User login
VAM Abstract Site Now Open
The abstract submission site for the 2019 Vascular Annual Meeting is now open. Submissions may be considered for the following programs: Scientific Session, Vascular and Endovascular Surgical Society (VESS), International Forum, International Fast Talk, Poster Competition and Interactive Poster. In addition to the International Forum and International Fast Talk, the international community has added two further opportunities to showcase research: The International Young Surgeon Competition and the International Poster Competition. This year the submission site is mobile friendly! Get more information on submission and policy guidelines here.
The abstract submission site for the 2019 Vascular Annual Meeting is now open. Submissions may be considered for the following programs: Scientific Session, Vascular and Endovascular Surgical Society (VESS), International Forum, International Fast Talk, Poster Competition and Interactive Poster. In addition to the International Forum and International Fast Talk, the international community has added two further opportunities to showcase research: The International Young Surgeon Competition and the International Poster Competition. This year the submission site is mobile friendly! Get more information on submission and policy guidelines here.
The abstract submission site for the 2019 Vascular Annual Meeting is now open. Submissions may be considered for the following programs: Scientific Session, Vascular and Endovascular Surgical Society (VESS), International Forum, International Fast Talk, Poster Competition and Interactive Poster. In addition to the International Forum and International Fast Talk, the international community has added two further opportunities to showcase research: The International Young Surgeon Competition and the International Poster Competition. This year the submission site is mobile friendly! Get more information on submission and policy guidelines here.
Oral immunotherapy desensitizes youth with peanut allergy
Peanut-allergic children and adolescents treated with a peanut-derived oral immunotherapy drug have shown significant improvements in response to a challenge dose of peanut protein, according to data presented at the annual meeting of the American College of Allergy, Asthma, and Immunology.
A phase 3 placebo-controlled study, published simultaneously Nov. 18 in the New England Journal of Medicine, randomized 551 individuals with peanut allergy to receive an escalating dose of AR101 – an investigational peanut-derived biologic oral immunotherapy drug – ranging from 0.5-300 mg daily, or placebo.
After 12 months, 67.2% of the 372 participants aged 4-17 years who received the immunotherapy drug were able to eat a dose of 600 mg or more of peanut protein with only mild symptoms, compared with 4% of the 124 participants aged 4-17 years in the placebo group.
The secondary endpoints were whether participants could tolerate either a 300 mg or 1,000 mg dose in the exit food challenge. For the 300 mg dose, 76.6% of the immunotherapy group and 8.1% of the placebo group were able to tolerate it, and for the 1,000 mg group, 50.3% of the immunotherapy group were able to tolerate it, compared with 2.4% of the placebo group.
During the exit food challenge, the severity of symptoms was significantly higher in the placebo group than in the treatment group. One-quarter of participants in the treatment group had at most moderate symptoms, compared with 59% in the placebo group. However, severe symptoms were experienced by 11% of the placebo group, compared with 5% of the treatment group.
One in 10 participants in the active group had to be treated with rescue epinephrine during the exit food challenge, compared with 53% of participants in the placebo group, and the number who required a second dose of rescue epinephrine was 1% and 15%, respectively.
“These data show that, in the context of a clinical trial, among participants 4-17 years of age, AR101 had immunomodulatory activity, raised the threshold dose of peanut exposure triggering the onset of clinically significant allergic symptoms (among participants having symptoms), during the double-blind, placebo-controlled exit food challenge, and attenuated the severity of those symptoms when they occurred,” wrote Brian P. Vickery, MD, of Emory University in Atlanta, and his coauthors.
The 55 participants aged 18-55 years were analyzed separately, and researchers found that for the 600 mg exit food test, the difference between the two groups did not reach statistical significance.
Apart from adverse events that occurred during the exit food challenge, the rate of adverse events was slightly higher in the treatment group compared to the placebo group (98.7% vs. 95.2%). The most common adverse events in the treatment arm were abdominal pain, vomiting, oral pruritis, and nausea.
The study was funded by Aimmune Therapeutics. Three authors were employees of or investigators for Aimmune Therapeutics and one also had a patent pending for oral immunotherapy for peanut allergy. Most authors declared funding, grants, consultancies, or other support from the pharmaceutical industry, including from some from Aimmune.
SOURCE: Vickery BP et al. N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMoa1812856.
Over the past decade, more case reports and small studies have suggested that the use of tiny and incrementally increasing amount of peanut could desensitize those who are allergic to peanuts. This study, which uses a product based on defatted peanut flour, has shown that by the end of the course of treatment, two-thirds of those treated could consume around four peanuts.
However, the treatment was associated with side effects, many participants needed treatment with epinephrine, and the study has not yet addressed concerns about the longer term side effects of sustained allergen consumption, such as eosinophilic esophagitis.
The question also still remains as to whether the allergen tolerance is long-lasting or whether it will need to be maintained with regular exposure.
Michael R. Perkin, PhD, is affiliated with the Population Health Research Unit at St George’s, University of London. These comments are taken from an accompanying editorial (N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMe1813314). No conflicts of interest were declared.
Over the past decade, more case reports and small studies have suggested that the use of tiny and incrementally increasing amount of peanut could desensitize those who are allergic to peanuts. This study, which uses a product based on defatted peanut flour, has shown that by the end of the course of treatment, two-thirds of those treated could consume around four peanuts.
However, the treatment was associated with side effects, many participants needed treatment with epinephrine, and the study has not yet addressed concerns about the longer term side effects of sustained allergen consumption, such as eosinophilic esophagitis.
The question also still remains as to whether the allergen tolerance is long-lasting or whether it will need to be maintained with regular exposure.
Michael R. Perkin, PhD, is affiliated with the Population Health Research Unit at St George’s, University of London. These comments are taken from an accompanying editorial (N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMe1813314). No conflicts of interest were declared.
Over the past decade, more case reports and small studies have suggested that the use of tiny and incrementally increasing amount of peanut could desensitize those who are allergic to peanuts. This study, which uses a product based on defatted peanut flour, has shown that by the end of the course of treatment, two-thirds of those treated could consume around four peanuts.
However, the treatment was associated with side effects, many participants needed treatment with epinephrine, and the study has not yet addressed concerns about the longer term side effects of sustained allergen consumption, such as eosinophilic esophagitis.
The question also still remains as to whether the allergen tolerance is long-lasting or whether it will need to be maintained with regular exposure.
Michael R. Perkin, PhD, is affiliated with the Population Health Research Unit at St George’s, University of London. These comments are taken from an accompanying editorial (N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMe1813314). No conflicts of interest were declared.
Peanut-allergic children and adolescents treated with a peanut-derived oral immunotherapy drug have shown significant improvements in response to a challenge dose of peanut protein, according to data presented at the annual meeting of the American College of Allergy, Asthma, and Immunology.
A phase 3 placebo-controlled study, published simultaneously Nov. 18 in the New England Journal of Medicine, randomized 551 individuals with peanut allergy to receive an escalating dose of AR101 – an investigational peanut-derived biologic oral immunotherapy drug – ranging from 0.5-300 mg daily, or placebo.
After 12 months, 67.2% of the 372 participants aged 4-17 years who received the immunotherapy drug were able to eat a dose of 600 mg or more of peanut protein with only mild symptoms, compared with 4% of the 124 participants aged 4-17 years in the placebo group.
The secondary endpoints were whether participants could tolerate either a 300 mg or 1,000 mg dose in the exit food challenge. For the 300 mg dose, 76.6% of the immunotherapy group and 8.1% of the placebo group were able to tolerate it, and for the 1,000 mg group, 50.3% of the immunotherapy group were able to tolerate it, compared with 2.4% of the placebo group.
During the exit food challenge, the severity of symptoms was significantly higher in the placebo group than in the treatment group. One-quarter of participants in the treatment group had at most moderate symptoms, compared with 59% in the placebo group. However, severe symptoms were experienced by 11% of the placebo group, compared with 5% of the treatment group.
