User login
Malpractice: Paid claims down, but average payment up
The rate of paid legal claims against physicians dropped by more than half between 1992 and 2014, but the average claim payment amount rose, according to an analysis of the National Practitioner Data Bank.
Adam C. Schaffer, MD, of Brigham and Women’s Hospital, Boston, and his colleagues examined paid claims from the National Practitioner Data Bank from Jan. 1, 1992, to Dec. 31, 2014, accounting for specialty. Dollar amounts were inflation-adjusted to 2014 dollars using the Consumer Price Index.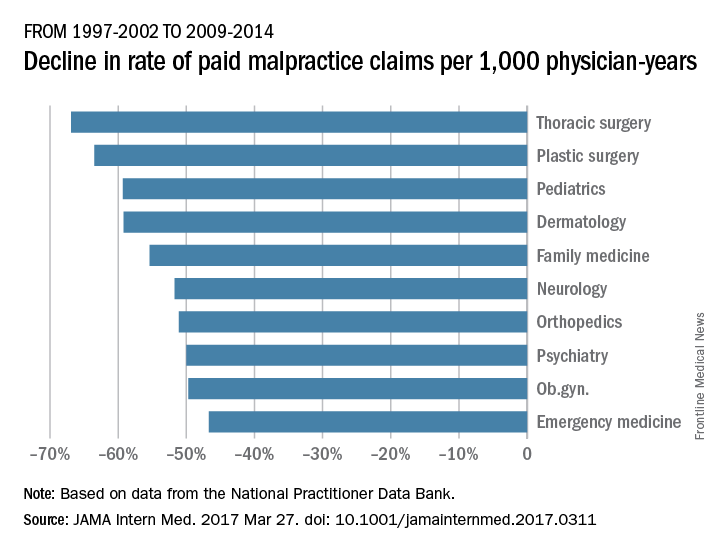
From 1992-1996 to 2009-2014, the rate of paid claims decreased by 56%, ranging from a 76% decrease in pediatrics to a 14% rate decrease in cardiology. The mean payment increased by 23% from $286,751 to $353,473 between 1992-1996 and 2009-2014. The increases ranged from $17,431 in general practice to $138,708 in pathology (JAMA Intern Med. 2017 Mar 27. doi: 10.1001/jamainternmed.2017.0311).
The most common allegation was diagnostic error, followed by surgical error and medication or treatment error. The proportion of paid claims attributable to diagnostic error varied widely and was highest in pathology and radiology. Plastic surgery had the highest percentage of paid claims related to surgical errors. Specialties with the highest percentage of paid claims related to medication/treatment errors were psychiatry, general practice, and pulmonology.
While prior data have shown the overall trend of declining paid malpractice claims and increasing average payments, this study is the first to examine such trends by medical specialty, said Dr. Schaffer.
“We think it is important to try to understand the reasons for the variation among specialties in characteristics of paid malpractice claims, as understanding the reasons for this variation may provide insights about how we can provide the safest care possible,” he said in an interview.
Dr. Schaffer said that he hopes the findings make physicians more aware of the malpractice landscape and aid their future practice decisions.
“Medical malpractice is an issue that concerns many physicians, and physicians’ perceptions of their liability risk can influence the decisions they make in caring for their patients,” he said. “By performing an analysis of a national database of paid medical malpractice claims broken down by specialty, we hope to provide physicians with data that give them an accurate picture of the medical malpractice environment in which they are practicing.”
[email protected]
On Twitter @legal_med
The rate of paid legal claims against physicians dropped by more than half between 1992 and 2014, but the average claim payment amount rose, according to an analysis of the National Practitioner Data Bank.
Adam C. Schaffer, MD, of Brigham and Women’s Hospital, Boston, and his colleagues examined paid claims from the National Practitioner Data Bank from Jan. 1, 1992, to Dec. 31, 2014, accounting for specialty. Dollar amounts were inflation-adjusted to 2014 dollars using the Consumer Price Index.
From 1992-1996 to 2009-2014, the rate of paid claims decreased by 56%, ranging from a 76% decrease in pediatrics to a 14% rate decrease in cardiology. The mean payment increased by 23% from $286,751 to $353,473 between 1992-1996 and 2009-2014. The increases ranged from $17,431 in general practice to $138,708 in pathology (JAMA Intern Med. 2017 Mar 27. doi: 10.1001/jamainternmed.2017.0311).
The most common allegation was diagnostic error, followed by surgical error and medication or treatment error. The proportion of paid claims attributable to diagnostic error varied widely and was highest in pathology and radiology. Plastic surgery had the highest percentage of paid claims related to surgical errors. Specialties with the highest percentage of paid claims related to medication/treatment errors were psychiatry, general practice, and pulmonology.
While prior data have shown the overall trend of declining paid malpractice claims and increasing average payments, this study is the first to examine such trends by medical specialty, said Dr. Schaffer.
“We think it is important to try to understand the reasons for the variation among specialties in characteristics of paid malpractice claims, as understanding the reasons for this variation may provide insights about how we can provide the safest care possible,” he said in an interview.
Dr. Schaffer said that he hopes the findings make physicians more aware of the malpractice landscape and aid their future practice decisions.
“Medical malpractice is an issue that concerns many physicians, and physicians’ perceptions of their liability risk can influence the decisions they make in caring for their patients,” he said. “By performing an analysis of a national database of paid medical malpractice claims broken down by specialty, we hope to provide physicians with data that give them an accurate picture of the medical malpractice environment in which they are practicing.”
[email protected]
On Twitter @legal_med
The rate of paid legal claims against physicians dropped by more than half between 1992 and 2014, but the average claim payment amount rose, according to an analysis of the National Practitioner Data Bank.
Adam C. Schaffer, MD, of Brigham and Women’s Hospital, Boston, and his colleagues examined paid claims from the National Practitioner Data Bank from Jan. 1, 1992, to Dec. 31, 2014, accounting for specialty. Dollar amounts were inflation-adjusted to 2014 dollars using the Consumer Price Index.
From 1992-1996 to 2009-2014, the rate of paid claims decreased by 56%, ranging from a 76% decrease in pediatrics to a 14% rate decrease in cardiology. The mean payment increased by 23% from $286,751 to $353,473 between 1992-1996 and 2009-2014. The increases ranged from $17,431 in general practice to $138,708 in pathology (JAMA Intern Med. 2017 Mar 27. doi: 10.1001/jamainternmed.2017.0311).
The most common allegation was diagnostic error, followed by surgical error and medication or treatment error. The proportion of paid claims attributable to diagnostic error varied widely and was highest in pathology and radiology. Plastic surgery had the highest percentage of paid claims related to surgical errors. Specialties with the highest percentage of paid claims related to medication/treatment errors were psychiatry, general practice, and pulmonology.
While prior data have shown the overall trend of declining paid malpractice claims and increasing average payments, this study is the first to examine such trends by medical specialty, said Dr. Schaffer.
“We think it is important to try to understand the reasons for the variation among specialties in characteristics of paid malpractice claims, as understanding the reasons for this variation may provide insights about how we can provide the safest care possible,” he said in an interview.
Dr. Schaffer said that he hopes the findings make physicians more aware of the malpractice landscape and aid their future practice decisions.
“Medical malpractice is an issue that concerns many physicians, and physicians’ perceptions of their liability risk can influence the decisions they make in caring for their patients,” he said. “By performing an analysis of a national database of paid medical malpractice claims broken down by specialty, we hope to provide physicians with data that give them an accurate picture of the medical malpractice environment in which they are practicing.”
