User login
Type 2 Diabetes Increases the Risk of Parkinson’s Disease
The risk is particularly high among younger patients and those with complications from diabetes.
Patients with type 2 diabetes mellitus have an increased risk of developing Parkinson’s disease later in life, according to an investigation published online ahead of print June 13 in Neurology. The magnitude of risk is greater in younger patients and in patients with complications from diabetes.
Investigators have hypothesized an association between diabetes and the risk of Parkinson’s disease, but studies of the potential link have had conflicting results. Thomas T. Warner, MD, PhD, Professor of Clinical Neurology at University College London (UCL), and colleagues conducted a retrospective cohort study to examine this question anew.
Analyzing a Nationwide Hospital Database
The researchers reviewed English national Hospital Episode Statistics and mortality data collected between 1999 and 2011 and created a cohort of 2,017,115 patients who had been admitted for hospital care with a diagnosis of type 2 diabetes. They created a reference cohort of 6,173,208 patients without diabetes who were admitted for minor medical and surgical procedures. Conditions in this cohort included sprains, inguinal hernia, bruising, and hip replacement. People with Parkinson’s disease, ischemic cerebral infarction, vascular parkinsonism, drug-induced secondary parkinsonism, and normal pressure hydrocephalus were excluded from the study. Dr. Warner and colleagues created multivariable Cox proportional hazard regression models to estimate the risk of subsequent Parkinson’s disease.
Participants with diabetes had a greater risk of a subsequent diagnosis of Parkinson’s disease than patients in the reference cohort (adjusted hazard ratio [HR], 1.32). In subgroup analyses, the researchers found that the risk was substantially higher among patients between ages 25 and 44 (adjusted HR, 3.81) and those with complicated diabetes (adjusted HR, 1.49). Genetic factors may exert a relatively greater effect on younger people, and this difference may account for the increased risk among younger participants with diabetes, said the authors.
The adjusted HR of Parkinson’s disease was 1.40 in patients with diabetes between ages 65 and 74 and 1.18 in those age 75 or older. “The association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes,” said Dr. Warner and colleagues.
No Adjustment for Potential Confounders
The large size of the database and the ability to exclude people with cerebrovascular disease and drug-induced and vascular parkinsonisms were among the study’s main strengths, according to the authors. Its weaknesses included an inability to adjust for potential confounders and the lack of clinical information about Parkinson’s disease ascertainment beyond routinely collected data.
The results could help researchers identify “new ways to treat or prevent the development of Parkinson’s disease, such as use of antidiabetes drugs to restore the brain’s insulin signaling,” said Dr. Warner. “A UCL-led study published last year found that a drug commonly used to treat diabetes shows promise in not only relieving Parkinson’s disease symptoms, but potentially altering the course of the disease itself. What we do not know is whether trying to treat people with type 2 diabetes better would reduce the risk of developing Parkinson’s disease.”
—Erik Greb
Suggested Reading
De Pablo-Fernandez E, Goldacre R, Pakpoor J, et al. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology. 2018 Jun 13 [Epub ahead of print].
The risk is particularly high among younger patients and those with complications from diabetes.
The risk is particularly high among younger patients and those with complications from diabetes.
Patients with type 2 diabetes mellitus have an increased risk of developing Parkinson’s disease later in life, according to an investigation published online ahead of print June 13 in Neurology. The magnitude of risk is greater in younger patients and in patients with complications from diabetes.
Investigators have hypothesized an association between diabetes and the risk of Parkinson’s disease, but studies of the potential link have had conflicting results. Thomas T. Warner, MD, PhD, Professor of Clinical Neurology at University College London (UCL), and colleagues conducted a retrospective cohort study to examine this question anew.
Analyzing a Nationwide Hospital Database
The researchers reviewed English national Hospital Episode Statistics and mortality data collected between 1999 and 2011 and created a cohort of 2,017,115 patients who had been admitted for hospital care with a diagnosis of type 2 diabetes. They created a reference cohort of 6,173,208 patients without diabetes who were admitted for minor medical and surgical procedures. Conditions in this cohort included sprains, inguinal hernia, bruising, and hip replacement. People with Parkinson’s disease, ischemic cerebral infarction, vascular parkinsonism, drug-induced secondary parkinsonism, and normal pressure hydrocephalus were excluded from the study. Dr. Warner and colleagues created multivariable Cox proportional hazard regression models to estimate the risk of subsequent Parkinson’s disease.
