User login
Longer hospitalization, greater care needed for depressed ischemic stroke patients
BALTIMORE – Patients admitted with acute ischemic stroke (AIS) with preexisting major depressive disorder (MDD) are less likely to die while in the hospital but are often hospitalized longer and are more likely to need specialty care when they are discharged, in comparison to similar patients without depression in the National Inpatient Sample.
“Our study displayed an increasing proportion of patients with MDD admitted due to AIS in the last decade with lower mortality but higher morbidity post stroke. In addition, there was less utilization of thrombolysis in this population,” study presenter Arpita Hazra, MD, Northwell Health, Jersey City, N.J., said at the annual meeting of the American Neurological Association.
Thrombolysis was carried out in fewer depressed patients than in those without depression (3.8% vs. 4.8%; P less than .001). The odds of death during hospitalization were 36% less for patients with MDD. However, patients with MDD tended to be hospitalized longer than nondepressed patients (median 3.6 vs. 3.4 days; P less than .001) and were nearly 40% more likely to require specialty care following discharge.
“There is a need to explore the reasons behind this disparity in outcomes and thrombolysis utilization in order to improve poststroke outcome in this vulnerable population,” Dr. Hazra said.
At face value, the observation of decreased mortality in AIS patients with preexisting MDD was surprising, according to Dr. Hazra. She suggested that this may reflect prior hospital treatment for these patients, because of other comorbidities associated with depression.
Dr. Hazra reported having no financial disclosures.
BALTIMORE – Patients admitted with acute ischemic stroke (AIS) with preexisting major depressive disorder (MDD) are less likely to die while in the hospital but are often hospitalized longer and are more likely to need specialty care when they are discharged, in comparison to similar patients without depression in the National Inpatient Sample.
“Our study displayed an increasing proportion of patients with MDD admitted due to AIS in the last decade with lower mortality but higher morbidity post stroke. In addition, there was less utilization of thrombolysis in this population,” study presenter Arpita Hazra, MD, Northwell Health, Jersey City, N.J., said at the annual meeting of the American Neurological Association.
Thrombolysis was carried out in fewer depressed patients than in those without depression (3.8% vs. 4.8%; P less than .001). The odds of death during hospitalization were 36% less for patients with MDD. However, patients with MDD tended to be hospitalized longer than nondepressed patients (median 3.6 vs. 3.4 days; P less than .001) and were nearly 40% more likely to require specialty care following discharge.
“There is a need to explore the reasons behind this disparity in outcomes and thrombolysis utilization in order to improve poststroke outcome in this vulnerable population,” Dr. Hazra said.
At face value, the observation of decreased mortality in AIS patients with preexisting MDD was surprising, according to Dr. Hazra. She suggested that this may reflect prior hospital treatment for these patients, because of other comorbidities associated with depression.
Dr. Hazra reported having no financial disclosures.
BALTIMORE – Patients admitted with acute ischemic stroke (AIS) with preexisting major depressive disorder (MDD) are less likely to die while in the hospital but are often hospitalized longer and are more likely to need specialty care when they are discharged, in comparison to similar patients without depression in the National Inpatient Sample.
“Our study displayed an increasing proportion of patients with MDD admitted due to AIS in the last decade with lower mortality but higher morbidity post stroke. In addition, there was less utilization of thrombolysis in this population,” study presenter Arpita Hazra, MD, Northwell Health, Jersey City, N.J., said at the annual meeting of the American Neurological Association.
Thrombolysis was carried out in fewer depressed patients than in those without depression (3.8% vs. 4.8%; P less than .001). The odds of death during hospitalization were 36% less for patients with MDD. However, patients with MDD tended to be hospitalized longer than nondepressed patients (median 3.6 vs. 3.4 days; P less than .001) and were nearly 40% more likely to require specialty care following discharge.
“There is a need to explore the reasons behind this disparity in outcomes and thrombolysis utilization in order to improve poststroke outcome in this vulnerable population,” Dr. Hazra said.
At face value, the observation of decreased mortality in AIS patients with preexisting MDD was surprising, according to Dr. Hazra. She suggested that this may reflect prior hospital treatment for these patients, because of other comorbidities associated with depression.
Dr. Hazra reported having no financial disclosures.
AT ANA 2016
Key clinical point:
Major finding: In-hospital mortality was significantly lower for depressed patients with acute ischemic stroke, compared with nondepressed patients, but depressed patients had a significantly longer length of hospitalization and higher rate of discharge to specialty care.
Data source: Study of 4.3 million hospital AIS-related admissions identified in the United States during 2002-2012 in the National Inpatient Sample.
Disclosures: Dr. Hazra reported having no financial disclosures.
OAK: Atezolizumab grows OS in advanced NSCLC
COPENHAGEN – The checkpoint inhibitor atezolizumab improved overall survival of advanced non–small-cell lung cancer, compared with docetaxel, primary analysis from the OAK trial shows.
In the first phase III trial of a monoclonal antibody directed against the programmed death 1 ligand (PD-L1) in non–small-lung cell lung cancer (NSCLC), median overall survival (OS) for patients assigned to atezolizumab (Tecentriq) was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, reported Fabrice Barlesi, MD, head of the multidisciplinary oncology and therapeutic innovations department at Aix-Marseille University and the Assistance Publique Hôpitaux de Marseille, France.
Atezolizumab was previously shown in the phase II POPLAR trial to significantly increase overall survival compared with docetaxel in second- or third-line therapy in patients with locally advanced or metastatic NSCLC who had disease progression after platinum-based chemotherapy.
The OAK trial investigators enrolled 1,225 patients with locally advanced or metastatic NSCLC following one or two prior lines of therapy that contained at least one platinum-based regimen. The patients could have any degree of PD-L1 expression.
Dr. Barlesi presented results of a prespecified intention-to-treat analysis of the first 850 patients enrolled.
Following stratification for PD-L1, histology, and prior chemotherapy regimens, they were randomly assigned to either atezolizumab 1,200 mg IV every 3 weeks or docetaxel 75 mg/m2 every 3 weeks. In the atezolizumab arm, therapy could be continued until disease progression or clinical benefit. In the docetaxel arm, therapy was continued until progression.
As noted, atezolizumab was associated with significantly better OS, the primary endpoint (13.8 vs. 9.6 months) with a hazard ratio (HR) of 0.73 (P = .0003). OS rates at 12 months were 55% in the atezolizumab arm vs. 41% in the docetaxel arm, and at 18 months the rates were 40% and 27%, respectively.
The investigators found that atezolizumab provided a benefit regardless of PD-L1 expression, with a hazard ratio of 0.74 (P = .0102) for the 55% of patients with PD-L1 expression on at least 1% of their tumor cells, and 0.75 (P = .0205) for patients with PD-L1 on less than 1% of tumor cells.
Median overall survival was greater with both drugs for patients with higher levels of PD-L1 expression, with a median of 15.7 months for those treated with atezolizumab and 10.3 months for those treated with docetaxel. In contrast, the respective median overall survival durations in the patients with less than 1% PD-L1 expression were 12.6 and 8.9 months.
