User login
Change in end-of-life cancer care imperative
With the passage of the Medicare Access and CHIP Reauthorization Act, changes to how cancer care is delivered are fast approaching. This legislation aims to reward value-based care and incentivize alternative payment models that prize quality. The shift from quantity-based to value-based reimbursement is motivated in part by the rising cost of health care as well as the growing demand from patients, employers, and payers to better understand the quality of care being delivered. In cancer care, one area of high-cost and questionable value being examined is aggressive care at the end of life.
The scientific pace of progress in cancer care is exciting, with 19 therapies approved or granted a new indication in 2015. New categories of drugs, such as immunotherapies, are changing how we treat patients. It is also a time of great change in how cancer care is being delivered in our clinics, hospitals, and academic institutions. We must be vigilant in learning from these experiments in care delivery to ensure that they deliver on their promise of value to patients.
Dr. Bobby Daly, Dr. Andrew Hantel, and Dr. Blase Polite are with the University of Chicago.
With the passage of the Medicare Access and CHIP Reauthorization Act, changes to how cancer care is delivered are fast approaching. This legislation aims to reward value-based care and incentivize alternative payment models that prize quality. The shift from quantity-based to value-based reimbursement is motivated in part by the rising cost of health care as well as the growing demand from patients, employers, and payers to better understand the quality of care being delivered. In cancer care, one area of high-cost and questionable value being examined is aggressive care at the end of life.
The scientific pace of progress in cancer care is exciting, with 19 therapies approved or granted a new indication in 2015. New categories of drugs, such as immunotherapies, are changing how we treat patients. It is also a time of great change in how cancer care is being delivered in our clinics, hospitals, and academic institutions. We must be vigilant in learning from these experiments in care delivery to ensure that they deliver on their promise of value to patients.
Dr. Bobby Daly, Dr. Andrew Hantel, and Dr. Blase Polite are with the University of Chicago.
With the passage of the Medicare Access and CHIP Reauthorization Act, changes to how cancer care is delivered are fast approaching. This legislation aims to reward value-based care and incentivize alternative payment models that prize quality. The shift from quantity-based to value-based reimbursement is motivated in part by the rising cost of health care as well as the growing demand from patients, employers, and payers to better understand the quality of care being delivered. In cancer care, one area of high-cost and questionable value being examined is aggressive care at the end of life.
The scientific pace of progress in cancer care is exciting, with 19 therapies approved or granted a new indication in 2015. New categories of drugs, such as immunotherapies, are changing how we treat patients. It is also a time of great change in how cancer care is being delivered in our clinics, hospitals, and academic institutions. We must be vigilant in learning from these experiments in care delivery to ensure that they deliver on their promise of value to patients.
Dr. Bobby Daly, Dr. Andrew Hantel, and Dr. Blase Polite are with the University of Chicago.
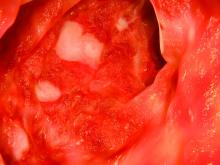
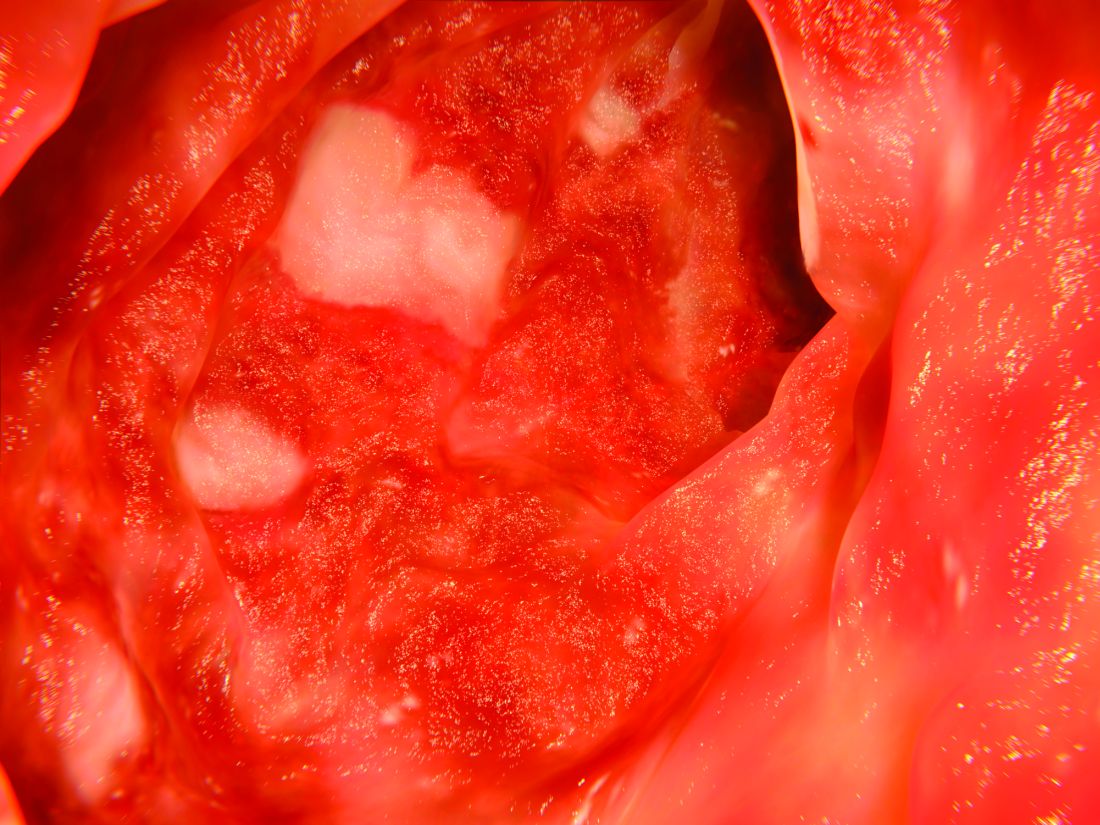
UC patients who failed infliximab gain from vedolizumab
Vedolizumab (Entyvio) was much more effective than placebo as a treatment for ulcerative colitis (UC) in a range of patients, researchers found in a follow-up analysis of trial data.
Patients who’d previously failed TNF-antagonist therapy with infliximab, however, fared worse at 6 and 52 weeks than those who hadn’t tried the treatment. Still, Brian G. Feagan, MD, of Western University, London, Ont., and fellow researchers report that vedolizumab had a “consistent benefit” in patients with moderate to severe active UC, regardless of whether their experience with TNF-antagonist therapy was poor or nonexistent. Remission rates at 52 weeks reached as high as 46.9%.
According to background information in the study, tumor necrosis factor–alpha (TNF) antagonist therapy has significantly improved treatment for ulcerative colitis, but many patients fail to respond. There is also a risk of infection.
The new study, published online Sept. 14 in Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.08.044), offers prespecified exploratory and post hoc analyses of vedolizumab vs. placebo in sets of UC patients from a phase III trial.
In a blinded group, 374 patients were randomly assigned to receive intravenous vedolizumab or placebo on days 1 and 15; 521 other patients in an open-label group received intravenous vedolizumab in the same fashion.
Patients in both groups who responded to vedolizumab at 6 weeks were randomly assigned to one of three groups: They continued vedolizumab every 8 weeks or every 4 weeks, or they received a placebo at week 6 for as many as 46 weeks. Patients who initially received the placebo either continued taking it over the maintenance period or stopped; those who didn’t respond to vedolizumab continued to get it every 4 weeks through week 52.
The new study focuses on 464 patients had not taken a TNF antagonist previously and 367 who’d failed TNF-antagonist therapy.
At week 6, 53.1% of the vedolizumab patients who hadn’t tried a TNF antagonist saw a clinical response, compared with 26.3% of counterparts who took a placebo (absolute difference, 26.4%; 95% confidence interval, 12.4-40.4; relative risk, 2.0; 95% CI, 1.3-3.0).
Among patients who had failed TNF antagonists, 39.0% saw a clinical response on vedolizumab at 6 weeks, and 20.6% saw one on placebo (absolute difference, 18.1%; 95% CI, 2.8-33.5; RR, 1.9; 95% CI, 1.1-3.2.). All those who’d failed TNF antagonists had taken infliximab because it was the only drug of its type available at the time of enrollment.
At week 52, the percentage of TNF antagonist–naive patients who were in remission was 46.9% in those on vedolizumab and 19.0% in those on placebo (absolute difference: 28.0%; 95% CI, 14.9-41.1; RR, 2.5; 95% CI, 1.5-4.0.) Among those who’d failed TNF-antagonist therapy, 36.1% of those on vedolizumab reached remission, and just 5.3% of those on placebo did (absolute difference, 29.5%; 95% CI, 12.8-46.1; RR, 6.6; 95% CI, 1.7-26.5.)
“These analyses show that vedolizumab had a consistent benefit for inducing and maintaining clinical response and remission in both TNF-naive and TNF-failure patients with moderately to severely active UC,” the authors write. “Further, no increased rates of serious infections were observed with vedolizumab treatment relative to placebo in either subgroup.”
The study authors caution against comparing the efficacy statistics in this study to the results of trials of TNF antagonists. While it’s “alluring” to make the comparison, they write, “We would caution against using such an approach to determine relative efficacy, given the confounding effects of differences in patient populations, outcome definitions, and, notably, trial design.”
They note that a study comparing the safety and efficacy of vedolizumab to adalimumab (Humira) in ulcerative colitis is underway (NCT02497469) – it’s now recruiting patients – and “the results of such studies will be critical in informing payers regarding the relative value of these agents.”
What’s next? The researchers note that TNF-antagonist alternatives to infliximab now exist. “Randomized trials should be performed comparing vedolizumab to the use of a second TNF antagonist in TNF -antagonist failure patients with adequate serum drug concentrations,” they write.
Millennium Pharmaceuticals (Takeda Pharmaceuticals) funded the clinical studies. Some of the study authors are employees of Takeda. Other study authors have received support from Takeda, Millennium, or both.
Vedolizumab (Entyvio) was much more effective than placebo as a treatment for ulcerative colitis (UC) in a range of patients, researchers found in a follow-up analysis of trial data.
Patients who’d previously failed TNF-antagonist therapy with infliximab, however, fared worse at 6 and 52 weeks than those who hadn’t tried the treatment. Still, Brian G. Feagan, MD, of Western University, London, Ont., and fellow researchers report that vedolizumab had a “consistent benefit” in patients with moderate to severe active UC, regardless of whether their experience with TNF-antagonist therapy was poor or nonexistent. Remission rates at 52 weeks reached as high as 46.9%.
According to background information in the study, tumor necrosis factor–alpha (TNF) antagonist therapy has significantly improved treatment for ulcerative colitis, but many patients fail to respond. There is also a risk of infection.
The new study, published online Sept. 14 in Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.08.044), offers prespecified exploratory and post hoc analyses of vedolizumab vs. placebo in sets of UC patients from a phase III trial.
In a blinded group, 374 patients were randomly assigned to receive intravenous vedolizumab or placebo on days 1 and 15; 521 other patients in an open-label group received intravenous vedolizumab in the same fashion.
Patients in both groups who responded to vedolizumab at 6 weeks were randomly assigned to one of three groups: They continued vedolizumab every 8 weeks or every 4 weeks, or they received a placebo at week 6 for as many as 46 weeks. Patients who initially received the placebo either continued taking it over the maintenance period or stopped; those who didn’t respond to vedolizumab continued to get it every 4 weeks through week 52.
The new study focuses on 464 patients had not taken a TNF antagonist previously and 367 who’d failed TNF-antagonist therapy.
At week 6, 53.1% of the vedolizumab patients who hadn’t tried a TNF antagonist saw a clinical response, compared with 26.3% of counterparts who took a placebo (absolute difference, 26.4%; 95% confidence interval, 12.4-40.4; relative risk, 2.0; 95% CI, 1.3-3.0).
