissions. We have been given tools and tips on how to most efficiently and effectively transition patients from the hospital setting to the outpatient world in order to limit the chances that they will “bounce back” to us, resulting in penalties to our hospital or health-care system. Tools such as Project BOOST and others help us to educate patients, transfer information effectively, follow up on patients post-discharge, and reconcile medications safely across the continuum of care. But without a competent and committed provider of care to “catch” the patient on the other side, we might just be dropping the ball yet again.
It is imperative as we look to safely transition patients into the next level of care that we, as hospitalists, get outside the box and begin to engage the community of care providers outside our walls, and yes, even outside of our control. We have been down this road before with other quality initiatives, which at first glance appeared to be outside our sphere of influence—such projects as post-operative antibiotic use and hospitalwide DVT prophylaxis. Given the right hospitalist leader, with the right set of leadership tools, these quality-improvement (QI) projects have been widely successful in many environments.
I would suggest that the issue of safe transitions for our patients is no different, and maybe more important, to the health and safety of our patients.
Solving the readmission problem on a local level requires an analytical approach, much like a “root-cause analysis.” We need to begin to examine the sources of our readmitted patients, as well as the routes of our discharged patients, and we need to ask ourselves if we are continuing to feed patients into the vicious circle that results in readmissions. Are there post-acute-care facilities in your area that are responsible for more than their fair share of patients returning to your service? If so, why do we continue to discharge patients to their care? Is it because we are pressured to lower length of stay, and any bed at the next level of care is better than another day in the acute-care hospital? At some point, this reasoning fails, and given the penalties coming soon, it may be better to begin to more discriminately discharge patients to facilities that provide higher-quality care and assist us in our goals to reduce unnecessary readmissions. Leading the charge in this endeavor also necessitates that we begin to engage those providers on the other side, making them aware of the quality data related to their facility and providing education and resources to assist them in improving their performance.
Realities of the Care Continuum
Several options pertaining to hospitalist groups are available. The first, already a large movement in our current marketplace, is to extend the current hospitalist group across the chasm and begin to deliver care in those post-acute facilities. Long-term acute care (LTAC) and skilled nursing facilities (SNF) are prime examples of this movement; the obvious advantage lies in the effective control of quality and efficient transfer of information that a single group can achieve when it extends to these facilities. Obviously, manpower issues and financial support are drawbacks in a model such as this.
More realistically, a group might consider taking a less aggressive approach to this problem. Educating care providers and assisting these facilities with QI projects would require fewer resources and might provide a higher return on investment (ROI) for your group and hospital. Engaging these physicians, nonphysician providers, and facility administrators is key to our ability to impact this problem. Demanding quality care for our discharged patients in terms of timeliness of follow-up, adherence to care paths, and responsiveness to changes in condition should be non-negotiable and factored into our development of referral patterns.
As our population of patients continues to be more acutely ill, and the level of care provided at post-acute care facilities continues to rise, our current reality is that a majority of these patients, at any given time, meet hospital admissions criteria. Preventing readmissions requires that post-acute care providers have mechanisms in place to stop the “knee-jerk” transfer to the emergency department, rather than attempt to evaluate and treat the patient in the facility. Interact II (http://interact2.net/index.aspx) is a resource that provides tools for post-acute-care facilities to use in monitoring their own internal data around acute-care transfers. It also provides tracking tools, communication strategies, advanced-care-planning tools, and clinical pathways for limiting the number of acute-care transfers. The reality is, once these patients end up in the emergency department, they are likely to be referred to us for consideration of readmission. The best way to stop this is to stop the transfer before it happens.
Demand Better
We, as hospitalists, need to begin to leverage our own “buying power” as it relates to the care of our patients post-discharge. We can start by educating and assisting care providers on a local level to improve compliance with well-known standards of care that prevent unnecessary readmissions. We need to be prepared to wield our collective weight as a specialty to demand from our post-acute care colleagues what has been demanded of us over the last several years: quality and value. Make no mistake—hospitalists have to get outside the box.
Dr. Harrington is an SHM board member and chief medical officer of Locum Leaders in Alpharetta, Ga.
As we enter a new era of health-care and payment reform, we are all keenly aware of the need to limit unnecessary readmissions. We have been given tools and tips on how to most efficiently and effectively transition patients from the hospital setting to the outpatient world in order to limit the chances that they will “bounce back” to us, resulting in penalties to our hospital or health-care system. Tools such as Project BOOST and others help us to educate patients, transfer information effectively, follow up on patients post-discharge, and reconcile medications safely across the continuum of care. But without a competent and committed provider of care to “catch” the patient on the other side, we might just be dropping the ball yet again.
It is imperative as we look to safely transition patients into the next level of care that we, as hospitalists, get outside the box and begin to engage the community of care providers outside our walls, and yes, even outside of our control. We have been down this road before with other quality initiatives, which at first glance appeared to be outside our sphere of influence—such projects as post-operative antibiotic use and hospitalwide DVT prophylaxis. Given the right hospitalist leader, with the right set of leadership tools, these quality-improvement (QI) projects have been widely successful in many environments.
I would suggest that the issue of safe transitions for our patients is no different, and maybe more important, to the health and safety of our patients.
Solving the readmission problem on a local level requires an analytical approach, much like a “root-cause analysis.” We need to begin to examine the sources of our readmitted patients, as well as the routes of our discharged patients, and we need to ask ourselves if we are continuing to feed patients into the vicious circle that results in readmissions. Are there post-acute-care facilities in your area that are responsible for more than their fair share of patients returning to your service? If so, why do we continue to discharge patients to their care? Is it because we are pressured to lower length of stay, and any bed at the next level of care is better than another day in the acute-care hospital? At some point, this reasoning fails, and given the penalties coming soon, it may be better to begin to more discriminately discharge patients to facilities that provide higher-quality care and assist us in our goals to reduce unnecessary readmissions. Leading the charge in this endeavor also necessitates that we begin to engage those providers on the other side, making them aware of the quality data related to their facility and providing education and resources to assist them in improving their performance.
Realities of the Care Continuum
Several options pertaining to hospitalist groups are available. The first, already a large movement in our current marketplace, is to extend the current hospitalist group across the chasm and begin to deliver care in those post-acute facilities. Long-term acute care (LTAC) and skilled nursing facilities (SNF) are prime examples of this movement; the obvious advantage lies in the effective control of quality and efficient transfer of information that a single group can achieve when it extends to these facilities. Obviously, manpower issues and financial support are drawbacks in a model such as this.
More realistically, a group might consider taking a less aggressive approach to this problem. Educating care providers and assisting these facilities with QI projects would require fewer resources and might provide a higher return on investment (ROI) for your group and hospital. Engaging these physicians, nonphysician providers, and facility administrators is key to our ability to impact this problem. Demanding quality care for our discharged patients in terms of timeliness of follow-up, adherence to care paths, and responsiveness to changes in condition should be non-negotiable and factored into our development of referral patterns.
As our population of patients continues to be more acutely ill, and the level of care provided at post-acute care facilities continues to rise, our current reality is that a majority of these patients, at any given time, meet hospital admissions criteria. Preventing readmissions requires that post-acute care providers have mechanisms in place to stop the “knee-jerk” transfer to the emergency department, rather than attempt to evaluate and treat the patient in the facility. Interact II (http://interact2.net/index.aspx) is a resource that provides tools for post-acute-care facilities to use in monitoring their own internal data around acute-care transfers. It also provides tracking tools, communication strategies, advanced-care-planning tools, and clinical pathways for limiting the number of acute-care transfers. The reality is, once these patients end up in the emergency department, they are likely to be referred to us for consideration of readmission. The best way to stop this is to stop the transfer before it happens.
Demand Better
We, as hospitalists, need to begin to leverage our own “buying power” as it relates to the care of our patients post-discharge. We can start by educating and assisting care providers on a local level to improve compliance with well-known standards of care that prevent unnecessary readmissions. We need to be prepared to wield our collective weight as a specialty to demand from our post-acute care colleagues what has been demanded of us over the last several years: quality and value. Make no mistake—hospitalists have to get outside the box.
Dr. Harrington is an SHM board member and chief medical officer of Locum Leaders in Alpharetta, Ga.
Project BOOST Study Documents Modest Impact on 30-Day Hospital Readmissions
Initial research on outcomes following Project BOOST (Better Outcomes for Older Adults through Safe Transitions) implementation shows modest improvement in rehospitalization rates. Moreover, some experts suggest the real problem might lie in using 30-day hospital readmissions, now a target for Medicare reimbursement penalties, as the quality metric for care transitions out of the hospital.
Study data showed a 2% absolute reduction in all-patient, 30-day readmission rates at 11 of the original 30 BOOST sites (to 12.7% from 14.7%), according to an article in the August issue of the Journal of Hospital Medicine.1
“Everybody has talked about readmissions as the quality target, but really it should be about improving transitions of care for the patient going home,” says Ashish Jha, MD, MPH, of the Harvard School of Public Health, Health Policy and Management. “If we’re going to use readmissions as our quality measure, maybe we’re set up to fail. Can we do care transitions better? Yes, we can. Can we do better quality measures? Yes. My take-home message is that we should get clearer on what we are trying to achieve.”
Project BOOST (www.hospitalmedicine.org/boost) has been a major quality initiative for SHM since 2008 and one of several national programs aimed at helping hospitals improve care-transitions processes and patient outcomes. BOOST offers participating sites an online toolkit of strategies and interventions, along with the support of an expert mentor.
“Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the authors conclude. But two accompanying editorials in the journal expressed disappointment with a lack of “robustness” to these results and lack of participation by BOOST sites.2,3 The editorials also acknowledge the challenges of multisite, voluntary research on a topic that, so far, has largely resisted validated, generalizable research outcomes demonstrating what really works in preventing readmissions.
“I think people want a silver bullet on this issue,” says lead author Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. “They want to be able to define an intervention to take care of all of the avoidable fraction of rehospitalizations. But I don’t think that’s possible. The disappointment may come from the fact that this is a more complicated issue than we thought.”
Dr. Hansen says data reporting was voluntary and uncompensated, and the BOOST research team is trying to facilitate better reporting from subsequent cohorts. He says one of BOOST’s unique aspects—tailoring interventions to local circumstances—could be a drawback to outcomes research. “We have to incorporate the diversity of experience into our research methods and our expectations,” he says.
Hospitalist Bradley Flansbaum, DO, MPH, FACP, SFHM, of Lenox Hill Hospital in New York City says BOOST reinforces many of things hospitalists should be doing to provide optimal discharges and transitions
.
