User login
Anticoagulants Safe With Enzyme-Inducing Meds for Epilepsy
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AES 2023
New Prior Auth Policy Tied to Delays, Discontinuation of Oral Cancer Meds
TOPLINE:
Imposing a new prior authorization requirement increased the likelihood that older patients with cancer will delay or stop filling their prescription for oral anticancer drugs, a new study showed.
METHODOLOGY:
- Prior authorization requirements, especially in oncology, continue to increase, but how these policies affect patients’ access to care remains less clear.
- Researchers analyzed Medicare Part D claims from 2010 to 2020 to assess the effects of prior authorization changes on prescriptions fills for 1 of 11 oral anticancer drugs.
- The study included 2495 patients filling a prescription for these medications prior to their health plan imposing a new prior authorization policy and 22,641 patients filling prescriptions for the same drugs with no change in prior authorization policy (control).
- Beneficiaries had at least three 30-day fills in the 120 days before the new prior authorization policy was established on January 1 and continued to be enrolled in the same plan 120 days after the policy change.
- The researchers focused on how often patients discontinued their therapy within 120 days following a prior authorization policy change, as well as the time to fill a prescription after this change.
TAKEAWAY:
- Patients subjected to a new prior authorization policy on an established drug had a sevenfold higher likelihood of stopping the drug within 120 days than those who had no change in prior authorization requirements (adjusted odds ratio, 7.1).
- The adjusted probability of discontinuing an oral cancer regimen within 120 days after an index date of January 1 (when most health plan policy changes occur) was 5.8% for those with a new prior authorization policy vs 1.4% for the control group.
- A new prior authorization requirement was also associated with an average 10-day delay to refill the first prescription following the policy change (P < .001).
- The probability of a delay of more than 30 days was 22% after a policy change vs 7% after no policy change.
IN PRACTICE:
“Our results suggest concerns about delayed and foregone care related to prior authorization are warranted,” the authors said. Overall, this study found that “prior authorization wasted time and undermined the policy priorities of access to care and oral anticancer drug adherence for patients who were regular users of a particular medication.”
SOURCE:
The study by Michael Anna Kyle, PhD, RN, and Nancy Keating, MD, MPH, with Harvard Medical School, Boston, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study did not look at patients starting new oral anticancer drugs, which may come with more complex prior authorization processes and create more significant access issues. The results are also limited to patients taking 1 of 11 oral anticancer agents in Medicare Part D.
DISCLOSURES:
Funding for the study was provided by the National Cancer Institute. The authors reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Imposing a new prior authorization requirement increased the likelihood that older patients with cancer will delay or stop filling their prescription for oral anticancer drugs, a new study showed.
METHODOLOGY:
- Prior authorization requirements, especially in oncology, continue to increase, but how these policies affect patients’ access to care remains less clear.
- Researchers analyzed Medicare Part D claims from 2010 to 2020 to assess the effects of prior authorization changes on prescriptions fills for 1 of 11 oral anticancer drugs.
- The study included 2495 patients filling a prescription for these medications prior to their health plan imposing a new prior authorization policy and 22,641 patients filling prescriptions for the same drugs with no change in prior authorization policy (control).
- Beneficiaries had at least three 30-day fills in the 120 days before the new prior authorization policy was established on January 1 and continued to be enrolled in the same plan 120 days after the policy change.
- The researchers focused on how often patients discontinued their therapy within 120 days following a prior authorization policy change, as well as the time to fill a prescription after this change.
TAKEAWAY:
- Patients subjected to a new prior authorization policy on an established drug had a sevenfold higher likelihood of stopping the drug within 120 days than those who had no change in prior authorization requirements (adjusted odds ratio, 7.1).
- The adjusted probability of discontinuing an oral cancer regimen within 120 days after an index date of January 1 (when most health plan policy changes occur) was 5.8% for those with a new prior authorization policy vs 1.4% for the control group.
- A new prior authorization requirement was also associated with an average 10-day delay to refill the first prescription following the policy change (P < .001).
- The probability of a delay of more than 30 days was 22% after a policy change vs 7% after no policy change.
IN PRACTICE:
“Our results suggest concerns about delayed and foregone care related to prior authorization are warranted,” the authors said. Overall, this study found that “prior authorization wasted time and undermined the policy priorities of access to care and oral anticancer drug adherence for patients who were regular users of a particular medication.”
SOURCE:
The study by Michael Anna Kyle, PhD, RN, and Nancy Keating, MD, MPH, with Harvard Medical School, Boston, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study did not look at patients starting new oral anticancer drugs, which may come with more complex prior authorization processes and create more significant access issues. The results are also limited to patients taking 1 of 11 oral anticancer agents in Medicare Part D.
DISCLOSURES:
Funding for the study was provided by the National Cancer Institute. The authors reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Imposing a new prior authorization requirement increased the likelihood that older patients with cancer will delay or stop filling their prescription for oral anticancer drugs, a new study showed.
METHODOLOGY:
- Prior authorization requirements, especially in oncology, continue to increase, but how these policies affect patients’ access to care remains less clear.
- Researchers analyzed Medicare Part D claims from 2010 to 2020 to assess the effects of prior authorization changes on prescriptions fills for 1 of 11 oral anticancer drugs.
- The study included 2495 patients filling a prescription for these medications prior to their health plan imposing a new prior authorization policy and 22,641 patients filling prescriptions for the same drugs with no change in prior authorization policy (control).
