User login
VIDEO: Fezolinetant drops testosterone levels in PCOS
CHICAGO – currently in development. The proof-of-concept phase 2 trial, which saw no concerning safety signals for the medication, fezolinetant, sets the stage for a larger, and perhaps longer, study to learn more about the neurokinin 3 receptor antagonist’s efficacy against PCOS.
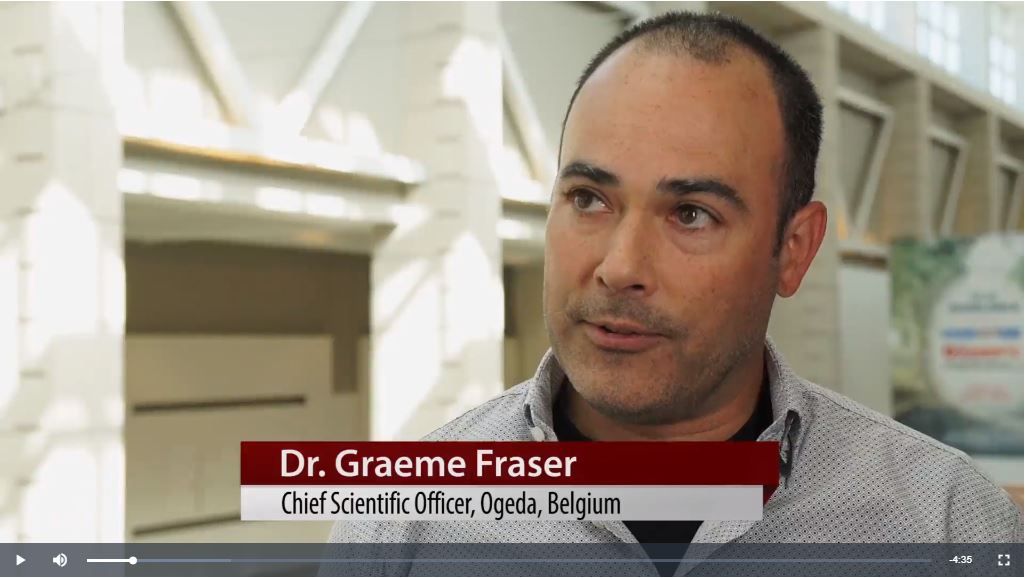
“The primary outcome for this phase 2 trial was to see if we could lower testosterone levels in these PCOS patients,” said Graeme Fraser, PhD, chief scientific officer for Ogeda, discussing the poster he and his colleagues presented at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Testosterone levels decreased: Measured at about 3 hours postdose, which is the approximate pharmacokinetic Cmax [maximum serum concentration], there was … a more than 30% decrease in testosterone levels,” said Dr. Fraser. By week 12, he said, “there was a very consistent decrease of 30% of testosterone levels. So that was quite good; we successfully hit the primary outcome.”
At 12 weeks, the higher dose of fezolinetant decreased testosterone by 0.64 nmol/L, compared with the 0.04 nmol/L seen with placebo (P less than .01).
Fezolinetant downregulates the activity of candy neurons in the hypothalamus, said Dr. Fraser in an interview. In turn, gonadotropin-releasing hormone (GNRH) pulse frequency is reduced, lowering luteinizing hormone (LH) levels. Since LH drives testosterone levels, this drops as well – a beneficial effect in PCOS, with its cardinal symptom of hyperandrogenism.
The double-blind, placebo-controlled study was conducted in Western Europe; 73 participants were randomized into three groups: 27 to a placebo group, 23 to a treatment group given 60 mg of fezolinetant once daily, and 23 to a treatment group given 180 mg of fezolinetant once daily. All groups received treatment for 12 weeks.
A total of 26 patients on placebo, 21 on 60-mg fezolinetant, and 17 on 180-mg fezolinetant completed the study. Patients who completed the study were included in the safety analysis, while all who took the study medication were included in the intent-to-treat analysis for primary and secondary outcome measures.
All participants had PCOS with hyperandrogenism, mean age was about 31 years, and about three quarters of enrollees were white, though local regulations restricted the collection of race and ethnicity data in some cases. One secondary outcome measure of the study was how fezolinetant affected the LH:FSH (follicle-stimulating hormone) ratio. “Patients with PCOS tend to have a very high GNRH [gonadotropin-releasing hormone] pulse frequency, which leads to a very high LH:FSH ratio,” said Dr. Fraser.
At baseline, the LH:FSH ratio was about 3. “With treatment, that ratio normalized to about 1, which is where it is in healthy women,” he said. The decrease in LH:FSH ratio occurred in a dose-dependent fashion and was statistically significant (P less than .001 for 180 mg fezolinetant versus placebo).
Dr. Fraser and his collaborators also tracked anti-müllerian hormone (AMH) levels, ovarian volume, and number of follicles. Although the investigators saw trends toward reduction in AMH levels and toward smaller ovarian volumes, these trends weren’t significant. “It was somewhat ambitious, I guess, to consider we’d hit these endpoints within 12 weeks,” he said. There were no serious drug-related adverse events.
“Most importantly, I would say, we did not get an increase in menses frequency, and, of course, that’s an important marker for fertility,” said Dr. Fraser. “There’s a debate in PCOS about whether this disease is driven by a malfunction in the brain or a malfunction in the ovaries. I guess on face value, perhaps this problem is in the ovaries.”
With the trends toward lower AMH and ovarian volumes, the research team is left wondering what would happen if the duration of therapy were extended. “Perhaps, the system would have been reset, and the frequency would have been restored. … So one thought that we have is to prolong the study in future trials and see if that could lead to an increase in fertility in PCOS,” said Dr. Fraser.
Fezolinetant is also being studied for menopause-related hot flashes in the United States and Europe.
Dr. Fraser is an employee of Ogeda, which sponsored the trial. Ogeda is a wholly owned subsidiary of Astelas.
SOURCE: Fraser G et al. ENDO 2018, Abstract SAT-305-LB.
CHICAGO – currently in development. The proof-of-concept phase 2 trial, which saw no concerning safety signals for the medication, fezolinetant, sets the stage for a larger, and perhaps longer, study to learn more about the neurokinin 3 receptor antagonist’s efficacy against PCOS.
“The primary outcome for this phase 2 trial was to see if we could lower testosterone levels in these PCOS patients,” said Graeme Fraser, PhD, chief scientific officer for Ogeda, discussing the poster he and his colleagues presented at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Testosterone levels decreased: Measured at about 3 hours postdose, which is the approximate pharmacokinetic Cmax [maximum serum concentration], there was … a more than 30% decrease in testosterone levels,” said Dr. Fraser. By week 12, he said, “there was a very consistent decrease of 30% of testosterone levels. So that was quite good; we successfully hit the primary outcome.”
At 12 weeks, the higher dose of fezolinetant decreased testosterone by 0.64 nmol/L, compared with the 0.04 nmol/L seen with placebo (P less than .01).
Fezolinetant downregulates the activity of candy neurons in the hypothalamus, said Dr. Fraser in an interview. In turn, gonadotropin-releasing hormone (GNRH) pulse frequency is reduced, lowering luteinizing hormone (LH) levels. Since LH drives testosterone levels, this drops as well – a beneficial effect in PCOS, with its cardinal symptom of hyperandrogenism.
The double-blind, placebo-controlled study was conducted in Western Europe; 73 participants were randomized into three groups: 27 to a placebo group, 23 to a treatment group given 60 mg of fezolinetant once daily, and 23 to a treatment group given 180 mg of fezolinetant once daily. All groups received treatment for 12 weeks.
A total of 26 patients on placebo, 21 on 60-mg fezolinetant, and 17 on 180-mg fezolinetant completed the study. Patients who completed the study were included in the safety analysis, while all who took the study medication were included in the intent-to-treat analysis for primary and secondary outcome measures.