The greatest benefit for atezolizumab was seen in patients with PD-L1 expression of 50% or more of tumor cells or 10% or more of tumor-infiltrating immune cells, with a median OS at a minimum follow-up of 19 months of 20.5 months vs. 8.9 months, translating into a hazard ratio for atezolizumab of 0.41 (P less than .0001).
An analysis of OS by histology showed HRs of 0.73 for atezolizumab for patients with either non-squamous tumors (P = .0015) or squamous tumors (P = .0383). In addition, an analysis of OS in selected subgroups showed a trend favoring atezolizumab in patients who had never smoked, who tend to have a worse prognosis than would current or former smokers.
In an analysis of progression-free survival, there was no significant difference between the groups (median 4.0 vs. 2.8 months).
Adverse events of grade 3 or greater of any cause were lower with atezolizumab, at 37% vs. 54%. There was one treatment-related death from respiratory infection in a patient who received docetaxel.
Invited discussant Naiyer Rizvi, MD, an oncologist at Columbia University Medical Center in New York, said that the OAK trial confirms that atezolizumab is an appropriate second-line therapy for unselected patients with advanced NSCLC, and should be explored for its potential survival benefits in never smokers.
COPENHAGEN – The checkpoint inhibitor atezolizumab improved overall survival of advanced non–small-cell lung cancer, compared with docetaxel, primary analysis from the OAK trial shows.
In the first phase III trial of a monoclonal antibody directed against the programmed death 1 ligand (PD-L1) in non–small-lung cell lung cancer (NSCLC), median overall survival (OS) for patients assigned to atezolizumab (Tecentriq) was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, reported Fabrice Barlesi, MD, head of the multidisciplinary oncology and therapeutic innovations department at Aix-Marseille University and the Assistance Publique Hôpitaux de Marseille, France.
Atezolizumab was previously shown in the phase II POPLAR trial to significantly increase overall survival compared with docetaxel in second- or third-line therapy in patients with locally advanced or metastatic NSCLC who had disease progression after platinum-based chemotherapy.
The OAK trial investigators enrolled 1,225 patients with locally advanced or metastatic NSCLC following one or two prior lines of therapy that contained at least one platinum-based regimen. The patients could have any degree of PD-L1 expression.
Dr. Barlesi presented results of a prespecified intention-to-treat analysis of the first 850 patients enrolled.
Following stratification for PD-L1, histology, and prior chemotherapy regimens, they were randomly assigned to either atezolizumab 1,200 mg IV every 3 weeks or docetaxel 75 mg/m2 every 3 weeks. In the atezolizumab arm, therapy could be continued until disease progression or clinical benefit. In the docetaxel arm, therapy was continued until progression.
As noted, atezolizumab was associated with significantly better OS, the primary endpoint (13.8 vs. 9.6 months) with a hazard ratio (HR) of 0.73 (P = .0003). OS rates at 12 months were 55% in the atezolizumab arm vs. 41% in the docetaxel arm, and at 18 months the rates were 40% and 27%, respectively.
The investigators found that atezolizumab provided a benefit regardless of PD-L1 expression, with a hazard ratio of 0.74 (P = .0102) for the 55% of patients with PD-L1 expression on at least 1% of their tumor cells, and 0.75 (P = .0205) for patients with PD-L1 on less than 1% of tumor cells.
Median overall survival was greater with both drugs for patients with higher levels of PD-L1 expression, with a median of 15.7 months for those treated with atezolizumab and 10.3 months for those treated with docetaxel. In contrast, the respective median overall survival durations in the patients with less than 1% PD-L1 expression were 12.6 and 8.9 months.
The greatest benefit for atezolizumab was seen in patients with PD-L1 expression of 50% or more of tumor cells or 10% or more of tumor-infiltrating immune cells, with a median OS at a minimum follow-up of 19 months of 20.5 months vs. 8.9 months, translating into a hazard ratio for atezolizumab of 0.41 (P less than .0001).
An analysis of OS by histology showed HRs of 0.73 for atezolizumab for patients with either non-squamous tumors (P = .0015) or squamous tumors (P = .0383). In addition, an analysis of OS in selected subgroups showed a trend favoring atezolizumab in patients who had never smoked, who tend to have a worse prognosis than would current or former smokers.
In an analysis of progression-free survival, there was no significant difference between the groups (median 4.0 vs. 2.8 months).
Adverse events of grade 3 or greater of any cause were lower with atezolizumab, at 37% vs. 54%. There was one treatment-related death from respiratory infection in a patient who received docetaxel.
Invited discussant Naiyer Rizvi, MD, an oncologist at Columbia University Medical Center in New York, said that the OAK trial confirms that atezolizumab is an appropriate second-line therapy for unselected patients with advanced NSCLC, and should be explored for its potential survival benefits in never smokers.
COPENHAGEN – The checkpoint inhibitor atezolizumab improved overall survival of advanced non–small-cell lung cancer, compared with docetaxel, primary analysis from the OAK trial shows.
In the first phase III trial of a monoclonal antibody directed against the programmed death 1 ligand (PD-L1) in non–small-lung cell lung cancer (NSCLC), median overall survival (OS) for patients assigned to atezolizumab (Tecentriq) was 13.8 months, compared with 9.6 months for patients assigned to docetaxel, reported Fabrice Barlesi, MD, head of the multidisciplinary oncology and therapeutic innovations department at Aix-Marseille University and the Assistance Publique Hôpitaux de Marseille, France.
Atezolizumab was previously shown in the phase II POPLAR trial to significantly increase overall survival compared with docetaxel in second- or third-line therapy in patients with locally advanced or metastatic NSCLC who had disease progression after platinum-based chemotherapy.
The OAK trial investigators enrolled 1,225 patients with locally advanced or metastatic NSCLC following one or two prior lines of therapy that contained at least one platinum-based regimen. The patients could have any degree of PD-L1 expression.
Dr. Barlesi presented results of a prespecified intention-to-treat analysis of the first 850 patients enrolled.
Following stratification for PD-L1, histology, and prior chemotherapy regimens, they were randomly assigned to either atezolizumab 1,200 mg IV every 3 weeks or docetaxel 75 mg/m2 every 3 weeks. In the atezolizumab arm, therapy could be continued until disease progression or clinical benefit. In the docetaxel arm, therapy was continued until progression.
As noted, atezolizumab was associated with significantly better OS, the primary endpoint (13.8 vs. 9.6 months) with a hazard ratio (HR) of 0.73 (P = .0003). OS rates at 12 months were 55% in the atezolizumab arm vs. 41% in the docetaxel arm, and at 18 months the rates were 40% and 27%, respectively.
The investigators found that atezolizumab provided a benefit regardless of PD-L1 expression, with a hazard ratio of 0.74 (P = .0102) for the 55% of patients with PD-L1 expression on at least 1% of their tumor cells, and 0.75 (P = .0205) for patients with PD-L1 on less than 1% of tumor cells.