- Beneficiaries had at least three 30-day fills in the 120 days before the new prior authorization policy was established on January 1 and continued to be enrolled in the same plan 120 days after the policy change.
- The researchers focused on how often patients discontinued their therapy within 120 days following a prior authorization policy change, as well as the time to fill a prescription after this change.
TAKEAWAY:
- Patients subjected to a new prior authorization policy on an established drug had a sevenfold higher likelihood of stopping the drug within 120 days than those who had no change in prior authorization requirements (adjusted odds ratio, 7.1).
- The adjusted probability of discontinuing an oral cancer regimen within 120 days after an index date of January 1 (when most health plan policy changes occur) was 5.8% for those with a new prior authorization policy vs 1.4% for the control group.
- A new prior authorization requirement was also associated with an average 10-day delay to refill the first prescription following the policy change (P < .001).
- The probability of a delay of more than 30 days was 22% after a policy change vs 7% after no policy change.
IN PRACTICE:
“Our results suggest concerns about delayed and foregone care related to prior authorization are warranted,” the authors said. Overall, this study found that “prior authorization wasted time and undermined the policy priorities of access to care and oral anticancer drug adherence for patients who were regular users of a particular medication.”
SOURCE:
The study by Michael Anna Kyle, PhD, RN, and Nancy Keating, MD, MPH, with Harvard Medical School, Boston, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study did not look at patients starting new oral anticancer drugs, which may come with more complex prior authorization processes and create more significant access issues. The results are also limited to patients taking 1 of 11 oral anticancer agents in Medicare Part D.
DISCLOSURES:
Funding for the study was provided by the National Cancer Institute. The authors reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
‘Left in the Dark’: Prior Authorization Erodes Trust, Costs More
Mark Lewis, MD, saw the pain in his patient’s body. The man’s gastrointestinal tumor had metastasized to his bones. Even breathing had become agonizing.
It was a Friday afternoon. Dr. Lewis could see his patient would struggle to make it through the weekend without some pain relief.
When this happens, “the clock is ticking,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Health in Salt Lake City, Utah. “A patient, especially one with more advanced disease, only has so much time to wait for care.”
Dr. Lewis sent in an electronic request for an opioid prescription to help ease his patient’s pain through the weekend. Once the prescription had gone through, Dr. Lewis told his patient the medication should be ready to pick up at his local pharmacy.
Dr. Lewis left work that Friday feeling a little lighter, knowing the pain medication would help his patient over the weekend.
Moments after walking into the clinic on Monday morning, Dr. Lewis received an unexpected message: “Your patient is in the hospital.”
The events of the weekend soon unfolded.
Dr. Lewis learned that when his patient went to the pharmacy to pick up his pain medication, the pharmacist told him the prescription required prior authorization.
The patient left the pharmacy empty-handed. Hours later, he was in the emergency room (ER) in extreme pain — the exact situation Dr. Lewis had been trying to avoid.
Dr. Lewis felt a sense of powerlessness in that moment.
“I had been left in the dark,” he said. The oncologist-patient relationship is predicated on trust and “that trust is eroded when I can’t give my patients the care they need,” he explained. “I can’t stand overpromising and underdelivering to them.”
Dr. Lewis had received no communication from the insurer that the prescription required prior authorization, no red flag that the request had been denied, and no notification to call the insurer.
Although physicians may need to tread carefully when prescribing opioids over the long term, “this was simply a prescription for 2-3 days of opioids for the exact patient who the drugs were developed to benefit,” Dr. Lewis said. But instead, “he ended up in ER with a pain crisis.”
Prior authorization delays like this often mean patients pay the price.
“These delays are not trivial,” Dr. Lewis said.
A recent study, presented at the ASCO Quality Care Symposium in October, found that among 3304 supportive care prescriptions requiring prior authorization, insurance companies denied 8% of requests, with final denials taking as long as 78 days. Among approved prescriptions, about 40% happened on the same day, while the remaining took anywhere from 1 to 54 days.
Denying or delaying necessary and cost-effective care, even briefly, can harm patients and lead to higher costs. A 2022 survey from the American Medical Association found that instead of reducing low-value care as insurance companies claim, prior authorization often leads to higher overall use of healthcare resources. More specifically, almost half of physicians surveyed said that prior authorization led to an ER visit or need for immediate care.
In this patient’s case, filling the opioid prescription that Friday would have cost no more than $300, possibly as little as $30. The ER visit to manage the patient’s pain crisis costs thousands.
The major issue overall, Dr. Lewis said, is the disconnect between the time spent waiting for prior authorization approvals and the necessity of these treatments. Dr. Lewis says even standard chemotherapy often requires prior authorization.
“The currency we all share is time,” Dr. Lewis said. “But it often feels like there’s very little urgency on insurance company side to approve a treatment, which places a heavy weight on patients and physicians.”
“It just shouldn’t be this hard,” he said.
A version of this article appeared on Medscape.com as part of the Gatekeepers of Care series on issues oncologists and people with cancer face navigating health insurance company requirements. Read more about the series here. Please email [email protected] to share experiences with prior authorization or other challenges receiving care.
Mark Lewis, MD, saw the pain in his patient’s body. The man’s gastrointestinal tumor had metastasized to his bones. Even breathing had become agonizing.
It was a Friday afternoon. Dr. Lewis could see his patient would struggle to make it through the weekend without some pain relief.