[email protected]
On Twitter @legal_med
FROM JAMA INTERNAL MEDICINE
Stroll Down the AmSECT Avenue
As the premier organization focused on extracorporeal circulation technology professionals, AmSECT encourages sharing of the latest trends and developments in the industry. When visiting the Exhibit Hall, stroll over to AmSECT Avenue and explore perfusion technologies and products – all in one place!
Walk the red carpet and meet with Abbott, Spectrum Medical, Medtronic, LivaNova, Terumo and more! See first-hand the latest in perfusion technology and advancements. You’ll gain knowledge of products that you can immediately take back to your facility, and other advancements that will help you remain current in the field of perfusion.
Stop by the AmSECT booth to discover how to keep learning after the conference concludes! AmSECT University provides a platform to facilitate perfusion-related education and earn continuing education units. The AmSECT team is available to answer your questions about AmSECT University and other benefits you will receive by becoming an AmSECT member.
As the premier organization focused on extracorporeal circulation technology professionals, AmSECT encourages sharing of the latest trends and developments in the industry. When visiting the Exhibit Hall, stroll over to AmSECT Avenue and explore perfusion technologies and products – all in one place!
Walk the red carpet and meet with Abbott, Spectrum Medical, Medtronic, LivaNova, Terumo and more! See first-hand the latest in perfusion technology and advancements. You’ll gain knowledge of products that you can immediately take back to your facility, and other advancements that will help you remain current in the field of perfusion.
Stop by the AmSECT booth to discover how to keep learning after the conference concludes! AmSECT University provides a platform to facilitate perfusion-related education and earn continuing education units. The AmSECT team is available to answer your questions about AmSECT University and other benefits you will receive by becoming an AmSECT member.
As the premier organization focused on extracorporeal circulation technology professionals, AmSECT encourages sharing of the latest trends and developments in the industry. When visiting the Exhibit Hall, stroll over to AmSECT Avenue and explore perfusion technologies and products – all in one place!
Walk the red carpet and meet with Abbott, Spectrum Medical, Medtronic, LivaNova, Terumo and more! See first-hand the latest in perfusion technology and advancements. You’ll gain knowledge of products that you can immediately take back to your facility, and other advancements that will help you remain current in the field of perfusion.
Stop by the AmSECT booth to discover how to keep learning after the conference concludes! AmSECT University provides a platform to facilitate perfusion-related education and earn continuing education units. The AmSECT team is available to answer your questions about AmSECT University and other benefits you will receive by becoming an AmSECT member.
Transplantation and ECMO feature prominently in Saturday session
Saturday’s course “Cardiothoracic Transplant and Mechanical Circulatory Support of Heart and Lung Failure: Mastery of the Management of End Stage Heart and Lung Disease” will explore novel techniques in heart and lung transplant, mechanical circulatory support, and extracorporeal membrane oxygenation (ECMO). The course is co-chaired by Matthew D. Bacchetta, MD, of Columbia University, Carmelo A. Milano, of Duke University, and Rich Walczak, CCP, FPP, also of Duke University.
The first session will focus on heart transplants, and will cover topics such as perfusion storage for transplantation, maintaining an ex-vivo heart, primary graft dysfunction and a talk from Yoshifumi Naka, MD, of Columbia University, who will provide first-hand accounts of using the latest durable centrifugal left ventricular assist device (LVAD).
“We’re going to get a very comprehensive update on the use of donation after cardiac death (DCD) lung transplantation, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. The course will close with a session on ECMO with Dr. Bacchetta presenting on ECMO bridge to transplantation (BTT), while other talks consider artificial lung development, ECMO transport, management of ambulation during ECMO, and ex vivo lung perfusion.
Dr. Bacchetta said, “We’ll be getting really good reviews from some of the best centers around the world.”
Saturday’s course “Cardiothoracic Transplant and Mechanical Circulatory Support of Heart and Lung Failure: Mastery of the Management of End Stage Heart and Lung Disease” will explore novel techniques in heart and lung transplant, mechanical circulatory support, and extracorporeal membrane oxygenation (ECMO). The course is co-chaired by Matthew D. Bacchetta, MD, of Columbia University, Carmelo A. Milano, of Duke University, and Rich Walczak, CCP, FPP, also of Duke University.
The first session will focus on heart transplants, and will cover topics such as perfusion storage for transplantation, maintaining an ex-vivo heart, primary graft dysfunction and a talk from Yoshifumi Naka, MD, of Columbia University, who will provide first-hand accounts of using the latest durable centrifugal left ventricular assist device (LVAD).
“We’re going to get a very comprehensive update on the use of donation after cardiac death (DCD) lung transplantation, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. The course will close with a session on ECMO with Dr. Bacchetta presenting on ECMO bridge to transplantation (BTT), while other talks consider artificial lung development, ECMO transport, management of ambulation during ECMO, and ex vivo lung perfusion.
Dr. Bacchetta said, “We’ll be getting really good reviews from some of the best centers around the world.”
Saturday’s course “Cardiothoracic Transplant and Mechanical Circulatory Support of Heart and Lung Failure: Mastery of the Management of End Stage Heart and Lung Disease” will explore novel techniques in heart and lung transplant, mechanical circulatory support, and extracorporeal membrane oxygenation (ECMO). The course is co-chaired by Matthew D. Bacchetta, MD, of Columbia University, Carmelo A. Milano, of Duke University, and Rich Walczak, CCP, FPP, also of Duke University.
The first session will focus on heart transplants, and will cover topics such as perfusion storage for transplantation, maintaining an ex-vivo heart, primary graft dysfunction and a talk from Yoshifumi Naka, MD, of Columbia University, who will provide first-hand accounts of using the latest durable centrifugal left ventricular assist device (LVAD).
“We’re going to get a very comprehensive update on the use of donation after cardiac death (DCD) lung transplantation, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. The course will close with a session on ECMO with Dr. Bacchetta presenting on ECMO bridge to transplantation (BTT), while other talks consider artificial lung development, ECMO transport, management of ambulation during ECMO, and ex vivo lung perfusion.
Dr. Bacchetta said, “We’ll be getting really good reviews from some of the best centers around the world.”
The 20th Annual C. Walton Lillehei Forum Award
Attendees will have the opportunity to hear about exceptional cardiothoracic research at Monday afternoon’s 20th Annual C. Walton Lillehei Resident Forum, which showcases top research by residents.
Co-chairs Dao Nguyen, MD, of the University of Miami Health System and Frederick Y. Chen, MD, PhD, of Tufts Medical Center, along with members of the AATS Research Scholarship Committee reviewed the submitted manuscripts and will judge the oral presentations to select the winner of a prestigious $5,000 cash prize. The competition is limited to original work presented by residents in cardiothoracic surgery and/or residents in general surgical training programs who are working in a cardiothoracic surgical laboratory or clinical rotation in North America.
“The competition is fierce as all the abstracts were of the highest scientific caliber and the committee had a hard time selecting the final top six to include in the final program,” he said. “It is a great honor for resident trainees to give a podium presentation of their research works at the top international meeting in the field of cardiothoracic surgery, such as the AATS.”
The individual presentations are the work of one to three years in the laboratory and represent the specialties’ best non-clinical research, Dr. Chen added.
“The top prize is the most prestigious prize for any postdoctoral research fellow in thoracic surgery and marks them as someone who will likely become a leader in the field,” he said.
Attendees will get the chance to hear a wide diversity of research on Monday, including two abstracts focused on cardiac surgery research, one of which centers on the genetic basis of aortic aneurysm, and another that focuses on cell therapy to rescue heart function following ischemic injury, Dr. Nguyen said. Four abstracts focus on thoracic surgery research, one of which relates to oncology, one that focuses on tissue engineering, one that pertains to lung transplantation, and one that applies the concept of lung perfusion to rehabilitate borderline lung donor graft to restore lung function affected by sepsis.