Participants with diabetes had a greater risk of a subsequent diagnosis of Parkinson’s disease than patients in the reference cohort (adjusted hazard ratio [HR], 1.32). In subgroup analyses, the researchers found that the risk was substantially higher among patients between ages 25 and 44 (adjusted HR, 3.81) and those with complicated diabetes (adjusted HR, 1.49). Genetic factors may exert a relatively greater effect on younger people, and this difference may account for the increased risk among younger participants with diabetes, said the authors.
The adjusted HR of Parkinson’s disease was 1.40 in patients with diabetes between ages 65 and 74 and 1.18 in those age 75 or older. “The association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes,” said Dr. Warner and colleagues.
No Adjustment for Potential Confounders
The large size of the database and the ability to exclude people with cerebrovascular disease and drug-induced and vascular parkinsonisms were among the study’s main strengths, according to the authors. Its weaknesses included an inability to adjust for potential confounders and the lack of clinical information about Parkinson’s disease ascertainment beyond routinely collected data.
The results could help researchers identify “new ways to treat or prevent the development of Parkinson’s disease, such as use of antidiabetes drugs to restore the brain’s insulin signaling,” said Dr. Warner. “A UCL-led study published last year found that a drug commonly used to treat diabetes shows promise in not only relieving Parkinson’s disease symptoms, but potentially altering the course of the disease itself. What we do not know is whether trying to treat people with type 2 diabetes better would reduce the risk of developing Parkinson’s disease.”
—Erik Greb
Suggested Reading
De Pablo-Fernandez E, Goldacre R, Pakpoor J, et al. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology. 2018 Jun 13 [Epub ahead of print].
Patients with type 2 diabetes mellitus have an increased risk of developing Parkinson’s disease later in life, according to an investigation published online ahead of print June 13 in Neurology. The magnitude of risk is greater in younger patients and in patients with complications from diabetes.
Investigators have hypothesized an association between diabetes and the risk of Parkinson’s disease, but studies of the potential link have had conflicting results. Thomas T. Warner, MD, PhD, Professor of Clinical Neurology at University College London (UCL), and colleagues conducted a retrospective cohort study to examine this question anew.
Analyzing a Nationwide Hospital Database
The researchers reviewed English national Hospital Episode Statistics and mortality data collected between 1999 and 2011 and created a cohort of 2,017,115 patients who had been admitted for hospital care with a diagnosis of type 2 diabetes. They created a reference cohort of 6,173,208 patients without diabetes who were admitted for minor medical and surgical procedures. Conditions in this cohort included sprains, inguinal hernia, bruising, and hip replacement. People with Parkinson’s disease, ischemic cerebral infarction, vascular parkinsonism, drug-induced secondary parkinsonism, and normal pressure hydrocephalus were excluded from the study. Dr. Warner and colleagues created multivariable Cox proportional hazard regression models to estimate the risk of subsequent Parkinson’s disease.
Participants with diabetes had a greater risk of a subsequent diagnosis of Parkinson’s disease than patients in the reference cohort (adjusted hazard ratio [HR], 1.32). In subgroup analyses, the researchers found that the risk was substantially higher among patients between ages 25 and 44 (adjusted HR, 3.81) and those with complicated diabetes (adjusted HR, 1.49). Genetic factors may exert a relatively greater effect on younger people, and this difference may account for the increased risk among younger participants with diabetes, said the authors.
The adjusted HR of Parkinson’s disease was 1.40 in patients with diabetes between ages 65 and 74 and 1.18 in those age 75 or older. “The association in elderly patients may be the consequence of disrupted insulin signaling secondary to additional lifestyle and environmental factors causing cumulative pathogenic brain changes,” said Dr. Warner and colleagues.
No Adjustment for Potential Confounders
The large size of the database and the ability to exclude people with cerebrovascular disease and drug-induced and vascular parkinsonisms were among the study’s main strengths, according to the authors. Its weaknesses included an inability to adjust for potential confounders and the lack of clinical information about Parkinson’s disease ascertainment beyond routinely collected data.