—Ashish Jha, MD, MPH, Harvard School of Public Health, Health Policy, and Management, Boston
“Like appropriate teaching and patient education, medication reconciliation, and setting up follow-up appointments,” says Dr. Flansbaum, a member of SHM’s Public Policy Committee and regular contributor to SHM’s Practice Management blog. “But if there was one thing I’d like hospitalists to take home from this research, it’s the cognitive dissonance—the challenge of matching the evidence with what the regulatory bodies expect from us and knowing that the evidence is falling short.
“As much as we might be held accountable for outcomes like readmissions, the reality is that we can’t control them. What we’re learning is that this is really hard to do.”
Amy Boutwell, MD, MPP, a hospitalist in Newton, Mass., and founder of Collaborative Healthcare Strategies, agrees transitions of care are difficult. However, she also thinks hospitals and hospitalists cannot wait for conclusive research that proves what works in preventing readmissions.
“The BOOST results reflect my own experience working with more than a hundred STAAR [State Action on Avoidable Readmissions] hospitals. We haven’t yet been able to sufficiently extract the data about readmissions from the field—and we need to figure out how to do that,” she says. “But when you look at the issue from a patient’s perspective and their desire for a safe transition, why would you not do the things recommended by Project BOOST and similar initiatives?”
Primary-care physicians (PCPs) need to know about major changes in a discharged patient’s plan of care in a timely manner, along with any results from pending lab tests, Dr. Boutwell explains. She emphasizes that patients and their caregivers need to be given clear discharge instructions when they leave the hospital.
“We have an obligation to do what we know to be best practices and standards of care. The BOOST toolkit of recommendations is very comprehensive and gives hospitals a lot of options to improve their internal processes,” Dr. Boutwell says. “It’s hard to argue against any of them, even if it’s hard to draw clear links between them and readmissions rates. These are the self-evident, basic tasks that I would want done for myself or my child or my parent, if we were in the hospital.”
Larry Beresford is a freelance writer in San Francisco.
References
- Hansen L, Greenwald J, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi: 10.1002/jhm.2054. Epub 2013 Jul 22.
- Auerbach A, Fang M, Glasheen J, Brotman D, O’Leary KJ, Horwitz LJ. BOOST: Evidence needing a lift. J Hosp Med. 2013;8(8):468-469. doi: 10.1002/jhm.2065. Epub 2013 Jul 22.
- Jha A. BOOST and readmissions: Thinking beyond the walls of the hospital. J Hosp Med. 2013;8(8):470-471. doi: 10.1002/jhm.2069. Epub 2013 Jul 22.
Initial research on outcomes following Project BOOST (Better Outcomes for Older Adults through Safe Transitions) implementation shows modest improvement in rehospitalization rates. Moreover, some experts suggest the real problem might lie in using 30-day hospital readmissions, now a target for Medicare reimbursement penalties, as the quality metric for care transitions out of the hospital.
Study data showed a 2% absolute reduction in all-patient, 30-day readmission rates at 11 of the original 30 BOOST sites (to 12.7% from 14.7%), according to an article in the August issue of the Journal of Hospital Medicine.1
“Everybody has talked about readmissions as the quality target, but really it should be about improving transitions of care for the patient going home,” says Ashish Jha, MD, MPH, of the Harvard School of Public Health, Health Policy and Management. “If we’re going to use readmissions as our quality measure, maybe we’re set up to fail. Can we do care transitions better? Yes, we can. Can we do better quality measures? Yes. My take-home message is that we should get clearer on what we are trying to achieve.”
Project BOOST (www.hospitalmedicine.org/boost) has been a major quality initiative for SHM since 2008 and one of several national programs aimed at helping hospitals improve care-transitions processes and patient outcomes. BOOST offers participating sites an online toolkit of strategies and interventions, along with the support of an expert mentor.
“Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the authors conclude. But two accompanying editorials in the journal expressed disappointment with a lack of “robustness” to these results and lack of participation by BOOST sites.2,3 The editorials also acknowledge the challenges of multisite, voluntary research on a topic that, so far, has largely resisted validated, generalizable research outcomes demonstrating what really works in preventing readmissions.
“I think people want a silver bullet on this issue,” says lead author Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. “They want to be able to define an intervention to take care of all of the avoidable fraction of rehospitalizations. But I don’t think that’s possible. The disappointment may come from the fact that this is a more complicated issue than we thought.”
Dr. Hansen says data reporting was voluntary and uncompensated, and the BOOST research team is trying to facilitate better reporting from subsequent cohorts. He says one of BOOST’s unique aspects—tailoring interventions to local circumstances—could be a drawback to outcomes research. “We have to incorporate the diversity of experience into our research methods and our expectations,” he says.
Hospitalist Bradley Flansbaum, DO, MPH, FACP, SFHM, of Lenox Hill Hospital in New York City says BOOST reinforces many of things hospitalists should be doing to provide optimal discharges and transitions
.
—Ashish Jha, MD, MPH, Harvard School of Public Health, Health Policy, and Management, Boston
“Like appropriate teaching and patient education, medication reconciliation, and setting up follow-up appointments,” says Dr. Flansbaum, a member of SHM’s Public Policy Committee and regular contributor to SHM’s Practice Management blog. “But if there was one thing I’d like hospitalists to take home from this research, it’s the cognitive dissonance—the challenge of matching the evidence with what the regulatory bodies expect from us and knowing that the evidence is falling short.
“As much as we might be held accountable for outcomes like readmissions, the reality is that we can’t control them. What we’re learning is that this is really hard to do.”
Amy Boutwell, MD, MPP, a hospitalist in Newton, Mass., and founder of Collaborative Healthcare Strategies, agrees transitions of care are difficult. However, she also thinks hospitals and hospitalists cannot wait for conclusive research that proves what works in preventing readmissions.
“The BOOST results reflect my own experience working with more than a hundred STAAR [State Action on Avoidable Readmissions] hospitals. We haven’t yet been able to sufficiently extract the data about readmissions from the field—and we need to figure out how to do that,” she says. “But when you look at the issue from a patient’s perspective and their desire for a safe transition, why would you not do the things recommended by Project BOOST and similar initiatives?”
Primary-care physicians (PCPs) need to know about major changes in a discharged patient’s plan of care in a timely manner, along with any results from pending lab tests, Dr. Boutwell explains. She emphasizes that patients and their caregivers need to be given clear discharge instructions when they leave the hospital.
“We have an obligation to do what we know to be best practices and standards of care. The BOOST toolkit of recommendations is very comprehensive and gives hospitals a lot of options to improve their internal processes,” Dr. Boutwell says. “It’s hard to argue against any of them, even if it’s hard to draw clear links between them and readmissions rates. These are the self-evident, basic tasks that I would want done for myself or my child or my parent, if we were in the hospital.”
Larry Beresford is a freelance writer in San Francisco.
References
- Hansen L, Greenwald J, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi: 10.1002/jhm.2054. Epub 2013 Jul 22.
- Auerbach A, Fang M, Glasheen J, Brotman D, O’Leary KJ, Horwitz LJ. BOOST: Evidence needing a lift. J Hosp Med. 2013;8(8):468-469. doi: 10.1002/jhm.2065. Epub 2013 Jul 22.
- Jha A. BOOST and readmissions: Thinking beyond the walls of the hospital. J Hosp Med. 2013;8(8):470-471. doi: 10.1002/jhm.2069. Epub 2013 Jul 22.
Initial research on outcomes following Project BOOST (Better Outcomes for Older Adults through Safe Transitions) implementation shows modest improvement in rehospitalization rates. Moreover, some experts suggest the real problem might lie in using 30-day hospital readmissions, now a target for Medicare reimbursement penalties, as the quality metric for care transitions out of the hospital.
Study data showed a 2% absolute reduction in all-patient, 30-day readmission rates at 11 of the original 30 BOOST sites (to 12.7% from 14.7%), according to an article in the August issue of the Journal of Hospital Medicine.1
“Everybody has talked about readmissions as the quality target, but really it should be about improving transitions of care for the patient going home,” says Ashish Jha, MD, MPH, of the Harvard School of Public Health, Health Policy and Management. “If we’re going to use readmissions as our quality measure, maybe we’re set up to fail. Can we do care transitions better? Yes, we can. Can we do better quality measures? Yes. My take-home message is that we should get clearer on what we are trying to achieve.”
Project BOOST (www.hospitalmedicine.org/boost) has been a major quality initiative for SHM since 2008 and one of several national programs aimed at helping hospitals improve care-transitions processes and patient outcomes. BOOST offers participating sites an online toolkit of strategies and interventions, along with the support of an expert mentor.
“Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the authors conclude. But two accompanying editorials in the journal expressed disappointment with a lack of “robustness” to these results and lack of participation by BOOST sites.2,3 The editorials also acknowledge the challenges of multisite, voluntary research on a topic that, so far, has largely resisted validated, generalizable research outcomes demonstrating what really works in preventing readmissions.
“I think people want a silver bullet on this issue,” says lead author Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. “They want to be able to define an intervention to take care of all of the avoidable fraction of rehospitalizations. But I don’t think that’s possible. The disappointment may come from the fact that this is a more complicated issue than we thought.”
Dr. Hansen says data reporting was voluntary and uncompensated, and the BOOST research team is trying to facilitate better reporting from subsequent cohorts. He says one of BOOST’s unique aspects—tailoring interventions to local circumstances—could be a drawback to outcomes research. “We have to incorporate the diversity of experience into our research methods and our expectations,” he says.
Hospitalist Bradley Flansbaum, DO, MPH, FACP, SFHM, of Lenox Hill Hospital in New York City says BOOST reinforces many of things hospitalists should be doing to provide optimal discharges and transitions
.
—Ashish Jha, MD, MPH, Harvard School of Public Health, Health Policy, and Management, Boston
“Like appropriate teaching and patient education, medication reconciliation, and setting up follow-up appointments,” says Dr. Flansbaum, a member of SHM’s Public Policy Committee and regular contributor to SHM’s Practice Management blog. “But if there was one thing I’d like hospitalists to take home from this research, it’s the cognitive dissonance—the challenge of matching the evidence with what the regulatory bodies expect from us and knowing that the evidence is falling short.
“As much as we might be held accountable for outcomes like readmissions, the reality is that we can’t control them. What we’re learning is that this is really hard to do.”
Amy Boutwell, MD, MPP, a hospitalist in Newton, Mass., and founder of Collaborative Healthcare Strategies, agrees transitions of care are difficult. However, she also thinks hospitals and hospitalists cannot wait for conclusive research that proves what works in preventing readmissions.
