User login
Three Clinical Studies Demonstrating Safety and Efficacy of Treatment for Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) in Adults
In this supplement to Internal Medicine News, Christine Frissora, MD provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
IBS-D diagnosis and treatment challenges
The role of microbial imbalance and altered gut microbiota
A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Internal Medicine News, Christine Frissora, MD provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
IBS-D diagnosis and treatment challenges
The role of microbial imbalance and altered gut microbiota
A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Internal Medicine News, Christine Frissora, MD provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
IBS-D diagnosis and treatment challenges
The role of microbial imbalance and altered gut microbiota
A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
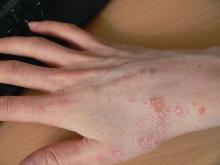
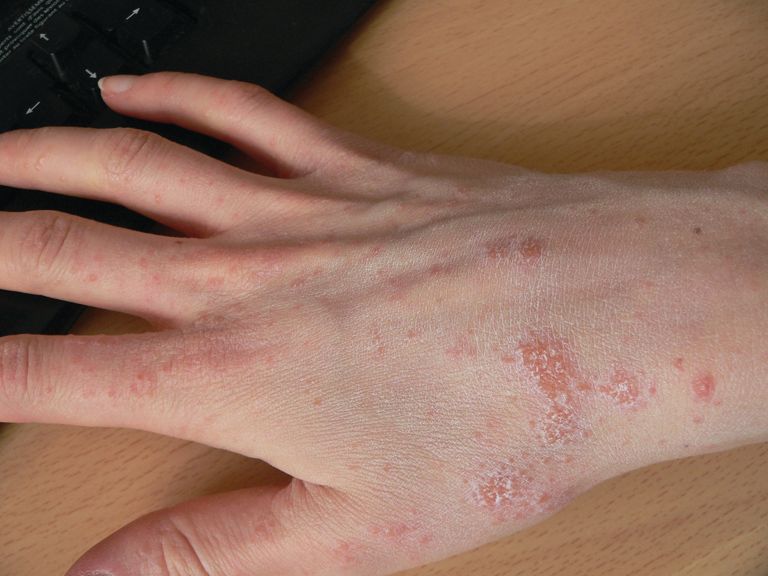
Relapsing scabies? Nails may hold a clue
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Pediatric relapse estimated as high as 66%.
Major finding: Nail scabies found in great toenail, not fingernails.
Study details: Observational multicenter prospective study of 47 pediatric patients with common scabies.
Disclosures: The authors had no relevant financial disclosures.
Source: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
Additional training may be warranted for clinicians administering DTaP
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
FROM PEDIATRICS
Key clinical point: No new or unexpected safety issues were found with DTaP.
Major finding: Nearly 90% of adverse events reported were not considered serious.
Study details: Large-scale data mining and records review from the Vaccine Adverse Event Reporting System.
Disclosures: The authors had no relevant financial disclosures. The study was funded by the Centers for Disease Control and Prevention and the Food and Drug Administration.
Source: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Population-Based Study Examining Utilization, Adverse Events, Length of Stay, and Cost
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).
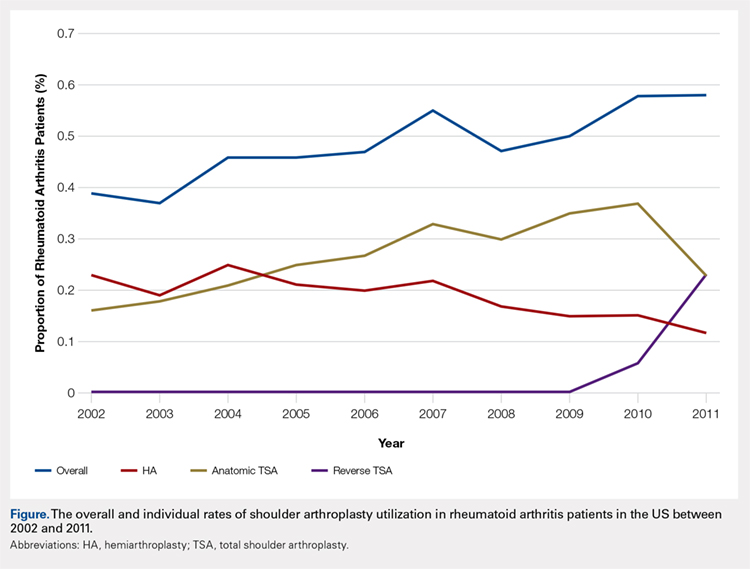
Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.
Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
TAKE-HOME POINTS
- There was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly TSA.
- There was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease.
- There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients.
- Non-RA patients had a significantly shorter length of stay.
- The utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Magnetic LES augmentation for Barrett’s regression debated
SEATTLE –
The study caught the attention of audience members – and raised a few eyebrows – at the 2018 World Congress of Endoscopic Surgery, where its results were presented, because the regression rate with the current standard operation for medically refractory gastroesophageal reflux – Nissen fundoplication – is only about 40%.
Lead investigator Evan Alicuben, MD, a general surgery resident at the university, cautioned that “longer-term follow-up is required to make a meaningful comparison with results following fundoplication.”
Fundoplication has been studied for decades, whereas the new study is likely the very first to look at the rates of Barrett’s regression after magnetic augmentation, and the 70% regression rate was based on postop endoscopies a median of 1.2 years after the procedure, not after the 5, 10, or even more years typically seen in fundoplication studies.
Magnetic sphincter augmentation (LINX Reflux Management System) was approved by the Food and Drug Administration in 2012 for reflux that persists despite maximum drug therapy. Patients have a band of magnetic titanium beads surgically placed around their LES; the band opens to let food pass, but tightens again to bolster the LES and prevent reflux.
The approach is gaining popularity. “We now know that it’s effective at controlling reflux symptoms, taking patients off proton pump inhibitors, and curing esophagitis,” at least in the short term. “One of the issues with [fundoplication] is that it may not last forever; the wrap comes undone or it slips. This device may give longer lasting” protection, Dr. Alicuben said.
