User login
Breakfast Based on Whey Protein May Help Manage Type 2 Diabetes
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.
NEW YORK (Reuters Health) - A breakfast rich in whey protein may help people with type 2 diabetes manage their illness better, new research from Israel suggests.
"Whey protein, a byproduct of cheese manufacturing, lowers postprandial glycemia more than other protein sources," said lead author Dr. Daniela Jakubowicz from Wolfson Medical Center at Tel Aviv University."
We found that in type 2 diabetes, increasing protein content at breakfast has a greater impact on weight loss, glycated hemoglobin (HbA1C), satiety and postprandial glycemia when the protein source is whey protein, compared with other protein sources, such as eggs, tuna and soy," she told Reuters Health by email.
Dr. Jakubowicz and her group presented their findings April 1 at ENDO 2016, the annual meeting of the Endocrine Society, in Boston.
They randomly assigned 48 overweight and obese patients with type 2 diabetes to one of three isocaloric diets. Over 12 weeks, everyone ate a large breakfast, a medium-sized lunch and a small dinner, but the amount and source of each group's breakfast proteins differed.
At breakfast, the 17 participants in the whey group ate 36 g of protein as part of a whey protein shake consisting of 40% carbohydrate, 40% protein and 20% fat. The 16 participants in the high-protein group ate 36 g of protein in the form of eggs, tuna and cheese (40% carbs; 40% protein; 20% fat). The 15 in the high-carbohydrate group ate 13 g of protein in ready-to-eat cereals (65% carbs; 15% protein; 20% fat).
All three diets included a 660 kcal breakfast, a 567 cal lunch and a 276 cal dinner, with the same composition at lunch and dinner.
After 12 weeks, the participants in the whey protein group lost the most weight (7.6 kg vs. 6.1 kg for participants in the high-protein group and 3.5 kg for those in the high-carbohydrate group (p<0.0001).
Participants on the whey protein diet were less hungry during the day and had lower glucose spikes after meals compared with those on the other two diets.
The drop in HbA1C was 11.5% in the whey group, 7.7% in the protein group and 4.6% in the carbohydrate group (p<0.0001). Compared with the carbohydrate group, the percentage drop in HbA1c was greater by 41% in the protein group and by 64% in the whey group (p<0.0001).
"Whey protein was consumed only at breakfast; however, the improvement of glucose, insulin and glucagon-like peptide 1 (GLP-1) was also observed after lunch and dinner. The mechanism of this persistent beneficial effect of whey protein needs further research," Dr. Jakubowicz said.
Co-author Dr. Julio Wainstein, also at Wolfson Medical Center, added by email, "Usually, patients with type 2 diabetes are treated with a combination of several antidiabetic drugs to achieve adequate glucose regulation and decrease HbA1c. Whey protein should be considered an important adjuvant in the management of type 2 diabetes."
"Furthermore," Dr. Wainstein added, "it is possible that by adding whey protein to the diet, glucose regulation might be achieved with less medication, which is a valuable advantage in type 2 diabetes treatment."
The study had no commercial funding, and the authors declared no conflicts of interest.
Combo could improve treatment of MM, team says

multiple myeloma
Combining a calcineurin inhibitor and a histone deacetylase (HDAC) inhibitor could improve the treatment of multiple myeloma (MM), according to researchers.
The team found that MM cells express high levels of the protein phosphatase PPP3CA, a subunit of calcineurin.
And combining the calcineurin inhibitor FK506 with the HDAC inhibitor panobinostat suppressed MM cell growth in vitro and decreased tumor growth in mouse models of MM.
Yoichi Imai, MD, PhD, of Tokyo Women’s Medical University in Japan, and colleagues conducted this research and reported the results in JCI Insight.
First, the team observed increased PPP3CA expression in MM cell lines and MM cells isolated from patients with advanced disease.
Then, the researchers found that panobinostat reduced PPP3CA expression in MM cell lines. And further investigation revealed that the drug induced degradation of PPP3CA through HSP90 inhibition.
When the team knocked down PPP3CA in MM cells, they observed a reduction in cell growth. And when they overexpressed PPP3CA, they observed enhanced MM cell growth.
The researchers noted that FK506 inhibits the association between PPP3CA and calcineurin B. Unfortunately, FK506 alone did not suppress the growth of MM cells in vitro.
However, when FK506 was given with panobinostat or the HDAC inhibitor ACY-1215, the researchers observed a greater reduction in MM cell growth than with either HDAC inhibitor alone.
Panobinostat and FK506 reduced the growth of MM cells that were t(4;14)-positive (KMS-11, KMS-18, and KMS-26) and t(4;14)-negative (U266 and KMS-12PE) more effectively than panobinostat alone.
In mice with MM, those treated with FK506 alone had tumor sizes similar to control mice. However, mice treated with panobinostat saw a decrease in tumor size. And this effect was enhanced by the addition of FK506.
The researchers observed reduced PPP3CA expression, enhanced histone H3 acetylation, and cleavage of caspase-3 in samples from panobinostat-treated mice. And FK506 augmented panobinostat-induced apoptosis.
The team said these results suggest that FK506 enhances the antimyeloma effect of panobinostat through PPP3CA reduction, which supports the importance of calcineurin in the pathogenesis of MM. ![]()

multiple myeloma
Combining a calcineurin inhibitor and a histone deacetylase (HDAC) inhibitor could improve the treatment of multiple myeloma (MM), according to researchers.
