User login
Therapy granted orphan designation to prevent GVHD
Image from PLOS ONE
The US Food and Drug Administration (FDA) has granted orphan drug designation to a programmed cellular immunotherapy known as ProTmune™.
The designation is for ProTmune to be used as graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
This indication covers a range of diseases, including hematologic malignancies and genetic disorders.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
About ProTmune
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood graft ex vivo with 2 small molecules—FT1050 and FT4145—to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mobilized peripheral blood graft is administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing allogeneic HSCT.
ProTmune was previously granted fast track designation from the FDA.
“The granting of both orphan drug and fast track designations for ProTmune validates the product candidate’s unique therapeutic potential to address life-threatening complications and improve the curative potential of allogeneic [HSCT],” said Scott Wolchko, president and chief executive officer of Fate Therapeutics.
“Graft-versus-host disease is a significant cause of morbidity and mortality in patients undergoing allogeneic [HSCT], and there are no FDA-approved therapies to prevent its occurrence. Through our development of ProTmune, we seek to transform the allogeneic [HSCT] paradigm by providing immunocompromised patients a therapeutically optimized donor graft containing immune cells with reduced alloreactivity and enhanced infection-fighting and anti-tumor properties.” ![]()

Image from PLOS ONE
The US Food and Drug Administration (FDA) has granted orphan drug designation to a programmed cellular immunotherapy known as ProTmune™.
The designation is for ProTmune to be used as graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
This indication covers a range of diseases, including hematologic malignancies and genetic disorders.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
About ProTmune
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood graft ex vivo with 2 small molecules—FT1050 and FT4145—to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mobilized peripheral blood graft is administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing allogeneic HSCT.
ProTmune was previously granted fast track designation from the FDA.
“The granting of both orphan drug and fast track designations for ProTmune validates the product candidate’s unique therapeutic potential to address life-threatening complications and improve the curative potential of allogeneic [HSCT],” said Scott Wolchko, president and chief executive officer of Fate Therapeutics.
“Graft-versus-host disease is a significant cause of morbidity and mortality in patients undergoing allogeneic [HSCT], and there are no FDA-approved therapies to prevent its occurrence. Through our development of ProTmune, we seek to transform the allogeneic [HSCT] paradigm by providing immunocompromised patients a therapeutically optimized donor graft containing immune cells with reduced alloreactivity and enhanced infection-fighting and anti-tumor properties.” ![]()

Image from PLOS ONE
The US Food and Drug Administration (FDA) has granted orphan drug designation to a programmed cellular immunotherapy known as ProTmune™.
The designation is for ProTmune to be used as graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
This indication covers a range of diseases, including hematologic malignancies and genetic disorders.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
About ProTmune
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood graft ex vivo with 2 small molecules—FT1050 and FT4145—to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mobilized peripheral blood graft is administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing allogeneic HSCT.
ProTmune was previously granted fast track designation from the FDA.
“The granting of both orphan drug and fast track designations for ProTmune validates the product candidate’s unique therapeutic potential to address life-threatening complications and improve the curative potential of allogeneic [HSCT],” said Scott Wolchko, president and chief executive officer of Fate Therapeutics.
“Graft-versus-host disease is a significant cause of morbidity and mortality in patients undergoing allogeneic [HSCT], and there are no FDA-approved therapies to prevent its occurrence. Through our development of ProTmune, we seek to transform the allogeneic [HSCT] paradigm by providing immunocompromised patients a therapeutically optimized donor graft containing immune cells with reduced alloreactivity and enhanced infection-fighting and anti-tumor properties.” ![]()
Cells might protect cancer patients from infection

receiving chemotherapy
Photo by Rhoda Baer
Researchers say they have discovered a type of macrophage that may protect against lung infections during chemotherapy.
These macrophages, found in the lungs of mice, were able to survive chemotherapy.
The macrophages could remove bacteria when activated by a vaccine, which improved survival in mice with lethal bacterial pneumonia that had received chemotherapy and were therefore depleted of neutrophils.
The researchers said these results suggest the cells—known as vaccine-induced macrophages (ViMs)— might be able to protect cancer patients from life-threatening infections.
“We have identified a new form of housekeeping macrophage in mice that may, in future, be harnessed to protect against lung infections—like bacterial pneumonia—that remain one of the greatest threats to survival of cancer patients during chemotherapy,” said Peter Murray, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Murray and his colleagues detailed this discovery in PNAS.
Working in a mouse model that mimics infection in chemotherapy-treated patients, the researchers found that vaccination protected mice from Pseudomonas aeruginosa pneumonia.
The quest to understand how such protection was possible in the absence of neutrophils led the team to discover ViMs.
The researchers found that ViMs were produced in the lungs following vaccination, proliferated locally, and could persist for at least a month.
Analyses suggested ViMs are closely related to alveolar macrophages, which originate in the embryo, reside in the air-exposed surfaces of alveoli, and are self-maintained in adults.
“All lines of cellular and molecular evidence in this study point to alveolar macrophages as the source of ViMs,” Dr Murray said.
However, unlike alveolar macrophages, the population of ViMs remained stable during chemotherapy and exhibited enhanced phagocytic activity.
When ViMs were transferred to unvaccinated mice depleted of neutrophils via chemotherapy, the animals were protected from lethal Pseudomonas infections.
“We now know that increasing the number of ViMs in the tissue can compensate for the immune deficit caused by chemotherapy,” said study author Akinobu Kamei, MD, of St. Jude Children’s Research Hospital.
“In this study, we relied on vaccination prior to chemotherapy. Going forward, we will explore other, more practical methods for use at the bedside to effectively induce tissue-resident macrophages like ViMs.”
