User login
Anticoagulation in dental surgery: Is it rude to interrupt?
When I was growing up, my mother frequently told me that it was rude to interrupt. Although she was referring to conversations, she may have been onto something bigger.
In the nearly three quarters of a century since their discovery, vitamin K antagonist anticoagulant drugs have been used by millions of patients to prevent heart attack and stroke. Before these patients undergo surgery, a decision to continue or interrupt anticoagulation must be made, weighing the risks of postsurgical hemorrhage with continuation of anticoagulation against the risks of stroke or other embolic complications with interruption of anticoagulation. Bleeding after dental surgery when anticoagulation is continued is rarely or never life-threatening. On the other hand, embolic complications of interrupting anticoagulation are almost always consequential and often lead to death or disability. Although consideration may be different for other types of surgery, there is no need to interrupt lifesaving anticoagulation for dental surgery.
EVIDENCE THAT SUPPORTS CONTINUING ANTICOAGULATION
As early as 1957, there were reports of prolonged postoperative bleeding after dental extractions in patients taking anticoagulants. But there were also reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. Since then, there has been a plethora of literature in this area.
A review published in 2000 showed that of more than 950 anticoagulated patients undergoing more than 2,400 dental surgical procedures (including simple and surgical extraction, alveoplasty, and gingival surgery), only 12 (< 1.3%) required more than local measures for hemostasis (eg, fresh-frozen plasma, vitamin K), and no patient died,1 leading to the conclusion that the bleeding risk was not significant in anticoagulated dental patients. Other studies and systematic reviews have also concluded that anticoagulation for dental procedures should not be interrupted.2,3 In a recent review of 83 studies, only 31 (0.6%) of 5,431 patients taking warfarin suffered bleeding complications requiring more than local measures for hemostasis; there were no fatalities.4
The risk of embolism
There have been many reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. A 2000 review of 575 cases in 526 patients whose anticoagulation was interrupted for dental procedures showed that 5 patients (0.9%) had a serious embolic complication, and 4 died.1 In a more recent review of 64 studies and more than 2,673 patients whose anticoagulation was interrupted for dental procedures, 22 patients (0.8%) suffered embolic complications, and 6 (0.2%) died of the complications.4 Of those with embolic complications, the interruption period was often not reported; however; the interruption ranged from 1 to 4 days. A 2003 systematic review by Dunn and Turpie found a 0.4% embolic complication rate when anticoagulation was interrupted for dental surgery.2
BLEEDING AFTER DENTAL SURGERY
Bleeding after dental surgery can occur with either anticoagulation continuation or interruption, and minor postoperative bleeding requiring additional local hemostatic methods occurs at about the same rate in anticoagulated patients as in those whose anticoagulation is interrupted.
In our recent literature review,4 about 6% of patients in whom anticoagulation was interrupted (and 7% in whom it was not interrupted) had minor bleeding requiring additional local hemostasis, and only 0.2% of patients required more than hemostatic measures (eg, vitamin K injection, plasma transfusion), the same rate found by Dunn and Turpie.2 All patients who required more than local hemostatic measures presumably made a full recovery, while at least 6 who suffered postoperative embolic complications died, and the rest may have had permanent disabilities.
Although bridging therapy with low-molecular-weight heparin can decrease the time without anticoagulation for a dental procedure to only 12 hours, it can be complicated to implement, and there appears to be no benefit in terms of the rates of bleeding or embolic complications. Of the 64 anticoagulation interruption studies,4 17 used heparin or low-molecular-weight heparin in conjunction with temporary warfarin interruption. In 210 instances of bridging therapy in 202 patients undergoing dental procedures, there were 2 embolic complications (1% of bridging cases) and 20 bleeding complications, with 3 (1.4%) requiring hemostasis beyond local measures.4
Many of the studies analyzed independently showed there was no significant difference in postoperative bleeding with:
- Anticoagulation continuation vs interruption for a few days
- Lower vs higher international normalized ratio (INR), including some over 4.0
- Surgical vs nonsurgical extraction
- Few vs many extractions.4
Some studies of anticoagulation and anticoagulation interruption for dental surgery had important limitations. Many of the anticoagulation studies excluded patients at high risk of bleeding, those with a high INR (> 4.0), and those with severe liver or kidney disease, and their exclusion could have lowered the incidence of bleeding complications. Many studies of anticoagulation interruption excluded patients at high risk of embolism, including patients with a previous embolic event and patients with an artificial heart valve, and this could have skewed the results lower for embolic complications.
WHY DO SOME CLINICIANS STILL RECOMMEND INTERRUPTION?
The choice seems clear: for dental surgery in anticoagulated patients, the small risk of a nonfatal bleeding complication in anticoagulated patients is outweighed by the small risk of a disabling or fatal embolic complication when anticoagulation is interrupted. Most authors have concluded that anticoagulation should be continued for dental surgery. Yet surveys of dentists and physicians have shown that many still recommend interrupting anticoagulation for dental surgery.5,6
Medical and dental association positions
The American Academy of Neurology7 and the American Dental Association8 recommend continuing anticoagulant medications for dental surgery. The American College of Chest Physicians also recommends continuing anticoagulation but in 2012 added an option to interrupt or decrease anticoagulation for 2 to 3 days for dental surgery.9 Their recommendation was based partly on the results of four controlled prospective studies10–13 comparing anticoagulated dental surgical patients with patients whose anticoagulation was interrupted. In each study, there were no embolic or bleeding complications requiring more than local methods for hemostasis in the interruption groups, leading the American College of Chest Physicians to conclude that brief anticoagulation interruption for dental surgery is safe and effective.
But the results of these studies actually argue against interrupting anticoagulation for dental surgery. In each study, rates of postoperative bleeding complications and blood loss were similar in both groups, and there were no embolic complications. The authors of each study independently concluded that anticoagulation should not be interrupted for dental surgery.
The optimal INR range for anticoagulation therapy is widely accepted as 2.0 to 3.0, and 2.5 to 3.5 for patients with a mechanical mitral valve.14 Interrupting warfarin anticoagulation for 2 or 3 days leads to a suboptimal INR. Patel et al15 studied the incidence of embolic complications (including stroke, non-central nervous system embolism, myocardial infarction, and vascular death) within 30 days in 7,082 patients taking warfarin with and without an interruption of therapy of at least 3 days (median 6 days). The observed rate of embolic events in those with temporary interruption (10.75 events per 100 patient-years) was more than double the rate in those without interruption (4.03 per 100 patient-years).15 However, this study was designed to compare rivaroxaban vs warfarin, not interrupting vs not interrupting warfarin.
A DECISION-TREE REANALYSIS
In 2010, Balevi published a decision-tree analysis that slightly favored withdrawing warfarin for dental surgery, but he stated that the analysis “can be updated in the future as more accurate and up-to-date data for each of the variables in the model become available.”16 Now that there are more accurate and up-to-date data, it is time to revisit this decision-tree analysis.
In Balevi’s analysis, major bleeding is not defined. But major bleeding after dental surgery should be defined as any bleeding requiring more than local measures for hemostasis. In calculating probabilities for the analysis, Balevi cited studies allegedly showing high incidences of major bleeding after dental extractions with warfarin continuation.17,18 There were some minor bleeding complications necessitating additional local measures for hemostasis in these studies, but no major bleeding complications at all in the warfarin- continuation or warfarin-interruption group. There were no significant bleeding events in either study, and the differences in bleeding rates were not significantly different between the two groups. In both studies, the authors concluded that warfarin interruption for dental surgery should be reconsidered.
Similarly, Balevi accurately asserted that there has never been a reported case of fatal bleeding after a dental procedure in an anticoagulated patient, but “for the sake of creating balance,”16 his decision-tree analysis uses a fatal bleeding probability of 1%, based on an estimated 1% risk for nondental procedures (eg, colorectal surgery, major abdominal surgery). It is unclear how a 1% incidence creates “balance,” but dental surgery is unlike other types of surgery, and that is one reason there has never been a documented postdental fatal hemorrhage in an anticoagulated patient. Major vessels are unlikely to be encountered, and bleeding sites are easily accessible to local hemostatic methods.
Balevi used an embolic complication incidence of 0.059% with warfarin interruption of 3 days. Perhaps he used such a low embolic probability because of his incorrect assertion that “there has been no reported case of a dental extraction causing a cardiovascular accident in a patient whose warfarin was temporarily discontinued.”16 In fact, our group has now identified at least 22 reported cases of embolic complications after temporary interruption of warfarin therapy in patients undergoing dental surgery.4 These included 12 embolic complications (3 fatal) after interruption periods from 1 to 5 days.19,20 In addition, there are numerous cases of embolic complications reported in patients whose warfarin was temporarily interrupted for other types of surgery.21,22
The literature shows that embolic complications after temporary warfarin interruption occur at a much higher rate than 0.059%. Many documented embolic complications have occurred after relatively long warfarin interruption periods (greater than 5 days), but many have occurred with much shorter interruptions. Wysokinski et al21 showed that there was a 1.1% incidence of thromboembolic events, more than 18 times greater than Balevi’s incidence, in patients whose warfarin was interrupted for 4 or 5 days with or without bridging therapy. One of these patients developed an occipital infarct within 3 days after stopping warfarin without bridging (for a nondental procedure). Garcia et al22 showed that of 984 warfarin therapy interruptions of 5 days or less, there were 4 embolic complications, a rate (0.4%) more than 6 times greater than that reported by Balevi.
Even if one were to accept a 0.059% embolic risk from interruption of warfarin, that would mean for every 1,700 warfarin interruptions for dental procedures, there would be one possibly fatal embolic complication. On the other hand, if 1,700 dental surgeries were performed without warfarin interruption, based on the literature, there may be some bleeding complications, but none would be fatal. If airline flights had a 0.059% chance of crashing, far fewer people would choose to fly. (There are 87,000 airline flights in the US per day. A 0.059% crash rate would mean there would be 51 crashes per day in the United States alone.)
But regardless of whether the embolic risk is 0.059% or 1%, the question comes down to whether an anticoagulated patient should be subjected to a small but significant risk of death or permanent disability (if anticoagulation is interrupted) or to a small risk of a bleeding complication (if anticoagulation is continued), when 100% of cases up until now have apparently resulted in a full recovery.
As a result, the decision-tree analysis was fatally flawed by grossly overestimating the incidence of fatal bleeding when warfarin is continued, and by grossly underestimating the incidence of embolic complications when warfarin is interrupted.
IS WARFARIN CONTINUATION ‘TROUBLESOME’?
An oral surgeon stated, “My experience and that of many of my colleagues is that even though bleeding is never life-threatening [emphasis mine], it can be difficult to control at therapeutic levels of anticoagulation and can be troublesome, especially for elderly patients.”23 The American College of Chest Physicians stated that postoperative bleeding after dental procedures can cause “anxiety and distress.”3 Patients with even minor postoperative bleeding can be anxious, but surely, postoperative stroke is almost always far more troublesome than postoperative bleeding, which has never been life-threatening. Although other types of surgery may be different, there is no need to interrupt lifesaving anticoagulation for innocuous dental surgery.
My mother was right—it can be rude to interrupt. Anticoagulation should not be interrupted for dental surgery.
- Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc 2000; 131:77–81.
- Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med 2003; 163:901–908.
- Nematullah A, Alabousi A, Blanas N, Douketis JD, Sutherland SE. Dental surgery for patients on anticoagulant therapy with warfarin: a systematic review and meta-analysis. J Can Dent Assoc 2009; 75:41.
- Wahl MJ, Pintos A, Kilham J, Lalla RV. Dental surgery in anticoagulated patients—stop the interruption. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119:136–157.
- van Diermen DE, van der Waal I, Hoogvliets MW, Ong FN, Hoogstraten J. Survey response of oral and maxillofacial surgeons on invasive procedures in patients using antithrombotic medication. Int J Oral Maxillofac Surg 2013; 42:502–507.
- Ward BB, Smith MH. Dentoalveolar procedures for the anticoagulated patient: literature recommendations versus current practice. J Oral Maxillofac Surg 2007; 65:1454–1460.
- Armstrong MJ, Gronseth G, Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:2065–2069.
- American Dental Association (ADA). Anticoagulant antiplatelet medications and dental procedures. www.ada.org/en/member-center/oral-health-topics/anticoagulant-antiplatelet-medications-and-dental-. Accessed May 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Campbell JH, Alvarado F, Murray RA. Anticoagulation and minor oral surgery: should the anticoagulation regimen be altered? J Oral Maxillofac Surg 2000; 58:131–135.
- Devani P, Lavery M, Howell CJT. Dental extractions in patients on warfarin: is alteration of anticoagulation regime necessary? Br J Oral Maxillofac Surg 1998; 36:107–111.
- Gaspar R, Brenner B, Ardekian L, Peled M, Laufer D. Use of tranexamic acid mouthwash to prevent postoperative bleeding in oral surgery patients on oral anticoagulant medication. Quintessence Int 1997; 28:375–379.
- Blinder D, Manor Y, Martinowitz U, Taicher S. Dental extractions in patients maintained on oral anticoagulant therapy: comparison of INR value with occurrence of postoperative bleeding. Int J Oral Maxillofac Surg 2001; 30:518–521.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Balevi B. Should warfarin be discontinued before a dental extraction? A decision-tree analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:691–697.
- Al-Mubarak S, Al-Ali N, Abou Rass M, et al. Evaluation of dental extractions, suturing and INR on postoperative bleeding of patients maintained on oral anticoagulant therapy. Br Dent J 2007; 203:E15.
- Evans IL, Sayers MS, Gibbons AJ, Price G, Snooks H, Sugar AW. Can warfarin be continued during dental extraction? Results of a randomized controlled trial. Br J Oral Maxillofac Surg 2002; 40:248–252.
- Yasaka M, Naritomi H, Minematsu K. Ischemic stroke associated with brief cessation of warfarin. Thromb Res 2006; 118:290–293.
- Akopov SE, Suzuki S, Fredieu A, Kidwell CS, Saver JL, Cohen SN. Withdrawal of warfarin prior to a surgical procedure: time to follow the guidelines? Cerbrovasc Dis 2005; 19:337–342.
- Wysokinski WE, McBane RD, Daniels PR, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc 2008; 83:639–645.
- Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med 2008; 168:63–69.
- Todd DW. Anticoagulated patients and oral surgery [letter]. Arch Intern Med 2003; 163:1242.
When I was growing up, my mother frequently told me that it was rude to interrupt. Although she was referring to conversations, she may have been onto something bigger.
In the nearly three quarters of a century since their discovery, vitamin K antagonist anticoagulant drugs have been used by millions of patients to prevent heart attack and stroke. Before these patients undergo surgery, a decision to continue or interrupt anticoagulation must be made, weighing the risks of postsurgical hemorrhage with continuation of anticoagulation against the risks of stroke or other embolic complications with interruption of anticoagulation. Bleeding after dental surgery when anticoagulation is continued is rarely or never life-threatening. On the other hand, embolic complications of interrupting anticoagulation are almost always consequential and often lead to death or disability. Although consideration may be different for other types of surgery, there is no need to interrupt lifesaving anticoagulation for dental surgery.
EVIDENCE THAT SUPPORTS CONTINUING ANTICOAGULATION
As early as 1957, there were reports of prolonged postoperative bleeding after dental extractions in patients taking anticoagulants. But there were also reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. Since then, there has been a plethora of literature in this area.
A review published in 2000 showed that of more than 950 anticoagulated patients undergoing more than 2,400 dental surgical procedures (including simple and surgical extraction, alveoplasty, and gingival surgery), only 12 (< 1.3%) required more than local measures for hemostasis (eg, fresh-frozen plasma, vitamin K), and no patient died,1 leading to the conclusion that the bleeding risk was not significant in anticoagulated dental patients. Other studies and systematic reviews have also concluded that anticoagulation for dental procedures should not be interrupted.2,3 In a recent review of 83 studies, only 31 (0.6%) of 5,431 patients taking warfarin suffered bleeding complications requiring more than local measures for hemostasis; there were no fatalities.4
The risk of embolism
There have been many reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. A 2000 review of 575 cases in 526 patients whose anticoagulation was interrupted for dental procedures showed that 5 patients (0.9%) had a serious embolic complication, and 4 died.1 In a more recent review of 64 studies and more than 2,673 patients whose anticoagulation was interrupted for dental procedures, 22 patients (0.8%) suffered embolic complications, and 6 (0.2%) died of the complications.4 Of those with embolic complications, the interruption period was often not reported; however; the interruption ranged from 1 to 4 days. A 2003 systematic review by Dunn and Turpie found a 0.4% embolic complication rate when anticoagulation was interrupted for dental surgery.2
BLEEDING AFTER DENTAL SURGERY
Bleeding after dental surgery can occur with either anticoagulation continuation or interruption, and minor postoperative bleeding requiring additional local hemostatic methods occurs at about the same rate in anticoagulated patients as in those whose anticoagulation is interrupted.
