User login
Integrated PrEP and ART prevents HIV transmission in couples
In committed couples, HIV transmission from positive partners dropped from an expected incidence of more than 5% to less than 0.5% per year when the uninfected partner used pre-exposure prophylaxis (PrEP) for the first 6 months of the infected partner’s antiretroviral therapy, according to a study in PLOS Medicine.
The open-label demonstration project on which the study reports involved 1,013 heterosexual HIV-1–serodiscordant couples in Kenya and Uganda (PLOS Med. 2016 Aug 23. doi: 10.1371/journal.pmed.1002099).
“To our knowledge, this study is one of the first and one of the largest demonstration projects to provide PrEP to a priority population at risk for HIV-1 outside of a clinical trial setting, and the findings demonstrate the feasibility and impact of using PrEP as a bridging strategy to sustained HIV-1 protection by ART [antiretroviral therapy]” in serodiscordant couples, said investigators led by Jared Baeten, MD, of the department of global health at the University of Washington, Seattle. Wide-scale roll-out “could have substantial effects in reducing the global burden of new HIV-1 infections,” Dr. Baeten and his coauthors concluded.
PrEP kept uninfected partners safe until ART reliably suppressed viral loads at about 6 months. Adherence to the regimen was about 85%, which was higher than in some clinical trials, perhaps because couples were being offered “a strategy with demonstrated safety and efficacy” instead of unproven products and placebo. Couples also recognized their elevated HIV risk, the research team said.
It was good to find out that the approach works in real-world settings in Africa, Dr. Baeten and his associates said, where the majority of the 2 million people infected each year live. Follow-up was less frequent than in trials, with brief counseling sessions “equivalent to what would be expected in public health settings” and HIV tests about every 4 months over a median of about a year. “This study shows that a practical delivery approach ... can virtually eliminate HIV1- transmission,” the researchers said.
There were just two HIV transmissions in the study, both in women with self-reported and objective evidence of interrupted PrEP use. Overall, the transmission incidence was 0.2/100 person-years. The investigators estimated there would have been 40 transmissions – 5.2/100 person-years – without the intervention.
Couples were recruited by community outreach in four cities and towns – Kisumu and Thika in Kenya, and Kabwohe and Kampala in Uganda. The investigators targeted couples at high risk for transmission, including those reporting unprotected sex and infected partners with plasma HIV-1 RNA levels of 50,000 copies/mL or more.
Almost all were married and living together. They reported a median of six sex acts per month, many unprotected. Partners were a median of about 30 years old, and 67% of uninfected partners were men.
The preferred ART regimen was tenofovir disoproxil fumarate (TDF), lamivudine, and efavirenz, with zidovudine and nevirapine as alternatives. PrEP was a combination emtricitabine/TDF 200 mg/300 mg once daily; it was provided at the study sites since PrEP was otherwise unavailable in Kenya and Uganda.
The National Institutes of Health, Bill and Melinda Gates Foundation, and U.S. Agency for International Development funded the work. Gilead Sciences donated the TDF for PrEP. Dr. Baeten had no disclosures; another author reported funding from Gilead for a TDF pharmacokinetics study, and has a patent pending for a formulation different from the drug used in the study.
In committed couples, HIV transmission from positive partners dropped from an expected incidence of more than 5% to less than 0.5% per year when the uninfected partner used pre-exposure prophylaxis (PrEP) for the first 6 months of the infected partner’s antiretroviral therapy, according to a study in PLOS Medicine.
The open-label demonstration project on which the study reports involved 1,013 heterosexual HIV-1–serodiscordant couples in Kenya and Uganda (PLOS Med. 2016 Aug 23. doi: 10.1371/journal.pmed.1002099).
“To our knowledge, this study is one of the first and one of the largest demonstration projects to provide PrEP to a priority population at risk for HIV-1 outside of a clinical trial setting, and the findings demonstrate the feasibility and impact of using PrEP as a bridging strategy to sustained HIV-1 protection by ART [antiretroviral therapy]” in serodiscordant couples, said investigators led by Jared Baeten, MD, of the department of global health at the University of Washington, Seattle. Wide-scale roll-out “could have substantial effects in reducing the global burden of new HIV-1 infections,” Dr. Baeten and his coauthors concluded.
PrEP kept uninfected partners safe until ART reliably suppressed viral loads at about 6 months. Adherence to the regimen was about 85%, which was higher than in some clinical trials, perhaps because couples were being offered “a strategy with demonstrated safety and efficacy” instead of unproven products and placebo. Couples also recognized their elevated HIV risk, the research team said.
It was good to find out that the approach works in real-world settings in Africa, Dr. Baeten and his associates said, where the majority of the 2 million people infected each year live. Follow-up was less frequent than in trials, with brief counseling sessions “equivalent to what would be expected in public health settings” and HIV tests about every 4 months over a median of about a year. “This study shows that a practical delivery approach ... can virtually eliminate HIV1- transmission,” the researchers said.
There were just two HIV transmissions in the study, both in women with self-reported and objective evidence of interrupted PrEP use. Overall, the transmission incidence was 0.2/100 person-years. The investigators estimated there would have been 40 transmissions – 5.2/100 person-years – without the intervention.
Couples were recruited by community outreach in four cities and towns – Kisumu and Thika in Kenya, and Kabwohe and Kampala in Uganda. The investigators targeted couples at high risk for transmission, including those reporting unprotected sex and infected partners with plasma HIV-1 RNA levels of 50,000 copies/mL or more.
Almost all were married and living together. They reported a median of six sex acts per month, many unprotected. Partners were a median of about 30 years old, and 67% of uninfected partners were men.
The preferred ART regimen was tenofovir disoproxil fumarate (TDF), lamivudine, and efavirenz, with zidovudine and nevirapine as alternatives. PrEP was a combination emtricitabine/TDF 200 mg/300 mg once daily; it was provided at the study sites since PrEP was otherwise unavailable in Kenya and Uganda.
The National Institutes of Health, Bill and Melinda Gates Foundation, and U.S. Agency for International Development funded the work. Gilead Sciences donated the TDF for PrEP. Dr. Baeten had no disclosures; another author reported funding from Gilead for a TDF pharmacokinetics study, and has a patent pending for a formulation different from the drug used in the study.
In committed couples, HIV transmission from positive partners dropped from an expected incidence of more than 5% to less than 0.5% per year when the uninfected partner used pre-exposure prophylaxis (PrEP) for the first 6 months of the infected partner’s antiretroviral therapy, according to a study in PLOS Medicine.
The open-label demonstration project on which the study reports involved 1,013 heterosexual HIV-1–serodiscordant couples in Kenya and Uganda (PLOS Med. 2016 Aug 23. doi: 10.1371/journal.pmed.1002099).
“To our knowledge, this study is one of the first and one of the largest demonstration projects to provide PrEP to a priority population at risk for HIV-1 outside of a clinical trial setting, and the findings demonstrate the feasibility and impact of using PrEP as a bridging strategy to sustained HIV-1 protection by ART [antiretroviral therapy]” in serodiscordant couples, said investigators led by Jared Baeten, MD, of the department of global health at the University of Washington, Seattle. Wide-scale roll-out “could have substantial effects in reducing the global burden of new HIV-1 infections,” Dr. Baeten and his coauthors concluded.
PrEP kept uninfected partners safe until ART reliably suppressed viral loads at about 6 months. Adherence to the regimen was about 85%, which was higher than in some clinical trials, perhaps because couples were being offered “a strategy with demonstrated safety and efficacy” instead of unproven products and placebo. Couples also recognized their elevated HIV risk, the research team said.
It was good to find out that the approach works in real-world settings in Africa, Dr. Baeten and his associates said, where the majority of the 2 million people infected each year live. Follow-up was less frequent than in trials, with brief counseling sessions “equivalent to what would be expected in public health settings” and HIV tests about every 4 months over a median of about a year. “This study shows that a practical delivery approach ... can virtually eliminate HIV1- transmission,” the researchers said.
There were just two HIV transmissions in the study, both in women with self-reported and objective evidence of interrupted PrEP use. Overall, the transmission incidence was 0.2/100 person-years. The investigators estimated there would have been 40 transmissions – 5.2/100 person-years – without the intervention.
Couples were recruited by community outreach in four cities and towns – Kisumu and Thika in Kenya, and Kabwohe and Kampala in Uganda. The investigators targeted couples at high risk for transmission, including those reporting unprotected sex and infected partners with plasma HIV-1 RNA levels of 50,000 copies/mL or more.
