User login
HL survivors have long-term risk of cardiovascular disease

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well. ![]()

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well. ![]()

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well. ![]()
MM drug met accelerated approval requirements

Photo courtesy of the CDC
Celgene Corporation has fulfilled the requirements for accelerated approval of pomalidomide (Pomalyst) in the US, based on results from the phase 3 MM-003 trial.
The trial showed that pomalidomide in combination with dexamethasone can improve survival in patients with relapsed or refractory multiple
myeloma (MM).
A drug can be granted accelerated approval in the US based on a surrogate endpoint thought to predict clinical benefit.
To retain approval from the US Food and Drug Administration (FDA), the drug must demonstrate an actual clinical benefit.
In 2013, the FDA granted pomalidomide accelerated approval for use in combination with dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor, and have demonstrated disease progression on or within 60 days of completing their last therapy.
The FDA’s approval was based on results from a phase 2 trial known as MM-002. The trial showed that pomalidomide plus dexamethasone can improve the overall response rate in relapsed/refractory MM patients when compared to pomalidomide alone.
About 29% of patients in the pomalidomide-dexamethasone arm achieved a partial response or better, compared to about 7% of patients in the pomalidomide-alone arm.
Now, results of the MM-003 trial have shown that pomalidomide plus low-dose dexamethasone can improve progression-free survival and overall survival in relapsed/refractory MM patients, when compared to high-dose dexamethasone alone.
The median progression-free survival was 3.6 months in the pomalidomide-dexamethasone arm and 1.8 months in the dexamethasone arm (P<0.001). And the median overall survival was 12.4 months and 8 months, respectively (P=0.009).
These outcomes suggest pomalidomide, in combination with dexamethasone, provides a clinical benefit for previously treated MM patients, which fulfills the requirements for accelerated approval. So the drug’s label has been updated to reflect his change.
For more details on pomalidomide, see the full prescribing information. ![]()

Photo courtesy of the CDC
Celgene Corporation has fulfilled the requirements for accelerated approval of pomalidomide (Pomalyst) in the US, based on results from the phase 3 MM-003 trial.
The trial showed that pomalidomide in combination with dexamethasone can improve survival in patients with relapsed or refractory multiple
myeloma (MM).
A drug can be granted accelerated approval in the US based on a surrogate endpoint thought to predict clinical benefit.
To retain approval from the US Food and Drug Administration (FDA), the drug must demonstrate an actual clinical benefit.
In 2013, the FDA granted pomalidomide accelerated approval for use in combination with dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor, and have demonstrated disease progression on or within 60 days of completing their last therapy.
The FDA’s approval was based on results from a phase 2 trial known as MM-002. The trial showed that pomalidomide plus dexamethasone can improve the overall response rate in relapsed/refractory MM patients when compared to pomalidomide alone.
About 29% of patients in the pomalidomide-dexamethasone arm achieved a partial response or better, compared to about 7% of patients in the pomalidomide-alone arm.
Now, results of the MM-003 trial have shown that pomalidomide plus low-dose dexamethasone can improve progression-free survival and overall survival in relapsed/refractory MM patients, when compared to high-dose dexamethasone alone.
The median progression-free survival was 3.6 months in the pomalidomide-dexamethasone arm and 1.8 months in the dexamethasone arm (P<0.001). And the median overall survival was 12.4 months and 8 months, respectively (P=0.009).
These outcomes suggest pomalidomide, in combination with dexamethasone, provides a clinical benefit for previously treated MM patients, which fulfills the requirements for accelerated approval. So the drug’s label has been updated to reflect his change.
For more details on pomalidomide, see the full prescribing information. ![]()

Photo courtesy of the CDC
Celgene Corporation has fulfilled the requirements for accelerated approval of pomalidomide (Pomalyst) in the US, based on results from the phase 3 MM-003 trial.
The trial showed that pomalidomide in combination with dexamethasone can improve survival in patients with relapsed or refractory multiple
myeloma (MM).
A drug can be granted accelerated approval in the US based on a surrogate endpoint thought to predict clinical benefit.
To retain approval from the US Food and Drug Administration (FDA), the drug must demonstrate an actual clinical benefit.
In 2013, the FDA granted pomalidomide accelerated approval for use in combination with dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor, and have demonstrated disease progression on or within 60 days of completing their last therapy.
The FDA’s approval was based on results from a phase 2 trial known as MM-002. The trial showed that pomalidomide plus dexamethasone can improve the overall response rate in relapsed/refractory MM patients when compared to pomalidomide alone.
About 29% of patients in the pomalidomide-dexamethasone arm achieved a partial response or better, compared to about 7% of patients in the pomalidomide-alone arm.
Now, results of the MM-003 trial have shown that pomalidomide plus low-dose dexamethasone can improve progression-free survival and overall survival in relapsed/refractory MM patients, when compared to high-dose dexamethasone alone.
The median progression-free survival was 3.6 months in the pomalidomide-dexamethasone arm and 1.8 months in the dexamethasone arm (P<0.001). And the median overall survival was 12.4 months and 8 months, respectively (P=0.009).
These outcomes suggest pomalidomide, in combination with dexamethasone, provides a clinical benefit for previously treated MM patients, which fulfills the requirements for accelerated approval. So the drug’s label has been updated to reflect his change.
For more details on pomalidomide, see the full prescribing information. ![]()
TCS Among Children with Pneumonia
National guidelines for the management of childhood pneumonia highlight the need for the development of objective outcome measures to inform clinical decision making, establish benchmarks of care, and compare treatments and interventions.[1] Time to clinical stability (TCS) is a measure reported in adult pneumonia studies that incorporates vital signs, ability to eat, and mental status to objectively assess readiness for discharge.[2, 3, 4] TCS has not been validated among children as it has in adults,[5, 6, 7, 8] although such measures could prove useful for assessing discharge readiness with applications in both clinical and research settings. The objective of our study was to test the performance of pediatric TCS measures among children hospitalized with pneumonia.
METHODS
Study Population
We studied children hospitalized with community‐acquired pneumonia at Monroe Carell Jr. Children's Hospital at Vanderbilt between January 6, 2010 and May 9, 2011. Study children were enrolled as part of the Centers for Disease Control & Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a prospective, population‐based study of community‐acquired pneumonia hospitalizations. Detailed enrollment criteria for the EPIC study were reported previously.[9] Institutional review boards at Vanderbilt University and the CDC approved this study. Informed consent was obtained from enrolled families.
Data Elements and Study Definitions
Baseline data, including demographics, illness history, comorbidities, and clinical outcomes (eg, length of stay [LOS], intensive care admission), were systematically and prospectively collected. Additionally, data for 4 physiologic parameters, including temperature, heart rate, respiratory rate, and use of supplemental oxygen were obtained from the electronic medical record. These parameters were measured at least every 6 hours from admission through discharge as part of routine care. Readmissions within 7 calendar days of discharge were also obtained from the electronic medical record.
Stability for each parameter was defined as follows: normal temperature (36.037.9C), normal respiratory and heart rates in accordance with Pediatric Advanced Life Support age‐based values (see Supporting Table 1 in the online version of this article),[10] and no administration of supplemental oxygen. If the last recorded value for a given parameter was abnormal, that parameter was considered unstable at discharge. Otherwise, the time and date of the last abnormal value for each parameter was subtracted from admission time and date to determine TCS for that parameter in hours.
To determine overall stability, we evaluated 4 combination TCS measures, each incorporating 2 individual parameters. All combinations included respiratory rate and need for supplemental oxygen, as these parameters are the most explicit clinical indicators of pneumonia. Stability for each combination measure was defined as normalization of all included measures.
Clinical Outcomes for the Combined TCS Measures
The 4 combined TCS measures were compared against clinical outcomes including hospital LOS (measured in hours) and an ordinal severity scale. The ordinal scale categorized children into 3 mutually exclusive groups as follows: nonsevere (hospitalization without need for intensive care or empyema requiring drainage), severe (intensive care admission without invasive mechanical ventilation or vasopressor support and no empyema requiring drainage), and very severe (invasive mechanical ventilation, vasopressor support, or empyema requiring drainage).
Statistical Analysis
Categorical and continuous variables were summarized using frequencies and percentages and median and interquartile range (IQR) values, respectively. Analyses were stratified by age (2 years, 24 years, 517 years). We also plotted summary statistics for the combined measures and LOS, and computed the median absolute difference between these measures for each level of the ordinal severity scale. Analyses were conducted using Stata 13 (StataCorp, College Station, TX).
RESULTS
Study Population
Among 336 children enrolled in the EPIC study at Vanderbilt during the study period, 334 (99.4%) with complete data were included. Median age was 33 months (IQR, 1480). Median LOS was 56.4 hours (IQR, 41.591.7). There were 249 (74.5%) children classified as nonsevere, 39 (11.7) as severe, and 46 (13.8) as very severe (for age‐based characteristics see Supporting Table 2 in the online version of this article). Overall, 12 (3.6%) children were readmitted within 7 days of discharge.
Individual Stability Parameters
Overall, 323 (96.7%) children had 1 parameter abnormal on admission. Respiratory rate (81.4%) was the most common abnormal parameter, followed by abnormal temperature (71.4%), use of supplemental oxygen (63.8%), and abnormal heart rate (54.4%). Overall, use of supplemental oxygen had the longest TCS, followed by respiratory rate (Table 1). In comparison, heart rate and temperature stabilized relatively quickly.
| Parameter | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | |||
|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | |
| ||||||
| Respiratory rate | 97 (74.6) | 38.6 (18.768.9) | 63 (70.0) | 31.6 (9.561.9) | 63 (62.4) | 24.3 (10.859.2) |
| Oxygen | 90 (69.2) | 39.5 (19.273.6) | 58 (64.4) | 44.2 (2477.6) | 61 (60.4) | 38.3 (1870.6) |
| Heart rate | 21 (16.2) | 4.5 (0.318.4) | 73 (81.1) | 21.8 (5.751.9) | 62 (61.4) | 18 (5.842.2) |
| Temperature | 101 (77.7) | 14.5 (4.545.3) | 61 (67.8) | 18.4 (2.842.8) | 62 (61.4) | 10.6 (0.834) |
Seventy children (21.0%) had 1 parameter abnormal at discharge, including abnormal respiratory rate in 13.7%, heart rate in 7.0%, and temperature in 3.3%. One child (0.3%) was discharged with supplemental oxygen. Ten children (3.0%) had 2 parameters abnormal at discharge. There was no difference in 7‐day readmissions for children with 1 parameter abnormal at discharge (1.4%) compared to those with no abnormal parameters at discharge (4.4%, P=0.253).