The team found that MM cells express high levels of the protein phosphatase PPP3CA, a subunit of calcineurin.
And combining the calcineurin inhibitor FK506 with the HDAC inhibitor panobinostat suppressed MM cell growth in vitro and decreased tumor growth in mouse models of MM.
Yoichi Imai, MD, PhD, of Tokyo Women’s Medical University in Japan, and colleagues conducted this research and reported the results in JCI Insight.
First, the team observed increased PPP3CA expression in MM cell lines and MM cells isolated from patients with advanced disease.
Then, the researchers found that panobinostat reduced PPP3CA expression in MM cell lines. And further investigation revealed that the drug induced degradation of PPP3CA through HSP90 inhibition.
When the team knocked down PPP3CA in MM cells, they observed a reduction in cell growth. And when they overexpressed PPP3CA, they observed enhanced MM cell growth.
The researchers noted that FK506 inhibits the association between PPP3CA and calcineurin B. Unfortunately, FK506 alone did not suppress the growth of MM cells in vitro.
However, when FK506 was given with panobinostat or the HDAC inhibitor ACY-1215, the researchers observed a greater reduction in MM cell growth than with either HDAC inhibitor alone.
Panobinostat and FK506 reduced the growth of MM cells that were t(4;14)-positive (KMS-11, KMS-18, and KMS-26) and t(4;14)-negative (U266 and KMS-12PE) more effectively than panobinostat alone.
In mice with MM, those treated with FK506 alone had tumor sizes similar to control mice. However, mice treated with panobinostat saw a decrease in tumor size. And this effect was enhanced by the addition of FK506.
The researchers observed reduced PPP3CA expression, enhanced histone H3 acetylation, and cleavage of caspase-3 in samples from panobinostat-treated mice. And FK506 augmented panobinostat-induced apoptosis.
The team said these results suggest that FK506 enhances the antimyeloma effect of panobinostat through PPP3CA reduction, which supports the importance of calcineurin in the pathogenesis of MM. ![]()

multiple myeloma
Combining a calcineurin inhibitor and a histone deacetylase (HDAC) inhibitor could improve the treatment of multiple myeloma (MM), according to researchers.
The team found that MM cells express high levels of the protein phosphatase PPP3CA, a subunit of calcineurin.
And combining the calcineurin inhibitor FK506 with the HDAC inhibitor panobinostat suppressed MM cell growth in vitro and decreased tumor growth in mouse models of MM.
Yoichi Imai, MD, PhD, of Tokyo Women’s Medical University in Japan, and colleagues conducted this research and reported the results in JCI Insight.
First, the team observed increased PPP3CA expression in MM cell lines and MM cells isolated from patients with advanced disease.
Then, the researchers found that panobinostat reduced PPP3CA expression in MM cell lines. And further investigation revealed that the drug induced degradation of PPP3CA through HSP90 inhibition.
When the team knocked down PPP3CA in MM cells, they observed a reduction in cell growth. And when they overexpressed PPP3CA, they observed enhanced MM cell growth.
The researchers noted that FK506 inhibits the association between PPP3CA and calcineurin B. Unfortunately, FK506 alone did not suppress the growth of MM cells in vitro.
However, when FK506 was given with panobinostat or the HDAC inhibitor ACY-1215, the researchers observed a greater reduction in MM cell growth than with either HDAC inhibitor alone.
Panobinostat and FK506 reduced the growth of MM cells that were t(4;14)-positive (KMS-11, KMS-18, and KMS-26) and t(4;14)-negative (U266 and KMS-12PE) more effectively than panobinostat alone.
In mice with MM, those treated with FK506 alone had tumor sizes similar to control mice. However, mice treated with panobinostat saw a decrease in tumor size. And this effect was enhanced by the addition of FK506.
The researchers observed reduced PPP3CA expression, enhanced histone H3 acetylation, and cleavage of caspase-3 in samples from panobinostat-treated mice. And FK506 augmented panobinostat-induced apoptosis.
The team said these results suggest that FK506 enhances the antimyeloma effect of panobinostat through PPP3CA reduction, which supports the importance of calcineurin in the pathogenesis of MM. ![]()
Drug corrects anemia in CKD patients

The investigational therapy roxadustat can effectively treat anemia in patients with chronic kidney disease (CKD) who are not on dialysis, according to a phase 2 study.
Roxadustat increased and maintained hemoglobin levels and decreased hepcidin levels in these patients, who had not received previous treatment with
erythropoiesis-stimulating agents and were treated with roxadustat regardless of their baseline iron repletion status.
In addition, researchers said there were no serious adverse events related to roxadustat.
Robert Provenzano, MD, of St. John Hospital and Medical Center in Detroit, Michigan, and his colleagues reported these results in the Clinical Journal of the American Society of Nephrology.
The study was sponsored by FibroGen, Inc., the company developing roxadustat in collaboration with AstraZeneca.
Roxadustat (FG-4592) is an oral, small-molecule inhibitor of hypoxia-inducible factor (HIF) prolyl hydroxylase activity. HIF is a transcription factor that induces the natural physiological response to conditions of low oxygen, “turning on” erythropoiesis and other protective pathways.
In this randomized, phase 2 study of roxadustat, 145 patients with anemia (hemoglobin < 10.5 g/dL at baseline) and non-dialysis CKD were randomized into 1 of 6 cohorts of approximately 24 patients.