“These research projects are at the cutting edge of innovation,” Dr. Nguyen said.
Monday’s forum will kick off with a research paper entitled “Mutations in ROBO4 Lead to the Development of Bicuspid Aortic Valve and Ascending Aortic Aneurysm,” presented by Hamza Aziz, MD, of Duke University.
Next, Jarrod Predina, MD, of the University of Pennsylvania, will present research that describes how “Targeted Near-Infrared Intraoperative Molecular Imaging Can Identify Residual Disease During Pulmonary Resection,” followed by an analysis on how “Delivery of Endothelial Progenitor Cells with Injectable Shear-Thinning Hydrogels Maintains Ventricular Geometry and Normalizes Dynamic Strain to Stabilize Cardiac Function Following Ischemic Injury,” presented by Ann C. Gaffey, MD, also of the University of Pennsylvania.
Attendees will also hear a presentation entitled “Targeted Cell Replacement in Human Lung Bioengineering,” by Brandon A. Guenthart, MD, of Columbia University. Stephen Chiu, MD, of Northwestern University, will then present his research paper called “Donor-derived Non-Classical Monocytes Mediate Primary Lung Allograft Dysfunction by Recruiting Recipient Neutrophils via Toll-Like Receptor-dependent Production of MIP-2 .”
Finally, attendees will hear about how “In Vivo Lung Perfusion Rehabilitates Sepsis-Induced Lung Injury,” presented by J. Hunter Mehaffey, MD, of the University of Virginia.
Dr. Nguyen encourages all meeting attendees to attend the forum to learn about the forefront of cardiothoracic surgery research performed by the future generation of the specialty and to “give the trainees all the support and accolades that they and their lab mentors all deserve.”
Attendees will have the opportunity to hear about exceptional cardiothoracic research at Monday afternoon’s 20th Annual C. Walton Lillehei Resident Forum, which showcases top research by residents.
Co-chairs Dao Nguyen, MD, of the University of Miami Health System and Frederick Y. Chen, MD, PhD, of Tufts Medical Center, along with members of the AATS Research Scholarship Committee reviewed the submitted manuscripts and will judge the oral presentations to select the winner of a prestigious $5,000 cash prize. The competition is limited to original work presented by residents in cardiothoracic surgery and/or residents in general surgical training programs who are working in a cardiothoracic surgical laboratory or clinical rotation in North America.
“The competition is fierce as all the abstracts were of the highest scientific caliber and the committee had a hard time selecting the final top six to include in the final program,” he said. “It is a great honor for resident trainees to give a podium presentation of their research works at the top international meeting in the field of cardiothoracic surgery, such as the AATS.”
The individual presentations are the work of one to three years in the laboratory and represent the specialties’ best non-clinical research, Dr. Chen added.
“The top prize is the most prestigious prize for any postdoctoral research fellow in thoracic surgery and marks them as someone who will likely become a leader in the field,” he said.
Attendees will get the chance to hear a wide diversity of research on Monday, including two abstracts focused on cardiac surgery research, one of which centers on the genetic basis of aortic aneurysm, and another that focuses on cell therapy to rescue heart function following ischemic injury, Dr. Nguyen said. Four abstracts focus on thoracic surgery research, one of which relates to oncology, one that focuses on tissue engineering, one that pertains to lung transplantation, and one that applies the concept of lung perfusion to rehabilitate borderline lung donor graft to restore lung function affected by sepsis.
“These research projects are at the cutting edge of innovation,” Dr. Nguyen said.
Monday’s forum will kick off with a research paper entitled “Mutations in ROBO4 Lead to the Development of Bicuspid Aortic Valve and Ascending Aortic Aneurysm,” presented by Hamza Aziz, MD, of Duke University.
Next, Jarrod Predina, MD, of the University of Pennsylvania, will present research that describes how “Targeted Near-Infrared Intraoperative Molecular Imaging Can Identify Residual Disease During Pulmonary Resection,” followed by an analysis on how “Delivery of Endothelial Progenitor Cells with Injectable Shear-Thinning Hydrogels Maintains Ventricular Geometry and Normalizes Dynamic Strain to Stabilize Cardiac Function Following Ischemic Injury,” presented by Ann C. Gaffey, MD, also of the University of Pennsylvania.
Attendees will also hear a presentation entitled “Targeted Cell Replacement in Human Lung Bioengineering,” by Brandon A. Guenthart, MD, of Columbia University. Stephen Chiu, MD, of Northwestern University, will then present his research paper called “Donor-derived Non-Classical Monocytes Mediate Primary Lung Allograft Dysfunction by Recruiting Recipient Neutrophils via Toll-Like Receptor-dependent Production of MIP-2 .”
Finally, attendees will hear about how “In Vivo Lung Perfusion Rehabilitates Sepsis-Induced Lung Injury,” presented by J. Hunter Mehaffey, MD, of the University of Virginia.
Dr. Nguyen encourages all meeting attendees to attend the forum to learn about the forefront of cardiothoracic surgery research performed by the future generation of the specialty and to “give the trainees all the support and accolades that they and their lab mentors all deserve.”
Attendees will have the opportunity to hear about exceptional cardiothoracic research at Monday afternoon’s 20th Annual C. Walton Lillehei Resident Forum, which showcases top research by residents.
Co-chairs Dao Nguyen, MD, of the University of Miami Health System and Frederick Y. Chen, MD, PhD, of Tufts Medical Center, along with members of the AATS Research Scholarship Committee reviewed the submitted manuscripts and will judge the oral presentations to select the winner of a prestigious $5,000 cash prize. The competition is limited to original work presented by residents in cardiothoracic surgery and/or residents in general surgical training programs who are working in a cardiothoracic surgical laboratory or clinical rotation in North America.
“The competition is fierce as all the abstracts were of the highest scientific caliber and the committee had a hard time selecting the final top six to include in the final program,” he said. “It is a great honor for resident trainees to give a podium presentation of their research works at the top international meeting in the field of cardiothoracic surgery, such as the AATS.”
The individual presentations are the work of one to three years in the laboratory and represent the specialties’ best non-clinical research, Dr. Chen added.
“The top prize is the most prestigious prize for any postdoctoral research fellow in thoracic surgery and marks them as someone who will likely become a leader in the field,” he said.
Attendees will get the chance to hear a wide diversity of research on Monday, including two abstracts focused on cardiac surgery research, one of which centers on the genetic basis of aortic aneurysm, and another that focuses on cell therapy to rescue heart function following ischemic injury, Dr. Nguyen said. Four abstracts focus on thoracic surgery research, one of which relates to oncology, one that focuses on tissue engineering, one that pertains to lung transplantation, and one that applies the concept of lung perfusion to rehabilitate borderline lung donor graft to restore lung function affected by sepsis.
“These research projects are at the cutting edge of innovation,” Dr. Nguyen said.
Monday’s forum will kick off with a research paper entitled “Mutations in ROBO4 Lead to the Development of Bicuspid Aortic Valve and Ascending Aortic Aneurysm,” presented by Hamza Aziz, MD, of Duke University.
Next, Jarrod Predina, MD, of the University of Pennsylvania, will present research that describes how “Targeted Near-Infrared Intraoperative Molecular Imaging Can Identify Residual Disease During Pulmonary Resection,” followed by an analysis on how “Delivery of Endothelial Progenitor Cells with Injectable Shear-Thinning Hydrogels Maintains Ventricular Geometry and Normalizes Dynamic Strain to Stabilize Cardiac Function Following Ischemic Injury,” presented by Ann C. Gaffey, MD, also of the University of Pennsylvania.