All participants had PCOS with hyperandrogenism, mean age was about 31 years, and about three quarters of enrollees were white, though local regulations restricted the collection of race and ethnicity data in some cases. One secondary outcome measure of the study was how fezolinetant affected the LH:FSH (follicle-stimulating hormone) ratio. “Patients with PCOS tend to have a very high GNRH [gonadotropin-releasing hormone] pulse frequency, which leads to a very high LH:FSH ratio,” said Dr. Fraser.
At baseline, the LH:FSH ratio was about 3. “With treatment, that ratio normalized to about 1, which is where it is in healthy women,” he said. The decrease in LH:FSH ratio occurred in a dose-dependent fashion and was statistically significant (P less than .001 for 180 mg fezolinetant versus placebo).
Dr. Fraser and his collaborators also tracked anti-müllerian hormone (AMH) levels, ovarian volume, and number of follicles. Although the investigators saw trends toward reduction in AMH levels and toward smaller ovarian volumes, these trends weren’t significant. “It was somewhat ambitious, I guess, to consider we’d hit these endpoints within 12 weeks,” he said. There were no serious drug-related adverse events.
“Most importantly, I would say, we did not get an increase in menses frequency, and, of course, that’s an important marker for fertility,” said Dr. Fraser. “There’s a debate in PCOS about whether this disease is driven by a malfunction in the brain or a malfunction in the ovaries. I guess on face value, perhaps this problem is in the ovaries.”
With the trends toward lower AMH and ovarian volumes, the research team is left wondering what would happen if the duration of therapy were extended. “Perhaps, the system would have been reset, and the frequency would have been restored. … So one thought that we have is to prolong the study in future trials and see if that could lead to an increase in fertility in PCOS,” said Dr. Fraser.
Fezolinetant is also being studied for menopause-related hot flashes in the United States and Europe.
Dr. Fraser is an employee of Ogeda, which sponsored the trial. Ogeda is a wholly owned subsidiary of Astelas.
SOURCE: Fraser G et al. ENDO 2018, Abstract SAT-305-LB.
CHICAGO – currently in development. The proof-of-concept phase 2 trial, which saw no concerning safety signals for the medication, fezolinetant, sets the stage for a larger, and perhaps longer, study to learn more about the neurokinin 3 receptor antagonist’s efficacy against PCOS.
“The primary outcome for this phase 2 trial was to see if we could lower testosterone levels in these PCOS patients,” said Graeme Fraser, PhD, chief scientific officer for Ogeda, discussing the poster he and his colleagues presented at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Testosterone levels decreased: Measured at about 3 hours postdose, which is the approximate pharmacokinetic Cmax [maximum serum concentration], there was … a more than 30% decrease in testosterone levels,” said Dr. Fraser. By week 12, he said, “there was a very consistent decrease of 30% of testosterone levels. So that was quite good; we successfully hit the primary outcome.”
At 12 weeks, the higher dose of fezolinetant decreased testosterone by 0.64 nmol/L, compared with the 0.04 nmol/L seen with placebo (P less than .01).
Fezolinetant downregulates the activity of candy neurons in the hypothalamus, said Dr. Fraser in an interview. In turn, gonadotropin-releasing hormone (GNRH) pulse frequency is reduced, lowering luteinizing hormone (LH) levels. Since LH drives testosterone levels, this drops as well – a beneficial effect in PCOS, with its cardinal symptom of hyperandrogenism.
The double-blind, placebo-controlled study was conducted in Western Europe; 73 participants were randomized into three groups: 27 to a placebo group, 23 to a treatment group given 60 mg of fezolinetant once daily, and 23 to a treatment group given 180 mg of fezolinetant once daily. All groups received treatment for 12 weeks.
A total of 26 patients on placebo, 21 on 60-mg fezolinetant, and 17 on 180-mg fezolinetant completed the study. Patients who completed the study were included in the safety analysis, while all who took the study medication were included in the intent-to-treat analysis for primary and secondary outcome measures.
All participants had PCOS with hyperandrogenism, mean age was about 31 years, and about three quarters of enrollees were white, though local regulations restricted the collection of race and ethnicity data in some cases. One secondary outcome measure of the study was how fezolinetant affected the LH:FSH (follicle-stimulating hormone) ratio. “Patients with PCOS tend to have a very high GNRH [gonadotropin-releasing hormone] pulse frequency, which leads to a very high LH:FSH ratio,” said Dr. Fraser.
At baseline, the LH:FSH ratio was about 3. “With treatment, that ratio normalized to about 1, which is where it is in healthy women,” he said. The decrease in LH:FSH ratio occurred in a dose-dependent fashion and was statistically significant (P less than .001 for 180 mg fezolinetant versus placebo).
Dr. Fraser and his collaborators also tracked anti-müllerian hormone (AMH) levels, ovarian volume, and number of follicles. Although the investigators saw trends toward reduction in AMH levels and toward smaller ovarian volumes, these trends weren’t significant. “It was somewhat ambitious, I guess, to consider we’d hit these endpoints within 12 weeks,” he said. There were no serious drug-related adverse events.
“Most importantly, I would say, we did not get an increase in menses frequency, and, of course, that’s an important marker for fertility,” said Dr. Fraser. “There’s a debate in PCOS about whether this disease is driven by a malfunction in the brain or a malfunction in the ovaries. I guess on face value, perhaps this problem is in the ovaries.”
With the trends toward lower AMH and ovarian volumes, the research team is left wondering what would happen if the duration of therapy were extended. “Perhaps, the system would have been reset, and the frequency would have been restored. … So one thought that we have is to prolong the study in future trials and see if that could lead to an increase in fertility in PCOS,” said Dr. Fraser.
Fezolinetant is also being studied for menopause-related hot flashes in the United States and Europe.
Dr. Fraser is an employee of Ogeda, which sponsored the trial. Ogeda is a wholly owned subsidiary of Astelas.
SOURCE: Fraser G et al. ENDO 2018, Abstract SAT-305-LB.
REPORTING FROM ENDO 2018
Match Day 2018: Ob.gyn. increases positions and matches
compared with 2017, according to the National Resident Matching Program (NRMP).
Ob.gyn. brought 1,336 first-year positions to the Match Day party this year and filled 78.7% of them with U.S. graduates. The overall fill rate of 99.6% is actually down from last year, when the specialty managed to fill all 1,288 positions offered. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
compared with 2017, according to the National Resident Matching Program (NRMP).
Ob.gyn. brought 1,336 first-year positions to the Match Day party this year and filled 78.7% of them with U.S. graduates. The overall fill rate of 99.6% is actually down from last year, when the specialty managed to fill all 1,288 positions offered. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
compared with 2017, according to the National Resident Matching Program (NRMP).
Ob.gyn. brought 1,336 first-year positions to the Match Day party this year and filled 78.7% of them with U.S. graduates. The overall fill rate of 99.6% is actually down from last year, when the specialty managed to fill all 1,288 positions offered. For all specialties, U.S. graduates filled 58.7% of the record-high 30,232 available spots, and the overall fill rate was 96.1%, the NRMP said in its 2018 Main Residency Match report.
Abdominal pain with high transaminases
A 54-year-old woman presents with severe abdominal pain lasting 3 hours. The pain came on suddenly and was 10/10 in severity. It was in her right upper quadrant radiating to her back. She has had a 50-pound weight loss in the past year. Her medications include sertraline, phentermine-topiramate, and simvastatin.
She is evaluated in the emergency department, and labs show the following: aspartate aminotransferase, 450; alanine aminotransferase, 500; alkaline phosphatase, 100; bilirubin, 1.2. She receives morphine for her pain with minimal relief. An ultrasound shows no gallstones and no dilated common bile duct (CBD).
Her pain resolves 3 hours after arriving in the ED. Repeat labs 15 minutes after pain resolution show the following: AST, 900; ALT, 1,000; alk phos, 130; bili, 1.2.
What is the most likely diagnosis?
A. Acetaminophen toxicity.
B. Hepatitis A.
C. Ischemic hepatitis.
D. Simvastatin.
E. Passage of gallstone.
The correct answer in this case is passage of a gallstone.
