User login
MSQC quality recommendations improve SSI in colectomy patients
The rate of patients decreased as hospitals implemented three specific care measures promoted by the Michigan Surgical Quality Collaborative, according to a study funded by the Blue Cross Blue Shield of Michigan.
With surgical site infections (SSIs) after colectomy associated with high morbidity as the second leading hospital acquired infection, and costing the health care system approximately $315 million annually, investment in this quality improvement program could ease a tremendous burden, according to Joceline V. Vu, MD, general surgery resident at the University of Michigan, Ann Arbor, and fellow investigators. The study was published in the Journal of the American College of Surgeons (2018 Jan;226[1]:91-99).
The study cohort included 5,742 colectomy patients at 1 of 52 hospitals associated with the Michigan Surgical Quality Collaborative (MSQC) between 2012 and 2016. Investigators assessed the use of the MSQC-recommended care bundle – cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and postoperative day-1 glucose less than or equal to 140 mg/dL – and the impact of the bundle components on surgical site infection (SSI).
Patients were also split into groups based on the use of perioperative treatments previously found to be associated with SSI improvement, which included the three treatments in the care bundle as well as postoperative normothermia, minimally invasive surgery, and operative duration defined as either less than or greater than 100 minutes.
Those who had received these perioperative measures were given one point for each measure received.
Of the total, 8.1% of patients received 0-1 point, 22.2% received 2 points, 31.7% received 3 points, 27.2% received 4 points, and 10.7% received 5-6 points.
Patients were split relatively evenly between male and female, and the majority of patients were white across all six SSI perioperative groups.
Patients with 0-1 point were more likely to be older than 65 years (56.4%), while those who received 5-6 points were more likely to be between 45 and 64 years old (45.6% [P less than .001]).
Hospitals increased the use of three of the promoted processes (cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and normoglycemia) during 2012-2016, and average use scores for patients went from 1.1 to 1.5 (P less than .001), according to investigators. As the rate of cefazolin/metronidazole and oral antibiotics with mechanical bowel preparation use rose from 18.6% and 42.9%, respectively, to 32.3% and 62.0% (P less than .001), SSI rates fell from 6.7% to 3.9% (P = .012) during the same period. The change in the normoglycemia rate (48.9% to 57.7%) was not significant (P = .112).
Hospitals that used all six recommended items saw a slightly decreased rate of SSI (r = –.39) between 2012 and 2016, according to Dr. Vu and her colleagues. Patients who received more measures had lower rates of complications, with SSI rates at 5.7% for those with 0-1 point, compared with 1.1% in those with 5-6 (P less than .001).
Rates of sepsis, pneumonia, emergency department visits, readmission, reoperation, and morbidity were also significantly lower.
“The MSQC and other collaborative quality improvement organizations represent a step toward this vision [of a learning health care system],” according to Dr. Vu and her colleagues. “Collaborating organizations can identify problems in care, adopt practice changes, and analyze the effect of those changes in a timely fashion.”
The findings of this study may be limited by a selection bias of how many bundle procedures a patient received based on comorbidities such as chronic obstructive pulmonary disease, hypertension, and obesity, which were all higher among those with fewer points. Investigators were also unable to discern causality of certain results because of the observational nature of the study. Hospitals included in the cohort volunteered to use the MSQC infrastructure, which may have limited the generalizability of the study,
Investigators reported no relevant financial disclosures. The study was funded by the Blue Cross Blue Shield of Michigan.
SOURCE: Vu, J V et al. J Am Coll Surg. 2018 Jan;226(1):91-9.
The rate of patients decreased as hospitals implemented three specific care measures promoted by the Michigan Surgical Quality Collaborative, according to a study funded by the Blue Cross Blue Shield of Michigan.
With surgical site infections (SSIs) after colectomy associated with high morbidity as the second leading hospital acquired infection, and costing the health care system approximately $315 million annually, investment in this quality improvement program could ease a tremendous burden, according to Joceline V. Vu, MD, general surgery resident at the University of Michigan, Ann Arbor, and fellow investigators. The study was published in the Journal of the American College of Surgeons (2018 Jan;226[1]:91-99).
The study cohort included 5,742 colectomy patients at 1 of 52 hospitals associated with the Michigan Surgical Quality Collaborative (MSQC) between 2012 and 2016. Investigators assessed the use of the MSQC-recommended care bundle – cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and postoperative day-1 glucose less than or equal to 140 mg/dL – and the impact of the bundle components on surgical site infection (SSI).
Patients were also split into groups based on the use of perioperative treatments previously found to be associated with SSI improvement, which included the three treatments in the care bundle as well as postoperative normothermia, minimally invasive surgery, and operative duration defined as either less than or greater than 100 minutes.
Those who had received these perioperative measures were given one point for each measure received.
Of the total, 8.1% of patients received 0-1 point, 22.2% received 2 points, 31.7% received 3 points, 27.2% received 4 points, and 10.7% received 5-6 points.
Patients were split relatively evenly between male and female, and the majority of patients were white across all six SSI perioperative groups.
Patients with 0-1 point were more likely to be older than 65 years (56.4%), while those who received 5-6 points were more likely to be between 45 and 64 years old (45.6% [P less than .001]).
Hospitals increased the use of three of the promoted processes (cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and normoglycemia) during 2012-2016, and average use scores for patients went from 1.1 to 1.5 (P less than .001), according to investigators. As the rate of cefazolin/metronidazole and oral antibiotics with mechanical bowel preparation use rose from 18.6% and 42.9%, respectively, to 32.3% and 62.0% (P less than .001), SSI rates fell from 6.7% to 3.9% (P = .012) during the same period. The change in the normoglycemia rate (48.9% to 57.7%) was not significant (P = .112).
Hospitals that used all six recommended items saw a slightly decreased rate of SSI (r = –.39) between 2012 and 2016, according to Dr. Vu and her colleagues. Patients who received more measures had lower rates of complications, with SSI rates at 5.7% for those with 0-1 point, compared with 1.1% in those with 5-6 (P less than .001).
Rates of sepsis, pneumonia, emergency department visits, readmission, reoperation, and morbidity were also significantly lower.
“The MSQC and other collaborative quality improvement organizations represent a step toward this vision [of a learning health care system],” according to Dr. Vu and her colleagues. “Collaborating organizations can identify problems in care, adopt practice changes, and analyze the effect of those changes in a timely fashion.”
The findings of this study may be limited by a selection bias of how many bundle procedures a patient received based on comorbidities such as chronic obstructive pulmonary disease, hypertension, and obesity, which were all higher among those with fewer points. Investigators were also unable to discern causality of certain results because of the observational nature of the study. Hospitals included in the cohort volunteered to use the MSQC infrastructure, which may have limited the generalizability of the study,
Investigators reported no relevant financial disclosures. The study was funded by the Blue Cross Blue Shield of Michigan.
SOURCE: Vu, J V et al. J Am Coll Surg. 2018 Jan;226(1):91-9.
The rate of patients decreased as hospitals implemented three specific care measures promoted by the Michigan Surgical Quality Collaborative, according to a study funded by the Blue Cross Blue Shield of Michigan.
With surgical site infections (SSIs) after colectomy associated with high morbidity as the second leading hospital acquired infection, and costing the health care system approximately $315 million annually, investment in this quality improvement program could ease a tremendous burden, according to Joceline V. Vu, MD, general surgery resident at the University of Michigan, Ann Arbor, and fellow investigators. The study was published in the Journal of the American College of Surgeons (2018 Jan;226[1]:91-99).
The study cohort included 5,742 colectomy patients at 1 of 52 hospitals associated with the Michigan Surgical Quality Collaborative (MSQC) between 2012 and 2016. Investigators assessed the use of the MSQC-recommended care bundle – cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and postoperative day-1 glucose less than or equal to 140 mg/dL – and the impact of the bundle components on surgical site infection (SSI).
Patients were also split into groups based on the use of perioperative treatments previously found to be associated with SSI improvement, which included the three treatments in the care bundle as well as postoperative normothermia, minimally invasive surgery, and operative duration defined as either less than or greater than 100 minutes.
Those who had received these perioperative measures were given one point for each measure received.
Of the total, 8.1% of patients received 0-1 point, 22.2% received 2 points, 31.7% received 3 points, 27.2% received 4 points, and 10.7% received 5-6 points.
Patients were split relatively evenly between male and female, and the majority of patients were white across all six SSI perioperative groups.
Patients with 0-1 point were more likely to be older than 65 years (56.4%), while those who received 5-6 points were more likely to be between 45 and 64 years old (45.6% [P less than .001]).
Hospitals increased the use of three of the promoted processes (cefazolin/metronidazole, oral antibiotics with mechanical bowel preparation, and normoglycemia) during 2012-2016, and average use scores for patients went from 1.1 to 1.5 (P less than .001), according to investigators. As the rate of cefazolin/metronidazole and oral antibiotics with mechanical bowel preparation use rose from 18.6% and 42.9%, respectively, to 32.3% and 62.0% (P less than .001), SSI rates fell from 6.7% to 3.9% (P = .012) during the same period. The change in the normoglycemia rate (48.9% to 57.7%) was not significant (P = .112).
Hospitals that used all six recommended items saw a slightly decreased rate of SSI (r = –.39) between 2012 and 2016, according to Dr. Vu and her colleagues. Patients who received more measures had lower rates of complications, with SSI rates at 5.7% for those with 0-1 point, compared with 1.1% in those with 5-6 (P less than .001).
Rates of sepsis, pneumonia, emergency department visits, readmission, reoperation, and morbidity were also significantly lower.
“The MSQC and other collaborative quality improvement organizations represent a step toward this vision [of a learning health care system],” according to Dr. Vu and her colleagues. “Collaborating organizations can identify problems in care, adopt practice changes, and analyze the effect of those changes in a timely fashion.”
The findings of this study may be limited by a selection bias of how many bundle procedures a patient received based on comorbidities such as chronic obstructive pulmonary disease, hypertension, and obesity, which were all higher among those with fewer points. Investigators were also unable to discern causality of certain results because of the observational nature of the study. Hospitals included in the cohort volunteered to use the MSQC infrastructure, which may have limited the generalizability of the study,
Investigators reported no relevant financial disclosures. The study was funded by the Blue Cross Blue Shield of Michigan.
SOURCE: Vu, J V et al. J Am Coll Surg. 2018 Jan;226(1):91-9.
Key clinical point: Promotion of quality improvement bundled process measures by regional programs is associated with lower SSI in colectomy patients.
Major finding: As use of bundled care processes increased, surgical site infections (SSI) decreased from 6.7% to 3.9% (P = .012) across 52 hospitals.
Study details: Observational study of 5,742 colectomy patients at 1 of 52 hospitals in the Michigan Surgical Quality Collaborative system between 2012 and 2016.
Disclosures: Investigators reported no relevant financial disclosures. The study was funded by the Blue Cross Blue Shield of Michigan.
Source: Vu, J V et al. J Am Coll Surg. 2018 Jan;226(1):91-99.
Running on empty: CHIP funding could run out Jan. 19 for some states
Some states are facing a mid-January loss of funding for their Children’s Health Insurance Program (CHIP) despite spending approved by Congress in late December that was expected to keep the program running for 3 months, federal health officials said Jan. 5.
The $2.85 billion was supposed to fund states’ CHIP programs through March 31. But some states will start running out of money after Jan. 19, according to the Centers for Medicare & Medicaid Services. The CMS did not say which states are likely to be affected first.
The latest estimates for when federal funding runs out could cause states to soon freeze enrollment and alert parents that the program could soon shut down.
The CHIP program provides health coverage to 9 million children from lower-income households that make too much money to qualify for Medicaid. Its federal authorization ended Oct. 1, and states were then forced to use unspent funds to carry them over while the House and Senate try to agree on a way to continue funding.
Congress extended funding on Dec. 21 and touted that states would have money to last while Congress worked on a long-term funding solution. But the CMS said on Jan. 5 it could only guarantee that the appropriation will be enough to fund all states through Jan. 19.
The CMS said the agency is in discussions with states to help deal with the funding shortfall.
“The funding … should carry all the states through January 19th based upon best estimates of state expenditures to date,” said CMS spokesman Johnathan Monroe. “However, due to a number of variables relating to state expenditure rates and reporting, we are unable to say with certainty whether there is enough funding for every state to continue its CHIP program through March 31, 2018.”
“States need to know whether they will need to find additional funding for children covered under the Medicaid CHIP program at a much lower federal matching rate, send letters to families, and re-program their eligibility systems,” said Lisa Dubay, a senior fellow at the Urban Institute. “Of course, the implications for families with CHIP-eligible children cannot be understated: Parents are worried that their children will lose coverage. And they should be.”
Although the program enjoys bipartisan support on Capitol Hill, the Republican-controlled House and Senate have for months been unable to agree on how to continue funding CHIP, which began in 1997.
The House plan includes a controversial funding provision – opposed by Democrats -– that takes millions of dollars from the Affordable Care Act’s Prevention and Public Health Fund and increases Medicare premiums for some higher-earning beneficiaries.