Attendees will also hear a presentation entitled “Targeted Cell Replacement in Human Lung Bioengineering,” by Brandon A. Guenthart, MD, of Columbia University. Stephen Chiu, MD, of Northwestern University, will then present his research paper called “Donor-derived Non-Classical Monocytes Mediate Primary Lung Allograft Dysfunction by Recruiting Recipient Neutrophils via Toll-Like Receptor-dependent Production of MIP-2 .”
Finally, attendees will hear about how “In Vivo Lung Perfusion Rehabilitates Sepsis-Induced Lung Injury,” presented by J. Hunter Mehaffey, MD, of the University of Virginia.
Dr. Nguyen encourages all meeting attendees to attend the forum to learn about the forefront of cardiothoracic surgery research performed by the future generation of the specialty and to “give the trainees all the support and accolades that they and their lab mentors all deserve.”
Cardiac surgery symposium critiques challenging cases
“This year’s Adult Cardiac Surgery Symposium will prove to be one of the most dynamic and liveliest to date,” said course chair Vinod H. Thourani, MD, in an interview. “Each session will have dedicated time for robust panel discussion and hopefully significant participant involvement,” he said.
Dr. Thourani, of Emory University, leads an expert roster of clinicians in the AATS/STS Adult Cardiac Surgery Symposium entitled “Excellence Through Knowledge.” The all-day Sunday format allows for an in-depth exploration that can benefit clinicians with a range of perspectives. The talks will include not only examples from clinical experience, but also “will provide the audience with the most up-to-date literature on a variety of topics relevant to the practice of cardiac surgery,” Dr. Thourani said.
The topics scheduled for discussion will include the management of short-term circulatory support for acute cardiac decompensation, aortic and mitral valve surgery, coronary artery disease, and management of aorta diseases, Dr. Thourani said.
Dr. Thourani highlighted some of specific topics that he considers especially hot this year: “New Surgical and Transcatheter Therapies for Right Ventricular Failure,” by Edward G. Soltesz, MD, of the Cleveland Clinic; “TAVR for the Treatment of Aortic Stenosis: Here Comes the Tsunami!!!,” by Michael J. Mack, MD, of the Baylor Health Care System; “Indications for Concomitant Tricuspid Valve Repair,” by Gilles D. Dreyfus, MD, of the Cardio Thoracic Centre of Monaco; “Making the Heat Team a Reality in Choosing Between CABG and PCI,” by Farouc A. Jaffer, MD, of Massachusetts General Hospital; and “Root Aneurysm with Bicuspid Aortic Valve: Spare or Replace,” by Tirone E. David, MD of Toronto General Hospital.
After a break for lunch, the symposium continues with a session on “Controversies in Mitral Valve Surgery.” Topics include not only indications for concomitant tricuspid valve repair, but also determinants for mitral valve repair or replacement and a discussion of atrial fibrillation in the setting of mitral valve disease.
“We are delighted that this symposium will not only have renowned surgeons, but also cardiologists and perfusionists that truly represent a heart team approach to these complex patients,” Dr. Thourani concluded.
“This year’s Adult Cardiac Surgery Symposium will prove to be one of the most dynamic and liveliest to date,” said course chair Vinod H. Thourani, MD, in an interview. “Each session will have dedicated time for robust panel discussion and hopefully significant participant involvement,” he said.
Dr. Thourani, of Emory University, leads an expert roster of clinicians in the AATS/STS Adult Cardiac Surgery Symposium entitled “Excellence Through Knowledge.” The all-day Sunday format allows for an in-depth exploration that can benefit clinicians with a range of perspectives. The talks will include not only examples from clinical experience, but also “will provide the audience with the most up-to-date literature on a variety of topics relevant to the practice of cardiac surgery,” Dr. Thourani said.
The topics scheduled for discussion will include the management of short-term circulatory support for acute cardiac decompensation, aortic and mitral valve surgery, coronary artery disease, and management of aorta diseases, Dr. Thourani said.
Dr. Thourani highlighted some of specific topics that he considers especially hot this year: “New Surgical and Transcatheter Therapies for Right Ventricular Failure,” by Edward G. Soltesz, MD, of the Cleveland Clinic; “TAVR for the Treatment of Aortic Stenosis: Here Comes the Tsunami!!!,” by Michael J. Mack, MD, of the Baylor Health Care System; “Indications for Concomitant Tricuspid Valve Repair,” by Gilles D. Dreyfus, MD, of the Cardio Thoracic Centre of Monaco; “Making the Heat Team a Reality in Choosing Between CABG and PCI,” by Farouc A. Jaffer, MD, of Massachusetts General Hospital; and “Root Aneurysm with Bicuspid Aortic Valve: Spare or Replace,” by Tirone E. David, MD of Toronto General Hospital.
After a break for lunch, the symposium continues with a session on “Controversies in Mitral Valve Surgery.” Topics include not only indications for concomitant tricuspid valve repair, but also determinants for mitral valve repair or replacement and a discussion of atrial fibrillation in the setting of mitral valve disease.
“We are delighted that this symposium will not only have renowned surgeons, but also cardiologists and perfusionists that truly represent a heart team approach to these complex patients,” Dr. Thourani concluded.
“This year’s Adult Cardiac Surgery Symposium will prove to be one of the most dynamic and liveliest to date,” said course chair Vinod H. Thourani, MD, in an interview. “Each session will have dedicated time for robust panel discussion and hopefully significant participant involvement,” he said.
Dr. Thourani, of Emory University, leads an expert roster of clinicians in the AATS/STS Adult Cardiac Surgery Symposium entitled “Excellence Through Knowledge.” The all-day Sunday format allows for an in-depth exploration that can benefit clinicians with a range of perspectives. The talks will include not only examples from clinical experience, but also “will provide the audience with the most up-to-date literature on a variety of topics relevant to the practice of cardiac surgery,” Dr. Thourani said.
The topics scheduled for discussion will include the management of short-term circulatory support for acute cardiac decompensation, aortic and mitral valve surgery, coronary artery disease, and management of aorta diseases, Dr. Thourani said.
Dr. Thourani highlighted some of specific topics that he considers especially hot this year: “New Surgical and Transcatheter Therapies for Right Ventricular Failure,” by Edward G. Soltesz, MD, of the Cleveland Clinic; “TAVR for the Treatment of Aortic Stenosis: Here Comes the Tsunami!!!,” by Michael J. Mack, MD, of the Baylor Health Care System; “Indications for Concomitant Tricuspid Valve Repair,” by Gilles D. Dreyfus, MD, of the Cardio Thoracic Centre of Monaco; “Making the Heat Team a Reality in Choosing Between CABG and PCI,” by Farouc A. Jaffer, MD, of Massachusetts General Hospital; and “Root Aneurysm with Bicuspid Aortic Valve: Spare or Replace,” by Tirone E. David, MD of Toronto General Hospital.
After a break for lunch, the symposium continues with a session on “Controversies in Mitral Valve Surgery.” Topics include not only indications for concomitant tricuspid valve repair, but also determinants for mitral valve repair or replacement and a discussion of atrial fibrillation in the setting of mitral valve disease.
“We are delighted that this symposium will not only have renowned surgeons, but also cardiologists and perfusionists that truly represent a heart team approach to these complex patients,” Dr. Thourani concluded.
New devices improve outcomes for pediatric patients
The smallest patients get a full day’s focus in the “Congenital Heart Disease Symposium: Innovations and Controversies in the Surgical Management of Congenital Heart Disease,” session on Sunday, April 30.
“This symposium is designed for participants to get up-to-date information about cardiothoracic surgery,” said Katsuhide Maeda, MD, of Stanford University Medical Center, in an interview.