“The main criticism is that it’s relatively new; people are still questioning it. The optimist in me wants to say that this is the answer we’ve been looking for; the pessimist [says] we need to wait to see what longer-term data show,” he said.
Barrett’s esophagus was confirmed by endoscopy in all 67 subjects before the magnets were placed, and each had at least one postop endoscopy.
At baseline, 29 had ultrashort-segment disease, which means there was no visible Barrett’s, but did have columnar epithelium with goblet cells on pathology. Thirty patients had short-segment disease, with up to 3 cm of visible involvement confirmed by pathology, while eight had long-segment disease, with involvement extending 3 cm or more.
Of the 67 patients, 48 had no evidence of Barrett’s after the procedure, for an overall regression rate of 71.6%. The regression rate was 82.8% in the ultrashort group (24/29); 73.3% in the short segment group (22/30); and 25% in the long segment group (2/8). Long-segment disease is notorious for persisting despite treatment; both patients had 3-cm lesions.
Among the 34 patients with two or more postop endoscopies, the regression rate was 73.5% (25).
There’s a lot of debate about whether ultrashort-segment disease is truly Barrett’s and whether it carries the same risk of malignant transformation, as one surgeon in the audience noted pointedly, worrying that including ultrashort patients oversold the results.
Dr. Alicuben countered that the regression rate remained strong even when ultrashort patients were excluded: 63% (24/38). “This is every bit as good if not better than the results of fundoplication,” another surgeon in the audience said.
The subjects were aged about 60 years, on average, with more men than women. Most had hiatal hernias, often measuring 3 cm or more. The mean body mass index was 27.3 kg/m2, but BMI ranged as high as 44.3.
Mean operative time was 66 minutes, and there were no major complications. None of the patients progressed to dysplasia or carcinoma. Median DeMeester scores fell from 35.3 to 9.2 after the operation in the 47 patients who had postop pH testing.
Surgeons have worried about esophageal erosion with the LINX system. A recent paper by Dr. Alicuben and his colleagues found 29 cases among almost 10,000 patients, which makes for an erosion rate of 0.3% at a median of about 2 years (J Gastrointest Surg. 2018 Apr 17. doi: 10.1007/s11605-018-3775-0).
About 500 LINX systems have been placed at the University of Southern California. Procedures in the study were performed between 2012 and late 2017.
Dr. Alicuben had no disclosures. Two investigators, including senior author John Lipham, MD, are paid consultants for Torax Medical, the maker of the LINX system, and Johnson & Johnson, which owns Torax through a subsidiary. There was no company funding for the review.
The World Congress of Endoscopic Surgery is hosted by the Society of American Gastrointestinal and Endoscopic Surgeons and the Canadian Association of General Surgeons
SOURCE: Alicuben E et al. WCE 2018, Abstract S095.
The enthusiasm and reservation surrounding the presented results supporting relatively high rates of regression of Barrett’s esophagus in patients undergoing LES magnetic sphincter augmentation are both well founded. Barrett’s regression, which has been observed to occur spontaneously as well as following antireflux interventions, is always a rich topic for debate. The reported rate of regression in this study being higher than that of complete fundoplication (Nissen fundoplication) is perplexing. The premise for the development of magnetic sphincter augmentation at a focal site was based on the theory that complete fundoplication is supraphysiologic, resulting in desired resolution of regurgitation with unwanted sequelae of dysphagia and bloat in a substantial number of patients. The development of magnetic sphincter augmentation was inspired by the concept that it would provide very reproducible control of regurgitation nearing that of complete fundoplication in a permanent fashion and do so at a focal point (< 1 cm) at the level of the lower esophageal sphincter, minimizing dysphagia and bloat. Since the design is one to replicate appropriate physiology without over treating the targeted reflux disease, theoretically any regression of Barrett’s esophagus should likewise approach but not exceed that of complete fundoplication.
I anticipate further studies will add to this rich debate. Any reservations about the results of this study should not overshadow the inherent advantages of magnetic sphincter augmentation. Its implantation is fairly straightforward to teach to surgeons who have a practice focused on antireflux surgery and due to the limited dissection/tissue mobilization required, most patients can return home a few hours after surgery and immediately resume a diet of solid foods.
Dr. Alicuban and colleagues discuss the small but real concern of erosion, however, another point of inherent concern is the binary function of the device. It is either implanted or not, there is no ability beyond endoscopic dilation to treat relative outflow obstruction and no means to convert the device to a “partial wrap.”
As we forge ahead with increasingly creative ways to address reflux disease the lessons we learn will contribute to the development of better medical, endoscopic and minimally invasive surgical technologies and the robust, civil debate seen here is something we can all enthusiastically anticipate.
Kevin M. Reavis, MD, FACS, is with the Division of Gastrointestinal and Minimally Invasive Surgery The Oregon Clinic; associate professor, Oregon Health & Science University, Portland, and President, Oregon Medical Society.
The enthusiasm and reservation surrounding the presented results supporting relatively high rates of regression of Barrett’s esophagus in patients undergoing LES magnetic sphincter augmentation are both well founded. Barrett’s regression, which has been observed to occur spontaneously as well as following antireflux interventions, is always a rich topic for debate. The reported rate of regression in this study being higher than that of complete fundoplication (Nissen fundoplication) is perplexing. The premise for the development of magnetic sphincter augmentation at a focal site was based on the theory that complete fundoplication is supraphysiologic, resulting in desired resolution of regurgitation with unwanted sequelae of dysphagia and bloat in a substantial number of patients. The development of magnetic sphincter augmentation was inspired by the concept that it would provide very reproducible control of regurgitation nearing that of complete fundoplication in a permanent fashion and do so at a focal point (< 1 cm) at the level of the lower esophageal sphincter, minimizing dysphagia and bloat. Since the design is one to replicate appropriate physiology without over treating the targeted reflux disease, theoretically any regression of Barrett’s esophagus should likewise approach but not exceed that of complete fundoplication.
