User login
Onychomatricoma: A Rare Case of Unguioblastic Fibroma of the Fingernail Associated With Trauma
Onychomatricoma (OM) is a rare benign neoplasm of the nail matrix. Even less common is its possible association with both trauma to the nail apparatus and onychomycosis. This case illustrates both of these findings.
Case Report
A 72-year-old white man presented to the dermatology clinic with a 26-year history of a thickened nail plate on the right third finger that had developed soon after a baseball injury. The patient reported that the nail was completely normal prior to the trauma. According to the patient, the distal aspect of the finger was directly hit by a baseball and subsequently was wrapped by the patient for a few weeks. The nail then turned black and eventually fell off. When the nail grew back, it appeared abnormal and in its current state. The patient stated the lesion was asymptomatic at the time of presentation.
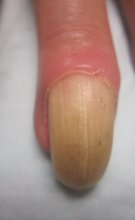
Physical examination revealed thickening, yellow discoloration, and transverse overcurvature of the nail plate on the right third finger with longitudinal ridging (Figure 1). A culture of the nail plate grew Chaetomium species. Application of topical clotrimazole for 3 months followed by a 6-week course of oral terbinafine produced no improvement. The patient then consented to a nail matrix incisional biopsy 6 months after initial presentation. After a digital nerve block was administered and a tourniquet of the proximal digit was applied, a nail avulsion was performed. Subsequently, a 3-mm punch biopsy was taken of the clinically apparent tumor in the nail matrix.
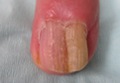
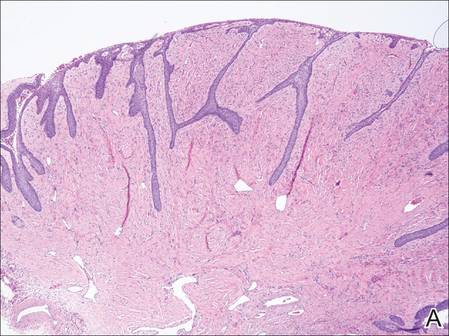
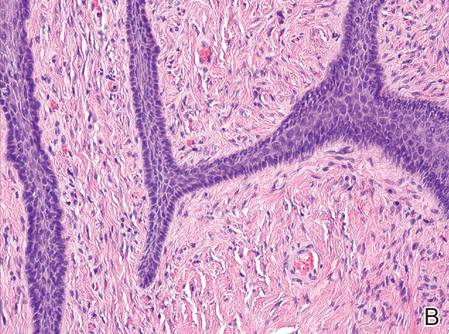
On microscopic examination of the removed tissue, a benign mixed epithelial and stromal proliferative lesion was noted. The basaloid epithelium, lacking a granular layer, arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component, which was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (Figure 2). The stromal component predominated over the epithelial component in this neoplasm. The nail was preserved in formalin and underwent hematoxylin and eosin staining. It was thickened and grossly showed filiform fibrous projections extending into the nail plate. Histologically, the nail displayed prominent oval clear channels. Periodic acid–Schiff staining was negative for fungal organisms.
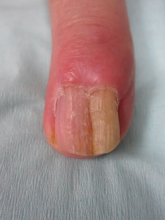
A diagnosis of unguioblastic fibroma–type OM was made. After receiving the diagnosis, expected course, and treatment options, the patient was offered conservative surgical excision but preferred clinical monitoring. At his last visit (6 months after the biopsy), the nail plate distal to the biopsy site had thinning and improvement, while the nail plate distal to the matrix that was not removed continued to show thickening, yellow discoloration, overcurvature, and longitudinal ridging (Figure 3).
| 
| |
Figure 2. The basaloid epithelium arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component (A)(H&E, original magnification ×2). At higher magnification, the stromal component was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (B)(H&E, original magnification ×10). | ||
|
|
Comment
Onychomatricoma is a rare tumor originating from the nail matrix. The tumor was first described by Baran and Kint1 in 1992 using the term onychomatrixoma, but later the term onychomatricoma became more widely used.2 Onychomatricomas are more common in adults (mean age, 48 years) and white individuals with no gender predilection.3,4 Fingernail involvement is twice as common as toenail involvement.3 Onychomatricoma is the only tumor that actively produces a nail plate.4
Clinically, OM presents with yellow discoloration along the entire nail plate and proximal splinter hemorrhages. It has a tendency toward transverse overcurvature of the nail plate with prominent longitudinal ridging.4 Trauma has been associated in at least 3 cases reported in the literature, though the association was sometimes weak.3,4 Xanthonychia and onychodystrophy of the nail are common.3 Pterygium, melanonychia, nail bleeding, and cutaneous horns have been reported but are rare.3-5 The tumor typically is painless with no radiographic bone involvement.3 Onychomycosis can be present,3 which may either be a predisposing factor for the tumor or secondary due to the deformed nail plate.4
When the nail plate is avulsed and the proximal nail fold is turned back, the matrix tumor is exposed. This polypoid and filiform tumor has characteristic fingerlike fibrokeratogenous projections extending from the nail matrix into the nail plate.3
Histologically, the tumor is fibroepithelial or biphasic with stromal and epithelial components. It has a lobulated and papillary growth pattern with 2 distinct areas that correspond to 2 anatomic zones.3 The base of the tumor corresponds to the proximal anatomic zone, which begins at the root of the nail and extends to the cuticle. This area is composed of V-shaped keratinous zones similar to the normal matrix. If the nail is removed prior to excision, these areas can be avulsed, leaving clear clefts. The superficial aspect of the tumor corresponds to the distal anatomic zone, which is located in the region of the lunula. This area is composed of multiple digitate or fingerlike projections with a fibrous core and a thick matrical epithelial covering.3 These digitations extend into small cavities in the nail plate, which can be visualized as clear channels or woodwormlike holes in hematoxylin and eosin–stained specimens. A biphasic fibrous stroma also can be observed with the superficial dermis being cellular with fibrillary collagen and the deep dermis more hypocellular with thicker collagen bundles.3,4
An analysis of keratins in the nail matrix, bed, and isthmus showed that OM has the capacity to recapitulate the entire nail unit with differentiation toward the nail bed and isthmus.6 It appears that the mesenchymal component has an inductive effect that can lead to complete epithelial onychogenic differentiation.6
Due to the histological differences among the described cases of OM in the literature, a new classification based on the spectrum of epithelial to stromal ratio of stromal cellularity and the extent of nuclear pleomorphism was proposed in 2004.7 The prominent feature of the unguioblastoma type of OM is epithelial, while the cellular stroma is the prominent feature in the unguioblastic fibroma type. Atypical unguioblastic fibroma refers to a tumor with increased mitotic activity and nuclear pleomorphism among the stroma.7
Most OM tumors follow a benign clinical course; however, complete excision is advised to include the normal nail matrix proximal to the lesion, which may prevent recurrence and serves as a primary treatment.
Conclusion
Onychomatricoma is a benign neoplasm of the nail matrix that may be triggered by trauma; however, due to the weak association, further observations and studies should be conducted to substantiate this possibility. Patients with the classic clinical presentation possibly may be spared a nail avulsion and biopsy. Onychomycosis occurs in the setting of OM, and culture and treatment are unlikely to change the appearance or course of this nail condition.
1. Baran R, Kint A. Onychomatrixoma. filamentous tufted tumour in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Haneke E, Franken J. Onychomatricoma. Dermatol Surg. 1995;21:984-987.
3. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):66-69.
4. Cañueto J, Santos-Briz Á, García JL, et al. Onychomatricoma: genome-wide analyses of a rare nail matrix tumor. J Am Acad Dermatol. 2011;64:573-578.