The results could help researchers identify “new ways to treat or prevent the development of Parkinson’s disease, such as use of antidiabetes drugs to restore the brain’s insulin signaling,” said Dr. Warner. “A UCL-led study published last year found that a drug commonly used to treat diabetes shows promise in not only relieving Parkinson’s disease symptoms, but potentially altering the course of the disease itself. What we do not know is whether trying to treat people with type 2 diabetes better would reduce the risk of developing Parkinson’s disease.”
—Erik Greb
Suggested Reading
De Pablo-Fernandez E, Goldacre R, Pakpoor J, et al. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology. 2018 Jun 13 [Epub ahead of print].
In T2DM, healthy lifestyle lowers CVD risk, mortality
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Eating a high-quality diet, not smoking, exercising for at least 150 minutes weekly, and drinking only moderate amounts of alcohol led to a statistically significant decrease in risk of cardiovascular disease among persons with type 2 diabetes mellitus.
Major finding: Over an average of 13.3 years of follow-up, the adjusted risk of CVD was 52% lower in participants with at least three of these healthy lifestyle factors compared with those with none (multivariate-adjusted HR, 0.48; 95% CI, 0.40-0.59).
Study details: Pooled analysis of data from 11,527 patients from the Nurses’ Health Study and the Health Professionals Follow-Up Study.
Disclosures: The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
Source: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76.
Implementing a health literacy assessment
Limited health literacy results in poor outcomes.
Hospitalists regularly treat patients with limited health literacy, and in many cases, the hospitalist may not even be aware of it. “Patients are unlikely to know or, more importantly, disclose their limited health literacy status,” according to a recent study.1 But hospitalists certainly see its effects: Limited health literacy often results in poor outcomes and high rates of readmittance.
“We know patients with limited health literacy are common and that they have poor health outcomes,” said study coauthor Robert Leverence, MD. “We also know there are ways to mitigate those outcomes. For that reason, we believe screening is important. In our study, we showed such routine screening is feasible in a large teaching hospital.”
The study describes the implementation of a hospital-wide routine health literacy assessment at an academic medical center initiated by nurses and applied to all adult inpatients. “We incorporated the health literacy screen and care plan into our electronic health record,” the authors wrote. “When a patient screens positive for limited health literacy, two automated responses are triggered: a one-time alert on chart entry for all users ... and a nursing care plan containing relevant educational recommendations.”
“To me it is a cringe-worthy event to give a 10-page AVS to a patient who can’t read,” Dr. Leverence added. “Health literacy screening allows us to tailor the discharge process to meet the needs of the individual patient. Once these patients are identified, then appropriate efforts can be efficiently deployed.”
Those efforts might include, at discharge, offering easy-to-read materials and teach-back, and having a caregiver in the room and a pharmacist performing bedside medication education.
Reference
1. Warring C, Pinkney J, Delvo-Favre E, et al. “Implementation of a Routine Health Literacy Assessment at an Academic Medical Center.” J Healthc Qual. doi: 10.1097/JHQ.0000000000000116
Limited health literacy results in poor outcomes.
Limited health literacy results in poor outcomes.
Hospitalists regularly treat patients with limited health literacy, and in many cases, the hospitalist may not even be aware of it. “Patients are unlikely to know or, more importantly, disclose their limited health literacy status,” according to a recent study.1 But hospitalists certainly see its effects: Limited health literacy often results in poor outcomes and high rates of readmittance.
“We know patients with limited health literacy are common and that they have poor health outcomes,” said study coauthor Robert Leverence, MD. “We also know there are ways to mitigate those outcomes. For that reason, we believe screening is important. In our study, we showed such routine screening is feasible in a large teaching hospital.”
The study describes the implementation of a hospital-wide routine health literacy assessment at an academic medical center initiated by nurses and applied to all adult inpatients. “We incorporated the health literacy screen and care plan into our electronic health record,” the authors wrote. “When a patient screens positive for limited health literacy, two automated responses are triggered: a one-time alert on chart entry for all users ... and a nursing care plan containing relevant educational recommendations.”
