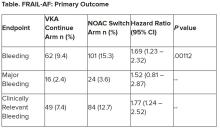
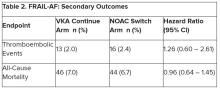
User login
Transient Skin Rippling in an Infant
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
The Diagnosis: Infantile Transient Smooth Muscle Contraction of the Skin
A diagnosis of infantile transient smooth muscle contraction of the skin (ITSMC) was made based on our patient’s clinical presentation and eliminating the diagnoses in the differential. No treatment ultimately was indicated, as episodes became less frequent over time.
The term infantile transient smooth muscle contraction of the skin was first proposed in 2013 by Torrelo et al,1 who described 9 newborns with episodic skin rippling occasionally associated with exposure to cold or friction. The authors postulated that ITSMC was the result of a transient contraction of the arrector pili smooth muscle fibers of the skin, secondary to autonomic immaturity, primitive reflexes, or smooth muscle hypersensitivity.1 Since this first description, ITSMC has remained a rarely reported and poorly understood phenomenon with rare identified cases in the literature.2,3 Clinical history and examination of infants with intermittent transient skin rippling help to distinguish ITSMC from other diagnoses without the need for biopsy, which is particularly undesirable in the pediatric population.
Congenital smooth muscle hamartoma is a benign proliferation of mature smooth muscle that also can arise from the arrector pili muscles.4 In contrast to ITSMC, a hamartoma does not clear; rather, it persists and grows proportionally with the child and is associated with overlying hyperpigmentation and hypertrichosis. The transient nature of ITSMC may be worrisome for mastocytoma; however, this condition presents as erythematous, yellow, red, or brown macules, papules, plaques, or nodules with a positive Darier sign.5 Although the differential diagnosis includes the shagreen patch characteristic of tuberous sclerosis, this irregular plaque typically is located on the lower back with overlying peau d’orange skin changes, and our patient lacked other features indicative of this condition.6 Becker nevus also remains a consideration in patients with rippled skin, but this entity typically becomes more notable at puberty and is associated with hyperpigmentation and hypertrichosis and is a type of smooth muscle hamartoma.4
Our case highlighted the unusual presentation of ITSMC, a condition that can easily go unrecognized, leading to unnecessary referrals and concern. Familiarity with this benign diagnosis is essential to inform prognosis and guide management.
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
- Torrelo A, Moreno S, Castro C, et al. Infantile transient smooth muscle contraction of the skin. J Am Acad Dermatol. 2013;69:498-500. doi:10.1016/j.jaad.2013.04.029
- Theodosiou G, Belfrage E, Berggård K, et al. Infantile transient smooth muscle contraction of the skin: a case report and literature review. Eur J Dermatol. 2021;31:260-261. doi:10.1684/ejd.2021.3996
- Topham C, Deacon DC, Bowen A, et al. More than goosebumps: a case of marked skin dimpling in an infant. Pediatr Dermatol. 2019;36:E71-E72. doi:10.1111/pde.13791
- Raboudi A, Litaiem N. Congenital smooth muscle hamartoma. StatPearls. StatPearls Publishing; 2022.
- Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46. doi:10.2174/1573396315666 181120163952
- Bongiorno MA, Nathan N, Oyerinde O, et al. Clinical characteristics of connective tissue nevi in tuberous sclerosis complex with special emphasis on shagreen patches. JAMA Dermatol. 2017;153:660-665. doi:10.1001/jamadermatol.2017.0298
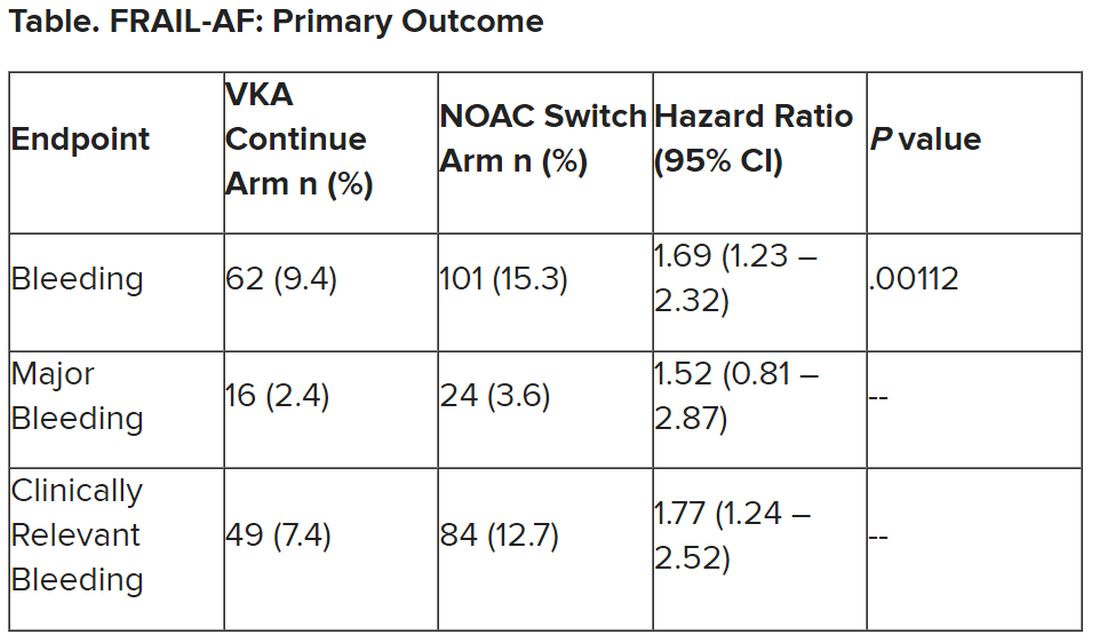
A healthy, full-term, 5-month-old infant boy presented to dermatology for evaluation of an intermittent, asymptomatic, rippled skin texture of the left thigh that resolved completely between flares. The parents noted fewer than 10 intermittent flares prior to the initial presentation at 5 months. Physical examination of the patient’s skin revealed no epidermal abnormalities, dermatographism, or subcutaneous nodules, and there was no positive Darier sign. A subsequent flare at 9 months of age occurred concurrently with fevers up to 39.4 °C (103 °F), and a corresponding photograph (quiz image) provided by the parents due to the intermittent and transient nature of the condition demonstrated an ill-defined, raised, rippled plaque on the left lateral thigh.
ADCs show early promise in NSCLC
This has led to a burgeoning interest in solid tumors, with over 100 clinical trials in progress. Non–small cell lung cancer (NSCLC) is no exception. In 2022, the Food and Drug Administration approved trastuzumab DXd for pretreated NSCLC patients with HER2-mutant tumors. Two others have lung cancer breakthrough therapy designations from the FDA, including patritumab deruxtecan (HER3-DXd) for EGFR-mutant NSCLC and telisotuzumab vedotin for NSCLC that overexpresses c-Met.