The cohorts had varying roxadustat starting doses (tiered weight and fixed amounts) and frequencies (2 and 3 times weekly), followed by hemoglobin maintenance with roxadustat 1 to 3 times weekly. The treatment duration was 16 or 24 weeks.
Results
Of the 143 patients evaluable for efficacy, 92% achieved a hemoglobin response—defined as a hemoglobin increase of > 1.0 g/dL from baseline and a hemoglobin of > 11.0 g/dL by the end of treatment (up to 16 weeks of treatment in 47 patients, and up to 24 weeks of treatment in 96 patients).
Generally, patients in all cohorts who received higher starting doses of roxadustat demonstrated earlier achievement of the hemoglobin response.
Roxadustat increased hemoglobin independently of the patients’ baseline iron repletion and inflammatory status, as measured by baseline C–reactive protein levels. Intravenous iron was not permitted throughout the study period, and 52.4% of patients were iron-replete at baseline.
Over 16 weeks of treatment, roxadustat decreased hepcidin levels by 16.9% (P=0.004), maintained reticulocyte hemoglobin content, and increased hemoglobin by a mean (±SD) of 1.83 (±0.09) g/dL (P<0.001).
After 8 weeks of roxadustat, total cholesterol levels decreased by a mean (±SD) of 26 (±30) mg/dL (P<0.001).
“In this study, anemia correction was achieved under a range of treatment options, including tiered-weight as well as fixed-starting-dose strategies,” Dr Provenzano said. “Correction of anemia and maintenance of hemoglobin response were seen at different dose frequencies—2 or 3 times weekly for achievement of hemoglobin response; 1, 2, or 3 times weekly for maintenance.”
“Secondary analyses showing decreases in hepcidin and increased iron utilization, as well as reductions in total cholesterol levels, suggest roxadustat consistently affects these parameters.”
Treatment-emergent adverse events were reported in 80% of all patients.
The most common events that occurred in more than 5% of patients were nausea (9.7%), diarrhea (8.3%), constipation (6.2%), vomiting (5.5%), peripheral edema (12.4%), urinary tract infection (9.7%), nasopharyngitis (9.0%), sinusitis (5.5%), dizziness (6.2%), headache (5.5%), and hypertension (7.6%). ![]()

The investigational therapy roxadustat can effectively treat anemia in patients with chronic kidney disease (CKD) who are not on dialysis, according to a phase 2 study.
Roxadustat increased and maintained hemoglobin levels and decreased hepcidin levels in these patients, who had not received previous treatment with
erythropoiesis-stimulating agents and were treated with roxadustat regardless of their baseline iron repletion status.
In addition, researchers said there were no serious adverse events related to roxadustat.
Robert Provenzano, MD, of St. John Hospital and Medical Center in Detroit, Michigan, and his colleagues reported these results in the Clinical Journal of the American Society of Nephrology.
The study was sponsored by FibroGen, Inc., the company developing roxadustat in collaboration with AstraZeneca.
Roxadustat (FG-4592) is an oral, small-molecule inhibitor of hypoxia-inducible factor (HIF) prolyl hydroxylase activity. HIF is a transcription factor that induces the natural physiological response to conditions of low oxygen, “turning on” erythropoiesis and other protective pathways.
In this randomized, phase 2 study of roxadustat, 145 patients with anemia (hemoglobin < 10.5 g/dL at baseline) and non-dialysis CKD were randomized into 1 of 6 cohorts of approximately 24 patients.
The cohorts had varying roxadustat starting doses (tiered weight and fixed amounts) and frequencies (2 and 3 times weekly), followed by hemoglobin maintenance with roxadustat 1 to 3 times weekly. The treatment duration was 16 or 24 weeks.
Results
Of the 143 patients evaluable for efficacy, 92% achieved a hemoglobin response—defined as a hemoglobin increase of > 1.0 g/dL from baseline and a hemoglobin of > 11.0 g/dL by the end of treatment (up to 16 weeks of treatment in 47 patients, and up to 24 weeks of treatment in 96 patients).
Generally, patients in all cohorts who received higher starting doses of roxadustat demonstrated earlier achievement of the hemoglobin response.
Roxadustat increased hemoglobin independently of the patients’ baseline iron repletion and inflammatory status, as measured by baseline C–reactive protein levels. Intravenous iron was not permitted throughout the study period, and 52.4% of patients were iron-replete at baseline.
Over 16 weeks of treatment, roxadustat decreased hepcidin levels by 16.9% (P=0.004), maintained reticulocyte hemoglobin content, and increased hemoglobin by a mean (±SD) of 1.83 (±0.09) g/dL (P<0.001).
After 8 weeks of roxadustat, total cholesterol levels decreased by a mean (±SD) of 26 (±30) mg/dL (P<0.001).
“In this study, anemia correction was achieved under a range of treatment options, including tiered-weight as well as fixed-starting-dose strategies,” Dr Provenzano said. “Correction of anemia and maintenance of hemoglobin response were seen at different dose frequencies—2 or 3 times weekly for achievement of hemoglobin response; 1, 2, or 3 times weekly for maintenance.”
“Secondary analyses showing decreases in hepcidin and increased iron utilization, as well as reductions in total cholesterol levels, suggest roxadustat consistently affects these parameters.”
Treatment-emergent adverse events were reported in 80% of all patients.
