User login
New and Noteworthy Information—October 2016
Resective surgery for epilepsy is cost-effective in the medium term, according to a study published online ahead of print September 5 in Epilepsia. A prospective cohort of adult patients with surgically remediable and medically intractable partial epilepsy was followed for more than five years in 15 French centers. During the second year of follow-up, the proportion of patients who had been completely seizure-free for the previous 12 months was 69.0% among participants who underwent surgery and 12.3% in the medical group. The respective rates of seizure freedom were 76.8% and 21% during the fifth year. Direct costs became significantly lower in the surgical group during the third year after surgery as a result of decreased antiepileptic drug use. Surgery became cost-effective between nine and 10 years after surgery.
The NIH Toolbox Cognitive Battery can assess important dimensions of cognition in persons with intellectual disabilities, and several tests may be useful for tracking response to interventions, according to a study published September 6 in the Journal of Neurodevelopmental Disorders. In separate pilot studies of patients with fragile X syndrome, Down syndrome, and idiopathic intellectual disabilities, researchers used the web-based NIH Toolbox Cognitive Battery to measure processing speed, executive function, episodic memory, word and letter reading, receptive vocabulary, and working memory. The test's feasibility was good to excellent for people above mental age 4 for all tests except list sorting. Test-retest stability was good to excellent. More extensive psychometric studies are needed to determine the battery's true utility as a set of outcome measures, said the researchers.
Graded aerobic treadmill testing is a safe, tolerable, and clinically valuable tool that can assist in the evaluation and management of pediatric sports-related concussion, according to a study published online ahead of print September 13 in the Journal of Neurosurgery: Pediatrics. Researchers conducted a retrospective chart review of 106 pediatric patients with sports-related concussion who were referred to a multidisciplinary pediatric concussion program and underwent graded aerobic treadmill testing between October 9, 2014, and February 11, 2016. Treadmill testing confirmed physiologic recovery in 96.9% of 65 patients tested, allowing successful return to play in 93.8% of patients. Of the 41 patients with physiologic post-concussion disorder who had complete follow-up and were treated with tailored submaximal exercise, 90.2% were classified as clinically improved and 80.5% successfully returned to sporting activities.
Exposure to MRI during the first trimester of pregnancy, compared with nonexposure, is not associated with increased risk of harm to the fetus or in early childhood, according to a study published September 6 in JAMA. Gadolinium MRI, however, was associated with an increased risk of rheumatologic, inflammatory, or infiltrative skin conditions, and stillbirth or neonatal death. The study included 1,424,105 deliveries. Researchers compared first-trimester MRI exposure to no MRI exposure. The adjusted relative risk of stillbirth, congenital anomalies, neoplasm, or vision or hearing loss for first-trimester MRI was not significantly higher, compared with no MRI exposure. Comparing gadolinium MRI with no MRI, the adjusted hazard ratio of any rheumatologic, inflammatory, or infiltrative skin condition for first-trimester MRI was 1.36, for an adjusted risk difference of 45.3 per 1,000 person-years.
In women in the United Kingdom, higher BMI is associated with increased risk of ischemic stroke, but decreased risk of hemorrhagic stroke, according to a study published online ahead of print September 7 in Neurology. Researchers recruited 1.3 million previously stroke-free women from the UK between 1996 and 2001 and followed them by record linkage for hospital admissions and deaths. Increased BMI was associated with an increased risk of ischemic stroke, but a decreased risk of hemorrhagic stroke. The BMI-associated trends for ischemic and hemorrhagic stroke were significantly different, but were not significantly different for intracerebral hemorrhage and subarachnoid hemorrhage. Published data from prospective studies showed consistently greater BMI-associated relative risks for ischemic stroke than hemorrhagic stroke, with most evidence before this study coming from Asian populations.
Data confirm the relevance of complement biomarkers in mild cognitive impairment (MCI) and Alzheimer's disease, according to a study published September 6 in the Journal of Alzheimer's Disease. Results also indicate the value of multiparameter models for disease prediction and stratification. Researchers studied 292 people to measure five complement proteins and four activation products in plasma from donors with MCI, those with Alzheimer's disease, and healthy controls. Only clusterin differed significantly between control and Alzheimer's disease plasma. Overall, a model combining clusterin with relevant covariables was highly predictive of disease. Clusterin, factor I, and terminal complement complex were significantly different between individuals with MCI who had converted to dementia one year later compared with nonconverters. A model combining these three analytes with informative covariables was highly predictive of conversion.
Prenatal exposure to levetiracetam or topiramate may not impair a child's thinking skills, according to a study published online ahead of print August 31 in Neurology. For this cross-sectional observational study, researchers followed women enrolled in the UK Epilepsy and Pregnancy Register. The women received monotherapy levetiracetam, topiramate, or valproate, or took no therapy. Physicians conducted assessor-blinded neuropsychologic assessments of the women's children between ages 5 and 9. In the adjusted analyses, prenatal exposure to levetiracetam and topiramate were not found to be associated with reductions in children's cognitive abilities, and adverse outcomes were not associated with increasing dose. Increasing the dose of valproate was associated with poorer full-scale IQ, verbal abilities, nonverbal abilities, and expressive language ability. The evidence base for newer antiepileptic drugs is limited, said the authors.
At six months, decompressive craniectomy in patients with traumatic brain injury (TBI) and refractory intracranial hypertension results in lower mortality and higher rates of vegetative state and severe disability, compared with medical care, according to a study published online ahead of print September 7 in the New England Journal of Medicine. Researchers randomly assigned 408 patients, ages 10 to 65, with TBI and refractory elevated intracranial pressure to undergo decompressive craniectomy or receive ongoing medical care. At six months, approximately 27% of patients who received a craniectomy had died, compared with 49% of patients who received medical management. Patients who survived after a craniectomy were more likely to be dependent on others for care. At 12 months, mortality was 30% among surgical patients and 52% among medical patients.
Contralaterally controlled functional electrical stimulation (CCFES) improves hand dexterity after stroke more than cyclic neuromuscular electrical stimulation (cNMES) does, according to a study published online ahead of print September 8 in Stroke. Researchers enrolled 80 patients with stroke and chronic moderate to severe upper extremity hemiparesis in the study. Participants were randomized to receive 10 sessions per week of CCFES- or cNMES-assisted hand-opening exercise at home, along with 20 sessions of functional task practice in the laboratory for 12 weeks. At six months post treatment, the CCFES group had an improvement of 4.6 on the Box and Block Test, compared with an improvement of 1.8 for the cNMES group. Fugl-Meyer performance and Arm Motor Abilities Test performance did not differ between groups, however.
Antipsychotic use is associated with higher risk of pneumonia, regardless of the choice of drug, according to a study published online ahead of print June 11 in Chest. Researchers investigated whether incident antipsychotic use or specific antipsychotics are related to higher risk of hospitalization or death due to pneumonia in the MEDALZ cohort. The cohort includes all persons who received a clinically verified diagnosis of Alzheimer's disease in Finland from 2005 to 2011. A matched comparison cohort without Alzheimer's disease was used to compare the magnitude of risk. Antipsychotic use was associated with higher risk of pneumonia in the Alzheimer's disease cohort and with somewhat higher risk in the comparison cohort. No major differences were observed between the most commonly used antipsychotics.
The FDA has allowed the marketing of two Trevo clot-retrieval devices as an initial therapy to reduce paralysis, speech difficulties, and other disabilities following ischemic stroke. The agency evaluated data from a clinical trial comparing 96 randomly selected patients treated with the Trevo device and t-PA and medical management with 249 patients who received only t-PA and medical management. Twenty-nine percent of patients treated with the Trevo device were functionally independent at three months after stroke, compared with 19% of patients who were not treated with the Trevo device. These devices should be used within six hours of symptom onset and only following treatment with a clot-dissolving drug, which needs to be given within three hours of symptom onset, said the FDA. Concentric Medical, headquartered in Mountain View, California, markets Trevo.
Class I evidence suggests that for boys with Duchenne muscular dystrophy, daily use of deflazacort or prednisone is effective in preserving muscle strength over a 12-week period, according to a study published online ahead of print August 26 in Neurology. This phase III, double-blind, randomized, placebo-controlled, multicenter study evaluated the muscle strength of 196 boys ages 5 to 15 with Duchenne muscular dystrophy during a 52-week period. Participants received deflazacort, prednisone, or placebo for 12 weeks. At week 13, patients continued active treatment or switched from placebo to active treatment. All treatment groups demonstrated significant improvement in muscle strength, compared with placebo, at 12 weeks. Participants taking prednisone had significantly more weight gain than other participants at 12 weeks and at 52 weeks.
The FDA has granted tentative approval to Supernus Pharmaceuticals's Supplemental New Drug Application (sNDA) requesting a label expansion for Trokendi XR (topiramate) to include prophylaxis of migraine headache in adults. The approval of the sNDA is tentative because the FDA has determined that the drug meets all of the required quality, safety, and efficacy standards for approval, but is subject to the pediatric exclusivity, which expires on March 28, 2017. Final approval may not be made effective until this exclusivity period has expired. The FDA also has granted final approval to expand the label for Trokendi XR for monotherapy treatment of partial onset seizures to include adults and pediatric patients age six and older, rather than age 10 and older. Supernus Pharmaceuticals is headquartered in Rockville, Maryland.
—Kimberly Williams
Resective surgery for epilepsy is cost-effective in the medium term, according to a study published online ahead of print September 5 in Epilepsia. A prospective cohort of adult patients with surgically remediable and medically intractable partial epilepsy was followed for more than five years in 15 French centers. During the second year of follow-up, the proportion of patients who had been completely seizure-free for the previous 12 months was 69.0% among participants who underwent surgery and 12.3% in the medical group. The respective rates of seizure freedom were 76.8% and 21% during the fifth year. Direct costs became significantly lower in the surgical group during the third year after surgery as a result of decreased antiepileptic drug use. Surgery became cost-effective between nine and 10 years after surgery.
The NIH Toolbox Cognitive Battery can assess important dimensions of cognition in persons with intellectual disabilities, and several tests may be useful for tracking response to interventions, according to a study published September 6 in the Journal of Neurodevelopmental Disorders. In separate pilot studies of patients with fragile X syndrome, Down syndrome, and idiopathic intellectual disabilities, researchers used the web-based NIH Toolbox Cognitive Battery to measure processing speed, executive function, episodic memory, word and letter reading, receptive vocabulary, and working memory. The test's feasibility was good to excellent for people above mental age 4 for all tests except list sorting. Test-retest stability was good to excellent. More extensive psychometric studies are needed to determine the battery's true utility as a set of outcome measures, said the researchers.
Graded aerobic treadmill testing is a safe, tolerable, and clinically valuable tool that can assist in the evaluation and management of pediatric sports-related concussion, according to a study published online ahead of print September 13 in the Journal of Neurosurgery: Pediatrics. Researchers conducted a retrospective chart review of 106 pediatric patients with sports-related concussion who were referred to a multidisciplinary pediatric concussion program and underwent graded aerobic treadmill testing between October 9, 2014, and February 11, 2016. Treadmill testing confirmed physiologic recovery in 96.9% of 65 patients tested, allowing successful return to play in 93.8% of patients. Of the 41 patients with physiologic post-concussion disorder who had complete follow-up and were treated with tailored submaximal exercise, 90.2% were classified as clinically improved and 80.5% successfully returned to sporting activities.
Exposure to MRI during the first trimester of pregnancy, compared with nonexposure, is not associated with increased risk of harm to the fetus or in early childhood, according to a study published September 6 in JAMA. Gadolinium MRI, however, was associated with an increased risk of rheumatologic, inflammatory, or infiltrative skin conditions, and stillbirth or neonatal death. The study included 1,424,105 deliveries. Researchers compared first-trimester MRI exposure to no MRI exposure. The adjusted relative risk of stillbirth, congenital anomalies, neoplasm, or vision or hearing loss for first-trimester MRI was not significantly higher, compared with no MRI exposure. Comparing gadolinium MRI with no MRI, the adjusted hazard ratio of any rheumatologic, inflammatory, or infiltrative skin condition for first-trimester MRI was 1.36, for an adjusted risk difference of 45.3 per 1,000 person-years.
In women in the United Kingdom, higher BMI is associated with increased risk of ischemic stroke, but decreased risk of hemorrhagic stroke, according to a study published online ahead of print September 7 in Neurology. Researchers recruited 1.3 million previously stroke-free women from the UK between 1996 and 2001 and followed them by record linkage for hospital admissions and deaths. Increased BMI was associated with an increased risk of ischemic stroke, but a decreased risk of hemorrhagic stroke. The BMI-associated trends for ischemic and hemorrhagic stroke were significantly different, but were not significantly different for intracerebral hemorrhage and subarachnoid hemorrhage. Published data from prospective studies showed consistently greater BMI-associated relative risks for ischemic stroke than hemorrhagic stroke, with most evidence before this study coming from Asian populations.
Data confirm the relevance of complement biomarkers in mild cognitive impairment (MCI) and Alzheimer's disease, according to a study published September 6 in the Journal of Alzheimer's Disease. Results also indicate the value of multiparameter models for disease prediction and stratification. Researchers studied 292 people to measure five complement proteins and four activation products in plasma from donors with MCI, those with Alzheimer's disease, and healthy controls. Only clusterin differed significantly between control and Alzheimer's disease plasma. Overall, a model combining clusterin with relevant covariables was highly predictive of disease. Clusterin, factor I, and terminal complement complex were significantly different between individuals with MCI who had converted to dementia one year later compared with nonconverters. A model combining these three analytes with informative covariables was highly predictive of conversion.
Prenatal exposure to levetiracetam or topiramate may not impair a child's thinking skills, according to a study published online ahead of print August 31 in Neurology. For this cross-sectional observational study, researchers followed women enrolled in the UK Epilepsy and Pregnancy Register. The women received monotherapy levetiracetam, topiramate, or valproate, or took no therapy. Physicians conducted assessor-blinded neuropsychologic assessments of the women's children between ages 5 and 9. In the adjusted analyses, prenatal exposure to levetiracetam and topiramate were not found to be associated with reductions in children's cognitive abilities, and adverse outcomes were not associated with increasing dose. Increasing the dose of valproate was associated with poorer full-scale IQ, verbal abilities, nonverbal abilities, and expressive language ability. The evidence base for newer antiepileptic drugs is limited, said the authors.
At six months, decompressive craniectomy in patients with traumatic brain injury (TBI) and refractory intracranial hypertension results in lower mortality and higher rates of vegetative state and severe disability, compared with medical care, according to a study published online ahead of print September 7 in the New England Journal of Medicine. Researchers randomly assigned 408 patients, ages 10 to 65, with TBI and refractory elevated intracranial pressure to undergo decompressive craniectomy or receive ongoing medical care. At six months, approximately 27% of patients who received a craniectomy had died, compared with 49% of patients who received medical management. Patients who survived after a craniectomy were more likely to be dependent on others for care. At 12 months, mortality was 30% among surgical patients and 52% among medical patients.
Contralaterally controlled functional electrical stimulation (CCFES) improves hand dexterity after stroke more than cyclic neuromuscular electrical stimulation (cNMES) does, according to a study published online ahead of print September 8 in Stroke. Researchers enrolled 80 patients with stroke and chronic moderate to severe upper extremity hemiparesis in the study. Participants were randomized to receive 10 sessions per week of CCFES- or cNMES-assisted hand-opening exercise at home, along with 20 sessions of functional task practice in the laboratory for 12 weeks. At six months post treatment, the CCFES group had an improvement of 4.6 on the Box and Block Test, compared with an improvement of 1.8 for the cNMES group. Fugl-Meyer performance and Arm Motor Abilities Test performance did not differ between groups, however.
Antipsychotic use is associated with higher risk of pneumonia, regardless of the choice of drug, according to a study published online ahead of print June 11 in Chest. Researchers investigated whether incident antipsychotic use or specific antipsychotics are related to higher risk of hospitalization or death due to pneumonia in the MEDALZ cohort. The cohort includes all persons who received a clinically verified diagnosis of Alzheimer's disease in Finland from 2005 to 2011. A matched comparison cohort without Alzheimer's disease was used to compare the magnitude of risk. Antipsychotic use was associated with higher risk of pneumonia in the Alzheimer's disease cohort and with somewhat higher risk in the comparison cohort. No major differences were observed between the most commonly used antipsychotics.
The FDA has allowed the marketing of two Trevo clot-retrieval devices as an initial therapy to reduce paralysis, speech difficulties, and other disabilities following ischemic stroke. The agency evaluated data from a clinical trial comparing 96 randomly selected patients treated with the Trevo device and t-PA and medical management with 249 patients who received only t-PA and medical management. Twenty-nine percent of patients treated with the Trevo device were functionally independent at three months after stroke, compared with 19% of patients who were not treated with the Trevo device. These devices should be used within six hours of symptom onset and only following treatment with a clot-dissolving drug, which needs to be given within three hours of symptom onset, said the FDA. Concentric Medical, headquartered in Mountain View, California, markets Trevo.