The Senate Finance Committee reached an agreement to extend the program for 5 years but did not unite around a plan on funding.
Before the CHIP funding extension on Dec. 21, Alabama said it would freeze enrollment Jan. 1 and shut down the program Jan. 31. Colorado, Connecticut, and Virginia sent letters to CHIP families warning that the program could soon end.
After the funding extension, Alabama put a hold on shutting down CHIP.
“Some states will begin exhausting all available funding earlier than others,” a CMS official said Jan. 5. “But the exact timing of when states will exhaust their funding is a moving target.”
Bruce Lesley, president of First Focus, a child advocacy group, said Congress should have known its short-term funding plan was not enough.
“The math never worked on the patch, as it only bought a few weeks,” he said. “Congress must get this finalized before Jan. 19.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Some states are facing a mid-January loss of funding for their Children’s Health Insurance Program (CHIP) despite spending approved by Congress in late December that was expected to keep the program running for 3 months, federal health officials said Jan. 5.
The $2.85 billion was supposed to fund states’ CHIP programs through March 31. But some states will start running out of money after Jan. 19, according to the Centers for Medicare & Medicaid Services. The CMS did not say which states are likely to be affected first.
The latest estimates for when federal funding runs out could cause states to soon freeze enrollment and alert parents that the program could soon shut down.
The CHIP program provides health coverage to 9 million children from lower-income households that make too much money to qualify for Medicaid. Its federal authorization ended Oct. 1, and states were then forced to use unspent funds to carry them over while the House and Senate try to agree on a way to continue funding.
Congress extended funding on Dec. 21 and touted that states would have money to last while Congress worked on a long-term funding solution. But the CMS said on Jan. 5 it could only guarantee that the appropriation will be enough to fund all states through Jan. 19.
The CMS said the agency is in discussions with states to help deal with the funding shortfall.
“The funding … should carry all the states through January 19th based upon best estimates of state expenditures to date,” said CMS spokesman Johnathan Monroe. “However, due to a number of variables relating to state expenditure rates and reporting, we are unable to say with certainty whether there is enough funding for every state to continue its CHIP program through March 31, 2018.”
“States need to know whether they will need to find additional funding for children covered under the Medicaid CHIP program at a much lower federal matching rate, send letters to families, and re-program their eligibility systems,” said Lisa Dubay, a senior fellow at the Urban Institute. “Of course, the implications for families with CHIP-eligible children cannot be understated: Parents are worried that their children will lose coverage. And they should be.”
Although the program enjoys bipartisan support on Capitol Hill, the Republican-controlled House and Senate have for months been unable to agree on how to continue funding CHIP, which began in 1997.
The House plan includes a controversial funding provision – opposed by Democrats -– that takes millions of dollars from the Affordable Care Act’s Prevention and Public Health Fund and increases Medicare premiums for some higher-earning beneficiaries.
The Senate Finance Committee reached an agreement to extend the program for 5 years but did not unite around a plan on funding.
Before the CHIP funding extension on Dec. 21, Alabama said it would freeze enrollment Jan. 1 and shut down the program Jan. 31. Colorado, Connecticut, and Virginia sent letters to CHIP families warning that the program could soon end.
After the funding extension, Alabama put a hold on shutting down CHIP.
“Some states will begin exhausting all available funding earlier than others,” a CMS official said Jan. 5. “But the exact timing of when states will exhaust their funding is a moving target.”
Bruce Lesley, president of First Focus, a child advocacy group, said Congress should have known its short-term funding plan was not enough.
“The math never worked on the patch, as it only bought a few weeks,” he said. “Congress must get this finalized before Jan. 19.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Some states are facing a mid-January loss of funding for their Children’s Health Insurance Program (CHIP) despite spending approved by Congress in late December that was expected to keep the program running for 3 months, federal health officials said Jan. 5.
The $2.85 billion was supposed to fund states’ CHIP programs through March 31. But some states will start running out of money after Jan. 19, according to the Centers for Medicare & Medicaid Services. The CMS did not say which states are likely to be affected first.
The latest estimates for when federal funding runs out could cause states to soon freeze enrollment and alert parents that the program could soon shut down.
The CHIP program provides health coverage to 9 million children from lower-income households that make too much money to qualify for Medicaid. Its federal authorization ended Oct. 1, and states were then forced to use unspent funds to carry them over while the House and Senate try to agree on a way to continue funding.
Congress extended funding on Dec. 21 and touted that states would have money to last while Congress worked on a long-term funding solution. But the CMS said on Jan. 5 it could only guarantee that the appropriation will be enough to fund all states through Jan. 19.
The CMS said the agency is in discussions with states to help deal with the funding shortfall.
“The funding … should carry all the states through January 19th based upon best estimates of state expenditures to date,” said CMS spokesman Johnathan Monroe. “However, due to a number of variables relating to state expenditure rates and reporting, we are unable to say with certainty whether there is enough funding for every state to continue its CHIP program through March 31, 2018.”
“States need to know whether they will need to find additional funding for children covered under the Medicaid CHIP program at a much lower federal matching rate, send letters to families, and re-program their eligibility systems,” said Lisa Dubay, a senior fellow at the Urban Institute. “Of course, the implications for families with CHIP-eligible children cannot be understated: Parents are worried that their children will lose coverage. And they should be.”
Although the program enjoys bipartisan support on Capitol Hill, the Republican-controlled House and Senate have for months been unable to agree on how to continue funding CHIP, which began in 1997.
The House plan includes a controversial funding provision – opposed by Democrats -– that takes millions of dollars from the Affordable Care Act’s Prevention and Public Health Fund and increases Medicare premiums for some higher-earning beneficiaries.
The Senate Finance Committee reached an agreement to extend the program for 5 years but did not unite around a plan on funding.
Before the CHIP funding extension on Dec. 21, Alabama said it would freeze enrollment Jan. 1 and shut down the program Jan. 31. Colorado, Connecticut, and Virginia sent letters to CHIP families warning that the program could soon end.
After the funding extension, Alabama put a hold on shutting down CHIP.
“Some states will begin exhausting all available funding earlier than others,” a CMS official said Jan. 5. “But the exact timing of when states will exhaust their funding is a moving target.”
Bruce Lesley, president of First Focus, a child advocacy group, said Congress should have known its short-term funding plan was not enough.
“The math never worked on the patch, as it only bought a few weeks,” he said. “Congress must get this finalized before Jan. 19.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
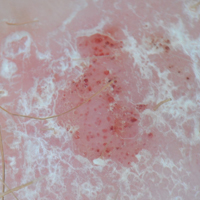
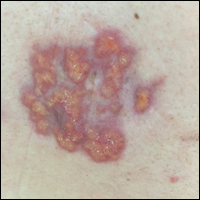
Purpuric Macule of the Right Axilla
The Diagnosis: Atypical Vascular Lesion
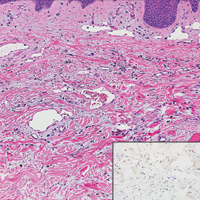
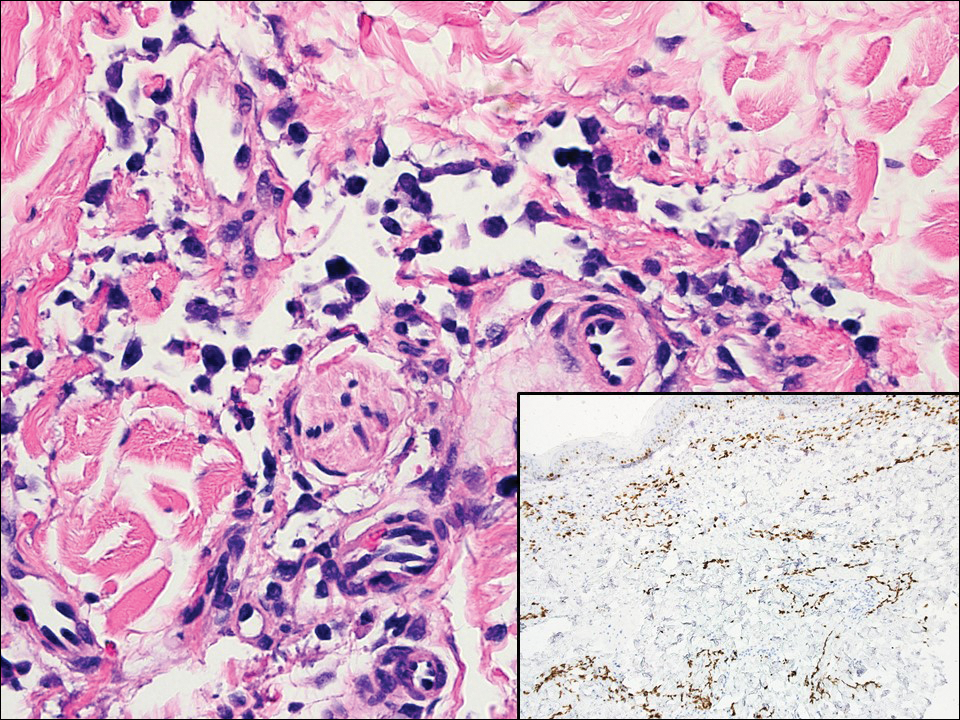
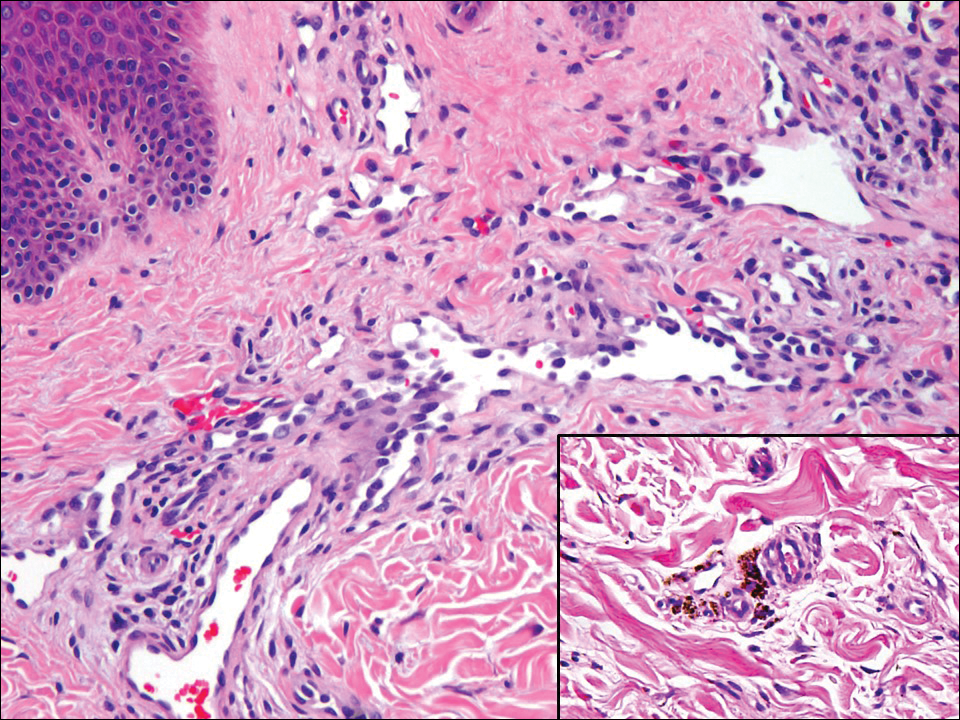
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.
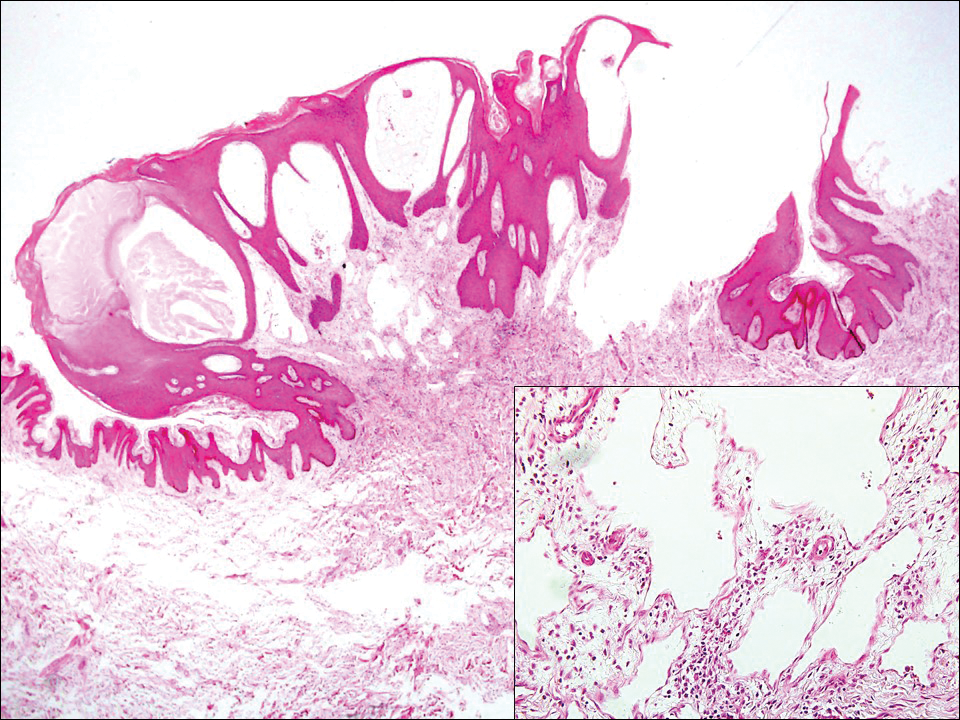
Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.
