User login
High incidence of treatment-resistant hypertension in SLE comes with high mortality
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Treatment-resistant hypertension is an important yet underappreciated comorbidity for clinicians to recognize in patients with systemic lupus erythematosus.
Major finding: The incidence rate of treatment-resistant hypertension was twice as great in patients with systemic lupus erythematosus compared with matched controls.
Study details: This retrospective, single-center study included 1,044 systemic lupus erythematosus patients and 5,241 matched controls.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
Source: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
Medical liability at sea
Question: Regarding medical care aboard cruise ships, which of the following is incorrect?
A. It is difficult to prove negligence because of jurisdictional issues.
B. The American College of Emergency Physicians has published practice guidelines.
C. Cruise line owners are immune from liability under the Barbetta rule.
D. The Franza decision may be a game changer.
E. Lawsuits are on the increase.
Answer: A. More and more people are cruising, with the number of passengers on North American lines reaching nearly 18 million in 2017, about one-third out of Florida. Medical illnesses and accidents predictably occur at sea, and unfortunately, substandard and negligent care occasionally follows some of these mishaps.
However, up until recently, courts have immunized cruise line owners from legal liability by relying on the so-called Barbetta rule, which is based on historical notions of limited resources at sea and the impossibility of exerting control over the conduct of a ship’s health care providers.
A 2007 Florida case is illustrative. The doctor aboard a Carnival cruise ship failed to diagnose acute appendicitis in a 14-year-old girl who had complained of several days of abdominal symptoms. As a result, the patient ruptured her appendix, and this eventually resulted in sterility. The parents sued the cruise line, which denied liability because the doctor was not an employee, a fact specifically disclosed in the cruise ticket.
Although the doctor’s contract stated that he was an independent contractor, the District Court of Appeal of Florida reasoned that, in a claim based on agency, it is the right of control rather than actual control itself that matters.
It therefore held that: “for purposes of fulfilling cruise line’s duty to exercise reasonable care, the ship’s doctor is an agent of cruise line whose negligence should be imputed to cruise line ... regardless of the contractual status ascribed to the doctor” and to the extent cruise ticket sought to limit cruise line’s liability for negligence of doctor, it was invalid.
However, the Florida Supreme Court quashed this decision, because federal maritime law has historically protected shipowners from liability flowing from the medical negligence of shipboard physicians.1
The Barbetta rule was named after a 1988 case in which Florence L. Barbetta suffered serious medical complications during a Mexican cruise out of Florida. The ship’s doctor was alleged to have been negligent for his failure to diagnose diabetes.2 The lower court dismissed the case, and the appellate court affirmed.
The appellate court noted that an impressive number of courts from many jurisdictions have, for almost 100 years, followed the same basic rule: If the doctor is negligent in treating a passenger, that negligence will not be imputed to the carrier unless the carrier itself was negligent in hiring the doctor.
Citing approvingly from another case, the appellate court noted: “[A] shipping company is not in the business of providing medical services to passengers; it does not possess the expertise requisite to supervise a physician or surgeon carried on board a ship as a convenience to passengers. A ship is not a floating hospital; a ship’s physician is an independent medical expert engaged on the basis of his professional qualifications and carried on board a ship for the convenience of passengers, who are free to contract with him for any medical services they may require.”
However, courts now appear ready to jettison this rule.
In the 2014 case of Franza v. Royal Caribbean Cruises, Ltd., a passenger fell and hit his head while his ship was at port in Bermuda.3 He died several days later, allegedly because of negligence and delay by the ship’s medical staff. The lower courts barred the lawsuit, based on Barbetta, but on appeal, the 11th Circuit Court reversed. It rejected the historical justifications for immunizing shipowners from liability, concluding that past reasons were no longer applicable to modern-day cruise ships.
The court wrote: “Here, the roots of the Barbetta rule snake back to a wholly different world. Instead of 19th-century steamships, we now confront state-of-the-art cruise ships that house thousands of people and operate as floating cities complete with well-stocked modern infirmaries and urgent-care centers. In place of independent doctors and nurses, we must now acknowledge that medical professionals routinely work for corporate masters. And whereas ships historically went ‘off the grid’ when they set sail, modern technology enables distant ships to communicate instantaneously with the mainland in meaningful ways.”
However, the injured person must still prove that the doctor or nurse was acting as a ship employee rather than an independent contractor. Some of the factors to be considered include whether the cruise line advertised its medical center or medical staff to passengers, whether it retained the right to hire and fire medical staff, and methods of payment.
This 2014 11th Circuit Court ruling has particular impact on Florida, because the state’s federal court system falls within its jurisdiction, and many cruise lines – such as Carnival, Celebrity, Disney, Norwegian, Royal Caribbean, and Silversea Cruises – are headquartered in Florida. Because most ocean liners hire medical professionals who are foreign nationals, seeking legal remedy against the individuals can be difficult.
As a result, suing the cruise line directly can serve as the simplest way to obtain compensation. Some major cruise lines have already seen an increase in personal injury lawsuits. According to Bloomberg Law, there were 164 such federal injury suits in 2016, 188 in 2017, and 83 cases in just the first 3 months of 2018.
The popular press recently highlighted the latest example of medical malpractice at sea. A cruise worker was awarded $3.34 million after a young, inexperienced doctor prescribed a large dose of intravenous promethazine for nausea. Instead of using 6.25 mg, the usual dose, he prescribed 25 mg, which was inadvertently injected into the ulnar artery. The caustic drug, known to injure vascular walls, caused severe damage with extravasation, and tissue swelling, ending in compartment syndrome.
Furthermore, he had to wait 24 hours before arriving in port for treatment. By then, his arm was gangrenous and had to be amputated. The defense had argued unsuccessfully that the plaintiff had a venous anomaly to account for the injury.4
These changes in the law augur well for the cruising public. The Franza decision puts cruise line owners on notice that they no longer enjoy blanket immunity, and will be held responsible for the negligence of their health care providers. Hopefully, this will ensure a more uniform and adequate standard of care.
The American College of Emergency Physicians has published specific guidelines regarding medical standards aboard cruise ships. In addition to having established medical policies and procedures, and a dedicated medical emergency telephone number, they must have at least one doctor available 24/7 to provide emergency medical care and maintain certain equipment on board, such as pulse oximeters, cardiac monitors, defibrillators, an EKG device, as well as a laboratory.
Norwegian Cruise Line has taken a step further: It recently installed the capability to consult in real time with the Cleveland Clinic for diagnostic and treatment advice.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Carlisle v. Carnival Corporation, et al., 953 So.2d 461, 2007.
2. Barbetta v. S/S Bermuda Star, 848 F.2d 1364 (5th Cir. 1988).
3. Franza v. Royal Caribbean Cruises Ltd., 772 F.3d 1225 (11th Cir. 2014).
4. Loncar v. NCL Bahamas, International Center for Dispute Resolution, Case # 01-16-0004-3640, June 13, 2018.
Question: Regarding medical care aboard cruise ships, which of the following is incorrect?
A. It is difficult to prove negligence because of jurisdictional issues.
B. The American College of Emergency Physicians has published practice guidelines.
C. Cruise line owners are immune from liability under the Barbetta rule.
D. The Franza decision may be a game changer.
E. Lawsuits are on the increase.
Answer: A. More and more people are cruising, with the number of passengers on North American lines reaching nearly 18 million in 2017, about one-third out of Florida. Medical illnesses and accidents predictably occur at sea, and unfortunately, substandard and negligent care occasionally follows some of these mishaps.
However, up until recently, courts have immunized cruise line owners from legal liability by relying on the so-called Barbetta rule, which is based on historical notions of limited resources at sea and the impossibility of exerting control over the conduct of a ship’s health care providers.
