User login
Measures predict outcomes of chronic HCV with compensated cirrhosis
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Tiny Lesion Turns Troublesome After Trauma
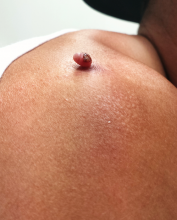
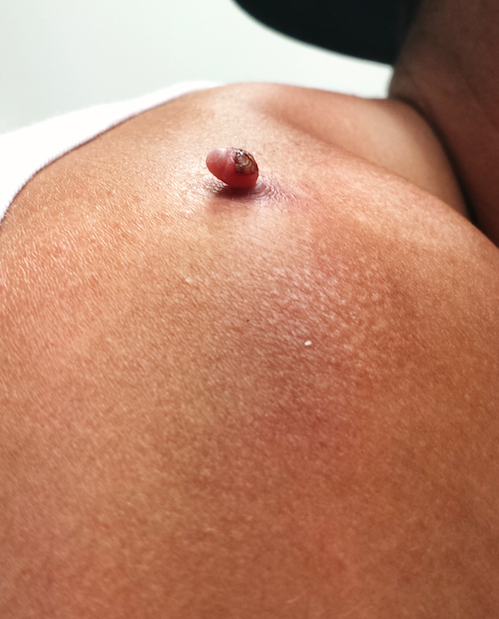
Four months ago, this 23-year-old man developed a lesion on his right shoulder. It appeared, as he recalls, over the course of a week and has subsequently grown. The lesion, which is now rather large, bleeds copiously with minor trauma. It causes only modest discomfort to the patient but considerable worry to his family.
The lesion was originally tiny and tag-like; the patient initially mistook it for a tick and tried to pull it off. Not only did that fail to work, it also seemed to irritate the lesion. At that point, it started to swell, eventually transforming into the lesion he presents with today.
The patient’s history is otherwise uneventful, and he reports taking no medications. He has had minimal sun exposure but says he tans easily when he does get some sun.
EXAMINATION
The lesion, measuring 5 mm x 2.5 mm, is a dark red and pedunculated papule on the crown of the right shoulder. It looks edematous and feels firm. The patient has otherwise unremarkable type IV skin.
Shave biopsy is performed, using a double-edged razor to make a shallow concave defect under the lesion. The wound is cauterized.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the clinical suspicion of pyogenic granuloma, which, ironically, is neither pyogenic nor granulomatous. The condition acquired this name more than 100 years ago, based on assumptions about its origin. Microscopic examination revealed the highly vascular nature of these lesions, showing a field full of circles that represented the truncated ends of bundles of capillaries and venules. Although they are also called lobular capillary hemangiomas, the term pyogenic granuloma (PG) is still more commonly used.
PGs commonly manifest in the patient’s second or third decade of life, typically on extremities, chest, and nipples. This patient’s story is typical: His lesion began as a tag or wart that he then traumatized, creating a situation in which the body attempts (in vain) to heal the wound. Undisturbed, nearly all PGs would eventually wither and resolve with minimal scarring; however, that process is prolonged when the patient fails to “leave it alone.” (This is especially true in the case of young children.)
While this presentation is characteristic, there are other circumstances in which PGs develop. One is as a consequence of taking certain medications (eg, retinoids, antiretrovirals, and certain chemotherapy drugs). PGs are also commonly seen in the oral cavity of pregnant women in the third trimester and on the end of the umbilical stump in many newborns. Ingrown toenails are another common site; PGs will appear as glistening red, friable buttons of vascular tissue in the perionychial skin adjacent to the affected portion of the nail.
Shave biopsy is standard in such cases, not only to produce a cure but also to establish, via pathologic examination of the tissue, the correct diagnosis. (Nodular melanoma is the most prominent item in the differential; to miss that diagnosis would have dire consequences.) In terms of treatment, mere cautery or cryotherapy will not work and excision is seldom necessary. A deep shave will capture the entire lesion; if any remains, electrodessication and curettage will take care of it. Prior to such procedures, the patient (and family) needs to understand that scarring and pigment loss will occur.
In cases associated with medications or with ingrown toenails, the “cure” would be to withdraw the offending medications—although this is not always advisable for other, obvious reasons.
TAKE-HOME LEARNING POINTS
• Pyogenic granulomas (PG) have nothing to do with infection, nor are they truly granulomatous.
• PGs bleed readily with minor trauma and are often swollen and occasionally painful.
• PGs appear to represent, in most cases, the body’s frustrated attempt to heal a wound, most often a prick or pinch of a pre-existing lesion (eg, tag or mole).
• PGs are also seen in other contexts, such as with use of certain medications or in association with pregnancy.
• If treatment is attempted, the resulting specimen must be submitted for pathologic examination, since other lesions can mimic PGs (of most concern, nodular melanoma).
Four months ago, this 23-year-old man developed a lesion on his right shoulder. It appeared, as he recalls, over the course of a week and has subsequently grown. The lesion, which is now rather large, bleeds copiously with minor trauma. It causes only modest discomfort to the patient but considerable worry to his family.
The lesion was originally tiny and tag-like; the patient initially mistook it for a tick and tried to pull it off. Not only did that fail to work, it also seemed to irritate the lesion. At that point, it started to swell, eventually transforming into the lesion he presents with today.
The patient’s history is otherwise uneventful, and he reports taking no medications. He has had minimal sun exposure but says he tans easily when he does get some sun.
EXAMINATION
The lesion, measuring 5 mm x 2.5 mm, is a dark red and pedunculated papule on the crown of the right shoulder. It looks edematous and feels firm. The patient has otherwise unremarkable type IV skin.
Shave biopsy is performed, using a double-edged razor to make a shallow concave defect under the lesion. The wound is cauterized.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the clinical suspicion of pyogenic granuloma, which, ironically, is neither pyogenic nor granulomatous. The condition acquired this name more than 100 years ago, based on assumptions about its origin. Microscopic examination revealed the highly vascular nature of these lesions, showing a field full of circles that represented the truncated ends of bundles of capillaries and venules. Although they are also called lobular capillary hemangiomas, the term pyogenic granuloma (PG) is still more commonly used.
PGs commonly manifest in the patient’s second or third decade of life, typically on extremities, chest, and nipples. This patient’s story is typical: His lesion began as a tag or wart that he then traumatized, creating a situation in which the body attempts (in vain) to heal the wound. Undisturbed, nearly all PGs would eventually wither and resolve with minimal scarring; however, that process is prolonged when the patient fails to “leave it alone.” (This is especially true in the case of young children.)
While this presentation is characteristic, there are other circumstances in which PGs develop. One is as a consequence of taking certain medications (eg, retinoids, antiretrovirals, and certain chemotherapy drugs). PGs are also commonly seen in the oral cavity of pregnant women in the third trimester and on the end of the umbilical stump in many newborns. Ingrown toenails are another common site; PGs will appear as glistening red, friable buttons of vascular tissue in the perionychial skin adjacent to the affected portion of the nail.
Shave biopsy is standard in such cases, not only to produce a cure but also to establish, via pathologic examination of the tissue, the correct diagnosis. (Nodular melanoma is the most prominent item in the differential; to miss that diagnosis would have dire consequences.) In terms of treatment, mere cautery or cryotherapy will not work and excision is seldom necessary. A deep shave will capture the entire lesion; if any remains, electrodessication and curettage will take care of it. Prior to such procedures, the patient (and family) needs to understand that scarring and pigment loss will occur.
In cases associated with medications or with ingrown toenails, the “cure” would be to withdraw the offending medications—although this is not always advisable for other, obvious reasons.
TAKE-HOME LEARNING POINTS
• Pyogenic granulomas (PG) have nothing to do with infection, nor are they truly granulomatous.
• PGs bleed readily with minor trauma and are often swollen and occasionally painful.
• PGs appear to represent, in most cases, the body’s frustrated attempt to heal a wound, most often a prick or pinch of a pre-existing lesion (eg, tag or mole).
• PGs are also seen in other contexts, such as with use of certain medications or in association with pregnancy.
• If treatment is attempted, the resulting specimen must be submitted for pathologic examination, since other lesions can mimic PGs (of most concern, nodular melanoma).
Four months ago, this 23-year-old man developed a lesion on his right shoulder. It appeared, as he recalls, over the course of a week and has subsequently grown. The lesion, which is now rather large, bleeds copiously with minor trauma. It causes only modest discomfort to the patient but considerable worry to his family.
The lesion was originally tiny and tag-like; the patient initially mistook it for a tick and tried to pull it off. Not only did that fail to work, it also seemed to irritate the lesion. At that point, it started to swell, eventually transforming into the lesion he presents with today.
The patient’s history is otherwise uneventful, and he reports taking no medications. He has had minimal sun exposure but says he tans easily when he does get some sun.
EXAMINATION
The lesion, measuring 5 mm x 2.5 mm, is a dark red and pedunculated papule on the crown of the right shoulder. It looks edematous and feels firm. The patient has otherwise unremarkable type IV skin.
Shave biopsy is performed, using a double-edged razor to make a shallow concave defect under the lesion. The wound is cauterized.
What is the diagnosis?
DISCUSSION
The pathology report confirmed the clinical suspicion of pyogenic granuloma, which, ironically, is neither pyogenic nor granulomatous. The condition acquired this name more than 100 years ago, based on assumptions about its origin. Microscopic examination revealed the highly vascular nature of these lesions, showing a field full of circles that represented the truncated ends of bundles of capillaries and venules. Although they are also called lobular capillary hemangiomas, the term pyogenic granuloma (PG) is still more commonly used.
PGs commonly manifest in the patient’s second or third decade of life, typically on extremities, chest, and nipples. This patient’s story is typical: His lesion began as a tag or wart that he then traumatized, creating a situation in which the body attempts (in vain) to heal the wound. Undisturbed, nearly all PGs would eventually wither and resolve with minimal scarring; however, that process is prolonged when the patient fails to “leave it alone.” (This is especially true in the case of young children.)
While this presentation is characteristic, there are other circumstances in which PGs develop. One is as a consequence of taking certain medications (eg, retinoids, antiretrovirals, and certain chemotherapy drugs). PGs are also commonly seen in the oral cavity of pregnant women in the third trimester and on the end of the umbilical stump in many newborns. Ingrown toenails are another common site; PGs will appear as glistening red, friable buttons of vascular tissue in the perionychial skin adjacent to the affected portion of the nail.
Shave biopsy is standard in such cases, not only to produce a cure but also to establish, via pathologic examination of the tissue, the correct diagnosis. (Nodular melanoma is the most prominent item in the differential; to miss that diagnosis would have dire consequences.) In terms of treatment, mere cautery or cryotherapy will not work and excision is seldom necessary. A deep shave will capture the entire lesion; if any remains, electrodessication and curettage will take care of it. Prior to such procedures, the patient (and family) needs to understand that scarring and pigment loss will occur.
In cases associated with medications or with ingrown toenails, the “cure” would be to withdraw the offending medications—although this is not always advisable for other, obvious reasons.
TAKE-HOME LEARNING POINTS
• Pyogenic granulomas (PG) have nothing to do with infection, nor are they truly granulomatous.
• PGs bleed readily with minor trauma and are often swollen and occasionally painful.
• PGs appear to represent, in most cases, the body’s frustrated attempt to heal a wound, most often a prick or pinch of a pre-existing lesion (eg, tag or mole).
• PGs are also seen in other contexts, such as with use of certain medications or in association with pregnancy.
• If treatment is attempted, the resulting specimen must be submitted for pathologic examination, since other lesions can mimic PGs (of most concern, nodular melanoma).
Studies shed new light on HSPC mobilization

in the bone marrow
Two new studies have revealed elements that are key to hematopoietic stem and progenitor cell (HSPC) mobilization.
In one study, investigators discovered that elevated levels of the peptide hormone angiotensin II increases HSPC mobilization in the context of vasculopathy and sickle cell disease (SCD).
In the other study, researchers found that p62, an autophagy regulator and signal organizer, is required to maintain HSPC retention in the bone marrow.
Jose Cancelas, MD, PhD, of the University of Cincinnati College of Medicine in Ohio, is the corresponding author on both studies.
In the first paper, published in Nature Communications, Dr Cancelas and his colleagues noted that patients with vasculopathies have an increase in circulating HSPCs.
“This phenomenon may represent a stress response contributing to vascular damage repair,” he said. “So the question becomes, how can we learn from these patients?”
Using mouse models of vasculopathy and vasculopathy-associated SCD, Dr Cancelas and his colleagues showed that acute and chronic elevated levels of angiotensin II resulted in an increased pool of HSPCs.
And when the researchers administered anti-angiotensin therapy, the pool of HSPCs decreased in mice and humans with SCD.
“These results indicate a new role for angiotensin in hematopoietic stem and progenitor cell trafficking under pathological conditions and define the hematopoietic consequences of anti-angiotensin therapy in vascular disease and sickle cell disease,” Dr Cancelas said.
“Every year, millions of patients receive anti-angiotensin therapies due to the harmful effects associated with chronic hyperangiotensinemia in cardiac, renal, or liver failure. Our study shows that this anti-angiotensin therapy modulates the levels of circulating stem cells and progenitors.”
In the second paper, published in Cell Reports, Dr Cancelas and his colleagues examined the role that p62 plays in HSPC mobilization.
The investigators found that, when p62 is lost in osteoblasts, mice develop a condition similar to osteoporosis in humans.
The osteoblasts cannot degrade inflammatory signals coming from macrophages. And as a consequence, the deficient osteoblasts secrete inflammatory signals that impair the retention of HSPCs in the bone marrow and allow their escape to the circulation.
Specifically, the team found that macrophages activate osteoblastic NF-kB, which results in osteopenia and HSPC egress. And p62 negatively regulates osteoblastic NF-kB activation.
Dr Cancelas noted that patients with inflammatory diseases often have osteopenia. So this research may provide insight into that phenomenon and help explain why patients with chronic inflammatory diseases have higher levels of circulating HSPCs. ![]()

in the bone marrow
Two new studies have revealed elements that are key to hematopoietic stem and progenitor cell (HSPC) mobilization.
In one study, investigators discovered that elevated levels of the peptide hormone angiotensin II increases HSPC mobilization in the context of vasculopathy and sickle cell disease (SCD).
In the other study, researchers found that p62, an autophagy regulator and signal organizer, is required to maintain HSPC retention in the bone marrow.
Jose Cancelas, MD, PhD, of the University of Cincinnati College of Medicine in Ohio, is the corresponding author on both studies.
In the first paper, published in Nature Communications, Dr Cancelas and his colleagues noted that patients with vasculopathies have an increase in circulating HSPCs.
“This phenomenon may represent a stress response contributing to vascular damage repair,” he said. “So the question becomes, how can we learn from these patients?”
Using mouse models of vasculopathy and vasculopathy-associated SCD, Dr Cancelas and his colleagues showed that acute and chronic elevated levels of angiotensin II resulted in an increased pool of HSPCs.
And when the researchers administered anti-angiotensin therapy, the pool of HSPCs decreased in mice and humans with SCD.
“These results indicate a new role for angiotensin in hematopoietic stem and progenitor cell trafficking under pathological conditions and define the hematopoietic consequences of anti-angiotensin therapy in vascular disease and sickle cell disease,” Dr Cancelas said.
“Every year, millions of patients receive anti-angiotensin therapies due to the harmful effects associated with chronic hyperangiotensinemia in cardiac, renal, or liver failure. Our study shows that this anti-angiotensin therapy modulates the levels of circulating stem cells and progenitors.”
In the second paper, published in Cell Reports, Dr Cancelas and his colleagues examined the role that p62 plays in HSPC mobilization.
The investigators found that, when p62 is lost in osteoblasts, mice develop a condition similar to osteoporosis in humans.
The osteoblasts cannot degrade inflammatory signals coming from macrophages. And as a consequence, the deficient osteoblasts secrete inflammatory signals that impair the retention of HSPCs in the bone marrow and allow their escape to the circulation.
Specifically, the team found that macrophages activate osteoblastic NF-kB, which results in osteopenia and HSPC egress. And p62 negatively regulates osteoblastic NF-kB activation.
Dr Cancelas noted that patients with inflammatory diseases often have osteopenia. So this research may provide insight into that phenomenon and help explain why patients with chronic inflammatory diseases have higher levels of circulating HSPCs. ![]()

in the bone marrow
Two new studies have revealed elements that are key to hematopoietic stem and progenitor cell (HSPC) mobilization.
In one study, investigators discovered that elevated levels of the peptide hormone angiotensin II increases HSPC mobilization in the context of vasculopathy and sickle cell disease (SCD).
In the other study, researchers found that p62, an autophagy regulator and signal organizer, is required to maintain HSPC retention in the bone marrow.
Jose Cancelas, MD, PhD, of the University of Cincinnati College of Medicine in Ohio, is the corresponding author on both studies.
In the first paper, published in Nature Communications, Dr Cancelas and his colleagues noted that patients with vasculopathies have an increase in circulating HSPCs.
“This phenomenon may represent a stress response contributing to vascular damage repair,” he said. “So the question becomes, how can we learn from these patients?”
Using mouse models of vasculopathy and vasculopathy-associated SCD, Dr Cancelas and his colleagues showed that acute and chronic elevated levels of angiotensin II resulted in an increased pool of HSPCs.
And when the researchers administered anti-angiotensin therapy, the pool of HSPCs decreased in mice and humans with SCD.
“These results indicate a new role for angiotensin in hematopoietic stem and progenitor cell trafficking under pathological conditions and define the hematopoietic consequences of anti-angiotensin therapy in vascular disease and sickle cell disease,” Dr Cancelas said.
“Every year, millions of patients receive anti-angiotensin therapies due to the harmful effects associated with chronic hyperangiotensinemia in cardiac, renal, or liver failure. Our study shows that this anti-angiotensin therapy modulates the levels of circulating stem cells and progenitors.”
In the second paper, published in Cell Reports, Dr Cancelas and his colleagues examined the role that p62 plays in HSPC mobilization.
The investigators found that, when p62 is lost in osteoblasts, mice develop a condition similar to osteoporosis in humans.
The osteoblasts cannot degrade inflammatory signals coming from macrophages. And as a consequence, the deficient osteoblasts secrete inflammatory signals that impair the retention of HSPCs in the bone marrow and allow their escape to the circulation.
Specifically, the team found that macrophages activate osteoblastic NF-kB, which results in osteopenia and HSPC egress. And p62 negatively regulates osteoblastic NF-kB activation.
Dr Cancelas noted that patients with inflammatory diseases often have osteopenia. So this research may provide insight into that phenomenon and help explain why patients with chronic inflammatory diseases have higher levels of circulating HSPCs. ![]()
EC supports continued use of ponatinib

Credit: Rhoda Baer
The European Commission (EC) has concluded that ponatinib (Iclusig) should continue to be prescribed in accordance with its already approved indications.
After trial results suggested the drug poses an increased risk of thrombotic events, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) conducted a review of available ponatinib data.
Results of that review suggested the benefits of ponatinib outweigh the risks. So the committee said the drug should be prescribed as indicated.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recently endorsed this recommendation, and, now, the EC has followed suit. The EC’s decision is legally binding.
Ponatinib is approved in the European Union to treat adults with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia who are resistant to dasatinib or nilotinib, who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
The drug is also approved to treat adults with Philadelphia-chromosome positive acute lymphoblastic leukemia who are resistant to dasatinib, who cannot tolerate dasatinib and subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
In October 2013, extended follow-up data from the PACE trial revealed that ponatinib-treated patients had a higher incidence of arterial and venous thrombotic events than was observed when the drug first gained approval. So one ponatinib trial was discontinued, and the rest were placed on partial clinical hold.
Soon after, ponatinib was pulled from the US market. The drug ultimately returned to the marketplace with new recommendations designed to decrease the risk of thrombotic events.
The EMA also revised its recommendations for ponatinib—discouraging use of the drug in certain patients, providing advice for managing comorbidities, and suggesting patient monitoring—but kept the drug on the market.
In October 2014, the PRAC concluded its 11-month review of ponatinib data, confirming that the benefit-risk profile of the drug was favorable in its approved indications and recommending that the indications remain unchanged.
However, the PRAC also said the risk of vascular occlusive events with ponatinib is likely dose-related. So the committee recommended that healthcare professionals monitor ponatinib-treated patients and consider dose reductions or discontinuing the drug in certain patients.
The CHMP endorsed these recommendations, and, now, the EC has as well. This is a legally binding decision for ponatinib to continue to be prescribed in Europe in accordance with its already approved indications.
Ponatinib is being developed by ARIAD Pharmaceuticals, Inc. ![]()

Credit: Rhoda Baer
The European Commission (EC) has concluded that ponatinib (Iclusig) should continue to be prescribed in accordance with its already approved indications.
After trial results suggested the drug poses an increased risk of thrombotic events, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) conducted a review of available ponatinib data.
Results of that review suggested the benefits of ponatinib outweigh the risks. So the committee said the drug should be prescribed as indicated.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recently endorsed this recommendation, and, now, the EC has followed suit. The EC’s decision is legally binding.
Ponatinib is approved in the European Union to treat adults with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia who are resistant to dasatinib or nilotinib, who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
The drug is also approved to treat adults with Philadelphia-chromosome positive acute lymphoblastic leukemia who are resistant to dasatinib, who cannot tolerate dasatinib and subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
In October 2013, extended follow-up data from the PACE trial revealed that ponatinib-treated patients had a higher incidence of arterial and venous thrombotic events than was observed when the drug first gained approval. So one ponatinib trial was discontinued, and the rest were placed on partial clinical hold.