Combination TCS Measures
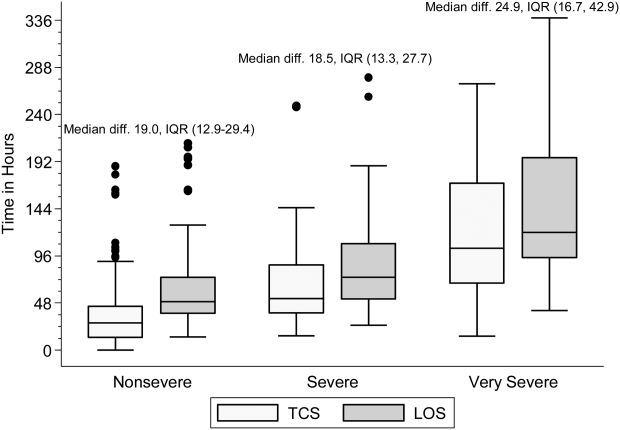
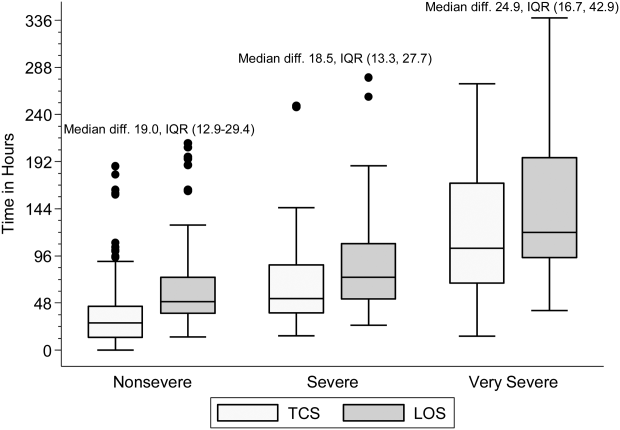
Within each age group, the percentage of children achieving stability was relatively consistent across the 4 combined TCS measures (Table 2); however, more children were considered unstable at discharge (and fewer classified as stable on admission) as the number of included parameters increased. More children 5 years of age reached stability (range, 80.0%85.6%) compared to children 5 years of age (range, 68.3%72.3%). We also noted increasing median TCS with increasing disease severity (Figure 1, P0.01) (see Supporting Fig. 1AC in the online version of this article); TCS was only slightly shorter than LOS across all 3 levels of the severity scale.
| TCS Measures | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | P Value | |||
|---|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | ||
| |||||||
| RR+O2 | 108 (83.1) | 40.5 (20.175.0) | 72 (80.0) | 39.6 (15.679.2) | 69 (68.3) | 30.4 (14.759.2) | 0.08 |
| RR+O2+HR | 109 (83.8) | 40.2 (19.573.9) | 73 (81.1) | 35.9 (15.977.6) | 68 (67.3) | 29.8 (17.256.6) | 0.11 |
| RR+O2+T | 110 (84.6) | 40.5 (20.770.1) | 77 (85.6) | 39.1 (18.477.6) | 73 (72.3) | 28.2 (14.744.7) | 0.03 |
| RR+O2+HR+T | 110 (84.6) | 40.5 (20.770.1) | 72 (80.0) | 39.7 (20.177.5) | 71 (70.3) | 29.2 (18.254) | 0.05 |
DISCUSSION
Our study demonstrates that longitudinal TCS measures consisting of routinely collected physiologic parameters may be useful for objectively assessing disease recovery and clinical readiness for discharge among children hospitalized with pneumonia. A simple TCS measure incorporating respiratory rate and oxygen requirement performed similarly to the more complex combinations and classified fewer children as unstable at discharge. However, we also note several challenges that deserve additional study prior to the application of a pediatric TCS measure in clinical and research settings.
Vital signs and supplemental oxygen use are used clinically to assess disease severity and response to therapy among children with acute respiratory illness. Because these objective parameters are routinely collected among hospitalized children, the systematization of these data could inform clinical decision making around hospital discharge. Similar to early warning scores used to detect impending clinical deterioration,[11] TCS measures, by signaling normalization of stability parameters in a consistent and objective manner, could serve as an early signal of readiness for discharge. However, maximizing the clinical utility of TCS would require embedding the process within the electronic health record, a tool that could also have implications for the Centers for Medicare and Medicaid Services' meaningful use regulations.[12]
TCS could also serve as an outcome measure in research and quality efforts. Increased disease severity was associated with longer TCS for the 4 combined measures; TCS also demonstrated strong agreement with LOS. Furthermore, TCS minimizes the influence of factors unrelated to disease that may impact LOS (eg, frequency of hospital rounds, transportation difficulties, or social impediments to discharge), an advantage when studying outcomes for research and quality benchmarking.
The percentage of children reaching stability and the median TCS for the combined measures demonstrated little variation within each age group, likely because respiratory rate and need for supplemental oxygen, 2 of the parameters with the longest individual time to stability, were also included in each of the combination measures. This suggests that less‐complex measures incorporating only respiratory rate and need for supplemental oxygen may be sufficient to assess clinical stability, particularly because these parameters are objectively measured and possess a direct physiological link to pneumonia. In contrast, the other parameters may be more often influenced by factors unrelated to disease severity.
Our study also highlights several shortcomings of the pediatric TCS measures. Despite use of published, age‐based reference values,[13] we noted wide variation in the achievement of stability across individual parameters, especially for children 5 years old. Overall, 21% of children had 1 abnormal parameter at discharge. Even the simplest combined measure classified 13.4% of children as unstable at discharge. Discharge with unstable parameters was not associated with 7‐day readmission, although our study was underpowered to detect small differences. Additional study is therefore needed to evaluate less restrictive cutoff values on calculated TCS and the impact of hospital discharge prior to reaching stability. In particular, relaxing the upper limit for normal respiratory rate in adolescents (16 breaths per minute) to more closely approximate the adult TCS parameter (24 breaths per minute) should be explored. Refinement and standardization of age‐based vital sign reference values specific to hospitalized children may also improve the performance of these measures.[14]
Several limitations deserve discussion. TCS parameters and readmission data were abstracted retrospectively from a single institution, and our findings may not be generalizable. Although clinical staff routinely measured these data, measurement variation likely exists. Nevertheless, such variation is likely systematic, limiting the impact of potential misclassification. TCS was calculated based on the last abnormal value for each parameter; prior fluctuations between normal and abnormal periods of stability were not captured. We were unable to assess room air oxygen saturations. Instead, supplemental oxygen use served as a surrogate for hypoxia. At our institution, oxygen therapy is provided for children with pneumonia to maintain oxygen saturations of 90% to 92%. We did not assess work of breathing (a marker of severe pneumonia) or ability to eat (a component of adult TCS measures). We initially considered the evaluation of intravenous fluids as a proxy for ability to eat (addition of this parameter to the 4 parameter TCS resulted in a modest increase in median time to stability, data not shown); however, we felt the lack of institutional policy and subjective nature of this parameter detracted from our study's objectives. Finally, we were not able to determine clinical readiness for discharge beyond the measurement of vital sign parameters. Therefore, prospective evaluation of the proposed pediatric TCS measures in broader populations will be important to build upon our findings, refine stability parameters, and test the utility of new parameters (eg, ability to eat, work of breathing) prior to use in clinical settings.
Our study provides an initial evaluation of TCS measures for assessing severity and recovery among children hospitalized with pneumonia. Similar to adults, such validated TCS measures may ultimately prove useful for improving the quality of both clinical care and research, although additional study to more clearly define stability criteria is needed prior to implementation.
Disclosures
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to Dr. Williams. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institutes of Health. Dr. Grijalva serves as a consultant to Glaxo‐Smith‐Kline and Pfizer outside of the scope of this article. Dr. Edwards is supported through grants from Novartis for the conduction of a Group B strep vaccine study and serves as the Chair of the Data Safety and Monitoring Data Committee for Influenza Study outside the scope of this article. Dr. Self reports grants from CareFusion, BioMerieux, Affinium Pharmaceuticals, Astute Medical, Crucell Holland BV, BRAHMS GmbH, Pfizer, Rapid Pathogen Screening, Venaxis, BioAegis Inc., Sphingotec GmbH, and Cempra Pharmaceuticals; personal fees from BioFire Diagnostics and Venaxis, Inc; and patent 13/632,874 (Sterile Blood Culture Collection System) pending; all outside the scope of this article.
- Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/research/data/hcup/index.html. Accessed February 1, 2014.
- , , , et al. Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457.
- , , , et al.; Neumofail Group. Reaching stability in community‐acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783–1790.
- , , , et al.; Community‐Acquired Pneumonia Organization. The pneumonia severity index predicts time to clinical stability in patients with community‐acquired pneumonia. Int J Tuberc Lung Dis. 2006;10:739–743.
- , , , , . Efficacy of corticosteroids in community‐acquired pneumonia: a randomized double‐blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982.
- , , , et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia. Chest 2003;124:1798–1804.
- , , , . A comparison between time to clinical stability in community‐acquired aspiration pneumonia and community‐acquired pneumonia. Intern Emerg Med. 2014;9:143–150.
- , , , et al.; Community‐Acquired Pneumonia Organization (CAPO) Investigators. A worldwide perspective of atypical pathogens in community‐acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093.
- , , , et al.; CDC EPIC Study Team. Community‐acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845.
- American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117:e989–e1004.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13:R135.
- Centers for Medicare and Medicaid Services. Regulations and guidance. EHR incentive programs. Available at: http://www.cms.gov/Regulations‐and‐Guidance/Legislation/EHRIncentivePrograms/index.html. Accessed February 20, 2015
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- , , , et al. Length of stay after reaching clinical stability drives hospital costs associated with adult community‐acquired pneumonia. Scand J Infect Dis. 2013;45:219–226.
National guidelines for the management of childhood pneumonia highlight the need for the development of objective outcome measures to inform clinical decision making, establish benchmarks of care, and compare treatments and interventions.[1] Time to clinical stability (TCS) is a measure reported in adult pneumonia studies that incorporates vital signs, ability to eat, and mental status to objectively assess readiness for discharge.[2, 3, 4] TCS has not been validated among children as it has in adults,[5, 6, 7, 8] although such measures could prove useful for assessing discharge readiness with applications in both clinical and research settings. The objective of our study was to test the performance of pediatric TCS measures among children hospitalized with pneumonia.
METHODS
Study Population
We studied children hospitalized with community‐acquired pneumonia at Monroe Carell Jr. Children's Hospital at Vanderbilt between January 6, 2010 and May 9, 2011. Study children were enrolled as part of the Centers for Disease Control & Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a prospective, population‐based study of community‐acquired pneumonia hospitalizations. Detailed enrollment criteria for the EPIC study were reported previously.[9] Institutional review boards at Vanderbilt University and the CDC approved this study. Informed consent was obtained from enrolled families.
Data Elements and Study Definitions
Baseline data, including demographics, illness history, comorbidities, and clinical outcomes (eg, length of stay [LOS], intensive care admission), were systematically and prospectively collected. Additionally, data for 4 physiologic parameters, including temperature, heart rate, respiratory rate, and use of supplemental oxygen were obtained from the electronic medical record. These parameters were measured at least every 6 hours from admission through discharge as part of routine care. Readmissions within 7 calendar days of discharge were also obtained from the electronic medical record.
Stability for each parameter was defined as follows: normal temperature (36.037.9C), normal respiratory and heart rates in accordance with Pediatric Advanced Life Support age‐based values (see Supporting Table 1 in the online version of this article),[10] and no administration of supplemental oxygen. If the last recorded value for a given parameter was abnormal, that parameter was considered unstable at discharge. Otherwise, the time and date of the last abnormal value for each parameter was subtracted from admission time and date to determine TCS for that parameter in hours.
To determine overall stability, we evaluated 4 combination TCS measures, each incorporating 2 individual parameters. All combinations included respiratory rate and need for supplemental oxygen, as these parameters are the most explicit clinical indicators of pneumonia. Stability for each combination measure was defined as normalization of all included measures.
Clinical Outcomes for the Combined TCS Measures
The 4 combined TCS measures were compared against clinical outcomes including hospital LOS (measured in hours) and an ordinal severity scale. The ordinal scale categorized children into 3 mutually exclusive groups as follows: nonsevere (hospitalization without need for intensive care or empyema requiring drainage), severe (intensive care admission without invasive mechanical ventilation or vasopressor support and no empyema requiring drainage), and very severe (invasive mechanical ventilation, vasopressor support, or empyema requiring drainage).
Statistical Analysis
Categorical and continuous variables were summarized using frequencies and percentages and median and interquartile range (IQR) values, respectively. Analyses were stratified by age (2 years, 24 years, 517 years). We also plotted summary statistics for the combined measures and LOS, and computed the median absolute difference between these measures for each level of the ordinal severity scale. Analyses were conducted using Stata 13 (StataCorp, College Station, TX).
RESULTS
Study Population
Among 336 children enrolled in the EPIC study at Vanderbilt during the study period, 334 (99.4%) with complete data were included. Median age was 33 months (IQR, 1480). Median LOS was 56.4 hours (IQR, 41.591.7). There were 249 (74.5%) children classified as nonsevere, 39 (11.7) as severe, and 46 (13.8) as very severe (for age‐based characteristics see Supporting Table 2 in the online version of this article). Overall, 12 (3.6%) children were readmitted within 7 days of discharge.