The possible approaches include using drugs or cytokines to induce protection in the immune-compromised host. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers say they have discovered a type of macrophage that may protect against lung infections during chemotherapy.
These macrophages, found in the lungs of mice, were able to survive chemotherapy.
The macrophages could remove bacteria when activated by a vaccine, which improved survival in mice with lethal bacterial pneumonia that had received chemotherapy and were therefore depleted of neutrophils.
The researchers said these results suggest the cells—known as vaccine-induced macrophages (ViMs)— might be able to protect cancer patients from life-threatening infections.
“We have identified a new form of housekeeping macrophage in mice that may, in future, be harnessed to protect against lung infections—like bacterial pneumonia—that remain one of the greatest threats to survival of cancer patients during chemotherapy,” said Peter Murray, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Murray and his colleagues detailed this discovery in PNAS.
Working in a mouse model that mimics infection in chemotherapy-treated patients, the researchers found that vaccination protected mice from Pseudomonas aeruginosa pneumonia.
The quest to understand how such protection was possible in the absence of neutrophils led the team to discover ViMs.
The researchers found that ViMs were produced in the lungs following vaccination, proliferated locally, and could persist for at least a month.
Analyses suggested ViMs are closely related to alveolar macrophages, which originate in the embryo, reside in the air-exposed surfaces of alveoli, and are self-maintained in adults.
“All lines of cellular and molecular evidence in this study point to alveolar macrophages as the source of ViMs,” Dr Murray said.
However, unlike alveolar macrophages, the population of ViMs remained stable during chemotherapy and exhibited enhanced phagocytic activity.
When ViMs were transferred to unvaccinated mice depleted of neutrophils via chemotherapy, the animals were protected from lethal Pseudomonas infections.
“We now know that increasing the number of ViMs in the tissue can compensate for the immune deficit caused by chemotherapy,” said study author Akinobu Kamei, MD, of St. Jude Children’s Research Hospital.
“In this study, we relied on vaccination prior to chemotherapy. Going forward, we will explore other, more practical methods for use at the bedside to effectively induce tissue-resident macrophages like ViMs.”
The possible approaches include using drugs or cytokines to induce protection in the immune-compromised host. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers say they have discovered a type of macrophage that may protect against lung infections during chemotherapy.
These macrophages, found in the lungs of mice, were able to survive chemotherapy.
The macrophages could remove bacteria when activated by a vaccine, which improved survival in mice with lethal bacterial pneumonia that had received chemotherapy and were therefore depleted of neutrophils.
The researchers said these results suggest the cells—known as vaccine-induced macrophages (ViMs)— might be able to protect cancer patients from life-threatening infections.
“We have identified a new form of housekeeping macrophage in mice that may, in future, be harnessed to protect against lung infections—like bacterial pneumonia—that remain one of the greatest threats to survival of cancer patients during chemotherapy,” said Peter Murray, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr Murray and his colleagues detailed this discovery in PNAS.
Working in a mouse model that mimics infection in chemotherapy-treated patients, the researchers found that vaccination protected mice from Pseudomonas aeruginosa pneumonia.
The quest to understand how such protection was possible in the absence of neutrophils led the team to discover ViMs.
The researchers found that ViMs were produced in the lungs following vaccination, proliferated locally, and could persist for at least a month.
Analyses suggested ViMs are closely related to alveolar macrophages, which originate in the embryo, reside in the air-exposed surfaces of alveoli, and are self-maintained in adults.
“All lines of cellular and molecular evidence in this study point to alveolar macrophages as the source of ViMs,” Dr Murray said.
However, unlike alveolar macrophages, the population of ViMs remained stable during chemotherapy and exhibited enhanced phagocytic activity.
When ViMs were transferred to unvaccinated mice depleted of neutrophils via chemotherapy, the animals were protected from lethal Pseudomonas infections.
“We now know that increasing the number of ViMs in the tissue can compensate for the immune deficit caused by chemotherapy,” said study author Akinobu Kamei, MD, of St. Jude Children’s Research Hospital.
“In this study, we relied on vaccination prior to chemotherapy. Going forward, we will explore other, more practical methods for use at the bedside to effectively induce tissue-resident macrophages like ViMs.”
The possible approaches include using drugs or cytokines to induce protection in the immune-compromised host. ![]()
Preserved slides provide insight into European malaria

Photo from the Institute
of Evolutionary Biology
A new study published in PNAS has provided insights regarding the origin and spread of European malaria.
The malaria-causing parasites Plasmodium vivax and Plasmodium falciparum were eradicated in Europe in the mid-twentieth century.
Now, researchers have recovered genetic data from European samples of malaria preserved on microscope slides in the 1940s.
The team performed second-generation sequencing on DNA extracted from 3 of the slides, which generated millions of sequences of malaria-causing parasites.
The researchers were then able to reconstruct the parasites’ mitochondrial genomes and compare them with those of present-day samples worldwide.
“The European sequence of P vivax is closely related to the most common strain currently found in Central and South America,” said study author Carles Lalueza-Fox, PhD, of the Institute of Evolutionary Biology (Consejo Superior de Investigaciones Científicas-Universitat Pompeu Fabra) in Barcelona, Spain.
“This suggests that the pathogen was introduced to the Americas by European colonists after Columbus. In contrast, the European sequence of P falciparum belongs to a strain which has only been found in India. This indicates that the pathogen of the most severe form of malaria was introduced to Europe from the Indian subcontinent, probably some 2500 years ago.”
The European samples the researchers analyzed were dated between 1942 and 1944. They originate from an old antimalarial center inaugurated in 1925 in Sant Jaume d’Enveja, on the Ebro Delta, located in Spain’s north-eastern region of Tarragona.