Median overall survival was greater with both drugs for patients with higher levels of PD-L1 expression, with a median of 15.7 months for those treated with atezolizumab and 10.3 months for those treated with docetaxel. In contrast, the respective median overall survival durations in the patients with less than 1% PD-L1 expression were 12.6 and 8.9 months.
The greatest benefit for atezolizumab was seen in patients with PD-L1 expression of 50% or more of tumor cells or 10% or more of tumor-infiltrating immune cells, with a median OS at a minimum follow-up of 19 months of 20.5 months vs. 8.9 months, translating into a hazard ratio for atezolizumab of 0.41 (P less than .0001).
An analysis of OS by histology showed HRs of 0.73 for atezolizumab for patients with either non-squamous tumors (P = .0015) or squamous tumors (P = .0383). In addition, an analysis of OS in selected subgroups showed a trend favoring atezolizumab in patients who had never smoked, who tend to have a worse prognosis than would current or former smokers.
In an analysis of progression-free survival, there was no significant difference between the groups (median 4.0 vs. 2.8 months).
Adverse events of grade 3 or greater of any cause were lower with atezolizumab, at 37% vs. 54%. There was one treatment-related death from respiratory infection in a patient who received docetaxel.
Invited discussant Naiyer Rizvi, MD, an oncologist at Columbia University Medical Center in New York, said that the OAK trial confirms that atezolizumab is an appropriate second-line therapy for unselected patients with advanced NSCLC, and should be explored for its potential survival benefits in never smokers.
AT ESMO 2016
Key clinical point: Atezolizumab improved overall survival (OS), compared with docetaxel, in patients with advanced non–small-cell lung cancer.
Major finding: Median OS for patients assigned to atezolizumab was 13.8 months, compared with 9.6 months for patients assigned to docetaxel.
Data source: Prespecified analysis of a randomized phase III trial in 850 patients with metastatic or recurrent NSCLC after platinum-based chemotherapy.
Disclosures: The OAK trial was funded by F. Hoffman-La Roche. Dr. Barlesi disclosed consulting fees from that company and others. Dr, Rizvi disclosed consulting for Roche and other companies.
Ultrasound-Guided Percutaneous Reconstruction of the Anterolateral Ligament: Surgical Technique
Tryptase gene variant linked to GI, joint, and skin symptoms
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
FROM NATURE GENETICS
Key clinical point:
Major finding: Increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene is associated with elevated basal serum tryptase and a collection of symptoms including irritable bowel syndrome, joint hypermobility, and autonomic dysfunction.
Data source: Study of 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Quality of outpatient care has not improved consistently since 2002
The clinical quality of outpatient care for adults in the United States did not improve consistently from 2002 to 2013, despite numerous local, regional, and national efforts to make it better, according to a report published online Oct. 17 in JAMA Internal Medicine.
“Current deficits in care continue to pose serious hazards to the health of the American public in the form of missed care opportunities as well as waste and potential harm from overuse,” said David M. Levine, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
Since a 2003 report showing that American adults received about half of the recommended health care services, many programs have sought to make improvements by expanding quality measurements and public reporting, increasing pay for performance, increasing value-based purchasing by payers, increasing the use of electronic medical records, improving coverage for recommended services, and expanding patient-centered medical homes.
Yet few data are available by which to gauge whether these efforts have been effective, the investigators noted (JAMA Intern Med. 2016 Oct 17; doi:10.1001/jamainternmed.2016.6217).
They analyzed data from a nationally representative annual outpatient survey to determine whether 46 indicators of care quality changed from 2002 to 2013. Sample sizes ranged from 20,679 to 26,509 adults per year, and that information was supplemented by responses from the patients’ clinicians, pharmacies, and employers.
Overall, rates of recommended treatment delivery showed an “anemic” improvement from 36% to 42%, with a few areas showing marked improvement while others showed little improvement or actual declines.
For example, rates of recommended medications for heart failure increased from 41% to 65%; rates for recommended statin therapy for stroke patients increased from 34% to 57%; and rates of recommended smoking-cessation counseling increased from 49% to 61%. However, rates of inappropriate antibiotic prescribing and inappropriate medications in the elderly both worsened.
Recommended colorectal cancer screening improved from 48% to 63%; but recommended breast cancer screening declined from 81% to 77%, and recommended cervical cancer screening declined from 90% to 86%.
Inappropriate cervical cancer screening in the elderly improved, but inappropriate colorectal cancer screening in the elderly worsened.
Of greatest concern, approximately half of elderly adults underwent inappropriate cancer screening when it was unlikely to prolong life. And approximately half of adults who saw a clinician for a viral illness received inappropriate antibiotics. In addition, approximately 15% who consulted a clinician for back pain received an inappropriate lumbar radiograph.
“These areas represent prime targets for efforts to improve the value of care delivered by eliminating services that have a neutral or negative impact on health,” Dr. Levine and his associates said.
The investigators emphasized that these data do not reflect changes resulting from the Affordable Care Act, which was implemented in late 2013. The ACA has encouraged many organizational changes, renewed the focus on primary care, and expanded health insurance coverage to 30 million more people. All of these changes are expected to improve overall health care quality, the study authors noted. But it is not yet known whether these changes will have their intended effect.
This study was supported in part by the National Institutes of Health and the Ryoichi Sasakawa Fellowship Fund. Dr. Levine and his associates reported having no relevant financial disclosures.
The study by Levine et al. had several limitations, so we still cannot say how good the overall quality of health care was in 2013.
The authors used quality measures that changed over time and weren’t valid across the entire decade of their study, which means the measures didn’t necessarily reflect the current best clinical practice. Also, 46 indicators is a relatively small number by which to assess quality of care, and they were chosen because they could be scored using administrative data rather than because of their importance.
Elizabeth A. McGlynn, PhD, is at Kaiser Permanente Research, Pasadena, Calif. She and her associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Levine’s report (JAMA Intern Med. 2016 Oct 17. doi: 10.1001/jamainternmed.2016.6233).
The study by Levine et al. had several limitations, so we still cannot say how good the overall quality of health care was in 2013.
The authors used quality measures that changed over time and weren’t valid across the entire decade of their study, which means the measures didn’t necessarily reflect the current best clinical practice. Also, 46 indicators is a relatively small number by which to assess quality of care, and they were chosen because they could be scored using administrative data rather than because of their importance.
Elizabeth A. McGlynn, PhD, is at Kaiser Permanente Research, Pasadena, Calif. She and her associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Levine’s report (JAMA Intern Med. 2016 Oct 17. doi: 10.1001/jamainternmed.2016.6233).
The study by Levine et al. had several limitations, so we still cannot say how good the overall quality of health care was in 2013.
The authors used quality measures that changed over time and weren’t valid across the entire decade of their study, which means the measures didn’t necessarily reflect the current best clinical practice. Also, 46 indicators is a relatively small number by which to assess quality of care, and they were chosen because they could be scored using administrative data rather than because of their importance.
Elizabeth A. McGlynn, PhD, is at Kaiser Permanente Research, Pasadena, Calif. She and her associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Levine’s report (JAMA Intern Med. 2016 Oct 17. doi: 10.1001/jamainternmed.2016.6233).