In our recent literature review,4 about 6% of patients in whom anticoagulation was interrupted (and 7% in whom it was not interrupted) had minor bleeding requiring additional local hemostasis, and only 0.2% of patients required more than hemostatic measures (eg, vitamin K injection, plasma transfusion), the same rate found by Dunn and Turpie.2 All patients who required more than local hemostatic measures presumably made a full recovery, while at least 6 who suffered postoperative embolic complications died, and the rest may have had permanent disabilities.
Although bridging therapy with low-molecular-weight heparin can decrease the time without anticoagulation for a dental procedure to only 12 hours, it can be complicated to implement, and there appears to be no benefit in terms of the rates of bleeding or embolic complications. Of the 64 anticoagulation interruption studies,4 17 used heparin or low-molecular-weight heparin in conjunction with temporary warfarin interruption. In 210 instances of bridging therapy in 202 patients undergoing dental procedures, there were 2 embolic complications (1% of bridging cases) and 20 bleeding complications, with 3 (1.4%) requiring hemostasis beyond local measures.4
Many of the studies analyzed independently showed there was no significant difference in postoperative bleeding with:
- Anticoagulation continuation vs interruption for a few days
- Lower vs higher international normalized ratio (INR), including some over 4.0
- Surgical vs nonsurgical extraction
- Few vs many extractions.4
Some studies of anticoagulation and anticoagulation interruption for dental surgery had important limitations. Many of the anticoagulation studies excluded patients at high risk of bleeding, those with a high INR (> 4.0), and those with severe liver or kidney disease, and their exclusion could have lowered the incidence of bleeding complications. Many studies of anticoagulation interruption excluded patients at high risk of embolism, including patients with a previous embolic event and patients with an artificial heart valve, and this could have skewed the results lower for embolic complications.
WHY DO SOME CLINICIANS STILL RECOMMEND INTERRUPTION?
The choice seems clear: for dental surgery in anticoagulated patients, the small risk of a nonfatal bleeding complication in anticoagulated patients is outweighed by the small risk of a disabling or fatal embolic complication when anticoagulation is interrupted. Most authors have concluded that anticoagulation should be continued for dental surgery. Yet surveys of dentists and physicians have shown that many still recommend interrupting anticoagulation for dental surgery.5,6
Medical and dental association positions
The American Academy of Neurology7 and the American Dental Association8 recommend continuing anticoagulant medications for dental surgery. The American College of Chest Physicians also recommends continuing anticoagulation but in 2012 added an option to interrupt or decrease anticoagulation for 2 to 3 days for dental surgery.9 Their recommendation was based partly on the results of four controlled prospective studies10–13 comparing anticoagulated dental surgical patients with patients whose anticoagulation was interrupted. In each study, there were no embolic or bleeding complications requiring more than local methods for hemostasis in the interruption groups, leading the American College of Chest Physicians to conclude that brief anticoagulation interruption for dental surgery is safe and effective.
But the results of these studies actually argue against interrupting anticoagulation for dental surgery. In each study, rates of postoperative bleeding complications and blood loss were similar in both groups, and there were no embolic complications. The authors of each study independently concluded that anticoagulation should not be interrupted for dental surgery.
The optimal INR range for anticoagulation therapy is widely accepted as 2.0 to 3.0, and 2.5 to 3.5 for patients with a mechanical mitral valve.14 Interrupting warfarin anticoagulation for 2 or 3 days leads to a suboptimal INR. Patel et al15 studied the incidence of embolic complications (including stroke, non-central nervous system embolism, myocardial infarction, and vascular death) within 30 days in 7,082 patients taking warfarin with and without an interruption of therapy of at least 3 days (median 6 days). The observed rate of embolic events in those with temporary interruption (10.75 events per 100 patient-years) was more than double the rate in those without interruption (4.03 per 100 patient-years).15 However, this study was designed to compare rivaroxaban vs warfarin, not interrupting vs not interrupting warfarin.
A DECISION-TREE REANALYSIS
In 2010, Balevi published a decision-tree analysis that slightly favored withdrawing warfarin for dental surgery, but he stated that the analysis “can be updated in the future as more accurate and up-to-date data for each of the variables in the model become available.”16 Now that there are more accurate and up-to-date data, it is time to revisit this decision-tree analysis.
In Balevi’s analysis, major bleeding is not defined. But major bleeding after dental surgery should be defined as any bleeding requiring more than local measures for hemostasis. In calculating probabilities for the analysis, Balevi cited studies allegedly showing high incidences of major bleeding after dental extractions with warfarin continuation.17,18 There were some minor bleeding complications necessitating additional local measures for hemostasis in these studies, but no major bleeding complications at all in the warfarin- continuation or warfarin-interruption group. There were no significant bleeding events in either study, and the differences in bleeding rates were not significantly different between the two groups. In both studies, the authors concluded that warfarin interruption for dental surgery should be reconsidered.
Similarly, Balevi accurately asserted that there has never been a reported case of fatal bleeding after a dental procedure in an anticoagulated patient, but “for the sake of creating balance,”16 his decision-tree analysis uses a fatal bleeding probability of 1%, based on an estimated 1% risk for nondental procedures (eg, colorectal surgery, major abdominal surgery). It is unclear how a 1% incidence creates “balance,” but dental surgery is unlike other types of surgery, and that is one reason there has never been a documented postdental fatal hemorrhage in an anticoagulated patient. Major vessels are unlikely to be encountered, and bleeding sites are easily accessible to local hemostatic methods.
Balevi used an embolic complication incidence of 0.059% with warfarin interruption of 3 days. Perhaps he used such a low embolic probability because of his incorrect assertion that “there has been no reported case of a dental extraction causing a cardiovascular accident in a patient whose warfarin was temporarily discontinued.”16 In fact, our group has now identified at least 22 reported cases of embolic complications after temporary interruption of warfarin therapy in patients undergoing dental surgery.4 These included 12 embolic complications (3 fatal) after interruption periods from 1 to 5 days.19,20 In addition, there are numerous cases of embolic complications reported in patients whose warfarin was temporarily interrupted for other types of surgery.21,22
The literature shows that embolic complications after temporary warfarin interruption occur at a much higher rate than 0.059%. Many documented embolic complications have occurred after relatively long warfarin interruption periods (greater than 5 days), but many have occurred with much shorter interruptions. Wysokinski et al21 showed that there was a 1.1% incidence of thromboembolic events, more than 18 times greater than Balevi’s incidence, in patients whose warfarin was interrupted for 4 or 5 days with or without bridging therapy. One of these patients developed an occipital infarct within 3 days after stopping warfarin without bridging (for a nondental procedure). Garcia et al22 showed that of 984 warfarin therapy interruptions of 5 days or less, there were 4 embolic complications, a rate (0.4%) more than 6 times greater than that reported by Balevi.
Even if one were to accept a 0.059% embolic risk from interruption of warfarin, that would mean for every 1,700 warfarin interruptions for dental procedures, there would be one possibly fatal embolic complication. On the other hand, if 1,700 dental surgeries were performed without warfarin interruption, based on the literature, there may be some bleeding complications, but none would be fatal. If airline flights had a 0.059% chance of crashing, far fewer people would choose to fly. (There are 87,000 airline flights in the US per day. A 0.059% crash rate would mean there would be 51 crashes per day in the United States alone.)
But regardless of whether the embolic risk is 0.059% or 1%, the question comes down to whether an anticoagulated patient should be subjected to a small but significant risk of death or permanent disability (if anticoagulation is interrupted) or to a small risk of a bleeding complication (if anticoagulation is continued), when 100% of cases up until now have apparently resulted in a full recovery.
As a result, the decision-tree analysis was fatally flawed by grossly overestimating the incidence of fatal bleeding when warfarin is continued, and by grossly underestimating the incidence of embolic complications when warfarin is interrupted.
IS WARFARIN CONTINUATION ‘TROUBLESOME’?
An oral surgeon stated, “My experience and that of many of my colleagues is that even though bleeding is never life-threatening [emphasis mine], it can be difficult to control at therapeutic levels of anticoagulation and can be troublesome, especially for elderly patients.”23 The American College of Chest Physicians stated that postoperative bleeding after dental procedures can cause “anxiety and distress.”3 Patients with even minor postoperative bleeding can be anxious, but surely, postoperative stroke is almost always far more troublesome than postoperative bleeding, which has never been life-threatening. Although other types of surgery may be different, there is no need to interrupt lifesaving anticoagulation for innocuous dental surgery.
My mother was right—it can be rude to interrupt. Anticoagulation should not be interrupted for dental surgery.
When I was growing up, my mother frequently told me that it was rude to interrupt. Although she was referring to conversations, she may have been onto something bigger.
In the nearly three quarters of a century since their discovery, vitamin K antagonist anticoagulant drugs have been used by millions of patients to prevent heart attack and stroke. Before these patients undergo surgery, a decision to continue or interrupt anticoagulation must be made, weighing the risks of postsurgical hemorrhage with continuation of anticoagulation against the risks of stroke or other embolic complications with interruption of anticoagulation. Bleeding after dental surgery when anticoagulation is continued is rarely or never life-threatening. On the other hand, embolic complications of interrupting anticoagulation are almost always consequential and often lead to death or disability. Although consideration may be different for other types of surgery, there is no need to interrupt lifesaving anticoagulation for dental surgery.
EVIDENCE THAT SUPPORTS CONTINUING ANTICOAGULATION
As early as 1957, there were reports of prolonged postoperative bleeding after dental extractions in patients taking anticoagulants. But there were also reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. Since then, there has been a plethora of literature in this area.
A review published in 2000 showed that of more than 950 anticoagulated patients undergoing more than 2,400 dental surgical procedures (including simple and surgical extraction, alveoplasty, and gingival surgery), only 12 (< 1.3%) required more than local measures for hemostasis (eg, fresh-frozen plasma, vitamin K), and no patient died,1 leading to the conclusion that the bleeding risk was not significant in anticoagulated dental patients. Other studies and systematic reviews have also concluded that anticoagulation for dental procedures should not be interrupted.2,3 In a recent review of 83 studies, only 31 (0.6%) of 5,431 patients taking warfarin suffered bleeding complications requiring more than local measures for hemostasis; there were no fatalities.4
The risk of embolism
There have been many reports of embolic complications in patients whose anticoagulation was interrupted for dental procedures. A 2000 review of 575 cases in 526 patients whose anticoagulation was interrupted for dental procedures showed that 5 patients (0.9%) had a serious embolic complication, and 4 died.1 In a more recent review of 64 studies and more than 2,673 patients whose anticoagulation was interrupted for dental procedures, 22 patients (0.8%) suffered embolic complications, and 6 (0.2%) died of the complications.4 Of those with embolic complications, the interruption period was often not reported; however; the interruption ranged from 1 to 4 days. A 2003 systematic review by Dunn and Turpie found a 0.4% embolic complication rate when anticoagulation was interrupted for dental surgery.2
BLEEDING AFTER DENTAL SURGERY
Bleeding after dental surgery can occur with either anticoagulation continuation or interruption, and minor postoperative bleeding requiring additional local hemostatic methods occurs at about the same rate in anticoagulated patients as in those whose anticoagulation is interrupted.
In our recent literature review,4 about 6% of patients in whom anticoagulation was interrupted (and 7% in whom it was not interrupted) had minor bleeding requiring additional local hemostasis, and only 0.2% of patients required more than hemostatic measures (eg, vitamin K injection, plasma transfusion), the same rate found by Dunn and Turpie.2 All patients who required more than local hemostatic measures presumably made a full recovery, while at least 6 who suffered postoperative embolic complications died, and the rest may have had permanent disabilities.
Although bridging therapy with low-molecular-weight heparin can decrease the time without anticoagulation for a dental procedure to only 12 hours, it can be complicated to implement, and there appears to be no benefit in terms of the rates of bleeding or embolic complications. Of the 64 anticoagulation interruption studies,4 17 used heparin or low-molecular-weight heparin in conjunction with temporary warfarin interruption. In 210 instances of bridging therapy in 202 patients undergoing dental procedures, there were 2 embolic complications (1% of bridging cases) and 20 bleeding complications, with 3 (1.4%) requiring hemostasis beyond local measures.4
Many of the studies analyzed independently showed there was no significant difference in postoperative bleeding with:
- Anticoagulation continuation vs interruption for a few days
- Lower vs higher international normalized ratio (INR), including some over 4.0
- Surgical vs nonsurgical extraction
- Few vs many extractions.4
Some studies of anticoagulation and anticoagulation interruption for dental surgery had important limitations. Many of the anticoagulation studies excluded patients at high risk of bleeding, those with a high INR (> 4.0), and those with severe liver or kidney disease, and their exclusion could have lowered the incidence of bleeding complications. Many studies of anticoagulation interruption excluded patients at high risk of embolism, including patients with a previous embolic event and patients with an artificial heart valve, and this could have skewed the results lower for embolic complications.
WHY DO SOME CLINICIANS STILL RECOMMEND INTERRUPTION?
The choice seems clear: for dental surgery in anticoagulated patients, the small risk of a nonfatal bleeding complication in anticoagulated patients is outweighed by the small risk of a disabling or fatal embolic complication when anticoagulation is interrupted. Most authors have concluded that anticoagulation should be continued for dental surgery. Yet surveys of dentists and physicians have shown that many still recommend interrupting anticoagulation for dental surgery.5,6
Medical and dental association positions
The American Academy of Neurology7 and the American Dental Association8 recommend continuing anticoagulant medications for dental surgery. The American College of Chest Physicians also recommends continuing anticoagulation but in 2012 added an option to interrupt or decrease anticoagulation for 2 to 3 days for dental surgery.9 Their recommendation was based partly on the results of four controlled prospective studies10–13 comparing anticoagulated dental surgical patients with patients whose anticoagulation was interrupted. In each study, there were no embolic or bleeding complications requiring more than local methods for hemostasis in the interruption groups, leading the American College of Chest Physicians to conclude that brief anticoagulation interruption for dental surgery is safe and effective.
But the results of these studies actually argue against interrupting anticoagulation for dental surgery. In each study, rates of postoperative bleeding complications and blood loss were similar in both groups, and there were no embolic complications. The authors of each study independently concluded that anticoagulation should not be interrupted for dental surgery.
The optimal INR range for anticoagulation therapy is widely accepted as 2.0 to 3.0, and 2.5 to 3.5 for patients with a mechanical mitral valve.14 Interrupting warfarin anticoagulation for 2 or 3 days leads to a suboptimal INR. Patel et al15 studied the incidence of embolic complications (including stroke, non-central nervous system embolism, myocardial infarction, and vascular death) within 30 days in 7,082 patients taking warfarin with and without an interruption of therapy of at least 3 days (median 6 days). The observed rate of embolic events in those with temporary interruption (10.75 events per 100 patient-years) was more than double the rate in those without interruption (4.03 per 100 patient-years).15 However, this study was designed to compare rivaroxaban vs warfarin, not interrupting vs not interrupting warfarin.
A DECISION-TREE REANALYSIS
In 2010, Balevi published a decision-tree analysis that slightly favored withdrawing warfarin for dental surgery, but he stated that the analysis “can be updated in the future as more accurate and up-to-date data for each of the variables in the model become available.”16 Now that there are more accurate and up-to-date data, it is time to revisit this decision-tree analysis.
In Balevi’s analysis, major bleeding is not defined. But major bleeding after dental surgery should be defined as any bleeding requiring more than local measures for hemostasis. In calculating probabilities for the analysis, Balevi cited studies allegedly showing high incidences of major bleeding after dental extractions with warfarin continuation.17,18 There were some minor bleeding complications necessitating additional local measures for hemostasis in these studies, but no major bleeding complications at all in the warfarin- continuation or warfarin-interruption group. There were no significant bleeding events in either study, and the differences in bleeding rates were not significantly different between the two groups. In both studies, the authors concluded that warfarin interruption for dental surgery should be reconsidered.