“The BOOST results reflect my own experience working with more than a hundred STAAR [State Action on Avoidable Readmissions] hospitals. We haven’t yet been able to sufficiently extract the data about readmissions from the field—and we need to figure out how to do that,” she says. “But when you look at the issue from a patient’s perspective and their desire for a safe transition, why would you not do the things recommended by Project BOOST and similar initiatives?”
Primary-care physicians (PCPs) need to know about major changes in a discharged patient’s plan of care in a timely manner, along with any results from pending lab tests, Dr. Boutwell explains. She emphasizes that patients and their caregivers need to be given clear discharge instructions when they leave the hospital.
“We have an obligation to do what we know to be best practices and standards of care. The BOOST toolkit of recommendations is very comprehensive and gives hospitals a lot of options to improve their internal processes,” Dr. Boutwell says. “It’s hard to argue against any of them, even if it’s hard to draw clear links between them and readmissions rates. These are the self-evident, basic tasks that I would want done for myself or my child or my parent, if we were in the hospital.”
Larry Beresford is a freelance writer in San Francisco.
References
- Hansen L, Greenwald J, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi: 10.1002/jhm.2054. Epub 2013 Jul 22.
- Auerbach A, Fang M, Glasheen J, Brotman D, O’Leary KJ, Horwitz LJ. BOOST: Evidence needing a lift. J Hosp Med. 2013;8(8):468-469. doi: 10.1002/jhm.2065. Epub 2013 Jul 22.
- Jha A. BOOST and readmissions: Thinking beyond the walls of the hospital. J Hosp Med. 2013;8(8):470-471. doi: 10.1002/jhm.2069. Epub 2013 Jul 22.
Wasteful Practices in Hospital Cardiac Services Identified
A recent article in the American Journal of Medical Quality reviewed 366 cardiac-related medical studies and 21 practice guidelines to identify eight measures of potential waste in hospital cardiac services.4 The wasteful measures included excess use of higher-cost implantable cardioverter-defibrillators and similar cardiac devices, the use of dual-chamber defibrillators rather than single-chamber devices, and excess lengths of stay in the hospital. The eight measures were validated with data from 261 hospitals.
The authors emphasize that their set of measures is not designed to determine clinical appropriateness but to highlight areas of potential overutilization that can be benchmarked with other hospitals.
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
A recent article in the American Journal of Medical Quality reviewed 366 cardiac-related medical studies and 21 practice guidelines to identify eight measures of potential waste in hospital cardiac services.4 The wasteful measures included excess use of higher-cost implantable cardioverter-defibrillators and similar cardiac devices, the use of dual-chamber defibrillators rather than single-chamber devices, and excess lengths of stay in the hospital. The eight measures were validated with data from 261 hospitals.
The authors emphasize that their set of measures is not designed to determine clinical appropriateness but to highlight areas of potential overutilization that can be benchmarked with other hospitals.
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
A recent article in the American Journal of Medical Quality reviewed 366 cardiac-related medical studies and 21 practice guidelines to identify eight measures of potential waste in hospital cardiac services.4 The wasteful measures included excess use of higher-cost implantable cardioverter-defibrillators and similar cardiac devices, the use of dual-chamber defibrillators rather than single-chamber devices, and excess lengths of stay in the hospital. The eight measures were validated with data from 261 hospitals.
The authors emphasize that their set of measures is not designed to determine clinical appropriateness but to highlight areas of potential overutilization that can be benchmarked with other hospitals.
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
Applied Psychology Improves Hand Hygiene in Hospitals
According to a recent New York Times report, hand-hygiene compliance in hospitals can be as low as 30%, with serious implications regarding hospital-acquired infections.1 While many initiatives have employed secret observers, vibrating badges, or hand-washing coaches, a Research, Innovations, and Clinical Vignettes (RIV) poster at HM13 outlined how a multidisciplinary committee at University of Colorado Hospital in Aurora applied principles of psychology to the challenge of compliance.2
The initiative focused on behavioral changes: surreptitiously auditing staff behaviors, real-time feedback, and immediate public corrections for observed nonadherence on the test unit from an infection-control champion. The study randomly assigned daily auditing responsibilities across all members of the nursing staff, including aides. Taking a page from soccer referees, auditors handed out red tickets to hygiene violators—and individually wrapped Life Savers to reinforce adherence.
When unprofessional behavior is the response to a verbal correction, leadership has to be prepared to act, explains hospitalist and lead author Ethan Cumbler, MD, FACP.
“We need to stop thinking about hospital staff and physicians as rational actors when it comes to hand hygiene, but as social animals who will respond to positive and negative reinforcements and group culture,” he says.
Noncompliant hand hygiene is largely unconscious behavior that needs to be brought to conscious attention but is amenable to change, Dr. Cumbler says, adding that “unit leadership steps in for repeated nonadherence or an unprofessional response to correction. We have never needed to intervene more than once with the same person.”
Hand-hygiene adherence reached 97% on the pilot unit in the second quarter of 2012 and has remained at that level, Dr. Cumbler says. Additionally, iatrogenic infections dropped to zero from 4.8 per 1,000 urinary catheter days, with bloodstream infections falling at a similar rate.
Similar results with hand-hygiene compliance have been reported at St. Mary’s Health Center in St. Louis, which has been testing a system that reminds nurses to wash their hands at various checkpoints in the hospital, tracking their compliance with a badge that turns green when registering the presence of hand sanitizer, thereby informing patients that the nurse’s hands are clean.
The system, developed by Biovigil Hygiene Technologies of Ann Arbor, Mich., started on two pilot units last year, where compliance has grown to 97% and 99%, respectively. System set-up can cost about $2,000 per patient room, plus monthly subscriptions per employee, but more hospitals in the system could sign on next year, reports the St. Louis Post-Dispatch.3
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
According to a recent New York Times report, hand-hygiene compliance in hospitals can be as low as 30%, with serious implications regarding hospital-acquired infections.1 While many initiatives have employed secret observers, vibrating badges, or hand-washing coaches, a Research, Innovations, and Clinical Vignettes (RIV) poster at HM13 outlined how a multidisciplinary committee at University of Colorado Hospital in Aurora applied principles of psychology to the challenge of compliance.2
The initiative focused on behavioral changes: surreptitiously auditing staff behaviors, real-time feedback, and immediate public corrections for observed nonadherence on the test unit from an infection-control champion. The study randomly assigned daily auditing responsibilities across all members of the nursing staff, including aides. Taking a page from soccer referees, auditors handed out red tickets to hygiene violators—and individually wrapped Life Savers to reinforce adherence.
When unprofessional behavior is the response to a verbal correction, leadership has to be prepared to act, explains hospitalist and lead author Ethan Cumbler, MD, FACP.
“We need to stop thinking about hospital staff and physicians as rational actors when it comes to hand hygiene, but as social animals who will respond to positive and negative reinforcements and group culture,” he says.
Noncompliant hand hygiene is largely unconscious behavior that needs to be brought to conscious attention but is amenable to change, Dr. Cumbler says, adding that “unit leadership steps in for repeated nonadherence or an unprofessional response to correction. We have never needed to intervene more than once with the same person.”
Hand-hygiene adherence reached 97% on the pilot unit in the second quarter of 2012 and has remained at that level, Dr. Cumbler says. Additionally, iatrogenic infections dropped to zero from 4.8 per 1,000 urinary catheter days, with bloodstream infections falling at a similar rate.
Similar results with hand-hygiene compliance have been reported at St. Mary’s Health Center in St. Louis, which has been testing a system that reminds nurses to wash their hands at various checkpoints in the hospital, tracking their compliance with a badge that turns green when registering the presence of hand sanitizer, thereby informing patients that the nurse’s hands are clean.
The system, developed by Biovigil Hygiene Technologies of Ann Arbor, Mich., started on two pilot units last year, where compliance has grown to 97% and 99%, respectively. System set-up can cost about $2,000 per patient room, plus monthly subscriptions per employee, but more hospitals in the system could sign on next year, reports the St. Louis Post-Dispatch.3
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
According to a recent New York Times report, hand-hygiene compliance in hospitals can be as low as 30%, with serious implications regarding hospital-acquired infections.1 While many initiatives have employed secret observers, vibrating badges, or hand-washing coaches, a Research, Innovations, and Clinical Vignettes (RIV) poster at HM13 outlined how a multidisciplinary committee at University of Colorado Hospital in Aurora applied principles of psychology to the challenge of compliance.2
The initiative focused on behavioral changes: surreptitiously auditing staff behaviors, real-time feedback, and immediate public corrections for observed nonadherence on the test unit from an infection-control champion. The study randomly assigned daily auditing responsibilities across all members of the nursing staff, including aides. Taking a page from soccer referees, auditors handed out red tickets to hygiene violators—and individually wrapped Life Savers to reinforce adherence.
When unprofessional behavior is the response to a verbal correction, leadership has to be prepared to act, explains hospitalist and lead author Ethan Cumbler, MD, FACP.
“We need to stop thinking about hospital staff and physicians as rational actors when it comes to hand hygiene, but as social animals who will respond to positive and negative reinforcements and group culture,” he says.
Noncompliant hand hygiene is largely unconscious behavior that needs to be brought to conscious attention but is amenable to change, Dr. Cumbler says, adding that “unit leadership steps in for repeated nonadherence or an unprofessional response to correction. We have never needed to intervene more than once with the same person.”
Hand-hygiene adherence reached 97% on the pilot unit in the second quarter of 2012 and has remained at that level, Dr. Cumbler says. Additionally, iatrogenic infections dropped to zero from 4.8 per 1,000 urinary catheter days, with bloodstream infections falling at a similar rate.
Similar results with hand-hygiene compliance have been reported at St. Mary’s Health Center in St. Louis, which has been testing a system that reminds nurses to wash their hands at various checkpoints in the hospital, tracking their compliance with a badge that turns green when registering the presence of hand sanitizer, thereby informing patients that the nurse’s hands are clean.
The system, developed by Biovigil Hygiene Technologies of Ann Arbor, Mich., started on two pilot units last year, where compliance has grown to 97% and 99%, respectively. System set-up can cost about $2,000 per patient room, plus monthly subscriptions per employee, but more hospitals in the system could sign on next year, reports the St. Louis Post-Dispatch.3
Larry Beresford is a freelance writer in San Francisco.
