User login
Long-Term Follow-Up of Ocrelizumab-Treated Patient With Relapsing MS
Five-year follow-up data show low levels of disability progression.
BERLIN—Switching from interferon beta-1a to ocrelizumab after two years at the start of the OPERA I and OPERA II open-label extension period was associated with a rapid reduction in annualized release rate, according to a report presented at ECTRIMS 2018. “Both patients who continued treatment with ocrelizumab as well as those who were switched from interferon beta-1a to ocrelizumab maintained their robust reduction in annualized relapse rate through the three-year follow-up of the open-label extension period,” said lead author Stephen L. Hauser, MD, Director of the UCSF Weill Institute for Neurosciences, University of California, San Francisco, and colleagues.
“After five years of follow-up, the proportion of patients with disability progression was lower in patients who initiated ocrelizumab treatment earlier, compared with patients who received initial interferon treatment before switching to ocrelizumab, showing that patients who initiated ocrelizumab two years earlier accrued significant and sustained reductions in disability progression compared with patients switching from interferon therapy.”
The efficacy and safety of ocrelizumab in relapsing multiple sclerosis (MS) were demonstrated in the 96-week double-blind control period of OPERA I and OPERA II, and results for the two-year follow-up of the pooled OPERA I and OPERA II open-label extension period have previously been reported. For this study, Dr. Hauser and colleagues sought to assess the efficacy of switching to or maintaining ocrelizumab therapy on clinical measures of disease activity and progression after three years of follow-up in the open-label extension period of the OPERA I and OPERA II phase III trials in relapsing MS.
At the start of the open-label extension period, patients continued ocrelizumab therapy or were switched from interferon beta-1a to ocrelizumab. The researchers analyzed adjusted annualized relapse rate (ARR), time to onset of 24-week confirmed disability progression, and change in adjusted mean Expanded Disability Status Scale (EDSS) score from baseline.
Overall, 88.6% of patients who entered the open-label extension completed year three of follow-up. Among patients who switched therapy, annualized release rate decreased from 0.20 in the year preswitch to 0.10, 0.08, and 0.07 at years one, two, and three postswitch. Those patients who continued on ocrelizumab maintained a low annualized relapse rate through the year prior to the open-label extension and the three years of the open-label extension period (0.13, 0.11, 0.08, and 0.07). In addition, those patients who continued on ocrelizumab versus those who switched therapy had lower proportions of patients with 24-week confirmed disability progression in the year preswitch and years one, two, and three of the open-label extension period (7.7%/12.0%, 10.1%/15.6%, 13.9%/18.1%, and 16.1%/21.3%).
This study was sponsored by F. Hoffmann-La Roche, and writing and editorial assistance was provided by Articulate Science, UK.
Five-year follow-up data show low levels of disability progression.
Five-year follow-up data show low levels of disability progression.
BERLIN—Switching from interferon beta-1a to ocrelizumab after two years at the start of the OPERA I and OPERA II open-label extension period was associated with a rapid reduction in annualized release rate, according to a report presented at ECTRIMS 2018. “Both patients who continued treatment with ocrelizumab as well as those who were switched from interferon beta-1a to ocrelizumab maintained their robust reduction in annualized relapse rate through the three-year follow-up of the open-label extension period,” said lead author Stephen L. Hauser, MD, Director of the UCSF Weill Institute for Neurosciences, University of California, San Francisco, and colleagues.
“After five years of follow-up, the proportion of patients with disability progression was lower in patients who initiated ocrelizumab treatment earlier, compared with patients who received initial interferon treatment before switching to ocrelizumab, showing that patients who initiated ocrelizumab two years earlier accrued significant and sustained reductions in disability progression compared with patients switching from interferon therapy.”
The efficacy and safety of ocrelizumab in relapsing multiple sclerosis (MS) were demonstrated in the 96-week double-blind control period of OPERA I and OPERA II, and results for the two-year follow-up of the pooled OPERA I and OPERA II open-label extension period have previously been reported. For this study, Dr. Hauser and colleagues sought to assess the efficacy of switching to or maintaining ocrelizumab therapy on clinical measures of disease activity and progression after three years of follow-up in the open-label extension period of the OPERA I and OPERA II phase III trials in relapsing MS.
At the start of the open-label extension period, patients continued ocrelizumab therapy or were switched from interferon beta-1a to ocrelizumab. The researchers analyzed adjusted annualized relapse rate (ARR), time to onset of 24-week confirmed disability progression, and change in adjusted mean Expanded Disability Status Scale (EDSS) score from baseline.
Overall, 88.6% of patients who entered the open-label extension completed year three of follow-up. Among patients who switched therapy, annualized release rate decreased from 0.20 in the year preswitch to 0.10, 0.08, and 0.07 at years one, two, and three postswitch. Those patients who continued on ocrelizumab maintained a low annualized relapse rate through the year prior to the open-label extension and the three years of the open-label extension period (0.13, 0.11, 0.08, and 0.07). In addition, those patients who continued on ocrelizumab versus those who switched therapy had lower proportions of patients with 24-week confirmed disability progression in the year preswitch and years one, two, and three of the open-label extension period (7.7%/12.0%, 10.1%/15.6%, 13.9%/18.1%, and 16.1%/21.3%).
This study was sponsored by F. Hoffmann-La Roche, and writing and editorial assistance was provided by Articulate Science, UK.
BERLIN—Switching from interferon beta-1a to ocrelizumab after two years at the start of the OPERA I and OPERA II open-label extension period was associated with a rapid reduction in annualized release rate, according to a report presented at ECTRIMS 2018. “Both patients who continued treatment with ocrelizumab as well as those who were switched from interferon beta-1a to ocrelizumab maintained their robust reduction in annualized relapse rate through the three-year follow-up of the open-label extension period,” said lead author Stephen L. Hauser, MD, Director of the UCSF Weill Institute for Neurosciences, University of California, San Francisco, and colleagues.
“After five years of follow-up, the proportion of patients with disability progression was lower in patients who initiated ocrelizumab treatment earlier, compared with patients who received initial interferon treatment before switching to ocrelizumab, showing that patients who initiated ocrelizumab two years earlier accrued significant and sustained reductions in disability progression compared with patients switching from interferon therapy.”
The efficacy and safety of ocrelizumab in relapsing multiple sclerosis (MS) were demonstrated in the 96-week double-blind control period of OPERA I and OPERA II, and results for the two-year follow-up of the pooled OPERA I and OPERA II open-label extension period have previously been reported. For this study, Dr. Hauser and colleagues sought to assess the efficacy of switching to or maintaining ocrelizumab therapy on clinical measures of disease activity and progression after three years of follow-up in the open-label extension period of the OPERA I and OPERA II phase III trials in relapsing MS.