Class I evidence suggests that for boys with Duchenne muscular dystrophy, daily use of deflazacort or prednisone is effective in preserving muscle strength over a 12-week period, according to a study published online ahead of print August 26 in Neurology. This phase III, double-blind, randomized, placebo-controlled, multicenter study evaluated the muscle strength of 196 boys ages 5 to 15 with Duchenne muscular dystrophy during a 52-week period. Participants received deflazacort, prednisone, or placebo for 12 weeks. At week 13, patients continued active treatment or switched from placebo to active treatment. All treatment groups demonstrated significant improvement in muscle strength, compared with placebo, at 12 weeks. Participants taking prednisone had significantly more weight gain than other participants at 12 weeks and at 52 weeks.
The FDA has granted tentative approval to Supernus Pharmaceuticals's Supplemental New Drug Application (sNDA) requesting a label expansion for Trokendi XR (topiramate) to include prophylaxis of migraine headache in adults. The approval of the sNDA is tentative because the FDA has determined that the drug meets all of the required quality, safety, and efficacy standards for approval, but is subject to the pediatric exclusivity, which expires on March 28, 2017. Final approval may not be made effective until this exclusivity period has expired. The FDA also has granted final approval to expand the label for Trokendi XR for monotherapy treatment of partial onset seizures to include adults and pediatric patients age six and older, rather than age 10 and older. Supernus Pharmaceuticals is headquartered in Rockville, Maryland.
—Kimberly Williams
Resective surgery for epilepsy is cost-effective in the medium term, according to a study published online ahead of print September 5 in Epilepsia. A prospective cohort of adult patients with surgically remediable and medically intractable partial epilepsy was followed for more than five years in 15 French centers. During the second year of follow-up, the proportion of patients who had been completely seizure-free for the previous 12 months was 69.0% among participants who underwent surgery and 12.3% in the medical group. The respective rates of seizure freedom were 76.8% and 21% during the fifth year. Direct costs became significantly lower in the surgical group during the third year after surgery as a result of decreased antiepileptic drug use. Surgery became cost-effective between nine and 10 years after surgery.
The NIH Toolbox Cognitive Battery can assess important dimensions of cognition in persons with intellectual disabilities, and several tests may be useful for tracking response to interventions, according to a study published September 6 in the Journal of Neurodevelopmental Disorders. In separate pilot studies of patients with fragile X syndrome, Down syndrome, and idiopathic intellectual disabilities, researchers used the web-based NIH Toolbox Cognitive Battery to measure processing speed, executive function, episodic memory, word and letter reading, receptive vocabulary, and working memory. The test's feasibility was good to excellent for people above mental age 4 for all tests except list sorting. Test-retest stability was good to excellent. More extensive psychometric studies are needed to determine the battery's true utility as a set of outcome measures, said the researchers.
Graded aerobic treadmill testing is a safe, tolerable, and clinically valuable tool that can assist in the evaluation and management of pediatric sports-related concussion, according to a study published online ahead of print September 13 in the Journal of Neurosurgery: Pediatrics. Researchers conducted a retrospective chart review of 106 pediatric patients with sports-related concussion who were referred to a multidisciplinary pediatric concussion program and underwent graded aerobic treadmill testing between October 9, 2014, and February 11, 2016. Treadmill testing confirmed physiologic recovery in 96.9% of 65 patients tested, allowing successful return to play in 93.8% of patients. Of the 41 patients with physiologic post-concussion disorder who had complete follow-up and were treated with tailored submaximal exercise, 90.2% were classified as clinically improved and 80.5% successfully returned to sporting activities.
Exposure to MRI during the first trimester of pregnancy, compared with nonexposure, is not associated with increased risk of harm to the fetus or in early childhood, according to a study published September 6 in JAMA. Gadolinium MRI, however, was associated with an increased risk of rheumatologic, inflammatory, or infiltrative skin conditions, and stillbirth or neonatal death. The study included 1,424,105 deliveries. Researchers compared first-trimester MRI exposure to no MRI exposure. The adjusted relative risk of stillbirth, congenital anomalies, neoplasm, or vision or hearing loss for first-trimester MRI was not significantly higher, compared with no MRI exposure. Comparing gadolinium MRI with no MRI, the adjusted hazard ratio of any rheumatologic, inflammatory, or infiltrative skin condition for first-trimester MRI was 1.36, for an adjusted risk difference of 45.3 per 1,000 person-years.
In women in the United Kingdom, higher BMI is associated with increased risk of ischemic stroke, but decreased risk of hemorrhagic stroke, according to a study published online ahead of print September 7 in Neurology. Researchers recruited 1.3 million previously stroke-free women from the UK between 1996 and 2001 and followed them by record linkage for hospital admissions and deaths. Increased BMI was associated with an increased risk of ischemic stroke, but a decreased risk of hemorrhagic stroke. The BMI-associated trends for ischemic and hemorrhagic stroke were significantly different, but were not significantly different for intracerebral hemorrhage and subarachnoid hemorrhage. Published data from prospective studies showed consistently greater BMI-associated relative risks for ischemic stroke than hemorrhagic stroke, with most evidence before this study coming from Asian populations.
Data confirm the relevance of complement biomarkers in mild cognitive impairment (MCI) and Alzheimer's disease, according to a study published September 6 in the Journal of Alzheimer's Disease. Results also indicate the value of multiparameter models for disease prediction and stratification. Researchers studied 292 people to measure five complement proteins and four activation products in plasma from donors with MCI, those with Alzheimer's disease, and healthy controls. Only clusterin differed significantly between control and Alzheimer's disease plasma. Overall, a model combining clusterin with relevant covariables was highly predictive of disease. Clusterin, factor I, and terminal complement complex were significantly different between individuals with MCI who had converted to dementia one year later compared with nonconverters. A model combining these three analytes with informative covariables was highly predictive of conversion.
Prenatal exposure to levetiracetam or topiramate may not impair a child's thinking skills, according to a study published online ahead of print August 31 in Neurology. For this cross-sectional observational study, researchers followed women enrolled in the UK Epilepsy and Pregnancy Register. The women received monotherapy levetiracetam, topiramate, or valproate, or took no therapy. Physicians conducted assessor-blinded neuropsychologic assessments of the women's children between ages 5 and 9. In the adjusted analyses, prenatal exposure to levetiracetam and topiramate were not found to be associated with reductions in children's cognitive abilities, and adverse outcomes were not associated with increasing dose. Increasing the dose of valproate was associated with poorer full-scale IQ, verbal abilities, nonverbal abilities, and expressive language ability. The evidence base for newer antiepileptic drugs is limited, said the authors.
At six months, decompressive craniectomy in patients with traumatic brain injury (TBI) and refractory intracranial hypertension results in lower mortality and higher rates of vegetative state and severe disability, compared with medical care, according to a study published online ahead of print September 7 in the New England Journal of Medicine. Researchers randomly assigned 408 patients, ages 10 to 65, with TBI and refractory elevated intracranial pressure to undergo decompressive craniectomy or receive ongoing medical care. At six months, approximately 27% of patients who received a craniectomy had died, compared with 49% of patients who received medical management. Patients who survived after a craniectomy were more likely to be dependent on others for care. At 12 months, mortality was 30% among surgical patients and 52% among medical patients.
Contralaterally controlled functional electrical stimulation (CCFES) improves hand dexterity after stroke more than cyclic neuromuscular electrical stimulation (cNMES) does, according to a study published online ahead of print September 8 in Stroke. Researchers enrolled 80 patients with stroke and chronic moderate to severe upper extremity hemiparesis in the study. Participants were randomized to receive 10 sessions per week of CCFES- or cNMES-assisted hand-opening exercise at home, along with 20 sessions of functional task practice in the laboratory for 12 weeks. At six months post treatment, the CCFES group had an improvement of 4.6 on the Box and Block Test, compared with an improvement of 1.8 for the cNMES group. Fugl-Meyer performance and Arm Motor Abilities Test performance did not differ between groups, however.
Antipsychotic use is associated with higher risk of pneumonia, regardless of the choice of drug, according to a study published online ahead of print June 11 in Chest. Researchers investigated whether incident antipsychotic use or specific antipsychotics are related to higher risk of hospitalization or death due to pneumonia in the MEDALZ cohort. The cohort includes all persons who received a clinically verified diagnosis of Alzheimer's disease in Finland from 2005 to 2011. A matched comparison cohort without Alzheimer's disease was used to compare the magnitude of risk. Antipsychotic use was associated with higher risk of pneumonia in the Alzheimer's disease cohort and with somewhat higher risk in the comparison cohort. No major differences were observed between the most commonly used antipsychotics.
The FDA has allowed the marketing of two Trevo clot-retrieval devices as an initial therapy to reduce paralysis, speech difficulties, and other disabilities following ischemic stroke. The agency evaluated data from a clinical trial comparing 96 randomly selected patients treated with the Trevo device and t-PA and medical management with 249 patients who received only t-PA and medical management. Twenty-nine percent of patients treated with the Trevo device were functionally independent at three months after stroke, compared with 19% of patients who were not treated with the Trevo device. These devices should be used within six hours of symptom onset and only following treatment with a clot-dissolving drug, which needs to be given within three hours of symptom onset, said the FDA. Concentric Medical, headquartered in Mountain View, California, markets Trevo.
Class I evidence suggests that for boys with Duchenne muscular dystrophy, daily use of deflazacort or prednisone is effective in preserving muscle strength over a 12-week period, according to a study published online ahead of print August 26 in Neurology. This phase III, double-blind, randomized, placebo-controlled, multicenter study evaluated the muscle strength of 196 boys ages 5 to 15 with Duchenne muscular dystrophy during a 52-week period. Participants received deflazacort, prednisone, or placebo for 12 weeks. At week 13, patients continued active treatment or switched from placebo to active treatment. All treatment groups demonstrated significant improvement in muscle strength, compared with placebo, at 12 weeks. Participants taking prednisone had significantly more weight gain than other participants at 12 weeks and at 52 weeks.
The FDA has granted tentative approval to Supernus Pharmaceuticals's Supplemental New Drug Application (sNDA) requesting a label expansion for Trokendi XR (topiramate) to include prophylaxis of migraine headache in adults. The approval of the sNDA is tentative because the FDA has determined that the drug meets all of the required quality, safety, and efficacy standards for approval, but is subject to the pediatric exclusivity, which expires on March 28, 2017. Final approval may not be made effective until this exclusivity period has expired. The FDA also has granted final approval to expand the label for Trokendi XR for monotherapy treatment of partial onset seizures to include adults and pediatric patients age six and older, rather than age 10 and older. Supernus Pharmaceuticals is headquartered in Rockville, Maryland.
—Kimberly Williams
Product News: October 2016
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Comfort Care
Carma Labs Inc introduces Carmex Comfort Care lip balm, a natural lip care product formulated with colloidal oatmeal and cold-pressed fruit oil to deliver soothing, long-lasting moisture and hydration. Colloidal oatmeal also possesses antioxidant and anti-inflammatory properties that benefit sensitive drug skin, especially on the lips. For more information, visit www.mycarmex.com.
Erelzi
Sandoz Inc, a Novartis Division, announces US Food and Drug Administration approval of Erelzi (etanercept-szzs) for all indications included in the reference product label: rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis. Erelzi is the second approved biosimilar from Sandoz. For more information, visit www.erelzi.com.
Loprox Cream
Medimetriks Pharmaceuticals, Inc, announces the launch of Loprox (ciclopirox) Cream 0.77% and the Loprox Cream Kit. Loprox Cream is a broad-spectrum therapy that treats 5 different skin infections from 6 different pathogens. It is indicated for the topical treatment of tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; candidiasis due to Candia albicans; and tinea (pityriasis) versicolor due to Malassezia furfur. Loprox Cream works quickly, usually within the first week (2 weeks for tinea versicolor), providing patients needed relief of pruritus and other symptoms. The Loprox Cream Kit includes Loprox Cream and Rehyla Hair + Body Cleanser for patient convenience. The cleanser hydrates and conditions and is gentle for daily use on the scalp and body. For more information, visit www.medimetriks.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Complementary medicine impedes children’s flu vaccination
Children treated with complementary and alternative medicine (CAM) therapies were significantly less likely to receive the annual influenza vaccine than those who didn’t use alternative medicine, based on data from 9,000 children aged 4-17 years in the Child Complementary and Alternative Medicine File of the 2012 National Health Interview Survey.
“CAM has been implicated as lending support to antivaccine/vaccine-hesitant viewpoints via criticism of vaccination, public health, and conventional medicine from adults using CAM, as well as from CAM practitioners and practitioners-in-training,” wrote William K. Bleser, Ph.D., and his colleagues at Pennsylvania State University, University Park.
No significant differences in vaccination rates were noted for children who had used three other categories of nonconventional care: biologically based therapies (such as herbal supplements), mind-body therapies (such as yoga), and multivitamins.
“There is an opportunity for U.S. public health, policy, and conventional medical professionals and educators to improve vaccine uptake and child health by better engaging both CAM and conventional medicine practitioners-in-training, parents of children using particular domains of CAM, and the CAM practitioners advising them,” the researchers said.
Find the full article in Pediatrics (2016. doi: 10.1542/peds.2015-4664).
Children treated with complementary and alternative medicine (CAM) therapies were significantly less likely to receive the annual influenza vaccine than those who didn’t use alternative medicine, based on data from 9,000 children aged 4-17 years in the Child Complementary and Alternative Medicine File of the 2012 National Health Interview Survey.
“CAM has been implicated as lending support to antivaccine/vaccine-hesitant viewpoints via criticism of vaccination, public health, and conventional medicine from adults using CAM, as well as from CAM practitioners and practitioners-in-training,” wrote William K. Bleser, Ph.D., and his colleagues at Pennsylvania State University, University Park.
No significant differences in vaccination rates were noted for children who had used three other categories of nonconventional care: biologically based therapies (such as herbal supplements), mind-body therapies (such as yoga), and multivitamins.
“There is an opportunity for U.S. public health, policy, and conventional medical professionals and educators to improve vaccine uptake and child health by better engaging both CAM and conventional medicine practitioners-in-training, parents of children using particular domains of CAM, and the CAM practitioners advising them,” the researchers said.
Find the full article in Pediatrics (2016. doi: 10.1542/peds.2015-4664).
Children treated with complementary and alternative medicine (CAM) therapies were significantly less likely to receive the annual influenza vaccine than those who didn’t use alternative medicine, based on data from 9,000 children aged 4-17 years in the Child Complementary and Alternative Medicine File of the 2012 National Health Interview Survey.
“CAM has been implicated as lending support to antivaccine/vaccine-hesitant viewpoints via criticism of vaccination, public health, and conventional medicine from adults using CAM, as well as from CAM practitioners and practitioners-in-training,” wrote William K. Bleser, Ph.D., and his colleagues at Pennsylvania State University, University Park.
No significant differences in vaccination rates were noted for children who had used three other categories of nonconventional care: biologically based therapies (such as herbal supplements), mind-body therapies (such as yoga), and multivitamins.
“There is an opportunity for U.S. public health, policy, and conventional medical professionals and educators to improve vaccine uptake and child health by better engaging both CAM and conventional medicine practitioners-in-training, parents of children using particular domains of CAM, and the CAM practitioners advising them,” the researchers said.
Find the full article in Pediatrics (2016. doi: 10.1542/peds.2015-4664).
What Makes Feedback Productive?
When my youngest daughter returns home from acting or dancing rehearsals, she talks about “notes” that she or the company received that day. Discussing them with her, I appreciate that giving notes to performers after rehearsal or even after a show is standard theater practice. The notes may be from the assistant stage director commenting on lines that were missed, mangled, or perfected. They also could be from the director concerning stage position or behaviors, or they may be about character development or a clarification about the emotions in a particular scene. They are written out as specific references to a certain line or segment of the script. Some directors write them on sticky memos so that they can actually be added to the actor’s script. Others keep their notes on index cards that can be sorted and handed out to the designated performer. My daughter works hard during the first part of the rehearsal process to get as few notes as possible, but at the end of the rehearsal process or during the run of the show, she likes getting notes as a reflection of how she is being perceived and to facilitate fine-tuning her performance.
Giving written notes in our offices to our colleagues, trainees, and staff after a day’s work is not likely to be productive; however, there are parts of this process that dermatologists can utilize. The notes give feedback that is timely and specific. They can be given to individuals or to the entire troupe. I also noticed that my daughter appeared to have a positive relationship with the note givers and looked for their feedback to improve her performance. When residents are on a procedural rotation with me, I endeavor to give them feedback every day about some part of their surgical technique to help them finesse their skills. I am not, however, as rigorous about giving feedback concerning other aspects of the practice, and so this editorial serves the purpose of reminding me that giving feedback is an important skill that we can and should use on a daily basis.