When this happens, “the clock is ticking,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Health in Salt Lake City, Utah. “A patient, especially one with more advanced disease, only has so much time to wait for care.”
Dr. Lewis sent in an electronic request for an opioid prescription to help ease his patient’s pain through the weekend. Once the prescription had gone through, Dr. Lewis told his patient the medication should be ready to pick up at his local pharmacy.
Dr. Lewis left work that Friday feeling a little lighter, knowing the pain medication would help his patient over the weekend.
Moments after walking into the clinic on Monday morning, Dr. Lewis received an unexpected message: “Your patient is in the hospital.”
The events of the weekend soon unfolded.
Dr. Lewis learned that when his patient went to the pharmacy to pick up his pain medication, the pharmacist told him the prescription required prior authorization.
The patient left the pharmacy empty-handed. Hours later, he was in the emergency room (ER) in extreme pain — the exact situation Dr. Lewis had been trying to avoid.
Dr. Lewis felt a sense of powerlessness in that moment.
“I had been left in the dark,” he said. The oncologist-patient relationship is predicated on trust and “that trust is eroded when I can’t give my patients the care they need,” he explained. “I can’t stand overpromising and underdelivering to them.”
Dr. Lewis had received no communication from the insurer that the prescription required prior authorization, no red flag that the request had been denied, and no notification to call the insurer.
Although physicians may need to tread carefully when prescribing opioids over the long term, “this was simply a prescription for 2-3 days of opioids for the exact patient who the drugs were developed to benefit,” Dr. Lewis said. But instead, “he ended up in ER with a pain crisis.”
Prior authorization delays like this often mean patients pay the price.
“These delays are not trivial,” Dr. Lewis said.
A recent study, presented at the ASCO Quality Care Symposium in October, found that among 3304 supportive care prescriptions requiring prior authorization, insurance companies denied 8% of requests, with final denials taking as long as 78 days. Among approved prescriptions, about 40% happened on the same day, while the remaining took anywhere from 1 to 54 days.
Denying or delaying necessary and cost-effective care, even briefly, can harm patients and lead to higher costs. A 2022 survey from the American Medical Association found that instead of reducing low-value care as insurance companies claim, prior authorization often leads to higher overall use of healthcare resources. More specifically, almost half of physicians surveyed said that prior authorization led to an ER visit or need for immediate care.
In this patient’s case, filling the opioid prescription that Friday would have cost no more than $300, possibly as little as $30. The ER visit to manage the patient’s pain crisis costs thousands.
The major issue overall, Dr. Lewis said, is the disconnect between the time spent waiting for prior authorization approvals and the necessity of these treatments. Dr. Lewis says even standard chemotherapy often requires prior authorization.
“The currency we all share is time,” Dr. Lewis said. “But it often feels like there’s very little urgency on insurance company side to approve a treatment, which places a heavy weight on patients and physicians.”
“It just shouldn’t be this hard,” he said.
A version of this article appeared on Medscape.com as part of the Gatekeepers of Care series on issues oncologists and people with cancer face navigating health insurance company requirements. Read more about the series here. Please email [email protected] to share experiences with prior authorization or other challenges receiving care.
Mark Lewis, MD, saw the pain in his patient’s body. The man’s gastrointestinal tumor had metastasized to his bones. Even breathing had become agonizing.
It was a Friday afternoon. Dr. Lewis could see his patient would struggle to make it through the weekend without some pain relief.
When this happens, “the clock is ticking,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Health in Salt Lake City, Utah. “A patient, especially one with more advanced disease, only has so much time to wait for care.”
Dr. Lewis sent in an electronic request for an opioid prescription to help ease his patient’s pain through the weekend. Once the prescription had gone through, Dr. Lewis told his patient the medication should be ready to pick up at his local pharmacy.
Dr. Lewis left work that Friday feeling a little lighter, knowing the pain medication would help his patient over the weekend.
Moments after walking into the clinic on Monday morning, Dr. Lewis received an unexpected message: “Your patient is in the hospital.”
The events of the weekend soon unfolded.
Dr. Lewis learned that when his patient went to the pharmacy to pick up his pain medication, the pharmacist told him the prescription required prior authorization.
The patient left the pharmacy empty-handed. Hours later, he was in the emergency room (ER) in extreme pain — the exact situation Dr. Lewis had been trying to avoid.
Dr. Lewis felt a sense of powerlessness in that moment.
“I had been left in the dark,” he said. The oncologist-patient relationship is predicated on trust and “that trust is eroded when I can’t give my patients the care they need,” he explained. “I can’t stand overpromising and underdelivering to them.”
Dr. Lewis had received no communication from the insurer that the prescription required prior authorization, no red flag that the request had been denied, and no notification to call the insurer.
Although physicians may need to tread carefully when prescribing opioids over the long term, “this was simply a prescription for 2-3 days of opioids for the exact patient who the drugs were developed to benefit,” Dr. Lewis said. But instead, “he ended up in ER with a pain crisis.”
Prior authorization delays like this often mean patients pay the price.
“These delays are not trivial,” Dr. Lewis said.
A recent study, presented at the ASCO Quality Care Symposium in October, found that among 3304 supportive care prescriptions requiring prior authorization, insurance companies denied 8% of requests, with final denials taking as long as 78 days. Among approved prescriptions, about 40% happened on the same day, while the remaining took anywhere from 1 to 54 days.
