User login
CT angiography boosts success in chronic total occlusion revascularization
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
WASHINGTON – When performed prior to revascularization, CT angiography almost doubles the likelihood of successful revascularization of chronic total occlusion relative to no CT angiography, according to a meta-analysis.
Because the meta-analysis relied primarily on retrospective data, the conclusion was characterized as hypothesis-generating. But the author, Wael Abuzeid, MD, an interventional cardiologist and assistant professor at Queen’s University, Kingston, Ont., suggested that there are several arguments to be made for pursuing a randomized trial.
This is not a new idea, according to results of a systematic review of the literature. Although only four articles met prespecified criteria for entry into the meta-analysis that was eventually conducted, Dr. Abuzeid and his coauthors found 424 articles on this subject in a search of three literature databases.
Only one of the four studies entered into the meta-analysis involved prospective data collection, but three of the four found a significant advantage for preprocedural CTA when compared with no CTA for procedural success, meaning restoration of blood flow in the target CTO. The fourth study also associated preprocedural CTA with improved procedural success, but the advantage did not reach statistical significance.
When the data were combined for a meta-analysis, the odds ratio for procedural success for conducting a preprocedural CTA relative to no preprocedural CTA was 1.89 with a significant 95% confidence interval (1.18-3.04; P less than .05).
The four studies were published during 2012-2015. Two of them used CTA with only a 64-slice capacity, raising the possibility that an even greater improvement in results would have been achieved with CTA using the greater resolutions now available.
There were several important limitations of the study, particularly the potential for selection bias from the nonrandomized designs in the studies evaluated, Dr. Abuzeid acknowledged. However, the most likely selection bias would be funneling of more challenging cases to preprocedural CTA, a potential disadvantage for CTA if this resulted in a patient population likely to have a poor outcome.
Currently, few centers employ preprocedural CTA in routine management of CTO, according to Dr. Abuzeid.
“CTA prior to revascularization in CTO is not a standard approach even in complex patients,” Dr. Abuzeid said. However, he believes an argument can already be made for preprocedural CTA in some types of challenging patients, such as those with particularly long target lesions. In such cases, CTA could provide advance information about negative distal remodeling and the likelihood that a retrograde approach might be needed.
“For the junior operator, I think the information provided by preprocedural CTA could be very useful in planning,” Dr. Abuzeid said.
The arguments against preprocedural CTA include higher radiation exposure and a delay in the time to procedure by adding the extra step of first conducting the imaging study. In addition to verifying that procedural success is achieved with preprocedural CTA, these issues are among those that deserve evaluation in a prospective study.
“The appropriate randomized trial would be conducted at high-volume centers with all comers, not just complex patients, randomized to undergo a preprocedural CTA or no preprocedural imaging,” Dr. Abuzeid suggested. He said the appropriate primary outcome is relative success at restoring Thrombolysis in Myocardial Infarction grade 2 flow, but suggested that other endpoints, such as procedure time and rate of complications, would be useful for determining the value of this approach.
Dr. Abuzeid reports having no financial relationships.
SOURCE: Abuzeid W. CRT 18.
REPORTING FROM CRT 2018
Key clinical point:
Major finding: In a meta-analysis of data from four studies, CT angiography almost doubled the likelihood of procedural success (OR 1.89; P less than .05).
Study details: A systematic review and meta-analysis of four studies.
Disclosures: Dr. Abuzeid reports having no financial relationships.
Source: Abuzaid W. CRT 18.
Match Day 2018: Internal medicine increases positions, matches
Internal medicine helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled about 4% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
Internal medicine brought 7,542 PGY-1 positions – more than any other specialty – to the Match Day party this year and filled 44.9% of them with U.S. graduates and 96.7% overall. Despite the increase in IM residency slots, however, the number and percentage of U.S. seniors matching declined, as it has done each year since 2015. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Internal medicine helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled about 4% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
Internal medicine brought 7,542 PGY-1 positions – more than any other specialty – to the Match Day party this year and filled 44.9% of them with U.S. graduates and 96.7% overall. Despite the increase in IM residency slots, however, the number and percentage of U.S. seniors matching declined, as it has done each year since 2015. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Internal medicine helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled about 4% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
Internal medicine brought 7,542 PGY-1 positions – more than any other specialty – to the Match Day party this year and filled 44.9% of them with U.S. graduates and 96.7% overall. Despite the increase in IM residency slots, however, the number and percentage of U.S. seniors matching declined, as it has done each year since 2015. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2018: Psychiatry increases positions and matches
as the specialty increased residency slots by 4.1% and accepted 3.3% more applicants, compared with 2017, according to the National Resident Matching Program.