Individual Stability Parameters
Overall, 323 (96.7%) children had 1 parameter abnormal on admission. Respiratory rate (81.4%) was the most common abnormal parameter, followed by abnormal temperature (71.4%), use of supplemental oxygen (63.8%), and abnormal heart rate (54.4%). Overall, use of supplemental oxygen had the longest TCS, followed by respiratory rate (Table 1). In comparison, heart rate and temperature stabilized relatively quickly.
| Parameter | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | |||
|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | |
| ||||||
| Respiratory rate | 97 (74.6) | 38.6 (18.768.9) | 63 (70.0) | 31.6 (9.561.9) | 63 (62.4) | 24.3 (10.859.2) |
| Oxygen | 90 (69.2) | 39.5 (19.273.6) | 58 (64.4) | 44.2 (2477.6) | 61 (60.4) | 38.3 (1870.6) |
| Heart rate | 21 (16.2) | 4.5 (0.318.4) | 73 (81.1) | 21.8 (5.751.9) | 62 (61.4) | 18 (5.842.2) |
| Temperature | 101 (77.7) | 14.5 (4.545.3) | 61 (67.8) | 18.4 (2.842.8) | 62 (61.4) | 10.6 (0.834) |
Seventy children (21.0%) had 1 parameter abnormal at discharge, including abnormal respiratory rate in 13.7%, heart rate in 7.0%, and temperature in 3.3%. One child (0.3%) was discharged with supplemental oxygen. Ten children (3.0%) had 2 parameters abnormal at discharge. There was no difference in 7‐day readmissions for children with 1 parameter abnormal at discharge (1.4%) compared to those with no abnormal parameters at discharge (4.4%, P=0.253).
Combination TCS Measures
Within each age group, the percentage of children achieving stability was relatively consistent across the 4 combined TCS measures (Table 2); however, more children were considered unstable at discharge (and fewer classified as stable on admission) as the number of included parameters increased. More children 5 years of age reached stability (range, 80.0%85.6%) compared to children 5 years of age (range, 68.3%72.3%). We also noted increasing median TCS with increasing disease severity (Figure 1, P0.01) (see Supporting Fig. 1AC in the online version of this article); TCS was only slightly shorter than LOS across all 3 levels of the severity scale.
| TCS Measures | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | P Value | |||
|---|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | ||
| |||||||
| RR+O2 | 108 (83.1) | 40.5 (20.175.0) | 72 (80.0) | 39.6 (15.679.2) | 69 (68.3) | 30.4 (14.759.2) | 0.08 |
| RR+O2+HR | 109 (83.8) | 40.2 (19.573.9) | 73 (81.1) | 35.9 (15.977.6) | 68 (67.3) | 29.8 (17.256.6) | 0.11 |
| RR+O2+T | 110 (84.6) | 40.5 (20.770.1) | 77 (85.6) | 39.1 (18.477.6) | 73 (72.3) | 28.2 (14.744.7) | 0.03 |
| RR+O2+HR+T | 110 (84.6) | 40.5 (20.770.1) | 72 (80.0) | 39.7 (20.177.5) | 71 (70.3) | 29.2 (18.254) | 0.05 |

DISCUSSION
Our study demonstrates that longitudinal TCS measures consisting of routinely collected physiologic parameters may be useful for objectively assessing disease recovery and clinical readiness for discharge among children hospitalized with pneumonia. A simple TCS measure incorporating respiratory rate and oxygen requirement performed similarly to the more complex combinations and classified fewer children as unstable at discharge. However, we also note several challenges that deserve additional study prior to the application of a pediatric TCS measure in clinical and research settings.
Vital signs and supplemental oxygen use are used clinically to assess disease severity and response to therapy among children with acute respiratory illness. Because these objective parameters are routinely collected among hospitalized children, the systematization of these data could inform clinical decision making around hospital discharge. Similar to early warning scores used to detect impending clinical deterioration,[11] TCS measures, by signaling normalization of stability parameters in a consistent and objective manner, could serve as an early signal of readiness for discharge. However, maximizing the clinical utility of TCS would require embedding the process within the electronic health record, a tool that could also have implications for the Centers for Medicare and Medicaid Services' meaningful use regulations.[12]
TCS could also serve as an outcome measure in research and quality efforts. Increased disease severity was associated with longer TCS for the 4 combined measures; TCS also demonstrated strong agreement with LOS. Furthermore, TCS minimizes the influence of factors unrelated to disease that may impact LOS (eg, frequency of hospital rounds, transportation difficulties, or social impediments to discharge), an advantage when studying outcomes for research and quality benchmarking.
The percentage of children reaching stability and the median TCS for the combined measures demonstrated little variation within each age group, likely because respiratory rate and need for supplemental oxygen, 2 of the parameters with the longest individual time to stability, were also included in each of the combination measures. This suggests that less‐complex measures incorporating only respiratory rate and need for supplemental oxygen may be sufficient to assess clinical stability, particularly because these parameters are objectively measured and possess a direct physiological link to pneumonia. In contrast, the other parameters may be more often influenced by factors unrelated to disease severity.
Our study also highlights several shortcomings of the pediatric TCS measures. Despite use of published, age‐based reference values,[13] we noted wide variation in the achievement of stability across individual parameters, especially for children 5 years old. Overall, 21% of children had 1 abnormal parameter at discharge. Even the simplest combined measure classified 13.4% of children as unstable at discharge. Discharge with unstable parameters was not associated with 7‐day readmission, although our study was underpowered to detect small differences. Additional study is therefore needed to evaluate less restrictive cutoff values on calculated TCS and the impact of hospital discharge prior to reaching stability. In particular, relaxing the upper limit for normal respiratory rate in adolescents (16 breaths per minute) to more closely approximate the adult TCS parameter (24 breaths per minute) should be explored. Refinement and standardization of age‐based vital sign reference values specific to hospitalized children may also improve the performance of these measures.[14]
Several limitations deserve discussion. TCS parameters and readmission data were abstracted retrospectively from a single institution, and our findings may not be generalizable. Although clinical staff routinely measured these data, measurement variation likely exists. Nevertheless, such variation is likely systematic, limiting the impact of potential misclassification. TCS was calculated based on the last abnormal value for each parameter; prior fluctuations between normal and abnormal periods of stability were not captured. We were unable to assess room air oxygen saturations. Instead, supplemental oxygen use served as a surrogate for hypoxia. At our institution, oxygen therapy is provided for children with pneumonia to maintain oxygen saturations of 90% to 92%. We did not assess work of breathing (a marker of severe pneumonia) or ability to eat (a component of adult TCS measures). We initially considered the evaluation of intravenous fluids as a proxy for ability to eat (addition of this parameter to the 4 parameter TCS resulted in a modest increase in median time to stability, data not shown); however, we felt the lack of institutional policy and subjective nature of this parameter detracted from our study's objectives. Finally, we were not able to determine clinical readiness for discharge beyond the measurement of vital sign parameters. Therefore, prospective evaluation of the proposed pediatric TCS measures in broader populations will be important to build upon our findings, refine stability parameters, and test the utility of new parameters (eg, ability to eat, work of breathing) prior to use in clinical settings.
Our study provides an initial evaluation of TCS measures for assessing severity and recovery among children hospitalized with pneumonia. Similar to adults, such validated TCS measures may ultimately prove useful for improving the quality of both clinical care and research, although additional study to more clearly define stability criteria is needed prior to implementation.
Disclosures
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to Dr. Williams. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institutes of Health. Dr. Grijalva serves as a consultant to Glaxo‐Smith‐Kline and Pfizer outside of the scope of this article. Dr. Edwards is supported through grants from Novartis for the conduction of a Group B strep vaccine study and serves as the Chair of the Data Safety and Monitoring Data Committee for Influenza Study outside the scope of this article. Dr. Self reports grants from CareFusion, BioMerieux, Affinium Pharmaceuticals, Astute Medical, Crucell Holland BV, BRAHMS GmbH, Pfizer, Rapid Pathogen Screening, Venaxis, BioAegis Inc., Sphingotec GmbH, and Cempra Pharmaceuticals; personal fees from BioFire Diagnostics and Venaxis, Inc; and patent 13/632,874 (Sterile Blood Culture Collection System) pending; all outside the scope of this article.
National guidelines for the management of childhood pneumonia highlight the need for the development of objective outcome measures to inform clinical decision making, establish benchmarks of care, and compare treatments and interventions.[1] Time to clinical stability (TCS) is a measure reported in adult pneumonia studies that incorporates vital signs, ability to eat, and mental status to objectively assess readiness for discharge.[2, 3, 4] TCS has not been validated among children as it has in adults,[5, 6, 7, 8] although such measures could prove useful for assessing discharge readiness with applications in both clinical and research settings. The objective of our study was to test the performance of pediatric TCS measures among children hospitalized with pneumonia.
METHODS
Study Population
We studied children hospitalized with community‐acquired pneumonia at Monroe Carell Jr. Children's Hospital at Vanderbilt between January 6, 2010 and May 9, 2011. Study children were enrolled as part of the Centers for Disease Control & Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a prospective, population‐based study of community‐acquired pneumonia hospitalizations. Detailed enrollment criteria for the EPIC study were reported previously.[9] Institutional review boards at Vanderbilt University and the CDC approved this study. Informed consent was obtained from enrolled families.
Data Elements and Study Definitions
Baseline data, including demographics, illness history, comorbidities, and clinical outcomes (eg, length of stay [LOS], intensive care admission), were systematically and prospectively collected. Additionally, data for 4 physiologic parameters, including temperature, heart rate, respiratory rate, and use of supplemental oxygen were obtained from the electronic medical record. These parameters were measured at least every 6 hours from admission through discharge as part of routine care. Readmissions within 7 calendar days of discharge were also obtained from the electronic medical record.
Stability for each parameter was defined as follows: normal temperature (36.037.9C), normal respiratory and heart rates in accordance with Pediatric Advanced Life Support age‐based values (see Supporting Table 1 in the online version of this article),[10] and no administration of supplemental oxygen. If the last recorded value for a given parameter was abnormal, that parameter was considered unstable at discharge. Otherwise, the time and date of the last abnormal value for each parameter was subtracted from admission time and date to determine TCS for that parameter in hours.
To determine overall stability, we evaluated 4 combination TCS measures, each incorporating 2 individual parameters. All combinations included respiratory rate and need for supplemental oxygen, as these parameters are the most explicit clinical indicators of pneumonia. Stability for each combination measure was defined as normalization of all included measures.
Clinical Outcomes for the Combined TCS Measures
The 4 combined TCS measures were compared against clinical outcomes including hospital LOS (measured in hours) and an ordinal severity scale. The ordinal scale categorized children into 3 mutually exclusive groups as follows: nonsevere (hospitalization without need for intensive care or empyema requiring drainage), severe (intensive care admission without invasive mechanical ventilation or vasopressor support and no empyema requiring drainage), and very severe (invasive mechanical ventilation, vasopressor support, or empyema requiring drainage).
Statistical Analysis
Categorical and continuous variables were summarized using frequencies and percentages and median and interquartile range (IQR) values, respectively. Analyses were stratified by age (2 years, 24 years, 517 years). We also plotted summary statistics for the combined measures and LOS, and computed the median absolute difference between these measures for each level of the ordinal severity scale. Analyses were conducted using Stata 13 (StataCorp, College Station, TX).
RESULTS
Study Population
Among 336 children enrolled in the EPIC study at Vanderbilt during the study period, 334 (99.4%) with complete data were included. Median age was 33 months (IQR, 1480). Median LOS was 56.4 hours (IQR, 41.591.7). There were 249 (74.5%) children classified as nonsevere, 39 (11.7) as severe, and 46 (13.8) as very severe (for age‐based characteristics see Supporting Table 2 in the online version of this article). Overall, 12 (3.6%) children were readmitted within 7 days of discharge.