At the annual World Conference on Lung Cancer, researchers outlined some of the latest developments in ADCs targeting the antigens HER3, trophoblast cell-surface antigen 2 (TROP-2), and the B7-H3 immunoregulatory protein, as single agents or in combination with immunotherapy. Following the presentations, discussant Helena Linardou, MD, PhD, emphasized the need for pharmacogenomics to predict toxicity and studies to understand resistance mechanisms. “ADCs are a new, rapidly evolving class of therapeutics, and I think that we will all have to be prepared for the future that is coming,” said Dr. Linardou, who is director of the 4th oncology department and the Comprehensive Clinical Trials Center at Metropolitan Hospital in Athens.
Researchers presented four ADC clinical trial updates.
Patritumab deruxtecan
Patritumab deruxtecan (Daiichi Sankyo) links a HER3 antibody to the topoisomerase I inhibitor deruxtecan (HER3-DXd). In the open-label, phase 2 HERTHENA-Lung01 trial, it was tested in patients with NSCLC EGFR-activation mutations, which occurs in 14%-38% of NSCLC cases. There are few options for these patients following failure of EGFR tyrosine kinase inhibitor therapy.
The study included 225 patients previously treated with EGFR tyrosine kinase inhibitors and platinum-based chemotherapy who received 5.6 mg/kg of HER3-DXd every 3 weeks. Over a median follow-up of 13.1 months and a median treatment duration of 5.5 months), 29.8% had a confirmed overall response (95% confidence interval, 23.9%-36.2%) with a median duration of 6.4 months (95% CI, 4.9-7.8). The median progression-free survival was 5.5 months (95% CI, 5.1-5.9), and the median OS was 11.9 months (95% CI, 11.2-13.1). The researchers noted similar outcomes among patients with different mechanisms of EGFR TKI resistance. The frequency of adverse events was similar to previous studies, with drug-related adverse events linked to treatment discontinuation of 7.1% interstitial lung disease in 5.3%. Among 30 patients with brain metastases, the confirmed intracranial response rate was 33.3% (95% CI, 17.3%-52.8%).
The study was published simultaneously online in the Journal of Clinical Oncology. (Abstract)
Datopotamab deruxtecan
PD-1/PD-L1 inhibitors are the first-line therapy for metastatic NSCLC, but there are efforts to improve outcomes through combination therapy. Datopotamab deruxtecan (Dato-DXd, Daiichi Sankyo, AstraZeneca) is an ADC targeting TROP2, and it has been shown in preclinical studies to enhance tumor responses to PD-1/PD-L1 inhibitors. The ADC improved progression-free survival (PFS), compared with docetaxel, in previously treated advanced or metastatic NSCLC.
In an interim analysis of the phase 1b TROPION-Lung04 study, researchers reported results from the combination of Dato-DXd with durvalumab with or without carboplatin. The study included 38 patients, some of whom had previously undergone treatment with immune checkpoint inhibitors. Nineteen patients received the doublet, and 14 received the carboplatin triplet. Grade 3 or higher treatment-emergent adverse events occurred in 42.1% of the doublet group and 71.4% of the triplet group. Interstitial lung disease occurred in 15.8% and 7.1% of the two groups, respectively. The objective response rate was 50.0% in the doublet group and 76.9% in the triplet group. The disease control rate was 92.9% and 92.3%, respectively. Durable responses occurred in both the first-line setting and the overall population. (Abstract)
Sacituzumab govitecan
Another ADC being tested with PD-1/PD-L1 inhibitors is sacituzumab govitecan (Trodelvy, Gilead), which has already received FDA approval for metastatic triple-negative breast cancer, pretreated HR+/HER2- metastatic breast cancer, and metastatic urothelial cancer. Like datopotamab, sacituzumab targets TROP-2. Researchers reported preliminary results from the phase 2 EVOKE-02 study, in which the ADC was combined with pembrolizumab for the first-line treatment of metastatic NSCLC. The overall response rate was 56% (95% CI, 42%-69%). Among patients with PD-L1 tumor proportion score (TPS) ≥ 50%, the ORR was 69% (95% CI, 49%-85%) and 44% (95% CI, 26%-62%) among those with TPS < 50%. The disease control rate was 86% (95% CI, 68%-96%) and 78% (95% CI, 60%-91%), respectively. The most frequent treatment-emergent adverse events (TEAEs) were diarrhea, anemia, and asthenia, and 18% of patients discontinued the study drug because of TEAEs. (Abstract)
Ifinatamab deruxtecan
Ifinatamab deruxtecan (Daiichi Sankyo) targets the B7-H3 antigen, which is an immunoregulatory protein that is overexpressed in many tumors. In the DS7300-A-J101 study, it was tested in patients with advanced or metastatic solid tumors, without selection for B7-H3 expression. A subgroup analysis of 22 patients with small cell lung cancer (SCLC) showed an ORR of 52.4 (95% CI, 29.8-74.3), a complete response of 4.8%, and a partial response in 47.6%. The median PFS was 5.6 months (95% CI, 3.9-8.1) and median OS was 12.2 months (95% CI, 6.4-not applicable). The most common treatment-emergent adverse events were nausea (59.1%), fatigue (50.0%), anemia (27.3%), vomiting (27.3%), and decreased appetite (22.7%). (Abstract)
This has led to a burgeoning interest in solid tumors, with over 100 clinical trials in progress. Non–small cell lung cancer (NSCLC) is no exception. In 2022, the Food and Drug Administration approved trastuzumab DXd for pretreated NSCLC patients with HER2-mutant tumors. Two others have lung cancer breakthrough therapy designations from the FDA, including patritumab deruxtecan (HER3-DXd) for EGFR-mutant NSCLC and telisotuzumab vedotin for NSCLC that overexpresses c-Met.
At the annual World Conference on Lung Cancer, researchers outlined some of the latest developments in ADCs targeting the antigens HER3, trophoblast cell-surface antigen 2 (TROP-2), and the B7-H3 immunoregulatory protein, as single agents or in combination with immunotherapy. Following the presentations, discussant Helena Linardou, MD, PhD, emphasized the need for pharmacogenomics to predict toxicity and studies to understand resistance mechanisms. “ADCs are a new, rapidly evolving class of therapeutics, and I think that we will all have to be prepared for the future that is coming,” said Dr. Linardou, who is director of the 4th oncology department and the Comprehensive Clinical Trials Center at Metropolitan Hospital in Athens.