One in 10 participants in the active group had to be treated with rescue epinephrine during the exit food challenge, compared with 53% of participants in the placebo group, and the number who required a second dose of rescue epinephrine was 1% and 15%, respectively.
“These data show that, in the context of a clinical trial, among participants 4-17 years of age, AR101 had immunomodulatory activity, raised the threshold dose of peanut exposure triggering the onset of clinically significant allergic symptoms (among participants having symptoms), during the double-blind, placebo-controlled exit food challenge, and attenuated the severity of those symptoms when they occurred,” wrote Brian P. Vickery, MD, of Emory University in Atlanta, and his coauthors.
The 55 participants aged 18-55 years were analyzed separately, and researchers found that for the 600 mg exit food test, the difference between the two groups did not reach statistical significance.
Apart from adverse events that occurred during the exit food challenge, the rate of adverse events was slightly higher in the treatment group compared to the placebo group (98.7% vs. 95.2%). The most common adverse events in the treatment arm were abdominal pain, vomiting, oral pruritis, and nausea.
The study was funded by Aimmune Therapeutics. Three authors were employees of or investigators for Aimmune Therapeutics and one also had a patent pending for oral immunotherapy for peanut allergy. Most authors declared funding, grants, consultancies, or other support from the pharmaceutical industry, including from some from Aimmune.
SOURCE: Vickery BP et al. N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMoa1812856.
Peanut-allergic children and adolescents treated with a peanut-derived oral immunotherapy drug have shown significant improvements in response to a challenge dose of peanut protein, according to data presented at the annual meeting of the American College of Allergy, Asthma, and Immunology.
A phase 3 placebo-controlled study, published simultaneously Nov. 18 in the New England Journal of Medicine, randomized 551 individuals with peanut allergy to receive an escalating dose of AR101 – an investigational peanut-derived biologic oral immunotherapy drug – ranging from 0.5-300 mg daily, or placebo.
After 12 months, 67.2% of the 372 participants aged 4-17 years who received the immunotherapy drug were able to eat a dose of 600 mg or more of peanut protein with only mild symptoms, compared with 4% of the 124 participants aged 4-17 years in the placebo group.
The secondary endpoints were whether participants could tolerate either a 300 mg or 1,000 mg dose in the exit food challenge. For the 300 mg dose, 76.6% of the immunotherapy group and 8.1% of the placebo group were able to tolerate it, and for the 1,000 mg group, 50.3% of the immunotherapy group were able to tolerate it, compared with 2.4% of the placebo group.
During the exit food challenge, the severity of symptoms was significantly higher in the placebo group than in the treatment group. One-quarter of participants in the treatment group had at most moderate symptoms, compared with 59% in the placebo group. However, severe symptoms were experienced by 11% of the placebo group, compared with 5% of the treatment group.
One in 10 participants in the active group had to be treated with rescue epinephrine during the exit food challenge, compared with 53% of participants in the placebo group, and the number who required a second dose of rescue epinephrine was 1% and 15%, respectively.
“These data show that, in the context of a clinical trial, among participants 4-17 years of age, AR101 had immunomodulatory activity, raised the threshold dose of peanut exposure triggering the onset of clinically significant allergic symptoms (among participants having symptoms), during the double-blind, placebo-controlled exit food challenge, and attenuated the severity of those symptoms when they occurred,” wrote Brian P. Vickery, MD, of Emory University in Atlanta, and his coauthors.
The 55 participants aged 18-55 years were analyzed separately, and researchers found that for the 600 mg exit food test, the difference between the two groups did not reach statistical significance.
Apart from adverse events that occurred during the exit food challenge, the rate of adverse events was slightly higher in the treatment group compared to the placebo group (98.7% vs. 95.2%). The most common adverse events in the treatment arm were abdominal pain, vomiting, oral pruritis, and nausea.
The study was funded by Aimmune Therapeutics. Three authors were employees of or investigators for Aimmune Therapeutics and one also had a patent pending for oral immunotherapy for peanut allergy. Most authors declared funding, grants, consultancies, or other support from the pharmaceutical industry, including from some from Aimmune.
SOURCE: Vickery BP et al. N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMoa1812856.
FROM ACAAI 2018
Key clinical point: Oral peanut immunotherapy can improve tolerance in patients aged 4-17 with peanut allergy.
Major finding: Among patients treated with oral peanut immunotherapy, 67.2% were able to tolerate 600 mg of peanut protein, compared with 4% of the placebo group.
Study details: A randomized, placebo-controlled phase 3 study in 551 individuals with peanut allergy.
Disclosures: The study was funded by Aimmune Therapeutics. Three authors were employees of or investigators for Aimmune Therapeutics, and one also had a patent pending for oral immunotherapy for peanut allergy. Most authors declared funding, grants, consultancies, or other support from the pharmaceutical industry, including from some from Aimmune.
Source: Vickery BP et al. N Engl J Med. 18 Nov 2018. doi: 10.1056/NEJMoa1812856.
Symptomatic hyperuricemia may respond to urate-lowering therapy
Persistent and nonepisodic foot pain in hyperuricemia is associated with ultrasound features of monosodium urate deposition within joints that is responsive to urate-lowering therapy, a case-control study shows.
, reported Yousef Mohammed Alammari, of the department of rheumatology, Tallaght University Hospital, Dublin, and associates.
Furthermore, the researchers wrote in Annals of the Rheumatic Diseases, their findings serve as “potential rationale” for reclassification of the ACR/EULAR 2015 gout classification criteria.
The research team noted that the gout classification criteria from the two rheumatology organizations require a history of a “prior episode of swelling, pain, or tenderness of a peripheral joint/bursa before confirmation either through MSU crystal identification in synovial fluid or through achieving a score of greater than 8 using a predefined scoring system of radiological, laboratory, and clinical features.”
Yet, emerging evidence suggests that foot pain could be a preclinical and clinical phase of gout that might occur prior to a first episodic gout attack, the investigators noted.
The current study involved 16 hyperuricemia individuals with persistent, nonepisodic foot pain who did not fulfill ACR/EULAR 2015 gout classification criteria but received febuxostat 80 mg once daily for 3 months. Controls were 15 individuals with asymptomatic hyperuricemia.
Results showed that double contour sign erosion and tophus occurred in 44%, 37%, and 37% of the cases, respectively, whereas no ultrasound features of gout were seen in the controls.
No significant difference in baseline serum urate was observed between the cases (450 plus or minus 18 mg/dL) and controls (426 plus or minus 7; P = nonsignificant), but at 1- and 3-month time points, serum urate fell in the cases (200 plus or minus 18; P less than 0.001 and 223 plus or minus 28; P less than less than 0.001).
In the cases, baseline 24-hour pain visual analogue score (65 plus or minus 4.9) reduced at 1 month (41 plus or minus 6.6; P = 0.001) and 3 months (33 plus or minus 7.2; P less than 0.001) of urate lowering therapy, as did the 7-day pain visual analogue score (70 plus or minus 4.7) scores (44 plus or minus 7.1; P less than 0.001 at 1 month; 38 plus or minus 8; P less than 0.001 at 3 months).
The Manchester Foot Pain and Disability Index also decreased at 1 month (21 plus or minus 2.9; P = 0.019) and 3 months (17 plus or minus 2.8; P = 0.012).
When the investigators grouped the cases according to the presence (n = 7) or absence (n = 9) of double contour sign on baseline ultrasound, no differences were observed for baseline pain scores.
However, after treatment with ultrasound, 24-hour pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months.
In addition, the researchers found, the 7-day pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months. No significant differences were seen between double contour positive and double contour negative patients in the Manchester Foot Pain and Disability Index or serum urate at 1 or 3 months of ultrasound.
The investigators noted that their findings indicated that persistent, nonepisodic foot pain in hyperuricemia was both associated with ultrasound features of monosodium urate deposition and was responsive to urate lowering therapy.