Denying or delaying necessary and cost-effective care, even briefly, can harm patients and lead to higher costs. A 2022 survey from the American Medical Association found that instead of reducing low-value care as insurance companies claim, prior authorization often leads to higher overall use of healthcare resources. More specifically, almost half of physicians surveyed said that prior authorization led to an ER visit or need for immediate care.
In this patient’s case, filling the opioid prescription that Friday would have cost no more than $300, possibly as little as $30. The ER visit to manage the patient’s pain crisis costs thousands.
The major issue overall, Dr. Lewis said, is the disconnect between the time spent waiting for prior authorization approvals and the necessity of these treatments. Dr. Lewis says even standard chemotherapy often requires prior authorization.
“The currency we all share is time,” Dr. Lewis said. “But it often feels like there’s very little urgency on insurance company side to approve a treatment, which places a heavy weight on patients and physicians.”
“It just shouldn’t be this hard,” he said.
A version of this article appeared on Medscape.com as part of the Gatekeepers of Care series on issues oncologists and people with cancer face navigating health insurance company requirements. Read more about the series here. Please email [email protected] to share experiences with prior authorization or other challenges receiving care.
Are Post-Meal Insulin Surges Beneficial?
Rapid surges in insulin following a meal are associated with favorable long-term cardiometabolic benefits, including improvements in beta cell function and a lower risk for the development of prediabetes or diabetes, contrary to some concerns of the surges being indicative of more negative effects.
“There are practitioners who subscribe to this notion of higher insulin levels being a bad thing, and sometimes are making recommendations to patients to limit their insulin fluctuations after the meal,” said first author Ravi Retnakaran, MD, an endocrinologist and Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at the Leadership Sinai Centre for Diabetes at Mount Sinai Hospital, Toronto, Ontario, in a press statement.
“But it’s not that simple,” he said. “We observed that a robust post-challenge insulin secretory response, once adjusted for glucose levels, is only associated with beneficial metabolic effects.”
The findings were published on December 13, 2023, in eClinicalMedicine, part of The Lancet Discovery Science.
Insulin levels increase after food consumption in the normal management of blood glucose; however, some research has suggested that more rapid spikes in insulin, especially after a high-carbohydrate meal, are linked to an anabolic state contributing to weight gain and insulin resistance.
As public awareness of those reports has grown, “patients are coming in concerned about the possibility of their insulin levels being high, and there is confusion about the physiology of these effects,” Dr. Retnakaran told this news organization.
However, other studies have shown that the effects of insulin surges are important relative to baseline factors, including ambient glycemia and, specifically, baseline glucose levels prior to a meal.
Therefore, a more appropriate assessment is to use a corrected insulin response, measuring insulin secretion at 30 minutes after an oral glucose challenge, in relation to baseline glucose levels, research has suggested.
To investigate the issue in a longitudinal context, Dr. Retnakaran and colleagues conducted a prospective cohort study of 306 pregnant women representing a full range of glucose tolerance, who were enrolled at a hospital in Toronto between October 2003 and March 2014.
The women received comprehensive cardiometabolic testing, including oral glucose tolerance tests at 1-year, 3-year, and 5-year postpartum, and their baseline post-challenge insulinemia was established using corrected insulin response at 1 year.
Over 4 years of follow-up, a progressive worsening of cardiometabolic factors was associated with higher tertiles of corrected insulin responses at baseline, including waist circumference (P = .016), high-density lipoprotein (P = .018), C-reactive protein (CRP; P = .006), and insulin sensitivity (P < .001).
However, those trends were also associated with progressively improved beta cell function (P < .001).
After adjustment in the longitudinal analysis for the clinical risk factors for diabetes, including age, ethnicity, family history of diabetes, and body mass index (BMI) at 1 year, a higher corrected insulin response tertile at baseline was independently associated with improved Insulin Secretion-Sensitivity Index-2 and insulinogenic index/insulin resistance index (IGI/HOMA-IR), as well as lower glycemia, as observed on fasting and 2-hour glucose at 3 years and 5 years (all P < .001).
The insulin response was meanwhile not associated with BMI, waist, lipids, CRP, or insulin sensitivity or resistance.
Importantly, the highest corrected insulin response tertile at 1-year postpartum was also significantly associated with a lower risk for prediabetes or diabetes than the lowest tertile at 3 years (adjusted OR [aOR], 0.19) as well as 5 years (aOR, 0.18).
“The real question in my mind was whether we had the statistical power to be able to demonstrate a longitudinal beneficial effect on glucose regulation, but we did,” Dr. Retnakaran told this news organization. “The results show lower prediabetes and diabetes among people who had the most robust postprandial insulin excursion at 1-year postpartum.”
While the unadjusted analyses at baseline showed adverse as well as favorable outcomes, “adjusted longitudinal analyses revealed consistent independent associations of higher complete insulin response with better beta cell function, lower glycemia, and lower risk of prediabetes or diabetes in the years thereafter,” the authors reported.
“This evidence should help push back concern around the postprandial insulin spike,” Dr. Retnakaran said.
Commenting on the study, James D. Johnson, PhD, a professor of cellular and physiological sciences and director of the Life Sciences Institute at the University of British Columbia, Canada, noted that “it is already well-known that the loss of postprandial first phase insulin secretion can be a key and early defect in the transition to prediabetes and type 2 diabetes. That is not new, but the confirmatory data are welcome,” he told this news organization.
