User login
Trends in Blood‐Product Transfusion
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
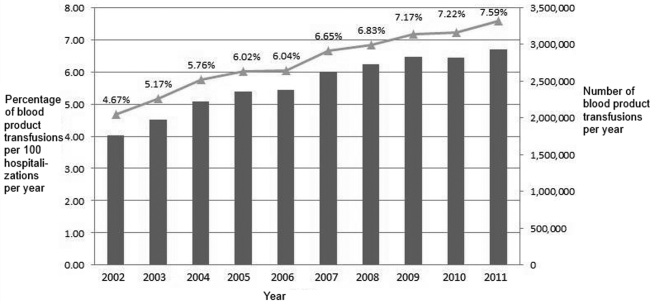
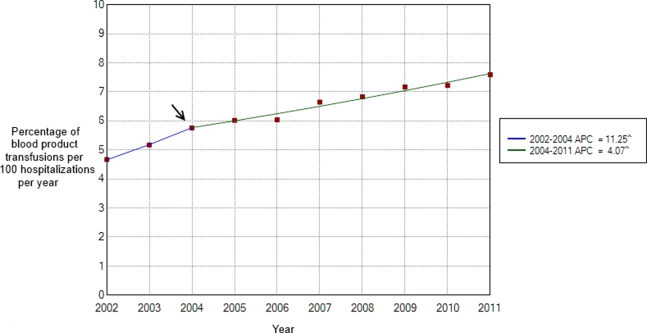
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).
Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).


Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
Although potentially life saving, blood‐product transfusion is costly and associated with transfusion‐related adverse events, including death on rare occasions. Studies in varied patient populations have demonstrated that a restrictive red blood cell transfusion strategy reduces the number of transfusion‐related adverse effects and can result in improved short‐term survival.[1, 2, 3] In 2011, more than 20 million blood products were transfused in the United States, which resulted in more than 50,000 transfusion‐related adverse reactions (0.24%).[4] With a mean cost of greater than $50 per unit of plasma and $500 per unit of apheresis platelets,[4] the cost of blood transfusion is well in excess of $1 billion per year. Blood‐product transfusion is the most frequent inpatient procedure,[5] and inpatient blood‐product transfusion contributes to the bulk of transfusions nationwide. To study the utilization of blood‐product transfusion in the inpatient population, we studied the temporal trend of inpatient blood‐product transfusions in the United States from 2002 to 2011 using data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.[4] The NIS, the largest inpatient care database in the United States, includes approximately a 20% stratified sample of US community hospital admissions and is weighted at discharge level to permit population‐level estimates.[6] We utilized this database to identify the total number of blood‐product transfusions and discharges between 2002 and 2011. We calculated the rate of all blood‐product transfusions, which include packed red blood cell, platelets, and other blood components, using the International Classification of DiseasesNinth Revision, Clinical Modification Procedural Clinical Classification Software code 222.[7] Trend analysis and calculation of average annual percent change were done using the Joinpoint Regression Program version 4.0.4 (National Cancer Institute, Bethesda, MD).[8] This software uses trend data and calculates the best fit lines to create the simplest joinpoint model that the data allow. The model can be expressed as a figure where several different multisegmented trend lines are connected together at the joinpoints. Trend over a fixed prespecified interval was computed as average annual percent change, and the Monte Carlo permutation method was used to test for apparent change in the trends.[9, 10] The study was exempted by the institutional review board of the University of Nebraska Medical Center.
Between 2002 and 2011, there were a total of 24,641,581 blood‐product transfusions among 389,761,571 hospitalizations. The rate of transfusion per 100 hospitalizations increased by 2.9% from 2002 to 2011 (4.6% in 2002 [n=1,767,111] to 7.5% in 2011 [n=2,929,312]) (Figure 1). The average annual percent change from 2002 to 2011 was 5.6% (95% confidence interval [CI]: 3.7‐7.6), which was statistically significant at P<0.05. A statistically significant change in trend (joinpoint) was observed in 2004. The annual percent change was 11.2% (95% CI: 0.323.4) from 2002 to 2004 and 4.1% (95% CI: 3.05.1) from 2004 to 2011, both of which were statistically significant at P<0.05 (Figure 2).


