User login
Looking Beyond Rest to Active and Targeted Treatments for Concussion
VANCOUVER—Prescribed rest is an important component of treating concussion, but it may not be the most appropriate intervention for all patients and may worsen symptoms in some cases, said Anthony P. Kontos, PhD, at the 68th Annual Meeting of the American Academy of Neurology (AAN).
Anthony P. Kontos, PhD
“We need to move the discussion on concussion toward more active and targeted treatments,” said Dr. Kontos, Research Director of the University of Pittsburgh Medical Center (UPMC) Sports Medicine Concussion Program.Concussion is a heterogeneous injury with varying clinical profiles and recovery trajectories. Approaches to treatment should account for these differences and involve multidisciplinary teams when necessary, he said.
In October 2015, Dr. Kontos, Michael “Micky” Collins, PhD, and David O. Okonkwo, MD, PhD, directed a meeting with 37 participants from the fields of neurology, neuropsychology, neurosurgery, primary care, athletic training, and physical therapy to create a summary agreement that can assist clinicians with concussion treatment.
Nineteen guests, including representatives from professional sports organizations, the military, and public health, also attended the Targeted Evaluation and Active Management (TEAM) Approach to Treating Concussion meeting. The National Football League and UPMC sponsored the meeting, which was held in Pittsburgh.
Consensus documents have predominantly focused on things like the various definitions of concussion, how to assess concussion, and how to manage it, said Dr. Kontos. “We really wanted to focus on more of that end point of treatment and potentially more active treatment,” he said.
The TEAM participants developed and agreed upon 17 statements, which they plan to publish. At the AAN meeting, Dr. Kontos provided a brief review of some of the statements and discussed them in the context of recent research.
Rest’s Benefits and Limitations
Physical and cognitive rest, as part of an individualized treatment plan, are currently “the foundation of sport-related concussion management,” according to National Collegiate Athletic Association interassociation concussion guidelines. Rest after concussion conserves needed energy in the brain and reduces the likelihood of second impact syndrome and other catastrophic events, Dr. Kontos said. Furthermore, some studies have suggested that rest improves recovery. Brown et al reported in 2014 that athletes who self-reported more cognitive activity after a concussion took longer to recover than those who reported less cognitive activity.
However, the evidence to support rest is limited. In 2013, the Institute of Medicine and National Research Council published a report on sports-related concussion in youth that found little evidence regarding the efficacy of rest following concussion or to inform the best timing and approach for return to activity. Their statement “still resonates now,” Dr. Kontos said. “There’s very little empirical data to support what we do with rest. It’s largely an across-the-board policy that’s not data-driven, and we need to change that.” The TEAM group agreed “there is limited empirical evidence for the effectiveness of prescribed physical and cognitive rest, with no multisite trials for prescribed rest following concussion.”
Prescribed rest can have psychologic consequences, including emotional distress, depression, and anxiety. Rest allows individuals time to ruminate on their injury, which can exacerbate symptoms in self-report. Individuals who somaticize are particularly vulnerable to this effect. Jeremy M. Root, MD, of Children’s National Medical Center in Washington, DC, Dr. Kontos, and colleagues reported in April in the Journal of Pediatrics that patients who had high somatization scores were approximately five to seven times more likely to report an increase in symptoms at two weeks and four weeks, compared with those who were not in the highest quartile of somatization.
In addition, patients who are prescribed rest may think, “Wow, I must have a really bad injury such that I can’t do anything for a week.” This contextual framing effect may also influence the outcome, said Dr. Kontos.
Thomas et al in 2015 published the results of a randomized controlled trial that found that, after a concussion, patients ages 11 to 22 who were prescribed five days’ rest reported more daily postconcussive symptoms, compared with patients who were prescribed two days’ rest with progressive return to activity. Symptoms peaked at four days, and differences between groups remained at 10 days. “They have higher symptoms when they’re told to rest longer than if they’re told to rest less,” Dr. Kontos said. Clinically, there was no significant difference between groups in neurocognitive or balance outcomes, however.
The effect of treatment on the number of postconcussive symptoms may not be that straightforward, however. When Dr. Kontos, Dr. Thomas, and colleagues reanalyzed the data to look at patients who only reported symptoms (eg, headache, nausea, dizziness) but did not otherwise have early signs of concussion (eg, loss of consciousness, posttraumatic amnesia, disorientation, confusion), the symptoms-only group reported more symptoms at 10 days when prescribed five days’ rest, compared with two days’ rest with progressive return to activity. Patients who had early signs of concussion, however, reported fewer symptoms when prescribed five days’ rest versus two days’ rest with progressive return to activity.
“We have a sort of dichotomy here. We don’t want to say rest is bad. It may be very good for these people who have a high organic level or severity to their injury, and we may need to think in terms of resting them longer, whereas these patients [with symptoms only] certainly need to get more active, probably earlier in the process,” Dr. Kontos said.
Activity and social interaction may provide benefits. Miller et al in 2013 reported that environmental enrichment, including cognitive, physical, and social activity, is associated with improved outcome and sparing of hippocampal atrophy in the chronic stages of traumatic brain injury.
The TEAM group agreed, “Active treatment strategies may be initiated early in recovery following concussion.” The group also agreed, “strict brain rest (eg, ‘cocoon’ therapy) is not indicated and may have detrimental effects on patients following concussion.”
A Heterogeneous Injury
A focal point of the TEAM meeting was the concept of various clinical profiles of concussion. The group agreed, “Concussions are characterized by diverse symptoms and impairments in function resulting in different clinical profiles and recovery trajectories.”
“We need to think in terms of what type of concussion does this individual have and is it multiple types,” such as cognitive-fatigue, vestibular, or ocular, said Dr. Kontos. “We don’t typically just see one of these.” For example, a patient may have a predominant vestibular concussion with some posttraumatic migraine and neck involvement. “Oftentimes we see misdiagnoses when people show up. They’ve been diagnosed with cognitive issues when in reality they’re having vision or oculomotor difficulties.”
There are many potential approaches to categorizing, classifying, or profiling concussion, including those that consider posttraumatic mood and migraine as modifying factors, he said.
Multidisciplinary Teams
In addition, the TEAM group stated, “thorough multidomain assessment is warranted to properly evaluate the clinical profiles of concussion.” Various experts may be needed to assess cognitive, exertional, oculomotor, vestibular, and other symptoms and impairment.
As part of a multidisciplinary team, a neurologist, neuropsychologist, or primary care physician could “serve as kind of a point guard, to use a basketball analogy,” said Dr. Kontos. When an aspect of a patient’s assessment or treatment needs to be addressed more in depth, such as with regard to medication, vestibular therapy, or imaging, the patient may be referred to experts in those areas. “We try to work as a team and work back through the point guard to coordinate that care system,” he said. Telemedicine might allow for multidisciplinary treatment in remote geographic areas where establishing multidisciplinary teams otherwise might not be feasible, Dr. Kontos noted.
“Pharmacological therapy may be indicated in selected circumstances to treat certain symptoms and impairments related to concussion,” the TEAM group agreed.There is “very little” evidence for medicine in concussion, and drugs can exacerbate symptoms in some situations, Dr. Kontos said. Randomized controlled trials will help researchers better understand medication’s role in treating concussion.
More Active Treatment
In particular, patients who do not receive appropriate management after a concussion and then go to a clinic several months later with chronic symptoms may benefit from more active approaches to treatment, such as brisk walking.
Dr. Kontos described the case of an ice hockey player who was prescribed rest following a first concussion. After resting, the athlete began a return-to-play protocol that focused on aerobic exertion with no dynamic movements. As soon as the player returned to the ice, however, dizziness and headache came flooding back.
Several months later, the athlete was referred to a concussion clinic. The patient underwent a thorough evaluation that included vestibular and oculomotor assessments. Clinicians determined that the athlete needed more active treatment, including vision training and walking with head movements. In three weeks, the athlete returned to the ice. About a week later, the athlete resumed full-contact ice hockey.
“Prescribing rest is not the only approach,” Dr. Kontos said. “We need to move the discussion in different directions. We need to be more active with certain people and we need to be more targeted with our approaches.”
—Jake Remaly
Suggested Reading
Brown NJ, Mannix RC, O’Brien MJ, et al. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299-304.
Miller LS, Colella B, Mikulis D, et al. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Front Hum Neurosci. 2013;7:506.
Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016 Apr 5 [Epub ahead of print].
Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
VANCOUVER—Prescribed rest is an important component of treating concussion, but it may not be the most appropriate intervention for all patients and may worsen symptoms in some cases, said Anthony P. Kontos, PhD, at the 68th Annual Meeting of the American Academy of Neurology (AAN).
Anthony P. Kontos, PhD
“We need to move the discussion on concussion toward more active and targeted treatments,” said Dr. Kontos, Research Director of the University of Pittsburgh Medical Center (UPMC) Sports Medicine Concussion Program.Concussion is a heterogeneous injury with varying clinical profiles and recovery trajectories. Approaches to treatment should account for these differences and involve multidisciplinary teams when necessary, he said.
In October 2015, Dr. Kontos, Michael “Micky” Collins, PhD, and David O. Okonkwo, MD, PhD, directed a meeting with 37 participants from the fields of neurology, neuropsychology, neurosurgery, primary care, athletic training, and physical therapy to create a summary agreement that can assist clinicians with concussion treatment.
Nineteen guests, including representatives from professional sports organizations, the military, and public health, also attended the Targeted Evaluation and Active Management (TEAM) Approach to Treating Concussion meeting. The National Football League and UPMC sponsored the meeting, which was held in Pittsburgh.
Consensus documents have predominantly focused on things like the various definitions of concussion, how to assess concussion, and how to manage it, said Dr. Kontos. “We really wanted to focus on more of that end point of treatment and potentially more active treatment,” he said.
The TEAM participants developed and agreed upon 17 statements, which they plan to publish. At the AAN meeting, Dr. Kontos provided a brief review of some of the statements and discussed them in the context of recent research.
Rest’s Benefits and Limitations
Physical and cognitive rest, as part of an individualized treatment plan, are currently “the foundation of sport-related concussion management,” according to National Collegiate Athletic Association interassociation concussion guidelines. Rest after concussion conserves needed energy in the brain and reduces the likelihood of second impact syndrome and other catastrophic events, Dr. Kontos said. Furthermore, some studies have suggested that rest improves recovery. Brown et al reported in 2014 that athletes who self-reported more cognitive activity after a concussion took longer to recover than those who reported less cognitive activity.
However, the evidence to support rest is limited. In 2013, the Institute of Medicine and National Research Council published a report on sports-related concussion in youth that found little evidence regarding the efficacy of rest following concussion or to inform the best timing and approach for return to activity. Their statement “still resonates now,” Dr. Kontos said. “There’s very little empirical data to support what we do with rest. It’s largely an across-the-board policy that’s not data-driven, and we need to change that.” The TEAM group agreed “there is limited empirical evidence for the effectiveness of prescribed physical and cognitive rest, with no multisite trials for prescribed rest following concussion.”
Prescribed rest can have psychologic consequences, including emotional distress, depression, and anxiety. Rest allows individuals time to ruminate on their injury, which can exacerbate symptoms in self-report. Individuals who somaticize are particularly vulnerable to this effect. Jeremy M. Root, MD, of Children’s National Medical Center in Washington, DC, Dr. Kontos, and colleagues reported in April in the Journal of Pediatrics that patients who had high somatization scores were approximately five to seven times more likely to report an increase in symptoms at two weeks and four weeks, compared with those who were not in the highest quartile of somatization.
In addition, patients who are prescribed rest may think, “Wow, I must have a really bad injury such that I can’t do anything for a week.” This contextual framing effect may also influence the outcome, said Dr. Kontos.
Thomas et al in 2015 published the results of a randomized controlled trial that found that, after a concussion, patients ages 11 to 22 who were prescribed five days’ rest reported more daily postconcussive symptoms, compared with patients who were prescribed two days’ rest with progressive return to activity. Symptoms peaked at four days, and differences between groups remained at 10 days. “They have higher symptoms when they’re told to rest longer than if they’re told to rest less,” Dr. Kontos said. Clinically, there was no significant difference between groups in neurocognitive or balance outcomes, however.
The effect of treatment on the number of postconcussive symptoms may not be that straightforward, however. When Dr. Kontos, Dr. Thomas, and colleagues reanalyzed the data to look at patients who only reported symptoms (eg, headache, nausea, dizziness) but did not otherwise have early signs of concussion (eg, loss of consciousness, posttraumatic amnesia, disorientation, confusion), the symptoms-only group reported more symptoms at 10 days when prescribed five days’ rest, compared with two days’ rest with progressive return to activity. Patients who had early signs of concussion, however, reported fewer symptoms when prescribed five days’ rest versus two days’ rest with progressive return to activity.
“We have a sort of dichotomy here. We don’t want to say rest is bad. It may be very good for these people who have a high organic level or severity to their injury, and we may need to think in terms of resting them longer, whereas these patients [with symptoms only] certainly need to get more active, probably earlier in the process,” Dr. Kontos said.
Activity and social interaction may provide benefits. Miller et al in 2013 reported that environmental enrichment, including cognitive, physical, and social activity, is associated with improved outcome and sparing of hippocampal atrophy in the chronic stages of traumatic brain injury.
The TEAM group agreed, “Active treatment strategies may be initiated early in recovery following concussion.” The group also agreed, “strict brain rest (eg, ‘cocoon’ therapy) is not indicated and may have detrimental effects on patients following concussion.”
A Heterogeneous Injury
A focal point of the TEAM meeting was the concept of various clinical profiles of concussion. The group agreed, “Concussions are characterized by diverse symptoms and impairments in function resulting in different clinical profiles and recovery trajectories.”
“We need to think in terms of what type of concussion does this individual have and is it multiple types,” such as cognitive-fatigue, vestibular, or ocular, said Dr. Kontos. “We don’t typically just see one of these.” For example, a patient may have a predominant vestibular concussion with some posttraumatic migraine and neck involvement. “Oftentimes we see misdiagnoses when people show up. They’ve been diagnosed with cognitive issues when in reality they’re having vision or oculomotor difficulties.”
There are many potential approaches to categorizing, classifying, or profiling concussion, including those that consider posttraumatic mood and migraine as modifying factors, he said.
Multidisciplinary Teams
In addition, the TEAM group stated, “thorough multidomain assessment is warranted to properly evaluate the clinical profiles of concussion.” Various experts may be needed to assess cognitive, exertional, oculomotor, vestibular, and other symptoms and impairment.
As part of a multidisciplinary team, a neurologist, neuropsychologist, or primary care physician could “serve as kind of a point guard, to use a basketball analogy,” said Dr. Kontos. When an aspect of a patient’s assessment or treatment needs to be addressed more in depth, such as with regard to medication, vestibular therapy, or imaging, the patient may be referred to experts in those areas. “We try to work as a team and work back through the point guard to coordinate that care system,” he said. Telemedicine might allow for multidisciplinary treatment in remote geographic areas where establishing multidisciplinary teams otherwise might not be feasible, Dr. Kontos noted.
“Pharmacological therapy may be indicated in selected circumstances to treat certain symptoms and impairments related to concussion,” the TEAM group agreed.There is “very little” evidence for medicine in concussion, and drugs can exacerbate symptoms in some situations, Dr. Kontos said. Randomized controlled trials will help researchers better understand medication’s role in treating concussion.
More Active Treatment
In particular, patients who do not receive appropriate management after a concussion and then go to a clinic several months later with chronic symptoms may benefit from more active approaches to treatment, such as brisk walking.
Dr. Kontos described the case of an ice hockey player who was prescribed rest following a first concussion. After resting, the athlete began a return-to-play protocol that focused on aerobic exertion with no dynamic movements. As soon as the player returned to the ice, however, dizziness and headache came flooding back.