Soon after, ponatinib was pulled from the US market. The drug ultimately returned to the marketplace with new recommendations designed to decrease the risk of thrombotic events.
The EMA also revised its recommendations for ponatinib—discouraging use of the drug in certain patients, providing advice for managing comorbidities, and suggesting patient monitoring—but kept the drug on the market.
In October 2014, the PRAC concluded its 11-month review of ponatinib data, confirming that the benefit-risk profile of the drug was favorable in its approved indications and recommending that the indications remain unchanged.
However, the PRAC also said the risk of vascular occlusive events with ponatinib is likely dose-related. So the committee recommended that healthcare professionals monitor ponatinib-treated patients and consider dose reductions or discontinuing the drug in certain patients.
The CHMP endorsed these recommendations, and, now, the EC has as well. This is a legally binding decision for ponatinib to continue to be prescribed in Europe in accordance with its already approved indications.
Ponatinib is being developed by ARIAD Pharmaceuticals, Inc. ![]()

Credit: Rhoda Baer
The European Commission (EC) has concluded that ponatinib (Iclusig) should continue to be prescribed in accordance with its already approved indications.
After trial results suggested the drug poses an increased risk of thrombotic events, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) conducted a review of available ponatinib data.
Results of that review suggested the benefits of ponatinib outweigh the risks. So the committee said the drug should be prescribed as indicated.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recently endorsed this recommendation, and, now, the EC has followed suit. The EC’s decision is legally binding.
Ponatinib is approved in the European Union to treat adults with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia who are resistant to dasatinib or nilotinib, who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
The drug is also approved to treat adults with Philadelphia-chromosome positive acute lymphoblastic leukemia who are resistant to dasatinib, who cannot tolerate dasatinib and subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation.
In October 2013, extended follow-up data from the PACE trial revealed that ponatinib-treated patients had a higher incidence of arterial and venous thrombotic events than was observed when the drug first gained approval. So one ponatinib trial was discontinued, and the rest were placed on partial clinical hold.
Soon after, ponatinib was pulled from the US market. The drug ultimately returned to the marketplace with new recommendations designed to decrease the risk of thrombotic events.
The EMA also revised its recommendations for ponatinib—discouraging use of the drug in certain patients, providing advice for managing comorbidities, and suggesting patient monitoring—but kept the drug on the market.
In October 2014, the PRAC concluded its 11-month review of ponatinib data, confirming that the benefit-risk profile of the drug was favorable in its approved indications and recommending that the indications remain unchanged.
However, the PRAC also said the risk of vascular occlusive events with ponatinib is likely dose-related. So the committee recommended that healthcare professionals monitor ponatinib-treated patients and consider dose reductions or discontinuing the drug in certain patients.
The CHMP endorsed these recommendations, and, now, the EC has as well. This is a legally binding decision for ponatinib to continue to be prescribed in Europe in accordance with its already approved indications.
Ponatinib is being developed by ARIAD Pharmaceuticals, Inc. ![]()
Combining bed nets and vaccines may worsen malaria risk

Credit: Caitlin Kleiboer
New research suggests that combining the use of malaria vaccines and insecticide-treated bed nets may actually increase the incidence of malaria.
The researchers used a mathematical model of malaria transmission to examine potential interactions between vaccines and bed nets.
They found that using insecticide-treated bed nets along with pre-erythrocytic vaccines (PEVs) or blood-stage vaccines (BSVs) increased the number of malaria cases.
However, using bed nets in conjunction with transmission-blocking vaccines (TBVs) resulted in fewer cases of malaria and increased the probability of eliminating the disease.
“The joint use of bed nets and vaccines will not always lead to consistent increases in the efficacy of malaria control,” said study author Mercedes Pascual, PhD, of the University of Michigan in Ann Arbor.
“Specifically, our study suggests that the combined use of some malaria vaccines with bed nets can lead to increased morbidity and mortality in older age classes.”
Dr Pascual and her colleagues described this research in Proceedings of the National Academy of Sciences.
The team noted that the malaria vaccine candidates currently under development fall into 3 categories, each focusing on a different stage of the malaria life cycle.
PEVs aim to reduce the chances that a person will be infected when bitten by a disease-carrying mosquito. BSVs don’t block infection but try to reduce the level of disease severity and the number of fatalities.
And TBVs don’t protect vaccinated individuals against infection or illness, but they prevent mosquitoes from spreading the disease to others after biting a vaccinated person.
Dr Pascual and her colleagues found that using bed nets in communities treated with BSVs can increase levels of morbidity—to levels even higher than those expected in the absence of nets. Furthermore, BSVs can’t promote malaria elimination on their own.
PEVs can promote malaria elimination, but the researchers found regions of decreased morbidity when PEV vaccination levels were low and increased morbidity when PEV vaccination levels were high.
This suggests that higher levels of PEV coverage and bed net use could result in malaria elimination, but it would involve crossing a peak of enhanced morbidity.
Finally, the researchers found that using bed nets in communities treated with TBVs always leads to significant decreases in morbidity and increases the probability of malaria elimination.
“Ironically, the vaccines that work best with bed nets are the ones that do not protect the vaccinated host—the bed net does that—but instead block transmission of malaria in mosquitoes that have found an opportunity to bite vaccinated hosts,” said study author Yael Artzy-Randrup, PhD, of the University of Amsterdam in The Netherlands.
Unraveling the interactions between bed nets and vaccines is especially challenging due to the complex and transient nature of malaria immunity, the researchers noted.
A child’s first malaria infection can result in severe, sometimes fatal, illness. If the child survives, he or she will gain partial immunity that reduces the risk of severe illness in the future.
Additional bites from infected mosquitoes can help the child retain that immunity, which would otherwise wane after 1 to 2 years. But the combination of bed nets and certain vaccines can undermine that natural immunity.
“This complexity is at the heart of why it has been so hard to develop any sort of malaria vaccine,” said study author Andrew Dobson, DPhil, of Princeton University in New Jersey. ![]()

Credit: Caitlin Kleiboer
New research suggests that combining the use of malaria vaccines and insecticide-treated bed nets may actually increase the incidence of malaria.
The researchers used a mathematical model of malaria transmission to examine potential interactions between vaccines and bed nets.
They found that using insecticide-treated bed nets along with pre-erythrocytic vaccines (PEVs) or blood-stage vaccines (BSVs) increased the number of malaria cases.
However, using bed nets in conjunction with transmission-blocking vaccines (TBVs) resulted in fewer cases of malaria and increased the probability of eliminating the disease.
“The joint use of bed nets and vaccines will not always lead to consistent increases in the efficacy of malaria control,” said study author Mercedes Pascual, PhD, of the University of Michigan in Ann Arbor.
“Specifically, our study suggests that the combined use of some malaria vaccines with bed nets can lead to increased morbidity and mortality in older age classes.”
Dr Pascual and her colleagues described this research in Proceedings of the National Academy of Sciences.
The team noted that the malaria vaccine candidates currently under development fall into 3 categories, each focusing on a different stage of the malaria life cycle.
PEVs aim to reduce the chances that a person will be infected when bitten by a disease-carrying mosquito. BSVs don’t block infection but try to reduce the level of disease severity and the number of fatalities.
And TBVs don’t protect vaccinated individuals against infection or illness, but they prevent mosquitoes from spreading the disease to others after biting a vaccinated person.
Dr Pascual and her colleagues found that using bed nets in communities treated with BSVs can increase levels of morbidity—to levels even higher than those expected in the absence of nets. Furthermore, BSVs can’t promote malaria elimination on their own.
PEVs can promote malaria elimination, but the researchers found regions of decreased morbidity when PEV vaccination levels were low and increased morbidity when PEV vaccination levels were high.
This suggests that higher levels of PEV coverage and bed net use could result in malaria elimination, but it would involve crossing a peak of enhanced morbidity.
Finally, the researchers found that using bed nets in communities treated with TBVs always leads to significant decreases in morbidity and increases the probability of malaria elimination.
“Ironically, the vaccines that work best with bed nets are the ones that do not protect the vaccinated host—the bed net does that—but instead block transmission of malaria in mosquitoes that have found an opportunity to bite vaccinated hosts,” said study author Yael Artzy-Randrup, PhD, of the University of Amsterdam in The Netherlands.
Unraveling the interactions between bed nets and vaccines is especially challenging due to the complex and transient nature of malaria immunity, the researchers noted.
A child’s first malaria infection can result in severe, sometimes fatal, illness. If the child survives, he or she will gain partial immunity that reduces the risk of severe illness in the future.
Additional bites from infected mosquitoes can help the child retain that immunity, which would otherwise wane after 1 to 2 years. But the combination of bed nets and certain vaccines can undermine that natural immunity.
“This complexity is at the heart of why it has been so hard to develop any sort of malaria vaccine,” said study author Andrew Dobson, DPhil, of Princeton University in New Jersey. ![]()

Credit: Caitlin Kleiboer
New research suggests that combining the use of malaria vaccines and insecticide-treated bed nets may actually increase the incidence of malaria.
The researchers used a mathematical model of malaria transmission to examine potential interactions between vaccines and bed nets.
They found that using insecticide-treated bed nets along with pre-erythrocytic vaccines (PEVs) or blood-stage vaccines (BSVs) increased the number of malaria cases.
However, using bed nets in conjunction with transmission-blocking vaccines (TBVs) resulted in fewer cases of malaria and increased the probability of eliminating the disease.
“The joint use of bed nets and vaccines will not always lead to consistent increases in the efficacy of malaria control,” said study author Mercedes Pascual, PhD, of the University of Michigan in Ann Arbor.
“Specifically, our study suggests that the combined use of some malaria vaccines with bed nets can lead to increased morbidity and mortality in older age classes.”
Dr Pascual and her colleagues described this research in Proceedings of the National Academy of Sciences.
The team noted that the malaria vaccine candidates currently under development fall into 3 categories, each focusing on a different stage of the malaria life cycle.
PEVs aim to reduce the chances that a person will be infected when bitten by a disease-carrying mosquito. BSVs don’t block infection but try to reduce the level of disease severity and the number of fatalities.
And TBVs don’t protect vaccinated individuals against infection or illness, but they prevent mosquitoes from spreading the disease to others after biting a vaccinated person.
Dr Pascual and her colleagues found that using bed nets in communities treated with BSVs can increase levels of morbidity—to levels even higher than those expected in the absence of nets. Furthermore, BSVs can’t promote malaria elimination on their own.
PEVs can promote malaria elimination, but the researchers found regions of decreased morbidity when PEV vaccination levels were low and increased morbidity when PEV vaccination levels were high.
This suggests that higher levels of PEV coverage and bed net use could result in malaria elimination, but it would involve crossing a peak of enhanced morbidity.
Finally, the researchers found that using bed nets in communities treated with TBVs always leads to significant decreases in morbidity and increases the probability of malaria elimination.
“Ironically, the vaccines that work best with bed nets are the ones that do not protect the vaccinated host—the bed net does that—but instead block transmission of malaria in mosquitoes that have found an opportunity to bite vaccinated hosts,” said study author Yael Artzy-Randrup, PhD, of the University of Amsterdam in The Netherlands.
Unraveling the interactions between bed nets and vaccines is especially challenging due to the complex and transient nature of malaria immunity, the researchers noted.
A child’s first malaria infection can result in severe, sometimes fatal, illness. If the child survives, he or she will gain partial immunity that reduces the risk of severe illness in the future.
Additional bites from infected mosquitoes can help the child retain that immunity, which would otherwise wane after 1 to 2 years. But the combination of bed nets and certain vaccines can undermine that natural immunity.
“This complexity is at the heart of why it has been so hard to develop any sort of malaria vaccine,” said study author Andrew Dobson, DPhil, of Princeton University in New Jersey. ![]()
Sofosbuvir and ribavirin effective in transplant patients with compensated recurrent HCV
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Sofosbuvir and ribavirin prevent HCV recurrence after liver transplantation
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Residents looking to work in larger cities
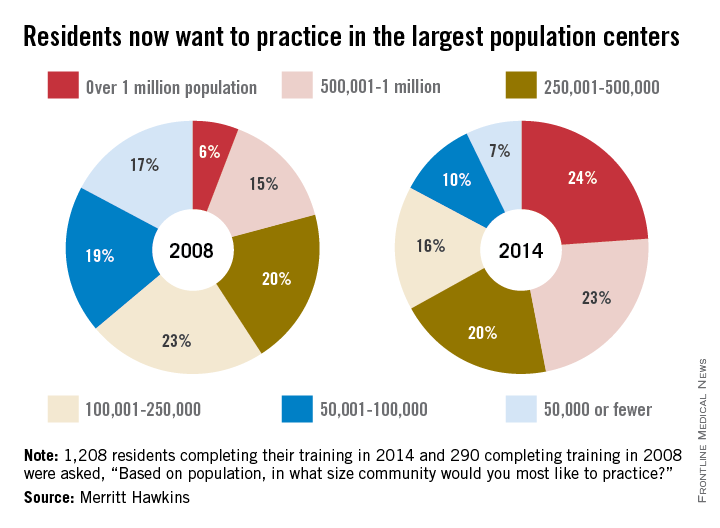
Since 2008, residents’ preference for their future practice location has shifted from smaller cities and rural areas to large population centers, according to findings reported by physician recruitment firm Merritt Hawkins.
In a survey of residents who completed their training in 2014, 24% said that they wanted to practice in a community with a population of more than 1 million, compared with 6% in 2008, while 23% of residents chose the next-highest level of population – 500,001 to 1 million – compared with 15% in 2008, according to Merritt Hawkins.
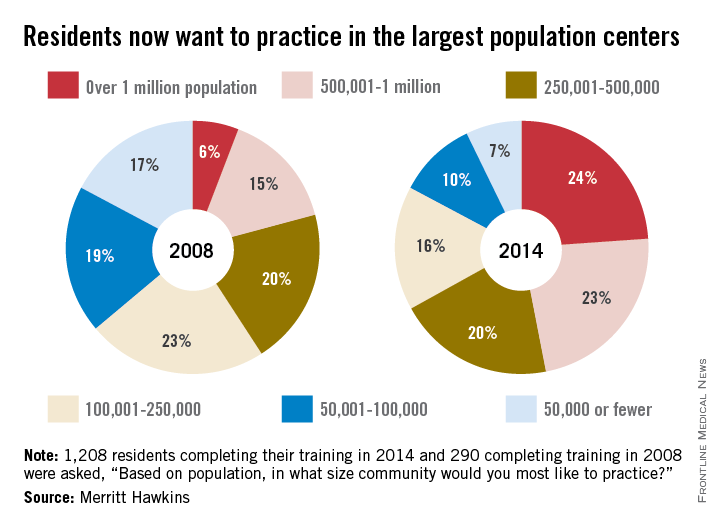
As for the smaller communities, residents who wanted to practice in a area of 50,000 or fewer dropped from 17% in 2008 to 7% in 2014. Support for communities of 50,001-100,000 fell from 19% in 2008 to 10% in 2014, the company said. Only 1% of residents wanted to practice in a community of 10,000 people or fewer in 2014.
Residents’ reservations about practicing in rural areas more often are related to their “concerns about being on a clinical ‘island’ without specialty support, information technology, and other resources than they may be about the amenities of rural communities,” Merritt Hawkins said in its analysis of the 1,208 survey responses.
Since 2008, residents’ preference for their future practice location has shifted from smaller cities and rural areas to large population centers, according to findings reported by physician recruitment firm Merritt Hawkins.
In a survey of residents who completed their training in 2014, 24% said that they wanted to practice in a community with a population of more than 1 million, compared with 6% in 2008, while 23% of residents chose the next-highest level of population – 500,001 to 1 million – compared with 15% in 2008, according to Merritt Hawkins.

As for the smaller communities, residents who wanted to practice in a area of 50,000 or fewer dropped from 17% in 2008 to 7% in 2014. Support for communities of 50,001-100,000 fell from 19% in 2008 to 10% in 2014, the company said. Only 1% of residents wanted to practice in a community of 10,000 people or fewer in 2014.
Residents’ reservations about practicing in rural areas more often are related to their “concerns about being on a clinical ‘island’ without specialty support, information technology, and other resources than they may be about the amenities of rural communities,” Merritt Hawkins said in its analysis of the 1,208 survey responses.
Since 2008, residents’ preference for their future practice location has shifted from smaller cities and rural areas to large population centers, according to findings reported by physician recruitment firm Merritt Hawkins.
In a survey of residents who completed their training in 2014, 24% said that they wanted to practice in a community with a population of more than 1 million, compared with 6% in 2008, while 23% of residents chose the next-highest level of population – 500,001 to 1 million – compared with 15% in 2008, according to Merritt Hawkins.

As for the smaller communities, residents who wanted to practice in a area of 50,000 or fewer dropped from 17% in 2008 to 7% in 2014. Support for communities of 50,001-100,000 fell from 19% in 2008 to 10% in 2014, the company said. Only 1% of residents wanted to practice in a community of 10,000 people or fewer in 2014.
Residents’ reservations about practicing in rural areas more often are related to their “concerns about being on a clinical ‘island’ without specialty support, information technology, and other resources than they may be about the amenities of rural communities,” Merritt Hawkins said in its analysis of the 1,208 survey responses.
Coexisting Frailty, Cognitive Impairment, and Heart Failure: Implications for Clinical Care
From the Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
Abstract
- Objective: To review some of the proposed pathways that increase frailty risk in older persons with heart failure and to discuss tools that may be used to assess for changes in physical and cognitive functioning in this population in order to assist with appropriate and timely intervention.
- Methods: Review of the literature.
- Results: Heart failure is the only cardiovascular disease that is increasing by epidemic proportions, largely due to an aging society and therapeutic advances in disease management. Because heart failure is largely a cardiogeriatric syndrome, age-related syndromes such as frailty and cognitive impairment are common in heart failure patients. Compared with age-matched counterparts, older adults with heart failure 4 to 6 times more likely to be frail or cognitively impaired. The reason for the high prevalence of frailty and cognitive impairment in this population is not well known but may likely reflect the synergistic effects of heart failure and aging, which may heighten vulnerability to stressors and accelerate loss of physiologic reserve. Despite the high prevalence of frailty and cognitive impairment in the heart failure population, these conditions are not routinely screened for in clinical practice settings and guidelines on optimal assessment strategies are lacking.
- Conclusion: Persons with heart failure are at an increased risk for frailty, which may worsen symptoms, impair self-management, and lead to worse heart failure outcomes. Early detection of frailty and cognitive impairment may be an opportunity for intervention and a key strategy for improving clinical outcomes in older adults with heart failure.
Approximately 5.7 million persons in the United States are diagnosed with heart failure, and the number of reported new cases is expected to increase to over 700,000 cases annually by the year 2040 [1]. This rising incidence is fueled by an aging population; by the year 2030, 1 in 5 Americans will be over 65 years of age [2]. Heart failure is prevalent among those 65 years of age and older and is the most common reason for hospitalization in this age-group. High readmission rates, approaching 50% over 6 months, are a major contributor to the the escalating economic burden associated with heart failure [3].
Persons with heart failure are more likely to be frail and experience cognitive impairment than their age-matched counterparts without heart failure. The reasons for this are not well known but may be related to hemodynamic, vascular, and inflammatory changes occurring as heart failure progresses. In this paper, we review the link between frailty and cognitive impairment in heart failure, instruments that may be useful for early detection, and interventions such as exercise that may be beneficial for attenuating both conditions.
Frailty in Heart Failure
Epidemiology
Frailty is a heightened vulnerability to stressors in the presence of low physiological reserve [4]. When exposed to stressors, persons who are frail have a much higher probability for disproportionate decompensation, negative events, functional decline, disability, and mortality [5]. Among persons with heart failure, frailty may predispose them to decompensate at a lower threshold, requiring more frequent hospitalizations. Persons with heart failure are more likely to be frail than their age-matched counterparts without heart failure [6,7].
Frailty is a powerful predictor of poor clinical outcomes and mortality in cardiovascular disease [8,9]. Compared with the non-frail, frail persons with heart failure have increased rates of mortality (16.9% vs 4.8%) and increased rates of heart failure hospitalization (20.5% vs 13.3%) [10]. Frailty has also been shown to predict falls, disability, and hospitalization in heart failure patients [6,9,11] and was found to have a negative linear relationship with health-related quality of life [12]. Frail heart failure patients are also more likely to have comorbidities such diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, depression, anemia, and chronic kidney disease [9,13].
Pathophysiology
There is significant overlap in the underlying pathological mechanisms of heart failure and frailty. Symptoms of heart failure, such as dyspnea, fatigue, and muscle loss, mirror components that occur with frailty. Further, cardiac cachexia, a metabolic syndrome in advanced heart failure characterized by a loss of muscle mass, is very similar to the sarcopenia that occurs in frailty.
