User login
Immune Thrombocytopenia
Introduction
Immune thrombocytopenia (ITP) is a common acquired autoimmune disease characterized by low platelet counts and an increased risk of bleeding. The incidence of ITP is approximately 3.3 per 100,000 adults.1 There is considerable controversy about all aspects of the disease, with little “hard” data on which to base decisions given the lack of randomized clinical trials to address most clinical questions. This article reviews the presentation and diagnosis of ITP and its treatment options and discusses management of ITP in specific clinical situations.
Pathogenesis and Epidemiology
ITP is caused by autoantibodies binding to platelet surface proteins, most often to the platelet receptor GP IIb/IIIa.2-4 These antibody-coated platelets then bind to Fc receptors in macrophages and are removed from circulation. The initiating event in ITP is unknown. It is speculated that the patient responds to a viral or bacterial infection by creating antibodies which cross-react with the platelet receptors. Continued exposure to platelets perpetuates the immune response. ITP that occurs in childhood appears to be an acute response to viral infection and usually resolves. ITP in adults may occur in any age group but is seen especially in young women.
Despite the increased platelet destruction that occurs in ITP, the production of new platelets often is not significantly increased. This is most likely due to lack of an increase in thrombopoietin, the predominant platelet growth factor.5
It had been thought that most adult patients who present with ITP go on to have a chronic course, but more recent studies have shown this is not the case. In modern series the percentage of patients who are “cured” with steroids ranges from 30% to 70%.6–9 In addition, it has been appreciated that even in patients with modest thrombocytopenia, no therapy is required if the platelet count remains higher than 30 × 103/µL. However, this leaves a considerable number of patients who will require chronic therapy.
Clinical Presentation
Presentation can range from a symptomatic patient with low platelets found on a routine blood count to a patient with massive bleeding. Typically, patients first present with petechiae (small bruises 1 mm in size) on the shins. True petechiae are seen only in severe thrombocytopenia. Patients will also report frequent bruising and bleeding from the gums. Patients with very low platelet counts will notice “wet purpura,” which is characterized by blood-filled bullae in the oral cavity. Life-threatening bleeding is a very unusual presenting sign unless other problems (trauma, ulcers) are present. The physical examination is only remarkable for stigmata of bleeding such as the petechiae. The presence of splenomegaly or lymphadenopathy weighs strongly against a diagnosis of ITP. Many patients with ITP will note fatigue when their platelets counts are lower.10
Diagnosis
Extremely low platelet counts with a normal blood smear and an otherwise healthy patient are diagnostic of ITP. The platelet count cutoff for considering ITP is 100 × 103/µL as the majority of patients with counts in the 100 to 150 × 103/µL range will not develop greater thrombocytopenia.11 Also, the platelet count decreases with age (9 × 103/µL per decade in one study), and this also needs to be factored into the evaluation.12 The finding of relatives with ITP should raise suspicion for congenital thrombocytopenia.13 One should question the patient carefully about drug exposure (see Drug-Induced Thrombocytopenia), especially about over-the-counter medicines, “natural” remedies, or recreational drugs.
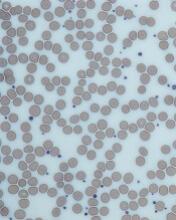
There is no laboratory test that rules in ITP; rather, it is a diagnosis of exclusion. The blood smear should be carefully examined for evidence of microangiopathic hemolytic anemias (schistocytes), bone marrow disease (blasts, teardrop cells), or any other evidence of a primary bone marrow disease. In ITP, the platelets can be larger than normal, but finding some platelets the size of red cells should raise the issue of congenital thrombocytopenia.14 Pseudo-thrombocytopenia, which is the clumping of platelets due to a reaction to the EDTA anticoagulant in the tube, should be excluded. The diagnosis is established by drawing the blood in a citrated (blue-top) tube to perform the platelet count. There is no role for antiplatelet antibody assay because this test lacks sensitivity and specificity. In a patient without a history of autoimmune disease or symptoms, empiric testing for autoimmune disease is not recommended.
Patients who present with ITP should be tested for both HIV and hepatitis C infection.15,16 These are the most common viral causes of secondary ITP, and both have prognostic and treatment implications. Some authorities also recommend checking thyroid function as hypothyroidism can present or aggravate the thrombocytopenia.
The role of bone marrow examination is controversial.17 Patients with a classic presentation of ITP (young woman, normal blood smear) do not require a bone marrow exam before therapy is initiated, although patients who do not respond to initial therapy should have a bone marrow aspiration. The rare entity amegakaryocytic thrombocytopenia can present with a clinical picture similar to that of ITP, but amegakaryocytic thrombocytopenia will not respond to steroids. Bone marrow aspiration reveals the absence of megakaryocytes in this entity. It is rare, however, that another hematologic disease is diagnosed in patients with a classic clinical presentation of ITP.
In the future, measurement of thrombopoietin and reticulated platelets may provide clues to the diagnosis.4 Patients with ITP paradoxically have normal or only mildly elevated thrombopoietin levels. The finding of a significantly elevated thrombopoietin level should lead to questioning of the diagnosis. One can also measure “reticulated platelets,” which are analogous to red cell reticulocytes. Patients with ITP (or any platelet destructive disorders) will have high levels of reticulated platelets. These tests are not recommended for routine evaluation, but may be helpful in difficult cases.
Treatment
In general, therapy in ITP should be guided by the patient’s signs of bleeding and not by unquestioning adherence to measuring platelet levels,15 as patients tolerate thrombocytopenia well. It is unusual to have life-threatening bleeding with platelet counts greater than 5 × 103/µL in the absence of mechanical lesions. Despite the low platelet count in patients with ITP, the overall mortality is estimated to be only 0.3% to 1.3%.18 It is sobering that in one study the rate of death from infections was twice as high as that from bleeding.19 Rare patients will have antibodies that interfere with the function of the platelet, and these patients can have profound bleeding with only modestly lowered platelet counts.20 A suggested cut-off for treating newly diagnosed patients is 30 × 103/µL.21
Initial Therapy
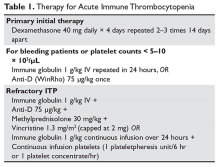
The primary therapy of ITP is glucocorticoids, either prednisone or dexamethasone. In the past prednisone at a dose of 60 to 80 mg/day was started at the time of diagnosis (Table 1).
For rapid induction of a response, there are 2 options. A single dose of intravenous immune globulin (IVIG) at 1 g/kg or intravenous anti-D immunoglobulin (anti-D) at 50 to 75 µg/kg can induce a response in more than 80% of patients in 24 to 48 hours.21,24 IVIG has several drawbacks. It can cause aseptic meningitis, and in patients with vascular disease the increased viscosity can induce ischemia. There is also a considerable fluid load delivered with the IVIG, and it needs to be given over several hours.
The use of anti-D is limited to Rh-positive patients who have not had a splenectomy. It should not be used in patients who are Coombs positive due to the risk of provoking more hemolysis. Rarely anti-D has been reported to cause a severe hemolytic disseminated intravascular coagulation syndrome (1:20,000 patients), which has led to restrictions in its use.25 Although the drug can be rapidly given over 15 minutes, due to these concerns current recommendations are now to observe patients for 8 hours after their dose and to perform a urine dipstick test for blood at 2, 4, and 8 hours. Concerns about this rare but serious side effect have led to a dramatic decrease in the use of anti-D.
For patients who are severely thrombocytopenic and do not respond to initial therapy, there are 2 options for raising the platelet counts. One is to use a combination of IVIG, methylprednisolone, vincristine, and/or anti-D.26 The combination of IVIG and anti-D may be synergistic since these agents block different Fc receptors. A response of 71% has been reported for this 3- or 4-drug combination in a series of 35 patients.26 The other option is to treat with a continuous infusion of platelets (1 unit over 6 hours) and IVIG 1 g/kg for 24 hours. Response rates of 62.7% have been reported with this combination, and this rapid rise in platelets can allow time for other therapies to take effect.27,28
Patients with severe thrombocytopenia who relapse with reduction of steroids or who do not respond to steroids have several options for further management. Repeated doses of IVIG can transiently raise the platelet count, and some patients may only need several courses of therapy over the course of many months. One study showed that 60% of patients could delay or defer therapy by receiving multiple doses of anti-D. However, 30% of patients did eventually receive splenectomy and 20% of patients required ongoing therapy with anti-D.29 In a randomized trial comparing early use of anti-D to steroids to avoid splenectomy, there was no difference in splenectomy rate (38% versus 42%).30 Finally, an option as mentioned above is to try a 6-month course of pulse dexamethasone 40 mg/day for 4 days, repeated every 28 days.
Options for Refractory ITP
There are multiple options for patients who do not respond to initial ITP therapies. These can be divided into several broad groups: curative therapies (splenectomy and rituximab), thrombopoietin receptor agonists, and anecdotal therapies.
Splenectomy
In patients with severe thrombocytopenia who do not respond or who relapse with lower doses of prednisone, splenectomy should be strongly considered. Splenectomy will induce a good response in 60% to 70% of patients and is durable in most patients. In 2 recently published reviews of splenectomy, the complete response rate was 67% and the total response rate was 88% to 90%%.8,31 Between 15% and 28% of patients relapsed over 5 years, with most recurrences occurring in the first 2 years. Splenectomy carries a short-term surgical risk, and the life-long risk of increased susceptibility to overwhelming sepsis is discussed below. However, the absolute magnitude of these risks is low and is often lower than the risks of continued prednisone therapy or of continued cytotoxic therapy.
Timing of splenectomy depends on the patient’s presentation. Most patients should be given a 6-month trial of steroids or other therapies before proceeding to splenectomy.31 However, patients who persist with severe thrombocytopenia despite initial therapies or who are suffering intolerable side effects from therapy should be considered sooner for splenectomy.31 In the George review, multiple factors such as responding to IVIG were found not to be predictive of response to splenectomy.8
Method of splenectomy appears not to matter.21 Rates of finding accessory spleens are just as high or higher with laparoscopic splenectomy and the patient can recover faster. In patients who are severely thrombocytopenic, open splenectomy can allow for quicker control of the vascular access of the spleen.
Rates of splenectomy in recent years have decreased for many reasons,32 including the acceptance of lower platelet counts in asymptomatic patients and the availability of alternative therapies such as rituximab. In addition, despite abundant data for good outcomes, there is a concern that splenectomy responses are not durable. Although splenectomy will not cure every patient with ITP, splenectomy is the therapy with the most patients, the longest follow-up, and the most consistent rate of cure, and it should be discussed with every ITP patient who does not respond to initial therapy and needs further treatment.
The risk of overwhelming sepsis varies by indications for splenectomy but appears to be about 1%.33,34 The use of pneumococcal vaccine and recognition of this syndrome have helped reduce the risk. Asplenic patients need to be counseled about the risk of overwhelming infections, should be vaccinated for pneumococcus, meningococcus, and Haemophilus influenzae, and should wear an ID bracelet.35–37 Patients previously vaccinated for pneumococcus should be re-vaccinated every 3 to 5 years. The role of prophylactic antibiotics in adults is controversial, but patients under the age of 18 should be on penicillin VK 250 mg orally twice daily.
Rituximab
Rituximab has been shown to be very active in ITP. Most studies used the standard dose of 375 mg/m2 weekly for 4 weeks, but other studies have shown that 1000 mg twice 14 days apart (ie, on days 1 and 15) resulted in the same response rate and may be more convenient for patients.38,39 The response time can vary, with patients either showing a rapid response or requiring up to 8 weeks for their counts to go up. Although experience is limited, the response seems to be durable, especially in those patients whose counts rise higher than 150 × 103/µL; in patients who relapse, a response can be re-induced with a repeat course. Overall the response rate for rituximab is about 60%, but only approximately 20% to 40% of patients will remain in long-term remission.40–42 There is no evidence yet that “maintenance” therapy or monitoring CD19/CD20 cells can help further the duration of remission.
Whether to give rituximab pre- or post-splenectomy is also uncertain. An advantage of presplenectomy rituximab is that many patients will achieve remission, delaying the need for surgery. Also, rituximab is a good option for patients whose medical conditions put them at high risk for complications with splenectomy. However, it is unknown whether rituximab poses any long-term risks, while the long-term risks of splenectomy are well-defined. Rituximab is the only curative option left for patients who have failed splenectomy and is a reasonable option for these patients.
There is an intriguing trial in which patients were randomly assigned to dexamethasone alone versus dexamethasone plus rituximab upon presentation with ITP; those who were refractory to dexamethasone alone received salvage therapy with dexamethasone plus rituximab.43 The dexamethasone plus rituximab group had an overall higher rate of sustained remission at 6 months than the dexamethasone group, 63% versus 36%. Interestingly, patients who failed their first course of dexamethasone but then were “salvaged” with dexamethasone/rituximab had a similar overall response rate of 56%, suggesting that saving the addition of rituximab for steroid failures may be an effective option.
Although not “chemotherapy,” rituximab is not without risks. Patients can develop infusion reactions, which can be severe in 1% to 2% of patients. In a meta-analysis the fatal reaction rate was 2.9%.40 Patients with chronic hepatitis B infections can experience reactivation with rituximab, and thus all patients should be screened before treatment. Finally, the very rare but devastating complication of progressive multifocal leukoencephalopathy has been reported.
Thrombopoietin Receptor Agonists
Although patients with ITP have low platelet counts, studies starting with Dameshek have shown that these patients also have reduced production of platelets.44 Despite the very low circulating platelet count, levels of the platelet growth factor thrombopoietin (TPO) are not raised.45 Seminal studies with recombinant TPO in the 1990s showed that ITP patients responded to thrombopoietin-stimulating protein, but the formation of anti-TPO antibodies halted trials with the first generation of these agents. Two TPO receptor agonists (TPO-RA) are approved for use in patients with ITP.
Romiplostim. Romiplostim is a peptibody, a combination of a peptide that binds and stimulates the TPO receptor and an Fc domain to extend its half-life.46 It is administered in a weekly subcutaneous dose starting at 1 to 3 µg/kg. Use of romiplostim in ITP patients produces a response rate of 80% to 88%, with 87% of patients being able to wean off or decrease other anti-ITP medications.47 In a long-term extension study, the response was again high at 87%.48 These studies have also shown a reduced incidence of bleeding.
The major side effect of romiplostim seen in clinical trials was marrow reticulin formation, which occurred in up to 5.6% of patients.47,48 The clinical course in these patients is the development of anemia and a myelophthisic blood smear with teardrop cells and nucleated red cells. These changes appear to reverse with cessation of the drug. The bone marrow shows increased reticulin formation but rarely, if ever, shows the collagen deposition seen with primary myelofibrosis.
Thrombosis has also been seen, with a rate of 0.08 to 0.1 cases per 100 patient-weeks,49 but it remains unclear if this is due to the drug, part of the natural history of ITP, or expected complications in older patients undergoing any type of medical therapy. Surprisingly, despite the low platelet counts, patients with ITP in one study had double the risk of venous thrombosis, demonstrating that ITP itself can be a risk factor for thrombosis.50 These trials have shown no long-term concerns for other clinical problems such as liver disease.
Eltrombopag. The other available TPO-RA is eltrombopag,51 an oral agent that stimulates the TPO receptor by binding the transmembrane domain and activating it. The drug is given orally starting at 50 mg/day (25 mg for patients of Asian ancestry or with liver disease) and can be dose escalated to 75 mg/day. The drug needs to be taken on an empty stomach. Eltrombopag has been shown to be effective in chronic ITP, with response rates of 59% to 80% and reduction in use of rescue medications.47,51,52 As with romiplostim, the incidence of bleeding was also decreased with eltrombopag in these trials.47,51
Clinical trials demonstrated that eltrombopag shares with romiplostim the risk for marrow fibrosis. A side effect unique to eltrombopag observed in these trials was a 3% to 7% incidence of elevated liver function tests.21,52 These abnormal findings appeared to resolve in most patients, but liver function tests need to be monitored in patients receiving eltrombopag.
Clinical use. The clearest indication for the use of TPO-RAs is in patients who have failed several therapies and remain symptomatic or are on intolerable doses of other medications such as prednisone. The clear benefits are their relative safety and high rates of success. The main drawback of TPO-RAs is the need for continuing therapy as the platelet count will return to baseline shortly after these agents are stopped. Currently there is no clear indication for one medication over the other. The advantages of romiplostim are great flexibility in dosing (1–10 µg/kg week) and no concerns about drug interaction. The current drawback of romiplostim is the Food and Drug Administration’s requirement for patients to receive the drug from a clinic and not at home. Eltrombopag offers the advantage of oral use, but it has a limited dose range and potential for drug interactions. Both agents have been associated with marrow reticulin formation, although in clinical use this risk appears to be very low.53
Other Options
In the literature there are numerous options for the treatment of ITP.54,55 Most of these studies are anecdotal, enrolled small number of patients, and sometimes included patients with mild thrombocytopenia, but these therapeutic options can be tried in patients who are refractory to standard therapies and have bleeding. The agents with the greatest amount of supporting data are danazol, vincristine, azathioprine, cyclophosphamide, and fostamatinib.
Danazol 200 mg 4 times daily is thought to downregulate the macrophage Fc receptor. The onset of action may be delayed and a therapeutic trial of up to 4 to 6 months is advised. Danazol is very effective in patients with antiphospholipid antibody syndrome who develop ITP and may be more effective in premenopausal women.56 Once a response is seen, danazol should be continued for 6 months and then an attempt to wean the patient off the agent should be made. A partial response can be seen in 70% to 90% of patients, but a complete response is rare.54
Vincristine 1.4 mg/m2 weekly has a low response rate, but if a response is going to occur, it will occur rapidly within 2 weeks. Thus, a prolonged trial of vincristine is not needed; if no platelet rise is seen in several weeks, the drug should be stopped. Again, partial responses are more common than complete response—50% to 63% versus 0% to 6%.54Azathioprine 150 mg orally daily, like danazol, demonstrates a delayed response and requires several months to assess for response. However, 19% to 25% of patients may have a complete response.54 It has been reported that the related agent mycophenolate 1000 mg twice daily is also effective in ITP.57
Cyclophosphamide 1 g/m2 intravenously repeated every 28 days has been reported to have a response rate of up to 40%.58 Although considered more aggressive, this is a standard immunosuppressive dose and should be considered in patients with very low platelet counts. Patients who have not responded to single-agent cyclophosphamide may respond to multi-agent chemotherapy with agents such as etoposide and vincristine plus cyclophosphamide.59
Fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, is currently under investigation for the treatment of ITP.60 This agent prevents phagocytosis of antibody-coated platelets by macrophages. In early studies fostamatinib has been well tolerated at a dose of 150 mg twice daily, with 75% of patients showing a response. Large phase 3 trials are underway, and if the earlier promising results hold up fostamatinib may be a novel option for refractory patients.
A Practical Approach to Refractory ITP
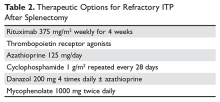
One approach is to divide patients into bleeders, or those with either very low platelet counts (< 5 × 103/µL) or who have had significant bleeding in the past, and nonbleeders, or those with platelet counts above 5 × 103/µL and no history of severe bleeding. Bleeders who do not respond adequately to splenectomy should first start with rituximab since it is not cytotoxic and is the only other “curative” therapy (Table 2).
Nonbleeders should be tried on danazol and other relatively safe agents. If this fails, rituximab or TPO-RAs can be considered. Before one considers cytotoxic therapy, the risk of the therapy must be weighed against the risk posed by the thrombocytopenia. The mortality from ITP is fairly low (5%) and is restricted to patients with severe disease. Patients with only moderate thrombocytopenia and no bleeding are better served with conservative management. There is little justification for the use of continuous steroid therapy in this group of patients given the long-term risks of this therapy.
Special Situations
Surgery
Patients with ITP who need surgery either for splenectomy or for other reasons should have their platelet counts raised to a level greater than 20 to 30 × 103/µL before surgery. Most patients with ITP have increased platelet function and will not have excessive bleeding with these platelet counts. For patients with platelet counts below this level, an infusion of immune globulin or anti-D may rapidly increase the platelet counts. If the surgery is elective, short-term use of TPO-RAs to raise the counts can also be considered.
Pregnancy
Up to 10% of pregnant women will develop low platelet counts during their pregnancy.61,62 The most common etiology is gestational thrombocytopenia, which is an exaggeration of the lowered platelet count seen in pregnancy. Counts may fall as low as 50 × 103/µL at the time of delivery. No therapy is required as the fetus is not affected and the mother does not have an increased risk of bleeding. Pregnancy complications such as HELLP syndrome and thrombotic microangiopathies also present with low platelet counts, but these can be diagnosed by history.61,63
Women with ITP can either develop the disease during pregnancy or have a worsening of the symptoms.64 Counts often drop dramatically during the first trimester. Early management should be conservative with low doses of prednisone to keep the count above 10 × 103/µL.21 Immunoglobulin is also effective,65 but there are rare reports of pulmonary edema. Rarely patients who are refractory will require splenectomy, which may be safely performed in the second trimester. For delivery the count should be greater than 30 × 103/µL and for an epidural greater than 50 × 103/µL.64 There are reports of the use of TPO-RAs in pregnancy, and this can be considered for refractory cases.66
Most controversy centers on management of the delivery. In the past it was feared that fetal thrombocytopenia could lead to intracranial hemorrhage, and Caesarean section was always recommended. It now appears that most cases of intracranial hemorrhage were due to alloimmune thrombocytopenia and not ITP. Furthermore, the nadir of the baby’s platelet count is not at birth but several days after. It appears the safest course is to proceed with a vaginal or C-section delivery determined by obstetrical indications and then immediately check the baby’s platelet count. If the platelet count is low in the neonate, immunoglobulin will raise the count. Since the neonatal thrombocytopenia is due to passive transfer of maternal antibody, the platelet destruction will abate in 4 to 6 weeks.
Pediatric Patients
The incidence of ITP in children is 2.2 to 5.3 per 100,000 children.1 There are several distinct differences in pediatric ITP. Most cases will resolve in weeks, with only a minority of patients transforming into chronic ITP (5%–10%). Also, the rates of serious bleeding are lower in children than in adults, with intracranial hemorrhage rates of 0.1% to 0.5% being seen.67 For most patients with no or mild bleeding, management now is observation alone regardless of platelet count because it is felt that the risks of therapies are higher than the risk of bleeding.21 For patients with bleeding, IVIG, anti-D, or a short course of steroids can be used. Given the risk of overwhelming sepsis, splenectomy is often deferred as long as possible. Rituximab is increasingly being used in children due to concerns about use of agents such a cyclophosphamide or azathioprine in children.68 Abundant data on use of TPO-RAs in children showing high response rates and safety support their use, and these should be considered in refractory ITP before any cytotoxic agent.69–71
Helicobacter Pylori Infection
There has been much interest in the relationship between H. pylori and ITP.16,72,73H. pylori infections have been associated with a variety of autoimmune diseases, and there is a confusing literature on this infection and ITP. Several meta-analyses have shown that eradication of H. pylori will result in an ITP response rate of 20% to 30%, but responses curiously appear to be limited to certain geographic areas such as Japan and Italy but not the United States. In patients with recalcitrant ITP, especially in geographic areas with high incidence, it may be worthwhile to check for H. pylori infection and treat accordingly if positive.
Drug-Induced Thrombocytopenia
Patients with drug-induced thrombocytopenia present with very low (< 10 × 103/µL) platelet counts 1 to 3 weeks after starting a new medication.74–76 In patients with a possible drug-induced thrombocytopenia, the primary therapy is to stop the suspect drug.77 If there are multiple new medications, the best approach is to stop any drug that has been strongly associated with thrombocytopenia (Table 3).74,78,79
Immune globulin, corticosteroids, or intravenous anti‑D have been suggested as useful in drug‑related thrombocytopenia. However, since most of these thrombocytopenic patients recover when the agent is cleared from the body, this therapy is probably not necessary and withholding treatment avoids exposing the patients to the adverse events associated with further therapy.
Evans Syndrome
Evans syndrome is defined as the combination of autoimmune hemolytic anemia (AIHA) and ITP.80,81 These cytopenias can present simultaneously or sequentially. Patients with Evans syndrome are thought to have a more severe disease process, to be more prone to bleeding, and to be more difficult to treat, but the rarity of this syndrome makes this hard to quantify.
The classic clinical presentation of Evans syndrome is severe anemia and thrombocytopenia. Children with Evans syndrome often have complex immunodeficiencies such as autoimmune lymphoproliferative syndrome.82,83 In adults, Evans syndrome most often complicates other autoimmune diseases such as lupus. There are increasing reports of Evans syndrome occurring as a complication of T-cell lymphomas. Often the autoimmune disease can predate the lymphoma diagnosis by months or even years.
In theory the diagnostic approach is straightforward by showing a Coombs-positive hemolytic anemia in the setting of a clinical diagnosis of immune thrombocytopenia. The blood smear will show spherocytes and a diminished platelet count. The presence of other abnormal red cell forms should raise the possibility of an alternative diagnosis. It is unclear how vigorously one should search for other underlying diseases. Many patients will already have the diagnosis of an underlying autoimmune disease. The presence of lymphadenopathy should raise concern for lymphoma.
Initial therapy is high-dose steroids (2 mg/kg/day). IVIG should be added if severe thrombocytopenia is present. Patients who cannot be weaned off prednisone or relapse after prednisone should be considered for splenectomy, although these patients are at higher risk of relapsing.80 Increasingly rituximab is being used with success.84,85 For patients who fail splenectomy and rituximab, aggressive immunosuppression should be considered. Increasing data support the benefits of sirolimus, and this should be considered for refractory patients.86 For patients with Evans syndrome due to underlying lymphoma, antineoplastic therapy often results in prompt resolution of the symptoms. Recurrence of the autoimmune cytopenias often heralds relapse.
1. Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol 2010;85:174–80.
2. McMillan R, Lopez-Dee J, Bowditch R. Clonal restriction of platelet-associated anti-GPIIb/IIIa autoantibodies in patients with chronic ITP. Thromb Haemost 2001;85:821–3.
3. Aster RH, George JN, McMillan R, Ganguly P. Workshop on autoimmune (idiopathic) thrombocytopenic purpura: Pathogenesis and new approaches to therapy. Am J Hematol 1998;58:231–4.
4. Toltl LJ, Arnold DM. Pathophysiology and management of chronic immune thrombocytopenia: focusing on what matters. Br J Haematol 2011;152:52–60.
5. Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1193–211.
6. Pamuk GE, Pamuk ON, Baslar Z, et al. Overview of 321 patients with idiopathic thrombocytopenic purpura. Retrospective analysis of the clinical features and response to therapy. Ann Hematol 2002;81:436–40.
7. Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med 1995;98:436–42.
8. Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004;104:2623–34.
9. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol 2016;136:101–7.
10. Newton JL, Reese JA, Watson SI, et al. Fatigue in adult patients with primary immune thrombocytopenia. Eur J Haematol 2011;86:420–9.
11. Stasi R, Amadori S, Osborn J, et al. Long-term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med 2006;3:e24.
12. Biino G, Balduini CL, Casula L, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica 2011;96:96–101.
13. Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004;103:390–8.
14. Geddis AE, Balduini CL. Diagnosis of immune thrombocytopenic purpura in children. Curr Opin Hematol 2007;14:520–5.
15. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86.
16. Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1275–97.
17. Jubelirer SJ, Harpold R. The role of the bone marrow examination in the diagnosis of immune thrombocytopenic purpura: case series and literature review. Clin Appl Thromb Hemost 2002;8:73–6.
18. George JN. Management of patients with refractory immune thrombocytopenic purpura. J Thromb Haemost 2006;4:1664–72.
19. Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001;97:2549–54.
20. McMillan R, Bowditch RD, Tani P, et al. A non-thrombocytopenic bleeding disorder due to an IgG4- kappa anti-GPIIb/IIIa autoantibody. Br J Haematol 1996;95:747–9.
21. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207.22. Mazzucconi MG, Fazi P, Bernasconi S, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood 2007;109:1401–7.
23. Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood 2016;127:296–302.
24. Newman GC, Novoa MV, Fodero EM, et al. A dose of 75 microg/kg/d of i.v. anti-D increases the platelet count more rapidly and for a longer period of time than 50 microg/kg/d in adults with immune thrombocytopenic purpura. Br J Haematol 2001;112:1076–8.