A 2007 Florida case is illustrative. The doctor aboard a Carnival cruise ship failed to diagnose acute appendicitis in a 14-year-old girl who had complained of several days of abdominal symptoms. As a result, the patient ruptured her appendix, and this eventually resulted in sterility. The parents sued the cruise line, which denied liability because the doctor was not an employee, a fact specifically disclosed in the cruise ticket.
Although the doctor’s contract stated that he was an independent contractor, the District Court of Appeal of Florida reasoned that, in a claim based on agency, it is the right of control rather than actual control itself that matters.
It therefore held that: “for purposes of fulfilling cruise line’s duty to exercise reasonable care, the ship’s doctor is an agent of cruise line whose negligence should be imputed to cruise line ... regardless of the contractual status ascribed to the doctor” and to the extent cruise ticket sought to limit cruise line’s liability for negligence of doctor, it was invalid.
However, the Florida Supreme Court quashed this decision, because federal maritime law has historically protected shipowners from liability flowing from the medical negligence of shipboard physicians.1
The Barbetta rule was named after a 1988 case in which Florence L. Barbetta suffered serious medical complications during a Mexican cruise out of Florida. The ship’s doctor was alleged to have been negligent for his failure to diagnose diabetes.2 The lower court dismissed the case, and the appellate court affirmed.
The appellate court noted that an impressive number of courts from many jurisdictions have, for almost 100 years, followed the same basic rule: If the doctor is negligent in treating a passenger, that negligence will not be imputed to the carrier unless the carrier itself was negligent in hiring the doctor.
Citing approvingly from another case, the appellate court noted: “[A] shipping company is not in the business of providing medical services to passengers; it does not possess the expertise requisite to supervise a physician or surgeon carried on board a ship as a convenience to passengers. A ship is not a floating hospital; a ship’s physician is an independent medical expert engaged on the basis of his professional qualifications and carried on board a ship for the convenience of passengers, who are free to contract with him for any medical services they may require.”
However, courts now appear ready to jettison this rule.
In the 2014 case of Franza v. Royal Caribbean Cruises, Ltd., a passenger fell and hit his head while his ship was at port in Bermuda.3 He died several days later, allegedly because of negligence and delay by the ship’s medical staff. The lower courts barred the lawsuit, based on Barbetta, but on appeal, the 11th Circuit Court reversed. It rejected the historical justifications for immunizing shipowners from liability, concluding that past reasons were no longer applicable to modern-day cruise ships.
The court wrote: “Here, the roots of the Barbetta rule snake back to a wholly different world. Instead of 19th-century steamships, we now confront state-of-the-art cruise ships that house thousands of people and operate as floating cities complete with well-stocked modern infirmaries and urgent-care centers. In place of independent doctors and nurses, we must now acknowledge that medical professionals routinely work for corporate masters. And whereas ships historically went ‘off the grid’ when they set sail, modern technology enables distant ships to communicate instantaneously with the mainland in meaningful ways.”
However, the injured person must still prove that the doctor or nurse was acting as a ship employee rather than an independent contractor. Some of the factors to be considered include whether the cruise line advertised its medical center or medical staff to passengers, whether it retained the right to hire and fire medical staff, and methods of payment.
This 2014 11th Circuit Court ruling has particular impact on Florida, because the state’s federal court system falls within its jurisdiction, and many cruise lines – such as Carnival, Celebrity, Disney, Norwegian, Royal Caribbean, and Silversea Cruises – are headquartered in Florida. Because most ocean liners hire medical professionals who are foreign nationals, seeking legal remedy against the individuals can be difficult.
As a result, suing the cruise line directly can serve as the simplest way to obtain compensation. Some major cruise lines have already seen an increase in personal injury lawsuits. According to Bloomberg Law, there were 164 such federal injury suits in 2016, 188 in 2017, and 83 cases in just the first 3 months of 2018.
The popular press recently highlighted the latest example of medical malpractice at sea. A cruise worker was awarded $3.34 million after a young, inexperienced doctor prescribed a large dose of intravenous promethazine for nausea. Instead of using 6.25 mg, the usual dose, he prescribed 25 mg, which was inadvertently injected into the ulnar artery. The caustic drug, known to injure vascular walls, caused severe damage with extravasation, and tissue swelling, ending in compartment syndrome.
Furthermore, he had to wait 24 hours before arriving in port for treatment. By then, his arm was gangrenous and had to be amputated. The defense had argued unsuccessfully that the plaintiff had a venous anomaly to account for the injury.4
These changes in the law augur well for the cruising public. The Franza decision puts cruise line owners on notice that they no longer enjoy blanket immunity, and will be held responsible for the negligence of their health care providers. Hopefully, this will ensure a more uniform and adequate standard of care.
The American College of Emergency Physicians has published specific guidelines regarding medical standards aboard cruise ships. In addition to having established medical policies and procedures, and a dedicated medical emergency telephone number, they must have at least one doctor available 24/7 to provide emergency medical care and maintain certain equipment on board, such as pulse oximeters, cardiac monitors, defibrillators, an EKG device, as well as a laboratory.
Norwegian Cruise Line has taken a step further: It recently installed the capability to consult in real time with the Cleveland Clinic for diagnostic and treatment advice.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Carlisle v. Carnival Corporation, et al., 953 So.2d 461, 2007.
2. Barbetta v. S/S Bermuda Star, 848 F.2d 1364 (5th Cir. 1988).
3. Franza v. Royal Caribbean Cruises Ltd., 772 F.3d 1225 (11th Cir. 2014).
4. Loncar v. NCL Bahamas, International Center for Dispute Resolution, Case # 01-16-0004-3640, June 13, 2018.
Question: Regarding medical care aboard cruise ships, which of the following is incorrect?
A. It is difficult to prove negligence because of jurisdictional issues.
B. The American College of Emergency Physicians has published practice guidelines.
C. Cruise line owners are immune from liability under the Barbetta rule.
D. The Franza decision may be a game changer.
E. Lawsuits are on the increase.
Answer: A. More and more people are cruising, with the number of passengers on North American lines reaching nearly 18 million in 2017, about one-third out of Florida. Medical illnesses and accidents predictably occur at sea, and unfortunately, substandard and negligent care occasionally follows some of these mishaps.
However, up until recently, courts have immunized cruise line owners from legal liability by relying on the so-called Barbetta rule, which is based on historical notions of limited resources at sea and the impossibility of exerting control over the conduct of a ship’s health care providers.
A 2007 Florida case is illustrative. The doctor aboard a Carnival cruise ship failed to diagnose acute appendicitis in a 14-year-old girl who had complained of several days of abdominal symptoms. As a result, the patient ruptured her appendix, and this eventually resulted in sterility. The parents sued the cruise line, which denied liability because the doctor was not an employee, a fact specifically disclosed in the cruise ticket.
Although the doctor’s contract stated that he was an independent contractor, the District Court of Appeal of Florida reasoned that, in a claim based on agency, it is the right of control rather than actual control itself that matters.
It therefore held that: “for purposes of fulfilling cruise line’s duty to exercise reasonable care, the ship’s doctor is an agent of cruise line whose negligence should be imputed to cruise line ... regardless of the contractual status ascribed to the doctor” and to the extent cruise ticket sought to limit cruise line’s liability for negligence of doctor, it was invalid.
However, the Florida Supreme Court quashed this decision, because federal maritime law has historically protected shipowners from liability flowing from the medical negligence of shipboard physicians.1
The Barbetta rule was named after a 1988 case in which Florence L. Barbetta suffered serious medical complications during a Mexican cruise out of Florida. The ship’s doctor was alleged to have been negligent for his failure to diagnose diabetes.2 The lower court dismissed the case, and the appellate court affirmed.
The appellate court noted that an impressive number of courts from many jurisdictions have, for almost 100 years, followed the same basic rule: If the doctor is negligent in treating a passenger, that negligence will not be imputed to the carrier unless the carrier itself was negligent in hiring the doctor.