Similarly, Balevi accurately asserted that there has never been a reported case of fatal bleeding after a dental procedure in an anticoagulated patient, but “for the sake of creating balance,”16 his decision-tree analysis uses a fatal bleeding probability of 1%, based on an estimated 1% risk for nondental procedures (eg, colorectal surgery, major abdominal surgery). It is unclear how a 1% incidence creates “balance,” but dental surgery is unlike other types of surgery, and that is one reason there has never been a documented postdental fatal hemorrhage in an anticoagulated patient. Major vessels are unlikely to be encountered, and bleeding sites are easily accessible to local hemostatic methods.
Balevi used an embolic complication incidence of 0.059% with warfarin interruption of 3 days. Perhaps he used such a low embolic probability because of his incorrect assertion that “there has been no reported case of a dental extraction causing a cardiovascular accident in a patient whose warfarin was temporarily discontinued.”16 In fact, our group has now identified at least 22 reported cases of embolic complications after temporary interruption of warfarin therapy in patients undergoing dental surgery.4 These included 12 embolic complications (3 fatal) after interruption periods from 1 to 5 days.19,20 In addition, there are numerous cases of embolic complications reported in patients whose warfarin was temporarily interrupted for other types of surgery.21,22
The literature shows that embolic complications after temporary warfarin interruption occur at a much higher rate than 0.059%. Many documented embolic complications have occurred after relatively long warfarin interruption periods (greater than 5 days), but many have occurred with much shorter interruptions. Wysokinski et al21 showed that there was a 1.1% incidence of thromboembolic events, more than 18 times greater than Balevi’s incidence, in patients whose warfarin was interrupted for 4 or 5 days with or without bridging therapy. One of these patients developed an occipital infarct within 3 days after stopping warfarin without bridging (for a nondental procedure). Garcia et al22 showed that of 984 warfarin therapy interruptions of 5 days or less, there were 4 embolic complications, a rate (0.4%) more than 6 times greater than that reported by Balevi.
Even if one were to accept a 0.059% embolic risk from interruption of warfarin, that would mean for every 1,700 warfarin interruptions for dental procedures, there would be one possibly fatal embolic complication. On the other hand, if 1,700 dental surgeries were performed without warfarin interruption, based on the literature, there may be some bleeding complications, but none would be fatal. If airline flights had a 0.059% chance of crashing, far fewer people would choose to fly. (There are 87,000 airline flights in the US per day. A 0.059% crash rate would mean there would be 51 crashes per day in the United States alone.)
But regardless of whether the embolic risk is 0.059% or 1%, the question comes down to whether an anticoagulated patient should be subjected to a small but significant risk of death or permanent disability (if anticoagulation is interrupted) or to a small risk of a bleeding complication (if anticoagulation is continued), when 100% of cases up until now have apparently resulted in a full recovery.
As a result, the decision-tree analysis was fatally flawed by grossly overestimating the incidence of fatal bleeding when warfarin is continued, and by grossly underestimating the incidence of embolic complications when warfarin is interrupted.
IS WARFARIN CONTINUATION ‘TROUBLESOME’?
An oral surgeon stated, “My experience and that of many of my colleagues is that even though bleeding is never life-threatening [emphasis mine], it can be difficult to control at therapeutic levels of anticoagulation and can be troublesome, especially for elderly patients.”23 The American College of Chest Physicians stated that postoperative bleeding after dental procedures can cause “anxiety and distress.”3 Patients with even minor postoperative bleeding can be anxious, but surely, postoperative stroke is almost always far more troublesome than postoperative bleeding, which has never been life-threatening. Although other types of surgery may be different, there is no need to interrupt lifesaving anticoagulation for innocuous dental surgery.
My mother was right—it can be rude to interrupt. Anticoagulation should not be interrupted for dental surgery.
- Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc 2000; 131:77–81.
- Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med 2003; 163:901–908.
- Nematullah A, Alabousi A, Blanas N, Douketis JD, Sutherland SE. Dental surgery for patients on anticoagulant therapy with warfarin: a systematic review and meta-analysis. J Can Dent Assoc 2009; 75:41.
- Wahl MJ, Pintos A, Kilham J, Lalla RV. Dental surgery in anticoagulated patients—stop the interruption. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119:136–157.
- van Diermen DE, van der Waal I, Hoogvliets MW, Ong FN, Hoogstraten J. Survey response of oral and maxillofacial surgeons on invasive procedures in patients using antithrombotic medication. Int J Oral Maxillofac Surg 2013; 42:502–507.
- Ward BB, Smith MH. Dentoalveolar procedures for the anticoagulated patient: literature recommendations versus current practice. J Oral Maxillofac Surg 2007; 65:1454–1460.
- Armstrong MJ, Gronseth G, Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:2065–2069.
- American Dental Association (ADA). Anticoagulant antiplatelet medications and dental procedures. www.ada.org/en/member-center/oral-health-topics/anticoagulant-antiplatelet-medications-and-dental-. Accessed May 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Campbell JH, Alvarado F, Murray RA. Anticoagulation and minor oral surgery: should the anticoagulation regimen be altered? J Oral Maxillofac Surg 2000; 58:131–135.
- Devani P, Lavery M, Howell CJT. Dental extractions in patients on warfarin: is alteration of anticoagulation regime necessary? Br J Oral Maxillofac Surg 1998; 36:107–111.
- Gaspar R, Brenner B, Ardekian L, Peled M, Laufer D. Use of tranexamic acid mouthwash to prevent postoperative bleeding in oral surgery patients on oral anticoagulant medication. Quintessence Int 1997; 28:375–379.
- Blinder D, Manor Y, Martinowitz U, Taicher S. Dental extractions in patients maintained on oral anticoagulant therapy: comparison of INR value with occurrence of postoperative bleeding. Int J Oral Maxillofac Surg 2001; 30:518–521.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Balevi B. Should warfarin be discontinued before a dental extraction? A decision-tree analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:691–697.
- Al-Mubarak S, Al-Ali N, Abou Rass M, et al. Evaluation of dental extractions, suturing and INR on postoperative bleeding of patients maintained on oral anticoagulant therapy. Br Dent J 2007; 203:E15.
- Evans IL, Sayers MS, Gibbons AJ, Price G, Snooks H, Sugar AW. Can warfarin be continued during dental extraction? Results of a randomized controlled trial. Br J Oral Maxillofac Surg 2002; 40:248–252.
- Yasaka M, Naritomi H, Minematsu K. Ischemic stroke associated with brief cessation of warfarin. Thromb Res 2006; 118:290–293.
- Akopov SE, Suzuki S, Fredieu A, Kidwell CS, Saver JL, Cohen SN. Withdrawal of warfarin prior to a surgical procedure: time to follow the guidelines? Cerbrovasc Dis 2005; 19:337–342.
- Wysokinski WE, McBane RD, Daniels PR, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc 2008; 83:639–645.
- Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med 2008; 168:63–69.
- Todd DW. Anticoagulated patients and oral surgery [letter]. Arch Intern Med 2003; 163:1242.
- Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc 2000; 131:77–81.
- Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med 2003; 163:901–908.
- Nematullah A, Alabousi A, Blanas N, Douketis JD, Sutherland SE. Dental surgery for patients on anticoagulant therapy with warfarin: a systematic review and meta-analysis. J Can Dent Assoc 2009; 75:41.
- Wahl MJ, Pintos A, Kilham J, Lalla RV. Dental surgery in anticoagulated patients—stop the interruption. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119:136–157.
- van Diermen DE, van der Waal I, Hoogvliets MW, Ong FN, Hoogstraten J. Survey response of oral and maxillofacial surgeons on invasive procedures in patients using antithrombotic medication. Int J Oral Maxillofac Surg 2013; 42:502–507.
- Ward BB, Smith MH. Dentoalveolar procedures for the anticoagulated patient: literature recommendations versus current practice. J Oral Maxillofac Surg 2007; 65:1454–1460.
- Armstrong MJ, Gronseth G, Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:2065–2069.
- American Dental Association (ADA). Anticoagulant antiplatelet medications and dental procedures. www.ada.org/en/member-center/oral-health-topics/anticoagulant-antiplatelet-medications-and-dental-. Accessed May 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Campbell JH, Alvarado F, Murray RA. Anticoagulation and minor oral surgery: should the anticoagulation regimen be altered? J Oral Maxillofac Surg 2000; 58:131–135.
- Devani P, Lavery M, Howell CJT. Dental extractions in patients on warfarin: is alteration of anticoagulation regime necessary? Br J Oral Maxillofac Surg 1998; 36:107–111.
- Gaspar R, Brenner B, Ardekian L, Peled M, Laufer D. Use of tranexamic acid mouthwash to prevent postoperative bleeding in oral surgery patients on oral anticoagulant medication. Quintessence Int 1997; 28:375–379.
- Blinder D, Manor Y, Martinowitz U, Taicher S. Dental extractions in patients maintained on oral anticoagulant therapy: comparison of INR value with occurrence of postoperative bleeding. Int J Oral Maxillofac Surg 2001; 30:518–521.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Balevi B. Should warfarin be discontinued before a dental extraction? A decision-tree analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:691–697.
- Al-Mubarak S, Al-Ali N, Abou Rass M, et al. Evaluation of dental extractions, suturing and INR on postoperative bleeding of patients maintained on oral anticoagulant therapy. Br Dent J 2007; 203:E15.
- Evans IL, Sayers MS, Gibbons AJ, Price G, Snooks H, Sugar AW. Can warfarin be continued during dental extraction? Results of a randomized controlled trial. Br J Oral Maxillofac Surg 2002; 40:248–252.
- Yasaka M, Naritomi H, Minematsu K. Ischemic stroke associated with brief cessation of warfarin. Thromb Res 2006; 118:290–293.
- Akopov SE, Suzuki S, Fredieu A, Kidwell CS, Saver JL, Cohen SN. Withdrawal of warfarin prior to a surgical procedure: time to follow the guidelines? Cerbrovasc Dis 2005; 19:337–342.
- Wysokinski WE, McBane RD, Daniels PR, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc 2008; 83:639–645.
- Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med 2008; 168:63–69.
- Todd DW. Anticoagulated patients and oral surgery [letter]. Arch Intern Med 2003; 163:1242.
The fifth vital sign: A complex story of politics and patient care
In this issue of the Journal, Dr. Marissa Galicia-Castillo discusses the use of opioids in older patients with persistent (formerly known as chronic) pain. Even though she devotes one and a half pages to the side effects of chronic opioid therapy, I am sure that in the current environment many readers will perceive her as expressing a surprisingly supportive tone regarding the use of these medications. The times have changed, and the difficulties and complexities of trying to help patients with ongoing pain have increased.
In the mid-1990s, the American Pain Society aggressively pushed the concept of pain as the fifth vital sign.1 Their stated goals included raising awareness that patients with pain were undertreated, in large part because in the Society’s opinion pain was not regularly assessed at physician office visits or even in the hospital after surgery. Half a decade later the Joint Commission and others hopped on this train, emphasizing that pain needs to be regularly assessed in all patients, that pain is a subjective measure, unlike the heart rate or blood pressure, and that physicians must accept and respect patient self-reporting of pain. Concurrent with these efforts was the enhanced promotion of pain medications—new highly touted and frequently prescribed narcotics as well as nonnarcotic medications re-marketed as analgesics. Opportunistically, or perhaps wielding inappropriate and sketchy influence, some drug manufacturers in the early 2000s funded publications and physician presentations to encourage the expanded use of opioids and other medications for pain control. In a recent CNN report on the opioid epidemic, it was noted that the Joint Commission published a book in 2000 for purchase by doctors as part of required continuing education seminars, and that the book cited studies claiming “there is no evidence that addiction is a significant issue when persons are given opioids for pain control.”2 According to the CNN report, the book was sponsored by a manufacturer of narcotic analgesics.2 Lack of evidence is not evidence supporting a lack of known concern.
Step forward in time, and pain control has become a measure of patient satisfaction, and thus potentially another physician and institutional rating score that can be linked to reimbursement. This despite reports suggesting that incorporation of this required pain scale did not actually improve the quality of pain management.3 I suspect that most of my peers function in the outpatient clinic as I do, without much interest in what was recorded on the intake pain scale, since I will be taking a more focused and detailed history from the patient if pain is any part of the reason for visiting with me. The goal of alleviating a patient’s pain, whenever reasonable, must always be on our agenda. Yet, while we need to respond to scores on a somewhat silly screening pain scale, the hurdles to prescribing analgesics are getting higher.
The latest data on opioid-related deaths are sobering and scary. Organized medicine must absolutely push to close the pain-pill mills, but is the link really so strong between thoughtful prescribing of short- or even long-term opioids and the escalating “epidemic” of opioid complications that we should not prescribe these drugs? Does the fact that we don’t have good data demonstrating long-term efficacy mean that these drugs are not effective in appropriately selected patients? Is it warranted to require regular database reviews of all patients who are prescribed these medications? Is it warranted, as one patient said to me, that she be treated like a potential criminal begging for drugs when her prescriptions are up, and that she be “looked at funny” by the pharmacist when she fills them?
An increasingly discussed concept is that of central generalization of pain, and patients who have this may be opioid-resistant and, perhaps, prone to developing opioid hyperalgesia. It has been studied in patients with fibromyalgia and is now felt by some to include patients with osteoarthritis and other initially localized painful conditions. Whether or not this concept ultimately turns out to be correct, it adds another dimension to our assessment of patients with pain.
The time has come to move past using a one-size-fits-all fifth vital sign (“How would you rate your pain on a scale of 1 to 10?”) and reflexively prescribing an opioid when pain is characterized as severe. But, if the patient truly needs the drug, we also need to move past not writing that prescription because of headlines and administrative hurdles. This is a much more complex story.
- American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995; 274:1874–1880.
- Moghe S. Opioid history: from ‘wonder drug’ to abuse epidemic. www.cnn.com/2016/05/12/health/opioid-addiction-history/. Accessed May 16, 2016.
- Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 2006; 21:607–612.
In this issue of the Journal, Dr. Marissa Galicia-Castillo discusses the use of opioids in older patients with persistent (formerly known as chronic) pain. Even though she devotes one and a half pages to the side effects of chronic opioid therapy, I am sure that in the current environment many readers will perceive her as expressing a surprisingly supportive tone regarding the use of these medications. The times have changed, and the difficulties and complexities of trying to help patients with ongoing pain have increased.
In the mid-1990s, the American Pain Society aggressively pushed the concept of pain as the fifth vital sign.1 Their stated goals included raising awareness that patients with pain were undertreated, in large part because in the Society’s opinion pain was not regularly assessed at physician office visits or even in the hospital after surgery. Half a decade later the Joint Commission and others hopped on this train, emphasizing that pain needs to be regularly assessed in all patients, that pain is a subjective measure, unlike the heart rate or blood pressure, and that physicians must accept and respect patient self-reporting of pain. Concurrent with these efforts was the enhanced promotion of pain medications—new highly touted and frequently prescribed narcotics as well as nonnarcotic medications re-marketed as analgesics. Opportunistically, or perhaps wielding inappropriate and sketchy influence, some drug manufacturers in the early 2000s funded publications and physician presentations to encourage the expanded use of opioids and other medications for pain control. In a recent CNN report on the opioid epidemic, it was noted that the Joint Commission published a book in 2000 for purchase by doctors as part of required continuing education seminars, and that the book cited studies claiming “there is no evidence that addiction is a significant issue when persons are given opioids for pain control.”2 According to the CNN report, the book was sponsored by a manufacturer of narcotic analgesics.2 Lack of evidence is not evidence supporting a lack of known concern.
Step forward in time, and pain control has become a measure of patient satisfaction, and thus potentially another physician and institutional rating score that can be linked to reimbursement. This despite reports suggesting that incorporation of this required pain scale did not actually improve the quality of pain management.3 I suspect that most of my peers function in the outpatient clinic as I do, without much interest in what was recorded on the intake pain scale, since I will be taking a more focused and detailed history from the patient if pain is any part of the reason for visiting with me. The goal of alleviating a patient’s pain, whenever reasonable, must always be on our agenda. Yet, while we need to respond to scores on a somewhat silly screening pain scale, the hurdles to prescribing analgesics are getting higher.