At the start of the open-label extension period, patients continued ocrelizumab therapy or were switched from interferon beta-1a to ocrelizumab. The researchers analyzed adjusted annualized relapse rate (ARR), time to onset of 24-week confirmed disability progression, and change in adjusted mean Expanded Disability Status Scale (EDSS) score from baseline.
Overall, 88.6% of patients who entered the open-label extension completed year three of follow-up. Among patients who switched therapy, annualized release rate decreased from 0.20 in the year preswitch to 0.10, 0.08, and 0.07 at years one, two, and three postswitch. Those patients who continued on ocrelizumab maintained a low annualized relapse rate through the year prior to the open-label extension and the three years of the open-label extension period (0.13, 0.11, 0.08, and 0.07). In addition, those patients who continued on ocrelizumab versus those who switched therapy had lower proportions of patients with 24-week confirmed disability progression in the year preswitch and years one, two, and three of the open-label extension period (7.7%/12.0%, 10.1%/15.6%, 13.9%/18.1%, and 16.1%/21.3%).
This study was sponsored by F. Hoffmann-La Roche, and writing and editorial assistance was provided by Articulate Science, UK.
Ongoing Neuronal Loss Is Greater in Secondary Progressive MS Than Primary Progressive MS
Serum levels of neurofilament light chain may be a prognostic biomarker of brain atrophy.
BERLIN—Levels of neurofilament light chain (NfL) indicate that patients with secondary progressive multiple sclerosis (MS) have more ongoing neuronal loss than patients with primary progressive MS of comparable age, both in the presence and in absence of gadolinium enhancing lesions, according to research presented at ECTRIMS 2018. In secondary progressive MS and primary progressive MS, NfL may serve as a prognostic marker of brain atrophy, said the investigators.
NfL is considered a blood biomarker for monitoring neuronal damage, disease activity, and treatment response in MS. Most studies of blood NfL have focused on patients with relapsing-remitting MS, and little is known about blood NfL levels in patients with progressive MS.
Jens Kuhle, MD, PhD, Head of the MS Center at University Hospital Basel in Switzerland, and colleagues, compared baseline blood NfL levels and assessed the prognostic potential of NfL for brain atrophy in patients with primary progressive MS and secondary progressive MS in placebo-controlled phase III trials of fingolimod (ie, INFORMS) and siponimod (ie, EXPAND).
The researchers retrospectively analyzed blood NfL levels in 1,452 patients with secondary progressive MS (mean age, 48.2; Expanded Disability Status Scale [EDSS], 5.4) and 378 patients with primary progressive MS (mean age, 48.7; EDSS, 4.6). They quantified NfL levels at baseline using single molecule array technology and grouped them into the categories of low (< 30 pg/mL), medium (30–60 pg/mL), and high (> 60 pg/mL). High and low baseline NfL categories were compared using Chi-square and Wilcoxon rank sum tests. Dr. Kuhle and colleagues examined the association of baseline NfL levels with MRI parameters by Spearman rank correlation (gadolinium enhancing lesion count, T2 lesion volume) and the Jonckheere Terpstra test (brain volume change).
NfL levels at baseline were higher in patients with secondary progressive MS than in patients with primary progressive MS (32.1 pg/mL vs 22.0 pg/mL). A similar trend was observed when patients of the same age were compared. Patients with secondary progressive MS had higher NfL levels than those with primary progressive MS.
Similarly, patients with no gadolinium enhancing lesions at baseline had NfL levels of 29.2 pg/mL and 21.0 pg/mL in secondary progressive MS and primary progressive MS, respectively, while patients with gadolinium enhancing lesions had NfL levels of 45.0 pg/mL in secondary progressive MS and 34.0 pg/mL in primary progressive MS. The gadolinium enhancing lesion count and T2 lesion volume at baseline correlated best with baseline NfL. In secondary progressive MS and primary progressive MS, high NfL at baseline was associated with higher percentage of brain volume loss at Month 12 (high NfL vs low NfL: −0.8% vs −0.2% in secondary progressive MS and −0.8% vs −0.4% in primary progressive MS) and at Month 24 (−1.5% vs −0.5% in secondary progressive MS and −1.9% vs −0.8% in primary progressive MS).
The study was funded by Novartis Pharma.
Serum levels of neurofilament light chain may be a prognostic biomarker of brain atrophy.
Serum levels of neurofilament light chain may be a prognostic biomarker of brain atrophy.
BERLIN—Levels of neurofilament light chain (NfL) indicate that patients with secondary progressive multiple sclerosis (MS) have more ongoing neuronal loss than patients with primary progressive MS of comparable age, both in the presence and in absence of gadolinium enhancing lesions, according to research presented at ECTRIMS 2018. In secondary progressive MS and primary progressive MS, NfL may serve as a prognostic marker of brain atrophy, said the investigators.
NfL is considered a blood biomarker for monitoring neuronal damage, disease activity, and treatment response in MS. Most studies of blood NfL have focused on patients with relapsing-remitting MS, and little is known about blood NfL levels in patients with progressive MS.
Jens Kuhle, MD, PhD, Head of the MS Center at University Hospital Basel in Switzerland, and colleagues, compared baseline blood NfL levels and assessed the prognostic potential of NfL for brain atrophy in patients with primary progressive MS and secondary progressive MS in placebo-controlled phase III trials of fingolimod (ie, INFORMS) and siponimod (ie, EXPAND).
The researchers retrospectively analyzed blood NfL levels in 1,452 patients with secondary progressive MS (mean age, 48.2; Expanded Disability Status Scale [EDSS], 5.4) and 378 patients with primary progressive MS (mean age, 48.7; EDSS, 4.6). They quantified NfL levels at baseline using single molecule array technology and grouped them into the categories of low (< 30 pg/mL), medium (30–60 pg/mL), and high (> 60 pg/mL). High and low baseline NfL categories were compared using Chi-square and Wilcoxon rank sum tests. Dr. Kuhle and colleagues examined the association of baseline NfL levels with MRI parameters by Spearman rank correlation (gadolinium enhancing lesion count, T2 lesion volume) and the Jonckheere Terpstra test (brain volume change).
NfL levels at baseline were higher in patients with secondary progressive MS than in patients with primary progressive MS (32.1 pg/mL vs 22.0 pg/mL). A similar trend was observed when patients of the same age were compared. Patients with secondary progressive MS had higher NfL levels than those with primary progressive MS.
Similarly, patients with no gadolinium enhancing lesions at baseline had NfL levels of 29.2 pg/mL and 21.0 pg/mL in secondary progressive MS and primary progressive MS, respectively, while patients with gadolinium enhancing lesions had NfL levels of 45.0 pg/mL in secondary progressive MS and 34.0 pg/mL in primary progressive MS. The gadolinium enhancing lesion count and T2 lesion volume at baseline correlated best with baseline NfL. In secondary progressive MS and primary progressive MS, high NfL at baseline was associated with higher percentage of brain volume loss at Month 12 (high NfL vs low NfL: −0.8% vs −0.2% in secondary progressive MS and −0.8% vs −0.4% in primary progressive MS) and at Month 24 (−1.5% vs −0.5% in secondary progressive MS and −1.9% vs −0.8% in primary progressive MS).