“Symptomatic hyperuricemia occurring prior to episodic gout therefore represents an earlier or alternative disease presentation,” they wrote. “Changes to the ACR/ EULAR classification criteria to include nonepisodic foot pain in the presence of [ultrasound] features of gout may increase the sensitivity of disease classification at an early stage, leading to improved future treatment strategies and long-term outcomes.”
No disclosures were declared.
SOURCE: Alammari YM et al. Ann Rheum Dis. 2018. doi: 10.1136/annrheumdis-2018-214305.
Persistent and nonepisodic foot pain in hyperuricemia is associated with ultrasound features of monosodium urate deposition within joints that is responsive to urate-lowering therapy, a case-control study shows.
, reported Yousef Mohammed Alammari, of the department of rheumatology, Tallaght University Hospital, Dublin, and associates.
Furthermore, the researchers wrote in Annals of the Rheumatic Diseases, their findings serve as “potential rationale” for reclassification of the ACR/EULAR 2015 gout classification criteria.
The research team noted that the gout classification criteria from the two rheumatology organizations require a history of a “prior episode of swelling, pain, or tenderness of a peripheral joint/bursa before confirmation either through MSU crystal identification in synovial fluid or through achieving a score of greater than 8 using a predefined scoring system of radiological, laboratory, and clinical features.”
Yet, emerging evidence suggests that foot pain could be a preclinical and clinical phase of gout that might occur prior to a first episodic gout attack, the investigators noted.
The current study involved 16 hyperuricemia individuals with persistent, nonepisodic foot pain who did not fulfill ACR/EULAR 2015 gout classification criteria but received febuxostat 80 mg once daily for 3 months. Controls were 15 individuals with asymptomatic hyperuricemia.
Results showed that double contour sign erosion and tophus occurred in 44%, 37%, and 37% of the cases, respectively, whereas no ultrasound features of gout were seen in the controls.
No significant difference in baseline serum urate was observed between the cases (450 plus or minus 18 mg/dL) and controls (426 plus or minus 7; P = nonsignificant), but at 1- and 3-month time points, serum urate fell in the cases (200 plus or minus 18; P less than 0.001 and 223 plus or minus 28; P less than less than 0.001).
In the cases, baseline 24-hour pain visual analogue score (65 plus or minus 4.9) reduced at 1 month (41 plus or minus 6.6; P = 0.001) and 3 months (33 plus or minus 7.2; P less than 0.001) of urate lowering therapy, as did the 7-day pain visual analogue score (70 plus or minus 4.7) scores (44 plus or minus 7.1; P less than 0.001 at 1 month; 38 plus or minus 8; P less than 0.001 at 3 months).
The Manchester Foot Pain and Disability Index also decreased at 1 month (21 plus or minus 2.9; P = 0.019) and 3 months (17 plus or minus 2.8; P = 0.012).
When the investigators grouped the cases according to the presence (n = 7) or absence (n = 9) of double contour sign on baseline ultrasound, no differences were observed for baseline pain scores.
However, after treatment with ultrasound, 24-hour pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months.
In addition, the researchers found, the 7-day pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months. No significant differences were seen between double contour positive and double contour negative patients in the Manchester Foot Pain and Disability Index or serum urate at 1 or 3 months of ultrasound.
The investigators noted that their findings indicated that persistent, nonepisodic foot pain in hyperuricemia was both associated with ultrasound features of monosodium urate deposition and was responsive to urate lowering therapy.
“Symptomatic hyperuricemia occurring prior to episodic gout therefore represents an earlier or alternative disease presentation,” they wrote. “Changes to the ACR/ EULAR classification criteria to include nonepisodic foot pain in the presence of [ultrasound] features of gout may increase the sensitivity of disease classification at an early stage, leading to improved future treatment strategies and long-term outcomes.”
No disclosures were declared.
SOURCE: Alammari YM et al. Ann Rheum Dis. 2018. doi: 10.1136/annrheumdis-2018-214305.
Persistent and nonepisodic foot pain in hyperuricemia is associated with ultrasound features of monosodium urate deposition within joints that is responsive to urate-lowering therapy, a case-control study shows.
, reported Yousef Mohammed Alammari, of the department of rheumatology, Tallaght University Hospital, Dublin, and associates.
Furthermore, the researchers wrote in Annals of the Rheumatic Diseases, their findings serve as “potential rationale” for reclassification of the ACR/EULAR 2015 gout classification criteria.
The research team noted that the gout classification criteria from the two rheumatology organizations require a history of a “prior episode of swelling, pain, or tenderness of a peripheral joint/bursa before confirmation either through MSU crystal identification in synovial fluid or through achieving a score of greater than 8 using a predefined scoring system of radiological, laboratory, and clinical features.”
Yet, emerging evidence suggests that foot pain could be a preclinical and clinical phase of gout that might occur prior to a first episodic gout attack, the investigators noted.
The current study involved 16 hyperuricemia individuals with persistent, nonepisodic foot pain who did not fulfill ACR/EULAR 2015 gout classification criteria but received febuxostat 80 mg once daily for 3 months. Controls were 15 individuals with asymptomatic hyperuricemia.
Results showed that double contour sign erosion and tophus occurred in 44%, 37%, and 37% of the cases, respectively, whereas no ultrasound features of gout were seen in the controls.
No significant difference in baseline serum urate was observed between the cases (450 plus or minus 18 mg/dL) and controls (426 plus or minus 7; P = nonsignificant), but at 1- and 3-month time points, serum urate fell in the cases (200 plus or minus 18; P less than 0.001 and 223 plus or minus 28; P less than less than 0.001).
In the cases, baseline 24-hour pain visual analogue score (65 plus or minus 4.9) reduced at 1 month (41 plus or minus 6.6; P = 0.001) and 3 months (33 plus or minus 7.2; P less than 0.001) of urate lowering therapy, as did the 7-day pain visual analogue score (70 plus or minus 4.7) scores (44 plus or minus 7.1; P less than 0.001 at 1 month; 38 plus or minus 8; P less than 0.001 at 3 months).
The Manchester Foot Pain and Disability Index also decreased at 1 month (21 plus or minus 2.9; P = 0.019) and 3 months (17 plus or minus 2.8; P = 0.012).
When the investigators grouped the cases according to the presence (n = 7) or absence (n = 9) of double contour sign on baseline ultrasound, no differences were observed for baseline pain scores.
However, after treatment with ultrasound, 24-hour pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months.
In addition, the researchers found, the 7-day pain visual analogue scores were significantly lower in double contour positive patients at 1 month and 3 months. No significant differences were seen between double contour positive and double contour negative patients in the Manchester Foot Pain and Disability Index or serum urate at 1 or 3 months of ultrasound.
The investigators noted that their findings indicated that persistent, nonepisodic foot pain in hyperuricemia was both associated with ultrasound features of monosodium urate deposition and was responsive to urate lowering therapy.
“Symptomatic hyperuricemia occurring prior to episodic gout therefore represents an earlier or alternative disease presentation,” they wrote. “Changes to the ACR/ EULAR classification criteria to include nonepisodic foot pain in the presence of [ultrasound] features of gout may increase the sensitivity of disease classification at an early stage, leading to improved future treatment strategies and long-term outcomes.”
No disclosures were declared.
SOURCE: Alammari YM et al. Ann Rheum Dis. 2018. doi: 10.1136/annrheumdis-2018-214305.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Persistent and nonepisodic foot pain in hyperuricemia is associated with ultrasound features of monosodium urate (MSU) deposition within joints that is responsive to urate-lowering therapy.
Major finding: Persistent, nonepisodic foot pain in hyperuricemia was both associated with ultrasound features of monosodium urate deposition and was responsive to ultrasound.
Study details: Case-control study involving 16 hyperuricemic individuals with persistent, nonepisodic foot pain who did not fulfill ACR/EULAR 2015 gout classification criteria.
Disclosures: No disclosures were declared.
Source: Alammari YM et al. Ann Rheum Dis. 2018. doi: 10.1136/annrheumdis-2018-214305.