There are many guides for giving feedback. The Center for Creative Leadership developed a feedback technique called Situation-Behavior-Impact (S-B-I).1 Similar to performance notes, it is simple, direct, and timely. Step 1: Capture the situation (S). Step 2: Describe the behavior (B). Step 3: Deliver the impact (I). For example, I have given the following feedback to many fellows when they are working with the resident: (S) “This morning when you two were finishing the repair, (B) you were talking about the lack of efficiency of the clinic in another hospital. (I) It made me uncomfortable because I believe the patient is the center of attention, and yet this was not a conversation that included him. I also worried that he would become nervous or anxious to hear about problems in a medical facility.” Another conversation could go: (S) “This morning with the patient with the eyelid tumor, (B) you told the patient that you would send the eye surgeon a photo so she could be prepared for the repair, and (I) I noticed the patient’s hands immediately relaxed.”
These are straightforward examples. There are more complicated situations that seem to require longer analysis; however, if we acquire the habit of immediate and specific feedback, there will be less need for more difficult conversations. Situation-Behavior-Impact is about behavior; it is not judgmental of the person, and it leaves room for the recipient to think about what happened without being defensive and to take action to create productive behaviors and improve performance. The Center for Creative Leadership recommends that feedback be framed as an observation, which further diminishes the development of a defensive rejection of the information.1
Feedback is such an important loop for all of us professionally and personally because it is the mechanism that gives us the opportunity to improve our performance, so why don’t we always hear it in a constructive thought-provoking way? Stone and Heen2 point out 3 triggers that escalate rejection of feedback: truth, relationship, and identity. They also can be described as immediate reactions: “You are wrong about your assessment,” “I don’t like you anyway,” and “You’re messing with who I am.” For those of you who want to up your game in any of your professional or personal arenas, Thanks for the Feedback: The Science and Art of Receiving Feedback Well2 will open you up to seek out and take in feedback. Feedback-seeking behavior has been linked to higher job satisfaction, greater creativity on the job, and faster adaptation to change, while negative feedback has been linked to improved job performance.3 Interestingly, it also helps in our personal lives; a husband’s openness to influence and
In an effort to decrease resistance to hearing feedback, there are proponents of the sandwich technique in which a positive comment is made, then the negative feedback is given, followed by another positive comment. In my experience, this technique does not work. First, you have to give some thought to the appropriate items to bring to the discussion, so the conversation might be delayed long enough to obscure the memory of the details involved in the situations. Second, if you employ it often, the receiver tenses up with the first positive comment, knowing a negative comment will ensue, and so he/she is primed to reject the feedback before it is even offered. Finally, it confuses the priorities for the conversation. However, working over time to give more positive feedback than negative feedback (an average of 4–5 to 1) allows for the development of trust and mutual respect and quiets the urge to immediately reject the negative messages. In my experience, positive feedback is especially effective in creating engagement as well as validating and promoting desirable behaviors. Physicians may have to work deliberately to offer positive feedback because it is more natural for us to diagnose problems than to identify good health.
What impresses me most about the theater culture surrounding notes is that giving and receiving feedback is an expected element of the artistic process. As practitioners, wouldn’t we as well as our patients benefit if the culture of medicine also expected that we were giving each other feedback on a daily basis?
- Weitzel SR. Feedback That Works: How to Build and Deliver Your Message. Greensboro, NC: Center for Creative Leadership; 2000.
- Stone D, Heen S. Thanks for the Feedback: The Science and Art of Receiving Feedback Well. New York, NY: Penguin Books; 2015:16-30.
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Carrère S, Buehlman KT, Gottman JM, et al. Predicting marital stability and divorce in newlywed couples. J Fam Psychol. 2000;14:42-58.
When my youngest daughter returns home from acting or dancing rehearsals, she talks about “notes” that she or the company received that day. Discussing them with her, I appreciate that giving notes to performers after rehearsal or even after a show is standard theater practice. The notes may be from the assistant stage director commenting on lines that were missed, mangled, or perfected. They also could be from the director concerning stage position or behaviors, or they may be about character development or a clarification about the emotions in a particular scene. They are written out as specific references to a certain line or segment of the script. Some directors write them on sticky memos so that they can actually be added to the actor’s script. Others keep their notes on index cards that can be sorted and handed out to the designated performer. My daughter works hard during the first part of the rehearsal process to get as few notes as possible, but at the end of the rehearsal process or during the run of the show, she likes getting notes as a reflection of how she is being perceived and to facilitate fine-tuning her performance.
Giving written notes in our offices to our colleagues, trainees, and staff after a day’s work is not likely to be productive; however, there are parts of this process that dermatologists can utilize. The notes give feedback that is timely and specific. They can be given to individuals or to the entire troupe. I also noticed that my daughter appeared to have a positive relationship with the note givers and looked for their feedback to improve her performance. When residents are on a procedural rotation with me, I endeavor to give them feedback every day about some part of their surgical technique to help them finesse their skills. I am not, however, as rigorous about giving feedback concerning other aspects of the practice, and so this editorial serves the purpose of reminding me that giving feedback is an important skill that we can and should use on a daily basis.
There are many guides for giving feedback. The Center for Creative Leadership developed a feedback technique called Situation-Behavior-Impact (S-B-I).1 Similar to performance notes, it is simple, direct, and timely. Step 1: Capture the situation (S). Step 2: Describe the behavior (B). Step 3: Deliver the impact (I). For example, I have given the following feedback to many fellows when they are working with the resident: (S) “This morning when you two were finishing the repair, (B) you were talking about the lack of efficiency of the clinic in another hospital. (I) It made me uncomfortable because I believe the patient is the center of attention, and yet this was not a conversation that included him. I also worried that he would become nervous or anxious to hear about problems in a medical facility.” Another conversation could go: (S) “This morning with the patient with the eyelid tumor, (B) you told the patient that you would send the eye surgeon a photo so she could be prepared for the repair, and (I) I noticed the patient’s hands immediately relaxed.”
These are straightforward examples. There are more complicated situations that seem to require longer analysis; however, if we acquire the habit of immediate and specific feedback, there will be less need for more difficult conversations. Situation-Behavior-Impact is about behavior; it is not judgmental of the person, and it leaves room for the recipient to think about what happened without being defensive and to take action to create productive behaviors and improve performance. The Center for Creative Leadership recommends that feedback be framed as an observation, which further diminishes the development of a defensive rejection of the information.1
Feedback is such an important loop for all of us professionally and personally because it is the mechanism that gives us the opportunity to improve our performance, so why don’t we always hear it in a constructive thought-provoking way? Stone and Heen2 point out 3 triggers that escalate rejection of feedback: truth, relationship, and identity. They also can be described as immediate reactions: “You are wrong about your assessment,” “I don’t like you anyway,” and “You’re messing with who I am.” For those of you who want to up your game in any of your professional or personal arenas, Thanks for the Feedback: The Science and Art of Receiving Feedback Well2 will open you up to seek out and take in feedback. Feedback-seeking behavior has been linked to higher job satisfaction, greater creativity on the job, and faster adaptation to change, while negative feedback has been linked to improved job performance.3 Interestingly, it also helps in our personal lives; a husband’s openness to influence and
In an effort to decrease resistance to hearing feedback, there are proponents of the sandwich technique in which a positive comment is made, then the negative feedback is given, followed by another positive comment. In my experience, this technique does not work. First, you have to give some thought to the appropriate items to bring to the discussion, so the conversation might be delayed long enough to obscure the memory of the details involved in the situations. Second, if you employ it often, the receiver tenses up with the first positive comment, knowing a negative comment will ensue, and so he/she is primed to reject the feedback before it is even offered. Finally, it confuses the priorities for the conversation. However, working over time to give more positive feedback than negative feedback (an average of 4–5 to 1) allows for the development of trust and mutual respect and quiets the urge to immediately reject the negative messages. In my experience, positive feedback is especially effective in creating engagement as well as validating and promoting desirable behaviors. Physicians may have to work deliberately to offer positive feedback because it is more natural for us to diagnose problems than to identify good health.
What impresses me most about the theater culture surrounding notes is that giving and receiving feedback is an expected element of the artistic process. As practitioners, wouldn’t we as well as our patients benefit if the culture of medicine also expected that we were giving each other feedback on a daily basis?
When my youngest daughter returns home from acting or dancing rehearsals, she talks about “notes” that she or the company received that day. Discussing them with her, I appreciate that giving notes to performers after rehearsal or even after a show is standard theater practice. The notes may be from the assistant stage director commenting on lines that were missed, mangled, or perfected. They also could be from the director concerning stage position or behaviors, or they may be about character development or a clarification about the emotions in a particular scene. They are written out as specific references to a certain line or segment of the script. Some directors write them on sticky memos so that they can actually be added to the actor’s script. Others keep their notes on index cards that can be sorted and handed out to the designated performer. My daughter works hard during the first part of the rehearsal process to get as few notes as possible, but at the end of the rehearsal process or during the run of the show, she likes getting notes as a reflection of how she is being perceived and to facilitate fine-tuning her performance.
Giving written notes in our offices to our colleagues, trainees, and staff after a day’s work is not likely to be productive; however, there are parts of this process that dermatologists can utilize. The notes give feedback that is timely and specific. They can be given to individuals or to the entire troupe. I also noticed that my daughter appeared to have a positive relationship with the note givers and looked for their feedback to improve her performance. When residents are on a procedural rotation with me, I endeavor to give them feedback every day about some part of their surgical technique to help them finesse their skills. I am not, however, as rigorous about giving feedback concerning other aspects of the practice, and so this editorial serves the purpose of reminding me that giving feedback is an important skill that we can and should use on a daily basis.
There are many guides for giving feedback. The Center for Creative Leadership developed a feedback technique called Situation-Behavior-Impact (S-B-I).1 Similar to performance notes, it is simple, direct, and timely. Step 1: Capture the situation (S). Step 2: Describe the behavior (B). Step 3: Deliver the impact (I). For example, I have given the following feedback to many fellows when they are working with the resident: (S) “This morning when you two were finishing the repair, (B) you were talking about the lack of efficiency of the clinic in another hospital. (I) It made me uncomfortable because I believe the patient is the center of attention, and yet this was not a conversation that included him. I also worried that he would become nervous or anxious to hear about problems in a medical facility.” Another conversation could go: (S) “This morning with the patient with the eyelid tumor, (B) you told the patient that you would send the eye surgeon a photo so she could be prepared for the repair, and (I) I noticed the patient’s hands immediately relaxed.”
These are straightforward examples. There are more complicated situations that seem to require longer analysis; however, if we acquire the habit of immediate and specific feedback, there will be less need for more difficult conversations. Situation-Behavior-Impact is about behavior; it is not judgmental of the person, and it leaves room for the recipient to think about what happened without being defensive and to take action to create productive behaviors and improve performance. The Center for Creative Leadership recommends that feedback be framed as an observation, which further diminishes the development of a defensive rejection of the information.1
Feedback is such an important loop for all of us professionally and personally because it is the mechanism that gives us the opportunity to improve our performance, so why don’t we always hear it in a constructive thought-provoking way? Stone and Heen2 point out 3 triggers that escalate rejection of feedback: truth, relationship, and identity. They also can be described as immediate reactions: “You are wrong about your assessment,” “I don’t like you anyway,” and “You’re messing with who I am.” For those of you who want to up your game in any of your professional or personal arenas, Thanks for the Feedback: The Science and Art of Receiving Feedback Well2 will open you up to seek out and take in feedback. Feedback-seeking behavior has been linked to higher job satisfaction, greater creativity on the job, and faster adaptation to change, while negative feedback has been linked to improved job performance.3 Interestingly, it also helps in our personal lives; a husband’s openness to influence and
In an effort to decrease resistance to hearing feedback, there are proponents of the sandwich technique in which a positive comment is made, then the negative feedback is given, followed by another positive comment. In my experience, this technique does not work. First, you have to give some thought to the appropriate items to bring to the discussion, so the conversation might be delayed long enough to obscure the memory of the details involved in the situations. Second, if you employ it often, the receiver tenses up with the first positive comment, knowing a negative comment will ensue, and so he/she is primed to reject the feedback before it is even offered. Finally, it confuses the priorities for the conversation. However, working over time to give more positive feedback than negative feedback (an average of 4–5 to 1) allows for the development of trust and mutual respect and quiets the urge to immediately reject the negative messages. In my experience, positive feedback is especially effective in creating engagement as well as validating and promoting desirable behaviors. Physicians may have to work deliberately to offer positive feedback because it is more natural for us to diagnose problems than to identify good health.
What impresses me most about the theater culture surrounding notes is that giving and receiving feedback is an expected element of the artistic process. As practitioners, wouldn’t we as well as our patients benefit if the culture of medicine also expected that we were giving each other feedback on a daily basis?
- Weitzel SR. Feedback That Works: How to Build and Deliver Your Message. Greensboro, NC: Center for Creative Leadership; 2000.
- Stone D, Heen S. Thanks for the Feedback: The Science and Art of Receiving Feedback Well. New York, NY: Penguin Books; 2015:16-30.
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Carrère S, Buehlman KT, Gottman JM, et al. Predicting marital stability and divorce in newlywed couples. J Fam Psychol. 2000;14:42-58.
- Weitzel SR. Feedback That Works: How to Build and Deliver Your Message. Greensboro, NC: Center for Creative Leadership; 2000.
- Stone D, Heen S. Thanks for the Feedback: The Science and Art of Receiving Feedback Well. New York, NY: Penguin Books; 2015:16-30.
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Carrère S, Buehlman KT, Gottman JM, et al. Predicting marital stability and divorce in newlywed couples. J Fam Psychol. 2000;14:42-58.
Walk the Talk: VA Mental Health Care Professionals’ Role in Promoting Physical Activity
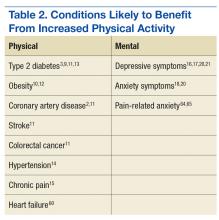
Physical activity is a key determinant of health. Low levels of activity are associated with onset of and poorer outcomes of many chronic health conditions (eg, obesity, coronary artery disease, type 2 diabetes mellitus, chronic pain, hypertension1-4) and with higher rates of mental health conditions (eg, depression, anxiety5-8).
Behavioral interventions (Table 1) can increase activity and improve physical health and mental health (Table 2).9-21 However, only 20% of adults in the U.S. meet federal recommendations for physical activity.22 The situation is particularly grim in the veteran population. Littman and colleagues found that veterans were less likely than nonveterans were to meet physical activity standards, and VA patients were even less likely than were non-VA veterans to meet the recommendations.23
Given that exercise can positively affect physical and mental health and that VA mental health care professionals (MHCPs) have training in motivational enhancement and behavior modification, these clinicians are well positioned to intervene. The question arises, though: How can VA MHCPs do more to effectively promote physical activity in veterans?
Addressing Physical Activity
There are numerous ways in which VA MHCPs can address physical activity with their patients. Several studies have demonstrated that physical activity interventions provided within primary care–mental health integration programs resulted in increased physical activity.28,29 The number of VA health care providers (HCPs) offering such programs is increasing, which could mean that behavioral health support for physical activity promotion could become easier for veterans to access.30
In addition, National Center for Health Promotion and Disease Prevention initiatives have led to an expansion of programs, such as the VA MOVE! Weight Management Program.31 Often cofacilitated by dieticians and MHCPs, MOVE! includes nutrition education, behavior modification, and physical activity promotion.32 Preliminary research suggests that MOVE! helps veterans lose weight and improve their health-related quality of life.33-36
Further, psychological and behavioral interventions can specifically target exercise and have been shown to increase physical activity, improve mood symptoms, and reduce health risk factors.9-21,37 However, little is known about the extent of exercise promotion in VA outpatient mental health services. For instance, some HCPs may educate patients about the benefits of physical activity, while others may facilitate physical activity scheduling, address barriers, and monitor, reinforce, and problem-solve physical activity goals.
Research also has supported the efficacy of technology-based interventions in physical activity promotion by MHCPs. These interventions include phone counseling, text messaging or smartphone application monitoring systems (including the MOVE! Coach mobile app), DVD-based approaches, and web-based interventions.38-42 However, these interventions may be most effective when complemented with face-to-face support (eg, psychotherapy, nutrition/exercise classes).43 Although MHCPs can promote physical activity in various ways, intensive focus on this target is not standard practice in many mental health care settings.