References
- Hartocollis A. With money at risk, hospitals push staff to wash hands. The New York Times website. Available at: http://www.nytimes.com/2013/05/29/nyregion/hospitals-struggle-to-get-workers-to-wash-their-hands.html?pagewanted=all&_r=0. Accessed May 28, 2013.
- Cumbler E, Castillo L, Satorie L, et al. Culture change in infection control: applying psychological principles to improve hand hygiene. J Nurs Care Qual. 2013 May 10 [Epub ahead of print].
- Bernhard B. High tech hand washing comes to St. Louis hospital. St. Louis Post-Dispatch website. Available at: http://www.stltoday.com/lifestyles/health-med-fit/health/high-tech-hand-washing-comes-to-st-louis-hospital/article_9379065d-85ff-5643-bae2-899254cb22fa.html. Accessed June 27, 2013.
- Lowe TJ, Partovian C, Kroch E, Martin J, Bankowitz R. Measuring cardiac waste: the Premier cardiac waste measures. Am J Med Qual. 2013 May 29 [Epub ahead of print].
- Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010. Healthcare Cost and Utilization Project website. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed July 15, 2013.
- Jackson Healthcare. Filling the void: 2013 physician outlook & practice trends. Jackson Healthcare website. Available at: http://www.jacksonhealthcare.com/media/193525/jc-2013physiciantrends-void_ebk0513.pdf. Accessed July 15, 2013.
Patient Satisfaction Surveys Not Accurate Measure of Hospitalists’ Performance
Feeling frustrated with your group’s patient-satisfaction performance? Wondering why your chief (fill in the blank) officer glazes over when you try to explain why your hospitalist group’s Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) scores for doctor communication are in a percentile rivaling the numeric age of your children?
It is likely that the C-suite administrator overseeing your hospitalist group has a portion of their pay based on HCAHPS or other patient-satisfaction (also called patient experience) scores. And for good reason: The Centers for Medicare & Medicaid Services (CMS) Hospital Value-Based Purchasing (HVBP) program that started Oct. 1, 2012, has placed your hospital’s Medicare reimbursement at risk based on its HCAHPS scores.
HVBP and Patient Satisfaction
Patient satisfaction will remain an important part of HVBP in the coming years. Table 1 (below) shows the domains that will be included in fiscal years 2014 (which starts Oct. 1, 2013), 2015, and 2016. Table 2 (below) depicts the percent weighting the patient-satisfaction domain will receive through 2016. You may recall that HVBP is a program in which all hospitals place 1% to 2% (2013 through 2017, starting at 1% and increasing each year by 0.25% so that by 2017%, it is 2%) of their CMS inpatient payments in a withhold pool and, based on performance, can make back some, all, or an amount in excess of the amount placed in the withhold pool.
Source: Federal Register Vol. 78, No. 91; May 10, 2013; Proposed Rules, pp. 27609-27622.
Source: Federal Register Vol.78, No.91; May 10, 2013; Proposed Rules, pp. 27609-27622.
End In Itself
A colleague of mine recently asked, “Is an increase in patient satisfaction associated with higher quality of care and better patient safety?” The point here: It doesn’t matter. Patient satisfaction is an end in itself, and we should strive to maximize it, or at least put ourselves in the place of the patient and design care accordingly.
For Hospitalists: A Starting Point
There is a conundrum for hospitalists vis-à-vis patient satisfaction. Follow this chain of logic: The hospitals at which we work are incented to perform well on the HCAHPS domains. Hospitals pay a lot for hospitalists. Hospitalists can impact many of the HCAHPS domains. So shouldn’t hospitalists be judged according to HCAHPS scores?
Yes and no.
HCAHPS as a survey is intended to measure a patient’s overall experience of receiving care in the hospital. For example, from the “Doctor Communication” domain, we have questions like “how often did doctors treat you with courtesy and respect?” And “how often did doctors explain things in a way you could understand?”
These questions, like all in HCAHPS, are not designed to get at individual doctor performance, or even performance of a group of doctors, such as hospitalists. Instead, they are designed to measure a patient’s overall experience with the hospitalization, and “Doctor Communication” questions are designed to assess satisfaction with “doctors” collectively.
The Need for Hospitalist-Specific Satisfaction Surveys
So while HCAHPS is not designed to measure hospitalist performance with regard to patient satisfaction, it is a reasonable interim step for hospitals to judge hospitalists according to HCAHPS. However, this should be a bridge to a strategy that adopts hospitalist-specific patient-satisfaction questionnaires in the future and not an end in itself.
Why? Perhaps the biggest reason is that HCAHPS scores are neither specific nor timely enough to form the basis of improvement efforts for hospitalists. If a hospitalist receives a low score on the “Doctor Communication” domain, the scores are likely to be three to nine months old. How can we legitimately assign (and then modify) behaviors based on those scores?
Further, because the survey is not built to measure patient satisfaction specifically with hospitalists, the results are unlikely to engender meaningful and sustained behavior change. Hospitalists I talk to are generally bewildered and confused by HCAHPS scores attributed to them or their groups. Even if they understand the scores, I almost never see true quality improvement (plan-do-study-act) based on specific HCAHPS results. Instead, I see hospitalists trying to adhere to “best practices,” with no adjustments made along the way based on performance.
Nearly all the prominent patient satisfaction vendors have developed a survey instrument specifically designed for hospitalists. Each has an approach to appropriately attribute performance to the hospitalist in question, and each has a battery of questions that is designed to capture patient satisfaction with the hospitalist. Although use of these surveys involves an added financial commitment, I submit that because hospitalists have an unparalleled proximity to hospitalized patients, such an investment is worthy of consideration and has an accompanying business case, thanks to HVBP. The results of these surveys may form the basis of legitimate, targeted feedback to hospitalists, who may then adjust their approach to patient interactions. Such performance improvement should result in improved HCAHPS scores.
In sum, hospitalists should pay close attention to patient satisfaction and embrace HCAHPS. However, we should be looking beyond HCAHPS to survey instruments that fairly and accurately measure our performance. Such surveys will be more widely accepted by the hospitalists they are measuring, and will allow hospitalists to perform meaningful quality improvement based on the results. Although hospitalist-specific surveys will require an investment, the increased patient satisfaction that results should be the basis of a favorable return on that investment.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. Email him at [email protected].
Feeling frustrated with your group’s patient-satisfaction performance? Wondering why your chief (fill in the blank) officer glazes over when you try to explain why your hospitalist group’s Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) scores for doctor communication are in a percentile rivaling the numeric age of your children?
It is likely that the C-suite administrator overseeing your hospitalist group has a portion of their pay based on HCAHPS or other patient-satisfaction (also called patient experience) scores. And for good reason: The Centers for Medicare & Medicaid Services (CMS) Hospital Value-Based Purchasing (HVBP) program that started Oct. 1, 2012, has placed your hospital’s Medicare reimbursement at risk based on its HCAHPS scores.
HVBP and Patient Satisfaction
Patient satisfaction will remain an important part of HVBP in the coming years. Table 1 (below) shows the domains that will be included in fiscal years 2014 (which starts Oct. 1, 2013), 2015, and 2016. Table 2 (below) depicts the percent weighting the patient-satisfaction domain will receive through 2016. You may recall that HVBP is a program in which all hospitals place 1% to 2% (2013 through 2017, starting at 1% and increasing each year by 0.25% so that by 2017%, it is 2%) of their CMS inpatient payments in a withhold pool and, based on performance, can make back some, all, or an amount in excess of the amount placed in the withhold pool.
Source: Federal Register Vol. 78, No. 91; May 10, 2013; Proposed Rules, pp. 27609-27622.
Source: Federal Register Vol.78, No.91; May 10, 2013; Proposed Rules, pp. 27609-27622.
End In Itself
A colleague of mine recently asked, “Is an increase in patient satisfaction associated with higher quality of care and better patient safety?” The point here: It doesn’t matter. Patient satisfaction is an end in itself, and we should strive to maximize it, or at least put ourselves in the place of the patient and design care accordingly.
For Hospitalists: A Starting Point
There is a conundrum for hospitalists vis-à-vis patient satisfaction. Follow this chain of logic: The hospitals at which we work are incented to perform well on the HCAHPS domains. Hospitals pay a lot for hospitalists. Hospitalists can impact many of the HCAHPS domains. So shouldn’t hospitalists be judged according to HCAHPS scores?
Yes and no.
HCAHPS as a survey is intended to measure a patient’s overall experience of receiving care in the hospital. For example, from the “Doctor Communication” domain, we have questions like “how often did doctors treat you with courtesy and respect?” And “how often did doctors explain things in a way you could understand?”
These questions, like all in HCAHPS, are not designed to get at individual doctor performance, or even performance of a group of doctors, such as hospitalists. Instead, they are designed to measure a patient’s overall experience with the hospitalization, and “Doctor Communication” questions are designed to assess satisfaction with “doctors” collectively.
The Need for Hospitalist-Specific Satisfaction Surveys
So while HCAHPS is not designed to measure hospitalist performance with regard to patient satisfaction, it is a reasonable interim step for hospitals to judge hospitalists according to HCAHPS. However, this should be a bridge to a strategy that adopts hospitalist-specific patient-satisfaction questionnaires in the future and not an end in itself.
Why? Perhaps the biggest reason is that HCAHPS scores are neither specific nor timely enough to form the basis of improvement efforts for hospitalists. If a hospitalist receives a low score on the “Doctor Communication” domain, the scores are likely to be three to nine months old. How can we legitimately assign (and then modify) behaviors based on those scores?
Further, because the survey is not built to measure patient satisfaction specifically with hospitalists, the results are unlikely to engender meaningful and sustained behavior change. Hospitalists I talk to are generally bewildered and confused by HCAHPS scores attributed to them or their groups. Even if they understand the scores, I almost never see true quality improvement (plan-do-study-act) based on specific HCAHPS results. Instead, I see hospitalists trying to adhere to “best practices,” with no adjustments made along the way based on performance.
Nearly all the prominent patient satisfaction vendors have developed a survey instrument specifically designed for hospitalists. Each has an approach to appropriately attribute performance to the hospitalist in question, and each has a battery of questions that is designed to capture patient satisfaction with the hospitalist. Although use of these surveys involves an added financial commitment, I submit that because hospitalists have an unparalleled proximity to hospitalized patients, such an investment is worthy of consideration and has an accompanying business case, thanks to HVBP. The results of these surveys may form the basis of legitimate, targeted feedback to hospitalists, who may then adjust their approach to patient interactions. Such performance improvement should result in improved HCAHPS scores.