Tegaderm eliminates corneal abrasions in robotic gynecologic surgery
LAS VEGAS – There hasn’t been a single corneal abrasion in 860 cases of gynecologic robotic surgery at the University of Texas, Austin, since surgeons and anesthesiologists there started sealing women’s eyes shut with a thick layer of ointment and Tegaderm, instead of the usual small squeeze of ointment and tape, according to Michael T. Breen, MD, a gynecologic surgeon at the university.
“Go back to your hospital, meet with your anesthesiologists, and see what you’re doing to protect your patients’ eyes,” Dr. Breen said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Slathered eyes and Tegaderm are now standard practice at the university. Before the switch was made, there were six corneal abrasions in 231 cases over 6 months. Two of those patients stayed longer in the hospital than they would have otherwise. The changes have eliminated the problem.
The impetus for the switch was a 42-year-old woman who had a robotic hysterectomy. The surgery went fine, but then Dr. Breen had to rush back to the recovery room. The woman was screaming in pain, not from her surgery, but from her left eye.
Corneal abrasions are a well-known risk of surgery because anesthesia decreases tear production and dries the eyes. Robotic gynecologic surgery increases the risk even more, because patients are under longer than with other approaches, and the steep Trendelenburg increases intraocular pressure and eye edema, especially with excess IV fluid.
And “believe it or not, having a pulse oximeter on the dominant hand [also] increases your risk of ocular injury,” Dr. Breen said. Sometimes, patients wake up, go to rub their eyes, and drag the device across their cornea, he said.
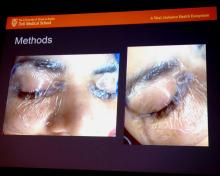
The screaming patient – who recovered without permanent damage – prompted Dr. Breen and his colleagues to turn to the literature for solutions. “One was a fully occlusive eye dressing, more than the tape we’ve all been accustomed to, with thick eye ointment application and Tegaderm applying positive pressure to the eye,” he said.
Dr. Breen showed his audience a slide of the setup. “It looks a little unorthodox, but this is how every one of our robotic patients now have their eyes protected. Thick gel which is then covered with a positive pressure Tegaderm,” he said.
, and placing the pulse ox on the nondominant hand. The team already had been decreasing IV fluids as part of their enhanced recovery after surgery protocol, and bringing patients out of steep Trendelenburg as soon as possible.
With the changes, “the rate of corneal abrasions decreased from 2.6% to 0% – and has stayed there,” Dr. Breen said.
There’ve been no allergic reactions to Tegaderm and no eyelid problems. “What we have seen with the simple taping is that, when it comes off, so does some of the eyelid, particularly with geriatric patients. We have not seen that with Tegaderm,” he said.
“Some people use goggles to protect the eyes, and we thought initially that the camera was hitting the face, so we used the Mayo stand to protect it from the camera,” but that didn’t turn out to be the problem, he said. “Goggles actually may make things worse.”
The project had no industry funding, and Dr. Breen had no relevant disclosures.
SOURCE: Breen MT et al. 2018 AAGL Global Congress, Abstract 16.
LAS VEGAS – There hasn’t been a single corneal abrasion in 860 cases of gynecologic robotic surgery at the University of Texas, Austin, since surgeons and anesthesiologists there started sealing women’s eyes shut with a thick layer of ointment and Tegaderm, instead of the usual small squeeze of ointment and tape, according to Michael T. Breen, MD, a gynecologic surgeon at the university.
“Go back to your hospital, meet with your anesthesiologists, and see what you’re doing to protect your patients’ eyes,” Dr. Breen said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Slathered eyes and Tegaderm are now standard practice at the university. Before the switch was made, there were six corneal abrasions in 231 cases over 6 months. Two of those patients stayed longer in the hospital than they would have otherwise. The changes have eliminated the problem.
The impetus for the switch was a 42-year-old woman who had a robotic hysterectomy. The surgery went fine, but then Dr. Breen had to rush back to the recovery room. The woman was screaming in pain, not from her surgery, but from her left eye.
Corneal abrasions are a well-known risk of surgery because anesthesia decreases tear production and dries the eyes. Robotic gynecologic surgery increases the risk even more, because patients are under longer than with other approaches, and the steep Trendelenburg increases intraocular pressure and eye edema, especially with excess IV fluid.
And “believe it or not, having a pulse oximeter on the dominant hand [also] increases your risk of ocular injury,” Dr. Breen said. Sometimes, patients wake up, go to rub their eyes, and drag the device across their cornea, he said.
The screaming patient – who recovered without permanent damage – prompted Dr. Breen and his colleagues to turn to the literature for solutions. “One was a fully occlusive eye dressing, more than the tape we’ve all been accustomed to, with thick eye ointment application and Tegaderm applying positive pressure to the eye,” he said.
Dr. Breen showed his audience a slide of the setup. “It looks a little unorthodox, but this is how every one of our robotic patients now have their eyes protected. Thick gel which is then covered with a positive pressure Tegaderm,” he said.
, and placing the pulse ox on the nondominant hand. The team already had been decreasing IV fluids as part of their enhanced recovery after surgery protocol, and bringing patients out of steep Trendelenburg as soon as possible.
With the changes, “the rate of corneal abrasions decreased from 2.6% to 0% – and has stayed there,” Dr. Breen said.
There’ve been no allergic reactions to Tegaderm and no eyelid problems. “What we have seen with the simple taping is that, when it comes off, so does some of the eyelid, particularly with geriatric patients. We have not seen that with Tegaderm,” he said.
“Some people use goggles to protect the eyes, and we thought initially that the camera was hitting the face, so we used the Mayo stand to protect it from the camera,” but that didn’t turn out to be the problem, he said. “Goggles actually may make things worse.”
The project had no industry funding, and Dr. Breen had no relevant disclosures.
SOURCE: Breen MT et al. 2018 AAGL Global Congress, Abstract 16.
LAS VEGAS – There hasn’t been a single corneal abrasion in 860 cases of gynecologic robotic surgery at the University of Texas, Austin, since surgeons and anesthesiologists there started sealing women’s eyes shut with a thick layer of ointment and Tegaderm, instead of the usual small squeeze of ointment and tape, according to Michael T. Breen, MD, a gynecologic surgeon at the university.
“Go back to your hospital, meet with your anesthesiologists, and see what you’re doing to protect your patients’ eyes,” Dr. Breen said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Slathered eyes and Tegaderm are now standard practice at the university. Before the switch was made, there were six corneal abrasions in 231 cases over 6 months. Two of those patients stayed longer in the hospital than they would have otherwise. The changes have eliminated the problem.
The impetus for the switch was a 42-year-old woman who had a robotic hysterectomy. The surgery went fine, but then Dr. Breen had to rush back to the recovery room. The woman was screaming in pain, not from her surgery, but from her left eye.
Corneal abrasions are a well-known risk of surgery because anesthesia decreases tear production and dries the eyes. Robotic gynecologic surgery increases the risk even more, because patients are under longer than with other approaches, and the steep Trendelenburg increases intraocular pressure and eye edema, especially with excess IV fluid.
And “believe it or not, having a pulse oximeter on the dominant hand [also] increases your risk of ocular injury,” Dr. Breen said. Sometimes, patients wake up, go to rub their eyes, and drag the device across their cornea, he said.
The screaming patient – who recovered without permanent damage – prompted Dr. Breen and his colleagues to turn to the literature for solutions. “One was a fully occlusive eye dressing, more than the tape we’ve all been accustomed to, with thick eye ointment application and Tegaderm applying positive pressure to the eye,” he said.
Dr. Breen showed his audience a slide of the setup. “It looks a little unorthodox, but this is how every one of our robotic patients now have their eyes protected. Thick gel which is then covered with a positive pressure Tegaderm,” he said.
, and placing the pulse ox on the nondominant hand. The team already had been decreasing IV fluids as part of their enhanced recovery after surgery protocol, and bringing patients out of steep Trendelenburg as soon as possible.
With the changes, “the rate of corneal abrasions decreased from 2.6% to 0% – and has stayed there,” Dr. Breen said.