Barriers to Physical Activity
Despite physical activity promotion efforts, patients struggle to implement and maintain physical activity recommendations.22,44,45 For many patients, exercise is a new or long abandoned activity, and instruction on how to exercise properly is needed.46 Lack of financial resources may limit access to a gym, trainer, or physical therapist.47 Some patients avoid exercise because of body image concerns, and many think they lack the self-discipline and time for exercise.46,48,49
Additional barriers to physical activity are pain, fatigue, and other physical symptoms.50-52 Obese patients may find physical activity less enjoyable and more uncomfortable.53 Some patients fear exercise will exacerbate medical problems or have negative physical consequences.51,52
Psychiatric symptoms and medication adverse effects are commonly reported barriers.54 Some patients with anxiety avoid physical activity because the resulting physiologic sensations (eg, rapid heart rate, sweating) are similar to anxiety symptoms.55 Patients with posttraumatic stress disorder (PTSD) are less likely to exercise, secondary to PTSD-related avoidance, even though they were physically active before their trauma.56-58 Some patients with depression avoid exercise and other activities because their symptoms (eg, fatigue, anhedonia) make it difficult for them to take action. Many patients put off exercise while waiting for relief of mental health symptoms, even though evidence suggests that physical activity may help improve those symptoms.5,6
These barriers often render ineffective the approach of simply recommending exercise or encouraging patients to exercise. Counseling alone may not be sufficient to effect meaningful change in exercise habits. Many effective physical activity interventions have both a counseling and exercise components,59-61 and research suggests that such interventions may be most effective when they include a form of experiential exercise.10
Clinician-Assisted Experiential Exercise
Exercise interventions may involve information dissemination, counseling, an experiential exercise program, or a combination of these activities. Research has yet to determine precisely which components are most effective. Given the barriers to adhering to exercise recommendations, however, exercise interventions that include an experiential component may be more likely to affect behavior change.
According to Sime, exercise therapy is the “practice of combining a program of exercise with traditional psychotherapy.”62 Sime outlined a 10-session approach to exercise therapy and suggested that walking with patients while engaging in psychotherapy can reduce barriers to change. This approach may be effective for several reasons. First, it models the recommendation to engage in activity despite not feeling well and often improves mood. Second, the experiential nature of the intervention gives the patient an immediate opportunity to physically feel the benefits of activity. Third, the experiential component is similar to experiential exercise interventions, which have been shown to improve chronic health problems, such as obesity, and it parallels in vivo exposure, which is highly effective in treating anxiety.10,63
Exposure to exercise also has been effective in treating chronic pain in patients who fear physical activity because they anticipate pain or reinjury. In patients with chronic low back pain, in vivo exposure reduced anxiety more than an education-only session did, and the result was improved participation in relevant daily activities.64 Results were sustained at the 6-month follow-up but only for patients who received in vivo exposure.65 Similarly, in vivo exposure to feared movements increased physical activity and reduced pain-related fear, catastrophizing, and disability in patients with chronic low back pain.65 These findings have implications for other chronic health problems. Particularly for patients who fear and avoid exercise, psychoeducation about exercise and opportunities to experience exercise in session may increase physical activity outside of therapy.10
Obstacles to Exercise Promotion
Mental health care providers may be reluctant to use experiential exercise interventions for a variety of reasons. Some fear that they or their patients might sustain an injury or an exacerbation of physical symptoms. In addition, some MHCPs have liability and safety concerns surrounding meetings with patients outside the office. And obtaining medical clearance requires extra time and energy.
Some MHCPs think that this type of experiential activity might cross a professional boundary. Others may wonder whether providing experiential exercise as part of mental health services is sufficiently evidence based or is a breach of standards of practice. Similarly, some MHCPs who use manual-based interventions are hesitant to stray from an evidence-based protocol and include experiential exercise in psychotherapy. Further, some MHCPs do not feel competent to provide such an intervention, given that it is not typically covered in their mental health care training, and they think that providing opportunities for experiential exercise falls outside their MHCP role. Last, some MHCPs are uncomfortable exercising on their own and thus may be particularly uncomfortable exercising in front of patients.
Promoting Physical Activity
Although significant, barriers to promotion of physical activity can be effectively reduced by taking the steps outlined in Table 3. First, MHCPs must reflect on their own past and present physical activity and on their readiness to provide clinician-assisted experiential exercise. In addition, MHCPs should explore nearby alternative resources for physical activity, share their findings with patients, and encourage patients to use these resources. Next, medical clearance for increased physical activity can be obtained from patients’ primary care physicians, and any physical activity recommendations or limitations can be reviewed and documented. Mental health care providers should then obtain patients’ informed consent, which involves discussing the potential risks and benefits of increased exercise and, if appropriate, collaborate with patients to reach an agreement to focus on physical activity as an important aspect of their work together. Any additional risks and benefits of clinician-assisted experiential exercise can be discussed, and the ways in which physical activity can be used in session (eg, “walk and talk therapy”; other exercises recommended by the medical team) can be reviewed. Further, MHCPs can clarify their role and discuss how clear boundaries will be maintained within the therapeutic relationship. Alternative VA and community services that can help increase physical activity should also be discussed.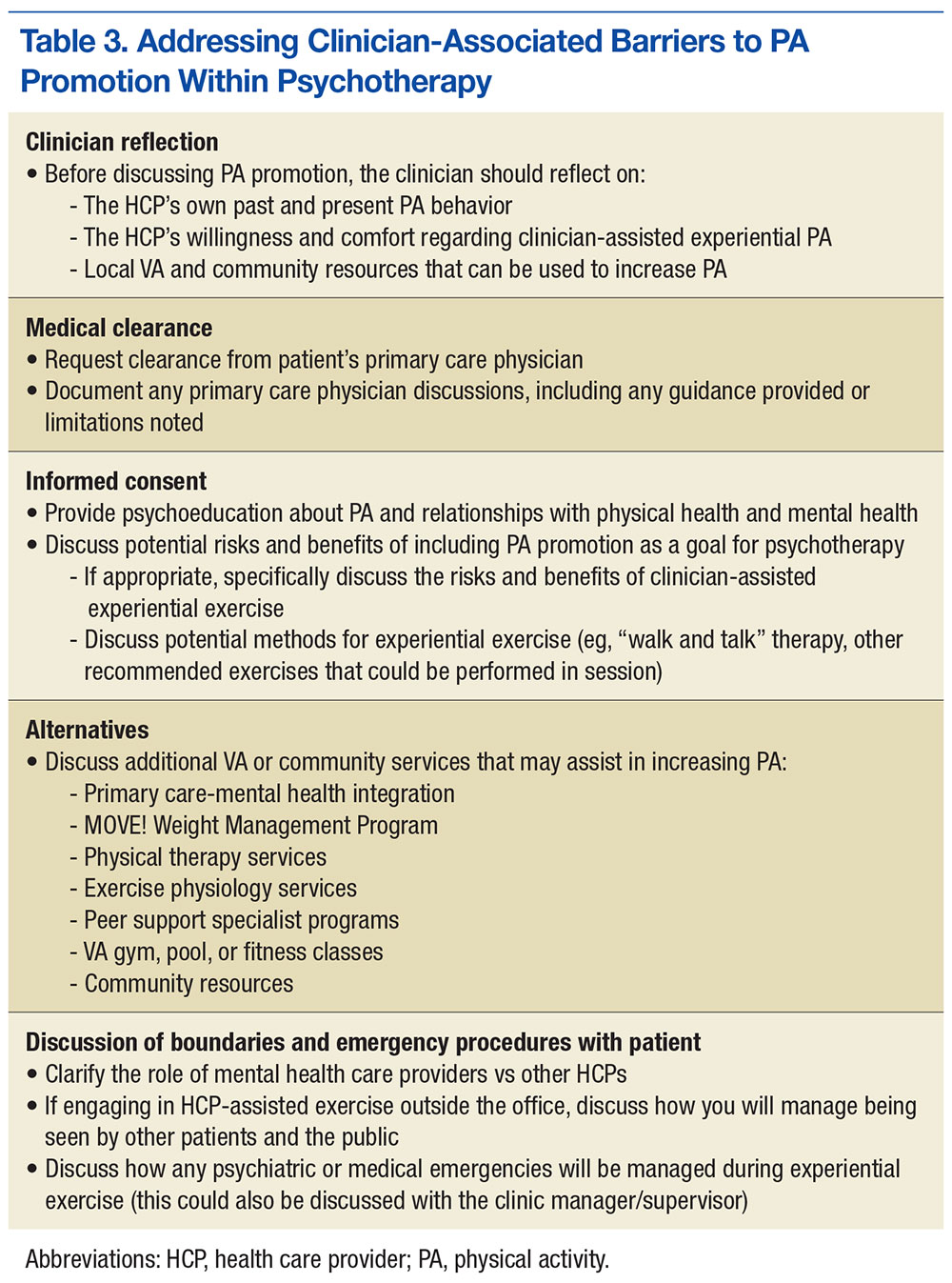
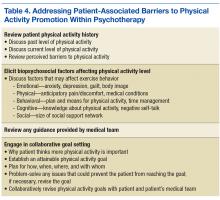
Once these steps are complete, MHCPs can address patient’s barriers to physical activity (Table 4). A discussion of the patient’s physical activity history is a good starting point. Biopsychosocial factors that can affect the ability to engage in and follow through with physical activity can then be explored, and HCPs and patients can set specific attainable physical activity goals. For instance, MHCPs can specify whether in-session clinic-assisted experiential exercise will be used and, if so, in what capacity. Last, physical activity goals can be revised periodically and revisited with the medical team.
Alternate Promotions
Mental health care providers also should consider involving other HCPs. Physical therapists and exercise physiologists are in a unique position to provide experiential exercise training. Some VA facilities include experiential exercise in their MOVE! program—veterans exercise together in the VA’s physical therapy gym while being monitored by a physical therapist.
Peer support specialists (PSPs) also are in a unique position to effectively provide experiential physical activity interventions at VA facilities. These PSPs are veterans who have physical or mental health problems but are far enough along in recovery to provide helpful services to other veterans with similar challenges.66 Recent organizational efforts have increased the presence of PSPs in VA clinics. Peer support specialists use aspects of their recovery to help other veterans, provide supportive counseling, and facilitate activity groups, such as walking, hiking, golfing, and photography groups. Further, PSPs may not have to deal with MHCPs’ concerns regarding scope of practice and clinical boundaries vis-à-vis exercise interventions.
Other VA and community programs provide ways for veterans to engage in experiential physical activities. Some VA facilities have pools and gyms that provide open hours for veterans; some even offer free HCP guidance. Clinics also occasionally provide transportation to community gyms that offer veterans discounted memberships. Team Red, White, and Blue (https://www.teamrwb.org), Veterans Expeditions, (http://www.vetexpeditions.com) and other community organizations promote veterans’ physical activity by organizing events, such as endurance races, fly fishing, and mountaineering. By staying up-to-date on local community services, MHCPs can facilitate opportunities for experiential exercise alongside the psychotherapy services they provide.
Although valuable resources exist, veterans nevertheless encounter obstacles to exercise. For instance, many VA HCPs do not include physical therapy as a standard part of the MOVE! program. Others offer physical therapy not as an integrated service but as a separate, optional service, and attendance requires more initiative. Often, veterans are referred for physical therapy only if they have sustained an injury. Even when physical therapy or exercise physiology services are offered, many veterans have difficulty following through. Reasons include anxiety, time constraints, difficulty managing multiple appointments, and negative beliefs about exercise and physical therapy (eg, it will make me hurt, physical therapy is only for people recovering from an injury). Last, some veterans are reluctant to engage in peer-led or non-VA exercise programs.
Future Research
This article highlights the need for research in several areas. First, it would be helpful to know the extent to which VA MHCPs are already promoting physical activity for their patients and the ways in which they are using experiential exercise interventions. Research also will help determine the extent to which experiential exercise interventions can be effective in treating mental and physical health conditions not listed in Table 2 and any conditions for which exercise interventions may be contraindicated. The effectiveness of exercise therapy, as described by Sime, also warrants more investigation with randomized clinical trials.62 Further, it would be useful to know more about the extent and effectiveness of other experiential exercise available to veterans, whether through the MOVE! program or through other VA or community resources. This would help HCPs understand how to best promote physical activity in veterans with chronic physical and mental health needs.
Conclusion
Most people fall short of recommended levels of physical activity, and this is especially true of veterans, who also are at higher risk for chronic physical and mental health problems.23,24 VA MHCPs are in a unique position to promote physical activity, and therapy programs that include experiential exercise may be particularly effective in helping veterans become and stay active. Other providers are well suited to provide experiential exercise opportunities, but MHCPs can simultaneously address the psychological factors that prevent veterans from engaging in exercise. Although VA MHCPs should continue to collaborate with other resources that can provide experiential exercise, they should also consider the potential benefit of experiential exercise within psychotherapy.
1. Turi BC, Codogno JS, Fernandes RA, Monteiro HL. Physical activity, adiposity and hypertension among patients of public healthcare system [in English, Portuguese]. Rev Bras Epidemiol. 2014;17(4):925-937.
2. Press V, Freestone I, George CF. Physical activity: the evidence of benefit in the prevention of coronary heart disease. QJM. 2003;96(4):245-251.
3. Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med. 2008;38(10):807-824.
4. Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241-2247.
5. Brunes A, Augestad L, Gudmundsdottir S. Personality, physical activity, and symptoms of anxiety and depression: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. 2013;48(5):745-756.
6. De Mello MT, Lemos Vde A, Antunes HK, Bittencourt L, Santos-Silva R, Tufik S. Relationship between physical activity and depression and anxiety symptoms: a population study. J Affect Disord. 2013;149(1-3):241-246.
7. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
8. Teixeira CM, Vasconcelos-Raposo J, Fernandes HM, Brustad RJ. Physical activity, depression and anxiety among the elderly. Soc Indic Res. 2013;113(1):307-318.
9. Avery L, Flynn D, Dombrowski SU, van Wersch A, Sniehotta FF, Trenell MI. Successful behavioural strategies to increase physical activity and improve glucose control in adults with type 2 diabetes. Diabet Med. 2015;32(8):1058-1062.
10. Craighead LW, Blum MD. Supervised exercise in behavioral treatment for moderate obesity. Behav Ther. 1989;20(1):49-59.
11. Gulliford MC, Charlton J, Bhattarai N, Charlton C, Rudisill C. Impact and cost-effectiveness of a universal strategy to promote physical activity in primary care: population-based cohort study and Markov model. Eur J Health Econ. 2014;15(4):341-351.
12. Muda SH, Kadir AA. The effectiveness of physical activity counseling in primary care clinic University Science Malaysia Hospital. Int Med J. 2006;13(4):249-253.
13. Plotnikoff RC, Pickering MA, Glenn N, et al. The effects of a supplemental, theory-based physical activity counseling intervention for adults with type 2 diabetes. J Phys Act Health. 2011;8(7):944-954.
14. Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43(10):1009-1023.
15. Tse MM, Vong SK, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J Clin Nurs. 2013;22(13-14):1843-1856.
16. Babyak M, Blumenthal JA, Herman S et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
17. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
18. Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nurs Res. 2010;59(3):224-231.
19. Lee C, Russell A. Effects of physical activity on emotional well-being among older Australian women: cross-sectional and longitudinal analyses. J Psychosom Res. 2003;54(2):155-160.
20. Martinsen EW. Physical activity in the prevention and treatment of anxiety and depression. Nord J Psychiatry. 2008;62(suppl 47):25-29.
21. Phillips WT, Kiernan M, King AC. Physical activity as a nonpharmacological treatment for depression: a review. Complement Health Pract Rev. 2003;8(2):1-14.
22. Centers for Disease Control and Prevention. Facts about physical activity. http://www.cdc.gov/physicalactivity/data/facts.htm. Updated May 23, 2014. Accessed August 22, 2016.
23. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
24. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25–64: United States, 2007–2010. NCHS Data Brief. http://www.cdc.gov/nchs/data/databriefs/db101.pdf. Published August 2012. Accessed August 22, 2016.
25. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
26. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA Health Care System. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
27. Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276(18):1473-1479.
28. Gagliardi AR, Abdallah F, Faulkner G, Ciliska D, Hicks A. Factors contributing to the effectiveness of physical activity counselling in primary care: a realist systematic review. Patient Educ Couns. 2015;98(4):412-419.
29. Hardcastle S, Blake N, Hagger M. The effectiveness of a motivational interviewing primary-care based intervention on physical activity and predictors of change in a disadvantaged community. J Behav Med. 2012;35(3):318-333.
30. Johnson-Lawrence V, Zivin K, Szymanski BR, Pfeiffer PN, McCarthy JF. VA primary care–mental health integration: patient characteristics and receipt of mental health services, 2008–2010. Psychiatr Serv. 2012;63(11):1137-1141.
31. U.S. Department of Veterans Affairs. MOVE! weight management program. http://www.move.va.gov. Updated August 1, 2016. Accessed August 22, 2016.
32. Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for veterans. Prev Chronic Dis. 2009;6(3):A98.
33. Dahn JR, Fitzpatrick SL, Llabre MM, et al. Weight management for veterans: examining change in weight before and after MOVE! Obesity (Silver Spring). 2011;19(5):977-981.
34. Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med. 2011;1(4):551-560.
35. Taft TH, Payvar S, Wool L. Effectiveness of the MOVE! program among African American veterans: weight loss and quality of life. Fed Pract. 2011;28(12):17-24.
36. Kahwati LC, Lewis MA, Kane H, et al. Best practices in the Veterans Health Administration’s MOVE! weight management program. Am J Prev Med. 2011;41(5):457-464.
37. Butryn M, Forman E, Hoffman K, Shaw J, Juarascio A. A pilot study of acceptance and commitment therapy for promotion of physical activity. J Phys Act Health. 2011;8(4):516-522.
38. Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med. 2002;22(3):177-183.
39. Prestwich A, Perugini M, Hurling R. Can the effects of implementation intentions on exercise be enhanced using text messages? Psychol Health. 2009;24(6):677-687.
40. U.S. Department of Veterans Affairs. What is MOVE! Coach? http://www.move.va.gov/moveCoachIntro.asp. Updated May 26, 2016. Accessed September 6, 2016.
41. Moffitt R, Mohr P. The efficacy of a self-managed acceptance and commitment therapy intervention DVD for physical activity initiation. Br J Health Psychol. 2015;20(1):115-129.
42. Duncan M, Vandelanotte C, Kolt GS, et al. Effectiveness of a web- and mobile phone–based intervention to promote physical activity and healthy eating in middle-aged males: randomized controlled trial of the ManUp study. J Med Internet Res. 2014;16(6):e136.
43. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28(4):320-329.
44. Jefferis BJ, Sartini C, Lee IM, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. 2014;14:382.
45. Stevinson C, Lydon A, Amir Z. Adherence to physical activity guidelines among cancer support group participants. Eur J Cancer Care (Engl). 2014;23(2):199-205.
46. Spector D, Battaglini C, Groff D. Perceived exercise barriers and facilitators among ethnically diverse breast cancer survivors. Oncol Nurs Forum. 2013;40(5):472-480.
47. Daly JM, Hartz AJ, Xu Y, et al. An assessment of attitudes, behaviors, and outcomes of patients with type 2 diabetes. J Am Board Fam Med. 2009;22(3):280-290.
48. Bautista L, Reininger B, Gay JL, Barroso CS, McCormick JB. Perceived barriers to exercise in Hispanic adults by level of activity. J Phys Act Health. 2011;8(7):916-925.
49. Asano M, Duquette P, Andersen R, Lapierre Y, Mayo N. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35(5):353-361.
50. Hefferon K, Murphy H, McLeod J, Mutrie N, Campbell A. Understanding barriers to exercise implementation 5-year post–breast cancer diagnosis: a large-scale qualitative study. Health Educ Res. 2013;28(5):843-856.
51. Crombez G, Vlaeyen J, Heuts P, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1-2):329-339.
52. Rogerson M, Murphy BM, Bird S, Morris T. “I don’t have the heart”: a qualitative study of barriers to and facilitators of physical activity for people with coronary heart disease and depressive symptoms. Int J Behav Nutr Phys Act. 2012;9:140.
53. Leone LA, Ward DS. A mixed methods comparison of perceived benefits and barriers to exercise between obese and nonobese women. J Phys Act Health. 2013;10(4):461-469.
54. Glover C, Ferron J, Whitley R. Barriers to exercise among people with severe mental illnesses. Psychiatr Rehabil J. 2013;36(1):45-47.
55. Sabourin B, Hilchey C, Lefaivre M, Watt M, Stewart S. Why do they exercise less? Barriers to exercise in high-anxiety-sensitive women. Cogn Behav Ther. 2011;40(3):206-215.
56. Hall KS, Hoerster KD, Yancy WS Jr. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103-115.
57. LeardMann CA, Kelton ML, Smith B, et al; Millennium Cohort Study Team. Prospectively assessed posttraumatic stress disorder and associated physical activity. Public Health Rep. 2011;126(3):371-383.
58. de Assis MA, de Mello MF, Scorza FA, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo). 2008;63(4):473-478.
59. Jimmy G, Martin BW. Implementation and effectiveness of a primary care based physical activity counselling scheme. Patient Educ Couns. 2005;56(3):323-331.
60. Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119-131.
61. van Koulil S, van Lankveld W, Kraaimaat FW, et al. Tailored cognitive-behavioral therapy and exercise training for high-risk patients with fibromyalgia. Arthritis Care Res (Hoboken). 2010;62(10):1377-1385.
62. Sime WE. Exercise therapy for stress management. In: Lehrer PM, Woolfolk RL, Sime WE, eds. Principles and Practice of Stress Management. 3rd ed. New York, NY: Guilford Press; 2007:333-359.
63. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York, NY: Oxford University Press; 2007.
64. de Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005;21(1):9-17.
65. Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18(4):251-261.
66. Chinman M, Young A, Hassell J, Davidson L. Toward the implementation of mental health consumer provider services. J Behav Health Serv Res. 2006;33(2):176-195.
Physical activity is a key determinant of health. Low levels of activity are associated with onset of and poorer outcomes of many chronic health conditions (eg, obesity, coronary artery disease, type 2 diabetes mellitus, chronic pain, hypertension1-4) and with higher rates of mental health conditions (eg, depression, anxiety5-8).
Behavioral interventions (Table 1) can increase activity and improve physical health and mental health (Table 2).9-21 However, only 20% of adults in the U.S. meet federal recommendations for physical activity.22 The situation is particularly grim in the veteran population. Littman and colleagues found that veterans were less likely than nonveterans were to meet physical activity standards, and VA patients were even less likely than were non-VA veterans to meet the recommendations.23
Given that exercise can positively affect physical and mental health and that VA mental health care professionals (MHCPs) have training in motivational enhancement and behavior modification, these clinicians are well positioned to intervene. The question arises, though: How can VA MHCPs do more to effectively promote physical activity in veterans?
Addressing Physical Activity
There are numerous ways in which VA MHCPs can address physical activity with their patients. Several studies have demonstrated that physical activity interventions provided within primary care–mental health integration programs resulted in increased physical activity.28,29 The number of VA health care providers (HCPs) offering such programs is increasing, which could mean that behavioral health support for physical activity promotion could become easier for veterans to access.30
In addition, National Center for Health Promotion and Disease Prevention initiatives have led to an expansion of programs, such as the VA MOVE! Weight Management Program.31 Often cofacilitated by dieticians and MHCPs, MOVE! includes nutrition education, behavior modification, and physical activity promotion.32 Preliminary research suggests that MOVE! helps veterans lose weight and improve their health-related quality of life.33-36
Further, psychological and behavioral interventions can specifically target exercise and have been shown to increase physical activity, improve mood symptoms, and reduce health risk factors.9-21,37 However, little is known about the extent of exercise promotion in VA outpatient mental health services. For instance, some HCPs may educate patients about the benefits of physical activity, while others may facilitate physical activity scheduling, address barriers, and monitor, reinforce, and problem-solve physical activity goals.
Research also has supported the efficacy of technology-based interventions in physical activity promotion by MHCPs. These interventions include phone counseling, text messaging or smartphone application monitoring systems (including the MOVE! Coach mobile app), DVD-based approaches, and web-based interventions.38-42 However, these interventions may be most effective when complemented with face-to-face support (eg, psychotherapy, nutrition/exercise classes).43 Although MHCPs can promote physical activity in various ways, intensive focus on this target is not standard practice in many mental health care settings.
Barriers to Physical Activity
Despite physical activity promotion efforts, patients struggle to implement and maintain physical activity recommendations.22,44,45 For many patients, exercise is a new or long abandoned activity, and instruction on how to exercise properly is needed.46 Lack of financial resources may limit access to a gym, trainer, or physical therapist.47 Some patients avoid exercise because of body image concerns, and many think they lack the self-discipline and time for exercise.46,48,49
Additional barriers to physical activity are pain, fatigue, and other physical symptoms.50-52 Obese patients may find physical activity less enjoyable and more uncomfortable.53 Some patients fear exercise will exacerbate medical problems or have negative physical consequences.51,52
Psychiatric symptoms and medication adverse effects are commonly reported barriers.54 Some patients with anxiety avoid physical activity because the resulting physiologic sensations (eg, rapid heart rate, sweating) are similar to anxiety symptoms.55 Patients with posttraumatic stress disorder (PTSD) are less likely to exercise, secondary to PTSD-related avoidance, even though they were physically active before their trauma.56-58 Some patients with depression avoid exercise and other activities because their symptoms (eg, fatigue, anhedonia) make it difficult for them to take action. Many patients put off exercise while waiting for relief of mental health symptoms, even though evidence suggests that physical activity may help improve those symptoms.5,6
These barriers often render ineffective the approach of simply recommending exercise or encouraging patients to exercise. Counseling alone may not be sufficient to effect meaningful change in exercise habits. Many effective physical activity interventions have both a counseling and exercise components,59-61 and research suggests that such interventions may be most effective when they include a form of experiential exercise.10
Clinician-Assisted Experiential Exercise
Exercise interventions may involve information dissemination, counseling, an experiential exercise program, or a combination of these activities. Research has yet to determine precisely which components are most effective. Given the barriers to adhering to exercise recommendations, however, exercise interventions that include an experiential component may be more likely to affect behavior change.
According to Sime, exercise therapy is the “practice of combining a program of exercise with traditional psychotherapy.”62 Sime outlined a 10-session approach to exercise therapy and suggested that walking with patients while engaging in psychotherapy can reduce barriers to change. This approach may be effective for several reasons. First, it models the recommendation to engage in activity despite not feeling well and often improves mood. Second, the experiential nature of the intervention gives the patient an immediate opportunity to physically feel the benefits of activity. Third, the experiential component is similar to experiential exercise interventions, which have been shown to improve chronic health problems, such as obesity, and it parallels in vivo exposure, which is highly effective in treating anxiety.10,63
Exposure to exercise also has been effective in treating chronic pain in patients who fear physical activity because they anticipate pain or reinjury. In patients with chronic low back pain, in vivo exposure reduced anxiety more than an education-only session did, and the result was improved participation in relevant daily activities.64 Results were sustained at the 6-month follow-up but only for patients who received in vivo exposure.65 Similarly, in vivo exposure to feared movements increased physical activity and reduced pain-related fear, catastrophizing, and disability in patients with chronic low back pain.65 These findings have implications for other chronic health problems. Particularly for patients who fear and avoid exercise, psychoeducation about exercise and opportunities to experience exercise in session may increase physical activity outside of therapy.10
Obstacles to Exercise Promotion
Mental health care providers may be reluctant to use experiential exercise interventions for a variety of reasons. Some fear that they or their patients might sustain an injury or an exacerbation of physical symptoms. In addition, some MHCPs have liability and safety concerns surrounding meetings with patients outside the office. And obtaining medical clearance requires extra time and energy.
Some MHCPs think that this type of experiential activity might cross a professional boundary. Others may wonder whether providing experiential exercise as part of mental health services is sufficiently evidence based or is a breach of standards of practice. Similarly, some MHCPs who use manual-based interventions are hesitant to stray from an evidence-based protocol and include experiential exercise in psychotherapy. Further, some MHCPs do not feel competent to provide such an intervention, given that it is not typically covered in their mental health care training, and they think that providing opportunities for experiential exercise falls outside their MHCP role. Last, some MHCPs are uncomfortable exercising on their own and thus may be particularly uncomfortable exercising in front of patients.
Promoting Physical Activity
Although significant, barriers to promotion of physical activity can be effectively reduced by taking the steps outlined in Table 3. First, MHCPs must reflect on their own past and present physical activity and on their readiness to provide clinician-assisted experiential exercise. In addition, MHCPs should explore nearby alternative resources for physical activity, share their findings with patients, and encourage patients to use these resources. Next, medical clearance for increased physical activity can be obtained from patients’ primary care physicians, and any physical activity recommendations or limitations can be reviewed and documented. Mental health care providers should then obtain patients’ informed consent, which involves discussing the potential risks and benefits of increased exercise and, if appropriate, collaborate with patients to reach an agreement to focus on physical activity as an important aspect of their work together. Any additional risks and benefits of clinician-assisted experiential exercise can be discussed, and the ways in which physical activity can be used in session (eg, “walk and talk therapy”; other exercises recommended by the medical team) can be reviewed. Further, MHCPs can clarify their role and discuss how clear boundaries will be maintained within the therapeutic relationship. Alternative VA and community services that can help increase physical activity should also be discussed.
Once these steps are complete, MHCPs can address patient’s barriers to physical activity (Table 4). A discussion of the patient’s physical activity history is a good starting point. Biopsychosocial factors that can affect the ability to engage in and follow through with physical activity can then be explored, and HCPs and patients can set specific attainable physical activity goals. For instance, MHCPs can specify whether in-session clinic-assisted experiential exercise will be used and, if so, in what capacity. Last, physical activity goals can be revised periodically and revisited with the medical team.
Alternate Promotions
Mental health care providers also should consider involving other HCPs. Physical therapists and exercise physiologists are in a unique position to provide experiential exercise training. Some VA facilities include experiential exercise in their MOVE! program—veterans exercise together in the VA’s physical therapy gym while being monitored by a physical therapist.
Peer support specialists (PSPs) also are in a unique position to effectively provide experiential physical activity interventions at VA facilities. These PSPs are veterans who have physical or mental health problems but are far enough along in recovery to provide helpful services to other veterans with similar challenges.66 Recent organizational efforts have increased the presence of PSPs in VA clinics. Peer support specialists use aspects of their recovery to help other veterans, provide supportive counseling, and facilitate activity groups, such as walking, hiking, golfing, and photography groups. Further, PSPs may not have to deal with MHCPs’ concerns regarding scope of practice and clinical boundaries vis-à-vis exercise interventions.
Other VA and community programs provide ways for veterans to engage in experiential physical activities. Some VA facilities have pools and gyms that provide open hours for veterans; some even offer free HCP guidance. Clinics also occasionally provide transportation to community gyms that offer veterans discounted memberships. Team Red, White, and Blue (https://www.teamrwb.org), Veterans Expeditions, (http://www.vetexpeditions.com) and other community organizations promote veterans’ physical activity by organizing events, such as endurance races, fly fishing, and mountaineering. By staying up-to-date on local community services, MHCPs can facilitate opportunities for experiential exercise alongside the psychotherapy services they provide.
Although valuable resources exist, veterans nevertheless encounter obstacles to exercise. For instance, many VA HCPs do not include physical therapy as a standard part of the MOVE! program. Others offer physical therapy not as an integrated service but as a separate, optional service, and attendance requires more initiative. Often, veterans are referred for physical therapy only if they have sustained an injury. Even when physical therapy or exercise physiology services are offered, many veterans have difficulty following through. Reasons include anxiety, time constraints, difficulty managing multiple appointments, and negative beliefs about exercise and physical therapy (eg, it will make me hurt, physical therapy is only for people recovering from an injury). Last, some veterans are reluctant to engage in peer-led or non-VA exercise programs.
Future Research
This article highlights the need for research in several areas. First, it would be helpful to know the extent to which VA MHCPs are already promoting physical activity for their patients and the ways in which they are using experiential exercise interventions. Research also will help determine the extent to which experiential exercise interventions can be effective in treating mental and physical health conditions not listed in Table 2 and any conditions for which exercise interventions may be contraindicated. The effectiveness of exercise therapy, as described by Sime, also warrants more investigation with randomized clinical trials.62 Further, it would be useful to know more about the extent and effectiveness of other experiential exercise available to veterans, whether through the MOVE! program or through other VA or community resources. This would help HCPs understand how to best promote physical activity in veterans with chronic physical and mental health needs.
Conclusion
Most people fall short of recommended levels of physical activity, and this is especially true of veterans, who also are at higher risk for chronic physical and mental health problems.23,24 VA MHCPs are in a unique position to promote physical activity, and therapy programs that include experiential exercise may be particularly effective in helping veterans become and stay active. Other providers are well suited to provide experiential exercise opportunities, but MHCPs can simultaneously address the psychological factors that prevent veterans from engaging in exercise. Although VA MHCPs should continue to collaborate with other resources that can provide experiential exercise, they should also consider the potential benefit of experiential exercise within psychotherapy.
Physical activity is a key determinant of health. Low levels of activity are associated with onset of and poorer outcomes of many chronic health conditions (eg, obesity, coronary artery disease, type 2 diabetes mellitus, chronic pain, hypertension1-4) and with higher rates of mental health conditions (eg, depression, anxiety5-8).
Behavioral interventions (Table 1) can increase activity and improve physical health and mental health (Table 2).9-21 However, only 20% of adults in the U.S. meet federal recommendations for physical activity.22 The situation is particularly grim in the veteran population. Littman and colleagues found that veterans were less likely than nonveterans were to meet physical activity standards, and VA patients were even less likely than were non-VA veterans to meet the recommendations.23
Given that exercise can positively affect physical and mental health and that VA mental health care professionals (MHCPs) have training in motivational enhancement and behavior modification, these clinicians are well positioned to intervene. The question arises, though: How can VA MHCPs do more to effectively promote physical activity in veterans?
Addressing Physical Activity
There are numerous ways in which VA MHCPs can address physical activity with their patients. Several studies have demonstrated that physical activity interventions provided within primary care–mental health integration programs resulted in increased physical activity.28,29 The number of VA health care providers (HCPs) offering such programs is increasing, which could mean that behavioral health support for physical activity promotion could become easier for veterans to access.30
In addition, National Center for Health Promotion and Disease Prevention initiatives have led to an expansion of programs, such as the VA MOVE! Weight Management Program.31 Often cofacilitated by dieticians and MHCPs, MOVE! includes nutrition education, behavior modification, and physical activity promotion.32 Preliminary research suggests that MOVE! helps veterans lose weight and improve their health-related quality of life.33-36
Further, psychological and behavioral interventions can specifically target exercise and have been shown to increase physical activity, improve mood symptoms, and reduce health risk factors.9-21,37 However, little is known about the extent of exercise promotion in VA outpatient mental health services. For instance, some HCPs may educate patients about the benefits of physical activity, while others may facilitate physical activity scheduling, address barriers, and monitor, reinforce, and problem-solve physical activity goals.