In sum, hospitalists should pay close attention to patient satisfaction and embrace HCAHPS. However, we should be looking beyond HCAHPS to survey instruments that fairly and accurately measure our performance. Such surveys will be more widely accepted by the hospitalists they are measuring, and will allow hospitalists to perform meaningful quality improvement based on the results. Although hospitalist-specific surveys will require an investment, the increased patient satisfaction that results should be the basis of a favorable return on that investment.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. Email him at [email protected].
Feeling frustrated with your group’s patient-satisfaction performance? Wondering why your chief (fill in the blank) officer glazes over when you try to explain why your hospitalist group’s Hospital Consumer Assessment of Healthcare Providers and System (HCAHPS) scores for doctor communication are in a percentile rivaling the numeric age of your children?
It is likely that the C-suite administrator overseeing your hospitalist group has a portion of their pay based on HCAHPS or other patient-satisfaction (also called patient experience) scores. And for good reason: The Centers for Medicare & Medicaid Services (CMS) Hospital Value-Based Purchasing (HVBP) program that started Oct. 1, 2012, has placed your hospital’s Medicare reimbursement at risk based on its HCAHPS scores.
HVBP and Patient Satisfaction
Patient satisfaction will remain an important part of HVBP in the coming years. Table 1 (below) shows the domains that will be included in fiscal years 2014 (which starts Oct. 1, 2013), 2015, and 2016. Table 2 (below) depicts the percent weighting the patient-satisfaction domain will receive through 2016. You may recall that HVBP is a program in which all hospitals place 1% to 2% (2013 through 2017, starting at 1% and increasing each year by 0.25% so that by 2017%, it is 2%) of their CMS inpatient payments in a withhold pool and, based on performance, can make back some, all, or an amount in excess of the amount placed in the withhold pool.
Source: Federal Register Vol. 78, No. 91; May 10, 2013; Proposed Rules, pp. 27609-27622.
Source: Federal Register Vol.78, No.91; May 10, 2013; Proposed Rules, pp. 27609-27622.
End In Itself
A colleague of mine recently asked, “Is an increase in patient satisfaction associated with higher quality of care and better patient safety?” The point here: It doesn’t matter. Patient satisfaction is an end in itself, and we should strive to maximize it, or at least put ourselves in the place of the patient and design care accordingly.
For Hospitalists: A Starting Point
There is a conundrum for hospitalists vis-à-vis patient satisfaction. Follow this chain of logic: The hospitals at which we work are incented to perform well on the HCAHPS domains. Hospitals pay a lot for hospitalists. Hospitalists can impact many of the HCAHPS domains. So shouldn’t hospitalists be judged according to HCAHPS scores?
Yes and no.
HCAHPS as a survey is intended to measure a patient’s overall experience of receiving care in the hospital. For example, from the “Doctor Communication” domain, we have questions like “how often did doctors treat you with courtesy and respect?” And “how often did doctors explain things in a way you could understand?”
These questions, like all in HCAHPS, are not designed to get at individual doctor performance, or even performance of a group of doctors, such as hospitalists. Instead, they are designed to measure a patient’s overall experience with the hospitalization, and “Doctor Communication” questions are designed to assess satisfaction with “doctors” collectively.
The Need for Hospitalist-Specific Satisfaction Surveys
So while HCAHPS is not designed to measure hospitalist performance with regard to patient satisfaction, it is a reasonable interim step for hospitals to judge hospitalists according to HCAHPS. However, this should be a bridge to a strategy that adopts hospitalist-specific patient-satisfaction questionnaires in the future and not an end in itself.
Why? Perhaps the biggest reason is that HCAHPS scores are neither specific nor timely enough to form the basis of improvement efforts for hospitalists. If a hospitalist receives a low score on the “Doctor Communication” domain, the scores are likely to be three to nine months old. How can we legitimately assign (and then modify) behaviors based on those scores?
Further, because the survey is not built to measure patient satisfaction specifically with hospitalists, the results are unlikely to engender meaningful and sustained behavior change. Hospitalists I talk to are generally bewildered and confused by HCAHPS scores attributed to them or their groups. Even if they understand the scores, I almost never see true quality improvement (plan-do-study-act) based on specific HCAHPS results. Instead, I see hospitalists trying to adhere to “best practices,” with no adjustments made along the way based on performance.
Nearly all the prominent patient satisfaction vendors have developed a survey instrument specifically designed for hospitalists. Each has an approach to appropriately attribute performance to the hospitalist in question, and each has a battery of questions that is designed to capture patient satisfaction with the hospitalist. Although use of these surveys involves an added financial commitment, I submit that because hospitalists have an unparalleled proximity to hospitalized patients, such an investment is worthy of consideration and has an accompanying business case, thanks to HVBP. The results of these surveys may form the basis of legitimate, targeted feedback to hospitalists, who may then adjust their approach to patient interactions. Such performance improvement should result in improved HCAHPS scores.
In sum, hospitalists should pay close attention to patient satisfaction and embrace HCAHPS. However, we should be looking beyond HCAHPS to survey instruments that fairly and accurately measure our performance. Such surveys will be more widely accepted by the hospitalists they are measuring, and will allow hospitalists to perform meaningful quality improvement based on the results. Although hospitalist-specific surveys will require an investment, the increased patient satisfaction that results should be the basis of a favorable return on that investment.
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is co-founder and past president of SHM. Email him at [email protected].
Hospitalists’ Capitol Hill Advocacy Effort Produces Results
On May 12, 113 hospitalists descended on Capitol Hill for “Hospitalists on the Hill 2013,” the public-advocacy highlight of SHM’s annual meeting. Hospitalists from all parts of the country engaged with congressional representatives in a daylong series of meet-and-greets that may seem to some people useless in the face of political obstinacy in Washington. But the trip worked.
Josh Boswell, SHM’s senior manager of government relations, reports many Hill Day objectives were achieved:
- The number of legislators co-sponsoring a bill regarding the “three-day observation rule” more than tripled in the House of Representatives and doubled in the Senate. SHM officials note that the added support has come from both political parties.
- A Congressional Budget Office (CBO) review of the bill has been formally requested by those legislators.
- A congressman from Washington state asked for—and received—a letter of support for a proposed measure, the Improved Health Care at a Lower Cost Act of 2013 (H.R. 1487).
- Multiple reports of continued dialogue between congressional staffers and SHM members nationwide. When planning the advocacy day, SHM officials noted that one of the most valuable results is creating relationships at the local level.
Observation Legislation
One of the three talking points hospitalists took into their legislative meetings was solving the dilemmas surrounding observation status. Currently, time spent on observation status does not count toward the required three consecutive overnights an inpatient needs to qualify for Medicare benefits at a skilled nursing facility (SNF).
Hospitalists have been pushing to change that rule, in large part by supporting the Improving Access to Medicare Coverage Act of 2013 (H.R. 1179, S. 569), sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio).1 In addition to the status reclassification, the proposal would establish a 90-day appeal period for those who have been denied the benefit.
The issue is important to hospitalists because of the penalties hospitals face for readmissions—and also in part because hospitalists increasingly are providing care at SNFs and other post-acute-care facilities. SHM says that after the Hill visits—and the ensuing follow-up communications—the number of co-sponsors in the House jumped to 70 from 22. The Senate version doubled its list of co-sponsors.
And, perhaps more important, a CBO analysis has been requested for the observation bills. That review, known as a CBO score, weighs the financial impacts of proposed laws and is considered a necessary precursor to successfully passing any legislation.
All in all, SHM was pleased with the progress on the observation-status bill and will continue to push for its passage, whether it is in this congressional session or the next.
“Rep. Courtney’s bill is now getting significant traction,” Boswell says. “Hospitalists should be proud to know this is in no small part due to their advocacy efforts.”
Political Networking
Hospitalist David Ramenofsky, MD, who works at Northwest Hospital and Medical Center in Seattle, wasn’t sure how much traction he was going to be able to generate at his first Hill Day. SHM had arranged meetings with the offices of three local politicians: Rep. Jim McDermott (D-Wash.), Sen. Patty Murray (D-Wash.), and Sen. Maria Cantwell (D-Wash.).
Dr. Ramenofsky sat with two of McDermott’s staffers, one of whom sounded knowledgeable and enthused about health-care-policy issues. Although the congressman couldn’t sit in on the meeting, he knew Dr. Ramenofsky’s name and took the time to say hello.
“It was really interesting to me that these staffers wanted to hear what I had to say and learn about my experience,” Dr. Ramenofsky adds. “My views may affect how they work with their bosses to make policy changes. It was surprising to me how much my opinions mattered to them.”
After the meeting and another briefing SHM arranged with another local hospitalist, McDermott reached out to SHM. He asked for support for his proposed bill to expand protections from anti-kickback laws and regulations, to provide safe harbor protection for gainsharing, and other incentive-payment systems.
SHM responded in July with a letter of support that thanked the congressman for his efforts.2
“We look forward to working with you,” the letter ended.
Dr. Ramenofsky says he’s proud his efforts led to a working relationship between his professional society and his local legislator. He says he’s looking forward to participating in future Hill Day activities and acting as a local liaison for SHM.
He laments that he has not received much post-meeting feedback from his discussions with the senators’ offices, but says he understands how busy politicians are. And a 1-for-3 showing is pretty good, given his status as a political novice.
“Given overall public perception of Congress, I’m amazed that my visits caused one of three offices to engage in further policy discussions with SHM,” he says. “I’m encouraged to remain engaged in political activities through SHM.”
Richard Quinn is a freelance writer in New Jersey.
References
- Society of Hospital Medicine. Letter to Congress members. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_ Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=33169. Accessed July 15, 2013.
- Society of Hospital Medicine. Letter to Congressman Jim McDermott. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34169. Accessed July 15, 2013.
On May 12, 113 hospitalists descended on Capitol Hill for “Hospitalists on the Hill 2013,” the public-advocacy highlight of SHM’s annual meeting. Hospitalists from all parts of the country engaged with congressional representatives in a daylong series of meet-and-greets that may seem to some people useless in the face of political obstinacy in Washington. But the trip worked.
Josh Boswell, SHM’s senior manager of government relations, reports many Hill Day objectives were achieved:
- The number of legislators co-sponsoring a bill regarding the “three-day observation rule” more than tripled in the House of Representatives and doubled in the Senate. SHM officials note that the added support has come from both political parties.
- A Congressional Budget Office (CBO) review of the bill has been formally requested by those legislators.
- A congressman from Washington state asked for—and received—a letter of support for a proposed measure, the Improved Health Care at a Lower Cost Act of 2013 (H.R. 1487).