Psychiatry brought 1,556 PGY-1 positions to the Match Day table this year and filled 63.1% of them with U.S. graduates – up for the fourth consecutive year – and 99.0% overall. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
as the specialty increased residency slots by 4.1% and accepted 3.3% more applicants, compared with 2017, according to the National Resident Matching Program.
Psychiatry brought 1,556 PGY-1 positions to the Match Day table this year and filled 63.1% of them with U.S. graduates – up for the fourth consecutive year – and 99.0% overall. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
as the specialty increased residency slots by 4.1% and accepted 3.3% more applicants, compared with 2017, according to the National Resident Matching Program.
Psychiatry brought 1,556 PGY-1 positions to the Match Day table this year and filled 63.1% of them with U.S. graduates – up for the fourth consecutive year – and 99.0% overall. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
Match Day 2018: Big increases for neurology
Neurology helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled over 12% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
The third consecutive year with a double-digit increase in PGY-1 positions enabled neurology to bring 552 slots to the Match Day party this year: 50.7% were filled with U.S. graduates and the overall fill rate was 97.6%. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.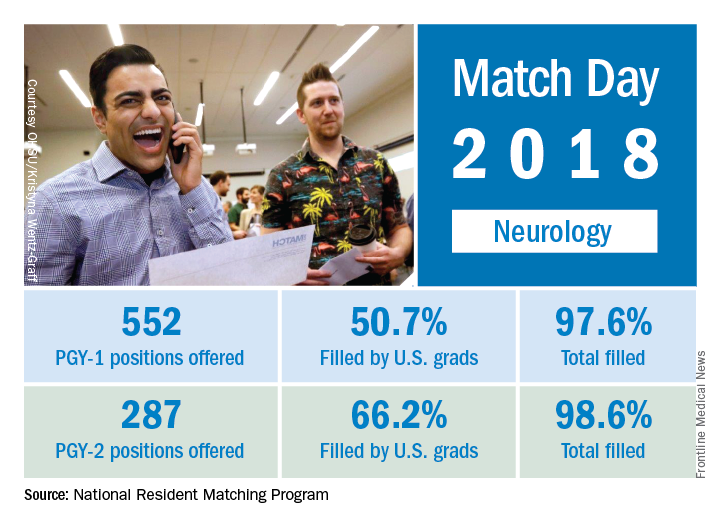
The total number of child neurology PGY-1 positions offered rose to 134, of which 96.3% were filled (75.4% by U.S. graduates). Another seven of eight PGY-2 child neurology positions were filled. Of 26 PGY-2 physician positions offered for child neurology, 12 were filled.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Neurology helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled over 12% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
The third consecutive year with a double-digit increase in PGY-1 positions enabled neurology to bring 552 slots to the Match Day party this year: 50.7% were filled with U.S. graduates and the overall fill rate was 97.6%. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
The total number of child neurology PGY-1 positions offered rose to 134, of which 96.3% were filled (75.4% by U.S. graduates). Another seven of eight PGY-2 child neurology positions were filled. Of 26 PGY-2 physician positions offered for child neurology, 12 were filled.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Neurology helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled over 12% more residency slots than in 2017, according to the National Resident Matching Program (NRMP).
The third consecutive year with a double-digit increase in PGY-1 positions enabled neurology to bring 552 slots to the Match Day party this year: 50.7% were filled with U.S. graduates and the overall fill rate was 97.6%. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
The total number of child neurology PGY-1 positions offered rose to 134, of which 96.3% were filled (75.4% by U.S. graduates). Another seven of eight PGY-2 child neurology positions were filled. Of 26 PGY-2 physician positions offered for child neurology, 12 were filled.
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total (up 4.7%) and PGY-1 positions (up 4.9%) filled, and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2018: A slow year for pediatrics
Pediatrics saw modest gains in residency slots and matches in what was the largest Match Day on record, according to the National Resident Matching Program (NRMP).