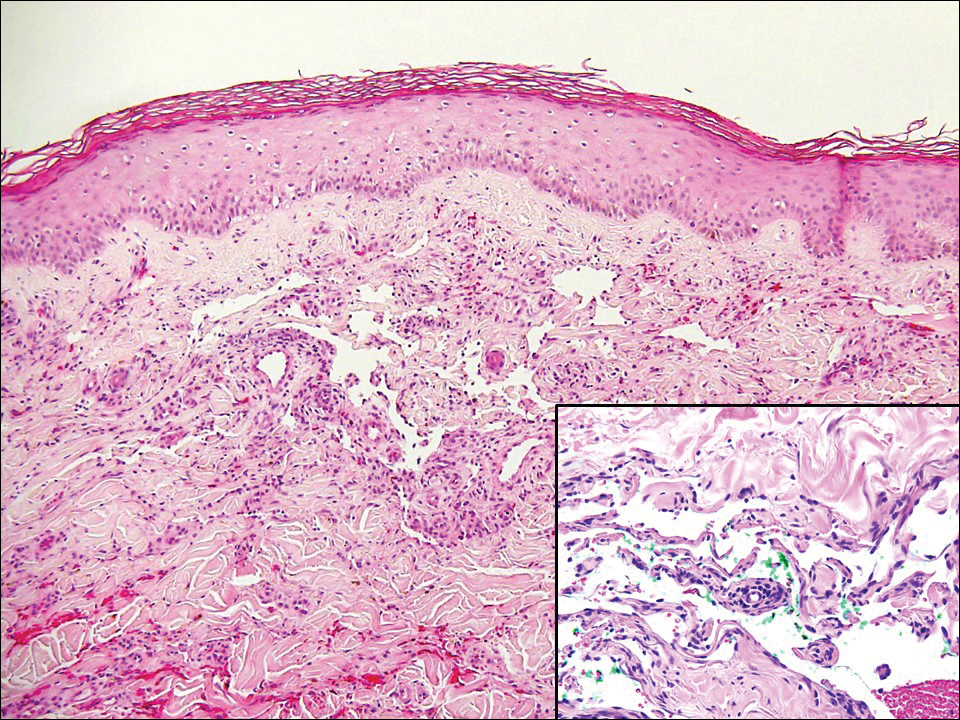
Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.
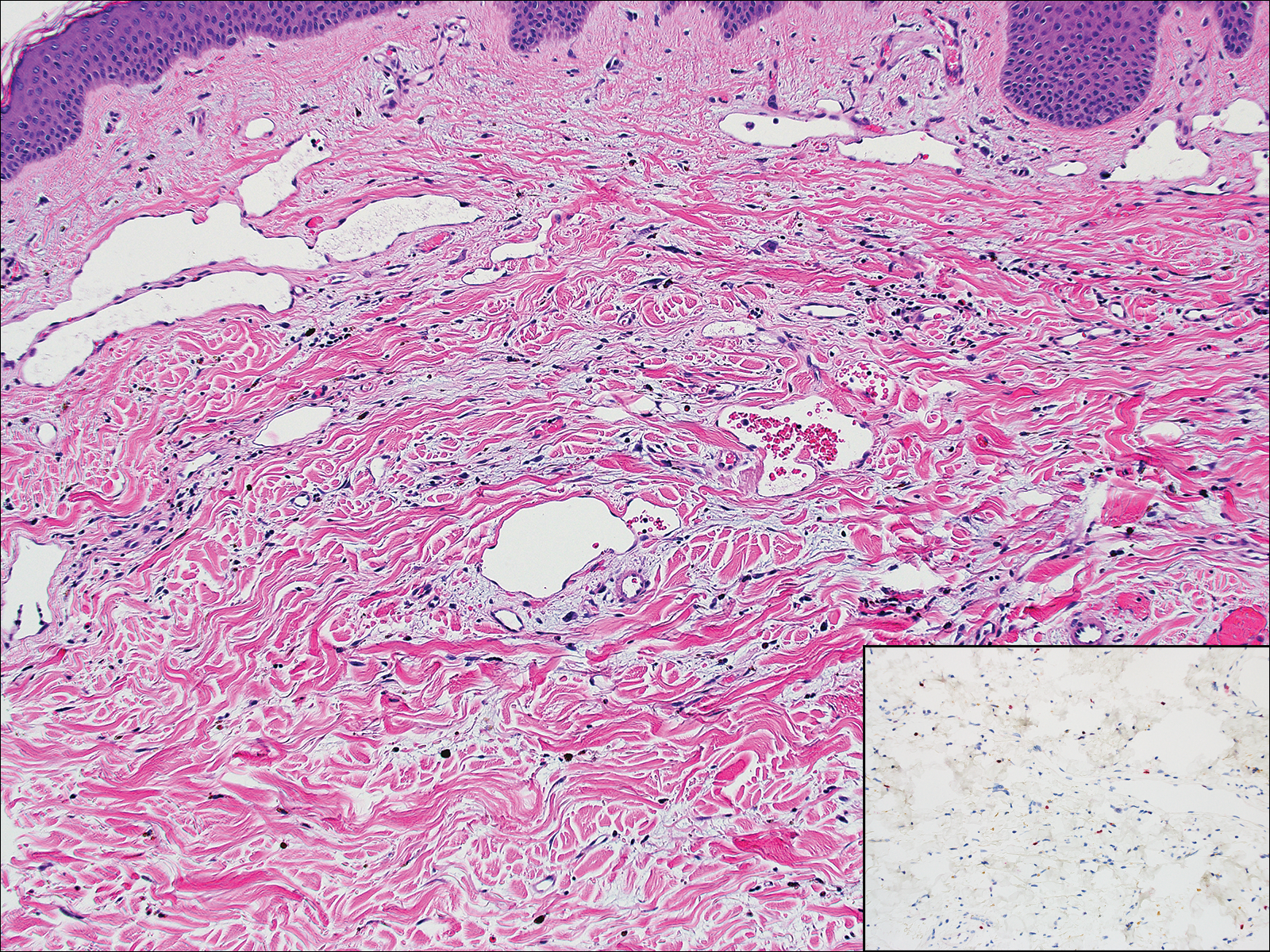
Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
A 67-year-old woman presented with a lesion on the medial aspect of the right axilla of 2 weeks' duration. The patient had a history of cancer of the right breast treated with a mastectomy and adjuvant radiation. She denied pain, bleeding, pruritus, or rapid growth, as well as any changes in medication or recent trauma. Physical examination revealed a 5-mm purpuric macule of the right axilla. A punch biopsy was performed. Amplification for the C-MYC gene was negative by fluorescence in situ hybridization.
A love of teaching: James Kim, MD
While James Kim, MD, did not originally begin medical school with a plan to become a hospitalist, he has embraced his current role wholeheartedly.
Since becoming board certified in both internal medicine and infectious diseases, Dr. Kim has welcomed the opportunity to be part of hospital medicine, which gives him the opportunity to pursue his other passion: teaching and mentoring.
“It’s not just what you know but how you convey what you know to other people,” said Dr. Kim. “While you might get useful information from a didactic teaching style, it’s important to ask questions to encourage the learner to think about not only what the right answer is but also what’s the thought process required to get the answer.”
As one of the newest additions to the editorial advisory board of The Hospitalist, Dr. Kim took time to tell us more about himself in a recent interview.
QUESTION: How did you find your career path in medicine?
ANSWER: I originally went into medical school thinking I was going to do pediatrics, but then I realized that I really enjoy talking to people and that I like the process of thinking through diagnoses, managing patients, and learning about what makes their circumstances unique.
Q: How did you get into hospital medicine?
A: When I finished my internal medicine residency, I thought I was going to do medical missions. However, I realized along the way that the care you need to provide in order to really make a difference in other countries requires a constant presence there – not just a week or two. So after my fellowship, I was searching for jobs and found a hospitalist position at the University of California, Los Angeles. When I saw it, I thought ‘Wow, I really miss doing inpatient medicine.’
Q: Since you started, what have been some of your favorite parts of hospital medicine?
A: When people come to you in the hospital setting, they are usually pretty sick. It is very satisfying when, through the course of a person’s hospital stay, we are able to come up with a plan that can get them acutely better.
Q: What do you think is the hardest part of hospital medicine?
A: I think one of the things that is most frustrating is when we are placed into a situation in which we are not necessarily doing medical work for a patient but are doing something more like social work. For instance, there are cases in which patients can not be on their own in the community, and there’s no family to take them in, so the hospital, on behalf of the state, has to take them in.
Q: What else do you do outside of hospitalist work?
A: Since I’ve finished medical school, I’ve always been in some kind of academia, which is not something I would have expected. But as time has gone by, I have really come to appreciate being in academia. I really enjoy teaching, and I also think that an academic institution kind of keeps me on my toes. I’m involved with interprofessional education at Emory, with teaching medical students, interns, and residents when I’m on teaching service, and obviously now I’m on The Hospitalist editorial board. I’m looking forward to keeping abreast of what’s hot in the world of hospital medicine.
Q: What are you excited about bringing to The Hospitalist editorial board?
A: I want to try to contribute ideas. I feel that even in my short time at Emory, I’ve gotten to know a few people who might be good resources for reporters to interview or even who might write articles themselves. I also think that seeing what is trending in the world of hospital medicine is a nice way of understanding the future direction of hospital medicine.
Q: What have you seen as being the biggest change in hospital medicine since you started?
A: I feel as though I’ve kept my head down and plowed forward through the first part of my career, but I think that, more than anything else, what I’ve noticed is bigger shifts within health care itself. I know that there’s a lot of consolidation going on. I think that there are many questions that are going to come up about how do we manage a health care system as complicated as America’s and how do we deliver optimal care to people especially when sometimes we end up in situations in which we don’t have all the resources that we would want to have because of circumstances.
Q: Do you see anything in particular on the horizon for hospital medicine?
A: I’ve noticed that there’s been more “hospitalist-ization” – if that’s even a term – of other medical services. At our institution, we already have an acute care service that is basically hospital medicine for general surgery. I think another thing that’s been kind of a hot topic recently is a point-of-care testing, including ultrasounds for line placements.
Q: Where do you see yourself in 10 years?
A: I really enjoy my work at Emory. I want to find more opportunities to teach. For example, I’ve already gotten involved in teaching physician assistant students about how to perform interviews and deliver presentations for attendings. A lot of serendipitous things have happened to me over time, so I think I will continue to teach, but I’m open to those opportunities that present themselves in the future.
Q: What’s the best book you’ve read recently and why?
A: “The Hero with a Thousand Faces,” by Joseph Campbell. This is a very well-known book – I think George Lucas made reference to it when he was writing Star Wars – but I think it was a great literary way to examine the hero’s journey. Once you read the book, and you then watch any kind of movie or read any other kind of adventure narrative, you can’t miss the pattern.
While James Kim, MD, did not originally begin medical school with a plan to become a hospitalist, he has embraced his current role wholeheartedly.
Since becoming board certified in both internal medicine and infectious diseases, Dr. Kim has welcomed the opportunity to be part of hospital medicine, which gives him the opportunity to pursue his other passion: teaching and mentoring.
“It’s not just what you know but how you convey what you know to other people,” said Dr. Kim. “While you might get useful information from a didactic teaching style, it’s important to ask questions to encourage the learner to think about not only what the right answer is but also what’s the thought process required to get the answer.”
As one of the newest additions to the editorial advisory board of The Hospitalist, Dr. Kim took time to tell us more about himself in a recent interview.
QUESTION: How did you find your career path in medicine?
ANSWER: I originally went into medical school thinking I was going to do pediatrics, but then I realized that I really enjoy talking to people and that I like the process of thinking through diagnoses, managing patients, and learning about what makes their circumstances unique.
Q: How did you get into hospital medicine?
A: When I finished my internal medicine residency, I thought I was going to do medical missions. However, I realized along the way that the care you need to provide in order to really make a difference in other countries requires a constant presence there – not just a week or two. So after my fellowship, I was searching for jobs and found a hospitalist position at the University of California, Los Angeles. When I saw it, I thought ‘Wow, I really miss doing inpatient medicine.’