The study was funded by Novartis Pharma.
BERLIN—Levels of neurofilament light chain (NfL) indicate that patients with secondary progressive multiple sclerosis (MS) have more ongoing neuronal loss than patients with primary progressive MS of comparable age, both in the presence and in absence of gadolinium enhancing lesions, according to research presented at ECTRIMS 2018. In secondary progressive MS and primary progressive MS, NfL may serve as a prognostic marker of brain atrophy, said the investigators.
NfL is considered a blood biomarker for monitoring neuronal damage, disease activity, and treatment response in MS. Most studies of blood NfL have focused on patients with relapsing-remitting MS, and little is known about blood NfL levels in patients with progressive MS.
Jens Kuhle, MD, PhD, Head of the MS Center at University Hospital Basel in Switzerland, and colleagues, compared baseline blood NfL levels and assessed the prognostic potential of NfL for brain atrophy in patients with primary progressive MS and secondary progressive MS in placebo-controlled phase III trials of fingolimod (ie, INFORMS) and siponimod (ie, EXPAND).
The researchers retrospectively analyzed blood NfL levels in 1,452 patients with secondary progressive MS (mean age, 48.2; Expanded Disability Status Scale [EDSS], 5.4) and 378 patients with primary progressive MS (mean age, 48.7; EDSS, 4.6). They quantified NfL levels at baseline using single molecule array technology and grouped them into the categories of low (< 30 pg/mL), medium (30–60 pg/mL), and high (> 60 pg/mL). High and low baseline NfL categories were compared using Chi-square and Wilcoxon rank sum tests. Dr. Kuhle and colleagues examined the association of baseline NfL levels with MRI parameters by Spearman rank correlation (gadolinium enhancing lesion count, T2 lesion volume) and the Jonckheere Terpstra test (brain volume change).
NfL levels at baseline were higher in patients with secondary progressive MS than in patients with primary progressive MS (32.1 pg/mL vs 22.0 pg/mL). A similar trend was observed when patients of the same age were compared. Patients with secondary progressive MS had higher NfL levels than those with primary progressive MS.
Similarly, patients with no gadolinium enhancing lesions at baseline had NfL levels of 29.2 pg/mL and 21.0 pg/mL in secondary progressive MS and primary progressive MS, respectively, while patients with gadolinium enhancing lesions had NfL levels of 45.0 pg/mL in secondary progressive MS and 34.0 pg/mL in primary progressive MS. The gadolinium enhancing lesion count and T2 lesion volume at baseline correlated best with baseline NfL. In secondary progressive MS and primary progressive MS, high NfL at baseline was associated with higher percentage of brain volume loss at Month 12 (high NfL vs low NfL: −0.8% vs −0.2% in secondary progressive MS and −0.8% vs −0.4% in primary progressive MS) and at Month 24 (−1.5% vs −0.5% in secondary progressive MS and −1.9% vs −0.8% in primary progressive MS).
The study was funded by Novartis Pharma.
Vitamin D Augments Glucocorticoid Efficacy
The vitamin enhances steroid efficacy via inhibition of mTORc1.
BERLIN—Vitamin D increases the therapeutic effects of glucocorticoids via an mTORc1–dependent upregulation of the glucocorticoid receptor, according to a report at ECTRIMS 2018. “These data suggest that efficacy of glucocorticoids in the treatment of multiple sclerosis (MS) relapses could be improved by mTORc1 inhibition,” said lead author Maud Bagnoud, a doctoral candidate from the Department for Biomedical Research at the University of Bern in Switzerland, and colleagues.
Glucocorticoids are the mainstay in the treatment of acute MS relapses. Still, disability increases in more than 40% of patients. Ms. Bagnoud and colleagues investigated the potential of vitamin D to enhance steroid efficacy for MS relapse therapy and the mechanisms involved.
The researchers analyzed vitamin D levels using an immunoassay in patients with stable MS (n = 56), patients with relapsing glucocorticoid-responsive MS (n = 30), and patients with relapsing glucocorticoid-resistant MS (n = 24). Gene expression of human T cells (microarrays, n = 112) were correlated with 25(OH)D3 levels. Glucocorticoid receptor protein was measured using ELISA. T cell apoptosis was analyzed by FACS. Myelin oligodendrocyte glycoprotein (MOG35-55) experimental autoimmune encephalomyelitis (EAE) was performed in wild type and knockout mice with T cell specific deficiency for glucocorticoid receptor/mTORc1. The investigators analyzed the relevance of the JNK-pathway in human T cells using a competitive JNK-inhibitor (SP600125).
Patients with glucocorticoid-resistant MS had a decreased vitamin D serum level, compared with patients with glucocorticoid-responsive MS or stable MS. This decreased level of vitamin D was associated with reduced expression of the glucocorticoid receptor in T cells. In vitro, vitamin D increased the concentration of glucocorticoid receptor protein in T cells in a dose-dependent manner. Focusing on T cells donated from patients with MS during glucocorticoid-resistant relapse, this glucocorticoid receptor upregulation by vitamin D increased T cell apoptosis by approximately 10%, if treated with vitamin D and glucocorticoids, compared with glucocorticoid monotherapy. Combination therapy ameliorated EAE disease course more efficiently than monotherapies did. This effect was dependent on the presence of the glucocorticoid receptor in T cells.
On a molecular level, vitamin D inhibited mTORc1 signal transduction in murine T cells in vitro. Furthermore, hypovitaminosis D was associated with reduced expression of the archetype mTORc1 inhibitor tuberous sclerosis complex 1 in human T cells. The upregulation of the glucocorticoid receptor by vitamin D as well as the functional vitamin D/glucocorticoid synergism observed in vitro and in vivo were absent in mice with mTORc1-deficient T cells. Pharmacologic inhibition of mTORc1 by everolimus augmented the effects of glucocorticoid treatment in wild type mice during EAE even more potently than vitamin D co-administration did.
No significant changes of proliferation or apoptosis by JNK-inhibition and MP co-incubation were observed in human T cells.
The vitamin enhances steroid efficacy via inhibition of mTORc1.
The vitamin enhances steroid efficacy via inhibition of mTORc1.
BERLIN—Vitamin D increases the therapeutic effects of glucocorticoids via an mTORc1–dependent upregulation of the glucocorticoid receptor, according to a report at ECTRIMS 2018. “These data suggest that efficacy of glucocorticoids in the treatment of multiple sclerosis (MS) relapses could be improved by mTORc1 inhibition,” said lead author Maud Bagnoud, a doctoral candidate from the Department for Biomedical Research at the University of Bern in Switzerland, and colleagues.