“To me it is a cringe-worthy event to give a 10-page AVS to a patient who can’t read,” Dr. Leverence added. “Health literacy screening allows us to tailor the discharge process to meet the needs of the individual patient. Once these patients are identified, then appropriate efforts can be efficiently deployed.”
Those efforts might include, at discharge, offering easy-to-read materials and teach-back, and having a caregiver in the room and a pharmacist performing bedside medication education.
Reference
1. Warring C, Pinkney J, Delvo-Favre E, et al. “Implementation of a Routine Health Literacy Assessment at an Academic Medical Center.” J Healthc Qual. doi: 10.1097/JHQ.0000000000000116
Hospitalists regularly treat patients with limited health literacy, and in many cases, the hospitalist may not even be aware of it. “Patients are unlikely to know or, more importantly, disclose their limited health literacy status,” according to a recent study.1 But hospitalists certainly see its effects: Limited health literacy often results in poor outcomes and high rates of readmittance.
“We know patients with limited health literacy are common and that they have poor health outcomes,” said study coauthor Robert Leverence, MD. “We also know there are ways to mitigate those outcomes. For that reason, we believe screening is important. In our study, we showed such routine screening is feasible in a large teaching hospital.”
The study describes the implementation of a hospital-wide routine health literacy assessment at an academic medical center initiated by nurses and applied to all adult inpatients. “We incorporated the health literacy screen and care plan into our electronic health record,” the authors wrote. “When a patient screens positive for limited health literacy, two automated responses are triggered: a one-time alert on chart entry for all users ... and a nursing care plan containing relevant educational recommendations.”
“To me it is a cringe-worthy event to give a 10-page AVS to a patient who can’t read,” Dr. Leverence added. “Health literacy screening allows us to tailor the discharge process to meet the needs of the individual patient. Once these patients are identified, then appropriate efforts can be efficiently deployed.”
Those efforts might include, at discharge, offering easy-to-read materials and teach-back, and having a caregiver in the room and a pharmacist performing bedside medication education.
Reference
1. Warring C, Pinkney J, Delvo-Favre E, et al. “Implementation of a Routine Health Literacy Assessment at an Academic Medical Center.” J Healthc Qual. doi: 10.1097/JHQ.0000000000000116
Impact of varicella vaccination on herpes zoster is not what was expected
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
REPORTING FROM ESPID 2018
Key clinical point: The exogenous boosting hypothesis that universal pediatric varicella vaccination would result in an increase in herpes zoster hasn’t been borne out by the U.S. experience.
Major finding: rather than accelerating as some had forecast.
Study details: This was a retrospective study of the annual incidence of varicella and herpes zoster during 1991-2016 in roughly one-fifth of the U.S. population.
Disclosures: The study was sponsored by Merck and presented by a company employee.
What Alaska can teach us about burnout
Some people have nightmares of giant rats attacking from their basements, others encounter monsters from a Stephen King novel. My nightmares are of clinic where time is the beast pursuing me. In my nightmares, I’m running late and can’t get to my next patient, or I’m trapped somehow and unable to get to clinic at all.

Time demands that I provide patients access quickly, start clinic on time, double my speed to make up for add-ins, or worse, late patients. Time is a constant, relentless monster, one that has apparently infiltrated my subconscious. Yet, time is relative.
Over Memorial Day weekend, my wife and I flew from San Diego to Alaska. Somewhere between those places time transforms – early summer in San Diego becomes early spring in Alaska.
When we landed, daffodils were in bloom, buds on the alders were just arriving, and the sun struggled to warm the air to 50 degrees. The daytime defied gravity: it was daylight by 4 a.m. and still so after 11 p.m. We were the first visitors this season in our little cabin near Seward.