5. Perrin C, Baran R. Onychomatricoma with dorsalpterygium: pathogenic mechanisms in 3 cases. J Am Acad Dermatol. 2008;59:990-994.
6. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
7. Ko CJ, Shi L, Barr RJ, et al. Unguioblastoma and unguioblastic fibroma—an expanded spectrum of onychomatricoma. J Cutan Pathol. 2004;31:307-311.
Onychomatricoma (OM) is a rare benign neoplasm of the nail matrix. Even less common is its possible association with both trauma to the nail apparatus and onychomycosis. This case illustrates both of these findings.
Case Report
A 72-year-old white man presented to the dermatology clinic with a 26-year history of a thickened nail plate on the right third finger that had developed soon after a baseball injury. The patient reported that the nail was completely normal prior to the trauma. According to the patient, the distal aspect of the finger was directly hit by a baseball and subsequently was wrapped by the patient for a few weeks. The nail then turned black and eventually fell off. When the nail grew back, it appeared abnormal and in its current state. The patient stated the lesion was asymptomatic at the time of presentation.
Physical examination revealed thickening, yellow discoloration, and transverse overcurvature of the nail plate on the right third finger with longitudinal ridging (Figure 1). A culture of the nail plate grew Chaetomium species. Application of topical clotrimazole for 3 months followed by a 6-week course of oral terbinafine produced no improvement. The patient then consented to a nail matrix incisional biopsy 6 months after initial presentation. After a digital nerve block was administered and a tourniquet of the proximal digit was applied, a nail avulsion was performed. Subsequently, a 3-mm punch biopsy was taken of the clinically apparent tumor in the nail matrix.
On microscopic examination of the removed tissue, a benign mixed epithelial and stromal proliferative lesion was noted. The basaloid epithelium, lacking a granular layer, arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component, which was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (Figure 2). The stromal component predominated over the epithelial component in this neoplasm. The nail was preserved in formalin and underwent hematoxylin and eosin staining. It was thickened and grossly showed filiform fibrous projections extending into the nail plate. Histologically, the nail displayed prominent oval clear channels. Periodic acid–Schiff staining was negative for fungal organisms.
A diagnosis of unguioblastic fibroma–type OM was made. After receiving the diagnosis, expected course, and treatment options, the patient was offered conservative surgical excision but preferred clinical monitoring. At his last visit (6 months after the biopsy), the nail plate distal to the biopsy site had thinning and improvement, while the nail plate distal to the matrix that was not removed continued to show thickening, yellow discoloration, overcurvature, and longitudinal ridging (Figure 3).

| 
| |
Figure 2. The basaloid epithelium arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component (A)(H&E, original magnification ×2). At higher magnification, the stromal component was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (B)(H&E, original magnification ×10). | ||
|
|
Comment
Onychomatricoma is a rare tumor originating from the nail matrix. The tumor was first described by Baran and Kint1 in 1992 using the term onychomatrixoma, but later the term onychomatricoma became more widely used.2 Onychomatricomas are more common in adults (mean age, 48 years) and white individuals with no gender predilection.3,4 Fingernail involvement is twice as common as toenail involvement.3 Onychomatricoma is the only tumor that actively produces a nail plate.4
Clinically, OM presents with yellow discoloration along the entire nail plate and proximal splinter hemorrhages. It has a tendency toward transverse overcurvature of the nail plate with prominent longitudinal ridging.4 Trauma has been associated in at least 3 cases reported in the literature, though the association was sometimes weak.3,4 Xanthonychia and onychodystrophy of the nail are common.3 Pterygium, melanonychia, nail bleeding, and cutaneous horns have been reported but are rare.3-5 The tumor typically is painless with no radiographic bone involvement.3 Onychomycosis can be present,3 which may either be a predisposing factor for the tumor or secondary due to the deformed nail plate.4
When the nail plate is avulsed and the proximal nail fold is turned back, the matrix tumor is exposed. This polypoid and filiform tumor has characteristic fingerlike fibrokeratogenous projections extending from the nail matrix into the nail plate.3
Histologically, the tumor is fibroepithelial or biphasic with stromal and epithelial components. It has a lobulated and papillary growth pattern with 2 distinct areas that correspond to 2 anatomic zones.3 The base of the tumor corresponds to the proximal anatomic zone, which begins at the root of the nail and extends to the cuticle. This area is composed of V-shaped keratinous zones similar to the normal matrix. If the nail is removed prior to excision, these areas can be avulsed, leaving clear clefts. The superficial aspect of the tumor corresponds to the distal anatomic zone, which is located in the region of the lunula. This area is composed of multiple digitate or fingerlike projections with a fibrous core and a thick matrical epithelial covering.3 These digitations extend into small cavities in the nail plate, which can be visualized as clear channels or woodwormlike holes in hematoxylin and eosin–stained specimens. A biphasic fibrous stroma also can be observed with the superficial dermis being cellular with fibrillary collagen and the deep dermis more hypocellular with thicker collagen bundles.3,4
An analysis of keratins in the nail matrix, bed, and isthmus showed that OM has the capacity to recapitulate the entire nail unit with differentiation toward the nail bed and isthmus.6 It appears that the mesenchymal component has an inductive effect that can lead to complete epithelial onychogenic differentiation.6
Due to the histological differences among the described cases of OM in the literature, a new classification based on the spectrum of epithelial to stromal ratio of stromal cellularity and the extent of nuclear pleomorphism was proposed in 2004.7 The prominent feature of the unguioblastoma type of OM is epithelial, while the cellular stroma is the prominent feature in the unguioblastic fibroma type. Atypical unguioblastic fibroma refers to a tumor with increased mitotic activity and nuclear pleomorphism among the stroma.7
Most OM tumors follow a benign clinical course; however, complete excision is advised to include the normal nail matrix proximal to the lesion, which may prevent recurrence and serves as a primary treatment.
Conclusion
Onychomatricoma is a benign neoplasm of the nail matrix that may be triggered by trauma; however, due to the weak association, further observations and studies should be conducted to substantiate this possibility. Patients with the classic clinical presentation possibly may be spared a nail avulsion and biopsy. Onychomycosis occurs in the setting of OM, and culture and treatment are unlikely to change the appearance or course of this nail condition.
Onychomatricoma (OM) is a rare benign neoplasm of the nail matrix. Even less common is its possible association with both trauma to the nail apparatus and onychomycosis. This case illustrates both of these findings.
Case Report
A 72-year-old white man presented to the dermatology clinic with a 26-year history of a thickened nail plate on the right third finger that had developed soon after a baseball injury. The patient reported that the nail was completely normal prior to the trauma. According to the patient, the distal aspect of the finger was directly hit by a baseball and subsequently was wrapped by the patient for a few weeks. The nail then turned black and eventually fell off. When the nail grew back, it appeared abnormal and in its current state. The patient stated the lesion was asymptomatic at the time of presentation.
Physical examination revealed thickening, yellow discoloration, and transverse overcurvature of the nail plate on the right third finger with longitudinal ridging (Figure 1). A culture of the nail plate grew Chaetomium species. Application of topical clotrimazole for 3 months followed by a 6-week course of oral terbinafine produced no improvement. The patient then consented to a nail matrix incisional biopsy 6 months after initial presentation. After a digital nerve block was administered and a tourniquet of the proximal digit was applied, a nail avulsion was performed. Subsequently, a 3-mm punch biopsy was taken of the clinically apparent tumor in the nail matrix.
