User login
Antibiotics may enhance mosquitoes’ ability to transmit malaria

Credit: James Gathany
Mosquitoes’ ability to transmit malaria may be affected by antibiotics in the blood of those they bite, according to research published in Nature Communications.
Feeding on blood that contained the antibiotics penicillin and streptomycin (PS) hindered bacterial growth in mosquitoes’ guts.
The mosquitoes also became more susceptible to malaria infection, exhibited improved fertility, and lived longer than mosquitoes whose blood meals did not contain PS.
Study investigators said these findings do not suggest people should avoid taking antibiotics. But the study does indicate that more research is needed to explore how different combinations of antibiotics affect mosquitoes’ ability to spread malaria.
“Antibiotics are a valuable weapon in the fight against malaria and other diseases,” said study author Mathilde Gendrin, PhD, of Imperial College London in the UK.
“Our study suggests that the presence of antibiotics in people’s blood may have hidden effects on the guts of mosquitoes when mosquitoes ingest that blood, and that these effects could alter the mosquitoes’ ability to transmit malaria.”
Dr Gendrin and her colleagues chose to evaluate the effects of PS because these antibiotics are not used to combat malaria and don’t directly affect malaria parasites.
The team began their research by supplementing blood with therapeutic concentrations of PS and feeding the blood to Anopheles gambiae mosquitoes.
The investigators monitored the bacterial load in the mosquito gut over 3 blood feeds offered every 3 days. And they found that the proliferation of bacteria typically seen at 24 hours after a blood meal was reduced by 70% when mosquitoes received blood containing PS.
Next, the researchers conducted 3 experiments to determine whether receiving blood containing PS would influence the mosquitoes’ susceptibility to malaria infection.
In the first experiment, the team fed mosquitoes blood from rodents infected with Plasmodium berghei before or after the animals received PS.
The investigators assessed infection by counting the proportion of mosquitoes carrying oocysts (prevalence) and the number of oocysts per mosquito (intensity). In the presence of PS, infection prevalence increased by 21% (P=5.10-7), and the median intensity doubled (P=0.041).
In the second experiment, the researchers assessed the effect of PS on infection with human parasites. They fed mosquitoes blood containing Plasmodium falciparum gametocytes (cultured in vitro) and supplemented the blood with PS or buffer.
Again, the team observed higher infection prevalence (P=0.0033) and intensity (P=0.00026) in the presence of PS.
In the third experiment, the investigators fed mosquitoes blood freshly drawn from children carrying P falciparum gametocytes. The blood was supplemented with PS or buffer.
Once more, the intensity of infection significantly increased in the presence of PS (P=0.02). The 6.3% increase in infection prevalence was not statistically significant (P=0.4), but the researchers said this was likely due to the reduced statistical power of the experiment.
The team’s final experiments assessed the effects of PS on mosquitoes’ fertility and survival.
Exposure to PS led to a 32% higher proportion of egg-laying females (P=0.0038) and a 53% higher average number of eggs per female (P=0.016), although the proportion of eggs hatching into larvae was not affected.
Mosquito survival increased significantly after the insects ingested PS-treated human blood. The P value was 0.017 for the first blood meal and 0.0016 for all 3 blood meals.
“We only looked at two antibiotics in our study, so this is early stage research, and we don’t know what it means for other antibiotics,” Dr Gendrin noted.
“It’s possible other antibiotics might have no effect, or that they might make it harder for mosquitoes to transmit malaria. We would like to see much more research carried out to further understand our findings and to explore how other antibiotics might affect bacteria in the guts of mosquitoes.”
“Ultimately, we hope that understanding any hidden effects of different antibiotics would mean we can combat the spread of malaria more effectively,” added study author George Christophides, PhD, also of Imperial College London.
“For example, if particular antibiotics make mosquitoes more able to transmit malaria, the use of these could be combined with supply of bed nets in order to reduce mosquito bites. Additionally, if a doctor prescribed such antibiotics to a malaria patient, they could combine this with a drug such as atovaquone that can both treat malaria and block its transmission.” ![]()

Credit: James Gathany
Mosquitoes’ ability to transmit malaria may be affected by antibiotics in the blood of those they bite, according to research published in Nature Communications.
Feeding on blood that contained the antibiotics penicillin and streptomycin (PS) hindered bacterial growth in mosquitoes’ guts.
The mosquitoes also became more susceptible to malaria infection, exhibited improved fertility, and lived longer than mosquitoes whose blood meals did not contain PS.
Study investigators said these findings do not suggest people should avoid taking antibiotics. But the study does indicate that more research is needed to explore how different combinations of antibiotics affect mosquitoes’ ability to spread malaria.
“Antibiotics are a valuable weapon in the fight against malaria and other diseases,” said study author Mathilde Gendrin, PhD, of Imperial College London in the UK.
“Our study suggests that the presence of antibiotics in people’s blood may have hidden effects on the guts of mosquitoes when mosquitoes ingest that blood, and that these effects could alter the mosquitoes’ ability to transmit malaria.”
Dr Gendrin and her colleagues chose to evaluate the effects of PS because these antibiotics are not used to combat malaria and don’t directly affect malaria parasites.
The team began their research by supplementing blood with therapeutic concentrations of PS and feeding the blood to Anopheles gambiae mosquitoes.
The investigators monitored the bacterial load in the mosquito gut over 3 blood feeds offered every 3 days. And they found that the proliferation of bacteria typically seen at 24 hours after a blood meal was reduced by 70% when mosquitoes received blood containing PS.
Next, the researchers conducted 3 experiments to determine whether receiving blood containing PS would influence the mosquitoes’ susceptibility to malaria infection.
In the first experiment, the team fed mosquitoes blood from rodents infected with Plasmodium berghei before or after the animals received PS.
The investigators assessed infection by counting the proportion of mosquitoes carrying oocysts (prevalence) and the number of oocysts per mosquito (intensity). In the presence of PS, infection prevalence increased by 21% (P=5.10-7), and the median intensity doubled (P=0.041).
In the second experiment, the researchers assessed the effect of PS on infection with human parasites. They fed mosquitoes blood containing Plasmodium falciparum gametocytes (cultured in vitro) and supplemented the blood with PS or buffer.
Again, the team observed higher infection prevalence (P=0.0033) and intensity (P=0.00026) in the presence of PS.
In the third experiment, the investigators fed mosquitoes blood freshly drawn from children carrying P falciparum gametocytes. The blood was supplemented with PS or buffer.
Once more, the intensity of infection significantly increased in the presence of PS (P=0.02). The 6.3% increase in infection prevalence was not statistically significant (P=0.4), but the researchers said this was likely due to the reduced statistical power of the experiment.
The team’s final experiments assessed the effects of PS on mosquitoes’ fertility and survival.
Exposure to PS led to a 32% higher proportion of egg-laying females (P=0.0038) and a 53% higher average number of eggs per female (P=0.016), although the proportion of eggs hatching into larvae was not affected.
Mosquito survival increased significantly after the insects ingested PS-treated human blood. The P value was 0.017 for the first blood meal and 0.0016 for all 3 blood meals.
“We only looked at two antibiotics in our study, so this is early stage research, and we don’t know what it means for other antibiotics,” Dr Gendrin noted.
“It’s possible other antibiotics might have no effect, or that they might make it harder for mosquitoes to transmit malaria. We would like to see much more research carried out to further understand our findings and to explore how other antibiotics might affect bacteria in the guts of mosquitoes.”
“Ultimately, we hope that understanding any hidden effects of different antibiotics would mean we can combat the spread of malaria more effectively,” added study author George Christophides, PhD, also of Imperial College London.
“For example, if particular antibiotics make mosquitoes more able to transmit malaria, the use of these could be combined with supply of bed nets in order to reduce mosquito bites. Additionally, if a doctor prescribed such antibiotics to a malaria patient, they could combine this with a drug such as atovaquone that can both treat malaria and block its transmission.” ![]()

Credit: James Gathany
Mosquitoes’ ability to transmit malaria may be affected by antibiotics in the blood of those they bite, according to research published in Nature Communications.
Feeding on blood that contained the antibiotics penicillin and streptomycin (PS) hindered bacterial growth in mosquitoes’ guts.
The mosquitoes also became more susceptible to malaria infection, exhibited improved fertility, and lived longer than mosquitoes whose blood meals did not contain PS.
Study investigators said these findings do not suggest people should avoid taking antibiotics. But the study does indicate that more research is needed to explore how different combinations of antibiotics affect mosquitoes’ ability to spread malaria.
“Antibiotics are a valuable weapon in the fight against malaria and other diseases,” said study author Mathilde Gendrin, PhD, of Imperial College London in the UK.
“Our study suggests that the presence of antibiotics in people’s blood may have hidden effects on the guts of mosquitoes when mosquitoes ingest that blood, and that these effects could alter the mosquitoes’ ability to transmit malaria.”
Dr Gendrin and her colleagues chose to evaluate the effects of PS because these antibiotics are not used to combat malaria and don’t directly affect malaria parasites.
The team began their research by supplementing blood with therapeutic concentrations of PS and feeding the blood to Anopheles gambiae mosquitoes.
The investigators monitored the bacterial load in the mosquito gut over 3 blood feeds offered every 3 days. And they found that the proliferation of bacteria typically seen at 24 hours after a blood meal was reduced by 70% when mosquitoes received blood containing PS.