Almost all were married and living together. They reported a median of six sex acts per month, many unprotected. Partners were a median of about 30 years old, and 67% of uninfected partners were men.
The preferred ART regimen was tenofovir disoproxil fumarate (TDF), lamivudine, and efavirenz, with zidovudine and nevirapine as alternatives. PrEP was a combination emtricitabine/TDF 200 mg/300 mg once daily; it was provided at the study sites since PrEP was otherwise unavailable in Kenya and Uganda.
The National Institutes of Health, Bill and Melinda Gates Foundation, and U.S. Agency for International Development funded the work. Gilead Sciences donated the TDF for PrEP. Dr. Baeten had no disclosures; another author reported funding from Gilead for a TDF pharmacokinetics study, and has a patent pending for a formulation different from the drug used in the study.
FROM PLOS MEDICINE
Key clinical point: In committed couples, HIV transmission from positive partners dropped from an expected incidence of more than 5% to less than 0.5% per year when the uninfected partner used pre-exposure prophylaxis for the first 6 months of the infected partner’s antiretroviral therapy.
Major finding: The transmission incidence was 0.2/100 person-years. The investigators estimated there would have been 40 transmissions – 5.2/100 person-years – without the intervention.
Data source: Open-label demonstration project with 1,013 heterosexual HIV-1–serodiscordant couples in Kenya and Uganda.
Disclosures: The National Institutes of Health, Bill and Melinda Gates Foundation, and U.S. Agency for International Development funded the work. Gilead Sciences donated the TDF for PrEP. Dr. Baeten had no disclosures; another author reported funding from Gilead for a TDF pharmacokinetics study and has a patent pending for a formulation different from the drug used in the study.
Better use of lab testing tools needed to beat HIV/AIDS
Major improvements in HIV laboratory capacity utilization are needed in low- and middle-income countries if the global UNAIDS 90-90-90 targets for HIV and AIDS diagnosis and treatment are to be met, according to a World Health Organization study.
The study, based on 3 years of WHO survey data from 127 countries (not including the United States), says achieving the 90-90-90 targets depends heavily on improved access to high-quality testing for early infant diagnosis and treatment monitoring. The availability of “cluster of differentiation 4” (CD4) and viral load testing instruments currently meets the needs of individuals living with HIV/AIDS, but the tests are not being utilized to their full potential.
“Expanding access to treatment requires high-quality HIV testing technologies, including CD4 to assess risk of disease progression, viral load testing to monitor treatment efficacy, early infant diagnosis to determine HIV-infection status in HIV-exposed children, and other monitoring capabilities within a tiered laboratory network,” wrote Vincent Habiyambere, MD, of the WHO in Geneva and his colleagues in a study published online Aug. 23 in PLOS Medicine.
Overall, 13.7% of existing CD4 testing capacity and 36.5% of existing viral load capacity were used in 2013. In addition, 7.4% of existing CD4 instruments and 10% of viral load instruments were not in use by the end of 2013 because of several factors including lack of reagents, improperly installed or destroyed equipment, and lack of staff training.
The survey results were limited by underreporting in some programs and the collection of data from the public sector only, the researchers noted. But the data suggest that “regardless of the need for point of care, it is clear that laboratory-based monitoring will remain a key component of HIV programmes now and in the future,” the authors said. “With laboratory systems in reporting countries expanding, a national laboratory strategic plan to strengthen services must be developed, implemented, and monitored by governments and their international partners.”
Read the full study in PLOS Medicine (doi:10.1371/journal.pmed.1002088).
Major improvements in HIV laboratory capacity utilization are needed in low- and middle-income countries if the global UNAIDS 90-90-90 targets for HIV and AIDS diagnosis and treatment are to be met, according to a World Health Organization study.
The study, based on 3 years of WHO survey data from 127 countries (not including the United States), says achieving the 90-90-90 targets depends heavily on improved access to high-quality testing for early infant diagnosis and treatment monitoring. The availability of “cluster of differentiation 4” (CD4) and viral load testing instruments currently meets the needs of individuals living with HIV/AIDS, but the tests are not being utilized to their full potential.
“Expanding access to treatment requires high-quality HIV testing technologies, including CD4 to assess risk of disease progression, viral load testing to monitor treatment efficacy, early infant diagnosis to determine HIV-infection status in HIV-exposed children, and other monitoring capabilities within a tiered laboratory network,” wrote Vincent Habiyambere, MD, of the WHO in Geneva and his colleagues in a study published online Aug. 23 in PLOS Medicine.
Overall, 13.7% of existing CD4 testing capacity and 36.5% of existing viral load capacity were used in 2013. In addition, 7.4% of existing CD4 instruments and 10% of viral load instruments were not in use by the end of 2013 because of several factors including lack of reagents, improperly installed or destroyed equipment, and lack of staff training.
The survey results were limited by underreporting in some programs and the collection of data from the public sector only, the researchers noted. But the data suggest that “regardless of the need for point of care, it is clear that laboratory-based monitoring will remain a key component of HIV programmes now and in the future,” the authors said. “With laboratory systems in reporting countries expanding, a national laboratory strategic plan to strengthen services must be developed, implemented, and monitored by governments and their international partners.”
Read the full study in PLOS Medicine (doi:10.1371/journal.pmed.1002088).
Major improvements in HIV laboratory capacity utilization are needed in low- and middle-income countries if the global UNAIDS 90-90-90 targets for HIV and AIDS diagnosis and treatment are to be met, according to a World Health Organization study.
The study, based on 3 years of WHO survey data from 127 countries (not including the United States), says achieving the 90-90-90 targets depends heavily on improved access to high-quality testing for early infant diagnosis and treatment monitoring. The availability of “cluster of differentiation 4” (CD4) and viral load testing instruments currently meets the needs of individuals living with HIV/AIDS, but the tests are not being utilized to their full potential.
“Expanding access to treatment requires high-quality HIV testing technologies, including CD4 to assess risk of disease progression, viral load testing to monitor treatment efficacy, early infant diagnosis to determine HIV-infection status in HIV-exposed children, and other monitoring capabilities within a tiered laboratory network,” wrote Vincent Habiyambere, MD, of the WHO in Geneva and his colleagues in a study published online Aug. 23 in PLOS Medicine.
Overall, 13.7% of existing CD4 testing capacity and 36.5% of existing viral load capacity were used in 2013. In addition, 7.4% of existing CD4 instruments and 10% of viral load instruments were not in use by the end of 2013 because of several factors including lack of reagents, improperly installed or destroyed equipment, and lack of staff training.
The survey results were limited by underreporting in some programs and the collection of data from the public sector only, the researchers noted. But the data suggest that “regardless of the need for point of care, it is clear that laboratory-based monitoring will remain a key component of HIV programmes now and in the future,” the authors said. “With laboratory systems in reporting countries expanding, a national laboratory strategic plan to strengthen services must be developed, implemented, and monitored by governments and their international partners.”
Read the full study in PLOS Medicine (doi:10.1371/journal.pmed.1002088).
FROM PLOS MEDICINE
AGA receives NIH funding for national FMT registry
After years of planning and behind-the-scenes discussions, the AGA Center for Gut Microbiome Research and Education is thrilled to announce that it has received NIH funding for the first-ever national registry documenting the use of fecal microbiota transplantation (FMT).
The AGA Fecal Microbiota Transplantation National Registry – which aims to begin collecting data in early 2017 – will gather clinical data from both stool donors and FMT recipients for the following purposes:
1. To assess short- and long-term safety.
2. To gather information on practice in the U.S. and assess effectiveness of the intervention.
3. To promote scientific investigation.
4. To aid practitioners and sponsors in satisfying regulatory requirements.
For more information, read the AGA press release announcing the registry. Stay tuned for additional information from the center on what this means for physicians practicing FMT and researchers studying FMT.
After years of planning and behind-the-scenes discussions, the AGA Center for Gut Microbiome Research and Education is thrilled to announce that it has received NIH funding for the first-ever national registry documenting the use of fecal microbiota transplantation (FMT).
The AGA Fecal Microbiota Transplantation National Registry – which aims to begin collecting data in early 2017 – will gather clinical data from both stool donors and FMT recipients for the following purposes:
1. To assess short- and long-term safety.
2. To gather information on practice in the U.S. and assess effectiveness of the intervention.
3. To promote scientific investigation.
4. To aid practitioners and sponsors in satisfying regulatory requirements.
For more information, read the AGA press release announcing the registry. Stay tuned for additional information from the center on what this means for physicians practicing FMT and researchers studying FMT.