25. Gaines AR. Acute onset hemoglobinemia and/or hemoglobinuria and sequelae following Rho(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 2000;95:2523–9.
26. Boruchov DM, Gururangan S, Driscoll MC, Bussel JB. Multiagent induction and maintenance therapy for patients with refractory immune thrombocytopenic purpura (ITP). Blood 2007;110:3526–31.
27. Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol 2008;83:122–5.
28. Olson SR, Chu C, Shatzel JJ, Deloughery TG. The “platelet boilermaker”: A treatment protocol to rapidly increase platelets in patients with immune-mediated thrombocytopenia. Am J Hematol 2016;91:E330–1.
29. Cooper N, Woloski BM, Fodero EM, et al. Does treatment with intermittent infusions of intravenous anti-D allow a proportion of adults with recently diagnosed immune thrombocytopenic purpura to avoid splenectomy? Blood 2002;99:1922–7.
30. George JN, Raskob GE, Vesely SK, et al. Initial management of immune thrombocytopenic purpura in adults: a randomized controlled trial comparing intermittent anti-D with routine care. Am J Hematol 2003;74:161–9.
31. Mikhael J, Northridge K, Lindquist K, et al. Short-term and long-term failure of laparoscopic splenectomy in adult immune thrombocytopenic purpura patients: a systematic review. Am J Hematol 2009;84:743–8.
32. Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol 2016;91:E267–72.
33. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664–73.
34. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect 2001;43:182–6.
35. Mileno MD, Bia FJ. The compromised traveler. Infect Dis Clin North Am 1998;12:369–412.
36. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. BMJ 1996;312:430–4.
37. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents 2003;21:181–8.
38. Tran H, Brighton T, Grigg A, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol 2014;167:243–51.
39. Mahevas M, Ebbo M, Audia S, et al. Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol 2013;88:858–61.
40. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007;146:25–33.
41. Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014;124:3228–36.
42. Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1653–61.
43. Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 2010;115:2755–62.
44. Dameshek W, Miller EB. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. Blood 1946;1:27–50.
45. Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med 2009;60:193–206.
46. Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006;355:1672–81.
47. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:641–8.
48. Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009;113:2161–71.
49. Gernsheimer TB, George JN, Aledort LM, et al. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J Thromb Haemost 2010;8:1372–82.
50. Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol 2011;152:360–2.
51. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007;357:2237–47.
52. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011;377:393–402.
53. Brynes RK, Orazi A, Theodore D, et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: Data from the EXTEND study. Am J Hematol 2015;90:598–601.
54. George JN, Kojouri K, Perdue JJ, Vesely SK. Management of patients with chronic, refractory idiopathic thrombocytopenic purpura. Semin Hematol 2000;37:290–8.
55. McMillan R. Therapy for adults with refractory chronic immune thrombocytopenic purpura. Ann Intern Med 1997;126:307–14.
56. Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, et al. Successful therapy with danazol in refractory autoimmune thrombocytopenia associated with rheumatic diseases. Br J Rheumatol 1997;36:1095–9.
57. Provan D, Moss AJ, Newland AC, Bussel JB. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol 2006;81:19–25.
58. Reiner A, Gernsheimer T, Slichter SJ. Pulse cyclophosphamide therapy for refractory autoimmune thrombocytopenic purpura. Blood 1995;85:351–8.
59. Figueroa M, Gehlsen J, Hammond D, et al. Combination chemotherapy in refractory immune thrombocytopenic purpura. N Engl J Med 1993;328:1226–9.
60. Newland A, Lee EJ, McDonald V, Bussel JB. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy 2 Oct 2017.
61. McCrae KR. Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program 2010;2010:397–402.
62. Gernsheimer T, McCrae KR. Immune thrombocytopenic purpura in pregnancy. Curr Opin Hematol 2007;14:574–80.
63. DeLoughery TG. Critical care clotting catastrophies. Crit Care Clin 2005;21:531–62.
64. Stavrou E, McCrae KR. Immune thrombocytopenia in pregnancy. Hematol Oncol Clin North Am 2009;23:1299–316.
65. Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016;128:1329–35.
66. Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood 2017;130:1097–103.
67. Psaila B, Petrovic A, Page LK, et al. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood 2009;114:4777–83.
68. Journeycake JM. Childhood immune thrombocytopenia: role of rituximab, recombinant thrombopoietin, and other new therapeutics. Hematology Am Soc Hematol Educ Program 2012;2012:444–9.
69. Zhang J, Liang Y, Ai Y, et al. Thrombopoietin-receptor agonists for children with immune thrombocytopenia: a systematic review. Expert Opin Pharmacother 2017;18:1543–51.
70. Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016;388:45–54.71. Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet 2015;386:1649–58.
72. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113:1231–40.
73. Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica 2009;94:850–6.
74. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580–7.
75. Reese JA, Li X, Hauben M, et al. Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood 2010;116:2127–33.
76. Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis and management. J Thromb Haemost 2009;7:911–8.
77. Zondor SD, George JN, Medina PJ. Treatment of drug-induced thrombocytopenia. Expert Opin Drug Saf 2002;1:173–80.
78. George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: A systematic review of published case reports. Ann Intern Med 1998;129:886–90.
79. Green D, Hougie C, Kazmier FJ, et al. Report of the working party on acquired inhibitors of coagulation: studies of the “lupus” anticoagulant. Thromb Haemost 1983;49:144–6.
80. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 2009;114:3167–72.
81. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology 2008;13:356–60.
82. Notarangelo LD. Primary immunodeficiencies (PIDs) presenting with cytopenias. Hematology Am Soc Hematol Educ Program 2009:139–43.
83. Martinez-Valdez L, Deya-Martinez A, Giner MT, et al. Evans syndrome as first manifestation of primary immunodeficiency in clinical practice. J Pediatr Hematol Oncol 2017;39:490–4.
84. Shanafelt TD, Madueme HL, Wolf RC, Tefferi A. Rituximab for immune cytopenia in adults: idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, and Evans syndrome. Mayo Clin Proc 2003;78:1340–6.
85. Mantadakis E, Danilatou V, Stiakaki E, Kalmanti M. Rituximab for refractory Evans syndrome and other immune-mediated hematologic diseases. Am J Hematol 2004;77:303–10.
86. Jasinski S, Weinblatt ME, Glasser CL. Sirolimus as an effective agent in the treatment of immune thrombocytopenia (ITP) and Evans syndrome (ES): a single institution’s experience. J Pediatr Hematol Oncol 2017;39:420–4.
Introduction
Immune thrombocytopenia (ITP) is a common acquired autoimmune disease characterized by low platelet counts and an increased risk of bleeding. The incidence of ITP is approximately 3.3 per 100,000 adults.1 There is considerable controversy about all aspects of the disease, with little “hard” data on which to base decisions given the lack of randomized clinical trials to address most clinical questions. This article reviews the presentation and diagnosis of ITP and its treatment options and discusses management of ITP in specific clinical situations.
Pathogenesis and Epidemiology
ITP is caused by autoantibodies binding to platelet surface proteins, most often to the platelet receptor GP IIb/IIIa.2-4 These antibody-coated platelets then bind to Fc receptors in macrophages and are removed from circulation. The initiating event in ITP is unknown. It is speculated that the patient responds to a viral or bacterial infection by creating antibodies which cross-react with the platelet receptors. Continued exposure to platelets perpetuates the immune response. ITP that occurs in childhood appears to be an acute response to viral infection and usually resolves. ITP in adults may occur in any age group but is seen especially in young women.
Despite the increased platelet destruction that occurs in ITP, the production of new platelets often is not significantly increased. This is most likely due to lack of an increase in thrombopoietin, the predominant platelet growth factor.5
It had been thought that most adult patients who present with ITP go on to have a chronic course, but more recent studies have shown this is not the case. In modern series the percentage of patients who are “cured” with steroids ranges from 30% to 70%.6–9 In addition, it has been appreciated that even in patients with modest thrombocytopenia, no therapy is required if the platelet count remains higher than 30 × 103/µL. However, this leaves a considerable number of patients who will require chronic therapy.
Clinical Presentation
Presentation can range from a symptomatic patient with low platelets found on a routine blood count to a patient with massive bleeding. Typically, patients first present with petechiae (small bruises 1 mm in size) on the shins. True petechiae are seen only in severe thrombocytopenia. Patients will also report frequent bruising and bleeding from the gums. Patients with very low platelet counts will notice “wet purpura,” which is characterized by blood-filled bullae in the oral cavity. Life-threatening bleeding is a very unusual presenting sign unless other problems (trauma, ulcers) are present. The physical examination is only remarkable for stigmata of bleeding such as the petechiae. The presence of splenomegaly or lymphadenopathy weighs strongly against a diagnosis of ITP. Many patients with ITP will note fatigue when their platelets counts are lower.10
Diagnosis
Extremely low platelet counts with a normal blood smear and an otherwise healthy patient are diagnostic of ITP. The platelet count cutoff for considering ITP is 100 × 103/µL as the majority of patients with counts in the 100 to 150 × 103/µL range will not develop greater thrombocytopenia.11 Also, the platelet count decreases with age (9 × 103/µL per decade in one study), and this also needs to be factored into the evaluation.12 The finding of relatives with ITP should raise suspicion for congenital thrombocytopenia.13 One should question the patient carefully about drug exposure (see Drug-Induced Thrombocytopenia), especially about over-the-counter medicines, “natural” remedies, or recreational drugs.
There is no laboratory test that rules in ITP; rather, it is a diagnosis of exclusion. The blood smear should be carefully examined for evidence of microangiopathic hemolytic anemias (schistocytes), bone marrow disease (blasts, teardrop cells), or any other evidence of a primary bone marrow disease. In ITP, the platelets can be larger than normal, but finding some platelets the size of red cells should raise the issue of congenital thrombocytopenia.14 Pseudo-thrombocytopenia, which is the clumping of platelets due to a reaction to the EDTA anticoagulant in the tube, should be excluded. The diagnosis is established by drawing the blood in a citrated (blue-top) tube to perform the platelet count. There is no role for antiplatelet antibody assay because this test lacks sensitivity and specificity. In a patient without a history of autoimmune disease or symptoms, empiric testing for autoimmune disease is not recommended.
Patients who present with ITP should be tested for both HIV and hepatitis C infection.15,16 These are the most common viral causes of secondary ITP, and both have prognostic and treatment implications. Some authorities also recommend checking thyroid function as hypothyroidism can present or aggravate the thrombocytopenia.
The role of bone marrow examination is controversial.17 Patients with a classic presentation of ITP (young woman, normal blood smear) do not require a bone marrow exam before therapy is initiated, although patients who do not respond to initial therapy should have a bone marrow aspiration. The rare entity amegakaryocytic thrombocytopenia can present with a clinical picture similar to that of ITP, but amegakaryocytic thrombocytopenia will not respond to steroids. Bone marrow aspiration reveals the absence of megakaryocytes in this entity. It is rare, however, that another hematologic disease is diagnosed in patients with a classic clinical presentation of ITP.
In the future, measurement of thrombopoietin and reticulated platelets may provide clues to the diagnosis.4 Patients with ITP paradoxically have normal or only mildly elevated thrombopoietin levels. The finding of a significantly elevated thrombopoietin level should lead to questioning of the diagnosis. One can also measure “reticulated platelets,” which are analogous to red cell reticulocytes. Patients with ITP (or any platelet destructive disorders) will have high levels of reticulated platelets. These tests are not recommended for routine evaluation, but may be helpful in difficult cases.
Treatment
In general, therapy in ITP should be guided by the patient’s signs of bleeding and not by unquestioning adherence to measuring platelet levels,15 as patients tolerate thrombocytopenia well. It is unusual to have life-threatening bleeding with platelet counts greater than 5 × 103/µL in the absence of mechanical lesions. Despite the low platelet count in patients with ITP, the overall mortality is estimated to be only 0.3% to 1.3%.18 It is sobering that in one study the rate of death from infections was twice as high as that from bleeding.19 Rare patients will have antibodies that interfere with the function of the platelet, and these patients can have profound bleeding with only modestly lowered platelet counts.20 A suggested cut-off for treating newly diagnosed patients is 30 × 103/µL.21
Initial Therapy
The primary therapy of ITP is glucocorticoids, either prednisone or dexamethasone. In the past prednisone at a dose of 60 to 80 mg/day was started at the time of diagnosis (Table 1).
For rapid induction of a response, there are 2 options. A single dose of intravenous immune globulin (IVIG) at 1 g/kg or intravenous anti-D immunoglobulin (anti-D) at 50 to 75 µg/kg can induce a response in more than 80% of patients in 24 to 48 hours.21,24 IVIG has several drawbacks. It can cause aseptic meningitis, and in patients with vascular disease the increased viscosity can induce ischemia. There is also a considerable fluid load delivered with the IVIG, and it needs to be given over several hours.
The use of anti-D is limited to Rh-positive patients who have not had a splenectomy. It should not be used in patients who are Coombs positive due to the risk of provoking more hemolysis. Rarely anti-D has been reported to cause a severe hemolytic disseminated intravascular coagulation syndrome (1:20,000 patients), which has led to restrictions in its use.25 Although the drug can be rapidly given over 15 minutes, due to these concerns current recommendations are now to observe patients for 8 hours after their dose and to perform a urine dipstick test for blood at 2, 4, and 8 hours. Concerns about this rare but serious side effect have led to a dramatic decrease in the use of anti-D.
For patients who are severely thrombocytopenic and do not respond to initial therapy, there are 2 options for raising the platelet counts. One is to use a combination of IVIG, methylprednisolone, vincristine, and/or anti-D.26 The combination of IVIG and anti-D may be synergistic since these agents block different Fc receptors. A response of 71% has been reported for this 3- or 4-drug combination in a series of 35 patients.26 The other option is to treat with a continuous infusion of platelets (1 unit over 6 hours) and IVIG 1 g/kg for 24 hours. Response rates of 62.7% have been reported with this combination, and this rapid rise in platelets can allow time for other therapies to take effect.27,28
Patients with severe thrombocytopenia who relapse with reduction of steroids or who do not respond to steroids have several options for further management. Repeated doses of IVIG can transiently raise the platelet count, and some patients may only need several courses of therapy over the course of many months. One study showed that 60% of patients could delay or defer therapy by receiving multiple doses of anti-D. However, 30% of patients did eventually receive splenectomy and 20% of patients required ongoing therapy with anti-D.29 In a randomized trial comparing early use of anti-D to steroids to avoid splenectomy, there was no difference in splenectomy rate (38% versus 42%).30 Finally, an option as mentioned above is to try a 6-month course of pulse dexamethasone 40 mg/day for 4 days, repeated every 28 days.
Options for Refractory ITP
There are multiple options for patients who do not respond to initial ITP therapies. These can be divided into several broad groups: curative therapies (splenectomy and rituximab), thrombopoietin receptor agonists, and anecdotal therapies.
Splenectomy
In patients with severe thrombocytopenia who do not respond or who relapse with lower doses of prednisone, splenectomy should be strongly considered. Splenectomy will induce a good response in 60% to 70% of patients and is durable in most patients. In 2 recently published reviews of splenectomy, the complete response rate was 67% and the total response rate was 88% to 90%%.8,31 Between 15% and 28% of patients relapsed over 5 years, with most recurrences occurring in the first 2 years. Splenectomy carries a short-term surgical risk, and the life-long risk of increased susceptibility to overwhelming sepsis is discussed below. However, the absolute magnitude of these risks is low and is often lower than the risks of continued prednisone therapy or of continued cytotoxic therapy.
Timing of splenectomy depends on the patient’s presentation. Most patients should be given a 6-month trial of steroids or other therapies before proceeding to splenectomy.31 However, patients who persist with severe thrombocytopenia despite initial therapies or who are suffering intolerable side effects from therapy should be considered sooner for splenectomy.31 In the George review, multiple factors such as responding to IVIG were found not to be predictive of response to splenectomy.8
Method of splenectomy appears not to matter.21 Rates of finding accessory spleens are just as high or higher with laparoscopic splenectomy and the patient can recover faster. In patients who are severely thrombocytopenic, open splenectomy can allow for quicker control of the vascular access of the spleen.
Rates of splenectomy in recent years have decreased for many reasons,32 including the acceptance of lower platelet counts in asymptomatic patients and the availability of alternative therapies such as rituximab. In addition, despite abundant data for good outcomes, there is a concern that splenectomy responses are not durable. Although splenectomy will not cure every patient with ITP, splenectomy is the therapy with the most patients, the longest follow-up, and the most consistent rate of cure, and it should be discussed with every ITP patient who does not respond to initial therapy and needs further treatment.
The risk of overwhelming sepsis varies by indications for splenectomy but appears to be about 1%.33,34 The use of pneumococcal vaccine and recognition of this syndrome have helped reduce the risk. Asplenic patients need to be counseled about the risk of overwhelming infections, should be vaccinated for pneumococcus, meningococcus, and Haemophilus influenzae, and should wear an ID bracelet.35–37 Patients previously vaccinated for pneumococcus should be re-vaccinated every 3 to 5 years. The role of prophylactic antibiotics in adults is controversial, but patients under the age of 18 should be on penicillin VK 250 mg orally twice daily.
Rituximab
Rituximab has been shown to be very active in ITP. Most studies used the standard dose of 375 mg/m2 weekly for 4 weeks, but other studies have shown that 1000 mg twice 14 days apart (ie, on days 1 and 15) resulted in the same response rate and may be more convenient for patients.38,39 The response time can vary, with patients either showing a rapid response or requiring up to 8 weeks for their counts to go up. Although experience is limited, the response seems to be durable, especially in those patients whose counts rise higher than 150 × 103/µL; in patients who relapse, a response can be re-induced with a repeat course. Overall the response rate for rituximab is about 60%, but only approximately 20% to 40% of patients will remain in long-term remission.40–42 There is no evidence yet that “maintenance” therapy or monitoring CD19/CD20 cells can help further the duration of remission.
Whether to give rituximab pre- or post-splenectomy is also uncertain. An advantage of presplenectomy rituximab is that many patients will achieve remission, delaying the need for surgery. Also, rituximab is a good option for patients whose medical conditions put them at high risk for complications with splenectomy. However, it is unknown whether rituximab poses any long-term risks, while the long-term risks of splenectomy are well-defined. Rituximab is the only curative option left for patients who have failed splenectomy and is a reasonable option for these patients.
There is an intriguing trial in which patients were randomly assigned to dexamethasone alone versus dexamethasone plus rituximab upon presentation with ITP; those who were refractory to dexamethasone alone received salvage therapy with dexamethasone plus rituximab.43 The dexamethasone plus rituximab group had an overall higher rate of sustained remission at 6 months than the dexamethasone group, 63% versus 36%. Interestingly, patients who failed their first course of dexamethasone but then were “salvaged” with dexamethasone/rituximab had a similar overall response rate of 56%, suggesting that saving the addition of rituximab for steroid failures may be an effective option.
Although not “chemotherapy,” rituximab is not without risks. Patients can develop infusion reactions, which can be severe in 1% to 2% of patients. In a meta-analysis the fatal reaction rate was 2.9%.40 Patients with chronic hepatitis B infections can experience reactivation with rituximab, and thus all patients should be screened before treatment. Finally, the very rare but devastating complication of progressive multifocal leukoencephalopathy has been reported.
Thrombopoietin Receptor Agonists
Although patients with ITP have low platelet counts, studies starting with Dameshek have shown that these patients also have reduced production of platelets.44 Despite the very low circulating platelet count, levels of the platelet growth factor thrombopoietin (TPO) are not raised.45 Seminal studies with recombinant TPO in the 1990s showed that ITP patients responded to thrombopoietin-stimulating protein, but the formation of anti-TPO antibodies halted trials with the first generation of these agents. Two TPO receptor agonists (TPO-RA) are approved for use in patients with ITP.
Romiplostim. Romiplostim is a peptibody, a combination of a peptide that binds and stimulates the TPO receptor and an Fc domain to extend its half-life.46 It is administered in a weekly subcutaneous dose starting at 1 to 3 µg/kg. Use of romiplostim in ITP patients produces a response rate of 80% to 88%, with 87% of patients being able to wean off or decrease other anti-ITP medications.47 In a long-term extension study, the response was again high at 87%.48 These studies have also shown a reduced incidence of bleeding.
The major side effect of romiplostim seen in clinical trials was marrow reticulin formation, which occurred in up to 5.6% of patients.47,48 The clinical course in these patients is the development of anemia and a myelophthisic blood smear with teardrop cells and nucleated red cells. These changes appear to reverse with cessation of the drug. The bone marrow shows increased reticulin formation but rarely, if ever, shows the collagen deposition seen with primary myelofibrosis.
Thrombosis has also been seen, with a rate of 0.08 to 0.1 cases per 100 patient-weeks,49 but it remains unclear if this is due to the drug, part of the natural history of ITP, or expected complications in older patients undergoing any type of medical therapy. Surprisingly, despite the low platelet counts, patients with ITP in one study had double the risk of venous thrombosis, demonstrating that ITP itself can be a risk factor for thrombosis.50 These trials have shown no long-term concerns for other clinical problems such as liver disease.
Eltrombopag. The other available TPO-RA is eltrombopag,51 an oral agent that stimulates the TPO receptor by binding the transmembrane domain and activating it. The drug is given orally starting at 50 mg/day (25 mg for patients of Asian ancestry or with liver disease) and can be dose escalated to 75 mg/day. The drug needs to be taken on an empty stomach. Eltrombopag has been shown to be effective in chronic ITP, with response rates of 59% to 80% and reduction in use of rescue medications.47,51,52 As with romiplostim, the incidence of bleeding was also decreased with eltrombopag in these trials.47,51
Clinical trials demonstrated that eltrombopag shares with romiplostim the risk for marrow fibrosis. A side effect unique to eltrombopag observed in these trials was a 3% to 7% incidence of elevated liver function tests.21,52 These abnormal findings appeared to resolve in most patients, but liver function tests need to be monitored in patients receiving eltrombopag.
Clinical use. The clearest indication for the use of TPO-RAs is in patients who have failed several therapies and remain symptomatic or are on intolerable doses of other medications such as prednisone. The clear benefits are their relative safety and high rates of success. The main drawback of TPO-RAs is the need for continuing therapy as the platelet count will return to baseline shortly after these agents are stopped. Currently there is no clear indication for one medication over the other. The advantages of romiplostim are great flexibility in dosing (1–10 µg/kg week) and no concerns about drug interaction. The current drawback of romiplostim is the Food and Drug Administration’s requirement for patients to receive the drug from a clinic and not at home. Eltrombopag offers the advantage of oral use, but it has a limited dose range and potential for drug interactions. Both agents have been associated with marrow reticulin formation, although in clinical use this risk appears to be very low.53
Other Options
In the literature there are numerous options for the treatment of ITP.54,55 Most of these studies are anecdotal, enrolled small number of patients, and sometimes included patients with mild thrombocytopenia, but these therapeutic options can be tried in patients who are refractory to standard therapies and have bleeding. The agents with the greatest amount of supporting data are danazol, vincristine, azathioprine, cyclophosphamide, and fostamatinib.
Danazol 200 mg 4 times daily is thought to downregulate the macrophage Fc receptor. The onset of action may be delayed and a therapeutic trial of up to 4 to 6 months is advised. Danazol is very effective in patients with antiphospholipid antibody syndrome who develop ITP and may be more effective in premenopausal women.56 Once a response is seen, danazol should be continued for 6 months and then an attempt to wean the patient off the agent should be made. A partial response can be seen in 70% to 90% of patients, but a complete response is rare.54
Vincristine 1.4 mg/m2 weekly has a low response rate, but if a response is going to occur, it will occur rapidly within 2 weeks. Thus, a prolonged trial of vincristine is not needed; if no platelet rise is seen in several weeks, the drug should be stopped. Again, partial responses are more common than complete response—50% to 63% versus 0% to 6%.54Azathioprine 150 mg orally daily, like danazol, demonstrates a delayed response and requires several months to assess for response. However, 19% to 25% of patients may have a complete response.54 It has been reported that the related agent mycophenolate 1000 mg twice daily is also effective in ITP.57
Cyclophosphamide 1 g/m2 intravenously repeated every 28 days has been reported to have a response rate of up to 40%.58 Although considered more aggressive, this is a standard immunosuppressive dose and should be considered in patients with very low platelet counts. Patients who have not responded to single-agent cyclophosphamide may respond to multi-agent chemotherapy with agents such as etoposide and vincristine plus cyclophosphamide.59
Fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, is currently under investigation for the treatment of ITP.60 This agent prevents phagocytosis of antibody-coated platelets by macrophages. In early studies fostamatinib has been well tolerated at a dose of 150 mg twice daily, with 75% of patients showing a response. Large phase 3 trials are underway, and if the earlier promising results hold up fostamatinib may be a novel option for refractory patients.
A Practical Approach to Refractory ITP
One approach is to divide patients into bleeders, or those with either very low platelet counts (< 5 × 103/µL) or who have had significant bleeding in the past, and nonbleeders, or those with platelet counts above 5 × 103/µL and no history of severe bleeding. Bleeders who do not respond adequately to splenectomy should first start with rituximab since it is not cytotoxic and is the only other “curative” therapy (Table 2).
Nonbleeders should be tried on danazol and other relatively safe agents. If this fails, rituximab or TPO-RAs can be considered. Before one considers cytotoxic therapy, the risk of the therapy must be weighed against the risk posed by the thrombocytopenia. The mortality from ITP is fairly low (5%) and is restricted to patients with severe disease. Patients with only moderate thrombocytopenia and no bleeding are better served with conservative management. There is little justification for the use of continuous steroid therapy in this group of patients given the long-term risks of this therapy.
Special Situations
Surgery
Patients with ITP who need surgery either for splenectomy or for other reasons should have their platelet counts raised to a level greater than 20 to 30 × 103/µL before surgery. Most patients with ITP have increased platelet function and will not have excessive bleeding with these platelet counts. For patients with platelet counts below this level, an infusion of immune globulin or anti-D may rapidly increase the platelet counts. If the surgery is elective, short-term use of TPO-RAs to raise the counts can also be considered.
Pregnancy
Up to 10% of pregnant women will develop low platelet counts during their pregnancy.61,62 The most common etiology is gestational thrombocytopenia, which is an exaggeration of the lowered platelet count seen in pregnancy. Counts may fall as low as 50 × 103/µL at the time of delivery. No therapy is required as the fetus is not affected and the mother does not have an increased risk of bleeding. Pregnancy complications such as HELLP syndrome and thrombotic microangiopathies also present with low platelet counts, but these can be diagnosed by history.61,63
Women with ITP can either develop the disease during pregnancy or have a worsening of the symptoms.64 Counts often drop dramatically during the first trimester. Early management should be conservative with low doses of prednisone to keep the count above 10 × 103/µL.21 Immunoglobulin is also effective,65 but there are rare reports of pulmonary edema. Rarely patients who are refractory will require splenectomy, which may be safely performed in the second trimester. For delivery the count should be greater than 30 × 103/µL and for an epidural greater than 50 × 103/µL.64 There are reports of the use of TPO-RAs in pregnancy, and this can be considered for refractory cases.66
Most controversy centers on management of the delivery. In the past it was feared that fetal thrombocytopenia could lead to intracranial hemorrhage, and Caesarean section was always recommended. It now appears that most cases of intracranial hemorrhage were due to alloimmune thrombocytopenia and not ITP. Furthermore, the nadir of the baby’s platelet count is not at birth but several days after. It appears the safest course is to proceed with a vaginal or C-section delivery determined by obstetrical indications and then immediately check the baby’s platelet count. If the platelet count is low in the neonate, immunoglobulin will raise the count. Since the neonatal thrombocytopenia is due to passive transfer of maternal antibody, the platelet destruction will abate in 4 to 6 weeks.
