User login
FDA urges caution with robotic devices in cancer surgery
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
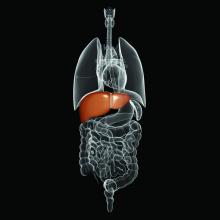
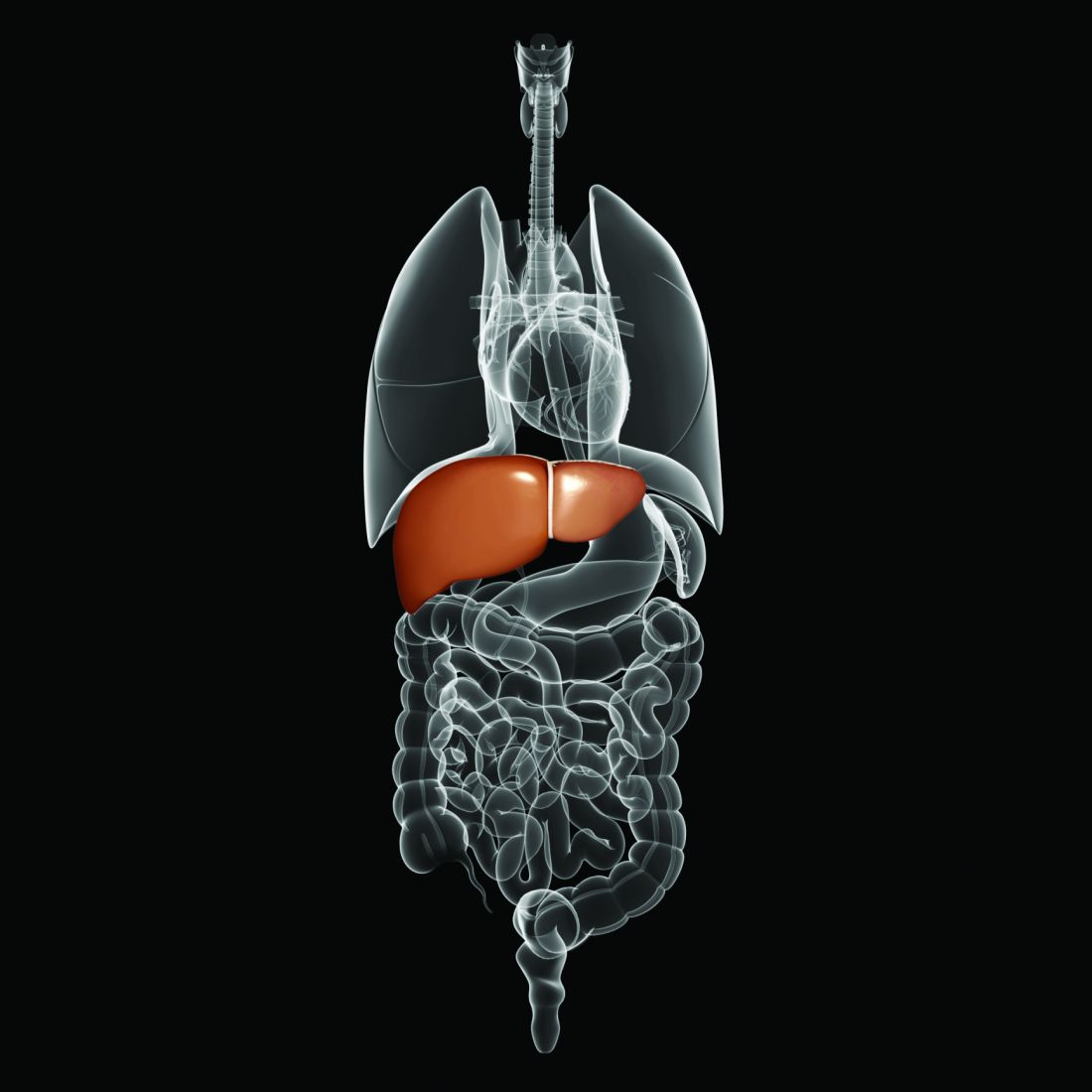
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
Barancik award winner probes role of fibrinogen in MS
DALLAS – The National Multiple Sclerosis society has awarded the 2018 Barancik Prize for Innovation in Multiple Sclerosis Research to Katerina Akassoglou, PhD.
Dr. Akassoglou laid out the course of research into the role of fibrinogen in multiple sclerosis and other neurodegenerative disorders during her talk at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Akassoglou and her collaborators questioned the received wisdom about what happens when the brain’s vasculature is disrupted, she said in a video interview. “Fibrinogen was studied for several decades as only a marker of blood-brain barrier disruption. We asked the question, ‘Is it possible that it’s not only a marker, but it also plays a role in disease pathogenesis?’ ”
Over the years, said Dr. Akassoglou, she and her collaborators have developed tools “to dissect this chicken-and-egg question.” A key answer came when the researchers found that when fibrinogen leaks through the vasculature into brain tissue, it can promote inflammatory processes. “At the same time, it induced pathogenic responses in brain immune cells, causing them to be toxic to neuronal cells.”