After years of planning and behind-the-scenes discussions, the AGA Center for Gut Microbiome Research and Education is thrilled to announce that it has received NIH funding for the first-ever national registry documenting the use of fecal microbiota transplantation (FMT).
The AGA Fecal Microbiota Transplantation National Registry – which aims to begin collecting data in early 2017 – will gather clinical data from both stool donors and FMT recipients for the following purposes:
1. To assess short- and long-term safety.
2. To gather information on practice in the U.S. and assess effectiveness of the intervention.
3. To promote scientific investigation.
4. To aid practitioners and sponsors in satisfying regulatory requirements.
For more information, read the AGA press release announcing the registry. Stay tuned for additional information from the center on what this means for physicians practicing FMT and researchers studying FMT.
Power morcellation dropped, abdominal hysterectomy increased after FDA warning
Electric power morcellation during hysterectomy declined sharply after the Food and Drug Administration discouraged use of the technique in April 2014 and then recommended against it for perimenopausal and postmenopausal women in November 2014. At the same time, use of abdominal hysterectomy increased, according to a new analysis.
The FDA took these actions because of concern that intraoperative morcellation could inadvertently expose healthy abdominal tissue to contamination from occult uterine malignancies. But some clinicians warned that avoiding morcellation would lead to a greater number of hysterectomies via laparotomy, with an attendant increase in surgical complications.
To assess the effect of the FDA recommendations, Jason D. Wright, MD, of the division of gynecologic oncology, Columbia University, New York, and his colleagues analyzed time trends in hysterectomy and morcellation during nine 3-month periods before and after the FDA announcements. They used information from a national database that covers more than 500 hospitals across the country, including in their analysis 203,520 women aged 18-95 years (mean age, 48 years) who underwent hysterectomy during the study period.
Among the 117,653 minimally invasive hysterectomies performed, the use of electric power morcellation rose slightly during 2013, peaking at 13.7% in the fourth quarter of that year. It then declined precipitously, to a low of 2.8% by the last 3-month period assessed, which was the first quarter of 2015. Simultaneously, the use of abdominal hysterectomy increased from 27.1% of procedures in early 2013 to 31.8% by the last 3-month period assessed, the researchers reported (JAMA 2016;316:877-8).
However, despite the increase in abdominal procedures, the complication rate did not change over time. It was 8.3% during the first quarter studied and 8.4% during the last. In fact, the rate of complications during abdominal hysterectomy also declined, from 18.4% to 17.6%. Similarly, the rate of complications during minimally invasive hysterectomy dropped from 4.4% to 4.1%, and the complication rate decreased from 4.7% to 4.2% during vaginal hysterectomy.
The researchers noted that the prevalence of uterine cancer, endometrial hyperplasia, other gynecologic cancers, and uterine tumors of indeterminate behavior were unchanged during the study period among women who underwent minimally invasive hysterectomy with power morcellation.
“The FDA warnings might result in a lower prevalence of cancer among women who underwent morcellation due to greater scrutiny on patient selection. However, the high rate of abnormal pathology after the warnings highlights the difficulty in the preoperative detection of uterine pathology,” the researchers wrote. “Continued caution is needed to limit the inadvertent morcellation of uterine pathology.”
The National Cancer Institute funded the study. The researchers reported having no relevant financial disclosures.
I read with interest “Trends in Use and Outcomes of Women Undergoing Hysterectomy With Electric Power Morcellation,” by Jason D. Wright and colleagues, published in JAMA. As expected, with the concerns raised by the FDA regarding electric power morcellation, there has been a statistically significant reduction in a laparoscopic approach to hysterectomy (59.2% to 56.2%) and an even more marked decrease in use of electric power morcellation (13.7% to 2.8%).

|
Dr. Charles E. Miller |
Interestingly, the increase in abdominal hysterectomy (27.1% to 31.8%) appears to be secondary not only to the reduction in minimally invasive gynecologic surgery, but vaginal hysterectomy as well. While the decrease in vaginal hysterectomy, is, in part, likely due to the trend away from this technique, it is probably also due to the concern of cutting up a potential sarcoma during the procedure to deliver the large fibroid uterus. This would be supported by the fact that the greatest percentage drop in vaginal hysterectomy occurred in Q1-Q2 2014 at the time the FDA issued its safety concern.
In a study by Matthew Siedhoff, MD, et al. (Am J Obstet Gynecol. 2015 May;212[5]:591.e1-8), utilizing a decision tree model, Dr. Siedhoff anticipated that severe complications would increase with conversion of a minimally invasive approach to laparotomy. While according to Wright et al., there is no increase noted in complications per se, there is no indication as to severity of complications. Thus, while overall complications have not increased, perhaps severe complications have, in fact, increased as anticipated by Siedhoff et al. Furthermore, impact on quality of life is not considered (i.e. hospitalization, convalescence at home, etc.) in this study, but is a well known difference between minimally invasive surgery and open surgery.
Charles E. Miller, MD, is a clinical associate professor at the University of Illinois, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. Dr. Miller reported that he is working on a study with Espiner Medical Ltd. to evaluate the safety and efficacy of a bag that is utilized for contained electric power morcellation. Karl Storz is sponsoring the study.
I read with interest “Trends in Use and Outcomes of Women Undergoing Hysterectomy With Electric Power Morcellation,” by Jason D. Wright and colleagues, published in JAMA. As expected, with the concerns raised by the FDA regarding electric power morcellation, there has been a statistically significant reduction in a laparoscopic approach to hysterectomy (59.2% to 56.2%) and an even more marked decrease in use of electric power morcellation (13.7% to 2.8%).

|
Dr. Charles E. Miller |
Interestingly, the increase in abdominal hysterectomy (27.1% to 31.8%) appears to be secondary not only to the reduction in minimally invasive gynecologic surgery, but vaginal hysterectomy as well. While the decrease in vaginal hysterectomy, is, in part, likely due to the trend away from this technique, it is probably also due to the concern of cutting up a potential sarcoma during the procedure to deliver the large fibroid uterus. This would be supported by the fact that the greatest percentage drop in vaginal hysterectomy occurred in Q1-Q2 2014 at the time the FDA issued its safety concern.
In a study by Matthew Siedhoff, MD, et al. (Am J Obstet Gynecol. 2015 May;212[5]:591.e1-8), utilizing a decision tree model, Dr. Siedhoff anticipated that severe complications would increase with conversion of a minimally invasive approach to laparotomy. While according to Wright et al., there is no increase noted in complications per se, there is no indication as to severity of complications. Thus, while overall complications have not increased, perhaps severe complications have, in fact, increased as anticipated by Siedhoff et al. Furthermore, impact on quality of life is not considered (i.e. hospitalization, convalescence at home, etc.) in this study, but is a well known difference between minimally invasive surgery and open surgery.
Charles E. Miller, MD, is a clinical associate professor at the University of Illinois, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. Dr. Miller reported that he is working on a study with Espiner Medical Ltd. to evaluate the safety and efficacy of a bag that is utilized for contained electric power morcellation. Karl Storz is sponsoring the study.
I read with interest “Trends in Use and Outcomes of Women Undergoing Hysterectomy With Electric Power Morcellation,” by Jason D. Wright and colleagues, published in JAMA. As expected, with the concerns raised by the FDA regarding electric power morcellation, there has been a statistically significant reduction in a laparoscopic approach to hysterectomy (59.2% to 56.2%) and an even more marked decrease in use of electric power morcellation (13.7% to 2.8%).

|
Dr. Charles E. Miller |
Interestingly, the increase in abdominal hysterectomy (27.1% to 31.8%) appears to be secondary not only to the reduction in minimally invasive gynecologic surgery, but vaginal hysterectomy as well. While the decrease in vaginal hysterectomy, is, in part, likely due to the trend away from this technique, it is probably also due to the concern of cutting up a potential sarcoma during the procedure to deliver the large fibroid uterus. This would be supported by the fact that the greatest percentage drop in vaginal hysterectomy occurred in Q1-Q2 2014 at the time the FDA issued its safety concern.