Researchers presented four ADC clinical trial updates.
Patritumab deruxtecan
Patritumab deruxtecan (Daiichi Sankyo) links a HER3 antibody to the topoisomerase I inhibitor deruxtecan (HER3-DXd). In the open-label, phase 2 HERTHENA-Lung01 trial, it was tested in patients with NSCLC EGFR-activation mutations, which occurs in 14%-38% of NSCLC cases. There are few options for these patients following failure of EGFR tyrosine kinase inhibitor therapy.
The study included 225 patients previously treated with EGFR tyrosine kinase inhibitors and platinum-based chemotherapy who received 5.6 mg/kg of HER3-DXd every 3 weeks. Over a median follow-up of 13.1 months and a median treatment duration of 5.5 months), 29.8% had a confirmed overall response (95% confidence interval, 23.9%-36.2%) with a median duration of 6.4 months (95% CI, 4.9-7.8). The median progression-free survival was 5.5 months (95% CI, 5.1-5.9), and the median OS was 11.9 months (95% CI, 11.2-13.1). The researchers noted similar outcomes among patients with different mechanisms of EGFR TKI resistance. The frequency of adverse events was similar to previous studies, with drug-related adverse events linked to treatment discontinuation of 7.1% interstitial lung disease in 5.3%. Among 30 patients with brain metastases, the confirmed intracranial response rate was 33.3% (95% CI, 17.3%-52.8%).
The study was published simultaneously online in the Journal of Clinical Oncology. (Abstract)
Datopotamab deruxtecan
PD-1/PD-L1 inhibitors are the first-line therapy for metastatic NSCLC, but there are efforts to improve outcomes through combination therapy. Datopotamab deruxtecan (Dato-DXd, Daiichi Sankyo, AstraZeneca) is an ADC targeting TROP2, and it has been shown in preclinical studies to enhance tumor responses to PD-1/PD-L1 inhibitors. The ADC improved progression-free survival (PFS), compared with docetaxel, in previously treated advanced or metastatic NSCLC.
In an interim analysis of the phase 1b TROPION-Lung04 study, researchers reported results from the combination of Dato-DXd with durvalumab with or without carboplatin. The study included 38 patients, some of whom had previously undergone treatment with immune checkpoint inhibitors. Nineteen patients received the doublet, and 14 received the carboplatin triplet. Grade 3 or higher treatment-emergent adverse events occurred in 42.1% of the doublet group and 71.4% of the triplet group. Interstitial lung disease occurred in 15.8% and 7.1% of the two groups, respectively. The objective response rate was 50.0% in the doublet group and 76.9% in the triplet group. The disease control rate was 92.9% and 92.3%, respectively. Durable responses occurred in both the first-line setting and the overall population. (Abstract)
Sacituzumab govitecan
Another ADC being tested with PD-1/PD-L1 inhibitors is sacituzumab govitecan (Trodelvy, Gilead), which has already received FDA approval for metastatic triple-negative breast cancer, pretreated HR+/HER2- metastatic breast cancer, and metastatic urothelial cancer. Like datopotamab, sacituzumab targets TROP-2. Researchers reported preliminary results from the phase 2 EVOKE-02 study, in which the ADC was combined with pembrolizumab for the first-line treatment of metastatic NSCLC. The overall response rate was 56% (95% CI, 42%-69%). Among patients with PD-L1 tumor proportion score (TPS) ≥ 50%, the ORR was 69% (95% CI, 49%-85%) and 44% (95% CI, 26%-62%) among those with TPS < 50%. The disease control rate was 86% (95% CI, 68%-96%) and 78% (95% CI, 60%-91%), respectively. The most frequent treatment-emergent adverse events (TEAEs) were diarrhea, anemia, and asthenia, and 18% of patients discontinued the study drug because of TEAEs. (Abstract)
Ifinatamab deruxtecan
Ifinatamab deruxtecan (Daiichi Sankyo) targets the B7-H3 antigen, which is an immunoregulatory protein that is overexpressed in many tumors. In the DS7300-A-J101 study, it was tested in patients with advanced or metastatic solid tumors, without selection for B7-H3 expression. A subgroup analysis of 22 patients with small cell lung cancer (SCLC) showed an ORR of 52.4 (95% CI, 29.8-74.3), a complete response of 4.8%, and a partial response in 47.6%. The median PFS was 5.6 months (95% CI, 3.9-8.1) and median OS was 12.2 months (95% CI, 6.4-not applicable). The most common treatment-emergent adverse events were nausea (59.1%), fatigue (50.0%), anemia (27.3%), vomiting (27.3%), and decreased appetite (22.7%). (Abstract)
This has led to a burgeoning interest in solid tumors, with over 100 clinical trials in progress. Non–small cell lung cancer (NSCLC) is no exception. In 2022, the Food and Drug Administration approved trastuzumab DXd for pretreated NSCLC patients with HER2-mutant tumors. Two others have lung cancer breakthrough therapy designations from the FDA, including patritumab deruxtecan (HER3-DXd) for EGFR-mutant NSCLC and telisotuzumab vedotin for NSCLC that overexpresses c-Met.
At the annual World Conference on Lung Cancer, researchers outlined some of the latest developments in ADCs targeting the antigens HER3, trophoblast cell-surface antigen 2 (TROP-2), and the B7-H3 immunoregulatory protein, as single agents or in combination with immunotherapy. Following the presentations, discussant Helena Linardou, MD, PhD, emphasized the need for pharmacogenomics to predict toxicity and studies to understand resistance mechanisms. “ADCs are a new, rapidly evolving class of therapeutics, and I think that we will all have to be prepared for the future that is coming,” said Dr. Linardou, who is director of the 4th oncology department and the Comprehensive Clinical Trials Center at Metropolitan Hospital in Athens.
Researchers presented four ADC clinical trial updates.
Patritumab deruxtecan
Patritumab deruxtecan (Daiichi Sankyo) links a HER3 antibody to the topoisomerase I inhibitor deruxtecan (HER3-DXd). In the open-label, phase 2 HERTHENA-Lung01 trial, it was tested in patients with NSCLC EGFR-activation mutations, which occurs in 14%-38% of NSCLC cases. There are few options for these patients following failure of EGFR tyrosine kinase inhibitor therapy.