Next, the researchers conducted 3 experiments to determine whether receiving blood containing PS would influence the mosquitoes’ susceptibility to malaria infection.
In the first experiment, the team fed mosquitoes blood from rodents infected with Plasmodium berghei before or after the animals received PS.
The investigators assessed infection by counting the proportion of mosquitoes carrying oocysts (prevalence) and the number of oocysts per mosquito (intensity). In the presence of PS, infection prevalence increased by 21% (P=5.10-7), and the median intensity doubled (P=0.041).
In the second experiment, the researchers assessed the effect of PS on infection with human parasites. They fed mosquitoes blood containing Plasmodium falciparum gametocytes (cultured in vitro) and supplemented the blood with PS or buffer.
Again, the team observed higher infection prevalence (P=0.0033) and intensity (P=0.00026) in the presence of PS.
In the third experiment, the investigators fed mosquitoes blood freshly drawn from children carrying P falciparum gametocytes. The blood was supplemented with PS or buffer.
Once more, the intensity of infection significantly increased in the presence of PS (P=0.02). The 6.3% increase in infection prevalence was not statistically significant (P=0.4), but the researchers said this was likely due to the reduced statistical power of the experiment.
The team’s final experiments assessed the effects of PS on mosquitoes’ fertility and survival.
Exposure to PS led to a 32% higher proportion of egg-laying females (P=0.0038) and a 53% higher average number of eggs per female (P=0.016), although the proportion of eggs hatching into larvae was not affected.
Mosquito survival increased significantly after the insects ingested PS-treated human blood. The P value was 0.017 for the first blood meal and 0.0016 for all 3 blood meals.
“We only looked at two antibiotics in our study, so this is early stage research, and we don’t know what it means for other antibiotics,” Dr Gendrin noted.
“It’s possible other antibiotics might have no effect, or that they might make it harder for mosquitoes to transmit malaria. We would like to see much more research carried out to further understand our findings and to explore how other antibiotics might affect bacteria in the guts of mosquitoes.”
“Ultimately, we hope that understanding any hidden effects of different antibiotics would mean we can combat the spread of malaria more effectively,” added study author George Christophides, PhD, also of Imperial College London.
“For example, if particular antibiotics make mosquitoes more able to transmit malaria, the use of these could be combined with supply of bed nets in order to reduce mosquito bites. Additionally, if a doctor prescribed such antibiotics to a malaria patient, they could combine this with a drug such as atovaquone that can both treat malaria and block its transmission.” ![]()
Immunotherapy moves into the breast cancer landscape
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Shrink Rap News: National study shows many offenders receive treatment only in prison
In a study published in the December 2014 issue of American Journal of Public Health, Jennifer M. Reingle Gonzalez, Ph.D., and Nadine M. Connell, Ph.D., of the University of Texas Health Science Center at Houston interviewed a nationally representative sample of all state and federal prisoners with psychiatric disorders to determine whether they had been screened for services at intake and to assess whether screening led to continuity of community treatment.
The subjects were chosen first by selecting a random sample of correctional facilities of varying sizes, from diverse geographic regions. From those facilities, 18,185 prisoners were chosen from among all those incarcerated on a single day in September 2002. Each subject was interviewed personally but also given computer-assisted interviews at different times to ensure recollection accuracy and the confidentiality of clinical data (Am. J. Public Health 2014;104:2328-33).
In the direct interview, each prisoner was asked if he or she had ever been diagnosed with a mental health condition such as depression, bipolar disorder, schizophrenia, posttraumatic stress disorder, an anxiety disorder, or a personality disorder. If any condition had ever been diagnosed, the inmates were then asked if they were taking a psychiatric medication upon admission to the facility. If they were in treatment at intake, they were asked if they had ever been on medication since that time. Finally, they were asked if they had had a medical examination while incarcerated.
Almost all prisoners (90%) were screened at intake and received a medical evaluation. The researchers found that 5,207 (26.2%) of the inmates received at least one lifetime mental health diagnosis, with depression being the most common. Eighteen percent reported taking medication for a psychiatric disorder at the time of intake, but of these, only about half were taking medication after incarceration. Medication continuance was twice as likely for schizophrenia as for depression, and those who received an intake screen were significantly more likely to be referred to a physician and receive medication. Notably, 27% of state and 16% of federal prisoners received medication only in prison.
The investigators attributed the discontinuity in treatment after incarceration to a lack of trained mental health professionals to diagnose and treat psychiatric disorders, and to a rise in prison populations out of proportion to available treatment services. The findings of this study supported the investigators’ recommendation for all facilities to employ intake screening to identify and refer prisoners in need of psychiatric care.
This research is important to forensic psychiatrists working in correctional systems and to those working as administrators or external court-appointed monitors, because it highlights the importance and efficacy of intake screening for prisoners with psychiatric disorders.
Unfortunately, traditional media coverage of this research was predictably provocative. Headlines blared: “Mental health care lacking in state and federal prisons.” The researchers themselves also implied that failure to continue medication in prison indicated some systemic deficiency. While an evaluation by a physician was correlated with the prescription of medication, this was not a perfect correlation. The authors did not discuss any of the valid reasons why medication might not be continued in prison.
The most common reason medication is not continued is that inmates are more likely to be abstinent from drugs and alcohol, and thus may require less or even no antidepressant medication after detoxification. This could account for as much as half of the treatment discontinuity, because as many as 20% of the prisoners had been diagnosed with depression. Also, evidence is mounting that indefinite medication may not be necessary for all conditions. Following a clinical assessment, a correctional physician may have determined that the prisoner had successfully completed maintenance therapy. The final reason why medication is not continued is that the inmate may simply have refused the offered treatment; some prisoners do choose to go without medication completely rather than risk a change to a different regimen.
All of this assumes that the subjects accurately reported both their psychiatric history and their mental health service contact following incarceration. Without access to current records or past documentation, this remains an open question. In my prison system, our electronic health record does contain some data provided by the public health care system. In rare cases, this information is consistent with what the patient reports, but this is the exception rather than the rule.
Finally, the most telling aspect of this research is what it reveals about free society care: A quarter of the inmates received treatment only in prison. That is the real finding worthy of a headline.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In a study published in the December 2014 issue of American Journal of Public Health, Jennifer M. Reingle Gonzalez, Ph.D., and Nadine M. Connell, Ph.D., of the University of Texas Health Science Center at Houston interviewed a nationally representative sample of all state and federal prisoners with psychiatric disorders to determine whether they had been screened for services at intake and to assess whether screening led to continuity of community treatment.
The subjects were chosen first by selecting a random sample of correctional facilities of varying sizes, from diverse geographic regions. From those facilities, 18,185 prisoners were chosen from among all those incarcerated on a single day in September 2002. Each subject was interviewed personally but also given computer-assisted interviews at different times to ensure recollection accuracy and the confidentiality of clinical data (Am. J. Public Health 2014;104:2328-33).
In the direct interview, each prisoner was asked if he or she had ever been diagnosed with a mental health condition such as depression, bipolar disorder, schizophrenia, posttraumatic stress disorder, an anxiety disorder, or a personality disorder. If any condition had ever been diagnosed, the inmates were then asked if they were taking a psychiatric medication upon admission to the facility. If they were in treatment at intake, they were asked if they had ever been on medication since that time. Finally, they were asked if they had had a medical examination while incarcerated.
Almost all prisoners (90%) were screened at intake and received a medical evaluation. The researchers found that 5,207 (26.2%) of the inmates received at least one lifetime mental health diagnosis, with depression being the most common. Eighteen percent reported taking medication for a psychiatric disorder at the time of intake, but of these, only about half were taking medication after incarceration. Medication continuance was twice as likely for schizophrenia as for depression, and those who received an intake screen were significantly more likely to be referred to a physician and receive medication. Notably, 27% of state and 16% of federal prisoners received medication only in prison.
The investigators attributed the discontinuity in treatment after incarceration to a lack of trained mental health professionals to diagnose and treat psychiatric disorders, and to a rise in prison populations out of proportion to available treatment services. The findings of this study supported the investigators’ recommendation for all facilities to employ intake screening to identify and refer prisoners in need of psychiatric care.
This research is important to forensic psychiatrists working in correctional systems and to those working as administrators or external court-appointed monitors, because it highlights the importance and efficacy of intake screening for prisoners with psychiatric disorders.
Unfortunately, traditional media coverage of this research was predictably provocative. Headlines blared: “Mental health care lacking in state and federal prisons.” The researchers themselves also implied that failure to continue medication in prison indicated some systemic deficiency. While an evaluation by a physician was correlated with the prescription of medication, this was not a perfect correlation. The authors did not discuss any of the valid reasons why medication might not be continued in prison.
The most common reason medication is not continued is that inmates are more likely to be abstinent from drugs and alcohol, and thus may require less or even no antidepressant medication after detoxification. This could account for as much as half of the treatment discontinuity, because as many as 20% of the prisoners had been diagnosed with depression. Also, evidence is mounting that indefinite medication may not be necessary for all conditions. Following a clinical assessment, a correctional physician may have determined that the prisoner had successfully completed maintenance therapy. The final reason why medication is not continued is that the inmate may simply have refused the offered treatment; some prisoners do choose to go without medication completely rather than risk a change to a different regimen.
All of this assumes that the subjects accurately reported both their psychiatric history and their mental health service contact following incarceration. Without access to current records or past documentation, this remains an open question. In my prison system, our electronic health record does contain some data provided by the public health care system. In rare cases, this information is consistent with what the patient reports, but this is the exception rather than the rule.