Individual Stability Parameters
Overall, 323 (96.7%) children had 1 parameter abnormal on admission. Respiratory rate (81.4%) was the most common abnormal parameter, followed by abnormal temperature (71.4%), use of supplemental oxygen (63.8%), and abnormal heart rate (54.4%). Overall, use of supplemental oxygen had the longest TCS, followed by respiratory rate (Table 1). In comparison, heart rate and temperature stabilized relatively quickly.
| Parameter | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | |||
|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | |
| ||||||
| Respiratory rate | 97 (74.6) | 38.6 (18.768.9) | 63 (70.0) | 31.6 (9.561.9) | 63 (62.4) | 24.3 (10.859.2) |
| Oxygen | 90 (69.2) | 39.5 (19.273.6) | 58 (64.4) | 44.2 (2477.6) | 61 (60.4) | 38.3 (1870.6) |
| Heart rate | 21 (16.2) | 4.5 (0.318.4) | 73 (81.1) | 21.8 (5.751.9) | 62 (61.4) | 18 (5.842.2) |
| Temperature | 101 (77.7) | 14.5 (4.545.3) | 61 (67.8) | 18.4 (2.842.8) | 62 (61.4) | 10.6 (0.834) |
Seventy children (21.0%) had 1 parameter abnormal at discharge, including abnormal respiratory rate in 13.7%, heart rate in 7.0%, and temperature in 3.3%. One child (0.3%) was discharged with supplemental oxygen. Ten children (3.0%) had 2 parameters abnormal at discharge. There was no difference in 7‐day readmissions for children with 1 parameter abnormal at discharge (1.4%) compared to those with no abnormal parameters at discharge (4.4%, P=0.253).
Combination TCS Measures
Within each age group, the percentage of children achieving stability was relatively consistent across the 4 combined TCS measures (Table 2); however, more children were considered unstable at discharge (and fewer classified as stable on admission) as the number of included parameters increased. More children 5 years of age reached stability (range, 80.0%85.6%) compared to children 5 years of age (range, 68.3%72.3%). We also noted increasing median TCS with increasing disease severity (Figure 1, P0.01) (see Supporting Fig. 1AC in the online version of this article); TCS was only slightly shorter than LOS across all 3 levels of the severity scale.
| TCS Measures | 2 Years, n=130 | 24 Years, n=90 | 517 Years, n=101 | P Value | |||
|---|---|---|---|---|---|---|---|
| No. (%)* | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | No. (%) | Median (IQR) TCS, h | ||
| |||||||
| RR+O2 | 108 (83.1) | 40.5 (20.175.0) | 72 (80.0) | 39.6 (15.679.2) | 69 (68.3) | 30.4 (14.759.2) | 0.08 |
| RR+O2+HR | 109 (83.8) | 40.2 (19.573.9) | 73 (81.1) | 35.9 (15.977.6) | 68 (67.3) | 29.8 (17.256.6) | 0.11 |
| RR+O2+T | 110 (84.6) | 40.5 (20.770.1) | 77 (85.6) | 39.1 (18.477.6) | 73 (72.3) | 28.2 (14.744.7) | 0.03 |
| RR+O2+HR+T | 110 (84.6) | 40.5 (20.770.1) | 72 (80.0) | 39.7 (20.177.5) | 71 (70.3) | 29.2 (18.254) | 0.05 |

DISCUSSION
Our study demonstrates that longitudinal TCS measures consisting of routinely collected physiologic parameters may be useful for objectively assessing disease recovery and clinical readiness for discharge among children hospitalized with pneumonia. A simple TCS measure incorporating respiratory rate and oxygen requirement performed similarly to the more complex combinations and classified fewer children as unstable at discharge. However, we also note several challenges that deserve additional study prior to the application of a pediatric TCS measure in clinical and research settings.
Vital signs and supplemental oxygen use are used clinically to assess disease severity and response to therapy among children with acute respiratory illness. Because these objective parameters are routinely collected among hospitalized children, the systematization of these data could inform clinical decision making around hospital discharge. Similar to early warning scores used to detect impending clinical deterioration,[11] TCS measures, by signaling normalization of stability parameters in a consistent and objective manner, could serve as an early signal of readiness for discharge. However, maximizing the clinical utility of TCS would require embedding the process within the electronic health record, a tool that could also have implications for the Centers for Medicare and Medicaid Services' meaningful use regulations.[12]
TCS could also serve as an outcome measure in research and quality efforts. Increased disease severity was associated with longer TCS for the 4 combined measures; TCS also demonstrated strong agreement with LOS. Furthermore, TCS minimizes the influence of factors unrelated to disease that may impact LOS (eg, frequency of hospital rounds, transportation difficulties, or social impediments to discharge), an advantage when studying outcomes for research and quality benchmarking.
The percentage of children reaching stability and the median TCS for the combined measures demonstrated little variation within each age group, likely because respiratory rate and need for supplemental oxygen, 2 of the parameters with the longest individual time to stability, were also included in each of the combination measures. This suggests that less‐complex measures incorporating only respiratory rate and need for supplemental oxygen may be sufficient to assess clinical stability, particularly because these parameters are objectively measured and possess a direct physiological link to pneumonia. In contrast, the other parameters may be more often influenced by factors unrelated to disease severity.
Our study also highlights several shortcomings of the pediatric TCS measures. Despite use of published, age‐based reference values,[13] we noted wide variation in the achievement of stability across individual parameters, especially for children 5 years old. Overall, 21% of children had 1 abnormal parameter at discharge. Even the simplest combined measure classified 13.4% of children as unstable at discharge. Discharge with unstable parameters was not associated with 7‐day readmission, although our study was underpowered to detect small differences. Additional study is therefore needed to evaluate less restrictive cutoff values on calculated TCS and the impact of hospital discharge prior to reaching stability. In particular, relaxing the upper limit for normal respiratory rate in adolescents (16 breaths per minute) to more closely approximate the adult TCS parameter (24 breaths per minute) should be explored. Refinement and standardization of age‐based vital sign reference values specific to hospitalized children may also improve the performance of these measures.[14]
Several limitations deserve discussion. TCS parameters and readmission data were abstracted retrospectively from a single institution, and our findings may not be generalizable. Although clinical staff routinely measured these data, measurement variation likely exists. Nevertheless, such variation is likely systematic, limiting the impact of potential misclassification. TCS was calculated based on the last abnormal value for each parameter; prior fluctuations between normal and abnormal periods of stability were not captured. We were unable to assess room air oxygen saturations. Instead, supplemental oxygen use served as a surrogate for hypoxia. At our institution, oxygen therapy is provided for children with pneumonia to maintain oxygen saturations of 90% to 92%. We did not assess work of breathing (a marker of severe pneumonia) or ability to eat (a component of adult TCS measures). We initially considered the evaluation of intravenous fluids as a proxy for ability to eat (addition of this parameter to the 4 parameter TCS resulted in a modest increase in median time to stability, data not shown); however, we felt the lack of institutional policy and subjective nature of this parameter detracted from our study's objectives. Finally, we were not able to determine clinical readiness for discharge beyond the measurement of vital sign parameters. Therefore, prospective evaluation of the proposed pediatric TCS measures in broader populations will be important to build upon our findings, refine stability parameters, and test the utility of new parameters (eg, ability to eat, work of breathing) prior to use in clinical settings.
Our study provides an initial evaluation of TCS measures for assessing severity and recovery among children hospitalized with pneumonia. Similar to adults, such validated TCS measures may ultimately prove useful for improving the quality of both clinical care and research, although additional study to more clearly define stability criteria is needed prior to implementation.
Disclosures
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to Dr. Williams. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institutes of Health. Dr. Grijalva serves as a consultant to Glaxo‐Smith‐Kline and Pfizer outside of the scope of this article. Dr. Edwards is supported through grants from Novartis for the conduction of a Group B strep vaccine study and serves as the Chair of the Data Safety and Monitoring Data Committee for Influenza Study outside the scope of this article. Dr. Self reports grants from CareFusion, BioMerieux, Affinium Pharmaceuticals, Astute Medical, Crucell Holland BV, BRAHMS GmbH, Pfizer, Rapid Pathogen Screening, Venaxis, BioAegis Inc., Sphingotec GmbH, and Cempra Pharmaceuticals; personal fees from BioFire Diagnostics and Venaxis, Inc; and patent 13/632,874 (Sterile Blood Culture Collection System) pending; all outside the scope of this article.
- Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/research/data/hcup/index.html. Accessed February 1, 2014.
- , , , et al. Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457.
- , , , et al.; Neumofail Group. Reaching stability in community‐acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783–1790.
- , , , et al.; Community‐Acquired Pneumonia Organization. The pneumonia severity index predicts time to clinical stability in patients with community‐acquired pneumonia. Int J Tuberc Lung Dis. 2006;10:739–743.
- , , , , . Efficacy of corticosteroids in community‐acquired pneumonia: a randomized double‐blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982.
- , , , et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia. Chest 2003;124:1798–1804.
- , , , . A comparison between time to clinical stability in community‐acquired aspiration pneumonia and community‐acquired pneumonia. Intern Emerg Med. 2014;9:143–150.
- , , , et al.; Community‐Acquired Pneumonia Organization (CAPO) Investigators. A worldwide perspective of atypical pathogens in community‐acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093.
- , , , et al.; CDC EPIC Study Team. Community‐acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845.
- American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117:e989–e1004.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13:R135.
- Centers for Medicare and Medicaid Services. Regulations and guidance. EHR incentive programs. Available at: http://www.cms.gov/Regulations‐and‐Guidance/Legislation/EHRIncentivePrograms/index.html. Accessed February 20, 2015
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- , , , et al. Length of stay after reaching clinical stability drives hospital costs associated with adult community‐acquired pneumonia. Scand J Infect Dis. 2013;45:219–226.
- Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/research/data/hcup/index.html. Accessed February 1, 2014.
- , , , et al. Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457.
- , , , et al.; Neumofail Group. Reaching stability in community‐acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783–1790.
- , , , et al.; Community‐Acquired Pneumonia Organization. The pneumonia severity index predicts time to clinical stability in patients with community‐acquired pneumonia. Int J Tuberc Lung Dis. 2006;10:739–743.
- , , , , . Efficacy of corticosteroids in community‐acquired pneumonia: a randomized double‐blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982.
- , , , et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia. Chest 2003;124:1798–1804.
- , , , . A comparison between time to clinical stability in community‐acquired aspiration pneumonia and community‐acquired pneumonia. Intern Emerg Med. 2014;9:143–150.
- , , , et al.; Community‐Acquired Pneumonia Organization (CAPO) Investigators. A worldwide perspective of atypical pathogens in community‐acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093.
- , , , et al.; CDC EPIC Study Team. Community‐acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845.
- American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117:e989–e1004.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13:R135.
- Centers for Medicare and Medicaid Services. Regulations and guidance. EHR incentive programs. Available at: http://www.cms.gov/Regulations‐and‐Guidance/Legislation/EHRIncentivePrograms/index.html. Accessed February 20, 2015
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- , , , et al. Length of stay after reaching clinical stability drives hospital costs associated with adult community‐acquired pneumonia. Scand J Infect Dis. 2013;45:219–226.
Drug can prevent bleeding in kids with hemophilia A
A recombinant factor VIII Fc fusion protein (rFVIIIFc/efmoroctocog alfa, Eloctate/Elocta) can prevent and control bleeding in previously treated children with severe hemophilia A, results of the phase 3 KIDS A-LONG study suggest.
Children who were previously receiving factor VIII prophylaxis saw their median annualized bleeding rate (ABR) decrease with rFVIIIFc, and close to half of the children on this study did not have any bleeding episodes while they were receiving rFVIIIFc.
None of the patients developed inhibitors to rFVIIIFc. And researchers said adverse events on this trial were typical of a pediatric hemophilia population.
The team reported these results in the Journal of Thrombosis and Haemostasis. The trial was sponsored by Biogen Idec and Sobi, the companies developing rFVIIIFc.
The study included 71 boys younger than 12 years of age who had severe hemophilia A. The patients had at least 50 prior exposure days to factor VIII and no history of factor VIII inhibitors.
The children were set to receive twice-weekly prophylactic infusions of rFVIIIFc, 25 IU/kg on day 1 and 50 IU/kg on day 4, but the researchers made adjustments to dosing as needed.