Among patients who had failed TNF antagonists, 39.0% saw a clinical response on vedolizumab at 6 weeks, and 20.6% saw one on placebo (absolute difference, 18.1%; 95% CI, 2.8-33.5; RR, 1.9; 95% CI, 1.1-3.2.). All those who’d failed TNF antagonists had taken infliximab because it was the only drug of its type available at the time of enrollment.
At week 52, the percentage of TNF antagonist–naive patients who were in remission was 46.9% in those on vedolizumab and 19.0% in those on placebo (absolute difference: 28.0%; 95% CI, 14.9-41.1; RR, 2.5; 95% CI, 1.5-4.0.) Among those who’d failed TNF-antagonist therapy, 36.1% of those on vedolizumab reached remission, and just 5.3% of those on placebo did (absolute difference, 29.5%; 95% CI, 12.8-46.1; RR, 6.6; 95% CI, 1.7-26.5.)
“These analyses show that vedolizumab had a consistent benefit for inducing and maintaining clinical response and remission in both TNF-naive and TNF-failure patients with moderately to severely active UC,” the authors write. “Further, no increased rates of serious infections were observed with vedolizumab treatment relative to placebo in either subgroup.”
The study authors caution against comparing the efficacy statistics in this study to the results of trials of TNF antagonists. While it’s “alluring” to make the comparison, they write, “We would caution against using such an approach to determine relative efficacy, given the confounding effects of differences in patient populations, outcome definitions, and, notably, trial design.”
They note that a study comparing the safety and efficacy of vedolizumab to adalimumab (Humira) in ulcerative colitis is underway (NCT02497469) – it’s now recruiting patients – and “the results of such studies will be critical in informing payers regarding the relative value of these agents.”
What’s next? The researchers note that TNF-antagonist alternatives to infliximab now exist. “Randomized trials should be performed comparing vedolizumab to the use of a second TNF antagonist in TNF -antagonist failure patients with adequate serum drug concentrations,” they write.
Millennium Pharmaceuticals (Takeda Pharmaceuticals) funded the clinical studies. Some of the study authors are employees of Takeda. Other study authors have received support from Takeda, Millennium, or both.
Vedolizumab (Entyvio) was much more effective than placebo as a treatment for ulcerative colitis (UC) in a range of patients, researchers found in a follow-up analysis of trial data.
Patients who’d previously failed TNF-antagonist therapy with infliximab, however, fared worse at 6 and 52 weeks than those who hadn’t tried the treatment. Still, Brian G. Feagan, MD, of Western University, London, Ont., and fellow researchers report that vedolizumab had a “consistent benefit” in patients with moderate to severe active UC, regardless of whether their experience with TNF-antagonist therapy was poor or nonexistent. Remission rates at 52 weeks reached as high as 46.9%.
According to background information in the study, tumor necrosis factor–alpha (TNF) antagonist therapy has significantly improved treatment for ulcerative colitis, but many patients fail to respond. There is also a risk of infection.
The new study, published online Sept. 14 in Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.08.044), offers prespecified exploratory and post hoc analyses of vedolizumab vs. placebo in sets of UC patients from a phase III trial.
In a blinded group, 374 patients were randomly assigned to receive intravenous vedolizumab or placebo on days 1 and 15; 521 other patients in an open-label group received intravenous vedolizumab in the same fashion.
Patients in both groups who responded to vedolizumab at 6 weeks were randomly assigned to one of three groups: They continued vedolizumab every 8 weeks or every 4 weeks, or they received a placebo at week 6 for as many as 46 weeks. Patients who initially received the placebo either continued taking it over the maintenance period or stopped; those who didn’t respond to vedolizumab continued to get it every 4 weeks through week 52.
The new study focuses on 464 patients had not taken a TNF antagonist previously and 367 who’d failed TNF-antagonist therapy.
At week 6, 53.1% of the vedolizumab patients who hadn’t tried a TNF antagonist saw a clinical response, compared with 26.3% of counterparts who took a placebo (absolute difference, 26.4%; 95% confidence interval, 12.4-40.4; relative risk, 2.0; 95% CI, 1.3-3.0).
Among patients who had failed TNF antagonists, 39.0% saw a clinical response on vedolizumab at 6 weeks, and 20.6% saw one on placebo (absolute difference, 18.1%; 95% CI, 2.8-33.5; RR, 1.9; 95% CI, 1.1-3.2.). All those who’d failed TNF antagonists had taken infliximab because it was the only drug of its type available at the time of enrollment.
At week 52, the percentage of TNF antagonist–naive patients who were in remission was 46.9% in those on vedolizumab and 19.0% in those on placebo (absolute difference: 28.0%; 95% CI, 14.9-41.1; RR, 2.5; 95% CI, 1.5-4.0.) Among those who’d failed TNF-antagonist therapy, 36.1% of those on vedolizumab reached remission, and just 5.3% of those on placebo did (absolute difference, 29.5%; 95% CI, 12.8-46.1; RR, 6.6; 95% CI, 1.7-26.5.)
“These analyses show that vedolizumab had a consistent benefit for inducing and maintaining clinical response and remission in both TNF-naive and TNF-failure patients with moderately to severely active UC,” the authors write. “Further, no increased rates of serious infections were observed with vedolizumab treatment relative to placebo in either subgroup.”
The study authors caution against comparing the efficacy statistics in this study to the results of trials of TNF antagonists. While it’s “alluring” to make the comparison, they write, “We would caution against using such an approach to determine relative efficacy, given the confounding effects of differences in patient populations, outcome definitions, and, notably, trial design.”
They note that a study comparing the safety and efficacy of vedolizumab to adalimumab (Humira) in ulcerative colitis is underway (NCT02497469) – it’s now recruiting patients – and “the results of such studies will be critical in informing payers regarding the relative value of these agents.”
What’s next? The researchers note that TNF-antagonist alternatives to infliximab now exist. “Randomized trials should be performed comparing vedolizumab to the use of a second TNF antagonist in TNF -antagonist failure patients with adequate serum drug concentrations,” they write.
Millennium Pharmaceuticals (Takeda Pharmaceuticals) funded the clinical studies. Some of the study authors are employees of Takeda. Other study authors have received support from Takeda, Millennium, or both.
Key clinical point: , but they didn’t fare as well as those who never tried TNF-antagonist therapy.
Major finding: Of UC patients who took vedolizumab for 52 weeks, 46.9% of those who’d never tried TNF-antagonist therapy were in remission, compared with 36.1% of those who failed infliximab.
Data source: Post hoc analysis of 831 patients with moderate to severe UC in a multicenter, randomized, phase III trial of vedolizumab vs. placebo.
Disclosures: Millennium Pharmaceuticals (Takeda Pharmaceuticals) funded the clinical studies. Some of the study authors are employees of Takeda. Other study authors have received support from Takeda, Millennium, or both.
Cabozantinib used as first-line therapy prolongs PFS for metastatic RCC
COPENHAGEN – For patients with previously untreated intermediate- or poor-risk renal cell carcinoma (RCC), cabozantanib offers a progression-free survival benefit better than that seen with sunitinib, the current standard of care, reported investigators in the phase II CABOSUN trial.
After a median follow-up of 20.8 months, progression-free survival (PFS) for patients assigned to cabozantinib (Canbometyx) the primary endpoint, was a median 8.2 months, compared with 5.6 months for patients assigned to sunitinib, reported Toni K. Choueiri, MD, from the Dana-Farber Cancer Institute in Boston.
“Cabozantinib represents a potential new treatment option for patients with untreated renal cell carcinoma,” Dr. Choueiri said at the European Society for Medical Oncology (ESMO) congress.
Sunitinib (Sutent), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) is the standard first-line therapy for patients who present with metastatic RCC. But patients with intermediate- or poor-risk metastatic RCC have shorter survival on sunitinib compared with patients with favorable-risk disease, Dr. Choueiri noted.
Resistance to VEGFR inhibitors in clear-cell RCC can arise through inactivation of the Von Hippel-Lindau (VHL) gene, which leads to upregulation of MET, AXL, and VEGF receptors.
Increased expression of MET and AXL has been associated with resistant to VEGFR inhibitors and poor clinical outcomes, he explained.
Cabozantib is an oral small-molecular inhibitor of multiple tyrosine kinases, including VEGF receptors, MET, and AXL. It is approved in the United States and Europe for use in the second-line setting following prior therapy with a VEGFR inhibitor.
Trial details
In the CABOSUN trial, 157 treatment-naive patients with clear-cell RCC with measurable disease, Eastern Cooperative Oncology Group performance status 0-2 and intermediate- or poor-risk disease according to International Metastatic Renal Cell Carcinoma Database (IMDC) criteria were randomly assigned to receive either oral cabaozantinib 60 mg daily for 6 week cycles, or oral sunitinib 50 mg daily on a standard schedule of 4 weeks on and 2 weeks off.
Tumors were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) every other cycle, and treatment was continued to the point of disease progression or intolerable toxicity.
A total of 79 patients were assigned to cabozantinib, 78 received it, and 13 were still on the drug at the time of the data cutoff. In all, 78 patients were assigned to sunitinib, 72 received it, and 2 were continuing treatment at last follow-up. The efficacy analysis was by intention-to-treat, and the safety analysis as-treated.
As noted, median PFS was 8.2.months for cabozantinib vs. 5.6 months for sunitinib, The hazard ratio for cabozatinib was 0.69 (P = .012). A subgroup analysis showed a trend favoring cabozantinib in each risk group, but this was not significant except among patients with bone metastases (HR, 0.51; 95% confidence interval, 0.29-0.90).
The overall response rate among patients on cabozantib was 46%, compared with 18% for sunitinib. Complete responses were seen in one patient in each group, partial responses in 35 and 13, respectively, stable disease in 26 and 28, and disease progression in 14 and 20 (data on 3 and 16 patients were not evaluable).
The overall survival analysis hinted at a benefit for cabozantinib (HR, 0.80), but this was not statistically significant.
All cause grade 3 or 4 adverse events occurred in 65% of patients in the cabozantinib arm and 68% in the sunitinib arm. Events occurring more frequently with cabozantinib were hypertension (28% vs. 22%), elevated alanine aminotranseferase (5% vs. 0%), anorexia (5% vs. 0%), palmar-plantar erythrodysethesia (8% vs. 4%), and weight loss (4% vs. 0%).
There were 3 deaths possibly, probably, or definitely related to treatment among 3 patients on cabozantinib and 2 on sunitinib.
Favorable data, but wait and see
“I think cabozantinib is superior to sunitinib in poor and intermediate risk metastatic renal cell cancer,” said invited discussant Bernard Escudier, MD, from the Gustave Roussy Cancer Center in Paris.
“Cabozantinib is a potent VEGF inhibitor and is probably also active in good risk patients, although we need to see the data in these patients,” he said.
He added that a phase 3 study is warranted to confirm these conclusions, and that he will likely wait until those data are available before moving patients to cabozantinib in the first line.
The trial was supported by the National Cancer Institute. Dr. Choueiri disclosed advising and institutional research funding from Exelixis, maker of cabozantinib, and Pfizer, maker of sunitinib. Dr. Escudier disclosed receiving honoraria from each company.
COPENHAGEN – For patients with previously untreated intermediate- or poor-risk renal cell carcinoma (RCC), cabozantanib offers a progression-free survival benefit better than that seen with sunitinib, the current standard of care, reported investigators in the phase II CABOSUN trial.
After a median follow-up of 20.8 months, progression-free survival (PFS) for patients assigned to cabozantinib (Canbometyx) the primary endpoint, was a median 8.2 months, compared with 5.6 months for patients assigned to sunitinib, reported Toni K. Choueiri, MD, from the Dana-Farber Cancer Institute in Boston.
“Cabozantinib represents a potential new treatment option for patients with untreated renal cell carcinoma,” Dr. Choueiri said at the European Society for Medical Oncology (ESMO) congress.