In a study by Matthew Siedhoff, MD, et al. (Am J Obstet Gynecol. 2015 May;212[5]:591.e1-8), utilizing a decision tree model, Dr. Siedhoff anticipated that severe complications would increase with conversion of a minimally invasive approach to laparotomy. While according to Wright et al., there is no increase noted in complications per se, there is no indication as to severity of complications. Thus, while overall complications have not increased, perhaps severe complications have, in fact, increased as anticipated by Siedhoff et al. Furthermore, impact on quality of life is not considered (i.e. hospitalization, convalescence at home, etc.) in this study, but is a well known difference between minimally invasive surgery and open surgery.
Charles E. Miller, MD, is a clinical associate professor at the University of Illinois, and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. Dr. Miller reported that he is working on a study with Espiner Medical Ltd. to evaluate the safety and efficacy of a bag that is utilized for contained electric power morcellation. Karl Storz is sponsoring the study.
Electric power morcellation during hysterectomy declined sharply after the Food and Drug Administration discouraged use of the technique in April 2014 and then recommended against it for perimenopausal and postmenopausal women in November 2014. At the same time, use of abdominal hysterectomy increased, according to a new analysis.
The FDA took these actions because of concern that intraoperative morcellation could inadvertently expose healthy abdominal tissue to contamination from occult uterine malignancies. But some clinicians warned that avoiding morcellation would lead to a greater number of hysterectomies via laparotomy, with an attendant increase in surgical complications.
To assess the effect of the FDA recommendations, Jason D. Wright, MD, of the division of gynecologic oncology, Columbia University, New York, and his colleagues analyzed time trends in hysterectomy and morcellation during nine 3-month periods before and after the FDA announcements. They used information from a national database that covers more than 500 hospitals across the country, including in their analysis 203,520 women aged 18-95 years (mean age, 48 years) who underwent hysterectomy during the study period.
Among the 117,653 minimally invasive hysterectomies performed, the use of electric power morcellation rose slightly during 2013, peaking at 13.7% in the fourth quarter of that year. It then declined precipitously, to a low of 2.8% by the last 3-month period assessed, which was the first quarter of 2015. Simultaneously, the use of abdominal hysterectomy increased from 27.1% of procedures in early 2013 to 31.8% by the last 3-month period assessed, the researchers reported (JAMA 2016;316:877-8).
However, despite the increase in abdominal procedures, the complication rate did not change over time. It was 8.3% during the first quarter studied and 8.4% during the last. In fact, the rate of complications during abdominal hysterectomy also declined, from 18.4% to 17.6%. Similarly, the rate of complications during minimally invasive hysterectomy dropped from 4.4% to 4.1%, and the complication rate decreased from 4.7% to 4.2% during vaginal hysterectomy.
The researchers noted that the prevalence of uterine cancer, endometrial hyperplasia, other gynecologic cancers, and uterine tumors of indeterminate behavior were unchanged during the study period among women who underwent minimally invasive hysterectomy with power morcellation.
“The FDA warnings might result in a lower prevalence of cancer among women who underwent morcellation due to greater scrutiny on patient selection. However, the high rate of abnormal pathology after the warnings highlights the difficulty in the preoperative detection of uterine pathology,” the researchers wrote. “Continued caution is needed to limit the inadvertent morcellation of uterine pathology.”
The National Cancer Institute funded the study. The researchers reported having no relevant financial disclosures.
Electric power morcellation during hysterectomy declined sharply after the Food and Drug Administration discouraged use of the technique in April 2014 and then recommended against it for perimenopausal and postmenopausal women in November 2014. At the same time, use of abdominal hysterectomy increased, according to a new analysis.
The FDA took these actions because of concern that intraoperative morcellation could inadvertently expose healthy abdominal tissue to contamination from occult uterine malignancies. But some clinicians warned that avoiding morcellation would lead to a greater number of hysterectomies via laparotomy, with an attendant increase in surgical complications.
To assess the effect of the FDA recommendations, Jason D. Wright, MD, of the division of gynecologic oncology, Columbia University, New York, and his colleagues analyzed time trends in hysterectomy and morcellation during nine 3-month periods before and after the FDA announcements. They used information from a national database that covers more than 500 hospitals across the country, including in their analysis 203,520 women aged 18-95 years (mean age, 48 years) who underwent hysterectomy during the study period.
Among the 117,653 minimally invasive hysterectomies performed, the use of electric power morcellation rose slightly during 2013, peaking at 13.7% in the fourth quarter of that year. It then declined precipitously, to a low of 2.8% by the last 3-month period assessed, which was the first quarter of 2015. Simultaneously, the use of abdominal hysterectomy increased from 27.1% of procedures in early 2013 to 31.8% by the last 3-month period assessed, the researchers reported (JAMA 2016;316:877-8).
However, despite the increase in abdominal procedures, the complication rate did not change over time. It was 8.3% during the first quarter studied and 8.4% during the last. In fact, the rate of complications during abdominal hysterectomy also declined, from 18.4% to 17.6%. Similarly, the rate of complications during minimally invasive hysterectomy dropped from 4.4% to 4.1%, and the complication rate decreased from 4.7% to 4.2% during vaginal hysterectomy.
The researchers noted that the prevalence of uterine cancer, endometrial hyperplasia, other gynecologic cancers, and uterine tumors of indeterminate behavior were unchanged during the study period among women who underwent minimally invasive hysterectomy with power morcellation.
“The FDA warnings might result in a lower prevalence of cancer among women who underwent morcellation due to greater scrutiny on patient selection. However, the high rate of abnormal pathology after the warnings highlights the difficulty in the preoperative detection of uterine pathology,” the researchers wrote. “Continued caution is needed to limit the inadvertent morcellation of uterine pathology.”
The National Cancer Institute funded the study. The researchers reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Electric power morcellation declined after the FDA recommended against using the technique during hysterectomy.
Major finding: Use of electric power morcellation peaked at 13.7% before the FDA recommendations, then declined to a low of 2.8%.
Data source: A retrospective database analysis involving 203,520 hysterectomies performed at more than 500 U.S. hospitals during 2013-2015.
Disclosures: The National Cancer Institute funded the study. The researchers reported having no relevant financial disclosures.
Herpes Zoster in Children
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
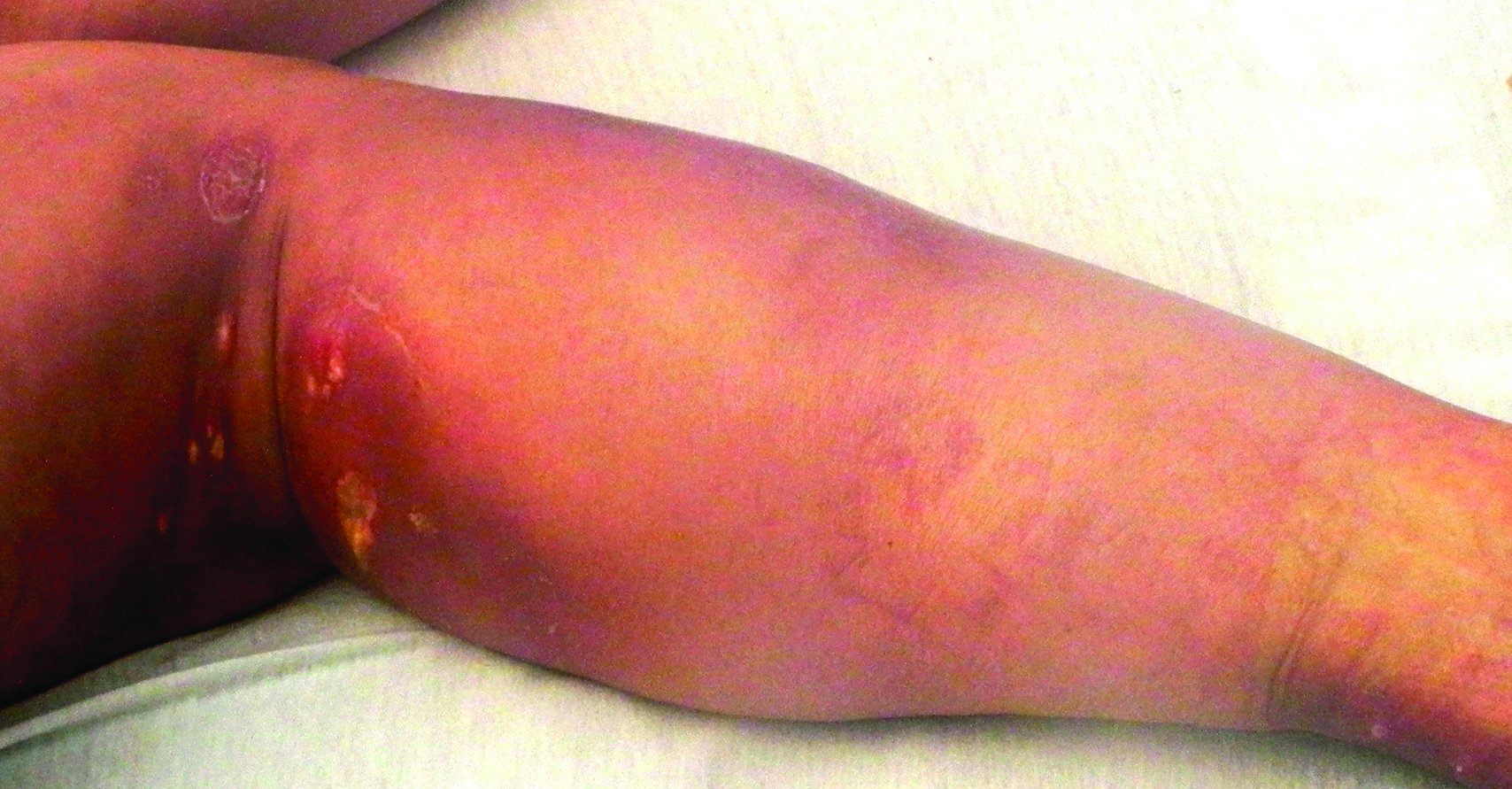
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated. Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated. Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated. Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Practice Points
- Herpes zoster (HZ) should be included in the differential diagnosis for children presenting with vesicular lesions in a dermatomal distribution and a history of varicella exposure.