However, with other data linking high insulin with adiposity and insulin resistance, “the nuance and subtleties are critical for us to understand the directions of the causality,” he said.
“It is quite possible that both of these models are true at different life stages and/or in different people. There may be more than one pathway to diabetes. This is the nature of science and progress.”
A key caveat is that with a specific cohort of pregnant women, the question remains of the generalizability to men and to those younger or older than childbearing age.
Nevertheless, “I think this is an interesting and important study,” Dr. Johnson said. “More data on this topic is always welcome, but I am not sure this will be the final say in this debate.”
The authors and Dr. Johnson had no disclosures to report.
A version of this article appeared on Medscape.com.
Rapid surges in insulin following a meal are associated with favorable long-term cardiometabolic benefits, including improvements in beta cell function and a lower risk for the development of prediabetes or diabetes, contrary to some concerns of the surges being indicative of more negative effects.
“There are practitioners who subscribe to this notion of higher insulin levels being a bad thing, and sometimes are making recommendations to patients to limit their insulin fluctuations after the meal,” said first author Ravi Retnakaran, MD, an endocrinologist and Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at the Leadership Sinai Centre for Diabetes at Mount Sinai Hospital, Toronto, Ontario, in a press statement.
“But it’s not that simple,” he said. “We observed that a robust post-challenge insulin secretory response, once adjusted for glucose levels, is only associated with beneficial metabolic effects.”
The findings were published on December 13, 2023, in eClinicalMedicine, part of The Lancet Discovery Science.
Insulin levels increase after food consumption in the normal management of blood glucose; however, some research has suggested that more rapid spikes in insulin, especially after a high-carbohydrate meal, are linked to an anabolic state contributing to weight gain and insulin resistance.
As public awareness of those reports has grown, “patients are coming in concerned about the possibility of their insulin levels being high, and there is confusion about the physiology of these effects,” Dr. Retnakaran told this news organization.
However, other studies have shown that the effects of insulin surges are important relative to baseline factors, including ambient glycemia and, specifically, baseline glucose levels prior to a meal.
Therefore, a more appropriate assessment is to use a corrected insulin response, measuring insulin secretion at 30 minutes after an oral glucose challenge, in relation to baseline glucose levels, research has suggested.
To investigate the issue in a longitudinal context, Dr. Retnakaran and colleagues conducted a prospective cohort study of 306 pregnant women representing a full range of glucose tolerance, who were enrolled at a hospital in Toronto between October 2003 and March 2014.
The women received comprehensive cardiometabolic testing, including oral glucose tolerance tests at 1-year, 3-year, and 5-year postpartum, and their baseline post-challenge insulinemia was established using corrected insulin response at 1 year.
Over 4 years of follow-up, a progressive worsening of cardiometabolic factors was associated with higher tertiles of corrected insulin responses at baseline, including waist circumference (P = .016), high-density lipoprotein (P = .018), C-reactive protein (CRP; P = .006), and insulin sensitivity (P < .001).
However, those trends were also associated with progressively improved beta cell function (P < .001).
After adjustment in the longitudinal analysis for the clinical risk factors for diabetes, including age, ethnicity, family history of diabetes, and body mass index (BMI) at 1 year, a higher corrected insulin response tertile at baseline was independently associated with improved Insulin Secretion-Sensitivity Index-2 and insulinogenic index/insulin resistance index (IGI/HOMA-IR), as well as lower glycemia, as observed on fasting and 2-hour glucose at 3 years and 5 years (all P < .001).
The insulin response was meanwhile not associated with BMI, waist, lipids, CRP, or insulin sensitivity or resistance.
Importantly, the highest corrected insulin response tertile at 1-year postpartum was also significantly associated with a lower risk for prediabetes or diabetes than the lowest tertile at 3 years (adjusted OR [aOR], 0.19) as well as 5 years (aOR, 0.18).
“The real question in my mind was whether we had the statistical power to be able to demonstrate a longitudinal beneficial effect on glucose regulation, but we did,” Dr. Retnakaran told this news organization. “The results show lower prediabetes and diabetes among people who had the most robust postprandial insulin excursion at 1-year postpartum.”
While the unadjusted analyses at baseline showed adverse as well as favorable outcomes, “adjusted longitudinal analyses revealed consistent independent associations of higher complete insulin response with better beta cell function, lower glycemia, and lower risk of prediabetes or diabetes in the years thereafter,” the authors reported.
“This evidence should help push back concern around the postprandial insulin spike,” Dr. Retnakaran said.
Commenting on the study, James D. Johnson, PhD, a professor of cellular and physiological sciences and director of the Life Sciences Institute at the University of British Columbia, Canada, noted that “it is already well-known that the loss of postprandial first phase insulin secretion can be a key and early defect in the transition to prediabetes and type 2 diabetes. That is not new, but the confirmatory data are welcome,” he told this news organization.
However, with other data linking high insulin with adiposity and insulin resistance, “the nuance and subtleties are critical for us to understand the directions of the causality,” he said.
“It is quite possible that both of these models are true at different life stages and/or in different people. There may be more than one pathway to diabetes. This is the nature of science and progress.”
A key caveat is that with a specific cohort of pregnant women, the question remains of the generalizability to men and to those younger or older than childbearing age.