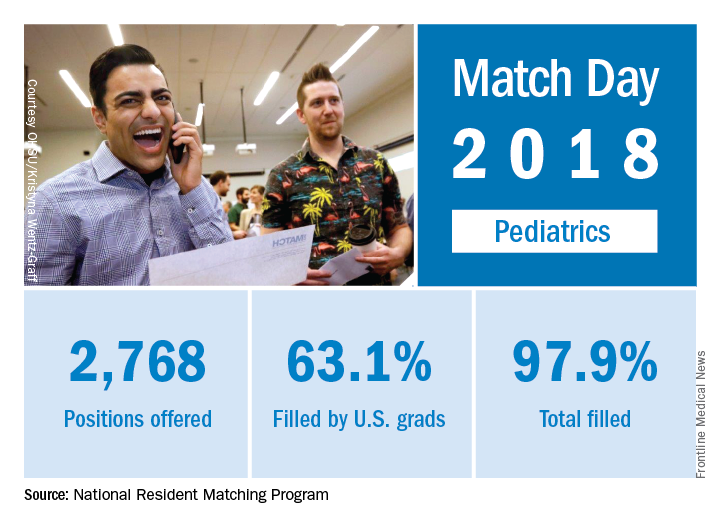
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Pediatrics saw modest gains in residency slots and matches in what was the largest Match Day on record, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Pediatrics saw modest gains in residency slots and matches in what was the largest Match Day on record, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2018: Surgery increases positions and matches
Surgery helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled 3% more residency slots than it did in 2017, according to the National Resident Matching Program (NRMP).
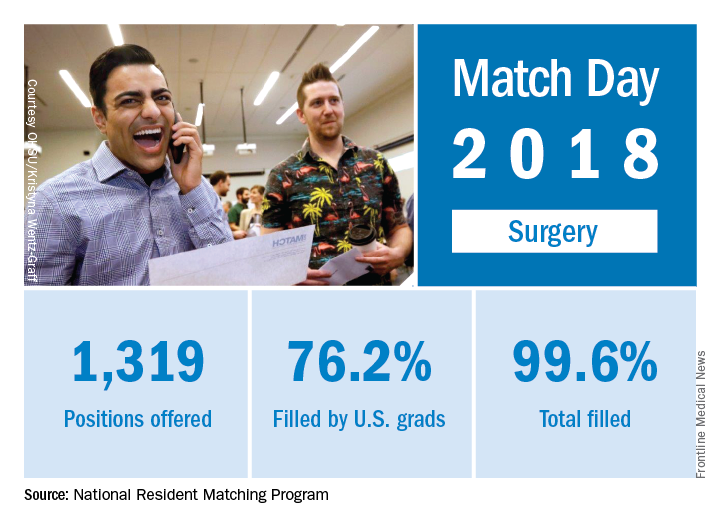
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Surgery helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled 3% more residency slots than it did in 2017, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Surgery helped to make the 2018 Main Residency Match the largest ever as the specialty offered and filled 3% more residency slots than it did in 2017, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2018: Family medicine increases positions, matches
as the specialty increased residency slots by 8% and accepted 9% more applicants, compared with 2017, according to the National Resident Matching Program (NRMP).
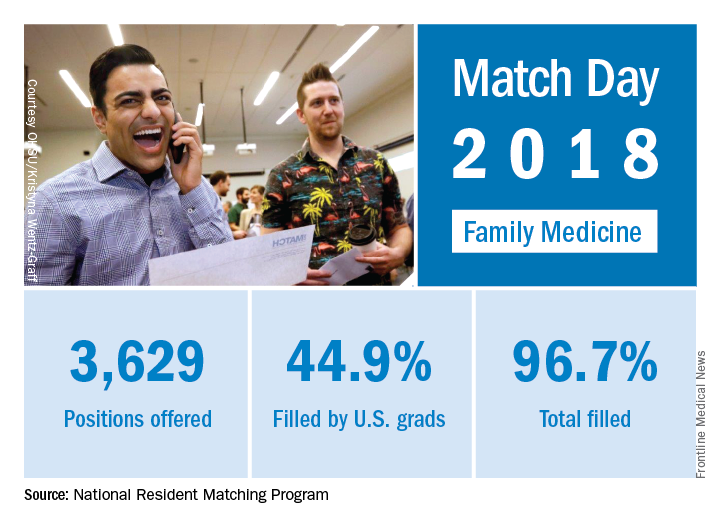
The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
as the specialty increased residency slots by 8% and accepted 9% more applicants, compared with 2017, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
as the specialty increased residency slots by 8% and accepted 9% more applicants, compared with 2017, according to the National Resident Matching Program (NRMP).

The 2018 Match also set new highs for total positions offered (up 4.4% over last year), total positions and PGY-1 positions filled (up 4.7% and 4.9%, respectively), and total applicants (up 1.7%). “The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Febuxostat increases cardiovascular mortality in CARES trial
ORLANDO – Long-term treatment with febuxostat in gout patients with comorbid cardiovascular disease conferred significantly higher risks of both cardiovascular death and all-cause mortality, compared with allopurinol, in the Food and Drug Administration–mandated postmarketing CARES trial, William B. White, MD, reported at the annual meeting of the American College of Cardiology.