“Using genetic models of depleting fibrinogen, or blocking its interactions with immune receptors, provided compelling data that this could be an important target for neurodegeneration and neuroinflammation,” said Dr. Akassoglou, professor of neurology at the University of California, San Francisco.
The problem came in trying to find a way to block or deplete fibrinogen inside central nervous system tissue without inactivating it throughout the circulation, since it’s essential for hemostasis.
A promising answer to this problem lies in an investigational monoclonal antibody that binds selectively to sites on fibrinogen that are activated in the brain but are not involved with fibrinogen’s usual role in the clotting cascade in the peripheral circulation, she said.
DALLAS – The National Multiple Sclerosis society has awarded the 2018 Barancik Prize for Innovation in Multiple Sclerosis Research to Katerina Akassoglou, PhD.
Dr. Akassoglou laid out the course of research into the role of fibrinogen in multiple sclerosis and other neurodegenerative disorders during her talk at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Akassoglou and her collaborators questioned the received wisdom about what happens when the brain’s vasculature is disrupted, she said in a video interview. “Fibrinogen was studied for several decades as only a marker of blood-brain barrier disruption. We asked the question, ‘Is it possible that it’s not only a marker, but it also plays a role in disease pathogenesis?’ ”
Over the years, said Dr. Akassoglou, she and her collaborators have developed tools “to dissect this chicken-and-egg question.” A key answer came when the researchers found that when fibrinogen leaks through the vasculature into brain tissue, it can promote inflammatory processes. “At the same time, it induced pathogenic responses in brain immune cells, causing them to be toxic to neuronal cells.”
“Using genetic models of depleting fibrinogen, or blocking its interactions with immune receptors, provided compelling data that this could be an important target for neurodegeneration and neuroinflammation,” said Dr. Akassoglou, professor of neurology at the University of California, San Francisco.
The problem came in trying to find a way to block or deplete fibrinogen inside central nervous system tissue without inactivating it throughout the circulation, since it’s essential for hemostasis.
A promising answer to this problem lies in an investigational monoclonal antibody that binds selectively to sites on fibrinogen that are activated in the brain but are not involved with fibrinogen’s usual role in the clotting cascade in the peripheral circulation, she said.
DALLAS – The National Multiple Sclerosis society has awarded the 2018 Barancik Prize for Innovation in Multiple Sclerosis Research to Katerina Akassoglou, PhD.
Dr. Akassoglou laid out the course of research into the role of fibrinogen in multiple sclerosis and other neurodegenerative disorders during her talk at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Akassoglou and her collaborators questioned the received wisdom about what happens when the brain’s vasculature is disrupted, she said in a video interview. “Fibrinogen was studied for several decades as only a marker of blood-brain barrier disruption. We asked the question, ‘Is it possible that it’s not only a marker, but it also plays a role in disease pathogenesis?’ ”
Over the years, said Dr. Akassoglou, she and her collaborators have developed tools “to dissect this chicken-and-egg question.” A key answer came when the researchers found that when fibrinogen leaks through the vasculature into brain tissue, it can promote inflammatory processes. “At the same time, it induced pathogenic responses in brain immune cells, causing them to be toxic to neuronal cells.”
“Using genetic models of depleting fibrinogen, or blocking its interactions with immune receptors, provided compelling data that this could be an important target for neurodegeneration and neuroinflammation,” said Dr. Akassoglou, professor of neurology at the University of California, San Francisco.
The problem came in trying to find a way to block or deplete fibrinogen inside central nervous system tissue without inactivating it throughout the circulation, since it’s essential for hemostasis.
A promising answer to this problem lies in an investigational monoclonal antibody that binds selectively to sites on fibrinogen that are activated in the brain but are not involved with fibrinogen’s usual role in the clotting cascade in the peripheral circulation, she said.
REPORTING FROM ACTRIMS FORUM 2019
From bedside to bench to bedside: Derisking MS research
DALLAS – Rhonda Voskuhl, MD, delivered the Kenneth P. Johnson Memorial lecture at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. In an hour-long talk, Dr. Voskuhl outlined her research approach, which she terms the “bedside to bench to bedside” strategy.
“My view has been that, when we start out research based on a molecule, we don’t really know for sure its clinical relevance. It’s kind of high risk, because you could go through a lot of work, for many years,” she said in a video interview. When work begins in vitro, the researcher runs the risk of proceeding from test tubes, to animal experiments, and then to people, “and then ultimately finding out that it’s not relevant,” said Dr. Voskuhl, director of the multiple sclerosis (MS) program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
“And so I just thought, to derisk this whole thing, I think I want to start at the end and then work backwards,” by choosing a physiologically relevant manifestation of MS, she said. “We know that there’s something there. ... All we have to do is figure it out.”