Several months later, the athlete was referred to a concussion clinic. The patient underwent a thorough evaluation that included vestibular and oculomotor assessments. Clinicians determined that the athlete needed more active treatment, including vision training and walking with head movements. In three weeks, the athlete returned to the ice. About a week later, the athlete resumed full-contact ice hockey.
“Prescribing rest is not the only approach,” Dr. Kontos said. “We need to move the discussion in different directions. We need to be more active with certain people and we need to be more targeted with our approaches.”
—Jake Remaly
VANCOUVER—Prescribed rest is an important component of treating concussion, but it may not be the most appropriate intervention for all patients and may worsen symptoms in some cases, said Anthony P. Kontos, PhD, at the 68th Annual Meeting of the American Academy of Neurology (AAN).
Anthony P. Kontos, PhD
“We need to move the discussion on concussion toward more active and targeted treatments,” said Dr. Kontos, Research Director of the University of Pittsburgh Medical Center (UPMC) Sports Medicine Concussion Program.Concussion is a heterogeneous injury with varying clinical profiles and recovery trajectories. Approaches to treatment should account for these differences and involve multidisciplinary teams when necessary, he said.
In October 2015, Dr. Kontos, Michael “Micky” Collins, PhD, and David O. Okonkwo, MD, PhD, directed a meeting with 37 participants from the fields of neurology, neuropsychology, neurosurgery, primary care, athletic training, and physical therapy to create a summary agreement that can assist clinicians with concussion treatment.
Nineteen guests, including representatives from professional sports organizations, the military, and public health, also attended the Targeted Evaluation and Active Management (TEAM) Approach to Treating Concussion meeting. The National Football League and UPMC sponsored the meeting, which was held in Pittsburgh.
Consensus documents have predominantly focused on things like the various definitions of concussion, how to assess concussion, and how to manage it, said Dr. Kontos. “We really wanted to focus on more of that end point of treatment and potentially more active treatment,” he said.
The TEAM participants developed and agreed upon 17 statements, which they plan to publish. At the AAN meeting, Dr. Kontos provided a brief review of some of the statements and discussed them in the context of recent research.
Rest’s Benefits and Limitations
Physical and cognitive rest, as part of an individualized treatment plan, are currently “the foundation of sport-related concussion management,” according to National Collegiate Athletic Association interassociation concussion guidelines. Rest after concussion conserves needed energy in the brain and reduces the likelihood of second impact syndrome and other catastrophic events, Dr. Kontos said. Furthermore, some studies have suggested that rest improves recovery. Brown et al reported in 2014 that athletes who self-reported more cognitive activity after a concussion took longer to recover than those who reported less cognitive activity.
However, the evidence to support rest is limited. In 2013, the Institute of Medicine and National Research Council published a report on sports-related concussion in youth that found little evidence regarding the efficacy of rest following concussion or to inform the best timing and approach for return to activity. Their statement “still resonates now,” Dr. Kontos said. “There’s very little empirical data to support what we do with rest. It’s largely an across-the-board policy that’s not data-driven, and we need to change that.” The TEAM group agreed “there is limited empirical evidence for the effectiveness of prescribed physical and cognitive rest, with no multisite trials for prescribed rest following concussion.”
Prescribed rest can have psychologic consequences, including emotional distress, depression, and anxiety. Rest allows individuals time to ruminate on their injury, which can exacerbate symptoms in self-report. Individuals who somaticize are particularly vulnerable to this effect. Jeremy M. Root, MD, of Children’s National Medical Center in Washington, DC, Dr. Kontos, and colleagues reported in April in the Journal of Pediatrics that patients who had high somatization scores were approximately five to seven times more likely to report an increase in symptoms at two weeks and four weeks, compared with those who were not in the highest quartile of somatization.
In addition, patients who are prescribed rest may think, “Wow, I must have a really bad injury such that I can’t do anything for a week.” This contextual framing effect may also influence the outcome, said Dr. Kontos.
Thomas et al in 2015 published the results of a randomized controlled trial that found that, after a concussion, patients ages 11 to 22 who were prescribed five days’ rest reported more daily postconcussive symptoms, compared with patients who were prescribed two days’ rest with progressive return to activity. Symptoms peaked at four days, and differences between groups remained at 10 days. “They have higher symptoms when they’re told to rest longer than if they’re told to rest less,” Dr. Kontos said. Clinically, there was no significant difference between groups in neurocognitive or balance outcomes, however.
The effect of treatment on the number of postconcussive symptoms may not be that straightforward, however. When Dr. Kontos, Dr. Thomas, and colleagues reanalyzed the data to look at patients who only reported symptoms (eg, headache, nausea, dizziness) but did not otherwise have early signs of concussion (eg, loss of consciousness, posttraumatic amnesia, disorientation, confusion), the symptoms-only group reported more symptoms at 10 days when prescribed five days’ rest, compared with two days’ rest with progressive return to activity. Patients who had early signs of concussion, however, reported fewer symptoms when prescribed five days’ rest versus two days’ rest with progressive return to activity.
“We have a sort of dichotomy here. We don’t want to say rest is bad. It may be very good for these people who have a high organic level or severity to their injury, and we may need to think in terms of resting them longer, whereas these patients [with symptoms only] certainly need to get more active, probably earlier in the process,” Dr. Kontos said.
Activity and social interaction may provide benefits. Miller et al in 2013 reported that environmental enrichment, including cognitive, physical, and social activity, is associated with improved outcome and sparing of hippocampal atrophy in the chronic stages of traumatic brain injury.
The TEAM group agreed, “Active treatment strategies may be initiated early in recovery following concussion.” The group also agreed, “strict brain rest (eg, ‘cocoon’ therapy) is not indicated and may have detrimental effects on patients following concussion.”
A Heterogeneous Injury
A focal point of the TEAM meeting was the concept of various clinical profiles of concussion. The group agreed, “Concussions are characterized by diverse symptoms and impairments in function resulting in different clinical profiles and recovery trajectories.”
“We need to think in terms of what type of concussion does this individual have and is it multiple types,” such as cognitive-fatigue, vestibular, or ocular, said Dr. Kontos. “We don’t typically just see one of these.” For example, a patient may have a predominant vestibular concussion with some posttraumatic migraine and neck involvement. “Oftentimes we see misdiagnoses when people show up. They’ve been diagnosed with cognitive issues when in reality they’re having vision or oculomotor difficulties.”
There are many potential approaches to categorizing, classifying, or profiling concussion, including those that consider posttraumatic mood and migraine as modifying factors, he said.
Multidisciplinary Teams
In addition, the TEAM group stated, “thorough multidomain assessment is warranted to properly evaluate the clinical profiles of concussion.” Various experts may be needed to assess cognitive, exertional, oculomotor, vestibular, and other symptoms and impairment.
As part of a multidisciplinary team, a neurologist, neuropsychologist, or primary care physician could “serve as kind of a point guard, to use a basketball analogy,” said Dr. Kontos. When an aspect of a patient’s assessment or treatment needs to be addressed more in depth, such as with regard to medication, vestibular therapy, or imaging, the patient may be referred to experts in those areas. “We try to work as a team and work back through the point guard to coordinate that care system,” he said. Telemedicine might allow for multidisciplinary treatment in remote geographic areas where establishing multidisciplinary teams otherwise might not be feasible, Dr. Kontos noted.
“Pharmacological therapy may be indicated in selected circumstances to treat certain symptoms and impairments related to concussion,” the TEAM group agreed.There is “very little” evidence for medicine in concussion, and drugs can exacerbate symptoms in some situations, Dr. Kontos said. Randomized controlled trials will help researchers better understand medication’s role in treating concussion.
More Active Treatment
In particular, patients who do not receive appropriate management after a concussion and then go to a clinic several months later with chronic symptoms may benefit from more active approaches to treatment, such as brisk walking.
Dr. Kontos described the case of an ice hockey player who was prescribed rest following a first concussion. After resting, the athlete began a return-to-play protocol that focused on aerobic exertion with no dynamic movements. As soon as the player returned to the ice, however, dizziness and headache came flooding back.
Several months later, the athlete was referred to a concussion clinic. The patient underwent a thorough evaluation that included vestibular and oculomotor assessments. Clinicians determined that the athlete needed more active treatment, including vision training and walking with head movements. In three weeks, the athlete returned to the ice. About a week later, the athlete resumed full-contact ice hockey.
“Prescribing rest is not the only approach,” Dr. Kontos said. “We need to move the discussion in different directions. We need to be more active with certain people and we need to be more targeted with our approaches.”
—Jake Remaly
Suggested Reading
Brown NJ, Mannix RC, O’Brien MJ, et al. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299-304.
Miller LS, Colella B, Mikulis D, et al. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Front Hum Neurosci. 2013;7:506.
Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016 Apr 5 [Epub ahead of print].
Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
Suggested Reading
Brown NJ, Mannix RC, O’Brien MJ, et al. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299-304.
Miller LS, Colella B, Mikulis D, et al. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Front Hum Neurosci. 2013;7:506.
Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016 Apr 5 [Epub ahead of print].
Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
Attention Deficit Therapy Improves Cognitive Deficits Associated With Epilepsy
VANCOUVER—Methylphenidate, a therapy approved for the treatment of attention deficit hyperactivity disorder (ADHD), lessens cognitive deficits associated with epilepsy, according to the results of a double-blind, placebo-controlled trial. Although the trial was small, the benefit was observed in multiple cognitive domains and persisted among patients who participated in an open-label extension after the double-blind portion of the study was completed.
“To the best of our knowledge, this is the first epilepsy study in adults using this type of established objective and standardized measures to evaluate multiple cognitive domains,” reported Jesse Adams, MD, who is completing a neuropsychiatry fellowship at Stanford University School of Medicine in California. The results of the trial, which was conducted with immediate-release methylphenidate, were presented by Dr. Adams at the 68th Annual Meeting of the American Academy of Neurology.
Thirty-five patients were enrolled and 31 completed the double-blind portion. Of those completing, 24 had focal seizure types, six had generalized seizure activity, and one had unclassified seizure activity. A broad array of seizure subtypes was represented. The age range of participants was 20 to 60 (median age, 35.3). The median duration of epilepsy was 12.5 years.
The study was conducted with a crossover design in three periods. Each patient received single dose placebo, 10 mg of methylphenidate, and 20 mg of methylphenidate in a random order one week apart. The primary cognitive measures were the Connors Continuous Performance Task (CPT), the Symbol-Digit Modalities Test (SDMT), and Medical College of Georgia Paragraph Memory Test (MCGPMT). These, along with additional cognitive tests employed as secondary measures, were administered at baseline and at the end of each period of treatment. Although not all differences reached statistical significance, cognitive performance on either dose of methylphenidate was consistently better than on placebo. The greatest difference was observed for the SDMT. Dr. Adams characterized the effect size in this measure as “moderate to large.” While other differences were more modest, the direction of benefit was consistently in favor of methylphenidate.
For example, an advantage was observed for CPT variables of hits, omissions, and detectability. No significant difference in cognitive performance could be detected when the two doses of methylphenidate were compared with each other.
Of the four patients who did not complete the double-blind portion of the study, one taking the 20-mg dose withdrew for cognitive problems, another taking the 20-mg was lost to follow-up, and one taking the 10-mg dose withdrew for agitation and tachycardia. A fourth participant who received 40 mg withdrew for tachycardia. However, the same patient participated in the open-label study on a lower dose without further complaints. Methylphenidate was otherwise well tolerated, although several patients, including those taking placebo, reported agitation.
At the end of the double-blind portion of the study, 30 participants elected to enter a four-week open-label extension. Patients were started on 5 mg or 10 mg with upward titration permitted as tolerated. Two patients left the extension before completion due to anxiety. However, testing at the end of this period continued to show improvements in cognitive function for those who remained on methylphenidate. In addition, improvement in a validated epilepsy quality-of-life instrument on methylphenidate was characterized as having “a large effect size.” Of the 28 patients who completed the open-label extension, 22 elected to continue taking methylphenidate.
Concern has been expressed about the potential for methylphenidate to trigger seizures, but this effect was not observed in this study. The seizure rate was not statistically different during the double-blind trial, compared with baseline.
—Theodore Bosworth
VANCOUVER—Methylphenidate, a therapy approved for the treatment of attention deficit hyperactivity disorder (ADHD), lessens cognitive deficits associated with epilepsy, according to the results of a double-blind, placebo-controlled trial. Although the trial was small, the benefit was observed in multiple cognitive domains and persisted among patients who participated in an open-label extension after the double-blind portion of the study was completed.
“To the best of our knowledge, this is the first epilepsy study in adults using this type of established objective and standardized measures to evaluate multiple cognitive domains,” reported Jesse Adams, MD, who is completing a neuropsychiatry fellowship at Stanford University School of Medicine in California. The results of the trial, which was conducted with immediate-release methylphenidate, were presented by Dr. Adams at the 68th Annual Meeting of the American Academy of Neurology.
Thirty-five patients were enrolled and 31 completed the double-blind portion. Of those completing, 24 had focal seizure types, six had generalized seizure activity, and one had unclassified seizure activity. A broad array of seizure subtypes was represented. The age range of participants was 20 to 60 (median age, 35.3). The median duration of epilepsy was 12.5 years.
The study was conducted with a crossover design in three periods. Each patient received single dose placebo, 10 mg of methylphenidate, and 20 mg of methylphenidate in a random order one week apart. The primary cognitive measures were the Connors Continuous Performance Task (CPT), the Symbol-Digit Modalities Test (SDMT), and Medical College of Georgia Paragraph Memory Test (MCGPMT). These, along with additional cognitive tests employed as secondary measures, were administered at baseline and at the end of each period of treatment. Although not all differences reached statistical significance, cognitive performance on either dose of methylphenidate was consistently better than on placebo. The greatest difference was observed for the SDMT. Dr. Adams characterized the effect size in this measure as “moderate to large.” While other differences were more modest, the direction of benefit was consistently in favor of methylphenidate.
For example, an advantage was observed for CPT variables of hits, omissions, and detectability. No significant difference in cognitive performance could be detected when the two doses of methylphenidate were compared with each other.
Of the four patients who did not complete the double-blind portion of the study, one taking the 20-mg dose withdrew for cognitive problems, another taking the 20-mg was lost to follow-up, and one taking the 10-mg dose withdrew for agitation and tachycardia. A fourth participant who received 40 mg withdrew for tachycardia. However, the same patient participated in the open-label study on a lower dose without further complaints. Methylphenidate was otherwise well tolerated, although several patients, including those taking placebo, reported agitation.
At the end of the double-blind portion of the study, 30 participants elected to enter a four-week open-label extension. Patients were started on 5 mg or 10 mg with upward titration permitted as tolerated. Two patients left the extension before completion due to anxiety. However, testing at the end of this period continued to show improvements in cognitive function for those who remained on methylphenidate. In addition, improvement in a validated epilepsy quality-of-life instrument on methylphenidate was characterized as having “a large effect size.” Of the 28 patients who completed the open-label extension, 22 elected to continue taking methylphenidate.
Concern has been expressed about the potential for methylphenidate to trigger seizures, but this effect was not observed in this study. The seizure rate was not statistically different during the double-blind trial, compared with baseline.
—Theodore Bosworth
VANCOUVER—Methylphenidate, a therapy approved for the treatment of attention deficit hyperactivity disorder (ADHD), lessens cognitive deficits associated with epilepsy, according to the results of a double-blind, placebo-controlled trial. Although the trial was small, the benefit was observed in multiple cognitive domains and persisted among patients who participated in an open-label extension after the double-blind portion of the study was completed.