Pediatric Patients
The incidence of ITP in children is 2.2 to 5.3 per 100,000 children.1 There are several distinct differences in pediatric ITP. Most cases will resolve in weeks, with only a minority of patients transforming into chronic ITP (5%–10%). Also, the rates of serious bleeding are lower in children than in adults, with intracranial hemorrhage rates of 0.1% to 0.5% being seen.67 For most patients with no or mild bleeding, management now is observation alone regardless of platelet count because it is felt that the risks of therapies are higher than the risk of bleeding.21 For patients with bleeding, IVIG, anti-D, or a short course of steroids can be used. Given the risk of overwhelming sepsis, splenectomy is often deferred as long as possible. Rituximab is increasingly being used in children due to concerns about use of agents such a cyclophosphamide or azathioprine in children.68 Abundant data on use of TPO-RAs in children showing high response rates and safety support their use, and these should be considered in refractory ITP before any cytotoxic agent.69–71
Helicobacter Pylori Infection
There has been much interest in the relationship between H. pylori and ITP.16,72,73H. pylori infections have been associated with a variety of autoimmune diseases, and there is a confusing literature on this infection and ITP. Several meta-analyses have shown that eradication of H. pylori will result in an ITP response rate of 20% to 30%, but responses curiously appear to be limited to certain geographic areas such as Japan and Italy but not the United States. In patients with recalcitrant ITP, especially in geographic areas with high incidence, it may be worthwhile to check for H. pylori infection and treat accordingly if positive.
Drug-Induced Thrombocytopenia
Patients with drug-induced thrombocytopenia present with very low (< 10 × 103/µL) platelet counts 1 to 3 weeks after starting a new medication.74–76 In patients with a possible drug-induced thrombocytopenia, the primary therapy is to stop the suspect drug.77 If there are multiple new medications, the best approach is to stop any drug that has been strongly associated with thrombocytopenia (Table 3).74,78,79
Immune globulin, corticosteroids, or intravenous anti‑D have been suggested as useful in drug‑related thrombocytopenia. However, since most of these thrombocytopenic patients recover when the agent is cleared from the body, this therapy is probably not necessary and withholding treatment avoids exposing the patients to the adverse events associated with further therapy.
Evans Syndrome
Evans syndrome is defined as the combination of autoimmune hemolytic anemia (AIHA) and ITP.80,81 These cytopenias can present simultaneously or sequentially. Patients with Evans syndrome are thought to have a more severe disease process, to be more prone to bleeding, and to be more difficult to treat, but the rarity of this syndrome makes this hard to quantify.
The classic clinical presentation of Evans syndrome is severe anemia and thrombocytopenia. Children with Evans syndrome often have complex immunodeficiencies such as autoimmune lymphoproliferative syndrome.82,83 In adults, Evans syndrome most often complicates other autoimmune diseases such as lupus. There are increasing reports of Evans syndrome occurring as a complication of T-cell lymphomas. Often the autoimmune disease can predate the lymphoma diagnosis by months or even years.
In theory the diagnostic approach is straightforward by showing a Coombs-positive hemolytic anemia in the setting of a clinical diagnosis of immune thrombocytopenia. The blood smear will show spherocytes and a diminished platelet count. The presence of other abnormal red cell forms should raise the possibility of an alternative diagnosis. It is unclear how vigorously one should search for other underlying diseases. Many patients will already have the diagnosis of an underlying autoimmune disease. The presence of lymphadenopathy should raise concern for lymphoma.
Initial therapy is high-dose steroids (2 mg/kg/day). IVIG should be added if severe thrombocytopenia is present. Patients who cannot be weaned off prednisone or relapse after prednisone should be considered for splenectomy, although these patients are at higher risk of relapsing.80 Increasingly rituximab is being used with success.84,85 For patients who fail splenectomy and rituximab, aggressive immunosuppression should be considered. Increasing data support the benefits of sirolimus, and this should be considered for refractory patients.86 For patients with Evans syndrome due to underlying lymphoma, antineoplastic therapy often results in prompt resolution of the symptoms. Recurrence of the autoimmune cytopenias often heralds relapse.
Introduction
Immune thrombocytopenia (ITP) is a common acquired autoimmune disease characterized by low platelet counts and an increased risk of bleeding. The incidence of ITP is approximately 3.3 per 100,000 adults.1 There is considerable controversy about all aspects of the disease, with little “hard” data on which to base decisions given the lack of randomized clinical trials to address most clinical questions. This article reviews the presentation and diagnosis of ITP and its treatment options and discusses management of ITP in specific clinical situations.
Pathogenesis and Epidemiology
ITP is caused by autoantibodies binding to platelet surface proteins, most often to the platelet receptor GP IIb/IIIa.2-4 These antibody-coated platelets then bind to Fc receptors in macrophages and are removed from circulation. The initiating event in ITP is unknown. It is speculated that the patient responds to a viral or bacterial infection by creating antibodies which cross-react with the platelet receptors. Continued exposure to platelets perpetuates the immune response. ITP that occurs in childhood appears to be an acute response to viral infection and usually resolves. ITP in adults may occur in any age group but is seen especially in young women.
Despite the increased platelet destruction that occurs in ITP, the production of new platelets often is not significantly increased. This is most likely due to lack of an increase in thrombopoietin, the predominant platelet growth factor.5
It had been thought that most adult patients who present with ITP go on to have a chronic course, but more recent studies have shown this is not the case. In modern series the percentage of patients who are “cured” with steroids ranges from 30% to 70%.6–9 In addition, it has been appreciated that even in patients with modest thrombocytopenia, no therapy is required if the platelet count remains higher than 30 × 103/µL. However, this leaves a considerable number of patients who will require chronic therapy.
Clinical Presentation
Presentation can range from a symptomatic patient with low platelets found on a routine blood count to a patient with massive bleeding. Typically, patients first present with petechiae (small bruises 1 mm in size) on the shins. True petechiae are seen only in severe thrombocytopenia. Patients will also report frequent bruising and bleeding from the gums. Patients with very low platelet counts will notice “wet purpura,” which is characterized by blood-filled bullae in the oral cavity. Life-threatening bleeding is a very unusual presenting sign unless other problems (trauma, ulcers) are present. The physical examination is only remarkable for stigmata of bleeding such as the petechiae. The presence of splenomegaly or lymphadenopathy weighs strongly against a diagnosis of ITP. Many patients with ITP will note fatigue when their platelets counts are lower.10
Diagnosis
Extremely low platelet counts with a normal blood smear and an otherwise healthy patient are diagnostic of ITP. The platelet count cutoff for considering ITP is 100 × 103/µL as the majority of patients with counts in the 100 to 150 × 103/µL range will not develop greater thrombocytopenia.11 Also, the platelet count decreases with age (9 × 103/µL per decade in one study), and this also needs to be factored into the evaluation.12 The finding of relatives with ITP should raise suspicion for congenital thrombocytopenia.13 One should question the patient carefully about drug exposure (see Drug-Induced Thrombocytopenia), especially about over-the-counter medicines, “natural” remedies, or recreational drugs.
There is no laboratory test that rules in ITP; rather, it is a diagnosis of exclusion. The blood smear should be carefully examined for evidence of microangiopathic hemolytic anemias (schistocytes), bone marrow disease (blasts, teardrop cells), or any other evidence of a primary bone marrow disease. In ITP, the platelets can be larger than normal, but finding some platelets the size of red cells should raise the issue of congenital thrombocytopenia.14 Pseudo-thrombocytopenia, which is the clumping of platelets due to a reaction to the EDTA anticoagulant in the tube, should be excluded. The diagnosis is established by drawing the blood in a citrated (blue-top) tube to perform the platelet count. There is no role for antiplatelet antibody assay because this test lacks sensitivity and specificity. In a patient without a history of autoimmune disease or symptoms, empiric testing for autoimmune disease is not recommended.
Patients who present with ITP should be tested for both HIV and hepatitis C infection.15,16 These are the most common viral causes of secondary ITP, and both have prognostic and treatment implications. Some authorities also recommend checking thyroid function as hypothyroidism can present or aggravate the thrombocytopenia.
The role of bone marrow examination is controversial.17 Patients with a classic presentation of ITP (young woman, normal blood smear) do not require a bone marrow exam before therapy is initiated, although patients who do not respond to initial therapy should have a bone marrow aspiration. The rare entity amegakaryocytic thrombocytopenia can present with a clinical picture similar to that of ITP, but amegakaryocytic thrombocytopenia will not respond to steroids. Bone marrow aspiration reveals the absence of megakaryocytes in this entity. It is rare, however, that another hematologic disease is diagnosed in patients with a classic clinical presentation of ITP.
In the future, measurement of thrombopoietin and reticulated platelets may provide clues to the diagnosis.4 Patients with ITP paradoxically have normal or only mildly elevated thrombopoietin levels. The finding of a significantly elevated thrombopoietin level should lead to questioning of the diagnosis. One can also measure “reticulated platelets,” which are analogous to red cell reticulocytes. Patients with ITP (or any platelet destructive disorders) will have high levels of reticulated platelets. These tests are not recommended for routine evaluation, but may be helpful in difficult cases.
Treatment
In general, therapy in ITP should be guided by the patient’s signs of bleeding and not by unquestioning adherence to measuring platelet levels,15 as patients tolerate thrombocytopenia well. It is unusual to have life-threatening bleeding with platelet counts greater than 5 × 103/µL in the absence of mechanical lesions. Despite the low platelet count in patients with ITP, the overall mortality is estimated to be only 0.3% to 1.3%.18 It is sobering that in one study the rate of death from infections was twice as high as that from bleeding.19 Rare patients will have antibodies that interfere with the function of the platelet, and these patients can have profound bleeding with only modestly lowered platelet counts.20 A suggested cut-off for treating newly diagnosed patients is 30 × 103/µL.21
Initial Therapy
The primary therapy of ITP is glucocorticoids, either prednisone or dexamethasone. In the past prednisone at a dose of 60 to 80 mg/day was started at the time of diagnosis (Table 1).
For rapid induction of a response, there are 2 options. A single dose of intravenous immune globulin (IVIG) at 1 g/kg or intravenous anti-D immunoglobulin (anti-D) at 50 to 75 µg/kg can induce a response in more than 80% of patients in 24 to 48 hours.21,24 IVIG has several drawbacks. It can cause aseptic meningitis, and in patients with vascular disease the increased viscosity can induce ischemia. There is also a considerable fluid load delivered with the IVIG, and it needs to be given over several hours.
The use of anti-D is limited to Rh-positive patients who have not had a splenectomy. It should not be used in patients who are Coombs positive due to the risk of provoking more hemolysis. Rarely anti-D has been reported to cause a severe hemolytic disseminated intravascular coagulation syndrome (1:20,000 patients), which has led to restrictions in its use.25 Although the drug can be rapidly given over 15 minutes, due to these concerns current recommendations are now to observe patients for 8 hours after their dose and to perform a urine dipstick test for blood at 2, 4, and 8 hours. Concerns about this rare but serious side effect have led to a dramatic decrease in the use of anti-D.
For patients who are severely thrombocytopenic and do not respond to initial therapy, there are 2 options for raising the platelet counts. One is to use a combination of IVIG, methylprednisolone, vincristine, and/or anti-D.26 The combination of IVIG and anti-D may be synergistic since these agents block different Fc receptors. A response of 71% has been reported for this 3- or 4-drug combination in a series of 35 patients.26 The other option is to treat with a continuous infusion of platelets (1 unit over 6 hours) and IVIG 1 g/kg for 24 hours. Response rates of 62.7% have been reported with this combination, and this rapid rise in platelets can allow time for other therapies to take effect.27,28
Patients with severe thrombocytopenia who relapse with reduction of steroids or who do not respond to steroids have several options for further management. Repeated doses of IVIG can transiently raise the platelet count, and some patients may only need several courses of therapy over the course of many months. One study showed that 60% of patients could delay or defer therapy by receiving multiple doses of anti-D. However, 30% of patients did eventually receive splenectomy and 20% of patients required ongoing therapy with anti-D.29 In a randomized trial comparing early use of anti-D to steroids to avoid splenectomy, there was no difference in splenectomy rate (38% versus 42%).30 Finally, an option as mentioned above is to try a 6-month course of pulse dexamethasone 40 mg/day for 4 days, repeated every 28 days.
Options for Refractory ITP
There are multiple options for patients who do not respond to initial ITP therapies. These can be divided into several broad groups: curative therapies (splenectomy and rituximab), thrombopoietin receptor agonists, and anecdotal therapies.
Splenectomy
In patients with severe thrombocytopenia who do not respond or who relapse with lower doses of prednisone, splenectomy should be strongly considered. Splenectomy will induce a good response in 60% to 70% of patients and is durable in most patients. In 2 recently published reviews of splenectomy, the complete response rate was 67% and the total response rate was 88% to 90%%.8,31 Between 15% and 28% of patients relapsed over 5 years, with most recurrences occurring in the first 2 years. Splenectomy carries a short-term surgical risk, and the life-long risk of increased susceptibility to overwhelming sepsis is discussed below. However, the absolute magnitude of these risks is low and is often lower than the risks of continued prednisone therapy or of continued cytotoxic therapy.
Timing of splenectomy depends on the patient’s presentation. Most patients should be given a 6-month trial of steroids or other therapies before proceeding to splenectomy.31 However, patients who persist with severe thrombocytopenia despite initial therapies or who are suffering intolerable side effects from therapy should be considered sooner for splenectomy.31 In the George review, multiple factors such as responding to IVIG were found not to be predictive of response to splenectomy.8
Method of splenectomy appears not to matter.21 Rates of finding accessory spleens are just as high or higher with laparoscopic splenectomy and the patient can recover faster. In patients who are severely thrombocytopenic, open splenectomy can allow for quicker control of the vascular access of the spleen.
Rates of splenectomy in recent years have decreased for many reasons,32 including the acceptance of lower platelet counts in asymptomatic patients and the availability of alternative therapies such as rituximab. In addition, despite abundant data for good outcomes, there is a concern that splenectomy responses are not durable. Although splenectomy will not cure every patient with ITP, splenectomy is the therapy with the most patients, the longest follow-up, and the most consistent rate of cure, and it should be discussed with every ITP patient who does not respond to initial therapy and needs further treatment.
The risk of overwhelming sepsis varies by indications for splenectomy but appears to be about 1%.33,34 The use of pneumococcal vaccine and recognition of this syndrome have helped reduce the risk. Asplenic patients need to be counseled about the risk of overwhelming infections, should be vaccinated for pneumococcus, meningococcus, and Haemophilus influenzae, and should wear an ID bracelet.35–37 Patients previously vaccinated for pneumococcus should be re-vaccinated every 3 to 5 years. The role of prophylactic antibiotics in adults is controversial, but patients under the age of 18 should be on penicillin VK 250 mg orally twice daily.
Rituximab
Rituximab has been shown to be very active in ITP. Most studies used the standard dose of 375 mg/m2 weekly for 4 weeks, but other studies have shown that 1000 mg twice 14 days apart (ie, on days 1 and 15) resulted in the same response rate and may be more convenient for patients.38,39 The response time can vary, with patients either showing a rapid response or requiring up to 8 weeks for their counts to go up. Although experience is limited, the response seems to be durable, especially in those patients whose counts rise higher than 150 × 103/µL; in patients who relapse, a response can be re-induced with a repeat course. Overall the response rate for rituximab is about 60%, but only approximately 20% to 40% of patients will remain in long-term remission.40–42 There is no evidence yet that “maintenance” therapy or monitoring CD19/CD20 cells can help further the duration of remission.
Whether to give rituximab pre- or post-splenectomy is also uncertain. An advantage of presplenectomy rituximab is that many patients will achieve remission, delaying the need for surgery. Also, rituximab is a good option for patients whose medical conditions put them at high risk for complications with splenectomy. However, it is unknown whether rituximab poses any long-term risks, while the long-term risks of splenectomy are well-defined. Rituximab is the only curative option left for patients who have failed splenectomy and is a reasonable option for these patients.
There is an intriguing trial in which patients were randomly assigned to dexamethasone alone versus dexamethasone plus rituximab upon presentation with ITP; those who were refractory to dexamethasone alone received salvage therapy with dexamethasone plus rituximab.43 The dexamethasone plus rituximab group had an overall higher rate of sustained remission at 6 months than the dexamethasone group, 63% versus 36%. Interestingly, patients who failed their first course of dexamethasone but then were “salvaged” with dexamethasone/rituximab had a similar overall response rate of 56%, suggesting that saving the addition of rituximab for steroid failures may be an effective option.
Although not “chemotherapy,” rituximab is not without risks. Patients can develop infusion reactions, which can be severe in 1% to 2% of patients. In a meta-analysis the fatal reaction rate was 2.9%.40 Patients with chronic hepatitis B infections can experience reactivation with rituximab, and thus all patients should be screened before treatment. Finally, the very rare but devastating complication of progressive multifocal leukoencephalopathy has been reported.
Thrombopoietin Receptor Agonists
Although patients with ITP have low platelet counts, studies starting with Dameshek have shown that these patients also have reduced production of platelets.44 Despite the very low circulating platelet count, levels of the platelet growth factor thrombopoietin (TPO) are not raised.45 Seminal studies with recombinant TPO in the 1990s showed that ITP patients responded to thrombopoietin-stimulating protein, but the formation of anti-TPO antibodies halted trials with the first generation of these agents. Two TPO receptor agonists (TPO-RA) are approved for use in patients with ITP.
Romiplostim. Romiplostim is a peptibody, a combination of a peptide that binds and stimulates the TPO receptor and an Fc domain to extend its half-life.46 It is administered in a weekly subcutaneous dose starting at 1 to 3 µg/kg. Use of romiplostim in ITP patients produces a response rate of 80% to 88%, with 87% of patients being able to wean off or decrease other anti-ITP medications.47 In a long-term extension study, the response was again high at 87%.48 These studies have also shown a reduced incidence of bleeding.
The major side effect of romiplostim seen in clinical trials was marrow reticulin formation, which occurred in up to 5.6% of patients.47,48 The clinical course in these patients is the development of anemia and a myelophthisic blood smear with teardrop cells and nucleated red cells. These changes appear to reverse with cessation of the drug. The bone marrow shows increased reticulin formation but rarely, if ever, shows the collagen deposition seen with primary myelofibrosis.
Thrombosis has also been seen, with a rate of 0.08 to 0.1 cases per 100 patient-weeks,49 but it remains unclear if this is due to the drug, part of the natural history of ITP, or expected complications in older patients undergoing any type of medical therapy. Surprisingly, despite the low platelet counts, patients with ITP in one study had double the risk of venous thrombosis, demonstrating that ITP itself can be a risk factor for thrombosis.50 These trials have shown no long-term concerns for other clinical problems such as liver disease.
Eltrombopag. The other available TPO-RA is eltrombopag,51 an oral agent that stimulates the TPO receptor by binding the transmembrane domain and activating it. The drug is given orally starting at 50 mg/day (25 mg for patients of Asian ancestry or with liver disease) and can be dose escalated to 75 mg/day. The drug needs to be taken on an empty stomach. Eltrombopag has been shown to be effective in chronic ITP, with response rates of 59% to 80% and reduction in use of rescue medications.47,51,52 As with romiplostim, the incidence of bleeding was also decreased with eltrombopag in these trials.47,51
Clinical trials demonstrated that eltrombopag shares with romiplostim the risk for marrow fibrosis. A side effect unique to eltrombopag observed in these trials was a 3% to 7% incidence of elevated liver function tests.21,52 These abnormal findings appeared to resolve in most patients, but liver function tests need to be monitored in patients receiving eltrombopag.
Clinical use. The clearest indication for the use of TPO-RAs is in patients who have failed several therapies and remain symptomatic or are on intolerable doses of other medications such as prednisone. The clear benefits are their relative safety and high rates of success. The main drawback of TPO-RAs is the need for continuing therapy as the platelet count will return to baseline shortly after these agents are stopped. Currently there is no clear indication for one medication over the other. The advantages of romiplostim are great flexibility in dosing (1–10 µg/kg week) and no concerns about drug interaction. The current drawback of romiplostim is the Food and Drug Administration’s requirement for patients to receive the drug from a clinic and not at home. Eltrombopag offers the advantage of oral use, but it has a limited dose range and potential for drug interactions. Both agents have been associated with marrow reticulin formation, although in clinical use this risk appears to be very low.53
Other Options
In the literature there are numerous options for the treatment of ITP.54,55 Most of these studies are anecdotal, enrolled small number of patients, and sometimes included patients with mild thrombocytopenia, but these therapeutic options can be tried in patients who are refractory to standard therapies and have bleeding. The agents with the greatest amount of supporting data are danazol, vincristine, azathioprine, cyclophosphamide, and fostamatinib.
Danazol 200 mg 4 times daily is thought to downregulate the macrophage Fc receptor. The onset of action may be delayed and a therapeutic trial of up to 4 to 6 months is advised. Danazol is very effective in patients with antiphospholipid antibody syndrome who develop ITP and may be more effective in premenopausal women.56 Once a response is seen, danazol should be continued for 6 months and then an attempt to wean the patient off the agent should be made. A partial response can be seen in 70% to 90% of patients, but a complete response is rare.54
Vincristine 1.4 mg/m2 weekly has a low response rate, but if a response is going to occur, it will occur rapidly within 2 weeks. Thus, a prolonged trial of vincristine is not needed; if no platelet rise is seen in several weeks, the drug should be stopped. Again, partial responses are more common than complete response—50% to 63% versus 0% to 6%.54Azathioprine 150 mg orally daily, like danazol, demonstrates a delayed response and requires several months to assess for response. However, 19% to 25% of patients may have a complete response.54 It has been reported that the related agent mycophenolate 1000 mg twice daily is also effective in ITP.57
Cyclophosphamide 1 g/m2 intravenously repeated every 28 days has been reported to have a response rate of up to 40%.58 Although considered more aggressive, this is a standard immunosuppressive dose and should be considered in patients with very low platelet counts. Patients who have not responded to single-agent cyclophosphamide may respond to multi-agent chemotherapy with agents such as etoposide and vincristine plus cyclophosphamide.59
Fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, is currently under investigation for the treatment of ITP.60 This agent prevents phagocytosis of antibody-coated platelets by macrophages. In early studies fostamatinib has been well tolerated at a dose of 150 mg twice daily, with 75% of patients showing a response. Large phase 3 trials are underway, and if the earlier promising results hold up fostamatinib may be a novel option for refractory patients.
A Practical Approach to Refractory ITP
One approach is to divide patients into bleeders, or those with either very low platelet counts (< 5 × 103/µL) or who have had significant bleeding in the past, and nonbleeders, or those with platelet counts above 5 × 103/µL and no history of severe bleeding. Bleeders who do not respond adequately to splenectomy should first start with rituximab since it is not cytotoxic and is the only other “curative” therapy (Table 2).
Nonbleeders should be tried on danazol and other relatively safe agents. If this fails, rituximab or TPO-RAs can be considered. Before one considers cytotoxic therapy, the risk of the therapy must be weighed against the risk posed by the thrombocytopenia. The mortality from ITP is fairly low (5%) and is restricted to patients with severe disease. Patients with only moderate thrombocytopenia and no bleeding are better served with conservative management. There is little justification for the use of continuous steroid therapy in this group of patients given the long-term risks of this therapy.
Special Situations
Surgery
Patients with ITP who need surgery either for splenectomy or for other reasons should have their platelet counts raised to a level greater than 20 to 30 × 103/µL before surgery. Most patients with ITP have increased platelet function and will not have excessive bleeding with these platelet counts. For patients with platelet counts below this level, an infusion of immune globulin or anti-D may rapidly increase the platelet counts. If the surgery is elective, short-term use of TPO-RAs to raise the counts can also be considered.
Pregnancy
Up to 10% of pregnant women will develop low platelet counts during their pregnancy.61,62 The most common etiology is gestational thrombocytopenia, which is an exaggeration of the lowered platelet count seen in pregnancy. Counts may fall as low as 50 × 103/µL at the time of delivery. No therapy is required as the fetus is not affected and the mother does not have an increased risk of bleeding. Pregnancy complications such as HELLP syndrome and thrombotic microangiopathies also present with low platelet counts, but these can be diagnosed by history.61,63
Women with ITP can either develop the disease during pregnancy or have a worsening of the symptoms.64 Counts often drop dramatically during the first trimester. Early management should be conservative with low doses of prednisone to keep the count above 10 × 103/µL.21 Immunoglobulin is also effective,65 but there are rare reports of pulmonary edema. Rarely patients who are refractory will require splenectomy, which may be safely performed in the second trimester. For delivery the count should be greater than 30 × 103/µL and for an epidural greater than 50 × 103/µL.64 There are reports of the use of TPO-RAs in pregnancy, and this can be considered for refractory cases.66
Most controversy centers on management of the delivery. In the past it was feared that fetal thrombocytopenia could lead to intracranial hemorrhage, and Caesarean section was always recommended. It now appears that most cases of intracranial hemorrhage were due to alloimmune thrombocytopenia and not ITP. Furthermore, the nadir of the baby’s platelet count is not at birth but several days after. It appears the safest course is to proceed with a vaginal or C-section delivery determined by obstetrical indications and then immediately check the baby’s platelet count. If the platelet count is low in the neonate, immunoglobulin will raise the count. Since the neonatal thrombocytopenia is due to passive transfer of maternal antibody, the platelet destruction will abate in 4 to 6 weeks.
Pediatric Patients
The incidence of ITP in children is 2.2 to 5.3 per 100,000 children.1 There are several distinct differences in pediatric ITP. Most cases will resolve in weeks, with only a minority of patients transforming into chronic ITP (5%–10%). Also, the rates of serious bleeding are lower in children than in adults, with intracranial hemorrhage rates of 0.1% to 0.5% being seen.67 For most patients with no or mild bleeding, management now is observation alone regardless of platelet count because it is felt that the risks of therapies are higher than the risk of bleeding.21 For patients with bleeding, IVIG, anti-D, or a short course of steroids can be used. Given the risk of overwhelming sepsis, splenectomy is often deferred as long as possible. Rituximab is increasingly being used in children due to concerns about use of agents such a cyclophosphamide or azathioprine in children.68 Abundant data on use of TPO-RAs in children showing high response rates and safety support their use, and these should be considered in refractory ITP before any cytotoxic agent.69–71
Helicobacter Pylori Infection
There has been much interest in the relationship between H. pylori and ITP.16,72,73H. pylori infections have been associated with a variety of autoimmune diseases, and there is a confusing literature on this infection and ITP. Several meta-analyses have shown that eradication of H. pylori will result in an ITP response rate of 20% to 30%, but responses curiously appear to be limited to certain geographic areas such as Japan and Italy but not the United States. In patients with recalcitrant ITP, especially in geographic areas with high incidence, it may be worthwhile to check for H. pylori infection and treat accordingly if positive.
Drug-Induced Thrombocytopenia
Patients with drug-induced thrombocytopenia present with very low (< 10 × 103/µL) platelet counts 1 to 3 weeks after starting a new medication.74–76 In patients with a possible drug-induced thrombocytopenia, the primary therapy is to stop the suspect drug.77 If there are multiple new medications, the best approach is to stop any drug that has been strongly associated with thrombocytopenia (Table 3).74,78,79
Immune globulin, corticosteroids, or intravenous anti‑D have been suggested as useful in drug‑related thrombocytopenia. However, since most of these thrombocytopenic patients recover when the agent is cleared from the body, this therapy is probably not necessary and withholding treatment avoids exposing the patients to the adverse events associated with further therapy.
Evans Syndrome
Evans syndrome is defined as the combination of autoimmune hemolytic anemia (AIHA) and ITP.80,81 These cytopenias can present simultaneously or sequentially. Patients with Evans syndrome are thought to have a more severe disease process, to be more prone to bleeding, and to be more difficult to treat, but the rarity of this syndrome makes this hard to quantify.
The classic clinical presentation of Evans syndrome is severe anemia and thrombocytopenia. Children with Evans syndrome often have complex immunodeficiencies such as autoimmune lymphoproliferative syndrome.82,83 In adults, Evans syndrome most often complicates other autoimmune diseases such as lupus. There are increasing reports of Evans syndrome occurring as a complication of T-cell lymphomas. Often the autoimmune disease can predate the lymphoma diagnosis by months or even years.
In theory the diagnostic approach is straightforward by showing a Coombs-positive hemolytic anemia in the setting of a clinical diagnosis of immune thrombocytopenia. The blood smear will show spherocytes and a diminished platelet count. The presence of other abnormal red cell forms should raise the possibility of an alternative diagnosis. It is unclear how vigorously one should search for other underlying diseases. Many patients will already have the diagnosis of an underlying autoimmune disease. The presence of lymphadenopathy should raise concern for lymphoma.