The clinical quality of outpatient care for adults in the United States did not improve consistently from 2002 to 2013, despite numerous local, regional, and national efforts to make it better, according to a report published online Oct. 17 in JAMA Internal Medicine.
“Current deficits in care continue to pose serious hazards to the health of the American public in the form of missed care opportunities as well as waste and potential harm from overuse,” said David M. Levine, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
Since a 2003 report showing that American adults received about half of the recommended health care services, many programs have sought to make improvements by expanding quality measurements and public reporting, increasing pay for performance, increasing value-based purchasing by payers, increasing the use of electronic medical records, improving coverage for recommended services, and expanding patient-centered medical homes.
Yet few data are available by which to gauge whether these efforts have been effective, the investigators noted (JAMA Intern Med. 2016 Oct 17; doi:10.1001/jamainternmed.2016.6217).
They analyzed data from a nationally representative annual outpatient survey to determine whether 46 indicators of care quality changed from 2002 to 2013. Sample sizes ranged from 20,679 to 26,509 adults per year, and that information was supplemented by responses from the patients’ clinicians, pharmacies, and employers.
Overall, rates of recommended treatment delivery showed an “anemic” improvement from 36% to 42%, with a few areas showing marked improvement while others showed little improvement or actual declines.
For example, rates of recommended medications for heart failure increased from 41% to 65%; rates for recommended statin therapy for stroke patients increased from 34% to 57%; and rates of recommended smoking-cessation counseling increased from 49% to 61%. However, rates of inappropriate antibiotic prescribing and inappropriate medications in the elderly both worsened.
Recommended colorectal cancer screening improved from 48% to 63%; but recommended breast cancer screening declined from 81% to 77%, and recommended cervical cancer screening declined from 90% to 86%.
Inappropriate cervical cancer screening in the elderly improved, but inappropriate colorectal cancer screening in the elderly worsened.
Of greatest concern, approximately half of elderly adults underwent inappropriate cancer screening when it was unlikely to prolong life. And approximately half of adults who saw a clinician for a viral illness received inappropriate antibiotics. In addition, approximately 15% who consulted a clinician for back pain received an inappropriate lumbar radiograph.
“These areas represent prime targets for efforts to improve the value of care delivered by eliminating services that have a neutral or negative impact on health,” Dr. Levine and his associates said.
The investigators emphasized that these data do not reflect changes resulting from the Affordable Care Act, which was implemented in late 2013. The ACA has encouraged many organizational changes, renewed the focus on primary care, and expanded health insurance coverage to 30 million more people. All of these changes are expected to improve overall health care quality, the study authors noted. But it is not yet known whether these changes will have their intended effect.
This study was supported in part by the National Institutes of Health and the Ryoichi Sasakawa Fellowship Fund. Dr. Levine and his associates reported having no relevant financial disclosures.
The clinical quality of outpatient care for adults in the United States did not improve consistently from 2002 to 2013, despite numerous local, regional, and national efforts to make it better, according to a report published online Oct. 17 in JAMA Internal Medicine.
“Current deficits in care continue to pose serious hazards to the health of the American public in the form of missed care opportunities as well as waste and potential harm from overuse,” said David M. Levine, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
Since a 2003 report showing that American adults received about half of the recommended health care services, many programs have sought to make improvements by expanding quality measurements and public reporting, increasing pay for performance, increasing value-based purchasing by payers, increasing the use of electronic medical records, improving coverage for recommended services, and expanding patient-centered medical homes.
Yet few data are available by which to gauge whether these efforts have been effective, the investigators noted (JAMA Intern Med. 2016 Oct 17; doi:10.1001/jamainternmed.2016.6217).
They analyzed data from a nationally representative annual outpatient survey to determine whether 46 indicators of care quality changed from 2002 to 2013. Sample sizes ranged from 20,679 to 26,509 adults per year, and that information was supplemented by responses from the patients’ clinicians, pharmacies, and employers.
Overall, rates of recommended treatment delivery showed an “anemic” improvement from 36% to 42%, with a few areas showing marked improvement while others showed little improvement or actual declines.
For example, rates of recommended medications for heart failure increased from 41% to 65%; rates for recommended statin therapy for stroke patients increased from 34% to 57%; and rates of recommended smoking-cessation counseling increased from 49% to 61%. However, rates of inappropriate antibiotic prescribing and inappropriate medications in the elderly both worsened.
Recommended colorectal cancer screening improved from 48% to 63%; but recommended breast cancer screening declined from 81% to 77%, and recommended cervical cancer screening declined from 90% to 86%.
Inappropriate cervical cancer screening in the elderly improved, but inappropriate colorectal cancer screening in the elderly worsened.
Of greatest concern, approximately half of elderly adults underwent inappropriate cancer screening when it was unlikely to prolong life. And approximately half of adults who saw a clinician for a viral illness received inappropriate antibiotics. In addition, approximately 15% who consulted a clinician for back pain received an inappropriate lumbar radiograph.
“These areas represent prime targets for efforts to improve the value of care delivered by eliminating services that have a neutral or negative impact on health,” Dr. Levine and his associates said.
The investigators emphasized that these data do not reflect changes resulting from the Affordable Care Act, which was implemented in late 2013. The ACA has encouraged many organizational changes, renewed the focus on primary care, and expanded health insurance coverage to 30 million more people. All of these changes are expected to improve overall health care quality, the study authors noted. But it is not yet known whether these changes will have their intended effect.
This study was supported in part by the National Institutes of Health and the Ryoichi Sasakawa Fellowship Fund. Dr. Levine and his associates reported having no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The clinical quality of outpatient care for adults in the United States did not improve consistently from 2002 to 2013.
Major finding: Approximately half of elderly adults underwent inappropriate cancer screening when it was unlikely to prolong life, approximately half of adults who saw a clinician for a viral illness received inappropriate antibiotics, and approximately 15% who consulted a clinician for back pain received an inappropriate lumbar radiograph.
Data source: An analysis of 10-year trends in quality of care based on data from nationally representative annual surveys of 20,679 to 26,509 adult outpatients.
Disclosures: This study was supported in part by the National Institutes of Health and the Ryoichi Sasakawa Fellowship Fund. Dr. Levine and his associates reported having no relevant financial disclosures.
Crusted Plaque in the Umbilicus
The Diagnosis: Sister Mary Joseph Nodule
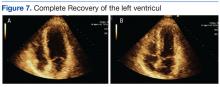
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.