In iterative fashion, Dr. Voskuhl proceeds from the clinical observation, “which we know is true, and then go to the laboratory bench and figure it out by doing a lot of manipulation and isolation of one thing versus another.”
“When you put this in the context of cell-specific and region-specific gene expression, to find disability-specific treatments,” Dr. Voskuhl said, this targeted approach helps address the fact that MS patients differ so much in their presentations and disease course.
“We know that, for example, the neuronal cells differ, and some of the neurotrophic cells differ from pathway to pathway; furthermore, what’s important is that oligocytes and astrocytes and dendrocytes have been shown to differ from one region of the brain and spinal cord to another. So these clearly would have different gene expression signatures, potentially posing different targets for treatment,” Dr. Voskuhl said.
DALLAS – Rhonda Voskuhl, MD, delivered the Kenneth P. Johnson Memorial lecture at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. In an hour-long talk, Dr. Voskuhl outlined her research approach, which she terms the “bedside to bench to bedside” strategy.
“My view has been that, when we start out research based on a molecule, we don’t really know for sure its clinical relevance. It’s kind of high risk, because you could go through a lot of work, for many years,” she said in a video interview. When work begins in vitro, the researcher runs the risk of proceeding from test tubes, to animal experiments, and then to people, “and then ultimately finding out that it’s not relevant,” said Dr. Voskuhl, director of the multiple sclerosis (MS) program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
“And so I just thought, to derisk this whole thing, I think I want to start at the end and then work backwards,” by choosing a physiologically relevant manifestation of MS, she said. “We know that there’s something there. ... All we have to do is figure it out.”
In iterative fashion, Dr. Voskuhl proceeds from the clinical observation, “which we know is true, and then go to the laboratory bench and figure it out by doing a lot of manipulation and isolation of one thing versus another.”
“When you put this in the context of cell-specific and region-specific gene expression, to find disability-specific treatments,” Dr. Voskuhl said, this targeted approach helps address the fact that MS patients differ so much in their presentations and disease course.
“We know that, for example, the neuronal cells differ, and some of the neurotrophic cells differ from pathway to pathway; furthermore, what’s important is that oligocytes and astrocytes and dendrocytes have been shown to differ from one region of the brain and spinal cord to another. So these clearly would have different gene expression signatures, potentially posing different targets for treatment,” Dr. Voskuhl said.
DALLAS – Rhonda Voskuhl, MD, delivered the Kenneth P. Johnson Memorial lecture at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. In an hour-long talk, Dr. Voskuhl outlined her research approach, which she terms the “bedside to bench to bedside” strategy.
“My view has been that, when we start out research based on a molecule, we don’t really know for sure its clinical relevance. It’s kind of high risk, because you could go through a lot of work, for many years,” she said in a video interview. When work begins in vitro, the researcher runs the risk of proceeding from test tubes, to animal experiments, and then to people, “and then ultimately finding out that it’s not relevant,” said Dr. Voskuhl, director of the multiple sclerosis (MS) program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
“And so I just thought, to derisk this whole thing, I think I want to start at the end and then work backwards,” by choosing a physiologically relevant manifestation of MS, she said. “We know that there’s something there. ... All we have to do is figure it out.”
In iterative fashion, Dr. Voskuhl proceeds from the clinical observation, “which we know is true, and then go to the laboratory bench and figure it out by doing a lot of manipulation and isolation of one thing versus another.”
“When you put this in the context of cell-specific and region-specific gene expression, to find disability-specific treatments,” Dr. Voskuhl said, this targeted approach helps address the fact that MS patients differ so much in their presentations and disease course.
“We know that, for example, the neuronal cells differ, and some of the neurotrophic cells differ from pathway to pathway; furthermore, what’s important is that oligocytes and astrocytes and dendrocytes have been shown to differ from one region of the brain and spinal cord to another. So these clearly would have different gene expression signatures, potentially posing different targets for treatment,” Dr. Voskuhl said.
REPORTING FROM ACTRIMS FORUM 2019
ASCO issues guideline for early detection, management of colorectal cancer
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
ASCO publishes new guideline for treatment, follow-up of early-stage colorectal cancer
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
What’s the price of rude behavior in the hospital?
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
REPORTING FROM CCC48
March 2019 Highlights
Developing an HCV vaccine faces significant challenges
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
FROM GASTROENTEROLOGY
Possible biomarkers found for progression to liver cancer in chronic HCV infection
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
FROM VIROLOGY
Umbilical cord allograft may boost diabetic foot ulcer healing
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
FROM INTERNATIONAL WOUND JOURNAL