Initial therapy is high-dose steroids (2 mg/kg/day). IVIG should be added if severe thrombocytopenia is present. Patients who cannot be weaned off prednisone or relapse after prednisone should be considered for splenectomy, although these patients are at higher risk of relapsing.80 Increasingly rituximab is being used with success.84,85 For patients who fail splenectomy and rituximab, aggressive immunosuppression should be considered. Increasing data support the benefits of sirolimus, and this should be considered for refractory patients.86 For patients with Evans syndrome due to underlying lymphoma, antineoplastic therapy often results in prompt resolution of the symptoms. Recurrence of the autoimmune cytopenias often heralds relapse.
1. Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol 2010;85:174–80.
2. McMillan R, Lopez-Dee J, Bowditch R. Clonal restriction of platelet-associated anti-GPIIb/IIIa autoantibodies in patients with chronic ITP. Thromb Haemost 2001;85:821–3.
3. Aster RH, George JN, McMillan R, Ganguly P. Workshop on autoimmune (idiopathic) thrombocytopenic purpura: Pathogenesis and new approaches to therapy. Am J Hematol 1998;58:231–4.
4. Toltl LJ, Arnold DM. Pathophysiology and management of chronic immune thrombocytopenia: focusing on what matters. Br J Haematol 2011;152:52–60.
5. Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1193–211.
6. Pamuk GE, Pamuk ON, Baslar Z, et al. Overview of 321 patients with idiopathic thrombocytopenic purpura. Retrospective analysis of the clinical features and response to therapy. Ann Hematol 2002;81:436–40.
7. Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med 1995;98:436–42.
8. Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004;104:2623–34.
9. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol 2016;136:101–7.
10. Newton JL, Reese JA, Watson SI, et al. Fatigue in adult patients with primary immune thrombocytopenia. Eur J Haematol 2011;86:420–9.
11. Stasi R, Amadori S, Osborn J, et al. Long-term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med 2006;3:e24.
12. Biino G, Balduini CL, Casula L, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica 2011;96:96–101.
13. Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004;103:390–8.
14. Geddis AE, Balduini CL. Diagnosis of immune thrombocytopenic purpura in children. Curr Opin Hematol 2007;14:520–5.
15. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86.
16. Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1275–97.
17. Jubelirer SJ, Harpold R. The role of the bone marrow examination in the diagnosis of immune thrombocytopenic purpura: case series and literature review. Clin Appl Thromb Hemost 2002;8:73–6.
18. George JN. Management of patients with refractory immune thrombocytopenic purpura. J Thromb Haemost 2006;4:1664–72.
19. Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001;97:2549–54.
20. McMillan R, Bowditch RD, Tani P, et al. A non-thrombocytopenic bleeding disorder due to an IgG4- kappa anti-GPIIb/IIIa autoantibody. Br J Haematol 1996;95:747–9.
21. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207.22. Mazzucconi MG, Fazi P, Bernasconi S, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood 2007;109:1401–7.
23. Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood 2016;127:296–302.
24. Newman GC, Novoa MV, Fodero EM, et al. A dose of 75 microg/kg/d of i.v. anti-D increases the platelet count more rapidly and for a longer period of time than 50 microg/kg/d in adults with immune thrombocytopenic purpura. Br J Haematol 2001;112:1076–8.
25. Gaines AR. Acute onset hemoglobinemia and/or hemoglobinuria and sequelae following Rho(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 2000;95:2523–9.
26. Boruchov DM, Gururangan S, Driscoll MC, Bussel JB. Multiagent induction and maintenance therapy for patients with refractory immune thrombocytopenic purpura (ITP). Blood 2007;110:3526–31.
27. Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol 2008;83:122–5.
28. Olson SR, Chu C, Shatzel JJ, Deloughery TG. The “platelet boilermaker”: A treatment protocol to rapidly increase platelets in patients with immune-mediated thrombocytopenia. Am J Hematol 2016;91:E330–1.
29. Cooper N, Woloski BM, Fodero EM, et al. Does treatment with intermittent infusions of intravenous anti-D allow a proportion of adults with recently diagnosed immune thrombocytopenic purpura to avoid splenectomy? Blood 2002;99:1922–7.
30. George JN, Raskob GE, Vesely SK, et al. Initial management of immune thrombocytopenic purpura in adults: a randomized controlled trial comparing intermittent anti-D with routine care. Am J Hematol 2003;74:161–9.
31. Mikhael J, Northridge K, Lindquist K, et al. Short-term and long-term failure of laparoscopic splenectomy in adult immune thrombocytopenic purpura patients: a systematic review. Am J Hematol 2009;84:743–8.
32. Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol 2016;91:E267–72.
33. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664–73.
34. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect 2001;43:182–6.
35. Mileno MD, Bia FJ. The compromised traveler. Infect Dis Clin North Am 1998;12:369–412.
36. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. BMJ 1996;312:430–4.
37. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents 2003;21:181–8.
38. Tran H, Brighton T, Grigg A, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol 2014;167:243–51.
39. Mahevas M, Ebbo M, Audia S, et al. Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol 2013;88:858–61.
40. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007;146:25–33.
41. Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014;124:3228–36.
42. Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1653–61.
43. Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 2010;115:2755–62.
44. Dameshek W, Miller EB. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. Blood 1946;1:27–50.
45. Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med 2009;60:193–206.
46. Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006;355:1672–81.
47. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:641–8.
48. Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009;113:2161–71.
49. Gernsheimer TB, George JN, Aledort LM, et al. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J Thromb Haemost 2010;8:1372–82.
50. Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol 2011;152:360–2.
51. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007;357:2237–47.
52. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011;377:393–402.
53. Brynes RK, Orazi A, Theodore D, et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: Data from the EXTEND study. Am J Hematol 2015;90:598–601.
54. George JN, Kojouri K, Perdue JJ, Vesely SK. Management of patients with chronic, refractory idiopathic thrombocytopenic purpura. Semin Hematol 2000;37:290–8.
55. McMillan R. Therapy for adults with refractory chronic immune thrombocytopenic purpura. Ann Intern Med 1997;126:307–14.
56. Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, et al. Successful therapy with danazol in refractory autoimmune thrombocytopenia associated with rheumatic diseases. Br J Rheumatol 1997;36:1095–9.
57. Provan D, Moss AJ, Newland AC, Bussel JB. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol 2006;81:19–25.
58. Reiner A, Gernsheimer T, Slichter SJ. Pulse cyclophosphamide therapy for refractory autoimmune thrombocytopenic purpura. Blood 1995;85:351–8.
59. Figueroa M, Gehlsen J, Hammond D, et al. Combination chemotherapy in refractory immune thrombocytopenic purpura. N Engl J Med 1993;328:1226–9.
60. Newland A, Lee EJ, McDonald V, Bussel JB. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy 2 Oct 2017.
61. McCrae KR. Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program 2010;2010:397–402.
62. Gernsheimer T, McCrae KR. Immune thrombocytopenic purpura in pregnancy. Curr Opin Hematol 2007;14:574–80.
63. DeLoughery TG. Critical care clotting catastrophies. Crit Care Clin 2005;21:531–62.
64. Stavrou E, McCrae KR. Immune thrombocytopenia in pregnancy. Hematol Oncol Clin North Am 2009;23:1299–316.
65. Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016;128:1329–35.
66. Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood 2017;130:1097–103.
67. Psaila B, Petrovic A, Page LK, et al. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood 2009;114:4777–83.
68. Journeycake JM. Childhood immune thrombocytopenia: role of rituximab, recombinant thrombopoietin, and other new therapeutics. Hematology Am Soc Hematol Educ Program 2012;2012:444–9.
69. Zhang J, Liang Y, Ai Y, et al. Thrombopoietin-receptor agonists for children with immune thrombocytopenia: a systematic review. Expert Opin Pharmacother 2017;18:1543–51.
70. Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016;388:45–54.71. Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet 2015;386:1649–58.
72. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113:1231–40.
73. Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica 2009;94:850–6.
74. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580–7.
75. Reese JA, Li X, Hauben M, et al. Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood 2010;116:2127–33.
76. Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis and management. J Thromb Haemost 2009;7:911–8.
77. Zondor SD, George JN, Medina PJ. Treatment of drug-induced thrombocytopenia. Expert Opin Drug Saf 2002;1:173–80.
78. George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: A systematic review of published case reports. Ann Intern Med 1998;129:886–90.
79. Green D, Hougie C, Kazmier FJ, et al. Report of the working party on acquired inhibitors of coagulation: studies of the “lupus” anticoagulant. Thromb Haemost 1983;49:144–6.
80. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 2009;114:3167–72.
81. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology 2008;13:356–60.
82. Notarangelo LD. Primary immunodeficiencies (PIDs) presenting with cytopenias. Hematology Am Soc Hematol Educ Program 2009:139–43.
83. Martinez-Valdez L, Deya-Martinez A, Giner MT, et al. Evans syndrome as first manifestation of primary immunodeficiency in clinical practice. J Pediatr Hematol Oncol 2017;39:490–4.
84. Shanafelt TD, Madueme HL, Wolf RC, Tefferi A. Rituximab for immune cytopenia in adults: idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, and Evans syndrome. Mayo Clin Proc 2003;78:1340–6.
85. Mantadakis E, Danilatou V, Stiakaki E, Kalmanti M. Rituximab for refractory Evans syndrome and other immune-mediated hematologic diseases. Am J Hematol 2004;77:303–10.
86. Jasinski S, Weinblatt ME, Glasser CL. Sirolimus as an effective agent in the treatment of immune thrombocytopenia (ITP) and Evans syndrome (ES): a single institution’s experience. J Pediatr Hematol Oncol 2017;39:420–4.
1. Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol 2010;85:174–80.
2. McMillan R, Lopez-Dee J, Bowditch R. Clonal restriction of platelet-associated anti-GPIIb/IIIa autoantibodies in patients with chronic ITP. Thromb Haemost 2001;85:821–3.
3. Aster RH, George JN, McMillan R, Ganguly P. Workshop on autoimmune (idiopathic) thrombocytopenic purpura: Pathogenesis and new approaches to therapy. Am J Hematol 1998;58:231–4.
4. Toltl LJ, Arnold DM. Pathophysiology and management of chronic immune thrombocytopenia: focusing on what matters. Br J Haematol 2011;152:52–60.
5. Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1193–211.
6. Pamuk GE, Pamuk ON, Baslar Z, et al. Overview of 321 patients with idiopathic thrombocytopenic purpura. Retrospective analysis of the clinical features and response to therapy. Ann Hematol 2002;81:436–40.
7. Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med 1995;98:436–42.
8. Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004;104:2623–34.
9. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol 2016;136:101–7.
10. Newton JL, Reese JA, Watson SI, et al. Fatigue in adult patients with primary immune thrombocytopenia. Eur J Haematol 2011;86:420–9.
11. Stasi R, Amadori S, Osborn J, et al. Long-term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med 2006;3:e24.
12. Biino G, Balduini CL, Casula L, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica 2011;96:96–101.
13. Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004;103:390–8.
14. Geddis AE, Balduini CL. Diagnosis of immune thrombocytopenic purpura in children. Curr Opin Hematol 2007;14:520–5.
15. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86.
16. Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am 2009;23:1275–97.
17. Jubelirer SJ, Harpold R. The role of the bone marrow examination in the diagnosis of immune thrombocytopenic purpura: case series and literature review. Clin Appl Thromb Hemost 2002;8:73–6.
18. George JN. Management of patients with refractory immune thrombocytopenic purpura. J Thromb Haemost 2006;4:1664–72.
19. Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001;97:2549–54.
20. McMillan R, Bowditch RD, Tani P, et al. A non-thrombocytopenic bleeding disorder due to an IgG4- kappa anti-GPIIb/IIIa autoantibody. Br J Haematol 1996;95:747–9.
21. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207.22. Mazzucconi MG, Fazi P, Bernasconi S, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood 2007;109:1401–7.
23. Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood 2016;127:296–302.
24. Newman GC, Novoa MV, Fodero EM, et al. A dose of 75 microg/kg/d of i.v. anti-D increases the platelet count more rapidly and for a longer period of time than 50 microg/kg/d in adults with immune thrombocytopenic purpura. Br J Haematol 2001;112:1076–8.
25. Gaines AR. Acute onset hemoglobinemia and/or hemoglobinuria and sequelae following Rho(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 2000;95:2523–9.
26. Boruchov DM, Gururangan S, Driscoll MC, Bussel JB. Multiagent induction and maintenance therapy for patients with refractory immune thrombocytopenic purpura (ITP). Blood 2007;110:3526–31.
27. Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol 2008;83:122–5.
28. Olson SR, Chu C, Shatzel JJ, Deloughery TG. The “platelet boilermaker”: A treatment protocol to rapidly increase platelets in patients with immune-mediated thrombocytopenia. Am J Hematol 2016;91:E330–1.
29. Cooper N, Woloski BM, Fodero EM, et al. Does treatment with intermittent infusions of intravenous anti-D allow a proportion of adults with recently diagnosed immune thrombocytopenic purpura to avoid splenectomy? Blood 2002;99:1922–7.
30. George JN, Raskob GE, Vesely SK, et al. Initial management of immune thrombocytopenic purpura in adults: a randomized controlled trial comparing intermittent anti-D with routine care. Am J Hematol 2003;74:161–9.
31. Mikhael J, Northridge K, Lindquist K, et al. Short-term and long-term failure of laparoscopic splenectomy in adult immune thrombocytopenic purpura patients: a systematic review. Am J Hematol 2009;84:743–8.
32. Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol 2016;91:E267–72.
33. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664–73.
34. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect 2001;43:182–6.
35. Mileno MD, Bia FJ. The compromised traveler. Infect Dis Clin North Am 1998;12:369–412.
36. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. BMJ 1996;312:430–4.
37. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents 2003;21:181–8.
38. Tran H, Brighton T, Grigg A, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol 2014;167:243–51.
39. Mahevas M, Ebbo M, Audia S, et al. Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol 2013;88:858–61.
40. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007;146:25–33.
41. Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014;124:3228–36.
42. Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1653–61.
43. Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 2010;115:2755–62.
44. Dameshek W, Miller EB. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. Blood 1946;1:27–50.
45. Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med 2009;60:193–206.
46. Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006;355:1672–81.
47. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:641–8.
48. Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009;113:2161–71.
49. Gernsheimer TB, George JN, Aledort LM, et al. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J Thromb Haemost 2010;8:1372–82.
50. Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol 2011;152:360–2.
51. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007;357:2237–47.
52. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011;377:393–402.
53. Brynes RK, Orazi A, Theodore D, et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: Data from the EXTEND study. Am J Hematol 2015;90:598–601.
54. George JN, Kojouri K, Perdue JJ, Vesely SK. Management of patients with chronic, refractory idiopathic thrombocytopenic purpura. Semin Hematol 2000;37:290–8.
55. McMillan R. Therapy for adults with refractory chronic immune thrombocytopenic purpura. Ann Intern Med 1997;126:307–14.
56. Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, et al. Successful therapy with danazol in refractory autoimmune thrombocytopenia associated with rheumatic diseases. Br J Rheumatol 1997;36:1095–9.
57. Provan D, Moss AJ, Newland AC, Bussel JB. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol 2006;81:19–25.
58. Reiner A, Gernsheimer T, Slichter SJ. Pulse cyclophosphamide therapy for refractory autoimmune thrombocytopenic purpura. Blood 1995;85:351–8.
59. Figueroa M, Gehlsen J, Hammond D, et al. Combination chemotherapy in refractory immune thrombocytopenic purpura. N Engl J Med 1993;328:1226–9.
60. Newland A, Lee EJ, McDonald V, Bussel JB. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy 2 Oct 2017.
61. McCrae KR. Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program 2010;2010:397–402.
62. Gernsheimer T, McCrae KR. Immune thrombocytopenic purpura in pregnancy. Curr Opin Hematol 2007;14:574–80.
63. DeLoughery TG. Critical care clotting catastrophies. Crit Care Clin 2005;21:531–62.
64. Stavrou E, McCrae KR. Immune thrombocytopenia in pregnancy. Hematol Oncol Clin North Am 2009;23:1299–316.
65. Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016;128:1329–35.
66. Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood 2017;130:1097–103.
67. Psaila B, Petrovic A, Page LK, et al. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood 2009;114:4777–83.
68. Journeycake JM. Childhood immune thrombocytopenia: role of rituximab, recombinant thrombopoietin, and other new therapeutics. Hematology Am Soc Hematol Educ Program 2012;2012:444–9.
69. Zhang J, Liang Y, Ai Y, et al. Thrombopoietin-receptor agonists for children with immune thrombocytopenia: a systematic review. Expert Opin Pharmacother 2017;18:1543–51.
70. Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016;388:45–54.71. Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet 2015;386:1649–58.
72. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113:1231–40.
73. Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica 2009;94:850–6.
74. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580–7.
75. Reese JA, Li X, Hauben M, et al. Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood 2010;116:2127–33.
76. Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis and management. J Thromb Haemost 2009;7:911–8.
77. Zondor SD, George JN, Medina PJ. Treatment of drug-induced thrombocytopenia. Expert Opin Drug Saf 2002;1:173–80.
78. George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: A systematic review of published case reports. Ann Intern Med 1998;129:886–90.
79. Green D, Hougie C, Kazmier FJ, et al. Report of the working party on acquired inhibitors of coagulation: studies of the “lupus” anticoagulant. Thromb Haemost 1983;49:144–6.
80. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 2009;114:3167–72.
81. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology 2008;13:356–60.
82. Notarangelo LD. Primary immunodeficiencies (PIDs) presenting with cytopenias. Hematology Am Soc Hematol Educ Program 2009:139–43.
83. Martinez-Valdez L, Deya-Martinez A, Giner MT, et al. Evans syndrome as first manifestation of primary immunodeficiency in clinical practice. J Pediatr Hematol Oncol 2017;39:490–4.
84. Shanafelt TD, Madueme HL, Wolf RC, Tefferi A. Rituximab for immune cytopenia in adults: idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, and Evans syndrome. Mayo Clin Proc 2003;78:1340–6.
85. Mantadakis E, Danilatou V, Stiakaki E, Kalmanti M. Rituximab for refractory Evans syndrome and other immune-mediated hematologic diseases. Am J Hematol 2004;77:303–10.
86. Jasinski S, Weinblatt ME, Glasser CL. Sirolimus as an effective agent in the treatment of immune thrombocytopenia (ITP) and Evans syndrome (ES): a single institution’s experience. J Pediatr Hematol Oncol 2017;39:420–4.
Hairy Cell Leukemia
Introduction
Hairy cell leukemia (HCL) is a rare chronic lymphoproliferative disorder, with only approximately 2000 new cases diagnosed in the United States each year.1 It is now recognized that there are 2 distinct categories of HCL, classic HCL (cHCL) and variant HCL (vHCL), with vHCL now classified as a separate entity under the World Health Organization Classification of Hematopoietic Tumors.2 For this reason, the 2 diseases will be discussed separately. However, they do bear many clinical and microscopic similarities and because of this were originally indistinguishable using diagnostic techniques previously available. Even in the modern era using immunophenotypic, molecular, and genetic testing, differentiating between the classic and variant disease subtypes is sometimes difficult.
For cHCL the median age of diagnosis is 55 years, with vHCL occurring in patients who are somewhat older; HCL has been described only in the adult population, with 1 exception.3,4 There is a 4:1 male predominance, and Caucasians are more frequently affected than other ethnic groups. While the cause of the disease remains largely unknown, it has been observed to occur more frequently in farmers and in persons exposed to pesticides and/or herbicides, petroleum products, and ionizing radiation.4 The Institute of Medicine recently updated their position regarding veterans and Agent Orange, stating that there is sufficient evidence of an association between herbicides and chronic lymphoid leukemias (including HCL) to consider these diseases linked to exposure.5 Familial forms have also been described that are associated with specific HLA haplotypes, indicating a possible hereditary component.6 Most likely, a combination of environmental and genetic factors ultimately contributes to the development of HCL.
In recent years enormous progress has been made with respect to new insights into the biology of cHCL and vHCL, with significant refinement of diagnostic criteria. In addition, tremendous advances have occurred in both treatment and supportive care regimens, which have resulted in a dramatically increased overall life expectancy as well as decreased disease-related morbidity. This has meant that more patients are affected by HCL over time and are more likely to require care for relapsed HCL or associated comorbidities. Although no curative treatment options exist outside of allogeneic transplantation, therapeutic improvements have resulted in patients with cHCL having a life expectancy similar to that of unaffected patients, increasing the need for vigilance to prevent foreseeable complications.
Biology and Patheogenisis
The family of HCLs are chronic B-cell malignancies that account for approximately 2% of all diagnosed leukemias.7 The first detailed characterization of HCL as a distinct clinical entity was performed by Dr. Bouroncle and colleagues at the Ohio State University in 1958.8 Originally called leukemic reticuloendotheliosis, it was renamed HCL following more detailed description of the unique morphology of these malignant cells.9 Significant advances have recently been made in identifying distinctive genetic, immunophenotypic, and morphologic features that distinguish HCL from other B-cell malignancies.
HCL B cells tend to accumulate in the bone marrow, splenic red pulp, and (in some cases) peripheral blood. Unlike other lymphoproliferative disorders, HCL only rarely results in lymphadenopathy. HCL derives its name from the distinct appearance of the malignant hairy cells (Figure). Morphologically, HCL cells are mature, small lymphoid B-cells with a round or oval nucleus and abundant pale blue cytoplasm. Irregular projections of cytoplasm and microvilli give the cells a serrated, “hairy” appearance.10 The biological significance of these fine hair-like projections remains unknown and is an area of ongoing investigation. Gene expression profiling has revealed that HCL B cells are most similar to splenic marginal zone B cells and memory B cells.11–13 A recent analysis of common genetic alterations in HCL suggests that the cell of origin is in fact the hematopoietic stem cell.14
Compared to other hematologic malignancies, the genomic profile of HCL is relatively stable, with few chromosomal defects or translocations observed. A seminal study by Tiacci and colleagues revealed that the BRAF V600E mutation was present in 47 out of 47 cHCL cases examined, results that have since been replicated by other groups, confirming that BRAF V600E is a hallmark mutation in cHCL.15 The BRAF V600E gain-of-function mutation results in constitutive activation of the serine-threonine protein kinase B-Raf, which regulates the mitogen-activated protein kinase (MAPK)/RAF-MEK-ERK pathway. Indeed, cHCL B cells have elevated MAPK signaling, leading to enhancement of growth and survival.16 This specific mutation in the BRAF gene is also seen in a number of solid tumor malignancies including melanoma and thyroid cancer, and represents a therapeutic target using BRAF inhibitors already developed to treat these malignancies.17 Testing for BRAF V600E by polymerase chain reaction or immunohistochemical staining is now routinely performed when HCL is suspected.
While BRAF V600E is identified in nearly all cases of cHCL, it is rare in vHCL.18 The variant type of HCL was classified as a distinct clinical entity in 2008 and can now often be distinguished from cHCL on the basis of BRAF mutational status, among other differences. Interestingly, in the rare cases of BRAF V600E–negative cHCL, other mutations in BRAF or downstream targets as well as aberrant activation of the RAF-MEK-ERK signaling cascade are observed, indicating that this pathway is critical in HCL and may still represent a viable therapeutic target. Expression of the IGHV4-34 immunoglobulin rearrangement, while more common in vHCL, has also been identified in 10% of cHCL cases and appears to confer poor prognosis.19 Other mutated genes that have been identified in HCL include CDKN1B, TP53, U2AF1, ARID1A, EZH2, and KDM6A.20
Classic HCL is characterized by the immunophenotypic expression of CD11c, CD25, CD103, and CD123, with kappa or lambda light chain restriction indicating clonality; HCL B cells are generally negative for CD5, CD10, CD23, CD27, and CD79b. In contrast, vHCL often lacks expression of CD25 and CD123.18 The B-cell receptor (BCR) is expressed on hairy cells and its activation promotes proliferation and survival in vitro.21 The role of BCR signaling in B-cell malignancies is increasingly recognized, and therapies that target the BCR and associated signaling molecules offer an attractive treatment strategy.22 HCL B cells also typically express CD19, CD20, CD22, CD79a, CD200, CD1d, and annexin A1. Tartrate-resistant acid phosphatase (TRAP) positivity by immunohistochemistry is a hallmark of cHCL. Interestingly, changes to the patient’s original immunophenotype have been observed following treatment and upon disease recurrence, highlighting the importance of tracking immunophenotype throughout the course of disease.
Diagnosis
Prior to the advent of annual screening evaluations with routine examination of complete blood counts (CBC), patients were most often diagnosed with HCL when they presented with symptoms of the disease such as splenomegaly, infections, or complications of anemia or thrombocytopenia.23 In the current era, patients are more likely to be incidentally diagnosed when they are found to have an abnormal value on a CBC. Any blood lineage may be affected and patients may have pancytopenia or isolated cytopenias. Of note, monocytopenia is a common finding in cHCL that is not entirely understood. The cells typical of cHCL do not usually circulate in the peripheral blood, but if present would appear as mature lymphocytes with villous cytoplasmic projections, pale blue cytoplasm, and reniform nuclei with open chromatin (Figure).9 Even if the morphologic examination is highly suggestive of HCL, additional testing is required to differentiate between cHCL, vHCL, and other hematologic malignancies which may also have cytoplasmic projections. A complete assessment of the immunophenotype, molecular profile, and cytogenetic features is required to arrive at this diagnosis.
The international Hairy Cell Leukemia Foundation recently published consensus guidelines for the diagnosis and treatment of HCL.24 These guidelines recommend that patients undergo examination of the peripheral blood for morphology and immunophenotyping and further recommend obtaining bone marrow core and aspirate biopsy samples for immunophenotyping via immunohistochemical staining and flow cytometry. The characteristic immunophenotype of cHCL is a population of monoclonal B lymphocytes which co-express CD19, CD20, CD11c, CD25, CD103, and CD123. Variant HCL is characterized by a very similar immunophenotype but is usually negative for CD25 and CD123. It is notable that CD25 positivity may be lost following treatment, and the absence of this marker should not be used as the sole basis of a cHCL versus vHCL diagnosis. Because marrow fibrosis in HCL may prevent a marrow aspirate from being obtained, many of the key diagnostic studies are performed on the core biopsy, including morphological evaluation and immunohistochemical stains such as CD20 (a pan-B cell antigen), annexin-1 (an anti-inflammatory protein expressed only in cHCL), and VE1 (a BRAF V600E stain).
As noted above, recurrent cytogenetic abnormalities have now been identified that may inform the diagnosis or prognosis of HCL. Next-generation sequencing and other testing of the genetic landscape are taking on a larger role in subtype differentiation, and it is likely that future guidelines will recommend evaluation for significant mutations. Given that BRAF V600E mutation status is a key feature of cHCL and is absent in vHCL, it is important to perform this testing at the time of diagnosis whenever possible. The mutation may be detected via VE1 immunohistochemical staining, allele-specific polymerase chain reaction, or next-generation sequencing. Other less sensitive tests exist but are utilized less frequently.
Minimal Residual Disease
There is currently no accepted standard for minimal residual disease (MRD) monitoring in HCL. While detection of MRD has been clearly associated with increased risk of disease progression, cHCL cells typically do not circulate in the peripheral blood, limiting the use of peripheral blood immunophenotyping for quantitative MRD assessment. For quantitative monitoring of marrow involvement by HCL, immunohistochemical staining of the bone marrow core biopsy is usually required. Staining may be performed for CD20, or, in patients who have received anti-CD20 therapy, DBA.44, VE-1, or CD79a. There is currently not a consensus regarding what level of disease involvement constitutes MRD. One group studied this issue and found that relapse could be predicted by evaluating MRD by percentage of positive cells in the marrow by immunohistochemical staining, with less than 1% involvement having the lowest risk for disease relapse and greater than 5% having the highest risk for disease relapse.25 A recent study evaluated MRD patterns in the peripheral blood of 32 cHCL patients who had completed frontline therapy. This group performed flow cytometry on the peripheral blood of patients at 1, 3, 6, and 12 months following therapy. All patients had achieved a complete response with initial therapy and peripheral blood MRD negativity at the completion of therapy. At a median follow-up of 100 months post therapy, 5 patients converted from peripheral blood–MRD negative to peripheral blood–MRD positive, and 6 patients developed overt disease progression. In all patients who progressed, progression was preceded by an increase in detectable peripheral blood MRD cells.26 Although larger studies are needed, peripheral blood flow cytometric monitoring for MRD may be a useful adjunct to predict ongoing response or impending relapse. In addition, newer, more sensitive methods of disease monitoring may ultimately supplant flow cytometry.