The most common events that occurred in more than 5% of patients were nausea (9.7%), diarrhea (8.3%), constipation (6.2%), vomiting (5.5%), peripheral edema (12.4%), urinary tract infection (9.7%), nasopharyngitis (9.0%), sinusitis (5.5%), dizziness (6.2%), headache (5.5%), and hypertension (7.6%). ![]()

The investigational therapy roxadustat can effectively treat anemia in patients with chronic kidney disease (CKD) who are not on dialysis, according to a phase 2 study.
Roxadustat increased and maintained hemoglobin levels and decreased hepcidin levels in these patients, who had not received previous treatment with
erythropoiesis-stimulating agents and were treated with roxadustat regardless of their baseline iron repletion status.
In addition, researchers said there were no serious adverse events related to roxadustat.
Robert Provenzano, MD, of St. John Hospital and Medical Center in Detroit, Michigan, and his colleagues reported these results in the Clinical Journal of the American Society of Nephrology.
The study was sponsored by FibroGen, Inc., the company developing roxadustat in collaboration with AstraZeneca.
Roxadustat (FG-4592) is an oral, small-molecule inhibitor of hypoxia-inducible factor (HIF) prolyl hydroxylase activity. HIF is a transcription factor that induces the natural physiological response to conditions of low oxygen, “turning on” erythropoiesis and other protective pathways.
In this randomized, phase 2 study of roxadustat, 145 patients with anemia (hemoglobin < 10.5 g/dL at baseline) and non-dialysis CKD were randomized into 1 of 6 cohorts of approximately 24 patients.
The cohorts had varying roxadustat starting doses (tiered weight and fixed amounts) and frequencies (2 and 3 times weekly), followed by hemoglobin maintenance with roxadustat 1 to 3 times weekly. The treatment duration was 16 or 24 weeks.
Results
Of the 143 patients evaluable for efficacy, 92% achieved a hemoglobin response—defined as a hemoglobin increase of > 1.0 g/dL from baseline and a hemoglobin of > 11.0 g/dL by the end of treatment (up to 16 weeks of treatment in 47 patients, and up to 24 weeks of treatment in 96 patients).
Generally, patients in all cohorts who received higher starting doses of roxadustat demonstrated earlier achievement of the hemoglobin response.
Roxadustat increased hemoglobin independently of the patients’ baseline iron repletion and inflammatory status, as measured by baseline C–reactive protein levels. Intravenous iron was not permitted throughout the study period, and 52.4% of patients were iron-replete at baseline.
Over 16 weeks of treatment, roxadustat decreased hepcidin levels by 16.9% (P=0.004), maintained reticulocyte hemoglobin content, and increased hemoglobin by a mean (±SD) of 1.83 (±0.09) g/dL (P<0.001).
After 8 weeks of roxadustat, total cholesterol levels decreased by a mean (±SD) of 26 (±30) mg/dL (P<0.001).
“In this study, anemia correction was achieved under a range of treatment options, including tiered-weight as well as fixed-starting-dose strategies,” Dr Provenzano said. “Correction of anemia and maintenance of hemoglobin response were seen at different dose frequencies—2 or 3 times weekly for achievement of hemoglobin response; 1, 2, or 3 times weekly for maintenance.”
“Secondary analyses showing decreases in hepcidin and increased iron utilization, as well as reductions in total cholesterol levels, suggest roxadustat consistently affects these parameters.”
Treatment-emergent adverse events were reported in 80% of all patients.
The most common events that occurred in more than 5% of patients were nausea (9.7%), diarrhea (8.3%), constipation (6.2%), vomiting (5.5%), peripheral edema (12.4%), urinary tract infection (9.7%), nasopharyngitis (9.0%), sinusitis (5.5%), dizziness (6.2%), headache (5.5%), and hypertension (7.6%). ![]()
A valuable string of PURLs
Since JFP’s launch of the PURL department in November of 2007, 122 PURLs—Priority Updates from the Research Literature—have been published. The Journal of Family Practice is the exclusive publication venue for these items. Because they have stood the test of time and are one of the more popular columns in JFP, I thought it would be worthwhile to describe the rigorous evaluation they undergo before they are published.
Many studies, but few PURLs. Each year, approximately 200,000 new human medical research studies are indexed on PubMed. Very few of these studies are pertinent to family medicine, however, and even fewer provide new patient-oriented evidence for primary care clinicians.
In 2005, the leaders of the Family Physician Inquiries Network (FPIN), which produces another popular JFP column, Clinical Inquiries, set about identifying high-priority research findings relevant to family medicine. A group of family physicians and librarians began combing the research literature monthly to find those rare randomized trials or high-quality observational studies that pertained to our specialty. To qualify as a PURL, a study had to meet 6 criteria. It had to be scientifically valid, relevant to family medicine, applicable in a medical care setting, immediately implementable, clinically meaningful, and practice changing. These criteria still stand today.
Making the cut. When a study is identified as a potential PURL, it is submitted to one of FPIN’s PURL review groups for a critical appraisal and rigorous peer review. If the group cannot convince the PURLs editors that the original research meets all 6 criteria, the study falls by the wayside. Most potential PURLs do not make the cut. I was one of the early PURL “divers,” and I was amazed at how few PURLs existed. Given the emphasis of research on subspecialties and the dearth of primary care research funding in the United States, I probably shouldn’t have been surprised.