On microscopic examination of the removed tissue, a benign mixed epithelial and stromal proliferative lesion was noted. The basaloid epithelium, lacking a granular layer, arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component, which was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (Figure 2). The stromal component predominated over the epithelial component in this neoplasm. The nail was preserved in formalin and underwent hematoxylin and eosin staining. It was thickened and grossly showed filiform fibrous projections extending into the nail plate. Histologically, the nail displayed prominent oval clear channels. Periodic acid–Schiff staining was negative for fungal organisms.
A diagnosis of unguioblastic fibroma–type OM was made. After receiving the diagnosis, expected course, and treatment options, the patient was offered conservative surgical excision but preferred clinical monitoring. At his last visit (6 months after the biopsy), the nail plate distal to the biopsy site had thinning and improvement, while the nail plate distal to the matrix that was not removed continued to show thickening, yellow discoloration, overcurvature, and longitudinal ridging (Figure 3).

| 
| |
Figure 2. The basaloid epithelium arose from the surface epithelial layer and formed a reticulated pattern extending into the stromal component (A)(H&E, original magnification ×2). At higher magnification, the stromal component was moderately cellular with spindle to fusiform nuclei dissecting between collagen bundles arranged in parallel arrays (B)(H&E, original magnification ×10). | ||
|
|
Comment
Onychomatricoma is a rare tumor originating from the nail matrix. The tumor was first described by Baran and Kint1 in 1992 using the term onychomatrixoma, but later the term onychomatricoma became more widely used.2 Onychomatricomas are more common in adults (mean age, 48 years) and white individuals with no gender predilection.3,4 Fingernail involvement is twice as common as toenail involvement.3 Onychomatricoma is the only tumor that actively produces a nail plate.4
Clinically, OM presents with yellow discoloration along the entire nail plate and proximal splinter hemorrhages. It has a tendency toward transverse overcurvature of the nail plate with prominent longitudinal ridging.4 Trauma has been associated in at least 3 cases reported in the literature, though the association was sometimes weak.3,4 Xanthonychia and onychodystrophy of the nail are common.3 Pterygium, melanonychia, nail bleeding, and cutaneous horns have been reported but are rare.3-5 The tumor typically is painless with no radiographic bone involvement.3 Onychomycosis can be present,3 which may either be a predisposing factor for the tumor or secondary due to the deformed nail plate.4
When the nail plate is avulsed and the proximal nail fold is turned back, the matrix tumor is exposed. This polypoid and filiform tumor has characteristic fingerlike fibrokeratogenous projections extending from the nail matrix into the nail plate.3
Histologically, the tumor is fibroepithelial or biphasic with stromal and epithelial components. It has a lobulated and papillary growth pattern with 2 distinct areas that correspond to 2 anatomic zones.3 The base of the tumor corresponds to the proximal anatomic zone, which begins at the root of the nail and extends to the cuticle. This area is composed of V-shaped keratinous zones similar to the normal matrix. If the nail is removed prior to excision, these areas can be avulsed, leaving clear clefts. The superficial aspect of the tumor corresponds to the distal anatomic zone, which is located in the region of the lunula. This area is composed of multiple digitate or fingerlike projections with a fibrous core and a thick matrical epithelial covering.3 These digitations extend into small cavities in the nail plate, which can be visualized as clear channels or woodwormlike holes in hematoxylin and eosin–stained specimens. A biphasic fibrous stroma also can be observed with the superficial dermis being cellular with fibrillary collagen and the deep dermis more hypocellular with thicker collagen bundles.3,4
An analysis of keratins in the nail matrix, bed, and isthmus showed that OM has the capacity to recapitulate the entire nail unit with differentiation toward the nail bed and isthmus.6 It appears that the mesenchymal component has an inductive effect that can lead to complete epithelial onychogenic differentiation.6
Due to the histological differences among the described cases of OM in the literature, a new classification based on the spectrum of epithelial to stromal ratio of stromal cellularity and the extent of nuclear pleomorphism was proposed in 2004.7 The prominent feature of the unguioblastoma type of OM is epithelial, while the cellular stroma is the prominent feature in the unguioblastic fibroma type. Atypical unguioblastic fibroma refers to a tumor with increased mitotic activity and nuclear pleomorphism among the stroma.7
Most OM tumors follow a benign clinical course; however, complete excision is advised to include the normal nail matrix proximal to the lesion, which may prevent recurrence and serves as a primary treatment.
Conclusion
Onychomatricoma is a benign neoplasm of the nail matrix that may be triggered by trauma; however, due to the weak association, further observations and studies should be conducted to substantiate this possibility. Patients with the classic clinical presentation possibly may be spared a nail avulsion and biopsy. Onychomycosis occurs in the setting of OM, and culture and treatment are unlikely to change the appearance or course of this nail condition.
1. Baran R, Kint A. Onychomatrixoma. filamentous tufted tumour in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Haneke E, Franken J. Onychomatricoma. Dermatol Surg. 1995;21:984-987.
3. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):66-69.
4. Cañueto J, Santos-Briz Á, García JL, et al. Onychomatricoma: genome-wide analyses of a rare nail matrix tumor. J Am Acad Dermatol. 2011;64:573-578.
5. Perrin C, Baran R. Onychomatricoma with dorsalpterygium: pathogenic mechanisms in 3 cases. J Am Acad Dermatol. 2008;59:990-994.
6. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
7. Ko CJ, Shi L, Barr RJ, et al. Unguioblastoma and unguioblastic fibroma—an expanded spectrum of onychomatricoma. J Cutan Pathol. 2004;31:307-311.
1. Baran R, Kint A. Onychomatrixoma. filamentous tufted tumour in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Haneke E, Franken J. Onychomatricoma. Dermatol Surg. 1995;21:984-987.
3. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):66-69.
4. Cañueto J, Santos-Briz Á, García JL, et al. Onychomatricoma: genome-wide analyses of a rare nail matrix tumor. J Am Acad Dermatol. 2011;64:573-578.
5. Perrin C, Baran R. Onychomatricoma with dorsalpterygium: pathogenic mechanisms in 3 cases. J Am Acad Dermatol. 2008;59:990-994.
6. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
7. Ko CJ, Shi L, Barr RJ, et al. Unguioblastoma and unguioblastic fibroma—an expanded spectrum of onychomatricoma. J Cutan Pathol. 2004;31:307-311.
Practice Points
- Onychomatricoma is a rare benign neoplasm of the nail matrix that actively produces a nail plate.
- Onychomatricoma should be in the differential diagnosis of a thickened discolored nail plate with transverse overcurvature.
- Onychomatricoma has been associated with onychomycosis and trauma to the nail apparatus.
Uterus transplant update: Fungal infection may have caused complication
The failure of the first U.S. uterus transplant may have been due a complication triggered by an infection of Candida, according to the Cleveland Clinic.
“Preliminary results suggest that the complication was due to an infection caused by an organism that is commonly found in a woman’s reproductive system,” officials at the Cleveland Clinic said in an April 8 statement. “The infection appears to have compromised the blood supply to the uterus, causing the need for its removal. There is an ongoing review of all the data and the team is modifying the protocol to reduce the chances of this complication occurring again in the future. The health of our patient is and has always been our primary concern.”
A team of surgeons at the Cleveland Clinic performed the first U.S. uterus transplant on a 26-year-old woman with uterine factor infertility on Feb. 24, but had to remove the transplanted uterus several days later following a sudden complication. The transplant is part of a study aimed at achieving pregnancy in women with uterine factor infertility. The study is still ongoing.