“To the best of our knowledge, this is the first epilepsy study in adults using this type of established objective and standardized measures to evaluate multiple cognitive domains,” reported Jesse Adams, MD, who is completing a neuropsychiatry fellowship at Stanford University School of Medicine in California. The results of the trial, which was conducted with immediate-release methylphenidate, were presented by Dr. Adams at the 68th Annual Meeting of the American Academy of Neurology.
Thirty-five patients were enrolled and 31 completed the double-blind portion. Of those completing, 24 had focal seizure types, six had generalized seizure activity, and one had unclassified seizure activity. A broad array of seizure subtypes was represented. The age range of participants was 20 to 60 (median age, 35.3). The median duration of epilepsy was 12.5 years.
The study was conducted with a crossover design in three periods. Each patient received single dose placebo, 10 mg of methylphenidate, and 20 mg of methylphenidate in a random order one week apart. The primary cognitive measures were the Connors Continuous Performance Task (CPT), the Symbol-Digit Modalities Test (SDMT), and Medical College of Georgia Paragraph Memory Test (MCGPMT). These, along with additional cognitive tests employed as secondary measures, were administered at baseline and at the end of each period of treatment. Although not all differences reached statistical significance, cognitive performance on either dose of methylphenidate was consistently better than on placebo. The greatest difference was observed for the SDMT. Dr. Adams characterized the effect size in this measure as “moderate to large.” While other differences were more modest, the direction of benefit was consistently in favor of methylphenidate.
For example, an advantage was observed for CPT variables of hits, omissions, and detectability. No significant difference in cognitive performance could be detected when the two doses of methylphenidate were compared with each other.
Of the four patients who did not complete the double-blind portion of the study, one taking the 20-mg dose withdrew for cognitive problems, another taking the 20-mg was lost to follow-up, and one taking the 10-mg dose withdrew for agitation and tachycardia. A fourth participant who received 40 mg withdrew for tachycardia. However, the same patient participated in the open-label study on a lower dose without further complaints. Methylphenidate was otherwise well tolerated, although several patients, including those taking placebo, reported agitation.
At the end of the double-blind portion of the study, 30 participants elected to enter a four-week open-label extension. Patients were started on 5 mg or 10 mg with upward titration permitted as tolerated. Two patients left the extension before completion due to anxiety. However, testing at the end of this period continued to show improvements in cognitive function for those who remained on methylphenidate. In addition, improvement in a validated epilepsy quality-of-life instrument on methylphenidate was characterized as having “a large effect size.” Of the 28 patients who completed the open-label extension, 22 elected to continue taking methylphenidate.
Concern has been expressed about the potential for methylphenidate to trigger seizures, but this effect was not observed in this study. The seizure rate was not statistically different during the double-blind trial, compared with baseline.
—Theodore Bosworth
Poor Olfaction May Predict Rapid Cognitive Decline in Parkinson’s Disease
VANCOUVER—Among patients with Parkinson’s disease, the lowest level of olfactory function is associated with subjective cognitive impairment and more rapid decline in global cognition, compared with higher levels of olfactory function, according to an analysis described at the 68th Annual Meeting of the American Academy of Neurology. Furthermore, a combination of CSF biomarkers and olfactory testing may increase neurologists’ ability to identify patients at highest risk for cognitive decline and progression to mild cognitive impairment (MCI).
These two biomarkers may reflect the dual pathology of Lewy bodies and amyloid plaques observed in Parkinson’s disease–related MCI and Parkinson’s disease dementia and come together to give a more complete clinical picture, said Michelle Fullard, MD, a fellow at the Parkinson’s Disease Research, Education, and Clinical Center at the Philadelphia Veterans Affairs Medical Center.
Michelle Fullard, MD
The rate and risk of cognitive decline in Parkinson’s disease vary from person to person, and biomarkers could help identify patients at highest risk for this outcome. Data suggest that olfaction reflects the Lewy pathology present in Parkinson’s disease. In addition, approximately one-third of patients with Parkinson’s disease dementia meet the pathologic criteria for Alzheimer’s disease. Increased levels of CSF tau and decreased levels of CSF amyloid beta are associated with cognitive impairment in this population.
The Parkinson’s Progression Markers Initiative
Dr. Fullard and colleagues sought to examine the association between baseline olfaction and measures of cognition in a cohort of 423 patients with early Parkinson’s disease. The researchers investigated whether olfaction alone or in combination with CSF biomarkers predicts cognitive decline and conversion to MCI. They analyzed data from the Parkinson’s Progression Markers Initiative, an observational cohort study of patients with Parkinson’s disease. Eligible participants were untreated at enrollment, had been diagnosed within two years of enrollment, and had dopamine transporter deficit on imaging.
All participants underwent various assessments at predetermined time points. Dr. Fullard and colleagues specifically examined the University of Pennsylvania Smell Identification Test (UPSIT), which was administered at baseline, and the Montreal Cognitive Assessment (MOCA), which was administered at baseline and annually thereafter. The investigators also analyzed CSF tau and CSF beta amyloid levels at baseline.
For the data analysis, the researchers grouped the cohort into tertiles according to olfactory function. They performed a cross-sectional analysis to investigate the association between olfaction and measures of cognition. A linear mixed-effects model was used to identify predictors of cognitive decline. Finally, the investigators used Cox proportional hazards models to investigate conversion to MCI.
UPSIT Predicted Cognitive Decline
Approximately 91% of participants had olfactory impairment. About 35% of patients were anosmic, and these patients constituted the lowest tertile of olfaction. This group tended to be older and had a higher proportion of males, compared with the other tertiles. Dr. Fullard and colleagues adjusted for age and gender in subsequent analyses. Education and disease duration were similar among the three tertiles. Average disease duration was between six to seven months for the population.
Participants in the lowest tertile were more likely to report subjective nonmotor symptoms than participants in the other tertiles, as measured by Part 1A of the Unified Parkinson’s Disease Rating Scale. Patients in the lowest tertile also were more likely to report subjective cognitive impairment, compared with the rest of the cohort. After the researchers adjusted the data for age and sex, they found that MOCA scores were similar among the three tertiles.
When Dr. Fullard and colleagues considered the UPSIT and the CSF biomarkers as continuous variables, they found that each was significantly associated with decline in MOCA score. For every one-point decrease in UPSIT score, the MOCA score declined by 0.02 points per year. Using a separate linear mixed-effects model, the investigators noted that patients in the lowest amyloid beta tertile tended to have more cognitive decline, but the result was not statistically significant. Participants with the highest tau–amyloid beta ratios, however, had significantly more cognitive decline than other participants.
Next, Dr. Fullard’s group categorized the patients as having low-, medium-, or high-risk profiles based on olfaction and CSF biomarkers. When they analyzed the composite of olfaction and amyloid beta, as well as the composite for olfaction and tau–amyloid beta ratio, they noted that patients with the highest risk profiles had significantly more cognitive decline, as measured by the MOCA.
Furthermore, the researchers observed that patients with the worst olfaction, as measured by the UPSIT, appeared to have a higher rate of conversion to MCI, but the result did not reach statistical significance. Similarly, participants with the highest tau–amyloid beta ratio appeared to have a higher rate of conversion to MCI, but the finding was not significant. When the investigators combined the biomarkers using the risk profiles, participants with the highest risk profiles were 79% more likely to develop MCI during the three-year study period than other participants.
—Erik Greb
Suggested Reading
Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
Ham JH, Lee JJ, Sunwoo MK, et al. Effect of olfactory impairment and white matter hyperintensities on cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:95-99.
Lee JE, Cho KH, Ham JH, et al. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(2):186-191.
VANCOUVER—Among patients with Parkinson’s disease, the lowest level of olfactory function is associated with subjective cognitive impairment and more rapid decline in global cognition, compared with higher levels of olfactory function, according to an analysis described at the 68th Annual Meeting of the American Academy of Neurology. Furthermore, a combination of CSF biomarkers and olfactory testing may increase neurologists’ ability to identify patients at highest risk for cognitive decline and progression to mild cognitive impairment (MCI).
These two biomarkers may reflect the dual pathology of Lewy bodies and amyloid plaques observed in Parkinson’s disease–related MCI and Parkinson’s disease dementia and come together to give a more complete clinical picture, said Michelle Fullard, MD, a fellow at the Parkinson’s Disease Research, Education, and Clinical Center at the Philadelphia Veterans Affairs Medical Center.
Michelle Fullard, MD
The rate and risk of cognitive decline in Parkinson’s disease vary from person to person, and biomarkers could help identify patients at highest risk for this outcome. Data suggest that olfaction reflects the Lewy pathology present in Parkinson’s disease. In addition, approximately one-third of patients with Parkinson’s disease dementia meet the pathologic criteria for Alzheimer’s disease. Increased levels of CSF tau and decreased levels of CSF amyloid beta are associated with cognitive impairment in this population.
The Parkinson’s Progression Markers Initiative
Dr. Fullard and colleagues sought to examine the association between baseline olfaction and measures of cognition in a cohort of 423 patients with early Parkinson’s disease. The researchers investigated whether olfaction alone or in combination with CSF biomarkers predicts cognitive decline and conversion to MCI. They analyzed data from the Parkinson’s Progression Markers Initiative, an observational cohort study of patients with Parkinson’s disease. Eligible participants were untreated at enrollment, had been diagnosed within two years of enrollment, and had dopamine transporter deficit on imaging.
All participants underwent various assessments at predetermined time points. Dr. Fullard and colleagues specifically examined the University of Pennsylvania Smell Identification Test (UPSIT), which was administered at baseline, and the Montreal Cognitive Assessment (MOCA), which was administered at baseline and annually thereafter. The investigators also analyzed CSF tau and CSF beta amyloid levels at baseline.
For the data analysis, the researchers grouped the cohort into tertiles according to olfactory function. They performed a cross-sectional analysis to investigate the association between olfaction and measures of cognition. A linear mixed-effects model was used to identify predictors of cognitive decline. Finally, the investigators used Cox proportional hazards models to investigate conversion to MCI.
UPSIT Predicted Cognitive Decline
Approximately 91% of participants had olfactory impairment. About 35% of patients were anosmic, and these patients constituted the lowest tertile of olfaction. This group tended to be older and had a higher proportion of males, compared with the other tertiles. Dr. Fullard and colleagues adjusted for age and gender in subsequent analyses. Education and disease duration were similar among the three tertiles. Average disease duration was between six to seven months for the population.
Participants in the lowest tertile were more likely to report subjective nonmotor symptoms than participants in the other tertiles, as measured by Part 1A of the Unified Parkinson’s Disease Rating Scale. Patients in the lowest tertile also were more likely to report subjective cognitive impairment, compared with the rest of the cohort. After the researchers adjusted the data for age and sex, they found that MOCA scores were similar among the three tertiles.
When Dr. Fullard and colleagues considered the UPSIT and the CSF biomarkers as continuous variables, they found that each was significantly associated with decline in MOCA score. For every one-point decrease in UPSIT score, the MOCA score declined by 0.02 points per year. Using a separate linear mixed-effects model, the investigators noted that patients in the lowest amyloid beta tertile tended to have more cognitive decline, but the result was not statistically significant. Participants with the highest tau–amyloid beta ratios, however, had significantly more cognitive decline than other participants.
Next, Dr. Fullard’s group categorized the patients as having low-, medium-, or high-risk profiles based on olfaction and CSF biomarkers. When they analyzed the composite of olfaction and amyloid beta, as well as the composite for olfaction and tau–amyloid beta ratio, they noted that patients with the highest risk profiles had significantly more cognitive decline, as measured by the MOCA.
Furthermore, the researchers observed that patients with the worst olfaction, as measured by the UPSIT, appeared to have a higher rate of conversion to MCI, but the result did not reach statistical significance. Similarly, participants with the highest tau–amyloid beta ratio appeared to have a higher rate of conversion to MCI, but the finding was not significant. When the investigators combined the biomarkers using the risk profiles, participants with the highest risk profiles were 79% more likely to develop MCI during the three-year study period than other participants.
—Erik Greb
VANCOUVER—Among patients with Parkinson’s disease, the lowest level of olfactory function is associated with subjective cognitive impairment and more rapid decline in global cognition, compared with higher levels of olfactory function, according to an analysis described at the 68th Annual Meeting of the American Academy of Neurology. Furthermore, a combination of CSF biomarkers and olfactory testing may increase neurologists’ ability to identify patients at highest risk for cognitive decline and progression to mild cognitive impairment (MCI).
These two biomarkers may reflect the dual pathology of Lewy bodies and amyloid plaques observed in Parkinson’s disease–related MCI and Parkinson’s disease dementia and come together to give a more complete clinical picture, said Michelle Fullard, MD, a fellow at the Parkinson’s Disease Research, Education, and Clinical Center at the Philadelphia Veterans Affairs Medical Center.
Michelle Fullard, MD
The rate and risk of cognitive decline in Parkinson’s disease vary from person to person, and biomarkers could help identify patients at highest risk for this outcome. Data suggest that olfaction reflects the Lewy pathology present in Parkinson’s disease. In addition, approximately one-third of patients with Parkinson’s disease dementia meet the pathologic criteria for Alzheimer’s disease. Increased levels of CSF tau and decreased levels of CSF amyloid beta are associated with cognitive impairment in this population.
The Parkinson’s Progression Markers Initiative
Dr. Fullard and colleagues sought to examine the association between baseline olfaction and measures of cognition in a cohort of 423 patients with early Parkinson’s disease. The researchers investigated whether olfaction alone or in combination with CSF biomarkers predicts cognitive decline and conversion to MCI. They analyzed data from the Parkinson’s Progression Markers Initiative, an observational cohort study of patients with Parkinson’s disease. Eligible participants were untreated at enrollment, had been diagnosed within two years of enrollment, and had dopamine transporter deficit on imaging.
All participants underwent various assessments at predetermined time points. Dr. Fullard and colleagues specifically examined the University of Pennsylvania Smell Identification Test (UPSIT), which was administered at baseline, and the Montreal Cognitive Assessment (MOCA), which was administered at baseline and annually thereafter. The investigators also analyzed CSF tau and CSF beta amyloid levels at baseline.
For the data analysis, the researchers grouped the cohort into tertiles according to olfactory function. They performed a cross-sectional analysis to investigate the association between olfaction and measures of cognition. A linear mixed-effects model was used to identify predictors of cognitive decline. Finally, the investigators used Cox proportional hazards models to investigate conversion to MCI.
UPSIT Predicted Cognitive Decline
Approximately 91% of participants had olfactory impairment. About 35% of patients were anosmic, and these patients constituted the lowest tertile of olfaction. This group tended to be older and had a higher proportion of males, compared with the other tertiles. Dr. Fullard and colleagues adjusted for age and gender in subsequent analyses. Education and disease duration were similar among the three tertiles. Average disease duration was between six to seven months for the population.
Participants in the lowest tertile were more likely to report subjective nonmotor symptoms than participants in the other tertiles, as measured by Part 1A of the Unified Parkinson’s Disease Rating Scale. Patients in the lowest tertile also were more likely to report subjective cognitive impairment, compared with the rest of the cohort. After the researchers adjusted the data for age and sex, they found that MOCA scores were similar among the three tertiles.
When Dr. Fullard and colleagues considered the UPSIT and the CSF biomarkers as continuous variables, they found that each was significantly associated with decline in MOCA score. For every one-point decrease in UPSIT score, the MOCA score declined by 0.02 points per year. Using a separate linear mixed-effects model, the investigators noted that patients in the lowest amyloid beta tertile tended to have more cognitive decline, but the result was not statistically significant. Participants with the highest tau–amyloid beta ratios, however, had significantly more cognitive decline than other participants.
Next, Dr. Fullard’s group categorized the patients as having low-, medium-, or high-risk profiles based on olfaction and CSF biomarkers. When they analyzed the composite of olfaction and amyloid beta, as well as the composite for olfaction and tau–amyloid beta ratio, they noted that patients with the highest risk profiles had significantly more cognitive decline, as measured by the MOCA.