Nevertheless, “I think this is an interesting and important study,” Dr. Johnson said. “More data on this topic is always welcome, but I am not sure this will be the final say in this debate.”
The authors and Dr. Johnson had no disclosures to report.
A version of this article appeared on Medscape.com.
Rapid surges in insulin following a meal are associated with favorable long-term cardiometabolic benefits, including improvements in beta cell function and a lower risk for the development of prediabetes or diabetes, contrary to some concerns of the surges being indicative of more negative effects.
“There are practitioners who subscribe to this notion of higher insulin levels being a bad thing, and sometimes are making recommendations to patients to limit their insulin fluctuations after the meal,” said first author Ravi Retnakaran, MD, an endocrinologist and Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at the Leadership Sinai Centre for Diabetes at Mount Sinai Hospital, Toronto, Ontario, in a press statement.
“But it’s not that simple,” he said. “We observed that a robust post-challenge insulin secretory response, once adjusted for glucose levels, is only associated with beneficial metabolic effects.”
The findings were published on December 13, 2023, in eClinicalMedicine, part of The Lancet Discovery Science.
Insulin levels increase after food consumption in the normal management of blood glucose; however, some research has suggested that more rapid spikes in insulin, especially after a high-carbohydrate meal, are linked to an anabolic state contributing to weight gain and insulin resistance.
As public awareness of those reports has grown, “patients are coming in concerned about the possibility of their insulin levels being high, and there is confusion about the physiology of these effects,” Dr. Retnakaran told this news organization.
However, other studies have shown that the effects of insulin surges are important relative to baseline factors, including ambient glycemia and, specifically, baseline glucose levels prior to a meal.
Therefore, a more appropriate assessment is to use a corrected insulin response, measuring insulin secretion at 30 minutes after an oral glucose challenge, in relation to baseline glucose levels, research has suggested.
To investigate the issue in a longitudinal context, Dr. Retnakaran and colleagues conducted a prospective cohort study of 306 pregnant women representing a full range of glucose tolerance, who were enrolled at a hospital in Toronto between October 2003 and March 2014.
The women received comprehensive cardiometabolic testing, including oral glucose tolerance tests at 1-year, 3-year, and 5-year postpartum, and their baseline post-challenge insulinemia was established using corrected insulin response at 1 year.
Over 4 years of follow-up, a progressive worsening of cardiometabolic factors was associated with higher tertiles of corrected insulin responses at baseline, including waist circumference (P = .016), high-density lipoprotein (P = .018), C-reactive protein (CRP; P = .006), and insulin sensitivity (P < .001).
However, those trends were also associated with progressively improved beta cell function (P < .001).
After adjustment in the longitudinal analysis for the clinical risk factors for diabetes, including age, ethnicity, family history of diabetes, and body mass index (BMI) at 1 year, a higher corrected insulin response tertile at baseline was independently associated with improved Insulin Secretion-Sensitivity Index-2 and insulinogenic index/insulin resistance index (IGI/HOMA-IR), as well as lower glycemia, as observed on fasting and 2-hour glucose at 3 years and 5 years (all P < .001).
The insulin response was meanwhile not associated with BMI, waist, lipids, CRP, or insulin sensitivity or resistance.
Importantly, the highest corrected insulin response tertile at 1-year postpartum was also significantly associated with a lower risk for prediabetes or diabetes than the lowest tertile at 3 years (adjusted OR [aOR], 0.19) as well as 5 years (aOR, 0.18).
“The real question in my mind was whether we had the statistical power to be able to demonstrate a longitudinal beneficial effect on glucose regulation, but we did,” Dr. Retnakaran told this news organization. “The results show lower prediabetes and diabetes among people who had the most robust postprandial insulin excursion at 1-year postpartum.”
While the unadjusted analyses at baseline showed adverse as well as favorable outcomes, “adjusted longitudinal analyses revealed consistent independent associations of higher complete insulin response with better beta cell function, lower glycemia, and lower risk of prediabetes or diabetes in the years thereafter,” the authors reported.
“This evidence should help push back concern around the postprandial insulin spike,” Dr. Retnakaran said.
Commenting on the study, James D. Johnson, PhD, a professor of cellular and physiological sciences and director of the Life Sciences Institute at the University of British Columbia, Canada, noted that “it is already well-known that the loss of postprandial first phase insulin secretion can be a key and early defect in the transition to prediabetes and type 2 diabetes. That is not new, but the confirmatory data are welcome,” he told this news organization.
However, with other data linking high insulin with adiposity and insulin resistance, “the nuance and subtleties are critical for us to understand the directions of the causality,” he said.
“It is quite possible that both of these models are true at different life stages and/or in different people. There may be more than one pathway to diabetes. This is the nature of science and progress.”
A key caveat is that with a specific cohort of pregnant women, the question remains of the generalizability to men and to those younger or older than childbearing age.
Nevertheless, “I think this is an interesting and important study,” Dr. Johnson said. “More data on this topic is always welcome, but I am not sure this will be the final say in this debate.”
The authors and Dr. Johnson had no disclosures to report.
A version of this article appeared on Medscape.com.
Circadian Blood Pressure Shifts Earlier in Children With Moderate to Severe OSA
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
What Causes One of Stroke’s Most Common Complications?
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
Newborn Recipient of Partial Heart Transplant Doing Well
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FDA Investigates Three Side Effects Reported With Weight Loss Drugs
or two other health problems.