Glucocorticoids are the mainstay in the treatment of acute MS relapses. Still, disability increases in more than 40% of patients. Ms. Bagnoud and colleagues investigated the potential of vitamin D to enhance steroid efficacy for MS relapse therapy and the mechanisms involved.
The researchers analyzed vitamin D levels using an immunoassay in patients with stable MS (n = 56), patients with relapsing glucocorticoid-responsive MS (n = 30), and patients with relapsing glucocorticoid-resistant MS (n = 24). Gene expression of human T cells (microarrays, n = 112) were correlated with 25(OH)D3 levels. Glucocorticoid receptor protein was measured using ELISA. T cell apoptosis was analyzed by FACS. Myelin oligodendrocyte glycoprotein (MOG35-55) experimental autoimmune encephalomyelitis (EAE) was performed in wild type and knockout mice with T cell specific deficiency for glucocorticoid receptor/mTORc1. The investigators analyzed the relevance of the JNK-pathway in human T cells using a competitive JNK-inhibitor (SP600125).
Patients with glucocorticoid-resistant MS had a decreased vitamin D serum level, compared with patients with glucocorticoid-responsive MS or stable MS. This decreased level of vitamin D was associated with reduced expression of the glucocorticoid receptor in T cells. In vitro, vitamin D increased the concentration of glucocorticoid receptor protein in T cells in a dose-dependent manner. Focusing on T cells donated from patients with MS during glucocorticoid-resistant relapse, this glucocorticoid receptor upregulation by vitamin D increased T cell apoptosis by approximately 10%, if treated with vitamin D and glucocorticoids, compared with glucocorticoid monotherapy. Combination therapy ameliorated EAE disease course more efficiently than monotherapies did. This effect was dependent on the presence of the glucocorticoid receptor in T cells.
On a molecular level, vitamin D inhibited mTORc1 signal transduction in murine T cells in vitro. Furthermore, hypovitaminosis D was associated with reduced expression of the archetype mTORc1 inhibitor tuberous sclerosis complex 1 in human T cells. The upregulation of the glucocorticoid receptor by vitamin D as well as the functional vitamin D/glucocorticoid synergism observed in vitro and in vivo were absent in mice with mTORc1-deficient T cells. Pharmacologic inhibition of mTORc1 by everolimus augmented the effects of glucocorticoid treatment in wild type mice during EAE even more potently than vitamin D co-administration did.
No significant changes of proliferation or apoptosis by JNK-inhibition and MP co-incubation were observed in human T cells.
BERLIN—Vitamin D increases the therapeutic effects of glucocorticoids via an mTORc1–dependent upregulation of the glucocorticoid receptor, according to a report at ECTRIMS 2018. “These data suggest that efficacy of glucocorticoids in the treatment of multiple sclerosis (MS) relapses could be improved by mTORc1 inhibition,” said lead author Maud Bagnoud, a doctoral candidate from the Department for Biomedical Research at the University of Bern in Switzerland, and colleagues.
Glucocorticoids are the mainstay in the treatment of acute MS relapses. Still, disability increases in more than 40% of patients. Ms. Bagnoud and colleagues investigated the potential of vitamin D to enhance steroid efficacy for MS relapse therapy and the mechanisms involved.
The researchers analyzed vitamin D levels using an immunoassay in patients with stable MS (n = 56), patients with relapsing glucocorticoid-responsive MS (n = 30), and patients with relapsing glucocorticoid-resistant MS (n = 24). Gene expression of human T cells (microarrays, n = 112) were correlated with 25(OH)D3 levels. Glucocorticoid receptor protein was measured using ELISA. T cell apoptosis was analyzed by FACS. Myelin oligodendrocyte glycoprotein (MOG35-55) experimental autoimmune encephalomyelitis (EAE) was performed in wild type and knockout mice with T cell specific deficiency for glucocorticoid receptor/mTORc1. The investigators analyzed the relevance of the JNK-pathway in human T cells using a competitive JNK-inhibitor (SP600125).
Patients with glucocorticoid-resistant MS had a decreased vitamin D serum level, compared with patients with glucocorticoid-responsive MS or stable MS. This decreased level of vitamin D was associated with reduced expression of the glucocorticoid receptor in T cells. In vitro, vitamin D increased the concentration of glucocorticoid receptor protein in T cells in a dose-dependent manner. Focusing on T cells donated from patients with MS during glucocorticoid-resistant relapse, this glucocorticoid receptor upregulation by vitamin D increased T cell apoptosis by approximately 10%, if treated with vitamin D and glucocorticoids, compared with glucocorticoid monotherapy. Combination therapy ameliorated EAE disease course more efficiently than monotherapies did. This effect was dependent on the presence of the glucocorticoid receptor in T cells.
On a molecular level, vitamin D inhibited mTORc1 signal transduction in murine T cells in vitro. Furthermore, hypovitaminosis D was associated with reduced expression of the archetype mTORc1 inhibitor tuberous sclerosis complex 1 in human T cells. The upregulation of the glucocorticoid receptor by vitamin D as well as the functional vitamin D/glucocorticoid synergism observed in vitro and in vivo were absent in mice with mTORc1-deficient T cells. Pharmacologic inhibition of mTORc1 by everolimus augmented the effects of glucocorticoid treatment in wild type mice during EAE even more potently than vitamin D co-administration did.
No significant changes of proliferation or apoptosis by JNK-inhibition and MP co-incubation were observed in human T cells.
What Are the Characteristics of Children With Poststroke Headache?
Researchers characterize poststroke headache in a pediatric population.
CHICAGO—Among children with pediatric stroke, older age at stroke onset, unilateral infarct location, and stroke etiologies of arteriopathy and chronic illness are associated with the development of poststroke headache, according to a retrospective study presented at the 47th Annual Meeting of the Child Neurology Society.
Poststroke headache is a common morbidity among patients with pediatric stroke, said Ana B. Chelse, MD, of Ann & Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine in Chicago, and colleagues. In addition, poststroke headache in children may increase health care utilization, including neuroimaging and hospital admission, the investigators said.
Research indicates that about 20% of children with stroke have headache one year after the stroke, but data about poststroke headache in children are limited.
To assess the prevalence of novel headache after pediatric stroke, the characteristics of patients with poststroke headache, and the association between poststroke headache and stroke recurrence, Dr. Chelse and colleagues conducted a single-center, retrospective study of children 30 days to 18 years old with stroke. The researchers used an internal database to identify patients with a radiographically confirmed stroke at Lurie Children’s Hospital between January 1, 2008, and December 31, 2016.
Patients with ischemic stroke with secondary hemorrhage were included in the study, but patients with primary intracerebral hemorrhage were not. The researchers obtained patients’ demographic characteristics, infarct location, headache history, emergency department visits, neuroimaging, hospital admissions, and headache treatment from medical records. They defined stroke recurrence as an acute neurologic deficit with evidence of new radiologically confirmed ischemia.