“Your hot water might take a bit to get hot,” our host, Jim, informed us. He wore a thick flannel shirt and Carhartt workman trousers. He leaned against the cabin’s door frame with one hand at the top and the other hanging from the weight of a DeWalt drill at his side. “I made these cabins myself,” he informed. I was anxious to move on, to unpack and start exploring, but every time I tried to break away from his conversation, he extended it. He shared how a cow and calf (that’s moose talk) had come through earlier that morning. Then he told us about working in the timber industry, starting by “pulling green chain” and working his way up to being the keeper of the saws. While he talked, I watched a raven drop down from the tall Sitka spruce to a branch just across from where we parked our car. Just behind Jim, the raven was not only watching, but also listening in on our conversation. Jim pointed, “That bit of snow over there was all that was left from the 12-foot-high snow earlier this year. It was an easy winter.” He then advised we should start our trip with a visit to Exit Glacier. It was reachable by road and an easy hike.
Staying upright on the steep trail to the glacier’s Harding Icefield concentrates the mind. Looking down and across the glacial outwash, I imagined how the ice once thousands of feet above my head carved a valley from rock. Ice compacted so completely and so deep that only blue light escapes. Indeed, a glacier is just a pile of unmelted snow, thousands of years in the making. The Kenai fjords, deep enough that humpback whales swim there, were carved from granite – at glacial speed. Some of the rocks there contain fossils all the way from the tropics. They were transported by the Pacific tectonic plate that has rotated counterclockwise from the equator to Alaska over millions of years – at tectonic speed. Life here has a way of sharpening your focus, allowing you to see perspective as exists in nature. Alaska is so old that an ob.gyn. could have seen his or her first patient here – a mother with a stillborn child – at the Upward Sun River, 11,500 years ago, where in 2013, the fossil remains of a late-term fetus dating back to that time was discovered. It is indeed relative.
After a long hike, a crispy, hot halibut sandwich, we made it back to our cabin. There was no WiFi or reliable cell service, no TV, no Netflix. We read in bed by daylight. I slept soundly, despite the bright light. No nightmares. No monsters.
The next morning, as I sipped my steaming coffee on our porch, the raven didn’t waste much time to stop by. He paused before coming nearly eye to eye on the roof of the firewood shed in front of me. He looked me up and down and cackled. Not a cawh, not warning me of my intrusion, but rather a vocalization. He just wanted to strike up a conversation with the first guest of the season. He had nothing but time.
On our last night, I lit a fire with wood Jim had cut for us (with help from lots of lighter fluid). Jim ambled over to say goodbye. When I mentioned we had a 2½ hour drive back to Anchorage, he said 3 hours wasn’t a long time for Alaskans. He’d made that drive many times when his kids were little just to take them to McDonald’s. I asked if he ever got burned out, living here. He gave a long pause, turning his chin up, letting the question sink in before constructing an answer. “Burned out? Huh. I don’t know. I guess like when I was pulling green chain in the saw mill. I was pretty tired by the end of the day. But that’s how we sleep so good in Alaska.”
He didn’t get it. In the lower 48, we rush, scramble, and hurry trying to outrun time. At the end, we’re burned out. In Alaska, they don’t know what burned out means. They do understand that time can’t be controlled or beaten. Rather, it is observed and appreciated. I hoped to bring a little of that perspective to clinic on Monday morning.
My recommendation to you if want to sleep better, with fewer nightmares, if you want to reduce your risk for burn out, then go to Alaska (or Montana, or Wyoming, or Idaho, or your backyard). .
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Some people have nightmares of giant rats attacking from their basements, others encounter monsters from a Stephen King novel. My nightmares are of clinic where time is the beast pursuing me. In my nightmares, I’m running late and can’t get to my next patient, or I’m trapped somehow and unable to get to clinic at all.

Time demands that I provide patients access quickly, start clinic on time, double my speed to make up for add-ins, or worse, late patients. Time is a constant, relentless monster, one that has apparently infiltrated my subconscious. Yet, time is relative.
Over Memorial Day weekend, my wife and I flew from San Diego to Alaska. Somewhere between those places time transforms – early summer in San Diego becomes early spring in Alaska.
When we landed, daffodils were in bloom, buds on the alders were just arriving, and the sun struggled to warm the air to 50 degrees. The daytime defied gravity: it was daylight by 4 a.m. and still so after 11 p.m. We were the first visitors this season in our little cabin near Seward.