The study included 225 patients previously treated with EGFR tyrosine kinase inhibitors and platinum-based chemotherapy who received 5.6 mg/kg of HER3-DXd every 3 weeks. Over a median follow-up of 13.1 months and a median treatment duration of 5.5 months), 29.8% had a confirmed overall response (95% confidence interval, 23.9%-36.2%) with a median duration of 6.4 months (95% CI, 4.9-7.8). The median progression-free survival was 5.5 months (95% CI, 5.1-5.9), and the median OS was 11.9 months (95% CI, 11.2-13.1). The researchers noted similar outcomes among patients with different mechanisms of EGFR TKI resistance. The frequency of adverse events was similar to previous studies, with drug-related adverse events linked to treatment discontinuation of 7.1% interstitial lung disease in 5.3%. Among 30 patients with brain metastases, the confirmed intracranial response rate was 33.3% (95% CI, 17.3%-52.8%).
The study was published simultaneously online in the Journal of Clinical Oncology. (Abstract)
Datopotamab deruxtecan
PD-1/PD-L1 inhibitors are the first-line therapy for metastatic NSCLC, but there are efforts to improve outcomes through combination therapy. Datopotamab deruxtecan (Dato-DXd, Daiichi Sankyo, AstraZeneca) is an ADC targeting TROP2, and it has been shown in preclinical studies to enhance tumor responses to PD-1/PD-L1 inhibitors. The ADC improved progression-free survival (PFS), compared with docetaxel, in previously treated advanced or metastatic NSCLC.
In an interim analysis of the phase 1b TROPION-Lung04 study, researchers reported results from the combination of Dato-DXd with durvalumab with or without carboplatin. The study included 38 patients, some of whom had previously undergone treatment with immune checkpoint inhibitors. Nineteen patients received the doublet, and 14 received the carboplatin triplet. Grade 3 or higher treatment-emergent adverse events occurred in 42.1% of the doublet group and 71.4% of the triplet group. Interstitial lung disease occurred in 15.8% and 7.1% of the two groups, respectively. The objective response rate was 50.0% in the doublet group and 76.9% in the triplet group. The disease control rate was 92.9% and 92.3%, respectively. Durable responses occurred in both the first-line setting and the overall population. (Abstract)
Sacituzumab govitecan
Another ADC being tested with PD-1/PD-L1 inhibitors is sacituzumab govitecan (Trodelvy, Gilead), which has already received FDA approval for metastatic triple-negative breast cancer, pretreated HR+/HER2- metastatic breast cancer, and metastatic urothelial cancer. Like datopotamab, sacituzumab targets TROP-2. Researchers reported preliminary results from the phase 2 EVOKE-02 study, in which the ADC was combined with pembrolizumab for the first-line treatment of metastatic NSCLC. The overall response rate was 56% (95% CI, 42%-69%). Among patients with PD-L1 tumor proportion score (TPS) ≥ 50%, the ORR was 69% (95% CI, 49%-85%) and 44% (95% CI, 26%-62%) among those with TPS < 50%. The disease control rate was 86% (95% CI, 68%-96%) and 78% (95% CI, 60%-91%), respectively. The most frequent treatment-emergent adverse events (TEAEs) were diarrhea, anemia, and asthenia, and 18% of patients discontinued the study drug because of TEAEs. (Abstract)
Ifinatamab deruxtecan
Ifinatamab deruxtecan (Daiichi Sankyo) targets the B7-H3 antigen, which is an immunoregulatory protein that is overexpressed in many tumors. In the DS7300-A-J101 study, it was tested in patients with advanced or metastatic solid tumors, without selection for B7-H3 expression. A subgroup analysis of 22 patients with small cell lung cancer (SCLC) showed an ORR of 52.4 (95% CI, 29.8-74.3), a complete response of 4.8%, and a partial response in 47.6%. The median PFS was 5.6 months (95% CI, 3.9-8.1) and median OS was 12.2 months (95% CI, 6.4-not applicable). The most common treatment-emergent adverse events were nausea (59.1%), fatigue (50.0%), anemia (27.3%), vomiting (27.3%), and decreased appetite (22.7%). (Abstract)
FROM WCLC 2023
Hydroxychloroquine blood level ‘sweet spot’ may maximize efficacy in lupus
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
Blueprint to curb postop opioids after pancreatic resection
TOPLINE:
Implementing a post-surgery protocol that has undergone incremental changes over time significantly reduced inpatient and discharge opioid volumes while maintaining pain control after pancreatic cancer surgery.
METHODOLOGY:
- To reduce opioid dependence, misuse, and diversion, Centers for Disease Control and Prevention guidelines emphasize strategies to minimize opioid prescribing for managing pain. Still, opioid prescribing following surgery remains common practice.
- In the current study,
- The study evaluated three sequential protocols implemented over a period of about 6 years, from 2016 to 2022.
- In the final version, a standardized three-drug nonopioid bundle (acetaminophen, celecoxib, and methocarbamol) was initiated intravenously in the recovery room, after which the patient was given oral agents on postoperative day 1.
- The primary outcome measure was inpatient and discharge opioid volume in oral morphine equivalents (OMEs) across the three pathways.
TAKEAWAY:
- Opioid use significantly decreased with each sequential pathway refinement.
- For inpatients, total OME decreased by more than 55% across the pathways from a median of 290 mg to 184 mg and finally to 129 mg (P < .001).
- Median discharge OME dropped from 150 mg to 25 mg and then to 0 mg across the pathways (P < .001).
- With the final version of the pathway, more than half of patients (52.5%) had opioid-free discharges, compared with only 7.2% in the first pathway. Pain scores remained stable at 3 or less; the number of postdischarge refill requests was unchanged.
IN PRACTICE:
“Our findings suggest that reduction of postoperative opioid dissemination through opioid-free discharge after pancreatectomy and other major cancer operations may be realistic and feasible by following this no-cost blueprint,” the authors concluded. In an accompanying editorial, Melissa Hogg, MD, from NorthShore University Health System in Evanston, Ill., said the “study inspired me to update our institution’s [early recovery after surgery] protocol to reduce and eliminate opioid prescriptions.”
SOURCE:
The study was led by Ching-Wei D. Tzeng, MD, of the University of Texas MD Anderson Cancer Center, Houston. It was published online in JAMA Surgery.
LIMITATIONS:
The study evaluated the opioid protocol at a single center, which may limit the generalizability of the findings. The researchers did not receive patient feedback on pain control expectations or postoperative quality of life.
DISCLOSURES:
Dr. Tzeng reported receiving consultant fees and a sponsored research agreement from PanTher outside the submitted work. Dr. Hogg reported receiving training and travel funds from Intuitive Money. No other disclosures or outside funding were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Implementing a post-surgery protocol that has undergone incremental changes over time significantly reduced inpatient and discharge opioid volumes while maintaining pain control after pancreatic cancer surgery.
METHODOLOGY:
- To reduce opioid dependence, misuse, and diversion, Centers for Disease Control and Prevention guidelines emphasize strategies to minimize opioid prescribing for managing pain. Still, opioid prescribing following surgery remains common practice.