CARES (Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout and Cardiovascular Disease) was a prospective, double-blind, 320-center North American clinical trial in which 6,190 patients were randomized to febuxostat (Uloric) at 40-80 mg once daily or 200-600 mg of allopurinol once daily. The postmarketing safety study was required by the FDA as a condition of marketing approval for febuxostat in light of preapproval evidence suggestive of a possible increased risk of cardiovascular events, explained Dr. White, professor of medicine and chief of the division of hypertension and clinical pharmacology at the University of Connecticut, Farmington.
The warning klaxon sounded when investigators scrutinized the individual components of the primary endpoint. They found that the 4.3% cardiovascular death rate in the febuxostat group was significantly higher than the 3.2% rate in the allopurinol group, representing a statistically significant 34% increase in relative risk. The event curves began to separate roughly 30 months into the trial. Moreover, all-cause mortality was also significantly increased in the febuxostat group, by a margin of 7.8% to 6.4%, for a 22% increase in risk.
The increased cardiovascular mortality in the febuxostat group was driven by a higher adjudicated sudden cardiac death rate: 2.7%, compared with 1.8% in the allopurinol group.
In a prespecified per protocol analysis of cardiovascular events occurring while patients were actually on treatment or within 1 month after discontinuation, the key findings remained unchanged: no between-group difference in the primary composite endpoint, but a 49% increase in the relative risk of cardiovascular death in the febuxostat-treated patients.
A hefty 45% of participants stopped taking their assigned drug early. Dr. White said this isn’t unusual; high dropout rates are common in clinical trials of patients with painful conditions. Because of the high lost-to-follow-up rate, however, the investigators hired a private investigator to scour the country looking for missed deaths among enrollees. This turned up an extra 199 deaths. When those were added to the total, all-cause mortality in the febuxostat group was no longer significantly higher than for allopurinol.
The puzzle over nonfatal events
A puzzling key study finding was that except for cardiovascular death, the other components of the primary composite endpoint – that is, nonfatal MI, nonfatal stroke, and urgent revascularization due to unstable angina – were all either neutral or numerically favored febuxostat.
“That’s been the biggest challenge in the trial: The nonfatal events didn’t go in the same direction as the fatal events. And we don’t have a real mechanism to explain why,” Dr. White told a panel of discussants.
“I scanned the medical literature over the last 4 decades, and I did not see another prospective, randomized, double-blind trial in which mortality was increased when none of the nonfatal events were increased. The finding is unique. Statistically there is only a 4% chance that the mortality finding is wrong,” the cardiologist said.
The CARES leadership included rheumatologists and nephrologists as well as cardiologists. Dr. White said he and the others were at a loss to come up with an explanation for the findings.
Patients in the febuxostat arm were significantly more likely to achieve serum urate levels below 6 and 5 mg/dL. Their flare rate was 0.68 events per person-year, similar to the 0.63 per person-year rate in the allopurinol group.
Among the pieces of the study puzzle: The majority of cardiovascular deaths occurred in patients who were no longer on therapy, yet investigators could find no evidence of a legacy effect. The mortality risk was 2.3-fold greater with febuxostat than with allopurinol among patients on NSAID therapy, but there was no significant between-group difference among patients not taking NSAIDs. There was a trend for more cardiovascular deaths with febuxostat than allopurinol among patients not on low-dose aspirin. And the cardiovascular mortality was 2.2-fold greater in the febuxostat arm than with allopurinol in patients on colchicine during the study.
Notably, prior to febuxostat’s marketing approval there were extensive studies of the drug’s potential effect on left ventricular function, thrombotic potential, possible arrhythmogenic effects, and impact upon atherosclerosis. Among these investigations was a QT-interval study conducted using febuxostat doses four times higher than the maximum therapeutic dose, which was prescient given the increased sudden cardiac death rate in the subsequent CARES trial. Yet no concerning signals were seen in any of this work, he continued.
“We’re still looking at some correlates that might have an impact. For example, my rheumatologist colleagues feel very strongly that we need to look really extensively at gout flares, even though rates were not that different between the two treatment groups. Gout flares are known to increase oxidative stress and perhaps cause temporary increases in endothelial dysfunction and possibly vasomotor abnormalities,” Dr. White said.
One would think, though, that if gout flares figured in the increase in cardiovascular mortality they would also have been associated with more urgent revascularization for unstable angina, when in fact the rate was actually numerically lower in the febuxostat group, he noted.