Our study demonstrates an overall increasing trend in the inpatient blood‐product transfusions over the past decade. However, the rate of increase seems to have slowed down since 2004. The National Blood Collection and Utilization Survey[4] demonstrated a decrease of 11.6% in the total number of all components transfused in the United States between 2008 and 2011. Our data are different from the survey, which also included blood transfusions in outpatient settings, emergency departments, and pediatric patients. The rising proportion of aging population with multiple comorbidities and cancers, increases in hematopoietic stem cell/solid organ transplants and chemotherapy, as well as widespread availability of blood products presumably contributed to the continued increase observed in our inpatient data after 2004. Nevertheless, the declining trend in the rate of the increased blood‐product transfusion usage seen after 2004 is encouraging. Increased awareness of restrictive transfusion strategy, coupled with efforts by professional bodies to improve the adoption of restrictive strategies, is most likely responsible for this.[3, 11, 12] As the clinical classification software procedure code 222 lumps together all the different types of blood products, we were unable to study the transfusion trend among each different type of blood products. In conclusion, further efforts need to be directed at increasing the awareness of clinicians, especially hospitalists, about the benefits of a restrictive transfusion policy and decreasing the rate of blood product use in the inpatient service. Furthermore, studies elaborating the patient population who are being transfused and the factors influencing the transfusion trends can provide useful insights to optimize blood‐product utilization and control resource consumption.
Disclosure
Nothing to report.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
- , , . Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
- , , , . Evidence review: periprocedural use of blood products. J Hosp Med. 2013;8(11):647–652.
- The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2013.
- , , , et al. HCUP facts and figures: statistics on hospital‐based care in the United States. 2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed January 2, 2014.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 15, 2013.
- HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM. Healthcare Cost and Utilization Project. 2009–2011. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 15, 2013.
- Joinpoint Regression Program, Version 4.0.4, December, 2014. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/download. Accessed December 25, 2013.
- , , , , . Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.
- , , , . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , . Patient‐centered blood management. J Hosp Med. 2014;9(1):60–65.
Bedside Interprofessional Rounds
Interprofessional collaborative care (IPCC) involves members from different professions working together to enhance communication, coordination, and healthcare quality.[1, 2, 3] Because several current healthcare policy initiatives include financial incentives for increased quality of care, there has been resultant interest in the implementation of IPCC in healthcare systems.[4, 5] Unfortunately, many hospitals have found IPCC difficult to achieve. Hospital‐based medicine units are complex, time‐constrained environments requiring a high degree of collaboration and mutual decision‐making between nurses, physicians, therapists, pharmacists, care coordinators, and patients. In addition, despite recommendations for interprofessional collaborative care, the implementation and assessment of IPCC within this environment has not been well studied.[6, 7]
On academic internal medicine services, the majority of care decisions occur during rounds. Although rounds provide a common structure, the participants, length, location, and agenda of rounds tend to vary by institution and individual physician preference.[8, 9, 10, 11] Traditionally, ward rounds occur mostly in hallways and conference rooms rather than the patient's bedside.[12] Additionally, during rounds, nurse‐physician collaboration occurs infrequently, estimated at <10% of rounding time.[13] Recently, an increased focus on quality, safety, and collaboration has inspired the investigation and implementation of new methods to increase interprofessional collaboration during rounds, but many of these interventions occurred away from the patient's bedside.[14, 15] One trial of bedside interprofessional rounds (BIRs) by Curley et al. suggested improvements in patient‐level outcomes (cost and length of stay) versus traditional physician‐based rounds.[16] Although interprofessional nurse‐physician rounds at patients' bedsides may represent an ideal process, limited work has investigated this activity.[17]
A prerequisite for successful and sustained integration of BIRs is a shared conceptualization among physicians and nurses regarding the process. Such a shared conceptualization would include perceptions of benefits and barriers to implementation.[18] Currently, such perceptions have not been measured. In this study, we sought to evaluate perceptions of front‐line care providers on inpatient units, specifically nursing staff, attending physicians, and housestaff physicians, regarding the benefits and barriers to BIRs.
METHODS
Study Design and Participants
In June 2013, we performed a cross‐sectional assessment of front‐line providers caring for patients on the internal medicine services in our academic hospital. Participants included medicine nursing staff in acute care and intermediate care units, medicine and combined medicine‐pediatrics housestaff physicians, and general internal medicine faculty physicians who supervised the housestaff physicians.
Study Setting
The study was conducted at a 378‐bed, university‐based, acute care teaching hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in2 units, a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). Both units are staffed by the general internal medicine physician teams. The academic medicine residency program consists of 69 internal medicine housestaff and 14 combined internal medicine‐pediatrics housestaff. Five teams, organized into 3 academic teaching teams and 2 nonteaching teams, provide care for all patients admitted to the medicine units. Teaching teams consist of 1 junior (postgraduate year [PGY]2) or senior (PGY34) housestaff member, 2 interns (PGY1), 2 medical students, and 1 attending physician.
There are several main features of BIRs in our medicine units. The rounding team of physicians alerts the assigned nurse about the start of rounds. In our main medicine unit, each doorway is equipped with a light that allows the physician team to indicate the start of the BIRs encounter. Case presentations by trainees occur either in the hallway or bedside, at the discretion of the attending physician. During bedside encounters, nurses typically contribute to the discussion about clinical status, decision making, patient concerns, and disposition. Patients are encouraged to contribute to the discussion and are provided the opportunity to ask questions.
For the purposes of this study, we specifically defined BIRs as: encounters that include the team of providers, at least 2 physicians plus a nurse or other care provider, discussing the case at the patient's bedside. In our prior work performed during the same time period as this study, we used the same definition to examine the incidence of and time spent in BIRs in both of our medicine units.[19] We found that 63% to 81% of patients in both units received BIRs. As a result, we assumed all nursing staff, attending physicians, and housestaff physicians had experienced this process, and their responses to this survey were contextualized in these experiences.
Survey Instrument
We developed a survey instrument specifically for this study. We derived items primarily from our prior qualitative work on physician‐based team bedside rounds and a literature review.[20, 21, 22, 23, 24, 25] For the benefits to BIRs, we developed items related to 5 domains, including factors related to the patient, education, communication/coordination/teamwork, efficiency and process, and outcomes.[20, 26] For the barriers to BIRs, we developed items related to 4 domains, including factors related to the patient, time, systems issues, and providers (nurses, attending physicians, and housestaff physicians).[22, 24, 25] We included our definition of BIRs into the survey instructions. We pilot tested the survey with 3 medicine faculty and 3 nursing staff and, based on our pilot, modified several questions to improve clarity. Primary demographic items in the survey included identification of provider role (nurses, attending physicians, or housestaff physicians) and years in the current role. Respondent preference for the benefits and barriers were investigated on a 7‐point scale (1=lowest response and 7=high response possible). Descriptive text was provided at the extremes (choice 1 and 7), but intermediary values (26) did not have descriptive cues.[27] As an incentive, the end of the survey provided respondents with an option for submitting their name to be entered into a raffle to win 1 of 50, $5 gift certificates to a coffee shop.
Prior to the end of the academic year in June 2013, we sent a survey link via e‐mail to all medicine nursing staff, housestaff physicians, and attending physicians. The email described the study and explained the voluntary nature of the work, and that informed consent would be implied by survey completion. Following the initial e‐mail, 3 additional weekly e‐mail reminders were sent by the lead investigator. The study was approved by the institutional review board at the Pennsylvania State College of Medicine.
Data Analysis
Descriptive statistics were used to examine the characteristics of the 3 respondent groups and combined totals for each survey item. The nonparametric Wilcoxon rank sum test was used to compare the average values between groups (nursing staff vs all physicians, attending physicians vs housestaff physicians) for both sets of survey variables (benefits and barriers). The nonparametric correlation statistical test Spearman rank was used to assess the degree of correlation between respondent groups for both survey variables. The data were analyzed using SAS 9.3 (SAS Institute, Cary, NC) and Stata/IC‐8 (StataCorp, College Station, Texas).
RESULTS
Of the 171 surveys sent, 149 participants completed surveys (response rate 87%). Responses were received from 53/58 nursing staff (91% response), 21/28 attending physicians (75% response), and 75/85 housestaff physicians (88% response). Table 1 describes the participant response demographics.
| Variable | Value |
|---|---|
| |
| Nursing staff, n=58, n (%) | 53 (36) |
| Intermediate care unit, n (%) | 14 (26) |
| General medicine ward, n (%) | 39 (74) |
| All day shifts, n (%) | 25 (47) |
| Mix of day and night shifts, n (%) | 32 (60) |
| Years of experience, mean (SD) | 7.4 (9) |
| Attending physicians, n=28, n (%) | 21 (14) |
| Years since residency graduation, mean (SD) | 10.5 (8) |
| No. of weeks in past year serving as teaching attending, mean (SD) | 9.1(8) |
| Housestaff physicians (n=85), n (%) | 75 (50) |
| Intern, n (%) | 28 (37) |
| Junior resident, n (%) | 25 (33) |
| Senior resident, n (%)a | 22 (29) |
Benefits of BIRs
Respondents' perceptions of the benefits of BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 2). Six of the 7 highest‐ranked benefits were related to communication, coordination, and teamwork, including improves communication between nurses and physicians, improves awareness of clinical issues that need to be addressed, and improves team‐building between nurses and physicians. Lowest‐ranked benefits were related to efficiency, process, and outcomes, including decreases patients' hospital length‐of‐stay, improves timeliness of consultations, and reduces ordering of unnecessary tests and treatments. Comparing mean values among the 3 groups, all 18 items showed statistical differences in response rates (all P values <0.05). Nursing staff reported more favorable ratings than both attending physicians and housestaff physicians for each of the 18 items, whereas attending physicians reported more favorable ratings than housestaff physicians in 16/18 items. The rank order among provider groups showed a high degree of correlation (r=0.92, P<0.001).
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, N=53, Mean (SD) | Attending Physicians, N=21, Mean (SD) | House staff Physicians, N=75, Mean (SD)b |
|---|---|---|---|---|---|
| |||||
| Improves communication between nurses and physicians. | CCT | 6.26 (1.11) | 6.74 (0.59)c | 6.52 (1.03)d | 5.85 (1.26) |
| Improves awareness of clinical issues needing to be addressed. | CCT | 6.05 (1.12) | 6.57 (0.64)c | 5.95 (1.07) | 5.71 (1.26) |
| Improves team‐building between nurses and physicians. | CCT | 6.03 (1.32) | 6.72 (0.60)c | 6.14 (1.11) | 5.52 (1.51) |
| Improves coordination of the patient's care. | CCT | 5.98 (1.34) | 6.60 (0.72)c | 6.00 (1.18) | 5.53 (1.55) |
| Improves nursing contributions to a patient's care plan. | CCT | 5.91 (1.25) | 6.47 (0.77)c | 6.14 (0.85) | 5.44 (1.43) |
| Improves quality of care delivered in our unit. | O | 5.72 (1.42) | 6.34 (0.83)c | 5.81 (1.33) | 5.25 (1.61) |
| Improves appreciation of the roles/contributions of other providers. | CCT | 5.69 (1.49) | 6.36 (0.86)c | 5.90 (1.04) | 5.16 (1.73) |
| Promotes shared decision making between patients and providers. | P | 5.62 (1.51) | 6.43 (0.77)c | 5.57 (1.40) | 5.05 (1.68) |
| Improves patients' satisfaction with their hospitalization. | P, O | 5.53 (1.40) | 6.15 (0.95)c | 5.38 (1.12) | 5.13 (1.58) |
| Provides more respect/dignity to patients. | P | 5.31 (1.55) | 6.23 (0.89)c | 5.10 (1.18) | 4.72 (1.71) |
| Decreases number of pages/phone calls between nurses and physicians. | EP | 5.28 (1.82) | 6.28 (0.93)c | 5.24 (1.30) | 4.57 (2.09) |
| Improves educational opportunities for housestaff/students. | E | 5.07 (1.77) | 6.08 (0.98)c | 4.81 (1.60) | 4.43 (1.93) |
| Improves the efficiency of your work. | EP | 5.01 (1.77) | 6.04 (1.13)c | 4.90 (1.30) | 4.31 (1.92) |
| Improves adherence to evidence‐based guidelines or interventions. | EP | 4.89 (1.79) | 6.06 (0.91)c | 4.00 (1.18) | 4.31 (1.97) |
| Improves the accuracy of your sign‐outs (or reports) to the next shift. | EP | 4.80 (1.99) | 6.30 (0.93)c | 4.05 (1.66) | 3.95 (2.01) |
| Reduces ordering of unnecessary tests and treatments. | O | 4.51 (1.86) | 5.77 (1.15)c | 3.86 (1.11) | 3.8 (1.97) |
| Improves the timeliness of consultations. | EP | 4.28 (1.99) | 5.66 (1.22)c | 3.24 (1.48) | 3.59 (2.02) |
| Decreases patients' hospital length of stay. | O | 4.15 (1.68) | 5.04 (1.24)c | 3.95 (1.16) | 3.57 (1.81) |
Barriers to BIRs
Respondents' perceptions of barriers to BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 3). The 6 highest‐ranked barriers were related to time, including nursing staff have limited time, the time required for bedside nurse‐physician encounters, and coordinating the start time of encounters with arrival of both physicians and nursing. The lowest‐ranked barriers were related to provider‐ and patient‐related factors, including patient lack of comfort with bedside nurse‐physician encounters, attending physicians/housestaff lack bedside skills, and attending physicians lack comfort with bedside nurse‐physician encounters. Comparing mean values between groups, 10 of 21 items showed statistical differences (P<0.05). The rank order among groups showed moderate correlation (nurses‐attending physicians r=0.62, nurses‐housestaff physicians r=0.76, attending physicians‐housestaff physicians r=0.82). A qualitative inspection of disparities among respondent groups highlighted that nursing staff were more likely to rank bedside rounds are not part of the unit's culture lower than physician groups.
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, n=53, Mean (SD) | Attending Physicians, n=21, Mean (SD) | Housestaff Physicians, n=75,b Mean (SD) |
|---|---|---|---|---|---|
| |||||
| Nursing staff have limited time. | T | 4.89 (1.34) | 4.96 (1.27) | 4.86 (1.65) | 4.85 (1.30) |
| Coordinating start time of encounters with arrival of physicians and nursing. | T | 4.80 (1.50) | 4.58 (1.43) | 5.24 (1.45) | 4.84 (1.55) |
| Housestaff have limited time. | T | 4.68 (1.47) | 4.56 (1.26) | 4.24 (1.81) | 4.89 (1.48) |
| Attending physicians have limited time. | T | 4.50 (1.49) | 4.81 (1.34) | 4.33 (1.65) | 4.34 (1.53) |
| Other acutely sick patients in unit. | T | 4.39 (1.42) | 4.79 (1.30)c | 4.52 (1.21) | 4.08 (1.49) |
| Time required for bedside nurse‐physician encounters. | T | 4.32 (1.55) | 4.85 (1.38)c | 3.62 (1.80) | 4.15 (1.49) |
| Lack of use of the pink‐rounding light to alert nursing staff. | S | 3.77 (1.75) | 4.71 (1.70)c | 3.48 (1.86) | 3.19 (1.46) |
| Patient not available (eg, off to test, getting bathed) | S | 3.74 (1.40) | 3.98 (1.28) | 4.52 (1.36)d | 3.35 (1.37) |
| Large team size. | S | 3.64 (1.74) | 3.12 (1.58)c | 3.95 (1.83) | 3.92 (1.77) |
| Patients in dispersed locations (eg, other units or in different hallways). | S | 3.64 (1.77) | 2.77 (1.55)c | 4.52 (1.83) | 4.00 (1.66) |
| Bedside nurse‐physician rounds are not part of the unit's culture. | S | 3.35 (1.94) | 2.25 (1.47)c | 4.76 (1.92) | 3.72 (1.85) |
| Limitations in physical facilities (eg, rooms too small, limited chairs). | S | 3.25 (1.71) | 2.71 (1.72) | 3.33 (1.71) | 3.59 (1.62) |
| Insufficient nurse engagement during bedside nurse‐physician encounters. | PR | 3.24 (1.63) | 2.71 (1.47)c | 3.67 (1.68) | 3.49 (1.65) |
| Patient on contact or respiratory isolation. | S | 3.20 (1.82) | 2.42 (1.67)c | 3.43 (1.63) | 3.69 (1.80) |
| Language barrier between providers and patients. | P | 2.69 (1.37) | 2.77 (1.39) | 2.57 (1.08) | 2.68 (1.43) |
| Privacy/sensitive patient issues. | P | 2.65 (1.45) | 2.27 (1.24) | 2.57 (1.33) | 2.93 (1.56) |
| Housestaff lack comfort with bedside nurse‐physician encounters. | PR | 2.55 (1.49) | 2.48 (1.15) | 2.67 (1.68) | 2.57 (1.65) |
| Nurses lack comfort with bedside nurse‐physician encounters. | PR | 2.45 (1.45) | 2.35 (1.27) | 2.48 (1.66) | 2.51 (1.53) |
| Attending physicians lack comfort with bedside nurse‐physician encounters. | PR | 2.35 (1.38) | 2.33 (1.25) | 2.33 (1.62) | 2.36 (1.41) |
| Attending physician/housestaff lack bedside skills (eg, history, exam). | PR | 2.34 (1.34) | 2.19 (1.19) | 2.85 (1.69) | 2.30 (1.32) |
| Patient lack of comfort with bedside nurse‐physician encounters. | P | 2.33 (1.48) | 2.23 (1.37) | 1.95 (1.32) | 2.5 (1.59) |
DISCUSSION
In this study, we sought to compare perceptions of nurses and physicians on the benefits and barriers to BIRs. Nursing staff ranked each benefit higher than physicians, though rank orders of specific benefits were highly correlated. Highest‐ranked benefits related to coordination and communication more than quality or process benefits. Across groups, the highest‐ranked barriers to BIRs were related to time, whereas the lowest‐ranked factors were related to provider and patient discomfort. These results highlight important similarities and differences in perceptions between front‐line providers.
The highest‐ranked benefits were related to improved interprofessional communication and coordination. Combining interprofessional team members during care delivery allows for integrated understanding of daily care plans and clinical issues, and fosters collaboration and a team‐based atmosphere.[1, 20, 26] The lowest‐ranked benefits were related to more tangible measures, including length of stay, timely consultations, and judicious laboratory ordering. This finding contrasts with the limited literature demonstrating increased efficiency in general medicine units practicing IPCC.[16] These rankings may reflect a poor understanding or self‐assessment of outcome measures by healthcare providers, representing a potential focus for educational initiatives. Future investigations using objective assessment methods of outcomes and collaboration will provide a more accurate understanding of these findings.
The highest‐ranked barriers were related to time and systems issues. Several studies of physician‐based bedside rounds have identified systems‐ and time‐related issues as primary limiting barriers.[22, 24] In units without colocalization of patients and providers, finding receptive times for BIRs can be difficult. Although time‐related issues could be addressed by decreasing patient‐provider ratios, these changes require substantial investment in resources. A reasonable degree of improvement in efficiency and coordination is expected following acclimation to BIRs or by addressing modifiable systems factors to increase this activity. Less costly interventions, such as tailoring provider schedules, prescheduling patient rounding times, and geographic colocalization of patients and providers may be more feasible. However, the clinical microsystems within which medicine patients are cared for are often chaotic and disorganized at the infrastructural and cultural levels, which may be less influenced by surface‐level interventions. Such interventions may be ward specific and require customization to individual team needs.
The lowest‐ranked barriers to BIRs were related to provider‐ and patient‐related factors, including comfort level of patients and providers. Prior work on bedside rounds has identified physicians who are apprehensive about performing bedside rounds, but those who experience this activity are more likely to be comfortable with it.[12, 28] Our results from a culture where BIRs occur on nearly two‐thirds of patients suggest provider discomfort is not a predominant barrier.[22, 29] Additionally, educators have raised concerns about patient discomfort with bedside rounds, but nearly all studies evaluating patients' perspectives reveal patient preference for bedside case presentations over activities occurring in alternative locations.[30, 31, 32] Little work has investigated patient preference for BIRs as per our definition; our participants do not believe patients are discomforted by BIRs, building upon evidence in the literature for patient preferences regarding bedside activities.
Nursing staff perceptions of the benefits and culture related to BIRs were more positive than physicians. We hypothesize several reasons for this disparity. First, nursing staff may have more experience with observing and understanding the positive impact of BIRs and therefore are more likely to understand the positive ramifications. Alternatively, nursing staff may be satisfied with active integration into traditional physician‐centric decisions. Additionally, the professional culture and educational foundation of the nursing culture is based upon a patient‐centered approach and therefore may be more aligned with the goals of BIRs. Last, physicians may have competing priorities, favoring productivity and didactic learning rather than interprofessional collaboration. Further investigation is required to understand differences between nurses and physicians, in addition to other providers integral to BIRs (eg, care coordinators, pharmacists). Regardless, during the implementation of interprofessional collaborative care models, our findings suggest initial challenges, and the focus of educational initiatives may necessitate acclimating physician groups to benefits identified by front‐line nursing staff.
There are several limitations to our study. We investigated the perceptions of medicine nurses and physicians in 1 teaching hospital, limiting generalizability to other specialties, other vital professional groups, and nonteaching hospitals. Additionally, BIRs has been a focus of our hospital for several years. Therefore, perceived barriers may differ in BIRs‐nave hospitals. Second, although pilot‐tested for content, the construct validity of the instrument was not rigorously assessed, and the instrument was not designed to measure benefits and barriers not explicitly identified during pilot testing. Last, although surveys were anonymous, the possibility of social desirability bias exists, thereby limiting accuracy.
For over a century, physician‐led rounds have been the preferred modality for point‐of‐care decision making.[10, 15, 32, 33] BIRs address our growing understanding of patient‐centered care. Future efforts should address the quality of collaboration and current hospital and unit structures hindering patient‐centered IPCC and patient outcomes.
Acknowledgements
The authors thank the medicine nursing staff and physicians for their dedication to patient‐centered care and willingness to participate in this study.
Disclosures: The Department of Medicine at the Penn State Hershey Medical Center provided funding for this project. There are no conflicts of interest to report.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009(3):CD000072.
- . Teamswork! Hosp Health Netw. 2012;86(3):24–27, 21.
- , , , . The coming of age for interprofessional education and practice. Am J Med. 2013;126(4):284–288.
- , . Payment incentives and integrated care delivery: levers for health system reform and cost containment. Inquiry. 2011;48(4):277–287.
- . Payment reform and the mission of academic medical centers. N Engl J Med. 2010;363(19):1784–1786.
- Josiah Macy Jr. Foundation. Transforming patient care: aligning interprofessional education and clinical practice redesign. In: Proceedings of the Josiah Macy Jr. Foundation Conference; January 17–20, 2013; Atlanta, GA.
- , , , . Bridging the quality chasm: interprofessional teams to the rescue? Am J Med. 2013;126(4):276–277.
- . Attending rounds: guidelines for teaching on the wards. J Gen Intern Med. 1992;7(1):68–75.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PloS One. 2010;5(6):e11246.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275–280.
- , . The challenge of innovation implementation. Acad Manage Rev. 1996;21(4):1055–1080.
- , , , . Ocular dipping in creutzfeldt‐jakob disease. J Clin Neurol. 2014;10(2):162–165.
- , , , et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326–333.
- , , , et al. The art of bedside rounds: a multi‐center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412–420.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , . Bedside teaching in medical education: a literature review. Perspec Med Educ. 2014;3(2):76–88.
- , , . Impediments to bed‐side teaching. Med Educ. 1998;32(2):159–162.
- , , , . Whither bedside teaching? A focus‐group study of clinical teachers. Acad Med. 2003;78(4):384–390.
- , . Staff preference for multidisciplinary rounding practices in the critical care setting. 2011. Paper presented at: Design July 6–10, 2011. Boston, MA. Available at: http://www.designandhealth.com/uploaded/documents/Awards‐and‐events/WCDH2011/Presentations/Friday/Session‐8/DianaAnderson.pdf. Accessed July 6, 2014.
- , . Health Measurement Scales: A Practical Guide to Their Development and Use. 2nd ed. New York, NY: Oxford University Press; 1995.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , , . The positive impact of portfolios on health care assistants' clinical practice. J Eval Clin Pract. 2008;14(1):172–174.
- , , , . The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273–1275.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
Interprofessional collaborative care (IPCC) involves members from different professions working together to enhance communication, coordination, and healthcare quality.[1, 2, 3] Because several current healthcare policy initiatives include financial incentives for increased quality of care, there has been resultant interest in the implementation of IPCC in healthcare systems.[4, 5] Unfortunately, many hospitals have found IPCC difficult to achieve. Hospital‐based medicine units are complex, time‐constrained environments requiring a high degree of collaboration and mutual decision‐making between nurses, physicians, therapists, pharmacists, care coordinators, and patients. In addition, despite recommendations for interprofessional collaborative care, the implementation and assessment of IPCC within this environment has not been well studied.[6, 7]
On academic internal medicine services, the majority of care decisions occur during rounds. Although rounds provide a common structure, the participants, length, location, and agenda of rounds tend to vary by institution and individual physician preference.[8, 9, 10, 11] Traditionally, ward rounds occur mostly in hallways and conference rooms rather than the patient's bedside.[12] Additionally, during rounds, nurse‐physician collaboration occurs infrequently, estimated at <10% of rounding time.[13] Recently, an increased focus on quality, safety, and collaboration has inspired the investigation and implementation of new methods to increase interprofessional collaboration during rounds, but many of these interventions occurred away from the patient's bedside.[14, 15] One trial of bedside interprofessional rounds (BIRs) by Curley et al. suggested improvements in patient‐level outcomes (cost and length of stay) versus traditional physician‐based rounds.[16] Although interprofessional nurse‐physician rounds at patients' bedsides may represent an ideal process, limited work has investigated this activity.[17]
A prerequisite for successful and sustained integration of BIRs is a shared conceptualization among physicians and nurses regarding the process. Such a shared conceptualization would include perceptions of benefits and barriers to implementation.[18] Currently, such perceptions have not been measured. In this study, we sought to evaluate perceptions of front‐line care providers on inpatient units, specifically nursing staff, attending physicians, and housestaff physicians, regarding the benefits and barriers to BIRs.
METHODS
Study Design and Participants
In June 2013, we performed a cross‐sectional assessment of front‐line providers caring for patients on the internal medicine services in our academic hospital. Participants included medicine nursing staff in acute care and intermediate care units, medicine and combined medicine‐pediatrics housestaff physicians, and general internal medicine faculty physicians who supervised the housestaff physicians.
Study Setting
The study was conducted at a 378‐bed, university‐based, acute care teaching hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in2 units, a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). Both units are staffed by the general internal medicine physician teams. The academic medicine residency program consists of 69 internal medicine housestaff and 14 combined internal medicine‐pediatrics housestaff. Five teams, organized into 3 academic teaching teams and 2 nonteaching teams, provide care for all patients admitted to the medicine units. Teaching teams consist of 1 junior (postgraduate year [PGY]2) or senior (PGY34) housestaff member, 2 interns (PGY1), 2 medical students, and 1 attending physician.
There are several main features of BIRs in our medicine units. The rounding team of physicians alerts the assigned nurse about the start of rounds. In our main medicine unit, each doorway is equipped with a light that allows the physician team to indicate the start of the BIRs encounter. Case presentations by trainees occur either in the hallway or bedside, at the discretion of the attending physician. During bedside encounters, nurses typically contribute to the discussion about clinical status, decision making, patient concerns, and disposition. Patients are encouraged to contribute to the discussion and are provided the opportunity to ask questions.
For the purposes of this study, we specifically defined BIRs as: encounters that include the team of providers, at least 2 physicians plus a nurse or other care provider, discussing the case at the patient's bedside. In our prior work performed during the same time period as this study, we used the same definition to examine the incidence of and time spent in BIRs in both of our medicine units.[19] We found that 63% to 81% of patients in both units received BIRs. As a result, we assumed all nursing staff, attending physicians, and housestaff physicians had experienced this process, and their responses to this survey were contextualized in these experiences.
Survey Instrument
We developed a survey instrument specifically for this study. We derived items primarily from our prior qualitative work on physician‐based team bedside rounds and a literature review.[20, 21, 22, 23, 24, 25] For the benefits to BIRs, we developed items related to 5 domains, including factors related to the patient, education, communication/coordination/teamwork, efficiency and process, and outcomes.[20, 26] For the barriers to BIRs, we developed items related to 4 domains, including factors related to the patient, time, systems issues, and providers (nurses, attending physicians, and housestaff physicians).[22, 24, 25] We included our definition of BIRs into the survey instructions. We pilot tested the survey with 3 medicine faculty and 3 nursing staff and, based on our pilot, modified several questions to improve clarity. Primary demographic items in the survey included identification of provider role (nurses, attending physicians, or housestaff physicians) and years in the current role. Respondent preference for the benefits and barriers were investigated on a 7‐point scale (1=lowest response and 7=high response possible). Descriptive text was provided at the extremes (choice 1 and 7), but intermediary values (26) did not have descriptive cues.[27] As an incentive, the end of the survey provided respondents with an option for submitting their name to be entered into a raffle to win 1 of 50, $5 gift certificates to a coffee shop.
Prior to the end of the academic year in June 2013, we sent a survey link via e‐mail to all medicine nursing staff, housestaff physicians, and attending physicians. The email described the study and explained the voluntary nature of the work, and that informed consent would be implied by survey completion. Following the initial e‐mail, 3 additional weekly e‐mail reminders were sent by the lead investigator. The study was approved by the institutional review board at the Pennsylvania State College of Medicine.
Data Analysis
Descriptive statistics were used to examine the characteristics of the 3 respondent groups and combined totals for each survey item. The nonparametric Wilcoxon rank sum test was used to compare the average values between groups (nursing staff vs all physicians, attending physicians vs housestaff physicians) for both sets of survey variables (benefits and barriers). The nonparametric correlation statistical test Spearman rank was used to assess the degree of correlation between respondent groups for both survey variables. The data were analyzed using SAS 9.3 (SAS Institute, Cary, NC) and Stata/IC‐8 (StataCorp, College Station, Texas).
RESULTS
Of the 171 surveys sent, 149 participants completed surveys (response rate 87%). Responses were received from 53/58 nursing staff (91% response), 21/28 attending physicians (75% response), and 75/85 housestaff physicians (88% response). Table 1 describes the participant response demographics.
| Variable | Value |
|---|---|
| |
| Nursing staff, n=58, n (%) | 53 (36) |
| Intermediate care unit, n (%) | 14 (26) |
| General medicine ward, n (%) | 39 (74) |
| All day shifts, n (%) | 25 (47) |
| Mix of day and night shifts, n (%) | 32 (60) |
| Years of experience, mean (SD) | 7.4 (9) |
| Attending physicians, n=28, n (%) | 21 (14) |
| Years since residency graduation, mean (SD) | 10.5 (8) |
| No. of weeks in past year serving as teaching attending, mean (SD) | 9.1(8) |
| Housestaff physicians (n=85), n (%) | 75 (50) |
| Intern, n (%) | 28 (37) |
| Junior resident, n (%) | 25 (33) |
| Senior resident, n (%)a | 22 (29) |
Benefits of BIRs
Respondents' perceptions of the benefits of BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 2). Six of the 7 highest‐ranked benefits were related to communication, coordination, and teamwork, including improves communication between nurses and physicians, improves awareness of clinical issues that need to be addressed, and improves team‐building between nurses and physicians. Lowest‐ranked benefits were related to efficiency, process, and outcomes, including decreases patients' hospital length‐of‐stay, improves timeliness of consultations, and reduces ordering of unnecessary tests and treatments. Comparing mean values among the 3 groups, all 18 items showed statistical differences in response rates (all P values <0.05). Nursing staff reported more favorable ratings than both attending physicians and housestaff physicians for each of the 18 items, whereas attending physicians reported more favorable ratings than housestaff physicians in 16/18 items. The rank order among provider groups showed a high degree of correlation (r=0.92, P<0.001).
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, N=53, Mean (SD) | Attending Physicians, N=21, Mean (SD) | House staff Physicians, N=75, Mean (SD)b |
|---|---|---|---|---|---|
| |||||
| Improves communication between nurses and physicians. | CCT | 6.26 (1.11) | 6.74 (0.59)c | 6.52 (1.03)d | 5.85 (1.26) |
| Improves awareness of clinical issues needing to be addressed. | CCT | 6.05 (1.12) | 6.57 (0.64)c | 5.95 (1.07) | 5.71 (1.26) |
| Improves team‐building between nurses and physicians. | CCT | 6.03 (1.32) | 6.72 (0.60)c | 6.14 (1.11) | 5.52 (1.51) |
| Improves coordination of the patient's care. | CCT | 5.98 (1.34) | 6.60 (0.72)c | 6.00 (1.18) | 5.53 (1.55) |
| Improves nursing contributions to a patient's care plan. | CCT | 5.91 (1.25) | 6.47 (0.77)c | 6.14 (0.85) | 5.44 (1.43) |
| Improves quality of care delivered in our unit. | O | 5.72 (1.42) | 6.34 (0.83)c | 5.81 (1.33) | 5.25 (1.61) |
| Improves appreciation of the roles/contributions of other providers. | CCT | 5.69 (1.49) | 6.36 (0.86)c | 5.90 (1.04) | 5.16 (1.73) |
| Promotes shared decision making between patients and providers. | P | 5.62 (1.51) | 6.43 (0.77)c | 5.57 (1.40) | 5.05 (1.68) |
| Improves patients' satisfaction with their hospitalization. | P, O | 5.53 (1.40) | 6.15 (0.95)c | 5.38 (1.12) | 5.13 (1.58) |
| Provides more respect/dignity to patients. | P | 5.31 (1.55) | 6.23 (0.89)c | 5.10 (1.18) | 4.72 (1.71) |
| Decreases number of pages/phone calls between nurses and physicians. | EP | 5.28 (1.82) | 6.28 (0.93)c | 5.24 (1.30) | 4.57 (2.09) |
| Improves educational opportunities for housestaff/students. | E | 5.07 (1.77) | 6.08 (0.98)c | 4.81 (1.60) | 4.43 (1.93) |
| Improves the efficiency of your work. | EP | 5.01 (1.77) | 6.04 (1.13)c | 4.90 (1.30) | 4.31 (1.92) |
| Improves adherence to evidence‐based guidelines or interventions. | EP | 4.89 (1.79) | 6.06 (0.91)c | 4.00 (1.18) | 4.31 (1.97) |
| Improves the accuracy of your sign‐outs (or reports) to the next shift. | EP | 4.80 (1.99) | 6.30 (0.93)c | 4.05 (1.66) | 3.95 (2.01) |
| Reduces ordering of unnecessary tests and treatments. | O | 4.51 (1.86) | 5.77 (1.15)c | 3.86 (1.11) | 3.8 (1.97) |
| Improves the timeliness of consultations. | EP | 4.28 (1.99) | 5.66 (1.22)c | 3.24 (1.48) | 3.59 (2.02) |
| Decreases patients' hospital length of stay. | O | 4.15 (1.68) | 5.04 (1.24)c | 3.95 (1.16) | 3.57 (1.81) |
Barriers to BIRs
Respondents' perceptions of barriers to BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 3). The 6 highest‐ranked barriers were related to time, including nursing staff have limited time, the time required for bedside nurse‐physician encounters, and coordinating the start time of encounters with arrival of both physicians and nursing. The lowest‐ranked barriers were related to provider‐ and patient‐related factors, including patient lack of comfort with bedside nurse‐physician encounters, attending physicians/housestaff lack bedside skills, and attending physicians lack comfort with bedside nurse‐physician encounters. Comparing mean values between groups, 10 of 21 items showed statistical differences (P<0.05). The rank order among groups showed moderate correlation (nurses‐attending physicians r=0.62, nurses‐housestaff physicians r=0.76, attending physicians‐housestaff physicians r=0.82). A qualitative inspection of disparities among respondent groups highlighted that nursing staff were more likely to rank bedside rounds are not part of the unit's culture lower than physician groups.
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, n=53, Mean (SD) | Attending Physicians, n=21, Mean (SD) | Housestaff Physicians, n=75,b Mean (SD) |
|---|---|---|---|---|---|
| |||||
| Nursing staff have limited time. | T | 4.89 (1.34) | 4.96 (1.27) | 4.86 (1.65) | 4.85 (1.30) |
| Coordinating start time of encounters with arrival of physicians and nursing. | T | 4.80 (1.50) | 4.58 (1.43) | 5.24 (1.45) | 4.84 (1.55) |
| Housestaff have limited time. | T | 4.68 (1.47) | 4.56 (1.26) | 4.24 (1.81) | 4.89 (1.48) |
| Attending physicians have limited time. | T | 4.50 (1.49) | 4.81 (1.34) | 4.33 (1.65) | 4.34 (1.53) |
| Other acutely sick patients in unit. | T | 4.39 (1.42) | 4.79 (1.30)c | 4.52 (1.21) | 4.08 (1.49) |
| Time required for bedside nurse‐physician encounters. | T | 4.32 (1.55) | 4.85 (1.38)c | 3.62 (1.80) | 4.15 (1.49) |
| Lack of use of the pink‐rounding light to alert nursing staff. | S | 3.77 (1.75) | 4.71 (1.70)c | 3.48 (1.86) | 3.19 (1.46) |
| Patient not available (eg, off to test, getting bathed) | S | 3.74 (1.40) | 3.98 (1.28) | 4.52 (1.36)d | 3.35 (1.37) |
| Large team size. | S | 3.64 (1.74) | 3.12 (1.58)c | 3.95 (1.83) | 3.92 (1.77) |
| Patients in dispersed locations (eg, other units or in different hallways). | S | 3.64 (1.77) | 2.77 (1.55)c | 4.52 (1.83) | 4.00 (1.66) |
| Bedside nurse‐physician rounds are not part of the unit's culture. | S | 3.35 (1.94) | 2.25 (1.47)c | 4.76 (1.92) | 3.72 (1.85) |
| Limitations in physical facilities (eg, rooms too small, limited chairs). | S | 3.25 (1.71) | 2.71 (1.72) | 3.33 (1.71) | 3.59 (1.62) |
| Insufficient nurse engagement during bedside nurse‐physician encounters. | PR | 3.24 (1.63) | 2.71 (1.47)c | 3.67 (1.68) | 3.49 (1.65) |
| Patient on contact or respiratory isolation. | S | 3.20 (1.82) | 2.42 (1.67)c | 3.43 (1.63) | 3.69 (1.80) |
| Language barrier between providers and patients. | P | 2.69 (1.37) | 2.77 (1.39) | 2.57 (1.08) | 2.68 (1.43) |
| Privacy/sensitive patient issues. | P | 2.65 (1.45) | 2.27 (1.24) | 2.57 (1.33) | 2.93 (1.56) |
| Housestaff lack comfort with bedside nurse‐physician encounters. | PR | 2.55 (1.49) | 2.48 (1.15) | 2.67 (1.68) | 2.57 (1.65) |
| Nurses lack comfort with bedside nurse‐physician encounters. | PR | 2.45 (1.45) | 2.35 (1.27) | 2.48 (1.66) | 2.51 (1.53) |
| Attending physicians lack comfort with bedside nurse‐physician encounters. | PR | 2.35 (1.38) | 2.33 (1.25) | 2.33 (1.62) | 2.36 (1.41) |
| Attending physician/housestaff lack bedside skills (eg, history, exam). | PR | 2.34 (1.34) | 2.19 (1.19) | 2.85 (1.69) | 2.30 (1.32) |
| Patient lack of comfort with bedside nurse‐physician encounters. | P | 2.33 (1.48) | 2.23 (1.37) | 1.95 (1.32) | 2.5 (1.59) |
DISCUSSION
In this study, we sought to compare perceptions of nurses and physicians on the benefits and barriers to BIRs. Nursing staff ranked each benefit higher than physicians, though rank orders of specific benefits were highly correlated. Highest‐ranked benefits related to coordination and communication more than quality or process benefits. Across groups, the highest‐ranked barriers to BIRs were related to time, whereas the lowest‐ranked factors were related to provider and patient discomfort. These results highlight important similarities and differences in perceptions between front‐line providers.
The highest‐ranked benefits were related to improved interprofessional communication and coordination. Combining interprofessional team members during care delivery allows for integrated understanding of daily care plans and clinical issues, and fosters collaboration and a team‐based atmosphere.[1, 20, 26] The lowest‐ranked benefits were related to more tangible measures, including length of stay, timely consultations, and judicious laboratory ordering. This finding contrasts with the limited literature demonstrating increased efficiency in general medicine units practicing IPCC.[16] These rankings may reflect a poor understanding or self‐assessment of outcome measures by healthcare providers, representing a potential focus for educational initiatives. Future investigations using objective assessment methods of outcomes and collaboration will provide a more accurate understanding of these findings.
The highest‐ranked barriers were related to time and systems issues. Several studies of physician‐based bedside rounds have identified systems‐ and time‐related issues as primary limiting barriers.[22, 24] In units without colocalization of patients and providers, finding receptive times for BIRs can be difficult. Although time‐related issues could be addressed by decreasing patient‐provider ratios, these changes require substantial investment in resources. A reasonable degree of improvement in efficiency and coordination is expected following acclimation to BIRs or by addressing modifiable systems factors to increase this activity. Less costly interventions, such as tailoring provider schedules, prescheduling patient rounding times, and geographic colocalization of patients and providers may be more feasible. However, the clinical microsystems within which medicine patients are cared for are often chaotic and disorganized at the infrastructural and cultural levels, which may be less influenced by surface‐level interventions. Such interventions may be ward specific and require customization to individual team needs.
The lowest‐ranked barriers to BIRs were related to provider‐ and patient‐related factors, including comfort level of patients and providers. Prior work on bedside rounds has identified physicians who are apprehensive about performing bedside rounds, but those who experience this activity are more likely to be comfortable with it.[12, 28] Our results from a culture where BIRs occur on nearly two‐thirds of patients suggest provider discomfort is not a predominant barrier.[22, 29] Additionally, educators have raised concerns about patient discomfort with bedside rounds, but nearly all studies evaluating patients' perspectives reveal patient preference for bedside case presentations over activities occurring in alternative locations.[30, 31, 32] Little work has investigated patient preference for BIRs as per our definition; our participants do not believe patients are discomforted by BIRs, building upon evidence in the literature for patient preferences regarding bedside activities.
Nursing staff perceptions of the benefits and culture related to BIRs were more positive than physicians. We hypothesize several reasons for this disparity. First, nursing staff may have more experience with observing and understanding the positive impact of BIRs and therefore are more likely to understand the positive ramifications. Alternatively, nursing staff may be satisfied with active integration into traditional physician‐centric decisions. Additionally, the professional culture and educational foundation of the nursing culture is based upon a patient‐centered approach and therefore may be more aligned with the goals of BIRs. Last, physicians may have competing priorities, favoring productivity and didactic learning rather than interprofessional collaboration. Further investigation is required to understand differences between nurses and physicians, in addition to other providers integral to BIRs (eg, care coordinators, pharmacists). Regardless, during the implementation of interprofessional collaborative care models, our findings suggest initial challenges, and the focus of educational initiatives may necessitate acclimating physician groups to benefits identified by front‐line nursing staff.
There are several limitations to our study. We investigated the perceptions of medicine nurses and physicians in 1 teaching hospital, limiting generalizability to other specialties, other vital professional groups, and nonteaching hospitals. Additionally, BIRs has been a focus of our hospital for several years. Therefore, perceived barriers may differ in BIRs‐nave hospitals. Second, although pilot‐tested for content, the construct validity of the instrument was not rigorously assessed, and the instrument was not designed to measure benefits and barriers not explicitly identified during pilot testing. Last, although surveys were anonymous, the possibility of social desirability bias exists, thereby limiting accuracy.
For over a century, physician‐led rounds have been the preferred modality for point‐of‐care decision making.[10, 15, 32, 33] BIRs address our growing understanding of patient‐centered care. Future efforts should address the quality of collaboration and current hospital and unit structures hindering patient‐centered IPCC and patient outcomes.
Acknowledgements
The authors thank the medicine nursing staff and physicians for their dedication to patient‐centered care and willingness to participate in this study.
Disclosures: The Department of Medicine at the Penn State Hershey Medical Center provided funding for this project. There are no conflicts of interest to report.
Interprofessional collaborative care (IPCC) involves members from different professions working together to enhance communication, coordination, and healthcare quality.[1, 2, 3] Because several current healthcare policy initiatives include financial incentives for increased quality of care, there has been resultant interest in the implementation of IPCC in healthcare systems.[4, 5] Unfortunately, many hospitals have found IPCC difficult to achieve. Hospital‐based medicine units are complex, time‐constrained environments requiring a high degree of collaboration and mutual decision‐making between nurses, physicians, therapists, pharmacists, care coordinators, and patients. In addition, despite recommendations for interprofessional collaborative care, the implementation and assessment of IPCC within this environment has not been well studied.[6, 7]
On academic internal medicine services, the majority of care decisions occur during rounds. Although rounds provide a common structure, the participants, length, location, and agenda of rounds tend to vary by institution and individual physician preference.[8, 9, 10, 11] Traditionally, ward rounds occur mostly in hallways and conference rooms rather than the patient's bedside.[12] Additionally, during rounds, nurse‐physician collaboration occurs infrequently, estimated at <10% of rounding time.[13] Recently, an increased focus on quality, safety, and collaboration has inspired the investigation and implementation of new methods to increase interprofessional collaboration during rounds, but many of these interventions occurred away from the patient's bedside.[14, 15] One trial of bedside interprofessional rounds (BIRs) by Curley et al. suggested improvements in patient‐level outcomes (cost and length of stay) versus traditional physician‐based rounds.[16] Although interprofessional nurse‐physician rounds at patients' bedsides may represent an ideal process, limited work has investigated this activity.[17]
A prerequisite for successful and sustained integration of BIRs is a shared conceptualization among physicians and nurses regarding the process. Such a shared conceptualization would include perceptions of benefits and barriers to implementation.[18] Currently, such perceptions have not been measured. In this study, we sought to evaluate perceptions of front‐line care providers on inpatient units, specifically nursing staff, attending physicians, and housestaff physicians, regarding the benefits and barriers to BIRs.
METHODS
Study Design and Participants
In June 2013, we performed a cross‐sectional assessment of front‐line providers caring for patients on the internal medicine services in our academic hospital. Participants included medicine nursing staff in acute care and intermediate care units, medicine and combined medicine‐pediatrics housestaff physicians, and general internal medicine faculty physicians who supervised the housestaff physicians.
Study Setting
The study was conducted at a 378‐bed, university‐based, acute care teaching hospital in central Pennsylvania. There are a total of 64 internal medicine beds located in2 units, a general medicine unit (44 beds, staffed by 60 nurses, nurse‐to‐patient ratio 1:4) and an intermediate care unit (20 beds, staffed by 41 nurses, nurse‐to‐patient ratio 1:3). Both units are staffed by the general internal medicine physician teams. The academic medicine residency program consists of 69 internal medicine housestaff and 14 combined internal medicine‐pediatrics housestaff. Five teams, organized into 3 academic teaching teams and 2 nonteaching teams, provide care for all patients admitted to the medicine units. Teaching teams consist of 1 junior (postgraduate year [PGY]2) or senior (PGY34) housestaff member, 2 interns (PGY1), 2 medical students, and 1 attending physician.
There are several main features of BIRs in our medicine units. The rounding team of physicians alerts the assigned nurse about the start of rounds. In our main medicine unit, each doorway is equipped with a light that allows the physician team to indicate the start of the BIRs encounter. Case presentations by trainees occur either in the hallway or bedside, at the discretion of the attending physician. During bedside encounters, nurses typically contribute to the discussion about clinical status, decision making, patient concerns, and disposition. Patients are encouraged to contribute to the discussion and are provided the opportunity to ask questions.
For the purposes of this study, we specifically defined BIRs as: encounters that include the team of providers, at least 2 physicians plus a nurse or other care provider, discussing the case at the patient's bedside. In our prior work performed during the same time period as this study, we used the same definition to examine the incidence of and time spent in BIRs in both of our medicine units.[19] We found that 63% to 81% of patients in both units received BIRs. As a result, we assumed all nursing staff, attending physicians, and housestaff physicians had experienced this process, and their responses to this survey were contextualized in these experiences.
Survey Instrument
We developed a survey instrument specifically for this study. We derived items primarily from our prior qualitative work on physician‐based team bedside rounds and a literature review.[20, 21, 22, 23, 24, 25] For the benefits to BIRs, we developed items related to 5 domains, including factors related to the patient, education, communication/coordination/teamwork, efficiency and process, and outcomes.[20, 26] For the barriers to BIRs, we developed items related to 4 domains, including factors related to the patient, time, systems issues, and providers (nurses, attending physicians, and housestaff physicians).[22, 24, 25] We included our definition of BIRs into the survey instructions. We pilot tested the survey with 3 medicine faculty and 3 nursing staff and, based on our pilot, modified several questions to improve clarity. Primary demographic items in the survey included identification of provider role (nurses, attending physicians, or housestaff physicians) and years in the current role. Respondent preference for the benefits and barriers were investigated on a 7‐point scale (1=lowest response and 7=high response possible). Descriptive text was provided at the extremes (choice 1 and 7), but intermediary values (26) did not have descriptive cues.[27] As an incentive, the end of the survey provided respondents with an option for submitting their name to be entered into a raffle to win 1 of 50, $5 gift certificates to a coffee shop.
Prior to the end of the academic year in June 2013, we sent a survey link via e‐mail to all medicine nursing staff, housestaff physicians, and attending physicians. The email described the study and explained the voluntary nature of the work, and that informed consent would be implied by survey completion. Following the initial e‐mail, 3 additional weekly e‐mail reminders were sent by the lead investigator. The study was approved by the institutional review board at the Pennsylvania State College of Medicine.
Data Analysis
Descriptive statistics were used to examine the characteristics of the 3 respondent groups and combined totals for each survey item. The nonparametric Wilcoxon rank sum test was used to compare the average values between groups (nursing staff vs all physicians, attending physicians vs housestaff physicians) for both sets of survey variables (benefits and barriers). The nonparametric correlation statistical test Spearman rank was used to assess the degree of correlation between respondent groups for both survey variables. The data were analyzed using SAS 9.3 (SAS Institute, Cary, NC) and Stata/IC‐8 (StataCorp, College Station, Texas).
RESULTS
Of the 171 surveys sent, 149 participants completed surveys (response rate 87%). Responses were received from 53/58 nursing staff (91% response), 21/28 attending physicians (75% response), and 75/85 housestaff physicians (88% response). Table 1 describes the participant response demographics.
| Variable | Value |
|---|---|
| |
| Nursing staff, n=58, n (%) | 53 (36) |
| Intermediate care unit, n (%) | 14 (26) |
| General medicine ward, n (%) | 39 (74) |
| All day shifts, n (%) | 25 (47) |
| Mix of day and night shifts, n (%) | 32 (60) |
| Years of experience, mean (SD) | 7.4 (9) |
| Attending physicians, n=28, n (%) | 21 (14) |
| Years since residency graduation, mean (SD) | 10.5 (8) |
| No. of weeks in past year serving as teaching attending, mean (SD) | 9.1(8) |
| Housestaff physicians (n=85), n (%) | 75 (50) |
| Intern, n (%) | 28 (37) |
| Junior resident, n (%) | 25 (33) |
| Senior resident, n (%)a | 22 (29) |
Benefits of BIRs
Respondents' perceptions of the benefits of BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 2). Six of the 7 highest‐ranked benefits were related to communication, coordination, and teamwork, including improves communication between nurses and physicians, improves awareness of clinical issues that need to be addressed, and improves team‐building between nurses and physicians. Lowest‐ranked benefits were related to efficiency, process, and outcomes, including decreases patients' hospital length‐of‐stay, improves timeliness of consultations, and reduces ordering of unnecessary tests and treatments. Comparing mean values among the 3 groups, all 18 items showed statistical differences in response rates (all P values <0.05). Nursing staff reported more favorable ratings than both attending physicians and housestaff physicians for each of the 18 items, whereas attending physicians reported more favorable ratings than housestaff physicians in 16/18 items. The rank order among provider groups showed a high degree of correlation (r=0.92, P<0.001).
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, N=53, Mean (SD) | Attending Physicians, N=21, Mean (SD) | House staff Physicians, N=75, Mean (SD)b |
|---|---|---|---|---|---|
| |||||
| Improves communication between nurses and physicians. | CCT | 6.26 (1.11) | 6.74 (0.59)c | 6.52 (1.03)d | 5.85 (1.26) |
| Improves awareness of clinical issues needing to be addressed. | CCT | 6.05 (1.12) | 6.57 (0.64)c | 5.95 (1.07) | 5.71 (1.26) |
| Improves team‐building between nurses and physicians. | CCT | 6.03 (1.32) | 6.72 (0.60)c | 6.14 (1.11) | 5.52 (1.51) |
| Improves coordination of the patient's care. | CCT | 5.98 (1.34) | 6.60 (0.72)c | 6.00 (1.18) | 5.53 (1.55) |
| Improves nursing contributions to a patient's care plan. | CCT | 5.91 (1.25) | 6.47 (0.77)c | 6.14 (0.85) | 5.44 (1.43) |
| Improves quality of care delivered in our unit. | O | 5.72 (1.42) | 6.34 (0.83)c | 5.81 (1.33) | 5.25 (1.61) |
| Improves appreciation of the roles/contributions of other providers. | CCT | 5.69 (1.49) | 6.36 (0.86)c | 5.90 (1.04) | 5.16 (1.73) |
| Promotes shared decision making between patients and providers. | P | 5.62 (1.51) | 6.43 (0.77)c | 5.57 (1.40) | 5.05 (1.68) |
| Improves patients' satisfaction with their hospitalization. | P, O | 5.53 (1.40) | 6.15 (0.95)c | 5.38 (1.12) | 5.13 (1.58) |
| Provides more respect/dignity to patients. | P | 5.31 (1.55) | 6.23 (0.89)c | 5.10 (1.18) | 4.72 (1.71) |
| Decreases number of pages/phone calls between nurses and physicians. | EP | 5.28 (1.82) | 6.28 (0.93)c | 5.24 (1.30) | 4.57 (2.09) |
| Improves educational opportunities for housestaff/students. | E | 5.07 (1.77) | 6.08 (0.98)c | 4.81 (1.60) | 4.43 (1.93) |
| Improves the efficiency of your work. | EP | 5.01 (1.77) | 6.04 (1.13)c | 4.90 (1.30) | 4.31 (1.92) |
| Improves adherence to evidence‐based guidelines or interventions. | EP | 4.89 (1.79) | 6.06 (0.91)c | 4.00 (1.18) | 4.31 (1.97) |
| Improves the accuracy of your sign‐outs (or reports) to the next shift. | EP | 4.80 (1.99) | 6.30 (0.93)c | 4.05 (1.66) | 3.95 (2.01) |
| Reduces ordering of unnecessary tests and treatments. | O | 4.51 (1.86) | 5.77 (1.15)c | 3.86 (1.11) | 3.8 (1.97) |
| Improves the timeliness of consultations. | EP | 4.28 (1.99) | 5.66 (1.22)c | 3.24 (1.48) | 3.59 (2.02) |
| Decreases patients' hospital length of stay. | O | 4.15 (1.68) | 5.04 (1.24)c | 3.95 (1.16) | 3.57 (1.81) |
Barriers to BIRs
Respondents' perceptions of barriers to BIRs are shown by mean value (between 1 and 7) for the total respondent pool and by each participant group (Table 3). The 6 highest‐ranked barriers were related to time, including nursing staff have limited time, the time required for bedside nurse‐physician encounters, and coordinating the start time of encounters with arrival of both physicians and nursing. The lowest‐ranked barriers were related to provider‐ and patient‐related factors, including patient lack of comfort with bedside nurse‐physician encounters, attending physicians/housestaff lack bedside skills, and attending physicians lack comfort with bedside nurse‐physician encounters. Comparing mean values between groups, 10 of 21 items showed statistical differences (P<0.05). The rank order among groups showed moderate correlation (nurses‐attending physicians r=0.62, nurses‐housestaff physicians r=0.76, attending physicians‐housestaff physicians r=0.82). A qualitative inspection of disparities among respondent groups highlighted that nursing staff were more likely to rank bedside rounds are not part of the unit's culture lower than physician groups.
| Survey Itema | Item Domain | Total, N=149, Mean (SD) | Nurses, n=53, Mean (SD) | Attending Physicians, n=21, Mean (SD) | Housestaff Physicians, n=75,b Mean (SD) |
|---|---|---|---|---|---|
| |||||
| Nursing staff have limited time. | T | 4.89 (1.34) | 4.96 (1.27) | 4.86 (1.65) | 4.85 (1.30) |
| Coordinating start time of encounters with arrival of physicians and nursing. | T | 4.80 (1.50) | 4.58 (1.43) | 5.24 (1.45) | 4.84 (1.55) |
| Housestaff have limited time. | T | 4.68 (1.47) | 4.56 (1.26) | 4.24 (1.81) | 4.89 (1.48) |
| Attending physicians have limited time. | T | 4.50 (1.49) | 4.81 (1.34) | 4.33 (1.65) | 4.34 (1.53) |
| Other acutely sick patients in unit. | T | 4.39 (1.42) | 4.79 (1.30)c | 4.52 (1.21) | 4.08 (1.49) |
| Time required for bedside nurse‐physician encounters. | T | 4.32 (1.55) | 4.85 (1.38)c | 3.62 (1.80) | 4.15 (1.49) |
| Lack of use of the pink‐rounding light to alert nursing staff. | S | 3.77 (1.75) | 4.71 (1.70)c | 3.48 (1.86) | 3.19 (1.46) |
| Patient not available (eg, off to test, getting bathed) | S | 3.74 (1.40) | 3.98 (1.28) | 4.52 (1.36)d | 3.35 (1.37) |
| Large team size. | S | 3.64 (1.74) | 3.12 (1.58)c | 3.95 (1.83) | 3.92 (1.77) |
| Patients in dispersed locations (eg, other units or in different hallways). | S | 3.64 (1.77) | 2.77 (1.55)c | 4.52 (1.83) | 4.00 (1.66) |
| Bedside nurse‐physician rounds are not part of the unit's culture. | S | 3.35 (1.94) | 2.25 (1.47)c | 4.76 (1.92) | 3.72 (1.85) |
| Limitations in physical facilities (eg, rooms too small, limited chairs). | S | 3.25 (1.71) | 2.71 (1.72) | 3.33 (1.71) | 3.59 (1.62) |
| Insufficient nurse engagement during bedside nurse‐physician encounters. | PR | 3.24 (1.63) | 2.71 (1.47)c | 3.67 (1.68) | 3.49 (1.65) |
| Patient on contact or respiratory isolation. | S | 3.20 (1.82) | 2.42 (1.67)c | 3.43 (1.63) | 3.69 (1.80) |
| Language barrier between providers and patients. | P | 2.69 (1.37) | 2.77 (1.39) | 2.57 (1.08) | 2.68 (1.43) |
| Privacy/sensitive patient issues. | P | 2.65 (1.45) | 2.27 (1.24) | 2.57 (1.33) | 2.93 (1.56) |
| Housestaff lack comfort with bedside nurse‐physician encounters. | PR | 2.55 (1.49) | 2.48 (1.15) | 2.67 (1.68) | 2.57 (1.65) |
| Nurses lack comfort with bedside nurse‐physician encounters. | PR | 2.45 (1.45) | 2.35 (1.27) | 2.48 (1.66) | 2.51 (1.53) |
| Attending physicians lack comfort with bedside nurse‐physician encounters. | PR | 2.35 (1.38) | 2.33 (1.25) | 2.33 (1.62) | 2.36 (1.41) |
| Attending physician/housestaff lack bedside skills (eg, history, exam). | PR | 2.34 (1.34) | 2.19 (1.19) | 2.85 (1.69) | 2.30 (1.32) |
| Patient lack of comfort with bedside nurse‐physician encounters. | P | 2.33 (1.48) | 2.23 (1.37) | 1.95 (1.32) | 2.5 (1.59) |
DISCUSSION
In this study, we sought to compare perceptions of nurses and physicians on the benefits and barriers to BIRs. Nursing staff ranked each benefit higher than physicians, though rank orders of specific benefits were highly correlated. Highest‐ranked benefits related to coordination and communication more than quality or process benefits. Across groups, the highest‐ranked barriers to BIRs were related to time, whereas the lowest‐ranked factors were related to provider and patient discomfort. These results highlight important similarities and differences in perceptions between front‐line providers.
The highest‐ranked benefits were related to improved interprofessional communication and coordination. Combining interprofessional team members during care delivery allows for integrated understanding of daily care plans and clinical issues, and fosters collaboration and a team‐based atmosphere.[1, 20, 26] The lowest‐ranked benefits were related to more tangible measures, including length of stay, timely consultations, and judicious laboratory ordering. This finding contrasts with the limited literature demonstrating increased efficiency in general medicine units practicing IPCC.[16] These rankings may reflect a poor understanding or self‐assessment of outcome measures by healthcare providers, representing a potential focus for educational initiatives. Future investigations using objective assessment methods of outcomes and collaboration will provide a more accurate understanding of these findings.
The highest‐ranked barriers were related to time and systems issues. Several studies of physician‐based bedside rounds have identified systems‐ and time‐related issues as primary limiting barriers.[22, 24] In units without colocalization of patients and providers, finding receptive times for BIRs can be difficult. Although time‐related issues could be addressed by decreasing patient‐provider ratios, these changes require substantial investment in resources. A reasonable degree of improvement in efficiency and coordination is expected following acclimation to BIRs or by addressing modifiable systems factors to increase this activity. Less costly interventions, such as tailoring provider schedules, prescheduling patient rounding times, and geographic colocalization of patients and providers may be more feasible. However, the clinical microsystems within which medicine patients are cared for are often chaotic and disorganized at the infrastructural and cultural levels, which may be less influenced by surface‐level interventions. Such interventions may be ward specific and require customization to individual team needs.
The lowest‐ranked barriers to BIRs were related to provider‐ and patient‐related factors, including comfort level of patients and providers. Prior work on bedside rounds has identified physicians who are apprehensive about performing bedside rounds, but those who experience this activity are more likely to be comfortable with it.[12, 28] Our results from a culture where BIRs occur on nearly two‐thirds of patients suggest provider discomfort is not a predominant barrier.[22, 29] Additionally, educators have raised concerns about patient discomfort with bedside rounds, but nearly all studies evaluating patients' perspectives reveal patient preference for bedside case presentations over activities occurring in alternative locations.[30, 31, 32] Little work has investigated patient preference for BIRs as per our definition; our participants do not believe patients are discomforted by BIRs, building upon evidence in the literature for patient preferences regarding bedside activities.
Nursing staff perceptions of the benefits and culture related to BIRs were more positive than physicians. We hypothesize several reasons for this disparity. First, nursing staff may have more experience with observing and understanding the positive impact of BIRs and therefore are more likely to understand the positive ramifications. Alternatively, nursing staff may be satisfied with active integration into traditional physician‐centric decisions. Additionally, the professional culture and educational foundation of the nursing culture is based upon a patient‐centered approach and therefore may be more aligned with the goals of BIRs. Last, physicians may have competing priorities, favoring productivity and didactic learning rather than interprofessional collaboration. Further investigation is required to understand differences between nurses and physicians, in addition to other providers integral to BIRs (eg, care coordinators, pharmacists). Regardless, during the implementation of interprofessional collaborative care models, our findings suggest initial challenges, and the focus of educational initiatives may necessitate acclimating physician groups to benefits identified by front‐line nursing staff.
There are several limitations to our study. We investigated the perceptions of medicine nurses and physicians in 1 teaching hospital, limiting generalizability to other specialties, other vital professional groups, and nonteaching hospitals. Additionally, BIRs has been a focus of our hospital for several years. Therefore, perceived barriers may differ in BIRs‐nave hospitals. Second, although pilot‐tested for content, the construct validity of the instrument was not rigorously assessed, and the instrument was not designed to measure benefits and barriers not explicitly identified during pilot testing. Last, although surveys were anonymous, the possibility of social desirability bias exists, thereby limiting accuracy.
For over a century, physician‐led rounds have been the preferred modality for point‐of‐care decision making.[10, 15, 32, 33] BIRs address our growing understanding of patient‐centered care. Future efforts should address the quality of collaboration and current hospital and unit structures hindering patient‐centered IPCC and patient outcomes.
Acknowledgements
The authors thank the medicine nursing staff and physicians for their dedication to patient‐centered care and willingness to participate in this study.
Disclosures: The Department of Medicine at the Penn State Hershey Medical Center provided funding for this project. There are no conflicts of interest to report.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009(3):CD000072.
- . Teamswork! Hosp Health Netw. 2012;86(3):24–27, 21.
- , , , . The coming of age for interprofessional education and practice. Am J Med. 2013;126(4):284–288.
- , . Payment incentives and integrated care delivery: levers for health system reform and cost containment. Inquiry. 2011;48(4):277–287.
- . Payment reform and the mission of academic medical centers. N Engl J Med. 2010;363(19):1784–1786.
- Josiah Macy Jr. Foundation. Transforming patient care: aligning interprofessional education and clinical practice redesign. In: Proceedings of the Josiah Macy Jr. Foundation Conference; January 17–20, 2013; Atlanta, GA.
- , , , . Bridging the quality chasm: interprofessional teams to the rescue? Am J Med. 2013;126(4):276–277.
- . Attending rounds: guidelines for teaching on the wards. J Gen Intern Med. 1992;7(1):68–75.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PloS One. 2010;5(6):e11246.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275–280.
- , . The challenge of innovation implementation. Acad Manage Rev. 1996;21(4):1055–1080.
- , , , . Ocular dipping in creutzfeldt‐jakob disease. J Clin Neurol. 2014;10(2):162–165.
- , , , et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326–333.
- , , , et al. The art of bedside rounds: a multi‐center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412–420.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , . Bedside teaching in medical education: a literature review. Perspec Med Educ. 2014;3(2):76–88.
- , , . Impediments to bed‐side teaching. Med Educ. 1998;32(2):159–162.
- , , , . Whither bedside teaching? A focus‐group study of clinical teachers. Acad Med. 2003;78(4):384–390.
- , . Staff preference for multidisciplinary rounding practices in the critical care setting. 2011. Paper presented at: Design July 6–10, 2011. Boston, MA. Available at: http://www.designandhealth.com/uploaded/documents/Awards‐and‐events/WCDH2011/Presentations/Friday/Session‐8/DianaAnderson.pdf. Accessed July 6, 2014.
- , . Health Measurement Scales: A Practical Guide to Their Development and Use. 2nd ed. New York, NY: Oxford University Press; 1995.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , , . The positive impact of portfolios on health care assistants' clinical practice. J Eval Clin Pract. 2008;14(1):172–174.
- , , , . The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273–1275.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009(3):CD000072.
- . Teamswork! Hosp Health Netw. 2012;86(3):24–27, 21.
- , , , . The coming of age for interprofessional education and practice. Am J Med. 2013;126(4):284–288.
- , . Payment incentives and integrated care delivery: levers for health system reform and cost containment. Inquiry. 2011;48(4):277–287.
- . Payment reform and the mission of academic medical centers. N Engl J Med. 2010;363(19):1784–1786.
- Josiah Macy Jr. Foundation. Transforming patient care: aligning interprofessional education and clinical practice redesign. In: Proceedings of the Josiah Macy Jr. Foundation Conference; January 17–20, 2013; Atlanta, GA.
- , , , . Bridging the quality chasm: interprofessional teams to the rescue? Am J Med. 2013;126(4):276–277.
- . Attending rounds: guidelines for teaching on the wards. J Gen Intern Med. 1992;7(1):68–75.
- , . Teaching at the bedside: a new model. Med Teach. 2003;25(2):127–130.
- . On bedside teaching. Ann Intern Med. 1997;126(3):217–220.
- , , , . Relationships of the location and content of rounds to specialty, institution, patient‐census, and team size. PloS One. 2010;5(6):e11246.
- , , , . Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105–110.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , . Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073–1079.
- , , . A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 suppl):AS4–AS12.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275–280.
- , . The challenge of innovation implementation. Acad Manage Rev. 1996;21(4):1055–1080.
- , , , . Ocular dipping in creutzfeldt‐jakob disease. J Clin Neurol. 2014;10(2):162–165.
- , , , et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326–333.
- , , , et al. The art of bedside rounds: a multi‐center qualitative study of strategies used by experienced bedside teachers. J Gen Intern Med. 2013;28(3):412–420.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , . Bedside teaching in medical education: a literature review. Perspec Med Educ. 2014;3(2):76–88.
- , , . Impediments to bed‐side teaching. Med Educ. 1998;32(2):159–162.
- , , , . Whither bedside teaching? A focus‐group study of clinical teachers. Acad Med. 2003;78(4):384–390.
- , . Staff preference for multidisciplinary rounding practices in the critical care setting. 2011. Paper presented at: Design July 6–10, 2011. Boston, MA. Available at: http://www.designandhealth.com/uploaded/documents/Awards‐and‐events/WCDH2011/Presentations/Friday/Session‐8/DianaAnderson.pdf. Accessed July 6, 2014.
- , . Health Measurement Scales: A Practical Guide to Their Development and Use. 2nd ed. New York, NY: Oxford University Press; 1995.
- , , . Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341–346.
- , , , , , . The positive impact of portfolios on health care assistants' clinical practice. J Eval Clin Pract. 2008;14(1):172–174.
- , , , . The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273–1275.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792–798.
- . Bedside rounds revisited. N Engl J Med. 1997;336(16):1174–1175.
© 2014 Society of Hospital Medicine
Inpatient Sleep Aid Utilization
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).
DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).

DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
Sleep is known to be poor among hospitalized patients for many reasons.[1] Patients may have pain, dyspnea, or other discomforts that prevent sleep. Diagnostic and therapeutic procedures, including medication administration and routine nursing care, may take place during normal sleep times. Environmental factors such as noise and light frequently remain at daytime levels during normal sleep times.[2] In response, patients frequently request pharmacological sleep aids. Unfortunately, the use of sleep medications has been linked to clinically relevant and detrimental outcomes such as delirium and falls, particularly in the elderly. For example, in the landmark study by Inouye et al., a multicomponent intervention was used to successfully reduce delirium in older (>70 years) hospitalized patients.[3] One of the successful components was nonpharmacological sleep promotion, which reduced the use of pharmacological sleep aids from 46% to 35% of patients. Most recently, Kolla and colleagues found zolpidem tartrate use to be a risk factor (odds ratio 4.37) for inpatient falls, a known risk factor for morbidity and increased healthcare costs.[4]
The scope of recent pharmacological sleep aid use in the inpatient setting is not well described. Frighetto and colleagues described the pattern of in‐hospital drug use more than 12 years ago, before the concerns above were described, with 29% of patients receiving a medication for sleep, mostly benzodiazepines.[5] In 2008, Bartick and colleagues also found a very high rate of sleep aid use (42% of patients), but additionally described in 2010 an intervention to minimize sleep disruption that successfully reduced sleep aid use by 38%.[6] Both concerns over side effects and sleep promotion efforts might have reduced the current rate of medication use. Therefore, we sought to evaluate the prescription and administration of pharmacological sleep aids in general medical and surgical inpatients at our institution. Using our electronic medical records, including preadmission and discharge medication records, we assessed new and continued usage of medications for sleep complaints following hospital admission.
METHODS
Patients and Design
Records were reviewed for all adult patient (18 years or older) admissions to 1 of 4 units (2 general medicine and 2 general surgical units) from January 1, 2013 to February 28, 2013. These units do not have specific policies to promote sleep, such as nocturnal noise and light reduction or clustering of care. Brigham and Women's Hospital (BWH) is a 793‐bed university‐affiliated teaching hospital. Approval for this retrospective chart review study was obtained from the Partners Healthcare Institutional Review Board.
BWH uses an in‐house electronic health record system, which gathers information from a wider healthcare system (Partners Healthcare). Medications, problem lists, and allergies are available from within‐system providers and prior encounters. Admitting physicians are also required to document a preadmission medication list. A computerized physician order entry (CPOE) system is used for all medication orders. Although standardized admission order sets are used, none of these sets contains a pharmacological sleep aid. There is decision support for geriatric patients (age >65 years) that may recommend reduced starting doses for some medications.[7]
Medications Monitored for Treatment of Sleep Complaints
Using our electronic medication ordering and administration system, each patient admission was reviewed for any medication that might be used for treatment of sleep complaints. The list of sleep medications was based on those commonly used for the outpatient treatment of insomnia, as well as others included based on the authors' experience as clinical inpatient pharmacists.[8, 9, 10] Admissions were reviewed for the following medications: first generation antihistamines (diphenhydramine, hydroxyzine), tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), serotonin‐norepinephrine reuptake inhibitor antidepressants (mirtazapine, trazodone, nefazodone), melatonin agonists (ramelteon), nonbenzodiazepine hypnotics (zolpidem tartrate, eszopiclone), benzodiazepines (oxazepam, temazepam, lorazepam, triazolam, diazepam), typical antipsychotics (haloperidol, fluphenazine, thioridazine, chlorpromazine), and atypical antipsychotics (quetiapine fumarate, ziprasidone, olanzapine, risperidone, aripiprazole). Melatonin, which is not regulated by the US Food and Drug Administration (FDA), cannot be prescribed using our CPOE system.
Determination of Medication Administration for Treatment of Sleep Complaints
The charts of patients receiving 1 or more of these monitored medications were then reviewed by the authors to determine if the medication was indeed prescribed for insomnia/sleep. Chart documents reviewed were the patient's problem list from outpatient provider notes; admission note, including past medical history and home medications; the preadmission medication list; and the inpatient daily progress note. The medication was considered to be used for sleep complaints (as opposed to another indication) when any of the additional following inclusion criteria were met: the medication was part of the patient's home medication regimen for insomnia, the medication order indicated that the medication was for insomnia/difficulty sleeping, or the medication was administered without a specific indication between the hours of 6 pm and 6 am. The medication was not considered to be used primarily as a sleep aid if any of the following were present (exclusion criteria): utilization for an as needed reason including anxiety, agitation, itching, nausea, muscle spasm; utilization for a documented disorder including depression, anxiety, schizophrenia, bipolar disorder, alcohol withdrawal, or epilepsy; intramuscular administration of olanzapine or ziprasidone; or topical administration of diphenhydramine.
Medication Administration Characteristics
For each medication that was administered for difficulty sleeping, the following data were documented: dose in milligrams, route of administration, time of administration, administration timing directions (eg, times 1 [x1], as needed [PRN], or standing), an increase or decrease in dose during hospital stay, documentation of the medication in discharge notes or discharge medications, and documentation of development of an allergy or adverse reaction due to the medication. Changes in dose were recorded. If a patient received more than 1 study medication, each individual medication and dose was evaluated for inclusion in the study analysis. Other data collected included the total number of days of exposure to each sleep aid during admission, the date of initiation, the total number of days of exposure to more than 1 sleep aid during admission, and the location of initiation of each individual sleep aid (eg, intensive care unit, medical floor). Appropriate time of administration was defined as sleep aid administration between 9 pm and 12 am.
RESULTS
Patients
During the 59‐day study period, there were 642 patients admitted to the study units. Two hundred seventy‐six patients received 1 of the monitored medications; however, 106 patients received the medication for an indication other than insomnia/difficulty sleeping. In 2 patients, incomplete records prevented ascertainment of the motivation for using the monitored medication. Thus, 168 patients (26.2%) were determined to have received a medication for sleep complaints and were included in the study analysis (Figure 1). Table 1 lists the characteristics of the 168 patients, of whom 10 had a prior documented sleep disorder such as insomnia (6 patients), restless leg syndrome (1 patient), and obstructive sleep apnea (3 patients). The rate of sleep medication use was lower, though not drastically so, in patients 65 years of age compared to those <65 years of age.