The Pumps for Kids, Infants, and Neonates (PumpKIN) trial is designed as a randomized, multicenter, two-arm study to evaluate an investigational ventricular assistance device (VAD) known as Jarvik 2015, comparing it to the EXCOR Pediatric VAD in children with heart failure. The study is designed to enroll 88 patients, 44 randomized to the Jarvik 2015 and 44 to the EXCOR.
The primary objectives of the PumpKIN trial, according to Clinicaltrials.gov description, are to determine the safety of the Jarvik 2015 based on evaluations of reported serious adverse events up to the first 180 days after implantation, and to assess benefits based on overall survival without severe neurologic impairment.
Symposium attendees can learn the latest information about outcomes as well as tips and techniques for device use in neonates and infants, Dr. Maeda said.
Some of the presentations featuring tips and techniques include “Device Innovations and Options for Biventrical Mechanical Circulatory Support,” by Mark Shepard, MD, of St. Louis Children’s Hospital; “Surgical Techniques for TAPVR,” by Christopher A. Caldarone, MD, of the Hospital for Sick Children, Toronto; and “The “Tweener” Arch - Front vs. Side,” by Charles D. Fraser, MD, of Texas Children’s Hospital.
Looking ahead, Dr. Maeda advised clinicians to keep watching the PumpKIN trial, which is set to begin in seven institutions in the United States and Canada. “This device may open a new door,” for pediatric heart surgery, he noted.
The smallest patients get a full day’s focus in the “Congenital Heart Disease Symposium: Innovations and Controversies in the Surgical Management of Congenital Heart Disease,” session on Sunday, April 30.
“This symposium is designed for participants to get up-to-date information about cardiothoracic surgery,” said Katsuhide Maeda, MD, of Stanford University Medical Center, in an interview.
The Pumps for Kids, Infants, and Neonates (PumpKIN) trial is designed as a randomized, multicenter, two-arm study to evaluate an investigational ventricular assistance device (VAD) known as Jarvik 2015, comparing it to the EXCOR Pediatric VAD in children with heart failure. The study is designed to enroll 88 patients, 44 randomized to the Jarvik 2015 and 44 to the EXCOR.
The primary objectives of the PumpKIN trial, according to Clinicaltrials.gov description, are to determine the safety of the Jarvik 2015 based on evaluations of reported serious adverse events up to the first 180 days after implantation, and to assess benefits based on overall survival without severe neurologic impairment.
Symposium attendees can learn the latest information about outcomes as well as tips and techniques for device use in neonates and infants, Dr. Maeda said.
Some of the presentations featuring tips and techniques include “Device Innovations and Options for Biventrical Mechanical Circulatory Support,” by Mark Shepard, MD, of St. Louis Children’s Hospital; “Surgical Techniques for TAPVR,” by Christopher A. Caldarone, MD, of the Hospital for Sick Children, Toronto; and “The “Tweener” Arch - Front vs. Side,” by Charles D. Fraser, MD, of Texas Children’s Hospital.
Looking ahead, Dr. Maeda advised clinicians to keep watching the PumpKIN trial, which is set to begin in seven institutions in the United States and Canada. “This device may open a new door,” for pediatric heart surgery, he noted.
The smallest patients get a full day’s focus in the “Congenital Heart Disease Symposium: Innovations and Controversies in the Surgical Management of Congenital Heart Disease,” session on Sunday, April 30.
“This symposium is designed for participants to get up-to-date information about cardiothoracic surgery,” said Katsuhide Maeda, MD, of Stanford University Medical Center, in an interview.
The Pumps for Kids, Infants, and Neonates (PumpKIN) trial is designed as a randomized, multicenter, two-arm study to evaluate an investigational ventricular assistance device (VAD) known as Jarvik 2015, comparing it to the EXCOR Pediatric VAD in children with heart failure. The study is designed to enroll 88 patients, 44 randomized to the Jarvik 2015 and 44 to the EXCOR.
The primary objectives of the PumpKIN trial, according to Clinicaltrials.gov description, are to determine the safety of the Jarvik 2015 based on evaluations of reported serious adverse events up to the first 180 days after implantation, and to assess benefits based on overall survival without severe neurologic impairment.
Symposium attendees can learn the latest information about outcomes as well as tips and techniques for device use in neonates and infants, Dr. Maeda said.
Some of the presentations featuring tips and techniques include “Device Innovations and Options for Biventrical Mechanical Circulatory Support,” by Mark Shepard, MD, of St. Louis Children’s Hospital; “Surgical Techniques for TAPVR,” by Christopher A. Caldarone, MD, of the Hospital for Sick Children, Toronto; and “The “Tweener” Arch - Front vs. Side,” by Charles D. Fraser, MD, of Texas Children’s Hospital.
Looking ahead, Dr. Maeda advised clinicians to keep watching the PumpKIN trial, which is set to begin in seven institutions in the United States and Canada. “This device may open a new door,” for pediatric heart surgery, he noted.
Multidisciplinary Teams Create Systems of Care
Experts from a variety of disciplines will come together to discuss topics related to preoperative, perioperative and postoperative care for cardiovascular surgery patients during Sunday’s full-day symposium, “Improving Systems of Care, Quality and Safety.”
“The AATS recognizes that the delivery of high-quality, patient-centered cardiovascular care is best achieved through the use of proficient multidisciplinary teams,” said course co-chair Katherine J. Hoercher, RN, FAHA, of the Cleveland Clinic. “This symposium, with its focus on interprofessional education, is an important mechanism for developing team-based care competencies that form the underpinning of high-functioning teams.”
By bringing together faculty and learners from multiple cardiovascular surgery professions, attendees can learn how effective collaboration and utilization of standardized processes can improve outcomes in systems of care, quality and safety, Ms. Hoercher said.
“The session should provide the audience with nuts-and-bolts approaches to everyday problems through a global view of the difficulties we face in consistently delivering the highest quality medical care,” added course co-chair Glenn J.R. Whitman, MD, of The Johns Hopkins Hospital.
The morning session “will address a variety of common problems that we all face in managing cardiac surgical patients,” Dr. Whitman said. “The topics are varied and include, for example, informed consent, optimizing preoperative surgical readiness, as well as specific aspects of postoperative management such as goal-directed resuscitation and approach to the cardiac arrest patient.”
Included in the morning presentations are four talks on a team approach to minimizing transfusions, including preadmission use of epoetin; preoperative evaluation and intraoperative management; perioperative management of blood preservation; and risks, recognition and management of post cardiopulmonary bypass hemorrhage.
The afternoon portion of the symposium “will continue the morning objective and focus on specific postoperative morbidities, including hyperglycemia, perioperative myocardial infarctions and strokes,” Dr. Whitman said.
The session will conclude with presentations from five nationally-renowned speakers. Douglas R. Johnston, MD, of the Cleveland Clinic, will discuss what surgical teams can learn from fighter pilots, special ops forces and other elite performers; Peter Pronovost, MD, of Johns Hopkins Medicine, and its Armstrong Institute for Patient Safety and Quality, will discuss organizational structure and process factors for improving cardiac surgery quality and safety; Don Goldmann, MD, of the Institute for Healthcare Improvement, will provide insights on the impact of the Medicare Access and CHIP Reauthorization Act (MACRA) on the delivery of cardiovascular care; David M. Shahian, MD, of Harvard Medical School, who oversees the Society of Thoracic Surgeons’ database, will talk about meaningful outcome measures in cardiac surgery; and Alex B. Haynes, MD, of Massachusetts General Hospital, will review surgical checklists and their association with decreased morbidity and mortality.
“This session should be of interest to all AATS meeting attendees as we navigate the ever-changing landscape of health care delivery,” Ms. Hoercher said. “The information presented will be something the audience can’t easily obtain elsewhere,” added Dr. Whitman.