Citing approvingly from another case, the appellate court noted: “[A] shipping company is not in the business of providing medical services to passengers; it does not possess the expertise requisite to supervise a physician or surgeon carried on board a ship as a convenience to passengers. A ship is not a floating hospital; a ship’s physician is an independent medical expert engaged on the basis of his professional qualifications and carried on board a ship for the convenience of passengers, who are free to contract with him for any medical services they may require.”
However, courts now appear ready to jettison this rule.
In the 2014 case of Franza v. Royal Caribbean Cruises, Ltd., a passenger fell and hit his head while his ship was at port in Bermuda.3 He died several days later, allegedly because of negligence and delay by the ship’s medical staff. The lower courts barred the lawsuit, based on Barbetta, but on appeal, the 11th Circuit Court reversed. It rejected the historical justifications for immunizing shipowners from liability, concluding that past reasons were no longer applicable to modern-day cruise ships.
The court wrote: “Here, the roots of the Barbetta rule snake back to a wholly different world. Instead of 19th-century steamships, we now confront state-of-the-art cruise ships that house thousands of people and operate as floating cities complete with well-stocked modern infirmaries and urgent-care centers. In place of independent doctors and nurses, we must now acknowledge that medical professionals routinely work for corporate masters. And whereas ships historically went ‘off the grid’ when they set sail, modern technology enables distant ships to communicate instantaneously with the mainland in meaningful ways.”
However, the injured person must still prove that the doctor or nurse was acting as a ship employee rather than an independent contractor. Some of the factors to be considered include whether the cruise line advertised its medical center or medical staff to passengers, whether it retained the right to hire and fire medical staff, and methods of payment.
This 2014 11th Circuit Court ruling has particular impact on Florida, because the state’s federal court system falls within its jurisdiction, and many cruise lines – such as Carnival, Celebrity, Disney, Norwegian, Royal Caribbean, and Silversea Cruises – are headquartered in Florida. Because most ocean liners hire medical professionals who are foreign nationals, seeking legal remedy against the individuals can be difficult.
As a result, suing the cruise line directly can serve as the simplest way to obtain compensation. Some major cruise lines have already seen an increase in personal injury lawsuits. According to Bloomberg Law, there were 164 such federal injury suits in 2016, 188 in 2017, and 83 cases in just the first 3 months of 2018.
The popular press recently highlighted the latest example of medical malpractice at sea. A cruise worker was awarded $3.34 million after a young, inexperienced doctor prescribed a large dose of intravenous promethazine for nausea. Instead of using 6.25 mg, the usual dose, he prescribed 25 mg, which was inadvertently injected into the ulnar artery. The caustic drug, known to injure vascular walls, caused severe damage with extravasation, and tissue swelling, ending in compartment syndrome.
Furthermore, he had to wait 24 hours before arriving in port for treatment. By then, his arm was gangrenous and had to be amputated. The defense had argued unsuccessfully that the plaintiff had a venous anomaly to account for the injury.4
These changes in the law augur well for the cruising public. The Franza decision puts cruise line owners on notice that they no longer enjoy blanket immunity, and will be held responsible for the negligence of their health care providers. Hopefully, this will ensure a more uniform and adequate standard of care.
The American College of Emergency Physicians has published specific guidelines regarding medical standards aboard cruise ships. In addition to having established medical policies and procedures, and a dedicated medical emergency telephone number, they must have at least one doctor available 24/7 to provide emergency medical care and maintain certain equipment on board, such as pulse oximeters, cardiac monitors, defibrillators, an EKG device, as well as a laboratory.
Norwegian Cruise Line has taken a step further: It recently installed the capability to consult in real time with the Cleveland Clinic for diagnostic and treatment advice.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Carlisle v. Carnival Corporation, et al., 953 So.2d 461, 2007.
2. Barbetta v. S/S Bermuda Star, 848 F.2d 1364 (5th Cir. 1988).
3. Franza v. Royal Caribbean Cruises Ltd., 772 F.3d 1225 (11th Cir. 2014).
4. Loncar v. NCL Bahamas, International Center for Dispute Resolution, Case # 01-16-0004-3640, June 13, 2018.
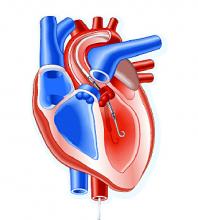
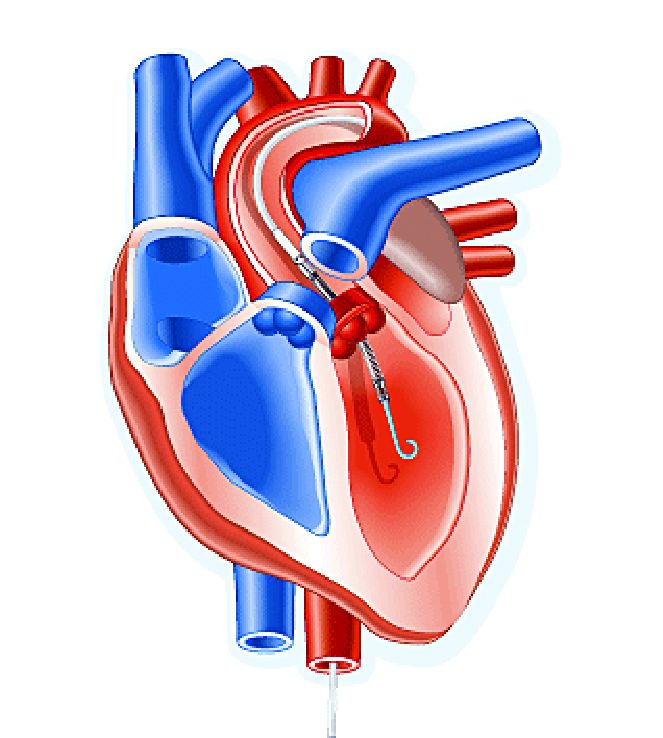
Impella heart pump may enable 30-minute reperfusion delay
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
CHICAGO – An investigative heart pump for unloading the left ventricle in patients who had an ST-elevation myocardial infarction (STEMI) yielded similar safety and efficacy outcomes with a 30-minute delay in reperfusion or the standard approach of immediate reperfusion.
That’s according to results of a pilot feasibility trial presented at the American Heart Association scientific sessions.
The trial, titled the DTU (Door to Unload)–STEMI trial, evaluated the Impella CP (Abiomed) device used for unloading the left ventricle (LV). “No prohibitive safety signals that would preclude proceeding to a larger pivotal study of left ventricle unloading and delaying reperfusion for 30 minutes were identified,” said principal investigator Navin Kapur, MD, of Tufts Medical Center.
The trial evaluated 50 patients who received the Impella device in two different groups: one that underwent immediate reperfusion after LV unloading, the other that had a 30-minute delay before reperfusion. The study found no significant difference in major adverse cardiovascular or cerebral events between the two groups (there were two in the delayed group vs. none in the immediate group), and no difference in infarct size increase as a percentage of LV mass at 30 days between the groups, Dr. Kapur said.
Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group, with door-to-unload times averaging around 60 minutes in both groups. “We were able to see successful enrollment and distribution across multiple sites and multiple operators, suggesting the feasibility of this approach,” Dr. Kapur said.
He noted “one of the most important messages” of the study was that no patients in either arm required percutaneous coronary intervention. “What this suggests is that, when we look at operator behavior, operators were comfortable with initiating LV unloading and waiting 30 minutes,” Dr. Kapur said.
The primary endpoint of the trial was to determine if delayed reperfusion led to an increase in infarct size. “We did not see that,” he noted. “And among patients with an anterior ST-segment elevation sum in leads V1-V4 of more than 6 mm Hg, infarct size normalized to the area at risk was significantly lower with 30 minutes of LV unloading before reperfusion, compared to LV unloading with immediate reperfusion.”