- Clinical diagnosis of HZ and herpes simplex virus can be aided by the use of viral polymerase chain reaction testing.
- Children with HZ should be monitored for the same possible complications as adults.
SAGE-547 for depression: Cause for caution and optimism
The importance of postpartum depression, both in terms of its prevalence and the need for appropriate screening and effective treatments, has become an increasingly important area of focus for clinicians, patients, and policymakers. This derives from more than a decade of data on the significant prevalence of the condition, with roughly 10% of women meeting the criteria for major or minor depression during the first 3-6 months post partum.
Over the last 5 years, interest has centered around establishing mechanisms for appropriate perinatal depression screening, most notably the January 2016 recommendation from the U.S. Preventive Services Task Force that all adults should be screened for depression, including the at-risk populations of pregnant and postpartum women. In 2015, the American College of Obstetricians and Gynecologists endorsed screening women for depression and anxiety symptoms at least once during the perinatal period using a validated tool. Unfortunately, we still lack data to support whether screening is effective in getting patients referred for treatment and if it leads to women accessing therapies that will actually get them well.
As we wait for that data and consider ways to best implement enhanced screening, it’s important to take stock of the available treatments for postpartum depression.
Seeking a rapid treatment
The current literature supports efficacy for nonpharmacologic therapies, such as interpersonal psychotherapy and cognitive-behavioral therapy, as well as several antidepressants. The efficacy of antidepressants – such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors – has been demonstrated for postpartum depression, but these agents carry the typical limitations and concerns in terms of side effects and the amount of time required to ascertain if there is benefit. While these are the same challenges seen in treating depression in general, the time to response – often 4-8 weeks – is particularly problematic for postpartum women where the impact of depression on maternal morbidity and child development is so critical.
The field has been clamoring for agents that work more quickly. One possibility in that area is ketamine, which is being studied as a rapid treatment in major depression. The National Institutes of Health also has an initiative underway called RAPID (Rapidly Acting Treatments for Treatment-Resistant Depression), aimed at identifying and testing pharmacologic and nonpharmacologic treatments that produce a response within days rather than weeks.
Recently, considerable interest has focused on SAGE-547, manufactured by Sage Therapeutics, which is a different type of antidepressant. The so-called neurosteriod is an allosteric modulator of the GABAA (gamma-aminobutyric acid type A) receptors. The product was granted fast-track status by the Food and Drug Administration to speed its development as a possible treatment for superrefractory status epilepticus, but it also is being studied for its potential in treating severe postpartum depression.
Approximately a year ago, there was preliminary evidence from an open-label study suggesting rapid response to SAGE-547 for women who received this medicine intravenously in a controlled hospital environment. And in July 2016, the manufacturer announced in a press release unpublished positive results from a small phase II controlled trial of SAGE-547 for the treatment of severe postpartum depression.
Specifically, this was a placebo-controlled, double-blind randomized trial for 21 women who had severe depressive symptoms with a baseline score of at least 26 on the Hamilton Rating Scale for Depression (HAM-D). For some of the women, postpartum depression was not of new onset, but rather was an extension of depression that had manifested no earlier than the third trimester of pregnancy. A total of 10 women received the drug, while 11 received placebo. Both groups received continuous intravenous infusion over a 60-hour period.
Consistent with the earlier report, participants receiving the active agent had a statistically significant reduction in HAM-D scores at 24 hours, compared with women who received the placebo. Seven out of 10 women who received the active drug achieved remission from depression at 60 hours, compared with only 1 of the 11 patients who received placebo. Even though the results derived from an extremely small sample, the signal for efficacy appears promising.
Of particular interest, there appeared to be a duration of benefit at 30 days’ follow-up. The medicine was well tolerated with no discontinuations due to adverse events, which were most commonly dizziness, sedation, or somnolence. The adverse events were about the same in both the drug and placebo groups.
Next steps
These early results have generated excitement, if not a “buzz,” in the field, given the rapid onset of antidepressant benefit and the apparent duration of the effect. But readers should be mindful that to date, the findings have not been peer reviewed and are available only through a company-issued press release. It is also noteworthy that on clinicaltrials.gov, the projected enrollment was 32, but 21 women enrolled. This may speak to the great difficulty in enrolling the sample and may ultimately reflect on the generalizability of the findings.
One significant challenge with SAGE-547 is the formulation. It’s hardly feasible for severely ill postpartum women to come to the hospital for 60 hours of treatment. The manufacturer will have to produce a reformulated compound that is able to sustain the efficacy signaled in this proof of concept study.
But even more importantly, we will need to see how the drug performs in a larger, rigorous phase IIB or phase III study to know if the signal of promise really translates into a potential viable treatment option for women with severe postpartum depression. When we have results from a randomized controlled trial with a substantially larger number of patients, then we’ll know whether the excitement is justified. It would be a significant advance for the field if this were to be the case.
The field of depression, in general, has been seeking an effective, rapid treatment for some time, and the role of neurosteriods has been spoken about for more than 2 decades. If postpartum women are in fact a subgroup who respond to this class of agents, then that would be an example of truly personalized medicine. But we won’t know that until the manufacturer does an appropriate large trial, which could take 2-4 years.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information and resources and conducts clinical care and research in reproductive mental health. He has no financial relationship with SAGE Therapeutics, but he has been a consultant to manufacturers of psychiatric medications.
The importance of postpartum depression, both in terms of its prevalence and the need for appropriate screening and effective treatments, has become an increasingly important area of focus for clinicians, patients, and policymakers. This derives from more than a decade of data on the significant prevalence of the condition, with roughly 10% of women meeting the criteria for major or minor depression during the first 3-6 months post partum.
Over the last 5 years, interest has centered around establishing mechanisms for appropriate perinatal depression screening, most notably the January 2016 recommendation from the U.S. Preventive Services Task Force that all adults should be screened for depression, including the at-risk populations of pregnant and postpartum women. In 2015, the American College of Obstetricians and Gynecologists endorsed screening women for depression and anxiety symptoms at least once during the perinatal period using a validated tool. Unfortunately, we still lack data to support whether screening is effective in getting patients referred for treatment and if it leads to women accessing therapies that will actually get them well.
As we wait for that data and consider ways to best implement enhanced screening, it’s important to take stock of the available treatments for postpartum depression.
Seeking a rapid treatment
The current literature supports efficacy for nonpharmacologic therapies, such as interpersonal psychotherapy and cognitive-behavioral therapy, as well as several antidepressants. The efficacy of antidepressants – such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors – has been demonstrated for postpartum depression, but these agents carry the typical limitations and concerns in terms of side effects and the amount of time required to ascertain if there is benefit. While these are the same challenges seen in treating depression in general, the time to response – often 4-8 weeks – is particularly problematic for postpartum women where the impact of depression on maternal morbidity and child development is so critical.