I anticipate further studies will add to this rich debate. Any reservations about the results of this study should not overshadow the inherent advantages of magnetic sphincter augmentation. Its implantation is fairly straightforward to teach to surgeons who have a practice focused on antireflux surgery and due to the limited dissection/tissue mobilization required, most patients can return home a few hours after surgery and immediately resume a diet of solid foods.
Dr. Alicuban and colleagues discuss the small but real concern of erosion, however, another point of inherent concern is the binary function of the device. It is either implanted or not, there is no ability beyond endoscopic dilation to treat relative outflow obstruction and no means to convert the device to a “partial wrap.”
As we forge ahead with increasingly creative ways to address reflux disease the lessons we learn will contribute to the development of better medical, endoscopic and minimally invasive surgical technologies and the robust, civil debate seen here is something we can all enthusiastically anticipate.
Kevin M. Reavis, MD, FACS, is with the Division of Gastrointestinal and Minimally Invasive Surgery The Oregon Clinic; associate professor, Oregon Health & Science University, Portland, and President, Oregon Medical Society.
The enthusiasm and reservation surrounding the presented results supporting relatively high rates of regression of Barrett’s esophagus in patients undergoing LES magnetic sphincter augmentation are both well founded. Barrett’s regression, which has been observed to occur spontaneously as well as following antireflux interventions, is always a rich topic for debate. The reported rate of regression in this study being higher than that of complete fundoplication (Nissen fundoplication) is perplexing. The premise for the development of magnetic sphincter augmentation at a focal site was based on the theory that complete fundoplication is supraphysiologic, resulting in desired resolution of regurgitation with unwanted sequelae of dysphagia and bloat in a substantial number of patients. The development of magnetic sphincter augmentation was inspired by the concept that it would provide very reproducible control of regurgitation nearing that of complete fundoplication in a permanent fashion and do so at a focal point (< 1 cm) at the level of the lower esophageal sphincter, minimizing dysphagia and bloat. Since the design is one to replicate appropriate physiology without over treating the targeted reflux disease, theoretically any regression of Barrett’s esophagus should likewise approach but not exceed that of complete fundoplication.
I anticipate further studies will add to this rich debate. Any reservations about the results of this study should not overshadow the inherent advantages of magnetic sphincter augmentation. Its implantation is fairly straightforward to teach to surgeons who have a practice focused on antireflux surgery and due to the limited dissection/tissue mobilization required, most patients can return home a few hours after surgery and immediately resume a diet of solid foods.
Dr. Alicuban and colleagues discuss the small but real concern of erosion, however, another point of inherent concern is the binary function of the device. It is either implanted or not, there is no ability beyond endoscopic dilation to treat relative outflow obstruction and no means to convert the device to a “partial wrap.”
As we forge ahead with increasingly creative ways to address reflux disease the lessons we learn will contribute to the development of better medical, endoscopic and minimally invasive surgical technologies and the robust, civil debate seen here is something we can all enthusiastically anticipate.
Kevin M. Reavis, MD, FACS, is with the Division of Gastrointestinal and Minimally Invasive Surgery The Oregon Clinic; associate professor, Oregon Health & Science University, Portland, and President, Oregon Medical Society.
SEATTLE –
The study caught the attention of audience members – and raised a few eyebrows – at the 2018 World Congress of Endoscopic Surgery, where its results were presented, because the regression rate with the current standard operation for medically refractory gastroesophageal reflux – Nissen fundoplication – is only about 40%.
Lead investigator Evan Alicuben, MD, a general surgery resident at the university, cautioned that “longer-term follow-up is required to make a meaningful comparison with results following fundoplication.”
Fundoplication has been studied for decades, whereas the new study is likely the very first to look at the rates of Barrett’s regression after magnetic augmentation, and the 70% regression rate was based on postop endoscopies a median of 1.2 years after the procedure, not after the 5, 10, or even more years typically seen in fundoplication studies.
Magnetic sphincter augmentation (LINX Reflux Management System) was approved by the Food and Drug Administration in 2012 for reflux that persists despite maximum drug therapy. Patients have a band of magnetic titanium beads surgically placed around their LES; the band opens to let food pass, but tightens again to bolster the LES and prevent reflux.
The approach is gaining popularity. “We now know that it’s effective at controlling reflux symptoms, taking patients off proton pump inhibitors, and curing esophagitis,” at least in the short term. “One of the issues with [fundoplication] is that it may not last forever; the wrap comes undone or it slips. This device may give longer lasting” protection, Dr. Alicuben said.
“The main criticism is that it’s relatively new; people are still questioning it. The optimist in me wants to say that this is the answer we’ve been looking for; the pessimist [says] we need to wait to see what longer-term data show,” he said.
Barrett’s esophagus was confirmed by endoscopy in all 67 subjects before the magnets were placed, and each had at least one postop endoscopy.
At baseline, 29 had ultrashort-segment disease, which means there was no visible Barrett’s, but did have columnar epithelium with goblet cells on pathology. Thirty patients had short-segment disease, with up to 3 cm of visible involvement confirmed by pathology, while eight had long-segment disease, with involvement extending 3 cm or more.
Of the 67 patients, 48 had no evidence of Barrett’s after the procedure, for an overall regression rate of 71.6%. The regression rate was 82.8% in the ultrashort group (24/29); 73.3% in the short segment group (22/30); and 25% in the long segment group (2/8). Long-segment disease is notorious for persisting despite treatment; both patients had 3-cm lesions.
Among the 34 patients with two or more postop endoscopies, the regression rate was 73.5% (25).
There’s a lot of debate about whether ultrashort-segment disease is truly Barrett’s and whether it carries the same risk of malignant transformation, as one surgeon in the audience noted pointedly, worrying that including ultrashort patients oversold the results.
Dr. Alicuben countered that the regression rate remained strong even when ultrashort patients were excluded: 63% (24/38). “This is every bit as good if not better than the results of fundoplication,” another surgeon in the audience said.
The subjects were aged about 60 years, on average, with more men than women. Most had hiatal hernias, often measuring 3 cm or more. The mean body mass index was 27.3 kg/m2, but BMI ranged as high as 44.3.