Finally, the most telling aspect of this research is what it reveals about free society care: A quarter of the inmates received treatment only in prison. That is the real finding worthy of a headline.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In a study published in the December 2014 issue of American Journal of Public Health, Jennifer M. Reingle Gonzalez, Ph.D., and Nadine M. Connell, Ph.D., of the University of Texas Health Science Center at Houston interviewed a nationally representative sample of all state and federal prisoners with psychiatric disorders to determine whether they had been screened for services at intake and to assess whether screening led to continuity of community treatment.
The subjects were chosen first by selecting a random sample of correctional facilities of varying sizes, from diverse geographic regions. From those facilities, 18,185 prisoners were chosen from among all those incarcerated on a single day in September 2002. Each subject was interviewed personally but also given computer-assisted interviews at different times to ensure recollection accuracy and the confidentiality of clinical data (Am. J. Public Health 2014;104:2328-33).
In the direct interview, each prisoner was asked if he or she had ever been diagnosed with a mental health condition such as depression, bipolar disorder, schizophrenia, posttraumatic stress disorder, an anxiety disorder, or a personality disorder. If any condition had ever been diagnosed, the inmates were then asked if they were taking a psychiatric medication upon admission to the facility. If they were in treatment at intake, they were asked if they had ever been on medication since that time. Finally, they were asked if they had had a medical examination while incarcerated.
Almost all prisoners (90%) were screened at intake and received a medical evaluation. The researchers found that 5,207 (26.2%) of the inmates received at least one lifetime mental health diagnosis, with depression being the most common. Eighteen percent reported taking medication for a psychiatric disorder at the time of intake, but of these, only about half were taking medication after incarceration. Medication continuance was twice as likely for schizophrenia as for depression, and those who received an intake screen were significantly more likely to be referred to a physician and receive medication. Notably, 27% of state and 16% of federal prisoners received medication only in prison.
The investigators attributed the discontinuity in treatment after incarceration to a lack of trained mental health professionals to diagnose and treat psychiatric disorders, and to a rise in prison populations out of proportion to available treatment services. The findings of this study supported the investigators’ recommendation for all facilities to employ intake screening to identify and refer prisoners in need of psychiatric care.
This research is important to forensic psychiatrists working in correctional systems and to those working as administrators or external court-appointed monitors, because it highlights the importance and efficacy of intake screening for prisoners with psychiatric disorders.
Unfortunately, traditional media coverage of this research was predictably provocative. Headlines blared: “Mental health care lacking in state and federal prisons.” The researchers themselves also implied that failure to continue medication in prison indicated some systemic deficiency. While an evaluation by a physician was correlated with the prescription of medication, this was not a perfect correlation. The authors did not discuss any of the valid reasons why medication might not be continued in prison.
The most common reason medication is not continued is that inmates are more likely to be abstinent from drugs and alcohol, and thus may require less or even no antidepressant medication after detoxification. This could account for as much as half of the treatment discontinuity, because as many as 20% of the prisoners had been diagnosed with depression. Also, evidence is mounting that indefinite medication may not be necessary for all conditions. Following a clinical assessment, a correctional physician may have determined that the prisoner had successfully completed maintenance therapy. The final reason why medication is not continued is that the inmate may simply have refused the offered treatment; some prisoners do choose to go without medication completely rather than risk a change to a different regimen.
All of this assumes that the subjects accurately reported both their psychiatric history and their mental health service contact following incarceration. Without access to current records or past documentation, this remains an open question. In my prison system, our electronic health record does contain some data provided by the public health care system. In rare cases, this information is consistent with what the patient reports, but this is the exception rather than the rule.
Finally, the most telling aspect of this research is what it reveals about free society care: A quarter of the inmates received treatment only in prison. That is the real finding worthy of a headline.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
A generalist approach to fertility preservation
More than 500,000 women of reproductive age (20-49) are diagnosed with cancer each year. Fortunately, given advances in timely detection and effective therapy, more than 83% of these women will survive at least 5 years (SEER Cancer Statistics Review, 1975-2011). As a result, more women are concerned about their child-bearing potential after undergoing chemotherapy or radiation, and as reproductive technology advances, the issue of fertility preservation becomes even more salient.
Potential candidates for fertility preservation
The importance of considering fertility preservation for any reproductive-aged woman with a new cancer diagnosis cannot be overemphasized. This is especially true for women who may be receiving gonadotoxic therapies, such as alkylating agents or abdominal/pelvic radiation.
To assess the risk of various cancer treatments to fertility, patients and providers can access the Fertility Risk Tool at www.livestrong.org. The Fertility Risk Tool compiles known data about the risk of amenorrhea from specific cancers, chemotherapy agents, and radiation treatments, based on the woman’s age. The risk for infertility is likely higher than the stated incidence of amenorrhea, but the chart provides an initial counseling tool for health care providers and an overview for patients.
Discussions regarding fertility preservation are essential for any woman from menarche through mid-40s. However, as technological advances are rapidly occurring in reproductive medicine, options such as ovarian tissue cryopreservation are becoming available for prepubescent girls. Counseling these young patients with a new cancer diagnosis should not be overlooked.
Fertility preservation options
In vitro fertilization (IVF) with embryo banking is currently the most successful fertility preservation option and the standard of care. The process involves an IVF cycle, including monitored ovarian stimulation, transvaginal oocyte retrieval, fertilization of the oocytes, and cryopreservation of embryos.
The entire process takes a minimum of 12 days, and usually starts shortly after the onset of menses. The embryos can survive for years in liquid nitrogen and the survival rate of frozen embryos is greater than 95%. The pregnancy rate per embryo transfer cycle depends on the age of the woman when the embryos were created, with women under age 35 having higher live-birth rates compared with women older than 42 (live-birth rates 42.4% versus 17.8%, respectively, according to national summary data from the Society for Assisted Reproductive Technology).
Another option, which may be more attractive for women without a committed male partner, involves egg banking. This process also involves ovarian stimulation and egg retrieval, but fertilization is not performed. Instead the oocytes are cryopreserved, often by way of a vitrification technique shown to have a higher percentage of oocytes that survive the thaw (Fertil. Steril. 2011;96:277-85).
While the data regarding live birth after egg banking are limited, studies have shown reassuring birth outcomes for more than 900 babies from this technology (Reprod. Biomed. Online 2009;18:769-76).
Timing of treatment must be coordinated with the help of an oncologist. For example, many women with breast cancer opt to have their oncological surgery and undergo ovarian stimulation during the 4-6 week recovery period before initiating chemotherapy.
Ovarian tissue banking is considered experimental, but may be the only option available for women who must initiate treatment immediately, or for prepubescent girls. This technology involves surgical removal of part of an ovary, which is divided into small sections and frozen. The options for reproductive potential may include in vitro maturation of the immature oocytes in the strips of ovarian tissue with subsequent fertilization in the laboratory versus transplantation of the segments of ovarian tissue with the goal of some restoration of ovarian function (Hum. Reprod. 2014;29:1931-40).
Another option is the use of a gonadotropin-releasing hormone (GnRH) agonist during therapy to induce a prepubertal state, with the hypothetical goal to decrease damage to immature oocytes. A recent meta-analysis of randomized trials found that suppression with a GnRH agonist during chemotherapy significantly decreased premature ovarian failure in young women (Cancer Treat. Rev. 2014;40:675-83).
To date, the available literature does not address the effect of GnRH agonist use on rates of infertility in cancer survivors.
Special considerations for hormone sensitive cancers
Women with hormone sensitive cancers, such as breast cancer, often have understandable concerns about preserving their fertility and the impact that ovarian stimulation and future pregnancy may have on their prognosis.
For breast cancer patients, an effective adjuvant treatment during ovarian stimulation is an aromatase inhibitor, which lowers the peak estrogen levels compared to a standard ovarian stimulation cycle, with a similar oocyte yield (J. Clin. Endocrinol. Metab. 2006;91:3885-90).
There does not appear to be a difference in recurrence of breast cancer in women who pursued egg or embryo banking versus those who did not (J. Clin. Oncol. 2008;26:2630-5). Even after subsequent successful pregnancies, recurrence risk in hormone sensitive cancers, including breast cancer, is not increased (Cancer 2004;100:465-9). It is important to note that many women with breast cancer are placed on tamoxifen for many years. These women may consider a surrogate or a “tamoxifen break” after consultation with their oncologists.
When to refer
In any pediatric or reproductive-aged woman with a new cancer diagnosis, it is important to have a conversation exploring fertility options as soon as possible after the diagnosis is made. An early referral yields more options prior to initiating treatment and more time for the woman to discuss and consider all approaches to preserving her fertility.
Dr. Mersereau is an associate professor at the University of North Carolina at Chapel Hill, and director of UNC’s Fertility Preservation Program. Dr. Hoff is a clinical instructor and a fellow in reproductive endocrinology and infertility at UNC-Chapel Hill.
More than 500,000 women of reproductive age (20-49) are diagnosed with cancer each year. Fortunately, given advances in timely detection and effective therapy, more than 83% of these women will survive at least 5 years (SEER Cancer Statistics Review, 1975-2011). As a result, more women are concerned about their child-bearing potential after undergoing chemotherapy or radiation, and as reproductive technology advances, the issue of fertility preservation becomes even more salient.