The investigators analyzed the data using chi-squared and Fisher’s exact tests. They also performed exploratory multiple logistic regression analyses that included predictors deemed significant in univariate analyses.
Of 183 patients, 45 (24.5%) had poststroke headache. Headache developed at an average of 11.7 months after stroke. In multiple logistic regression analysis, older age and unilateral infarct location were associated with poststroke headache, as were stroke etiologies of arteriopathy (odds ratio [OR], 7.28) or chronic illness (OR, 1.90). Twenty-one patients (11.4%) had a recurrent stroke during the study period. Poststroke headache was associated with stroke recurrence in a univariate analysis, but the association did not reach statistical significance after multiple logistic regression. This association is “uncertain but potentially important,” Dr. Chelse and colleagues said.
Researchers characterize poststroke headache in a pediatric population.
Researchers characterize poststroke headache in a pediatric population.
CHICAGO—Among children with pediatric stroke, older age at stroke onset, unilateral infarct location, and stroke etiologies of arteriopathy and chronic illness are associated with the development of poststroke headache, according to a retrospective study presented at the 47th Annual Meeting of the Child Neurology Society.
Poststroke headache is a common morbidity among patients with pediatric stroke, said Ana B. Chelse, MD, of Ann & Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine in Chicago, and colleagues. In addition, poststroke headache in children may increase health care utilization, including neuroimaging and hospital admission, the investigators said.
Research indicates that about 20% of children with stroke have headache one year after the stroke, but data about poststroke headache in children are limited.
To assess the prevalence of novel headache after pediatric stroke, the characteristics of patients with poststroke headache, and the association between poststroke headache and stroke recurrence, Dr. Chelse and colleagues conducted a single-center, retrospective study of children 30 days to 18 years old with stroke. The researchers used an internal database to identify patients with a radiographically confirmed stroke at Lurie Children’s Hospital between January 1, 2008, and December 31, 2016.
Patients with ischemic stroke with secondary hemorrhage were included in the study, but patients with primary intracerebral hemorrhage were not. The researchers obtained patients’ demographic characteristics, infarct location, headache history, emergency department visits, neuroimaging, hospital admissions, and headache treatment from medical records. They defined stroke recurrence as an acute neurologic deficit with evidence of new radiologically confirmed ischemia.
The investigators analyzed the data using chi-squared and Fisher’s exact tests. They also performed exploratory multiple logistic regression analyses that included predictors deemed significant in univariate analyses.
Of 183 patients, 45 (24.5%) had poststroke headache. Headache developed at an average of 11.7 months after stroke. In multiple logistic regression analysis, older age and unilateral infarct location were associated with poststroke headache, as were stroke etiologies of arteriopathy (odds ratio [OR], 7.28) or chronic illness (OR, 1.90). Twenty-one patients (11.4%) had a recurrent stroke during the study period. Poststroke headache was associated with stroke recurrence in a univariate analysis, but the association did not reach statistical significance after multiple logistic regression. This association is “uncertain but potentially important,” Dr. Chelse and colleagues said.
CHICAGO—Among children with pediatric stroke, older age at stroke onset, unilateral infarct location, and stroke etiologies of arteriopathy and chronic illness are associated with the development of poststroke headache, according to a retrospective study presented at the 47th Annual Meeting of the Child Neurology Society.
Poststroke headache is a common morbidity among patients with pediatric stroke, said Ana B. Chelse, MD, of Ann & Robert H. Lurie Children’s Hospital and Northwestern University Feinberg School of Medicine in Chicago, and colleagues. In addition, poststroke headache in children may increase health care utilization, including neuroimaging and hospital admission, the investigators said.
Research indicates that about 20% of children with stroke have headache one year after the stroke, but data about poststroke headache in children are limited.
To assess the prevalence of novel headache after pediatric stroke, the characteristics of patients with poststroke headache, and the association between poststroke headache and stroke recurrence, Dr. Chelse and colleagues conducted a single-center, retrospective study of children 30 days to 18 years old with stroke. The researchers used an internal database to identify patients with a radiographically confirmed stroke at Lurie Children’s Hospital between January 1, 2008, and December 31, 2016.
Patients with ischemic stroke with secondary hemorrhage were included in the study, but patients with primary intracerebral hemorrhage were not. The researchers obtained patients’ demographic characteristics, infarct location, headache history, emergency department visits, neuroimaging, hospital admissions, and headache treatment from medical records. They defined stroke recurrence as an acute neurologic deficit with evidence of new radiologically confirmed ischemia.
The investigators analyzed the data using chi-squared and Fisher’s exact tests. They also performed exploratory multiple logistic regression analyses that included predictors deemed significant in univariate analyses.
Of 183 patients, 45 (24.5%) had poststroke headache. Headache developed at an average of 11.7 months after stroke. In multiple logistic regression analysis, older age and unilateral infarct location were associated with poststroke headache, as were stroke etiologies of arteriopathy (odds ratio [OR], 7.28) or chronic illness (OR, 1.90). Twenty-one patients (11.4%) had a recurrent stroke during the study period. Poststroke headache was associated with stroke recurrence in a univariate analysis, but the association did not reach statistical significance after multiple logistic regression. This association is “uncertain but potentially important,” Dr. Chelse and colleagues said.
ACR and EULAR draft classification criteria for IgG4-related disease
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.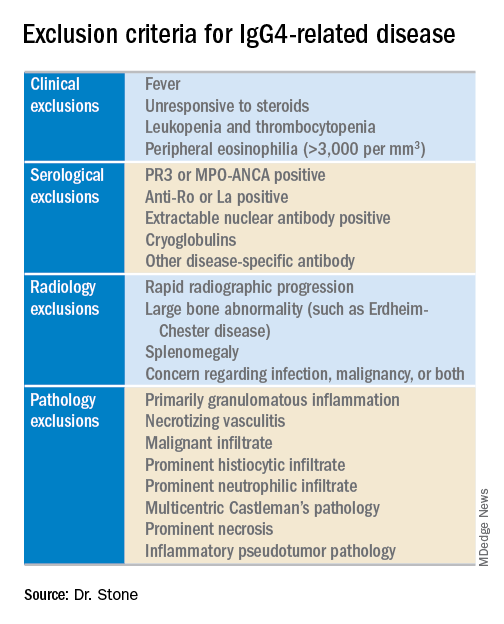
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.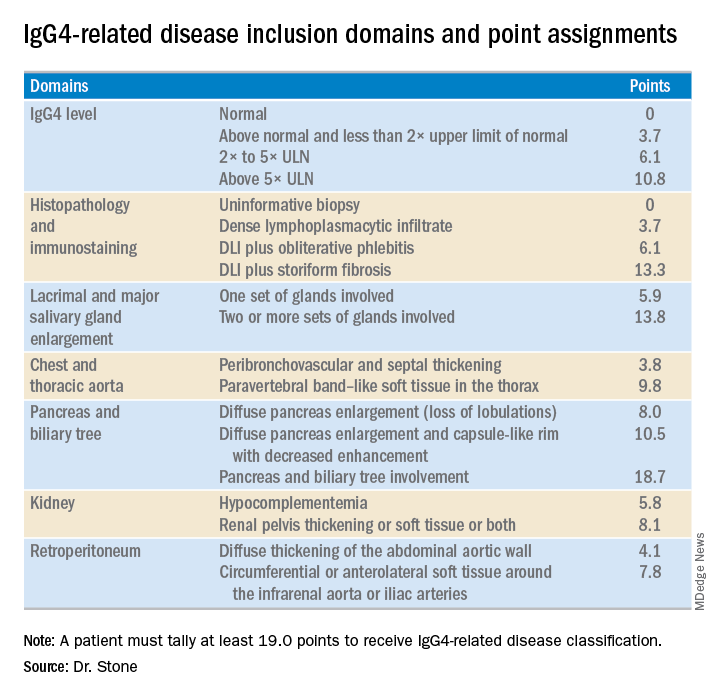
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
REPORTING FROM THE ACR ANNUAL MEETING
Clone of COPD and Asthma Supplement
COPD and Asthma Update


"COPD and Asthma Update" is a clinical aid for PCPs to further understand and manage patients with COPD or asthma. Click here to read the supplement, then click the buttons below for supplementary materials to each chapter.