Mean operative time was 66 minutes, and there were no major complications. None of the patients progressed to dysplasia or carcinoma. Median DeMeester scores fell from 35.3 to 9.2 after the operation in the 47 patients who had postop pH testing.
Surgeons have worried about esophageal erosion with the LINX system. A recent paper by Dr. Alicuben and his colleagues found 29 cases among almost 10,000 patients, which makes for an erosion rate of 0.3% at a median of about 2 years (J Gastrointest Surg. 2018 Apr 17. doi: 10.1007/s11605-018-3775-0).
About 500 LINX systems have been placed at the University of Southern California. Procedures in the study were performed between 2012 and late 2017.
Dr. Alicuben had no disclosures. Two investigators, including senior author John Lipham, MD, are paid consultants for Torax Medical, the maker of the LINX system, and Johnson & Johnson, which owns Torax through a subsidiary. There was no company funding for the review.
The World Congress of Endoscopic Surgery is hosted by the Society of American Gastrointestinal and Endoscopic Surgeons and the Canadian Association of General Surgeons
SOURCE: Alicuben E et al. WCE 2018, Abstract S095.
SEATTLE –
The study caught the attention of audience members – and raised a few eyebrows – at the 2018 World Congress of Endoscopic Surgery, where its results were presented, because the regression rate with the current standard operation for medically refractory gastroesophageal reflux – Nissen fundoplication – is only about 40%.
Lead investigator Evan Alicuben, MD, a general surgery resident at the university, cautioned that “longer-term follow-up is required to make a meaningful comparison with results following fundoplication.”
Fundoplication has been studied for decades, whereas the new study is likely the very first to look at the rates of Barrett’s regression after magnetic augmentation, and the 70% regression rate was based on postop endoscopies a median of 1.2 years after the procedure, not after the 5, 10, or even more years typically seen in fundoplication studies.
Magnetic sphincter augmentation (LINX Reflux Management System) was approved by the Food and Drug Administration in 2012 for reflux that persists despite maximum drug therapy. Patients have a band of magnetic titanium beads surgically placed around their LES; the band opens to let food pass, but tightens again to bolster the LES and prevent reflux.
The approach is gaining popularity. “We now know that it’s effective at controlling reflux symptoms, taking patients off proton pump inhibitors, and curing esophagitis,” at least in the short term. “One of the issues with [fundoplication] is that it may not last forever; the wrap comes undone or it slips. This device may give longer lasting” protection, Dr. Alicuben said.
“The main criticism is that it’s relatively new; people are still questioning it. The optimist in me wants to say that this is the answer we’ve been looking for; the pessimist [says] we need to wait to see what longer-term data show,” he said.
Barrett’s esophagus was confirmed by endoscopy in all 67 subjects before the magnets were placed, and each had at least one postop endoscopy.
At baseline, 29 had ultrashort-segment disease, which means there was no visible Barrett’s, but did have columnar epithelium with goblet cells on pathology. Thirty patients had short-segment disease, with up to 3 cm of visible involvement confirmed by pathology, while eight had long-segment disease, with involvement extending 3 cm or more.
Of the 67 patients, 48 had no evidence of Barrett’s after the procedure, for an overall regression rate of 71.6%. The regression rate was 82.8% in the ultrashort group (24/29); 73.3% in the short segment group (22/30); and 25% in the long segment group (2/8). Long-segment disease is notorious for persisting despite treatment; both patients had 3-cm lesions.
Among the 34 patients with two or more postop endoscopies, the regression rate was 73.5% (25).
There’s a lot of debate about whether ultrashort-segment disease is truly Barrett’s and whether it carries the same risk of malignant transformation, as one surgeon in the audience noted pointedly, worrying that including ultrashort patients oversold the results.
Dr. Alicuben countered that the regression rate remained strong even when ultrashort patients were excluded: 63% (24/38). “This is every bit as good if not better than the results of fundoplication,” another surgeon in the audience said.
The subjects were aged about 60 years, on average, with more men than women. Most had hiatal hernias, often measuring 3 cm or more. The mean body mass index was 27.3 kg/m2, but BMI ranged as high as 44.3.
Mean operative time was 66 minutes, and there were no major complications. None of the patients progressed to dysplasia or carcinoma. Median DeMeester scores fell from 35.3 to 9.2 after the operation in the 47 patients who had postop pH testing.
Surgeons have worried about esophageal erosion with the LINX system. A recent paper by Dr. Alicuben and his colleagues found 29 cases among almost 10,000 patients, which makes for an erosion rate of 0.3% at a median of about 2 years (J Gastrointest Surg. 2018 Apr 17. doi: 10.1007/s11605-018-3775-0).
About 500 LINX systems have been placed at the University of Southern California. Procedures in the study were performed between 2012 and late 2017.
Dr. Alicuben had no disclosures. Two investigators, including senior author John Lipham, MD, are paid consultants for Torax Medical, the maker of the LINX system, and Johnson & Johnson, which owns Torax through a subsidiary. There was no company funding for the review.
The World Congress of Endoscopic Surgery is hosted by the Society of American Gastrointestinal and Endoscopic Surgeons and the Canadian Association of General Surgeons
SOURCE: Alicuben E et al. WCE 2018, Abstract S095.
REPORTING FROM WCE 2018
Key clinical point: Magnetic lower esophageal sphincter augmentation might offer an easier and more effective fix for gastroesophageal reflux than the current standard, Nissen fundoplication.
Major finding: The overall regression rate of Barrett’s esophagus topped 70%.
Study details: Review of 67 patients
Disclosures: There was no industry funding, and the presenter had no disclosures. Two authors are consultants for Torax Medical, the company that makes the device.
Source: Alicuben E et al. WCE 2018, Abstract S095
Monoclonal antibody reduced alpha-synuclein levels in Parkinson’s patients
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
FROM JAMA NEUROLOGY
Key clinical point: Treatment with a monoclonal antibody for alpha-synuclein known as PRX002 was safe and effective in a preliminary study of Parkinson’s disease patients.