The next step is to initiate a pivotal trial of the device, Dr. Kapur said. “The findings from the DTU-STEMI pilot trial will inform the pivotal trial based on preclinical data showing that LV unloading attenuates myocardial ischemia and also preconditions the myocardium to allow it to be more receptive to reperfusion with a reduction in reperfusion injury,” he said. The pivotal trial will have two similar arms: one using the standard of care of immediate reperfusion and the other utilizing the 30-minute delay.
In his discussion of the DTU-STEMI trial, Holger Thiele, MD, of the Leipzig (Germany) Heart Institute and the University of Leipzig, expressed concern with the lack of a standard-of-care group in the trial. “Thus, the primary efficacy endpoint on infarct size cannot be reliably compared,” he said. “Based on the small sample size, there’s no reliable information on safety.”
Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic, and MD Start. Dr. Thiele had no financial disclosures.
SOURCE: Kapur NK et al. AHA scientific sessions, LBCT-19578
REPORTING FROM AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Door-to-balloon times averaged 73 minutes in the immediate reperfusion group and 97 minutes in the delayed reperfusion group.
Study details: A phase 1, randomized, exploratory safety and feasibility trial in 50 patients with anterior STEMI to left ventricle unloading using the Impella CP followed by immediate reperfusion versus delayed reperfusion after 30 minutes of unloading.
Disclosures: Dr. Kapur reported financial relationships with Abiomed, Boston Scientific, Abbott, Medtronic and MD Start/Precardia.
Source: Kapur NK et al. AHA scientific sessions, LBCT-19578.
Novel formulation for childhood ADHD
Also today, CARE MD protocol could help treat somatic disorders, children with poor cardiorespiratory fitness have a higher risk of type 2 diabetes, and ganglion stimulation boosts cerebral blood flow and improves stroke outcomes.
Amazon Alexa
Apple Podcasts
Google Podcasts
Also today, CARE MD protocol could help treat somatic disorders, children with poor cardiorespiratory fitness have a higher risk of type 2 diabetes, and ganglion stimulation boosts cerebral blood flow and improves stroke outcomes.
Amazon Alexa
Apple Podcasts
Google Podcasts
Also today, CARE MD protocol could help treat somatic disorders, children with poor cardiorespiratory fitness have a higher risk of type 2 diabetes, and ganglion stimulation boosts cerebral blood flow and improves stroke outcomes.
Amazon Alexa
Apple Podcasts
Google Podcasts
Triplet produces ‘unprecedented’ ORR in PI-refractory MM
Phase 2 results suggest a three-drug regimen may improve response rates in patients with proteasome inhibitor (PI)-refractory multiple myeloma (MM).
The combination—nelfinavir, bortezomib, and dexamethasone (NeVd)—produced an objective response rate (ORR) of 65% in this trial.
In comparison, past studies have shown response rates of 24% to 36% in the PI-refractory MM population.1,2,3
“The unprecedented ORR of NeVd observed in this heavily pretreated, multi-refractory setting warrants further investigation to explore the potential of nelfinavir in combination with PIs . . . ,” Christoph Driessen, of Kantonsspital St. Gallen in Switzerland, and his colleagues wrote in a letter to Blood.
The researchers noted that NeVd previously demonstrated activity in a phase 1 trial of MM patients who were refractory to both bortezomib and lenalidomide.
For the phase 2 study (NCT02188537), Dr. Driessen and his colleagues tested NeVd in MM patients who were refractory to their most recent PI-containing regimen and were previously exposed to or intolerant of at least one immunomodulatory drug.
The 34 patients had a median age of 67 (range, 42-82). Sixty-two percent were male, 38% had poor-risk cytogenetics, and most had a performance status of 0 (59%) or 1 (32%).
All patients had received lenalidomide, 47% had prior pomalidomide, 76% had prior high-dose chemotherapy, and 76% had a prior transplant.
All patients were bortezomib-refractory, 79% were refractory to a PI and lenalidomide, 44% were refractory to a PI and pomalidomide, and 38% were refractory to a PI, lenalidomide, and pomalidomide.
In this study, the patients received:
- Nelfinavir at 2500 mg on days 1 to 14 twice daily
- Bortezomib at 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11
- Dexamethasone at 20 mg orally on days 1 to 2, 4 to 5, 8 to 9, and 11 to 12.
The patients were treated for up to six 21-day cycles. They received a median of 4.5 cycles (range, 1-6).
Results
The ORR was 65%, with 17 partial responses (PRs) and five very good PRs. Three patients had a minimal response, and four had stable disease.
The ORR (PR or better) was:
- 77% in patients with poor-risk cytogenetics
- 67% in patients with fewer than five prior therapies
- 63% in patients with five or more prior therapies
- 70% in patients refractory to bortezomib and lenalidomide
- 60% in patients refractory to bortezomib and pomalidomide
- 62% in patients refractory to bortezomib, lenalidomide, and pomalidomide.
The researchers said the clinical benefit of NeVd may have been underestimated in this trial because patients were only able to receive six cycles of treatment on study due to a lack of external funding support.
“The time course of paraprotein levels also suggests that individual patients might potentially have experienced myeloma control if NeVd had been continued until progression,” the researchers wrote.
Five patients did receive more than six cycles of NeVd on a compassionate-use basis. In all, 27 patients received additional anti-myeloma treatment during follow-up.
The median progression-free survival was 12 weeks for the entire cohort and 16 weeks among patients who achieved a PR or better.
The median overall survival was 12 months, and 17 patients had died as of November 2016.
The researchers said the most common adverse events in this trial were anemia (97%), thrombocytopenia (82%), hypertension (53%), diarrhea (47%), fatigue (38%), and dyspnea (35%).
There were four deaths during treatment—three due to septicemia and one from heart failure. Three of the deaths were associated with underlying pneumonia.
“Although this mortality rate is consistent with the background mortality among patients with heavily pretreated, refractory myeloma, these findings suggest that prophylactic antibiotic therapy should be considered in those with low neutrophil counts and/or advanced age undergoing NeVd treatment,” the researchers wrote.
This work was supported by the Swiss Group for Clinical Cancer Research, the Swiss State Secretariat for Education, Research and Innovation, the Rising Tide Foundation for Clinical Cancer Research, and the Gateway for Cancer Research.
Most of the researchers declared no competing financial interests, but one reported personal fees from Celgene, Takeda, Amgen, Novartis, and Janssen-Cilag that were unrelated to this trial.
1. Lokhorst HM et al. N Engl J Med. 2015; 373(13):1207-1219.
2. Dimopoulos MA et al. Blood. 2016;128(4): 497-503.
Phase 2 results suggest a three-drug regimen may improve response rates in patients with proteasome inhibitor (PI)-refractory multiple myeloma (MM).
The combination—nelfinavir, bortezomib, and dexamethasone (NeVd)—produced an objective response rate (ORR) of 65% in this trial.
In comparison, past studies have shown response rates of 24% to 36% in the PI-refractory MM population.1,2,3
“The unprecedented ORR of NeVd observed in this heavily pretreated, multi-refractory setting warrants further investigation to explore the potential of nelfinavir in combination with PIs . . . ,” Christoph Driessen, of Kantonsspital St. Gallen in Switzerland, and his colleagues wrote in a letter to Blood.
The researchers noted that NeVd previously demonstrated activity in a phase 1 trial of MM patients who were refractory to both bortezomib and lenalidomide.
For the phase 2 study (NCT02188537), Dr. Driessen and his colleagues tested NeVd in MM patients who were refractory to their most recent PI-containing regimen and were previously exposed to or intolerant of at least one immunomodulatory drug.
The 34 patients had a median age of 67 (range, 42-82). Sixty-two percent were male, 38% had poor-risk cytogenetics, and most had a performance status of 0 (59%) or 1 (32%).
All patients had received lenalidomide, 47% had prior pomalidomide, 76% had prior high-dose chemotherapy, and 76% had a prior transplant.