Furthermore, the researchers observed that patients with the worst olfaction, as measured by the UPSIT, appeared to have a higher rate of conversion to MCI, but the result did not reach statistical significance. Similarly, participants with the highest tau–amyloid beta ratio appeared to have a higher rate of conversion to MCI, but the finding was not significant. When the investigators combined the biomarkers using the risk profiles, participants with the highest risk profiles were 79% more likely to develop MCI during the three-year study period than other participants.
—Erik Greb
Suggested Reading
Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
Ham JH, Lee JJ, Sunwoo MK, et al. Effect of olfactory impairment and white matter hyperintensities on cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:95-99.
Lee JE, Cho KH, Ham JH, et al. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(2):186-191.
Suggested Reading
Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
Ham JH, Lee JJ, Sunwoo MK, et al. Effect of olfactory impairment and white matter hyperintensities on cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:95-99.
Lee JE, Cho KH, Ham JH, et al. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(2):186-191.
Adjunctive Everolimus Reduces Seizures Associated With Tuberous Sclerosis Complex
VANCOUVER—Adjunctive treatment with everolimus significantly reduces seizure frequency in patients with treatment-resistant seizures associated with tuberous sclerosis complex (TSC), according to a trial described at the 68th Annual Meeting of the American Academy of Neurology. The drug appears not to be associated with unexpected adverse events in this indication.
“Results from this [study] … suggest that one can use everolimus as an antiepilepsy therapy, which treats the underlying cause of seizures,” said Jacqueline A. French, MD, Professor of Neurology at NYU Langone Comprehensive Epilepsy Center in New York City. “Whether it in fact is also disease-modifying for epilepsy and [whether] continued therapy will produce a continued improvement remain to be seen.”
Comparing Two Drug Concentrations With Placebo
She and her colleagues conducted a randomized, double-blind, placebo-controlled trial of everolimus in patients with TSC. Eligible participants were between ages 2 and 65 and had at least 16 treatment-resistant seizures in the eight-week baseline phase. Participants also had to be taking one to three antiepileptic drugs at stable concentrations for four weeks before enrollment, as well as through the study’s eight-week baseline phase.
Participants were randomized to adjunctive treatment with placebo, a low serum concentration of everolimus (ie, 3 to 7 ng/mL), or a high serum concentration of everolimus (ie, 9 to 15 ng/mL). Concentrations were titrated during the first eight weeks to achieve stable serum concentrations of treatment. A 12-week maintenance phase followed. Seizures were counted during the titration and maintenance phases. At the end of the maintenance phase, patients had the option of continuing in the extension phase of the trial, which is ongoing. The primary end points were percentage seizure reduction and 50% responder rate.
In all, 119 patients received placebo, 117 received low-concentration everolimus, and 130 received high-concentration everolimus. The treatment arms were evenly matched, in terms of age and gender. Approximately half of participants were female. Five patients in the placebo arm discontinued the study, compared with seven in the low-concentration group and eight in the high-concentration group. Five discontinuations in the low-concentration group resulted from adverse events, including stomatitis, anxiety, immunodeficiency, and pyrexia. Four discontinuations in the high-concentration group resulted from adverse events, including mouth ulceration, neutropenia, pneumonia, and stomatitis. Most patients chose to enter the extension phase.
The study population was highly resistant to treatment. Approximately half of participants had failed more than six antiepileptic drugs before enrollment, and about half were taking three antiepileptic drugs at enrollment. Median baseline seizure frequency per 28 days was higher than the requirement for enrollment. Several participants were receiving treatment with vagus nerve stimulation or the ketogenic diet. Patients had various seizure types and syndromes.
Treatment Yielded Clinically Meaningful Improvement
The median percent seizure reduction was significantly greater with low-concentration everolimus (29.3%) and high-concentration everolimus (39.6%), compared with placebo (14.9%). The 50% responder rate also was significantly greater with low-concentration everolimus (28.2%) and high-concentration everolimus (40%), compared with placebo (15.1%).
Approximately 5% of participants in the low-concentration group became seizure-free, along with 3.8% of participants in the high-concentration group versus 0.8% for placebo. Patients receiving placebo had a greater likelihood of seizure worsening, compared with patients receiving everolimus. Participants who received everolimus were more likely to improve, compared with controls. Approximately 15% of the patients in the high-concentration group had a 75% to 100% improvement in seizures.
Approximately 17% of participants in the low-concentration group and 23% of participants in the high-concentration group had severe or life-threatening adverse events versus 10.9% on placebo. The most common adverse events were stomatitis, diarrhea, mouth ulceration, nasal pharyngitis, and upper respiratory tract infection.
“This study demonstrated a statistically significant and clinically meaningful reduction in seizure frequency in patients with treatment-resistant seizures associated with TSC,” said Dr. French. “As we learn more, we find that there are more causes of epilepsy that relate to abnormalities in the mTOR pathway, including … some focal cortical dysplasias,” she continued. Although Dr. French was discussing a cohort of patients with tuberous sclerosis, “it could be that this pathway and interventions in this pathway will be very important for other common forms of epilepsy as well.”
—Erik Greb
Suggested Reading
Fukumura S, Watanabe T, Takayama R, et al. Everolimus treatment for an early infantile subependymal giant cell astrocytoma with tuberous sclerosis complex. J Child Neurol. 2015;30(9):1192-1195.
Tran LH, Zupanc ML. Long-term everolimus treatment in individuals with tuberous sclerosis complex: a review of the current literature. Pediatr Neurol. 2015;53(1):23-30.
VANCOUVER—Adjunctive treatment with everolimus significantly reduces seizure frequency in patients with treatment-resistant seizures associated with tuberous sclerosis complex (TSC), according to a trial described at the 68th Annual Meeting of the American Academy of Neurology. The drug appears not to be associated with unexpected adverse events in this indication.
“Results from this [study] … suggest that one can use everolimus as an antiepilepsy therapy, which treats the underlying cause of seizures,” said Jacqueline A. French, MD, Professor of Neurology at NYU Langone Comprehensive Epilepsy Center in New York City. “Whether it in fact is also disease-modifying for epilepsy and [whether] continued therapy will produce a continued improvement remain to be seen.”
Comparing Two Drug Concentrations With Placebo
She and her colleagues conducted a randomized, double-blind, placebo-controlled trial of everolimus in patients with TSC. Eligible participants were between ages 2 and 65 and had at least 16 treatment-resistant seizures in the eight-week baseline phase. Participants also had to be taking one to three antiepileptic drugs at stable concentrations for four weeks before enrollment, as well as through the study’s eight-week baseline phase.
Participants were randomized to adjunctive treatment with placebo, a low serum concentration of everolimus (ie, 3 to 7 ng/mL), or a high serum concentration of everolimus (ie, 9 to 15 ng/mL). Concentrations were titrated during the first eight weeks to achieve stable serum concentrations of treatment. A 12-week maintenance phase followed. Seizures were counted during the titration and maintenance phases. At the end of the maintenance phase, patients had the option of continuing in the extension phase of the trial, which is ongoing. The primary end points were percentage seizure reduction and 50% responder rate.
In all, 119 patients received placebo, 117 received low-concentration everolimus, and 130 received high-concentration everolimus. The treatment arms were evenly matched, in terms of age and gender. Approximately half of participants were female. Five patients in the placebo arm discontinued the study, compared with seven in the low-concentration group and eight in the high-concentration group. Five discontinuations in the low-concentration group resulted from adverse events, including stomatitis, anxiety, immunodeficiency, and pyrexia. Four discontinuations in the high-concentration group resulted from adverse events, including mouth ulceration, neutropenia, pneumonia, and stomatitis. Most patients chose to enter the extension phase.
The study population was highly resistant to treatment. Approximately half of participants had failed more than six antiepileptic drugs before enrollment, and about half were taking three antiepileptic drugs at enrollment. Median baseline seizure frequency per 28 days was higher than the requirement for enrollment. Several participants were receiving treatment with vagus nerve stimulation or the ketogenic diet. Patients had various seizure types and syndromes.
Treatment Yielded Clinically Meaningful Improvement
The median percent seizure reduction was significantly greater with low-concentration everolimus (29.3%) and high-concentration everolimus (39.6%), compared with placebo (14.9%). The 50% responder rate also was significantly greater with low-concentration everolimus (28.2%) and high-concentration everolimus (40%), compared with placebo (15.1%).
Approximately 5% of participants in the low-concentration group became seizure-free, along with 3.8% of participants in the high-concentration group versus 0.8% for placebo. Patients receiving placebo had a greater likelihood of seizure worsening, compared with patients receiving everolimus. Participants who received everolimus were more likely to improve, compared with controls. Approximately 15% of the patients in the high-concentration group had a 75% to 100% improvement in seizures.
Approximately 17% of participants in the low-concentration group and 23% of participants in the high-concentration group had severe or life-threatening adverse events versus 10.9% on placebo. The most common adverse events were stomatitis, diarrhea, mouth ulceration, nasal pharyngitis, and upper respiratory tract infection.
“This study demonstrated a statistically significant and clinically meaningful reduction in seizure frequency in patients with treatment-resistant seizures associated with TSC,” said Dr. French. “As we learn more, we find that there are more causes of epilepsy that relate to abnormalities in the mTOR pathway, including … some focal cortical dysplasias,” she continued. Although Dr. French was discussing a cohort of patients with tuberous sclerosis, “it could be that this pathway and interventions in this pathway will be very important for other common forms of epilepsy as well.”
—Erik Greb
VANCOUVER—Adjunctive treatment with everolimus significantly reduces seizure frequency in patients with treatment-resistant seizures associated with tuberous sclerosis complex (TSC), according to a trial described at the 68th Annual Meeting of the American Academy of Neurology. The drug appears not to be associated with unexpected adverse events in this indication.
“Results from this [study] … suggest that one can use everolimus as an antiepilepsy therapy, which treats the underlying cause of seizures,” said Jacqueline A. French, MD, Professor of Neurology at NYU Langone Comprehensive Epilepsy Center in New York City. “Whether it in fact is also disease-modifying for epilepsy and [whether] continued therapy will produce a continued improvement remain to be seen.”
Comparing Two Drug Concentrations With Placebo
She and her colleagues conducted a randomized, double-blind, placebo-controlled trial of everolimus in patients with TSC. Eligible participants were between ages 2 and 65 and had at least 16 treatment-resistant seizures in the eight-week baseline phase. Participants also had to be taking one to three antiepileptic drugs at stable concentrations for four weeks before enrollment, as well as through the study’s eight-week baseline phase.
Participants were randomized to adjunctive treatment with placebo, a low serum concentration of everolimus (ie, 3 to 7 ng/mL), or a high serum concentration of everolimus (ie, 9 to 15 ng/mL). Concentrations were titrated during the first eight weeks to achieve stable serum concentrations of treatment. A 12-week maintenance phase followed. Seizures were counted during the titration and maintenance phases. At the end of the maintenance phase, patients had the option of continuing in the extension phase of the trial, which is ongoing. The primary end points were percentage seizure reduction and 50% responder rate.
In all, 119 patients received placebo, 117 received low-concentration everolimus, and 130 received high-concentration everolimus. The treatment arms were evenly matched, in terms of age and gender. Approximately half of participants were female. Five patients in the placebo arm discontinued the study, compared with seven in the low-concentration group and eight in the high-concentration group. Five discontinuations in the low-concentration group resulted from adverse events, including stomatitis, anxiety, immunodeficiency, and pyrexia. Four discontinuations in the high-concentration group resulted from adverse events, including mouth ulceration, neutropenia, pneumonia, and stomatitis. Most patients chose to enter the extension phase.
The study population was highly resistant to treatment. Approximately half of participants had failed more than six antiepileptic drugs before enrollment, and about half were taking three antiepileptic drugs at enrollment. Median baseline seizure frequency per 28 days was higher than the requirement for enrollment. Several participants were receiving treatment with vagus nerve stimulation or the ketogenic diet. Patients had various seizure types and syndromes.
Treatment Yielded Clinically Meaningful Improvement
The median percent seizure reduction was significantly greater with low-concentration everolimus (29.3%) and high-concentration everolimus (39.6%), compared with placebo (14.9%). The 50% responder rate also was significantly greater with low-concentration everolimus (28.2%) and high-concentration everolimus (40%), compared with placebo (15.1%).
Approximately 5% of participants in the low-concentration group became seizure-free, along with 3.8% of participants in the high-concentration group versus 0.8% for placebo. Patients receiving placebo had a greater likelihood of seizure worsening, compared with patients receiving everolimus. Participants who received everolimus were more likely to improve, compared with controls. Approximately 15% of the patients in the high-concentration group had a 75% to 100% improvement in seizures.
Approximately 17% of participants in the low-concentration group and 23% of participants in the high-concentration group had severe or life-threatening adverse events versus 10.9% on placebo. The most common adverse events were stomatitis, diarrhea, mouth ulceration, nasal pharyngitis, and upper respiratory tract infection.
“This study demonstrated a statistically significant and clinically meaningful reduction in seizure frequency in patients with treatment-resistant seizures associated with TSC,” said Dr. French. “As we learn more, we find that there are more causes of epilepsy that relate to abnormalities in the mTOR pathway, including … some focal cortical dysplasias,” she continued. Although Dr. French was discussing a cohort of patients with tuberous sclerosis, “it could be that this pathway and interventions in this pathway will be very important for other common forms of epilepsy as well.”
—Erik Greb
Suggested Reading
Fukumura S, Watanabe T, Takayama R, et al. Everolimus treatment for an early infantile subependymal giant cell astrocytoma with tuberous sclerosis complex. J Child Neurol. 2015;30(9):1192-1195.
Tran LH, Zupanc ML. Long-term everolimus treatment in individuals with tuberous sclerosis complex: a review of the current literature. Pediatr Neurol. 2015;53(1):23-30.
Suggested Reading
Fukumura S, Watanabe T, Takayama R, et al. Everolimus treatment for an early infantile subependymal giant cell astrocytoma with tuberous sclerosis complex. J Child Neurol. 2015;30(9):1192-1195.
Tran LH, Zupanc ML. Long-term everolimus treatment in individuals with tuberous sclerosis complex: a review of the current literature. Pediatr Neurol. 2015;53(1):23-30.
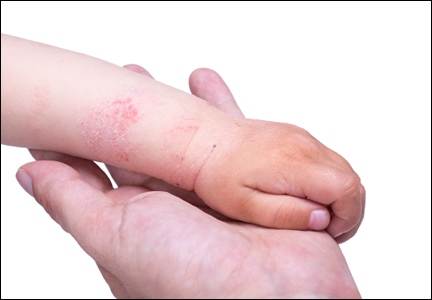
A Practical Overview of Pediatric Atopic Dermatitis, Part 3: Differential Diagnosis, Comorbidities, and Measurement of Disease Burden
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
Practice Points
- Atopic dermatitis (AD) has a variety of comorbidities including psychosocial disorders, obesity, and infection.
- A variety of skin conditions can mimic AD.
- Atopic dermatitis can be complicated by coinfections.
Everything You Want to Know About Living with a Hospitalist
As a hospitalist, patient satisfaction is top of mind. Then there’s another group of people whose satisfaction is also paramount: your family. What do they have to say about life with a hospitalist? You’re about to find out!
The Hospitalist asked family members of David Pressel, MD, PhD, a pediatric hospitalist at A.I. DuPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist, for their impressions, and wife Karen and son Rob’s honest answers (and gentle ribbings) show that for whatever ups and downs life may bring, being part of a hospitalist’s family is full of rewards and lots of love. Of course, that’s not to say they didn’t have some suggestions for improvements.