Sunitinib (Sutent), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) is the standard first-line therapy for patients who present with metastatic RCC. But patients with intermediate- or poor-risk metastatic RCC have shorter survival on sunitinib compared with patients with favorable-risk disease, Dr. Choueiri noted.
Resistance to VEGFR inhibitors in clear-cell RCC can arise through inactivation of the Von Hippel-Lindau (VHL) gene, which leads to upregulation of MET, AXL, and VEGF receptors.
Increased expression of MET and AXL has been associated with resistant to VEGFR inhibitors and poor clinical outcomes, he explained.
Cabozantib is an oral small-molecular inhibitor of multiple tyrosine kinases, including VEGF receptors, MET, and AXL. It is approved in the United States and Europe for use in the second-line setting following prior therapy with a VEGFR inhibitor.
Trial details
In the CABOSUN trial, 157 treatment-naive patients with clear-cell RCC with measurable disease, Eastern Cooperative Oncology Group performance status 0-2 and intermediate- or poor-risk disease according to International Metastatic Renal Cell Carcinoma Database (IMDC) criteria were randomly assigned to receive either oral cabaozantinib 60 mg daily for 6 week cycles, or oral sunitinib 50 mg daily on a standard schedule of 4 weeks on and 2 weeks off.
Tumors were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) every other cycle, and treatment was continued to the point of disease progression or intolerable toxicity.
A total of 79 patients were assigned to cabozantinib, 78 received it, and 13 were still on the drug at the time of the data cutoff. In all, 78 patients were assigned to sunitinib, 72 received it, and 2 were continuing treatment at last follow-up. The efficacy analysis was by intention-to-treat, and the safety analysis as-treated.
As noted, median PFS was 8.2.months for cabozantinib vs. 5.6 months for sunitinib, The hazard ratio for cabozatinib was 0.69 (P = .012). A subgroup analysis showed a trend favoring cabozantinib in each risk group, but this was not significant except among patients with bone metastases (HR, 0.51; 95% confidence interval, 0.29-0.90).
The overall response rate among patients on cabozantib was 46%, compared with 18% for sunitinib. Complete responses were seen in one patient in each group, partial responses in 35 and 13, respectively, stable disease in 26 and 28, and disease progression in 14 and 20 (data on 3 and 16 patients were not evaluable).
The overall survival analysis hinted at a benefit for cabozantinib (HR, 0.80), but this was not statistically significant.
All cause grade 3 or 4 adverse events occurred in 65% of patients in the cabozantinib arm and 68% in the sunitinib arm. Events occurring more frequently with cabozantinib were hypertension (28% vs. 22%), elevated alanine aminotranseferase (5% vs. 0%), anorexia (5% vs. 0%), palmar-plantar erythrodysethesia (8% vs. 4%), and weight loss (4% vs. 0%).
There were 3 deaths possibly, probably, or definitely related to treatment among 3 patients on cabozantinib and 2 on sunitinib.
Favorable data, but wait and see
“I think cabozantinib is superior to sunitinib in poor and intermediate risk metastatic renal cell cancer,” said invited discussant Bernard Escudier, MD, from the Gustave Roussy Cancer Center in Paris.
“Cabozantinib is a potent VEGF inhibitor and is probably also active in good risk patients, although we need to see the data in these patients,” he said.
He added that a phase 3 study is warranted to confirm these conclusions, and that he will likely wait until those data are available before moving patients to cabozantinib in the first line.
The trial was supported by the National Cancer Institute. Dr. Choueiri disclosed advising and institutional research funding from Exelixis, maker of cabozantinib, and Pfizer, maker of sunitinib. Dr. Escudier disclosed receiving honoraria from each company.
COPENHAGEN – For patients with previously untreated intermediate- or poor-risk renal cell carcinoma (RCC), cabozantanib offers a progression-free survival benefit better than that seen with sunitinib, the current standard of care, reported investigators in the phase II CABOSUN trial.
After a median follow-up of 20.8 months, progression-free survival (PFS) for patients assigned to cabozantinib (Canbometyx) the primary endpoint, was a median 8.2 months, compared with 5.6 months for patients assigned to sunitinib, reported Toni K. Choueiri, MD, from the Dana-Farber Cancer Institute in Boston.
“Cabozantinib represents a potential new treatment option for patients with untreated renal cell carcinoma,” Dr. Choueiri said at the European Society for Medical Oncology (ESMO) congress.
Sunitinib (Sutent), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) is the standard first-line therapy for patients who present with metastatic RCC. But patients with intermediate- or poor-risk metastatic RCC have shorter survival on sunitinib compared with patients with favorable-risk disease, Dr. Choueiri noted.
Resistance to VEGFR inhibitors in clear-cell RCC can arise through inactivation of the Von Hippel-Lindau (VHL) gene, which leads to upregulation of MET, AXL, and VEGF receptors.
Increased expression of MET and AXL has been associated with resistant to VEGFR inhibitors and poor clinical outcomes, he explained.
Cabozantib is an oral small-molecular inhibitor of multiple tyrosine kinases, including VEGF receptors, MET, and AXL. It is approved in the United States and Europe for use in the second-line setting following prior therapy with a VEGFR inhibitor.
Trial details
In the CABOSUN trial, 157 treatment-naive patients with clear-cell RCC with measurable disease, Eastern Cooperative Oncology Group performance status 0-2 and intermediate- or poor-risk disease according to International Metastatic Renal Cell Carcinoma Database (IMDC) criteria were randomly assigned to receive either oral cabaozantinib 60 mg daily for 6 week cycles, or oral sunitinib 50 mg daily on a standard schedule of 4 weeks on and 2 weeks off.
Tumors were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) every other cycle, and treatment was continued to the point of disease progression or intolerable toxicity.
A total of 79 patients were assigned to cabozantinib, 78 received it, and 13 were still on the drug at the time of the data cutoff. In all, 78 patients were assigned to sunitinib, 72 received it, and 2 were continuing treatment at last follow-up. The efficacy analysis was by intention-to-treat, and the safety analysis as-treated.
As noted, median PFS was 8.2.months for cabozantinib vs. 5.6 months for sunitinib, The hazard ratio for cabozatinib was 0.69 (P = .012). A subgroup analysis showed a trend favoring cabozantinib in each risk group, but this was not significant except among patients with bone metastases (HR, 0.51; 95% confidence interval, 0.29-0.90).
The overall response rate among patients on cabozantib was 46%, compared with 18% for sunitinib. Complete responses were seen in one patient in each group, partial responses in 35 and 13, respectively, stable disease in 26 and 28, and disease progression in 14 and 20 (data on 3 and 16 patients were not evaluable).
The overall survival analysis hinted at a benefit for cabozantinib (HR, 0.80), but this was not statistically significant.
All cause grade 3 or 4 adverse events occurred in 65% of patients in the cabozantinib arm and 68% in the sunitinib arm. Events occurring more frequently with cabozantinib were hypertension (28% vs. 22%), elevated alanine aminotranseferase (5% vs. 0%), anorexia (5% vs. 0%), palmar-plantar erythrodysethesia (8% vs. 4%), and weight loss (4% vs. 0%).
There were 3 deaths possibly, probably, or definitely related to treatment among 3 patients on cabozantinib and 2 on sunitinib.
Favorable data, but wait and see
“I think cabozantinib is superior to sunitinib in poor and intermediate risk metastatic renal cell cancer,” said invited discussant Bernard Escudier, MD, from the Gustave Roussy Cancer Center in Paris.
“Cabozantinib is a potent VEGF inhibitor and is probably also active in good risk patients, although we need to see the data in these patients,” he said.
He added that a phase 3 study is warranted to confirm these conclusions, and that he will likely wait until those data are available before moving patients to cabozantinib in the first line.
The trial was supported by the National Cancer Institute. Dr. Choueiri disclosed advising and institutional research funding from Exelixis, maker of cabozantinib, and Pfizer, maker of sunitinib. Dr. Escudier disclosed receiving honoraria from each company.
AT ESMO 2016
Key clinical point:
Major finding: Median progression-free survival for patients assigned to cabozantinib was 8.2 months, compared with 5.6 months for patients assigned to sunitinib,
Data source: Randomized phase II trial involving 157 patients with previously untreated metastatic RCC.
Disclosures: The trial was supported by the National Cancer Institute. Dr. Choueiri disclosed advising and institutional research funding from Exelixis, maker of cabozantinib, and Pfizer, maker of sunitinib. Dr. Escudier disclosed receiving honoraria from each company.
Hepatitis Outlook: September 2016
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
A recent study suggested that hepatitis B virus (HBV)-DNA testing should be considered routinely in monitoring drug-induced liver injury, and also in other clinical implications associated with occult hepatitis B virus infection.
German researchers said that stopping or interrupting treatment with nucleos(t)ide analogues in noncirrhotic patients with HBeAg-negative chronic hepatitis B should be further investigated as a strategy to accelerate HBsAg loss.
Despite its high variability, the hepatitis C virus (HCV) is under strict evolutionary constraints, most probably to keep its genes and proteins functional during the replication cycle, according to a study in Virus Evolution.
The high burden of chronic hepatitis B virus infection in Asian countries is a major challenge for the incorporation of national programs to prevent chronic HBV complications within health care systems, according to a meta-analysis in the International Journal of Infectious Diseases.
Recent research suggests that a transition to newer direct acting antivirals is urgently needed in Rwanda and sub-Saharan Africa more generally to improve treatment outcomes for hepatitis C virus–infected patients.
Ribavirin is an effective treatment for hepatitis E virus (HEV) infection, according to a recent review study, but further studies are required to determine which other antiviral agents are of clinical utility in treating HEV in the minority of patients who do not respond to ribavirin.
Robust HEV-specific T-cell responses generated during acute disease predominantly target open reading frame 2 (ORF2), according to a study in Hepatology, but decline in magnitude and polyfunctionality over time. The authors said that defining HEV T-cell targets will be important for the investigation of HEV-associated autoimmune disease.
A recent study indicated that the variability in estimates of spontaneous viral clearance of hepatitis C virus infection – between HIV-positive men who have sex with men and people who inject drugs – has an impact on HIV coinfection and HCV reinfection.
A French study found that the rate of patients failing to return for the results of HIV, HBV, HCV, and syphilis testing is a problem, but the use of currently available technologies, such as phone texting, might be a partial solution in conjunction with rapid tests for diagnosis.
In chronic hepatitis C virus infection, the preferential association of HCV with B cells is mediated by the complement system, mainly through complement receptor 2 (CD21), in conjunction with the CD19 and CD81 complex, according to a study in Hepatology.
An Italian study found that virological response at week 8 of pegylated interferon and ribavirin therapy in hepatitis C–infected children could be considered a reliable predictor of sustained virologic response.
A recent study found that interferon treatment increased, rather than decreased, hepatitis C virus long double-stranded RNA (dsRNA) in patients. The authors said this unexpected finding suggests that HCV produces dsRNA in response to interferon, potentially to antagonize antiviral defenses.
A vaccine study in Argentina found that single-dose universal hepatitis A immunization in 1-year-old children resulted in sustained immunologic protection for up to 9 years.
A Japanese study found that CD14+ monocyte-derived galectin-9 increases NK cell cytotoxicity in chronic hepatitis C virus infection, which might be associated with liver injury and persistent infection.
Sofosbuvir and ribavirin for 6 weeks was not effective among people with recent hepatitis C virus infection, according to results from the DARE-C II study.
A study in Hepatology found that a single broadly neutralizing antibody can prevent acute hepatitis C virus infection without inducing the emergence of resistance-associated variants (RAVs), and may complement direct-acting antivirals to reduce the emergence of RAVs.
Vaccination with DTPa-HBV-IPV/Hib (diphtheria-tetanus-acellular pertussis–hepatitis B–inactivated poliovirus/Haemophilus influenza type b) in infancy induces sustained seroprotection and immune memory against hepatitis B virus, a recent study found, as revealed by the strong anamnestic response to the hepatitis B vaccine challenge in 12- to 13-year-old adolescents.