Frailty, characterized by an increased physiologic vulnerability to stressors, may predispose frail persons with heart failure to exacerbation and worsening of heart failure due to greater susceptibility to the harmful pathophysiologic processes in heart failure, such as inflammation and autonomic dysfunction. Proposed pathophysiologic pathways in frailty include free radicals and oxidative stress, cumulative DNA damage, decreased telomere length, and nuclear fragmentation [14,15]. Frailty has been associated with low-grade chronic inflammation and increased inflammatory cytokines, such as C-reactive protein, tumor necrosis factor–alpha (TNFα), interleukin-6 (IL-6)and fibrinogen [16–18]. Heart failure also is associated with a low-grade and chronic cardiac inflammatory response that is correlated with disease progression [19].
Inflammation. IL-6 is detectable in a higher proportion of persons who are frail compared to non-frail [16] and is the most highly correlated biomarker with frailty. In addition, among those with detectable IL-6 levels, those categorized as frail have higher IL-6 levels compared to those who are non-frail [16,20]. Individuals categorized as frail were found to have significantly higher levels of TNFα than those who were non-frail [16,20]. Increased IL-6 levels are associated with decreased muscle strength, while increased TNFα levels are associated with decreased skeletal muscle protein synthesis [21,22], thus contributing to frailty.
Oxidative stress. Protein carbonyls result from protein oxidation promoted by reactive oxygen species and are markers of oxidative stress. Protein carbonylation is implicated in the pathogenesis of the loss of skeletal muscle mass; high serum protein carbonyls are associated with poor grip strength [23]. 8-OHdG is an oxidized nucleoside indicative of oxidative damage to DNA and a measure of oxidative stress. Accumulation of 8-OHdG in skeletal muscle leads to loss of muscle mass and is associated with decreased hand grip strength in the elderly [24]. Higher serum levels of 8-OHdG are present in older adults who are frail as compared to those who are non-frail [25].
Measurement of Frailty in the Clinical Setting
Frailty has been conceptualized in a number of studies using different models and measures; however, there continues to be a lack of consensus on the definition and operationalization of frailty. Prior research has led to the development of several validated models of frailty that have demonstrated good prediction of adverse outcomes in older adults. Some models, such as the Fried phenotype [6], focus solely on the physical dimension, while other models take a multidimensional approach.Single-item measures (eg, gait speed, 6-minute walk test, handgrip strength) are also commonly used to screen for frailty, but a frailty measure that incorporates more than 1 physical dimension may be more sensitive and reliable. In our opinion, the ideal measure of frailty would consist of a brief assessment that can be serially performed in most clinical practice settings that can identify change in function over time. The incorporation of sensitive physical function measures that can detect frailty early has the potential to slow physical function decline by preserving physiological thresholds.
Cognitive Impairment in Heart Failure
Epidemiology
Cognitive impairment occurs frequently in patients with heart failure, and the presence of cognitive impairment in persons with heart failure has been shown to heighten risk for adverse clinical outcomes, disability, poor quality of life, and mortality [26,27]. Heart failure negatively influences cognitive functioning in most domains [28–32]. The most common domains adversely affected by heart failure and aging are memory and executive function. Deficits in these domains can substantially diminish patient ability to carry out essential self-care behaviors [30,32].
The most common form of cognitive impairment seen in patients with heart failure is mild cognitive impairment (MCI), which is a measurable deficit with memory or another core cognitive domain. Up to 60% of persons with heart failure have been reported to have MCI. Patients with MCI have cognitive deficits that are more pronounced than those seen in normal aging, but lack other symptoms of dementia, such as impaired judgment or reasoning. MCI often will not impede patients’ ability to carry out the activities of daily living (ADLs) independently, but patients may have difficulty in performing some instrumental activities of daily living (IADLs), such as remembering medications, scheduling provider appointments. Dementia, a decline in cognitive ability severe enough to hinder an individual’s ability to perform ADLs or IADLs or engage in social activities or occupational responsibilities, occurs in approximately 25% of persons with heart failure [33].
Persons with heart failure have a fourfold greater likelihood of developing CI than persons without heart failure. Several cohort studies have shown that persons with heart failure had lower performance on cognitive tests than individuals without heart failure [34,35] and were 50% more likely to progress to dementia.
Assessment Tools
Although a comprehensive neurocognitive battery would aid in detecting cognitive impairment in heart failure, few clinical practice settings have the resources to perform such a detailed and time-consuming measurement. Most studies in heart failure have relied on global screening questionnaires such as the Mini-Mental State Examination (MMSE) [36] to assess cognitive functioning in persons with heart failure and in other cardiovascular disorders. Global cognitive measures, however, often lack sensitivity for detecting subtle cognitive deficits such as seen in MCI [28–30]. Screening that measures executive function may be the most beneficial for busy clinical settings, since declines in this domain are well established as contributing to poor outcomes in persons with heart failure.
The Montreal Cognitive Assessment (MoCA) is a rapid screening test designed to detect MCI. It assesses different cognitive domains, including attention, memory, language, and executive function [37]. The MoCA lends itself to use in clinical setting because it is brief, requires little training to administer, and is easy to score. This instrument has been used successfully to assess MCI in persons with heart failure and may be more sensitive than the MMSE in identifying clinically relevant cognitive dysfunction. In 2013 study, Cameron et al [38] administered the MMSE and MoCA to 93 hospitalized heart failure patients and found that the MoCA classified 41% of patients as cognitively impaired that were not classified using the MMSE. For persons with a vascular cognitive deficit, the MMSE has been portrayed as an inadequate screening test due to lack of sensitivity for visuospatial and executive function deficits. Because the MoCA was designed to be more sensitive to such deficits, it may be a superior screening method for persons with heart failure. Although previous studies support the use of the MoCA in persons with heart failure, more research is needed in larger, more diverse heart failure samples with a wide range of cognitive deficits.
A Reasonable Clinical Assessment Approach
Considering the link between heart failure, frailty, and MCI, incorporating simple physical performance measures with cognitive screening may be an effective strategy to identify persons at risk for frailty. Two clinically relevant physical performance-based measures of frailty are proposed: the Fried phenotype (mentioned earlier) and the Short Physical Performance Battery (SBBP). In addition, cognitive screening using the MoCA is recommended as part of the routine examination for determining possible MCI or more severe cognitive deficits. The predictive validity of measuring physical frailty is enhanced when cognitive impairment is included in the assessment [36,39].
The performance-based measures included in this review have previously demonstrated excellent psychometric properties as well as sensitivity for change that is clinically meaningful. Minimal detectable change (MDC), a threshold score that refers to the minimal amount of change outside of error that reflects true change by a patient between 2 time points (rather than variation in measurement), is important for interpreting level of risk for frailty and is included for each instrument [40,41]. If a more brief frailty examination is needed, cut-points for gait speed and handgrip have been used effectively in a number of studies as a threshold for determining frailty, including in older patients with cardiovascular disease and in heart failure [8,42,43].
Fried Frailty Phenotype
The Fried phenotype is an appropriate method of measuring frailty in a clinical setting due to its wide application across diverse populations and consistent identification of adverse outcomes [44]. This model is derived from a frailty model proposed by Fried et al [6] in which a phenotypic cycle exists that includes disease, sarcopenia, decreased walking speed, chronic undernutrition, decreased total energy expenditure, senescent musculoskeletal changes, decreased resting metabolic rate, weight loss and decreased maximal oxygen consumption. Frailty exists when a critical mass of these cycle components are identified in an individual [6].
To validate the model, Fried et al used data from the Cardiovascular Health Study and used the model to show association with a 3-year and 7-year incidence of mobility and ADL disability among 4317 community-dwelling men and women aged 65 years and older, independent of comorbidities. Several studies have directly tested the frailty phenotype model alone and in comparison to other models of frailty in large prospective studies across different populations, such as the Survey of Health, Aging and Retirement in Europe (SHARE) [45], the European Male Aging Study [46], and the Canadian Health Study of Aging [47]. While these studies found the prevalence of frailty to vary across the populations, they all validated the Fried model and found no significant differences in the predictive ability of the Fried model and other models of frailty. The Frailty Consensus conference evaluated the different models of frailty and determined that the Fried model is a validated construct of frailty and is acceptable for use in the identification of individuals who are frail or likely to become frail [48]. Thus, the Fried et al frailty phenotype model is considered to be a standard measure of frailty in older individuals.
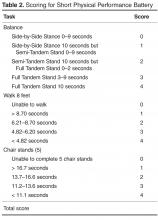
Short Physical Performance Battery
In other chronic illness populations, the SPPB has also been used as a predictor of outcomes before, during, or after hospitalization. Valpato et al [53], for example, used the SPPB to assess older adults (mean age, 78 yr) admitted to the hospital with a diagnosis of heart failure (64%), pneumonia (13%), chronic obstructive pulmonary disease (16%), or minor stroke (6.6%) at admission (baseline) and discharge. Patients with the lowest SPPB quartile scores at hospital discharge had a fivefold greater risk of rehospitalization or mortality compared to the highest quartile. In addition, those who had an early decline in SPPB scores 1 month after hospital discharge had greater limitations in performing activities of daily living and a significantly greater probability of being re-hospitalized or death during the 1-year follow-up period. These studies suggest that the SPPB at the first follow-up outpatient visit following hospital discharge may be beneficial for identifying need for further intervention or the need for more frequent follow-up care. Although the SPPB is not part of the Fried et al phenotype, it may provide additional information concerning risk for falls and lower extremity strength that may be beneficial in the evaluation of some persons with heart failure [54]. The SPPB along with instructions and normative data are available for clinical use at no charge at www.grc.nia.nih.gov/branches/ledb/sppb/index.htm.
Interventions for Frailty in Heart Failure
Interventions to address frailty have included exercise training, comprehensive geriatric assessment and management services, social support systems, nutrition, and drugs; however, few intervention studies have examined frailty in heart failure [8]. Restoration of physical function through aerobic exercise and resistance training has shown benefit in frail older adults [55–57] and in persons with heart failure [58]. Maintaining and/or restoring physical function through aerobic and resistance exercise training may be the key to preventing further decline or potentially reversing frailty in older adults with heart failure.
Aerobic exercise has been shown to be beneficial for both frail older adults and frail persons with heart failure [18]. In a study of community-dwelling frail older adults aged 65 and older, a combined aerobic and resistance exercise intervention, performed over 16 weeks, demonstrated significant improvement in frailty scores during the 1-year follow-up in contrast to worsening frailty measures in the control group [57].
Older adults with heart failure experience a much lower exercise tolerance largely due to a 50% to 75% decrease in aerobic capacity in addition to the well-known alterations in peripheral musculoskeletal performance that contribute to fatigue and greater symptom severity. Aerobic exercise has been shown to be beneficial for most heart failure patients by altering the peripheral and central mechanisms, such as inflammatory cytokines, that contribute to heart failure exacerbations, worsened symptom severity, and poor clinical outcomes [59–62].Lower rates of hospitalization, improved physical function, and enhanced health-related quality of life are reported in heart failure patients who routinely exercise [59]. Resistance training has been shown to improve physical function in frail older adults [55]. Further, the use of TheraBand exercise bands in resistance training demonstrated improvement in physical function among frail older adults [56].
Exercise also appears to exert a positive effect on cognition, particularly executive functioning, and may also have a protective effect against cognitive decline with aging and among those with heart failure. The underlying mechanism for improvement in cognition remains poorly understood but is likely related to improved cardiac function, cerebral perfusion, and oxygenation, although this has not been clearly established. Larson et al (2006) evaluated the frequency of participation in a variety of physical activities (eg, walking, bicycling and swimming) over 6 years in 1740 older adults [63]. Older adults who exercised more than 3 times per week during initial assessment were 34% less likely to be diagnosed with dementia than those who exercised fewer than 3 times per week. Several meta-analyses in recent years have shown a consistent and positive relationship between aerobic exercise and cognition [64,65]. Importantly, findings from meta-analyses have shown a moderate effect size (> 0.5) from aerobic training, which was similar for normal and cognitively impaired adults [64].
Implications for Clinical Care
A systematic assessment performed periodically using physical and cognitive measures that may identify prefrailty may be the best strategy for preventing further functional loss, limitations, and disability in persons with heart failure. Persons with heart failure ideally should be evaluated annually for physical function, since a decline has been consistently shown to be a strong predictor of adverse health outcomes, disability, and death [6,66]. Cognitive function should also be assessed routinely in persons with heart failure, particularly when first diagnosed, when changes in treatment regimen occur, and with worsening disease severity, since these events have been shown to occur before changes in cognition [31]. Incorporating geriatric performance-based measures in heart failure management would allow for more treatment strategies aimed at improving physical function, cognitive outcomes, and quality of life. Further, identifying frailty in heart failure is an important component of clinical decision-making when determining if a patient can tolerate therapies such as implantable defibrillators, cardiac resynchronization therapy, or left ventricular assist device placement.
In older adults, performance measures are well established and commonly used as part of geriatric assessment to evaluate physical and cognitive functioning. Performance-based measures may be particularly beneficial in older adults with heart failure to monitor serial changes in physical function. Performance measures in clinical settings require staff time but little training, space, equipment, or risk. As performance measures become more common in practice settings, MDC thresholds may need to be re-evaluated based on the characteristics of the population [67].
For persons with heart failure whose screening outcomes suggest MCI, more comprehensive neuropsychological testing should be available as well as provision of resources to optimize functional independence. Early identification of impaired cognition may lower risk of poor self-management through simplification of medication regimens or providing resources to help manage other regimens essential for optimal heart failure care. It is also important to recognize that depressive symptoms are common in persons with heart failure and are highly correlated with cognitive impairment in this population. Screening for depressive symptoms therefore, may also enhance identification of persons with heart failure at risk for frailty [4,28].
Conclusion
Effective appraisal and development of effective interventions are essential in older adults with heart failure who are at high risk for frailty and cognitive impairment. This will become increasingly important as the population ages and the incidence of heart failure rises proportionately. Although curative treatments for frailty and cognitive impairment are not available, interdisciplinary interventions such as exercise and comprehensive geriatric assessment may improve outcomes in older persons with heart failure [68]. Information gained from objective, simple, inexpensive physical performance measures, when used in combination with cognitive screening, may enhance the ability to evaluate change that signal onset of frailty or cognitive impairment [54,69,70]. The high morbidity and mortality associated with frailty and cognitive impairment indicate that it should be a priority for future research as a strategy to improve clinical outcomes, enhance quality of life, and lower health care costs in this growing population.
Corresponding author: Rebecca Gary, PhD, RN, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA 30322, [email protected].
Funding/support: B. Butts was partially funded for this work through National Institutes of Health/National
Institute of Nursing Research Grant #T32NR012715.
1. Velagaleti RS and Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem. Cardiol Clin 2007;25:487–95.
2. Vincent GK, Velkoff VA. The next four decades. The older population in the United States: 2010 to 2050. United States Census Bureau Report No: P25-1138. U.S. Department of Commerce; May 2010.
3. Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat. J Am Coll Cardiol 2008;52:435–7.
4. Gary R. Evaluation of frailty in older adults with cardiovascular disease: incorporating physical performance measures. J Cardiovasc Nurs 2012;27:120–131.
5. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Age Res Rev 2013;12:719–36.
6. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56:M146–M156.
7. Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56:M158–66.
8. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–21.
9. Cacciatore F, Abete P, Maella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2008;35:723–30.
10. Lupón J, González B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Españ Cardiol 2008;61:835–42.
11. Rich MW. Heart failure in the oldest patients: the impact of comorbid conditions. Am J Geriatr Cardiol 2007;14:134–41.
12. Buck HG, Riegel B. The impact of frailty on health related quality of life in heart failure. Eur J Cardiovasc Nurs 2011;10:159–66
13. Boxer RS, Shah KB Kenny AM. Frailty and prognosis in advanced heart failure. Curr Opin Supp Pall Care 2014;8:25–9.
14. Afilalo J, Sebag IA, Chalifour LE, et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol 2007;293:H1451–6.
15. Walston J. Frailty—the search for underlying causes. Sci Aging Know Environ 2004;2004:e4.
16. Hubbard RE, O’Mahony MS, Savva GM, et al. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13:3103–9.
17. Hubbard RE Woodhouse KW. Frailty, inflammation and the elderly. Biogerontol 2010;11:635–41.
18. Baptista G, Dupuy A-M, Jaussent A, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Rad Res 2012;46:1108–14.
19. Abbate A. The heart on fire: Inflammasome and cardiomyopathy. Exper Physiol 2013;98:385.
20. Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Age Devel 2012;133:456–66.
21. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–46.
22. Toth MJ, Matthews DE, Tracy RP and Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am j Physiol Endocrin Metab 2005;288:E883–91.
23. Howard C, Ferrucci L, Sun K, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol 2007;103:17–20.
24. Muzembo BA, Nagano Y, Eitoku M, et al. A cross-sectional assessment of oxidative DNA damage and muscle strength among elderly people living in the community. Envir Health Prev Med 2014;19:21–9.
25. Wu I-C, Shiesh S-C, Kuo P-H and Lin X-Z. High oxidative stress is correlated with frailty in elderly Chinese. J Am Geriatr Soc 2009;57:1666–71.
26. Alosco ML, Spitznagel MB, Cohen R, et al. Cognitive impairment is independently associated with reduced instrumental activities of daily living in persons with heart failure. J Cardiovasc Nurs 2012;27:44–50.
27. Feola M, Rosso GL, Peano M, et al. Correlation between cognitive impairment and prognostic parameters in patients with congestive heart failure. Arch Med Res 2007;38:234–9.
28. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nurs Res 2010;59:127–39.
29. Pressler SJ, Kim J, Riley P, et al. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Cardiac Fail 2010;16:750–60.
30. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs 2010;25:189–98.
31. Hajduk AM, Lemon SC, Mcmanus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol 2013; 24:407–16.
32. Dardiotis E, Giamouzis G, Mastrogiannis D, et al. Cognitive impairment in heart failure. Cardiol Res Prac 2012; 2012:595821.
33. Petersen RC and O’brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–54.
34. Hjelm C, Dahl A, Broström A, et al. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs 2012; 21:994–1003.
35. Almeida OP, Garrido GJ, Beer C, et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J 2012;33:1769–76.
36. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
37. Nasveddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699.
38. Cameron J, Worrall-Carter L, Page K, et al. Screening for mild cognitive impairment in patients with heart failure: Montreal cognitive assessment versus mini mental state exam. Eur J Cardiovasc Nurs 2013;12:252–60.
39. Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 57:453–61.
40. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Am Geriatr Soc 2006;54:743–9.
41. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 2009;13:538–44.
42. Abellan Van Kan G, Rolland Y, Houles M, et al. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–86.
43. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging 2009;13:724–8.
44. Gary R. Evaluation of frailty in older adults with cardiovascular disease. J Cardiovasc Nurs 2012;27:120–31.
45. Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: evidence from 620+ community-dwelling Europeans living in 11 countries. BMC Geriatr 2013;13:1–9.
46. Ravinrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS). Arch Gerontol Geriatr 2013;57:360–8.
47. Rockwood K, Andrew M and Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol Med Sci 2007;62:738–43.
48. Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7.
49. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94.
50. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61.
51. Di Bari M, Pozzi C, Cavallini Mc, et al. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol 2004;44:1601–08.
52. Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Cardiac Failure 2010; 16:390–5.
53. Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89–96.
54. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51:314–22.
55. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 2012; 50:1921–8.
56. Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehab 2000;81:960–5.
57. Yamada M, Arai H, Sonoda T and Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc 2012;13:507–11.
58. Gary RA, Cress ME, Higgins MK, et al. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: a pilot study. J Cardiovasc Nurs 2012;27:418–30.
59. De Meirelles L, Matsuura C, Resende AD, et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients. Eur J Prev Cardiol 2014;21:1225–32.
60. Feiereisen P, Vaillant M, Gilson G, Delagardelle C. Effects of different training modalities on circulating anabolic/catabolic markers in chronic heart failure. J Cardiopulm Rehab Prev 2013;33:303–8.
61. Smart NA, Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail 2011;17:110–4.
62. Nunes RB, Alves JP, Kessler LP, Lago PD. Aerobic exercsie improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clin Chest Med 2013;68:876–82.
63. Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–8.
64. Colcombe S and Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003;14:125–30.
65. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehab 2004; 85:1694–704.
66. Bautmans I, Vanpuyvelde K, Mets T. Sarcopenia and functional decline: pathophysiology, prevention and therapy. Acta Clinica Belgica 2009;64:303–16.
67. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55A:M221–M231.
68. Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11:342–8.
69. Harkness K, Heckman GA, Mckelvie RS. The older patient with heart failure: high risk for frailty and cognitive impairment. Expert Rev Cardiovasc Ther 2012;10:779–95.
70. Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging 2010;5:259–70.
From the Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
Abstract
- Objective: To review some of the proposed pathways that increase frailty risk in older persons with heart failure and to discuss tools that may be used to assess for changes in physical and cognitive functioning in this population in order to assist with appropriate and timely intervention.
- Methods: Review of the literature.
- Results: Heart failure is the only cardiovascular disease that is increasing by epidemic proportions, largely due to an aging society and therapeutic advances in disease management. Because heart failure is largely a cardiogeriatric syndrome, age-related syndromes such as frailty and cognitive impairment are common in heart failure patients. Compared with age-matched counterparts, older adults with heart failure 4 to 6 times more likely to be frail or cognitively impaired. The reason for the high prevalence of frailty and cognitive impairment in this population is not well known but may likely reflect the synergistic effects of heart failure and aging, which may heighten vulnerability to stressors and accelerate loss of physiologic reserve. Despite the high prevalence of frailty and cognitive impairment in the heart failure population, these conditions are not routinely screened for in clinical practice settings and guidelines on optimal assessment strategies are lacking.