All patients were bortezomib-refractory, 79% were refractory to a PI and lenalidomide, 44% were refractory to a PI and pomalidomide, and 38% were refractory to a PI, lenalidomide, and pomalidomide.
In this study, the patients received:
- Nelfinavir at 2500 mg on days 1 to 14 twice daily
- Bortezomib at 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11
- Dexamethasone at 20 mg orally on days 1 to 2, 4 to 5, 8 to 9, and 11 to 12.
The patients were treated for up to six 21-day cycles. They received a median of 4.5 cycles (range, 1-6).
Results
The ORR was 65%, with 17 partial responses (PRs) and five very good PRs. Three patients had a minimal response, and four had stable disease.
The ORR (PR or better) was:
- 77% in patients with poor-risk cytogenetics
- 67% in patients with fewer than five prior therapies
- 63% in patients with five or more prior therapies
- 70% in patients refractory to bortezomib and lenalidomide
- 60% in patients refractory to bortezomib and pomalidomide
- 62% in patients refractory to bortezomib, lenalidomide, and pomalidomide.
The researchers said the clinical benefit of NeVd may have been underestimated in this trial because patients were only able to receive six cycles of treatment on study due to a lack of external funding support.
“The time course of paraprotein levels also suggests that individual patients might potentially have experienced myeloma control if NeVd had been continued until progression,” the researchers wrote.
Five patients did receive more than six cycles of NeVd on a compassionate-use basis. In all, 27 patients received additional anti-myeloma treatment during follow-up.
The median progression-free survival was 12 weeks for the entire cohort and 16 weeks among patients who achieved a PR or better.
The median overall survival was 12 months, and 17 patients had died as of November 2016.
The researchers said the most common adverse events in this trial were anemia (97%), thrombocytopenia (82%), hypertension (53%), diarrhea (47%), fatigue (38%), and dyspnea (35%).
There were four deaths during treatment—three due to septicemia and one from heart failure. Three of the deaths were associated with underlying pneumonia.
“Although this mortality rate is consistent with the background mortality among patients with heavily pretreated, refractory myeloma, these findings suggest that prophylactic antibiotic therapy should be considered in those with low neutrophil counts and/or advanced age undergoing NeVd treatment,” the researchers wrote.
This work was supported by the Swiss Group for Clinical Cancer Research, the Swiss State Secretariat for Education, Research and Innovation, the Rising Tide Foundation for Clinical Cancer Research, and the Gateway for Cancer Research.
Most of the researchers declared no competing financial interests, but one reported personal fees from Celgene, Takeda, Amgen, Novartis, and Janssen-Cilag that were unrelated to this trial.
1. Lokhorst HM et al. N Engl J Med. 2015; 373(13):1207-1219.
2. Dimopoulos MA et al. Blood. 2016;128(4): 497-503.
Phase 2 results suggest a three-drug regimen may improve response rates in patients with proteasome inhibitor (PI)-refractory multiple myeloma (MM).
The combination—nelfinavir, bortezomib, and dexamethasone (NeVd)—produced an objective response rate (ORR) of 65% in this trial.
In comparison, past studies have shown response rates of 24% to 36% in the PI-refractory MM population.1,2,3
“The unprecedented ORR of NeVd observed in this heavily pretreated, multi-refractory setting warrants further investigation to explore the potential of nelfinavir in combination with PIs . . . ,” Christoph Driessen, of Kantonsspital St. Gallen in Switzerland, and his colleagues wrote in a letter to Blood.
The researchers noted that NeVd previously demonstrated activity in a phase 1 trial of MM patients who were refractory to both bortezomib and lenalidomide.
For the phase 2 study (NCT02188537), Dr. Driessen and his colleagues tested NeVd in MM patients who were refractory to their most recent PI-containing regimen and were previously exposed to or intolerant of at least one immunomodulatory drug.
The 34 patients had a median age of 67 (range, 42-82). Sixty-two percent were male, 38% had poor-risk cytogenetics, and most had a performance status of 0 (59%) or 1 (32%).
All patients had received lenalidomide, 47% had prior pomalidomide, 76% had prior high-dose chemotherapy, and 76% had a prior transplant.
All patients were bortezomib-refractory, 79% were refractory to a PI and lenalidomide, 44% were refractory to a PI and pomalidomide, and 38% were refractory to a PI, lenalidomide, and pomalidomide.
In this study, the patients received:
- Nelfinavir at 2500 mg on days 1 to 14 twice daily
- Bortezomib at 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11
- Dexamethasone at 20 mg orally on days 1 to 2, 4 to 5, 8 to 9, and 11 to 12.
The patients were treated for up to six 21-day cycles. They received a median of 4.5 cycles (range, 1-6).
Results
The ORR was 65%, with 17 partial responses (PRs) and five very good PRs. Three patients had a minimal response, and four had stable disease.
The ORR (PR or better) was:
- 77% in patients with poor-risk cytogenetics
- 67% in patients with fewer than five prior therapies
- 63% in patients with five or more prior therapies
- 70% in patients refractory to bortezomib and lenalidomide
- 60% in patients refractory to bortezomib and pomalidomide
- 62% in patients refractory to bortezomib, lenalidomide, and pomalidomide.
The researchers said the clinical benefit of NeVd may have been underestimated in this trial because patients were only able to receive six cycles of treatment on study due to a lack of external funding support.
“The time course of paraprotein levels also suggests that individual patients might potentially have experienced myeloma control if NeVd had been continued until progression,” the researchers wrote.
Five patients did receive more than six cycles of NeVd on a compassionate-use basis. In all, 27 patients received additional anti-myeloma treatment during follow-up.
The median progression-free survival was 12 weeks for the entire cohort and 16 weeks among patients who achieved a PR or better.
The median overall survival was 12 months, and 17 patients had died as of November 2016.
The researchers said the most common adverse events in this trial were anemia (97%), thrombocytopenia (82%), hypertension (53%), diarrhea (47%), fatigue (38%), and dyspnea (35%).
There were four deaths during treatment—three due to septicemia and one from heart failure. Three of the deaths were associated with underlying pneumonia.
“Although this mortality rate is consistent with the background mortality among patients with heavily pretreated, refractory myeloma, these findings suggest that prophylactic antibiotic therapy should be considered in those with low neutrophil counts and/or advanced age undergoing NeVd treatment,” the researchers wrote.
This work was supported by the Swiss Group for Clinical Cancer Research, the Swiss State Secretariat for Education, Research and Innovation, the Rising Tide Foundation for Clinical Cancer Research, and the Gateway for Cancer Research.
Most of the researchers declared no competing financial interests, but one reported personal fees from Celgene, Takeda, Amgen, Novartis, and Janssen-Cilag that were unrelated to this trial.
1. Lokhorst HM et al. N Engl J Med. 2015; 373(13):1207-1219.
2. Dimopoulos MA et al. Blood. 2016;128(4): 497-503.
Elderly NHL patients have higher NRM after HSCT
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
Americans concerned about cost of cancer care
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
A recent survey suggests Americans are nearly as worried about the cost of a cancer diagnosis as they are about dying from cancer.
The cost of cancer care was a top concern even among people who had no prior experience with cancer.
At the same time, cancer patients/survivors admitted to delaying or forgoing care due to costs, and caregivers reported taking “dramatic” actions to pay for their loved one’s care.
These are findings from the American Society of Clinical Oncology (ASCO)’s second annual National Cancer Opinion Survey.
The survey was conducted online by The Harris Poll from July 10, 2018, to August 10, 2018. It included 4,887 U.S. adults age 18 and older—1,001 of whom have or had cancer.
Cost among top concerns
Death and pain/suffering were the top concerns related to a cancer diagnosis. Fifty-four percent of respondents said death would be one of their greatest concerns if they were diagnosed with cancer, and the same percentage rated pain/suffering a top concern.