Potential candidates for fertility preservation
The importance of considering fertility preservation for any reproductive-aged woman with a new cancer diagnosis cannot be overemphasized. This is especially true for women who may be receiving gonadotoxic therapies, such as alkylating agents or abdominal/pelvic radiation.
To assess the risk of various cancer treatments to fertility, patients and providers can access the Fertility Risk Tool at www.livestrong.org. The Fertility Risk Tool compiles known data about the risk of amenorrhea from specific cancers, chemotherapy agents, and radiation treatments, based on the woman’s age. The risk for infertility is likely higher than the stated incidence of amenorrhea, but the chart provides an initial counseling tool for health care providers and an overview for patients.
Discussions regarding fertility preservation are essential for any woman from menarche through mid-40s. However, as technological advances are rapidly occurring in reproductive medicine, options such as ovarian tissue cryopreservation are becoming available for prepubescent girls. Counseling these young patients with a new cancer diagnosis should not be overlooked.
Fertility preservation options
In vitro fertilization (IVF) with embryo banking is currently the most successful fertility preservation option and the standard of care. The process involves an IVF cycle, including monitored ovarian stimulation, transvaginal oocyte retrieval, fertilization of the oocytes, and cryopreservation of embryos.
The entire process takes a minimum of 12 days, and usually starts shortly after the onset of menses. The embryos can survive for years in liquid nitrogen and the survival rate of frozen embryos is greater than 95%. The pregnancy rate per embryo transfer cycle depends on the age of the woman when the embryos were created, with women under age 35 having higher live-birth rates compared with women older than 42 (live-birth rates 42.4% versus 17.8%, respectively, according to national summary data from the Society for Assisted Reproductive Technology).
Another option, which may be more attractive for women without a committed male partner, involves egg banking. This process also involves ovarian stimulation and egg retrieval, but fertilization is not performed. Instead the oocytes are cryopreserved, often by way of a vitrification technique shown to have a higher percentage of oocytes that survive the thaw (Fertil. Steril. 2011;96:277-85).
While the data regarding live birth after egg banking are limited, studies have shown reassuring birth outcomes for more than 900 babies from this technology (Reprod. Biomed. Online 2009;18:769-76).
Timing of treatment must be coordinated with the help of an oncologist. For example, many women with breast cancer opt to have their oncological surgery and undergo ovarian stimulation during the 4-6 week recovery period before initiating chemotherapy.
Ovarian tissue banking is considered experimental, but may be the only option available for women who must initiate treatment immediately, or for prepubescent girls. This technology involves surgical removal of part of an ovary, which is divided into small sections and frozen. The options for reproductive potential may include in vitro maturation of the immature oocytes in the strips of ovarian tissue with subsequent fertilization in the laboratory versus transplantation of the segments of ovarian tissue with the goal of some restoration of ovarian function (Hum. Reprod. 2014;29:1931-40).
Another option is the use of a gonadotropin-releasing hormone (GnRH) agonist during therapy to induce a prepubertal state, with the hypothetical goal to decrease damage to immature oocytes. A recent meta-analysis of randomized trials found that suppression with a GnRH agonist during chemotherapy significantly decreased premature ovarian failure in young women (Cancer Treat. Rev. 2014;40:675-83).
To date, the available literature does not address the effect of GnRH agonist use on rates of infertility in cancer survivors.
Special considerations for hormone sensitive cancers
Women with hormone sensitive cancers, such as breast cancer, often have understandable concerns about preserving their fertility and the impact that ovarian stimulation and future pregnancy may have on their prognosis.
For breast cancer patients, an effective adjuvant treatment during ovarian stimulation is an aromatase inhibitor, which lowers the peak estrogen levels compared to a standard ovarian stimulation cycle, with a similar oocyte yield (J. Clin. Endocrinol. Metab. 2006;91:3885-90).
There does not appear to be a difference in recurrence of breast cancer in women who pursued egg or embryo banking versus those who did not (J. Clin. Oncol. 2008;26:2630-5). Even after subsequent successful pregnancies, recurrence risk in hormone sensitive cancers, including breast cancer, is not increased (Cancer 2004;100:465-9). It is important to note that many women with breast cancer are placed on tamoxifen for many years. These women may consider a surrogate or a “tamoxifen break” after consultation with their oncologists.
When to refer
In any pediatric or reproductive-aged woman with a new cancer diagnosis, it is important to have a conversation exploring fertility options as soon as possible after the diagnosis is made. An early referral yields more options prior to initiating treatment and more time for the woman to discuss and consider all approaches to preserving her fertility.
Dr. Mersereau is an associate professor at the University of North Carolina at Chapel Hill, and director of UNC’s Fertility Preservation Program. Dr. Hoff is a clinical instructor and a fellow in reproductive endocrinology and infertility at UNC-Chapel Hill.
More than 500,000 women of reproductive age (20-49) are diagnosed with cancer each year. Fortunately, given advances in timely detection and effective therapy, more than 83% of these women will survive at least 5 years (SEER Cancer Statistics Review, 1975-2011). As a result, more women are concerned about their child-bearing potential after undergoing chemotherapy or radiation, and as reproductive technology advances, the issue of fertility preservation becomes even more salient.
Potential candidates for fertility preservation
The importance of considering fertility preservation for any reproductive-aged woman with a new cancer diagnosis cannot be overemphasized. This is especially true for women who may be receiving gonadotoxic therapies, such as alkylating agents or abdominal/pelvic radiation.
To assess the risk of various cancer treatments to fertility, patients and providers can access the Fertility Risk Tool at www.livestrong.org. The Fertility Risk Tool compiles known data about the risk of amenorrhea from specific cancers, chemotherapy agents, and radiation treatments, based on the woman’s age. The risk for infertility is likely higher than the stated incidence of amenorrhea, but the chart provides an initial counseling tool for health care providers and an overview for patients.
Discussions regarding fertility preservation are essential for any woman from menarche through mid-40s. However, as technological advances are rapidly occurring in reproductive medicine, options such as ovarian tissue cryopreservation are becoming available for prepubescent girls. Counseling these young patients with a new cancer diagnosis should not be overlooked.
Fertility preservation options
In vitro fertilization (IVF) with embryo banking is currently the most successful fertility preservation option and the standard of care. The process involves an IVF cycle, including monitored ovarian stimulation, transvaginal oocyte retrieval, fertilization of the oocytes, and cryopreservation of embryos.
The entire process takes a minimum of 12 days, and usually starts shortly after the onset of menses. The embryos can survive for years in liquid nitrogen and the survival rate of frozen embryos is greater than 95%. The pregnancy rate per embryo transfer cycle depends on the age of the woman when the embryos were created, with women under age 35 having higher live-birth rates compared with women older than 42 (live-birth rates 42.4% versus 17.8%, respectively, according to national summary data from the Society for Assisted Reproductive Technology).
Another option, which may be more attractive for women without a committed male partner, involves egg banking. This process also involves ovarian stimulation and egg retrieval, but fertilization is not performed. Instead the oocytes are cryopreserved, often by way of a vitrification technique shown to have a higher percentage of oocytes that survive the thaw (Fertil. Steril. 2011;96:277-85).
While the data regarding live birth after egg banking are limited, studies have shown reassuring birth outcomes for more than 900 babies from this technology (Reprod. Biomed. Online 2009;18:769-76).
Timing of treatment must be coordinated with the help of an oncologist. For example, many women with breast cancer opt to have their oncological surgery and undergo ovarian stimulation during the 4-6 week recovery period before initiating chemotherapy.
Ovarian tissue banking is considered experimental, but may be the only option available for women who must initiate treatment immediately, or for prepubescent girls. This technology involves surgical removal of part of an ovary, which is divided into small sections and frozen. The options for reproductive potential may include in vitro maturation of the immature oocytes in the strips of ovarian tissue with subsequent fertilization in the laboratory versus transplantation of the segments of ovarian tissue with the goal of some restoration of ovarian function (Hum. Reprod. 2014;29:1931-40).
Another option is the use of a gonadotropin-releasing hormone (GnRH) agonist during therapy to induce a prepubertal state, with the hypothetical goal to decrease damage to immature oocytes. A recent meta-analysis of randomized trials found that suppression with a GnRH agonist during chemotherapy significantly decreased premature ovarian failure in young women (Cancer Treat. Rev. 2014;40:675-83).
To date, the available literature does not address the effect of GnRH agonist use on rates of infertility in cancer survivors.
Special considerations for hormone sensitive cancers
Women with hormone sensitive cancers, such as breast cancer, often have understandable concerns about preserving their fertility and the impact that ovarian stimulation and future pregnancy may have on their prognosis.
For breast cancer patients, an effective adjuvant treatment during ovarian stimulation is an aromatase inhibitor, which lowers the peak estrogen levels compared to a standard ovarian stimulation cycle, with a similar oocyte yield (J. Clin. Endocrinol. Metab. 2006;91:3885-90).
There does not appear to be a difference in recurrence of breast cancer in women who pursued egg or embryo banking versus those who did not (J. Clin. Oncol. 2008;26:2630-5). Even after subsequent successful pregnancies, recurrence risk in hormone sensitive cancers, including breast cancer, is not increased (Cancer 2004;100:465-9). It is important to note that many women with breast cancer are placed on tamoxifen for many years. These women may consider a surrogate or a “tamoxifen break” after consultation with their oncologists.
When to refer
In any pediatric or reproductive-aged woman with a new cancer diagnosis, it is important to have a conversation exploring fertility options as soon as possible after the diagnosis is made. An early referral yields more options prior to initiating treatment and more time for the woman to discuss and consider all approaches to preserving her fertility.