The median average weekly rFVIIIFc prophylactic dose was 88.11 IU kg. About 90% of the patients were on twice-weekly dosing at the end of the study. Seventy-four percent of patients were able to reduce their dosing frequency with rFVIIIFc compared to factor VIII prophylaxis.
About 46% of patients did not have any bleeding events on study. The median ABR was 1.96 overall and 0 for spontaneous and traumatic bleeding episodes, as well as for spontaneous joint bleeding episodes.
Among patients who were previously receiving factor VIII prophylaxis, their median ABR decreased with rFVIIIFc. For children younger than 6 years of age, the median ABR fell from 1.50 to 0. For children ages 6 through 11, the median ABR fell from 2.50 to 2.01.
About 86% of patients had at least one adverse event while on rFVIIIFc, but none of them discontinued treatment as a result.
Two non-serious events (myalgia and erythematous rash) were considered related to rFVIIIFc. And 5 patients experienced 7 serious adverse events that were not related to treatment. ![]()
A recombinant factor VIII Fc fusion protein (rFVIIIFc/efmoroctocog alfa, Eloctate/Elocta) can prevent and control bleeding in previously treated children with severe hemophilia A, results of the phase 3 KIDS A-LONG study suggest.
Children who were previously receiving factor VIII prophylaxis saw their median annualized bleeding rate (ABR) decrease with rFVIIIFc, and close to half of the children on this study did not have any bleeding episodes while they were receiving rFVIIIFc.
None of the patients developed inhibitors to rFVIIIFc. And researchers said adverse events on this trial were typical of a pediatric hemophilia population.
The team reported these results in the Journal of Thrombosis and Haemostasis. The trial was sponsored by Biogen Idec and Sobi, the companies developing rFVIIIFc.
The study included 71 boys younger than 12 years of age who had severe hemophilia A. The patients had at least 50 prior exposure days to factor VIII and no history of factor VIII inhibitors.
The children were set to receive twice-weekly prophylactic infusions of rFVIIIFc, 25 IU/kg on day 1 and 50 IU/kg on day 4, but the researchers made adjustments to dosing as needed.
The median average weekly rFVIIIFc prophylactic dose was 88.11 IU kg. About 90% of the patients were on twice-weekly dosing at the end of the study. Seventy-four percent of patients were able to reduce their dosing frequency with rFVIIIFc compared to factor VIII prophylaxis.
About 46% of patients did not have any bleeding events on study. The median ABR was 1.96 overall and 0 for spontaneous and traumatic bleeding episodes, as well as for spontaneous joint bleeding episodes.
Among patients who were previously receiving factor VIII prophylaxis, their median ABR decreased with rFVIIIFc. For children younger than 6 years of age, the median ABR fell from 1.50 to 0. For children ages 6 through 11, the median ABR fell from 2.50 to 2.01.
About 86% of patients had at least one adverse event while on rFVIIIFc, but none of them discontinued treatment as a result.
Two non-serious events (myalgia and erythematous rash) were considered related to rFVIIIFc. And 5 patients experienced 7 serious adverse events that were not related to treatment. ![]()
A recombinant factor VIII Fc fusion protein (rFVIIIFc/efmoroctocog alfa, Eloctate/Elocta) can prevent and control bleeding in previously treated children with severe hemophilia A, results of the phase 3 KIDS A-LONG study suggest.
Children who were previously receiving factor VIII prophylaxis saw their median annualized bleeding rate (ABR) decrease with rFVIIIFc, and close to half of the children on this study did not have any bleeding episodes while they were receiving rFVIIIFc.
None of the patients developed inhibitors to rFVIIIFc. And researchers said adverse events on this trial were typical of a pediatric hemophilia population.
The team reported these results in the Journal of Thrombosis and Haemostasis. The trial was sponsored by Biogen Idec and Sobi, the companies developing rFVIIIFc.
The study included 71 boys younger than 12 years of age who had severe hemophilia A. The patients had at least 50 prior exposure days to factor VIII and no history of factor VIII inhibitors.
The children were set to receive twice-weekly prophylactic infusions of rFVIIIFc, 25 IU/kg on day 1 and 50 IU/kg on day 4, but the researchers made adjustments to dosing as needed.
The median average weekly rFVIIIFc prophylactic dose was 88.11 IU kg. About 90% of the patients were on twice-weekly dosing at the end of the study. Seventy-four percent of patients were able to reduce their dosing frequency with rFVIIIFc compared to factor VIII prophylaxis.
About 46% of patients did not have any bleeding events on study. The median ABR was 1.96 overall and 0 for spontaneous and traumatic bleeding episodes, as well as for spontaneous joint bleeding episodes.
Among patients who were previously receiving factor VIII prophylaxis, their median ABR decreased with rFVIIIFc. For children younger than 6 years of age, the median ABR fell from 1.50 to 0. For children ages 6 through 11, the median ABR fell from 2.50 to 2.01.
About 86% of patients had at least one adverse event while on rFVIIIFc, but none of them discontinued treatment as a result.
Two non-serious events (myalgia and erythematous rash) were considered related to rFVIIIFc. And 5 patients experienced 7 serious adverse events that were not related to treatment. ![]()
Chemotherapy drugs recalled in US

Photo by Bill Branson
The pharmaceutical company Mylan is conducting a US-wide recall of several injectable chemotherapy drugs.
Testing of retention samples revealed foreign particulate matter in lots of gemcitabine, carboplatin, methotrexate, and cytarabine. So Mylan issued a
recall of these lots to the hospital/user level.
To date, Mylan has not received any reports of adverse events related to this recall. However, administering injectables that contain foreign particulates can have severe consequences.
Intrathecal administration could result in a life-threatening adverse event or permanent impairment of a body function. Intravenous administration has the potential to damage and/or obstruct blood vessels, which could induce emboli, particularly in the lungs. Intravenous injection can also result in local inflammation, phlebitis, allergic response, and/or embolization in the body and infection.
Intra-arterial administration could result in damage to blood vessels in the distal extremities or organs. And intramuscular administration could result in foreign-body inflammatory response, with local pain, swelling, and possible long-term granuloma formation.
Recall details
The following drugs are included in this recall:
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801396; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801401; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 0069-3857-10; Lot number: 7801089; Expiration date: 07/2015
- Gemcitabine for Injection, USP 2 g; 100 mL; NDC number: 67457-463-02; Lot number: 7801222; Expiration date: 03/2016
- Gemcitabine for Injection, USP 1 g; 50 mL; NDC number: 67457-462-01; Lot number: 7801273; Expiration date: 05/2016
- Carboplatin Injection 10 mg/mL; 100 mL; NDC number: 67457-493-46; Lot number: 7801312; Expiration date: 06/2015
- Methotrexate Injection, USP 25 mg/mL; 2 mL (5 x 2 mL); NDC number: 0069-0146-02; Lot number: 7801082; Expiration date: 07/2015
- Cytarabine Injection 20 mg/mL; 5 mL (10 x 5mL); NDC number: 0069-0152-02; Lot number: 7801050; Expiration date: 05/2015.
Gemcitabine for Injection, USP 200 mg is an intravenously administered product indicated for the treatment of ovarian cancer, breast cancer, non-small cell lung cancer, and pancreatic cancer. These lots were distributed in the US between February 18, 2014, and December 19, 2014, and were manufactured and packaged by Agila Onco Therapies Limited, a Mylan company. Lot 7801089 is packaged with a Pfizer Injectable label.
Carboplatin Injection 10 mg/mL is an intravenously administered product indicated for the treatment of advanced ovarian carcinoma. The lot was distributed in the US between August 11, 2014, and October 7, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Mylan Institutional label.
Methotrexate Injection, USP 25 mg/mL can be administered intramuscularly, intravenously, intra-arterially, or intrathecally and is indicated for certain neoplastic diseases, severe psoriasis, and adult rheumatoid arthritis. The lot was distributed in the US between January 16, 2014, and March 25, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Pfizer Injectables label.
Cytarabine Injection can be administered intravenously or intrathecally and in combination with other approved anticancer drugs. Cytarabine is indicated for remission induction in acute non-lymphocytic leukemia in adults and pediatric patients. The lot was distributed in the US between May 02, 2014, and July 24, 2014, and was manufactured and packaged by Agila Onco Therapies Limited, a Mylan company located in Bangalore, India, and is packaged with a Pfizer Injectables label.
Mylan is notifying its distributors and customers by letter and is arranging for the return of all recalled products. Distributors, retailers, hospitals, clinics, and physicians with the recalled products should stop using them and return them to the place of purchase.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 1-800-796-9526 or [email protected], Monday through Friday from 8 am to 5 pm EST.
Consumers should contact their physicians or healthcare providers if they have experienced any problems that may be related to using these drugs.
Adverse reactions or quality problems related to the use of these product may be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Photo by Bill Branson
The pharmaceutical company Mylan is conducting a US-wide recall of several injectable chemotherapy drugs.
Testing of retention samples revealed foreign particulate matter in lots of gemcitabine, carboplatin, methotrexate, and cytarabine. So Mylan issued a
recall of these lots to the hospital/user level.
To date, Mylan has not received any reports of adverse events related to this recall. However, administering injectables that contain foreign particulates can have severe consequences.
Intrathecal administration could result in a life-threatening adverse event or permanent impairment of a body function. Intravenous administration has the potential to damage and/or obstruct blood vessels, which could induce emboli, particularly in the lungs. Intravenous injection can also result in local inflammation, phlebitis, allergic response, and/or embolization in the body and infection.
Intra-arterial administration could result in damage to blood vessels in the distal extremities or organs. And intramuscular administration could result in foreign-body inflammatory response, with local pain, swelling, and possible long-term granuloma formation.
Recall details
The following drugs are included in this recall:
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801396; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801401; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 0069-3857-10; Lot number: 7801089; Expiration date: 07/2015
- Gemcitabine for Injection, USP 2 g; 100 mL; NDC number: 67457-463-02; Lot number: 7801222; Expiration date: 03/2016
- Gemcitabine for Injection, USP 1 g; 50 mL; NDC number: 67457-462-01; Lot number: 7801273; Expiration date: 05/2016
- Carboplatin Injection 10 mg/mL; 100 mL; NDC number: 67457-493-46; Lot number: 7801312; Expiration date: 06/2015
- Methotrexate Injection, USP 25 mg/mL; 2 mL (5 x 2 mL); NDC number: 0069-0146-02; Lot number: 7801082; Expiration date: 07/2015
- Cytarabine Injection 20 mg/mL; 5 mL (10 x 5mL); NDC number: 0069-0152-02; Lot number: 7801050; Expiration date: 05/2015.
Gemcitabine for Injection, USP 200 mg is an intravenously administered product indicated for the treatment of ovarian cancer, breast cancer, non-small cell lung cancer, and pancreatic cancer. These lots were distributed in the US between February 18, 2014, and December 19, 2014, and were manufactured and packaged by Agila Onco Therapies Limited, a Mylan company. Lot 7801089 is packaged with a Pfizer Injectable label.
Carboplatin Injection 10 mg/mL is an intravenously administered product indicated for the treatment of advanced ovarian carcinoma. The lot was distributed in the US between August 11, 2014, and October 7, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Mylan Institutional label.
Methotrexate Injection, USP 25 mg/mL can be administered intramuscularly, intravenously, intra-arterially, or intrathecally and is indicated for certain neoplastic diseases, severe psoriasis, and adult rheumatoid arthritis. The lot was distributed in the US between January 16, 2014, and March 25, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Pfizer Injectables label.
Cytarabine Injection can be administered intravenously or intrathecally and in combination with other approved anticancer drugs. Cytarabine is indicated for remission induction in acute non-lymphocytic leukemia in adults and pediatric patients. The lot was distributed in the US between May 02, 2014, and July 24, 2014, and was manufactured and packaged by Agila Onco Therapies Limited, a Mylan company located in Bangalore, India, and is packaged with a Pfizer Injectables label.