Forty-four percent of respondents said paying for cancer treatment would be a top concern, and 45% said the same about the financial impact of a cancer diagnosis on their family. When combined, financial issues were a top concern for 57% of respondents.
Paying for treatment was a top concern for:
- 36% of respondents who had/have cancer
- 51% of caregivers
- 43% of people with no prior cancer experience.
The financial impact on family was a top concern for:
- 39% of respondents who had/have cancer
- 55% of caregivers
- 42% of people with no prior cancer experience.
Cutting costs
Sixty-one percent of caregivers surveyed said they or another relative have taken a “dramatic” step to help pay for their loved one’s care, including:
- Dipping into savings accounts (35%)
- Working extra hours (23%)
- Taking an early withdrawal from a retirement account or college fund (14%)
- Postponing retirement (14%)
- Taking out a second mortgage or other type of loan (13%)
- Taking an additional job (13%)
- Selling family heirlooms (9%).
Twenty percent of cancer patients/survivors said they have taken actions to reduce treatment costs, including:
- Delaying scans (7%)
- Skipping or delaying appointments (7%)
- Skipping doses of prescribed treatment (6%)
- Postponing or not filling prescriptions (5%)
- Refusing treatment (3%).
“Patients are right to be concerned about the financial impact of a cancer diagnosis on their families,” said Richard L. Schilsky, MD, ASCO’s chief medical officer.
“It’s clear that high treatment costs are taking a serious toll not only on patients, but also on the people who care for them. If a family member has been diagnosed with cancer, the sole focus should be helping them get well. Instead, Americans are worrying about affording treatment, and, in many cases, they’re making serious personal sacrifices to help pay for their loved ones’ care.”
Beware bacteremia suspicious of colon cancer
Clinical question: Is bacteremia from certain microbes associated with colorectal cancer?
Background: Streptococcus bovis bacteremia is classically associated with colorectal cancer. A number of other bacterial species have been found in colorectal cancer microbiota and may even exert oncogenic effects. However, it is not known whether bacteremia from these microbes is associated with colorectal cancer.
Study design: Retrospective cohort study.
Setting: Public hospitals in Hong Kong.
Synopsis: Using the Clinical Data Analysis and Reporting System (representing greater than 90% of inpatient services provided in Hong Kong), researchers identified 15,215 patients with bacteremia from 11 genera of bacteria known to be present in the colorectal cancer microbiota, including Bacteroides, Clostridium, Filifactor, Fusobacterium, Gemella, Granulicatella, Parvimonas, Peptostreptococcus, Prevotella, Solobacterium, and Streptococcus. Compared with matched controls without bacteremia, a higher proportion of exposed patients had a subsequent diagnosis of colorectal cancer (1.69% vs. 1.16%; hazard ratio, 1.72; 95% confidence interval, 1.40-2.12). Bacteremia with other organisms was not associated with colorectal cancer, and bacteremia with the preidentified organisms was not associated with other types of non–colorectal cancer or nonmalignant gastrointestinal diseases, with the exception of a few genera commonly associated with diverticulitis.
Given the observational nature of this study, no causal relationship can be established. It is not clear if these species are involved in the oncogenesis of colorectal cancer or if colorectal tumors merely serve as a site of entry for bacteria into the bloodstream.
Bottom line: Bacteremia with certain bacterial genera is associated with colon cancer and should prompt consideration of colonoscopy to evaluate for malignancy.
Citation: Kwong TNY et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018 Aug;155(2):383-90.
Dr. Scarpato is clinical instructor in the division of hospital medicine, University of Colorado, Denver.
Clinical question: Is bacteremia from certain microbes associated with colorectal cancer?
Background: Streptococcus bovis bacteremia is classically associated with colorectal cancer. A number of other bacterial species have been found in colorectal cancer microbiota and may even exert oncogenic effects. However, it is not known whether bacteremia from these microbes is associated with colorectal cancer.
Study design: Retrospective cohort study.
Setting: Public hospitals in Hong Kong.
Synopsis: Using the Clinical Data Analysis and Reporting System (representing greater than 90% of inpatient services provided in Hong Kong), researchers identified 15,215 patients with bacteremia from 11 genera of bacteria known to be present in the colorectal cancer microbiota, including Bacteroides, Clostridium, Filifactor, Fusobacterium, Gemella, Granulicatella, Parvimonas, Peptostreptococcus, Prevotella, Solobacterium, and Streptococcus. Compared with matched controls without bacteremia, a higher proportion of exposed patients had a subsequent diagnosis of colorectal cancer (1.69% vs. 1.16%; hazard ratio, 1.72; 95% confidence interval, 1.40-2.12). Bacteremia with other organisms was not associated with colorectal cancer, and bacteremia with the preidentified organisms was not associated with other types of non–colorectal cancer or nonmalignant gastrointestinal diseases, with the exception of a few genera commonly associated with diverticulitis.
Given the observational nature of this study, no causal relationship can be established. It is not clear if these species are involved in the oncogenesis of colorectal cancer or if colorectal tumors merely serve as a site of entry for bacteria into the bloodstream.
Bottom line: Bacteremia with certain bacterial genera is associated with colon cancer and should prompt consideration of colonoscopy to evaluate for malignancy.
Citation: Kwong TNY et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018 Aug;155(2):383-90.
Dr. Scarpato is clinical instructor in the division of hospital medicine, University of Colorado, Denver.
Clinical question: Is bacteremia from certain microbes associated with colorectal cancer?
Background: Streptococcus bovis bacteremia is classically associated with colorectal cancer. A number of other bacterial species have been found in colorectal cancer microbiota and may even exert oncogenic effects. However, it is not known whether bacteremia from these microbes is associated with colorectal cancer.
Study design: Retrospective cohort study.
Setting: Public hospitals in Hong Kong.
Synopsis: Using the Clinical Data Analysis and Reporting System (representing greater than 90% of inpatient services provided in Hong Kong), researchers identified 15,215 patients with bacteremia from 11 genera of bacteria known to be present in the colorectal cancer microbiota, including Bacteroides, Clostridium, Filifactor, Fusobacterium, Gemella, Granulicatella, Parvimonas, Peptostreptococcus, Prevotella, Solobacterium, and Streptococcus. Compared with matched controls without bacteremia, a higher proportion of exposed patients had a subsequent diagnosis of colorectal cancer (1.69% vs. 1.16%; hazard ratio, 1.72; 95% confidence interval, 1.40-2.12). Bacteremia with other organisms was not associated with colorectal cancer, and bacteremia with the preidentified organisms was not associated with other types of non–colorectal cancer or nonmalignant gastrointestinal diseases, with the exception of a few genera commonly associated with diverticulitis.
Given the observational nature of this study, no causal relationship can be established. It is not clear if these species are involved in the oncogenesis of colorectal cancer or if colorectal tumors merely serve as a site of entry for bacteria into the bloodstream.
Bottom line: Bacteremia with certain bacterial genera is associated with colon cancer and should prompt consideration of colonoscopy to evaluate for malignancy.
Citation: Kwong TNY et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018 Aug;155(2):383-90.
Dr. Scarpato is clinical instructor in the division of hospital medicine, University of Colorado, Denver.
Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.
Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).
DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.