The field has been clamoring for agents that work more quickly. One possibility in that area is ketamine, which is being studied as a rapid treatment in major depression. The National Institutes of Health also has an initiative underway called RAPID (Rapidly Acting Treatments for Treatment-Resistant Depression), aimed at identifying and testing pharmacologic and nonpharmacologic treatments that produce a response within days rather than weeks.
Recently, considerable interest has focused on SAGE-547, manufactured by Sage Therapeutics, which is a different type of antidepressant. The so-called neurosteriod is an allosteric modulator of the GABAA (gamma-aminobutyric acid type A) receptors. The product was granted fast-track status by the Food and Drug Administration to speed its development as a possible treatment for superrefractory status epilepticus, but it also is being studied for its potential in treating severe postpartum depression.
Approximately a year ago, there was preliminary evidence from an open-label study suggesting rapid response to SAGE-547 for women who received this medicine intravenously in a controlled hospital environment. And in July 2016, the manufacturer announced in a press release unpublished positive results from a small phase II controlled trial of SAGE-547 for the treatment of severe postpartum depression.
Specifically, this was a placebo-controlled, double-blind randomized trial for 21 women who had severe depressive symptoms with a baseline score of at least 26 on the Hamilton Rating Scale for Depression (HAM-D). For some of the women, postpartum depression was not of new onset, but rather was an extension of depression that had manifested no earlier than the third trimester of pregnancy. A total of 10 women received the drug, while 11 received placebo. Both groups received continuous intravenous infusion over a 60-hour period.
Consistent with the earlier report, participants receiving the active agent had a statistically significant reduction in HAM-D scores at 24 hours, compared with women who received the placebo. Seven out of 10 women who received the active drug achieved remission from depression at 60 hours, compared with only 1 of the 11 patients who received placebo. Even though the results derived from an extremely small sample, the signal for efficacy appears promising.
Of particular interest, there appeared to be a duration of benefit at 30 days’ follow-up. The medicine was well tolerated with no discontinuations due to adverse events, which were most commonly dizziness, sedation, or somnolence. The adverse events were about the same in both the drug and placebo groups.
Next steps
These early results have generated excitement, if not a “buzz,” in the field, given the rapid onset of antidepressant benefit and the apparent duration of the effect. But readers should be mindful that to date, the findings have not been peer reviewed and are available only through a company-issued press release. It is also noteworthy that on clinicaltrials.gov, the projected enrollment was 32, but 21 women enrolled. This may speak to the great difficulty in enrolling the sample and may ultimately reflect on the generalizability of the findings.
One significant challenge with SAGE-547 is the formulation. It’s hardly feasible for severely ill postpartum women to come to the hospital for 60 hours of treatment. The manufacturer will have to produce a reformulated compound that is able to sustain the efficacy signaled in this proof of concept study.
But even more importantly, we will need to see how the drug performs in a larger, rigorous phase IIB or phase III study to know if the signal of promise really translates into a potential viable treatment option for women with severe postpartum depression. When we have results from a randomized controlled trial with a substantially larger number of patients, then we’ll know whether the excitement is justified. It would be a significant advance for the field if this were to be the case.
The field of depression, in general, has been seeking an effective, rapid treatment for some time, and the role of neurosteriods has been spoken about for more than 2 decades. If postpartum women are in fact a subgroup who respond to this class of agents, then that would be an example of truly personalized medicine. But we won’t know that until the manufacturer does an appropriate large trial, which could take 2-4 years.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information and resources and conducts clinical care and research in reproductive mental health. He has no financial relationship with SAGE Therapeutics, but he has been a consultant to manufacturers of psychiatric medications.
The importance of postpartum depression, both in terms of its prevalence and the need for appropriate screening and effective treatments, has become an increasingly important area of focus for clinicians, patients, and policymakers. This derives from more than a decade of data on the significant prevalence of the condition, with roughly 10% of women meeting the criteria for major or minor depression during the first 3-6 months post partum.
Over the last 5 years, interest has centered around establishing mechanisms for appropriate perinatal depression screening, most notably the January 2016 recommendation from the U.S. Preventive Services Task Force that all adults should be screened for depression, including the at-risk populations of pregnant and postpartum women. In 2015, the American College of Obstetricians and Gynecologists endorsed screening women for depression and anxiety symptoms at least once during the perinatal period using a validated tool. Unfortunately, we still lack data to support whether screening is effective in getting patients referred for treatment and if it leads to women accessing therapies that will actually get them well.
As we wait for that data and consider ways to best implement enhanced screening, it’s important to take stock of the available treatments for postpartum depression.
Seeking a rapid treatment
The current literature supports efficacy for nonpharmacologic therapies, such as interpersonal psychotherapy and cognitive-behavioral therapy, as well as several antidepressants. The efficacy of antidepressants – such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors – has been demonstrated for postpartum depression, but these agents carry the typical limitations and concerns in terms of side effects and the amount of time required to ascertain if there is benefit. While these are the same challenges seen in treating depression in general, the time to response – often 4-8 weeks – is particularly problematic for postpartum women where the impact of depression on maternal morbidity and child development is so critical.
The field has been clamoring for agents that work more quickly. One possibility in that area is ketamine, which is being studied as a rapid treatment in major depression. The National Institutes of Health also has an initiative underway called RAPID (Rapidly Acting Treatments for Treatment-Resistant Depression), aimed at identifying and testing pharmacologic and nonpharmacologic treatments that produce a response within days rather than weeks.
Recently, considerable interest has focused on SAGE-547, manufactured by Sage Therapeutics, which is a different type of antidepressant. The so-called neurosteriod is an allosteric modulator of the GABAA (gamma-aminobutyric acid type A) receptors. The product was granted fast-track status by the Food and Drug Administration to speed its development as a possible treatment for superrefractory status epilepticus, but it also is being studied for its potential in treating severe postpartum depression.
Approximately a year ago, there was preliminary evidence from an open-label study suggesting rapid response to SAGE-547 for women who received this medicine intravenously in a controlled hospital environment. And in July 2016, the manufacturer announced in a press release unpublished positive results from a small phase II controlled trial of SAGE-547 for the treatment of severe postpartum depression.
Specifically, this was a placebo-controlled, double-blind randomized trial for 21 women who had severe depressive symptoms with a baseline score of at least 26 on the Hamilton Rating Scale for Depression (HAM-D). For some of the women, postpartum depression was not of new onset, but rather was an extension of depression that had manifested no earlier than the third trimester of pregnancy. A total of 10 women received the drug, while 11 received placebo. Both groups received continuous intravenous infusion over a 60-hour period.
Consistent with the earlier report, participants receiving the active agent had a statistically significant reduction in HAM-D scores at 24 hours, compared with women who received the placebo. Seven out of 10 women who received the active drug achieved remission from depression at 60 hours, compared with only 1 of the 11 patients who received placebo. Even though the results derived from an extremely small sample, the signal for efficacy appears promising.
Of particular interest, there appeared to be a duration of benefit at 30 days’ follow-up. The medicine was well tolerated with no discontinuations due to adverse events, which were most commonly dizziness, sedation, or somnolence. The adverse events were about the same in both the drug and placebo groups.
Next steps
These early results have generated excitement, if not a “buzz,” in the field, given the rapid onset of antidepressant benefit and the apparent duration of the effect. But readers should be mindful that to date, the findings have not been peer reviewed and are available only through a company-issued press release. It is also noteworthy that on clinicaltrials.gov, the projected enrollment was 32, but 21 women enrolled. This may speak to the great difficulty in enrolling the sample and may ultimately reflect on the generalizability of the findings.
One significant challenge with SAGE-547 is the formulation. It’s hardly feasible for severely ill postpartum women to come to the hospital for 60 hours of treatment. The manufacturer will have to produce a reformulated compound that is able to sustain the efficacy signaled in this proof of concept study.
But even more importantly, we will need to see how the drug performs in a larger, rigorous phase IIB or phase III study to know if the signal of promise really translates into a potential viable treatment option for women with severe postpartum depression. When we have results from a randomized controlled trial with a substantially larger number of patients, then we’ll know whether the excitement is justified. It would be a significant advance for the field if this were to be the case.
The field of depression, in general, has been seeking an effective, rapid treatment for some time, and the role of neurosteriods has been spoken about for more than 2 decades. If postpartum women are in fact a subgroup who respond to this class of agents, then that would be an example of truly personalized medicine. But we won’t know that until the manufacturer does an appropriate large trial, which could take 2-4 years.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information and resources and conducts clinical care and research in reproductive mental health. He has no financial relationship with SAGE Therapeutics, but he has been a consultant to manufacturers of psychiatric medications.