Discussant Athena Poppas, MD, director of the Lifespan Cardiovascular Institute at Rhode Island Hospital, Providence, said she couldn’t determine how much of the increased cardiovascular mortality in the febuxostat patients was due to the drug and how much resulted from the suboptimal use of guideline-directed medical therapy across both study arms. At baseline, only 60% of study participants – all by definition at high cardiovascular risk – were on aspirin, just under 75% were on lipid-lowering therapy, 58% were on a beta blocker, and 70% were on a renin-angiotensin system blocker, even though the majority of subjects had stage 3 chronic kidney disease.
Implications of findings and FDA’s next steps
Another discussant, C. Noel Bairey Merz, MD, called the CARES findings “curious.” But despite the lack of a plausible mechanistic explanation for the results, she said, the implications are clear.
“I would conclude that because your modified intention-to-treat as well as your per protocol analyses were consistent for the death endpoint, then despite the high dropout rate, that finding is relatively robust and probably should be used to inform policy,” said Dr. Merz, director of the Women’s Heart Center and the Preventive and Rehabilitative Cardiac Center in the Cedars-Sinai Heart Institute and professor of medicine at the University of California, Los Angeles.
At a press conference, Dr. White said the FDA will spend several months poring over the CARES results and that it would be premature to speculate on what action the agency might take on febuxostat. The drug is prescribed far less frequently than allopurinol for the nation’s estimated 8.2 million gout patients.
“I would certainly be concerned about our findings. However, rheumatologists take care of very ill patients and all the drugs they use have morbidity. So if they’re having substantial efficacy and the person has been on febuxostat for 8 years, I suspect they’re going to continue to give that drug to him,” he said.
Simultaneously with Dr. White’s presentation at ACC 2018, the CARES results were published in the New England Journal of Medicine (N Engl J Med. 2018 Mar 12. doi: 10.1056/NEJMoa1710895).
The CARES trial was funded by Takeda. Dr. White reported serving as a consultant to that company and Novartis and receiving research funding from the National Institutes of Health.
SOURCE: White W et al. ACC 18.
ORLANDO – Long-term treatment with febuxostat in gout patients with comorbid cardiovascular disease conferred significantly higher risks of both cardiovascular death and all-cause mortality, compared with allopurinol, in the Food and Drug Administration–mandated postmarketing CARES trial, William B. White, MD, reported at the annual meeting of the American College of Cardiology.
CARES (Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout and Cardiovascular Disease) was a prospective, double-blind, 320-center North American clinical trial in which 6,190 patients were randomized to febuxostat (Uloric) at 40-80 mg once daily or 200-600 mg of allopurinol once daily. The postmarketing safety study was required by the FDA as a condition of marketing approval for febuxostat in light of preapproval evidence suggestive of a possible increased risk of cardiovascular events, explained Dr. White, professor of medicine and chief of the division of hypertension and clinical pharmacology at the University of Connecticut, Farmington.
The warning klaxon sounded when investigators scrutinized the individual components of the primary endpoint. They found that the 4.3% cardiovascular death rate in the febuxostat group was significantly higher than the 3.2% rate in the allopurinol group, representing a statistically significant 34% increase in relative risk. The event curves began to separate roughly 30 months into the trial. Moreover, all-cause mortality was also significantly increased in the febuxostat group, by a margin of 7.8% to 6.4%, for a 22% increase in risk.
The increased cardiovascular mortality in the febuxostat group was driven by a higher adjudicated sudden cardiac death rate: 2.7%, compared with 1.8% in the allopurinol group.
In a prespecified per protocol analysis of cardiovascular events occurring while patients were actually on treatment or within 1 month after discontinuation, the key findings remained unchanged: no between-group difference in the primary composite endpoint, but a 49% increase in the relative risk of cardiovascular death in the febuxostat-treated patients.
A hefty 45% of participants stopped taking their assigned drug early. Dr. White said this isn’t unusual; high dropout rates are common in clinical trials of patients with painful conditions. Because of the high lost-to-follow-up rate, however, the investigators hired a private investigator to scour the country looking for missed deaths among enrollees. This turned up an extra 199 deaths. When those were added to the total, all-cause mortality in the febuxostat group was no longer significantly higher than for allopurinol.
The puzzle over nonfatal events
A puzzling key study finding was that except for cardiovascular death, the other components of the primary composite endpoint – that is, nonfatal MI, nonfatal stroke, and urgent revascularization due to unstable angina – were all either neutral or numerically favored febuxostat.
“That’s been the biggest challenge in the trial: The nonfatal events didn’t go in the same direction as the fatal events. And we don’t have a real mechanism to explain why,” Dr. White told a panel of discussants.