Mylan is notifying its distributors and customers by letter and is arranging for the return of all recalled products. Distributors, retailers, hospitals, clinics, and physicians with the recalled products should stop using them and return them to the place of purchase.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 1-800-796-9526 or [email protected], Monday through Friday from 8 am to 5 pm EST.
Consumers should contact their physicians or healthcare providers if they have experienced any problems that may be related to using these drugs.
Adverse reactions or quality problems related to the use of these product may be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Photo by Bill Branson
The pharmaceutical company Mylan is conducting a US-wide recall of several injectable chemotherapy drugs.
Testing of retention samples revealed foreign particulate matter in lots of gemcitabine, carboplatin, methotrexate, and cytarabine. So Mylan issued a
recall of these lots to the hospital/user level.
To date, Mylan has not received any reports of adverse events related to this recall. However, administering injectables that contain foreign particulates can have severe consequences.
Intrathecal administration could result in a life-threatening adverse event or permanent impairment of a body function. Intravenous administration has the potential to damage and/or obstruct blood vessels, which could induce emboli, particularly in the lungs. Intravenous injection can also result in local inflammation, phlebitis, allergic response, and/or embolization in the body and infection.
Intra-arterial administration could result in damage to blood vessels in the distal extremities or organs. And intramuscular administration could result in foreign-body inflammatory response, with local pain, swelling, and possible long-term granuloma formation.
Recall details
The following drugs are included in this recall:
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801396; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 67457-464-20; Lot number: 7801401; Expiration date: 08/2016
- Gemcitabine for Injection, USP 200 mg; 10 mL; NDC number: 0069-3857-10; Lot number: 7801089; Expiration date: 07/2015
- Gemcitabine for Injection, USP 2 g; 100 mL; NDC number: 67457-463-02; Lot number: 7801222; Expiration date: 03/2016
- Gemcitabine for Injection, USP 1 g; 50 mL; NDC number: 67457-462-01; Lot number: 7801273; Expiration date: 05/2016
- Carboplatin Injection 10 mg/mL; 100 mL; NDC number: 67457-493-46; Lot number: 7801312; Expiration date: 06/2015
- Methotrexate Injection, USP 25 mg/mL; 2 mL (5 x 2 mL); NDC number: 0069-0146-02; Lot number: 7801082; Expiration date: 07/2015
- Cytarabine Injection 20 mg/mL; 5 mL (10 x 5mL); NDC number: 0069-0152-02; Lot number: 7801050; Expiration date: 05/2015.
Gemcitabine for Injection, USP 200 mg is an intravenously administered product indicated for the treatment of ovarian cancer, breast cancer, non-small cell lung cancer, and pancreatic cancer. These lots were distributed in the US between February 18, 2014, and December 19, 2014, and were manufactured and packaged by Agila Onco Therapies Limited, a Mylan company. Lot 7801089 is packaged with a Pfizer Injectable label.
Carboplatin Injection 10 mg/mL is an intravenously administered product indicated for the treatment of advanced ovarian carcinoma. The lot was distributed in the US between August 11, 2014, and October 7, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Mylan Institutional label.
Methotrexate Injection, USP 25 mg/mL can be administered intramuscularly, intravenously, intra-arterially, or intrathecally and is indicated for certain neoplastic diseases, severe psoriasis, and adult rheumatoid arthritis. The lot was distributed in the US between January 16, 2014, and March 25, 2014, and was packaged by Agila Onco Therapies Limited, a Mylan company, with a Pfizer Injectables label.
Cytarabine Injection can be administered intravenously or intrathecally and in combination with other approved anticancer drugs. Cytarabine is indicated for remission induction in acute non-lymphocytic leukemia in adults and pediatric patients. The lot was distributed in the US between May 02, 2014, and July 24, 2014, and was manufactured and packaged by Agila Onco Therapies Limited, a Mylan company located in Bangalore, India, and is packaged with a Pfizer Injectables label.
Mylan is notifying its distributors and customers by letter and is arranging for the return of all recalled products. Distributors, retailers, hospitals, clinics, and physicians with the recalled products should stop using them and return them to the place of purchase.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 1-800-796-9526 or [email protected], Monday through Friday from 8 am to 5 pm EST.
Consumers should contact their physicians or healthcare providers if they have experienced any problems that may be related to using these drugs.
Adverse reactions or quality problems related to the use of these product may be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()
Fractional laser resurfacing plus ALA-PDT upped AK clearance
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
AT LASER 2015
Key clinical point: Performing fractional CO2 laser resurfacing before aminolevulinic acid blue light photodynamic therapy cleared significantly more actinic keratoses than ALA-PDT alone.
Major finding: For the 30-minute incubation group, laser plus ALA-PDT cleared 94% of actinic keratoses, compared with 82% for ALA-PDT alone.
Data source: A randomized, single-blinded, split-face study of 20 patients.
Disclosures: Deka made the fractional CO2 laser tested in the study and DUSA Pharmaceuticals made the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
CDK inhibitor proves active against AML, ALL

PHILADELPHIA—Preclinical research suggests a cyclin-dependent kinase (CDK) inhibitor is active against acute leukemias, particularly those with mixed-lineage leukemia rearrangements (MLL-r).
CYC065 selectively inhibits CDK2, which drives cell-cycle transition and activates major DNA double-strand break repair pathways; CDK5, which drives metastatic spread; and CDK9, which regulates the transcription of genes important for the proliferation and survival of malignant cells.
Experiments have shown that CYC065 exhibits activity against acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), with and without MLL-r.
Daniella Zheleva, PhD, and her colleagues described these experiments in a poster at the AACR Annual Meeting 2015 (abstract 1650). All of the researchers are employees of Cyclacel Ltd., the company developing CYC065.
The researchers tested CYC065 in a panel of AML cell lines with wild-type MLL (HEL, HL60, Kasumi-1, KG-1, OCI-AML5, and PL21) and MLL-r (EOL-1, ML-2, MOLM-13, MV4-11, Nomo-1, OCI-AML2, and THP-1).
They found that MLL-r cell lines were “highly sensitive” to CYC065, but the sensitivity of cell lines with wild-type MLL correlated with the level of Bcl-2 family proteins. In the wild-type cell lines, IC50/70/90 values were correlated with BclXL and inversely correlated with Bak.
Six-hour pulse treatment of CYC065 at 0.5 µM to 1 µM was sufficient to cause 90% or greater cell death in sensitive cell lines. And cell lines with reduced sensitivity to the drug could be targeted by exposure to 10-hour pulse treatments of CYC065, or to CYC065 in combination with short pulses of Bcl-2 inhibitors.
The researchers observed “potent antitumor activity” when they administered CYC065 in AML xenograft models.
In an EOL-1 model, the median tumor growth inhibition on day 19 was 97% for mice that received CYC065 at 40 mg/kg (every day on days 1-5 and 8-12), 95% for mice that received CYC065 at 20 mg/kg every day on days 1-5 and 8-12), and 41% for mice that received cytarabine at 100 mg/kg (every day on days 1-5).
In the HL60 model, the median tumor growth inhibition on day 11 was 90% for mice that received CYC065 at 70 mg/kg (every day on days 1-5 and 8-12). And 2 mice achieved a complete response to treatment.
The researchers also found that CYC065 synergizes with cytarabine, particularly when CYC065 is given first. In fact, the combination could overcome the cytarabine resistance observed in the MV4-11 cell line.
CYC065 was “strongly synergistic” with Bcl2/BclXL inhibitors as well, the researchers said. CYC065 synergized with ABT-199, ABT-263, and ABT-737 in both AML cell lines (THP-1 and HEL) and ALL cell lines (Jurkat and SEM).
The researchers said the potent in vitro and in vivo activity of CYC065 and the ability to combine the drug with other antileukemic agents suggest that it may have therapeutic potential in AML and ALL. ![]()

PHILADELPHIA—Preclinical research suggests a cyclin-dependent kinase (CDK) inhibitor is active against acute leukemias, particularly those with mixed-lineage leukemia rearrangements (MLL-r).
CYC065 selectively inhibits CDK2, which drives cell-cycle transition and activates major DNA double-strand break repair pathways; CDK5, which drives metastatic spread; and CDK9, which regulates the transcription of genes important for the proliferation and survival of malignant cells.
Experiments have shown that CYC065 exhibits activity against acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), with and without MLL-r.
Daniella Zheleva, PhD, and her colleagues described these experiments in a poster at the AACR Annual Meeting 2015 (abstract 1650). All of the researchers are employees of Cyclacel Ltd., the company developing CYC065.
The researchers tested CYC065 in a panel of AML cell lines with wild-type MLL (HEL, HL60, Kasumi-1, KG-1, OCI-AML5, and PL21) and MLL-r (EOL-1, ML-2, MOLM-13, MV4-11, Nomo-1, OCI-AML2, and THP-1).
They found that MLL-r cell lines were “highly sensitive” to CYC065, but the sensitivity of cell lines with wild-type MLL correlated with the level of Bcl-2 family proteins. In the wild-type cell lines, IC50/70/90 values were correlated with BclXL and inversely correlated with Bak.
Six-hour pulse treatment of CYC065 at 0.5 µM to 1 µM was sufficient to cause 90% or greater cell death in sensitive cell lines. And cell lines with reduced sensitivity to the drug could be targeted by exposure to 10-hour pulse treatments of CYC065, or to CYC065 in combination with short pulses of Bcl-2 inhibitors.
The researchers observed “potent antitumor activity” when they administered CYC065 in AML xenograft models.
In an EOL-1 model, the median tumor growth inhibition on day 19 was 97% for mice that received CYC065 at 40 mg/kg (every day on days 1-5 and 8-12), 95% for mice that received CYC065 at 20 mg/kg every day on days 1-5 and 8-12), and 41% for mice that received cytarabine at 100 mg/kg (every day on days 1-5).
In the HL60 model, the median tumor growth inhibition on day 11 was 90% for mice that received CYC065 at 70 mg/kg (every day on days 1-5 and 8-12). And 2 mice achieved a complete response to treatment.
The researchers also found that CYC065 synergizes with cytarabine, particularly when CYC065 is given first. In fact, the combination could overcome the cytarabine resistance observed in the MV4-11 cell line.
CYC065 was “strongly synergistic” with Bcl2/BclXL inhibitors as well, the researchers said. CYC065 synergized with ABT-199, ABT-263, and ABT-737 in both AML cell lines (THP-1 and HEL) and ALL cell lines (Jurkat and SEM).
The researchers said the potent in vitro and in vivo activity of CYC065 and the ability to combine the drug with other antileukemic agents suggest that it may have therapeutic potential in AML and ALL. ![]()

PHILADELPHIA—Preclinical research suggests a cyclin-dependent kinase (CDK) inhibitor is active against acute leukemias, particularly those with mixed-lineage leukemia rearrangements (MLL-r).
CYC065 selectively inhibits CDK2, which drives cell-cycle transition and activates major DNA double-strand break repair pathways; CDK5, which drives metastatic spread; and CDK9, which regulates the transcription of genes important for the proliferation and survival of malignant cells.
Experiments have shown that CYC065 exhibits activity against acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), with and without MLL-r.
Daniella Zheleva, PhD, and her colleagues described these experiments in a poster at the AACR Annual Meeting 2015 (abstract 1650). All of the researchers are employees of Cyclacel Ltd., the company developing CYC065.
The researchers tested CYC065 in a panel of AML cell lines with wild-type MLL (HEL, HL60, Kasumi-1, KG-1, OCI-AML5, and PL21) and MLL-r (EOL-1, ML-2, MOLM-13, MV4-11, Nomo-1, OCI-AML2, and THP-1).
They found that MLL-r cell lines were “highly sensitive” to CYC065, but the sensitivity of cell lines with wild-type MLL correlated with the level of Bcl-2 family proteins. In the wild-type cell lines, IC50/70/90 values were correlated with BclXL and inversely correlated with Bak.