When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
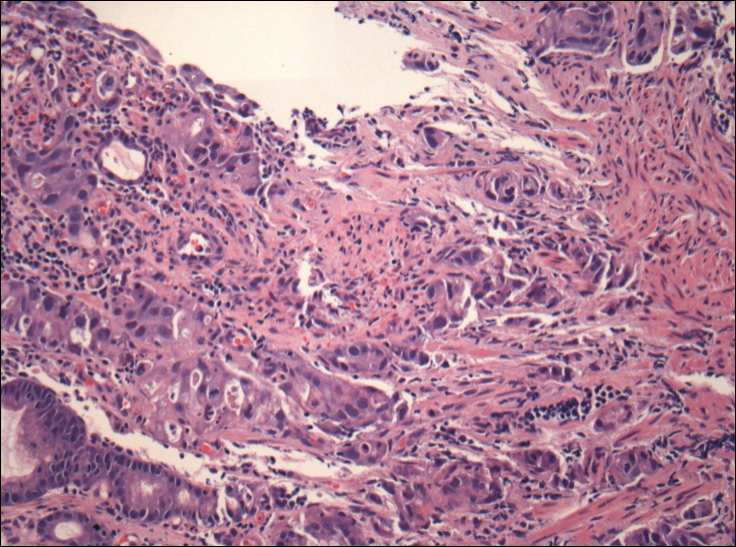
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
The Diagnosis: Sister Mary Joseph Nodule
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.

When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
The Diagnosis: Sister Mary Joseph Nodule
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.

When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
A 74-year-old man presented to our outpatient dermatology clinic with an asymptomatic umbilical lesion of unknown duration. The patient believed the lesion was a scar resulting from a prior laparoscopic repair of an umbilical hernia. However, the patient reported epigastric abdominal pain and diarrhea of 1 month's duration that he believed was due to the stomach flu. The patient denied fever, chills, loss of appetite, or weight loss. History was remarkable for hypertension, hyperlipidemia, coronary artery disease, chronic kidney disease, and emphysema. The patient had a surgical history of percutaneous transluminal coronary angioplasty in addition to the laparoscopic umbilical hernia repair. The patient's medications included pantoprazole, ondansetron, diphenoxylate-atropine as needed, amlodipine, lisinopril-hydrochlorothiazide, simvastatin, and aspirin. Physical examination revealed a 1×2-cm pink, nodular, firm plaque with crust at the umbilicus that was tender on palpation. A shave biopsy of the umbilicus was performed and sent for both pathological and immunohistochemical analysis.
Postinflammatory erythema
The troubling, frustrating part of acne: the persistent acne scars that are often a prolonged battle for most of our patients. We have many techniques to deal with postinflammatory hyperpigmentation (PIH). However, postinflammatory erythema (PIE), the erythematous scars often seen in acne and other inflammatory skin conditions, is not well understood. And despite its pervasive nature, very little data exist that identify its etiology and effective treatment options.
Inflammatory acne scars are not all the same. PIH, often seen with Fitzpatrick skin types III-VI, is related to brown spots, not red spots. Hyperpigmentation is caused by an excess production of melanin. There are treatments for PIH in our armamentarium – such as microdermabrasion, chemical peels, hydroquinone, and vitamin C – that inhibit melanogenesis and blend the skin discoloration.
In contrast, PIE is characterized by pink, red, and sometimes purple-appearing vascular neogenesis seen most often with skin types I-III after an inflammatory skin condition resolves, and is often seen in cystic acne.
The term postinflammatory erythema was initially introduced in the dermatology literature in 2013 by Bae-Harboe et al. to describe erythema often seen after the resolution of inflammatory acne or other inflammatory skin conditions.1 It is not to be confused with the erythema and telangiectasias seen in erythematotelangiectatic rosacea, which is a separate entity.
In my practice, microneedling has also been effective in reducing PIE. Although this may seem counterintuitive because of the bleeding associated with the microneedling process, microneedling-induced skin tissue injury and neocollagenesis have been clinically shown to improve the abnormal vascular proliferation that occurs in PIE. Similar techniques can be used with fractional resurfacing lasers. However, no studies have specifically evaluated the erythematous component of acne scars treated with fractionated lasers.
Topical preparations containing brimonidine (Mirvaso), azelaic acid, and green tea, as well as oral nicotinamide, can have a temporary effect on reducing skin erythema.
However, very little data or clinical studies are available on treatments for PIE, and there are no well-studied preparations with long-term efficacy data. Studies are needed to provide better clinical guidelines for treatment methods and alternatives to treatments, including topical and systemic medications.
References
1. J Clin Aesthet Dermatol. 2013 Sep;6(9):46-7.
2. J Am Acad Dermatol. 2009 May;60(5):801-7.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
The troubling, frustrating part of acne: the persistent acne scars that are often a prolonged battle for most of our patients. We have many techniques to deal with postinflammatory hyperpigmentation (PIH). However, postinflammatory erythema (PIE), the erythematous scars often seen in acne and other inflammatory skin conditions, is not well understood. And despite its pervasive nature, very little data exist that identify its etiology and effective treatment options.
Inflammatory acne scars are not all the same. PIH, often seen with Fitzpatrick skin types III-VI, is related to brown spots, not red spots. Hyperpigmentation is caused by an excess production of melanin. There are treatments for PIH in our armamentarium – such as microdermabrasion, chemical peels, hydroquinone, and vitamin C – that inhibit melanogenesis and blend the skin discoloration.
In contrast, PIE is characterized by pink, red, and sometimes purple-appearing vascular neogenesis seen most often with skin types I-III after an inflammatory skin condition resolves, and is often seen in cystic acne.
The term postinflammatory erythema was initially introduced in the dermatology literature in 2013 by Bae-Harboe et al. to describe erythema often seen after the resolution of inflammatory acne or other inflammatory skin conditions.1 It is not to be confused with the erythema and telangiectasias seen in erythematotelangiectatic rosacea, which is a separate entity.
In my practice, microneedling has also been effective in reducing PIE. Although this may seem counterintuitive because of the bleeding associated with the microneedling process, microneedling-induced skin tissue injury and neocollagenesis have been clinically shown to improve the abnormal vascular proliferation that occurs in PIE. Similar techniques can be used with fractional resurfacing lasers. However, no studies have specifically evaluated the erythematous component of acne scars treated with fractionated lasers.
Topical preparations containing brimonidine (Mirvaso), azelaic acid, and green tea, as well as oral nicotinamide, can have a temporary effect on reducing skin erythema.
However, very little data or clinical studies are available on treatments for PIE, and there are no well-studied preparations with long-term efficacy data. Studies are needed to provide better clinical guidelines for treatment methods and alternatives to treatments, including topical and systemic medications.
References
1. J Clin Aesthet Dermatol. 2013 Sep;6(9):46-7.
2. J Am Acad Dermatol. 2009 May;60(5):801-7.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
The troubling, frustrating part of acne: the persistent acne scars that are often a prolonged battle for most of our patients. We have many techniques to deal with postinflammatory hyperpigmentation (PIH). However, postinflammatory erythema (PIE), the erythematous scars often seen in acne and other inflammatory skin conditions, is not well understood. And despite its pervasive nature, very little data exist that identify its etiology and effective treatment options.
Inflammatory acne scars are not all the same. PIH, often seen with Fitzpatrick skin types III-VI, is related to brown spots, not red spots. Hyperpigmentation is caused by an excess production of melanin. There are treatments for PIH in our armamentarium – such as microdermabrasion, chemical peels, hydroquinone, and vitamin C – that inhibit melanogenesis and blend the skin discoloration.