Major finding: Significant reductions in free serum alpha-synuclein levels persisted at 85 days after the third infusion in the 3-mg/kg through 60-mg/kg dose groups.
Study details: The data come from a randomized, phase 1b trial of 80 adults aged 40-80 years with Parkinson’s disease.
Disclosures: The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
Source: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
TNF inhibitor linked to one-third drop in total mortality
AMSTERDAM – Patients treated with a tumor necrosis factor inhibitor for any indication had their mortality rate cut by about one third, compared with the general population, in a combined analysis of safety findings from 78 trials that involved nearly 30,000 patients.
This first indication that treatment with a tumor necrosis factor inhibitor (TNFi) significantly cut overall mortality only became apparent because of the very large number of patients and patient-years of treatment analyzed, and is likely a real effect – not an artifact – that’s probably linked in part to the anti-inflammatory effect from treatment and its favorable impact on cardiovascular disease events, Gerd R. Burmester, MD, said at the European Congress of Rheumatology.
The cut in overall mortality might also partially result from a “healthy cohort effect,” in which patients enrolled in trials pay more attention to their diet and other aspects of a healthy lifestyle, compared with the general population. But Dr. Burmester cited the recent results from the CANTOS trial that showed treatment with the anti-inflammatory drug canakinumab (Ilaris) was linked with a significant 12% relative reduction in cardiovascular death, myocardial infarction, and stroke (New Engl J Med. 2017 Sept 21;377[12]:1119-31).
“It may be that the anticytokine effect of TNFi works the same way as canakinumab,” Dr. Burmester said in an interview.
The results also confirmed previous reports, based on trial data from fewer numbers of TNFi-treated patients, of low rates of serious infections and malignancies, said Dr. Burmester, professor and director of the department of rheumatology and clinical immunology at Charité Medical University in Berlin.
The data he presented came from both randomized trials and open-label studies of adalimumab (Humira) conducted in several countries worldwide through the end of 2016. The various studies enrolled a total of 29,987 patients treated with adalimumab for 56,951 patient-years who had any of 11 different diseases, including rheumatologic, gastrointestinal, and dermatologic diseases. The most common condition treated in the studies was rheumatoid arthritis (in 33 of the 78 studies), followed by psoriasis (13 studies), and Crohn’s disease (11 studies).
The studies included 9,363 patients treated for at least 2 years, and 4,003 patients treated for at least 5 years. The median duration of adalimumab exposure was 0.7 years and the maximum exposure was just over 12 years.
The overall rate of serious infections in treated patients was 3.7 per 100 patient-years. The most common serious infections were pneumonia, at a rate of 0.6 per 100 patient-years, followed by cellulitis, at a rate of 0.2 per 100 patient-years. Active tuberculosis infections also occurred at a rate of 0.2 per 100 patient-years. Malignancies occurred at a rate of 0.6 per 100 patient-years. These rates were similar to those reported by Dr. Burmester and his associates in 2013 using data from a small pool of patients – 23,458 – enrolled in 71 studies of adalimumab (Ann Rheum Dis. 2013 Apr;72[4]:517-24).
In the current study, Dr. Burmester and his coauthors analyzed the observed mortality rate of the adalimumab-treated patients against the mortality rates for the general populations in the various countries in which the studies were run, based on World Health Organization statistics for the period 1997-2006, and adjusted so that the age and sex of the comparison general populations matched the age and sex of the treated patients. This analysis showed an overall, statistically significant mortality reduction in patients receiving adalimumab of 35%, which was consistent in both the subgroups of men and women.
The observed mortality reduction linked with TNFi treatment is likely a class effect, Dr. Burmester said, although similar analyses have not been conducted using data from patients treated with other TNFis. So far, he has been unsuccessful in getting similar, large-scale trial data from manufacturers of other TNFis that he has approached, but Dr. Burmester said he hopes to eventually receive these data so that he can perform an even larger analysis.
The study was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Burmester has been a consultant to and speaker on behalf of AbbVie, as well as for Bristol Myers Squibb, Merk, Pfizer, Roche, and UCB.
SOURCE: Burmester GR et al. Ann Rheum Dis. 2018;77(Suppl 2):165. Abstract OP0233.
AMSTERDAM – Patients treated with a tumor necrosis factor inhibitor for any indication had their mortality rate cut by about one third, compared with the general population, in a combined analysis of safety findings from 78 trials that involved nearly 30,000 patients.
This first indication that treatment with a tumor necrosis factor inhibitor (TNFi) significantly cut overall mortality only became apparent because of the very large number of patients and patient-years of treatment analyzed, and is likely a real effect – not an artifact – that’s probably linked in part to the anti-inflammatory effect from treatment and its favorable impact on cardiovascular disease events, Gerd R. Burmester, MD, said at the European Congress of Rheumatology.
The cut in overall mortality might also partially result from a “healthy cohort effect,” in which patients enrolled in trials pay more attention to their diet and other aspects of a healthy lifestyle, compared with the general population. But Dr. Burmester cited the recent results from the CANTOS trial that showed treatment with the anti-inflammatory drug canakinumab (Ilaris) was linked with a significant 12% relative reduction in cardiovascular death, myocardial infarction, and stroke (New Engl J Med. 2017 Sept 21;377[12]:1119-31).
“It may be that the anticytokine effect of TNFi works the same way as canakinumab,” Dr. Burmester said in an interview.
The results also confirmed previous reports, based on trial data from fewer numbers of TNFi-treated patients, of low rates of serious infections and malignancies, said Dr. Burmester, professor and director of the department of rheumatology and clinical immunology at Charité Medical University in Berlin.
The data he presented came from both randomized trials and open-label studies of adalimumab (Humira) conducted in several countries worldwide through the end of 2016. The various studies enrolled a total of 29,987 patients treated with adalimumab for 56,951 patient-years who had any of 11 different diseases, including rheumatologic, gastrointestinal, and dermatologic diseases. The most common condition treated in the studies was rheumatoid arthritis (in 33 of the 78 studies), followed by psoriasis (13 studies), and Crohn’s disease (11 studies).