Six-hour pulse treatment of CYC065 at 0.5 µM to 1 µM was sufficient to cause 90% or greater cell death in sensitive cell lines. And cell lines with reduced sensitivity to the drug could be targeted by exposure to 10-hour pulse treatments of CYC065, or to CYC065 in combination with short pulses of Bcl-2 inhibitors.
The researchers observed “potent antitumor activity” when they administered CYC065 in AML xenograft models.
In an EOL-1 model, the median tumor growth inhibition on day 19 was 97% for mice that received CYC065 at 40 mg/kg (every day on days 1-5 and 8-12), 95% for mice that received CYC065 at 20 mg/kg every day on days 1-5 and 8-12), and 41% for mice that received cytarabine at 100 mg/kg (every day on days 1-5).
In the HL60 model, the median tumor growth inhibition on day 11 was 90% for mice that received CYC065 at 70 mg/kg (every day on days 1-5 and 8-12). And 2 mice achieved a complete response to treatment.
The researchers also found that CYC065 synergizes with cytarabine, particularly when CYC065 is given first. In fact, the combination could overcome the cytarabine resistance observed in the MV4-11 cell line.
CYC065 was “strongly synergistic” with Bcl2/BclXL inhibitors as well, the researchers said. CYC065 synergized with ABT-199, ABT-263, and ABT-737 in both AML cell lines (THP-1 and HEL) and ALL cell lines (Jurkat and SEM).
The researchers said the potent in vitro and in vivo activity of CYC065 and the ability to combine the drug with other antileukemic agents suggest that it may have therapeutic potential in AML and ALL. ![]()
Drug that fell short in prostate cancer could treat MM
PHILADELPHIA—A drug that has fallen short of expectations in clinical trials of prostate cancer may be effective for treating multiple myeloma (MM), according to research presented at the AACR Annual Meeting 2015.
The drug, tasquinimod, inhibits the function of S100A9, a pro-inflammatory protein that is elevated in MM, prostate cancer, and other malignancies.
Researchers found that tasquinimod can reduce tumor growth and improve survival in mouse models of MM. And these effects are associated with reduced angiogenesis in the bone marrow.
Cindy Lin, PhD, of the Wistar Institute in Philadelphia, Pennsylvania, and her colleagues presented these findings as abstract 1364.* The research was supported by Active Biotech and Ipsen, the companies developing tasquinimod.
Dr Lin noted that tasquinimod has already been tested in clinical trials of prostate cancer and initially appeared to be very effective. However, recent results from a phase 3 trial suggested the drug does not confer a favorable risk-benefit ratio for this population.
So Active Biotech and Ipsen decided to discontinue all trials of tasquinimod in prostate cancer. But the preclinical results observed in MM suggest tasquinimod may hold promise for treating these patients.
Activity in MM
Dr Lin said previous preclinical experiments revealed that myeloid-derived suppressor cells are involved in regulating MM progression, and these cells produce S100A9.
Tasquinimod is a quinoline-3-carboxamide derivative that binds to S100A9 and inhibits interaction with its receptors. So Dr Lin and her colleagues decided to investigate the antitumor effect of the drug in mouse models of MM.
The researchers initially tested tasquinimod in a syngeneic MM model, randomizing mice to treatment or control. Mice in the treatment group received tasquinimod at 30 mg/kg/day in their drinking water for 28 days.
Tasquinimod significantly improved survival in this model (P<0.005). All control mice died within 30 days of tumor inoculation, but about 40% of tasquinimod-treated mice were still alive more than 80 days out.
The researchers then tested tasquinimod in xenograft models of human MM. The drug significantly reduced tumor size in both H929 (P=0.0042) and RPMI-8226 models (P=0.0003). Treatment significantly improved survival in the H929 (P=0.0008) and RPMI-8226 models as well (P=0.0243).
Dr Lin said she and her colleagues did not see any side effects of treatment in any of the mice.
Investigating the mechanism
To determine if the antitumor effect of tasquinimod is, in fact, mediated through inhibition of S100A9, the researchers administered the drug to S100A9-knockout mice with MM. Tasquinimod did not improve survival in these mice, which suggests its anti-MM effects are mediated through S100A9 inhibition.
“To try and investigate some of the mechanisms of how survival is improved in tasquinimod-treated, tumor-bearing mice, we looked at a variety of different things, including angiogenesis,” Dr Lin said.
“We used CD31 immunohistochemistry to look at angiogenesis, and, in the untreated mice, we didn’t see a lot of staining. But in the tumor-bearing mice, there was a lot more staining [P=0.0231]. And when we gave the mice tasquinimod, angiogenesis was significantly decreased [P<0.0001].”
The researchers also looked at different angiogenic factors. And they found that, compared to control-treated mice with MM, tumor-bearing mice that received tasquinimod had a significant decrease in serum levels of VEGF, FGF2, tissue factor, and endoglin.
The team is now assessing the effects of S100A9 and tasquinimod on megakaryocytes and platelets, 2 of the major cell populations that promote angiogenesis. ![]()
*Information in the abstract differs from that presented at the meeting.
PHILADELPHIA—A drug that has fallen short of expectations in clinical trials of prostate cancer may be effective for treating multiple myeloma (MM), according to research presented at the AACR Annual Meeting 2015.
The drug, tasquinimod, inhibits the function of S100A9, a pro-inflammatory protein that is elevated in MM, prostate cancer, and other malignancies.
Researchers found that tasquinimod can reduce tumor growth and improve survival in mouse models of MM. And these effects are associated with reduced angiogenesis in the bone marrow.
Cindy Lin, PhD, of the Wistar Institute in Philadelphia, Pennsylvania, and her colleagues presented these findings as abstract 1364.* The research was supported by Active Biotech and Ipsen, the companies developing tasquinimod.
Dr Lin noted that tasquinimod has already been tested in clinical trials of prostate cancer and initially appeared to be very effective. However, recent results from a phase 3 trial suggested the drug does not confer a favorable risk-benefit ratio for this population.
So Active Biotech and Ipsen decided to discontinue all trials of tasquinimod in prostate cancer. But the preclinical results observed in MM suggest tasquinimod may hold promise for treating these patients.
Activity in MM
Dr Lin said previous preclinical experiments revealed that myeloid-derived suppressor cells are involved in regulating MM progression, and these cells produce S100A9.
Tasquinimod is a quinoline-3-carboxamide derivative that binds to S100A9 and inhibits interaction with its receptors. So Dr Lin and her colleagues decided to investigate the antitumor effect of the drug in mouse models of MM.
The researchers initially tested tasquinimod in a syngeneic MM model, randomizing mice to treatment or control. Mice in the treatment group received tasquinimod at 30 mg/kg/day in their drinking water for 28 days.
Tasquinimod significantly improved survival in this model (P<0.005). All control mice died within 30 days of tumor inoculation, but about 40% of tasquinimod-treated mice were still alive more than 80 days out.
The researchers then tested tasquinimod in xenograft models of human MM. The drug significantly reduced tumor size in both H929 (P=0.0042) and RPMI-8226 models (P=0.0003). Treatment significantly improved survival in the H929 (P=0.0008) and RPMI-8226 models as well (P=0.0243).
Dr Lin said she and her colleagues did not see any side effects of treatment in any of the mice.
Investigating the mechanism
To determine if the antitumor effect of tasquinimod is, in fact, mediated through inhibition of S100A9, the researchers administered the drug to S100A9-knockout mice with MM. Tasquinimod did not improve survival in these mice, which suggests its anti-MM effects are mediated through S100A9 inhibition.
“To try and investigate some of the mechanisms of how survival is improved in tasquinimod-treated, tumor-bearing mice, we looked at a variety of different things, including angiogenesis,” Dr Lin said.
“We used CD31 immunohistochemistry to look at angiogenesis, and, in the untreated mice, we didn’t see a lot of staining. But in the tumor-bearing mice, there was a lot more staining [P=0.0231]. And when we gave the mice tasquinimod, angiogenesis was significantly decreased [P<0.0001].”
The researchers also looked at different angiogenic factors. And they found that, compared to control-treated mice with MM, tumor-bearing mice that received tasquinimod had a significant decrease in serum levels of VEGF, FGF2, tissue factor, and endoglin.
The team is now assessing the effects of S100A9 and tasquinimod on megakaryocytes and platelets, 2 of the major cell populations that promote angiogenesis. ![]()
*Information in the abstract differs from that presented at the meeting.
PHILADELPHIA—A drug that has fallen short of expectations in clinical trials of prostate cancer may be effective for treating multiple myeloma (MM), according to research presented at the AACR Annual Meeting 2015.
The drug, tasquinimod, inhibits the function of S100A9, a pro-inflammatory protein that is elevated in MM, prostate cancer, and other malignancies.
Researchers found that tasquinimod can reduce tumor growth and improve survival in mouse models of MM. And these effects are associated with reduced angiogenesis in the bone marrow.
Cindy Lin, PhD, of the Wistar Institute in Philadelphia, Pennsylvania, and her colleagues presented these findings as abstract 1364.* The research was supported by Active Biotech and Ipsen, the companies developing tasquinimod.
Dr Lin noted that tasquinimod has already been tested in clinical trials of prostate cancer and initially appeared to be very effective. However, recent results from a phase 3 trial suggested the drug does not confer a favorable risk-benefit ratio for this population.
So Active Biotech and Ipsen decided to discontinue all trials of tasquinimod in prostate cancer. But the preclinical results observed in MM suggest tasquinimod may hold promise for treating these patients.
Activity in MM
Dr Lin said previous preclinical experiments revealed that myeloid-derived suppressor cells are involved in regulating MM progression, and these cells produce S100A9.
Tasquinimod is a quinoline-3-carboxamide derivative that binds to S100A9 and inhibits interaction with its receptors. So Dr Lin and her colleagues decided to investigate the antitumor effect of the drug in mouse models of MM.
The researchers initially tested tasquinimod in a syngeneic MM model, randomizing mice to treatment or control. Mice in the treatment group received tasquinimod at 30 mg/kg/day in their drinking water for 28 days.
Tasquinimod significantly improved survival in this model (P<0.005). All control mice died within 30 days of tumor inoculation, but about 40% of tasquinimod-treated mice were still alive more than 80 days out.
The researchers then tested tasquinimod in xenograft models of human MM. The drug significantly reduced tumor size in both H929 (P=0.0042) and RPMI-8226 models (P=0.0003). Treatment significantly improved survival in the H929 (P=0.0008) and RPMI-8226 models as well (P=0.0243).
Dr Lin said she and her colleagues did not see any side effects of treatment in any of the mice.
Investigating the mechanism
To determine if the antitumor effect of tasquinimod is, in fact, mediated through inhibition of S100A9, the researchers administered the drug to S100A9-knockout mice with MM. Tasquinimod did not improve survival in these mice, which suggests its anti-MM effects are mediated through S100A9 inhibition.
“To try and investigate some of the mechanisms of how survival is improved in tasquinimod-treated, tumor-bearing mice, we looked at a variety of different things, including angiogenesis,” Dr Lin said.
“We used CD31 immunohistochemistry to look at angiogenesis, and, in the untreated mice, we didn’t see a lot of staining. But in the tumor-bearing mice, there was a lot more staining [P=0.0231]. And when we gave the mice tasquinimod, angiogenesis was significantly decreased [P<0.0001].”
The researchers also looked at different angiogenic factors. And they found that, compared to control-treated mice with MM, tumor-bearing mice that received tasquinimod had a significant decrease in serum levels of VEGF, FGF2, tissue factor, and endoglin.
The team is now assessing the effects of S100A9 and tasquinimod on megakaryocytes and platelets, 2 of the major cell populations that promote angiogenesis. ![]()
*Information in the abstract differs from that presented at the meeting.
Using cervical length screening to predict preterm birth
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
One of the key indicators of a nation’s health is how well it can care for its young. Despite many advances in medical care and improvements in access to care, infant mortality remains a significant concern worldwide. According to the World Health Organization, the leading cause of death among children under age 5 is preterm birth complications. With an estimated 15 million babies born prematurely (prior to 37 weeks’ gestation) globally each year, it is vital for ob.gyns. to uncover ways to predict, diagnose early, and treat the causes of preterm birth.