On Twitter @maryellenny
The failure of the first U.S. uterus transplant may have been due a complication triggered by an infection of Candida, according to the Cleveland Clinic.
“Preliminary results suggest that the complication was due to an infection caused by an organism that is commonly found in a woman’s reproductive system,” officials at the Cleveland Clinic said in an April 8 statement. “The infection appears to have compromised the blood supply to the uterus, causing the need for its removal. There is an ongoing review of all the data and the team is modifying the protocol to reduce the chances of this complication occurring again in the future. The health of our patient is and has always been our primary concern.”
A team of surgeons at the Cleveland Clinic performed the first U.S. uterus transplant on a 26-year-old woman with uterine factor infertility on Feb. 24, but had to remove the transplanted uterus several days later following a sudden complication. The transplant is part of a study aimed at achieving pregnancy in women with uterine factor infertility. The study is still ongoing.
On Twitter @maryellenny
The failure of the first U.S. uterus transplant may have been due a complication triggered by an infection of Candida, according to the Cleveland Clinic.
“Preliminary results suggest that the complication was due to an infection caused by an organism that is commonly found in a woman’s reproductive system,” officials at the Cleveland Clinic said in an April 8 statement. “The infection appears to have compromised the blood supply to the uterus, causing the need for its removal. There is an ongoing review of all the data and the team is modifying the protocol to reduce the chances of this complication occurring again in the future. The health of our patient is and has always been our primary concern.”
A team of surgeons at the Cleveland Clinic performed the first U.S. uterus transplant on a 26-year-old woman with uterine factor infertility on Feb. 24, but had to remove the transplanted uterus several days later following a sudden complication. The transplant is part of a study aimed at achieving pregnancy in women with uterine factor infertility. The study is still ongoing.
On Twitter @maryellenny
VIDEO: More routine use of unilateral thyroidectomy advocated for papillary thyroid microcarcinoma
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ENDO 2016
Key clinical point: Unilateral thryoidectomy should be reconsidered as a routine strategy in treatment of papillary thyroid microcarcinoma.
Major finding: Data compiled from over 80 years at a single institution indicates the value of unilateral thyroidectomy in terms of recurrence and morbidity.
Data source: Retrospective analysis of data from 1,153 adult patients.
Disclosures: Dr. Hay had no disclosures.
New vulvar cancer guidelines stress regional disease control
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network has issued new guidelines for the diagnosis and management of vulvar cancer.
Vulvar cancers are rare neoplasms, with an estimated U.S. annual incidence of 5,950 cases, and 1,110 deaths. The majority of cases (about 90%) are of squamous cell histology.
Treatment of vulvar cancer has evolved from en bloc resections used throughout most of the 20th century, to more refined techniques, said Dr. Benjamin E. Greer, professor of gynecological oncology at the University of Washington in Seattle.
“In the 1980s, we started to modify treatment to reduce morbidity,” he said at the annual conference of the National Comprehensive Cancer Network.
With older, more radical techniques, groin breakdown, leg edema, and impaired sexual function were common post-surgery consequences. Current practice, however, is to perform regional lymph node management for unilateral cancers, radical local excision rather than en bloc resections, separate groin incisions, lymphatic mapping, radiation, chemotherapy, and, if necessary, exenteration, Dr. Greer noted.
The guidelines note that adequate surgical margins – 1 to 2 cm – at the time of primary surgery appear to be essential for reducing risk of local recurrence, and that if margins are within 8 mm of tumor, the surgeon should consider re-excision or adjuvant radiation.
Lymph node status is the most important determinant of survival, with historical reports showing overall survival following surgery of 70% to 80% among patients with negative nodes, compared with 30% to 40% of those with positive nodes, he said.
Evaluation of bilateral inguinofemoral groin nodes should be performed in patients with lesions in the vulvar midline, and ipsilateral groin node evaluation should be performed for those with lateral lesions lying more than 2 cm from the vulvar midline. Additionally, select patients may require sentinel lymph node biopsy, the guidelines state.
Unilateral carcinomas of the vulva can be treated with limited radical vulvectomy and ipsilateral inguinal femoral node dissection. Lymph node dissection can be performed through a separate incision. For patients with positive nodes, adjuvant radiation may aid in disease control. Patients with inoperable carcinomas are recommended to receive radiation and chemotherapy.
Radiation for vulvar cancer
“For early stage tumors, adjuvant radiotherapy is an effective treatment modality that significantly decreases recurrence, especially in surgically resected groins, and it leads to improvement in relapse-free and overall survival,” said Dr. Wui-Jin Koh, medical director for radiation oncology at the Fred Hutchinson Cancer Research Center in Seattle.
Concurrent chemotherapy and radiation may provide additional therapeutic benefit, especially for patients with advanced, unresectable tumors, and it may help to address systemic risk in patients with multiple positive lymph nodes, Dr. Koh said.
The guidelines state that radiation can be given with external beam radiation delivered via a 3D-conformal or intensity modulated (IMRT) technique, with brachytherapy boost for some tumors where the anatomy permits.
“Careful attention should be taken to ensure adequate tumor coverage by combining clinical examination, imaging findings, and appropriate nodal volumes at risk to define the target volume,” the guideline states.
For adjuvant therapy, doses of 50.4 Gy divided in 1.8 Gy fractions should be delivered once daily 5 days per week, with minimal treatment breaks.
For treatment of unresectable tumors, doses range from 59.4 Gy to 64.8 Gy in 1.8 Gy fractions, with a boost dose to approximately 70 Gy for large lymph nodes in select cases.
Residual disease
The decision to provide additional treatment following surgery is based on whether the patient is clinically negative for residual tumor at the primary site and nodes.
“If one has negative margins and negative nodes? Observation, absolutely,” Dr. Koh said. “If one has positive margins for invasive disease, our recommendation is to re-excise and not go straight to radiation, and if one can do it and get negative margins, again observe the majority of them.”
“Use radiation very judiciously,” he added. “Only if patients have positive margins or have unresectable primary disease do we routinely recommend radiation.”
Locally advanced disease
For patients who cannot be treated with conventional or sphincter-sparing, organ preserving surgery upfront, the recommendation is to provide chemoradiation, with initial radiation to the primary site, groins, and pelvis, and concurrent week cisplatin at a dose of 30-40 mg/m2 per week. The recommended radiation doses are 45 Gy to at-risk, microscopic clinical tumor volume, and 57.6 to 60 Gy to gross tumor volume (primary site and nodes).
“If one uses IMRT, you need to be very generous with the volumes,” Dr. Koh said.
The panelists also recommend re-imaging and re-evaluating patients 6 to 8 weeks after the completion of chemoradiation, with possible resection or biopsy of the primary tumor site, and limited groin resection of imaged residual disease.
For patients with clearly node-positive disease, “my general preference is to give upfront chemoradiation therapy to avoid delay of primary therapy, and then resect residual nodes after the chemoradiation is done,” he said.
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network has issued new guidelines for the diagnosis and management of vulvar cancer.
Vulvar cancers are rare neoplasms, with an estimated U.S. annual incidence of 5,950 cases, and 1,110 deaths. The majority of cases (about 90%) are of squamous cell histology.
Treatment of vulvar cancer has evolved from en bloc resections used throughout most of the 20th century, to more refined techniques, said Dr. Benjamin E. Greer, professor of gynecological oncology at the University of Washington in Seattle.
“In the 1980s, we started to modify treatment to reduce morbidity,” he said at the annual conference of the National Comprehensive Cancer Network.