For Hospitalists’ Spouses Everywhere
Marrying a doctor was never on my to-do list. In fact, my list specified quite the opposite; I was never going to marry a physician. My stereotypical perception of the lives of physicians included long hours, too much stress, no family time, guaranteed interruptions at social events, calls at all hours of the day, never enough sleep—you get the picture. I imagined too many headaches to make being a “doctor’s wife” in the slightest bit enticing. I wanted no part of it, and besides, I had my own career to think about.
But then I met my husband, and my list went out the window.
Still, after a couple decades of negotiating a balance between the demands of his job (see above) and the demands of his family, there are things I’d like to say to him. So here goes. Hospitalists, take note.
There Are Only 24 Hours in a Day
How many times have you called to say, “I’ll be leaving the hospital in 10 minutes”? How long did it take for me to realize that relying on that kind of statement was crazy? I’m embarrassed to say that it took me way longer than it should have to come to that understanding. After many overcooked dinners and missed social events, I finally realized that your anticipated departure held no validity and I could only trust that you had left the hospital when you called from the car with the wheels rolling. Fortunately, you were more astute than I and changed your communication habits rather quickly, although the timing of said notifications still does not always take traffic into account and could use some work.
Still, I recognize that “leaving the hospital” is really just a physical indicator of your location, not necessarily a reflection of your state of mind. When you get home, please tell me if you still have work to do (notes, email, patient follow-up) or if you are done for the day. I suppose second-guessing your clinical decisions and calling the hospital to check on patients are unavoidable, but give me a clue whether I should actually expect you fully home to join the rest of the family—or if you will just be working at the home “nursing station” all evening. The burden of healthcare in America doesn’t fall on just you. If you can’t figure out what is wrong with a patient or don’t know what to do, you have many colleagues who can help.
Please remember that you are only one person. Don’t think that if there is a staffing shortage to fill, the responsibility for working is yours. Your colleagues are wonderful and, almost without exception, are happy to pitch in to help carry the extra load. The same goes with holidays; you don’t need to work more than everyone else. I know you are not a slacker. If you try to spread the load when you manage patient care and work schedules, you will have a happier spouse. Remember, a happy wife is a happy life. (I’m sure there is an analogous saying for your colleagues’ husbands and partners.)
Along those same lines, please limit the moonlighting you choose to do. My preconceived idea of a physician’s salary was very different from your reality. You are a pediatrician, an academic pediatrician. Having said that, we lead a wonderful life. We have what we need and have been very happy without the fanciness of some of our neighbors. Although the extra income is nice, I’d rather see more of you than more money. Besides, we just wrote the last check for college tuition, and I’m sure the boys will never ask us for money again.
Being Grumpy (No, Not the Dwarf)
My thoughts on moonlighting lead me perfectly to a discussion of your frame of mind: your mood. By definition, your patients are seriously ill hospitalized children. The bursting hospital census, the acuity of your patients, and the relative craziness of some of their parents invariably elevate your stress level. This, in turn, drives more frequent calls to the hospital and time on the computer all hours of the day or night. This does not allow for a restful sleep, when you sleep at all. I may be biased, but I think you are in the minority of hospitalists who bring their jobs home. Not that I’m complaining too loudly; this is who you are and why I love you, but if you haven’t noticed, when you are on service you tend to get grumpy. Think about this: If you’re not on call, why not turn off your pager, turn off your phone, and leave email alone?
Given the pressures inherent in your job, please tell me again why you would want to moonlight. Moonlighting means even longer hours, more stress, and less sleep for you, all of which make you grumpier and, as a result, tend to make me grumpy.
No, thank you.
Everyone we know has some form of “honey-do” list, whether intended for himself or herself, a spouse, or a professional. I know it makes you feel like a competent husband and man to do things around the house, but here’s a bit of advice: Let me hire someone else. Keep in mind that contractors were invented for good reason. The aggravation you’ll have trying to fit whatever project we’ve contemplated into your schedule will be dwarfed by the aggravation I’ll have when you can’t. I’ve never heard you ruminate about not cutting the lawn after we hired the landscaper and you got rid of the lawnmower.
The same goes for quality. Do you really think you did anywhere near as good a job replacing the leaking toilet as a real plumber? Should we talk about the breakfast room light fixture? Do you want me to continue?
My annoyance probably lessened any satisfaction you derived by completing these projects yourself. You should always keep the Pressel money-management credo forefront in your mind: “You earn it, I spend it.” Please let me do my job.
Let Me See If “The Doctor” Is In
Please leave the professor at the office; don’t talk too much medicine when you are not at work. Your trainees might need to hear all the minute details of whatever medical issue is at hand, but your family and friends do not. Most of those close to us chose careers outside of medicine a long time ago and probably don’t want to change direction now. Why do you think they call me for medical advice? It’s not because I’m a better doctor but because they know they’ll hear one of two things:
- I’ll tell them I don’t have a clue and they should ask you; or
- I’ll answer their questions in a tenth of the time that it would have taken you. And we’re talking easy questions because, while I’ve listened to you speak to medical students and residents for the last 20 years, we both know I am not a doctor.
Nevertheless, I do pretty well even with some of the hard questions, if I say so myself. Don’t worry though, there’s no need for concern. Please know that I am not practicing with your license.
Relative to the home practice of medicine, it’s OK to look in our kids’ ears! You must remember the huge fight we had when our son exhibited all of the classic signs of an ear infection and you refused to examine his ears. I know you agonize when you make a clinical error with a patient, but this was just an ear infection. I would have taken him to a real doctor if he was sick enough to merit consideration of what you were worried about missing (brain abscess or meningitis). Really? If I had known how to work your otoscope back then, I would have looked in his ears myself. I’m still not sure how treating minor illnesses in our children is different from the same thing with children of our friends.
You have a perfectly reasonable excuse to be exhausted, yet you are often embarrassed when you fall asleep at our friends’ houses during social events. But the truth is they consider it a mark of true friendship when you go missing before dessert is served. When we were still new in the area and someone would realize that you had disappeared, I was mortified. I quickly realized though that our friends would all rather you and I join them than stay home entirely. No one is offended to find you asleep on the sofa (and your disappearance is now almost expected). To tell you the truth, I’m not sure anyone misses your conversation.
Meetings make the world go round, and your attendance is obligatory at many, even if you’d rather not attend. When I was still working, someone came up with the idea of a stand-up meeting. It was a brilliant idea that made meeting participants use the time more efficiently. Why don’t you propose that some of your administrative meetings be run that way rather than depending on me to page you, “Dr. Pressel, we need you urgently in room 23!”? Sorry I’m calling you out on this, but I’m not always available at the exact time you’ve specified that you want to be interrupted. Besides, it is sometimes amusing to hear that you fell asleep at some senior hospital administrator’s meeting.
I started this by writing that I never wanted to marry a physician, but the last quarter century with you has been the adventure of a lifetime. I just sometimes ask myself, “Why didn’t he become a dermatologist?” TH
Karen Pressel is the wife of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
What I Want My Hospitalist Father to Know
Let me start out by saying that I think you have a great job and I am proud of you. But there are some things you should know. I’ll begin with the good ones.
We lead a very comfortable life, and I am grateful for all that you do for me. You don’t need to remind me, though, every time you manually scoop poop from some constipated kid that it pays for the roof over my head, clothes on my back, and my expensive university education.
I get it.
Even so, having a parent who is a physician is way better than having a parent who is, say, an accountant. I don’t need help with my taxes, but it sure is nice to get some quick medical advice when I have a rash. I even still trust you after you missed my broken arm when I was in sixth grade. Do me a favor though: Just tell me what it is and how I can fix the problem. Save the lecture on the pathophysiology, epidemiology, and differential diagnosis for your residents and medical students. It’s only poison ivy.
When we were growing up, you always gave us a “case of the week.” There were some consistent themes, and I’ve never been sure if these patients were real or fake. Most were either adolescent girls with belly pain or children experiencing bizarre spells who ended up being intoxicated from some ingestion. Was there supposed to be a not-so-subtle message here not to use drugs and to choose my romantic interests carefully?
I actually enjoy hearing about interesting patients, although maybe you could vary the cases, focusing more on human-interest situations rather than on complex technical patients. Relative to the human-interest stories, shouldn’t some of the names parents give to their children be considered child abuse? You probably don’t know, but in Iceland, there is a government Naming Committee that actually maintains a list of approved children’s names.
I know you have to take both clinical and administrative calls. When you get a medical call while we’re having dinner, would you please go somewhere else to talk? Hearing you ask about a patient’s diarrhea when we are eating sort of ruins my appetite.
Similarly, please let me vet topics before you discuss them with my friends. You have some cool stories, but Dad, I’m not sure my friends want to hear about child abuse or vaginal discharge. I will say that the absolute best phone calls you get occur when, after 22 years of Pressel Medical School, I’m able to make the diagnosis or give the correct advice (sometimes faster than your medical students and residents).
Let’s talk about what you learned from me. Though you may not agree, you should think of all the times you found me annoying, particularly when I was a pain-in-the-ass kid, as CME. Over the years, I gave you regular opportunities to enhance your knowledge of child development and to improve your parenting skills—things that undoubtedly continue to help you as a pediatrician.
I like visiting you in the hospital. I know you enjoy showing me off. When you introduce me to your coworkers, it’s OK if you tell them I’m not going to medical school. Still, you should know that I fully intend to repay all that you have done.
Hearing from you about all that happens in a hospital, I can understand why you never want to be a patient. I’ll do my best to ensure you don’t get admitted to a hospital and are able to die peacefully at home. You can count on your loving son, Dad. I’ll be sure you don’t have a hospitalist with you at the end. TH
Rob Pressel is the son of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
As a hospitalist, patient satisfaction is top of mind. Then there’s another group of people whose satisfaction is also paramount: your family. What do they have to say about life with a hospitalist? You’re about to find out!
The Hospitalist asked family members of David Pressel, MD, PhD, a pediatric hospitalist at A.I. DuPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist, for their impressions, and wife Karen and son Rob’s honest answers (and gentle ribbings) show that for whatever ups and downs life may bring, being part of a hospitalist’s family is full of rewards and lots of love. Of course, that’s not to say they didn’t have some suggestions for improvements.
For Hospitalists’ Spouses Everywhere
Marrying a doctor was never on my to-do list. In fact, my list specified quite the opposite; I was never going to marry a physician. My stereotypical perception of the lives of physicians included long hours, too much stress, no family time, guaranteed interruptions at social events, calls at all hours of the day, never enough sleep—you get the picture. I imagined too many headaches to make being a “doctor’s wife” in the slightest bit enticing. I wanted no part of it, and besides, I had my own career to think about.
But then I met my husband, and my list went out the window.
Still, after a couple decades of negotiating a balance between the demands of his job (see above) and the demands of his family, there are things I’d like to say to him. So here goes. Hospitalists, take note.
There Are Only 24 Hours in a Day
How many times have you called to say, “I’ll be leaving the hospital in 10 minutes”? How long did it take for me to realize that relying on that kind of statement was crazy? I’m embarrassed to say that it took me way longer than it should have to come to that understanding. After many overcooked dinners and missed social events, I finally realized that your anticipated departure held no validity and I could only trust that you had left the hospital when you called from the car with the wheels rolling. Fortunately, you were more astute than I and changed your communication habits rather quickly, although the timing of said notifications still does not always take traffic into account and could use some work.
Still, I recognize that “leaving the hospital” is really just a physical indicator of your location, not necessarily a reflection of your state of mind. When you get home, please tell me if you still have work to do (notes, email, patient follow-up) or if you are done for the day. I suppose second-guessing your clinical decisions and calling the hospital to check on patients are unavoidable, but give me a clue whether I should actually expect you fully home to join the rest of the family—or if you will just be working at the home “nursing station” all evening. The burden of healthcare in America doesn’t fall on just you. If you can’t figure out what is wrong with a patient or don’t know what to do, you have many colleagues who can help.
Please remember that you are only one person. Don’t think that if there is a staffing shortage to fill, the responsibility for working is yours. Your colleagues are wonderful and, almost without exception, are happy to pitch in to help carry the extra load. The same goes with holidays; you don’t need to work more than everyone else. I know you are not a slacker. If you try to spread the load when you manage patient care and work schedules, you will have a happier spouse. Remember, a happy wife is a happy life. (I’m sure there is an analogous saying for your colleagues’ husbands and partners.)
Along those same lines, please limit the moonlighting you choose to do. My preconceived idea of a physician’s salary was very different from your reality. You are a pediatrician, an academic pediatrician. Having said that, we lead a wonderful life. We have what we need and have been very happy without the fanciness of some of our neighbors. Although the extra income is nice, I’d rather see more of you than more money. Besides, we just wrote the last check for college tuition, and I’m sure the boys will never ask us for money again.
Being Grumpy (No, Not the Dwarf)
My thoughts on moonlighting lead me perfectly to a discussion of your frame of mind: your mood. By definition, your patients are seriously ill hospitalized children. The bursting hospital census, the acuity of your patients, and the relative craziness of some of their parents invariably elevate your stress level. This, in turn, drives more frequent calls to the hospital and time on the computer all hours of the day or night. This does not allow for a restful sleep, when you sleep at all. I may be biased, but I think you are in the minority of hospitalists who bring their jobs home. Not that I’m complaining too loudly; this is who you are and why I love you, but if you haven’t noticed, when you are on service you tend to get grumpy. Think about this: If you’re not on call, why not turn off your pager, turn off your phone, and leave email alone?
Given the pressures inherent in your job, please tell me again why you would want to moonlight. Moonlighting means even longer hours, more stress, and less sleep for you, all of which make you grumpier and, as a result, tend to make me grumpy.
No, thank you.
Everyone we know has some form of “honey-do” list, whether intended for himself or herself, a spouse, or a professional. I know it makes you feel like a competent husband and man to do things around the house, but here’s a bit of advice: Let me hire someone else. Keep in mind that contractors were invented for good reason. The aggravation you’ll have trying to fit whatever project we’ve contemplated into your schedule will be dwarfed by the aggravation I’ll have when you can’t. I’ve never heard you ruminate about not cutting the lawn after we hired the landscaper and you got rid of the lawnmower.
The same goes for quality. Do you really think you did anywhere near as good a job replacing the leaking toilet as a real plumber? Should we talk about the breakfast room light fixture? Do you want me to continue?
My annoyance probably lessened any satisfaction you derived by completing these projects yourself. You should always keep the Pressel money-management credo forefront in your mind: “You earn it, I spend it.” Please let me do my job.
Let Me See If “The Doctor” Is In
Please leave the professor at the office; don’t talk too much medicine when you are not at work. Your trainees might need to hear all the minute details of whatever medical issue is at hand, but your family and friends do not. Most of those close to us chose careers outside of medicine a long time ago and probably don’t want to change direction now. Why do you think they call me for medical advice? It’s not because I’m a better doctor but because they know they’ll hear one of two things:
- I’ll tell them I don’t have a clue and they should ask you; or
- I’ll answer their questions in a tenth of the time that it would have taken you. And we’re talking easy questions because, while I’ve listened to you speak to medical students and residents for the last 20 years, we both know I am not a doctor.
Nevertheless, I do pretty well even with some of the hard questions, if I say so myself. Don’t worry though, there’s no need for concern. Please know that I am not practicing with your license.
Relative to the home practice of medicine, it’s OK to look in our kids’ ears! You must remember the huge fight we had when our son exhibited all of the classic signs of an ear infection and you refused to examine his ears. I know you agonize when you make a clinical error with a patient, but this was just an ear infection. I would have taken him to a real doctor if he was sick enough to merit consideration of what you were worried about missing (brain abscess or meningitis). Really? If I had known how to work your otoscope back then, I would have looked in his ears myself. I’m still not sure how treating minor illnesses in our children is different from the same thing with children of our friends.