The center’s head, Ildefonso Canicio, spent decades treating malaria sufferers who worked in the area’s rice fields and ultimately contracted the disease himself.
Following Dr Canicio’s death in 1961, some of his slides, which were used for diagnostic purposes, were saved from destruction when they were recognized by his descendants, who allowed them to be used in the current study.
“It is still possible to see malaria-carrying parasites on the slides when they are studied under the microscope,” Dr Lalueza-Fox said. “However, the quantity of the pathogen’s DNA available in a single drop of blood is very limited, and when you add to that the issue of poor preservation after 70 years, it is clear why this type of study has never been carried out.”
Still, the researchers said this study has shown that historic specimens can be an important source of insight into the genetics of extinct or eradicated pathogens.
“Analyzing the nuclear genome in these pathogens will allow us to know more about the mutations which have made current-day strains resistant to different drugs, given that the European Plasmodium which has been retrieved is older than all of these treatments,” said study author Pere Gelabert, also of the Institute of Evolutionary Biology. ![]()

Photo from the Institute
of Evolutionary Biology
A new study published in PNAS has provided insights regarding the origin and spread of European malaria.
The malaria-causing parasites Plasmodium vivax and Plasmodium falciparum were eradicated in Europe in the mid-twentieth century.
Now, researchers have recovered genetic data from European samples of malaria preserved on microscope slides in the 1940s.
The team performed second-generation sequencing on DNA extracted from 3 of the slides, which generated millions of sequences of malaria-causing parasites.
The researchers were then able to reconstruct the parasites’ mitochondrial genomes and compare them with those of present-day samples worldwide.
“The European sequence of P vivax is closely related to the most common strain currently found in Central and South America,” said study author Carles Lalueza-Fox, PhD, of the Institute of Evolutionary Biology (Consejo Superior de Investigaciones Científicas-Universitat Pompeu Fabra) in Barcelona, Spain.
“This suggests that the pathogen was introduced to the Americas by European colonists after Columbus. In contrast, the European sequence of P falciparum belongs to a strain which has only been found in India. This indicates that the pathogen of the most severe form of malaria was introduced to Europe from the Indian subcontinent, probably some 2500 years ago.”
The European samples the researchers analyzed were dated between 1942 and 1944. They originate from an old antimalarial center inaugurated in 1925 in Sant Jaume d’Enveja, on the Ebro Delta, located in Spain’s north-eastern region of Tarragona.
The center’s head, Ildefonso Canicio, spent decades treating malaria sufferers who worked in the area’s rice fields and ultimately contracted the disease himself.
Following Dr Canicio’s death in 1961, some of his slides, which were used for diagnostic purposes, were saved from destruction when they were recognized by his descendants, who allowed them to be used in the current study.
“It is still possible to see malaria-carrying parasites on the slides when they are studied under the microscope,” Dr Lalueza-Fox said. “However, the quantity of the pathogen’s DNA available in a single drop of blood is very limited, and when you add to that the issue of poor preservation after 70 years, it is clear why this type of study has never been carried out.”
Still, the researchers said this study has shown that historic specimens can be an important source of insight into the genetics of extinct or eradicated pathogens.
“Analyzing the nuclear genome in these pathogens will allow us to know more about the mutations which have made current-day strains resistant to different drugs, given that the European Plasmodium which has been retrieved is older than all of these treatments,” said study author Pere Gelabert, also of the Institute of Evolutionary Biology. ![]()

Photo from the Institute
of Evolutionary Biology
A new study published in PNAS has provided insights regarding the origin and spread of European malaria.
The malaria-causing parasites Plasmodium vivax and Plasmodium falciparum were eradicated in Europe in the mid-twentieth century.
Now, researchers have recovered genetic data from European samples of malaria preserved on microscope slides in the 1940s.
The team performed second-generation sequencing on DNA extracted from 3 of the slides, which generated millions of sequences of malaria-causing parasites.
The researchers were then able to reconstruct the parasites’ mitochondrial genomes and compare them with those of present-day samples worldwide.
“The European sequence of P vivax is closely related to the most common strain currently found in Central and South America,” said study author Carles Lalueza-Fox, PhD, of the Institute of Evolutionary Biology (Consejo Superior de Investigaciones Científicas-Universitat Pompeu Fabra) in Barcelona, Spain.
“This suggests that the pathogen was introduced to the Americas by European colonists after Columbus. In contrast, the European sequence of P falciparum belongs to a strain which has only been found in India. This indicates that the pathogen of the most severe form of malaria was introduced to Europe from the Indian subcontinent, probably some 2500 years ago.”
The European samples the researchers analyzed were dated between 1942 and 1944. They originate from an old antimalarial center inaugurated in 1925 in Sant Jaume d’Enveja, on the Ebro Delta, located in Spain’s north-eastern region of Tarragona.
The center’s head, Ildefonso Canicio, spent decades treating malaria sufferers who worked in the area’s rice fields and ultimately contracted the disease himself.
Following Dr Canicio’s death in 1961, some of his slides, which were used for diagnostic purposes, were saved from destruction when they were recognized by his descendants, who allowed them to be used in the current study.
“It is still possible to see malaria-carrying parasites on the slides when they are studied under the microscope,” Dr Lalueza-Fox said. “However, the quantity of the pathogen’s DNA available in a single drop of blood is very limited, and when you add to that the issue of poor preservation after 70 years, it is clear why this type of study has never been carried out.”
Still, the researchers said this study has shown that historic specimens can be an important source of insight into the genetics of extinct or eradicated pathogens.
“Analyzing the nuclear genome in these pathogens will allow us to know more about the mutations which have made current-day strains resistant to different drugs, given that the European Plasmodium which has been retrieved is older than all of these treatments,” said study author Pere Gelabert, also of the Institute of Evolutionary Biology. ![]()
EMA and FDA collaborate to combat rare diseases

Photo courtesy of the FDA
The European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have set up a new working group, or “cluster,” on rare diseases.