Risk Stratification
Although much progress has been made in the risk stratification profiling of hematologic malignancies in general, HCL has unfortunately lagged behind in this effort. The most recent risk stratification analysis was performed in 1982 by Jansen and colleagues.27 This group of researchers performed a retrospective analysis of 391 HCL patients treated at 22 centers. One of the central questions in their analysis was survival time from diagnosis in patients who had not yet undergone splenectomy (a standard treatment at the time). This group consisted of a total of 154 patients. As this study predated modern pathological and molecular testing, clinical and laboratory features were examined, and these mostly consisted of physical exam findings and analysis of the peripheral blood. This group found that several factors influenced the survival of these patients, including duration of symptoms prior to diagnosis, the degree of splenomegaly, hemoglobin level, and number of hairy cells in the peripheral blood. However, because of interobserver variation for the majority of these variables, only hemoglobin and spleen size were included in the proportional hazard model. Using only these 2 variables, the authors were able to determine 3 clinical stages for HCL (Table 1). The stages were found to correlate with median survival: patients with stage 1 disease had a median survival not reached at 72 months, but patients with stage 2 disease had a median survival of 18 months, which decreased to only 12 months in patients with stage 3 disease.
Because the majority of patients with HCL in the modern era will be diagnosed prior to reaching stage 3, a risk stratification system incorporating clinical features, laboratory parameters, and molecular and genetic testing is of considerable interest and is a subject of ongoing research. Ultimately, the goal will be to identify patients at higher risk of early relapse so that more intensive therapies can be applied to initial treatment that will result in longer treatment-free intervals.
Treatment
Because there is no curative treatment for either cHCL or vHCL outside allogeneic transplantation, and it is not clear that early treatment leads to better outcomes in HCL, patients do not always receive treatment at the time of diagnosis or relapse. The general consensus is that patients should be treated if there is a declining trend in hematologic parameters or they experience symptoms from the disease.24 Current consensus guidelines recommend treatment when any of the following hematologic parameters are met: hemoglobin less than 11 g/dL, platelet count less than 100 × 103/µL, or absolute neutrophil count less than 1000/µL.24 These parameters are surrogate markers that indicate compromised bone marrow function. Cytopenias may also be caused by splenomegaly, and symptomatic splenomegaly with or without cytopenias is an indication for treatment. A small number of patients with HCL (approximately 10%) do not require immediate therapy after diagnosis and are monitored by their provider until treatment is indicated.
First-Line Therapy
Despite advances in targeted therapies for HCL, because no treatment has been shown to extend the treatment-free interval longer than chemotherapy, treatment with a purine nucleoside analog is usually the recommended first-line therapy. This includes either cladribine or pentostatin. Both agents appear to be equally effective, and the choice of therapy is determined by the treating physician based on his or her experience. Cladribine administration has been studied using a number of different schedules and routes: intravenous continuous infusion (0.1 mg/kg) for 7 days, intravenous infusion (0.14 mg/kg/day) over 2 hours on a 5-day regimen, or alternatively subcutaneously (0.1–0.14 mg/kg/day) on a once-per-day or once-per-week regimen (Table 2).28,29
Unlike cHCL, vHCL remains difficult to treat and early disease progression is common. The best outcomes have been seen in patients who have received combination chemo-immunotherapy such as purine nucleoside analog therapy plus rituximab or bendamustine plus rituximab.31 One pilot study of bendamustine plus rituximab in 12 patients found an overall response rate of 100%, with the majority of patients achieving a complete response.31 For patients who achieved a complete response, the median duration of response had not been reached, but patients achieving only a partial response had a median duration of response of only 20 months, indicating there is a subgroup of patients who will require a different treatment approach.32 A randomized phase 2 trial of rituximab with either pentostatin or bendamustine is ongoing.33
Assessment of Response
Response assessment involves physical examination for estimation of spleen size, assessment of hematologic parameters, and a bone marrow biopsy for evaluation of marrow response. It is recommended that the bone marrow biopsy be performed 4 to 6 months following cladribine administration, or after completion of 12 doses of pentostatin. Detailed response assessment criteria are shown in Table 3.
Second-Line Therapy
Although the majority of patients treated with purine analogs will achieve durable remissions, approximately 40% of patients will eventually require second-line therapy. Criteria for treatment at relapse are the same as the criteria for initial therapy, including symptomatic disease or progressive anemia, thrombocytopenia, or neutropenia. The choice of treatment is based on clinical parameters and the duration of the previous remission. If the initial remission was longer than 65 months and the patient is eligible to receive chemotherapy, re-treatment with initial therapy is recommended. For a remission between 24 and 65 months, re-treatment with a purine analog combined with an anti-CD20 monoclonal antibody may be considered.34 If the first remission is shorter than 24 months, confirmation of the original diagnosis as well as consideration for testing for additional mutations with therapeutic targets (BRAF V600E, MAP2K1) should be considered before a treatment decision is made. For these patients, alternative therapies, including investigational agents, should be considered.24
Monoclonal antibody therapy has been studied in both the up-front setting and in relapsed or refractory HCL.35 An initial study of 15 patients with relapsed HCL found an overall response rate of 80%, with 8 patients achieving a complete response. A subsequent study of 26 patients who relapsed after cladribine therapy found an overall response rate of 80%, with a complete response rate of 32%. Median relapse-free survival was 27 months.36 Ravandi and others studied rituximab in the up-front setting in combination with cladribine, and found an overall response rate of 100%, including in patients with vHCL. At the time of publication of the study results, the median survival had not been reached.37 As has been seen with other lymphoid malignancies, concurrent therapy with rituximab appears to enhance the activity of the agent with which it is combined. While its use in the up-front setting remains an area of active investigation, there is a clear role for chemo-immunotherapy in the relapsed setting.
In patients with cHCL, excellent results including complete remissions have been reported with the use of BRAF inhibitors, both as a single agent and when combined with anti-CD20 therapy. The 2 commercially available BRAF inhibitors are vemurafenib and dabrafenib, and both have been tested in relapsed cHCL.38,39 The first study of vemurafenib was reported by Tiacci and colleagues, who found an overall response rate of 96% after a median of 8 weeks and a 100% response rate after a median of 12 weeks, with complete response rates up to 42%.38 The median relapse-free survival was 23 months (decreasing to only 6 months in patients who achieved only a partial remission), indicating that these agents will likely need to be administered in combination with other effective therapies with non-overlapping toxicities. Vemurafenib has been administered concurrently with rituximab, and preliminary results of this combination therapy showed early rates of complete responses.40 Dabrafenib has been reported for use as a single agent in cHCL and clinical trials are underway evaluating its efficacy when administered with trametinib, a MEK inhibitor.39,41 Of note, patients receiving BRAF inhibitors frequently develop cutaneous complications of RAF inhibition including cutaneous squamous cell carcinomas and keratoacanthomas, and close dermatologic surveillance is required.
Variant HCL does not harbor the BRAF V600E mutation, but up to half of patients have been found to have mutations of MAP2K1, which upregulates MEK1 expression.42 Trametinib is approved by the US Food and Drug Administration for the treatment of patients with melanoma at a dose of 2 mg orally daily, and has been successfully used to treat 1 patient with vHCL.43 Further evaluation of this targeted therapy is underway.
Ibrutinib, a Bruton tyrosine kinase inhibitor, and moxetumomab pasudotox, an immunotoxin conjugate, are currently being studied in National Institutes of Health–sponsored multi-institutional trials for patients with HCL. Ibrutinib is administered orally at 420 mg per day until relapse.44 Moxetumomab pasudotox was tested at different doses between 5 and 50 μg/kg intravenously every other day for 3 doses for up to 16 cycles unless they experienced disease progression or developed neutralizing antibodies.45 Both agents have been shown to have significant activity in cHCL and vHCL and will likely be included in the treatment armamentarium once trials are completed. Second-line therapy options are summarized in Table 4.
Complications and Supportive Care
The complications of HCL may be separated into the pre-, intra-, and post-treatment periods. At the time of diagnosis and prior to the initiation of therapy, marrow infiltration by HCL frequently leads to cytopenias which cause symptomatic anemia, infection, and/or bleeding complications. Many patients develop splenomegaly, which may further lower the blood counts and which is experienced as abdominal fullness or distention, with early satiety leading to weight loss. Patients may also experience constitutional symptoms with fatigue, fevers in the absence of infection, and unintentional weight loss even without splenomegaly.
For patients who initiate therapy with purine nucleoside analogs, the early part of treatment is associated with the greatest risk of morbidity and mortality. Chemotherapy leads to both immunosuppression (altered cellular immunity) as well as myelosuppression. Thus, patients who are already in need of treatment because of disease-related cytopenias will experience an abrupt and sometimes significant decline in the peripheral blood counts. The treatment period prior to recovery of neutrophils requires the greatest vigilance. Because patients are profoundly immunocompromised, febrile neutropenia is a common complication leading to hospital admission and the cause is often difficult to identify. Treatment with broad-spectrum antibiotics, investigation for opportunistic and viral infections, and considerations for antifungal prophylaxis or therapy are required in this setting. It is recommended that all patients treated with purine nucleoside analogs receive prophylactic antimicrobials for herpes simplex virus and varicella zoster virus, as well as prophylaxis against Pneumocystis jirovecii. Unfortunately, growth factor support has not proven successful in this patient population but is not contraindicated.46
Following successful completion of therapy, patients may remain functionally immunocompromised for a significant period of time even with a normal neutrophil count. Monitoring of the CD4 count may help to determine when prophylactic antimicrobials may be discontinued. A CD4 count greater than 200 cells/µL is generally considered to be adequate for prevention of opportunistic infections. Although immunizations have not been well studied in HCL, it is recommended that patients receive annual influenza immunizations as well as age-appropriate immunizations against Streptococcus pneumoniae and other infectious illnesses as indicated. Live viral vaccines such as the currently available herpes zoster vaccine can lead to infections in this patient population and are not recommended.
Like many hematologic malignancies, HCL may be associated with comorbid conditions related to immune dysfunction. There is a known association with an increased risk of second primary malignancies, which may predate the diagnosis of HCL.47 Therefore, it is recommended that patients continue annual cancer screenings as well as undergo prompt evaluation for potential symptoms of second malignancies. In addition, it is thought that there may be an increased risk for autoimmune disorders such as inflammatory arthritis or immune-mediated cytopenias. One case-control study found a possible association between autoimmune diseases and HCL, noting that at times these diseases are diagnosed concurrently.48 However, because of the rarity of the disease it has been difficult to quantify these associated conditions in a systematic way. There is currently an international patient data registry under development for the systematic study of HCL and its complications which may answer many of these questions.
Survivorship and quality of life are important considerations in chronic diseases. It is not uncommon for patients to develop anxiety related to the trauma of diagnosis and treatment, especially when intensive care has been required. Patients may have lingering fears regarding concerns of developing infections due to exposure to ill persons or fears regarding risk of relapse and need for re-treatment. A proactive approach with partnership with psychosocial oncology may be of benefit, especially when symptoms of post-traumatic stress disorder are evident.
Conclusion
HCL is a rare, chronic lymphoid malignancy that is now subclassified into classic and variant HCL. Further investigations into the disease subtypes will allow more precise disease definitions, and these studies are underway. Renewed efforts toward updated risk stratification and clinical staging systems will be important aspects of these investigations. Refinements in treatment and supportive care have resulted in greatly improved overall survival, which has translated into larger numbers of people living with HCL. However, new treatment paradigms for vHCL are needed as the progression-free survival in this disease remains significantly lower than that of cHCL. Future efforts toward understanding survivorship issues and management of long-term treatment and disease-related complications will be critical for ensuring good quality of life for patients living with HCL.
1. Teras LR, Desantis DE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
2. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008.
3. Yetgin S, Olcay L, Yenicesu I, et al. Relapse in hairy cell leukemia due to isolated nodular skin infiltration. Pediatr Hematol Oncol 2001;18:415–7.
4. Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:175–9.
5. Veterans and agent orange: update 2014. Mil Med 2017;182:1619–20.
6. Villemagne B, Bay JO, Tournilhac O, et al. Two new cases of familial hairy cell leukemia associated with HLA haplotypes A2, B7, Bw4, Bw6. Leuk Lymphoma 2005;46:243–5.
7. Chandran R, Gardiner SK, Smith SD, Spurgeon SE. Improved survival in hairy cell leukaemia over three decades: a SEER database analysis of prognostic factors. Br J Haematol 2013;163:407–9.
8. Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood 1958;13:609–30.
9. Schrek R, Donnelly WJ. “Hairy” cells in blood in lymphoreticular neoplastic disease and “flagellated” cells of normal lymph nodes. Blood 1966;27:199–211.
10. Polliack A, Tadmor T. Surface topography of hairy cell leukemia cells compared to other leukemias as seen by scanning electron microscopy. Leuk Lymphoma 2011;52 Suppl 2:14–7.
11. Miranda RN, Cousar JB, Hammer RD, et al. Somatic mutation analysis of IgH variable regions reveals that tumor cells of most parafollicular (monocytoid) B-cell lymphoma, splenic marginal zone B-cell lymphoma, and some hairy cell leukemia are composed of memory B lymphocytes. Hum Pathol 1999;30:306–12.
12. Vanhentenrijk V, Tierens A, Wlodarska I, et al. V(H) gene analysis of hairy cell leukemia reveals a homogeneous mutation status and suggests its marginal zone B-cell origin. Leukemia 2004;18:1729–32.
13. Basso K, Liso A, Tiacci E, et al. Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. J Exp Med 2004;199:59–68.
14. Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci Transl Med 2014;6:238ra71.
15. Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305–15.
16. Kamiguti AS, Harris RJ, Slupsky JR, et al. Regulation of hairy-cell survival through constitutive activation of mitogen-activated protein kinase pathways. Oncogene 2003;22:2272–84.
17. Rahman MA, Salajegheh A, Smith RA, Lam AK. BRAF inhibitors: From the laboratory to clinical trials. Crit Rev Oncol Hematol 2014;90:220–32.
18. Shao H, Calvo KR, Gronborg M, et al. Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria. Leuk Res 2013;37:401–9.
19. Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood 2012;119:3330–2.
20. Jain P, Pemmaraju N, Ravandi F. Update on the biology and treatment options for hairy cell leukemia. Curr Treat Options Oncol 2014;15:187–209.
21. Sivina M, Kreitman RJ, Arons E, et al. The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: a new therapeutic approach. Br J Haematol 2014;166:177–88.
22. Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood 2015;126:842–50.
23. Andritsos LA, Grever MR. Historical overview of hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:166–74.
24. Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017;129:553–60.
25. Mhawech-Fauceglia P, Oberholzer M, Aschenafi S, et al. Potential predictive patterns of minimal residual disease detected by immunohistochemistry on bone marrow biopsy specimens during a long-term follow-up in patients treated with cladribine for hairy cell leukemia. Arch Pathol Lab Med 2006;130:374–7.
26. Ortiz-Maldonado V, Villamor N, Baumann T, et al., Is there a role for minimal residual disease monitoring in the management of patients with hairy-cell leukaemia? Br J Haematol 2017 Aug 18.
27. Jansen J, Hermans J. Clinical staging system for hairy-cell leukemia. Blood 1982;60:571–7.
28. Grever MR, Lozanski G. Modern strategies for hairy cell leukemia. J Clin Oncol 2011;29:583–90.
29. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
30. Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol 1995;13:974–82.
31. Kreitman RJ, Wilson W, Calvo KR, et al. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin Cancer Res 2013;19:6873–81.
32. Burotto M, Stetler-Stevenson M, Arons E, et al. Bendamustine and rituximab in relapsed and refractory hairy cell leukemia. Clin Cancer Res 2013;19:6313–21.
33. Randomized phase II trial of rituximab with either pentostatin or bendamustine for multiply relapsed or refractory hairy cell leukemia. 2017 [cited 2017 Oct 26]; NCT01059786. https://clinicaltrials.gov/ct2/show/NCT01059786.
34. Else M, Dearden CE, Matutes E, et al. Rituximab with pentostatin or cladribine: an effective combination treatment for hairy cell leukemia after disease recurrence. Leuk Lymphoma 2011;52 Suppl 2:75–8.
35. Thomas DA, O’Brien S, Bueso-Ramos C, et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood 2003;102:3906–11.
36. Zenhäusern R, Simcock M, Gratwohl A, et al. Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98). Haematologica 2008;93(9):1426–8.
37. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
38. Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med 2015;373:1733–47.
39. Blachly JS, Lozanski G, Lucas DM, et al. Cotreatment of hairy cell leukemia and melanoma with the BRAF inhibitor dabrafenib. J Natl Compr Canc Netw 2015;13:9–13.
40. Tiacci E, De Carolis L, Zaja F, et al. Vemurafenib plus rituximab in hairy cell leukemia: a promisingchemotherapy-free regimen for relapsed or refractory patients. Blood 2016;128:1.
41. A phase II, open-label study in subjects with BRAF V600E-mutated rare cancers with several histologies to investigate the clinical efficacy and safety of the combination therapy of dabrafenib and trametinib. 2017 [cited 2017 Oct 26]; NCT02034110. https://clinicaltrials.gov/ct2/show/NCT02034110.
42. Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet 2014;46:8–10.
43. Andritsos LA, Grieselhuber NR, Anghelina M, et al. Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma 2017 Sep 18:1–4.
44. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497–506.
45. Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012;30:1822–8.
46. Saven A, Burian C, Adusumalli J, Koziol JA. Filgrastim for cladribine-induced neutropenic fever in patients with hairy cell leukemia. Blood 1999;93:2471–7.
47. Cornet E, Tomowiak C, Tanguy-Schmidt A, et al. Long-term follow-up and second malignancies in 487 patients with hairy cell leukaemia. Br J Haematol 2014;166:390–400.
48. Anderson LA, Engels EA. Autoimmune conditions and hairy cell leukemia: an exploratory case-control study. J Hematol Oncol 2010;3:35.
Introduction
Hairy cell leukemia (HCL) is a rare chronic lymphoproliferative disorder, with only approximately 2000 new cases diagnosed in the United States each year.1 It is now recognized that there are 2 distinct categories of HCL, classic HCL (cHCL) and variant HCL (vHCL), with vHCL now classified as a separate entity under the World Health Organization Classification of Hematopoietic Tumors.2 For this reason, the 2 diseases will be discussed separately. However, they do bear many clinical and microscopic similarities and because of this were originally indistinguishable using diagnostic techniques previously available. Even in the modern era using immunophenotypic, molecular, and genetic testing, differentiating between the classic and variant disease subtypes is sometimes difficult.
For cHCL the median age of diagnosis is 55 years, with vHCL occurring in patients who are somewhat older; HCL has been described only in the adult population, with 1 exception.3,4 There is a 4:1 male predominance, and Caucasians are more frequently affected than other ethnic groups. While the cause of the disease remains largely unknown, it has been observed to occur more frequently in farmers and in persons exposed to pesticides and/or herbicides, petroleum products, and ionizing radiation.4 The Institute of Medicine recently updated their position regarding veterans and Agent Orange, stating that there is sufficient evidence of an association between herbicides and chronic lymphoid leukemias (including HCL) to consider these diseases linked to exposure.5 Familial forms have also been described that are associated with specific HLA haplotypes, indicating a possible hereditary component.6 Most likely, a combination of environmental and genetic factors ultimately contributes to the development of HCL.
In recent years enormous progress has been made with respect to new insights into the biology of cHCL and vHCL, with significant refinement of diagnostic criteria. In addition, tremendous advances have occurred in both treatment and supportive care regimens, which have resulted in a dramatically increased overall life expectancy as well as decreased disease-related morbidity. This has meant that more patients are affected by HCL over time and are more likely to require care for relapsed HCL or associated comorbidities. Although no curative treatment options exist outside of allogeneic transplantation, therapeutic improvements have resulted in patients with cHCL having a life expectancy similar to that of unaffected patients, increasing the need for vigilance to prevent foreseeable complications.
Biology and Patheogenisis
The family of HCLs are chronic B-cell malignancies that account for approximately 2% of all diagnosed leukemias.7 The first detailed characterization of HCL as a distinct clinical entity was performed by Dr. Bouroncle and colleagues at the Ohio State University in 1958.8 Originally called leukemic reticuloendotheliosis, it was renamed HCL following more detailed description of the unique morphology of these malignant cells.9 Significant advances have recently been made in identifying distinctive genetic, immunophenotypic, and morphologic features that distinguish HCL from other B-cell malignancies.
HCL B cells tend to accumulate in the bone marrow, splenic red pulp, and (in some cases) peripheral blood. Unlike other lymphoproliferative disorders, HCL only rarely results in lymphadenopathy. HCL derives its name from the distinct appearance of the malignant hairy cells (Figure). Morphologically, HCL cells are mature, small lymphoid B-cells with a round or oval nucleus and abundant pale blue cytoplasm. Irregular projections of cytoplasm and microvilli give the cells a serrated, “hairy” appearance.10 The biological significance of these fine hair-like projections remains unknown and is an area of ongoing investigation. Gene expression profiling has revealed that HCL B cells are most similar to splenic marginal zone B cells and memory B cells.11–13 A recent analysis of common genetic alterations in HCL suggests that the cell of origin is in fact the hematopoietic stem cell.14
Compared to other hematologic malignancies, the genomic profile of HCL is relatively stable, with few chromosomal defects or translocations observed. A seminal study by Tiacci and colleagues revealed that the BRAF V600E mutation was present in 47 out of 47 cHCL cases examined, results that have since been replicated by other groups, confirming that BRAF V600E is a hallmark mutation in cHCL.15 The BRAF V600E gain-of-function mutation results in constitutive activation of the serine-threonine protein kinase B-Raf, which regulates the mitogen-activated protein kinase (MAPK)/RAF-MEK-ERK pathway. Indeed, cHCL B cells have elevated MAPK signaling, leading to enhancement of growth and survival.16 This specific mutation in the BRAF gene is also seen in a number of solid tumor malignancies including melanoma and thyroid cancer, and represents a therapeutic target using BRAF inhibitors already developed to treat these malignancies.17 Testing for BRAF V600E by polymerase chain reaction or immunohistochemical staining is now routinely performed when HCL is suspected.
While BRAF V600E is identified in nearly all cases of cHCL, it is rare in vHCL.18 The variant type of HCL was classified as a distinct clinical entity in 2008 and can now often be distinguished from cHCL on the basis of BRAF mutational status, among other differences. Interestingly, in the rare cases of BRAF V600E–negative cHCL, other mutations in BRAF or downstream targets as well as aberrant activation of the RAF-MEK-ERK signaling cascade are observed, indicating that this pathway is critical in HCL and may still represent a viable therapeutic target. Expression of the IGHV4-34 immunoglobulin rearrangement, while more common in vHCL, has also been identified in 10% of cHCL cases and appears to confer poor prognosis.19 Other mutated genes that have been identified in HCL include CDKN1B, TP53, U2AF1, ARID1A, EZH2, and KDM6A.20
Classic HCL is characterized by the immunophenotypic expression of CD11c, CD25, CD103, and CD123, with kappa or lambda light chain restriction indicating clonality; HCL B cells are generally negative for CD5, CD10, CD23, CD27, and CD79b. In contrast, vHCL often lacks expression of CD25 and CD123.18 The B-cell receptor (BCR) is expressed on hairy cells and its activation promotes proliferation and survival in vitro.21 The role of BCR signaling in B-cell malignancies is increasingly recognized, and therapies that target the BCR and associated signaling molecules offer an attractive treatment strategy.22 HCL B cells also typically express CD19, CD20, CD22, CD79a, CD200, CD1d, and annexin A1. Tartrate-resistant acid phosphatase (TRAP) positivity by immunohistochemistry is a hallmark of cHCL. Interestingly, changes to the patient’s original immunophenotype have been observed following treatment and upon disease recurrence, highlighting the importance of tracking immunophenotype throughout the course of disease.
Diagnosis
Prior to the advent of annual screening evaluations with routine examination of complete blood counts (CBC), patients were most often diagnosed with HCL when they presented with symptoms of the disease such as splenomegaly, infections, or complications of anemia or thrombocytopenia.23 In the current era, patients are more likely to be incidentally diagnosed when they are found to have an abnormal value on a CBC. Any blood lineage may be affected and patients may have pancytopenia or isolated cytopenias. Of note, monocytopenia is a common finding in cHCL that is not entirely understood. The cells typical of cHCL do not usually circulate in the peripheral blood, but if present would appear as mature lymphocytes with villous cytoplasmic projections, pale blue cytoplasm, and reniform nuclei with open chromatin (Figure).9 Even if the morphologic examination is highly suggestive of HCL, additional testing is required to differentiate between cHCL, vHCL, and other hematologic malignancies which may also have cytoplasmic projections. A complete assessment of the immunophenotype, molecular profile, and cytogenetic features is required to arrive at this diagnosis.
The international Hairy Cell Leukemia Foundation recently published consensus guidelines for the diagnosis and treatment of HCL.24 These guidelines recommend that patients undergo examination of the peripheral blood for morphology and immunophenotyping and further recommend obtaining bone marrow core and aspirate biopsy samples for immunophenotyping via immunohistochemical staining and flow cytometry. The characteristic immunophenotype of cHCL is a population of monoclonal B lymphocytes which co-express CD19, CD20, CD11c, CD25, CD103, and CD123. Variant HCL is characterized by a very similar immunophenotype but is usually negative for CD25 and CD123. It is notable that CD25 positivity may be lost following treatment, and the absence of this marker should not be used as the sole basis of a cHCL versus vHCL diagnosis. Because marrow fibrosis in HCL may prevent a marrow aspirate from being obtained, many of the key diagnostic studies are performed on the core biopsy, including morphological evaluation and immunohistochemical stains such as CD20 (a pan-B cell antigen), annexin-1 (an anti-inflammatory protein expressed only in cHCL), and VE1 (a BRAF V600E stain).
As noted above, recurrent cytogenetic abnormalities have now been identified that may inform the diagnosis or prognosis of HCL. Next-generation sequencing and other testing of the genetic landscape are taking on a larger role in subtype differentiation, and it is likely that future guidelines will recommend evaluation for significant mutations. Given that BRAF V600E mutation status is a key feature of cHCL and is absent in vHCL, it is important to perform this testing at the time of diagnosis whenever possible. The mutation may be detected via VE1 immunohistochemical staining, allele-specific polymerase chain reaction, or next-generation sequencing. Other less sensitive tests exist but are utilized less frequently.
Minimal Residual Disease
There is currently no accepted standard for minimal residual disease (MRD) monitoring in HCL. While detection of MRD has been clearly associated with increased risk of disease progression, cHCL cells typically do not circulate in the peripheral blood, limiting the use of peripheral blood immunophenotyping for quantitative MRD assessment. For quantitative monitoring of marrow involvement by HCL, immunohistochemical staining of the bone marrow core biopsy is usually required. Staining may be performed for CD20, or, in patients who have received anti-CD20 therapy, DBA.44, VE-1, or CD79a. There is currently not a consensus regarding what level of disease involvement constitutes MRD. One group studied this issue and found that relapse could be predicted by evaluating MRD by percentage of positive cells in the marrow by immunohistochemical staining, with less than 1% involvement having the lowest risk for disease relapse and greater than 5% having the highest risk for disease relapse.25 A recent study evaluated MRD patterns in the peripheral blood of 32 cHCL patients who had completed frontline therapy. This group performed flow cytometry on the peripheral blood of patients at 1, 3, 6, and 12 months following therapy. All patients had achieved a complete response with initial therapy and peripheral blood MRD negativity at the completion of therapy. At a median follow-up of 100 months post therapy, 5 patients converted from peripheral blood–MRD negative to peripheral blood–MRD positive, and 6 patients developed overt disease progression. In all patients who progressed, progression was preceded by an increase in detectable peripheral blood MRD cells.26 Although larger studies are needed, peripheral blood flow cytometric monitoring for MRD may be a useful adjunct to predict ongoing response or impending relapse. In addition, newer, more sensitive methods of disease monitoring may ultimately supplant flow cytometry.