The latest data on opioid-related deaths are sobering and scary. Organized medicine must absolutely push to close the pain-pill mills, but is the link really so strong between thoughtful prescribing of short- or even long-term opioids and the escalating “epidemic” of opioid complications that we should not prescribe these drugs? Does the fact that we don’t have good data demonstrating long-term efficacy mean that these drugs are not effective in appropriately selected patients? Is it warranted to require regular database reviews of all patients who are prescribed these medications? Is it warranted, as one patient said to me, that she be treated like a potential criminal begging for drugs when her prescriptions are up, and that she be “looked at funny” by the pharmacist when she fills them?
An increasingly discussed concept is that of central generalization of pain, and patients who have this may be opioid-resistant and, perhaps, prone to developing opioid hyperalgesia. It has been studied in patients with fibromyalgia and is now felt by some to include patients with osteoarthritis and other initially localized painful conditions. Whether or not this concept ultimately turns out to be correct, it adds another dimension to our assessment of patients with pain.
The time has come to move past using a one-size-fits-all fifth vital sign (“How would you rate your pain on a scale of 1 to 10?”) and reflexively prescribing an opioid when pain is characterized as severe. But, if the patient truly needs the drug, we also need to move past not writing that prescription because of headlines and administrative hurdles. This is a much more complex story.
In this issue of the Journal, Dr. Marissa Galicia-Castillo discusses the use of opioids in older patients with persistent (formerly known as chronic) pain. Even though she devotes one and a half pages to the side effects of chronic opioid therapy, I am sure that in the current environment many readers will perceive her as expressing a surprisingly supportive tone regarding the use of these medications. The times have changed, and the difficulties and complexities of trying to help patients with ongoing pain have increased.
In the mid-1990s, the American Pain Society aggressively pushed the concept of pain as the fifth vital sign.1 Their stated goals included raising awareness that patients with pain were undertreated, in large part because in the Society’s opinion pain was not regularly assessed at physician office visits or even in the hospital after surgery. Half a decade later the Joint Commission and others hopped on this train, emphasizing that pain needs to be regularly assessed in all patients, that pain is a subjective measure, unlike the heart rate or blood pressure, and that physicians must accept and respect patient self-reporting of pain. Concurrent with these efforts was the enhanced promotion of pain medications—new highly touted and frequently prescribed narcotics as well as nonnarcotic medications re-marketed as analgesics. Opportunistically, or perhaps wielding inappropriate and sketchy influence, some drug manufacturers in the early 2000s funded publications and physician presentations to encourage the expanded use of opioids and other medications for pain control. In a recent CNN report on the opioid epidemic, it was noted that the Joint Commission published a book in 2000 for purchase by doctors as part of required continuing education seminars, and that the book cited studies claiming “there is no evidence that addiction is a significant issue when persons are given opioids for pain control.”2 According to the CNN report, the book was sponsored by a manufacturer of narcotic analgesics.2 Lack of evidence is not evidence supporting a lack of known concern.
Step forward in time, and pain control has become a measure of patient satisfaction, and thus potentially another physician and institutional rating score that can be linked to reimbursement. This despite reports suggesting that incorporation of this required pain scale did not actually improve the quality of pain management.3 I suspect that most of my peers function in the outpatient clinic as I do, without much interest in what was recorded on the intake pain scale, since I will be taking a more focused and detailed history from the patient if pain is any part of the reason for visiting with me. The goal of alleviating a patient’s pain, whenever reasonable, must always be on our agenda. Yet, while we need to respond to scores on a somewhat silly screening pain scale, the hurdles to prescribing analgesics are getting higher.
The latest data on opioid-related deaths are sobering and scary. Organized medicine must absolutely push to close the pain-pill mills, but is the link really so strong between thoughtful prescribing of short- or even long-term opioids and the escalating “epidemic” of opioid complications that we should not prescribe these drugs? Does the fact that we don’t have good data demonstrating long-term efficacy mean that these drugs are not effective in appropriately selected patients? Is it warranted to require regular database reviews of all patients who are prescribed these medications? Is it warranted, as one patient said to me, that she be treated like a potential criminal begging for drugs when her prescriptions are up, and that she be “looked at funny” by the pharmacist when she fills them?
An increasingly discussed concept is that of central generalization of pain, and patients who have this may be opioid-resistant and, perhaps, prone to developing opioid hyperalgesia. It has been studied in patients with fibromyalgia and is now felt by some to include patients with osteoarthritis and other initially localized painful conditions. Whether or not this concept ultimately turns out to be correct, it adds another dimension to our assessment of patients with pain.
The time has come to move past using a one-size-fits-all fifth vital sign (“How would you rate your pain on a scale of 1 to 10?”) and reflexively prescribing an opioid when pain is characterized as severe. But, if the patient truly needs the drug, we also need to move past not writing that prescription because of headlines and administrative hurdles. This is a much more complex story.
- American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995; 274:1874–1880.
- Moghe S. Opioid history: from ‘wonder drug’ to abuse epidemic. www.cnn.com/2016/05/12/health/opioid-addiction-history/. Accessed May 16, 2016.
- Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 2006; 21:607–612.
- American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995; 274:1874–1880.
- Moghe S. Opioid history: from ‘wonder drug’ to abuse epidemic. www.cnn.com/2016/05/12/health/opioid-addiction-history/. Accessed May 16, 2016.
- Mularski RA, White-Chu F, Overbay D, et al. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 2006; 21:607–612.
Opioids for persistent pain in older adults
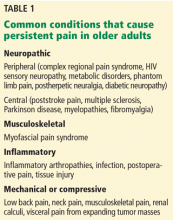
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
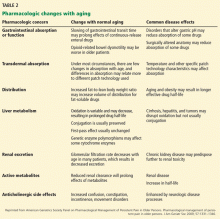
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
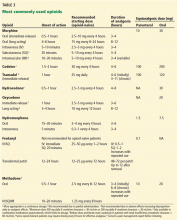
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
KEY POINTS
- Treatment of persistent pain in older adults presents several challenges.
- Often, persistent pain is underrecognized and undertreated, impairing function and reducing quality of life.
- A combination of pharmacologic and nonpharmacologic strategies is needed to address the multiple factors contributing to pain and manage it effectively.
- The World Health Organization’s three-step ladder is valuable for treating persistent pain in older adults.
- Although nonopioids are the first-line treatments for persistent pain, opioids are also important to provide safe and effective pain management in older adults.
Navigating pneumococcal vaccination in adults
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
Streptococcus pneumoniae (the “pneumococcus”) causes a variety of clinical syndromes that range from otitis media to bacteremia, meningitis, and pneumonia. Hardest hit are immunocompromised people and those at the extremes of age. Therefore, preventing disease through pneumococcal vaccination is very important in these groups.
This review summarizes the current guidelines from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) for pneumococcal immunization in adults.
STRIKES THE VERY YOUNG, VERY OLD, AND IMMUNOCOMPROMISED
Invasive pneumococcal disease is defined as infection in which S pneumoniae can be found in a normally sterile site such as the cerebrospinal fluid or blood, and it includes bacteremic pneumonia.1 By far the most common type of pneumococcal disease is pneumonia, followed by bacteremia and meningitis (Figure 1)2,3; about 25% of patients with pneumococcal pneumonia also have bacteremia.2
Invasive pneumococcal disease most often occurs in children age 2 and younger, adults age 65 and older, and people who are immunocompromised. In 2010, the incidence was 3.8 per 100,000 in people ages 18 to 34 but was 10 times higher in the elderly and those with compromised immunity.1
Even now that vaccines are available, invasive pneumococcal disease continues to cause 4,000 deaths per year in the United States.1
TWO INACTIVATED VACCINES
S pneumoniae is a gram-positive coccus with an outer capsule composed of polysaccharides that protect the bacterium from being ingested and killed by host phagocytic cells. Some 91 serotypes of this organism have been identified on the basis of genetic differences in capsular polysaccharide composition.
Currently, two inactivated vaccines are available that elicit antibody responses to the most common pneumococcal serotypes that infect humans.
- PPSV23 (pneumococcal polysaccharide vaccine-23, or Pneumovax 23) contains purified capsular polysaccharides from 23 pneumococcal serotypes.
- PCV13 (pneumococcal conjugate vaccine-13, or Prevnar 13) contains purified capsular polysaccharides from 13 serotypes that are covalently bound to (conjugated with) a carrier protein.
PPSV23 AND PCV13 ARE NOT THE SAME
Apart from the number of serotypes covered, the two vaccines differ in important ways. Both of them elicit a B-cell-mediated immune response, but only PCV13 produces a T-cell-dependent response, which is essential for maturation of the B-cell response and development of immune memory.
PPSV23 generally provides 3 to 5 years of immunity, and repeat doses do not offer additive or “boosted” protection. It is ineffective in children under 2 years of age.
Pneumococcal conjugate vaccine has been available since 2000 for children starting at 2 months of age. Since then it has directly reduced the incidence of invasive pneumococcal disease in children and indirectly in adults. The impact on pneumococcal disease rates in adults has probably been related to reduction in rates of pneumococcal nasopharyngeal carriage in children, another unique benefit of conjugated vaccines.3
In December 2011, the US Food and Drug Administration (FDA) approved PCV13 for adults on the basis of immunologic studies and anticipation that clinical efficacy would be similar to that observed in children.
HOW EFFECTIVE ARE THEY?
The efficacy and safety of PPSV23 and PCV13 have been studied in a variety of patient populations. Though antibody responses to PCV13 were similar to or better than those with PPSV23, no studies of specific correlations between immunologic responses and disease outcomes are available.4,5
In large studies in healthy adults, both vaccines reduced the incidence of invasive pneumococcal disease. A study in more than 47,000 adults age 65 and older showed a significant reduction in pneumococcal bacteremia (hazard ratio 0.56, 95% confidence interval 0.33–0.93) in those who received PPSV23 compared with those who received placebo.6 However, PPSV23 was not effective in preventing nonbacteremic and noninvasive pneumococcal community-acquired pneumonia when all bacterial serotypes were considered.6
In a placebo-controlled trial in more than 84,000 people age 65 and older, PCV13 prevented both nonbacteremic and bacteremic community-acquired pneumococcal pneumonia due to serotypes included in the vaccine (relative risk reduction 45%, P < .007) and overall invasive pneumococcal disease due to serotypes included in the vaccine (relative risk reduction 70%, P < .001).7
Both vaccines have also demonstrated efficacy in immunocompromised adults. Several studies showed an equivalent or superior antibody response to a seven-valent pneumococcal conjugate vaccine (PCV7, which has been replaced by PCV13) compared with PPSV23 in adults with human immunodeficiency virus (HIV) infection.8,9 While specific clinical studies of the efficacy of PCV13 among immunocompromised people are not available, a study of vaccination with PCV7 in 496 people in Malawi, of whom 88% were infected with HIV, found that the vaccine was effective in preventing invasive pneumococcal disease (hazard ratio 26%, 95% confidence interval 0.10–0.70).10
AT-RISK PATIENT POPULATIONS
Since both PPSV23 and PCV13 are approved for use in adults, it is important to understand appropriate indications for their use. The ACIP recommends pneumococcal vaccination in adults at an increased risk of invasive pneumococcal disease: ie, people age 65 and older, at-risk people ages 19 to 64, and people who are immunocompromised or asplenic.
A more robust antibody response has been shown with PCV13 compared to PPSV23 in healthy people.5 Of note, when PPSV23 is given before PCV13, there is a diminished immune response to PCV13.11,12 Therefore, unvaccinated people who will receive both PCV13 and PPSV23 should be given the conjugate vaccine PCV13 first. (See Commonly asked questions.)
ADULTS AGE 65 AND OLDER: ONE DOSE EACH OF PCV13 AND PPSV23
Before September 2014, the ACIP recommended one dose of PPSV23 for adults age 65 and older to prevent invasive pneumococcal disease.13 With evidence that PCV13 also produces an antibody response and is clinically effective against pneumococcal pneumonia in older people, the ACIP now recommends that all adults age 65 and older receive one dose of PCV13 and one dose of PPSV23.3, 14
Based on antibody studies, the ACIP recommends giving PCV13 first and PPSV23 12 months after.11,12 Patients who received PPSV23 at age 65 or older should receive PCV13 at least 1 year after PPSV23 (Figure 2).3,14 Patients who had previously received one dose of PPSV23 before age 65 who are now age 65 or older should receive one dose of PCV13 at least 1 year after PPSV23 and an additional dose of PPSV23 at least 5 years after the first dose of PPSV23 and at least 1 year after the dose of PCV13.3 Patients who received a dose of PCV13 before age 65 do not need an additional dose after age 65.
The Centers for Medicare and Medicaid Services have updated the reimbursement for pneumococcal vaccines to include both PCV13 and PPSV23. Patients can receive one dose of pneumococcal vaccine followed by a different, second pneumococcal vaccine at least 11 full months after the month in which the first pneumococcal vaccine was administered.15
AT-RISK PATIENTS AGES 19 TO 64
Before 2012, the ACIP recommended that patients at risk, including immunocompromised patients and those without a spleen, with cerebrospinal fluid leaks, or with cochlear implants, receive only PPSV23 before age 65.13 In 2010, 50% of cases of invasive pneumococcal disease in immunocompromised adults were due to serotypes contained in PCV13.16 Additionally, according to CDC data from 2013, in adults ages 19 to 64 at risk of pneumococcal disease, only 21.2% had received pneumococcal vaccine.17 With information on epidemiology, safety, and efficacy, as well as expanded FDA approval of PCV13 for adults in 2011, the ACIP updated its guidelines for pneumococcal immunization of adults with immunocompromising conditions in October 2012.16 The updated guidelines now include giving PCV13 to adults at increased risk of invasive pneumococcal disease.16
Adults under age 65 at risk of invasive pneumococcal disease can be further divided into those who are immunocompetent with comorbid conditions, and those with cochlear implants or cerebrospinal fluid leak. (Table 1).16
Patients with cochlear implants or cerebrospinal fluid leaks should receive one dose of PCV13 followed by one dose of PPSV23 8 weeks later. If PPSV23 is given first in this group, PCV13 can be given 1 year later.
Immunocompetent patients with comorbid conditions, including cigarette smoking, chronic heart, liver, or lung disease, asthma, cirrhosis, and diabetes mellitus, should receive one dose of PPSV23 before age 65 (Table 1).16
IMMUNOCOMPROMISED AND ASPLENIC PATIENTS
Immunocompromised patients at risk for invasive pneumococcal disease include patients with functional or anatomic asplenia or immunocompromising conditions such as HIV infection, chronic renal failure, generalized malignancy, solid organ transplant, iatrogenic immunosuppression (eg, due to corticosteroid therapy), and other immunocompromising conditions.16 Patients on corticosteroid therapy are considered immunosuppressed if they take 20 mg or more of prednisone daily (or an equivalent corticosteroid dose) for at least 14 days.16 These immunocompromised patients should receive one dose of PCV13, followed by a PPSV23 dose 8 weeks later and a second PPSV23 dose 5 years after the first.16
The time between vaccinations is also important. If PCV13 is given first, PPSV23 can be given after at least 8 weeks. If PPSV23 is given first, PCV13 should be given after 12 months. The time between PPSV23 doses is 5 years (Figure 3).16
ADDRESSING BARRIERS TO PNEUMOCOCCAL VACCINATION
In 2013, only 59.7% of adults age 65 and older and 21.1% of younger, at-risk adults with immunocompromising conditions had received pneumococcal vaccination.17 Healthcare providers have the opportunity to improve pneumococcal vaccination rates. The National Foundation for Infectious Diseases (www.nfid.org) summarized challenges in vaccinating at-risk patients and recommended strategies to overcome barriers.18
Challenges include the cost of vaccine coverage, limited time (with competing priorities during office appointments or hospitalizations), patient refusal, and knowledge gaps.
Strategies to overcome barriers include incorporating vaccination into protocols and procedures; educating healthcare providers and patients about pneumococcal disease, vaccines, costs, and reimbursement; engaging nonclinical staff members; and monitoring local vaccination rates. However, the most important factor affecting whether adults are vaccinated is whether the healthcare provider recommends it.
AN OPPORTUNITY TO IMPROVE
In the last 30 years, great strides have been made in recognizing and preventing pneumococcal disease, but challenges remain. Adherence to the new ACIP guidelines for pneumococcal vaccination in immunocompromised, at risk and elderly patients is important in reducing invasive pneumococcal disease.
Healthcare providers have the opportunity to improve pneumococcal vaccination rates at outpatient appointments to decrease the burden of invasive pneumococcal disease in at-risk populations. A comprehensive understanding of the guideline recommendations for pneumococcal vaccination can aid the provider in identifying patients who are eligible for vaccination.