A new FDA report listed potential links between the medications and alopecia, aspiration, or suicidal ideation, CBS News reported. The investigation centers on reports of the health problems among people taking GLP-1 receptor agonists, some of which are Ozempic, Wegovy, Mounjaro, and Zepbound. The drugs are used to treat diabetes and overweight or obesity.
An investigation by the FDA doesn’t mean that the FDA has concluded a risk exists, the FDA’s webpage for risk evaluation cautions.
“It means that FDA has identified a potential safety issue, but it does not mean that FDA has identified a causal relationship between the drug and the listed risk,” the FDA site states.
Possible next steps after an investigation could include updating drug labels with new information, putting a risk management plan in place to prevent or manage the health risks, or gathering more information.
“The FDA monitors the safety of drugs throughout their life cycle,” even after the drugs are approved. In addition, the FDA uses “surveillance and risk assessment programs to identify and evaluate adverse events that did not appear during the drug development process,” FDA spokesperson Chanapa Tantibanchachai said in an email published by multiple news outlets.
Although an investigation may lead to no changes in how a drug is regulated by the FDA, this isn’t the first time that the popular medicines have landed on the FDA’s radar for safety reevaluation. Last year, the label for the drug Ozempic was updated to acknowledge reports of intestinal obstructions, CBS News reported.
European regulators are also looking into reports of suicidal thoughts among people taking GLP-1 receptor agonists, although no link has been established.
Concerns about aspiration during surgery resulted in the American Society of Anesthesiologists advising in June that people should stop taking GLP-1 receptor agonists before they have elective surgeries.
“While there is currently a lack of scientific data on how GLP-1 receptor agonists affect patients having surgery and interact with anesthesia, we’ve received anecdotal reports that the delay in stomach emptying could be associated with an increased risk of regurgitation and aspiration of food into the airways and lungs during general anesthesia and deep sedation,” the society’s president, Michael W. Champeau, MD, said in a statement at the time.
According to CBS News, the FDA’s drug reporting system links the medications to 201 reports of suicide or suicidal ideation, 18 reports that mention aspiration, and 422 reports that mention alopecia.
Novo Nordisk, whose portfolio includes Wegovy and Ozempic, told CNN that it works with the FDA to monitor safety and is aware of the reports of side effects.
“Novo Nordisk stands behind the safety and efficacy of all of our GLP-1RA medicines when they are used as indicated and when they are taken under the care of a licensed healthcare professional,” the company said in a statement to CNN.
A spokesperson for Eli Lilly, which makes Mounjaro and Zepbound, told CBS News in a statement, “Currently, the FDA is reviewing data on certain potential risks for GLP-1 receptor agonist medicines. Patient safety is our priority, and we are collaborating with the FDA on these potential signals.”
A version of this article appeared on WebMD.com .
or two other health problems.
A new FDA report listed potential links between the medications and alopecia, aspiration, or suicidal ideation, CBS News reported. The investigation centers on reports of the health problems among people taking GLP-1 receptor agonists, some of which are Ozempic, Wegovy, Mounjaro, and Zepbound. The drugs are used to treat diabetes and overweight or obesity.
An investigation by the FDA doesn’t mean that the FDA has concluded a risk exists, the FDA’s webpage for risk evaluation cautions.
“It means that FDA has identified a potential safety issue, but it does not mean that FDA has identified a causal relationship between the drug and the listed risk,” the FDA site states.
Possible next steps after an investigation could include updating drug labels with new information, putting a risk management plan in place to prevent or manage the health risks, or gathering more information.
“The FDA monitors the safety of drugs throughout their life cycle,” even after the drugs are approved. In addition, the FDA uses “surveillance and risk assessment programs to identify and evaluate adverse events that did not appear during the drug development process,” FDA spokesperson Chanapa Tantibanchachai said in an email published by multiple news outlets.
Although an investigation may lead to no changes in how a drug is regulated by the FDA, this isn’t the first time that the popular medicines have landed on the FDA’s radar for safety reevaluation. Last year, the label for the drug Ozempic was updated to acknowledge reports of intestinal obstructions, CBS News reported.
European regulators are also looking into reports of suicidal thoughts among people taking GLP-1 receptor agonists, although no link has been established.
Concerns about aspiration during surgery resulted in the American Society of Anesthesiologists advising in June that people should stop taking GLP-1 receptor agonists before they have elective surgeries.
“While there is currently a lack of scientific data on how GLP-1 receptor agonists affect patients having surgery and interact with anesthesia, we’ve received anecdotal reports that the delay in stomach emptying could be associated with an increased risk of regurgitation and aspiration of food into the airways and lungs during general anesthesia and deep sedation,” the society’s president, Michael W. Champeau, MD, said in a statement at the time.
According to CBS News, the FDA’s drug reporting system links the medications to 201 reports of suicide or suicidal ideation, 18 reports that mention aspiration, and 422 reports that mention alopecia.
Novo Nordisk, whose portfolio includes Wegovy and Ozempic, told CNN that it works with the FDA to monitor safety and is aware of the reports of side effects.
“Novo Nordisk stands behind the safety and efficacy of all of our GLP-1RA medicines when they are used as indicated and when they are taken under the care of a licensed healthcare professional,” the company said in a statement to CNN.