Holding their value. I reviewed all 122 PURLs this week and am proud to say that nearly all still provide highly pertinent, practice-changing information for family physicians and other primary care clinicians. For a quick review of our string of PURLs, go to www.jfponline.com, select “Articles” in the banner, and then “PURLs,” and read the short practice changer box for each one. I guarantee it will be time well spent!
If you would like to become part of the PURLs process, either by nominating or reviewing a PURL, please contact the PURLs Project Manager at [email protected].
Since JFP’s launch of the PURL department in November of 2007, 122 PURLs—Priority Updates from the Research Literature—have been published. The Journal of Family Practice is the exclusive publication venue for these items. Because they have stood the test of time and are one of the more popular columns in JFP, I thought it would be worthwhile to describe the rigorous evaluation they undergo before they are published.
Many studies, but few PURLs. Each year, approximately 200,000 new human medical research studies are indexed on PubMed. Very few of these studies are pertinent to family medicine, however, and even fewer provide new patient-oriented evidence for primary care clinicians.
In 2005, the leaders of the Family Physician Inquiries Network (FPIN), which produces another popular JFP column, Clinical Inquiries, set about identifying high-priority research findings relevant to family medicine. A group of family physicians and librarians began combing the research literature monthly to find those rare randomized trials or high-quality observational studies that pertained to our specialty. To qualify as a PURL, a study had to meet 6 criteria. It had to be scientifically valid, relevant to family medicine, applicable in a medical care setting, immediately implementable, clinically meaningful, and practice changing. These criteria still stand today.
Making the cut. When a study is identified as a potential PURL, it is submitted to one of FPIN’s PURL review groups for a critical appraisal and rigorous peer review. If the group cannot convince the PURLs editors that the original research meets all 6 criteria, the study falls by the wayside. Most potential PURLs do not make the cut. I was one of the early PURL “divers,” and I was amazed at how few PURLs existed. Given the emphasis of research on subspecialties and the dearth of primary care research funding in the United States, I probably shouldn’t have been surprised.
Holding their value. I reviewed all 122 PURLs this week and am proud to say that nearly all still provide highly pertinent, practice-changing information for family physicians and other primary care clinicians. For a quick review of our string of PURLs, go to www.jfponline.com, select “Articles” in the banner, and then “PURLs,” and read the short practice changer box for each one. I guarantee it will be time well spent!
If you would like to become part of the PURLs process, either by nominating or reviewing a PURL, please contact the PURLs Project Manager at [email protected].
Since JFP’s launch of the PURL department in November of 2007, 122 PURLs—Priority Updates from the Research Literature—have been published. The Journal of Family Practice is the exclusive publication venue for these items. Because they have stood the test of time and are one of the more popular columns in JFP, I thought it would be worthwhile to describe the rigorous evaluation they undergo before they are published.
Many studies, but few PURLs. Each year, approximately 200,000 new human medical research studies are indexed on PubMed. Very few of these studies are pertinent to family medicine, however, and even fewer provide new patient-oriented evidence for primary care clinicians.
In 2005, the leaders of the Family Physician Inquiries Network (FPIN), which produces another popular JFP column, Clinical Inquiries, set about identifying high-priority research findings relevant to family medicine. A group of family physicians and librarians began combing the research literature monthly to find those rare randomized trials or high-quality observational studies that pertained to our specialty. To qualify as a PURL, a study had to meet 6 criteria. It had to be scientifically valid, relevant to family medicine, applicable in a medical care setting, immediately implementable, clinically meaningful, and practice changing. These criteria still stand today.
Making the cut. When a study is identified as a potential PURL, it is submitted to one of FPIN’s PURL review groups for a critical appraisal and rigorous peer review. If the group cannot convince the PURLs editors that the original research meets all 6 criteria, the study falls by the wayside. Most potential PURLs do not make the cut. I was one of the early PURL “divers,” and I was amazed at how few PURLs existed. Given the emphasis of research on subspecialties and the dearth of primary care research funding in the United States, I probably shouldn’t have been surprised.
Holding their value. I reviewed all 122 PURLs this week and am proud to say that nearly all still provide highly pertinent, practice-changing information for family physicians and other primary care clinicians. For a quick review of our string of PURLs, go to www.jfponline.com, select “Articles” in the banner, and then “PURLs,” and read the short practice changer box for each one. I guarantee it will be time well spent!
If you would like to become part of the PURLs process, either by nominating or reviewing a PURL, please contact the PURLs Project Manager at [email protected].
Increased syncopal episodes post surgery • Dx?
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
Diabetes update: Your guide to the latest ADA standards
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
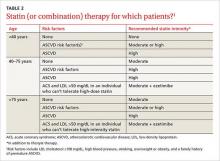
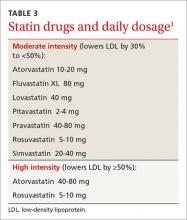
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Is platelet-rich plasma right for your patient?
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
Improving your care of patients with spinal cord injury/disease
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
From The Journal of Family Practice | 2016;65(5):302-306,308-309.
Thromboprophylaxis efficacy similar before and after colorectal surgery
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
The findings also raise questions about the fairness of financial penalties imposed by the Centers for Medicare & Medicaid Services for perioperative venous thromboembolism, Dr. Karen Zaghiyan of Cedars Sinai Medical Center, Los Angeles said at the annual meeting of the American Surgical Association.