Risk Stratification
Although much progress has been made in the risk stratification profiling of hematologic malignancies in general, HCL has unfortunately lagged behind in this effort. The most recent risk stratification analysis was performed in 1982 by Jansen and colleagues.27 This group of researchers performed a retrospective analysis of 391 HCL patients treated at 22 centers. One of the central questions in their analysis was survival time from diagnosis in patients who had not yet undergone splenectomy (a standard treatment at the time). This group consisted of a total of 154 patients. As this study predated modern pathological and molecular testing, clinical and laboratory features were examined, and these mostly consisted of physical exam findings and analysis of the peripheral blood. This group found that several factors influenced the survival of these patients, including duration of symptoms prior to diagnosis, the degree of splenomegaly, hemoglobin level, and number of hairy cells in the peripheral blood. However, because of interobserver variation for the majority of these variables, only hemoglobin and spleen size were included in the proportional hazard model. Using only these 2 variables, the authors were able to determine 3 clinical stages for HCL (Table 1). The stages were found to correlate with median survival: patients with stage 1 disease had a median survival not reached at 72 months, but patients with stage 2 disease had a median survival of 18 months, which decreased to only 12 months in patients with stage 3 disease.
Because the majority of patients with HCL in the modern era will be diagnosed prior to reaching stage 3, a risk stratification system incorporating clinical features, laboratory parameters, and molecular and genetic testing is of considerable interest and is a subject of ongoing research. Ultimately, the goal will be to identify patients at higher risk of early relapse so that more intensive therapies can be applied to initial treatment that will result in longer treatment-free intervals.
Treatment
Because there is no curative treatment for either cHCL or vHCL outside allogeneic transplantation, and it is not clear that early treatment leads to better outcomes in HCL, patients do not always receive treatment at the time of diagnosis or relapse. The general consensus is that patients should be treated if there is a declining trend in hematologic parameters or they experience symptoms from the disease.24 Current consensus guidelines recommend treatment when any of the following hematologic parameters are met: hemoglobin less than 11 g/dL, platelet count less than 100 × 103/µL, or absolute neutrophil count less than 1000/µL.24 These parameters are surrogate markers that indicate compromised bone marrow function. Cytopenias may also be caused by splenomegaly, and symptomatic splenomegaly with or without cytopenias is an indication for treatment. A small number of patients with HCL (approximately 10%) do not require immediate therapy after diagnosis and are monitored by their provider until treatment is indicated.
First-Line Therapy
Despite advances in targeted therapies for HCL, because no treatment has been shown to extend the treatment-free interval longer than chemotherapy, treatment with a purine nucleoside analog is usually the recommended first-line therapy. This includes either cladribine or pentostatin. Both agents appear to be equally effective, and the choice of therapy is determined by the treating physician based on his or her experience. Cladribine administration has been studied using a number of different schedules and routes: intravenous continuous infusion (0.1 mg/kg) for 7 days, intravenous infusion (0.14 mg/kg/day) over 2 hours on a 5-day regimen, or alternatively subcutaneously (0.1–0.14 mg/kg/day) on a once-per-day or once-per-week regimen (Table 2).28,29
Unlike cHCL, vHCL remains difficult to treat and early disease progression is common. The best outcomes have been seen in patients who have received combination chemo-immunotherapy such as purine nucleoside analog therapy plus rituximab or bendamustine plus rituximab.31 One pilot study of bendamustine plus rituximab in 12 patients found an overall response rate of 100%, with the majority of patients achieving a complete response.31 For patients who achieved a complete response, the median duration of response had not been reached, but patients achieving only a partial response had a median duration of response of only 20 months, indicating there is a subgroup of patients who will require a different treatment approach.32 A randomized phase 2 trial of rituximab with either pentostatin or bendamustine is ongoing.33
Assessment of Response
Response assessment involves physical examination for estimation of spleen size, assessment of hematologic parameters, and a bone marrow biopsy for evaluation of marrow response. It is recommended that the bone marrow biopsy be performed 4 to 6 months following cladribine administration, or after completion of 12 doses of pentostatin. Detailed response assessment criteria are shown in Table 3.
Second-Line Therapy
Although the majority of patients treated with purine analogs will achieve durable remissions, approximately 40% of patients will eventually require second-line therapy. Criteria for treatment at relapse are the same as the criteria for initial therapy, including symptomatic disease or progressive anemia, thrombocytopenia, or neutropenia. The choice of treatment is based on clinical parameters and the duration of the previous remission. If the initial remission was longer than 65 months and the patient is eligible to receive chemotherapy, re-treatment with initial therapy is recommended. For a remission between 24 and 65 months, re-treatment with a purine analog combined with an anti-CD20 monoclonal antibody may be considered.34 If the first remission is shorter than 24 months, confirmation of the original diagnosis as well as consideration for testing for additional mutations with therapeutic targets (BRAF V600E, MAP2K1) should be considered before a treatment decision is made. For these patients, alternative therapies, including investigational agents, should be considered.24
Monoclonal antibody therapy has been studied in both the up-front setting and in relapsed or refractory HCL.35 An initial study of 15 patients with relapsed HCL found an overall response rate of 80%, with 8 patients achieving a complete response. A subsequent study of 26 patients who relapsed after cladribine therapy found an overall response rate of 80%, with a complete response rate of 32%. Median relapse-free survival was 27 months.36 Ravandi and others studied rituximab in the up-front setting in combination with cladribine, and found an overall response rate of 100%, including in patients with vHCL. At the time of publication of the study results, the median survival had not been reached.37 As has been seen with other lymphoid malignancies, concurrent therapy with rituximab appears to enhance the activity of the agent with which it is combined. While its use in the up-front setting remains an area of active investigation, there is a clear role for chemo-immunotherapy in the relapsed setting.
In patients with cHCL, excellent results including complete remissions have been reported with the use of BRAF inhibitors, both as a single agent and when combined with anti-CD20 therapy. The 2 commercially available BRAF inhibitors are vemurafenib and dabrafenib, and both have been tested in relapsed cHCL.38,39 The first study of vemurafenib was reported by Tiacci and colleagues, who found an overall response rate of 96% after a median of 8 weeks and a 100% response rate after a median of 12 weeks, with complete response rates up to 42%.38 The median relapse-free survival was 23 months (decreasing to only 6 months in patients who achieved only a partial remission), indicating that these agents will likely need to be administered in combination with other effective therapies with non-overlapping toxicities. Vemurafenib has been administered concurrently with rituximab, and preliminary results of this combination therapy showed early rates of complete responses.40 Dabrafenib has been reported for use as a single agent in cHCL and clinical trials are underway evaluating its efficacy when administered with trametinib, a MEK inhibitor.39,41 Of note, patients receiving BRAF inhibitors frequently develop cutaneous complications of RAF inhibition including cutaneous squamous cell carcinomas and keratoacanthomas, and close dermatologic surveillance is required.
Variant HCL does not harbor the BRAF V600E mutation, but up to half of patients have been found to have mutations of MAP2K1, which upregulates MEK1 expression.42 Trametinib is approved by the US Food and Drug Administration for the treatment of patients with melanoma at a dose of 2 mg orally daily, and has been successfully used to treat 1 patient with vHCL.43 Further evaluation of this targeted therapy is underway.
Ibrutinib, a Bruton tyrosine kinase inhibitor, and moxetumomab pasudotox, an immunotoxin conjugate, are currently being studied in National Institutes of Health–sponsored multi-institutional trials for patients with HCL. Ibrutinib is administered orally at 420 mg per day until relapse.44 Moxetumomab pasudotox was tested at different doses between 5 and 50 μg/kg intravenously every other day for 3 doses for up to 16 cycles unless they experienced disease progression or developed neutralizing antibodies.45 Both agents have been shown to have significant activity in cHCL and vHCL and will likely be included in the treatment armamentarium once trials are completed. Second-line therapy options are summarized in Table 4.
Complications and Supportive Care
The complications of HCL may be separated into the pre-, intra-, and post-treatment periods. At the time of diagnosis and prior to the initiation of therapy, marrow infiltration by HCL frequently leads to cytopenias which cause symptomatic anemia, infection, and/or bleeding complications. Many patients develop splenomegaly, which may further lower the blood counts and which is experienced as abdominal fullness or distention, with early satiety leading to weight loss. Patients may also experience constitutional symptoms with fatigue, fevers in the absence of infection, and unintentional weight loss even without splenomegaly.
For patients who initiate therapy with purine nucleoside analogs, the early part of treatment is associated with the greatest risk of morbidity and mortality. Chemotherapy leads to both immunosuppression (altered cellular immunity) as well as myelosuppression. Thus, patients who are already in need of treatment because of disease-related cytopenias will experience an abrupt and sometimes significant decline in the peripheral blood counts. The treatment period prior to recovery of neutrophils requires the greatest vigilance. Because patients are profoundly immunocompromised, febrile neutropenia is a common complication leading to hospital admission and the cause is often difficult to identify. Treatment with broad-spectrum antibiotics, investigation for opportunistic and viral infections, and considerations for antifungal prophylaxis or therapy are required in this setting. It is recommended that all patients treated with purine nucleoside analogs receive prophylactic antimicrobials for herpes simplex virus and varicella zoster virus, as well as prophylaxis against Pneumocystis jirovecii. Unfortunately, growth factor support has not proven successful in this patient population but is not contraindicated.46
Following successful completion of therapy, patients may remain functionally immunocompromised for a significant period of time even with a normal neutrophil count. Monitoring of the CD4 count may help to determine when prophylactic antimicrobials may be discontinued. A CD4 count greater than 200 cells/µL is generally considered to be adequate for prevention of opportunistic infections. Although immunizations have not been well studied in HCL, it is recommended that patients receive annual influenza immunizations as well as age-appropriate immunizations against Streptococcus pneumoniae and other infectious illnesses as indicated. Live viral vaccines such as the currently available herpes zoster vaccine can lead to infections in this patient population and are not recommended.
Like many hematologic malignancies, HCL may be associated with comorbid conditions related to immune dysfunction. There is a known association with an increased risk of second primary malignancies, which may predate the diagnosis of HCL.47 Therefore, it is recommended that patients continue annual cancer screenings as well as undergo prompt evaluation for potential symptoms of second malignancies. In addition, it is thought that there may be an increased risk for autoimmune disorders such as inflammatory arthritis or immune-mediated cytopenias. One case-control study found a possible association between autoimmune diseases and HCL, noting that at times these diseases are diagnosed concurrently.48 However, because of the rarity of the disease it has been difficult to quantify these associated conditions in a systematic way. There is currently an international patient data registry under development for the systematic study of HCL and its complications which may answer many of these questions.
Survivorship and quality of life are important considerations in chronic diseases. It is not uncommon for patients to develop anxiety related to the trauma of diagnosis and treatment, especially when intensive care has been required. Patients may have lingering fears regarding concerns of developing infections due to exposure to ill persons or fears regarding risk of relapse and need for re-treatment. A proactive approach with partnership with psychosocial oncology may be of benefit, especially when symptoms of post-traumatic stress disorder are evident.
Conclusion
HCL is a rare, chronic lymphoid malignancy that is now subclassified into classic and variant HCL. Further investigations into the disease subtypes will allow more precise disease definitions, and these studies are underway. Renewed efforts toward updated risk stratification and clinical staging systems will be important aspects of these investigations. Refinements in treatment and supportive care have resulted in greatly improved overall survival, which has translated into larger numbers of people living with HCL. However, new treatment paradigms for vHCL are needed as the progression-free survival in this disease remains significantly lower than that of cHCL. Future efforts toward understanding survivorship issues and management of long-term treatment and disease-related complications will be critical for ensuring good quality of life for patients living with HCL.
Introduction
Hairy cell leukemia (HCL) is a rare chronic lymphoproliferative disorder, with only approximately 2000 new cases diagnosed in the United States each year.1 It is now recognized that there are 2 distinct categories of HCL, classic HCL (cHCL) and variant HCL (vHCL), with vHCL now classified as a separate entity under the World Health Organization Classification of Hematopoietic Tumors.2 For this reason, the 2 diseases will be discussed separately. However, they do bear many clinical and microscopic similarities and because of this were originally indistinguishable using diagnostic techniques previously available. Even in the modern era using immunophenotypic, molecular, and genetic testing, differentiating between the classic and variant disease subtypes is sometimes difficult.
For cHCL the median age of diagnosis is 55 years, with vHCL occurring in patients who are somewhat older; HCL has been described only in the adult population, with 1 exception.3,4 There is a 4:1 male predominance, and Caucasians are more frequently affected than other ethnic groups. While the cause of the disease remains largely unknown, it has been observed to occur more frequently in farmers and in persons exposed to pesticides and/or herbicides, petroleum products, and ionizing radiation.4 The Institute of Medicine recently updated their position regarding veterans and Agent Orange, stating that there is sufficient evidence of an association between herbicides and chronic lymphoid leukemias (including HCL) to consider these diseases linked to exposure.5 Familial forms have also been described that are associated with specific HLA haplotypes, indicating a possible hereditary component.6 Most likely, a combination of environmental and genetic factors ultimately contributes to the development of HCL.
In recent years enormous progress has been made with respect to new insights into the biology of cHCL and vHCL, with significant refinement of diagnostic criteria. In addition, tremendous advances have occurred in both treatment and supportive care regimens, which have resulted in a dramatically increased overall life expectancy as well as decreased disease-related morbidity. This has meant that more patients are affected by HCL over time and are more likely to require care for relapsed HCL or associated comorbidities. Although no curative treatment options exist outside of allogeneic transplantation, therapeutic improvements have resulted in patients with cHCL having a life expectancy similar to that of unaffected patients, increasing the need for vigilance to prevent foreseeable complications.
Biology and Patheogenisis
The family of HCLs are chronic B-cell malignancies that account for approximately 2% of all diagnosed leukemias.7 The first detailed characterization of HCL as a distinct clinical entity was performed by Dr. Bouroncle and colleagues at the Ohio State University in 1958.8 Originally called leukemic reticuloendotheliosis, it was renamed HCL following more detailed description of the unique morphology of these malignant cells.9 Significant advances have recently been made in identifying distinctive genetic, immunophenotypic, and morphologic features that distinguish HCL from other B-cell malignancies.
HCL B cells tend to accumulate in the bone marrow, splenic red pulp, and (in some cases) peripheral blood. Unlike other lymphoproliferative disorders, HCL only rarely results in lymphadenopathy. HCL derives its name from the distinct appearance of the malignant hairy cells (Figure). Morphologically, HCL cells are mature, small lymphoid B-cells with a round or oval nucleus and abundant pale blue cytoplasm. Irregular projections of cytoplasm and microvilli give the cells a serrated, “hairy” appearance.10 The biological significance of these fine hair-like projections remains unknown and is an area of ongoing investigation. Gene expression profiling has revealed that HCL B cells are most similar to splenic marginal zone B cells and memory B cells.11–13 A recent analysis of common genetic alterations in HCL suggests that the cell of origin is in fact the hematopoietic stem cell.14
Compared to other hematologic malignancies, the genomic profile of HCL is relatively stable, with few chromosomal defects or translocations observed. A seminal study by Tiacci and colleagues revealed that the BRAF V600E mutation was present in 47 out of 47 cHCL cases examined, results that have since been replicated by other groups, confirming that BRAF V600E is a hallmark mutation in cHCL.15 The BRAF V600E gain-of-function mutation results in constitutive activation of the serine-threonine protein kinase B-Raf, which regulates the mitogen-activated protein kinase (MAPK)/RAF-MEK-ERK pathway. Indeed, cHCL B cells have elevated MAPK signaling, leading to enhancement of growth and survival.16 This specific mutation in the BRAF gene is also seen in a number of solid tumor malignancies including melanoma and thyroid cancer, and represents a therapeutic target using BRAF inhibitors already developed to treat these malignancies.17 Testing for BRAF V600E by polymerase chain reaction or immunohistochemical staining is now routinely performed when HCL is suspected.
While BRAF V600E is identified in nearly all cases of cHCL, it is rare in vHCL.18 The variant type of HCL was classified as a distinct clinical entity in 2008 and can now often be distinguished from cHCL on the basis of BRAF mutational status, among other differences. Interestingly, in the rare cases of BRAF V600E–negative cHCL, other mutations in BRAF or downstream targets as well as aberrant activation of the RAF-MEK-ERK signaling cascade are observed, indicating that this pathway is critical in HCL and may still represent a viable therapeutic target. Expression of the IGHV4-34 immunoglobulin rearrangement, while more common in vHCL, has also been identified in 10% of cHCL cases and appears to confer poor prognosis.19 Other mutated genes that have been identified in HCL include CDKN1B, TP53, U2AF1, ARID1A, EZH2, and KDM6A.20
Classic HCL is characterized by the immunophenotypic expression of CD11c, CD25, CD103, and CD123, with kappa or lambda light chain restriction indicating clonality; HCL B cells are generally negative for CD5, CD10, CD23, CD27, and CD79b. In contrast, vHCL often lacks expression of CD25 and CD123.18 The B-cell receptor (BCR) is expressed on hairy cells and its activation promotes proliferation and survival in vitro.21 The role of BCR signaling in B-cell malignancies is increasingly recognized, and therapies that target the BCR and associated signaling molecules offer an attractive treatment strategy.22 HCL B cells also typically express CD19, CD20, CD22, CD79a, CD200, CD1d, and annexin A1. Tartrate-resistant acid phosphatase (TRAP) positivity by immunohistochemistry is a hallmark of cHCL. Interestingly, changes to the patient’s original immunophenotype have been observed following treatment and upon disease recurrence, highlighting the importance of tracking immunophenotype throughout the course of disease.
Diagnosis
Prior to the advent of annual screening evaluations with routine examination of complete blood counts (CBC), patients were most often diagnosed with HCL when they presented with symptoms of the disease such as splenomegaly, infections, or complications of anemia or thrombocytopenia.23 In the current era, patients are more likely to be incidentally diagnosed when they are found to have an abnormal value on a CBC. Any blood lineage may be affected and patients may have pancytopenia or isolated cytopenias. Of note, monocytopenia is a common finding in cHCL that is not entirely understood. The cells typical of cHCL do not usually circulate in the peripheral blood, but if present would appear as mature lymphocytes with villous cytoplasmic projections, pale blue cytoplasm, and reniform nuclei with open chromatin (Figure).9 Even if the morphologic examination is highly suggestive of HCL, additional testing is required to differentiate between cHCL, vHCL, and other hematologic malignancies which may also have cytoplasmic projections. A complete assessment of the immunophenotype, molecular profile, and cytogenetic features is required to arrive at this diagnosis.
The international Hairy Cell Leukemia Foundation recently published consensus guidelines for the diagnosis and treatment of HCL.24 These guidelines recommend that patients undergo examination of the peripheral blood for morphology and immunophenotyping and further recommend obtaining bone marrow core and aspirate biopsy samples for immunophenotyping via immunohistochemical staining and flow cytometry. The characteristic immunophenotype of cHCL is a population of monoclonal B lymphocytes which co-express CD19, CD20, CD11c, CD25, CD103, and CD123. Variant HCL is characterized by a very similar immunophenotype but is usually negative for CD25 and CD123. It is notable that CD25 positivity may be lost following treatment, and the absence of this marker should not be used as the sole basis of a cHCL versus vHCL diagnosis. Because marrow fibrosis in HCL may prevent a marrow aspirate from being obtained, many of the key diagnostic studies are performed on the core biopsy, including morphological evaluation and immunohistochemical stains such as CD20 (a pan-B cell antigen), annexin-1 (an anti-inflammatory protein expressed only in cHCL), and VE1 (a BRAF V600E stain).
As noted above, recurrent cytogenetic abnormalities have now been identified that may inform the diagnosis or prognosis of HCL. Next-generation sequencing and other testing of the genetic landscape are taking on a larger role in subtype differentiation, and it is likely that future guidelines will recommend evaluation for significant mutations. Given that BRAF V600E mutation status is a key feature of cHCL and is absent in vHCL, it is important to perform this testing at the time of diagnosis whenever possible. The mutation may be detected via VE1 immunohistochemical staining, allele-specific polymerase chain reaction, or next-generation sequencing. Other less sensitive tests exist but are utilized less frequently.
Minimal Residual Disease
There is currently no accepted standard for minimal residual disease (MRD) monitoring in HCL. While detection of MRD has been clearly associated with increased risk of disease progression, cHCL cells typically do not circulate in the peripheral blood, limiting the use of peripheral blood immunophenotyping for quantitative MRD assessment. For quantitative monitoring of marrow involvement by HCL, immunohistochemical staining of the bone marrow core biopsy is usually required. Staining may be performed for CD20, or, in patients who have received anti-CD20 therapy, DBA.44, VE-1, or CD79a. There is currently not a consensus regarding what level of disease involvement constitutes MRD. One group studied this issue and found that relapse could be predicted by evaluating MRD by percentage of positive cells in the marrow by immunohistochemical staining, with less than 1% involvement having the lowest risk for disease relapse and greater than 5% having the highest risk for disease relapse.25 A recent study evaluated MRD patterns in the peripheral blood of 32 cHCL patients who had completed frontline therapy. This group performed flow cytometry on the peripheral blood of patients at 1, 3, 6, and 12 months following therapy. All patients had achieved a complete response with initial therapy and peripheral blood MRD negativity at the completion of therapy. At a median follow-up of 100 months post therapy, 5 patients converted from peripheral blood–MRD negative to peripheral blood–MRD positive, and 6 patients developed overt disease progression. In all patients who progressed, progression was preceded by an increase in detectable peripheral blood MRD cells.26 Although larger studies are needed, peripheral blood flow cytometric monitoring for MRD may be a useful adjunct to predict ongoing response or impending relapse. In addition, newer, more sensitive methods of disease monitoring may ultimately supplant flow cytometry.
Risk Stratification
Although much progress has been made in the risk stratification profiling of hematologic malignancies in general, HCL has unfortunately lagged behind in this effort. The most recent risk stratification analysis was performed in 1982 by Jansen and colleagues.27 This group of researchers performed a retrospective analysis of 391 HCL patients treated at 22 centers. One of the central questions in their analysis was survival time from diagnosis in patients who had not yet undergone splenectomy (a standard treatment at the time). This group consisted of a total of 154 patients. As this study predated modern pathological and molecular testing, clinical and laboratory features were examined, and these mostly consisted of physical exam findings and analysis of the peripheral blood. This group found that several factors influenced the survival of these patients, including duration of symptoms prior to diagnosis, the degree of splenomegaly, hemoglobin level, and number of hairy cells in the peripheral blood. However, because of interobserver variation for the majority of these variables, only hemoglobin and spleen size were included in the proportional hazard model. Using only these 2 variables, the authors were able to determine 3 clinical stages for HCL (Table 1). The stages were found to correlate with median survival: patients with stage 1 disease had a median survival not reached at 72 months, but patients with stage 2 disease had a median survival of 18 months, which decreased to only 12 months in patients with stage 3 disease.
Because the majority of patients with HCL in the modern era will be diagnosed prior to reaching stage 3, a risk stratification system incorporating clinical features, laboratory parameters, and molecular and genetic testing is of considerable interest and is a subject of ongoing research. Ultimately, the goal will be to identify patients at higher risk of early relapse so that more intensive therapies can be applied to initial treatment that will result in longer treatment-free intervals.
Treatment
Because there is no curative treatment for either cHCL or vHCL outside allogeneic transplantation, and it is not clear that early treatment leads to better outcomes in HCL, patients do not always receive treatment at the time of diagnosis or relapse. The general consensus is that patients should be treated if there is a declining trend in hematologic parameters or they experience symptoms from the disease.24 Current consensus guidelines recommend treatment when any of the following hematologic parameters are met: hemoglobin less than 11 g/dL, platelet count less than 100 × 103/µL, or absolute neutrophil count less than 1000/µL.24 These parameters are surrogate markers that indicate compromised bone marrow function. Cytopenias may also be caused by splenomegaly, and symptomatic splenomegaly with or without cytopenias is an indication for treatment. A small number of patients with HCL (approximately 10%) do not require immediate therapy after diagnosis and are monitored by their provider until treatment is indicated.
First-Line Therapy
Despite advances in targeted therapies for HCL, because no treatment has been shown to extend the treatment-free interval longer than chemotherapy, treatment with a purine nucleoside analog is usually the recommended first-line therapy. This includes either cladribine or pentostatin. Both agents appear to be equally effective, and the choice of therapy is determined by the treating physician based on his or her experience. Cladribine administration has been studied using a number of different schedules and routes: intravenous continuous infusion (0.1 mg/kg) for 7 days, intravenous infusion (0.14 mg/kg/day) over 2 hours on a 5-day regimen, or alternatively subcutaneously (0.1–0.14 mg/kg/day) on a once-per-day or once-per-week regimen (Table 2).28,29
Unlike cHCL, vHCL remains difficult to treat and early disease progression is common. The best outcomes have been seen in patients who have received combination chemo-immunotherapy such as purine nucleoside analog therapy plus rituximab or bendamustine plus rituximab.31 One pilot study of bendamustine plus rituximab in 12 patients found an overall response rate of 100%, with the majority of patients achieving a complete response.31 For patients who achieved a complete response, the median duration of response had not been reached, but patients achieving only a partial response had a median duration of response of only 20 months, indicating there is a subgroup of patients who will require a different treatment approach.32 A randomized phase 2 trial of rituximab with either pentostatin or bendamustine is ongoing.33
Assessment of Response
Response assessment involves physical examination for estimation of spleen size, assessment of hematologic parameters, and a bone marrow biopsy for evaluation of marrow response. It is recommended that the bone marrow biopsy be performed 4 to 6 months following cladribine administration, or after completion of 12 doses of pentostatin. Detailed response assessment criteria are shown in Table 3.
Second-Line Therapy
Although the majority of patients treated with purine analogs will achieve durable remissions, approximately 40% of patients will eventually require second-line therapy. Criteria for treatment at relapse are the same as the criteria for initial therapy, including symptomatic disease or progressive anemia, thrombocytopenia, or neutropenia. The choice of treatment is based on clinical parameters and the duration of the previous remission. If the initial remission was longer than 65 months and the patient is eligible to receive chemotherapy, re-treatment with initial therapy is recommended. For a remission between 24 and 65 months, re-treatment with a purine analog combined with an anti-CD20 monoclonal antibody may be considered.34 If the first remission is shorter than 24 months, confirmation of the original diagnosis as well as consideration for testing for additional mutations with therapeutic targets (BRAF V600E, MAP2K1) should be considered before a treatment decision is made. For these patients, alternative therapies, including investigational agents, should be considered.24
Monoclonal antibody therapy has been studied in both the up-front setting and in relapsed or refractory HCL.35 An initial study of 15 patients with relapsed HCL found an overall response rate of 80%, with 8 patients achieving a complete response. A subsequent study of 26 patients who relapsed after cladribine therapy found an overall response rate of 80%, with a complete response rate of 32%. Median relapse-free survival was 27 months.36 Ravandi and others studied rituximab in the up-front setting in combination with cladribine, and found an overall response rate of 100%, including in patients with vHCL. At the time of publication of the study results, the median survival had not been reached.37 As has been seen with other lymphoid malignancies, concurrent therapy with rituximab appears to enhance the activity of the agent with which it is combined. While its use in the up-front setting remains an area of active investigation, there is a clear role for chemo-immunotherapy in the relapsed setting.
In patients with cHCL, excellent results including complete remissions have been reported with the use of BRAF inhibitors, both as a single agent and when combined with anti-CD20 therapy. The 2 commercially available BRAF inhibitors are vemurafenib and dabrafenib, and both have been tested in relapsed cHCL.38,39 The first study of vemurafenib was reported by Tiacci and colleagues, who found an overall response rate of 96% after a median of 8 weeks and a 100% response rate after a median of 12 weeks, with complete response rates up to 42%.38 The median relapse-free survival was 23 months (decreasing to only 6 months in patients who achieved only a partial remission), indicating that these agents will likely need to be administered in combination with other effective therapies with non-overlapping toxicities. Vemurafenib has been administered concurrently with rituximab, and preliminary results of this combination therapy showed early rates of complete responses.40 Dabrafenib has been reported for use as a single agent in cHCL and clinical trials are underway evaluating its efficacy when administered with trametinib, a MEK inhibitor.39,41 Of note, patients receiving BRAF inhibitors frequently develop cutaneous complications of RAF inhibition including cutaneous squamous cell carcinomas and keratoacanthomas, and close dermatologic surveillance is required.