Chronic hepatitis C virus infection appears to disrupt the milieu of soluble inflammatory mediators even after viral clearance, investigators found, indicating HCV cure does not lead to complete immunological restitution.
Among the largest U.S. community-based real-world cohort of Asian chronic hepatitis C virus genotype 6 patients treated with all-oral sofosbuvir/ledipasvir without ribavirin, sustained viral response at 12 weeks was similar to SVR12 reported in clinical trials, validating current HCV genotype 6 treatment guideline recommendations.
Lower serum albumin and higher AST appear to be important mortality risk factors in HIV/HCV-coinfection, but much less so in HIV-monoinfected individuals, reported a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus and suffering from progressive liver disease, which investigators said underscores the need for close monitoring and for earlier and more effective HCV treatment.
Chinese investigators have developed and validated a novel classification and regression tree (CART) analysis model that they say is superior to the model for end-stage liver disease (MELD) for predicting 3-month mortality of patients with acute-on-chronic hepatitis B liver failure.
A study in Lancet Infectious Diseases confirmed that injection drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
A recent study suggested that hepatitis B virus (HBV)-DNA testing should be considered routinely in monitoring drug-induced liver injury, and also in other clinical implications associated with occult hepatitis B virus infection.
German researchers said that stopping or interrupting treatment with nucleos(t)ide analogues in noncirrhotic patients with HBeAg-negative chronic hepatitis B should be further investigated as a strategy to accelerate HBsAg loss.
Despite its high variability, the hepatitis C virus (HCV) is under strict evolutionary constraints, most probably to keep its genes and proteins functional during the replication cycle, according to a study in Virus Evolution.
The high burden of chronic hepatitis B virus infection in Asian countries is a major challenge for the incorporation of national programs to prevent chronic HBV complications within health care systems, according to a meta-analysis in the International Journal of Infectious Diseases.
Recent research suggests that a transition to newer direct acting antivirals is urgently needed in Rwanda and sub-Saharan Africa more generally to improve treatment outcomes for hepatitis C virus–infected patients.
Ribavirin is an effective treatment for hepatitis E virus (HEV) infection, according to a recent review study, but further studies are required to determine which other antiviral agents are of clinical utility in treating HEV in the minority of patients who do not respond to ribavirin.
Robust HEV-specific T-cell responses generated during acute disease predominantly target open reading frame 2 (ORF2), according to a study in Hepatology, but decline in magnitude and polyfunctionality over time. The authors said that defining HEV T-cell targets will be important for the investigation of HEV-associated autoimmune disease.
A recent study indicated that the variability in estimates of spontaneous viral clearance of hepatitis C virus infection – between HIV-positive men who have sex with men and people who inject drugs – has an impact on HIV coinfection and HCV reinfection.
A French study found that the rate of patients failing to return for the results of HIV, HBV, HCV, and syphilis testing is a problem, but the use of currently available technologies, such as phone texting, might be a partial solution in conjunction with rapid tests for diagnosis.
In chronic hepatitis C virus infection, the preferential association of HCV with B cells is mediated by the complement system, mainly through complement receptor 2 (CD21), in conjunction with the CD19 and CD81 complex, according to a study in Hepatology.
An Italian study found that virological response at week 8 of pegylated interferon and ribavirin therapy in hepatitis C–infected children could be considered a reliable predictor of sustained virologic response.
A recent study found that interferon treatment increased, rather than decreased, hepatitis C virus long double-stranded RNA (dsRNA) in patients. The authors said this unexpected finding suggests that HCV produces dsRNA in response to interferon, potentially to antagonize antiviral defenses.
A vaccine study in Argentina found that single-dose universal hepatitis A immunization in 1-year-old children resulted in sustained immunologic protection for up to 9 years.
A Japanese study found that CD14+ monocyte-derived galectin-9 increases NK cell cytotoxicity in chronic hepatitis C virus infection, which might be associated with liver injury and persistent infection.
Sofosbuvir and ribavirin for 6 weeks was not effective among people with recent hepatitis C virus infection, according to results from the DARE-C II study.
A study in Hepatology found that a single broadly neutralizing antibody can prevent acute hepatitis C virus infection without inducing the emergence of resistance-associated variants (RAVs), and may complement direct-acting antivirals to reduce the emergence of RAVs.
Vaccination with DTPa-HBV-IPV/Hib (diphtheria-tetanus-acellular pertussis–hepatitis B–inactivated poliovirus/Haemophilus influenza type b) in infancy induces sustained seroprotection and immune memory against hepatitis B virus, a recent study found, as revealed by the strong anamnestic response to the hepatitis B vaccine challenge in 12- to 13-year-old adolescents.
Chronic hepatitis C virus infection appears to disrupt the milieu of soluble inflammatory mediators even after viral clearance, investigators found, indicating HCV cure does not lead to complete immunological restitution.
Among the largest U.S. community-based real-world cohort of Asian chronic hepatitis C virus genotype 6 patients treated with all-oral sofosbuvir/ledipasvir without ribavirin, sustained viral response at 12 weeks was similar to SVR12 reported in clinical trials, validating current HCV genotype 6 treatment guideline recommendations.
Lower serum albumin and higher AST appear to be important mortality risk factors in HIV/HCV-coinfection, but much less so in HIV-monoinfected individuals, reported a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus and suffering from progressive liver disease, which investigators said underscores the need for close monitoring and for earlier and more effective HCV treatment.
Chinese investigators have developed and validated a novel classification and regression tree (CART) analysis model that they say is superior to the model for end-stage liver disease (MELD) for predicting 3-month mortality of patients with acute-on-chronic hepatitis B liver failure.
A study in Lancet Infectious Diseases confirmed that injection drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
A recent study suggested that hepatitis B virus (HBV)-DNA testing should be considered routinely in monitoring drug-induced liver injury, and also in other clinical implications associated with occult hepatitis B virus infection.
German researchers said that stopping or interrupting treatment with nucleos(t)ide analogues in noncirrhotic patients with HBeAg-negative chronic hepatitis B should be further investigated as a strategy to accelerate HBsAg loss.
Despite its high variability, the hepatitis C virus (HCV) is under strict evolutionary constraints, most probably to keep its genes and proteins functional during the replication cycle, according to a study in Virus Evolution.
The high burden of chronic hepatitis B virus infection in Asian countries is a major challenge for the incorporation of national programs to prevent chronic HBV complications within health care systems, according to a meta-analysis in the International Journal of Infectious Diseases.
Recent research suggests that a transition to newer direct acting antivirals is urgently needed in Rwanda and sub-Saharan Africa more generally to improve treatment outcomes for hepatitis C virus–infected patients.
Ribavirin is an effective treatment for hepatitis E virus (HEV) infection, according to a recent review study, but further studies are required to determine which other antiviral agents are of clinical utility in treating HEV in the minority of patients who do not respond to ribavirin.
Robust HEV-specific T-cell responses generated during acute disease predominantly target open reading frame 2 (ORF2), according to a study in Hepatology, but decline in magnitude and polyfunctionality over time. The authors said that defining HEV T-cell targets will be important for the investigation of HEV-associated autoimmune disease.
A recent study indicated that the variability in estimates of spontaneous viral clearance of hepatitis C virus infection – between HIV-positive men who have sex with men and people who inject drugs – has an impact on HIV coinfection and HCV reinfection.
A French study found that the rate of patients failing to return for the results of HIV, HBV, HCV, and syphilis testing is a problem, but the use of currently available technologies, such as phone texting, might be a partial solution in conjunction with rapid tests for diagnosis.
In chronic hepatitis C virus infection, the preferential association of HCV with B cells is mediated by the complement system, mainly through complement receptor 2 (CD21), in conjunction with the CD19 and CD81 complex, according to a study in Hepatology.
An Italian study found that virological response at week 8 of pegylated interferon and ribavirin therapy in hepatitis C–infected children could be considered a reliable predictor of sustained virologic response.
A recent study found that interferon treatment increased, rather than decreased, hepatitis C virus long double-stranded RNA (dsRNA) in patients. The authors said this unexpected finding suggests that HCV produces dsRNA in response to interferon, potentially to antagonize antiviral defenses.
A vaccine study in Argentina found that single-dose universal hepatitis A immunization in 1-year-old children resulted in sustained immunologic protection for up to 9 years.
A Japanese study found that CD14+ monocyte-derived galectin-9 increases NK cell cytotoxicity in chronic hepatitis C virus infection, which might be associated with liver injury and persistent infection.
Sofosbuvir and ribavirin for 6 weeks was not effective among people with recent hepatitis C virus infection, according to results from the DARE-C II study.
A study in Hepatology found that a single broadly neutralizing antibody can prevent acute hepatitis C virus infection without inducing the emergence of resistance-associated variants (RAVs), and may complement direct-acting antivirals to reduce the emergence of RAVs.
Vaccination with DTPa-HBV-IPV/Hib (diphtheria-tetanus-acellular pertussis–hepatitis B–inactivated poliovirus/Haemophilus influenza type b) in infancy induces sustained seroprotection and immune memory against hepatitis B virus, a recent study found, as revealed by the strong anamnestic response to the hepatitis B vaccine challenge in 12- to 13-year-old adolescents.
Chronic hepatitis C virus infection appears to disrupt the milieu of soluble inflammatory mediators even after viral clearance, investigators found, indicating HCV cure does not lead to complete immunological restitution.
Among the largest U.S. community-based real-world cohort of Asian chronic hepatitis C virus genotype 6 patients treated with all-oral sofosbuvir/ledipasvir without ribavirin, sustained viral response at 12 weeks was similar to SVR12 reported in clinical trials, validating current HCV genotype 6 treatment guideline recommendations.
Lower serum albumin and higher AST appear to be important mortality risk factors in HIV/HCV-coinfection, but much less so in HIV-monoinfected individuals, reported a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus and suffering from progressive liver disease, which investigators said underscores the need for close monitoring and for earlier and more effective HCV treatment.
Chinese investigators have developed and validated a novel classification and regression tree (CART) analysis model that they say is superior to the model for end-stage liver disease (MELD) for predicting 3-month mortality of patients with acute-on-chronic hepatitis B liver failure.
A study in Lancet Infectious Diseases confirmed that injection drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B.
[email protected]
On Twitter @richpizzi
Kevin Conrad, MD, MBA, Brings His Passion for Problem Solving to TH’s Editorial Board
Kevin Conrad, MD, MBA, could always picture being a doctor, given that he enjoyed the sciences and wanted a job where he could work with people. He has more trouble figuring out how people don’t enjoy the detective work that comes with medicine.
“I have a hard time imagining what people do besides people in the sciences,” he says. “In sciences, you deal with facts, you deal with numbers, you deal with data, and you put it together and you come to conclusions. And to me, that seemed like a career pathway as opposed to, say, a field like law or writing or whatnot. I could never wrap my head around what they actually do in a given day.”
Dr. Conrad’s passion for the sciences hasn’t waned yet. He serves as the medical director of community affairs and healthy policy at Ochsner Health Systems of New Orleans, where he focuses on systems improvement. He published his first book this year, Absolute Hospital Medicine Review: An Intensive Question and Answer Guide, and is working on his second tome.
And this year, he was named one of the eight new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist.
Question: What led you to a more hands-on medical field as opposed to being a basic or translational scientist?
Answer: I had some early exposure to lab work in high school and college and saw what they did and saw how they were sort of confined to labs for long periods of time and said, “No, I think that I would rather be kind of out there, combining science as well as interacting with people in the field.”
Q: When you started residency, was it clear to you that hospital medicine was where you wanted to go, or were you looking at a few different options?
A: Hospital medicine combined my interests in internal medicine, which is sort of a broad overview of all aspects of medicine and healthcare as well as being a little bit more intensive, a little bit more action-oriented. The patients tend to be a little bit more ill, require a little bit more acute attention, and that appealed to me as opposed to my training in internal medicine.