This means the EMA and FDA will hold regular meetings via teleconference to share information on the regulation of medicines for rare diseases.
The cluster will provide a forum for the confidential exchange of draft documents, proposed policies, and detailed information supporting the scientific basis for decision-making on medicine development.
The agencies will exchange information on topics such as:
- The design of clinical trials in small populations and the use of statistical analysis methods
- The selection and validation of trial endpoints
- Preclinical evidence to support development programs
- The design of post-marketing studies—in particular, in the context of early access mechanisms such as the EMA’s conditional marketing authorization and the FDA’s accelerated approval
- Risk management strategies for long-term safety issues with medicines for rare diseases.
The first meeting of the rare diseases cluster took place on September 23, 2016. The cluster will initially meet once a month via teleconference and will be chaired jointly by the FDA and EMA.
The creation of this cluster is the latest step in the EMA’s and FDA’s wider objective to expand and reinforce international collaboration.
The clusters established by the agencies focus on areas where the parties involved could benefit from an intensified exchange of information and strengthened collaboration.
The existing EMA/FDA clusters address issues related to patient engagement, biosimilars, orphan medicines, cancer drugs, medicines for children, and pharmacovigilance, among other topics. ![]()

Photo courtesy of the FDA
The European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have set up a new working group, or “cluster,” on rare diseases.
This means the EMA and FDA will hold regular meetings via teleconference to share information on the regulation of medicines for rare diseases.
The cluster will provide a forum for the confidential exchange of draft documents, proposed policies, and detailed information supporting the scientific basis for decision-making on medicine development.
The agencies will exchange information on topics such as:
- The design of clinical trials in small populations and the use of statistical analysis methods
- The selection and validation of trial endpoints
- Preclinical evidence to support development programs
- The design of post-marketing studies—in particular, in the context of early access mechanisms such as the EMA’s conditional marketing authorization and the FDA’s accelerated approval
- Risk management strategies for long-term safety issues with medicines for rare diseases.
The first meeting of the rare diseases cluster took place on September 23, 2016. The cluster will initially meet once a month via teleconference and will be chaired jointly by the FDA and EMA.
The creation of this cluster is the latest step in the EMA’s and FDA’s wider objective to expand and reinforce international collaboration.
The clusters established by the agencies focus on areas where the parties involved could benefit from an intensified exchange of information and strengthened collaboration.
The existing EMA/FDA clusters address issues related to patient engagement, biosimilars, orphan medicines, cancer drugs, medicines for children, and pharmacovigilance, among other topics. ![]()

Photo courtesy of the FDA
The European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have set up a new working group, or “cluster,” on rare diseases.
This means the EMA and FDA will hold regular meetings via teleconference to share information on the regulation of medicines for rare diseases.
The cluster will provide a forum for the confidential exchange of draft documents, proposed policies, and detailed information supporting the scientific basis for decision-making on medicine development.
The agencies will exchange information on topics such as:
- The design of clinical trials in small populations and the use of statistical analysis methods
- The selection and validation of trial endpoints
- Preclinical evidence to support development programs
- The design of post-marketing studies—in particular, in the context of early access mechanisms such as the EMA’s conditional marketing authorization and the FDA’s accelerated approval
- Risk management strategies for long-term safety issues with medicines for rare diseases.
The first meeting of the rare diseases cluster took place on September 23, 2016. The cluster will initially meet once a month via teleconference and will be chaired jointly by the FDA and EMA.
The creation of this cluster is the latest step in the EMA’s and FDA’s wider objective to expand and reinforce international collaboration.
The clusters established by the agencies focus on areas where the parties involved could benefit from an intensified exchange of information and strengthened collaboration.
The existing EMA/FDA clusters address issues related to patient engagement, biosimilars, orphan medicines, cancer drugs, medicines for children, and pharmacovigilance, among other topics. ![]()
Can TEE find septal defects in conotruncal repair?
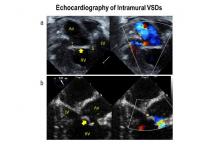
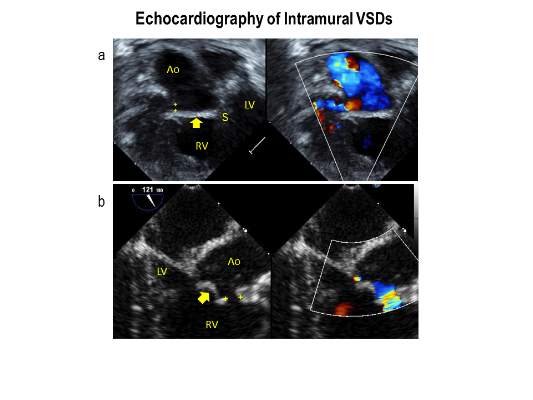
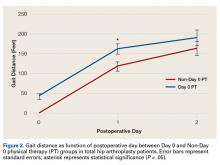
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Intraoperative transesophageal echocardiography has modest sensitivity but high specificity for detecting ventricular septal defects after repair of conotruncal anomalies.
Major finding: TEE is useful for identifying most VSDs during the index operation, providing the opportunity to repair the defects during the index operation.
Data source: A single-institution database of 337 patients who had operations to repair conotruncal anomalies between January 2006 and June 2013.
Disclosures: Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Transcatheter mitral valve therapy at ‘event horizon’
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
Channeling Bob Dylan’s “The Times They Are A-Changin’” in his invited commentary, W. Randolph Chitwood Jr., MD, of East Carolina University in Greenville, N.C., called Dr. Badhwar’s expert opinion “the clarion call for cardiac surgeons to become engaged in this rapidly evolving parade.”