“I scanned the medical literature over the last 4 decades, and I did not see another prospective, randomized, double-blind trial in which mortality was increased when none of the nonfatal events were increased. The finding is unique. Statistically there is only a 4% chance that the mortality finding is wrong,” the cardiologist said.
The CARES leadership included rheumatologists and nephrologists as well as cardiologists. Dr. White said he and the others were at a loss to come up with an explanation for the findings.
Patients in the febuxostat arm were significantly more likely to achieve serum urate levels below 6 and 5 mg/dL. Their flare rate was 0.68 events per person-year, similar to the 0.63 per person-year rate in the allopurinol group.
Among the pieces of the study puzzle: The majority of cardiovascular deaths occurred in patients who were no longer on therapy, yet investigators could find no evidence of a legacy effect. The mortality risk was 2.3-fold greater with febuxostat than with allopurinol among patients on NSAID therapy, but there was no significant between-group difference among patients not taking NSAIDs. There was a trend for more cardiovascular deaths with febuxostat than allopurinol among patients not on low-dose aspirin. And the cardiovascular mortality was 2.2-fold greater in the febuxostat arm than with allopurinol in patients on colchicine during the study.
Notably, prior to febuxostat’s marketing approval there were extensive studies of the drug’s potential effect on left ventricular function, thrombotic potential, possible arrhythmogenic effects, and impact upon atherosclerosis. Among these investigations was a QT-interval study conducted using febuxostat doses four times higher than the maximum therapeutic dose, which was prescient given the increased sudden cardiac death rate in the subsequent CARES trial. Yet no concerning signals were seen in any of this work, he continued.
“We’re still looking at some correlates that might have an impact. For example, my rheumatologist colleagues feel very strongly that we need to look really extensively at gout flares, even though rates were not that different between the two treatment groups. Gout flares are known to increase oxidative stress and perhaps cause temporary increases in endothelial dysfunction and possibly vasomotor abnormalities,” Dr. White said.
One would think, though, that if gout flares figured in the increase in cardiovascular mortality they would also have been associated with more urgent revascularization for unstable angina, when in fact the rate was actually numerically lower in the febuxostat group, he noted.
Discussant Athena Poppas, MD, director of the Lifespan Cardiovascular Institute at Rhode Island Hospital, Providence, said she couldn’t determine how much of the increased cardiovascular mortality in the febuxostat patients was due to the drug and how much resulted from the suboptimal use of guideline-directed medical therapy across both study arms. At baseline, only 60% of study participants – all by definition at high cardiovascular risk – were on aspirin, just under 75% were on lipid-lowering therapy, 58% were on a beta blocker, and 70% were on a renin-angiotensin system blocker, even though the majority of subjects had stage 3 chronic kidney disease.
Implications of findings and FDA’s next steps
Another discussant, C. Noel Bairey Merz, MD, called the CARES findings “curious.” But despite the lack of a plausible mechanistic explanation for the results, she said, the implications are clear.
“I would conclude that because your modified intention-to-treat as well as your per protocol analyses were consistent for the death endpoint, then despite the high dropout rate, that finding is relatively robust and probably should be used to inform policy,” said Dr. Merz, director of the Women’s Heart Center and the Preventive and Rehabilitative Cardiac Center in the Cedars-Sinai Heart Institute and professor of medicine at the University of California, Los Angeles.
At a press conference, Dr. White said the FDA will spend several months poring over the CARES results and that it would be premature to speculate on what action the agency might take on febuxostat. The drug is prescribed far less frequently than allopurinol for the nation’s estimated 8.2 million gout patients.
“I would certainly be concerned about our findings. However, rheumatologists take care of very ill patients and all the drugs they use have morbidity. So if they’re having substantial efficacy and the person has been on febuxostat for 8 years, I suspect they’re going to continue to give that drug to him,” he said.
Simultaneously with Dr. White’s presentation at ACC 2018, the CARES results were published in the New England Journal of Medicine (N Engl J Med. 2018 Mar 12. doi: 10.1056/NEJMoa1710895).
The CARES trial was funded by Takeda. Dr. White reported serving as a consultant to that company and Novartis and receiving research funding from the National Institutes of Health.
SOURCE: White W et al. ACC 18.
ORLANDO – Long-term treatment with febuxostat in gout patients with comorbid cardiovascular disease conferred significantly higher risks of both cardiovascular death and all-cause mortality, compared with allopurinol, in the Food and Drug Administration–mandated postmarketing CARES trial, William B. White, MD, reported at the annual meeting of the American College of Cardiology.