There’ve been no allergic reactions to Tegaderm and no eyelid problems. “What we have seen with the simple taping is that, when it comes off, so does some of the eyelid, particularly with geriatric patients. We have not seen that with Tegaderm,” he said.
“Some people use goggles to protect the eyes, and we thought initially that the camera was hitting the face, so we used the Mayo stand to protect it from the camera,” but that didn’t turn out to be the problem, he said. “Goggles actually may make things worse.”
The project had no industry funding, and Dr. Breen had no relevant disclosures.
SOURCE: Breen MT et al. 2018 AAGL Global Congress, Abstract 16.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: “The rate of corneal abrasions decreased from 2.6% to 0% – and has stayed there.”
Major finding: Of the 860 cases of gynecologic robotic surgery at the University of Texas, Austin, there has not been a single case of corneal abrasion since the switch.
Study details: Quality improvement project at the university.
Disclosures: The project had no industry funding, and Dr. Breen had no relevant disclosures.
Source: Breen MT et al. 2018 AAGL Global Congress, Abstract 16.
Careful follow-up is key in lupus psychosis
Psychosis, a rare manifestation of systemic lupus erythematosus (SLE), generally appears early in the disease course. But treatment can lead to good outcomes for most patients with careful follow-up, according to a large international study.
The findings “confirm and expand upon the results of previous cross-sectional and historical studies of psychosis in SLE,” John G. Hanly, MD, and his associates wrote in Arthritis & Rheumatology.
The prospective study involved 1,826 patients enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network who were seen at 31 centers across 10 countries, including the United States and Canada.
Patients were followed for an average of 7.4 years, the majority were women (88.8%), almost half the cohort were white (48.8%), and the mean age was 35.1 years, reported Dr. Hanly, of Dalhousie University, Halifax, N.S., and his associates.
The researchers used the American College of Rheumatology definition of psychosis: delusions or hallucinations without insight; (ii) causing clinical distress or impairment in social, occupational or other relevant areas of functioning; (iii) disturbance should not occur exclusively during delirium; (iv) not better accounted for by another mental disorder.
During study follow-up, 28 patients experienced 31 psychotic events (1.53%); 26 of these patients had one event, one patient had two, and one had three events, Dr. Hanly and his associates said.
Using two attribution models, the investigators found that most psychotic events were directly attributed to SLE, and (80%) had their first episode either in the year prior to or within 3 years following a diagnosis of SLE.
The factors positively associated with psychosis on multivariate analysis were African ancestry (hazard ratio, 4.59; 95% confidence interval, 1.79-11.76), previous SLE neuropsychiatric events (HR, 3.59; 95% CI, 1.16-11.14), male sex (HR 3, 95% CI, 1.20-7.50), and younger age at the time of SLE diagnosis (per 10 years, HR, 1.45; 95% CI. 1.01-2.07).
More than 80% of the psychotic events had resolved by the second annual assessment after onset of the event, the investigators reported.
In terms of impact on quality of life, all subscales of the 36-Item Short-Form Health Survey (SF-36) were negatively affected in patients with lupus psychosis.
However, the investigators said.
“Psychosis is an infrequent manifestation of [neuropsychiatric SLE]. Generally, it occurs early after SLE onset and has a significant negative impact on health status,” they concluded. “As determined by patient and physician report, the short- and long-term outlook is good for most patients, though careful follow-up is required.”
Dr. Hanly disclosed grant support from the Canadian Institutes of Health. His associates reported financial support from several entities, including the Basque Government, the Danish Rheumatism Association, the Hopkins Lupus Cohort, and the National Institute for Health Research/Wellcome Trust Birmingham Clinical Research Facility.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2018. doi: 10.1002/art.40764.
Psychosis, a rare manifestation of systemic lupus erythematosus (SLE), generally appears early in the disease course. But treatment can lead to good outcomes for most patients with careful follow-up, according to a large international study.
The findings “confirm and expand upon the results of previous cross-sectional and historical studies of psychosis in SLE,” John G. Hanly, MD, and his associates wrote in Arthritis & Rheumatology.
The prospective study involved 1,826 patients enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network who were seen at 31 centers across 10 countries, including the United States and Canada.
Patients were followed for an average of 7.4 years, the majority were women (88.8%), almost half the cohort were white (48.8%), and the mean age was 35.1 years, reported Dr. Hanly, of Dalhousie University, Halifax, N.S., and his associates.
The researchers used the American College of Rheumatology definition of psychosis: delusions or hallucinations without insight; (ii) causing clinical distress or impairment in social, occupational or other relevant areas of functioning; (iii) disturbance should not occur exclusively during delirium; (iv) not better accounted for by another mental disorder.
During study follow-up, 28 patients experienced 31 psychotic events (1.53%); 26 of these patients had one event, one patient had two, and one had three events, Dr. Hanly and his associates said.
Using two attribution models, the investigators found that most psychotic events were directly attributed to SLE, and (80%) had their first episode either in the year prior to or within 3 years following a diagnosis of SLE.
The factors positively associated with psychosis on multivariate analysis were African ancestry (hazard ratio, 4.59; 95% confidence interval, 1.79-11.76), previous SLE neuropsychiatric events (HR, 3.59; 95% CI, 1.16-11.14), male sex (HR 3, 95% CI, 1.20-7.50), and younger age at the time of SLE diagnosis (per 10 years, HR, 1.45; 95% CI. 1.01-2.07).
More than 80% of the psychotic events had resolved by the second annual assessment after onset of the event, the investigators reported.
In terms of impact on quality of life, all subscales of the 36-Item Short-Form Health Survey (SF-36) were negatively affected in patients with lupus psychosis.
However, the investigators said.
“Psychosis is an infrequent manifestation of [neuropsychiatric SLE]. Generally, it occurs early after SLE onset and has a significant negative impact on health status,” they concluded. “As determined by patient and physician report, the short- and long-term outlook is good for most patients, though careful follow-up is required.”
Dr. Hanly disclosed grant support from the Canadian Institutes of Health. His associates reported financial support from several entities, including the Basque Government, the Danish Rheumatism Association, the Hopkins Lupus Cohort, and the National Institute for Health Research/Wellcome Trust Birmingham Clinical Research Facility.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2018. doi: 10.1002/art.40764.
Psychosis, a rare manifestation of systemic lupus erythematosus (SLE), generally appears early in the disease course. But treatment can lead to good outcomes for most patients with careful follow-up, according to a large international study.
The findings “confirm and expand upon the results of previous cross-sectional and historical studies of psychosis in SLE,” John G. Hanly, MD, and his associates wrote in Arthritis & Rheumatology.
The prospective study involved 1,826 patients enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network who were seen at 31 centers across 10 countries, including the United States and Canada.
Patients were followed for an average of 7.4 years, the majority were women (88.8%), almost half the cohort were white (48.8%), and the mean age was 35.1 years, reported Dr. Hanly, of Dalhousie University, Halifax, N.S., and his associates.
The researchers used the American College of Rheumatology definition of psychosis: delusions or hallucinations without insight; (ii) causing clinical distress or impairment in social, occupational or other relevant areas of functioning; (iii) disturbance should not occur exclusively during delirium; (iv) not better accounted for by another mental disorder.
During study follow-up, 28 patients experienced 31 psychotic events (1.53%); 26 of these patients had one event, one patient had two, and one had three events, Dr. Hanly and his associates said.
Using two attribution models, the investigators found that most psychotic events were directly attributed to SLE, and (80%) had their first episode either in the year prior to or within 3 years following a diagnosis of SLE.
The factors positively associated with psychosis on multivariate analysis were African ancestry (hazard ratio, 4.59; 95% confidence interval, 1.79-11.76), previous SLE neuropsychiatric events (HR, 3.59; 95% CI, 1.16-11.14), male sex (HR 3, 95% CI, 1.20-7.50), and younger age at the time of SLE diagnosis (per 10 years, HR, 1.45; 95% CI. 1.01-2.07).