In 376 consecutive adult patients undergoing laparoscopic or open major colorectal surgery who had no occult preoperative deep vein thrombosis (DVT) on lower extremity venous duplex scan and who were randomized to preoperative or postoperative chemical thromboprophylaxis (CTP) with 5,000 U of subcutaneous heparin, no differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery, Dr. Zaghiyan said.
“There was no significant difference in our primary outcome – early postoperative VTE [venous thromboembolism] – in patients managed with postoperative or preoperative prophylaxis,” she said, noting that three patients in each group developed asymptomatic intraoperative DVT, and two additional patients in the postoperative treatment group developed asymptomatic DVT between postoperative day 0 and 2.
Two additional patients in the postoperative treatment group developed clinically significant DVT between postoperative day 2 and 30.
“Both patients had a complicated prolonged hospital course, and developed DVT while still hospitalized. This difference still did not reach statistical significance, and there were no post-discharge DVT or PEs [pulmonary embolisms] in the entire cohort,” she said.
Bleeding complications, including estimated blood loss and number receiving transfusion, were similar in the two groups, she said, noting that no patients developed heparin-induced thrombocytopenia, and that hospital stay, readmissions, and overall complications were similar between the two groups.
Study subjects had a mean age of 53 years, and 52% were women. The preoperative- and postoperative treatment groups were similar with respect to demographics and preoperative characteristics. They underwent lower extremity venous duplex just prior to surgery, immediately after surgery in the recovery room, on day 2 after surgery, and subsequently as clinically indicated.
Thromboprophylaxis in the preoperative treatment group was given in the “pre-op holding area” then 8 hours after surgery and every 8 hours thereafter until discharge. Thromboprophylaxis in the postoperative treatment group was given within 24 hours after surgery, and then every 8 hours until discharge.
Preoperative and postoperative CTP were equally safe and effective, and since occult preoperative DVT is twice as common as postoperative DVT, occurring in a surprising 4% of patients in this study, the findings support preoperative scans and anticoagulation based on the results – especially in older patients and those with comorbid disease, Dr. Zaghiyan said.
The findings could help improve patients care; although VTE prevention and chemical prophylaxis in colorectal surgery have been extensively studied, current guidelines are vague, with both the American College of Chest Physicians and the Surgical Care Improvement Project recommending that prophylaxis be initiated 24 hours prior to or after major colorectal surgery, she said.
The findings could also help avoid CMS penalties for postoperatively identified VTE,” she added.
Further, those penalties may not be supported by the clinical data; in this study, the majority of early postoperative DVTs were unpreventable, with no additional protection provided with preoperative prophylaxis, she explained.
“CMS should reevaluate the financial penalties, taking preventability into account,” she said.
Dr. Zaghiyan reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Lower extremity duplex scans should be performed prior to colorectal surgery, and anticoagulation should be tailored to the result, findings from a randomized clinical trial suggest.
Major finding: No differences were seen with respect to the primary outcome of venous thromboembolism within 48 hours of surgery in patients treated with pre- or post-operative chemical thromboprophylaxis.
Data source: A randomized clinical trial of 376 patients.
Disclosures: Dr. Zaghiyan reported having no disclosures.
Surgery for PHPT improves sleep quality
BALTIMORE – Research into how primary hyperparathyroidism and parathyroidectomy affect sleep quality has been limited, but investigators at the Medical College of Wisconsin, Milwaukee, reported that primary hyperparathyroidism does indeed disrupt sleep patterns and that curative surgery can improve sleep quality in a third of patients.
“Today, most patients with primary hyperparathyroidism have what is considered asymptomatic disease,” Justin La reported at the annual meeting of the American Association of Endocrine Surgeons. “However, recent studies demonstrate that many of these asymptomatic patients commonly exhibit neuropsychological problems, including sleep disturbances.” Mr. La is a fourth-year medical student at the Medical College of Wisconsin.
This prospective study, led by Dr. Tina Yen, recruited patients between June 2013 and September 2015 and compared 110 patients who had parathyroidectomy for primary hyperparathyroidism (PHPT) with 45 controls who had thyroidectomy for benign euthyroid disease between June 2013 and September 2015.
“Multiple studies, including recent meta-analyses, have demonstrated lower quality of life in patients with primary hyperparathyroidism and have suggested that patients, regardless of symptoms or degree of hypercalcemia, report varying degrees of improvement after parathyroidectomy,” Mr. La said. “In contrast there is a relative paucity of literature on the effects of primary hyperparathyroidism on sleep quality and changes after parathyroidectomy.”
He noted studies from both the University of Texas M.D. Anderson Cancer Center, Houston, and the University of Wisconsin–Madison had demonstrated a 44%-63% incidence of sleep disturbance preoperatively and improvement postoperatively in patients with PHPT who had parathyroidectomy (Endocr Pract. 2007 Jul-Aug;13:338-44; World J Surg. 2014 Mar;38:542-8; Surgery. 2009 Dec;146:1116-22).
“However, these studies were limited by small sample sizes and lack of a control group,” La said.
The latest study had subjects complete questionnaires inquiring about quality of life and sleep patterns at three different intervals: before surgery; and 1 and 6 months after surgery. The study used the Medical Outcomes Study SF-36 to assess quality of life and the Pittsburgh Sleep Quality Index (PSQI) to evaluate sleep quality. The PSQI rates sleep quality on a scale of 0 to 21; a score of 5 or higher indicates poor sleep quality.