“I hope many of the AATS meeting attendees, both surgeons and non-surgeons, will join us at what will be an excellent educational opportunity,” Ms. Hoercher said.
Experts from a variety of disciplines will come together to discuss topics related to preoperative, perioperative and postoperative care for cardiovascular surgery patients during Sunday’s full-day symposium, “Improving Systems of Care, Quality and Safety.”
“The AATS recognizes that the delivery of high-quality, patient-centered cardiovascular care is best achieved through the use of proficient multidisciplinary teams,” said course co-chair Katherine J. Hoercher, RN, FAHA, of the Cleveland Clinic. “This symposium, with its focus on interprofessional education, is an important mechanism for developing team-based care competencies that form the underpinning of high-functioning teams.”
By bringing together faculty and learners from multiple cardiovascular surgery professions, attendees can learn how effective collaboration and utilization of standardized processes can improve outcomes in systems of care, quality and safety, Ms. Hoercher said.
“The session should provide the audience with nuts-and-bolts approaches to everyday problems through a global view of the difficulties we face in consistently delivering the highest quality medical care,” added course co-chair Glenn J.R. Whitman, MD, of The Johns Hopkins Hospital.
The morning session “will address a variety of common problems that we all face in managing cardiac surgical patients,” Dr. Whitman said. “The topics are varied and include, for example, informed consent, optimizing preoperative surgical readiness, as well as specific aspects of postoperative management such as goal-directed resuscitation and approach to the cardiac arrest patient.”
Included in the morning presentations are four talks on a team approach to minimizing transfusions, including preadmission use of epoetin; preoperative evaluation and intraoperative management; perioperative management of blood preservation; and risks, recognition and management of post cardiopulmonary bypass hemorrhage.
The afternoon portion of the symposium “will continue the morning objective and focus on specific postoperative morbidities, including hyperglycemia, perioperative myocardial infarctions and strokes,” Dr. Whitman said.
The session will conclude with presentations from five nationally-renowned speakers. Douglas R. Johnston, MD, of the Cleveland Clinic, will discuss what surgical teams can learn from fighter pilots, special ops forces and other elite performers; Peter Pronovost, MD, of Johns Hopkins Medicine, and its Armstrong Institute for Patient Safety and Quality, will discuss organizational structure and process factors for improving cardiac surgery quality and safety; Don Goldmann, MD, of the Institute for Healthcare Improvement, will provide insights on the impact of the Medicare Access and CHIP Reauthorization Act (MACRA) on the delivery of cardiovascular care; David M. Shahian, MD, of Harvard Medical School, who oversees the Society of Thoracic Surgeons’ database, will talk about meaningful outcome measures in cardiac surgery; and Alex B. Haynes, MD, of Massachusetts General Hospital, will review surgical checklists and their association with decreased morbidity and mortality.
“This session should be of interest to all AATS meeting attendees as we navigate the ever-changing landscape of health care delivery,” Ms. Hoercher said. “The information presented will be something the audience can’t easily obtain elsewhere,” added Dr. Whitman.
“I hope many of the AATS meeting attendees, both surgeons and non-surgeons, will join us at what will be an excellent educational opportunity,” Ms. Hoercher said.
Experts from a variety of disciplines will come together to discuss topics related to preoperative, perioperative and postoperative care for cardiovascular surgery patients during Sunday’s full-day symposium, “Improving Systems of Care, Quality and Safety.”
“The AATS recognizes that the delivery of high-quality, patient-centered cardiovascular care is best achieved through the use of proficient multidisciplinary teams,” said course co-chair Katherine J. Hoercher, RN, FAHA, of the Cleveland Clinic. “This symposium, with its focus on interprofessional education, is an important mechanism for developing team-based care competencies that form the underpinning of high-functioning teams.”
By bringing together faculty and learners from multiple cardiovascular surgery professions, attendees can learn how effective collaboration and utilization of standardized processes can improve outcomes in systems of care, quality and safety, Ms. Hoercher said.
“The session should provide the audience with nuts-and-bolts approaches to everyday problems through a global view of the difficulties we face in consistently delivering the highest quality medical care,” added course co-chair Glenn J.R. Whitman, MD, of The Johns Hopkins Hospital.
The morning session “will address a variety of common problems that we all face in managing cardiac surgical patients,” Dr. Whitman said. “The topics are varied and include, for example, informed consent, optimizing preoperative surgical readiness, as well as specific aspects of postoperative management such as goal-directed resuscitation and approach to the cardiac arrest patient.”
Included in the morning presentations are four talks on a team approach to minimizing transfusions, including preadmission use of epoetin; preoperative evaluation and intraoperative management; perioperative management of blood preservation; and risks, recognition and management of post cardiopulmonary bypass hemorrhage.
The afternoon portion of the symposium “will continue the morning objective and focus on specific postoperative morbidities, including hyperglycemia, perioperative myocardial infarctions and strokes,” Dr. Whitman said.
The session will conclude with presentations from five nationally-renowned speakers. Douglas R. Johnston, MD, of the Cleveland Clinic, will discuss what surgical teams can learn from fighter pilots, special ops forces and other elite performers; Peter Pronovost, MD, of Johns Hopkins Medicine, and its Armstrong Institute for Patient Safety and Quality, will discuss organizational structure and process factors for improving cardiac surgery quality and safety; Don Goldmann, MD, of the Institute for Healthcare Improvement, will provide insights on the impact of the Medicare Access and CHIP Reauthorization Act (MACRA) on the delivery of cardiovascular care; David M. Shahian, MD, of Harvard Medical School, who oversees the Society of Thoracic Surgeons’ database, will talk about meaningful outcome measures in cardiac surgery; and Alex B. Haynes, MD, of Massachusetts General Hospital, will review surgical checklists and their association with decreased morbidity and mortality.
“This session should be of interest to all AATS meeting attendees as we navigate the ever-changing landscape of health care delivery,” Ms. Hoercher said. “The information presented will be something the audience can’t easily obtain elsewhere,” added Dr. Whitman.
“I hope many of the AATS meeting attendees, both surgeons and non-surgeons, will join us at what will be an excellent educational opportunity,” Ms. Hoercher said.
ACC/AHA vs. USPSTF statin guidelines
The American College of Cardiology/American Heart Association guidelines for preventive statin therapy would cover an estimated 9.3 million more U.S. adults than would the U.S. Preventive Services Task Force guidelines, according to a report published online April 18 in JAMA.
Most of this difference can be attributed to younger adults and those with diabetes, for whom the ACC/AHA guidelines recommend statin therapy but the USPSTF guidelines do not, said Neha J. Pagidipati, MD, of the Duke Clinical Research Institute, Durham, N.C., and her associates.
They compared the proportion of patients who would be eligible for primary prevention statin therapy according to these two sets of guidelines by applying the eligibility criteria to a sample of 3,416 participants in the nationally representative 2009-2014 National Health and Nutrition Examination Survey (NHANES). These participants were 40-75 years of age, were free of cardiovascular disease, and had triglyceride levels of 400 mg/dL or less. A total of 747 (21.5%) were taking lipid-lowering medication at the time of the survey.
If the USPSTF guidelines (JAMA. 2016 Nov 15;316[19]:1997-2007)were fully implemented in this cohort, 15.8% more of the participants would be taking statin therapy. In contrast, if the ACC/AHA guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-4) were fully implemented in this cohort, 24.3% more would be taking statins.
A total of 8.9% of the cohort would be recommended for statin therapy under the ACC/AHA guidelines but not the USPSTF guidelines. Most of this discrepancy could be attributed to the youngest adults and to those with diabetes. The ACC/AHA guidelines were much more likely to recommend statins to patients aged 40-59 years. Such patients have a relatively low 10-year risk of cardiovascular events (7.0%) but a much higher longer-term risk of 34.6% at 30 years, the investigators said.