In contrast, PIE is characterized by pink, red, and sometimes purple-appearing vascular neogenesis seen most often with skin types I-III after an inflammatory skin condition resolves, and is often seen in cystic acne.
The term postinflammatory erythema was initially introduced in the dermatology literature in 2013 by Bae-Harboe et al. to describe erythema often seen after the resolution of inflammatory acne or other inflammatory skin conditions.1 It is not to be confused with the erythema and telangiectasias seen in erythematotelangiectatic rosacea, which is a separate entity.
In my practice, microneedling has also been effective in reducing PIE. Although this may seem counterintuitive because of the bleeding associated with the microneedling process, microneedling-induced skin tissue injury and neocollagenesis have been clinically shown to improve the abnormal vascular proliferation that occurs in PIE. Similar techniques can be used with fractional resurfacing lasers. However, no studies have specifically evaluated the erythematous component of acne scars treated with fractionated lasers.
Topical preparations containing brimonidine (Mirvaso), azelaic acid, and green tea, as well as oral nicotinamide, can have a temporary effect on reducing skin erythema.
However, very little data or clinical studies are available on treatments for PIE, and there are no well-studied preparations with long-term efficacy data. Studies are needed to provide better clinical guidelines for treatment methods and alternatives to treatments, including topical and systemic medications.
References
1. J Clin Aesthet Dermatol. 2013 Sep;6(9):46-7.
2. J Am Acad Dermatol. 2009 May;60(5):801-7.
Dr. Talakoub and Dr. Wesley are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
Delayed ICU Transfer Affects Mortality and Length of Stay
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
IV Fluid Can Save Lives in Hemodynamically Stable Patients with Sepsis
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Epinephrine-Induced Takotsubo Cardiomyopathy
Takotsubo cardiomyopathy (TCM) is a transient left ventricular (LV) wall motion abnormality often associated with acute medical illnesses, catastrophic life events, and intense physical or emotional stress.1,2 Takotsubo cardiomyopathy also is known as stress-induced transient cardiomyopathy, apical ballooning syndrome, ampulla cardiomyopathy, and broken heart syndrome. The first reported case occurred in 1990 in Japan and accounts for 2% of all suspected acute coronary syndromes. Postmenopausal women seem to be at a higher risk for developing the disease, as about 90% of takotsubo cardiomyopathy occurs in postmenopausal women.3
Diagnosis of TCM is difficult, and there is an ongoing debate about the diagnostic criteria. The Mayo Clinic proposed the following criteria: (1) transient hypokinesis, akinesis, or dyskinesis of the LV mid-segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often but not always present; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) absence of pheochromocytoma and myocarditis.4
Depending on the part of the LV involved, TCM can be classified into apical ballooning variant (most common), an inverted or reverse takotsubo variant (basal akinesis with hyper dynamic apex), or a midventricular takotsubo variant.5
Several investigators have proposed the role of catecholamine in the pathogeneses of TCM.6,7 Previous case reports have shown that the clinical circumstances with elevated catecholamine can result in TCM.8,9 The authors recently encountered a case of a patient who developed apical ballooning TCM after an inadvertent injection of a high dose of epinephrine.
Case Report
A 60-year-old man with a history of hypertension, alcohol abuse, tobacco use, non-Hodgkin lymphoma, and recently diagnosed squamous cell carcinoma of the right glossopalatine fossa was admitted to the chemotherapy infusion clinic at the Kansas City VAMC to receive his first dose of cetuximab. Forty-five minutes after starting the test-dose infusion, the patient developed shortness of breath along with hives on his trunk and extremities. His blood pressure was 80/50 mm Hg; heart rate, 100 bpm; 16 breaths per minute respiration rate; and oxygen saturation by pulse oximetry was 90% on ambient air. The patient reported no chest pain, and a cardiac examination was unremarkable without wheezing on lung auscultation.
A clinical diagnosis of acute anaphylaxis reaction was considered; the test dose of cetuximab was discontinued, and the patient was given 50 mg of diphenhydramine, followed by an inadvertent administration of 1 mg of epinephrine intravenously (recommended dose 0.1 mg). After 3 minutes of administration of the medication, the patient’s blood pressure increased to190/110 with a pulse rate of 120 bpm.
An electrocardiogram (EKG) showed ST elevation in the precordial and inferior leads consistent with inferior and anterior myocardial injury (Figure 1). Pertinent laboratory studies revealed normal serum potassium (4.4 mEq/L), elevated serum glucose (219 mg/dL), and elevated troponin-I, (1.180 ng/mL; reference range 0-0.3 ng/mL). An emergent transthoracic echocardiogram showed a LV ejection fraction of 40% with akinesis involving the apical anterior, mid and distal anteroseptal, and anterolateral wall with no significant valvular abnormalities (Figures 2 and 3).
Given these echocardiographic findings, persistent EKG changes, and elevated troponin levels, an emergent coronary angiography along with left ventriculography was performed. The left ventriculogram demonstrated apical ballooning consistent with TCM (Figures 4 and 5). The left epicardial coronary arteries did not reveal any obstructive disease (Figure 6).
The subsequent hospital course was unremarkable, and the patient was discharged on guideline-directed medications for systolic dysfunction (ie, beta blockers and angiotensin-converting-enzyme inhibitors). Serum levels of epinephrine were not checked. A follow-up echocardiogram performed after 8 weeks showed complete recovery of the LV function (Figures 7a and 7b). The temporal sequence of events was highly suggestive of TCM, possibly due to the supratherapeutic dose of epinephrine.
Discussion
The pathogenesis of stress-induced cardiomyopathy is not fully understood. Various hypotheses have been suggested, including catecholamine excess, coronary vasospasm, microvascular dysfunction, and dynamic midcavity or LV outflow tract obstruction.1,10 The hypothesis implicating catecholamine excess in the pathogenesis of TCM is supported by animal studies.7 These studies suggest that catecholamines possibly play a role by “direct toxicity to myocardial cells” as evidenced by histologic findings of myofibrillar degeneration, contraction band necrosis, and leukocyte infiltration in harvested hearts from experimental animals and patients undergoing autopsy.7,11 Similarly, cultured cardiomyocytes exposed to high doses of epinephrine showed apoptic changes. Pretreatment of these cells with α- and β-adrenoreceptor blockers significantly attenuated these changes.7
However, myocardial necrosis alone cannot explain the entire picture, because most patients with TCM regain full recovery of LV function within several days of the event, and the degree of LV dysfunction is inconsistent with the mild elevations in cardiac
The role of estrogen also has been implicated in the pathogenesis of TCM. From a prevalence standpoint, almost 90% of TCM occurs in postmenopausal women.11,12 Estrogen plays a critical role in protecting the myocardium, possibly by down regulating β-adrenergic receptors. Postmenopausal women who do not receive estrogen replacement therapy lose this protection and may be more vulnerable to the catecholamine surge that is associated with stress.11 Overall, the catecholamines play a central role in the pathogenesis. This case suggests that exogenous administration of epinephrine in suprapharmacologic doses can be sufficient to replicate the stressful situation and induce TCM.