Adult pneumococcal immunization rates are low due to missed opportunities. Healthcare providers can improve these rates by viewing every patient encounter as a chance to provide vaccination.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance report (ABCs). ABCs Report: Streptococcus pneumoniae, 2010. www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. Accessed May 13, 2016.
- Said MA, Johnson, HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. Plos One 2013; 8:e60273.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al; Infectious Disease Clinical Research Program HIV Working Group. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010: 202:1114–1125.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults. Vaccine 2013; 31:3577–3584.
- Jackson LA, Neuzil KM, Yu O, et al; Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747–1755.
- Bonten M, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125.
- Lesprit P, Pedrono G, Molina JM, et al; ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boosted pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434.
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553.
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2013; 31:3585–3593.
- Greenberg RN, Gurtman A, French RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 2014; 32:2364–2374.
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010: 59:1102–1106.
- Kobayashi M, Bennett NM, Gierke R, et al. Centers for Disease Control and Prevention (CDC). Intervals between PCV13 and PPSV23; Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2015;64:944-947.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. Modifications to Medicare Part B coverage of pneumococcal vaccinations. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9051.pdf. Accessed May 13, 2016.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W; National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med 2012; 124:71–79.
- Centers for Disease Control and Prevention (CDC). Vaccines and immunizations. PCV13 (pneumococcal conjugate) vaccine. Recommendations, scenarios and Q&As for healthcare professionals about PCV13 for adults. www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm. Accessed May 13, 2016.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Pneumococcal vaccines (PCV13 and PPSV23). www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp. Accessed May 13, 2016.
KEY POINTS
- At highest risk of invasive pneumococcal disease are people who are immunocompromised, very young, or very old.
- Pneumococcal polysaccharide vaccine-23 (PPSV23) covers more serotypes of S pneumoniae than pneumococcal conjugate vaccine-13 (PCV13), but the latter induces a stronger antibody response.
- The combination of both vaccines in sequence produces a better antibody response than either vaccine alone.
- The Advisory Committee on Immunization Practices now recommends that immunocompromised and asplenic adults who need pneumococcal vaccination receive both vaccines, preferably PCV13 first, followed by PPSV23 8 weeks later. Those who have already received PPSV23 can receive PCV13 after at least 1 year has passed.
- People with asplenia or immunocompromising conditions should receive a second dose of PPSV23 at least 5 years after the first dose.
- Vaccination schedules and information are available from the US Centers for Disease Control and Prevention at www.cdc.gov.
Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
For some patients with chronic pancreatitis, the best option is to remove the entire pancreas. This does not necessarily doom the patient to diabetes mellitus, because we can harvest the islet cells and reinsert them so that, lodged in the liver, they can continue making insulin. However, this approach is underemphasized in the general medical literature and is likely underutilized in the United States.
Here, we discuss chronic pancreatitis, the indications for and contraindications to this procedure, its outcomes, and the management of patients who undergo it.
CHRONIC PANCREATITIS IS PROGRESSIVE AND PAINFUL
Chronic pancreatitis is a progressive condition characterized by chronic inflammation, irreversible fibrosis, and scarring, resulting in loss of both exocrine and endocrine tissue.
According to a National Institutes of Health database, pancreatitis is the seventh most common digestive disease diagnosis on hospitalization, with annual healthcare costs exceeding $3 billion.1 Although data are scarce, by some estimates the incidence of chronic pancreatitis ranges from 4 to 14 per 100,000 person-years, and the prevalence ranges from 26.4 to 52 per 100,000.2–4 Moreover, a meta-analysis5 found that acute pancreatitis progresses to chronic pancreatitis in 10% of patients who have a first episode of acute pancreatitis and in 36% who have recurrent episodes.
Historically, alcoholism was and still is the most common cause of chronic pancreatitis, contributing to 60% to 90% of cases in Western countries.6,7 However, cases due to nonalcoholic causes have been increasing, and in more than one-fourth of patients, no identifiable cause is found.6,8 Smoking is an independent risk factor.6,8,9 Some cases can be linked to genetic abnormalities, particularly in children.10
The clinical manifestations of chronic pancreatitis include exocrine pancreatic insufficiency (leading to malnutrition and steatorrhea), endocrine insufficiency (causing diabetes mellitus), and intractable pain.11 Pain is the predominant clinical symptom early in the disease and is often debilitating and difficult to manage. Uncontrolled pain has a devastating impact on quality of life and may become complicated by narcotic dependence.
The pain of chronic pancreatitis is often multifactorial, with mechanisms that include increased intraductal pressure from obstruction of the pancreatic duct, pancreatic ischemia, neuronal injury, and neuroimmune interactions between neuronal processes and chronic inflammation.12
Treatment: Medical and surgical
In chronic pancreatitis, the aim of treatment is to alleviate deficiencies of exocrine and endocrine function and mitigate the pain. Patients who smoke or drink alcohol should be strongly encouraged to quit.
Loss of exocrine function is mainly managed with oral pancreatic enzyme supplements, and diabetes control is often attained with insulin therapy.13 Besides helping digestion, pancreatic enzyme therapy in the form of nonenteric tablets may also reduce pain and pancreatitis attacks.14 The mechanism may be by degrading cholecystokinin-releasing factor in the duodenum, lowering cholecystokinin levels and thereby reducing pain.12
Nonnarcotic analgesics are often the first line of therapy for pain management, but many patients need narcotic analgesics. Along with narcotics, adjunctive agents such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, and gabapentinoids have been used to treat chronic pancreatitis pain, but with limited success.15
In patients for whom medical pain management has failed, one can consider another option, such as nerve block, neurolysis, or endoscopic or surgical therapy. Neuromodulators are often prescribed by pain clinics.15 Percutaneous and endoscopic celiac ganglion blocks can be an option but rarely achieve substantial or permanent pain relief, and the induced transient responses (on average 2 to 4 months) often cannot be repeated.14–17
Surgical options to relieve pain try to preserve pancreatic function and vary depending on the degree of severity and nature of pancreatic damage. In broad terms, the surgical procedures can be divided into two types:
- Drainage procedures (eg, pseudocyst drainage; minimally invasive endoscopic duct drainage via sphincterotomy or stent placement, or both; pancreaticojejunostomy)
- Resectional procedures (eg, distal pancreatectomy, isolated head resection, pancreaticoduodenectomy, Whipple procedure, total pancreatectomy).
In carefully selected patients, total pancreatectomy can be offered to remove the cause of the pain.18 This procedure is most often performed in patients who have small-duct disease or a genetic cause or for whom other surgical procedures have failed.11
HISTORY OF THE PROCEDURE
Islet cell transplantation grew out of visionary work by Paul Lacy and David Scharp at the University of Washington at Seattle, whose research focused on isolating and transplanting islet cells in rodent models. The topic has been reviewed by Jahansouz et al.19 In the 1970s, experiments in pancreatectomized dogs showed that transplanting unpurified pancreatic islet tissue that was dispersed by collagenase digestion into the spleen or portal vein could prevent diabetes.20,21 In 1974, the first human trials of transplanting islet cells were conducted, using isolated islets from cadaveric donors to treat diabetes.19
In the past, pancreatectomy was performed to treat painful chronic pancreatitis, but it was viewed as undesirable because removing the gland would inevitably cause insulin-dependent diabetes.22 That changed in 1977 at the University of Minnesota, with the first reported islet cell autotransplant after pancreatectomy. The patient remained pain-free and insulin-independent long-term.23 This seminal case showed that endocrine function could be preserved by autotransplant of islets prepared from the excised pancreas.24
In 1992, Pyzdrowski et al25 reported that intrahepatic transplant of as few as 265,000 islets was enough to prevent the need for insulin therapy. Since this technique was first described, there have been many advances, and now more than 30 centers worldwide do it.
PRIMARY INDICATION: INTRACTABLE PAIN
Interest has been growing in using total pancreatectomy and islet autotransplant to treat recurrent acute pancreatitis, chronic pancreatitis, and hereditary pancreatitis. The rationale is that removing the offending tissue eliminates pancreatitis, pain, and cancer risk, while preserving and replacing the islet cells prevents the development of brittle diabetes with loss of insulin and glucagon.26
Proposed criteria for total pancreatectomy and islet autotransplant
Bellin et al14 proposed five criteria for patient selection for this procedure based on imaging studies, pancreatic function tests, and histopathology to detect pancreatic fibrosis. Patients must fulfill all five of the following criteria:
Criterion 1. Diagnosis of chronic pancreatitis, based on chronic abdominal pain lasting more than 6 months with either at least one of the following:
- Pancreatic calcifications on computed tomography
- At least two of the following: four or more of nine criteria on endoscopic ultrasonography described by Catalano et al,27 a compatible ductal or parenchymal abnormality on secretin magnetic resonance cholangiopancreatography; abnormal endoscopic pancreatic function test (peak HCO2 ≤ 80 mmol/L)
- Histopathologically confirmed diagnosis of chronic pancreatitis
- Compatible clinical history and documented hereditary pancreatitis (PRSS1 gene mutation)
OR
- History of recurrent acute pancreatitis (more than one episode of characteristic pain associated with imaging diagnostic of acute pancreatitis or elevated serum amylase or lipase > 3 times the upper limit of normal).
Criterion 2. At least one of the following:
- Daily narcotic dependence
- Pain resulting in impaired quality of life, which may include inability to attend school, recurrent hospitalizations, or inability to participate in usual age-appropriate activities.
Criterion 3. Complete evaluation with no reversible cause of pancreatitis present or untreated.
Criterion 4. Failure to respond to maximal medical and endoscopic therapy.
Criterion 5. Adequate islet cell function (nondiabetic or C-peptide-positive). Patients with C-peptide-negative diabetes meeting criteria 1 to 4 are candidates for total pancreatectomy alone.
The primary goal is to treat intractable pain and improve quality of life in selected patients with chronic pancreatitis or recurrent acute pancreatitis when endoscopic and prior surgical therapies have failed, and whose impairment due to pain is substantial enough to accept the risk of postoperative insulin-dependent diabetes and lifelong commitment to pancreatic enzyme replacement therapy.15,26 Patients with a known genetic cause of chronic pancreatitis should be offered special consideration for the procedure, as their disease is unlikely to remit.
CONTRAINDICATIONS
Total pancreatectomy and islet autotransplant should not be performed in patients with active alcoholism, illicit drug use, or untreated or poorly controlled psychiatric illnesses that could impair the patient’s ability to adhere to a complicated postoperative medical regimen.
A poor support network may be a relative contraindication in view of the cost and complexity of diabetic and pancreatic enzyme replacement therapy.18,26
Islet cell autotransplant is contraindicated in patients with conditions such as C-peptide-negative or type 1 diabetes or a history of portal vein thrombosis, portal hypertension, significant liver disease, high-risk cardiopulmonary disease, or pancreatic cancer (Table 1).26
WHEN TO CONSIDER REFERRAL FOR THIS PROCEDURE
The choice of total pancreatectomy and islet autotransplant vs conventional surgery must be individualized on the basis of each patient’s anatomy, comorbidities, symptom burden, presence or degree of diabetes, and rate of disease progression. The most important factors to consider in determining the need for and timing of this procedure are the patient’s pain, narcotic requirements, and impaired ability to function.26
Sooner rather than later?
An argument can be made for performing this procedure sooner in the course of the disease rather than later when all else has failed. First, prolonged pain can result in central sensitization, in which the threshold for perceiving pain is lowered by damage to the nociceptive neurons from repeated stimulation and inflammation.28
Further, prolonged opioid therapy can lead to opioid-induced hyperalgesia, which may also render patients more sensitive to pain and aggravate their preexisting pain.26,28
In addition, although operative drainage procedures and partial resections are often considered the gold standard for chronic pancreatitis management, patients who undergo partial pancreatectomy or lateral pancreaticojejunostomy (Puestow procedure) have fewer islet cells left to harvest (about 50% fewer) if they subsequently undergo total pancreatectomy and islet cell autotransplant.22,26
Therefore, performing this procedure earlier may help the patient avoid chronic pain syndromes and complications of chronic opioid use, including hyperalgesia, and give the best chance of harvesting enough islet cells to prevent or minimize diabetes afterward.11
REMOVING THE PANCREAS, RETURNING THE ISLET CELLS
During this procedure, the blood supply to the pancreas must be preserved until just before its removal to minimize warm ischemia of the islet cells.18,29 Although there are several surgical variations, a pylorus-preserving total pancreatectomy with duodenectomy is typically performed, usually with splenectomy to preserve perfusion to the body and tail.30
The resected pancreas is taken to the islet isolation laboratory. There, the pancreatic duct is cannulated to fill the organ with a cold collagenase solution, followed by gentle mechanical dispersion using the semiautomated Ricordi method,31 which separates the islet cells from the exocrine tissue.32
The number of islet cells is quantified as islet equivalents; 1 islet equivalent is equal to the volume of an islet with a diameter of 150 µm. Islet equivalents per kilogram of body weight is the unit commonly used to report the graft amount transplanted.33
After digestion, the islet cells can be purified or partially purified by a gradient separation method using a Cobe 2991 cell processor (Terumo Corporation, Tokyo, Japan),34 or can be transplanted as an unpurified preparation. In islet cell autotransplant for chronic pancreatitis, purification is not always necessary due to the small tissue volume extracted from the often atrophic and fibrotic pancreas.32 The decision to purify depends on the postdigest tissue volume; usually, a tissue volume greater than 0.25 mL/kg body weight is an indication to at least partially purify.18,35
The final preparation is returned to the operating room, and after heparin is given, the islets are infused into the portal system using a stump of the splenic vein, or alternatively through direct puncture of the portal vein or cannulation of the umbilical vein.32,36 If the portal vein pressure reaches 25 cm H2O, the infusion is stopped and the remaining islets can be placed in the peritoneal cavity or elsewhere.18 Transplant of the islets into the liver or peritoneum allows the islets to secrete insulin into the hepatic portal circulation, which is the route used by the native pancreas.22
CONTROLLING GLUCOSE DURING AND AFTER THE PROCEDURE
Animal studies have shown that hyperglycemia impairs islet revascularization,37 and glucose toxicity may cause dysfunction and structural lesions of the transplanted islets.11,38
Therefore, during and after the procedure, most centers maintain euglycemia by an intravenous insulin infusion and subsequently move to subcutaneous insulin when the patient starts eating again. Some centers continue insulin at discharge and gradually taper it over months, even in patients who can possibly achieve euglycemia without it.
OUTCOMES
Many institutions have reported their clinical outcomes in terms of pain relief, islet function, glycemic control, and improvement of quality of life. The largest series have been from the University of Minnesota, Leicester General Hospital, the University of Cincinnati, and the Medical University of South Carolina.
Insulin independence is common but wanes with time
The ability to achieve insulin independence after islet autotransplant appears to be related to the number of islets transplanted, with the best results when more than 2,000 or 3,000 islet equivalents/kg are transplanted.39,40
Sutherland et al18 reported that of 409 patients who underwent islet cell autotransplant at the University of Minnesota (the largest series reported to date), 30% were insulin-independent at 3 years, 33% had partial graft function (defined by positive C-peptide), and 82% achieved a mean hemoglobin A1c of less than 7%. However, in the subset who received more than 5,000 islet equivalents/kg, nearly three-fourths of patients were insulin-independent at 3 years.
The Leicester General Hospital group presented long-term data on 46 patients who underwent total pancreatectomy and islet cell autotransplant. Twelve of the 46 had shown periods of insulin independence for a median of 16.5 months, and 5 remained insulin-free at the time of the publication.41 Over the 10 years of follow-up, insulin requirements and hemoglobin A1c increased notably. However, all of the patients tested C-peptide-positive, suggesting long-lasting graft function.
Most recently, the University of Cincinnati group reported long-term data on 116 patients. The insulin independence rate was 38% at 1 year, decreasing to 27% at 5 years. The number of patients with partial graft function was 38% at 1 year and 35% at 5 years.42
Thus, all three institutions confirmed that the autotransplanted islets continue to secrete insulin long-term, but that function decreases over time.
Pancreatectomy reduces pain
Multiple studies have shown that total pancreatectomy reduces pain in patients with chronic pancreatitis. Ahmad et al43 reported a marked reduction in narcotic use (mean morphine equivalents 206 mg/day before surgery, compared with 90 mg after), and a 58% reduction in pain as demonstrated by narcotic independence.