Q: Since you started, what have been some of your favorite parts of hospital medicine?
A: When people come to you in the hospital setting, they are usually pretty sick. It is very satisfying when, through the course of a person’s hospital stay, we are able to come up with a plan that can get them acutely better.
Q: What do you think is the hardest part of hospital medicine?
A: I think one of the things that is most frustrating is when we are placed into a situation in which we are not necessarily doing medical work for a patient but are doing something more like social work. For instance, there are cases in which patients can not be on their own in the community, and there’s no family to take them in, so the hospital, on behalf of the state, has to take them in.
Q: What else do you do outside of hospitalist work?
A: Since I’ve finished medical school, I’ve always been in some kind of academia, which is not something I would have expected. But as time has gone by, I have really come to appreciate being in academia. I really enjoy teaching, and I also think that an academic institution kind of keeps me on my toes. I’m involved with interprofessional education at Emory, with teaching medical students, interns, and residents when I’m on teaching service, and obviously now I’m on The Hospitalist editorial board. I’m looking forward to keeping abreast of what’s hot in the world of hospital medicine.
Q: What are you excited about bringing to The Hospitalist editorial board?
A: I want to try to contribute ideas. I feel that even in my short time at Emory, I’ve gotten to know a few people who might be good resources for reporters to interview or even who might write articles themselves. I also think that seeing what is trending in the world of hospital medicine is a nice way of understanding the future direction of hospital medicine.
Q: What have you seen as being the biggest change in hospital medicine since you started?
A: I feel as though I’ve kept my head down and plowed forward through the first part of my career, but I think that, more than anything else, what I’ve noticed is bigger shifts within health care itself. I know that there’s a lot of consolidation going on. I think that there are many questions that are going to come up about how do we manage a health care system as complicated as America’s and how do we deliver optimal care to people especially when sometimes we end up in situations in which we don’t have all the resources that we would want to have because of circumstances.
Q: Do you see anything in particular on the horizon for hospital medicine?
A: I’ve noticed that there’s been more “hospitalist-ization” – if that’s even a term – of other medical services. At our institution, we already have an acute care service that is basically hospital medicine for general surgery. I think another thing that’s been kind of a hot topic recently is a point-of-care testing, including ultrasounds for line placements.
Q: Where do you see yourself in 10 years?
A: I really enjoy my work at Emory. I want to find more opportunities to teach. For example, I’ve already gotten involved in teaching physician assistant students about how to perform interviews and deliver presentations for attendings. A lot of serendipitous things have happened to me over time, so I think I will continue to teach, but I’m open to those opportunities that present themselves in the future.
Q: What’s the best book you’ve read recently and why?
A: “The Hero with a Thousand Faces,” by Joseph Campbell. This is a very well-known book – I think George Lucas made reference to it when he was writing Star Wars – but I think it was a great literary way to examine the hero’s journey. Once you read the book, and you then watch any kind of movie or read any other kind of adventure narrative, you can’t miss the pattern.
While James Kim, MD, did not originally begin medical school with a plan to become a hospitalist, he has embraced his current role wholeheartedly.
Since becoming board certified in both internal medicine and infectious diseases, Dr. Kim has welcomed the opportunity to be part of hospital medicine, which gives him the opportunity to pursue his other passion: teaching and mentoring.
“It’s not just what you know but how you convey what you know to other people,” said Dr. Kim. “While you might get useful information from a didactic teaching style, it’s important to ask questions to encourage the learner to think about not only what the right answer is but also what’s the thought process required to get the answer.”
As one of the newest additions to the editorial advisory board of The Hospitalist, Dr. Kim took time to tell us more about himself in a recent interview.
QUESTION: How did you find your career path in medicine?
ANSWER: I originally went into medical school thinking I was going to do pediatrics, but then I realized that I really enjoy talking to people and that I like the process of thinking through diagnoses, managing patients, and learning about what makes their circumstances unique.
Q: How did you get into hospital medicine?
A: When I finished my internal medicine residency, I thought I was going to do medical missions. However, I realized along the way that the care you need to provide in order to really make a difference in other countries requires a constant presence there – not just a week or two. So after my fellowship, I was searching for jobs and found a hospitalist position at the University of California, Los Angeles. When I saw it, I thought ‘Wow, I really miss doing inpatient medicine.’
Q: Since you started, what have been some of your favorite parts of hospital medicine?
A: When people come to you in the hospital setting, they are usually pretty sick. It is very satisfying when, through the course of a person’s hospital stay, we are able to come up with a plan that can get them acutely better.
Q: What do you think is the hardest part of hospital medicine?
A: I think one of the things that is most frustrating is when we are placed into a situation in which we are not necessarily doing medical work for a patient but are doing something more like social work. For instance, there are cases in which patients can not be on their own in the community, and there’s no family to take them in, so the hospital, on behalf of the state, has to take them in.
Q: What else do you do outside of hospitalist work?
A: Since I’ve finished medical school, I’ve always been in some kind of academia, which is not something I would have expected. But as time has gone by, I have really come to appreciate being in academia. I really enjoy teaching, and I also think that an academic institution kind of keeps me on my toes. I’m involved with interprofessional education at Emory, with teaching medical students, interns, and residents when I’m on teaching service, and obviously now I’m on The Hospitalist editorial board. I’m looking forward to keeping abreast of what’s hot in the world of hospital medicine.
Q: What are you excited about bringing to The Hospitalist editorial board?
A: I want to try to contribute ideas. I feel that even in my short time at Emory, I’ve gotten to know a few people who might be good resources for reporters to interview or even who might write articles themselves. I also think that seeing what is trending in the world of hospital medicine is a nice way of understanding the future direction of hospital medicine.
Q: What have you seen as being the biggest change in hospital medicine since you started?
A: I feel as though I’ve kept my head down and plowed forward through the first part of my career, but I think that, more than anything else, what I’ve noticed is bigger shifts within health care itself. I know that there’s a lot of consolidation going on. I think that there are many questions that are going to come up about how do we manage a health care system as complicated as America’s and how do we deliver optimal care to people especially when sometimes we end up in situations in which we don’t have all the resources that we would want to have because of circumstances.
Q: Do you see anything in particular on the horizon for hospital medicine?
A: I’ve noticed that there’s been more “hospitalist-ization” – if that’s even a term – of other medical services. At our institution, we already have an acute care service that is basically hospital medicine for general surgery. I think another thing that’s been kind of a hot topic recently is a point-of-care testing, including ultrasounds for line placements.
Q: Where do you see yourself in 10 years?
A: I really enjoy my work at Emory. I want to find more opportunities to teach. For example, I’ve already gotten involved in teaching physician assistant students about how to perform interviews and deliver presentations for attendings. A lot of serendipitous things have happened to me over time, so I think I will continue to teach, but I’m open to those opportunities that present themselves in the future.
Q: What’s the best book you’ve read recently and why?
A: “The Hero with a Thousand Faces,” by Joseph Campbell. This is a very well-known book – I think George Lucas made reference to it when he was writing Star Wars – but I think it was a great literary way to examine the hero’s journey. Once you read the book, and you then watch any kind of movie or read any other kind of adventure narrative, you can’t miss the pattern.
Prior biologic exposure doesn’t diminish ixekizumab’s efficacy
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.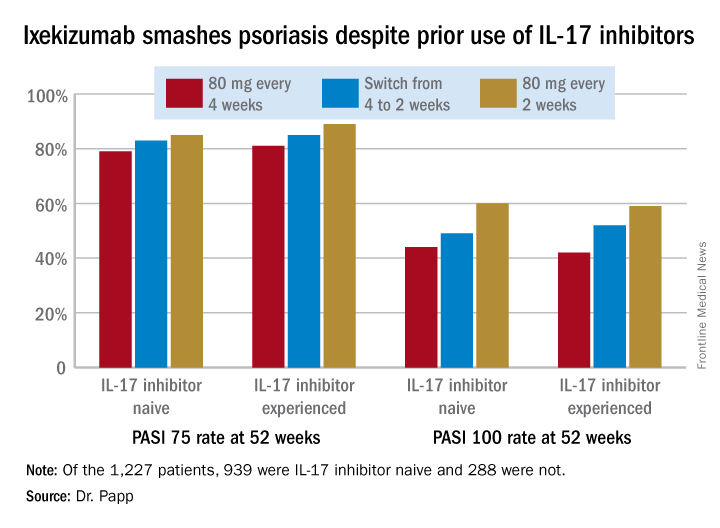
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
REPORTING FROM THE EADV CONGRESS
GENEVA – Psoriasis patients switched to ixekizumab after previous exposure to another interleukin-17 inhibitor respond as well as those who are IL-17 antagonist naive, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This finding is of importance in real-world clinical practice because it’s not at all uncommon for psoriasis patients on one biologic to have to switch to another because of insufficient efficacy, side effects, or a change in insurance coverage. Physicians would like to know what sort of responses can be expected to whatever agent they prescribe next.
However, that was not a problem in this secondary analysis of a large clinical trial whose primary purpose was to evaluate the relative safety and efficacy of ixekizumab (Taltz) when dosed every 2 weeks versus every 4 weeks.
“I think what we have seen here is a very compelling story: , nor for that matter does it appear to impact safety,” the dermatologist said.
He reported on 1,227 patients with moderate to severe plaque psoriasis who were randomized to ixekizumab at 80 mg every 2 or 4 weeks following an initial 160 mg loading dose. Among those who started out on ixekizumab every 4 weeks, 306 patients got a per-protocol dose adjustment to biweekly therapy because of an insufficient response to monthly dosing as defined by a Physician’s Global Assessment score of 2 or more on two consecutive office visits during study weeks 12-40.
A total of 939 patients were IL-17 inhibitor naive. The other 288 had previously been on the IL-17 antagonists brodalumab (Siliq) or secukinumab (Cosentyx). The two groups had similar baseline demographics with the exception that the experienced cohort had on average a 22.2-year duration of psoriasis, 3.7 years more than IL-17 antagonist-naive patients.
In an intent-to-treat analysis, Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses at week 52 didn’t differ significantly between the IL-17 inhibitor-naive and -experienced groups. In fact, patients with prior exposure to other IL-17 antagonists showed a consistent trend for slightly higher response rates (see graphic).
It was clear from this analysis that dosing ixekizumab every 2 weeks provides significantly better efficacy than was dosing every 4 weeks, Dr. Papp noted. Yet the approved dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.
No new safety issues arose in this study. The only difference between the naive and experienced groups was a lower rate of allergic reactions/hypersensitivity in the experienced group. For example, in patients on ixekizumab every 2 weeks for the entire 52-week study period the incidence of such reactions was 11.5% in the IL-17 antagonist-naive group, compared with 4.1% in the experienced cohort. This isn’t really surprising, according to Dr. Papp.
“Most injection site reactions occur in the newbies,” he said.
The study was sponsored by Eli Lilly. Dr. Papp serves as a consultant and/or adviser to Lilly and numerous other pharmaceutical companies involved in the development of dermatologic therapies.
Updosing omalizumab for chronic urticaria pays off
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
GENEVA – In real-world clinical practice, roughly – and for those who don’t, three-quarters will respond upon updosing to 450 or 600 mg every 4 weeks.
That’s the key message of an open-label study of 286 patients with chronic spontaneous urticaria (CSU) conducted at 15 hospitals by the Catalan and Balearic Chronic Urticaria Network (XUrCB), Jorge Spertino, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In three published, pivotal, phase 3 randomized trials, the clinical response rate to omalizumab at 300 mg every 4 weeks, as defined by a weekly 7-day Urticaria Activity Score (UAS7) of 6 or less at 12 weeks, was 52% in ASTERIA I, 66% in ASTERIA II, and 52% in GLACIAL. But patients enrolled in formal randomized trials are often quite different from the broader group encountered in daily practice, and the Spanish dermatologists wanted to know if updosing in suboptimal responders was safe and effective. It turns out that it certainly is, according to Dr. Spertino of Teknon Medical Center in Barcelona.
The treatment algorithm followed by the XUrCB investigators was that, if after six doses at the approved dose of 300 mg every 4 weeks a patient didn’t have good control of disease activity, the dose was increased to 450 mg every 4 weeks. If after three doses at that level, there still wasn’t good control of the CSU, the dose was further increased to 600 mg every 4 weeks.
As in the pivotal phase 3 clinical trials, the XUrCB group defined good control of disease activity as a UAS7 score of 6 or less in accord with a study that demonstrated such a score on the 0- to 42-point UAS7 correlates well with minimal or no patient symptoms (Br J Dermatol. 2017 Oct;177[4]:1093-1101).
At baseline, the mean age of the 286 CSU patients was 44.6 years and the mean UAS7 score was 26.5; 74% were women. Forty-seven percent of patients experienced angioedema and 33% had inducible urticaria, most commonly brought forth by pressure or dermographism. One-third of patients had previously been on cyclosporine and half of the patients had a high d-dimer level.