- In the current study,
- The study evaluated three sequential protocols implemented over a period of about 6 years, from 2016 to 2022.
- In the final version, a standardized three-drug nonopioid bundle (acetaminophen, celecoxib, and methocarbamol) was initiated intravenously in the recovery room, after which the patient was given oral agents on postoperative day 1.
- The primary outcome measure was inpatient and discharge opioid volume in oral morphine equivalents (OMEs) across the three pathways.
TAKEAWAY:
- Opioid use significantly decreased with each sequential pathway refinement.
- For inpatients, total OME decreased by more than 55% across the pathways from a median of 290 mg to 184 mg and finally to 129 mg (P < .001).
- Median discharge OME dropped from 150 mg to 25 mg and then to 0 mg across the pathways (P < .001).
- With the final version of the pathway, more than half of patients (52.5%) had opioid-free discharges, compared with only 7.2% in the first pathway. Pain scores remained stable at 3 or less; the number of postdischarge refill requests was unchanged.
IN PRACTICE:
“Our findings suggest that reduction of postoperative opioid dissemination through opioid-free discharge after pancreatectomy and other major cancer operations may be realistic and feasible by following this no-cost blueprint,” the authors concluded. In an accompanying editorial, Melissa Hogg, MD, from NorthShore University Health System in Evanston, Ill., said the “study inspired me to update our institution’s [early recovery after surgery] protocol to reduce and eliminate opioid prescriptions.”
SOURCE:
The study was led by Ching-Wei D. Tzeng, MD, of the University of Texas MD Anderson Cancer Center, Houston. It was published online in JAMA Surgery.
LIMITATIONS:
The study evaluated the opioid protocol at a single center, which may limit the generalizability of the findings. The researchers did not receive patient feedback on pain control expectations or postoperative quality of life.
DISCLOSURES:
Dr. Tzeng reported receiving consultant fees and a sponsored research agreement from PanTher outside the submitted work. Dr. Hogg reported receiving training and travel funds from Intuitive Money. No other disclosures or outside funding were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Implementing a post-surgery protocol that has undergone incremental changes over time significantly reduced inpatient and discharge opioid volumes while maintaining pain control after pancreatic cancer surgery.
METHODOLOGY:
- To reduce opioid dependence, misuse, and diversion, Centers for Disease Control and Prevention guidelines emphasize strategies to minimize opioid prescribing for managing pain. Still, opioid prescribing following surgery remains common practice.
- In the current study,
- The study evaluated three sequential protocols implemented over a period of about 6 years, from 2016 to 2022.
- In the final version, a standardized three-drug nonopioid bundle (acetaminophen, celecoxib, and methocarbamol) was initiated intravenously in the recovery room, after which the patient was given oral agents on postoperative day 1.
- The primary outcome measure was inpatient and discharge opioid volume in oral morphine equivalents (OMEs) across the three pathways.
TAKEAWAY:
- Opioid use significantly decreased with each sequential pathway refinement.
- For inpatients, total OME decreased by more than 55% across the pathways from a median of 290 mg to 184 mg and finally to 129 mg (P < .001).
- Median discharge OME dropped from 150 mg to 25 mg and then to 0 mg across the pathways (P < .001).
- With the final version of the pathway, more than half of patients (52.5%) had opioid-free discharges, compared with only 7.2% in the first pathway. Pain scores remained stable at 3 or less; the number of postdischarge refill requests was unchanged.
IN PRACTICE:
“Our findings suggest that reduction of postoperative opioid dissemination through opioid-free discharge after pancreatectomy and other major cancer operations may be realistic and feasible by following this no-cost blueprint,” the authors concluded. In an accompanying editorial, Melissa Hogg, MD, from NorthShore University Health System in Evanston, Ill., said the “study inspired me to update our institution’s [early recovery after surgery] protocol to reduce and eliminate opioid prescriptions.”
SOURCE:
The study was led by Ching-Wei D. Tzeng, MD, of the University of Texas MD Anderson Cancer Center, Houston. It was published online in JAMA Surgery.
LIMITATIONS:
The study evaluated the opioid protocol at a single center, which may limit the generalizability of the findings. The researchers did not receive patient feedback on pain control expectations or postoperative quality of life.
DISCLOSURES:
Dr. Tzeng reported receiving consultant fees and a sponsored research agreement from PanTher outside the submitted work. Dr. Hogg reported receiving training and travel funds from Intuitive Money. No other disclosures or outside funding were reported.
A version of this article appeared on Medscape.com.
FROM JAMA SURGERY
Company submits supplemental NDA for topical atopic dermatitis treatment
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
Universal anxiety screening recommendation is a good start
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Diabetes patients satisfied with continuous glucose monitors
TOPLINE:
However, significant proportions also reported concerns about accuracy under certain circumstances and about skin problems.
METHODOLOGY:
Researchers did an online survey of 504 people with type 1 diabetes from the T1D Exchange and 101 with type 2 diabetes from the Dynata database.
TAKEAWAY:
- The Dexcom G6 device was used by 60.7% of all current CGM users, including 69% of those with type 1 diabetes vs. 12% with type 2 diabetes.
- People with type 2 diabetes were more likely to use older Dexcom versions (G4/G5) (32%) or Abbott’s FreeStyle Libre systems (35%).
- Overall, 90% agreed that most sensors were accurate, but just 79% and 78%, respectively, were satisfied with sensor performance on the first and last day of wear.
- Moreover, 42% suspected variations in accuracy from sensor to sensor, and 32% continue to perform finger-stick monitoring more than six times a week.
- Individuals with type 2 diabetes were more likely than those with type 1 diabetes to be concerned about poor sensor performance affecting confidence in making diabetes management decisions (52% vs. 19%).
- Over half reported skin reactions and/or pain with the sensors (53.7% and 55.4%, respectively).
- Concerns about medications affecting sensor accuracy were more common among those with type 2 vs. type 1 diabetes (65% vs. 29%).
- Among overall concerns about substances or situations affecting sensor accuracy, the top choice (47%) was dehydration (despite a lack of supportive published literature), followed by pain medications (43%), cold/flu medications (32%), and coffee (24%).
- Inaccurate/false alarms negatively affected daily life for 36% of participants and diabetes management for 34%.
IN PRACTICE:
“CGM is a game-changing technology and has evolved in the past decade to overcome many technical and usability obstacles. Our survey suggests that there remain areas for further improvement ... Mistrust in CGM performance was more common than expected.”
SOURCE:
The study was done by Elizabeth Holt, of LifeScan, and colleagues. It was published in Clinical Diabetes.
LIMITATIONS:
- The databases used to recruit study participants may not be representative of the entire respective patient populations.