“Compared to the preoperative scores, sleep scores after parathyroidectomy were lower, signifying better sleep quality among the 105 patients who completed 1-month postoperative surveys and the 94 patients who completed the 6-month surveys,” La said.
Before surgery, PHPT patients had worse sleep quality than their thyroid counterparts with PSQI scores of 8.1 vs. 5.3, respectively. After surgery, sleep quality scores between the two groups were similar, with mean PSQI scores of 6.3 vs. 5.3 at 1 month after surgery for the parathyroid and thyroid groups, respectively, and 5.8 vs. 4.6 for the two groups at 6 months.
Also, the proportion of patients in both groups who had poor sleep quality after surgery showed no statistical difference. At 1 month after surgery, 50% of patients in the parathyroid group and 40% in the thyroid group continued to have poor sleep quality, La said. However, when comparing preoperative with postoperative sleep scores, 37% in the parathyroid group had a noticeable improvement in their sleep scores, while only 10% of the thyroid group demonstrated improvement.
The researchers also evaluated physical and mental function in the two groups. “Preoperative overall health status was significantly worse in the parathyroid group,” La said. At 1 and 6 months after parathyroidectomy, only two physical components, physical functioning and bodily pain, remained worse in the PHPT patients. Compared with preoperative scores, PHPT patients showed statistically significant improvement in all four mental components at both postoperative periods. “In contrast, the thyroid group demonstrated no significant changes in the preoperative to postoperative scores in all eight components,” La said.
“Our study adds to the body of literature suggesting that asymptomatic patients with primary hyperparathyroidism are unlikely to be truly asymptomatic,” La said. “All patients with primary hyperparathyroidism should be referred for surgical consultation, particularly those with neurocognitive symptoms.”
He also said that patients should be counseled that improvement in sleep quality and quality of life, if they are to occur, typically are seen within 1 month after surgery.
Mr. La, Dr. Yen, and the study coauthors had no relationships to disclose.
BALTIMORE – Research into how primary hyperparathyroidism and parathyroidectomy affect sleep quality has been limited, but investigators at the Medical College of Wisconsin, Milwaukee, reported that primary hyperparathyroidism does indeed disrupt sleep patterns and that curative surgery can improve sleep quality in a third of patients.
“Today, most patients with primary hyperparathyroidism have what is considered asymptomatic disease,” Justin La reported at the annual meeting of the American Association of Endocrine Surgeons. “However, recent studies demonstrate that many of these asymptomatic patients commonly exhibit neuropsychological problems, including sleep disturbances.” Mr. La is a fourth-year medical student at the Medical College of Wisconsin.
This prospective study, led by Dr. Tina Yen, recruited patients between June 2013 and September 2015 and compared 110 patients who had parathyroidectomy for primary hyperparathyroidism (PHPT) with 45 controls who had thyroidectomy for benign euthyroid disease between June 2013 and September 2015.
“Multiple studies, including recent meta-analyses, have demonstrated lower quality of life in patients with primary hyperparathyroidism and have suggested that patients, regardless of symptoms or degree of hypercalcemia, report varying degrees of improvement after parathyroidectomy,” Mr. La said. “In contrast there is a relative paucity of literature on the effects of primary hyperparathyroidism on sleep quality and changes after parathyroidectomy.”
He noted studies from both the University of Texas M.D. Anderson Cancer Center, Houston, and the University of Wisconsin–Madison had demonstrated a 44%-63% incidence of sleep disturbance preoperatively and improvement postoperatively in patients with PHPT who had parathyroidectomy (Endocr Pract. 2007 Jul-Aug;13:338-44; World J Surg. 2014 Mar;38:542-8; Surgery. 2009 Dec;146:1116-22).
“However, these studies were limited by small sample sizes and lack of a control group,” La said.
The latest study had subjects complete questionnaires inquiring about quality of life and sleep patterns at three different intervals: before surgery; and 1 and 6 months after surgery. The study used the Medical Outcomes Study SF-36 to assess quality of life and the Pittsburgh Sleep Quality Index (PSQI) to evaluate sleep quality. The PSQI rates sleep quality on a scale of 0 to 21; a score of 5 or higher indicates poor sleep quality.
“Compared to the preoperative scores, sleep scores after parathyroidectomy were lower, signifying better sleep quality among the 105 patients who completed 1-month postoperative surveys and the 94 patients who completed the 6-month surveys,” La said.
Before surgery, PHPT patients had worse sleep quality than their thyroid counterparts with PSQI scores of 8.1 vs. 5.3, respectively. After surgery, sleep quality scores between the two groups were similar, with mean PSQI scores of 6.3 vs. 5.3 at 1 month after surgery for the parathyroid and thyroid groups, respectively, and 5.8 vs. 4.6 for the two groups at 6 months.
Also, the proportion of patients in both groups who had poor sleep quality after surgery showed no statistical difference. At 1 month after surgery, 50% of patients in the parathyroid group and 40% in the thyroid group continued to have poor sleep quality, La said. However, when comparing preoperative with postoperative sleep scores, 37% in the parathyroid group had a noticeable improvement in their sleep scores, while only 10% of the thyroid group demonstrated improvement.