Dr. Mersereau is an associate professor at the University of North Carolina at Chapel Hill, and director of UNC’s Fertility Preservation Program. Dr. Hoff is a clinical instructor and a fellow in reproductive endocrinology and infertility at UNC-Chapel Hill.
Doctors are easy targets for threats and attacks
Buried under news of the terrible Charlie Hebdo terrorism murders was another serious attack. A doctor was shot and killed by an angry patient at a Texas VA hospital, who then took his own life.
I’m not trying to belittle the tragedy in Paris, but instead point out that medicine can be more hazardous than many realize.
We don’t intentionally try to offend, but in a field like this, it’s impossible to please everyone. People get upset that I can’t cure them or find a cause for their (medically unexplainable) symptoms, or won’t give them as many narcotics as they want. The unhappy ones never come back, or post an angry review on Yelp, or send a nasty letter, or some combination of the above.
But, occasionally, we get threats. They’re rare in an office practice, though I suspect surprisingly common in emergency department work. Most are empty threats to sue, but occasionally my staff and I get threatened with physical harm. While most are simply words, there’s really no easy way of knowing who will or won’t actually snap and carry them out.
We live in a society where guns are common, easily obtained, and affordable. So anyone might have one. Unless your office has a metal detector or does pat downs, you’re at risk (at least hypothetically). Putting up a sign that says “no guns allowed” isn’t going to stop anyone. Neither do laws to protect health professionals. Those who have decided to harm others don’t worry about such things.
For that matter, I have several patients who usually have a gun on them. Sometimes concealed, sometimes obvious. Does it bother me? Not at all. They’re all polite and pleasant, and I understand their reason for keeping one on hand.
But doctors, unfortunately, are easy targets for the irrational and armed. The shooting in El Paso occurred in a government hospital with armed security, and that certainly didn’t make a difference. We generally keep predictable hours, park in the same spaces, and our offices aren’t locked up. We do a job where trust is assumed, because people are coming to us for help and we’re here for their benefit.
Is there an answer? I know doctors who keep a handgun under their coats, or in their desks. In a perfect world, they wouldn’t need it, but our world is far from it. Being a doctor, whether you’re on the front line in the emergency department or hidden in a nameless medical plaza, can still be a dangerous business.
Medicine is a surprising field to think of as a hazardous one, but these days, sadly, it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Buried under news of the terrible Charlie Hebdo terrorism murders was another serious attack. A doctor was shot and killed by an angry patient at a Texas VA hospital, who then took his own life.
I’m not trying to belittle the tragedy in Paris, but instead point out that medicine can be more hazardous than many realize.
We don’t intentionally try to offend, but in a field like this, it’s impossible to please everyone. People get upset that I can’t cure them or find a cause for their (medically unexplainable) symptoms, or won’t give them as many narcotics as they want. The unhappy ones never come back, or post an angry review on Yelp, or send a nasty letter, or some combination of the above.
But, occasionally, we get threats. They’re rare in an office practice, though I suspect surprisingly common in emergency department work. Most are empty threats to sue, but occasionally my staff and I get threatened with physical harm. While most are simply words, there’s really no easy way of knowing who will or won’t actually snap and carry them out.
We live in a society where guns are common, easily obtained, and affordable. So anyone might have one. Unless your office has a metal detector or does pat downs, you’re at risk (at least hypothetically). Putting up a sign that says “no guns allowed” isn’t going to stop anyone. Neither do laws to protect health professionals. Those who have decided to harm others don’t worry about such things.
For that matter, I have several patients who usually have a gun on them. Sometimes concealed, sometimes obvious. Does it bother me? Not at all. They’re all polite and pleasant, and I understand their reason for keeping one on hand.
But doctors, unfortunately, are easy targets for the irrational and armed. The shooting in El Paso occurred in a government hospital with armed security, and that certainly didn’t make a difference. We generally keep predictable hours, park in the same spaces, and our offices aren’t locked up. We do a job where trust is assumed, because people are coming to us for help and we’re here for their benefit.
Is there an answer? I know doctors who keep a handgun under their coats, or in their desks. In a perfect world, they wouldn’t need it, but our world is far from it. Being a doctor, whether you’re on the front line in the emergency department or hidden in a nameless medical plaza, can still be a dangerous business.
Medicine is a surprising field to think of as a hazardous one, but these days, sadly, it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Buried under news of the terrible Charlie Hebdo terrorism murders was another serious attack. A doctor was shot and killed by an angry patient at a Texas VA hospital, who then took his own life.
I’m not trying to belittle the tragedy in Paris, but instead point out that medicine can be more hazardous than many realize.
We don’t intentionally try to offend, but in a field like this, it’s impossible to please everyone. People get upset that I can’t cure them or find a cause for their (medically unexplainable) symptoms, or won’t give them as many narcotics as they want. The unhappy ones never come back, or post an angry review on Yelp, or send a nasty letter, or some combination of the above.
But, occasionally, we get threats. They’re rare in an office practice, though I suspect surprisingly common in emergency department work. Most are empty threats to sue, but occasionally my staff and I get threatened with physical harm. While most are simply words, there’s really no easy way of knowing who will or won’t actually snap and carry them out.
We live in a society where guns are common, easily obtained, and affordable. So anyone might have one. Unless your office has a metal detector or does pat downs, you’re at risk (at least hypothetically). Putting up a sign that says “no guns allowed” isn’t going to stop anyone. Neither do laws to protect health professionals. Those who have decided to harm others don’t worry about such things.
For that matter, I have several patients who usually have a gun on them. Sometimes concealed, sometimes obvious. Does it bother me? Not at all. They’re all polite and pleasant, and I understand their reason for keeping one on hand.
But doctors, unfortunately, are easy targets for the irrational and armed. The shooting in El Paso occurred in a government hospital with armed security, and that certainly didn’t make a difference. We generally keep predictable hours, park in the same spaces, and our offices aren’t locked up. We do a job where trust is assumed, because people are coming to us for help and we’re here for their benefit.
Is there an answer? I know doctors who keep a handgun under their coats, or in their desks. In a perfect world, they wouldn’t need it, but our world is far from it. Being a doctor, whether you’re on the front line in the emergency department or hidden in a nameless medical plaza, can still be a dangerous business.
Medicine is a surprising field to think of as a hazardous one, but these days, sadly, it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Transfusion linked to bad outcomes in percutaneous peripheral vascular interventions
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: High institutional blood transfusion rates in conjunction with percutaneous interventions for peripheral arterial disease can be sharply and safely lowered through a focused quality improvement program.
Major finding: The average periprocedural transfusion rate at 44 Michigan hospitals fell from 6.6% to 3.2% in response to the performance improvement program.
Data source: A retrospective analysis of prospectively gathered data on 18,127 Michigan patients who underwent nonhybrid percutaneous interventions for peripheral arterial disease.
Disclosures: The study was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network. The presenter reported having no financial conflicts.
Imaging reveals how HSPCs interact with niche
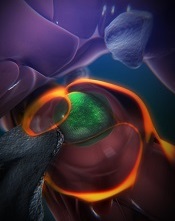
(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()

(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()

(green) in a zebrafish
Boston Children’s Hospital
Using a zebrafish model and enhanced imaging, a group of researchers discovered how hematopoietic stem and progenitor cells (HSPCs) interact with their niche.
Subsequent imaging in mice showed that HSPCs behaved the same way in mammals, which suggests similar results might be observed in humans.
In fact, the researchers are already using the results of this study to inform research on hematopoietic stem cell transplants.
“The same process occurs during a bone marrow transplant as occurs in the body naturally,” said senior study investigator Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“Our direct visualization gives us a series of steps to target, and, in theory, we can look for drugs that affect every step of that process.”
He and his colleagues described this research in a paper published in Cell, as well as in two animations on YouTube, one that’s general and one more technical.
“Stem cell and bone marrow transplants are still very much a black box,” said study author Owen Tamplin, PhD, also of Boston Children’s Hospital.
“Cells are introduced into a patient, and, later on, we can measure recovery of their blood system, but what happens in between can’t be seen. Now, we have a system where we can actually watch that middle step.”
The researchers already knew that HSPCs bud off from cells in the aorta, then circulate in the body until they find a niche where they’re prepped for creating blood.
With the current study, the team observed how this niche forms, using time-lapse imaging of naturally transparent zebrafish embryos and a genetic modification that tagged the HSPCs green.
On arrival in its niche (in the tail in zebrafish), the newborn HSPC attaches itself to the blood vessel wall. There, chemical signals prompt it to squeeze itself through the wall and into a space just outside the blood vessel. Other cells begin to interact with the HSPC, and nearby endothelial cells wrap themselves around it.
“We think that is the beginning of making a stem cell happy in its niche, like a mother cuddling a baby,” Dr Zon said.
As the HSPC is being “cuddled,” it’s brought into contact with a nearby stromal cell that helps keep it attached.
The “cuddling” was reconstructed from confocal and electron microscopy images of the zebrafish taken during this stage. Through a series of image slices, the researchers were able to reassemble the whole 3D structure—HSPC, cuddling endothelial cells, and stromal cells.
“Nobody’s ever visualized live how a stem cell interacts with its niche,” Dr Zon said. “This is the first time we get a very high-resolution view of the process.”