Q: What about the intensity of hospital medicine appeals to you?
A: As opposed to sitting down with a patient in an office setting, I think, in hospital medicine, I like the idea that you’re called from one semi-emergency to another and that you have to think quickly on your feet and move on to the new task. And the new tasks come in rapid sequence: You have one problem that you fix and then you’re called to do another one, and each and every day, it will tend to be sort of a different set of problems.
Q: What was the motivation to write the book and now working on the second?
A: I wanted to share my experience, and I felt I was in a position where I could not only share my personal practice experience as well as sort of collate the other material that has been written and published in hospital medicine. I also think there is a need right now to continue to define what hospital medicine is and show what we’re experts at as well as show our value to the system. We have an ambiguous practice, and people still aren’t quite sure what we do and what we’re expert at. So I think it’s our task to showcase this is what we do well, this is what we do better than other people, and this is our value to the system.
Q: So what is hospitalists’ value, and what are they experts in?
A: We’re experts at systems, hospital systems. … We understand better than anyone what goes on in the hospital, the physicians’ practice, the nursing practice, the administrative practice. … I mean, we’re there. We’re on the floors. No one has a better insight into the function of the hospital than the hospitalists. I think the other unique role we have is we see trends that other specialties don’t. We don’t really own our patients. Our patients come and go, but we tend to have a unique view of the practice of medicine that we see trends maybe before other specialists. … We see some of the failures of other systems.
Q: What is your least favorite part of being a hospitalist?
A: The clerical work. I think I probably speak for most people in that the clerical work is needed. It has become an increasing part of our practice in that now we spend a great deal of our time obviously in front of computers as opposed to at the bedside. The electronic medical record, which is good, which has served us well, becomes a greater and bigger part of our day, and so we find that it is too much a part of my day. I’m not saying it’s not needed because I think it has improved the quality of care we deliver, but it’s not something that you imagine that you should be doing as a physician. You are spending most of your day in front of a computer as opposed to most of the day with patients.
Q: What’s your favorite part of the job?
A: It has changed over my career. I think it probably started out in education. I enjoyed teaching medical students and residents, and certainly, that’s still a part of it, but then I think it has evolved into a little bit more of an academic interest. I just published my first book this past year and am working on my second book, and that has become sort of my bigger focus now. TH
Richard Quinn is a freelance writer in New Jersey.
Kevin Conrad, MD, MBA, could always picture being a doctor, given that he enjoyed the sciences and wanted a job where he could work with people. He has more trouble figuring out how people don’t enjoy the detective work that comes with medicine.
“I have a hard time imagining what people do besides people in the sciences,” he says. “In sciences, you deal with facts, you deal with numbers, you deal with data, and you put it together and you come to conclusions. And to me, that seemed like a career pathway as opposed to, say, a field like law or writing or whatnot. I could never wrap my head around what they actually do in a given day.”
Dr. Conrad’s passion for the sciences hasn’t waned yet. He serves as the medical director of community affairs and healthy policy at Ochsner Health Systems of New Orleans, where he focuses on systems improvement. He published his first book this year, Absolute Hospital Medicine Review: An Intensive Question and Answer Guide, and is working on his second tome.
And this year, he was named one of the eight new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist.
Question: What led you to a more hands-on medical field as opposed to being a basic or translational scientist?
Answer: I had some early exposure to lab work in high school and college and saw what they did and saw how they were sort of confined to labs for long periods of time and said, “No, I think that I would rather be kind of out there, combining science as well as interacting with people in the field.”
Q: When you started residency, was it clear to you that hospital medicine was where you wanted to go, or were you looking at a few different options?
A: Hospital medicine combined my interests in internal medicine, which is sort of a broad overview of all aspects of medicine and healthcare as well as being a little bit more intensive, a little bit more action-oriented. The patients tend to be a little bit more ill, require a little bit more acute attention, and that appealed to me as opposed to my training in internal medicine.
Q: What about the intensity of hospital medicine appeals to you?
A: As opposed to sitting down with a patient in an office setting, I think, in hospital medicine, I like the idea that you’re called from one semi-emergency to another and that you have to think quickly on your feet and move on to the new task. And the new tasks come in rapid sequence: You have one problem that you fix and then you’re called to do another one, and each and every day, it will tend to be sort of a different set of problems.
Q: What was the motivation to write the book and now working on the second?
A: I wanted to share my experience, and I felt I was in a position where I could not only share my personal practice experience as well as sort of collate the other material that has been written and published in hospital medicine. I also think there is a need right now to continue to define what hospital medicine is and show what we’re experts at as well as show our value to the system. We have an ambiguous practice, and people still aren’t quite sure what we do and what we’re expert at. So I think it’s our task to showcase this is what we do well, this is what we do better than other people, and this is our value to the system.
Q: So what is hospitalists’ value, and what are they experts in?
A: We’re experts at systems, hospital systems. … We understand better than anyone what goes on in the hospital, the physicians’ practice, the nursing practice, the administrative practice. … I mean, we’re there. We’re on the floors. No one has a better insight into the function of the hospital than the hospitalists. I think the other unique role we have is we see trends that other specialties don’t. We don’t really own our patients. Our patients come and go, but we tend to have a unique view of the practice of medicine that we see trends maybe before other specialists. … We see some of the failures of other systems.
Q: What is your least favorite part of being a hospitalist?
A: The clerical work. I think I probably speak for most people in that the clerical work is needed. It has become an increasing part of our practice in that now we spend a great deal of our time obviously in front of computers as opposed to at the bedside. The electronic medical record, which is good, which has served us well, becomes a greater and bigger part of our day, and so we find that it is too much a part of my day. I’m not saying it’s not needed because I think it has improved the quality of care we deliver, but it’s not something that you imagine that you should be doing as a physician. You are spending most of your day in front of a computer as opposed to most of the day with patients.
Q: What’s your favorite part of the job?
A: It has changed over my career. I think it probably started out in education. I enjoyed teaching medical students and residents, and certainly, that’s still a part of it, but then I think it has evolved into a little bit more of an academic interest. I just published my first book this past year and am working on my second book, and that has become sort of my bigger focus now. TH
Richard Quinn is a freelance writer in New Jersey.
Kevin Conrad, MD, MBA, could always picture being a doctor, given that he enjoyed the sciences and wanted a job where he could work with people. He has more trouble figuring out how people don’t enjoy the detective work that comes with medicine.
“I have a hard time imagining what people do besides people in the sciences,” he says. “In sciences, you deal with facts, you deal with numbers, you deal with data, and you put it together and you come to conclusions. And to me, that seemed like a career pathway as opposed to, say, a field like law or writing or whatnot. I could never wrap my head around what they actually do in a given day.”
Dr. Conrad’s passion for the sciences hasn’t waned yet. He serves as the medical director of community affairs and healthy policy at Ochsner Health Systems of New Orleans, where he focuses on systems improvement. He published his first book this year, Absolute Hospital Medicine Review: An Intensive Question and Answer Guide, and is working on his second tome.
And this year, he was named one of the eight new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist.
Question: What led you to a more hands-on medical field as opposed to being a basic or translational scientist?
Answer: I had some early exposure to lab work in high school and college and saw what they did and saw how they were sort of confined to labs for long periods of time and said, “No, I think that I would rather be kind of out there, combining science as well as interacting with people in the field.”
Q: When you started residency, was it clear to you that hospital medicine was where you wanted to go, or were you looking at a few different options?
A: Hospital medicine combined my interests in internal medicine, which is sort of a broad overview of all aspects of medicine and healthcare as well as being a little bit more intensive, a little bit more action-oriented. The patients tend to be a little bit more ill, require a little bit more acute attention, and that appealed to me as opposed to my training in internal medicine.
Q: What about the intensity of hospital medicine appeals to you?
A: As opposed to sitting down with a patient in an office setting, I think, in hospital medicine, I like the idea that you’re called from one semi-emergency to another and that you have to think quickly on your feet and move on to the new task. And the new tasks come in rapid sequence: You have one problem that you fix and then you’re called to do another one, and each and every day, it will tend to be sort of a different set of problems.
Q: What was the motivation to write the book and now working on the second?
A: I wanted to share my experience, and I felt I was in a position where I could not only share my personal practice experience as well as sort of collate the other material that has been written and published in hospital medicine. I also think there is a need right now to continue to define what hospital medicine is and show what we’re experts at as well as show our value to the system. We have an ambiguous practice, and people still aren’t quite sure what we do and what we’re expert at. So I think it’s our task to showcase this is what we do well, this is what we do better than other people, and this is our value to the system.
Q: So what is hospitalists’ value, and what are they experts in?
A: We’re experts at systems, hospital systems. … We understand better than anyone what goes on in the hospital, the physicians’ practice, the nursing practice, the administrative practice. … I mean, we’re there. We’re on the floors. No one has a better insight into the function of the hospital than the hospitalists. I think the other unique role we have is we see trends that other specialties don’t. We don’t really own our patients. Our patients come and go, but we tend to have a unique view of the practice of medicine that we see trends maybe before other specialists. … We see some of the failures of other systems.
Q: What is your least favorite part of being a hospitalist?
A: The clerical work. I think I probably speak for most people in that the clerical work is needed. It has become an increasing part of our practice in that now we spend a great deal of our time obviously in front of computers as opposed to at the bedside. The electronic medical record, which is good, which has served us well, becomes a greater and bigger part of our day, and so we find that it is too much a part of my day. I’m not saying it’s not needed because I think it has improved the quality of care we deliver, but it’s not something that you imagine that you should be doing as a physician. You are spending most of your day in front of a computer as opposed to most of the day with patients.
Q: What’s your favorite part of the job?
A: It has changed over my career. I think it probably started out in education. I enjoyed teaching medical students and residents, and certainly, that’s still a part of it, but then I think it has evolved into a little bit more of an academic interest. I just published my first book this past year and am working on my second book, and that has become sort of my bigger focus now. TH
Richard Quinn is a freelance writer in New Jersey.
CHMP recommends conditional approval of drug for CLL

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of venetoclax (Venclyxto™).
The CHMP is recommending that venetoclax receive conditional marketing authorization to treat adults with chronic lymphocytic leukemia (CLL) who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with an inhibitor of the B-cell receptor pathway.
The CHMP is also recommending the conditional authorization of venetoclax as a treatment for adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemo immunotherapy and treatment with an inhibitor of the B-cell receptor pathway.
The European Commission (EC) will review the CHMP’s opinion and is expected to make a final decision about venetoclax in late 2016.
If the EC follows the CHMP’s recommendations, venetoclax will become the first BCL-2 inhibitor approved for use in Europe. The authorization will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, and Norway.
Conditional marketing authorization represents an expedited path for approval. The EC grants conditional marketing authorization to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
Conditional marketing authorization is granted before pivotal registration studies of a product are completed, but the company developing the product is required to complete post-marketing studies showing that the product provides a clinical benefit.
Venetoclax is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently approved for use in Argentina, Canada, Puerto Rico, and the US. The drug is being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse events was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()
Editorial: Transitioning to College with Epilepsy
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health , in Royal Oak, Michigan.
Starting college is both an exciting and stressful experience for new students. For people with epilepsy, going to college raises some specific and unique concerns compared to those without the condition . The purpose of this article is to look at some of the challenges for people with epilepsy and some potential solutions. The overall theme is that it is better to be prepared ahead of time.
The main goal of the person with epilepsy at college is to ensure that a breakthrough seizure does not occur, as this could lead to serious consequences (injuries, sprains, strains, and a change in lifestyle). However, it is equally important to ensure that an individual with epilepsy has a quality life.
For most students, attending college is one of life’s milestones. College is a new environment, with some new pressures that include academic success, making new friends (fitting in), participation in extracurricular activities (both academic and not), and taking personal responsibility of what may considered basic yet essential daily activities, such as meal preparation, laundry, etc.
Some of these issues may be of lesser concern, depending on the distance from college to home; however the struggle to maintain independence is common regardless how far away the college campus is from home.