|
Dr. W. Randolph Chitwood Jr. |
The evidence supporting the safety and efficacy of transcatheter aortic valve replacement (TAVR) is already strong, Dr. Chitwood noted. “It seems reasonable to suspect that the evolving pathway for the development of transcatheter mitral valve replacement (TMVR) could recapitulate the success of TAVR, with each generation having improved results,” he said (J Thorac Cardiovasc Surg. 2016;152:336-7).
Cardiac surgeons need to develop alternate access platforms and acquire the skills to use the new generation of transcatheter mitral devices, Dr. Chitwood said. The expert opinion “should encourage cardiac surgeons to become members of a heart team,” he said. “Guidewire skills are at the pinnacle of necessity to remain a player in this new world.”
Dr. Chitwood’s advice to colleagues: “Then you better start swimming or you’ll sink like a stone, For the times they are a-changin’.”
Dr. Chitwood disclosed he is a consultant to Direct Flow Medical and co-principal investigator for the Edwards Lifesciences Transform Trial.
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
As investigational transcatheter mitral valve therapies continue to explode onto the scene, cardiac surgeons must act now to seize and assert their place in the multidisciplinary team with interventional, imaging, and heart failure colleagues to deliver these treatments to people with complex mitral valve regurgitation, an expert opinion report in the August issue of the Journal of Thoracic and Cardiovascular Surgery states (J Thorac Cardiovasc Surg. 2016;152:330-6).
“There is a growing population of patients with primary and secondary mitral regurgitation underserved by surgical therapy because of comorbid risk,” Vinay Badhwar, MD, of West Virginia University and his colleagues said. “This has led to a tremendous activity of device development.”
With more than 25 different transcatheter mitral valve devices in development (MitraClip, Abbott Vascular, is the only FDA-approved transcatheter for primary mitral regurgitation [MR]), cardiac surgeons will soon have the tools to offer transcatheter mitral valve repair (TMVr) and transcatheter mitral valve replacement (TMVR) to more complex patients who have MR along with other health problems. Today about half of those patients do not get surgery because they are too frail, Dr. Badhwar and his colleagues said.
The authors used the astrophysical phrase “event horizon” to define the current state of transcatheter mitral valve therapies – “a point of no return.” They expect surgery to remain the treatment of choice for MR for the next 10 years. “However, as our patient cohorts become increasingly more complex and transcatheter mitral therapies more facile, the day when this will become a daily clinical reality will soon be upon us,” Dr. Badhwar and his colleagues said.
The multidisciplinary team approach will be integral in achieving the full potential of transcatheter mitral valve replacement or repair, Dr. Badhwar and his coauthors said. While surgery is the most effective treatment for primary MR, cardiac surgeons are challenged to introduce transcatheter treatments in patients who have other health problems. “The best way to adjudicate innovative surgical and interventional mitral therapies is through a robust collaboration within a well-functioning heart team that includes not only a cardiac surgeon and interventional cardiologist but also an imaging specialist,” the authors said.
The time to reach out to those other specialties is now, before those investigational devices start emerging from the development pipeline, Dr. Badhwar and his colleagues said. “This will soon enable the team-based mitral specialist to be facile in safely transitioning patients from open mitral surgery to TMVr or TMVR as most appropriate for durable long-term outcomes.”
Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign, and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Transcatheter mitral valve repair and replacement technology has reached a critical point that requires cardiac surgeons to assume their place in a multidisciplinary team.
Major finding: One transcatheter device is commercially available in the United States and more than 25 companies have devices in development.
Data source: Review of 22 published reports on transcatheter mitral valve technology.
Disclosures: Dr. Badhwar disclosed he is an uncompensated member of the Abbott Vascular advisory board. Coauthor Vinod Thourani, MD, disclosed relationships with Edwards Lifesciences, Medtronic Cardiovascular, Abbott Vascular, St. Jude Medical, Mitralign and AtriCure. Coauthor Michael Mack, MD, serves on the Edwards Lifesciences steering committee Partner Trial, and is an uncompensated co-principal investigator of the Abbott Vascular Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy Trial.
Case Study - Partial Epilepsy With and Without Secondary Generalization
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health System, in Royal Oak, Michigan.
A 55-year-old woman with a past medical history of hypertension was brought to a local emergency department by ambulance after a co-worker noticed her repeating the phrase “I have to close the store, I have to close the store” to herself after the store was already closed. The patient did not recall this time period.
In the emergency room a drug screen was negative and a computed tomography (CT) scan of the head was negative. A routine EEG was normal. A diagnosis of a seizure was made and the patient was started on levetiracetam (Keppra) 1000 mg PO q12hrs.
The patient had a consultation with an epileptologist the following week. The consultation revealed that the patient family noticed that she had periods where she would stare, her speech would not make sense, and it would appear as though she was “chewing something.” The patient denied such symptoms. The patient’s family believed these symptoms had been present for at least 5 years, but thought they were due to tiredness. When questioned, the patient recalled at least 2 instances where she has fallen asleep in bed and woken up on the floor.
When asked about potential head trauma, the patient stated she was involved in a car accident 7 months ago, but the cause was not determined. The patient was driving, there was no inclement weather, and her car veered off the road and into the ditch. The patient was not intoxicated. She was not injured as a result of the accident.
Diagnosis: partial epilepsy with and without secondary generalization.
Questions and Discussion:
- Although the patient only recently received an official diagnosis of seizure, it is likely that she was having seizures for many years (at least 5 years per history).
- Because of the long patient history with seizures, the more accurate diagnosis for this patient is complex partial epilepsy with secondary generalization.