More than 80% of the psychotic events had resolved by the second annual assessment after onset of the event, the investigators reported.
In terms of impact on quality of life, all subscales of the 36-Item Short-Form Health Survey (SF-36) were negatively affected in patients with lupus psychosis.
However, the investigators said.
“Psychosis is an infrequent manifestation of [neuropsychiatric SLE]. Generally, it occurs early after SLE onset and has a significant negative impact on health status,” they concluded. “As determined by patient and physician report, the short- and long-term outlook is good for most patients, though careful follow-up is required.”
Dr. Hanly disclosed grant support from the Canadian Institutes of Health. His associates reported financial support from several entities, including the Basque Government, the Danish Rheumatism Association, the Hopkins Lupus Cohort, and the National Institute for Health Research/Wellcome Trust Birmingham Clinical Research Facility.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2018. doi: 10.1002/art.40764.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Psychosis, a rare manifestation of systemic lupus erythematosus, is treatable with good short- and long-term outcomes.
Major finding: Twenty-eight patients experienced 31 psychotic events (1.53%); 80% had their first episode either in the year prior to or within 3 years of an SLE diagnosis.
Study details: Prospective study involving 1,826 patients from the Systemic Lupus International Collaborating Clinics network seen at 31 centers across 10 countries.
Disclosures: Dr. Hanly disclosed grant support from the Canadian Institutes of Health. His associates reported financial support from several entities, including the Basque Government, the Danish Rheumatism Association, the Hopkins Lupus Cohort, and the National Institute for Health Research/Wellcome Trust Birmingham Clinical Research Facility.Source: Hanly JG et al. Arthritis Rheumatol. 2018. doi: 10.1002/art.40764.
Use CARE MD protocol to treat somatic disorders
SAN DIEGO – Patients with somatic symptom and related disorders can get better in primary care when clinicians use a few key strategies, according to an expert.
When it comes to these disorders, “a lot of our anxiety and gallows humor comes from a place of not knowing what we want to do, how we can treat these individuals,” said Matthew Reed, MD, MPH, a psychiatrist and pain specialist at the University of California, Irvine. “It became more appealing once I learned a little bit more about what was going on with some of these disorders and how I might be able to intervene.”
Dr. Reed spoke at Pain Care for Primary Care, held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Throughout his presentation, Dr. Reed drew upon the listing of somatic symptom and related disorders as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Among the disorders he discussed:
Somatic symptom disorder
This disorder causes significant distress because of at least one somatic symptom and is persistent (lasting over 6 months). A medical illness might be present, and depression and anxiety are common.
“This diagnosis says you have pathology – this somatic disorder – which is making your life miserable,” Dr. Reed said.
He’s often thrilled when patients also have comorbid depression and/or anxiety. “You treat that primary issue, and the somatic disorder melts away,” he said. “It’s harder when you have the pure version of somatic disorder, and there is no anxiety or depressive disorder.”
Illness anxiety disorder (formerly known as hypochondriasis)
Patients with this disorder have been preoccupied about having a serious illness for at least 6 months but do not have somatic symptoms. Reassurance typically is not effective, Dr. Reed said. In response to a statement such as “nothing’s wrong,” he said, patients might reply with a statement along the lines of “something’s wrong because I’m suffering.”
Patients with this condition can command “high rates of medical utilization, often pretty inappropriate,” especially if they are VIPs, he said.
Conversion disorder (also known as functional neurological symptom disorder)
This is less common than the other disorders. Patients with this condition develop “one or more symptoms of altered voluntary motor or sensory function” that’s “not better explained by another medical condition.”
A “listening ear” and physical therapy can prove helpful, Dr. Reed said.
Factitious disorder
Patients with this condition falsify or induce signs of illness, impairment, or injury. “There’s more of an overt feigning of symptoms in order to maintain that sick role,” said Dr. Reed, who cautioned that some people with this condition actually might be suffering.
Protocol can guide treatment
How can these conditions be treated in patients? Dr. Reed pointed to the protocol discussed by Robert McCarron, DO, and embedded in the mnemonic “CARE MD”: Cognitive-behavioral therapy (CBT)/consultation, regular visits, empathy, med-psych interface, and do no harm.
“Reassure them that you value them,” he said. Under CARE MD, the idea of multiple visits is to help the patient develop coping strategies and stop overusing medical care.
Return visits should not be too frequent, Dr. Reed said, and they should be short. Physicians must remember to take care of themselves and other patients, he said, and not spend too much time with these patients. “We need to be compassionate,” he said, but “we don’t need to be compassionate in a way that we don’t have our sanity after clinic.”
As for CBT, Dr. Reed likes to suggest it in a way that doesn’t aggravate patients who are sensitive to the idea that their condition is all in their heads.
Physicians, he said, can say: “Wow, this is really affecting your life. You have 17 specialists working on you. You’ll continue to see them, I know. But I worry. I look at your chart, and we’re missing a whole area of treatment.”
He then mentions CBT. “Other providers may have told you about it,” he’ll say. “I’ve seen such good benefits with CBT, even with patients who were in motor vehicle accidents. It doesn’t matter where it’s coming from. This CBT seems to work.”
Ideally, he said, patients agree to try it.
Dr. Reed had no disclosures.
SAN DIEGO – Patients with somatic symptom and related disorders can get better in primary care when clinicians use a few key strategies, according to an expert.
When it comes to these disorders, “a lot of our anxiety and gallows humor comes from a place of not knowing what we want to do, how we can treat these individuals,” said Matthew Reed, MD, MPH, a psychiatrist and pain specialist at the University of California, Irvine. “It became more appealing once I learned a little bit more about what was going on with some of these disorders and how I might be able to intervene.”
Dr. Reed spoke at Pain Care for Primary Care, held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Throughout his presentation, Dr. Reed drew upon the listing of somatic symptom and related disorders as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Among the disorders he discussed:
Somatic symptom disorder
This disorder causes significant distress because of at least one somatic symptom and is persistent (lasting over 6 months). A medical illness might be present, and depression and anxiety are common.
“This diagnosis says you have pathology – this somatic disorder – which is making your life miserable,” Dr. Reed said.
He’s often thrilled when patients also have comorbid depression and/or anxiety. “You treat that primary issue, and the somatic disorder melts away,” he said. “It’s harder when you have the pure version of somatic disorder, and there is no anxiety or depressive disorder.”
Illness anxiety disorder (formerly known as hypochondriasis)
Patients with this disorder have been preoccupied about having a serious illness for at least 6 months but do not have somatic symptoms. Reassurance typically is not effective, Dr. Reed said. In response to a statement such as “nothing’s wrong,” he said, patients might reply with a statement along the lines of “something’s wrong because I’m suffering.”
Patients with this condition can command “high rates of medical utilization, often pretty inappropriate,” especially if they are VIPs, he said.
Conversion disorder (also known as functional neurological symptom disorder)
This is less common than the other disorders. Patients with this condition develop “one or more symptoms of altered voluntary motor or sensory function” that’s “not better explained by another medical condition.”
A “listening ear” and physical therapy can prove helpful, Dr. Reed said.
Factitious disorder
Patients with this condition falsify or induce signs of illness, impairment, or injury. “There’s more of an overt feigning of symptoms in order to maintain that sick role,” said Dr. Reed, who cautioned that some people with this condition actually might be suffering.
Protocol can guide treatment
How can these conditions be treated in patients? Dr. Reed pointed to the protocol discussed by Robert McCarron, DO, and embedded in the mnemonic “CARE MD”: Cognitive-behavioral therapy (CBT)/consultation, regular visits, empathy, med-psych interface, and do no harm.
“Reassure them that you value them,” he said. Under CARE MD, the idea of multiple visits is to help the patient develop coping strategies and stop overusing medical care.
Return visits should not be too frequent, Dr. Reed said, and they should be short. Physicians must remember to take care of themselves and other patients, he said, and not spend too much time with these patients. “We need to be compassionate,” he said, but “we don’t need to be compassionate in a way that we don’t have our sanity after clinic.”