Next, the cuddled HSPC begins dividing. One daughter cell leaves the niche, while the other stays. Eventually, all the HSPCs leave and begin colonizing their future site of blood production. (In zebrafish, this is in the kidney, which is similar to mammalian bone marrow.)
Additional imaging in mice revealed evidence that HSPCs go through much the same process in mammals, which makes it likely in humans too.
These detailed observations are already informing the Zon lab’s attempt to improve stem cell transplants. By conducting a chemical screen in large numbers of zebrafish embryos, the researchers found that the compound lycorine promotes interaction between the HSPC and its niche, leading to greater numbers of HSPCs in the adult fish. ![]()
IPT doesn’t seem to benefit children with anemia

one of the trials took place
Credit: Gabrielle Tenenbaum
Intermittent preventive antimalarial treatment (IPT) does not provide much benefit for anemic children living in malaria-endemic regions, results of a review indicate.
Researchers reviewed 6 randomized, controlled trials and found that IPT, doses of antimalarial drugs given at regular intervals in case children had contracted malaria, did lead to an improvement in hemoglobin levels.
But this did not translate to a reduction in the incidence of anemia or the rate of death and hospitalization compared to children who received placebo.
“While we did note small benefits in hemoglobin levels when treating anemic children with IPT, there was no detectable effect on the number of deaths or hospital admissions,” said review author Mwaka Athuman, of Ifakara Health Institute in Dodoma, Tanzania, Africa.
“However, 3 of the trials were carried out in areas where malaria transmission was low, so any estimate of the protective effect of IPT would be expected to be modest. The summary of the evidence will assist people forming policy guidance as to whether IPT is worthwhile and provide a basis for researchers to consider whether additional studies are needed.”
The researchers reported these findings in the Cochrane Database of Systematic Reviews.
The team reviewed 6 trials that included a total of 3847 children with anemia. Three trials were conducted in areas of low malaria endemicity and 3 in areas of moderate-to-high endemicity.
In all trials, there was a group of children who received IPT and a control group receiving placebo. In some trials, children also received iron supplements, and this was taken into consideration when the researchers analyzed the data.
Data from 4 of the trials showed that IPT did increase the mean change in hemoglobin levels from baseline to follow-up at 12 weeks—on average, by 0.32 g/dL. The reviewers dubbed this moderate-quality evidence.
Results from the same 4 trials showed that the mean hemoglobin at 12 weeks’ follow-up was, on average, 0.35 g/dL higher in the IPT group than in the placebo group. This was considered low-quality evidence.
Regardless of improvements in hemoglobin, there was no significant difference in the number of children who had anemia at 12 weeks. The median risk of anemia across 4 trials was 579 per 1000 in the placebo group and 561 per 1000 in the IPT group. This was considered moderate-quality evidence.
Similarly, there was no significant difference in the rate of death and hospitalization at 6 months between children who received IPT and those who received placebo.
The median risk of both events combined was 34 per 1000 in the placebo group and 31 per 1000 in the IPT group. This was based on data from 3 trials and was considered moderate-quality evidence.
For all of these outcomes, there was no significant difference between children who received iron and those who did not, and there was no difference between children living in regions of low malaria endemicity and those living in regions of moderate-to-high malaria endemicity. ![]()

one of the trials took place
Credit: Gabrielle Tenenbaum
Intermittent preventive antimalarial treatment (IPT) does not provide much benefit for anemic children living in malaria-endemic regions, results of a review indicate.
Researchers reviewed 6 randomized, controlled trials and found that IPT, doses of antimalarial drugs given at regular intervals in case children had contracted malaria, did lead to an improvement in hemoglobin levels.
But this did not translate to a reduction in the incidence of anemia or the rate of death and hospitalization compared to children who received placebo.
“While we did note small benefits in hemoglobin levels when treating anemic children with IPT, there was no detectable effect on the number of deaths or hospital admissions,” said review author Mwaka Athuman, of Ifakara Health Institute in Dodoma, Tanzania, Africa.
“However, 3 of the trials were carried out in areas where malaria transmission was low, so any estimate of the protective effect of IPT would be expected to be modest. The summary of the evidence will assist people forming policy guidance as to whether IPT is worthwhile and provide a basis for researchers to consider whether additional studies are needed.”
The researchers reported these findings in the Cochrane Database of Systematic Reviews.
The team reviewed 6 trials that included a total of 3847 children with anemia. Three trials were conducted in areas of low malaria endemicity and 3 in areas of moderate-to-high endemicity.
In all trials, there was a group of children who received IPT and a control group receiving placebo. In some trials, children also received iron supplements, and this was taken into consideration when the researchers analyzed the data.
Data from 4 of the trials showed that IPT did increase the mean change in hemoglobin levels from baseline to follow-up at 12 weeks—on average, by 0.32 g/dL. The reviewers dubbed this moderate-quality evidence.
Results from the same 4 trials showed that the mean hemoglobin at 12 weeks’ follow-up was, on average, 0.35 g/dL higher in the IPT group than in the placebo group. This was considered low-quality evidence.
Regardless of improvements in hemoglobin, there was no significant difference in the number of children who had anemia at 12 weeks. The median risk of anemia across 4 trials was 579 per 1000 in the placebo group and 561 per 1000 in the IPT group. This was considered moderate-quality evidence.
Similarly, there was no significant difference in the rate of death and hospitalization at 6 months between children who received IPT and those who received placebo.
The median risk of both events combined was 34 per 1000 in the placebo group and 31 per 1000 in the IPT group. This was based on data from 3 trials and was considered moderate-quality evidence.
For all of these outcomes, there was no significant difference between children who received iron and those who did not, and there was no difference between children living in regions of low malaria endemicity and those living in regions of moderate-to-high malaria endemicity. ![]()

one of the trials took place
Credit: Gabrielle Tenenbaum
Intermittent preventive antimalarial treatment (IPT) does not provide much benefit for anemic children living in malaria-endemic regions, results of a review indicate.
Researchers reviewed 6 randomized, controlled trials and found that IPT, doses of antimalarial drugs given at regular intervals in case children had contracted malaria, did lead to an improvement in hemoglobin levels.
But this did not translate to a reduction in the incidence of anemia or the rate of death and hospitalization compared to children who received placebo.
“While we did note small benefits in hemoglobin levels when treating anemic children with IPT, there was no detectable effect on the number of deaths or hospital admissions,” said review author Mwaka Athuman, of Ifakara Health Institute in Dodoma, Tanzania, Africa.
“However, 3 of the trials were carried out in areas where malaria transmission was low, so any estimate of the protective effect of IPT would be expected to be modest. The summary of the evidence will assist people forming policy guidance as to whether IPT is worthwhile and provide a basis for researchers to consider whether additional studies are needed.”
The researchers reported these findings in the Cochrane Database of Systematic Reviews.
The team reviewed 6 trials that included a total of 3847 children with anemia. Three trials were conducted in areas of low malaria endemicity and 3 in areas of moderate-to-high endemicity.
In all trials, there was a group of children who received IPT and a control group receiving placebo. In some trials, children also received iron supplements, and this was taken into consideration when the researchers analyzed the data.
Data from 4 of the trials showed that IPT did increase the mean change in hemoglobin levels from baseline to follow-up at 12 weeks—on average, by 0.32 g/dL. The reviewers dubbed this moderate-quality evidence.
Results from the same 4 trials showed that the mean hemoglobin at 12 weeks’ follow-up was, on average, 0.35 g/dL higher in the IPT group than in the placebo group. This was considered low-quality evidence.
Regardless of improvements in hemoglobin, there was no significant difference in the number of children who had anemia at 12 weeks. The median risk of anemia across 4 trials was 579 per 1000 in the placebo group and 561 per 1000 in the IPT group. This was considered moderate-quality evidence.
Similarly, there was no significant difference in the rate of death and hospitalization at 6 months between children who received IPT and those who received placebo.
The median risk of both events combined was 34 per 1000 in the placebo group and 31 per 1000 in the IPT group. This was based on data from 3 trials and was considered moderate-quality evidence.
For all of these outcomes, there was no significant difference between children who received iron and those who did not, and there was no difference between children living in regions of low malaria endemicity and those living in regions of moderate-to-high malaria endemicity. ![]()
Investigation of simulated saline continues

Credit: FDA
The US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are still investigating multiple instances of Wallcur’s simulated intravenous (IV) saline products being administered to patients.
So far, more than 40 patients have received infusions of Wallcur’s simulated IV saline solution, Practi-0.9% sodium chloride solution, which is intended for training purposes only. The product is not sterile and should not be injected in humans or animals.
There have been many adverse events associated with the infusions, including fever, chills, tremors, and headache. Some patients were hospitalized, and there has been 1 death, although it’s not clear if this death is directly related to the product.
Adverse events have been reported in 7 states: Florida, Georgia, Idaho, Louisiana, North Carolina, New York, and Colorado.
The FDA, in partnership with the CDC, has collected samples of Wallcur Practi 0.9% sodium chloride solution from clinics and distributors. These products are being tested to determine if they caused the adverse events observed in patients.
In addition, Wallcur has initiated a voluntary recall of Practi-0.9% sodium chloride IV solutions.
Most medical facilities that received the product said they were unaware that the IV solution bags were simulation products. However, at least one clinic recognized the Wallcur product was a simulation product upon receipt and returned it to the distributor.
The FDA said it is working with distributors who sold the simulated IV products and clinics that purchased and administered the products from Wallcur to determine how these products entered the supply chain and were administered to patients.