- Does a normal routine EEG change the diagnosis?
- No. An EEG can be normal in a patient with epilepsy.
- What further testing should be ordered?
- The standard of care is to use dedicated magnetic resonance imaging (MRI) testing MRI-protocol for epilepsy.
- What was the likely cause of the car accident?
- A seizure was the likely cause of the car accident.
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health System, in Royal Oak, Michigan.
A 55-year-old woman with a past medical history of hypertension was brought to a local emergency department by ambulance after a co-worker noticed her repeating the phrase “I have to close the store, I have to close the store” to herself after the store was already closed. The patient did not recall this time period.
In the emergency room a drug screen was negative and a computed tomography (CT) scan of the head was negative. A routine EEG was normal. A diagnosis of a seizure was made and the patient was started on levetiracetam (Keppra) 1000 mg PO q12hrs.
The patient had a consultation with an epileptologist the following week. The consultation revealed that the patient family noticed that she had periods where she would stare, her speech would not make sense, and it would appear as though she was “chewing something.” The patient denied such symptoms. The patient’s family believed these symptoms had been present for at least 5 years, but thought they were due to tiredness. When questioned, the patient recalled at least 2 instances where she has fallen asleep in bed and woken up on the floor.
When asked about potential head trauma, the patient stated she was involved in a car accident 7 months ago, but the cause was not determined. The patient was driving, there was no inclement weather, and her car veered off the road and into the ditch. The patient was not intoxicated. She was not injured as a result of the accident.
Diagnosis: partial epilepsy with and without secondary generalization.
Questions and Discussion:
- Although the patient only recently received an official diagnosis of seizure, it is likely that she was having seizures for many years (at least 5 years per history).
- Because of the long patient history with seizures, the more accurate diagnosis for this patient is complex partial epilepsy with secondary generalization.
- Does a normal routine EEG change the diagnosis?
- No. An EEG can be normal in a patient with epilepsy.
- What further testing should be ordered?
- The standard of care is to use dedicated magnetic resonance imaging (MRI) testing MRI-protocol for epilepsy.
- What was the likely cause of the car accident?
- A seizure was the likely cause of the car accident.
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health System, in Royal Oak, Michigan.
A 55-year-old woman with a past medical history of hypertension was brought to a local emergency department by ambulance after a co-worker noticed her repeating the phrase “I have to close the store, I have to close the store” to herself after the store was already closed. The patient did not recall this time period.
In the emergency room a drug screen was negative and a computed tomography (CT) scan of the head was negative. A routine EEG was normal. A diagnosis of a seizure was made and the patient was started on levetiracetam (Keppra) 1000 mg PO q12hrs.
The patient had a consultation with an epileptologist the following week. The consultation revealed that the patient family noticed that she had periods where she would stare, her speech would not make sense, and it would appear as though she was “chewing something.” The patient denied such symptoms. The patient’s family believed these symptoms had been present for at least 5 years, but thought they were due to tiredness. When questioned, the patient recalled at least 2 instances where she has fallen asleep in bed and woken up on the floor.
When asked about potential head trauma, the patient stated she was involved in a car accident 7 months ago, but the cause was not determined. The patient was driving, there was no inclement weather, and her car veered off the road and into the ditch. The patient was not intoxicated. She was not injured as a result of the accident.
Diagnosis: partial epilepsy with and without secondary generalization.
Questions and Discussion:
- Although the patient only recently received an official diagnosis of seizure, it is likely that she was having seizures for many years (at least 5 years per history).
- Because of the long patient history with seizures, the more accurate diagnosis for this patient is complex partial epilepsy with secondary generalization.
- Does a normal routine EEG change the diagnosis?
- No. An EEG can be normal in a patient with epilepsy.
- What further testing should be ordered?
- The standard of care is to use dedicated magnetic resonance imaging (MRI) testing MRI-protocol for epilepsy.
- What was the likely cause of the car accident?
- A seizure was the likely cause of the car accident.
MRSA patients report signs of stigma tied to illness
About half of individuals infected with methicillin-resistant Staphylococcus aureus report feeling stigmatized in interactions with hospital staff, data from a survey of 61 adult patients show.
“Hospital care for people who carry MRSA calls for a dedicated and patient-centered approach in both the way the care is delivered ... as well as the way the care is organized at the institutional level,” wrote Babette Rump, MD, of the Regional Health Service Utrecht region, Zeist, the Netherlands, and her coauthors (J Hosp Infect. 2016. doi: 10.1016/j.jhin.2016.09.010). “Prevention of unnecessary intrusive measures, while as the same time applying appropriate precautionary measures, is key to successful and respectful MRSA management.”
Dr. Rump and her associates set out to identify and quantify stigma tied to MRSA and “explore its association with mental health within a country with a MRSA ‘search and destroy’ policy.” In the Netherlands and Scandinavian countries, this policy includes isolating MRSA carriers, wearing personal protective equipment, and disinfecting the room after patients are discharged (Antimicrob Resist Infect Control. 2014 Jan 15;3[1]3). The U.S. Centers for Disease Control and Prevention, in its 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings, recommends similar methods, including application of infection control precautions during patient care and environmental measures, such as cleaning and disinfection of the patient care environment and dedicated single-patient use of noncritical equipment.
In the current study, 60-item questionnaires were provided to all adult patients at two hospitals and two regional health services who had acquired MRSA between Oct. 1, 2013, and April 1, 2014. Stigma was assessed using the 40-item Berger HIV Stigma Scale, reported Dr. Rump.
Overall, 56% of survey respondents reported stigma, including 14% who reported clear stigma and 42% who reported suggestive stigma. The remaining 44% reported no stigma. A total of 80% of the patients received MRSA eradication treatment, which was strongly associated with higher stigma, the researchers noted.