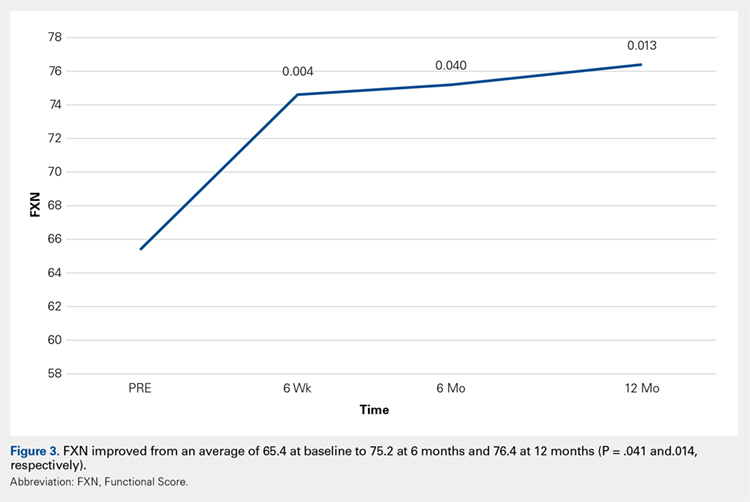
A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14
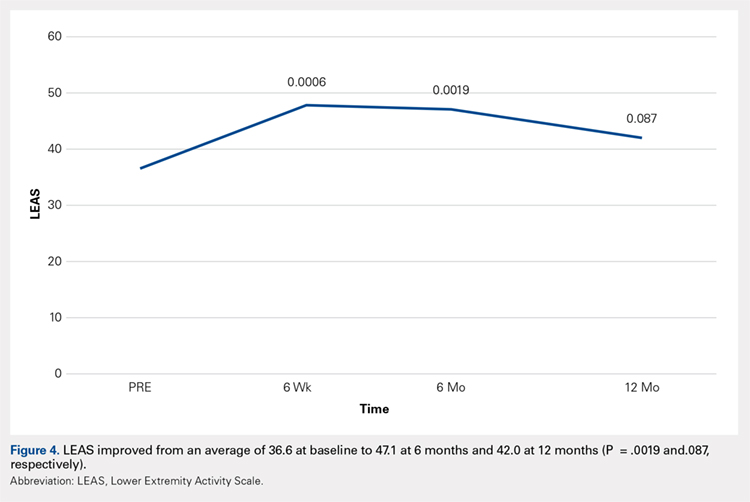
Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
TAKE-HOME POINTS
- Severe knee osteoarthritis causes pain and limits functions in a substantial proportion of the aging population.
- Total knee arthroplasty is often recommended in this group of patients when conservative management has failed.
- Many patients in this group continue to seek a nonsurgical option for this process.
- Autologous, micro-fractured, minimally manipulated adipose tissue is easy to harvest, and injection into a knee joint resulted in significant improvement in pain and function for at least 12 months in this study population.
- This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population.
Monalizumab-cetuximab combo shows promise in advanced head and neck SCC
WASHINGTON – and associated with deep and durable responses in patients with recurrent or metastatic squamous cell carcinoma (SCC) of the head and neck, according to data from an ongoing cohort expansion study.
As of Aug. 21, 2018, the primary study endpoint of overall response rate in 40 evaluable patients enrolled in the single-arm, nonrandomized phase 1/2 study was 27.5%, Roger Cohen, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
There were 11 confirmed responses, including 1 complete response and 10 partial responses at a median follow-up of 8 months, said Dr. Cohen, a professor of medicine at the Hospital of the University of Pennsylvania and director of clinical research at the Abramson Cancer Center, Philadelphia.
Median progression-free survival and overall survival were 5.0 months and 10.3 months, respectively.
“We observed responses in patients who were naive to immunotherapy, as well as patients who had received and progressed after immunotherapy. We observed responses in patients who were platinum resistant, and we also saw responses in [human papillomavirus (HPV)]–positive and –negative patients,” he said, adding that responses occurred relatively early at a median of 1.6 months, and that there was little difference between those who had and had not received prior immunotherapy with programmed death-1 (PD-1) antibodies.
A number of the responses, as well as the stable diseases, were durable for “a considerable period of time.” The median duration of response was 5.6 months, he said.
Study participants were mainly middle-aged men with recurrent or metastatic HPV-positive or -negative advanced disease and “decent” performance status. They received monalizumab at a dose of 10 mg/kg every 2 weeks plus cetuximab at the labeled loading dose of 400 mg/m2 once weekly then 250 mg/m2 once weekly. All had progressed after prior platinum-based chemotherapy and had no more than two prior lines of therapy in the recurrent/metastatic setting; 17 (43%) had prior anti–programmed death-ligand 1 (PD-L1) therapy, 5 (13%) had prior cetuximab, but none of those patients were cetuximab resistant.
They were treated until disease progression or unacceptable toxicity and were assessed every 8 weeks for response per Response Evaluation Criteria in Solid Tumors (RECIST) criteria, he said.
This treatment combination was shown in the phase 1 portion of the study to have a favorable safety profile, and the safety profile was confirmed in this expansion cohort; adverse events related to the combination were dominated by EGFR antibody–related side effects in the skin, as well as hypomagnesemia. Most adverse events associated with monalizumab were grade 1-2.
“Serious adverse events are in the single digits,” Dr. Cohen said.
Monalizumab is a first-in-class humanized immunoglobulin-G4 monoclonal antibody against the human natural killer group 2A (NKG2A), which is the receptor for the NKG2A ligand, HLA-E.
“The HLA-E NKG2A diad shuts down NK cells and tumor-infiltrating CD8-positive T-cells,” he explained, adding that “the concept behind the antibody is that by blocking the interaction of the receptor for the ligand you can reduce this inhibitory signaling by NK cells and thereby unleash their ability to target tumor.”
Cetuximab is an established and approved EGFR inhibitor for the treatment of patients with head and neck SCC who progress after platinum-based chemotherapy. It has been associated with a 13% response rate.
“The therapeutic hypothesis is that dual targeting with these two antibodies will allow us to realize greater antitumor activity in head and neck cancer than is seen with cetuximab alone,” he said, later adding that “the combination of monalizumab and cetuximab results in early, deep, and durable responses in patients with squamous cell cancer of the head and neck ... and the activity indeed is higher than cetuximab alone, compared with historical data.”
Additionally, the safety is acceptable, and preliminary translational analyses do show some immunological proof-of-concept – mainly that infiltration of the tumor stroma with NK and CD8-positive T cells is correlated with response, he said.
“Importantly, this study is continuing to enroll patients, and taking account of the ever-changing landscape in the treatment of patients with advanced cancer, we are going to enroll another 40 patients, except this time we will require them to be platinum, as well as PD-1 antibody exposed. These patients still represent an enormous unmet medical need.
“We think these results do warrant further development of the combo of monalizumab and cetuximab in patients with advanced SCC of the head and neck,” he concluded.
Dr. Cohen reported receiving consulting fees and/or research funding from Cantargia, Celldex, Genocea, Innate, HEAT, Kyntherapeutics, Merck, Takeda Macrogenics, and Tmunity.
SOURCE: Cohen R et al. SITC 2018, Abstract 051.
WASHINGTON – and associated with deep and durable responses in patients with recurrent or metastatic squamous cell carcinoma (SCC) of the head and neck, according to data from an ongoing cohort expansion study.
As of Aug. 21, 2018, the primary study endpoint of overall response rate in 40 evaluable patients enrolled in the single-arm, nonrandomized phase 1/2 study was 27.5%, Roger Cohen, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
There were 11 confirmed responses, including 1 complete response and 10 partial responses at a median follow-up of 8 months, said Dr. Cohen, a professor of medicine at the Hospital of the University of Pennsylvania and director of clinical research at the Abramson Cancer Center, Philadelphia.
Median progression-free survival and overall survival were 5.0 months and 10.3 months, respectively.
“We observed responses in patients who were naive to immunotherapy, as well as patients who had received and progressed after immunotherapy. We observed responses in patients who were platinum resistant, and we also saw responses in [human papillomavirus (HPV)]–positive and –negative patients,” he said, adding that responses occurred relatively early at a median of 1.6 months, and that there was little difference between those who had and had not received prior immunotherapy with programmed death-1 (PD-1) antibodies.
A number of the responses, as well as the stable diseases, were durable for “a considerable period of time.” The median duration of response was 5.6 months, he said.