- Conclusion: Persons with heart failure are at an increased risk for frailty, which may worsen symptoms, impair self-management, and lead to worse heart failure outcomes. Early detection of frailty and cognitive impairment may be an opportunity for intervention and a key strategy for improving clinical outcomes in older adults with heart failure.
Approximately 5.7 million persons in the United States are diagnosed with heart failure, and the number of reported new cases is expected to increase to over 700,000 cases annually by the year 2040 [1]. This rising incidence is fueled by an aging population; by the year 2030, 1 in 5 Americans will be over 65 years of age [2]. Heart failure is prevalent among those 65 years of age and older and is the most common reason for hospitalization in this age-group. High readmission rates, approaching 50% over 6 months, are a major contributor to the the escalating economic burden associated with heart failure [3].
Persons with heart failure are more likely to be frail and experience cognitive impairment than their age-matched counterparts without heart failure. The reasons for this are not well known but may be related to hemodynamic, vascular, and inflammatory changes occurring as heart failure progresses. In this paper, we review the link between frailty and cognitive impairment in heart failure, instruments that may be useful for early detection, and interventions such as exercise that may be beneficial for attenuating both conditions.
Frailty in Heart Failure
Epidemiology
Frailty is a heightened vulnerability to stressors in the presence of low physiological reserve [4]. When exposed to stressors, persons who are frail have a much higher probability for disproportionate decompensation, negative events, functional decline, disability, and mortality [5]. Among persons with heart failure, frailty may predispose them to decompensate at a lower threshold, requiring more frequent hospitalizations. Persons with heart failure are more likely to be frail than their age-matched counterparts without heart failure [6,7].
Frailty is a powerful predictor of poor clinical outcomes and mortality in cardiovascular disease [8,9]. Compared with the non-frail, frail persons with heart failure have increased rates of mortality (16.9% vs 4.8%) and increased rates of heart failure hospitalization (20.5% vs 13.3%) [10]. Frailty has also been shown to predict falls, disability, and hospitalization in heart failure patients [6,9,11] and was found to have a negative linear relationship with health-related quality of life [12]. Frail heart failure patients are also more likely to have comorbidities such diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, depression, anemia, and chronic kidney disease [9,13].
Pathophysiology
There is significant overlap in the underlying pathological mechanisms of heart failure and frailty. Symptoms of heart failure, such as dyspnea, fatigue, and muscle loss, mirror components that occur with frailty. Further, cardiac cachexia, a metabolic syndrome in advanced heart failure characterized by a loss of muscle mass, is very similar to the sarcopenia that occurs in frailty.
Frailty, characterized by an increased physiologic vulnerability to stressors, may predispose frail persons with heart failure to exacerbation and worsening of heart failure due to greater susceptibility to the harmful pathophysiologic processes in heart failure, such as inflammation and autonomic dysfunction. Proposed pathophysiologic pathways in frailty include free radicals and oxidative stress, cumulative DNA damage, decreased telomere length, and nuclear fragmentation [14,15]. Frailty has been associated with low-grade chronic inflammation and increased inflammatory cytokines, such as C-reactive protein, tumor necrosis factor–alpha (TNFα), interleukin-6 (IL-6)and fibrinogen [16–18]. Heart failure also is associated with a low-grade and chronic cardiac inflammatory response that is correlated with disease progression [19].
Inflammation. IL-6 is detectable in a higher proportion of persons who are frail compared to non-frail [16] and is the most highly correlated biomarker with frailty. In addition, among those with detectable IL-6 levels, those categorized as frail have higher IL-6 levels compared to those who are non-frail [16,20]. Individuals categorized as frail were found to have significantly higher levels of TNFα than those who were non-frail [16,20]. Increased IL-6 levels are associated with decreased muscle strength, while increased TNFα levels are associated with decreased skeletal muscle protein synthesis [21,22], thus contributing to frailty.
Oxidative stress. Protein carbonyls result from protein oxidation promoted by reactive oxygen species and are markers of oxidative stress. Protein carbonylation is implicated in the pathogenesis of the loss of skeletal muscle mass; high serum protein carbonyls are associated with poor grip strength [23]. 8-OHdG is an oxidized nucleoside indicative of oxidative damage to DNA and a measure of oxidative stress. Accumulation of 8-OHdG in skeletal muscle leads to loss of muscle mass and is associated with decreased hand grip strength in the elderly [24]. Higher serum levels of 8-OHdG are present in older adults who are frail as compared to those who are non-frail [25].
Measurement of Frailty in the Clinical Setting
Frailty has been conceptualized in a number of studies using different models and measures; however, there continues to be a lack of consensus on the definition and operationalization of frailty. Prior research has led to the development of several validated models of frailty that have demonstrated good prediction of adverse outcomes in older adults. Some models, such as the Fried phenotype [6], focus solely on the physical dimension, while other models take a multidimensional approach.Single-item measures (eg, gait speed, 6-minute walk test, handgrip strength) are also commonly used to screen for frailty, but a frailty measure that incorporates more than 1 physical dimension may be more sensitive and reliable. In our opinion, the ideal measure of frailty would consist of a brief assessment that can be serially performed in most clinical practice settings that can identify change in function over time. The incorporation of sensitive physical function measures that can detect frailty early has the potential to slow physical function decline by preserving physiological thresholds.
Cognitive Impairment in Heart Failure
Epidemiology
Cognitive impairment occurs frequently in patients with heart failure, and the presence of cognitive impairment in persons with heart failure has been shown to heighten risk for adverse clinical outcomes, disability, poor quality of life, and mortality [26,27]. Heart failure negatively influences cognitive functioning in most domains [28–32]. The most common domains adversely affected by heart failure and aging are memory and executive function. Deficits in these domains can substantially diminish patient ability to carry out essential self-care behaviors [30,32].
The most common form of cognitive impairment seen in patients with heart failure is mild cognitive impairment (MCI), which is a measurable deficit with memory or another core cognitive domain. Up to 60% of persons with heart failure have been reported to have MCI. Patients with MCI have cognitive deficits that are more pronounced than those seen in normal aging, but lack other symptoms of dementia, such as impaired judgment or reasoning. MCI often will not impede patients’ ability to carry out the activities of daily living (ADLs) independently, but patients may have difficulty in performing some instrumental activities of daily living (IADLs), such as remembering medications, scheduling provider appointments. Dementia, a decline in cognitive ability severe enough to hinder an individual’s ability to perform ADLs or IADLs or engage in social activities or occupational responsibilities, occurs in approximately 25% of persons with heart failure [33].
Persons with heart failure have a fourfold greater likelihood of developing CI than persons without heart failure. Several cohort studies have shown that persons with heart failure had lower performance on cognitive tests than individuals without heart failure [34,35] and were 50% more likely to progress to dementia.
Assessment Tools
Although a comprehensive neurocognitive battery would aid in detecting cognitive impairment in heart failure, few clinical practice settings have the resources to perform such a detailed and time-consuming measurement. Most studies in heart failure have relied on global screening questionnaires such as the Mini-Mental State Examination (MMSE) [36] to assess cognitive functioning in persons with heart failure and in other cardiovascular disorders. Global cognitive measures, however, often lack sensitivity for detecting subtle cognitive deficits such as seen in MCI [28–30]. Screening that measures executive function may be the most beneficial for busy clinical settings, since declines in this domain are well established as contributing to poor outcomes in persons with heart failure.
The Montreal Cognitive Assessment (MoCA) is a rapid screening test designed to detect MCI. It assesses different cognitive domains, including attention, memory, language, and executive function [37]. The MoCA lends itself to use in clinical setting because it is brief, requires little training to administer, and is easy to score. This instrument has been used successfully to assess MCI in persons with heart failure and may be more sensitive than the MMSE in identifying clinically relevant cognitive dysfunction. In 2013 study, Cameron et al [38] administered the MMSE and MoCA to 93 hospitalized heart failure patients and found that the MoCA classified 41% of patients as cognitively impaired that were not classified using the MMSE. For persons with a vascular cognitive deficit, the MMSE has been portrayed as an inadequate screening test due to lack of sensitivity for visuospatial and executive function deficits. Because the MoCA was designed to be more sensitive to such deficits, it may be a superior screening method for persons with heart failure. Although previous studies support the use of the MoCA in persons with heart failure, more research is needed in larger, more diverse heart failure samples with a wide range of cognitive deficits.
A Reasonable Clinical Assessment Approach
Considering the link between heart failure, frailty, and MCI, incorporating simple physical performance measures with cognitive screening may be an effective strategy to identify persons at risk for frailty. Two clinically relevant physical performance-based measures of frailty are proposed: the Fried phenotype (mentioned earlier) and the Short Physical Performance Battery (SBBP). In addition, cognitive screening using the MoCA is recommended as part of the routine examination for determining possible MCI or more severe cognitive deficits. The predictive validity of measuring physical frailty is enhanced when cognitive impairment is included in the assessment [36,39].
The performance-based measures included in this review have previously demonstrated excellent psychometric properties as well as sensitivity for change that is clinically meaningful. Minimal detectable change (MDC), a threshold score that refers to the minimal amount of change outside of error that reflects true change by a patient between 2 time points (rather than variation in measurement), is important for interpreting level of risk for frailty and is included for each instrument [40,41]. If a more brief frailty examination is needed, cut-points for gait speed and handgrip have been used effectively in a number of studies as a threshold for determining frailty, including in older patients with cardiovascular disease and in heart failure [8,42,43].
Fried Frailty Phenotype
The Fried phenotype is an appropriate method of measuring frailty in a clinical setting due to its wide application across diverse populations and consistent identification of adverse outcomes [44]. This model is derived from a frailty model proposed by Fried et al [6] in which a phenotypic cycle exists that includes disease, sarcopenia, decreased walking speed, chronic undernutrition, decreased total energy expenditure, senescent musculoskeletal changes, decreased resting metabolic rate, weight loss and decreased maximal oxygen consumption. Frailty exists when a critical mass of these cycle components are identified in an individual [6].
To validate the model, Fried et al used data from the Cardiovascular Health Study and used the model to show association with a 3-year and 7-year incidence of mobility and ADL disability among 4317 community-dwelling men and women aged 65 years and older, independent of comorbidities. Several studies have directly tested the frailty phenotype model alone and in comparison to other models of frailty in large prospective studies across different populations, such as the Survey of Health, Aging and Retirement in Europe (SHARE) [45], the European Male Aging Study [46], and the Canadian Health Study of Aging [47]. While these studies found the prevalence of frailty to vary across the populations, they all validated the Fried model and found no significant differences in the predictive ability of the Fried model and other models of frailty. The Frailty Consensus conference evaluated the different models of frailty and determined that the Fried model is a validated construct of frailty and is acceptable for use in the identification of individuals who are frail or likely to become frail [48]. Thus, the Fried et al frailty phenotype model is considered to be a standard measure of frailty in older individuals.
Short Physical Performance Battery
In other chronic illness populations, the SPPB has also been used as a predictor of outcomes before, during, or after hospitalization. Valpato et al [53], for example, used the SPPB to assess older adults (mean age, 78 yr) admitted to the hospital with a diagnosis of heart failure (64%), pneumonia (13%), chronic obstructive pulmonary disease (16%), or minor stroke (6.6%) at admission (baseline) and discharge. Patients with the lowest SPPB quartile scores at hospital discharge had a fivefold greater risk of rehospitalization or mortality compared to the highest quartile. In addition, those who had an early decline in SPPB scores 1 month after hospital discharge had greater limitations in performing activities of daily living and a significantly greater probability of being re-hospitalized or death during the 1-year follow-up period. These studies suggest that the SPPB at the first follow-up outpatient visit following hospital discharge may be beneficial for identifying need for further intervention or the need for more frequent follow-up care. Although the SPPB is not part of the Fried et al phenotype, it may provide additional information concerning risk for falls and lower extremity strength that may be beneficial in the evaluation of some persons with heart failure [54]. The SPPB along with instructions and normative data are available for clinical use at no charge at www.grc.nia.nih.gov/branches/ledb/sppb/index.htm.
Interventions for Frailty in Heart Failure
Interventions to address frailty have included exercise training, comprehensive geriatric assessment and management services, social support systems, nutrition, and drugs; however, few intervention studies have examined frailty in heart failure [8]. Restoration of physical function through aerobic exercise and resistance training has shown benefit in frail older adults [55–57] and in persons with heart failure [58]. Maintaining and/or restoring physical function through aerobic and resistance exercise training may be the key to preventing further decline or potentially reversing frailty in older adults with heart failure.
Aerobic exercise has been shown to be beneficial for both frail older adults and frail persons with heart failure [18]. In a study of community-dwelling frail older adults aged 65 and older, a combined aerobic and resistance exercise intervention, performed over 16 weeks, demonstrated significant improvement in frailty scores during the 1-year follow-up in contrast to worsening frailty measures in the control group [57].
Older adults with heart failure experience a much lower exercise tolerance largely due to a 50% to 75% decrease in aerobic capacity in addition to the well-known alterations in peripheral musculoskeletal performance that contribute to fatigue and greater symptom severity. Aerobic exercise has been shown to be beneficial for most heart failure patients by altering the peripheral and central mechanisms, such as inflammatory cytokines, that contribute to heart failure exacerbations, worsened symptom severity, and poor clinical outcomes [59–62].Lower rates of hospitalization, improved physical function, and enhanced health-related quality of life are reported in heart failure patients who routinely exercise [59]. Resistance training has been shown to improve physical function in frail older adults [55]. Further, the use of TheraBand exercise bands in resistance training demonstrated improvement in physical function among frail older adults [56].
Exercise also appears to exert a positive effect on cognition, particularly executive functioning, and may also have a protective effect against cognitive decline with aging and among those with heart failure. The underlying mechanism for improvement in cognition remains poorly understood but is likely related to improved cardiac function, cerebral perfusion, and oxygenation, although this has not been clearly established. Larson et al (2006) evaluated the frequency of participation in a variety of physical activities (eg, walking, bicycling and swimming) over 6 years in 1740 older adults [63]. Older adults who exercised more than 3 times per week during initial assessment were 34% less likely to be diagnosed with dementia than those who exercised fewer than 3 times per week. Several meta-analyses in recent years have shown a consistent and positive relationship between aerobic exercise and cognition [64,65]. Importantly, findings from meta-analyses have shown a moderate effect size (> 0.5) from aerobic training, which was similar for normal and cognitively impaired adults [64].
Implications for Clinical Care
A systematic assessment performed periodically using physical and cognitive measures that may identify prefrailty may be the best strategy for preventing further functional loss, limitations, and disability in persons with heart failure. Persons with heart failure ideally should be evaluated annually for physical function, since a decline has been consistently shown to be a strong predictor of adverse health outcomes, disability, and death [6,66]. Cognitive function should also be assessed routinely in persons with heart failure, particularly when first diagnosed, when changes in treatment regimen occur, and with worsening disease severity, since these events have been shown to occur before changes in cognition [31]. Incorporating geriatric performance-based measures in heart failure management would allow for more treatment strategies aimed at improving physical function, cognitive outcomes, and quality of life. Further, identifying frailty in heart failure is an important component of clinical decision-making when determining if a patient can tolerate therapies such as implantable defibrillators, cardiac resynchronization therapy, or left ventricular assist device placement.
In older adults, performance measures are well established and commonly used as part of geriatric assessment to evaluate physical and cognitive functioning. Performance-based measures may be particularly beneficial in older adults with heart failure to monitor serial changes in physical function. Performance measures in clinical settings require staff time but little training, space, equipment, or risk. As performance measures become more common in practice settings, MDC thresholds may need to be re-evaluated based on the characteristics of the population [67].
For persons with heart failure whose screening outcomes suggest MCI, more comprehensive neuropsychological testing should be available as well as provision of resources to optimize functional independence. Early identification of impaired cognition may lower risk of poor self-management through simplification of medication regimens or providing resources to help manage other regimens essential for optimal heart failure care. It is also important to recognize that depressive symptoms are common in persons with heart failure and are highly correlated with cognitive impairment in this population. Screening for depressive symptoms therefore, may also enhance identification of persons with heart failure at risk for frailty [4,28].
Conclusion
Effective appraisal and development of effective interventions are essential in older adults with heart failure who are at high risk for frailty and cognitive impairment. This will become increasingly important as the population ages and the incidence of heart failure rises proportionately. Although curative treatments for frailty and cognitive impairment are not available, interdisciplinary interventions such as exercise and comprehensive geriatric assessment may improve outcomes in older persons with heart failure [68]. Information gained from objective, simple, inexpensive physical performance measures, when used in combination with cognitive screening, may enhance the ability to evaluate change that signal onset of frailty or cognitive impairment [54,69,70]. The high morbidity and mortality associated with frailty and cognitive impairment indicate that it should be a priority for future research as a strategy to improve clinical outcomes, enhance quality of life, and lower health care costs in this growing population.
Corresponding author: Rebecca Gary, PhD, RN, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA 30322, [email protected].
Funding/support: B. Butts was partially funded for this work through National Institutes of Health/National
Institute of Nursing Research Grant #T32NR012715.
From the Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
Abstract
- Objective: To review some of the proposed pathways that increase frailty risk in older persons with heart failure and to discuss tools that may be used to assess for changes in physical and cognitive functioning in this population in order to assist with appropriate and timely intervention.
- Methods: Review of the literature.
- Results: Heart failure is the only cardiovascular disease that is increasing by epidemic proportions, largely due to an aging society and therapeutic advances in disease management. Because heart failure is largely a cardiogeriatric syndrome, age-related syndromes such as frailty and cognitive impairment are common in heart failure patients. Compared with age-matched counterparts, older adults with heart failure 4 to 6 times more likely to be frail or cognitively impaired. The reason for the high prevalence of frailty and cognitive impairment in this population is not well known but may likely reflect the synergistic effects of heart failure and aging, which may heighten vulnerability to stressors and accelerate loss of physiologic reserve. Despite the high prevalence of frailty and cognitive impairment in the heart failure population, these conditions are not routinely screened for in clinical practice settings and guidelines on optimal assessment strategies are lacking.
- Conclusion: Persons with heart failure are at an increased risk for frailty, which may worsen symptoms, impair self-management, and lead to worse heart failure outcomes. Early detection of frailty and cognitive impairment may be an opportunity for intervention and a key strategy for improving clinical outcomes in older adults with heart failure.
Approximately 5.7 million persons in the United States are diagnosed with heart failure, and the number of reported new cases is expected to increase to over 700,000 cases annually by the year 2040 [1]. This rising incidence is fueled by an aging population; by the year 2030, 1 in 5 Americans will be over 65 years of age [2]. Heart failure is prevalent among those 65 years of age and older and is the most common reason for hospitalization in this age-group. High readmission rates, approaching 50% over 6 months, are a major contributor to the the escalating economic burden associated with heart failure [3].
Persons with heart failure are more likely to be frail and experience cognitive impairment than their age-matched counterparts without heart failure. The reasons for this are not well known but may be related to hemodynamic, vascular, and inflammatory changes occurring as heart failure progresses. In this paper, we review the link between frailty and cognitive impairment in heart failure, instruments that may be useful for early detection, and interventions such as exercise that may be beneficial for attenuating both conditions.
Frailty in Heart Failure
Epidemiology
Frailty is a heightened vulnerability to stressors in the presence of low physiological reserve [4]. When exposed to stressors, persons who are frail have a much higher probability for disproportionate decompensation, negative events, functional decline, disability, and mortality [5]. Among persons with heart failure, frailty may predispose them to decompensate at a lower threshold, requiring more frequent hospitalizations. Persons with heart failure are more likely to be frail than their age-matched counterparts without heart failure [6,7].
Frailty is a powerful predictor of poor clinical outcomes and mortality in cardiovascular disease [8,9]. Compared with the non-frail, frail persons with heart failure have increased rates of mortality (16.9% vs 4.8%) and increased rates of heart failure hospitalization (20.5% vs 13.3%) [10]. Frailty has also been shown to predict falls, disability, and hospitalization in heart failure patients [6,9,11] and was found to have a negative linear relationship with health-related quality of life [12]. Frail heart failure patients are also more likely to have comorbidities such diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, depression, anemia, and chronic kidney disease [9,13].
Pathophysiology
There is significant overlap in the underlying pathological mechanisms of heart failure and frailty. Symptoms of heart failure, such as dyspnea, fatigue, and muscle loss, mirror components that occur with frailty. Further, cardiac cachexia, a metabolic syndrome in advanced heart failure characterized by a loss of muscle mass, is very similar to the sarcopenia that occurs in frailty.
Frailty, characterized by an increased physiologic vulnerability to stressors, may predispose frail persons with heart failure to exacerbation and worsening of heart failure due to greater susceptibility to the harmful pathophysiologic processes in heart failure, such as inflammation and autonomic dysfunction. Proposed pathophysiologic pathways in frailty include free radicals and oxidative stress, cumulative DNA damage, decreased telomere length, and nuclear fragmentation [14,15]. Frailty has been associated with low-grade chronic inflammation and increased inflammatory cytokines, such as C-reactive protein, tumor necrosis factor–alpha (TNFα), interleukin-6 (IL-6)and fibrinogen [16–18]. Heart failure also is associated with a low-grade and chronic cardiac inflammatory response that is correlated with disease progression [19].