The patient has had weight loss, which increases the risk of gallstone formation, and the pain pattern is consistent with passage of a gallstone through the common bile duct.
I have seen a number of cases where the diagnosis was missed when the lab pattern is similar to the labs in this case. The high transaminases and the absence of significant alkaline phosphatase elevation can be confusing. We are taught in our medical training that alkaline phosphatase is a lab value that goes up with obstruction, and that transaminases are liver injury labs. What are the data on liver labs in the setting of acute obstruction as seen with the passage of a gallstone?
Frederick Kiechle, MD, and colleagues reported that alkaline phosphatase levels, either alone or in conjunction with bilirubin levels, were not useful in determining the presence of common bile duct stones.1 Ming-Hsun Yang et al. found that normal gamma-glutamyl transferase results had the highest negative predictive value for the presence of a common bile duct stone (97%).2 The sensitivity for ultrasound detection of CBD stone in this study was only 35%.
Keun Soo Ahn and colleagues found that, in patients with symptomatic CBD stones, the average AST was 275, and the average ALT was 317 – about six to seven times the upper limit of normal for these lab tests.3 In the same study, the average alkaline phosphatase was 213, which is about twice the upper limit of normal.
Sometimes, extremely high transaminase elevations can occur with choledocholithiasis. Saroja Bangaru et al. reported on a case series of patients who all had transaminase values greater than 1,000 with symptomatic choledocholithiasis.4 All of the patients had normal or just mildly elevated alkaline phosphatase levels.
Rahul Nathwani, MD, and colleagues also reported on a series of 16 patients with choledocholithiasis and transaminase levels greater than 1,000.5 All patients were symptomatic, and the average alkaline phosphatase levels were 2.5 times the upper limit of normal.
Ala Sharara, MD, et al. looked at 40 patients in a retrospective study of patients found to have choledocholithiasis who presented within 12 hours of pain onset.6 Levels of AST and ALT both significantly correlated with duration of pain (P less than .001), whereas there was no significant correlation with alkaline phosphatase and bilirubin levels.
Pearl: AST and ALT elevations in patients with acute abdominal pain could be due to choledocholithiasis, even if there are minimal or no abnormalities in alkaline phosphatase. Marked elevations (greater than 1,000) can occur.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Emerg Med. 1985 Nov;3(6):556-60.
2. Surg Endosc. 2008 Jul;22(7):1620-4.
3. World J Surg. 2016 Aug;40(8):1925-31.
4. J Clin Gastroenterol. 2017 Sep;51(8):728-33.
5. Am J Gastroenterol. 2005 Feb;100(2):295-8.
6. Clin Gastroenterol Hepatol. 2010 Dec;8(12):1077-82.
A 54-year-old woman presents with severe abdominal pain lasting 3 hours. The pain came on suddenly and was 10/10 in severity. It was in her right upper quadrant radiating to her back. She has had a 50-pound weight loss in the past year. Her medications include sertraline, phentermine-topiramate, and simvastatin.
She is evaluated in the emergency department, and labs show the following: aspartate aminotransferase, 450; alanine aminotransferase, 500; alkaline phosphatase, 100; bilirubin, 1.2. She receives morphine for her pain with minimal relief. An ultrasound shows no gallstones and no dilated common bile duct (CBD).
Her pain resolves 3 hours after arriving in the ED. Repeat labs 15 minutes after pain resolution show the following: AST, 900; ALT, 1,000; alk phos, 130; bili, 1.2.
What is the most likely diagnosis?
A. Acetaminophen toxicity.
B. Hepatitis A.
C. Ischemic hepatitis.
D. Simvastatin.
E. Passage of gallstone.
The correct answer in this case is passage of a gallstone.
The patient has had weight loss, which increases the risk of gallstone formation, and the pain pattern is consistent with passage of a gallstone through the common bile duct.
I have seen a number of cases where the diagnosis was missed when the lab pattern is similar to the labs in this case. The high transaminases and the absence of significant alkaline phosphatase elevation can be confusing. We are taught in our medical training that alkaline phosphatase is a lab value that goes up with obstruction, and that transaminases are liver injury labs. What are the data on liver labs in the setting of acute obstruction as seen with the passage of a gallstone?
Frederick Kiechle, MD, and colleagues reported that alkaline phosphatase levels, either alone or in conjunction with bilirubin levels, were not useful in determining the presence of common bile duct stones.1 Ming-Hsun Yang et al. found that normal gamma-glutamyl transferase results had the highest negative predictive value for the presence of a common bile duct stone (97%).2 The sensitivity for ultrasound detection of CBD stone in this study was only 35%.
Keun Soo Ahn and colleagues found that, in patients with symptomatic CBD stones, the average AST was 275, and the average ALT was 317 – about six to seven times the upper limit of normal for these lab tests.3 In the same study, the average alkaline phosphatase was 213, which is about twice the upper limit of normal.
Sometimes, extremely high transaminase elevations can occur with choledocholithiasis. Saroja Bangaru et al. reported on a case series of patients who all had transaminase values greater than 1,000 with symptomatic choledocholithiasis.4 All of the patients had normal or just mildly elevated alkaline phosphatase levels.
Rahul Nathwani, MD, and colleagues also reported on a series of 16 patients with choledocholithiasis and transaminase levels greater than 1,000.5 All patients were symptomatic, and the average alkaline phosphatase levels were 2.5 times the upper limit of normal.
Ala Sharara, MD, et al. looked at 40 patients in a retrospective study of patients found to have choledocholithiasis who presented within 12 hours of pain onset.6 Levels of AST and ALT both significantly correlated with duration of pain (P less than .001), whereas there was no significant correlation with alkaline phosphatase and bilirubin levels.
Pearl: AST and ALT elevations in patients with acute abdominal pain could be due to choledocholithiasis, even if there are minimal or no abnormalities in alkaline phosphatase. Marked elevations (greater than 1,000) can occur.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Emerg Med. 1985 Nov;3(6):556-60.
2. Surg Endosc. 2008 Jul;22(7):1620-4.
3. World J Surg. 2016 Aug;40(8):1925-31.
4. J Clin Gastroenterol. 2017 Sep;51(8):728-33.
5. Am J Gastroenterol. 2005 Feb;100(2):295-8.
6. Clin Gastroenterol Hepatol. 2010 Dec;8(12):1077-82.
A 54-year-old woman presents with severe abdominal pain lasting 3 hours. The pain came on suddenly and was 10/10 in severity. It was in her right upper quadrant radiating to her back. She has had a 50-pound weight loss in the past year. Her medications include sertraline, phentermine-topiramate, and simvastatin.
She is evaluated in the emergency department, and labs show the following: aspartate aminotransferase, 450; alanine aminotransferase, 500; alkaline phosphatase, 100; bilirubin, 1.2. She receives morphine for her pain with minimal relief. An ultrasound shows no gallstones and no dilated common bile duct (CBD).
Her pain resolves 3 hours after arriving in the ED. Repeat labs 15 minutes after pain resolution show the following: AST, 900; ALT, 1,000; alk phos, 130; bili, 1.2.
What is the most likely diagnosis?
A. Acetaminophen toxicity.
B. Hepatitis A.
C. Ischemic hepatitis.
D. Simvastatin.
E. Passage of gallstone.
The correct answer in this case is passage of a gallstone.
The patient has had weight loss, which increases the risk of gallstone formation, and the pain pattern is consistent with passage of a gallstone through the common bile duct.
I have seen a number of cases where the diagnosis was missed when the lab pattern is similar to the labs in this case. The high transaminases and the absence of significant alkaline phosphatase elevation can be confusing. We are taught in our medical training that alkaline phosphatase is a lab value that goes up with obstruction, and that transaminases are liver injury labs. What are the data on liver labs in the setting of acute obstruction as seen with the passage of a gallstone?