While the challenges to infant health could be considered more of an issue in developing countries, here in the United States, the Centers for Disease Control and Prevention estimates that 1 in 9 babies is born prematurely. Preterm birth-related causes of death (i.e., breathing and feeding problems and disabilities) accounted for 35% of all infant deaths in 2010.
The World Health Organization (WHO) lists the United States as one of the top 10 countries with the greatest number of preterm births, despite the fact that we spend approximately 17.1% of our gross domestic product in total health care expenditures – the highest rate among our peer nations.
In the April 2014 edition of Master Class, we discussed one of the primary causes of preterm birth, bacterial infections, and specifically the need for ob.gyns. to rigorously screen patients for asymptomatic bacteriuria, which can lead to pyelonephritis. This month, we examine another biologic marker of preterm birth, cervical length.
Seminal studies of transvaginal sonography to measure cervical length during pregnancy and predict premature birth were published more than 2 decades ago. This work showed that a short cervix at 24 and 28 weeks’ gestation predicted preterm birth. Since then, clinical studies have demonstrated the utility of cervical length screening in women with prior preterm pregnancies. In the last decade, three large, randomized human trials have examined the usefulness of universal cervical length screening (Am. J. Obstet. Gynecol. 2012;207:101-6). However, the results of these trials have given practitioners a confusing picture of the predictability of this biologic marker.
Given the complexity of the “to screen or not to screen” issue, we have devoted this Master Class to a discussion on the role of cervical length screening and the prediction of preterm birth. Our guest author this month is Dr. Erika Werner, an assistant professor in ob.gyn (maternal-fetal medicine) in the department of obstetrics and gynecology at Brown University, in Providence, R.I., and an expert in the area of preterm birth.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
The benefits, costs of universal cervical length screening
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.
Rates of preterm birth in the United States have been falling since 2006, but the rates of early preterm birth in singletons (those under 34 weeks’ gestation), specifically, have not trended downward as dramatically as have late preterm birth in singletons (34-36 weeks). According to 2015 data from the National Vital Statistics Reports, the rate of early preterm births is still 3.4% in all pregnancies and 2.7% among singletons.
While the number of neonates born before 37 weeks of gestation remains high – approximately 11% in 2013 – and signifies a continuing public health problem, the rate of early preterm birth is particularly concerning because early preterm birth is more significantly associated with neonatal mortality, long-term morbidity and extended neonatal intensive care unit stays, all leading to increased health care expenditures.
Finding predictors for preterm birth that are stronger than traditional clinical factors has long been a goal of ob.gyns. because the vast majority of all spontaneous preterm births occur to women without known risk factors (i.e., multiple gestations or prior preterm birth).
Cervical length in the midtrimester is now a well-verified predictor of preterm birth, for both low- and high-risk women. Furthermore, vaginal progesterone has been shown to be a safe and beneficial intervention for women with no known risk factors who are diagnosed with a shortened cervical length (< 2 cm), and cervical cerclage has been suggested to reduce the risk of preterm birth for women with a history of prior preterm birth who also have a shortened cervical length.
Some are now advocating universal cervical length screening for women with singleton gestations, but before universal screening is mandated, the downstream effect of such a change in practice must be considered.
Backdrop to screening
Cervical length measurement was first investigated more than 25 years ago as a possible predictor of preterm birth. In 1996, a prospective multicenter study of almost 3,000 women with singleton pregnancies showed that the risk of preterm delivery is inversely and directly related to the length of the cervix, as measured with vaginal ultrasonography (N. Engl. J. Med. 1996;334:567-72).
In fact, at 24 weeks’ gestation, every 1 mm of additional cervical length equates to a significant decrease in preterm birth risk (odds ratio, 0.91). Several other studies, in addition to the landmark 1996 study, have similarly demonstrated this inverse relationship between preterm birth risk and cervical length between 18 and 24 weeks’ gestation.
However, the use of cervical measurement did not achieve widespread use until more than a decade later, when researchers began to identify interventions that could prolong pregnancy if a short cervix was diagnosed in the second trimester.
For example, Dr. E.B. Fonseca’s study of almost 25,000 asymptomatic pregnant women, demonstrated that daily vaginal progesterone reduced the risk of spontaneous delivery before 34 weeks by approximately 44% in women identified with a cervical length of 1.5 cm or less (N. Engl. J. Med. 2007;357:462-9). The vast majority of the women in this study had singleton pregnancies.
Shortly thereafter, Dr. S.S. Hassan and her colleagues completed a similar trial in women with singleton gestations and transvaginal cervical lengths between 1.0 and 2.0 cm at 20-23 weeks’ gestation. In this trial, nightly progesterone gel (with 90 mg progesterone per application) was associated with a 45% reduction in preterm birth before 33 weeks and a 38% reduction in preterm birth before 35 weeks (Ultrasound. Obstet. Gynecol. 2011;38:18-31).
A meta-analysis led by Dr. Roberto Romero, which included the Fonseca and Hassan trials, looked specifically at 775 women with a midtrimester cervical length of 2.5 cm or less. Women with a singleton gestation who had no history of preterm birth had a 40% reduction in the rate of early preterm birth when they were treated with vaginal progesterone (Am. J. Obstet. Gynecol. 2012;206:124-e1-19).
The benefits of identifying a short cervix likely extend to women with a history of prior preterm birth. A patient-level meta-analysis published in 2011 demonstrated that cervical cerclage placement was associated with a significant reduction in preterm birth before 35 weeks’ gestation in women with singleton gestations, previous spontaneous preterm birth, and cervical length less than 2.5 cm before 24 weeks’ gestation (Obstet. Gynecol. 2011;117:663-71).
The possible benefits of diagnosing and intervening for a shortened cervix have tipped many experts and clinicians toward the practice of universal cervical length screening of all singleton pregnancies. Research has shown that we can accurately obtain a cervical-length measurement before 24 weeks, and that we have effective and safe interventions for cases of short cervix: cerclage in women with a history of preterm birth who are already receiving progesterone, and vaginal progesterone in women without such a history.
Screening certainties and doubts
In 2011, my colleagues and I compared the cost effectiveness of two approaches to preterm birth prevention in low-risk pregnancies: no screening versus a single transvaginal ultrasound cervical-length measurement in all asymptomatic, low-risk singleton pregnant individuals between 18 and 24 weeks’ gestation.
In our model, women identified as having a cervical length less than 1.5 cm would be offered vaginal progesterone. Based on published data, we assumed there would be a 92% adherence rate, and a 45% reduction in deliveries before 34 weeks with progesterone treatment.
We found that in low-risk pregnancies, universal transvaginal cervical-length ultrasound screening and progesterone intervention would be cost effective and in many cases cost saving. We estimated that screening would prevent 248 early preterm births – as well as 22 neonatal deaths or neonates with long-term neurologic deficits – per 100,000 deliveries.
Our sensitivity analyses showed that screening remained cost saving under a range of clinical scenarios, including varied preterm birth rates and predictive values of a shortened cervix. Screening was not cost saving, but remained cost effective, when the expense of a transvaginal ultrasound scan exceeds $187 or when vaginal progesterone is assumed to reduce the risk of early preterm delivery by less than 20% (Ultrasound Obstet. Gynecol. 2011;38;32-37).
Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine support mandated universal transvaginal ultrasound cervical length screening. Both organizations state, however, that the approach may be considered in women with singleton gestations without prior spontaneous preterm birth.
Interestingly, Thomas Jefferson University in Philadelphia, which uses a universal screening program for singleton gestations without prior preterm birth, has recently published data that complicate the growing trend toward universal cervical length screening.
The Philadelphia clinicians followed a strategy whereby women with a transvaginal cervical length of 2 cm or less were prescribed vaginal progesterone (90 mg vaginal progesterone gel, or 200 mg micronized progesterone gel capsules). Those with a cervical length between approximately 2 cm and 2.5 cm were asked to return for a follow-up cervical length measurement before 24 weeks’ gestation.
What they found in this cohort was surprising: a rate of short cervix that is significantly lower than what previous research has shown.
Among those screened, 0.8% of women had a cervical length of 2 cm or less on an initial transvaginal ultrasonogram. Previously, a prevalence of 1%-2% for an even shorter cervical length (less than 1.5 cm) was fairly consistent in the literature.
As Dr. Kelly M. Orzechowski and her colleagues point out, the low incidence of short cervix “raises questions regarding whether universal transvaginal ultrasonogram cervical length screening in low-risk asymptomatic women is beneficial” (Obstet. Gynecol. 2014;124:520-5).
In our 2011 cost-effectiveness analysis, we found that screening was no longer a cost-saving practice when the incidence of cervical length less than 1.5 cm falls below 0.8%. Screening remained cost effective, however.
Recently, we found that if the Philadelphia protocol is followed and the U.S. population has an incidence of shortened cervix similar to that described by Dr. Orzechowski and her colleagues, universal cervical length screening in low-risk singleton pregnancies is cost effective but not cost saving. Furthermore, we found several additional plausible situations in this unpublished analysis in which universal screening ceased to be cost effective.
Thus, before we move to a strategy of mandated universal screening, we need better population-based estimates of the incidence of short cervix in a truly low-risk population.
We also must consider the future costs of progesterone. It is possible that costs may increase significantly if vaginal progesterone wins approval from the Food and Drug Administration for this indication.
Finally, if universal cervical length screening is to become the standard of care, we need policies in place to prevent misuse of the screening technology that would inevitably drive up costs without improving outcomes. For example, we must ensure that one cervical length measurement does not transition into serial cervical length measurements over the course of pregnancy, since measurement after 24 weeks has limited clinical utility. Similarly, progesterone use for a cervical length less than or equal to 2.0 cm cannot progress to progesterone for anyone approaching 2.0 cm (i.e. 2.5 cm or even 3 cm) as there is no evidence to suggest a benefit for women with longer cervixes.
Over time, it would be beneficial to have additional data on how best to manage patients who have a cervical length of 2 cm-2.5 cm before 24 weeks’ gestation. Many of us ask these women to return for a follow-up measurement and some may prescribe progesterone. However, we lack evidence for either approach; while a cervical length measurement less than 2.5 cm is clearly associated with an increased risk of preterm birth, the benefit of treatment has been demonstrated only with a cervical length of 2 cm or less.
Today and the future
For women with a history of preterm birth, cervical length screening is now routine. For low-risk pregnant women – those without a history of previous spontaneous preterm delivery – various approaches are currently taken. Most physicians recommend assessing the cervical length transabdominally at the time of the 18-20-week ultrasound, and proceeding to transvaginal ultrasonography if the cervical length is less than 3 cm or 3.5 cm.
To reliably image the cervix with transabdominal ultrasound, it should be performed with a full bladder and with the understanding that the cervix appears longer (6 mm longer, on average) when the bladder is full (Aust. N. Z. J. Obstet. Gynaecol. 2014;54:250-55).
Transvaginal ultrasound has been widely recognized as a sensitive and reproducible method for detecting shortened cervical length. Overall, this tool has several advantages over the transabdominal approach. However, the lack of universal access to transvaginal ultrasound and to consistently reliable cervical length measurements have been valid concerns of those who oppose universal transvaginal ultrasound cervical length screening.
Such concerns likely will lessen over time as transvaginal ultrasound continues to become more pervasive. Several years ago, the Perinatal Quality Foundation set standards for measuring the cervix and launched the Cervical Length Education and Review (CLEAR) program. When sonographers and physicians obtain training and credentialing, there appears to be only a 5%-10% intraobserver variability in cervical length measurement. (The PQF’s initial focus in 2005 was the Nuchal Translucency Quality Review program.)
Increasingly, I believe, transvaginal ultrasound cervical length measurement will be utilized to identify women at high risk for early preterm birth so that low-risk women can receive progesterone and high-risk women (those with a history of preterm birth) can be considered as candidates for cerclage placement. In the process, the quality of clinical care as well as the quality of our research data will improve. Whether and when such screening will become universal, however, is still uncertain.
Dr. Werner reported that she has no financial disclosures relevant to this Master Class.