Supplementary Materials:

Supplemental Materials are joint copyright © 2018 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
COPD and Asthma Update


"COPD and Asthma Update" is a clinical aid for PCPs to further understand and manage patients with COPD or asthma. Click here to read the supplement, then click the buttons below for supplementary materials to each chapter.
Supplementary Materials:


Supplemental Materials are joint copyright © 2018 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
COPD and Asthma Update


"COPD and Asthma Update" is a clinical aid for PCPs to further understand and manage patients with COPD or asthma. Click here to read the supplement, then click the buttons below for supplementary materials to each chapter.
Supplementary Materials:


Supplemental Materials are joint copyright © 2018 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
Venetoclax/HMA combo still safe, effective for elderly with AML
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
In patients aged 65 years and older with acute myeloid leukemia (AML), the combination of venetoclax (Venclexta) and a hypomethylating agent had good efficacy and was well tolerated, according to updated results from a phase 1b dose-escalation and expansion trial.
At a median of 8.9 months of study, the overall response rate (ORR) among all treated patients was 68%, with a median duration of complete remission (CR) plus CR with incomplete count recovery (CRi) of 11.3 months, reported Courtney DiNardo, MD, from the University of Texas MD Anderson Center in Houston and her colleagues.
“Venetoclax in combination with azacitidine or decitabine was well tolerated, with similar safety profiles within all arms of the dose escalation and expansion phases in elderly patients with previously untreated AML ineligible for standard induction therapy,” they wrote in a paper published in Blood.
At the 2017 European Hematology Association Congress, the investigators reported that the combined rate of complete remission CR and CRi was 60% among patients with poor-risk cytogenetics and 78% among patients with intermediate-risk disease. In addition, the drug combination was effective among patients with both primary de novo AML (68%) and secondary AML (related to myelodysplasia or myeloproliferative neoplasms or previous therapy; 73%).
In this, the most recent analysis, Dr. DiNardo and her colleagues reported on follow-up of 145 patients aged 65 and older with treatment-naive AML who were not eligible for intensive chemotherapy regimens used for younger adults. The median age was 74 years. Approximately half of all patients (49%) had poor-risk cytogenetics.
The patients were treated with either decitabine or azacitidine plus venetoclax at a dose of either 400 mg or 800 mg. Decitabine was dosed at 20 mg/m2 intravenously on days 1-5 of a 28-day cycle. Azacitidine was dosed at 75 mg/m2 subcutaneously on days 1-7 of every cycle.
The median time on study was 8.9 months. Among all patients treated at all doses, 67% had either a CR or CRi. The combined CR/CRi rate in patients treated at the 400 mg dose of venetoclax was 73%.
The CR/CRi rate for patients with poor-risk cytogenetics was 60%, and the rate for patients aged 75 years and older was 65%.
Among all patients, the median duration of CR/CRi was 11.3 months, and median overall survival was 17.5 months. In the 400 mg venetoclax cohort, the median duration of CR/CRi was 12.5 months, with the median OS not reached at the time of data cutoff.
Adverse events occurring in 30% or more of patients included constipation, diarrhea, vomiting, nausea, fatigue, febrile neutropenia, hypokalemia, decreased appetite, and decreased white blood cell count. There were no reported cases of the tumor lysis syndrome, a known complication of venetoclax therapy.
Venetoclax plus decitabine or azacitidine was effective in high-risk subgroups, including patients aged 75 years and older, those with poor cytogenetic risk, and those with secondary AML, the investigators noted.
“Though these observations are drawn from a relatively small subset of patients, the remission rates achieved by our low-intensity regimen are encouraging in light of the traditionally lower remission rates in the elderly AML population (40%-50%) compared with young patients receiving chemotherapy (60%-70%) and the relatively short duration of these remissions,” Dr. DiNardo and her colleagues wrote.
A phase 3 trial is currently underway comparing venetoclax at the 400 mg dose plus azacitidine with azacitidine alone in treatment-naive patients with AML who are ineligible for standard induction therapy.
The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
SOURCE: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
FROM BLOOD
Key clinical point:
Major finding: At a median time of study of 8.9 months, the overall response rate among all treated patients was 68%.
Study details: Follow-up of a phase 1b dose-escalation and expansion cohort of 145 patients aged 65 years and older with treatment-naive AML.
Disclosures: The trial was supported by AbbVie and Genentech. Dr. DiNardo and multiple coauthors disclosed relationships with AbbVie, Genentech, and other companies.
Source: DiNardo C et al. Blood. 2018 Oct 25. doi: 10.1182/blood-2018-08-868752.
DEA Reclassifies Epidiolex as Schedule V
The regulatory action acknowledges the drug’s medical use and clears the way for it to be marketed.
WASHINGTON, DC
The DEA’s final rescheduling order is limited to drugs approved by the FDA that contain cannabis-derived CBD and no more than 0.1% tetrahydrocannabinol (THC). In practice, this means that the rescheduling currently applies only to Epidiolex, since this is the only formulation of CBD that has received FDA approval.
A Low Potential for Abuse
“We are pleased that the DEA has placed Epidiolex in the lowest restriction schedule, because it will help ensure that patients with LGS and Dravet syndrome, two of the most debilitating forms of epilepsy, can access this important new treatment option through their physicians,” said GW Pharmaceutical’s Chief Executive Officer Justin Gover in a statement.