The studies included 9,363 patients treated for at least 2 years, and 4,003 patients treated for at least 5 years. The median duration of adalimumab exposure was 0.7 years and the maximum exposure was just over 12 years.
The overall rate of serious infections in treated patients was 3.7 per 100 patient-years. The most common serious infections were pneumonia, at a rate of 0.6 per 100 patient-years, followed by cellulitis, at a rate of 0.2 per 100 patient-years. Active tuberculosis infections also occurred at a rate of 0.2 per 100 patient-years. Malignancies occurred at a rate of 0.6 per 100 patient-years. These rates were similar to those reported by Dr. Burmester and his associates in 2013 using data from a small pool of patients – 23,458 – enrolled in 71 studies of adalimumab (Ann Rheum Dis. 2013 Apr;72[4]:517-24).
In the current study, Dr. Burmester and his coauthors analyzed the observed mortality rate of the adalimumab-treated patients against the mortality rates for the general populations in the various countries in which the studies were run, based on World Health Organization statistics for the period 1997-2006, and adjusted so that the age and sex of the comparison general populations matched the age and sex of the treated patients. This analysis showed an overall, statistically significant mortality reduction in patients receiving adalimumab of 35%, which was consistent in both the subgroups of men and women.
The observed mortality reduction linked with TNFi treatment is likely a class effect, Dr. Burmester said, although similar analyses have not been conducted using data from patients treated with other TNFis. So far, he has been unsuccessful in getting similar, large-scale trial data from manufacturers of other TNFis that he has approached, but Dr. Burmester said he hopes to eventually receive these data so that he can perform an even larger analysis.
The study was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Burmester has been a consultant to and speaker on behalf of AbbVie, as well as for Bristol Myers Squibb, Merk, Pfizer, Roche, and UCB.
SOURCE: Burmester GR et al. Ann Rheum Dis. 2018;77(Suppl 2):165. Abstract OP0233.
AMSTERDAM – Patients treated with a tumor necrosis factor inhibitor for any indication had their mortality rate cut by about one third, compared with the general population, in a combined analysis of safety findings from 78 trials that involved nearly 30,000 patients.
This first indication that treatment with a tumor necrosis factor inhibitor (TNFi) significantly cut overall mortality only became apparent because of the very large number of patients and patient-years of treatment analyzed, and is likely a real effect – not an artifact – that’s probably linked in part to the anti-inflammatory effect from treatment and its favorable impact on cardiovascular disease events, Gerd R. Burmester, MD, said at the European Congress of Rheumatology.
The cut in overall mortality might also partially result from a “healthy cohort effect,” in which patients enrolled in trials pay more attention to their diet and other aspects of a healthy lifestyle, compared with the general population. But Dr. Burmester cited the recent results from the CANTOS trial that showed treatment with the anti-inflammatory drug canakinumab (Ilaris) was linked with a significant 12% relative reduction in cardiovascular death, myocardial infarction, and stroke (New Engl J Med. 2017 Sept 21;377[12]:1119-31).
“It may be that the anticytokine effect of TNFi works the same way as canakinumab,” Dr. Burmester said in an interview.
The results also confirmed previous reports, based on trial data from fewer numbers of TNFi-treated patients, of low rates of serious infections and malignancies, said Dr. Burmester, professor and director of the department of rheumatology and clinical immunology at Charité Medical University in Berlin.
The data he presented came from both randomized trials and open-label studies of adalimumab (Humira) conducted in several countries worldwide through the end of 2016. The various studies enrolled a total of 29,987 patients treated with adalimumab for 56,951 patient-years who had any of 11 different diseases, including rheumatologic, gastrointestinal, and dermatologic diseases. The most common condition treated in the studies was rheumatoid arthritis (in 33 of the 78 studies), followed by psoriasis (13 studies), and Crohn’s disease (11 studies).
The studies included 9,363 patients treated for at least 2 years, and 4,003 patients treated for at least 5 years. The median duration of adalimumab exposure was 0.7 years and the maximum exposure was just over 12 years.
The overall rate of serious infections in treated patients was 3.7 per 100 patient-years. The most common serious infections were pneumonia, at a rate of 0.6 per 100 patient-years, followed by cellulitis, at a rate of 0.2 per 100 patient-years. Active tuberculosis infections also occurred at a rate of 0.2 per 100 patient-years. Malignancies occurred at a rate of 0.6 per 100 patient-years. These rates were similar to those reported by Dr. Burmester and his associates in 2013 using data from a small pool of patients – 23,458 – enrolled in 71 studies of adalimumab (Ann Rheum Dis. 2013 Apr;72[4]:517-24).
In the current study, Dr. Burmester and his coauthors analyzed the observed mortality rate of the adalimumab-treated patients against the mortality rates for the general populations in the various countries in which the studies were run, based on World Health Organization statistics for the period 1997-2006, and adjusted so that the age and sex of the comparison general populations matched the age and sex of the treated patients. This analysis showed an overall, statistically significant mortality reduction in patients receiving adalimumab of 35%, which was consistent in both the subgroups of men and women.
The observed mortality reduction linked with TNFi treatment is likely a class effect, Dr. Burmester said, although similar analyses have not been conducted using data from patients treated with other TNFis. So far, he has been unsuccessful in getting similar, large-scale trial data from manufacturers of other TNFis that he has approached, but Dr. Burmester said he hopes to eventually receive these data so that he can perform an even larger analysis.
The study was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Burmester has been a consultant to and speaker on behalf of AbbVie, as well as for Bristol Myers Squibb, Merk, Pfizer, Roche, and UCB.
SOURCE: Burmester GR et al. Ann Rheum Dis. 2018;77(Suppl 2):165. Abstract OP0233.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Major finding: Patients on a TNF inhibitor had 35% fewer deaths, compared with the age- and sex-matched general population.
Study details: Post-hoc analysis of data from 29,987 patients treated with adalimumab in 78 studies.