“Your hot water might take a bit to get hot,” our host, Jim, informed us. He wore a thick flannel shirt and Carhartt workman trousers. He leaned against the cabin’s door frame with one hand at the top and the other hanging from the weight of a DeWalt drill at his side. “I made these cabins myself,” he informed. I was anxious to move on, to unpack and start exploring, but every time I tried to break away from his conversation, he extended it. He shared how a cow and calf (that’s moose talk) had come through earlier that morning. Then he told us about working in the timber industry, starting by “pulling green chain” and working his way up to being the keeper of the saws. While he talked, I watched a raven drop down from the tall Sitka spruce to a branch just across from where we parked our car. Just behind Jim, the raven was not only watching, but also listening in on our conversation. Jim pointed, “That bit of snow over there was all that was left from the 12-foot-high snow earlier this year. It was an easy winter.” He then advised we should start our trip with a visit to Exit Glacier. It was reachable by road and an easy hike.
Staying upright on the steep trail to the glacier’s Harding Icefield concentrates the mind. Looking down and across the glacial outwash, I imagined how the ice once thousands of feet above my head carved a valley from rock. Ice compacted so completely and so deep that only blue light escapes. Indeed, a glacier is just a pile of unmelted snow, thousands of years in the making. The Kenai fjords, deep enough that humpback whales swim there, were carved from granite – at glacial speed. Some of the rocks there contain fossils all the way from the tropics. They were transported by the Pacific tectonic plate that has rotated counterclockwise from the equator to Alaska over millions of years – at tectonic speed. Life here has a way of sharpening your focus, allowing you to see perspective as exists in nature. Alaska is so old that an ob.gyn. could have seen his or her first patient here – a mother with a stillborn child – at the Upward Sun River, 11,500 years ago, where in 2013, the fossil remains of a late-term fetus dating back to that time was discovered. It is indeed relative.
After a long hike, a crispy, hot halibut sandwich, we made it back to our cabin. There was no WiFi or reliable cell service, no TV, no Netflix. We read in bed by daylight. I slept soundly, despite the bright light. No nightmares. No monsters.
The next morning, as I sipped my steaming coffee on our porch, the raven didn’t waste much time to stop by. He paused before coming nearly eye to eye on the roof of the firewood shed in front of me. He looked me up and down and cackled. Not a cawh, not warning me of my intrusion, but rather a vocalization. He just wanted to strike up a conversation with the first guest of the season. He had nothing but time.
On our last night, I lit a fire with wood Jim had cut for us (with help from lots of lighter fluid). Jim ambled over to say goodbye. When I mentioned we had a 2½ hour drive back to Anchorage, he said 3 hours wasn’t a long time for Alaskans. He’d made that drive many times when his kids were little just to take them to McDonald’s. I asked if he ever got burned out, living here. He gave a long pause, turning his chin up, letting the question sink in before constructing an answer. “Burned out? Huh. I don’t know. I guess like when I was pulling green chain in the saw mill. I was pretty tired by the end of the day. But that’s how we sleep so good in Alaska.”
He didn’t get it. In the lower 48, we rush, scramble, and hurry trying to outrun time. At the end, we’re burned out. In Alaska, they don’t know what burned out means. They do understand that time can’t be controlled or beaten. Rather, it is observed and appreciated. I hoped to bring a little of that perspective to clinic on Monday morning.
My recommendation to you if want to sleep better, with fewer nightmares, if you want to reduce your risk for burn out, then go to Alaska (or Montana, or Wyoming, or Idaho, or your backyard). .
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Some people have nightmares of giant rats attacking from their basements, others encounter monsters from a Stephen King novel. My nightmares are of clinic where time is the beast pursuing me. In my nightmares, I’m running late and can’t get to my next patient, or I’m trapped somehow and unable to get to clinic at all.

Time demands that I provide patients access quickly, start clinic on time, double my speed to make up for add-ins, or worse, late patients. Time is a constant, relentless monster, one that has apparently infiltrated my subconscious. Yet, time is relative.
Over Memorial Day weekend, my wife and I flew from San Diego to Alaska. Somewhere between those places time transforms – early summer in San Diego becomes early spring in Alaska.
When we landed, daffodils were in bloom, buds on the alders were just arriving, and the sun struggled to warm the air to 50 degrees. The daytime defied gravity: it was daylight by 4 a.m. and still so after 11 p.m. We were the first visitors this season in our little cabin near Seward.