| Patients | Value |
|---|---|
| |
| Age, y | 57.919.8a |
| Age 65 years | 70 (41.7) |
| Age 64 years | 98 (58.3) |
| Female, n (%) | 97 (57.7) |
| Ethnicity, n (%) | |
| Caucasian | 114 (67.9) |
| Black | 18 (10.7) |
| Hispanic | 13 (7.7) |
| Other | 23 (13.7) |
| Admitted to floor from: | |
| Emergency department | 95 (56.5) |
| Operating room | 30 (17.9) |
| Transferred from outside hospital | 35 (20.8) |
| Intensive care unit | 8 (4.8) |
| Admission service, n (%) | |
| Medical | 109 (64.9) |
| Surgical | 59 (35.1) |
| Hospital length of stay | 10.516.0a |
| Known sleep disorder | |
| Insomnia | 6 (3.6) |
| Restless leg syndrome | 1 (0.6) |
| Obstructive sleep apnea | 3(1.8) |
Medications Used for Treatment of Sleep Complaints
Of the 25 monitored medications, 13 were administered to patients for sleep during the study period. The most commonly administered medications (percent of patients, median dose, absolute dose range) were trazodone (30.4%, 50 mg, 12.5450 mg), lorazepam (24.4%, 0.5 mg, 0.25 mg2 mg), and zolpidem tartrate (17.9%, 10 mg, 2.5 mg10 mg) (Table 2). As only a few of these medications (diphenhydramine, ramelteon, temazepam, triazolam, zolpidem) have a formal FDA indication for insomnia, most patients (72%) were treated using an off‐label medication. Although the types of medication used did not vary substantially between young and old patients, the median doses and ranges were lower in the elderly. Admitting service did not substantially influence the medication or dose chosen for sleep complaints (data not shown).
| Medication | All Patients, N=168, n (%) | Patients <65 Years Old, n=98, n (%) | Patients >65 Years Old, n=70, n (%) |
|---|---|---|---|
| |||
| Trazodoneb | 51 (30.4) | 29 (29.6) | 22 (31.4) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5450 | 25450 | 12.5200 |
| Lorazepamc | 41 (24.4) | 24 (24.5) | 17 (24.3) |
| Median dose | 0.5 | 1 | 0.25 |
| Dose range | 0.252 | 0.252 | 0.251 |
| Zolpidem tartratec | 30 (17.9) | 20 (20.4) | 10 (14.3) |
| Median dose | 10 | 10 | 5 |
| Dose range | 2.510 | 2.510 | 2.510 |
| Quetiapine fumarate | 21 (12.5) | 9 (9.2) | 12 (17.1) |
| Median dose | 50 | 50 | 25 |
| Dose range | 12.5300 | 12.5300 | 12.5100 |
| Haloperidol | 18 (10.7) | 7 (7.1) | 11 (15.7) |
| Median dose | 1 | 5 | 1 |
| Dose range | 0.2510 | 0.510 | 0.251 |
| Diphenhydraminec | 16 (9.5) | 12 (12.2) | 4 (5.7) |
| Median dose | 25 | 25 | 12.5 |
| Dose range | 12.550 | 12.550 | 12.525 |
| Mirtazapine | 7 (4.2) | 3 (3.1) | 4 (5.7) |
| Median dose | 15 | 30 | 7.5 |
| Dose range | 7.545 | 7.530 | 7.545 |
| Olanzapine | 5 (3.6) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 2.5 |
| Dose range | 2.512.5 | 512.5 | 2.52.5 |
| Amitriptyline | 5 (3.0) | 4 (4.1) | 1 (1.4) |
| Median dose | 25 | 25 | 25 |
| Dose range | 25100 | 25100 | |
| Diazepam | 5 (3.0) | 3 (3.1) | 2 (2.9) |
| Median dose | 5 | 5 | 10 |
| Dose range | 510 | ||
| Oxazepam | 2 (1.2) | 0 | 2 (2.9) |
| Median dose | 10 | 10 | |
| Dose range | 1010 | 1010 | |
| Temazepamc | 1 (0.6) | 0 | 1 (1.4) |
| Median dose | 15 | 15 | |
| Dose range | |||
| Hydroxyzine | 1 (0.6) | 1 (1.0) | 0 |
| Median dose | 50 | 50 | |
| Dose range | |||
Initiation, Duration, and Changes to Medications for Treatment of Sleep Complaints
None of the medication orders were part of a standardized order set. The sleep medication for the majority of patients (n=108, 64.3%) was initiated during their time on the study units (general inpatient hospital wards). Most patients (n=90, 53.6%) were ordered for a sleep aid within 48 hours of admission to the hospital. The patients who received medication for sleep had a median length of stay of 6 (interquartile range [IQR], 311) days on the study units, and received medication for a median of 2 (IQR, 15) days (Table 3). One hundred twenty patients (71.4%) were continued on a sleep aid until discharge. Essentially the same percentage of patients experienced an increase (14.9%) or decrease (14.9%) in the dose of their sleep aid during admission. Although most patients received 1 medication for sleep throughout their admission, almost one‐quarter of the patients were given 2 or more medications for sleep during their admission to the floor, sometimes including multiple medications on the same night.
| Variable | Patients, N=168 |
|---|---|
| |
| Total sleep aids each patient received during hospital length of stay, n (%) | |
| 1 sleep aid | 132 (78.6) |
| 2 sleep aids | 28 (16.7) |
| 3 sleep aids | 6 (3.6) |
| 4 sleep aids | 2 (1.2) |
| Patients who received multiple sleep aids for 1 or more days during hospital length of stay, n (%) | 20 (11.9) |
| Length of stay on study units, d | 6 [310.75]a |
| Length of sleep aid therapy on study units, d | 2 [15]a |
Of patients not known to be previously on sleep aid therapy, 40 (34.4%) of them were discharged home on a sleep aid.
Medication Administration Characteristics
Sleep medications were prescribed most frequently as standing orders (63.7%), rather than x1 (17.7%) or PRN (18.6%). Although the majority of sleep medications were administered between the hours of 9 pm and 12 am, more than 35% of doses were given outside of this range (Figure 2).