If college is far from home, it may be better for the individual with epilepsy to establish care with a local neurologist, or preferably, a local epileptologist. This is helpful in case of emergency. Ideally, prescriptions should be transferred to a nearby pharmacy before refills are due. It is also beneficial to coordinate follow up visits if needed with the original neurologist/epileptologist at term breaks and vacations. If there is an increase in seizure frequency or side effects patients should contact their physician as soon as possible and not simply wait for the next trip home. It is advisable for people with epilepsy to keep a wallet card or similar such item with them that lists medications names and doses as well as emergency contact numbers. If the patient is traveling by air or bus, mediations should be packed in hand luggage, not checked baggage, to avoid the possible of delayed arrival at the destination.
As class times may differ in a college environment, individuals with epilepsy should ensure they remain complaint with their medication and not miss doses. This may mean remembering to carry the medication with them, and keeping an extra supply at the dormitory/residence in case of emergency.
For individuals with epilepsy, stressors, such as decreased sleep/sleep deprivation, missed meals, over exertion, dehydration can lead to breakthrough seizures (even in those who are compliant with their antiepileptic drugs [AEDs]). Sleep deprivation and missed meals can be somewhat common in a college atmosphere, especially around examination time. People with epilepsy should ensure they get adequate sleep, do not miss meals, and are adequately hydrated (more so when participating in sports).
Use of alcohol and or illicit substances should be avoided as these can reduce seizure threshold, reduce the effectiveness of AEDs, and lead to breakthrough seizures.
For people with epilepsy, there may be an underlying anxiety of if and when a breakthrough seizure may occur. However, it is important to note that for the college student, this fear should not prevent him or her from achieving their full potential.
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health , in Royal Oak, Michigan.
Starting college is both an exciting and stressful experience for new students. For people with epilepsy, going to college raises some specific and unique concerns compared to those without the condition . The purpose of this article is to look at some of the challenges for people with epilepsy and some potential solutions. The overall theme is that it is better to be prepared ahead of time.
The main goal of the person with epilepsy at college is to ensure that a breakthrough seizure does not occur, as this could lead to serious consequences (injuries, sprains, strains, and a change in lifestyle). However, it is equally important to ensure that an individual with epilepsy has a quality life.
For most students, attending college is one of life’s milestones. College is a new environment, with some new pressures that include academic success, making new friends (fitting in), participation in extracurricular activities (both academic and not), and taking personal responsibility of what may considered basic yet essential daily activities, such as meal preparation, laundry, etc.
Some of these issues may be of lesser concern, depending on the distance from college to home; however the struggle to maintain independence is common regardless how far away the college campus is from home.
If college is far from home, it may be better for the individual with epilepsy to establish care with a local neurologist, or preferably, a local epileptologist. This is helpful in case of emergency. Ideally, prescriptions should be transferred to a nearby pharmacy before refills are due. It is also beneficial to coordinate follow up visits if needed with the original neurologist/epileptologist at term breaks and vacations. If there is an increase in seizure frequency or side effects patients should contact their physician as soon as possible and not simply wait for the next trip home. It is advisable for people with epilepsy to keep a wallet card or similar such item with them that lists medications names and doses as well as emergency contact numbers. If the patient is traveling by air or bus, mediations should be packed in hand luggage, not checked baggage, to avoid the possible of delayed arrival at the destination.
As class times may differ in a college environment, individuals with epilepsy should ensure they remain complaint with their medication and not miss doses. This may mean remembering to carry the medication with them, and keeping an extra supply at the dormitory/residence in case of emergency.
For individuals with epilepsy, stressors, such as decreased sleep/sleep deprivation, missed meals, over exertion, dehydration can lead to breakthrough seizures (even in those who are compliant with their antiepileptic drugs [AEDs]). Sleep deprivation and missed meals can be somewhat common in a college atmosphere, especially around examination time. People with epilepsy should ensure they get adequate sleep, do not miss meals, and are adequately hydrated (more so when participating in sports).
Use of alcohol and or illicit substances should be avoided as these can reduce seizure threshold, reduce the effectiveness of AEDs, and lead to breakthrough seizures.
For people with epilepsy, there may be an underlying anxiety of if and when a breakthrough seizure may occur. However, it is important to note that for the college student, this fear should not prevent him or her from achieving their full potential.
Nikesh Ardeshna, MD
Dr. Ardeshna is the Medical Director of Adult Epilepsy Services at Royal Oak Hospital, Beaumont Health , in Royal Oak, Michigan.
Starting college is both an exciting and stressful experience for new students. For people with epilepsy, going to college raises some specific and unique concerns compared to those without the condition . The purpose of this article is to look at some of the challenges for people with epilepsy and some potential solutions. The overall theme is that it is better to be prepared ahead of time.
The main goal of the person with epilepsy at college is to ensure that a breakthrough seizure does not occur, as this could lead to serious consequences (injuries, sprains, strains, and a change in lifestyle). However, it is equally important to ensure that an individual with epilepsy has a quality life.
For most students, attending college is one of life’s milestones. College is a new environment, with some new pressures that include academic success, making new friends (fitting in), participation in extracurricular activities (both academic and not), and taking personal responsibility of what may considered basic yet essential daily activities, such as meal preparation, laundry, etc.
Some of these issues may be of lesser concern, depending on the distance from college to home; however the struggle to maintain independence is common regardless how far away the college campus is from home.
If college is far from home, it may be better for the individual with epilepsy to establish care with a local neurologist, or preferably, a local epileptologist. This is helpful in case of emergency. Ideally, prescriptions should be transferred to a nearby pharmacy before refills are due. It is also beneficial to coordinate follow up visits if needed with the original neurologist/epileptologist at term breaks and vacations. If there is an increase in seizure frequency or side effects patients should contact their physician as soon as possible and not simply wait for the next trip home. It is advisable for people with epilepsy to keep a wallet card or similar such item with them that lists medications names and doses as well as emergency contact numbers. If the patient is traveling by air or bus, mediations should be packed in hand luggage, not checked baggage, to avoid the possible of delayed arrival at the destination.
As class times may differ in a college environment, individuals with epilepsy should ensure they remain complaint with their medication and not miss doses. This may mean remembering to carry the medication with them, and keeping an extra supply at the dormitory/residence in case of emergency.
For individuals with epilepsy, stressors, such as decreased sleep/sleep deprivation, missed meals, over exertion, dehydration can lead to breakthrough seizures (even in those who are compliant with their antiepileptic drugs [AEDs]). Sleep deprivation and missed meals can be somewhat common in a college atmosphere, especially around examination time. People with epilepsy should ensure they get adequate sleep, do not miss meals, and are adequately hydrated (more so when participating in sports).
Use of alcohol and or illicit substances should be avoided as these can reduce seizure threshold, reduce the effectiveness of AEDs, and lead to breakthrough seizures.
For people with epilepsy, there may be an underlying anxiety of if and when a breakthrough seizure may occur. However, it is important to note that for the college student, this fear should not prevent him or her from achieving their full potential.
Adjuvant sunitinib offers DFS edge in high-risk RCC
COPENHAGEN – In patients with high-risk locoregional clear cell renal cell (RCC) carcinoma, adjuvant therapy with sunitinib significantly prolonged disease-free survival, compared with placebo, reported investigators in a randomized, phase III trial.
Among 615 patients with clear cell RCC at high risk for recurrence following nephrectomy, the median duration of disease-free survival (DFS) by blinded central review was 6.8 years for patients treated with sunitinib (Sutent) for 1 year, compared with 5.6 years for patients assigned to placebo, reported Alain Ravaud, MD, PhD from Hôpital Saint André in Bordeaux, France.
“These results represent a major step forward in the clinical management of clear cell renal cell carcinoma,” he said at the European Society for Medical Oncology Congress.
The study was published simultaneously online in the New England Journal of Medicine.
Approximately 16% of all patients with RCC have locoregional (stage III) disease, which is associated with a 5-year survival rate of 53%. Up to 40% of patients with locoregional disease will have a relapse with metastases after nephrectomy, indicating a need for effective adjuvant therapy to reduce the risk of relapse in this population, Dr. Ravaud said.
However, to date, adjuvant therapy with either cytokines, hormones, immunotherapy, or radiotherapy has not been successful at preventing relapse, and the ASSURE trial, comparing sunitinib and sorafenib (Nexavar) with placebo showed that neither of the tyrosine kinase inhibitors (TKIs) improved survival, and each necessitated discontinuations due to toxicity, despite dose reductions, Dr. Ravaud noted.
The trial reported here, S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy), had different results, however.
A total of 615 patients with high-risk RCC were enrolled. High-risk disease was defined as stage T3, NO or Nx, M0, any Furhman grade and any Eastern Cooperative Oncology Group (ECOG) performance status; T4, N0 or NX, M0, any Fuhrman/ECOG status; or any T, N1-N2, M0, any Fuhrman/ECOG status.
Patients were stratified by risk groups, ECOG status, and country, then randomized to receive either sunitinib 50 mg daily or placebo on a 4 weeks–on, 2 weeks–off schedule for 1 year or until recurrence, unacceptable toxicity, or withdrawal of consent.
Central Reviewers Yes, Investigators No
As noted before, the duration of median DFS, the primary endpoint, was 14 months longer with sunitinib, translating into a hazard ratio (HR) of 0.76 (P = .03). This determination of DFS was performed by central reviewers blinded to treatment type.
However, DFS rates by investigators review, while numerically different between trial arms (6.5 years for sunitinib vs. 4.5 years in the placebo group), were not statistically significant (HR, 0.81, P = .08).
Overall survival data were not mature at the time of the data cutoff, with the median not reached in either group.
Treatment-emergent adverse events were seen in 99.7% of the patients in the sunitinib group and in 88.5% of controls. Investigators attributed adverse events to treatment in 98.4% of patients in the sunitinib arm and 75.7% of those in the placebo arm.
The most common adverse events in the sunitinib group were diarrhea, palmar-plantar erythrodysesthesia, hypertension, fatigue, and nausea.
Grade 3 or higher adverse events were reported in 194 patients (63.4%) in the sunitinib group and in 66 (21.7%) in the placebo group.
Interestingly, there were fewer dose reductions in the sunitinib group (34.3% vs. 46.4%) and fewer dose interruptions (2.0% vs. 13.2%), although more patients on sunitinib discontinued because of adverse events (28.1% vs. 5.6%).
The most common cause of death in each group was RCC, which accounted for 47 of 62 deaths (75.8%) in the sunitinib arm and 47 of 64 (73.4%) in the placebo arm. No death was attributed to treatment-associated toxicities.
Patients on sunitinib also had generally lower scores on quality of life scales, but the between-group differences did not reach the prespecified minimally important difference of 10 patients except for the domains of diarrhea and loss of appetite.
But having said that, he noted that, in S-TRAC as in ASSURE, the study was negative going by investigator rather than central review, and that the positive HR for central review in S-TRAC was near the significance boundary.
Overall, the quality of evidence supporting sunitinib in the adjuvant setting is weak, with benefits barely outweighing harms, and that the cost of the therapy, in light of the above, is high, Dr. Bex said.
He added that he would need to see mature overall survival data from S-TRAC and ASSURE, further results from other ongoing trials with sunitinib, and a positive meta-analysis of studies of vascular endothelial growth factor receptor tyrosine kinase inhibitors in the adjuvant setting before he would change his mind on the use of sunitinib and related agents.
The trial was supported by Pfizer. Dr. Ravaud and Dr. Bex disclosed consulting and research grants from Pfizer and other companies.
COPENHAGEN – In patients with high-risk locoregional clear cell renal cell (RCC) carcinoma, adjuvant therapy with sunitinib significantly prolonged disease-free survival, compared with placebo, reported investigators in a randomized, phase III trial.