“Given that half of all [cardiovascular] events in men and one-third in women occur before age 65 years, reliance on 10-year risk alone may miss many younger individuals who could potentially benefit from long-term statin therapy,” they noted (JAMA 2017 Apr 18. doi: 10.1001/jama.2017.3416).
Further analysis broke down which adults the ACC/AHA guidelines covered, in contrast to the USPSTF guidelines: younger male smokers, younger men with dyslipidemia, younger men with high LDL-cholesterol levels, younger women with obesity, and older men who didn’t have high LDL-cholesterol levels.
Extrapolating these findings to the general U.S. population, “there could be an estimated 17.1 million vs 26.4 million U.S. adults with a new recommendation for statin therapy, based on the USPSTF recommendations vs the ACC/AHA guideline recommendations, respectively – an estimated difference of 9.3 million individuals,” Dr. Pagidipati and her associates wrote.
“Alternative approaches to augmenting risk-based cholesterol guidelines, including those that explicitly incorporate potential benefit of therapy, should be considered,” they added.
This study was supported by the Duke Clinical Research Institute. Dr. Pagidipati reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.
The American College of Cardiology/American Heart Association guidelines for preventive statin therapy would cover an estimated 9.3 million more U.S. adults than would the U.S. Preventive Services Task Force guidelines, according to a report published online April 18 in JAMA.
Most of this difference can be attributed to younger adults and those with diabetes, for whom the ACC/AHA guidelines recommend statin therapy but the USPSTF guidelines do not, said Neha J. Pagidipati, MD, of the Duke Clinical Research Institute, Durham, N.C., and her associates.
They compared the proportion of patients who would be eligible for primary prevention statin therapy according to these two sets of guidelines by applying the eligibility criteria to a sample of 3,416 participants in the nationally representative 2009-2014 National Health and Nutrition Examination Survey (NHANES). These participants were 40-75 years of age, were free of cardiovascular disease, and had triglyceride levels of 400 mg/dL or less. A total of 747 (21.5%) were taking lipid-lowering medication at the time of the survey.
If the USPSTF guidelines (JAMA. 2016 Nov 15;316[19]:1997-2007)were fully implemented in this cohort, 15.8% more of the participants would be taking statin therapy. In contrast, if the ACC/AHA guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-4) were fully implemented in this cohort, 24.3% more would be taking statins.
A total of 8.9% of the cohort would be recommended for statin therapy under the ACC/AHA guidelines but not the USPSTF guidelines. Most of this discrepancy could be attributed to the youngest adults and to those with diabetes. The ACC/AHA guidelines were much more likely to recommend statins to patients aged 40-59 years. Such patients have a relatively low 10-year risk of cardiovascular events (7.0%) but a much higher longer-term risk of 34.6% at 30 years, the investigators said.
“Given that half of all [cardiovascular] events in men and one-third in women occur before age 65 years, reliance on 10-year risk alone may miss many younger individuals who could potentially benefit from long-term statin therapy,” they noted (JAMA 2017 Apr 18. doi: 10.1001/jama.2017.3416).
Further analysis broke down which adults the ACC/AHA guidelines covered, in contrast to the USPSTF guidelines: younger male smokers, younger men with dyslipidemia, younger men with high LDL-cholesterol levels, younger women with obesity, and older men who didn’t have high LDL-cholesterol levels.
Extrapolating these findings to the general U.S. population, “there could be an estimated 17.1 million vs 26.4 million U.S. adults with a new recommendation for statin therapy, based on the USPSTF recommendations vs the ACC/AHA guideline recommendations, respectively – an estimated difference of 9.3 million individuals,” Dr. Pagidipati and her associates wrote.
“Alternative approaches to augmenting risk-based cholesterol guidelines, including those that explicitly incorporate potential benefit of therapy, should be considered,” they added.
This study was supported by the Duke Clinical Research Institute. Dr. Pagidipati reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.
The American College of Cardiology/American Heart Association guidelines for preventive statin therapy would cover an estimated 9.3 million more U.S. adults than would the U.S. Preventive Services Task Force guidelines, according to a report published online April 18 in JAMA.
Most of this difference can be attributed to younger adults and those with diabetes, for whom the ACC/AHA guidelines recommend statin therapy but the USPSTF guidelines do not, said Neha J. Pagidipati, MD, of the Duke Clinical Research Institute, Durham, N.C., and her associates.
They compared the proportion of patients who would be eligible for primary prevention statin therapy according to these two sets of guidelines by applying the eligibility criteria to a sample of 3,416 participants in the nationally representative 2009-2014 National Health and Nutrition Examination Survey (NHANES). These participants were 40-75 years of age, were free of cardiovascular disease, and had triglyceride levels of 400 mg/dL or less. A total of 747 (21.5%) were taking lipid-lowering medication at the time of the survey.
If the USPSTF guidelines (JAMA. 2016 Nov 15;316[19]:1997-2007)were fully implemented in this cohort, 15.8% more of the participants would be taking statin therapy. In contrast, if the ACC/AHA guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-4) were fully implemented in this cohort, 24.3% more would be taking statins.
A total of 8.9% of the cohort would be recommended for statin therapy under the ACC/AHA guidelines but not the USPSTF guidelines. Most of this discrepancy could be attributed to the youngest adults and to those with diabetes. The ACC/AHA guidelines were much more likely to recommend statins to patients aged 40-59 years. Such patients have a relatively low 10-year risk of cardiovascular events (7.0%) but a much higher longer-term risk of 34.6% at 30 years, the investigators said.
“Given that half of all [cardiovascular] events in men and one-third in women occur before age 65 years, reliance on 10-year risk alone may miss many younger individuals who could potentially benefit from long-term statin therapy,” they noted (JAMA 2017 Apr 18. doi: 10.1001/jama.2017.3416).
Further analysis broke down which adults the ACC/AHA guidelines covered, in contrast to the USPSTF guidelines: younger male smokers, younger men with dyslipidemia, younger men with high LDL-cholesterol levels, younger women with obesity, and older men who didn’t have high LDL-cholesterol levels.
Extrapolating these findings to the general U.S. population, “there could be an estimated 17.1 million vs 26.4 million U.S. adults with a new recommendation for statin therapy, based on the USPSTF recommendations vs the ACC/AHA guideline recommendations, respectively – an estimated difference of 9.3 million individuals,” Dr. Pagidipati and her associates wrote.
“Alternative approaches to augmenting risk-based cholesterol guidelines, including those that explicitly incorporate potential benefit of therapy, should be considered,” they added.
This study was supported by the Duke Clinical Research Institute. Dr. Pagidipati reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: The ACC/AHA guidelines for preventive statin therapy would cover an estimated 9 million more U.S. adults than the USPSTF guidelines.
Major finding: 8.9% of the 3,416 NHANES participants would be recommended for statin therapy under the ACC/AHA guidelines but not the USPSTF guidelines.
Data source: An analysis of the difference in eligibility for preventive statin therapy between two sets of guidelines using data for 3,416 participants in the nationally representative NHANES survey in 2009-2014.
Disclosures: This study was supported by the Duke Clinical Research Institute. Dr. Pagidipati reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.
Medial Patellofemoral Ligament Repair
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Beautiful Boston Welcomes AATS Members
Boston offers its visitors an unrivaled combination of American history, culture, and entertainment. Theater and performance art make an active home in the city and its environs, and there is much to do and see during the AATS Centennial meeting this year,
In entertainment venues, on Saturday, April 29, the acclaimed Alvin Ailey Dance Company will be beforming at the Wang Theater, and Sunday, April 30, the Celebrity Series of Boston will present an evening with Broadway and television star, Kristin Chenoweth at Boston Symphony Hall; and throughout the meeting, the Boston Lyric Opera will be performing Mozaert’s “The Marriage of Figaro.”