Frederick Kiechle, MD, and colleagues reported that alkaline phosphatase levels, either alone or in conjunction with bilirubin levels, were not useful in determining the presence of common bile duct stones.1 Ming-Hsun Yang et al. found that normal gamma-glutamyl transferase results had the highest negative predictive value for the presence of a common bile duct stone (97%).2 The sensitivity for ultrasound detection of CBD stone in this study was only 35%.
Keun Soo Ahn and colleagues found that, in patients with symptomatic CBD stones, the average AST was 275, and the average ALT was 317 – about six to seven times the upper limit of normal for these lab tests.3 In the same study, the average alkaline phosphatase was 213, which is about twice the upper limit of normal.
Sometimes, extremely high transaminase elevations can occur with choledocholithiasis. Saroja Bangaru et al. reported on a case series of patients who all had transaminase values greater than 1,000 with symptomatic choledocholithiasis.4 All of the patients had normal or just mildly elevated alkaline phosphatase levels.
Rahul Nathwani, MD, and colleagues also reported on a series of 16 patients with choledocholithiasis and transaminase levels greater than 1,000.5 All patients were symptomatic, and the average alkaline phosphatase levels were 2.5 times the upper limit of normal.
Ala Sharara, MD, et al. looked at 40 patients in a retrospective study of patients found to have choledocholithiasis who presented within 12 hours of pain onset.6 Levels of AST and ALT both significantly correlated with duration of pain (P less than .001), whereas there was no significant correlation with alkaline phosphatase and bilirubin levels.
Pearl: AST and ALT elevations in patients with acute abdominal pain could be due to choledocholithiasis, even if there are minimal or no abnormalities in alkaline phosphatase. Marked elevations (greater than 1,000) can occur.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Emerg Med. 1985 Nov;3(6):556-60.
2. Surg Endosc. 2008 Jul;22(7):1620-4.
3. World J Surg. 2016 Aug;40(8):1925-31.
4. J Clin Gastroenterol. 2017 Sep;51(8):728-33.
5. Am J Gastroenterol. 2005 Feb;100(2):295-8.
6. Clin Gastroenterol Hepatol. 2010 Dec;8(12):1077-82.
New tool improves hand-off communications
Transitions of care can be rife with communications issues – and subsequent adverse events. They are also a place where hospitalists can take the lead in making improvements.
“They are the team leaders, typically,” said Ana Pujols McKee, MD, the executive vice president and chief medical officer for The Joint Commission. “The hospitalist really owns this process of the transfer of this accurate information.”
To help, The Joint Commission has issued a new Sentinel Event Alert, which provides seven recommendations to improve the communication failures that can occur when patients are transitioned from one caregiver to another, as well as a Targeted Solutions Tool to put the recommendations into action.
“Every organization is challenged in communicating accurate and timely information regarding patients,” Dr. McKee said. “One of the riskiest transitions that patients go through is when they change levels of care from ICU to med-surg, or from the ER to ICU, OR to ICU, med-surg to home, and home to home care. All of those transitions inherently carry a certain amount of risk and are deeply reliant on the transfer of the right information at the right time to the right person.”
These resources reflect what The Joint Commission has found: “The knowledge that we now have is that one of the defects that occurs in this transitioning is that – I’ll speak of sender and receiver – the information that is sent is always sent from the perspective of what the sender thinks is important, not the information the receiver needs to manage that patient safely.”
The tool uses the principles of Lean Six Sigma and change management, and organizations can use it to identify their opportunities for improvement and develop strategies to address their specific root causes in their organization.
“It’s a self-guided tool,” Dr. McKee said. “Organizations have reduced errors significantly in using this tool. I think if the hospitalist community takes this on, that would really help transform how we do transitions of care.”
Transitions of care can be rife with communications issues – and subsequent adverse events. They are also a place where hospitalists can take the lead in making improvements.
“They are the team leaders, typically,” said Ana Pujols McKee, MD, the executive vice president and chief medical officer for The Joint Commission. “The hospitalist really owns this process of the transfer of this accurate information.”
To help, The Joint Commission has issued a new Sentinel Event Alert, which provides seven recommendations to improve the communication failures that can occur when patients are transitioned from one caregiver to another, as well as a Targeted Solutions Tool to put the recommendations into action.
“Every organization is challenged in communicating accurate and timely information regarding patients,” Dr. McKee said. “One of the riskiest transitions that patients go through is when they change levels of care from ICU to med-surg, or from the ER to ICU, OR to ICU, med-surg to home, and home to home care. All of those transitions inherently carry a certain amount of risk and are deeply reliant on the transfer of the right information at the right time to the right person.”
These resources reflect what The Joint Commission has found: “The knowledge that we now have is that one of the defects that occurs in this transitioning is that – I’ll speak of sender and receiver – the information that is sent is always sent from the perspective of what the sender thinks is important, not the information the receiver needs to manage that patient safely.”
The tool uses the principles of Lean Six Sigma and change management, and organizations can use it to identify their opportunities for improvement and develop strategies to address their specific root causes in their organization.
“It’s a self-guided tool,” Dr. McKee said. “Organizations have reduced errors significantly in using this tool. I think if the hospitalist community takes this on, that would really help transform how we do transitions of care.”
Transitions of care can be rife with communications issues – and subsequent adverse events. They are also a place where hospitalists can take the lead in making improvements.
“They are the team leaders, typically,” said Ana Pujols McKee, MD, the executive vice president and chief medical officer for The Joint Commission. “The hospitalist really owns this process of the transfer of this accurate information.”
To help, The Joint Commission has issued a new Sentinel Event Alert, which provides seven recommendations to improve the communication failures that can occur when patients are transitioned from one caregiver to another, as well as a Targeted Solutions Tool to put the recommendations into action.
“Every organization is challenged in communicating accurate and timely information regarding patients,” Dr. McKee said. “One of the riskiest transitions that patients go through is when they change levels of care from ICU to med-surg, or from the ER to ICU, OR to ICU, med-surg to home, and home to home care. All of those transitions inherently carry a certain amount of risk and are deeply reliant on the transfer of the right information at the right time to the right person.”
These resources reflect what The Joint Commission has found: “The knowledge that we now have is that one of the defects that occurs in this transitioning is that – I’ll speak of sender and receiver – the information that is sent is always sent from the perspective of what the sender thinks is important, not the information the receiver needs to manage that patient safely.”
The tool uses the principles of Lean Six Sigma and change management, and organizations can use it to identify their opportunities for improvement and develop strategies to address their specific root causes in their organization.
“It’s a self-guided tool,” Dr. McKee said. “Organizations have reduced errors significantly in using this tool. I think if the hospitalist community takes this on, that would really help transform how we do transitions of care.”
Popular vaginal dryness products don’t beat placebos
based on data from a randomized trial of more than 300 patients suffering from genitourinary syndrome of menopause (GSM), a constellation of symptoms including pain on vaginal penetration and vaginal dryness.
“Surveys of postmenopausal women demonstrate a preference for effective, nonhormonal therapies, often due to safety concerns,” wrote Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston, and her colleagues. The report was published in JAMA Internal Medicine. The researchers randomized 302 postmenopausal women with GSM 1:1:1 to a Vagifem 10-microgram estradiol tablet and placebo gel, a placebo tablet and Replens gel, or a placebo tablet and a placebo gel.
The average age of the women was 61 years, 88% were white, and 81% were sexually active.
The primary outcome was a decrease in the most bothersome symptoms reported by the women after 12 weeks of treatment. The most common of these were pain on penetration (60%) and vulvovaginal dryness (21%).
After 12 weeks, the women reported no significant difference in most bothersome symptoms between estradiol or moisturizing gel, compared with placebo products (P = .25 and P = .31, respectively). The average improvement in symptom scores was similar between the estradiol tablet and placebo tablet (P = .64) and between the moisturizer and placebo gels (P = .17).
The study was limited by several factors including the homogenous population and the absence of a head-to-head comparison of treatments, the researchers noted. However, the results suggest that more research is needed about genitourinary syndrome of menopause, but that a nonprescription lubricating gel may be an appropriate estrogen-free choice, and that “treatment choice should be based on individual patient preferences regarding cost and formulation,” they said.