As for CBT, Dr. Reed likes to suggest it in a way that doesn’t aggravate patients who are sensitive to the idea that their condition is all in their heads.
Physicians, he said, can say: “Wow, this is really affecting your life. You have 17 specialists working on you. You’ll continue to see them, I know. But I worry. I look at your chart, and we’re missing a whole area of treatment.”
He then mentions CBT. “Other providers may have told you about it,” he’ll say. “I’ve seen such good benefits with CBT, even with patients who were in motor vehicle accidents. It doesn’t matter where it’s coming from. This CBT seems to work.”
Ideally, he said, patients agree to try it.
Dr. Reed had no disclosures.
SAN DIEGO – Patients with somatic symptom and related disorders can get better in primary care when clinicians use a few key strategies, according to an expert.
When it comes to these disorders, “a lot of our anxiety and gallows humor comes from a place of not knowing what we want to do, how we can treat these individuals,” said Matthew Reed, MD, MPH, a psychiatrist and pain specialist at the University of California, Irvine. “It became more appealing once I learned a little bit more about what was going on with some of these disorders and how I might be able to intervene.”
Dr. Reed spoke at Pain Care for Primary Care, held by the American Pain Society and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Throughout his presentation, Dr. Reed drew upon the listing of somatic symptom and related disorders as outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Among the disorders he discussed:
Somatic symptom disorder
This disorder causes significant distress because of at least one somatic symptom and is persistent (lasting over 6 months). A medical illness might be present, and depression and anxiety are common.
“This diagnosis says you have pathology – this somatic disorder – which is making your life miserable,” Dr. Reed said.
He’s often thrilled when patients also have comorbid depression and/or anxiety. “You treat that primary issue, and the somatic disorder melts away,” he said. “It’s harder when you have the pure version of somatic disorder, and there is no anxiety or depressive disorder.”
Illness anxiety disorder (formerly known as hypochondriasis)
Patients with this disorder have been preoccupied about having a serious illness for at least 6 months but do not have somatic symptoms. Reassurance typically is not effective, Dr. Reed said. In response to a statement such as “nothing’s wrong,” he said, patients might reply with a statement along the lines of “something’s wrong because I’m suffering.”
Patients with this condition can command “high rates of medical utilization, often pretty inappropriate,” especially if they are VIPs, he said.
Conversion disorder (also known as functional neurological symptom disorder)
This is less common than the other disorders. Patients with this condition develop “one or more symptoms of altered voluntary motor or sensory function” that’s “not better explained by another medical condition.”
A “listening ear” and physical therapy can prove helpful, Dr. Reed said.
Factitious disorder
Patients with this condition falsify or induce signs of illness, impairment, or injury. “There’s more of an overt feigning of symptoms in order to maintain that sick role,” said Dr. Reed, who cautioned that some people with this condition actually might be suffering.
Protocol can guide treatment
How can these conditions be treated in patients? Dr. Reed pointed to the protocol discussed by Robert McCarron, DO, and embedded in the mnemonic “CARE MD”: Cognitive-behavioral therapy (CBT)/consultation, regular visits, empathy, med-psych interface, and do no harm.
“Reassure them that you value them,” he said. Under CARE MD, the idea of multiple visits is to help the patient develop coping strategies and stop overusing medical care.
Return visits should not be too frequent, Dr. Reed said, and they should be short. Physicians must remember to take care of themselves and other patients, he said, and not spend too much time with these patients. “We need to be compassionate,” he said, but “we don’t need to be compassionate in a way that we don’t have our sanity after clinic.”
As for CBT, Dr. Reed likes to suggest it in a way that doesn’t aggravate patients who are sensitive to the idea that their condition is all in their heads.
Physicians, he said, can say: “Wow, this is really affecting your life. You have 17 specialists working on you. You’ll continue to see them, I know. But I worry. I look at your chart, and we’re missing a whole area of treatment.”
He then mentions CBT. “Other providers may have told you about it,” he’ll say. “I’ve seen such good benefits with CBT, even with patients who were in motor vehicle accidents. It doesn’t matter where it’s coming from. This CBT seems to work.”
Ideally, he said, patients agree to try it.
Dr. Reed had no disclosures.
REPORTING FROM PAIN CARE FOR PRIMARY CARE
Smaller assistant ports mean less prolapse repair pain
LAS VEGAS – Patients experience less right-sided pain after laparoscopic sacrocolpopexy when surgeons use an 8-mm assistant port instead of a 12-mm port, results from a small trial show.
In the trial, conducted at Loyola University Medical Center in Maywood, Ill.,17 women were randomized to undergo the procedure with an 8-mm assistant port, and 18 with a 12-mm port, both on the right side of the abdomen.
Overall, pain severity was low at 2 weeks postop in both groups at just over 1 point on a 10-point visual analogue scale and not statistically different. However, patients who had a 12-mm assistant port were more likely to report right-sided pain, compared with those who had an 8-mm assistant port (60% vs.18%, P = 0.027).
“I saw a lot of these patients in the clinic at 2 weeks, and even though the overall pain score was low, they kept complaining about a dull, achy pain on the right side. They were using ibuprofen, and some of them had even restricted [their activities] because they were afraid they were going to pop a stitch,” said study lead Yufan B. Chen, MD, a urogynecology fellow at Loyola.
He said the research team thinks the difference in port-site pain “is clinically significant. . Since our study ended, we have stopped using the 12-mm port in most of our cases; we use the 8-mm port for the assistant,” he said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The tradeoff was that there were more needle struggles with the 8-mm port, 3.1/case vs. 0.6/case (P = .004). Surgeons found inserting and withdrawing the Gore-Tex needle through the smaller port more difficult. But there were no needle losses in either group and no differences in operative time – an average of about 95 minutes.
“The larger port size essentially benefits the surgeon more than the patient. This is kind of a common theme in minimally invasive surgery where less is actually more,” he said. Since the study, “we have identified why we have challenges with the needle transport; it’s usually because the needle gets bent, so we just unbend it a little bit with our needle drivers before we remove it,” Dr. Chen said.
Even so, “there may be a role in using the 12-mm port when you have assistants who are not [that] experienced,” he said.
Laparoscopic sacrocolpopexy is done through four ports at Loyola: a 12-mm umbilical port for the scope; two 5-mm ports on the left for the primary surgeon; and the right side port for the assistant, through which the Gore-Tex needle is passed.
In the study, there were no demographic or preop differences between the two groups of women. About 60% were white, with a mean age of 61 years. About 10% of the women had had prior abdominal surgery.
The study received no industry funding, and the investigators had no disclosures.
SOURCE: Chen YB et al. 2018 AAGL Global Congress, Abstract 197.
LAS VEGAS – Patients experience less right-sided pain after laparoscopic sacrocolpopexy when surgeons use an 8-mm assistant port instead of a 12-mm port, results from a small trial show.
In the trial, conducted at Loyola University Medical Center in Maywood, Ill.,17 women were randomized to undergo the procedure with an 8-mm assistant port, and 18 with a 12-mm port, both on the right side of the abdomen.
Overall, pain severity was low at 2 weeks postop in both groups at just over 1 point on a 10-point visual analogue scale and not statistically different. However, patients who had a 12-mm assistant port were more likely to report right-sided pain, compared with those who had an 8-mm assistant port (60% vs.18%, P = 0.027).
“I saw a lot of these patients in the clinic at 2 weeks, and even though the overall pain score was low, they kept complaining about a dull, achy pain on the right side. They were using ibuprofen, and some of them had even restricted [their activities] because they were afraid they were going to pop a stitch,” said study lead Yufan B. Chen, MD, a urogynecology fellow at Loyola.
He said the research team thinks the difference in port-site pain “is clinically significant. . Since our study ended, we have stopped using the 12-mm port in most of our cases; we use the 8-mm port for the assistant,” he said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The tradeoff was that there were more needle struggles with the 8-mm port, 3.1/case vs. 0.6/case (P = .004). Surgeons found inserting and withdrawing the Gore-Tex needle through the smaller port more difficult. But there were no needle losses in either group and no differences in operative time – an average of about 95 minutes.