With older, more radical techniques, groin breakdown, leg edema, and impaired sexual function were common post-surgery consequences. Current practice, however, is to perform regional lymph node management for unilateral cancers, radical local excision rather than en bloc resections, separate groin incisions, lymphatic mapping, radiation, chemotherapy, and, if necessary, exenteration, Dr. Greer noted.
The guidelines note that adequate surgical margins – 1 to 2 cm – at the time of primary surgery appear to be essential for reducing risk of local recurrence, and that if margins are within 8 mm of tumor, the surgeon should consider re-excision or adjuvant radiation.
Lymph node status is the most important determinant of survival, with historical reports showing overall survival following surgery of 70% to 80% among patients with negative nodes, compared with 30% to 40% of those with positive nodes, he said.
Evaluation of bilateral inguinofemoral groin nodes should be performed in patients with lesions in the vulvar midline, and ipsilateral groin node evaluation should be performed for those with lateral lesions lying more than 2 cm from the vulvar midline. Additionally, select patients may require sentinel lymph node biopsy, the guidelines state.
Unilateral carcinomas of the vulva can be treated with limited radical vulvectomy and ipsilateral inguinal femoral node dissection. Lymph node dissection can be performed through a separate incision. For patients with positive nodes, adjuvant radiation may aid in disease control. Patients with inoperable carcinomas are recommended to receive radiation and chemotherapy.
Radiation for vulvar cancer
“For early stage tumors, adjuvant radiotherapy is an effective treatment modality that significantly decreases recurrence, especially in surgically resected groins, and it leads to improvement in relapse-free and overall survival,” said Dr. Wui-Jin Koh, medical director for radiation oncology at the Fred Hutchinson Cancer Research Center in Seattle.
Concurrent chemotherapy and radiation may provide additional therapeutic benefit, especially for patients with advanced, unresectable tumors, and it may help to address systemic risk in patients with multiple positive lymph nodes, Dr. Koh said.
The guidelines state that radiation can be given with external beam radiation delivered via a 3D-conformal or intensity modulated (IMRT) technique, with brachytherapy boost for some tumors where the anatomy permits.
“Careful attention should be taken to ensure adequate tumor coverage by combining clinical examination, imaging findings, and appropriate nodal volumes at risk to define the target volume,” the guideline states.
For adjuvant therapy, doses of 50.4 Gy divided in 1.8 Gy fractions should be delivered once daily 5 days per week, with minimal treatment breaks.
For treatment of unresectable tumors, doses range from 59.4 Gy to 64.8 Gy in 1.8 Gy fractions, with a boost dose to approximately 70 Gy for large lymph nodes in select cases.
Residual disease
The decision to provide additional treatment following surgery is based on whether the patient is clinically negative for residual tumor at the primary site and nodes.
“If one has negative margins and negative nodes? Observation, absolutely,” Dr. Koh said. “If one has positive margins for invasive disease, our recommendation is to re-excise and not go straight to radiation, and if one can do it and get negative margins, again observe the majority of them.”
“Use radiation very judiciously,” he added. “Only if patients have positive margins or have unresectable primary disease do we routinely recommend radiation.”
Locally advanced disease
For patients who cannot be treated with conventional or sphincter-sparing, organ preserving surgery upfront, the recommendation is to provide chemoradiation, with initial radiation to the primary site, groins, and pelvis, and concurrent week cisplatin at a dose of 30-40 mg/m2 per week. The recommended radiation doses are 45 Gy to at-risk, microscopic clinical tumor volume, and 57.6 to 60 Gy to gross tumor volume (primary site and nodes).
“If one uses IMRT, you need to be very generous with the volumes,” Dr. Koh said.
The panelists also recommend re-imaging and re-evaluating patients 6 to 8 weeks after the completion of chemoradiation, with possible resection or biopsy of the primary tumor site, and limited groin resection of imaged residual disease.
For patients with clearly node-positive disease, “my general preference is to give upfront chemoradiation therapy to avoid delay of primary therapy, and then resect residual nodes after the chemoradiation is done,” he said.
HOLLYWOOD, FLA. – The National Comprehensive Cancer Network has issued new guidelines for the diagnosis and management of vulvar cancer.
Vulvar cancers are rare neoplasms, with an estimated U.S. annual incidence of 5,950 cases, and 1,110 deaths. The majority of cases (about 90%) are of squamous cell histology.
Treatment of vulvar cancer has evolved from en bloc resections used throughout most of the 20th century, to more refined techniques, said Dr. Benjamin E. Greer, professor of gynecological oncology at the University of Washington in Seattle.
“In the 1980s, we started to modify treatment to reduce morbidity,” he said at the annual conference of the National Comprehensive Cancer Network.
With older, more radical techniques, groin breakdown, leg edema, and impaired sexual function were common post-surgery consequences. Current practice, however, is to perform regional lymph node management for unilateral cancers, radical local excision rather than en bloc resections, separate groin incisions, lymphatic mapping, radiation, chemotherapy, and, if necessary, exenteration, Dr. Greer noted.
The guidelines note that adequate surgical margins – 1 to 2 cm – at the time of primary surgery appear to be essential for reducing risk of local recurrence, and that if margins are within 8 mm of tumor, the surgeon should consider re-excision or adjuvant radiation.
Lymph node status is the most important determinant of survival, with historical reports showing overall survival following surgery of 70% to 80% among patients with negative nodes, compared with 30% to 40% of those with positive nodes, he said.
Evaluation of bilateral inguinofemoral groin nodes should be performed in patients with lesions in the vulvar midline, and ipsilateral groin node evaluation should be performed for those with lateral lesions lying more than 2 cm from the vulvar midline. Additionally, select patients may require sentinel lymph node biopsy, the guidelines state.
Unilateral carcinomas of the vulva can be treated with limited radical vulvectomy and ipsilateral inguinal femoral node dissection. Lymph node dissection can be performed through a separate incision. For patients with positive nodes, adjuvant radiation may aid in disease control. Patients with inoperable carcinomas are recommended to receive radiation and chemotherapy.
Radiation for vulvar cancer
“For early stage tumors, adjuvant radiotherapy is an effective treatment modality that significantly decreases recurrence, especially in surgically resected groins, and it leads to improvement in relapse-free and overall survival,” said Dr. Wui-Jin Koh, medical director for radiation oncology at the Fred Hutchinson Cancer Research Center in Seattle.
Concurrent chemotherapy and radiation may provide additional therapeutic benefit, especially for patients with advanced, unresectable tumors, and it may help to address systemic risk in patients with multiple positive lymph nodes, Dr. Koh said.
The guidelines state that radiation can be given with external beam radiation delivered via a 3D-conformal or intensity modulated (IMRT) technique, with brachytherapy boost for some tumors where the anatomy permits.
“Careful attention should be taken to ensure adequate tumor coverage by combining clinical examination, imaging findings, and appropriate nodal volumes at risk to define the target volume,” the guideline states.
For adjuvant therapy, doses of 50.4 Gy divided in 1.8 Gy fractions should be delivered once daily 5 days per week, with minimal treatment breaks.
For treatment of unresectable tumors, doses range from 59.4 Gy to 64.8 Gy in 1.8 Gy fractions, with a boost dose to approximately 70 Gy for large lymph nodes in select cases.
Residual disease
The decision to provide additional treatment following surgery is based on whether the patient is clinically negative for residual tumor at the primary site and nodes.
“If one has negative margins and negative nodes? Observation, absolutely,” Dr. Koh said. “If one has positive margins for invasive disease, our recommendation is to re-excise and not go straight to radiation, and if one can do it and get negative margins, again observe the majority of them.”
“Use radiation very judiciously,” he added. “Only if patients have positive margins or have unresectable primary disease do we routinely recommend radiation.”