Variant HCL does not harbor the BRAF V600E mutation, but up to half of patients have been found to have mutations of MAP2K1, which upregulates MEK1 expression.42 Trametinib is approved by the US Food and Drug Administration for the treatment of patients with melanoma at a dose of 2 mg orally daily, and has been successfully used to treat 1 patient with vHCL.43 Further evaluation of this targeted therapy is underway.
Ibrutinib, a Bruton tyrosine kinase inhibitor, and moxetumomab pasudotox, an immunotoxin conjugate, are currently being studied in National Institutes of Health–sponsored multi-institutional trials for patients with HCL. Ibrutinib is administered orally at 420 mg per day until relapse.44 Moxetumomab pasudotox was tested at different doses between 5 and 50 μg/kg intravenously every other day for 3 doses for up to 16 cycles unless they experienced disease progression or developed neutralizing antibodies.45 Both agents have been shown to have significant activity in cHCL and vHCL and will likely be included in the treatment armamentarium once trials are completed. Second-line therapy options are summarized in Table 4.
Complications and Supportive Care
The complications of HCL may be separated into the pre-, intra-, and post-treatment periods. At the time of diagnosis and prior to the initiation of therapy, marrow infiltration by HCL frequently leads to cytopenias which cause symptomatic anemia, infection, and/or bleeding complications. Many patients develop splenomegaly, which may further lower the blood counts and which is experienced as abdominal fullness or distention, with early satiety leading to weight loss. Patients may also experience constitutional symptoms with fatigue, fevers in the absence of infection, and unintentional weight loss even without splenomegaly.
For patients who initiate therapy with purine nucleoside analogs, the early part of treatment is associated with the greatest risk of morbidity and mortality. Chemotherapy leads to both immunosuppression (altered cellular immunity) as well as myelosuppression. Thus, patients who are already in need of treatment because of disease-related cytopenias will experience an abrupt and sometimes significant decline in the peripheral blood counts. The treatment period prior to recovery of neutrophils requires the greatest vigilance. Because patients are profoundly immunocompromised, febrile neutropenia is a common complication leading to hospital admission and the cause is often difficult to identify. Treatment with broad-spectrum antibiotics, investigation for opportunistic and viral infections, and considerations for antifungal prophylaxis or therapy are required in this setting. It is recommended that all patients treated with purine nucleoside analogs receive prophylactic antimicrobials for herpes simplex virus and varicella zoster virus, as well as prophylaxis against Pneumocystis jirovecii. Unfortunately, growth factor support has not proven successful in this patient population but is not contraindicated.46
Following successful completion of therapy, patients may remain functionally immunocompromised for a significant period of time even with a normal neutrophil count. Monitoring of the CD4 count may help to determine when prophylactic antimicrobials may be discontinued. A CD4 count greater than 200 cells/µL is generally considered to be adequate for prevention of opportunistic infections. Although immunizations have not been well studied in HCL, it is recommended that patients receive annual influenza immunizations as well as age-appropriate immunizations against Streptococcus pneumoniae and other infectious illnesses as indicated. Live viral vaccines such as the currently available herpes zoster vaccine can lead to infections in this patient population and are not recommended.
Like many hematologic malignancies, HCL may be associated with comorbid conditions related to immune dysfunction. There is a known association with an increased risk of second primary malignancies, which may predate the diagnosis of HCL.47 Therefore, it is recommended that patients continue annual cancer screenings as well as undergo prompt evaluation for potential symptoms of second malignancies. In addition, it is thought that there may be an increased risk for autoimmune disorders such as inflammatory arthritis or immune-mediated cytopenias. One case-control study found a possible association between autoimmune diseases and HCL, noting that at times these diseases are diagnosed concurrently.48 However, because of the rarity of the disease it has been difficult to quantify these associated conditions in a systematic way. There is currently an international patient data registry under development for the systematic study of HCL and its complications which may answer many of these questions.
Survivorship and quality of life are important considerations in chronic diseases. It is not uncommon for patients to develop anxiety related to the trauma of diagnosis and treatment, especially when intensive care has been required. Patients may have lingering fears regarding concerns of developing infections due to exposure to ill persons or fears regarding risk of relapse and need for re-treatment. A proactive approach with partnership with psychosocial oncology may be of benefit, especially when symptoms of post-traumatic stress disorder are evident.
Conclusion
HCL is a rare, chronic lymphoid malignancy that is now subclassified into classic and variant HCL. Further investigations into the disease subtypes will allow more precise disease definitions, and these studies are underway. Renewed efforts toward updated risk stratification and clinical staging systems will be important aspects of these investigations. Refinements in treatment and supportive care have resulted in greatly improved overall survival, which has translated into larger numbers of people living with HCL. However, new treatment paradigms for vHCL are needed as the progression-free survival in this disease remains significantly lower than that of cHCL. Future efforts toward understanding survivorship issues and management of long-term treatment and disease-related complications will be critical for ensuring good quality of life for patients living with HCL.
1. Teras LR, Desantis DE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
2. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008.
3. Yetgin S, Olcay L, Yenicesu I, et al. Relapse in hairy cell leukemia due to isolated nodular skin infiltration. Pediatr Hematol Oncol 2001;18:415–7.
4. Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:175–9.
5. Veterans and agent orange: update 2014. Mil Med 2017;182:1619–20.
6. Villemagne B, Bay JO, Tournilhac O, et al. Two new cases of familial hairy cell leukemia associated with HLA haplotypes A2, B7, Bw4, Bw6. Leuk Lymphoma 2005;46:243–5.
7. Chandran R, Gardiner SK, Smith SD, Spurgeon SE. Improved survival in hairy cell leukaemia over three decades: a SEER database analysis of prognostic factors. Br J Haematol 2013;163:407–9.
8. Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood 1958;13:609–30.
9. Schrek R, Donnelly WJ. “Hairy” cells in blood in lymphoreticular neoplastic disease and “flagellated” cells of normal lymph nodes. Blood 1966;27:199–211.
10. Polliack A, Tadmor T. Surface topography of hairy cell leukemia cells compared to other leukemias as seen by scanning electron microscopy. Leuk Lymphoma 2011;52 Suppl 2:14–7.
11. Miranda RN, Cousar JB, Hammer RD, et al. Somatic mutation analysis of IgH variable regions reveals that tumor cells of most parafollicular (monocytoid) B-cell lymphoma, splenic marginal zone B-cell lymphoma, and some hairy cell leukemia are composed of memory B lymphocytes. Hum Pathol 1999;30:306–12.
12. Vanhentenrijk V, Tierens A, Wlodarska I, et al. V(H) gene analysis of hairy cell leukemia reveals a homogeneous mutation status and suggests its marginal zone B-cell origin. Leukemia 2004;18:1729–32.
13. Basso K, Liso A, Tiacci E, et al. Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. J Exp Med 2004;199:59–68.
14. Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci Transl Med 2014;6:238ra71.
15. Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305–15.
16. Kamiguti AS, Harris RJ, Slupsky JR, et al. Regulation of hairy-cell survival through constitutive activation of mitogen-activated protein kinase pathways. Oncogene 2003;22:2272–84.
17. Rahman MA, Salajegheh A, Smith RA, Lam AK. BRAF inhibitors: From the laboratory to clinical trials. Crit Rev Oncol Hematol 2014;90:220–32.
18. Shao H, Calvo KR, Gronborg M, et al. Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria. Leuk Res 2013;37:401–9.
19. Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood 2012;119:3330–2.
20. Jain P, Pemmaraju N, Ravandi F. Update on the biology and treatment options for hairy cell leukemia. Curr Treat Options Oncol 2014;15:187–209.
21. Sivina M, Kreitman RJ, Arons E, et al. The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: a new therapeutic approach. Br J Haematol 2014;166:177–88.
22. Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood 2015;126:842–50.
23. Andritsos LA, Grever MR. Historical overview of hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:166–74.
24. Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017;129:553–60.
25. Mhawech-Fauceglia P, Oberholzer M, Aschenafi S, et al. Potential predictive patterns of minimal residual disease detected by immunohistochemistry on bone marrow biopsy specimens during a long-term follow-up in patients treated with cladribine for hairy cell leukemia. Arch Pathol Lab Med 2006;130:374–7.
26. Ortiz-Maldonado V, Villamor N, Baumann T, et al., Is there a role for minimal residual disease monitoring in the management of patients with hairy-cell leukaemia? Br J Haematol 2017 Aug 18.
27. Jansen J, Hermans J. Clinical staging system for hairy-cell leukemia. Blood 1982;60:571–7.
28. Grever MR, Lozanski G. Modern strategies for hairy cell leukemia. J Clin Oncol 2011;29:583–90.
29. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
30. Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol 1995;13:974–82.
31. Kreitman RJ, Wilson W, Calvo KR, et al. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin Cancer Res 2013;19:6873–81.
32. Burotto M, Stetler-Stevenson M, Arons E, et al. Bendamustine and rituximab in relapsed and refractory hairy cell leukemia. Clin Cancer Res 2013;19:6313–21.
33. Randomized phase II trial of rituximab with either pentostatin or bendamustine for multiply relapsed or refractory hairy cell leukemia. 2017 [cited 2017 Oct 26]; NCT01059786. https://clinicaltrials.gov/ct2/show/NCT01059786.
34. Else M, Dearden CE, Matutes E, et al. Rituximab with pentostatin or cladribine: an effective combination treatment for hairy cell leukemia after disease recurrence. Leuk Lymphoma 2011;52 Suppl 2:75–8.
35. Thomas DA, O’Brien S, Bueso-Ramos C, et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood 2003;102:3906–11.
36. Zenhäusern R, Simcock M, Gratwohl A, et al. Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98). Haematologica 2008;93(9):1426–8.
37. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
38. Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med 2015;373:1733–47.
39. Blachly JS, Lozanski G, Lucas DM, et al. Cotreatment of hairy cell leukemia and melanoma with the BRAF inhibitor dabrafenib. J Natl Compr Canc Netw 2015;13:9–13.
40. Tiacci E, De Carolis L, Zaja F, et al. Vemurafenib plus rituximab in hairy cell leukemia: a promisingchemotherapy-free regimen for relapsed or refractory patients. Blood 2016;128:1.
41. A phase II, open-label study in subjects with BRAF V600E-mutated rare cancers with several histologies to investigate the clinical efficacy and safety of the combination therapy of dabrafenib and trametinib. 2017 [cited 2017 Oct 26]; NCT02034110. https://clinicaltrials.gov/ct2/show/NCT02034110.
42. Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet 2014;46:8–10.
43. Andritsos LA, Grieselhuber NR, Anghelina M, et al. Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma 2017 Sep 18:1–4.
44. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497–506.
45. Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012;30:1822–8.
46. Saven A, Burian C, Adusumalli J, Koziol JA. Filgrastim for cladribine-induced neutropenic fever in patients with hairy cell leukemia. Blood 1999;93:2471–7.
47. Cornet E, Tomowiak C, Tanguy-Schmidt A, et al. Long-term follow-up and second malignancies in 487 patients with hairy cell leukaemia. Br J Haematol 2014;166:390–400.
48. Anderson LA, Engels EA. Autoimmune conditions and hairy cell leukemia: an exploratory case-control study. J Hematol Oncol 2010;3:35.
1. Teras LR, Desantis DE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
2. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008.
3. Yetgin S, Olcay L, Yenicesu I, et al. Relapse in hairy cell leukemia due to isolated nodular skin infiltration. Pediatr Hematol Oncol 2001;18:415–7.
4. Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:175–9.
5. Veterans and agent orange: update 2014. Mil Med 2017;182:1619–20.
6. Villemagne B, Bay JO, Tournilhac O, et al. Two new cases of familial hairy cell leukemia associated with HLA haplotypes A2, B7, Bw4, Bw6. Leuk Lymphoma 2005;46:243–5.
7. Chandran R, Gardiner SK, Smith SD, Spurgeon SE. Improved survival in hairy cell leukaemia over three decades: a SEER database analysis of prognostic factors. Br J Haematol 2013;163:407–9.
8. Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood 1958;13:609–30.
9. Schrek R, Donnelly WJ. “Hairy” cells in blood in lymphoreticular neoplastic disease and “flagellated” cells of normal lymph nodes. Blood 1966;27:199–211.
10. Polliack A, Tadmor T. Surface topography of hairy cell leukemia cells compared to other leukemias as seen by scanning electron microscopy. Leuk Lymphoma 2011;52 Suppl 2:14–7.
11. Miranda RN, Cousar JB, Hammer RD, et al. Somatic mutation analysis of IgH variable regions reveals that tumor cells of most parafollicular (monocytoid) B-cell lymphoma, splenic marginal zone B-cell lymphoma, and some hairy cell leukemia are composed of memory B lymphocytes. Hum Pathol 1999;30:306–12.
12. Vanhentenrijk V, Tierens A, Wlodarska I, et al. V(H) gene analysis of hairy cell leukemia reveals a homogeneous mutation status and suggests its marginal zone B-cell origin. Leukemia 2004;18:1729–32.
13. Basso K, Liso A, Tiacci E, et al. Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. J Exp Med 2004;199:59–68.
14. Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci Transl Med 2014;6:238ra71.
15. Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305–15.
16. Kamiguti AS, Harris RJ, Slupsky JR, et al. Regulation of hairy-cell survival through constitutive activation of mitogen-activated protein kinase pathways. Oncogene 2003;22:2272–84.
17. Rahman MA, Salajegheh A, Smith RA, Lam AK. BRAF inhibitors: From the laboratory to clinical trials. Crit Rev Oncol Hematol 2014;90:220–32.
18. Shao H, Calvo KR, Gronborg M, et al. Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria. Leuk Res 2013;37:401–9.
19. Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood 2012;119:3330–2.
20. Jain P, Pemmaraju N, Ravandi F. Update on the biology and treatment options for hairy cell leukemia. Curr Treat Options Oncol 2014;15:187–209.
21. Sivina M, Kreitman RJ, Arons E, et al. The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: a new therapeutic approach. Br J Haematol 2014;166:177–88.
22. Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood 2015;126:842–50.
23. Andritsos LA, Grever MR. Historical overview of hairy cell leukemia. Best Pract Res Clin Haematol 2015;28:166–74.
24. Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017;129:553–60.
25. Mhawech-Fauceglia P, Oberholzer M, Aschenafi S, et al. Potential predictive patterns of minimal residual disease detected by immunohistochemistry on bone marrow biopsy specimens during a long-term follow-up in patients treated with cladribine for hairy cell leukemia. Arch Pathol Lab Med 2006;130:374–7.
26. Ortiz-Maldonado V, Villamor N, Baumann T, et al., Is there a role for minimal residual disease monitoring in the management of patients with hairy-cell leukaemia? Br J Haematol 2017 Aug 18.
27. Jansen J, Hermans J. Clinical staging system for hairy-cell leukemia. Blood 1982;60:571–7.
28. Grever MR, Lozanski G. Modern strategies for hairy cell leukemia. J Clin Oncol 2011;29:583–90.
29. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
30. Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol 1995;13:974–82.
31. Kreitman RJ, Wilson W, Calvo KR, et al. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin Cancer Res 2013;19:6873–81.
32. Burotto M, Stetler-Stevenson M, Arons E, et al. Bendamustine and rituximab in relapsed and refractory hairy cell leukemia. Clin Cancer Res 2013;19:6313–21.
33. Randomized phase II trial of rituximab with either pentostatin or bendamustine for multiply relapsed or refractory hairy cell leukemia. 2017 [cited 2017 Oct 26]; NCT01059786. https://clinicaltrials.gov/ct2/show/NCT01059786.
34. Else M, Dearden CE, Matutes E, et al. Rituximab with pentostatin or cladribine: an effective combination treatment for hairy cell leukemia after disease recurrence. Leuk Lymphoma 2011;52 Suppl 2:75–8.
35. Thomas DA, O’Brien S, Bueso-Ramos C, et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood 2003;102:3906–11.
36. Zenhäusern R, Simcock M, Gratwohl A, et al. Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98). Haematologica 2008;93(9):1426–8.
37. Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011;118:3818–23.
38. Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med 2015;373:1733–47.
39. Blachly JS, Lozanski G, Lucas DM, et al. Cotreatment of hairy cell leukemia and melanoma with the BRAF inhibitor dabrafenib. J Natl Compr Canc Netw 2015;13:9–13.
40. Tiacci E, De Carolis L, Zaja F, et al. Vemurafenib plus rituximab in hairy cell leukemia: a promisingchemotherapy-free regimen for relapsed or refractory patients. Blood 2016;128:1.
41. A phase II, open-label study in subjects with BRAF V600E-mutated rare cancers with several histologies to investigate the clinical efficacy and safety of the combination therapy of dabrafenib and trametinib. 2017 [cited 2017 Oct 26]; NCT02034110. https://clinicaltrials.gov/ct2/show/NCT02034110.
42. Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet 2014;46:8–10.
43. Andritsos LA, Grieselhuber NR, Anghelina M, et al. Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma 2017 Sep 18:1–4.
44. Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497–506.
45. Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012;30:1822–8.
46. Saven A, Burian C, Adusumalli J, Koziol JA. Filgrastim for cladribine-induced neutropenic fever in patients with hairy cell leukemia. Blood 1999;93:2471–7.
47. Cornet E, Tomowiak C, Tanguy-Schmidt A, et al. Long-term follow-up and second malignancies in 487 patients with hairy cell leukaemia. Br J Haematol 2014;166:390–400.
48. Anderson LA, Engels EA. Autoimmune conditions and hairy cell leukemia: an exploratory case-control study. J Hematol Oncol 2010;3:35.
Genomic Testing in Women with Early-Stage Hormone Receptor–Positive, HER2-Negative Breast Cancer
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
IHS Awards Funding to Behavioral Health Programs
Indian Health Service (IHS) has awarded grants worth $16.5 million to 4 behavioral health programs serving American Indians and Alaska Natives. The programs are Substance Abuse and Suicide Prevention (SASP), the Domestic Violence Prevention Program (DVPP), the Behavioral Health Integration Initiative (BH21), and Preventing Alcohol-Related Deaths (PARD).
Related: Development and Implementation of a Homeless Mobile Medical/Mental Veteran Intervention
Substance Abuse and Suicide Prevention provides culturally appropriate prevention and early intervention strategies aimed at reducing suicide and substance use and misuse among Native youth. Projects implement evidence-based, practice-based, and emerging practices to build resiliency, foster positive development, and promote family engagement.
The DVPP funding will expand outreach and increase awareness of domestic and sexual violence, provide victim advocacy, intervention, case coordination, policy development, community response teams, community and school education programs, and forensic health care services.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
The BH21 is a new funding opportunity at IHS. It assists awardees in planning, developing, implementing, and evaluating behavioral health integration with primary care.
Preventing Alcohol-Related Deaths also is a new program. Funding increases access to social detoxification, evaluation, stabilization, and fostering patient readiness for and entry into treatment.
Indian Health Service (IHS) has awarded grants worth $16.5 million to 4 behavioral health programs serving American Indians and Alaska Natives. The programs are Substance Abuse and Suicide Prevention (SASP), the Domestic Violence Prevention Program (DVPP), the Behavioral Health Integration Initiative (BH21), and Preventing Alcohol-Related Deaths (PARD).
Related: Development and Implementation of a Homeless Mobile Medical/Mental Veteran Intervention
Substance Abuse and Suicide Prevention provides culturally appropriate prevention and early intervention strategies aimed at reducing suicide and substance use and misuse among Native youth. Projects implement evidence-based, practice-based, and emerging practices to build resiliency, foster positive development, and promote family engagement.
The DVPP funding will expand outreach and increase awareness of domestic and sexual violence, provide victim advocacy, intervention, case coordination, policy development, community response teams, community and school education programs, and forensic health care services.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
The BH21 is a new funding opportunity at IHS. It assists awardees in planning, developing, implementing, and evaluating behavioral health integration with primary care.
Preventing Alcohol-Related Deaths also is a new program. Funding increases access to social detoxification, evaluation, stabilization, and fostering patient readiness for and entry into treatment.
Indian Health Service (IHS) has awarded grants worth $16.5 million to 4 behavioral health programs serving American Indians and Alaska Natives. The programs are Substance Abuse and Suicide Prevention (SASP), the Domestic Violence Prevention Program (DVPP), the Behavioral Health Integration Initiative (BH21), and Preventing Alcohol-Related Deaths (PARD).
Related: Development and Implementation of a Homeless Mobile Medical/Mental Veteran Intervention
Substance Abuse and Suicide Prevention provides culturally appropriate prevention and early intervention strategies aimed at reducing suicide and substance use and misuse among Native youth. Projects implement evidence-based, practice-based, and emerging practices to build resiliency, foster positive development, and promote family engagement.
The DVPP funding will expand outreach and increase awareness of domestic and sexual violence, provide victim advocacy, intervention, case coordination, policy development, community response teams, community and school education programs, and forensic health care services.
Related: New Center of Excellence to Lead Research of “Signature Wounds”
The BH21 is a new funding opportunity at IHS. It assists awardees in planning, developing, implementing, and evaluating behavioral health integration with primary care.
Preventing Alcohol-Related Deaths also is a new program. Funding increases access to social detoxification, evaluation, stabilization, and fostering patient readiness for and entry into treatment.
Group develops MM-specific CAR T-cell therapy
Researchers say they have identified a multiple myeloma (MM)-specific target for chimeric antigen receptor (CAR) T-cell therapy.
The team unearthed an MM-specific monoclonal antibody (mAb) and designed a CAR that incorporates a fragment derived from that mAb.
The resulting CAR T-cell therapy exhibited anti-MM activity in vitro and in vivo but did not have a negative effect on normal hematopoietic cells.
The researchers described this work in Nature Medicine.
The team believed that new antigen epitopes could be discovered by thoroughly searching for cancer-specific mAbs and characterizing the antigens they recognize.
“We applied this strategy to identify novel therapeutic targets for multiple myeloma . . . ,” explained study author Naoki Hosen, MD, PhD, of Osaka University in Osaka, Japan.
The researchers screened more than 10,000 anti-MM mAb clones and identified MMG49 as an MM-specific mAb that recognizes a subset of integrin β7 molecules.
The team found that MMG49 reacted to MM cells—but not to normal hematopoietic cells—in MM patient samples.
This prompted the researchers to design a CAR that incorporates a fragment derived from MMG49.
When tested in vitro, MMG49 CAR T cells exhibited cytotoxic activity against cell lines expressing the MMG49 epitope and primary MM cell from patients’ bone marrow. However, MMG49 CAR T cells did not have cytotoxic effects on MMG49-negative cells or normal hematopoietic cells.
The researchers said MM cells were completely eradicated by MMG49 CAR T cells. But the therapy did not kill T cells, even when integrin β7 was activated by MAdCAM-1 and CXCL12.
In mouse models of MM, MMG49 CAR T cells significantly prolonged survival when compared to a CD19 CAR T-cell therapy. MMG49 CAR T cells eradicated MM cells and significantly decreased tumor burden in some mice, but some mice relapsed.
MMG49 CAR T cells did not affect normal hematopoietic cells in the mice, and the researchers said there were no unexpected side effects with the treatment.
“Our results also demonstrate that the active conformer of integrin β7 can serve as an immunotherapeutic target against MM, even though the expression of the protein itself is not specific to MM,” said study author Yukiko Matsunaga, PhD, of Princess Margaret Cancer Centre, University Health Network, in Toronto, Canada.
“Therefore, it’s highly plausible that there are other cancer immunotherapeutic targets that have yet to be identified in many cell-surface proteins that undergo conformational changes, even if the expression of the proteins themselves is not cancer-specific.” ![]()
Researchers say they have identified a multiple myeloma (MM)-specific target for chimeric antigen receptor (CAR) T-cell therapy.
The team unearthed an MM-specific monoclonal antibody (mAb) and designed a CAR that incorporates a fragment derived from that mAb.
The resulting CAR T-cell therapy exhibited anti-MM activity in vitro and in vivo but did not have a negative effect on normal hematopoietic cells.
The researchers described this work in Nature Medicine.
The team believed that new antigen epitopes could be discovered by thoroughly searching for cancer-specific mAbs and characterizing the antigens they recognize.
“We applied this strategy to identify novel therapeutic targets for multiple myeloma . . . ,” explained study author Naoki Hosen, MD, PhD, of Osaka University in Osaka, Japan.
The researchers screened more than 10,000 anti-MM mAb clones and identified MMG49 as an MM-specific mAb that recognizes a subset of integrin β7 molecules.
The team found that MMG49 reacted to MM cells—but not to normal hematopoietic cells—in MM patient samples.
This prompted the researchers to design a CAR that incorporates a fragment derived from MMG49.
When tested in vitro, MMG49 CAR T cells exhibited cytotoxic activity against cell lines expressing the MMG49 epitope and primary MM cell from patients’ bone marrow. However, MMG49 CAR T cells did not have cytotoxic effects on MMG49-negative cells or normal hematopoietic cells.
The researchers said MM cells were completely eradicated by MMG49 CAR T cells. But the therapy did not kill T cells, even when integrin β7 was activated by MAdCAM-1 and CXCL12.
In mouse models of MM, MMG49 CAR T cells significantly prolonged survival when compared to a CD19 CAR T-cell therapy. MMG49 CAR T cells eradicated MM cells and significantly decreased tumor burden in some mice, but some mice relapsed.
MMG49 CAR T cells did not affect normal hematopoietic cells in the mice, and the researchers said there were no unexpected side effects with the treatment.
“Our results also demonstrate that the active conformer of integrin β7 can serve as an immunotherapeutic target against MM, even though the expression of the protein itself is not specific to MM,” said study author Yukiko Matsunaga, PhD, of Princess Margaret Cancer Centre, University Health Network, in Toronto, Canada.
“Therefore, it’s highly plausible that there are other cancer immunotherapeutic targets that have yet to be identified in many cell-surface proteins that undergo conformational changes, even if the expression of the proteins themselves is not cancer-specific.” ![]()
Researchers say they have identified a multiple myeloma (MM)-specific target for chimeric antigen receptor (CAR) T-cell therapy.
The team unearthed an MM-specific monoclonal antibody (mAb) and designed a CAR that incorporates a fragment derived from that mAb.
The resulting CAR T-cell therapy exhibited anti-MM activity in vitro and in vivo but did not have a negative effect on normal hematopoietic cells.
The researchers described this work in Nature Medicine.
The team believed that new antigen epitopes could be discovered by thoroughly searching for cancer-specific mAbs and characterizing the antigens they recognize.
“We applied this strategy to identify novel therapeutic targets for multiple myeloma . . . ,” explained study author Naoki Hosen, MD, PhD, of Osaka University in Osaka, Japan.
The researchers screened more than 10,000 anti-MM mAb clones and identified MMG49 as an MM-specific mAb that recognizes a subset of integrin β7 molecules.
The team found that MMG49 reacted to MM cells—but not to normal hematopoietic cells—in MM patient samples.
This prompted the researchers to design a CAR that incorporates a fragment derived from MMG49.
When tested in vitro, MMG49 CAR T cells exhibited cytotoxic activity against cell lines expressing the MMG49 epitope and primary MM cell from patients’ bone marrow. However, MMG49 CAR T cells did not have cytotoxic effects on MMG49-negative cells or normal hematopoietic cells.
The researchers said MM cells were completely eradicated by MMG49 CAR T cells. But the therapy did not kill T cells, even when integrin β7 was activated by MAdCAM-1 and CXCL12.
In mouse models of MM, MMG49 CAR T cells significantly prolonged survival when compared to a CD19 CAR T-cell therapy. MMG49 CAR T cells eradicated MM cells and significantly decreased tumor burden in some mice, but some mice relapsed.
MMG49 CAR T cells did not affect normal hematopoietic cells in the mice, and the researchers said there were no unexpected side effects with the treatment.
“Our results also demonstrate that the active conformer of integrin β7 can serve as an immunotherapeutic target against MM, even though the expression of the protein itself is not specific to MM,” said study author Yukiko Matsunaga, PhD, of Princess Margaret Cancer Centre, University Health Network, in Toronto, Canada.
“Therefore, it’s highly plausible that there are other cancer immunotherapeutic targets that have yet to be identified in many cell-surface proteins that undergo conformational changes, even if the expression of the proteins themselves is not cancer-specific.” ![]()
Mogamulizumab BLA receives priority review
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for mogamulizumab.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4 that is being developed by Kyowa Hakko Kirin Co., Ltd.