“Your hot water might take a bit to get hot,” our host, Jim, informed us. He wore a thick flannel shirt and Carhartt workman trousers. He leaned against the cabin’s door frame with one hand at the top and the other hanging from the weight of a DeWalt drill at his side. “I made these cabins myself,” he informed. I was anxious to move on, to unpack and start exploring, but every time I tried to break away from his conversation, he extended it. He shared how a cow and calf (that’s moose talk) had come through earlier that morning. Then he told us about working in the timber industry, starting by “pulling green chain” and working his way up to being the keeper of the saws. While he talked, I watched a raven drop down from the tall Sitka spruce to a branch just across from where we parked our car. Just behind Jim, the raven was not only watching, but also listening in on our conversation. Jim pointed, “That bit of snow over there was all that was left from the 12-foot-high snow earlier this year. It was an easy winter.” He then advised we should start our trip with a visit to Exit Glacier. It was reachable by road and an easy hike.
Staying upright on the steep trail to the glacier’s Harding Icefield concentrates the mind. Looking down and across the glacial outwash, I imagined how the ice once thousands of feet above my head carved a valley from rock. Ice compacted so completely and so deep that only blue light escapes. Indeed, a glacier is just a pile of unmelted snow, thousands of years in the making. The Kenai fjords, deep enough that humpback whales swim there, were carved from granite – at glacial speed. Some of the rocks there contain fossils all the way from the tropics. They were transported by the Pacific tectonic plate that has rotated counterclockwise from the equator to Alaska over millions of years – at tectonic speed. Life here has a way of sharpening your focus, allowing you to see perspective as exists in nature. Alaska is so old that an ob.gyn. could have seen his or her first patient here – a mother with a stillborn child – at the Upward Sun River, 11,500 years ago, where in 2013, the fossil remains of a late-term fetus dating back to that time was discovered. It is indeed relative.
After a long hike, a crispy, hot halibut sandwich, we made it back to our cabin. There was no WiFi or reliable cell service, no TV, no Netflix. We read in bed by daylight. I slept soundly, despite the bright light. No nightmares. No monsters.
The next morning, as I sipped my steaming coffee on our porch, the raven didn’t waste much time to stop by. He paused before coming nearly eye to eye on the roof of the firewood shed in front of me. He looked me up and down and cackled. Not a cawh, not warning me of my intrusion, but rather a vocalization. He just wanted to strike up a conversation with the first guest of the season. He had nothing but time.
On our last night, I lit a fire with wood Jim had cut for us (with help from lots of lighter fluid). Jim ambled over to say goodbye. When I mentioned we had a 2½ hour drive back to Anchorage, he said 3 hours wasn’t a long time for Alaskans. He’d made that drive many times when his kids were little just to take them to McDonald’s. I asked if he ever got burned out, living here. He gave a long pause, turning his chin up, letting the question sink in before constructing an answer. “Burned out? Huh. I don’t know. I guess like when I was pulling green chain in the saw mill. I was pretty tired by the end of the day. But that’s how we sleep so good in Alaska.”
He didn’t get it. In the lower 48, we rush, scramble, and hurry trying to outrun time. At the end, we’re burned out. In Alaska, they don’t know what burned out means. They do understand that time can’t be controlled or beaten. Rather, it is observed and appreciated. I hoped to bring a little of that perspective to clinic on Monday morning.
My recommendation to you if want to sleep better, with fewer nightmares, if you want to reduce your risk for burn out, then go to Alaska (or Montana, or Wyoming, or Idaho, or your backyard). .
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
When the Poisoned Risk Poisoning Others: Fatal Sodium Azide Overdose
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
We're on Instagram!
We're now on Instagram, a social media app designed for sharing photos and videos from a smartphone. Be sure to follow us!
We're now on Instagram, a social media app designed for sharing photos and videos from a smartphone. Be sure to follow us!
We're now on Instagram, a social media app designed for sharing photos and videos from a smartphone. Be sure to follow us!
Is the rise of suicide a medical or societal issue?