- Multiple reports of continued dialogue between congressional staffers and SHM members nationwide. When planning the advocacy day, SHM officials noted that one of the most valuable results is creating relationships at the local level.
Observation Legislation
One of the three talking points hospitalists took into their legislative meetings was solving the dilemmas surrounding observation status. Currently, time spent on observation status does not count toward the required three consecutive overnights an inpatient needs to qualify for Medicare benefits at a skilled nursing facility (SNF).
Hospitalists have been pushing to change that rule, in large part by supporting the Improving Access to Medicare Coverage Act of 2013 (H.R. 1179, S. 569), sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio).1 In addition to the status reclassification, the proposal would establish a 90-day appeal period for those who have been denied the benefit.
The issue is important to hospitalists because of the penalties hospitals face for readmissions—and also in part because hospitalists increasingly are providing care at SNFs and other post-acute-care facilities. SHM says that after the Hill visits—and the ensuing follow-up communications—the number of co-sponsors in the House jumped to 70 from 22. The Senate version doubled its list of co-sponsors.
And, perhaps more important, a CBO analysis has been requested for the observation bills. That review, known as a CBO score, weighs the financial impacts of proposed laws and is considered a necessary precursor to successfully passing any legislation.
All in all, SHM was pleased with the progress on the observation-status bill and will continue to push for its passage, whether it is in this congressional session or the next.
“Rep. Courtney’s bill is now getting significant traction,” Boswell says. “Hospitalists should be proud to know this is in no small part due to their advocacy efforts.”
Political Networking
Hospitalist David Ramenofsky, MD, who works at Northwest Hospital and Medical Center in Seattle, wasn’t sure how much traction he was going to be able to generate at his first Hill Day. SHM had arranged meetings with the offices of three local politicians: Rep. Jim McDermott (D-Wash.), Sen. Patty Murray (D-Wash.), and Sen. Maria Cantwell (D-Wash.).
Dr. Ramenofsky sat with two of McDermott’s staffers, one of whom sounded knowledgeable and enthused about health-care-policy issues. Although the congressman couldn’t sit in on the meeting, he knew Dr. Ramenofsky’s name and took the time to say hello.
“It was really interesting to me that these staffers wanted to hear what I had to say and learn about my experience,” Dr. Ramenofsky adds. “My views may affect how they work with their bosses to make policy changes. It was surprising to me how much my opinions mattered to them.”
After the meeting and another briefing SHM arranged with another local hospitalist, McDermott reached out to SHM. He asked for support for his proposed bill to expand protections from anti-kickback laws and regulations, to provide safe harbor protection for gainsharing, and other incentive-payment systems.
SHM responded in July with a letter of support that thanked the congressman for his efforts.2
“We look forward to working with you,” the letter ended.
Dr. Ramenofsky says he’s proud his efforts led to a working relationship between his professional society and his local legislator. He says he’s looking forward to participating in future Hill Day activities and acting as a local liaison for SHM.
He laments that he has not received much post-meeting feedback from his discussions with the senators’ offices, but says he understands how busy politicians are. And a 1-for-3 showing is pretty good, given his status as a political novice.
“Given overall public perception of Congress, I’m amazed that my visits caused one of three offices to engage in further policy discussions with SHM,” he says. “I’m encouraged to remain engaged in political activities through SHM.”
Richard Quinn is a freelance writer in New Jersey.
References
- Society of Hospital Medicine. Letter to Congress members. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_ Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=33169. Accessed July 15, 2013.
- Society of Hospital Medicine. Letter to Congressman Jim McDermott. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34169. Accessed July 15, 2013.
On May 12, 113 hospitalists descended on Capitol Hill for “Hospitalists on the Hill 2013,” the public-advocacy highlight of SHM’s annual meeting. Hospitalists from all parts of the country engaged with congressional representatives in a daylong series of meet-and-greets that may seem to some people useless in the face of political obstinacy in Washington. But the trip worked.
Josh Boswell, SHM’s senior manager of government relations, reports many Hill Day objectives were achieved:
- The number of legislators co-sponsoring a bill regarding the “three-day observation rule” more than tripled in the House of Representatives and doubled in the Senate. SHM officials note that the added support has come from both political parties.
- A Congressional Budget Office (CBO) review of the bill has been formally requested by those legislators.
- A congressman from Washington state asked for—and received—a letter of support for a proposed measure, the Improved Health Care at a Lower Cost Act of 2013 (H.R. 1487).
- Multiple reports of continued dialogue between congressional staffers and SHM members nationwide. When planning the advocacy day, SHM officials noted that one of the most valuable results is creating relationships at the local level.
Observation Legislation
One of the three talking points hospitalists took into their legislative meetings was solving the dilemmas surrounding observation status. Currently, time spent on observation status does not count toward the required three consecutive overnights an inpatient needs to qualify for Medicare benefits at a skilled nursing facility (SNF).
Hospitalists have been pushing to change that rule, in large part by supporting the Improving Access to Medicare Coverage Act of 2013 (H.R. 1179, S. 569), sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio).1 In addition to the status reclassification, the proposal would establish a 90-day appeal period for those who have been denied the benefit.
The issue is important to hospitalists because of the penalties hospitals face for readmissions—and also in part because hospitalists increasingly are providing care at SNFs and other post-acute-care facilities. SHM says that after the Hill visits—and the ensuing follow-up communications—the number of co-sponsors in the House jumped to 70 from 22. The Senate version doubled its list of co-sponsors.
And, perhaps more important, a CBO analysis has been requested for the observation bills. That review, known as a CBO score, weighs the financial impacts of proposed laws and is considered a necessary precursor to successfully passing any legislation.
All in all, SHM was pleased with the progress on the observation-status bill and will continue to push for its passage, whether it is in this congressional session or the next.
“Rep. Courtney’s bill is now getting significant traction,” Boswell says. “Hospitalists should be proud to know this is in no small part due to their advocacy efforts.”
Political Networking
Hospitalist David Ramenofsky, MD, who works at Northwest Hospital and Medical Center in Seattle, wasn’t sure how much traction he was going to be able to generate at his first Hill Day. SHM had arranged meetings with the offices of three local politicians: Rep. Jim McDermott (D-Wash.), Sen. Patty Murray (D-Wash.), and Sen. Maria Cantwell (D-Wash.).
Dr. Ramenofsky sat with two of McDermott’s staffers, one of whom sounded knowledgeable and enthused about health-care-policy issues. Although the congressman couldn’t sit in on the meeting, he knew Dr. Ramenofsky’s name and took the time to say hello.
“It was really interesting to me that these staffers wanted to hear what I had to say and learn about my experience,” Dr. Ramenofsky adds. “My views may affect how they work with their bosses to make policy changes. It was surprising to me how much my opinions mattered to them.”
After the meeting and another briefing SHM arranged with another local hospitalist, McDermott reached out to SHM. He asked for support for his proposed bill to expand protections from anti-kickback laws and regulations, to provide safe harbor protection for gainsharing, and other incentive-payment systems.
SHM responded in July with a letter of support that thanked the congressman for his efforts.2
“We look forward to working with you,” the letter ended.
Dr. Ramenofsky says he’s proud his efforts led to a working relationship between his professional society and his local legislator. He says he’s looking forward to participating in future Hill Day activities and acting as a local liaison for SHM.
He laments that he has not received much post-meeting feedback from his discussions with the senators’ offices, but says he understands how busy politicians are. And a 1-for-3 showing is pretty good, given his status as a political novice.
“Given overall public perception of Congress, I’m amazed that my visits caused one of three offices to engage in further policy discussions with SHM,” he says. “I’m encouraged to remain engaged in political activities through SHM.”
Richard Quinn is a freelance writer in New Jersey.
References
- Society of Hospital Medicine. Letter to Congress members. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_ Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=33169. Accessed July 15, 2013.
- Society of Hospital Medicine. Letter to Congressman Jim McDermott. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34169. Accessed July 15, 2013.
Goals, Patient-Centered Decisions Drive Hospitalist Julianna Lindsey

Growing up on a farm in rural Kentucky could have led to a career in the family business for Julianna Lindsey, MD, MBA, FHM. Except she knew at an early age that she wanted to be a doctor.
“My family physician was very influential on my decision to become a physician,” she says. “[He] mentored and encouraged me from a young age; it was very powerful for me.”
Dr. Lindsey earned bachelor’s degrees in biomedical science from the University of South Alabama and biochemistry from Western Kentucky University. She graduated from the University of Kentucky College of Medicine and completed her internal-medicine residency at the University of Kentucky. In 2011, she earned her master’s in business administration from the University of Tennessee.
Immediately following residency, she worked for the Veterans Affairs Medical Center in Lexington, Ky., as an ED physician. In 2002, she latched on to a career in HM when she and her husband, a gastroenterologist, relocated to Knoxville, Tenn. She recently launched a startup company, Synergy Surgicalists, with two orthopedic surgeons, and also provides process-improvement and leadership-development consulting.
She says she was told early in her career to know your goals and stay focused.
“That has been the guiding light for me throughout my career,” says Dr. Lindsey, one of nine new Team Hospitalist members, The Hospitalist’s volunteer editorial advisory group. “My goal is to make medical care better and safer for hospitalized patients. We increasingly need to figure out how to do that with fewer and fewer resources. Regardless, we can never move backward on delivering better and safer care to patients.”
Question: How did you decide to become a hospitalist?
Answer: I have always been drawn to the practice of acute-care medicine. I enjoy taking care of patients and their families in their times of need. From the purely diagnostic standpoint, I very much enjoy the critical decision-making required in the diagnosis and treatment of the acutely ill patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the opportunity to “dig in” and positively affect processes and patient outcomes throughout hospitals.
Q: What do you dislike most?
A: Fighting the “scope creep” that is continually pushing on us as hospitalists. Hospitalists are constantly being asked to admit patients whose problems are outside the scope of our practice as medically trained physicians. A few examples of this include acute surgical abdomens, intracranial hemorrhages, and blunt-trauma cases.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The explosion of hospitalist programs throughout the country. Hospitalists programs are now even being built by payors and long-term-care facilities.
Q: For group leaders, why is it important for you to continue seeing patients?
A: In order to improve upon a process, you must know the process; to truly know the process, you must live the process. If you are not at the bedside delivering care to patients, there will be a disconnect between you, as a leader, and your physicians, who are at the bedside delivering care.
Q: What are your interests outside of patient care?