Disclosures: The study was sponsored by AbbVie, the company that markets adalimumab (Humira). Dr. Burmester has been a consultant to and speaker on behalf of AbbVie, as well as for Bristol Myers Squibb, Merk, Pfizer, Roche, and UCB.
Source: Burmester GR et al. Ann Rheum Dis. 2018;77(Suppl 2):165. Abstract OP0233.
Watch for substance use risks among never-deployed reservists
SAN DIEGO – Reserve soldiers who have never been deployed are just as likely to have poor outcomes related to mental health and substance use as their peers who have been deployed, results from a novel survey suggest.
“We tend to focus on screening and intervention efforts for soldiers who have been deployed and who have experienced combat, but we should also focus our intervention and screening efforts on soldiers who have never been deployed,” lead study author Rachel A. Hoopsick, MPH, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “Both are at risk for substance use and mental illness.”
Measures included the Non-Deployment Emotions Questionnaire, the Alcohol Use Disorders Identification Test, the National Institute on Drug Abuse Modified Alcohol, Smoking and Substance Involvement Screening Test 2.0, and the Marital Adjustment Test. The researchers used separate models to examine the relationship between nondeployment emotions and alcohol problems, frequent heavy drinking, current nonmedical use of prescription drugs, and current illicit drug use, and controlled for years of military service, number of military friends in the social network, and marital satisfaction.
Ms. Hoopsick reported results from 121 never-deployed, male soldiers who completed the survey at baseline and at a 1-year follow-up. Their mean age was 30 years, 76% were non-Hispanic white, and 84% had at least some college education. In addition, the soldiers had served a mean of 5.5 years, had a mean of 0.6 military friends in the social network, and had a mean marital satisfaction score of 114 out of 158 possible points.
On responses to the Non-Deployment Emotions Questionnaire, 65% of nondeployed soldiers felt guilt, 56% felt decreased value within his unit, 50% felt decreased camaraderie within his unit, and 50% felt decreased connectedness within his unit for having never been deployed. “We did anticipate that some of these soldiers were going to have poor outcomes related to their nondeployment emotions, but it was surprising to see how many of the soldiers did express having negative emotions related to having never been deployed,” Ms. Hoopsick said. After the researchers controlled for years of military service, the number of military friends in the soldier’s social network, and marital satisfaction, more negative nondeployment emotions were associated with a greater likelihood of alcohol problems (adjusted risk ratio, 1.06), frequent heavy drinking (ARR, 1.03), and current nonmedical use of prescription drugs (ARR, 1.21).
She acknowledged certain limitations of the study, including its cross-sectional nature. she said.
The National Institute on Drug Abuse provided funding to Ms. Hoopsick’s mentor and coauthor, Gregory G. Homish, PhD, and the study received additional funding from the National Center for Advancing Translational Sciences and the Health Resources and Services Administration.
SAN DIEGO – Reserve soldiers who have never been deployed are just as likely to have poor outcomes related to mental health and substance use as their peers who have been deployed, results from a novel survey suggest.
“We tend to focus on screening and intervention efforts for soldiers who have been deployed and who have experienced combat, but we should also focus our intervention and screening efforts on soldiers who have never been deployed,” lead study author Rachel A. Hoopsick, MPH, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “Both are at risk for substance use and mental illness.”
Measures included the Non-Deployment Emotions Questionnaire, the Alcohol Use Disorders Identification Test, the National Institute on Drug Abuse Modified Alcohol, Smoking and Substance Involvement Screening Test 2.0, and the Marital Adjustment Test. The researchers used separate models to examine the relationship between nondeployment emotions and alcohol problems, frequent heavy drinking, current nonmedical use of prescription drugs, and current illicit drug use, and controlled for years of military service, number of military friends in the social network, and marital satisfaction.
Ms. Hoopsick reported results from 121 never-deployed, male soldiers who completed the survey at baseline and at a 1-year follow-up. Their mean age was 30 years, 76% were non-Hispanic white, and 84% had at least some college education. In addition, the soldiers had served a mean of 5.5 years, had a mean of 0.6 military friends in the social network, and had a mean marital satisfaction score of 114 out of 158 possible points.
On responses to the Non-Deployment Emotions Questionnaire, 65% of nondeployed soldiers felt guilt, 56% felt decreased value within his unit, 50% felt decreased camaraderie within his unit, and 50% felt decreased connectedness within his unit for having never been deployed. “We did anticipate that some of these soldiers were going to have poor outcomes related to their nondeployment emotions, but it was surprising to see how many of the soldiers did express having negative emotions related to having never been deployed,” Ms. Hoopsick said. After the researchers controlled for years of military service, the number of military friends in the soldier’s social network, and marital satisfaction, more negative nondeployment emotions were associated with a greater likelihood of alcohol problems (adjusted risk ratio, 1.06), frequent heavy drinking (ARR, 1.03), and current nonmedical use of prescription drugs (ARR, 1.21).
She acknowledged certain limitations of the study, including its cross-sectional nature. she said.
The National Institute on Drug Abuse provided funding to Ms. Hoopsick’s mentor and coauthor, Gregory G. Homish, PhD, and the study received additional funding from the National Center for Advancing Translational Sciences and the Health Resources and Services Administration.
SAN DIEGO – Reserve soldiers who have never been deployed are just as likely to have poor outcomes related to mental health and substance use as their peers who have been deployed, results from a novel survey suggest.
“We tend to focus on screening and intervention efforts for soldiers who have been deployed and who have experienced combat, but we should also focus our intervention and screening efforts on soldiers who have never been deployed,” lead study author Rachel A. Hoopsick, MPH, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “Both are at risk for substance use and mental illness.”
Measures included the Non-Deployment Emotions Questionnaire, the Alcohol Use Disorders Identification Test, the National Institute on Drug Abuse Modified Alcohol, Smoking and Substance Involvement Screening Test 2.0, and the Marital Adjustment Test. The researchers used separate models to examine the relationship between nondeployment emotions and alcohol problems, frequent heavy drinking, current nonmedical use of prescription drugs, and current illicit drug use, and controlled for years of military service, number of military friends in the social network, and marital satisfaction.