The study was funded by the National Institutes of Health/National Institute on Aging. Dr. Mitchell is a consultant for Symbiomix Therapeutics, and coauthors reported grant support from Bayer and having served on a scientific advisory board for Sermonix.
SOURCE: Mitchell C et al. JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0116.
The double-negative finding of the study suggests a potential change in clinical practice as to the value of estrogen for postmenopausal women, Alison J. Huang, MD, and Deborah Grady, MD, wrote in an editorial.
“Based on the results of this study, women and their physicians may want to take this one step further and conclude that postmenopausal women experiencing vulvovaginal symptoms should choose the cheapest moisturizer or lubricant available over the counter – at least until new evidence arises to suggest that there is any benefit to doing otherwise,” they said. The study compared popular active treatments – an estradiol tablet and a nonhormonal moisturizing gel – with placebo and not with each other, which could be considered a limitation, they said. However, the similar effectiveness of the treatments to placebo support a choice of treatments for vulvovaginal symptoms based on cost and patient preference for a particular formulation, they noted (JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0094).
Dr. Huang and Dr. Grady are affiliated with the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System. Dr. Huang disclosed research grants from Pfizer and Astellas Pharma. Dr. Grady has served as a consultant to MenoGeniX.
The double-negative finding of the study suggests a potential change in clinical practice as to the value of estrogen for postmenopausal women, Alison J. Huang, MD, and Deborah Grady, MD, wrote in an editorial.
“Based on the results of this study, women and their physicians may want to take this one step further and conclude that postmenopausal women experiencing vulvovaginal symptoms should choose the cheapest moisturizer or lubricant available over the counter – at least until new evidence arises to suggest that there is any benefit to doing otherwise,” they said. The study compared popular active treatments – an estradiol tablet and a nonhormonal moisturizing gel – with placebo and not with each other, which could be considered a limitation, they said. However, the similar effectiveness of the treatments to placebo support a choice of treatments for vulvovaginal symptoms based on cost and patient preference for a particular formulation, they noted (JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0094).
Dr. Huang and Dr. Grady are affiliated with the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System. Dr. Huang disclosed research grants from Pfizer and Astellas Pharma. Dr. Grady has served as a consultant to MenoGeniX.
The double-negative finding of the study suggests a potential change in clinical practice as to the value of estrogen for postmenopausal women, Alison J. Huang, MD, and Deborah Grady, MD, wrote in an editorial.
“Based on the results of this study, women and their physicians may want to take this one step further and conclude that postmenopausal women experiencing vulvovaginal symptoms should choose the cheapest moisturizer or lubricant available over the counter – at least until new evidence arises to suggest that there is any benefit to doing otherwise,” they said. The study compared popular active treatments – an estradiol tablet and a nonhormonal moisturizing gel – with placebo and not with each other, which could be considered a limitation, they said. However, the similar effectiveness of the treatments to placebo support a choice of treatments for vulvovaginal symptoms based on cost and patient preference for a particular formulation, they noted (JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0094).
Dr. Huang and Dr. Grady are affiliated with the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System. Dr. Huang disclosed research grants from Pfizer and Astellas Pharma. Dr. Grady has served as a consultant to MenoGeniX.
based on data from a randomized trial of more than 300 patients suffering from genitourinary syndrome of menopause (GSM), a constellation of symptoms including pain on vaginal penetration and vaginal dryness.
“Surveys of postmenopausal women demonstrate a preference for effective, nonhormonal therapies, often due to safety concerns,” wrote Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston, and her colleagues. The report was published in JAMA Internal Medicine. The researchers randomized 302 postmenopausal women with GSM 1:1:1 to a Vagifem 10-microgram estradiol tablet and placebo gel, a placebo tablet and Replens gel, or a placebo tablet and a placebo gel.
The average age of the women was 61 years, 88% were white, and 81% were sexually active.
The primary outcome was a decrease in the most bothersome symptoms reported by the women after 12 weeks of treatment. The most common of these were pain on penetration (60%) and vulvovaginal dryness (21%).
After 12 weeks, the women reported no significant difference in most bothersome symptoms between estradiol or moisturizing gel, compared with placebo products (P = .25 and P = .31, respectively). The average improvement in symptom scores was similar between the estradiol tablet and placebo tablet (P = .64) and between the moisturizer and placebo gels (P = .17).
The study was limited by several factors including the homogenous population and the absence of a head-to-head comparison of treatments, the researchers noted. However, the results suggest that more research is needed about genitourinary syndrome of menopause, but that a nonprescription lubricating gel may be an appropriate estrogen-free choice, and that “treatment choice should be based on individual patient preferences regarding cost and formulation,” they said.
The study was funded by the National Institutes of Health/National Institute on Aging. Dr. Mitchell is a consultant for Symbiomix Therapeutics, and coauthors reported grant support from Bayer and having served on a scientific advisory board for Sermonix.
SOURCE: Mitchell C et al. JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0116.
based on data from a randomized trial of more than 300 patients suffering from genitourinary syndrome of menopause (GSM), a constellation of symptoms including pain on vaginal penetration and vaginal dryness.
“Surveys of postmenopausal women demonstrate a preference for effective, nonhormonal therapies, often due to safety concerns,” wrote Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston, and her colleagues. The report was published in JAMA Internal Medicine. The researchers randomized 302 postmenopausal women with GSM 1:1:1 to a Vagifem 10-microgram estradiol tablet and placebo gel, a placebo tablet and Replens gel, or a placebo tablet and a placebo gel.
The average age of the women was 61 years, 88% were white, and 81% were sexually active.
The primary outcome was a decrease in the most bothersome symptoms reported by the women after 12 weeks of treatment. The most common of these were pain on penetration (60%) and vulvovaginal dryness (21%).
After 12 weeks, the women reported no significant difference in most bothersome symptoms between estradiol or moisturizing gel, compared with placebo products (P = .25 and P = .31, respectively). The average improvement in symptom scores was similar between the estradiol tablet and placebo tablet (P = .64) and between the moisturizer and placebo gels (P = .17).
The study was limited by several factors including the homogenous population and the absence of a head-to-head comparison of treatments, the researchers noted. However, the results suggest that more research is needed about genitourinary syndrome of menopause, but that a nonprescription lubricating gel may be an appropriate estrogen-free choice, and that “treatment choice should be based on individual patient preferences regarding cost and formulation,” they said.
The study was funded by the National Institutes of Health/National Institute on Aging. Dr. Mitchell is a consultant for Symbiomix Therapeutics, and coauthors reported grant support from Bayer and having served on a scientific advisory board for Sermonix.
SOURCE: Mitchell C et al. JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0116.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Estradiol tablets had no increased benefit, compared with placebo, for relieving postmenopausal vulvovaginal symptoms.
Major finding: The improvement in vaginal discomfort after 12 weeks was not significantly different between an estradiol tablet and placebo (P = .64) or between a popular vaginal moisturizer and placebo (P = .17).
Study details: The data come from a randomized, clinical trial of 302 postmenopausal women.
Disclosures: The study was funded by the National Institutes of Health/National Institute on Aging. Dr. Mitchell is a consultant for Symbiomix Therapeutics, and coauthors reported grant support from Bayer and having served on a scientific advisory board for Sermonix.
Source: Mitchell C et al. JAMA Intern Med. 2018 Mar. doi: 10.1001/jamainternmed.2018.0116.
How the ADA shapes health care
Question: After many years of diabetes, a 60-year-old office worker develops nephropathy followed by end-stage renal disease, and now requires dialysis. He has opted for peritoneal dialysis rather than hemodialysis, so that he does not have to be away from the workplace for treatment. His diabetes is insulin requiring, and he has occasional hypoglycemic reactions. Although he qualifies for Social Security disability benefits, he prefers to continue working full time. The employer is considering terminating him.
Which of the following is best?