The researchers also evaluated physical and mental function in the two groups. “Preoperative overall health status was significantly worse in the parathyroid group,” La said. At 1 and 6 months after parathyroidectomy, only two physical components, physical functioning and bodily pain, remained worse in the PHPT patients. Compared with preoperative scores, PHPT patients showed statistically significant improvement in all four mental components at both postoperative periods. “In contrast, the thyroid group demonstrated no significant changes in the preoperative to postoperative scores in all eight components,” La said.
“Our study adds to the body of literature suggesting that asymptomatic patients with primary hyperparathyroidism are unlikely to be truly asymptomatic,” La said. “All patients with primary hyperparathyroidism should be referred for surgical consultation, particularly those with neurocognitive symptoms.”
He also said that patients should be counseled that improvement in sleep quality and quality of life, if they are to occur, typically are seen within 1 month after surgery.
Mr. La, Dr. Yen, and the study coauthors had no relationships to disclose.
BALTIMORE – Research into how primary hyperparathyroidism and parathyroidectomy affect sleep quality has been limited, but investigators at the Medical College of Wisconsin, Milwaukee, reported that primary hyperparathyroidism does indeed disrupt sleep patterns and that curative surgery can improve sleep quality in a third of patients.
“Today, most patients with primary hyperparathyroidism have what is considered asymptomatic disease,” Justin La reported at the annual meeting of the American Association of Endocrine Surgeons. “However, recent studies demonstrate that many of these asymptomatic patients commonly exhibit neuropsychological problems, including sleep disturbances.” Mr. La is a fourth-year medical student at the Medical College of Wisconsin.
This prospective study, led by Dr. Tina Yen, recruited patients between June 2013 and September 2015 and compared 110 patients who had parathyroidectomy for primary hyperparathyroidism (PHPT) with 45 controls who had thyroidectomy for benign euthyroid disease between June 2013 and September 2015.
“Multiple studies, including recent meta-analyses, have demonstrated lower quality of life in patients with primary hyperparathyroidism and have suggested that patients, regardless of symptoms or degree of hypercalcemia, report varying degrees of improvement after parathyroidectomy,” Mr. La said. “In contrast there is a relative paucity of literature on the effects of primary hyperparathyroidism on sleep quality and changes after parathyroidectomy.”
He noted studies from both the University of Texas M.D. Anderson Cancer Center, Houston, and the University of Wisconsin–Madison had demonstrated a 44%-63% incidence of sleep disturbance preoperatively and improvement postoperatively in patients with PHPT who had parathyroidectomy (Endocr Pract. 2007 Jul-Aug;13:338-44; World J Surg. 2014 Mar;38:542-8; Surgery. 2009 Dec;146:1116-22).
“However, these studies were limited by small sample sizes and lack of a control group,” La said.
The latest study had subjects complete questionnaires inquiring about quality of life and sleep patterns at three different intervals: before surgery; and 1 and 6 months after surgery. The study used the Medical Outcomes Study SF-36 to assess quality of life and the Pittsburgh Sleep Quality Index (PSQI) to evaluate sleep quality. The PSQI rates sleep quality on a scale of 0 to 21; a score of 5 or higher indicates poor sleep quality.
“Compared to the preoperative scores, sleep scores after parathyroidectomy were lower, signifying better sleep quality among the 105 patients who completed 1-month postoperative surveys and the 94 patients who completed the 6-month surveys,” La said.
Before surgery, PHPT patients had worse sleep quality than their thyroid counterparts with PSQI scores of 8.1 vs. 5.3, respectively. After surgery, sleep quality scores between the two groups were similar, with mean PSQI scores of 6.3 vs. 5.3 at 1 month after surgery for the parathyroid and thyroid groups, respectively, and 5.8 vs. 4.6 for the two groups at 6 months.
Also, the proportion of patients in both groups who had poor sleep quality after surgery showed no statistical difference. At 1 month after surgery, 50% of patients in the parathyroid group and 40% in the thyroid group continued to have poor sleep quality, La said. However, when comparing preoperative with postoperative sleep scores, 37% in the parathyroid group had a noticeable improvement in their sleep scores, while only 10% of the thyroid group demonstrated improvement.
The researchers also evaluated physical and mental function in the two groups. “Preoperative overall health status was significantly worse in the parathyroid group,” La said. At 1 and 6 months after parathyroidectomy, only two physical components, physical functioning and bodily pain, remained worse in the PHPT patients. Compared with preoperative scores, PHPT patients showed statistically significant improvement in all four mental components at both postoperative periods. “In contrast, the thyroid group demonstrated no significant changes in the preoperative to postoperative scores in all eight components,” La said.
“Our study adds to the body of literature suggesting that asymptomatic patients with primary hyperparathyroidism are unlikely to be truly asymptomatic,” La said. “All patients with primary hyperparathyroidism should be referred for surgical consultation, particularly those with neurocognitive symptoms.”
He also said that patients should be counseled that improvement in sleep quality and quality of life, if they are to occur, typically are seen within 1 month after surgery.
Mr. La, Dr. Yen, and the study coauthors had no relationships to disclose.
AT AAES 2016
Key clinical point: A large proportion of “asymptomatic” patients with primary hyperparathyroidism (PHPT) actually have sleep disturbances.
Major finding: Sleep scores a month after parathyroidectomy were found to improve in 50% of patients with PHPT.
Data source: Single institution, prospective study of 155 patients comparing sleep patterns in patients with PHPT and thyroid controls.
Disclosures: Mr. La and his coauthors reported having no financial disclosures.