Conclusion
The authors’ case report describes the induction of apical ballooning TCM by administration of supratherapeutic doses of epinephrine and supports the role of catecholamines in the pathophysiology of TCM. It is hoped that this report will help clinicians to suspect, recognize, and manage this disease and be aware of possible iatrogenic causes.
1. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602-609.
2. Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548.
3. Parodi G, Del Pace S, Carrabba N, et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99(2):182-185.
4. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35(4):240-243.
5. Kawai S, Kitabatake A, Tomoike H; Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71(6):990-992.
6. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22-29.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706.
8. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009;53(15):1320-1325.
9. Wedekind H, Möller K, Scholz KH. Tako-tsubo cardiomyopathy. Incidence in patients with acute coronary syndrome. [in German]. Herz. 2006;31(4):339-346.
10. Khullar M, Datta BN, Wahi PL, Chakravarti RN. Catecholamine-induced experimental cardiomyopathy—a histopathological, histochemical and ultrastructural study. Indian Heart J. 1989;41(5):307-313.
11. Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(suppl 1):S117-S119.
12. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472-479.
Takotsubo cardiomyopathy (TCM) is a transient left ventricular (LV) wall motion abnormality often associated with acute medical illnesses, catastrophic life events, and intense physical or emotional stress.1,2 Takotsubo cardiomyopathy also is known as stress-induced transient cardiomyopathy, apical ballooning syndrome, ampulla cardiomyopathy, and broken heart syndrome. The first reported case occurred in 1990 in Japan and accounts for 2% of all suspected acute coronary syndromes. Postmenopausal women seem to be at a higher risk for developing the disease, as about 90% of takotsubo cardiomyopathy occurs in postmenopausal women.3
Diagnosis of TCM is difficult, and there is an ongoing debate about the diagnostic criteria. The Mayo Clinic proposed the following criteria: (1) transient hypokinesis, akinesis, or dyskinesis of the LV mid-segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often but not always present; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) absence of pheochromocytoma and myocarditis.4
Depending on the part of the LV involved, TCM can be classified into apical ballooning variant (most common), an inverted or reverse takotsubo variant (basal akinesis with hyper dynamic apex), or a midventricular takotsubo variant.5
Several investigators have proposed the role of catecholamine in the pathogeneses of TCM.6,7 Previous case reports have shown that the clinical circumstances with elevated catecholamine can result in TCM.8,9 The authors recently encountered a case of a patient who developed apical ballooning TCM after an inadvertent injection of a high dose of epinephrine.
Case Report
A 60-year-old man with a history of hypertension, alcohol abuse, tobacco use, non-Hodgkin lymphoma, and recently diagnosed squamous cell carcinoma of the right glossopalatine fossa was admitted to the chemotherapy infusion clinic at the Kansas City VAMC to receive his first dose of cetuximab. Forty-five minutes after starting the test-dose infusion, the patient developed shortness of breath along with hives on his trunk and extremities. His blood pressure was 80/50 mm Hg; heart rate, 100 bpm; 16 breaths per minute respiration rate; and oxygen saturation by pulse oximetry was 90% on ambient air. The patient reported no chest pain, and a cardiac examination was unremarkable without wheezing on lung auscultation.
A clinical diagnosis of acute anaphylaxis reaction was considered; the test dose of cetuximab was discontinued, and the patient was given 50 mg of diphenhydramine, followed by an inadvertent administration of 1 mg of epinephrine intravenously (recommended dose 0.1 mg). After 3 minutes of administration of the medication, the patient’s blood pressure increased to190/110 with a pulse rate of 120 bpm.
An electrocardiogram (EKG) showed ST elevation in the precordial and inferior leads consistent with inferior and anterior myocardial injury (Figure 1). Pertinent laboratory studies revealed normal serum potassium (4.4 mEq/L), elevated serum glucose (219 mg/dL), and elevated troponin-I, (1.180 ng/mL; reference range 0-0.3 ng/mL). An emergent transthoracic echocardiogram showed a LV ejection fraction of 40% with akinesis involving the apical anterior, mid and distal anteroseptal, and anterolateral wall with no significant valvular abnormalities (Figures 2 and 3).
Given these echocardiographic findings, persistent EKG changes, and elevated troponin levels, an emergent coronary angiography along with left ventriculography was performed. The left ventriculogram demonstrated apical ballooning consistent with TCM (Figures 4 and 5). The left epicardial coronary arteries did not reveal any obstructive disease (Figure 6).
The subsequent hospital course was unremarkable, and the patient was discharged on guideline-directed medications for systolic dysfunction (ie, beta blockers and angiotensin-converting-enzyme inhibitors). Serum levels of epinephrine were not checked. A follow-up echocardiogram performed after 8 weeks showed complete recovery of the LV function (Figures 7a and 7b). The temporal sequence of events was highly suggestive of TCM, possibly due to the supratherapeutic dose of epinephrine.
Discussion
The pathogenesis of stress-induced cardiomyopathy is not fully understood. Various hypotheses have been suggested, including catecholamine excess, coronary vasospasm, microvascular dysfunction, and dynamic midcavity or LV outflow tract obstruction.1,10 The hypothesis implicating catecholamine excess in the pathogenesis of TCM is supported by animal studies.7 These studies suggest that catecholamines possibly play a role by “direct toxicity to myocardial cells” as evidenced by histologic findings of myofibrillar degeneration, contraction band necrosis, and leukocyte infiltration in harvested hearts from experimental animals and patients undergoing autopsy.7,11 Similarly, cultured cardiomyocytes exposed to high doses of epinephrine showed apoptic changes. Pretreatment of these cells with α- and β-adrenoreceptor blockers significantly attenuated these changes.7
However, myocardial necrosis alone cannot explain the entire picture, because most patients with TCM regain full recovery of LV function within several days of the event, and the degree of LV dysfunction is inconsistent with the mild elevations in cardiac
The role of estrogen also has been implicated in the pathogenesis of TCM. From a prevalence standpoint, almost 90% of TCM occurs in postmenopausal women.11,12 Estrogen plays a critical role in protecting the myocardium, possibly by down regulating β-adrenergic receptors. Postmenopausal women who do not receive estrogen replacement therapy lose this protection and may be more vulnerable to the catecholamine surge that is associated with stress.11 Overall, the catecholamines play a central role in the pathogenesis. This case suggests that exogenous administration of epinephrine in suprapharmacologic doses can be sufficient to replicate the stressful situation and induce TCM.
Conclusion
The authors’ case report describes the induction of apical ballooning TCM by administration of supratherapeutic doses of epinephrine and supports the role of catecholamines in the pathophysiology of TCM. It is hoped that this report will help clinicians to suspect, recognize, and manage this disease and be aware of possible iatrogenic causes.