A. The Americans with Disabilities Act prohibits job discrimination against patients with disabilities, so long as they are otherwise qualified for every aspect of the job.
B. Renal insufficiency and diabetes are considered disabilities under the ADA.
C. The employer is obligated to provide full accommodation to enable this employee to continue working.
D. If the accommodations needed for a disabled person are unreasonable, or prove too disruptive or expensive, then the employer is not obligated to provide them.
E. This patient should simply retire and enjoy his SS disability benefits.
Answer: D. Enacted in 1990, the Americans with Disabilities Act seeks to provide clear, strong, consistent, and enforceable standards for ending discrimination against individuals with disabilities.1 The main thrust of the ADA, Title I, is to protect otherwise qualified workers with permanent disabilities from losing their jobs or seeking one, so long as they are qualified to perform the essential (not necessarily all) functions of the job.
In addition, the law prohibits discrimination against people with disabilities from accessing public accommodations (Title III), which include doctors’ offices and health care facilities, as well as restaurants, retail stores, etc. Other areas under the purview of the omnibus ADA include transportation, communications, and access to state and local government programs and services.
The Equal Employment Opportunity Commission (EEOC) enforces Title I of the ADA, the section that deals with job discrimination. Its compliance manual sets out guidelines for determining whether an individual in fact has a disability.
The word “disability” has three components, and the term is not synonymous with “impairment.” However, a disability begins with having an impairment, defined as a physiological disorder affecting one or more of a number of body systems or a mental or psychological disorder.
An example given by the EEOC: If a person cannot find a job because that person has the equivalent of a second-grade education and therefore cannot read, that person does not have an impairment for purposes of the ADA. If, however, that person cannot read because of severe dyslexia, that person has an impairment. Likewise, being overweight is not considered an impairment (unless due to an underlying physical condition, e.g., hypothyroidism), although extreme obesity in excess of 100% ideal body weight is.
Having determined that an impairment exists, the next step in the analysis is to ascertain if the impairment limits one or more “major life activities.” These have classically included activities such as caring for oneself, performing manual tasks, walking, seeing, hearing, speaking, and breathing.
Third, the limitation must be substantial, meaning sufficiently severe, compared with what an average person is capable of doing. According to the EEOC, a mild type 2 diabetes patient on diet treatment alone and no other restriction has an impairment; but the impairment does not substantially limit any of his major life activities. On the other hand, some impairments are so severe that there is no doubt they substantially limit major life activities, e.g., insulin-dependent diabetes, legal blindness, deafness, manic-depressive syndrome, alcoholism, and HIV infection.
There is litigation aplenty over these issues.
In its seminal 1988 case, the U.S. Supreme Court provided the analytical steps listed above in arriving at its holding that, under the ADA, asymptomatic HIV infection is a disability.2 The case involved a dentist who was sued when he declined to treat an HIV-positive female patient in the office, offering instead to treat her in a hospital without any additional charge. A dental office, like a doctor’s office, is recognized as a place of public accommodations, and therefore falls under the protection of Title III of the ADA.
The court first considered whether HIV infection was a physical impairment. Second, it identified the major life activity upon which the plaintiff relied (reproduction and childbearing) and determined whether it constituted a major life activity under the ADA. Third, it tied the two statutory phrases together, and asked whether the impairment substantially limited these major life activities.
The court held that, in light of the immediacy with which the HIV virus begins to damage the infected person’s white blood cells and the severity of the disease, it is an impairment from the moment of infection, even if the patient was asymptomatic. It also ruled that the HIV infection substantially limited her ability to reproduce in two independent ways. First, a woman infected with HIV who tries to conceive a child imposes on the man a significant risk of becoming infected, and second, an infected woman risks infecting her child during gestation and childbirth, i.e., perinatal transmission.
In 2004, a case reached the U.S. Third Circuit Court of Appeals regarding Cathy Fiscus, an employee at a Walmart Sam’s Club warehouse store in Pittsburgh, who faced being terminated after 12 years at her job. A lower U.S. district court had ruled in favor of the company, agreeing with Walmart that the woman’s end-stage renal disease had not left her significantly limited in a major life activity. Ms. Fiscus sought a reasonable accommodation from her employer during the period of her peritoneal dialysis, which required her to self administer the 45-minute dialysis process at the workplace. Walmart initially agreed, but later declined. The appeals court overturned the lower court’s ruling, writing, “A physical impairment that limits an individual’s ability to cleanse and eliminate body waste does impair a major life activity.”3
Not all conditions are covered by the ADA’s definition of disability. The list includes temporary physical or mental impairments, current illegal drug use, predisposition to illness, personality traits, advanced age, and pregnancy, to name a few.
To avoid running afoul of the ADA, an employer is required to make “reasonable accommodations” for the disabled employee. This refers to practices that allow a disabled person to perform the essential functions of the job.
Examples of reasonable accommodations include making existing facilities readily accessible to and usable by individuals with disabilities, restructuring jobs, modifying work schedules, and providing qualified readers or interpreters.
A “qualified individual with a disability” is an individual with a disability who, “with or without reasonable accommodation,” can perform the essential functions of the employment position in question. A person is not a qualified individual with a disability, however, if he or she cannot satisfy the basic attendance requirements of a position.
Employers are not required to offer any and all accommodations, such as those that are disruptive to the business, overly burdensome, or prohibitively expensive. Providing a clean and private area in the workplace for self-administered peritoneal dialysis fluid exchange would likely qualify as a reasonable accommodation that should be offered, absent some compelling reason not to.
The protection given by the ADA may be suspended if the condition poses a direct threat, defined as “a significant risk to the health or safety of others that cannot be eliminated by a modification of policies, practices, or procedures, or by the provision of auxiliary aids or services.”4 The U.S. Supreme Court has noted that this should be assessed by the objective reasonableness of the views of health care professionals.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Americans with Disabilities Act of 1990 (ADA), 104 Stat. 327, 42 U. S. C. § 12101 et seq.
2. Bragdon v. Abbott et al. 524 U.S. 624 (1998).
3. Cathy A. Fiscus v. Wal-Mart Stores Inc., 385 F.3d 378 (3d Cir. 2004).
4. 42 U. S. C. § 12182(b)(3).
5. Available at www.kidney.org/atoz/content/employersguide.
Question: After many years of diabetes, a 60-year-old office worker develops nephropathy followed by end-stage renal disease, and now requires dialysis. He has opted for peritoneal dialysis rather than hemodialysis, so that he does not have to be away from the workplace for treatment. His diabetes is insulin requiring, and he has occasional hypoglycemic reactions. Although he qualifies for Social Security disability benefits, he prefers to continue working full time. The employer is considering terminating him.
Which of the following is best?
A. The Americans with Disabilities Act prohibits job discrimination against patients with disabilities, so long as they are otherwise qualified for every aspect of the job.
B. Renal insufficiency and diabetes are considered disabilities under the ADA.
C. The employer is obligated to provide full accommodation to enable this employee to continue working.
D. If the accommodations needed for a disabled person are unreasonable, or prove too disruptive or expensive, then the employer is not obligated to provide them.
E. This patient should simply retire and enjoy his SS disability benefits.
Answer: D. Enacted in 1990, the Americans with Disabilities Act seeks to provide clear, strong, consistent, and enforceable standards for ending discrimination against individuals with disabilities.1 The main thrust of the ADA, Title I, is to protect otherwise qualified workers with permanent disabilities from losing their jobs or seeking one, so long as they are qualified to perform the essential (not necessarily all) functions of the job.
In addition, the law prohibits discrimination against people with disabilities from accessing public accommodations (Title III), which include doctors’ offices and health care facilities, as well as restaurants, retail stores, etc. Other areas under the purview of the omnibus ADA include transportation, communications, and access to state and local government programs and services.
The Equal Employment Opportunity Commission (EEOC) enforces Title I of the ADA, the section that deals with job discrimination. Its compliance manual sets out guidelines for determining whether an individual in fact has a disability.