In the University of Minnesota series, 85% of the 409 patients had less pain at 2 years, and 59% were able to stop taking narcotics.18
The University of Cincinnati group reported a narcotic independence rate of 55% at 1 year, which continued to improve to 73% at 5 years.42
Although the source of pain is removed, pain persists or recurs in 10% to 20% of patients after total pancreatectomy and islet cell autotransplant, showing that the pathogenesis of pain is complex, and some uncertainty exists about it.26
Quality of life
Reports evaluating health-related quality of life after total pancreatectomy and islet autotransplant are limited.
The University of Cincinnati group reported the long-term outcomes of quality of life as measured by the Short Form 36 Health Survey.42 Ninety-two percent of patients reported overall improvement in their health at 1 year, and 85% continued to report improved health more than 5 years after the surgery.
In a series of 20 patients, 79% to 90% reported improvements in the seven various domains of the Pain Disability Index. In addition, 60% showed improvement in depression and 70% showed improvement in anxiety. The greatest improvements were in those who had not undergone prior pancreatic surgery, who were younger, and in those with higher levels of preoperative pain.30
Similarly, in a series of 74 patients, the Medical University of South Carolina group reported significant improvement in physical and mental health components of the Short Form 12 Health Survey and an associated decrease in daily narcotic requirements. Moreover, the need to start or increase the dose of insulin after the surgery was not associated with a lower quality of life.44
OFF-SITE ISLET CELL ISOLATION
Despite the positive outcomes in terms of pain relief and insulin independence in many patients after total pancreatectomy and islet cell autotransplant, few medical centers have an on-site islet-processing facility. Since the mid-1990s, a few centers have been able to circumvent this limitation by working with off-site islet cell isolation laboratories.45,46
The University of California, Los Angeles, first reported on a series of nine patients who received autologous islet cells after near-total or total pancreatectomy using a remote islet cell isolation facility, with results comparable to those of other large institutions.45
Similarly, the procedure has been performed at Cleveland Clinic since 2007 with the collaboration of an off-site islet cell isolation laboratory at the University of Pittsburgh. A cohort study from this collaboration published in 2015 showed that in 36 patients (mean follow-up 28 months, range 3–26 months), 33% were insulin-independent, with a C-peptide-positive rate of 70%. This is the largest cohort to date from a center utilizing an off-site islet isolation facility.47
In view of the positive outcomes at these centers, lack of a local islet-processing facility should no longer be a barrier to total pancreatectomy and islet cell autotransplant.
PATIENT CARE AFTER THE PROCEDURE
A multidisciplinary team is an essential component of the postoperative management of patients who undergo total pancreatectomy and islet cell autotransplant.
For patients who had been receiving narcotics for a long time before surgery or who were receiving frequent doses, an experienced pain management physician should be involved in the patient’s postoperative care.
Because islet function can wane over time, testing for diabetes should be done at least annually for the rest of the patient’s life and should include fasting plasma glucose, hemoglobin A1c, and C-peptide, along with self-monitored blood glucose.26
All patients who have surgically induced exocrine insufficiency are at risk of malabsorption and fat-soluble vitamin deficiencies.48 Hence, lifelong pancreatic enzyme replacement therapy is mandatory. Nutritional monitoring should include assessment of steatorrhea, body composition, and fat-soluble vitamin levels (vitamins A, D, and E) at least every year.26 Patients with chronic pancreatitis are at increased risk for low bone density from malabsorption of vitamin D and calcium; therefore, it is recommended that a dual-energy x-ray absorptiometry bone density scan be obtained.26,49
Patients who undergo splenectomy as part of their procedure will require appropriate precautions and ongoing vaccinations as recommended by the US Centers for Disease Control and Prevention.26,50,51
WHAT TO EXPECT FOR THE FUTURE
The National Institute of Diabetes and Digestive and Kidney Diseases has reviewed the potential future research directions for total pancreatectomy and islet autotransplant.15
Patient selection remains challenging despite the availability of criteria15 and guidelines.26 More research is needed to better assess preoperative beta-cell function and to predict postoperative outcomes. Mixed meal-tolerance testing is adopted by most clinical centers to predict posttransplant beta-cell function. The use of arginine instead of glucagon in a stimulation test for insulin and C-peptide response has been validated and may allow more accurate assessment.52,53
Another targeted area of research is the advancement of safety and metabolic outcomes. Techniques to minimize warm ischemic time and complications are being evaluated. Islet isolation methods that yield more islets, reduce beta-cell apoptosis, and can isolate islets from glands with malignancy should be further investigated.54 Further, enhanced islet infusion methods that achieve lower portal venous pressures and minimize portal vein thrombosis are needed.
Unfortunately, the function of transplanted islet grafts declines over time. This phenomenon is at least partially attributed to the immediate blood-mediated inflammatory response,55,56 along with islet hypoxia,57 leading to islet apoptosis. Research on different strategies is expanding our knowledge in islet engraftment and posttransplant beta-cell apoptosis, with the expectation that the transplanted islet lifespan will increase. Alternative transplant sites with low inflammatory reaction, such as the omental pouch,58 muscle,59 and bone marrow,60 have shown encouraging data. Other approaches, such as adjuvant anti-inflammatory agents and heparinization, have been proposed.15
Research into complications is also of clinical importance. There is growing attention to hypoglycemia unrelated to exogenous insulin use in posttransplant patients. One hypothesis is that glucagon secretion, a counterregulatory response to hypoglycemia, is defective if the islet cells are transplanted into the liver, and that implanting them into another site may avoid this effect.61
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
- Everhart JE. Pancreatitis. In: Everhart JE, editor. The Burden of Digestive Diseases in the United States. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of
- Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2008. www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burden-digestive-diseases-in-united-states-report.aspx. Accessed May 10, 2016.
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199.
- Lévy P, Barthet M, Mollard BR, Amouretti M, Marion-Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 2006; 30:838–844.
- Hirota M, Shimosegawa T, Masamune A, et al; Research Committee of Intractable Pancreatic Diseases. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496.
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015; 149:1490–1500.e1.
- Coté GA, Yadav D, Slivka A, et al; North American Pancreatitis Study Group. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon 2014; 60:530–550.
- Frulloni L, Gabbrielli A, Pezzilli R, et al; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 2009; 41:311–317.
- Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999; 44:1303–1311.
- Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015; 166:890–896.e1.
- Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87:1477–1501.
- Lieb JG 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther 2009; 29:706–719.
- Lin YK, Johnston PC, Arce K, Hatipoglu BA. Chronic pancreatitis and diabetes mellitus. Curr Treat Options Gastroenterol 2015; 13:319–331.
- Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas 2014; 43:1163–1171.
- Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part II: diagnosis, complications, and management. Dis Mon 2015; 61:5–37.
- Warshaw AL, Banks PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776.
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol 2010; 24:323–335.
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012; 214:409–426.
- Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of beta-cell replacement therapy in diabetes mellitus: islet cell transplantation. J Transplant 2011; 2011:247959.
- Kretschmer GJ, Sutherland DE, Matas AJ, Cain TL, Najarian JS. Autotransplantation of pancreatic islets without separation of exocrine and endocrine tissue in totally pancreatectomized dogs. Surgery 1977; 82:74–81.
- Kretschmer GJ, Sutherland DR, Matas AJ, Payne WD, Najarian JS. Autotransplantation of pancreatic fragments to the portal vein and spleen of totally pancreatectomized dogs: a comparative evaluation. Ann Surg 1978; 187:79–86.
- Bellin MD, Sutherland DE, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hosp Pract (1995) 2012; 40:80–87.
- Najarian JS, Sutherland DE, Baumgartner D, et al. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192:526–542.
- Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58:365–382.
- Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327:220–226.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14:27–35.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587.
- Bramis K, Gordon-Weeks AN, Friend PJ, et al. Systematic review of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Br J Surg 2012; 99:761–766.
- Walsh RM, Saavedra JR, Lentz G, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012; 16:1469–1477.
- Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(suppl 1):140–142.
- Witkowski P, Savari O, Matthews JB. Islet autotransplantation and total pancreatectomy. Adv Surg 2014; 48:223–233.
- Bellin MD, Beilman GJ, Dunn TB, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas 2013; 42:317–321.
- Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011; 91:508–514.
- Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(suppl 1):143–145.
- Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:793–799.
- Andersson A, Korsgren O, Jansson L. Intraportally transplanted pancreatic islets revascularized from hepatic arterial system. Diabetes 1989; 38(suppl 1):192–195.
- Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15:442–455.
- Bellin MD, Carlson AM, Kobayashi T, et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008; 47:37–44.
- White SA, Davies JE, Pollard C, et al. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg 2001; 233:423–431.
- Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008; 37:282–287.
- Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg 2014; 260:659–667.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201:680–687.
- Dorlon M, Owczarski S, Wang H, Adams D, Morgan K. Increase in postoperative insulin requirements does not lead to decreased quality of life after total pancreatectomy with islet cell autotransplantation for chronic pancreatitis. Am Surg 2013; 79:676–680.
- Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg 2015; 150:118–124.
- Rabkin JM, Olyaei AJ, Orloff SL, et al. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg 1999; 177:423–427.
- Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab 2015; 100:1765–1770.
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg 1991; 214:131–140.
- Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012; 41:1119–1124.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–e100.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Robertson RP, Raymond RH, Lee DS, et al; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of beta-cell function. Am J Physiol Endocrinol Metab 2014; 307:E720–E727.
- Robertson RP, Bogachus LD, Oseid E, et al. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015; 64:565–572.
- Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep 2014; 14:512.
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014; 14:428–437.
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004; 53:2815–2823.
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 2011; 60:2350–2353.
- Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9:91–104.
- Sterkers A, Hubert T, Gmyr V, et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant 2013; 13:891–898.
- Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes 2013; 62:3523–3531.
- Bellin MD, Parazzoli S, Oseid E, et al. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 2014; 14:1880–1886.
KEY POINTS
- Chronic pancreatitis is caused by inflammation and results in progressive, irreversible loss of both exocrine and endocrine function.
- Total pancreatectomy with islet cell autotransplant is a definitive treatment for chronic pancreatitis, with most patients reporting less pain and better quality of life.
- Patients who have undergone this procedure need lifelong pancreatic enzyme replacement therapy along with surveillance for and treatment of diabetes.
- Research in this field is expanding our knowledge, from altered physiology to patient selection to improvement in islet yield and survival.
Methylphenidate tied to greater risk of arrhythmia in children, adolescents
Children and adolescents with attention-deficit/hyperactivity disorder who are prescribed methylphenidate to manage their conditions stand at a higher risk for arrhythmia and other, more serious, cardiac conditions.
In a study published in the BMJ, Ju-Young Shin, Ph.D., and colleagues examined records of 1,224 patients aged 17 years and younger from a nationwide South Korean health insurance database submitted between January 2007 and December 2011. All of the patients had experienced a cardiovascular event and at least one recorded prescription for methylphenidate to treat attention-deficit/hyperactivity disorder (ADHD) (BMJ 2016;353:i2550 doi:10.1136/bmj.i2550).
Of the 1,224 subjects, 864 (70.5%) had experienced arrhythmias, and the mean duration of exposure to methylphenidate was 0.5 years. During periods of methylphenidate treatment, subjects had an increased risk of arrhythmia, as Dr. Shin and coinvestigators calculated an adjusted incidence rate ratio of 1.61 (95% confidence interval, 1.48-1.74). This incidence rate ratio jumped up to 2.01 (95% CI, 1.74-2.31) during the first 3 days of methylphenidate treatment.
The risk was even higher for subjects with congenital heart disease; this subgroup had an adjusted incidence rate ratio of 3.49 (95% CI, 2.33-5.22), compared with 1.34 (95% CI, 1.23-1.46) in patients without it. Both the median age of first exposure to methylphenidate and occurrence of the first cardiac event were in patients aged 11-13 years, reported Dr. Shin of the Centre for Clinical Epidemiology at Jewish General Hospital and McGill University, both in Montreal, and colleagues in Australia and Korea.
“These results are consistent with the biological plausibility that the mechanism of action relates to the effect of methylphenidate on the heart rate,” the authors concluded. “Delayed effects would be expected with myocardial infarction, while more immediate effects would be expected with arrhythmias, as we observed.”
Dr. Shin and colleagues cited several limitations. For example, coding mistakes and incomplete records could not be ruled out in their study. In addition, they said, the “outcome measures were limited to patients with diagnoses of cardiovascular adverse events, and we could have missed outcomes not diagnosed.”
Nevertheless, they said, in light of the increased use of methylphenidate to treat ADHD across the globe, the benefits of using the drug “should be carefully weighed against potential cardiovascular risks of these drugs in children and adolescents.”
Two of the authors disclosed receiving support via fellowships from Australia’s National Health and Medical Research Council.
Children and adolescents with attention-deficit/hyperactivity disorder who are prescribed methylphenidate to manage their conditions stand at a higher risk for arrhythmia and other, more serious, cardiac conditions.
In a study published in the BMJ, Ju-Young Shin, Ph.D., and colleagues examined records of 1,224 patients aged 17 years and younger from a nationwide South Korean health insurance database submitted between January 2007 and December 2011. All of the patients had experienced a cardiovascular event and at least one recorded prescription for methylphenidate to treat attention-deficit/hyperactivity disorder (ADHD) (BMJ 2016;353:i2550 doi:10.1136/bmj.i2550).
Of the 1,224 subjects, 864 (70.5%) had experienced arrhythmias, and the mean duration of exposure to methylphenidate was 0.5 years. During periods of methylphenidate treatment, subjects had an increased risk of arrhythmia, as Dr. Shin and coinvestigators calculated an adjusted incidence rate ratio of 1.61 (95% confidence interval, 1.48-1.74). This incidence rate ratio jumped up to 2.01 (95% CI, 1.74-2.31) during the first 3 days of methylphenidate treatment.
The risk was even higher for subjects with congenital heart disease; this subgroup had an adjusted incidence rate ratio of 3.49 (95% CI, 2.33-5.22), compared with 1.34 (95% CI, 1.23-1.46) in patients without it. Both the median age of first exposure to methylphenidate and occurrence of the first cardiac event were in patients aged 11-13 years, reported Dr. Shin of the Centre for Clinical Epidemiology at Jewish General Hospital and McGill University, both in Montreal, and colleagues in Australia and Korea.
“These results are consistent with the biological plausibility that the mechanism of action relates to the effect of methylphenidate on the heart rate,” the authors concluded. “Delayed effects would be expected with myocardial infarction, while more immediate effects would be expected with arrhythmias, as we observed.”
Dr. Shin and colleagues cited several limitations. For example, coding mistakes and incomplete records could not be ruled out in their study. In addition, they said, the “outcome measures were limited to patients with diagnoses of cardiovascular adverse events, and we could have missed outcomes not diagnosed.”
Nevertheless, they said, in light of the increased use of methylphenidate to treat ADHD across the globe, the benefits of using the drug “should be carefully weighed against potential cardiovascular risks of these drugs in children and adolescents.”
Two of the authors disclosed receiving support via fellowships from Australia’s National Health and Medical Research Council.
Children and adolescents with attention-deficit/hyperactivity disorder who are prescribed methylphenidate to manage their conditions stand at a higher risk for arrhythmia and other, more serious, cardiac conditions.
In a study published in the BMJ, Ju-Young Shin, Ph.D., and colleagues examined records of 1,224 patients aged 17 years and younger from a nationwide South Korean health insurance database submitted between January 2007 and December 2011. All of the patients had experienced a cardiovascular event and at least one recorded prescription for methylphenidate to treat attention-deficit/hyperactivity disorder (ADHD) (BMJ 2016;353:i2550 doi:10.1136/bmj.i2550).
Of the 1,224 subjects, 864 (70.5%) had experienced arrhythmias, and the mean duration of exposure to methylphenidate was 0.5 years. During periods of methylphenidate treatment, subjects had an increased risk of arrhythmia, as Dr. Shin and coinvestigators calculated an adjusted incidence rate ratio of 1.61 (95% confidence interval, 1.48-1.74). This incidence rate ratio jumped up to 2.01 (95% CI, 1.74-2.31) during the first 3 days of methylphenidate treatment.
The risk was even higher for subjects with congenital heart disease; this subgroup had an adjusted incidence rate ratio of 3.49 (95% CI, 2.33-5.22), compared with 1.34 (95% CI, 1.23-1.46) in patients without it. Both the median age of first exposure to methylphenidate and occurrence of the first cardiac event were in patients aged 11-13 years, reported Dr. Shin of the Centre for Clinical Epidemiology at Jewish General Hospital and McGill University, both in Montreal, and colleagues in Australia and Korea.