You have a perfectly reasonable excuse to be exhausted, yet you are often embarrassed when you fall asleep at our friends’ houses during social events. But the truth is they consider it a mark of true friendship when you go missing before dessert is served. When we were still new in the area and someone would realize that you had disappeared, I was mortified. I quickly realized though that our friends would all rather you and I join them than stay home entirely. No one is offended to find you asleep on the sofa (and your disappearance is now almost expected). To tell you the truth, I’m not sure anyone misses your conversation.
Meetings make the world go round, and your attendance is obligatory at many, even if you’d rather not attend. When I was still working, someone came up with the idea of a stand-up meeting. It was a brilliant idea that made meeting participants use the time more efficiently. Why don’t you propose that some of your administrative meetings be run that way rather than depending on me to page you, “Dr. Pressel, we need you urgently in room 23!”? Sorry I’m calling you out on this, but I’m not always available at the exact time you’ve specified that you want to be interrupted. Besides, it is sometimes amusing to hear that you fell asleep at some senior hospital administrator’s meeting.
I started this by writing that I never wanted to marry a physician, but the last quarter century with you has been the adventure of a lifetime. I just sometimes ask myself, “Why didn’t he become a dermatologist?” TH
Karen Pressel is the wife of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
What I Want My Hospitalist Father to Know
Let me start out by saying that I think you have a great job and I am proud of you. But there are some things you should know. I’ll begin with the good ones.
We lead a very comfortable life, and I am grateful for all that you do for me. You don’t need to remind me, though, every time you manually scoop poop from some constipated kid that it pays for the roof over my head, clothes on my back, and my expensive university education.
I get it.
Even so, having a parent who is a physician is way better than having a parent who is, say, an accountant. I don’t need help with my taxes, but it sure is nice to get some quick medical advice when I have a rash. I even still trust you after you missed my broken arm when I was in sixth grade. Do me a favor though: Just tell me what it is and how I can fix the problem. Save the lecture on the pathophysiology, epidemiology, and differential diagnosis for your residents and medical students. It’s only poison ivy.
When we were growing up, you always gave us a “case of the week.” There were some consistent themes, and I’ve never been sure if these patients were real or fake. Most were either adolescent girls with belly pain or children experiencing bizarre spells who ended up being intoxicated from some ingestion. Was there supposed to be a not-so-subtle message here not to use drugs and to choose my romantic interests carefully?
I actually enjoy hearing about interesting patients, although maybe you could vary the cases, focusing more on human-interest situations rather than on complex technical patients. Relative to the human-interest stories, shouldn’t some of the names parents give to their children be considered child abuse? You probably don’t know, but in Iceland, there is a government Naming Committee that actually maintains a list of approved children’s names.
I know you have to take both clinical and administrative calls. When you get a medical call while we’re having dinner, would you please go somewhere else to talk? Hearing you ask about a patient’s diarrhea when we are eating sort of ruins my appetite.
Similarly, please let me vet topics before you discuss them with my friends. You have some cool stories, but Dad, I’m not sure my friends want to hear about child abuse or vaginal discharge. I will say that the absolute best phone calls you get occur when, after 22 years of Pressel Medical School, I’m able to make the diagnosis or give the correct advice (sometimes faster than your medical students and residents).
Let’s talk about what you learned from me. Though you may not agree, you should think of all the times you found me annoying, particularly when I was a pain-in-the-ass kid, as CME. Over the years, I gave you regular opportunities to enhance your knowledge of child development and to improve your parenting skills—things that undoubtedly continue to help you as a pediatrician.
I like visiting you in the hospital. I know you enjoy showing me off. When you introduce me to your coworkers, it’s OK if you tell them I’m not going to medical school. Still, you should know that I fully intend to repay all that you have done.
Hearing from you about all that happens in a hospital, I can understand why you never want to be a patient. I’ll do my best to ensure you don’t get admitted to a hospital and are able to die peacefully at home. You can count on your loving son, Dad. I’ll be sure you don’t have a hospitalist with you at the end. TH
Rob Pressel is the son of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
As a hospitalist, patient satisfaction is top of mind. Then there’s another group of people whose satisfaction is also paramount: your family. What do they have to say about life with a hospitalist? You’re about to find out!
The Hospitalist asked family members of David Pressel, MD, PhD, a pediatric hospitalist at A.I. DuPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist, for their impressions, and wife Karen and son Rob’s honest answers (and gentle ribbings) show that for whatever ups and downs life may bring, being part of a hospitalist’s family is full of rewards and lots of love. Of course, that’s not to say they didn’t have some suggestions for improvements.
For Hospitalists’ Spouses Everywhere
Marrying a doctor was never on my to-do list. In fact, my list specified quite the opposite; I was never going to marry a physician. My stereotypical perception of the lives of physicians included long hours, too much stress, no family time, guaranteed interruptions at social events, calls at all hours of the day, never enough sleep—you get the picture. I imagined too many headaches to make being a “doctor’s wife” in the slightest bit enticing. I wanted no part of it, and besides, I had my own career to think about.
But then I met my husband, and my list went out the window.
Still, after a couple decades of negotiating a balance between the demands of his job (see above) and the demands of his family, there are things I’d like to say to him. So here goes. Hospitalists, take note.
There Are Only 24 Hours in a Day
How many times have you called to say, “I’ll be leaving the hospital in 10 minutes”? How long did it take for me to realize that relying on that kind of statement was crazy? I’m embarrassed to say that it took me way longer than it should have to come to that understanding. After many overcooked dinners and missed social events, I finally realized that your anticipated departure held no validity and I could only trust that you had left the hospital when you called from the car with the wheels rolling. Fortunately, you were more astute than I and changed your communication habits rather quickly, although the timing of said notifications still does not always take traffic into account and could use some work.
Still, I recognize that “leaving the hospital” is really just a physical indicator of your location, not necessarily a reflection of your state of mind. When you get home, please tell me if you still have work to do (notes, email, patient follow-up) or if you are done for the day. I suppose second-guessing your clinical decisions and calling the hospital to check on patients are unavoidable, but give me a clue whether I should actually expect you fully home to join the rest of the family—or if you will just be working at the home “nursing station” all evening. The burden of healthcare in America doesn’t fall on just you. If you can’t figure out what is wrong with a patient or don’t know what to do, you have many colleagues who can help.
Please remember that you are only one person. Don’t think that if there is a staffing shortage to fill, the responsibility for working is yours. Your colleagues are wonderful and, almost without exception, are happy to pitch in to help carry the extra load. The same goes with holidays; you don’t need to work more than everyone else. I know you are not a slacker. If you try to spread the load when you manage patient care and work schedules, you will have a happier spouse. Remember, a happy wife is a happy life. (I’m sure there is an analogous saying for your colleagues’ husbands and partners.)
Along those same lines, please limit the moonlighting you choose to do. My preconceived idea of a physician’s salary was very different from your reality. You are a pediatrician, an academic pediatrician. Having said that, we lead a wonderful life. We have what we need and have been very happy without the fanciness of some of our neighbors. Although the extra income is nice, I’d rather see more of you than more money. Besides, we just wrote the last check for college tuition, and I’m sure the boys will never ask us for money again.
Being Grumpy (No, Not the Dwarf)
My thoughts on moonlighting lead me perfectly to a discussion of your frame of mind: your mood. By definition, your patients are seriously ill hospitalized children. The bursting hospital census, the acuity of your patients, and the relative craziness of some of their parents invariably elevate your stress level. This, in turn, drives more frequent calls to the hospital and time on the computer all hours of the day or night. This does not allow for a restful sleep, when you sleep at all. I may be biased, but I think you are in the minority of hospitalists who bring their jobs home. Not that I’m complaining too loudly; this is who you are and why I love you, but if you haven’t noticed, when you are on service you tend to get grumpy. Think about this: If you’re not on call, why not turn off your pager, turn off your phone, and leave email alone?
Given the pressures inherent in your job, please tell me again why you would want to moonlight. Moonlighting means even longer hours, more stress, and less sleep for you, all of which make you grumpier and, as a result, tend to make me grumpy.
No, thank you.
Everyone we know has some form of “honey-do” list, whether intended for himself or herself, a spouse, or a professional. I know it makes you feel like a competent husband and man to do things around the house, but here’s a bit of advice: Let me hire someone else. Keep in mind that contractors were invented for good reason. The aggravation you’ll have trying to fit whatever project we’ve contemplated into your schedule will be dwarfed by the aggravation I’ll have when you can’t. I’ve never heard you ruminate about not cutting the lawn after we hired the landscaper and you got rid of the lawnmower.
The same goes for quality. Do you really think you did anywhere near as good a job replacing the leaking toilet as a real plumber? Should we talk about the breakfast room light fixture? Do you want me to continue?
My annoyance probably lessened any satisfaction you derived by completing these projects yourself. You should always keep the Pressel money-management credo forefront in your mind: “You earn it, I spend it.” Please let me do my job.
Let Me See If “The Doctor” Is In
Please leave the professor at the office; don’t talk too much medicine when you are not at work. Your trainees might need to hear all the minute details of whatever medical issue is at hand, but your family and friends do not. Most of those close to us chose careers outside of medicine a long time ago and probably don’t want to change direction now. Why do you think they call me for medical advice? It’s not because I’m a better doctor but because they know they’ll hear one of two things:
- I’ll tell them I don’t have a clue and they should ask you; or
- I’ll answer their questions in a tenth of the time that it would have taken you. And we’re talking easy questions because, while I’ve listened to you speak to medical students and residents for the last 20 years, we both know I am not a doctor.
Nevertheless, I do pretty well even with some of the hard questions, if I say so myself. Don’t worry though, there’s no need for concern. Please know that I am not practicing with your license.
Relative to the home practice of medicine, it’s OK to look in our kids’ ears! You must remember the huge fight we had when our son exhibited all of the classic signs of an ear infection and you refused to examine his ears. I know you agonize when you make a clinical error with a patient, but this was just an ear infection. I would have taken him to a real doctor if he was sick enough to merit consideration of what you were worried about missing (brain abscess or meningitis). Really? If I had known how to work your otoscope back then, I would have looked in his ears myself. I’m still not sure how treating minor illnesses in our children is different from the same thing with children of our friends.
You have a perfectly reasonable excuse to be exhausted, yet you are often embarrassed when you fall asleep at our friends’ houses during social events. But the truth is they consider it a mark of true friendship when you go missing before dessert is served. When we were still new in the area and someone would realize that you had disappeared, I was mortified. I quickly realized though that our friends would all rather you and I join them than stay home entirely. No one is offended to find you asleep on the sofa (and your disappearance is now almost expected). To tell you the truth, I’m not sure anyone misses your conversation.
Meetings make the world go round, and your attendance is obligatory at many, even if you’d rather not attend. When I was still working, someone came up with the idea of a stand-up meeting. It was a brilliant idea that made meeting participants use the time more efficiently. Why don’t you propose that some of your administrative meetings be run that way rather than depending on me to page you, “Dr. Pressel, we need you urgently in room 23!”? Sorry I’m calling you out on this, but I’m not always available at the exact time you’ve specified that you want to be interrupted. Besides, it is sometimes amusing to hear that you fell asleep at some senior hospital administrator’s meeting.
I started this by writing that I never wanted to marry a physician, but the last quarter century with you has been the adventure of a lifetime. I just sometimes ask myself, “Why didn’t he become a dermatologist?” TH
Karen Pressel is the wife of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
What I Want My Hospitalist Father to Know
Let me start out by saying that I think you have a great job and I am proud of you. But there are some things you should know. I’ll begin with the good ones.
We lead a very comfortable life, and I am grateful for all that you do for me. You don’t need to remind me, though, every time you manually scoop poop from some constipated kid that it pays for the roof over my head, clothes on my back, and my expensive university education.
I get it.
Even so, having a parent who is a physician is way better than having a parent who is, say, an accountant. I don’t need help with my taxes, but it sure is nice to get some quick medical advice when I have a rash. I even still trust you after you missed my broken arm when I was in sixth grade. Do me a favor though: Just tell me what it is and how I can fix the problem. Save the lecture on the pathophysiology, epidemiology, and differential diagnosis for your residents and medical students. It’s only poison ivy.
When we were growing up, you always gave us a “case of the week.” There were some consistent themes, and I’ve never been sure if these patients were real or fake. Most were either adolescent girls with belly pain or children experiencing bizarre spells who ended up being intoxicated from some ingestion. Was there supposed to be a not-so-subtle message here not to use drugs and to choose my romantic interests carefully?
I actually enjoy hearing about interesting patients, although maybe you could vary the cases, focusing more on human-interest situations rather than on complex technical patients. Relative to the human-interest stories, shouldn’t some of the names parents give to their children be considered child abuse? You probably don’t know, but in Iceland, there is a government Naming Committee that actually maintains a list of approved children’s names.
I know you have to take both clinical and administrative calls. When you get a medical call while we’re having dinner, would you please go somewhere else to talk? Hearing you ask about a patient’s diarrhea when we are eating sort of ruins my appetite.
Similarly, please let me vet topics before you discuss them with my friends. You have some cool stories, but Dad, I’m not sure my friends want to hear about child abuse or vaginal discharge. I will say that the absolute best phone calls you get occur when, after 22 years of Pressel Medical School, I’m able to make the diagnosis or give the correct advice (sometimes faster than your medical students and residents).
Let’s talk about what you learned from me. Though you may not agree, you should think of all the times you found me annoying, particularly when I was a pain-in-the-ass kid, as CME. Over the years, I gave you regular opportunities to enhance your knowledge of child development and to improve your parenting skills—things that undoubtedly continue to help you as a pediatrician.
I like visiting you in the hospital. I know you enjoy showing me off. When you introduce me to your coworkers, it’s OK if you tell them I’m not going to medical school. Still, you should know that I fully intend to repay all that you have done.
Hearing from you about all that happens in a hospital, I can understand why you never want to be a patient. I’ll do my best to ensure you don’t get admitted to a hospital and are able to die peacefully at home. You can count on your loving son, Dad. I’ll be sure you don’t have a hospitalist with you at the end. TH
Rob Pressel is the son of David Pressel, MD, PhD, a pediatric hospitalist at A.I. duPont Hospital for Children in Wilmington, Del., and a former member of Team Hospitalist.
Clarifying the Roles of Hospitalist and PCP
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
I explain my role as a hospitalist and my connection to the patient’s primary care physician (PCP) on first meeting the patient. I look for ways to reinforce this throughout the hospitalization.
Why I Do It
Even when I was hospitalized at my own institution, it was difficult for me to remember all of the providers involved in my care and their roles. My injuries and the large number of doctors caring for me interfered with my ability to absorb this information. I imagine that this is amplified for patients who have little or no experience with the medical system and are unfamiliar with the role that we play in their care.
During a recent initiative to improve the patient experience at my institution, we found it difficult to collect specific feedback on individual providers because many patients did not know their inpatient doctors’ names, frequently referencing their PCPs when asked for feedback on their care. This is common: A 2009 study showed that 75% of patients were unable to name the inpatient physician in charge of their care. Of those who could identify a name, only 40% correctly identified a member of their primary inpatient team, often identifying the PCP or a specialist instead.1
Clarifying our role on the care team, identifying ourselves as the point person for questions or concerns, and reinforcing our relationship with the PCP can help engender trust in the relationship, eliminate confusion, and improve the patient experience.
How I Do It
After introducing myself, I explain to patients that I will notify their PCP of the admission, and I state that I will be acting as the head of the inpatient team on behalf of their PCP. I often explain that most PCPs do not see their own patients in the hospital.
When multiple teams or house staff are involved in care, I clarify my role in relation to other team members. I look for opportunities throughout the hospitalization to reinforce this. For example, I tell patients when I have updated their PCP on significant events, and I clarify my role in simple terms, such as “quarterback,” when there are multiple subspecialists involved in care. I try to avoid terms like “attending,” which are often meaningless to patients.