CARES (Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout and Cardiovascular Disease) was a prospective, double-blind, 320-center North American clinical trial in which 6,190 patients were randomized to febuxostat (Uloric) at 40-80 mg once daily or 200-600 mg of allopurinol once daily. The postmarketing safety study was required by the FDA as a condition of marketing approval for febuxostat in light of preapproval evidence suggestive of a possible increased risk of cardiovascular events, explained Dr. White, professor of medicine and chief of the division of hypertension and clinical pharmacology at the University of Connecticut, Farmington.
The warning klaxon sounded when investigators scrutinized the individual components of the primary endpoint. They found that the 4.3% cardiovascular death rate in the febuxostat group was significantly higher than the 3.2% rate in the allopurinol group, representing a statistically significant 34% increase in relative risk. The event curves began to separate roughly 30 months into the trial. Moreover, all-cause mortality was also significantly increased in the febuxostat group, by a margin of 7.8% to 6.4%, for a 22% increase in risk.
The increased cardiovascular mortality in the febuxostat group was driven by a higher adjudicated sudden cardiac death rate: 2.7%, compared with 1.8% in the allopurinol group.
In a prespecified per protocol analysis of cardiovascular events occurring while patients were actually on treatment or within 1 month after discontinuation, the key findings remained unchanged: no between-group difference in the primary composite endpoint, but a 49% increase in the relative risk of cardiovascular death in the febuxostat-treated patients.
A hefty 45% of participants stopped taking their assigned drug early. Dr. White said this isn’t unusual; high dropout rates are common in clinical trials of patients with painful conditions. Because of the high lost-to-follow-up rate, however, the investigators hired a private investigator to scour the country looking for missed deaths among enrollees. This turned up an extra 199 deaths. When those were added to the total, all-cause mortality in the febuxostat group was no longer significantly higher than for allopurinol.
The puzzle over nonfatal events
A puzzling key study finding was that except for cardiovascular death, the other components of the primary composite endpoint – that is, nonfatal MI, nonfatal stroke, and urgent revascularization due to unstable angina – were all either neutral or numerically favored febuxostat.
“That’s been the biggest challenge in the trial: The nonfatal events didn’t go in the same direction as the fatal events. And we don’t have a real mechanism to explain why,” Dr. White told a panel of discussants.
“I scanned the medical literature over the last 4 decades, and I did not see another prospective, randomized, double-blind trial in which mortality was increased when none of the nonfatal events were increased. The finding is unique. Statistically there is only a 4% chance that the mortality finding is wrong,” the cardiologist said.
The CARES leadership included rheumatologists and nephrologists as well as cardiologists. Dr. White said he and the others were at a loss to come up with an explanation for the findings.
Patients in the febuxostat arm were significantly more likely to achieve serum urate levels below 6 and 5 mg/dL. Their flare rate was 0.68 events per person-year, similar to the 0.63 per person-year rate in the allopurinol group.
Among the pieces of the study puzzle: The majority of cardiovascular deaths occurred in patients who were no longer on therapy, yet investigators could find no evidence of a legacy effect. The mortality risk was 2.3-fold greater with febuxostat than with allopurinol among patients on NSAID therapy, but there was no significant between-group difference among patients not taking NSAIDs. There was a trend for more cardiovascular deaths with febuxostat than allopurinol among patients not on low-dose aspirin. And the cardiovascular mortality was 2.2-fold greater in the febuxostat arm than with allopurinol in patients on colchicine during the study.
Notably, prior to febuxostat’s marketing approval there were extensive studies of the drug’s potential effect on left ventricular function, thrombotic potential, possible arrhythmogenic effects, and impact upon atherosclerosis. Among these investigations was a QT-interval study conducted using febuxostat doses four times higher than the maximum therapeutic dose, which was prescient given the increased sudden cardiac death rate in the subsequent CARES trial. Yet no concerning signals were seen in any of this work, he continued.
“We’re still looking at some correlates that might have an impact. For example, my rheumatologist colleagues feel very strongly that we need to look really extensively at gout flares, even though rates were not that different between the two treatment groups. Gout flares are known to increase oxidative stress and perhaps cause temporary increases in endothelial dysfunction and possibly vasomotor abnormalities,” Dr. White said.
One would think, though, that if gout flares figured in the increase in cardiovascular mortality they would also have been associated with more urgent revascularization for unstable angina, when in fact the rate was actually numerically lower in the febuxostat group, he noted.