A: I believe the success—or failure—of a hospital, physician group, corporation, etc. is directly related to leadership. I enjoy leadership development because I see that as “mission critical” to the success of delivering better and safer patient care in any health-care system. As physicians, most of us never receive meaningful leadership training, yet are expected to come out of residency ready to lead. I enjoy providing physicians the tools to lead effectively. It makes the careers of physician leaders more fulfilling, as well as the careers of those physicians who are “following.”
Q: What is your biggest professional challenge?
A: Continuing to provide better and safer patient care with diminishing resources.
Q: What is your biggest professional reward?
A: Making a difference in the lives of patients. It is very rewarding to me to be able to come into a hospital and put processes in place, then actually see the risk-adjusted mortality rates improve. One of my teams’ biggest wins was taking over an HM program in a hospital with a mortality rate of 4, and seeing that mortality rate cut literally in half within six months.
Q: When you aren’t working, what is important to you?
A: My husband and children are the most important aspect of my life. My husband is a gastroenterologist; we have been married for 13 years. We have two healthy, happy kiddos ages 8 and 10.
Q: What’s next professionally? Where do you see yourself in 10 years?
A: I am partnering with two orthopedic surgeons in a startup company, Synergy Surgicalists. Our company mirrors the hospitalist model utilizing general and orthopedic surgeons. It’s very exciting to have the opportunity to bring value to hospitals and patients on a larger scale. Also, for the immediate future, I have accepted the role of interim executive medical director for hospital medicine for University of Texas Southwestern and Parkland hospitals. We are completely restructuring those programs in preparation for moving into two beautiful new (and very large) hospitals. I’m very excited about working with a truly excellent group of physicians and leaders while we are recruiting a permanent executive director and expanding our ranks.
Q: If you weren’t a doctor, what would you be doing right now?
A: I cannot imagine not being a physician. I suppose if pressed, I imagine I would have landed somewhere in the financial industry. I am also a musician, but have a hard time seeing myself employed in that industry.
Q: What’s the best book you’ve read recently?
A: “Widow Walk” by Gerard LaSalle. He is a physician author who pens a beautiful story. It’s just an enjoyable read of American historical fiction set in the Pacific Northwest.
Q: How many Apple products do you interface with in a given week?
A: Sadly, I interface with 11 (11!) different Apple products in any given week. (Even sadder: I just came into an iPod Shuffle, so I’m up to 12 … )
Q: What’s next in your Netflix queue?
A: “Fringe,” Season 2, Episode 19.
Richard Quinn is a freelance writer in New Jersey.

Growing up on a farm in rural Kentucky could have led to a career in the family business for Julianna Lindsey, MD, MBA, FHM. Except she knew at an early age that she wanted to be a doctor.
“My family physician was very influential on my decision to become a physician,” she says. “[He] mentored and encouraged me from a young age; it was very powerful for me.”
Dr. Lindsey earned bachelor’s degrees in biomedical science from the University of South Alabama and biochemistry from Western Kentucky University. She graduated from the University of Kentucky College of Medicine and completed her internal-medicine residency at the University of Kentucky. In 2011, she earned her master’s in business administration from the University of Tennessee.
Immediately following residency, she worked for the Veterans Affairs Medical Center in Lexington, Ky., as an ED physician. In 2002, she latched on to a career in HM when she and her husband, a gastroenterologist, relocated to Knoxville, Tenn. She recently launched a startup company, Synergy Surgicalists, with two orthopedic surgeons, and also provides process-improvement and leadership-development consulting.
She says she was told early in her career to know your goals and stay focused.
“That has been the guiding light for me throughout my career,” says Dr. Lindsey, one of nine new Team Hospitalist members, The Hospitalist’s volunteer editorial advisory group. “My goal is to make medical care better and safer for hospitalized patients. We increasingly need to figure out how to do that with fewer and fewer resources. Regardless, we can never move backward on delivering better and safer care to patients.”
Question: How did you decide to become a hospitalist?
Answer: I have always been drawn to the practice of acute-care medicine. I enjoy taking care of patients and their families in their times of need. From the purely diagnostic standpoint, I very much enjoy the critical decision-making required in the diagnosis and treatment of the acutely ill patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the opportunity to “dig in” and positively affect processes and patient outcomes throughout hospitals.
Q: What do you dislike most?
A: Fighting the “scope creep” that is continually pushing on us as hospitalists. Hospitalists are constantly being asked to admit patients whose problems are outside the scope of our practice as medically trained physicians. A few examples of this include acute surgical abdomens, intracranial hemorrhages, and blunt-trauma cases.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The explosion of hospitalist programs throughout the country. Hospitalists programs are now even being built by payors and long-term-care facilities.
Q: For group leaders, why is it important for you to continue seeing patients?
A: In order to improve upon a process, you must know the process; to truly know the process, you must live the process. If you are not at the bedside delivering care to patients, there will be a disconnect between you, as a leader, and your physicians, who are at the bedside delivering care.
Q: What are your interests outside of patient care?
A: I believe the success—or failure—of a hospital, physician group, corporation, etc. is directly related to leadership. I enjoy leadership development because I see that as “mission critical” to the success of delivering better and safer patient care in any health-care system. As physicians, most of us never receive meaningful leadership training, yet are expected to come out of residency ready to lead. I enjoy providing physicians the tools to lead effectively. It makes the careers of physician leaders more fulfilling, as well as the careers of those physicians who are “following.”
Q: What is your biggest professional challenge?
A: Continuing to provide better and safer patient care with diminishing resources.
Q: What is your biggest professional reward?
A: Making a difference in the lives of patients. It is very rewarding to me to be able to come into a hospital and put processes in place, then actually see the risk-adjusted mortality rates improve. One of my teams’ biggest wins was taking over an HM program in a hospital with a mortality rate of 4, and seeing that mortality rate cut literally in half within six months.
Q: When you aren’t working, what is important to you?
A: My husband and children are the most important aspect of my life. My husband is a gastroenterologist; we have been married for 13 years. We have two healthy, happy kiddos ages 8 and 10.
Q: What’s next professionally? Where do you see yourself in 10 years?
A: I am partnering with two orthopedic surgeons in a startup company, Synergy Surgicalists. Our company mirrors the hospitalist model utilizing general and orthopedic surgeons. It’s very exciting to have the opportunity to bring value to hospitals and patients on a larger scale. Also, for the immediate future, I have accepted the role of interim executive medical director for hospital medicine for University of Texas Southwestern and Parkland hospitals. We are completely restructuring those programs in preparation for moving into two beautiful new (and very large) hospitals. I’m very excited about working with a truly excellent group of physicians and leaders while we are recruiting a permanent executive director and expanding our ranks.
Q: If you weren’t a doctor, what would you be doing right now?
A: I cannot imagine not being a physician. I suppose if pressed, I imagine I would have landed somewhere in the financial industry. I am also a musician, but have a hard time seeing myself employed in that industry.
Q: What’s the best book you’ve read recently?
A: “Widow Walk” by Gerard LaSalle. He is a physician author who pens a beautiful story. It’s just an enjoyable read of American historical fiction set in the Pacific Northwest.
Q: How many Apple products do you interface with in a given week?
A: Sadly, I interface with 11 (11!) different Apple products in any given week. (Even sadder: I just came into an iPod Shuffle, so I’m up to 12 … )
Q: What’s next in your Netflix queue?
A: “Fringe,” Season 2, Episode 19.
Richard Quinn is a freelance writer in New Jersey.

Growing up on a farm in rural Kentucky could have led to a career in the family business for Julianna Lindsey, MD, MBA, FHM. Except she knew at an early age that she wanted to be a doctor.
“My family physician was very influential on my decision to become a physician,” she says. “[He] mentored and encouraged me from a young age; it was very powerful for me.”
Dr. Lindsey earned bachelor’s degrees in biomedical science from the University of South Alabama and biochemistry from Western Kentucky University. She graduated from the University of Kentucky College of Medicine and completed her internal-medicine residency at the University of Kentucky. In 2011, she earned her master’s in business administration from the University of Tennessee.
Immediately following residency, she worked for the Veterans Affairs Medical Center in Lexington, Ky., as an ED physician. In 2002, she latched on to a career in HM when she and her husband, a gastroenterologist, relocated to Knoxville, Tenn. She recently launched a startup company, Synergy Surgicalists, with two orthopedic surgeons, and also provides process-improvement and leadership-development consulting.
She says she was told early in her career to know your goals and stay focused.
“That has been the guiding light for me throughout my career,” says Dr. Lindsey, one of nine new Team Hospitalist members, The Hospitalist’s volunteer editorial advisory group. “My goal is to make medical care better and safer for hospitalized patients. We increasingly need to figure out how to do that with fewer and fewer resources. Regardless, we can never move backward on delivering better and safer care to patients.”
Question: How did you decide to become a hospitalist?
Answer: I have always been drawn to the practice of acute-care medicine. I enjoy taking care of patients and their families in their times of need. From the purely diagnostic standpoint, I very much enjoy the critical decision-making required in the diagnosis and treatment of the acutely ill patient.
Q: What do you like most about working as a hospitalist?
A: I enjoy the opportunity to “dig in” and positively affect processes and patient outcomes throughout hospitals.
Q: What do you dislike most?
A: Fighting the “scope creep” that is continually pushing on us as hospitalists. Hospitalists are constantly being asked to admit patients whose problems are outside the scope of our practice as medically trained physicians. A few examples of this include acute surgical abdomens, intracranial hemorrhages, and blunt-trauma cases.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The explosion of hospitalist programs throughout the country. Hospitalists programs are now even being built by payors and long-term-care facilities.
Q: For group leaders, why is it important for you to continue seeing patients?
A: In order to improve upon a process, you must know the process; to truly know the process, you must live the process. If you are not at the bedside delivering care to patients, there will be a disconnect between you, as a leader, and your physicians, who are at the bedside delivering care.
Q: What are your interests outside of patient care?
A: I believe the success—or failure—of a hospital, physician group, corporation, etc. is directly related to leadership. I enjoy leadership development because I see that as “mission critical” to the success of delivering better and safer patient care in any health-care system. As physicians, most of us never receive meaningful leadership training, yet are expected to come out of residency ready to lead. I enjoy providing physicians the tools to lead effectively. It makes the careers of physician leaders more fulfilling, as well as the careers of those physicians who are “following.”
Q: What is your biggest professional challenge?
A: Continuing to provide better and safer patient care with diminishing resources.
Q: What is your biggest professional reward?
A: Making a difference in the lives of patients. It is very rewarding to me to be able to come into a hospital and put processes in place, then actually see the risk-adjusted mortality rates improve. One of my teams’ biggest wins was taking over an HM program in a hospital with a mortality rate of 4, and seeing that mortality rate cut literally in half within six months.