Inflammation. IL-6 is detectable in a higher proportion of persons who are frail compared to non-frail [16] and is the most highly correlated biomarker with frailty. In addition, among those with detectable IL-6 levels, those categorized as frail have higher IL-6 levels compared to those who are non-frail [16,20]. Individuals categorized as frail were found to have significantly higher levels of TNFα than those who were non-frail [16,20]. Increased IL-6 levels are associated with decreased muscle strength, while increased TNFα levels are associated with decreased skeletal muscle protein synthesis [21,22], thus contributing to frailty.
Oxidative stress. Protein carbonyls result from protein oxidation promoted by reactive oxygen species and are markers of oxidative stress. Protein carbonylation is implicated in the pathogenesis of the loss of skeletal muscle mass; high serum protein carbonyls are associated with poor grip strength [23]. 8-OHdG is an oxidized nucleoside indicative of oxidative damage to DNA and a measure of oxidative stress. Accumulation of 8-OHdG in skeletal muscle leads to loss of muscle mass and is associated with decreased hand grip strength in the elderly [24]. Higher serum levels of 8-OHdG are present in older adults who are frail as compared to those who are non-frail [25].
Measurement of Frailty in the Clinical Setting
Frailty has been conceptualized in a number of studies using different models and measures; however, there continues to be a lack of consensus on the definition and operationalization of frailty. Prior research has led to the development of several validated models of frailty that have demonstrated good prediction of adverse outcomes in older adults. Some models, such as the Fried phenotype [6], focus solely on the physical dimension, while other models take a multidimensional approach.Single-item measures (eg, gait speed, 6-minute walk test, handgrip strength) are also commonly used to screen for frailty, but a frailty measure that incorporates more than 1 physical dimension may be more sensitive and reliable. In our opinion, the ideal measure of frailty would consist of a brief assessment that can be serially performed in most clinical practice settings that can identify change in function over time. The incorporation of sensitive physical function measures that can detect frailty early has the potential to slow physical function decline by preserving physiological thresholds.
Cognitive Impairment in Heart Failure
Epidemiology
Cognitive impairment occurs frequently in patients with heart failure, and the presence of cognitive impairment in persons with heart failure has been shown to heighten risk for adverse clinical outcomes, disability, poor quality of life, and mortality [26,27]. Heart failure negatively influences cognitive functioning in most domains [28–32]. The most common domains adversely affected by heart failure and aging are memory and executive function. Deficits in these domains can substantially diminish patient ability to carry out essential self-care behaviors [30,32].
The most common form of cognitive impairment seen in patients with heart failure is mild cognitive impairment (MCI), which is a measurable deficit with memory or another core cognitive domain. Up to 60% of persons with heart failure have been reported to have MCI. Patients with MCI have cognitive deficits that are more pronounced than those seen in normal aging, but lack other symptoms of dementia, such as impaired judgment or reasoning. MCI often will not impede patients’ ability to carry out the activities of daily living (ADLs) independently, but patients may have difficulty in performing some instrumental activities of daily living (IADLs), such as remembering medications, scheduling provider appointments. Dementia, a decline in cognitive ability severe enough to hinder an individual’s ability to perform ADLs or IADLs or engage in social activities or occupational responsibilities, occurs in approximately 25% of persons with heart failure [33].
Persons with heart failure have a fourfold greater likelihood of developing CI than persons without heart failure. Several cohort studies have shown that persons with heart failure had lower performance on cognitive tests than individuals without heart failure [34,35] and were 50% more likely to progress to dementia.
Assessment Tools
Although a comprehensive neurocognitive battery would aid in detecting cognitive impairment in heart failure, few clinical practice settings have the resources to perform such a detailed and time-consuming measurement. Most studies in heart failure have relied on global screening questionnaires such as the Mini-Mental State Examination (MMSE) [36] to assess cognitive functioning in persons with heart failure and in other cardiovascular disorders. Global cognitive measures, however, often lack sensitivity for detecting subtle cognitive deficits such as seen in MCI [28–30]. Screening that measures executive function may be the most beneficial for busy clinical settings, since declines in this domain are well established as contributing to poor outcomes in persons with heart failure.
The Montreal Cognitive Assessment (MoCA) is a rapid screening test designed to detect MCI. It assesses different cognitive domains, including attention, memory, language, and executive function [37]. The MoCA lends itself to use in clinical setting because it is brief, requires little training to administer, and is easy to score. This instrument has been used successfully to assess MCI in persons with heart failure and may be more sensitive than the MMSE in identifying clinically relevant cognitive dysfunction. In 2013 study, Cameron et al [38] administered the MMSE and MoCA to 93 hospitalized heart failure patients and found that the MoCA classified 41% of patients as cognitively impaired that were not classified using the MMSE. For persons with a vascular cognitive deficit, the MMSE has been portrayed as an inadequate screening test due to lack of sensitivity for visuospatial and executive function deficits. Because the MoCA was designed to be more sensitive to such deficits, it may be a superior screening method for persons with heart failure. Although previous studies support the use of the MoCA in persons with heart failure, more research is needed in larger, more diverse heart failure samples with a wide range of cognitive deficits.
A Reasonable Clinical Assessment Approach
Considering the link between heart failure, frailty, and MCI, incorporating simple physical performance measures with cognitive screening may be an effective strategy to identify persons at risk for frailty. Two clinically relevant physical performance-based measures of frailty are proposed: the Fried phenotype (mentioned earlier) and the Short Physical Performance Battery (SBBP). In addition, cognitive screening using the MoCA is recommended as part of the routine examination for determining possible MCI or more severe cognitive deficits. The predictive validity of measuring physical frailty is enhanced when cognitive impairment is included in the assessment [36,39].
The performance-based measures included in this review have previously demonstrated excellent psychometric properties as well as sensitivity for change that is clinically meaningful. Minimal detectable change (MDC), a threshold score that refers to the minimal amount of change outside of error that reflects true change by a patient between 2 time points (rather than variation in measurement), is important for interpreting level of risk for frailty and is included for each instrument [40,41]. If a more brief frailty examination is needed, cut-points for gait speed and handgrip have been used effectively in a number of studies as a threshold for determining frailty, including in older patients with cardiovascular disease and in heart failure [8,42,43].
Fried Frailty Phenotype
The Fried phenotype is an appropriate method of measuring frailty in a clinical setting due to its wide application across diverse populations and consistent identification of adverse outcomes [44]. This model is derived from a frailty model proposed by Fried et al [6] in which a phenotypic cycle exists that includes disease, sarcopenia, decreased walking speed, chronic undernutrition, decreased total energy expenditure, senescent musculoskeletal changes, decreased resting metabolic rate, weight loss and decreased maximal oxygen consumption. Frailty exists when a critical mass of these cycle components are identified in an individual [6].
To validate the model, Fried et al used data from the Cardiovascular Health Study and used the model to show association with a 3-year and 7-year incidence of mobility and ADL disability among 4317 community-dwelling men and women aged 65 years and older, independent of comorbidities. Several studies have directly tested the frailty phenotype model alone and in comparison to other models of frailty in large prospective studies across different populations, such as the Survey of Health, Aging and Retirement in Europe (SHARE) [45], the European Male Aging Study [46], and the Canadian Health Study of Aging [47]. While these studies found the prevalence of frailty to vary across the populations, they all validated the Fried model and found no significant differences in the predictive ability of the Fried model and other models of frailty. The Frailty Consensus conference evaluated the different models of frailty and determined that the Fried model is a validated construct of frailty and is acceptable for use in the identification of individuals who are frail or likely to become frail [48]. Thus, the Fried et al frailty phenotype model is considered to be a standard measure of frailty in older individuals.
Short Physical Performance Battery
In other chronic illness populations, the SPPB has also been used as a predictor of outcomes before, during, or after hospitalization. Valpato et al [53], for example, used the SPPB to assess older adults (mean age, 78 yr) admitted to the hospital with a diagnosis of heart failure (64%), pneumonia (13%), chronic obstructive pulmonary disease (16%), or minor stroke (6.6%) at admission (baseline) and discharge. Patients with the lowest SPPB quartile scores at hospital discharge had a fivefold greater risk of rehospitalization or mortality compared to the highest quartile. In addition, those who had an early decline in SPPB scores 1 month after hospital discharge had greater limitations in performing activities of daily living and a significantly greater probability of being re-hospitalized or death during the 1-year follow-up period. These studies suggest that the SPPB at the first follow-up outpatient visit following hospital discharge may be beneficial for identifying need for further intervention or the need for more frequent follow-up care. Although the SPPB is not part of the Fried et al phenotype, it may provide additional information concerning risk for falls and lower extremity strength that may be beneficial in the evaluation of some persons with heart failure [54]. The SPPB along with instructions and normative data are available for clinical use at no charge at www.grc.nia.nih.gov/branches/ledb/sppb/index.htm.
Interventions for Frailty in Heart Failure
Interventions to address frailty have included exercise training, comprehensive geriatric assessment and management services, social support systems, nutrition, and drugs; however, few intervention studies have examined frailty in heart failure [8]. Restoration of physical function through aerobic exercise and resistance training has shown benefit in frail older adults [55–57] and in persons with heart failure [58]. Maintaining and/or restoring physical function through aerobic and resistance exercise training may be the key to preventing further decline or potentially reversing frailty in older adults with heart failure.
Aerobic exercise has been shown to be beneficial for both frail older adults and frail persons with heart failure [18]. In a study of community-dwelling frail older adults aged 65 and older, a combined aerobic and resistance exercise intervention, performed over 16 weeks, demonstrated significant improvement in frailty scores during the 1-year follow-up in contrast to worsening frailty measures in the control group [57].
Older adults with heart failure experience a much lower exercise tolerance largely due to a 50% to 75% decrease in aerobic capacity in addition to the well-known alterations in peripheral musculoskeletal performance that contribute to fatigue and greater symptom severity. Aerobic exercise has been shown to be beneficial for most heart failure patients by altering the peripheral and central mechanisms, such as inflammatory cytokines, that contribute to heart failure exacerbations, worsened symptom severity, and poor clinical outcomes [59–62].Lower rates of hospitalization, improved physical function, and enhanced health-related quality of life are reported in heart failure patients who routinely exercise [59]. Resistance training has been shown to improve physical function in frail older adults [55]. Further, the use of TheraBand exercise bands in resistance training demonstrated improvement in physical function among frail older adults [56].
Exercise also appears to exert a positive effect on cognition, particularly executive functioning, and may also have a protective effect against cognitive decline with aging and among those with heart failure. The underlying mechanism for improvement in cognition remains poorly understood but is likely related to improved cardiac function, cerebral perfusion, and oxygenation, although this has not been clearly established. Larson et al (2006) evaluated the frequency of participation in a variety of physical activities (eg, walking, bicycling and swimming) over 6 years in 1740 older adults [63]. Older adults who exercised more than 3 times per week during initial assessment were 34% less likely to be diagnosed with dementia than those who exercised fewer than 3 times per week. Several meta-analyses in recent years have shown a consistent and positive relationship between aerobic exercise and cognition [64,65]. Importantly, findings from meta-analyses have shown a moderate effect size (> 0.5) from aerobic training, which was similar for normal and cognitively impaired adults [64].
Implications for Clinical Care
A systematic assessment performed periodically using physical and cognitive measures that may identify prefrailty may be the best strategy for preventing further functional loss, limitations, and disability in persons with heart failure. Persons with heart failure ideally should be evaluated annually for physical function, since a decline has been consistently shown to be a strong predictor of adverse health outcomes, disability, and death [6,66]. Cognitive function should also be assessed routinely in persons with heart failure, particularly when first diagnosed, when changes in treatment regimen occur, and with worsening disease severity, since these events have been shown to occur before changes in cognition [31]. Incorporating geriatric performance-based measures in heart failure management would allow for more treatment strategies aimed at improving physical function, cognitive outcomes, and quality of life. Further, identifying frailty in heart failure is an important component of clinical decision-making when determining if a patient can tolerate therapies such as implantable defibrillators, cardiac resynchronization therapy, or left ventricular assist device placement.
In older adults, performance measures are well established and commonly used as part of geriatric assessment to evaluate physical and cognitive functioning. Performance-based measures may be particularly beneficial in older adults with heart failure to monitor serial changes in physical function. Performance measures in clinical settings require staff time but little training, space, equipment, or risk. As performance measures become more common in practice settings, MDC thresholds may need to be re-evaluated based on the characteristics of the population [67].
For persons with heart failure whose screening outcomes suggest MCI, more comprehensive neuropsychological testing should be available as well as provision of resources to optimize functional independence. Early identification of impaired cognition may lower risk of poor self-management through simplification of medication regimens or providing resources to help manage other regimens essential for optimal heart failure care. It is also important to recognize that depressive symptoms are common in persons with heart failure and are highly correlated with cognitive impairment in this population. Screening for depressive symptoms therefore, may also enhance identification of persons with heart failure at risk for frailty [4,28].
Conclusion
Effective appraisal and development of effective interventions are essential in older adults with heart failure who are at high risk for frailty and cognitive impairment. This will become increasingly important as the population ages and the incidence of heart failure rises proportionately. Although curative treatments for frailty and cognitive impairment are not available, interdisciplinary interventions such as exercise and comprehensive geriatric assessment may improve outcomes in older persons with heart failure [68]. Information gained from objective, simple, inexpensive physical performance measures, when used in combination with cognitive screening, may enhance the ability to evaluate change that signal onset of frailty or cognitive impairment [54,69,70]. The high morbidity and mortality associated with frailty and cognitive impairment indicate that it should be a priority for future research as a strategy to improve clinical outcomes, enhance quality of life, and lower health care costs in this growing population.
Corresponding author: Rebecca Gary, PhD, RN, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA 30322, [email protected].
Funding/support: B. Butts was partially funded for this work through National Institutes of Health/National
Institute of Nursing Research Grant #T32NR012715.
1. Velagaleti RS and Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem. Cardiol Clin 2007;25:487–95.
2. Vincent GK, Velkoff VA. The next four decades. The older population in the United States: 2010 to 2050. United States Census Bureau Report No: P25-1138. U.S. Department of Commerce; May 2010.
3. Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat. J Am Coll Cardiol 2008;52:435–7.
4. Gary R. Evaluation of frailty in older adults with cardiovascular disease: incorporating physical performance measures. J Cardiovasc Nurs 2012;27:120–131.
5. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Age Res Rev 2013;12:719–36.
6. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56:M146–M156.
7. Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56:M158–66.
8. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–21.
9. Cacciatore F, Abete P, Maella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2008;35:723–30.
10. Lupón J, González B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Españ Cardiol 2008;61:835–42.
11. Rich MW. Heart failure in the oldest patients: the impact of comorbid conditions. Am J Geriatr Cardiol 2007;14:134–41.
12. Buck HG, Riegel B. The impact of frailty on health related quality of life in heart failure. Eur J Cardiovasc Nurs 2011;10:159–66
13. Boxer RS, Shah KB Kenny AM. Frailty and prognosis in advanced heart failure. Curr Opin Supp Pall Care 2014;8:25–9.
14. Afilalo J, Sebag IA, Chalifour LE, et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol 2007;293:H1451–6.
15. Walston J. Frailty—the search for underlying causes. Sci Aging Know Environ 2004;2004:e4.
16. Hubbard RE, O’Mahony MS, Savva GM, et al. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13:3103–9.
17. Hubbard RE Woodhouse KW. Frailty, inflammation and the elderly. Biogerontol 2010;11:635–41.
18. Baptista G, Dupuy A-M, Jaussent A, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Rad Res 2012;46:1108–14.
19. Abbate A. The heart on fire: Inflammasome and cardiomyopathy. Exper Physiol 2013;98:385.
20. Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Age Devel 2012;133:456–66.
21. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–46.
22. Toth MJ, Matthews DE, Tracy RP and Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am j Physiol Endocrin Metab 2005;288:E883–91.
23. Howard C, Ferrucci L, Sun K, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol 2007;103:17–20.
24. Muzembo BA, Nagano Y, Eitoku M, et al. A cross-sectional assessment of oxidative DNA damage and muscle strength among elderly people living in the community. Envir Health Prev Med 2014;19:21–9.
25. Wu I-C, Shiesh S-C, Kuo P-H and Lin X-Z. High oxidative stress is correlated with frailty in elderly Chinese. J Am Geriatr Soc 2009;57:1666–71.
26. Alosco ML, Spitznagel MB, Cohen R, et al. Cognitive impairment is independently associated with reduced instrumental activities of daily living in persons with heart failure. J Cardiovasc Nurs 2012;27:44–50.
27. Feola M, Rosso GL, Peano M, et al. Correlation between cognitive impairment and prognostic parameters in patients with congestive heart failure. Arch Med Res 2007;38:234–9.
28. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nurs Res 2010;59:127–39.
29. Pressler SJ, Kim J, Riley P, et al. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Cardiac Fail 2010;16:750–60.
30. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs 2010;25:189–98.
31. Hajduk AM, Lemon SC, Mcmanus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol 2013; 24:407–16.
32. Dardiotis E, Giamouzis G, Mastrogiannis D, et al. Cognitive impairment in heart failure. Cardiol Res Prac 2012; 2012:595821.
33. Petersen RC and O’brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–54.
34. Hjelm C, Dahl A, Broström A, et al. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs 2012; 21:994–1003.
35. Almeida OP, Garrido GJ, Beer C, et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J 2012;33:1769–76.
36. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
37. Nasveddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699.
38. Cameron J, Worrall-Carter L, Page K, et al. Screening for mild cognitive impairment in patients with heart failure: Montreal cognitive assessment versus mini mental state exam. Eur J Cardiovasc Nurs 2013;12:252–60.
39. Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 57:453–61.
40. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Am Geriatr Soc 2006;54:743–9.
41. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 2009;13:538–44.
42. Abellan Van Kan G, Rolland Y, Houles M, et al. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–86.
43. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging 2009;13:724–8.
44. Gary R. Evaluation of frailty in older adults with cardiovascular disease. J Cardiovasc Nurs 2012;27:120–31.
45. Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: evidence from 620+ community-dwelling Europeans living in 11 countries. BMC Geriatr 2013;13:1–9.
46. Ravinrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS). Arch Gerontol Geriatr 2013;57:360–8.
47. Rockwood K, Andrew M and Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol Med Sci 2007;62:738–43.
48. Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7.
49. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94.
50. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61.
51. Di Bari M, Pozzi C, Cavallini Mc, et al. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol 2004;44:1601–08.
52. Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Cardiac Failure 2010; 16:390–5.
53. Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89–96.
54. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51:314–22.
55. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 2012; 50:1921–8.
56. Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehab 2000;81:960–5.
57. Yamada M, Arai H, Sonoda T and Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc 2012;13:507–11.
58. Gary RA, Cress ME, Higgins MK, et al. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: a pilot study. J Cardiovasc Nurs 2012;27:418–30.
59. De Meirelles L, Matsuura C, Resende AD, et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients. Eur J Prev Cardiol 2014;21:1225–32.
60. Feiereisen P, Vaillant M, Gilson G, Delagardelle C. Effects of different training modalities on circulating anabolic/catabolic markers in chronic heart failure. J Cardiopulm Rehab Prev 2013;33:303–8.
61. Smart NA, Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail 2011;17:110–4.
62. Nunes RB, Alves JP, Kessler LP, Lago PD. Aerobic exercsie improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clin Chest Med 2013;68:876–82.
63. Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–8.
64. Colcombe S and Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003;14:125–30.
65. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehab 2004; 85:1694–704.
66. Bautmans I, Vanpuyvelde K, Mets T. Sarcopenia and functional decline: pathophysiology, prevention and therapy. Acta Clinica Belgica 2009;64:303–16.
67. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55A:M221–M231.
68. Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11:342–8.
69. Harkness K, Heckman GA, Mckelvie RS. The older patient with heart failure: high risk for frailty and cognitive impairment. Expert Rev Cardiovasc Ther 2012;10:779–95.
70. Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging 2010;5:259–70.
1. Velagaleti RS and Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem. Cardiol Clin 2007;25:487–95.
2. Vincent GK, Velkoff VA. The next four decades. The older population in the United States: 2010 to 2050. United States Census Bureau Report No: P25-1138. U.S. Department of Commerce; May 2010.
3. Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat. J Am Coll Cardiol 2008;52:435–7.
4. Gary R. Evaluation of frailty in older adults with cardiovascular disease: incorporating physical performance measures. J Cardiovasc Nurs 2012;27:120–131.
5. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Age Res Rev 2013;12:719–36.
6. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56:M146–M156.
7. Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56:M158–66.
8. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–21.
9. Cacciatore F, Abete P, Maella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2008;35:723–30.
10. Lupón J, González B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Españ Cardiol 2008;61:835–42.
11. Rich MW. Heart failure in the oldest patients: the impact of comorbid conditions. Am J Geriatr Cardiol 2007;14:134–41.
12. Buck HG, Riegel B. The impact of frailty on health related quality of life in heart failure. Eur J Cardiovasc Nurs 2011;10:159–66
13. Boxer RS, Shah KB Kenny AM. Frailty and prognosis in advanced heart failure. Curr Opin Supp Pall Care 2014;8:25–9.
14. Afilalo J, Sebag IA, Chalifour LE, et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol 2007;293:H1451–6.
15. Walston J. Frailty—the search for underlying causes. Sci Aging Know Environ 2004;2004:e4.
16. Hubbard RE, O’Mahony MS, Savva GM, et al. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13:3103–9.
17. Hubbard RE Woodhouse KW. Frailty, inflammation and the elderly. Biogerontol 2010;11:635–41.
18. Baptista G, Dupuy A-M, Jaussent A, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Rad Res 2012;46:1108–14.
19. Abbate A. The heart on fire: Inflammasome and cardiomyopathy. Exper Physiol 2013;98:385.
20. Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Age Devel 2012;133:456–66.
21. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–46.
22. Toth MJ, Matthews DE, Tracy RP and Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am j Physiol Endocrin Metab 2005;288:E883–91.
23. Howard C, Ferrucci L, Sun K, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol 2007;103:17–20.
24. Muzembo BA, Nagano Y, Eitoku M, et al. A cross-sectional assessment of oxidative DNA damage and muscle strength among elderly people living in the community. Envir Health Prev Med 2014;19:21–9.
25. Wu I-C, Shiesh S-C, Kuo P-H and Lin X-Z. High oxidative stress is correlated with frailty in elderly Chinese. J Am Geriatr Soc 2009;57:1666–71.
26. Alosco ML, Spitznagel MB, Cohen R, et al. Cognitive impairment is independently associated with reduced instrumental activities of daily living in persons with heart failure. J Cardiovasc Nurs 2012;27:44–50.
27. Feola M, Rosso GL, Peano M, et al. Correlation between cognitive impairment and prognostic parameters in patients with congestive heart failure. Arch Med Res 2007;38:234–9.
28. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nurs Res 2010;59:127–39.
29. Pressler SJ, Kim J, Riley P, et al. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Cardiac Fail 2010;16:750–60.
30. Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs 2010;25:189–98.
31. Hajduk AM, Lemon SC, Mcmanus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol 2013; 24:407–16.
32. Dardiotis E, Giamouzis G, Mastrogiannis D, et al. Cognitive impairment in heart failure. Cardiol Res Prac 2012; 2012:595821.
33. Petersen RC and O’brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–54.
34. Hjelm C, Dahl A, Broström A, et al. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs 2012; 21:994–1003.
35. Almeida OP, Garrido GJ, Beer C, et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J 2012;33:1769–76.
36. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
37. Nasveddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699.
38. Cameron J, Worrall-Carter L, Page K, et al. Screening for mild cognitive impairment in patients with heart failure: Montreal cognitive assessment versus mini mental state exam. Eur J Cardiovasc Nurs 2013;12:252–60.
39. Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 57:453–61.
40. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Am Geriatr Soc 2006;54:743–9.
41. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 2009;13:538–44.
42. Abellan Van Kan G, Rolland Y, Houles M, et al. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–86.
43. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging 2009;13:724–8.
44. Gary R. Evaluation of frailty in older adults with cardiovascular disease. J Cardiovasc Nurs 2012;27:120–31.
45. Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: evidence from 620+ community-dwelling Europeans living in 11 countries. BMC Geriatr 2013;13:1–9.
46. Ravinrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS). Arch Gerontol Geriatr 2013;57:360–8.
47. Rockwood K, Andrew M and Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol Med Sci 2007;62:738–43.
48. Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7.
49. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94.
50. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61.
51. Di Bari M, Pozzi C, Cavallini Mc, et al. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol 2004;44:1601–08.
52. Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Cardiac Failure 2010; 16:390–5.
53. Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89–96.
54. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51:314–22.
55. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 2012; 50:1921–8.
56. Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehab 2000;81:960–5.
57. Yamada M, Arai H, Sonoda T and Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc 2012;13:507–11.
58. Gary RA, Cress ME, Higgins MK, et al. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: a pilot study. J Cardiovasc Nurs 2012;27:418–30.
59. De Meirelles L, Matsuura C, Resende AD, et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients. Eur J Prev Cardiol 2014;21:1225–32.
60. Feiereisen P, Vaillant M, Gilson G, Delagardelle C. Effects of different training modalities on circulating anabolic/catabolic markers in chronic heart failure. J Cardiopulm Rehab Prev 2013;33:303–8.
61. Smart NA, Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail 2011;17:110–4.
62. Nunes RB, Alves JP, Kessler LP, Lago PD. Aerobic exercsie improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clin Chest Med 2013;68:876–82.
63. Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–8.
64. Colcombe S and Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003;14:125–30.
65. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehab 2004; 85:1694–704.
66. Bautmans I, Vanpuyvelde K, Mets T. Sarcopenia and functional decline: pathophysiology, prevention and therapy. Acta Clinica Belgica 2009;64:303–16.
67. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55A:M221–M231.
68. Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11:342–8.
69. Harkness K, Heckman GA, Mckelvie RS. The older patient with heart failure: high risk for frailty and cognitive impairment. Expert Rev Cardiovasc Ther 2012;10:779–95.
70. Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging 2010;5:259–70.
Changing Hospital Visiting Policies: From Families as “Visitors” to Families as Partners
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken, Ms. Kaufman, and Ms. Johnson), Anne Arundel Medical Center, Annapolis, MD (Dr. Perkins), Contra Costa Regional Medical Center & Health Centers, Martinez, CA (Ms. Benepal, Ms. Roth, and Vidant Health, Greenville, NC (Ms. Dutton and Ms. Jones).
Abstract
- Objective: To describe a campaign to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care.
- Methods: Descriptive report.
- Results: Many hospitals still have “visiting” hours that limit family presence, often counter to patient preferences. To change the concept of families as visitors and eliminate restrictive hospital visiting policies, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families, calling on all hospitals to welcome families 24 hours a day and transform their policies and approaches to care so that patients’ families and loved ones are included in care and decision making, according to patient preferences. As part of the campaign, IPFCC recognized 12 hospitals that exemplify success in eliminating restrictive visiting policies and have changed the concept of families from “visitors” to partners. Leaders at these hospitals attest to the benefits of the changes through improved experience of care and other outcomes. Three exemplar hospitals are highlighted in this article and share their processes of change as well as key learnings and outcomes.
- Conclusion: Hospital policies and practices that encourage and support families as partners in care are essential to patients’ health, well-being, and safety.
Many families are restricted from the bedsides of loved ones because of hospital visiting policies [1–3]. Restrictive policies are often based on long-held beliefs that the presence and participation of families interferes with care, exhausts patients, is a burden to families, spreads infection, or violates HIPAA. However, there is no evidence to support those beliefs. In fact, isolating patients at their most vulnerable time from the people who know them best places them at risk for medical error, emotional harm, inconsistencies in care, and lack of preparedness for transitions in care [4,5]. Jackie Gruzenski’s story “Behind a Locked Door” (printed below) affectingly describes the impact of restrictive policies on a couple's last days.
Fortunately, a growing number of hospitals are lifting these restrictions. But opening the door is not enough. Hospitals need to change the concept of families as “visitors” to families as partners in care. Changing policies is a foundational step in creating a patient- and family-centered culture where families are recognized as essential to patients’ health and well-being and where they are respected as allies for quality and safety.
In response to this critical need for change, in June 2014 the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families. IPFCC, founded in 1992, is a nonprofit organization that provides essential leadership to advance the understanding and practice of patient- and family-centered care [6]. Emphasizing the importance of family presence and participation to quality and safety, the campaign seeks to eliminate restrictive “visiting” policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference [7]. The goal of the campaign is to change visiting policies in 1000 hospitals by 2017. Partnering with IPFCC in this initiative are the American Society for Healthcare Risk Management, American Association of Critical Care Nurses, National Partnership for Women & Families, New Yorkers for Patient and Family Empowerment, Health In Aging Foundation, and the Canadian Foundation for Healthcare Improvement.
Anne Arundel Medical Center
A regional not-for-profit hospital founded in 1902, Anne Arundel Medical Center in Annapolis, MD, provides acute inpatient and outpatient care to residents of 4 counties in Maryland. A 380-bed facility, Anne Arundel has a cancer institute, heart and vascular institute, joint center, spine center, and a women’s and children’s center. In April 2011, the hospital completed a $424 million expansion project, which included a pediatric emergency room, an expanded general emergency room, 50 new patient beds, and 8 new operating rooms.
In 2010, based on a desire to concretely implement the principles of patient- and family-centered care, leaders at Anne Arundel began working with patient and family advisors and initiated a process to change the hospital’s restrictive visiting policy. Now, there are no restrictions on family presence anywhere in the hospital, from ICUs to medical/surgical units to other clinical areas. Patients have the power to choose who they want to stay with them—24 hours a day, 7 days a week. According to Anne Arundel’s policy, each patient determines who is defined as “family.” A “Revisiting Visiting” task force, comprising support staff, providers, and patient and family advisors, worked for 9 months to develop the new family presence policy and support its implementation.
With Anne Arundel leadership encouragement and support, patient and family advisors participated in all phases of the development and implementation of the new family presence policy and in other ways to advance the practice of patient- and family-centered care. The advisors also participated in the process to change the way nurse change of shift report was conducted, and they made recommendations for changes in the directional signs throughout the hospital. New signs, featuring a pineapple (a symbol of hospitality) and the words “Welcome Families” replaced old ones displaying the former restrictive visiting policy.
Supporting patient and family involvement in transitions in care is an integral aspect of implementing family presence policies and practices. Through an “Always Events” grant from the Picker Institute (for information about the Always Events program, see www.ihi.org/Engage/Initiatives/PatientFamilyCenteredCare/Pages/AlwaysEvents.aspx), patient and family advisors, staff, and providers at Anne Arundel developed the SMART discharge protocol, which includes a simple 5-item checklist that is reviewed and discussed with the patient and family prior to discharge. SMART is an acronym for Signs, Medications, Appointments, Results, and Talk. In its work, the SMART team built on current evidence, created urgency and expectation for use with patients, families, and caregivers, disseminated findings, and promoted the protocol as a national standard. The tool is available at www.ihi.org/resources/Pages/Tools/SMARTDischargeProtocol.aspx.
According to Anne Arundel’s COO and CNO, Sherry Perkins, a critical part of the change process was to first understand staff’s fears and then learn what the evidence says. For example, with regard to the impact of additional family presence on infection control, they learned that family presence did not pose additional infection control concerns.
In 2009, there were no patient and family advisors volunteering at Anne Arundel. In 2014, there are approximately 80 advisors. Since 2009, the overall HCAHPS rating of the hospital has gone from 75.4% to 82% (the national average is 70%). While patient satisfaction scores have previously been in the top deciles at Anne Arundel, they have consistently risen since expanding family presence and implementing additional patient- and family-centered strategies.
Contra Costa Regional Medical Center and Health Centers
Contra Costa Health Services in Martinez, CA, includes Contra Costa Regional Medical Center and 10 health centers as part of a comprehensive county health system. Its 164-bed public hospital is dedicated to offering services that are welcoming, accessible, safe and respectful for everyone.
Like many hospitals in the country, for years Contra Costa Regional Medical Center restricted the hours when family members and loved ones could visit patients. However, the hospital’s medical staff often felt uncomfortable that they had to usher family and care partners away from patients when visiting hours were over. Anna Roth, Contra Costa’s CEO, recalls an incident that caused great anguish and contributed to the hospital’s decision to eliminate its restrictive visiting policy. A young boy whose grandfather was in the ICU was denied visitation. The grandfather, who had raised him, passed away, with the two having had no chance to say goodbye. Roth said that the incident hit home for her and the entire staff, and they knew they could do better. “Our old policies treated family members like visitors, until we realized that we are the visitors in people’s lives, not the other way around,” she noted.
In 2012, the hospital established an interdisciplinary team to transform its existing visiting policy into a “welcoming policy.” The team comprised the director of inpatient nursing, the chief of nursing, the chief of security, a public health program specialist, the facilities manager, and a patient partner. The new policy would support family presence 24/7 as well as change the concept of families as “visitors.”
Over the course of a year, the team designed the framework for a 3-day pilot and developed a check-in process to help loved ones gain access to patients after regular hospital hours. Working closely with nursing leadership, front-line staff, patient partners, and security, the team made necessary adjustments to the policy throughout the pilot period. The pilot was well-received and the policy was implemented soon after.
In the policy’s first year, more than 7000 family members and care partners were able to be with their loved ones between 8 pm and 6 am, the time period formerly restricted. The feedback from staff, patients and loved ones has been overwhelmingly positive. Front-line nurses are currently strengthening their skills and confidence in conducting change of shift report at bedside with patients and families. Other “welcoming” measures have also been implemented, including making signage more user-friendly, providing comfortable chairs to sleep in, and installing new vending machines with healthy snacks and drinks.
Vidant Health
Vidant Health serves 1.4 million people in a 29-county region of eastern North Carolina and comprises over 70 primary and specialty physician office practices, a 900-bed academic medical center, 7 community hospitals, an ambulatory surgery center, and home health and hospice services. Vidant Health’s efforts to advance a culture of patient- and family-centered care began in the late 1990s in the James and Connie Maynard Children’s Hospital and the regional rehabilitation center, but this culture did not spread consistently throughout Vidant Health, leading to differing experiences of care for patients and families.
The Vidant Health executive team and senior leaders heard about these inconsistencies firsthand in the spring of 2007 when an employee shared her family’s experiences during her brother’s ICU stay. Christie Odom described how the visitation policy restricted her family’s access to her brother to 15-minute increments, 6 times a day, which led to heightened fear and anxiety for her brother, family, and friends and impeded patient and family engagement in care and decision-making. Odom’s brother died alone with no family by his side. A physical therapist, Odom observed that in the regional rehabilitation center, families were partners in care, yet in the adult ICU, they were visitors.
After hearing Christie’s story, the system’s executive team, board of directors, and physician leaders made a commitment to eliminate these restrictive visitation guidelines. Leaders understood that this would require the organization’s culture to change from viewing patients and families as passive recipients of care to recognizing them as partners. Subsequently, the commitment to patient-family partnerships was imbedded in key documents including the corporation’s strategic and 5-year quality plans. An Office of Patient-Family Experience was established at Vidant Medical Center in 2008 and, a year later, a corporate office was established to guide system transformation. Emphasis was placed on building a solid team of patient-family advisors and staff champions. An initial focus was to replace the restrictive visitation policy with family presence guidelines. A key tenet of these guidelines is that patients define who their family members are and how they should be included in care and decision-making.
This policy and practice change provided the impetus for ongoing evolution of patient-family partnerships. Patient-family advisors are now integrated across the health care system. They serve on performance improvement teams, make safety rounds, serve as faculty in education programs, interview applicants for key positions, and develop and edit patient education materials. The outcomes achieved by this system transformation are evidenced in exceptional HCAHPS performance, significant reductions in serious safety events and hospital acquired infections and national recognition for commitment to patient-family partnerships.
Conclusion
Changing the concept of families as visitors to families as partners in care, according to patient preference, is foundational to advancing the practice of patient- and family-centered care and to building a safe, high-quality, cost-effective system of care. In 2009, Lucian Leape and colleagues envisioned a transformed health care culture in which “the family is respected as part of the care team—never visitors—in every area of the hospital, including the emergency department and the intensive care unit” [8]. A 2014 report by the National Patient Safety Foundation’s Lucian Leape Institute affirmed, “patients and families can play a critical role in preventing medical errors and reducing harm” [9].
Many hospitals still do not encourage family presence and participation and do not embrace the concept of families as true partners in care. But as demonstrated by the actions of the exemplar hospitals described here, it is possible to make this critical culture shift. The exemplar hospitals understand the importance of partnering with patients’ families instead of treating them as outsiders who are interfering in their loved one’s care. These hospitals are proving that giving patients the access they want to their loved ones actually helps themget better.
Through its campaign Better Together: Partnering with Families, IPFCC challenges hospitals across the United States and Canada to pledge to join this important initiative. Now is the time for all hospitals to embrace family presence and participation and to welcome families and other care partners 24 hours a day, 7 days a week.
Hospitals are invited to join this initiative. Steps to begin the change process may be found at www.ipfcc.org/advance/topics/better-together-pledge.html. Also available at IPFCC website is the Better Together toolkit, other materials and information that support the initiative, and a complete list of the exemplar hospitals and their processes and policies. The toolkit includes an organizational self-assessment, sample processes and policies, videos, and guides for families and staff to use in developing partnership. It is available at no charge at www.ipfcc.org/bettertogether/.
Corresponding author: Beverley H. Johnson, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814, [email protected].
Behind a Locked Door
This is our story as I remember it.
One day I came home from work, and my husband was confused. In all the years we’d been married, I’d never known him to be confused. He was sitting in the family room, and he looked frightened.
I said, “Bill, what’s going on?” He said, “Well, I was just going to get up and go look outside for Joey.” I said, “Bill, Joey’s not here.” He insisted, “Oh yes, we just came back from vacation. Joey just went outside, and I was going to check on him.”
I was alarmed and said, “Bill, I think you’re a little confused. I’m concerned because this morning you told me you had a headache. Maybe we should go to the hospital.” He resisted, but I simply told him, “Bill, if something was to happen to you, I might be held responsible because I didn’t do what was in your best interest. We can come home if everything is okay.”
And so we went to the emergency room, where we learned that my husband had a small bleed in his brain. We were told that we needed to go to another hospital that had a neurosurgeon on staff. My husband was transported by ambulance to a hospital about a mile away, and I followed him there in our car.
I want to stop here and tell you a bit about my husband and about myself. My name is Jackie Gruzenski, and I am a nurse involved in the field of psychiatric nursing. My husband’s name was Dr. William Gruzenski. He was a psychiatrist for forty years, and he was a chief medical officer for the last twelve years of his career.
Bill was a very good doctor and a very good husband. And toward the end of his life, he realized that all of his degrees, along with money and material possessions, didn’t matter. They were nothing. He just wanted to have me with him. We loved each other very deeply, and we wanted to share our last days and moments together, but I’m getting ahead of myself.
When we got to the second hospital, my husband was in the emergency room from about 7:00 p.m. until about 6:00 a.m. the next morning. At some point, he started to develop a hypertensive crisis, and the staff could not bring his blood pressure down. They started an IV medication, which required that he be monitored closely in the intensive care unit (ICU).
Of course, I went with him as he was transferred from emergency to ICU. When we got to the ICU door, I was told, “Now, just go into the waiting room. We’re going to settle your husband, and then we’ll come and get you.”
I was a nervous wreck while I waited. I knew my husband had been pretty sick while in emergency. What if he got more confused? What if he lost even this current level of functioning and wouldn’t
remember me? The longer I waited, the more my anxiety grew.
The waiting room was a small area, with chairs around the perimeter, except by the locked door. After an hour with no news, I saw a phone on the wall and called. I said to the voice on the other end, “My name is Mrs. Gruzenski. I was informed that my husband was going to be settled and that someone would come and get me.”
The next thing I knew a young, perky nurse came out, greeted me, and then directed me totally away from my husband, away from the door to the ICU, to a little room. She proceeded to give me the strict policies and procedures for the ICU, including that visitation was allowed only four times a day for thirty minutes each time.
Not believing what I was hearing, I said, “But my husband is going to be worried that I am not with him. We are the center of each other’s lives—we are only apart when we are at work!” Her response was, “Well, you can’t be with him. Those are the rules.”
I lived ten miles away. What was I supposed to do between these widely spaced thirty-minute visits? I felt I had to play by the rules. I was afraid that if I questioned too much or was abrupt with someone, they would treat my husband meanly. And because he was behind a locked door, I would never know.
I didn’t know what else to do and so, shortly before 8:00 a.m., I went home to get some rest. Ironically, just after I got home and started to settle after our long night in the ER, I got a flurry of calls from different residents who wanted information about my husband. They never said, “Come over and visit. He’s missing you.” They called because they needed the information I could give them, but they kept me locked out.
When I was able to have my first visit the next day, my husband asked where I had been. I explained that there were very limited visiting hours. This prompted my husband to speak to his nurse and say, “You know, she’s not a visitor. She’s my wife!” But he was informed that didn’t matter, that there were rules, and that I was a visitor and had to be treated as a visitor.
The rule trumped both of us and what we wanted. The rule meant he had to suffer alone. This was an accredited hospital, but in my view it was archaic. Staff hid behind the rules rather than using their heads and their hearts.
Over the next few days, I saw that my not being there with my husband was leading to more and more distress for him. As he became more ill, he would not allow the nurses to wash him, and he would not eat their food. He was doing everything he could do to get the staff’s attention to revisit the visiting restrictions. If I’d been allowed to stay, I’m sure I could have helped with feeding, with bathing, and with toileting. I’m certain I could have calmed him and helped lower his blood pressure.
I was treated as though I was an enemy, but all I wanted was to be with him, to share the last days of his life. I had always been his anchor. I was the person who navigated the everyday waters of his life. The hospital’s rules meant that he was adrift, and I was lost.
During his hospitalization, he was not afforded the respect he had given to all his patients and the nurses and doctors he had worked with each day. For example, the ICU staff never asked him how he would like to be addressed. They called him “Bill” when he should have been addressed as “Doctor Gruzenski.” He wouldn’t have thought of calling a resident by his first name, and there were only a few people in his life, his inner circle of family and friends, who called him “Bill.”
One day, I actually witnessed one doctor refer to my husband not even as “Bill” but as “Billy.”
I followed this doctor out of the ICU and challenged him saying “Would you think you were valued as a medical professional, and that your life had meant something if, in forty years’ time, someone called you ‘Billy’? ‘Billy’ is what you call some young boy you like, not someone who is sixty-eight years old and is a dignified gentleman and physician.”