During the FDA advisory committee meeting for the approval of Epidiolex to treat LGS and Dravet syndrome, the FDA and GW Pharmaceuticals concluded that the potential to abuse CBD was low, since it does not contain THC, the primary psychoactive component of cannabis.
All other marijuana products are currently classified as Schedule I, along with illegal substances such as heroin and cocaine.
An Established Medical Use
Epidiolex had received fast track and rare pediatric designations from the FDA for LGS and Dravet syndrome; the approval was based on three pivotal randomized, double-blind, placebo-controlled clinical trials. The drug met its primary end point of reduced seizure frequency in all trials when added to standard of care for patients with drug-resistant LGS and those with Dravet syndrome.
Safety evaluations assessed data from 1,756 patients and found that the 20 deaths seen during the study period were not clearly linked to Epidiolex and may be expected for children with severe seizure disorders.
In supplementary information accompanying the order, the DEA’s Acting Administrator Uttam Dhillon noted that the FDA’s approval of Epidiolex means that “it has a currently accepted medical use in treatment for purposes of the Controlled Substances Act [CSA]. Accordingly, Epidiolex no longer meets the criteria for placement in Schedule I of the CSA.” Schedule I drugs do not have a currently accepted medical use.
Schedule V drugs, according to the DEA, are defined as “drugs with lower potential for abuse than Schedule IV and [that] consist of preparations containing limited quantities of certain narcotics.” Other Schedule V drugs include cough medicine with less than 200 mg of codeine or per 100 mL, antidiarrheal medications, pregabalin, and the antiepileptic drugs brivaracetam and lacosamide. “Schedule V drugs represent the least potential for abuse,” according to the DEA.
Initial dosing recommendations for Epidiolex are to titrate to a dose of 10 mg/kg/day. Dose adjustments to 20 mg/kg/day are permissible, depending on clinical response and tolerability. The manufacturer has submitted a marketing agreement to the European Medicines Agency and has received orphan drug designation for Epidiolex in the treatment of tuberous sclerosis complex.
—Kari Oakes
The regulatory action acknowledges the drug’s medical use and clears the way for it to be marketed.
The regulatory action acknowledges the drug’s medical use and clears the way for it to be marketed.
WASHINGTON, DC
The DEA’s final rescheduling order is limited to drugs approved by the FDA that contain cannabis-derived CBD and no more than 0.1% tetrahydrocannabinol (THC). In practice, this means that the rescheduling currently applies only to Epidiolex, since this is the only formulation of CBD that has received FDA approval.
A Low Potential for Abuse
“We are pleased that the DEA has placed Epidiolex in the lowest restriction schedule, because it will help ensure that patients with LGS and Dravet syndrome, two of the most debilitating forms of epilepsy, can access this important new treatment option through their physicians,” said GW Pharmaceutical’s Chief Executive Officer Justin Gover in a statement.
During the FDA advisory committee meeting for the approval of Epidiolex to treat LGS and Dravet syndrome, the FDA and GW Pharmaceuticals concluded that the potential to abuse CBD was low, since it does not contain THC, the primary psychoactive component of cannabis.
All other marijuana products are currently classified as Schedule I, along with illegal substances such as heroin and cocaine.
An Established Medical Use
Epidiolex had received fast track and rare pediatric designations from the FDA for LGS and Dravet syndrome; the approval was based on three pivotal randomized, double-blind, placebo-controlled clinical trials. The drug met its primary end point of reduced seizure frequency in all trials when added to standard of care for patients with drug-resistant LGS and those with Dravet syndrome.
Safety evaluations assessed data from 1,756 patients and found that the 20 deaths seen during the study period were not clearly linked to Epidiolex and may be expected for children with severe seizure disorders.
In supplementary information accompanying the order, the DEA’s Acting Administrator Uttam Dhillon noted that the FDA’s approval of Epidiolex means that “it has a currently accepted medical use in treatment for purposes of the Controlled Substances Act [CSA]. Accordingly, Epidiolex no longer meets the criteria for placement in Schedule I of the CSA.” Schedule I drugs do not have a currently accepted medical use.
Schedule V drugs, according to the DEA, are defined as “drugs with lower potential for abuse than Schedule IV and [that] consist of preparations containing limited quantities of certain narcotics.” Other Schedule V drugs include cough medicine with less than 200 mg of codeine or per 100 mL, antidiarrheal medications, pregabalin, and the antiepileptic drugs brivaracetam and lacosamide. “Schedule V drugs represent the least potential for abuse,” according to the DEA.
Initial dosing recommendations for Epidiolex are to titrate to a dose of 10 mg/kg/day. Dose adjustments to 20 mg/kg/day are permissible, depending on clinical response and tolerability. The manufacturer has submitted a marketing agreement to the European Medicines Agency and has received orphan drug designation for Epidiolex in the treatment of tuberous sclerosis complex.
—Kari Oakes
WASHINGTON, DC
The DEA’s final rescheduling order is limited to drugs approved by the FDA that contain cannabis-derived CBD and no more than 0.1% tetrahydrocannabinol (THC). In practice, this means that the rescheduling currently applies only to Epidiolex, since this is the only formulation of CBD that has received FDA approval.
A Low Potential for Abuse
“We are pleased that the DEA has placed Epidiolex in the lowest restriction schedule, because it will help ensure that patients with LGS and Dravet syndrome, two of the most debilitating forms of epilepsy, can access this important new treatment option through their physicians,” said GW Pharmaceutical’s Chief Executive Officer Justin Gover in a statement.
During the FDA advisory committee meeting for the approval of Epidiolex to treat LGS and Dravet syndrome, the FDA and GW Pharmaceuticals concluded that the potential to abuse CBD was low, since it does not contain THC, the primary psychoactive component of cannabis.
All other marijuana products are currently classified as Schedule I, along with illegal substances such as heroin and cocaine.
An Established Medical Use
Epidiolex had received fast track and rare pediatric designations from the FDA for LGS and Dravet syndrome; the approval was based on three pivotal randomized, double-blind, placebo-controlled clinical trials. The drug met its primary end point of reduced seizure frequency in all trials when added to standard of care for patients with drug-resistant LGS and those with Dravet syndrome.
Safety evaluations assessed data from 1,756 patients and found that the 20 deaths seen during the study period were not clearly linked to Epidiolex and may be expected for children with severe seizure disorders.
In supplementary information accompanying the order, the DEA’s Acting Administrator Uttam Dhillon noted that the FDA’s approval of Epidiolex means that “it has a currently accepted medical use in treatment for purposes of the Controlled Substances Act [CSA]. Accordingly, Epidiolex no longer meets the criteria for placement in Schedule I of the CSA.” Schedule I drugs do not have a currently accepted medical use.