Sporotrichoid Fluctuant Nodules
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
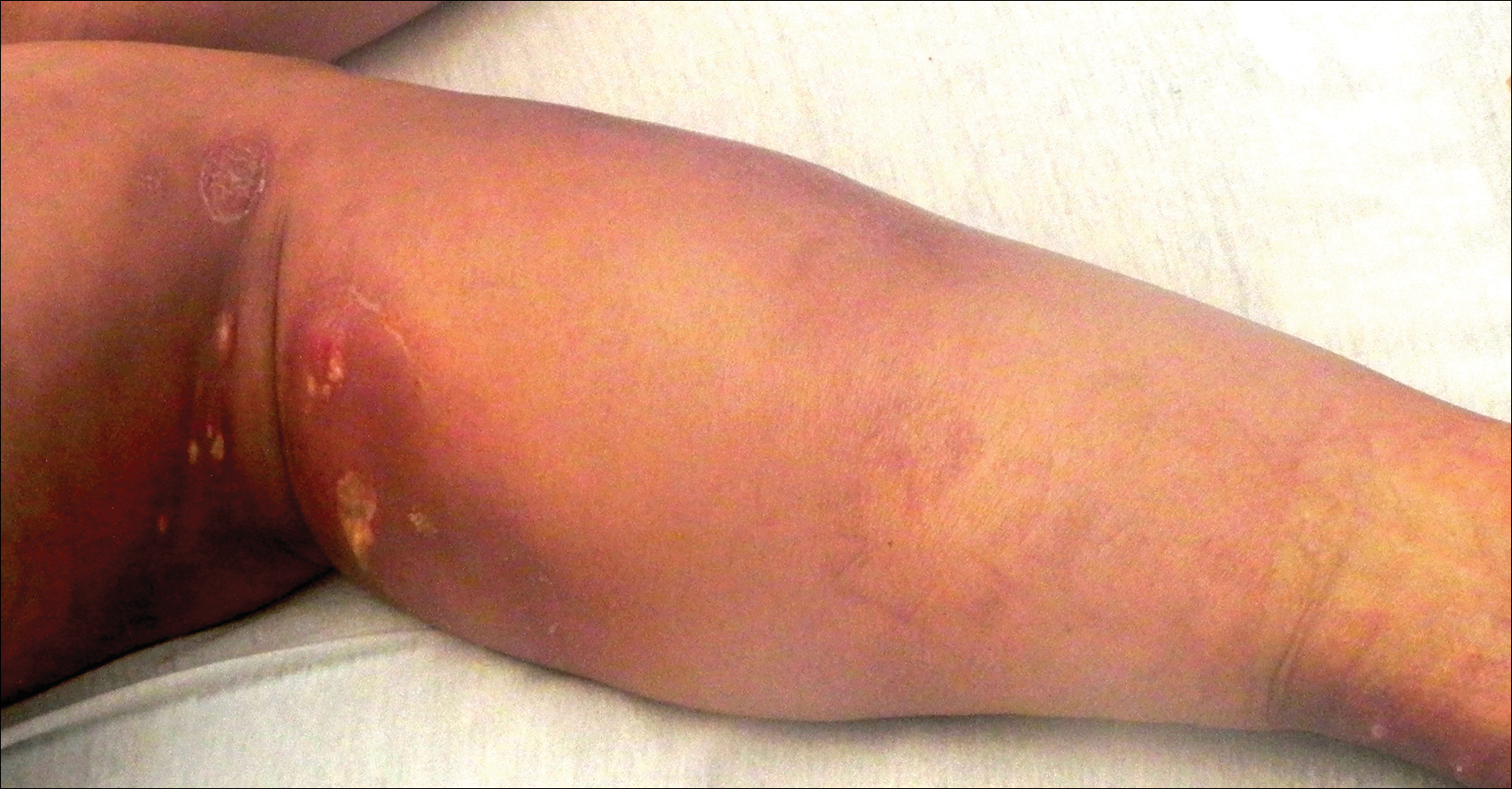
A woman in her 50s presented with low-grade subjective intermittent fevers and painful draining ulcerations on the legs of 7 months’ duration. Her medical history was remarkable for polymyositis and interstitial lung disease managed with prednisone and mycophenolate mofetil. While living in Taiwan, she developed lower extremity abscesses and persistent fevers. The patient denied any skin injuries or exposure to animals or brackish water. Mycophenolate mofetil was discontinued, and she was treated with multiple antibiotics alone and in combination without improvement, including amoxicillin–clavulanic acid, levofloxacin, azithromycin, moxifloxacin, rifampin, rifabutin, and ethambutol. She returned to the United States for evaluation. Physical examination revealed ulcerations with purulent drainage and interconnected sinus tracts with rare fluctuant nodules along the right leg. A single similar lesion was present on the right chest wall. There was no clinical evidence of disseminated disease.
Product News: 08 2016
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Impact of Acne Vulgaris on Quality of Life and Self-esteem
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.
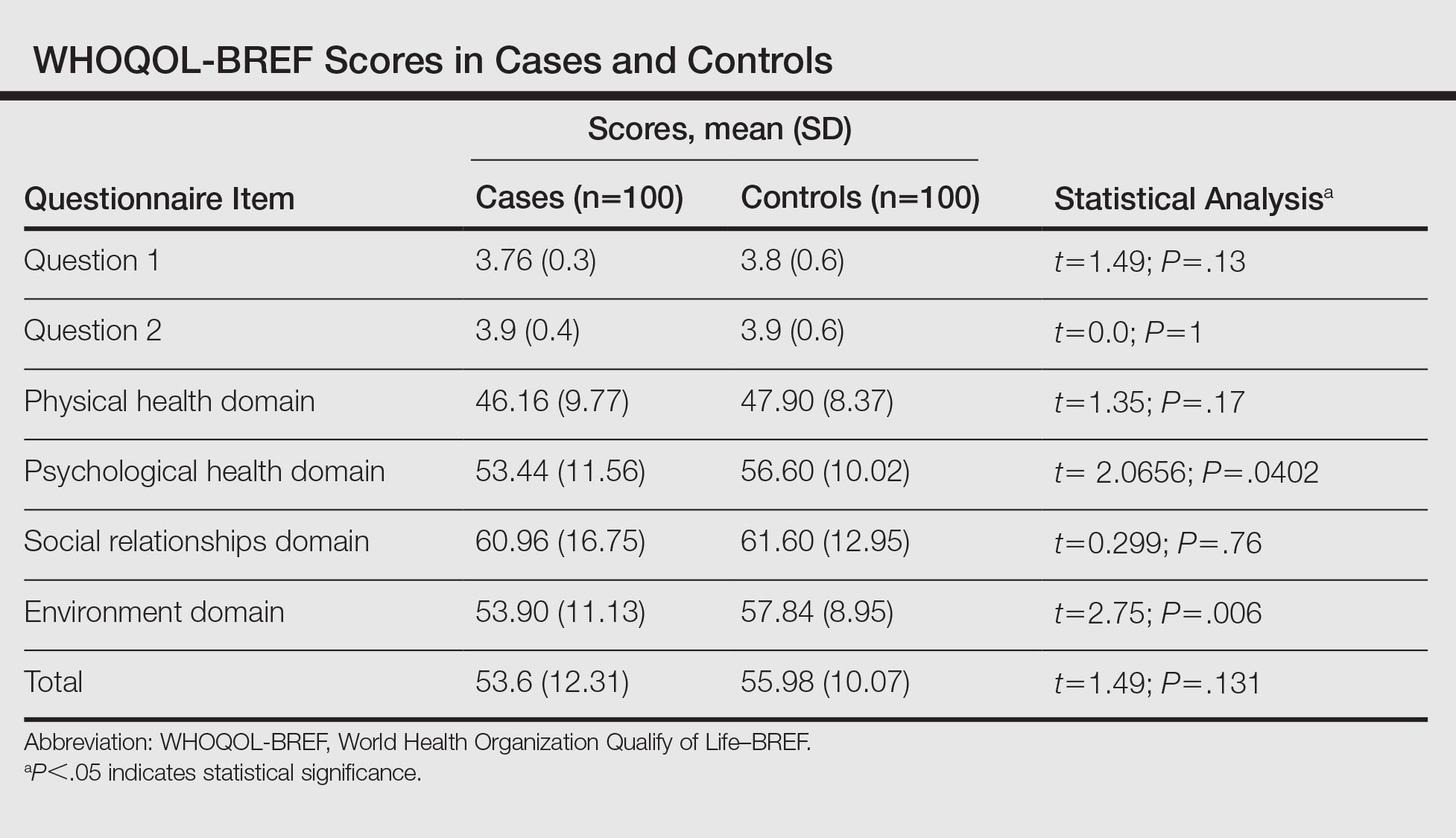
The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.