- Exercise wasn’t given as an option for affecting CGM accuracy, which might partly explain the dehydration finding.
DISCLOSURES:
Funding for this study and preparation of the manuscript were provided by LifeScan Inc. Two authors are LifeScan employees, and two others currently work for the T1D Exchange.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, significant proportions also reported concerns about accuracy under certain circumstances and about skin problems.
METHODOLOGY:
Researchers did an online survey of 504 people with type 1 diabetes from the T1D Exchange and 101 with type 2 diabetes from the Dynata database.
TAKEAWAY:
- The Dexcom G6 device was used by 60.7% of all current CGM users, including 69% of those with type 1 diabetes vs. 12% with type 2 diabetes.
- People with type 2 diabetes were more likely to use older Dexcom versions (G4/G5) (32%) or Abbott’s FreeStyle Libre systems (35%).
- Overall, 90% agreed that most sensors were accurate, but just 79% and 78%, respectively, were satisfied with sensor performance on the first and last day of wear.
- Moreover, 42% suspected variations in accuracy from sensor to sensor, and 32% continue to perform finger-stick monitoring more than six times a week.
- Individuals with type 2 diabetes were more likely than those with type 1 diabetes to be concerned about poor sensor performance affecting confidence in making diabetes management decisions (52% vs. 19%).
- Over half reported skin reactions and/or pain with the sensors (53.7% and 55.4%, respectively).
- Concerns about medications affecting sensor accuracy were more common among those with type 2 vs. type 1 diabetes (65% vs. 29%).
- Among overall concerns about substances or situations affecting sensor accuracy, the top choice (47%) was dehydration (despite a lack of supportive published literature), followed by pain medications (43%), cold/flu medications (32%), and coffee (24%).
- Inaccurate/false alarms negatively affected daily life for 36% of participants and diabetes management for 34%.
IN PRACTICE:
“CGM is a game-changing technology and has evolved in the past decade to overcome many technical and usability obstacles. Our survey suggests that there remain areas for further improvement ... Mistrust in CGM performance was more common than expected.”
SOURCE:
The study was done by Elizabeth Holt, of LifeScan, and colleagues. It was published in Clinical Diabetes.
LIMITATIONS:
- The databases used to recruit study participants may not be representative of the entire respective patient populations.
- Exercise wasn’t given as an option for affecting CGM accuracy, which might partly explain the dehydration finding.
DISCLOSURES:
Funding for this study and preparation of the manuscript were provided by LifeScan Inc. Two authors are LifeScan employees, and two others currently work for the T1D Exchange.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, significant proportions also reported concerns about accuracy under certain circumstances and about skin problems.
METHODOLOGY:
Researchers did an online survey of 504 people with type 1 diabetes from the T1D Exchange and 101 with type 2 diabetes from the Dynata database.
TAKEAWAY:
- The Dexcom G6 device was used by 60.7% of all current CGM users, including 69% of those with type 1 diabetes vs. 12% with type 2 diabetes.
- People with type 2 diabetes were more likely to use older Dexcom versions (G4/G5) (32%) or Abbott’s FreeStyle Libre systems (35%).
- Overall, 90% agreed that most sensors were accurate, but just 79% and 78%, respectively, were satisfied with sensor performance on the first and last day of wear.
- Moreover, 42% suspected variations in accuracy from sensor to sensor, and 32% continue to perform finger-stick monitoring more than six times a week.
- Individuals with type 2 diabetes were more likely than those with type 1 diabetes to be concerned about poor sensor performance affecting confidence in making diabetes management decisions (52% vs. 19%).
- Over half reported skin reactions and/or pain with the sensors (53.7% and 55.4%, respectively).
- Concerns about medications affecting sensor accuracy were more common among those with type 2 vs. type 1 diabetes (65% vs. 29%).
- Among overall concerns about substances or situations affecting sensor accuracy, the top choice (47%) was dehydration (despite a lack of supportive published literature), followed by pain medications (43%), cold/flu medications (32%), and coffee (24%).
- Inaccurate/false alarms negatively affected daily life for 36% of participants and diabetes management for 34%.
IN PRACTICE:
“CGM is a game-changing technology and has evolved in the past decade to overcome many technical and usability obstacles. Our survey suggests that there remain areas for further improvement ... Mistrust in CGM performance was more common than expected.”
SOURCE:
The study was done by Elizabeth Holt, of LifeScan, and colleagues. It was published in Clinical Diabetes.
LIMITATIONS:
- The databases used to recruit study participants may not be representative of the entire respective patient populations.
- Exercise wasn’t given as an option for affecting CGM accuracy, which might partly explain the dehydration finding.
DISCLOSURES:
Funding for this study and preparation of the manuscript were provided by LifeScan Inc. Two authors are LifeScan employees, and two others currently work for the T1D Exchange.
A version of this article first appeared on Medscape.com.
Steady VKA therapy beats switch to NOAC in frail AFib patients: FRAIL-AF
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Novel ADC offers hope in heavily pretreated NSCLC
SINGAPORE – Heavily pretreated patients with EGFR-mutated non–small cell lung cancer (NSCLC) may experience a clinically meaningful benefit with the antibody-drug conjugate (ADC) patritumab deruxtecan (HER3-DXd), new phase 2 trial results suggest.
In the trial, almost 30% of patients receiving HER3-DXd achieved an objective response, and patients’ median overall survival approached 1 year.
HER3-DXd has “emerged as a promising therapy” in this patient population, “for whom available treatment options provide only limited efficacy,” concluded lead study author Helena A. Yu, MD, from Memorial Sloan Kettering Cancer Center, New York City, who presented findings from the HERTHENA-Lung01 trial at the 2023 World Conference on Lung Cancer.
The results were simultaneously published in the Journal of Clinical Oncology.
Acquired resistance to therapy among heavily pretreated patients with EGFR-mutated NSCLC is “universal,” Dr. Yu explained. The mechanisms of resistance to first-line osimertinib are also “diverse” and hard to identify. Salvage therapies after failed EGFR tyrosine kinase inhibitor (TKI) therapy and platinum-based chemotherapy offer “only a limited and transient and clinical benefit,” she said.
And with limited treatment options available to patients resistant to TKIs, there is a “high unmet medical need” for new therapies, said Helena Linardou, MD, PhD, who was not involved in the study.
HER3-DXd consists of a fully human anti-HER3 immunoglobulin G1 monoclonal antibody (patritumab) attached to a topoisomerase I inhibitor payload (DXd) via a tetrapeptide-based cleavable linker.
HER3, Dr. Linardou explained, is a “biologically and clinically important target” in NSCLC. It is highly expressed in NSCLC, upregulated in TKI-resistant EGFR-mutated NSCLC, and is associated with a poor prognosis.