Schedule V drugs, according to the DEA, are defined as “drugs with lower potential for abuse than Schedule IV and [that] consist of preparations containing limited quantities of certain narcotics.” Other Schedule V drugs include cough medicine with less than 200 mg of codeine or per 100 mL, antidiarrheal medications, pregabalin, and the antiepileptic drugs brivaracetam and lacosamide. “Schedule V drugs represent the least potential for abuse,” according to the DEA.
Initial dosing recommendations for Epidiolex are to titrate to a dose of 10 mg/kg/day. Dose adjustments to 20 mg/kg/day are permissible, depending on clinical response and tolerability. The manufacturer has submitted a marketing agreement to the European Medicines Agency and has received orphan drug designation for Epidiolex in the treatment of tuberous sclerosis complex.
—Kari Oakes
FDA approval of powerful opioid tinged with irony
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
Rapid Sequence MRI May Hold Advantages for Neonates With Seizures
The imaging modality may reduce patients’ exposure to radiation and sedation.
CHICAGO—Use of rapid sequence MRI in the evaluation of neonates with seizures and no hypoxic-ischemic encephalopathy (HIE) is associated with reduced number of CT and MRI scans and similar length of hospital stay and cost, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. “The potential reduction in radiation and sedation exposure associated with CTs and regular MRIs makes rapid sequence MRI an attractive imaging modality in this population,” said Theresa M. Czech, MD, and Andrea C. Pardo, MD, attending physicians at Ann & Robert H. Lurie Children’s Hospital of Chicago. Dr. Czech is an Instructor in Pediatrics, and Dr. Pardo is an Assistant Professor of Pediatrics, both at Northwestern University Feinberg School of Medicine in Chicago.
Neurologists routinely use brain imaging to identify the etiologies of neonatal seizures. Rapid sequence MRI “can reduce the risk of radiation associated with CT or the need for procedural sedation associated with regular MRIs,” said Drs. Czech and Pardo. “Our goal was to determine whether the implementation of a protocol recommending the use of rapid sequence MRI was associated with increased efficiency in the evaluation of neonates with seizures.” Rapid sequence MRI consisted of three plane ultrafast T2 sequences with gradient echo and diffusion weighted sequences.
The researchers compared outcomes before and after the implementation of a protocol recommending the use of rapid sequence MRI. The primary outcome was hospital length of stay. Secondary outcomes included the use of other imaging modalities (ie, head ultrasound, CT, and MRI), cost of imaging, and cost of hospitalization. They excluded neonates with clinical evidence of HIE. Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using the chi-squared test.
In all, 95 patients (gestational age, 39 weeks; 63% male) met inclusion criteria—47 in the preintervention group and 48 in the postintervention group. The groups had similar demographics and severity of illness. Implementation of the protocol-guided rapid sequence MRI was associated with decreased use of CT (34% vs 10%) and full MRI (85% vs 62%). Use of head ultrasound (28% vs 12%), length of hospital stay, and costs were not significantly different between groups.
The imaging modality may reduce patients’ exposure to radiation and sedation.
The imaging modality may reduce patients’ exposure to radiation and sedation.
CHICAGO—Use of rapid sequence MRI in the evaluation of neonates with seizures and no hypoxic-ischemic encephalopathy (HIE) is associated with reduced number of CT and MRI scans and similar length of hospital stay and cost, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. “The potential reduction in radiation and sedation exposure associated with CTs and regular MRIs makes rapid sequence MRI an attractive imaging modality in this population,” said Theresa M. Czech, MD, and Andrea C. Pardo, MD, attending physicians at Ann & Robert H. Lurie Children’s Hospital of Chicago. Dr. Czech is an Instructor in Pediatrics, and Dr. Pardo is an Assistant Professor of Pediatrics, both at Northwestern University Feinberg School of Medicine in Chicago.
Neurologists routinely use brain imaging to identify the etiologies of neonatal seizures. Rapid sequence MRI “can reduce the risk of radiation associated with CT or the need for procedural sedation associated with regular MRIs,” said Drs. Czech and Pardo. “Our goal was to determine whether the implementation of a protocol recommending the use of rapid sequence MRI was associated with increased efficiency in the evaluation of neonates with seizures.” Rapid sequence MRI consisted of three plane ultrafast T2 sequences with gradient echo and diffusion weighted sequences.
The researchers compared outcomes before and after the implementation of a protocol recommending the use of rapid sequence MRI. The primary outcome was hospital length of stay. Secondary outcomes included the use of other imaging modalities (ie, head ultrasound, CT, and MRI), cost of imaging, and cost of hospitalization. They excluded neonates with clinical evidence of HIE. Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using the chi-squared test.
In all, 95 patients (gestational age, 39 weeks; 63% male) met inclusion criteria—47 in the preintervention group and 48 in the postintervention group. The groups had similar demographics and severity of illness. Implementation of the protocol-guided rapid sequence MRI was associated with decreased use of CT (34% vs 10%) and full MRI (85% vs 62%). Use of head ultrasound (28% vs 12%), length of hospital stay, and costs were not significantly different between groups.
CHICAGO—Use of rapid sequence MRI in the evaluation of neonates with seizures and no hypoxic-ischemic encephalopathy (HIE) is associated with reduced number of CT and MRI scans and similar length of hospital stay and cost, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. “The potential reduction in radiation and sedation exposure associated with CTs and regular MRIs makes rapid sequence MRI an attractive imaging modality in this population,” said Theresa M. Czech, MD, and Andrea C. Pardo, MD, attending physicians at Ann & Robert H. Lurie Children’s Hospital of Chicago. Dr. Czech is an Instructor in Pediatrics, and Dr. Pardo is an Assistant Professor of Pediatrics, both at Northwestern University Feinberg School of Medicine in Chicago.
Neurologists routinely use brain imaging to identify the etiologies of neonatal seizures. Rapid sequence MRI “can reduce the risk of radiation associated with CT or the need for procedural sedation associated with regular MRIs,” said Drs. Czech and Pardo. “Our goal was to determine whether the implementation of a protocol recommending the use of rapid sequence MRI was associated with increased efficiency in the evaluation of neonates with seizures.” Rapid sequence MRI consisted of three plane ultrafast T2 sequences with gradient echo and diffusion weighted sequences.
The researchers compared outcomes before and after the implementation of a protocol recommending the use of rapid sequence MRI. The primary outcome was hospital length of stay. Secondary outcomes included the use of other imaging modalities (ie, head ultrasound, CT, and MRI), cost of imaging, and cost of hospitalization. They excluded neonates with clinical evidence of HIE. Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using the chi-squared test.
In all, 95 patients (gestational age, 39 weeks; 63% male) met inclusion criteria—47 in the preintervention group and 48 in the postintervention group. The groups had similar demographics and severity of illness. Implementation of the protocol-guided rapid sequence MRI was associated with decreased use of CT (34% vs 10%) and full MRI (85% vs 62%). Use of head ultrasound (28% vs 12%), length of hospital stay, and costs were not significantly different between groups.