The word “disability” has three components, and the term is not synonymous with “impairment.” However, a disability begins with having an impairment, defined as a physiological disorder affecting one or more of a number of body systems or a mental or psychological disorder.
An example given by the EEOC: If a person cannot find a job because that person has the equivalent of a second-grade education and therefore cannot read, that person does not have an impairment for purposes of the ADA. If, however, that person cannot read because of severe dyslexia, that person has an impairment. Likewise, being overweight is not considered an impairment (unless due to an underlying physical condition, e.g., hypothyroidism), although extreme obesity in excess of 100% ideal body weight is.
Having determined that an impairment exists, the next step in the analysis is to ascertain if the impairment limits one or more “major life activities.” These have classically included activities such as caring for oneself, performing manual tasks, walking, seeing, hearing, speaking, and breathing.
Third, the limitation must be substantial, meaning sufficiently severe, compared with what an average person is capable of doing. According to the EEOC, a mild type 2 diabetes patient on diet treatment alone and no other restriction has an impairment; but the impairment does not substantially limit any of his major life activities. On the other hand, some impairments are so severe that there is no doubt they substantially limit major life activities, e.g., insulin-dependent diabetes, legal blindness, deafness, manic-depressive syndrome, alcoholism, and HIV infection.
There is litigation aplenty over these issues.
In its seminal 1988 case, the U.S. Supreme Court provided the analytical steps listed above in arriving at its holding that, under the ADA, asymptomatic HIV infection is a disability.2 The case involved a dentist who was sued when he declined to treat an HIV-positive female patient in the office, offering instead to treat her in a hospital without any additional charge. A dental office, like a doctor’s office, is recognized as a place of public accommodations, and therefore falls under the protection of Title III of the ADA.
The court first considered whether HIV infection was a physical impairment. Second, it identified the major life activity upon which the plaintiff relied (reproduction and childbearing) and determined whether it constituted a major life activity under the ADA. Third, it tied the two statutory phrases together, and asked whether the impairment substantially limited these major life activities.
The court held that, in light of the immediacy with which the HIV virus begins to damage the infected person’s white blood cells and the severity of the disease, it is an impairment from the moment of infection, even if the patient was asymptomatic. It also ruled that the HIV infection substantially limited her ability to reproduce in two independent ways. First, a woman infected with HIV who tries to conceive a child imposes on the man a significant risk of becoming infected, and second, an infected woman risks infecting her child during gestation and childbirth, i.e., perinatal transmission.
In 2004, a case reached the U.S. Third Circuit Court of Appeals regarding Cathy Fiscus, an employee at a Walmart Sam’s Club warehouse store in Pittsburgh, who faced being terminated after 12 years at her job. A lower U.S. district court had ruled in favor of the company, agreeing with Walmart that the woman’s end-stage renal disease had not left her significantly limited in a major life activity. Ms. Fiscus sought a reasonable accommodation from her employer during the period of her peritoneal dialysis, which required her to self administer the 45-minute dialysis process at the workplace. Walmart initially agreed, but later declined. The appeals court overturned the lower court’s ruling, writing, “A physical impairment that limits an individual’s ability to cleanse and eliminate body waste does impair a major life activity.”3
Not all conditions are covered by the ADA’s definition of disability. The list includes temporary physical or mental impairments, current illegal drug use, predisposition to illness, personality traits, advanced age, and pregnancy, to name a few.
To avoid running afoul of the ADA, an employer is required to make “reasonable accommodations” for the disabled employee. This refers to practices that allow a disabled person to perform the essential functions of the job.
Examples of reasonable accommodations include making existing facilities readily accessible to and usable by individuals with disabilities, restructuring jobs, modifying work schedules, and providing qualified readers or interpreters.
A “qualified individual with a disability” is an individual with a disability who, “with or without reasonable accommodation,” can perform the essential functions of the employment position in question. A person is not a qualified individual with a disability, however, if he or she cannot satisfy the basic attendance requirements of a position.
Employers are not required to offer any and all accommodations, such as those that are disruptive to the business, overly burdensome, or prohibitively expensive. Providing a clean and private area in the workplace for self-administered peritoneal dialysis fluid exchange would likely qualify as a reasonable accommodation that should be offered, absent some compelling reason not to.
The protection given by the ADA may be suspended if the condition poses a direct threat, defined as “a significant risk to the health or safety of others that cannot be eliminated by a modification of policies, practices, or procedures, or by the provision of auxiliary aids or services.”4 The U.S. Supreme Court has noted that this should be assessed by the objective reasonableness of the views of health care professionals.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Americans with Disabilities Act of 1990 (ADA), 104 Stat. 327, 42 U. S. C. § 12101 et seq.
2. Bragdon v. Abbott et al. 524 U.S. 624 (1998).
3. Cathy A. Fiscus v. Wal-Mart Stores Inc., 385 F.3d 378 (3d Cir. 2004).
4. 42 U. S. C. § 12182(b)(3).
5. Available at www.kidney.org/atoz/content/employersguide.
Question: After many years of diabetes, a 60-year-old office worker develops nephropathy followed by end-stage renal disease, and now requires dialysis. He has opted for peritoneal dialysis rather than hemodialysis, so that he does not have to be away from the workplace for treatment. His diabetes is insulin requiring, and he has occasional hypoglycemic reactions. Although he qualifies for Social Security disability benefits, he prefers to continue working full time. The employer is considering terminating him.
Which of the following is best?
A. The Americans with Disabilities Act prohibits job discrimination against patients with disabilities, so long as they are otherwise qualified for every aspect of the job.
B. Renal insufficiency and diabetes are considered disabilities under the ADA.
C. The employer is obligated to provide full accommodation to enable this employee to continue working.
D. If the accommodations needed for a disabled person are unreasonable, or prove too disruptive or expensive, then the employer is not obligated to provide them.
E. This patient should simply retire and enjoy his SS disability benefits.
Answer: D. Enacted in 1990, the Americans with Disabilities Act seeks to provide clear, strong, consistent, and enforceable standards for ending discrimination against individuals with disabilities.1 The main thrust of the ADA, Title I, is to protect otherwise qualified workers with permanent disabilities from losing their jobs or seeking one, so long as they are qualified to perform the essential (not necessarily all) functions of the job.
In addition, the law prohibits discrimination against people with disabilities from accessing public accommodations (Title III), which include doctors’ offices and health care facilities, as well as restaurants, retail stores, etc. Other areas under the purview of the omnibus ADA include transportation, communications, and access to state and local government programs and services.
The Equal Employment Opportunity Commission (EEOC) enforces Title I of the ADA, the section that deals with job discrimination. Its compliance manual sets out guidelines for determining whether an individual in fact has a disability.
The word “disability” has three components, and the term is not synonymous with “impairment.” However, a disability begins with having an impairment, defined as a physiological disorder affecting one or more of a number of body systems or a mental or psychological disorder.
An example given by the EEOC: If a person cannot find a job because that person has the equivalent of a second-grade education and therefore cannot read, that person does not have an impairment for purposes of the ADA. If, however, that person cannot read because of severe dyslexia, that person has an impairment. Likewise, being overweight is not considered an impairment (unless due to an underlying physical condition, e.g., hypothyroidism), although extreme obesity in excess of 100% ideal body weight is.
Having determined that an impairment exists, the next step in the analysis is to ascertain if the impairment limits one or more “major life activities.” These have classically included activities such as caring for oneself, performing manual tasks, walking, seeing, hearing, speaking, and breathing.
Third, the limitation must be substantial, meaning sufficiently severe, compared with what an average person is capable of doing. According to the EEOC, a mild type 2 diabetes patient on diet treatment alone and no other restriction has an impairment; but the impairment does not substantially limit any of his major life activities. On the other hand, some impairments are so severe that there is no doubt they substantially limit major life activities, e.g., insulin-dependent diabetes, legal blindness, deafness, manic-depressive syndrome, alcoholism, and HIV infection.
There is litigation aplenty over these issues.