In my hospitalist group, we help to reinforce our role and identity by providing a business card that includes a headshot. TH
Dr. Moore is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School, both in Boston. She is a member of SHM’s Patient Experience Committee.
Reference
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
I explain my role as a hospitalist and my connection to the patient’s primary care physician (PCP) on first meeting the patient. I look for ways to reinforce this throughout the hospitalization.
Why I Do It
Even when I was hospitalized at my own institution, it was difficult for me to remember all of the providers involved in my care and their roles. My injuries and the large number of doctors caring for me interfered with my ability to absorb this information. I imagine that this is amplified for patients who have little or no experience with the medical system and are unfamiliar with the role that we play in their care.
During a recent initiative to improve the patient experience at my institution, we found it difficult to collect specific feedback on individual providers because many patients did not know their inpatient doctors’ names, frequently referencing their PCPs when asked for feedback on their care. This is common: A 2009 study showed that 75% of patients were unable to name the inpatient physician in charge of their care. Of those who could identify a name, only 40% correctly identified a member of their primary inpatient team, often identifying the PCP or a specialist instead.1
Clarifying our role on the care team, identifying ourselves as the point person for questions or concerns, and reinforcing our relationship with the PCP can help engender trust in the relationship, eliminate confusion, and improve the patient experience.
How I Do It
After introducing myself, I explain to patients that I will notify their PCP of the admission, and I state that I will be acting as the head of the inpatient team on behalf of their PCP. I often explain that most PCPs do not see their own patients in the hospital.
When multiple teams or house staff are involved in care, I clarify my role in relation to other team members. I look for opportunities throughout the hospitalization to reinforce this. For example, I tell patients when I have updated their PCP on significant events, and I clarify my role in simple terms, such as “quarterback,” when there are multiple subspecialists involved in care. I try to avoid terms like “attending,” which are often meaningless to patients.
In my hospitalist group, we help to reinforce our role and identity by providing a business card that includes a headshot. TH
Dr. Moore is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School, both in Boston. She is a member of SHM’s Patient Experience Committee.
Reference
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
I explain my role as a hospitalist and my connection to the patient’s primary care physician (PCP) on first meeting the patient. I look for ways to reinforce this throughout the hospitalization.
Why I Do It
Even when I was hospitalized at my own institution, it was difficult for me to remember all of the providers involved in my care and their roles. My injuries and the large number of doctors caring for me interfered with my ability to absorb this information. I imagine that this is amplified for patients who have little or no experience with the medical system and are unfamiliar with the role that we play in their care.
During a recent initiative to improve the patient experience at my institution, we found it difficult to collect specific feedback on individual providers because many patients did not know their inpatient doctors’ names, frequently referencing their PCPs when asked for feedback on their care. This is common: A 2009 study showed that 75% of patients were unable to name the inpatient physician in charge of their care. Of those who could identify a name, only 40% correctly identified a member of their primary inpatient team, often identifying the PCP or a specialist instead.1
Clarifying our role on the care team, identifying ourselves as the point person for questions or concerns, and reinforcing our relationship with the PCP can help engender trust in the relationship, eliminate confusion, and improve the patient experience.
How I Do It
After introducing myself, I explain to patients that I will notify their PCP of the admission, and I state that I will be acting as the head of the inpatient team on behalf of their PCP. I often explain that most PCPs do not see their own patients in the hospital.
When multiple teams or house staff are involved in care, I clarify my role in relation to other team members. I look for opportunities throughout the hospitalization to reinforce this. For example, I tell patients when I have updated their PCP on significant events, and I clarify my role in simple terms, such as “quarterback,” when there are multiple subspecialists involved in care. I try to avoid terms like “attending,” which are often meaningless to patients.
In my hospitalist group, we help to reinforce our role and identity by providing a business card that includes a headshot. TH
Dr. Moore is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School, both in Boston. She is a member of SHM’s Patient Experience Committee.
Reference
- Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201.
AYAs still fare worse than kids with leukemia, lymphoma

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia

patient and her father
Photo by Rhoda Baer
Adolescents and young adults (AYAs) are less likely than children to survive 8 relatively common types of cancer, according to a long-running study of cancer survival across Europe.
The study showed that AYAs had significantly worse survival rates than children if they were diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin or non-Hodgkin lymphoma (NHL), and 4 types of solid tumor malignancies.
The study’s authors say that variations in survival between age groups are due to a number of factors, including delays in diagnosis and treatment, a lack of treatment guidelines and clinical trials specifically for AYAs, and differences in the biology of some cancers.
“The good news is that the number of children, adolescents, and young adults surviving for at least 5 years after diagnosis has risen steadily over time in Europe,” said author Annalisa Trama, PhD, of The National Institute of Cancer (Istituto Nazionale dei Tumori: Fondazione IRCCS) in Milan, Italy.
“Across all cancers, the level of improvement is similar in these age groups. This contrasts with earlier results that adolescents and young adults diagnosed up to the 1990s were lagging behind children in terms of survival.”
“However, we found that adolescents and young adults still tend to die earlier than children for several cancers common to these age groups, particularly blood cancers like leukemias and non-Hodgkin’s lymphoma.”
Dr Trama and her colleagues reported these findings in The Lancet Oncology.
The researchers compared survival between AYAs (ages 15 to 39), children (ages 0 to 14), and adults (ages 40 to 69) who were diagnosed from 2000 to 2007 and followed up to at least 2008.
The team analyzed data from population-based cancer registries covering all or part of 27 European countries* and estimated 5-year survival for 56,505 cancer cases in children; 312,483 in AYAs; and 3,567,383 in adults. The researchers also analyzed changes in survival over time from 1999 to 2007.
For AYAs, survival at 5 years from diagnosis for all cancers combined was 82% for 2005-2007, which is up from 79% for 1999-2001 (P<0.0001). In children, survival improved from 76% to 79% over the same time period (P<0.0001).
Survival improved significantly in children and AYAs for ALL (P<0.0001) and NHL (P<0.0001 in AYAs and P=0.023 in children). On the other hand, between 1999 and 2007, survival rates remained unchanged for AYAs with AML (around 50%).
Overall, AYAs had slightly better 5-year survival than children because they were diagnosed more often with cancers with fairly good prognoses—Hodgkin lymphoma, NHL, germ cell tumors, melanoma, thyroid cancer, and breast cancer.
However, the overall survival rates conceal differences between specific cancers. Survival was significantly worse for AYAs than for children when it came to 8 relatively common cancers affecting both age groups:
- ALL—55.6% for AYAs and 85.8% for children (P<0.0001)
- AML—49.8% and 60.5%, respectively (P<0.0001)
- Hodgkin lymphoma—92.9% and 95.1%, respectively (P<0.0001)
- NHL—77.4% and 83.0%, respectively (P<0.0001)
- Astrocytomas—46.4% and 61.9%, respectively (P<0.0001)
- Ewing’s sarcoma of bone—49.3% and 66.6%, respectively (P<0.0001)
- Rhabdomyosarcoma—37.8% and 66.6%, respectively (P<0.0001)
- Osteosarcoma—61.5% and 66.8%, respectively (P=0.011).
AYAs had a survival advantage over adults for almost all major cancers affecting both age groups, supporting the idea that younger patients with few other illnesses are likely to fare better than older patients.
There are only 2 types of cancer for which AYAs were at a survival disadvantage—breast (83.5% vs 87.0%) and prostate (79.9% vs 89.8%).
Dr Trama and her colleagues pointed out that this analysis pre-dates recent initiatives to improve outcomes for AYAs that have been implemented in several European countries.
“The European Network for Teenagers and Young Adults with Cancer is advocating collaboration between pediatric and adult oncologists, greater access to clinical trials and research to improve treatments for this specific age group, as well as developing adolescent and young adult-specific practice guidelines, encouraging healthier lifestyles and the greater involvement of patients and patients support groups,” Dr Trama said.
“This study will provide an important starting point from which to evaluate whether these initiatives will reduce the gulf in survival between European adolescents and young adults and children with cancer.” ![]()
*Finland, Iceland, Norway, Sweden, England, Ireland, Northern Ireland, Scotland, Wales, Austria, Belgium, France, Germany, Netherlands, Switzerland, Croatia, Italy, Malta, Portugal, Slovenia, Spain, Bulgaria, Estonia, Latvia, Lithuania, Poland, and Slovakia
Acute Pancreatitis
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure 48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | 101 |
| Renal serum creatinine (mg/dL) | 1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | 90, fluid responsive | 90, not fluid responsive | 90, pH 7.3 | 90, pH 7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate 120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.
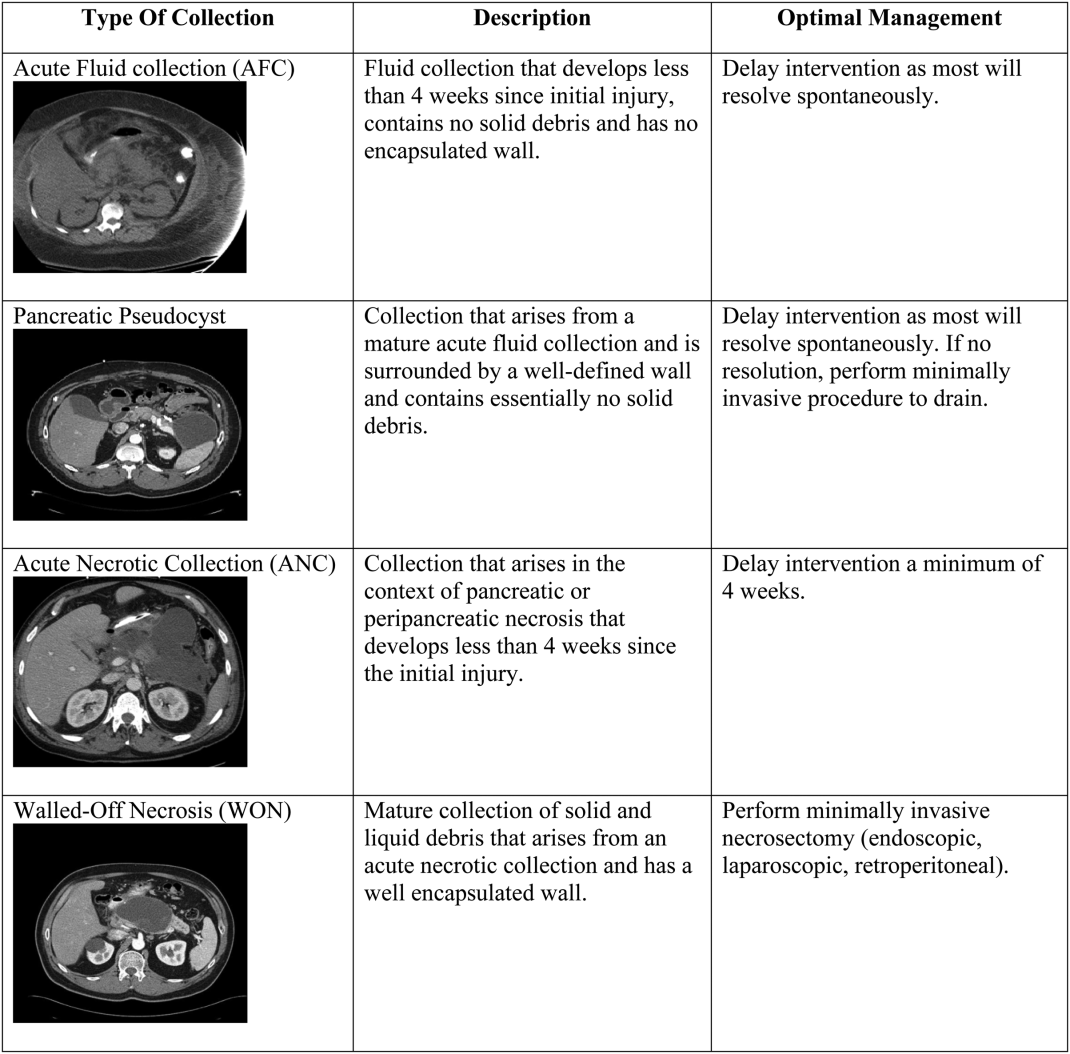
The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure 48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | 101 |
| Renal serum creatinine (mg/dL) | 1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | 90, fluid responsive | 90, not fluid responsive | 90, pH 7.3 | 90, pH 7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate 120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.

The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
A 55‐year‐old man presents with colicky right upper quadrant pain radiating to his back for 12 hours. He does not use ethanol and has no familial or personal history of pancreatic disease. Pertinent laboratory values include: white blood cell count 23.6 103/L; hemoglobin 16.2 g/dL; blood urea nitrogen (BUN) 52 mg/dL; aspartate aminotransferase 110 U/L; alanine aminotransferase 272 U/L; alkaline phosphatase 432 U/L; total bilirubin 4.3 mg/dL; amylase 2230 U/L; lipase 1623 U/L. He is afebrile, normotensive, and not hypoxic, but his respiratory rate is 30. He has voluntary guarding with palpation of the abdomen, decreased bowel sounds, and decreased breath sounds at the left lung base. A transabdominal ultrasound of the right upper quadrant reveals cholelithiaisis without choledocholithiasis. There is mild peripancreatic stranding and the head is slightly edematous.