Q: When you aren’t working, what is important to you?
A: My husband and children are the most important aspect of my life. My husband is a gastroenterologist; we have been married for 13 years. We have two healthy, happy kiddos ages 8 and 10.
Q: What’s next professionally? Where do you see yourself in 10 years?
A: I am partnering with two orthopedic surgeons in a startup company, Synergy Surgicalists. Our company mirrors the hospitalist model utilizing general and orthopedic surgeons. It’s very exciting to have the opportunity to bring value to hospitals and patients on a larger scale. Also, for the immediate future, I have accepted the role of interim executive medical director for hospital medicine for University of Texas Southwestern and Parkland hospitals. We are completely restructuring those programs in preparation for moving into two beautiful new (and very large) hospitals. I’m very excited about working with a truly excellent group of physicians and leaders while we are recruiting a permanent executive director and expanding our ranks.
Q: If you weren’t a doctor, what would you be doing right now?
A: I cannot imagine not being a physician. I suppose if pressed, I imagine I would have landed somewhere in the financial industry. I am also a musician, but have a hard time seeing myself employed in that industry.
Q: What’s the best book you’ve read recently?
A: “Widow Walk” by Gerard LaSalle. He is a physician author who pens a beautiful story. It’s just an enjoyable read of American historical fiction set in the Pacific Northwest.
Q: How many Apple products do you interface with in a given week?
A: Sadly, I interface with 11 (11!) different Apple products in any given week. (Even sadder: I just came into an iPod Shuffle, so I’m up to 12 … )
Q: What’s next in your Netflix queue?
A: “Fringe,” Season 2, Episode 19.
Richard Quinn is a freelance writer in New Jersey.
Intravenous Immunoglobulin Most Common Retreatment Approach for Refractory Kawasaki Disease
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Proposed Bill Would Open Door to Gainsharing Arrangements for Hospitals, Physicians
There is little dispute in the potential for cost savings when gainsharing arrangements incentivize things like product standardization, substitution of lower-cost products, and, most notably for hospitalists, medically appropriate decreases in length of stay. However, well-meaning but overly inclusive federal law makes the legal risk of establishing these arrangements so great that providers recoil at the prospect.
This doesn’t mean that gainsharing isn’t occurring. Currently, Medicare accountable-care organizations (ACOs) have been granted official waivers to establish such arrangements; smaller-scale pilot projects implemented by Medicare also have been granted similar waivers in the past. As availability is limited to participants within officially sanctioned programs, most providers are not able to tap into these cost-saving efforts, though this has not been for lack of trying.
Hospitals and physicians are engaging in a number of clinical joint ventures that have spurred them to seek their own gainsharing waivers by approaching the Office of the Inspector General (OIG). The OIG is the arm of the U.S. Department of Health and Human Services charged with enforcing the applicable laws affecting gainsharing. The OIG responded by cautioning that gainsharing arrangements violate the Social Security Act’s “Civil Monetary Penalty” prohibition against limitation of services to publicly insured patients, in addition to violating the federal Anti-Kickback Law and possibly the “Stark” law. Nonetheless, the OIG concluded it would not impose sanctions for the violations. In short, the OIG declared the proposals illegal but gave the go-ahead. The caveat, of course, is that these opinions are nonbinding, so providers remain understandably timid.
As a result, gainsharing currently remains more or less out of reach for those not participating in a Medicare ACO. This makes little sense at a time when Medicare and the entire health-care system are focusing on how to deliver high-quality, cost-conscious care. For example, if hospitalists are capable of reducing length of stay without detriment to the patient, they should not be legally prohibited from sharing any of the resulting cost savings. Fortunately, U.S. Rep. Jim McDermott (D-Wash.) agrees with this sentiment and has introduced legislation to address the problem.
McDermott introduced the Improved Health Care at Lower Cost Act of 2013 (H.R. 1487) in April. It seeks to exempt monetary incentive payments made by hospitals to physicians from federal anti-kickback and other sanctions. Such exemptions, or safe harbors, would be automatically granted to gainsharing arrangements that meet a pre-determined set of requirements. This means no formal application process or participation in a specific federal program would be required.
Passage of the bill would be a major step in the right direction for providers lacking the resources to navigate legal minefields or establish a full-scale ACO. If well-implemented, it could also generate significant cost savings for Medicare.
It is for these reasons that SHM supports H.R. 1487 and looks forward to working with McDermott in securing its passage.
In the coming months, members of SHM’s Grassroots Network will be encouraging Congress to make this important change to facilitate practice arrangements that provide high-value coordinated care for patients. Stay informed and take action when SHM issues Legislative Action Alerts by signing up for the Grassroots Network at www.hospitalmedicine.org/grassroots.
Josh Boswell is SHM’s senior manager of government relations.
There is little dispute in the potential for cost savings when gainsharing arrangements incentivize things like product standardization, substitution of lower-cost products, and, most notably for hospitalists, medically appropriate decreases in length of stay. However, well-meaning but overly inclusive federal law makes the legal risk of establishing these arrangements so great that providers recoil at the prospect.
This doesn’t mean that gainsharing isn’t occurring. Currently, Medicare accountable-care organizations (ACOs) have been granted official waivers to establish such arrangements; smaller-scale pilot projects implemented by Medicare also have been granted similar waivers in the past. As availability is limited to participants within officially sanctioned programs, most providers are not able to tap into these cost-saving efforts, though this has not been for lack of trying.
Hospitals and physicians are engaging in a number of clinical joint ventures that have spurred them to seek their own gainsharing waivers by approaching the Office of the Inspector General (OIG). The OIG is the arm of the U.S. Department of Health and Human Services charged with enforcing the applicable laws affecting gainsharing. The OIG responded by cautioning that gainsharing arrangements violate the Social Security Act’s “Civil Monetary Penalty” prohibition against limitation of services to publicly insured patients, in addition to violating the federal Anti-Kickback Law and possibly the “Stark” law. Nonetheless, the OIG concluded it would not impose sanctions for the violations. In short, the OIG declared the proposals illegal but gave the go-ahead. The caveat, of course, is that these opinions are nonbinding, so providers remain understandably timid.
As a result, gainsharing currently remains more or less out of reach for those not participating in a Medicare ACO. This makes little sense at a time when Medicare and the entire health-care system are focusing on how to deliver high-quality, cost-conscious care. For example, if hospitalists are capable of reducing length of stay without detriment to the patient, they should not be legally prohibited from sharing any of the resulting cost savings. Fortunately, U.S. Rep. Jim McDermott (D-Wash.) agrees with this sentiment and has introduced legislation to address the problem.
McDermott introduced the Improved Health Care at Lower Cost Act of 2013 (H.R. 1487) in April. It seeks to exempt monetary incentive payments made by hospitals to physicians from federal anti-kickback and other sanctions. Such exemptions, or safe harbors, would be automatically granted to gainsharing arrangements that meet a pre-determined set of requirements. This means no formal application process or participation in a specific federal program would be required.
Passage of the bill would be a major step in the right direction for providers lacking the resources to navigate legal minefields or establish a full-scale ACO. If well-implemented, it could also generate significant cost savings for Medicare.
It is for these reasons that SHM supports H.R. 1487 and looks forward to working with McDermott in securing its passage.
In the coming months, members of SHM’s Grassroots Network will be encouraging Congress to make this important change to facilitate practice arrangements that provide high-value coordinated care for patients. Stay informed and take action when SHM issues Legislative Action Alerts by signing up for the Grassroots Network at www.hospitalmedicine.org/grassroots.
Josh Boswell is SHM’s senior manager of government relations.
There is little dispute in the potential for cost savings when gainsharing arrangements incentivize things like product standardization, substitution of lower-cost products, and, most notably for hospitalists, medically appropriate decreases in length of stay. However, well-meaning but overly inclusive federal law makes the legal risk of establishing these arrangements so great that providers recoil at the prospect.
This doesn’t mean that gainsharing isn’t occurring. Currently, Medicare accountable-care organizations (ACOs) have been granted official waivers to establish such arrangements; smaller-scale pilot projects implemented by Medicare also have been granted similar waivers in the past. As availability is limited to participants within officially sanctioned programs, most providers are not able to tap into these cost-saving efforts, though this has not been for lack of trying.
Hospitals and physicians are engaging in a number of clinical joint ventures that have spurred them to seek their own gainsharing waivers by approaching the Office of the Inspector General (OIG). The OIG is the arm of the U.S. Department of Health and Human Services charged with enforcing the applicable laws affecting gainsharing. The OIG responded by cautioning that gainsharing arrangements violate the Social Security Act’s “Civil Monetary Penalty” prohibition against limitation of services to publicly insured patients, in addition to violating the federal Anti-Kickback Law and possibly the “Stark” law. Nonetheless, the OIG concluded it would not impose sanctions for the violations. In short, the OIG declared the proposals illegal but gave the go-ahead. The caveat, of course, is that these opinions are nonbinding, so providers remain understandably timid.
As a result, gainsharing currently remains more or less out of reach for those not participating in a Medicare ACO. This makes little sense at a time when Medicare and the entire health-care system are focusing on how to deliver high-quality, cost-conscious care. For example, if hospitalists are capable of reducing length of stay without detriment to the patient, they should not be legally prohibited from sharing any of the resulting cost savings. Fortunately, U.S. Rep. Jim McDermott (D-Wash.) agrees with this sentiment and has introduced legislation to address the problem.
McDermott introduced the Improved Health Care at Lower Cost Act of 2013 (H.R. 1487) in April. It seeks to exempt monetary incentive payments made by hospitals to physicians from federal anti-kickback and other sanctions. Such exemptions, or safe harbors, would be automatically granted to gainsharing arrangements that meet a pre-determined set of requirements. This means no formal application process or participation in a specific federal program would be required.
Passage of the bill would be a major step in the right direction for providers lacking the resources to navigate legal minefields or establish a full-scale ACO. If well-implemented, it could also generate significant cost savings for Medicare.
It is for these reasons that SHM supports H.R. 1487 and looks forward to working with McDermott in securing its passage.
In the coming months, members of SHM’s Grassroots Network will be encouraging Congress to make this important change to facilitate practice arrangements that provide high-value coordinated care for patients. Stay informed and take action when SHM issues Legislative Action Alerts by signing up for the Grassroots Network at www.hospitalmedicine.org/grassroots.
Josh Boswell is SHM’s senior manager of government relations.