While Sodium Chloride 0.9% Injection (normal saline) has been in short supply, the FDA has been working with manufacturers to end the shortage.
The FDA has allowed the temporary distribution of additional IV normal saline from alternate sources: Fresenius Kabi USA, Baxter Healthcare Corp., and B. Braun Medical Inc. Currently, normal saline is available from several manufacturers, as posted on the FDA’s website.
FDA recommendations
The FDA is encouraging healthcare providers to ensure IV solution simulation products are removed from office inventory to eliminate the possible injection of Wallcur simulated products into patients.
Providers should visually inspect all current IV saline solution bags to ensure none of the bags are labeled “Wallcur,” “Practi-products,” “For clinical simulation,” or “Not for use in human or animal patients.”
If you have products labeled with any of these words or suspect you may have received other products intended for training purposes, separate simulation products from existing inventory, and contact your distributor for directions on how to return these products.
If you have received Wallcur Practi-products by mistake, please contact the distributor, or Wallcur, LLC of San Diego for return instructions.
Consider reviewing your office procedures and make sure there are procedures in place to visually inspect all future shipments of normal saline products to ensure they are for clinical use.
If you suspect that any Wallcur training IV products may have been administered to a patient, whether or not the incident has resulted in an adverse event:
- Evaluate all potentially exposed patients with new or ongoing symptoms
- Use appropriate treatment
- Report suspected cases to the state health department
- Report any adverse events following the use of these products to the FDA’s MedWatch program online or at 1-800-332-1088.
Patients who believe they received an injection of Wallcur simulated IV solution should contact their healthcare provider.
Patients who received simulated IV saline experienced fever, chills, muscle aches, and headaches almost immediately upon injection, and some required hospitalization. In most reported cases, these signs and symptoms were immediately recognized, and patients received appropriate medical attention.
Wholesalers, distributors, and suppliers of IV saline products should inspect their inventory to ensure they are not distributing simulated products as clinical-use products.
If you suspect you may have distributed Wallcur simulated IV solution to clients by mistake, immediately attempt to recall the products and warn clients of the potential risks. You should also contact Wallcur and your distributor and file a report with the FDA’s MedWatch program. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are still investigating multiple instances of Wallcur’s simulated intravenous (IV) saline products being administered to patients.
So far, more than 40 patients have received infusions of Wallcur’s simulated IV saline solution, Practi-0.9% sodium chloride solution, which is intended for training purposes only. The product is not sterile and should not be injected in humans or animals.
There have been many adverse events associated with the infusions, including fever, chills, tremors, and headache. Some patients were hospitalized, and there has been 1 death, although it’s not clear if this death is directly related to the product.
Adverse events have been reported in 7 states: Florida, Georgia, Idaho, Louisiana, North Carolina, New York, and Colorado.
The FDA, in partnership with the CDC, has collected samples of Wallcur Practi 0.9% sodium chloride solution from clinics and distributors. These products are being tested to determine if they caused the adverse events observed in patients.
In addition, Wallcur has initiated a voluntary recall of Practi-0.9% sodium chloride IV solutions.
Most medical facilities that received the product said they were unaware that the IV solution bags were simulation products. However, at least one clinic recognized the Wallcur product was a simulation product upon receipt and returned it to the distributor.
The FDA said it is working with distributors who sold the simulated IV products and clinics that purchased and administered the products from Wallcur to determine how these products entered the supply chain and were administered to patients.
While Sodium Chloride 0.9% Injection (normal saline) has been in short supply, the FDA has been working with manufacturers to end the shortage.
The FDA has allowed the temporary distribution of additional IV normal saline from alternate sources: Fresenius Kabi USA, Baxter Healthcare Corp., and B. Braun Medical Inc. Currently, normal saline is available from several manufacturers, as posted on the FDA’s website.
FDA recommendations
The FDA is encouraging healthcare providers to ensure IV solution simulation products are removed from office inventory to eliminate the possible injection of Wallcur simulated products into patients.
Providers should visually inspect all current IV saline solution bags to ensure none of the bags are labeled “Wallcur,” “Practi-products,” “For clinical simulation,” or “Not for use in human or animal patients.”
If you have products labeled with any of these words or suspect you may have received other products intended for training purposes, separate simulation products from existing inventory, and contact your distributor for directions on how to return these products.
If you have received Wallcur Practi-products by mistake, please contact the distributor, or Wallcur, LLC of San Diego for return instructions.
Consider reviewing your office procedures and make sure there are procedures in place to visually inspect all future shipments of normal saline products to ensure they are for clinical use.
If you suspect that any Wallcur training IV products may have been administered to a patient, whether or not the incident has resulted in an adverse event:
- Evaluate all potentially exposed patients with new or ongoing symptoms
- Use appropriate treatment
- Report suspected cases to the state health department
- Report any adverse events following the use of these products to the FDA’s MedWatch program online or at 1-800-332-1088.
Patients who believe they received an injection of Wallcur simulated IV solution should contact their healthcare provider.
Patients who received simulated IV saline experienced fever, chills, muscle aches, and headaches almost immediately upon injection, and some required hospitalization. In most reported cases, these signs and symptoms were immediately recognized, and patients received appropriate medical attention.
Wholesalers, distributors, and suppliers of IV saline products should inspect their inventory to ensure they are not distributing simulated products as clinical-use products.
If you suspect you may have distributed Wallcur simulated IV solution to clients by mistake, immediately attempt to recall the products and warn clients of the potential risks. You should also contact Wallcur and your distributor and file a report with the FDA’s MedWatch program. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are still investigating multiple instances of Wallcur’s simulated intravenous (IV) saline products being administered to patients.
So far, more than 40 patients have received infusions of Wallcur’s simulated IV saline solution, Practi-0.9% sodium chloride solution, which is intended for training purposes only. The product is not sterile and should not be injected in humans or animals.
There have been many adverse events associated with the infusions, including fever, chills, tremors, and headache. Some patients were hospitalized, and there has been 1 death, although it’s not clear if this death is directly related to the product.
Adverse events have been reported in 7 states: Florida, Georgia, Idaho, Louisiana, North Carolina, New York, and Colorado.
The FDA, in partnership with the CDC, has collected samples of Wallcur Practi 0.9% sodium chloride solution from clinics and distributors. These products are being tested to determine if they caused the adverse events observed in patients.
In addition, Wallcur has initiated a voluntary recall of Practi-0.9% sodium chloride IV solutions.
Most medical facilities that received the product said they were unaware that the IV solution bags were simulation products. However, at least one clinic recognized the Wallcur product was a simulation product upon receipt and returned it to the distributor.
The FDA said it is working with distributors who sold the simulated IV products and clinics that purchased and administered the products from Wallcur to determine how these products entered the supply chain and were administered to patients.
While Sodium Chloride 0.9% Injection (normal saline) has been in short supply, the FDA has been working with manufacturers to end the shortage.
The FDA has allowed the temporary distribution of additional IV normal saline from alternate sources: Fresenius Kabi USA, Baxter Healthcare Corp., and B. Braun Medical Inc. Currently, normal saline is available from several manufacturers, as posted on the FDA’s website.
FDA recommendations
The FDA is encouraging healthcare providers to ensure IV solution simulation products are removed from office inventory to eliminate the possible injection of Wallcur simulated products into patients.
Providers should visually inspect all current IV saline solution bags to ensure none of the bags are labeled “Wallcur,” “Practi-products,” “For clinical simulation,” or “Not for use in human or animal patients.”
If you have products labeled with any of these words or suspect you may have received other products intended for training purposes, separate simulation products from existing inventory, and contact your distributor for directions on how to return these products.
If you have received Wallcur Practi-products by mistake, please contact the distributor, or Wallcur, LLC of San Diego for return instructions.
Consider reviewing your office procedures and make sure there are procedures in place to visually inspect all future shipments of normal saline products to ensure they are for clinical use.
If you suspect that any Wallcur training IV products may have been administered to a patient, whether or not the incident has resulted in an adverse event:
- Evaluate all potentially exposed patients with new or ongoing symptoms
- Use appropriate treatment
- Report suspected cases to the state health department
- Report any adverse events following the use of these products to the FDA’s MedWatch program online or at 1-800-332-1088.
Patients who believe they received an injection of Wallcur simulated IV solution should contact their healthcare provider.
Patients who received simulated IV saline experienced fever, chills, muscle aches, and headaches almost immediately upon injection, and some required hospitalization. In most reported cases, these signs and symptoms were immediately recognized, and patients received appropriate medical attention.
Wholesalers, distributors, and suppliers of IV saline products should inspect their inventory to ensure they are not distributing simulated products as clinical-use products.
If you suspect you may have distributed Wallcur simulated IV solution to clients by mistake, immediately attempt to recall the products and warn clients of the potential risks. You should also contact Wallcur and your distributor and file a report with the FDA’s MedWatch program. ![]()
Platelet transfusions may increase risk of death

Platelet transfusions may increase the risk of death in patients with platelet consumptive disorders, results of a large study suggest.
Investigators observed a 2-fold increase in the risk of death among patients with thrombotic thrombocytopenic purpura (TTP) who received platelet transfusions and a 5-fold increase among transfused patients with heparin-induced thrombocytopenia (HIT).
There was no increased risk among patients with immune thrombocytopenia (ITP), however.
Aaron Tobian, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in Blood. They were previously presented at the 2014 AABB Annual Meeting.