Sixty-five percent of patients achieved good disease control on omalizumab at 300 mg every 4 weeks. Of the 99 patients (35%) who didn’t, 20 patients stopped treatment at their dermatologist’s request because their symptoms remained uncontrolled on the approved dose. But 59 of the 79 who updosed obtained good disease control: 43 on a dose of 450 mg and 16 on a dose of 600 mg.
In multivariate analysis, two predictors of treatment success with updosing were identified: previous treatment with cyclosporine and obesity. Among patients previously on cyclosporine – a marker for more severe disease – only 21% achieved a UAS7 score of 6 or less on the approved dose, while 41% did so upon updosing. And obesity was associated with a 3.7-fold increased likelihood of a favorable response to updosing after lack of treatment success at the approved dose.
Neither a high d-dimer or serum IgE level, baseline UAS7 score, gender, associated angioedema, nor inducible urticaria was significantly associated with an increased treatment success rate upon updosing.
Updosing proved to be safe. All adverse events were mild and infrequent, consisting of headache, local injection site reactions, and arthromyalgia s, each occurring in 1%-2% of patients. Frequencies were similar in updosed patients and those on the approved dosing schedule.
Session cochair Jorgen Serup, MD, DMsc, congratulated Dr. Spertino for supplying physicians with “very-much-needed data.”
“This is very convincing data and highly clinically relevant for those of us who have these patients in our practices,” said Dr. Serup, professor of dermatology at Copenhagen University.
Dr. Spertino reported having no financial conflicts of interest regarding his presentation.
SOURCE: Spertino J et al. EADV Congress
REPORTING FROM THE EADV CONGRESS
Key clinical point: Updosing of omalizumab to a maximum of twice the approved dose is safe and effective in chronic spontaneous urticaria patients unresponsive to the licensed dose.
Major finding: Upon updosing, 75% of nonresponders to the approved dose achieved good disease control with no increase in adverse events.
Study details: This multicenter study of an omalizumab updosing algorithm included 286 patients with chronic spontaneous urticaria.
Disclosures: The study presenter reported having no financial conflicts.
Two MS diagnostic criteria found to have similar accuracy
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria showed accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis, a retrospective study found.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration is given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” wrote researchers led by Massimo Filippi, MD. The report was published Dec. 21, 2017, in The Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment and more studies should be done to evaluate the effect of including optic nerve assessment as an additional DIS [dissemination in space] criterion.”
Dr. Filippi, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University, Milan, and his coauthors at eight centers reported that of the 368 patients, 189 (51%) developed clinically definite MS at the last evaluation, which occurred at a median of 50 months. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs. 93%, respectively), similar specificity (33% vs. 32%), and similar area under the curve values (AUC, 0.62 vs. 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (it stood at 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity fell to 26% and AUC dropped to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (e.g., using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite multiple sclerosis.
The study was funded by the U.K. MS Society, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, and the Dutch MS Research Foundation. The authors reported having numerous financial disclosures with the pharmaceutical industry.
SOURCE: Filippi M et al., Lancet Neurol. 2017 Dec 21. doi: 10.1016/S1474-4422(17)30469-6.
As multiple sclerosis diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, which in turn has helped clinicians establish earlier diagnosis. In an editorial published online Dec. 21, 2017, in The Lancet Neurology (doi: 10.1016/S1474-4422(17)30459-3), Anne H. Cross, MD, and Robert N. Naismith, MD, point out that while the study by Dr. Filippi et al. showed that for both sets of MRI criteria sensitivity was greater than specificity for predicting clinically definite multiple sclerosis, the modest specificity is cause for concern. They cited one study (Neurology 2016;87:1393-9) that emphasized the importance of not misdiagnosing other CNS diseases as multiple sclerosis. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” wrote Dr. Cross and Dr. Naismith, both with the department of neurology at Washington University, St. Louis. “[Seventy percent] of the 110 individuals had received disease-modifying therapy and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with non-specific neurological symptoms.”
The authors noted that vascular and other diseases can cause MRI abnormalities that could meet the 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) recommendations or the 2010 and 2017 McDonald MRI criteria. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for dissemination in time, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, they advise clinicians to weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses.
Dr. Cross has received consulting fees from AbbVie, Bayer, Biogen, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Mallinckodt, Novartis, and Teva. Dr. Naismith has consulted for Acorda, Alkermes, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Teva.
As multiple sclerosis diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, which in turn has helped clinicians establish earlier diagnosis. In an editorial published online Dec. 21, 2017, in The Lancet Neurology (doi: 10.1016/S1474-4422(17)30459-3), Anne H. Cross, MD, and Robert N. Naismith, MD, point out that while the study by Dr. Filippi et al. showed that for both sets of MRI criteria sensitivity was greater than specificity for predicting clinically definite multiple sclerosis, the modest specificity is cause for concern. They cited one study (Neurology 2016;87:1393-9) that emphasized the importance of not misdiagnosing other CNS diseases as multiple sclerosis. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” wrote Dr. Cross and Dr. Naismith, both with the department of neurology at Washington University, St. Louis. “[Seventy percent] of the 110 individuals had received disease-modifying therapy and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with non-specific neurological symptoms.”
The authors noted that vascular and other diseases can cause MRI abnormalities that could meet the 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) recommendations or the 2010 and 2017 McDonald MRI criteria. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for dissemination in time, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, they advise clinicians to weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses.
Dr. Cross has received consulting fees from AbbVie, Bayer, Biogen, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Mallinckodt, Novartis, and Teva. Dr. Naismith has consulted for Acorda, Alkermes, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Teva.
As multiple sclerosis diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, which in turn has helped clinicians establish earlier diagnosis. In an editorial published online Dec. 21, 2017, in The Lancet Neurology (doi: 10.1016/S1474-4422(17)30459-3), Anne H. Cross, MD, and Robert N. Naismith, MD, point out that while the study by Dr. Filippi et al. showed that for both sets of MRI criteria sensitivity was greater than specificity for predicting clinically definite multiple sclerosis, the modest specificity is cause for concern. They cited one study (Neurology 2016;87:1393-9) that emphasized the importance of not misdiagnosing other CNS diseases as multiple sclerosis. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” wrote Dr. Cross and Dr. Naismith, both with the department of neurology at Washington University, St. Louis. “[Seventy percent] of the 110 individuals had received disease-modifying therapy and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with non-specific neurological symptoms.”
The authors noted that vascular and other diseases can cause MRI abnormalities that could meet the 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) recommendations or the 2010 and 2017 McDonald MRI criteria. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for dissemination in time, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, they advise clinicians to weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses.
Dr. Cross has received consulting fees from AbbVie, Bayer, Biogen, EMD Serono, Genentech/Roche, Genzyme/Sanofi, Mallinckodt, Novartis, and Teva. Dr. Naismith has consulted for Acorda, Alkermes, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Teva.
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria showed accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis, a retrospective study found.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration is given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” wrote researchers led by Massimo Filippi, MD. The report was published Dec. 21, 2017, in The Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment and more studies should be done to evaluate the effect of including optic nerve assessment as an additional DIS [dissemination in space] criterion.”
Dr. Filippi, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University, Milan, and his coauthors at eight centers reported that of the 368 patients, 189 (51%) developed clinically definite MS at the last evaluation, which occurred at a median of 50 months. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs. 93%, respectively), similar specificity (33% vs. 32%), and similar area under the curve values (AUC, 0.62 vs. 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (it stood at 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity fell to 26% and AUC dropped to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (e.g., using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite multiple sclerosis.
The study was funded by the U.K. MS Society, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, and the Dutch MS Research Foundation. The authors reported having numerous financial disclosures with the pharmaceutical industry.
SOURCE: Filippi M et al., Lancet Neurol. 2017 Dec 21. doi: 10.1016/S1474-4422(17)30469-6.
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria showed accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis, a retrospective study found.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration is given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” wrote researchers led by Massimo Filippi, MD. The report was published Dec. 21, 2017, in The Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment and more studies should be done to evaluate the effect of including optic nerve assessment as an additional DIS [dissemination in space] criterion.”
Dr. Filippi, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University, Milan, and his coauthors at eight centers reported that of the 368 patients, 189 (51%) developed clinically definite MS at the last evaluation, which occurred at a median of 50 months. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs. 93%, respectively), similar specificity (33% vs. 32%), and similar area under the curve values (AUC, 0.62 vs. 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (it stood at 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity fell to 26% and AUC dropped to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (e.g., using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite multiple sclerosis.
The study was funded by the U.K. MS Society, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, and the Dutch MS Research Foundation. The authors reported having numerous financial disclosures with the pharmaceutical industry.
SOURCE: Filippi M et al., Lancet Neurol. 2017 Dec 21. doi: 10.1016/S1474-4422(17)30469-6.
FROM THE LANCET NEUROLOGY
Key clinical point:
Major finding: The 2016 MAGNIMS criteria and 2010 McDonald criteria performed similarly for predicting clinically definite multiple sclerosis (a sensitivity of 91% and 93%, respectively, and a specificity of 33% and 32%).
Study details: A retrospective study of 368 patients with clinically isolated syndrome.
Disclosures: The study was funded by the U.K. MS Society, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, and the Dutch MS Research Foundation. The study authors reported having numerous financial disclosures.
Source: Filippi M et al., Lancet Neurol. 2017 Dec 21. doi: 10.1016/S1474-4422(17)30469-6.
Scaly Pink Patches: Differentiating Psoriasis From Basal Cell Carcinoma
Dermoscopy increases diagnostic accuracy in the analysis of skin growths.1,2 Recently the use of dermoscopy has broadened to include inflammatory dermatoses and skin infections.3 To substantiate the value of dermoscopy in assessing psoriasis, we performed a systematic review of the literature and briefly reviewed 31 articles. We also report a case that highlights the differences between psoriasis and basal cell carcinoma (BCC) under dermoscopic examination, and we discuss the literature on the dermoscopic findings of psoriasis with an emphasis on the relative sensitivities and specificities of dermoscopic findings for psoriasis and for BCC.
Case Report
A 63-year-old man with psoriasis and a history of BCC presented for follow-up of psoriasis, which was well-controlled on etanercept. The physical examination was remarkable for scaly pink papules scattered on the trunk and extremities. A new larger red-pink patch was located on the left lower back (Figure 1). Dermoscopic evaluation of the new patch revealed shiny white lines and branching blood vessels (Figure 2).
Comment
The clinical morphology of psoriasis and BCC can be similar, and dermoscopy can help in differentiating between the 2 conditions.