Among 615 patients with clear cell RCC at high risk for recurrence following nephrectomy, the median duration of disease-free survival (DFS) by blinded central review was 6.8 years for patients treated with sunitinib (Sutent) for 1 year, compared with 5.6 years for patients assigned to placebo, reported Alain Ravaud, MD, PhD from Hôpital Saint André in Bordeaux, France.
“These results represent a major step forward in the clinical management of clear cell renal cell carcinoma,” he said at the European Society for Medical Oncology Congress.
The study was published simultaneously online in the New England Journal of Medicine.
Approximately 16% of all patients with RCC have locoregional (stage III) disease, which is associated with a 5-year survival rate of 53%. Up to 40% of patients with locoregional disease will have a relapse with metastases after nephrectomy, indicating a need for effective adjuvant therapy to reduce the risk of relapse in this population, Dr. Ravaud said.
However, to date, adjuvant therapy with either cytokines, hormones, immunotherapy, or radiotherapy has not been successful at preventing relapse, and the ASSURE trial, comparing sunitinib and sorafenib (Nexavar) with placebo showed that neither of the tyrosine kinase inhibitors (TKIs) improved survival, and each necessitated discontinuations due to toxicity, despite dose reductions, Dr. Ravaud noted.
The trial reported here, S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy), had different results, however.
A total of 615 patients with high-risk RCC were enrolled. High-risk disease was defined as stage T3, NO or Nx, M0, any Furhman grade and any Eastern Cooperative Oncology Group (ECOG) performance status; T4, N0 or NX, M0, any Fuhrman/ECOG status; or any T, N1-N2, M0, any Fuhrman/ECOG status.
Patients were stratified by risk groups, ECOG status, and country, then randomized to receive either sunitinib 50 mg daily or placebo on a 4 weeks–on, 2 weeks–off schedule for 1 year or until recurrence, unacceptable toxicity, or withdrawal of consent.
Central Reviewers Yes, Investigators No
As noted before, the duration of median DFS, the primary endpoint, was 14 months longer with sunitinib, translating into a hazard ratio (HR) of 0.76 (P = .03). This determination of DFS was performed by central reviewers blinded to treatment type.
However, DFS rates by investigators review, while numerically different between trial arms (6.5 years for sunitinib vs. 4.5 years in the placebo group), were not statistically significant (HR, 0.81, P = .08).
Overall survival data were not mature at the time of the data cutoff, with the median not reached in either group.
Treatment-emergent adverse events were seen in 99.7% of the patients in the sunitinib group and in 88.5% of controls. Investigators attributed adverse events to treatment in 98.4% of patients in the sunitinib arm and 75.7% of those in the placebo arm.
The most common adverse events in the sunitinib group were diarrhea, palmar-plantar erythrodysesthesia, hypertension, fatigue, and nausea.
Grade 3 or higher adverse events were reported in 194 patients (63.4%) in the sunitinib group and in 66 (21.7%) in the placebo group.
Interestingly, there were fewer dose reductions in the sunitinib group (34.3% vs. 46.4%) and fewer dose interruptions (2.0% vs. 13.2%), although more patients on sunitinib discontinued because of adverse events (28.1% vs. 5.6%).
The most common cause of death in each group was RCC, which accounted for 47 of 62 deaths (75.8%) in the sunitinib arm and 47 of 64 (73.4%) in the placebo arm. No death was attributed to treatment-associated toxicities.
Patients on sunitinib also had generally lower scores on quality of life scales, but the between-group differences did not reach the prespecified minimally important difference of 10 patients except for the domains of diarrhea and loss of appetite.
But having said that, he noted that, in S-TRAC as in ASSURE, the study was negative going by investigator rather than central review, and that the positive HR for central review in S-TRAC was near the significance boundary.
Overall, the quality of evidence supporting sunitinib in the adjuvant setting is weak, with benefits barely outweighing harms, and that the cost of the therapy, in light of the above, is high, Dr. Bex said.
He added that he would need to see mature overall survival data from S-TRAC and ASSURE, further results from other ongoing trials with sunitinib, and a positive meta-analysis of studies of vascular endothelial growth factor receptor tyrosine kinase inhibitors in the adjuvant setting before he would change his mind on the use of sunitinib and related agents.
The trial was supported by Pfizer. Dr. Ravaud and Dr. Bex disclosed consulting and research grants from Pfizer and other companies.
COPENHAGEN – In patients with high-risk locoregional clear cell renal cell (RCC) carcinoma, adjuvant therapy with sunitinib significantly prolonged disease-free survival, compared with placebo, reported investigators in a randomized, phase III trial.
Among 615 patients with clear cell RCC at high risk for recurrence following nephrectomy, the median duration of disease-free survival (DFS) by blinded central review was 6.8 years for patients treated with sunitinib (Sutent) for 1 year, compared with 5.6 years for patients assigned to placebo, reported Alain Ravaud, MD, PhD from Hôpital Saint André in Bordeaux, France.
“These results represent a major step forward in the clinical management of clear cell renal cell carcinoma,” he said at the European Society for Medical Oncology Congress.
The study was published simultaneously online in the New England Journal of Medicine.
Approximately 16% of all patients with RCC have locoregional (stage III) disease, which is associated with a 5-year survival rate of 53%. Up to 40% of patients with locoregional disease will have a relapse with metastases after nephrectomy, indicating a need for effective adjuvant therapy to reduce the risk of relapse in this population, Dr. Ravaud said.
However, to date, adjuvant therapy with either cytokines, hormones, immunotherapy, or radiotherapy has not been successful at preventing relapse, and the ASSURE trial, comparing sunitinib and sorafenib (Nexavar) with placebo showed that neither of the tyrosine kinase inhibitors (TKIs) improved survival, and each necessitated discontinuations due to toxicity, despite dose reductions, Dr. Ravaud noted.
The trial reported here, S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy), had different results, however.
A total of 615 patients with high-risk RCC were enrolled. High-risk disease was defined as stage T3, NO or Nx, M0, any Furhman grade and any Eastern Cooperative Oncology Group (ECOG) performance status; T4, N0 or NX, M0, any Fuhrman/ECOG status; or any T, N1-N2, M0, any Fuhrman/ECOG status.
Patients were stratified by risk groups, ECOG status, and country, then randomized to receive either sunitinib 50 mg daily or placebo on a 4 weeks–on, 2 weeks–off schedule for 1 year or until recurrence, unacceptable toxicity, or withdrawal of consent.
Central Reviewers Yes, Investigators No
As noted before, the duration of median DFS, the primary endpoint, was 14 months longer with sunitinib, translating into a hazard ratio (HR) of 0.76 (P = .03). This determination of DFS was performed by central reviewers blinded to treatment type.
However, DFS rates by investigators review, while numerically different between trial arms (6.5 years for sunitinib vs. 4.5 years in the placebo group), were not statistically significant (HR, 0.81, P = .08).
Overall survival data were not mature at the time of the data cutoff, with the median not reached in either group.
Treatment-emergent adverse events were seen in 99.7% of the patients in the sunitinib group and in 88.5% of controls. Investigators attributed adverse events to treatment in 98.4% of patients in the sunitinib arm and 75.7% of those in the placebo arm.
The most common adverse events in the sunitinib group were diarrhea, palmar-plantar erythrodysesthesia, hypertension, fatigue, and nausea.
Grade 3 or higher adverse events were reported in 194 patients (63.4%) in the sunitinib group and in 66 (21.7%) in the placebo group.
Interestingly, there were fewer dose reductions in the sunitinib group (34.3% vs. 46.4%) and fewer dose interruptions (2.0% vs. 13.2%), although more patients on sunitinib discontinued because of adverse events (28.1% vs. 5.6%).
The most common cause of death in each group was RCC, which accounted for 47 of 62 deaths (75.8%) in the sunitinib arm and 47 of 64 (73.4%) in the placebo arm. No death was attributed to treatment-associated toxicities.
Patients on sunitinib also had generally lower scores on quality of life scales, but the between-group differences did not reach the prespecified minimally important difference of 10 patients except for the domains of diarrhea and loss of appetite.
But having said that, he noted that, in S-TRAC as in ASSURE, the study was negative going by investigator rather than central review, and that the positive HR for central review in S-TRAC was near the significance boundary.
Overall, the quality of evidence supporting sunitinib in the adjuvant setting is weak, with benefits barely outweighing harms, and that the cost of the therapy, in light of the above, is high, Dr. Bex said.
He added that he would need to see mature overall survival data from S-TRAC and ASSURE, further results from other ongoing trials with sunitinib, and a positive meta-analysis of studies of vascular endothelial growth factor receptor tyrosine kinase inhibitors in the adjuvant setting before he would change his mind on the use of sunitinib and related agents.
The trial was supported by Pfizer. Dr. Ravaud and Dr. Bex disclosed consulting and research grants from Pfizer and other companies.
AT ESMO 2016
Key clinical point: S-TRAC is the first clinical trial to show a benefit of adjuvant drug therapy in renal cell carcinoma.
Major finding: The median duration of disease-free survival by central review was 6.8 years for patients randomized to sunitinib vs. 5.6 years for those randomized to placebo.
Data source: Phase III trial of adjuvant therapy following nephrectomy in 615 patients with high-risk clear cell RCC.
Disclosures: The trial was supported by Pfizer. Dr. Ravaud and Dr. Bex disclosed consulting and research grants from Pfizer and other companies.
MACRA final rule exempts many more doctors
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Procalcitonin helps ID pneumonia patients needing intubation
Procalcitonin can help predict which patients with community-acquired pneumonia may require intubation for respiratory failure during a hospital admission.
Compared to those with undetectable levels, patients with a procalcitonin of 5 ng/mL were three times more likely to require invasive respiratory support, and those with a 10 ng/mL level were five times more likely, reported Wesley Self, MD, and his colleagues (Chest. 2016;150[4]:819-28. doi: 10.1016/j.chest.2016.04.010).
While predictive accuracy isn’t good enough to merit use of procalcitonin as a stand-alone test, adding it to existing clinical management tools “is likely to improve identification of patients needing intensive care,” wrote Dr. Self of Vanderbilt University, Nashville, and his colleagues. “An elevated procalcitonin level may help identify these patients without overt clinical signs of impending respiratory failure or shock but who would benefit from early [intensive care unit] admission.”
The team examined serum procalcitonin as a biomarker in a subgroup of patients included in the Etiology of Pneumonia in the Community (EPIC) study of adults hospitalized with community-acquire pneumonia. The primary outcome was the need for invasive respiratory and/or vasopressor support (IRVS) within 72 hours.
Secondarily, they looked at whether adding procalcitonin boosted the performance of accepted pneumonia risk scores, including the American Thoracic Society minor criteria (ATS).
The cohort comprised 1,770 patients with a median age of 57 years. Of these, 115 (6.5%) needed IRVS within 72 hours of admission. Almost 16% were admitted directly into an intensive care unit; almost 7% experienced a delayed transfer from a medical unit into an ICU. The in-hospital mortality was about 2%.
Most (1,642) had an ATS score of less than 3; among these, about 5% needed IRVS. The remainder had a score of 3 or higher; about 30% required IRVS. All had procalcitonin levels pulled at admission. The levels were significantly higher among patients who required IRVS than those who didn’t (1.43 ng/mL vs. 0.14 ng/mL).
A multivariate analysis found that procalcitonin was strongly associated with the risk of IRVS. In patients with undetectable levels, the risk was 4%. At 5-10 ng/mL, the overall risk of IRVS was about 14%. Every 1-ng/mL increase in this range boosted the risk of IRVS by 1%-2%.
Adding the measurement of procalcitonin to traditional pneumonia severity risk scores significantly improved the patients’ performance. When stratified by ATS minor criteria, the risk of IRVS was 4.7% among low-risk patients. That decreased to 2.4% with the addition of undetectable procalcitonin, and increased to 12% with the addition of a 10 ng/mL level.
Conversely, without considering procalcitonin, ATS high-risk patients had almost a 30% risk of IRVS. Among these high-risk patients, IRVS risk dropped to 13% with undetectable procalcitonin and increased to 36% with high procalcitonin.