The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.

The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
Practice Points
- Grading of acne will help with appropriate treatment, thus reducing the adverse psychological effects of the condition.
- Acne severity has a negative impact on quality of life and self-esteem.
- A sympathetic approach and basic psychosomatic treatment are necessary in the management of acne.
Younger age, inconsistency of care affect antiviral adherence in hepatitis B
Patients with chronic hepatitis B who are aged under 35 years or do not see the same clinician consistently are more likely to not take their antiviral medication, finds the largest study of its kind to date.
According to the authors of the research led by Nicole Allard, PhD, from the WHO collaborative Centre for Viral Hepatitis and the Peter Doherty Institute for Infection and Immunity in Melbourne, their findings illustrate the need for clinicians to reinforce the importance of medication adherence at each patient consultation.
Furthermore, “providing more flexible and convenient care arrangements, reminder systems, and working with the individual to maximize medication adherence should be a routine part of clinical practice,” they wrote in their paper published in the Journal of Viral Hepatitis. Previous research had shown that adherence to antiviral therapy in the treatment of hepatitis B was associated with improved patient outcomes. Suboptimal adherence could lead to antiviral resistance or antiviral breakthrough, potentially leading to liver cirrhosis and liver cancer.
Understanding poor adherence in clinical settings, together with the factors associated with lower adherence, was important in informing efforts for promoting adherence, the research team said (J Viral Hepat. 2016. doi: 10.1111/jvh.12582).
In their retrospective study they assessed the pharmacy records of 1,026 patients who were dispensed antiviral therapy for hepatitis B across four Australian public hospital outpatient clinics between 2010 and 2013.
They discovered that one in five patients were “nonadherent” to therapy based on a medication possession ratio of less than 0.90 – a cutoff shown in previous studies to be correlated with virologic breakthrough.
One significant factor that affected adherence to medication was being aged under 35 years (P = .002), the research team reported.
This finding was similar to other studies of chronic diseases that had shown younger patients were at a higher risk of nonadherence, the authors noted.
“Recognition of the challenges faced by a younger person regularly taking medication at a time in their lives when they feel well is important,” they wrote.
Poor adherence was also associated with seeing multiple clinicians over shorter periods of time.
“While hospitals accommodate trainees and staffing changes occur regularly, seeking to maximize the consistency of clinical care delivery may be an important strategy to support better adherence and optimizing care for individuals,” they said.
Factors such as variations in the amount of medication, duration of treatment, sex, and cultural background did not affect adherence to medication.
“Adherence is a dynamic process and needs to be addressed regularly in a nonjudgmental way working with individuals towards improved health,” they concluded.
Patients with chronic hepatitis B who are aged under 35 years or do not see the same clinician consistently are more likely to not take their antiviral medication, finds the largest study of its kind to date.
According to the authors of the research led by Nicole Allard, PhD, from the WHO collaborative Centre for Viral Hepatitis and the Peter Doherty Institute for Infection and Immunity in Melbourne, their findings illustrate the need for clinicians to reinforce the importance of medication adherence at each patient consultation.
Furthermore, “providing more flexible and convenient care arrangements, reminder systems, and working with the individual to maximize medication adherence should be a routine part of clinical practice,” they wrote in their paper published in the Journal of Viral Hepatitis. Previous research had shown that adherence to antiviral therapy in the treatment of hepatitis B was associated with improved patient outcomes. Suboptimal adherence could lead to antiviral resistance or antiviral breakthrough, potentially leading to liver cirrhosis and liver cancer.
Understanding poor adherence in clinical settings, together with the factors associated with lower adherence, was important in informing efforts for promoting adherence, the research team said (J Viral Hepat. 2016. doi: 10.1111/jvh.12582).
In their retrospective study they assessed the pharmacy records of 1,026 patients who were dispensed antiviral therapy for hepatitis B across four Australian public hospital outpatient clinics between 2010 and 2013.
They discovered that one in five patients were “nonadherent” to therapy based on a medication possession ratio of less than 0.90 – a cutoff shown in previous studies to be correlated with virologic breakthrough.
One significant factor that affected adherence to medication was being aged under 35 years (P = .002), the research team reported.
This finding was similar to other studies of chronic diseases that had shown younger patients were at a higher risk of nonadherence, the authors noted.
“Recognition of the challenges faced by a younger person regularly taking medication at a time in their lives when they feel well is important,” they wrote.
Poor adherence was also associated with seeing multiple clinicians over shorter periods of time.
“While hospitals accommodate trainees and staffing changes occur regularly, seeking to maximize the consistency of clinical care delivery may be an important strategy to support better adherence and optimizing care for individuals,” they said.
Factors such as variations in the amount of medication, duration of treatment, sex, and cultural background did not affect adherence to medication.
“Adherence is a dynamic process and needs to be addressed regularly in a nonjudgmental way working with individuals towards improved health,” they concluded.
Patients with chronic hepatitis B who are aged under 35 years or do not see the same clinician consistently are more likely to not take their antiviral medication, finds the largest study of its kind to date.
According to the authors of the research led by Nicole Allard, PhD, from the WHO collaborative Centre for Viral Hepatitis and the Peter Doherty Institute for Infection and Immunity in Melbourne, their findings illustrate the need for clinicians to reinforce the importance of medication adherence at each patient consultation.
Furthermore, “providing more flexible and convenient care arrangements, reminder systems, and working with the individual to maximize medication adherence should be a routine part of clinical practice,” they wrote in their paper published in the Journal of Viral Hepatitis. Previous research had shown that adherence to antiviral therapy in the treatment of hepatitis B was associated with improved patient outcomes. Suboptimal adherence could lead to antiviral resistance or antiviral breakthrough, potentially leading to liver cirrhosis and liver cancer.
Understanding poor adherence in clinical settings, together with the factors associated with lower adherence, was important in informing efforts for promoting adherence, the research team said (J Viral Hepat. 2016. doi: 10.1111/jvh.12582).
In their retrospective study they assessed the pharmacy records of 1,026 patients who were dispensed antiviral therapy for hepatitis B across four Australian public hospital outpatient clinics between 2010 and 2013.
They discovered that one in five patients were “nonadherent” to therapy based on a medication possession ratio of less than 0.90 – a cutoff shown in previous studies to be correlated with virologic breakthrough.
One significant factor that affected adherence to medication was being aged under 35 years (P = .002), the research team reported.
This finding was similar to other studies of chronic diseases that had shown younger patients were at a higher risk of nonadherence, the authors noted.
“Recognition of the challenges faced by a younger person regularly taking medication at a time in their lives when they feel well is important,” they wrote.
Poor adherence was also associated with seeing multiple clinicians over shorter periods of time.
“While hospitals accommodate trainees and staffing changes occur regularly, seeking to maximize the consistency of clinical care delivery may be an important strategy to support better adherence and optimizing care for individuals,” they said.
Factors such as variations in the amount of medication, duration of treatment, sex, and cultural background did not affect adherence to medication.
“Adherence is a dynamic process and needs to be addressed regularly in a nonjudgmental way working with individuals towards improved health,” they concluded.
FROM THE JOURNAL OF VIRAL HEPATITIS
Key clinical point: Adherence to antiviral medication is a dynamic process that clinicians need to address regularly in a nonjudgmental way at each consultation, particularly in younger patients. Providing flexible and convenient care arrangements that foster continuity of care will also improve adherence to medication.
Major finding: One in five patients with hepatitis B were nonadherent to antiviral medications. Younger age (under 35) (P = .002), and inconsistency of care predicted nonadherence.
Data Source: A retrospective study of the pharmacy records of 1,026 patients who were attending a public hospital clinic and were prescribed antiviral therapy for hepatitis B.
Disclosures: Dr. Nicole Allard is supported by an APA scholarship from the University of Melbourne. Funding for the project was provided by the Royal Melbourne Hospital lottery grant. Dr. Allard declared no conflicts of interest in the last 3 years. Several of the authors in the group reported financial ties to pharmaceutical companies.