Locally advanced disease
For patients who cannot be treated with conventional or sphincter-sparing, organ preserving surgery upfront, the recommendation is to provide chemoradiation, with initial radiation to the primary site, groins, and pelvis, and concurrent week cisplatin at a dose of 30-40 mg/m2 per week. The recommended radiation doses are 45 Gy to at-risk, microscopic clinical tumor volume, and 57.6 to 60 Gy to gross tumor volume (primary site and nodes).
“If one uses IMRT, you need to be very generous with the volumes,” Dr. Koh said.
The panelists also recommend re-imaging and re-evaluating patients 6 to 8 weeks after the completion of chemoradiation, with possible resection or biopsy of the primary tumor site, and limited groin resection of imaged residual disease.
For patients with clearly node-positive disease, “my general preference is to give upfront chemoradiation therapy to avoid delay of primary therapy, and then resect residual nodes after the chemoradiation is done,” he said.
AT THE NCCN ANNUAL CONFERENCE
Key clinical point: Nodal status is an important determinant of survival of patients with vulvar carcinomas.
Major finding: Historically, reported overall survival following surgery is 70% to 80% among patients with negative nodes, compared with 30% to 40% of those with positive nodes.
Data source: Review of new clinical guidelines for the management of patients with vulvar cancer.
Disclosures: Dr. Greer and Dr. Koh reported having no relevant clinical disclosures.
WATCH: Why Teaching Hospital Medicine Can Be a Rewarding Career
Two academic hospitalists talk about why they teach, what they're learning from their students, and what they see as the future of hospital medicine. Since academic HM is the new-hospitalist pipeline, hearing what they're seeing in their student and resident trainee corps is a snapshot of HM's sustainability.
Two academic hospitalists talk about why they teach, what they're learning from their students, and what they see as the future of hospital medicine. Since academic HM is the new-hospitalist pipeline, hearing what they're seeing in their student and resident trainee corps is a snapshot of HM's sustainability.
Two academic hospitalists talk about why they teach, what they're learning from their students, and what they see as the future of hospital medicine. Since academic HM is the new-hospitalist pipeline, hearing what they're seeing in their student and resident trainee corps is a snapshot of HM's sustainability.
Less symptomatic patients ‘worse off’ after knee surgery
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AMSTERDAM – Patients with milder knee osteoarthritis symptoms or better quality of life before undergoing total knee replacement surgery gained less benefit from the surgery than did those who had more severe symptoms in two separate analyses of British and U.S. patients.
Additional evidence from total knee replacements (TKRs) performed on U.S. participants of the Osteoarthritis Initiative also suggest that as the use of TKR has increased to include less symptomatic patients, the overall cost-effectiveness of the procedure has declined.
“Knee replacements are one of those interventions that are known to be very effective and very cost-effective,” Rafael Pinedo-Villanueva, Ph.D., of the University of Oxford (England) said during his presentation of National Health Service data from England at the World Congress on Osteoarthritis. Indeed, knee replacements are associated with significant improvements in pain, function, and quality of life, he said, but that is if you look at the mean values.
As the deciles for baseline knee pain and function decrease in severity, there are diminishing mean improvements and an increasing proportion of patients who do worse after the operation, he reported. Up to 17% of patients had unchanged or improved knee pain scores and up to 27% had lower quality of life scores. If minimally important differences were considered, these percentages rose to 40% and 48% of patients being worse off, respectively.
“The significant improvements seem to be overshadowing what happens to those patients who are doing worse,” Dr. Pinedo-Villanueva suggested. “So essentially cost-effectiveness is being driven by the magnitude of the change in those who do improve, and we don’t really see much about what is happening to those who are doing worse.”
Of over 215,000 records of knee replacement collected from all patients undergoing TKR in England during 2008-2012, Dr. Pinedo-Villanueva and his study coauthors found 117,844 had data on pre- and post-operative knee pain assessed using the Oxford Knee Score (OKS) and quality of life measured with the EQ-5D instrument. The majority of replacements were in women (55%) and almost three quarters of patients had one or no comorbidities. Overall, the mean change in OKS was 15 points, improving from 19 to 34 (the higher the score the lesser the knee pain). EQ-5D scores also improved by a mean difference of 0.30 (from 0.41 to 0.70 where 1.0 is perfect health). Although the vast majority of patients had improved OKS and EQ-5D scores after surgery, unchanged or decreased scores were seen in 8% and 22% of patients, respectively.
“As we breakdown these data by deciles of baseline pain and function we see clearly that those starting at the lower decile improved the most, and that’s to be expected; they’ve a lot more to improve than the ones that came into the operation at the higher decile,” Dr. Pinedo-Villanueva said at the Congress, sponsored by the Osteoarthritis Research Society International. But there were patients who fared worse at every decile, he noted.
Dr. Pinedo-Villanueva concluded that outcome prediction models were needed to try to reduce the number of patients who are apparently worse off after knee replacement and improve the efficiency of resource allocation.
The value of TKR in a contemporary U.S. population was the focus of a separate presentation by Dr. Bart Ferket of Mount Sinai Hospital in New York. Dr. Ferket reported the results of a study looking at the impact of TKR on patients’ quality of life, lifetime costs, and quality-adjusted life years (QALYs) while varying the use of TKR by patients’ functional status at baseline.
“In the United States, the rate at which total knee replacement is performed has doubled in the last two decades,” Dr. Ferket observed. This “disproportionate” increase has been attributed to expanding the eligibility criteria to include less symptomatic patients.
Using data collected over an 8-year period on 1,327 participants from the Osteoarthritis Initiative, Dr. Ferket and his associates at Mount Sinai and Erasmus University Medical Center in Rotterdam (The Netherlands) discovered that the increased uptake of TKR might have affected the likely benefit and reduced the overall cost-effectiveness of the procedure.
At baseline, 17% of the participants, who all had knee osteoarthritis, had had a prior knee replacement.
Quality of life measured on the physical component scores (PCS) of the 12-item Short Form (SF-12) were generally improved after TKR but decreased in those who did not have a knee replaced. The effect on the mental component of the SF-12 was less clear, with possibly a decrease seen in some patients. Changes on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) showed a considerable benefit for knee replacement and there was a general decrease in pain medication over time in those who had surgery. The overall effect was more pronounced if patients with greater baseline symptoms were considered.
Cost-effectiveness modeling showed that reserving TKR for more seriously affected patients may make it more economically attractive. The QALYs gained from TKR was about 11 but as the number of QALYs increased, so did the relative lifetime cost, with increasing incremental cost-effectiveness ratios (ICERs) as SF-12 PCS rose. ICERs were around $143,000, $160,000, $217,000, $385,000, and $1,175,000 considering patients with SF-12 PCS of less than 30, 35, 40, 45, and 50, respectively.
“The more lenient the eligibility criteria are, the higher the effectiveness, but also the higher the costs,” Dr. Ferket said. “The most cost-effective scenarios are actually more restrictive that what is currently seen in current practice in the U.S.”
Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
AT OARSI 2016
Key clinical point: Patients with milder osteoarthritis can fare worse after knee replacement surgery, and the cost-effectiveness of the procedure is lower.
Major finding: Knee pain and quality of life scores were unchanged or worse after the operation in 8% and 22% of patients, respectively.
Data source: Two separate studies looking at the value of knee replacement in patients with knee osteoarthritis.
Disclosures: Dr. Pinedo-Villanueva and Dr. Ferket reported having no financial disclosures.