DISCUSSION
Our results confirm the continued frequent use of pharmacological sleep aids in the hospital setting, even in the elderly, despite recent concerns regarding the use of certain sleep medications. Additional, novel findings of our study are: (1) medications used for sleep complaints in the hospital are frequently those without a formal indication for sleep, (2) medications for sleep complaints are frequently administered too early or too late at night to be consistent with good sleep hygiene, and (3) many patients never previously on a medication for insomnia are discharged with a prescription for a sleep aid.
Despite recent warnings regarding side effects especially in the elderly, our rate of medication use has only slightly improved from prior reports from more than a decade ago.[3, 5] This high rate of use is likely due to a combination of patient, clinician, and environmental factors. In our sample, the sleep aid orders were not part of an order set; thus, the orders were either the result of patient request or in response to a patient report of poor sleep. Patients and clinicians may perceive medications for sleep as highly effective and safe, despite evidence to the contrary. Another factor is that both patients and clinicians may be unaware of nonpharmacological interventions that might improve sleep. Similarly, hospital environmental factors (noise, light) may be so disruptive as to preclude these interventions or opportunities for adequate sleep. Thus, the continued high use of medication for sleep is due in part to the lack of patient and clinician education and the difficulty in changing the hospital environment and culture, especially with only limited data on the value of sleep during recovery from illness.
Clinicians typically receive little training regarding sleep or its importance. In fact, most clinicians do not assess or communicate about the patient's quality of sleep.[11] Many may not know that there is little evidence of benefit of pharmacological sleep aids in the hospital. For example, a recent report found, in contrast to the authors' hypothesis, no changes in sleep architecture or duration using 10 mg of zolpidem tartrate in postoperative patients.[12] In our study, we found that some patients required an increase in the dose of their medications or were transitioned to a different sleep aid class (suggesting that sleep aids were ineffective). Alternatively, some patients' sleep aids were discontinued during hospital admission, again, likely due to perceived ineffectiveness or perhaps side effects. It is possible that the effectiveness of these medications might be influenced by timing of administration. This too was variable in our study. Proper clinician education around sleep hygiene might prevent early medication administration (which might lead to middle‐of‐the‐night awakenings) or delayed administration (which will delay the sleep phase), as was frequently seen in our cohort.
Despite emerging evidence of the importance of sleep in maintaining adequate immune, cardiovascular, and cognitive function, there are limited data regarding the benefits of sleep during acute illness.[13, 14, 15, 16] In the absence of compelling data, promoting sleep in the hospital has been difficult. The successful interventions used by Inouye and Bartick and their colleagues to minimize sedative hypnotic use included: a bedtime routine (eg, milk or herbal tea, relaxation tapes or music, back massage, toilet at bedtime), unit‐wide noise‐reduction strategies (eg, silent pill crushers, vibrating beepers, quiet hallways, and noise‐monitoring equipment that alerted staff above a certain decibel level), and schedule adjustments to allow sleep (eg, rescheduling of medications, intravenous fluids, and procedures), all of which require substantial clinician care or may not be possible in more acutely ill patients. Although such changes might be costly, sleep promotion and minimization of sleep aids continues to be part of a strategy that reduces delirium, hospital costs, and hospital length of stay.[16] From a patient perspective, many are interested in nondrug alternatives, especially those who have never used medications before, but few are told of them.[17]
Novel findings of our study include the types of medications used for sleep in the hospital. We found that a variety of medications and classes of medications were prescribed by clinicians for sleep complaints during hospitalization. This variability is due to a number of factors including the lack of rigorous data in this area, well‐established guidelines, or clinician education. We speculate that the high rates of use of nonbenzodiazepine and non‐ gamma‐aminobutyric acid (GABA)ergic agents, such as trazodone and quetiapine, reflect concerns about the use of medications such as zolpidem. Conversely, this means patients are increasingly treated with medications without formal FDA labeling for sleep. It does appear at least that the median doses of medication prescribed were lower in those over age 65 years compared to younger patients, although we cannot determine whether this reflects physician awareness or effective decision support used during computerized order entry. The geriatric decision support recommends a reduced dose for some (eg, trazodone, haloperidol) but not all (eg, quetiapine, lorazepam) of the monitored medications.
We found that many patients, even those who were never previously known to have insomnia, were discharged with a prescription for a sleep medication. Our study design is limited in assessing whether this prescription was needed or not, that is, whether or not the patient will have insomnia (sleep difficulty despite adequate opportunity for sleep) after hospital discharge. However, other studies have suggested that acute illness can be a precipitant for insomnia. Some of this literature has focused on patients in the intensive care unit, but it seems reasonable that patients on general medical and surgical wards (not having come through the intensive care units) might also be at risk for insomnia.[18] A study by Zisberg and colleagues in an elderly Israeli cohort found hospitalization (even without intensive care unit stay) to be both a starting point and a stopping point for chronic sleep medication use.[19] Alternatively, patients may not continue to suffer from insomnia after discharge, and thus the prescription for a sleep aid is inappropriate, as it is likely to have no benefit but may carry risk.[20] Regardless of whether hospital‐acquired insomnia persists past discharge, our findings suggest that some patients will start on chronic medication use for insomnia. Importantly, these patients may have limited understanding of the reason for their prescription, medication risks and benefits, and are unlikely to receive guidance on sleep hygiene or referral to a sleep specialist if needed. In our case, the high rate of prescriptions likely reflects the way in which inpatient medications can be added as a discharge medication automatically. This represents an area for improvement at our institution.
Limitations
This retrospective, single‐center study has several limitations. First, although our results are specific to our institution, our use of pharmacological sleep aids is similar to those previously reported in the literature. Our results are consistent in some ways with the changing trends in outpatient management of insomnia, in which trazodone and quetiapine are now frequently used. Second, we rely on the medical record for prior documentation of insomnia and/or use of medications for insomnia. However, our rate of prior diagnosis of insomnia or medication use of 8.3% is consistent with epidemiological studies.[21] Third, in this retrospective study we may have included some medications in our analysis that may have been given for indications other than sleep promotion, such as medications for anxiety or agitation. However, sleep promotion may have been an intended benefit of the medication choice. Fourth, we did not follow patients after discharge to know whether they continued with sleep medication use outside the hospital. Finally, in this retrospective chart review, we focused on utilization metrics, not on efficacy (which we can only infer) or adverse effects, such as altered mental status or falls. Moreover, we did not compare those patients who did and who did not receive any medication for sleep. However, such work will be crucial in future studies.
CONCLUSIONS
Despite increasing evidence of risks such as delirium or falls, pharmacological sleep aid use in hospitalized patients, even the elderly, remains common. A variety of medications are used, with variable administration times, which likely reflects the few rigorous studies or guidelines for the use of pharmacological sleep aids in hospitalized patients. Many patients not known to be on medications for sleep before admission leave the hospital with a sleep aid prescription. Our results suggest the need to better understand the factors that contribute to the high rate of sleep aid use in hospitalized patients. Clinician education regarding sleep, and nonpharmacological strategies to improve sleep in the hospital, are also needed.
Disclosure: Nothing to report.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
- , , , . Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008;3(6):473–482.
- . Sleep and the sleep environment of older adults in acute care settings. J Gerontol Nurs. 2008;34(6):15–21.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6.
- , , , , , . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17.
- , , , , . Decrease in as‐needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20–E24.
- , , , , , . Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165(7):802–807.
- , , , . National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349.
- , . Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718–722.
- , . Ten‐year trends in the pharmacological treatment of insomnia. Sleep. 1999;22(3):371–375.
- , , , . How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342–347.
- , , . Postoperative sleep disturbances after zolpidem treatment in fast‐track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326.
- , , . Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50–54.
- , , , , , . Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10(5):643–653.
- , , , et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186.
- , , , et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–226.
- , , , , . Hospitalized patients' preference in the treatment of insomnia: pharmacological versus non‐pharmacological. Can J Clin Pharmacol. 2003;10(2):89–92.
- , , , et al. Post‐discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13(8):1106–1109.
- , , , , , . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–576.
- , , , et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134.
- , . Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484.
© 2014 Society of Hospital Medicine
Pediatric Hospital Medicine 2014: Clinical Competence for the Community Hospitalist
Presenters: Julia Aquino, Elisabeth Schainker, Michelle Hofmann
Summary: The panel began by laying the foundation for why competency assessment of community pediatric hospitalists is important, based on the Pediatric Hospital Medicine Core Competencies published in the Journal of Hospital Medicine. Throughout the presentation, the panel used their own personal experience of developing a neonatal competency assessment program to demonstrate how to build a successful, comprehensive program for community pediatric hospitalists.
The presenters outlined the key goals of a competency assessment program, including:
• Feedback about both strengths and weaknesses should be provided in order to reinforce learned knowledge and guide future learning;
• Programs should assess not just clinical, but also non-clinical skills, such as communication, patient safety, and continuous quality improvement; and
• Assessment should include formative assessment, or “low stakes” monitoring of learning, as well as summative assessment, in which there is a “high stakes” consequence for not demonstrating competency. An example of summative assessment would be the current NRP testing: if you do not pass the test, you do not receive NRP certification.
The panel then described the need to build a competency assessment program based on the concepts of Miller’s Triangle of assessment. The bottom two layers of the triangle include standardized evaluations of a user’s knowledge, while the top two layers are comprised of practice-based assessment.
The bottom layer of the triangle assesses a user’s knowledge through test questions. The next level up tests a user’s ability to apply knowledge, for example, the current PALS case-based learning. The third level evaluates a user’s ability to show or demonstrate knowledge, such as through simulation training. The final or top level assesses the user actually doing, or performing the task, such as through direct observation.
The presenters pointed out the relative strengths and weaknesses of each level, namely, as one moves up the pyramid, the levels of assessment demonstrate increasing levels of competency, but come at the expense of increasing work or cost.
Key Takeaways
A comprehensive competency assessment program should:
(1) Evaluate clinical and non-clinical skills, based on the Pediatric Hospital Medicine Core Competencies;
(2) Incorporate both “low stakes” formative and “high stakes” summative assessment; and
(3) Include standardized assessments as well as practice-based evaluations, based on the concepts of Miller’s Triangle. TH
Dr. O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Presenters: Julia Aquino, Elisabeth Schainker, Michelle Hofmann
Summary: The panel began by laying the foundation for why competency assessment of community pediatric hospitalists is important, based on the Pediatric Hospital Medicine Core Competencies published in the Journal of Hospital Medicine. Throughout the presentation, the panel used their own personal experience of developing a neonatal competency assessment program to demonstrate how to build a successful, comprehensive program for community pediatric hospitalists.
The presenters outlined the key goals of a competency assessment program, including:
• Feedback about both strengths and weaknesses should be provided in order to reinforce learned knowledge and guide future learning;
• Programs should assess not just clinical, but also non-clinical skills, such as communication, patient safety, and continuous quality improvement; and
• Assessment should include formative assessment, or “low stakes” monitoring of learning, as well as summative assessment, in which there is a “high stakes” consequence for not demonstrating competency. An example of summative assessment would be the current NRP testing: if you do not pass the test, you do not receive NRP certification.
The panel then described the need to build a competency assessment program based on the concepts of Miller’s Triangle of assessment. The bottom two layers of the triangle include standardized evaluations of a user’s knowledge, while the top two layers are comprised of practice-based assessment.
The bottom layer of the triangle assesses a user’s knowledge through test questions. The next level up tests a user’s ability to apply knowledge, for example, the current PALS case-based learning. The third level evaluates a user’s ability to show or demonstrate knowledge, such as through simulation training. The final or top level assesses the user actually doing, or performing the task, such as through direct observation.
The presenters pointed out the relative strengths and weaknesses of each level, namely, as one moves up the pyramid, the levels of assessment demonstrate increasing levels of competency, but come at the expense of increasing work or cost.
Key Takeaways
A comprehensive competency assessment program should:
(1) Evaluate clinical and non-clinical skills, based on the Pediatric Hospital Medicine Core Competencies;
(2) Incorporate both “low stakes” formative and “high stakes” summative assessment; and
(3) Include standardized assessments as well as practice-based evaluations, based on the concepts of Miller’s Triangle. TH
Dr. O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Presenters: Julia Aquino, Elisabeth Schainker, Michelle Hofmann
Summary: The panel began by laying the foundation for why competency assessment of community pediatric hospitalists is important, based on the Pediatric Hospital Medicine Core Competencies published in the Journal of Hospital Medicine. Throughout the presentation, the panel used their own personal experience of developing a neonatal competency assessment program to demonstrate how to build a successful, comprehensive program for community pediatric hospitalists.
The presenters outlined the key goals of a competency assessment program, including:
• Feedback about both strengths and weaknesses should be provided in order to reinforce learned knowledge and guide future learning;
• Programs should assess not just clinical, but also non-clinical skills, such as communication, patient safety, and continuous quality improvement; and
• Assessment should include formative assessment, or “low stakes” monitoring of learning, as well as summative assessment, in which there is a “high stakes” consequence for not demonstrating competency. An example of summative assessment would be the current NRP testing: if you do not pass the test, you do not receive NRP certification.
The panel then described the need to build a competency assessment program based on the concepts of Miller’s Triangle of assessment. The bottom two layers of the triangle include standardized evaluations of a user’s knowledge, while the top two layers are comprised of practice-based assessment.
The bottom layer of the triangle assesses a user’s knowledge through test questions. The next level up tests a user’s ability to apply knowledge, for example, the current PALS case-based learning. The third level evaluates a user’s ability to show or demonstrate knowledge, such as through simulation training. The final or top level assesses the user actually doing, or performing the task, such as through direct observation.
The presenters pointed out the relative strengths and weaknesses of each level, namely, as one moves up the pyramid, the levels of assessment demonstrate increasing levels of competency, but come at the expense of increasing work or cost.
Key Takeaways
A comprehensive competency assessment program should:
(1) Evaluate clinical and non-clinical skills, based on the Pediatric Hospital Medicine Core Competencies;
(2) Incorporate both “low stakes” formative and “high stakes” summative assessment; and
(3) Include standardized assessments as well as practice-based evaluations, based on the concepts of Miller’s Triangle. TH
Dr. O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Plan helped atrial fibrillation patients safely switch oral anticoagulants
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Following a prescribed transition plan when switching oral anticoagulants protected most AF patients from strokes and major bleeding.
Major finding: Thirty days after transitioning to a new oral anticoagulant, 98.7% of patients who were switched from high-dose edoxaban had at least one therapeutic INR, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin. Post-transition rates of stroke and major bleeding also were similar among all three groups.
Data source: Randomized, open-label study of 13,642 patients with AF from the ENGAGE AF-TIMI 48 trial. At the end of the trial, 68.2% of patients transitioned to an open-label vitamin K antagonist, and 31.2% were switched to new oral anticoagulant.
Disclosures: Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
NSAIDs can target LSCs in AML