The company is seeking FDA approval for mogamulizumab as a treatment for patients with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
The FDA expects to make a decision on the BLA by June 4, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The BLA for mogamulizumab is supported by data from the MAVORIC study, the largest global randomized clinical trial of systemic therapy in CTCL.
MAVORIC is a phase 3 trial in which researchers evaluated mogamulizumab and an active comparator in 372 patients with CTCL who had failed at least 1 prior systemic treatment. The study was conducted in the US, Europe, Japan, and Australia.
Results from this trial are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 817).
The FDA previously granted mogamulizumab breakthrough therapy designation as a treatment for CTCL patients who have received at least 1 prior systemic therapy.
Breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for mogamulizumab.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4 that is being developed by Kyowa Hakko Kirin Co., Ltd.
The company is seeking FDA approval for mogamulizumab as a treatment for patients with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
The FDA expects to make a decision on the BLA by June 4, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The BLA for mogamulizumab is supported by data from the MAVORIC study, the largest global randomized clinical trial of systemic therapy in CTCL.
MAVORIC is a phase 3 trial in which researchers evaluated mogamulizumab and an active comparator in 372 patients with CTCL who had failed at least 1 prior systemic treatment. The study was conducted in the US, Europe, Japan, and Australia.
Results from this trial are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 817).
The FDA previously granted mogamulizumab breakthrough therapy designation as a treatment for CTCL patients who have received at least 1 prior systemic therapy.
Breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for mogamulizumab.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4 that is being developed by Kyowa Hakko Kirin Co., Ltd.
The company is seeking FDA approval for mogamulizumab as a treatment for patients with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
The FDA expects to make a decision on the BLA by June 4, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The BLA for mogamulizumab is supported by data from the MAVORIC study, the largest global randomized clinical trial of systemic therapy in CTCL.
MAVORIC is a phase 3 trial in which researchers evaluated mogamulizumab and an active comparator in 372 patients with CTCL who had failed at least 1 prior systemic treatment. The study was conducted in the US, Europe, Japan, and Australia.
Results from this trial are scheduled to be presented at the 2017 ASH Annual Meeting (abstract 817).
The FDA previously granted mogamulizumab breakthrough therapy designation as a treatment for CTCL patients who have received at least 1 prior systemic therapy.
Breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
FDA grants priority review to NDA for avatrombopag
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for avatrombopag.
Avatrombopag is a second-generation thrombopoietin receptor agonist that is intended to address the limitations of existing treatments for thrombocytopenia.
With this NDA, Dova Pharmaceuticals, Inc., is seeking approval of avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure.
The FDA expects to make a decision on the NDA by May 21, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Phase 3 trials
The NDA submission for avatrombopag is supported by 2 identically designed phase 3 trials, ADAPT 1 and ADAPT 2. Results from these trials were presented at the 2017 American Association for the Study of Liver Disease (AASLD) Meeting last month (abstract 217).
The studies randomized 435 patients with thrombocytopenia and chronic liver disease who were scheduled to undergo a procedure.
Patients with low baseline platelet counts (<40x 109/L) were randomized to receive 60 mg of avatrombopag or placebo daily for 5 days.
Patients with higher baseline platelet counts (40 to <50 x 109/L) were randomized to receive 40 mg of avatrombopag or placebo daily for 5 days.
Patients underwent their procedures 5 to 8 days after their last dose of avatrombopag.
In ADAPT-1, 85 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 78 controls completed the study.
In ADAPT-2, 68 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 68 controls completed the study.
Efficacy
The primary efficacy endpoint of these trials was the proportion of patients who did not require any bleeding rescue up to 7 days post-procedure. Bleeding rescue included platelet transfusion, fresh frozen plasma, cryoprecipitate, vitamin K (phytonadione), desmopressin, recombinant activated factor VII, aminocaproicacid, tranexamic acid, whole blood transfusion, packed red cell transfusion, surgical intervention, or interventional radiology.
In ADAPT-1, the primary endpoint was achieved by 66% of patients who received avatrombopag at 60 mg and 23% of those who received placebo in the low-platelet-count cohort (P<0.0001). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 38% of controls in the higher-platelet-count cohort (P<0.0001).
In ADAPT-2, the primary endpoint was achieved by 69% of patients who received avatrombopag at 60 mg and 35% of those who received placebo in the low-platelet-count cohort (P<0.0006). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 33% of controls in the higher- platelet-count cohort (P<0.0001).
A secondary efficacy endpoint was the proportion of patients achieving the target platelet count (≥50 x 109/L).
In ADAPT-1, this endpoint was met by 69% of patients who received avatrombopag at 60 mg and 4% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 88% of patients who received avatrombopag at 40 mg and 21% of controls in the higher-platelet-count cohort (P<0.0001)
In ADAPT-2, this endpoint was met by 67% of patients who received avatrombopag at 60 mg and 7% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 93% of patients who received avatrombopag at 40 mg and 39% of controls in the higher-platelet-count cohort (P<0.0001).
Safety
The researchers pooled safety data from the 2 trials.
Treatment-emergent adverse events (AEs) occurred in 58.2% of controls and 56% of avatrombopag-treated patients in the low-platelet-count cohort (60 mg). Treatment-emergent AEs also occurred in 50.8% of controls and 51.3% of avatrombopag-treated patients in the higher-platelet-count cohort (40 mg).
The most frequently reported treatment-emergent AEs were pyrexia, abdominal pain, nausea, headache, diarrhea, and fatigue.
One patient experienced partial portal vein thrombosis that was considered non-serious and potentially related to avatrombopag.
Treatment-related AEs occurred in 17.6% of controls and 11.3% of avatrombopag-treated patients in the low-platelet-count cohort. Treatment-related AEs also occurred in 6.2% of controls and 7% of avatrombopag-treated patients in the higher-platelet-count cohort.
Serious AEs occurred in 13.2%, 6.9%, 3.1%, and 7.8%, respectively.
There were 3 deaths—2 in the 40 mg avatrombopag arm in ADAPT-1 and 1 in the control group in ADAPT-2. None of the deaths was considered treatment-related.
Future directions
Dova Pharmaceuticals, Inc., is planning to explore the potential use of avatrombopag in a broader population of patients with thrombocytopenia. This includes patients undergoing surgical procedures associated with a high risk of bleeding and patients who develop thrombocytopenia after receiving chemotherapy.
In addition, the company is exploring a potential regulatory approval pathway for avatrombopag for the treatment of adults with chronic immune thrombocytopenic purpura based on results from a completed phase 3 trial in this patient population. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for avatrombopag.
Avatrombopag is a second-generation thrombopoietin receptor agonist that is intended to address the limitations of existing treatments for thrombocytopenia.
With this NDA, Dova Pharmaceuticals, Inc., is seeking approval of avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure.
The FDA expects to make a decision on the NDA by May 21, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Phase 3 trials
The NDA submission for avatrombopag is supported by 2 identically designed phase 3 trials, ADAPT 1 and ADAPT 2. Results from these trials were presented at the 2017 American Association for the Study of Liver Disease (AASLD) Meeting last month (abstract 217).
The studies randomized 435 patients with thrombocytopenia and chronic liver disease who were scheduled to undergo a procedure.
Patients with low baseline platelet counts (<40x 109/L) were randomized to receive 60 mg of avatrombopag or placebo daily for 5 days.
Patients with higher baseline platelet counts (40 to <50 x 109/L) were randomized to receive 40 mg of avatrombopag or placebo daily for 5 days.
Patients underwent their procedures 5 to 8 days after their last dose of avatrombopag.
In ADAPT-1, 85 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 78 controls completed the study.
In ADAPT-2, 68 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 68 controls completed the study.
Efficacy
The primary efficacy endpoint of these trials was the proportion of patients who did not require any bleeding rescue up to 7 days post-procedure. Bleeding rescue included platelet transfusion, fresh frozen plasma, cryoprecipitate, vitamin K (phytonadione), desmopressin, recombinant activated factor VII, aminocaproicacid, tranexamic acid, whole blood transfusion, packed red cell transfusion, surgical intervention, or interventional radiology.
In ADAPT-1, the primary endpoint was achieved by 66% of patients who received avatrombopag at 60 mg and 23% of those who received placebo in the low-platelet-count cohort (P<0.0001). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 38% of controls in the higher-platelet-count cohort (P<0.0001).
In ADAPT-2, the primary endpoint was achieved by 69% of patients who received avatrombopag at 60 mg and 35% of those who received placebo in the low-platelet-count cohort (P<0.0006). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 33% of controls in the higher- platelet-count cohort (P<0.0001).
A secondary efficacy endpoint was the proportion of patients achieving the target platelet count (≥50 x 109/L).
In ADAPT-1, this endpoint was met by 69% of patients who received avatrombopag at 60 mg and 4% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 88% of patients who received avatrombopag at 40 mg and 21% of controls in the higher-platelet-count cohort (P<0.0001)
In ADAPT-2, this endpoint was met by 67% of patients who received avatrombopag at 60 mg and 7% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 93% of patients who received avatrombopag at 40 mg and 39% of controls in the higher-platelet-count cohort (P<0.0001).
Safety
The researchers pooled safety data from the 2 trials.
Treatment-emergent adverse events (AEs) occurred in 58.2% of controls and 56% of avatrombopag-treated patients in the low-platelet-count cohort (60 mg). Treatment-emergent AEs also occurred in 50.8% of controls and 51.3% of avatrombopag-treated patients in the higher-platelet-count cohort (40 mg).
The most frequently reported treatment-emergent AEs were pyrexia, abdominal pain, nausea, headache, diarrhea, and fatigue.
One patient experienced partial portal vein thrombosis that was considered non-serious and potentially related to avatrombopag.
Treatment-related AEs occurred in 17.6% of controls and 11.3% of avatrombopag-treated patients in the low-platelet-count cohort. Treatment-related AEs also occurred in 6.2% of controls and 7% of avatrombopag-treated patients in the higher-platelet-count cohort.
Serious AEs occurred in 13.2%, 6.9%, 3.1%, and 7.8%, respectively.
There were 3 deaths—2 in the 40 mg avatrombopag arm in ADAPT-1 and 1 in the control group in ADAPT-2. None of the deaths was considered treatment-related.
Future directions
Dova Pharmaceuticals, Inc., is planning to explore the potential use of avatrombopag in a broader population of patients with thrombocytopenia. This includes patients undergoing surgical procedures associated with a high risk of bleeding and patients who develop thrombocytopenia after receiving chemotherapy.
In addition, the company is exploring a potential regulatory approval pathway for avatrombopag for the treatment of adults with chronic immune thrombocytopenic purpura based on results from a completed phase 3 trial in this patient population. ![]()
The US Food and Drug Administration (FDA) has granted priority review to the new drug application (NDA) for avatrombopag.
Avatrombopag is a second-generation thrombopoietin receptor agonist that is intended to address the limitations of existing treatments for thrombocytopenia.
With this NDA, Dova Pharmaceuticals, Inc., is seeking approval of avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure.
The FDA expects to make a decision on the NDA by May 21, 2018.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Phase 3 trials
The NDA submission for avatrombopag is supported by 2 identically designed phase 3 trials, ADAPT 1 and ADAPT 2. Results from these trials were presented at the 2017 American Association for the Study of Liver Disease (AASLD) Meeting last month (abstract 217).
The studies randomized 435 patients with thrombocytopenia and chronic liver disease who were scheduled to undergo a procedure.
Patients with low baseline platelet counts (<40x 109/L) were randomized to receive 60 mg of avatrombopag or placebo daily for 5 days.
Patients with higher baseline platelet counts (40 to <50 x 109/L) were randomized to receive 40 mg of avatrombopag or placebo daily for 5 days.
Patients underwent their procedures 5 to 8 days after their last dose of avatrombopag.
In ADAPT-1, 85 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 78 controls completed the study.
In ADAPT-2, 68 patients completed treatment with avatrombopag at 60 mg, 55 completed treatment with avatrombopag at 40 mg, and 68 controls completed the study.
Efficacy
The primary efficacy endpoint of these trials was the proportion of patients who did not require any bleeding rescue up to 7 days post-procedure. Bleeding rescue included platelet transfusion, fresh frozen plasma, cryoprecipitate, vitamin K (phytonadione), desmopressin, recombinant activated factor VII, aminocaproicacid, tranexamic acid, whole blood transfusion, packed red cell transfusion, surgical intervention, or interventional radiology.
In ADAPT-1, the primary endpoint was achieved by 66% of patients who received avatrombopag at 60 mg and 23% of those who received placebo in the low-platelet-count cohort (P<0.0001). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 38% of controls in the higher-platelet-count cohort (P<0.0001).
In ADAPT-2, the primary endpoint was achieved by 69% of patients who received avatrombopag at 60 mg and 35% of those who received placebo in the low-platelet-count cohort (P<0.0006). The endpoint was also achieved by 88% of patients who received avatrombopag at 40 mg and 33% of controls in the higher- platelet-count cohort (P<0.0001).
A secondary efficacy endpoint was the proportion of patients achieving the target platelet count (≥50 x 109/L).
In ADAPT-1, this endpoint was met by 69% of patients who received avatrombopag at 60 mg and 4% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 88% of patients who received avatrombopag at 40 mg and 21% of controls in the higher-platelet-count cohort (P<0.0001)
In ADAPT-2, this endpoint was met by 67% of patients who received avatrombopag at 60 mg and 7% of controls in the low-platelet-count cohort (P<0.0001). It was also met by 93% of patients who received avatrombopag at 40 mg and 39% of controls in the higher-platelet-count cohort (P<0.0001).
Safety
The researchers pooled safety data from the 2 trials.
Treatment-emergent adverse events (AEs) occurred in 58.2% of controls and 56% of avatrombopag-treated patients in the low-platelet-count cohort (60 mg). Treatment-emergent AEs also occurred in 50.8% of controls and 51.3% of avatrombopag-treated patients in the higher-platelet-count cohort (40 mg).
The most frequently reported treatment-emergent AEs were pyrexia, abdominal pain, nausea, headache, diarrhea, and fatigue.
One patient experienced partial portal vein thrombosis that was considered non-serious and potentially related to avatrombopag.
Treatment-related AEs occurred in 17.6% of controls and 11.3% of avatrombopag-treated patients in the low-platelet-count cohort. Treatment-related AEs also occurred in 6.2% of controls and 7% of avatrombopag-treated patients in the higher-platelet-count cohort.
Serious AEs occurred in 13.2%, 6.9%, 3.1%, and 7.8%, respectively.
There were 3 deaths—2 in the 40 mg avatrombopag arm in ADAPT-1 and 1 in the control group in ADAPT-2. None of the deaths was considered treatment-related.
Future directions
Dova Pharmaceuticals, Inc., is planning to explore the potential use of avatrombopag in a broader population of patients with thrombocytopenia. This includes patients undergoing surgical procedures associated with a high risk of bleeding and patients who develop thrombocytopenia after receiving chemotherapy.
In addition, the company is exploring a potential regulatory approval pathway for avatrombopag for the treatment of adults with chronic immune thrombocytopenic purpura based on results from a completed phase 3 trial in this patient population. ![]()
Acute Bronchitis
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Treatment-resistant depression boosts early mortality
PARIS – Young adults with treatment-resistant depression have more than double the risk of all-cause mortality, compared with their peers with major depressive disorder that’s not treatment resistant, Johan Reutfors, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Across the full age spectrum of adults with treatment-resistant depression, however, the magnitude of the increased risk associated with treatment-resistant depression is less extreme than in the 18- to 29-year-olds. Yet, the moderate overall 11% increased risk of all-cause mortality in adults with treatment-resistant depression, compared with those with non–treatment-resistant major depressive disorder remains both statistically significant and clinically meaningful, observed Dr. Reutfors, a psychiatrist at the Karolinska Institute in Stockholm.
During a mean 4.1 years of follow-up, 4,662 patients died. The all-cause mortality adjusted for age, gender, and a history of substance use disorders or self-harm was 2.17-fold greater in 18- to 29-year-olds who had treatment-resistant depression, compared with those with nonresistant major depressive disorder, 1.51-fold greater in 30- to 49-year-olds with treatment-resistant depression, and 1.18-fold greater in 50- to 69-year-olds with treatment-resistant depression.
In contrast, patients aged 70 or older with treatment-resistant depression had a significant 17% lower risk of all-cause mortality than those with non-TRD major depression. In an interview, Dr. Reutfors said this apparent protective effect was probably tied to survival bias.
“If you have lived so long that perhaps during your lifetime you’ve already had many depressive episodes, maybe only the healthier ones have survived,” he explained.
The all-cause mortality in patients with treatment-resistant depression and a history of self-harm was 37% greater than in patients without treatment-resistant depression.
The causes of excess mortality in the treatment-resistant depression group were quite different in the younger and older patients. In younger patients, the increased mortality was attributed mainly to suicides and accidents. In the older group, where the degree of excess risk was more modest, a variety of fatal illnesses figured more prominently.
The study was supported by Janssen. Dr. Reutfors reported having served as a paid speaker for Eli Lilly and receiving unrestricted grant support from Schering-Plough.
PARIS – Young adults with treatment-resistant depression have more than double the risk of all-cause mortality, compared with their peers with major depressive disorder that’s not treatment resistant, Johan Reutfors, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Across the full age spectrum of adults with treatment-resistant depression, however, the magnitude of the increased risk associated with treatment-resistant depression is less extreme than in the 18- to 29-year-olds. Yet, the moderate overall 11% increased risk of all-cause mortality in adults with treatment-resistant depression, compared with those with non–treatment-resistant major depressive disorder remains both statistically significant and clinically meaningful, observed Dr. Reutfors, a psychiatrist at the Karolinska Institute in Stockholm.
During a mean 4.1 years of follow-up, 4,662 patients died. The all-cause mortality adjusted for age, gender, and a history of substance use disorders or self-harm was 2.17-fold greater in 18- to 29-year-olds who had treatment-resistant depression, compared with those with nonresistant major depressive disorder, 1.51-fold greater in 30- to 49-year-olds with treatment-resistant depression, and 1.18-fold greater in 50- to 69-year-olds with treatment-resistant depression.
In contrast, patients aged 70 or older with treatment-resistant depression had a significant 17% lower risk of all-cause mortality than those with non-TRD major depression. In an interview, Dr. Reutfors said this apparent protective effect was probably tied to survival bias.
“If you have lived so long that perhaps during your lifetime you’ve already had many depressive episodes, maybe only the healthier ones have survived,” he explained.
The all-cause mortality in patients with treatment-resistant depression and a history of self-harm was 37% greater than in patients without treatment-resistant depression.
The causes of excess mortality in the treatment-resistant depression group were quite different in the younger and older patients. In younger patients, the increased mortality was attributed mainly to suicides and accidents. In the older group, where the degree of excess risk was more modest, a variety of fatal illnesses figured more prominently.
The study was supported by Janssen. Dr. Reutfors reported having served as a paid speaker for Eli Lilly and receiving unrestricted grant support from Schering-Plough.
PARIS – Young adults with treatment-resistant depression have more than double the risk of all-cause mortality, compared with their peers with major depressive disorder that’s not treatment resistant, Johan Reutfors, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Across the full age spectrum of adults with treatment-resistant depression, however, the magnitude of the increased risk associated with treatment-resistant depression is less extreme than in the 18- to 29-year-olds. Yet, the moderate overall 11% increased risk of all-cause mortality in adults with treatment-resistant depression, compared with those with non–treatment-resistant major depressive disorder remains both statistically significant and clinically meaningful, observed Dr. Reutfors, a psychiatrist at the Karolinska Institute in Stockholm.
During a mean 4.1 years of follow-up, 4,662 patients died. The all-cause mortality adjusted for age, gender, and a history of substance use disorders or self-harm was 2.17-fold greater in 18- to 29-year-olds who had treatment-resistant depression, compared with those with nonresistant major depressive disorder, 1.51-fold greater in 30- to 49-year-olds with treatment-resistant depression, and 1.18-fold greater in 50- to 69-year-olds with treatment-resistant depression.
In contrast, patients aged 70 or older with treatment-resistant depression had a significant 17% lower risk of all-cause mortality than those with non-TRD major depression. In an interview, Dr. Reutfors said this apparent protective effect was probably tied to survival bias.
“If you have lived so long that perhaps during your lifetime you’ve already had many depressive episodes, maybe only the healthier ones have survived,” he explained.
The all-cause mortality in patients with treatment-resistant depression and a history of self-harm was 37% greater than in patients without treatment-resistant depression.
The causes of excess mortality in the treatment-resistant depression group were quite different in the younger and older patients. In younger patients, the increased mortality was attributed mainly to suicides and accidents. In the older group, where the degree of excess risk was more modest, a variety of fatal illnesses figured more prominently.
The study was supported by Janssen. Dr. Reutfors reported having served as a paid speaker for Eli Lilly and receiving unrestricted grant support from Schering-Plough.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Young adult Swedes with treatment-resistant major depressive disorder have an all-cause mortality nearly 2.2-fold higher than their peers with non–treatment-resistant major depression.
Data source: This retrospective cohort study used Swedish national databases to look at all-cause mortality in more than 127,000 adults under psychiatric care for major depressive disorder.
Disclosures: The study was supported by Janssen. The presenter reported having served as a paid speaker for Eli Lilly and receiving unrestricted grant support from Schering-Plough.
Delayed HIV diagnoses still substantial for some at-risk groups
HIV diagnoses are coming sooner after infection, increasing physicians’ ability to treat and prevent the spread of HIV, according to a new Centers for Disease Control and Prevention (CDC) Vital Signs report.
As HIV testing has increased, the percentage of people aware of their HIV infection has also steadily grown. As of 2014, 85% of people living with HIV in the United States were aware of their infection. This knowledge allows individuals to seek antiretroviral treatment to suppress the virus, which decreases morbidity and mortality while reducing the risk of sexual transmission to others; knowing one’s HIV status, then, is incredibly important, clinicians say, because people who are unaware that they are HIV positive account for approximately 40% of ongoing transmissions.
While HIV testing has led to a reduction of HIV infection in the total population, several groups that are at a high risk of HIV infection are not getting tested as often as they should. According to the report, 29% of men who have sex with men, 42% of intravenous drugs users, and 59% of heterosexuals did not report having been tested within the past 12 months. Of the risk groups mentioned, at least two-thirds of people in each group had seen a health care provider in the last year. Heterosexual men are at particular risk of going undiagnosed because they are less likely to see a health care provider. This has led to half of heterosexual men who have HIV going undiagnosed for 5 years or more, the report notes.
Health care providers can improve testing by discussing HIV with patients and explaining that HIV testing is a routine part of any patient’s health care. Physicians should routinely test all patients aged 13-64 years, the CDC says. Testing should be emphasized in patients in high-risk groups; these patients should be tested at least once a year. Sexually active gay and bisexual men should ideally be tested every 3-6 months. Pregnant women, or those looking to become pregnant, should be tested to as soon as possible. If a pregnant woman is in a high-risk population, she should be tested again in the third trimester.
If a patient tests positive for HIV, the CDC report says it is important for clinicians connect them with treatment options and discuss prevention of transmission. The earlier a person begins HIV treatment, the greater the benefits will be. As part of the treatment, clinicians should encourage patients to stay on antiretroviral care to reduce the viral load in the body to either very low (less than 200 copies/mL) or undetectable levels.
“HIV is being diagnosed more quickly, the number of people who have the virus under control is up, and annual infections are down. So while we celebrate our progress, we pledge to work together to end this epidemic forever,” said CDC Director Brenda Fitzgerald, MD, in a statement.
HIV diagnoses are coming sooner after infection, increasing physicians’ ability to treat and prevent the spread of HIV, according to a new Centers for Disease Control and Prevention (CDC) Vital Signs report.
As HIV testing has increased, the percentage of people aware of their HIV infection has also steadily grown. As of 2014, 85% of people living with HIV in the United States were aware of their infection. This knowledge allows individuals to seek antiretroviral treatment to suppress the virus, which decreases morbidity and mortality while reducing the risk of sexual transmission to others; knowing one’s HIV status, then, is incredibly important, clinicians say, because people who are unaware that they are HIV positive account for approximately 40% of ongoing transmissions.
While HIV testing has led to a reduction of HIV infection in the total population, several groups that are at a high risk of HIV infection are not getting tested as often as they should. According to the report, 29% of men who have sex with men, 42% of intravenous drugs users, and 59% of heterosexuals did not report having been tested within the past 12 months. Of the risk groups mentioned, at least two-thirds of people in each group had seen a health care provider in the last year. Heterosexual men are at particular risk of going undiagnosed because they are less likely to see a health care provider. This has led to half of heterosexual men who have HIV going undiagnosed for 5 years or more, the report notes.
Health care providers can improve testing by discussing HIV with patients and explaining that HIV testing is a routine part of any patient’s health care. Physicians should routinely test all patients aged 13-64 years, the CDC says. Testing should be emphasized in patients in high-risk groups; these patients should be tested at least once a year. Sexually active gay and bisexual men should ideally be tested every 3-6 months. Pregnant women, or those looking to become pregnant, should be tested to as soon as possible. If a pregnant woman is in a high-risk population, she should be tested again in the third trimester.
If a patient tests positive for HIV, the CDC report says it is important for clinicians connect them with treatment options and discuss prevention of transmission. The earlier a person begins HIV treatment, the greater the benefits will be. As part of the treatment, clinicians should encourage patients to stay on antiretroviral care to reduce the viral load in the body to either very low (less than 200 copies/mL) or undetectable levels.
“HIV is being diagnosed more quickly, the number of people who have the virus under control is up, and annual infections are down. So while we celebrate our progress, we pledge to work together to end this epidemic forever,” said CDC Director Brenda Fitzgerald, MD, in a statement.
HIV diagnoses are coming sooner after infection, increasing physicians’ ability to treat and prevent the spread of HIV, according to a new Centers for Disease Control and Prevention (CDC) Vital Signs report.
As HIV testing has increased, the percentage of people aware of their HIV infection has also steadily grown. As of 2014, 85% of people living with HIV in the United States were aware of their infection. This knowledge allows individuals to seek antiretroviral treatment to suppress the virus, which decreases morbidity and mortality while reducing the risk of sexual transmission to others; knowing one’s HIV status, then, is incredibly important, clinicians say, because people who are unaware that they are HIV positive account for approximately 40% of ongoing transmissions.
While HIV testing has led to a reduction of HIV infection in the total population, several groups that are at a high risk of HIV infection are not getting tested as often as they should. According to the report, 29% of men who have sex with men, 42% of intravenous drugs users, and 59% of heterosexuals did not report having been tested within the past 12 months. Of the risk groups mentioned, at least two-thirds of people in each group had seen a health care provider in the last year. Heterosexual men are at particular risk of going undiagnosed because they are less likely to see a health care provider. This has led to half of heterosexual men who have HIV going undiagnosed for 5 years or more, the report notes.
Health care providers can improve testing by discussing HIV with patients and explaining that HIV testing is a routine part of any patient’s health care. Physicians should routinely test all patients aged 13-64 years, the CDC says. Testing should be emphasized in patients in high-risk groups; these patients should be tested at least once a year. Sexually active gay and bisexual men should ideally be tested every 3-6 months. Pregnant women, or those looking to become pregnant, should be tested to as soon as possible. If a pregnant woman is in a high-risk population, she should be tested again in the third trimester.
If a patient tests positive for HIV, the CDC report says it is important for clinicians connect them with treatment options and discuss prevention of transmission. The earlier a person begins HIV treatment, the greater the benefits will be. As part of the treatment, clinicians should encourage patients to stay on antiretroviral care to reduce the viral load in the body to either very low (less than 200 copies/mL) or undetectable levels.
“HIV is being diagnosed more quickly, the number of people who have the virus under control is up, and annual infections are down. So while we celebrate our progress, we pledge to work together to end this epidemic forever,” said CDC Director Brenda Fitzgerald, MD, in a statement.
FROM CDC VITAL SIGNS REPORT
Key clinical point:
Major finding: Approximately 15% of those living with HIV in 2015 were unaware of their infection and had a median diagnosis delay of 3 years.
Data source: Data of 39,720 individuals reported to the CDC’s National HIV Surveillance System from 50 states and the District of Columbia in 2015.
Disclosures: No conflicts of interest were reported.