Written comments provided by 40 patients (68%) along with the questionnaires “offer valuable insights to set the focus for improvement,” the researchers said.
The most frequent comments involved patients’ perceived organizational problems with the hospital (8 patients), lack of staff knowledge (4 patients), as well as little attention paid to patient perspectives (4 patients) and unnecessarily intrusive treatments (3 patients). Also of note, 5 patients blamed and 2 “shamed” the hospital as their source of MRSA.
The results were limited by several factors, including the small study size, the researchers wrote. However, the findings suggest that “a substantial proportion of people that carry MRSA experience signs of stigma and that anticipation on MRSA-associated stigma is warranted,” they said.
The researchers had no financial conflicts to disclose.
About half of individuals infected with methicillin-resistant Staphylococcus aureus report feeling stigmatized in interactions with hospital staff, data from a survey of 61 adult patients show.
“Hospital care for people who carry MRSA calls for a dedicated and patient-centered approach in both the way the care is delivered ... as well as the way the care is organized at the institutional level,” wrote Babette Rump, MD, of the Regional Health Service Utrecht region, Zeist, the Netherlands, and her coauthors (J Hosp Infect. 2016. doi: 10.1016/j.jhin.2016.09.010). “Prevention of unnecessary intrusive measures, while as the same time applying appropriate precautionary measures, is key to successful and respectful MRSA management.”
Dr. Rump and her associates set out to identify and quantify stigma tied to MRSA and “explore its association with mental health within a country with a MRSA ‘search and destroy’ policy.” In the Netherlands and Scandinavian countries, this policy includes isolating MRSA carriers, wearing personal protective equipment, and disinfecting the room after patients are discharged (Antimicrob Resist Infect Control. 2014 Jan 15;3[1]3). The U.S. Centers for Disease Control and Prevention, in its 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings, recommends similar methods, including application of infection control precautions during patient care and environmental measures, such as cleaning and disinfection of the patient care environment and dedicated single-patient use of noncritical equipment.
In the current study, 60-item questionnaires were provided to all adult patients at two hospitals and two regional health services who had acquired MRSA between Oct. 1, 2013, and April 1, 2014. Stigma was assessed using the 40-item Berger HIV Stigma Scale, reported Dr. Rump.
Overall, 56% of survey respondents reported stigma, including 14% who reported clear stigma and 42% who reported suggestive stigma. The remaining 44% reported no stigma. A total of 80% of the patients received MRSA eradication treatment, which was strongly associated with higher stigma, the researchers noted.
Written comments provided by 40 patients (68%) along with the questionnaires “offer valuable insights to set the focus for improvement,” the researchers said.
The most frequent comments involved patients’ perceived organizational problems with the hospital (8 patients), lack of staff knowledge (4 patients), as well as little attention paid to patient perspectives (4 patients) and unnecessarily intrusive treatments (3 patients). Also of note, 5 patients blamed and 2 “shamed” the hospital as their source of MRSA.
The results were limited by several factors, including the small study size, the researchers wrote. However, the findings suggest that “a substantial proportion of people that carry MRSA experience signs of stigma and that anticipation on MRSA-associated stigma is warranted,” they said.
The researchers had no financial conflicts to disclose.
About half of individuals infected with methicillin-resistant Staphylococcus aureus report feeling stigmatized in interactions with hospital staff, data from a survey of 61 adult patients show.
“Hospital care for people who carry MRSA calls for a dedicated and patient-centered approach in both the way the care is delivered ... as well as the way the care is organized at the institutional level,” wrote Babette Rump, MD, of the Regional Health Service Utrecht region, Zeist, the Netherlands, and her coauthors (J Hosp Infect. 2016. doi: 10.1016/j.jhin.2016.09.010). “Prevention of unnecessary intrusive measures, while as the same time applying appropriate precautionary measures, is key to successful and respectful MRSA management.”
Dr. Rump and her associates set out to identify and quantify stigma tied to MRSA and “explore its association with mental health within a country with a MRSA ‘search and destroy’ policy.” In the Netherlands and Scandinavian countries, this policy includes isolating MRSA carriers, wearing personal protective equipment, and disinfecting the room after patients are discharged (Antimicrob Resist Infect Control. 2014 Jan 15;3[1]3). The U.S. Centers for Disease Control and Prevention, in its 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings, recommends similar methods, including application of infection control precautions during patient care and environmental measures, such as cleaning and disinfection of the patient care environment and dedicated single-patient use of noncritical equipment.
In the current study, 60-item questionnaires were provided to all adult patients at two hospitals and two regional health services who had acquired MRSA between Oct. 1, 2013, and April 1, 2014. Stigma was assessed using the 40-item Berger HIV Stigma Scale, reported Dr. Rump.
Overall, 56% of survey respondents reported stigma, including 14% who reported clear stigma and 42% who reported suggestive stigma. The remaining 44% reported no stigma. A total of 80% of the patients received MRSA eradication treatment, which was strongly associated with higher stigma, the researchers noted.
Written comments provided by 40 patients (68%) along with the questionnaires “offer valuable insights to set the focus for improvement,” the researchers said.
The most frequent comments involved patients’ perceived organizational problems with the hospital (8 patients), lack of staff knowledge (4 patients), as well as little attention paid to patient perspectives (4 patients) and unnecessarily intrusive treatments (3 patients). Also of note, 5 patients blamed and 2 “shamed” the hospital as their source of MRSA.
The results were limited by several factors, including the small study size, the researchers wrote. However, the findings suggest that “a substantial proportion of people that carry MRSA experience signs of stigma and that anticipation on MRSA-associated stigma is warranted,” they said.