After promising phase 1 data, Dr. Yu and colleagues conducted a phase 2 trial in patients with advanced EGFR-mutated NSCLC who had progressed on systematic therapy and had received EGFR TKI and platinum-based chemotherapy. Patients could have active or previously treated asymptomatic brain metastases.
Patients were initially randomized either to a fixed-dose arm of HER3-DXd 5.6 mg/kg once every 3 weeks or an uptitration arm with doses escalating from 3.2 mg/kg to 4.8 mg/kg to 6.4 mg/kg over three cycles. However, Dr. Yu noted, enrollment in the uptitration arm closed early based on a “benefit-risk assessment.”
The current findings focus on the 225 patients in the fixed-dose arm.
About half of patients had a history of central nervous system metastasis, and patients had a median of three prior lines of systemic therapy. Most patients (92.9%) had previously received a third-generation EGFR TKI, about 40% had received immunotherapy, and all had received platinum-based chemotherapy.
After a median follow-up of 18.9 months, the confirmed objective response rate with HER3-DXd across the whole patient population was 29.8%. The median duration of response was 6.4 months, median progression-free survival was 5.5 months, and median overall survival was 11.9 months.
The results were virtually identical when looking only at patients who had received a third-generation EGFR TKI versus any EGFR TKI. Response rates were also similar regardless of the driver of EGFR TKI resistance.
Among the 30 patients with measurable brain metastases at baseline, 33.3% had a confirmed objective response to therapy. In this group, the disease control rate was 76.7% and the intracranial duration of response was 8.4 months.
To identify biomarkers of response to HER3-DXd, the team stratified the patients by baseline tumor HER3 membrane H-scores. Dr. Yu and colleagues found no differences in the likelihood of having a complete or partial response, stable disease, or progressive disease based on HER3 expression at study entry.
Looking at the safety of the ADC, only 7.1% of patients experienced a treatment-emergent adverse event associated with treatment discontinuation, but close to half of patients (45.3%) experienced a grade 3 or higher treatment-emergent adverse event. Interstitial lung disease, for instance, occurred in 5.3% of patients, including one patient who died.
The safety profile of HER3-DXd in this population appeared to be consistent with previous reports, Dr. Yu noted.
Overall, the findings suggest that “HER3-DXd provided clinically meaningful and durable efficacy” in patients with advanced EGFR-mutant NSCLC that has progressed after EGFR TKI and platinum-based chemotherapy, Dr. Yu said.
“Efficacy was observed across diverse mechanisms of resistance and across a broad range of pretreatment tumor HER3 expression” and the ADC demonstrated “clinically meaningful intracranial antitumor activity,” she added.
Dr. Linardou agreed that the current results suggest that HER3-DXd was associated with a “meaningful and durable efficacy,” including in patients with intracranial metastases, and she pointed to its “easy dosing schedule” and activity across patient subgroups.
However, she noted that, despite the researchers’ best efforts with the data available, “we don’t have a biomarker of activity,” which is a “general issue with ADCs.”
Still, Dr. Linardou said, “HER3 is now a clinically actionable therapeutic target, and this is a great bonus.”
The study was sponsored by Daiichi Sankyo. Dr. Yu declared relationships with AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics, Taiho Oncology, and others. Other authors declare numerous relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – Heavily pretreated patients with EGFR-mutated non–small cell lung cancer (NSCLC) may experience a clinically meaningful benefit with the antibody-drug conjugate (ADC) patritumab deruxtecan (HER3-DXd), new phase 2 trial results suggest.
In the trial, almost 30% of patients receiving HER3-DXd achieved an objective response, and patients’ median overall survival approached 1 year.
HER3-DXd has “emerged as a promising therapy” in this patient population, “for whom available treatment options provide only limited efficacy,” concluded lead study author Helena A. Yu, MD, from Memorial Sloan Kettering Cancer Center, New York City, who presented findings from the HERTHENA-Lung01 trial at the 2023 World Conference on Lung Cancer.
The results were simultaneously published in the Journal of Clinical Oncology.
Acquired resistance to therapy among heavily pretreated patients with EGFR-mutated NSCLC is “universal,” Dr. Yu explained. The mechanisms of resistance to first-line osimertinib are also “diverse” and hard to identify. Salvage therapies after failed EGFR tyrosine kinase inhibitor (TKI) therapy and platinum-based chemotherapy offer “only a limited and transient and clinical benefit,” she said.
And with limited treatment options available to patients resistant to TKIs, there is a “high unmet medical need” for new therapies, said Helena Linardou, MD, PhD, who was not involved in the study.
HER3-DXd consists of a fully human anti-HER3 immunoglobulin G1 monoclonal antibody (patritumab) attached to a topoisomerase I inhibitor payload (DXd) via a tetrapeptide-based cleavable linker.
HER3, Dr. Linardou explained, is a “biologically and clinically important target” in NSCLC. It is highly expressed in NSCLC, upregulated in TKI-resistant EGFR-mutated NSCLC, and is associated with a poor prognosis.
After promising phase 1 data, Dr. Yu and colleagues conducted a phase 2 trial in patients with advanced EGFR-mutated NSCLC who had progressed on systematic therapy and had received EGFR TKI and platinum-based chemotherapy. Patients could have active or previously treated asymptomatic brain metastases.
Patients were initially randomized either to a fixed-dose arm of HER3-DXd 5.6 mg/kg once every 3 weeks or an uptitration arm with doses escalating from 3.2 mg/kg to 4.8 mg/kg to 6.4 mg/kg over three cycles. However, Dr. Yu noted, enrollment in the uptitration arm closed early based on a “benefit-risk assessment.”
The current findings focus on the 225 patients in the fixed-dose arm.
About half of patients had a history of central nervous system metastasis, and patients had a median of three prior lines of systemic therapy. Most patients (92.9%) had previously received a third-generation EGFR TKI, about 40% had received immunotherapy, and all had received platinum-based chemotherapy.
After a median follow-up of 18.9 months, the confirmed objective response rate with HER3-DXd across the whole patient population was 29.8%. The median duration of response was 6.4 months, median progression-free survival was 5.5 months, and median overall survival was 11.9 months.
The results were virtually identical when looking only at patients who had received a third-generation EGFR TKI versus any EGFR TKI. Response rates were also similar regardless of the driver of EGFR TKI resistance.
Among the 30 patients with measurable brain metastases at baseline, 33.3% had a confirmed objective response to therapy. In this group, the disease control rate was 76.7% and the intracranial duration of response was 8.4 months.