My husband earned the title of “Doctor.” He attended four years of medical school, one year of internship, four years as a resident psychiatrist, and he was board certified in psychiatry. He had earned respect by exceeding all the societal standards for being addressed as “Doctor.” These achievements should not be washed away once you are hospitalized. In fact, I believe my husband might have felt a little safer if he had been addressed as “Doctor.”
My husband was in the ICU for eight of the last sixteen days of his life, and there were lots of missed opportunities for us. He wanted me there more than I was allowed. We missed time together we could have had. I feel it was a very cruel thing that was done to us.
We both knew the gravity of his condition, and my husband wanted quality of life, not quantity. I was a large part of the quality he wanted, but I was locked out for the greater part of his last days.
After my husband died, I felt I had to do something so that what happened to us wouldn’t happen to anyone else. I wrote letters to the chief executive officer of the hospital. I wrote to the chief of the medical staff. I wrote to the chief of nursing. And I wrote to the chaplain. The only person I ever heard from was the chaplain. No one apologized or said they would change the rules.
I believe more harm comes when family are not actively involved, and research is proving my belief is sound. And so I will continue to tell my story. I hope that if I tell it enough times, maybe people who write the rules in hospitals will realize that loved ones are advocates, not visitors.
I will never stop advocating for the elimination of visiting hours.
Reprinted from Crocker L, Johnson B. Privileged presence. Personal stories of connections in health care. 2nd ed. Boulder, CO: Bull Publishing; 2014.
1. American Association of Critical-Care Nurses. Practice alert: Family presence: Visitation in the adult ICU. Accessed at www.aacn.org/WD/practice/docs/practicealerts/family-visitation-adult-icu-practicealert.pdf.
2. New Yorkers for Patient & Family Empowerment and the New York Public Interest Research Group. Sick, scared and separated from loved ones: A report on NYS hospital visiting policies and how patient-centered approaches can promote wellness and safer healthcare. August 2012. Accessed at www.patientandfamily/default.html.
3. Liu V, Read JL, Scruth E, Cheng E. Visitation policies and practices in US ICUs. Crit Care 2013;17:R71.
4. Institute for Patient- and Family-Centered Care. Facts and figures about family presence and participation. Accessed at www.ipfcc.org/advance/topics/Better-Together-Facts-and-Figures.pdf.
5. Better together: partnering with families. Changing the concept of families as visitors bibliography. Accessed at www.ipfcc.org/advance/topics/Changing-the-Concept-of-Families-as-Visitors-Bibliography.pdf.
6. Institute for Patient- and Family-Centered Care. Accessed at www.ipfcc.org/about/index.html.
7. Institute for Patient- and Family-Centered Care. Better together: Partnering with families. Accessed at www.ipfcc.org/advance/topics/better-together.html.
8. Leape L, Berwick D, Clancy C, et al; Lucian Leape Institute at the National Patient Safety Foundation. Transforming healthcare: a safety imperative. Qual Saf Health Care 2009;18:424–8.
9. The National Patient Safety Foundation’s Lucien Leape Institute. Safety is personal: Partnering with patients and families for the safest care. Report of the Roundtable on Consumer Engagement in Patient Safety. Boston: National Patient Safety Foundation; 2014.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken, Ms. Kaufman, and Ms. Johnson), Anne Arundel Medical Center, Annapolis, MD (Dr. Perkins), Contra Costa Regional Medical Center & Health Centers, Martinez, CA (Ms. Benepal, Ms. Roth, and Vidant Health, Greenville, NC (Ms. Dutton and Ms. Jones).
Abstract
- Objective: To describe a campaign to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care.
- Methods: Descriptive report.
- Results: Many hospitals still have “visiting” hours that limit family presence, often counter to patient preferences. To change the concept of families as visitors and eliminate restrictive hospital visiting policies, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families, calling on all hospitals to welcome families 24 hours a day and transform their policies and approaches to care so that patients’ families and loved ones are included in care and decision making, according to patient preferences. As part of the campaign, IPFCC recognized 12 hospitals that exemplify success in eliminating restrictive visiting policies and have changed the concept of families from “visitors” to partners. Leaders at these hospitals attest to the benefits of the changes through improved experience of care and other outcomes. Three exemplar hospitals are highlighted in this article and share their processes of change as well as key learnings and outcomes.
- Conclusion: Hospital policies and practices that encourage and support families as partners in care are essential to patients’ health, well-being, and safety.
Many families are restricted from the bedsides of loved ones because of hospital visiting policies [1–3]. Restrictive policies are often based on long-held beliefs that the presence and participation of families interferes with care, exhausts patients, is a burden to families, spreads infection, or violates HIPAA. However, there is no evidence to support those beliefs. In fact, isolating patients at their most vulnerable time from the people who know them best places them at risk for medical error, emotional harm, inconsistencies in care, and lack of preparedness for transitions in care [4,5]. Jackie Gruzenski’s story “Behind a Locked Door” (printed below) affectingly describes the impact of restrictive policies on a couple's last days.
Fortunately, a growing number of hospitals are lifting these restrictions. But opening the door is not enough. Hospitals need to change the concept of families as “visitors” to families as partners in care. Changing policies is a foundational step in creating a patient- and family-centered culture where families are recognized as essential to patients’ health and well-being and where they are respected as allies for quality and safety.
In response to this critical need for change, in June 2014 the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families. IPFCC, founded in 1992, is a nonprofit organization that provides essential leadership to advance the understanding and practice of patient- and family-centered care [6]. Emphasizing the importance of family presence and participation to quality and safety, the campaign seeks to eliminate restrictive “visiting” policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference [7]. The goal of the campaign is to change visiting policies in 1000 hospitals by 2017. Partnering with IPFCC in this initiative are the American Society for Healthcare Risk Management, American Association of Critical Care Nurses, National Partnership for Women & Families, New Yorkers for Patient and Family Empowerment, Health In Aging Foundation, and the Canadian Foundation for Healthcare Improvement.
Anne Arundel Medical Center
A regional not-for-profit hospital founded in 1902, Anne Arundel Medical Center in Annapolis, MD, provides acute inpatient and outpatient care to residents of 4 counties in Maryland. A 380-bed facility, Anne Arundel has a cancer institute, heart and vascular institute, joint center, spine center, and a women’s and children’s center. In April 2011, the hospital completed a $424 million expansion project, which included a pediatric emergency room, an expanded general emergency room, 50 new patient beds, and 8 new operating rooms.
In 2010, based on a desire to concretely implement the principles of patient- and family-centered care, leaders at Anne Arundel began working with patient and family advisors and initiated a process to change the hospital’s restrictive visiting policy. Now, there are no restrictions on family presence anywhere in the hospital, from ICUs to medical/surgical units to other clinical areas. Patients have the power to choose who they want to stay with them—24 hours a day, 7 days a week. According to Anne Arundel’s policy, each patient determines who is defined as “family.” A “Revisiting Visiting” task force, comprising support staff, providers, and patient and family advisors, worked for 9 months to develop the new family presence policy and support its implementation.
With Anne Arundel leadership encouragement and support, patient and family advisors participated in all phases of the development and implementation of the new family presence policy and in other ways to advance the practice of patient- and family-centered care. The advisors also participated in the process to change the way nurse change of shift report was conducted, and they made recommendations for changes in the directional signs throughout the hospital. New signs, featuring a pineapple (a symbol of hospitality) and the words “Welcome Families” replaced old ones displaying the former restrictive visiting policy.
Supporting patient and family involvement in transitions in care is an integral aspect of implementing family presence policies and practices. Through an “Always Events” grant from the Picker Institute (for information about the Always Events program, see www.ihi.org/Engage/Initiatives/PatientFamilyCenteredCare/Pages/AlwaysEvents.aspx), patient and family advisors, staff, and providers at Anne Arundel developed the SMART discharge protocol, which includes a simple 5-item checklist that is reviewed and discussed with the patient and family prior to discharge. SMART is an acronym for Signs, Medications, Appointments, Results, and Talk. In its work, the SMART team built on current evidence, created urgency and expectation for use with patients, families, and caregivers, disseminated findings, and promoted the protocol as a national standard. The tool is available at www.ihi.org/resources/Pages/Tools/SMARTDischargeProtocol.aspx.
According to Anne Arundel’s COO and CNO, Sherry Perkins, a critical part of the change process was to first understand staff’s fears and then learn what the evidence says. For example, with regard to the impact of additional family presence on infection control, they learned that family presence did not pose additional infection control concerns.
In 2009, there were no patient and family advisors volunteering at Anne Arundel. In 2014, there are approximately 80 advisors. Since 2009, the overall HCAHPS rating of the hospital has gone from 75.4% to 82% (the national average is 70%). While patient satisfaction scores have previously been in the top deciles at Anne Arundel, they have consistently risen since expanding family presence and implementing additional patient- and family-centered strategies.
Contra Costa Regional Medical Center and Health Centers
Contra Costa Health Services in Martinez, CA, includes Contra Costa Regional Medical Center and 10 health centers as part of a comprehensive county health system. Its 164-bed public hospital is dedicated to offering services that are welcoming, accessible, safe and respectful for everyone.
Like many hospitals in the country, for years Contra Costa Regional Medical Center restricted the hours when family members and loved ones could visit patients. However, the hospital’s medical staff often felt uncomfortable that they had to usher family and care partners away from patients when visiting hours were over. Anna Roth, Contra Costa’s CEO, recalls an incident that caused great anguish and contributed to the hospital’s decision to eliminate its restrictive visiting policy. A young boy whose grandfather was in the ICU was denied visitation. The grandfather, who had raised him, passed away, with the two having had no chance to say goodbye. Roth said that the incident hit home for her and the entire staff, and they knew they could do better. “Our old policies treated family members like visitors, until we realized that we are the visitors in people’s lives, not the other way around,” she noted.
In 2012, the hospital established an interdisciplinary team to transform its existing visiting policy into a “welcoming policy.” The team comprised the director of inpatient nursing, the chief of nursing, the chief of security, a public health program specialist, the facilities manager, and a patient partner. The new policy would support family presence 24/7 as well as change the concept of families as “visitors.”
Over the course of a year, the team designed the framework for a 3-day pilot and developed a check-in process to help loved ones gain access to patients after regular hospital hours. Working closely with nursing leadership, front-line staff, patient partners, and security, the team made necessary adjustments to the policy throughout the pilot period. The pilot was well-received and the policy was implemented soon after.
In the policy’s first year, more than 7000 family members and care partners were able to be with their loved ones between 8 pm and 6 am, the time period formerly restricted. The feedback from staff, patients and loved ones has been overwhelmingly positive. Front-line nurses are currently strengthening their skills and confidence in conducting change of shift report at bedside with patients and families. Other “welcoming” measures have also been implemented, including making signage more user-friendly, providing comfortable chairs to sleep in, and installing new vending machines with healthy snacks and drinks.
Vidant Health
Vidant Health serves 1.4 million people in a 29-county region of eastern North Carolina and comprises over 70 primary and specialty physician office practices, a 900-bed academic medical center, 7 community hospitals, an ambulatory surgery center, and home health and hospice services. Vidant Health’s efforts to advance a culture of patient- and family-centered care began in the late 1990s in the James and Connie Maynard Children’s Hospital and the regional rehabilitation center, but this culture did not spread consistently throughout Vidant Health, leading to differing experiences of care for patients and families.
The Vidant Health executive team and senior leaders heard about these inconsistencies firsthand in the spring of 2007 when an employee shared her family’s experiences during her brother’s ICU stay. Christie Odom described how the visitation policy restricted her family’s access to her brother to 15-minute increments, 6 times a day, which led to heightened fear and anxiety for her brother, family, and friends and impeded patient and family engagement in care and decision-making. Odom’s brother died alone with no family by his side. A physical therapist, Odom observed that in the regional rehabilitation center, families were partners in care, yet in the adult ICU, they were visitors.
After hearing Christie’s story, the system’s executive team, board of directors, and physician leaders made a commitment to eliminate these restrictive visitation guidelines. Leaders understood that this would require the organization’s culture to change from viewing patients and families as passive recipients of care to recognizing them as partners. Subsequently, the commitment to patient-family partnerships was imbedded in key documents including the corporation’s strategic and 5-year quality plans. An Office of Patient-Family Experience was established at Vidant Medical Center in 2008 and, a year later, a corporate office was established to guide system transformation. Emphasis was placed on building a solid team of patient-family advisors and staff champions. An initial focus was to replace the restrictive visitation policy with family presence guidelines. A key tenet of these guidelines is that patients define who their family members are and how they should be included in care and decision-making.
This policy and practice change provided the impetus for ongoing evolution of patient-family partnerships. Patient-family advisors are now integrated across the health care system. They serve on performance improvement teams, make safety rounds, serve as faculty in education programs, interview applicants for key positions, and develop and edit patient education materials. The outcomes achieved by this system transformation are evidenced in exceptional HCAHPS performance, significant reductions in serious safety events and hospital acquired infections and national recognition for commitment to patient-family partnerships.
Conclusion
Changing the concept of families as visitors to families as partners in care, according to patient preference, is foundational to advancing the practice of patient- and family-centered care and to building a safe, high-quality, cost-effective system of care. In 2009, Lucian Leape and colleagues envisioned a transformed health care culture in which “the family is respected as part of the care team—never visitors—in every area of the hospital, including the emergency department and the intensive care unit” [8]. A 2014 report by the National Patient Safety Foundation’s Lucian Leape Institute affirmed, “patients and families can play a critical role in preventing medical errors and reducing harm” [9].
Many hospitals still do not encourage family presence and participation and do not embrace the concept of families as true partners in care. But as demonstrated by the actions of the exemplar hospitals described here, it is possible to make this critical culture shift. The exemplar hospitals understand the importance of partnering with patients’ families instead of treating them as outsiders who are interfering in their loved one’s care. These hospitals are proving that giving patients the access they want to their loved ones actually helps themget better.
Through its campaign Better Together: Partnering with Families, IPFCC challenges hospitals across the United States and Canada to pledge to join this important initiative. Now is the time for all hospitals to embrace family presence and participation and to welcome families and other care partners 24 hours a day, 7 days a week.
Hospitals are invited to join this initiative. Steps to begin the change process may be found at www.ipfcc.org/advance/topics/better-together-pledge.html. Also available at IPFCC website is the Better Together toolkit, other materials and information that support the initiative, and a complete list of the exemplar hospitals and their processes and policies. The toolkit includes an organizational self-assessment, sample processes and policies, videos, and guides for families and staff to use in developing partnership. It is available at no charge at www.ipfcc.org/bettertogether/.
Corresponding author: Beverley H. Johnson, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814, [email protected].
Behind a Locked Door
This is our story as I remember it.
One day I came home from work, and my husband was confused. In all the years we’d been married, I’d never known him to be confused. He was sitting in the family room, and he looked frightened.
I said, “Bill, what’s going on?” He said, “Well, I was just going to get up and go look outside for Joey.” I said, “Bill, Joey’s not here.” He insisted, “Oh yes, we just came back from vacation. Joey just went outside, and I was going to check on him.”
I was alarmed and said, “Bill, I think you’re a little confused. I’m concerned because this morning you told me you had a headache. Maybe we should go to the hospital.” He resisted, but I simply told him, “Bill, if something was to happen to you, I might be held responsible because I didn’t do what was in your best interest. We can come home if everything is okay.”
And so we went to the emergency room, where we learned that my husband had a small bleed in his brain. We were told that we needed to go to another hospital that had a neurosurgeon on staff. My husband was transported by ambulance to a hospital about a mile away, and I followed him there in our car.
I want to stop here and tell you a bit about my husband and about myself. My name is Jackie Gruzenski, and I am a nurse involved in the field of psychiatric nursing. My husband’s name was Dr. William Gruzenski. He was a psychiatrist for forty years, and he was a chief medical officer for the last twelve years of his career.
Bill was a very good doctor and a very good husband. And toward the end of his life, he realized that all of his degrees, along with money and material possessions, didn’t matter. They were nothing. He just wanted to have me with him. We loved each other very deeply, and we wanted to share our last days and moments together, but I’m getting ahead of myself.
When we got to the second hospital, my husband was in the emergency room from about 7:00 p.m. until about 6:00 a.m. the next morning. At some point, he started to develop a hypertensive crisis, and the staff could not bring his blood pressure down. They started an IV medication, which required that he be monitored closely in the intensive care unit (ICU).
Of course, I went with him as he was transferred from emergency to ICU. When we got to the ICU door, I was told, “Now, just go into the waiting room. We’re going to settle your husband, and then we’ll come and get you.”
I was a nervous wreck while I waited. I knew my husband had been pretty sick while in emergency. What if he got more confused? What if he lost even this current level of functioning and wouldn’t
remember me? The longer I waited, the more my anxiety grew.
The waiting room was a small area, with chairs around the perimeter, except by the locked door. After an hour with no news, I saw a phone on the wall and called. I said to the voice on the other end, “My name is Mrs. Gruzenski. I was informed that my husband was going to be settled and that someone would come and get me.”
The next thing I knew a young, perky nurse came out, greeted me, and then directed me totally away from my husband, away from the door to the ICU, to a little room. She proceeded to give me the strict policies and procedures for the ICU, including that visitation was allowed only four times a day for thirty minutes each time.
Not believing what I was hearing, I said, “But my husband is going to be worried that I am not with him. We are the center of each other’s lives—we are only apart when we are at work!” Her response was, “Well, you can’t be with him. Those are the rules.”
I lived ten miles away. What was I supposed to do between these widely spaced thirty-minute visits? I felt I had to play by the rules. I was afraid that if I questioned too much or was abrupt with someone, they would treat my husband meanly. And because he was behind a locked door, I would never know.
I didn’t know what else to do and so, shortly before 8:00 a.m., I went home to get some rest. Ironically, just after I got home and started to settle after our long night in the ER, I got a flurry of calls from different residents who wanted information about my husband. They never said, “Come over and visit. He’s missing you.” They called because they needed the information I could give them, but they kept me locked out.
When I was able to have my first visit the next day, my husband asked where I had been. I explained that there were very limited visiting hours. This prompted my husband to speak to his nurse and say, “You know, she’s not a visitor. She’s my wife!” But he was informed that didn’t matter, that there were rules, and that I was a visitor and had to be treated as a visitor.
The rule trumped both of us and what we wanted. The rule meant he had to suffer alone. This was an accredited hospital, but in my view it was archaic. Staff hid behind the rules rather than using their heads and their hearts.
Over the next few days, I saw that my not being there with my husband was leading to more and more distress for him. As he became more ill, he would not allow the nurses to wash him, and he would not eat their food. He was doing everything he could do to get the staff’s attention to revisit the visiting restrictions. If I’d been allowed to stay, I’m sure I could have helped with feeding, with bathing, and with toileting. I’m certain I could have calmed him and helped lower his blood pressure.
I was treated as though I was an enemy, but all I wanted was to be with him, to share the last days of his life. I had always been his anchor. I was the person who navigated the everyday waters of his life. The hospital’s rules meant that he was adrift, and I was lost.
During his hospitalization, he was not afforded the respect he had given to all his patients and the nurses and doctors he had worked with each day. For example, the ICU staff never asked him how he would like to be addressed. They called him “Bill” when he should have been addressed as “Doctor Gruzenski.” He wouldn’t have thought of calling a resident by his first name, and there were only a few people in his life, his inner circle of family and friends, who called him “Bill.”
One day, I actually witnessed one doctor refer to my husband not even as “Bill” but as “Billy.”
I followed this doctor out of the ICU and challenged him saying “Would you think you were valued as a medical professional, and that your life had meant something if, in forty years’ time, someone called you ‘Billy’? ‘Billy’ is what you call some young boy you like, not someone who is sixty-eight years old and is a dignified gentleman and physician.”
My husband earned the title of “Doctor.” He attended four years of medical school, one year of internship, four years as a resident psychiatrist, and he was board certified in psychiatry. He had earned respect by exceeding all the societal standards for being addressed as “Doctor.” These achievements should not be washed away once you are hospitalized. In fact, I believe my husband might have felt a little safer if he had been addressed as “Doctor.”
My husband was in the ICU for eight of the last sixteen days of his life, and there were lots of missed opportunities for us. He wanted me there more than I was allowed. We missed time together we could have had. I feel it was a very cruel thing that was done to us.
We both knew the gravity of his condition, and my husband wanted quality of life, not quantity. I was a large part of the quality he wanted, but I was locked out for the greater part of his last days.
After my husband died, I felt I had to do something so that what happened to us wouldn’t happen to anyone else. I wrote letters to the chief executive officer of the hospital. I wrote to the chief of the medical staff. I wrote to the chief of nursing. And I wrote to the chaplain. The only person I ever heard from was the chaplain. No one apologized or said they would change the rules.
I believe more harm comes when family are not actively involved, and research is proving my belief is sound. And so I will continue to tell my story. I hope that if I tell it enough times, maybe people who write the rules in hospitals will realize that loved ones are advocates, not visitors.
I will never stop advocating for the elimination of visiting hours.
Reprinted from Crocker L, Johnson B. Privileged presence. Personal stories of connections in health care. 2nd ed. Boulder, CO: Bull Publishing; 2014.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken, Ms. Kaufman, and Ms. Johnson), Anne Arundel Medical Center, Annapolis, MD (Dr. Perkins), Contra Costa Regional Medical Center & Health Centers, Martinez, CA (Ms. Benepal, Ms. Roth, and Vidant Health, Greenville, NC (Ms. Dutton and Ms. Jones).