Credit: Robert Paulson
Preclinical research suggests that non-steroidal anti-inflammatory drugs (NSAIDs) might help prevent relapse in acute myeloid leukemia (AML).
NSAIDs inhibit 5-lipoxygenase (5-LO), and researchers found this enzyme plays a key role in the survival of leukemic stem cells (LSCs).
In cell cultures and mouse models of AML, NSAIDs selectively and efficiently attacked LSCs.
The researchers detailed these results in Cancer Research.
“These results provide the basis for the potential implementation of 5-LO-inhibitors as stem cell therapeutic agents for a sustained AML cure, although this must be investigated further in preclinical and clinical studies,” said study author Martin Ruthardt, MD, of Goethe University in Frankfurt, Germany.
Recent research suggested 5-LO is critical to the maintenance of LSCs in chronic myeloid leukemia. So Dr Ruthardt and his colleagues hypothesized that 5-LO might be a therapeutic target for AML.
To test that theory, the researchers inhibited 5-LO in a PML/RARα -positive model of AML. As LSC models, the team used Sca-1+/lin- murine hematopoietic stem and progenitor cells (HSPCs), which were retrovirally transduced with PML/RARα.
The group found that inhibiting 5-LO with the NSAIDs CJ-13,610 and zileuton reduced the stem cell capacity of PML/RARα-expressing HSPCs. The NSAIDs also inhibited Wnt signaling.
On the other hand, targeted genetic inhibition of 5-LO did not recapitulate the NSAIDs’ effects on leukemogenic potential and the aberrant stem cell capacity induced by PML/RARα.
Further investigation revealed that Wnt and LSC inhibition is mediated by the enzymatically inactive form of 5-LO, which hinders the nuclear translocation of ß-catenin. So it seems 5-LO inhibitors also inhibit Wnt signaling due to the generation of a catalytically inactive form of 5-LO, which assumes a new function.
“[T]here are plans for further molecular biological studies with the objective of understanding exactly how the 5-LO inhibitors act on the leukemic cells,” said study author Thorsten J. Maier, MD, PhD, of Aarhus University in Denmark. ![]()