Adding the biomarker level to the ATS system improved its ability to correctly classify patients, the team said. “Using at least 3 ATS minor criteria alone to indicate high risk, 77 (4.4%) of the 1,770 total patients were misclassified as low-risk and experienced IRVS. Including procalcitonin of at least 0.83 ng/ml in addition … as a high-risk indicator reduced the number of patients with IRVS misclassified as low risk to 44 (2.5%). Adding procalcitonin of at least 0.83 ng/mL as a high-risk indicator resulted in 370 additional patients being classified as high risk, with 33 correctly classified as having IRVS.”
Dr. Self reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @Alz_Gal
While measuring procalcitonin adds valuable information to pneumonia risk stratification schemes, this process lacks the accuracy needed to enable the diagnosis of pneumonia as a stand-alone test, Daiana Stolz, MD, MPH, FCCP, wrote in an editorial.
“This study further supports the notion that procalcitonin has a limited prognostic accuracy as a stand-alone test. It also does not seem to outperform the risk estimation of a combination of clinical and laboratorial parameters. However, it also emphasizes its potential to capture nuances elusive to the clinical assessment, which do not seem to be consistently reflected even in elaborated severity scores recommended for clinical routine use,” she said (Chest. 2016;150[4]:769-71. doi: 10.1016/j.chest.2016.07.017).
[Procalcitonin] values vary according to the pneumonia severity and this association is stronger than the one between disease severity and other clinical and laboratory variables,” she said.
Risk scores like the ATS system can be weak, “in particular with regard to positive predictive values,” Dr. Stolz wrote. And this is an important issue. “It is clear that patients fulfilling major criteria (endotracheal intubation and mechanical ventilation; shock-requiring vasopressors) should be considered for ICU admission; however, there is still controversy about the value of the minor criteria. ICU care is costly and a limited resource worldwide.”
She called for “a randomized study evaluating the outcome and cost-effectiveness of a procalcitonin-refined clinical score in severe [community acquired pneumonia].”
Dr. Stolz is a pulmonologist at the University Hospital Basel, Switzerland. She reported financial relationships with several pharmaceutical companies.
While measuring procalcitonin adds valuable information to pneumonia risk stratification schemes, this process lacks the accuracy needed to enable the diagnosis of pneumonia as a stand-alone test, Daiana Stolz, MD, MPH, FCCP, wrote in an editorial.
“This study further supports the notion that procalcitonin has a limited prognostic accuracy as a stand-alone test. It also does not seem to outperform the risk estimation of a combination of clinical and laboratorial parameters. However, it also emphasizes its potential to capture nuances elusive to the clinical assessment, which do not seem to be consistently reflected even in elaborated severity scores recommended for clinical routine use,” she said (Chest. 2016;150[4]:769-71. doi: 10.1016/j.chest.2016.07.017).
[Procalcitonin] values vary according to the pneumonia severity and this association is stronger than the one between disease severity and other clinical and laboratory variables,” she said.
Risk scores like the ATS system can be weak, “in particular with regard to positive predictive values,” Dr. Stolz wrote. And this is an important issue. “It is clear that patients fulfilling major criteria (endotracheal intubation and mechanical ventilation; shock-requiring vasopressors) should be considered for ICU admission; however, there is still controversy about the value of the minor criteria. ICU care is costly and a limited resource worldwide.”
She called for “a randomized study evaluating the outcome and cost-effectiveness of a procalcitonin-refined clinical score in severe [community acquired pneumonia].”
Dr. Stolz is a pulmonologist at the University Hospital Basel, Switzerland. She reported financial relationships with several pharmaceutical companies.
While measuring procalcitonin adds valuable information to pneumonia risk stratification schemes, this process lacks the accuracy needed to enable the diagnosis of pneumonia as a stand-alone test, Daiana Stolz, MD, MPH, FCCP, wrote in an editorial.
“This study further supports the notion that procalcitonin has a limited prognostic accuracy as a stand-alone test. It also does not seem to outperform the risk estimation of a combination of clinical and laboratorial parameters. However, it also emphasizes its potential to capture nuances elusive to the clinical assessment, which do not seem to be consistently reflected even in elaborated severity scores recommended for clinical routine use,” she said (Chest. 2016;150[4]:769-71. doi: 10.1016/j.chest.2016.07.017).
[Procalcitonin] values vary according to the pneumonia severity and this association is stronger than the one between disease severity and other clinical and laboratory variables,” she said.
Risk scores like the ATS system can be weak, “in particular with regard to positive predictive values,” Dr. Stolz wrote. And this is an important issue. “It is clear that patients fulfilling major criteria (endotracheal intubation and mechanical ventilation; shock-requiring vasopressors) should be considered for ICU admission; however, there is still controversy about the value of the minor criteria. ICU care is costly and a limited resource worldwide.”
She called for “a randomized study evaluating the outcome and cost-effectiveness of a procalcitonin-refined clinical score in severe [community acquired pneumonia].”
Dr. Stolz is a pulmonologist at the University Hospital Basel, Switzerland. She reported financial relationships with several pharmaceutical companies.
Procalcitonin can help predict which patients with community-acquired pneumonia may require intubation for respiratory failure during a hospital admission.
Compared to those with undetectable levels, patients with a procalcitonin of 5 ng/mL were three times more likely to require invasive respiratory support, and those with a 10 ng/mL level were five times more likely, reported Wesley Self, MD, and his colleagues (Chest. 2016;150[4]:819-28. doi: 10.1016/j.chest.2016.04.010).
While predictive accuracy isn’t good enough to merit use of procalcitonin as a stand-alone test, adding it to existing clinical management tools “is likely to improve identification of patients needing intensive care,” wrote Dr. Self of Vanderbilt University, Nashville, and his colleagues. “An elevated procalcitonin level may help identify these patients without overt clinical signs of impending respiratory failure or shock but who would benefit from early [intensive care unit] admission.”
The team examined serum procalcitonin as a biomarker in a subgroup of patients included in the Etiology of Pneumonia in the Community (EPIC) study of adults hospitalized with community-acquire pneumonia. The primary outcome was the need for invasive respiratory and/or vasopressor support (IRVS) within 72 hours.
Secondarily, they looked at whether adding procalcitonin boosted the performance of accepted pneumonia risk scores, including the American Thoracic Society minor criteria (ATS).
The cohort comprised 1,770 patients with a median age of 57 years. Of these, 115 (6.5%) needed IRVS within 72 hours of admission. Almost 16% were admitted directly into an intensive care unit; almost 7% experienced a delayed transfer from a medical unit into an ICU. The in-hospital mortality was about 2%.
Most (1,642) had an ATS score of less than 3; among these, about 5% needed IRVS. The remainder had a score of 3 or higher; about 30% required IRVS. All had procalcitonin levels pulled at admission. The levels were significantly higher among patients who required IRVS than those who didn’t (1.43 ng/mL vs. 0.14 ng/mL).
A multivariate analysis found that procalcitonin was strongly associated with the risk of IRVS. In patients with undetectable levels, the risk was 4%. At 5-10 ng/mL, the overall risk of IRVS was about 14%. Every 1-ng/mL increase in this range boosted the risk of IRVS by 1%-2%.
Adding the measurement of procalcitonin to traditional pneumonia severity risk scores significantly improved the patients’ performance. When stratified by ATS minor criteria, the risk of IRVS was 4.7% among low-risk patients. That decreased to 2.4% with the addition of undetectable procalcitonin, and increased to 12% with the addition of a 10 ng/mL level.
Conversely, without considering procalcitonin, ATS high-risk patients had almost a 30% risk of IRVS. Among these high-risk patients, IRVS risk dropped to 13% with undetectable procalcitonin and increased to 36% with high procalcitonin.
Adding the biomarker level to the ATS system improved its ability to correctly classify patients, the team said. “Using at least 3 ATS minor criteria alone to indicate high risk, 77 (4.4%) of the 1,770 total patients were misclassified as low-risk and experienced IRVS. Including procalcitonin of at least 0.83 ng/ml in addition … as a high-risk indicator reduced the number of patients with IRVS misclassified as low risk to 44 (2.5%). Adding procalcitonin of at least 0.83 ng/mL as a high-risk indicator resulted in 370 additional patients being classified as high risk, with 33 correctly classified as having IRVS.”
Dr. Self reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @Alz_Gal
Procalcitonin can help predict which patients with community-acquired pneumonia may require intubation for respiratory failure during a hospital admission.
Compared to those with undetectable levels, patients with a procalcitonin of 5 ng/mL were three times more likely to require invasive respiratory support, and those with a 10 ng/mL level were five times more likely, reported Wesley Self, MD, and his colleagues (Chest. 2016;150[4]:819-28. doi: 10.1016/j.chest.2016.04.010).
While predictive accuracy isn’t good enough to merit use of procalcitonin as a stand-alone test, adding it to existing clinical management tools “is likely to improve identification of patients needing intensive care,” wrote Dr. Self of Vanderbilt University, Nashville, and his colleagues. “An elevated procalcitonin level may help identify these patients without overt clinical signs of impending respiratory failure or shock but who would benefit from early [intensive care unit] admission.”
The team examined serum procalcitonin as a biomarker in a subgroup of patients included in the Etiology of Pneumonia in the Community (EPIC) study of adults hospitalized with community-acquire pneumonia. The primary outcome was the need for invasive respiratory and/or vasopressor support (IRVS) within 72 hours.
Secondarily, they looked at whether adding procalcitonin boosted the performance of accepted pneumonia risk scores, including the American Thoracic Society minor criteria (ATS).
The cohort comprised 1,770 patients with a median age of 57 years. Of these, 115 (6.5%) needed IRVS within 72 hours of admission. Almost 16% were admitted directly into an intensive care unit; almost 7% experienced a delayed transfer from a medical unit into an ICU. The in-hospital mortality was about 2%.
Most (1,642) had an ATS score of less than 3; among these, about 5% needed IRVS. The remainder had a score of 3 or higher; about 30% required IRVS. All had procalcitonin levels pulled at admission. The levels were significantly higher among patients who required IRVS than those who didn’t (1.43 ng/mL vs. 0.14 ng/mL).
A multivariate analysis found that procalcitonin was strongly associated with the risk of IRVS. In patients with undetectable levels, the risk was 4%. At 5-10 ng/mL, the overall risk of IRVS was about 14%. Every 1-ng/mL increase in this range boosted the risk of IRVS by 1%-2%.
Adding the measurement of procalcitonin to traditional pneumonia severity risk scores significantly improved the patients’ performance. When stratified by ATS minor criteria, the risk of IRVS was 4.7% among low-risk patients. That decreased to 2.4% with the addition of undetectable procalcitonin, and increased to 12% with the addition of a 10 ng/mL level.
Conversely, without considering procalcitonin, ATS high-risk patients had almost a 30% risk of IRVS. Among these high-risk patients, IRVS risk dropped to 13% with undetectable procalcitonin and increased to 36% with high procalcitonin.
Adding the biomarker level to the ATS system improved its ability to correctly classify patients, the team said. “Using at least 3 ATS minor criteria alone to indicate high risk, 77 (4.4%) of the 1,770 total patients were misclassified as low-risk and experienced IRVS. Including procalcitonin of at least 0.83 ng/ml in addition … as a high-risk indicator reduced the number of patients with IRVS misclassified as low risk to 44 (2.5%). Adding procalcitonin of at least 0.83 ng/mL as a high-risk indicator resulted in 370 additional patients being classified as high risk, with 33 correctly classified as having IRVS.”
Dr. Self reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @Alz_Gal
FROM CHEST
Key clinical point:
Major finding: At a procalcitonin level of 5-10 ng/mL, the overall risk of invasive respiratory and/or vasopressor support was about 14%.
Data source: The analysis comprised 1,770 patients.
Disclosures: Dr. Self reported financial relationships with several pharmaceutical companies.