A spokesperson for Eli Lilly, which makes Mounjaro and Zepbound, told CBS News in a statement, “Currently, the FDA is reviewing data on certain potential risks for GLP-1 receptor agonist medicines. Patient safety is our priority, and we are collaborating with the FDA on these potential signals.”
A version of this article appeared on WebMD.com .
or two other health problems.
A new FDA report listed potential links between the medications and alopecia, aspiration, or suicidal ideation, CBS News reported. The investigation centers on reports of the health problems among people taking GLP-1 receptor agonists, some of which are Ozempic, Wegovy, Mounjaro, and Zepbound. The drugs are used to treat diabetes and overweight or obesity.
An investigation by the FDA doesn’t mean that the FDA has concluded a risk exists, the FDA’s webpage for risk evaluation cautions.
“It means that FDA has identified a potential safety issue, but it does not mean that FDA has identified a causal relationship between the drug and the listed risk,” the FDA site states.
Possible next steps after an investigation could include updating drug labels with new information, putting a risk management plan in place to prevent or manage the health risks, or gathering more information.
“The FDA monitors the safety of drugs throughout their life cycle,” even after the drugs are approved. In addition, the FDA uses “surveillance and risk assessment programs to identify and evaluate adverse events that did not appear during the drug development process,” FDA spokesperson Chanapa Tantibanchachai said in an email published by multiple news outlets.
Although an investigation may lead to no changes in how a drug is regulated by the FDA, this isn’t the first time that the popular medicines have landed on the FDA’s radar for safety reevaluation. Last year, the label for the drug Ozempic was updated to acknowledge reports of intestinal obstructions, CBS News reported.
European regulators are also looking into reports of suicidal thoughts among people taking GLP-1 receptor agonists, although no link has been established.
Concerns about aspiration during surgery resulted in the American Society of Anesthesiologists advising in June that people should stop taking GLP-1 receptor agonists before they have elective surgeries.
“While there is currently a lack of scientific data on how GLP-1 receptor agonists affect patients having surgery and interact with anesthesia, we’ve received anecdotal reports that the delay in stomach emptying could be associated with an increased risk of regurgitation and aspiration of food into the airways and lungs during general anesthesia and deep sedation,” the society’s president, Michael W. Champeau, MD, said in a statement at the time.
According to CBS News, the FDA’s drug reporting system links the medications to 201 reports of suicide or suicidal ideation, 18 reports that mention aspiration, and 422 reports that mention alopecia.
Novo Nordisk, whose portfolio includes Wegovy and Ozempic, told CNN that it works with the FDA to monitor safety and is aware of the reports of side effects.
“Novo Nordisk stands behind the safety and efficacy of all of our GLP-1RA medicines when they are used as indicated and when they are taken under the care of a licensed healthcare professional,” the company said in a statement to CNN.
A spokesperson for Eli Lilly, which makes Mounjaro and Zepbound, told CBS News in a statement, “Currently, the FDA is reviewing data on certain potential risks for GLP-1 receptor agonist medicines. Patient safety is our priority, and we are collaborating with the FDA on these potential signals.”
A version of this article appeared on WebMD.com .
Evidence Grows for SGLT2 Inhibitors in Rheumatology
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
Asymptomatic Violaceous Plaques on the Face and Back
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1
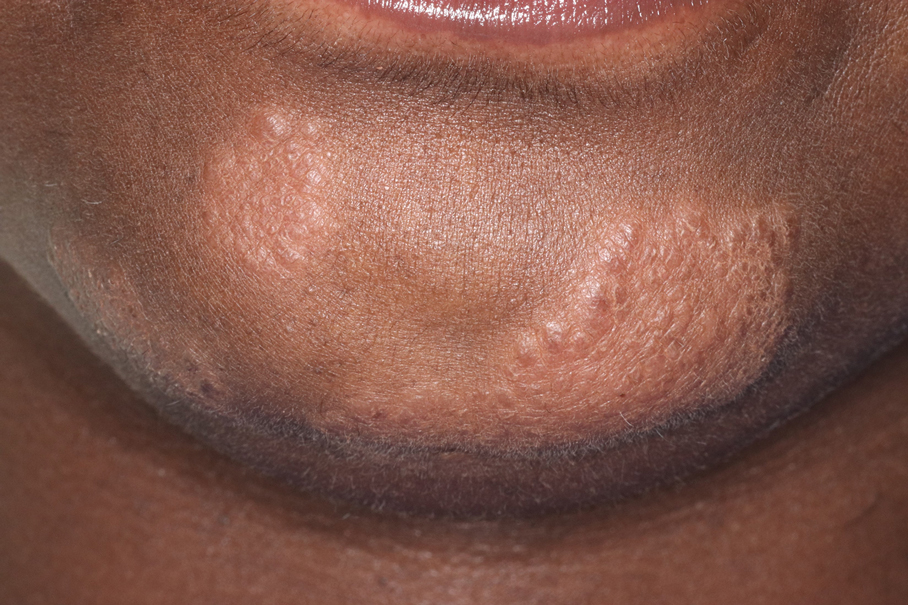
Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1

Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1

Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
A 35-year-old Black woman presented to dermatology as a new patient for evaluation of an asymptomatic rash that had enlarged and spread to involve both the face and back over the last 4 months. She had not tried any treatments. She had no notable medical history and was uncertain of her family history. Physical examination showed indurated, flesh-colored to violaceous plaques around the alar-facial groove (top), nasal tip, chin, and back (bottom). The mucosae and nails were not involved.