Credit: Robert Paulson
Preclinical research suggests that non-steroidal anti-inflammatory drugs (NSAIDs) might help prevent relapse in acute myeloid leukemia (AML).
NSAIDs inhibit 5-lipoxygenase (5-LO), and researchers found this enzyme plays a key role in the survival of leukemic stem cells (LSCs).
In cell cultures and mouse models of AML, NSAIDs selectively and efficiently attacked LSCs.
The researchers detailed these results in Cancer Research.
“These results provide the basis for the potential implementation of 5-LO-inhibitors as stem cell therapeutic agents for a sustained AML cure, although this must be investigated further in preclinical and clinical studies,” said study author Martin Ruthardt, MD, of Goethe University in Frankfurt, Germany.
Recent research suggested 5-LO is critical to the maintenance of LSCs in chronic myeloid leukemia. So Dr Ruthardt and his colleagues hypothesized that 5-LO might be a therapeutic target for AML.
To test that theory, the researchers inhibited 5-LO in a PML/RARα -positive model of AML. As LSC models, the team used Sca-1+/lin- murine hematopoietic stem and progenitor cells (HSPCs), which were retrovirally transduced with PML/RARα.
The group found that inhibiting 5-LO with the NSAIDs CJ-13,610 and zileuton reduced the stem cell capacity of PML/RARα-expressing HSPCs. The NSAIDs also inhibited Wnt signaling.
On the other hand, targeted genetic inhibition of 5-LO did not recapitulate the NSAIDs’ effects on leukemogenic potential and the aberrant stem cell capacity induced by PML/RARα.
Further investigation revealed that Wnt and LSC inhibition is mediated by the enzymatically inactive form of 5-LO, which hinders the nuclear translocation of ß-catenin. So it seems 5-LO inhibitors also inhibit Wnt signaling due to the generation of a catalytically inactive form of 5-LO, which assumes a new function.
“[T]here are plans for further molecular biological studies with the objective of understanding exactly how the 5-LO inhibitors act on the leukemic cells,” said study author Thorsten J. Maier, MD, PhD, of Aarhus University in Denmark. ![]()

Credit: Robert Paulson
Preclinical research suggests that non-steroidal anti-inflammatory drugs (NSAIDs) might help prevent relapse in acute myeloid leukemia (AML).
NSAIDs inhibit 5-lipoxygenase (5-LO), and researchers found this enzyme plays a key role in the survival of leukemic stem cells (LSCs).
In cell cultures and mouse models of AML, NSAIDs selectively and efficiently attacked LSCs.
The researchers detailed these results in Cancer Research.
“These results provide the basis for the potential implementation of 5-LO-inhibitors as stem cell therapeutic agents for a sustained AML cure, although this must be investigated further in preclinical and clinical studies,” said study author Martin Ruthardt, MD, of Goethe University in Frankfurt, Germany.
Recent research suggested 5-LO is critical to the maintenance of LSCs in chronic myeloid leukemia. So Dr Ruthardt and his colleagues hypothesized that 5-LO might be a therapeutic target for AML.
To test that theory, the researchers inhibited 5-LO in a PML/RARα -positive model of AML. As LSC models, the team used Sca-1+/lin- murine hematopoietic stem and progenitor cells (HSPCs), which were retrovirally transduced with PML/RARα.
The group found that inhibiting 5-LO with the NSAIDs CJ-13,610 and zileuton reduced the stem cell capacity of PML/RARα-expressing HSPCs. The NSAIDs also inhibited Wnt signaling.
On the other hand, targeted genetic inhibition of 5-LO did not recapitulate the NSAIDs’ effects on leukemogenic potential and the aberrant stem cell capacity induced by PML/RARα.
Further investigation revealed that Wnt and LSC inhibition is mediated by the enzymatically inactive form of 5-LO, which hinders the nuclear translocation of ß-catenin. So it seems 5-LO inhibitors also inhibit Wnt signaling due to the generation of a catalytically inactive form of 5-LO, which assumes a new function.
“[T]here are plans for further molecular biological studies with the objective of understanding exactly how the 5-LO inhibitors act on the leukemic cells,” said study author Thorsten J. Maier, MD, PhD, of Aarhus University in Denmark. ![]()
Expediting drug approvals may compromise safety

Credit: CDC
The introduction of expedited drug approvals in the US coincides with an increase in black box warnings and market withdrawals, a new study shows.
The researchers could not establish a causal link between the events, but they still believe physicians and patients should exercise caution when considering the use of drugs approved since 1992.
That’s when Congress passed a law allowing the Food and Drug Administration (FDA) to collect fees to expedite drug approvals.
The researchers found that drugs approved after the law—the Prescription Drug User Fee Act (PDUFA)—was enacted were significantly more likely than previously approved drugs to acquire a black box warning or be withdrawn for safety reasons.
“Our findings raise concern that the FDA is rushing its review of new drugs and allowing potentially unsafe medicines onto the market,” said Karen Lasser, MD, MPH, of Boston University School of Medicine and Boston Medical Center.
“As a primary care doctor, I’m wary of prescribing brand new drugs unless they’re really a breakthrough, since their full risks are often unknown. And patients should be wary too.”
Dr Lasser and her colleagues expressed this viewpoint and described the research supporting it in Health Affairs.
The researchers collected information on new molecular entities (active ingredients that have never before been marketed in the US in any form) approved by the FDA between 1975 and 2009.
Of these 748 drugs, 114 (15.2%) received one or more black box warnings, and 32 (4.3%) were withdrawn from the market for safety reasons.
Very few of the 32 withdrawn drugs had clearly unique benefits at the time of approval, but all had unique risks that eventually led to their withdrawal.
Half of all black box warnings appeared after a drug had been on the market for 12 years, and safety withdrawals have occurred as late as 30 years after a drug’s initial release.
Drugs approved after the enactment of PDUFA were significantly more likely to receive a black box warning or withdrawal than drugs approved before PDUFA’s enactment—26.7 out of 100 drugs vs 21.2 out of 100 drugs (P<0.05) at up to 16 years of follow-up.
Since the law was enacted, the average approval time for all drugs has fallen from 34 months to 16 months.
“Since PDUFA, the review times for the drugs that are eventually banned have decreased enormously,” said study author Sidney Wolfe, MD, founder of Public Citizen’s Health Research Group and author of Worst Pills, Best Pills.
“These shorter review times, combined with increased FDA authority to require further studies after approval—rather than settling safety issues before approval—possibly contributes to the increased rate of withdrawals and black box warnings.”
The researchers noted that they could not determine with certainty whether PDUFA caused the increase in drug withdrawals and black box warnings. It’s possible that other factors caused or contributed to the decrease in safety observed in recent years. ![]()

Credit: CDC
The introduction of expedited drug approvals in the US coincides with an increase in black box warnings and market withdrawals, a new study shows.
The researchers could not establish a causal link between the events, but they still believe physicians and patients should exercise caution when considering the use of drugs approved since 1992.
That’s when Congress passed a law allowing the Food and Drug Administration (FDA) to collect fees to expedite drug approvals.
The researchers found that drugs approved after the law—the Prescription Drug User Fee Act (PDUFA)—was enacted were significantly more likely than previously approved drugs to acquire a black box warning or be withdrawn for safety reasons.
“Our findings raise concern that the FDA is rushing its review of new drugs and allowing potentially unsafe medicines onto the market,” said Karen Lasser, MD, MPH, of Boston University School of Medicine and Boston Medical Center.
“As a primary care doctor, I’m wary of prescribing brand new drugs unless they’re really a breakthrough, since their full risks are often unknown. And patients should be wary too.”
Dr Lasser and her colleagues expressed this viewpoint and described the research supporting it in Health Affairs.
The researchers collected information on new molecular entities (active ingredients that have never before been marketed in the US in any form) approved by the FDA between 1975 and 2009.
Of these 748 drugs, 114 (15.2%) received one or more black box warnings, and 32 (4.3%) were withdrawn from the market for safety reasons.
Very few of the 32 withdrawn drugs had clearly unique benefits at the time of approval, but all had unique risks that eventually led to their withdrawal.
Half of all black box warnings appeared after a drug had been on the market for 12 years, and safety withdrawals have occurred as late as 30 years after a drug’s initial release.
Drugs approved after the enactment of PDUFA were significantly more likely to receive a black box warning or withdrawal than drugs approved before PDUFA’s enactment—26.7 out of 100 drugs vs 21.2 out of 100 drugs (P<0.05) at up to 16 years of follow-up.
Since the law was enacted, the average approval time for all drugs has fallen from 34 months to 16 months.
“Since PDUFA, the review times for the drugs that are eventually banned have decreased enormously,” said study author Sidney Wolfe, MD, founder of Public Citizen’s Health Research Group and author of Worst Pills, Best Pills.
“These shorter review times, combined with increased FDA authority to require further studies after approval—rather than settling safety issues before approval—possibly contributes to the increased rate of withdrawals and black box warnings.”
The researchers noted that they could not determine with certainty whether PDUFA caused the increase in drug withdrawals and black box warnings. It’s possible that other factors caused or contributed to the decrease in safety observed in recent years. ![]()

Credit: CDC
The introduction of expedited drug approvals in the US coincides with an increase in black box warnings and market withdrawals, a new study shows.
The researchers could not establish a causal link between the events, but they still believe physicians and patients should exercise caution when considering the use of drugs approved since 1992.
That’s when Congress passed a law allowing the Food and Drug Administration (FDA) to collect fees to expedite drug approvals.
The researchers found that drugs approved after the law—the Prescription Drug User Fee Act (PDUFA)—was enacted were significantly more likely than previously approved drugs to acquire a black box warning or be withdrawn for safety reasons.
“Our findings raise concern that the FDA is rushing its review of new drugs and allowing potentially unsafe medicines onto the market,” said Karen Lasser, MD, MPH, of Boston University School of Medicine and Boston Medical Center.
“As a primary care doctor, I’m wary of prescribing brand new drugs unless they’re really a breakthrough, since their full risks are often unknown. And patients should be wary too.”
Dr Lasser and her colleagues expressed this viewpoint and described the research supporting it in Health Affairs.
The researchers collected information on new molecular entities (active ingredients that have never before been marketed in the US in any form) approved by the FDA between 1975 and 2009.
Of these 748 drugs, 114 (15.2%) received one or more black box warnings, and 32 (4.3%) were withdrawn from the market for safety reasons.
Very few of the 32 withdrawn drugs had clearly unique benefits at the time of approval, but all had unique risks that eventually led to their withdrawal.
Half of all black box warnings appeared after a drug had been on the market for 12 years, and safety withdrawals have occurred as late as 30 years after a drug’s initial release.
Drugs approved after the enactment of PDUFA were significantly more likely to receive a black box warning or withdrawal than drugs approved before PDUFA’s enactment—26.7 out of 100 drugs vs 21.2 out of 100 drugs (P<0.05) at up to 16 years of follow-up.
Since the law was enacted, the average approval time for all drugs has fallen from 34 months to 16 months.
“Since PDUFA, the review times for the drugs that are eventually banned have decreased enormously,” said study author Sidney Wolfe, MD, founder of Public Citizen’s Health Research Group and author of Worst Pills, Best Pills.
“These shorter review times, combined with increased FDA authority to require further studies after approval—rather than settling safety issues before approval—possibly contributes to the increased rate of withdrawals and black box warnings.”
The researchers noted that they could not determine with certainty whether PDUFA caused the increase in drug withdrawals and black box warnings. It’s possible that other factors caused or contributed to the decrease in safety observed in recent years. ![]()
Mice are suitable models for inflammatory conditions, study suggests