Discussant Athena Poppas, MD, director of the Lifespan Cardiovascular Institute at Rhode Island Hospital, Providence, said she couldn’t determine how much of the increased cardiovascular mortality in the febuxostat patients was due to the drug and how much resulted from the suboptimal use of guideline-directed medical therapy across both study arms. At baseline, only 60% of study participants – all by definition at high cardiovascular risk – were on aspirin, just under 75% were on lipid-lowering therapy, 58% were on a beta blocker, and 70% were on a renin-angiotensin system blocker, even though the majority of subjects had stage 3 chronic kidney disease.
Implications of findings and FDA’s next steps
Another discussant, C. Noel Bairey Merz, MD, called the CARES findings “curious.” But despite the lack of a plausible mechanistic explanation for the results, she said, the implications are clear.
“I would conclude that because your modified intention-to-treat as well as your per protocol analyses were consistent for the death endpoint, then despite the high dropout rate, that finding is relatively robust and probably should be used to inform policy,” said Dr. Merz, director of the Women’s Heart Center and the Preventive and Rehabilitative Cardiac Center in the Cedars-Sinai Heart Institute and professor of medicine at the University of California, Los Angeles.
At a press conference, Dr. White said the FDA will spend several months poring over the CARES results and that it would be premature to speculate on what action the agency might take on febuxostat. The drug is prescribed far less frequently than allopurinol for the nation’s estimated 8.2 million gout patients.
“I would certainly be concerned about our findings. However, rheumatologists take care of very ill patients and all the drugs they use have morbidity. So if they’re having substantial efficacy and the person has been on febuxostat for 8 years, I suspect they’re going to continue to give that drug to him,” he said.
Simultaneously with Dr. White’s presentation at ACC 2018, the CARES results were published in the New England Journal of Medicine (N Engl J Med. 2018 Mar 12. doi: 10.1056/NEJMoa1710895).
The CARES trial was funded by Takeda. Dr. White reported serving as a consultant to that company and Novartis and receiving research funding from the National Institutes of Health.
SOURCE: White W et al. ACC 18.
REPORTING FROM ACC 18
Key clinical point: Gout patients on febuxostat were 34% more likely to die of cardiovascular causes than were those on allopurinol.
Major finding: Death due to cardiovascular causes occurred in 4.3% of febuxostat-treated patients and 3.2% assigned to allopurinol.
Study details: This prospective, randomized, double-blind, 320-center clinical trial included nearly 6,200 gout patients with comorbid cardiovascular disease.
Disclosures: The FDA-mandated CARES trial was sponsored by Takeda. The study presenter is a consultant to the company.
Source: White W et al. ACC 18.
Prevalence and Impact of Self-Citation in Academic Orthopedic Surgery
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
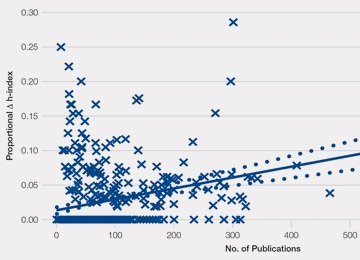
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.
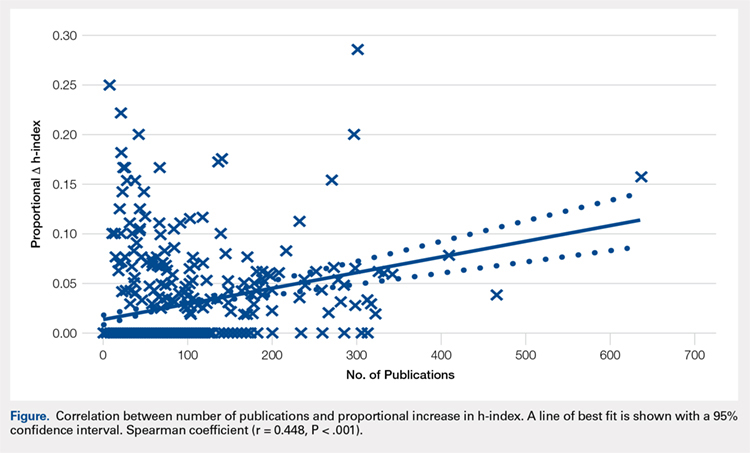
Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.

Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.

Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
TAKE-HOME POINTS
- In all, 83.8% of orthopedic surgeons cite previous work at least once.
- Self-citations account for only 5.8% of all citations.
- Including self-citations increases the mean h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001).
- The magnitude of increase in h-index via self-citation is proportional to the career number of publications.
- Overall, while prevalent, the practice of self-citation has minimal impact on an academic orthopedic surgeon’s h-index.
Uterosacral ligament colpopexy: The way we do it
The way we do it

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
The way we do it
The way we do it
This video is brought to you by