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Invasive cardiology sets starting salary standard
A 5% increase in average starting salary for the 2017-2018 recruiting year enabled invasive cardiologists to replace orthopedic surgeons as the top physician earners, according to physician recruitment firm Merritt Hawkins.
Invasive cardiologists who started new jobs in the past year received an average starting salary of $590,000, compared with $563,000 in 2016-2017, while orthopedic surgeons took an 8% cut as their starting salaries dropped from $579,000 to $533,000, Merritt Hawkins reported in its 2018 Review of Physician and Advanced Practitioners Recruiting Incentives.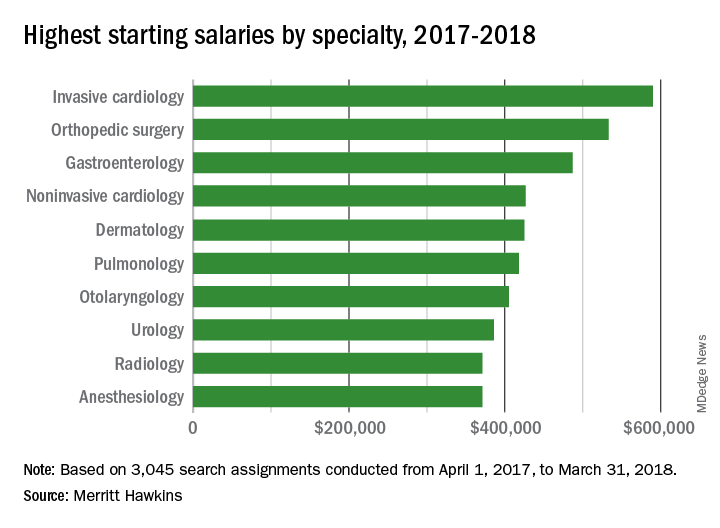
Besides the orthopedic surgeons, specialists who saw considerable drops in pay from 2016 to 2017 included otolaryngologists (–13%), radiologists (–15%), and urologists (–16%), according to the report.
“Demand for specialists is being driven upward by population aging and other factors. Average salaries, however, do not always correspond to increases in demand, at least not initially, as the market needs time to adjust to changing supply and demand dynamics,” Merritt Hawkins noted.
A 5% increase in average starting salary for the 2017-2018 recruiting year enabled invasive cardiologists to replace orthopedic surgeons as the top physician earners, according to physician recruitment firm Merritt Hawkins.
Invasive cardiologists who started new jobs in the past year received an average starting salary of $590,000, compared with $563,000 in 2016-2017, while orthopedic surgeons took an 8% cut as their starting salaries dropped from $579,000 to $533,000, Merritt Hawkins reported in its 2018 Review of Physician and Advanced Practitioners Recruiting Incentives.
Besides the orthopedic surgeons, specialists who saw considerable drops in pay from 2016 to 2017 included otolaryngologists (–13%), radiologists (–15%), and urologists (–16%), according to the report.
“Demand for specialists is being driven upward by population aging and other factors. Average salaries, however, do not always correspond to increases in demand, at least not initially, as the market needs time to adjust to changing supply and demand dynamics,” Merritt Hawkins noted.
A 5% increase in average starting salary for the 2017-2018 recruiting year enabled invasive cardiologists to replace orthopedic surgeons as the top physician earners, according to physician recruitment firm Merritt Hawkins.
Invasive cardiologists who started new jobs in the past year received an average starting salary of $590,000, compared with $563,000 in 2016-2017, while orthopedic surgeons took an 8% cut as their starting salaries dropped from $579,000 to $533,000, Merritt Hawkins reported in its 2018 Review of Physician and Advanced Practitioners Recruiting Incentives.
Besides the orthopedic surgeons, specialists who saw considerable drops in pay from 2016 to 2017 included otolaryngologists (–13%), radiologists (–15%), and urologists (–16%), according to the report.
“Demand for specialists is being driven upward by population aging and other factors. Average salaries, however, do not always correspond to increases in demand, at least not initially, as the market needs time to adjust to changing supply and demand dynamics,” Merritt Hawkins noted.
Size can matter: Laparoscopic hysterectomy for the very large uterus

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by