Abstract
- Objective: To describe a campaign to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care.
- Methods: Descriptive report.
- Results: Many hospitals still have “visiting” hours that limit family presence, often counter to patient preferences. To change the concept of families as visitors and eliminate restrictive hospital visiting policies, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families, calling on all hospitals to welcome families 24 hours a day and transform their policies and approaches to care so that patients’ families and loved ones are included in care and decision making, according to patient preferences. As part of the campaign, IPFCC recognized 12 hospitals that exemplify success in eliminating restrictive visiting policies and have changed the concept of families from “visitors” to partners. Leaders at these hospitals attest to the benefits of the changes through improved experience of care and other outcomes. Three exemplar hospitals are highlighted in this article and share their processes of change as well as key learnings and outcomes.
- Conclusion: Hospital policies and practices that encourage and support families as partners in care are essential to patients’ health, well-being, and safety.
Many families are restricted from the bedsides of loved ones because of hospital visiting policies [1–3]. Restrictive policies are often based on long-held beliefs that the presence and participation of families interferes with care, exhausts patients, is a burden to families, spreads infection, or violates HIPAA. However, there is no evidence to support those beliefs. In fact, isolating patients at their most vulnerable time from the people who know them best places them at risk for medical error, emotional harm, inconsistencies in care, and lack of preparedness for transitions in care [4,5]. Jackie Gruzenski’s story “Behind a Locked Door” (printed below) affectingly describes the impact of restrictive policies on a couple's last days.
Fortunately, a growing number of hospitals are lifting these restrictions. But opening the door is not enough. Hospitals need to change the concept of families as “visitors” to families as partners in care. Changing policies is a foundational step in creating a patient- and family-centered culture where families are recognized as essential to patients’ health and well-being and where they are respected as allies for quality and safety.
In response to this critical need for change, in June 2014 the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families. IPFCC, founded in 1992, is a nonprofit organization that provides essential leadership to advance the understanding and practice of patient- and family-centered care [6]. Emphasizing the importance of family presence and participation to quality and safety, the campaign seeks to eliminate restrictive “visiting” policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference [7]. The goal of the campaign is to change visiting policies in 1000 hospitals by 2017. Partnering with IPFCC in this initiative are the American Society for Healthcare Risk Management, American Association of Critical Care Nurses, National Partnership for Women & Families, New Yorkers for Patient and Family Empowerment, Health In Aging Foundation, and the Canadian Foundation for Healthcare Improvement.
Anne Arundel Medical Center
A regional not-for-profit hospital founded in 1902, Anne Arundel Medical Center in Annapolis, MD, provides acute inpatient and outpatient care to residents of 4 counties in Maryland. A 380-bed facility, Anne Arundel has a cancer institute, heart and vascular institute, joint center, spine center, and a women’s and children’s center. In April 2011, the hospital completed a $424 million expansion project, which included a pediatric emergency room, an expanded general emergency room, 50 new patient beds, and 8 new operating rooms.
In 2010, based on a desire to concretely implement the principles of patient- and family-centered care, leaders at Anne Arundel began working with patient and family advisors and initiated a process to change the hospital’s restrictive visiting policy. Now, there are no restrictions on family presence anywhere in the hospital, from ICUs to medical/surgical units to other clinical areas. Patients have the power to choose who they want to stay with them—24 hours a day, 7 days a week. According to Anne Arundel’s policy, each patient determines who is defined as “family.” A “Revisiting Visiting” task force, comprising support staff, providers, and patient and family advisors, worked for 9 months to develop the new family presence policy and support its implementation.
With Anne Arundel leadership encouragement and support, patient and family advisors participated in all phases of the development and implementation of the new family presence policy and in other ways to advance the practice of patient- and family-centered care. The advisors also participated in the process to change the way nurse change of shift report was conducted, and they made recommendations for changes in the directional signs throughout the hospital. New signs, featuring a pineapple (a symbol of hospitality) and the words “Welcome Families” replaced old ones displaying the former restrictive visiting policy.
Supporting patient and family involvement in transitions in care is an integral aspect of implementing family presence policies and practices. Through an “Always Events” grant from the Picker Institute (for information about the Always Events program, see www.ihi.org/Engage/Initiatives/PatientFamilyCenteredCare/Pages/AlwaysEvents.aspx), patient and family advisors, staff, and providers at Anne Arundel developed the SMART discharge protocol, which includes a simple 5-item checklist that is reviewed and discussed with the patient and family prior to discharge. SMART is an acronym for Signs, Medications, Appointments, Results, and Talk. In its work, the SMART team built on current evidence, created urgency and expectation for use with patients, families, and caregivers, disseminated findings, and promoted the protocol as a national standard. The tool is available at www.ihi.org/resources/Pages/Tools/SMARTDischargeProtocol.aspx.
According to Anne Arundel’s COO and CNO, Sherry Perkins, a critical part of the change process was to first understand staff’s fears and then learn what the evidence says. For example, with regard to the impact of additional family presence on infection control, they learned that family presence did not pose additional infection control concerns.
In 2009, there were no patient and family advisors volunteering at Anne Arundel. In 2014, there are approximately 80 advisors. Since 2009, the overall HCAHPS rating of the hospital has gone from 75.4% to 82% (the national average is 70%). While patient satisfaction scores have previously been in the top deciles at Anne Arundel, they have consistently risen since expanding family presence and implementing additional patient- and family-centered strategies.
Contra Costa Regional Medical Center and Health Centers
Contra Costa Health Services in Martinez, CA, includes Contra Costa Regional Medical Center and 10 health centers as part of a comprehensive county health system. Its 164-bed public hospital is dedicated to offering services that are welcoming, accessible, safe and respectful for everyone.
Like many hospitals in the country, for years Contra Costa Regional Medical Center restricted the hours when family members and loved ones could visit patients. However, the hospital’s medical staff often felt uncomfortable that they had to usher family and care partners away from patients when visiting hours were over. Anna Roth, Contra Costa’s CEO, recalls an incident that caused great anguish and contributed to the hospital’s decision to eliminate its restrictive visiting policy. A young boy whose grandfather was in the ICU was denied visitation. The grandfather, who had raised him, passed away, with the two having had no chance to say goodbye. Roth said that the incident hit home for her and the entire staff, and they knew they could do better. “Our old policies treated family members like visitors, until we realized that we are the visitors in people’s lives, not the other way around,” she noted.
In 2012, the hospital established an interdisciplinary team to transform its existing visiting policy into a “welcoming policy.” The team comprised the director of inpatient nursing, the chief of nursing, the chief of security, a public health program specialist, the facilities manager, and a patient partner. The new policy would support family presence 24/7 as well as change the concept of families as “visitors.”
Over the course of a year, the team designed the framework for a 3-day pilot and developed a check-in process to help loved ones gain access to patients after regular hospital hours. Working closely with nursing leadership, front-line staff, patient partners, and security, the team made necessary adjustments to the policy throughout the pilot period. The pilot was well-received and the policy was implemented soon after.
In the policy’s first year, more than 7000 family members and care partners were able to be with their loved ones between 8 pm and 6 am, the time period formerly restricted. The feedback from staff, patients and loved ones has been overwhelmingly positive. Front-line nurses are currently strengthening their skills and confidence in conducting change of shift report at bedside with patients and families. Other “welcoming” measures have also been implemented, including making signage more user-friendly, providing comfortable chairs to sleep in, and installing new vending machines with healthy snacks and drinks.
Vidant Health
Vidant Health serves 1.4 million people in a 29-county region of eastern North Carolina and comprises over 70 primary and specialty physician office practices, a 900-bed academic medical center, 7 community hospitals, an ambulatory surgery center, and home health and hospice services. Vidant Health’s efforts to advance a culture of patient- and family-centered care began in the late 1990s in the James and Connie Maynard Children’s Hospital and the regional rehabilitation center, but this culture did not spread consistently throughout Vidant Health, leading to differing experiences of care for patients and families.
The Vidant Health executive team and senior leaders heard about these inconsistencies firsthand in the spring of 2007 when an employee shared her family’s experiences during her brother’s ICU stay. Christie Odom described how the visitation policy restricted her family’s access to her brother to 15-minute increments, 6 times a day, which led to heightened fear and anxiety for her brother, family, and friends and impeded patient and family engagement in care and decision-making. Odom’s brother died alone with no family by his side. A physical therapist, Odom observed that in the regional rehabilitation center, families were partners in care, yet in the adult ICU, they were visitors.
After hearing Christie’s story, the system’s executive team, board of directors, and physician leaders made a commitment to eliminate these restrictive visitation guidelines. Leaders understood that this would require the organization’s culture to change from viewing patients and families as passive recipients of care to recognizing them as partners. Subsequently, the commitment to patient-family partnerships was imbedded in key documents including the corporation’s strategic and 5-year quality plans. An Office of Patient-Family Experience was established at Vidant Medical Center in 2008 and, a year later, a corporate office was established to guide system transformation. Emphasis was placed on building a solid team of patient-family advisors and staff champions. An initial focus was to replace the restrictive visitation policy with family presence guidelines. A key tenet of these guidelines is that patients define who their family members are and how they should be included in care and decision-making.
This policy and practice change provided the impetus for ongoing evolution of patient-family partnerships. Patient-family advisors are now integrated across the health care system. They serve on performance improvement teams, make safety rounds, serve as faculty in education programs, interview applicants for key positions, and develop and edit patient education materials. The outcomes achieved by this system transformation are evidenced in exceptional HCAHPS performance, significant reductions in serious safety events and hospital acquired infections and national recognition for commitment to patient-family partnerships.
Conclusion
Changing the concept of families as visitors to families as partners in care, according to patient preference, is foundational to advancing the practice of patient- and family-centered care and to building a safe, high-quality, cost-effective system of care. In 2009, Lucian Leape and colleagues envisioned a transformed health care culture in which “the family is respected as part of the care team—never visitors—in every area of the hospital, including the emergency department and the intensive care unit” [8]. A 2014 report by the National Patient Safety Foundation’s Lucian Leape Institute affirmed, “patients and families can play a critical role in preventing medical errors and reducing harm” [9].
Many hospitals still do not encourage family presence and participation and do not embrace the concept of families as true partners in care. But as demonstrated by the actions of the exemplar hospitals described here, it is possible to make this critical culture shift. The exemplar hospitals understand the importance of partnering with patients’ families instead of treating them as outsiders who are interfering in their loved one’s care. These hospitals are proving that giving patients the access they want to their loved ones actually helps themget better.
Through its campaign Better Together: Partnering with Families, IPFCC challenges hospitals across the United States and Canada to pledge to join this important initiative. Now is the time for all hospitals to embrace family presence and participation and to welcome families and other care partners 24 hours a day, 7 days a week.
Hospitals are invited to join this initiative. Steps to begin the change process may be found at www.ipfcc.org/advance/topics/better-together-pledge.html. Also available at IPFCC website is the Better Together toolkit, other materials and information that support the initiative, and a complete list of the exemplar hospitals and their processes and policies. The toolkit includes an organizational self-assessment, sample processes and policies, videos, and guides for families and staff to use in developing partnership. It is available at no charge at www.ipfcc.org/bettertogether/.
Corresponding author: Beverley H. Johnson, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814, [email protected].
Behind a Locked Door
This is our story as I remember it.
One day I came home from work, and my husband was confused. In all the years we’d been married, I’d never known him to be confused. He was sitting in the family room, and he looked frightened.
I said, “Bill, what’s going on?” He said, “Well, I was just going to get up and go look outside for Joey.” I said, “Bill, Joey’s not here.” He insisted, “Oh yes, we just came back from vacation. Joey just went outside, and I was going to check on him.”
I was alarmed and said, “Bill, I think you’re a little confused. I’m concerned because this morning you told me you had a headache. Maybe we should go to the hospital.” He resisted, but I simply told him, “Bill, if something was to happen to you, I might be held responsible because I didn’t do what was in your best interest. We can come home if everything is okay.”
And so we went to the emergency room, where we learned that my husband had a small bleed in his brain. We were told that we needed to go to another hospital that had a neurosurgeon on staff. My husband was transported by ambulance to a hospital about a mile away, and I followed him there in our car.
I want to stop here and tell you a bit about my husband and about myself. My name is Jackie Gruzenski, and I am a nurse involved in the field of psychiatric nursing. My husband’s name was Dr. William Gruzenski. He was a psychiatrist for forty years, and he was a chief medical officer for the last twelve years of his career.
Bill was a very good doctor and a very good husband. And toward the end of his life, he realized that all of his degrees, along with money and material possessions, didn’t matter. They were nothing. He just wanted to have me with him. We loved each other very deeply, and we wanted to share our last days and moments together, but I’m getting ahead of myself.
When we got to the second hospital, my husband was in the emergency room from about 7:00 p.m. until about 6:00 a.m. the next morning. At some point, he started to develop a hypertensive crisis, and the staff could not bring his blood pressure down. They started an IV medication, which required that he be monitored closely in the intensive care unit (ICU).
Of course, I went with him as he was transferred from emergency to ICU. When we got to the ICU door, I was told, “Now, just go into the waiting room. We’re going to settle your husband, and then we’ll come and get you.”
I was a nervous wreck while I waited. I knew my husband had been pretty sick while in emergency. What if he got more confused? What if he lost even this current level of functioning and wouldn’t
remember me? The longer I waited, the more my anxiety grew.
The waiting room was a small area, with chairs around the perimeter, except by the locked door. After an hour with no news, I saw a phone on the wall and called. I said to the voice on the other end, “My name is Mrs. Gruzenski. I was informed that my husband was going to be settled and that someone would come and get me.”
The next thing I knew a young, perky nurse came out, greeted me, and then directed me totally away from my husband, away from the door to the ICU, to a little room. She proceeded to give me the strict policies and procedures for the ICU, including that visitation was allowed only four times a day for thirty minutes each time.
Not believing what I was hearing, I said, “But my husband is going to be worried that I am not with him. We are the center of each other’s lives—we are only apart when we are at work!” Her response was, “Well, you can’t be with him. Those are the rules.”
I lived ten miles away. What was I supposed to do between these widely spaced thirty-minute visits? I felt I had to play by the rules. I was afraid that if I questioned too much or was abrupt with someone, they would treat my husband meanly. And because he was behind a locked door, I would never know.
I didn’t know what else to do and so, shortly before 8:00 a.m., I went home to get some rest. Ironically, just after I got home and started to settle after our long night in the ER, I got a flurry of calls from different residents who wanted information about my husband. They never said, “Come over and visit. He’s missing you.” They called because they needed the information I could give them, but they kept me locked out.
When I was able to have my first visit the next day, my husband asked where I had been. I explained that there were very limited visiting hours. This prompted my husband to speak to his nurse and say, “You know, she’s not a visitor. She’s my wife!” But he was informed that didn’t matter, that there were rules, and that I was a visitor and had to be treated as a visitor.
The rule trumped both of us and what we wanted. The rule meant he had to suffer alone. This was an accredited hospital, but in my view it was archaic. Staff hid behind the rules rather than using their heads and their hearts.
Over the next few days, I saw that my not being there with my husband was leading to more and more distress for him. As he became more ill, he would not allow the nurses to wash him, and he would not eat their food. He was doing everything he could do to get the staff’s attention to revisit the visiting restrictions. If I’d been allowed to stay, I’m sure I could have helped with feeding, with bathing, and with toileting. I’m certain I could have calmed him and helped lower his blood pressure.
I was treated as though I was an enemy, but all I wanted was to be with him, to share the last days of his life. I had always been his anchor. I was the person who navigated the everyday waters of his life. The hospital’s rules meant that he was adrift, and I was lost.
During his hospitalization, he was not afforded the respect he had given to all his patients and the nurses and doctors he had worked with each day. For example, the ICU staff never asked him how he would like to be addressed. They called him “Bill” when he should have been addressed as “Doctor Gruzenski.” He wouldn’t have thought of calling a resident by his first name, and there were only a few people in his life, his inner circle of family and friends, who called him “Bill.”
One day, I actually witnessed one doctor refer to my husband not even as “Bill” but as “Billy.”
I followed this doctor out of the ICU and challenged him saying “Would you think you were valued as a medical professional, and that your life had meant something if, in forty years’ time, someone called you ‘Billy’? ‘Billy’ is what you call some young boy you like, not someone who is sixty-eight years old and is a dignified gentleman and physician.”
My husband earned the title of “Doctor.” He attended four years of medical school, one year of internship, four years as a resident psychiatrist, and he was board certified in psychiatry. He had earned respect by exceeding all the societal standards for being addressed as “Doctor.” These achievements should not be washed away once you are hospitalized. In fact, I believe my husband might have felt a little safer if he had been addressed as “Doctor.”
My husband was in the ICU for eight of the last sixteen days of his life, and there were lots of missed opportunities for us. He wanted me there more than I was allowed. We missed time together we could have had. I feel it was a very cruel thing that was done to us.
We both knew the gravity of his condition, and my husband wanted quality of life, not quantity. I was a large part of the quality he wanted, but I was locked out for the greater part of his last days.
After my husband died, I felt I had to do something so that what happened to us wouldn’t happen to anyone else. I wrote letters to the chief executive officer of the hospital. I wrote to the chief of the medical staff. I wrote to the chief of nursing. And I wrote to the chaplain. The only person I ever heard from was the chaplain. No one apologized or said they would change the rules.
I believe more harm comes when family are not actively involved, and research is proving my belief is sound. And so I will continue to tell my story. I hope that if I tell it enough times, maybe people who write the rules in hospitals will realize that loved ones are advocates, not visitors.
I will never stop advocating for the elimination of visiting hours.
Reprinted from Crocker L, Johnson B. Privileged presence. Personal stories of connections in health care. 2nd ed. Boulder, CO: Bull Publishing; 2014.
1. American Association of Critical-Care Nurses. Practice alert: Family presence: Visitation in the adult ICU. Accessed at www.aacn.org/WD/practice/docs/practicealerts/family-visitation-adult-icu-practicealert.pdf.
2. New Yorkers for Patient & Family Empowerment and the New York Public Interest Research Group. Sick, scared and separated from loved ones: A report on NYS hospital visiting policies and how patient-centered approaches can promote wellness and safer healthcare. August 2012. Accessed at www.patientandfamily/default.html.
3. Liu V, Read JL, Scruth E, Cheng E. Visitation policies and practices in US ICUs. Crit Care 2013;17:R71.
4. Institute for Patient- and Family-Centered Care. Facts and figures about family presence and participation. Accessed at www.ipfcc.org/advance/topics/Better-Together-Facts-and-Figures.pdf.
5. Better together: partnering with families. Changing the concept of families as visitors bibliography. Accessed at www.ipfcc.org/advance/topics/Changing-the-Concept-of-Families-as-Visitors-Bibliography.pdf.
6. Institute for Patient- and Family-Centered Care. Accessed at www.ipfcc.org/about/index.html.
7. Institute for Patient- and Family-Centered Care. Better together: Partnering with families. Accessed at www.ipfcc.org/advance/topics/better-together.html.
8. Leape L, Berwick D, Clancy C, et al; Lucian Leape Institute at the National Patient Safety Foundation. Transforming healthcare: a safety imperative. Qual Saf Health Care 2009;18:424–8.
9. The National Patient Safety Foundation’s Lucien Leape Institute. Safety is personal: Partnering with patients and families for the safest care. Report of the Roundtable on Consumer Engagement in Patient Safety. Boston: National Patient Safety Foundation; 2014.
1. American Association of Critical-Care Nurses. Practice alert: Family presence: Visitation in the adult ICU. Accessed at www.aacn.org/WD/practice/docs/practicealerts/family-visitation-adult-icu-practicealert.pdf.
2. New Yorkers for Patient & Family Empowerment and the New York Public Interest Research Group. Sick, scared and separated from loved ones: A report on NYS hospital visiting policies and how patient-centered approaches can promote wellness and safer healthcare. August 2012. Accessed at www.patientandfamily/default.html.
3. Liu V, Read JL, Scruth E, Cheng E. Visitation policies and practices in US ICUs. Crit Care 2013;17:R71.
4. Institute for Patient- and Family-Centered Care. Facts and figures about family presence and participation. Accessed at www.ipfcc.org/advance/topics/Better-Together-Facts-and-Figures.pdf.
5. Better together: partnering with families. Changing the concept of families as visitors bibliography. Accessed at www.ipfcc.org/advance/topics/Changing-the-Concept-of-Families-as-Visitors-Bibliography.pdf.
6. Institute for Patient- and Family-Centered Care. Accessed at www.ipfcc.org/about/index.html.
7. Institute for Patient- and Family-Centered Care. Better together: Partnering with families. Accessed at www.ipfcc.org/advance/topics/better-together.html.
8. Leape L, Berwick D, Clancy C, et al; Lucian Leape Institute at the National Patient Safety Foundation. Transforming healthcare: a safety imperative. Qual Saf Health Care 2009;18:424–8.
9. The National Patient Safety Foundation’s Lucien Leape Institute. Safety is personal: Partnering with patients and families for the safest care. Report of the Roundtable on Consumer Engagement in Patient Safety. Boston: National Patient Safety Foundation; 2014.