NATURAL HISTORY
Acute pancreatitis (AP) is a common cause for emergency room presentation, resulting in over 280,000 hospital admissions in the United States at a cost of nearly $3 billion dollars annually.[1] In its mildest form it may require a 2‐ to 5‐day hospital stay and an uncomplicated discharge. In more severe cases, such as in the setting of pancreatic necrosis and/or the development of organ failure, hospitalization can feature a much longer and complicated hospital course.[2]
|
| AP is now classified as mild, moderately acute, or severe based on the presence of local complications and/or persistent organ failure. |
| Lactated Ringer's solution should be used in all patients as the resuscitative fluid in AP |
| Aggressive fluid resuscitation is critical (defined as 250500 mL/h), especially in the first 24 hours of admission. |
| Enteric feeding should be attempted within the first 72 hours of admission and can be given orally with a low‐fat diet. |
| Antibiotics should not be used unless there is documented infection; prophylactic antibiotics to treat necrotizing AP are not beneficial. |
| New definitions of pancreatic fluid collections determine optimal therapy. |
| Medical therapy for infected pancreatic necrosis should be attempted prior to necrosectomy. |
| Alternatives to open necrosectomy, such as endoscopic or retroperitoneal debridement, are preferred in cases of unstable infected pancreatic necrosis. |
DIAGNOSTIC CRITERIA AND CLASSIFICATION
AP is diagnosed by the patient having 2 out of the following 3 criteria: (1) classic clinical symptoms with abdominal pain consistent with AP (2) serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or (3) characteristic findings from abdominal imaging.[3] It is important for the hospitalist to recognize that patients can have AP with normal serum amylase and/or lipase levels, as long as their clinical symptoms and imaging exam are consistent with the disease.[4] It is also important to recognize that amylase and/or lipase elevation is not 100% specific for pancreatitis; alternate conditions that elevate amylase levels include renal insufficiency, intestinal ischemia and obstruction, macroamylasemia, and multiple medications, whereas lipase elevations can be seen in spontaneous bacterial peritonitis, intestinal ischemia, and esophagitis.[5]
AP is classified as either mild (absence of organ failure or local complications), moderate (local complications and/or transient organ failure 48 hours) or severe (persistent organ failure >48 hours).[3] Organ failure is defined by the modified Marshall score, and local complications include pancreatic fluid collections, pancreatic and peripancreatic necrosis, and vascular thrombosis (Table 2).[6]
| Organ System Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| |||||
| Respiratory (PaO2/FiO2) | >400 | 301400 | 201300 | 101200 | 101 |
| Renal serum creatinine (mg/dL) | 1.4 | 1.41.8 | 1.93.6 | 3.74.9 | >4.9 |
| Cardiovascular systolic blood pressure (mm Hg) | >90 | 90, fluid responsive | 90, not fluid responsive | 90, pH 7.3 | 90, pH 7.2 |
ETIOLOGY
Transiently obstructing gallstones, thought to account for about 50% of cases, are the most common cause of AP. The rising prevalence of obesity, which is a known risk factor for AP due to the corresponding increase in the frequency of gallstones, suggests that this will continue to be the leading cause going forward.[7] Alcohol use is associated with both acute and chronic pancreatitis; however, the extent to which it is a primary cause of AP is uncertain.[8] Trauma, medications, hypercalcemia, and hypertriglyceridemia must also be considered; however, they are much less common. AP from endoscopic retrograde cholangiopancreatography (ERCP) occurs following 5% of procedures and from endoscopic ultrasound (EUS) fine‐needle aspiration following 1%. Although several medications are clearly associated with AP, many that were previously invoked seem less likely.[9] Immunoglobulin G (IgG) 4related systemic disease, although rare, is becoming more recognized and should be considered when the more common etiologies are ruled out. Finally, it is controversial whether anatomic findings such as pancreatic divisum and functional disorders such as sphincter of Oddi dysfunction cause AP.[10]
Identifying the cause of an acute episode remains important, as subsequent treatment strategies can be tailored to help prevent recurrence. A thorough personal history, including prior gallbladder disease, alcohol use, and medications is strongly recommended. Basic laboratory studies including liver function tests, serum calcium and triglycerides, as well as a right upper quadrant ultrasound are indicated in all patients presenting with AP.[1] Idiopathic AP is not uncommon. Given the increasing awareness of genetic factors, potential role of advanced endoscopy, and higher risk of recurrence in this group, patients with idiopathic AP should be referred to specialized centers of expertise.[4]
PROGNOSTICATION
Most cases of AP are mild and do not require prolonged hospitalization; however, because 5% of hospitalized patients will die from this disease, prognostic criteria are needed to determine high‐risk cases.[11] Multiple systems have been developed (Bedside Index for Severity in Acute Pancreatitis, Ranson's, Acute Physiology and Chronic Health Evaluation II, Computed Tomography Severity Index), but all have had difficulty achieving accuracy in a user‐friendly tool; because of this, hospitalists should instead focus on the individual laboratory parameters that correlate with pathophysiologic derangement. Elevations in BUN and hematocrit indicate hypovolemia, leukocytosis, and fluid sequestration are indicators of the inflammatory cascade. Creatinine, elevated liver tests, and hypoxia are indicators of organ damage. Low calcium is reflective of fat necrosis saponification (endorgan damage) and also an indicator of hypovolemia. Essentially, the prediction of severity depends on identifying indications of endorgan damage in a timely manner and can be performed through a combination of age, known comorbidities, physical exam, and basic laboratory testing.[12]
ADDITIONAL INITIAL IMAGING
Although sensitive and specific for AP, routine computed tomography (CT) imaging for all patients presenting with suspected AP is not indicated. The diagnosis is often clear on a clinical and lab basis alone, and most patients with AP will improve within 48 hours.[13] CT or magnetic resonance imaging (MRI) can be considered for patients with an unclear diagnosis and indeterminate ultrasound or in those who are not improving within the first 48 to72 hours after presentation. This additional imaging can help make an alternative diagnosis or detect an early complication such as pancreatic necrosis. CT is preferred; however, MRI may be utilized if there is a high suspicion for biliary stones that were not seen on ultrasound or when CT is indicated but impaired renal function precludes its use.[4] In patients presenting with recurrent idiopathic AP, EUS is recommended to evaluate for an occult malignancy or microlithiasis.[14]
INITIAL CLINICAL MANAGEMENT
Without evidence of either (1) ascending cholangitis or (2) proven choledocholithiasis with clinical decompensation and worsening liver tests, ERCP should not be performed and management should be focused on supportive care, pain control, and monitoring prognostic information regarding severity. The initial management of AP should include fluid replacement with lactated Ringer's (LR) solution at 5‐10 mL/kg/h to achieve noninvasive parameters of a heart rate 120, mean arterial pressure 65 to 85 mm Hg, and urine output >0.5 to 1 mL/kg/h. LR decreases the incidence of the systemic inflammatory response syndrome (SIRS) by 80% compared with normal saline.[4, 15] Early and sufficient fluid replacement is associated with decreased rates of SIRS and organ failure, whereas under‐resuscitation has been associated with necrosis and increased mortality. In the first 48 to 72 hours of admission, frequent assessment of hemoglobin (HgB) and BUN, as well as urine output measurements, should be obtained to make sure fluid resuscitation is adequate.[4] Intravenous fluid replacement should continue in the hospital until the patient can adequately maintain appropriate fluid intake orally. Prophylactic antimicrobial therapy is not indicated in initial cases of AP, unless there are clear signs of an underlying infection. Pain control is essential, and efforts at reintroducing oral feeding should be initiated once the pain is decreasing. There are no randomized trials that have identified an optimal narcotic‐based pain regimen. On a daily basis, a complete blood count, renal function, and liver function should be measured. There is no reason to continue measuring serum amylase or lipase, as it may not be elevated in some instances in AP, and its fluctuation is not indicative of a change in clinical status.
Case Management Strategy
The patient has mild AP based on lack of organ failure and local complications and is admitted to the regular medical floor. The etiology appears to be due to cholelithiasis, but the patient does not have cholangitis, so ERCP was not considered, and antibiotics were not started. Aggressive fluid resuscitation with lactated Ringer's is started at a rate of 350 mL/h, and BUN and HgB are monitored every 8 hours to make sure that these levels are decreasing. The patient is placed on a low‐fat diet and encouraged to eat as tolerated. Further imaging is not ordered at this time.
Hospital Day 3
The patient's liver tests have normalized, but the BUN continues to rise (82 mg/dL) despite aggressive fluid resuscitation with LR. He remains afebrile and normotensive, but is now hypoxic and requiring nasal cannula oxygen at 4 L/min to maintain his oxygen saturation above 90%. His abdominal pain is controlled with intravenous opiates, but he is not hungry or able to eat. With these changes in his clinical course, a CT scan is performed, which demonstrates acute peripancreatic necrosis centered on the head of the pancreas.
PERSISTENT ORGAN FAILURE AND PANCREATIC NECROSIS
Generally, patients with severe AP (persistent organ failure >48 hours following admission) should be followed in the intensive care unit for effective monitoring and support.
Pancreatic necrosis is defined as a diffuse or focal area of nonviable pancreatic parenchyma >3 cm in size or >30% of the pancreas.[1] Extrapancreatic necrosis can also be present, and is associated with adverse outcomes such as organ failure.[16] Pancreatic and extrapancreatic necrosis can be sterile or infected. The presence of infection does not necessarily increase the risk of subsequent organ failure.
FEEDING
In patients with mild pancreatitis, oral feeding with a low‐fat solid diet can be initiated when nausea, vomiting, and pain have resolved.[1] A randomized controlled trial demonstrated that patients who receive oral feeding earlier in the course of their stay have a shorter length of stay and fewer complications.[17] In patients with evolving AP who unable to tolerate oral feeding, enteral tube feeding either via nasogastric or nasojejunal routes should be initiated to support the intestinal biome and prevent bacterial translocation from the gut to the pancreas. Nasogastric feeding appears to be as safe as nasojejunal feeding.[18] Parenteral nutrition should only be used as a second‐line therapy if adequate caloric requirements cannot be maintained via an enteral route given the increased rate of infections and mortality when compared with nasoenteric feeding.[19] The most recent study on when to start enteric feeding in patients at high risk for complications demonstrates no benefit from starting nasoenteric feeding within the first 24 hours of admission compared to starting an oral diet at 72 hours.[20]
INTRA‐ABDOMINAL COMPARTMENT SYNDROME
A sometimes overlooked consequence of aggressive fluid resuscitation can be the development of intra‐abdominal compartment syndrome, which is defined as new organ dysfunction with concomitant intra‐abdominal pressure measurements >20 mm Hg. Patients with an increasingly tense abdomen, oliguria, or increasing ventilator requirements should have intravesical pressures measured with a urinary catheter. Initial treatment consists of decreasing the fluid resuscitation rate along with supportive measures such as reducing ventilator tidal volume and placing nasogastric and rectal tubes; if not successful, surgical decompression is indicated.
SUBSPECIALIST INVOLVEMENT
The majority of mild AP cases can effectively be managed by hospitalists, and there is no evidence that subspecialist involvement improves important clinical outcomes in mild disease. The need for subspecialty input should be based on the need for a procedure such as ERCP or collaborative care if the patient develops more acute complications requiring ongoing critical care support or decisions centered on sampling of fluid collections and/or necrosectomy.
Case Management Strategy
The patient is transferred to the intensive care unit for closer monitoring of his hemodynamic and respiratory status. His LR is held at 250 mL/h and his BUN is checked every 8 hours. He undergoes serial abdominal exams and twice‐daily bladder pressure measurements to evaluate for intra‐abdominal compartment syndrome. Antibiotics continue to be held as there is no evidence of pancreatic or extrapancreatic infection. A nasogastric tube is placed and enteral feeding begun with a low‐fat formulation and advanced as tolerated. The gastroenterology service is consulted to assist in management.
Hospital Day 17
With optimal intensive care unit monitoring of fluid status, early initiation of enteral feeding, and management of pain, the patient's vital signs have normalized and is he is transferred to the medical ward and is tolerating a clear liquid diet. In the next 48 hours, he becomes febrile. Urinalysis is unremarkable and blood cultures show no growth. Given continued fevers without a clear source, a CT scan of the abdomen is obtained. It demonstrates formation of a necrotic collection.
DEFINITION AND MANAGEMENT OF PANCREATIC FLUID COLLECTIONS
There are 4 main types of pancreatic collections, which include acute fluid collections, acute necrotic collections, pseudocysts, and walled off necrosis (Figure 1).[3] Acute fluid collections (AFC) develop less than 4 weeks after an episode of interstitial pancreatitis. They are found in the pancreatic parenchyma or peripancreatic tissue and usually resolve without requiring intervention. When a fluid collection develops in the context of pancreatic necrosis, it is known as an acute necrotic collection. If an AFC does not resolve in 4 weeks and develops an encapsulated wall that lacks solid debris, it is characterized as a pseudocyst. Pseudocysts are usually extrapancreatic, but occasionally can be intrapancreatic as a result of a disrupted pancreatic duct. Walled off necrosis (WON) occurs after 4 weeks, contains solid debris, and occurs only in the context of necrotizing pancreatitis.

The most important strategy for the hospitalist in managing AFC is to delay intervention as long as possible.[14, 21, 22] This decision generally requires multidisciplinary input (for example with gastroenterology, surgical, and infectious diseases consultative services), as any intervention performed prematurely may lead to significant morbidity and occasional mortality. The vast majority of AFCs and pseudocysts will resolve spontaneously. In addition, most ANCs can be allowed to mature beyond the time of the initial hospitalization and can be managed as an outpatient if/when they proceed to WON.
INFECTED PANCREATIC NECROSIS
In the last decade, the paradigm for managing infected pancreatic necrosis has shifted dramatically. It is no longer necessary to sample the pancreas to make the diagnosis of infected pancreatic necrosis. In most cases, a careful history, clinical examination, and imaging should be able to make the diagnosis.[1, 23] Historically, open necrosectomy/debridement was the standard for the treatment of infected necrosis, but due to increased mortality, this practice has been abandoned. Currently, it is recommended that in stable patients, a course of pancreas‐penetrating antibiotics (such as meropenem) can be tried to allow for better organization of the inflammatory reaction. Subsequently, if the patient remains ill and the infected necrosis has not resolved, minimally invasive necrosectomy, via a variety of techniques such as endoscopy, laparoscopy, or a video‐assisted retroperitoneal approach, should be employed before considering any open surgery. Minimally invasive techniques have the advantages of not only being as successful as open surgery, but also have lower complication rates.[24]
Case Management Strategy
In the setting of fevers and a necrotic fluid collection, the patient is empirically started on meropenem. The pancreatic fluid collection has caused pressure on the stomach, which has led to nausea and vomiting, but he has tolerated continued enteral feeding via a nasogastric tube.
Hospital Day 29
The patient undergoes successful direct endoscopic necrosectomy on hospital day 29 after a repeat CT scan demonstrates complete maturation of the walled off pancreatic necrosis. Following the procedure, his nausea resolves and he is able to tolerate transition to a low‐fat diet.
OTHER COMPLICATIONS
Prior to discharge, it is important to consider other possible complications that may have arisen. New onset glucose intolerance or diabetes, thrombosis of the portal vasculature, and/or splenic aneurysm development can all occur several weeks into the hospitalization. The hospitalist must be aware of clinical clues such as new‐onset ascites due to thrombosis of the superior mesenteric vein.
PREVENTING READMISSIONS
Patients presenting with acute pancreatitis have a 30‐day readmission rate around 20%.[25] Prognostic factors that reduce the risk of readmission include patient tolerating a solid diet, absence of other gastrointestinal symptoms (nausea, vomiting, or diarrhea), and well‐controlled pain. The presence of pancreatic necrosis and the necessity for antimicrobial therapy increase the risk of readmission.[25] In terms of modifiable risk factors, risk of readmission has been correlated with alcohol as etiology of index hospitalization and tobacco abuse. Careful attention to addressing alcohol use and abuse as well as the challenging transition from acute to chronic pain control for patients with chronic pancreatitis is essential, as it is often recurrent pain and possibly not pancreatitis per se that may be the most common reason for hospital readmission. Finally, cholecystectomy for biliary AP should be performed prior to discharge; if this is not feasible, short‐interval outpatient follow‐up for surgery is imperative.
Management Strategy
The patient undergoes an uneventful laparoscopic cholecystectomy on hospital day 35. He is discharged to a skilled nursing facility with physical and occupational rehabilitation services. He has follow‐up scheduled with the gastroenterology service in 2 weeks. His case highlights many of the potential complications of acute pancreatitis and the major updates to management of this common illness (Table 1).
Disclosure
Nothing to report.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
- , , , et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415.
- , , . Acute pancreatitis. BMJ. 2014;349:g4859.
- , , , et al. Classification of acute pancreatitis‐2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
- , . Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281.
- , , , et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;2010:614–620.
- , , , et al. Acute pancreatitis with normal serum lipase: a case series. JOP. 2010;11:369–372.
- , , , et al. Body mass index and the risk and prognosis of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2011;23(12):1136–1143.
- , , , et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19(5):638–647.
- , , , et al. Drug‐induced pancreatitis. Curr Gastroenterol Rep. 2012;14:131–138.
- , . Pancreas divisum does not cause pancreatitis, but associates with CFTR mutations. Am J Gastroenterol. 2012;107:318–320.
- , , , et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744.
- , . Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- , , . Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15.
- , , , et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194.
- , , , et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62(10):1475–1480.
- , , . A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–345.
- , , , et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis. A non‐inferiority randomized controlled trial. Pancreas. 2012;41:153–159.
- , , , . Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
- , , , et al. Early versus on‐demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–1993.
- , , , et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–1263.
- , , , et al. Endoscopic necrosectomy in necrotizing pancreatitis: indication is the key. Gut. 2010;59:1587.
- , , . Management of acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2014;8(6):1–8.
- , . Evidence‐based management of acute pancreatitis. Curr Treat Options Gastroenterol. 2014;9(2):175–180.
- , , , et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol. 2011;9(2):175–180.
Darkened skin, vomiting, and salt cravings in a teenager • Dx?
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.