In its seminal 1988 case, the U.S. Supreme Court provided the analytical steps listed above in arriving at its holding that, under the ADA, asymptomatic HIV infection is a disability.2 The case involved a dentist who was sued when he declined to treat an HIV-positive female patient in the office, offering instead to treat her in a hospital without any additional charge. A dental office, like a doctor’s office, is recognized as a place of public accommodations, and therefore falls under the protection of Title III of the ADA.
The court first considered whether HIV infection was a physical impairment. Second, it identified the major life activity upon which the plaintiff relied (reproduction and childbearing) and determined whether it constituted a major life activity under the ADA. Third, it tied the two statutory phrases together, and asked whether the impairment substantially limited these major life activities.
The court held that, in light of the immediacy with which the HIV virus begins to damage the infected person’s white blood cells and the severity of the disease, it is an impairment from the moment of infection, even if the patient was asymptomatic. It also ruled that the HIV infection substantially limited her ability to reproduce in two independent ways. First, a woman infected with HIV who tries to conceive a child imposes on the man a significant risk of becoming infected, and second, an infected woman risks infecting her child during gestation and childbirth, i.e., perinatal transmission.
In 2004, a case reached the U.S. Third Circuit Court of Appeals regarding Cathy Fiscus, an employee at a Walmart Sam’s Club warehouse store in Pittsburgh, who faced being terminated after 12 years at her job. A lower U.S. district court had ruled in favor of the company, agreeing with Walmart that the woman’s end-stage renal disease had not left her significantly limited in a major life activity. Ms. Fiscus sought a reasonable accommodation from her employer during the period of her peritoneal dialysis, which required her to self administer the 45-minute dialysis process at the workplace. Walmart initially agreed, but later declined. The appeals court overturned the lower court’s ruling, writing, “A physical impairment that limits an individual’s ability to cleanse and eliminate body waste does impair a major life activity.”3
Not all conditions are covered by the ADA’s definition of disability. The list includes temporary physical or mental impairments, current illegal drug use, predisposition to illness, personality traits, advanced age, and pregnancy, to name a few.
To avoid running afoul of the ADA, an employer is required to make “reasonable accommodations” for the disabled employee. This refers to practices that allow a disabled person to perform the essential functions of the job.
Examples of reasonable accommodations include making existing facilities readily accessible to and usable by individuals with disabilities, restructuring jobs, modifying work schedules, and providing qualified readers or interpreters.
A “qualified individual with a disability” is an individual with a disability who, “with or without reasonable accommodation,” can perform the essential functions of the employment position in question. A person is not a qualified individual with a disability, however, if he or she cannot satisfy the basic attendance requirements of a position.
Employers are not required to offer any and all accommodations, such as those that are disruptive to the business, overly burdensome, or prohibitively expensive. Providing a clean and private area in the workplace for self-administered peritoneal dialysis fluid exchange would likely qualify as a reasonable accommodation that should be offered, absent some compelling reason not to.
The protection given by the ADA may be suspended if the condition poses a direct threat, defined as “a significant risk to the health or safety of others that cannot be eliminated by a modification of policies, practices, or procedures, or by the provision of auxiliary aids or services.”4 The U.S. Supreme Court has noted that this should be assessed by the objective reasonableness of the views of health care professionals.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Americans with Disabilities Act of 1990 (ADA), 104 Stat. 327, 42 U. S. C. § 12101 et seq.
2. Bragdon v. Abbott et al. 524 U.S. 624 (1998).
3. Cathy A. Fiscus v. Wal-Mart Stores Inc., 385 F.3d 378 (3d Cir. 2004).
4. 42 U. S. C. § 12182(b)(3).
5. Available at www.kidney.org/atoz/content/employersguide.
VAM Registration Now Open
Registration and housing for the 2018 Vascular Annual Meeting are now open. Register today for VAM, June 20 to 23 in Boston, including looking over housing options. Following a full day of postgraduate courses, VESS abstracts, workshops and international programming on Wednesday, June 20, abstract-based scientific sessions will open June 21 and continue to June 23. The Exhibit Hall will be open June 21 to 22.
Registration and housing for the 2018 Vascular Annual Meeting are now open. Register today for VAM, June 20 to 23 in Boston, including looking over housing options. Following a full day of postgraduate courses, VESS abstracts, workshops and international programming on Wednesday, June 20, abstract-based scientific sessions will open June 21 and continue to June 23. The Exhibit Hall will be open June 21 to 22.
Registration and housing for the 2018 Vascular Annual Meeting are now open. Register today for VAM, June 20 to 23 in Boston, including looking over housing options. Following a full day of postgraduate courses, VESS abstracts, workshops and international programming on Wednesday, June 20, abstract-based scientific sessions will open June 21 and continue to June 23. The Exhibit Hall will be open June 21 to 22.
Catch the Innovation Spirit: Register for VRIC
Register today for this year’s Vascular Research Initiatives Conference, May 9, in San Francisco. The theme “Road to Innovation, Invention and Enterprise,” is reflected in the Translational Panel presentation, "Road to Entrepreneurship." Also part of VRIC are four abstract sessions, on stem cells and regeneration, PAD, vascular endothelium and thrombosis and vascular inflammation and injury.
Register today for this year’s Vascular Research Initiatives Conference, May 9, in San Francisco. The theme “Road to Innovation, Invention and Enterprise,” is reflected in the Translational Panel presentation, "Road to Entrepreneurship." Also part of VRIC are four abstract sessions, on stem cells and regeneration, PAD, vascular endothelium and thrombosis and vascular inflammation and injury.
Register today for this year’s Vascular Research Initiatives Conference, May 9, in San Francisco. The theme “Road to Innovation, Invention and Enterprise,” is reflected in the Translational Panel presentation, "Road to Entrepreneurship." Also part of VRIC are four abstract sessions, on stem cells and regeneration, PAD, vascular endothelium and thrombosis and vascular inflammation and injury.
ABS announces Continuous Certification Program
The American Board of Surgery has announced the details of its new Continuous Certification Program, shaped by surgeon feedback and designed to provide greater value, flexibility and convenience in maintaining ABS board certification. Instead of taking one recertification exam every 10 years, surgeons will use the new program to demonstrate their surgical knowledge on a continual basis. General surgeons will follow the new assessment this year; members of other ABS specialties will do so over the next few years.
The American Board of Surgery has announced the details of its new Continuous Certification Program, shaped by surgeon feedback and designed to provide greater value, flexibility and convenience in maintaining ABS board certification. Instead of taking one recertification exam every 10 years, surgeons will use the new program to demonstrate their surgical knowledge on a continual basis. General surgeons will follow the new assessment this year; members of other ABS specialties will do so over the next few years.
The American Board of Surgery has announced the details of its new Continuous Certification Program, shaped by surgeon feedback and designed to provide greater value, flexibility and convenience in maintaining ABS board certification. Instead of taking one recertification exam every 10 years, surgeons will use the new program to demonstrate their surgical knowledge on a continual basis. General surgeons will follow the new assessment this year; members of other ABS specialties will do so over the next few years.
Women, Leadership Training Grant Deadline Extended to March 21
The application for the SVS Women’s Leadership Training Grant has been extended a week, to March 21. This grant seeks to identify female surgeons who want to sharpen their leadership skills. It provides a $5,000 award to help defray costs for travel, hotel accommodations and registration expenses to attend relevant courses and/or other leadership training opportunities and activities.
The application for the SVS Women’s Leadership Training Grant has been extended a week, to March 21. This grant seeks to identify female surgeons who want to sharpen their leadership skills. It provides a $5,000 award to help defray costs for travel, hotel accommodations and registration expenses to attend relevant courses and/or other leadership training opportunities and activities.
The application for the SVS Women’s Leadership Training Grant has been extended a week, to March 21. This grant seeks to identify female surgeons who want to sharpen their leadership skills. It provides a $5,000 award to help defray costs for travel, hotel accommodations and registration expenses to attend relevant courses and/or other leadership training opportunities and activities.