To identify biomarkers of response to HER3-DXd, the team stratified the patients by baseline tumor HER3 membrane H-scores. Dr. Yu and colleagues found no differences in the likelihood of having a complete or partial response, stable disease, or progressive disease based on HER3 expression at study entry.
Looking at the safety of the ADC, only 7.1% of patients experienced a treatment-emergent adverse event associated with treatment discontinuation, but close to half of patients (45.3%) experienced a grade 3 or higher treatment-emergent adverse event. Interstitial lung disease, for instance, occurred in 5.3% of patients, including one patient who died.
The safety profile of HER3-DXd in this population appeared to be consistent with previous reports, Dr. Yu noted.
Overall, the findings suggest that “HER3-DXd provided clinically meaningful and durable efficacy” in patients with advanced EGFR-mutant NSCLC that has progressed after EGFR TKI and platinum-based chemotherapy, Dr. Yu said.
“Efficacy was observed across diverse mechanisms of resistance and across a broad range of pretreatment tumor HER3 expression” and the ADC demonstrated “clinically meaningful intracranial antitumor activity,” she added.
Dr. Linardou agreed that the current results suggest that HER3-DXd was associated with a “meaningful and durable efficacy,” including in patients with intracranial metastases, and she pointed to its “easy dosing schedule” and activity across patient subgroups.
However, she noted that, despite the researchers’ best efforts with the data available, “we don’t have a biomarker of activity,” which is a “general issue with ADCs.”
Still, Dr. Linardou said, “HER3 is now a clinically actionable therapeutic target, and this is a great bonus.”
The study was sponsored by Daiichi Sankyo. Dr. Yu declared relationships with AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics, Taiho Oncology, and others. Other authors declare numerous relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – Heavily pretreated patients with EGFR-mutated non–small cell lung cancer (NSCLC) may experience a clinically meaningful benefit with the antibody-drug conjugate (ADC) patritumab deruxtecan (HER3-DXd), new phase 2 trial results suggest.
In the trial, almost 30% of patients receiving HER3-DXd achieved an objective response, and patients’ median overall survival approached 1 year.
HER3-DXd has “emerged as a promising therapy” in this patient population, “for whom available treatment options provide only limited efficacy,” concluded lead study author Helena A. Yu, MD, from Memorial Sloan Kettering Cancer Center, New York City, who presented findings from the HERTHENA-Lung01 trial at the 2023 World Conference on Lung Cancer.
The results were simultaneously published in the Journal of Clinical Oncology.
Acquired resistance to therapy among heavily pretreated patients with EGFR-mutated NSCLC is “universal,” Dr. Yu explained. The mechanisms of resistance to first-line osimertinib are also “diverse” and hard to identify. Salvage therapies after failed EGFR tyrosine kinase inhibitor (TKI) therapy and platinum-based chemotherapy offer “only a limited and transient and clinical benefit,” she said.
And with limited treatment options available to patients resistant to TKIs, there is a “high unmet medical need” for new therapies, said Helena Linardou, MD, PhD, who was not involved in the study.
HER3-DXd consists of a fully human anti-HER3 immunoglobulin G1 monoclonal antibody (patritumab) attached to a topoisomerase I inhibitor payload (DXd) via a tetrapeptide-based cleavable linker.
HER3, Dr. Linardou explained, is a “biologically and clinically important target” in NSCLC. It is highly expressed in NSCLC, upregulated in TKI-resistant EGFR-mutated NSCLC, and is associated with a poor prognosis.
After promising phase 1 data, Dr. Yu and colleagues conducted a phase 2 trial in patients with advanced EGFR-mutated NSCLC who had progressed on systematic therapy and had received EGFR TKI and platinum-based chemotherapy. Patients could have active or previously treated asymptomatic brain metastases.
Patients were initially randomized either to a fixed-dose arm of HER3-DXd 5.6 mg/kg once every 3 weeks or an uptitration arm with doses escalating from 3.2 mg/kg to 4.8 mg/kg to 6.4 mg/kg over three cycles. However, Dr. Yu noted, enrollment in the uptitration arm closed early based on a “benefit-risk assessment.”
The current findings focus on the 225 patients in the fixed-dose arm.
About half of patients had a history of central nervous system metastasis, and patients had a median of three prior lines of systemic therapy. Most patients (92.9%) had previously received a third-generation EGFR TKI, about 40% had received immunotherapy, and all had received platinum-based chemotherapy.
After a median follow-up of 18.9 months, the confirmed objective response rate with HER3-DXd across the whole patient population was 29.8%. The median duration of response was 6.4 months, median progression-free survival was 5.5 months, and median overall survival was 11.9 months.
The results were virtually identical when looking only at patients who had received a third-generation EGFR TKI versus any EGFR TKI. Response rates were also similar regardless of the driver of EGFR TKI resistance.
Among the 30 patients with measurable brain metastases at baseline, 33.3% had a confirmed objective response to therapy. In this group, the disease control rate was 76.7% and the intracranial duration of response was 8.4 months.
To identify biomarkers of response to HER3-DXd, the team stratified the patients by baseline tumor HER3 membrane H-scores. Dr. Yu and colleagues found no differences in the likelihood of having a complete or partial response, stable disease, or progressive disease based on HER3 expression at study entry.
Looking at the safety of the ADC, only 7.1% of patients experienced a treatment-emergent adverse event associated with treatment discontinuation, but close to half of patients (45.3%) experienced a grade 3 or higher treatment-emergent adverse event. Interstitial lung disease, for instance, occurred in 5.3% of patients, including one patient who died.
The safety profile of HER3-DXd in this population appeared to be consistent with previous reports, Dr. Yu noted.
Overall, the findings suggest that “HER3-DXd provided clinically meaningful and durable efficacy” in patients with advanced EGFR-mutant NSCLC that has progressed after EGFR TKI and platinum-based chemotherapy, Dr. Yu said.
“Efficacy was observed across diverse mechanisms of resistance and across a broad range of pretreatment tumor HER3 expression” and the ADC demonstrated “clinically meaningful intracranial antitumor activity,” she added.
Dr. Linardou agreed that the current results suggest that HER3-DXd was associated with a “meaningful and durable efficacy,” including in patients with intracranial metastases, and she pointed to its “easy dosing schedule” and activity across patient subgroups.
However, she noted that, despite the researchers’ best efforts with the data available, “we don’t have a biomarker of activity,” which is a “general issue with ADCs.”
Still, Dr. Linardou said, “HER3 is now a clinically actionable therapeutic target, and this is a great bonus.”
The study was sponsored by Daiichi Sankyo. Dr. Yu declared relationships with AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics, Taiho Oncology, and others. Other authors declare numerous relationships.
A version of this article first appeared on Medscape.com.
AT IASLC 2023