Takotsubo cardiomyopathy (TCM) is a transient left ventricular (LV) wall motion abnormality often associated with acute medical illnesses, catastrophic life events, and intense physical or emotional stress.1,2 Takotsubo cardiomyopathy also is known as stress-induced transient cardiomyopathy, apical ballooning syndrome, ampulla cardiomyopathy, and broken heart syndrome. The first reported case occurred in 1990 in Japan and accounts for 2% of all suspected acute coronary syndromes. Postmenopausal women seem to be at a higher risk for developing the disease, as about 90% of takotsubo cardiomyopathy occurs in postmenopausal women.3
Diagnosis of TCM is difficult, and there is an ongoing debate about the diagnostic criteria. The Mayo Clinic proposed the following criteria: (1) transient hypokinesis, akinesis, or dyskinesis of the LV mid-segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often but not always present; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) absence of pheochromocytoma and myocarditis.4
Depending on the part of the LV involved, TCM can be classified into apical ballooning variant (most common), an inverted or reverse takotsubo variant (basal akinesis with hyper dynamic apex), or a midventricular takotsubo variant.5
Several investigators have proposed the role of catecholamine in the pathogeneses of TCM.6,7 Previous case reports have shown that the clinical circumstances with elevated catecholamine can result in TCM.8,9 The authors recently encountered a case of a patient who developed apical ballooning TCM after an inadvertent injection of a high dose of epinephrine.
Case Report
A 60-year-old man with a history of hypertension, alcohol abuse, tobacco use, non-Hodgkin lymphoma, and recently diagnosed squamous cell carcinoma of the right glossopalatine fossa was admitted to the chemotherapy infusion clinic at the Kansas City VAMC to receive his first dose of cetuximab. Forty-five minutes after starting the test-dose infusion, the patient developed shortness of breath along with hives on his trunk and extremities. His blood pressure was 80/50 mm Hg; heart rate, 100 bpm; 16 breaths per minute respiration rate; and oxygen saturation by pulse oximetry was 90% on ambient air. The patient reported no chest pain, and a cardiac examination was unremarkable without wheezing on lung auscultation.
A clinical diagnosis of acute anaphylaxis reaction was considered; the test dose of cetuximab was discontinued, and the patient was given 50 mg of diphenhydramine, followed by an inadvertent administration of 1 mg of epinephrine intravenously (recommended dose 0.1 mg). After 3 minutes of administration of the medication, the patient’s blood pressure increased to190/110 with a pulse rate of 120 bpm.
An electrocardiogram (EKG) showed ST elevation in the precordial and inferior leads consistent with inferior and anterior myocardial injury (Figure 1). Pertinent laboratory studies revealed normal serum potassium (4.4 mEq/L), elevated serum glucose (219 mg/dL), and elevated troponin-I, (1.180 ng/mL; reference range 0-0.3 ng/mL). An emergent transthoracic echocardiogram showed a LV ejection fraction of 40% with akinesis involving the apical anterior, mid and distal anteroseptal, and anterolateral wall with no significant valvular abnormalities (Figures 2 and 3).
Given these echocardiographic findings, persistent EKG changes, and elevated troponin levels, an emergent coronary angiography along with left ventriculography was performed. The left ventriculogram demonstrated apical ballooning consistent with TCM (Figures 4 and 5). The left epicardial coronary arteries did not reveal any obstructive disease (Figure 6).
The subsequent hospital course was unremarkable, and the patient was discharged on guideline-directed medications for systolic dysfunction (ie, beta blockers and angiotensin-converting-enzyme inhibitors). Serum levels of epinephrine were not checked. A follow-up echocardiogram performed after 8 weeks showed complete recovery of the LV function (Figures 7a and 7b). The temporal sequence of events was highly suggestive of TCM, possibly due to the supratherapeutic dose of epinephrine.
Discussion
The pathogenesis of stress-induced cardiomyopathy is not fully understood. Various hypotheses have been suggested, including catecholamine excess, coronary vasospasm, microvascular dysfunction, and dynamic midcavity or LV outflow tract obstruction.1,10 The hypothesis implicating catecholamine excess in the pathogenesis of TCM is supported by animal studies.7 These studies suggest that catecholamines possibly play a role by “direct toxicity to myocardial cells” as evidenced by histologic findings of myofibrillar degeneration, contraction band necrosis, and leukocyte infiltration in harvested hearts from experimental animals and patients undergoing autopsy.7,11 Similarly, cultured cardiomyocytes exposed to high doses of epinephrine showed apoptic changes. Pretreatment of these cells with α- and β-adrenoreceptor blockers significantly attenuated these changes.7
However, myocardial necrosis alone cannot explain the entire picture, because most patients with TCM regain full recovery of LV function within several days of the event, and the degree of LV dysfunction is inconsistent with the mild elevations in cardiac
The role of estrogen also has been implicated in the pathogenesis of TCM. From a prevalence standpoint, almost 90% of TCM occurs in postmenopausal women.11,12 Estrogen plays a critical role in protecting the myocardium, possibly by down regulating β-adrenergic receptors. Postmenopausal women who do not receive estrogen replacement therapy lose this protection and may be more vulnerable to the catecholamine surge that is associated with stress.11 Overall, the catecholamines play a central role in the pathogenesis. This case suggests that exogenous administration of epinephrine in suprapharmacologic doses can be sufficient to replicate the stressful situation and induce TCM.
Conclusion
The authors’ case report describes the induction of apical ballooning TCM by administration of supratherapeutic doses of epinephrine and supports the role of catecholamines in the pathophysiology of TCM. It is hoped that this report will help clinicians to suspect, recognize, and manage this disease and be aware of possible iatrogenic causes.
1. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602-609.
2. Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548.
3. Parodi G, Del Pace S, Carrabba N, et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99(2):182-185.
4. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35(4):240-243.
5. Kawai S, Kitabatake A, Tomoike H; Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71(6):990-992.
6. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22-29.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706.
8. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009;53(15):1320-1325.
9. Wedekind H, Möller K, Scholz KH. Tako-tsubo cardiomyopathy. Incidence in patients with acute coronary syndrome. [in German]. Herz. 2006;31(4):339-346.
10. Khullar M, Datta BN, Wahi PL, Chakravarti RN. Catecholamine-induced experimental cardiomyopathy—a histopathological, histochemical and ultrastructural study. Indian Heart J. 1989;41(5):307-313.
11. Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(suppl 1):S117-S119.
12. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472-479.
1. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602-609.
2. Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548.
3. Parodi G, Del Pace S, Carrabba N, et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99(2):182-185.
4. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35(4):240-243.
5. Kawai S, Kitabatake A, Tomoike H; Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71(6):990-992.
6. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22-29.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706.
8. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009;53(15):1320-1325.
9. Wedekind H, Möller K, Scholz KH. Tako-tsubo cardiomyopathy. Incidence in patients with acute coronary syndrome. [in German]. Herz. 2006;31(4):339-346.
10. Khullar M, Datta BN, Wahi PL, Chakravarti RN. Catecholamine-induced experimental cardiomyopathy—a histopathological, histochemical and ultrastructural study. Indian Heart J. 1989;41(5):307-313.
11. Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(suppl 1):S117-S119.
12. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472-479.