“These results are consistent with the biological plausibility that the mechanism of action relates to the effect of methylphenidate on the heart rate,” the authors concluded. “Delayed effects would be expected with myocardial infarction, while more immediate effects would be expected with arrhythmias, as we observed.”
Dr. Shin and colleagues cited several limitations. For example, coding mistakes and incomplete records could not be ruled out in their study. In addition, they said, the “outcome measures were limited to patients with diagnoses of cardiovascular adverse events, and we could have missed outcomes not diagnosed.”
Nevertheless, they said, in light of the increased use of methylphenidate to treat ADHD across the globe, the benefits of using the drug “should be carefully weighed against potential cardiovascular risks of these drugs in children and adolescents.”
Two of the authors disclosed receiving support via fellowships from Australia’s National Health and Medical Research Council.
FROM THE BMJ
Key clinical point: Use of methylphenidate to treat children with attention-deficit/hyperactivity disorder (ADHD) can lead to higher risk for arrhythmia, especially in the first 3 days of use.
Major finding: The risk of arrhythmia increased during periods of treatment with methylphenidate when compared with other treatment periods (incidence rate ratio, 1.61), and reached 2.01 during the first 1-3 days of methylphenidate treatment.
Data source: A retrospective case series analysis of 1,224 patients at or younger than 17 years of age, who had at least one recorded cardiovascular event and one prescription for methylphenidate.
Disclosures: Two coauthors disclosed receiving support via fellowships from Australia’s National Health and Medical Research Council.
A Pragmatic Approach to Melanoma Screening in Collaboration With Primary Care Providers
In 2009, the US Preventive Services Task Force issued an I statement for routine skin cancer screening, noting a lack of evidence to support the balance of benefits and harms from screening,1 a recommendation that is likely to be upheld this year. As dermatologists and melanoma specialists, we have abundant anecdotal evidence of the value of screening; however, population-based screening performed exclusively by dermatologists is not practical. There are approximately 170,000,000 adults 35 years and older and only 9600 practicing dermatologists in the United States, requiring each dermatologist to screen nearly 18,000 individuals per year to meet the needs of the population.
Only 8% to 15% of people in the United States report having received a recent skin examination by a physician.2,3 Partnering with our primary care provider (PCP) colleagues has the potential to reach more patients and to improve skin cancer screening rates more rapidly. The workforce in primary care is substantially larger than dermatology by approximately 30-fold, and PCPs are more likely than dermatologists to practice in rural areas, thus reaching patients with limited access to dermatologists. Skin cancer screening can be included in the routine PCP visit, reducing the need for an additional physician visit for the patient. Patients visit their PCP more frequently as they age, which parallels the risk for developing and dying from melanoma and also provides an opportunity to introduce skin cancer education and screening to a population at higher risk who may not otherwise seek it on their own.4 Providing PCPs with the training and tools to perform melanoma screening shifts the responsibility of initiating screening from the patient alone to a shared responsibility of patient and provider. Dermatologists, in turn, need to be available to examine those patients found to have a suspicious lesion, treat newly diagnosed skin cancer, and follow those patients at highest risk of developing skin cancer, including those who are immunosuppressed, have multiple atypical moles, or have a personal or family history of melanoma.
Evidence from the SCREEN (Skin Cancer Research to provide Evidence for Effectiveness of Screening in Northern Germany) project supports PCP-based screening. In the 5 years following a 1-year pilot screening program, there was nearly a 50% reduction in melanoma mortality.5 Unfortunately, these encouraging results were not confirmed once the pilot project was translated into a national skin cancer screening program.6 However, there are lessons to be learned from the German project and we propose that PCP-led screening is feasible and practical in the United States and we currently have a pilot program in our institution, the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania).
In the SCREEN project and in routine practice across the United States, screening is primarily driven by patients. Generally, higher-risk patients such as men and the elderly are the least likely group to seek skin cancer screening. In our program, PCPs are offered training in skin cancer screening using a validated web-based program and alerted through the electronic health record to offer skin cancer screening annually to patients 35 years and older who present for routine primary care visits.7 This approach reduces self-referral bias by promoting physician initiation rather than patient initiation of screening, which can occur while the patient is already in the PCP’s office.
Melanoma thickness can be measured among screened patients, unscreened patients, and historic controls and compared to determine if this approach is effective. Health care utilization data can help to inform us if this approach leads to more skin biopsies and procedures or to an increased rate of dermatology referrals. As health care payment and delivery models evolve, there is greater emphasis on outcomes and team-based care. We believe that this approach will allow us to form effective teams of PCPs, dermatologists, and other experts in melanoma, public health, and informatics to reduce melanoma mortality in a cost-effective manner.
- U.S. Preventive Services Task Force. Screening for skin cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:188-193.
- Saraiya M, Hall HI, Thompson T, et al. Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39:308-314.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. CDC website. http://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated April 27, 2016. Accessed May 4, 2016.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Weinstock M. INFORMED: melanoma and skin cancer early detection. Skinsight website. http://www.skinsight.com/info/for_professionals/skin-cancer-detection-informed/skin-cancer-education. Accessed May 12, 2016.
In 2009, the US Preventive Services Task Force issued an I statement for routine skin cancer screening, noting a lack of evidence to support the balance of benefits and harms from screening,1 a recommendation that is likely to be upheld this year. As dermatologists and melanoma specialists, we have abundant anecdotal evidence of the value of screening; however, population-based screening performed exclusively by dermatologists is not practical. There are approximately 170,000,000 adults 35 years and older and only 9600 practicing dermatologists in the United States, requiring each dermatologist to screen nearly 18,000 individuals per year to meet the needs of the population.
Only 8% to 15% of people in the United States report having received a recent skin examination by a physician.2,3 Partnering with our primary care provider (PCP) colleagues has the potential to reach more patients and to improve skin cancer screening rates more rapidly. The workforce in primary care is substantially larger than dermatology by approximately 30-fold, and PCPs are more likely than dermatologists to practice in rural areas, thus reaching patients with limited access to dermatologists. Skin cancer screening can be included in the routine PCP visit, reducing the need for an additional physician visit for the patient. Patients visit their PCP more frequently as they age, which parallels the risk for developing and dying from melanoma and also provides an opportunity to introduce skin cancer education and screening to a population at higher risk who may not otherwise seek it on their own.4 Providing PCPs with the training and tools to perform melanoma screening shifts the responsibility of initiating screening from the patient alone to a shared responsibility of patient and provider. Dermatologists, in turn, need to be available to examine those patients found to have a suspicious lesion, treat newly diagnosed skin cancer, and follow those patients at highest risk of developing skin cancer, including those who are immunosuppressed, have multiple atypical moles, or have a personal or family history of melanoma.
Evidence from the SCREEN (Skin Cancer Research to provide Evidence for Effectiveness of Screening in Northern Germany) project supports PCP-based screening. In the 5 years following a 1-year pilot screening program, there was nearly a 50% reduction in melanoma mortality.5 Unfortunately, these encouraging results were not confirmed once the pilot project was translated into a national skin cancer screening program.6 However, there are lessons to be learned from the German project and we propose that PCP-led screening is feasible and practical in the United States and we currently have a pilot program in our institution, the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania).
In the SCREEN project and in routine practice across the United States, screening is primarily driven by patients. Generally, higher-risk patients such as men and the elderly are the least likely group to seek skin cancer screening. In our program, PCPs are offered training in skin cancer screening using a validated web-based program and alerted through the electronic health record to offer skin cancer screening annually to patients 35 years and older who present for routine primary care visits.7 This approach reduces self-referral bias by promoting physician initiation rather than patient initiation of screening, which can occur while the patient is already in the PCP’s office.
Melanoma thickness can be measured among screened patients, unscreened patients, and historic controls and compared to determine if this approach is effective. Health care utilization data can help to inform us if this approach leads to more skin biopsies and procedures or to an increased rate of dermatology referrals. As health care payment and delivery models evolve, there is greater emphasis on outcomes and team-based care. We believe that this approach will allow us to form effective teams of PCPs, dermatologists, and other experts in melanoma, public health, and informatics to reduce melanoma mortality in a cost-effective manner.
In 2009, the US Preventive Services Task Force issued an I statement for routine skin cancer screening, noting a lack of evidence to support the balance of benefits and harms from screening,1 a recommendation that is likely to be upheld this year. As dermatologists and melanoma specialists, we have abundant anecdotal evidence of the value of screening; however, population-based screening performed exclusively by dermatologists is not practical. There are approximately 170,000,000 adults 35 years and older and only 9600 practicing dermatologists in the United States, requiring each dermatologist to screen nearly 18,000 individuals per year to meet the needs of the population.
Only 8% to 15% of people in the United States report having received a recent skin examination by a physician.2,3 Partnering with our primary care provider (PCP) colleagues has the potential to reach more patients and to improve skin cancer screening rates more rapidly. The workforce in primary care is substantially larger than dermatology by approximately 30-fold, and PCPs are more likely than dermatologists to practice in rural areas, thus reaching patients with limited access to dermatologists. Skin cancer screening can be included in the routine PCP visit, reducing the need for an additional physician visit for the patient. Patients visit their PCP more frequently as they age, which parallels the risk for developing and dying from melanoma and also provides an opportunity to introduce skin cancer education and screening to a population at higher risk who may not otherwise seek it on their own.4 Providing PCPs with the training and tools to perform melanoma screening shifts the responsibility of initiating screening from the patient alone to a shared responsibility of patient and provider. Dermatologists, in turn, need to be available to examine those patients found to have a suspicious lesion, treat newly diagnosed skin cancer, and follow those patients at highest risk of developing skin cancer, including those who are immunosuppressed, have multiple atypical moles, or have a personal or family history of melanoma.
Evidence from the SCREEN (Skin Cancer Research to provide Evidence for Effectiveness of Screening in Northern Germany) project supports PCP-based screening. In the 5 years following a 1-year pilot screening program, there was nearly a 50% reduction in melanoma mortality.5 Unfortunately, these encouraging results were not confirmed once the pilot project was translated into a national skin cancer screening program.6 However, there are lessons to be learned from the German project and we propose that PCP-led screening is feasible and practical in the United States and we currently have a pilot program in our institution, the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania).
In the SCREEN project and in routine practice across the United States, screening is primarily driven by patients. Generally, higher-risk patients such as men and the elderly are the least likely group to seek skin cancer screening. In our program, PCPs are offered training in skin cancer screening using a validated web-based program and alerted through the electronic health record to offer skin cancer screening annually to patients 35 years and older who present for routine primary care visits.7 This approach reduces self-referral bias by promoting physician initiation rather than patient initiation of screening, which can occur while the patient is already in the PCP’s office.
Melanoma thickness can be measured among screened patients, unscreened patients, and historic controls and compared to determine if this approach is effective. Health care utilization data can help to inform us if this approach leads to more skin biopsies and procedures or to an increased rate of dermatology referrals. As health care payment and delivery models evolve, there is greater emphasis on outcomes and team-based care. We believe that this approach will allow us to form effective teams of PCPs, dermatologists, and other experts in melanoma, public health, and informatics to reduce melanoma mortality in a cost-effective manner.
- U.S. Preventive Services Task Force. Screening for skin cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:188-193.
- Saraiya M, Hall HI, Thompson T, et al. Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39:308-314.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. CDC website. http://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated April 27, 2016. Accessed May 4, 2016.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Weinstock M. INFORMED: melanoma and skin cancer early detection. Skinsight website. http://www.skinsight.com/info/for_professionals/skin-cancer-detection-informed/skin-cancer-education. Accessed May 12, 2016.
- U.S. Preventive Services Task Force. Screening for skin cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:188-193.
- Saraiya M, Hall HI, Thompson T, et al. Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39:308-314.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- Centers for Disease Control and Prevention. Ambulatory care use and physician office visits. CDC website. http://www.cdc.gov/nchs/fastats/physician-visits.htm. Updated April 27, 2016. Accessed May 4, 2016.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Weinstock M. INFORMED: melanoma and skin cancer early detection. Skinsight website. http://www.skinsight.com/info/for_professionals/skin-cancer-detection-informed/skin-cancer-education. Accessed May 12, 2016.
Actinic Keratosis as a Marker of Field Cancerization in Excision Specimens of Cutaneous Malignancies
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).
Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.