Results of a new study contradict previous research suggesting mice do not make suitable models for human inflammatory conditions.
The original study, published in PNAS in February 2013, indicated that genomic responses to different acute inflammatory stressors—trauma, burns, sepsis, and infection—are highly similar in humans but poorly reproduced in corresponding mouse models.
The new study, published in PNAS yesterday, suggests that is not the case.
The original study was conducted by Junhee Seok, PhD, of Northwestern University, and his colleagues. It garnered a lot of attention from the scientific community and the general public, reigniting the debate over mouse models’ suitability for medical research.
Tsuyoshi Miyakawa, PhD, of Fujita Health University in Japan, was among those who argued that mice are suitable models, and Dr Seok’s findings were likely incorrect.
So Dr Miyakawa and his colleague, Keizo Takao, PhD, of the National Institute for Physiological Sciences in Japan, reanalyzed the data from Dr Seok’s study using the bioinformatics tool NextBio.
Dr Seok’s group had compared the expression levels of genes that were altered in a particular human condition between humans and mice.
A comparison of the genomic response between humans and mice, including those genes altered in one species but not in another, obscures the correlation between homologous genes of humans and mice to nearly 0, as the team showed.
The group’s comparison of the gene expression patterns between human burn victims and mouse models of burns, trauma, sepsis, and infection revealed a Pearson’s correlation coefficient (R) that ranged from 0.14 to 0.28. And the percentage of genes whose expression changed in the same direction was 55% to 61%.
In the new analysis based on the same data, Drs Miyakawa and Takao found the R values ranged from 0.36 to 0.59. And 77% to 93% of the genes changed in the same directions between the human condition and the mouse models.
Non-parametric ranking analysis using NextBio showed the pattern of the gene expression changes in mouse models was highly similar to that in human burn conditions—a significant correlation (P = 6.5 x 10-11 to 1.2 x 10-35).
Drs Miyakawa and Takao noted that many molecular pathways are commonly dysregulated in human diseases and mouse models. And focusing on the commonalities between human diseases and mouse models will allow us to derive useful information for studying the pathophysiology and pathogenesis of human diseases, as well as aid treatment development. ![]()

Results of a new study contradict previous research suggesting mice do not make suitable models for human inflammatory conditions.
The original study, published in PNAS in February 2013, indicated that genomic responses to different acute inflammatory stressors—trauma, burns, sepsis, and infection—are highly similar in humans but poorly reproduced in corresponding mouse models.
The new study, published in PNAS yesterday, suggests that is not the case.
The original study was conducted by Junhee Seok, PhD, of Northwestern University, and his colleagues. It garnered a lot of attention from the scientific community and the general public, reigniting the debate over mouse models’ suitability for medical research.
Tsuyoshi Miyakawa, PhD, of Fujita Health University in Japan, was among those who argued that mice are suitable models, and Dr Seok’s findings were likely incorrect.
So Dr Miyakawa and his colleague, Keizo Takao, PhD, of the National Institute for Physiological Sciences in Japan, reanalyzed the data from Dr Seok’s study using the bioinformatics tool NextBio.
Dr Seok’s group had compared the expression levels of genes that were altered in a particular human condition between humans and mice.
A comparison of the genomic response between humans and mice, including those genes altered in one species but not in another, obscures the correlation between homologous genes of humans and mice to nearly 0, as the team showed.
The group’s comparison of the gene expression patterns between human burn victims and mouse models of burns, trauma, sepsis, and infection revealed a Pearson’s correlation coefficient (R) that ranged from 0.14 to 0.28. And the percentage of genes whose expression changed in the same direction was 55% to 61%.
In the new analysis based on the same data, Drs Miyakawa and Takao found the R values ranged from 0.36 to 0.59. And 77% to 93% of the genes changed in the same directions between the human condition and the mouse models.
Non-parametric ranking analysis using NextBio showed the pattern of the gene expression changes in mouse models was highly similar to that in human burn conditions—a significant correlation (P = 6.5 x 10-11 to 1.2 x 10-35).
Drs Miyakawa and Takao noted that many molecular pathways are commonly dysregulated in human diseases and mouse models. And focusing on the commonalities between human diseases and mouse models will allow us to derive useful information for studying the pathophysiology and pathogenesis of human diseases, as well as aid treatment development. ![]()

Results of a new study contradict previous research suggesting mice do not make suitable models for human inflammatory conditions.
The original study, published in PNAS in February 2013, indicated that genomic responses to different acute inflammatory stressors—trauma, burns, sepsis, and infection—are highly similar in humans but poorly reproduced in corresponding mouse models.
The new study, published in PNAS yesterday, suggests that is not the case.
The original study was conducted by Junhee Seok, PhD, of Northwestern University, and his colleagues. It garnered a lot of attention from the scientific community and the general public, reigniting the debate over mouse models’ suitability for medical research.
Tsuyoshi Miyakawa, PhD, of Fujita Health University in Japan, was among those who argued that mice are suitable models, and Dr Seok’s findings were likely incorrect.
So Dr Miyakawa and his colleague, Keizo Takao, PhD, of the National Institute for Physiological Sciences in Japan, reanalyzed the data from Dr Seok’s study using the bioinformatics tool NextBio.
Dr Seok’s group had compared the expression levels of genes that were altered in a particular human condition between humans and mice.
A comparison of the genomic response between humans and mice, including those genes altered in one species but not in another, obscures the correlation between homologous genes of humans and mice to nearly 0, as the team showed.
The group’s comparison of the gene expression patterns between human burn victims and mouse models of burns, trauma, sepsis, and infection revealed a Pearson’s correlation coefficient (R) that ranged from 0.14 to 0.28. And the percentage of genes whose expression changed in the same direction was 55% to 61%.
In the new analysis based on the same data, Drs Miyakawa and Takao found the R values ranged from 0.36 to 0.59. And 77% to 93% of the genes changed in the same directions between the human condition and the mouse models.
Non-parametric ranking analysis using NextBio showed the pattern of the gene expression changes in mouse models was highly similar to that in human burn conditions—a significant correlation (P = 6.5 x 10-11 to 1.2 x 10-35).
Drs Miyakawa and Takao noted that many molecular pathways are commonly dysregulated in human diseases and mouse models. And focusing on the commonalities between human diseases and mouse models will allow us to derive useful information for studying the pathophysiology and pathogenesis of human diseases, as well as aid treatment development. ![]()
Pathologist Emmanuel Farber dies at 95

Emmanuel Farber, MD, PhD, a renowned pathologist who made fundamental contributions to our understanding of chemical carcinogenesis, passed away on August 3 at the age of 95.
Dr Farber’s studies in experimental pathology demonstrated that chemical carcinogens are capable of binding to nucleic acids, in turn generating specific DNA adducts.
These early studies led to the observation that chemical carcinogenesis is a sequential process.
Dr Farber later proved this theory by showing that cancer could be induced through a series of step-by-step chemical treatments in the liver. He served on the Surgeon General’s first Advisory Committee on Smoking and Health from 1961 to 1964.
The committee was responsible for issuing the 1964 Surgeon General’s Report, which has now done more to prevent tobacco-related disease than any other preventive measure.
Throughout his career, Dr Farber promoted the concept that to understand carcinogenesis, one must also understand the cellular, genetic, metabolic, and molecular changes that are occurring during the process. This mindset, along with Dr Farber’s energy and enthusiasm in exploring the nature of cancer, has served as a source of inspiration and guidance for cancer researchers worldwide.
Dr Farber was born in Toronto, Canada, on October 19, 1918. He obtained his medical degree from the Faculty of Medicine, University of Toronto in 1942.
After completing his residency training in pathology at the Hamilton General Hospital in Ontario, Canada, he served in the Royal Canadian Army Medical Corps and later obtained a doctorate in biochemistry from the University of California, Berkeley.
His academic career began at Tulane University in New Orleans, Louisiana. It continued with his appointment as Professor and Chairman of Pathology and Professor of Biochemistry at the University of Pittsburgh School of Medicine and at the Fels Research Institute, Temple University School of Medicine, in Philadelphia, Pennsylvania, where he was Professor of Pathology and Biochemistry and Director of the Institute.
In 1975, Dr Farber moved back to his native city to take the post of Professor and Chairman of the Department of Pathology and Professor in the Department of Biochemistry at the University of Toronto. At his death, he held the title of Chairman Emeritus and Professor in the Department of Pathology at the University of Toronto.
Dr Farber is survived by his daughter Naomi Farber, son-in-law Steven Grosby, and grandson Samuel Grosby, who wish to extend their sincere appreciation to those who enriched his personal and professional life and joined his tireless search for scientific truth. ![]()

Emmanuel Farber, MD, PhD, a renowned pathologist who made fundamental contributions to our understanding of chemical carcinogenesis, passed away on August 3 at the age of 95.
Dr Farber’s studies in experimental pathology demonstrated that chemical carcinogens are capable of binding to nucleic acids, in turn generating specific DNA adducts.
These early studies led to the observation that chemical carcinogenesis is a sequential process.
Dr Farber later proved this theory by showing that cancer could be induced through a series of step-by-step chemical treatments in the liver. He served on the Surgeon General’s first Advisory Committee on Smoking and Health from 1961 to 1964.
The committee was responsible for issuing the 1964 Surgeon General’s Report, which has now done more to prevent tobacco-related disease than any other preventive measure.
Throughout his career, Dr Farber promoted the concept that to understand carcinogenesis, one must also understand the cellular, genetic, metabolic, and molecular changes that are occurring during the process. This mindset, along with Dr Farber’s energy and enthusiasm in exploring the nature of cancer, has served as a source of inspiration and guidance for cancer researchers worldwide.
Dr Farber was born in Toronto, Canada, on October 19, 1918. He obtained his medical degree from the Faculty of Medicine, University of Toronto in 1942.
After completing his residency training in pathology at the Hamilton General Hospital in Ontario, Canada, he served in the Royal Canadian Army Medical Corps and later obtained a doctorate in biochemistry from the University of California, Berkeley.
His academic career began at Tulane University in New Orleans, Louisiana. It continued with his appointment as Professor and Chairman of Pathology and Professor of Biochemistry at the University of Pittsburgh School of Medicine and at the Fels Research Institute, Temple University School of Medicine, in Philadelphia, Pennsylvania, where he was Professor of Pathology and Biochemistry and Director of the Institute.
In 1975, Dr Farber moved back to his native city to take the post of Professor and Chairman of the Department of Pathology and Professor in the Department of Biochemistry at the University of Toronto. At his death, he held the title of Chairman Emeritus and Professor in the Department of Pathology at the University of Toronto.
Dr Farber is survived by his daughter Naomi Farber, son-in-law Steven Grosby, and grandson Samuel Grosby, who wish to extend their sincere appreciation to those who enriched his personal and professional life and joined his tireless search for scientific truth. ![]()

Emmanuel Farber, MD, PhD, a renowned pathologist who made fundamental contributions to our understanding of chemical carcinogenesis, passed away on August 3 at the age of 95.
Dr Farber’s studies in experimental pathology demonstrated that chemical carcinogens are capable of binding to nucleic acids, in turn generating specific DNA adducts.
These early studies led to the observation that chemical carcinogenesis is a sequential process.
Dr Farber later proved this theory by showing that cancer could be induced through a series of step-by-step chemical treatments in the liver. He served on the Surgeon General’s first Advisory Committee on Smoking and Health from 1961 to 1964.
The committee was responsible for issuing the 1964 Surgeon General’s Report, which has now done more to prevent tobacco-related disease than any other preventive measure.
Throughout his career, Dr Farber promoted the concept that to understand carcinogenesis, one must also understand the cellular, genetic, metabolic, and molecular changes that are occurring during the process. This mindset, along with Dr Farber’s energy and enthusiasm in exploring the nature of cancer, has served as a source of inspiration and guidance for cancer researchers worldwide.
Dr Farber was born in Toronto, Canada, on October 19, 1918. He obtained his medical degree from the Faculty of Medicine, University of Toronto in 1942.
After completing his residency training in pathology at the Hamilton General Hospital in Ontario, Canada, he served in the Royal Canadian Army Medical Corps and later obtained a doctorate in biochemistry from the University of California, Berkeley.
His academic career began at Tulane University in New Orleans, Louisiana. It continued with his appointment as Professor and Chairman of Pathology and Professor of Biochemistry at the University of Pittsburgh School of Medicine and at the Fels Research Institute, Temple University School of Medicine, in Philadelphia, Pennsylvania, where he was Professor of Pathology and Biochemistry and Director of the Institute.
In 1975, Dr Farber moved back to his native city to take the post of Professor and Chairman of the Department of Pathology and Professor in the Department of Biochemistry at the University of Toronto. At his death, he held the title of Chairman Emeritus and Professor in the Department of Pathology at the University of Toronto.
Dr Farber is survived by his daughter Naomi Farber, son-in-law Steven Grosby, and grandson Samuel Grosby, who wish to extend their sincere appreciation to those who enriched his personal and professional life and joined his tireless search for scientific truth. ![]()
Meet Dr. Leslie Baumann at the Summer AAD meeting
You love Dr. Baumann’s Cosmeceutical Critique column in Skin & Allergy News, and soon that content will be expanded in a new book entitled "Cosmeceuticals and Cosmetic Ingredients," available this November via Amazon.com. Meet Dr. Baumann at the Skin Disease Education Foundation (SDEF)/Skin & Allergy News Booth #1500 from 12:00-12:30 p.m. on Saturday, Aug. 9 at the 2014 Summer Academy Meeting in Chicago. And be sure to pick up a copy of her latest column from Skin & Allergy News, the leading news publication for aesthetic, medical, and surgical dermatology.
In addition, you can visit Dr. Baumann at her booth (#1716) during the meeting.
Read Dr. Baumann’s columns online at edermatologynews.com.
You love Dr. Baumann’s Cosmeceutical Critique column in Skin & Allergy News, and soon that content will be expanded in a new book entitled "Cosmeceuticals and Cosmetic Ingredients," available this November via Amazon.com. Meet Dr. Baumann at the Skin Disease Education Foundation (SDEF)/Skin & Allergy News Booth #1500 from 12:00-12:30 p.m. on Saturday, Aug. 9 at the 2014 Summer Academy Meeting in Chicago. And be sure to pick up a copy of her latest column from Skin & Allergy News, the leading news publication for aesthetic, medical, and surgical dermatology.
In addition, you can visit Dr. Baumann at her booth (#1716) during the meeting.
Read Dr. Baumann’s columns online at edermatologynews.com.
You love Dr. Baumann’s Cosmeceutical Critique column in Skin & Allergy News, and soon that content will be expanded in a new book entitled "Cosmeceuticals and Cosmetic Ingredients," available this November via Amazon.com. Meet Dr. Baumann at the Skin Disease Education Foundation (SDEF)/Skin & Allergy News Booth #1500 from 12:00-12:30 p.m. on Saturday, Aug. 9 at the 2014 Summer Academy Meeting in Chicago. And be sure to pick up a copy of her latest column from Skin & Allergy News, the leading news publication for aesthetic, medical, and surgical dermatology.
In addition, you can visit Dr. Baumann at her booth (#1716) during the meeting.
Read Dr. Baumann’s columns online at edermatologynews.com.