Ms. Hoopsick reported results from 121 never-deployed, male soldiers who completed the survey at baseline and at a 1-year follow-up. Their mean age was 30 years, 76% were non-Hispanic white, and 84% had at least some college education. In addition, the soldiers had served a mean of 5.5 years, had a mean of 0.6 military friends in the social network, and had a mean marital satisfaction score of 114 out of 158 possible points.
On responses to the Non-Deployment Emotions Questionnaire, 65% of nondeployed soldiers felt guilt, 56% felt decreased value within his unit, 50% felt decreased camaraderie within his unit, and 50% felt decreased connectedness within his unit for having never been deployed. “We did anticipate that some of these soldiers were going to have poor outcomes related to their nondeployment emotions, but it was surprising to see how many of the soldiers did express having negative emotions related to having never been deployed,” Ms. Hoopsick said. After the researchers controlled for years of military service, the number of military friends in the soldier’s social network, and marital satisfaction, more negative nondeployment emotions were associated with a greater likelihood of alcohol problems (adjusted risk ratio, 1.06), frequent heavy drinking (ARR, 1.03), and current nonmedical use of prescription drugs (ARR, 1.21).
She acknowledged certain limitations of the study, including its cross-sectional nature. she said.
The National Institute on Drug Abuse provided funding to Ms. Hoopsick’s mentor and coauthor, Gregory G. Homish, PhD, and the study received additional funding from the National Center for Advancing Translational Sciences and the Health Resources and Services Administration.
REPORTING FROM CPDD 2018
Key clinical point: All military personnel, regardless of deployment status, could be at risk for substance use.
Major finding:. More negative nondeployment emotions were associated with a greater likelihood of alcohol problems (adjusted risk ratio, 1.06), frequent heavy drinking (ARR, 1.03), and current nonmedical use of prescription drugs (ARR, 1.21).
Study details: Responses from 121 never-deployed, male U.S. Army Reserve/National Guard soldiers who completed surveys at baseline and at a 1-year follow-up.
Disclosures: The National Institute on Drug Abuse provided funding to Ms. Hoopsick’s mentor and coauthor, Gregory G. Homish, PhD, and the study received additional funding from the National Center for Advancing Translational Sciences and the Health Resources and Services Administration.
Launching the Moderate to Severe Asthma Center of Excellence
The American College of Chest Physicians (CHEST) announces a new partnership with Medscape focused on supporting physicians in addressing the challenges of diagnosing and treating moderate to severe asthma. The Moderate to Severe Asthma Center of Excellence will provide news, expert commentary, and insights on challenging cases to physicians specializing in chest medicine, allergy, primary care, pediatrics, and emergency medicine.
Medscape is a leading source of clinical news, health information, and point-of-care tools for physicians and health-care professionals. This new Center of Excellence available on Medscape.com will explore the diagnostic, therapeutic, and prevention strategies associated with moderate to severe asthma, including the latest research and breakthroughs. Topics will include challenges in classifying and diagnosing disease; risks, benefits, and barriers to treatment; and impact on patients’ quality of life.
Don’t miss Dr. Aaron Holley’s video on “Diagnosing Severe Asthma: ‘Not as Easy as It Sounds’ ”
Visit the Moderate to Severe Asthma Center of Excellence at https://www.medscape.com/resource/moderate-severe-asthma
The American College of Chest Physicians (CHEST) announces a new partnership with Medscape focused on supporting physicians in addressing the challenges of diagnosing and treating moderate to severe asthma. The Moderate to Severe Asthma Center of Excellence will provide news, expert commentary, and insights on challenging cases to physicians specializing in chest medicine, allergy, primary care, pediatrics, and emergency medicine.
Medscape is a leading source of clinical news, health information, and point-of-care tools for physicians and health-care professionals. This new Center of Excellence available on Medscape.com will explore the diagnostic, therapeutic, and prevention strategies associated with moderate to severe asthma, including the latest research and breakthroughs. Topics will include challenges in classifying and diagnosing disease; risks, benefits, and barriers to treatment; and impact on patients’ quality of life.
Don’t miss Dr. Aaron Holley’s video on “Diagnosing Severe Asthma: ‘Not as Easy as It Sounds’ ”
Visit the Moderate to Severe Asthma Center of Excellence at https://www.medscape.com/resource/moderate-severe-asthma
The American College of Chest Physicians (CHEST) announces a new partnership with Medscape focused on supporting physicians in addressing the challenges of diagnosing and treating moderate to severe asthma. The Moderate to Severe Asthma Center of Excellence will provide news, expert commentary, and insights on challenging cases to physicians specializing in chest medicine, allergy, primary care, pediatrics, and emergency medicine.
Medscape is a leading source of clinical news, health information, and point-of-care tools for physicians and health-care professionals. This new Center of Excellence available on Medscape.com will explore the diagnostic, therapeutic, and prevention strategies associated with moderate to severe asthma, including the latest research and breakthroughs. Topics will include challenges in classifying and diagnosing disease; risks, benefits, and barriers to treatment; and impact on patients’ quality of life.
Don’t miss Dr. Aaron Holley’s video on “Diagnosing Severe Asthma: ‘Not as Easy as It Sounds’ ”
Visit the Moderate to Severe Asthma Center of Excellence at https://www.medscape.com/resource/moderate-severe-asthma
Galectin-3: A new post-MI prognostic biomarker?
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
REPORTING FROM ACC 18
Key clinical point: Galectin-3 level post-MI is a potent long-term predictor of both heart failure and mortality independent of known prognostic markers.
Major finding: Post-MI patients in the top tertile of circulating galectin-3 were at an adjusted 2.4-fold increased mortality risk and a 2.05-fold greater risk of developing heart failure compared with those in the lowest tertile.
Study details: This prospective population-based cohort study included 1,401 MI patients followed for a mean of 5.3 years.
Disclosures: The National Institutes of Health supported the study. The presenter reported having no financial conflicts of interest.