Inhibitor could overcome TKI resistance in Ph+ B-ALL

Results of preclinical research indicate that combining 2 kinase inhibitors may be a promising treatment strategy for Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL).
Researchers found that combining a tyrosine kinase inhibitor (TKI) and an inhibitor of focal adhesion kinase (FAK) was “remarkably effective” against Ph+ B-ALL in vitro and in vivo.
The TKI dasatinib and the FAK inhibitor VS-4718 decreased leukemic cell survival and adhesion, inhibited tumor growth, and prolonged survival in mouse models of Ph+ B-ALL.
Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their results in JCI Insight.
The researchers noted that patients with Ph+ ALL have shown resistance to TKI therapy, and this resistance has been tied to alterations in IKZF1.
As FAK expression is elevated in IKZF1-mutated leukemias, the team speculated that adding a FAK inhibitor to TKI therapy might lead to better results.
First, the researchers set out to confirm that FAK is overexpressed in Ph+ B-ALL. Their experiments revealed upregulation of the FAK pathway in Ph+ B-ALL cells, with further overexpression of FAK in IKZF1-mutated Ph+ B-ALL cells.
When they inhibited FAK with VS-4718, the team observed decreases in the survival, clonogenicity, and adhesion of IKZF1-mutated Ph+ B-ALL cells from both mice and humans.
Next, the researchers found that VS-4718 synergizes with the TKI dasatinib in vitro and in vivo.
In in vitro experiments with both mouse and human Ph+ B-ALL cells, the combination decreased cell survival and adhesion and inhibited downstream targets of FAK.
In mouse models of Ph+ B-ALL, VS-4718 proved ineffective when given alone.
However, the researchers said the combination of VS-4718 and dasatinib “dramatically” decreased leukemic burden and extended the lives of mice.
In fact, 1 long-term survivor achieved a complete remission that endured after treatment was stopped.
The researchers said these results suggest that targeting FAK with VS-4718 can overcome the deleterious effects of FAK overexpression in Ph+ B-ALL, potentiating responsiveness to TKIs. ![]()

Results of preclinical research indicate that combining 2 kinase inhibitors may be a promising treatment strategy for Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL).
Researchers found that combining a tyrosine kinase inhibitor (TKI) and an inhibitor of focal adhesion kinase (FAK) was “remarkably effective” against Ph+ B-ALL in vitro and in vivo.
The TKI dasatinib and the FAK inhibitor VS-4718 decreased leukemic cell survival and adhesion, inhibited tumor growth, and prolonged survival in mouse models of Ph+ B-ALL.
Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their results in JCI Insight.
The researchers noted that patients with Ph+ ALL have shown resistance to TKI therapy, and this resistance has been tied to alterations in IKZF1.
As FAK expression is elevated in IKZF1-mutated leukemias, the team speculated that adding a FAK inhibitor to TKI therapy might lead to better results.
First, the researchers set out to confirm that FAK is overexpressed in Ph+ B-ALL. Their experiments revealed upregulation of the FAK pathway in Ph+ B-ALL cells, with further overexpression of FAK in IKZF1-mutated Ph+ B-ALL cells.
When they inhibited FAK with VS-4718, the team observed decreases in the survival, clonogenicity, and adhesion of IKZF1-mutated Ph+ B-ALL cells from both mice and humans.
Next, the researchers found that VS-4718 synergizes with the TKI dasatinib in vitro and in vivo.
In in vitro experiments with both mouse and human Ph+ B-ALL cells, the combination decreased cell survival and adhesion and inhibited downstream targets of FAK.
In mouse models of Ph+ B-ALL, VS-4718 proved ineffective when given alone.
However, the researchers said the combination of VS-4718 and dasatinib “dramatically” decreased leukemic burden and extended the lives of mice.
In fact, 1 long-term survivor achieved a complete remission that endured after treatment was stopped.
The researchers said these results suggest that targeting FAK with VS-4718 can overcome the deleterious effects of FAK overexpression in Ph+ B-ALL, potentiating responsiveness to TKIs. ![]()

Results of preclinical research indicate that combining 2 kinase inhibitors may be a promising treatment strategy for Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia (Ph+ B-ALL).
Researchers found that combining a tyrosine kinase inhibitor (TKI) and an inhibitor of focal adhesion kinase (FAK) was “remarkably effective” against Ph+ B-ALL in vitro and in vivo.
The TKI dasatinib and the FAK inhibitor VS-4718 decreased leukemic cell survival and adhesion, inhibited tumor growth, and prolonged survival in mouse models of Ph+ B-ALL.
Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their results in JCI Insight.
The researchers noted that patients with Ph+ ALL have shown resistance to TKI therapy, and this resistance has been tied to alterations in IKZF1.
As FAK expression is elevated in IKZF1-mutated leukemias, the team speculated that adding a FAK inhibitor to TKI therapy might lead to better results.
First, the researchers set out to confirm that FAK is overexpressed in Ph+ B-ALL. Their experiments revealed upregulation of the FAK pathway in Ph+ B-ALL cells, with further overexpression of FAK in IKZF1-mutated Ph+ B-ALL cells.
When they inhibited FAK with VS-4718, the team observed decreases in the survival, clonogenicity, and adhesion of IKZF1-mutated Ph+ B-ALL cells from both mice and humans.
Next, the researchers found that VS-4718 synergizes with the TKI dasatinib in vitro and in vivo.
In in vitro experiments with both mouse and human Ph+ B-ALL cells, the combination decreased cell survival and adhesion and inhibited downstream targets of FAK.
In mouse models of Ph+ B-ALL, VS-4718 proved ineffective when given alone.
However, the researchers said the combination of VS-4718 and dasatinib “dramatically” decreased leukemic burden and extended the lives of mice.
In fact, 1 long-term survivor achieved a complete remission that endured after treatment was stopped.
The researchers said these results suggest that targeting FAK with VS-4718 can overcome the deleterious effects of FAK overexpression in Ph+ B-ALL, potentiating responsiveness to TKIs. ![]()
Postoperative Clostridium Difficile Infection Associated with Number of Antibiotics, Surgical Procedure Complexity
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Clinical question: What are the factors that increase risk of Clostridium difficile infection (CDI) in postoperative patients?
Background: CDI has become an important infectious etiology for morbidity, lengthy and costly hospital admissions, and mortality. This study focused on the risks for postoperative patients to be infected with C. diff. Awareness of the risk factors for CDI allows for processes to be implemented that can decrease the rate of infection.
Study design: Retrospective, observational study.
Setting: Multiple Veterans Health Administration surgery programs.
Synopsis: The study investigated 468,386 surgical procedures in 134 surgical programs in 12 subspecialties over a four-year period. Overall, the postoperative CDI rate was 0.4% per year. Rates were higher in emergency or complex procedures, older patients, patients with longer preoperative hospital stays, and those who received three or more classes of antibiotics. CDI in postoperative patients was associated with five times higher risk of mortality, a 12 times higher risk of morbidity, and longer hospital stays (17.9 versus 3.6 days) compared with those without CDI. Further studies with a larger population size will confirm the findings of this study.
The study was conducted on middle-aged to elderly male veterans, and it can only be assumed that these results will translate to other populations. Nevertheless, CDI can lead to significant morbidity and mortality, and the study reinforces the importance of infection control and prevention to reduce CDI incidence and disease severity.
Bottom line: Postoperative CDI is significantly associated with the number of postoperative antibiotics, surgical procedure complexity, preoperative length of stay, and patient comorbidities.
Citation: Li X, Wilson M, Nylander W, Smith T, Lynn M, Gunnar W. Analysis of morbidity and mortality outcomes in postoperative Clostridium difficile infection in the Veterans Health Administration. JAMA Surg. 2015;25:1-9.