The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF HOSPITAL INFECTION
Key clinical point: Adults with methicillin-resistant Staphylococcus aureus (MRSA) are susceptible to stigma.
Major finding: Approximately half (56%) of adults being treated for MRSA reported stigma associated with their illness.
Data source: A cross-sectional study including 61 adults with MRSA.
Disclosures: The researchers had no financial conflicts to disclose.
A Modified Levering Technique for Removing a Broken Solid Intramedullary Tibial Nail: A Technical Tip
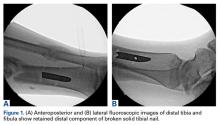
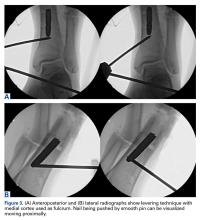
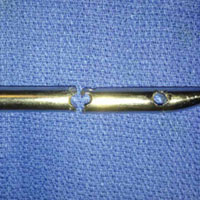
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In both elective and revision surgery, removal of retained hardware can be unpredictable. Broken hardware, whether identified before or during surgery, presents a significant challenge. Cases often require enlisting a large variety of equipment and techniques that often result in larger dissection and potential for wider soft-tissue or bony destruction. Broken intramedullary devices, located entirely within the cortices of bone, pose unique challenges.1,2 Various techniques have been used to remove broken cannulated nails.1-9 There is, however, a paucity of techniques for removing broken solid nails from within the tibia.1,2 Moreover, many of these techniques require significant metaphyseal and cortical bone destruction that may compromise the integrity of the long bone.1,3,9 In this article, we describe a modified technique for removal of a broken solid nail, with minimal cortical bone destruction, in the setting of a tibial nonunion.
Technique
A 23-year-old man presented with a symptomatic valgus nonunion of the tibia, which had been treated with a solid intramedullary 9-mm nail (Orthofix). The patient was taken to the operative theater for nonunion takedown and exchanged reamed intramedullary nailing. The proximal fragment of the anterograde intramedullary nail was removed in standard fashion using the Winquist Universal Extraction Set (Shukla Medical). When threading the extractor into the proximal aspect of the nail, we found it helpful to leave one of the cross-locks in place to prevent nail rotation.10 Inspection of the removed nail revealed a fracture of the device at the more proximal of 2 distal cross-locks (Figures 1A, 1B, 2).
To remove the distal fragment of the nail, we used a 5.0-mm smooth Steinmann pin. After cross-lock removal, the pin was placed unicortically through the distal medial cortex at the tip of the retained implant. The distal nail fragment was pushed proximally using the pin as a lever with the interposed cortical bone serving as a fulcrum (Figures 3A, 3B).
Discussion
Removal of broken solid intramedullary tibial nails presents orthopedic surgeons with a unique challenge. We have described a technique that modifies and incorporates previously described techniques while exploiting available surgical windows to facilitate hardware removal. This technique obviates the need for further bony and soft-tissue dissection, potentially mitigating surgical morbidity.
Other techniques for removing broken solid intramedullary devices have been reported. Krettek and colleagues7 described a technique in which the short distal fragment of a broken solid femoral intramedullary nail was removed with use of retrograde levering through a cortical window just proximal to the articular surface. The same window was then used for anterograde nail removal with a small Hohmann retractor serving as a guide. This technique is limited by the need for a large bony window, which potentially creates a stress riser within the distal segment. In addition, a short, distal nail fragment is required in order to facilitate manipulation through the metaphyseal bone. This technique is more readily used within the distal femur, given the large metaphyseal volume, in contrast with the distal tibial metaphysis. Giannoudis and colleagues1 described a method (for both tibia and femur) in which the intramedullary canal was proximally reamed to permit retrograde removal of an anterograde nail. The authors described reaming the canal to 4 mm larger than the nail to create access for a cleaning trephine and then a ratcheting extractor. This technique can be easily applied to the tibia or femur but requires special equipment that may not be readily available. Other retrograde techniques for the femur8 are not as suitable for the tibia, as they would cause significant chondral damage to the tibiotalar joint.
In developing our technique, which includes modifications of other methods, we used cortical windows, levering, and anterograde reaming to permit removal of a broken solid fragment through a nonunion site and with minimal additional destruction of bone. Although an existing cortical window was used, the newly created cortical window was significantly smaller than windows used in other techniques, and it avoids the articular surface. This technique can be performed with common, readily accessible equipment, which may be helpful in situations in which broken nails are encountered unexpectedly. In summary, this simple, safe, and effective technique uses standard equipment to preserve bone, decrease operative time, and alleviate surgeon frustration in complicated hardware removal surgeries.
Am J Orthop. 2016;45(6):E352-E354. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.
1. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
2. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008;16(2):113-120.
3. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
4. Dawson GR Jr, Stader RO. Extractor for removing broken stuck intramedullary nail. Am J Orthop Surg. 1968;10(6):150-151.
5. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
6. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
7. Krettek C, Schandelmaier P, Tscherne H. Removal of a broken solid femoral nail: a simple push-out technique. A case report. J Bone Joint Surg Am. 1997;79(2):247-251.
8. Milia MJ, Vincent AB, Bosse MJ. Retrograde removal of an incarcerated solid titanium femoral nail after subtrochanteric fracture. J Orthop Trauma. 2003;17(7):521-524.
9. Whalley H, Thomas G, Hull P, Porter K. Surgeon versus metalwork—tips to remove a retained intramedullary nail fragment. Injury. 2009;40(7):783-789.
10. Smith G, Khan A, Marsh A. A novel way to remove a broken intramedullary nail. Ann R Coll Surg Engl. 2012;94(8):605.
Does Accelerated Physical Therapy After Elective Primary Hip and Knee Arthroplasty Facilitate Early Discharge?
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.