Study participants were mainly middle-aged men with recurrent or metastatic HPV-positive or -negative advanced disease and “decent” performance status. They received monalizumab at a dose of 10 mg/kg every 2 weeks plus cetuximab at the labeled loading dose of 400 mg/m2 once weekly then 250 mg/m2 once weekly. All had progressed after prior platinum-based chemotherapy and had no more than two prior lines of therapy in the recurrent/metastatic setting; 17 (43%) had prior anti–programmed death-ligand 1 (PD-L1) therapy, 5 (13%) had prior cetuximab, but none of those patients were cetuximab resistant.
They were treated until disease progression or unacceptable toxicity and were assessed every 8 weeks for response per Response Evaluation Criteria in Solid Tumors (RECIST) criteria, he said.
This treatment combination was shown in the phase 1 portion of the study to have a favorable safety profile, and the safety profile was confirmed in this expansion cohort; adverse events related to the combination were dominated by EGFR antibody–related side effects in the skin, as well as hypomagnesemia. Most adverse events associated with monalizumab were grade 1-2.
“Serious adverse events are in the single digits,” Dr. Cohen said.
Monalizumab is a first-in-class humanized immunoglobulin-G4 monoclonal antibody against the human natural killer group 2A (NKG2A), which is the receptor for the NKG2A ligand, HLA-E.
“The HLA-E NKG2A diad shuts down NK cells and tumor-infiltrating CD8-positive T-cells,” he explained, adding that “the concept behind the antibody is that by blocking the interaction of the receptor for the ligand you can reduce this inhibitory signaling by NK cells and thereby unleash their ability to target tumor.”
Cetuximab is an established and approved EGFR inhibitor for the treatment of patients with head and neck SCC who progress after platinum-based chemotherapy. It has been associated with a 13% response rate.
“The therapeutic hypothesis is that dual targeting with these two antibodies will allow us to realize greater antitumor activity in head and neck cancer than is seen with cetuximab alone,” he said, later adding that “the combination of monalizumab and cetuximab results in early, deep, and durable responses in patients with squamous cell cancer of the head and neck ... and the activity indeed is higher than cetuximab alone, compared with historical data.”
Additionally, the safety is acceptable, and preliminary translational analyses do show some immunological proof-of-concept – mainly that infiltration of the tumor stroma with NK and CD8-positive T cells is correlated with response, he said.
“Importantly, this study is continuing to enroll patients, and taking account of the ever-changing landscape in the treatment of patients with advanced cancer, we are going to enroll another 40 patients, except this time we will require them to be platinum, as well as PD-1 antibody exposed. These patients still represent an enormous unmet medical need.
“We think these results do warrant further development of the combo of monalizumab and cetuximab in patients with advanced SCC of the head and neck,” he concluded.
Dr. Cohen reported receiving consulting fees and/or research funding from Cantargia, Celldex, Genocea, Innate, HEAT, Kyntherapeutics, Merck, Takeda Macrogenics, and Tmunity.
SOURCE: Cohen R et al. SITC 2018, Abstract 051.
WASHINGTON – and associated with deep and durable responses in patients with recurrent or metastatic squamous cell carcinoma (SCC) of the head and neck, according to data from an ongoing cohort expansion study.
As of Aug. 21, 2018, the primary study endpoint of overall response rate in 40 evaluable patients enrolled in the single-arm, nonrandomized phase 1/2 study was 27.5%, Roger Cohen, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
There were 11 confirmed responses, including 1 complete response and 10 partial responses at a median follow-up of 8 months, said Dr. Cohen, a professor of medicine at the Hospital of the University of Pennsylvania and director of clinical research at the Abramson Cancer Center, Philadelphia.
Median progression-free survival and overall survival were 5.0 months and 10.3 months, respectively.
“We observed responses in patients who were naive to immunotherapy, as well as patients who had received and progressed after immunotherapy. We observed responses in patients who were platinum resistant, and we also saw responses in [human papillomavirus (HPV)]–positive and –negative patients,” he said, adding that responses occurred relatively early at a median of 1.6 months, and that there was little difference between those who had and had not received prior immunotherapy with programmed death-1 (PD-1) antibodies.
A number of the responses, as well as the stable diseases, were durable for “a considerable period of time.” The median duration of response was 5.6 months, he said.
Study participants were mainly middle-aged men with recurrent or metastatic HPV-positive or -negative advanced disease and “decent” performance status. They received monalizumab at a dose of 10 mg/kg every 2 weeks plus cetuximab at the labeled loading dose of 400 mg/m2 once weekly then 250 mg/m2 once weekly. All had progressed after prior platinum-based chemotherapy and had no more than two prior lines of therapy in the recurrent/metastatic setting; 17 (43%) had prior anti–programmed death-ligand 1 (PD-L1) therapy, 5 (13%) had prior cetuximab, but none of those patients were cetuximab resistant.
They were treated until disease progression or unacceptable toxicity and were assessed every 8 weeks for response per Response Evaluation Criteria in Solid Tumors (RECIST) criteria, he said.
This treatment combination was shown in the phase 1 portion of the study to have a favorable safety profile, and the safety profile was confirmed in this expansion cohort; adverse events related to the combination were dominated by EGFR antibody–related side effects in the skin, as well as hypomagnesemia. Most adverse events associated with monalizumab were grade 1-2.
“Serious adverse events are in the single digits,” Dr. Cohen said.
Monalizumab is a first-in-class humanized immunoglobulin-G4 monoclonal antibody against the human natural killer group 2A (NKG2A), which is the receptor for the NKG2A ligand, HLA-E.
“The HLA-E NKG2A diad shuts down NK cells and tumor-infiltrating CD8-positive T-cells,” he explained, adding that “the concept behind the antibody is that by blocking the interaction of the receptor for the ligand you can reduce this inhibitory signaling by NK cells and thereby unleash their ability to target tumor.”
Cetuximab is an established and approved EGFR inhibitor for the treatment of patients with head and neck SCC who progress after platinum-based chemotherapy. It has been associated with a 13% response rate.
“The therapeutic hypothesis is that dual targeting with these two antibodies will allow us to realize greater antitumor activity in head and neck cancer than is seen with cetuximab alone,” he said, later adding that “the combination of monalizumab and cetuximab results in early, deep, and durable responses in patients with squamous cell cancer of the head and neck ... and the activity indeed is higher than cetuximab alone, compared with historical data.”
Additionally, the safety is acceptable, and preliminary translational analyses do show some immunological proof-of-concept – mainly that infiltration of the tumor stroma with NK and CD8-positive T cells is correlated with response, he said.
“Importantly, this study is continuing to enroll patients, and taking account of the ever-changing landscape in the treatment of patients with advanced cancer, we are going to enroll another 40 patients, except this time we will require them to be platinum, as well as PD-1 antibody exposed. These patients still represent an enormous unmet medical need.
“We think these results do warrant further development of the combo of monalizumab and cetuximab in patients with advanced SCC of the head and neck,” he concluded.
Dr. Cohen reported receiving consulting fees and/or research funding from Cantargia, Celldex, Genocea, Innate, HEAT, Kyntherapeutics, Merck, Takeda Macrogenics, and Tmunity.
SOURCE: Cohen R et al. SITC 2018, Abstract 051.
REPORTING FROM SITC 2018
Key clinical point: Monalizumab + cetuximab is safe, active in recurrent or metastatic SCC of the head and neck.
Major finding: Overall response rate in 40 evaluable patients was 27.5%,with 1 CR and 10 PRs at 8 weeks.
Study details: A cohort expansion of 40 patients in the single-arm, non-randomized phase 1/2 study.
Disclosures: Dr. Cohen reported receiving consulting fees and/or research funding from Cantargia, Celldex, Genocea, Innate, HEAT, Kyntherapeutics, Merck, Takeda Macrogenics, and Tmunity.
Source: Cohen R et al. SITC 2018, Abstract 051.