Research also has supported the efficacy of technology-based interventions in physical activity promotion by MHCPs. These interventions include phone counseling, text messaging or smartphone application monitoring systems (including the MOVE! Coach mobile app), DVD-based approaches, and web-based interventions.38-42 However, these interventions may be most effective when complemented with face-to-face support (eg, psychotherapy, nutrition/exercise classes).43 Although MHCPs can promote physical activity in various ways, intensive focus on this target is not standard practice in many mental health care settings.
Barriers to Physical Activity
Despite physical activity promotion efforts, patients struggle to implement and maintain physical activity recommendations.22,44,45 For many patients, exercise is a new or long abandoned activity, and instruction on how to exercise properly is needed.46 Lack of financial resources may limit access to a gym, trainer, or physical therapist.47 Some patients avoid exercise because of body image concerns, and many think they lack the self-discipline and time for exercise.46,48,49
Additional barriers to physical activity are pain, fatigue, and other physical symptoms.50-52 Obese patients may find physical activity less enjoyable and more uncomfortable.53 Some patients fear exercise will exacerbate medical problems or have negative physical consequences.51,52
Psychiatric symptoms and medication adverse effects are commonly reported barriers.54 Some patients with anxiety avoid physical activity because the resulting physiologic sensations (eg, rapid heart rate, sweating) are similar to anxiety symptoms.55 Patients with posttraumatic stress disorder (PTSD) are less likely to exercise, secondary to PTSD-related avoidance, even though they were physically active before their trauma.56-58 Some patients with depression avoid exercise and other activities because their symptoms (eg, fatigue, anhedonia) make it difficult for them to take action. Many patients put off exercise while waiting for relief of mental health symptoms, even though evidence suggests that physical activity may help improve those symptoms.5,6
These barriers often render ineffective the approach of simply recommending exercise or encouraging patients to exercise. Counseling alone may not be sufficient to effect meaningful change in exercise habits. Many effective physical activity interventions have both a counseling and exercise components,59-61 and research suggests that such interventions may be most effective when they include a form of experiential exercise.10
Clinician-Assisted Experiential Exercise
Exercise interventions may involve information dissemination, counseling, an experiential exercise program, or a combination of these activities. Research has yet to determine precisely which components are most effective. Given the barriers to adhering to exercise recommendations, however, exercise interventions that include an experiential component may be more likely to affect behavior change.
According to Sime, exercise therapy is the “practice of combining a program of exercise with traditional psychotherapy.”62 Sime outlined a 10-session approach to exercise therapy and suggested that walking with patients while engaging in psychotherapy can reduce barriers to change. This approach may be effective for several reasons. First, it models the recommendation to engage in activity despite not feeling well and often improves mood. Second, the experiential nature of the intervention gives the patient an immediate opportunity to physically feel the benefits of activity. Third, the experiential component is similar to experiential exercise interventions, which have been shown to improve chronic health problems, such as obesity, and it parallels in vivo exposure, which is highly effective in treating anxiety.10,63
Exposure to exercise also has been effective in treating chronic pain in patients who fear physical activity because they anticipate pain or reinjury. In patients with chronic low back pain, in vivo exposure reduced anxiety more than an education-only session did, and the result was improved participation in relevant daily activities.64 Results were sustained at the 6-month follow-up but only for patients who received in vivo exposure.65 Similarly, in vivo exposure to feared movements increased physical activity and reduced pain-related fear, catastrophizing, and disability in patients with chronic low back pain.65 These findings have implications for other chronic health problems. Particularly for patients who fear and avoid exercise, psychoeducation about exercise and opportunities to experience exercise in session may increase physical activity outside of therapy.10
Obstacles to Exercise Promotion
Mental health care providers may be reluctant to use experiential exercise interventions for a variety of reasons. Some fear that they or their patients might sustain an injury or an exacerbation of physical symptoms. In addition, some MHCPs have liability and safety concerns surrounding meetings with patients outside the office. And obtaining medical clearance requires extra time and energy.
Some MHCPs think that this type of experiential activity might cross a professional boundary. Others may wonder whether providing experiential exercise as part of mental health services is sufficiently evidence based or is a breach of standards of practice. Similarly, some MHCPs who use manual-based interventions are hesitant to stray from an evidence-based protocol and include experiential exercise in psychotherapy. Further, some MHCPs do not feel competent to provide such an intervention, given that it is not typically covered in their mental health care training, and they think that providing opportunities for experiential exercise falls outside their MHCP role. Last, some MHCPs are uncomfortable exercising on their own and thus may be particularly uncomfortable exercising in front of patients.
Promoting Physical Activity
Although significant, barriers to promotion of physical activity can be effectively reduced by taking the steps outlined in Table 3. First, MHCPs must reflect on their own past and present physical activity and on their readiness to provide clinician-assisted experiential exercise. In addition, MHCPs should explore nearby alternative resources for physical activity, share their findings with patients, and encourage patients to use these resources. Next, medical clearance for increased physical activity can be obtained from patients’ primary care physicians, and any physical activity recommendations or limitations can be reviewed and documented. Mental health care providers should then obtain patients’ informed consent, which involves discussing the potential risks and benefits of increased exercise and, if appropriate, collaborate with patients to reach an agreement to focus on physical activity as an important aspect of their work together. Any additional risks and benefits of clinician-assisted experiential exercise can be discussed, and the ways in which physical activity can be used in session (eg, “walk and talk therapy”; other exercises recommended by the medical team) can be reviewed. Further, MHCPs can clarify their role and discuss how clear boundaries will be maintained within the therapeutic relationship. Alternative VA and community services that can help increase physical activity should also be discussed.
Once these steps are complete, MHCPs can address patient’s barriers to physical activity (Table 4). A discussion of the patient’s physical activity history is a good starting point. Biopsychosocial factors that can affect the ability to engage in and follow through with physical activity can then be explored, and HCPs and patients can set specific attainable physical activity goals. For instance, MHCPs can specify whether in-session clinic-assisted experiential exercise will be used and, if so, in what capacity. Last, physical activity goals can be revised periodically and revisited with the medical team.
Alternate Promotions
Mental health care providers also should consider involving other HCPs. Physical therapists and exercise physiologists are in a unique position to provide experiential exercise training. Some VA facilities include experiential exercise in their MOVE! program—veterans exercise together in the VA’s physical therapy gym while being monitored by a physical therapist.
Peer support specialists (PSPs) also are in a unique position to effectively provide experiential physical activity interventions at VA facilities. These PSPs are veterans who have physical or mental health problems but are far enough along in recovery to provide helpful services to other veterans with similar challenges.66 Recent organizational efforts have increased the presence of PSPs in VA clinics. Peer support specialists use aspects of their recovery to help other veterans, provide supportive counseling, and facilitate activity groups, such as walking, hiking, golfing, and photography groups. Further, PSPs may not have to deal with MHCPs’ concerns regarding scope of practice and clinical boundaries vis-à-vis exercise interventions.
Other VA and community programs provide ways for veterans to engage in experiential physical activities. Some VA facilities have pools and gyms that provide open hours for veterans; some even offer free HCP guidance. Clinics also occasionally provide transportation to community gyms that offer veterans discounted memberships. Team Red, White, and Blue (https://www.teamrwb.org), Veterans Expeditions, (http://www.vetexpeditions.com) and other community organizations promote veterans’ physical activity by organizing events, such as endurance races, fly fishing, and mountaineering. By staying up-to-date on local community services, MHCPs can facilitate opportunities for experiential exercise alongside the psychotherapy services they provide.
Although valuable resources exist, veterans nevertheless encounter obstacles to exercise. For instance, many VA HCPs do not include physical therapy as a standard part of the MOVE! program. Others offer physical therapy not as an integrated service but as a separate, optional service, and attendance requires more initiative. Often, veterans are referred for physical therapy only if they have sustained an injury. Even when physical therapy or exercise physiology services are offered, many veterans have difficulty following through. Reasons include anxiety, time constraints, difficulty managing multiple appointments, and negative beliefs about exercise and physical therapy (eg, it will make me hurt, physical therapy is only for people recovering from an injury). Last, some veterans are reluctant to engage in peer-led or non-VA exercise programs.
Future Research
This article highlights the need for research in several areas. First, it would be helpful to know the extent to which VA MHCPs are already promoting physical activity for their patients and the ways in which they are using experiential exercise interventions. Research also will help determine the extent to which experiential exercise interventions can be effective in treating mental and physical health conditions not listed in Table 2 and any conditions for which exercise interventions may be contraindicated. The effectiveness of exercise therapy, as described by Sime, also warrants more investigation with randomized clinical trials.62 Further, it would be useful to know more about the extent and effectiveness of other experiential exercise available to veterans, whether through the MOVE! program or through other VA or community resources. This would help HCPs understand how to best promote physical activity in veterans with chronic physical and mental health needs.
Conclusion
Most people fall short of recommended levels of physical activity, and this is especially true of veterans, who also are at higher risk for chronic physical and mental health problems.23,24 VA MHCPs are in a unique position to promote physical activity, and therapy programs that include experiential exercise may be particularly effective in helping veterans become and stay active. Other providers are well suited to provide experiential exercise opportunities, but MHCPs can simultaneously address the psychological factors that prevent veterans from engaging in exercise. Although VA MHCPs should continue to collaborate with other resources that can provide experiential exercise, they should also consider the potential benefit of experiential exercise within psychotherapy.
1. Turi BC, Codogno JS, Fernandes RA, Monteiro HL. Physical activity, adiposity and hypertension among patients of public healthcare system [in English, Portuguese]. Rev Bras Epidemiol. 2014;17(4):925-937.
2. Press V, Freestone I, George CF. Physical activity: the evidence of benefit in the prevention of coronary heart disease. QJM. 2003;96(4):245-251.
3. Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med. 2008;38(10):807-824.
4. Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241-2247.
5. Brunes A, Augestad L, Gudmundsdottir S. Personality, physical activity, and symptoms of anxiety and depression: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. 2013;48(5):745-756.
6. De Mello MT, Lemos Vde A, Antunes HK, Bittencourt L, Santos-Silva R, Tufik S. Relationship between physical activity and depression and anxiety symptoms: a population study. J Affect Disord. 2013;149(1-3):241-246.
7. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
8. Teixeira CM, Vasconcelos-Raposo J, Fernandes HM, Brustad RJ. Physical activity, depression and anxiety among the elderly. Soc Indic Res. 2013;113(1):307-318.
9. Avery L, Flynn D, Dombrowski SU, van Wersch A, Sniehotta FF, Trenell MI. Successful behavioural strategies to increase physical activity and improve glucose control in adults with type 2 diabetes. Diabet Med. 2015;32(8):1058-1062.
10. Craighead LW, Blum MD. Supervised exercise in behavioral treatment for moderate obesity. Behav Ther. 1989;20(1):49-59.
11. Gulliford MC, Charlton J, Bhattarai N, Charlton C, Rudisill C. Impact and cost-effectiveness of a universal strategy to promote physical activity in primary care: population-based cohort study and Markov model. Eur J Health Econ. 2014;15(4):341-351.
12. Muda SH, Kadir AA. The effectiveness of physical activity counseling in primary care clinic University Science Malaysia Hospital. Int Med J. 2006;13(4):249-253.
13. Plotnikoff RC, Pickering MA, Glenn N, et al. The effects of a supplemental, theory-based physical activity counseling intervention for adults with type 2 diabetes. J Phys Act Health. 2011;8(7):944-954.
14. Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43(10):1009-1023.
15. Tse MM, Vong SK, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J Clin Nurs. 2013;22(13-14):1843-1856.
16. Babyak M, Blumenthal JA, Herman S et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
17. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
18. Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nurs Res. 2010;59(3):224-231.
19. Lee C, Russell A. Effects of physical activity on emotional well-being among older Australian women: cross-sectional and longitudinal analyses. J Psychosom Res. 2003;54(2):155-160.
20. Martinsen EW. Physical activity in the prevention and treatment of anxiety and depression. Nord J Psychiatry. 2008;62(suppl 47):25-29.
21. Phillips WT, Kiernan M, King AC. Physical activity as a nonpharmacological treatment for depression: a review. Complement Health Pract Rev. 2003;8(2):1-14.
22. Centers for Disease Control and Prevention. Facts about physical activity. http://www.cdc.gov/physicalactivity/data/facts.htm. Updated May 23, 2014. Accessed August 22, 2016.
23. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
24. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25–64: United States, 2007–2010. NCHS Data Brief. http://www.cdc.gov/nchs/data/databriefs/db101.pdf. Published August 2012. Accessed August 22, 2016.
25. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
26. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA Health Care System. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
27. Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276(18):1473-1479.
28. Gagliardi AR, Abdallah F, Faulkner G, Ciliska D, Hicks A. Factors contributing to the effectiveness of physical activity counselling in primary care: a realist systematic review. Patient Educ Couns. 2015;98(4):412-419.
29. Hardcastle S, Blake N, Hagger M. The effectiveness of a motivational interviewing primary-care based intervention on physical activity and predictors of change in a disadvantaged community. J Behav Med. 2012;35(3):318-333.
30. Johnson-Lawrence V, Zivin K, Szymanski BR, Pfeiffer PN, McCarthy JF. VA primary care–mental health integration: patient characteristics and receipt of mental health services, 2008–2010. Psychiatr Serv. 2012;63(11):1137-1141.
31. U.S. Department of Veterans Affairs. MOVE! weight management program. http://www.move.va.gov. Updated August 1, 2016. Accessed August 22, 2016.
32. Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for veterans. Prev Chronic Dis. 2009;6(3):A98.
33. Dahn JR, Fitzpatrick SL, Llabre MM, et al. Weight management for veterans: examining change in weight before and after MOVE! Obesity (Silver Spring). 2011;19(5):977-981.
34. Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med. 2011;1(4):551-560.
35. Taft TH, Payvar S, Wool L. Effectiveness of the MOVE! program among African American veterans: weight loss and quality of life. Fed Pract. 2011;28(12):17-24.
36. Kahwati LC, Lewis MA, Kane H, et al. Best practices in the Veterans Health Administration’s MOVE! weight management program. Am J Prev Med. 2011;41(5):457-464.
37. Butryn M, Forman E, Hoffman K, Shaw J, Juarascio A. A pilot study of acceptance and commitment therapy for promotion of physical activity. J Phys Act Health. 2011;8(4):516-522.
38. Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med. 2002;22(3):177-183.
39. Prestwich A, Perugini M, Hurling R. Can the effects of implementation intentions on exercise be enhanced using text messages? Psychol Health. 2009;24(6):677-687.
40. U.S. Department of Veterans Affairs. What is MOVE! Coach? http://www.move.va.gov/moveCoachIntro.asp. Updated May 26, 2016. Accessed September 6, 2016.
41. Moffitt R, Mohr P. The efficacy of a self-managed acceptance and commitment therapy intervention DVD for physical activity initiation. Br J Health Psychol. 2015;20(1):115-129.
42. Duncan M, Vandelanotte C, Kolt GS, et al. Effectiveness of a web- and mobile phone–based intervention to promote physical activity and healthy eating in middle-aged males: randomized controlled trial of the ManUp study. J Med Internet Res. 2014;16(6):e136.
43. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28(4):320-329.
44. Jefferis BJ, Sartini C, Lee IM, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. 2014;14:382.
45. Stevinson C, Lydon A, Amir Z. Adherence to physical activity guidelines among cancer support group participants. Eur J Cancer Care (Engl). 2014;23(2):199-205.
46. Spector D, Battaglini C, Groff D. Perceived exercise barriers and facilitators among ethnically diverse breast cancer survivors. Oncol Nurs Forum. 2013;40(5):472-480.
47. Daly JM, Hartz AJ, Xu Y, et al. An assessment of attitudes, behaviors, and outcomes of patients with type 2 diabetes. J Am Board Fam Med. 2009;22(3):280-290.
48. Bautista L, Reininger B, Gay JL, Barroso CS, McCormick JB. Perceived barriers to exercise in Hispanic adults by level of activity. J Phys Act Health. 2011;8(7):916-925.
49. Asano M, Duquette P, Andersen R, Lapierre Y, Mayo N. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35(5):353-361.
50. Hefferon K, Murphy H, McLeod J, Mutrie N, Campbell A. Understanding barriers to exercise implementation 5-year post–breast cancer diagnosis: a large-scale qualitative study. Health Educ Res. 2013;28(5):843-856.