Sports fans need not be disappointed, especially baseball fans, as Fenway Park, the home of the Boston Red Sox, will be hosting the Chicago Cubs, April 28-30, and the Baltimore Orioles, May 1-4.
As every American knows, Boston has a very rich history. The Freedom Trail links 16 historically significant sites of the Revolutionary era by a red brick path and has organized walking tours. There are also popular tours of Boston breweries and a special Saturday Chocolate Walking Tour of the Back Bay.
Boston also has many world-renowned museums ranging from the Museum of Fine arts to the Museum of Science (where the delightful Butterfly Garden opens April 28), the Computer Museum, and the JFK Library and Museum. During the meeting, the Museum of Fine Arts is featuring a special ongoing exhibit of the legendary French artist, Henri Matisse. The New England Aquarium on April 28 begins a special focus on Tentacles, featuring the giant Pacific octopus and its many relations.
For more active artistic fare, the annual meeting coincides with the popular Boston ArtWeek, an award-winning festival featuring more than 150 unique and creative experiences that are participatory, interactive, or offer behind-the-scenes access to artists or the creative process. It is located at 270 Tremont Street
Photowalks Tours offers walking tours with guides of the various landmakrs and neighborhoods of Boston, allowing photo opportunites, exercise, and the chance to learn about city history and culture, including such locations as Beacon Hill and the Waterfront.
For those with a macabre sense of history, take the “Ghosts & Gravestones” tour, through Boston’s most chilling sites and haunted places. The tour is performed with the cooperation of the city’s Historic Burying Grounds Initiative, and a portion of the ticket price goes toward maintaining Boston’s historic cemeteries.
For more standard historical fare, two famous ships are available for touriing: the USS Constitution (“Old Ironsides,” the oldest commissioned warship afloat in the world), and the Boston Tea Party Ship.
For foodies, and those of us who just have to eat, the city has restaurants for every taste although seafood, of course, is the specialty cuisine. For the best in dining, visit www.bostonmagazine.com/best-restaurants-in-boston.
For more info and links to restaurants, shopping, and suggestions on things to do in the Boston area during your visit, go to www.BostonUSA.com, or www.bostoncalendar.com.
Boston offers its visitors an unrivaled combination of American history, culture, and entertainment. Theater and performance art make an active home in the city and its environs, and there is much to do and see during the AATS Centennial meeting this year,
In entertainment venues, on Saturday, April 29, the acclaimed Alvin Ailey Dance Company will be beforming at the Wang Theater, and Sunday, April 30, the Celebrity Series of Boston will present an evening with Broadway and television star, Kristin Chenoweth at Boston Symphony Hall; and throughout the meeting, the Boston Lyric Opera will be performing Mozaert’s “The Marriage of Figaro.”
Sports fans need not be disappointed, especially baseball fans, as Fenway Park, the home of the Boston Red Sox, will be hosting the Chicago Cubs, April 28-30, and the Baltimore Orioles, May 1-4.
As every American knows, Boston has a very rich history. The Freedom Trail links 16 historically significant sites of the Revolutionary era by a red brick path and has organized walking tours. There are also popular tours of Boston breweries and a special Saturday Chocolate Walking Tour of the Back Bay.
Boston also has many world-renowned museums ranging from the Museum of Fine arts to the Museum of Science (where the delightful Butterfly Garden opens April 28), the Computer Museum, and the JFK Library and Museum. During the meeting, the Museum of Fine Arts is featuring a special ongoing exhibit of the legendary French artist, Henri Matisse. The New England Aquarium on April 28 begins a special focus on Tentacles, featuring the giant Pacific octopus and its many relations.
For more active artistic fare, the annual meeting coincides with the popular Boston ArtWeek, an award-winning festival featuring more than 150 unique and creative experiences that are participatory, interactive, or offer behind-the-scenes access to artists or the creative process. It is located at 270 Tremont Street
Photowalks Tours offers walking tours with guides of the various landmakrs and neighborhoods of Boston, allowing photo opportunites, exercise, and the chance to learn about city history and culture, including such locations as Beacon Hill and the Waterfront.
For those with a macabre sense of history, take the “Ghosts & Gravestones” tour, through Boston’s most chilling sites and haunted places. The tour is performed with the cooperation of the city’s Historic Burying Grounds Initiative, and a portion of the ticket price goes toward maintaining Boston’s historic cemeteries.
For more standard historical fare, two famous ships are available for touriing: the USS Constitution (“Old Ironsides,” the oldest commissioned warship afloat in the world), and the Boston Tea Party Ship.
For foodies, and those of us who just have to eat, the city has restaurants for every taste although seafood, of course, is the specialty cuisine. For the best in dining, visit www.bostonmagazine.com/best-restaurants-in-boston.
For more info and links to restaurants, shopping, and suggestions on things to do in the Boston area during your visit, go to www.BostonUSA.com, or www.bostoncalendar.com.
Boston offers its visitors an unrivaled combination of American history, culture, and entertainment. Theater and performance art make an active home in the city and its environs, and there is much to do and see during the AATS Centennial meeting this year,
In entertainment venues, on Saturday, April 29, the acclaimed Alvin Ailey Dance Company will be beforming at the Wang Theater, and Sunday, April 30, the Celebrity Series of Boston will present an evening with Broadway and television star, Kristin Chenoweth at Boston Symphony Hall; and throughout the meeting, the Boston Lyric Opera will be performing Mozaert’s “The Marriage of Figaro.”
Sports fans need not be disappointed, especially baseball fans, as Fenway Park, the home of the Boston Red Sox, will be hosting the Chicago Cubs, April 28-30, and the Baltimore Orioles, May 1-4.
As every American knows, Boston has a very rich history. The Freedom Trail links 16 historically significant sites of the Revolutionary era by a red brick path and has organized walking tours. There are also popular tours of Boston breweries and a special Saturday Chocolate Walking Tour of the Back Bay.
Boston also has many world-renowned museums ranging from the Museum of Fine arts to the Museum of Science (where the delightful Butterfly Garden opens April 28), the Computer Museum, and the JFK Library and Museum. During the meeting, the Museum of Fine Arts is featuring a special ongoing exhibit of the legendary French artist, Henri Matisse. The New England Aquarium on April 28 begins a special focus on Tentacles, featuring the giant Pacific octopus and its many relations.
For more active artistic fare, the annual meeting coincides with the popular Boston ArtWeek, an award-winning festival featuring more than 150 unique and creative experiences that are participatory, interactive, or offer behind-the-scenes access to artists or the creative process. It is located at 270 Tremont Street
Photowalks Tours offers walking tours with guides of the various landmakrs and neighborhoods of Boston, allowing photo opportunites, exercise, and the chance to learn about city history and culture, including such locations as Beacon Hill and the Waterfront.
For those with a macabre sense of history, take the “Ghosts & Gravestones” tour, through Boston’s most chilling sites and haunted places. The tour is performed with the cooperation of the city’s Historic Burying Grounds Initiative, and a portion of the ticket price goes toward maintaining Boston’s historic cemeteries.
For more standard historical fare, two famous ships are available for touriing: the USS Constitution (“Old Ironsides,” the oldest commissioned warship afloat in the world), and the Boston Tea Party Ship.
For foodies, and those of us who just have to eat, the city has restaurants for every taste although seafood, of course, is the specialty cuisine. For the best in dining, visit www.bostonmagazine.com/best-restaurants-in-boston.
For more info and links to restaurants, shopping, and suggestions on things to do in the Boston area during your visit, go to www.BostonUSA.com, or www.bostoncalendar.com.