“Because these conditions are so rare, they’re difficult to study,” Dr Tobian noted. “There was some suggestion that transfusion may be harmful in these conditions, but it really was not known until now.”
“Our study is the first one to show that platelet transfusions are frequently administered to patients with ITP, HIT, and TTP, and that they’re associated with higher odds of arterial blood clots and mortality in TTP and HIT.”
For this study, Dr Tobian and his colleagues analyzed data from the Nationwide Inpatient Sample, a federal database that contains billing records for about 20% of all patients treated and discharged at about 1000 US community hospitals in 47 states.
The database contains information on about 8 million inpatient hospitalizations per year nationwide. The study covered the years 2007 to 2011.
During that time, there were 79,980 hospital admissions for ITP, 10,624 for TTP, and 6332 for HIT. Platelet transfusions were reported in 10.1% of hospitalizations for TTP, 7.1% for HIT, and 25.8% for ITP.
“Our analysis found no significantly increased risks from platelet transfusions in ITP,” said study author Ruchika Goel, MD, also of Johns Hopkins.
“But in TTP, a platelet transfusion increased the odds of a potentially lethal arterial blood clot more than 5-fold and doubled the odds of a heart attack.”
Specifically, in an age- and gender-adjusted analysis, the odds ratio (OR) for arterial thrombosis was 5.8, and the OR for acute myocardial infarction (AMI) was 2.0 in TTP patients who received platelet transfusions. The OR for stroke was 1.6, and the OR for venous thrombosis was 1.1.
Similarly, HIT patients had an increased risk of arterial thrombosis (OR=3.4) and AMI (OR=1.9) if they received a platelet transfusion. But they did not have an increased risk of venous thrombosis (OR=0.8) or stroke (OR=0.5).
Platelet transfusions among ITP patients were not significantly associated with venous thrombosis (OR=1.3), arterial thrombosis (OR=0.3), AMI (OR=1.3), or stroke (OR=1.3) after adjustment for age and gender.
The all-cause, in-hospital mortality rates were 8.8% for TTP patients, 3.4% for HIT patients, and 1.4% for ITP patients.
Patients with TTP and HIT had a significantly increased risk of all-cause mortality if they received platelet transfusions, with age- and gender-adjusted ORs of 2.0 and 5.2, respectively. But platelet transfusions were not significantly associated with mortality in ITP patients, with an OR of 1.1.
The investigators said they were surprised at the prevalence of platelet transfusions in this patient population, in spite of some practitioners’ concerns about the risks.
But Dr Tobian noted that, in some cases, doctors may not know a patient has a platelet disorder until they see the potentially deadly reaction to the transfusion. And in other cases, the transfusion may be used as a last resort.
He and his colleagues believe that, for patients with HIT and TTP, platelet transfusions should be reserved “only for severe, life-threatening bleeding refractory to other therapies or major surgery.” ![]()

Platelet transfusions may increase the risk of death in patients with platelet consumptive disorders, results of a large study suggest.
Investigators observed a 2-fold increase in the risk of death among patients with thrombotic thrombocytopenic purpura (TTP) who received platelet transfusions and a 5-fold increase among transfused patients with heparin-induced thrombocytopenia (HIT).
There was no increased risk among patients with immune thrombocytopenia (ITP), however.
Aaron Tobian, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in Blood. They were previously presented at the 2014 AABB Annual Meeting.
“Because these conditions are so rare, they’re difficult to study,” Dr Tobian noted. “There was some suggestion that transfusion may be harmful in these conditions, but it really was not known until now.”
“Our study is the first one to show that platelet transfusions are frequently administered to patients with ITP, HIT, and TTP, and that they’re associated with higher odds of arterial blood clots and mortality in TTP and HIT.”
For this study, Dr Tobian and his colleagues analyzed data from the Nationwide Inpatient Sample, a federal database that contains billing records for about 20% of all patients treated and discharged at about 1000 US community hospitals in 47 states.
The database contains information on about 8 million inpatient hospitalizations per year nationwide. The study covered the years 2007 to 2011.
During that time, there were 79,980 hospital admissions for ITP, 10,624 for TTP, and 6332 for HIT. Platelet transfusions were reported in 10.1% of hospitalizations for TTP, 7.1% for HIT, and 25.8% for ITP.
“Our analysis found no significantly increased risks from platelet transfusions in ITP,” said study author Ruchika Goel, MD, also of Johns Hopkins.
“But in TTP, a platelet transfusion increased the odds of a potentially lethal arterial blood clot more than 5-fold and doubled the odds of a heart attack.”
Specifically, in an age- and gender-adjusted analysis, the odds ratio (OR) for arterial thrombosis was 5.8, and the OR for acute myocardial infarction (AMI) was 2.0 in TTP patients who received platelet transfusions. The OR for stroke was 1.6, and the OR for venous thrombosis was 1.1.
Similarly, HIT patients had an increased risk of arterial thrombosis (OR=3.4) and AMI (OR=1.9) if they received a platelet transfusion. But they did not have an increased risk of venous thrombosis (OR=0.8) or stroke (OR=0.5).
Platelet transfusions among ITP patients were not significantly associated with venous thrombosis (OR=1.3), arterial thrombosis (OR=0.3), AMI (OR=1.3), or stroke (OR=1.3) after adjustment for age and gender.
The all-cause, in-hospital mortality rates were 8.8% for TTP patients, 3.4% for HIT patients, and 1.4% for ITP patients.
Patients with TTP and HIT had a significantly increased risk of all-cause mortality if they received platelet transfusions, with age- and gender-adjusted ORs of 2.0 and 5.2, respectively. But platelet transfusions were not significantly associated with mortality in ITP patients, with an OR of 1.1.
The investigators said they were surprised at the prevalence of platelet transfusions in this patient population, in spite of some practitioners’ concerns about the risks.
But Dr Tobian noted that, in some cases, doctors may not know a patient has a platelet disorder until they see the potentially deadly reaction to the transfusion. And in other cases, the transfusion may be used as a last resort.
He and his colleagues believe that, for patients with HIT and TTP, platelet transfusions should be reserved “only for severe, life-threatening bleeding refractory to other therapies or major surgery.” ![]()

Platelet transfusions may increase the risk of death in patients with platelet consumptive disorders, results of a large study suggest.
Investigators observed a 2-fold increase in the risk of death among patients with thrombotic thrombocytopenic purpura (TTP) who received platelet transfusions and a 5-fold increase among transfused patients with heparin-induced thrombocytopenia (HIT).
There was no increased risk among patients with immune thrombocytopenia (ITP), however.
Aaron Tobian, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in Blood. They were previously presented at the 2014 AABB Annual Meeting.
“Because these conditions are so rare, they’re difficult to study,” Dr Tobian noted. “There was some suggestion that transfusion may be harmful in these conditions, but it really was not known until now.”
“Our study is the first one to show that platelet transfusions are frequently administered to patients with ITP, HIT, and TTP, and that they’re associated with higher odds of arterial blood clots and mortality in TTP and HIT.”
For this study, Dr Tobian and his colleagues analyzed data from the Nationwide Inpatient Sample, a federal database that contains billing records for about 20% of all patients treated and discharged at about 1000 US community hospitals in 47 states.
The database contains information on about 8 million inpatient hospitalizations per year nationwide. The study covered the years 2007 to 2011.
During that time, there were 79,980 hospital admissions for ITP, 10,624 for TTP, and 6332 for HIT. Platelet transfusions were reported in 10.1% of hospitalizations for TTP, 7.1% for HIT, and 25.8% for ITP.
“Our analysis found no significantly increased risks from platelet transfusions in ITP,” said study author Ruchika Goel, MD, also of Johns Hopkins.
“But in TTP, a platelet transfusion increased the odds of a potentially lethal arterial blood clot more than 5-fold and doubled the odds of a heart attack.”
Specifically, in an age- and gender-adjusted analysis, the odds ratio (OR) for arterial thrombosis was 5.8, and the OR for acute myocardial infarction (AMI) was 2.0 in TTP patients who received platelet transfusions. The OR for stroke was 1.6, and the OR for venous thrombosis was 1.1.
Similarly, HIT patients had an increased risk of arterial thrombosis (OR=3.4) and AMI (OR=1.9) if they received a platelet transfusion. But they did not have an increased risk of venous thrombosis (OR=0.8) or stroke (OR=0.5).
Platelet transfusions among ITP patients were not significantly associated with venous thrombosis (OR=1.3), arterial thrombosis (OR=0.3), AMI (OR=1.3), or stroke (OR=1.3) after adjustment for age and gender.
The all-cause, in-hospital mortality rates were 8.8% for TTP patients, 3.4% for HIT patients, and 1.4% for ITP patients.
Patients with TTP and HIT had a significantly increased risk of all-cause mortality if they received platelet transfusions, with age- and gender-adjusted ORs of 2.0 and 5.2, respectively. But platelet transfusions were not significantly associated with mortality in ITP patients, with an OR of 1.1.
The investigators said they were surprised at the prevalence of platelet transfusions in this patient population, in spite of some practitioners’ concerns about the risks.
But Dr Tobian noted that, in some cases, doctors may not know a patient has a platelet disorder until they see the potentially deadly reaction to the transfusion. And in other cases, the transfusion may be used as a last resort.
He and his colleagues believe that, for patients with HIT and TTP, platelet transfusions should be reserved “only for severe, life-threatening bleeding refractory to other therapies or major surgery.” ![]()