“The larger port size essentially benefits the surgeon more than the patient. This is kind of a common theme in minimally invasive surgery where less is actually more,” he said. Since the study, “we have identified why we have challenges with the needle transport; it’s usually because the needle gets bent, so we just unbend it a little bit with our needle drivers before we remove it,” Dr. Chen said.
Even so, “there may be a role in using the 12-mm port when you have assistants who are not [that] experienced,” he said.
Laparoscopic sacrocolpopexy is done through four ports at Loyola: a 12-mm umbilical port for the scope; two 5-mm ports on the left for the primary surgeon; and the right side port for the assistant, through which the Gore-Tex needle is passed.
In the study, there were no demographic or preop differences between the two groups of women. About 60% were white, with a mean age of 61 years. About 10% of the women had had prior abdominal surgery.
The study received no industry funding, and the investigators had no disclosures.
SOURCE: Chen YB et al. 2018 AAGL Global Congress, Abstract 197.
LAS VEGAS – Patients experience less right-sided pain after laparoscopic sacrocolpopexy when surgeons use an 8-mm assistant port instead of a 12-mm port, results from a small trial show.
In the trial, conducted at Loyola University Medical Center in Maywood, Ill.,17 women were randomized to undergo the procedure with an 8-mm assistant port, and 18 with a 12-mm port, both on the right side of the abdomen.
Overall, pain severity was low at 2 weeks postop in both groups at just over 1 point on a 10-point visual analogue scale and not statistically different. However, patients who had a 12-mm assistant port were more likely to report right-sided pain, compared with those who had an 8-mm assistant port (60% vs.18%, P = 0.027).
“I saw a lot of these patients in the clinic at 2 weeks, and even though the overall pain score was low, they kept complaining about a dull, achy pain on the right side. They were using ibuprofen, and some of them had even restricted [their activities] because they were afraid they were going to pop a stitch,” said study lead Yufan B. Chen, MD, a urogynecology fellow at Loyola.
He said the research team thinks the difference in port-site pain “is clinically significant. . Since our study ended, we have stopped using the 12-mm port in most of our cases; we use the 8-mm port for the assistant,” he said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The tradeoff was that there were more needle struggles with the 8-mm port, 3.1/case vs. 0.6/case (P = .004). Surgeons found inserting and withdrawing the Gore-Tex needle through the smaller port more difficult. But there were no needle losses in either group and no differences in operative time – an average of about 95 minutes.
“The larger port size essentially benefits the surgeon more than the patient. This is kind of a common theme in minimally invasive surgery where less is actually more,” he said. Since the study, “we have identified why we have challenges with the needle transport; it’s usually because the needle gets bent, so we just unbend it a little bit with our needle drivers before we remove it,” Dr. Chen said.
Even so, “there may be a role in using the 12-mm port when you have assistants who are not [that] experienced,” he said.
Laparoscopic sacrocolpopexy is done through four ports at Loyola: a 12-mm umbilical port for the scope; two 5-mm ports on the left for the primary surgeon; and the right side port for the assistant, through which the Gore-Tex needle is passed.
In the study, there were no demographic or preop differences between the two groups of women. About 60% were white, with a mean age of 61 years. About 10% of the women had had prior abdominal surgery.
The study received no industry funding, and the investigators had no disclosures.
SOURCE: Chen YB et al. 2018 AAGL Global Congress, Abstract 197.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Patients experienced less pain at 2 weeks with a smaller port; there are also more needle struggles, but that can be easily fixed.
Major finding: Patients who had a 12-mm assistant port were more likely to report right-sided pain, compared with those who received an 8- mm assistant port (60% vs. 18%, P = 0.027).
Study details: Randomized trial with 35 women.
Disclosures: The study received no industry funding, and the investigators had no disclosures.
Source: Chen YB et al. 2018 AAGL Global Congress, Abstract 197.
Try to normalize albumin before laparoscopic hysterectomy
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Antibiotics backed as standard of care for myomectomies
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
LAS VEGAS – The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not, in a review of 1,433 cases at Massachusetts General Hospital and Brigham and Women’s Hospital, Boston.
That is despite the fact that antibiotic cases were longer – 155 minutes vs. 89 minutes – and had more blood loss, 200 ml vs. 117 ml. Antibiotic cases also had larger specimen weights – 346 g vs. 176 g – and were more likely to have the uterine cavity entered, 30.2% vs. 14.4%.
“Surgical site infections were more common in the no-antibiotics group despite these being less complex cases.” There was “nearly a fivefold increased odds of surgical site infection or any infectious complication when no antibiotics were given,” after controlling for infection risk factors, including smoking and diabetes, said investigator Nisse V. Clark, MD, a minimally invasive gynecologic surgeon affiliated with Massachusetts General Hospital.
There are no perioperative antibiotic guidelines for myomectomies; maybe there should be. Almost 94% of the women in the review did receive antibiotics at the Harvard-affiliated hospitals, but the nationwide average has been pegged at about two-thirds, she said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
The antibiotic cases usually received a cephalosporin before surgery, and were about evenly about evenly split between abdominal, robotic, and laparoscopic approaches.
About one-third of the 90 women (6.3%) who did not get antibiotics had hysteroscopic procedures in which antibiotics usually are not given because the peritoneal cavity is not breeched. Most of the rest, however, were laparoscopic cases. It’s unknown why they weren’t given antibiotics. In her own practice, Dr. Clark said preop antibiotics are the rule for laparoscopic myomectomies.
The surgical site infection difference was driven largely by higher incidences of pelvic abscesses and other organ space infections in the no-antibiotic group.
The only significant demographic difference between the two groups was that women who received antibiotics were slightly younger (mean 38 versus 39.7 years).
In addition to diabetes and smoking, the team adjusted for age, surgery route, body mass index, uterine entry, intraoperative complications, and myoma weight in their multivariate analysis. Still, women in the no-antibiotic group were 4.59 times more likely to have a surgical site infection, 4.76 more likely to have any infectious complication, and almost 8 times more likely to have a major infectious complication. All of the findings were statistically significant.
The study had no industry funding, and Dr. Clark had no disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: A Boston study suggests that even low-risk cases benefit from antibiotics.
Major finding: The surgical site infection rate was 2.9% among women who received perioperative antibiotics for fibroid surgery, but 7.8% among those who did not.
Study details: Review of 1,433 myomectomies at two academic medical centers.
Disclosures: The study had no industry funding, and Dr. Clark had no disclosures.
CHMP backs blinatumomab for MRD
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding marketing authorization for blinatumomab (Blincyto) to include treatment of minimal residual disease (MRD).
The CHMP has recommended approval for blinatumomab as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission with MRD greater than or equal to 0.1%.
The CHMP had originally adopted a negative opinion on extending the use of blinatumomab to these patients. However, the committee re-examined its opinion and reversed that decision.
The CHMP has requested that Amgen, the company developing blinatumomab, provide results from ongoing studies to support the new approval.
The CHMP’s recommendations are reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of CHMP recommendations.
Blinatumomab is already EC-approved as monotherapy for:
- Adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
- Pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
CHMP’s reversal of opinion
In July, the CHMP recommended against approving blinatumomab to treat patients with MRD based on data from the BLAST trial. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab produced MRD negativity in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP complied.
During the re-examination, the CHMP reviewed all the data and consulted a group of experts.
The experts concluded, and the CHMP agreed, that, although there is no strong evidence of improved survival, the available data indicate a good response to blinatumomab, with around 78% of patients becoming MRD-negative after treatment.
The CHMP also noted that MRD-positive patients have a high risk of relapse and few treatment options.
Therefore, the committee concluded that the benefits of blinatumomab outweigh its risks in this patient population.
The CHMP recommended granting the change to the marketing authorization but also requested that Amgen provide data from ongoing studies of blinatumomab in MRD-positive patients, once those data are available.