Most Postoperative Readmissions Due to Patient Factors
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
Clinical question: What is the etiology of 30-day readmissions in postoperative patients?
Background: As the focus of healthcare changes to a quality-focused model, readmissions impact physicians, reimbursements, and patients. Understanding the cause of readmissions becomes essential to preventing them. The etiology of 30-day readmissions in postoperative patients has not specifically been studied.
Study design: Retrospective analysis.
Setting: Academic tertiary-care center.
Synopsis: Using administrative claims data, an analysis of 22,559 patients who underwent a major surgical procedure between 2009 and 2013 was performed. A total of 56 surgeons within eight surgical subspecialties were analyzed, showing that variation in 30-day readmissions was largely due to patient-specific factors (82.8%) while only a minority were attributable to surgical subspecialty (14.5%) and individual surgeon levels (2.8%). Factors associated with readmission included race/ethnicity, comorbidities, postoperative complications, and extended length of stay.
Further studies within this area will need to be conducted focusing on one specific subspecialty and one surgeon to exclude confounding factors. Additional meta-analysis can then compare these individual studies. A larger population and multiple care centers will also further validate the findings. Understanding the cause of the readmissions in postoperative patients can prevent further readmissions, improve quality of care, and decrease healthcare costs. If patient factors are identified as a major cause for readmissions in postoperative patients, changes in preoperative management may need to be made.
Bottom line: Postoperative readmissions are more dependent on patient factors than surgeon- or surgical subspecialty-specific factors.
Citation: Gani F, Lucas DJ, Kim Y, Schneider EB, Pawlik TM. Understanding variation in 30-day surgical readmission in the era of accountable care: effect of the patient, surgeon, and surgical subspecialties. JAMA Surg. 2015;150(11):1042-1049.
U.S. Hospitals Should Prepare for "ransomeware" Attacks by Cyber Criminals
(Reuters) - U.S. hospitals should brace for a surge in "ransomware" attacks by cyber criminals who infect and shut down computer networks, then demand payment in return for unlocking them, a non-profit healthcare group warned on Friday.
The Health Information Trust Alliance conducted a study of some 30 mid-sized U.S. hospitals late last year and found that 52 percent of them were infected with malicious software, HITRUST Chief Executive Daniel Nutkis told Reuters.
The most common type of malware was ransomware, Nutkis said, which was present in 35 percent of the hospitals included in the study of network traffic conducted by security software maker Trend Micro Inc.
Ransomware is malicious software that locks up data in computers and leaves messages demanding payment to recover the data. Last month, Hollywood Presbyterian Hospital in Los Angeles paid a ransom of $17,000 to regain access to its systems.
This week, an attack on MedStar Health forced the largest healthcare provider in Washington, D.C., to shut down much of its computer network. The Baltimore Sun reported a ransom of $18,500 was sought. MedStar declined to comment.
HITRUST said it expects such attacks to become more frequent because ransomware has turned into a profitable business for cyber criminals.
The results of the study, which HITRUST has yet to share with the public, demonstrate that hackers have moved away from focusing on stealing patient data, Nutkis said.
"If stuff isn't working, they move on. If stuff is working, they keep doing it," said Nutkis. "Organizations that are paying have considered their options, and unfortunately they don't have a lot of options."
Extortion has become more popular with cyber criminals because it is seen as a way to generate fast money, said Larry Whiteside, a healthcare expert with cyber security firm Optiv.
Stealing healthcare data is far more labour intensive, requiring attackers to keep their presence in a victim's network undetected for months as they steal data, then they need to find buyers, he added.
"With ransomware I'm going to get paid immediately," Whiteside said.
Frisco, Texas-based HITRUST's board includes executives from Anthem, Health Care Services, Humana, UnitedHealth and Walgreens.
(Reuters) - U.S. hospitals should brace for a surge in "ransomware" attacks by cyber criminals who infect and shut down computer networks, then demand payment in return for unlocking them, a non-profit healthcare group warned on Friday.
The Health Information Trust Alliance conducted a study of some 30 mid-sized U.S. hospitals late last year and found that 52 percent of them were infected with malicious software, HITRUST Chief Executive Daniel Nutkis told Reuters.
The most common type of malware was ransomware, Nutkis said, which was present in 35 percent of the hospitals included in the study of network traffic conducted by security software maker Trend Micro Inc.
Ransomware is malicious software that locks up data in computers and leaves messages demanding payment to recover the data. Last month, Hollywood Presbyterian Hospital in Los Angeles paid a ransom of $17,000 to regain access to its systems.
This week, an attack on MedStar Health forced the largest healthcare provider in Washington, D.C., to shut down much of its computer network. The Baltimore Sun reported a ransom of $18,500 was sought. MedStar declined to comment.
HITRUST said it expects such attacks to become more frequent because ransomware has turned into a profitable business for cyber criminals.
The results of the study, which HITRUST has yet to share with the public, demonstrate that hackers have moved away from focusing on stealing patient data, Nutkis said.
"If stuff isn't working, they move on. If stuff is working, they keep doing it," said Nutkis. "Organizations that are paying have considered their options, and unfortunately they don't have a lot of options."
Extortion has become more popular with cyber criminals because it is seen as a way to generate fast money, said Larry Whiteside, a healthcare expert with cyber security firm Optiv.
Stealing healthcare data is far more labour intensive, requiring attackers to keep their presence in a victim's network undetected for months as they steal data, then they need to find buyers, he added.
"With ransomware I'm going to get paid immediately," Whiteside said.
Frisco, Texas-based HITRUST's board includes executives from Anthem, Health Care Services, Humana, UnitedHealth and Walgreens.
(Reuters) - U.S. hospitals should brace for a surge in "ransomware" attacks by cyber criminals who infect and shut down computer networks, then demand payment in return for unlocking them, a non-profit healthcare group warned on Friday.
The Health Information Trust Alliance conducted a study of some 30 mid-sized U.S. hospitals late last year and found that 52 percent of them were infected with malicious software, HITRUST Chief Executive Daniel Nutkis told Reuters.
The most common type of malware was ransomware, Nutkis said, which was present in 35 percent of the hospitals included in the study of network traffic conducted by security software maker Trend Micro Inc.
Ransomware is malicious software that locks up data in computers and leaves messages demanding payment to recover the data. Last month, Hollywood Presbyterian Hospital in Los Angeles paid a ransom of $17,000 to regain access to its systems.
This week, an attack on MedStar Health forced the largest healthcare provider in Washington, D.C., to shut down much of its computer network. The Baltimore Sun reported a ransom of $18,500 was sought. MedStar declined to comment.
HITRUST said it expects such attacks to become more frequent because ransomware has turned into a profitable business for cyber criminals.
The results of the study, which HITRUST has yet to share with the public, demonstrate that hackers have moved away from focusing on stealing patient data, Nutkis said.
"If stuff isn't working, they move on. If stuff is working, they keep doing it," said Nutkis. "Organizations that are paying have considered their options, and unfortunately they don't have a lot of options."
Extortion has become more popular with cyber criminals because it is seen as a way to generate fast money, said Larry Whiteside, a healthcare expert with cyber security firm Optiv.
Stealing healthcare data is far more labour intensive, requiring attackers to keep their presence in a victim's network undetected for months as they steal data, then they need to find buyers, he added.
"With ransomware I'm going to get paid immediately," Whiteside said.
Frisco, Texas-based HITRUST's board includes executives from Anthem, Health Care Services, Humana, UnitedHealth and Walgreens.