Literature Search on Dermoscopy and Psoriasis
We performed a PubMed search of articles indexed for MEDLINE to review the published literature on dermoscopy and psoriasis. Two reviewers (C.H. and L.C.) searched for psoriasis paired with the terms dermoscopy or dermatoscopy or epiluminescence microscopy. Only English-language articles published between 1996 and 2016 were included in the search. Articles that focused solely on confocal microscopy were excluded. Article titles and abstracts were evaluated and articles that omitted mention of dermoscopy and psoriasis were excluded, yielding a total of 31 articles. Of these articles, only 2 discussed the specificity or sensitivity of the dermoscopic findings of psoriasis.4,5 Most of the articles were case reports and descriptive cross-sectional studies. The reports addressed multiple subtypes of psoriasis, but reports on psoriasis vulgaris and scalp psoriasis were most common (Table). Lallas et al6 provided a comprehensive descriptive review of the main findings on dermoscopy for psoriasis and other inflammatory skin conditions, but it lacked a comparison between psoriasis and BCC or data on the sensitivity and specificity of the findings. Two studies reported sensitivity and specificity values for the dermoscopic findings of psoriasis.4,5 Pan et al5 reported a 98% diagnostic probability of psoriasis if red dots, homogeneous vascular pattern, and a light red background are all present. Additionally, they reported that the presence of 4 of 6 criteria for BCC—scattered vascular pattern, arborizing microvessels, telangiectatic or atypical vessels, milky-pink background, and brown dots⁄globules—yielded a diagnostic probability of 99%.5 Similarly, Lallas et al6 demonstrated that the presence of dotted vessels alone is not sufficient to presume a diagnosis of psoriasis, as this finding can be seen in other inflammatory skin conditions. However, “the combination of regularly distributed dotted vessels over a light red background associated with diffuse white scales was highly predictive of [plaque psoriasis] and allowed a correct diagnosis with 88.0% specificity and 84.9% sensitivity.”4 Figure 3 shows a dermoscopic image of plaque psoriasis that demonstrates these findings. The remaining literature corroborated this evidence, with the most commonly reported dermoscopic findings of psoriasis being red dots, red globules, glomerular vessels (also known as twisted capillary loops), red globular ring
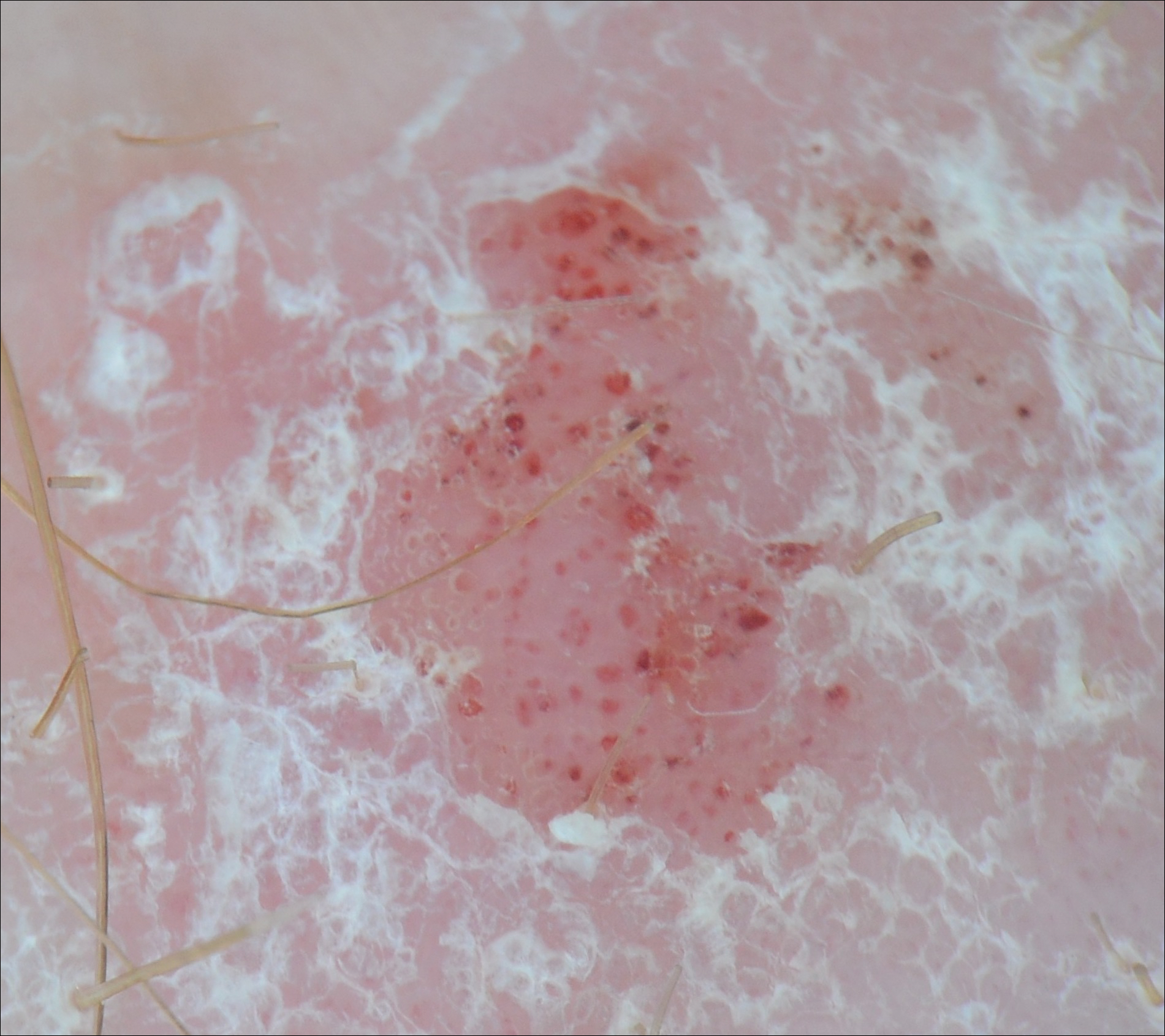
Dermoscopy and BCC
Much has been published on the dermoscopic findings of BCC.5,13-15 The dermoscopic findings of BCC include large blue-gray ovoid nests, leaflike areas, spoke-wheel–like areas, arborizing vessels (telangiectasia), and ulceration.15 Superficial BCC is characterized by short fine or arborizing telangiectasia, shallow erosions, and shiny white areas.15 The positive predictive value of dermoscopy in BCC is as high as 97%.16 Additionally, multiple studies report a sensitivity of 95% to 99%5,13,14 and a specificity of 79% to 99% in the use of dermoscopy for identifying BCC. According to Pan et al,5 the most sensitive finding for BCC is a scattered vascular pattern (97%), while the most specific finding is arborizing microvessels (99%).
Utility of Dermoscopy
Our case of a 63-year-old man with a history of psoriasis and BCC highlights the usefulness of dermoscopy in accurately determining the features of each condition. Additionally, dermoscopy aids in differentiating between psoriasis and squamous cell carcinoma. In contrast to the dotted vessels seen in psoriasis, squamous cell carcinomas often have peripheral hairpin (glomerular) vessels.17
If future reports confirm dermoscopy’s utility in accurately diagnosing psoriasis, fewer biopsies may be needed when evaluating patients with new rashes. Furthermore, dermoscopy may expedite treatment of psoriasis (as it can for malignant conditions) by obviating the wait for pathology results currently needed to initiate systemic treatment. For patients with psoriasis who also have sun-damaged skin, dermoscopy may assist in differentiating pink patches and plaques of psoriasis from skin cancer, such as superficial BCCs, which often have shiny white lines not seen in psoriasis.15
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Lallas A, Giacomel J, Argenziano G, et al. Dermoscopy in general dermatology: practical tips for the clinician. Br J Dermatol. 2014;170:514-526.
- Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198-1205.
- Pan Y, Chamberlain AJ, Bailey M, et al. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque–features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268-274.
- Lallas A, Apalla Z, Argenziano G, et al. Dermoscopic pattern of psoriatic lesions on specific body sites. Dermatology. 2014;228:250-254.
- Almeida MC, Romiti R, Doche I, et al. Psoriatic scarring alopecia. An Bras Dermatol. 2013;88:29-31.
- Zalaudek I, Argenziano G. Dermoscopy subpatterns of inflammatory skin disorders. Arch Dermatol. 2006;142:808.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
- Vázquez-López F, Zaballos P, Fueyo-Casado A, et al. A dermoscopy subpattern of plaque-type psoriasis: red globular rings. Arch Dermatol. 2007;143:1612.
- Lacarrubba F, Nasca MR, Micali G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J Am Acad Dermatol. 2009;61:1084-1086.
- Liebman TN, Wang SQ. Detection of early basal cell carcinoma with dermoscopy in a patient with psoriasis. Dermatol Online J. 2011;17:12.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Marghoob AA, Malvehy J, Braun RP, eds. An Atlas of Dermoscopy. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Nelson SA, Scope A, Rishpon A, et al. Accuracy and confidence in the clinical diagnosis of basal cell cancer using dermoscopy and reflex confocal microscopy. Int J Dermatol. 2016;55:1351-1356.
- Zalaudek I, Kreusch J, Giacomel J, et al. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. melanocytic skin tumors. J Am Acad Dermatol. 2010;63:361-374.
Dermoscopy increases diagnostic accuracy in the analysis of skin growths.1,2 Recently the use of dermoscopy has broadened to include inflammatory dermatoses and skin infections.3 To substantiate the value of dermoscopy in assessing psoriasis, we performed a systematic review of the literature and briefly reviewed 31 articles. We also report a case that highlights the differences between psoriasis and basal cell carcinoma (BCC) under dermoscopic examination, and we discuss the literature on the dermoscopic findings of psoriasis with an emphasis on the relative sensitivities and specificities of dermoscopic findings for psoriasis and for BCC.
Case Report
A 63-year-old man with psoriasis and a history of BCC presented for follow-up of psoriasis, which was well-controlled on etanercept. The physical examination was remarkable for scaly pink papules scattered on the trunk and extremities. A new larger red-pink patch was located on the left lower back (Figure 1). Dermoscopic evaluation of the new patch revealed shiny white lines and branching blood vessels (Figure 2).


Comment
The clinical morphology of psoriasis and BCC can be similar, and dermoscopy can help in differentiating between the 2 conditions.
Literature Search on Dermoscopy and Psoriasis
We performed a PubMed search of articles indexed for MEDLINE to review the published literature on dermoscopy and psoriasis. Two reviewers (C.H. and L.C.) searched for psoriasis paired with the terms dermoscopy or dermatoscopy or epiluminescence microscopy. Only English-language articles published between 1996 and 2016 were included in the search. Articles that focused solely on confocal microscopy were excluded. Article titles and abstracts were evaluated and articles that omitted mention of dermoscopy and psoriasis were excluded, yielding a total of 31 articles. Of these articles, only 2 discussed the specificity or sensitivity of the dermoscopic findings of psoriasis.4,5 Most of the articles were case reports and descriptive cross-sectional studies. The reports addressed multiple subtypes of psoriasis, but reports on psoriasis vulgaris and scalp psoriasis were most common (Table). Lallas et al6 provided a comprehensive descriptive review of the main findings on dermoscopy for psoriasis and other inflammatory skin conditions, but it lacked a comparison between psoriasis and BCC or data on the sensitivity and specificity of the findings. Two studies reported sensitivity and specificity values for the dermoscopic findings of psoriasis.4,5 Pan et al5 reported a 98% diagnostic probability of psoriasis if red dots, homogeneous vascular pattern, and a light red background are all present. Additionally, they reported that the presence of 4 of 6 criteria for BCC—scattered vascular pattern, arborizing microvessels, telangiectatic or atypical vessels, milky-pink background, and brown dots⁄globules—yielded a diagnostic probability of 99%.5 Similarly, Lallas et al6 demonstrated that the presence of dotted vessels alone is not sufficient to presume a diagnosis of psoriasis, as this finding can be seen in other inflammatory skin conditions. However, “the combination of regularly distributed dotted vessels over a light red background associated with diffuse white scales was highly predictive of [plaque psoriasis] and allowed a correct diagnosis with 88.0% specificity and 84.9% sensitivity.”4 Figure 3 shows a dermoscopic image of plaque psoriasis that demonstrates these findings. The remaining literature corroborated this evidence, with the most commonly reported dermoscopic findings of psoriasis being red dots, red globules, glomerular vessels (also known as twisted capillary loops), red globular ring


Dermoscopy and BCC
Much has been published on the dermoscopic findings of BCC.5,13-15 The dermoscopic findings of BCC include large blue-gray ovoid nests, leaflike areas, spoke-wheel–like areas, arborizing vessels (telangiectasia), and ulceration.15 Superficial BCC is characterized by short fine or arborizing telangiectasia, shallow erosions, and shiny white areas.15 The positive predictive value of dermoscopy in BCC is as high as 97%.16 Additionally, multiple studies report a sensitivity of 95% to 99%5,13,14 and a specificity of 79% to 99% in the use of dermoscopy for identifying BCC. According to Pan et al,5 the most sensitive finding for BCC is a scattered vascular pattern (97%), while the most specific finding is arborizing microvessels (99%).
Utility of Dermoscopy
Our case of a 63-year-old man with a history of psoriasis and BCC highlights the usefulness of dermoscopy in accurately determining the features of each condition. Additionally, dermoscopy aids in differentiating between psoriasis and squamous cell carcinoma. In contrast to the dotted vessels seen in psoriasis, squamous cell carcinomas often have peripheral hairpin (glomerular) vessels.17
If future reports confirm dermoscopy’s utility in accurately diagnosing psoriasis, fewer biopsies may be needed when evaluating patients with new rashes. Furthermore, dermoscopy may expedite treatment of psoriasis (as it can for malignant conditions) by obviating the wait for pathology results currently needed to initiate systemic treatment. For patients with psoriasis who also have sun-damaged skin, dermoscopy may assist in differentiating pink patches and plaques of psoriasis from skin cancer, such as superficial BCCs, which often have shiny white lines not seen in psoriasis.15
Dermoscopy increases diagnostic accuracy in the analysis of skin growths.1,2 Recently the use of dermoscopy has broadened to include inflammatory dermatoses and skin infections.3 To substantiate the value of dermoscopy in assessing psoriasis, we performed a systematic review of the literature and briefly reviewed 31 articles. We also report a case that highlights the differences between psoriasis and basal cell carcinoma (BCC) under dermoscopic examination, and we discuss the literature on the dermoscopic findings of psoriasis with an emphasis on the relative sensitivities and specificities of dermoscopic findings for psoriasis and for BCC.
Case Report
A 63-year-old man with psoriasis and a history of BCC presented for follow-up of psoriasis, which was well-controlled on etanercept. The physical examination was remarkable for scaly pink papules scattered on the trunk and extremities. A new larger red-pink patch was located on the left lower back (Figure 1). Dermoscopic evaluation of the new patch revealed shiny white lines and branching blood vessels (Figure 2).


Comment
The clinical morphology of psoriasis and BCC can be similar, and dermoscopy can help in differentiating between the 2 conditions.