51. Crombez G, Vlaeyen J, Heuts P, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1-2):329-339.
52. Rogerson M, Murphy BM, Bird S, Morris T. “I don’t have the heart”: a qualitative study of barriers to and facilitators of physical activity for people with coronary heart disease and depressive symptoms. Int J Behav Nutr Phys Act. 2012;9:140.
53. Leone LA, Ward DS. A mixed methods comparison of perceived benefits and barriers to exercise between obese and nonobese women. J Phys Act Health. 2013;10(4):461-469.
54. Glover C, Ferron J, Whitley R. Barriers to exercise among people with severe mental illnesses. Psychiatr Rehabil J. 2013;36(1):45-47.
55. Sabourin B, Hilchey C, Lefaivre M, Watt M, Stewart S. Why do they exercise less? Barriers to exercise in high-anxiety-sensitive women. Cogn Behav Ther. 2011;40(3):206-215.
56. Hall KS, Hoerster KD, Yancy WS Jr. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103-115.
57. LeardMann CA, Kelton ML, Smith B, et al; Millennium Cohort Study Team. Prospectively assessed posttraumatic stress disorder and associated physical activity. Public Health Rep. 2011;126(3):371-383.
58. de Assis MA, de Mello MF, Scorza FA, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo). 2008;63(4):473-478.
59. Jimmy G, Martin BW. Implementation and effectiveness of a primary care based physical activity counselling scheme. Patient Educ Couns. 2005;56(3):323-331.
60. Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119-131.
61. van Koulil S, van Lankveld W, Kraaimaat FW, et al. Tailored cognitive-behavioral therapy and exercise training for high-risk patients with fibromyalgia. Arthritis Care Res (Hoboken). 2010;62(10):1377-1385.
62. Sime WE. Exercise therapy for stress management. In: Lehrer PM, Woolfolk RL, Sime WE, eds. Principles and Practice of Stress Management. 3rd ed. New York, NY: Guilford Press; 2007:333-359.
63. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York, NY: Oxford University Press; 2007.
64. de Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005;21(1):9-17.
65. Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18(4):251-261.
66. Chinman M, Young A, Hassell J, Davidson L. Toward the implementation of mental health consumer provider services. J Behav Health Serv Res. 2006;33(2):176-195.
1. Turi BC, Codogno JS, Fernandes RA, Monteiro HL. Physical activity, adiposity and hypertension among patients of public healthcare system [in English, Portuguese]. Rev Bras Epidemiol. 2014;17(4):925-937.
2. Press V, Freestone I, George CF. Physical activity: the evidence of benefit in the prevention of coronary heart disease. QJM. 2003;96(4):245-251.
3. Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med. 2008;38(10):807-824.
4. Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241-2247.
5. Brunes A, Augestad L, Gudmundsdottir S. Personality, physical activity, and symptoms of anxiety and depression: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. 2013;48(5):745-756.
6. De Mello MT, Lemos Vde A, Antunes HK, Bittencourt L, Santos-Silva R, Tufik S. Relationship between physical activity and depression and anxiety symptoms: a population study. J Affect Disord. 2013;149(1-3):241-246.
7. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
8. Teixeira CM, Vasconcelos-Raposo J, Fernandes HM, Brustad RJ. Physical activity, depression and anxiety among the elderly. Soc Indic Res. 2013;113(1):307-318.
9. Avery L, Flynn D, Dombrowski SU, van Wersch A, Sniehotta FF, Trenell MI. Successful behavioural strategies to increase physical activity and improve glucose control in adults with type 2 diabetes. Diabet Med. 2015;32(8):1058-1062.
10. Craighead LW, Blum MD. Supervised exercise in behavioral treatment for moderate obesity. Behav Ther. 1989;20(1):49-59.
11. Gulliford MC, Charlton J, Bhattarai N, Charlton C, Rudisill C. Impact and cost-effectiveness of a universal strategy to promote physical activity in primary care: population-based cohort study and Markov model. Eur J Health Econ. 2014;15(4):341-351.
12. Muda SH, Kadir AA. The effectiveness of physical activity counseling in primary care clinic University Science Malaysia Hospital. Int Med J. 2006;13(4):249-253.
13. Plotnikoff RC, Pickering MA, Glenn N, et al. The effects of a supplemental, theory-based physical activity counseling intervention for adults with type 2 diabetes. J Phys Act Health. 2011;8(7):944-954.
14. Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43(10):1009-1023.
15. Tse MM, Vong SK, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J Clin Nurs. 2013;22(13-14):1843-1856.
16. Babyak M, Blumenthal JA, Herman S et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
17. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
18. Conn VS. Anxiety outcomes after physical activity interventions: meta-analysis findings. Nurs Res. 2010;59(3):224-231.
19. Lee C, Russell A. Effects of physical activity on emotional well-being among older Australian women: cross-sectional and longitudinal analyses. J Psychosom Res. 2003;54(2):155-160.
20. Martinsen EW. Physical activity in the prevention and treatment of anxiety and depression. Nord J Psychiatry. 2008;62(suppl 47):25-29.
21. Phillips WT, Kiernan M, King AC. Physical activity as a nonpharmacological treatment for depression: a review. Complement Health Pract Rev. 2003;8(2):1-14.
22. Centers for Disease Control and Prevention. Facts about physical activity. http://www.cdc.gov/physicalactivity/data/facts.htm. Updated May 23, 2014. Accessed August 22, 2016.
23. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
24. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25–64: United States, 2007–2010. NCHS Data Brief. http://www.cdc.gov/nchs/data/databriefs/db101.pdf. Published August 2012. Accessed August 22, 2016.
25. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
26. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA Health Care System. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
27. Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276(18):1473-1479.
28. Gagliardi AR, Abdallah F, Faulkner G, Ciliska D, Hicks A. Factors contributing to the effectiveness of physical activity counselling in primary care: a realist systematic review. Patient Educ Couns. 2015;98(4):412-419.
29. Hardcastle S, Blake N, Hagger M. The effectiveness of a motivational interviewing primary-care based intervention on physical activity and predictors of change in a disadvantaged community. J Behav Med. 2012;35(3):318-333.
30. Johnson-Lawrence V, Zivin K, Szymanski BR, Pfeiffer PN, McCarthy JF. VA primary care–mental health integration: patient characteristics and receipt of mental health services, 2008–2010. Psychiatr Serv. 2012;63(11):1137-1141.
31. U.S. Department of Veterans Affairs. MOVE! weight management program. http://www.move.va.gov. Updated August 1, 2016. Accessed August 22, 2016.
32. Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for veterans. Prev Chronic Dis. 2009;6(3):A98.
33. Dahn JR, Fitzpatrick SL, Llabre MM, et al. Weight management for veterans: examining change in weight before and after MOVE! Obesity (Silver Spring). 2011;19(5):977-981.
34. Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med. 2011;1(4):551-560.
35. Taft TH, Payvar S, Wool L. Effectiveness of the MOVE! program among African American veterans: weight loss and quality of life. Fed Pract. 2011;28(12):17-24.
36. Kahwati LC, Lewis MA, Kane H, et al. Best practices in the Veterans Health Administration’s MOVE! weight management program. Am J Prev Med. 2011;41(5):457-464.
37. Butryn M, Forman E, Hoffman K, Shaw J, Juarascio A. A pilot study of acceptance and commitment therapy for promotion of physical activity. J Phys Act Health. 2011;8(4):516-522.
38. Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med. 2002;22(3):177-183.
39. Prestwich A, Perugini M, Hurling R. Can the effects of implementation intentions on exercise be enhanced using text messages? Psychol Health. 2009;24(6):677-687.
40. U.S. Department of Veterans Affairs. What is MOVE! Coach? http://www.move.va.gov/moveCoachIntro.asp. Updated May 26, 2016. Accessed September 6, 2016.
41. Moffitt R, Mohr P. The efficacy of a self-managed acceptance and commitment therapy intervention DVD for physical activity initiation. Br J Health Psychol. 2015;20(1):115-129.
42. Duncan M, Vandelanotte C, Kolt GS, et al. Effectiveness of a web- and mobile phone–based intervention to promote physical activity and healthy eating in middle-aged males: randomized controlled trial of the ManUp study. J Med Internet Res. 2014;16(6):e136.
43. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28(4):320-329.
44. Jefferis BJ, Sartini C, Lee IM, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. 2014;14:382.
45. Stevinson C, Lydon A, Amir Z. Adherence to physical activity guidelines among cancer support group participants. Eur J Cancer Care (Engl). 2014;23(2):199-205.
46. Spector D, Battaglini C, Groff D. Perceived exercise barriers and facilitators among ethnically diverse breast cancer survivors. Oncol Nurs Forum. 2013;40(5):472-480.
47. Daly JM, Hartz AJ, Xu Y, et al. An assessment of attitudes, behaviors, and outcomes of patients with type 2 diabetes. J Am Board Fam Med. 2009;22(3):280-290.
48. Bautista L, Reininger B, Gay JL, Barroso CS, McCormick JB. Perceived barriers to exercise in Hispanic adults by level of activity. J Phys Act Health. 2011;8(7):916-925.
49. Asano M, Duquette P, Andersen R, Lapierre Y, Mayo N. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35(5):353-361.
50. Hefferon K, Murphy H, McLeod J, Mutrie N, Campbell A. Understanding barriers to exercise implementation 5-year post–breast cancer diagnosis: a large-scale qualitative study. Health Educ Res. 2013;28(5):843-856.
51. Crombez G, Vlaeyen J, Heuts P, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1-2):329-339.
52. Rogerson M, Murphy BM, Bird S, Morris T. “I don’t have the heart”: a qualitative study of barriers to and facilitators of physical activity for people with coronary heart disease and depressive symptoms. Int J Behav Nutr Phys Act. 2012;9:140.
53. Leone LA, Ward DS. A mixed methods comparison of perceived benefits and barriers to exercise between obese and nonobese women. J Phys Act Health. 2013;10(4):461-469.
54. Glover C, Ferron J, Whitley R. Barriers to exercise among people with severe mental illnesses. Psychiatr Rehabil J. 2013;36(1):45-47.
55. Sabourin B, Hilchey C, Lefaivre M, Watt M, Stewart S. Why do they exercise less? Barriers to exercise in high-anxiety-sensitive women. Cogn Behav Ther. 2011;40(3):206-215.
56. Hall KS, Hoerster KD, Yancy WS Jr. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103-115.
57. LeardMann CA, Kelton ML, Smith B, et al; Millennium Cohort Study Team. Prospectively assessed posttraumatic stress disorder and associated physical activity. Public Health Rep. 2011;126(3):371-383.
58. de Assis MA, de Mello MF, Scorza FA, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo). 2008;63(4):473-478.
59. Jimmy G, Martin BW. Implementation and effectiveness of a primary care based physical activity counselling scheme. Patient Educ Couns. 2005;56(3):323-331.
60. Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119-131.
61. van Koulil S, van Lankveld W, Kraaimaat FW, et al. Tailored cognitive-behavioral therapy and exercise training for high-risk patients with fibromyalgia. Arthritis Care Res (Hoboken). 2010;62(10):1377-1385.
62. Sime WE. Exercise therapy for stress management. In: Lehrer PM, Woolfolk RL, Sime WE, eds. Principles and Practice of Stress Management. 3rd ed. New York, NY: Guilford Press; 2007:333-359.
63. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York, NY: Oxford University Press; 2007.
64. de Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005;21(1):9-17.
65. Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18(4):251-261.
66. Chinman M, Young A, Hassell J, Davidson L. Toward the implementation of mental health consumer provider services. J Behav Health Serv Res. 2006;33(2):176-195.
Mentally Stimulating Lifestyle May Protect Cognition From the Effects of a Poor Diet
TORONTO—A mentally stimulating lifestyle may help to increase resilience to cognitive decline despite an unhealthy diet, according to research presented at the 2016 Alzheimer’s Association International Conference. “Our results show the role higher educational attainment, mentally stimulating work, and social engagement can play in protecting your brain from cognitive decline, counteracting some negative effects of an unhealthy diet,” said Matthew Parrott, PhD, of Baycrest Health Sciences in Toronto.
Previous studies have linked poor diet to cognitive decline. In addition, factors such as education and social engagement may be associated with cognitive reserve (ie, the ability of the brain to withstand damage and maintain function). To determine whether the relationship between diet quality and cognitive function depends on an individual’s level of cognitive reserve, Dr. Parrott and colleagues conducted a study in community-dwelling older adults.
Researchers analyzed data from a subset of 351 healthy older adults enrolled in the Quebec Longitudinal Study of Nutrition and Successful Aging (NuAge). Participants’ mean age was 74. Participants were free from cognitive impairment and had to be able to climb one flight of stairs or walk a city block unassisted and without stopping. They were followed for three years. Participants were classified as having a low or high level of cognitive reserve based on their years of formal education, the social and data complexity of the occupation at which they worked the longest, and current participation in social leisure activities.
At baseline, investigators assessed the diet of participants over the previous 12 months using a semi-quantitative food frequency questionnaire. Each person was then assigned a diet score based on adherence to a Western dietary pattern (eg, consumption of red and processed meats, potatoes, white bread, and prepackaged foods and sweets), which was defined as a poor diet.
Investigators quantified cognitive reserve as a binary composite score based on educational attainment, occupational complexity, and social engagement. Education was defined as self-reported formal education. Social engagement was measured by a social activities score from the Elderly Activities Inventory questionnaire. Occupational complexity was based on participants’ self-reported longest serving occupation. Cognitive function was measured using the Modified Mini-Mental State Examination.
Overall, poor diet was associated with more cognitive decline. Data suggested, however, that a mentally stimulating lifestyle, or high cognitive reserve, protected older adults with a poor diet against cognitive decline. The researchers concluded that older adults who had a mentally stimulating lifestyle and limited their consumption of unhealthy foods appeared to be at the least at risk of dementia, compared with other participants. Mental stimulation seemed to build cognitive reserve, which may potentially lower the risk of developing dementia despite a poor diet. Since the Western diet was associated with worse baseline cognitive function in those with high cognitive reserve, mental stimulation did not seem to provide complete protection from an unhealthy diet. After three years, however, people with low cognitive reserve and a poor diet performed the worst.
“I think the take-home message is that older adults with a low lifetime history of mental stimulation or cognitive reserve should be especially vigilant to limit consumption of foods associated with a typical Western diet because they appear most vulnerable to the adverse influence of a poor diet on cognitive function,” said Dr. Parrott. “Next steps include trying to understand whether these results are limited to specific cognitive domains (eg, memory and executive function) and to link this protective effect to biomarkers and indicators of neuropathology.”
—Erica Robinson
Suggested Reading
Jackson PA, Pialoux V, Corbett D, et al. Promoting brain health through exercise and diet in older adults: a physiological perspective. J Physiol. 2016;594(16):4485-4498.
TORONTO—A mentally stimulating lifestyle may help to increase resilience to cognitive decline despite an unhealthy diet, according to research presented at the 2016 Alzheimer’s Association International Conference. “Our results show the role higher educational attainment, mentally stimulating work, and social engagement can play in protecting your brain from cognitive decline, counteracting some negative effects of an unhealthy diet,” said Matthew Parrott, PhD, of Baycrest Health Sciences in Toronto.
Previous studies have linked poor diet to cognitive decline. In addition, factors such as education and social engagement may be associated with cognitive reserve (ie, the ability of the brain to withstand damage and maintain function). To determine whether the relationship between diet quality and cognitive function depends on an individual’s level of cognitive reserve, Dr. Parrott and colleagues conducted a study in community-dwelling older adults.
Researchers analyzed data from a subset of 351 healthy older adults enrolled in the Quebec Longitudinal Study of Nutrition and Successful Aging (NuAge). Participants’ mean age was 74. Participants were free from cognitive impairment and had to be able to climb one flight of stairs or walk a city block unassisted and without stopping. They were followed for three years. Participants were classified as having a low or high level of cognitive reserve based on their years of formal education, the social and data complexity of the occupation at which they worked the longest, and current participation in social leisure activities.
At baseline, investigators assessed the diet of participants over the previous 12 months using a semi-quantitative food frequency questionnaire. Each person was then assigned a diet score based on adherence to a Western dietary pattern (eg, consumption of red and processed meats, potatoes, white bread, and prepackaged foods and sweets), which was defined as a poor diet.
Investigators quantified cognitive reserve as a binary composite score based on educational attainment, occupational complexity, and social engagement. Education was defined as self-reported formal education. Social engagement was measured by a social activities score from the Elderly Activities Inventory questionnaire. Occupational complexity was based on participants’ self-reported longest serving occupation. Cognitive function was measured using the Modified Mini-Mental State Examination.
Overall, poor diet was associated with more cognitive decline. Data suggested, however, that a mentally stimulating lifestyle, or high cognitive reserve, protected older adults with a poor diet against cognitive decline. The researchers concluded that older adults who had a mentally stimulating lifestyle and limited their consumption of unhealthy foods appeared to be at the least at risk of dementia, compared with other participants. Mental stimulation seemed to build cognitive reserve, which may potentially lower the risk of developing dementia despite a poor diet. Since the Western diet was associated with worse baseline cognitive function in those with high cognitive reserve, mental stimulation did not seem to provide complete protection from an unhealthy diet. After three years, however, people with low cognitive reserve and a poor diet performed the worst.