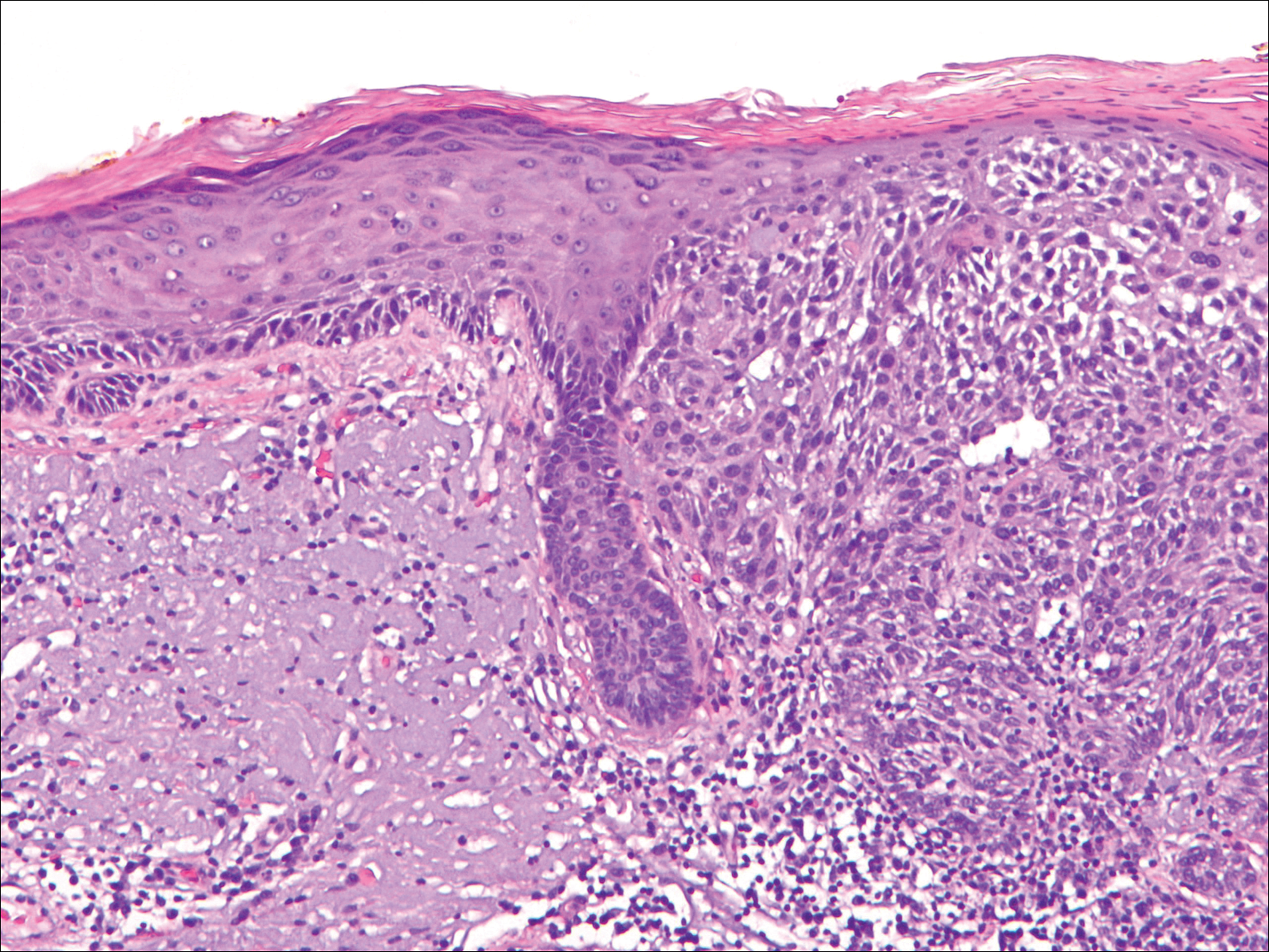
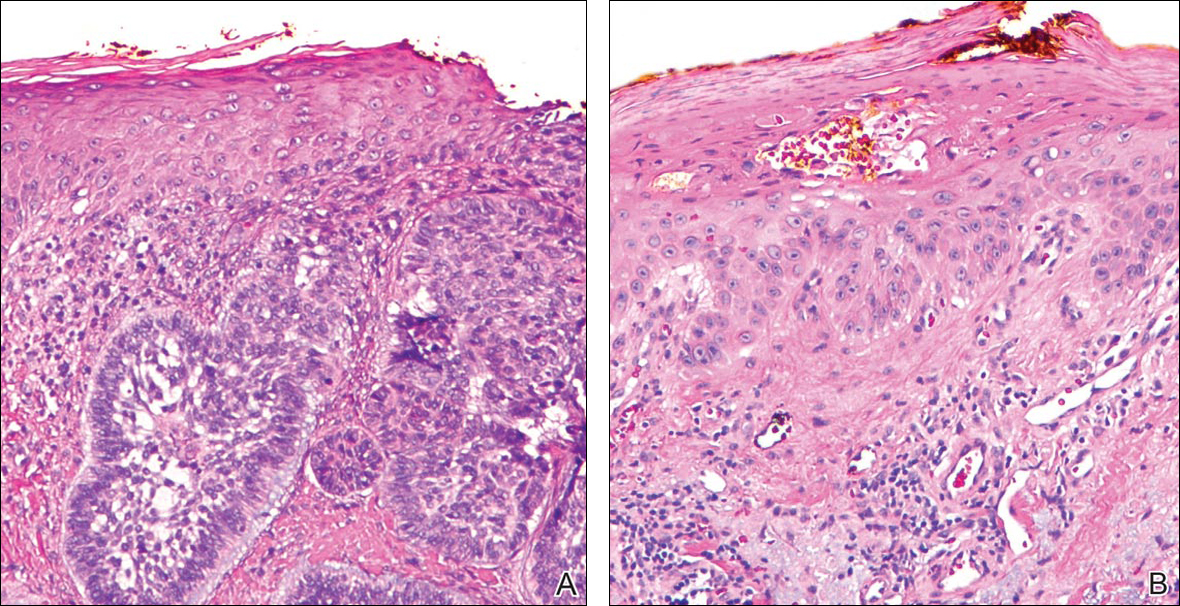
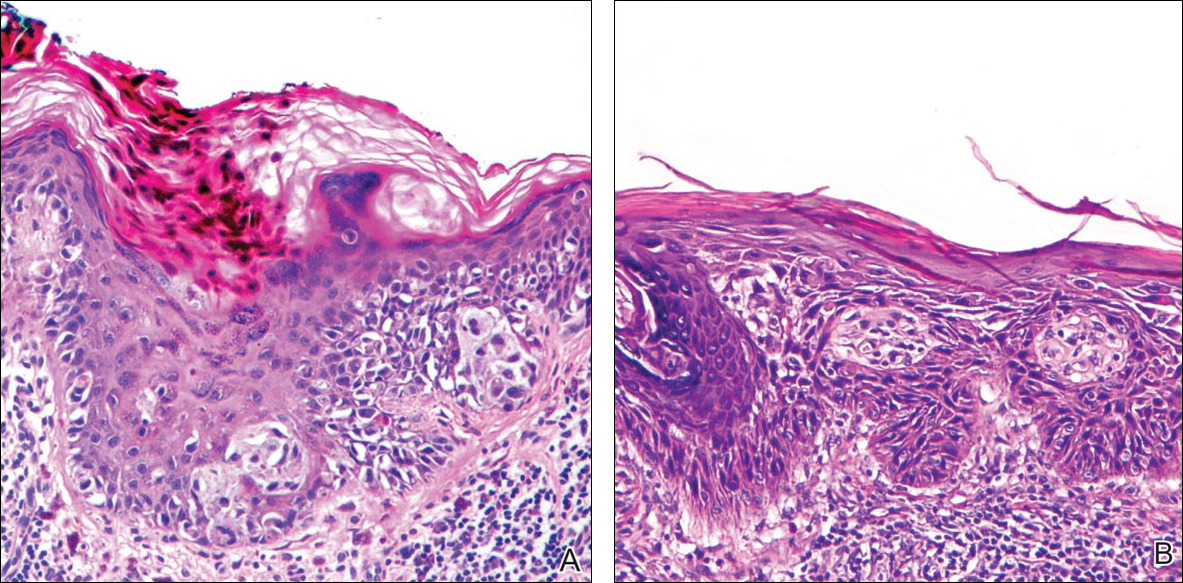
Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).
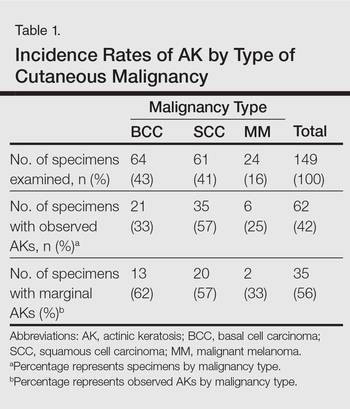
Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.
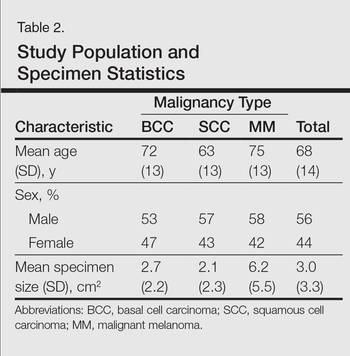
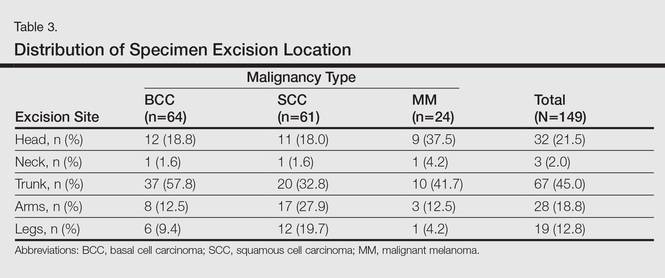
A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
Practice Points
- Clinically apparent and subclinical actinic keratoses usually are present in patients, a concept known as field cancerization, and it is important to treat both types of lesions.
- Actinic keratoses are present in the field of cutaneous malignancies, including basal cell carcinoma, squamous cell carcinoma, and melanoma.
Groundhog Day
Early in my career, an attorney asked me to review a malpractice case. “The question,” he said, “is whether liquid nitrogen is the standard of care for treating warts.”
Come again?
A young woman had visited an academic center, where a physician froze her warts. These blistered and turned purple. Alarmed, she went to an ER, where doctors admitted her for cellulitis and gave her intravenous antibiotics for several days.
I later submitted an essay based on this case to my malpractice insurer’s Risk Management Contest, in which I called for better communication between treating doctors and those who follow up. I placed second and got a check from my malpractice insurer. How cool is that?
I hadn’t thought about that case in years, until last month. Earvin is a late-middle-aged gent with psoriasis on his elbows and liver spots on his chest. He asked me to freeze them, as I had done before.
After lunch 2 days later, my secretary buzzed me. “The man on the phone is very unhappy,” she said. “He says he called this morning and no one called back. He says if he doesn’t hear from you, his next call will be to his attorney.”
I phoned Earvin. My apology for not having gotten the message before was met with frigid hostility. “That’s unconscionable,” he said.
“My skin swelled up like a balloon,” he told me. “I know what to expect with freezing, and this never happened before. I think this time you froze me with a heavy hand. When I didn’t hear from you, I went to an urgent care clinic down the street. They said it was infected and prescribed an antibiotic ointment. I had to wait an hour, and the ointment cost $49.”
I held my breath. “Actually,” I said, “I don’t think it’s infected.”
“They said it was infected,” said Earvin. “It is swollen. I’m afraid it is going to scar. The cream cost $49.”
Straining to keep my voice even, I replied, “Would you like me to have a look at it?”
“I live too far,” he said. “I don’t have a ride. Maybe I will come tomorrow if someone can take me.”
Later that day I called him back. “I live the next town over from you,” I said. “I will stop by on my way home.” He agreed, and told me the color of his house.
Earvin let me in looking less angry than fearful. One glance at his chest told me what I needed to know: There was no infection. His skin looked like what skin looks like after it’s been frozen. Other treated areas – the ones Earvin wasn’t worried about – looked the same to me, though clearly not to him. “This one was worse,” he said. “It’s gone down since this morning.”
After examining Earvin with my cell phone flashlight, I sat down across from him. “Let me be clear,” I said. “You are not infected. Some areas frozen at the same time in the same way blister more briskly than others. In the end they heal fine, and so will yours. You’re not going to scar.”
Earvin relaxed a little. “I was afraid I’d done something I didn’t need to. My mother had skin cancer on her chest, not far from where this one is. My skin was discolored and swollen. The doctor at urgent care said it was infected.” There was a pause, as Earvin slumped in his chair. “I feel better,” he allowed.
There it was, the whole package – thinking that was full of hopelessly muddled categories. Infection? Scarring? Skin cancer? Yes to all? And there was moral anxiety – disappointment in himself for doing something needless and now having to pay the price – as well as anger at me as the instrument of retribution. Who knows what else? There were so many strands, hopelessly coiled, impossible to disentangle.
And all wrapped up in elemental terror. What have I done? What has he done?
Earvin did not need treatment. He needed a hug. Metaphorically, that is what I gave him.
So much angst, so much anger, so much fear, so little time. To be frank, it gets tiring.
People like to quote the philosopher George Santayana. He said that those who cannot remember the past are condemned to repeat it.
I like to paraphrase the even greater philosopher Bill Murray of “Groundhog Day”: Those who do remember the past are condemned to repeat it anyway.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Early in my career, an attorney asked me to review a malpractice case. “The question,” he said, “is whether liquid nitrogen is the standard of care for treating warts.”
Come again?
A young woman had visited an academic center, where a physician froze her warts. These blistered and turned purple. Alarmed, she went to an ER, where doctors admitted her for cellulitis and gave her intravenous antibiotics for several days.
I later submitted an essay based on this case to my malpractice insurer’s Risk Management Contest, in which I called for better communication between treating doctors and those who follow up. I placed second and got a check from my malpractice insurer. How cool is that?
I hadn’t thought about that case in years, until last month. Earvin is a late-middle-aged gent with psoriasis on his elbows and liver spots on his chest. He asked me to freeze them, as I had done before.
After lunch 2 days later, my secretary buzzed me. “The man on the phone is very unhappy,” she said. “He says he called this morning and no one called back. He says if he doesn’t hear from you, his next call will be to his attorney.”
I phoned Earvin. My apology for not having gotten the message before was met with frigid hostility. “That’s unconscionable,” he said.
“My skin swelled up like a balloon,” he told me. “I know what to expect with freezing, and this never happened before. I think this time you froze me with a heavy hand. When I didn’t hear from you, I went to an urgent care clinic down the street. They said it was infected and prescribed an antibiotic ointment. I had to wait an hour, and the ointment cost $49.”
I held my breath. “Actually,” I said, “I don’t think it’s infected.”
“They said it was infected,” said Earvin. “It is swollen. I’m afraid it is going to scar. The cream cost $49.”
Straining to keep my voice even, I replied, “Would you like me to have a look at it?”
“I live too far,” he said. “I don’t have a ride. Maybe I will come tomorrow if someone can take me.”
Later that day I called him back. “I live the next town over from you,” I said. “I will stop by on my way home.” He agreed, and told me the color of his house.
Earvin let me in looking less angry than fearful. One glance at his chest told me what I needed to know: There was no infection. His skin looked like what skin looks like after it’s been frozen. Other treated areas – the ones Earvin wasn’t worried about – looked the same to me, though clearly not to him. “This one was worse,” he said. “It’s gone down since this morning.”
After examining Earvin with my cell phone flashlight, I sat down across from him. “Let me be clear,” I said. “You are not infected. Some areas frozen at the same time in the same way blister more briskly than others. In the end they heal fine, and so will yours. You’re not going to scar.”
Earvin relaxed a little. “I was afraid I’d done something I didn’t need to. My mother had skin cancer on her chest, not far from where this one is. My skin was discolored and swollen. The doctor at urgent care said it was infected.” There was a pause, as Earvin slumped in his chair. “I feel better,” he allowed.
There it was, the whole package – thinking that was full of hopelessly muddled categories. Infection? Scarring? Skin cancer? Yes to all? And there was moral anxiety – disappointment in himself for doing something needless and now having to pay the price – as well as anger at me as the instrument of retribution. Who knows what else? There were so many strands, hopelessly coiled, impossible to disentangle.
And all wrapped up in elemental terror. What have I done? What has he done?
Earvin did not need treatment. He needed a hug. Metaphorically, that is what I gave him.
So much angst, so much anger, so much fear, so little time. To be frank, it gets tiring.
People like to quote the philosopher George Santayana. He said that those who cannot remember the past are condemned to repeat it.
I like to paraphrase the even greater philosopher Bill Murray of “Groundhog Day”: Those who do remember the past are condemned to repeat it anyway.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Early in my career, an attorney asked me to review a malpractice case. “The question,” he said, “is whether liquid nitrogen is the standard of care for treating warts.”
Come again?
A young woman had visited an academic center, where a physician froze her warts. These blistered and turned purple. Alarmed, she went to an ER, where doctors admitted her for cellulitis and gave her intravenous antibiotics for several days.
I later submitted an essay based on this case to my malpractice insurer’s Risk Management Contest, in which I called for better communication between treating doctors and those who follow up. I placed second and got a check from my malpractice insurer. How cool is that?
I hadn’t thought about that case in years, until last month. Earvin is a late-middle-aged gent with psoriasis on his elbows and liver spots on his chest. He asked me to freeze them, as I had done before.
After lunch 2 days later, my secretary buzzed me. “The man on the phone is very unhappy,” she said. “He says he called this morning and no one called back. He says if he doesn’t hear from you, his next call will be to his attorney.”
I phoned Earvin. My apology for not having gotten the message before was met with frigid hostility. “That’s unconscionable,” he said.
“My skin swelled up like a balloon,” he told me. “I know what to expect with freezing, and this never happened before. I think this time you froze me with a heavy hand. When I didn’t hear from you, I went to an urgent care clinic down the street. They said it was infected and prescribed an antibiotic ointment. I had to wait an hour, and the ointment cost $49.”
I held my breath. “Actually,” I said, “I don’t think it’s infected.”
“They said it was infected,” said Earvin. “It is swollen. I’m afraid it is going to scar. The cream cost $49.”
Straining to keep my voice even, I replied, “Would you like me to have a look at it?”
“I live too far,” he said. “I don’t have a ride. Maybe I will come tomorrow if someone can take me.”
Later that day I called him back. “I live the next town over from you,” I said. “I will stop by on my way home.” He agreed, and told me the color of his house.
Earvin let me in looking less angry than fearful. One glance at his chest told me what I needed to know: There was no infection. His skin looked like what skin looks like after it’s been frozen. Other treated areas – the ones Earvin wasn’t worried about – looked the same to me, though clearly not to him. “This one was worse,” he said. “It’s gone down since this morning.”
After examining Earvin with my cell phone flashlight, I sat down across from him. “Let me be clear,” I said. “You are not infected. Some areas frozen at the same time in the same way blister more briskly than others. In the end they heal fine, and so will yours. You’re not going to scar.”
Earvin relaxed a little. “I was afraid I’d done something I didn’t need to. My mother had skin cancer on her chest, not far from where this one is. My skin was discolored and swollen. The doctor at urgent care said it was infected.” There was a pause, as Earvin slumped in his chair. “I feel better,” he allowed.
There it was, the whole package – thinking that was full of hopelessly muddled categories. Infection? Scarring? Skin cancer? Yes to all? And there was moral anxiety – disappointment in himself for doing something needless and now having to pay the price – as well as anger at me as the instrument of retribution. Who knows what else? There were so many strands, hopelessly coiled, impossible to disentangle.
And all wrapped up in elemental terror. What have I done? What has he done?
Earvin did not need treatment. He needed a hug. Metaphorically, that is what I gave him.
So much angst, so much anger, so much fear, so little time. To be frank, it gets tiring.
People like to quote the philosopher George Santayana. He said that those who cannot remember the past are condemned to repeat it.
I like to paraphrase the even greater philosopher Bill Murray of “Groundhog Day”: Those who do remember the past are condemned to repeat it anyway.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Silicone Arthroplasty After Ankylosis of Proximal Interphalangeal Joints in Rheumatoid Arthritis: A Case Report
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.