Literature Search on Dermoscopy and Psoriasis
We performed a PubMed search of articles indexed for MEDLINE to review the published literature on dermoscopy and psoriasis. Two reviewers (C.H. and L.C.) searched for psoriasis paired with the terms dermoscopy or dermatoscopy or epiluminescence microscopy. Only English-language articles published between 1996 and 2016 were included in the search. Articles that focused solely on confocal microscopy were excluded. Article titles and abstracts were evaluated and articles that omitted mention of dermoscopy and psoriasis were excluded, yielding a total of 31 articles. Of these articles, only 2 discussed the specificity or sensitivity of the dermoscopic findings of psoriasis.4,5 Most of the articles were case reports and descriptive cross-sectional studies. The reports addressed multiple subtypes of psoriasis, but reports on psoriasis vulgaris and scalp psoriasis were most common (Table). Lallas et al6 provided a comprehensive descriptive review of the main findings on dermoscopy for psoriasis and other inflammatory skin conditions, but it lacked a comparison between psoriasis and BCC or data on the sensitivity and specificity of the findings. Two studies reported sensitivity and specificity values for the dermoscopic findings of psoriasis.4,5 Pan et al5 reported a 98% diagnostic probability of psoriasis if red dots, homogeneous vascular pattern, and a light red background are all present. Additionally, they reported that the presence of 4 of 6 criteria for BCC—scattered vascular pattern, arborizing microvessels, telangiectatic or atypical vessels, milky-pink background, and brown dots⁄globules—yielded a diagnostic probability of 99%.5 Similarly, Lallas et al6 demonstrated that the presence of dotted vessels alone is not sufficient to presume a diagnosis of psoriasis, as this finding can be seen in other inflammatory skin conditions. However, “the combination of regularly distributed dotted vessels over a light red background associated with diffuse white scales was highly predictive of [plaque psoriasis] and allowed a correct diagnosis with 88.0% specificity and 84.9% sensitivity.”4 Figure 3 shows a dermoscopic image of plaque psoriasis that demonstrates these findings. The remaining literature corroborated this evidence, with the most commonly reported dermoscopic findings of psoriasis being red dots, red globules, glomerular vessels (also known as twisted capillary loops), red globular ring


Dermoscopy and BCC
Much has been published on the dermoscopic findings of BCC.5,13-15 The dermoscopic findings of BCC include large blue-gray ovoid nests, leaflike areas, spoke-wheel–like areas, arborizing vessels (telangiectasia), and ulceration.15 Superficial BCC is characterized by short fine or arborizing telangiectasia, shallow erosions, and shiny white areas.15 The positive predictive value of dermoscopy in BCC is as high as 97%.16 Additionally, multiple studies report a sensitivity of 95% to 99%5,13,14 and a specificity of 79% to 99% in the use of dermoscopy for identifying BCC. According to Pan et al,5 the most sensitive finding for BCC is a scattered vascular pattern (97%), while the most specific finding is arborizing microvessels (99%).
Utility of Dermoscopy
Our case of a 63-year-old man with a history of psoriasis and BCC highlights the usefulness of dermoscopy in accurately determining the features of each condition. Additionally, dermoscopy aids in differentiating between psoriasis and squamous cell carcinoma. In contrast to the dotted vessels seen in psoriasis, squamous cell carcinomas often have peripheral hairpin (glomerular) vessels.17
If future reports confirm dermoscopy’s utility in accurately diagnosing psoriasis, fewer biopsies may be needed when evaluating patients with new rashes. Furthermore, dermoscopy may expedite treatment of psoriasis (as it can for malignant conditions) by obviating the wait for pathology results currently needed to initiate systemic treatment. For patients with psoriasis who also have sun-damaged skin, dermoscopy may assist in differentiating pink patches and plaques of psoriasis from skin cancer, such as superficial BCCs, which often have shiny white lines not seen in psoriasis.15
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Lallas A, Giacomel J, Argenziano G, et al. Dermoscopy in general dermatology: practical tips for the clinician. Br J Dermatol. 2014;170:514-526.
- Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198-1205.
- Pan Y, Chamberlain AJ, Bailey M, et al. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque–features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268-274.
- Lallas A, Apalla Z, Argenziano G, et al. Dermoscopic pattern of psoriatic lesions on specific body sites. Dermatology. 2014;228:250-254.
- Almeida MC, Romiti R, Doche I, et al. Psoriatic scarring alopecia. An Bras Dermatol. 2013;88:29-31.
- Zalaudek I, Argenziano G. Dermoscopy subpatterns of inflammatory skin disorders. Arch Dermatol. 2006;142:808.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
- Vázquez-López F, Zaballos P, Fueyo-Casado A, et al. A dermoscopy subpattern of plaque-type psoriasis: red globular rings. Arch Dermatol. 2007;143:1612.
- Lacarrubba F, Nasca MR, Micali G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J Am Acad Dermatol. 2009;61:1084-1086.
- Liebman TN, Wang SQ. Detection of early basal cell carcinoma with dermoscopy in a patient with psoriasis. Dermatol Online J. 2011;17:12.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Marghoob AA, Malvehy J, Braun RP, eds. An Atlas of Dermoscopy. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Nelson SA, Scope A, Rishpon A, et al. Accuracy and confidence in the clinical diagnosis of basal cell cancer using dermoscopy and reflex confocal microscopy. Int J Dermatol. 2016;55:1351-1356.
- Zalaudek I, Kreusch J, Giacomel J, et al. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. melanocytic skin tumors. J Am Acad Dermatol. 2010;63:361-374.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Lallas A, Giacomel J, Argenziano G, et al. Dermoscopy in general dermatology: practical tips for the clinician. Br J Dermatol. 2014;170:514-526.
- Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198-1205.
- Pan Y, Chamberlain AJ, Bailey M, et al. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque–features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268-274.
- Lallas A, Apalla Z, Argenziano G, et al. Dermoscopic pattern of psoriatic lesions on specific body sites. Dermatology. 2014;228:250-254.
- Almeida MC, Romiti R, Doche I, et al. Psoriatic scarring alopecia. An Bras Dermatol. 2013;88:29-31.
- Zalaudek I, Argenziano G. Dermoscopy subpatterns of inflammatory skin disorders. Arch Dermatol. 2006;142:808.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
- Vázquez-López F, Zaballos P, Fueyo-Casado A, et al. A dermoscopy subpattern of plaque-type psoriasis: red globular rings. Arch Dermatol. 2007;143:1612.
- Lacarrubba F, Nasca MR, Micali G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J Am Acad Dermatol. 2009;61:1084-1086.
- Liebman TN, Wang SQ. Detection of early basal cell carcinoma with dermoscopy in a patient with psoriasis. Dermatol Online J. 2011;17:12.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Marghoob AA, Malvehy J, Braun RP, eds. An Atlas of Dermoscopy. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Nelson SA, Scope A, Rishpon A, et al. Accuracy and confidence in the clinical diagnosis of basal cell cancer using dermoscopy and reflex confocal microscopy. Int J Dermatol. 2016;55:1351-1356.
- Zalaudek I, Kreusch J, Giacomel J, et al. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. melanocytic skin tumors. J Am Acad Dermatol. 2010;63:361-374.
Practice Points
- Dermoscopy has been largely utilized for the evaluation of malignant lesions. It also is gaining traction in the evaluation of inflammatory dermatoses.
- Early distinction between basal cell carcinoma and psoriasis is important for both treatment options and health care costs.
Irregular Yellow-Brown Plaques on the Trunk and Thighs
The Diagnosis: Necrobiotic Xanthogranuloma
A 4-mm punch biopsy was performed for routine stain with hematoxylin and eosin. The differential diagnosis included sarcoidosis, necrobiosis lipoidica, xanthoma disseminatum, and multicentric reticulohistiocytosis. Histopathologic examination demonstrated a dermal infiltrate of foamy histiocytes and neutrophils (Figure). There were surrounding areas of degenerated collagen containing numerous cholesterol clefts. After clinical pathologic correlation, a diagnosis of necrobiotic xanthogranuloma (NXG) was elucidated.
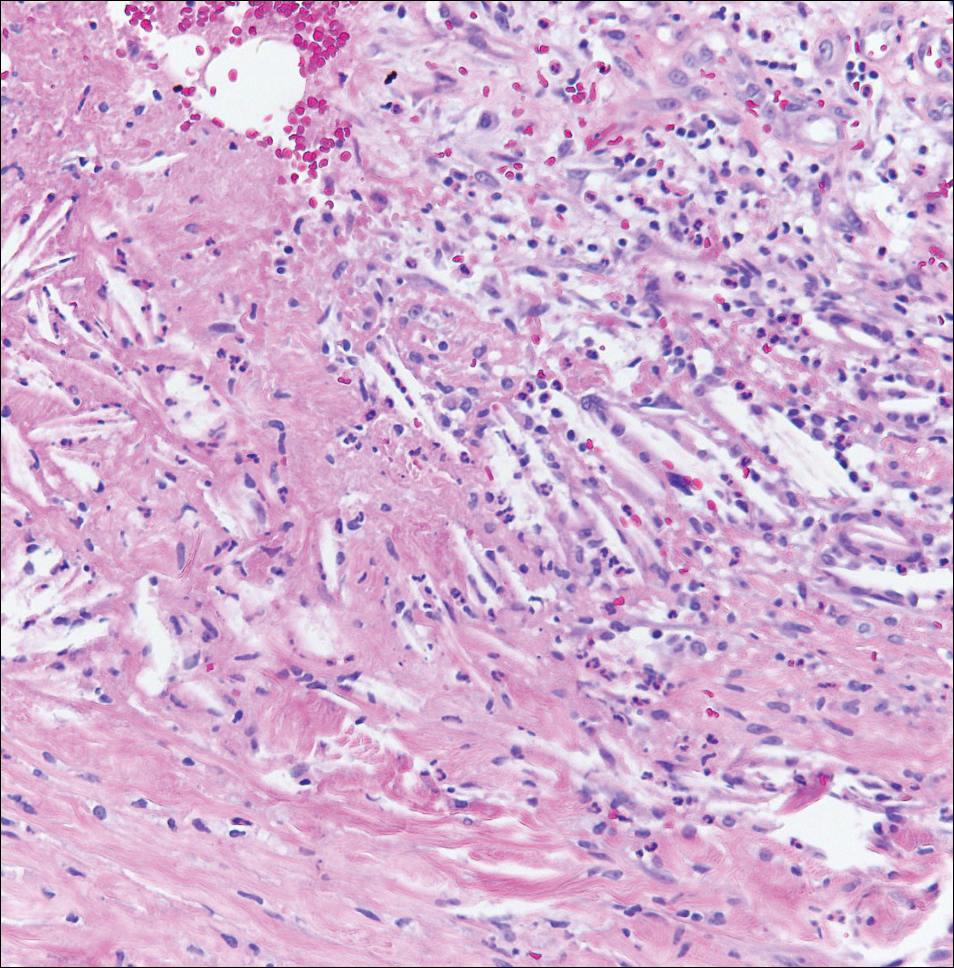
The patient was referred to general surgery for elective excision of 1 or more of the lesions. Excision of an abdominal lesion was performed without complication. After several months, a new lesion reformed within the excisional scar that also was consistent with NXG. At further dermatologic visits, a trial of intralesional corticosteroids was attempted to the largest lesions with modest improvement. In addition, follow-up with hematology and oncology was recommended for routine surveillance of the known blood dyscrasia.
Necrobiotic xanthogranuloma is a multisystem non-Langerhans cell histiocytic disease. Clinically, NXG is characterized by infiltrative plaques and ulcerative nodules. Lesions may appear red, brown, or yellow with associated atrophy and telangiectasia.1 Koch et al2 described a predilection for granuloma formation within preexisting scars. Periorbital location is the most common cutaneous site of involvement of NXG, seen in 80% of cases, but the trunk and extremities also may be involved.1,3 Approximately half of those with periocular involvement experience ocular symptoms including prop- tosis, blepharoptosis, and restricted eye movements.4 The onset of NXG most commonly is seen in middle age.
Characteristic systemic associations have been reported in the setting of NXG. More than 20% of patients may exhibit hepatomegaly. Hematologic abnormalities, hyperlipidemia, and cryoglobulinemia also may be seen.1 In addition, a monoclonal gammopathy of uncertain significance is found in more than 80% of NXG cases. The IgG κ light chain is most commonly identified.2 A foreign body reaction is incited by the immunoglobulin-lipid complex, which is thought to contribute to the formation of cutaneous lesions. There may be associated plasma cell dyscrasia such as multiple myeloma or B-cell lymphoma in approximately 13% of cases.2 Evaluation for underlying plasma cell dyscrasia or lymphoproliferative disorder should be performed regularly with serum protein electrophoresis or immunofixation electrophoresis, and in some cases full-body imaging with computed tomography or magnetic resonance imaging may be warranted.1
Treatment of NXG often is unsuccessful. Surgical excision, systemic immunosuppressive agents, electron beam radiation, and destructive therapies such as cryotherapy may be trialed, often with little success.1 Cutaneous regression has been reported with combination treatment of high-dose dexamethasone and high-dose lenalidomide.5
- Efebera Y, Blanchard E, Allam C, et al. Complete response to thalidomide and dexamethasone in a patient with necrobiotic xanthogranuloma associated with monoclonal gammopathy: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2011;11:298-302.