“I think the take-home message is that older adults with a low lifetime history of mental stimulation or cognitive reserve should be especially vigilant to limit consumption of foods associated with a typical Western diet because they appear most vulnerable to the adverse influence of a poor diet on cognitive function,” said Dr. Parrott. “Next steps include trying to understand whether these results are limited to specific cognitive domains (eg, memory and executive function) and to link this protective effect to biomarkers and indicators of neuropathology.”
—Erica Robinson
Suggested Reading
Jackson PA, Pialoux V, Corbett D, et al. Promoting brain health through exercise and diet in older adults: a physiological perspective. J Physiol. 2016;594(16):4485-4498.
TORONTO—A mentally stimulating lifestyle may help to increase resilience to cognitive decline despite an unhealthy diet, according to research presented at the 2016 Alzheimer’s Association International Conference. “Our results show the role higher educational attainment, mentally stimulating work, and social engagement can play in protecting your brain from cognitive decline, counteracting some negative effects of an unhealthy diet,” said Matthew Parrott, PhD, of Baycrest Health Sciences in Toronto.
Previous studies have linked poor diet to cognitive decline. In addition, factors such as education and social engagement may be associated with cognitive reserve (ie, the ability of the brain to withstand damage and maintain function). To determine whether the relationship between diet quality and cognitive function depends on an individual’s level of cognitive reserve, Dr. Parrott and colleagues conducted a study in community-dwelling older adults.
Researchers analyzed data from a subset of 351 healthy older adults enrolled in the Quebec Longitudinal Study of Nutrition and Successful Aging (NuAge). Participants’ mean age was 74. Participants were free from cognitive impairment and had to be able to climb one flight of stairs or walk a city block unassisted and without stopping. They were followed for three years. Participants were classified as having a low or high level of cognitive reserve based on their years of formal education, the social and data complexity of the occupation at which they worked the longest, and current participation in social leisure activities.
At baseline, investigators assessed the diet of participants over the previous 12 months using a semi-quantitative food frequency questionnaire. Each person was then assigned a diet score based on adherence to a Western dietary pattern (eg, consumption of red and processed meats, potatoes, white bread, and prepackaged foods and sweets), which was defined as a poor diet.
Investigators quantified cognitive reserve as a binary composite score based on educational attainment, occupational complexity, and social engagement. Education was defined as self-reported formal education. Social engagement was measured by a social activities score from the Elderly Activities Inventory questionnaire. Occupational complexity was based on participants’ self-reported longest serving occupation. Cognitive function was measured using the Modified Mini-Mental State Examination.
Overall, poor diet was associated with more cognitive decline. Data suggested, however, that a mentally stimulating lifestyle, or high cognitive reserve, protected older adults with a poor diet against cognitive decline. The researchers concluded that older adults who had a mentally stimulating lifestyle and limited their consumption of unhealthy foods appeared to be at the least at risk of dementia, compared with other participants. Mental stimulation seemed to build cognitive reserve, which may potentially lower the risk of developing dementia despite a poor diet. Since the Western diet was associated with worse baseline cognitive function in those with high cognitive reserve, mental stimulation did not seem to provide complete protection from an unhealthy diet. After three years, however, people with low cognitive reserve and a poor diet performed the worst.
“I think the take-home message is that older adults with a low lifetime history of mental stimulation or cognitive reserve should be especially vigilant to limit consumption of foods associated with a typical Western diet because they appear most vulnerable to the adverse influence of a poor diet on cognitive function,” said Dr. Parrott. “Next steps include trying to understand whether these results are limited to specific cognitive domains (eg, memory and executive function) and to link this protective effect to biomarkers and indicators of neuropathology.”
—Erica Robinson
Suggested Reading
Jackson PA, Pialoux V, Corbett D, et al. Promoting brain health through exercise and diet in older adults: a physiological perspective. J Physiol. 2016;594(16):4485-4498.
Evaluation of Cortical Lesions Could Improve Diagnosis of MS
LONDON—Evaluation of cortical lesions improves the specificity of the diagnostic criteria for multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Assessment of cortical lesions, in concert with current McDonald criteria, also preserved a high level of diagnostic sensitivity and accuracy in a multicentric cohort of patients with clinically isolated syndrome, reported Paolo Preziosa, MD, Neuroimaging Research Unit at the Institute of Experimental Neurology and Division of Neuroscience at San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and his research colleagues.
Since the publication of the 2010 revised McDonald criteria, new data regarding the application of MRI for the diagnosis of MS have become available. In a single-center study, adding the assessment of cortical lesions was shown to modify the diagnostic algorithm, resulting in higher specificity.
In the present study, Dr. Preziosa and colleagues sought to test the performance of different sets of imaging criteria, including the assessment of cortical lesions, for the development of MS in a multicentric cohort of patients with clinically isolated syndrome.
The researchers analyzed brain double inversion recovery and brain and cord T2-weighted and post-contrast T1-weighted sequences acquired from 72 patients with clinically isolated syndrome from five European centers (Barcelona, Belgrade, Mainz, Milan, and Verona) within three months and after 12 months from disease onset. Patients were followed clinically for 24 or more months or until the development of clinically defined MS. Median follow-up was 24.2 months. Sensitivity, specificity, and accuracy of the different dissemination in space MRI criteria for the development of MS were tested.
At follow-up, 65 patients (90%) had clinically and/or radiologically definite MS. The sensitivity of all criteria was high (McDonald 2005, 83%; McDonald 2010, 92%; Filippi 2010, 80%). Specificity of Filippi 2010 was higher (67%), compared with the others (50% for McDonald 2005 and 2010). The accuracy of all criteria was high (McDonald 2005, 81%; McDonald 2010, 89%; Filippi 2010, 79%).
“The detection of cortical lesions in vivo using MRI should be considered in future clinical trials,” Dr. Preziosa said.
—Glenn S. Williams
Suggested Reading
Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75(22):1988-1994.
LONDON—Evaluation of cortical lesions improves the specificity of the diagnostic criteria for multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Assessment of cortical lesions, in concert with current McDonald criteria, also preserved a high level of diagnostic sensitivity and accuracy in a multicentric cohort of patients with clinically isolated syndrome, reported Paolo Preziosa, MD, Neuroimaging Research Unit at the Institute of Experimental Neurology and Division of Neuroscience at San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and his research colleagues.
Since the publication of the 2010 revised McDonald criteria, new data regarding the application of MRI for the diagnosis of MS have become available. In a single-center study, adding the assessment of cortical lesions was shown to modify the diagnostic algorithm, resulting in higher specificity.
In the present study, Dr. Preziosa and colleagues sought to test the performance of different sets of imaging criteria, including the assessment of cortical lesions, for the development of MS in a multicentric cohort of patients with clinically isolated syndrome.
The researchers analyzed brain double inversion recovery and brain and cord T2-weighted and post-contrast T1-weighted sequences acquired from 72 patients with clinically isolated syndrome from five European centers (Barcelona, Belgrade, Mainz, Milan, and Verona) within three months and after 12 months from disease onset. Patients were followed clinically for 24 or more months or until the development of clinically defined MS. Median follow-up was 24.2 months. Sensitivity, specificity, and accuracy of the different dissemination in space MRI criteria for the development of MS were tested.
At follow-up, 65 patients (90%) had clinically and/or radiologically definite MS. The sensitivity of all criteria was high (McDonald 2005, 83%; McDonald 2010, 92%; Filippi 2010, 80%). Specificity of Filippi 2010 was higher (67%), compared with the others (50% for McDonald 2005 and 2010). The accuracy of all criteria was high (McDonald 2005, 81%; McDonald 2010, 89%; Filippi 2010, 79%).
“The detection of cortical lesions in vivo using MRI should be considered in future clinical trials,” Dr. Preziosa said.
—Glenn S. Williams
Suggested Reading
Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75(22):1988-1994.
LONDON—Evaluation of cortical lesions improves the specificity of the diagnostic criteria for multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS). Assessment of cortical lesions, in concert with current McDonald criteria, also preserved a high level of diagnostic sensitivity and accuracy in a multicentric cohort of patients with clinically isolated syndrome, reported Paolo Preziosa, MD, Neuroimaging Research Unit at the Institute of Experimental Neurology and Division of Neuroscience at San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and his research colleagues.
Since the publication of the 2010 revised McDonald criteria, new data regarding the application of MRI for the diagnosis of MS have become available. In a single-center study, adding the assessment of cortical lesions was shown to modify the diagnostic algorithm, resulting in higher specificity.
In the present study, Dr. Preziosa and colleagues sought to test the performance of different sets of imaging criteria, including the assessment of cortical lesions, for the development of MS in a multicentric cohort of patients with clinically isolated syndrome.
The researchers analyzed brain double inversion recovery and brain and cord T2-weighted and post-contrast T1-weighted sequences acquired from 72 patients with clinically isolated syndrome from five European centers (Barcelona, Belgrade, Mainz, Milan, and Verona) within three months and after 12 months from disease onset. Patients were followed clinically for 24 or more months or until the development of clinically defined MS. Median follow-up was 24.2 months. Sensitivity, specificity, and accuracy of the different dissemination in space MRI criteria for the development of MS were tested.
At follow-up, 65 patients (90%) had clinically and/or radiologically definite MS. The sensitivity of all criteria was high (McDonald 2005, 83%; McDonald 2010, 92%; Filippi 2010, 80%). Specificity of Filippi 2010 was higher (67%), compared with the others (50% for McDonald 2005 and 2010). The accuracy of all criteria was high (McDonald 2005, 81%; McDonald 2010, 89%; Filippi 2010, 79%).
“The detection of cortical lesions in vivo using MRI should be considered in future clinical trials,” Dr. Preziosa said.
—Glenn S. Williams
Suggested Reading
Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75(22):1988-1994.
Smoking, vitamin D deficiency linked to early MS disability
LONDON – Severe vitamin D deficiency and current smoking predicted accumulated disability in patients with clinically isolated syndrome, which can be a precursor to the development of multiple sclerosis.
Prospectively collected data from the ongoing Barcelona clinically isolated syndrome (CIS) cohort, which includes more than 1,000 patients with CIS, showed that patients with a serum vitamin D below 8.0 ng/mL (9% of 503 patient samples) had more than double the risk for accumulated disability when compared with those who had higher vitamin D levels. The hazard ratio for disability with severely low vitamin D levels was 2.3 (P = .049) in an analysis adjusted for the potential confounding factors of patients’ sex and age, the number of baseline T2 lesions, receipt of disease-modifying treatment, CIS topography, and oligoclonal bands. Disability accumulation was defined as an Expanded Disability Status Scale score of 3.0 or more.
However, neither vitamin D deficiency nor current smoking predicted the conversion of CIS to clinically definite multiple sclerosis (CDMS), said María Zuluaga, MD, of the Centre d’Esclerosi Múltiple de Catalunya at Vall d’Hebron University Hospital in Barcelona.
Environmental factors such as vitamin D levels and smoking have been purported to play a role in the development of CIS to CDMS, Dr. Zuluaga explained at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood samples collected within 6 months of a diagnosis of CIS were examined and vitamin D deficiency defined as normal, mild, moderate, or severe based on serum 25-hydroxy vitamin D of greater than 20, 16-20, 8-15, and less that 8 ng/mL.
Levels of the nicotine metabolite cotinine in the blood were used as a proxy for current smoking. Cotinine has a half-life of around 20 hours and smokers – active or passive – have a level of 14 ng/mL or more while nonsmokers have a level of less than 14 ng/mL.
The study received no commercial funding. Dr. Zuluaga reported having no conflict of interest related to the study.
LONDON – Severe vitamin D deficiency and current smoking predicted accumulated disability in patients with clinically isolated syndrome, which can be a precursor to the development of multiple sclerosis.
Prospectively collected data from the ongoing Barcelona clinically isolated syndrome (CIS) cohort, which includes more than 1,000 patients with CIS, showed that patients with a serum vitamin D below 8.0 ng/mL (9% of 503 patient samples) had more than double the risk for accumulated disability when compared with those who had higher vitamin D levels. The hazard ratio for disability with severely low vitamin D levels was 2.3 (P = .049) in an analysis adjusted for the potential confounding factors of patients’ sex and age, the number of baseline T2 lesions, receipt of disease-modifying treatment, CIS topography, and oligoclonal bands. Disability accumulation was defined as an Expanded Disability Status Scale score of 3.0 or more.
However, neither vitamin D deficiency nor current smoking predicted the conversion of CIS to clinically definite multiple sclerosis (CDMS), said María Zuluaga, MD, of the Centre d’Esclerosi Múltiple de Catalunya at Vall d’Hebron University Hospital in Barcelona.
Environmental factors such as vitamin D levels and smoking have been purported to play a role in the development of CIS to CDMS, Dr. Zuluaga explained at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood samples collected within 6 months of a diagnosis of CIS were examined and vitamin D deficiency defined as normal, mild, moderate, or severe based on serum 25-hydroxy vitamin D of greater than 20, 16-20, 8-15, and less that 8 ng/mL.
Levels of the nicotine metabolite cotinine in the blood were used as a proxy for current smoking. Cotinine has a half-life of around 20 hours and smokers – active or passive – have a level of 14 ng/mL or more while nonsmokers have a level of less than 14 ng/mL.
The study received no commercial funding. Dr. Zuluaga reported having no conflict of interest related to the study.
LONDON – Severe vitamin D deficiency and current smoking predicted accumulated disability in patients with clinically isolated syndrome, which can be a precursor to the development of multiple sclerosis.
Prospectively collected data from the ongoing Barcelona clinically isolated syndrome (CIS) cohort, which includes more than 1,000 patients with CIS, showed that patients with a serum vitamin D below 8.0 ng/mL (9% of 503 patient samples) had more than double the risk for accumulated disability when compared with those who had higher vitamin D levels. The hazard ratio for disability with severely low vitamin D levels was 2.3 (P = .049) in an analysis adjusted for the potential confounding factors of patients’ sex and age, the number of baseline T2 lesions, receipt of disease-modifying treatment, CIS topography, and oligoclonal bands. Disability accumulation was defined as an Expanded Disability Status Scale score of 3.0 or more.
However, neither vitamin D deficiency nor current smoking predicted the conversion of CIS to clinically definite multiple sclerosis (CDMS), said María Zuluaga, MD, of the Centre d’Esclerosi Múltiple de Catalunya at Vall d’Hebron University Hospital in Barcelona.
Environmental factors such as vitamin D levels and smoking have been purported to play a role in the development of CIS to CDMS, Dr. Zuluaga explained at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood samples collected within 6 months of a diagnosis of CIS were examined and vitamin D deficiency defined as normal, mild, moderate, or severe based on serum 25-hydroxy vitamin D of greater than 20, 16-20, 8-15, and less that 8 ng/mL.
Levels of the nicotine metabolite cotinine in the blood were used as a proxy for current smoking. Cotinine has a half-life of around 20 hours and smokers – active or passive – have a level of 14 ng/mL or more while nonsmokers have a level of less than 14 ng/mL.
The study received no commercial funding. Dr. Zuluaga reported having no conflict of interest related to the study.
Key clinical point:
Major finding: Patients with a serum 25-hydroxy vitamin D level of less than 8.0 ng/mL showed an increased risk for disability in a multivariate analysis (HR, 2.3; P = .049). Nonsmokers were significantly less likely to have disability progression (HR, 0.4; P = .002).
Data source: Barcelona CIS cohort of 1,127 individuals
Disclosures: The study received no commercial funding. Dr. Zuluaga reported having no conflict of interest related to the study.
Can Treating Neuroinflammation in REM Sleep Behavior Disorder Delay Parkinson’s Disease Onset?
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C-PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putamenal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C-PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putamenal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C-PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putamenal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
How Do Diet, Exercise, and Supplements Affect Parkinson’s Disease Progression?
PORTLAND, OR—Among patients with Parkinson’s disease, eating foods common in a Mediterranean diet and exercising regularly are associated with reduced rates of Parkinson’s disease progression, according to the results of a natural history study described at the Fourth World Parkinson Congress.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep. The researchers observed a dose-response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should ... know that they can make choices that affect outcomes.”
—Jake Remaly
PORTLAND, OR—Among patients with Parkinson’s disease, eating foods common in a Mediterranean diet and exercising regularly are associated with reduced rates of Parkinson’s disease progression, according to the results of a natural history study described at the Fourth World Parkinson Congress.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep. The researchers observed a dose-response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should ... know that they can make choices that affect outcomes.”
—Jake Remaly
PORTLAND, OR—Among patients with Parkinson’s disease, eating foods common in a Mediterranean diet and exercising regularly are associated with reduced rates of Parkinson’s disease progression, according to the results of a natural history study described at the Fourth World Parkinson Congress.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep. The researchers observed a dose-response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should ... know that they can make choices that affect outcomes.”
—Jake Remaly