- Koch PS, Goerdt S, Géraud C. Erythematous papules, plaques, and nodular lesions on the trunk and within preexisting scars. JAMA Dermatol. 2013;149:1103-1104.
- Kerstetter J, Wang J. Adult orbital xanthogranulomatous disease: a review with emphasis on etiology, systemic associations, diagnostic tools, and treatment. Dermatol Clin. 2015;33:457-463.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Dholaria BR, Cappel M, Roy V. Necrobiotic xanthogranuloma associated with monoclonal gammopathy: successful treatment with lenalidomide and dexamethasone [published online Jan 27, 2016]. Ann Hematol. 2016;95:671-672.
The Diagnosis: Necrobiotic Xanthogranuloma
A 4-mm punch biopsy was performed for routine stain with hematoxylin and eosin. The differential diagnosis included sarcoidosis, necrobiosis lipoidica, xanthoma disseminatum, and multicentric reticulohistiocytosis. Histopathologic examination demonstrated a dermal infiltrate of foamy histiocytes and neutrophils (Figure). There were surrounding areas of degenerated collagen containing numerous cholesterol clefts. After clinical pathologic correlation, a diagnosis of necrobiotic xanthogranuloma (NXG) was elucidated.

The patient was referred to general surgery for elective excision of 1 or more of the lesions. Excision of an abdominal lesion was performed without complication. After several months, a new lesion reformed within the excisional scar that also was consistent with NXG. At further dermatologic visits, a trial of intralesional corticosteroids was attempted to the largest lesions with modest improvement. In addition, follow-up with hematology and oncology was recommended for routine surveillance of the known blood dyscrasia.
Necrobiotic xanthogranuloma is a multisystem non-Langerhans cell histiocytic disease. Clinically, NXG is characterized by infiltrative plaques and ulcerative nodules. Lesions may appear red, brown, or yellow with associated atrophy and telangiectasia.1 Koch et al2 described a predilection for granuloma formation within preexisting scars. Periorbital location is the most common cutaneous site of involvement of NXG, seen in 80% of cases, but the trunk and extremities also may be involved.1,3 Approximately half of those with periocular involvement experience ocular symptoms including prop- tosis, blepharoptosis, and restricted eye movements.4 The onset of NXG most commonly is seen in middle age.
Characteristic systemic associations have been reported in the setting of NXG. More than 20% of patients may exhibit hepatomegaly. Hematologic abnormalities, hyperlipidemia, and cryoglobulinemia also may be seen.1 In addition, a monoclonal gammopathy of uncertain significance is found in more than 80% of NXG cases. The IgG κ light chain is most commonly identified.2 A foreign body reaction is incited by the immunoglobulin-lipid complex, which is thought to contribute to the formation of cutaneous lesions. There may be associated plasma cell dyscrasia such as multiple myeloma or B-cell lymphoma in approximately 13% of cases.2 Evaluation for underlying plasma cell dyscrasia or lymphoproliferative disorder should be performed regularly with serum protein electrophoresis or immunofixation electrophoresis, and in some cases full-body imaging with computed tomography or magnetic resonance imaging may be warranted.1
Treatment of NXG often is unsuccessful. Surgical excision, systemic immunosuppressive agents, electron beam radiation, and destructive therapies such as cryotherapy may be trialed, often with little success.1 Cutaneous regression has been reported with combination treatment of high-dose dexamethasone and high-dose lenalidomide.5
The Diagnosis: Necrobiotic Xanthogranuloma
A 4-mm punch biopsy was performed for routine stain with hematoxylin and eosin. The differential diagnosis included sarcoidosis, necrobiosis lipoidica, xanthoma disseminatum, and multicentric reticulohistiocytosis. Histopathologic examination demonstrated a dermal infiltrate of foamy histiocytes and neutrophils (Figure). There were surrounding areas of degenerated collagen containing numerous cholesterol clefts. After clinical pathologic correlation, a diagnosis of necrobiotic xanthogranuloma (NXG) was elucidated.

The patient was referred to general surgery for elective excision of 1 or more of the lesions. Excision of an abdominal lesion was performed without complication. After several months, a new lesion reformed within the excisional scar that also was consistent with NXG. At further dermatologic visits, a trial of intralesional corticosteroids was attempted to the largest lesions with modest improvement. In addition, follow-up with hematology and oncology was recommended for routine surveillance of the known blood dyscrasia.
Necrobiotic xanthogranuloma is a multisystem non-Langerhans cell histiocytic disease. Clinically, NXG is characterized by infiltrative plaques and ulcerative nodules. Lesions may appear red, brown, or yellow with associated atrophy and telangiectasia.1 Koch et al2 described a predilection for granuloma formation within preexisting scars. Periorbital location is the most common cutaneous site of involvement of NXG, seen in 80% of cases, but the trunk and extremities also may be involved.1,3 Approximately half of those with periocular involvement experience ocular symptoms including prop- tosis, blepharoptosis, and restricted eye movements.4 The onset of NXG most commonly is seen in middle age.
Characteristic systemic associations have been reported in the setting of NXG. More than 20% of patients may exhibit hepatomegaly. Hematologic abnormalities, hyperlipidemia, and cryoglobulinemia also may be seen.1 In addition, a monoclonal gammopathy of uncertain significance is found in more than 80% of NXG cases. The IgG κ light chain is most commonly identified.2 A foreign body reaction is incited by the immunoglobulin-lipid complex, which is thought to contribute to the formation of cutaneous lesions. There may be associated plasma cell dyscrasia such as multiple myeloma or B-cell lymphoma in approximately 13% of cases.2 Evaluation for underlying plasma cell dyscrasia or lymphoproliferative disorder should be performed regularly with serum protein electrophoresis or immunofixation electrophoresis, and in some cases full-body imaging with computed tomography or magnetic resonance imaging may be warranted.1
Treatment of NXG often is unsuccessful. Surgical excision, systemic immunosuppressive agents, electron beam radiation, and destructive therapies such as cryotherapy may be trialed, often with little success.1 Cutaneous regression has been reported with combination treatment of high-dose dexamethasone and high-dose lenalidomide.5
- Efebera Y, Blanchard E, Allam C, et al. Complete response to thalidomide and dexamethasone in a patient with necrobiotic xanthogranuloma associated with monoclonal gammopathy: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2011;11:298-302.
- Koch PS, Goerdt S, Géraud C. Erythematous papules, plaques, and nodular lesions on the trunk and within preexisting scars. JAMA Dermatol. 2013;149:1103-1104.
- Kerstetter J, Wang J. Adult orbital xanthogranulomatous disease: a review with emphasis on etiology, systemic associations, diagnostic tools, and treatment. Dermatol Clin. 2015;33:457-463.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Dholaria BR, Cappel M, Roy V. Necrobiotic xanthogranuloma associated with monoclonal gammopathy: successful treatment with lenalidomide and dexamethasone [published online Jan 27, 2016]. Ann Hematol. 2016;95:671-672.
- Efebera Y, Blanchard E, Allam C, et al. Complete response to thalidomide and dexamethasone in a patient with necrobiotic xanthogranuloma associated with monoclonal gammopathy: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2011;11:298-302.
- Koch PS, Goerdt S, Géraud C. Erythematous papules, plaques, and nodular lesions on the trunk and within preexisting scars. JAMA Dermatol. 2013;149:1103-1104.
- Kerstetter J, Wang J. Adult orbital xanthogranulomatous disease: a review with emphasis on etiology, systemic associations, diagnostic tools, and treatment. Dermatol Clin. 2015;33:457-463.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Dholaria BR, Cappel M, Roy V. Necrobiotic xanthogranuloma associated with monoclonal gammopathy: successful treatment with lenalidomide and dexamethasone [published online Jan 27, 2016]. Ann Hematol. 2016;95:671-672.
A 40-year-old man presented with tender lesions on the back, abdomen, and thighs of 10 years' duration. His medical history was remarkable for follicular lymphoma treated with chemotherapy and a monoclonal gammopathy of uncertain significance diagnosed 5 years after the onset of skin symptoms. Physical examination revealed numerous irregularly shaped, yellow plaques on the back, abdomen, and thighs with overlying telangiectasia. A single lesion was noted to extend from a scar.
Rituximab may outperform some other first-line multiple sclerosis treatments
Rituximab was associated with a lower drug discontinuation rate versus all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (RRMS) in a retrospective study of patient data from a Swedish multiple sclerosis registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to results of the study, which appeared online Jan. 8 in JAMA Neurology.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for RRMS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. RRMS is not an approved indication for rituximab in the United States, whereas across Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The addition of new DMTs for RRMS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (that is, interferon beta and glatiramer acetate) within 2 years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that 494 included who received a diagnosis of RRMS between January 1, 2012, and October 31, 2015.
The largest subset of patients (n = 215) received injectable DMTs, while the rest received rituximab (n = 120), dimethyl fumarate (n = 86), natalizumab (Tysabri; n = 50), fingolimod (Gilenya; n = 17), or another treatment (n = 6), according to data in the report.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs, study authors found. Compared with rituximab, the hazard ratios for drug discontinuation after adjusting for covariates and propensity score were 11.4 (95% confidence interval, 4.7-27.4) for injectable DMTs, 15.1 (95% CI, 3.9-58.0) for dimethyl fumarate, 5.9 (95% CI, 1.5-23.4) for fingolimod, and 11.3 (95% CI 3.2-39.4) for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, relapse rates and gadolinium-enhancing lesions with rituximab were less frequent, but the authors said those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
SOURCE: Granqvist M et al. JAMA Neurol. 2018 Jan 8. doi: 10.1001/jamaneurol.2017.4011
Rituximab was associated with a lower drug discontinuation rate versus all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (RRMS) in a retrospective study of patient data from a Swedish multiple sclerosis registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to results of the study, which appeared online Jan. 8 in JAMA Neurology.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for RRMS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. RRMS is not an approved indication for rituximab in the United States, whereas across Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The addition of new DMTs for RRMS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (that is, interferon beta and glatiramer acetate) within 2 years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that 494 included who received a diagnosis of RRMS between January 1, 2012, and October 31, 2015.
The largest subset of patients (n = 215) received injectable DMTs, while the rest received rituximab (n = 120), dimethyl fumarate (n = 86), natalizumab (Tysabri; n = 50), fingolimod (Gilenya; n = 17), or another treatment (n = 6), according to data in the report.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs, study authors found. Compared with rituximab, the hazard ratios for drug discontinuation after adjusting for covariates and propensity score were 11.4 (95% confidence interval, 4.7-27.4) for injectable DMTs, 15.1 (95% CI, 3.9-58.0) for dimethyl fumarate, 5.9 (95% CI, 1.5-23.4) for fingolimod, and 11.3 (95% CI 3.2-39.4) for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, relapse rates and gadolinium-enhancing lesions with rituximab were less frequent, but the authors said those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
SOURCE: Granqvist M et al. JAMA Neurol. 2018 Jan 8. doi: 10.1001/jamaneurol.2017.4011
Rituximab was associated with a lower drug discontinuation rate versus all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (RRMS) in a retrospective study of patient data from a Swedish multiple sclerosis registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to results of the study, which appeared online Jan. 8 in JAMA Neurology.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for RRMS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. RRMS is not an approved indication for rituximab in the United States, whereas across Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The addition of new DMTs for RRMS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (that is, interferon beta and glatiramer acetate) within 2 years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that 494 included who received a diagnosis of RRMS between January 1, 2012, and October 31, 2015.
The largest subset of patients (n = 215) received injectable DMTs, while the rest received rituximab (n = 120), dimethyl fumarate (n = 86), natalizumab (Tysabri; n = 50), fingolimod (Gilenya; n = 17), or another treatment (n = 6), according to data in the report.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs, study authors found. Compared with rituximab, the hazard ratios for drug discontinuation after adjusting for covariates and propensity score were 11.4 (95% confidence interval, 4.7-27.4) for injectable DMTs, 15.1 (95% CI, 3.9-58.0) for dimethyl fumarate, 5.9 (95% CI, 1.5-23.4) for fingolimod, and 11.3 (95% CI 3.2-39.4) for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, relapse rates and gadolinium-enhancing lesions with rituximab were less frequent, but the authors said those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
SOURCE: Granqvist M et al. JAMA Neurol. 2018 Jan 8. doi: 10.1001/jamaneurol.2017.4011
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: Rituximab-treated patients had significantly lower rates of discontinuation, compared with injectable DMTs, fingolimod, natalizumab, and dimethyl fumarate. Relapse rates with rituximab were lower than they were with injectable DMTs and dimethyl fumarate.
Data source: Retrospective cohort study of data from a Swedish multiple sclerosis registry that included 494 patients with a diagnosis of RRMS.
Disclosures: The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
Source: Granqvist M et al. JAMA Neurol. 2018 Jan 8. doi: 10.1001/jamaneurol.2017.4011